Continued Development of Artificial Insemination Technology in Alpacas Rare & Natural Animal Fibres R&D Program
|
|
|
- Ashlyn Gray
- 6 years ago
- Views:
Transcription
1 Continued Development of Artificial Insemination Technology in Alpacas RIRDC Publication No. 08/057 Rare & Natural Animal Fibres R&D Program RIRDC Innovation for rural Australia covers.indd 1 16/06/2008 8:45:21 AM
2
3 Continued Development of Artificial Insemination Technology in Alpacas by Katherine M. Morton, Jane L. Vaughan and W.M. Chis Maxwell June 2008 RIRDC Publication No 08/057 RIRDC Project No US-138A i
4 2008 Rural Industries Research and Development Corporation. All rights reserved. ISBN ISSN The Continued Development of Atificial Insemination Technology in Alpacas Publication No. 08/057 Project No. US-138A The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable regions. You must not rely on any information contained in this publication without taking specialist advice relevant to your particular circumstances. While reasonable care has been taken in preparing this publication to ensure that information is true and correct, the Commonwealth of Australia gives no assurance as to the accuracy of any information in this publication. The Commonwealth of Australia, the Rural Industries Research and Development Corporation (RIRDC), the authors or contributors expressly disclaim, to the maximum extent permitted by law, all responsibility and liability to any person, arising directly or indirectly from any act or omission, or for any consequences of any such act or omission, made in reliance on the contents of this publication, whether or not caused by any negligence on the part of the Commonwealth of Australia, RIRDC, the authors or contributors. The Commonwealth of Australia does not necessarily endorse the views in this publication. This publication is copyright. Apart from any use as permitted under the Copyright Act 1968, all other rights are reserved. However, wide dissemination is encouraged. Requests and inquiries concerning reproduction and rights should be addressed to the RIRDC Publications Manager on phone Researcher Contact Details Professor WM Chis Maxwell Centre for Advanced Technologies in Animal Genetics and Reproduction (ReproGen), Faculty of Veterinary Science, The University of Sydney, NSW, 2006 Phone: Fax: chism@vetsci.usyd.edu.au Dr. Katherine M Morton Centre for Advanced Technologies in Animal Genetics and Reproduction (ReproGen), Faculty of Veterinary Science, The University of Sydney, NSW, 2006 Phone: Fax: kmorton@vetsci.usyd.edu.au Dr. Jane Vaughan Criagenesis PO Box 406 OCEAN GROVE VIC Vaughan@ava.com.au In submitting this report, the researcher has agreed to RIRDC publishing this material in its edited form. RIRDC Contact Details Rural Industries Research and Development Corporation Level 2, 15 National Circuit BARTON ACT 2600 PO Box 4776 KINGSTON ACT 2604 Phone: Fax: rirdc@rirdc.gov.au. Web: Published in June 2008 ii ii
5 Foreword Alpaca fibre is soft, fine and light and feels 2-3 microns finer than wool. The animals are plantigrade (compared with sheep that are digitigrade) and spread their weight over a greater surface area of ground (Ordoñez 1994). They do not require tail docking or mulesing as they have a hairless perineum. They defecate in communal dung piles so that gastrointestinal parasite burdens are lower and the necessity for anthelmintic drenching is much reduced (Carmichael 1999). Alpacas are nutritionally more efficient than sheep and require less protein and energy (a 65 kg alpaca requires the same amount of maintenance feed as a 40 kg sheep; San Martin et al. 1989). Alpacas do not require sophisticated fencing and guard their offspring against predators such as dogs and foxes (Sumar 1983). Alpacas, along with llamas, guanacos, vicugnas, Bactrian and Dromedary camels make up the Camelidae family. Their reproductive physiology is considerably different from that of other domestic animals, and remains poorly understood. The difficulties in collection of high quality semen during prolonged mating, and handling and preservation of viscous semen, have precluded the development of artificial insemination (AI) technology in this species. Development of AI will facilitate the more widespread use of superior sires, and reduce the dependence of the Australian industry on mobile matings, thereby increasing the welfare of the males and reducing the risk of the spread of Johne s disease, lice and other communicable diseases and pests. Moreover, the use of AI will result in genetic gain, which would be unlikely to occur using conventional breeding programs, through the widespread use of sires of high genetic value (both national and international) and increase the rate at which superior genetics are disseminated. There are major gaps in knowledge about many aspects of artificial breeding in alpacas. The primary aims of this project were to (1) develop and establish the technology for collection, processing and preservation of alpaca semen, and (2) develop and establish the technology for insemination of alpaca semen. These were identified as objectives in the RIRDC Rare Natural Animal Fibre Research and Development Plan The outcomes were an optimum method for semen collection and AI of alpacas under Australian conditions, the education of alpaca breeders in the benefits of using AI technology in their own herds to hasten improvement of fibre quality via a presentation at the Australian Alpaca Association Inc (AAA). National Conference and publication of an article in the AAA Conference proceedings, and the production of a manual that can be used by small ruminant artificial breeding centres and practising veterinary practitioners to provide commercial AI programs to alpaca breeders. This project made considerable advances in collection, liquefaction and preservation of alpaca semen, demonstrated the limitations imposed by the viscous seminal plasma and highlighted the need for future research into seminal plasma viscosity, which remains the key problem for the development of AI technology. The efficient and reliable methods for liquid storage and cryopreservation of alpaca semen will also hasten the commercialisation of AI. Overall, the knowledge gained during this project has made a significant contribution to the scientific field, and the results challenge many previously held perceptions associated with the preservation of alpaca semen. The report comprises a literature review, covering semen characteristics, preservation and AI in camelids with particular reference to alpacas, a full description of the methods used in this project (which can be used as a practical manual by breeders and practitioners contemplating artificial breeding in alpacas), seven experimental chapters describing semen collection, assessment of sperm function, liquefaction of semen, liquid storage, cryopreservation, AI and sperm sexing. The experimental chapters can be read as stand-alone reports and are followed by a general discussion of the results and a consideration of the implications and recommendations arising from the work. The appendices to the report also provide detailed protocols, equipment and publication lists that may be useful for scientists and practitioners. iii
6 This project was funded by RIRDC Core Funds provided by the Australian Government and partly from a voluntary levy from the Alpaca Industry. This report, an addition to RIRDC s diverse range of over 1800 research publications, forms part of our Rare Natural Animal Fibres R&D program, which aims to develop artificial insemination technology in alpacas. Most of our publications are available for viewing, downloading or purchasing online through our website: downloads at purchases at Peter O Brien Managing Director Rural Industries Research and Development Corporation iv
7 Acknowledgments The authors wish to thank the Australian Alpaca Association (AAA), in particular Iona McKinnon and Wendy Jones for their continued enthusiasm, faith, generosity and support which was instrumental in completing this project. Jill Nicholas (Belgrave Park Alpacas), Daniel and Diane McAusland, Janie Hicks (Coolaroo Alpaca Stud), Daphne and Keith Gregory (Chiverton Alpacas), Merilyn Matthews and Geoff Pearson (Daisy Banks Alpacas), Dianne Rutter (Keiana Lodge Alpacas) and Roger Curvey (Waratah Alpacas) are thanked for their generous donation and loan of animals for semen collection. Penny and John Pittard (Currabungla Alpacas), Iona McKinnon (Turra Stud), Daphne and Keith Gregory (Chiverton Alpacas), Joy (Stud), Dr. Ian and Harriet Davison (Illawarra Alpacas) and those at the Castration days are thanked for their kind donation of alpaca testes. Dr. Ian and Harriet Davison are also thanked for their kind provision of animals, facilities and generous hospitality for the field trial experiment. Staff and students from The University of Sydney are thanked for their assistance and contributions to the project. Associate Professor Peter Thompson is thanked for statistical analysis, Mr. Byron Biffin and Mr. Keith Tribe for alpaca handling, husbandry and maintenance. Dr. Roslyn Bathgate is thanked for assistance with cryopreservation of epididymal sperm and Dr. Monica Ruckholdt for operation of the MoFlo and assistance with alpaca sperm-sexing. Students, in particular Michael Bertoldo, Kristie Bailey, Zamira Gibb, Jorge Reyna and Sarah Wilson provided valuable contributions to semen collection, performing experiments and technical assistance. Special thanks go to Ms. Kim Heasman for blood collection, developing and validating the alpaca cortisol and testosterone assays, and for her enthusiasm, excellent technical assistance, and support throughout the project. Professor Ahmed Tibary (Washington State University), Dr. Lulu Skidmore (Camel Reproduction Centre, Dubai) and Dr. Marcelo Ratto (University of Saskatchewan and Universidad Austral de Chile) are thanked for their advice and enthusiasm with the project. The authors also wish to express their thanks to Dr. Peter McInnes, Mrs. June Murphy and the Rare Animal Natural Fibres Committee for their continued and enthusiastic support. This research was supported by funding from the Rural Industries Research and Development Corporation (Project US-138A) and the Australian Alpaca Association Inc. v
8 Abbreviations AI artificial insemination APVMA Australian pesticides and veterinary medicine authority AQIS Australian quarantine inspection service AV artificial vagina BSA bovine serum albumin CAMP cyclic adenosine monophosphate CAT catalase CASA computer assisted sperm analysis CM cleavage medium CO 2 carbon dioxide COC cumulus oocyte complex DNA deoxyribonucleic acid DMSO dimethyl sulphoxide EEJ electroejaculation ET embryo transfer FBS foetal bovine serum FITC fluoresceun-isothiocynate-conjugated to peanut agglutinin FSH follicle stimulating hormone G gauge g grams g gravity GnRH gonadotrophin releasing hormone H33342 Hoechst h hours Hepes N-(2-hydroxyethyl) piperizine-n -(2-ethanesulphonic acid) H 2 O water HSA human serum albumin i.m. intramuscular I.U. international units IVC in vitro culture IVF in vitro fertilisation IVM in vitro maturation IVP in vitro production kg kilograms kda kilodaltons L litres LH luteinising hormone M199 medium 199 M molar mm millimolar mg milligrams min minutes MI metaphase I MII metaphase II ml millilitre mosmol milli-osmole MOET multiple ovulation and embryo transfer nm nanometers ODB oestradiol benzoate PBS phosphate buffered saline ph hydrogen ion concentration, -log 10 of. PMSG pregnant mare serum gonadotrophin RIA radioimmunoassay vi
9 ROS SAC SB s.e.m. SOD SOF sperm SS TCM μg μl μm μm UV vs. v:v w:v X sperm Y sperm reactive oxygen species South American camelid sperm buffer standard error of the mean superoxide dismutase synthetic oviduct fluid spermatozoa sheep serum tissue culture medium microgram microlitre micron micromolar ultraviolet versus volume: volume ratio weight: volume ratio X-chromosome bearing spermatozoa Y-chromosome bearing spermatozoa vii
10 Contents Foreword... iii Acknowledgments... v Abbreviations... vi Executive Summary... x Introduction... 1 Objectives Review of the literature Introduction Reproductive anatomy and physiology of the male alpaca Semen collection Characteristics of camelid semen Semen preservation Artificial insemination Concluding remarks and required research Methodology Use of animals in research Animals Location of research and facilities Training of males for semen collection Semen collection Mating length Semen assessment Sperm assessments Dilution of semen Liquefaction of semen Liquid storage of semen Sperm cryopreservation Sperm thawing Artificial insemination Statistical analysis Collection of alpaca semen Introduction Materials and methods Results Discussion Assessment of sperm function and integrity Introduction Materials and methods Results Discussion Liquefaction of alpaca semen Introduction Materials and Methods Results Discussion Liquid-storage of alpaca sperm Introduction Materials and methods Results Discussion viii
11 7. Cryopreservation of alpaca sperm Introduction Materials and methods Results Discussion Artificial Insemination Introduction Materials and methods Results Discussion Preliminary development of sperm-sexing technology in alpacas Introduction Materials and methods Results Discussion General discussion of results Implications Recommendations Appendices Appendix I: List and source of laboratory equipment Appendix II: List and source of laboratory chemicals Appendix III: Semen collection worksheet Appendix IV: List and composition of diluents Appendix V: Company contact details Appendix VI: Protocol for the production of AV liners Appendix VII: Protocol for the collection of alpaca semen Appendix VIII: Protocol for the assessment of alpaca sperm acrosome integrity using FITC-PNA Appendix IX: Protocol for the liquid storage of alpaca semen Appendix X: Protocol for the cryopreservation of alpaca sperm Appendix XI: List of publications Appendix XII: List of literature obtained by the research team References ix
12 Executive Summary Introduction The aim of this project was to develop the technology for artificial insemination (AI) in alpacas by establishing efficient and reliable methods for the collection, processing, preservation and artificial insemination of alpaca semen, continuing the work of RIRDC project AAA-1A (publication 03/104). What the report is about This report provides information about the artificial breeding of alpacas, including: a literature review of semen characteristics, preservation and to increase the knowledge of the attributes of camelid reproductive physiology, and previous attempts to collect, preserve and AI camelids; detailed information about semen handling and preservation procedures to increase the knowledge of breeders and veterinarians about these procedures and the impact that semen handling can have on semen quality; descriptive methodologies of the procedures used to collect, assess, preserve and AI semen to increase understanding of these procedures; information about sperm anatomy and physiology to increase the knowledge of the effects of semen preservation procedures on sperm physiology and function; detailed descriptions of the experiments conducted to determine the optimal semen collection procedure for alpacas in Australia; information about different types of sperm assessment and their relative merits; detailed descriptions of the various mechanical and enzymatic methods which can be used to liquefy alpaca semen; descriptions of the effects of different parameters such as temperature, dilution rate and diluent on the viability of sperm during liquid storage; descriptions of the experiments conducted to develop a method to cryoprese (store frozen) alpaca semen; a description of the AI procedure and results after AI with preserved semen; and a description of the sperm-sexing procedure and its application to alpacas. Who the report is targeted at The information contained in this report is aimed at breeders and veterinarians in the Australian alpaca industry, and camelid scientists. Background information about sperm assessment, physiology and preservation is provided to increase the knowledge of breeders and practitioners who may not have experience with this topic. Background Alpacas are members of the camelid family along with llamas, guanacos, vicugnas, Bactrian and Dromedary camels. Their reproductive physiology is considerably different to other domestic animals and remains poorly understood. Alpacas have a long generation interval as males do not reach puberty until 2 3 years of age and gestation is long (11.5 months). Mating is in sternal recumbency for up to 90 minutes. Alpacas ejaculate throughout copulation and produce an ejaculate of low volume, low sperm concentration and high viscosity (reviewed by Bravo et al. 2000c). Development of AI will facilitate the more widespread use of superior sires, and reduce the dependence of the Australian industry on mobile matings, thereby increasing the welfare of the males and reducing the risk of the spread of Johne s disease, lice and other communicable diseases and pests. Moreover, the use of AI will result in genetic gain which would be unlikely to occur using conventional breeding programs. The increased use of superior alpaca males, facilitated by AI, will increase the rate of genetic gain, similar to that achieved in the sheep and goat industries (Evans and Maxwell 1987). The development of artificial insemination technology in camelids was attempted as early as the 1960s (Fernandez-Baca and Novoa 1968) and more recently in Australia (Vaughan et al. 2003a), x
13 Europe (McEvoy et al. 1992b; Burgel et al. 2000) and North and South America (Bravo et al. 1996b; 1997a,b; 1999; Ratto et al. 1999; Bravo et al. 2000a; Raymundo et al. 2000). However, the results have been disappointing, owing to problems with semen collection and the viscous nature of camelid semen, which make evaluation, dilution and preservation difficult. These limitations have been emphasised by Vaughan et al. (2003a) and international camelid laboratories that were unable to consistently and reliably collect and process high quality camelid semen. Aims and objectives Project US 138A The continued development of artificial insemination technology in alpacas aimed to further develop and establish the technology for collection, processing, preservation and AI of alpaca semen, which has been identified as an objective in the RIRDC Rare Natural Animal Fibre Research and Development Plan The overall objective was to improve the ability of breeders and veterinarians to apply AI techniques to genetically improve Australian alpacas. Methods used The literature was reviewed to determine the current state of semen preservation in camelids. Male alpacas were then trained for semen collection and a semen collection facility was established at the University s Animal Reproduction Unit at Camden, NSW. Numerous preliminary experiments were then conducted based on instrumental designs which incorporated new ideas as well as those already established in the literature. Thereafter, a group of experiments were conducted around each objective to determine the method that produced the highest quality semen. These experiments examined, among other factors, a redesigned mannequin, redesigned artificial vagina (AV) liners, an artificial cervix added into the AV, the presence of females during semen collection, the collection of semen into a diluent, and supplementation of the diluent with the antioxidant catalase. The review of the literature revealed that many of the sophisticated tests used to assess sperm from cattle, sheep and pigs had not been developed for alpacas. In the former species, sperm are often subjected to a barrage of tests to determine their physiological status and provide as much information as possible about the effects of various procedures. Very few tests have been established for alpacas, and those articles provided only limited information about the effects of certain procedures on sperm viability. A number of different objective and subjective assessment methods were applied to alpaca sperm. The useful tests were then incorporated into the rest of the experiments to provide greater information and more definitive results. No published full-length studies on the liquid storage of alpaca semen, and only a few studies on liquid storing camelid semen, were found in the literature. In the current project, basic parameters, such as temperature, dilution rate and diluents were established for alpaca sperm. Based on this preliminary work, experiments were designed to determine the optimal diluent, dilution rate and temperature for the liquid storage of alpaca semen. While the literature yielded slightly more studies on alpaca sperm cryopreservation, the results of these studies were disappointing and many of the basic parameters were not properly described. Therefore, experiments were conducted to determine the optimal diluent, packaging, freezing rate, cryoprotectant concentration and supplementation for alpaca sperm during freezing and thawing. Because of the difficulty of obtaining consistent sperm quality in ejaculated semen, epididymal sperm, which had not been exposed to the inhibitory effects of the viscous seminal plasma, were used as a model in many of the experiments. Although epididymal sperm are unlikely to be used in practice, except in special circumstances, they do provide a clear indication sperm function specifically, as opposed to that of the semen as a whole. Many previous studies referred to the variation in semen parameters within and between alpaca males, although none specifically investigated this variation. An experiment was therefore conducted to examine the variation within and between males, and the effect of season on semen quality. Methods developed to cryopreserve epididymal alpaca sperm were assessed in a field trial, in which alpaca females were inseminated with frozen-thawed sperm. Facilities for the separation of X- and Y-sperm were available during the project, and an experiment was conducted to examine the possibility of applying this sperm sexing technology to alpacas. xi
14 Results and key findings This report presents new research, which developed an improved collection method that reliably and consistently produces high quality semen. Collection of high quality semen was possible using a redesigned mannequin containing an AV which was fitted with a straight silicone liner and a collecting vessel containing 0.5 ml Tris diluent supplemented with 200 or 600 units/ml catalase. New methods were developed for subjective and objective assessment of alpaca sperm, most importantly an acrosome-specific stain to assess acrosomal integrity. Methods for objective assessment of motility and motility patterns by computer assisted sperm analysis (CASA), and simultaneous assessment of viability and acrosome integrity using fluorescent stains, were also developed. A modified heterologous oocyte binding assay using in vitro matured sheep oocytes was established to facilitate in vitro assessment of sperm function and fertility. A number of mechanical and enzymatic semen liquefaction methods were also studied. Addition of enzymes during or after semen collection were assessed and found to be either detrimental to sperm function or ineffective at liquefying semen. Similar results were observed for most mechanical methods including centrifugation, density gradient centrifugation and needling. However, pipetting of semen during dilution with handling medium reduced semen viscosity without detrimental effects on sperm motility or integrity. The highest sperm motility was obtained when semen was liquefied during dilution (1:4) with Biladyl medium (Minitüb, Germany) and stored at 4 C. Sperm remained viable for periods up to 96 h although optimal storage times remained within 48 h, but preferably 24 h of collection. Sperm diluted in Lactose diluent and pellet-frozen on dry ice had superior post-thaw survival compared with the other methods examined. We were unable to obtain pregnancies after transcervical AI of alpaca females with frozen-thawed epididymal sperm at an induced ovulation. Nevertheless, we identified the first step on the roadmap to a practical method for alpaca AI, namely that these sperm are capable of binding with oocytes in a heterologous in vitro assay. This key finding needs to be extended with further research on the inhibitory components in seminal plasma and methods for deeper deposition of sperm at AI. The application of sperm-sexing technology to alpacas was also investigated. The DNA difference between X- and Y- chromosome bearing sperm in alpacas was determined to be 3.8 %. Based on this difference, X- and Y-chromosome bearing sperm were successfully separated, highlighting the potential to apply this technology to alpacas in the future. Implications for relevant stakeholders This project made considerable advances in collection, liquefaction and preservation of alpaca semen, demonstrated the limitations imposed by the viscous seminal plasma and highlighted the need for future research into seminal plasma viscosity, which remains the key problem for the development of AI technology. Nevertheless, efficient and reliable methods for semen collection, harvesting epididymal sperm, liquid storage and cryopreservation were established during this project which will hasten the commercialisation of AI with liquid-stored semen. Overall, the knowledge gained during this project has made a significant contribution to the scientific field, and the results challenge many previously held perceptions associated with the preservation of alpaca semen. Recommendations It is first recommended that breeders and practitioners adopt the new sperm collection, assessment and processing methods developed during this project and detailed in this report. Second, the results presented in this report highlight the need for a sustained, long-term research plan. Specifically, future research is required to determine the origin and constituents of the viscous seminal plasma in camelid semen, further determine the effects of alpaca seminal plasma on sperm viability and functional integrity, further investigate the effects of liquid storage and cryopreservation on sperm functional integrity using computer assisted sperm analysis (CASA) and sophisticated sperm assessments, and develop an appropriate AI procedure and investigate the appropriate site of semen deposition, sperm dose and timing of insemination after ovulation. xii
15 AI in alpacas will facilitate the widespread use of sires of high genetic value (both national and international) and increase the rate at which superior genetics are disseminated. While offspring have been born from AI with both fresh and frozen-thawed sperm the efficiency is low. Improvements to liquid and frozen sperm preservation techniques would certainly increase AI efficiency. However, it is doubtful that any major increases in efficiency (to the levels required for commercialisation) will occur until there is a fundamental understanding of the components of the seminal plasma, which limits both the function and recovery of sperm from the alpaca ejaculate. In many ejaculates, a high proportion of the sperm are unavailable for processing and storage because they are either bound up in the viscous seminal plasma and/or inhibited by its components. Epididymal sperm do not have these restrictions, because they have yet to be exposed to the seminal plasma, which is naturally mixed with the sperm at the time of ejaculation. In this project, we have been able to demonstrate methods to recover these epididymal sperm from castrated males, and to process and store them, with their function maintained for up to 96 h or indefinitely, for liquid and a frozen storage, respectively. We were unable to obtain in vivo fertility after transcervical insemination of female alpacas with frozen-thawed epididymal sperm, but we obtained preliminary evidence that they may be able to bind to oocytes in vitro. The commercial use of epididymal semen is unlikely to be practicable, apart from circumstances such as the death or serious injury/disease of a particularly valuable male, or in the event of sperm harvest from genetically valuable clones, as its recovery is a surgical procedure. However, epididymal sperm do provide a good model for determining the innate fertilising capacity of camelid sperm, unsullied by the inhibiting factors in seminal plasma. It is therefore recommended that studies continue on determining the fertilising capacity of both epididymal and ejaculated sperm, after liquid and frozen storage, using in vitro and in vivo techniques. Given the difficulties of obtaining consistent fertility to date, and the limited numbers of animals available for research, it may be that a laparoscopic or mini-laparotomy technique needs to be developed, to enable deposition of stored semen close to the site of fertilisation, probably in the oviduct. Such a procedure might be more costly than current transcervical insemination methods, although the latter still require induction of ovulation, sedation and individual handling of females, but a more reliable AI technique might allow high fertility to be obtained from the semen of highly valuable males deposited in high value females. The key problem for storage of semen from all camelids remains the viscous seminal plasma. If the industry is to achieve the practical application of liquid and frozen sperm storage, and a viable system of AI in camelids, particularly llamas and alpacas, the following basic questions must be answered: (1) What are the sources and constituents of the viscous seminal plasma? and (2) What is the basic protein content, structure and function of camelid seminal plasma? Analysing the source and constituents responsible for semen viscosity is critical to developing methods to overcome or circumvent the deleterious effects of the seminal plasma. This has been achieved in other species with problematic seminal plasma, for example the goat, but has either been ignored or considered too difficult in camelids. The major problem for goat semen preservation is the bulbourethral gland secretion (BUS) responsible for deterioration of sperm diluted in skimmed milk or in media containing egg yolk. The component from BUS responsible for this effect was identified as a kda glycoprotein lipase (Busgp60) belonging to the pancreatic lipase related protein 2 family (GoPLRP2). This enzyme was able to hydrolyse both triolein and milk triglycerides into free fatty acids which strongly inhibit the motility and damage the membranes of buck sperm. The solutions to this problem, developed in France and by our group during the 1980s, have been one of the most significant advances for the European goat industry over the past 50 years, leading to the widespread application of AI in dairy goats. Therefore, the main recommendation from this project is to undertake fundamental studies on the viscous seminal plasma of camelids with the objective of answering the two questions posed above. A successful outcome to such a study would be a major step along the roadmap to the widespread commercialisation of AI for the Australian alpaca industry. xiii
16 Introduction The project was broken down into a number of steps to achieve these aims: 1. Develop consistent and reliable collection of semen by optimising the mannequin design and investigating the effects on semen quality of different artificial vagina designs, modifications to the semen collection vessel and its contents, and the management of males and females during collection; 2. Develop new methods to assess the function and integrity of alpaca sperm, including sperm motility, acrosome integrity, sperm viability and in vitro fertility; 3. Develop methods to liquify alpaca semen mechanically or by enzymes; 4. Optimise liquid storage of alpaca semen by investigating diluents, storage temperatures, dilution rates and removal of seminal plasma; 5. Optimise cryopreservation of alpaca sperm using an epididymal model and investigates the effects of cryodiluent, packaging, freezing rate, supplements and liquid storage prior to freezing; 6. Artificially inseminate females with frozen-thawed alpaca sperm; and 7. Undertake preliminary development of sperm-sexing technology in alpacas. Artificial insemination (AI) in alpacas is the subject of research in several countries. First attempts at AI occurred in the 1960s in Peru (Fernandez-Baca and Calderon 1966; Calderon et al. 1968) and camels (Elliot 1961). Since then, studies in semen collection and artificial insemination have been performed in alpacas and other South American camelids in Australia (Gunn 1999), North and South America (Bravo et al. 1997b; Ratto et al. 1999; Huanca and Gauly 2001), Britain (McEvoy et al. 1992) and Germany (Burgel et al. 1999; 2000). Concentrated efforts at AI in camels have occurred in the last 20 years in an attempt to improve quality and production of milk and meat, in response to higher stakes in camel racing and increased interest in breed conservation (Purohit 1999). The reproductive physiology of alpacas differs to that of other domestic animals and remains poorly understood. Males mate in sternal recumbency for approximately 20 min and ejaculate many times during this period (Lichtenwalner et al. 1996a,b). Each ejaculate consists of low volume, high viscosity semen containing a low sperm concentration (Sumar 1983; McEvoy et al. 1994). The fertility of alpaca males declines with increasing numbers of consecutive matings (Bravo et al. 1997a). Gestation length is about 11.5 months, twins are rare and males reach puberty from 1 to 3 years of age (Bravo and Johnson 1994). Generation intervals are relatively long because males are slow to sexually mature and females exhibit an extended gestation, so conventional breeding results in slower genetic gain in comparison to other fibre-producing domestic species such as sheep and goats. Development of AI is aimed at increasing the use of superior alpaca males as multiple doses of semen will be derived from each ejaculate. AI will reduce the need for mobile matings and therefore reduce the risk of injury and transport-associated diseases in elite alpaca males and significantly reduce the risk of transmission of Johne s disease, lice and other infectious agents among alpaca farms. AI will also allow more humane and economic importation of superior genotypes from overseas and increase the efficiency of dissemination of superior alpaca genotypes around Australia. In other fibre industries, such as Merino sheep and Angora goat production, assisted breeding is being used to improve fibre quality more rapidly than would otherwise be possible by natural mating. There are no established AI services world-wide for alpacas. The reasons include: the difficulties of funding and sustaining research in Latin America the difficulty in semen collection the mucoid characteristic of alpaca semen which precludes the easy adaptation of AI technology from other species the characteristic of induced ovulation in female alpacas 1
17 the lack of techniques to define reproductive excellence in male alpacas the lack of proven techniques to store alpaca semen in chilled or frozen form the difficulty of transcervical pipette passage of some females The Australian alpaca industry is endeavouring to become commercially viable by producing large quantities of high quality fibre. There are currently 35,500 alpacas in Australia (J. Rothque, Australian Alpaca Association, personal communication). These animals are shorn annually and their fibre is processed for yarn, clothing and bedding. The national herd has an average fibre diameter of 28 microns (B. McGregor, Victorian Dept Natural Resources and Environment, personal communication). 84 % of the 1999/2000 Australian alpaca clip had a diameter greater than 25 microns, 27 % of the clip was white and each animal produced an average of 1.25 kg skirted fleece (Holt 2001). The Australian Alpaca Co-operative currently pays AU$ 45/kg clean fibre for fibre diameter less than 20 microns, but only AU$ 5/kg clean fibre for fibre with a diameter of microns. Breeders must place maximum selection pressure on males and combine this with rapid and broad dissemination of appropriate genotypes to achieve the goal of producing high quality fibre. The increased use of alpaca males with superior genotypes via artificial insemination would allow a faster reduction in fibre diameter, increased fibre density per animal and greater control of fibre colour in the national alpaca herd. Producers would benefit with improved quality and increased quantity of alpaca fibre in their herds. Many alpaca farmers do not own a male suitable for breeding as the Australian alpaca industry is based on hundreds of properties each with less than 20 animals. Semen is not yet available for reliable, cost effective artificial insemination (Burgel et al. 1999). Farmers that do not own a male currently use selected males owned by other breeders to improve the fibre quality of their animals. Therefore, the Australian alpaca industry relies heavily on mobile or trailer matings to disseminate throughout the national alpaca herd genotypes perceived to be superior for producing fine fibre. This method involves one alpaca, usually the male, being transported to the farm where mating is to occur. The farm manager determines the time of mating in both mobile matings and on-farm pen-matings. This project aimed to improve the ability of breeders and veterinarians to apply AI techniques to overcome the need for mobile mating and to assist genetic improvement of alpacas in Australia. The report that follows comprises a literature review, covering semen characteristics, preservation and AI in camelids with particular reference to alpacas (Chapter 1), a full description of the methods used in this project, which can be used as a practical manual by breeders and practitioners contemplating artificial breeding in alpacas (Chapter 2), seven experimental chapters describing our work on semen collection (Chapter 3), assessment of sperm function (Chapter 4), liquefaction of alpaca semen (Chapter 5), liquid storage of sperm (Chapter 6), cryopreservation of sperm (Chapter 7), artificial insemination (Chapter 8) and sperm sexing (Chapter 9). These experimental chapters can be read as stand-alone reports and, as a consequence, contain some necessary repetition. The experimental chapters are followed by a general discussion of the results and a consideration of the implications and recommendations arising from the work carried out in this project. The appendices to the report also provide detailed protocols, equipment and publication lists that may be useful for scientists and practitioners. 2
18 Objectives Project US-138A, The continued development of artificial insemination technology in alpacas continued the work of RIRDC project AAA-1A (publication 03/104; Vaughan et al. 2003a) and had two main objectives. The first objective was to further develop and establish the technology for collection, processing and preservation of alpaca semen. The second objective was to further develop and establish the technology for insemination of alpaca semen. The outcomes from these objectives were: an optimum method for semen collection and AI of alpacas under Australian conditions the education of alpaca breeders in the benefits of using AI technology in their own herds to hasten improvement of fibre quality via a presentation at the Australian Alpaca Association Inc. National Conference and publication of an article in the AAA Conference proceedings the production of a manual that can be used by small ruminant artificial breeding centres and practising veterinary practitioners to provide commercial artificial insemination programs to alpaca breeders. 3
19 1. Review of the literature 1.1. Introduction The Camelidae family belong to the order Artiodactyla that includes all even-toed mammals and includes the Antilocapridae (antelopes), Bovidae (cattle and bovids), Cervidae (deer), Giraffidae (giraffes), Hippopotamidae (hippopotamuses), Ovidae (sheep and goats) and Suidae (pigs) families. Members of the Artiodactyla families exist throughout the world, except Antarctica, Australia and New Zealand (Table 1.1). Camelidae belong to the sub-order, Tylopoda (padded foot animals) and consist of three genera: Camelus, Lama and Vicugna. There are six species in the Camelidae family and all have 37 pairs of chromosomes. The general features of the members of the Camelidae family are summarised in Table 1.1. The functional anatomy and the reproductive physiology of the Camelidae family is similar for the Old (Camelus genus) and New World (Lama and Vicugna genus) camelids despite their evolutionary divergence. Both New and Old World camelids inhabit harsh climates, albeit on different continents. Camelidae reproductive physiology results in a naturally low reproductive rate. Reasons for this include age at puberty (late maturation), long gestation interval, seasonality of breeding, induced ovulation, failure of ovulation, low sperm production, and in camels a long period of lactation-related anoestrus leading to long intervals between births, high incidence of reproductive (genital tract) abnormalities and high rates of embryonic mortality during the hotter months (Al-Qarawi 2005). The various aspects of Camelidae general and reproductive anatomy and physiology have been extensively reviewed (Elwishy 1987, 1988; Fernandez-Baca 1993; Smith et al. 1994; Tibary and Memon 1999a,b; Al-Eknah 2000; Skidmore 2000a,b; Vaughan et al. 2003a; Skidmore 2005; Tibary and Vaughan 2006; Vaughan and Tibary 2006). This review is adapted from RIRDC project AAA- 1A (Vaughan et al. 2003a) and was updated at the commencement of this project. A list of literature obtained during this project is presented in Appendix XII. Preservation of semen permits the easy transport of semen from superior sires, storage of semen from superior sires beyond the lifespan of the donor, prevention and control of disease and increase in the efficiency of breeding. The desire to preserve semen leads to an extensive period of empirical research to determine the optimal freezing diluent for sperm and refine processing, freezing and thawing methods (Salamon and Maxwell 1995a). 4
20 Table 1.1. General overview of Camelidae family (adapted from Vaughan 2001), derived from Chen et al. (1985), Elwishy (1987), Wheeler (1995) and Bravo et al. (2000c). 5 Old world New World Characteristic Bactrian camel Dromedary camel Alpaca Llama Guanaco Vicuña Genus species Camelus Bactrianus Camelus dromedarius Vicugna pacos Lama glama Lama guanicoe Vicugna vicugna Alshan, Indian, Cara (short wool) Breeds Kazakh, Mongolian, > 50 breeds # Huacaya Chaku (long wool) Suri Sunite, Xinjiang Suria Distribution and population Asian continent: China to central Asia and a small population in Northern India Saharan and sub- Saharan Africa, Middle East, Asian subcontinent and Australia Peru, Bolivia, Chile, Argentina, USA, UK, Australia Peru, Bolivia, Chile, Argentina, USA, UK, Australia Argentina, Chile, Peru, Bolivia, Australia, UK, UAE 300,00 in South America Peru, Chile, Bolivia, Argentina 2 million worldwide 3.5 million in 2.0 million in South 20 million worldwide South America America Life expectancy years Live weight (kg) Height at shoulder (cm) Description Single or multicoloured from white through to black and grey Fibre, meat, Fibre, meat, skins skins Light brown with white belly, darker head (grey black)? Rich chestnut colour white belly and white bib Products Fibre, meat and milk Meat, milk, skins, Fibre quality is faeces (fuel) mediocre Fibre Fibre diameter (μm) Age at first breeding (m) Female: 12 Male: Gestation length (m) Ovarian weight (g) Testes weight (g) Additional animals located in zoos around the world. # Whether these are actual breeds or variants of the same type has not been established (*Tibary and Anouassi 1997).
21 1.2. Reproductive anatomy and physiology of the male alpaca Puberty Male camelids are usually born with descended testes that are small, soft and difficult to palpate (Sumar 1983; Elwishy 1988; Bravo 1995; Fowler 1998). The mechanism of testicular descent has not been described (Tibary and Memon 1999a). Plasma testosterone levels are basal (60-90 pg/ml; Bravo 1995, 2002) and adhesions exist between the penis and prepuce (Sumar 1983; Johnson 1989; Fernandez-Baca 1993). As males mature, the testes enlarge (Table 1.2) and plasma testosterone levels increase to more than 1000 pg/ml (at approximately 20 months of age in the majority of alpacas; Bravo 1995, 2002). Rising concentrations of testosterone allow the animal to grow and put on body condition, develop secondary sexual characteristics and apparently breakdown peno-preputial adhesions (Sumar 1983; Elwishy 1988; Fernandez-Baca 1993; Bravo and Johnson 1994; Bravo 1995; Fowler 1998; Brown 2000). There is variation in the age of puberty in different camelid species (Table 1.3). Seminiferous tubules may develop lumina and commence spermatogenesis in alpacas from 12 months of age (Montalvo et al. 1979; Galloway 2000). However, the onset of sexual maturity is often determined by the age at which the penile adhesions disappear and males become capable of a full erection, rather than the time at which viable sperm are produced (Figure 1.1; Smith 1999b). It has been observed in alpacas that at 1 year of age 8-12 % of males, at 2 years of age % of males and at 3 years of age % of males have lost peno-preputial adhesions (Sumar 1983; Fernandez-Baca 1993; Fowler 1998; Smith 1999b; Bravo et al. 2000c). Table 1.2. Testicular size and weight in relation to age and body weight in alpacas (Bravo 1995), adapted from Vaughan et al Age (years) Testicular dimensions (cm) Testicular weight (g) x x x Body weight (kg) Detachment begins at the tip of the penis, at approximately months of age in the majority of males; half the glans is free at 18 months of age and the penis is completely free of adhesions between 21 and 26 months of age (Bravo and Johnson 1994; Bravo 1995). The variation in age at which peno-preputial adhesions are lost may be partially explained by plane of nutrition (Fernandez- Baca 1993) as there is a correlation between body size and mean testicular length (Galloway 2000). The wide variation in testicular size at any one age or body size suggests that other factors, probably genetic, are also important (Galloway 2000). Alpacas are considered to have reached full sexual development at 5 years of age, at approximately 63 kg body weight (Sumar 1983). Table 1.3. Age at which puberty attained in camelids. Reference Species Age (years) capable of breeding Montalvo et al Alpaca years Galloway 2000 Alpaca 1 3 Sumar 1983 Alpaca/ llama 1 3 Fowler 1998 Alpaca/llama 1 3 Johnson 1989 Llama Novoa 1970 Vicuña 1 Elwishy 198 Dromedary 3 4 Arthur 1992 Camel 5 6
22 1.2.2 Anatomy Prepuce The prepuce is triangular, flattened side-to-side, non-pendulous and situated midline in the inguinal region (Fowler 1998; Smith 1999b). There are two rudimentary teats on either side near its caudal border. The preputial orifice is directed caudo-ventrally when relaxed (Fowler 1998). Well developed muscles (lateral preputial muscles and cranial and caudal preputial muscles) can direct the prepuce forwards for erection and backwards for urination (Elwishy 1988; Johnson 1989) Penis Camelids have a fibroelastic penis with a prescrotal sigmoid flexure that allows retraction of the penis into the prepuce in the non-erect state (Figure 1.2; Novoa 1970; Sumar 1983; Elwishy 1988; Bravo 1995; Fowler 1998; Smith 1999b). The retractor penis muscles are attached to the ventral convexity of the sigmoid flexure of the penis (Tibary and Memon 1999a). The erect penis is cm long in alpacas (Sumar 1983; Bravo and Johnson 1994) and cm long in llamas (Fowler 1998). Eighteen to 25 cm of the penis extends beyond the prepuce in the erect state (Johnson 1989). Erectile tissue consists of two corpora cavernosa at the root of the penis that converge into a single corpus cavernosum in the body of the penis (Elwishy 1988). The urethra is incorporated ventrally in a groove of the corpus spongiosum (Elwishy 1988). The three cavernous bodies of the penis are surrounded by thick fibrous tunica albuginea (5-7 mm thick in camels; (Elwishy 1988). The glans penis is 9-12 cm long (Fowler 1998). It tapers from a 2 cm diameter fibrous structure proximally, to 1 cm diameter distally, and ends in a single rounded cartilaginous projection, the urethral process (Sumar 1983; Elwishy 1988). The process is 10 mm long in alpacas, curves in a clockwise direction (Sumar 1983) and may play a role in directing the penis through the cervix during copulation (Johnson 1989; Bravo 1995; Fowler 1998) and dispersing semen in the uterus (Smith 1999b). The urethral opening of the glans is at the base of the cartilaginous process (Fowler 1998; Smith 1999b). There is a dorsal urethral diverticulum at the level of the pelvic symphysis that prevents passage of a catheter into the bladder (Fowler 1998; Smith 1999b) Testes The testes of camelids are located in a non-pendulous scrotum without a defined neck, forming a subanal protuberance comparable to boars (Sumar 1983; Elwishy 1988). Normally, both testes are small and elliptical, similar in size, turgid on palpation and move freely within the scrotum (Sumar 1983; Bravo 1995; Fowler 1998). The long-axis of each testis is oblique, with a caudodorsal, cranioventral orientation (Sumar 1983). The histology of camelid testes has been described (Delhon and Lawzewitsch 1987) and is similar to that of other livestock species (Fowler 1998; Hafez and Hafez 2001). Spermatogenesis has been divided into eight stages in camelids (Delhon and Lawzewitsch 1987) with development beginning with spermatogonia lying in the outermost regions of seminiferous tubules and progressing to primary and secondary spermatocytes, spermatids and spermatozoa, which lie adjacent to the lumen of the seminiferous tubules (Galloway, unpublished). Sertoli cells line the seminiferous tubules and contribute to fluid production by the tubule (Hafez and Hafez 2001). Leydig cells lie between the seminiferous tubules and secrete testosterone into the testicular veins and lymphatic vessels (Delhon and Lawzewitsch 1987; Hafez and Hafez 2001). Histological examination of camel testes (Ismail 1988) revealed that camels have abundant Leydig cells (which constitute 28.2 % of testicular volume) and seminiferous tubules that constitute 68.3 % 7
23 of the whole testicular parenchyma. The average width of the tubules is μm, and their length is 1138 m, with tubules reaching their maximum diameter in camels at years of age. These data also demonstrate that gonadal sperm reserves increase with age and peak in camels aged years, then decrease gradually until 20 years of age (Ismail 1988). Seminiferous tubules in alpacas have a diameter of μm (Bravo et al. 2000c), and in llamas a mean (± SD) diameter of ± 19.8 μm (Delhon and Lawzewitsch 1987). The complete process of spermatogenesis in bulls takes approximately 60 days. The seminiferous epithelium cycle takes days and four to five cycles of the seminiferous epithelium are required. Sperm take another eight or nine days to travel through the epididymis. Therefore, the testes may take more than 2 months to recover from damage resulting from an injury such as hyperthermia or systemic illness (Entwistle 2002). It is not known how long the process of spermatogenesis takes in alpacas. Daily sperm production varies between species from million per gram of testicular tissue (Hafez and Hafez 2001) but is considerably lower in camelids than other farm animals, which may be a result of smaller testicular size, lower contribution of the parenchyma to testes weight (owing to the thick tunica albuginea), lower contribution of the seminiferous tubules to parenchyma weight (owing to abundant interstitium) or shorter seminiferous tubules (Ismail 1988). These conclusions are speculative as the real relationship between testes weight, fertile sperm production in camelids is unknown. Sperm production may be correlated with testicular size in camelids (Tibary and Memon 1999a; Galloway 2000). As testicular weight and size increase in bulls, so too does daily sperm output and fertility (Entwistle 2002); presumably this occurs in alpacas too. Testicular size is highly heritable in bulls and is also related to fertility of his female relatives (Entwistle 2002). Camelid testes are relatively small and sperm production is low (Smith 1999b)and the estimated daily sperm production in camels is 8.1 x 10 6 per day in spring and 4.2 x 10 6 per day in summer (Bravo et al. 2000c). Each adult testis in the alpaca measures approximately 4-5 cm long, cm wide and weighs 15-18g (Tables 1.4, 1.5), thus accounting for % of body weight (Sumar 1983; Bravo 1995), in comparison with the testes of rams (1.4 % of body weight) and bulls (0.18 % of body weight; (Entwistle 2002). Three-year-old camels have testes 7-10 cm long that each weigh g (Novoa 1970). Under normal conditions of spermatogenesis, seminferous tubules and the tail of the epididymides are distended with spermatozoa and fluid, giving tissues tone and resilience upon palpation (Entwistle 2002). Poor tone and resilience or excessive firmness suggests abnormalities of testicular and/or epididymal function. Vascular rather than muscular thermoregulation of the testes may be important in camelids because of the close apposition of the scrotum to the body (Elwishy 1988). The testicular artery is convoluted and coiled. The pampiniform plexus of the testicular veins enmeshes the artery and allows arterial blood entering the testis to be cooled by venous blood leaving the testis via a counter-current cooling mechanism (Hafez 1993). The muscular structures of the scrotum and spermatic cord (dartos and cremaster muscles) elevate the testes, with associated thickening of scrotal skin, during cold weather and relax slightly during warm weather (Bravo and Johnson 1994). The skin of the scrotum is relatively thick and may be an adaptation to protect the testes from accidental castration during malemale fighting (Fowler 1998). Efferent ducts run from the testis into the head of the epididymis (Bravo 1995). 8
24 Table 1.4. Mean (range) testicular size (cm) and weight (g) in alpacas, llamas and vicuñas (adapted from Sumar 1983, Fowler 1998 and Bravo 2002). Age (months) Alpaca Llamas Vicuña (n=158) (n=54) (n=6) Size (cm) Weight (g) Size (cm) Weight (g) Size (cm) x x 1.4 na 0.7 x x x x x x x x x x x x na x x x 1.4 Sires Fowler et al x x 3.3 na 3.3 x 1.9 Sires Sumar x Table 1.5. Mean and range of testicular lengths (cm) in alpacas of different ages (Galloway 2000). Age (months) Number of Mean testicle length males (cm) Range (cm) <12 (4-11) Testicular defects including hypoplasia, cryptorchidism and ectopic testes have been reported in camelids (Sumar 1983; Elwishy 1988; Johnson 1989; Fowler 1998; Galloway 2000) and are considered as genetic faults, though this has yet to be proven (Sumar 1983). Testicular hypoplasia is the most common testicular defect observed in alpacas (Sumar 1983). In a Peruvian study, 10 % (385/3807) of all males examined exhibited some form of hypoplasia (Sumar 1983) while in an Australian study, 5 % (4/73) males exhibited testicular hypoplasia (Galloway 2000). Hypoplasia may be bilateral or unilateral, and ranges from mild (varying degrees of spermatogenic activity) to severe (Sertoli cells only) histologically (Sumar 1983; Elwishy 1988) Hypoplastic alpaca testes weigh 7-12 g and are firmer than normal (Sumar 1983). Cryptorchid testes are small, soft, undescended testes that are usually situated close to the internal inguinal ring (Sumar 1983). Left testes are more commonly affected than right testes (Sumar 1983, Perkins et al. 1996). Ectopic testes are situated outside the scrotal sac perineally, on the medial thigh or lateral to the penis (Sumar 1983). Ectopic testes are small (8-12 g in weight), flabby and more common on the left side (Sumar 1983). 9
25 In an extensive examination of post-mortem material, (Sumar 1983) observed 1-5 mm diameter unilateral and bilateral cystic structures in the head of the epididymis and/or in the body of the testis. It is thought that the cysts are derived from paramesonephric ducts (Sumar 1983). Acquired testicular problems in male camelids include testicular trauma secondary to fighting and biting and scrotal oedema and reduced sperm production and libido secondary to heat stress (Johnson 1989; Fowler 1998; Smith 1999b). South American camelids possess a non-pendulous scrotum and appear poorly adapted to heat-sensitive testicular thermoregulation (Pugh 1999). Thermoregulation may be complicated by excessive scrotal fat in obese males (Pugh 1999). Infertility due to heat stress is usually temporary, and fertility generally returns days after the initial stress (Johnson 1989; Pugh 1999) Epididymides The epididymis is small and closely adherent to the testis (Smith 1999b). It consists of a head (caput), body (corpus) and tail (cauda; Tibary and Memon 1999a). The head is larger than the tail (Bravo 1995). The head of the epididymis lies cranioventral to the testis, the body extends medially and dorsocaudally to the tail which lies dorsal to the testis (Fowler 1998). The head and body are sites of sperm maturation, while the tail is associated with sperm storage (Elwishy 1988). Histology of the epididymis is similar to other species (Delhon and Lawzewitsch 1994; Smith 1999b). Distal movement of the cytoplasmic droplet occurs as sperm transit through the epididymis (Tibary and Memon 1999a). A distal cytoplasmic droplet remains in more than 60 % of sperm in the terminal segment (Elwishy 1988; Tibary and Memon 1999a) and is lost when sperm reach the ductus deferens (Delhon and Lawzewitsch 1994). The ductus deferens is 40 cm long and 2-3 mm wide in the llama (Bravo and Johnson 1994; Smith 1999b) and cm long in the camel (Bravo et al. 2000c). It runs from the tail of the epididymis, through the inguinal ring to the urethra just caudal to the neck of the bladder, where there is a small, poorly defined ampulla 2-4 mm in diameter (Bravo and Johnson 1994; Bravo 2002). The lack of/small ampulla may explain why electroejaculation does not work well in camelids as the ampulla is unable to act as a site of sperm storage (Bravo et al. 2000c). Moreover, epididymal sperm reserves are low in camelids partly due to the relatively small cauda epididymis (Tibary and Vaughan 2006). This may explain the rapid decline in sperm numbers in ejaculates when males are mated frequently (Bravo et al. 1997a) Accessory sex glands The prostate is H-shaped and firmly attached to the dorsolateral aspect of the urethra, near the trigone of the bladder (Sumar 1983; Bravo and Johnson 1994; Bravo 1995; Fowler 1998; Smith 1999b). It is 3-4 cm wide and 1-2 cm high in llamas (Fowler 1998; Smith 1999b) and 3.7 x 5 cm in camels (Novoa 1970). Seasonal changes in weight are correlated with testicular activity (Elwishy 1988). The two bulbourethral glands are ovoid and located lateral to the terminal part of pelvic urethra at the ischial arch at the root of the penis (Sumar 1983; Johnson 1989; Bravo 1995), 7-8 cm caudal to the prostate in the alpaca (Smith 1999b). They measure 1 cm in alpacas and up to 2 cm in llamas (Fowler 1998; Smith 1999b). Each gland is partly covered by the bulbocavernosis muscle and they exhibit seasonal variation in size (Elwishy 1988). These glands are most likely to produce the viscous material found in the ejaculate of camelids (Bravo 1995). Vesicular glands (seminal vesicles) are not found in camelids (Sumar 1983; Elwishy 1988; Johnson 1989; Bravo and Johnson 1994; Smith 1999b). 10
26 Figure 1.1. Diagram of preputial adhesions of South American camelids (A = preputial adhesions, B = prepuce, C = penis; Fowler 1998). Figure 1.2. Diagram of the lateral view of male camelid genitalia (Fowler 1998). 11
27 Seasonality of reproduction Old World camelids are highly seasonal breeders, and have a well-defined breeding (rutting) season which coincides with the cool winter months (Table 1.6; Deen et al. 2005). During the rutting season, male camels undergo behavioural as well as endrocrine changes (Yagil and Etzion 1980): they cease being docile, become restless and aggressive, mark their territory, attack humans, often bite strangers, grind their teeth, salivate excessively and make a whistling sound (Deen et al. 2005). Males intermittently extrude their soft palate, urinate on their tails, and whisked the urine on their back and an acrid-smelling fluid is secreted from their poll glands (Deen et al. 2005). These behavioural changes are associated with a marked increase in plasma cortisol, follicle stimulation hormone (FSH), luteinizing hormone (LH), and testosterone, while prolactin decreases (Azouz et al. 1992). Spermatogenesis occurs throughout the year in all camelids (Elwishy 1988; Arthur 1992) but changes with the rutting and non-rutting seasons. Seasonal variations in sperm production are based on geographical origin (hence nutrition, climate and other environmental factors), herd management, extent of domestication and social structure of the group of camelids occur (Novoa 1970; Elwishy 1988; McEvoy et al. 1994). During the breeding season, testicular weight increases (Elwishy 1988) due to increased development of interstitial tissue and increased diameter of seminiferous tubules. The mean paired testes weight in Dromedary camels is g in the nonbreeding season and g in the breeding season (Elwishy 1988). From December to March, seminiferous tubules are 183μm in diameter and contain 5-6 layers of germ cells in various stages of differentiation. However after March, degeneration occurs, tubules became devoid of sperm and seminiferous tubules decrease to 131 μm diameter (Novoa 1970). Spermatogenesis also increases as do epididymal sperm reserves (Abdel-Raouf et al. 1975) and semen collected during the non-breeding season has a lower ph, lower concentration of sperm, reduced sperm motility and a higher proportion of dead sperm, sperm abnormalities and acrosome damage (Tibary and Anouassi 1997) than that collecting during the breeding season. Furthermore, the ability of camel sperm to penetrate she-camel cervical mucus is reduced during the non-breeding season (Amer et al. 2006). Generally, New World, or South American camelids are considered less seasonal, although there is a defined breeding season in South America which extends from November to May (Spring Autumn; Bravo 2002). In the USA and Australia, however, New World camelids are not considered seasonal breeders and are capable of mating and giving birth year round (Smith et al. 1994). Behaviour changes exhibited by male camels in the rutting season do not occur in New World camelids in South America (Novoa 1970) although seasonal changes in testosterone have been observed (Table 1.7). Camelids in the USA do not display seasonal differences in plasma testosterone concentration, LH pulse frequency or testicular size (Smith 1999a). In the non-breeding season, there are differences in the longitudinal and transverse diameters of testes of vicuña living in the Andes. There are also differences in the diameter of seminiferous tubules, and Leydig cell nuclei diameters, and a reduction in the proportion of tubules that contain spermatozoa in winter compared with summer (Urquieta et al. 1994). Plasma testosterone levels are also reduced during the non-breeding season for New World camelids living in South America (Table 1.7). February and March are the peak breeding months for alpacas in Peru following summer rains and increased pasture growth (Garnica et al. 1993; Raymundo et al. 2000). In the USA, no seasonal differences were noted in plasma testosterone concentrations, LH pulse frequency or testicular size in a group of males monitored for 12 months (Smith 1999b). 12
28 Table 1.6. Breeding seasons for New and Old-World camelids in different locations (adapted from Vaughan 2001). Species Location Breeding season Reference Alpaca Peru December March San-Martin et al Alpaca New Zealand September April Knight et al Alpaca/Llama South America January April Fernandez-Baca 1993 Alpaca/Llama Peru December March Smith et al Alpaca/Llama USA Year round Smith et al Llama USA Year round Smith 1999a Llama Peru November March Bravo 2002 Vicuña Chile/Peru February May Urquieta et al Bactrian camel China December April Chen et al Dromedary Somalia April May Mares 1954 Dromedary Egypt December May Shalash 1965 Dromedary Sudan March August Musa and Abusineina 1978 Dromedary Sudan May June Homeida et al Dromedary Tunisia December March Minoia et al Dromedary Morocco November April Sghiri and Driancourt 1999 Dromedary United Arab Emirates November - February Skidmore 2003 Dromedary India December mid-may Deen et al Dromedary Saudi Arabia December March Tibary and Anouassi 1997 Dromedary Kenya Continuous Tibary and Anouassi 1997 Plasma testosterone concentrations also vary between seasons (Fowler 1998). When male alpacas are separated from females as a management practice in Peru, testosterone concentrations are 3900 pg/ml compared with 9000 pg/ml in the breeding season. In the USA, where males are mated all year, plasma testosterone fluctuate between 900 and 1200 pg/ml throughout the year (Fowler 1998) Table 1.7. Concentration of plasma testosterone for camelids during the different seasons. Season Alpaca Dromedary Llama Vicuña (pg/ml) (ng/ml) (pg/ml) (pg/ml) Bravo 2002 Tibary and Anouassi 1997 Bravo 2002 Bravo 2002 Summer ± ± ± 52.7 Autumn ± ± ± 14.9 Winter ± ± ± 73.7 Spring ± ± ± Mating behaviour and copulation Mating behaviour can be seen in South American camelid males weeks to months after birth when they try to mount females or other juveniles and begin play-fighting (Fowler 1998; Smith 1999b). Androgen production increases significantly between 18 and 24 months of age and usually coincides with sperm production and increased libido (Smith 1999b). Males fight to assert their dominance over other males, using their neck, chest and canines to establish superiority (Fowler 1998). There is no evidence that experience affects the behaviour of alpaca males. However, there is individual variation in libido among males and variation in the number of females mated (Pollard et al. 1995). 13
29 Males in their first season behave in a similar manner to those with more experience. The mating behaviour exhibited by a SAC male may be divided into courting and copulatory phases. The courting phase occurs when the male actively chases the female and may only last a few seconds, as some females sit immediately, or up to 10 minutes, but attempts longer than 4 minutes usually result in no mating (England et al. 1971). The length of this phase may be influenced by the level of libido of the male (Fowler 1998). The mating behaviour of the female is mainly submissive, but a male may mount and push the female on the withers with his front legs. Males may exhibit flehmen by elevating their head and lip after sniffing the dung pile (Fowler 1998). The copulatory phase occurs when the female adopts the copulatory position of sternal recumbency with legs tucked beneath the body (Fernandez-Baca et al. 1970; England et al. 1971). The male straddles the female, grips her shoulders with his elbows, and his metatarsi rest flat on the ground, lateral to those of the female (Novoa 1970). Penile erection commences at this stage to allow intromission (Fowler 1998). The penis may have a corkscrew end as it searches the perineum for the vulval opening (Fowler 1998; Tibary and Memon 1999a). Repositioning of the pelvis of the male may occur to allow intromission via searching of the penis (England et al. 1971). When intromission is successfully accomplished, the pelvis of the male is close to the pelvis of the female (Fowler 1998). Males show their excitement with trembling ears, tail flipping up and down, nostrils dilating and constricting and by vocalisation (Novoa 1970). The characteristic guttural sound made by males during mating, or orgling, is reported to be an integral aspect of the preovulatory LH release (Bravo 1994). During copulation, the male penetrates the cervix with his penis, and deposits semen during multiple ejaculations into both uterine horns using mild thrusting movements, as evidenced by uterine palpation via laparotomy during copulation (Franco et al. 1981; Bravo 1994, 2002). Examination of the uterus 24 hours after copulation has revealed haemorrhage, inflammation, oedema and hyperaemia of the endometrium, possibly due to penile trauma or an inflammatory reaction to seminal plasma (Bravo et al. 1996a; Velasquez and Novoa 1999; Bravo 2002). Inflammation of the uterus may last 3 to 4 days and be more severe following multiple matings (Bravo 2002). When male and female alpacas are kept separately and allowed to copulate at infrequent intervals, both sexes remain sexually active all year (Sumar 1996). At the start of the breeding season in Peru, when males are first placed in a paddock with females, they show intense copulatory activity for the first 3 days, copulating up to 18 times per day (Sumar 1983; Fernandez-Baca 1993). However, continuous association of male and female SAC somehow inhibits the breeding activity of males to the point of complete cessation of sexual activity in spite of receptive females (Fernandez-Baca 1993). If inactive males are then placed in with a new herd of females, they resume sexual activity (Fernandez-Baca 1993). It is not known what factors are responsible for the inhibitory effect on male libido resulting from continued exposure to females and what restarts breeding activity (Fernandez- Baca 1993). However, nutrition, management and environmental factors (temperature, relative humidity, light) or olfactory cues may influence the central nervous system centres that control reproductive behaviour (Brown 2000) Fertility Under conditions of natural mating, pregnancy and subsequent parturition rates following a single mating have been reported as low (Sumar 1996) and less efficient than other farm species (Wiepz and Chapman 1985). However, there have been reports of 21 out of 28 (75 %) female llamas pregnant after two matings 4-8 hours apart (Adams et al. 1990), a 46 % parturition rate in single-mated llamas (Condorena et al. 1988) and 34 out of 70 (49 %) females conceiving after a single natural mating, regardless of ovarian follicular diameter (Vaughan et al. 2003b). These rates compare favourably with conception rates in other domestic livestock and suggest that unsuitable reproductive management, nutritional deficiencies and inbreeding contribute to low fertility (Parraguez et al. 1997). 14
30 The sperm concentration required for successful fertilisation and pregnancy is not known, but intracornual semen deposition of semen during copulation may be an adaptation to overcome relatively low sperm concentrations in ejaculates (Brown 2000). To minimise the number of matings per conception it has been recommended to select males with large testicular size on the assumption that the direct relationship between testis size and sperm production in other domestic livestock also occurs in camelids (Sumar 1983; Smith 1999b; Brown 2000; Galloway 2000). Mean testicular length (the average length of left and right testes) is correlated with testicular weight (Galloway 2000) and may be used as a simple means of assessing testicular size in alpacas. Mean testicular length may be used to estimate the likelihood of sperm production in alpacas (Table 1.8; Galloway 2000). Bravo (1995) describes a trial in which male llamas with small testes (3.0 x 1.9 cm) achieved a 45 % pregnancy rate, while males with larger testes (4.8 x 3.2) achieved a 75 % pregnancy rate. Pugh (1999) observed male llamas with testes 3.5 x 2.9 cm achieved 21/30 (70 %) pregnancies, while males with smaller testes (2.5 x 2.2 cm) only achieved 12/30 (40 %) pregnancies. Table 1.8. Development of testicular function in alpacas with testicles of different sizes (Galloway 2000). Mean testicular length (cm) Proportion of males (%) % Testicular tissue containing elongated spermatids < < < 10 > > 60 Body size has also been correlated with mean testicular length and suggests that nutrition is one important factor in determining testicular size (Galloway 2000). Precocious behaviour, mating ability (no peno-preputial adhesions) and sperm production in yearlings and are also desirable traits in selection programs (Sumar 1983; Galloway 2000). The number of matings performed in one day by a male influences fertility. Bravo et al. (1997c) observed that male alpacas bred 2 or 4 times per day achieved a 77 % pregnancy rate, while those bred 6 times per day achieved a 59 % pregnancy rate. It was proposed that rates declined in response to exhaustion of sperm reserves (Bravo et al. 1997c). The number of consecutive days on which matings occur also influences fertility (Bravo et al. 1997c). Copulation time decreased over consecutive breeding days and the percent pregnant decreased from 71 % on Day 1 of breeding, to 50 % by Day 8 and 0 % on Day 9 (Bravo et al. 1997c). Three to 4 matings per day for 4 to 5 days does not compromise fertility in male alpacas (Bravo 1995) Copulation length Copulation usually lasts minutes, with a range of 5-65 minutes (Table 1.9). The length of copulation is determined by the male and is affected by breed, age, season and frequency of use (Tibary and Memon 1999a). The presence of other camelids will influence length of copulation (England et al. 1971; Johnson 1989). Studies of paddock matings show the average duration of copulation in alpaca herds varies based on animal interactions. Males in herds without other males may average 20 minutes compared with 15 minutes in herds with several males; males mating yearling females may average 15 minutes compared with multiparous females 22 minutes (Escobar 1984; Pollard et al. 1995). Interruption/short duration of copulation in multi-sire herds was attributed 15
31 to normal male territorial fighting, as males are vulnerable to attack during mating (Escobar 1984). Knight et al. (1992) observed that the copulation of maximum duration was not always the first mating when males performed more than one mating per day but others have noted that subsequent matings are shorter than the first mating of the day (Pollard et al. 1995; Bravo et al. 1997a; Vaughan 2001). Various studies have shown matings to be longer in autumn than spring (England et al. 1971; Knight et al. 1992; Pollard et al. 1995). There is no correlation between mating duration and conception rate (Tibary and Memon 1999a). Table 1.9. Duration of copulation in camelids. Reference Species Average duration of mating (min) Range (min) Fernandez-Baca and Novoa 1968 Alpaca 5-50 San Martin et al Alpaca Fernandez-Baca et al Alpaca 21.9 ± 1.2 (n=44) Pollard et al Alpaca Pen mating 18.7 Paddock mating (single sire) 11.3 First mating 28.3 Subsequent mating 12.2 Knight et al Alpaca Autumn multiple matings per day 9.8 ± 0.8 Autumn single mating per day 15.7 ± 2.0 Spring multiple matings per day 8.7 ± 1.0 Spring single mating per day 9.6 ± 0.7 Pollard et al Alpaca Pollard et al Alpaca 16.4 ± 0.65 (n=150) Spring Autumn 18.5 Bravo et al. 1997a Alpaca 1st mating 17.9 ± 0.7 (n=71) 2nd mating 14.1 ± 0.9 (n=34) 3rd mating 13.9 ± 1.4 (n=15) average 15.3 (n=120) Bravo et al. 1997c Alpaca 2 matings/d/male 14.6 ± matings/d/male 11.6 ± matings/d/male 10.3 ±0.5 Paolicchi et al Alpaca 20 Vaughan 2001 Alpaca 1st mating 22.7 ± 1.1 (n=48) nd mating 14.8 ± 1.6 (n=20) ± 8.5 December 22.6 England et al Llama February March 21.8 April-May 26.3 Johnson 1989 Llama Bravo et al Llama 18 (n=4) Bravo 1994 Llama 10d post-partum 24.7 (n=17) 20days post-partum 20.5 (n=17) 30days post-partum 24.9 (n=22) Lichtenwalner et al. 1996a Llama 21.7 ± 7.8 Parraguez et al Llama >12 Alpaca Chen et al Bactrian 3 Elias et al Dromedary 5.2 ± 1.2 Marie and Anouassi 1986 Dromedary 5-10 Marie and Anouassi 1987 Dromedary ovulatory 8.3 ± non-ovulatory 10.0 ± Elwishy 1987 Dromedary
32 Characteristics of ejaculation Ejaculation occurs early during copulation as pregnancies have been observed after matings of 5-8 minutes duration (Fernandez-Baca et al. 1970; Vaughan et al. 2003b) and 8 of 26 sperm collections had sperm within 10 minutes of the start of copulation (Lichtenwalner et al. 1996b). Lichtenwalner et al. (1996a) observed that there were ± 61.0 urethral pulses per copulation at a rate of 6 per minute when describing the nature of ejaculation in llamas. There were 19 clusters (one whole body strain plus 4-5 urethral pulses) per copulation, 20 seconds long and equivalent to approximately 1 cluster per minute during copulation. Each cluster was equivalent to one distinct ejaculation. Therefore, there were multiple distinct ejaculations per prolonged copulation, few urethral pulses between clusters and 22 whole body strains, 816 pelvic thrusts and 53 repositions (which occurred between clusters; Lichtenwalner et al. 1996a). Sperm emission occurs throughout copulation and sperm concentration increases with time (Lichtenwalner et al. 1996b). Bravo (2002) observed that urethral contractions were evenly distributed throughout copulation but only measured an average of 40 in alpacas and 63 in llamas (range ) Semen collection Various methods have been used to collect semen from camelids to allow analysis of semen characteristics (Table 1.10). It is difficult to collect semen because of the mating posture, duration of copulation, intrauterine deposition of semen and the viscous nature of the ejaculate (Bravo and Johnson 1994; Bravo et al. 2000c). The use of intravaginal sheaths or condoms and intravaginal sponges interfere with normal ejaculation and result in shorter duration of mating, continuous movement of the male, frothing of semen and damage to sperm (San Martin et al. 1968; Sumar 1983; Johnson 1989; McEvoy et al. 1992, 1994; Bravo 1995). Creation of a urethral fistula required delicate surgery and long-term wound management (von Kubicek 1974). Retrieval of semen from the vagina after copulation and epididymal aspiration do not provide representative ejaculates (McEvoy et al. 1994; Bravo 1995). Disadvantages of electroejaculation include the requirement for heavy sedation or general anaesthetic, urine contamination of semen, inability to assess libido, variable sperm concentration and welfare issues (Calderon et al. 1968; Sumar 1983; Johnson 1989; McEvoy et al. 1994; Bourke et al. 1995; Bravo 1995; Giuliano et al. 2002). The use of an artificial vagina (AV) in combination with either a receptive female (Gauly and Leidinger 1996; Huanca and Gauly 2001), a dummy (Garnica et al. 1993; Bravo et al. 1997a,b; Davalos and Olazabal 2002) or half-dummy mount (Lichtenwalner et al. 1996b) in South American camelids has yielded the most representative copulation times and mating behaviour. There is no urine contamination of semen and assessment of sexual behaviour is possible (Lichtenwalner et al. 1996b). Therefore, the AV method of semen collection presumably provides semen samples most representative of natural mating. One author has noted that semen composition (ejaculate volume, sperm concentration, percentage of live sperm) varies significantly depending on whether a receptive female or a dummy is used with the AV (Davalos and Olazabal 2002). However, another has noted that use of an AV with a dummy or live-mount produced consistent results once males are trained (Adams and Ratto 2001). The dummy mount is constructed of a wooden frame in the shape of a recumbent female, covered by an alpaca hide, with a hole in the rear to accommodate the AV (Garnica et al. 1993; Lichtenwalner et al. 1996b; Bravo et al. 1997b). The AV may be constructed of PVC pipe or reinforced rubber tube (25-30 cm long, 5-7 cm diameter) and lined with a latex liner (Figure 1.3; Lichtenwalner et al. 1996b; Bravo et al. 1997b). Various workers have noted the importance of placing a stricture around the latex liner of the AV to simulate the presence of a cervix (Figure 1.4; Fowler 1989; Garnica et al. 1993; Bravo 1995; Bravo et al. 1997b). 17
33 Figure 1.3. Diagram of an artificial vagina used in Dromedary camels (Bravo et al. 2000a). Figure 1.4. Diagram of a modified artificial vagina used to collect semen from alpacas (Bravo et al. 1997b). Warm water is placed between the inner latex liner and the outer tube (Garnica et al. 1993; Lichtenwalner et al. 1996b; Bravo et al. 1997b). The temperature of the AV should be between C (Von Baer et al. 1999, Bravo et al. 2000a) and 45 C (Garnica et al. 1993, 1995). The AV is wrapped in a heating pad (Fowler 1989; Bravo 1995; Bravo et al. 1997b) or warm water is continuously pumped through it (Lichtenwalner et al. 1996b) to maintain temperature. Males are trained to mount a dummy by first exposing them to receptive females and mating pairs of alpacas as a stimulus (Garnica et al. 1993; Bravo et al. 1997a,b). Not all males will ejaculate into an AV at every attempt. Complete ejaculates were recovered in 108 of 132 attempts (82 %) in llamas (Aller 2001) and 203 of 272 attempts (75 %) in Dromedaries (Deen et al. 2003). 18
34 1.4. Characteristics of camelid semen Ejaculates from camelids are characterised by low volume, high viscosity, low numbers of sperm and the parameters are highly variable between individual males, and between ejaculates collected from the same male. This can potentially lead to problems in obtaining ejaculates of acceptable quality for sperm preservation and artificial insemination (Gauly and Leidinger 1996; Von Baer and Hellemann 1999; Raymundo et al. 2000). Semen parameters for camelids are summarised in Table Semen consistency ranges from translucent to creamy white, depending on sperm concentration and often a greyish colour is observed (Tibary and Memon 1999a). In alpacas, the majority of the ejaculate (88.5 %) consists of seminal plasma, and the remaining 11.5 % consists of sperm (Garnica et al. 1993). Semen volume, sperm concentration, percentage of live sperm, percentage of normal sperm, and duration of copulation have been observed to vary among alpaca males, however, oscillation and ph did not differ between males (Bravo et al. 1997b). The volume of each ejaculate in alpacas averages 1-2 ml and ranges from less than 1 ml up to approximately 7 ml (von Kubicek 1974; Fowler 1989; Garnica et al. 1995). Volumes are usually lower when semen is collected by electroejaculation than with use of an AV (Tibary and Memon 1999a). Semen is usually described as milky- or creamywhite in colour (Garnica et al. 1993; Bravo et al. 1997a,b) depending on sperm concentration (Tibary and Memon 1999a). Alpaca semen consists of 11.5 % sperm and 88.5 % seminal fluid by volume (Garnica et al. 1993). The specific gravity of llama semen is 1.1 ± 0.0 g/ml (Lichtenwalner et al. 1996b). Alpaca semen is very viscous (Garnica et al. 1993; Bravo et al. 1997a,b), making it difficult to handle (e.g. pipetting, smearing on a slide), difficult to determine parameters such as sperm concentration and sperm motility, and difficult to dilute (Garnica et al. 1993; Tibary and Memon 1999a). Viscosity may be indirectly calculated by measuring the distance of a known volume of semen that is drawn upwards from a glass slide by a pipette (Bravo et al. 2000b). The degree of viscosity varies between males (Tibary and Memon 1999a) and decreases with increasing number of ejaculates on any day (Bravo et al. 1997a). Semen liquefies 23 hours (range 8-30 hours) after collection (Bravo 1994). Sperm concentration varies from approximately 30,000 up to 150 million per ml in alpacas (Bravo 1995; Garnica et al. 1995; Gauly and Leidinger 1996). Such wide variations are attributed to differences in males, semen collection methods and ejaculate number (Tibary and Memon 1999a). Concentration is determined after liquefaction of semen, using a haemocytometer (Bravo et al. 1997a). The ph of camelid semen ranges between 7.2 and 8.6 (Bravo et al. 1997a; Von Baer and Hellemann 1998). The movement of camelid sperm in undiluted semen is best described as oscillatory as most sperm move back and forth and only 5 to 10 % of individual sperm actively progress forwards (Bravo et al. 1997a,b; Neely and Bravo 1997; Bravo et al. 2000b). Sperm increase their progressive motility as the ejaculate becomes more liquid (Tibary and Memon 1999a). Muscular contractility and ciliary motion of the female reproductive tract are probably more important mechanisms of sperm transport during the transfer of sperm from the site of deposition in the uterus to the site of fertilisation in the oviduct, but sperm motility is essential for passage through the uterotubal junction and penetration of the cumulus cells and zona pellucida of the ovum (Hafez 1993). Some workers have noted that ejaculates are not fractionated in alpacas and llamas so semen is uniform in quality from start to end of copulation (Sumar 1983). Others, however, have observed that sperm concentration, percentage of oscillatory sperm, percentage of live sperm and percentage of normal sperm increased with time during a single copulation in alpacas and llamas (Lichtenwalner et al. 1996b; Bravo et al. 2002). Bravo et al. (1997a) noted that sperm concentration, ejaculatory volume, percent live, percent normal and motility, decreased with increasing number of ejaculations/day but ph did not (Bravo et al. 1997a; Bravo 2002). Ejaculatory volume, sperm concentration, percentage of live sperm and duration of copulation were different on different days of semen collection (Bravo et al. 1997a). 19
35 Table Methods of collecting camelid semen and characteristics of camelid semen (adapted from Vaughan et al. 2003a). 20 Reference Species Ejaculate characteristics Sperm characteristics Collection Copulation Sperm conc. Live sperm Normal method length Colour Consistency Volume ph (x10 6 ml -1 Motility (%) ) (%) sperm (%) Mogrovejo 1952 Alpaca Condom milky viscous Low 41 von Kubicek 1974 Alpaca Urethral fistula Sucapuca 1991 Alpaca Condom Slow Garnica et al Alpaca AV dummy Milky - creamy viscous 1.7 ± Bravo 1994 Alpaca Bravo 1995 Alpaca EEJ Average AV dummy Milky viscous Oscillatory Bravo et al. 1997a Alpaca AV 17.9±0.7 viscous 1.6 ± ± ± ± ± ± 0.9 Bravo et al. 1997b Alpaca AV 21.8 Milky white viscous 1.9 ± ± ± ± ± 2.1 Fowler and Bravo 1998 Alpaca Milky - creamy Gelatinous 1.9 ± ± ± ± ± 2.1 Bravo et al. 2000b Alpaca AV dummy viscous Raymundo et al Alpaca AV dummy 28.4 ± 8.4 viscous 2.6 ± ± ± 22.6 Bravo 2002 Alpaca AV dummy Translucent - milky Semiviscous Flores et al Alpaca AV female 12.3 ± 7.2 Opalescent - white 1.8 ± High AV dummy & 16.8 ± ± ± ± ± Davalos and Olazabal female Alpaca 2002 AV dummy 15.9 ± ± ± ± ± no female Intravaginal viscous 17.7 sheath McEvoy et al. 1992a Llama EEJ viscous 120 Epididymal aspiration 257 Bravo 1995 Llama EEJ Milky - Highly Slight to ± 30.8 creamy viscous vigorous Gauly and Leidinger 1996 Llama AV and Light grey dummy milky High viscous Lichtenwalner et al. 1996b Llama Dummy fitted over female 32.4 ± 12.0 viscous 3.0 ± ± ± ± ± 18.5 Fowler and Bravo 1998 Llama White - gelatinous 1.6 ± ± ± ± 7.3
36 21 Reference Species Collection method Ejaculate characteristics Copulation length Colour Consistency Volume ph cream Sperm conc. (x10 6 ml -1 ) Sperm characteristics Live sperm Motility (%) (%) Normal sperm (%) Von Baer and Hellemann AV and Llama 1998 female 3.5 ± ± ± ± 22.3 Huanca and Gauly 2001 Llama AV and female 9.1 ± ± ± ± ± 22.3 Aller 2001 Llama AV and Spumous and dummy/femal 18.5 ± 5.2 viscous e 2.2 ± ± ± ± ± ± 12.4 Bravo 2002 Llama AV and Translucent - Semi - dummy creamy viscous Aller et al Llama AV 2.2 ± ± ± ± ± 12.3 Giuliano et al Llama AV 2.45 ± ± ± ± ± 16.7 Giuliano et al Vicuña EEJ n/a whitish viscous <10 % Tingari et al Dromedary EEJ Elwishy 1988 Dromedary Light grey - milky viscous Davalos et al 1989 Dromedary AV and dummy 15.9 ± ± ± ± ± Abdel-Rahim and El Nazier 1992 Dromedary AV Anouassi et al Dromedary AV gelatinous Light grey - AV Musa et al Dromedary milky viscous EEJ Hassan et al Dromedary AV with White 10 min female creamy viscous Abdel Rahim 1997 Dromedary Badamdorj et al Dromedary Al-Eknah et al Dromedary AV with female AV and female 6. 5 ± ± 1.9 Al-Qarawi et al Dromedary EEJ n/a Whitish - creamy 5.5 ± ± Light-grey Milky-white Viscous 3-6 ml ± ± ± ± AV and 4.5 ± 10.2 Deen et al Dromedary 4.3 ± ± 1.1 female seconds Zhao et al Bactrian AV 4.7 ± ± ± 2.3
37 Biochemical characteristics The biochemical analysis of seminal plasma is provided in Table Citric acid (mean 4.3 mg/dl, range mg/dl) and fructose (mean 5.0 mg/dl, range mg/dl) concentrations in alpaca semen were found to be independent of age and much lower compared with bulls, rams, boars, stallions and Dromedary camels possibly due to a lack of vesicular glands (Garnica et al. 1995). High fructose and citric acid concentrations have been noted in camels (Elwishy 1988). High glucose concentrations may serve as the primary energy source for sperm (Garnica et al. 1995). Data from Bactrian camels (Chen et al. 1985; Xu et al. 1985; Zhao et al. 1992; Pan et al. 2001, 2003) and alpacas (Adams et al. 2005; Ratto et al. 2005, 2006) indicate that camelid semen contains a factor that induces ovulation. Seminal plasma induced ovulation in females following placement into the vagina or uterus without mating (Chen et al. 1985; Xu et al. 1985; Sumar 1994) or by intramuscular injection (Chen et al. 1985; Pan et al. 1992; Adams and Ratto 2001). The biochemical composition of the factor is unknown but is different to GnRH (Zhao et al. 1992; Paolicchi et al. 1999). Mating behaviour may have some augmentative effect on the semen-induced ovulations (Fernandez- Baca et al. 1970; Chen et al. 1985). Contrary to these findings, Anouassi et al. (1992) found that although insemination of whole semen induced ovulation in some Dromedary camels, ovulation and pregnancy rates were significantly higher in those artificially inseminated and mated by a vasectomised male. Table Biochemical characteristics of camelid semen (derived from El-Manna et al. 1986; Zhao et al. 1992; Garnica et al. 1993; Bravo et al. 2000c; Agarwal et al. 2004). Components Alpaca # Alpaca* Bactrian Dromedary Llama Citric acid (mg/dl) 4.3 ± ± 1.16 Chloride (meq/l) 348 ± ± ± ± 10 Calcium (mg/dl) 18 ± 1 18 ± 3 13 ± 5 - Phosphate (inorganic) (mg/dl) 12 ± ± ± 1 10 ±? Glucose (mg/dl) 7 ± ± ± ± ± 0.3 Fructose (mg/dl) 6.0 ± ± ± 0.2 Lipids (mg/dl) 86 ± ± ± 5 Phospholipids (mg/dl) 29 ± 1 29 ± 1 - Total Nitrogen (mg/dl) 548 ± ± ± 23 Total Protein (g/dl) 3.0 ± ± ± ± 0.1 Albumin (g/dl) 2.0 ± ± Globulins (g/dl) 1.0 ± ± Cholesterol (mg/dl) 19.5 ± 0.72 Potassium (meq/l) 16.7 ± 0.72 Sodium (meq/l) ± ± 1.6 # Three year old, *Six year old Sperm morphology The length of camelid sperm is smaller than that of the bull, buffalo, ram, ass and stallion sperm, but is longer than boar sperm (Tibary and Anouassi 1997). Dromedary and Llama sperm are of similar length, at around 50 μm (Table 1.12), 15 % shorter than Bactrian sperm, which is related to the length of the principal piece of the tail. The head of camelid sperm is elliptical rather than ovoid (Tibary and Memon 1999a) and llama sperm have shorter heads than Bactrian and Dromedary sperm (Merlian et al. 1979). Ultrastructural 22
38 studies reveal that Dromedary sperm are similar to those of other mammalian sperm but have a special configuration of the head, components of the neck region, and altered disposition of the mitochondrial sheath and the fibrous sheath of the flagellum (Tingari 1991). Table Dimensions of camelid sperm (adapted from Merlian et al. 1979; Buendia et al. 2002) Parameter (μm) Alpaca Bactrian Dromedary Llama Total length 42.9 ± ± ± 2.2 Head length 6.1 ± ± ± ± 0.5 Head width 3.6 ± ± ± ± 0.1 Midpiece length 6.2 ± ± ± 1.6 Tail length 30.8 ± ± ± Abnormalities of sperm Various abnormalities have been reported in camelid sperm, with variations in frequency among species (Table 1.13). All sperm abnormalities found in other domestic livestock may be found in camelids. However, the frequency of abnormalities is higher in camelid semen than would be considered acceptable for other domestic species (Tibary and Memon 1999a). High levels of head abnormalities include tailless, small, large, pyriform, tapering and double heads (Bravo et al. 1997a,b; Buendia et al. 2002; Flores et al. 2002). Abnormal midpieces of sperm include thick midpieces, proximal droplets and uneven or absent mitochondrial sheets (Lichtenwalner et al. 1996b; Von Baer and Hellemann 1999). Tail abnormalities include bent, double, coiled, kinked tails (Lichtenwalner et al. 1996b; Bravo et al. 1997a,b; Giuliano et al. 2002). The proportion of morphological abnormalities is highly variable for llama sperm, and the most frequently observed abnormalities concern the head (20 ± 1.9%) and acrosome (13 ± 1.2%), and the incidence of cytoplasmic droplets averages 11.1 ± 1.2 % (Lichtenwalner et al. 1996a). Frequency of semen collection affects the proportion of abnormal sperm. Bravo et al. (1997b) reported that the percentage of abnormal sperm heads and tails increased with the frequency of semen collection on any day. However, sperm abnormalities did not change on different days of collection. Flores et al. (2002) observed a high number of abnormal sperm in ejaculates collected 45 and 90 days after the previous mating. There were twice as many tailless sperm heads in ejaculates collected after 90 days of sexual rest compared with 45 days. It is not known what effects sperm abnormalities have on fertility (Tibary and Memon 1999a). 23
39 Table Camelid sperm abnormalities. 24 Reference Species % Abnormal heads (range) % Tailless (range) % Cytoplasmic droplets (range) % Abnormal acrosomes (range) % Abnormal midpieces (range) % Abnormal tails (range) % Total (range) Bravo et al. 1997a Alpaca 6.7 ± ± ± 0.8 ( ) ( ) ( ) Bravo et al. 1997b Alpaca 3.8 ± 0.8 ( ) Davalos et al Alpaca 15 Flores et al Alpaca ± 15.2 ( ) Lichtenwalner et al ± ± ± ± ± ± ± 18.5 Llama 1996b ( ) ( ) ( ) ( ) ( ) ( ) ( ) Gauly et al Llama 5.8 Von Baer et al Llama 34.9 ± 19 Aller 2001 Llama 12.3 ± ± ± ± Giuliano et al Llama 7.2 ± ± ± ± 13.9 Giuliano et al Vicuña Musa et al Dromedary
40 Sperm transport in the female reproductive tract Few studies are available on the transport of sperm in the female reproductive tract following intrauterine deposition of semen in camelids (Bravo et al. 1996a). There appears to be no difference in sperm numbers travelling up the left and right uterine horns (Bravo et al. 1996a). Despite the fact that 98 % of pregnancies are carried in the left uterine horn, ovulations occur with equal frequency from both ovaries (Fernandez-Baca et al. 1973). A high percentage of sperm (77 %) is found at the uterotubal junctions or in the oviducts 6 hours after mating in the alpaca. Ninety-nine percent of sperm have entered the oviducts 12 hours after mating, and 33 % are at the isthmus in anticipation of fertilisation. Maximum sperm concentration at each isthmus occurs 18 hours after mating. The uterotubal junctions are the sites of sperm storage hours post-mating. The endometrium is oedematous, hyperaemic and inflamed between 6 and 30 hours post-copulation (Bravo et al. 1996a). Nothing is known about camelid sperm longevity. However, ovulation occurs approximately 30 hours after the mating stimulus (Huanca et al. 2001), so sperm survival is certainly longer than this. Presumably, sperm capacitation and acrosome activation occurs during this period, possibly when sperm reach the isthmus (Hafez 1993) Semen preservation Semen liquefaction The first difficulty in handling alpaca semen is removing it from the AV and liquefying the ejaculate. Semen liquefies naturally 23 hours (range 8-30 hours) after collection (Bravo 1994). Various methods of liquefaction of semen, such as the use of enzymes (Bravo et al. 1999; Callo et al. 1999; Bravo et al. 2000b; Bravo 2002) and mechanical stirring (A Niasari-Naslaji, personal communication), have been used with some success to simplify handling and allow dilution, extension and freezing of semen. Collagenase, fibrinolysin, hyaluronidase and trypsin have all been used to reduce viscosity of SAC semen (Bravo et al. 2000b). Collagenase apparently had the least effect on sperm viability but all enzymes damaged the anterior part of the acrosome in 6-8 % of sperm (Bravo et al. 2000b) Semen dilution and liquid storage and cryopreservation Various methods that have been used to liquefy, dilute, extend and freeze camelid semen (Table 1.15 and 1.16), and their respective success rates. Adequate sperm viability must be maintained during semen handling and processing to achieve pregnancies. Longevity of sperm viability is probably improved by storage at 4-5 C compared with higher temperatures due to reduced metabolic activity (Evans and Maxwell 1987). When semen was stored at 35 C, motility decreased almost linearly with storage time: after 30 minutes 42 % sperm were motile, after 1 hour 33 % sperm were motile, after 2 hours 16 % sperm were motile, after 3 hours 5.5 % sperm were motile and after 6 hours only 1.8 % sperm remained motile (Raymundo et al. 2000). In horses, rapid cooling of semen from 37 C to 20 C does not adversely affect sperm motility. However, cooling rates from 20 C to 4-8 C are more critical. The current recommendation is to cool semen at a rate of 0.5 to 1.0 C per minute to maximise motility (Evans and Maxwell 1987). Sperm retain better motility when stored at 4-6 C compared with 0-2 C using milk-based extenders (Batellier et al. 2001). Oxidative stress is one of the primary causes of sperm deterioration (Batellier et al. 2001). Semen extenders contain compounds that protect sperm outside the reproductive tract and during chilling and freezing (Evans and Maxwell 1987). Lipoproteins, such as those found in egg yolk and milk, protect sperm against cold shock by stabilising cellular membranes. Energy is provided to sperm by addition of metabolisable substrates such as glucose, fructose or lactose. Antibiotics may be 25
41 added to semen extenders to control bacterial growth. Osmotic pressure ( mosm/l) and ph of extenders need to be controlled to maximise sperm survival (Evans and Maxwell 1987). Dilution of semen with extender varies with sperm concentration of the ejaculate. More dilute semen may be diluted 1:1 (v:v) whereas more concentrated samples may be diluted 1:2 or 1:3 with extender. It may be advantageous to add 1-2 ml extender to the AV prior to semen collection to assist sperm preservation during prolonged copulation (Niasari-Naslaji pers. comm.). Sperm factors that are important for fertilisation include progressive motility, normal metabolism, and intact cellular membranes, acrosomal enzymes and nucleoproteins. These factors must be preserved during successful freezing of semen. Freezing may damage sperm by altering membrane structure, osmotic shock, dehydration, salt toxicity, intracellular ice formation, fluctuation in cellular volume/surface area or metabolic imbalance. Bravo (2002) claims that Tris buffer combined with egg yolk is the most promising diluent to maintain sperm viability during dilution, refrigeration, freezing and thawing of semen. Numerous workers have attempted freezing camelid semen using a two-step dilution with glycerol, and slow freezing in liquid nitrogen vapour (Bravo et al. 1996b; Valdivia et al. 1999; Bravo et al. 2000c). Thawing of straws is in a water bath at C for periods ranging from seconds to minutes (Table 1.16). 26
42 Table Liquid-storage of camelid semen 27 Reference Species Diluent and dilution rate Storage Initial Sperm motility (%) and (semen: diluent) temp ( C) motility (%) storage time (h) Citrate, glucose, egg yolk Chilled 48 h: 70 % Bravo 2002 Alpaca Skim milk (not 24 h: 68 % Glucose, citrate specified) 24 h: 60 % Vaughan et al. 2003a Alpaca Triladyl, 1: h: 50 %; 48 h: 45 % Ratto et al Tris, citrate, fructose + 20 % EY 72 h: 30 % Llama 5 Kenney s 72 h: 50 % (epididymal) Colorado 72 h: 20 % Huanca and Gauly 2001 Llama BSA, glucose, H2O 24 h: 21 %; 48 h: 14 %; 72 h: 5 57 % Diluted 1:1 8.7% Lactose (11 %) Egg yolk, diluted 1:1 Specific results not specified, Not specified Lactose best diluent Giuliano et al. 2006b Llama Tris-citrate-egg yolk 5 PBS-llama serum skim milk-glucose Skidmore, pers. comm. Dromedary Dilute semen 1:1 with Green buffer 4 Hassan et al Dromedary Tris, citric acid, glucose, mannose 10 h: 2 60 diluent; diluted 1:1 24 h: 0 Tris, citric acid, glucose, mannose, 4 10 h: 0 60 Orvus ES paste, diluted 1:1 24 h: 0 Lactose fructose, diluted 1:1 10 h: h: 0 Tibary and Anouassi 1997 Dromedary Lactose (11 %) 1:1 Laciphos 1:3 5 Morfeld et al Dromedary Lactose (11 %), egg yolk (20 %), 48 h: 30 % % glycerol (8 %) Lactose (11 %), egg yolk (20 %), 48 h: 30 % DMSO (8 %) Fructose (11 %), egg yolk (20 %), glycerol (8 %) No reduction in motility after 48 h storage
43 28 Reference Vyas et al Deen et al Wani et al Species Dromedary Dromedary Dromedary (epididymal) Diluent and dilution rate (semen: diluent) Fructose (11 %), egg yolk (20 %), DMSO (8 %) Glucose (11 %), egg yolk (20 %), glycerol (8 %) Glucose (11 %), egg yolk (20 %), DMSO (8 %) Lactose (11 %), egg yolk Tris (details not specified Tris Storage temp ( C) 5 4 Initial motility (%) % Sperm motility (%) and storage time (h) 48 h: 30 % 48 h: 30 % 48 h: 30 % Motilities not specified, Tris superior for storage at 24, 48 and 72 h. 24 h: % 48 h: % 96 h: % 24 h: 0 % Biociphos Tris-tes-egg yolk 8 d: 41.4 ± 8.9 % Tris-lactose-egg yolk 8 d: 41.6 ± 10.3% 4 Citrate-egg yolk 8 d: 0 Sucrose-egg yolk 8 d: 0 Tris-fructose-egg yolk 8 d: 0 Tris-tes-egg yolk 8 d: 36.6 ± 9.0 % Tris-lactose-egg yolk 8 d: 47.0 ± 4.9 % Citrate-egg yolk 8 d: 0 Sucrose-egg yolk 23 8 d: 0 Tris-fructose-egg yolk 8 d: 13.6 ± 9.1 % Niasari-Naslaji et al. 2006a Bactrian Tris (214.6 mm), citric acid (64.2 mm), glucose (66.6 mm), fructose (49.9 mm), Egg yolk 20 % Diluted 1:10 Lactose (277.5 mm) Egg yolk 20 % Diluted 1: % 24 h: 40 % 31.0 % 24 h: 0 %
44 29 Reference Zhao et al Species Bactrian Diluent and dilution rate (semen: diluent) Storage temp ( C) Initial motility (%) Sperm motility (%) and storage time (h) Sucrose (292.1 mm) 24 h: 0 % Egg yolk 20 % Diluted 1: % Green Buffer (IMV) 24 h: 35 % Egg yolk 20 % Diluted 1: % SYG-1 (see table 1.16) 8 h: 18.9 ± 4.1 SYG-2 8 h: 40.2 ± 5.3 Tris 8 h: 4.2 ± 3.2 SCDE 8 h: 2.7 ± ± 5.3 Stallion 8 h: 12.1 ± 3.2 Swine 8 h: 20.6 ± 5.4 Ram 8 h: 7.9 ± 3.8 Buck 8 h: 2.7 ± 1.3
45 Table Diluents and cryoprotectants used for the cryopreservation of camelid semen. 30 Reference Bravo et al. 1996b Bravo et al. 2000a Vaughan et al. 2003a Valdivia et al Species and sperm type Alpaca Alpaca (epididymal) Alpaca Alpaca Diluent and cryoprotectant Freezing methods Post-thaw motility (%) Citrate-glucose-EY-G Egg yolk 20 % Glycerol Two-step dilution No dilution rate given! Citrate Semen diluted 1:3 with Green buffer Egg yolk 20 % Glycerol (final [ ] 7 %) Two-step dilution Semen diluted 1:3 with Biladyl Egg yolk 20 % Glycerol (final [ ] 7 %) Two-step dilution? Glycerol 6 or 7 % Egg yolk 0.25 ml straw Store at 4 C for 1 h, Straws frozen 5 cm above LN 2 vapour and plunged in LN ml straws Store at 4 C for 1 h, Straws frozen 5 cm above LN 2 vapour and plunged in LN ml straws Diluted semen cooled to 4 C over 1.5 h Straws placed 10 cm above LN 2 vapour for 30 min 0.5 ml straws Diluted semen cooled to 4 C over 1.5 h Straws placed 10 cm above LN 2 vapour for 30 min Store at 4 C for 1 h - 10 C for 20 min - 20 C for 15 min - 50 C for 15 min Initial: 85 % Post-thaw: 46.7 % Results not given Biladyl: 21.3 ± 3.3 % Green buffer: 17.4 ± 1.7 % Biladyl: 21.3 ± 3.3 % Green buffer: 17.4 ± 1.7 % Perez 2005 Alpaca Tris-yolk extender Two-step dilution Egg yolk? % Glycerol (final [ ] 6 %) BSA-glucose-H 2 O Egg yolk -10 % Glycerol 2.5% (final [ ] not spec.) plunge in LN 2 Store at 4 C for 1 h - 20 C for 30 min - 90 C for 30 min plunge in LN ml straws over LN 2 Initial motility not specified Post-thaw = 22.5
46 31 Reference Species and sperm type Diluent and cryoprotectant Freezing methods Post-thaw motility (%) BSA-glucose-H 2 O Egg yolk -10 % Glycerol 5.0% (final [ ] not spec.) BSA-glucose-H 2 O Egg yolk -10 % Glycerol 7.5% (final [ ] not spec.) BSA-glucose-H 2 O Egg yolk -20 % Glycerol 2.5% (final [ ] not spec.) BSA-glucose-H 2 O Egg yolk -20 % Glycerol 5.0% (final [ ] not spec.) BSA-glucose-H 2 O Egg yolk -20 % Glycerol 7.5% (final [ ] not spec.) BSA-glucose-H 2 O Egg yolk -30 % Glycerol 2.5% (final [ ] not spec.) BSA-glucose-H 2 O Egg yolk -30 % Glycerol 5.0% (final [ ] not spec.) BSA-glucose-H 2 O Egg yolk -30 % Glycerol 7.5% (final [ ] not spec.) Post-thaw = 27.7 Post-thaw = 34.4 Post-thaw = 31.4 Post-thaw = 39.2 Post-thaw = 41.0 Post-thaw = 32.4 Post-thaw = 36.2 Post-thaw = 38.7
47 32 Reference Santiani et al Graham et al 1978 in Brown review McEvoy et al. 1992a Bravo et al. 1996b Species and sperm type Alpaca Llama Llama Llama Diluent and cryoprotectant Freezing methods Post-thaw motility (%) Tris g Citrate g Glucose g Egg yolk 20 % Glycerol 7 % final [ ] Per 10 ml Tris g Citrate g Glucose g Egg yolk 20 % Ethylene glycol 7 % final [ ] Skim milk 10 ml Fructose g Egg yolk 5 % Glycerol 7 % final [ ] Skim milk 10 ml Fructose g Egg yolk 5 % Ethylene glycol 7 % final [ ] Diluted 1:8 in Tris-egg yolk diluent Glycerol Semen diluted 1:2 with Triladyl (20 % EY) Citrate-glucose-EY-G Egg yolk 20 % Glycerol Two-step dilution No dilution rate given! Aller 1999 Llama Tris-citrate-fructose Tris diluent with EDTA? Llama ± Equex Von Baer and Hellemann ml straws Semen diluted 1:1 with extender and cooled to 5 C (-1 C/3 min). Semen further diluted 1:1 with extender (with cryoprotectant), equilibrated for 30 min. Straws exposed (height not specified) to LN 2 vapour for 15 min Pellets 0.5 ml straw Semen cooled to 5 C Straws suspended 7 cm above LN 2 vapour and plunged into LN ml straw Store at 4 C for 1 h, Straws frozen 5 cm above LN 2 vapour and plunged in LN ml straw Straws held over LN 2 vapour Initial motility = 72 % Post thaw: 4 % Post thaw: 1.0 % Post thaw: 15.3 % Post thaw: 20 % Successful Initial: 85 Post-thaw: 45
48 33 Reference Burgel et al Aller 2001 Species and sperm type Llama Llama Diluent and cryoprotectant Freezing methods Post-thaw motility (%) Biociphos-Plus Glycerol (final [ ] not spec.) Na-Citrate-fructose-EY Glycerol (final [ ] not spec.) Tris extender Glycerol (final [ ] not spec.) Citrate diluent Egg yolk (20 %) Glycerol (final [ ] 5 %) Two-step diluent PBS-Llama serum (40%) Egg yolk 5 % Glycerol (final [ ] 2.5 %) Two-step diluent 0.25 and 0.5 ml straw Diluted semen cooled to 5 degrees Semen frozen: 10 to 30 C at 40 C min to - 50 C at 60 C min to 130 C at 80 C min -1 Pre-freeze motility: 56.0 ±17.3 Biociphos 0.25 ml: 2.4 ± 4.0 % 0.5 ml: 3.7 ± 5.8 % Pre-freeze motility: 56.0 ±17.3 Citrate: 0.25 ml: 20.7 ± 9.3 % 0.5 ml: 29.1 ± 8.6 % plunged in LN 2 Pre-freeze motility: 56.0 ± ml straws Semen cooled to 5 C at 0.25 to 0.5 C min -1 Semen frozen: 5 to 50 C at 11 C min to 80 C at 6 C min to 120 C at 8 C min -1 plunge in to LN and 0.5 ml straws Tris: 0.25 ml: 22.4 ± 12.1 % 0.5 ml: 27.1 ± 14.5 % Burgel et al Llama Semen diluted 1:1 or 1:2 Biociphos-Plus Glycerol (final [ ] not spec.) Diluted semen cooled to 5 degrees Semen frozen: 10 to 30 C at 40 C min to - 50 C at 60 C min to 130 C at 80 C min -1 Post-thaw motility <10 % for all groups (specific % not given) Aller et al Llama Citrate-glucose-EY Egg yolk 15 % Glycerol (final [ ] 6? %) DMSO (final [ ] 8? %) Two-step dilution plunged in LN ml straw Semen cooled to 5 C at 0.25 to 0.5 C min -1 Semen frozen: 5 to 50 C at 11 C min -1 Fresh mot = 54.3± 10.5 Frozen-thawed = 20.4±7.5
49 Reference Graham et al 1978 Hassan paper Anouassi et al Musa et al Species and sperm type Diluent and cryoprotectant Freezing methods Post-thaw motility (%) - 50 to 80 C at 6 C min to 120 C at 8 C min -1 plunge in to LN 2 Dromedary Raffinose glycerol yolk Pellets on dry ice Dromedary Dromedary Lactose (11 %) Egg yolk- 20 % Lactose (11 %) Egg yolk- 20 % Glycerol (final [ ] 2 %) Equex (final [ ] 0.5 %) 4.0 ml straws Diluted semen cooled to 15 C over 2.5 h Straws held over LN 2 vapour for 20 min and plunged Successful pre-freeze = 60 % Post-thaw = 50 % 34 Hassan et al Dromedary Tris, citric acid, glucose, mannose diluent [ ] not spec Tris, citric acid, glucose, mannose, Orvus ES paste Lactose fructose Semen diluted 1:1 with diluent, equilibrated (6 h) and loaded in to 0.5 ml straws and frozen using standard LN static vapour technique Straws size not specified Semen cooled to 5 C over 1h, held at 5 C for 2 h Pre-freeze = 60 % Post-thaw = 2 % Pre-freeze = 60 % Post-thaw = 0 % Pre-freeze = 60 % Post-thaw = 35 % (Tibary and Anouassi 1997) Niasari-Naslaji pers. Comm. Dromedary camel Dromedary Green buffer Egg yolk (20 %) Glycerol (final [ ] 3 %) Two-step dilution Green buffer Egg yolk (20 %) Straws held 4 cm above LN 2 vapour for 10 min and plunged in LN ml straws Diluted semen cooled to 4 C over 2h, diluted with glycerolated portion, held at 4 C for 30 min. Straws held above LN 2 vapour for 20 min then plunged into LN ml straws Diluted semen cooled to 4 C over 2h, diluted with glycerolated portion, held at 4 C for 30 min.
50 Reference Species and sperm type Diluent and cryoprotectant Freezing methods Post-thaw motility (%) 35 Skidmore pers. comm. (Deen and Sahani 2000) (Deen et al. 2003) Morfeld et al Dromedary camel Dromedary Dromedary Lactose (11 %) Egg yolk (20 %) Glycerol (final [ ] 3 %) Tris (3.028 g) Citric acid (1.675 g) Fructose (1.25 g) per 100 ml Egg yolk -20 % Glycerol (final [ ] %) Fructose (11 %) Egg yolk (20 %), Glycerol (8 %) final [ ] 2 % Straws held above LN 2 vapour for 20 min then plunged into LN ml straws Diluted semen cooled to 15 C over 2.5h. Glycerolated portion added and semen cooled to 5 C Straws held above LN 2 vapour for 20 min then plunged into LN 2. Semen diluted 1:1 and cooled to 4 C over 2 h. Semen further diluted 1:1 1:5 and loaded into 2 ml cryoampoules 4 to 15 C at 1 C min to 60 C at 4 C min to 100 C at 20 C min -1 plunge in to LN 2 Semen diluted 1:4, cooled to 4 C over 1 h. Diluted semen loaded into 0.25 and 0.5 ml straws, equilibrated for 1 h. Straws frozen over LN 2 vapour (20 min, height not specified) and plunged into LN 2. 11/26 samples showed post-thaw motility >20 % Pre-freeze motility: % Post-thaw: 0.25 ml straw. Thawed at 20 C = 21.7 % 32 C = 37.5 % 50 C = 45 % 0.5 ml straw, thawed at 20 C = 23.3 % 32 C = 43 % 50 C = 42.3 %
51 Reference Species and sperm type Diluent and cryoprotectant Freezing methods Post-thaw motility (%) Fructose (11 %) Egg yolk (20 %) Glycerol (12 %) final [ ] 3 % Pre-freeze motility: % Post-thaw: 0.25 ml straw. Thawed at 20 C = 5.0 % 32 C = 16.7 % 50 C = 0 % 0.5 ml straw, thawed at 20 C = 18.3 % 32 C = 31.7 % 50 C = 22.5 % 36 Chen et al Bactrian Two-step method Semen diluted 1:1 with SYG-2 [Sucrose (12 % sol w:v)-egg yolk (10 %)-Glycerol (3.5 %)] Further diluted 1:2-3 with SYG-3 [12 % sucrose solution, 20 % EY and 7 % Glycerol]. Diluted semen (1.5 ml) placed in ampoule and equilibrated at 37, 20 and 10 C for 10 min. Ampoules frozen above LN surface: 3 cm for 3 min 2 cm for 2 min 1 cm for 1 min plunge in to LN ml straws Semen cooled to 5 C at 0.25 to 0.5 C min -1 Initial motility = 0.8 Post-thaw motility = 0.43 Zhao et al Bactrian Sucrose (12 %) Egg yolk 20 % Glycerol (final [ ] 3 %?) Semen frozen: 5 to 50 C at 11 C min to 80 C at 6 C min to 120 C at 8 C min -1 plunge in to LN 2
52 Reference Species and sperm type Diluent and cryoprotectant Freezing methods Post-thaw motility (%) Semen diluted (ratio not spec) and held at 37 C for 30 min. 37 Zhao et al Bactrian SYG-1 12 % sucrose solution Egg-yolk (10 %) Glycerol (final [ ] 3.5%) SYG-2 12 % sucrose solution Egg-yolk (20 %) Glycerol (final [ ] 7.0%) Tris 3.28 g Citric acid monohydrate-1.25 g Fructose 1.7 g Egg yolk (20 %) Glycerol (final [ ] 7%) Sodium citrate (dihydrate) 2.32 g Egg yolk (20 %) Glycerol (final [ ] 7%) Stallion extender Sucrose (11 % solution) Egg yolk 13 % Glycerol (final [ ] 5%) Swine extender Glucose (80% solution) Egg yolk (20 %) Glycerol (3 %) Diluted semen slowly cooled to 4 C over 4 h and loaded into 2.0 ml glass ampoules Ampoules frozen over liquid nitrogen vapour: 2 cm above for 3 min 2 cm above for 2 min 1 cm above for 1 min plunge in LN Semen diluted (ratio not spec) and held at 37 C for 30 min. Diluted semen slowly cooled to 4 C over 4 h, loaded into 2.0 ml glass ampoules and held at 4 C for 10 min Ampoules frozen over liquid nitrogen vapour: 2 cm above for 3 min 2 cm above for 2 min 1 cm above for 1 min plunge in LN Post-thaw = 42.6 ± 4.1 Post-thaw = 61.5 ± 4.9 Post-thaw = 22.4 ± 3.9 Post-thaw = 24.8 ± 2.6 Post-thaw = 41.8 ± 2.7 Post-thaw = 45.4 ± 2.7
53 Reference Species and sperm type Diluent and cryoprotectant Freezing methods Post-thaw motility (%) Ram extender Tris g Citric acid monohydrate g Egg yolk (18 %) Glycerol (6 %) [Evans and Maxwell] Buck extender Tris g Citric acid monohydrate g Egg yolk (3 %) Glycerol (6 %) [Evans and Maxwell] Post-thaw = 25.8 ± 2.6 Post-thaw 23.2 ±
54 1.6. Artificial insemination Induction of ovulation Methods of inducing ovulation in alpacas have been discussed thoroughly elsewhere (Vaughan 2001), and a summary of the commonly used methods is provided in Table Intramuscular injections of 8 μg buserelin (Bourke et al. 1992, 1995), iu hcg (Adam et al. 1989) and 2-5 mg LH (Taylor et al. 2000; Huanca et al. 2001) have been used to consistently induce ovulation approximately 30 hours after injection. Table Synchronisation and induction of ovulation for AI and ET in camelids Reference Species Synchronisation and induction of ovulation Success Adams et al Alpaca Seminal plasma injection im (93 %) 93 Bravo et al Alpaca 750 IU hcg (Chorulon) AI 24 h post-induction Bourke et al Llama 750 IU hcg im at mating Mating intact male - 80 % 80% ovulated 750 IU hcg 91 % 91 8 ug GnRH analogue (Buserelin) - 90 % 90 Adam et al Llama Mating + hcg 90 % 90 Mating + GnRH 81 % 81 Seminal plasma injection im (100 %) 100 GnRH (83 %) % donors had no follicles 16.6 % (n=2 donors had no embryos) Aller et al Llama Day 0 CIDR + 2 mg ODB Mated on Day µg Buserelin recovery rate = 84.6 % (22/26) Bourke et al Llama (guanaco and llama embryos) Natural cycle 8 µg Buserelin Flushed 6-8 d after ovulation Location of semen deposition and time of insemination relative to ovulation Synchronous ovulation and insemination is required to achieve high conception rates. In some species, high conception rates occur when sperm are in the oviduct just before ovulation (Hafez 1993). If sperm are placed into the tract too early, they will lose viability prior to ovulation, and if sperm are placed into the tract after ovulation, the ovum may lose viability before fertilisation can occur (Hafez 1993). In an early study, the highest proportion of fertilised ova resulted when intrauterine AI in alpacas was 35 to 45 hours after induction of ovulation using either a vasectomised male or intramuscular hcg (Calderon et al. 1968). More recently, AI hours after induction of ovulation has resulted in acceptable conception rates (Table 1.16; Pacheco 1996; Quispe 1996a; Apaza et al. 1999). 39
55 The advent of transrectal ultrasound for monitoring ovarian activity allows the observation of ovulation after synchronisation treatments (Vaughan 2001). To date, however, there have been no studies using this technology to determine the appropriate timing of semen deposition relative to actual ovulation in camelid AI Numbers of sperm required per AI dose The sperm dose required for successful fertilisation and pregnancy is not known in camelids (Brown 2000). Ewes require 100 million fresh sperm using cervical AI for acceptable conception rates, but only 20 million frozen/thawed sperm when intrauterine AI is used (Evans and Maxwell 1987). Pregnancies have been obtained in alpacas and llamas after inseminating 8-26 million sperm transcervically (Table 1.18; Quispe 1996a; Aller 1999). Intracornual semen deposition of semen during copulation in camelids may be an adaptation to overcome the relatively low sperm concentrations (Brown 2000). Very little is known about the optimum number of sperm for fertility after AI of alpacas, and when it should be deposited. This deficiency in knowledge becomes even more important if the sperm have been compromised by liquid or frozen preservation Method of delivering sperm into uterus Bravo et al. (1997b) compared the techniques of transcervical and laparoscopic AI in alpacas using fresh, undiluted semen and achieved similar conception rates. Transcervical AI would appear to be a simpler, less-invasive procedure, but has limitations of rectal/pelvic capacity to allow transrectal manual stabilisation of the cervix when passing the AI pipette through the cervix, and the difficulty of locating the external os of the cervix during this procedure. Bravo (2002) describes the need for patience and dexterity to pass a pipette through the cervix into the uterus. Laparoscopic AI requires restraint of the female in a crate, sedation with or without a general anaesthetic, and expensive laparoscopic equipment. An investigation of alternative AI methods particularly when using cryopreserved sperm, may lead to improved fertility results. The laparoscopic AI method developed in sheep to overcome the cervical barrier to fertility when using frozen-thawed semen (Evans and Maxwell 1987). Due to the limited number of sperm available for preservation in alpacas and the questionable fertility after AI, it may be that an alternative or semi-surgical insemination technique such as deep uterine or oviducal deposition may be required in order to obtain acceptable fertility. 40
56 Table Artificial insemination with fresh and preserved semen in camelids (adapted from Vaughan et al. 2003a). 41 Reference Species Sperm type Dose (x10 6 ) Fernandez-Baca and Calderon 1966 Alpaca Vicuña Fresh whole EEJ semen Calderon et al Alpaca Fresh, whole Not spec. Fernandez-Baca and Novoa 1968 cited by Bravo 2002 Leyva et al 1979 cited by Bravo 2002 Alpaca Fresh, whole Alpacas and Llamas Fresh, whole EEJ semen Time (h) after induction of ovulation Immediately after mating with a vasectomised male after vasectomised male and hcg Vasectomised male used to induce ovulation hcg ± vasectomised male and used to induce ovulation Location Pregnancy rate (%) Transcervical, semen deposited at bifurcation of two uterine horns Transcervical or intravaginal with speculum Transcervical, semen deposited it uterine horns 1/42 live births 75% for AI h post ovulation 2.4 % (42 females inseminated) 30.8 % (94 females inseminated) hcg induced females = 48 % vasectomised male induced females = 11 % 67 % (40 females inseminated) 57 % Bravo et al cited by Bravo 2002 Alpaca Fresh, whole hcg Laparoscopy or transcervical Bravo and Quispe 1995 Diluted Alpaca hcg cited by Bravo 2002 (citrate) (80 females inseminated) Bravo et al. 1996b Alpaca and Frozen 26 % hcg Llamas (citrate) (19 females inseminated) de la Vega 1996 Alpaca Diluted 30 h after mating with (PBS) vasectomised male Transcervical 40 % (54/133) Pacheco 1996 Alpaca Fresh, whole GnRH 24 h before AI Transcervical 60 % (48/80) pregnant Quispe 1996b Alpacas Fresh 8 (motile) 36 h Transcervical 57 % Diluted 69.6 % (Tris buffer) (25 females inseminated) Bravo and Castilla 1997 Liquid stored 66.7 % Alpacas? GnRH? cited by Bravo 2002 (Tris buffer) (25 females inseminated) Frozen 68 % (Tris buffer) (25 females inseminated) Bravo et al. 1997b Alpaca Fresh whole hcg given at AI Transcervical or Laparoscopic Transcervical: 40 (8/20) Lap AI: 50 % (10/20)
57 42 Reference Species Sperm type Dose (x10 6 ) Apaza et al Alpaca Frozen Not specified Time (h) after induction of ovulation h post GnRH Transcervical Location Pregnancy rate (%) Uterine horns (both): 37 % (11/30) Uterine body: 27 (4/15) 53 % 67 % Bravo et al Alpaca Fresh hcg Transcervical 62 % Bravo et al 1999 cited Frozen hcg 25 % Alpaca? by Bravo 2002 (Tris buffer) GnRH (190 females inseminated) Aller 1999 Llama Fresh h post-gnrh Transcervical 22 % (5/23) Bravo et al. 2000a Alpaca 37.5 % based on refusal to Frozen 10?? (epididymal) mate 21 post-ai Bravo et al. 2000c Alpaca Llama Frozen Transcervical? 5/19 pregnant (26 %) Aller 2001 Llama Fresh 5/23 (22 %) 5 24 h after 8 ug Buserelin Frozen 2/5 (40 %) Aller et al Llama Fresh Transcervical into horn ipsilateral 21.7 % (n=23) h after 8 ug Buserelin Frozen to follicle > 7 mm 7.9 % (3/39) Perez 2005 Alpaca Diluted fresh n.s. Vasectomised male Transcervical (4/11) Perez 2005 Alpaca Frozen n.s. Vasectomised male Transcervical 25.0 (5/20) 23.4 ± 2.59 h No pregnancies Giuliano et al. 2006a Llama 8 µg Buserelin (10 females inseminated) Transcervical, semen deposited at 28.0 ± 1.3 h post Liquid stored 51.5 ± 49.9 the uterotubal junction ipsilateral Buserelin (8 µg), (lactose) 9 to the ovary with the dominant 23 % immediately post follicle (13 females inseminated) ovulation (detected by ultrasonography) Anouassi et al Dromedary Fresh diluted 100 Fresh- whole Freshdiluted 100 Females mated to vasectomised male Trans-cervical or uterine deposition 2/ 10 ovulated ½ preg 6 inseminated 3 ovulated 1 pregnant 6/10 ovulated 5/6 pregnant
58 43 Reference Species Sperm type Dose (x10 6 Time (h) after ) induction of ovulation Location Pregnancy rate (%) Musa et al Dromedary Frozen 24 h Transcervical Not specified Fresh diluted Green buffer: 47 % Green Buffer 300 Laciphos: 53 % Laciphos (motile) Kenney s: 0 % Kenney s Skidmore and Billah 100 Dromedary Green buffer 2001 (motile) 25 h post GnRH Transcervical 10 Green buffer 200 (motile) 22 Green buffer Niasari-Naslaji et al Deen et al Niasari-Naslaji pers comm. in Vaughan et al 2003 Dromedary Dromedary Dromedary Fresh Fresh Liquid-stored Frozen Fresh (motile) 160 (motile) 24 h after GnRH Vaginal - - Transcervical 300 (motile) Chen et al Bactrian Frozen? Bravo et al. 2000c Bactrian Frozen h after GnRH (24 ug) or hcg (3000 iu) Inseminated when follicles reached 1.2 cm Transcervical Transcervical Transcervical AI twice at 24 h interval 5/12 (42 %) pregnant at Day (4/10) 0 (0/10) 8 (1/13) Inseminated twice in 24 h Overall 29/31 = 93.5 % 285/300 (95 %)
59 1.7. Concluding remarks and required research The preceding review has described the reproductive physiology of male camelids, with particular reference to alpacas where possible. The review concluded with a section on the current knowledge of semen preservation and artificial insemination in camelids. They are major gaps in knowledge about many aspects of artificial breeding in camelids. Of particular importance to alpaca AI, the following have yet to be determined: 1. Methods to consistently collect high quality semen 2. Methods to determine functional integrity of alpaca sperm 3. Methods to release the sperm from the highly viscous seminal plasma in ejaculated semen, so that they may be preserved after storage 4. Fundamental media requirements for liquid and frozen preservation of sperm 5. The optimum site and timing (relative to ovulation) of semen deposition 6. The optimum dose of sperm (fresh and preserved) for AI 7. If alpaca sperm can be used for other artificial reproductive technologies, such as sperm sexing. The primary aims of this project were to further develop the technology for semen collection, preservation and artificial insemination in alpacas, which have been identified as objectives in the RIRDC Rare Natural Animal Fibre Research and Development Plan The project was broken down into a number of steps to achieve these aims: 1. Consistent and reliable collection of semen by optimising the mannequin design and investigating the effects of different AV liners, cervix, semen collection vessel, presence of females and addition of diluent to the collecting container on semen quality parameters 2. Development of new methods to assess function and integrity of alpaca sperm including objective methods to assess sperm motility, acrosome integrity, sperm viability and in vitro fertility 3. Liquefaction of alpaca semen using mechanical and enzymatic methods 4. Optimising liquid storage of alpaca semen by investigating the effects of diluent, temperature, dilution rate and removal of seminal plasma 5. Optimising cryopreservation of alpaca sperm using an epididymal model and investigating the effects of cryodiluent, packaging, freezing rate, supplement addition and liquid storage prior to freezing 6. Artificial insemination of females with frozen-thawed alpaca sperm 7. Preliminary development of sperm-sexing technology in alpacas Each of the milestones is represented by a separate chapter (Chapters 3-9) in this report. 44
60 2. Methodology 2.1. Use of animals in research All research complied with the Animal Research Act 1985, Animal Research Regulation 2005, and the Australian Code of Practice for Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council 1990), and all procedures described herein were approved by The University of Sydney s Animal Ethics Committee (approval number N00/7-2005/2/4138) prior to the commencement of the project Animals Source of animals Males alpacas (n=16; aged: 7.03 ± 0.6 yr, range: ) were loaned, or donated, to The University of Sydney for this project by alpaca studs located in NSW and Victoria. Two female alpacas were also loaned to The University of Sydney by alpaca studs in NSW for use in semen collection experiments Maintenance of animals Male alpacas were maintained in two groups of eight in paddocks at The University of Sydney s Animal Reproduction Unit, Cobbitty, NSW Australia (Figure 2.1). Alpacas were exposed to natural day length and ambient temperatures. Males were maintained on native pastures and supplemented with Lucerne hay (Medicago sativa), commercially formulated alpaca pellets and had ad libitum access to water. Figure 2.1. Male alpacas in their paddock Female alpacas were also maintained at The University of Sydney s Animal Reproduction Unit, Cobbitty, NSW, Australia. Female alpacas were exposed to natural day length and ambient temperatures and had ad libitum access to water. Female alpacas were maintained on native pastures and shared their paddock with pregnant and lambing ewes, and lambs. Female alpacas were supplemented with Lucerne hay in their paddock, and a mixture of alpaca pellets and commercially formulated horse stud mix during semen collection. Male and females alpacas were weighed fortnightly to monitor body weight, were shorn annually and had routine husbandry procedures performed as required. 45
61 2.3. Location of research and facilities Facilities used to conduct research were located at the Camperdown and Camden (Cobbitty) campuses of the Faculty of Veterinary Science, The University of Sydney. Facilities at The University of Sydney s Animal Reproduction Unit, Cobbitty, NSW, Australia (latitude 30'50''S) were used to maintain animals, collect and initially assess semen. Further semen assessments and preservation were conducted in the andrology laboratories in the Gunn Building, Faculty of Veterinary Science, Camperdown campus Semen collection facilities The semen collection shed was designed to permit access to each pen, and movement of alpacas and people through the shed without disturbing other mating males. The shed consisted of four semen collection pens, two on either side of the shed, a central walkway, a holding pen and small storage area. Pens were designed to be large enough for males moving around freely (i.e. turning around in a circle), and permit the entry of people without disturbing the male. A small storage area was located at the back of the shed for the mannequins and semen collection equipment. The floor in the collecting shed was covered with carpet mats to prevent contamination of semen with dirt, dust and other material. The side panels of the collecting shed were covered with Hessian fabric to prevent males from distracting each other. The scales for weighing the alpacas were fitted in the middle of the walkway to permit weighing of alpacas without stress. Animals were accustomed to walking over the scales by permitting them free access to the scales and allowing them to adjust to their presence Laboratory facilities Cryopreservation of epididymal and ejaculated sperm, sperm-sexing and objective sperm assessments were conducted at the Andrology laboratories in the Gunn Building, Faculty of Veterinary Science, Camperdown campus. A list of laboratory equipment and chemicals used throughout this study are presented in Appendix I and II respectively Maintenance of the laboratory Many factors determine the success of semen collection; the sperm s immediate milieu is of utmost importance, as are the surrounding conditions. Not all factors within a laboratory can be controlled, but they can be monitored, and fluctuations reduced to a minimum. Precautions were taken to maintain a clean and, where possible, sterile environment for all procedures. All working areas were regularly cleaned and maintained dust-free. When necessary working areas were sterilised with 70 % (v:v) ethanol. Temperature within the laboratories was maintained between 25 to 35 C and, in laboratories with air conditioning, the temperature was maintained at 26 to 28 C. To minimise fluctuations in the temperature and atmosphere encountered by sperm, removal from the storage environment for observation was kept to a minimum and all procedures were performed as quickly as possible. To minimise fluctuations in sperm temperatures, all microscopes were equipped with warm stages (Lec Instruments Co. Ltd. Scoresby, Vic) set at 39 ± 0.25 C. Two controlled atmosphere incubators were used for the culture of gametes in this study [Jouan (model number IG150) Jouan S.A. Saint-Herblaine, France, and Mini-incubator (model number MINC-1000) Cook IVF, Brisbane, Australia] and were checked daily for temperature fluctuations, gas and water levels. Gas for the Jouan and Mini-incubator was purchased from BOC Gas (Industrial 46
62 compressed CO 2, gas code: O81, BOC Gas Australia Pty. Ltd. North Ryde, NSW, Australia) and Linde Gas Pty. Ltd. (Yenora, NSW, Australia) respectively. As the Mini-incubator was primarily used for gamete culture, each batch of gas was analysed at a National Association of Testing Authorities accredited laboratory, and supplied as 6.0 ± 1.0 % CO 2, 5.0 ± 1.0 % O 2, 89 % N 2. Incubators were regularly cleaned with 0.1 % (v:v) 7X (Bacto Laboratories Pty. Ltd, Liverpool, NSW, Australia) and rinsed with water purified by a reverse osmosis and deionisation system (MQ-water; Millipore, North Ryde, NSW), then sprayed with 70 % (v:v) ethanol and allowed to dry. Incubators were dismantled (shelves and interior removed) bi-annually and thoroughly cleaned with a 0.1 % (v:v) solution of Roccal (Benzalkonium Chloride, B-6295; Sigma, St. Louis MO, USA), rinsed with MQwater, sprayed with 70 % (v:v) ethanol and allowed to dry. Incubators were reset for temperature and gas and allowed to equilibrate overnight before use. During the culture of gametes, medium was overlaid with oil (Sydney IVF culture oil K-SICO-200, Cook IVF, Brisbane, Australia), which also minimised fluctuations in the ph of the medium Cleaning of glassware and plastics Re-usable plastic ware was kept separate to general laboratory equipment and prepared for use by soaking overnight in 0.1 % (v:v) 7X detergent, followed by three rinses in reverse-osmosis (RO) water. Plastics were dried at 37 C, covered with aluminium foil, and sterilised by autoclaving at 120 C for 20 min. Glassware used to prepare media and diluents were prepared by overnight soaking in 0.01 % (v:v) 7X, then rinsed three times in RO water and three times in Milli-Q water, dried and heat sterilised by dry heat (200 C for 4-6 h). Tissue-grade dishes were used during gamete culture (Nalge Nunc, Illinois, USA) and were purchased pre-sterilised, by gamma irradiation, from the manufacturers. Pipette tips (Interpath Services, Caringbah, NSW) were placed in pipette boxes and autoclaved as described above Preparation and storage of media and diluents All media and diluents were prepared using analytical or tissue grade, and where possible embryo tested reagents, supplied from Sigma (St. Louis, MO, USA) unless stated otherwise in the relevant section. The water used to prepare media and diluents was produced in-house using an ultra filtration high-purity ion-exchange system comprising a carbon cartridge, two mixed bed ion exchange resin cartridges, an organics extraction cartridge and a UF-type pyrogen extraction cartridge before sterilisation using a 0.22 μm filter (Milli-Q water, Milli-Q Reagent Water System, Millipore). During times when the Milli-Q system did not reach the desired specifications (>18 mω) tissue-grade water was purchased (Trace Biosciences, Castle Hill, NSW) and stored at 4 C for no more than one month (as storage times longer than this have been shown to reduce gamete survival; Nagao et al. 1995). Media for washing and culture of gametes were supplemented with several antibiotics (described in the relevant sections), which were prepared using Milli-Q water, as a 100x strength stock solution and frozen at -20 C until use. Media were prepared directly from chemicals or from stock solutions and sterilised by filtering through a 0.22 μm filter (Millipore Australia Pty. Ltd, North Ryde NSW) prior to storage in gamma radiation sterilised disposable plastic flasks (Falcon flasks: 50 ml ; 250 ml , Bacto Laboratories). Media were stored at 4 C until use and, unless specified, for no more than one week with the addition of amino acids, hormones, and sera performed on the day of medium use, to reduce deterioration of compounds in the medium during refrigeration. Semen diluents were prepared according to the manufacturers instructions. After the addition of egg yolk (20 % v:v, unless stated otherwise) diluents were ultracentrifuged (10,000 g; 30 min) to clarify the egg yolk. The supernatant was then divided in to 10 ml aliquots and stored at - 20 C until use. On the day of use, diluents were thawed and warmed to 35 or 37 C by placing in a water bath. Diluents were mixed thoroughly by inverting prior to use. 47
63 The osmolarity of each medium was determined using a freezing point depression osmometer (Advanced Instruments, Norwood, MA, USA) and adjustments to the osmolarity of the medium were performed using Milli-Q water or NaCl. If the volume of Milli-Q water required for the adjustment of the osmolarity was greater than 5.0 % of the total volume of the medium, the batch was discarded and a new one prepared. The ph of each medium was determined after formulation and in the unequilibrated state, where appropriate, using disposable ph strips (Sigma) which had previously been compared with the bench top ph meter (Hanna Instruments; Australian Scientific, Kotara, NSW). Adjustments to ph were made using sterile solutions of 0.1 M sodium hydroxide and 0.1 M hydrochloric acid (prepared in Milli-Q water). Bovine serum albumin (BSA; Fraction V) supplied by Sigma-Aldrich (Sydney, Australia) was present as the protein source in some of the semen diluents. BSA was imported by Sigma-Aldrich as an unregistered veterinary medicine and its use was therefore restricted to in vitro only by AQIS and the Australian Pharmaceutical and Veterinary Medicines Authority (APVMA). Research conducted by recognised research institutes are permitted under the APVMA small scale trial permit (PER 7250) to use unregistered veterinary medicines in vivo, under strict conditions. Human serum albumin (HSA) was used as the protein source during the in vitro culture of gametes, and was present media obtained from Sydney IVF (Cook IVF). HSA was of pharmaceutical grade approved for human intravenous use and rigorously screened for HIV, Hepatitis B, Hepatitis C and other pathogens (Scandinavian IVF Science, Göteborg, Sweden). HSA was supplied as a 20 % (w:v) solution (200 mg ml -1 in saline) and incorporated into media formulations to provide a final concentration of 10 mg ml -1. During gamete culture, the medium was overlaid with mineral oil (Sydney IVF culture oil, Cook IVF). The overlay of culture medium with mineral oil is commonplace during in vitro embryo production, although it has been suggested that the oil could potentially partition its inherent toxicants into the culture medium. The risk of the oil contaminants reaching the culture medium is increased as the surface area of medium in contact with the oil increases relative to the volume. To avoid this, and equilibrate the oil, it was filtered and extensively washed by underlying the oil with cleavage medium, and storing at 39 C and 6 % CO 2 environment for at least two days prior to use Training of males for semen collection Initial on-farm Males were initially exposed to the mannequins on-farm and those which demonstrated interest in the mannequin were selected for training. The training involved the development and reinforcement of the male conditioned reflex to mate with the mannequin in the collection shed in the presence of people Semen collection For semen collection, males were brought from their paddock to the shed via a laneway. The males then entered the holding area at the rear of the shed where they had access to kikuyu based pasture, and a mixture of alpaca pellets and stud mix (approx. 500 g/hd/d). From the holding area the males entered the holding pen at the rear of the collection shed where a gate prevented them from entering the central walkway. When the gate was opened, males entered the central walkway and selected a pen. Once the male entered the pen the gate was secured behind them and the next male allowed out of the holding yard. After the male had finished mating, the pen gate was opened and the male directed down the central walkway, out of the collection shed and back to the laneway (Figure 2.3). This process was repeated until semen was collected from all males. Semen was collected from males twice weekly during experiments using a modified sheep artificial vagina (AV). 48
64 Figure 2.3. Male alpacas in the laneway after semen collection Preparation and assembly of AV The AV was lined with a purpose-made silicone tube approximately 3 cm wide and 5 mm thick. The ends of the silicone liner were then folded back over the AV and secured with rubber bands to form a water-tight chamber between the inside of the AV and the silicone liner. One hundred and fifty ml of hot water (60 C) was then loaded into a 50 ml syringe and squirted in the water chamber via the valve. A camel collecting glass was then filled with 37 C water and the end capped with a rubber bung and a foam insulation sleeve placed around the collecting glass. A schematic diagram of the constructed AV is presented in Figure 2.4. Figure 2.4. Schematic diagram of the alpaca AV (not to scale). The AV was partially inflated with air by blowing in the air valve (to simulate pressure inside the female reproductive tract) and the whole unit was wrapped in an electric blanket (set to approx. 32 C; Figure 2.5) to minimise heat loss during the copulatory period. 49
65 Figure 2.5. The assembled AV and foam covered collecting cone prior to wrapping with the electric blanket (seen unfolded under the AV) The outer end of the AV was then lubricated with non-spermicidal lubricant (Minitube ) and secured inside the mannequin (Figures 2.6 and 2.7) using elastic straps. Figure 2.6. Underside view of the AV fitted inside the mannequin. Males were then permitted access to the mannequin, and allowed to continue mating until they voluntarily desisted, which was displayed by standing and showing no further interest in the mannequin. Figure 2.7. The AV mounted inside the mannequin ready for semen collection. 50
66 After the male exited the pen, the AV was retrieved and semen was shaken down in to the collecting container from the silicone liner. The air was then released from the AV and the collecting container removed and stored in a 37 C incubator or 35 C water bath until assessment (< 5 min after removal of the AV from the mannequin). The liner was then removed from the AV and the water tipped out. The collecting container with the semen was then taken to the laboratory for assessment Mating length Mating length was measured using a stopwatch. Mating duration was defined as the time between the male mounting the mannequin and standing up from the mannequin for more than one minute. Frequent repositioning was observed in some males and not in others. Re-positioning involving standing up and immediately remounting the mannequin was included in the mating duration Semen assessment Semen assessments were conducted in the field laboratory at the Anima Reproduction Unit, Cobbitty. Semen was collected in the collecting shed and the collecting container covered with paraffin film and transported in a foam esky (Livingstone, NSW, Australia) to the laboratory (Figures 2.8 and 2.9). Figure 2.8. The laboratory used for initial assessment of semen. Semen was assessed as soon as possible after collection, and maintained at 35 C in the water bath. Semen was assessed as described below and data recorded on the semen collection and assessment worksheet (Appendix III). Figure 2.9. The working area used for initial semen assessment. 51
67 Semen volume Semen volume was assessed immediately after collection using the graduations on the collecting cone after the AV was shaken in a downwards fashion to ensure that no semen was left clinging to the silicone liner Semen viscosity Objective and precise measurements of viscosity can be achieved using a microviscosimeter or by measuring the time it takes for a known volume of semen to run out of a pipette which has been calibrated using oils of standard viscosities. Mortimer (1994) states that these methods are clearly excessive and consequently objective measurement and definition of human semen viscosity are uncommon. In human IVF clinics, the generally accepted method for measuring viscosity is subjective, based on the length of thread produced by semen as it runs out of the pipette used to measure the ejaculate (Mortimer 1994). A modification of this procedure is to draw semen into a pipette, pipette part of the semen onto a slide, draw the pipette upwards forming a thread of semen and measure the length of the thread. Semen viscosity was measured by removing 50 μl of semen using a pipette and placing 25 μl onto a glass slide. The pipette was slowly raised to form a thread of semen which was pulled slowly until it broke. The distance between the pipette and the slide at the time that the thread broke was measured using a ruler (Figure 2.10). Figure Measurement of viscous alpaca semen by thread formation Semen ph Semen ph was measured using disposable ph strips (Sigma-Aldrich) and recorded in increments of 0.5 ph units. An aliquot of semen was removed from the ejaculated and placed on the ph strip and allowed to settle. Strips were examined once the indicators had stopped changing colour (approx sec). As semen ph increases with time after ejaculation (Mortimer 1994), ph was measured as soon as practicable Semen osmolarity Fifty μl of neat semen was removed from the ejaculate with a pipette, and placed in a micro centrifuge tube (Bacto Laboratories) and stored at 4 C until assessment. Semen osmolarity was measured using a freezing point depression osmometer (described above) within two weeks of semen collection. 52
68 2.8. Sperm assessments Sperm motility Sperm motility was assessed subjectively as described by Evans and Maxwell (1987). Briefly, 10 µl of neat or diluted semen was placed onto a pre-warmed slide (Livingstone, NSW, Australia) and covered with a 22 x 22 mm, pre-warmed cover slip (Marienfeld, Germany). At least four fields of sperm were viewed using phase contrast microscopy under 100x magnification, and scored as a percentage of progressively motile sperm in 5 % increments Sperm concentration The concentration of sperm was assessed by diluting 10 μl of neat semen with 90 μl of 3 % (w:v) NaCl solution. Ten μl of this solution was then placed on each side of a prepared haemocytometer (Improved Naebauer, Weber, London U.K.). The sperm in the grid on each side of the chamber were counted, with the average from both sides being used to calculate the number of sperm ml -1 in the original sample. Counts with > 10 % disparity were rejected and a fresh preparation made and counted Sperm viability Sperm viability was assessed using the Eosin-Nigrosin stain (Evans and Maxwell 1987). Briefly, equal volumes of semen and staining solution (approx. 10 μl each) were placed on a clean, warm microscope slide, mixed and smeared into a thin film along the slide. Smears were allowed to dry before examination. Stained slides were examined using phase contrast microscopy (magnification x 400) or oil immersion (x 1000). At least 100 sperm per slide were counted. Sperm which did not take up the stain had intact plasma membranes and were considered live; sperm which stained did not have intact plasma membranes and were considered dead Sperm morphology Neat or diluted semen was fixed by placing 50 μl in a light-proof micro centrifuge tube and diluting 1:1 with 8 % (v:v) gluteraldehyde in PBS (Sigma-Aldrich). Samples were then mixed thoroughly by vortexing for sec and were then stored at 4 C in the dark until assessment. For examination, 10 μl fixed sperm suspension were placed on a glass slide and covered with a coverslip. Stained slides were examined using phase contrast microscopy (magnification x 400) or oil immersion (x 1000). At least 100 sperm per slide were counted and were divided into six categories (Figure 2.11): Normal Head abnormalities including both size and shape related abnormalities: large, small, tapering, pyriform, amorphous, vacuolated, double head Midpiece abnormalities: absent tail, non-inserted or bent tail, distended or irregular midpiece, abnormally thin midpiece Tail abnormalities including short, hairpin, multiple, broken, irregular width, coiled The presence of cytoplasmic droplets. 53
69 Figure Sperm morphology diagram from Evans and Maxwell (1987) Dilution of semen Semen dilution was performed as soon as possible after collection and routine examination. Both the semen and the diluent were brought to the same temperature, by placing in a 30 C water bath at the time of dilution, taking care to ensure that water did not enter either the semen or the diluent. Diluent was added drop wise to the semen, not semen to the diluent as this can cause shock to sperm and reduce their motility. Care was taken to ensure that the correct amount of diluent was added and that the diluent and semen were properly mixed Liquefaction of semen At the onset of this study there was no established procedure for the liquefaction of alpaca semen. Detailed methodologies for the liquefaction procedures tested have been included in the relevant chapter Liquid storage of semen A number of different procedures were tested for the liquid storage of alpaca semen and detailed methodologies have been included in the relevant chapter. Unless specified otherwise, semen was collected according to the final protocol established (see Chapter 3) without diluent in the collecting container. Semen was assessed and then diluted with the appropriate diluent (1:4). Tubes containing diluted semen were placed in a foil-wrapped boar semen bottle containing 100 ml of 35 C water and placed in the 4 C refrigerator. Diluted semen was cooled to the desired temperature (4 C unless specified) over 2 hours. Semen was stored at 4 C and assessed at 24 h intervals until the completion of the storage period. The composition of the diluents used is presented in Appendix IV Sperm cryopreservation Procedures for the cryopreservation of alpaca sperm had not been established at the onset of this project. Numerous investigations of various methods for the cryopreservation of alpaca sperm were conducted and a detailed description of procedures is presented in the relevant chapter. Unless specified otherwise, diluted semen was placed in a foil wrapped water bath (as described for liquid storage and placed on a foam block inside a walk-in 4 C cold room. Diluted sperm were chilled to 4 54
70 C over 2 h, and then frozen as pellets on dry-ice (unless stated otherwise) using the method described by Evans and Maxwell (1987). Briefly, diluted semen (250 μl) was carefully placed (using cold pipettes) onto depressions in the dry ice (solid CO 2 ; - 79 C; Figure 2.12) which had been made previously. Care was taken to ensure that the depressions were not too shallow as the depth of the depressions can have a significant effect on the freezing rate (and therefore survival) of sperm. Pellets were then left to freeze on the surface of the dry ice for 2-3 min until the pellets became opaque. Frozen pellets were then transferred into a small esky containing liquid nitrogen (LN 2 ), loaded into pre-labelled glass test tubes and transferred to goblets for long-term storage in LN 2. b Figure Creating depressions in the dry ice (a) and freezing diluted semen as pellets in the depressions (b) from Evans and Maxwell (1987) Sperm thawing Unless otherwise specified, sperm frozen as pellets were thawed by removing two pellets from liquid nitrogen and placing them in a clean glass test-tube (Figure 2.13). The tubes were then vigorously agitated in a 37 C water bath until the semen melted. Straws were removed from liquid nitrogen and thawed by agitating in a 37 C water bath for 30 sec or 1 min for 0.25 ml and 0.5 ml straws respectively. Figure Thawing pellet frozen semen from Evans and Maxwell (1987) Artificial insemination A detailed description of the methodology used for artificial insemination is provided in the relevant chapter Statistical analysis Analysis of data was performed by Associate Professor Peter Thomson, Dr Adrienne Kirby and Dr Katherine Morton. All statistical analyses were performed using GenStat (version 9.0; Ceanet, Brisbane, Australia). Data were tested for equal variance and normality before analysis. Data were transformed where necessary prior to analysis and P values < 0.05 were considered significant. Specific details regarding analysis are present in the relevant chapters. 55
71 3. Collection of alpaca semen 3.1. Introduction Camelid reproductive physiology is considerably different to other domestic and wildlife species, presenting many challenges for the establishment of a reliable and efficient method of semen collection. Compared with other species, such as sheep, cattle, horses and pigs, there are few reports on the successful collection of camelid semen. In most cases, semen was obtained by either electroejaculation or copulation with a mannequin (see the Chapter 1). Copulation with a mannequin must be considered the only viable option for the development of AI technology owing to welfare implications of collecting semen by electroejaculation. The duration of copulation, penetration of the cervix, intracornual semen deposition and ejaculation throughout copulation (dribble ejaculating) deposition of the semen present major challenges to the collection of high quality semen. Maintenance of semen collection equipment at a constant temperature throughout the extended duration of copulation alone is a challenge. Furthermore, there is the potential for latex toxicity when sperm are exposed to latex for extended periods of time (up to 90 min in alpacas) and semen ejaculated within the first few minutes of copulation may be exposed to air and other contaminants, which may be detrimental to semen quality. The difficulty of collecting high quality camelid semen is one of the reasons that artificial insemination technology is poorly developed. It is unlikely that this technology will be further refined until a reliable method of semen collection is developed. Considerable advances were made during RIRDC project AAA-1A. However, two important issues were raised: the difficulty of controlling the environment inside the AV with the possibility of a reduction in semen quality during prolonged copulation striking a balance between the improved semen quality parameters with increased mating length, and the deleterious effects of prolonged exposure of semen to latex AV liners These issues must be overcome before high quality semen can be collected. Furthermore, understanding the relationship between semen quality and mating length, and characterising the sources of variation in semen quality, are paramount to facilitating the development of AI in alpacas. The aim of the work reported in this chapter was to determine the optimal semen collection parameters. The relationship between semen quality parameters and sources of variation are discussed in a subsequent chapter (Chapter 6). Construction of the second mannequin was deemed integral to determining optimal semen collection parameters. Re-designing the mannequin and artificial vagina (AV) to mimic more closely the female reproductive tract was hypothesised to increase the quality and reduce the variation in quality of collected semen. The research presented in this chapter aimed to determine the optimal semen collection procedure in alpacas by investigating: the design of the mannequin the design of the artificial vagina, specifically the liner and the semen collection vessel the presence of females during semen collection addition of various diluents to the collection vessel addition of catalase to the collection vessel Materials and methods For all experiments, mating length, semen viscosity, semen volume, sperm motility, sperm concentration, acrosome integrity and sperm morphology were assessed as described in the Methodologies section. Males were randomly allocated to pens and treatment groups to remove potential bias. 56
72 Design of mannequin and AV liners Details regarding the background, development and testing of the mannequin and AV liners has been included in the results section to provide a full overview of the process leading to the establishment of the semen collection method Effect of a cervix included in artificial vagina, on semen quality For this experiment a cervix was incorporated into the AV using commercially available rubber insulation foam (Clark Rubber, Bayswater, Vic, Australia) and semen was collected from males (n=9; n=37 ejaculates) Effect of semen collecting vessel on semen quality To determine the most appropriate collection vessel, semen was collected (n=11 males; n=39 ejaculates) using AVs fitted with either a glass camel collecting cone (Camel, IMV Technologies, L Aigle, France), or a baby s bottle fitted with a 15 ml (Bottle 15 ml tube) or 50 ml centrifuge tube (Bottle 50 ml tube). Glass camel-collecting containers (Figure 3.1) are expensive (160 AUD inc. GST) and given their location and construction (glass) could easily be broken. Figure 3.1. IMV glass camel collecting cone. Figure 3.2. Baby s bottle system developed at The University of Sydney. As an alternative, we developed a system consisting of a plastic baby s bottle with a 15 ml centrifuge tube suspended inside (Figure 3.2). The baby s bottle system was modified to contain a 50 ml centrifuge tube, as we theorised that the viscous semen would flow more freely through the wider neck Effect of the presence of females during semen collection on semen quality Semen was collected (n=13 males; n=119 ejaculates) in the presence or absence of females Effect of diluent addition to the collecting container on semen quality Semen was collected (n=12 males; n=51 ejaculates) into a camel collecting glass containing no diluent (Control) or 500 µl of UHT-skim milk, Androhep (Minitube, Germany) or Salamon s liquid storage diluent (Evans and Maxwell, 1987; modified for alpaca sperm by the addition of 0.3 % w:v BSA) Effect of collecting semen into medium containing catalase Semen was collected (n=7 males; n=14 ejaculates) into camel collecting containers with 500 µl of modified Salamon s diluent containing 0 (control), 100, 200 or 600 units of catalase. 57
73 Statistical analyses Statistical differences in mating length and the semen quality parameters assessed were determined using a linear mixed model (REML) procedure in GenStat (Release 7.2, Ceanet, Brisbane, Australia) with P<0.05 considered significant. For all experiments, the model for each of the traits took the form: Trait = Mean + Replicate + Treatment + Male + ε For all experiments, data transformations were performed according to the information presented in Table 3.1. Table 3.1. Transformation of data prior to statistical analysis. Where data contained zero values the values were adjusted by adding the smallest whole unit of the parameter measured (i.e. addition of 1% or 1 in count data). Note: logit(y) = loge[p/(100-p)], logit(y;+1) = loge[(p+1)/(100-p+1)] Experiment Semen quality parameter Cervix addition Collection vessel Presence of females Diluent addition Catalase supplementation Mating length log(y) log(y) log(y) log(y) log(y) Semen volume y y y y y Semen viscosity log(y+1) log(y+1) log(y+1) log(y+1) Sperm concentration log(y) log(y) log(y) log(y+1) log(y) Total no. sperm in ejaculate log(y) log(y) log(y) log(y) log(y) Sperm motility logit(y,+1) logit(y,+1) logit(y,+1) logit(y,+1) logit(y) Acrosome integrity logit(y,+1) logit(y,+1) logit(y,+1) logit(y,+1) logit(y) Morphologically normal logit(y) logit(y) logit(y,+1) logit(y) logit(y) Head abnormalities logit(y,+1) logit(y,+1) logit(y,+1) logit(y,+1) logit(y,+1) Midpiece abnormalities logit(y) logit(y,+1) logit(y,+1) logit(y) logit(y) Tail abnormalities logit(y,+1) logit(y,+1) logit(y,+1) logit(y,+1) logit(y) Cytoplasmic droplet logit(y) logit(y,+1) logit(y,+1) logit(y) logit(y) 3.3. Results Design of mannequin The external dimensions of live females (n=5) were measured and incorporated into the specifications of the mannequin (Figure 3.3). Figure 3.3 Side view of the mannequin The mannequin was constructed from wood, high-density foam, fibreglass, and was covered with wool. The base of the mannequin consisted of two parallel longitudinal pieces of wood measuring 95 58
74 cm x 40 cm. These pieces were then covered with another piece of wood to mimic the back and ribs. A door was created in the side of the mannequin to permit easy insertion/removal of the AV which rested on a shelf inside the mannequin. The wooden frame was then filled with fibreglass. The external surface was covered with a highdensity foam, and then merino wool (Figure 3.4). The wool surface was attached with Velcro to facilitate removal and cleaning. The head/neck section of the mannequin was constructed in a similar fashion. However, low-density foam was used to reduce the weight of the mannequin. The neck joins the body at the front of the mannequin to allow easy removal, and to permit movement during mating. Figure 3.4 The completed mannequin The capacity of the AV to retain heat, and therefore keep sperm alive, was tested at Coolaroo Alpaca Stud, Mittagong, NSW, in June (approx. ambient temperature 13 C). The temperature of the water in the AV was measured every 5 min for 45 min. The temperature fell sharply within the first 20 min (Figure 3.5) demonstrating that the insulation contained within the previous mannequin was not sufficient to maintain the required temperature during outdoor collections in the colder months of the year Indoor Outdoors Temp Time (mins) Figure 3.5 Temperature of water in the AV when collections were performed indoors or outdoors. 59
75 Additional insulation was therefore included in the new mannequin, ensuring that the temperature of the AV did not decrease during the collection process, thereby permitting indoor and outdoor collections all year round. The new mannequin was tested at Belbourie Alpaca Stud and at the University s Animal Reproduction Unit during August and September Most males found the mannequin too high and too wide and consequently did not adopt a natural mating position (Figure 3.6). Figure 3.6. A male attempting to mate the new style mannequin. Figure 3.7. Alpacas naturally mating in a pen Figure 3.6. Clearly demonstrates an unnatural mating position compared with the positions adopted for natural mating (Figure 3.7) and mating with the previous mannequin (Figure 3.8). Figure 3.8. Mating with the previous mannequin To rectify this unnatural mating posture, smaller versions of the mannequin (n=3) were constructed in November The double insulation was retained to help maintain the temperature of the AV during the collection period. Testing at the University s Animal Reproduction Unit (Cobbitty) demonstrated adequate heat retention (Figure 3.9). These three smaller mannequins and one original design (Consuela) were utilised throughout the remainder of the project. 60
76 Temp ( o C) Without Time (mins) Electric blanket Figure 3.9. Temperature of the water in the jacket of the IMV collecting glass inside the new mannequin with ( ) or without ( ) the electric blanket surrounding the AV Design of AV liners Given the length of copulation, and the perceived effect of the design of the AV on the subsequent quality of semen, we decided to re-design the AV to include a vagina, cervix and uterus. The length of the liner was also increased from 40 to 51 cm and a three-ring cervix was included. Three designs for liners were created. The first was the straight liner used by Dr. Vaughan. The second was similar to the design of the first liner, but with the addition of a cervix (Figure 3.10). The third was designed with variable diameter and a cervix (Figure 3.11), which incorporated the descriptions and dimensions of the female reproductive tract reported by Drs. Julio Sumar, Walter Bravo and Wilfredo Huanca. Figure Specifications of the two constant diameter silicone liners: straight no cervix, straight with cervix detail (inset) 61
77 Figure 3.11 Specifications for variable diameter silicone liner with a cervix. Initially the liners were made from latex. However, as the males would not continue mating with latex liners for more than 1-2 minutes and there was still some suggestion of latex toxicity in the literature, alternative approaches were investigated. Particular batches of latex have been shown to be more toxic to ram sperm than others (WMC Maxwell, unpublished). Given the inherent concerns regarding latex, and continual need for toxicity testing of new batches, silicone was the only material considered suitable for liner production. Silicone liners were made in house at The University of Sydney using medical grade silicone (Prosil 8 ; Barnes Products Pty. Ltd.; Bankstown, NSW, Australia). Initial attempts to produce liners failed as the mixture did not set. Several pilot studies to avoid potential toxicity problems (which can occur with residues from the production process) were therefore conducted to determine the optimal production process. Moulds were initially built using wood. However, when the first layer of silicone was put into the oven to dry the silicone began to bubble resulting in a rough surface. We then approached a casting company to produce aluminium moulds. Different temperatures and drying times were tested using the aluminium moulds. Extended periods of drying resulted in a rigid liner, which broke easily, while shorter periods produced soft, smooth and stretchy liners. Drying of silicone in commercial drying ovens at 140 C, rather than the 25 C was recommended by Barnes Pty. Ltd. as optimal for Prosil 8. Prosil 8 was discontinued in 2006 and the remainder of the liners were produced using Prosil 20 (the closest product to Prosil 8 ). These liners were hand made, and produced according to the procedure described in Appendix VI. The final silicone liners are presented in Figure Figure 3.12 Silicone liners produced using an aluminium mould. 62
78 Field testing of the liners revealed that those containing a cervix resulted in the display of abnormal mating behaviour by the males. The most prominent behaviour was the withdrawal of the penis from the AV, standing up followed by sitting down and attempting to re-mate the mannequin. This fidgeting and overall discomfort suggested that the cervix did not adequately mimic that of a real female. The second liner with the constant diameter and cervix was tolerated by the males and they did not display any abnormal behaviour. However, no sperm were found in any of the ejaculates collected with this liner. Semen collections made with the first liner (constant diameter, no cervix) were highly successful. The males displayed normal mating behaviour and sperm were present in the majority of the ejaculates. While the design allowed the penis to come in contact with the collection glass, the ejaculate characteristics observed (presented in Table 3.2) were similar to those previously described in the literature (see Chapter 1). Given these results, the decision was made to use the straight liner with no cervix and investigate the addition of an artificial cervix in to the AV itself. Table 3.2. Quality parameters of fresh undiluted alpaca semen after collection using an artificial vagina with the constant diameter liner without a cervix. Parameter Mean ± SEM Range Mating length (min) ± Semen volume (ml) 1.9 ± Sperm motility (%) 44.9 ± Sperm concentration (x10 6 /ml) 71.2 ± ± Effect of an artificial cervix into the artificial vagina on semen quality Parameters for semen collected using an AV with or without a cervix are presented in Table 3.3. Mating length was significantly reduced by the addition of a cervix, although sperm midpiece and tail abnormalities were reduced. Table 3.3. Quality parameters of fresh undiluted alpaca semen after collection using an artificial vagina with or without a cervix. Data are mean ± SEM, values within a row with a different superscript are significantly different (P<0.05). Parameter Control Cervix Mating length (min) 23.1 ± 4.4 a 21.9 ± 2.4 b Semen volume (ml) 1.5 ± ± 0.2 Semen viscosity (mm) 15.1 ± ± 5.3 Sperm concentration (x10 6 /ml) 58.1 ± ± 11.2 Total no. sperm in ejaculate (x10 6 ) 87.6 ± ± 15.6 Sperm motility (%) 27.8 ± ± 6.1 Acrosome integrity (%) 87.7 ± ± 2.9 Morphologically normal (%) 55.3 ± ±4.9 Head abnormalities (%) 1.5 ± ± 1.9 Midpiece abnormalities (%) 13.0 ± 1.9 a 8.9 ± 1.3 b Tail abnormalities (%) 15.4 ± 4.6 a 7.9 ± 2.9 b Cytoplasmic droplets (%) 14.8 ± ±
79 Effect of semen collection vessel on semen quality During semen collection it was observed that the 15 ml tube became clogged with frothy semen and semen was backed up in to the AV, which made collection even more difficult. There were no significant differences in mating length, semen volume, or sperm motility between the types of collecting vessel (Table 3.4). However, sperm concentration was higher when semen was collected with the camel container, or the 50 ml tube inside the baby s bottle when compared with the 15 ml tube. Higher proportions of morphologically normal sperm were observed for semen collected with the camel collecting container compared with the 50 ml tube, and lower numbers of cytoplasmic droplets were seen when semen was collected with the camel collecting container and 50 ml tube compared with the 15 ml tube. Table 3.4. Quality parameters of fresh undiluted alpaca semen after collection using an artificial vagina and a camel collecting container, or a baby bottle with a 15 or 50 ml tube. Data are mean ± SEM, values within a row with a different superscript are significantly different (P<0.05). Parameter Camel container Bottle 15 ml Bottle 50 ml Mating length (min) 24.0 ± ± ± 4.0 Semen volume (ml) 1.6 ± ± ± 0.4 Semen viscosity (mm) Sperm concentration (x10 6 /ml) ± 21.4 a 64.6 ± 20.6 b 99.2 ± 15.2 a Total no. sperm in ejaculate (x10 6 ) ± ± ± 50.5 Sperm motility (%) 32.7 ± ± ± 5.7 Acrosome integrity (%) 94.6 ± ± ± 1.5 Morphologically normal (%) 70.4 ± 5.7 a 66.5 ± 6.6 a,c 64.1 ± 3.7 b,c Head abnormalities (%) ± ± 0.4 Midpiece abnormalities (%) ± ± 0.7 Tail abnormalities (%) ± ± 3.3 Cytoplasmic droplets (%) 8.0 ± 2.4 a 16.3 ± 3.5 b 3.0 ± 2.5 a During this experiment we observed that water entered both the 15 and 50 ml tubes inside the baby bottles. We attempted to seal the leak by permanently gluing the tubes to the teats using Araldite and Plumber s silicone. During toxicity testing sperm motility declined significantly after exposure to Araldite and Plumber s silicone. Eventually this system was abandoned in favour of the new design of camel collecting cone. The new design had a fluted end which did not detach from the AV as readily as the previous design and was therefore less likely to be broken. The second edition of these camel collecting glasses were used throughout the remainder of the project. 64
80 Figure The second version of the IMV camel collecting glass featuring the fluted/bulbous end Effect of the presence of females during semen collection on semen quality There were no significant differences in mating length, semen viscosity, semen volume, sperm motility or concentration when semen was collected in the presence or absence of females (Table 3.5). However, the presence of the females altered the males behaviour, rendering them aggressive and difficult to handle. The males became accustomed to mating in the presence of other males (Figure 3.13) and were far less aggressive when the females were absent. Table 3.5. Quality parameters of fresh undiluted alpaca semen after collection in the presence or absence of female alpacas. Data are mean ± SEM, values within a row with a different superscript are significantly different (P<0.05). Parameter Absence Presence Mating length (min) 28.4 ± ± 2.0 Semen volume (ml) 3.0 ± ± 0.2 Semen viscosity (mm) 16.0 ± ± 2.1 Sperm concentration (x10 6 /ml) 82.6 ± ± 1.3 Total no. sperm in ejaculate (x10 6 ) ± ± 28.0 Sperm motility (%) 25.3 ± ± 3.7 Acrosome integrity (%) 82.0 ± ± 2.1 Morphologically normal (%) 67.5 ± ± 2.1 Head abnormalities (%) 0.9 ± ± 0.2 Midpiece abnormalities (%) 9.2 ± ± 0.9 Tail abnormalities (%) 7.0 ± ± 1.0 Cytoplasmic droplets (%) 15.0 ± ±
81 Figure Semen collection in the absence of female alpacas Effect of diluent in the collecting vessel on semen quality The collection of semen into a collecting glass containing Salamon s diluent significantly increased sperm motility (Table 3.6) compared with no diluent (control), Androhep, or skim-milk diluents. While collection of semen into Androhep and Skim milk diluents reduced semen viscosity (P<0.05) compared semen collected with no (control) or Salamon s diluents. Acrosome integrity was higher for semen collected in Skim-milk diluent compared with Androhep, Salamon s or no diluent. Table 3.6. Quality parameters of fresh alpaca semen after collection using an artificial vagina and collecting glass containing no diluent (Control), or Androhep, Salamon s or Skim-milk diluents. Data are mean ± SEM, values within a row with a different superscript are significantly different (P<0.05). Parameter Control Androhep Salamon s Skim-milk Mating length (min) 19.6 ± ± ± ± 2.0 Semen volume (ml) 1.3 ± ± ± ± 0.9 Semen viscosity (mm) 15.8 ± 5.0 a 8.2 ± 2.9 b 10.8 ± 3.3 a 6.9 ± 3.2 b Sperm concentration (x10 6 /ml) 60.0 ± ± ± ± 20.8 Total no. sperm in ejaculate (x10 6 ) 76.4 ± ± ± ± 21.9 Sperm motility (%) 45.5 ± 8.7 a 40.3 ± 6.6 a 60.1 ± 6.7 b 37.5 ± 8.0 a Acrosome integrity (%) 77.6 ± 3.5 a 80.6 ± 4.4 a 82.3 ± 4.8 a 88.4 ± 3.2 b Morphologically normal (%) 57.3 ± ± ± ± 4.9 Head abnormalities (%) 3.5 ± ± ± ± 0.6 Midpiece abnormalities (%) 10.8 ± ± ± ± 2.4 Tail abnormalities (%) 16.7 ± ± ± ± 4.1 Cytoplasmic droplets (%) 14.8 ± ± ± ± Effect of collecting semen into medium containing catalase on semen quality Collection of semen into Salamon s diluent containing 200 unit ml of Catalase significantly reduced sperm concentration but total number of sperm in the ejaculate was unaffected (Table 3.7). Semen viscosity was reduced, and the proportion of normal sperm increased for semen collected into Salamon s diluent and 200 or 600 units ml of Catalase compared with 0 or 100 units. The proportion of sperm with cytoplasmic droplets was significantly reduced for semen collected into any concentration of catalase compared with the control. 66
82 Table 3.7. Quality parameters of fresh alpaca semen after collection using an artificial vagina and collecting glass containing Salamon s diluent supplemented with 0 (control), 100, 200 or 600 units of catalase. Data are mean ± SEM, values within a row with a different superscript are significantly different (P<0.05). Parameter Catalase concentration (units ml -1 ) 0 (Control) Semen volume (ml) 1.6 ± ± ± ± 0.3 Semen viscosity (mm) 9.3 ± 3.5 a 10.0 ± 5.8 a 2.0 ±1.2 b 5.0 ± 2.9 b Sperm concentration (x10 6 /ml) ± 21.5 a 81.4 ± 32.3 a ± ± 70.4 a Total no. sperm in ejaculate (x10 6 ) ± ± ± 24.9 b ± 51.4 Sperm motility (%) 50.0 ± ± ± ± 0.0 Acrosome integrity (%) 81.0 ± ± ± ± 14.5 Morphologically normal (%) 54.7 ± 5.3 a 53.0 ± 5.4 a 66.8 ± 8.8 b 68.3 ± 4.8 b Head abnormalities (%) 2.3 ± ± ± ± 1.8 Midpiece abnormalities (%) 9.0 ± 2.6 a 6.5 ± 2.1 a 4.8 ± 0.3 b 5.0 ± 2.0 b Tail abnormalities (%) 8.3 ± ± ± ± 1.5 Cytoplasmic droplets (%) 21.3 ± 1.76 a 14.0 ± 1.5 b 13.5 ± 5.6 b 14.0 ± 3.6 b 3.4 Discussion Semen was successfully collected using the new method developed and outlined in this chapter, and the modifications to the mannequin and AV liner resolved the two crucial issues raised during RIRDC project AAA-1A. Semen characteristics were similar to previous reports (Bravo et al. 1997b; Vaughan et al. 2003a). Semen was also highly viscous, and not fractionated, concurring with Sumar (1983), Bravo et al. (1997b) and Vaughan et al. (2003a). However, some authors have observed that some semen parameters increased with mating length (Lichtenwalner et al. 1996b; Bravo 2002). Throughout all experiment both oscillatory and forward progressive motility were observed, contrasting with Garnica et al. (1993), Bravo et al. (1997b), Bravo et al. (2000a) and Vaughan et al. (2003a) who observed only oscillatory motion. Sperm swimming at greater than one body length per second were considered to be forward progressive, and those which displayed twitching, circular or non-progressive motility were considered to display oscillatory motion. Generally, oscillatory motility was observed in samples of higher viscosity and lower viscosity samples displayed forward progressive motility. In contrast, the majority of New World camelid literature reports no increase in motility concomitant with viscosity reduction (Bravo et al. 2000b) but some of the Old World camel literature reports that upon liquefaction individual sperm gain motility (Tibary and Memon 1999a). The beat frequency of sea urchin sperm flagella is significantly affected by the viscosity of the surrounding environment reducing with increasing environmental viscosity (Pate and Brokaw 1980). Interestingly the type and nature of the macromolecules used to increase viscosity affected the movement of sea urchin sperm flagella, but it was reduced for all increased viscosities. Inhibition or reduction of alpaca sperm flagella movement whilst in the presence of the viscous seminal plasma may result in the typical oscillatory motion of sperm. However, Bravo et al. (1996b) did not observe a simultaneous shift in motility patterns when semen was liquefied. Sperm motility did change from oscillatory to forward progressive before freezing, but the authors did not qualify the stage at which this shift was observed, i.e. after dilution but before cooling to 4 C, or after cooling to 4 C. Moreover, Bravo et al. (2000a) observed that epididymal sperm did not display forward progressive motility despite having no contact with the viscous seminal plasma, and the effect of semen viscosity on alpaca sperm motility remains to be elucidated. In addition, the function of the viscous seminal plasma remains to be elucidated but it is generally believed to be related to the length of time between mating and ovulation (approx 36 h; Bravo et al. 1997b). Recent studies by Ratto (2005) have demonstrated the presence of an ovulation inducing factor (OIF) in the seminal plasma of llamas and 67
83 alpacas but it appears unrelated to viscosity. Further detail about the viscosity and liquefaction of alpaca semen is presented in Chapter 4. The frothy nature of alpaca semen described by a number of workers (Von Baer and Hellemann 1998; Aller et al. 2003; Vaughan et al. 2003a; Giuliano et al. 2007) was also observed during the current project. We hypothesised that the froth is caused by the males retrieving and reinserting their penises into the collection container, resembling their behaviour during natural mating which results in trauma to the uterine lining and aids in the induction of ovulation (Ratto 2005). Giuliano et al. (2007) observed that only ejaculates collected by electroejaculation did not contain foam. The authors suggested that the rotation movement of the penis in the AV whips the seminal plasma and produces the foam (froth). Von Baer and Hellemann (1998) discarded ejaculates with foam and Giuliano et al. (2007) discarded ejaculates with more than 40 % foam but the reasons for this remain unclear. Ejaculates with foam were utilised by Aller et al. (2003) and Vaughan et al. (2003a) and the present study, and have been successfully liquid stored (Morton et al., unpublished) and cryopreserved (Aller et al. 2003). One possible reason for not utilising ejaculates containing foam is the variable time required to decant the foam (ranging from 1 min 24 h; Giuliano et al. 2007) which results in an imprecise evaluation of semen characteristics. In addition, the latter commented on the possible negative effects of the foam on sperm viability. In the present study, ejaculates containing foam were left to stand (range 5 60 min, depending on the initial amount of froth) to allow dispersion, which did not appear to have any adverse effects on semen quality. There is a wide variation in the reported motility of alpaca sperm (see Chapter 1). This may reflect differences in the quality of the semen collected. However, it is more likely to be a reflection of the subjective assessment of sperm motility. To standardize motility assessment, and reduce the variation in motility scoring, all team members underwent training, and undertook yearly refresher courses. Despite these precautions, there will always be assessor variation when motility is determined subjectively. The objective assessment of sperm motility and motility patterns by computer assisted sperm analysis (CASA) can overcome this although it has yet to be applied to alpaca sperm. The objective measurement of alpaca sperm characteristics is dealt with in Chapter 5. The majority of the literature reports the sperm concentration to be in millions per ml, but a notable exception are the studies of Raymundo et al. (2000) and Davalos and Olazabal (2002) who reported sperm concentrations in the 10s 100s of thousands. The generally accepted nomenclature for sperm concentration is millions, or billions per ml. It remains to be seen whether the low sperm concentration reported by the latter authors were related to environment and resulted from decreased spermatogenesis during the non-breeding season or times of decreased nutrition, or an experimental artefact (e.g. sperm sticking to the inside of containers 1 ) or a miscalculation during determination of sperm concentration. The incorporation of an artificial cervix in the AV did not significantly alter the quality of the semen collected. The major difference observed was a reduction in the proportion of sperm with midpiece and tail abnormalities. Previously, the number of tailless sperm was shown to increased between after 45 and 90 days of sexual rest (Flores et al. 2002) but other factors influencing sperm morphology have yet to be identified. Bravo et al. (1997b) included a stricture which resembled a cervix and a heating pad to keep the entire AV warm and concluded that these two improvements resulted in semen which was as close as possible to a natural ejaculate. In combination, both of these features improved the quality of the collected semen although it is not possible to distinguish the relative contributions of the stricture and the heating pad. Collection of alpaca semen using an IMV glass camel collecting container significantly increased the sperm concentration and proportion of morphologically normal sperm in an ejaculate compared with the control group. Despite their glass construction and concerns about fragility, only one camel semen collection container was broken during the course of the project (during cleaning in the laboratory). 1 Gametes contain surface charges which causes them to stick to the surface of plastic and glass. Generally, the addition of protein macromolecules such as serum albumin prevents them from becoming sticky. 68
84 Given the increased quality of semen collected using these containers, it is recommended that they be used and where possible the second edition of these containers with the fluted top. Davalos and Olazabal (2002) collected semen from 10 male alpacas three times a week over a 3 month period with an AV fitted inside a mannequin with or without a receptive female. Semen collected in the presence of a female had a greater volume (1.73 ± 0.09 ml vs ± 0.04 ml), sperm concentration (57.5 ± 10 4 ml vs ± 4.3 x 10 4 ml), sperm motility (68.9 ± 4.9 % vs ± 5.3 %) and proportion of live sperm (72.1 ± 1.9 % vs ± 4.2 %) but mating length and the proportion of abnormal sperm were not altered compared with that collected without a female. In contrast, our results demonstrated that there was no effect of the presence of females on semen quality parameters. One important difference between the two studies was that only one of the females used in the present study was receptive. Without a herd of females to select from, it was not always possible to have a receptive female present. One of the two females donated to the current project was over 20 years of age. Throughout the project, this female was ignored, in favour of the younger female, by all the males. Differences in the results obtained in these two studies may also be attributable to the housing and environment of the animals, although sufficient detail is not provided by Davalos and Olazabal (2002) to permit a comparison. The housing of males and females together or separately can affect reproductive parameters (known as bio-stimulation, reviewed by Walkden-Brown et al. 1999). Biostimulation has received particular attention in small ruminants as it is frequently used in reproductive management. The introduction of rams to anoestrous ewes is widely used to induce ovulation out of season (known as the ram effect) and in rams, this results in increased LH and testosterone concentrations (Ungerfeld and Silva 2004). The differences in the findings of Davalos and Olazabal (2002) and the present study may be explained by a hembra effect. During the present study, male and female alpacas were housed within sight and smell of each other. However, there are insufficient details provided in the study of Davalos and Olazabal (2002) to determine the presence of a hembra effect. Nevertheless, the increase in the quality of semen collected in the presence of females was likely the result of a female induced increase in pituitary activity. The stimulation of male llama libido by exposure to females prior to, and during semen collection is commonplace (Aller et al. 1997). Increased male libido prior to semen collection appears to be an important determinant of the quality of the semen collected. However, further research, specifically investigating the possibility of a hembra effect and the use of receptive females during semen collection is required to elucidate the mechanisms responsible for the increase in semen quality. An interesting observation of the present study was the change in the males behaviour during semen collection in the presence of females. This alone would have necessitated the removal of the females, during semen collection. Generally, and subsequent to the removal of the females the males were docile and easily handled during semen collection. Small spats between males were fairly common. The spats consisted of laying back of the ears and snaking of the neck, although they occasionally escalated to spitting and generally resulted from lower ranking males trying to push aside higher ranking males. In order to avoid any potential effects of stress on semen quality, the males were collected in the same order throughout the project. Where possible, all four pens were utilised simultaneously and the four highest ranking males were allowed access to the mannequins first. The males became quickly accustomed to the order of semen collection, and the four highest ranking males would wait at the internal gate while the lower ranking males remained in a holding pen or in the laneway outside the shed. The extended time of mating during semen collection from alpacas results in the small volume of semen being exposure to high temperatures and atmospheric conditions for periods up to 90 min. This could reduce the quality of the semen collected as the liquid can evaporate, changing the ratio of the constituents and altering semen osmolarity and ph. Furthermore, the sperm s requirements for energy substrates may also be sub-optimal. The addition of a diluent to the collecting vessel has been suggested (Bravo et al. 2000c) as a possible way to improve, or prevent deterioration in camelid semen quality. In this chapter, the addition of three diluents to the collecting vessel was tested. Androhep, Salamon s and Skim-milk diluent were chosen as they had all had been successfully used with alpaca or camelid semen (Androhep: Morton et al. 2006, Skim-milk: Santiani et al. 2005; Salamon s: Morton 69
85 et al. 2006). When semen was collected in the presence of a diluent there was a trend for sperm concentration and the total number of sperm in the ejaculate to increase, although the increase remained below the level required for statistical significance (P>0.05). This trend may have resulted from a rinsing action of the diluent. The protein components of the diluents may have altered the surface charges of the sperm and unstick them from the glass collecting vessel. Collection of goat semen into 10 ml Tris diluent improved sperm motility and acrosome integrity after a 3 hr thermo resistance test, compared with those collected in a smaller volume (1 ml; Yamashiro et al. 2006). The authors concluded that the reduction in the contact between the sperm and the seminal plasma reduced the damage by various seminal plasma components, such as goat seminal plasma proteins (GSP) which reduces the GSP protein-induced damage to sperm at ejaculation. There are few reports in the literature on collecting semen into diluent, as the ability to collect high quality semen has not necessitated the research. In the present study, collection of semen into Androhep or Skim-milk diluent significantly reduced semen viscosity but neither improved any other semen quality parameter. Semen collected into Salamon s diluent contained sperm with significantly higher motility than the other groups. Whilst the overall motility of the sperm was increased there was also a shift in the pattern of sperm motility. Salamon s diluent was modified by the addition of protein (in the form of BSA) which is a routine component of sperm handling media. There is some evidence that BSA causes an influx of Ca 2+ into the sperm plasma membrane and decreases the cholesterol and phospholipid ratio in the outer membrane of the acrosome (Davis et al. 1980), which has led to the use of BSA as a capacitating agent. Capacitated sperm display hyperactived motility and sperm collected in Salamon s diluent displayed a motility which was visually similar, suggesting the level of BSA in the medium may have a capacitating effect, but CASA analysis would be required to confirm this. These results demonstrate that there are no deleterious effects of collecting semen into diluents, and that sperm motility and concentration are generally increased when a diluent is present. However, more diluents need to be tested before any definitive conclusions can be drawn. For domestic species such as sheep, cattle, pigs and horses the preferred diluents for each species are well known, but this information is lacking with camelids. Detailed factorial studies examining the effects of diluting (or collecting) alpaca semen with different diluents on sperm motility (both proportion of motile sperm, and pattern of motility) and functional integrity are required to determine the most appropriate media for alpaca sperm. In addition to the sub-optimal conditions experienced by sperm during lengthy semen collection procedures, the exposure of sperm to air and oxygen during collection, and aeration of the semen by the movement of the penis may also have deleterious effects on semen quality. Long periods of exposure to air, particularly oxygen, increases sperm metabolism which results in the accumulation of lactic acid in the semen, which in turn reduces ph below optimum levels and reduces the viability of the sperm (Evans and Maxwell 1987). As sperm die and begin to decay reactive oxygen species (ROS) are produced which then reduce the viability of the remaining live sperm. Antioxidants, specifically catalase, can ameliorate the deleterious effects of ROS and lipid peroxidation, and extend the viable lifespan of sperm during preservation in cattle (Shannon and Curson 1982) and sheep (Maxwell and Stojanov 1996). Addition of catalase to the collection vessel may modify or reduce damage due to ROS and lipid peroxidation caused by the length of exposure to atmospheric environments and aeration of semen during alpaca semen collection. The effects of antioxidants on camelid semen have yet to be determined but previous studies have generally found catalase beneficial (Maxwell and Stojanov 1996) while the actions of other antioxidants remain somewhat more equivocal (Maxwell and Stojanov 1996). In the present study, semen was collected into diluents supplemented with catalase in an attempt to reduce the effects of oxidative stress on semen quality and sperm viability. The higher concentrations of catalase (200 and 600 units/ml) reduced semen viscosity and increased the proportion of morphologically normal sperm. However, no effect of catalase was observed on the motility of alpaca sperm which suggests that either there is little oxidative damage to alpaca sperm or that lipid peroxidation damage occurs through mechanisms which could not be reversed by catalase. One of the first reports of oxidative (Macleod 1943) reported that incubation of human sperm under high oxygen conditions reduced motility which could be 70
86 reversed by the addition of catalase to the incubation medium. This loss in motility is thought to be caused by peroxidative damage to the sperm plasma membrane (Jones et al. 1979). Human sperm are particularly susceptible to oxidative stress as their plasma membranes are enriched with unsaturated fatty acids (Jones 1979) which are essential to give the membrane the fluidity required to participate in fertilization (Aitken and Krausz 2001). The fatty acid double bonds are also the site attacked by ROS (Aitken and Krausz 2001) leading to a loss in sperm viability as a result of changes in membrane fluidity. Furthermore, the high content of free iron and copper in human seminal plasma catalyses the lipid peroxidation cascade. The susceptibility and extent of lipid peroxidation damage to alpaca sperm during semen collection remains unknown as the fatty acid content of the sperm plasma membranes and free iron and copper content of the seminal plasma remain to be investigated. Further research examining the level of ROS in seminal plasma and the fatty acid composition of the plasma membrane is required to determined the susceptibility and extend of lipid peroxidation damage to alpaca sperm. In all the experiments presented in this chapter, a high level of intra, and inter male variation was observed. This has been previously described in the literature for camelids (Buendia et al. 2002; Flores et al. 2002; Vaughan et al. 2003a; Giuliano et al. 2007) and appears to be higher than the variation observed for males of other species. In order to understand this variation, normal semen quality parameters for alpacas under Australian conditions, must be determined. This is essential to determine acceptable minimum parameters for semen preservation. In AAA-1A (Vaughan et al. 2003a) stated that Semen needs to be collected and examined from many more clinically normal alpacas of known fertility to define the limits of semen characteristics that indicate normal reproductive function and high fertility. While Vaughan et al. (2003a) were referring to the importance of using the normal semen quality parameters to determine if particular semen samples meet minimum standards required for cryopreservation, this is equally true if some of the sources of variation are to be elucidated and quantified. In order to determine the normal semen parameters for alpacas a large number of ejaculate needs to be collected and characterised. Whilst this project has access to a number of males their fertility is unknown or untested. This has prevented the linking of these normal semen parameters with minimum standards for cryopreservation. The experiments conducted in this Chapter represent significant advances in determining optimal semen collection parameters. The best semen collection used of a modified mannequin containing an artificial vagina fitted with a straight silicone liner, a glass IMV camel collecting container with 0.5 ml Salamon s diluent. Furthermore, the two major impediments to the development of a reliable semen collection procedure, outlined in AAA-1A have been overcome. In a commercial context, the construction of a mannequin with similar dimensions to that used in AAA-1A but with additional thermal insulation requires no investment in equipment over and above that required for the previous model of the mannequin and is highly practical commercially. However, making the silicone AV liners by hand is time consuming, labour intensive and therefore expensive. The method, developed at The University of Sydney, is presented in full in Appendix VI. Briefly, the silicone mixture is painted onto a mould (in our case a metal vacuum cleaner pipe) and dried in a heated oven. The need to paint around silicone layers onto the mould extends the time required to produce each liner. While production of silicone AV liners on a commercial scale by hand is not practical, those made commercially lack the required elasticity. Alternatives for the production of these liners are i) establish a collaboration with a commercial moulding company, and ii) utilise the existing skills in the alpaca industry to develop a to produce the required AI equipment and disposables, through a small research grant from the AAA R&D sub-committee. The company and the AAA would retain the profits which could then be disseminated according to the research priorities of the AAA. In summary, the research presented in the present study outlines an efficient method of collecting high quality semen using an AV. The incorporation of a cervix-like stricture, or collection of semen in the presence of females did not improve semen quality but addition of Tris diluent supplemented with 200 or 600 units/ml catalase to the collecting glass reduced semen viscosity and increased semen quality, which are two of the major obstacles to the development of AI in alpacas. Further research is required before widespread use of AI in camelids. However, collection of lower viscosity, higher quality semen using the methods outlined in the current study is a significant advancement which facilitates the further development of AI technology in alpacas. 71
87 4. Assessment of sperm function and integrity 4.1. Introduction The accurate measurement of basic semen parameters is vital if sperm function and integrity are to be determined. These measurements are also required for all experimental work and subsequent preservation of semen. Assessment of semen parameters, such as volume, ph, viscosity, sperm concentration and motility, is routine in most human and domestic animal andrology laboratories. Semen assessment is used as an indication of sperm fertility although no single test accurately predicts the fertility of a sperm sample. Nevertheless, the examination of a number of parameters provides clues to the fertilising potential of the sperm. Procedures for handling and assessing sperm from livestock species such as cattle, horses, sheep and pigs are well established. Originally, many of the methods were developed for field use, but over the years have become more sophisticated. Recent developments include the application of objective rather than subjective assessment; these include computer assisted sperm analysis (CASA) for estimating sperm motility and fluorescence activated cell sorting (FACS) assessment of sperm membrane status and integrity. Other recent developments include the use of specific rather than general stains to assess sperm membrane status. Giemsa stain was first used to assess acrosome integrity in ram sperm in 1975 and subsequently has been used in ram and camelid sperm assessment. More recently, acrosome status has been measured using fluorescent stains conjugated to lectins, which specifically bind to glycoprotein contained within the acrosomal matrix. A lack of reliable and accurate methods for the assessment of alpaca sperm is a major impediment to the development of artificial reproductive technologies. In particular, the viscous seminal plasma precludes the adaption of existing methods for handling and assessment of semen from other domestic livestock species. The development of methods for assessing alpaca semen and the application of sophisticated tests to diagnose damage to sperm during preservation procedures are paramount to development, optimisation and commercialisation of semen preservation and AI. Many of the existing methods for camelids rely on many procedures which are considered outdated and are rarely utilised in other species. Furthermore, the adaption of more sophisticated tests for use with alpaca sperm would provide additional information about their functional integrity. A brief summary of the types and methods for semen assessments are presented in Table
88 Table 4.1. Commonly used objective and subjective methods of semen and sperm analysis. Assessment Motility Acrosome integrity Sperm morphology Method Visualising under microscope Giemsa, Spermac, FITC stains counted manually Eosinnigrosin Subjective Parameters assessed/speed Forward progressive motility, Circular motility Sperm divided into acrosome intact or damaged populations (subjective determination) Sperm manually (subjectively) assigned to categories Objective Parameters Method assessed/speed Computer Summarised in assisted Table 4.2 sperm analyser (CASA) FITC-PNA stain assessed by flow cytometry Sperm divided into acrosome intact and damaged populations but the categorisation is objective Application to camelids Camels (Al-Qarawi et al. 2002) Giemsa & Spermac stains with subjective determination Subjective only Sperm vitality (measurement of plasma membrane integrity) DNA integrity Hypo osmotic swelling test (HOS) Eosinnigrosin, H33258, Propidium Iodide exclusion Staining with acrodine orange HOS: sperm tails manually assessed for swelling pattern. Eosinnigrosin/H33258/PI : sperm manually assessed for stain exclusion Sperm manually divided into populations of single (stains orange-red) and double (stains green) stranded DNA H33258 Propidium Iodide Sybr-14 exclusion Acrodine orange stain Sperm stained and stain exclusion assessed objectively (FACS assessment) Sperm fluorescence (green/ orangered) assessed manually using a FACS Subjective only N/a The aim of the research presented in this chapter was to establish methods for handling and assessing alpaca sperm, specifically the development of an acrosome specific stain Materials and methods Collection and sources of sperm and semen Semen was collected from adult males as described in Chapter 2 and epididymal sperm were harvested as described in Chapter 8. Semen and sperm were then used to develop procedures for handling and assessment. As most of the procedures were developed using trial and error, methodologies are presented in the results section and the protocol for the final procedure presented in the Appendices. 73
89 4.3. Results General handling of alpaca semen The most suitable method for handling of raw semen (whilst still in the collecting glass) was to cover the top of the collecting glass with Parafilm (to prevent evaporation and contamination with debris) and transport the glass in a foam esky (with a lid to prevent UV light damage to sperm). Short duration (<5 min) handling/transport of alpaca semen was undertaken in the esky. Storage of neat alpaca semen in a 37 C water bath resulted in a reduced lifespan compared with immersion of samples in a C water bath. Androhep (Minitube, Germany), originally developed for the liquid storage of boar semen, was an appropriate medium for the general dilution and handling of alpaca sperm. Androhep is commercially available, cheap ($13 per litre) and has been used at the University of Sydney for the processing and preservation of sheep, cattle and pig sperm Assessment of sperm motility Examination of motility subjectively using the procedures described in Chapter 2 is well established. The use of a phase contrast microscope with a 20x objective is recommended owing to the small head size of alpaca sperm. While the subjective motility assessment technique is simple and practical in the field, motility scores often vary between technicians within the same laboratory, and technicians from different laboratories. The development of computer assisted sperm analysis (CASA; Figure 4.1) has lead to more objective and repeatable measurement of sperm motility. Moreover, recently the pattern of sperm motility has received much attention in the scientific community as several studies have demonstrated relationships between sperm motility patterns and sperm fertility (Mortimer 1994). Assessment of sperm motility is complex, relies on many mathematical algorithms (Mortimer 1994) and is arduous to manually calculate. Development of computer systems which analyse sperm trajectory and patterns of motion have resulted in the widespread application of this type of sperm assessment. Recent research has highlighted the importance not only of the proportion of motile sperm but also of the pattern of motility in relation to the achievement of normal fertility. Figure 4.1. CASA analysis of sperm from Vyt (2007). 74
90 Figure 4.2. Derivation of the different sperm velocities from a track reconstructed at 30 frames per second. Circles denote head centroid positions (open circles = non-apex points; closed circles = apex points), and the solid line joining them is the path followed by the sperm head that is used to calculate the curvilinear velocity (VCL). The average path (in this case visually appraised) used to calculate the average path velocity (VAP)is shown by the dotted line; the broken line joining the first and last points of the track is the straight-line path used to calculate the straight-line velocity (VSL). One measurement of the instantaneous velocity is shown by the brace (Mortimer 1994). CASA measures a number of motility parameters outlined in Table 4.2. These are now routinely used in many laboratories as one of the end point assessments in experiments. CASA analysis has been applied previously in the camel (Tibary and Memnon 1999) but not to alpaca sperm, although there are no published reports in the scientific literature about factors which affect the motility patterns of camel sperm. Our aim was to apply CASA analysis to alpaca sperm, and to investigate the pattern of motility post-thaw. Table 4.2. Parameters measured by CASA (definitions from Mortimer 1994). See Figure 4.2 to aid in description explanations. Parameter Abbreviation Description of measurement Average path velocity (μms 1 ) VAP The velocity along the average path of the spermatozoon Straight line velocity (μms 1 ) VSL The linear or progression velocity of the cell. Calculated from the straight line distance between the start and the end of the observed track. Curvilinear velocity (μms 1 ) VCL Calculated from the sum of the straight lines joining the sequential positions of the sperm head along the spermatozoon s track Beat cross frequency (Hz) BCF The number of times the curvilinear track crosses the average path. Amplitude of lateral head Calculated from the amplitudes of the heads lateral deviation ALH displacement (μm) about the cell s axis of progression Linearity (%) LIN Is calculated by dividing the VSL/VCL x 100 Straightness (%) STR Is calculated by dividing the VSL/VAP x 100 Objective assessment of alpaca sperm was conducted using a computer assisted sperm analyser (CASA; HTM-IVOS v. 12; Hamilton-Thorne, Beverly, USA). Semen or sperm samples (5.5 µl) were placed on CASA slides (Cell Vu, Millennium Sciences Corp., NY, USA; pre-warmed to 37 C) and covered with a 22 x 22 mm cover slip before immediate transfer to the CASA. 75
91 Motility characteristics were determined by assessment of at least three randomly selected microscopic fields (>150 spermatozoa per sample) utilising factory CASA settings (boar) at an image sampling frequency of 60Hz. While the CASA settings were successfully modified for use with alpaca sperm, the CASA confused cells with a similar shape to alpaca sperm heads, resulting in an artificially low estimate of motility. While there were still discrepancies between the % motility recorded after subjective and objective analysis, the CASA did accurately track the motility patterns of the swimming sperm. The results of CASA assessment of frozen-thawed epididymal sperm are presented in Table 4.3. Table 4.3. Results of CASA analysis of epididymal alpaca sperm motility after freezing and thawing Parameter Mean ± sem Range Average path velocity (μms 1 ) 45.4 ± Straight line velocity (μms 1 ) 28.8 ± Curvilinear velocity (μms 1 ) ± Beat cross frequency (Hz) 27.9 ± Amplitude of lateral head displacement (μm) 5.2 ± Straightness (%) 64.4 ± Linearity (%) 30.9 ± Length 8.2 ± Sperm concentration (x10 6 ) 29.6 ± Motility (%) 6.6 ± Progressive motility (%) 0.9 ± Concentration of motile sperm (x 10 6 ) 1.7 ± Concentration of progressively motility sperm (x 10 6 ) 0.2 ± Assessment of acrosome integrity Giemsa stain Previous studies have used stains such as Eosin-nigrosin, Giemsa and Hancock s stain to assess the acrosome integrity of camelid sperm (Tibary and Anouassi 1997; Bravo 2002; Vaughan et al. 2003a; Santiani et al. 2005). While these stains were originally developed as live/dead sperm discriminators, none of them do not bind specifically to the contents of the acrosome and may therefore vary in accuracy and specificity. Initial attempts to develop and validate a protocol for the use of Giemsa stain were based on the procedure described by Santiani et al. (2005). Briefly, the authors air-dried 25 μl smears of sperm suspensions for 20 min. Slides were then stained with 20 % Giemsa (Merck, Darmstadt, Germany) for 40 min. Stained slides were then washed in distilled water, air-dried at room temperature for 30 min, mounted with Permount and covered with a cover slip. Sperm cells (at least 200) were classified as having intact or damaged acrosomes according to the following staining patterns: 1) sperm with blue or dark-blue post-acrosomal region and a pink or purple acrosomal region were considered to have intact acrosomes, while those with a blue or dark-blue post-acrosomal region and a white acrosomal region were considered to have damaged or non-intact acrosomes (Figure 4.2 and 4.3). 76
92 Figure 4.2. Appearance of acrosome intact sperm (left) and acrosome detached sperm (right) after successful staining using Giemsa. Figure 4.3. Giemsa stained sperm from Santiani et al. (2005), (A): Dead spermatozoa with intact acrosome; (B): Dead spermatozoa with detached acrosome; (C): Viable spermatozoa with detached acrosome; (D): Viable spermatozoa with intact acrosome. Our experience with Giemsa is that the stain is not able to penetrate unfixed sperm. We investigated various fixation methods and staining times. We concluded that the fixing of air-dried smeared semen slides in ethanol overnight (either at room temperature or at 4 C) followed by overnight staining in 20 % (w:v) Giemsa produced the best results. However, sperm heads accepted the stain to varying degrees and leaching of the stain into the seminal plasma could be observed over time, giving unreliable results. Furthermore, there was considerable variation between sources of Giemsa. We conclude that Giemsa is not a reliable staining method to observe acrosome integrity in alpaca sperm Spermac stain After staining with Spermac stain, the sperm acrosome appears dark green, the nucleus red, the equatorial region pale green and the midpiece and tail green (Figure 4.4). Spermac has been used to assess the integrity of llama sperm (Von Baer and Hellemann 1999). The manufacturer s instructions for use of Spermac state that viscous semen can interfere with the staining procedure and they recommend the dilution of semen (1:1) with citrate buffer prior to smearing slides. In our experience, the dilution of semen 1:1, 1:2, or 1:4 with citrate buffer or Salamon s diluent does not overcome the problem of viscous seminal plasma. All samples were faintly stained with a dark background which interfered with interpretation of sperm morphology and integrity. 77
93 Figure 4.4. Appearance of acrosome intact sperm (right) and acrosome detached sperm (left) after successful staining using Spermac (from Fluorescein-conjugated peanut agglutinin (FITC-PNA) Assessment of sperm acrosome integrity is routinely achieved using a fluorescein-conjugated peanut agglutinin (FITC-PNA) which stains the β-d-galactosyl residues which are found exclusively in the acrosomal membrane (Mortimer et al. 1990) and is therefore an acrosome-specific stain. After staining with FITC-PNA, sperm with an intact acrosome display green fluorescence over the head whilst those with a detached acrosome display green fluorescence over the equatorial region of the head (Figure 4.5). Figure 4.5. Representation of an acrosome-intact (left) and non-intact (right) sperm. Our preliminary studies suggested that the gelatinous seminal plasma interfered with the penetration of the FTIC-PNA stain into the acrosome (Figure 4.6). However, further investigations were conducted 78
94 which determined the importance of fixing the sperm in 96 % ethanol for 30 seconds prior to staining. The final protocol for the assessment of ejaculated and epididymal alpaca sperm is presented in Appendix VIII. Figure 4.6. Frozen-thawed epididymal alpaca sperm stained with FITC-PNA Heterologous oocyte binding assay At present, there are no reliable methods of assessing the fertility of alpaca sperm in vitro. In vivo assessment of fertility can be achieved using natural mating or artificial insemination but both require large amounts of resources and are time consuming and expensive. In vitro methods to assess fertility include in vitro fertilization (which has yet to be fully developed for alpacas), homologous or heterologous oocyte binding or penetration assays. The zona free hamster sperm penetration is a frequently used human sperm assessment (Mortimer 1994). These binding assays investigate the potential for sperm to associate with and bind to the oocyte s outer coating, the zona pellucida, and can be used to assess different types of sperm (fresh, liquid or frozen preserved), assess fertility of different doses of sperm etc. Heterologous oocyte binding assays are used in species where there is an absence of effective in vitro fertilization procedures, such as the horse and the alpaca. They can be performed with fresh immature, in vitro matured or salt-stored oocytes (Clulow et al. 2006). Recently in our laboratory a heterologous oocyte binding assay (using salt stored bovine oocytes) was developed to assess the fertility of unsorted and sex-sorted frozenthawed stallion sperm (Clulow et al. 2006). This assay was modified in an attempt to develop an assay for the assessment of fertility in alpaca sperm. Briefly, oocytes were aspirated from abattoir-sourced cow ovaries using a needle and syringe as described by Morton et al. (2004). Cumulus cells were then removed from the oocytes by incubation in 0.03% hyaluronidase (H4272, Sigma) and oocytes salt-stored (in 0.75MMgCl 2 6H 2 O, 0.5M(NH 4 ) 2 SO 4, 0.2mM ZnCl 2, 40mM HEPES and 0.1 mg/ml PVA; Clulow et al. 2006). Oocytes were then washed in fertilization medium to remove all traces of salt storage medium, which is toxic to sperm. Groups of oocytes (n=20) were then placed in 0.5 ml wells of fertilization medium (covered with 0.2 ml embryo culture oil). Pellets of frozen epididymal alpaca sperm were then thawed, washed (300 g; 10 min), resuspended in Androhep and added to the wells containing the oocytes. Sperm and oocytes were then co-incubated for 3 h before removal from the fertilization wells, washing (to remove sperm only loosely associated with the zona) and wet mounting. The number of sperm bound to the zona pellucida was then counted using an Olympus BHS phase contrast microscope (400 magnification). Data from replicates using salt-stored bovine oocytes revealed an absence of alpaca sperm bound to the zona pellucida. Studies were continued using in vitro matured ovine oocytes, rather than immature salt-stored bovine oocytes, as allowing oocytes to undergo the maturation process may facilitate the binding of alpaca sperm to the oocyte s zona. 79
95 Ovine oocytes were harvested from abattoir sourced ovaries and matured in vitro as described by (Morton et al. 2004,2005a-e; Morton et al. 2006). Frozen-thawed epididymal sperm were added to the well containing the in vitro matured ovine oocytes. Sperm and oocytes were then co-incubated for 4-6 hours until assessment of the number of sperm bound to the zona pellucida. During the initial states of the establishment of the oocyte binding assay, approximately ½ a pellet of frozen-thawed sperm were incubated with 20 oocytes. Alpaca sperm readily bound to in vitro matured ovine oocytes, unlike the salt-stored bovine oocytes. The number of sperm bound to oocytes is presented in Table 4.4 and stallion sperm bound to saltstored bovine oocytes are shown in Figure 4.7 after staining with Hoechst Table 4.4. Number of frozen-thawed epididymal alpaca sperm bound to the zona pellucida of in vitro matured ovine oocytes. No. sperm bound to the zona No. oocytes Frozen-thawed epididymal sperm were observed to remain motile after 4-6 h of co-incubation with the oocytes, even in the presence of glycerol, egg yolk and the cryodiluent, and motile sperm were observed in the wet mount. Figure 4.7. Hoechst stained equine spermatozoa attached to salt stored bovine oocytes at 200 x magnification under UV light (Clulow 2006). These results demonstrate the establishment of a heterologous oocyte binding assay which has the potential to be used to assess the fertility of alpaca sperm in vitro. 80
96 4.4. Discussion The main findings from these studies were: 1. Establishment of general procedures for the handling of alpaca sperm (short term storage/transport in an esky, immersion of samples in a C not 37 C water bath, and dilution in Androhep ). 2. Alpaca sperm can be reliably assessed by CASA provided it is properly freed from the viscous seminal plasma. 3. Despite previous reports, Giemsa stain is unsatisfactory for the assessment of alpaca sperm viability or membrane status. 4. Lectin based (FITC-PNA) staining is a reliable method for the assessment of alpaca sperm acrosome status. 5. A heterologous oocyte binding assay was developed for the assessment of alpaca sperm fertility in vitro. The results of these studies confirm that the general handling procedure required for alpaca semen is similar to that used in other species, particularly the ram (Evans and Maxwell 1987). Initial measurements of sperm motion examined sperm motility as well as sperm vigour (subjectively assessed on a 0-5 scale; Evans and Maxwell 1987) but the advent of computer assisted semen analysis (CASA) technology allows for substantially more parameters to be simultaneously measured (Table 4.2). Developed approximately two decades ago, CASA technology is able to provide powerful insights into sperm function as well as semen heterogeneity (subpopulations of sperm that exist within the ejaculate; Holt et al. 2007). CASA is now routinely used around the world in andrology laboratories for the assessment of human semen and in many artificial breeding centres to assess semen from domestic animal species (bull, boar, ram and stallion). More recently, CASA analysis has been applied to wildlife species including the Mohar gazelle, common marmoset, and grey short-tailed opossum (Holt et al. 2007). The application of CASA to commercial semen preservation focuses on the insights into sperm function which, in conjunction with other assessments of sperm function and integrity (acrosome integrity, ability to bind to a zona pellucida, etc.) are used to compare different methods of semen preservation and draw conclusions about the most appropriate method. Various kinematic parameters of bull sperm have been correlated with in vivo fertility, and the importance of straight-line velocity to the fertilising capacity of sperm has also been highlighted in bulls, humans and boars (discussed by Gillan et al. 2008). Gillan et al. (2008) demonstrated that a combination of semen assessments (including CASA) accounted for 60 % of the variation in fertility, highlighting the importance of their routine use in the context of commercial artificial breeding centres. The majority of studies on camelid semen have used Giemsa or other non-acrosome-specific stains, such as Hancock s or flourochromes 6-carboxifluorescein diacetate (CDFA; Giuliano et al. 2007), to determine sperm viability. Previous reports in the literature indicate post-thaw acrosome integrity of less than 20 % for all groups after Giemsa staining (Santiani et al. 2005) and 50 % after staining with Spermac (Von Baer and Hellemann 1999). These estimates are substantially lower than the high proportion of post-thaw acrosome integrity observed during this project (Chapter 7), suggesting that they were either inaccurate or compromised by artefacts associated with the method. Moreover, Wani et al. (2007) reported a sharp decline in acrosome integrity of camel semen liquid stored for 12 h, whereas the results of our studies (Chapter 6) did not observe a decrease in acrosome integrity until 72 h after the onset of liquid storage. The development of an accurate and reliable method of assessing sperm acrosome integrity (using FITC-PNA) is an important step for the preservation of semen in alpacas. Acrosome damage, or reaction, during liquid storage is one of its major deleterious consequences, and an effective method for assessment of acrosome status in alpaca sperm is integral to developing appropriate methods for semen preservation in both the liquid and frozen state. Heterologous, homologous and other binding assays (salt-stored eggs, hemi-zona assays, etc.) are widely used in species where there is difficulty in obtaining large numbers of females (such as wildlife 81
97 species and horses) and in species where there are no established protocols for the production of embryos in vitro (also used to assess sperm fertility). The development of an in vitro test for the fertility of alpaca sperm is a major advancement for research aiming to improve the efficiency of semen preservation. During the development of methods to preserve sheep semen, numerous large scale field fertility trials were conducted to examine the effect of various diluents, cryoprotects, freezing methods, and supplements on the resultant fertility of preserved semen (see Salamon and Maxwell 1995b for a description of the field fertility trials). Differences between semen preservation methods often result in small differences in field fertility which necessitates the use of large numbers of females and it is common for ewes to be used in a field trial. However, the difficulty in obtaining enough female alpacas precludes such large-scale field fertility trials, which would be otherwise necessary to determine the effects of various semen preservation methods on sperm fertility. The assessment of sperm fertility using a heterologous oocyte binding assay partially overcomes this limitation, although there is no final substitute for in vivo fertility, and provides researchers with the opportunity to efficiently (with respect to cost, labour and resources) estimate the fertility of preserved alpaca semen. A wide range of functional tests are now available for the assessment of livestock sperm (Gillan et al. 2005). In the past, many laboratory methods used to evaluate semen quality had not correlated highly with fertilizing capacity. The discovery of a variety of fluorochromes and compounds conjugated to fluorescent probes has enabled a more widespread analysis of sperm attributes, and in conjunction with the flow cytometer, permit the evaluation of a large number of spermatozoa. A number of characteristics of sperm integrity, viability and function can be assessed by flow cytometry. The DNA status of spermatozoa has been determined using the metachromatic properties of acridine orange (AO). AO staining, when used in the sperm chromatin structure assay (SCSA1), correlates with fertility in a number of species. DNA fragmentation can also be assessed using the terminal deoxynucleotidyl transferase-mediated dutp nick-end labeling (TUNEL) assay, which identifies DNA strand breaks by labeling free 30-OH termini with modified nucleotides. The status of the sperm acrosome can be determined using fluorescently labeled lectins and LysoTracker Green DND-26, a fluorescent acidotropic probe. Capacitation status has been observed through calcium-mediated changes using chlortetracycline (CTC) or by changes in membrane fluidity monitored by the binding of the fluorescent amphiphilic probe, Merocyanine 540. Fluorescently labeled annexin-v, C6NBD and Ro can also be used to detect changes in membrane phospholipid distribution. Cell viability can be determined using the propensity of propidium iodide (PI), ethidium homodimer-1 (EthD-1) or Yo-Pro-1 to permeate damaged membranes. These are generally more adaptable to clinical flow cytometry than the bisbenzimide membrane impermeable stain, Hoechst 33258, which excites in the ultraviolet range and requires UV laser equipment. Mitochondrial function can be determined using rhodamine 123 (R123) and MitoTracker Green FM (MITO) and 5,50,6,60-tetrachloro-1,10,3,30-tetraethylbenzimidazolylcarbocyanine iodide (JC-1). Many of the techniques mentioned above have yet to be adapted or applied to camelid sperm. However, given the limitations on filed assessment of fertility, they may prove useful for its laboratory assessment. Many of these techniques are most efficiently applied using flow cytometry, which is a tool that may also be used in the future to monitor many new potential markers of sperm function. This technology is only now being introduced, to a limited extent, to commercial artificial breeding laboratories for cattle AI, so there is some time before it may be applied in camelids beyond research. Nevertheless, a new method has been developed as part of this project to reliable identify alpaca sperm acrosome status, using a readily available fluorochrome, which could, in the future, be assessed using flow cytometric techniques. For the moment, the method is now available for use by practitioners in field laboratories using standard fluorescence microscopy. 82
98 5. Liquefaction of alpaca semen 5.1. Introduction The highly viscous nature of camelid semen represents a further challenge to the development of AI technology. Many researchers cite the viscous nature of camelid semen as the major hindrance which prevents the development of semen preservation and AI technology. Viscous semen is not unique to camelids, in fact it is common among mammals, although the mechanisms appear to vary widely (Robert and Cagnon 1999). The physiological significance of coagulating semen is still under discussion in the literature, and the formation of a vaginal plug in some species is believed to be essential in preventing outflow of semen from the female s vagina (rodents, primates) or cervix (pig). In rodents, the plug is thought to prevent subsequent insemination of sperm by non-dominant males. Vaginal plugs may also represent a reservoir and contribute to the gradual release of sperm in species where the female does not have a long cervix and cervical mucus, and semen is deposited directly in to the uterus (Robert and Cagnon 1999). Following ejaculation, human semen spontaneously coagulates into a semi-solid gelatinous mass. Liquefaction is also spontaneously in normal human semen, owing to the presence of chymotrypsin (Cohen and Aafjes 1982), trypsin (Mortimer 1994) and a chymotrypsin-like protease secreted from the prostate, known as prostate-specific antigen (PSA; Robert and Cagnon 1999). However, a proportion of human semen samples are hyperviscous. The clinical significance of altered (i.e. hyperviscous) semen viscosity remains uncertain. However, Mortimer (1994) suggests that it may be associated with a reduction in human fertility by virtue of its impairment of sperm movement and, therefore, penetration of cervical mucus. Furthermore, obtaining high sperm yields (recovery rates) from hyperviscous semen samples remains a major problem (Mortimer 1994). Hyperviscous human semen samples have been liquefied by mechanical and enzymatic methods. Mechanical methods include needling (the passing of semen back and forth through a needle), mixing viscous semen with culture medium, loading on to a Percoll (or Puresperm ) gradient and centrifuging (known as gradient density centrifugation). Mixing of the culture medium with the semen is achieved by swirling the container, non-vigorous agitation or gentle pipetting through a wide-bore serological of Pasteur pipette. Viscous human semen has been treated with hydrolytic enzymes such as bromelain (Tucker et al. 1990), chymotrypsin (Tucker et al. 1990; Bollendorf et al. 1994), papain (Pattinson et al. 1990b), subtilisin (Pattinson et al. 1990b) and trypsin (Cohen and Aafjes 1982). Trypsin was employed in early studies but workers have more recently favoured Chymotrypsin. Enzymatic methods have been used previously to liquefy alpaca semen (Bravo et al. 1999; Callo et al. 1999; Bravo et al. 2000b). Bravo et al. (2000) used collagenase, fibrinolysin, hyaluronidase and trypsin for liquefaction of alpaca and llama semen and reported few detrimental effects, while others observed enzyme treatment to be highly toxic to camel sperm (Skidmore, pers. comm.). Needling (Santiani et al. 2005) and other methods have been used also to liquefy alpaca semen prior to liquid or frozen preservation but the authors have failed to quantify the effects on semen viscosity, sperm motility and sperm functional integrity, or have not reported their results adequately. Proteolytic enzymes that have been used to treat semen may have detrimental effects on sperm motility, function and integrity. Before enzyme liquefaction of alpaca semen could be applied in a commercial setting, studies specifically investigating the effects of various liquefaction methods on sperm function and viability must be conducted. Parameters such as: Appropriate treatment time: during semen collection, or added post-collection in the laboratory Enzyme: Bromelain, Chymotrypsin, Collagenase, Hyaluronidase, Papain, Trypsin Enzyme concentration Exposure time: min Method of enzyme removal, if necessary 83
99 The experiments presented in this chapter aimed to develop an efficient method of liquefying alpaca semen. A number of different mechanical methods, such as centrifugation, gradient centrifugation, needling and pipetting, were examined. Investigations of enzymatic methods included the collection of semen into enzyme-supplemented diluent, and the addition of various concentrations of enzymes after semen collection. In addition, the effect of exposure time on semen viscosity, sperm motility and functional integrity was assessed Materials and Methods Unless otherwise specified, semen was collected for the experiments presented in this Chapter using an AV fitted inside a mannequin as described in Chapter Centrifugation For centrifugation, semen was diluted 1:4 (v:v, semen:diluent) with Androhep and centrifuged at 600 g for 7 min. The supernatant was then removed (and discarded) and the pellet resuspended in 500 μl Androhep. Sperm motility, acrosome integrity and concentration were assessed as described in Chapter Gradient centrifugation Separation of the motile sperm from the immotile sperm and seminal plasma using PureSperm gradients is routinely used in other species such as humans, cattle, horses, pigs and sheep. PureSperm gradients are constructed as described in Figure 5.1. Figure 5.1. Schematic diagram of PureSperm gradients from For most species the semen is layered onto gradients made with 90 : 45 or 80 : 40 % PureSperm. Gradient centrifugation has not been previously applied to alpaca semen and our preliminary studies demonstrated that the standard 90 : 45% gradient may not be the optimal proportion for the separation of alpaca sperm. Both the proportions and volume of each layer alter sperm recovery rate. An experiment was performed to compare gradients made up of 90 and 45 %, and 45 and 22.5 % PureSperm with 0.5 or 1.0 ml layers. For the experiment, ml of diluted semen was layered on top of a PureSperm gradient and centrifuged at 600 g for 20 min. The pellet was then resuspended in 200 µl Androhep. Sperm motility, acrosome integrity and concentration were assessed as described in Chapter 2. 84
100 Needling Semen was loaded into a 2.5 ml syringe attached to a small gauge (26 30) needle. The plunger was placed in the syringe and pressure applied, forcing the semen through the needle. Sperm motility, acrosome integrity and concentration were assessed as described in Chapter Pipetting Semen was collected (n=8 males; n=36 ejaculates) and liquefied by gentle pipetting. Gentle pipetting was performed using a 1 ml pipette during dilution with handling medium. Semen was mixed by depressing and releasing the plunger on a 1 ml Finpette (in a similar way to that used to remove cumulus cells from matured oocytes in preparation for in vitro fertilisation). After initial medium addition, semen was clearly visible as an opaque blob within the diluent. Gentle pipetting was ceased when the opaque blob was no longer visible. Sperm motility and acrosome integrity were then assessed as described in Chapter Collection of semen into enzyme supplemented diluent Semen was collected (n=11 males; n=33 ejaculates) using a mannequin fitted with an AV containing 500 µl of modified Salamon s diluent containing no enzymes (Control), Papain or Chymotrypsin in the collecting vessel. Mating length, semen viscosity, semen volume, sperm motility, concentration, acrosome integrity and morphology were assessed as described in Chapter Enzyme addition after semen collection Semen was collected (n=4 males; n=12 ejaculates) and semen volume, semen viscosity, sperm motility, sperm concentration, acrosome integrity and morphology were assessed immediately after collection Semen was then diluted 1:1 (semen:diluent) with Androhep containing either 1, 2, 4 or 8 mg ml -1 Papain, Collagenase or Trypsin (final enzyme concentrations 0.5, 1.0, 2.0 and 4.0 mg ml -1 ). Semen viscosity, sperm motility and acrosome integrity were then assessed at 5, 10, 20 and 40 min after enzyme addition as described in Chapter Removal of enzymes after seminal plasma viscosity reduction A further experiment was conducted to determine the effectiveness of PureSperm gradients to remove enzymes and seminal plasma after enzyme treatment. Semen was collected (n=11 males; n=33 ejaculates) using a mannequin fitted with an AV containing 500 µl of modified Salamon s diluent containing no enzymes (Control), Papain or Chymotrypsin in the collecting vessel. Mating length, semen viscosity, semen volume, sperm motility, concentration, acrosome integrity and morphology were assessed as described in Chapter 2. Enzyme treated semen was processed through a 45 : 22.5 % PureSperm gradient, as described above. Sperm motility, acrosome integrity and concentration were assessed as described in Chapter Results Centrifugation Removal of the supernatant (containing the seminal plasma) was difficult and resulted in turbulence which disrupted the sperm pellet. Sperm motility was similar before (50.0 ± 5.8 %) and after (66.7 ± 6.7 %) centrifugation and ± 33.4 % of sperm were recovered. While centrifugation may be a viable option, removing the supernatant takes considerable care and precision and there is the potential to lose significant numbers of sperm. 85
101 Gradient centrifugation (PureSperm ) The results clearly show that sperm motility and sperm recovery rates were higher using a gradient based on 45 and 22.5 % than 90 and 45 % PureSperm layers (Table 5.1). There was no significant difference for sperm motility and recovery rate using between 0.5 or 1.0 ml layers of 45 : 22.5% PureSperm. Table 5.1. Sperm motility and recovery rate after centrifugation through PureSperm gradients consisting of 90 : 45, and 45 : 22.5% PureSperm layers of 0.5 or 1.0 ml. Data are mean ± SEM, values within a rows (A,B) or a column (a,b) with a different superscript are significantly different (P<0.05). PureSperm gradient (%) Layer volume (ml) Pre- PureSperm Sperm Acrosome motility integrity (%) (%) Sperm motility (%) Post- PureSperm Acrosome Sperm recovery integrity rate (% 1 ) (%) ± 6.5 B,a 82.9 ± 3.6 a 17.3 ± 5.5 a 90: ± 4.7 B,a 83.0 ± 3.5 a 19.1 ± 4.9 a 41.8 ± 6.5 A 86.2 ± ± 5.5 A,b 88.8 ± 1.4 a 68.5 ± 9.8 b 45 : ± 6.5 A,b 81.9 ± 2.2 a 70.2 ± 14.1 b 1 Sperm recovery rate was calculated by dividing the total number of sperm recovered from the pellet after centrifuging by the total number of sperm layered on top of the PureSperm gradient Needling Needling was not efficient at reducing semen viscosity. It was not considered a viable method for liquefying alpaca semen as liquefaction was not complete and semen parameters were altered Pipetting Semen viscosity, sperm motility and acrosome integrity prior to and after liquefaction of semen by gentle pipetting are presented in Table 5.2. After gentle pipetting, sperm motility was increased compared with non-pipetted controls. In most cases there was a decrease in the proportion of sperm displaying oscillatory motion, and an increase in forward progressive motion, after pipetting. There was no significant difference in sperm acrosome integrity after pipetting and dilution in Androhep, compared with unpipetted semen, but semen viscosity decreased and sperm motility increased. Table 5.2. Semen characteristics after semen liquefaction by pipetting and dilution with Androhep. Data are mean ± SEM, values across columns (before and after pipetting) with a different superscript are significantly different (P<0.05). Diluent Semen viscosity (mm) Before dilution Sperm motility (%) Acrosome integrity (%) After pipetting and dilution Semen viscosity (mm) Sperm motility (%) Acrosome integrity Androhep 11.7 ± 1.8 a 48.6 ± 3.2 a 92.4 ± ± 0.3 b 62.8 ± 1.4 b 89.4 ± 0.9 (%) 86
102 Effect of enzyme treatment during semen collection Mating length, semen volume, sperm motility and sperm concentration were similar (P>0.05) between treatments. Semen viscosity was significantly reduced (P<0.05) by collecting into containers with diluent containing Papain (Table 5.2) compared with the Control and Chymotrypsin. Table 5.2. Semen characteristics after the collection of alpaca semen into containers with diluent (Control) containing Papain or Chymotrypsin. Parameter Control Chymotrypsin Papain Mating length (min) 17.0 ± ± ± 4.2 Semen volume (ml) 1.1 ± ± ± 0.1 Semen viscosity (mm) 10.8 ± 3.0 a 11.3 ± 4.9 a 1.3 ± 1.0 b Sperm concentration (x10 6 /ml) ± ± ± 44.7 Total no. sperm in ejaculate (x10 6 ) 99.6 ± ± ± 14.7 Sperm motility (%) 40.5 ± ± ± 7.1 Acrosome integrity (%) 85.0 ± 2.6 a 74.0 ± 12.3 a 41.6 ± 14.3 b Morphologically normal (%) 56.6 ± 3.4 a 68.8 ± 5.3 b 64.6 ± 5.5 b Head abnormalities (%) 2.4 ± ± ± 0.4 Midpiece abnormalities (%) 5.6 ± ± ± 1.4 Tail abnormalities (%) 14.6 ± 2.9 a 8.2 ± 2.4 b 7.3 ± 1.9 b Cytoplasmic droplets (%) 17.4 ± ± ± Enzyme addition after semen collection Both Papain and Trypsin were effective at reducing semen viscosity but Papain was more effective at lower concentrations (Table 5.3). Sperm motility was severely reduced by Collagenase treatment but was not affected by Papain and Trypsin treatment (Table 5.4). Interestingly, it was observed that motility changed from oscillatory to forward progressive after the breakdown of the seminal plasma. Table 5.3. Semen viscosity (mm) after treatment of neat semen with 0.5, 1.0, 2.0 and 4.0 mg ml -1 Collagenase, Papain and Trypsin. Data are mean ± SEM, values within a row (A,B) or column (a,b) with a different superscript are significantly different (P<0.05). Enzyme Conc Times (min) Control 3.3 ± 0.8 a,a 3.3 ± 0.7 a,a 1.8 ± 0.5 a,b 1.4 ± 0.5 a,b,b Collagenase ± 0.8 a,a 2.4 ± 0.8 a,b,a 2.2 ± 0.7 a,a 1.7 ± 0.7 a,a Collagenase ± 0.7 a,a 1.9 ± 0.7 b,a 1.7 ± 0.7 a,b,b 1.4 ± 0.7 a,b,b Collagenase ± 0.6 a,b,a 1.9 ± 0.6 b,a 1.8 ± 0.7 a,b,a,b 0.8 ± 0.5 a,b,b Collagenase ± 0.6 c,a,b 2.1 ± 0.7 a,b,a 0.8 ± 0.4 b,c,b 0.3 ± 0.2 b,c,b Papain ± 0.4 c,d,a 0.0 ± 0.0 c,a 0.0 ± 0.0 c,a 0.0 ± 0.0 c,a Papain ± 0.3 c,d,a 0.0 ± 0.0 c,a 0.0 ± 0.0 c,a 0.0 ± 0.0 c,a Papain ± 0.4 d,a 0.3 ± 0.3 c,a 0.5 ± 0.5 c,a 0.6 ± 0.6 b,c,a Papain ± 0.0 d,a 0.7 ± 0.5 c,a 0.0 ± 0.0 c,a 0.0 ± 0.0 c,a Trypsin ± 0.8 a,a 2.8 ± 0.6 a,b,a 2.0 ± 0.7 a,a,b 1.5 ± 0.5 a,b Trypsin ± 0.8 a,a 3.1 ± 0.8 a,a 2.5 ± 0.8 a,a 1.2 ± 0.6 a,b,b Trypsin ± 0.6 a,a 2.3 ± 0.7 a,b,a 1.6 ± 0.6 a,b,a 0.4 ± 0.3 b,c,b Trypsin ± 0.3 b,a 1.7 ± 0.5 b,a 0.6 ± 0.3 c,b 0.2 ± 0.2 b,c,b 87
103 Table 5.4. Sperm motility (%) after treatment of neat semen with 0.5, 1.0, 2.0 and 4.0 mg ml -1 Collagenase, Papain and Trypsin. Data are mean ± SEM, values within a row (A,B) or column (a,b) with a different superscript are significantly different (P<0.05). Enzyme Conc Time (min) Control 49.0 ± 3.6 a,a 32.3 ± 4.1 a,a 16.9 ± 4.5 a,b 8.7 ± 2.8 a,b Collagenase ± 1.1 b,a 0.0 ± 0.0 b,b 0.0 ± 0.0 b,b 0.0 ± 0.0 b,b Collagenase ± 1.2 b,a 0.6 ± 0.6 b,b 0.7 ± 0.7 b,b 0.4 ± 0.4 b,b Collagenase ± 0.6 c,a 0.0 ± 0.0 b,b 0.0 ± 0.0 b,b 0.0 ± 0.0 b,b Collagenase ± 0.4 d,a 0.0 ± 0.0 b,a 0.0 ± 0.0 b,a 0.0 ± 0.0 b,a Papain ± 3.2 a,a 30.4 ± 3.8 a,a 16.5 ± 4.2 a,b 7.4 ± 2.3 a,c,e,b Papain ± 4.0 a,a 27.3 ± 2.9 a,a,b 16.7 ± 4.4 a,b,c 8.8 ± 2.5 a,c,e,c Papain ± 3.2 a,a 25.8 ± 4.7 a,a,b 15.0 ± 4.3 a,b 7.7 ± 3.0 a,c,e,c Papain ± 4.1 a,a 26.3 ± 4.7 a,a,b 15.4 ± 3.4 a,b 10.2 ± 2.6 a,e,b Trypsin ± 2.8 a,a 28.9 ± 4.0 a,a,b 15.8 ± 3.8 a,b 8.3 ± 3.1 c,d,c Trypsin ± 3.2 a,a 25.0 ± 4.2 a,a,b 12.9 ± 3.2 a,b 7.3 ± 2.7 c,d,c Trypsin ± 3.0 a,a 23.8 ± 3.2 a,a,b 14.2 ± 2.6 a,b,c 7.4 ± 1.4 c,e,c Trypsin ± 2.9 a,a 25.6 ± 4.2 a,a,b 17.3 ± 3.3 a,a,b 10.0 ± 1.7 e,b Table 5.5. Sperm acrosome integrity (%) after treatment of neat semen with 0.5, 1.0, 2.0 and 4.0 mg ml -1 Collagenase, Papain and Trypsin. Data are mean ± SEM, values within a row (A,B) or column (a,b) with a different superscript are significantly different (P<0.05). Enzyme Conc Time (min) Control 90.6 ± 1.9 a,a 86.2 ± 2.4 a,b,a 87.7 ± 3.5 a,a 76.7 ± 6.7 a,b,b Collagenase ± 2.0 a,a 87.2 ± 2.7 a,b,a 87.2 ± 3.5 a,a 79.3 ± 6.7 a,b,a Collagenase ± 2.3 a,a 89.7 ± 1.4 a,a 75.9 ± 9.8 a,b,a 75.7 ± 8.7 a,b,a Collagenase ± 2.6 a,a 89.0 ± 1.8 a,b,a 90.9 ± 2.5 a,a 81.1 ± 6.1 a,b,a Collagenase ± 2.7 a,a 85.0 ± 5.4 a,b,a 88.2 ± 3.0 a,a 90.1 ± 1.4 a,a Papain ± 8.5 a,b,a 36.2 ± 8.7 b,c,a 41.0 ± 9.0 a,b,a 29.3 ± 9.9 b,a Papain ± 9.7 a,b,a 40.7 ± 10.1 c,a 37.3 ± 8.3 b,c,a 12.7 ± 5.4 b,b Papain ± 8.8 a,b,a 15.9 ± 8.9 d,b 25.4 ± 8.1 c,b 16.3 ± 7.2 b,a,b Papain ± 9.1 b,a 18.0 ± 8.8 d,b 20.6 ± 11.3 d,b 5.6 ± 4.4 c,c Trypsin ± 2.5 a,a 85.3 ± 2.6 a,b,a 75.4 ± 5.9 a,a 74.6 ± 5.6 a,b,a Trypsin ± 3.7 a,a 88.3 ± 1.5 a,a 86.2 ± 4.0 a,a 70.1 ± 9.2 a,b,a Trypsin ± 10.2 a,b,a 78.9 ± 10.5 a,b,c,a 73.9 ± 10.2 a,b,a 73.9 ± 10.6 a,b,a Trypsin ± 12.3 a,b,a 63.2 ± 11.0 a,b,c,a 65.0 ± 13.0 b,a 62.7 ± 12.3 b,a Removal of enzymes after seminal plasma viscosity reduction Sperm motility after Puresperm gradient processing and sperm recovery rates were similar for all treatments (Table 5.6) suggesting the gradients may be an efficient method of removing enzymes from semen. 88
104 Table 5.6. Sperm motility and recovery rate after centrifugation of enzyme-treated diluted semen through 45: 22.5 % PureSperm gradients. Enzyme Pre-PureSperm Sperm Acrosome motility (%) integrity (%) Post-PureSperm Sperm Acrosome motility (%) integrity (%) Sperm recovery rate (% 1 ) Control 38.3 ± ± 2.6 a 48.3 ± ± 3.7 a 46.0 ± 11.5 Chymotrypsin 41.0 ± ± 12.3 a 52.0 ± ± 6.2 b 49.3 ± 12.4 Papain 37.5 ± ± 14.3 b 52.5 ± ± 4.7 c 53.2 ± Sperm recovery rate was calculated by dividing the total number of sperm recovered by the total number of sperm layered on top of the PureSperm gradient Discussion A number of mechanical and enzymatic methods for reducing semen viscosity were evaluated. Centrifugation was not effective for separating sperm from the viscous seminal plasma as the turbulence created during sperm removal resulted in significant loss of cells and only 60 % were recovered. Furthermore, different centrifugation times and speeds would be required for ejaculates of different viscosities. Given the variation in viscosity between males and ejaculates, centrifugation would become a costly and time-consuming procedure in a commercial artificial breeding setting. Mortimer (1994) emphasises that centrifugal pelleting of unselected sperm populations must be avoided under all circumstances where the sperm are to be tested for their function or used for therapeutic purposes. Moreover, Ferre et al. (2000) observed that centrifugation was deleterious to llama sperm motility (not cent: 31.6 %, cent: 26.6 %) and 1 hr (not cent: 25.0: cent: 5.0 %). Gradient centrifugation using 45: 22.5 % PureSperm was more effective in recovering sperm from the viscous seminal plasma than the 90: 45 % gradients traditionally used for the separation of boar, cattle, and sheep sperm. No differences were detected when the volume of the gradient was varied. Therefore, as the cost of Puresperm is very high (approx. $2000 per litre), all subsequent experiments were conducted using 0.5 ml gradients. Constructing gradients is time-consuming and requires a high level of precision, otherwise the layers merge and the effectiveness of the gradient is reduced. Coupled with the cost of the Puresperm, this precludes the commercial application of the procedure at present. Recovery rates for alpaca sperm after Puresperm gradient processing were comparable to other species, but only 70 % of the sperm were recovered and, given the low number of sperm in alpaca ejaculates, loss of approximately 30 % represents a significant reduction in the number of cells available for preservation. Given these disadvantages, several methods of in-situ viscosity reduction such as needling, pipetting and vortexing were next examined. Needling was ineffective for reducing the viscosity of alpaca semen, despite previous reports to the contrary (Santiani et al. 2005). Needling has been successful for partial liquefaction of human semen, although it is not recommended for samples where sperm will be assessed for functionality or integrity (Mortimer 1994) as the high shear forces experienced by sperm are deleterious and result in a marked deterioration in sperm motility (Knuth et al. 1986). Vortexing and pipetting break the seminal plasma down in situ (in the presence of the sperm). One important difference between these methods is the potential for the loss of significant numbers of sperm during the removal of the seminal plasma. Vortexing of alpaca semen does not reduce semen viscosity and may be highly detrimental to alpaca sperm (Vaughan et al. 2003a), so we did not investigate it as a method of reducing semen viscosity in the current project. However, gentle pipetting of semen diluted with Androhep was effective in reducing semen viscosity without compromising sperm acrosome integrity or sperm motility. This is considered the simplest and most effective method 89
105 available to date for viscosity reduction, although it is not completely effective and does not remove any other detrimental effects of the seminal plasma which is still present after the procedure. Collection of semen into Tris diluent containing the proteolytic enzyme papain significantly reduced semen viscosity but the same procedure with medium containing chymotrypsin was not effective. Collection of semen into media containing an enzyme did not affect sperm motility but acrosome integrity was reduced for samples collected into Tris containing papain. Similarly, when papain was added to neat semen after collection semen, viscosity was eliminated almost immediately, sperm motility was not compromised but acrosome integrity was reduced. The reduction in semen viscosity and acrosome integrity occurred in a dose dependant manner, with less acrosome damage occurring when 0.5 mg/ml of papain was added to the semen. Compared with papain, collagenase and trypsin did not cause as much damage to sperm acrosomes nor were they as effective in reducing semen viscosity. Sperm motility declined over time for all treatments including the control but collagenase had an instantaneous effect on sperm motility with those in the majority of samples being immobilised almost immediately after addition of the enzyme. Trypsin has less effect on sperm motility than collagenase, and less effect on acrosome integrity, but was not as efficient at reducing semen viscosity as papain. Removal of papain after collection of semen into Tris diluent containing papain was hypothesised as a way of reducing the damage to the sperm acrosome but this was ineffective as the data showed that the damage had already occurred (Table 5.4). These results suggest that collection of semen into diluent containing enzymes is not a suitable method for reducing semen viscosity in alpacas. Removal of enzymes after a shorter exposure to papain, such as addition of papain in the laboratory, may be more effective. Damage to sperm membrane integrity was observed after as little as 10 min exposure to the enzymes (Table 5.5) suggesting that the exposure period to papain must be less than this. Addition of papain to neat semen (or semen collected in Tris diluent, Chapter 3) followed by immediate centrifugation and resuspension in diluent (without enzymes) might be an effective method of reducing semen viscosity but it is time consuming and labour intensive. The results of our studies examining the effect of enzymes on alpaca semen viscosity contradict those of Bravo et al. (1999) and Bravo et al. (2000b). Their studies used trypsin (0.3, 0.6, 1.25 and 2.5 % solutions; Bravo et al. 1999), collagenase (1, 5, 10 and 20 mg/ml; Bravo et al and 0.25 mg/ml; Bravo et al. 2000b), fibrinolysin (Bravo et al. 2000b) and hyaluronidase (Bravo et al. 2000b) to liquefy alpaca and/or llama semen. Bravo et al. (2000b) reported that all concentration of collagenase and trypsin reduced semen viscosity and Bravo et al. (1999) reported that once semen was in contact with the enzymes, viscosity was eliminated. Bravo et al. (2000b) reported that, despite reduction in semen viscosity, sperm motility remained oscillatory after enzyme addition and did not display progressive motility. Our results, in the contrary, indicated that sperm motility became more progressive as a consequence of enzyme treatment. This could be explained by the presence of a sperm motility inhibitor in alpaca semen, similar to that present in human semen (Robert and Gagnon 1995). This 52 kda seminal plasma sperm motility inhibitor (SMI) is present at ejaculation and rapidly transformed into smaller masses during liquefaction (Robert and Gagnon 1994). The presence of a SMI in alpaca semen has yet to be confirmed, although it could have resulted from an evolutionary need to maintain the sperm in a quiescent state from ejaculation until ovulation takes place. Other studies examining the effects of enzymes have reported a number of interesting, and often contradictory findings. Deen et al. (2003) added α-chymotrypsin to camel semen and reported that motility was not increased but failed to quantify the effects on seminal plasma viscosity or sperm ultra structure. Hyperviscous human semen samples are routinely treated with chymotrypsin and no differences have been observed in sperm motility, membrane integrity or morphology between samples liquefied by dilution and pipetting or treatment with chymotrypsin (Zavos et al. 1997), but (Shinohara et al. 1985) demonstrated that chymotrypsin treatment of hamster sperm accelerated the 90
106 sperm acrosome reaction. Treatment of human semen with chymotrypsin reduced penetration of zona free hamster oocytes (Pattinson et al. 1990b) but did not reduce the pregnancy rate after intrauterine insemination (IUI; Tucker et al. 1990) and increased the in vitro fertilizing ability of viscous human semen (Cohen and Aafjes 1982). Unfortunately, chymotrypsin was not effective in reducing semen viscosity in the concentrations examined in the present study. Treatment of Capuchin money semen with trypsin and hyaluronidase did not cause any adverse effects on sperm motility, vigour and acrosome integrity, but enzymes only partially liquefied the semen (Rodrigues de Paz et al. 2006). Treatment of bovine sperm with trypsin adversely affected sperm motility (Wheeler and Seidel 1989; Silva et al. 1999) and accelerated the acrosome reaction of hamster sperm (Shinohara et al. 1985). Penetration of human sperm into zona free hamster oocytes was not reduced by trypsin treatment (Pattinson et al. 1990b) and treatment of sperm with trypsin has been observed to increase oocyte penetration (Dandekar and Gordon 1975; Cohen and Aafjes 1982). A dose dependent reduction in sperm vitality was observed after incubation with papain (Pattinson et al. 1990a). Concentrations over 25 U/mL reduced sperm motility and papain treatment altered cell membranes (measured by eosin exclusion; Pattinson et al. 1990a) and papain treated (50 U/ml) sperm displayed impaired penetration of zona-free hamster oocytes (Pattinson et al. 1990b). Of the enzymes tested by Pattinson et al. (1990a,b), trypsin exhibited the most restricted specificity of the enzymes, being directed towards lysyl and arginyl residues, chymotrypsin was also quite specific in hydrolysing phenylalanyl tyrosyl and tryptophanyl residues. The activity of papain is broader than either trypsin or chymotrypsin, being capable of cleaving the peptide bonds adjacent to most amino acids. This may explain the strong efficacy of papain to liquefy semen in the present study, and to release sperm motility from the bonds of its containment by the viscous gel. The interval between semen collection and liquefaction in vitro necessitates the development of a method to liquefy alpaca semen. Various method of semen liquefaction exist, although the method of choice must be reliable, easy (in a commercial setting), cost and time-effective and not reduce sperm viability or function. In this project, enzyme treatment had some effects on sperm function although the nature and degree of the effect varied according to the enzyme used. Despite the deleterious effect of papain on sperm integrity, papain was highly effective in reducing semen viscosity. Given the restrictions on animal-derived products in diluents designed for in vivo use, the use of non-animal enzymes may be beneficial. Papain is derived from plants and remains effective at lower concentrations. Thus, perseverance in determining the optimum concentration, exposure time and removal methods is warranted. The favourable outcomes demonstrated by the use of papain also proved that the viscous component of seminal plasma was highly susceptible to liquefaction by proteolytic enzymes, thus proving that it has a major protein component. It has been reported in the literature that the viscous semen is composed of sulphated mucopolysaccharides secreted by the bulbourethral gland during the rutting season (El-Naggar and Abdel-Raouf 1977). Based on the lack of evidence for this claim, and in addition to studies determining the optimal use of proteolytic enzymes such as papain, we believe that studies are urgently required to confirm the findings of El-Naggar and Abdel-Raouf (1977). Further studies are also required to identify the components responsible for the viscosity before effective, efficient and commercially viable methods for the liquefaction of alpaca semen can be developed. 91
107 6. Liquid-storage of alpaca sperm 6.1. Introduction Artificial insemination (AI) facilitates the widespread use of genetics from superior sires, thereby increasing the rate of genetic gain and is an important tool for animal breeding programs. There is considerable interest in the use of AI in camelid breeding programs. At present the lack of efficient methods to preserve the fertilising lifespan of semen in liquid or frozen form prevent the widespread use of AI in camelids. Successful semen storage is dependant on the reversible reduction in metabolic activity and motility, which prolongs the fertilising lifespan of sperm. This can be achieved by cryopreserving or liquid storing sperm. Cryopreservation of sperm in camelids is inefficient, primarily owing to a lack of knowledge regarding camelid sperm physiology and the viscous nature of the seminal plasma (Bravo et al. 2000c). As camelid sperm are generally not tolerant of freezing and thawing procedures, liquid storage of sperm may facilitate the development of AI technology. There have been few reports on the liquid storage of camelid semen and the optimal conditions for liquid storage remain to be elucidated. Studies published to date have generally reported poor results (Dromedary camel: Hassan et al. 1995; Deen et al. 2004; llama: Huanca and Gauly 2001; Giuliano et al. 2006b). However, pregnancies have been achieved after AI with liquid-stored semen in Dromedary camels (Skidmore 2005) and llamas (Giuliano et al. 2006a) but the efficiency is still below commercially acceptable levels. During liquid storage, the metabolic activity of sperm is reduced by storage at a reduced temperature, in a slightly acidic environment, gassing diluents with CO 2 or N 2 under anaerobic conditions (Vishwanath and Shannon 2000). However, optimal storage conditions are species-specific, reflecting differences in sperm physiology and preferences for ph buffers, diluent tonicity, energy sources, tolerance to cold shock and susceptibility to oxidative damage. These parameters have yet to be thoroughly investigated for alpacas and development of the optimal method of liquid storage will depend on first determining the optimal diluent, storage temperature and dilution rate. The aim of the work presented in this chapter was to investigate the effects on sperm function of i) different diluents (Androhep, Biladyl, Lactose, Salamon s and Triladyl ) and storage temperatures (4 and 15 C), ii) dilution rate (1:1, 1:2, and 1:4 v:v) and interactions between dilution rate and storage temperature, and iii) removal of seminal plasma and dead sperm prior to liquid storage. We also iv) aimed to determine if epididymal alpaca sperm could be stored in a liquid state Materials and methods Procedures described herein were approved by The University of Sydney s Animal Ethics Committee and performed in accordance with the Australian Code of Practice for Care and Use of Animals for Scientific Purposes Animals Male alpacas (n=16; 7.03 ± 0.6 years old) were maintained under field conditions at The University of Sydney s Animal Reproduction Unit, Cobbitty, NSW, Australia for the duration of the experimental period (June to November 2006). Alpacas were maintained on native pastures, exposed to natural day length and ambient temperatures and supplemented with Lucerne hay (Medicago sativa) and commercially formulated alpaca pellets as required. 92
108 Reagents and media Chemicals used were of analytical grade and, where possible, cell culture tested by the manufacturer. All media components were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise stated. Androhep, Biladyl (fraction A only) and Triladyl (Minitüb, Tiefenbach, Germany) were reconstituted according to the manufacturers instructions. Lactose diluent consisted of 11 % (w:v) lactose as described by Morton et al. (2007a). Salamon s diluent was modified from that described by Evans and Maxwell (1987) and consisted of 300 mm Tris (hydroxymethyl)aminomethane, 27.8 mm fructose, 94.7 mm citric acid. All diluents were prepared using tissue-culture grade water filtered (0.22 μm, Millipore). Diluents were then supplemented with 20 % (v/v) hens egg yolk, and ultracentrifuged (10,000 g; 30 min). Diluents were then aliquoted (10 ml) and stored frozen at 20 C until use (within 8 weeks) Semen collection Semen was collected as previously described by Morton et al. (2007a). Briefly, semen was collected using a rubber sheep AV (IMV Technologies, L Aigle, France) with a silicone liner and a camel collecting glass (IMV Technologies). The artificial vagina (AV) was then wrapped in an electric heating pad (Bodyzone TM Heat Pad HP700; Breville, Botany, NSW, Australia), mounted inside the wooden mannequin and placed in the semen collection pen. A male alpaca was then allowed to enter the pen and mate with the mannequin. After mating ceased, the AV was removed from the mannequin. Viscous semen contained within the AV was forced into the collecting glass by repeated, sharp downwards thrusts. Collecting glasses were stored in a 35 C water bath until evaluation and dilution (within 10 min of collection) Semen evaluation and assessment of sperm characteristics Ejaculate volume, semen viscosity, sperm concentration, sperm motility, acrosome status and morphology were then determined on neat semen samples. Semen evaluation and sperm assessments were performed as described by Morton et al. (2007a). Graduations on the collecting glass were used to determine ejaculate volume and the thread formation technique (Bravo et al. 2000b) was used to evaluate semen viscosity. Briefly, 50 μl neat semen were removed from the ejaculate using a micropipette and approximately 25 μl was pipetted on to a glass slide. A thread of semen was formed by slowly raising the pipette. The distance between the pipette and the slide at the time the thread broke was measured using a ruler and recorded as the viscosity. Sperm motility patterns were classified subjectively as either oscillatory or progressive. Sperm travelling at greater than one body length per second were considered to display forward progressive motility, and sperm which did not display forward motility were considered oscillatory. Sperm motility was estimated subjectively to the nearest 5 % by examining five fields of the neat or diluted semen sample (10 μl) placed on a pre-warmed slide using phase contrast microscopy (X 100, Olympus, Tokyo, Japan; Evans and Maxwell, 1987). Sperm concentration was determined using a haemocytometer as described by Evans and Maxwell (1987). Acrosome integrity was assessed by fluorescent isothiocyanate-conjugated peanut agglutin (FITC- PNA) as described by Morton et al. (2007a). Briefly, an aliquot of sperm was smeared on a slide, air dried and fixed for 30 sec in 96 % (v:v) ethanol. Slides were then stored at 4 C for 2-4 wk. Immediately before assessment slides were stained with 100 μg/ml FITC-PNA (Sigma-Aldrich) in phosphate buffered saline (Sigma-Aldrich) for 30 min in a humid 37 C atmosphere. Slides were then rinsed with PBS to remove excess stain and placed in the dark at 37 C to dry. Fade retardant consisting of 90 % (v:v) glycerol (Sigma-Aldrich), 10 % (v:v) PBS (Sigma-Aldrich) and 0.1 % (w:v) p-phenylenediamine (Sigma-Aldrich) was then placed on the stained area of the slide and covered with a cover slip. Sperm were considered acrosome intact if the acrosome stained green while those with no 93
109 staining or a single band of green staining at the equatorial segment were considered as having nonintact acrosomes Pilot study Semen was collected from alpaca males (n = 8; 2 ejaculates per male) and assessed for quality parameters. Semen was then divided into three aliquots and left neat or diluted 1:1 or 1:2 (v:v, semen:diluent) with Androhep (without clarified egg-yolk). Aliquots of neat or diluted semen were placed in a beaker of 35 C water (same temperature as the semen) and liquid stored in a portable fridge (approx. 15 C) as displayed in Figure 6.1. Sperm motility was examined immediately after dilution and one and two hours after the onset of liquid storage Effect of diluent and temperature Semen was collected from alpaca males (n = 6; 4 ejaculates per male) and assessed for quality parameters. Semen was then divided into five aliquots and diluted 1:4 (v/v) with Androhep, Biladyl, Lactose, Salamon s and Triladyl diluents. Dilution was performed drop wise, and after the addition of approximately the same volume of diluent as the semen (1:1, v/v dilution), parts of the viscous semen were clearly visible as opaque masses within the diluted semen. Viscous semen was gently pipetted with a 1.0 ml pipette to break up the viscous semen and facilitate proper mixing of the diluent. Pipetting ceased when the opaque mass was no longer visible which coincides with a reduction in semen viscosity (Chapter 5). Aliquots of diluted semen were divided into two parts which were stored at 4 and 15 C. Sperm motility and acrosome integrity were assessed immediately after collection, dilution (0 h) and 24, 48, 72 and 96 h after liquid storage Effect of dilution rate Semen was collected from alpaca males (n = 4; 3 ejaculates per male) and assessed for quality parameters. Semen was then divided into three aliquots and diluted as described above 1:1, 1:2 and 1:4 (v:v, semen:diluent) with Biladyl. Diluted semen was then divided into two parts which were stored at 4 and 15 C. Sperm motility and acrosome integrity were assessed immediately after collection, dilution (0 h) and 24, 48, 72 and 96 h after liquid storage Effect of seminal plasma removal Semen was collected (n=3 males; n=3 ejaculates) and semen volume, semen viscosity, sperm motility, sperm concentration, acrosome integrity and morphology were assessed immediately after collection Semen was then diluted 1:4 with Biladyl and divided into three aliquots: control (dilution only), washed (dilution, centrifugation and resuspension in Biladyl ), PureSperm gradient processed (dilution, PureSperm processing, washing and resuspension in Biladyl. For washing, sperm were centrifuged at 300 g for 10 min, the supernatant (containing diluent and seminal plasma) was removed and discarded. The pellet, containing motile and immotile sperm was then resuspended to the same final volume as the control samples with Biladyl. For PureSperm processing, diluted sperm were layered on top of a 22.5 : 45 % Puresperm gradient and centrifuged (600 g; 20 min) as described in Chapter 5. The sperm pellet was the resuspended in 500 μl Biladyl and washed (as described above). Diluted sperm were then stored at 15 C for 48 h. Sperm motility, acrosome integrity and morphology were examined at 0, 24 and 48 h after the onset of liquid storage. 94
110 Liquid storage of epididymal sperm Based on the results of Experiments 1 and 2, a pilot study was conducted to investigate the liquid storage of epididymal alpaca sperm. Testes and epididymides and testes from castrated alpaca males (n=10, age: 32.5 ± 1.8 mo) were transported overnight to the laboratory using the method described by Morton et al. (2007a). Briefly, testes and epididymides were wrapped in gauze, which had previously been soaked in phosphate buffered saline (Sigma, St Louis, MO, USA), and placed in a foam esky on top of a cold pack for transport to the laboratory overnight. Upon arrival at the laboratory, the epididymides were cleaned, excess tissue was removed and the surface blood vessels were punctured and the blood wiped away. Epididymides were then minced with a scalpel and the sperm were allowed to swim out for 30 min into 4.0 ml warm Androhep (AH; Minitüb, Tiefenbach, Germany) in 60 mm Petri dishes (Bacto Laboratories, Liverpool, Australia) placed on heated (37 C) stages. Sperm suspensions were centrifuged (300 g; 10 min), the supernatant removed and the resultant pellet resuspended to a final volume of 1 ml with Biladyl (as described in Experiment 1 and 2 and cooled to 4 C over 2 h (-0.14 C/min). Cooled sperm were then stored at 4 C for 24 h. Sperm motility and acrosome integrity were assessed immediately after collection (post harvest) and 24 h after the onset of liquid storage Variation between males Semen was collected from 10 adult alpaca males (A - J) and liquid-stored during a variety of experiments. Data were then pooled by male and used to generate box plots. Box plots were chosen to display results as a large volume of information can be clearly conveyed in a simple diagram. The box plots display the variation between ejaculates from the same male (intramale variation), and between ejaculates from different males (intermale variation). Box plots are a convenient diagrammatic method to represent groups of data and are based on five values the smallest observation, lower quartile, median, upper quartile and the largest observation. The box contains the middle 50 % of the data and the upper and lower edges of the box indicate the 75 th (upper) and 25 th (lower) percentile of the data while the line inside the box represents the median value. The vertical lines (or whiskers) extend beyond both edges of the box as far as the minimum and maximum values. The distance between the various parts of the box are used to help indicate variation in a population. Maximum value observed Upper edge 75 th percentile Median value Lower edge 25 th percentile Minimum value observed Figure 6.1. Sample box plot (from 95
111 Statistical analysis Statistical differences were determined after logit transformation of sperm motility and acrosome integrity data. Data were analysed using a linear mixed model (REML) procedure and means compared using least significant differences (LSD) in GenStat (Release 7.2, Ceanet, Brisbane, Australia) with P<0.05 considered significant Results Pilot study The first attempts to store alpaca sperm in Androhep in a portable refrigerator were based on a protocol developed for the transport of boar semen at The University of Sydney (Figure 6.2). a b Figure 6.2. Portable refrigerator (a) used to for liquid storage, b) diluted alpaca sperm in refrigerator. Preliminary experiments were conducted to establish the appropriate dilution rate. Alpaca semen was stored neat or after dilution (1:1 or 1:2) with Androhep but this was largely unsuccessful. Preliminary attempts to store alpaca sperm in a portable refrigerator in a medium such as Androhep without egg yolk resulted in a rapid loss of motility with sperm dying within two to three hours after the onset of storage (Table 6.1). Table 6.1. Motility of alpaca sperm during cooling to 15 C Dilution rate Sperm motility (%) 0 h 2 h Neat (control) 26.1 ± ± 1.7 1: ± ± 2.9 1: ± ± 6.0 The results from the pilot study suggested that 1:2 dilution is superior to 1:1 dilution, and that further investigation of dilution rates was warranted. Generally, semen from other species stored at 15 C is not supplemented with egg yolk but the rapid loss in alpaca sperm viability suggested that the inclusion of egg yolk in the medium might be beneficial. Moreover, the temperature of the portable refrigerator varied, and the decision was made to use a standard 15 C refrigerator routinely used to store pig semen at the University of Sydney (Figure 6.3). 96
112 Figure 6.3. Refrigerator used to store alpaca sperm at 15 C Effect of diluent and temperature Data for the motility of sperm liquid-stored at 4 or 15 C after dilution in Androhep, Biladyl, Lactose, Salamon s, and Triladyl are presented in Table 6.2. Interactions between the diluent and time (P<0.001) and temperature and time (P<0.001) on sperm motility were observed but there was no interaction between diluent and temperature (P=0.13). Overall, there was a trend for sperm motility to be higher after storage at 4 C compared with 15 C (P=0.089) and motility of liquid-stored sperm was higher after storage in Biladyl than in all the other diluents. Motility was similar for sperm liquid-stored in Triladyl and Lactose, but was reduced for Androhep and Salamon s diluents (P<0.05). Motility had declined for sperm stored at 4 and 15 C in Androhep, Lactose, Salamon s and sperm stored at 15 C in Triladyl by 24 h after the onset of liquid storage (P<0.05). By contrast, sperm motility did not decline until 48 h for sperm stored at 4 and 15 C in Biladyl, and 4 C in Triladyl (P<0.05). Motility was similar for sperm stored at 4 and 15 C at 24 and 48 h but was higher at 72 and 96 h for sperm stored at 4 C (P<0.05). Table 6.2. Motility of ejaculated alpaca sperm after storage at 4 or 15 C for up to 96 h after dilution in Androhep, Biladyl, Lactose, Salamon s or Triladyl diluents. Data are mean ± SEM, values within a row (A,B) or column (a,b) with a different superscript are significantly different (P<0.05). Diluent Androhep Biladyl Lactose Salamon s Triladyl Storage time (h) Temp ( C) ± 1.6 B,a 5.3 ± 0.2 B,a 2.1 ± 1.1 C,a 1.5 ± 1.1 C,a 48.6 ± 2.7 A,a ± 2.9 B,b 8.6 ± 1.8 B,a,b 0.0 C,b 0.0 C,b ± 3.3 A,c 16.2 ± 2.9 B,b 6.5 ± 2.4 C,c 1.9 ± 1.9 D,a,b 53.3 ± 2.2 A,a ± 3.9 A,b,c 14.8 ± 2.6 B,b 6.1 ± 3.0 C,c 0.4 ± 0.4 D,a,b ± 2.6 B,b 10.0 ± 2.1 B,a,b 2.9 ± 1.6 C,a,c 0.4 ± 0.4 D,a,b 39.5 ± 2.2 A,a ± 2.9 B,b,c 9.3 ± 2.2 C,a,b 1.4 ± 1.4 D,b 0.4 ± 0.4 D,a,b ± 1.2 B,a 4.9 ± 0.1 B,a 0.0 C,b 0.0 C,b 48.1 ± 2.2 A,a ± 2.8 B,a,b 6.7 ± 1.2 B,a,b 0.4 ± 0.4 C,b 0.0 C,b ± 3.9 A,b,c 12.9 ± 2.8 B,a,b 2.1 ± 1.2 C,a,c 0.8 ± 0.8 D,a,b 52.6 ± 1.7 A,a ± 3.9 B,b,c 11.2 ± 2.8 C,a,b 0.4 ± 0.4 D,b 0.0 D,b Data on the acrosome integrity of sperm liquid stored at 4 or 15 C after dilution in Androhep, Biladyl, Lactose, Salamon s, and Triladyl are presented in Table 6.3. There was no effect of 97
113 diluent or temperature on acrosome integrity, although interactions were detected between the effects of diluent and time (P=0.005) and temperature and time (P<0.001). Overall, a decline in acrosome integrity was not observed until 72 h after the onset of liquid storage (P=0.05) and the decline was similar for sperm liquid stored at 4 or 15 C. Table 6.3. Acrosome integrity of ejaculated alpaca sperm stored at 4 or 15 C for up to 96 h in Androhep, Biladyl, Lactose, Salamon s or Triladyl diluents. Data are mean ± SEM, values within a row (A,B) or column (a,b) with a different superscript are significantly different (P<0.05). Diluent Temp ( C) Storage time (h) Androhep ± 1.3 A,a 87.5 ± 3.1 A,a 59.3 ± 5.9 B,a 48.0 ± 20.5 B,a,b 88.4 ± 1.4 A,a ± 0.9 A,a 88.0 ± 2.3 A,a 57.0 ± 19.8 B,a 50.3 ± 7.9 B,a Biladyl ± 1.7 A,a 78.4 ± 3.0 A,a 74.0 ± 15.0 A,b 62.0 ± 12.0 A,a 90.8 ± 1.4 A,a ± 1.8 A,a 80.1 ± 3.5 A,a 55.5 ± 19.1 B,a,b 38.0 ± 0.0 B,b Lactose ± 1.4 A,a 85.0 ± 2.7 A,a 50.0 ± 0.0 B,a 46.0 ± 21.6 B,a,b 85.3 ± 3.4 A,a ± 4.3 A,a 79.8 ± 3.7 A,a 56.3 ± 8.6 a,b nd Salamon s ±2.3 A,a 85.0 ± 2.2 A,a 9.0 ± 0.0 B,c nd 87.5 ± 1.4 A,a ± 5.8 A,a 82.5 ± 3.2 A,a 5.0 ± 0.0 B,c nd Triladyl ± 3.5 A,a 84.6 ± 2.9 A,a 47.0 ± 23.7 B,a nd 91.9 ± 1.3 A,a ± 1.1 A,a 81.4 ± 3.5 A,a 25.0 ± 24.0 B,a nd Nd = not determined due to slide damage Effect of dilution rate Data for the motility of sperm liquid-stored at 4 or 15 C after 1:1, 1:2, or 1:4 (v:v) dilution in Biladyl, are presented in Table 6.4. Sperm motility was affected by dilution rate and storage time (P<0.001) but not by storage temperature (P=0.79), and there were no interactions. Motility was higher for liquid-stored sperm after a 1:4 than a 1:2 or 1:1 dilution, and after 1:2 than 1:1 dilution (P<0.05). For all dilution rates and temperatures, sperm motility declined (P<0.05) by 24 h after the onset of liquid storage. Table 6.4. Motility of ejaculated alpaca sperm diluted 1:1, 1:2, or 1:4 (v:v) with Biladyl and liquid stored at 4 or 15 C for 48 h. Data are means ± SEM. Data are mean ± SEM, values within a row (A,B) or column (a,b) with a different superscript are significantly different (P<0.05). Dilution rate 1:1 1:2 1:4 Temp ( C) Storage time (h) ± 3.2 B,a 11.8 ± 3.8 B,a,b 2.6 ± 2.1 C,a 47.8 ± 4.2 A,a ± 3.9 B,b 16.4 ± 4.1 B,a 3.1 ± 1.8 C,a,b ± 3.3 A,a 34.5 ± 4.0 B,b,c 19.7 ± 4.7 C,a 6.6 ± 3.1 D,a,b ± 4.2 B,b 21.2 ± 5.0 C,a 6.3 ± 2.8 D,a,b ± 3.5 B,c 31.1 ± 5.4 C,b 9.2 ± 3.9 D,b ± 3.1 A,a 39.8 ± 3.4 B,c 25.4 ± 5.2 B,a,b 7.8 ± 3.5 C,a,b Data on the acrosome integrity of sperm liquid-stored at 4 or 15 C after 1:1, 1:2, or 1:4 (v/v) dilution in Biladyl are presented in Table 6.5. There was no influence of dilution rate or storage temperature 98
114 and no interactions. Sperm acrosome integrity declined with time (P=0.005) and was reduced 72 h after the onset of liquid storage. Table 6.5. Acrosome integrity of ejaculated alpaca sperm diluted 1:1, 1:2, or 1:4 (v/v) with Biladyl and liquid stored at 4 or 15 C for 48 h. Data are mean ± SEM, values within a row (A,B) or column (a,b) with a different superscript are significantly different (P<0.05). Dilution rate 1:1 1:2 1:4 Temp ( C) Storage time (h) ± 1.7 A,a 80.2 ± 2.0 A,a 60.5 ± 2.4 A,a 86.7 ± 2.7 A,a ± 1.8 A,a 78.7 ± 2.2 A,a 56.2 ± 2.2 B,a ± 1.8 A,a 79.4 ± 2.0 A,a 52.0 ± 7.8 B,a ± 1.4 A,a 86.2 ± 2.7 A,a 76.9 ± 3.2 A,a 58.8 ± 2.2 A,a ± 1.1 A,a 78.7 ± 2.4 A,a 68.2 ± 1.9 A,a ± 2.1 A,a 88.9 ± 1.6 A,a 77.4 ± 3.0 A,a 54.5 ± 7.8 A,a Effect of seminal plasma removal Data on the motility of alpaca sperm immediately after dilution, PureSperm gradient centrifugation or washing, and after 24, 48 and 72 h liquid storage are presented in Table 6.6. Immediately after processing and at all stages of liquid storage (24, 48 and 72 h) motility was similar for semen diluted, PureSperm gradient centrifuged and washed. Table 6.6. Mean ± SEM motility (%) for ejaculated alpaca sperm after dilution (Control), washing (Wash) or PureSperm gradient (PureSperm ) processing prior to liquid storage at 15 C. Data are mean ± SEM, values within a row (A,B) or column (a,b) with a different superscript are significantly different (P<0.05). Treatment Storage time (h) Dilution 46.3 ± 3.7 A,a 28.3 ± 10.9 A,a 4.3 ± 1.6 B,a 0.8 ± 0.8 C,a PureSperm 46.7 ± 4.5 A,a 20.0 ± 5.0 B,a 1.7 ± 1.7 B,a 0.7 ± 0.7 C,a Wash 45.0 ± 9.3 A,a 18.7 ± 5.8 B,a 0.0 ± 0.0 C,a 0.0 ± 0.0 C,a Acrosome integrity data immediately after dilution, PureSperm gradient centrifugation or washing, and after 24, 48 and 72 h of liquid storage are presented in Table 6.7. Immediately after processing and at all stages of liquid storage (24, 48 and 72 h) sperm acrosome integrity was similar for semen diluted, PureSperm gradient centrifuged and washed. 99
115 Table 6.7. Acrosome integrity of ejaculated alpaca sperm after dilution (1:4, v:v) of neat semen (Dilution), or sperm centrifuged (washed), or processed through a PureSperm gradient (PureSperm ) to remove the seminal plasma prior to liquid storage. Data are mean ± SEM, values within a row (A,B) or column (a,b) with a different superscript are significantly different (P<0.05). Treatment Storage time (h) Dilution 91.5 ± 4.5 A,a 88.7 ± 2.3 A,a 77.0 ± 0.0 A,B,a 66.3 ± 2.0 B,a PureSperm 91.3 ± 1.9 A,a 87.2 ± 8.0 A,a 75.0 ± 0.0 A,a 64.4 ± 4.0 A,a Wash 87.0 ± 3.6 A,a 78.0 ± 9.0 A,a 69.0 ± 4.0 A,a 61.2 ± 2.0 B,a Liquid storage of epididymal sperm Motility and acrosome integrity data for epididymal alpaca sperm after harvest and storage at 4 C are presented in Tables 6.8 and 6.9, respectively. Motility and acrosome integrity were similar immediately after harvest and 24 h after the onset of liquid storage (P>0.05). Table 6.8. Motility of epididymal alpaca sperm liquid stored in Biladyl at 4 C for h. Data are single observations for males, and means ± SEM are presented in the overall row. Male Motility (%) post harvest liquid stored Pooled Overall 53.0 ± ± Sperm were pooled from the supernatants after centrifugation of sperm from individual males 100
116 Table 6.9. Acrosome integrity of epididymal alpaca sperm liquid stored in Biladyl at 4 C for h. Data are single observations for males, and means ± SEM are presented in the overall row. Male Acrosome integrity (%) post harvest liquid stored Pooled Overall 92.3 ± ± Sperm were pooled from the supernatants after centrifugation of sperm from individual males Variation between males Data on the motility of sperm from alpaca males (A - J) immediately after dilution, and at 24, 48 and 72 h of liquid storage are presented in Figures 6.4, 6.5, 6.6, and 6.7, respectively. Considerable differences in sperm motility both within and between males was observed at all time points (Figures ) indicating a high level of variability. Sperm motility was less variable in some males (E and G) compared with other males (B and C) and survival during liquid storage was longer for some males (A, D, F, H, and J) compared with other males (B, C, G, and I). Despite these differences, sperm samples from some males were observed to have more than 40 % progressive motility after 72 h of liquid storage (males A, F and H). Figure 6.4. Motility of ejaculated alpaca sperm from 10 males (A J) immediately after dilution, prior to liquid storage. 101
117 Figure 7.5. Motility of ejaculated alpaca sperm from 10 males (A J) after liquid-storage for 24 hours. Figure 6.6. Motility of ejaculated alpaca sperm from 10 males (A J) after liquid-storage for 48 hours. 102
118 Figure 6.6. Motility of ejaculated alpaca sperm from 10 males (A J) after liquid-storage for 72 hours. 6.4 Discussion The results presented in this chapter demonstrate that alpaca semen can be liquid stored at 4 or 15 C. Sperm retained acceptable rates of motility after 48 h of liquid storage and remained motile for 96 h (data not presented). Biladyl was the most suitable of the diluents tested, and dilution at a rate of 1:4 with this medium produced superior results compared with lower dilution rates. Furthermore, alpaca epididymal sperm were liquid-stored at 4 C for 24 h without a loss of motility or acrosome integrity. Variation in the effectiveness of diluents in preserving the longevity and fertilising capacity of sperm during liquid storage can be attributed to their different constituents. Three of the diluents examined in this study were Tris-based (Biladyl, Triladyl, and Salamon s), while Androhep and Lactose media were based on Hepes (a zwitterionic buffer) and the sugar lactose, respectively. Tris-based diluents are commonly used for the liquid storage of bull (Vishwanath and Shannon 2000), buck (Leboeuf et al. 2000) and ram semen (Evans and Maxwell 1987), and have been used for liquid storage of alpaca (Vaughan et al. 2003a), camel (Vyas et al. 1998; Deen et al. 2004; Wani et al. 2005; Niasari-Naslaji et al. 2006a) and llama sperm (Ratto et al. 1999; Giuliano et al. 2006b). Sperm motility was higher after storage in Biladyl compared with Androhep, Lactose, Salamon s or Triladyl. Interestingly, the survival of sperm stored in Biladyl, Triladyl and Salamon s diluents differed markedly, despite the similarities in their constituents. Salamon s diluent comprises 300 mm Tris (hydroxymethyl)aminomethane, 94.7 mm citric acid and 27.8 mm fructose while Biladyl is a two-step extender based on Triladyl, comprising 100 mm Tris, 33 mm citric acid, 28 mm fructose solution with antibiotics. This suggests that the reduced Tris and citric acid in Biladyl and Triladyl may be responsible for the improvement in longevity for sperm stored in these media compared to Salamon s diluent. 103
119 In contrast to the results of the present study, Vaughan et al. (2003a) concluded that Triladyl was superior to Biladyl for liquid storage of ejaculated alpaca semen. However, full details of the experiment and analyses were not presented. Vyas et al. (1998) concluded that Tris was superior to Lactose diluent for the liquid storage of Dromedary camel semen at 5 C, and Deen et al. (2004) concluded that Tris was superior to Biociphos, as sperm remained motile for up to 96 h in the former compared with only 24 h for the latter medium. Survival of Dromedary camel sperm in Tris-tes egg yolk and Tris-lactose egg yolk was reported to be superior to that in citrate egg yolk, sucrose egg yolk and Tris-fructose egg yolk diluents (Wani et al. 2005). Moreover, Niasari-Naslaji et al. (2006a) observed that Tris and Camel Green Buffer (zwitterionic buffered, IMV USA) resulted in superior motility of liquid stored Bactrian camel sperm compared with lactose and sucrose diluents. Liquid storage of llama semen at 5 C was superior after dilution in Lactose diluent compared with Tris, PBS (supplemented with 40 % llama serum) and skim-milk diluents (Giuliano et al. 2006b). Taken together, these results suggest that the survival of camelid semen is generally better after storage in Tris diluents than diluents based on other organic buffers (such as citrate), sugars (glucose, lactose or sucrose) and milk proteins. However, as mentioned previously, the amount of Tris in the Tris based diluents confounds comparison, particularly as the amount is often not specified. Further studies are clearly required to determine the optimal levels of Tris, and other constituents in diluents for the liquid storage of alpaca semen. Until the present study, the longevity of camelid semen liquid-stored at different temperatures had not been investigated. Previous studies have stored the semen at 5 C only (alpaca: Vaughan et al. 2003a; Bactrian camel: Niasari-Naslaji et al. 2007; Dromedary camel: Deen et al. 2004; llama: Ratto et al. 1999; Huanca and Gauly 2001; Giuliano et al. 2006a,b) or failed to comment on sperm survival after Dromedary camel epididymal sperm were liquid stored at 4 and 23 C (Wani et al. 2005). The results of the present study demonstrate successful liquid storage of alpaca semen at 15 C. Furthermore, storage of alpaca sperm for up to 48 h was equally efficient at 4 and 15 C. A temperature of 4 C is recommended for storage periods exceeding 48 h, although the survival of liquid stored alpaca sperm beyond 48 h was poor in the present study. Survival of llama sperm after 72 h of liquid storage has been demonstrated previously by Huanca and Gauly (2001) and Ratto et al. (1999) who utilised ejaculated and epididymal samples, respectively. Moreover, liquid stored Dromedary camel epididymal sperm retained motility for 8 days during liquid storage at 4 and 23 C. In the present study, alpaca sperm were still motile after 96 h of liquid storage. While the mean motility was low (< 5 %), the motility of individual samples ranged between 0 and 20 % demonstrating high levels of variation between males. Motilities of 50 % or more were observed in individual samples after 72 h of liquid storage, which is encouraging given the lack of research investigating the liquid storage of alpaca and other camelid semen. However, improvements to the longevity of liquid stored semen are required and its fertilizing capacity is yet to be determined under the storage conditions developed in this study. Various dilution rates have been utilised in the liquid storage of camelid semen. Llama semen is most frequently diluted 1:1 (Huanca and Gauly 2001; Giuliano et al. 2006a,b) whilst Bactrian and Dromedary camel semen have been diluted 1:10 (Niasari-Naslaji et al. 2006a) and 1:3 (Vyas et al. 1998; Deen et al. 2004), respectively. However, no direct comparisons have been made. The results from our study demonstrate that survival of alpaca semen after 1:4 dilution is superior to a 1:2 and 1:1 dilution. These results are in agreement with the anecdotal observations of Vaughan et al. (2003a) who suggested a 1:4 dilution for alpaca semen, and the observations of Rigby et al. (2001) who concluded higher dilution rates were beneficial for stallion semen, and reduced the negative effects on the survival of liquid stored stallion sperm (Jasko et al. 1991). Increased dilution rates, above 1:4, may be beneficial although further research is required to confirm this. In a series of experiments, Niasari-Naslaji et al. (2006a) demonstrated the impact of diluent osmolarity and ph of the survival of Bactrian camel sperm stored at 4 C. Tris diluents with an osmolarity of mosm/kg provided acceptable sperm survival whilst those with osmolarities of 290 or 390 mosm/kg yielded poor survival rates. Furthermore, sperm liquid stored in Tris diluent with a ph of 104
120 6.9 were motile 24 h after the onset of storage, whereas a ph of 5.5, 6, 7.5, 7.9 or 8.9 resulted in a loss of sperm motility between 4 and 12 h after the onset of liquid storage. The increased survival of Bactrian camel sperm under slightly acidic conditions (ph 6.9 at the onset of liquid storage) would be expected, given the relationship between sperm metabolism and ph identified by Koellikker (1856; cited by Vishwanath and Shannon 2000). Interestingly, storage of alpaca semen at slightly acidic conditions in Androhep or Lactose diluents did not improve sperm survival in the present study. However, adjustment of the ph of the optimum diluent from the present study (Biladyl ) was not investigated, and this medium might be more beneficial for alpaca sperm longevity during liquid storage if the ph were made slightly acidic. Sperm motility declined throughout liquid storage in the present study with the most prominent reduction in motility occurring during the first 24 h, agreeing with Giuliano et al. (2006b) who observed a significant reduction to llama sperm motility during 24 h of liquid storage. Vaughan et al. (2003a) observed high sperm motility (45 50 %) for sperm liquid stored for 24 h in Biladyl and Triladyl but the authors did not disclose the initial motility of the samples thereby preventing a comparison of the loss in motility during the first 24 h of storage. Vaughan et al. (2003a) also reported 10 and 5% reductions in sperm motility between 24 and 48 h of liquid storage in Biladyl and Triladyl, respectively. This is similar to the results of the present study, where a 15 % reduction in sperm motility was observed between 24 and 48 h of liquid storage. Interestingly, the loss in motility between harvesting and 24 h of liquid storage was minimal for epididymal sperm in the present study. Previously, epididymal alpaca sperm have been stored in lactose diluent at 4 C for 24 h with less than a 5 % reduction in sperm motility (Chapter 7). Ratto et al. (1999) also reported motilities of % for epididymal llama sperm stored for 72 h at 5 C. These results demonstrate that epididymal alpaca and llama sperm, unlike ejaculated sperm, tolerate liquid storage procedures, owing to the exposure of the latter to the viscous seminal plasma. Epididymal alpaca sperm also tolerate freezing and thawing better than ejaculated sperm, retaining approximately % of the original motility after thawing (Morton et al. 2007a). Furthermore, epididymal alpaca sperm which were transported overnight to the laboratory, liquid stored at 4 C for 24 and then frozen and thawed, retained % of their original motility (Chapter 7) demonstrating their robustness. The recent use of epididymal models to develop semen preservation techniques in alpacas has challenged perceptions about alpaca sperm and further highlighted the viscous seminal plasma as a major hindrance to the development of assisted reproductive technologies in alpacas and other camelids. In summary, the research presented in this chapter demonstrated successful liquid storage of ejaculated alpaca semen at both 4 and 15 C, and epididymal sperm at 4 C. Dilution of semen at 1:4 in Biladyl for liquid storage is advantageous whilst storage temperatures of 4 or 15 C for periods up to 48 h yielded similar results. However, a temperature of 4 C is recommended for storage periods greater than 48 h. Despite these advances, further research is required to improve the longevity and determine the fertilizing capacity of liquid stored ejaculated alpaca semen. 105
121 7. Cryopreservation of alpaca sperm 7.1. Introduction Artificial insemination is widely used in cattle, horse, sheep and pig breeding and production programs. Semen cryopreservation is a valuable tool for the preservation of genetic resources. It has facilitated the widespread use of AI in cattle, horses and sheep and permits the widespread dissemination of superior genetic material. While there is considerable interest in the application of AI technology in camelids, this is limited as semen cryopreservation has not been well developed (Bravo et al. 2000c). Attempts to apply semen cryopreservation and AI methods developed for other livestock to camelids have met with little success owing to their difficult reproductive physiology, particularly the length of copulation which makes semen collection challenging. Camelid semen is generally of poor quality and is highly viscous making handling, dilution and assessment problematic and there is little knowledge of camelid sperm physiology. Previous attempts to cryopreserve alpaca sperm have met with little success. A number of parameters affect the efficiency of semen cryopreservation. These include the diluent (buffer, sugar/energy source and other additives such as Equex STM paste), cryoprotectant (type and concentration), length of exposure of sperm to the cryoprotectant, temperature of cryoprotectant exposure, packaging during freezing and rate of freezing/thawing. Glycerol is the most commonly used cryoprotectant (Holt 2000) and is often the first examined when establishing sperm cryopreservation procedures for new species. The optimal level of glycerol in semen freezing diluents is highly species-specific, ranging from 1.75 % for murine to 20 % for marsupial sperm (Holt 2000). The majority of studies examining the cryopreservation of camelid sperm have employed media and procedures which have a final glycerol concentration of 6-7 % (alpacas: Santiani et al. 2005; Bactrian camels: Zhao et al. 1996; Niasari-Naslaji et al. 2007; llamas: Aller et al. 2003). However, poor postthaw recovery combined with the loss of the majority of initial (pre-freeze) motility during cryopreservation (alpacas: Valdivia et al. 2000; Vaughan et al. 2003a; Santiani et al. 2005; Dromedary camels: Hassan et al. 1995; llamas: McEvoy et al. 1992a; Burgel et al. 2000, 2001) suggests that the current methods for cryopreserving camelid semen are suboptimal and further research is required. An efficient method of cryopreserving epididymal alpaca sperm has been developed recently which utilises a final glycerol concentration of 3 % (Morton et al. 2007a) suggesting that in previous experiments using of 6-7 % glycerol, the optimal level required by alpaca sperm may have been exceeded. However, critical studies examining the glycerol concentration during the cryopreservation of alpaca sperm have yet to be undertaken. Sodium dodecyl sulphate (SDS) in the form of Equex STM and Orvus ES paste has been used as an additive to cryodiluents to enhance the protective effect of egg yolk by breaking down the lipid and making it more accessible to the sperm membrane (Pontbriand et al. 1989). SDS in the diluent has proved beneficial for the cryopreservation of sperm from a number of domestic and wildlife species (Holt 2000). While SDS has been used for cryopreservation of alpaca (Morton et al. 2007a), camel (Musa et al. 1992) and llama sperm (Von Baer and Hellemann 1999), its effects on alpaca sperm function during and after cryopreservation have not been critically examined. While the advantages of utilising frozen semen in breeding programmes are clear, considerable research is required to determine the optimal freezing and thawing process. Successfully cryopreservation and thawing of ejaculated alpaca semen were achieved during RIRDC project AAA- 1A although it was noted that the viscous nature of alpaca semen precludes even mixing of semen with extender, ensures marked heterogeneity of the solution to be cryopreserved and may explain poor 106
122 post-thaw motility rates in camelids compared with species with less viscous semen. Clearly, the viscous nature of the seminal plasma and the resulting heterogeneity of the semen solution reduce the overall success of freezing and thawing semen and influences interpretation of the results. Therefore, epididymal sperm, which have not been exposed to the viscous seminal plasma, were chosen to investigate the foundations of the freezing and thawing process. Epididymal sperm, which tolerate cryopreservation and retain a high proportion of initial motility after freezing and thawing have been used as a model for establishing and refining cryopreservation methods for alpaca semen (Morton et al. 2007a). Using the epididymal sperm model, we re-examined some of the areas identified as requiring further research during RIRDC project AAA-1A. These included: defining males that have semen that consistently freezes well and the variation that exists amongst males in this respect defining ejaculates before processing that will have good freezability the relative benefits of adding surfactant/other compounds to the extender to reduce frothing of semen in the AV, and facilitate more efficient interaction with sperm plasma membranes (ie allow better mixing of semen with extender) types of extender best suited to alpacas addition of buffer to control egg yolk fluctuations and carbon dioxide absorption final glycerol percentage of extended semen equilibration time of glycerol and semen prior to freezing whether there is a need to dilute the concentration of glycerol after thawing to reduce its toxic effects on sperm. freezing semen in straws/pellets of different volumes height of freezing above liquid nitrogen (ie rate of freezing) These areas were divided into two groups, reflecting those which constituted the primary and secondary aspects of the freezing and thawing protocol. The primary aspects are those, which are used to establish a good working procedure whilst the secondary aspects are those that are used to refine, or improve, the procedure. Primary considerations when establishing a freezing procedure are: the diluent (based on Tris, citrate, lactose, or skim-milk) packaging type (pellets or straws and size of pellets or straws) freezing rate (determined by pellet size, straw size and height above liquid nitrogen) final percentage of glycerol (ranging from 3 25 % depending on species) The use of cryoprotectants other than glycerol and the selection of a non-animal based protein (not milk or egg yolk) were classified as secondary aspects to be considered after a robust freezing and thawing procedure was established. The addition of glycerol at physiological or room temperature was not investigated as glycerol is not required for survival of alpaca sperm during cooling to, or liquidstorage at 4 C (Chapter 6). However, the addition of glycerol at higher temperatures (35 C) does not appear deleterious to alpaca sperm. The aims of the experiments presented in this Chapter were to develop an efficient method of cryopreserving alpaca sperm based on the highly efficient epididymal sperm model. Epididymal sperm could be used to create gene banks for vicuña and guanaco, which are listed as threatened species in Appendix II of CITES (2005), and for sperm from deceased high genetic value male alpacas and racing camels. A number of experiments were performed in order to establish an efficient method of cryopreservation. The experiments consisted of: Experiment 1: Comparison of different diluents Experiment 2: Comparison of freeze rates and packages Experiment 3: Comparison glycerol concentration Experiment 4: Comparison of Equex addition 107
123 7.2. Materials and methods Animals and experimental design Procedures described herein were approved by The University of Sydney s Animal Ethics Committee. For Experiment 1, epididymides and testes from castrated alpaca males (n=10, age: 32.9 ± 5.8 mo) were wrapped in gauze, which had previously been soaked in phosphate buffered saline (Sigma, St Louis, MO, USA), and placed in a foam esky on top of a cold pack for transport to the laboratory overnight. Epididymal sperm were then harvested, washed and divided into three aliquots for dilution in Citrate, Tris and Lactose based cryodiluents, cooled to 4 C and pellet-frozen. For Experiment 2, epididymides and testes from castrated alpaca males (n=10, age: 22.5 ± 0.9 mo) were transported overnight to the laboratory using the method described for Experiment 1. Harvested epididymal sperm were washed, diluted with Lactose cryodiluent and cooled to 4 C. Cooled sperm were then frozen as pellets or in 0.25 or 0.5 ml straws on dry ice, or in 0.25 or 0.5 ml straws over liquid nitrogen vapour. For both experiments, sperm from individual males were kept separate throughout all procedures and motility and acrosome integrity were assessed immediately after harvest, cooling to 4 C, and at 0 and 3 h after thawing. For Experiment 3, epididymides and testes from castrated alpaca males (n=11, age: 33.9 ± 40 mo) were transported overnight to the laboratory using the method described for Experiment 1. Harvested epididymal sperm were washed, diluted with Lactose cooling extender and cooled to 4 C. Cooled sperm were then further diluted (2:1, v:v) with Lactose cryodiluent containing 6, 9, or 12 % glycerol. Sperm were then frozen as pellets and stored in liquid nitrogen vapour. For Experiment 4, epididymides and testes from castrated alpaca males (n=10, age: 32.5 ± 1.8 mo) were transported overnight to the laboratory using the method described above. Harvested epididymal sperm were washed, diluted in Biladyl (part A, no glycerol), cooled, and stored at 4 C for h. Cooled sperm were then further diluted with Biladyl containing 6 % glycerol with or without 2 % Equex STM paste. Cooled sperm were then frozen as pellets and stored in liquid nitrogen. For all experiments, sperm from individual males were kept separate throughout all procedures (unless otherwise specified) and motility and acrosome integrity were assessed immediately after harvest, cooling to 4 C, and at 0 and 3 h after thawing Sperm harvesting Upon arrival at the laboratory, excess tissue was removed from the epididymides. The epididymides were thoroughly cleaned, surface blood vessels were punctured and the blood wiped away. Epididymides were then minced with a scalpel and the sperm were allowed to swim out for 30 min into 4.0 ml warm Androhep (AH; Minitüb, Tiefenbach, Germany) in 60 mm Petri dishes (Bacto Laboratories, Liverpool, Australia) placed on heated (37 C) stages Cryopreservation of epididymal sperm Comparison of different cryodiluents For Experiment 1, sperm were harvested from males (n=10, age: 32.9 ± 5.8 mo) and suspensions were centrifuged at 300 g for 10 min and the pellet resuspended in 1.5 ml AH. A two-step cryopreservation method was employed for Citrate and Lactose diluents, and a one-step method for the Tris based diluent. Sperm samples were divided into three aliquots and diluted 1:1 with Citrate or Lactose cooling diluents, or 1:2 with Tris freezing diluent. Citrate cooling diluent consisted of a 80.6 mm sodium citrate, 33.3 mm glucose solution supplemented with 20 % (v:v) hens egg yolk (Bravo et al. 1996). 108
124 Citrate freezing diluent consisted of the cooling diluent supplemented with 5% (v:v) glycerol. Lactose cooling diluent consisted of 11 % (w:v) Lactose solution supplemented with 20 % egg yolk. Lactose freezing diluent consisted of the cooling diluent supplemented with 9 % (v:v) glycerol and 1.5 % (v:v) Equex STM paste (IMV, L Aigle, France) as previously described by Bathgate et al. (2006). Tris cryodiluent consisted of 360 mm Tris, mm citric acid, and 33.3 mm glucose solution supplemented with 20 % egg yolk and 6 % glycerol (Evans and Maxwell 1987). All diluents were adjusted to have a final glycerol concentration of 3 % (v:v). Diluted sperm were cooled to 4 C over 2 h ( C/min). The Citrate and Lactose diluted sperm were further diluted 2:1 with Lactose freezing extender or 2:3 with Citrate freezing extender. Cooled sperm were then frozen as pellets (250 µl) on dry ice as described by Evans and Maxwell (1987) Effect of packaging and freeze rate on post-thaw survival For Experiment 2, sperm were harvested from males (n=10, age: 22.5 ± 0.9 mo) and frozen using the two-step Lactose method as described for Experiment 1. Briefly, sperm suspensions were centrifuged (300 g; 10 min) and the pellet resuspended to a final volume of 2 ml with cooling extender and cooled to 4 C over 2 h (-0.14 C/min). Cooled sperm were further diluted with 1.0 ml freezing extender and frozen as pellets (250 μl) on dry ice, or loaded into 0.25 ml or 0.5 ml straws and frozen on dry ice (5 min) or in liquid nitrogen vapour. For the later freezing method, straws were suspended 8.0 cm above the surface of the liquid nitrogen for 10 min, and then 4 cm above the surface for 5 min. Straws were then plunged into liquid nitrogen and stored until use Comparison of 2, 3, and 4 % final glycerol concentration For Experiment 3, sperm were harvested from males (n= 11, age: 33.9 ± 3.9 mo) and frozen using the two-step Lactose method as described for Experiment 1. Briefly, sperm suspensions were centrifuged (300 g, 10 min) and the pellet resuspended in 2.0 ml of 11 % (w;v) Lactose solution supplemented with 20 % egg yolk and cooled to 4 C over 2 h ( C/min). Diluted sperm were then divided into three aliquots and further diluted 2:1 (v;v) with Lactose freezing diluent which consisted of Lactose cooling diluent supplemented with 1.5 % (v;v) Equex STM paste (IMV, L Aigle, France) and either 6, 9 or 12 % (v;v) glycerol (Sigma-Aldrich; final glycerol concentration 2, 3 or 4 %). Cooled sperm were then frozen as pellets (250 µl) on dry ice as described by Evans and Maxwell (1987) and stored in liquid nitrogen Addition of Equex STM paste For Experiment 4, sperm were harvested from males (n=10, age: 32.5 ± 1.8 mo) and frozen in two steps using the tris-based medium Biladyl (Minitüb, Tiefenbach, Germany). Briefly, sperm suspensions were centrifuged (300 g; 10 min) and the pellet resuspended with 1.0 ml Biladyl (part A, no glycerol), cooled to 4 C over 2 h (-0.14 C/min) and stored at that temperature for h. Cooled sperm were then divided into two aliquots and further diluted with 1.0 ml Biladyl (part B, 6 % glycerol, 3 % final glycerol concentration) or Biladyl (part B) supplemented with 2.0 % (v:v) Equex STM paste (final Equex concentration 1 % v;v) and pellet-frozen as described as above Thawing of sperm Sperm frozen as pellets were thawed, two at a time, in clean glass test tubes by vigorous agitation in a 37 C water bath until the pellets melted. Straws were thawed by agitating in a 37 C water bath for 30 sec or 1 min for 0.25 ml and 0.5 ml straws, respectively. Thawed sperm were then washed by centrifugation (300 g; 10 min), the pellet resuspended in 0.5 ml warm Androhep and maintained at 37 C. 109
125 7.2.5 Assessment of sperm Subjective assessments of motility were made by placing a 10 μl aliquot of resuspended sperm on a pre-warmed slide and covering with a warmed coverslip as described by Evans and Maxwell (1987). Motility was examined using phase contrast microscopy (X 200, Olympus, Tokyo, Japan) immediately after harvest (post-harvest), cooling to 4 C, and immediately (0 h) and three h (3 h) after thawing. Acrosome integrity was assessed by fluorescent isothiocyanate-conjugated peanut agglutin (FITC- PNA) as described by Morton et al. (2007a) with modifications. Briefly, an aliquot of sperm was smeared on a slide and air dried. Slides were then stored at 4 C for 2-4 wk. Immediately before assessment slides were stained with 100 μg/ml FITC-PNA (Sigma; St Louis, MO, USA) in phosphate buffered saline (Sigma) for 30 min in a humid 37 C atmosphere. Slides were then rinsed with PBS to remove excess stain and placed in the dark at 37 C to dry. Fade retardant consisting of 90 % (v:v) glycerol, 10 % (v:v) PBS (Sigma) and 0.1 % (w:v) p-phenylenediamine was then placed on the stained area of the slide and covered with a cover slip. Sperm were considered acrosome intact if the acrosome stained green while those with no staining or a single band of green staining at the equatorial segment were considered as having non-intact acrosomes Statistical analysis Statistical differences in the proportion of motile sperm and intact acrosomes were determined by ANOVA after arcsine transformation of data and means compared by least significant differences (LSD) using GenStat (version 9, Ceanet, Brisbane, Australia) with P<0.05 considered significant Results Comparison of different cryodiluents Sperm were successfully harvested from the epididymides of all males. Opaque white fluid was observed leaking into the medium after mincing of the epididymides. The fluid was not viscous and the sperm therein displayed forward progressive motility (46.9 ± 4.5 %). Considerable variation was observed between the epididymides of different males. On average ± 22.2 x 10 6 sperm per male were recovered but this ranged from x Sperm motility also varied between males, ranging from %. After dilution and cooling to 4 C, motility was higher for sperm diluted in Lactose than Citrate or Tris diluents. Sperm motility was also higher immediately after freezing and thawing for sperm frozen in Lactose compared with Tris or Citrate diluents (P<0.05; Table 7.1) but was higher at 3 h post-thaw for sperm frozen in Lactose than Citrate but not Tris. Table 7.1. Motility of epididymal alpaca sperm after harvesting (post-harvest), cooling to 4 C (Cooled), and immediately (0 h) and three h (3 h) after freezing and thawing in Citrate, Lactose and Tris based diluents. Data are means ± SEM. Values with a different superscript are significantly different (P<0.05). Motility (%) Diluent Post-harvest Cooled Post-thaw 0 h 3 h Citrate 12.5 ± 3.8 a 6.9 ± 2.3 a 0.9 ± 0.6 a Lactose 46.9 ± ± 5.1 b 18.2 ± 5.7 b 3.2 ± 1.6 b Tris 18.8 ± 4.1 a 11.3 ± 3.0 a 1.4 ± 0.8 a,b 110
126 Acrosome integrity after harvesting was 90.6 ± 1.5 %, and was higher after cooling for sperm diluted in Lactose and Tris than for Citrate cryodiluents (Table 7.2). Immediately after thawing (0h post-thaw) acrosome integrity was higher for sperm frozen in Tris than Citrate cryodiluents but was not different for sperm frozen in Lactose. Three hours after thawing acrosome integrity was similar for sperm frozen in the three diluents. Table 7.2. Acrosome integrity of epididymal alpaca sperm after harvesting (post-harvest), cooling to 4 C (Cooled), and immediately (0 h) and three h (3 h) after freezing and thawing in Citrate, Lactose and Tris based diluents. Data are means ± SEM. Values with a different superscript are significantly different (P<0.05). Acrosome integrity (%) Diluent Post-harvest Cooled Post-thaw 0 h 3 h Citrate 86.4 ± 0.8 a 79.0 ± 1.0 a 75.0 ± 1.6 a Lactose 90.6 ± ± 0.5 b 80.0 ± 2.2 a,b 79.0 ± 0.9 a Tris 92.4 ± 1.1 b 83.4 ± 2.2 b 76.1 ± 1.4 a Effect of packaging and freeze rate on post-thaw survival As in Experiment 1, sperm were successfully harvested from the epididymides of all males, with a mean motility of 47.7 ± 5.0 % and ranged between % for individual males. On average ± 9.6 x 10 6 (range: x 10 6 ) sperm were recovered per male. Motility of sperm after cooling to 4 C was similar to immediately after harvest (data not presented). Immediately after thawing (0 h), motility was higher for sperm frozen as pellets than all other groups (P<0.05; Table 7.3). Motility immediately after thawing was higher for sperm frozen in 0.5 ml straws in liquid nitrogen vapour compared with 0.25 ml straws in LN and 0.25 and 0.5 ml straws frozen on dry ice (P<0.05). However, motility was similar between groups by 3 h post-thaw (P>0.05). Table 7.3. Motility of epididymal alpaca sperm after harvesting (post-harvest), and immediately (0 h) and three h (3 h) after freezing and thawing in pellets, or in 0.25 ml and 0.5 ml straws frozen on dry ice or in liquid nitrogen vapour. Data are means ± SEM. Values within the same column with a different superscript differ (P<0.05). Freeze method Post-thaw 0 h 3 h Pellets 27.0 ± 5.2 a 1.0 ± 0.7 a Dry ice 0.25 ml 5.5 ± 1.1 b 0.6 ± 0.4 a 0.5 ml 5.0 ± 1.6 b 0.5 ± 0.5 a LN 2 vapour 0.25 ml 8.6 ± 1.8 b 0.6 ± 0.5 a 0.5 ml 10.0 ± 2.4 c 0.5 ± 0.5 a Acrosome integrity was similar immediately after harvest (91.2 ± 3.1 %) and cooling to 4 C (89.3 ± 3.8 %). After thawing, acrosome integrity was similar between groups at 0 h and 3 h post-thaw (P>0.05; Table 7.4). Table 7.4. Acrosome integrity of epididymal alpaca sperm after harvesting (post-harvest), and immediately (0 h) and three h (3 h) after freezing and thawing in pellets, or in 0.25 ml and 0.5 ml 111
127 straws frozen on dry ice or in liquid nitrogen vapour. Data are means ± SEM. Values within the same column with a different superscript differ (P<0.05). Freeze method Post-thaw 0 h 3 h Pellets 87.5 ± 1.2 a 83.3 ± 2.5 a Dry ice 0.25 ml 88.3 ± 1.1 a 82.3 ± 1.7 a 0.5 ml 81.0 ± 2.2 a 78.3 ± 2.1 a LN 2 vapour 0.25 ml 89.8 ± 1.6 a 83.0 ± 2.0 a 0.5 ml 89.0 ± 1.5 a 79.0 ± 1.9 a Comparison of 2, 3, and 4 % final glycerol concentration Opaque white fluid containing sperm was successfully harvested from the epididymides of all males. Immediately after harvesting sperm displayed forward progressive motility. Sperm motility was 52.7 ± 3.3 % and ranged between %. The mean number of sperm harvested was ± 44.5 x 10 6 and ranged between x 10 6 Sperm motility was similar after harvesting and cooling to 4 C (Table 7.5). Immediately, and three hours after freezing and thawing sperm motility was similar for sperm frozen in lactose cryodiluent supplemented with 2, 3 or 4 % glycerol (P>0.05, Table 8.5) Table 7.5. Motility of epididymal alpaca sperm after harvesting (post-harvest), cooling to 4 C (Cooled), and immediately (0 h) and three h (3 h) after freezing and thawing in lactose diluents supplemented with 2, 3 or 4 % (v:v) glycerol. Data are means ± SEM. Values with a different superscript are significantly different (P<0.05). Motility (%) Glycerol conc. (%) Post-harvest Cooled Post-thaw 0 h 3 h ± ± ± ± ± ± ± ± 0.5 Acrosome integrity after harvesting was 89.3 ± 3.3 %, and was similar after sperm diluted in lactose cryodiluents were cooled to 4 C (Table 7.6). Immediately after thawing (0h post-thaw) acrosome integrity was similar for sperm frozen lactose cryodiluents supplemented with 2, 3, or 4 % glycerol but three hours after thawing was higher (P<0.05) for sperm frozen in lactose cryodiluent supplemented with 3 and 4 % than 2 % glycerol. Table 7.6. Acrosome integrity of epididymal alpaca sperm after harvesting (post-harvest), cooling to 4 C (Cooled), and immediately (0 h) and three h (3 h) after freezing and thawing in lactose and cryodiluent supplemented with 2, 3 or 4 % (v:v) glycerol. Data are means ± SEM. Values with a different superscript are significantly different (P<0.05). Glycerol conc. Acrosome integrity (%) (%) Post-harvest Cooled Post-thaw 0 h 3 h ± ± 2.7 a ± ± ± ± 3.8 b ± ± 4.1 b 112
128 The proportion of motility retained (% of original motility) after freezing and thawing was similar (P>0.05) for sperm frozen in lactose diluent supplemented with 2 (30.8 ± 8.3 %), 3 (37.7 ± 10.0 %) or 4 % glycerol (35.5 ± 11.9 %) Addition of Equex STM paste The epididymides of all males were observed to contain sperm. The mean number of sperm recovered was ± 43.5 x 10 6 sperm per male were recovered but this ranged from 49.5 to x 10 6 for individual males. Sperm motility also varied between males ranging from % but the means sperm motility was 46.9 ± 4.5 % and sperm displayed forward progressive motility. Sperm motility was similar after harvest, cooling to 4 C, and storage at 4 C for h (Table 8.7). There was no difference in sperm motility at 0 or 3 h post-thaw when sperm were frozen in Biladyl or Biladyl supplemented with Equex STM paste (P>0.05; Table 7.7). Table 7.7. Motility of epididymal alpaca sperm after harvesting (post-harvest), cooling to 4 C (Cooled), and immediately (0 h) and three h (3 h) after freezing and thawing in Biladyl supplemented with or without Equex STM paste. Data are means ± SEM. Values with a different superscript are significantly different (P<0.05). Motility (%) Treatment Post-harvest Cooled Post-thaw 0 h 3 h Control 14.4 ± 2.1 a 0.0 ± 0.0 a 53.0 ± ± 1.4 Equex 21.5 ± 3.5 b 0.0 ± 0.0 a Furthermore, sperm frozen and thawed in the presence of Equex retained 32.4 ± 5.2 % of their prefreeze motility compared with 45.8 ± 7.2 % of motility retained if Equex was omitted during freezing and thawing (P<0.05). Acrosome integrity after harvesting was 92.3 ± 2.0 %, and was similar after cooling to 4 C (Table 7.8). Immediately (0h) and three hours after thawing acrosome integrity was similar for sperm frozen in Biladyl and Biladyl supplemented with Equex STM paste (P>0.05, Table 8.8). Table 7.8. Acrosome integrity of epididymal alpaca sperm after harvesting (post-harvest), cooling to 4 C (Cooled), and immediately (0 h) and three h (3 h) after freezing and thawing in Biladyl with or without Equex supplementation. Data are means ± SEM. Values with a different superscript are significantly different (P<0.05). Acrosome integrity (%) Diluent Post-harvest Cooled Post-thaw 0 h 3 h Control 89.6 ± ± ± ± 0.64 Equex 91.1 ± ±
129 7.4. Discussion In these studies, an efficient method was developed for the cryopreservation of epididymal alpaca sperm after overnight transport to the laboratory. Post-thaw sperm recovery was superior in Lactose compared with Citrate and Tris cryodiluents. Moreover, pellet-frozen sperm had higher post-thaw motility compared with 0.25 and 0.5 ml straws frozen on dry ice or over liquid nitrogen vapour. Moreover, we described a method for successfully cryopreserving epididymal alpaca sperm utilizing 3-4 % final glycerol and 1 % final Equex STM paste concentration in the cryodiluent and demonstrated, for the first time, that epididymal alpaca sperm can be liquid-stored prior to cryopreservation. Cryopreservation of epididymal alpaca sperm in 3 4 % glycerol improved the proportion of intact acrosomes at 3 h post-thaw, compared with a lower glycerol concentration, despite the lack of effect on post-thaw motility. Moreover, sperm frozen in the presence of Equex STM had higher post-thaw motility than those frozen in its absence. Compared with other domestic livestock species such as cattle, sheep and pigs, there have been few investigations of freezing methods for ejaculated or epididymal alpaca sperm. Cryopreservation of camelid semen has generally met with poor success. Reported post-thaw motilities have been <10 % (llama: Burgel et al. 2000, 2001; Dromedary camel: Hassan et al. 1995), 6 20 % (alpaca: Vaughan et al. 2003a; Santiani et al. 2005; llama: Burgel et al. 2000), or % (Bactrian camel: Zhao et al. 2004; Dromedary camel: Hassan et al. 1995; llama: Burgel et al. 2000; Aller et al. 2003). Post thaw motility has rarely exceeded 40 % for camelid semen, although post thaw motility in excess of 45 % has been reported for alpaca sperm by Bravo et al. (1996b) and 60 % for Bactrian camel sperm by Zhao et al. (2004). The majority of investigations have focused on AI with frozen-thawed semen and have neglected to determine the optimal freezing and thawing processes. Consequently, the packaging, cryodiluent type and concentration of the cryoprotectant as well as the freezing and thawing rates remain to be elucidated for alpaca, and other members of the Camelidae family. Dilution with sucrose-egg yolk-glycerol (Zhao et al. 2004) medium and freezing in glass ampoules (2 ml) has been the preferred method for semen from Bactrian camels, while Dromedary semen has been packaged in 0.25 ml straws after dilution in Green Buffer (IVM), or 4.0 ml straws after dilution with Lactose cryodiluent (Bravo et al. 2000b). Freezing of alpaca and llama semen has been conducted in citrate (Aller et al. 2003; Bravo et al. 1996; 2000a; Burgel et al. 2000), Biladyl (Vaughan et al. 2003), Green Buffer (Vaughan et al. 2003), Triladyl (McEvoy et al. 1992; Vaughan et al. 2003), and Tris (Burgel et al. 2000; Santiani et al. 2005; Von Baer and Hellemann 1999) cryodiluents. Using Citrate cryodiluent, Bravo et al. (1996) reported one of the more successful methods of cryopreservation alpaca sperm. High rates of sperm survival and an overall motility of 46.7 % after thawing were reported, with 54.9 % of initial motility retained after freezing and thawing. However, the details provided by the authors are not sufficient to permit replication of the methods. In a subsequent study, and the only report on cryopreservation of epididymal alpaca sperm, Bravo et al. (2000a) froze epididymal sperm from five llamas and four alpacas using the same method as for ejaculated semen (Bravo et al. 1996). Sperm from two of the five llamas did not survive freezing and thawing, and the post-thaw motility of sperm from one alpaca was 5 %. The authors stated that the other samples were motile post-thaw, but did not present motility data. Subsequent studies have not met with similar success to those of Bravo et al. (1996). Far lower post-thaw motility of alpaca and llama sperm has been reported using Citrate cryodiluent (12.5 % present study; % Aller et al. 2003; Burgel et al. 2000). Other diluents used to cryopreserve alpaca and llama sperm have also resulted in low post-thaw motilities (Biladyl: 21.3 % Vaughan et al. 2003; Green buffer: 17.4 % Vaughan et al. 2003; Skim-milk: % Santiani et al. 2005; Triladyl: 10 % McEvoy et al. 1992, Vaughan et al. 2003; Tris: 19 %: present study; % Burgel et al. 2000). Lactose-based cryodiluents have not previously been used to freeze alpaca sperm but have been applied successfully 114
130 in camel sperm freezing (Anouassi et al. 1992; Bravo et al. 2000; Musa et al. 1992) and are routinely used for boar sperm cryopreservation (Johnson et al. 2000). Alpaca and llama sperm have been generally frozen in smaller volumes than camel semen, with the majority of studies using 0.25 ml straws frozen in liquid nitrogen vapour (Bravo et al. 1996; 2000a,b). It would be expected that post-thaw motility would be improved for smaller straws, as they have a comparatively larger surface area to volume ratio than larger straws. This reduces variation in the freezing and thawing rates within the straw (Bwanga et al. 1999). However, Burgel et al. (2000) and Vaughan et al. (2003) concluded that post-thaw motility of llama and alpaca sperm, respectively, was superior with 0.5 compared with 0.25 ml straws. In contrast, the post-thaw quality of Dromedary semen was improved by freezing in 0.25 ml compared with 4.0 ml straws (Bravo et al. 2000b). Pellet freezing provides superior post-thaw motility of ram sperm compared to straw freezing (Maxwell et al. 1995) and substantially increased the post-thaw motility of epididymal alpaca sperm compared with both 0.25 and 0.5 ml straws, in the present study. While pellet freezing is clearly more efficient, the requirements of the AI technique ultimately influence, if not dictate, the choice of freezing technique (Holt 2000) resulting in the majority of sperm being frozen in straws, and for camels, large volume straws (4.0 ml) owing to the volume of seminal plasma required to induce ovulation (Bravo et al. 2000b). The majority of studies on cryopreservation of camelid semen have utilized higher glycerol concentrations than in the present study, traditionally in the vicinity of 6 7 % final concentration, but have reported poor post-thaw motility (Zhao et al. 1996; Aller et al. 2003; Santiani et al. 2005; Niasari-Naslaji et al. 2007). In a recent study, Niasari-Naslaji et al. (2007) compared 4, 6 and 8 % final glycerol concentrations for the cryopreservation of Bactrian camel semen. The highest post thaw motility (35 %, compared with 74 % motility post collection) was obtained when the freezing diluent contained 6 % glycerol. These results are in agreement with a previous study (Zhao et al. 1996) which concluded that 7 % final glycerol concentration was superior to 3.5 % for the cryopreservation of Bactrian camel semen. Nevertheless, lower final glycerol concentrations (3 %: Deen et al. 2003) have been successfully used for cryopreservation of Dromedary camel semen. Cryopreservation of alpaca semen with higher glycerol concentrations (7 %) has resulted in post-thaw motilities of < 20 % (Vaughan et al. 2003a; Santiani et al. 2005). These studies, taken together with the results of the present study, suggest that lower glycerol concentrations, in the range of 3 4 %, may be optimal for the cryopreservation of alpaca sperm. However, further studies testing wider ranges of glycerol concentrations are required before a definitive conclusion can be reached. Addition of sodium dodecyl sulphate (SDS) in the form of Equex or Orvus ES paste to the cryodiluent is routine for boar sperm, and has previously been demonstrated to improve post-thaw sperm quality in Eld s deer, elephants, greater kudu, scimitar-horned oryx, wildebeest and zebra (Holt 2000). Equex and Orvus ES may be beneficial to camelid sperm during cryopreservation, and have been used in cryodiluents for camel (Musa et al. 1992) and alpaca (Lactose diluent in present study) sperm. The inclusion of Equex STM paste in the cryodiluent was beneficial for frozen-thawed epididymal alpaca sperm, concurring with previous reports (Holt 2000). The active component of Equex STM is sodium dodecyl sulphate (SDS) which is a water soluble anionic detergent. SDS denatures membrane proteins and can solubilise biological membranes completely at high concentrations (Helenius et al. 1979). The precise mechanisms by which SDS (added in the form of Equex STM or Orvus ES paste) improves sperm cryosurvival remain unknown. However, it has been demonstrated that SDS is only beneficial in the presence of egg yolk, suggesting that it functions by altering the tertiary structure of the egg yolk lipoproteins (Pursel et al. 1978). In the present study, post thaw motility was higher when sperm were frozen and thawed in the presence of Equex STM while acrosome integrity was unaffected. Previous studies have observed benefits from SDS in both acrosome integrity (Axner et al. 2004) and post thaw motility (Akourki et al. 2004). However, in contrast with the results of the present study, the inclusion of 0.5 % v:v Equex STM during the cryopreservation of llama semen failed to demonstrate a beneficial effect (Von Baer 115
131 and Hellemann 1999). Further studies on Equex STM as a cryodiluent additive for the frozen preservation of epididymal alpaca are warranted to determine the optimum concentration of this additive. The initial motility of the epididymal sperm in the present study was high considering the interval between castration and harvesting (16 24 h). Epididymal sperm are relatively easy to handle and dilute, being free from the viscous seminal plasma that impedes progress in developing efficient methods for preserving ejaculated alpaca sperm. Forward progressive motion was displayed by epididymal sperm immediately after harvest, in contrast to the oscillatory motility of ejaculated sperm. By contrast, Bravo et al. (2000a) reported the absence of forward progressive motility for alpaca or llama epididymal sperm. The effects of centrifugation on camelid sperm have not been extensively investigated but Ferre et al. (2000) reported that centrifugation (300 g; 10 min) was highly detrimental to the longevity of fresh diluted llama sperm. Therefore, the post-thaw survival of epididymal alpaca sperm may be improved by the removal of the pre-freeze centrifugation step. Parrilla et al. (2006) employed sedimentation instead of centrifugation to concentrate sex-sorted boar sperm. Given the beneficial effect of sedimentation on sex-sorted boar sperm reported by the authors, attempts to modify this technique for inclusion during cryopreservation of alpaca sperm may be warranted. A high proportion of the initial motility of sperm was retained after freezing and thawing in Experiment 1 (range: %), Experiment 2 (range: %), Experiment 3 (range: %) and Experiment 4 ( %), demonstrating the efficacy of cryopreserving epididymal alpaca sperm after overnight transport to the laboratory. Interestingly, the proportion of initial motility retained in Experiment 2 was similar to Experiment 1, despite the additional overnight liquid storage step, suggesting that epididymal alpaca sperm are tolerant of liquid storage at 4 C and this extended period of storage does not adversely affect survival after subsequent cryopreservation. These observations suggest that alpaca sperm may be resistant to cold shock although further larger scale studies employing both ejaculated and epididymal alpaca sperm are required to confirm this hypothesis. Furthermore, the minimal loss in motility after cryopreservation of epididymal alpaca sperm when pellet-frozen in Lactose cryodiluent was unexpected, given the lack of knowledge about freezing camelid sperm and the general perception that camelid sperm do not survive well after freezing and thawing (Vaughan et al. 2003). These results highlight the need for systematic investigation of the effects of the various freezing and thawing processes using ejaculated alpaca sperm, similar to those described by Salamon and Maxwell (1995), which will undoubtedly improve the survival of ejaculated alpaca sperm after freezing and thawing, and lead to the development of efficient sperm preservation procedures for alpacas and eventually all camelids. In conclusion, this research demonstrates the feasibility of harvesting and cryopreserving epididymal alpaca sperm with a long interval between castration and harvesting. Pellet-freezing epididymal alpaca sperm in Lactose cryodiluent produces superior post-thaw survival compared with Citrate and Tris based cryodiluents, and freezing in 0.25 and 0.5 ml straws. In addition, the research presented in this study demonstrated the beneficial effect of Equex STM on the function of frozen-thawed epididymal alpaca sperm. In addition, we showed for the first time that the sperm could be stored in a chilled state overnight, prior to cryopreservation, without any loss of function, suggesting that they are tolerant of liquid storage. Furthermore, this research challenges the perception that alpaca sperm is highly susceptible to cold shock and does not tolerate cryopreservation. While these results represent a major step forward in the preservation of sperm from alpacas, further research is required to determine whether the sperm retain fertilizing capacity after cryopreservation. 116
132 8. Artificial Insemination 8.1. Introduction Successful commercial development of artificial insemination (AI) for the alpaca industry would facilitate the widespread use of sires of high genetic value, both nationally and internationally, and increase the rate at which superior genetics are disseminated. The first demonstration of AI in alpacas was by Fernandez-Baca and Calderon (1966) using fresh whole Vicuña semen (collected by electroejaculation). These authors reported the birth of one offspring after the AI of 42 females. Subsequent studies have reported fertility rates ranging from % (fresh semen), % (liquid stored semen) and % (frozen semen) after the AI of alpacas and llamas. While several studies have demonstrated the feasibility of AI, and offspring have been born after AI with fresh, liquid stored and frozen thawed sperm in camelid species, the efficiency of AI remains very low and results vary widely between studies (see Table 1.18). Many authors have highlighted the lack of techniques for the preservation of semen and for inducing ovulation as limiting factors in the commercial development of AI in alpacas and other camelids. There have been recent improvements in the techniques for inducing ovulation (Vaughan 2001, 2002; Vaughan et al. 2003a), semen collection (Vaughan et al. 2003a; this report) and preservation of semen (this report; Morton et al. 2007a,b,c). However, the fertilizing capacity of liquid stored and cryopreserved sperm has yet to be determined. Using the improvements to the collection and preservation of semen developed during this project, and the latest methods for induction of ovulation, the objective of this chapter was to achieve pregnancies after the AI of females with frozen-thawed epididymal sperm. Epididymal sperm were used (1) as they have not been in contact with the viscous seminal plasma, identified as a potential negative factor affecting sperm function, and would demonstrate the specific fertilizing capacity of the sperm, independent of semen, and (2) methods for their freezing and thawing with preserved post-thaw function had already been demonstrated (Chapter 7) Materials and methods Animals and follicular synchronization and induction of ovulation Twenty adult female alpacas were allocated to the experiment, 10 for insemination with frozen-thawed epididymal sperm and 10 for natural mating (controls). Ten females were allocated in August (replicate 1) and 10 in September 2007 (replicate 2). Control matings were undertaken by the same 5 males (1 female each for replicates 1 and 2, n = 2 per male). Synchronisation of follicular wave emergence and induction of ovulation was performed in the females using synthetic prostaglandins (Juramate ; Jurox Pty Ltd, Newcastle, NSW) and gonadotrophin-releasing hormone analogue (Receptal, Intervet, Bendigo East, VIC) according to the regime described in to Table 8.1. Table 8.1. Regime to synchronise follicular growth and induce ovulation in alpacas Day Treatment -13 Cloprostenol [0.8 ml Juramate im] -12 Buserelin 40 μg [1.0 ml Receptal im] -2 Cloprostenol [0.8 ml Juramate im] -1 Buserelin 40 μg [1.0 ml Receptal im] 0 Ovulation 117
133 Ultrasonography Ultrasonography was performed as described by (Vaughan et al. 2003a). Briefly, females were sedated 10 min prior to ultrasound with 0.7 ml Torbugesic (Butorphonol; 10 mg/ml, Fort Dodge Animal Health, Baulkham Hills, NSW), 1.3 ml Xylazine (Rompun, 20 mg/ml; Bayer Health Care, Pymble NSW). Females were restrained in sternal recumbency and examined by transrectal ultrasonography using an Aloka SSD-500 with 7.5 MHz linear array transducer (Aloka Co. Japan; Figure 8.1) to enable selection of animals with a newly emerged dominant follicle with a diameter of 7 to 10 mm, 6 to 8 days after new wave emergence. Figure 8.1. Examination of follicular status using transrectal ultrasonography The ovaries of each female were examined by transrectal ultrasonography at the time of AI. Females that were inseminated were examined for pregnancy by ultrasound again approximately 6 weeks after AI Sperm cryopreservation Epididymal sperm were pellet-frozen using lactose and Biladyl diluents as described in Chapter 7. Sperm were thawed in a water bath (37 C; Figure 8.2) until the pellets melted. The inseminate was loaded into cervical AI pipettes (as used for sheep, Evans and Maxwell, 1987) connected to a 10 ml tuberculin syringe by a 5 cm length of silicone tubing and inseminated according to the technique described below. Figure 8.2. Field laboratory for thawing and preparation of semen for AI. 118
134 8.2.4 Insemination technique Artificial insemination was performed according to the technique described by Vaughan et al. (2003a). Briefly, females were restrained in sternal recumbency and the tail held out of the way (Figure 8.3). Figure 8.3. Restraint of female in preparation of insemination The perineum was cleaned with paper towel and alcohol. A small, gloved, lubricated hand was placed into the rectum and used to stabilise the cervix. The AI pipette was then passed through the vulva, vagina and cervix, as depicted in Figures 8.4b, c and d, into the uterine horn ipsilateral to the ovary containing the dominant follicle. When the pipette was positioned at the tip of the uterine horn, the plunger on the syringe was depressed, depositing the sperm from the pipette into the uterus (Figure 8d). The AI pipette was then withdrawn, the perineum wiped clean with paper towel and the female released. Artificial insemination was performed approximately 24 hours after induction of ovulation, approximately 4 to 6 hours before the predicted time of ovulation, as assessed by the size and time of disappearance of the dominant follicle. Each female received 1.5 ml of thawed inseminate containing million motile spermatozoa. Control natural matings were also performed 24 hours after induction of ovulation. The five females inseminated for each replicate were allowed to naturally mate with a randomly allocated male in purpose-built individual mating yards (Figure 8.4a). Males were allowed to continue mating until ejaculation was completed (approximately minutes). Figure 8.4a,b,c and d. Natural mating and artificial insemination of females 119
135 8.3. Results Ovarian follicles were observed by transrectal ultrasonography initially at 22 h (replicate 2) and 24 h (replicate 1) and again at 27 h (replicate 2) and 29 h (replicate 1). Visualisation of ovarian follicles before and after ovulation is presented in Figure 8.5. Nine of the 10 females ovulated, three females had ovulated and six females ovulated between h post-buseralin. Once ovulation was observed, sperm were thawed, assessed under the microscope (Figure 8.6) and females inseminated. Figure 8.5. Ovarian follicles prior (left) and post (right) ovulation Figure 8.6. Frozen-thawed epididymal sperm (x 200) immediately prior to insemination The post-thaw motility, number of sperm inseminated and pregnancy results are presented in Table 8.2. Table 8.2. Number of sperm inseminated, post-thaw motility of sperm and pregnancy results after natural mating (control) or artificial insemination. Treatment No. No. sperm inseminated Sperm motility No. pregnancies females (x10 6 ml) (%) (%) Natural mating 10 Not determined Not determined 4 (40 %) Artificial insemination % 0 (0 %) 120
136 8.4. Discussion This experiment represents the first attempt to obtain pregnancies after AI of camelids with epididymal sperm that has been frozen and thawed. Epididymal sperm were used in this experiment because of the difficulty of obtaining and processing sufficient quantities of ejaculated sperm. We have already demonstrated that epididymal alpaca sperm retain their motility and function for several hours after freezing and thawing, using processing methods developed as part of this project (Chapter 7). Epididymal sperm were originally chosen as they were readily available from castrated males and had not been in contact with the viscous seminal plasma, identified as a potential negative factor affecting sperm function, which makes sperm extraction from the ejaculate and processing difficult. Our objective was to demonstrate the specific fertilizing capacity of the sperm, independent of their seminal plasma, in order to test the hypothesis that the seminal plasma was the main barrier to progress in the development of semen preservation methods. Unfortunately, no pregnancies were obtained in this preliminary experiment, so we were not able to come to any conclusion regarding this hypothesis. That is, it was neither proven nor disproven. A number of factors, other than the sperm themselves, determine the success of an AI programme, such as the success of synchronizing follicular growth and ovulation, timing of insemination relative to ovulation, sperm dose and where the sperm are deposited within the female reproductive tract. Considerable research, in the order of 20 years, has occurred in domestic animal species, such as sheep, cattle and pigs to determine the optimal methods to synchronise females, induce ovulation, and determine the optimal AI conditions. Nevertheless, research into these areas in camelids is lacking, and is difficult and resource-consuming due to the unusual reproductive physiology of camelids compared with other livestock species. Given the small numbers of animals available for experimentation in Australia, and the early stage of development of the industry limiting large-scale research, it was not possible to include other important, and potentially confounding, parameters in the current field experiment. It would have been particularly important to examine different times of insemination relative to ovulation, a range of sperm doses and different sites of insemination. Such work remains to be done when resources are available and the fertilizing capacity of frozen alpaca sperm have been confirmed. Nevertheless, we were able to monitor ovulation in the female alpacas subjected to AI, and we were able to identify large dominant follicles (Figure 8.6) and thus determine the time approximate time of ovulation. Based on this information, ovulation was successfully induced 24 to 30 hours after intramuscular injection of Buserelin. These findings are consistent with other workers (see Section 1.5.5; Bourke et al. 1992, 1995; Huanca et al. 2001). However, the low pregnancy rate obtained in the controls (40 % pregnant) does cast some doubt on the efficacy of the synchronization protocol used in this trial, or on our chosen timing of mating and insemination. Synchronous ovulation and insemination is required to achieve high conception rates. In some species, high conception rates occur when sperm are in the oviduct just before ovulation (Hafez 1993). Fertility with cryopreserved semen in domestic livestock may be high providing the insemination is made four hours before ovulation (Watson 2000). Fertility with fresh semen is maintained over a much longer period. If sperm are placed into the tract too early, they will lose viability prior to ovulation, and if sperm are placed into the tract after ovulation, the ovum may lose viability before fertilisation can occur (Hafez 1993). In the present trial, AI was performed 24 hours after induction of ovulation, approximately 4 to 6 hours before the predicted time of ovulation. In an early alpaca study, the highest proportion of fertilised ova resulted when intrauterine AI with fresh semen was 35 to 45 hours after induction of ovulation using either a vasectomised male or intramuscular hcg (Calderon et al. 1968). More recently, AI hours after induction of ovulation in alpacas has been reported to result in acceptable conception rates with fresh and frozen semen (Table 1.18; Pacheco 1996; Quispe 1996a; 121
137 Apaza et al. 1999). Despite these reports, we were unable to obtain any pregnancies with frozen sperm, or satisfactory pregnancies from natural matings using established synchronization methods. Bravo et al. (1997b), using fresh, undiluted semen compared the techniques of transcervical and laparoscopic AI in alpacas and achieved similar conception rates. Transcervical AI would appear to be a simpler, less-invasive procedure, but it has limitations of rectal/pelvic capacity to allow transrectal manual stabilisation of the cervix when passing the AI pipette through the cervix, and the difficulty of locating the external os of the cervix during this procedure. Bravo (2002) describes the need for patience and dexterity to pass a pipette through the cervix into the uterus. Laparoscopic AI requires restraint of the female in a crate, sedation with or without a general anaesthetic, and expensive laparoscopic equipment. Transcervical deposition of semen into the tip of the uterine horn ipsilateral to the ovary bearing the dominant follicle proved to be a simple and efficient technique for AI, and was based on previous reports on the success of this technique using fresh and liquid stored semen (Vaughan et al. 2003a). Given the poor success in this experiment, however, it may be necessary in the future to develop a deeper, semi-surgical method of AI for distributing semen from genetically valuable males. Surgical deposition of sperm in the uterine horns by laparoscopy is now the standard method of AI in sheep even using frozen semen; the procedure is used by veterinarians to inseminate some 1.5 million ewes annually in Australia (Evans and Maxwell 1987). Once the fertilizing capacity of frozen-thawed alpaca sperm has been established, it may be necessary to develop a similar method to deposit the inseminate into the utero-tubal junction or oviducts, in order to obtain satisfactory fertility from frozen sperm. The lack of pregnancies following insemination of approximately 300 million sperm following extension and freezing in Biladyl A/B, indicates that further studies are required to increase the percentage of active sperm post-thaw, to refine the timing of AI in relation to ovulation, to determine the numbers of sperm (chilled and frozen) required for conception and the optimum method of delivery of sperm into the female reproductive tract to ensure high conception rates. While other workers have obtained pregnancies using extended fresh and chilled semen, they have achieved few pregnancies using frozen-thawed semen (see Table 1.18). Bravo et al. (2000c) reported one of the best results so far with frozen thawed semen with 5 young born to 19 inseminated females. Semen characteristics post-thaw in that study were similar to those reported here. Other workers have had little success in terms of pregnancies with either chilled or frozen semen in alpacas or llamas (Aller 2001). Some apparently good results are reported in the literature in different camelid species (Table 1.18). Details of the precise techniques used are sometimes missing. Authors rarely report negative results and so the overall picture of fertility with frozen-thawed semen in camelids remains unclear. There is a significant relationship between sperm number per dose of semen and fertility (Rodriguez- Martinez et al. 2001) but it may be possible to reduce the number of sperm per dose through refining techniques of collection, processing and AI, and therefore increase the number of doses per ejaculate. There is a threshold number of viable sperm per AI-dose for individual males, below which the fewer viable sperm inseminated, the greater the risk that fertility will be compromised (Foote et al. 1993). A sufficient number of fully competent sperm capable of achieving fertilisation at the time of ovulation are required in the oviduct to achieve pregnancy (Watson 2000). Two hundred million cryopreserved sperm placed at the cervix, 20 million cryopreserved sperm placed into the uterus or 1 million cryopreserved sperm placed into the oviduct are needed to achieve more than 50 % fertility in sheep (Maxwell 1986; Maxwell et al. 1993). Fertility declines exponentially when sperm number or quality is reduced. All these parameters remain to be established in the alpaca. Induction of ovulation was achieved in the present experiment, confirming previous reports by Vaughan (2001). However, despite retained sperm function after freezing and thawing, no pregnancies were obtained in conjunction with timed transcervical intrauterine insemination of females with frozen-thawed epididymal sperm. Because of the large number of potential variables affecting fertility 122
138 after AI of alpacas, which were not examined here due to limited resources, the application of frozen semen for AI cannot yet be recommended without further research. This research needs to initially determine the fertilizing capacity of frozen-thawed alpaca sperm. Because of the severe limitations of the viscous seminal plasma in ejaculated semen, it is recommended that this work be done on epididymal sperm, based on the methods developed in the current project. The fertilizing capacity should be determined through an in vitro fertilization (IVF) system, either by direct IVF of fresh alpaca oocytes or, more practically, through a sperm binding and penetration test. Such tests have been developed in our laboratory using salt-stored oocytes either from the same or another species. For example, we have developed a successful heterologous in vitro assay using salt-stored bovine oocytes, through which we were able to establish the fertilizing capacity of sex-sorted frozen-thawed stallion sperm. Importantly, further development of AI with frozen semen in alpacas will also depend on a solution to the problem of the viscous seminal plasma in the ejaculate, so that freezing technology can be transferred from epididymal to ejaculated sperm. 123
139 9. Preliminary development of spermsexing technology in alpacas 9.1. Introduction Methods to control the sex of offspring have been reported as far back as BC by Hippocrates, who believed that male children developed on the right, and females on the left side of the uterus and therefore the appropriate sexual position (to deposit semen in the correct side of the uterus) would give rise to the desired sex. Since then a number of methods for selecting the sex of offspring have been tried, included the timing of intercourse relative to the menstrual cycle, maternal diet, and ligation or removal of the appropriate testis (removal of the left if the couple desired male children and vice versa). These unsuccessful methods for selecting the sex of children have been extensively reviewed (Shettles and Rorvik 1984; Zarutskie et al. 1989; Windsor et al. 1993; Winston 1993). However, it was not until the discovery that mammalian sex is determined by the penetration of the egg by an X or Y chromosome bearing sperm at fertilisation that sperm separation methods were investigated to select the sex of offspring. A number of methods have been suggested as aids to sex preselection including separation of X- and Y-spermatozoa based on motility (Ericsson et al. 1973), density, sperm head volume (Cui and Matthews 1993), surface charge (Kaneko et al. 1984) and cell surface antigens (Hendriksen et al. 1993). However, the only reliable methods for the production of pre-sexed embryos/offspring are the use of flow cytometry to separate X- and Y-chromosome bearing sperm, or the biopsy and diagnosis of embryonic sex by polymerase chain reaction (PCR). One disadvantage of embryo biopsy and sexing by PCR is that embryos of both sex are produced, and those of the undesired sex are discarded, thereby reducing efficiency. Sperm sexing by flow cytometry is therefore more efficient for producing offspring and embryos of a pre-determined sex, as only those of the desired sex are produced. The separation of sperm into X- and Y-chromosome bearing populations by flow cytometry ( sperm sexing ) is an emerging reproductive technology that has resulted in the birth of pre-sexed offspring in a number of species using AI, IVF or MOET (Maxwell et al. 2004). The basis for separating sperm using flow cytometry is the larger size, and therefore higher DNA content of the X chromosome (Moruzzi 1979). The greater the difference between the DNA content in the X- and Y-chromosome bearing spermatozoa the more easily and efficiently the populations can be resolved and separated using a modified flow cytometer. Most domestic animal species have a DNA content difference of between % (Johnson 2000b). For the flow cytometer to correctly interpret the difference in the DNA content between the X- and Y- chromosome bearing sperm the cells must be correctly aligned as they pass through the cytometer. Before sperm can be separated by the flow cytometry they must be stained with a fluorescent dye which binds to the minor groove of the DNA helix (Hoechst 33342; Johnson et al. 1987). After staining the sperm are then pushed through the cytometer in single file passed a UV laser which causes the Hoechst dye to fluoresce. Based on the amount of fluorescence the cytometer applies a positive or negative electrical charge to the sperm surface. The sperm then pass next to electromagnets, are attracted to their opposite charge, and are separated into two populations. The application of sperm sexing technology for a new species involves the development of speciesspecific protocols for staining, sorting and post-sorting preservation. Preliminary development of this technology, the separation of X- and Y-chromosome bearing spermatozoa, has recently occurred in buffalo (Lu et al. 2006), goats (Parrilla et al. 2004) and non-human primates (O'Brien et al. 2005a,b) and pre-sexed offspring produced after artificial insemination (AI) with sex-sorted frozen thawed sperm in elk (Schenk and DeGrofft 2003) and dolphins (O'Brien and Robeck 2006). However, sperm- 124
140 sexing has yet to be applied to camelids. Given the different prices for male and female alpacas there is considerable interest in the development of this technology. The aims of this study were to i) optimize the Hoechst (H33342) staining concentration for the flow cytometric separation of X and Y alpaca sperm nuclei, ii) separate alpaca sperm nuclei into high purity (> 90%) populations bearing the X- and Y-chromosome, and iii) determine the DNA difference between the X- and Y-bearing sperm in alpacas Materials and methods Animals and experimental design Semen was collected from adult male alpacas, and sperm nuclei were stained with different concentrations of H33342 and subjected to flow cytometric separation. Data for the proportion of orientated sperm (those able to be sorted), flow rate (speed of sperm passage through the machine) and sort rate (speed of separation of sperm into X- and Y-populations) H33342 were recorded for the different H33342 concentrations. The purity of the separated populations was determined by re-sort analysis and the difference in the DNA content of the X- and Y-bearing spermatozoa was determined. Sperm nuclei rather than whole spermatozoa were used for all analyses to ensure a more accurate determination of the DNA content of the X- and Y-bearing spermatozoa by removing interference from the sperm midpiece and tail. The whole procedure was replicated on four separate occasions Semen collection Semen was collected according to the procedure described in Chapter Preparation of sperm nuclei samples Sperm suspensions were washed by centrifugation (700 g, 6 min), the supernatant removed and the pellet resuspended in 2 ml Androhep. Samples were then sonicated using a Branson Sonifier 250 (Branson Ultrasonics, Danbury, CT, USA) at 70 % power until >95 % of the midpieces and tails were removed (approx. 30 sec). Samples were then centrifuged (700 g, 45 min) through a Percoll gradient modified for alpaca sperm (1 ml 45 %; 1 ml 22.5 %; Sigma-Aldrich, Sydney, Australia). The pellet was resuspended in Androhep to a concentration of 100 x 10 6 /ml. Samples were then divided into four aliquots for staining with 36, 54, 72 or 90 μm H33342 (Sigma-Aldrich) and incubated for 1 h at 34 C. Samples were rotated every 15 min to ensure even staining and filtered through 35 μm cell strainer tubes (Falcon 2235, Bacto Laboratories, Franklin Lakes, NJ, USA) prior to analysis Flow cytometric sorting Stained sperm nuclei were separated into X- and Y-chromosome bearing populations using a high speed cell sorter (SX-MoFlo ; DakoCytomation Inc., Fort Collins, Co, USA) modified for sperm sorting (Rens et al. 1998a; Johnson and Welch 1999), running a diode pumped solid state pulse laser (Vanguard 350 HMD-355; Spectra Physics, Mountain View, CA, USA), operating at 40 psi with PBSsheath fluid (Sigma-Aldrich). Gates were placed around the correctly oriented sperm nuclei to achieve purities greater than 90 % in each of the enriched X- and Y-chromosome-bearing sperm populations. Ten ml centrifuge tubes containing 0.25 ml Androhep were used to collect sorted sperm nuclei (a minimum of 15,000 events per male). 125
141 Determination of difference in total DNA between X- and Y-chromosomebearing sperm The SX-MoFlo was used to measure the fluorescence intensity of the X- and Y-bearing sperm populations and the difference in the DNA content (percentage separation of the fluorescent peaks representing the two populations) was then determined using the formula: Difference = 100 [X Y/0.5 (X + Y)] Where X = X-bearing sperm nuclei mean fluorescence and Y= Y-bearing sperm nuclei mean fluorescence (Garner et al. 1983) Assessment of sperm sorting accuracy Reanalysis of sorted samples was used to evaluate the accuracy of sorting. For sort reanalysis, an aliquot of the sorted sample containing a minimum of 5 x 10 6 sperm nuclei was re-stained with 17.8 μm H33342, incubated for 20 min at 34 C and analysed using the SX-MoFlo (a minimum 10,000 events were analysed per sample). The proportions of X- and Y-bearing spermatozoa were determined by a mathematical model (Welch and Johnson 1999) Statistical analysis Data for the percentage of correctly oriented sperm nuclei were arcsine transformed prior to analysis. Statistical differences in the percentage of correctly oriented sperm nuclei and nuclei sorting rates were determined by ANOVA and means compared using least significant differences (LSD) in GenStat 9.0 (Ceanet, Brisbane, Australia) Results Effect of H33342 staining concentration on sorting parameters Sperm nuclei from all males and all stain concentrations could be resolved into two populations representing the X- and Y-chromosome bearing sperm. Resolution, as indicated by the depth of the split on the histogram output, of X- and Y-sperm nuclei populations was improved at higher (72 and 90 μm) H33342 concentrations. Examples of histogram and dot plot outputs for different H33342 concentrations are presented in Figure
142 a) b) c) d) Figure 9.1. Flow cytometric dot plot and histogram outputs showing fluorescent signals generated by alpaca sperm nuclei stained with a) 36, b) 54, c) 72 and d) 90 μm H Fluorescent signals from correctly oriented sperm nuclei are shown in region 1 (R1) of the dot plot output are displayed in the histogram output. Hoechst staining concentration did not influence the percentage of correctly oriented sperm nuclei (Table 9.1). The speed of sperm nuclei sorting was moderate and was not altered by H33342 stain concentration (Table 9.1). Table 9.1. Percentage of correctly oriented sperm nuclei and speed of sperm nuclei sorting (sperm nuclei/sec) after flow cytometric analysis of alpaca sperm nuclei stained with μm Hoechst Data are mean ± SEM. 127
143 Hoechst concentration (μm) Correctly oriented sperm nuclei (%) Speed of sperm nuclei sorting (sperm/sec) ± ± ± ± ± ± ± ± 127 There were no significant differences There were differences in the proportion of correctly oriented sperm nuclei (range: 33.2 ± 4.3 to 53.5 ± 4.3) and the speed of nuclei sorting (range: ± to ± 116.0) between the males (P<0.05) Sort reanalysis and DNA difference between X- and Y-bearing sperm nuclei A flow cytometric histogram generated from fluorescent signals produced by an alpaca X- and Y- chromosome bearing nuclei enriched population is displayed in Figure 9.2. Difference of 3.8 % in DNA content between X and Y alpaca chromosomes. Y-chromosome bearing sperm X-chromosome bearing sperm Figure 9.2. Calculation of the DNA difference between X and Y-chromosome bearing alpaca sperm nuclei. 128
144 A flow cytometric dot plot and histogram generated from fluorescent signals produced by an alpaca Y- chromosome bearing nuclei enriched population is displayed in Figure 9.3. The mean purity of the separated X- and Y-sperm nuclei populations was 96.6 ± 0.7 % and 96.1 ± 1.1 %, respectively, and the mean percentage difference in DNA content between X and Y-bearing sperm for alpacas was 3.81 ± 0.06 %. Figure 9.3. Flow cytometric dot plots and histogram outputs for sort reanalysis procedures showing fluorescent signals generated by alpaca Y-chromosome bearing nuclei enriched populations (97 % purity) Discussion This study represents the first application of sperm sexing technology to new world camelids and demonstrates that alpaca sperm nuclei can be separated successfully into high purity X and Y populations. Further research is required to optimize preparation and sorting procedures before the technique could be combined with AI or in vitro fertilization (IVF) for the production of pre-sexed alpaca offspring. There is considerable interest from livestock producers in the production of offspring of a predetermined sex. Pre-selecting the sex of offspring can increase the rate of genetic progress of flocks and herds, and has advantages for management and efficient production of livestock, particularly the production of female replacement dairy heifers and male and female crossbred lines in the pig industry. Camelids contribute to many economies through meat, milk and fibre production (Tibary et al. 2006) and there is considerable scope for the application of sex-preselection in the various camelid industries. The camel dairy industry would benefit from increased proportions of female replacement dairy camels. Given the differential prices for male and female llamas, alpacas and guanacos, fibre producers would have the option of producing offspring of the sex they desire. Sperm-sexing technology can also be used as a potential population management strategy for wildlife species with a single-sex dominated social structure (Maxwell et al. 2004) and could potentially be applied to populations of threatened camelids such as the vicuña. While the results of the present study demonstrate the potential for the application of sperm sexing technology to camelids, improvements in the efficiency of the procedure as well as the development of 129
145 other reproductive technologies such as AI and IVF must occur. Reproductive technologies are generally not well developed in camelids as their reproductive physiology is vastly different from other domestic species. Camelid semen is highly viscous (Bravo et al. 2000c) which has precluded the adoption of technologies developed for other species. While AI has been used to produce offspring from camelids (reviewed by Bravo et al. 2000c) the application of this technology remains restricted as the efficiency is far below commercially acceptable levels. The proportion of correctly oriented whole sperm, the difference in DNA content between the X- and Y sperm and the proportion of viable spermatozoa are the major determinants of the speed of sorting sperm. The 3.8 % DNA difference between the X and Y-chromosome bearing sperm in alpacas is sufficient to permit rapid sorting of whole sperm, but an increase in the efficiency of the sperm sexing procedure is required in order to maximize sort rates. The proportion of correctly oriented sperm nuclei found in the present study was also moderate and needs to improve if an increase in the efficiency of the sexing procedure is to occur. Sperm morphology is assumed to have a major effect on the efficiency of sexing sperm and the heterogeneous nature of human sperm heads is cited as one factor limiting the improvements in the efficiency of sexing human sperm (Vidal et al. 1998). Sperm with morphological abnormalities such as coiled tails (Rens et al. 1998b), and microcephalic and macrocephalic heads (O'Brien et al. 2005a) produce fluorescence intensity signals which are outside the region of normal fluorescence. O'Brien et al. (2005a) hypothesise that sperm with irregular and amorphous heads may produce fluorescence intensity signals within the normal range and remain within the population that are separated. High levels of sperm morphological abnormalities are present in alpaca ejaculates (approx. 30 %), with head and tail abnormalities accounting for 28 % and 42.0 % of the abnormalities, respectively (Bravo et al. 1997a). Given these proportions of abnormal sperm, their removal prior to flow cytometric sex-sorting may prove beneficial. Pre-sort processing to remove dead, immotile and morphologically abnormal sperm is routinely employed during sex-sorting of human sperm in the form of either washing, density gradient centrifugation and glass wool filtration (Fugger 1999). Density gradient centrifugation has been used previously to improve the quality and resolution of gorilla (O'Brien et al. 2005a) and human (Fugger 1999) spermatozoa. Given the moderate level of sperm nuclei orientation observed in the present study, and the sperm morphological abnormalities present in alpaca ejaculates, further studies on the application of pre-sort processing using density gradient separation seem warranted. Moderate sperm nuclei sorting rates (around 1300 nuclei/sec) were obtained in the present study and are similar to those reported during the development phase of this technology for other species (O'Brien et al. 2005b). However, for commercial application, sort rates of around 15 million sperm per hour are required (Maxwell et al. 2004). The DNA difference between the X and Y chromosome in alpacas was determined to be 3.81 ± 0.06%, which is similar to the 3.3 % reported by Johnson (2000a) for camels. This is similar to the difference in cattle and sheep sperm (3.8 and 4.2 % respectively; Johnson 2000a), which have all been sorted successfully and used to produce offspring of a pre-determined sex. In conclusion, the present study showed that standard methodology could be applied to the high-purity sorting of X- and Y-chromosome bearing sperm nuclei in alpacas. These results demonstrate the potential to produce pre-sexed alpaca offspring using flow cytometrically sex-sorted sperm. However, further research is required to optimise the pre-sort processing, develop protocols for the preservation of sperm post-sorting and increase the efficiency of current AI procedures. 130
146 10. General discussion of results Project US-138A, The continued development of artificial insemination technology in alpacas continued the work of RIRDC project AAA-1A (publication 03/104) and had two main objectives. The first objective was to further develop and establish the technology for collection, processing and preservation of alpaca semen. The second objective was to further develop and establish the technology for insemination of alpaca semen. In addressing these overall objectives, a number of aspects of AI technology in alpacas, developed during project AAA-1A, we adopted for use in the current project. These included the general set-up and training of males for semen collection, the utilising of methods for induction and synchronisation of ovulation in females, and methods for transcervical insemination of alpacas. In addition, this project sought to answer some very important questions and issues raised during AAA-1A namely: The difficulty of controlling the environment inside the artificial vagina (AV) used for semen collection, possibly resulting in reduced semen quality during prolonged copulation. The temperature of the AV decreased over time despite the presence of a heat pad around the outside. The detrimental effect on sperm survival of prolonged contact of semen with the latex liner (Aller 2001). Whether or not seminal plasma should be removed from the ejaculate. The viscous nature of alpaca semen, which precludes proper mixing of the semen with extender and results in heterogeneous solutions and poor post-storage sperm survival. The need to develop a simple, accurate method of predicting field fertility of frozen-thawed alpaca semen. The type of extender for semen freezing. The final glycerol concentration in the medium for freezing alpaca sperm. Freezing of semen in pellets or straws of different volumes. Moreover, this project generated considerable data regarding the collection, characterisation, preservation and AI of alpaca sperm. The outcomes of this project are summarised below: Further optimisation of the semen collection procedure resulting in a significant improvement in the quality of ejaculated alpaca sperm collected using a mannequin and artificial vagina. Design of a new mannequin with sufficient insulation to be used indoors and outdoors. Production of silicone AV liners to remove the deleterious effects of prolonged contact of semen with latex liners. Demonstration that an artificial cervix is not required in the AV during semen collection. Demonstration of the effect of the collection vessel on semen quality. Demonstration that the presence of females during semen collection is not required. Demonstration that collection of semen into a Tris-based diluent improves the quality of alpaca semen collected with an AV. Development of optimal laboratory handling and assessment methods for of alpaca sperm. Development of a sperm acrosome integrity assessment method using staining with FITC-PNA. Demonstration that, contrary to previous reports, Giemsa stain is not suitable for assessment of alpaca sperm. Application of CASA to alpaca sperm assessment. Preliminary development of a heterologous oocyte-binding assay for alpaca sperm. Demonstration that epididymal alpaca sperm retain functional integrity after freezing and thawing, and bind to in vitro matured sheep oocytes in vitro. 131
147 Demonstration that centrifugation and needling are not effective methods to reduce the viscosity of alpaca semen. Development of a gradient centrifugation method to separate alpaca sperm from viscous semen. Development of a method to reduce alpaca semen viscosity using gentle pipetting. Determination that the enzymes collagenase, chymotrypsin, papain and trypsin are able to reduce the viscosity of alpaca seminal plasma but that they also alter the membrane properties of sperm. Demonstration of the high protein content in the viscous semen of the alpaca. Confirmation that Biladyl and Triladyl are the most suitable diluents for liquid storage of alpaca semen. Demonstration that liquid storage of semen at 4 C is preferential to 15 C. Demonstration that 1:4 is the most appropriate dilution rate for the liquid storage of alpaca semen. First demonstration that alpaca epididymal sperm may be liquid-stored for 24 h without loss of motility or functional integrity. Demonstration that epididymal sperm do not require glycerol during cooling to, or liquid storage at 4 C. Longest retained viability to date of alpaca sperm during liquid storage (96 hours). Demonstration of considerable inter- and intra-male variation in the longevity of liquid stored semen. The first successful cryopreservation of epididymal alpaca sperm with retained viability post thaw. Superior survival of alpaca sperm cryopreserved in lactose compared with Tris- or citrate-based diluents. Demonstration that pellet freezing of alpaca sperm is superior to freezing in 0.25 or 0.5 ml straws. Recommended levels of cryoprotectants in media for cryopreservation of alpaca sperm established: 3 4 % glycerol and 1 % Equex STM paste. Development of an overnight transport protocol for testes from castrated males, with subsequent efficient harvesting and cryopreservation of epididymal sperm. Demonstration that epididymal sperm can be transported overnight, then liquid stored for 24 h prior to cryopreservation, without reduction in survival (compared with unstored sperm). Demonstration that epididymal alpaca sperm are highly tolerant of cryopreservation with around 35 % of original motility retained after freezing and thawing Paving the way for the development of sex sorting of alpaca sperm: establishment of the DNA difference between X and Y-chromosome-bearing sperm (3.8 %) and demonstration that sperm sexing technology can be applied to alpacas and sperm nuclei can be successfully separated into X- and Y-chromosome population with >90 % accuracy. From these outcomes, we have been able to provide a manual of standard methods for a number of routine procedures associated with the collection and processing of alpaca semen. In particular, training of males, semen collection, maintenance of semen quality after collection, dilution, cooling and liquid storage of semen are now well established procedures. The potential of more advanced procedures, such as cryopreservation and sex-sorting of sperm, have also been demonstrated. However, a number of important goals remain to be achieved, particularly successful and routine in vivo fertility after AI with preserved sperm. The characteristics of alpaca semen observed and recorded during this project were similar to those previously described by other workers (Table 1.10). Furthermore, considerable variation was observed, both within and between males for all semen parameters. Ejaculates of high quality were more suited to liquid storage and survived longer than those of lower quality. While the relationship between semen quality and survival after preservation is well established, there are currently no guidelines on what constitutes a high quality alpaca ejaculate. Vaughan et al. (2003a) state that Semen needs to be collected and examined from many more clinically normal alpacas of known fertility to define the limits of semen characteristics that indicate normal reproductive function and high fertility. Despite a further two years of semen collection and assessment as part of the current project, it is our opinion that this statement still holds true. Considerable research is required to 132
148 determine the average parameters of an alpaca ejaculate under Australian conditions, as well as to define parameters for good, acceptable and poor quality ejaculates. Moreover, the effects of nutrition, season and other environmental factors on semen quality must also be elucidated. Nevertheless, through the collection and assessment of many ejaculates from a standard group of males, we were able to further define the quantum of variation in ejaculate quality and confirm that this is an important limitation to the development of reproductive technology in the alpaca, compared with other small ruminants. Between and within male ejaculate quality also varies considerably in the equine species, and this has sometimes necessitated the development of tailor-made media and methods for semen preservation. This may be a necessary area for research in the future if functional sperm need to be stored from particularly valuable males. Vaughan et al. (2003a) also described a number of relationships between various semen parameters and the management of the collection process. For example, when ejaculates were collected every 3 or 4 days there was a trend for higher concentration of sperm per ejaculate and greater semen volume, than shorter or longer collection frequencies. If semen preservation and artificial insemination are to be commercially viable in Australia there must be a clear understanding of the relationships between semen parameters and male management (ie interval between semen collections) so that recommendations can be made regarding the regime for semen collection in a commercial setting. Therefore, further research, re-examining relationships between various ejaculate parameters is also required. Significant practical advances have been made in the assessment of alpaca semen in the current project (Chapter 4) although the high cost of equipment such as the CASA and FACS machine prevent their widespread use in assessing alpaca semen in the field. However, in the commercial setting of a semen preservation centre, these machines are now becoming available for routine assessment of bovine and ovine semen, particularly in Europe. They are currently available in Australia in several reference laboratories, and research correlating sperm parameters (such as motility patterns, metabolism, etc.) with field fertility could occur. The correlation of sperm fertility with a parameter which can be assessed in the laboratory remains one of the ultimate aims of andrologists. Fertility cannot be correlated to a simple assessment such as motility alone, but recently Gillan et al. (2008) described groups of assessments which were highly correlated with sperm fertility. In the latter study, the performance of frozen-thawed spermatozoa from 10 Holstein bulls in a range of in vitro diagnostic tests and the relationship with adjusted in vivo fertility data was determined. The tests included an assessment of motility (subjective and computer-assisted), morphology, concentration, viability, acrosomal and chromatin integrity conducted immediately post-thaw and after swim-up, in conjunction with membrane status (CTC staining) and migration in an artificial cervical mucus. Adjusted in vivo fertility correlated with subjectively assessed post-thaw motility (r=0.672), post-thaw straight-line velocity (r=0.636), post-thaw sperm morphology (r=-0.762), post-thaw sperm viability (r=0.635), the concentration of spermatozoa after swim-up (r=0.649), sperm morphology after swimup (r=-0.687), the number of spermatozoa migrating 10 mm into artificial cervical mucus (r=0.632) and the distance migrated by the vanguard spermatozoon in artificial mucus (r=0.701). A stepwise regression analysis identified tests which, when combined, produced models with a strong correlation (R 2 >0.9) to fertility. Although such data would be almost impossible to develop for camelids in Australia, given the lack of organised artificial breeding, such studies point to the value of high standards of semen assessment, and the utility of the data collected as a predictor of the potential fertility of males. The interval between semen collection and liquefaction in vitro necessitates the development of a method to liquefy alpaca semen. Various method of semen liquefaction exist, although the method of choice must be reliable, easy (in a commercial setting), cost and time-effective and not reduce sperm viability or function. A number of mechanical and enzymatic methods for reducing semen viscosity were evaluated. Centrifugation and needling were not effective, gradient centrifugation using 45 : 22.5 % PureSperm was more effective in recovering sperm from the viscous seminal plasma but the cost of Puresperm is too high to justify its use commercially. Gentle pipetting of semen diluted with Androhep was effective in reducing semen viscosity without compromising sperm acrosome 133
149 integrity or sperm motility. This method is simple but it is not completely effective and does not remove any other detrimental effects of the seminal plasma which is still present after the procedure. Treatment of semen with enzymes (either during semen collection or added afterwards in the lab) had some effects on sperm function although the nature and degree of the effect varied according to the enzyme used, in contradiction to the results of Bravo et al. (1999) and Bravo et al. (2000b). However, the favourable outcomes demonstrated by the use of papain also proved that the viscous component of seminal plasma was highly susceptible to liquefaction by proteolytic enzymes, thus proving that it has a major protein component. It has been reported in the literature that the viscous semen is composed of sulphated mucopolysaccharides secreted by the bulbourethral gland during the rutting season (El- Naggar and Abdel-Raouf 1977). Based on the lack of evidence for this claim, and in addition to studies determining the optimal use of proteolytic enzymes such as papain, we believe that studies are urgently required to confirm the findings of El-Naggar and Abdel-Raouf (1977). Further studies are also required to identify the components responsible for the viscosity before effective, efficient and commercially viable methods for the liquefaction of alpaca semen can be developed. Results from the liquid storage of alpaca semen confirmed the earlier findings of (Vaughan et al. 2003a) that Triadyl was the most promising diluent. After several studies examining the effect of dilution rate, storage temperature and presence of seminal plasma on the survival of sperm after liquid storage, the optimal method for alpaca semen was confirmed to be a 1:4 dilution (semen: diluent) with Triadyl and storage at 4 C (see Appendix IX for a detailed protocol for the liquid storage of semen). In this report, diluents were made with clarified egg yolk (achieved by ultracentrifugation to remove egg yolk particles) to facilitate the visualization of the sperm during liquid storage. In a commercial setting, clarification of egg yolk is time-consuming and expensive (ultracentrifuges cost > $100,000) and is therefore not recommended. Therefore, the use of egg yolk-free liquid storage media such as Androhep and Androhep EnduraGuard would be advantageous but survival of alpaca sperm stored these diluents has not been satisfactory. In this study, sperm motilities of > 50 % were observed in individual ejaculates after 72 h of storage, although the average values were lower (owing to storage of poorer quality ejaculates). Selection of males with superior semen quality, resulting in the storage of only high quality ejaculates, will improve the proportion of motile sperm after liquid storage. Further improvements to sperm survival might also be obtained if the sperm were stored under anaerobic conditions (for example, in an atmosphere of carbon dioxide) or with the addition of antioxidants such as catalase (Vishwanath and Shannon 2000). Routinely, motilities of 45 % were observed after h of liquid storage, indicating that the use of liquid stored semen for AI in Australia will be possible in the not too distant future. Nevertheless, more work is required to determine the optimal timing of insemination, sperm dose and site of sperm deposition before commercialization of AI with liquid stored semen, or preserved semen in general. In the field fertility trial conducted at the end of the current project, no pregnancies were obtained after transcervical insemination of alpaca females with frozen-thawed epididymal sperm at an induced oestrus. Induction of ovulation was achieved, confirming previous reports by Vaughan (2001). However, despite retained sperm function after freezing and thawing, we were unable to properly test the in vivo fertility of frozen-thawed epididymal sperm. We made some progress towards determining the fertilizing capacity of these sperm through the development of a heterologous in vitro binding assay (Chapter 4). However, these were preliminary results, and future research needs to initially determine the fertilizing capacity of frozen-thawed alpaca sperm. Because of the severe limitations of the viscous seminal plasma in ejaculated semen, it is recommended that this work be done on epididymal sperm, based on the methods developed in the current project. The fertilizing capacity should be determined through an in vitro fertilization (IVF) system, either by direct IVF of fresh alpaca oocytes or, more practically, through a sperm binding and penetration test. Importantly, further development of AI with frozen semen in alpacas will also depend on a solution to the problem of the 134
150 viscous seminal plasma in the ejaculate, so that freezing technology can be transferred from epididymal to ejaculated sperm. While important advances have been made in semen collection and preservation by Vaughan et al. (2003a) and during this project, the key problem for the storage of semen from all camelids remains the viscous seminal plasma. If the industry is to achieve the practical application of liquid and frozen sperm storage, and a viable system of artificial insemination in camelids, particularly llamas and alpacas, the following basic questions must be answered: (1) What are the sources and constituents of the viscous seminal plasma? and (2) What is the basic protein content, structure and function of camelid seminal plasma? Analysing the source and constituents responsible for semen viscosity is critical to developing methods to overcome or circumvent the deleterious effect of the seminal plasma. This has been achieved in other species with problematic seminal plasma, for example the goat, but has either been ignored or considered too difficult in camelids. The studies undertaken during this project did indicate that the viscous component of alpaca seminal plasma is susceptible to proteolytic enzymes, and is therefore likely proteinaceous in nature. However, all attempts to disrupt or dissolve (liquefy) the gel failed to provide sperm of a quality equivalent to those derived from the epididymides, which had not been exposed to seminal plasma. Further progress on the removal of this viscosity will depend on the identification of its origin and biochemical nature. 135
151 11. Implications In evaluating the implications of the practical research undertaken in the current project and RIRDC project AAA-1A, it is important to note that research on alpaca reproduction in Australia is currently very difficult. There are a number of reasons for this. Principally, the numbers of females and males available for research is limited, both by price/value and availability. The alpaca industry has been generous with offers of animals and facilities but these have always been constrained by the relatively high value of the individual animals. Moreover, there are particular ethical and welfare limitations to the application of ART: females require sedation and skilled individual handling for common reproductive tasks, such as transrectal ultrasound and transcervical insemination. In cattle and sheep, such procedures are routine, requiring less intervention, minimal skill and less time/labour. Such issues not only require careful consideration of the welfare of the individual animals but also limit the number of animals and procedures that can be carried out in a given time. A positive side of these limitations, and particularly the high value of individual animals, is that high cost and labour intensive procedures may be more affordable, or have a better benefit-cost ratio, in alpacas compared with other small ruminants. It may well be worthwhile developing a semi-surgical inseminations technique to allow the use of frozen semen AI in valuable females using semen from males of high genetic value. Thus, the implications of the current research project are that some significant barriers still need to be overcome before AI can be routine in the alpaca industry. With these important considerations in mind, a roadmap for the research and application of AI in the Australian alpaca industry might look something like this: 1. Confirm the in vitro and in vivo fertilising capacity of both liquid and frozen-stored epididymal alpaca semen: a. In vitro using laboratory-based in vitro fertilisation procedures: this will confirm whether or not the sperm cells actually retain normal egg binding and penetration capacity after preservation and in the absence of seminal plasma. b. In vivo by using semi-surgical laparoscopic techniques, such as those developed in our laboratory for the sheep model, to deposit the preserved sperm close to the site of fertilization in the oviducts: this will confirm whether or not the sperm cells actually retain normal fertilizing capacity after preservation and in the absence of seminal plasma. 2. Determine the sources and constituents of the viscous seminal plasma of camelids and identify its basic protein content, structure and function: this will lead to the development of an artificial but non-toxic method for disrupting or liquefying the seminal plasma immediately after ejaculation, so that ejaculated sperm may be utilised routinely for preservation and in vivo inseminations. The end of this roadmap would be, at worst, an effective but high-cost method for successful AI of alpacas with preserved sperm for a wide range of males and, at best, semen preservation and AI procedures as routine as those currently available in the sheep and goat industries. Both outcomes would undoubtedly provide a significant boost to the genetic improvement of alpacas in Australia. 136
152 12. Recommendations AI in alpacas will facilitate the widespread use of sires of high genetic value (both national and international) and increase the rate at which superior genetics are disseminated. While offspring have been born from AI with both fresh and frozen-thawed sperm (Table 1.18) the efficiency is low. Improvements to liquid and frozen sperm preservation techniques would certainly increase AI efficiency. However, it is doubtful that any major increases in efficiency (to the levels required for commercialisation) will occur until there is a fundamental understanding of the components of the seminal plasma, which limits both the function and recovery of sperm from the alpaca ejaculate. In many ejaculates, a high proportion of the sperm are unavailable for processing and storage because they are either bound up in the viscous seminal plasma and/or inhibited by its components. Epididymal sperm do not have these restrictions, because they have yet to be exposed to the seminal plasma, which is naturally mixed with the sperm at the time of ejaculation. In this project, we have been able to demonstrate methods to recover these epididymal sperm from castrated males, and to process and store them, with their function maintained for up to 96 hours or indefinitely, for liquid and a frozen storage, respectively. We were unable to obtain in vivo fertility after transcervical insemination of female alpacas with frozen-thawed epididymal sperm, but we obtained preliminary evidence that they may be able to bind to oocytes in vitro. The commercial use of epididymal semen is unlikely to be practicable, apart from particular circumstances such as the death or serious injury/disease of a particularly valuable male, or in the event of sperm harvest from genetically valuable clones, as its recovery is a surgical procedure. However, epididymal sperm do provide a good model for determining the innate fertilising capacity of camelid sperm, unsullied by the inhibiting factors in seminal plasma. It is therefore recommended that studies continue on determining the fertilising capacity of both epididymal and ejaculated sperm, after liquid and frozen storage, using in vitro and in vivo techniques. Given the difficulties of obtaining consistent fertility to date, and the limited numbers of animals available for research, it may be that a laparoscopic or mini-laparotomy technique needs to be developed, to enable deposition of stored semen close to the site of fertilisation, probably in the oviduct. Such a procedure might be more costly than current transcervical insemination methods, although the latter still require induction of ovulation, sedation and individual handling of females, but a more reliable AI technique might allow high fertility to be obtained from the semen of highly valuable males deposited in high value females. We made some progress towards determining the fertilizing capacity of epididymal sperm through the development of a heterologous in vitro binding assay (Chapter 4). However, these were preliminary results, and future research needs to initially determine the fertilizing capacity of frozen-thawed alpaca sperm. Because of the severe limitations of the viscous seminal plasma in ejaculated semen, it is recommended that this work be done on epididymal sperm, based on the methods developed in the current project. The fertilizing capacity should be determined through an in vitro fertilization (IVF) system, either by direct IVF of fresh alpaca oocytes or, more practically, through a sperm binding and penetration test. Importantly, further development of AI with frozen semen in alpacas will also depend on a solution to the problem of the viscous seminal plasma in the ejaculate, so that freezing technology can be transferred from epididymal to ejaculated sperm. The key problem for storage of semen from all camelids remains the viscous seminal plasma. If the industry is to achieve the practical application of liquid and frozen sperm storage, and a viable system of AI in camelids, particularly llamas and alpacas, the following basic questions must be answered: (1) What are the sources and constituents of the viscous seminal plasma? and (2) What is the basic protein content, structure and function of camelid seminal plasma? Analysing the source and constituents responsible for semen viscosity is critical to developing methods to overcome or circumvent the deleterious effects of the seminal plasma. This has been achieved in other species with problematic seminal plasma, for example the goat, but has either been ignored or considered too difficult in 137
153 camelids. The major problem for goat semen preservation is the bulbourethral gland secretion (BUS) responsible for deterioration of sperm diluted in skimmed milk or in media containing egg yolk. The component from BUS responsible for this effect was identified as a kda glycoprotein lipase (Busgp60) belonging to the pancreatic lipase related protein 2 family (GoPLRP2). This enzyme was able to hydrolyse both triolein and milk triglycerides into free fatty acids which strongly inhibit the motility and damage the membranes of buck sperm. The solutions to this problem, developed in France and by our group during the 1980s, have been one of the most significant advances for the European goat industry over the past 50 years, leading to the widespread application of AI in dairy goats. The main recommendation from this project, therefore, is to undertake fundamental studies on the viscous seminal plasma of alpacas with the objective of answering the two questions posed above. A successful outcome to such a study would be a major step along the roadmap to the widespread commercialisation of AI for the Australian alpaca industry. 138
154 13. Appendices Appendix I: List and source of laboratory equipment Equipment (product no.) Artificial insemination gun Artificial insemination sheath Boar semen bottle Bovine AV casting Callipers Camel collecting glass CASA Centrifuge tubes (15 ml) Sarstedt yellow top Controlled rate freezing machine Coverslips 22 x 22 mm, no. 1.5 (560056) Coverslips 18 x 18 mm, no. 1.5 (560041) Dry ice (CO 2 ) Esky Filter paper (Whatman no. 1) Goblets Haemocytometer Heating pads Incubator Liquid Nitrogen Mannequins Microscope phase contrast Microscope fluorescent Micro centrifuge tubes (clear) Microscope lens wipes MiniLube (17116/7010) Needles Osmometer Pipettes Pipette tips (Greiner; blue) Pipette tips (Greiner; yellow) Petri dishes (35 mm) Manufacturer/Supplier Laboratory equipment IMV Technologies (available through Pacific Vet) IMV Technologies (available through Pacific Vet) Minitüb used as water jackets during semen cooling IMV Technologies (available through Pacific Vet) Bunnings IMV Technologies (available through Pacific Vet) Hamilton Thorne Livingstone International Pty Ltd Planar Kryo 10 Series III, Quantum Scientific, Rydalmere, NSW, Australia Australian Scientific Australian Scientific BOC gasses. Livingstone Bacto Laboratories, Liverpool, NSW, Australia Pacific vet Improved Naebauer, Weber, London United Kingdom Breville Thermoline (bench top; ungassed) Small Thermoline incs LN 2 was delivered fortnightly by Ace Cryogenics, when necessary additional LN 2 was obtained from The University of Sydney Chemistry Department. Manufactured at The University of Sydney according to the dimensions described in Chapter 3. Olympus, Tokyo, Japan Olympus, Tokyo, Japan ThermoTrace Livingstone International Pty Ltd Minitüb, Germany Livingstone International Pty Ltd Freezing point depression osmometer, Advanced Instruments, Norwood, Massachusetts, USA. Gilson or Finpette brand from Bacto Laboratories Interpath Services Pty Ltd PO Box 340 Heidelberg West 3081 Interpath Services Pty Ltd PO Box 340 Heidelberg West 3081 Bacto Laboratories 139
155 Petri dishes (100 mm) Bacto Laboratories Portable car fridge Waeco International, Burleigh Heads, Queensland, Australia Scalpel blades Livingstone International Pty Ltd Surgical instruments Livingstone International Pty Ltd Sheep artificial vagina Pacific vet Straw sealer Heat-sealer, Packmatic Australia, Blacktown, NSW, Australia SX-MoFlo Dako-cytmoation Syringes Livingstone International Pty Ltd Sharps disposal containers Livingstone International Pty Ltd Slides (plain glass; 75 x 25 mm) Livingstone International Pty Ltd Slides (Starfrost) Livingstone International Pty Ltd Spermac stain (15405/0000) Minitub Straws 0.25 ml IMV Straws 0.5 ml IMV Syringes Livingstone International Pty Ltd Tongs Livingstone International Pty Ltd Transfer pipettes Bacto Laboratories Plastic cell culture tubes 6 ml (352001) Bacto Laboratories Plastic cell culture tubes 14 ml (352003) Bacto Laboratories Test tubes glass 12 x 75 mm (TTRL 12x 75) Livingstone International Pty Ltd Test tubes glass 13 x 100 mm(ttrl 13 x 100) Livingstone International Pty Ltd Thermometer (digital) HI98509 Bacto Laboratories Ultracentrifuge Becton Dickenson Becton Coulter Australia Pty Ltd Ultracentrifuge tubes # Unit D/24 College Street Gladesville NSW 2111 Ph: Vortex (model 34524) Snijders press-to-mix vortex, Australian Instrument Services, Bayswater, Victoria, Australia Warm trays LEC details # Tubes dependant on the ultracentrifuge rotor 140
156 Appendix II: List and source of laboratory chemicals Chemical/Equipment (product no.) Manufacturer/Supplier Laboratory chemicals Bromelain (B-4882) Sigma-Aldrich chemical company Bovine serum albumin (Fraction V; A9647) Sigma-Aldrich chemical company Citric Acid (monohydrate; C7129) Sigma-Aldrich chemical company Collagenase (C9722) Sigma-Aldrich chemical company α-chymotrypsin (CHY5S) Sigma-Aldrich chemical company Acridine Orange (A-1301) Sigma Caffeine (anhydrous; C-0750) Sigma Catalase (C-9322) Sigma-Aldrich chemical company (in vitro use only) Eosin Sigma-Aldrich chemical company Ethylene glycol (E-9129) Sigma-Aldrich chemical company Equex STM Paste IMV, L Aigle, France, or Nova Chemicals Lectin from pisum sativum (FITC-PNA; L-0770) Sigma-Aldrich chemical company Fructose (F-0127) Sigma-Aldrich chemical company Galactose (G-5388) Sigma-Aldrich chemical company Gentamycin sulfate (G-3632) Sigma-Aldrich chemical company Giemsa stain (G-9641) Sigma-Aldrich chemical company Glucose (G-8270) Sigma-Aldrich chemical company Glutaraldehyde Sigma-Aldrich chemical company Glycerol (G-2025) Sigma-Aldrich chemical company Bisbenzimide Hoechst (B-2261) Sigma-Aldrich chemical company Kanamycin monosulfate (K-1377) Sigma-Aldrich chemical company Mineral oil (embryo tested; M-5310) Sigma-Aldrich chemical company Lactose (L-3625) Sigma-Aldrich chemical company Nigrosin Sigma-Aldrich chemical company Neomycin sulfate (N-6386) Sigma-Aldrich chemical company Papain (P-5306) Sigma-Aldrich chemical company Dulbecco s PBS powder (D-5652) Sigma-Aldrich chemical company PBS tablets (BR14) Oxoid Percoll (cell culture tested; P-4937) Sigma-Aldrich chemical company Penicillin G (sodium salt; P-3032) Sigma-Aldrich chemical company ph strips Sigma-Aldrich chemical company p-phenylenediamine Sigma-Aldrich chemical company Propidium iodide (P-4170) Sigma-Aldrich chemical company 1,2-Propanediol (Propylene glycol; P-1009) Sigma-Aldrich chemical company Barnes Products Pty Ltd Prosil PO Box 393, Padstow, NSW (SR-PROSIL20-T) Ph: Fax: Puresperm 100 Nidacon Tek Event Sodium azide (S-8032) Sigma-Aldrich chemical company Sodium bicarbonate (S-1554) Sigma-Aldrich chemical company Sodium citrate Sigma-Aldrich chemical company Sodium chloride (S-5886) Sigma-Aldrich chemical company Sodium phosphate (dibasic anhydrous; S-5136) Sigma-Aldrich chemical company Spermac stain Streptomycin sulfate (S-6501) Sigma-Aldrich chemical company Superoxide dismutase (S-8160) Sigma-Aldrich chemical company Trizma base (T-1503) Tris(hydroxymethyl)aminomethane Sigma-Aldrich chemical company Trypsin (T-9935) Sigma-Aldrich chemical company Triton-X 100 (T-9284) Sigma-Aldrich chemical company 141
157 Appendix III: Semen collection worksheet Experiment: Date: Male: Mating assessments AV Temp: Collecting container H 2 O bath temp: AV configuration: Mating duration: Start: Finish: Length: Libido: Time to mount mannequin: Mating comments: Semen assessments Semen appearance: clear / milky / creamy Semen volume (minus foam): Semen viscosity (mm): Semen Osmolarity: Sample number (if required): Foam: present / absent Semen ph: Sperm assessments Motility: Concentration: x 10 6 /ml Viability: Live: Acrosome integrity: Intact: Morphology: Normal: Head abnormalities: Mid-piece abnorm: Tail abnorm: Cytoplasmic droplet: Motility type: oscillatory / forward progressive Dead: Damaged: 142
158 Appendix IV: List and composition of diluents For quality control relating to manufacture of media see Methodologies Diluent Manufacturer/Supplier Composition Androhep (product no /2001) Androhep Enduraguard (product no /2010) Biladyl A/B (product no /004) Camel Buffer Citrate Equipro (product no /2001) Lactose Salamon s cryodiluent (1+2 dilution) Salamon s liquid storage diluent Triladyl (product no ) Minitüb, Germany Minitüb, Germany Minitüb, Germany IMV USA Manufacturer at The University of Sydney according to Evans and Maxwell (1987) Minitüb, Germany Manufacturer at The University of Sydney according to Morton et al Manufacturer at The University of Sydney according to Evans and Maxwell (1987) Manufacturer at The University of Sydney according to Evans and Maxwell (1987) Minitüb, Germany # prior to adjustment by commercial suppliers and commercial confidentiality Glucose 26.0 g, HEPES 9.0 g, EDTA (triplex) 2.4 g, Sodium citrate (dihydrate), 8.0 g, Sodium bicarbonate (NaHCO ) 1.2 g, BSA (fraction V) g per litre (Weitze, 1990) # Not known Fraction A: 100 mm Tris, 33 mm Citric acid, 28 mm Fructose solution with antibiotics (Tylosin, Gentamycin, Lincomycin, Spectinomycin) and egg yolk (20 % v:v) Fraction B: 100 mm Tris, 33 mm Citric acid, 28 mm Fructose solution with antibiotics (Tylosin, Gentamycin, Lincomycin, Spectinomycin) egg yolk (20 % v:v), and 13.6 % (v:v) glycerol Clear camel buffer non-glycerolated. Green camel buffer glycerolated. Constituents not known Fraction A: 80.6 mm sodium citrate, 33.3 mm glucose solution supplemented with 20 % (v:v) hens egg yolk. Fraction B: 80.6 mm sodium citrate, 33.3 mm glucose solution supplemented with 20 % (v:v) hens egg yolk and 5 % (v:v) glycerol. Defined medium based on specific caseinates derived from various fractions of milk casein, sugars and buffers. Fraction A: Lactose solution (11 %; w:v) supplemented with 20 % (v:v) hens egg yolk. Fraction B: Lactose solution (11 %; w:v) supplemented with 20 % (v:v) hens egg yolk and 9 % (v:v) glycerol and 1.5 % (v:v) Equex STM paste. 360 mm Tris, mm citric acid, 33.3 mm glucose solution supplemented with 20 % hens egg yolk (v:v) and 6 % (v:v) glycerol. 300 mm Tris, 94.7 mm citric acid, 27.8 mm fructose solution supplemented with 20 % hens egg yolk (v:v). Tris, citric acid, fructose, antibiotics, egg yolk (20 % v:v), glycerol, tylosin, gentamycin, spectinomycin and lincomycin, exact proportions not specified. 143
159 Minitüb products are available in Australia from: Minitüb Australia Pty. Ltd. Alan Smith, General Manager P.O. Box 218 Sebastopol, Vic 3356 Phone: Fax: Web: Pacific Vet Unit 1, Herald St. Cheltenham, Vic 3129 Phone: Fax:
160 Appendix V: Company contact details Bacto Laboratories Pty. Ltd. P.O. Box 295 Liverpool NSW 2170 Phone: Fax: Cryologic Pty Ltd Dr Anna Skroce, product manager 54 Geddes St Mulgrave Vic 3170 Phone: Fax: Web: Genetics Australia Woolpack Rd Bacchus Marsh Vic 3340 Phone: 03 Fax: 03 HD Scientific Trevor Hinwood 10/28 Garling Rd Kings Park, NSW 2148 Phone: Fax: IMV International Corporation th Avenue North Maple Grove, MN, 55369, USA Phone: Fax: Web: Invitrogen Hanna Lampinen PO Box 4296, Mulgrave, Vic, 3170 Phone: Fax: Web: Interpath Australia Pty. Ltd. 44 Crammond Bvd Caringbah NSW 2229 Phone: Fax: John Morris Scientific Pty. Ltd. PO Box 447 Willoughby NSW 2068 Phone: Fax: LEC Instruments Lauri Aaltonen, Director 8 Doris Court, Scoresby, Vic, 3179 Phone: Fax: Lauri@lecinstruments.com Web: Life Technologies (GibcoBRL) Andrew Szentirmay PO Box 4296, Mulgrave, Vic, 3170 Phone: Fax: aszentir@lifetech.com Livingstone International Pty. Ltd Epsom Rd Rosebury NSW 2018 Phone: Fax: Minitüb Australia Pty. Ltd. Alan Smith, General Manager P.O. Box 218 Sebastopol, Vic 3356 Phone: Fax: infor@minitube.com.au Web: Minitüb Abfüll-u. Labortechnik GmbH & Co. KG Dr. Monika Esch, Sales Manager Hauptstaβe 41 Tiefenbach, D-84184, Germany Phone: Fax: esch@minitube.de Web: Nidacon Tek-event Regine Regal P.O. Box 569, Round Corner NSW 2158 Phone: Fax: regine@tekevent.com Web: 145
161 Oxoid Australia Pty. Ltd. P.O. Box 220 West Heidelberg Vic 3081 Phone: Fax: Pacific Vet Unit 1, Herald St. Cheltenham, Vic 3129 Phone: Fax: Sarstedt Australia Pty. Ltd. P.O. Box 90 Ingle Farm SA 5098 Phone: Fax: Sigma-Aldrich Chemical Company Pty. Ltd. P.O. Box 970 Castle Hill NSW 2154 Phone: Fax:
162 Appendix VI: Protocol for the production of AV liners Setup: Mould (vacuum cleaner pipes) Prosil 20 Paint brushes Petri dishes (120 mm) Prosil 20 preparation: Weight 100 g of Prosil 20 part A into a 120 mm Petri dish weight 100 g of Prosil 20 part B into a 120 mm Petri dish Combine parts A and B in the deeper half of the Petri dish and mix well with a paint brush Liner production protocol: Set up the oven at 100 C and turn on (allow min to warm) Prepare Prosil 20 Spray the moulds with 70 % (v:v) ethanol and dry with a kim wipe or other lint free tissue. Place mould in the oven for 5 min to warm Paint a thin layer of Prosil 20 onto the mould with a paint brush and place in the oven for 30 min to dry. It is important that each layer be thinly painted on, thick layers reduce the elasticity of the finished liner. Check the layer and if dry add a second layer, otherwise return to oven for min. Add successive layers until the desired thickness (10-12 layers) After the last layer has been added, return the mould to the oven for 1 hour at 110 C. Remove the mould from the oven and place in a bucket of cold water for 10 min to cool down. Peel off the liners from the moulds by rolling down the mould. Unroll the liner, hang it on a wooden pole (to prevent the inside of the liner sticking) and store in the liner box. Washing liners: Silicone liners can be hand washed in a non-pyrogenic detergent such as 7X or Pyroneg at concentrations of 0.01 % (v:v or w:v respectively) After use, rise the liner with warm water as soon as possible Wash by immersing the liner in warm water and detergent (see above) and gently agitate Immersion and agitation in warm water are generally sufficient to remove trace amount of semen. If liners are particularly dirty, gently scrub the inside of the liner with a babies brush. Rinse well in RO and Milli-Q water Hang vertical (ensuring that the liner is an open cylinder not collapsed) and peg one end of the liner on the tea-towel (or similar) rack 147
163 Appendix VII: Protocol for the collection of alpaca semen Equipment: Rubber artificial vaginas IMV Camel collecting glasses Silicone liners Rubber bands Minilube Electric blankets Catheter bag ties Esky Semen collection worksheets & pen Timers Setup: In Lab: 1. Turn on water bath and power pack connected to the microscopes and warming stages. 2. Boil kettle and fill water container. 3. Collect liners, camel collecting glasses, esky, timers, worksheets, pen etc and take to the shed In Shed: 1. Adjust boiled water to 60 C and 37 C and place in appropriate incubators 2. Fill water-jacket on the camel collecting glass and place in 40 C incubator 3. Get down mannequins and plug in electric blankets 4. Make up AVs (see below) and take to semen collection pen 5. At pen, wrap the AV in the electric blanket and tie with a catheter bag strap and place inside mannequin. Tie the AV into the mannequin with a catheter bag strap to prevent the AV slipping backwards 6. Set up paperwork, re-set timers and place feed into the feed tin 7. Collect males from paddock into the holding pen and close gate Alpaca artificial vagina set-up: 1. Place the silicone liner into the AV and fold back approx. 1.5 inches of the liner over the end of the shorter side of the AV (when measuring the distance between the tap and the end of the AV) 2. Fold approx. 1.5 inches of the liner over the other end of the AV and pull out the overhang of the silicone liner (this is where the collecting glass attaches). 3. Secure ends with elastic bands by folding around 2-3 times, depending on type of elastic bands. 4. Check that the elastic bands are tightly around the ends of the AV this is important to prevent water and air leaking out of the AV. 5. Fill the AV with approx. 180 ml of 60 C water. 6. Insert the camel collecting glass into the overhanging section of liner by stretching and secure with an elastic band if necessary 7. Open the top part of the tap and blow-up the AV with air 8. Check the pressure of the AV and make sure it has enough pressure (a snug fit for a pinkie finger) 9. Depress the plunger on the Minilube container 1-2 times and smear the minilube around the entrance to the AV. Semen collection procedure: 1. After the AVs are fitted into the mannequin let the male in the pen. 2. Start timer once male has begun orgling and sat down on the mannequin 3. Record information and observation on the worksheet - during mating observe behaviour and check how fidgety the males are how many times they get on and off the mannequin (record behaviour on semen collection sheet) 4. After male has finished mating, stop timer and untie the AV from the mannequin. 5. Shake the AV so that the semen in the liner is forced down into the collection tube 6. Remove the camel collecting glass and place in the esky with the appropriate worksheet and transport to the lab 7. Dismantle the AV, pour out the water, and place the liner in the washing bucket. 148
164 Appendix VIII: Protocol for the assessment of alpaca sperm acrosome integrity using FITC-PNA References: Morton, KM, Bathgate R, Evans G, & Maxwell WMC 2007, Cryopreservation of epididymal alpaca (Vicugna pacos) sperm: a comparison of Citrate, Tris and Lactose based diluents, and pellets and straws. Reprod Fertil Dev, Media/Stain Prep: PNA: 1. Add PBS to lyophilized stock to make 1mg/ml solution (Sigma L-7381; lectin from Arachis hypogaea, FITC labelled). 2. Store in freezer 20ul aliquots in 1.5ml micro centrifuge tubes wrapped in foil to protect from light 3. Thaw 20ul aliquot just before use and dilute with 480ul PBS (final working concentration is 10ug/ml). 4. Re-freeze any working solution that is not used (mark on tube date of re-freeze and that it is a working solution). UCD Mounting medium: 1. Into a foil covered 10 ml place 0.5ml of 100x penstrep stock (ie 750mg Penicillin-G and 500mg streptomycin made up to 100ml in Milli-Q water freeze in 1ml aliquots in eppendorfs) 2. Add 5mg p-phenylenediamine (= 0.1% w/v) (Sigma P6001, free base) 3. Add 4.5ml glycerol. 4. Adjust to ph 8.0 using ph strips. 5. Store as 100ul aliquots in 0.5ml foil-covered micro centrifuge tubes in freezer. Specimen preparation and staining: 1. Label slide with a pencil (pen ink is dissolved by ethanol) with date, male, experiment/treatment and other necessary information 2. Make slide smears by placing 5 10 μl sample on slide (if concentrated (ie >400 x 10 6 /ml) slowly dilute sample with appropriate medium if possible) 3. The smear is made by placing a clean slide near the sample until it comes into contact with the sample; the clean slide is then dragged down the sample slide so that the sample follows the moving slide 4. Air dry the smear for 5 min on the bench 5. Dip slides into 96 % (v:v) ethanol for 30s to fix, and air dry on bench 6. Place slides in a slide box and store in fridge at 4 C until assessment. Ideal storage time is less than 4 weeks but slides can be stored for as long as 18 months 7. For staining, place 30 μl of PNA working solution on the centre of the slide and roll the slide from side to side to spread the PNA solution so it covers a large area of the slide 8. Mark the boundaries of the PNA solution with a pen 9. Place slides in the humidified bench top incubator and dry for 30 min at 38 ºC (the incubator can be humidified by placing a large surface area container with water in the bottom) 10. Rinse slides by dipping in a 50 ml tube of PBS (without antibiotics). Dip slides twice if the concentration of sperm is high, or once if the sperm sample is dilute [change PBS dipping tube regularly to avoid cross contamination between experiments and samples] 11. Air-dry slides (or dry in a non-humidified incubator) and place 5 μl of UCD mounting medium on slide, cover with clean 20 mm x 20 mm coverslip (or larger coverslip if sperm sample is dilute) DO NOT put excess mounting medium on slide (it should not ooze out sides are coverslip has settled) 12. Evaluate sperm using fluorescent light on the Olympus BHS microscope (fitted with phase contrast and fluorescent optics) at either magnification x 400 (40 x objective; alpaca, bovids, 149
165 sheep, pigs) or x 1000 (100 x objective; cats, primates, rhinos) [Excitation beam passed through a nm band pass filter with a supplementary EY 510 nm dichroic mirror and an additional 490 nm barrier filter] 13. Use low-level bright field microscopy (white light) in conjunction with fluorescence to enable proper visualisation. Only assess sperm where the entire head can be clearly visualized 14. Assess a minimum of 100 sperm per slide 15. Staining patterns with PNA: Bright green head and/or apical ridge = acrosome intact Bright green, patchy head = partially reacted (or dissolving of acrosomal membrane) Very faint-to no green stain over entire head = non-intact ie acrosome reacted Bright green band across equatorial segment = non-intact ie acrosome reacted 150
166 Appendix IX: Protocol for the liquid storage of alpaca semen References: Morton KM, Gibb Z, Wilson SJ, Bertoldo M, Evans G & Maxwell WMC 2007, Effect of diluent, dilution rate and storage temperature on longevity and functional integrity of liquid stored alpaca (Vicugna pacos) sperm. Reprod Fertil Dev (submitted) Equipment: Equipment for semen collection (see protocol) Microscope Fridge (4 or 15 C) Misc. lab equipment Media & Prep: Media: 1. Prepare diluent for liquid storage as described in Appendix IV and Chapter Thaw diluent (if frozen) in water bath until diluent is the same temperature as the semen 3. Ensure that diluent is thoroughly mixed by inverting tube 5 10 times, slowly taking care not to shake. Preparation: 1. Turn on all equipment in the lab and allow it to warm/cool to the desired temperature 2. Ensure that there are sufficient yellow top tubes and foil covered boar semen AI bottles for the volume of semen to be stored 3. Label the required number of yellow top tubes, starsted slides and amber tubes with appropriate details (male, date, time, diluent, dilution rate, etc) 4. Set up 2 nd water bath to provide warm water for inside the boar semen preservation bottles Specimen preparation: 1. Collect semen according to the semen collection protocol 2. Assess semen parameters according to semen collection worksheet (or experimental design). If semen has been collected into a diluent other than the one to be used for liquid storage this must be removed before diluting the semen. To remove collection diluent, centrifuge semen for 7 min at 600 g, remove and discard the supernatant. 3. Pay particular attention to semen volume (or sperm concentration, if using concentration as a basis for diluting semen). 4. If neat semen is collected, or semen is still viscous dilute semen 1:1 (or add approximately 1.0 ml for centrifuged semen) and gently pipette with a 1.0 ml pipette until the viscosity is reduced. 5. Examine sperm motility; if sperm display forward progressive motility continue with sperm dilution. If sperm are still oscillatory, continue gentle pipetting until an increase in forward progressive motility is observed. 6. Dilute semen stepwise ensuring that small drops of diluent are added and thoroughly mixed before any additional diluent is added. 7. Once sperm are fully diluted re-assess sperm motility, acrosome integrity and sperm morphology (and another other assessments according to experimental design). 8. Place tube of diluted sperm into a boar semen AI bottle (previously filled with warm water up to the marker line) and place in the 4 C fridge. 9. Open the fridge and examine the semen only as required. Opening the fridge door too frequently severely reduces the longevity of liquid stored semen! 151
167 Appendix X: Protocol for the cryopreservation of alpaca sperm Equipment: Surgical instruments and scalpels (one epididymal kit per person) Dry ice (remember to order before noon the day before testes arrive) Petri dishes 3 and 6 cm Ruler Setup: In Lab: 1. Turn on water bath and power pack connected to the microscopes and warming stages. 2. Thaw Androhep (without egg yolk) 3. Set up epididymal kits, paper towels dishes etc 4. Collect liners, camel collecting glasses, esky, timers, worksheets, pen etc and take to the shed In Shed: 8. Unwrap testes and trim off excess tissue 9. Dissect epididymis away from the testis 10. Place epididymis in a 3 or 6 cm Petri dish, depending on size, and pipette 2-3 ml over the epididymis (to prevent it drying out) 11. Mince the epididymis into small pieces with a scalpel and place the dish on a warm stage (the heat helps the sperm to swim out). Leave sperm to swim out for min. 12. Repeat for other epididymis 13. Measure and weigh the testes 14. Cut the testes in half and place in a red top histology pot (label with male s name/iar/tag number and the date). 15. After 30 min, pipette 5 L of Androhep from a Petri dish and examine for the presence of motile sperm. If motile sperm are present, aspirate Androhep and sperm from the Petri dishes and place in a yellow top tube. 16. Add an additional 2-3 ml of fluid into the Petri dish, place on a warm stage for min. This step can be repeated while motile sperm are recovered from the testes pieces. 17. Sperm can be frozen in a number of small batches, or held until all sperm are recovered and frozen in one batch. For individual batches, centrifuge sperm immediately after aspiration into a yellow top tube and for a bulk freeze wait until all sperm are harvested. 18. Harvest sperm, those in yellow top tubes are placed in a foam tube rack and held at room temperature until centrifugation. 19. Centrifuge the desired number of yellow top tubes (containing sperm) for 10 min at 300 g 20. Remove the supernatant and resuspend the sperm pellet in 1.0 ml of freezing (or required volume if using sperm concentration as basis for freezing). 21. Place yellow top tube in a boar semen AI bottle (previously filled to the line with warm water) 22. Place boar semen bottle in the cold room on top of the foam esky lid and leave to cool for 2 h. 23. When sperm is cooled to 4 C add the second part of the extender (containing glycerol) if using a 2-step freezing protocol. Mix by inverting the tube several times, taking care not to shake. 24. Remove dry ice from the esky and make depressions using the metal tool. 25. Pipette 250 μl of cooled semen into the depression; repeat until 10 μl semen remains (reserve this for checking motility). 26. Once pellets look opaque (approx. 2-3 min on dry ice) move them into liquid nitrogen and store in labelled test tubes. 27. Place test tubes in the appropriate liquid nitrogen storage container 28. Repeat until all semen is frozen. 29. Collect tubes containing 10 μl diluted sperm and assess motility (allow samples to warm to 37 C). 152
168 Appendix XI: List of publications Scientific (refereed) publications: Full-papers Morton, KM, Bathgate, R, Evans, G, & Maxwell, WMC 2007, Cryopreservation of epididymal alpaca (Vicugna pacos) sperm: a comparison of Citrate, Tris and Lactose based diluents, and pellets and straws Reprod. Fertil. Dev. vol. 19, pp Morton, KM, Evans, G, & Maxwell, WMC 2007, Effect of glycerol concentration, Equex STM supplementation and liquid storage prior to freezing on the survival and functional integrity of frozenthawed epididymal alpaca (Vicugna pacos) sperm Anim. Reprod. Sci. (submitted) Morton, KM, Gibb, Z, Wilson, SJ, Bertoldo, M, Evans, G, & Maxwell, WMC 2007, Effect of diluent, dilution rate and storage temperature on longevity and functional integrity of liquid stored alpaca (Vicugna pacos) sperm Reprod. Fertil. Dev. (submitted) Morton, KM, Rückholdt, M, Evans, G, & Maxwell, WMC 2007, Quantification of the DNA difference, and separation of X- and Y-bearing sperm in alpacas (Vicugna pacos) Reprod. Domest. Anim. (in press). Morton, KM, Thompson, P, Bailey, K, Evans, G, & Maxwell, WMC 2007, Quality parameters for alpaca (Vicugna pacos) semen are affected by semen collection procedure Reprod. Fertil. Dev. (submitted). Abstracts Maxwell, WMC, Leahy, T, Morton, KM, Marti, J, & Evans, G 2007, Seminal plasma effects on ruminant and camelid sperm function during processing for storage and sex-sorting Reprod. Domest. Anim. vol. 42, pp. 60. Bailey, KJ, Morton, KM, Bathgate, R, Evans, G, & Maxwell, WMC 2006, Effect of alpaca age on testes growth and development, sperm production and post-thaw sperm survival: preliminary results in JL Juengel, JF Murray & MF Smith (eds) Reproduction in Domestic Ruminants VI, Nottingham University Press, Nottingham, UK, pp 472. Morton, KM, Bathgate, R, Evans, G, & Maxwell, WMC 2006, A comparison of three diluents for the cryopreservation of epididymal alpaca sperm Reprod. Domest. Anim. vol. 41 pp Morton, KM., Bathgate, R, Evans, G, & Maxwell, WMC 2006, Cryopreservation of epididymal alpaca sperm in pellets and straws frozen on dry ice or in liquid nitrogen vapour in JL Juengel, JF Murray & MF Smith (eds) Reproduction in Domestic Ruminants VI, Nottingham University Press, Nottingham, UK, pp 471. Morton, KM, Rückholdt, M, Evans, G, & Maxwell, WMC 2006, Preliminary development of sperm sexing technology in alpacas (Lama pacos) in Proceedings of the 1 st International Society of Camelid Research and Development pp
169 Editorially reviewed publications: Conference abstracts Leahy, T, Morton, KM, Maxwell, WMC & Evans, G 2006, Peculiarities and problems in alpaca reproduction in Proceedings of the 5 th Annual Postgraduate Research Conference. Faculty of Veterinary Science, The University of Sydney, Australia. Reyna, J, Morton, KM, Evans, G & Maxwell, WMC 2005, Collection and evaluation of alpaca sperm in Proceedings of the 4 th Annual Postgraduate Research Conference. Faculty of Veterinary Science, The University of Sydney, Australia. Conference papers Morton, KM & Maxwell, WMC 2006, The continued development of AI technology in alpacas, in Proceedings of the Australian Alpaca Association National Conference, Adelaide, Australia, pp Magazine articles Morton, KM 2007, Camelid research at Sydney University, Llama Lines Spring Morton, KM 2007, Sydney University Camelid research, Alpacas Australia 52, Morton, KM 2006, Alpaca Research Update in Alpaca Hmmm (Central Coast and Hunter Region Newsletter). Issue 9, Summer Morton, KM 2006, The 1 st Conference of the International Society of Camelid Research and Development in Alpaca Hmmm (Central Coast and Hunter Region Newsletter). Issue 11, Winter Morton, KM 2006, Highlights from Sydney University Alpaca Research in Alpacas Australia 50, Morton, KM 2006, Highlights from Sydney University Alpaca Research in Alpacas Australia 49, 13- Morton, KM 2006, Testicular science and Llama Association of Australia, Llama lines Summer Morton, KM 2005, Epididymal sperm freezing in alpacas in Alpacas Australia. Magazine of the Australian Alpaca Association. Reyna, J 2005, Alpaca breeding in Peru and perspectives for the future in Alpacas Australia, New Zealand Alpaca, The International Camelid Quarterly, and World Alpacas. Reyna, J 2005, Alpaca female reproductive physiology in Alpacas Australia, New Zealand Alpaca, The International Camelid Quarterly, and World Alpacas. Reyna, J 2005, Artificial insemination in Alpacas in Alpacas Australia, New Zealand Alpaca, The International Camelid Quarterly, and World Alpacas. Reyna, J 2005, Desarrollo y perspectives de la crianza de alpacas en Australia in Revista Peruana Agronoticias. Reyna, J 2005, The origin and evolution of South American camelids in Alpacas Australia, New Zealand Alpaca, The International Camelid Quarterly, and World Alpacas. 154
170 Oral presentations and speeches Maxwell, WMC, Leahy, T, Morton, KM, Marti, J, & Evans, G 2007, Seminal plasma effects on ruminant and camelid sperm function during processing for storage and sex-sorting, Paper presented to the European Society of Domestic Animal Reproduction, Celle, Germany, September (INVITED SPEAKER). Leahy, T, Morton, KM, Maxwell, WMC & Evans, G 2006, Peculiarities and problems in alpaca reproduction paper presented to the Fifth Annual Postgraduate Research Conference. Faculty of Veterinary Science, The University of Sydney, Australia. Morton, KM 2006, Alpaca Research at The University of Sydney, Paper presented at the Australian Alpaca Associated Central Coast and Highlands Region December Meeting. Morton KM & Maxwell, WMC 2006, The continued development of AI technology in alpacas, Paper presented to the Australian Alpaca Association National Conference, Adelaide, Australia. Morton, KM, Ruckholdt, M, Evans, G, & Maxwell, WMC 2006, Preliminary development of sperm sexing technology in alpacas (Lama pacos), Paper presented to the First International Society of Camelid Research and Development, Al-Ain, United Arab Emirates, April. Morton, KM 2005, Alpaca Research at The University of Sydney, Paper presented at the Australian Alpaca Associated Hawkesbusy Region October Meeting. Reyna, J 2005, Artificial insemination in Alpacas Paper presented at the Alpaca Fling, Belbourie Alpaca Stud, May. Reyna, J, Morton, KM, Evans, G & Maxwell, WMC 2005, Collection and evaluation of alpaca sperm, Paper presented to the Fourth Annual Postgraduate Research Conference. Faculty of Veterinary Science, The University of Sydney, Australia. Reyna, J, Morton, KM, Vaughan, J, Evans, G, & Maxwell, WMC 2005, Artificial Insemination in Alpacas, Paper presented at the Coolaroo Alpaca Stud Workshop, April. Press releases Morton, KM 2005, Alpaca Research at The University of Sydney, Published in all AAA regional newsletters. Artificially Breeding Alpacas. Roundhouse, newsletter of the Faculty of Veterinary Science, The University of Sydney. 155
171 Appendix XII: List of literature obtained by the research team Aba, M 2006, Estado actual del conocimiento sobre las particularidades reproductivas de las hembras de camélidos sudamericanos. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Aba, MA, Bravo, PW, Forsberg, M and Kindahl, H 1997, 'Endocrine changes during early pregnancy in the alpaca', Anim. Reprod. Sci. vol. 47, pp Aba, MA, Chaves, MG, Aguero, A et al. 2000, Endocrine and structural changes during follicular waves in llamas. Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Aba, MA and Forsberg, M 1995, 'Heterologous radioimmunoassay for llama and alpaca luteinizing hormone with a monoclonal antibody, an equine standard and a human tracer', Acta Vet. Scand. vol. 36, pp Aba, MA, Forsberg, M, Kindahl, H, Sumar, J and Edqvist, LE 1995, 'Endocrine changes after mating in pregnant and non-pregnant llamas and alpacas', Acta Vet. Scand. vol. 36, pp Aba, MA, Kindahl, H, Forsberg, M, Quiroga, M and Auza, N 2000, 'Levels of progesterone and changes in prostaglandin F2 alpha release during luteolysis and early pregnancy in llamas and the effect of treatment with flunixin meglumine', Anim. Reprod. Sci. vol. 59, pp Aba, MA, Miragaya, MH, Chaves, MG et al. 2005, 'Effect of exogenous progesterone and ecg treatment on ovarian follicular dynamics in vicuñas (Vicugna vicugna)', Anim. Reprod. Sci. vol. 86, pp Aba, MA, Quiroga, MA, Auza, N, Forsberg, M and Kindahl, H 1999, 'Control of ovarian activity in Llamas (Lama glama) with medroxyprogesterone acetate', Reprod. Domest. Anim. vol. 34, pp Aba, MA, Sumar, J, Kindahl, H, Forsberg, M and Edqvist, LE 1998, 'Plasma concentrations of 15-ketodihydro- PGF2 alpha, progesterone, oestrone sulphate, oestradiol-17 beta and cortisol during late gestation, parturition and the early post partum period in llamas and alpacas', Anim. Reprod. Sci. vol. 50, pp Abdel-Rahim, HA and El Nazier, AT 1992, 'Studies on the sexual behaviour of the Dromedary camel', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992, R & W Publications Ltd, Newmarket, UK. Abdel-Raouf, M, Fateh El-Bab, MR and Owaida, MM 1975, 'Studies on reproduction in the camel (Camelu dromedarius) V. Morphology of the testis in relation to age and season', J. Reprod. Fert. vol. 43, pp Abdel Rahim, SEA 1997, 'Studies on the age of puberty of male camels (Camelus dromedarius) in Saudi Arabia', Vet. J. vol. 154, pp Abdo, GA, Hemeida, NA, Zaki, K, Forsberg, M and Kindahl, H 2000, Interrelationship of progesterone, oestradiol-17b and prostaglandin F2a during different stages of camel parturition (Camelus dromedarius). Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Abdoon, ASS 2001, 'Factors affecting follicular population, oocyte yield and quality in camels (Camelus dromedarius) ovary with special reference to maturation time in vitro', Anim. Reprod. Sci. vol. 66, pp Abdoon, ASS, Kandil, OM, Berisha, B, Kliem, H and Schams, D 2007, 'Morphology of Dromedary camel oocytes and their ability to spontaneous and chemical pathenogenetic activation', Reprod. Domest. Anim. vol. 42, pp Adam, CL, Bourka, DA, Kyle, CE, Young, P and McEvoy, TG 1992, 'Ovulation and embryo recovery in the llama', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd, Newmarket, UK. Adam, CL, Moir, CE and Shiach, P 1989, 'Plasma progesterone concentrations in pregnant and non-pregnant llamas (Lama glama)', Vet. Rec. vol. 125, pp Adams, GP, Griffin, PG and Ginther, OJ 1989, 'In situ morphologic dynamics of ovaries, uterus and cervix in llamas', Biol. Reprod. vol. 41, pp Adams, GP, Ratto, M and Singh, J 2003, 'Breeding synchronization in llamas', The International Camelid Quarterly vol. 2, pp Adams, GP and Ratto, MH 2001, 'Reproductive biotechnology in South American camelids', Rev. de Investigaciones Veterinarias del Peru vol. 1, pp Adams, GP, Ratto, MH, Huanca, W and Jaswant, S 2005, 'Ovulation-inducing factor in the seminal plasma of alpacas and llamas', Biol. Reprod. vol. 73, pp Adams, GP, Sumar, J and Ginther, OJ 1990, 'Effects of lactational and reproductive status on ovarian follicular waves in llamas (Lama glama)', J. Reprod. Fertil. vol. 90, pp Adams, GP, Sumar, J and Ginther, OJ 1991, 'Form and function of the corpus luteum in llamas', Anim. Reprod. Sci. vol. 24, pp Adams, GP, Sumar, J and Ginther, OJ 1991, 'Hemorrhagic ovarian follicles in llamas', Theriogenology vol. 35, pp Agag, MA 1996, 'A development of an electronic camel bibliography on camel reproduction', J. Camel Prac. Res. vol. 3, pp
172 Agarwal, SP, Khanna, ND, Agarwal, VK and Dwaraknath, PK 1987, 'Circulating levels of estrogen and progesterone in female camel (Camelus dromedarius) during pregnancy.' Theriogenology vol. 28, pp Agarwal, SP, Rai, AK and Khanna, ND 1992, 'Hormonal studies in post partum female camels and their neonates', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd, Newmarket, UK. Agarwal, SP, Rai, AK and Khanna, ND 1997, 'Seasonal variation in the concentration of steroid hormones in seminal plasma of camel', Indian Vet. J. vol. 74, pp Agarwal, SP, Rai, AK, Vyas, S and Khanna, ND 1996, 'Augmentation of early reproduction through hormonal therapy in camel heifers', Int. J. Amin. Sci. vol. 11, pp Agarwal, VK, Ram, L, Rai, AK, Khanna, ND and Agarwal, SP 2004, 'Physical and biochemical attributes of camel semen', J. Camel Science vol. 1, pp Aguero, A, Chaves, MG, Capdevielle, EF, Russo, AF and Aba, MA 2001, Superovulacion en llamas: comparacion de dos tratamientos', Invest. Vet. vol. 3, pp Aguero, A, Miragaya, M, Ferrer, MS et al 'Follicular dynamics in Vicuña vicuña', Theriogenology pp Ahmadi, MR, Nazifi, S, Ghaisari, HR and Radmehr, M 2005, 'Evaulation of reproductive cycle with cervical and uterine cytology in Iranian Dromedary camels', Comp. Clinical Path. vol. 14, pp Akingbemi, BT and Aire, TA 1991, 'Testicular dimensions and sperm reserves in the camel (Camelus dromedarius) in Nigeria', B. Anim. Health Prod. Africa vol. 39, pp Al-Ajlan, A and Bailey, GS 1997, 'Purification and partial characterization of camel anionic chymotrypsin', Arch. Biochemistry and Biophysics vol. 348, pp Al-Eknah, M, Homeida, AM, Dafalla, EA, Galil, AKA and Al-Tahir, AY 1997, 'Uterine activity after induction of hypocalcaemia in the ovariectomized camel (Camelus dromedarius)', Vet. Res. Commun. vol. 21, pp Al-Eknah, M, Homeida, N and Al-Haider, A 2001, 'A new method for semen collection by artificial vagina from the Dromedary camel', J. Camel Prac. Res. vol. 8, pp Al-Eknah, MM 2000, 'Reproduction in old world camels', Anim. Reprod. Sci. vol. 60/61, pp Al-Eknah, MM, Dafalla, EA, Homeida, AM, Galil, AKA and Al-Taher, AY 1993, 'Spontaneous uterine activity during the oestrous cycle of the camel (Camelus dromedarius)', Anim. Reprod. Sci. vol. 32, pp Al-Eknah, MM, Homeida, AM, Ramadan, RO, Al-Modhi, FA and Al-Busadah, KA 2001, 'Pregnancy dependence on ovarian progesterone in the camel (Camelus dromedaries)', Emirates J. Agr. Sci. vol. 13, pp Al-Qarawi, AA 2001, 'Intratesticular morphometric, cellular and endrocrine changes around the pubertal period in Dromedary camels', Vet. J. vol. 162, pp Al-Qarawi, AA 2005, 'Infertility in the Dromedary bull: a review of causes, relations and implications', Anim. Reprod. Sci. vol. 87, pp Al-Qarawi, AA, Abdel-Rahman, HA, El-Belely, MS and El-Mougy, SA 2000, 'Age-related changes in plasma testosterone concentrations and genital organs content of bulk and trace elements in the male Dromedary camel', Anim. Reprod. Sci. vol. 62, pp Al-Qarawi, AA, Abdel-Rahman, HA, El-Mougy, SA and El-Belely, MS 2002, 'Use of a new computerized system for evaluation of spermatozoal motility and velocity characteristics in relation to fertility levels in Dromedary bulls', Anim. Reprod. Sci. vol. 74, pp Al-Qarawi, AA, Omar, HM, Abdel-Rahim, HA, El-Mougy, SA and El-Belely, MS 2005, 'Trypanosomiasisinduced infertility in Dromedary (Camelus dromedarius) bulls: changes in plasma steroids concentration and semen characteristics', Anim. Reprod. Sci. vol. 84, pp Al Qarawi, AA and El Belely, MS 2004, 'Intratesticular morphometric, cellular and endocrine changes in Dromedary bulls exhibiting azoospermia. ' Vet. J. vol. 167, pp Alarcon, V, Sumar, J, Riera, GS and Foote, WC 1990, 'Comparison of three methods of pregnancy diagnosis in alpacas and llamas', Theriogenology vol. 34, pp Alfaro, CO 2004, Aplicacion de la tecnica del ADN polimorfico amplificado al Azar (RAPD) en el estudio molecular de Lama pacos. Facultad de farmacia y bioquimica. Lima, Peru, Universidad Nacional Mayor De San Marcos: 68. Alfuraija, MM and Moussa, IA 1995, 'Gonadotrophin-releasing hormone-like factors inthe seminal plasma of the Dromedary camel (Camelus dromedarius)', Theriogenology vol. 43, pp Ali, BH 1988, 'A survey of some drugs commonly used in the camel', Vet. Res. Commun. vol. 12, pp Ali, HA, Tingari, MD and Moniem, KA 1978, 'On the morphology of the accessory male grlands and histochemistry of the ampulla ductus deferentis of the camel', J. Anat. vol. 125, pp Aller, J, Alberio, JR and Rebuffi, G 1998, Diagnostico y edad de gestacion determinadas por palpacion rectal y ultrasonografia en llamas (Lama glama)', Arch. Zootec. vol. 47, pp Aller, J, Ferre, L, Rebuffi, G and Alberio, R 1997a, 'Inseminación artificial en llamas', Rev. Vet. Arg. vol. 16, pp
173 Aller, JF 1999, Inseminacion artificial en llamas en la Puna. Proceedings of the II Congreso Mundial sobre Camelidos Cusco, Peru. Aller, JF 2001, 'Cryopreservation of semen and artificial insemination in South Amercian camelids', International Foundation for Sciences Final Report Aller, JF and Alberio, RH 1996, Dinamica folicular en llamas en la epoca ontono-invernal', Rev. Arg. de Prod. Anim. vol. 16, pp Aller, JF, Alberio, RH and Rebuffi, G 1996, 'Relacion entre palpacion rectal y ecografia para determinar tamano de gestacion en llamas', Rev. Arg. de Prod. Anim. vol. 16, pp Aller, JF, Cancino, AK, Rebuffi, GE and Alberio, RH 2002 Transferencia de embriones vitrificados de llama (Lama glama) en el altiplano Argentino', Vet. Argentina vol. 19, pp Aller, JF, Ferre, L, Rebuffi, GE and Alberio, RH 1997, Recoleccion de semen de llama (Lama glama) en la Puna Argentina', Vet. Argentina vol. 14, pp Aller, JF, Rebuffi, GE and Cancino, AK 2002, 'Superovulation response to progestogen treatment in vicuña (Vicugna vicugna) in semicaptive conditions.' Theriogenology vol. 57, pp. 576 (abstract). Aller, JF, Rebuffi, GE, Cancino, AK and Alberio, JR 2003, 'Fetal mortality diagnosis by ultrasound in the vicuña (Vicugna vicugna)', Reprod. Fertil. Dev. vol. 15, pp Aller, JF, Rebuffi, GE, Cancino, AK and Alberio, RH 2002, 'Successful transfer of vitrified llama (Lama glama) embryos', Anim. Reprod. Sci. vol. 73, pp Aller, JF, Rebuffi, GE, Cancino, AK and Alberio, RH 2003, Influencia de la criopreservacion sobre la motilidad, viabilidad y fertilidad de espermatozoides de llama (Lama glama)', Arch. Zootec. vol. 52, pp Anouassi, A, Adnani, M and Raed, E 1992, 'Artificial insemination in the camel requires induction of ovulation to achieve pregnancy', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992, R & W Publications Ltd, Newmarket, UK. Anouassi, A and Combarnous, Y 1991, 'Development of radioimmunoassay and enzyme immunoassays for luteinizing hormone in dromedaries (Camelus dromedarius)', J. Reprod. Fertil. vol. 93, pp Aparicio, M, Leyva, VV, Novoa, CM and Garcia, WV 2003, 'Effecto de la copulacion durantre el celo postovulatorio en la mortalidad embrionaria en alpacas', Rev. Int. Vet. Peru vol. 14, pp Apaza, N, Alarcon, V, Huanca, T and Cardenas, O 1999, Avances sobre la inseminacion artificial con semen congelado an alpacas. Proceedings of the II Congreso Mundial sobre Camlidos Cusco, Peru. Apichela, S, Jimenez-Diaz, M, Schuster, S, Sinowatz, F and Miceli, DC 2006, 'Vicuña oviduct mucosa: Ultrastructure and lectin affinity', Small Ruminant Res. vol. 66 pp Arainga, MR, Leyva, VV, Garcia, WV and Franco, LE 2003, 'Efecto de la GnRH en el proceso del reconocimiento maternal de la prenez sobre la supervivencia embrionaria en alpacas', Rev. Int. Vet. Peru vol. 14, pp Arthur, GH 1992, 'An overview of reproduction in the camelids', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd, Newmarket, UK. Azouz, A, Ateia, MZ, Shawky, H, Zakaria, AD and Farahat, AA 1992, 'Hormonal changes during rutting and the non-breeding season in male Dromedary camels', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd, Newmarket, UK. Bailey, KJ, Morton, KM, Bathgate, R, Evans, G and Maxwell, WMC 2006, ' Effect of alpaca age on testes growth and development, sperm production and post-thaw sperm survival: preliminary results', in. JL Juengel, JF Murray and MF Smith (eds), Reproduction in Domestic Ruminants VI, Nottingham University Press, Nottingham, UK. Barril, A, Villagran, K, Von Baer, A and M., B 2006, 'Evaluacion del comportamiento in vivo del semen de llama y busqueda de un factor proteico que incidiria en la licuefaccion del plasma seminal', Int. J. Morphol. vol. 24 Suppl. 1, pp Barrington, GM, Allen, AJ, Parish, SM and Tibary, A 2006, 'Biosecurity and biocontainment in alpaca operations', Small Ruminant Res. vol. 61, pp Bianchi, CP, Cavilla, MV, Jorgensen, E and Aba, MA 2006a, Sensibilidad del cuerpo lúteo (CL) a las prostaglandinas en función del momento de aplicación en llamas. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Bianchi, CP, Cavilla, MV, Jorgensen, E and Aba, MA 2006b, Sensibilidad del cuerpo luteo a las prostaglandinas en funcion del momento de aplicacion en llamas. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Bianchi, CP, Meikle, A, Sartore, I, Gonzalez, F and Aba, MA 2007, 'Uterine estrogen receptor alpha and progesterone receptop during the follicular and luteal phase in llamas', Anim. Reprod. Sci. vol. 99, pp
174 Billah, M and Skidmore, JA 1992, 'The collection, evaluation and deep freezing of Dromedary camel semen', in WR Allan, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proceedings of the First International Camel Conference, Dubai, 2nd - 6th February 1992, R & W Publications Ltd, Newmarket, UK. Bono, G, Dahir, MA, Comin, A and Jumale, MA 1989, 'Plasma LH, corticoid and sex steroid variations in Camels (Camelus dromedarius) in relation to seasonal climatic changes', Anim. Reprod. Sci. vol. 21, pp Bou, SG, Pang, YF, Zhang, SL and Xue, XX 1993, 'Preliminary study on in vitro fertilization in the domestic camel (Camelus Bactrianus). [Chinese]', Chinese J. Zool. vol. 28, pp Bourke, D, Adam, K, Kyle, C and al, e 1994, 'Ovarian response to PMSG and FSH in llamas.' European Symp on South American Camelids vol 1, pp Bourke, DA, Adam, CL and Kyle, CE 1991, 'Sucsessful pregnancy following non-surgical embryo transfer in llamas.' Vet. Rec. vol, pp. 68. Bourke, DA, Adam, CL, Kyle, CE, McEvoy, TG and Young, P 1992, 'Ovulation, superovulation and embryo recovery in llamas.' Proceedings of the 12th International Congress on Animal Reproduction, The Hague vol. 1, pp Bourke, DA, Adam, CL, Kyle, CE, Young, P and McEvoy, TG 1992, 'Superovulation and embryo transfer in the llama', in WR Allan, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proceedings of the First International Camel Conference, Dubai, 2nd - 6th February 1992, R & W Publications Ltd, Newmarket, UK. Bourke, DA, Kyle, CE, McEvoy, TG, Young, P and Adam, CL 1995, 'Advanced reproductive technologies in South American camelids.' Proceedings of the 2nd European Symposium on South American Camelids vol. 1, pp Bourke, DA, Kyle, CE, McEvoy, TG, Young, P and Adam, CL 1995, 'Recipient synchronisation and embryo transfer in South American camelids', Theriogenology vol. 43, pp Bourke, DA, Kyle, CE, McEvoy, TG, Young, P and Adam, CL 1995, 'Superovulatory responses to ecg in llamas (Lama glama)', Theriogenology vol. 44, pp Bravo, PW 1993, 'Basic reproductive physiology in female South American camelids', in (eds), Zoo and Wild Animal Medicine W.B. Saunders Company, Philadelphia USA. Bravo, PW 1994, 'Reproductive endocrinology of llamas and alpacas', Vet. Clin. N. Am. Food A. vol. 10, pp Bravo, PW 1995, 'Physiology of reproduction and fertility evaluation in the male alpaca', Proceedings of Post Graduate Foundation in Veterinary Science vol. 257, pp Bravo, PW 1996, 'Relationship between testicle size and fertility', The Alpaca Registry Journal vol. 1, pp Bravo, PW 2000, 'Male lamoid semen evaluation.' in The Ohio State University College of Veterinary Medicine Presents Camelid Medicine, Surgery and Reproduction, Ohio State University.College of Veterinary Medicine, Columbus, Ohio. Bravo, PW 2002, An insight into the development of freezing semen in South American camelids. Columbus, Ohio, USA. Bravo, PW 2002, The Reproductive Process of South American Camelids. Seagull Printing, Salt Lake City, Utah, USA. Bravo, PW, 'Breeding sound examination of the male alpaca', Alpaca World Magazine Bravo, PW, Alarcon, V and Bondurant, RH 2000, Epididymal spermatozoa characteristics and its use on artificial insemination of llamas and alpacas. Paper presented to Proc. 14th International Congress on Animal Reproduction Stockholm, Sweden. Bravo, PW, Bazan, PJ, Troedsson, MHT, Villalta, PR and Garnica, JP 1996, 'Induction of parturition in alpacas and subsequent survival of neonates.' J. Am. Vet. Med. Assoc. vol. 209, pp Bravo, PW, Ccallo, M and Garnica, J 2000, 'The effect of enzymes on semen viscosity in llamas and alpacas', Small Ruminant Res. vol. 38, pp Bravo, PW, Cosio, E, Alarcon, V and Ordonez, C 2004, The first days of alpaca embryo. Paper presented to 15 th International Congress on Animal Reproduction, Porto. Bravo, PW, Flores, D and Ordonez, C 1997, 'Effect of repeated collection on semen characteristics of alpacas', Biol. Reprod. vol. 57, pp Bravo, PW, Flores, U, Garnica, J and Ordonez, C 1997, 'Collection of semen and artificial insemination of alpacas', Theriogenology vol. 47, pp Bravo, PW, Fowler, ME and Lasley, BL 1994, 'The postpartum llama: fertility after parturition', Biol. Reprod. vol. 51, pp Bravo, PW, Fowler, ME, Stabenfeldt, GH and Lasley, B 1990, 'Endocrine responses in the llama to copulation', Theriogenology vol. 33, pp Bravo, PW, Fowler, ME, Stabenfeldt, GH and Lasley, BL 1990, 'Ovarian follicular dynamics in the llama', Biol. Reprod. vol. 43, pp Bravo, PW, Fowlers, ME and Stabenfeldt, GE 1992, Testis growth and testosterone concentration in the male llama. Paper presented to the 12th International Congress on Animal Reproduction 159
175 Bravo, PW, Garnica, J and Aviles, E 2001, 'Cortisol concentrations in the perinatal and weaning periods of alpacas', Anim. Reprod. Sci. vol. 67, pp Bravo, PW, Garnica, J and Fowler, ME 1997, 'Immunoglobulin G concentrations in periparturient llamas, alpacas and their crias', Small Ruminant Res. vol. 26, pp Bravo, PW and Johnson, LW 1994, 'Reproductive physiology of the male camelid', Vet. Clin. N. Am. Food A. vol. 10, pp Bravo, PW, Lasley, BL and Fowler, ME 1995, 'Resumption of ovarian follicular activity and uterine involution in the postpartum llama', Theriogenology vol. 44, pp Bravo, PW, Mayta, MM and Ordonez, CA 2000, 'Growth of the conceptus in alpacas', Am. J. Vet. Res. vol. 61, pp Bravo, PW, Mellado, W, Ampuero, E and Alarcon, V 1997, 'Effect of daily breeding on fertility of male alpaca', Andrology vol. 9, pp Bravo, PW, Moscoso, J, Ordonez, C and Alarcon, V 1996, 'Transport of spermatozoa and ova in female alpaca', Anim. Reprod. Sci. vol. 43, pp Bravo, PW, Moscoso, R, Alarcon, V and Ordonez, C 2002, 'Ejaculatory process and related semen characteristics', Arch. Androl. vol. 48, pp Bravo, PW, Ordonez, C and Alarcon, V 1996, Processing and freezing of semen of alpacas and llamas. Paper presented to 13th International Congress on Animal Reproduction Sydney, Australia. Bravo, PW, Pacheco, C, Quispe, G, Vilcapaza, L and Ordonez, C 1999, 'Degelification of alpaca semen and the effect of dilution rates on artificial insemination outcome', Arch. Androl. vol. 43, pp Bravo, PW, Pezo, D and Alarcon, V 1995, 'Evaluation of early reproductive performance in the postpartum alpaca by progesterone concentrations', Anim. Reprod. Sci. vol. 39, pp Bravo, PW, Skidmore, JA and Zhao, XX 2000, 'Reproductive aspects and storage of semen in Camelidae', Anim. Reprod. Sci. vol. 62, pp Bravo, PW, Solis, P, Ordonez, C and Alarcon, V 1997, 'Fertility of the male alpaca: effect of daily consecutive breeding', Anim. Reprod. Sci. vol. 46, pp Bravo, PW, Stabenfeldt, GH, Fowler, ME and Lasley, BL 1991, 'Urinary steroids in the periparturient and postpartum periods through early pregnancy in llamas', Theriogenology vol. 36, pp Bravo, PW, Stabenfeldt, GH, Fowler, ME and Lasley, BL 1992, 'Pituitary response to repeated copulation and/or gonadotropin-releasing hormone administration in llamas and alpacas', Biol. Reprod. vol. 47, pp Bravo, PW, Stabenfeldt, GH, Fowler, ME and Lasley, BL 1993, 'Ovarian and endocrine patterns associated with reproductive abnormalities in llamas and alpacas', J. Am. Vet. Med. Assoc. vol. 202, pp Bravo, PW, Stabenfeldt, GH, Lasley, BL and Fowler, ME 1991, 'The effect of ovarian follicle size on pituitary and ovarian responses to copulation in domesticated South American camelids', Biol. Reprod. vol. 45, pp Bravo, PW, Stewart, DR, Lasley, BL and Fowler, ME 1996, 'Hormonal indicators of pregnancy in llamas and alpacas', J. Am. Vet. Med. Assoc. vol. 208, pp Bravo, PW and Sumar, J 1989, 'Laparoscopic examination of the ovarian activity in alpacas', Anim. Reprod. Sci. vol. 21, pp Bravo, PW and Sumar, J 1991, 'Evaluation of intra abdominal vasectomy in llamas and alpacas.' J. Am. Vet. Med. Assoc. vol. 199, pp Bravo, PW, Tsutsui, T and Lasley, BL 1995, 'Dose response to equine chorionic gonadotropin and subsequent ovulation in llamas', Small Ruminant Res. vol. 18, pp Bravo, PW and Varela, MH 1993, 'Prenatal development of the alpaca (Lama pacos)', Anim. Reprod. Sci. vol. 32, pp Brogliatti, GM, Palasz, AT and Adams, GP 1996, 'Ultrasound-guided transvaginal follicle aspiration and oocyte collection in llamas (Lama glama)', Theriogenology vol. 45, pp Brogliatti, GM, Palasz, AT, Rodriguez-Martinez, H, Mapletoft, RJ and Adams, GP 2000, 'Transvaginal collection and ultrastructure of llama (Lama glama) oocytes', Theriogenology vol. 54, pp Brown, BW 2000, 'A review on reproduction in South American camelids', Anim. Reprod. Sci. vol. 58, pp Buendia, P, Soler, C, Paolicchi, F et al. 2002, 'Morphometric characterization and classification of alpaca sperm heads using the Sperm-Class Analyzer(R) computer-assisted system', Theriogenology vol. 57, pp Burgel, H, Erhardt, G and Gauly, M 1999, 'Cryopreservation of llama (Lama glama) semen', Proceedings of the II Congreso Mundial sobre Camelidos, Cusco, Peru pp. 82. Burgel, H, Erhardt, G and Gauly, M 2000, 'Cryopreservation of llama (Lama glama) semen', Reprod. Domest. Anim. vol. 35, pp. 26 (abstract). Burgel, H, Erhardt, G and Gauly, M 2001, 'Cryopreservation of llama (Lama glama) spermatozoa with an eggyolk-free extender', in M Gerken and C Renieri (eds), Progress in South American Camelids Research Wageningen Pers, Wageningen Netherlands. 160
176 Bustamante, AV, Zambelli, A, De Lamo, DA, von Thungen, J and Vidal-Rioja, L 2002, 'Genetic variability of guanaco and llama populations in Argentina', Small Ruminant Res. vol. 44, pp Calderon, W, Sumar, J and Franco, E 1968, 'Advances en la inseminacion artificial de las alpacas', Rev. de la Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos, Lima, Peru. vol. 22, pp Callo, M, Garnica, J and Bravo, PW 1999, Efecto de la fibrinolisina, hialuronidasa, colagenasa y tripsina sobre la viscosidad del semen en alpacas. Proceedings of the II Congreso Mundial sobre Camelidos Cusco, Peru. Carmichael, IH 1999, ' Internal parasitism in alpacas in southern Australia.' Proceedings of the Australian Camelid Veterinary Association Conference pp Carretero, MI, Chaves, MG, Agüero, A and Miragaya, MH 2006, Sincronización de la onda folicular mediante la administración de progesterona y benzoato de estradiol inyectables en la especie Lama glama. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Carrión, OR, Carreón, O, Zambrano, V and Olivera, M 2006, Tridimensionalidad microvascular del testículo de la alpaca. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Cavalcanti, SMC and Knowlton, FF 1998, 'Evaluation of physical and behavioural traits of llamas associated with aggressiveness toward sheep-threatening canids.' Appl. Anim. Behav. Sci. vol. 61, pp Cavilla, MV, Bianchi, CP, Waimann, E, Aguilera, MF and Aba, MA 2006, Perfil de progesterona plasmática y actividad folicular en llamas tratadas con un dispositivo intravaginal comercial conteniendo 0,78 g de progesterona. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Cervantes, MP 2004, Estudio del efecto del estadio del desarrollo folicular al momento de la monta sobre la ovulacion y sobrevivencia embrionaria en alpacas. Faculdad de medicina veterinaria Lima, Peru, Universidad Nacional Mayor De San Marcos: 43. Cervantes, MP, Huanca, W, Palomino, JM and Huanca, T 2004, Embryo mortality and its relation with the phase of follicular development at mating in alpaca. Proceeings of the 15th International Congress on Animal Reproduction Chaudhary, ZI 1995, 'Artificial insemination in the camel: problems and prospects. A review', J. Camel Prac. Res. vol. 2, pp Chaudhary, ZI 2000, 'Reproduction in the Dromedary camel', in TK Ghlaot (ed), Selected topics on camelids The Camelid Publishers, Bikaner India. Chaves, MG 2006, Comparación de la eficiencia reproductiva aplicada a la producción de embriones en la llama (Lama glama). Proceedings of IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Chaves, MG, Aba, M, Aguero, A et al. 2002, 'Ovarian follicular wave pattern and the effect of exogenous progesterone on follicular activity in non-mated llamas', Anim. Reprod. Sci. vol. 69, pp Chen, BX, Yuen, ZX and Pan, GW 1985, 'Semen-induced ovulation in the Bactrian camel (Camelus Bactrianus)', J. Reprod. Fert. vol. 74, pp Chen, BX, Zhao, XX and Huang, YM 1993, 'Freezing semen and AI in the Bactrian camel (Camelus Bactrianus)', Etudes et Syntheses de l'iemvt vol. 41, pp Coila Añasco, P, Mamani, G and Sapana, R 2006, Relación entre la morfología, motilidad y vigor espermático del semen con la actividad enzimática de las transaminasas GOT y GTP del plasma seminal de alpacas. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Coila Añasco, P, Quina, E, Calsin, B and Valdivia, RS 2006, Actividad enzimatica de las fosfatasas acidayalcalina en el plasma seminal de alpacas (Vicugna pacos). Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Conde, P, Herrera, C, Chaves, MG et al. 2006, Producción in vitro de embriones de llama mediante las técnicas de ICSI y FIV. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Conde, PA, Herrera, C, Trasorras, VL et al. 2007, 'In vitro production of llama (Lama glama) embryos by IVF and ICSI with fresh semen', Anim. Reprod. Sci. pp. in press. Condorena, A, N. and Velasco N, J 1978, Comparacion de dos sistemas de empadre en la alpaca', Memoria, Asociacion Latinoamericana de Produccion Animal vol. 13, pp Condorena, A, Sumar, J, Franco, E and Alarcon, V 1988, Largo de gestacion en llamas (Lama glama). Proceedings of the XI Congreso Panamericano de Ciencias Veterinarias, Lima, Peru. Cordero, A, Genovese, P, Vega, R et al. 2006, Hallazgo de granulomas espermáticos en las cabezas de epidídimos de un alpaca. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Cordero, A, Huanca, W and Huanca, T 2000, Clinical and histological characteristics of testicles of the alpaca male from birth to 12 months. Proceeedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Cordero, A, San Martin, F, Echevarrie, L et al. 1993, 'Progesterona plasmatica en alpacas y llamas: temperatras y tiempo previos a la centrifugation', Investigaciones pecuarias vol. 6, pp Correa, JE, Ratto, MH and Gatica, R 1994, 'Actividad estral y respuesta ovarica en alpacas y llamas tratadas con progesterona y gonadotrofinas', Arch. Med.Vet. vol. 26, pp
177 Correa, JE, Ratto, MH and Gatica, R 1997, 'Superovulation in llamas (Lama glama) with pfsh and equine chorionic gonadotrophin used individually or in combination', Anim. Reprod. Sci. vol. 46, pp Correa, JE, Ratto, MH, Ladrix, R and Gatica, R 1992, ' Obtencion de prenez en una llama por transferencia de embriones', Arch. Med. Vet. vol. 24, pp Correa, JE, Ratto, MV and Gatica, R 1994, 'Actividad estral y respuesta ovarica en alpacas y llamas tratadas con progesterona y gonadotrofinas', Arch. Med. Vet. vol. 26, pp Cristian, R, Álvarez, C, Rodríguez, V, Uipan, P and Valdivia, M 2006, Interacción homóloga del sistema proacrosina/acrosina con glicoproteínas de la zona pelúcida de alpaca (Vicugna pacos). Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Cristofori, F, Aria, G, Vincenti, L et al. 1989, 'Mating-dependent endocrinological variations in female Camelus dromedarius', Anim. Reprod. Sci. vol. 21, pp Davalos, R and Olazabal, JL 2002, 'Evaluacion de dos formas de collection de semen en alpacas', Rev. Int. Vet. Peru vol. 13, pp Davalos, RR 2006, Estudio de la producción espermática total de testículos de alpaca. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Davis, GH, Dodds, KG, Moore, GH and Bruce, GD 1997, 'Seasonal effects on gestation length and birth weight in alpacas', Anim. Reprod. Sci. vol. 46, pp Deen, A and Sahani, MS 2000, 'Preliminary attempts to collect and cryopreserve camel semen', J. Camel Prac. Res. vol. 7, pp Deen, A, Sumant, V, Mamta, J and Sahani, MS 2002, 'A note on effect of carting on libido and semen production in camel (Camelus dromedarius)', J. Camel Prac. Res. vol. 9, pp Deen, A, Sumant, V, Mamta, J and Sahani, MS 2004, 'Explanation of no or low sperm motility in camel semen', Israel J. Vet. Med. vol. 59, pp Deen, A, Sumant, V, Mamta, J and Sahani, MS 2004, 'Refrigeratory preservation of camel semen', J. Camel Prac. Res. vol. 11, pp Deen, A, Sumant, V and Sahani, MS 2003, 'Semen collection, cryopreservation and artificial insemination in the Dromedary camel', Anim. Reprod. Sci. vol. 77, pp Deen, A, Sumant, V and Sahani, MS 2005a, 'Problems of artificial insemination in dromedarius camel - failure of ovulation and entrapment of spermatozoa in gelatinous camel semen', Vet. Arhiv. vol. 75, pp Deen, A, Vyas, S and Sahani, MS 2005, 'Testosterone profiles in the camel (C. dromedarius) during the rutting season', Israel J. Vet. Med. vol. 60. Del Campo, CH, Berland, M and Del Campo, MR 1991, 'Arterias de útero y ovarios en Alpacas (Lama pacos)', Revista Chilena de Anatomía vol. 9, pp. 26 (abstract). Del Campo, CH, Del Campo, MR, Donoso, X, Berland, M and Mapletoft, R 1993, 'Fecundación in vivo e in vitro con espermatozoides del epidídimo de Llama (Lama glama) en Camélidos Sudamericanos.' Ciencia e Investigación Agraria vol. 20, pp. 90. Del Campo, CH, Vits, L and Berland, M 1993, 'Alteraciones genitales en Lamas y Alpacas de interés en programas de reproducción y producción', Ciencia e Investigación Agraria vol. 20, pp. 87. Del Campo, MR and Del Campo, CH 1998, 'Reproduction in South American camelids: basic and applied aspects', in. K Ostensson and WG Vale (eds), Proceedings of the 4th SIPAR follow-up seminar on animal reproduction and biotechnology for Latin America, Belem-Para-Brazil, 8-20 February, 1998: Volume 1., Swedish University of Agricultural Sciences, Uppsala, Sweden. Del Campo, MR, Del Campo, CH, Adams, GP and Mapletoft, RJ 1995, 'The application of new reproductive technologies to South American camelids', Theriogenology vol. 43, pp Del Campo, MR, Del Campo, CH, Donoso, MX and Berland, M 1994, 'In vitro fertilisation of llama (Lama glama) follicular oocytes', Theriogenology vol. 41, pp Del Campo, MR, Del Campo, CH, Donoso, MX, Berland, M and Mapletoft, RJ 1994, 'In vitro fertilization and development of llama (Lama glama) oocytes using epididymal spermatozoa and oviductal cell co-culture', Theriogenology vol. 41, pp Del Campo, MR, Donoso, MX, Del Campo, CH and al, e 1992, 'In vitro maturation of llama (Lama glama) oocytes', Proceedings of the 12th International Congress on Animal Reproduction pp Delhon, GA and Lawzewitsch, I 1994, 'Ductus epididymis compartments and morphology of epididymal spermtaozoa in llamas', Anat. Histol. Embryol. vol. 23, pp Delhon, GA and Lawzewitsch, Iv 1987, 'Reproduction in the male llama (Lama glama), a South American camelid. I. Spermatogenesis and organization of the intertubular space of the mature testis', Acta Anat. vol. 129, pp Director, A 2004, Evaluation of llama (Lama glama) semen obtained by electroejaculation or using an artificial vagina. Proceedings of the 15th International Congress on Animal Reproduction. Dixit, VP, Mehta, SN, Georgie, GC and Singal, SP 1987, 'Serum testosterone and cortisol in rutting and non rutting Indian male camels', Indian J. Anim. Sci. vol. 57, pp
178 El-Hassanein, E 2003, 'An invention for easy semen collection from Dromedary calems, El-Hassanein camel dummy', in. GP Adams and L Skidmore (eds), Recent Advances in Camelid Reproduction International Veterinary Information Service, Ithaca, New York, USA. El-Manna, MM, Tingari, MD and Ahmed, AK 1986, 'Studies on camel semen II. Biochemical characteristics', Anim. Reprod. Sci. vol. 12, pp El-Naggar, M and Abdel-Raouf, M 1976, 'Studies on reproduction in camels (Camelus dromedarius). VII. The acid and the alkaline phosphatase activities of the seminal plasma', Indian Vet. J. vol. 53, pp El-Naggar, M and Abdel-Raouf, M 1977, 'Studies on reproduction in camels (Camelus dromedarius). VIII. The electrophoretic pattern and the amino acid content of the seminal plasma protein', Indian Vet. J. vol. 54, pp El Allali, K, Achaaban, MR, Vivien-Roels, B et al. 2005, 'Seasonal variations in the nycthemeral rhythm of plasma melatonin in the camel (Camelus dromedarius)', J. Pineal Res. vol. 39 pp Elias, E, Bedrak, E and Cohen, D 1985, 'Induction of oestrus in the camel (Camelus dromedarius) during seasonal anoestrus', J. Reprod. Fertil. vol. 74, pp Elias, E, Bedrak, E and Yagil, R 1984, 'Estradiol concentration in the serum of the one-humped camel (Camelus dromedarius) during various reproductive stages', General and Comparative Endocrinology vol. 56, pp Elliot, FI 1961, 'Artificial insemination of a Bactrian camel (Camelus Bactrianus)', Int. Zoo Yearbook vol. 3, pp Elwishy, AB 1987, 'Reproduction in the female Dromedary (Camelus dromedarius): a review', Anim. Reprod. Sci. vol. 15, pp Elwishy, AB 1988, 'Reproduction in the male Dromedary (Camelus dromedarius): a review', Anim. Reprod. Sci. vol. 17, pp Elwishy, AB 1988, 'A study on the genital organs of the female Dromedary (Camelus dromedarius)', J. Reprod. Fertil. vol. 82, pp ElWishy, AB 1992, 'Functional morphology of the ovaries of the Dromedary camel', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd, Newmarket, UK. Elwishy, AB and Omar, AM 1975, 'On the relation between testes size and sperm reserves in the one-humped camel (Camelus dromedarius)', Beit. Trop. Landwirt. vol. 13, pp England, BG, Foote, WC, Cardozo, AG, Matthews, DH and Riera, S 1971, 'Oestrus and mating behaviour in the llama (Lama glama). ' Animal Behaviour vol. 19, pp Escobar, RC 1984, 'Mating, parturition', in. R Hennig (eds), The Llama, Animal Breeding and Production of South American Camelids, Talleres Graficos de ABRIL, Lima, Peru. Feder, FH, Gonzalez, H and Arias, P 1999, 'Comparative histological study of the female reproductive system in the llama (Lama glama). II. Oviduct, uterus, cervix, vagina.' Anat. Histol. Embryol. vol. 28, pp Fernandez-Baca, S 1993, 'Manipulation of reproductive functions in male and female New World camelids', Anim. Reprod. Sci. vol. 33, pp Fernandez-Baca, S and Calderon, W 1966, 'Methods of collection of semen in the alpaca', Rev. de la Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos, Lima, Peru. vol. 13, pp Fernandez-Baca, S, Hansel, W and Novoa, C 1970, 'Corpus luteum function in the alpaca', Biol. Reprod. vol. 3, pp Fernandez-Baca, S, Hansel, W and Novoa, C 1970, 'Embryonic mortality in the alpaca', Biol. Reprod. vol. 3, pp Fernandez-Baca, S, Hansel, W, Saatman, R, Sumar, J and Novoa, C 1979, 'Differential luteolytic effects of right and left uterine horns in the alpaca', Biol. Reprod. vol. 20, pp Fernandez-Baca, S, Madden, DH and Novoa, C 1970, 'Effect of different mating stimuli on induction of ovulation in the alpaca', J. Reprod. Fertil. vol. 22, pp Fernandez-Baca, S and Novoa, C 1968, 'Primer ensayo de inseminacion artificial (Lama pacos) con semen de vicuña (Vicugna vicugna).' Rev. Fac. Med. Vet. Lima vol. 22, pp. 9. Fernandez-Baca, S, Sumar, J and Novoa, C 1976, 'Actividad functional del ovario y cuerno uterino en la alpaca', Memoria, Asociacion Latinoamericana de Produccion Animal vol. 11, pp. 70. Fernandez-Baca, S, Sumar, J, Novoa, C and Leyva, V 1973, 'Relacion entre la ubicacion del cuerpo luteo y la localizacion del embrion en la alpaca', Rev. de Investigaciones Pecuarias, Peru vol. 2, pp Ferre, L, Malik, TG, Nigro, H, Aller, J and Alberio, R 1996, 'Produccion de semen de llama bajo dos frecuencias de colecta en primavera', Rev. Arg. de Prod. Anim. vol. 16, pp Ferre, LB, Malik de Tchara, G, Aller, JF and Alberio, JR 2000, Effects of diluent, seminal plasma and centrifugation on quality traits of llama semen. Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Ferre, LB and Werkmeister, A 1996, Desarrollo de una vagina artificial termoelectrica para la colecta de semen en camelidos (resultados preliminares)', Rev. Arg. de Prod. Anim. vol. 16, pp
179 Ferrer, MS, Aguero, A, Chaves, MG, Russo, AF and Rutter, B 2002, 'Sincronizacion de la onda folicular mediante el uso de buserelina en la llama (Lama glama)', InVet - Investigacion Veterinaria vol. 4, pp Ferrer, MS, Aguero, A, Flores, M and Rutter, B 1999, 'Follicular dynamics control in llamas (Lama glama) using intravaginal sponges with Medroxiprogesterone', J. Camel Prac. Res. pp Flores, F 2002, Determinacion del perfil proteico y presencia de anticuerpos en el plasma seminal de la llama (Lama glama). San Pablo, Universidad Catolica Boliviana: 99. Flores, P, Garcia-Huidobro, J, Munoz, C, Bustos-Obregon, E and Urquieta, B 2002, 'Alpaca semen characteristics previous to a mating period', Anim. Reprod. Sci. vol. 72, pp Fowler, ME 1989, Medicine and surgery of South American camelids: llama, alpaca, vicuña, guanaco. Iowa State University Press, Iowa, USA. Fowler, ME 1997, 'Evolutionary history and differences between camelids and ruminants', J. Camel Prac. Res. vol. 4, pp Fowler, ME 1997, 'Medical management of South American camelids in North America', J. Camel Prac. Res. vol. 4, pp Fowler, ME 1998, Medicine and surgery of South American camelids: llama, alpaca, vicuña, guanaco. Iowa State University Press, Iowa, USA. Fowler, ME and Bravo, PW 1998, 'Reproduction', in Medicine and Surgery of South American Camelids 2nd Edition Iowa State University Press, Iowa, USA. Franco, E, Sumar, J and Varela, M 1981, 'Eyaculacion en la alpaca (Lama pacos)', Proceedings of IV Convencion Internacional sobre Camelidos Sudamericanos. Corporacion Nacional Forestal. Instituto de la Patagonia, Chile, Punta Arenas Galloway, DB 2000, The development of the testicles in alpacas in Australia. Proceedings of the Australian Alpaca Association Conference Canberra, Australia. Garcia Pereira, FL, Greene, SA, McEwen, MM and Keegan, R 2006, 'Analgesia and anesthesia in camelids', Small Ruminant Res. vol. 61, pp Garcia, W, Pezo, D, San Martin, F, Olazabal, J and Franco, F 2005, Manual del tecnico alpaquero Cusco, Peru. Garnica, J, Achata, R and Bravo, PW 1993, 'Physical and biochemical characteristics of alpaca semen', Anim. Reprod. Sci. vol. 32, pp Garnica, J, Flores, E and Bravo, PW 1995, 'Citric acid and fructose concentrations in seminal plasma of the alpaca', Small Ruminant Res. vol. 18, pp Gatica, R, Ratto, MH, Schuler, C et al. 1994, 'Nacimiento en una llama por Transferencia de embriones', Agricultura Técnica. vol. 1, pp Gatica, R, Ratto, MH, Shuler, C and al, e 1994, 'Cria obtenida en una llama (Lama glama) por transferencia de embriones.' Agricultura.Tecnica (Chile) vol. 54, pp Gatica, RG, Ratto, MF, Schuler, CC et al. 1994, 'Cria obtenida en un llama (Lama glama) por transferencia de embriones', Agricultura Tecnica (Santiago) vol. 54, pp Gauly, M 1997, 'Reproduction in South American camelids. A review. [German] Die Reproduktionsphysiologie von Neuweltkameliden. Ubersichtsreferat', Tierarztliche Praxis vol. 25, pp Gauly, M 1997, 'Reproductive physiology in New World camelids. Review', Tierarztliche Praxis vol. 25, pp Gauly, M 1997, 'Seasonal changes in semen characters and serum concentrations of testosterone, oestradiol-17 beta, thyroxine and triiodothyronine in male llamas (Lama glama) in Central Europe. [German]', Justus- Liebig-Universitat, Fachbereich Veterinarmedizin, Giessen, Germany. Gauly, M, Erhardt, G and Dzapo, V 1997, 'Annual changes in serum levels of thyroid hormones in male llamas (Lama glama) and their correlation with reproduction parameters', J. Camel Prac. Res. vol. 4, pp Gauly, M and Leidinger, H 1996, Semen quality, characteristic volume distribution and hypo-osmotic sensitivity of spermatozoa of Lama glama and Lama guanicoe. Proceedings of the 2nd European Symposium on South American camelids. Gauna, CD, Riesco, O, Williamson, D et al. 2003, Resultados preliminares en el uso de diluyentes para semen de camélidos sudamericanos. Proceedings of the 3rd Congreso de la Asociación Latinoamericana d Especialistas en Pequeños Rumiantes y Camélidos Sudamericanos, Viña del Mar (Chile). Gazitua, FJ, Corradini, P, Ferrando, G, Raggi, LA and Parraguez, VH 2001, 'Prediction of gestational age by ultrasonic fetometry in llamas (Lama glama) and alpacas (Lama pacos)', Anim. Reprod. Sci. vol. 66, pp Gibbons, A, Cueto, MI, Iovannitti, B and Lanuza, G 1996, 'Ovulacion por induccion hormonal exogena en guanaco (Lama guanicoe)', Rev. Arg. de Prod. Animal vol. 16, pp Gigli, I, Russo, A and Aguero, A 2006, 'Consideraciones sobre la dinamica ovaria en equino, bovino y camelidos sudamericanos', InVet - Investigacion Veterinaria vol. 8, pp
180 Giuliano, S, Chaves, MG, Director, A, Trasorras, V and Miragaya, M 2006, Inseminación artificial con semen refrigerado en llamas. Resultados preliminares. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Giuliano, S, Director, A, Gambarotta, M, Trasorras, V and Miragaya, M 2006, Influencia de diluyentes en semen refrigerado a 5ºC durante 24 hs. en la especie Lama glama. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Giuliano, S, Director, A, Gambarotta, M, Trasorras, V and Miragaya, M 2007, 'Collection method, season and individual variation on seminal characteristics in the llama (Lama glama)', Anim. Reprod. Sci. pp. in press. Giuliano, SM, Spirito, SE, Maragaya, MH et al. 2002, 'Electroejaculation and seminal parameters in vicuña (Vicugna vicugna)', Theriogenology vol. 57, pp Gomez, G, Ratto, MH, Berland, M, Wolter, M and Adams, GP 2002, Superstimulatory response and oocyte collection in Alpacas', Theriogenology vol. pp Gonzáles, V, Copa, S, Guzman, F and Cortez, G 2006, Técnica de inducción de ovulación en llamas e inseminación con semen fresco en Oruro, Bolivia. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. González, M, Huanca, T and Huanca, W 2006, Efecto del estradiol en la sobrevivencia embrionaria en alpacas y llamas. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. González, M, Huanca, T and Huanca, W 2006, Evaluación del efecto de dilutores en la motilidad de espermatozoides de semen de alpacas. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Gordon, I 1997, 'Controlled Reproduction in Camelids', in Controlled Reproduction in Horses, Deer and Camelids CAB International, Oxford, UK. Goswami, P, Gupta, ML and Purohit, GN 2003, 'Physical attributes of camel semen during different months under rural conditions', Indian J. Anim. Sci. vol. 73, pp Goswami, P, Purohit, GN and Gupta, ML 2004, 'Biometry of camel (Camelus dromedarius) spermatozoa and their morphological abnormalities', Indian J. Anim. Sci. vol. 74, pp Grace, ND, Hill, FI, Death, AF and Wyeth, TK 1994, 'A preliminary study of copper supplementation in alpacas (Lama pacos)', New Zeal. Vet. J. vol. 42, pp Guerrero, CA, Alva, J, Vega, I, Hernandez, J and Rojas, M 1973, 'Algunos aspectos biologicos y patologicos del Lamanema chavezi en alpacas, Lama pacos', Rev. de Investigaciones Pecuarias (IVITA) Universidad Nacional Mayor de San Marcos. vol. 2, pp Gunn, I 1999, 'Alpaca field trials', Rural Industries Research and Development Corporation Project. Guzman, F, Gonzáles, V, Copa, S and Cortez, G 2006, Técnica de colección de semen en llamas (Lama glama) para inseminación artificial con semen fresco. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Hafez, ESE and Hafez, B 2001, 'Reproductive parameters of male Dromedary and Bactrian camels', Arch. Androl. vol. 46, pp Hamali, H and Tajik, P 2003, 'Assessment of camel sperm motility obtained from cauda epididymis in Green buffer with or without egg yolk. [Persian]', J. Faculty of Vet. Med., University of Tehran vol. 58, pp Hassan, MM, Saeed, M and Rizwan ul, M 1995, 'Semen collection by artificial vagina and cryopreservation of camel (Camelus dromedarius) spermatozoa', Pakistan Vet. J. vol. 15, pp Heath, E, Mostafa, MS and Karasek, S 1986, 'Ultrastructure of camel (Camelus dromedarius) spermatozoa', Anat. Histol. Embryol. vol. 15, pp Hegazy, AA, Ali, A, Al-Eknah, M and Ismail, S 2004, 'Studies on pituitary-ovarian axis in the female camel with special reference to cystic and inactive ovaries', J. Camel Science vol. 1, pp Hemeida, NA, Al-Eknah, MM, Ismail, ST and Al-Haider, AK 2001, 'A new technique for collection of semen from Dromedary camels', Emirates J. Agr. Sci. vol. 13, pp Herrera, EA, Riquelme, RA, Sanhueza, EM, Raggi, LA and Llanos, AJ 2002, 'Use of fetal biometry to determine fetal age in late pregnancy in llamas', Anim. Reprod. Sci. vol. 74, pp Hill, FI, Thompson, KG and Grace, ND 1994, 'Rickets in alpacas (Lama pacos) in New Zealand', New Zeal. Vet. J. vol. 42, pp Hoffman, E and Fowler, ME 1995, The alpaca book. Clay Press, Herald, California, USA. Hombach-Klonisch, S, Abd-Elnaeim, M, Skidmore, JA et al. 2000, 'Ruminant relaxin in the pregnant onehumped camel (Camelus dromedarius)', Biol. Reprod. vol. 62, pp Homeida, AM 1990, 'A gonadotrophin-like bioactivity of the serum of pregnant camel (Camelus dromedarius) ', Anim. Reprod. Sci. vol. 23, pp Homeida, AM, Khalil, MGR and Taha, AAM 1988, 'Plasma concentrations of progesterone, oestrogens, testosterone and Lh-like activity during the oestrus cycle of the camel (Camelus dromedarius)', J. Reprod. Fertil. vol. 83, pp
181 Homeida, AM, Taha, AAM, Khalil, MGR and Hoppen, HO 1991, 'Secretion of LH and progesterone after intravenous adminisatration of GnRH in the camel (Camelus dromedarius)', Anim. Reprod. Sci. vol. 25, pp Hopkins, D 2004, 'Alpaca embryo transfer-the present situation', in The International Alpaca Handbook Alpaca Consulting Services of Australia, Norwood, S.A. Huanca, T, Apaza, N and Huanca, W 2006, Evaluación del efecto edad en el comportamiento reproductivo de alpacas machos. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Huanca, T, Cardenas, O, Huanca, W, Sapana, R and Cordero, A 2000, Effect of ecg and hcg on ovarian response in alpaca. Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Huanca, T, Cordero, A and Huanca, W 2006, Aplicación de un análogo de GnRH (Acetato de buserelina) y segundo servicio y su efecto sobre la tasa de concepción en llamas. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Huanca, W, Ampuero, A, Camacho, J and Cordero, A 2000, Testicular and seminal characteristics in Alpaca. Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Huanca, W, Cardenas, O, Olazabal, C, Ratto, M and Adams, GP 2001, 'Efecto hormonal y empadre sobre el intervalo a la ovulacion en llamas', Rev. Int. Vet. Peru Suplemento vol. 1, pp Huanca, W and Gauly, M 2001, 'Conservacion de semen refrigerado de llamas.' Rev. Inv. Vet. Peru vol. 1, pp Huanca, W, Gonzalez M., Cordero, A and Huanca, T 2006, Comportamiento reproductivo de donadoras de embriones, después de un protocolo de súper ovulación en llamas. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Huanca, W, Ratto, M, Cordero, A et al. 2005, 'Evaluación de un tratamiento de superovulación en la respuesta ovárica y tasa de preñez en llamas', Archivos Latinoamericanos de Produccion Animal vol. 13, pp. 24. Huanca, W, Ratto, M, Cordero, A et al. 2006, Respuesta ovárica y transferencia de embriones en llamas y alpacas en la zona altoandina del Perú. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Huanca, W, Ratto, MH, Santiani, A, Cordero, A and Huanca, T 2004, 'Embryo Transfer in camelids: Study of a reliable superovulatory treatment in llamas', Proceeings of the 4th European Symposium on South American Camelids. Hull, S and Cameron, T 2004, 'An introduction to alpaca sex-focus on the female', in The International Alpaca Handbook Alpaca Consulting services of Australia, Norwood, S.A. Hussein, FM, Bahgat Noseir, M, El-Bawab, IE and Paccamonti, DL 1991, 'Prenatal assessment of weight and dimensions of the camel conceptus (Camelus dromedarius)', Anim. Reprod. Sci. vol. 26, pp Ismail, AA, Radwan, YM and El-Mougy, SA 1988, 'Gonadotropins and testosterone concentrations in the follicular fluid of she-camel', Indian Vet. J. vol. 65, pp Ismail, AA, Siam, AA, El-Nahla, A and Abuzeag, SMM 1998, Synchronization of estrus in the she-camel. Proceedings of the 3rd Annual Meeting for Animal Production Under Arid Conditions, Al-Ain, UAE. Ismail, ST 1987, 'A review of reproduction in the female camel (Camelus dromedarius)', Theriogenology vol. 28, pp Ismail, ST 1988, 'Reproduction in the male Dromedary (Camelus dromedarius)', Theriogenology vol. 29, pp Ismail, ST, Al-Eknah, MM and Hemeida, NA 2007, 'Superovulation trial for embryo transfer in the camel (Camelus dromedarius)', King Faisel University Website. Ismail, ST, Azab, GA, Abdoon, ASS and El-Belely, MS 1993, 'New approach for superovulation and embryo recovery in the one humped camel', Vet. Med. J. Giza vol. 41, pp Johnson, LW 1989, 'Llama reproduction', Vet. Clin. N. Am. Food A. vol. 5, pp Jones, CJP, Abd-Elnaeim, M, Bevilacqua, E, Oliveira, LV and Leiser, R 2002, 'Comparison of uteroplacental glycosylation in the camel (Camelus dromedarius) and alpaca (Lama pacos)', Reproduction vol. 123, pp Kadwell, M, Fernandez, M, Stanley, HF et al. 2001, 'Genetic analysis reveals the wild ancestors of the llama and the alpaca', Proc. Roy. Soc. London - Series B vol. 268, pp Kafi, M, Mesbah, F, Nili, H and Khalili, A 2005, 'Chronological and ultrastructural changes in camel (Camelus dromedarius) oocytes during in vitro maturation', Theriogenology vol. 63, pp Kafi, M, Nili, H and Mesbah, F 2000, Timing of nuclear progression and cumulus cell expansion during in vitro maturation of Dromedary camel oocytes. Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Kaufmann, BA 2004, 'Reproductive performance of camels (Camelus dromedarius) under pastoral management and its influence on herd development', Livestock Prod. Sci. pp. in press. Khan, AA and Kohli, IS 1973, 'A note on collection of semen from camel with the help of an artificial vagina', Indian J. Anim. Sci. vol , pp
182 Khan, AA and Kohli, IS 1973, 'A note on the sexual behaviour of male camel (Camelus dromedarius)', Indian J. Anim. Sci. vol , pp Khan, AK and Kohli, IS 1973, 'A note on biometrics of the camel spermatozoa (Camelus dromedarius)', Indian J. Anim. Sci. vol. 43, pp Khatir, H and Anouassi, A 2005, 'The first Dromedary (Camelus dromedarius) offspring obtained from in vitro matured, in vitro fertilized and in vitro cultured abattoir-derived oocytes', Theriogenology vol. pp.. Khatir, H, Anouassi, A and Tibary, A 2004, 'Production of Dromedary (Camelus dromedarius) embryos by IVM and IVF and co-culture with oviductal or granulosa cells', Theriogenology vol. 62, pp Khatir, H, Anouassi, A and Tibary, A 2005, 'In vitro and in vivo developmental competence of Dromedary (Camelus dromedarius) embryos produced in vitro using two culture systems (mksomaa and oviductal cells)', Reprod. Domest. Anim. vol. 40, pp Khatir, H, Anouassi, A and Tibary, A 2007, 'Effect of follicular size on in vitro developmental competence of oocytes and viability of embryos after transfer in the Dromedary (Camelus dromedarius)', Anim. Reprod. Sci. vol. 99, pp Khatir, H, Anouassi, A and Tibary, A 2007, 'Quality and developmental ability of Dromedary (Camelus dromedarius) embryos obtained by IVM/IVF, in vivo matured/ivf or in vivo matured/fertilized oocytes', Reprod. Domest. Anim. in press. King, MR, Hendrickson, DA, Southwood, LL, Trumble, TN and Johnson, LW 1998, 'Laparoscopic ovariectomy in two standing llamas', J. Am. Vet. Med. Assoc. vol. 213, pp Knight, T, Death, A and Wyeth, T 1995, 'Photoperiodic control of the time of parturition in alpacas (Lama pacos)', Anim. Reprod. Sci. vol. 39, pp Knight, TW, Death, A, Wyeth, T and Hill, F 1992, 'Effects of GnRH and of single versus multiple mating on the conception rate in alpacas', Proc. New Zealand Soc. Anim. Prod. vol. 52, pp Knight, TW, Ridland, M, Scott, I, Death, AF and Wyeth, TK 1995, 'Foetal mortality at different stages of gestation in alpacas (Lama pacos) and the associated changes in progesterone concentrations', Anim. Reprod. Sci. vol. 40, pp Kubicek, J 1974, 'Semen collection in the alpaca via a urethral fistula. [German] Samenentnahme beim Alpaka durch eine Harnrohrenfistel', Zeitschrift fur Tierzuchtung und Zuchtungsbiologie vol. 90, pp Lattanzi, M, Santos, C, Chaves, G et al. 2002, 'Cryopreservation of llama (Lama glama) embryos by slow freezing and vitrification', Theriogenology vol. 57, pp Leahy, T, Morton, KM, Maxwell, WMC and Evans, G 2006, 'Peculiarities and problems in alpaca reproduction', Proceedings of the 5th Annual Postgraduate Research Conference. Faculty of Veterinary Science, The University of Sydney, Australia Lichtenwalner, AB, Woods, GL and Weber, JA 1996, 'Ejaculatory pattern of llamas during copulation', Theriogenology vol. 46, pp Lichtenwalner, AB, Woods, GL and Weber, JA 1996, 'Seminal collection, seminal characteristics and pattern of ejaculation in llamas', Theriogenology vol. 46, pp Lichtenwalner, AB, Woods, GL and Weber, JA 1998, 'Male llama choice between receptive and nonreceptive females', Appl. Anim. Behav. Sci. vol. 59, pp Lupidio, MC, Gomez, SA, Castro, ANC and Ghezzi, MD 2006, Micro-estructura de la porcion diseminada de la glandula prostatica de la Llama (Lama glama). Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Lupidio, MC, Gomez, SA, Castro, ANC and Ghezzi, MD 2006, Microestructura del ovario de la Llama (Lama glama). Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Mahamat, H, Olaho-Mukani, W, Mboloi, MM, Guya, SO and Krombaritis, GE 1997, 'Pregnancy diagnosis in the Dromedary camel (Camelus dromedarius) based on a competitive progesterone enzyme linked immunosorbent assay (ELISA)', J. Camel Prac. Res. vol. 4, pp. 11 ref. Marai, IFM and Zeidan, AEB 2007, 'Artificial insemination in Camelidae', Trop. Subtropical Agroecosystems vol. 7, pp Marie, M and Anouassi, A 1986, 'Mating-induced luteinizing hormone surge and ovulation in the female camel (Camelus dromedarius)', Biol. Reprod. vol. 35, pp Marie, M and Anouassi, A 1987, 'Induction of luteal activity and progesterone secretion in the nonpregnant onehumped camel (Camelus dromedarius)', J. Reprod. Fertil. vol. 80, pp Maxwell, WMC, Leahy, T, Morton, KM, Marti, J and Evans, G 2007, 'Seminal plasma effects on ruminant and camelid sperm function during processing for storage and sex-sorting', Reprod. Domest. Anim. vol. 42, pp. 60. McEvoy, TG, Bourke, DA and Adam, CL 1994, 'Recent advances in understanding and controlling the reproductive biology of South American camelids.' Proc Assisted Reproductive Technology and Andrology Meeting, Spain vol. 5, pp
183 McEvoy, TG, Kyle, CE, Slater, D, Adam, CL and Bourke, DA 1992, 'Collection, evaluation and cryopreservation of llama semen.' J. Reprod. Fertil. Abstract Series vol. 9, pp. 48. McEvoy, TG, Kyle, CE, Young, P, Adam, CL and Bourke, D 1992, 'Aspects of artificial breeding and establishment of pregnancy in South American Camelids', Proceedings of the 12th International Congress on Animal Reproduction, The Hague vol. 4, pp McKinnon, AO and Tinson, AH 1992, 'Embryo transfer in Dromedary camels', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992, R & W Publications Ltd, Newmarket, UK. McKinnon, AO, Tinson, AH and Nation, G 1994, 'Embryo transfer in Dromedary camels', Theriogenology vol. 41, pp Medina Suca, G, Catacora, N and Olivera, L 2006, Niveles hormonales, evaluación de semen y espermatogénesis en alpacas. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Merkt, H, Rath, D, Musa, B and El-Naggar, MA 1990, Reproduction in Camels: a review. FAO Animal Production and Health Paper 82 Rome, Italy, Food and Agriculture Organization of the United Nations. Merlian, CP, Sikes, JD, Read, BW, Boever, WJ and Knox, D 1979, 'Comparative characteristics of spermatozoa and semen from a Bactrian camel, Dromedary camel and llama', J. Zoo Anim. Med. vol. 10, pp Mesbah, F, Kafi, M and Nili, H 2000, Recovery and morphological correlates of camel (Camelus dromedarius) oocytes. Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Mesbah, SF, Kafi, M, Nili, H and Nasr-Esfahani, MH 2004, 'Spontaneous parthenogenesis and development of camel (Camelus dromedarius) oocytes', Vet. Rec. vol. 155, pp Minoia, P, Moslah, M, Localandra, GM, Khorchani, T and Zarrilli, A 1992, 'Induction of oestrus and management of reproduction in the female Dromedary camel', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd, Newmarket, UK. Miragaya, MH, Aba, MA, Capdevielle, EF et al. 2004, 'Follicular activity and hormonal secretory profile in vicuña (Vicugna vicugna)', Theriogenology vol. 61, pp Miragaya, MH, Chaves, MG and Aguero, A 2006, 'Reproductive biotechnology in South American Camelids', Small Ruminant Res. vol. 91, pp Miragaya, MH, Chaves, MG, Capdevielle, EF et al. 2002, 'In vitro maturation of llama (Lama glama) oocytes obtained surgically using follicle aspiration', Theriogenology vol. 57, pp Mobarak, AM, El Gaafary, MAH, Ewais, MS and Ammar, SMS 1980, 'Prenatal development of the rumen of the Dromedary camel in relation to CVR length and body weight', J. Egyptian Vet. Med. Assoc. vol. 40, pp Mogrovejo, SD 1952, Estudios del semen de la alpaca. Facultad de medicina veterinaria Lima, Peru, University Nac Mayor San Marcos: 37. Montalvo, C, Cevallos, E and Copaira, M 1979, 'Estudio microscopico del parenquima testicular de la alpaca durante las estaciones del ano', Res Proyectos de Investigacion, Periodo Univ Nac Mayor San Marcos, Lima pp. 37. Morrow, CK, Barrington, GM, Johnson, LW and Hendrickson, DA 1998, 'How to perform laparoscopic ovariectomy in llamas and alpacas', Vet. Med. U.S. vol. 93, pp Morton, KM 2005, 'Epididymal sperm freezing in alpacas', 'Alpacas Australia'. Magazine of the Australian Alpaca Association, December issue. Morton, KM, Bathgate, R, Evans, G and Maxwell, WMC 2006, 'A comparison of three diluents for the cryopreservation of epididymal alpaca sperm', Reprod. Domest. Anim. vol. 41, pp Morton, KM, Bathgate, R, Evans, G and Maxwell, WMC 2006, 'Cryopreservation of epididymal alpaca sperm in pellets and straws frozen on dry ice or in liquid nitrogen vapour.' in JL Juengel, JF Murray and MF Smith (eds), Reproduction in Domestic Ruminants VI, Nottingham University Press, Nottingham, UK. Morton, KM, Bathgate, R, Evans, G and Maxwell, WMC 2007, 'Cryopreservation of epididymal alpaca (Vicugna pacos) sperm: a comparison of Citrate, Tris and Lactose based diluents, and pellets and straws.' Reprod. Fertil. Dev. vol. 19, pp Morton, KM, Evans, G and Maxwell, WMC 2007, 'Effect of glycerol concentration, Equex STM supplementation and liquid storage prior to freezing on the survival and functional integrity of frozen-thawed epididymal alpaca (Vicugna pacos) sperm. ' Anim. Reprod. Sci. pp. submitted. Morton, KM, Gibb, Z, Wilson, SJ et al. 2007, 'Effect of diluent, dilution rate and storage temperature on longevity and functional integrity of liquid stored alpaca (Vicugna pacos) sperm. ' Reprod. Fertil. Dev. pp. submitted. Morton, KM and Maxwell, WMC 2006, 'The continued development of AI technology in alpacas', Proc. Aust. Alpaca Assoc. Conference vol. 1, pp
184 Morton, KM, Ruckholdt, M, Evans, G and Maxwell, WMC 2006, 'Preliminary development of sperm sexing technology in alpacas (Lama pacos)', in Proceedings of the 1st International Society of Camelid Research and Development, Al Ain, UAE. Morton, KM, Ruckholdt, M, Evans, G and Maxwell, WMC 2007, 'Quantification of the DNA difference, and separation of X- and Y-bearing sperm in alpacas (Vicugna pacos)', Reprod. Domest. Anim. pp. in press. Morton, KM, Thomson, PC, Bailey, KJ, Evans, G and Maxwell, WMC 2007, 'Quality parameters for alpaca (Vicugna pacos) semen are affected by semen collection procedure', Reprod. Fertil. Dev. pp. submitted. Mosaferi, S, Niasari-Naslaji, A, Abarghani, A, Gharahdaghi, AA and Gerami, A 2005, 'Biophysical and biochemical characteristics of Bactrian camel semen collected by artificial vagina', Theriogenology vol. 63, pp Mosaferi, S, Niasari-Naslaji, A, Bahmani, N et al. 2006, 'Comparing different levels of osmolarity of sucrose extender on the viability of spermatozoa in Bactrian camel (Camelus Bactrianus).' Reprod. Fertil. Dev. vol. 18, pp Mosaferi, S, Niasari-Naslaji, N, Gharahdaghi, AA et al. 2005, 'Comparing different levels of osmolarity and ph of lactose extender on the viability of spermatozoa in the Bactrian camel (Camelus Bactrianus)', Reprod. Fertil. Dev. vol. 17, pp Moslah, M, Minoia, P, Lacalandra, GM, Khorchani, T and Zarrilli, A 1992, 'Hormonal stimulation of libido and reproductive function in the male Dromedary camel: clinical observations', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd, Newmarket, UK. Musa, B, Merkt, H, Hago, B, Hoppen, HO and Sieme, H 1993, 'The female camel (Camelus dromedarius) and artificial insemination', Etudes et Syntheses de l'iemvt vol. 41, pp Musa, B, Sieme, H, Merkt, H and Hago, BED 1992, 'Artificial insemination in Dromedary camels', in in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd, Newmarket, UK. Musa, B, Sieme, H, Merkt, H et al. 1993, 'Manipulation of reproductive functions in male and female camelids', Anim. Reprod. Sci. vol. 33, pp Musa, BE 2004, 'Reproduction in the male', in. FK Al-Ani (eds), Camel: Management and Diseases Faculty of Veterinary Medicine, Baghdad University, Baghdad, Iraq. Nagy, P, Jutka, J and Wernery, U 2005, 'Incidence of spontaneous ovulation and development of the corpus luteum in non-mated Dromedary camels (Camelus dromedarius)', Theriogenology vol. 64, pp Neely, DP and Bravo, WP 1997, 'Reproductive evaluation and infertility in the male llama and alpaca', in RS Younguist (eds), Current Therapy in Large Animal Theriogenology. Niasari-Naslaji, A, Mosaferi, S, Bahmani, N et al. 2007, 'Semen cryopreservation in Bactrian camel (Camelus Bactrianus) using SHOTOR diluent: Effects of cooling rates and glycerol concentrations ', Theriogenology vol. 68, pp Niasari-Naslaji, A, Mosaferi, S, Bahmani, N et al. 2006, 'Effectiveness of a tris-based extender (SHOTOR diluent) for the preservation of Bactrian camel (Camelus Bactrianus) semen.' Cryobiology vol. 53, pp Niasari-Naslaji, A, Mosaferi, S, Gharahdaghi, AA et al. 2006, 'Comparison between two extenders for cryopreservation of Bactrian camel semen', Reprod. Fertil. Dev. vol. 18, pp Niasari-Naslaji, N, Mosaferi, S, Gharahdaghi, AA et al. 2005, 'A novel extender for preservation of Bactrian camel (Camelus Bactrianus) semen', Reprod. Fertil. Dev. vol. 17, pp Niasari-Naslaji, N, Sarhaddi, F, Nazari, M and razavi, K 2001, 'Vaginal artifical insemination in maiden Dromedary camel-a novel approach', Theriogenology vol., pp Nili, H, Mesbah, F and Kafi, M 2000, Ultrastructural features of immature culturable camel (Camelus dromedarius) oocytes. Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Nili, H, Mesbah, F, Kafi, M and Esfahani, MHN 2004, 'Light and transmission electron microscopy of immature camelus dromedarius oocyte', Anat. Histol. Embryol. vol. 33, pp Nourani, H and Khodakaram-Tafti, A 2004, 'Pathological study of ovaries of non-pregnant camels (Camelus dromedarius) slaughtered in Iran', J. Camel Prac. Res. vol. 11, pp Novoa, C 1970, 'Reproduction in Camelidae', J. Reprod. Fertil. vol. 22, pp Novoa, C and Leyva, V 1996, 'Reproducción en alpacas y llamas.' Publicación Científica Nº 26. IVITA- UNMSM. Lima. pp. 32. Nowshari, MA 2005, 'The effect of harvesting technique on efficiency of oocyte collection and different maturation media on the nuclear maturation of oocytes in camels (Camelus dromedarius)', Theriogenology vol. 63, pp Nowshari, MA and Ali, SA 2005, 'Effect of season and gonadotropins on the superovulatory response in camel (Camelus dromedarius)', Theriogenology vol. 64, pp Nowshari, MA, Ali, SA and Shazia, S 2005, 'Offspring resulting from transfer of cryopreserved embryos in camel (Camelus dromedarius)', Theriogenology vol. 63, pp
185 Nowshari, MA and Wani, NA, 'Camelid embryo development in vitro: effects of protein supplement in maturation medium and subsequent culture in two different media on fertilization and development', Reprod. Fertil. Dev. pp Omer, MA, Bashandy, MM, Dessouky, IM, Hanafi, EM and Ahmed, WM 2000, Endometritis in camel: comparative studies between early and late post partum periods. Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Ordoñez, TH 1994, 'Llamas, llama production and llama nutrition in the Ecuador highlands.' Journal of Arid Environments vol. 26, pp Osman, AM and El-Azab, EA 1974, 'Gonadal and epididymal sperm reserves in the camel, Camelus dromedarius', J. Reprod. Fertil. vol. 38, pp Osman, DI 1986, 'The intratesticular excurrent ducts of the camel (Camelus dromedarius)', Anim. Reprod. Sci. vol. 10, pp Osman, DI and Ploen, L 1986, 'Fine structure of epididymal spermatozoa in the camel (Camelus dromedarius)', Anim. Reprod. Sci. vol. 10, pp Osman, DI and Ploen, L 1986, 'Fine structure of sertoli cells in the camel (Camelus dromedarius)', Anim. Reprod. Sci. vol. 10, pp Osman, DI and Ploen, L 1986, 'Spermatogenesis in the camel (Camelus dromedarius)', Anim. Reprod. Sci. vol. 10, pp Palasz, AT 2000, 'Effect of day of collection and of permeating cryoprotectants on llama (Lama glama) embryos and trophoblastic vesicles', Theriogenology vol. 53, pp Palasz, AT, Rodriguez-martinez, H and Brogliatti, GM 1997, 'The ultrastructure of llama (Lama glama) blastocysts', Theriogenology vol. 47, pp Palomino, CJ, Cervantes M. and W., H 2006, Perfiles de Progesterona, tamaño del cuerpo lúteo y sobrevivencia embrionaria en llamas (Lama glama) con aplicación de estradiol. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Palomino, CJ, Huanca, W and Cervantes, M 2006, Efecto de la aplicación de estradiol sobre la sobrevivencia embrionaria en llamas (Lama glama). Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Palomino, JM 2004, Estudio del efecto de la aplicacion de estradiol y progesterone sobre la sobrevivencia embrionarie en llamas. Facultad de medicina veterinaria Lima, Peru, Universidad Nacional Mayor De San Marcos. Pan, G, Chen, Z, Liu, X et al. 2001, 'Isolation and purification of the ovulation-inducing factor from seminal plasma in the Bactrian camel (Camelus Bactrianus)', Theriogenology vol. 55, pp Pan, G, Xie, Q, Cai, X et al. 2003, 'Study on ovulation inducing mechanism in Bactrian camel II target organ of ovulation inducing factor (OIF) and its metabolic dynamics in Bactrian camel. [Chinese]', Sci. Agr. vol. 36, pp Pan, GW, Zhao, XX, Chen, B-H et al. 1992, 'The ovulation-inducing effect of seminal plasma in the Bactrian camel', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992, R & W Publications Ltd, Newmarket, UK. Pan, GW, Zhao, XX, Chen, BH et al. 1986, 'Ovulation inducing effect of seminal plasma injected intramuscularly in the Bactrian camel. [Chinese]', Sci. Agr. vol. 2, pp Paolicchi, F, Bustos Obregon, E, Alberio, R and Urquieta, B 1996, Respuesta hipofisiaria en alpacas tratadas con GnRH: analisis de la concentracion plasmatica de hormona luteinizante mediante un radioinmunoanalisis heterologo', Rev. Arg. de Prod. Anim. vol. 16, pp Paolicchi, F, Urquieta, B, del Valle, B and Bustos, OE 1996, Actividad biologica del plasma seminal de alpaca: Estimula para la prooduccion de LH por celulas gonadotropas', Rev. Arg. de Prod. Anim. vol. 16, pp Paolicchi, F, Urquieta, B, Del Valle, L and Bustos-Obregon, E 1999, 'Biological activity of the seminal plasma of alpacas: stimulus for the production of LH by pituitary cells', Anim. Reprod. Sci. vol. 54, pp Parraguez, VH, Cortez, S, Gazitua, FJ et al. 1997, 'Early pregnancy diagnosis in alpaca (Lama pacos) and llama (Lama glama) by ultrasound', Anim. Reprod. Sci. vol. 47, pp Parraguez, VH, Gazitua, FJ, Cortez, S and Raggi, LA 1996, Estudio ultrasonografico de la gestacion an alpacas (Lama pacos): Resultados preliminares', Rev. Arg. de Prod. Anim. vol. 16, pp Pérez Durand, M, Garcia, W, Capcha, A and Pacheco, J 2006, Evaluación de la capacidad fecundante de los espermatoziodes (conducto deferente) conservado en alpacas. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Perez, MG 2005, Perspectivas de la inseminacion artificial en camelidos sudamericanos., Universidad Nacional del Altiplano, Puno-Peru. Pollard, JC, Littlejohn, RP and Moore, GH 1995, 'Seasonal and other factors affecting the sexual behaviour of alpacas', Anim. Reprod. Sci. vol. 37, pp
186 Pollard, JC, Littlejohn, RP and Scott, IC 1994, 'The effects of mating on the sexual receptivity of female alpacas', Anim. Reprod. Sci. vol. 34, pp Pollard, JC, Moore, GH and Littlejohn, RP 1991, 'The sexual behaviour of alpacas imported to New Zealand from Chile. ' Proceedings of the New Zealand Society of Animal Production vol. 51, pp Powell, SA 2000, The relationship of estradiol to embryo-maternal interactions in the Llama, Oregon State University. PhD: 118. Powell, SA, Smith, BB, Timm, KI and Menino, J, A.R. 2007, 'Estradiol production by preimplantation blastocysts and increased serum progesterone following estradiol treatment in llamas', Anim. Reprod. Sci. vol. 102, pp Pugh, DG 1999, 'Male lama reproductive evaluation', Proceeings of the Society for Theriogenology pp Pugh, DG 2000, 'Reproductive evaluation of the male llama.' in The Ohio State University College of Veterinary Medicine Presents Camelid Medicine, Surgery and Reproduction, Ohio State University. College of Veterinary Medicine, Columbus, Ohio. Pugh, DG and Montes, AJ 1994, 'Advanced reproductive technologies in South American camelids', Vet. Clin. N. Am. Food A. vol. 10, pp Pugh, DG, Waldridge, B and Wenzel, JGW 1999, 'Trace mineral nutrition in llamas', J. Camel Prac. Res. vol. 6, pp Purohit, GN 1999, 'Biotechnologies in camelid reproduction: current status and future prospectives', J. Camel Prac. Res. vol. 6, pp Purohit, GN, Mahesh, D and Sharma, SS 1999, 'Efficiency of three methods of oocyte recovery in the Dromedary camel (Camelus dromedarius)', Indian J. Anim. Sci. vol. 69, pp Quispe, G 1996, Inseminacion artificial en alpacas (Lama pacos) con semen diluido a differentes concentraciones. Fac Agron Zootec. Cusco, Peru, Univ San Antonio Abad: 103 Raggi, LA, Ferrando, G, Parraguez, VH, MacNiven, V and Urquieta, B 1999, 'Plasma progesterone in alpaca (Lama pacos) during pregnancy, parturition and early postpartum', Anim. Reprod. Sci. vol. 54, pp Raggi, SLA 2000, 'Advances in research and management of llamas (Lama glama) and alpaca (Lama pacos) in Chile', Cien. Invest. Agraria, vol. 27, pp Rai, AK, Sharma, N and Khanna, ND 1995, 'Ovarian activity during breeding and non breeding seasons in Indian camel', Indian J. Anim. Sci. vol. 65, pp Rai, AK, Sharma, N, Manivannan, B and Khanna, ND 1997, 'Camel semen during breeding and non-breeding seasons', Indian J. Anim. Sci. vol. 67, pp Ranga, A and Rai, AK 1992, 'Scanning electron microscopic study of camel spermatozoa', Indian J. Anim. Sci. vol. 62, pp Ratto, M, Berland, M, Huanca, W, Jaswant, S and Adams, GP 2005, 'In vitro and in vivo maturation of llama oocytes', Theriogenology vol. 63, pp Ratto, M, Gomez, C, Berland, M and Adams, GP 2007, 'Effect of ovarian superstimulation on COC collection and maturation in alpacas', Anim. Reprod. Sci. vol. 97, pp Ratto, M, Huanca, W, Huanca, T, Singh, J and Adams, G 2004, 'Ovulation inducing factor in seminal plasma: species comparision and molecular weight determination', Biol. Reprod. Suppl. pp Ratto, M, Huanca, W, Singh, J and Adams, GP 2006, 'Comparison on the effect of natural mating, LH, and GnRH on interval to ovulation and luteal function in llamas', Anim. Reprod. Sci. vol. 91, pp Ratto, MH 1995, Induction of superovulation in Llamas. Valdivia, Chile, Universidad Austral de Chile. Ratto, MH 2005, Ovarian follicular synchronisation, ovulation and oocyte development in llamas and alpacas. Department of Veterinary Biomedical Sciences Saskatoon, Canada, University of Saskatchewan: 168. Ratto, MH, Berland, M and Adams, GP 2002, 'Ovarian superstimulation and ultrasound-guided oocyte collection in llamas', Theriogenology vol. 57, pp Ratto, MH, Berland, M, Gomez, C et al, 1999, 'In vitro maturation of llama oocytes', II World Congress of South American Camelids, Cusco, Peru pp. 80. Ratto, MH and G.P., A 2003, 'Embryo technologies in the llama', in. RS Youngquist (eds), Chapter in Current Therapy in Large Animal Theriogenology WB Saunders Co, Philadelphia, USA. Ratto, MH, Gatica, R and Correa, JE 1997, 'Timing of mating and ovarian response in llamas (Lama glama) treated with pfsh', Anim. Reprod. Sci. vol. 48, pp Ratto, MH, Huanca, W, Singh, J and Adams, GP 2005, 'Local versus systemic effect of ovulation-inducing factor in the seminal plasma of alpacas.' Reprod. Biol. Endocrinol. vol. 3, pp Ratto, MH, Huanca, W, Singh, J and Adams, GP 2006, 'Comparison of the effect of ovulation-inducing factor (OIF) in the seminal plasma of llamas, alpacas and bulls', Theriogenology vol. 66, pp Ratto, MH, Singh, J and Adams, GP, 'Follicular wave synchronisation and fixed-time mating in llamas', Theriogenology vol., pp Ratto, MH, Singh, J, Huanca, W and Adams, GP 2003, 'Ovarian follicular wave synchronization and pregnancy rate after fixed-time natural mating in llamas', Theriogenology vol. 60, pp
187 Ratto, MH, Wolter, M and Berland, M 1999, Refrigeration of epididymal sperm from lama with three different extenders. Proceedings of the II Congreso Mundial sobre Camelidos Cusco, Peru. Raymundo, F, Huanca, W, Huanca, T, Huerta, S and Cordero, A 2006, 'Efecto De Tres Dilutores En La Conservacion Del Semen De Alpacas ', Rev Inv Vet Perú vol. 17, pp Raymundo, F, Huanca, W, Huertas, S and Gauly, M 2000, Influence of different extenders on the motility in alpaca (Lama pacos) semen. Proceedings of the 2nd Int Camelid Conference Agroeconomics of Camelid Farming Almaty, Kazakhstan. Reyes, S, Robinson, TF and Silcox, RW 2003, 'Estudio de extraccion de semen en macho alpacas', RELAN vol. 2, pp Reyna, J, Morton, KM, Evans, G and Maxwell, WMC 2005, Collection and evaluation of alpaca sperm. Proceedings of the 4th Annual Postgraduate Research Conference. Faculty of Veterinary Science, The University of Sydney, Australia.. Riveros, JL, Araya, M, Urquieta, B, Bonacic, C and Bas, MF 2006, Relación entre el tamaño folicular ovárico y la conducta de estro en hembras guanaco paridas y no gestantes en cautiverio en la zona central de Chile. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Roldán-Olarte, M, Apichela, S, Valdecantos, P et al. 2006, Expresion del gen del activador del plasminogeno tipo uroquinasa en celulas del epitelio oviducal de Vicuña Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Sahani, MS, Vyas, S and Deen, A 2003, 'Improvement in reproductive efficiency in farm camels under hot arid region.' Indian J. Anim. Reprod. vol. 24, pp Saleh, A-MM, Alameldin, MA, Abdelmoniem, ME, Hassouna, EM and Wrobel, K-H 2002, 'On the intrinsic innervation of the epididymis of the camel (Camelus dromedarius)', Ann. Anat. vol. 184, pp Salem, HAH, Serur, BH and Amer, HA 1997, 'Oestradiol, progesterone and thyroxine in follicular fluids of normal, cystic and atretic follicles of non-pregnant camels in Saudi Arabia', J. Camel Prac. Res. vol. 4, pp. 20 ref. Salinas, J, Bravo, PW, Ordonez, C, Zapata, B and Alarcon, B 1999, 'Niveles optimos de glicerina en la congelacion de semen en alpacas (Lama pacos)', Resumenes de Investigacion en Camelidos Sudamericanos, Cusco, Peru pp. 24. San Martin, HF 1996, Nutricion de camelidos sudamericanos y su relacion con la reproduccion', Rev. Arg. de Prod. Anim. vol. 16, pp San Martin, M, Copaira, M, Zuniga, J et al. 1968, 'Aspects of reproduction in the alpaca', J. Reprod. Fertil. vol. 16, pp San Martin, M, de la Vega, E and Gonzalez, SC 1960/62, 'Actividad mitogenica y estadios precoces de la oogenesis en el ovario de alpacas' Facultad de Medicina Veterinaria Revista, Universidad Nacional Mayor de San Marcos, vol. 15, pp Sanchez, AE and Correa, JE 1995, 'Descripcion ecografica preliminar de prostata y glandulas bulbouretrales en tres machos llama', Arch. Med. Vet. vol. 27, pp Sansinena, M, Taylor, P, Taylor, S, Denniston, RS and Godke, RA 2000, 'Production of interspecies nuclear transfer embryos using male and female llama (Lama glama) cell lines', Theriogenology pp Sansinena, MJ 2004, Somatic cell interspecies nuclear transfer, Louisiana State University. PhD: 186. Sansinena, MJ, Taylor, SA, Taylor, PJ, Denniston, RS and Godke, RA 2003, 'Production of nuclear transfer llama (Lama glama) embryos from in vitro matured llama oocytes', Cloning & Stem Cells vol. 5, pp Sansinena, MJ, Taylor, SA, Taylor, PJ et al. 2007, 'In vitro production of llama (Lama glama) embryos by intracytoplasmic sperm injection: Effect of chemical activiation treatments and culture conditions.' Anim. Reprod. Sci. vol. 99, pp Santiani, A, Huanca, W, Sapana, R et al. 2005, 'Effects on the quality of frozen-thawed alpaca (Lama pacos) semen using two different cryoprotectants and extenders', Asian J. Androl. vol. 7, pp Santiani, A, Leya, VV and Garcia, WV 2002, 'Effecto inhibitorio de la progesterona sobre el desarrollo de la onda folicular en llamas', Rev. Int. Vet. Peru vol. 13, pp Schwalm, A, Gauly, M, Erhardt, G and Bergmann, M 2007, 'Changes in testicular histology and sperm quality in llamas (Lama glama) following exposure to high ambient temperature', Theriogenology vol. 67, pp Schwarzenberger, F, Speckbacher, G and Bamberg, E 1995, 'Plasma and fecal progestagen evaluations during and after the breeding season of the female vicuña (Vicuña vicuña)', Theriogenology vol. 43, pp Sepulveda, BN and Risopatron, GJ 1993, 'Antecedentes sobre la ganaderia de camelidos en el sur de Chile', Arch. Med. Vet. vol. 25, pp Sghiri, A and Driancourt, MA 1999, 'Seasonal effects on fertility and ovarian follicular growth and maturation in camels (Camelus dromedarius)', Anim. Reprod. Sci. vol. 55, pp Shereif, NA and Tinson, AH 2000, 'Genetics and breeding methods in the domedary camels', in. TK Gahlot and J Singh (eds), Selected topics on Camelids The Camelid Publishers, Bikaner. 172
188 Sieme, H, Merkt, H, Musa, B, Hago, B and Willem, T 1990, 'Liquid and deep freeze preservation of camel semen using different extenders and methods', Proceedings of the workshop - Is it possible to improve the reproductive performance of the camel, Paris, France. Sieme, H, Merkt, H, Musa, B, Hago, BED and Willem, T 1993, 'Liquid and deep freeze preservation of camel semen using different extenders and methods', Etudes et Syntheses de l'iemvt vol. 41, pp Singh, J, Purohit, GN and Pareek, PK 2001, 'Seminal plasma electrolytes in the Dromedary camel and their correlation with seminal quality', Indian J. Anim. Sci. vol. 71, pp Singh, UB and Bharadwaj, MB 1978, ' Histological and histochemical studies on the testis of camel (Camelus dromedarius) during the various seasons and ages. Part II.' Acta Anat. vol. 101, pp Skidmore, J, Allen, WR, Cooper, MJ et al. 1992, 'The recovery and transfer of embryos in the Dromedary camel: results of preliminary experiments', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992, R & W Publications Ltd, Newmarket, UK. Skidmore, J, Boyle, MS, Cran, D and Allen, WR 1989, 'Micromanipulation of equine embryos to produce monozygotic twins', Equine Vet. J. vol. Supplement 8, pp Skidmore, JA 2000, 'Anatomy of the camel reproductive tract', in. L Skidmore and GP Adams (eds), Recent Advances in Camelid Reproduction, International Veterinary Information Service (IVIS), Ithaca, New York, USA. Skidmore, JA 2000, 'Embryo Transfer', in. JA Skidmore (eds), Lecture notes in the short course in reproduction in the Dromedary camel, International Veterinary Information Service, Ithaca, New York, USA. Skidmore, JA 2000, 'Ovarian kinetics and control of ovulation', in L Skidmore and GP Adams (eds), Recent Advances in Camelid Reproduction, International Veterinary Information Service (IVIS), Ithaca, New York, USA. Skidmore, JA 2000, 'Reproductive physiology of the male camel', in Lecture Notes for the Short Course in Reproduction in the Dromedary Camel International Veterinary Information Service. Skidmore, JA 2003, 'The main challenges facing camel reproduction research in the 21st century.' in BK Campbell, R Webb, H Dobson and C Doberska (eds), Reproduction in Domestic Ruminats V, Cambridge University Press, Cambridge. Skidmore, JA 2005, 'Reproduction in Dromedary camels: an update', Anim. Reprod. vol. 2, pp Skidmore, JA, Allen, WR and Heap, RB 1994, 'Oestrogen synthesis by the peri-implantation conceptus of the one-humped camel (Camelus dromedarius)', J. Reprod. Fertil. vol. 101, pp Skidmore, JA, Allen, WR and Heap, RB 1997, 'Maternal recognition of pregnancy in the Dromedary camel', J. Camel Prac. Res. vol. 4, pp Skidmore, JA and Billah, A 2005, 'Comparison of pregnancy rates in Dromedary camels (Camelus dromedarius) after deep intra-uterine versus cervical insemination', Theriogenology. Skidmore, JA and Billah, M 2005, 'Assisted reproduction in Dromedary camels. (NATO Science Series: Life and Behavioural Sciences, Vol.362)', in B Faye and P Esenov (eds), Desertification Combat and Food Safety: the added value of camel producers, Ashkabad, Turkmenistan, April 2004 IOS Press, Amsterdam, Netherlands. Skidmore, JA and Billah, M 2005, 'Embryo transfer in the Dromedary camel (Camelus dromedarius) using asynchronous, meclofenamic acid-treated recipients', Reprod. Fertil. Dev. vol. 17, pp Skidmore, JA, Billah, M and Allen, WR 1992, 'Ultrasonographic and videoendoscopic monitoring of early foetal development in the Dromedary camel.' in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992, R & W Publications Ltd, Newmarket, UK. Skidmore, JA, Billah, M and Allen, WR 1995, 'The ovarian follicular wave pattern in the mated and non-mated Dromedary camel (Camelus dromedarius)', J. Reprod. Fertil. Suppl. vol. 49, pp Skidmore, JA, Billah, M and Allen, WR 1996, 'The ovarian follicular wave pattern and induction of ovulation in the mated and non-mated one-humped camel (Camelus dromedarius)', J. Reprod. Fertil. vol. 106, pp Skidmore, JA, Billah, M and Allen, WR 1996, 'Patterns of hormone secretion throughout pregnancy in the onehumped Camel (Camelus dromedarius)', Reprod. Fertil. Dev. vol. 8, pp Skidmore, JA, Billah, M and Allen, WR 1997, 'The ovarian follicular wave pattern and control of ovulation in the mated and non-mated female Dromedary camel (Camelus dromedarius)', J. Camel Prac. Res. vol. 4, pp Skidmore, JA, Billah, M and Allen, WR 2000, 'Using modern reproductive technologies such as embryo transfer and artificial insemination to improve the reproductive potential of Dromedary camels', Rev. Elev. Med. Vet. Pay. vol. 53, pp Skidmore, JA, Billah, M and Allen, WR 2002, 'Investigation of factors affecting pregnancy rate after embryo transfer in the Dromedary camel', Reprod. Fertil. Dev. vol. 14, pp
189 Skidmore, JA, Billah, M, Allen, WR and Short, RV 1999, 'Modern reproductive methods to hybridize old and new world camelids: Camelus dromedarius x Lama guanicoe', Reprod. Fertil. Dev. vol. 34, pp Skidmore, JA, Billah, M, Allen, WR and Short, RV 2000, 'Using modern reproductive methods to hybridize Old and New World Camelids: Camelus dromedariusxlama guanicoe', Rev. Elev. Med. Vet. Pay. vol. 53, pp Skidmore, JA, Billah, M, Binns, M, Short, RV and Allen, WR 1999, 'Hybridizing Old and New World camelids: Camelus dromedarius X Lama guanicoe', Proc. Roy. Soc. London B. Bio. vol. 266, pp Skidmore, JA, Billah, M and Loskutoff, NM 2004, 'Developmental competence in vitro and in vivo of cryopreserved hatched blastocysts fro mthe domedary camel (Camelus dromedarius)', Reprod. Fertil. Dev. vol. 16, pp Skidmore, JA, Billah, M and Loskutoff, NM 2005, 'Comparison of two different methods for the vitrification of hatched blastocysts from the Dromedary camel (Camelus dromedarius) ', Reprod. Fertil. Dev. vol. 17, pp Skidmore, JA, Billah, M, Short, RV and Allen, WR 2001, 'Assisted reproductive techniques for hybridization of camelids', Reprod. Fertil. Dev. vol. 13, pp Skidmore, JA and Loskutoff, NM 1999, 'Developmental competence in vitro and in vivo of cryopreserved expanding blastocysts from the Dromedary camel (Camelus dromedarius).' Theriogenology vol. 51, pp Skidmore, JA, Starbuck, GR, Lamming, GE and Allen, WR 1998, 'Control of luteolysis in the one-humped camel (Camelus dromedarius)', J. Reprod. Fertil. vol. 114, pp Skidmore, JA, Wooding, FBP and Allen, WR 1996, 'Implantation and early placentation in the one-humped camel (Camelus dromedarius)', Placenta vol. 17, pp Skidmore, JA, Wooding, FBP and Allen, WR 1997, 'Placentation during the first 60 days of gestation in the Dromedary camel (Camelus dromedarius)', J. Camel Prac. Res. vol. 4, pp. 14. Skidmore, L 2004, 'Artifical Insemination', in Lecture notes for the short course in reproduction in the Dromedary camel International Veterinary Information Service, Ithaca, New York, USA. Smith, BB 1999, 'Overview of reproduction in the male llama and alpaca' Proceedings of the Society for Theriogenology pp Smith, BB and Hollingshead, NC 2001, 'Use of fecal progesterone metabolites as an alternative method of pregnancy diagnosis in the llama and alpaca', in. M Gerken and C Renieri (eds), Progress in South American Camelids Research Wageningen Pers, Wageningen Netherlands. Smith, CL 1991, 'Exploring llama reproduction', J. Am. Vet. Med. Assoc. vol. 199, pp Smith, CL, Peter, AT and Pugh, DG 1994, 'Reproduction in llamas and alpacas: a review', Theriogenology vol. 41, pp Srikandakumar, A, Johnson, EH, Mahgoub, O, Kadim, LT and Al-Ajmi, DS 2001, 'Anatomy and histology of the female reproductive tract of the Arabian camel', Emirates J. Agr. Sci. vol. 13, pp Steven, DH, Burton, GJ, Sumar, J and Nathanielsz, PW 1980, 'Ultrastructural observations on the placenta of the alpaca (Lama pacos)', Placenta vol. 1, pp Sucapuca, V 1991, Caracteristicas fisicas del semen de la alpaca obtenido por el metodo del preservativo, Puno, Peru, Univ Nac Altiplano: 49. Sumant, V, Purohit, GN, Pareek, PK and Sahani, MS 2002, 'Ultrasonographic imaging to monitor early pregnancy in the camel (Camelus dromedarius)', Rev. Elev. Med. Vet. Pay. vol. 55, pp Sumar, J 1983, Studies on reproductive pathology in alpacas. Faculty of Veterinary Medicine Uppsala, Sweden, University of Agricultural Sciences: 90. Sumar, J 1988, 'Removal of the ovaries or ablation of the corpus luteum and its effect on the maintenance of gestation in the alpaca and llama', Acta Vet. Scand. vol. 83, pp Sumar, J 1994, 'Effects of various ovulation induction stimuli in alpacas and llamas', J. Arid Environments vol. 26, pp Sumar, J and Bravo, PW 1991, 'In situ observation of the ovaries of llamas and alpacas by use of a laparoscopic technique', J. Am. Vet. Med. Assoc. vol. 199, pp Sumar, J, Bravo, PW and Foote, WC 1993, 'Sexual receptivity and time of ovulation in alpacas', Small Ruminant Res. vol. 11, pp Sumar, J, Fredriksson, G, Alarcon, V, Kindahl, H and Edqvist, LE 1988, 'Levels of 15-keto-13,14-dihydro- PGF2 alpha, progesterone and oestradiol-17 beta after induced ovulation in llamas and alpacas', Acta Vet. Scand. vol. 29, pp Sumar, JB 1996, 'Reproduction in llamas and alpacas', Anim. Reprod. Sci. vol. 42, pp Sumar, JB 1999, 'Reproduction in female South American domestic camelids', J. Reprod. Fertil. Suppl. vol. 54, pp Sumar, JK 1993, 'Efectos de los estimulos de induccion en ia ovulacion de alpacas y llamas', Investigaciones pecuarias vol. 6, pp. 7 pages. 174
190 Sumar, KJ 2001, 'Reproduction and herd fertility in domestic camelids', in M Gerken and C Renieri (eds), Progress in South American Camelids Research Proceedings of the 3rd European Symposium, Gottingen, Germany, May Sutmoller, P 1999, 'Risk of disease transmission by llama embryos', Rev. Sc. Tech.OIE vol. 18, pp Sutmoller, P and Taylor, P 1998, 'An assessment of the risk of disease transmission by llama embryos', in Proc. One Hundred and Second Annual Meeting of the United States Animal Health Association, Minneapolis, Minnesota, USA, 3-9 October, 1998, United States Animal Health Association, Richmond. Tanco, VM, Ratto, MH, Lazzarotto, M and Adams, GP 2007, 'Dose response to ovulation-inducing factor (OIF) in llamas ', Theriogenology pp. in press. Taylor, P, Taylor, S, James, AN, Denniston, RS and Godke, RA 2001, 'Alpaca offspring born after cross species embryo transfer to llama recipients', Theriogenology vol. 55, pp Taylor, P, Taylor, S, James, AN and Godke, RA 2000, 'Successful commercial embryo transfer in the llama (Lama glama)', Theriogenology vol. 53, pp Taylor, P, Taylor, S, Sansinena, M and Godke, RA 2006, 'Llama glama pregnancies from vitrified/warmed blastocysts using a novel coaxial cryoprotectant microinjection system.' Reprod. Fertil. Dev. vol. 18, pp Tibary, A 2004, 'Breeding management and pregnancy', in The International Alpaca Handbook Alpaca Consulting Services of Australia, Norwood, South Australia. Tibary, A 2004, 'Improving reproductive efficiency in alpacas, reproductive patterns in the female and male', in The International Alpaca Handbook Alpaca Consulting Services of Australia, Norwood, South Australia. Tibary, A 2006, Infertility in the Female Lamoid. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Tibary, A 2006, Infertility in the Male Lamoid. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Tibary, A, Abdelhaq, A and Abdelmalek, S 2005, 'Factors affecting reproductive performance of camels at the herd and individual level, in. B Faye and P Esenov (eds), Desertification Combat and Food Safety: the added value of camel producers, Ashkabad, Turkmenistan, April 2004 IOS Press, Amsterdam, Netherlands. Tibary, A and Anouassi, A 1996, 'Ultrasonographic changes of the reproductive tract in the female camel (Camelus dromedarius) during the follicular cycle and pregnancy', J. Camel Prac. Res. vol. 3, pp Tibary, A and Anouassi, A 1997, Theriogenology in Camelidae. Actes Editions, Rabat, Morocco. Tibary, A and Anouassi, A 2000, 'Reproductive disorders in the female camelid', in JA Skidmore and GP Adams (eds), Recent Advances in Camelid Reproduction International Veterinary Information Service. Tibary, A and Anouassi, A 2000, 'Reproductive disorders in the male Camelid', in JA Skidmore and GP Adams (eds), Recent Advances in Camelid Reproduction International Veterinary Information Service. Tibary, A and Anouassi, A 2000, 'Ultrasonography of the gentical tract in camels (Camelus dromedarius and Camelus Bactrianus)', in TK Gahlot (eds), Selected topics on Camelids The Camelid Publishers, Bikaner, India. Tibary, A and Anouassi, A 2001, 'Uterine infections in Camelidae', Vet. Sci. Tomorrow vol. 3, pp Tibary, A and Anouassi, A 2004, 'Standing castration in camels', J. Camel Prac. Res. vol. 11, pp Tibary, A, Anouassi, A and Khatir, H 2005, 'Update on reproductive biotechnologies in small ruminants and camelids', Theriogenology. Tibary, A, Anouassi, A and Memon, MA 2001, 'An approach to the diagnosis of infertility in camelids: retrospective study in alpaca, llamas and camels', J. Camel Prac. Res. vol. 8, pp Tibary, A, Fite, C, Anouassi, A and Sghiri, A 2006, 'Infectious causes of reproductive loss in camelids', Theriogenology vol. 66, pp Tibary, A and Memon, MA 1999, 'Reproduction in the male South American Camelidae', J. Camel Prac. Res. vol. 6, pp Tibary, A and Memon, MA 1999, 'Reproductive physiology in the female South American camelidae', J. Camel Prac. Res. vol. 6, pp Tibary, A and Memon, MA 2004, 'Reproduction in the male South American camelidae', in TK Gahlot (eds), Selected research on camelid physiology and nutrition The Camelid Publishers, Bikaner, India. Tibary, A and Vaughan, JL 2006, 'Reproductive physiology and infertility in male South American camelids: A review and clinical observations', Small Ruminant Res. vol. 61, pp Tingari, MD 1979, 'Observations on the fine structire of the testicular interstitial cells in the camel (Camelus dromedarius)', Q. J. Exp. Phys. vol. 64, pp Tingari, MD 1989, 'The fine structure of the epithelial lining of the epididymis of the camel (Camelus dromedarius) with special reference to regional differences', J. Anat. vol. 165, pp Tingari, MD 1991, 'Studies on camel semen. III. Ultrastructure of the spermatozoon', Anim. Reprod. Sci. vol. 26, pp Tingari, MD, El-Manna, MM, Rahim, ATA, Ahmed, AK and Hamad, MH 1986, 'Studies on camel semen. I. Electroejaculation and some aspects of semen characteristics', Anim. Reprod. Sci. vol. 12, pp
191 Tingari, MD and Moniem, KA 1979, 'On the regional histology and histochemistry of the epididymis of the camel (Camelus dromedarius)', J. Reprod. Fertil. vol. 57, pp Tingari, MD, Rahma, BA and Saad, AH 1984, 'Studies on the poll glands of the one-humped camel in relation to reproductive activity I: seasonal morphological and histochemical changes', J. Anat. vol. 138, pp Tingari, MD, Ramos, AS, Gaili, ESE, Rahma, BA and Saad, AH 1984, 'Morphology of the testis of the onehumped camel in relation to reproductive activity', J. Anat. vol. 139, pp Tinson, AH, Harrison, B, King, B et al. 2001, 'Production of pre-sexed racing camel offpsring using embryo biopsy and detection of camelid sex specific polynucleic acid sequences.' Emirates J. Agr. Sci. vol. 13, pp Tinson, AH, Kuhad, KS, Singh, K and Al-Masri, J 2000, 'Surgical technique to control sand masturbation in male racing camels', J. Camel Prac. Res. vol. 7, pp Tinson, AH, Kuhad, KS, Singh, K et al. 2001, 'Twinning in camels', Emirates J. Agr. Sci. vol. 13, pp Tinson, AH and McKinnon, AO 1992, 'Ultrasonography of the reproductive tract of the female Dromedary camel', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992, R & W Publications Ltd, Newmarket, UK. Tinson, AH, McKinnon, AO, Singh, AK, Kuhad, KS and Sambyal, R 2001, 'Oocyte collection techniques in the Dromedary camel', Emirates J. Agr. Sci. vol. 13, pp Tinson, AH and Singh, K 1998, Embryo transfer in the camel, can it be applied to field conditions with realistic costs? Proceedings of the 3rd Annual Meeting for Animal Production Under Arid Conditions Al-Ain, UAE. Tinson, AH, Singh, K and Kuhad, KS 2000, 'Large scale management of camels for embryo transfer', J. Camel Prac. Res. vol. 7, pp Torner, H, Heleil, B, Alm, H et al. 2003, 'Changes in cumulus-oocyte complexes of pregnant and non-pregnant camels (Camelus dromedarius) during maturation in vitro', Theriogenology vol. 60, pp Urquieta, B, Cepeda, R, Caceres, JE, Raggi, LA and Rojas, JR 1994, 'Seasonal variation in some reproductive parameters of the male vicuña in the high Andes of Northern Chile.' J. Arid Environments vol. 26, pp Urquieta, B, Flores, P, Munoz, C, Bustos-Obregon, E and Garcia-Huidobro, J 2005, 'Alpaca semen characteristics under free and directed mounts during a mating period.' Anim. Reprod. Sci. vol. 90, pp Valdivia, EOd, Sumar K, J, Casas P, J and Ponce, J 1979, 'Induccion y sincronizacion del parto en la alpaca', Memoria, Asociacion Latinoamericana de Produccion Animal vol , pp Valdivia, M, Alvarez, C, Rodriguez, C et al. 2007, 'Molecular recognition of fresh and frozen alpaca spermatozoas with zona pellucida', Biol. Reprod. Suppl. Valdivia, M, Canorio, N, Carillo, E and Uipan, P 2005, 'Effects of cryoprotectants on alpacas spermatozoa during cooling process', Biol. Reprod. Suppl., pp Valdivia, M, Manosalva, I, Quispe, J et al. 2003, 'Effects of permeating cryoprotectants on survive and motility in alpaca spermatozoa', Biol. Reprod. Suppl. vol. 68, pp Valdivia, M, Ruiz, M, Bermudez, L et al. 1999, Criopreservacion de semen de alpacas. Proceedings of the II Congreso Mundial sobre Camelidos Cusco, Peru. Valdivia, M, Suyo, M, Manosalva, I et al. 2000, 'Cryopreservation and immunoreactivity of proacrosin/acrosin system in alpaca spermatozoa.' Biol. Reprod. Suppl. vol. 62, pp Vásquez, M, Huanca, W, Huanca, T, Ratto, M and Adams, G 2006, Fracción del plasma seminal como inductor de ovulación en llamas (Lama glama). Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Vaughan, JL 2001, Control of ovarian follicular growth in the alpaca (Lama pacos). Animal Sciences and Production Group Central Queensland University. Vaughan, JL 2002, Improving the efficiency of reproduction and breeding in alpacas. RIRDC project; no. UCQ- 11A. Rural Industries Research and Development Corporation, Barton, A.C.T. Vaughan, JL 2003, 'Artificial insemination in Alpacas', Alpaca World Magazine Vaughan, JL 2004, 'Artificial insemination in Alpacas', in The International Alpaca Handbook Alpaca Consulting Services of Australia, Norwood, South Australia. Vaughan, JL, D'Occhio, MJ and Macmillan, KL 2000, Ovarian follicular wave inter-wave intervals in alpacas. Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Vaughan, JL, Galloway, D and Hopkins, D 2003, Artificial insemination in alpacas (Lama pacos). RIRDC project; no. AAA-1A, Rural Industries Research and Development Corporation, Barton ACT. Vaughan, JL, Macmillan, KL, Anderson, GA and D'Occhio, MJ 2003, 'Effects of mating behaviour and the ovarian follicular state of female alpacas on conception', Aust. Vet. J. vol. 81, pp Vaughan, JL, Macmillan, KL and D'Occhio, MJ 2004, 'Ovarian follicular wave characteristics in alpacas', Anim. Reprod. Sci. vol. 80, pp Vaughan, JL and Tibary, A 2006, 'Reproduction in female South American camelids: A review and clinical observations', Small Ruminant Res. vol. 61, pp
192 Von Baer, A, Del Campo, MR, Donoso, X et al. 2002, 'Vitrification and cold storage of llama (Lama glama) hatched blastocysts', Theriogenology vol. 57, pp Von Baer, L and Hellemann, C 1998, 'Variables seminales en llama (Lama glama)', Arch. Med. Vet. vol. 30, pp Von Baer, L and Hellemann, C 1999, 'Cryopreservation of llama (Lama glama) semen', Reprod. Domest. Anim. vol. 34, pp Von Baer, L and Hellemann, C 2001, 'Collection, evaluation and cryopreservation of llama (L. glama) semen, in M Gerken and C Renieri (eds), Progress in South American Camelids Research Wageningen Pers, Wageningen Netherlands. von Kubicek, J 1974, 'Semen collection in alpaca with a urethral fistula [German]', Zeitschrift Tierzuchtung und Zuchtungsbiologie vol. 90, pp Vyas, S, Goswami, P, Rai, AK and Khanna, ND 1998, 'Use of tris and lactose extenders in preservation of camel semen at refrigerated temperature', Indian Vet. J. vol. 75, pp Vyas, S, Rai, AK, Goswami, PK et al. 2004, 'Superovulatory response and embryo recovery after treatment with different gonadotrophins during induced luteal phase in Camelus dromedarius', Trop. Anim. Health Pro. vol. 36, pp Vyas, S, Rai, AK and Khanna, ND 1998, 'Treatment of cystic ovarian degeneration in Indian camel (Camelus dromedarius)', Indian Vet. J. vol. 75, pp Vyas, S, Rai, AK, Sahani, MS and Khanna, ND 2004, 'Use of real-time ultrasonography for control of follicular activity and pregnancy diagnosis in the one humped camel (Camelus dromedarius) during the non-breeding season', Anim. Reprod. Sci. vol. 84, pp Vyas, S and Sahani, MS 2000, 'Real-time ultrasonography of ovaries and breeding of the one-humped camel (Camelus dromedarius) during the early postpartum period.' Anim. Reprod. Sci. vol. 59, pp Vyas, S, Sharma, N, Bissa, UK, Chirania, BL and Bishnoi, BL 1999, 'Effect of Prostaglandin F2 alpha on induction of parturition in she camel (Camelus dromedarius)', Indian J. Anim. Reprod. vol. 20, pp. 73. Vyas, S, Singh, AK, Goswami, P et al. 1998, 'Superovulation and non-surgical embryo flushing in Indian camel (Camelus dromedarius)', Int. J. Amin. Sci. vol. 13, pp Wani, N, Nowshari, M and Wernery, U 2005, 'Storage of camel (Camelus dromedarius) epididymal spermatozoa in different extenders and at different temperatures', Biol. Reprod. Supplement 1 vol. 73, pp Wani, NA, Billa, M and Skidmore, JA 2007, 'Studies on liquefaction and storage of ejaculated Dromedary camel (Camelus dromedarius) semen', Anim. Reprod. Sci. pp. in press. Wani, NA and Nowshari, MA 2005, 'Effect of heparin and calcium ionophore on acrosome reactoin in epididymal spermatozoa of Dromedary camel (Camelus dromedarius)', Reprod. Fertil. Dev. pp Wani, NA and Nowshari, MA 2005, 'Kinetics of nuclear maturation and effect of holding ovaries at room temperature on in vitro maturation of camel (Camelus dromedarius) oocytes', Theriogenology vol. 64, pp Wani, NA and Wani, GM 2003, 'Use of spermatozoa from epididymus of slaughtered rams for in vitro fertilization of ovine oocytes', Indian J. Anim. Sci. vol. 73, pp Wani, NA, Wernery, U and Nowshari, MA 2004, 'Chronological events of in vitro maturation in camel (Camelus dromedarius) oocytes. ' Reprod. Fertil. Dev. vol. 16, pp Wernery, U and Kumar, BN 1994, 'Reproductive disorders in Dromedary camels due to infectious causes and its treatment', J. Camel Prac. Res. vol. 1, pp. 7 ref. Wheeler, JC 1995, 'Evolution and present situation of the South American Camelidae', Biol. J. Linn. Soc. vol. 54, pp Wiepz, DW and Chapman, RJ 1985, 'Non-surgical embryo transfer and live birth in a llama', Theriogenology vol. 24, pp Wilber, G 2006, Superovulacion en alpacas y recuperacion de embriones mediante un metodo quirurgico. Proceedings of the IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Willmen, T, Sieme, H, Merkt, H et al. 1992, 'Manipulation of reproductive functions in the male camelvariations of hormone concentration and semen quality after hormonal intervention', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd- 6th February 1992., R & W Publications Ltd, Newmarket, UK. Willmen, T, Sieme, H, Merkt, H et al. 1993, 'Semen preservation and hormonal effects on semen quality in the camel', Reprod. Domest. Anim. vol. 28, pp Woodling, FBP, Ozturk, M, Skidmore, JA and W.R., A 2003, 'Developmental changes in localization of steroid synthesis enzymes in camel placenta', Reproduction vol. 126, pp Xu, YS, Wang, HY, Zeng, GQ, Jiang, GT and Gao, YH 1985, 'Hormone concentrations before and after semeninduced ovulation in the Bactrian camel (Camelus Bactrianus)', J. Reprod. Fertil. vol. 74, pp Yagil, R 2006, 'Reproductive processes in camels (Camelus dromedarius)', Israel J. Vet. Med. vol. 61, pp
193 Yagil, R and Etzion, Z 1980, 'Hormonal and behavioural patterns in the male camel (Camelus dromedarius)', J. Reprod. Fertil. vol. 58, pp Zhao, X-X, Huang, YM and Chen, B-X 1994, 'Artificial insemination and pregnancy diagnosis in the Bactrian camel (Camelus Bactrianus)', J. Arid Environments vol. 26, pp Zhao, XX 2000, 'Reproduction in the Bactrian camel', in TK Ghalot and Singh (eds), Selected topics on camelids, The Camelid Publishers, Bikaner, India. Zhao, XX 2000, 'Semen characteristics and artifical insemination in the Bactrian Camel', in. JA Skidmore and GP Adams (eds), Recent Advances in Camelid reproduction International Veterinary Information Service. Zhao, XX, Huang, HY and Chen, BX 1992, 'Studies on the semen characteristics of Bactrian camel semen.' Chin. J. Anim. Sci. vol. 28, pp Zhao, XX, Huang, YM and Chen, BX 1992, 'Biological activity of gonadotrophin-releasing hormone-like factors in the seminal plasma of the Bactrian camel', in WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992, R & W Publications Ltd, Newmarket, UK. Zhao, XX, Huang, YM, Nie, QC, Zhang, YK and Chen, BX 1996, 'Effect of different extenders on motility, survival and acrosomal integrity of camel spermatozoa frozen in ampoules', J. Camel Prac. Res. vol. 3, pp Zhao, XX, Huang, YM, Nie, QC, Zhang, YK and Chen, BX 2004, 'Effect of different extenders on motility survival and acrosomal integrity of camel spermatozoa frozen in ampoules', J. Camel Science vol. 1, pp Zhao, XX, Li, XL and Chen, BX 2001, 'Isolation of ovulation-inducing factors in the seminal plasma of Bactrian camel (Camelus Bactrianus) by DEAE-cellulose chromatography.' Reprod. Domest. Anim. vol. 36, pp Zhao, XX, Zhang, Y and Chen, BX 1998, 'Serum progesterone and 17b estradiol concentrations during pregnancy of Bactrian camels (Camelus Bactrianus)', Theriogenology vol. 50, pp Zhao, XX, Zhang, Y and Chen, BX 2001, 'Preliminary observations on the concentrations of serum luteinizing hormone and follicle-stimulating hormone during gestation in Bactrian camels (Camelus Bactrianus)', Vet. Res. Commun. vol. 25, pp Zia-ur-Rahman, NA, Bukhari, SA, Akhtar, N and Haq, IU 2006, 'Serum hormonal, electrolytes and trace elements profiling in the rutting and non-rutting one humped male camel (Camelus dromedarius)' Anim. Reprod. Sci. pp. in press. 178
194 14. References Abdel-Rahim, HA and El Nazier, AT 1992, 'Studies on the sexual behaviour of the Dromedary camel', in. WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference R&W Publications Ltd, Newmarket, UK. Abdel-Raouf, M, Fateh El-Bab, MR and Owaida, MM 1975, 'Studies on reproduction in the camel (Camelu dromedarius) V. Morphology of the testis in relation to age and season', J. Reprod. Fert. vol. 43, pp Abdel Rahim, SEA 1997, 'Studies on the age of puberty of male camels (Camelus dromedarius) in Saudi Arabia', Vet. J. vol. 154, pp Adam, CL, Bourka, DA, Kyle, CE, Young, P and McEvoy, TG 1992, 'Ovulation and embryo recovery in the llama', in. WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference R & W Publications Ltd, Newmarket, UK. Adam, CL, Moir, CE and Shiach, P 1989, 'Plasma progesterone concentrations in pregnant and non-pregnant llamas (Lama glama)', Vet. Rec. vol. 125, pp Adams, GP, Griffin, PG and Ginther, OJ 1989, 'In situ morphologic dynamics of ovaries, uterus and cervix in llamas', Biol. Reprod. vol. 41, pp Adams, GP and Ratto, MH 2001, 'Reproductive biotechnology in South American camelids', Rev. de Investigaciones Veterinarias del Peru vol. 1, pp Adams, GP, Ratto, MH, Huanca, W and Jaswant, S 2005, 'Ovulation-inducing factor in the seminal plasma of alpacas and llamas', Biol. Reprod. vol. 73, pp Adams, GP, Sumar, J and Ginther, OJ 1990, 'Effects of lactational and reproductive status on ovarian follicular waves in llamas (Lama glama)', J. Reprod. Fertil. vol. 90, pp Agarwal, VK, Ram, L, Rai, AK, Khanna, ND and Agarwal, SP 2004, 'Physical and biochemical attributes of camel semen', J. Camel Science vol. 1, pp Aitken, RJ and Krausz, C 2001, 'Oxidative stress, DNA damage and the Y chromosome', Reproduction vol. 122, pp Akourki, A, Gil, L, Echegaray, A et al. 2004, 'Effect of the extender supplement equex-stm on cryopreserved semen in the Assaf sheep ', Cryoletters vol. 25, pp Al-Eknah, M, Homeida, N and Al-Haider, A 2001, 'A new method for semen collection by artificial vagina from the Dromedary camel', J. Camel Prac. Res. vol. 8, pp Al-Eknah, MM 2000, 'Reproduction in old world camels', Anim. Reprod. Sci. vol. 60/61, pp Al-Qarawi, AA 2005, 'Infertility in the Dromedary bull: a review of causes, relations and implications', Anim. Reprod. Sci. vol. 87, pp Al-Qarawi, AA, Abdel-Rahman, HA, El-Mougy, SA and El-Belely, MS 2002, 'Use of a new computerized system for evaluation of spermatozoal motility and velocity characteristics in relation to fertility levels in Dromedary bulls', Anim. Reprod. Sci. vol. 74, pp Aller, JF 1999, Inseminacion artificial en llamas en la Puna.Paper presented to Proc II Congreso Mundial sobre Camelidos Cusco, Peru. Aller, JF 2001, 'Cryopreservation of semen and artificial insemination in South Amercian camelids', International Foundation for Sciences Final Report Aller, JF, Ferre, L, Rebuffi, GE and Alberio, RH 1997, 'Semen collection in llamas (Lama glama) from the Argentinian Puna. [Spanish] Recoleccion de semen de llama (Lama glama) en la Puna Argentina', Vet. Argentina vol. 14, pp Aller, JF, Rebuffi, GE, Cancino, AK and Alberio, RH 2002, 'Successful transfer of vitrified llama (Lama glama) embryos', Anim. Reprod. Sci. vol. 73, pp Aller, JF, Rebuffi, GE, Cancino, AK and Alberio, RH 2003, 'Influence of cryopreservation on the motility, viability and fertility of llama (Lama glama) spermatozoa. [Spanish] Influencia de la criopreservacion sobre la motilidad, viabilidad y fertilidad de espermatozoides de llama (Lama glama)', Arch. Zootec. vol. 52, pp Amer, HA, Zeidan, AEB and Ahmadi, EAA 2006, 'Effect of different seasons and ages on semen quality, hormonal activities and sperm penetrating ability in Dromedary camel (Camelus dromedarius).' Reprod. Domest. Anim. vol. 41, pp. 2. Anouassi, A, Adnani, M and Raed, E 1992, 'Artificial insemination in the camel requires induction of ovulation to achieve pregnancy', in. WR Allen, Higgins A. J., IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd, Newmarket, UK. Apaza, N, Alarcon, V, Huanca, T and Cardenas, O 1999, Avances sobre la inseminacion artificial con semen congelado an alpacas.paper presented to Proc II Congreso Mundial sobre Camlidos Cusco, Peru. 179
195 Arthur, GH 1992, 'An overview of reproduction in the camelids', in. WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference R & W Publications Ltd, Newmarket, UK. Axner, E, Hermansson, U and Linde-Forsberg, C 2004, 'The effect of Equex STM paste and sperm morphology on post-thaw survival of cat epididymal spermatozoa', Anim. Reprod. Sci. vol. 84, pp Azouz, A, Ateia, MZ, Shawky, H, Zakaria, AD and Farahat, AA 1992, 'Hormonal changes during rutting and the non-breeding season in male Dromedary camels', in. WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference R&W Publications Ltd, Newmarket, UK. Batellier, F, Vidament, M, Fauquant, J et al. 2001, 'Advances in cooled semen technology', Anim. Reprod. Sci. vol. 68, pp Bollendorf, A, Check, JH, Katsoff, D and Fedele, A 1994, 'The use of chymotrypsin/galactose to treat spermatozoa bound with anti-sperm antibodies prior to intra-uterine insemination', Hum. Reprod. vol. 9, pp Bourke, DA, Adam, CL and Kyle, CE 1991, 'Sucsessful pregnancy following non-surgical embryo transfer in llamas.' Vet. Rec. vol.??, pp. 68. Bourke, DA, Adam, CL, Kyle, CE, McEvoy, TG and Young, P 1992, 'Ovulation, superovulation and embryo recovery in llamas.' Proceedings of the 12th International Congress on Animal Reproduction, The Hague vol. 1, pp Bourke, DA, Kyle, CE, McEvoy, TG, Young, P and Adam, CL 1995, 'Advanced reproductive technologies in South American camelids.' Proceedings of the 2nd European Symposium on South American Camelids vol. 1, pp Bourke, DA, Kyle, CE, McEvoy, TG, Young, P and Adam, CL 1995, 'Recipient synchronisation and embryo transfer in South American camelids', Theriogenology vol. 43, pp Bravo, PW 1994, 'Reproductive endocrinology of llamas and alpacas', Vet. Clin. N. Am. Food A. vol. 10, pp Bravo, PW 1995, 'Physiology of reproduction and fertility evaluation in the male alpaca', Proceedings of Post Graduate Foundation in Veterinary Science vol. 257, pp Bravo, PW 2002, The Reproductive Process of South American Camelids. Seagull Printing, Salt Lake City, Utah, USA. Bravo, PW, Alarcon, V and Bondurant, RH 2000a, Epididymal spermatozoa characteristics and its use on artificial insemination of llamas and alpacas.paper presented to Proc. 14th International Congress on Animal Reproduction Stockholm, Sweden. Bravo, PW, Ccallo, M and Garnica, J 2000b, 'The effect of enzymes on semen viscosity in llamas and alpacas', Small Ruminant Res. vol. 38, pp Bravo, PW, Flores, D and Ordonez, C 1997a, 'Effect of repeated collection on semen characteristics of alpacas', Biol. Reprod. vol. 57, pp Bravo, PW, Flores, U, Garnica, J and Ordonez, C 1997b, 'Collection of semen and artificial insemination of alpacas', Theriogenology vol. 47, pp Bravo, PW, Fowler, ME, Stabenfeldt, GH and Lasley, B 1990, 'Endocrine responses in the llama to copulation', Theriogenology vol. 33, pp Bravo, PW and Johnson, LW 1994, 'Reproductive physiology of the male camelid', Vet. Clin. N. Am. Food A. vol. 10, pp Bravo, PW, Moscoso, J, Ordonez, C and Alarcon, V 1996a, 'Transport of spermatozoa and ova in female alpaca', Anim. Reprod. Sci. vol. 43, pp Bravo, PW, Moscoso, R, Alarcon, V and Ordonez, C 2002, 'Ejaculatory process and related semen characteristics', Arch. Androl. vol. 48, pp Bravo, PW, Ordonez, C and Alarcon, V 1996b, Processing and freezing of semen of alpacas and llamas.paper presented to Proc 13th International Congress on Animal Reproduction Sydney, Australia. Bravo, PW, Pacheco, C, Quispe, G, Vilcapaza, L and Ordonez, C 1999, 'Degelification of alpaca semen and the effect of dilution rates on artificial insemination outcome', Arch. Androl. vol. 43, pp Bravo, PW, Skidmore, JA and Zhao, XX 2000c, 'Reproductive aspects and storage of semen in Camelidae', Anim. Reprod. Sci. vol. 62, pp Bravo, PW, Solis, P, Ordonez, C and Alarcon, V 1997c, 'Fertility of the male alpaca: effect of daily consecutive breeding', Anim. Reprod. Sci. vol. 46, pp Brown, BW 2000, 'A review on reproduction in South American camelids', Anim. Reprod. Sci. vol. 58, pp Buendia, P, Soler, C, Paolicchi, F et al. 2002, 'Morphometric characterization and classification of alpaca sperm heads using the Sperm-Class Analyzer(R) computer-assisted system', Theriogenology vol. 57, pp Burgel, H, Erhardt, G and Gauly, M 1999, 'Cryopreservation of llama (Lama glama) semen. ' Proc II Congreso Mundial sobre Camelidos, Cusco, Peru pp
196 Burgel, H, Erhardt, G and Gauly, M 2000, 'Cryopreservation of llama (Lama glama) semen', Reprod. Domest. Anim. vol. 35, pp. 26 (abstract). Burgel, H, Erhardt, G and Gauly, M 2001, 'Cryopreservation of llama (Lama glama) spermatozoa with an eggyolk-free extender. (EAAP Publication No. 105)', in. M Gerken and C Renieri (eds), Progress in South American Camelids Research Wageningen Pers, Wageningen Netherlands. Calderon, W, Sumar, J and Franco, E 1968, 'Advances en la inseminacion artificial de las alpacas (Lama pacos) [Spanish]', Rev. de la Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos, Lima, Peru. vol. 22, pp Callo, M, Garnica, J and Bravo, PW 1999, Efecto de la fibrinolisina, hialuronidasa, colagenasa y tripsina sobre la viscosidad del semen en alpacas.paper presented to Proceedings of the 2nd Congreso Mundial sobre Camelidos Cusco, Peru. Carmichael, IH 1999, ' Internal parasitism in alpacas in southern Australia.' Proceedings of the Australian Camelid Veterinary Association Conference pp Chen, BX, Yuen, ZX and Pan, GW 1985, 'Semen-induced ovulation in the Bactrian camel (Camelus Bactrianus)', J. Reprod. Fert. vol. 74, pp Chen, BX, Zhao, XX and Huang, YM 1993, 'Freezing semen and AI in the Bactrian camel (Camelus Bactrianus)', Etudes et Syntheses de l'iemvt vol. 41, pp Clulow, JR 2006, Development of sperm sorting for sex-preselection of stallion spermatozoa Faculty of Veterinary Science. Sydney, The University of Sydney. PhD thesis, 261 pages. Clulow, JR, Evans, G, Morris, LHA and Maxwell, WMC 2006, 'In vitro function of sex-sorted and non-sorted fresh and frozen stallion spermatozoa', Anim. Reprod. Sci. vol. 94, pp Cohen, J and Aafjes, JH 1982, 'Proteolytic enzmyes stimulate human spermaotozoal motility and in vitro hamster egg penetration', Life Sci vol. 30, pp Condorena, A, N.,, Sumar, J, Franco, E and Alarcon, V 1988, Largo de gestacion en llamas (Lama glama).paper presented to XI Congreso Panamericano de Ciencias Veterinarias, Lima, Peru. Cui, K-H and Matthews, CD 1993, 'Human X larger than Y sperms.' Nature vol. 366, pp Dandekar, PV and Gordon, M 1975, 'Electron microscope evaluation of rabbit eggs exposed to spermatozoa treated with capacitating agents', J. Reprod. Fertil. vol. 44, pp Davalos, R and Olazabal, JL 2002, 'Evaluacion de dos formas de collection de semen en alpacas', Rev. Int. Vet. Peru vol. 13, pp Davis, BK, Byrne, R and Bedigian, K 1980, 'Studies on the mechanism of capacitation; Albumin-mediated changes in plasma membrane lipids during in vitro incubation of rat sperm cells', Proc. Nat. Acad. Sci. USA vol. 77, pp de la Vega, D 1996, Efecto de la concentration espermatica y la hora de inseminacion artificial con semenfresco sobre el porcentaje de gestacion en alpacas. MVZ Thesis, Faculdad Medicina Veterinaria Universidad Nacional Altiplano, Peru 54. Deen, A and Sahani, MS 2000, 'Preliminary attempts to collect and cryopreserve camel semen', J. Camel Prac. Res. vol. 7, pp Deen, A, Sumant, V, Mamta, J and Sahani, MS 2004, 'Refrigeratory preservation of camel semen', J. Camel Prac. Res. vol. 11, pp Deen, A, Sumant, V and Sahani, MS 2003, 'Semen collection, cryopreservation and artificial insemination in the Dromedary camel', Anim. Reprod. Sci. vol. 77, pp Deen, A, Vyas, S and Sahani, MS 2005, 'Testosterone profiles in the camel (C. dromedarius) during the rutting season', Israel J. Vet. Med.? vol. 60, pp.??? Delhon, GA and Lawzewitsch, I 1994, 'Ductus epididymis compartments and morphology of epididymal spermtaozoa in llamas', Anat. Histol. Embryol. vol. 23, pp Delhon, GA and Lawzewitsch, Iv 1987, 'Reproduction in the male llama (Lama glama), a South American camelid. I. Spermatogenesis and organization of the intertubular space of the mature testis', Acta Anat. vol. 129, pp El-Manna, MM, Tingari, MD and Ahmed, AK 1986, 'Studies on camel semen II. Biochemical characteristics', Anim. Reprod. Sci. vol. 12, pp El-Naggar, M and Abdel-Raouf, M 1977, 'Studies on reproduction in camels (Camelus dromedarius). VIII. The electrophoretic pattern and the amino acid content of the seminal plasma protein', Indian Vet. J. vol. 54, pp Elias, E, Bedrak, E and Cohen, D 1985, 'Induction of oestrus in the camel (Camelus dromedarius) during seasonal anoestrus', J. Reprod. Fertil. vol. 74, pp Elliot, FI 1961, 'Artificial insemination of a Bactrian camel (Camelus Bactrianus)', Int. Zoo Yearbook vol. 3, pp Elwishy, AB 1987, 'Reproduction in the female Dromedary (Camelus dromedarius): a review', Anim. Reprod. Sci. vol. 15, pp
197 Elwishy, AB 1988, 'Reproduction in the male Dromedary (Camelus dromedarius): a review', Anim. Reprod. Sci. vol. 17, pp England, BG, Foote, WC, Cardozo, AG, Matthews, DH and Riera, S 1971, 'Oestrus and mating behaviour in the llama (Lama glama). ' Animal Behaviour vol. 19, pp Entwistle, K 2002, 'Bull reproductive anatomy and physiology', in (eds), Bull Fertility: Selection and Management in Australia, Australian Association of Cattle Veterinarians. Ericsson, RJ, Langevin, CN and Nishino, M 1973, 'Isolation of fractions rich in human Y sperm.' Nature vol. 246, pp Escobar, RC 1984, 'Mating, parturition', in. R Hennig (eds), The Llama, Animal Breeding and Production of South American Camelids, Talleres Graficos de ABRIL, Lima, Peru. Evans, G and Maxwell, WMC 1987, Salamon's artificial insemination of sheep and goats. Butterworths, Sydney. Fernandez-Baca, S 1993, 'Manipulation of reproductive functions in male and female New World camelids', Anim. Reprod. Sci. vol. 33, pp Fernandez-Baca, S and Calderon, W 1966, 'Methods of collection of semen in the alpaca', Rev. de la Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos, Lima, Peru. vol. 13, pp Fernandez-Baca, S, Madden, DH and Novoa, C 1970, 'Effect of different mating stimuli on induction of ovulation in the alpaca', J. Reprod. Fertil. vol. 22, pp Fernandez-Baca, S and Novoa, C 1968, 'Primer ensayo de inseminacion artificial (Lama pacos) con semen de vicuña (Vicugna vicugna).' Rev. Fac. Med. Vet. Lima vol. 22, pp. 9. Fernandez-Baca, S, Sumar, J, Novoa, C and Leyva, V 1973, 'Relationship between the location of the corpus luteum and that of the embryo in the alpaca. [Spanish] Relacion entre la ubicacion del cuerpo luteo y la localizacion del embrion en la alpaca', Rev. de Investigaciones Pecuarias, Peru vol. 2, pp Ferre, LB, Malik de Tchara, G, Aller, JF and Alberio, JR 2000, Effects of diluent, seminal plasma and centrifugation on quality traits of llama semen.paper presented to Proceedings of the 14th International Congress on Animal Reproduction Stockholm, Sweden. Flores, P, Garcia-Huidobro, J, Munoz, C, Bustos-Obregon, E and Urquieta, B 2002, 'Alpaca semen characteristics previous to a mating period', Anim. Reprod. Sci. vol. 72, pp Flores, P, Garcia-Huidobro, J, Munoz, C, Bustos-Obregon, E and Urquieta, B 2002, 'Alpaca semen characteristics previous to a mating period', Anim. Reprod. Sci. vol. 72, pp Fowler, ME 1989, Medicine and surgery of South American camelids : llama, alpaca, vicuña, guanaco. Iowa State University Press, Ames. Fowler, ME 1998, Medicine and surgery of South American camelids: llama, alpaca, vicuña, guanaco. Iowa State University Press., Iowa, USA. Fowler, ME and Bravo, PW 1998, 'Reproduction', in (eds), Medicine and Surgery of South American Camelids 2nd Edition Iowa State University Press, Iowa, USA. Franco, E, Sumar, J and Varela, M 1981, 'Eyaculacion en la alpaca (Lama pacos)', Proceedings of IV Convencion Internacional sobre Camelidos Sudamericanos. Corporacion Nacional Forestal. Instituto de la Patagonia, Chile, Punta Arenas Fugger, EF 1999, 'Clinical experience with flow cytometric separation of human X- and Y-chromosome bearing spermatozoa', Theriogenology vol. 52, pp Galloway, DB 2000, The development of the testicles in alpacas in Australia.Paper presented to Proceedings of the Australian Alpaca Association Conference Canberra, Australia. Garner, DL, Gledhill, BL, Pinkel, D et al. 1983, 'Quantification of the X- and Y-chromosome-bearing spermatozoa of domestic animals.' Biol. Reprod. vol. 28, pp Garnica, J, Achata, R and Bravo, PW 1993, 'Physical and biochemical characteristics of alpaca semen', Anim. Reprod. Sci. vol. 32, pp Garnica, J, Achata, R and Bravo, PW 1993, 'Physical and biochemical characteristics of alpaca semen', Anim. Reprod. Sci. vol. 32, pp Garnica, J, Flores, E and Bravo, PW 1995, 'Citric acid and fructose concentrations in seminal plasma of the alpaca', Small Ruminant Res. vol. 18, pp Gauly, M and Leidinger, H 1996, Semen quality, characteristic volume distribution and hypo-osmotic sensitivity of spermatozoa of Lama glama and Lama guanicoe.paper presented to Proceedings of the 2nd European Symposium on South American camelids Gillan, L, Evans, G and Maxwell, WMC 2005, 'Flow cytometric evaluation of sperm parameters in relation to fertility potential', Theriogenology vol. 63, pp Gillan, L, Kroetsch, T, Maxwell, WMC and Evans, G 2008, 'Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls.' Anim. Reprod. Sci. vol. 103, pp
198 Giuliano, S, Chaves, MG, Director, A, Trasorras, V and Miragaya, M 2006a, Inseminación artificial con semen refrigerado en llamas. Resultados preliminares.paper presented to Proc. IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Giuliano, S, Director, A, Gambarotta, M, Trasorras, V and Miragaya, M 2006b, Influencia de diluyentes en semen refrigerado a 5ºC durante 24 hs. en la especie Lama glama.paper presented to Proc. IV Congreso Mundial sobre Camelidos, Santa Maria, Argentina. Giuliano, S, Director, A, Gambarotta, M, Trasorras, V and Miragaya, M 2007, 'Collection method, season and individual variation on seminal characteristics in the llama (Lama glama)', Anim. Reprod. Sci. pp. in press. Giuliano, SM, Spirito, SE, Maragaya, MH et al. 2002, 'Electroejaculation and seminal parameters in vicuña (Vicugna vicugna)', Theriogenology vol. 57, pp Gunn, I 1999, 'Alpaca field trials', Rural Industries Research and Development Corporation Project. Hafez, ESE 1993, Reproduction in Farm Animals. Lea & Febiger, Baltimore. Hafez, ESE and Hafez, B 2001, 'Reproductive parameters of male Dromedary and Bactrian camels', Arch. Androl. vol. 46, pp Hassan, MM, Saeed, M and Rizwan ul, M 1995, 'Semen collection by artificial vagina and cryopreservation of camel (Camelus dromedarius) spermatozoa', Pakistan Vet. J. vol. 15, pp Helenius, A, McCaslin, DR, Fries, E and Tandford, C 1979, 'Properties of detergents. ' Meth Enzymol vol. 56, pp Hendriksen, PJM, Tieman, M, Van Der Lende, T and Johnson, LA 1993, 'Binding of anti-h-y monoclonal antibodies to separated X and Y chromosome bearing porcine and bovine sperm.' Mol. Reprod. Dev. vol. 35, pp Holt, C 2001, 'Statistically speaking.' Alpacas Australia vol. 35, pp Holt, WV 2000, 'Basic aspects of frozen storage of semen', Anim. Reprod. Sci. vol. 62, pp Homeida, AM, Khalil, MGR and Taha, AAM 1988, 'Plasma concentrations of progesterone, oestrogens, testosterone and Lh-like activity during the oestrus cycle of the camel (Camelus dromedarius)', J. Reprod. Fertil. vol. 83, pp Huanca, W, Cardenas, O, Olazabal, C, Ratto, M and Adams, GP 2001, 'Efecto hormonal y empadre sobre el intervalo a la ovulacion en llamas', Rev. Int. Vet. Peru Suplemento vol. 1, pp Huanca, W and Gauly, M 2001, 'Conservacion de semen refrigerado de llamas.' Rev. Inv. Vet. Peru vol. 1, pp Ismail, ST 1988, 'Reproduction in the male Dromedary (Camelus dromedarius)', Theriogenology vol. 29, pp Jasko, DJ, Moran, DM, Farlin, ME and Squires, EL 1991, 'Effects of seminal plasma dilution or removal on spermatozoal motion characteristics of cooled stallion semen', Theriogenology vol. 35, pp Johnson, LA 2000a, 'Sexing mammalian sperm for production of offspring: the state-of-the-art', Anim. Reprod. Sci. vol. 60/61, pp Johnson, LA 2000b, 'Sexing mammalian sperm for production of offspring: the state-of-the-art', Anim. Reprod. Sci. vol. 60/61, pp Johnson, LA, Flook, JP and Look, MV 1987, 'Flow cytometry of X and Y chromosome-bearing sperm for DNA using an improved preparation method and staining with Hoechst 33342', Gamete Res. vol. 17, pp Johnson, LA and Welch, GR 1999, 'Sex preselection: high-speed flow cytometric sorting of X and Y sperm for maximum efficiency', Theriogenology vol. 52, pp Johnson, LW 1989, 'Llama reproduction', Vet. Clin. N. Am. Food A. vol. 5, pp Jones, R, Mann, T and Sherins, RJ 1979, 'Peroxidative breakdown of phospholipids in human spermatozoa: spermicidal effects of fatty acid peroxides and protective action of seminal plasma.' Fertil. Steril. vol. 31, pp Kaneko, S, Oshio, S, Kobayahi, T, Iizuka, R and Mohri, H 1984, 'Human X- and Y-bearing sperm differ in cell surface sialic acid content.' Biochem. Biophys. Res. Commun. vol. 124, pp Knight, TW, Death, A, Wyeth, T and Hill, F 1992, 'Effects of GnRH and of single versus multiple mating on the conception rate in alpacas', Proc. New Zealand Soc. Anim. Prod. vol. 52, pp Knight, TW, Death, A, Wyeth, T and Hill, F 1992, 'Effects of GnRH and of single versus multiple mating on the conception rate in alpacas', Proc. New Zealand Soc. Anim. Prod. vol. 52, pp Knuth, UA, Menkveld, R, Stander, FSH et al. 1986, 'Bias to routine semen analysis by uncontrolled changes in laboratory environment - detection by long-term sampling of monthly means for quality control.' Int. J. Androl vol. 12, pp Leboeuf, B, Restall, B and Salamon, S 2000, 'Production and storage of goat semen for artificial insemination ', Anim. Reprod. Sci. vol. 68, pp Lichtenwalner, AB, Woods, GL and Weber, JA 1996a, 'Ejaculatory pattern of llamas during copulation', Theriogenology vol. 46, pp Lichtenwalner, AB, Woods, GL and Weber, JA 1996b, 'Seminal collection, seminal characteristics and pattern of ejaculation in llamas', Theriogenology vol. 46, pp
199 Lu, YQ, Zhang, M, Meng, B et al. 2006, 'Identification of X- and Y-chromosome bearing buffalo (Bubalus bubalis) sperm', Anim. Reprod. Sci. vol. 95, pp Macleod, J 1943, 'The role of oxygen in the metabolism and motility of human spermatozoa', Am. J. Physiol. vol. 138, pp Mares, RG 1954, 'Animal husbandry, animal industry and animal disease in the Somali land protectorate', Br. Vet. J. vol. 110, pp Marie, M and Anouassi, A 1986, 'Mating-induced luteinizing hormone surge and ovulation in the female camel (Camelus dromedarius)', Biol. Reprod. vol. 35, pp Marie, M and Anouassi, A 1987, 'Induction of luteal activity and progesterone secretion in the nonpregnant onehumped camel (Camelus dromedarius)', J. Reprod. Fertil. vol. 80, pp Maxwell, WMC 1986, 'Artificial insemination of ewes with frozen-thawed semen at a synchronized oestrus. 2. Effect of dose of spermatozoa and site of intrauterine insemination on fertility.' Anim. Reprod. Sci. vol. 10, pp Maxwell, WMC, Evans, G, Hollinshead, FK et al. 2004, 'Integration of sperm sexing into the ART toolbox.' Anim. Reprod. Sci. vol , pp Maxwell, WMC, Evans, G, Rhodes, SL, Hillard, MA and Bindon, BM 1993, 'Fertility of superovulated ewes after intrauterine or oviducal insemination with low numbers of fresh or frozen-thawed spermatozoa', Reprod. Fertil. Dev. vol. 5, pp Maxwell, WMC and Stojanov, T 1996, 'Liquid storage of ram semen in the absence or presence of some antioxidants.' Reprod. Fertil. Dev. vol. 8, pp McEvoy, TG, Bourke, DA and Adam, CL 1994, 'Recent advances in understanding and controlling the reproductive biology of South American camelids.' Proc Assisted Reproductive Technology and Andrology Meeting, Spain vol. 5, pp McEvoy, TG, Kyle, CE, Slater, D, Adam, CL and Bourke, DA 1992, 'Collection, evaluation and cryopreservation of llama semen.' J. Reprod. Fertil. Abstract Series vol. 9, pp. 48. McEvoy, TG, Kyle, CE, Slater, D, Adam, CL and Bourke, DA 1992a, 'Collection, evaluation and cryopreservation of llama semen.' J. Reprod. Fertil. Abstract Series vol. 9, pp. 48. McEvoy, TG, Kyle, CE, Young, P, Adam, CL and Bourke, D 1992b, 'Aspects of artificial breeding and establishment of pregnancy in South American Camelids', Proceedings of the 12th International Congress on Animal Reproduction, The Hague vol. 4, pp Merlian, CP, Sikes, JD, Read, BW, Boever, WJ and Knox, D 1979, 'Comparative characteristics of spermatozoa and semen from a Bactrian camel, Dromedary camel and llama', J. Zoo Anim. Med. vol. 10, pp Minoia, P, Moslah, M, Localandra, GM, Khorchani, T and Zarrilli, A 1992, 'Induction of oestrus and management of reproduction in the female Dromedary camel', in. WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd, Newmarket, UK. Mogrovejo, SD 1952, Estudios del semen de la alpaca. Facultad de medicina veterinaria Lima, Peru, University Nac Mayor San Marcos: 37. Montalvo, C, Cevallos, E and Copaira, M 1979, 'Estudio microscopico del parenquima testicular de la alpaca durante las estaciones del ano', Res Proyectos de Investigacion, Periodo Univ Nac Mayor San Marcos, Lima pp. 37. Morfeld, KA, Skidmore, JA, Billah, AM and Loskutoff, NM 2003, 'Effect of various cryoprotectants and methods for the short- and long-term preservation of sperm from the Dromedary camel (Camelus dromedarius)', Theriogenology vol. 59, pp Mortimer, D 1994, Practical Laboratory Andrology. Oxford University Press, Oxford, UK. Mortimer, D, Curtis, EF and Camenzind, AR 1990, 'Combined use of fluorescent peanut agglutinin lectin and Hoechst to monitor the acrosomal status and viability of human spermatozoa', Hum. Reprod. vol. 5, pp Morton, KM, Bathgate, R, Evans, G and Maxwell, WMC 2006, 'A comparison of three diluents for the cryopreservation of epididymal alpaca sperm', Reprod. Domest. Anim. vol. 41, pp. 329 (abstract). Morton, KM, Bathgate, R, Evans, G and Maxwell, WMC 2007a, 'Cryopreservation of epididymal alpaca (Vicugna pacos) sperm: a comparison of Citrate, Tris and Lactose based diluents, and pellets and straws.' Reprod. Fertil. Dev. vol. 19, pp Morton, KM, Catt, SL, Hollinshead, FK, Maxwell, WMC and Evans, G 2004, 'Production of lambs after the transfer of fresh and cryopreserved in vitro produced embryos from prepubertal lamb oocytes and unsorted and sex-sorted frozen-thawed spermatozoa.' Reprod. Domest. Anim. vol. 39, pp Morton, KM, Catt, SL, Hollinshead, FK, Maxwell, WMC and Evans, G 2005a, 'The effect of gamete coincubation time during in vitro fertilisation with frozen-thawed unsorted and sex-sorted ram spermatozoa on the development of in vitro matured adult and prepubertal ewe oocytes', Theriogenology vol. 64, pp
200 Morton, KM, Catt, SL, Maxwell, WMC and Evans, G 2005b, 'Effects of lamb age, hormone stimulation and response to hormone stimulation on the yield and in vitro developmental competence of prepubertal lamb oocytes.' Reprod. Fertil. Dev. vol. 17, pp Morton, KM, Catt, SL, Maxwell, WMC and Evans, G 2005c, 'An efficient method of ovarian stimulation and in vitro embryo production from prepubertal lambs.' Reprod. Fertil. Dev. vol. 17, pp Morton, KM, Catt, SL, Maxwell, WMC and Evans, G 2005d, 'In vitro and in vivo developmental capabilities and kinetics of in vitro development of in vitro matured oocytes from adult, unstimulated and hormone stimulated prepubertal ewes.' Theriogenology vol. 64, pp Morton, KM, de Graaf, SP, Campbell, A et al. 2005e, 'Repeated oocyte pick up and in vitro embryo production from adult ewes with and without FSH treatment', Reprod. Domest. Anim. vol. 40, pp Morton, KM, Evans, G and Maxwell, WMC 2007b, 'Effect of glycerol concentration, Equex STM supplementation and liquid storage prior to freezing on the survival and functional integrity of frozen-thawed epididymal alpaca (Vicugna pacos) sperm. ' Anim. Reprod. Sci. pp. submitted. Morton, KM, Gibb, Z, Wilson, SJ et al. 2007c, 'Effect of diluent, dilution rate and storage temperature on longevity and functional integrity of liquid stored alpaca (Vicugna pacos) sperm. ' Reprod. Fertil. Dev. submitted. Morton, KM, Rowe, AR, Maxwell, WMC and Evans, G 2006, 'In vitro and in vivo survival of bisected in vitro produced embryos derived from frozen-thawed unsorted, and frozen-thawed sex-sorted and re-frozen-thawed ram spermatozoa.' Theriogenology vol. 65, pp Morton, KM, Thomson, PC, Bailey, KJ, Evans, G and Maxwell, WMC 2007, 'Quality parameters for alpaca (Vicugna pacos) semen are affected by semen collection procedure', Reprod. Fertil. Dev. pp. submitted. Moruzzi, JF 1979, 'Selecting a mammalian species for the separation of X- and Y-chromosome bearing spermatozoa', J. Reprod. Fertil. vol. 57, pp Musa, B and Abusineina, ME 1978, 'Clinical pregnancy in the camel and comparison with bovine pregnancy', Vet. Rec. vol. 102, pp Musa, B, Sieme, H, Merkt, H and Hago, BED 1992, 'Artificial insemination in Dromedary camels', in. WR Allen, HA J., IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd-6th February 1992., R & W Publications Ltd,, Newmarket, UK. Musa, B, Sieme, H, Merkt, H et al. 1993, 'Manipulation of reproductive functions in male and female camelids', Anim. Reprod. Sci. vol. 33, pp Nagao, Y, Saeki, K, Hoshi, M, Takahashi, Y and Kanagawa, H 1995, 'The effects of water quality on in vitro fertilisation and development of bovine oocytes in protein-free medium', Theriogenology vol. 44, pp National Health and Medical Research Council, CSIROAAC 1990, Australian code of practice for the care and use of animals for scientific purposes. Neely, DP and Bravo, WP 1997, 'Reproductive evaluation and infertility in the male llama and alpaca', in. RS Younguist (eds), Current Therapy in Large Animal Theriogenology. Niasari-Naslaji, A, Mosaferi, S, Bahmani, N et al. 2007, 'Semen cryopreservation in Bactrian camel (Camelus Bactrianus) using SHOTOR diluent: Effects of cooling rates and glycerol concentrations ', Theriogenology vol. 68, pp Niasari-Naslaji, A, Mosaferi, S, Bahmani, N et al. 2006a, 'Effectiveness of a tris-based extender (SHOTOR diluent) for the preservation of Bactrian camel (Camelus Bactrianus) semen.' Cryobiology vol. 53, pp Niasari-Naslaji, A, Mosaferi, S, Gharahdaghi, AA et al. 2006b, 'Comparison between two extenders for cryopreservation of Bactrian camel semen', Reprod. Fertil. Dev. vol. 18, pp. 161 (abstract). Niasari-Naslaji, N, Mosaferi, S, Gharahdaghi, AA et al. 2005, 'A novel extender for preservation of Bactrian camel (Camelus Bactrianus) semen', Reprod. Fertil. Dev. vol. 17, pp Niasari-Naslaji, N, Sarhaddi, F, Nazari, M and razavi, K 2001, 'Vaginal artifical insemination in maiden Dromedary camel-a novel approach', Theriogenology vol.??, pp Novoa, C 1970, 'Reproduction in Camelidae', J. Reprod. Fertil. vol. 22, pp O'Brien, JK and Robeck, TR 2006, 'Development of sperm sexing and associated assisted reproductive technology for sex preselection of captive bottlenose dolphins (Tursiops truncatus)', Reprod. Fertil. Dev. vol. 18, pp O'Brien, JK, Stojanov, T, Crichton, EG et al. 2005a, 'Flow cytometric sorting of fresh and frozen-thawed spermatozoa in the Western Lowland Gorilla (Gorilla gorilla gorilla)', Am. J. Primatol. vol. 66, pp O'Brien, JK, Stojanov, T, Heffernan, SJ et al. 2005b, 'Flow cytometric sorting of non-human primate spermatozoa', Theriogenology vol. 63, pp Ordoñez, TH 1994, 'Llamas, llama production and llama nutrition in the Ecuador highlands.' Journal of Arid Environments vol. 26, pp Pacheco, C 1996, Efecto de la tripsina y colagenasa sobre el acrosoma espermatozoide y su relacion con le fertilidad del semen de alpaca. MVZ Thesis, Prog Med Vet Zootec Univ Catol Santa Maria, Arequipa, Peru 185
201 Pacheco, C 1996, Efecto de la tripsina y colagenasa sobre el acrosoma espermatozoide y su relacion con le fertilidad del semen de alpaca. MVZ Thesis, Prog Med Vet Zootec Univ Catol Santa Maria, Arequipa, Peru Pan, G, Chen, Z, Liu, X et al. 2001, 'Isolation and purification of the ovulation-inducing factor from seminal plasma in the Bactrian camel (Camelus Bactrianus)', Theriogenology vol. 55, pp Pan, G, Xie, Q, Cai, X et al. 2003, 'Study on ovulation inducing mechanism in Bactrian camel II target organ of ovulation inducing factor (OIF) and its metabolic dynamics in Bactrian camel. [Chinese]', Sci. Agr. vol. 36, pp Pan, GW, Zhao, XX, Chen, B-H et al. 1992, 'The ovulation-inducing effect of seminal plasma in the Bactrian camel', in (eds), Proc. First International Camel Conference R&W Publications Ltd,, Newmarket, UK. Paolicchi, F, Urquieta, B, Del Valle, L and Bustos-Obregon, E 1999, 'Biological activity of the seminal plasma of alpacas: stimulus for the production of LH by pituitary cells', Anim. Reprod. Sci. vol. 54, pp Parraguez, VH, Cortez, S, Gazitua, FJ et al. 1997, 'Early pregnancy diagnosis in alpaca (Lama pacos) and llama (Lama glama) by ultrasound', Anim. Reprod. Sci. vol. 47, pp Parrilla, I, Vazquez, JM, Roca, J and Martinez, EM 2004, 'Flow cytometry identification of X- and Y- chromosome bearing goat spermatozoa', Reprod. Domest. Anim. vol. 39, pp Pate, EF and Brokaw, CJ 1980, 'Movement of spermatozoa in viscous environments', J. Exp. Biol. vol. 88, pp Pattinson, HA, Mortimer, D, Curtis, EF, Leader, A and Taylor, PJ 1990a, 'Treatment of spermagglutination with proteolytic enzmyes. I. Sperm motility, viability, longevity and successful disagglutination', Hum. Reprod. vol. 5, pp Pattinson, HA, Mortimer, D and Taylor, PJ 1990b, 'Treatment of spermagglutination with proteolytic enzymes. II. Sperm function after enzymatic disagglutination', Hum. Reprod. vol. 5, pp Perez, MG 2005, Perspectivas de la inseminacion artificial en camelidos sudamericanos., Universidad Nacional del Altiplano, Puno-Peru. Pollard, JC, Littlejohn, RP and Moore, GH 1995, 'Seasonal and other factors affecting the sexual behaviour of alpacas', Anim. Reprod. Sci. vol. 37, pp Pollard, JC, Littlejohn, RP and Scott, IC 1994, 'The effects of mating on the sexual receptivity of female alpacas', Anim. Reprod. Sci. vol. 34, pp Pollard, JC, Moore, GH and Littlejohn, RP 1991, 'The sexual behaviour of alpacas imported to New Zealand from Chile. ' Proceedings of the New Zealand Society of Animal Production vol. 51, pp Pontbriand, D, Howard, JG, Schieve, MC, Stuart, LD and Wildt, DE 1989, 'Effect of cryoprotective diluent and method of freeze-thawing on survival and acrosomal integrity of ram spermatozoa', Cryobiology vol. 26, pp Pugh, DG 1999, 'Male lama reproductive evaluation', Proceeings of the Society for Theriogenology pp Purohit, GN 1999, 'Biotechnologies in camelid reproduction: current status and future prospectives', J. Camel Prac. Res. vol. 6, pp Pursel, VG, Schulman, LL and Johnson, LA 1978, 'Effect of orvus es paste on acrosome morphology, motility and fertilising capacity of frozen-thawed boar sperm.' Journal of Animal Science vol. 47, pp Quispe, G 1996a, Inseminacion artificial en alpacas (Lama pacos) con semen diluido a differentes concentraciones. Fac Agron Zootec. Cusco, Peru, Univ San Antonio Abad: 103 Quispe, G 1996b, Insemination artificial en alpacas (Lama pacos) con semen diluido a differentes concentraciones: I. Zootec Thesis, Faculty Agron Zootec. Cusco, Peru, University National San Antonion Adad: 103. Ratto, MH 2005, Ovarian follicular synchronisation, ovulation and oocyte development in llamas and alpacas. Department of Veterinary Biomedical Sciences Saskatoon, Canada, University of Saskatchewan: 168. Ratto, MH, Huanca, W, Singh, J and Adams, GP 2005, 'Local versus systemic effect of ovulation-inducing factor in the seminal plasma of alpacas.' Reprod. Biol. Endocrinol. vol. 3, pp Ratto, MH, Huanca, W, Singh, J and Adams, GP 2006, 'Comparison of the effect of ovulation-inducing factor (OIF) in the seminal plasma of llamas, alpacas and bulls', Theriogenology vol. 66, pp Ratto, MH, Wolter, M and Berland, M 1999, Refrigeration of epididymal sperm from lama with three different extenders.paper presented to Proceedings of the 2nd Congreso Mundial sobre Camelidos Cusco, Peru. Raymundo, F, Huanca, W, Huertas, S and Gauly, M 2000, Influence of different extenders on the motility in alpaca (Lama pacos) semen.paper presented to Proc 2nd Int Camelid Conference Agroeconomics of Camelid Farming Almaty, Kazakhstan. Rens, W, Welch, G and Johnson, L 1998a, 'A novel nozzle for more efficient sperm orientation to improve sperm sorting efficiency of X and Y chromosome bearing sperm.' Cytometry vol. 33, pp Rens, W, Welch, GR, Houch, DW, van Oven, CH and Johnson, LA 1998b, 'Slit-scan flow cytometry for consistent high resolution DNA analysis of X- and Y-chromosome bearing sperm', Cytometry vol. 25, pp Rigby, SL, Brinsko, SP, Cochran, M et al. 2001, 'Advances in cooled semen technologies: seminal plasma and semen extender', Anim. Reprod. Sci. vol. 68, pp
202 Robert, M and Cagnon, C 1999, 'Semenogelin I: a coagulum forming, multifunctional seminal vesicle protein', Cell. Mol. Life Sci. vol. 55, pp Robert, M and Gagnon, C 1994, 'Sperm motility inhibitor from human seminal plasma: presence of a precursor molecule in seminal vesicle fluid and its molecular processing after ejaculation', Int. J. Androl. vol. 17, pp Robert, M and Gagnon, C 1995, 'Sperm motility inhibitor from human seminal plasma: association with semen coagulum', Hum. Reprod. vol. 10, pp Rodrigues de Paz, RC, Zacariotti, RL, Teixeira, RH, Alcindo de Barros, M and Guimaraes, V 2006, 'O efeito das enzimas hialuronidase e tripsina na liquefacao do semen de macacos preos (Cebus apella)', Braz. J. Vet. Res. Anim. Sci., Sao Paulo vol. 43, pp Salamon, S and Maxwell, WMC 1995a, 'Frozen storage of ram semen I. Processing, freezing, thawing and fertility after cervical insemination', Anim. Reprod. Sci. vol. 37, pp Salamon, S and Maxwell, WMC 1995b, 'Frozen storage of ram semen II. Causes of low fertility after cervical insemination and methods of improvement.' Anim. Reprod. Sci. vol. 38, pp San-Martin, M, Copaira, M, Zuniga, J et al. 1968, 'Aspects of reproduction in the alpaca', J. Reprod. Fertil. vol. 16, pp San Martin, M, Copaira, M, Zuniga, J et al. 1968, 'Aspects of reproduction in the alpaca', J. Reprod. Fertil. vol. 16, pp Santiani, A, Huanca, W, Sapana, R et al. 2005, 'Effects on the quality of frozen-thawed alpaca (Lama pacos) semen using two different cryoprotectants and extenders', Asian J. Androl. vol. 7, pp Schenk, JL and DeGrofft, DL 2003, 'Insemination of cow elk with sexed frozen semen', Theriogenology vol. 59, pp. 514 (abstract). Sghiri, A and Driancourt, MA 1999, 'Seasonal effects on fertility and ovarian follicular growth and maturation in camels (Camelus dromedarius)', Anim. Reprod. Sci. vol. 55, pp Shalash, MR 1965, 'Some reproductive aspects in the female camel', World Rev. Anim. Prod. vol. 4, pp Shannon, P and Curson, B 1982, 'Kinetics of the aromatic l-amino acid oxidase from dead bovine spermatozoa and the effect of catalase on fertility of diluted bovine semen stored at 5 C and ambient temperatures', J. Reprod. Fert. vol. 64, pp Shettles, CB and Rorvik, D 1984, How to choose the sex of your baby. Harper Collins Publishers Pty. Ltd., Sydney, Australia. Shinohara, H, Yanagimachi, R and Srivastava, PN 1985, 'Enhancement of the acrosome reaction of hamster spermatozoa by proteolytic enzmyes, kallikrein, trypsin and chymotrypsin', Gamete Res. vol. 11, pp Silva, N, Solana, A and Castro, JM 1999, 'Evaluation of the effects of different typrsin treatments on semen quality after BHV-1 inactivation, and a comparison of the results before and after freezing, assessed by a computer image analyzer', Anim. Reprod. Sci. vol. 54, pp Skidmore, JA 2000a, 'Anatomy of the camel reproductive tract', in. L Skidmore and GP Adams (eds), Recent Advances in Camelid Reproduction, International Veterinary Information Service (IVIS), Ithaca, New York, USA. Skidmore, JA 2000b, 'Reproductive physiology of the male camel', in (eds), Lecture Notes for the Short Course in Reproduction in the Dromedary Camel International Veterinary Information Service ( Skidmore, JA 2003, 'The main challenges facing camel reproduction research in the 21st century.' in. BK Campbell, R Webb, H Dobson and C Doberska (eds), Reproduction in Domestic Ruminats V Society for Reproduction and Fertility, Cambridge. Skidmore, JA 2005, 'Reproduction in Dromedary camels: an update', Anim. Reprod. vol. 2, pp Skidmore, JA and Billah, M 2001, Assisted reproduction in the Dromedary camel (Camelus dromedarius).paper presented to Proceedings of the 1st International Symposium on Assisted Reproductive Technology for the Conservation and Genetic Management of Wildlife Smith, BB 1999a, 'Overview of reproduction in the male llama and alpaca', Proc of the Society for Theriogenology pp Smith, BB 1999b, 'Overview of reproduction in the male llama and alpaca. ' Proceedings of the Society for Theriogenology pp Smith, CL, Peter, AT and Pugh, DG 1994, 'Reproduction in llamas and alpacas: a review', Theriogenology vol. 41, pp Sucapuca, V 1991, Caracteristicas fisicas del semen de la alpaca obtenido por el metodo del preservativo.. Puno, Peru, Univ Nac Altiplano: 49. Sumar, J 1983, Studies on reproductive pathology in alpacas. Faculty of Veterinary Medicine Uppsala, Sweden, University of Agricultural Sciences: 90. Sumar, J 1983, Studies on reproductive pathology in alpacas. Faculty of Veterinary Medicine Uppsala, Sweden, University of Agricultural Sciences: 90. Sumar, J 1983, Studies on reproductive pathology in alpacas. Faculty of Veterinary Medicine Uppsala, Sweden, University of Agricultural Sciences:
203 Sumar, J 1994, 'Effects of various ovulation induction stimuli in alpacas and llamas', J. Arid Environments vol. 26, pp Sumar, JB 1996, 'Reproduction in llamas and alpacas', Anim. Reprod. Sci. vol. 42, pp Taylor, P, Taylor, S, James, AN and Godke, RA 2000, 'Successful commercial embryo transfer in the llama (Lama glama)', Theriogenology vol. 53, pp Tibary, A and Anouassi, A 1997, Theriogenology in Camelidae. Actes Editions, Rabat, Morocco. Tibary, A, Fite, C, Anouassi, A and Sghiri, A 2006, 'Infectious causes of reproductive loss in camelids', Theriogenology vol. 66, pp Tibary, A and Memon, MA 1999a, 'Reproduction in the male South American Camelidae', J. Camel Prac. Res. vol. 6, pp Tibary, A and Memon, MA 1999b, 'Reproductive physiology in the female South American camelidae', J. Camel Prac. Res. vol. 6, pp Tibary, A and Vaughan, JL 2006, 'Reproductive physiology and infertility in male South American camelids: A review and clinical observations', Small Ruminant Res. vol. 61, pp Tingari, MD 1991, 'Studies on camel semen. III. Ultrastructure of the spermatozoon', Anim. Reprod. Sci. vol. 26, pp Tingari, MD, El-Manna, MM, Rahim, ATA, Ahmed, AK and Hamad, MH 1986, 'Studies on camel semen. I. Electroejaculation and some aspects of semen characteristics', Anim. Reprod. Sci. vol. 12, pp Tucker, M, Wright, G, Bishop, F et al. 1990, 'Chymotrypsin in semen preparation for ARTA', Mol. Androl. vol. 2, pp Ungerfeld, R and Silva, L 2004, 'Ewe effect: endocrine and testicular changes in experienced adult and inexperienced young Corriedale rams used for the ram effect', Anim. Reprod. Sci. vol. 80, pp Urquieta, B, Cepeda, R, Caceres, JE, Raggi, LA and Rojas, JR 1994, 'Seasonal variation in some reproductive parameters of the male vicuña in the high Andes of Northern Chile.' J. Arid Environments vol. 26, pp Valdivia, M, Ruiz, M, Bermudez, L et al. 1999, Criopreservacion de semen de alpacas.paper presented to Proc of the 2nd Congreso Mundial sobre Camelidos Cusco, Peru. Valdivia, M, Suyo, M, Manosalva, I et al. 2000, 'Cryopreservation and immunoreactivity of proacrosin/acrosin system in alpaca spermatozoa.' Biol. Reprod. Suppl. vol. 62, pp Vaughan, JL 2001, Control of ovarian follicular growth in the alpaca (Lama pacos). Animal Sciences and Production Group Central Queensland University. Vaughan, JL 2002, Improving the efficiency of reproduction and breeding in alpacas. RIRDC project; no. UCQ- 11A.. Barton, A.C.T., Rural Industries Research and Development Corporation: viii, 20. Vaughan, JL, Galloway, D and Hopkins, D 2003a, Artificial insemination in alpacas (Lama pacos). RIRDC project; no. AAA-1A., Rural Industries Research and Development Corporation: 90. Vaughan, JL, Macmillan, KL, Anderson, GA and D'Occhio, MJ 2003b, 'Effects of mating behaviour and the ovarian follicular state of female alpacas on conception', Aust. Vet. J. vol. 81, pp Vaughan, JL and Tibary, A 2006, 'Reproduction in female South American camelids: A review and clinical observations', Small Ruminant Res. vol. 61, pp Velasquez, CV and Novoa, MC 1999, 'Superovulacion con PMSG aplicada fase folicular y fase luteal en alpacas', Rev. Int. Vet. Peru vol. 10, pp Vidal, F, Fugger, EF, Blanco, J et al. 1998, 'Efficiency of MicroSort flow cytometry for producing sperm populations enriched in X- or Y-chromosome haplotypes: a blind trial assessed by double and triple colour fluorescent in-situ hybridization', Hum. Reprod. vol. 13, pp Vishwanath, R and Shannon, P 2000, 'Storage of bovine semen in liquid and frozen state', Anim. Reprod. Sci. vol. 62, pp Von Baer, L and Hellemann, C 1998, 'Semen characteristics in the llama (Lama glama). [Spanish] Variables seminales en llama (Lama glama)', Arch. Med. Vet. vol. 30, pp Von Baer, L and Hellemann, C 1999, 'Cryopreservation of llama (Lama glama) semen', Reprod. Domest. Anim. vol. 34, pp Von Baer, L and Hellemann, C 1999, 'Cryopreservation of llama (Lama glama) semen', Reprod. Domest. Anim. vol. 34, pp von Kubicek, J 1974, 'Semen collection in alpaca with a urethral fistula [German]', Zeitschrift Tierzuchtung und Zuchtungsbiologie vol. 90, pp Vyas, S, Goswami, P, Rai, AK and Khanna, ND 1998, 'Use of tris and lactose extenders in preservation of camel semen at refrigerated temperature', Indian Vet. J. vol. 75, pp Vyt, P 2007, Examination and storage of liquid porcine semen. Faculty of Veterinary Medicine, Ghent University. Doctor of Veterinary Medicine: 162. Walkden-Brown, SW, Martin, GB and Restall, BJ 1999, 'Role of male-female interaction in regulating reproduction in sheep and goats.' J. Reprod. Fertil. vol. Suppl. 52, pp Wani, N, Nowshari, M and Wernery, U 2005, 'Storage of camel (Camelus dromedarius) epididymal spermatozoa in different extenders and at different temperatures', Biol. Reprod. Supplement 1 vol. 73, pp
204 Wani, NA, Billa, M and Skidmore, JA 2007, 'Studies on liquefaction and storage of ejaculated Dromedary camel (Camelus dromedarius) semen', Anim. Reprod. Sci. pp. in press. Watson, PF 2000, 'The causes of reduced fertility with cryopreserved semen', Anim. Reprod. Sci. vol , pp Welch, GR and Johnson, LA 1999, 'Sex preselection: laboratory validation of the sperm sex ratio of flow sorted X- and Y-sperm by sort reanalysis for DNA', Theriogenology vol. 52, pp Wheeler, JC 1995, 'Evolution and present situation of the South American Camelidae', Biol. J. Linn. Soc. vol. 54, pp Wheeler, MB and Seidel, GEJ 1989, 'Capacitation of bovine spermatozoa by lysophospholipids and trypsin', Gamete Res. vol. 22, pp Wiepz, DW and Chapman, RJ 1985, 'Non-surgical embryo transfer and live birth in a llama', Theriogenology vol. 24, pp Windsor, DP, Evans, G and White, IG 1993, 'Sex predetermination by separation of X and Y chromosome bearing sperm: a review', Reprod. Fertil. Dev. vol. 5, pp Winston, R 1993, Getting pregnant. Pan Books Ltd., London, UK. Xu, YS, Wang, HY, Zeng, GQ, Jiang, GT and Gao, YH 1985, 'Hormone concentrations before and after semeninduced ovulation in the Bactrian camel (Camelus Bactrianus)', J. Reprod. Fertil. vol. 74, pp Yagil, R and Etzion, Z 1980, 'Hormonal and behavioural patterns in the male camel (Camelus dromedarius)', J. Reprod. Fertil. vol. 58, pp Yamashiro, H, Kumamoto, K, Wang, H, Yamashita, Y and Terada, T 2006, 'Effect of semen collection in extender solution on the characteristics of goat spermatozoa', J. Reprod. Dev. vol. 52, pp Zarutskie, PW, Muller, CH, Magone, M and Soules, MR 1989, 'The clinical relevance of sex selection techniques', Fertil. Steril. vol. 52, pp Zavos, PM, Zarmakoupis-Zavos, PN and Correa, JR 1997, 'Effect of treatment of seminal viscosity difficulties with α-chymotrypsin on the recovery of spermatozoa for assisted reproductive technologies: comparison between the SpermPrep TM filtration and Percoll gradient centrifugation methods', Middle East Fert. Soc. J. vol. 2, pp Zhao, X-X, Huang, YM and Chen, B-X 1994, 'Artificial insemination and pregnancy diagnosis in the Bactrian camel (Camelus Bactrianus)', J. Arid Environments vol. 26, pp Zhao, XX, Huang, HY and Chen, BX 1992, 'Studies on the semen characteristics of Bactrian camel semen.' Chin. J. Anim. Sci. vol. 28, pp Zhao, XX, Huang, YM and Chen, BX 1992, 'Biological activity of gonadotrophin-releasing hormone-like factors in the seminal plasma of the Bactrian camel', in. WR Allen, AJ Higgins, IG Mayhew, DH Snow and JF Wade (eds), Proc. First International Camel Conference, Dubai, 2nd - 6th February 1992., R&W Publications Ltd,, Newmarket, UK. Zhao, XX, Huang, YM, Nie, QC, Zhang, YK and Chen, BX 1996, 'Effect of different extenders on motility, survival and acrosomal integrity of camel spermatozoa frozen in ampoules', J. Camel Prac. Res. vol. 3, pp Zhao, XX, Huang, YM, Nie, QC, Zhang, YK and Chen, BX 2004, 'Effect of different extenders on motility survival and acrosomal integrity of camel spermatozoa frozen in ampoules', J. Camel Science vol. 1, pp
205 Continued Development of Artificial Insemination Technology in Alpacas By K Morton, J Vaughan and W Maxwell RIRDC Pub. No. 08/057 This project discusses collection, liquefaction and preservation of alpaca semen, demonstrates the limitations imposed by the viscous seminal plasma and highlights the need for future research into seminal plasma viscosity, which remains the key problem for the development of artificial insemination (AI) technology. It has a literature review, covering semen characteristics, preservation and AI in camelids with particular reference to alpacas, a full description of the methods used in this project which can be used as a practical manual by breeders and practitioners contemplating artificial breeding in alpacas seven experimental chapters describing semen collection, assessment of sperm function, liquefaction of semen, liquid storage, cryopreservation, AI and sperm sexing. The experimental chapters can be read as stand-alone reports and are followed by a general discussion of the results and a consideration of the implications and recommendations arising from the work. The appendices provide detailed protocols, equipment and publication lists that may be useful for scientists and practitioners. RIRDC s Rare Natural Animal Fibres R&D program aims to facilitate the development of new and established industries based on rare natural fibres. The program incorporates cashmere, mohair, alpaca fibre, camel hair and other rare fibre research and development projects. The Rural Industries Research and Development Corporation (RIRDC) manages and funds priority research and translates results into practical outcomes for industry. Our business is about new products and services and better ways of producing them. Most of the information we produce can be downloaded for free from our website: RIRDC books can be purchased by phoning or online at: Contact RIRDC: Level 2 15 National Circuit Barton ACT 2600 PO Box 4776 Kingston ACT 2604 This publication can be viewed at our website All RIRDC books can be purchased from: Ph: Fax: rirdc@rirdc.gov.au web: RIRDC Innovation for rural Australia covers.indd 2 16/06/2008 8:45:27 AM
The continued development of artificial insemination technologies in alpacas
 The continued development of artificial insemination technologies in alpacas Katherine M. Morton and W.M. Chis Maxwell 1 1 Centre for Advanced Technologies in Animal Genetics and Reproduction (ReproGen),
The continued development of artificial insemination technologies in alpacas Katherine M. Morton and W.M. Chis Maxwell 1 1 Centre for Advanced Technologies in Animal Genetics and Reproduction (ReproGen),
Semen-induced ovulation in the bactrian camel (Camelus bactrianus)
 Semen-induced ovulation in the bactrian camel (Camelus bactrianus) B. X. Chen, Z. X. Yuen and G. W. Pan Department of Veterinary Medicine, Gansu Agricultural University, Wuwei, Gansu and *Haixi Institute
Semen-induced ovulation in the bactrian camel (Camelus bactrianus) B. X. Chen, Z. X. Yuen and G. W. Pan Department of Veterinary Medicine, Gansu Agricultural University, Wuwei, Gansu and *Haixi Institute
WBC / ICAR 2008 Satellite Meeting on Camelid Reproduction
 Close window to return to IVIS www.ivis.org WBC / ICAR 2008 Satellite Meeting on Camelid Reproduction 12-13 July, 2008, Budapest, Hungary Program and Extended stracts Peter Nagy; Gyula Huszenicza; Judit
Close window to return to IVIS www.ivis.org WBC / ICAR 2008 Satellite Meeting on Camelid Reproduction 12-13 July, 2008, Budapest, Hungary Program and Extended stracts Peter Nagy; Gyula Huszenicza; Judit
Small Ruminant Reproductive Management Workshop
 Small Ruminant Reproductive Management Workshop Animal Nutrition and Physiology Center, North Dakota State University Sponsors: American Sheep and Goat Center, North Dakota State University, University
Small Ruminant Reproductive Management Workshop Animal Nutrition and Physiology Center, North Dakota State University Sponsors: American Sheep and Goat Center, North Dakota State University, University
Male Reproduction Organs. 1. Testes 2. Epididymis 3. Vas deferens 4. Urethra 5. Penis 6. Prostate 7. Seminal vesicles 8. Bulbourethral glands
 Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Cryopreservation of epididymal alpaca (Vicugna pacos) sperm: a comparison of citrate-, Tris- and lactose-based diluents and pellets and straws
 CSIRO PUBLISHING Reproduction, Fertility and Development, 2007, 19, 792 796 www.publish.csiro.au/journals/rfd Cryopreservation of epididymal alpaca (Vicugna pacos) sperm: a comparison of citrate-, Tris-
CSIRO PUBLISHING Reproduction, Fertility and Development, 2007, 19, 792 796 www.publish.csiro.au/journals/rfd Cryopreservation of epididymal alpaca (Vicugna pacos) sperm: a comparison of citrate-, Tris-
UNDERSTANDING EMBRYO-TRANSFER (ET) A GUIDE TO THE BENEFIT OF ET IN YOUR HERD
 UNDERSTANDING EMBRYO-TRANSFER (ET) A GUIDE TO THE BENEFIT OF ET IN YOUR HERD Embryo Transfer allows one superior cow to produce a greater number of calves than normal in her lifetime TABLE OF CONTENTS
UNDERSTANDING EMBRYO-TRANSFER (ET) A GUIDE TO THE BENEFIT OF ET IN YOUR HERD Embryo Transfer allows one superior cow to produce a greater number of calves than normal in her lifetime TABLE OF CONTENTS
Human Anatomy Unit 3 REPRODUCTIVE SYSTEM
 Human Anatomy Unit 3 REPRODUCTIVE SYSTEM In Anatomy Today Male Reproductive System Gonads = testes primary organ responsible for sperm production development/maintenan ce of secondary sex characteristics
Human Anatomy Unit 3 REPRODUCTIVE SYSTEM In Anatomy Today Male Reproductive System Gonads = testes primary organ responsible for sperm production development/maintenan ce of secondary sex characteristics
Male Reproductive System. Dr Maan Al-Abbasi PhD, MSc, MBChB, MD
 Male Reproductive System Dr Maan Al-Abbasi PhD, MSc, MBChB, MD Learning Objectives 1. Describe the General Anatomy of the Male Reproductive System 2. Identify the structures that are related to the prostate.
Male Reproductive System Dr Maan Al-Abbasi PhD, MSc, MBChB, MD Learning Objectives 1. Describe the General Anatomy of the Male Reproductive System 2. Identify the structures that are related to the prostate.
Outline. Male Reproductive System Testes and Sperm Hormonal Regulation
 Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Unit B Understanding Animal Body Systems. Lesson 7 Understanding Animal Reproduction
 Unit B Understanding Animal Body Systems Lesson 7 Understanding Animal Reproduction 1 Terms Anestrus Artificial insemination Castration Cervix Copulation Diestrus Egg Ejaculation Estrous cycle Estrus Fertilization
Unit B Understanding Animal Body Systems Lesson 7 Understanding Animal Reproduction 1 Terms Anestrus Artificial insemination Castration Cervix Copulation Diestrus Egg Ejaculation Estrous cycle Estrus Fertilization
Unit B Understanding Animal Body Systems. Lesson 6 Anatomy and Physiology of Animal Reproduction Systems
 Unit B Understanding Animal Body Systems Lesson 6 Anatomy and Physiology of Animal Reproduction Systems 1 Terms Alimentary canal Bladder Cervix Clitoris Cloaca Copulation Cowper s gland Epididymis Fallopian
Unit B Understanding Animal Body Systems Lesson 6 Anatomy and Physiology of Animal Reproduction Systems 1 Terms Alimentary canal Bladder Cervix Clitoris Cloaca Copulation Cowper s gland Epididymis Fallopian
EMBRYO TRANSFER ANIMAL SCIENCE 8818-B INTRODUCTION
 ANIMAL SCIENCE 8818-B EMBRYO TRANSFER INTRODUCTION Embryo transfer* is a process by which an embryo is collected from a donor female and then transferred into a recipient female where the embryo completes
ANIMAL SCIENCE 8818-B EMBRYO TRANSFER INTRODUCTION Embryo transfer* is a process by which an embryo is collected from a donor female and then transferred into a recipient female where the embryo completes
List of Equipment, Tools, Supplies, and Facilities:
 Unit B: Understanding Animal Body Systems Lesson 6: Anatomy and Physiology of Animal Reproductive Systems Student Learning Objectives: Instruction in this lesson should result in students achieving the
Unit B: Understanding Animal Body Systems Lesson 6: Anatomy and Physiology of Animal Reproductive Systems Student Learning Objectives: Instruction in this lesson should result in students achieving the
Primary sex organs (gonads): testes and ovaries. Accessory reproductive organs: ducts, glands, and external genitalia
 Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
The Reproductive System
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
- production of two types of gametes -- fused at fertilization to form zygote
 Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
Chapter 28: REPRODUCTIVE SYSTEM: MALE
 Chapter 28: REPRODUCTIVE SYSTEM: MALE I. FUNCTIONAL ANATOMY (Fig. 28.1) A. Testes: glands which produce male gametes, as well as glands producing testosterone 2. Seminiferous tubules (Fig.28.3; 28.5) a.
Chapter 28: REPRODUCTIVE SYSTEM: MALE I. FUNCTIONAL ANATOMY (Fig. 28.1) A. Testes: glands which produce male gametes, as well as glands producing testosterone 2. Seminiferous tubules (Fig.28.3; 28.5) a.
The Reproductive System
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
Reproductive System Purpose General Structures Male Structures Functions Female Anatomy Structures Functions Clinical Applications
 The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
Chapter 14 The Reproductive System
 Biology 12 Name: Reproductive System Per: Date: Chapter 14 The Reproductive System Complete using BC Biology 12, page 436-467 14. 1 Male Reproductive System pages 440-443 1. Distinguish between gametes
Biology 12 Name: Reproductive System Per: Date: Chapter 14 The Reproductive System Complete using BC Biology 12, page 436-467 14. 1 Male Reproductive System pages 440-443 1. Distinguish between gametes
The Reproductive System
 16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
REPRODUCCIÓN. La idea fija. Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
Male Reproductive System
 21-1 21-2 Reproductive System Male Reproductive System Genital Tract In males the testes, held outside the body in the scrotum (optimum temp of about 35 0 C), produce sperm. Sperm mature in coiled tubes
21-1 21-2 Reproductive System Male Reproductive System Genital Tract In males the testes, held outside the body in the scrotum (optimum temp of about 35 0 C), produce sperm. Sperm mature in coiled tubes
What are the main functions of the male reproductive system? 1. Produce sperm 2. Deposit sperm into the female 3. Provide a pathway for the removal
 What are the main functions of the male reproductive system? 1. Produce sperm 2. Deposit sperm into the female 3. Provide a pathway for the removal of urine Where is sperm produced? -In the 2 testes What
What are the main functions of the male reproductive system? 1. Produce sperm 2. Deposit sperm into the female 3. Provide a pathway for the removal of urine Where is sperm produced? -In the 2 testes What
Sperm production. Sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production. Sperm production. Controlling sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Male Reproductive Structures I. Overview A. Main functions: 1. Produce a haploid male gamete (sperm) 2. Deposit sperm in the female so fertilization
 Male Reproductive Structures I. Overview A. Main functions: 1. Produce a haploid male gamete (sperm) 2. Deposit sperm in the female so fertilization may occur! A. Scrotum 1. Muscular pouch that holds the
Male Reproductive Structures I. Overview A. Main functions: 1. Produce a haploid male gamete (sperm) 2. Deposit sperm in the female so fertilization may occur! A. Scrotum 1. Muscular pouch that holds the
Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature
 REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
Objectives: 1. Review male & female reproductive anatomy 2. Gametogenesis & steroidogenesis 3. Reproductive problems
 CH. 15 - REPRODUCTIVE SYSTEM Objectives: 1. Review male & female reproductive anatomy 2. Gametogenesis & steroidogenesis 3. Reproductive problems 3. Male Reproductive anatomy and physiology. Testes = paired
CH. 15 - REPRODUCTIVE SYSTEM Objectives: 1. Review male & female reproductive anatomy 2. Gametogenesis & steroidogenesis 3. Reproductive problems 3. Male Reproductive anatomy and physiology. Testes = paired
describe the parts and function of semen and the glands that contribute to it
 You need to be able to: describe spermatogenesis (How is sperm made?) describe the anatomy of a sperm describe the parts and function of semen and the glands that contribute to it How is sperm made? Spermatogenesis
You need to be able to: describe spermatogenesis (How is sperm made?) describe the anatomy of a sperm describe the parts and function of semen and the glands that contribute to it How is sperm made? Spermatogenesis
Male Reproductive System
 Male Reproductive System The male reproductive system consists of a number of sex organs that are part of the reproductive process. The following sections describe the function of each part of the male
Male Reproductive System The male reproductive system consists of a number of sex organs that are part of the reproductive process. The following sections describe the function of each part of the male
Papain and its inhibitor E-64 reduce camelid semen viscosity without impairing sperm function and improve post-thaw motility rates
 Papain and its inhibitor E-64 reduce camelid semen viscosity without impairing sperm function and improve post-thaw motility rates by Kershaw, C.M., Evans, G., Rodney, R. and Maxwell, W.M.C. Copyright,
Papain and its inhibitor E-64 reduce camelid semen viscosity without impairing sperm function and improve post-thaw motility rates by Kershaw, C.M., Evans, G., Rodney, R. and Maxwell, W.M.C. Copyright,
Assessing the Risks of Transmitting OJD in the Semen of Rams by Artificial Insemination
 Assessing the Risks of Transmitting OJD in the Semen of Rams by Artificial Insemination Project number OJD.012 Final Report prepared for MLA by: Central Tablelands Rural Lands Protection Board Meat & Livestock
Assessing the Risks of Transmitting OJD in the Semen of Rams by Artificial Insemination Project number OJD.012 Final Report prepared for MLA by: Central Tablelands Rural Lands Protection Board Meat & Livestock
Study Guide Answer Key Reproductive System
 Biology 12 Human Biology Textbook: BC Biology 12 Study Guide Answer Key Reproductive System 1. Distinguish between a gamete and a gonad using specific examples from the male and female systems. Gonads
Biology 12 Human Biology Textbook: BC Biology 12 Study Guide Answer Key Reproductive System 1. Distinguish between a gamete and a gonad using specific examples from the male and female systems. Gonads
COMPARISON OF KAMPONG AND COMMERCIAL CHICKEN EGG-BASED EXTENDERS ON CRYOPRESERVED GOAT SPERM MOVEMENT CHARACTERISTICS
 COMPARISON OF KAMPONG AND COMMERCIAL CHICKEN EGG-BASED EXTENDERS ON CRYOPRESERVED GOAT SPERM MOVEMENT CHARACTERISTICS Janice, C.W.K. 1, Kanwal, K.D.S. 1*, Wan Khadijah, W.E. 2 and Abdullah, R.B. 2 1 School
COMPARISON OF KAMPONG AND COMMERCIAL CHICKEN EGG-BASED EXTENDERS ON CRYOPRESERVED GOAT SPERM MOVEMENT CHARACTERISTICS Janice, C.W.K. 1, Kanwal, K.D.S. 1*, Wan Khadijah, W.E. 2 and Abdullah, R.B. 2 1 School
DISSECTION 8: URINARY AND REPRODUCTIVE SYSTEMS
 8546d_c01_1-42 6/25/02 4:32 PM Page 38 mac48 Mac 48: 420_kec: 38 Cat Dissection DISSECTION 8: URINARY AND REPRODUCTIVE SYSTEMS Typically, the urinary and reproductive systems are studied together, because
8546d_c01_1-42 6/25/02 4:32 PM Page 38 mac48 Mac 48: 420_kec: 38 Cat Dissection DISSECTION 8: URINARY AND REPRODUCTIVE SYSTEMS Typically, the urinary and reproductive systems are studied together, because
Chapter 22 The Reproductive System (I)
 Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Male Reproductive System Dr. Gary Mumaugh
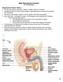 Male Reproductive System Dr. Gary Mumaugh Reproductive System Basics Primary sex organs (gonads) testes in males, ovaries in females Gonads produce sex cells called gametes (gametes means spouses) and
Male Reproductive System Dr. Gary Mumaugh Reproductive System Basics Primary sex organs (gonads) testes in males, ovaries in females Gonads produce sex cells called gametes (gametes means spouses) and
Unit 15 ~ Learning Guide
 Unit 15 ~ Learning Guide Name: INSTRUCTIONS Complete the following notes and questions as you work through the related lessons. You are required to have this package completed BEFORE you write your unit
Unit 15 ~ Learning Guide Name: INSTRUCTIONS Complete the following notes and questions as you work through the related lessons. You are required to have this package completed BEFORE you write your unit
Male Reproductive System
 Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following hormones controls the release of anterior pituitary gonadotropins? A) LH
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following hormones controls the release of anterior pituitary gonadotropins? A) LH
Select the Sex of Your Next Calf Prior to Mating: Using Sexed Semen 1
 AN163 Select the Sex of Your Next Calf Prior to Mating: Using Sexed Semen 1 Gary R. Hansen 2 Introduction Through recent advances in reproductive technology, scientists have the ability to pre-select the
AN163 Select the Sex of Your Next Calf Prior to Mating: Using Sexed Semen 1 Gary R. Hansen 2 Introduction Through recent advances in reproductive technology, scientists have the ability to pre-select the
The Reproductive System. Presenter: Dr. Jim Hurrell
 The Reproductive System Presenter: Dr. Jim Hurrell A Warm Welcome from My Faculty TEAM and Me!!! 2 The Pledge of Allegiance 3 Veterinary Technician/NURSE Oath 4 5 Come Hang Out with Us in Our Awesome Facebook
The Reproductive System Presenter: Dr. Jim Hurrell A Warm Welcome from My Faculty TEAM and Me!!! 2 The Pledge of Allegiance 3 Veterinary Technician/NURSE Oath 4 5 Come Hang Out with Us in Our Awesome Facebook
Chapter 36 Active Reading Guide Reproduction and Development
 Name: AP Biology Mr. Croft Chapter 36 Active Reading Guide Reproduction and Development Section 1 1. Distinguish between sexual reproduction and asexual reproduction. 2. Which form of reproduction: a.
Name: AP Biology Mr. Croft Chapter 36 Active Reading Guide Reproduction and Development Section 1 1. Distinguish between sexual reproduction and asexual reproduction. 2. Which form of reproduction: a.
1. Both asexual and sexual reproduction occur in the animal kingdom
 1. Both asexual and sexual reproduction occur in the animal kingdom Asexual reproduction involves the formation of individuals whose genes all come from one parent. There is no fusion of sperm and egg.
1. Both asexual and sexual reproduction occur in the animal kingdom Asexual reproduction involves the formation of individuals whose genes all come from one parent. There is no fusion of sperm and egg.
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
AI and its Influence on Production Efficiency
 AI and its Influence on Production Efficiency Christianne E. Glossop - Malmesbury, Wiltshire, England 2007 Introduction AI in pigs is by no means a new technique. Methods for semen collection and processing,
AI and its Influence on Production Efficiency Christianne E. Glossop - Malmesbury, Wiltshire, England 2007 Introduction AI in pigs is by no means a new technique. Methods for semen collection and processing,
PELVIS II: FUNCTION TABOOS (THE VISCERA) Defecation Urination Ejaculation Conception
 PELVIS II: FUNCTION TABOOS (THE VISCERA) Defecation Urination Ejaculation Conception REVIEW OF PELVIS I Pelvic brim, inlet Pelvic outlet True pelvis-- --viscera Tilt forward Mid-sagital views-- --how the
PELVIS II: FUNCTION TABOOS (THE VISCERA) Defecation Urination Ejaculation Conception REVIEW OF PELVIS I Pelvic brim, inlet Pelvic outlet True pelvis-- --viscera Tilt forward Mid-sagital views-- --how the
STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM
 Unit 7A STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM LEARNING OBJECTIVES 1. Learn the structures of the male reproductive system. 2. Learn the functions of the male reproductive system. 3. Learn
Unit 7A STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM LEARNING OBJECTIVES 1. Learn the structures of the male reproductive system. 2. Learn the functions of the male reproductive system. 3. Learn
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM 1. The male reproductive system is made up of the following structures, EXCEPT: a. prostate; b. testicle; c. spermatic ducts; d. vestibular bulbs; e. seminal vesicles. 2.The testicle:
MALE REPRODUCTIVE SYSTEM 1. The male reproductive system is made up of the following structures, EXCEPT: a. prostate; b. testicle; c. spermatic ducts; d. vestibular bulbs; e. seminal vesicles. 2.The testicle:
a. the tail disappears b. they become spermatids c. they undergo capacitation d. they have been stored in the uterus for several days
 (2 points each) Multiple Choice. Read each question thoroughly before answering. From the choices available, choose the answer that is the most correct. Place all answers on the accompanying answer sheet.
(2 points each) Multiple Choice. Read each question thoroughly before answering. From the choices available, choose the answer that is the most correct. Place all answers on the accompanying answer sheet.
Chapter 26: Reproductive Systems. Male 11/29/2015. Male reproductive system is composed of... BIO 218 Fall Gonads (testes)
 Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Advanced Non-Cycling Program. Health
 Advanced Non-Cycling Program Health Why Treat Non-Cycling Cows? Treating cows that have not been detected in oestrus ( non-cycling ) prior to the planned start of mating with DIB-Synch provides a return
Advanced Non-Cycling Program Health Why Treat Non-Cycling Cows? Treating cows that have not been detected in oestrus ( non-cycling ) prior to the planned start of mating with DIB-Synch provides a return
Objectives: 1. Review male & female reproductive anatomy 2. Gametogenesis & steroidogenesis 3. Reproductive problems
 CH. 15 - REPRODUCTIVE SYSTEM Objectives: 1. Review male & female reproductive anatomy 2. Gametogenesis & steroidogenesis 3. Reproductive problems Review of Male Reproductive Anatomy Fig 15.9 Vas deferens
CH. 15 - REPRODUCTIVE SYSTEM Objectives: 1. Review male & female reproductive anatomy 2. Gametogenesis & steroidogenesis 3. Reproductive problems Review of Male Reproductive Anatomy Fig 15.9 Vas deferens
Reproductive System. Where it all begins
 Reproductive System Where it all begins When it comes the reproductive anatomy of my gender, I would rate my knowledge (1 very poor, 10 excellent) When it comes the reproductive anatomy of the opposite
Reproductive System Where it all begins When it comes the reproductive anatomy of my gender, I would rate my knowledge (1 very poor, 10 excellent) When it comes the reproductive anatomy of the opposite
Male reproduction. Cross section of Human Testis ผศ.ดร.พญ.ส ว ฒณ ค ปต ว ฒ ภาคว ชาสร รว ทยา คณะแพทยศาสตร ศ ร ราชพยาบาล 1. Aims
 Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
AUSTRALIAN AND NEW ZEALAND COLLEGE OF VETERINARY SCIENTISTS. FELLOWSHIP GUIDELINES Animal Reproduction (Dog and Cat)
 Approved 1999 AUSTRALIAN AND NEW ZEALAND COLLEGE OF VETERINARY SCIENTISTS FELLOWSHIP GUIDELINES Animal Reproduction (Dog and Cat) ELIGIBILITY 1. The candidate shall meet the eligibility prerequisites for
Approved 1999 AUSTRALIAN AND NEW ZEALAND COLLEGE OF VETERINARY SCIENTISTS FELLOWSHIP GUIDELINES Animal Reproduction (Dog and Cat) ELIGIBILITY 1. The candidate shall meet the eligibility prerequisites for
6.7 IN. Continuity through Reproduction. What are the differences between male and female gametes? Discuss their formation and physical attributes.
 6.7 IN What are the differences between male and female gametes? Discuss their formation and physical attributes. Males - 4 sperm per parent cell; Females - 1 ovum per parent cell Sperm - motile (tail);
6.7 IN What are the differences between male and female gametes? Discuss their formation and physical attributes. Males - 4 sperm per parent cell; Females - 1 ovum per parent cell Sperm - motile (tail);
Sample Provincial exam Q s: Reproduction
 Sample Provincial exam Q s: Reproduction 11. Functions Testosterone Makes the male sex organs function normally, and also inhibits hypothalamus s release of GnRH and thus LH & FSH and thus testosterone
Sample Provincial exam Q s: Reproduction 11. Functions Testosterone Makes the male sex organs function normally, and also inhibits hypothalamus s release of GnRH and thus LH & FSH and thus testosterone
ANATOMY AND PHYSIOLOGY HOMEWORK CHAPTER 15 AND 16
 ANATOMY AND PHYSIOLOGY HOMEWORK CHAPTER 15 AND 16 Name Identify the following: 1) The ureter is indicated by letter 2) The renal pyramid is indicated by letter 3) The fibrous capsule is indicated by letter
ANATOMY AND PHYSIOLOGY HOMEWORK CHAPTER 15 AND 16 Name Identify the following: 1) The ureter is indicated by letter 2) The renal pyramid is indicated by letter 3) The fibrous capsule is indicated by letter
In domestic animals, we have limited period of estrus (sexual receptivity) and the term estrous
 REPRODUCTIVE CYCLES 1. Estrous cycle 2. Menstrual cycle In domestic animals, we have limited period of estrus (sexual receptivity) and the term estrous cycle is used. The onset of proestrus defines the
REPRODUCTIVE CYCLES 1. Estrous cycle 2. Menstrual cycle In domestic animals, we have limited period of estrus (sexual receptivity) and the term estrous cycle is used. The onset of proestrus defines the
Semen Characteristics and Artificial Insemination in the Bactrian Camel (25 Sep 2000)
 In: Recent Advances in Camelid Reproduction, Skidmore J.A. and Adams G.P. (Eds.) Publisher: International Veterinary Information Service (www.ivis.org) Semen Characteristics and Artificial Insemination
In: Recent Advances in Camelid Reproduction, Skidmore J.A. and Adams G.P. (Eds.) Publisher: International Veterinary Information Service (www.ivis.org) Semen Characteristics and Artificial Insemination
DATE: NAME: CLASS: Chapter 14 Test
 Multiple Choice Questions Decide which of the choices best completes the statement or answers the question. Locate that question number on the separate answer sheet provided. Use the procedure described
Multiple Choice Questions Decide which of the choices best completes the statement or answers the question. Locate that question number on the separate answer sheet provided. Use the procedure described
by Bergero and Cynthia Préfontaine photos by Shary B. Akers Introduction
 by Bergero and Cynthia Préfontaine photos by Shary B. Akers Introduction The embryo transfer (ET= embryo transfer) is a breeding method or reproductive technology, where an embryo is flushed from a donor
by Bergero and Cynthia Préfontaine photos by Shary B. Akers Introduction The embryo transfer (ET= embryo transfer) is a breeding method or reproductive technology, where an embryo is flushed from a donor
1 st International Symposium on Bison Health
 Faculty of Veterinary Medicine 1 st International Symposium on Bison Health Assisted Reproductive Technologies in Bison Robert McCorkell June 26, 2015 Artificial Insemination Frozen semen Estrus synchronization
Faculty of Veterinary Medicine 1 st International Symposium on Bison Health Assisted Reproductive Technologies in Bison Robert McCorkell June 26, 2015 Artificial Insemination Frozen semen Estrus synchronization
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM
 LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
Draft. Draft. 2. The system of breeding which breeds a registered male to a registered female animal of the same breed is:
 Student Name: Draft Teacher: Date: District: Wake County Assessment: 9_12 Agriculture AA21 - Animal Science I Test 4 Description: Test 7: Reproduction & Genetics Form: 501 Draft 1. Superior traits of offspring
Student Name: Draft Teacher: Date: District: Wake County Assessment: 9_12 Agriculture AA21 - Animal Science I Test 4 Description: Test 7: Reproduction & Genetics Form: 501 Draft 1. Superior traits of offspring
AP Biology Ch ANIMAL REPRODUCTION. Using only what you already know (you cannot look up anything) complete the chart below.
 AP Biology Ch. 46 - ANIMAL REPRODUCTION Using only what you already know (you cannot look up anything) complete the chart below. I. Overview of Animal Reproduction A. Both asexual and sexual reproduction
AP Biology Ch. 46 - ANIMAL REPRODUCTION Using only what you already know (you cannot look up anything) complete the chart below. I. Overview of Animal Reproduction A. Both asexual and sexual reproduction
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Bull BreedingSoundness Evaluation
 Bull BreedingSoundness Evaluation Scott Norman (BVSc, PhD, DipACT, GCEd) Registered Specialist in Veterinary Reproduction Associate Professor in Theriogenology, School of Animal and Veterinary Sciences
Bull BreedingSoundness Evaluation Scott Norman (BVSc, PhD, DipACT, GCEd) Registered Specialist in Veterinary Reproduction Associate Professor in Theriogenology, School of Animal and Veterinary Sciences
Lab #9: Kidney: Gross Anatomy & Histology
 Name Date Lab #9: Kidney: Gross Anatomy & Histology Lab #10: Male Reproductive System: Human Models & Histology Lab #11: Female Reproductive System: Human Models & Histology Stuff to Know Dr. L. Bacha
Name Date Lab #9: Kidney: Gross Anatomy & Histology Lab #10: Male Reproductive System: Human Models & Histology Lab #11: Female Reproductive System: Human Models & Histology Stuff to Know Dr. L. Bacha
Small Ruminant Embryo Transfer. - Opportunities and Challenges A Practitioner s perspective Brian W. McOnie
 Small Ruminant Embryo Transfer - Opportunities and Challenges A Practitioner s perspective Brian W. McOnie Benefits of embryo transfer Embryo transfer (ET) technology gained commercial prominence in the
Small Ruminant Embryo Transfer - Opportunities and Challenges A Practitioner s perspective Brian W. McOnie Benefits of embryo transfer Embryo transfer (ET) technology gained commercial prominence in the
Health Science: the structures & functions of the reproductive system
 Health Science: the structures & functions of the reproductive BELLWORK 1. List (4) careers that are r/t the Reproductive, Urinary, and Endocrine Systems 2. Copy down the following terms: -ologist = one
Health Science: the structures & functions of the reproductive BELLWORK 1. List (4) careers that are r/t the Reproductive, Urinary, and Endocrine Systems 2. Copy down the following terms: -ologist = one
Time / days. Explain how the release of FSH is controlled by negative feedback.
 1. The graph shows the changes in concentration of the hormones responsible for controlling the menstrual cycle. A Hormone concentration Oestrogen B C 0 14 28 Time / days WD Phillips and TJ Chilton A Level
1. The graph shows the changes in concentration of the hormones responsible for controlling the menstrual cycle. A Hormone concentration Oestrogen B C 0 14 28 Time / days WD Phillips and TJ Chilton A Level
Proper steps for bull semen dilution and freezing. IMV Technologies France
 Proper steps for bull semen dilution and freezing IMV Technologies France Introduction Since Polge reported the first successful cryopreservation of spermatozoa in 1949, spermatozoa from many mammalian
Proper steps for bull semen dilution and freezing IMV Technologies France Introduction Since Polge reported the first successful cryopreservation of spermatozoa in 1949, spermatozoa from many mammalian
Human Reproduction. Human Reproductive System. Scrotum. Male Reproductive System
 Human Reproductive System Human Reproduction Chapter 41 Contraceptives Scrotum Testes Epididymus Vas Deferens Seminal Vesicles Prostate Gland Bulbourethral Gland Penis Scrotum Sac of smooth muscle tissue
Human Reproductive System Human Reproduction Chapter 41 Contraceptives Scrotum Testes Epididymus Vas Deferens Seminal Vesicles Prostate Gland Bulbourethral Gland Penis Scrotum Sac of smooth muscle tissue
1. Describe the importance and process of animal reproduction. 2. List the sexual classification of animals for major species.
 Unit B: Understanding Animal Body Systems Lesson 7: Understanding Animal Reproduction Student Learning Objectives: Instruction in this lesson should result in students achieving the following objectives:
Unit B: Understanding Animal Body Systems Lesson 7: Understanding Animal Reproduction Student Learning Objectives: Instruction in this lesson should result in students achieving the following objectives:
Feeding Alpacas. to Enhance Reproduction and. Fleece Quality. RIRDC article 11/111
 Feeding Alpacas to Enhance Reproduction and Fleece Quality RIRDC article 11/111 by D. Blache, J. Vaughan, S.K. Maloney, J.T.B. Milton Summarised by Sarah Busby What the report is about In Australia, alpacas
Feeding Alpacas to Enhance Reproduction and Fleece Quality RIRDC article 11/111 by D. Blache, J. Vaughan, S.K. Maloney, J.T.B. Milton Summarised by Sarah Busby What the report is about In Australia, alpacas
New Assisted Reproductive Techniques for Horses. Dirk K. Vanderwall, DVM, PhD, Dipl. ACT
 New Assisted Reproductive Techniques for Horses Dirk K. Vanderwall, DVM, PhD, Dipl. ACT Northwest Equine Reproduction Laboratory Department of Animal and Veterinary Science Center for Reproductive Biology
New Assisted Reproductive Techniques for Horses Dirk K. Vanderwall, DVM, PhD, Dipl. ACT Northwest Equine Reproduction Laboratory Department of Animal and Veterinary Science Center for Reproductive Biology
Study Regarding Age-Related Morphometric Features of Buffalo Sperm
 Available online at www.sciencedirect.com ScienceDirect Agriculture and Agricultural Science Procedia 6 ( 2015 ) 272 276 ST26733, International Conference "Agriculture for Life, Life for Agriculture" Study
Available online at www.sciencedirect.com ScienceDirect Agriculture and Agricultural Science Procedia 6 ( 2015 ) 272 276 ST26733, International Conference "Agriculture for Life, Life for Agriculture" Study
Chapter 14 Reproduction Review Assignment
 Date: Mark: _/45 Chapter 14 Reproduction Review Assignment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Use the diagram above to answer the next question.
Date: Mark: _/45 Chapter 14 Reproduction Review Assignment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Use the diagram above to answer the next question.
Transforming IVF and Biological Separations Presentation by Executive Chairman, Alison Coutts
 Transforming IVF and Biological Separations Presentation by Executive Chairman, Alison Coutts NuSep (ASX:NSP) key facts No. shares 237,606,002 Market Cap. $7.1 million (@3 cps) No. of shareholders Approx.
Transforming IVF and Biological Separations Presentation by Executive Chairman, Alison Coutts NuSep (ASX:NSP) key facts No. shares 237,606,002 Market Cap. $7.1 million (@3 cps) No. of shareholders Approx.
MULTIPLE CHOICE: match the term(s) or description with the appropriate letter of the structure.
 Chapter 27 Exam Due NLT Thursday, July 31, 2015 Name MULTIPLE CHOICE: match the term(s) or description with the appropriate letter of the structure. Figure 27.1 Using Figure 27.1, match the following:
Chapter 27 Exam Due NLT Thursday, July 31, 2015 Name MULTIPLE CHOICE: match the term(s) or description with the appropriate letter of the structure. Figure 27.1 Using Figure 27.1, match the following:
Web Activity: Simulation Structures of the Female Reproductive System
 differentiate. The epididymis is a coiled tube found along the outer edge of the testis where the sperm mature. 3. Testosterone is a male sex hormone produced in the interstitial cells of the testes. It
differentiate. The epididymis is a coiled tube found along the outer edge of the testis where the sperm mature. 3. Testosterone is a male sex hormone produced in the interstitial cells of the testes. It
Fertility Preservation for Trans Women: Sperm Banking
 Fertility Preservation for Trans Women: Sperm Banking A PUBLICATION OF FAIRFAX CRYOBANK About the Author Michelle Ottey, PhD, HCLD is the Director of Operations for Fairfax Cryobank and Cryogenic Laboratories,
Fertility Preservation for Trans Women: Sperm Banking A PUBLICATION OF FAIRFAX CRYOBANK About the Author Michelle Ottey, PhD, HCLD is the Director of Operations for Fairfax Cryobank and Cryogenic Laboratories,
Examining Breeding Soundness of Beef Bulls
 Examining Breeding Soundness of Beef Bulls A herd bull that will serve a higher percentage of cows during a limited breeding season is essential to a successful cow-calf operation. In many of these operations,
Examining Breeding Soundness of Beef Bulls A herd bull that will serve a higher percentage of cows during a limited breeding season is essential to a successful cow-calf operation. In many of these operations,
o Production of genetically identical offspring from one parent o E.g. - Bacteria Reproduce by binary fission a cell to divide into 2
 Reproduction (IGCSE Biology Syllabus 2016-2018) Asexual Reproduction o Production of genetically identical offspring from one parent o E.g. - Bacteria Reproduce by binary fission a cell to divide into
Reproduction (IGCSE Biology Syllabus 2016-2018) Asexual Reproduction o Production of genetically identical offspring from one parent o E.g. - Bacteria Reproduce by binary fission a cell to divide into
TECH EXTENSION. How low can we go? Finding a practical path to produce more pigs from fewer and better boars.
 TECH EXTENSION How low can we go? Finding a practical path to produce more pigs from fewer and better boars www.fastgenetics.com Artificial insemination (AI) has become the standard breeding method for
TECH EXTENSION How low can we go? Finding a practical path to produce more pigs from fewer and better boars www.fastgenetics.com Artificial insemination (AI) has become the standard breeding method for
VM 744 THERIOGENOLOGY Spring 2013
 VM 744 THERIOGENOLOGY Spring 2013 Credits-3 Coordinator: Patrick M., DVM, PhD, Diplomate ACT Department of Clinical Sciences Office: Equine Reproduction Laboratory Phone: 491-8626 email: pmccue@colostate.edu
VM 744 THERIOGENOLOGY Spring 2013 Credits-3 Coordinator: Patrick M., DVM, PhD, Diplomate ACT Department of Clinical Sciences Office: Equine Reproduction Laboratory Phone: 491-8626 email: pmccue@colostate.edu
Animal Reproductive Systems. Chapter 42
 Animal Reproductive Systems Chapter 42 Impacts, Issues Male or Female? Body or Genes? Body and genes don t always match male or female characteristics also depend on hormones mutations can result in intersex
Animal Reproductive Systems Chapter 42 Impacts, Issues Male or Female? Body or Genes? Body and genes don t always match male or female characteristics also depend on hormones mutations can result in intersex
A report for the Rural Industries Research and Development Corporation. by Dr. David B. Boyle. September 2003
 NEW FOWL POX VACCINE EVALUATION Evaluation of fowl pox (FPV) strains free of reticuloendotheliosis virus as vaccines for use in Australian poultry flocks A report for the Rural Industries Research and
NEW FOWL POX VACCINE EVALUATION Evaluation of fowl pox (FPV) strains free of reticuloendotheliosis virus as vaccines for use in Australian poultry flocks A report for the Rural Industries Research and
Histology of Male Reproductive system (1)
 Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Animal Reproduction Chapter 46. Fission. Budding. Parthenogenesis. Fragmentation 11/27/2017
 Animal Reproduction Chapter 46 Both asexual and sexual reproduction occur in the animal kingdom Sexual reproduction is the creation of an offspring by fusion of a male gamete (sperm) and female gamete
Animal Reproduction Chapter 46 Both asexual and sexual reproduction occur in the animal kingdom Sexual reproduction is the creation of an offspring by fusion of a male gamete (sperm) and female gamete
Why Reproduce? In order to ensure the continuation of the species and the continuation of life in general by producing offspring
 HUMAN REPRODUCTION Why Reproduce? In order to ensure the continuation of the species and the continuation of life in general by producing offspring Asexual vs Sexual Reproduction Remember: Asexual reproduction:
HUMAN REPRODUCTION Why Reproduce? In order to ensure the continuation of the species and the continuation of life in general by producing offspring Asexual vs Sexual Reproduction Remember: Asexual reproduction:
Reproductive Endocrinology. Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007
 Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Reversible Conditions Organising More Information semen analysis Male Infertility at Melbourne IVF Fertility Preservation
 Male Infertility Understanding fertility in men Conceiving a baby depends on a number of factors, including healthy sperm. After a woman s age, this can be the biggest issue. Reproduction, although simple
Male Infertility Understanding fertility in men Conceiving a baby depends on a number of factors, including healthy sperm. After a woman s age, this can be the biggest issue. Reproduction, although simple
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
