Clinical Policy Title: Cecostomy for fecal incontinence
|
|
|
- Sandra Terry
- 5 years ago
- Views:
Transcription
1 Clinical Policy Title: Cecostomy for fecal incontinence Clinical Policy Number: Effective Date: July 1, 2016 Initial Review Date: February 17, 2016 Most Recent Review Date: April 19, 2017 Next Review Date: April 2018 Policy contains: Chronic constipation. Fecal incontinence (FI). Open and percutaneous cecostomy. Related policies: CP# Injectable bulking agents for fecal incontinence ABOUT THIS POLICY: Select Health of South Carolina has developed clinical policies to assist with making coverage determinations. Select Health of South Carolina s clinical policies are based on guidelines from established industry sources, such as the Centers for Medicare & Medicaid Services (CMS), state regulatory agencies, the American Medical Association (AMA), medical specialty professional societies, and peerreviewed professional literature. These clinical policies along with other sources, such as plan benefits and state and federal laws and regulatory requirements, including any state- or plan-specific definition of medically necessary, and the specific facts of the particular situation are considered by Select Health of South Carolina when making coverage determinations. In the event of conflict between this clinical policy and plan benefits and/or state or federal laws and/or regulatory requirements, the plan benefits and/or state and federal laws an d/or regulatory requirements shall control. Select Health of South Carolina s clinical policies are for informational purposes only and not intended as medical advice or to direct treatment. Physicians and other health care providers are solely responsible for the treatment decisions for their patients. Select Health of South Carolina s clinical policies are reflective of evidence-based medicine at the time of review. As medical science evolves, Select Health of South Carolina will update its clinical policies as necessary. Select Health of South Carolina s clinical policies are not guarantees of payment. Coverage policy Select Health of South Carolina considers the use of cecostomy to be clinically proven and, therefore, medically necessary for treatment of fecal incontinence (FI) when all of the following criteria are met: Persons ages 4 years or older. Unresponsive to conservative treatment for relieving the bowels for at least a 60-day period. Conservative treatment consists of at least two of the following: - Biofeedback. - Lifestyle and dietary modifications. - Bowel habit interventions. - Anal plugs. - Pelvic floor muscle training. - Rectal irrigation. - Drug therapy. - Electrostimulation. For the purpose of either: - Facilitating an antegrade continence enema (ACE) in persons with FI secondary to neurologic disease. 1
2 - Providing cecal decompression for patients with chronic refractory constipation, chronic colonic pseudo-obstruction, or colonic obstruction. Limitations: All other clinical indications are not medically necessary. Absolute contraindications to cecostomy include previous abdominal surgical procedures; active peritonitis, colitis, or ileocolitis; uncorrectable coagulopathy; bowel ischemia; and excessive abdominal wall fat. Relative contraindications include recent gastrointestinal bleeding, hemodynamic instability, ascites, respiratory compromise, and certain anatomic alterations. For patients receiving anticoagulant or antiplatelet therapy: International Normalized Ratio (INR) less than 1.5. Platelet count greater than 50,000/µL. Alternative covered services: Lifestyle and dietary modifications. Bowel habit interventions. Anal plugs. Pelvic floor muscle training. Rectal irrigation. Drug therapy (e.g., bulk-forming agents [fibers], emollient stool softeners, rapidly acting lubricants, prokinetics, laxatives, osmotic agents, and prosecretory drugs). Electrostimulation. Other surgical or minimally invasive procedures (e.g., colostomy, artificial bowel sphincter, or dynamic graciloplasty). Background FI is a debilitating symptom resulting from deficits in factors that control bowel function. Organic causes include neurogenic disorders, inflammatory disorders, obstetric trauma, and anorectal anomalies. Functional causes encompass bowel disturbances, most commonly constipation with or without fecal impaction or overflow diarrhea, without evidence of a structural or biochemical explanation (Bharucha, 2015). Definitions of FI vary according to target population (adults versus children), symptoms, symptom duration, and criteria used (Bharucha, 2015; Paquette, 2015; Dobson, 2009). A working definition from 2
3 the American Society of Colon and Rectal Surgeons (ASCRS) encompasses several factors: The uncontrolled passage of feces or gas over at least one month s duration, in an individual of at least 4 years of age, who had previously achieved control (Paquette, 2015). FI is a clinical diagnosis primarily based on history and examination, and may include anal manometry, anal ultrasound, colonic transit study, magnetic resonance imaging (MRI), defecography, flexible sigmoidoscopy or colonoscopy, and anal electromyography (EMG) (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK], 2016). Initial treatment typically involves one or more of the following conservative approaches: dietary modifications, medications (laxatives and suppositories), rectal irrigation, bowel training, pelvic floor exercises, biofeedback, manual disimpaction, and electrostimulation (NIDDK, 2016). Surgery may be indicated for FI refractory to conservative treatment or for colonic pseudo-obstruction. Cecostomy: Cecostomy is the creation of an opening in the cecum to facilitate an ACE or to provide cecal decompression (Itkin, 2011). The procedure involves a standard colonoscopy preparation followed by placement of a temporary decompressive or lavage cecostomy tube (C-tube) surgically or percutaneously with endoscopic or image guidance. Fluoroscopically-guided percutaneous cecostomy is performed according to the technique first described by Chait, et al., in treating FI in children (Chait, 1997; Itkin, 2011). The cecostomy tube/catheter used in this procedure has received marketing approval as a Class II device (U.S. Food and Drug Administration, 2016). For open cecostomy, the hospital length of stay ranges from five days to 10 days. Patients undergoing percutaneous cecostomy typically have a shorter hospital stay. Approximately one week after the procedure, the patient begins self-administering ACEs through the C-tube, and an individualized irrigation routine is established. After six weeks, the temporary catheter is exchanged for a semipermanent, low-profile cecostomy catheter designed to accommodate different lengths of subcutaneous tissue. This is an outpatient procedure performed by a gastroenterologist, colorectal surgeon, or interventional radiologist over a wire with fluoroscopic guidance, without sedation or antibiotic coverage. Replacement of the semipermanent catheters is performed annually. Searches Select Health of South Carolina searched PubMed and the databases of: UK National Health Services Centre for Reviews and Dissemination. Agency for Healthcare Research and Quality s National Guideline Clearinghouse and other evidence-based practice centers. The Centers for Medicare & Medicaid Services (CMS). 3
4 We conducted searches on February 23, Search terms were: cecostomy [MeSH] and free text terms cecostomy and caecostomy. We included: Systematic reviews, which pool results from multiple studies to achieve larger sample sizes and greater precision of effect estimation than in smaller primary studies. Systematic reviews use predetermined transparent methods to minimize bias, effectively treating the review as a scientific endeavor, and are thus rated highest in evidence-grading hierarchies. Guidelines based on systematic reviews. Economic analyses, such as cost-effectiveness, and benefit or utility studies (but not simple cost studies), reporting both costs and outcomes sometimes referred to as efficiency studies which also rank near the top of evidence hierarchies. Findings We identified three systematic reviews or evidence reports, three evidence-based guidelines, two new case series, one retrospective cohort study, and no economic studies for this policy. The evidence consists of largely single-institution, retrospective case series without comparators. One retrospective cohort study compared the Malone ACE (MACE) procedure to a cecostomy button in adults and children with neurogenic bowel dysfunction (Hoy, 2013). Both surgical and percutaneous cecostomy procedures have been reported in the literature, but the number of studies favors percutaneous placement. For ACE delivery, the Chait Trapdoor catheter was used in the majority of patients. The overall quality of the evidence is low, and the evidence lacks prospective comparison to other surgical alternatives and clearly defined, consistent inclusion criteria and outcome measures. The evidence suggests that cecostomy may improve some symptoms of chronic refractory constipation with FI and pseudo-obstruction. The effect on quality of life (QOL) was inconsistent. Minor complications associated with the procedure were common and similar to those seen with percutaneous gastrostomy and percutaneous feeding tubes, including local infection at the wound or ACE site, catheter-related complications, and the development of granulation tissue. These complications were usually mild and easily treatable, and no major complications associated with the procedure were reported. Limited evidence suggests no difference in complication rates between endoscopic and radiologic placement (Itkin, 2011). However, prospective comparative studies are needed to determine how cecostomy compares with other surgical or minimally invasive procedures (e.g., colostomy, artificial bowel sphincter or dynamic graciloplasty) in functional outcomes, post-procedural complications and QOL. One Cochrane review found a striking lack of high-quality randomized controlled trials (RCTs) on FI surgery, with existing RCTs focusing on sacral neuromodulation and injectable bulking agents (Brown, 2013). Therefore, clinical research provides limited guidance for use of alternative surgical procedures such as cecostomy. 4
5 Improved patient selection criteria are needed to select the appropriate patients with chronic refractory FI for the respective open and minimally invasive procedures. Clinical indications for adults included chronic colonic pseudo-obstruction, colonic obstruction, chronic refractory constipation with FI, acute traumatic anal sphincter rupture, major defect in the external anal sphincter in the presence of gross FI and rectal prolapse. Cecostomy was used in children mainly with neurologic disease (e.g., spina bifida, spinal cord injury, cerebral palsy) that results in severe refractory FI. Absolute contraindications to C-tube placement include previous abdominal surgical procedures; active peritonitis, colitis, and ileocolitis; uncorrectable coagulopathy; bowel ischemia; and excessive abdominal wall fat. Relative contraindications include recent gastrointestinal bleeding, hemodynamic instability, ascites, respiratory compromise and certain anatomic alterations (Hayes, 2011a; Hayes, 2011b; Itkin, 2011). According to one multidisciplinary guideline, percutaneous cecostomy is indicated for patients with neurologic disease that results in FI to facilitate cleansing enemas and for treatment of chronic refractory constipation, chronic colonic pseudo-obstruction and colonic obstruction (Itkin, 2011). The ASCRS guideline mentions cecostomy, noting the limited evidence supporting the procedure, but recommends neither for nor against its use (Paquette, 2015). The demand for percutaneous cecostomy for FI may increase, despite a lack of high-quality evidence. Both surgeons and patients may demand it, since the procedure may delay the need for more invasive surgical treatments. The Society of Interventional Radiology (SIR) and American Gastroenterological Association (AGA) Institute issued recommendations for pre-procedural risk assessment to identify candidates for cecostomy (Itkin, 2011). Pre-procedure assessment involves evaluation of procedural risk from bleeding and patient s probability of a thromboembolic complication occurring should anticoagulation or antiplatelet therapy be stopped before the procedure. Cecostomy is considered a high-risk procedure. Table 1 presents risk determination based on patient condition (Itkin, 2011). Table 1. SIR and AGA determination of risk for patients receiving anticoagulant or antiplatelet therapy based on clinical condition. Level of risk Patients receiving anticoagulant therapy Patients receiving antiplatelet therapy Low risk Aortic metal valve. Coronary artery disease (CAD) without Atrial fibrillation without valvular disease. stents. Xenograft valve. CAD with drug-eluting stents > 12 months Deep vein thrombosis > three months after out. event. CAD with bare stents > one month out. Cerebrovascular accident. Arteriosclerotic peripheral vascular disease. High risk Mitral metal valve. CAD with drug-eluting stents < 12 months Atrial fibrillation with prosthetic valve. out. Atrial fibrillation with mitral valve stenosis. CAD with bare stents < one month out. Deep venous thrombosis < three months 5
6 Level of risk Patients receiving anticoagulant therapy Patients receiving antiplatelet therapy after event. Thrombophilia syndromes. AGA recommendations that apply to cecostomy for a patient with a low-risk condition are (Itkin, 2011): If on warfarin, warfarin should be stopped five days before the procedure. The INR should be checked on the day of the procedure and should be confirmed to be lower than 1.5. Warfarin may be started later on the night of the procedure, with INR checked one week later. Clopidogrel therapy should be discontinued seven days before the procedure, with aspirin therapy continued. If the patient is not receiving aspirin, aspirin therapy should be started as the patient discontinues receiving clopidogrel. AGA recommendations that apply to cecostomy for a patient with a high-risk condition are (Itkin, 2011): Warfarin should be stopped five days before the procedure. A therapeutic dose of low molecular weight heparin should be substituted, with the dose withheld on the morning of the procedure. On the night of the procedure, warfarin should be restarted at the full therapeutic dose. For clopidogrel therapy, the clinician should discuss the necessity of the procedure first with the primary care physician, as risk is significant. If the procedure is deemed to be essential, clopidogrel should be stopped seven days before surgery and the patient given aspirin therapy in the interim. Clopidogrel therapy may be restarted on the morning after the procedure. SIR recommendations for cecostomy include (Itkin, 2011): INR: If greater than 1.5, correct until it is less than 1.5. Policy updates: Platelets: If platelet count is lower than 50,000/µL, administer transfusion until the count is greater than 50,000/µL. Clopidogrel: Withhold for five days before the procedure. Aspirin: Do not withhold. Low molecular weight heparin (therapeutic dose): Withhold one dose before the procedure. None. Summary of clinical evidence: 6
7 Citation Hayes (2015) Cecostomy for slow transit constipation in adults Khan (2015) Percutaneous C-tube in the management of FI in children DeFreest (2014) Laparoscopic-assisted percutaneous cecostomy for ACE Hoy (2013) MACE versus cecostomy button in patients with neurogenic bowel dysfunction Hayes (2011a, updated 2012, archived 2014) Cecostomy for treatment of FI in adults Content, Methods, Recommendations Search and summary report without analysis of one case series (21 patients), one retrospective single-center study (eight patients), one retrospective case series (54 patients) and one retrospective standardized questionnaire (23 patients). Overall quality: low. Retrospective, single-arm studies, lacking clear descriptions of patient populations and outcomes. Percutaneous endoscopic cecostomy (PEC) placement is safe and achieved satisfying functional and quality of life results in 75% of patients. PEC removed in 25% of patients because of chronic wound pain. Comparable outcomes with PEC or surgically or fluoroscopically placed cecostomy in some patients with recurrent colonic pseudoobstruction or chronic intractable constipation. Insufficient published evidence to assess the safety and/or impact on health outcomes or patient management of cecostomy for treatment of slow transit constipation. Retrospective case series of 290 children (mean age 10.1 years) with FI who underwent percutaneous C-tube placement and subsequent tube management between June 1994 and March 2009 and followed until March Technical success: tube placement (98%), exchange to a low-profile tube (92%). After routine exchanges to low-profile tubes, a minority of patients experienced problems and most problems were minor. Authors conclusions: The percutaneous cecostomy procedure is feasible and safe for FI in the pediatric population. Single center retrospective series of 16 children (eight boys). Mean age at procedure was 11 years (range 6 16 years). Diagnoses were functional constipation with soiling (14 patients), incontinence after surgery for Hirschsprung's disease (one patient), and constipation secondary to mitochondrial disease (one patient). Seven had significant developmental or psychiatric problems. Mean follow-up after initial cecostomy = 22 months (range, 6 51 months). Authors conclusions: Laparoscopic-assisted percutaneous cecostomy has an excellent safety profile and patient comfort. The procedure is simple, secure, and reversible. Cessation of fecal soiling with no need for diapers achieved in half of the patients. Associated psychiatric or behavioral problems may predict poor response to ACE. Retrospective chart review of patients who underwent MACE (26 patients) or cecostomy (23 patients) at the University of Alberta between January 2006 and January No significant differences between MACE and cecostomy button with respect to FI or complication rates at one year follow-up. Each approach poses unique challenges. Systematic review of three small retrospective case series (48 total patients). PEC performed in two studies, open surgery in one study for colonic decompression or delivery of ACE (using Chait device or gastrostomy button). 7
8 Citation Hayes (2011b, updated 2012, archived 2014) Cecostomy for treatment of FI in children Content, Methods, Recommendations Overall quality: very low. Retrospective and uncontrolled with serious methodological flaws, used subjective study outcomes, and had incomplete analysis of data. Cecostomy is feasible in adults with FI. Cecostomy may improve some symptoms of chronic refractory constipation with FI and pseudo-obstruction. The effect on QOL was inconsistent. Complications with either procedure were mild with no need for surgery Systematic review of one prospective case series and five retrospective case series (378 total children) with chronic refractory constipation or chronic FI secondary to a variety of diagnoses. PEC or open surgical technique used. Chait trapdoor catheter was used for ACE delivery in the majority of patients. Follow-up one month to seven years. Overall quality: low. Retrospective design, no control groups and use of subjective outcome measures; no well-designed prospective trials. Procedural complications were relatively minor and treatable related to tube placement, integrity or infection. Most frequently reported: granulation tissue (range 4% to 68%) and leakage along the button (range 42% to 48%). Limited evidence that PEC may improve symptoms of chronic FI in children by reducing the number of FI episodes per week and improving some measures of QOL. Relative efficacy and safety of PEC or surgical cecostomy and more definitive patient selection criteria are needed. References Professional society guidelines/other: 510(k) Premarket Notification database searched using 510(k) number K FDA website. Accessed February 23, Fecal incontinence. National Institute of Diabetes and Digestive and Kidney Diseases website. Accessed February 23, Itkin M, DeLegge MH, Fang JC, et al. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Gastroenterology. 2011; 141(2): Paquette IM, Varma MG, Kaiser AM, Steele SR, Rafferty JF. The American Society of Colon and Rectal Surgeons' Clinical Practice Guideline for the Treatment of Fecal Incontinence. Dis Colon Rectum. 2015; 58(7):
9 Rao SS, American College of Gastroenterology Practice Parameters C. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 2004; 99(8): Peer-reviewed references: Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015; 110(1): Brown SR, Wadhawan H, Nelson RL. Surgery for faecal incontinence in adults. Cochrane Database Syst Rev. 2013; 7: Cd Chait PG, Shandling B, Richards HM, Connolly BL. Fecal incontinence in children: treatment with percutaneous cecostomy tube placement a prospective study. Radiology. 1997; 203(3): DeFreest L, Smith J, Whyte C. Laparoscopic-assisted percutaneous cecostomy for antegrade continence enema. J Laparoendosc Adv Surg Tech A. 2014; 24(4): Dobson P, Rogers J. Assessing and treating faecal incontinence in children. Nurs Stand. 2009; 24(2): 49 56; quiz 58, 60. Frattini JC, Nogueras JJ. Slow Transit Constipation: A Review of a Colonic Functional Disorder. Clinics in Colon and Rectal Surgery. 2008; 21(2): Hayes Inc., Hayes Medical Technology Report. Cecostomy for children. Lansdale, Pa. Hayes Inc.; 2011 [Updated Archived 2014].(a) Hayes Inc., Hayes Medical Technology Report. Cecostomy-adults. Lansdale, Pa. Hayes Inc.; 2011 [Updated Archived 2014].(b) Hayes Inc., Hayes Medical Technology Report. Search and summary. slow transit. Lansdale, Pa. Hayes Inc.; Hoy NY, Metcalfe P, Kiddoo DA. Outcomes following fecal continence procedures in patients with neurogenic bowel dysfunction. J Urol. 2013; 189(6): Khan WU, Satkunasingham J, Moineddin R, et al. The percutaneous cecostomy tube in the management of fecal incontinence in children. J Vasc Interv Radiol. 2015; 26(2): CMS National Coverage Determinations (NCDs): 9
10 No NCDs identified as of the writing of this policy. Local Coverage Determinations (LCDs): No LCDs identified as of the writing of this policy. Commonly submitted codes Below are the most commonly submitted codes for the service(s)/item(s) subject to this policy. This is not an exhaustive list of codes. Providers are expected to consult the appropriate coding manuals and bill accordingly. CPT Code Description Comments Laparoscopy, surgical, colostomy or skin level cecostomy Placement of enterostomy or cecostomy for decompression Insertion of cecostomy or other colonic tube, percutaneous ICD-10 Code Description Comments K59.00 Constipation, unspecified K59.01 Slow transit constipation K59.02 Outlet dysfunction constipation K59.04 Chronic idiopathic constipation K59.09 Chronic constipation R15.9 Fecal incontinence NOS HCPCS Level II Code N/A Description Comments 10
Clinical Policy Title: Cecostomy for fecal incontinence
 Clinical Policy Title: Cecostomy for fecal incontinence Clinical Policy Number: 08.01.06 Effective Date: July 1, 2016 Initial Review Date: February 17, 2016 Most Recent Review Date: March 6, 2018 Next
Clinical Policy Title: Cecostomy for fecal incontinence Clinical Policy Number: 08.01.06 Effective Date: July 1, 2016 Initial Review Date: February 17, 2016 Most Recent Review Date: March 6, 2018 Next
Clinical Policy Title: Injectable bulking agents fecal incontinence
 Clinical Policy Title: Injectable bulking agents fecal incontinence Clinical policy number: 08.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date: June 5, 2018
Clinical Policy Title: Injectable bulking agents fecal incontinence Clinical policy number: 08.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date: June 5, 2018
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Injectable bulking agents for fecal incontinence
 Clinical Policy Title: Injectable bulking agents for fecal incontinence Clinical policy number: 08.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date: June
Clinical Policy Title: Injectable bulking agents for fecal incontinence Clinical policy number: 08.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date: June
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Cardiac rehabilitation
 Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
Clinical Policy Title: Genicular nerve block
 Clinical Policy Title: Genicular nerve block Clinical Policy Number: 14.01.10 Effective Date: October 1, 2017 Initial Review Date: September 21, 2017 Most Recent Review Date: October 19, 2017 Next Review
Clinical Policy Title: Genicular nerve block Clinical Policy Number: 14.01.10 Effective Date: October 1, 2017 Initial Review Date: September 21, 2017 Most Recent Review Date: October 19, 2017 Next Review
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Management of Neurogenic Bowel Dysfunction. Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders
 Management of Neurogenic Bowel Dysfunction Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders DEFECATION Delivery of colon contents to the rectum Rectal compliance
Management of Neurogenic Bowel Dysfunction Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders DEFECATION Delivery of colon contents to the rectum Rectal compliance
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Strep testing
 Clinical Policy Title: Strep testing Clinical Policy Number: 07.01.09 Effective Date: December 1, 2017 Initial Review Date: October 19, 2017 Most Recent Review Date: November 16, 2017 Next Review Date:
Clinical Policy Title: Strep testing Clinical Policy Number: 07.01.09 Effective Date: December 1, 2017 Initial Review Date: October 19, 2017 Most Recent Review Date: November 16, 2017 Next Review Date:
Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering)
 Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering) Clinical Policy Number: 17.02.02 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent
Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering) Clinical Policy Number: 17.02.02 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent
Fecal Incontinence. What is fecal incontinence?
 Scan for mobile link. Fecal Incontinence Fecal incontinence is the inability to control the passage of waste material from the body. It may be associated with constipation or diarrhea and typically occurs
Scan for mobile link. Fecal Incontinence Fecal incontinence is the inability to control the passage of waste material from the body. It may be associated with constipation or diarrhea and typically occurs
PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS. A08/S/d Colorectal: Faecal Incontinence (Adult)
 A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
Clinical Policy Title: Computerized gait analysis
 Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: May 1, 2018 Next Review Date:
Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: May 1, 2018 Next Review Date:
Clinical Policy Title: Computerized gait analysis
 Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: June 22, 2017 Next Review Date:
Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: June 22, 2017 Next Review Date:
Patients on anticoagulant or antiplatelet therapy undergoing elective endoscopic procedures
 This is an official Northern Trust policy and should not be edited in any way Patients on anticoagulant or antiplatelet therapy undergoing elective endoscopic procedures Reference Number: NHSCT/11/454
This is an official Northern Trust policy and should not be edited in any way Patients on anticoagulant or antiplatelet therapy undergoing elective endoscopic procedures Reference Number: NHSCT/11/454
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening
 Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Clinical Policy Title: Zoster (shingles) vaccine
 Clinical Policy Title: Zoster (shingles) vaccine Clinical Policy Number: 18.02.10 Effective Date: June 1, 2018 Initial Review Date: April 10, 2018 Most Recent Review Date: May 1, 2018 Next Review Date:
Clinical Policy Title: Zoster (shingles) vaccine Clinical Policy Number: 18.02.10 Effective Date: June 1, 2018 Initial Review Date: April 10, 2018 Most Recent Review Date: May 1, 2018 Next Review Date:
Peristeen and the Neurogenic Bowel Dysfunction Score (NBD) for pediatric patients with spina bifida
 Peristeen and the Neurogenic Bowel Dysfunction Score (NBD) for pediatric patients with spina bifida Coloplast develops products and services that make life easier for people with very personal and private
Peristeen and the Neurogenic Bowel Dysfunction Score (NBD) for pediatric patients with spina bifida Coloplast develops products and services that make life easier for people with very personal and private
Duc M. Vo, MD, FACS Northwest Surgical Specialists
 Duc M. Vo, MD, FACS Northwest Surgical Specialists Disclosures none Outline Definition Etiologies Exam findings Additional testing Medical management Surgical options What is fecal incontinence? Recurrent
Duc M. Vo, MD, FACS Northwest Surgical Specialists Disclosures none Outline Definition Etiologies Exam findings Additional testing Medical management Surgical options What is fecal incontinence? Recurrent
ACG Clinical Guideline: Management of Benign Anorectal Disorders
 ACG Clinical Guideline: Management of Benign Anorectal Disorders Arnold Wald, MD, MACG 1, Adil E. Bharucha, MBBS, MD 2, Bard C. Cosman, MD, MPH, FASCRS 3 and William E. Whitehead, PhD, MACG 4 1 Division
ACG Clinical Guideline: Management of Benign Anorectal Disorders Arnold Wald, MD, MACG 1, Adil E. Bharucha, MBBS, MD 2, Bard C. Cosman, MD, MPH, FASCRS 3 and William E. Whitehead, PhD, MACG 4 1 Division
Oral anti-thrombotic therapy-management in patients requiring endoscopy
 Oral anti-thrombotic therapy-management in patients requiring endoscopy Management of anti-thrombotic therapy in patients requiring endoscopy This guideline suggests appropriate management of patients
Oral anti-thrombotic therapy-management in patients requiring endoscopy Management of anti-thrombotic therapy in patients requiring endoscopy This guideline suggests appropriate management of patients
Policy #: 049 Latest Review Date: April 2009 Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
 Name of Policy: Pulsed Irrigation Evacuation (PIE) Policy #: 049 Latest Review Date: April 2009 Category: DME Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
Name of Policy: Pulsed Irrigation Evacuation (PIE) Policy #: 049 Latest Review Date: April 2009 Category: DME Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Ketamine for treatment-resistant depression
 Clinical Policy Title: Ketamine for treatment-resistant depression Clinical Policy Number: 00.02.13 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: January
Clinical Policy Title: Ketamine for treatment-resistant depression Clinical Policy Number: 00.02.13 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: January
Description. Section: Medicine Effective Date: April 15, 2016 Subsection: Medicine Original Policy Date: June 7, Page: 1 of 6.
 Section: Medicine Effective Date: April 15, 2016 Page: 1 of 6 Last Review Status/Date: March 2016 Description Radiofrequency (RF) energy has been investigated as a minimally invasive treatment of fecal
Section: Medicine Effective Date: April 15, 2016 Page: 1 of 6 Last Review Status/Date: March 2016 Description Radiofrequency (RF) energy has been investigated as a minimally invasive treatment of fecal
SACRAL NERVE STIMULATION FOR EXPERIENCE IN CHILDREN
 SACRAL NERVE STIMULATION FOR COLORECTAL DISEASES: EXPERIENCE IN CHILDREN C. LOUIS-BORRIONE - JM. GUYS TIMONE-ENFANTS MARSEILLE SACRAL NEUROMODULATION IN CHILDREN 26 : Humphreys et al - 23 children with
SACRAL NERVE STIMULATION FOR COLORECTAL DISEASES: EXPERIENCE IN CHILDREN C. LOUIS-BORRIONE - JM. GUYS TIMONE-ENFANTS MARSEILLE SACRAL NEUROMODULATION IN CHILDREN 26 : Humphreys et al - 23 children with
Motility Disorders. Pelvic Floor. Colorectal Center for Functional Bowel Disorders (N = 701) January 2010 November 2011
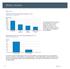 Motility Disorders Pelvic Floor Colorectal Center for Functional Bowel Disorders (N = 71) January 21 November 211 New Patients 35 3 25 2 15 1 5 Constipation Fecal Incontinence Rectal Prolapse Digestive-Genital
Motility Disorders Pelvic Floor Colorectal Center for Functional Bowel Disorders (N = 71) January 21 November 211 New Patients 35 3 25 2 15 1 5 Constipation Fecal Incontinence Rectal Prolapse Digestive-Genital
Description. Section: Medicine Effective Date: April 15, 2017 Subsection: Medicine Original Policy Date: June 7, Page: 1 of 6.
 Page: 1 of 6 Last Review Status/Date: March 2017 Description Radiofrequency energy has been investigated as a minimally invasive treatment of fecal incontinence, in a procedure referred to as the Secca
Page: 1 of 6 Last Review Status/Date: March 2017 Description Radiofrequency energy has been investigated as a minimally invasive treatment of fecal incontinence, in a procedure referred to as the Secca
Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab
 Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab Clinical Policy Number: 01.01.03 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent
Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab Clinical Policy Number: 01.01.03 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent
An effective and minimally invasive bridge between conservative therapy and invasive surgery for BCD (bowel control disorder).
 An effective and minimally invasive bridge between conservative therapy and invasive surgery for BCD (bowel control disorder). Mederi Therapeutics has developed this kit to help you raise awareness of
An effective and minimally invasive bridge between conservative therapy and invasive surgery for BCD (bowel control disorder). Mederi Therapeutics has developed this kit to help you raise awareness of
Populations Interventions Comparators Outcomes Individuals: With fecal incontinence
 Protocol Biofeedback as a Treatment of Fecal Incontinence or Constipation (20164) Medical Benefit Effective Date: 07/01/13 Next Review Date: 03/19 Preauthorization No Review Dates: 09/07, 09/08, 09/09,
Protocol Biofeedback as a Treatment of Fecal Incontinence or Constipation (20164) Medical Benefit Effective Date: 07/01/13 Next Review Date: 03/19 Preauthorization No Review Dates: 09/07, 09/08, 09/09,
Chapter 31 Bowel Elimination
 Chapter 31 Bowel Elimination Defecation Defecation: the act of expelling feces from the body Peristalsis: rhythmic contractions of intestinal smooth muscle to facilitate defecation Gastrocolic reflex:
Chapter 31 Bowel Elimination Defecation Defecation: the act of expelling feces from the body Peristalsis: rhythmic contractions of intestinal smooth muscle to facilitate defecation Gastrocolic reflex:
PREPARING FOR ANORECTOAL MANOMETRY. ManoScan Anorectal Manometry System
 PREPARING FOR ANORECTOAL MANOMETRY ManoScan Anorectal Manometry System WHAT IS ANORECTAL MANOMETRY? Anorectal manometry is a test used to evaluate the function and coordination of the sphincter and pelvic
PREPARING FOR ANORECTOAL MANOMETRY ManoScan Anorectal Manometry System WHAT IS ANORECTAL MANOMETRY? Anorectal manometry is a test used to evaluate the function and coordination of the sphincter and pelvic
Unraveling childhood constipation: Pathophysiology, diagnostics and treatment Mugie, S.M.
 UvA-DARE (Digital Academic Repository) Unraveling childhood constipation: Pathophysiology, diagnostics and treatment Mugie, S.M. Link to publication Citation for published version (APA): Mugie, S. M. (2014).
UvA-DARE (Digital Academic Repository) Unraveling childhood constipation: Pathophysiology, diagnostics and treatment Mugie, S.M. Link to publication Citation for published version (APA): Mugie, S. M. (2014).
GI Physiology - Investigating and treating patients with pelvic floor dysfunction. Lynne Smith Department of GI Physiology NGH Sheffield
 GI Physiology - Investigating and treating patients with pelvic floor dysfunction Lynne Smith Department of GI Physiology NGH Sheffield Aims o o o To give an overview of lower GI investigations To demonstrate
GI Physiology - Investigating and treating patients with pelvic floor dysfunction Lynne Smith Department of GI Physiology NGH Sheffield Aims o o o To give an overview of lower GI investigations To demonstrate
Clinical Policy Title: Bloodless heart transplant
 Clinical Policy Title: Bloodless heart transplant Clinical Policy Number: 05.03.05 Effective Date: July 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next Review Date:
Clinical Policy Title: Bloodless heart transplant Clinical Policy Number: 05.03.05 Effective Date: July 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next Review Date:
Transanal Radiofrequency Treatment of Fecal Incontinence
 Medical Policy Manual Surgery, Policy No. 129 Transanal Radiofrequency Treatment of Fecal Incontinence Next Review: December 2018 Last Review: December 2017 Effective: February 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Surgery, Policy No. 129 Transanal Radiofrequency Treatment of Fecal Incontinence Next Review: December 2018 Last Review: December 2017 Effective: February 1, 2018 IMPORTANT REMINDER
Clinical Policy: Fecal Incontinence Treatments Reference Number: PA.CP.MP.137
 Clinical Policy: Fecal Incontinence Treatments Reference Number: PA.CP.MP.137 Effective Date: 01/18 Last Review Date: 12/16 Coding Implications Revision Log Description Fecal incontinence defined as the
Clinical Policy: Fecal Incontinence Treatments Reference Number: PA.CP.MP.137 Effective Date: 01/18 Last Review Date: 12/16 Coding Implications Revision Log Description Fecal incontinence defined as the
Tertiary, regional and local pelvic floor service providers: the future. model? Andrew Williams
 Tertiary, regional and local pelvic floor service providers: the future Andrew Williams model? Pelvic Floor Unit Guy s and St Thomas NHS Foundation Trust Background 23% women suffer at least one pelvic
Tertiary, regional and local pelvic floor service providers: the future Andrew Williams model? Pelvic Floor Unit Guy s and St Thomas NHS Foundation Trust Background 23% women suffer at least one pelvic
Anti-Reflux Surgery in Cerebral Palsy Patients
 Anti-Reflux Surgery in Cerebral Palsy Patients Cecostomy for Bowel Management Surgery for Prenatally Identified Congenital Lung Lesions Dr. Mike Giacomantonio IWK Health Centre, Halifax, NS G. E. Reflux
Anti-Reflux Surgery in Cerebral Palsy Patients Cecostomy for Bowel Management Surgery for Prenatally Identified Congenital Lung Lesions Dr. Mike Giacomantonio IWK Health Centre, Halifax, NS G. E. Reflux
Premier Health Plan considers Intravascular Ultrasound (IVUS) for Coronary Vessels medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL MP.091.PH - Intravascular Ultrasound for Coronary Vessels This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
Premier Health Plan POLICY AND PROCEDURE MANUAL MP.091.PH - Intravascular Ultrasound for Coronary Vessels This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
A Case of Fecal Incontinence: Medical and Interventional Treatment Options
 A Case of Fecal Incontinence: Medical and Interventional Treatment Options HPI JP is a 69 year-old F with a 12-month history of FI. Her symptoms began after a colonoscopy She has been experiencing passive
A Case of Fecal Incontinence: Medical and Interventional Treatment Options HPI JP is a 69 year-old F with a 12-month history of FI. Her symptoms began after a colonoscopy She has been experiencing passive
Clinical Policy Title: Ear tubes (tympanostomy)
 Clinical Policy Title: Ear tubes (tympanostomy) Clinical Policy Number: 11.03.05 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next
Clinical Policy Title: Ear tubes (tympanostomy) Clinical Policy Number: 11.03.05 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next
Local Coverage Determination for Colorectal Cancer Screening (L29796)
 Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Clinical Policy Title: Breast cancer index genetic testing
 Clinical Policy Title: Breast cancer index genetic testing Clinical Policy Number: 02.01.22 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2016
Clinical Policy Title: Breast cancer index genetic testing Clinical Policy Number: 02.01.22 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2016
Clinical Policy Title: Discography
 Clinical Policy Title: Discography Clinical Policy Number: 03.01.01 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2017 Next Review Date: October
Clinical Policy Title: Discography Clinical Policy Number: 03.01.01 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2017 Next Review Date: October
Clinical Policy Title: Subtalar arthroereisis (implant)
 Clinical Policy Title: Subtalar arthroereisis (implant) Clinical Policy Number: 14.03.05 Effective Date: April 1, 2017 Initial Review Date: August 17, 2016 Most Recent Review Date: September 21, 2017 Next
Clinical Policy Title: Subtalar arthroereisis (implant) Clinical Policy Number: 14.03.05 Effective Date: April 1, 2017 Initial Review Date: August 17, 2016 Most Recent Review Date: September 21, 2017 Next
TREATMENT SOCIETY GUIDELINES FOR CONSTIPATION: WHAT IS NEW? FUNCTIONAL CONSTIPATION
 SOCIETY GUIDELINES FOR CONSTIPATION: WHAT IS NEW? Samuel Nurko MD MPH Center for Motility and Functional Gastrointestinal Disorders FUNCTIONAL CONSTIPATION One of the most common functional GI disorders
SOCIETY GUIDELINES FOR CONSTIPATION: WHAT IS NEW? Samuel Nurko MD MPH Center for Motility and Functional Gastrointestinal Disorders FUNCTIONAL CONSTIPATION One of the most common functional GI disorders
15. Prevention of UTI and lifestyle modifications
 15. Prevention of UTI and lifestyle modifications Key questions: Does improving poor voiding habits help prevent UTI recurrence? Does improving constipation help prevent UTI recurrence? Does increasing
15. Prevention of UTI and lifestyle modifications Key questions: Does improving poor voiding habits help prevent UTI recurrence? Does improving constipation help prevent UTI recurrence? Does increasing
Transanal Radiofrequency Treatment of Fecal Incontinence
 2.01.58 Transanal Radiofrequency Treatment of Fecal Incontinence Section 2.0 Medicine Effective Date January 30, 2015 Subsection Original Policy Date July 6, 2012 Next Review Date January 2016 Description
2.01.58 Transanal Radiofrequency Treatment of Fecal Incontinence Section 2.0 Medicine Effective Date January 30, 2015 Subsection Original Policy Date July 6, 2012 Next Review Date January 2016 Description
A70.4 Insertion of neurostimulator electrodes into peripheral nerve Z12.2 Posterior tibial nerve R15.X Faecal incontinence
 The National Institute for Health and Clinical Excellence (NICE) has issued full guidance to the NHS in England, Wales, Scotland and Northern Ireland on Percutaneous tibial nerve stimulation (PTNS) for
The National Institute for Health and Clinical Excellence (NICE) has issued full guidance to the NHS in England, Wales, Scotland and Northern Ireland on Percutaneous tibial nerve stimulation (PTNS) for
Clinical Policy Title: Spinal cord stimulators for chronic pain
 Clinical Policy Title: Spinal cord stimulators for chronic pain Clinical Policy Number: 03.03.01 Effective Date: October 1, 2014 Initial Review Date: March 19, 2014 Most Recent Review Date: April 19, 2017
Clinical Policy Title: Spinal cord stimulators for chronic pain Clinical Policy Number: 03.03.01 Effective Date: October 1, 2014 Initial Review Date: March 19, 2014 Most Recent Review Date: April 19, 2017
Percutaneous Cecostomy Tube Placement
 Information About Your Child s Procedure Percutaneous Cecostomy Tube Placement Read this form so you understand the procedure and its risks. Please ask questions about anything you do not understand. What
Information About Your Child s Procedure Percutaneous Cecostomy Tube Placement Read this form so you understand the procedure and its risks. Please ask questions about anything you do not understand. What
Common Gastrointestinal Problems in the Elderly
 Common Gastrointestinal Problems in the Elderly Brian Viviano, D.O. Objectives Understand the pathophysiology, clinical manifestations, diagnosis and management of GI diseases of the elderly. Differentiate
Common Gastrointestinal Problems in the Elderly Brian Viviano, D.O. Objectives Understand the pathophysiology, clinical manifestations, diagnosis and management of GI diseases of the elderly. Differentiate
Clinical Policy: Fecal Calprotectin Assay Reference Number: CP.MP.135
 Clinical Policy: Reference Number: CP.MP.135 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.135 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Transanal colonic irrigation has recently become an
 ORIGINAL CONTRIBUTION Long-Term Outcome and Safety of Transanal Irrigation for Constipation and Fecal Incontinence Peter Christensen, Ph.D. 1,2 & Klaus Krogh, D.M.Sci. 2 & Steen Buntzen, D.M.Sci. 1 Fariborz
ORIGINAL CONTRIBUTION Long-Term Outcome and Safety of Transanal Irrigation for Constipation and Fecal Incontinence Peter Christensen, Ph.D. 1,2 & Klaus Krogh, D.M.Sci. 2 & Steen Buntzen, D.M.Sci. 1 Fariborz
Manuel Castella MD PhD Hospital Clínic, University of
 Manuel Castella MD PhD Hospital Clínic, University of Barcelona mcaste@clinic.ub.es @mcastellamd www.escardio.org/guidelines European Heart Journal - doi:10.1093/eurheartj/ehw210 Providing integrated care
Manuel Castella MD PhD Hospital Clínic, University of Barcelona mcaste@clinic.ub.es @mcastellamd www.escardio.org/guidelines European Heart Journal - doi:10.1093/eurheartj/ehw210 Providing integrated care
who where symptoms? colon cancer facts affected? what
 who Over 130,000 new cases diagnosed each year is Greater than 50,000 deaths annually attributable to colon cancer Second leading cause of cancer death in the U.S. Equal risk in men and women Women over
who Over 130,000 new cases diagnosed each year is Greater than 50,000 deaths annually attributable to colon cancer Second leading cause of cancer death in the U.S. Equal risk in men and women Women over
Efficacy of Peristeen transanal irrigation system for neurogenic bowel in the pediatric population: Preliminary findings
 Efficacy of Peristeen transanal irrigation system for neurogenic bowel in the pediatric population: Preliminary findings Tiffany Gordon, MSN, RN, CRRN, CPN David Vandersteen, MD John Belew RN, PhD Gillette
Efficacy of Peristeen transanal irrigation system for neurogenic bowel in the pediatric population: Preliminary findings Tiffany Gordon, MSN, RN, CRRN, CPN David Vandersteen, MD John Belew RN, PhD Gillette
Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders
 Original article doi:10.1111/j.1463-1318.2011.02797.x Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders D. S. Y. Chan*, A. Saklani, P. R. Shah, M. Lewis and P. N. Haray
Original article doi:10.1111/j.1463-1318.2011.02797.x Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders D. S. Y. Chan*, A. Saklani, P. R. Shah, M. Lewis and P. N. Haray
Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207
 Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207 Effective Date: 03/05 Last Review Date: 10/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207 Effective Date: 03/05 Last Review Date: 10/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy Title: Pharmocogenetic testing for warfarin (Coumadin ) sensitivity
 Clinical Policy Title: Pharmocogenetic testing for warfarin (Coumadin ) sensitivity Clinical Policy Number: 02.01.13 Effective Date: September 1, 2013 Initial Review Date: May 15, 2013 Most Recent Review
Clinical Policy Title: Pharmocogenetic testing for warfarin (Coumadin ) sensitivity Clinical Policy Number: 02.01.13 Effective Date: September 1, 2013 Initial Review Date: May 15, 2013 Most Recent Review
Robotic Ventral Rectopexy
 Robotic Ventral Rectopexy What is a robotic ventral rectopexy? The term rectopexy refers to an operation in which the rectum (the part of the bowel nearest the anus) is put back into its normal position
Robotic Ventral Rectopexy What is a robotic ventral rectopexy? The term rectopexy refers to an operation in which the rectum (the part of the bowel nearest the anus) is put back into its normal position
ACCIDENTAL BOWEL LEAKAGE: A PRACTICAL APPROACH TO EVALUATION. Tristi W. Muir, MD Chair, Department of OB/GYN Houston Methodist Hospital
 ACCIDENTAL BOWEL LEAKAGE: A PRACTICAL APPROACH TO EVALUATION Tristi W. Muir, MD Chair, Department of OB/GYN Houston Methodist Hospital Accidental Bowel Leakage What Gets the Woman into Your Office 67%
ACCIDENTAL BOWEL LEAKAGE: A PRACTICAL APPROACH TO EVALUATION Tristi W. Muir, MD Chair, Department of OB/GYN Houston Methodist Hospital Accidental Bowel Leakage What Gets the Woman into Your Office 67%
2/5/2016. Evolving Surgical Treatment Approaches for Fecal Incontinence in Women: An Evidence and Cased-Based Approach
 Evolving Surgical Treatment Approaches for Fecal Incontinence in Women: An Evidence and Cased-Based Approach Holly E Richter, PhD, MD, FACOG, FACS J Marion Sims Professor Obstetrics and Gynecology Professor
Evolving Surgical Treatment Approaches for Fecal Incontinence in Women: An Evidence and Cased-Based Approach Holly E Richter, PhD, MD, FACOG, FACS J Marion Sims Professor Obstetrics and Gynecology Professor
Use of gatekeeper in obese patients with fecal incontinence before bariatric surgery, is it improving the results?
 International Surgery Journal Ibrahim AAM. Int Surg J. 2017 Nov;4(11):3594-3598 http://www.ijsurgery.com pissn 2349-3305 eissn 2349-2902 Original Research Article DOI: http://dx.doi.org/10.18203/2349-2902.isj20174876
International Surgery Journal Ibrahim AAM. Int Surg J. 2017 Nov;4(11):3594-3598 http://www.ijsurgery.com pissn 2349-3305 eissn 2349-2902 Original Research Article DOI: http://dx.doi.org/10.18203/2349-2902.isj20174876
Clinical Policy Title: Fecal biomarkers in inflammatory bowel disease
 Clinical Policy Title: Fecal biomarkers in inflammatory bowel disease Clinical Policy Number: 08.01.08 Effective Date: April 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: November
Clinical Policy Title: Fecal biomarkers in inflammatory bowel disease Clinical Policy Number: 08.01.08 Effective Date: April 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: November
CONSTIPATION. Atan Baas Sinuhaji
 CONSTIPATION Atan Baas Sinuhaji Sub Division of Pediatrics Gastroentero-Hepatolgy Department of ChildHealth,School of Medicine University of Sumatera Utara MEDAN DEFECATION REGULAR PATTERN CONSTIPATION
CONSTIPATION Atan Baas Sinuhaji Sub Division of Pediatrics Gastroentero-Hepatolgy Department of ChildHealth,School of Medicine University of Sumatera Utara MEDAN DEFECATION REGULAR PATTERN CONSTIPATION
FECAL INCONTINENCE. John H. Winston, III, M.D., M.B.A.
 FECAL INCONTINENCE John H. Winston, III, M.D., M.B.A. Diplomate, American Board of Colon & Rectal Surgery Diplomate, American Board of Surgery www.colorectalsurgeryservices.com Fecal Incontinence (FI)
FECAL INCONTINENCE John H. Winston, III, M.D., M.B.A. Diplomate, American Board of Colon & Rectal Surgery Diplomate, American Board of Surgery www.colorectalsurgeryservices.com Fecal Incontinence (FI)
Sympathetic Electrical Stimulation Therapy for Chronic Pain
 Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
Bowel Function After Spinal Cord Injury
 Bowel Function After Spinal Cord Injury A resource for individuals with SCI and their supporters This presentation is based on SCI Model Systems research and was developed with support from the National
Bowel Function After Spinal Cord Injury A resource for individuals with SCI and their supporters This presentation is based on SCI Model Systems research and was developed with support from the National
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND (TRUS)
 MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
Treatments for Fecal Incontinence A Review of the Research for Adults
 Treatments for Fecal Incontinence A Review of the Research for Adults e Is This Information Right for Me? This information is right for you if: Your health care professional* said you or your loved one
Treatments for Fecal Incontinence A Review of the Research for Adults e Is This Information Right for Me? This information is right for you if: Your health care professional* said you or your loved one
MEDICAL POLICY SUBJECT: BIOFEEDBACK
 MEDICAL POLICY PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Clinical Policy Title: Room humidifiers
 Clinical Policy Title: Room humidifiers Clinical Policy Number: 17.02.05 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review Date:
Clinical Policy Title: Room humidifiers Clinical Policy Number: 17.02.05 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review Date:
Clinical Policy Title: Platelet rich plasma
 Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2017 Next Review
Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2017 Next Review
Clinical Policy Title: Prothrombin international normalized ratio self-testing
 Clinical Policy Title: Prothrombin international normalized ratio self-testing Clinical Policy Number: 05.01.02 Effective Date: July 1, 2013 Initial Review Date: April 23, 2013 Most Recent Review Date:
Clinical Policy Title: Prothrombin international normalized ratio self-testing Clinical Policy Number: 05.01.02 Effective Date: July 1, 2013 Initial Review Date: April 23, 2013 Most Recent Review Date:
Clinical problems related to GI involvement in SSc
 Clinical problems related to GI involvement in SSc Incontinence Abdominal pain/distension Gastro-oesophageal Diarrhoea Weight loss/al Issues Constipation Management of incontinence Establish diagnosis
Clinical problems related to GI involvement in SSc Incontinence Abdominal pain/distension Gastro-oesophageal Diarrhoea Weight loss/al Issues Constipation Management of incontinence Establish diagnosis
Sacral Nerve Stimulation for Faecal Incontinence
 Sacral Nerve Stimulation for Faecal Incontinence Questions & Answers GLASGOW COLORECTAL CENTRE Ross Hall Hospital 221 Crookston Road Glasgow G52 3NQ e-mail: info@colorectalcentre.co.uk Ph: Main hospital
Sacral Nerve Stimulation for Faecal Incontinence Questions & Answers GLASGOW COLORECTAL CENTRE Ross Hall Hospital 221 Crookston Road Glasgow G52 3NQ e-mail: info@colorectalcentre.co.uk Ph: Main hospital
Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT)
 Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT) Clinical Policy Number: 09.01.02 Effective Date: September 1, 2013 Initial Review Date: February 18, 2013 Most
Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT) Clinical Policy Number: 09.01.02 Effective Date: September 1, 2013 Initial Review Date: February 18, 2013 Most
Aging Persons with Intellectual Developmental Disorders (IDD): Constipation KEYPOINTS OVERVIEW
 Aging Persons with Intellectual Developmental Disorders (IDD): Constipation KEYPOINTS A major medical conditions that commonly is seen among persons with IDD and may lead to serious complications is constipation.
Aging Persons with Intellectual Developmental Disorders (IDD): Constipation KEYPOINTS A major medical conditions that commonly is seen among persons with IDD and may lead to serious complications is constipation.
Constipation. What is constipation? What is the criteria for having constipation? What are the different types of constipation?
 What is constipation? is defined as having a bowel movement less than 3 times per week. It is usually associated with hard stools or difficulty passing stools. You may have pain while passing stools or
What is constipation? is defined as having a bowel movement less than 3 times per week. It is usually associated with hard stools or difficulty passing stools. You may have pain while passing stools or
Saint-Petersburg State Pediatric Medical University, Saint-Petersburg, Russia
 Saint-Petersburg State Pediatric Medical University, Saint-Petersburg, Russia Modern technologies in treatment of fecal incontinence in children Komissarov Igor Alexeevich- Ph.D, M.D, Prof. Kolesnikova
Saint-Petersburg State Pediatric Medical University, Saint-Petersburg, Russia Modern technologies in treatment of fecal incontinence in children Komissarov Igor Alexeevich- Ph.D, M.D, Prof. Kolesnikova
Long-Term Bowel Symptoms Following Corrective Surgery
 HIRSCHSPRUNG'S DISEASE Samuel Nurko MD MPH Center for Motility and Functional Gastrointestinal Disorders Children s Hospital Medical Center, Boston Ma Long-Term Bowel Symptoms Following Corrective Surgery
HIRSCHSPRUNG'S DISEASE Samuel Nurko MD MPH Center for Motility and Functional Gastrointestinal Disorders Children s Hospital Medical Center, Boston Ma Long-Term Bowel Symptoms Following Corrective Surgery
Novel Options for the Management of Fecal Incontinence
 Novel Options for the Management of Fecal Incontinence Arnold Wald, MD, MACG University of Wisconsin School of Medicine and Public Health, Madison WI ANORECTAL CONTINENCE MECHANISMS Reservoir Elements
Novel Options for the Management of Fecal Incontinence Arnold Wald, MD, MACG University of Wisconsin School of Medicine and Public Health, Madison WI ANORECTAL CONTINENCE MECHANISMS Reservoir Elements
Corporate Medical Policy
 Corporate Medical Policy Biofeedback as a Treatment of Pain File Name: Origination: Last CAP Review: Next CAP Review: Last Review: Biofeedback_as_a_treatment_of_pain 2/2017 5/2018 5/2019 5/2018 Description
Corporate Medical Policy Biofeedback as a Treatment of Pain File Name: Origination: Last CAP Review: Next CAP Review: Last Review: Biofeedback_as_a_treatment_of_pain 2/2017 5/2018 5/2019 5/2018 Description
Clinical Policy Title: Bone growth stimulators for non-healing fractures
 Clinical Policy Title: Bone growth stimulators for non-healing fractures Clinical Policy Number: 14.02.03 Effective Date: January 1, 2015 Initial Review Date: July 16, 2014 Most Recent Review Date: March
Clinical Policy Title: Bone growth stimulators for non-healing fractures Clinical Policy Number: 14.02.03 Effective Date: January 1, 2015 Initial Review Date: July 16, 2014 Most Recent Review Date: March
Clinical Policy Title: Tactile breast imaging
 Clinical Policy Title: Tactile breast imaging Clinical Policy Number: 05.01.07 Effective Date: February 1, 2018 Initial Review Date: November 16, 2017 Most Recent Review Date: January 11, 2018 Next Review
Clinical Policy Title: Tactile breast imaging Clinical Policy Number: 05.01.07 Effective Date: February 1, 2018 Initial Review Date: November 16, 2017 Most Recent Review Date: January 11, 2018 Next Review
MEDICAL POLICY SUBJECT: BULKING AGENTS FOR TREATMENT OF URINARY OR FECAL INCONTINENCE. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: BULKING AGENTS FOR (ARCHIVED DATE: 05/28/09-, EDITED DATE: 05/27/10, 05/19/11, 05/24/12, 05/23/13) PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific
MEDICAL POLICY SUBJECT: BULKING AGENTS FOR (ARCHIVED DATE: 05/28/09-, EDITED DATE: 05/27/10, 05/19/11, 05/24/12, 05/23/13) PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific
Clinical Policy: Gastric Electrical Stimulation Reference Number: CP.MP.40
 Clinical Policy: Reference Number: CP.MP.40 Effective Date: 09/09 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.40 Effective Date: 09/09 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
