Infection Control Manual
|
|
|
- Maurice Shepherd
- 6 years ago
- Views:
Transcription
1 This plicy has been adpted by UNC Health Care fr its use in infectin cntrl. It is prvided t yu as infrmatin nly. Infectin Cntrl Manual Plicy Name The Preventin f Intravascular Catheter- Related Infectins Plicy Number Date this Versin Effective August 2017 Respnsible fr Cntent Hspital Epidemilgy I. Descriptin Describes the infectin cntrl guidelines t prevent intravenus catheter-related infectins. Table f Cntents I. Descriptin... 1 II. Ratinale... 1 III. Plicy... 1 A. General Recmmendatins fr All Intravascular Catheters... 1 B. Additinal Recmmendatins fr Peripheral Venus Catheters... 7 C. Additinal Recmmendatins fr Central Venus Catheters... 9 D. Management f Parenteral Nutritin (PN) E. Peripheral Arterial Catheters F. Recmmendatins fr Umbilical Catheters G. Special Cnsideratins Dcumentatin H. IV-Related Infectins I. Respnsibility Statement IV. References V. Reviewed/Apprved by VI. Original Plicy Date and Revisins Appendix 1: Quick Reference Timing fr Tubing Changes Appendix 2: Quick Reference: Hang Time Reference fr Parenteral Fluids II. Ratinale Intravascular catheters prvide a rute fr micrrganisms t enter the vascular system bypassing nrmal skin defense mechanisms and putting the patient at risk fr lcal and systemic infectius cmplicatins. Strict adherence t the guidelines in this plicy can reduce the risk f a vascular catheter infectin. III. Plicy A. General Recmmendatins fr All Intravascular Catheters Fr additinal guidelines, refer t Nursing Plicies: Peripheral Intravenus Device and Venipuncture Central Venus Access Device (CVAD) Care and Maintenance Parenteral Nutritin 1. Health Care Wrker Educatin and Training Initial and nging educatin and training f health care wrkers wh manage intravascular catheters is cnducted by the Venus Access Team, Nursing Department, and Nursing Practice and Prfessinal Develpment. Educatin includes indicatins fr the use f and prcedures fr the insertin and maintenance f intravascular devices, and apprpriate infectin cntrl measures t prevent Page 1 f 17
2 intravascular catheter-related infectins. Hspital Epidemilgy persnnel will cnduct surveillance fr intravascular device-related infectins t determine infectin rates, mnitr trends in thse rates, and assist in identifying lapses in infectin cntrl practices. 2. Hand Hygiene Wash hands fr a minimum f 15 secnds using an antiseptic-cntaining prduct (e.g., 2% chlrhexidine glucnate [CHG]) befre palpating, inserting, changing, remving, r dressing any intravascular device. A waterless alchl-based hand rub (ABHR) is an acceptable alternative t antiseptic sap and water if hands are nt visibly siled. The use f glves des nt replace the need fr hand hygiene. 3. Patient Assessment a. Mnitr the catheter site per nursing plicy, visually r by palpatin thrugh the intact dressing. When the catheter is used fr cntinuus infusin, assess the site hurly fr pediatric patients (ages 12 and under) and every 4 hurs fr adults. A med-lcked catheter site is assessed every 8 hurs in Adults and every 6 hurs in Pediatrics and befre use. If the patient has tenderness at the insertin site, fever withut bvius surce, r ther manifestatin suggesting lcal r bldstream infectin, the dressing shuld be remved t allw thrugh examinatin f the site. If signs f infectin are present, ntify the patient s licensed independent practitiner (LIP). b. Recrd the date and time f catheter insertin in the patient recrd. c. D nt rutinely perfrm surveillance cultures f patients r devices used fr intravascular access. 4. Catheter Remval Discntinue an intravascular device as sn as it is n lnger clinically indicated. There shuld be a daily assessment fr need f CVADs and the device remved when n lnger clinically indicated. 5. Skin Antisepsis a. Disinfect clean skin with an apprpriate antiseptic befre catheter insertin and at the time f dressing change. A 2% chlrhexidine glucnate and alchl preparatin is preferred. Alternatively, 70% alchl, 10% pvidne-idine, r 2% tincture f idine may be used. The antiseptic shuld be liberally applied and allwed t dry prir t catheter insertin. Allw pvidne-idine t remain n the skin fr at least 2 minutes r lnger if nt yet dry befre inserting the catheter. In general, the antiseptic shuld nt be remved with alchl r sterile water. When wearing clean glves fr PIV catheter insertin, d nt tuch the access site after applicatin f antiseptic. Fr patients in NCCC, nn-tinted Chlraprep is used fr skin preparatin fr PIV and PICC insertin except fr thse babies weighing 1000 grams r less. Fr patients 1000 grams and less, Betadine is used fr the first week f life and then Chlraprep is used. Prep slutin is remved with sterile water after catheter has been inserted. b. D nt apply rganic slvents (e.g., acetne r ether) t the skin befre insertin r during dressing change. Page 2 f 17
3 c. If a tpical skin anesthetic is used, the manufacturer s recmmendatins shuld be fllwed and the agent applied in a manner t prevent cntaminatin f the cntainer. d. When changing the central catheter dressing, use clean glves t remve the ld dressing and sterile glves t apply the new dressing, perfrming hand hygiene befre applying glves and then between glve change. A mask is wrn fr the dressing prcedure. e. If the patient is diaphretic, r if the site is bleeding r zing, use sterile gauze held in place by either a transparent dressing r tape. The dressing must be changed every 48 hurs. f. Immediately remve IV site dressings and apply a new dressing whenever the dressing becmes damp, lsened, r siled. D nt reinfrce lse dressings by adding gauze r tape. g. D nt rutinely replace IV site dressings fr pediatric patients when the risk f disldging the catheter utweighs the benefit f the dressing change. h. D nt submerge the catheter under water (e.g., tub baths r swimming). Shwering is permitted if precautins are taken t reduce the likelihd f intrducing rganisms int the catheter; e.g., use an impermeable cvering fr the catheter and cnnecting device during the shwer. i. D nt use tpical antibitic intment t the insertin site during dressing changes because f the ptential t prmte fungal infectins and antimicrbial resistance. 6. Intravascular Devices a. Administratin Set i. Replace administratin sets including secndary sets, add n devices, and all needleless access devices every 96 hurs ii. Change tubing if a catheter related infectin is suspected r dcumented. iii. Extensin tubing attached t the catheter shuld be treated as part f the catheter iv. Tubing/bag cnnectin must be prepped with alchl swab fr 15 secnds and allwed t dry prir t remving ld tubing and spiking new bag. v. Any left ver, unused IV set-ups shuld be discarded when expired. vi. Change IV tubing whenever cntaminatin is suspected (e.g., uncapped end f tubing falls n the flr r bed b. Tubing i. Tubing shuld be changed every 96 hurs ii. Exceptin: Lipid- Free Parenteral Nutritin (PN) tubing Adults: Change tubing every 24 hurs Pediatrics: Change tubing at least every 96 hurs Lipids and Lipid Cntaining Parenteral Nutritin (PN) Page 3 f 17
4 iii. iv. Prpfl Bld c. Prime & Spike Adults & Pediatrics: Change tubing every 24 hurs Change tubing every12 hurs D nt exceed 4 hurs fr infusin duratin Dispse f all bld prducts and administratin tubing after 4 hurs. Nte: D nt infuse multiple units thrugh the same bld administratin tubing unless yu are certain all units will cmpletely infuse in less than 4 hurs. After 4 hurs f use, the bld administratin tubing filter becmes full f prduct debris and will decrease the flw rate and damage the red cells. Vaspressrs r ther Life Supprting Medicatins Fr critically ill patients wh are receiving vaspressrs r ther life supprting medicatins a manifld stpcck system, it is allwable t delay changing the manifld. When the patient s cnditin imprves, the manifld shuld be changed at the same interval as the remainder f the administratin sets. Label IV tubing with the date the IV tubing is changed, catheter gauge and initials. It is nt necessary t change the IV fluids at the time the tubing is changed unless the bag has been hanging fr 96 hurs r cntaminatin is suspected. i. Preperative, areas (PCS), GI prcedures, and utpatient infusin areas are allwed t spike and prime IV sets up t 96 hurs. ii. iii. Ready-t-use IV set-ups must be maintained in a secured manner until used. Any left ver, unused IV set-ups shuld be discarded when expired. d. Suspected Infectins i. Change all IV tubing whenever a catheter is remved due t a suspected catheter-related infectin. e. The bag f IV fluid (including PN) must be changed if sterility f the bag is cmprmised during entry r re-spiking. f. Using a circular mtin (like juicing an range), vigrusly cleanse needleless access prts with an alchl swab fr 15 secnds prir t accessing. Fr sequential access, fllw the manufacture s recmmendatin fr use. OneLink needless access devices require cleansing the device fr each access, including sequential accesses. Refer t NURS 0074: Central Venus Access Device Care & Maintenance Plicy fr further infrmatin. Page 4 f 17
5 g. The IV system shuld remain a clsed system. If tubing must be discnnected, use aseptic technique t prevent cntaminatin. The catheter must be capped with a needleless access prt and the administratin set tubing clsed with a sterile plastic cap. 7. Pressure Mnitring Systems (Arterial and Venus) a. Keep sterile all devices and fluids that cme int cntact with the fluid f the pressure mnitring circuit (e.g., calibratin devices, heparinized saline). b. Minimize the number f manipulatins and entries int the pressure-mnitring system. Use a clsed-flush system (i.e., cntinuus flush), rather than an pen system (i.e., ne that requires a syringe and stpcck), t maintain the patency f the pressure-mnitring catheters. If stpccks are used, treat them as sterile and cver them with a sterile cap r syringe when nt in use. c. When the pressure-mnitring system is accessed thrugh a rubber diaphragm rather than a stpcck, cleanse the diaphragm with an apprpriate antiseptic befre and after accessing the system. d. Maintain line patency with nrmal saline r heparinized nrmal saline per unit/pharmacy prtcl. e. Replace the pressure mnitring system every 96 hurs. f. D nt administer dextrse-cntaining slutins r parenteral nutritin fluids thrugh the pressure mnitring circuit. 8. Preparatin and Quality Cntrl f Intravenus Admixtures a. Admix all parenteral fluids in the Pharmacy under a laminar-flw hd using aseptic technique. Clinically urgent IV admixtures prepared by nursing must be replaced as sn as pssible by fluids prepared by pharmacy and may hang fr a maximum f 24 hurs. Strict aseptic technique must be used when preparing these slutins. Refer t the Pharmacy Infectin Cntrl Plicy. b. Check all cntainers f parenteral fluid fr visible turbidity, leaks, cracks, particulate matter, and the manufacturer's expiratin date befre use and discard if present r slutin expired. In additin t patient identificatin, a distinctive supplementary label shuld be attached t each parenteral nutritin admixture (PN) stating, at a minimum; vlume f slutin, the additives and their dsages, the date and time f cmpunding, the expiratin date and time. c. Medicatin Vials i. Cleanse the rubber diaphragm f single dse medicatin vials with alchl and allw t dry prir t entering. Single use vials shuld be accessed nly nce using a sterile syringe and needleless access device. Any unused medicatin shuld be apprpriately discarded. ii. Fr multi-dse vials, refer t the UNC Health Care Operatinal Plicy: Medicatin Management: Use f Multi-Dse Vials f Parenteral Medicatins in Acute Care and Ambulatry Care Envirnments. iii. If a sterile access device (vial adaptr) is used, a new sterile syringe must be used fr each access. Cleanse the access prt with alchl fr 15 secnds prir t accessing. d. Prpfl Page 5 f 17
6 Strict aseptic prcedures are required during preparatin and administratin f prpfl. If prpfl is administered directly frm the vial, administratin shuld be cmpleted within 12 hurs after the vial is spiked. The tubing and any unused prpfl shuld be discarded after 12 hurs. Always disinfect the vial rubber stpper with 70% alchl and allw t dry prir t spiking. If prpfl emulsin is transferred t a syringe r ther cntainer prir t use, administratin shuld be started and cmpleted within 6 hurs after the cntainer is pened. Prpfl is a single-use parenteral prduct. 9. Hang Time fr Parenteral Fluids These guidelines apply t the bag f fluid nly. See Intravascular Device Tubing (sectin III.A.6.) fr frequency f administratin set replacement. a. Cmmercially prepared parenteral fluids (e.g. nrmal saline, D5%) can hang fr a maximum fr 96 hurs. b. Admixed by pharmacy (under a laminar flw hd) parenteral fluids hd can hang fr a maximum f 48 hurs. Beynd use dating allws the admixed fluids t hang fr 48 hurs after the bag is spiked. c. Parenteral fluids admixed n the unit must be replaced as sn as pssible by thse prepared in pharmacy, but may hang fr a maximum f 24 hurs. These fluids include all medicatin drips and syringe pump infusins, prepared by the nurse n the patient care unit. The type f medicatin, the manufacturer, r Pharmacy may indicate a shrter expiratin time. d. Parenteral nutritin fluids (PN)/lipids shuld be cmpleted within 24 hurs r n lnger than specified expiratin n label. e. Infusins f bld and bld prducts shuld be cmpleted within 4 hurs f hanging. f. Epidural fluids may hang n lnger than 48 hurs. g. Patient Cntrlled Analgesia (PCA) i. Cmmercially prepared PCA fluids can hang per the manufacturers recmmendatins f 96 hurs ii. 10. In-Line Filters PCA fluids admixed in pharmacy can hang fr a maximum f 48 hurs D nt rutinely use filters fr infectin preventin purpses. In-line filters are used with PN t prevent infusin f precipitates. 11. Prphylactic Antimicrbials D nt rutinely administer antimicrbials fr prphylaxis f catheter clnizatin r bldstream infectin befre insertin r during use f an intravascular device Prphylactic Antibitic Lck Therapy might be cnsidered in high-risk patients t prevent CLABSIs in lines that are infrequently accessed such as dialysis and chemtherapy lines. The Antibitic Lck Therapy prtcl may be accessed via Pharmacy s Intranet website. Page 6 f 17
7 B. Additinal Recmmendatins fr Peripheral Venus Catheters 1. Selectin f Catheters a. Select catheters based n the intended purpse and duratin f use, knwn cmplicatins (e.g., phlebitis and infiltratin), and experience at the institutin. Use a Tefln catheter r a plyurethane catheter which are preferred ver a steel needle. b. Avid the use f steel needles fr the administratin f fluids/medicatins that may cause tissue necrsis if extravasatin ccurs. 2. Selectin f Insertin Site a. Cnsideratins fr selectin f insertin site include: i. Maintenance f asepsis and risk f infectin ii. Risk f mechanical cmplicatins iii. Patient-specific factrs (e.g., preexisting catheters, anatmic defrmity) iv. Security v. Cmfrt b. Majr risk factrs fr infectin The density f skin flra at the insertin site and risk f thrmbphlebitis are majr risk factrs fr infectin. Lwer extremity insertin sites are assciated with higher risk f infectin than upper arm extremity sites, in adult patients. c. Insertin site selectin based n age i. In adults, use an upper extremity site in preference t ne n a lwer extremity fr catheter insertin (e.g., hand, wrist, and arm). Replace a catheter inserted in a lwer extremity site t an upper extremity site as sn as pssible. ii. In pediatric patients (1-12 years f age) insert the catheter int the veins f the hand, wrists, r frearm. At the request f the patient s physician, lwer extremities may als be used but this is recmmended fr nn-ambulatry children nly and when n ther site is available. Refer t Nursing Prcedure: Peripheral Intravenus Device and Venipuncture. iii. In children <1 year f age, insert the catheter preferably in the superficial veins f the hands, arms, feet, r legs. Scalp veins (in nenates r yung infants) can be used as the catheter insertin site. 3. Catheter Insertin a. Perfrm hand hygiene befre putting n glves. Wear clean glves during catheter insertin. D nt cut ff glve s fingertip. b. Refer t sectin III.A.5.f this plicy fr skin preparatin. 4. Catheter-Site Dressing a. Peripheral venus/arterial catheters are dressed with a sterile transparent dressing. If the peripheral venus catheter cannt be rtated t a new site every 7 days, the dressing shuld be changed every 7 days r whenever damp, Page 7 f 17
8 lsened r siled. Change dressing accrding t the Nursing PIV Device and Venipuncture Plicy. b. Central venus catheters (including Peripherally Inserted Central Catheters PICC s) are dressed using a sterile transparent dressing (e.g., Tegaderm, Srbaview ). The dressing is changed every 7 days, as lng as it remains clean, dry and intact. Change the dressing whenever damp, lsened r siled. i. If the patient is diaphretic, r if the site is bleeding r zing, use sterile gauze held in place by either a transparent dressing r tape. The dressing must be changed every 48 hurs. c. Refer t nursing plicy Central Venus Access Device (CVAD) Care & Maintenance. d. Fr central venus catheters placed in the internal jugular vein, a SORBAVIEW IJ dressing is recmmended. e. A CHG-impregnated patch (e.g. Bipatch) shuld be used n central venus access devices and midline catheters fr inpatients unless cntraindicated. Cntraindicatins include sensitivity t prduct, umbilical catheter, and use n PICC lines with premature infants in NCCC. The patch shuld be placed arund the insertin site within 24 hurs f catheter placement. If the dressing must be changed prir t the 7 day time perid, the CHG-impregnated patch als shuld be changed at that time. 5. Catheter Change a. In all patients >12 years f age, change peripheral venus catheters and rtate peripheral venus sites every 7 days r sner based n clinical assessment. b. In pediatric patients (ages 12 and under), catheters are nt rutinely rtated. c. In adults, remve catheters inserted under emergency cnditins, where breaks in aseptic technique are likely t have ccurred. Insert a new catheter at a different site as sn as feasible (<24 hurs). d. Remve peripheral venus catheters and ntify the patient s physician when the patient develps signs f phlebitis, purulent thrmbphlebitis r cellulitis (i.e., warmth, tenderness, erythema, palpable venus crd) at the insertin site and when patient develps an IV related bacteremia. 6. Medlcks: peripheral and central a. Cleanse the medlck access prt with alchl fr 15 secnds prir t accessing. b. Rutinely flush peripheral venus medlcks per nursing plicy. c. Replace the medlck peripheral venus catheter every 7 days. d. All infectin cntrl guidelines fr peripheral venus catheters and central venus catheters receiving a cntinuus infusin apply t medlcks that are used fr intermittent infusin. e. Medlcking IVs fr patient transprt: Shuld nly be dne fr patient safety reasns, nt cnvenience If necessary fr patient safety, the prcess shuld be dne aseptically Page 8 f 17
9 7. Midline Catheters Sterile dead end cap placed n end f line (d nt cnnect IV tubing back int the needleless access prt). Fr recnnectin, needless access device (e.g. Onelink) shuld be disinfected with alchl prir t recnnectin. a. Midline catheters are inserted by a Venus Access Team Registered Nurse and are used fr patients wh are receiving is-smtic, nn-irritating IV medicatins fr greater than 6 days. b. Standard peripheral catheters d nt extend beynd the axillary regin (d nt enter a central vein). These catheters have a lwer rate f phlebitis than shrt peripheral catheters and are nt rutinely changed. c. The site dressing is changed every 7 days (transparent dressing) r whenever damp, lsened, r siled. d. Fr care and maintenance f Midline Catheters refer t Nursing Plicy NURS 0605: Midline Catheter: Adults. 8. Catheter Site Care D nt apply tpic antimicrbial r antiseptic intment/cream t the insertin site f peripheral venus catheters. C. Additinal Recmmendatins fr Central Venus Catheters (Including PICC, Pulmnary Artery Catheters in Adult and Pediatric Patients) 1. Patients/family member shuld be educated regarding measures t prevent catheterassciated infectins and dcumented. 2. Selectin f Catheters a. Use a catheter with the minimum number f prts r lumens essential fr the management f the patient. b. A peripherally inserted central catheter (PICC) may be indicated when the duratin f therapy is expected t exceed 6 days. c. Use tunneled catheters (i.e., Pwerlines, Hickman r Brviac) r implantable vascular access devices (i.e., prts) when lng-term vascular access (>30 days) is anticipated. Use ttally implantable access devices fr lng-term intermittent venus access. Use a tunneled catheter fr frequent r cntinuus access. 3. Selectin f Insertin Site In adult patients, the risk and benefits f different insertin sites (e.g., subclavian vein vs. internal jugular vein) must be cnsidered n an individual basis with regard t infectius and nninfectius cmplicatins. Avid using the femral vein fr central venus access in adult patients when the catheter is placed under planned and cntrlled cnditins. 4. Catheter Insertin a. Central catheters shuld be inserted with maximal sterile barrier technique (e.g., patient draped in a sterile drape frm head t te) using sterile equipment. This includes sterile glves, sterile gwn, hair cvering and a surgical mask with eye prtectin. Page 9 f 17
10 b. A checklist can be used as a reminder fr imprtant elements f insertin. Here is a link t the Central Line Insertin Check List als, the triple lumen and intrduce kits cntain check lists in the plastic sleeves t reference. c. Needle-guided access using ultrasund technlgy shuld be used fr CVAD insertin whenever pssible t reduce the risk f mechanical cmplicatins and infectin. Transilluminatrs may als be used fr catheter insertin. If used n intact skin, this equipment may be disinfected between use n different patients using an EPA-apprved cleanser/disinfectant prduct (e.g., Metriguard, Sani- Wipes). If this equipment is used n nn-intact skin (e.g., a burn wund), it requires high-level disinfectin (e.g., Cidex). If ultrasund gel is applied t the skin within the sterile field, sterile gel must be used (available in single-use packets). When using ultrasund technlgy within a sterile field, a sterile prbe cver is required. d. When inserting the catheter, a wide field shuld be prepped using a 2% chlrhexidine glucnate and alchl slutin (e.g. Chlraprep) unless there is a cntraindicatin in which case 70% alchl r 10% pvidne idine may be used. See umbilical catheter sectin fr exceptins in nenates. Slutins shuld be allwed t air dry. e. Use a sterile sleeve t prtect pulmnary artery catheters during insertin. f. The catheter site shuld be dressed immediately after insertin. i. Temprary (<30 days) nn-tunneled catheters (e.g., triple lumen catheters) shuld be dressed immediately after insertin. This includes catheters placed in Vascular Interventinal Radilgy (VIR) and the Operating Rm (OR). ii. iii. 5. Catheter Changes Lng term tunneled catheters (e.g., brviacs, hickmans) are dressed as a central line. Patients admitted t the hspitals with existing catheters shuld have a new catheter dressing placed within 24 hurs f admissin. Implanted prts are dressed as a surgical wund immediately after placement. When the prt is accessed with a nn-cring needle (e.g., Prt Safety Needles), the site is dressed as a central line. The dressing and nn-cring needle are changed every 7 days. a. D nt rutinely replace temprary central venus catheters r PICCs as a methd t prevent catheter-related infectins. b. D nt rutinely replace pulmnary artery catheters mre frequently than 7 days as a methd t prevent catheter-related infectin. c. D nt remve CVC r PICCs slely because f fever. Use clinical judgment regarding the apprpriateness f remving the catheter if there is evidence f infectin elsewhere. Obtain bth central line and peripheral bld cultures t assist in decisin-making. d. Guidewire Exchange i. Rutine guidewire exchange f nntunneled catheters has nt been shwn t reduce CRBSI and shuld be avided. Page 10 f 17
11 ii. iii. Use a guidewire exchange t replace a malfunctining nn-tunneled catheter if there is n evidence f infectin and the risk f inserting a catheter int a new site is unacceptably high. This prcedure is cmpleted using the same aseptic technique as used t place a new central line. Use full barrier precautins (haircver, mask, sterile gwn, glves, twels, drape) and site preparatin as if placing a new catheter in a new site. Use a new set f sterile glves prir t handling the new catheter. e. D nt use guidewire-assisted catheter exchange whenever catheter-related sepsis is dcumented r tunnel/iv site infectin is present. If the patient requires cntinued vascular access, remve the implicated catheter and replace it with anther catheter at a new site. 6. Flush Slutins and Anticagulants a. Patients with a psitive HIT test (heparin induced thrmbcytpenia) r pending HIT test result shuld nt receive heparin. Use f a psitive r neutral pressure cap is recmmended. b. Use cmmercially prepared flush slutins. 7. Additinal Recmmendatins fr Hemdialysis/Apheresis Catheters a. Selectin f Catheter Use cuffed tunneled central venus catheters fr hemdialysis if the perid f temprary access is anticipated t be prlnged (i.e., >3 weeks). Use a fistula r graft instead f a central catheter fr permanent access. b. Selectin f Insertin Site Use the jugular r femral vein rather than a subclavian vein t avid venus stensis. c. Catheter Insertin Whenever pssible, these catheters shuld be placed in Vascular Interventinal Radilgy r the Operating Rm. If, under emergent cnditins, the catheter must be placed utside these areas, fllw the insertin guidelines fr central venus catheters prvided in this plicy. d. Catheter Changes D nt rutinely replace hemdialysis catheters as a methd t prevent catheterrelated infectin. There is n rutine change required fr hemdialysis/apheresis catheters. Refer t the catheter change sectin fr Central Venus Catheters in this plicy fr additinal infrmatin regarding catheter changes. e. Additinal Guidelines i. Catheters, shunts, fistulas, femral, subclavian, r ther vascular access catheters (e.g., ECMO cannulas) will be cared fr using meticulus aseptic technique. Hemdialysis Plicies and the Apheresis Catheter Plicies describe in detail care fr these devices. ECMO vascular access catheter care is perfrmed fllwing the guidelines in the ECMO Nursing Plicy. Page 11 f 17
12 ii. Only hemdialysis staff/nephrlgists may access catheters used fr dialysis. The nly exceptin t this plicy wuld be in the event f a life threatening medical emergency where rapid vascular access is required fr resuscitatin. If the catheter is being cnsidered fr ther purpses, the Nephrlgy Cnsult Service shuld be cntacted. iii. Hemdialysis and Apheresis staff will perfrm catheter dressing changes n treatment days when indicated. When dressing changes are perfrmed by ther clinical persnnel, fllw the guidelines prvided in this plicy and nursing plicy. D. Management f Parenteral Nutritin (PN) Althugh parenteral nutritin can be a lifesaving therapeutic mdality, cmplicatins are pssible. Reprted cmplicatins include metablic derangements, hepatic injury, sepsis, thrmbsis f central veins, and extravasatin f fluid. Hwever, the mst frequently nted serius cmplicatins f parenteral nutritin have been metablic derangements and sepsis. Refer t the PN Nursing Plicy fr additinal guidelines. 1. Guidelines fr Administratin f Parenteral Nutritin (PN) Therapy a. Parenteral nutritin therapy (PN) is initiated nly when indicated by the patient's clinical requirements. The need fr parenteral nutritin must be balanced against the risks inherent in such therapy. b. Parenteral nutritin therapy (PN) shuld be prescribed and clsely supervised by a prvider wh is thrughly versed in the techniques and risks f such therapy. c. Adult patients receiving Parenteral Nutritin therapy (PN) at UNC Health Care are verseen by a team f individuals with a particular interest and expertise in the field f parenteral nutritin. This team includes a nurse, pharmacist, dietitian and physician wh versee the metablic mnitring f adult patients receiving PN and teach sund infectin cntrl principles and techniques t physicians and nurses charged with the care f parenteral systems. d. Fr patients wh are receiving PN n a lng term basis, discnnectin at the PN tubing/catheter junctin may be necessary under the fllwing circumstances: i. PN Cycling: PN can run ver shrter time perids, such as 12, 14, 16, r 20 hurs. Each time perid is called a cycle. If tubing must be discnnected, use aseptic technique t prevent cntaminatin. The catheter must be capped with a needleless access prt and the administratin set tubing clsed with a sterile plastic cap. The tubing may be reattached t the catheter junctin as lng as aseptic technique is used, n cntaminatin is suspected and the PN bag has nt expired past 24 hurs. ii. Central line change and n infectin is suspected: The discnnected tubing must be capped with a sterile device using aseptic technique. After the catheter is changed and placement verified by x-ray, the tubing may be reattached t the catheter junctin as lng as aseptic technique is used and n cntaminatin is suspected. iii. Central line change and infectin is suspected: new tubing must be attached t the PN bag. The bag/spike cnnectin must be prepped fr 15 secnds with alchl and allwed t dry prir t remving the ld tubing. Spike the bag with the new tubing using aseptic technique. If the bag was cntaminated during re-spiking, the ld bag f PN fluids will need t be Page 12 f 17
13 replaced with a new PN bag frm Pharmacy as sn as available and new tubing attached. Fr TPN prvided by Adult TPN/PEN Service if line infectin is suspected, discard all tubing and PN bags and receive rders frm prvider fr dextrse cntaining slutin (e.g. D5%) e. See Administratin Sets Abve fr descriptin f tubing change guidelines fr PNPN administratin shuld be discntinued immediately if signs f extravasatin are bserved. Apprpriate measures t prevent hypglycemia with lss f central PN shuld be instituted simultaneusly. f. The physician must be ntified f signs f inflammatin, erythema r purulent drainage at the IV catheter site. Hwever, catheters may be the surce f septicemia even if lcal signs f inflammatin are nt present. g. In sme instances, parenteral Nutritin may als be delivered via peripheral IV catheters. h. Once PN is started thrugh a lumen d nt use that lumen fr any ther purpse (e.g., administratin f fluids, bld/bld prducts). D nt rtate the PN lumen. PN catheters remain in place fr lng perids f time and have the highest risk f infectin. Exceptins may be made with an LIP s rder. E. Peripheral Arterial Catheters 1. Arterial Catheter Insertin a. A cap, mask, sterile glves, and a large sterile fenestrated drape shuld be used during peripheral arterial catheter insertin. i. Fr peripherally inserted arterial catheters in the nenatal ppulatin, minimally sterile glves and mask is required during insertin. b. The site is prepped and dressed using guidelines fr peripheral venus catheters. c. Use dispsable, rather than reusable, transducer assemblies. d. Replace transducers at 96 hur intervals. Replace ther cmpnents f the system (e.g., tubing, cntinuus flush device, flush slutin) at the time the transducer is replaced. 2. Arterial Catheter Changes a. Replace arterial catheters nly when there is a clinical indicatin. F. Recmmendatins fr Umbilical Catheters 1. Catheter Insertin/Care a. Umbilical arterial and venus catheters are placed using sterile technique with sterile barrier, gwns, masks, and glves. Hair and beards must be cvered. b. Cleanse the umbilical insertin site with an antiseptic befre catheter insertin. Avid tincture f idine because f the ptential effect n the thyrid. Pvidne idine may be used. c. Dressings are nt rutinely applied. d. Add lw dse heparin t fluid infused thrugh umbilical artery catheters. Page 13 f 17
14 e. D nt use tpical antibitic intment r creams n an umbilical catheter insertin site. f. Replace IV tubing and all add n devices at least every 72 hurs; lipid-cntaining lines are changed every 24 hurs. g. Cmplete infusins f lipid cntaining fluids within 24 hurs f hanging the fluid. 2. Catheter Changes a. Umbilical catheters shuld be remved as sn as n lnger essential fr medical management r fr any signs f catheter-related bldstream infectin. b. Umbilical catheters are nt rutinely changed. c. Optimally, remve umbilical arterial catheters within 5 days. Umbilical venus catheters can be left in place up t 14 days if managed aseptically. G. Special Cnsideratins Dcumentatin Licensed Independent Practitiners (LIPs) and/r nurses shuld dcument the fllwing: 1. Placement f IV lines under nnsterile cnditins such as in emergencies. 2. Use f hemdialysis, PN catheters fr ther purpses. 3. Inability t change a catheter despite knwn r prbable catheter sepsis. 4. Inability t fllw recmmended line change times (e.g., peripheral IV every 96 hurs). 5. Any line related cmplicatins (e.g., phlebitis, extravasatin with tissue damage, sepsis). 6. Educatin f patients and caregivers f patients ging hme with central line catheters is dcumented in the electrnic medical recrd. Management f Stpcck Prts 1. Stpccks shuld be used nly when abslutely necessary, as in the care f critically ill patients. 2. Prep all prts with alchl and let dry prir t access 3. Stpcck prts must be cvered with a sterile cap. Never reuse an ld cap. Stpccks n venus lines (nt arterial lines) shuld be capped with a needleless access cap and all accesses shuld be thrugh the cap. 4. Flush stpcck immediately if bld is seen in the prt. H. IV-Related Infectins 1. Ntify the LIP if there is a suspicin f site infectin. Clean site and cver with a small cclusive sterile dressing. 2. If an IV system is t be discntinued because f suspected IV-related infectin, such as purulent thrmbphlebitis r bacteremia, the skin at the skin-cannula junctin shuld be cleaned with CHG/alchl allwing 30 secnd cntact time and allwed t dry befre cannula remval, and the cannula shuld be cultured using a semiquantitative technique. a. Ntify Hspital Epidemilgy f any suspected cntaminatin f IV fluids. Page 14 f 17
15 b. If cntaminatin f fluid is cnfirmed, the implicated bttle and the remaining units f the implicated lt shuld be saved, and the lt numbers f fluid and additives shuld be recrded. c. If intrinsic cntaminatin (cntaminatin during manufacturing) is suspected, the lcal health authrities, CDC, and the U.S. Fd and Drug Administratin shuld be ntified immediately. I. Respnsibility Statement Implementatin f this plicy is the respnsibility f Nursing service line directrs, Vascular Access Team, Nutritin Supprt Service, and Medical Staff. IV. References CDC Guidelines fr the Preventin f Intravascular Catheter-Related Infectins, Infusin Nurses Sciety. Infusin Nursing Standards f Practice. Jurnal f Infusin Nursing. Jan/Feb Infusin Nurses Sciety. (2011) Plicies & Prcedures fr Infusin Nursing, 4 th Editin. V. Reviewed/Apprved by Hspital Infectin Cntrl Cmmittee VI. Original Plicy Date and Revisins Revised n Apr 2005, July 2007, May 2010, May 2013, May 2016, Aug 2017 rev Page 15 f 17
16 Appendix 1: Quick Reference Timing fr Tubing Changes Tubing Adult Patients Pediatric Patients Ntes Regular Fluids (e.g. D5%, NS, secndary set) Parenteral Nutritin w/ut lipids Every 96 Hurs Every 96 Hurs Discard if Cntaminated Every 24 Hurs At Least Every 96 Hurs Lipids & Lipid cntaining PN (e.g. 3 in 1) Every 24 Hurs Every 24 Hurs Prpfl Every 12 Hurs Every 12 Hurs Bld Prducts Discard after 4 Hurs Discard after 4 Hurs Nte: D nt infuse multiple units thrugh the same bld administratin tubing unless yu are certain all units will cmpletely infuse in less than 4 hurs. Page 16 f 17
17 Appendix 2: Quick Reference: Hang Time Reference fr Parenteral Fluids Parenteral Fluid Hang Time Ntes Cmmercially Manufactured Max 96 Hurs As lng as n additins by pharmacy (e.g. nrmal saline, D5%) Admixed by Pharmacy Max 48 Hurs Beynd Use Dating allws admixed fluids t hand fr 48 hurs after the bag is spiked. Admixed by Nurse n Unit Max 24 Hurs Shuld be replaced as sn as pssible Bld Prducts Max 4 Hurs Cmplete within 4 hurs Epidural Fluids Max 48 Hurs Must be replaced within 48 hurs. Parenteral Nutritin/Lipids Max 24 hurs Shuld be cmplete within 24 hurs r n lnger than specified expiratin date Patient Cntrlled Analgesia Max 96 hurs Must be cnsistent with manufacturers recmmendatins. Page 17 f 17
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018
 Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
1.11 INSULIN INFUSION PUMP MANAGEMENT INPATIENT
 WOMEN AND NEWBORN HEALTH SERVICE CLINICAL GUIDELINES SECTION A: GUIDELINES RELEVANT TO OBSTETRICS AND GYNAECOLOGY 1 STANDARD PROTOCOLS 1.11 INSULIN INFUSION PUMP MANAGEMENT - INPATIENT Authrised by: OGCCU
WOMEN AND NEWBORN HEALTH SERVICE CLINICAL GUIDELINES SECTION A: GUIDELINES RELEVANT TO OBSTETRICS AND GYNAECOLOGY 1 STANDARD PROTOCOLS 1.11 INSULIN INFUSION PUMP MANAGEMENT - INPATIENT Authrised by: OGCCU
SECTION O. MEDICATIONS
 SECTION O. MEDICATIONS 1. NUMBER OF MEDICA TIONS (Recrd the number f different medicatins used in the last 7 days; enter "0" if nne used) O1. Number f Medicatins (7-day lk back) Intent: Prcess: Cding:
SECTION O. MEDICATIONS 1. NUMBER OF MEDICA TIONS (Recrd the number f different medicatins used in the last 7 days; enter "0" if nne used) O1. Number f Medicatins (7-day lk back) Intent: Prcess: Cding:
Infection Control Guidelines for Cabin Crew Members on Commercial Aircraft
 Infectin Cntrl Guidelines fr Cabin Crew Members n Cmmercial Aircraft PURPOSE These guidelines prvide cabin crew members (flight attendants) with practical measures t prtect themselves, passengers, and
Infectin Cntrl Guidelines fr Cabin Crew Members n Cmmercial Aircraft PURPOSE These guidelines prvide cabin crew members (flight attendants) with practical measures t prtect themselves, passengers, and
Bariatric Surgery FAQs for Employees in the GRMC Group Health Plan
 Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Percutaneous Nephrolithotomy (PCNL)
 Percutaneus Nephrlithtmy (PCNL) What is a percutaneus nephrlithtmy? is the mst effective f the cmmnly perfrmed prcedures fr kidney stnes. It is the best prcedure fr large and cmplex stnes. T perfrm this
Percutaneus Nephrlithtmy (PCNL) What is a percutaneus nephrlithtmy? is the mst effective f the cmmnly perfrmed prcedures fr kidney stnes. It is the best prcedure fr large and cmplex stnes. T perfrm this
Annex III. Amendments to relevant sections of the Product Information
 Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Infection Control. Three Ways Germs Are Spread Germs are spread in the environment three ways: direct contact, indirect contact, and droplet spread.
 Infectin Cntrl Infectin cntrl is preventing the spread f germs that cause illness and infectin. Infectin cntrl starts with understanding germs and hw they are spread. Abut Germs Everyne cmes in cntact
Infectin Cntrl Infectin cntrl is preventing the spread f germs that cause illness and infectin. Infectin cntrl starts with understanding germs and hw they are spread. Abut Germs Everyne cmes in cntact
PREPHERALLY INSERTED VENOUS CATHETERS (MIDLINE CATHETERS)
 CVC MID REV12/2016 PREPHERALLY INSERTED VENOUS CATHETERS (MIDLINE CATHETERS) Instructins fr use Intended use: Sterile single use device indicated t be placed peripherally in the upper arm veins fr use
CVC MID REV12/2016 PREPHERALLY INSERTED VENOUS CATHETERS (MIDLINE CATHETERS) Instructins fr use Intended use: Sterile single use device indicated t be placed peripherally in the upper arm veins fr use
CLINICAL MEDICAL POLICY
 Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
BLOOD BORNE PATHOGENS
 BLOOD BORNE PATHOGENS GALVESTON ISD ANNUAL TRAINING 2018-2019 Galvestn Independent Schl District Special Prgrams/ECH Health Services Required Training Ò Training is required by the Texas Department f Health
BLOOD BORNE PATHOGENS GALVESTON ISD ANNUAL TRAINING 2018-2019 Galvestn Independent Schl District Special Prgrams/ECH Health Services Required Training Ò Training is required by the Texas Department f Health
SURGICAL NOTE. Surgical Recommendations to Optimize Femoral/Iliac Artery Cannulation
 SURGICAL NOTE Surgical Recmmendatins t Optimize Femral/Iliac Artery Cannulatin Due t its size, lcatin, and ease f access, the femral artery is frequently used fr bld pressure catheter placement. Less frequently,
SURGICAL NOTE Surgical Recmmendatins t Optimize Femral/Iliac Artery Cannulatin Due t its size, lcatin, and ease f access, the femral artery is frequently used fr bld pressure catheter placement. Less frequently,
Methadone Maintenance Treatment for Opioid Dependence
 POLICY STATEMENT Methadne Maintenance Treatment fr Opiid Dependence APPROVED BY COUNCIL: May 2010 PUBLICATION DATE: Dialgue, Issue 2, 2010 Disclaimer: As f May 19, 2018 physicians n lnger require an exemptin
POLICY STATEMENT Methadne Maintenance Treatment fr Opiid Dependence APPROVED BY COUNCIL: May 2010 PUBLICATION DATE: Dialgue, Issue 2, 2010 Disclaimer: As f May 19, 2018 physicians n lnger require an exemptin
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION
 CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
Completing the NPA online Patient Safety Incident Report form: 2016
 Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
INSTRUCTIONS FOR USE ZINBRYTA (zin-bry-tuh) (daclizumab) Injection, for Subcutaneous Use Single-Dose Prefilled Syringe 150 mg
 INSTRUCTIONS FOR USE ZINBRYTA (zin-bry-tuh) (daclizumab) Injectin, fr Subcutaneus Use Single-Dse Prefilled Syringe 150 mg Read this Instructins fr Use befre yu start using ZINBRYTA and each time yu get
INSTRUCTIONS FOR USE ZINBRYTA (zin-bry-tuh) (daclizumab) Injectin, fr Subcutaneus Use Single-Dse Prefilled Syringe 150 mg Read this Instructins fr Use befre yu start using ZINBRYTA and each time yu get
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
H1N1 Influenza 09 Guidance for Residential Aged Care
 H1N1 Influenza 09 Guidance fr Residential Aged Care 11 June 2009 As knwledge abut H1N1 Influenza 09 develps, further advice will be prvided. Please check www.healthemergency.gv.au fr the latest infrmatin.
H1N1 Influenza 09 Guidance fr Residential Aged Care 11 June 2009 As knwledge abut H1N1 Influenza 09 develps, further advice will be prvided. Please check www.healthemergency.gv.au fr the latest infrmatin.
Health Screening Record: Entry Level Due: August 1st MWF 150 Entry Year
 Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Solid Organ Transplant Benefits to Change for Texas Medicaid
 Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Cancer Association of South Africa (CANSA)
 Cancer Assciatin f Suth Africa (CANSA) Fact Sheet and Psitin Statement n Cannabis in Suth Africa Intrductin Cannabis is a drug that cmes frm Indian hemp plants such as Cannabis sativa and Cannabis indica.
Cancer Assciatin f Suth Africa (CANSA) Fact Sheet and Psitin Statement n Cannabis in Suth Africa Intrductin Cannabis is a drug that cmes frm Indian hemp plants such as Cannabis sativa and Cannabis indica.
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Louisiana Healthcare Connections Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
MANAGEMENT OF INTRAVASCULAR (IV) LINES AND THERAPY. All GCC Countries
 TITLE/DESCRIPTION: MANAGEMENT OF INTRAVASCULAR (IV) LINES AND THERAPY INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL CENTRE
TITLE/DESCRIPTION: MANAGEMENT OF INTRAVASCULAR (IV) LINES AND THERAPY INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL CENTRE
US Public Health Service Clinical Practice Guidelines for PrEP
 Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
1. Obtain the Catheter (choose one of 3 types below):
 Checklist fr Insertin and Setup f an ICP Mnitring Device Befre prceeding, cntact neursurgery t identify the type f catheter t be inserted. The catheter type will define the equipment requirements and preparatin
Checklist fr Insertin and Setup f an ICP Mnitring Device Befre prceeding, cntact neursurgery t identify the type f catheter t be inserted. The catheter type will define the equipment requirements and preparatin
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Intravenus Vancmycin Use in Adults Intermittent (Pulsed) Infusin Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment
Intravenus Vancmycin Use in Adults Intermittent (Pulsed) Infusin Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Managed Health Services (MHS)
 Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
School Medication Authorization Form. School Grade Teacher. Emergency Phone No: To be completed by the student's physician: Name of Medication:
 Schl Medicatin Authrizatin Frm Student's Name Address Birth Date Hme Phne Schl Grade Teacher Emergency Phne N: T be cmpleted by the student's physician: Name f Medicatin: Dsage Frequency Time t be given
Schl Medicatin Authrizatin Frm Student's Name Address Birth Date Hme Phne Schl Grade Teacher Emergency Phne N: T be cmpleted by the student's physician: Name f Medicatin: Dsage Frequency Time t be given
ABIOpure TM Total RNA (version 2.0)
 ABIOpure TM Ttal RNA (versin 2.0) Bld Extractin Handbk Cat N: M541RP50-B FOR RESEARCH USE ONLY Table f Cntents Cntents Page Kit Cmpnents 3 Precautins 3 Stability & Strage 4 General Descriptin 4 Limitatins
ABIOpure TM Ttal RNA (versin 2.0) Bld Extractin Handbk Cat N: M541RP50-B FOR RESEARCH USE ONLY Table f Cntents Cntents Page Kit Cmpnents 3 Precautins 3 Stability & Strage 4 General Descriptin 4 Limitatins
Patrick J McGahan, MD Orthopaedic Surgeon Specializing in Sports Medicine/Shoulder Reconstruction Surgery Instructions Hip
 Patrick J McGahan, MD Orthpaedic Surgen Specializing in Sprts Medicine/Shulder Recnstructin 2801 K St, Ste 330, Sacrament, CA, 95816 (p) 916-733-5049 (f) 916-733-8914 www.patrickmcgahanmd.cm Befre Surgery
Patrick J McGahan, MD Orthpaedic Surgen Specializing in Sprts Medicine/Shulder Recnstructin 2801 K St, Ste 330, Sacrament, CA, 95816 (p) 916-733-5049 (f) 916-733-8914 www.patrickmcgahanmd.cm Befre Surgery
Rate Lock Policy. Contents
 Rate Lck Plicy Cntents Rate Lcks... 2 Rate Lck Cnfirmatin... 2 Lck Term... 2 Pre-Lck... 2 Maximum Qualified Rate... 3 Extensins... 3 Cst t Extend... 3 Relcks... 4 Re-Negtiatin r Flat Dwn Plicy... 4 Prgram
Rate Lck Plicy Cntents Rate Lcks... 2 Rate Lck Cnfirmatin... 2 Lck Term... 2 Pre-Lck... 2 Maximum Qualified Rate... 3 Extensins... 3 Cst t Extend... 3 Relcks... 4 Re-Negtiatin r Flat Dwn Plicy... 4 Prgram
Immunisation and Disease Prevention Policy
 Immunisatin and Disease Preventin Plicy Quality Area 2: Children s Health and Safety 2.1 Each child s health is prmted 2.1.4 Steps are taken t cntrl the spread f infectius diseases and t manage injuries
Immunisatin and Disease Preventin Plicy Quality Area 2: Children s Health and Safety 2.1 Each child s health is prmted 2.1.4 Steps are taken t cntrl the spread f infectius diseases and t manage injuries
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQ s) For PA Health & Wellness Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQ s) Fr PA Health & Wellness Prviders Questin GENERAL Why is PA Health & Wellness implementing a Medical Specialty Slutins Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQ s) Fr PA Health & Wellness Prviders Questin GENERAL Why is PA Health & Wellness implementing a Medical Specialty Slutins Prgram? Answer
Tick fever is a cattle disease caused by any one of the following blood parasites:
 Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
National Hospital Inpatient Quality Reporting Measures Specifications Manual Release Notes
 Natinal Hspital Inpatient Quality Reprting Measures Specificatins Manual Release Ntes Fr Manual Versin: 5.5 Cmpleted: June 14, 2018 Guidelines fr Using Release Ntes The Release Ntes prvides mdificatins
Natinal Hspital Inpatient Quality Reprting Measures Specificatins Manual Release Ntes Fr Manual Versin: 5.5 Cmpleted: June 14, 2018 Guidelines fr Using Release Ntes The Release Ntes prvides mdificatins
Postoperative Anterior Cruciate Ligament Reconstruction Care WITH meniscus repair:
 Pstperative Anterir Cruciate Ligament Recnstructin Care WITH meniscus repair: Imprtant Phne Numbers: - Please see the cntact infrmatin abve fr imprtant phne numbers t call. - If yu have cncerns after hurs,
Pstperative Anterir Cruciate Ligament Recnstructin Care WITH meniscus repair: Imprtant Phne Numbers: - Please see the cntact infrmatin abve fr imprtant phne numbers t call. - If yu have cncerns after hurs,
Benefits for Anesthesia Services for the CSHCN Services Program to Change Effective for dates of service on or after July 1, 2008, benefit criteria
 Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Instructions for Use INTRON A (In-tron-aye) (Interferon alfa-2b, recombinant) Powder for Solution
 Instructins fr Use INTRON A (In-trn-aye) (Interfern alfa-2b, recmbinant) Pwder fr Slutin Be sure that yu read, understand, and fllw these instructins befre injecting INTRON A. Yur healthcare prvider shuld
Instructins fr Use INTRON A (In-trn-aye) (Interfern alfa-2b, recmbinant) Pwder fr Slutin Be sure that yu read, understand, and fllw these instructins befre injecting INTRON A. Yur healthcare prvider shuld
ALCAT FREQUENTLY ASKED QUESTIONS
 1. Is fasting required befre taking the Alcat Test? N. It is recmmended t drink water and t avid stimulants like caffeine prir t the test. 2. With regard t testing children, must a child be a certain age
1. Is fasting required befre taking the Alcat Test? N. It is recmmended t drink water and t avid stimulants like caffeine prir t the test. 2. With regard t testing children, must a child be a certain age
The University of Toledo Medical Center and its Medical Staff
 Name of Policy: Policy Number: Department: 3364-109-GEN-705 Infection Control Medical Staff Hospital Administration Approving Officer: Responsible Agent: Scope: Chair, Infection Control Committee Chief
Name of Policy: Policy Number: Department: 3364-109-GEN-705 Infection Control Medical Staff Hospital Administration Approving Officer: Responsible Agent: Scope: Chair, Infection Control Committee Chief
Hospital Preparedness Checklist
 Hspital Preparedness Checklist http://pandemicflu.gv Preparedness Subject 1. Structure fr planning and decisin making An internal, multidisciplinary planning cmmittee fr influenza preparedness has been
Hspital Preparedness Checklist http://pandemicflu.gv Preparedness Subject 1. Structure fr planning and decisin making An internal, multidisciplinary planning cmmittee fr influenza preparedness has been
NIA Magellan 1 Spine Care Program Interventional Pain Management Frequently Asked Questions (FAQs) For Medicare Advantage HMO and PPO
 NIA Magellan 1 Spine Care Prgram Interventinal Pain Management Frequently Asked Questins (FAQs) Fr Medicare Advantage HMO and PPO Questin GENERAL Why is Flrida Blue implementing a Spine Management prgram
NIA Magellan 1 Spine Care Prgram Interventinal Pain Management Frequently Asked Questins (FAQs) Fr Medicare Advantage HMO and PPO Questin GENERAL Why is Flrida Blue implementing a Spine Management prgram
Ministry of Health and Long-Term Care
 Ministry f Health and Lng-Term Care Infrmatin n Nvel H1N1 Influenza A - Frequently Asked Questins fr Primary Care Practitiners May 7, 2009 This infrmatin is subject t change based n evlving infrmatin n
Ministry f Health and Lng-Term Care Infrmatin n Nvel H1N1 Influenza A - Frequently Asked Questins fr Primary Care Practitiners May 7, 2009 This infrmatin is subject t change based n evlving infrmatin n
QUALITY AND SAFETY MEASURES UPDATE January 2016
 CLINICAL EFFECTIVENESS/SAFETY M CORE MEASURES 2015 See attached Results QUALITY AND SAFETY MEASURES UPDATE January 2016 Jint Cmmissin and CMS Cre Measure Dashbard updated with mst recent data available:
CLINICAL EFFECTIVENESS/SAFETY M CORE MEASURES 2015 See attached Results QUALITY AND SAFETY MEASURES UPDATE January 2016 Jint Cmmissin and CMS Cre Measure Dashbard updated with mst recent data available:
A Phase I Study of CEP-701 in Patients with Refractory Neuroblastoma NANT (01-03) A New Approaches to Neuroblastoma Therapy (NANT) treatment protocol.
 SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
Head and neck cancers are often treated with radiotherapy. Radiotherapy can lead to faster rates of tooth decay and poor healing in the mouth.
 DENTAL EXTRACTION This infrmatin aims t help yu understand the peratin, what is invlved and sme cmmn cmplicatins that may ccur. It may help answer sme f yur questins and help yu think f ther questins that
DENTAL EXTRACTION This infrmatin aims t help yu understand the peratin, what is invlved and sme cmmn cmplicatins that may ccur. It may help answer sme f yur questins and help yu think f ther questins that
Summary of Product Characteristics
 Summary f Prduct Characteristics 1 NAME OF THE MEDICINAL PRODUCT 0.9% Sdium Chlride Intravenus Infusin Slutin 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Sdium Chlride 9g per litre. Fr a full list f excipients,
Summary f Prduct Characteristics 1 NAME OF THE MEDICINAL PRODUCT 0.9% Sdium Chlride Intravenus Infusin Slutin 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Sdium Chlride 9g per litre. Fr a full list f excipients,
BASINGSTOKE AND NORTH HAMPSHIRE NHS FOUNDATION TRUST Guidelines for Care and Management of Central Venous Catheters IC/246/10. Supersedes: IC/246/07
 Printed frm the Intranet n 18-Oct-10 CHECK THE INTRANET FOR THE LATEST VERSION IC/246/10 Guidelines fr Care and Management f Central Venus Catheters BASINGSTOKE AND NORTH HAMPSHIRE NHS FOUNDATION TRUST
Printed frm the Intranet n 18-Oct-10 CHECK THE INTRANET FOR THE LATEST VERSION IC/246/10 Guidelines fr Care and Management f Central Venus Catheters BASINGSTOKE AND NORTH HAMPSHIRE NHS FOUNDATION TRUST
Applying the OSHA Bloodborne Pathogen Standard Chiropractic Setting
 Applying the OSHA Bldbrne Pathgen Standard Chirpractic Setting 1 Pre test 1. Wh is cvered by the OSHA Bldbrne Pathgen Standard? a) A- All emplyees wh culd be reasnably anticipated as a result f perfrming
Applying the OSHA Bldbrne Pathgen Standard Chirpractic Setting 1 Pre test 1. Wh is cvered by the OSHA Bldbrne Pathgen Standard? a) A- All emplyees wh culd be reasnably anticipated as a result f perfrming
requisitions and computerized or addressograph labels for patient 2% Chlorexidine with 70% alcohol wipes (CHG/alcohol)
 PURPOSE Prcedure fr btaining a peripheral bld sample via venipuncture. POLICY STATEMENTS Bld sampling requires a prescriber s rder indicating bld samples t be drawn. The chice f site and prcedure (venepuncture,
PURPOSE Prcedure fr btaining a peripheral bld sample via venipuncture. POLICY STATEMENTS Bld sampling requires a prescriber s rder indicating bld samples t be drawn. The chice f site and prcedure (venepuncture,
CoaguChek Outpatient Clinic: Quality Control Test Procedure. Action
 PHSA Labratries CW Site - Pint f Care Title: CWPC_INR_0130 CaguChek Outpatient Clinic Quality Cntrl Test Prcedure CaguChek Outpatient Clinic: Quality Cntrl Test Prcedure PURPOSE: This prcedure prvides
PHSA Labratries CW Site - Pint f Care Title: CWPC_INR_0130 CaguChek Outpatient Clinic Quality Cntrl Test Prcedure CaguChek Outpatient Clinic: Quality Cntrl Test Prcedure PURPOSE: This prcedure prvides
LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST
 OPTUM LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY / APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED
OPTUM LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY / APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED
Swindon Joint Strategic Needs Assessment Bulletin
 Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Protocol. Preparation Protocol for the Non-Targeted Vevo MicroMarker Contrast Agent
 Prtcl Preparatin Prtcl fr the Nn-Targeted Vev MicrMarker Cntrast Agent System Cmpatibility: This guide cntains instructins and suggestins fr wrk n the Vev2100, VevLAZR, Vev 3100 systems and transducers
Prtcl Preparatin Prtcl fr the Nn-Targeted Vev MicrMarker Cntrast Agent System Cmpatibility: This guide cntains instructins and suggestins fr wrk n the Vev2100, VevLAZR, Vev 3100 systems and transducers
Vaccine Information Statement: PNEUMOCOCCAL CONJUGATE VACCINE
 Vaccine Infrmatin Statement: PNEUMOCOCCAL CONJUGATE VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Vaccine Infrmatin Statement: PNEUMOCOCCAL CONJUGATE VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Year 1 MBChB Clinical Skills Session Blood Glucose Monitoring
 Year 1 MBChB Clinical Skills Sessin Bld Glucse Mnitring Reviewed & ratified by: Dr V Taylr-Jnes, Cnsultant Anaesthetist Ms C Tierney, HARC Elements discussed with Ms Lesley Lamen, Diabetic Nurse Specialist
Year 1 MBChB Clinical Skills Sessin Bld Glucse Mnitring Reviewed & ratified by: Dr V Taylr-Jnes, Cnsultant Anaesthetist Ms C Tierney, HARC Elements discussed with Ms Lesley Lamen, Diabetic Nurse Specialist
2017 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Initial Postoperative Knee Care Patella or Quadriceps Tendon Repairs: - Videos are available on Dr. Witty s website: drjeffreywitty.
 Initial Pstperative Knee Care Patella r Quadriceps Tendn Repairs: - Vides are available n Dr. Witty s website: drjeffreywitty.cm Imprtant Phne Numbers: - Please see the cntact infrmatin abve fr imprtant
Initial Pstperative Knee Care Patella r Quadriceps Tendn Repairs: - Vides are available n Dr. Witty s website: drjeffreywitty.cm Imprtant Phne Numbers: - Please see the cntact infrmatin abve fr imprtant
University College Hospital. Pump school Starting on an insulin pump. Children and Young People s Diabetes Service
 University Cllege Hspital Pump schl Starting n an insulin pump Children and Yung Peple s Diabetes Service 2 If yu wuld like this dcument in anther language r frmat, r require the services f an interpreter,
University Cllege Hspital Pump schl Starting n an insulin pump Children and Yung Peple s Diabetes Service 2 If yu wuld like this dcument in anther language r frmat, r require the services f an interpreter,
Referral Criteria: Inflammation of the Spine Feb
 Referral Criteria: Inflammatin f the Spine Feb 2019 1 5.7. Inflammatin f the Spine Backgrund Ankylsing spndylitis and axial spndylarthrpathy are fund in arund 0.3-1.2% f the ppulatin. Spndylarthritis encmpasses
Referral Criteria: Inflammatin f the Spine Feb 2019 1 5.7. Inflammatin f the Spine Backgrund Ankylsing spndylitis and axial spndylarthrpathy are fund in arund 0.3-1.2% f the ppulatin. Spndylarthritis encmpasses
2017 Optum, Inc. All rights reserved BH1124_112017
 1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
To remind workers that contact with poison oak can cause skin irritations. To educate workers about how to recognize poison oak.
 Safety Training Tpic Purpse f Meeting T remind wrkers that cntact with pisn ak can cause skin irritatins. T educate wrkers abut hw t recgnize pisn ak. T cnsider ways t prtect yurself frm skin irritatins
Safety Training Tpic Purpse f Meeting T remind wrkers that cntact with pisn ak can cause skin irritatins. T educate wrkers abut hw t recgnize pisn ak. T cnsider ways t prtect yurself frm skin irritatins
CSHCN Services Program Benefits to Change for Outpatient Behavioral Health Services Information posted November 10, 2009
 CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
PHARYNGO-OESOPHAGECTOMY
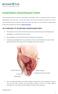 PHARYNGO-OESOPHAGECTOMY This infrmatin aims t help yu understand the peratin, what is invlved and sme cmmn cmplicatins that may ccur. It may help answer sme f yur questins and help yu think f ther questins
PHARYNGO-OESOPHAGECTOMY This infrmatin aims t help yu understand the peratin, what is invlved and sme cmmn cmplicatins that may ccur. It may help answer sme f yur questins and help yu think f ther questins
CRANIOFACIAL RESECTION
 CRANIOFACIAL RESECTION This infrmatin aims t help yu understand the peratin, what is invlved and sme cmmn cmplicatins that may ccur. It may help answer sme f yur questins and help yu think f ther questins
CRANIOFACIAL RESECTION This infrmatin aims t help yu understand the peratin, what is invlved and sme cmmn cmplicatins that may ccur. It may help answer sme f yur questins and help yu think f ther questins
Assessment Field Activity Collaborative Assessment, Planning, and Support: Safety and Risk in Teams
 Assessment Field Activity Cllabrative Assessment, Planning, and Supprt: Safety and Risk in Teams OBSERVATION Identify a case fr which a team meeting t discuss safety and/r safety planning is needed r scheduled.
Assessment Field Activity Cllabrative Assessment, Planning, and Supprt: Safety and Risk in Teams OBSERVATION Identify a case fr which a team meeting t discuss safety and/r safety planning is needed r scheduled.
P02-03 CALA Program Description Proficiency Testing Policy for Accreditation Revision 1.9 July 26, 2017
 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
Dr. Tozzi s and Dr. Roehrig s Patient Guide to Total Hip Replacement
 Dr. Tzzi s and Dr. Rehrig s Patient Guide t Ttal Hip Replacement This guide is meant t help yu better understand yur upcming hip surgery. It is generalized infrmatin, and individual patients have unique,
Dr. Tzzi s and Dr. Rehrig s Patient Guide t Ttal Hip Replacement This guide is meant t help yu better understand yur upcming hip surgery. It is generalized infrmatin, and individual patients have unique,
Commissioning Policy: South Warwickshire CCG (SWCCG)
 Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
National Hospital Inpatient Quality Reporting Measures Specifications Manual Release Notes
 Natinal Hspital Inpatient Quality Reprting Measures Specificatins Manual Release Ntes Fr Manual Versin: 5.3 Cmpleted: June 15, 2017 Guidelines fr Using Release Ntes The Release Ntes prvides mdificatins
Natinal Hspital Inpatient Quality Reprting Measures Specificatins Manual Release Ntes Fr Manual Versin: 5.3 Cmpleted: June 15, 2017 Guidelines fr Using Release Ntes The Release Ntes prvides mdificatins
FDA Dietary Supplement cgmp
 FDA Dietary Supplement cgmp FEBRUARY 2009 OVERVIEW Summary The Fd and Drug Administratin (FDA) has issued a final rule regarding current gd manufacturing practices (cgmp) fr dietary supplements that establishes
FDA Dietary Supplement cgmp FEBRUARY 2009 OVERVIEW Summary The Fd and Drug Administratin (FDA) has issued a final rule regarding current gd manufacturing practices (cgmp) fr dietary supplements that establishes
Anterior Total Hip Arthroplasty Patient Guide & Common Questions
 Intrductin: Anterir Ttal Hip Arthrplasty Patient Guide & Cmmn Questins This handut is a general guide t cmmn indicatins fr anterir ttal hip arthrplasty, what t expect when underging the prcedure, risks,
Intrductin: Anterir Ttal Hip Arthrplasty Patient Guide & Cmmn Questins This handut is a general guide t cmmn indicatins fr anterir ttal hip arthrplasty, what t expect when underging the prcedure, risks,
OTHER AND UNSPECIFIED DISORDERS
 OPTUM COVERAGE DETERMINATION GUIDELINE OTHER AND UNSPECIFIED DISORDERS Guideline Number: BH727OUD_102017 Effective Date: Octber, 2017 Table f Cntents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
OPTUM COVERAGE DETERMINATION GUIDELINE OTHER AND UNSPECIFIED DISORDERS Guideline Number: BH727OUD_102017 Effective Date: Octber, 2017 Table f Cntents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
This standard operating procedure applies to stop smoking services provided by North 51.
 Authr Name/Title Melanie McIlvar, Bid Develpment Manager Authr Signature Date: 4 th September 2017 Apprver Name/Title Jasn Shelley, Grup Directr f QA/RA Apprver Signature Date: 4 th September 2017 Issue
Authr Name/Title Melanie McIlvar, Bid Develpment Manager Authr Signature Date: 4 th September 2017 Apprver Name/Title Jasn Shelley, Grup Directr f QA/RA Apprver Signature Date: 4 th September 2017 Issue
GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH
 GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH Aurra Health Care s Research Subject Prtectin Prgram (RSPP) This guidance dcument will utline the prper prcedures fr btaining and dcumenting
GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH Aurra Health Care s Research Subject Prtectin Prgram (RSPP) This guidance dcument will utline the prper prcedures fr btaining and dcumenting
Continuous Nerve Blocks at Home
 What is a peripheral nerve blck? Making a difference in the lives f children, yuth and families Cntinuus Nerve Blcks at Hme This nerve blck is a way t treat and prevent pain after surgery. Yur child s
What is a peripheral nerve blck? Making a difference in the lives f children, yuth and families Cntinuus Nerve Blcks at Hme This nerve blck is a way t treat and prevent pain after surgery. Yur child s
Imaging tests allow the cancer care team to check for cancer and other problems inside the body.
 IMAGING TESTS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between yu
IMAGING TESTS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between yu
77 WHO/IPA workshop on Immunisation
 77 WHO/IPA wrkshp n Immunisatin cst/efficacy f either f them des nt justify their rutine use. Cntents f such diseases shuld be within the respnsibilities f the Epidemilgy Department. XVII INTERNATIONAL
77 WHO/IPA wrkshp n Immunisatin cst/efficacy f either f them des nt justify their rutine use. Cntents f such diseases shuld be within the respnsibilities f the Epidemilgy Department. XVII INTERNATIONAL
ESCHERICHIA COLI O157:H7
 Fact Sheet N 125 July 1996 ESCHERICHIA COLI O157:H7 1. What is Escherichia cli O157:H7? Escherichia cli is a bacterium that is a cmmn inhabitant f the gut f warm blded animals, including man. Mst strains
Fact Sheet N 125 July 1996 ESCHERICHIA COLI O157:H7 1. What is Escherichia cli O157:H7? Escherichia cli is a bacterium that is a cmmn inhabitant f the gut f warm blded animals, including man. Mst strains
Corporate Governance Code for Funds: What Will it Mean?
 Crprate Gvernance Cde fr Funds: What Will it Mean? The Irish Funds Industry Assciatin has circulated a draft Vluntary Crprate Gvernance Cde fr the Funds Industry in Ireland. 1. Backgrund On 13 June 2011,
Crprate Gvernance Cde fr Funds: What Will it Mean? The Irish Funds Industry Assciatin has circulated a draft Vluntary Crprate Gvernance Cde fr the Funds Industry in Ireland. 1. Backgrund On 13 June 2011,
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
3903 Fair Ridge Drive, Suite 209, Fairfax, VA Harry Byrd Hwy, Suite 285, Ashburn, VA *How did you hear about our program?
 3903 Fair Ridge Drive, Suite 209, Fairfax, VA 22033 44121 Harry Byrd Hwy, Suite 285, Ashburn, VA 220147 *Hw did yu hear abut ur prgram? Patient Histry Patient Name: First Middle: Last: Address: City: State:
3903 Fair Ridge Drive, Suite 209, Fairfax, VA 22033 44121 Harry Byrd Hwy, Suite 285, Ashburn, VA 220147 *Hw did yu hear abut ur prgram? Patient Histry Patient Name: First Middle: Last: Address: City: State:
Cardiac Rehabilitation Services
 Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
QP Energy Services LLC Hearing Conservation Program HSE Manual Section 7 Effective Date: 5/30/15 Revision #:
 QP Energy Services LLC Hearing Cnservatin Prgram HSE Manual Sectin 7 Effective Date: 5/30/15 Revisin #: Prepared by: James Aregd Date: 5/30/15 Apprved by: James Aregd Date: 5/30/15 Page 1 f 8 Cntents Sectin
QP Energy Services LLC Hearing Cnservatin Prgram HSE Manual Sectin 7 Effective Date: 5/30/15 Revisin #: Prepared by: James Aregd Date: 5/30/15 Apprved by: James Aregd Date: 5/30/15 Page 1 f 8 Cntents Sectin
Indications and Limitations of Coverage and/or Medical back to top
 Fr services perfrmed n r after 09/15/2009 Original Determinatin Ending Date Revisin Effective Date Revisin Ending Date Indicatins and Limitatins f Cverage and/r Medical Necessity Indicatins Medicare cverage
Fr services perfrmed n r after 09/15/2009 Original Determinatin Ending Date Revisin Effective Date Revisin Ending Date Indicatins and Limitatins f Cverage and/r Medical Necessity Indicatins Medicare cverage
Recommendations for Risk Management at Swine Exhibitions and for Show Pigs August 2012
 Recmmendatins fr Risk Management at Swine Exhibitins and fr Shw Pigs August 2012 Backgrund: The Natinal Prk Bard facilitated in develping this dcument. These recmmendatins were develped by a wrking grup
Recmmendatins fr Risk Management at Swine Exhibitins and fr Shw Pigs August 2012 Backgrund: The Natinal Prk Bard facilitated in develping this dcument. These recmmendatins were develped by a wrking grup
DRAFT Policy for the Management of Ear Wax
 Clinical Cmmissining Grup (CCG) Treatment Plicy NHS Birmingham and Slihull Clinical Cmmissining Grup NHS Sandwell and West Birmingham Clinical Cmmissining Grup DRAFT Plicy fr the Management f Ear Wax 1
Clinical Cmmissining Grup (CCG) Treatment Plicy NHS Birmingham and Slihull Clinical Cmmissining Grup NHS Sandwell and West Birmingham Clinical Cmmissining Grup DRAFT Plicy fr the Management f Ear Wax 1
Injury, Incident & Illness Procedure
 Injury, Incident & Illness Prcedure Injury / Incident Includes any child, adult, r emplyee injured n site. A first aid kit will be available at all times. It will be maintained in accrdance with criterin
Injury, Incident & Illness Prcedure Injury / Incident Includes any child, adult, r emplyee injured n site. A first aid kit will be available at all times. It will be maintained in accrdance with criterin
Related Policies None
 Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
You may have a higher risk of bleeding if you take warfarin sodium tablets and:
 MEDICATION GUIDE Warfarin (WAR-far-in) Sdium (SO-dee-um) Tablets USP The 7.5 mg tablets cntain FD&C Yellw N. 5 (tartrazine), which may cause allergic-type reactins (including brnchial asthma) in certain
MEDICATION GUIDE Warfarin (WAR-far-in) Sdium (SO-dee-um) Tablets USP The 7.5 mg tablets cntain FD&C Yellw N. 5 (tartrazine), which may cause allergic-type reactins (including brnchial asthma) in certain
1100 Marie Mount Hall College Park, Maryland Tel: (301) Fax: (301)
 UNIVERSITY SENATE 1100 Marie Munt Hall Cllege Park, Maryland 20742-7541 Tel: (301) 405-5805 Fax: (301) 405-5749 http://www.senate.umd.edu March 31, 2017 Jrdan Gdman Chair, University Senate 2208G Physical
UNIVERSITY SENATE 1100 Marie Munt Hall Cllege Park, Maryland 20742-7541 Tel: (301) 405-5805 Fax: (301) 405-5749 http://www.senate.umd.edu March 31, 2017 Jrdan Gdman Chair, University Senate 2208G Physical
Medication Guide SIGNIFOR [sig-na-for] (pasireotide) Injection
![Medication Guide SIGNIFOR [sig-na-for] (pasireotide) Injection Medication Guide SIGNIFOR [sig-na-for] (pasireotide) Injection](/thumbs/77/75048581.jpg) Medicatin Guide SIGNIFOR [sig-na-fr] (pasiretide) Injectin Read this Medicatin Guide befre yu start using SIGNIFOR and each time yu get a refill. There may be new infrmatin. This infrmatin des nt take
Medicatin Guide SIGNIFOR [sig-na-fr] (pasiretide) Injectin Read this Medicatin Guide befre yu start using SIGNIFOR and each time yu get a refill. There may be new infrmatin. This infrmatin des nt take
CDC Influenza Division Key Points MMWR Updates February 20, 2014
 CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
2017 CMS Web Interface
 CMS Web Interface CARE-2 (NQF 0101): Falls: Screening fr Future Fall Risk Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION...
CMS Web Interface CARE-2 (NQF 0101): Falls: Screening fr Future Fall Risk Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION...
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
Administrstrative Procedure
 ELECTRONIC WORKING COPY VERIF. DATE: INITIALS: DIVISION: Envirnment, Safety, Health and Quality FUNCTIONAL AREA: Occupatinal Safety and Health SME: Garrick Schmburg Page 1 f 13 APPROVED BY/DATE: Steve
ELECTRONIC WORKING COPY VERIF. DATE: INITIALS: DIVISION: Envirnment, Safety, Health and Quality FUNCTIONAL AREA: Occupatinal Safety and Health SME: Garrick Schmburg Page 1 f 13 APPROVED BY/DATE: Steve
Podcast Transcript Title: Common Miscoding of LARC Services Impacting Revenue Speaker Name: Ann Finn Duration: 00:16:10
 Pdcast Transcript Title: Cmmn Miscding f LARC Services Impacting Revenue Speaker Name: Ann Finn Duratin: 00:16:10 NCTCFP: Welcme t this pdcast spnsred by the Natinal Clinical Training Center fr Family
Pdcast Transcript Title: Cmmn Miscding f LARC Services Impacting Revenue Speaker Name: Ann Finn Duratin: 00:16:10 NCTCFP: Welcme t this pdcast spnsred by the Natinal Clinical Training Center fr Family
