ABSTRACT. The purpose of this research was to investigate the role of TGF-β in the luteinizing
|
|
|
- Robert Blankenship
- 5 years ago
- Views:
Transcription
1 ABSTRACT SRIPERUMBUDUR, RAJAGOPAL. The Role of TGF-β in Luteinization in the Pig. (Under the direction of Dr. John E. Gadsby). The purpose of this research was to investigate the role of TGF-β in the luteinizing follicle and to examine the expression and localization of TGF-β and components of its signaling pathway in the peri-ovulatory porcine follicle. It is well known that the LH-surge causes ovulation of follicles and initiates luteinization. There is also evidence from our own studies as well from the literature suggesting that TGF-β may also play a role in luteinization. In the first study we proposed to examine the expression of TGF-β ligands (i.e. TGF-β1, 2 and 3) and components of its signaling pathway {the TGF-β receptors, TβRI and II, the Smad [the vertebrate homologue of Drosophila Mad (Mad =Mothers against decapentaplegic) and the related Caenorhabditis elegans gene Sma] proteins, Smad 2, 3, 4, 6 and 7, and the Smad Anchor for Receptor Activation, (SARA)} in porcine follicles induced to luteinize in vivo with hcg treatment. Pre-pubertal pigs were injected with a half dose of PG-600 (200 I.U. ecg and 100 I.U. hcg) to induce ovarian follicular development and then injected with hcg 3 days later to induce ovulation. Ovaries were collected surgically at 0, 1, 12, 24, 48 and 96 hours following hcg treatment, and used for RNA and protein extraction, and for immunohistochemistry. Semi-quantitative RT-PCR analysis was used to assess mrna expression of components of the TGF-β signaling pathway. Our data revealed that hcg caused up-regulation of mrna expression of TGF-β3 at 12h and TβRII at 96h versus 0h control. However, protein expression in these samples, though mirroring mrna expression for TβRII, was not significantly different compared to the 0h control. Using immunohistochemistry, we also localized TGF-β1 expression to granulosa cells (GC), TGF-
2 β2 to theca cells (TC) in the pre-ovulatory follicle, TGF-β1 and TGF-β2 expression in developing luteal cells (LC) in the post-ovulatory follicle, and TβRI and TβRII expression in GC, TC and LC of the pre- and post-ovulatory follicle. Since hcg, whose actions simulate those of naturally-occurring LH, upregulates some components of the TGF-β signaling pathway and induces luteinization, we suggest that TGF-β signaling may play a role in mediating luteinization that occurs during the normal cycle. In the second study, we proposed to examine the expression of TGF-β ligands (i.e. TGF-β1, 2 and 3) and components of its signaling pathway (the TGF-β receptors, TβRI and II, the Smad proteins, Smad 2, 3, 4, 6, 7 and SARA) in porcine granulosa cells induced to luteinize in vitro with either LH/IGF-1, various doses of TGF-β1 or a combination of the two treatments. Granulosa cells were obtained from slaughterhouse porcine follicles and were placed into culture in M199 containing 10% fetal bovine serum on collagen I coated culture dishes. Granulosa cell cultures were treated with either LH (250 ng/ml) / IGF-I (10 ng/ml), varying doses of TGFβ-1 (10 and 100 ng/ml) or with a combination of the two treatments (LH/IGF-1 + TGF-β1 10 ng/ml or LH/IGF-1 + TGF-β1 100ng/ml). Progesterone levels were measured using radioimmunoassay, and semi-quantitative RT-PCR analysis was used to assess mrna expression of components of the TGF-β signaling pathway in granulosa cells. Our data revealed that the combination treatment of LH/IGF-1 and TGF-β1 (10 ng/ml) increased progesterone production in the granulosa cells, compared to the individual treatments themselves, suggesting a possible synergistic effect of LH and TGF-β1 in luteinization of the cells. It also appeared that LH/IGF-I treatment may upregulate most components of the TGF-β signaling pathway, indicating the possibility that TGF-β may at least in part mediate the luteinizing action of LH/IGF-I. Though we cannot draw definite
3 conclusions because of the limited number of viable cultures, the preliminary results are very interesting, nonetheless. Because TGF-β1 synergizes with LH/IGF-1 in increasing progesterone production and LH/IGF-1 may upregulate components of the TGF-β signaling pathway in luteinizing granulosa cells, we suggest that TGF-β signaling may play a role in mediating the LH-induced luteinization.
4 THE ROLE OF TGF-β IN LUTEINIZATION IN THE PIG By RAJAGOPAL SRIPERUMBUDUR A dissertation submitted to the Graduate Faculty of North Carolina State University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy COMPARATIVE BIOMEDICAL SCIENCES Raleigh, North Carolina 2006 APPROVED BY: Dr. Charlotte E. Farin Dr. William L. Flowers Dr. John E. Gadsby Chair of Advisory Committee Dr. Jorge A. Piedrahita
5 DEDICATION This work is dedicated to my family and friends - my parents, Narasimhan and Vanjula Sriperumbudur, who have encouraged me from my childhood to always follow my dreams and to do what I enjoy doing; to my wife, Priya Narayanan, who has been completely supportive of me and has endured so many sacrifices, and without whose encouragement, I would not have been able to complete my Ph.D.; to my daughter Sumitra, whose birth has brought me immense joy and the motivation to move ahead with my work; to my late brother Madhusudanan Sriperumbudur, who will forever remain a source of inspiration to me; to all my friends who believed in me. Finally, I would be remiss not to acknowledge the countless pigs that made my research possible. ii
6 BIOGRAPHY The author grew up in Chennai (formerly Madras), India. He received his DVM degree in 2000 from the Virginia-Maryland Regional College of Veterinary Medicine, Blacksburg, VA. He started his Ph.D. at the North Carolina State University College of Veterinary Medicine, Raleigh in August In August 2001, he transferred to Dr. Gadsby s lab where he has worked since then. iii
7 ACKNOWLEDGMENTS The author would like to express his heartfelt gratitude to the members of his advisory committee. Dr. John Gadsby, his major advisor, was instrumental in providing guidance and support during the entire course of his study at North Carolina State University. Without his constant guidance and encouragement, this work would not have been possible. The other members of the committee, Drs. Char Farin, William Flowers and Jorge Piedrahita have also been extremely helpful throughout, and have always been available to answer questions or address concerns. The author wishes to thank Drs. Peter Farin, William Flowers, Maria Correa and Mr. Derek Coombs for their help with statistical analysis. The author is also thankful to Ms. Leah Zorrilla, Vickie Hedgepeth, and all faculty members and friends at the NCSU CVM for their friendship and technical support. The author is grateful to Dr. Neil Olson, Research Dean, for providing the CVM stipend during the first four years in his program, and to Dr. Prema Arasu, Director of Graduate Programs, for providing encouragement and advice whenever needed. The author also gratefully thanks Mr. Curtis Powell and staff of the NCSU Swine Education Unit for supplying the animals, staff of the City Packing slaughterhouse for providing me with tissue for my in vitro study, and finally, Ms. Monica Mattmüller of the NCSU CVM Histopathology lab for all her assistance with immunohistochemistry. iv
8 TABLE OF CONTENTS page LIST OF TABLES... viii LIST OF FIGURES...x LITERATURE REVIEW...1 Follicular development...1 Ovulation...5 Luteinization and Corpus luteum...6 Progesterone production and the CL...9 Regulation of the CL formation and function...10 Members of the TGF-β superfamily in the ovary...13 TGF-β members in primordial and primary follicles...14 TGF-β members in primary to antral stage follicles...15 TGF-β members in ovulation and luteinization...17 TGF-β superfamily...20 TGF-β structure and activation...21 Overview of TGF-β signaling...22 TGF-β receptors...22 Mechanisms of transforming growth factor-β receptor activation...23 Structural requirements for transforming growth factor-b receptor activation...24 Smads...25 Structure of Smads...25 Smad signaling...26 v
9 The function of the Smad anchor for receptor activation...27 Summary of literature review...27 References...30 TGF-β ligands, receptors and Smad expression in peri-ovulatory porcine follicles...46 Abstract...47 Introduction...48 Methods and materials...51 Reagents...51 Animals and tissue collection...51 Semi-quantitative analysis of mrna expression patterns of TGF-β ligands, receptors and Smad proteins...52 Western Blotting...54 Immunohistochemistry...55 Statistical analysis...56 Results...57 Discussion...60 References...78 Expression of TGF-β and components of its signaling pathway in cultured porcine granulosa cells: a possible role of TGF-β in luteinization?...81 Summary...81 Introduction...83 vi
10 Methods and materials...84 Granulosa cell isolation and culture...85 Progesterone RIA...86 Semi-quantitative RT-PCR...87 Results...89 Discussion...92 References General conclusions References Appendix vii
11 LIST OF TABLES TGF-β ligands, receptors and Smad expression in peri-ovulatory porcine follicles 1. Primers used for semi-quantitative RT-PCR Summary table of ovarian characteristics at ovariectomy across all animals...67 Expression of TGF-β and components of its signaling pathway in cultured porcine granulosa cells: a possible role of TGF-β in luteinization? 1. Summary of the number of cell cultures attempted, number of cultures for which progesterone values were obtained and the number of cultures used for mrna expression Primers used for semi-q RT-PCR Progesterone values (ng/ml) expressed as means ± SEM from four granulosa cell cultures Raw normalized densities of amplicon bands used to calculate mean densities for graphs in figures 2 and 3, and raw progesterone values (last row) Fold increases in normalized mrna expression over control in granulosa cell cultures Correlation between progesterone values and enzymes involved in progesterone synthesis Multiple correlation between TGF-β components and progesterone values and mrna expression of pro-luteinization enzymes viii
12 Appendix A1. List of genes upregulated by at least 2 fold compared to the 0h control after hcg treatment from cdna expression analysis ix
13 LIST OF FIGURES LITERATURE REVIEW 1. An overview of TGF-β signaling...29 TGF-β ligands, receptors and Smad expression in peri-ovulatory porcine follicles 1. mrna expression patterns of some LH-responsive genes in hcg-treated follicles mrna expression patterns of TGF-β ligands and receptors in hcg-treated follicles mrna expression patterns of Stimulatory Smads, SARA and inhibitory Smads in hcgtreated follicles Example of an ethidium bromide-stained agarose gel of Smad3 amplification products from hcg-treated follicles mrna expression patterns of TGF-β ligands in granulosa and theca compartments of hcg-treated follicles mrna expression patterns of TGF-β receptors in granulosa and theca compartments of hcg-treated follicles Protein expression patterns of TGF-β ligands and receptors in hcg-treated follicles Protein expression patterns of Smad3 and psmad3 in hcg-treated follicles Representative Western blots of TGF-β ligands, receptors, Smad3 and psmad Immunohistochemical localization of TGF-β ligands and receptors in hcg-treated follicles...77 Expression of TGF-β and components of its signaling pathway in cultured porcine granulosa cells: a possible role of TGF-β in luteinization? x
14 1. Effect of treatment on progesterone levels in granulosa cell cultures mrna expression patterns of LH-responsive genes in luteinizing granulosa cells in vitro mrna expression patterns of some TGF-β pathway components and LH-responsive genes in luteinizing granulosa cells in vitro General conclusions 1. Possible cell model for TGF-β augmenting LH in promoting luteinization xi
15 LITERATURE REVIEW The first part of this section takes one through all the stages of folliculogenesis, from the primordial germ cell stage to ovulation and luteinization. The second part discusses the role of TGF-β superfamily members in folliculogenesis and luteinization. Follicular development Development of the follicle begins soon after conception in the female fetus. After the the primordial germ cells migrate to the gonadal ridge, they are called oogonia [1].A layer of mesenchymal cells then surround the oogonia to form oogonial clusters or syncytia [2]. Depending on the species, the oogonia undergo a predetermined number of mitotic divisions until they enter meiosis to give rise to oocytes [1]. This occurs usually around mid to late gestation, depending on the species [1]. Follicle formation starts when the mesenchymal cells surrounding the oogonia transform into flattened granulosa cells [3], to give rise to primordial follicles. In rodents, follicular assembly begins immediately after birth, while in primates and domestic animals, it occurs around mid to late gestation [4]. Meiosis marks the end of replication of female germ cells; thus, all the oocytes that a female will have in her lifetime are set at this stage [2]. After initiation of meiosis, the primary oocyte progresses through leptotene, zygotene and pachytene stages of the first prophase, before arresting in the diplotene/dictyate stage until it receives further signals to resume growth [1]. In the pig, there is evidence that the oogonial to oocyte transformation continues after birth [5] Primordial follicle activation to resume growth is marked by the transformation of the granulosa cells from flattened into cuboidal shape and is also accompanied by granulosa cell proliferation [6]. The primordial to primary follicle transition might take a few days for some follicles, or much longer, up to a few years, for other follicles, depending on the order 1
16 in which they were formed [207]. It is evident from the vast literature available on follicle development that there is a myriad of endocrine and paracrine factors that can signal the follicle to resume development, although how they act singly or together to induce the resumption of follicular growth remains to be worked out. Among these signals are growth factors like insulin and insulin-like growth factors (IGFs), transforming growth factor (TGF)- α, members of the transforming growth factor-beta superfamily such as TGF-β, bone morphogenic proteins (BMPs) inhibin, activin, anti Müllerian hormone (AMH), and growth differentiation factors (GDFs), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), cytokines like tumor necrosis factor-α (TNF-α), and intra-ovarian steroid hormones like estrogen (E 2 ) and progesterone (P 4 ) [3, 6-10]. These signals initiate a multitude of signaling cascades to bring about changes in gene expression, which in turn cause follicle selection, and growth and differentiation of the follicular components. As the primordial follicles resume growth, they leave the pool of resting follicles and become primary follicles; this transition is irreversible, and the follicle is now commited to growth, ending in either atresia or ovulation [3]. The mechanism of selection of certain follicles to proceed to ovulation, while the majority undergoes atresia is poorly understood. However, recent research suggests that local factors produced by the oocyte and the cells surrounding it influence the growth and development of one another (e.g. c-kit/kit ligand) [11]. The oocyte has been shown to be the source of some of the growth factors and cytokines mentioned earlier (e.g. GDF-9, BMP-15) that may play a role in follicle growth and development [9]. The initial follicle growth is thought to be gonadotropin-independent [11, 12] because granulosa cells at this stage do not express LH [13] or FSH [14] receptors. In the hamster, however, there is evidence for the presence of FSH receptors in small primary 2
17 and secondary follicles [15]. The accumulation of mitochondria, and smooth and rough endoplasmic reticulum in the bovine oocyte at the primary follicle stage provides a clue to the increasing energy and synthetic requirements of the oocyte for growth [16] When the follicle develops two or more layers of granulosa cells, it is called a secondary or preantral follicle [17]; at this stage, the granulosa cells proliferate, and the theca cells which arise from interstitial stromal cells, form a layer around the granulosa layer. Also at this stage, oocyte RNA synthesis is first detectable [18]. Also during this stage, the oocyte secretes a glycoprotein structure around it, called the zona pellucida. The granulosa cells form gap junctions with each other and with the oocyte via connexin proteins [19, 20]. These gap junctions enable critical communication between the oocyte and the granulosa cells. Thus, the oocyte can promote the growth and differentiation of the granulosa cells surrounding it, while the granulosa cells can signal the growth and differentiation of the oocyte [9, 21]. The cells surrounding the oocyte continue to proliferate and differentiate into two layers of theca cells, theca interna and externa, which surround the granulosa cells [22]. At this stage, the follicle may become receptive to FSH [23, 24] and LH [23, 24], as indicated by the appearance of their receptors on granulosa and theca cells [25]. Estrogen is also thought to play a role at this stage of follicle development; in the rat, estrogen receptors ERα and ERβ have been found in theca and granulosa cells respectively of primary follicles [26]. Hypophysectomized rats, and mutant mice lacking FSHR, LHR or ERα exhibit follicles arrested at the preantral stage [27]. Thus, it is clear that gonadotropins and estrogen are necessary for normal follicular growth beyond this stage, and especially the final, rapid phase of preovulatory follicle development. Environmental factors such as nutrition also influence follicle development at this stage [28]. In the pig, it is known that starting at the preantral 3
18 stage, when theca interna cells appear [29], the granulosa cells with their FSH and estradiol receptors also proliferate. Under the influence of FSH and estradiol, theca interna cells acquire LH receptors [30]. Based on rodent models, it is clear that theca cells, under LH influence, convert cholesterol to androstenedione, which then diffuses across the basement membrane to granulosa cells [31]. The granulosa cells possess P450aromatase, used in converting the androstenedione to estradiol [30]. The difference between the rodent and the pig is that the porcine theca interna cells also possess aromatase activity and hence are capable of producing estradiol [32]. The rapid increase in steroid biosynthesis is thought to result in the increase and accumulation of follicular fluid, forming the antral cavity, and consequently, the follicle is now called an antral follicle. Antral fluid production increases with increasing follicular vascularization and permeability of blood vessels [17]. The factors responsible for the formation of the antrum are not clear, but in vitro studies in rodent, porcine and bovine follicles suggest that FSH [33, 34], LH [35], activin [36], Kit ligand [37], EGF [38] may all be involved. The antral follicle has a layer of granulosa cells closely associated with the oocyte (together called the cumulus-oocyte complex or COC), with the antral fluid separating the COC from the other granulosa cells (called mural granulosa cells) along the inside of the follicular wall [22]. The oocyte acquires the ability to resume nuclear maturation, and the competency to undergo fertilization and cell cleavage [17]. As the antral follicles continue to develop, one or more of these follicles, depending on the species and breed of animal, is (are) selected to ovulate and these are called dominant follicles. Again, factors responsible for this selection step are not clearly understood, but it has been shown that dominant follicles have increased estradiol and inhibin activity [39], 4
19 which, via a negative feedback on the hypothalamo-pituitary axis, control FSH secretion (FSH levels decline to levels below what is necessary for further follicular selection) and thus inhibit the growth of subordinate follicles [40, 41]. At the same time, granulosa cells acquire LH receptors that are necessary for continued development [42]. The dominant follicle(s) with LH receptors continue their growth and development, while the subordinate follicles undergo atresia [42, 43]. Whereas FSH promotes rapid growth of GCs in the preantral follicles by the regulation and/or induction of genes involved in cell cycle control, LH promotes the exit from cell cycle and terminal differentiation of the GCs in the mature follicle, and induces genes related to ovulation and luteinization [25]. A very interesting fact is that both of these hormones act via seemingly identical camp signaling pathways, and through structurally related receptors, but yet produce completely different outcomes. Ovulation The process of mature ovarian follicle rupture to release a fertilizable oocyte is termed ovulation [44]. The main stimulus for ovulation is a surge in LH, which brings about change in expression of multiple genes, some leading to follicle rupture and oocyte release and some leading to subsequent luteinization of the follicle remnant [44]. The timing of the expression of these genes which include A Disintegrin-like And Metalloprotease domain with ThromboSpondin type I (ADAMTS) members, Metallothionein (MT)-I, epidermal growth factor (EGF)-like factors and CGMP-dependent protein kinase II, is absolutely crucial; if the luteinization genes are turned on before the ovulation genes, then the granulosa and theca cells start to luteinize before the oocyte can be released, thus resulting in a failure of ovulation ([45-50]. The genes responsible for ovulation are thought to bring about (i) locally increased flow of blood to the follicle, causing inflammatory cells like leukocytes and 5
20 macrophages to invade the follicle [51]; (ii) increased expression of proteolytic enzymes such as ADAMTS-1 and cathepsin L [44], plasminogen activator [52], the matrix metalloproteinases MMP2, MMP9 and MMP 13 [53], all of which digest the extracellular matrix of the follicle, thus weakening the follicular wall and facilitating rupture [54, 55]; and (iii) activation of adenylyl cyclase/protein kinase A pathways, which in turn cause the expression of genes involved in ovulation, like progesterone receptor, cyclooxygenase (COX)-2, the CAAT enhancer binding protein, prostaglandin endoperoxidase synthase-2 etc. [44, 53]; the targets of progesterone receptor, in turn, include plasminogen activators, matrix metalloproteinases (MMPs) and their inhibitors (tissue inhibitors of MMPs; TIMPs) [53, 56-60]), all of which help to degrade the extracellular matrix around the follicle, while the TIMPs are thought to protect other growing follicles from the degrading actions of the MMPs [52]. With the help of all these factors, the COC is released from the follicle. Luteinization and the corpus luteum The corpus luteum (CL), an endocrine gland, develops from the follicle tissue remnants after ovulation, by a process called luteinization. The CL is the source of progesterone, the steroid hormone responsible for creating a uterine environment conducive to maintaining pregnancy. If pregnancy does not occur, the CL undergoes a process of regression, at which time progesterone production declines (functional luteolysis), and ultimately the CL itself degenerates (structural luteolysis), a process mediated by prostaglandin F 2α [61]. Luteolysis brings an end to the lifespan of the CL, and initiates the process of follicular maturation, ovulation and formation of a new CL of the next cycle. In pregnant animals, the CL is maintained throughout the length of the gestation and is the major source of progesterone in several species [61]. In the pig, the CL is required for the 6
21 entire duration of pregnancy to ensure progesterone availability and pregnancy maintenance. The role of progesterone for maintenance of pregnancy is underlined by the fact that the hormone, by itself, can support gestation in ovariectomized gilts [62]. In the pig, ovulation occurs hours after the LH surge [63]. In sows, this time of ovulation after the LH surge is extremely variable, reported anywhere from 26 to 34 hours [64]. After ovulation, the follicle undergoes hyperemic and hypertrophic changes [62, 65]; the granulosa cell layer is thrown into extensive folds around the follicular antrum; there is breakdown of the basement membrane and the theca cells are carried by the vascular and connective tissue into the core of the developing CL along these folds. The development of vascular supply proceeds in centripetal as well as lateral directions [66]. The hypertrophic changes in the granulosa cells and the proliferation of endothelial cells in the CL is phenomenally rapid, even exceeding similar changes in some of the most malignant tumors [65]. All these changes transform the fluid-filled follicle into a solid mass of cells that comprise the CL, within a few days (4-6) days after ovulation. Analyses of the cell types in the CL in the guinea pig [67], sheep [68, 69] and the cow [70] have yielded information about the composition of the CL during various stages of its development. Based on the classification described by others [61, 69, 70], it is accepted that the cell types of the CL can be broadly divided into steroidogenic and non-steroidogenic components. The steroidogenic cells can be further subdivided into small luteal cells (SLC) and large luteal cells (LLC). Endothelial cells, fibroblasts, pericytes and macrophages form the non steroidogenic component [68, 70]. It is generally believed that in the pig, the theca cells are the precursors to the SLC, whereas the granulosa cells give rise to the LLC [62, 71]. Studies in the sheep and cow indicate that the SLCs may transform into the LLCs later during the luteal phase [61]. 7
22 The LLCs are characterized by their larger size (>26-31 μm in sheep) and round shape, and by the presence of abundant rough and smooth endoplasmic reticulum, numerous mitochondria, lipid droplets and secretory granules in the cytoplasm, all characteristics of a steroidogenically active cell type [70]. Although the LLCs make up about a third of the CL volume, they account for over 80 % of progesterone secretion at peak production (mid luteal phase) [68, 70]. The SLC constitute around 20 % of the CL volume; together, the LLCs and the SLCs roughly make up about 35 % of the total cell number in the CL [68, 72]. During CL development, the LLCs increase in diameter by 3 to 4 fold, though the total cell number of LLCs remains fairly constant [68]. The LLCs, but not the SLCs, also possess PGF 2α receptors, and hence appear to mediate the luteolytic actions of PGF 2α [73] The SLCs are about a third of the size of the LLCs in domestic ruminants, and are irregularly shaped, and have a lot of smooth endoplasmic reticulum, lipid droplets, a lot less rough endoplasmic reticulum and secretory granules compared to the LLCs [68, 70]. The SLCs maintain a constant size throughout the luteal phase, but increase in cell number by up to five fold [68]. The SLCs, but not the LLCs, possess LH receptors; therefore, they are able to respond to LH and increase their otherwise low basal P4 production [74]. In the pig, it is thought that endothelial cells, usually found lining the capillary lumina, migrate from capillaries into the developing CL in apparent response to signals like VEGF produced by granulosa and theca cells [75]. But the factors that signal the other nonsteroidogenic cell types to migrate into the developing CL are unknown. The numbers of endothelial cells and pericytes increase almost 4-fold during CL development, and they also contribute to around 50 % of the total cell number, and around % of the total luteal volume [68, 70]. 8
23 Luteinization is also generally associated with the loss of cytochrome P450aromatase expression in most species, and with the onset of synthesis of progesterone in significant quantities [62]. In the pig, however, aromatase activity is never completely lost and the porcine CL continues to synthesize small quantities of estradiol [76-78]. Steroid production switches from predominantly estradiol production by the follicle, to predominantly progesterone production by the CL. There is also a concomitant upregulation in the amounts of other critical components involved in progesterone synthetic pathway low density lipoprotein (LDL) receptor, the cholesterol side chain cleavage enzyme P450scc, and 3β- HSD, another enzyme involved in progesterone synthesis, as well as the cholesterol transport protein StAR (Steroidogenic Acute Regulatory protein) [79, 80]. Progesterone production by the CL Cholesterol, in the form of low-density and high-density lipoproteins (LDL and HDL), is used by the CL as the substrate for steroid production [81]. Of the two kinds of lipoproteins, LDL is the major substrate used by the porcine CL [80]. Steroidogenic cells take up LDL or HDL when it binds to its receptor or biding protein on the cell membrane [82]. After being transported to the cytosol, the LDL or HDL can be broken down to yield free cholesterol to be used in the next step of steroid synthesis, incorporated into the lipid bilayered cell membrane or be stored in the cytosol as lipid droplets [83]. The cholesterol to be used for steroid synthesis is transported to the outer membrane of the mitochondria with the help of sterol carrier protein 2 (SCP2)[80]. Another protein called the steroidogenic acute regulatory protein, StAR, transports the cholesterol from the outer to the inner mitochondrial membrane, and this transport step is considered to be a rate-limiting step in progesterone synthesis [84]. The enzyme P450scc, present in the 9
24 inner mitochondrial membrane, cleaves the cholesterol side chain to yield pregnenolone, which is then transported to the smooth endoplasmic reticulum; here, it is converted to progesterone by the enzyme 3β-HSD [61]. In the pig, as the CL continues to develop, progesterone production increases exponentially, peaking between days 8-12 after ovulation [63]. Progesterone levels decrease thereafter, and by day 16, they are reduced to approximately one twelfth of that measured on day 8 as CL undergoes regression [63]. Regulation of CL formation and function The initial stimulus for luteinization is believed to be the LH surge, and LH initiates a number of downstream events that result in successful ovulation and the formation and maintenance of the CL. There have been a number of models employed to study the process of luteinization, both in-vivo and in-vitro [85]. Although these models have increased our understanding of the process of luteinization, the regulatory mechanisms inducing luteinization still remain unclear. It is clear, however, that the process begins before ovulation, at the cellular level [86]. At the genetic level, a number of genes have been identified, mostly from rodent studies [44, 45, 47, 48, 86-91], and a recent study in primates [92], as being involved in luteinization. Based on these studies, the genes can be classified into major classes such as (i) genes involved in steroidogenesis P450scc, StAR, LDLr, 3β-HSD, P450aromatase, 17β-HSD etc. The genes involved in progesterone synthesis such as P450scc, StAR, LDLr, and 3β-HSD and estrogen degradation such as 17β- HSD are upregulated, and the ones involved in estrogen synthesis such as P450aromatase are downregulated, as luteinization proceeds [80, 88, 93, 94]. 10
25 (ii) genes involved in extra cellular matrix modeling ADAMTS-1, Metallothionein (MT)-1, Tissue Inhibitor of Metallo Proteinase (TIMP-1) etc. ADAMTS-1, a metalloproteinase, is upregulated around ovulation and early luteinization, possibly contributing to the rupture of the follicle and tissue remodeling that transforms the follicle into the CL; TIMP-1, an inhibitor of metalloproteinases like ADAMTS-1, and MT-1, are both upregulated after ovulation and possibly help to moderate the degradative actions of metalloproteinases, mitigate the local inflammatory reaction caused by ovulation, help in healing and promote angiogenesis [45, 90] (iii) genes involved in inflammatory processes and angiogenesis: for example, there is evidence that the expression of the enzyme cycloxygenase-2 (COX-2) is induced by the LH surge [95]. Along with lipoxygenase enzymes, COX-2, catalyzes the arachidonic acid pathway, the interruption of which has been shown to reduce the rate of ovulation and luteinization [96, 97]. Message levels of VEGF, a potent angiogenic factor, has been shown to remain constant during the luteal phase and decline only during luteal regression in the mare [98]. In the porcine CL, VEGF receptor 1 (VEGFR-1) mrna levels increase from low levels after ovulation, to highest levels in mid to late luteal phase [99]. (iv) genes that help in maintaining CL function: during the luteal phase, insulin and IGF-I, which are present in the circulating blood, are believed to help in the luteinization process and the support of the CL [62]. In particular, increased IGF- 1 expression/blood levels, IGF-type I receptor and the steroidogenic response to 11
26 IGF-1, correlates with the early stages of CL development (days 4-10), strongly suggesting a role of IGF-1 in this process in the pig [ ]. (v) genes involved in cell cycle regulation: luteinization marks the exit of granulosa and theca cells from the cell cycle [62]. In this respect, luteinization can be defined as the terminal differentiation of the follicular cells and marks the transition from a proliferative to a differentiated state. The differentiation of the follicle into CL is also marked by the phenomenal rate of tissue growth and cellular proliferation [61]. The proliferation is evident from mitotic markers such as the proliferating cell nuclear antigen (PCNA) [62] during early CL development, but the labeling index gradually decreases and eventually is undetectable in the LLC over the lifespan of the CL [105]. Thus, it is thought that terminal differentiation occurs in the porcine CL, with increasing restriction in the proliferating capacity over time. Recent evidence [53, 106] shows that in the early CL in rodents, mitosis is initiated by members of the retinoblastoma gene family such as prb, p107 and p130 [107] but in the late CL, these proteins are inactivated, thereby inactivating positive cell cycle regulators such as cyclins D1, D2 and E. Another factor known to be involved in regulation of the cell cycle is p27 Kip1, a member of the family of cyclin-dependent kinases. It is an inhibitor of the cell cycle progression and in knockout studies in mice, it has been shown that p27 levels decrease after the ovulatory surge of LH, thereby preventing granulosa cells from proliferating and facilitate their differentiation into luteal cells [108] (vi) genes that regulate the transcription of other genes: gene transcription plays an important role in CL formation [62]. This process involves chromatin 12
27 modification, coactivator recruitment, and synthesis and activation of transcription factors. It is well known that the LH surge increases camp levels, which in turn activate the protein kinase A pathway and phosphorylation of the camp response binding protein (CREB) [106]. Among other genes, the ones for P450scc and StAR transcription, are activated by CREB. The transcription of several genes is also stimulated by VEGF, including its own receptors [109]. Members of the TGF-β superfamily in the ovary Members of the TGF-β superfamily are involved in every stage of follicular development, ovulation and luteinization [110, 111]. Because of their pleiotropic roles in these processes, they are described in this section separately. These members include bone morphogenic proteins (BMPs), TGF-βs, growth differentiation factors (GDFs), anti- Mullerian hormone (AMH), activins, inhibin, etc. Most data on the role of TGF-β family members in the ovary come from studies in rodent species, but recently, a number of studies point to their role as intraovarian factors in other species such as the sheep, cat, cow, primates etc. A review of the existing literature shows that TGF-β superfamily ligands and components of their signaling pathways are expressed both by oocytes and granulosa and theca cells, and that this varies not only between species, but also with the stage of follicle, making the task of determining the precise role of each member extremely difficult. But the clear message that emerges from all of this evidence is that these proteins play key roles in all aspects of follicle development, from the development of the primordial follicle to formation of the CL. They are involved in granulosa and theca cell proliferation and atresia, steroidogenesis, gonadotropin receptor expression, oocyte maturation, ovulation and 13
28 luteinization (as reviewed in [111]). The following few sections illustrate the patterns of stage-dependent expression of these proteins during folliculogenesis. TGF-β members in primordial and primary follicles In rats, it has been shown that intra bursal administration of BMP-7 decreases the number of primordial follicles, while increasing the number of follicles from later stages such as primary, secondary and antral follicles [112]. In another rat study [113], BMP-4 produced similar results in a whole ovary-organ culture system; primary follicles increased while primordial follicles decreased. In the same study, it was also found that when ovaries were exposed to a neutralizing antibody to BMP-4, they were much smaller than controls, with accompanying changes such as progressive loss of oocytes and primordial follicles and also increased cellular apoptosis, eventually resulting in loss of ovarian tissue morphology. Other TGF-β superfamily members have been shown to be expressed specifically in oocytes in the primordial and primary stage follicles. In rodents and ruminants, GDF-9, BMP-15 and BMP-6 have been identified in oocytes [ ]. The receptors for these ligands have been identified in the pre-granulosa and granulosa cells, indicating that these cells are targets of signaling by these ligands. Mice null for gdf-9 gene are infertile and have follicles arrested at the primary stage [119, 120]. In another study [121], GDF-9 treatment of rat follicles in vitro caused progression of follicle growth from early to late primary stage. Sheep with homozygous mutations in the bmp-15 or gdf-9 genes are infertile [122, 123]. It has been shown that AMH inhibits the initiation of primordial follicle growth in the mouse, and is expressed by granulosa cells of primary stage follicles onward [124]. In another study, mice with targeted deletion of the amh gene exhibited abnormally increased rate of primordial follicle recruitment [125]. These findings support the idea that AMH 14
29 secreted by growing primary stage follicles and all the way up to pre-ovulatory stage follicles suppress the premature recruitment of primordial follicles. TGF-β members in primary to antral stage follicles Several members of the TGF-β superfamily are thought to be involved in the growth of primary follicles to secondary and antral follicles. In vitro studies in rodents [121, ] have shown that exposing ovaries to GDF-9 promotes primary follicle progression. In other studies, in mice null for GDF-9 [119], and in sheep with homozygous mutations in the gdf-9 gene [129], follicle development did not proceed beyond the primary follicle stage. Sheep with homozygous mutations in the bmp-15 gene are also completely infertile with follicle development not proceeding beyond the primordial follicle stage [129, 130]. Thus, GDF-9 and BMP-15 seem to promote transition of primary follicles to later stages. In the rat, thecal cells from the primary stage onward express BMP-4 and BMP-7 [131]. Taken together with the study mentioned above, where intrabursal administration of BMP-7 caused an increase in the transition of primordial to primary stage follicles and up, these data suggest that thecal cell-derived BMP-7 likely causes growth of granulosa cells. As the follicle reaches the antral stage, more BMP types are expressed in addition to the ones mentioned above, and the expression varies within different compartments of the follicle: it has been shown from studies in the rodent [117, 131], human [132], sheep [133] and cow [134], that BMP-6, BMP-15 and GDF-9 are expressed in the oocyte, BMP-2, BMP-5 and BMP-6 are expressed by granulosa cells, and BMP-2, BMP-3b, BMP-4 and BMP-7 are expressed by theca cells. Rat studies have shown that BMP-4, BMP-6, BMP-7, BMP-15 and GDF-9 attenuate the effects of FSH on granulosa cells from antral follicles through unknown mechanisms [112, 135, 136]. In sheep [123], GDF-9 and BMP-15, alone and in combination, 15
30 suppressed FSH-stimulated progesterone production and caused granulosa cells to proliferate. In the pig, BMP-2 reduced FSH-stimulated progesterone production by granulosa cells [137]. In the cow, BMP-4, BMP-6 and BMP-7 also suppressed basal as well as LHinduced androgen production by theca cells [138] Activin βa and βb subunits have also been detected in primary stage follicles onward in many species including human [139, 140]. Though the exact role of activin in follicle development is still unclear, there is some evidence that it may promote granulosa cell proliferation and differentiation (as reviewed in [141]). In the antral follicle however, activin A has been shown to accelerate oocyte maturation and improve oocyte developmental competence [142, 143]. On the other hand, AMH is thought to have a negative effect on primordial follicle recruitment, as seen earlier. It is also known that AMH inhibits FSHdependent growth of late preantral follicles in cultured mouse ovaries [144]. Message and protein from TGF-β ligands (TGF-β1, TGF-β2, TGF-β3) and receptors (type-i and type-ii) have been detected first at the preantral follicle stage of several species, including the rat [145], cow [146], sheep [147] and human [148], but there is species to species as well as spatio-temporal expression variation and the role of these proteins is still unclear. In the antral follicle of the rat [149], TGF-β and in the human [150], TGF-β1 and 2 have been shown to be produced by both theca and granulosa cells. In sheep, cows and pigs [11], TGF-β1 is mainly produced by thecal cells of antral follicles. In the rat study, TGF-β (the specific ligand unknown) was shown to be expressed in theca cells and exhibited proliferative and cytodifferentiative actions on granulosa cells [151]. In another rat study, TGF-β was shown to increase FSH receptor mrna and protein [152]. In pig studies, it was established that TGF-β is produced by thecal cells [153] and inhibits proliferation of 16
31 granulosa cells, in contrast to the rat study [154, 155]. The actions of TGF-β on granulosa cells has been further substantiated by the discovery of TGF-β receptors on porcine granulosa cells [156]. More evidence that pig granulosa cells may not produce TGF-β comes the studies of Mulheron and coworkers [157], who showed that TGF-β2 mrna was not expressed by these cells. TGF-β members in ovulation and luteinization In most species, inhibin/activin subunit expression is down-regulated after ovulation and during CL formation [111]. Follicular expression of BMP-2, BMP-3b, BMP-4, BMP-6, BMP-7, BMP-15 and GDF-9 is also downregulated in the rat after ovulation [131, 135]. It is thought that these factors inhibit luteinization in the follicle until ovulation has occurred. Mouse luteal cells express both TGF-β1 and TGF-β2 [158], and so does the rat luteal macrophage [159]. In another rat study, prolactin, a progesterone production enhancing peptide, and TGF-β had similar effects on cultured luteal cells; they both suppressed 20α- HSD, whose activity decreases progesterone [160]. In this study, it was shown that prolactin also stimulates TGF-β2 expression in luteal macrophages. In another study, human granulosa-lutein cells treated with TGF-β1 showed decreased apoptosis [161]. Taken together, it appears that TGF-β, in concert with prolactin, supports CL function by increasing progesterone and decreasing apoptosis. Although there is evidence of expression of TGF-β ligands and its signaling pathway components during follicular development, there is very little information on the exact role of these proteins on ovulation and luteinization. The following studies suggest the possible role of TGF-β in ovulation and luteinization: 17
32 (i) When immature mice were sequentially treated with FSH for 3 consecutive days and hcg on the fourth day[162], it was shown that the receptors TβRI and TβRII were immunolocalized to both theca and granulosa cells, the oocyte stained for TβRI only while Smads were present in all cell types, with Smad 4 staining the strongest. Protein and mrna expression analysis showed that TGF-β2, TβRI, Smad 2 and Smad 4 were upregulated, whereas that of Smad 6 was downregulated, in response to the gonadotropins (FSH and LH). This study thus shows that components of the TGF-β pathway are upregulated in the periovulatory follicle in response to LH and FSH. We used a similar model for our study with pre-pubertal pigs treated sequentially with gonadotropins (P.G.600, a combination of hcg and ecg, followed by hcg 3 days later). Our findings appear in the next chapter. (ii) In the marmoset, TGF-β1 and TβRII were co-immunolocalized with the luteinization marker 3β-HSD in granulosa and theca cells of periovulatory follicles; on the other hand, the expression of TGF-β1 and TβRII was absent in atretic follicles [163]. In another marmoset study, when large, non-luteinized follicles were cultured and induced to luteinize with treatments known to induce luteinization, such as LH, TGF-β and camp, increased expression of TβRII was observed, along with other expected changes such as breakdown of basement membrane of the follicle, increased expression of 3β-HSD, and decreased expression of connexin-43 (an ovarian gap junction protein that decreases during luteinization) and TβRII [164]. The consistent, increased expression of TβRII in luteinizing cells only prompted the authors to start using TβRII as a marker of luteinization in subsequent studies. (iii) In a follow up study to the studies described in (ii) above, [165], the authors monitored the effects of Tumor Necrosis Factor (TNF)-α (which is known to have an 18
33 inhibitory effect on luteinization), on porcine granulosa cells in vitro and monitored the cells for luteinization. TNF-α downregulated TβRII along with other luteinization-inhibition parameters such as decreased progesterone receptor expression, and influenced the balance between proliferation and apoptosis. (iv) In the chicken, TGF-β1, on its own or in combination with FSH, increased the expression of FSH receptor and LH receptor in undifferentiated granulosa cells; the increase in LH receptor sensitizes the granulosa cells to LH action by increasing P450scc and StAR expression, thus increasing the cells ability to produce more progesterone [166]. (v) The presence of TGF-β1 and 2 in murine luteal cells [158] and rat luteal macrophages [159] has been reported. A few studies have also indicated the possible role of TGF-β in the CL: Prolactin, a luteotropic hormone, and TGF-β increased progesterone production in cultured rat luteal cells by suppressing 20α-HSD expression, and in rat macrophage cells, prolactin stimulated TGF-β expression [160]. Also, in the same study, when the luteal cells were treated with an anti-tgf-β antibody, the suppressive action of prolactin on 20α-HSD was decreased, thus indicating that the luteotropic actions of prolactin may partly be mediated by TGF-β. (vi) More evidence about the role of TGF-β in CL formation and maintenance comes from two studies in the rhesus monkey. In the first study, when CLs at various stages of pregnancy were examined for the presence of StAR, TGF-β1 and TβRII mrna and protein, it was found that their expression progressively increased during early pregnancy, when the CL was being formed and was functional, while in the regressing CL, their expression decreased [167]. Also, in this study, Interferon (IFN)-γ, a luteolytic cytokine decreased the expression of all three luteotropic proteins mentioned above. In a follow-up study, CL 19
34 development was induced in the rhesus monkey using ecg/hcg treatment, and the expression of TGF-β1, TβRI, TβRII and StAR was examined in CLs at various stages of development using immunohistochemistry and in-situ hybridization [168]. It was found that their expression increased to reach peak levels when the CLs were functional, and decreased when the CL regressed, and IFN-γ reduced their expression. These studies strongly suggest that TGF-β and its receptors may play a role in CL formation and maintenance of its function. All of the above data support the view that TGF-β working via TβRI and TβRII plays a role in maintaining luteal function in these species. In the sheep however, a study shows that TGF-β1 and TGF-β2 inhibit progesterone production in cultured granulosa cells [147], suggesting that TGF-β may be suppressing luteinization in this species. Before describing our studies to investigate the role of TGF-β in luteinization in the pig, a review of TGF-β and its signaling pathways is provided below. TGF-β superfamily Members of the transforming growth factor (TGF)-β superfamily are important regulators of cellular processes in many cell types [169]. The members of this superfamily are structurally and functionally similar, and influence such crucial processes as cell growth and differentiation, early embryonic development, extracellular matrix deposition, immunoregulation, cell motility and apoptosis [170]. Some of the prominent members of the superfamily are TGF-β1-3, bone morphogenic proteins (BMPs), growth and differentiation factor (GDF), Müllerian inhibiting substance (MIS), activins and inhibins. 20
35 For the purposes of describing the signal transduction mechanisms of members of the TGF-β superfamily, the prototype member, TGF-β, will be considered here, especially as it is the focus of the studies described in this thesis. TGF-β structure and activation TGF-β has three structurally similar isoforms, TGF-β1-3, which are encoded by three distinct genes in mammals [171]. They all have similar bioactivity in vitro [169], with TGFβ1 being the most prevalent isoform [172]. When initially synthesized, the precursor TGF-β protein is large, consisting of around amino acids [169]. The precursor protein is comprised of three domains: 1) an N-terminal signal domain called the latency associated peptide (LAP, 249 amino acids), 2) a signal peptide (SP, 29 amino acids) and 3) a C-terminal domain (112 amino acids) [173]. The C-terminal fragment is small and forms the mature portion of the protein, which is involved in triggering intracellular signal transduction. The signal domain functions to target the precursor to the secretory pathways, while the prodomain is thought to help in folding, dimerization and regulation of the biological activity of the C-terminal fragment. The mature TGF-β molecule usually has 7-9 cysteine residues, and exists as a homodimer formed by disulfide bonds [174]. The TGF-β precursor is thought to be activated through different mechanisms [173, 175], as described below. (i) The LAP can be cleaved by proteases, or (ii) a conformational change in the LAP can be induced by interaction with integrins or (iii) the non-covalent bonds between the LAP and the mature peptide can be broken [173]. Once the mature peptide is released, it can interact with its receptor to induce signaling. 21
36 Overview of TGF-β signaling Members of the TGF-β superfamily use common mechanisms to signal to the nucleus [170]. They bind to membrane receptors possessing a cytoplasmic serine/threonine kinase domain. Once the ligand binds to its receptor, the ligand-receptor complex phosphorylates members of the Smad (the vertebrate homologue of Drosophila Mad (Mad =Mothers against decapentaplegic ) and the related Caenorhabditis elegans gene Sma) or MAPK (Mitogen Activate Protein Kinase) protein families. These protein families can in turn translocate to the nucleus themselves, or phosphorylate downstream targets which can move to the nucleus and control target gene expression [169, 170]. There is now evidence to suggest that the Smad and the MAPK pathways intersect [169]. A simplified schematic of TGF-β signaling is given in figure 1. The components of the TGF-β signaling pathway are discussed in detail in the following paragraphs. TGF-β receptors Signaling by TGF β is initiated by its binding to its receptor complex. All members of the TGF-β superfamily transduce their signals through two serine/threonine transmembrane receptors, called Type I and Type II receptors and their downstream effectors, the Smad proteins [173, 176]. To date, five type II receptors and seven type I receptors have been discovered in mammals, each binding to different ligands [177]. The type II receptor TGF β RII (TβRII) binds only to TGF β isoforms, with a higher affinity for TGF β1 and TGF β3, and a lower affinity for TGF β2. The kinase domain of the type II receptor is the most conserved domain [177]. Of the type I receptors, ALK5, ALK1 and ALK2 can transmit TGF-β signaling, but ALK5 (also known as TβRI) is the major type I receptor and like TβRII, is a single 22
37 transmembrane serine/threonine kinase [177]. The receptors TβRI and TβRII receptors are structurally similar, although TβRI does have a shorter extracellular domain, a much shorter C-terminal tail, and a highly conserved glycine and serine (GS)-rich region immediately preceding the kinase domain. The SGSGLP amino acid sequence is considered to be a signature of TβRI. The phosphorylation of serines and threonines in the GS domain by TβRII is required for the activation of TβRI and TGF β signaling [177]. There are also type III or accessory receptors for TGF-β signaling, and include betaglycan and endoglin [177]. The biological functions of both betaglycan and endoglin are not clear. It was thought that betaglycan may function to present the TGF βs, especially TGF β2, to TβRII [178]. More recently, it has been shown that betaglycan is responsible for endocardial cell transformation (Brown et al., 1999). In addition, some cell lines can secrete soluble betaglycan, corresponding to the extracellular domain of betaglycan, which can inhibit the binding of TGF β to the TβRI-TβRII complex [179]. Supporting evidence comes from studies showing that this soluble betaglycan can inhibit tumorigenesis and metastasis of MDA-MB-231 human breast cancer cells by reducing the activity of TGF β1 and TGF β2 secreted by these cells [180]. Mechanisms of transforming growth factor-β receptor activation All Type I and Type II TGF β receptors exist as homodimers in the absence of ligand [177, 181]. For TGF β receptors, TGF β binds poorly TβRI to alone [182]; TGF β must first bind to the TβRII homodimer, which then recruits TβRI to form a heterotetrameric complex [183, 184]. TβRII is constitutively hyperphosphorylated at several serine residues [183, 185]. The formation of TGF β receptor heterotetrameric complex results in the 23
38 phosphorylation of serine and threonine residues in the TβRI GS domain by TβRII [183]. The phosphorylated TβRI then becomes activated, and signals are transduced to cytoplasmic targets [186, 187]. Since TGF β2 has only low affinity for TβRII, the binding of TGF β2 to Type III receptors can facilitate the binding of TGF β2 to TβRII, which, in turn, can recruit TβRI to form an active receptor complex [188, 189]. However, TGF β2 can also directly bind to TGF β receptor complexes in the absence of RIII in some cell types [ ]. Structural requirements for transforming growth factor-β receptor activation Specific domains in TβRI and TβRII are important for TGF β signaling [170]. The extracellular domains of both receptors are required for ligand binding [193, 194]. The cytoplasmic domains of both TβRI and TβRII are required for the dimerization of the receptors, which is essential for TGF β signaling [187, 194, 195]. In addition, the kinase domain of TβRII is required for TβRII to phosphorylate TβRI, while the kinase domain of TβRI is required for TβRI to phosphorylate downstream targets [183, 194, 196, 197]. It is also possible that TβRII can directly phosphorylate other downstream targets. The glycine and serine-rich GS domain is essential for the activation of TβRI by TβRII [183, 196] Some of the serine and threonine residues in the Type I and Type II receptors are important for TGF β receptor signaling as well [177]. TβRII primarily autophosphorylates threonines in vitro, but serines in vivo [183]. TβRII autophosphorylates at least three serine residues: Ser409 and Ser416 in the protein kinase domain and Ser213 in the juxtamembrane domain [187]. Phosphorylation of Ser213 and Ser409 is essential for TβRII kinase signaling, while phosphorylation of Ser416 has been shown to inhibit TβRII function [187]. 24
39 Phosphorylation of TβRII has also been observed on Ser551 and Ser553 of the C- terminus and Ser225, Ser228, and Ser229 in the juxtamembrane domain [183, 198]. Since C- terminal tail deletion did not affect TβRII actions [194, 197], the phosphorylation of the C- terminal tail is not thought to play a critical role in TβRII signaling. However, it is still not known whether the phosphorylation of the serine residues in the juxtamembrane domain plays a role in TβRII signaling. In vitro, TβRII can autophosphorylate on Tyr259 in the kinase domain, Tyr336 in subdomain V, and Try424 in subdomain VIII [199]. The role of TβRII tyrosine autophosphorylation in TGF β signaling is not clear. TβRII also phosphorylates TβRI at four sites in the GS domain (Thr186, Ser187, Ser189, and Ser191) and at Ser165 in the juxtamembrane domain. The phosphorylation of Ser165 was thought to be the direct effect of TβRII, and may modulate the signaling specificity of TGF β receptors [198]. Smads Smads can be divided into three groups based upon their structure and function. The first group, referred to as receptor-activated Smads (RSmads), includes Smads 1-3, 5, and 8. The RSmads specific for TGF-β signaling are Smad2 and Smad3. The second group consists of the single member Smad 4, and is referred to as common-partner Smad (Co-Smad). The last group is referred to as the inhibitory Smads, including Smads 6 and 7 [170]. Structure of Smads Smads share extensive sequence homology in two distinct regions, an N-terminal domain (called MH1 for MAD-homology 1) and a C-terminal domain (called MH2), while the proline-rich linker regions are poorly conserved. The MH1 domain is highly conserved among RSmads and Co-Smad, but not in the inhibitory Smads. The RSmads, but not the Co- 25
40 Smad, also have a characteristic serine-rich SXS motif in their MH2 domain. The phosphorylation of the two serine residues in the SXS motif of the MH2 domain activates the RSmad [200, 201]. Smad signaling The general mechanisms mediating TGF β receptor activation of Smads are as follows: binding of the ligand to the TGF β receptor complex induces the phosphorylation of RSmads. Generally, Smads 1, 5, and 8 can be phosphorylated by BMPs, while TGF β and activin can phosphorylate Smad2 and Smad3. It is known that TGF β can also phosphorylate Smad1 in Hs578T human breast cancer cells and IEC 4-1 cells [169]. In addition, it has been shown that TGF β can phosphorylate Smad5 in human hematopoietic cells [202]. The active TβRI phosphorylates the RSmad at the serine-rich sequence SXS in the C- terminal tail of the MH2 domain, thus activating it. Smad4 does not have this SXS motif, and hence is not phosphorylated by TβRI [169]. Structural analysis suggests that the inability of Smad4 to bind to ΤβRI is due to a lack of a basic, positively-charged surface patch at the MH2 domain of Smad4 [203]. The phosphorylation of RSmad causes conformational changes and results in its dissociation from TβR1, as well as increases its affinity for the CoSmad, and thus, facilitating the assembly of the RSmad-CoSmad complex [201]. There is evidence that the RSmad-CoSmad complex is a heterotrimer, made of two RSmads and the CoSmad [204]. It is thought that after activation and trimerization, the Smad complex can translocate to the nucleus. Once in the nucleus, the Smad complex either can directly bind to DNA or can associate with other transcription factors to induce target gene transcription [170, 192, 197]. Moreover, Smad signaling can be modulated by many Smad-associated proteins and by other signaling pathways, including the Ras/MAPK pathways [199]. In the 26
41 linker region of RSmads, there are several consensus MAPK phosphorylation sites that are responsible for crosstalk between the Smad and MAPK pathways [169, 205].The inhibitory Smads, 6 and 7, appear to block the activation of RSmads by TGF β receptors [183]. The function of the Smad anchor for receptor activation After ligand binding, RSmads can transiently associate with Type I receptors with the help of the auxiliary protein, Smad anchor for receptor activation (SARA) [196]. The SARA protein contains a FYVE (a double zinc finger) domain, which can bind to phosphatidylinositol-3-phosphate in the lipid layer endosomal membrane. There is also a peptide sequence of SARA that can interact with the RSmads [206]. TβRI-mediated phosphorylation of SARA-bound RSmad is more efficient in SARA-rich endosomes ([201]. The role of SARA as a regulator of TGF-β signaling has been demonstrated in studies where a mutation in SARA in Mv1Lu or HepG2 cell cultures caused disruption of TGF-β signaling [206] Summary of literature review The control of follicle development involves the interaction of multiple hormone and peptide signaling cascades, and the differential expression of various genes, the details of which are just beginning to be understood but are still unclear. The literature review illustrates the complexity of these processes and lists the majority of factors known to date that are thought to be involved in the control of folliculogenesis, through various stages of follicle development, ovulation and luteinization. Of particular interest in our laboratory is the control of the follicular to luteal transition. 27
42 Members of the TGF-β superfamily have been shown to be involved at all stages of follicle development. There is evidence that the prototypical member of this superfamily, TGF-β, is involved in pre-antral to antral follicle development and ovulation. But there are only a few studies that have examined the role of this cytokine in the post-ovulatory follicle. Even among these studies, data for the pig is limited. Preliminary studies from our laboratory indicated that TGF-β2 precursor as being one of the genes that was upregulated very early in response to the pre-ovulatory surge of LH. This observation led us to believe that TGF-β may be involved in the process of luteinization. To test our hypothesis, the following research was performed. In the first study, the message and protein expression of TGF-β and components of its signaling pathway was examined in peri-ovulatory porcine follicles at various time points after hcg administration. In the second study, the expression of TGF-β and components of its signaling pathway were examined in granulosa cells induced to luteinize in vitro with LH/IGF-1. The effects of TGFβ alone or in combination with LH/IGF-1 on luteinization were also examined in by monitoring progesterone production and mrna expression of key enzymes and proteins involved in progesterone production. 28
43 Figure 1. An overview of TGF-β signaling (Courtesy: Sigma-Aldrich) 29
44 REFERENCES 1. Picton HM. Activation of follicle development: the primordial follicle. Theriogenology 2001; 55: Smitz JE, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction 2002; 123: Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology 2003; 144: Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci 2003; 78: Fulka J, Kopecny V, Trebichavsky I. Studies on oogenesis in the early postnatal pig ovary. Biol Reprod 1972; 6: Fair T. Follicular oocyte growth and acquisition of developmental competence. Anim Reprod Sci 2003; 78: Filicori M, Cognigni GE, Samara A, Melappioni S, Perri T, Cantelli B, Parmegiani L, Pelusi G, DeAloysio D. The use of LH activity to drive folliculogenesis: exploring uncharted territories in ovulation induction. Hum Reprod Update 2002; 8: Fortune JE, Rivera GM, Yang MY. Follicular development: the role of the follicular microenvironment in selection of the dominant follicle. Anim Reprod Sci 2004; 82-83: Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci 2004; 82-83: Zeleznik AJ. The physiology of follicle selection. Reprod Biol Endocrinol 2004; 2: McNatty KP, Heath DA, Lundy T, Fidler AE, Quirke L, O'Connell A, Smith P, Groome N, Tisdall DJ. Control of early ovarian follicular development. J Reprod Fertil Suppl 1999; 54: Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Mol Cell Endocrinol 2000; 163: McNatty KP, Fidler AE, Juengel JL, Quirke LD, Smith PR, Heath DA, Lundy T, O'Connell A, Tisdall DJ. Growth and paracrine factors regulating follicular formation and cellular function. Mol Cell Endocrinol 2000; 163:
45 14. Oktay K, Briggs D, Gosden RG. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab 1997; 82: Roy SK, Wang SC, Greenwald GS. Radioreceptor and autoradiographic analysis of FSH, hcg and prolactin binding sites in primary to antral hamster follicles during the periovulatory period. J Reprod Fertil 1987; 79: Fair T, Hulshof SC, Hyttel P, Greve T, Boland M. Oocyte ultrastructure in bovine primordial to early tertiary follicles. Anat Embryol (Berl) 1997; 195: van den Hurk R, Zhao J. Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles. Theriogenology 2005; 63: Fair T, Hulshof SC, Hyttel P, Greve T, Boland M. Nucleus ultrastructure and transcriptional activity of bovine oocytes in preantral and early antral follicles. Mol Reprod Dev 1997; 46: McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000; 21: Amleh A, Dean J. Mouse genetics provides insight into folliculogenesis, fertilization and early embryonic development. Hum Reprod Update 2002; 8: Hunter MG, Brankin V, Quinn RL, Ferguson EM, Edwards SA, Ashworth CJ. Oocyte-somatic cell-endocrine interactions in pigs. Domest Anim Endocrinol 2005; 29: Driancourt MA. Follicular dynamics in sheep and cattle. Theriogenology 1991; 35: van den Hurk R, Bevers MM, Dieleman SJ. Comparative endocrinology and reproduction. 1999; p Van den Hurk R, Abir R, Telfer EE, Bevers MM. Primate and bovine immature oocytes and follicles as sources of fertilizable oocytes. Hum Reprod Update 2000; 6: Conti M. Specificity of the cyclic adenosine 3',5'-monophosphate signal in granulosa cell function. Biol Reprod 2002; 67: Palter SF, Tavares AB, Hourvitz A, Veldhuis JD, Adashi EY. Are estrogens of import to primate/human ovarian folliculogenesis? Endocr Rev 2001; 22: Robker RL, Richards JS. Hormonal control of the cell cycle in ovarian cells: proliferation versus differentiation. Biol Reprod 1998; 59: Garnsworthy PC, Webb R. The influence of nutrition on fertility in dairy cows. 1999;
46 29. Foxcroft GR, Hunter MG, Grant SA. The physiology of follicular maturation in the pig. Acta Physiol Pol 1989; 40: Ainsworth L, Tsang BK, Downey BR, Marcus GJ. The synthesis and actions of steroids and prostaglandins during follicular maturation in the pig. J Reprod Fertil Suppl 1990; 40: Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the 'two-cell, two-gonadotrophin' model revisited. Mol Cell Endocrinol 1994; 100: Tsang BK, Ainsworth L, Downey BR, Marcus GJ. Differential production of steroids by dispersed granulosa and theca interna cells from developing preovulatory follicles of pigs. J Reprod Fertil 1985; 74: Hartshorne GM. In vitro culture of ovarian follicles. Rev Reprod 1997; 2: Mao J, Wu G, Smith MF, McCauley TC, Cantley TC, Prather RS, Didion BA, Day BN. Effects of culture medium, serum type, and various concentrations of follicle-stimulating hormone on porcine preantral follicular development and antrum formation in vitro. Biol Reprod 2002; 67: Cortvrindt R, Hu Y, Smitz J. Recombinant luteinizing hormone as a survival and differentiation factor increases oocyte maturation in recombinant follicle stimulating hormone-supplemented mouse preantral follicle culture. Hum Reprod 1998; 13: Zhao J, Taverne MA, van der Weijden GC, Bevers MM, van den Hurk R. Effect of activin A on in vitro development of rat preantral follicles and localization of activin A and activin receptor II. Biol Reprod 2001; 65: Driancourt MA, Reynaud K, Cortvrindt R, Smitz J. Roles of KIT and KIT LIGAND in ovarian function. Rev Reprod 2000; 5: Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod 2000; 62: Fortune JE, Rivera GM, Evans AC, Turzillo AM. Differentiation of dominant versus subordinate follicles in cattle. Biol Reprod 2001; 65: Ireland JJ. Control of follicular growth and development. J Reprod Fertil Suppl 1987; 34: Hunter MG, Robinson RS, Mann GE, Webb R. Endocrine and paracrine control of follicular development and ovulation rate in farm species. Anim Reprod Sci 2004; 82-83:
47 42. Webb R, Nicholas B, Gong JG, Campbell BK, Gutierrez CG, Garverick HA, Armstrong DG. Mechanisms regulating follicular development and selection of the dominant follicle. Reprod Suppl 2003; 61: Campbell BK, Scaramuzzi RJ, Webb R. Control of antral follicle development and selection in sheep and cattle. J Reprod Fertil Suppl 1995; 49: Robker RL, Russell DL, Yoshioka S, Sharma SC, Lydon JP, O'Malley BW, Espey LL, Richards JS. Ovulation: a multi-gene, multi-step process. Steroids 2000; 65: Espey LL, Ujioka T, Okamura H, Richards JS. Metallothionein-1 messenger RNA transcription in steroid-secreting cells of the rat ovary during the periovulatory period. Biol Reprod 2003; 68: Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem 2003; 278: Richards JS, Hernandez-Gonzalez I, Gonzalez-Robayna I, Teuling E, Lo Y, Boerboom D, Falender AE, Doyle KH, LeBaron RG, Thompson V, Sandy JD. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: evidence for specific and redundant patterns during ovulation. Biol Reprod 2005; 72: Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol 2006; 20: Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 2006; 20: Sriraman V, Rudd MD, Lohmann SM, Mulders SM, Richards JS. Cyclic guanosine 5'- monophosphate-dependent protein kinase II is induced by luteinizing hormone and progesterone receptor-dependent mechanisms in granulosa cells and cumulus oocyte complexes of ovulating follicles. Mol Endocrinol 2006; 20: Norman RJ, Brannstrom M. White cells and the ovary--incidental invaders or essential effectors? J Endocrinol 1994; 140: Tsafriri A, Reich R. Molecular aspects of mammalian ovulation. Exp Clin Endocrinol Diabetes 1999; 107: Richards JS, Russell DL, Robker RL, Dajee M, Alliston TN. Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol 1998; 145:
48 54. Espey LL. Ovulation as an inflammatory reaction--a hypothesis. Biol Reprod 1980; 22: Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod 1994; 50: Curry TE,Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: Simpson KS, Komar CM, Curry TE,Jr. Localization and expression of tissue inhibitor of metalloproteinase-4 in the immature gonadotropin-stimulated and adult rat ovary. Biol Reprod 2003; 68: Jo M, Thomas LE, Wheeler SE, Curry TE,Jr. Membrane type 1-matrix metalloproteinase (MMP)-associated MMP-2 activation increases in the rat ovary in response to an ovulatory dose of human chorionic gonadotropin. Biol Reprod 2004; 70: Jo M, Curry TE,Jr. Regulation of matrix metalloproteinase-19 messenger RNA expression in the rat ovary. Biol Reprod 2004; 71: Smedts AM, Curry TE,Jr. Expression of basigin, an inducer of matrix metalloproteinases, in the rat ovary. Biol Reprod 2005; 73: Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev 2000; 80: Murphy BD, Gevry N, Ruiz-Cortes T, Cote F, Downey BR, Sirois J. Formation and early development of the corpus luteum in pigs. Reprod Suppl 2001; 58: Anderson LL, Dyck GW, Mori H, Henricks DM, Melampy RM. Ovarian function in pigs following hypophysial stalk transection or hypophysectomy. Am J Physiol 1967; 212: Soede NM, Helmond FA, Kemp B. Periovulatory profiles of oestradiol, LH and progesterone in relation to oestrus and embryo mortality in multiparous sows using transrectal ultrasonography to detect ovulation. J Reprod Fertil 1994; 101: Tamanini C, De Ambrogi M. Angiogenesis in developing follicle and corpus luteum. Reprod Domest Anim 2004; 39: Corner GW. On the origin of the corpus luteum of the sow from both granulosa and theca interna. American Journal of Anatomy 1919; 27: Azmi TI, O'Shea JD, Bruce NW, Rodgers RJ. Morphometry of the functional and regressing corpus luteum of the guinea pig. Anat Rec 1984; 210:
49 68. Farin CE, Moeller CL, Sawyer HR, Gamboni F, Niswender GD. Morphometric analysis of cell types in the ovine corpus luteum throughout the estrous cycle. Biol Reprod 1986; 35: Farin CE, Sawyer HR, Niswender GD. Analysis of cell types in the corpus luteum of the sheep. J Reprod Fertil Suppl 1989; 37: Wiltbank MC. Cell types and hormonal mechanisms associated with mid-cycle corpus luteum function. J Anim Sci 1994; 72: Alila HW, Hansel W. Origin of different cell types in the bovine corpus luteum as characterized by specific monoclonal antibodies. Biol Reprod 1984; 31: Rodgers RJ, O'Shea JD, Bruce NW. Morphometric analysis of the cellular composition of the ovine corpus luteum. J Anat 1984; 138 ( Pt 4): Pate JL, Condon WA. Effects of prostaglandin F2 alpha on agonist-induced progesterone production in cultured bovine luteal cells. Biol Reprod 1984; 31: Niswender GD, Juengel JL, McGuire WJ, Belfiore CJ, Wiltbank MC. Luteal function: the estrous cycle and early pregnancy. Biol Reprod 1994; 50: Barboni B, Turriani M, Galeati G, Spinaci M, Bacci ML, Forni M, Mattioli M. Vascular endothelial growth factor production in growing pig antral follicles. Biol Reprod 2000; 63: Lemon M, Loir M. Steroid release in vitro by two luteal cell types in the corpus luteum of the pregnant sow. J Endocrinol 1977; 72: Conley AJ, Howard HJ, Slanger WD, Ford JJ. Steroidogenesis in the preovulatory porcine follicle. Biol Reprod 1994; 51: Slomczynska M, Duda M, Sl zak K. The expression of androgen receptor, cytochrome P450 aromatase and FSH receptor mrna in the porcine ovary. Folia Histochem Cytobiol 2001; 39: Juengel JL, Guy MK, Tandeski TR, McGuire WJ, Niswender GD. Steady-state concentrations of messenger ribonucleic acid encoding cytochrome P450 side-chain cleavage and 3 beta-hydroxysteroid dehydrogenase/delta 5,delta 4 isomerase in ovine corpora lutea during the estrous cycle. Biol Reprod 1994; 51: LaVoie HA, Benoit AM, Garmey JC, Dailey RA, Wright DJ, Veldhuis JD. Coordinate developmental expression of genes regulating sterol economy and cholesterol side-chain cleavage in the porcine ovary. Biol Reprod 1997; 57: Pate JL, Condon WA. Effects of serum and lipoproteins on steroidogenesis in cultured bovine luteal cells. Mol Cell Endocrinol 1982; 28:
50 82. Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986; 232: Johnson WJ, Phillips MC, Rothblat GH. Lipoproteins and cellular cholesterol homeostasis. Subcell Biochem 1997; 28: Stocco DM. A StAR search: implications in controlling steroidgenesis. Biol Reprod 1997; 56: Murphy BD. Models of luteinization. Biol Reprod 2000; 63: McRae RS, Johnston HM, Mihm M, O'Shaughnessy PJ. Changes in mouse granulosa cell gene expression during early luteinization. Endocrinology 2005; 146: Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev 1994; 15: Fitzpatrick SL, Carlone DL, Robker RL, Richards JS. Expression of aromatase in the ovary: down-regulation of mrna by the ovulatory luteinizing hormone surge. Steroids 1997; 62: Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27kip1. Mol Endocrinol 1998; 12: Espey LL, Yoshioka S, Russell DL, Robker RL, Fujii S, Richards JS. Ovarian expression of a disintegrin and metalloproteinase with thrombospondin motifs during ovulation in the gonadotropin-primed immature rat. Biol Reprod 2000; 62: Espey LL, Richards JS. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod 2002; 67: Stouffer RL, Xu F, Duffy DM. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci 2007; 12: Meduri G, Vu Hai MT, Jolivet A, Takemori S, Kominami S, Driancourt MA, Milgrom E. Comparison of cellular distribution of LH receptors and steroidogenic enzymes in the porcine ovary. J Endocrinol 1996; 148: Pescador N, Houde A, Stocco DM, Murphy BD. Follicle-stimulating hormone and intracellular second messengers regulate steroidogenic acute regulatory protein messenger ribonucleic acid in luteinized porcine granulosa cells. Biol Reprod 1997; 57: Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Dore M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update 2004; 10: Downey BR, Mootoo JE, Doyle SE. A role for lipoxygenase metabolites of arachidonic acid in porcine ovulation. Anim Reprod Sci 1998; 49:
51 97. Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res 2002; 57: Al-zi'abi MO, Watson ED, Fraser HM. Angiogenesis and vascular endothelial growth factor expression in the equine corpus luteum. Reproduction 2003; 125: Boonyaprakob U, Gadsby JE, Hedgpeth V, Routh P, Almond GW. Expression and localization of vascular endothelial growth factor and its receptors in pig corpora lutea during the oestrous cycle. Reproduction 2003; 126: Gadsby JE, Lovdal JA, Samaras S, Barber JS, Hammond JM. Expression of the messenger ribonucleic acids for insulin-like growth factor-i and insulin-like growth factor binding proteins in porcine corpora lutea. Biol Reprod 1996; 54: Ge Z, Nicholson WE, Plotner DM, Farin CE, Gadsby JE. Insulin-like growth factor I receptor mrna and protein expression in pig corpora lutea. J Reprod Fertil 2000; 120: Ge Z, Miller E, Nicholson WE, Hedgpeth V, Gadsby JE. Insulin-like growth factor (IGF)-1 and IGF binding proteins -2, -3, -4, -5 in porcine corpora lutea during the estrus cycle; evidence for inhibitory actions of IGFBP-3. Domestic Animal Endocrinology 2003; 25: Miller EA, Ge Z, Hedgpeth V, Gadsby JE. Steroidogenic responses of pig corpora lutea to insulin-like growth factor I (IGF-I) throughout the oestrous cycle. Reproduction 2003; 125: Gadsby JE, Rose L, Sriperumbudur R, Ge Z. The role of intra-luteal factors in the control of the porcine corpus luteum. In: Control of Pig Reproduction VII; 2006; Netherlands Ricke WA, Redmer DA, Reynolds LP. Growth and cellular proliferation of pig corpora lutea throughout the oestrous cycle. J Reprod Fertil 1999; 117: Richards JS. Graafian follicle function and luteinization in nonprimates. J Soc Gynecol Investig 2001; 8:S Classon M, Dyson N. p107 and p130: versatile proteins with interesting pockets. Exp Cell Res 2001; 264: Jirawatnotai S, Moons DS, Stocco CO, Franks R, Hales DB, Gibori G, Kiyokawa H. The cyclin-dependent kinase inhibitors p27kip1 and p21cip1 cooperate to restrict proliferative life span in differentiating ovarian cells. J Biol Chem 2003; 278: Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res 2001; 49:
52 110. Knight PG, Glister C. Local roles of TGF-beta superfamily members in the control of ovarian follicle development. Anim Reprod Sci 2003; 78: Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006; 132: Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod 2001; 65: Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 2003; 69: McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol 1995; 9: Bodensteiner KJ, Clay CM, Moeller CL, Sawyer HR. Molecular cloning of the ovine Growth/Differentiation factor-9 gene and expression of growth/differentiation factor-9 in ovine and bovine ovaries. Biol Reprod 1999; 60: Jaatinen R, Laitinen MP, Vuojolainen K, Aaltonen J, Louhio H, Heikinheimo K, Lehtonen E, Ritvos O. Localization of growth differentiation factor-9 (GDF-9) mrna and protein in rat ovaries and cdna cloning of rat GDF-9 and its novel homolog GDF-9B. Mol Cell Endocrinol 1999; 156: Elvin JA, Yan C, Matzuk MM. Oocyte-expressed TGF-beta superfamily members in female fertility. Mol Cell Endocrinol 2000; 159: McNatty KP, Juengel JL, Wilson T, Galloway SM, Davis GH. Genetic mutations influencing ovulation rate in sheep. Reprod Fertil Dev 2001; 13: Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996; 383: Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol 1998; 204: Nilsson EE, Skinner MK. Growth and differentiation factor-9 stimulates progression of early primary but not primordial rat ovarian follicle development. Biol Reprod 2002; 67: Galloway SM, Gregan SM, Wilson T, McNatty KP, Juengel JL, Ritvos O, Davis GH. Bmp15 mutations and ovarian function. Mol Cell Endocrinol 2002; 191:
53 123. McNatty KP, Smith P, Moore LG, Reader K, Lun S, Hanrahan JP, Groome NP, Laitinen M, Ritvos O, Juengel JL. Oocyte-expressed genes affecting ovulation rate. Mol Cell Endocrinol 2005; 234: Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002; 143: Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-mullerian hormone in the mouse ovary. Endocrinology 1999; 140: Hayashi M, McGee EA, Min G, Klein C, Rose UM, van Duin M, Hsueh AJ. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology 1999; 140: Wang XF, Lin HY, Ng-Eaton E, Downward J, Lodish HF, Weinberg RA. Expression cloning and characterization of the TGF-beta type III receptor. Cell 1991; 67: Wang J, Roy SK. Growth differentiation factor-9 and stem cell factor promote primordial follicle formation in the hamster: modulation by follicle-stimulating hormone. Biol Reprod 2004; 70: Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod 2004; 70: Juengel JL, Hudson NL, Heath DA, Smith P, Reader KL, Lawrence SB, O'Connell AR, Laitinen MP, Cranfield M, Groome NP, Ritvos O, McNatty KP. Growth differentiation factor 9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep. Biol Reprod 2002; 67: Erickson GF, Shimasaki S. The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod Biol Endocrinol 2003; 1: Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 2004; 25: Juengel JL, McNatty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update 2005; 11: Glister C, Kemp CF, Knight PG. Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction 2004; 127:
54 135. Shimasaki S, Zachow RJ, Li D, Kim H, Iemura S, Ueno N, Sampath K, Chang RJ, Erickson GF. A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci U S A 1999; 96: Otsuka F, Yao Z, Lee T, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15. Identification of target cells and biological functions. J Biol Chem 2000; 275: Brankin V, Quinn RL, Webb R, Hunter MG. Evidence for a functional bone morphogenetic protein (BMP) system in the porcine ovary. Domest Anim Endocrinol 2005; 28: Glister C, Richards SL, Knight PG. Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology 2005; 146: Rabinovici J, Goldsmith PC, Roberts VJ, Vaughan J, Vale W, Jaffe RB. Localization and secretion of inhibin/activin subunits in the human and subhuman primate fetal gonads. J Clin Endocrinol Metab 1991; 73: Drummond AE, Le MT, Ethier JF, Dyson M, Findlay JK. Expression and localization of activin receptors, Smads, and beta glycan to the postnatal rat ovary. Endocrinology 2002; 143: Matzuk MM, Kumar TR, Shou W, Coerver KA, Lau AL, Behringer RR, Finegold MJ. Transgenic models to study the roles of inhibins and activins in reproduction, oncogenesis, and development. Recent Prog Horm Res 1996; 51:123-54; discussion Sadatsuki M, Tsutsumi O, Yamada R, Muramatsu M, Taketani Y. Local regulatory effects of activin A and follistatin on meiotic maturation of rat oocytes. Biochem Biophys Res Commun 1993; 196: Silva CC, Knight PG. Modulatory actions of activin-a and follistatin on the developmental competence of in vitro-matured bovine oocytes. Biol Reprod 1998; 58: Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001; 142: Teerds KJ, Dorrington JH. Immunohistochemical localization of transforming growth factor-beta 1 and -beta 2 during follicular development in the adult rat ovary. Mol Cell Endocrinol 1992; 84:R
55 146. Nilsson EE, Doraiswamy V, Skinner MK. Transforming growth factor-beta isoform expression during bovine ovarian antral follicle development. Mol Reprod Dev 2003; 66: Juengel JL, Bibby AH, Reader KL, Lun S, Quirke LD, Haydon LJ, McNatty KP. The role of transforming growth factor-beta (TGF-beta) during ovarian follicular development in sheep. Reprod Biol Endocrinol 2004; 2: Roy SK, Kole AR. Ovarian transforming growth factor-beta (TGF-beta) receptors: invitro effects of follicle stimulating hormone, epidermal growth factor and TGF-beta on receptor expression in human preantral follicles. Mol Hum Reprod 1998; 4: Findlay JK, Drummond AE, Dyson ML, Baillie AJ, Robertson DM, Ethier JF. Recruitment and development of the follicle; the roles of the transforming growth factor-beta superfamily. Mol Cell Endocrinol 2002; 191: Chegini N, Flanders KC. Presence of transforming growth factor-beta and their selective cellular localization in human ovarian tissue of various reproductive stages. Endocrinology 1992; 130: Skinner MK, Keski-Oja J, Osteen KG, Moses HL. Ovarian thecal cells produce transforming growth factor-beta which can regulate granulosa cell growth. Endocrinology 1987; 121: Dunkel L, Tilly JL, Shikone T, Nishimori K, Hsueh AJ. Follicle-stimulating hormone receptor expression in the rat ovary: increases during prepubertal development and regulation by the opposing actions of transforming growth factors beta and alpha. Biol Reprod 1994; 50: Gangrade BK, May JV. The production of transforming growth factor-beta in the porcine ovary and its secretion in vitro. Endocrinology 1990; 127: May JV, Frost JP, Schomberg DW. Differential effects of epidermal growth factor, somatomedin-c/insulin-like growth factor I, and transforming growth factor-beta on porcine granulosa cell deoxyribonucleic acid synthesis and cell proliferation. Endocrinology 1988; 123: Mondschein JS, Canning SF, Hammond JM. Effects of transforming growth factorbeta on the production of immunoreactive insulin-like growth factor I and progesterone and on [3H]thymidine incorporation in porcine granulosa cell cultures. Endocrinology 1988; 123: Goddard I, Hendrick JC, Benahmed M, Morera AM. Transforming growth factor beta receptor expression in cultured porcine granulosa cells. Mol Cell Endocrinol 1995; 115: Mulheron GW, Mulheron JG, Danielpour D, Schomberg DW. Porcine granulosa cells do not express transforming growth factor-beta 2 (TGF-beta 2) messenger ribonucleic acid: 41
56 molecular basis for their inability to produce TGF-beta activity comparable to that of rat granulosa cells. Endocrinology 1992; 131: Ghiglieri C, Khatchadourian C, Tabone E, Hendrick JC, Benahmed M, Menezo Y. Immunolocalization of transforming growth factor-beta 1 and transforming growth factorbeta 2 in the mouse ovary during gonadotrophin-induced follicular maturation. Hum Reprod 1995; 10: Matsuyama S, Takahashi M. Immunoreactive (ir)-transforming growth factor (TGF)- beta in rat corpus luteum: ir-tgf beta is expressed by luteal macrophages. Endocr J 1995; 42: Matsuyama S, Shiota K, Takahashi M. Possible role of transforming growth factorbeta as a mediator of luteotropic action of prolactin in rat luteal cell cultures. Endocrinology 1990; 127: Matsubara H, Ikuta K, Ozaki Y, Suzuki Y, Suzuki N, Sato T, Suzumori K. Gonadotropins and cytokines affect luteal function through control of apoptosis in human luteinized granulosa cells. J Clin Endocrinol Metab 2000; 85: Gueripel X, Benahmed M, Gougeon A. Sequential gonadotropin treatment of immature mice leads to amplification of transforming growth factor beta action, via upregulation of receptor-type 1, Smad 2 and 4, and downregulation of Smad 6. Biol Reprod 2004; 70: Wehrenberg U, Giebel J, Rune GM. Possible involvement of transforming growth factor-beta 1 and transforming growth factor-beta receptor type II during luteinization in the marmoset ovary. Tissue Cell 1998; 30: Wehrenberg U, Rune GM. Spontaneous luteinization of antral marmoset follicles in vitro. Mol Hum Reprod 2000; 6: Prange-Kiel J, Kreutzkamm C, Wehrenberg U, Rune GM. Role of tumor necrosis factor in preovulatory follicles of swine. Biol Reprod 2001; 65: Johnson AL, Bridgham JT, Woods DC. Cellular mechanisms and modulation of activin A- and transforming growth factor beta-mediated differentiation in cultured hen granulosa cells. Biol Reprod 2004; 71: Chen XL, Gao HJ, Wei P, Song XX, Hu ZY, Liu YX. Regulatory effect of IFNgamma on expression of TGF-beta 1, T beta R-II, and StAR in corpus luteum of pregnant rhesus monkey. Acta Pharmacol Sin 2003; 24: Gao H, Chen XL, Zhang ZH, Song XX, Hu ZY, Liu YX. IFN-gamma and TNF-alpha inhibit expression of TGF-beta-1, its receptors TBETAR-I and TBETAR-II in the corpus luteum of PMSG/hCG treated rhesus monkey. Front Biosci 2005; 10:
57 169. Yue J, Mulder KM. Transforming growth factor-beta signal transduction in epithelial cells. Pharmacol Ther 2001; 91: Massague J. TGF-beta signal transduction. Annu Rev Biochem 1998; 67: Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci 2004; 35: Seyedin SM, Thomas TC, Thompson AY, Rosen DM, Piez KA. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc Natl Acad Sci U S A 1985; 82: Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocr Rev 2005; 26: Sun PD, Davies DR. The cystine-knot growth-factor superfamily. Annu Rev Biophys Biomol Struct 1995; 24: Lawrence DA. Latent-TGF-beta: an overview. Mol Cell Biochem 2001; 219: Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 2006; 177. Derynck R, Feng XH. TGF-beta receptor signaling. Biochim Biophys Acta 1997; 1333:F Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem 1992; 267: Lopez-Casillas F, Cheifetz S, Doody J, Andres JL, Lane WS, Massague J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell 1991; 67: Bandyopadhyay A, Zhu Y, Cibull ML, Bao L, Chen C, Sun L. A soluble transforming growth factor beta type III receptor suppresses tumorigenicity and metastasis of human breast cancer MDA-MB-231 cells. Cancer Res 1999; 59: Chen RH, Derynck R. Homomeric interactions between type II transforming growth factor-beta receptors. J Biol Chem 1994; 269: Franzen P, ten Dijke P, Ichijo H, Yamashita H, Schulz P, Heldin CH, Miyazono K. Cloning of a TGF beta type I receptor that forms a heteromeric complex with the TGF beta type II receptor. Cell 1993; 75: Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature 1994; 370:
58 184. Attisano L, Wrana JL, Montalvo E, Massague J. Activation of signalling by the activin receptor complex. Mol Cell Biol 1996; 16: Mathews LS, Vale WW. Characterization of type II activin receptors. Binding, processing, and phosphorylation. J Biol Chem 1993; 268: Wieser R, Wrana JL, Massague J. GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J 1995; 14: Luo K, Lodish HF. Signaling by chimeric erythropoietin-tgf-beta receptors: homodimerization of the cytoplasmic domain of the type I TGF-beta receptor and heterodimerization with the type II receptor are both required for intracellular signal transduction. EMBO J 1996; 15: Lopez-Casillas F, Wrana JL, Massague J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell 1993; 73: Moustakas A, Lin HY, Henis YI, Plamondon J, O'Connor-McCourt MD, Lodish HF. The transforming growth factor beta receptors types I, II, and III form hetero-oligomeric complexes in the presence of ligand. J Biol Chem 1993; 268: Mulder KM, Segarini PR, Morris SL, Ziman JM, Choi HG. Role of receptor complexes in resistance or sensitivity to growth inhibition by TGF beta in intestinal epithelial cell clones. J Cell Physiol 1993; 154: Zhou GH, Sechrist GL, Brattain MG, Mulder KM. Clonal heterogeneity of the sensitivity of human colon carcinoma cell lines to TGE beta isoforms. J Cell Physiol 1995; 165: Zhou GH, Sechrist GL, Periyasamy S, Brattain MG, Mulder KM. Transforming growth factor beta isoform-specific differences in interactions with type I and II transforming growth factor beta receptors. Cancer Res 1995; 55: Okadome T, Yamashita H, Franzen P, Moren A, Heldin CH, Miyazono K. Distinct roles of the intracellular domains of transforming growth factor-beta type I and type II receptors in signal transduction. J Biol Chem 1994; 269: Feng XH, Filvaroff EH, Derynck R. Transforming growth factor-beta (TGF-beta)- induced down-regulation of cyclin A expression requires a functional TGF-beta receptor complex. Characterization of chimeric and truncated type I and type II receptors. J Biol Chem 1995; 270: Feng XH, Derynck R. Ligand-independent activation of transforming growth factor (TGF) beta signaling pathways by heteromeric cytoplasmic domains of TGF-beta receptors. J Biol Chem 1996; 271:
59 196. Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell 1992; 71: Carcamo J, Zentella A, Massague J. Disruption of transforming growth factor beta signaling by a mutation that prevents transphosphorylation within the receptor complex. Mol Cell Biol 1995; 15: Souchelnytskyi S, ten Dijke P, Miyazono K, Heldin CH. Phosphorylation of Ser165 in TGF-beta type I receptor modulates TGF-beta1-induced cellular responses. EMBO J 1996; 15: Lawler S, Feng XH, Chen RH, Maruoka EM, Turck CW, Griswold-Prenner I, Derynck R. The type II transforming growth factor-beta receptor autophosphorylates not only on serine and threonine but also on tyrosine residues. J Biol Chem 1997; 272: Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGFbeta family signalling. Nature 2003; 425: Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003; 113: Bruno E, Horrigan SK, Van Den Berg D, Rozler E, Fitting PR, Moss ST, Westbrook C, Hoffman R. The Smad5 gene is involved in the intracellular signaling pathways that mediate the inhibitory effects of transforming growth factor-beta on human hematopoiesis. Blood 1998; 91: Wu G, Chen YG, Ozdamar B, Gyuricza CA, Chong PA, Wrana JL, Massague J, Shi Y. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science 2000; 287: Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 2005; 21: Mulder KM. Role of Ras and Mapks in TGFbeta signaling. Cytokine Growth Factor Rev 2000; 11: Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 1998; 95: Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991; 124:
60 TGF-β ligands, receptors and Smad expression in peri-ovulatory porcine follicles Rajagopal Sriperumbudur, Leah M. Zorrilla, and John E. Gadsby Department of Biomedical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC Acknowledgements: This project was supported by USDA Grant no and by a stipend to Rajagopal Sriperumbudur from the NCSU College of Veterinary Medicine. The authors thank Drs. William Flowers and Peter Farin for their help with statistical analysis, Dr. Jorge Piedrahita for the usage of reagents and equipment, Ms. Monica Mattmüller for her assistance with immunohistochemistry, and Mr. Curtis Powell and the staff of NCSU Swine Education Unit for supply and care the animals, and for daily heat checking. 46
61 ABSTRACT It is well known that the LH-surge causes ovulation of follicles and initiates luteinization. There is also evidence from our own studies as well from the literature suggesting that TGF-β may also play a role in luteinization. In this study we proposed to examine the expression of TGF-β ligands (i.e. TGF-β1, 2 and 3) and components of its signaling pathway (the TGF-β receptors, TβRI and II, the Smad proteins, Smad 2, 3, 4, 6 and 7, and the Smad Anchor for Receptor Activation, SARA) in porcine follicles induced to luteinize in vivo with hcg treatment. Pre-pubertal pigs were injected with PG-600 to induce ovarian follicular development and hcg 3 days later to induce ovulation. Ovulation consistently occurred between 24 and 48h post-hcg in all of the treated animals. Ovaries were collected surgically at 0, 1, 12, 24, 48 and 96 hours following hcg treatment, and used for RNA and protein extraction, and for immunohistochemistry. Expression of mrna for the components of the TGF-β signaling pathway was measured using semi-quantitative RT-PCR. Our data revealed that hcg caused upregulation of mrna expression for TGF-β3 at 12h, and TβRII at 96h, (versus 0h control) while there was not a significant change in expression versus the 0h control in these and the other components of the TGF-β signaling pathway at all other times. However, protein expression of TGF-β1, TGF-β2, TβRI and TβRII in these samples was not significantly different compared to the 0 h control. Using immunohistochemistry, we also localized TGF-β1 expression to granulosa cells (GC), TGF-β2 to theca cells (TC) in the pre-ovulatory follicle, TGF-β1 and TGF-β2 expression in luteal cells (LC) in the post-ovulatory follicle and TβRI and TβRII expression in GC, TC and LC of the pre- and post-ovulatory follicle. Since hcg, whose actions simulate those of naturally-occurring LH, up-regulates some components of the TGF-β signaling pathway, and 47
62 induces luteinization, we suggest that TGF-β signaling may play a role in mediating the LHsurge induced luteinization that occurs during the normal cycle. INTRODUCTION The processes of ovulation and luteinization are initiated by a surge of luteinizing hormone (LH). The LH surge sets off a cascade of events, including rapid changes in the expression of genes within the follicle that are involved in these processes, such as those for proinflammatory cytokines like interleukin-1b and its receptor, interleukin-1br [1], prostaglandins (i.e. endoperoxidase synthase-2) [2], angiogenic factors such as vascular endothelial growth factor [3], metalloproteinases that degrade follicular connective tissue [4, 5], and components of the steroidogenic pathway facilitating increased progesterone production, such as steroidogenic acute regulatory protein [6]. In response to the LH surge following ovulation, the steroidogenic cells of the follicle, the granulosa and theca cells, undergo luteinization and differentiate into the large and small (respectively) luteal cells of the corpus luteum (CL) [7] through a process of terminal differentiation [8, 9]. Grossly, ovulation is marked by rupture of the follicle and release of the oocyte (ovulation), and this is followed by a transient hyperemia, (i.e., in which the follicle fills with blood), and a gradual replacement of blood with the developing CL tissue via extensive hypertrophy of the granulosa and theca cells, and remodeling of extracellular matrix [9-12]. In addition, new blood vessels grow into the ruptured follicle (neovascularization), which involves endothelial cell invasion and growth, and invasion by macrophages [9]. In rodents, the LH surge also causes granulosa and theca cells to immediately exit from the cell cycle [8, 9]. This might not be entirely true in the pig, where there is evidence of continued mitosis into the luteal phase, with gradual loss of proliferation [13]. During the post-ovulatory period, follicular steroidogenesis switches from producing predominantly estrogen to producing predominantly 48
63 progesterone, and this change is accomplished by changes in expression of enzymes that regulate steroidogenesis. These changes include decreased protein and message expression of aromatase, and increased protein and message expression of P450scc, 3β-HSD and StAR [14-17]. Although the LH surge is known to cause ovulation and luteinization, complete details of the pathways and mechanisms by which these processes occur remain largely unidentified. From preliminary studies carried out in our laboratory using cdna array analysis (Sriperumbudur and Gadsby, Appendix 1), we had detected elevated transforming growth factor β-2 (TGF-β2) precursor mrna expression in porcine follicles treated with hcg (in vivo), suggesting a possible role for TGF-β in ovulation or luteinization in the pig. Although there is considerable evidence for the expression and actions of TGF-β and members of the TGF-β superfamily in follicle development, much less is known about the possible roles for TGF-β in follicle ovulation and luteinization, or in the corpus luteum itself. Clues to the possible involvement of TGF-β in luteinization come from but a handful of studies: (i) FSH treatment followed by hcg caused the increase in mrna and protein for TGFβ2, TβRI, the stimulatory Smads, Smad 2 and Smad 4 and decrease in mrna and protein for the inhibitory Smad 6 in immature mice [18], suggesting that components of the TGF-β pathway are upregulated in the peri-ovulatory follicle in response to FSH and LH. (ii) In a marmoset study, TβRII immunolocalization was consistently observed only in luteinizing cells, and the expression increased as luteinization progressed [19], prompting the authors to start using TβRII as a marker of luteinization in subsequent studies. (iii) In a follow up study to the studies described in (ii) above, [20], luteolytic TNF-α downregulated TβRII and decreased progesterone receptor expression in porcine granulosa cells in vitro. 49
64 (iv) In the chicken, TGF-β1, on its own or in combination with FSH, increased the expression of FSH receptor and LH receptor in undifferentiated granulosa cells; the increase in LH receptor sensitized the granulosa cells to LH action leading to increased P450scc and StAR expression, thus increasing the cells ability to produce more progesterone [21]. (v) Prolactin, a luteotropic hormone, and TGF-β increased progesterone production in cultured rat luteal cells by suppressing 20α-HSD expression, and in rat macrophage cells, prolactin stimulated TGF-β expression [22]. Also, in the same study, when the luteal cells were treated with an anti-tgf-β antibody, the suppressive action of prolactin on 20α-HSD was decreased, thus indicating that the luteotropic actions of prolactin may partly be mediated by TGF-β. (vi) More evidence about the role of TGF-β in CL formation and maintenance comes from two studies in the rhesus monkey. In the first study, when CLs at various stages of pregnancy were examined for the presence of StAR, TGF-β1 and TβRII mrna and protein, it was found that their expression progressively increased during early pregnancy, when the CL was being formed and was functional, while in the regressing CL, their expression decreased [23]. In a follow-up study, CL development was induced in the rhesus monkey using ecg/hcg treatment, and the expression of StAR, TGF-β1, TβRI and TβRII was examined in CLs at various stages of development using immunohistochemistry and in-situ hybridization [24]. It was found that their expression increased to reach peak levels when the CLs were functional, and decreased when the CL regressed. Treating the animals with the luteolytic cytokines IFN-γ and TNF-α also reduced their expression. These studies strongly suggest that TGF-β and its receptors may play a role in CL formation and maintenance of its function. 50
65 All of the above data support the view that TGF-β working via TβRI and TβRII plays a role in maintaining luteal function in these species. Thus, based on the aforementioned literature indicating a possible role for TGF-β in luteinization and the corpus luteum, we proposed to examine the role of TGF-β in ovulation and luteinization in the pig by characterizing the temporal and spatial patterns of expression of TGFβ ligands, receptors and Smad proteins involved in the TGF-β signaling pathway in porcine follicles in response to hcg (given to mimic the naturally occurring pre-ovulatory surge of LH), in vivo. METHODS AND MATERIALS Reagents All buffers and reagents, unless otherwise specified, were purchased from Sigma Chemical Co (St. Louis, MO) or Fisher Scientific (Clackamas, OR). Animals and Tissue collection All animals were housed at the NCSU Swine Educational Unit, Lake Wheeler Rd, Raleigh, NC. All animal care, handling and treatments, as well as surgery and post-surgical recovery, were approved according by the NCSU Institutional Animal Care and Use Committee day old prepubertal (Landrace/Yorkshire X Duroc/Hampshire hybrid) gilts were treated with a half dose of PG-600 (200 I.U. ecg plus 100 I.U. hcg, i.m.; Intervet, UK) per animal to promote follicle growth and maturation. Seventy two hours later, the animals were given 300 IU hcg (i.m.; Sigma) to induce ovulation and luteinization. Ovaries were surgically harvested at different times (n=7 animals for 0, 1, 24 and 48h time points; n=5 animals for the 12h time point; n=4 for the 96h time point) after hcg administration. The study was performed over a two 51
66 month period in the Spring. On any given day, surgeries were performed on animals at random and different time points post-hcg, with a maximum of four surgeries per day. Vascular, periovulatory follicles were dissected from the ovaries and were flash-frozen in liquid nitrogen for subsequent mrna and protein analysis. Follicles to be used for immunohistochemistry (n=2 follicles from different animals per time point) were fixed in 10% neutral-buffered formalin for 48h, then stored in 70% ethanol until further processing. Two follicles per time point for 0h, 12h, 24h and 48h were also used for separation into enriched granulosa and theca cell compartments by using a modification of the procedure described in [25]. Briefly, follicles were bisected, the follicle lining grasped at its base with sterile forceps and removed from the follicle by everting; granulosa cells were scraped for 2 min using a sterile inoculation loop into 250 μl sterile M-199 (Cambrex Bioscience, Baltimore, MD). The granulosa cell suspension was labeled GC and flash frozen; the remaining follicle lining, consisting mostly of theca cells, was labeled TC and flash frozen. Semi-quantitative analysis of mrna expression patterns of TGF-β ligands, receptors and Smad proteins: Total RNA was extracted from follicle tissue and the separated GC and TC fractions with TRI-Reagent (MRC, Inc., Cincinnati, OH) and the RNAqueous kit (Ambion, Inc., Austin, TX) and 100 μg of total RNA was subsequently DNAse-treated with Turbo-DNA free enzyme (Ambion) to minimize genomic DNA contamination. Total RNA was reverse transcribed to generate cdna using Omniscript RT kit (Qiagen, Valencia, CA). To ensure that there was no genomic contamination, -RT controls for all samples were also included in the reverse transcription reaction. To determine the linear phase of amplification, a pooled RNA sample across all samples was reverse transcribed and PCR was performed with each primer pair. The 52
67 pool was constructed by using 3 μg of total RNA from each sample and reverse transcribing to obtain the pool cdna. Amplification by PCR was performed on the pool cdna using porcinespecific primers (Sigma-Genosys, Woodlands, TX) for TGF-β ligands, receptors and Smad proteins (See Table 1 for primer sequences and amplicon sizes) for a total of 40 cycles, and 2 tubes per sample were removed every 5 cycles beginning with cycle 20. Amplicons were visualized on ethidium bromide-stained 2 % agarose gels and band densities were determined using the Alpha Imager gel documentation system (Alpha Innotech, San Leandro, CA). The densities were then graphed to determine the number of cycles which corresponded to the linear phase of amplification for each primer pair. Nucleotide sequencing of all PCR products was performed at the Gene Technologies Labarotories, Texas A & M University (College Station, TX) and compared with existing porcine sequences from the GenBank database. All gene products were confirmed as authentic based on published nucleotide sequences. Thus, for individual samples, PCR was performed under the following conditions: all samples were placed in AB biosystem s 9600 thermocycler for 3 min at 94ºC (initial denaturation), denaturation at 94ºC for 30 seconds. The standard PCR program included annealing at 50ºC for 30 seconds, extension at 72ºC for 60 seconds for appropriate numbers of cycles (specified in Table 1) for each primer, and a final extension at 72ºC for 5 minutes. Semi-quantitative analysis was performed on PCR products obtained during the linear phase of PCR amplification, after normalizing levels of gene expression to expression levels of Histone (H)2A, as a housekeeping gene. Absence of amplicons bands for the RT samples confirmed the absence of genomic DNA contamination (data not shown). The intra-assay CV for PCR assays ranged from 2.6% to 13.7%. To prove that our animal model resulted in luteinization, we also examined several other genes (e.g. Cyclin D2, Aromatase, and StAR), which are known to be responsive to LH. 53
68 Western blotting Protein expression of some of the components of the TGF-β signaling pathway in hcg treated follicles was determined using Western blot analysis. Briefly, follicles were homogenized in RIPA lysis buffer (sc-24948, Santa Cruz Biotechnology Inc., Santa Cruz, CA; 30 mm TrisHCl, ph 7.4, 0.1% SDS, 150 mm NaCl, 1% NP-40, 0.5% Deoxycholate, 2 mm EDTA with sodium orthovanadate, PMSF and protease inhibitor cocktail) and centrifuged to remove cell debris. The protein concentration of the lysates was determined using micro BCA protein assay (Pierce, Rockford, IL). Thirty μg of total protein (per sample) was separated on mini 4-12 % Bis- Tris gels (Invitrogen, Carlsbad, CA) at 200V for 30 minutes at room temperature under reducing conditions. Following electrophoresis, the gel-resolved proteins were (semi-dry) transferred onto PVDF membranes using the XCell II blot module (Invitrogen, Carlsbad, CA) at 30V for 1h at room temperature. Nonspecific binding sites were then blocked in tris-buffered saline with 0.2% Tween in PBS with 5% milk and probed overnight at 4ºC with primary antibody (1:200 for TGFβ pathway components, 1:5000 for β-actin). Primary antibodies used were TGF-β1 (sc-146), TGF-β2 (sc-90), TβRI (sc-398), TβRII (sc-400), Smad3 (sc-8332), p-smad3 (sc-11769) (all polyclonal, SantaCruz) and the housekeeping protein β-actin (ab8224, polyclonal, Abcam, Amherst, MA). Specificity of the antibodies was verified using blocking peptides (Santa Cruz); the primary antibody was incubated with its blocking peptide (1:5) for 1 h at room temperature immediately before probing the membrane. Porcine specific antibodies were not available for TGF-β3 and Smads 2, 4, 6 and 7. Following incubation with the primary antibody, the membrane was washed with wash solution (0.2% Tween in PBS) and incubated at room temperature for 1 h with the secondary antibody. Appropriate (anti-rabbit for sc-146, sc-90, sc- 398, sc-400, sc-8332, sc-11769, Santa Cruz, 1:20,000 dilution and anti-mouse for ab8224, 54
69 Sigma, 1:20,000 dilution) secondary antibodies were used to bind to the primary antibody. The membranes were washed four times for 10 minutes each in was solution. Protein bands were visualized by chemiluminescence using Luminol reagent (sc-2048, SantaCruz) in the Lumi Imager system (Roche) and quantified after normalizing to β-actin, using the Alpha Imager gel documentation system (Alpha Innotech, San Leandro, CA). Immunohistochemistry: Fixed tissue stored in 70% EtOH was embedded in paraffin and cut into 5 μm sections. Sections on slides were deparaffinized in xylene and hydrated in alcohol series in the following sequence: 1. Incubation of sections in Xylene: 2 to 3 changes, 5 min. each % absolute ethanol: 2 changes, 3 min. each 3. 95% ethanol: 2 changes, 3 min. each 4. 80% ethanol: 3 min % ethanol: 3 min. 6. Rinsing with distilled water: 2 changes, 3 min. each. To unmask the antigen, slides were immersed in 10 mm sodium citrate buffer for 20 minutes at 95ºC. Sections were cooled in the sodium citrate buffer for 10 min at RT, then rinsed in di H 2 O. Endogenous peroxidase was neutralized by incubating sections in 3% H 2 O 2 for 10 min at RT. Sections were rinsed in running water for 2min, then placed in PBS briefly. Sections were blocked in 5% goat serum (Biogenex, San Ramon, CA) for 20 min at RT and then incubated with primary antibody (Santa Cruz, conc. 1:50 for TGF-β1 and TGF-β2, and 1:500 for TβRI and TβRII) overnight at 4ºC. For negative controls, the primary antibody was replaced 55
70 with rabbit IgG at the same dilution. Sections were washed with PBS (3X5min) at RT and then incubated with biotinylated secondary Ab (Biogenex, San Ramon, CA) for 2 h at RT at a concentration of 1:40. After washing with PBS (3X5min) at RT, sections were labeled with avidin HRP (Biogenex) for 20 min at RT at a concentration of 1:40. They were then washed with PBS, 3X5min, and incubated with diaminobenzidine until color developed (30 sec-2min). After rinsing with di H 2 O, nuclei were counterstained with hematoxylin blue mix for 15 sec-1min. Sections were finally washed in di H 2 O for 2min, quickly dehydrated in alcohol series and xylene and coverslipped with mounting medium. Photographs of the sections were taken using the Leica DM5000 compound fluorescent/dic microscope and Simple PCI software (Compix Inc., Sewickley, PA). Statistical Analysis All data for expression of mrna were expressed as the ratio of mrna of interest to H2A mrna. Expression of protein was expressed as the ratio of protein of interest to β-actin. The normalized data for message and protein expression are presented as least squares means ± SEM (except for data for the separated GC and TC fractions, which are expressed as raw means ± SEM) for each time point examined. Comparison of message and protein levels was performed using one-way analysis of variance using the GLM procedure, followed by Scheffe multiple comparison test (SAS, Cary, NC). Differences were considered statistically significant at the 95 % confidence level (p<0.05). For the protein data, statistical analyses showed that there was a significant replicate effect for TβRII and Smad3, most likely due to one outlying replicate. Statistical analyses were not performed on data from the separated fractions. 56
71 RESULTS mrna expression in whole follicles: Luteinization-associated genes: The levels of mrna expression for the cell cycle regulatory protein, Cyclin D2, and two critical regulators of steroidogenesis, P450aromatase and StAR, are shown in Figure 1. No significant changes in Cyclin D2 were observed. Aromatase expression showed some dramatic changes in response to hcg, and declined significantly to reach lowest levels at 48h and 96h post-hcg. Finally, StAR expression showed a biphasic response, with highest levels at 0h, significantly declining at 1h, 12h and 24h post-hcg, increasing again at 48h and 96h, post-hcg, as luteinization proceeded. TGF-β ligands: As shown in the top panel of Figure 2, there were no statistically significant changes in the expression of either TGF-β1 or TGF-β2 mrnas over time post-hcg treatment. However, TGF-β3 mrna expression did show significant increase at 12h post-hcg, compared with all other time points (Fig.2, top panel) TGF-β receptors: The levels of expression of mrna for the TGF-β receptors in porcine follicles over time post-hcg are shown in the bottom panel of Figure 2. There were no statistically significant changes in TGF-β receptor type I (TβRI) expression, but the type II receptor (TβRII) did display significantly higher expression at 96h post-hcg; at this time point, the follicles are postovulatory and luteinization is progressing (Figure 2, bottom panel). Smads and regulatory protein SARA: 57
72 There were no significant changes in the expression of mrnas for the stimulatory Smads 2, 3, 4 and the Smad anchor for receptor activation, SARA (Fig. 3, top panel), or the inhibitory Smads 6, 7 (Fig. 3, bottom panel) in response to hcg. A representative gel demonstrating Smad3 amplification products is shown in figure 4. mrna expression in separated granulosa and theca cells: TGF-β ligands: As shown in the top and middle panels of Figure 5, mrna expression of TGF-β1 and TGF-β2 appeared to be greater in the TC fraction compared to the GC fraction. No definitive patterns of expression were apparent in the mrna expression pattern of TGF-β3, as seen in the bottom panel of Figure 5. TGF-β receptors: The levels of expression of mrna for the type I TGF-β receptor in the separated fractions over time post-hcg is shown in the top panel of Figure 6. There was no apparent pattern of expression for TβRI mrna in the two cell types. TβRII levels, shown in the bottom panel of Figure 6, appeared to increase progressively with time post-hcg in both GC and TC fractions starting with the 12h time point. Also, TβRII levels appear to be greater in the TC fraction compared to the GC fraction. Protein expression: TGF-β ligands: The levels of protein expression from Western Blot analyses of TGF-β1 and TGF-β2 (Fig. 7, top panel), are shown. No significant differences (p value = for TGF-β1, for TGF-β2) in the protein levels of either TGF-β ligand were observed. TGF-β receptors: 58
73 The protein expression levels of TβRI and TβRII (Fig. 7, bottom panel) also did not vary significantly over time after hcg treatment, but there was a numerical increase in expression of TβRII at 96h, again when the follicles are undergoing luteinization (p value = ). The numerical increase in TβRI was not significant because of a high p value (0.6746) Smad3, phospho-smad3: There were no significant changes in the expression of protein for Smad 3 and phosphorylated Smad3, although psmad3 appeared to increase at 12h post-hcg (Fig. 8) (p value = for Smad3, for psmad3). Representative Western blots for all the proteins examined are shown in figure 9. For all proteins examined, pre-incubation of each antibody with its respective blocking peptide eliminated the antibody signal (data not shown). Immunohistochemistry: In pre-ovulatory follicles (0h post-hcg), TGF-β1 was most highly expressed in granulosa cells (GC) while TGF-β2 was localized mainly to theca cells (TC); (Fig. 10). TβRI and TβRII were shown to be expressed to the same degree in both granulosa and theca cells in pre-ovulatory follicles (0h post-hcg); (Fig. 10). At both 48h and 96h post-hcg (i.e., post-ovulation), it was hard to distinguish the theca cells from the granulosa cells, and thus the cells were labeled as LC (luteinizing cells). Both TGF-β1 and TGF-β2 continue to show expression in luteinizing cells at 48h and 96h post-hcg. Similarly, both TβRI and TβRII were immunolocalized to luteinizing cells of post-ovulatory follicles (48h and 96h post-hcg; Figure 10). 59
74 DISCUSSION In this study, we explored the expression patterns of TGF-β ligands, TGF-β receptors and post-receptor signaling mediators (Smads and SARA) in porcine follicles which had been stimulated to develop in immature (pre-pubertal) gilts with PG-600 and then induced to ovulate and luteinize with hcg. PG-600 has been shown by others [26] to reliably produce a normal (i.e. not superovulated) number of ovulated follicles which go on to luteinize and develop into corpora lutea (Table 2). The data presented in Table 2 also indicated that hcg-treated follicles consistently ovulated between 24h and 48h, which is close to the approximately 40h time frame for ovulation post LH-surge in cycling gilts [27]. In addition, the expression patterns of luteinization associated-genes presented in Fig. 1 confirmed that in this model, porcine follicles did indeed undergo ovulation and luteinization. We examined these genes to provide a positive control for the other genes being examined in this study. These data support the view that hcgtreated follicles obtained from our model underwent critical changes in steroidogenic enzymes and other proteins consistent with those previously described in the pig, rodent and cow [14, 15, 28, 29]. These data also reflect the critical change in steroidogenic potential of peri-ovulatory follicles from predominantly estrogen secreting structures before the LH-surge, to the predominantly progesterone secreting luteinizing follicle/developing corpus luteum. The lack of change in the mrna expression patterns of CyclinD2 is in agreement that in the pig, unlike in rodents, follicular granulosa and theca cells continue to undergo cell division following the LHsurge [30]. In this study, we described some important changes in TGF-β ligand and TGF-β receptor expression in porcine follicles following hcg treatment. Specifically, we showed a significant elevation of TGF-β3 mrna expression at 12h post-hcg. Furthermore, we showed a significant 60
75 increase in the type II TGF-β receptor mrna at 96h post-hcg. These data suggest that hcginduced ovulation and luteinization is associated with increased expression of TGF-β3 and its receptor TβRII, leading us to speculate that TGF-β may play a role in mediating the luteinizing actions of LH (or hcg). In addition, our observation that levels of TGF-β3 mrna increased significantly following hcg exposure in the follicle, might suggest a specific role for this isoform early in the peri-ovulatory period in the pig. However, monitoring protein expression of TβRII did not show the change reflected in its mrna expression. Since a porcine-specific antibody for TGF-β3 was not available, its protein expression pattern could not be examined; should one become available in the near future, it can be used to verify if the protein expression pattern follows the mrna expression pattern, only after which the possible role of TGF-β3 in ovulation and luteinization will be clearer. However, the protein expression for the active form of Smad3, the phosphorylated Smad3, showed a tendency to increase at 12h post-hcg, suggesting that the TGF-β pathway may be active at this time point. To our knowledge, this is the first report of variable expression of TGF-β3 mrna in the luteinizing follicle in the pig; only one other study reports expression of this isoform in the cow in the pre-antral and antral stage follicle [31]. The expression patterns of mrna for the TGF-β ligands and receptors from the separated GC and TC compartments did not show any changes over time post-hcg. But there appeared to be differences in the levels of expression of TGF-β1, TGF-β2 and TβRII in the two compartments; all three components seemed to be expressed greater in the TC compartment. Though not significant, these data provide a clue that TGF-β pathway components may be differentially expressed in these compartments. A possible explanation as to why changes in 61
76 mrna expression seen in whole follicles are not reflected in the separated components can be due to the small sample number. With regard to the possible functions of TGF-β in the periovulatory porcine follicle, it was surprising that we could not detect any statistical differences in the expression of any of the Smads or SARA, at either mrna or protein levels. Perhaps the most sensitive test of Smad activation is protein phosphorylation, and yet even here we could not observe any differences post-hcg that might haven been anticipated with TGF-β actions, especially at 48h and 96 h. It is possible that despite an increase in ligand (TGF-β3) and receptor (TβRII) mrna levels, it is neither reflected in the protein levels of TβRII, nor the activation of the downstream components of the TGF-β pathway. There is the possibility of post-transcriptional regulation where the increase in TβRII mrna levels does translate into increased protein levels. Future studies may be designed to test this hypothesis. Finally, we observed some interesting patterns in the immunolocalization of TGF-β ligands and the receptors in peri-ovulatory porcine follicles. It was very interesting to observe the contrasting expression patterns of TGF-β1 and TGF-β2 in the steroidogenic components of the follicle. TGF-β1 was strongly expressed by granulosa cells but not theca cells of the preovulatory follicle. In contrast, TGF-β2 expression was just the opposite with strong expression in the theca cells but not in the granulosa cells. After ovulation, the distinction between granulosa and theca cells could not be made and the cells were labeled luteinizing cells (LC). At 48 and 96h, the LCs expressed both ligands. The receptors ΤβRΙ and II were expressed in both theca and granulosa, as well as in luteinizing cells in the post-ovulatory follicle. It was interesting to note that these observations were different from the mrna expression observed in the separated theca and granulosa compartments, where TGF-β1 and TGF-β2 mrna expression appeared to 62
77 be higher in the theca compartment. Previous studies have shown that theca cells from immature pigs are the source of TGF-β in culture [32, 33], but these studies do not mention the specific TGF-β isoform examined. Another study showed that porcine granulosa cells do not express TGF-β2 mrna [34]. This observation may explain the low levels of TGF-β2 protein expression in granulosa cells in our immunohistochemical studies. Thus, our findings demonstrate for the first time differential protein expression of TGF-β ligands within the peri-ovulatory porcine follicle. In summary, we have presented evidence that TGF-β3 and its receptor TβRII are upregulated at the mrna level particularly in post-ovulatory, luteinizing porcine follicles. This is not reflected in the protein levels of TβRII, but there was a tendency for psmad3 protein levels to increase at 12h post-hcg. This leads us to suggest that TGF-β3 and its receptor, ΤβRII are regulated at the post-transcriptional level, and hcg may not stimulate the TGF-β pathway. On the other hand, TGF-β has been shown to increase the expression of LH receptors in immature granulosa cells in the chicken [21]and promote their differentiation and increase progesterone production. Thus, TGF-β may promote luteinization indirectly by enhancing the effects of LH. In addition, based on the immunohistochemical localization of TGF-β ligands and their receptors, we suggest that TGF-β may have both autocrine as well as paracrine actions within periovulatory follicles, that may account its proposed luteinizing actions. 63
78 FIGURE LEGENDS Figure 1. mrna expression of some LH-responsive genes in hcg-treated follicles at 0 h (control), 1 h, 12 h, 24 h, 48 h and 96 h post-hcg Figure 2. mrna expression of TGF-β ligands (top panel) and receptors (bottom panel). Figure 3. mrna expression of stimulatory Smads and SARA (top panel) and inhibitory Smads (bottom panel). For figures 1-3, quantification of mrna was performed using semi-quantitative PCR, with band densities expressed as the least squares mean ± SEM (n = 4-7 animals per time) of ratios relative to H2A. Data were analyzed using PROC GLM ANOVA, followed by Scheffe Multiple Comparison Test using SAS. Means for each gene with asterisks indicate significant differences (p<0.05; versus 0h post-hcg) over time post-hcg. Figure 4. Representative ethidium bromide-stained agarose gel of Smad3 amplification product from hcg-treated follicles. Lane 1, base pair marker (100 bp ladder); Lane 2 and 3, 0h sample; Lane 4, 1h sample; Lane 5, 12h sample; Lane 6, 24h sample; Lane 7, 48h sample; Lane 8, 96h sample; Lane 9, -RT control. The expected amplicon size for Smad3 is 541 bp. Figures 5 and 6. mrna expression of TGF-β1 (Fig.5, top panel), TGF-β2 (Fig. 5, middle panel) and TGF-β3 (Fig.5, bottom panel), TβRI (Fig.6, top panel) and TβRII (Fig. 7, bottom panel) in separated granulosa and theca cells from hcg treated follicles at 0 h (control), 12 h, 24 h, and 48 64
79 h post-hcg. Quantification of mrna was performed using semi-quantitative PCR, with band densities expressed as the mean ± SEM (n = 2 animals per time) of ratios relative to H2A. Figures 7 and 8. Protein expression of TGF-β ligands (Fig.7, top panel), receptors (Fig. 7, bottom panel), Smad 3 and phosphorylated Smad 3 (Fig. 8) in hcg-treated follicles at 0 h (control), 1 h, 12 h, 24 h, 48 h and 96 h post-hcg. Quantification of protein was performed using Western blot analyses, with band densities expressed as the least squares mean ± SEM (n = 4-7 animals per time) of ratios relative to β-actin. Data were analyzed using PROC GLM ANOVA, followed by Scheffe Multiple Comparison Test using SAS. None of the means were significantly (based on p<0.05; versus 0h post-hcg) different over time post- hcg. Figure 9. Representative Western blots from hcg-treated follicles. Top panel, TGF-β ligands TGF-β1 and TGF-β2. Middle panel, TGF-β receptors TβRI and TβRII. Bottom panel, Smad3 and psmad3. The expected size of each product is given for each protein adjacent to its blot. Figure 10. Immunohistochemical localization of TGF-β1 (1 st row), TGF-β2 (2 nd row), TβRI (3 rd row) and TβRII (4 th row) in representative sections from hcg treated follicles at 0 h (control), 48 h and 96 h post-hcg. For the negative control (last row), the primary antibody was substituted with normal goat serum. The antrum (A), granulosa cells (GC), theca cells (TC) and luteinizing cells (LC) are labeled. Magnification 200 x. 65
80 Table 1. Primers used for semi-q RT-PCR Gene Primer sequence (5-3 ) TGF-β1 Extension cycles F:CTGTGTCTGTCCACCATTCATTTG R:CAACTTTGCTATGTCTGTCTCCCC Product size (bp) TGF-β2 TGF-β3 TβRI TβRII Smad2 Smad3 Smad4 Smad6 Smad7 SARA H2A Cyclin-D2 Aromatase F:CATCTCCTGCTAATGTTGTTGCC R:CGGTTCTAAATCCTGGGACACG F:CAGACTGGAAGATGCTTGGAGC R:CTTAGAGGGGTCAGGAGAGGAAAC F:GCAGTATGTCTTCTAGCCTGCCT R:TTCGCAGGCAGCTAACTGTATCC F:CTGAGGCAGAACACATCTGAGC R:TCGCTGTGCAGGTGTGCAATGC F:GAAGAGAAGTGGTGTGAGAAAGCAG R:AATACTGGAGGCAAAACTGGTGTC F:TGGAGGAGGTGGAGAAATCAGAAC R:CACACTCGCTTGCTCACTGTAATC F:CCTGAGTATTGGTGTTCCATTGC R:TGATGCTCTGCCTTGGGTAATC F:CGTCAGCATCTTCTACGACCTACC R:TATCTGGGGAGGGTTCTTCCG F:TACTGGGAGGAGAAGACGAGAGTG R:TGGCTGACTTGATGAAGATGGG F:TCCACATTTCTTCTCTCCCACTTC R:CAGTTTCTGAGTCACAGCCACAAC F:AGGACGACTAGCCATGGACGTGTG R:CCACCACCAGCAATTGTAGCCTTG F:CCCCTACCCCCTTCAAAAACTC R:TGTTGTTGACACTTCTGCTCCTCAC F:CATCACCAAGCACCTGGACAAG R:TAGTTCCTCTTCAACCTGGGGG StAR F: GAGTGGTTCCATTCTTCCAGAGC R: CAACTATCCCTTCCATCCGTCTC
81 Table 2. Summary table of ovarian characteristics at ovariectomy across all animals Time post-hcg 0h 1h 12h 24h 48h 96h Number of unovulated follicles Number of Corpora Lutea
82 CyclinD2 Aromatase StAR mrna levels normalized to H2A * * * 0h 1h 12h 24h 48h 96h Time post-hcg Figure 1. mrna expression patterns of some LH-responsive genes in hcg-treated follicles 68
83 mrna levels normalized to H2A TGFβ1 TGFb1 TGFβ2 TGFb2 TGFb3 TGFβ3 * 0h 1h 12h 24h 48h 96h Time post-hcg TB TβRI TBRII TβRII mrna levels normalized to H2A h 1h 12h 24h 48h 96h Time post-hcg * Figure 2. mrna expression patterns of TGF-β ligands and receptors in hcg-treated follicles 69
84 Smad2 Smad3 Smad4 SARA mrna levels normalized to H2A h 1h 12h 24h 48h 96h Time post-hcg Smad6 Smad7 mrna levels normalized to H2A h 1h 12h 24h 48h 96h Time post-hcg Figure 3. mrna expression patterns of stimulatory Smads, SARA and inhibitory Smads in hcgtreated follicles 70
85 Figure 4. Example of an ethidium bromide-stained agarose gel of Smad3 amplification products from hcg-treated follicles 71
86 GC TC TGF-b1 β1 mrna expression normalized to H2A h 12h 24h 48h Time post-hcg GC TC TGF-b2 β2 mrna expression normalized to H2A h 12h 24h 48h Time post-hcg GC TC TGF-b3 β3 mrna expression normalized to H2A h 12h 24h 48h Tim e post-hcg Figure 5. mrna expression patterns of TGF-β ligands in granulosa and theca compartments separated from hcg-treated follicles 72
87 GC TC TbRI β mrna expression normalized to H2A h 12h 24h 48h Time post-hcg GC TC TbRII β mrna expression normalized to H2A h 12h 24h 48h Time post-hcg Figure 6. mrna expression patterns of TGF-β receptors in granulosa and theca compartments separated from hcg-treated follicles 73
88 TGFβ1 TGFb1 TGFb2 TGFβ2 Protein levels normalized to β- b-actin h 1h 12h 24h 48h 96h Time post-hcg TBRI TβRI TBRII TβRII Protein levels normalized to β- b-actin h 1h 12h 24h 48h 96h Time post-hcg Figure 7. Protein expression of TGF-β ligands and receptors in hcg-treated follicles 74
89 Smad3 psmad3 Protein levels normalized to b-actin β h 1h 12h 24h 48h 96h Time post-hcg Figure 8. Protein expression of Smad3 and p-smad3 in hcg-treated follicles 75
90 TGFβ1 TGFβ2 44 kda 47 kda TβRI TβRII 53 kda 70 kda Smad3 psmad3 65 kda 50 kda Figure 9. Representative Western blots of TGF-β ligands, receptors, Smad3 and psmad3 76
91 77
REPRODUCTIVE CYCLE OF FEMALE MAMMAL
 REPRODUCTIVE CYCLE OF FEMALE MAMMAL Fig. 8-12 Secondary follicles growing follicles increase in number of layers of granulosa cells Tertiary follicles maturing follicles antrum formation fluid filled space
REPRODUCTIVE CYCLE OF FEMALE MAMMAL Fig. 8-12 Secondary follicles growing follicles increase in number of layers of granulosa cells Tertiary follicles maturing follicles antrum formation fluid filled space
OVARY The surface of the ovary is covered with surface epithelium
 OVARY Cow The ovary, or female gonad, is: 1. an exocrine gland, producing oocytes 2. an endocrine gland, secreting hormones, i.e., estrogen and progesterone OVARY OVARY The surface of the ovary is covered
OVARY Cow The ovary, or female gonad, is: 1. an exocrine gland, producing oocytes 2. an endocrine gland, secreting hormones, i.e., estrogen and progesterone OVARY OVARY The surface of the ovary is covered
The intra-follicular molecular biology mandating advancement of egg retrieval in some women
 The intra-follicular molecular biology mandating advancement of egg retrieval in some women David H. Barad, USA Director of Assisted Reproductive Technology, The Center for Human Reproduction New York
The intra-follicular molecular biology mandating advancement of egg retrieval in some women David H. Barad, USA Director of Assisted Reproductive Technology, The Center for Human Reproduction New York
Animal Science 434! Tonic and Preovulatory Surge of GnRH! Tonic and Preovulatory Surge of GnRH! Lecture 11: The Follicular Phase of the Estrous Cycle!
 Tonic and Preovulatory Surge of GnRH! Animal Science 434! Lecture 11: The Follicular Phase of the Estrous Cycle!! (-)! Hypothalamus! GnRH! Estradiol! (-)! Tonic and Preovulatory Surge of GnRH! Anterior!
Tonic and Preovulatory Surge of GnRH! Animal Science 434! Lecture 11: The Follicular Phase of the Estrous Cycle!! (-)! Hypothalamus! GnRH! Estradiol! (-)! Tonic and Preovulatory Surge of GnRH! Anterior!
The reproductive lifespan
 The reproductive lifespan Reproductive potential Ovarian cycles Pregnancy Lactation Male Female Puberty Menopause Age Menstruation is an external indicator of ovarian events controlled by the hypothalamicpituitary
The reproductive lifespan Reproductive potential Ovarian cycles Pregnancy Lactation Male Female Puberty Menopause Age Menstruation is an external indicator of ovarian events controlled by the hypothalamicpituitary
GENERAL SUMMARY Corpus luteum is a transient endocrine structure formed from the ruptured ovarian follicle. Its main function is to secrete P 4, a pro
 Corpus luteum is a transient endocrine structure formed from the ruptured ovarian follicle. Its main function is to secrete P 4, a pro-gestational hormone, essential for establishment and maintenance of
Corpus luteum is a transient endocrine structure formed from the ruptured ovarian follicle. Its main function is to secrete P 4, a pro-gestational hormone, essential for establishment and maintenance of
THE MENSTRUAL CYCLE INA S. IRABON, MD, FPOGS, FPSRM, FPSGE OBSTETRICS AND GYNECOLOGY REPRODUCTIVE ENDOCRINOLOGY AND INFERTILITY
 THE MENSTRUAL CYCLE INA S. IRABON, MD, FPOGS, FPSRM, FPSGE OBSTETRICS AND GYNECOLOGY REPRODUCTIVE ENDOCRINOLOGY AND INFERTILITY REFERENCE Comprehensive Gynecology 7 th edition, 2017 (Lobo RA, Gershenson
THE MENSTRUAL CYCLE INA S. IRABON, MD, FPOGS, FPSRM, FPSGE OBSTETRICS AND GYNECOLOGY REPRODUCTIVE ENDOCRINOLOGY AND INFERTILITY REFERENCE Comprehensive Gynecology 7 th edition, 2017 (Lobo RA, Gershenson
Two important cells in female are the theca cells and the granulose cells. Granulosa cells are affected by the two gonadotropin hormones; FSH and LH.
 1 UGS physiology sheet #13 lecture 3 Dr.Saleem Khresha. Now we will start discussing the female reproductive system Ovarian Steroids Two important cells in female are the theca cells and the granulose
1 UGS physiology sheet #13 lecture 3 Dr.Saleem Khresha. Now we will start discussing the female reproductive system Ovarian Steroids Two important cells in female are the theca cells and the granulose
Chapter 27 The Reproductive System. MDufilho
 Chapter 27 The Reproductive System 1 Figure 27.19 Events of oogenesis. Before birth Meiotic events 2n Oogonium (stem cell) Mitosis Follicle development in ovary Follicle cells Oocyte 2n Primary oocyte
Chapter 27 The Reproductive System 1 Figure 27.19 Events of oogenesis. Before birth Meiotic events 2n Oogonium (stem cell) Mitosis Follicle development in ovary Follicle cells Oocyte 2n Primary oocyte
CASE 41. What is the pathophysiologic cause of her amenorrhea? Which cells in the ovary secrete estrogen?
 CASE 41 A 19-year-old woman presents to her gynecologist with complaints of not having had a period for 6 months. She reports having normal periods since menarche at age 12. She denies sexual activity,
CASE 41 A 19-year-old woman presents to her gynecologist with complaints of not having had a period for 6 months. She reports having normal periods since menarche at age 12. She denies sexual activity,
Female Reproductive Physiology. Dr Raelia Lew CREI, FRANZCOG, PhD, MMed, MBBS Fertility Specialist, Melbourne IVF
 Female Reproductive Physiology Dr Raelia Lew CREI, FRANZCOG, PhD, MMed, MBBS Fertility Specialist, Melbourne IVF REFERENCE Lew, R, Natural History of ovarian function including assessment of ovarian reserve
Female Reproductive Physiology Dr Raelia Lew CREI, FRANZCOG, PhD, MMed, MBBS Fertility Specialist, Melbourne IVF REFERENCE Lew, R, Natural History of ovarian function including assessment of ovarian reserve
ABSTRACT. Key words: ovulation, ovary, human, follicle, collagen, MMP and TIMP. ISBN-10: ISBN-13:
 HUMAN OVULATION Studies on collagens, gelatinases and tissue inhibitors of metalloproteinases Anna Karin Lind Department of Obstetrics and Gynecology Institute of Clinical Sciences Sahlgrenska University
HUMAN OVULATION Studies on collagens, gelatinases and tissue inhibitors of metalloproteinases Anna Karin Lind Department of Obstetrics and Gynecology Institute of Clinical Sciences Sahlgrenska University
Ovarian follicular development in cattle
 Ovarian follicular development in cattle John P Kastelic Professor of Theriogenology Head, Department of Production Animal Health University of Calgary Calgary, Alberta, Canada Overview Prenatal development
Ovarian follicular development in cattle John P Kastelic Professor of Theriogenology Head, Department of Production Animal Health University of Calgary Calgary, Alberta, Canada Overview Prenatal development
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
Embryology Lecture # 4
 1 Quick Review: Oogenesis : - Oogonia start appear in the ovary when the age of the fetus 1 is th (5 week). - Then the Oogonia transformed into 1ry Oocyte. - 1ry Oocyte is surrounded by a follicle (cover).
1 Quick Review: Oogenesis : - Oogonia start appear in the ovary when the age of the fetus 1 is th (5 week). - Then the Oogonia transformed into 1ry Oocyte. - 1ry Oocyte is surrounded by a follicle (cover).
Mohammad. Renad zakaria ---
 13 Mohammad Renad zakaria --- Before we start: - I didn t follow the record order, for organizing purposes. - I added extra information from our text box which is Guyton 12 th edition, pages 987-997, actually
13 Mohammad Renad zakaria --- Before we start: - I didn t follow the record order, for organizing purposes. - I added extra information from our text box which is Guyton 12 th edition, pages 987-997, actually
REPRODUCCIÓN. La idea fija. Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
Biology of Reproduction- Zool 346 Exam 2
 Biology of Reproduction- Zool 346 Exam 2 ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER. Some critical words are boldfaced. This exam is 7 pages long.
Biology of Reproduction- Zool 346 Exam 2 ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER. Some critical words are boldfaced. This exam is 7 pages long.
FREE-ROAMING HORSE AND BURRO FERTILITY CONTROL WORKSHOP Albuquerque, NM November 8, 2018
 FREE-ROAMING HORSE AND BURRO FERTILITY CONTROL WORKSHOP Albuquerque, NM November 8, 2018 Current Contraceptive Use pzp GonaCon Porcine Zona Pellucida Antibodies to ZP3 Cons: Requires boosters Continuous
FREE-ROAMING HORSE AND BURRO FERTILITY CONTROL WORKSHOP Albuquerque, NM November 8, 2018 Current Contraceptive Use pzp GonaCon Porcine Zona Pellucida Antibodies to ZP3 Cons: Requires boosters Continuous
Obesity, Metabolic Hormone Signaling, and Granulosa Cell Gene Expression
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Theses and Dissertations in Animal Science Animal Science Department 12-2010 Obesity, Metabolic Hormone Signaling, and Granulosa
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Theses and Dissertations in Animal Science Animal Science Department 12-2010 Obesity, Metabolic Hormone Signaling, and Granulosa
Animal and Veterinary Science Department University of Idaho. REGULATION OF REPRODUCTION AVS 222 (Instructor: Dr. Amin Ahmadzadeh) Chapter 5
 Animal and Veterinary Science Department University of Idaho REGULATION OF REPRODUCTION AVS 222 (Instructor: Dr. Amin Ahmadzadeh) Chapter 5 I. DEFINITIONS A. Endocrine Gland B. Hormone Chemical messenger
Animal and Veterinary Science Department University of Idaho REGULATION OF REPRODUCTION AVS 222 (Instructor: Dr. Amin Ahmadzadeh) Chapter 5 I. DEFINITIONS A. Endocrine Gland B. Hormone Chemical messenger
The role of growth factors in regulating cellular events during ovarian follicular development Leon J. Spicer
 The role of growth factors in regulating cellular events during ovarian follicular development Leon J. Spicer Department of Animal Science, Oklahoma State University, Stillwater, OK USA SESSION #54 EAAP
The role of growth factors in regulating cellular events during ovarian follicular development Leon J. Spicer Department of Animal Science, Oklahoma State University, Stillwater, OK USA SESSION #54 EAAP
Regulation of Ovarian Follicular Development in Primates: Facts and Hypotheses
 0163-769X/96/$03.00/0 Endocrine Reviews Copyright 1996 by The Endocrine Society Vol. 17, No. 2 Printed in U.S.A. Regulation of Ovarian Follicular Development in Primates: Facts and Hypotheses ALAIN GOUGEON
0163-769X/96/$03.00/0 Endocrine Reviews Copyright 1996 by The Endocrine Society Vol. 17, No. 2 Printed in U.S.A. Regulation of Ovarian Follicular Development in Primates: Facts and Hypotheses ALAIN GOUGEON
Endocrine control of female reproductive function
 Medicine School of Women s & Children s Health Discipline of Obstetrics & Gynaecology Endocrine control of female reproductive function Kirsty Walters, PhD Fertility Research Centre, School of Women s
Medicine School of Women s & Children s Health Discipline of Obstetrics & Gynaecology Endocrine control of female reproductive function Kirsty Walters, PhD Fertility Research Centre, School of Women s
Reproductive Endocrinology. Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007
 Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Reproduction. Mammalian Ovary. Amitabh Krishna; Raj Kamal Srivastava Arnab Banerjee
 Reproduction Mammalian Ovary Amitabh Krishna; Raj Kamal Srivastava Arnab Banerjee Department of Zoology, Banaras Hindu University, Varanasi-221005, India Contents Introduction Development of the ovary
Reproduction Mammalian Ovary Amitabh Krishna; Raj Kamal Srivastava Arnab Banerjee Department of Zoology, Banaras Hindu University, Varanasi-221005, India Contents Introduction Development of the ovary
Raoul Orvieto. The Chaim Sheba Medical Center Tel Hashomer, Israel. Declared no potential conflict of interest
 Raoul Orvieto The Chaim Sheba Medical Center Tel Hashomer, Israel Declared no potential conflict of interest LH in antagonist cycles; is the story really written? Raoul Orvieto M.D. Israel Overview Role
Raoul Orvieto The Chaim Sheba Medical Center Tel Hashomer, Israel Declared no potential conflict of interest LH in antagonist cycles; is the story really written? Raoul Orvieto M.D. Israel Overview Role
AnS 214 SI Multiple Choice Set 4 Weeks 10/14-10/23
 AnS 214 SI Multiple Choice Set 4 Weeks 10/14-10/23 The following multiple choice questions pertain to material covered in the last two weeks' lecture sets. Answering the following questions will aid your
AnS 214 SI Multiple Choice Set 4 Weeks 10/14-10/23 The following multiple choice questions pertain to material covered in the last two weeks' lecture sets. Answering the following questions will aid your
Endocrinology of the Female Reproductive Axis
 Endocrinology of the Female Reproductive Axis girlontheriver.com Geralyn Lambert-Messerlian, PhD, FACB Professor Women and Infants Hospital Alpert Medical School at Brown University Women & Infants BROWN
Endocrinology of the Female Reproductive Axis girlontheriver.com Geralyn Lambert-Messerlian, PhD, FACB Professor Women and Infants Hospital Alpert Medical School at Brown University Women & Infants BROWN
Embryology 3. Spermatogenesis:
 Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Reproductive System. Testes. Accessory reproductive organs. gametogenesis hormones. Reproductive tract & Glands
 Reproductive System Testes gametogenesis hormones Accessory reproductive organs Reproductive tract & Glands transport gametes provide nourishment for gametes Hormonal regulation in men Hypothalamus - puberty
Reproductive System Testes gametogenesis hormones Accessory reproductive organs Reproductive tract & Glands transport gametes provide nourishment for gametes Hormonal regulation in men Hypothalamus - puberty
GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1
 GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1 1. The endocrine system consists of glands that secrete chemical signals, called hormones, into the blood. In addition, other organs and cells
GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1 1. The endocrine system consists of glands that secrete chemical signals, called hormones, into the blood. In addition, other organs and cells
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Age-Related Changes in Major Ovarian Follicle Wave Dynamics: Morphologic and Endocrinologic Characteristics
 Age-Related Changes in Major Ovarian Follicle Wave Dynamics: Morphologic and Endocrinologic Characteristics A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfillment of
Age-Related Changes in Major Ovarian Follicle Wave Dynamics: Morphologic and Endocrinologic Characteristics A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfillment of
Investigation: The Human Menstrual Cycle Research Question: How do hormones control the menstrual cycle?
 Investigation: The Human Menstrual Cycle Research Question: How do hormones control the menstrual cycle? Introduction: The menstrual cycle (changes within the uterus) is an approximately 28-day cycle that
Investigation: The Human Menstrual Cycle Research Question: How do hormones control the menstrual cycle? Introduction: The menstrual cycle (changes within the uterus) is an approximately 28-day cycle that
10.7 The Reproductive Hormones
 10.7 The Reproductive Hormones December 10, 2013. Website survey?? QUESTION: Who is more complicated: men or women? The Female Reproductive System ovaries: produce gametes (eggs) produce estrogen (steroid
10.7 The Reproductive Hormones December 10, 2013. Website survey?? QUESTION: Who is more complicated: men or women? The Female Reproductive System ovaries: produce gametes (eggs) produce estrogen (steroid
Concept : effects by treatment
 QuickTime and a TIFF (LZW) decompressor are needed to see this picture. ESHRE CAMPUS 2010 MARIBOR INFLUENCE of stimulation on oocyte quality? Johan Smitz, MD, PhD UZBrussel Vrije Universiteit Brussel Brussels,
QuickTime and a TIFF (LZW) decompressor are needed to see this picture. ESHRE CAMPUS 2010 MARIBOR INFLUENCE of stimulation on oocyte quality? Johan Smitz, MD, PhD UZBrussel Vrije Universiteit Brussel Brussels,
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
A Tale of Three Hormones: hcg, Progesterone and AMH
 A Tale of Three Hormones: hcg, Progesterone and AMH Download the Ferring AR ipad/iphone app from the Apple Store: http://bit.ly/1okk74m Human Ovarian Steroidogenesis and Gonadotrophin Stimulation Johan
A Tale of Three Hormones: hcg, Progesterone and AMH Download the Ferring AR ipad/iphone app from the Apple Store: http://bit.ly/1okk74m Human Ovarian Steroidogenesis and Gonadotrophin Stimulation Johan
Reproduction and Development. Female Reproductive System
 Reproduction and Development Female Reproductive System Outcomes 5. Identify the structures in the human female reproductive system and describe their functions. Ovaries, Fallopian tubes, Uterus, Endometrium,
Reproduction and Development Female Reproductive System Outcomes 5. Identify the structures in the human female reproductive system and describe their functions. Ovaries, Fallopian tubes, Uterus, Endometrium,
Folliculogenesis: Physiology and pathophysiology
 DIMITRIOS LOUTRADIS Professor of Obstetrics and Gynaecology Head of 1st Department of Obstetrics and Gynaecology University of Athens Medical School Alexandra Hospital Folliculogenesis: Physiology and
DIMITRIOS LOUTRADIS Professor of Obstetrics and Gynaecology Head of 1st Department of Obstetrics and Gynaecology University of Athens Medical School Alexandra Hospital Folliculogenesis: Physiology and
Chapter 14 Reproduction Review Assignment
 Date: Mark: _/45 Chapter 14 Reproduction Review Assignment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Use the diagram above to answer the next question.
Date: Mark: _/45 Chapter 14 Reproduction Review Assignment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Use the diagram above to answer the next question.
Gametogenesis. Dr Corinne de Vantéry Arrighi Dr Hervé Lucas
 WHO Collaborating Center for Research in Human Reproduction Clinic for Infertility and Gynecological Endocrinology University Hospital, Geneva, Switzerland Gametogenesis Dr Corinne de Vantéry Arrighi Dr
WHO Collaborating Center for Research in Human Reproduction Clinic for Infertility and Gynecological Endocrinology University Hospital, Geneva, Switzerland Gametogenesis Dr Corinne de Vantéry Arrighi Dr
Reproductive Hormones
 Reproductive Hormones Male gonads: testes produce male sex cells! sperm Female gonads: ovaries produce female sex cells! ovum The union of male and female sex cells during fertilization produces a zygote
Reproductive Hormones Male gonads: testes produce male sex cells! sperm Female gonads: ovaries produce female sex cells! ovum The union of male and female sex cells during fertilization produces a zygote
Physiology of Male Reproductive System
 Physiology of Male Reproductive System the anterior pituitary gland serves as the primary control of reproductive function at puberty Ant Pituitary secretes FSH & large amounts of LH (ICSH) FSH & LH cause
Physiology of Male Reproductive System the anterior pituitary gland serves as the primary control of reproductive function at puberty Ant Pituitary secretes FSH & large amounts of LH (ICSH) FSH & LH cause
Fertility Diagnostics
 Fertility Diagnostics Fertility hormones measured on PATHFAST For internal use only Diagnostics PATHFAST Chemiluminescence-immuno-analyzer 1 Content: page 1. Fertility hormones - general aspects 1.1 Reproductive
Fertility Diagnostics Fertility hormones measured on PATHFAST For internal use only Diagnostics PATHFAST Chemiluminescence-immuno-analyzer 1 Content: page 1. Fertility hormones - general aspects 1.1 Reproductive
Reproductive physiology
 Reproductive physiology Sex hormones: Androgens Estrogens Gestagens Learning objectives 86 (also 90) Sex Genetic sex Gonadal sex Phenotypic sex XY - XX chromosomes testes - ovaries external features Tha
Reproductive physiology Sex hormones: Androgens Estrogens Gestagens Learning objectives 86 (also 90) Sex Genetic sex Gonadal sex Phenotypic sex XY - XX chromosomes testes - ovaries external features Tha
Female Reproductive System. Lesson 10
 Female Reproductive System Lesson 10 Learning Goals 1. What are the five hormones involved in the female reproductive system? 2. Understand the four phases of the menstrual cycle. Human Reproductive System
Female Reproductive System Lesson 10 Learning Goals 1. What are the five hormones involved in the female reproductive system? 2. Understand the four phases of the menstrual cycle. Human Reproductive System
BIOSYNTHESIS OF STEROID HORMONES
 BIOSYNTHESIS OF STEROID HORMONES Sri Widia A Jusman Department of Biochemistry & Molecular Biology FMUI sw/steroidrepro/inter/08 1 STEROID HORMONES Progestins (21 C) Glucocorticoids (21 C) Mineralocorticoids
BIOSYNTHESIS OF STEROID HORMONES Sri Widia A Jusman Department of Biochemistry & Molecular Biology FMUI sw/steroidrepro/inter/08 1 STEROID HORMONES Progestins (21 C) Glucocorticoids (21 C) Mineralocorticoids
I. Endocrine System & Hormones Figure 1: Human Endocrine System
 I. Endocrine System & Hormones Figure 1: Human Endocrine System Endocrine System: a) Endocrine glands are ductless since they lack specific vessels for the transport of hormones throughout the body. Instead,
I. Endocrine System & Hormones Figure 1: Human Endocrine System Endocrine System: a) Endocrine glands are ductless since they lack specific vessels for the transport of hormones throughout the body. Instead,
Role of oocyte-secreted factors in prevention of cumulus cell apoptosis and enhancement of oocyte developmental competence
 Role of oocyte-secreted factors in prevention of cumulus cell apoptosis and enhancement of oocyte developmental competence Tamer Hussein, MScMed Research Centre for Reproductive Health, Discipline of Obstetrics
Role of oocyte-secreted factors in prevention of cumulus cell apoptosis and enhancement of oocyte developmental competence Tamer Hussein, MScMed Research Centre for Reproductive Health, Discipline of Obstetrics
REPRODUCTION & GENETICS. Hormones
 REPRODUCTION & GENETICS Hormones http://www.youtube.com/watch?v=np0wfu_mgzo Objectives 2 Define what hormones are; Compare and contrast the male and female hormones; Explain what each hormone in the mail
REPRODUCTION & GENETICS Hormones http://www.youtube.com/watch?v=np0wfu_mgzo Objectives 2 Define what hormones are; Compare and contrast the male and female hormones; Explain what each hormone in the mail
Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature
 REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
Hormone Balance - Female Report SAMPLE. result graph based on Luteal Phase. result graph based on Luteal Phase
 Patient Name: Patient DOB: Gender: Physician: Test Hormone Balance - Female Report SAMPLE Grote, Mary Jane Batch Number: B6437 2/16/1954 Accession Number: N52281 F Date Received: 2/3/2015 Any Lab Test
Patient Name: Patient DOB: Gender: Physician: Test Hormone Balance - Female Report SAMPLE Grote, Mary Jane Batch Number: B6437 2/16/1954 Accession Number: N52281 F Date Received: 2/3/2015 Any Lab Test
Page 1. A wide variety of ovarian abnormalities are encountered in clinical practice
 A wide variety of ovarian abnormalities are encountered in clinical practice Common Problems Anovulatory follicles Persistent anovulatory follicles Hemorrhagic/Luteinized follicles Persistent corpus luteum
A wide variety of ovarian abnormalities are encountered in clinical practice Common Problems Anovulatory follicles Persistent anovulatory follicles Hemorrhagic/Luteinized follicles Persistent corpus luteum
Abstracts for the KSAR and JSAR Joint Symposium. Fertility control in female domestic animals: From basic understanding to application
 Abstracts for the KSAR and JSAR Joint Symposium Fertility control in female domestic animals: From basic understanding to application Current Research Orientation in Livestock Reproduction in Korea Choong-Saeng
Abstracts for the KSAR and JSAR Joint Symposium Fertility control in female domestic animals: From basic understanding to application Current Research Orientation in Livestock Reproduction in Korea Choong-Saeng
Chemical Classification of Hormones
 Steroid Hormones Chemical Classification of Hormones Hormones are chemical messengers that transport signals from one cell to another There are 4 major chemical classes of hormones steroid hormones - i.e.
Steroid Hormones Chemical Classification of Hormones Hormones are chemical messengers that transport signals from one cell to another There are 4 major chemical classes of hormones steroid hormones - i.e.
Reproduction. AMH Anti-Müllerian Hormone. Analyte Information
 Reproduction AMH Anti-Müllerian Hormone Analyte Information - 1-2011-01-11 AMH Anti-Müllerian Hormone Introduction Anti-Müllerian Hormone (AMH) is a glycoprotein dimer composed of two 72 kda monomers 1.
Reproduction AMH Anti-Müllerian Hormone Analyte Information - 1-2011-01-11 AMH Anti-Müllerian Hormone Introduction Anti-Müllerian Hormone (AMH) is a glycoprotein dimer composed of two 72 kda monomers 1.
Web Activity: Simulation Structures of the Female Reproductive System
 differentiate. The epididymis is a coiled tube found along the outer edge of the testis where the sperm mature. 3. Testosterone is a male sex hormone produced in the interstitial cells of the testes. It
differentiate. The epididymis is a coiled tube found along the outer edge of the testis where the sperm mature. 3. Testosterone is a male sex hormone produced in the interstitial cells of the testes. It
Biochimica et Biophysica Acta
 Biochimica et Biophysica Acta 1822 (2012) 1896 1912 Contents lists available at SciVerse ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbadis Review Molecular control
Biochimica et Biophysica Acta 1822 (2012) 1896 1912 Contents lists available at SciVerse ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbadis Review Molecular control
ROLE OF HORMONAL ASSAY IN DIAGNOSING PCOD DR GAANA SREENIVAS (JSS,MYSURU)
 ROLE OF HORMONAL ASSAY IN DIAGNOSING PCOD DR GAANA SREENIVAS (JSS,MYSURU) In 1935, Stein and Leventhal described 7 women with bilateral enlarged PCO, amenorrhea or irregular menses, infertility and masculinizing
ROLE OF HORMONAL ASSAY IN DIAGNOSING PCOD DR GAANA SREENIVAS (JSS,MYSURU) In 1935, Stein and Leventhal described 7 women with bilateral enlarged PCO, amenorrhea or irregular menses, infertility and masculinizing
Why Cycle Control?" Manipulating Ovulation and Estrous Synchronization" Manipulating Ovulation" Cattle" Principle of PGF 2α Use"
 Why Cycle Control?" Manipulating Ovulation and Estrous Synchronization" John Parrish 1. Group females for parturition: " a) Decrease labor, calving period Reduce calving season" b) More uniform weaning
Why Cycle Control?" Manipulating Ovulation and Estrous Synchronization" John Parrish 1. Group females for parturition: " a) Decrease labor, calving period Reduce calving season" b) More uniform weaning
Female Reproduction 1 and 2: The Menstrual Cycle R.J. Witorsch, Ph.D.
 Female Reproduction 1 and 2: The Menstrual Cycle R.J. Witorsch, Ph.D. OBJECTIVES: At the end of this block of lectures, the student should be able to: 1. Describe the functional anatomy of ovary with particular
Female Reproduction 1 and 2: The Menstrual Cycle R.J. Witorsch, Ph.D. OBJECTIVES: At the end of this block of lectures, the student should be able to: 1. Describe the functional anatomy of ovary with particular
Reproductive FSH. Analyte Information
 Reproductive FSH Analyte Information 1 Follicle-stimulating hormone Introduction Follicle-stimulating hormone (FSH, also known as follitropin) is a glycoprotein hormone secreted by the anterior pituitary
Reproductive FSH Analyte Information 1 Follicle-stimulating hormone Introduction Follicle-stimulating hormone (FSH, also known as follitropin) is a glycoprotein hormone secreted by the anterior pituitary
Human Anatomy and Physiology - Problem Drill 16: The Endocrine System
 Human Anatomy and Physiology - Problem Drill 16: The Endocrine System Question No. 1 of 10 The endocrine system is made up of a number of organs and glands. Which one of the following is not an organ or
Human Anatomy and Physiology - Problem Drill 16: The Endocrine System Question No. 1 of 10 The endocrine system is made up of a number of organs and glands. Which one of the following is not an organ or
9.4 Regulating the Reproductive System
 9.4 Regulating the Reproductive System The Reproductive System to unite a single reproductive cell from a female with a single reproductive cell from a male Both male and female reproductive systems include
9.4 Regulating the Reproductive System The Reproductive System to unite a single reproductive cell from a female with a single reproductive cell from a male Both male and female reproductive systems include
Phases of the Ovarian Cycle
 OVARIAN CYCLE An ovary contains many follicles, and each one contains an immature egg called an oocyte. A female is born with as many as 2 million follicles, but the number is reduced to 300,000 to 400,000
OVARIAN CYCLE An ovary contains many follicles, and each one contains an immature egg called an oocyte. A female is born with as many as 2 million follicles, but the number is reduced to 300,000 to 400,000
Reproductive cyclicity 19. Introduction. Page 1. repro and its story lines. Male repro: a simpler way of control
 Reproductive cyclicity 19 Male repro: a simpler way of control Menstrual cycles: ovary / uterine anatomy and cell types, follicular phase, ovulation, luteal phase, cyclicity Race events: removal of P4
Reproductive cyclicity 19 Male repro: a simpler way of control Menstrual cycles: ovary / uterine anatomy and cell types, follicular phase, ovulation, luteal phase, cyclicity Race events: removal of P4
Reproductive Cyclicity. Reproductive cycles are present so that offspring are presented at a time providing maximal survival
 Reproductive Cyclicity Reproductive cycles are present so that offspring are presented at a time providing maximal survival Proximate Factors control adult reproductive activity photoperiod ambient temperature
Reproductive Cyclicity Reproductive cycles are present so that offspring are presented at a time providing maximal survival Proximate Factors control adult reproductive activity photoperiod ambient temperature
Endocrinology laboratory Department of Zoology Kalyani University Kalyani, West Bengal India
 Epidermal growth factor (EGF) promotes ovarian steroidogenesis and epidermal growth factor receptor (EGFR) signaling is required for gonadotropin-induced steroid production in common carp Cyprinus carpio
Epidermal growth factor (EGF) promotes ovarian steroidogenesis and epidermal growth factor receptor (EGFR) signaling is required for gonadotropin-induced steroid production in common carp Cyprinus carpio
The menstrual Cycle. François Pralong
 The menstrual Cycle François Pralong Services d Endocrinologie, Diabétologie et Métabolisme, Hôpitaux Universitaires de Genève et Lausanne Centre des Maladies CardioVasculaires et Métaboliques, Lausanne
The menstrual Cycle François Pralong Services d Endocrinologie, Diabétologie et Métabolisme, Hôpitaux Universitaires de Genève et Lausanne Centre des Maladies CardioVasculaires et Métaboliques, Lausanne
ABSTRACT. RODRIGUEZ, KARINA FLORES. Molecular Mechanisms of Gonadotropin-Induced. Oocyte Maturation. (Under the direction of Dr. Charlotte E.
 ABSTRACT RODRIGUEZ, KARINA FLORES. Molecular Mechanisms of Gonadotropin-Induced Oocyte Maturation. (Under the direction of Dr. Charlotte E. Farin) In vitro maturation of oocytes is routinely utilized for
ABSTRACT RODRIGUEZ, KARINA FLORES. Molecular Mechanisms of Gonadotropin-Induced Oocyte Maturation. (Under the direction of Dr. Charlotte E. Farin) In vitro maturation of oocytes is routinely utilized for
1. During the follicular phase of the ovarian cycle, the hypothalamus releases GnRH.
 1. During the follicular phase of the ovarian cycle, the hypothalamus releases GnRH. 2. This causes the anterior pituitary to secrete small quantities of FSH and LH. 3. At this time, the follicles in the
1. During the follicular phase of the ovarian cycle, the hypothalamus releases GnRH. 2. This causes the anterior pituitary to secrete small quantities of FSH and LH. 3. At this time, the follicles in the
LH activity administration during the
 LH activity administration during the luteal-follicular transition Richard Fleming On behalf of the Luveris Pre-treatment group University of Glasgow Scotland Androgens have a Paracrine action in the Early
LH activity administration during the luteal-follicular transition Richard Fleming On behalf of the Luveris Pre-treatment group University of Glasgow Scotland Androgens have a Paracrine action in the Early
Nutritional and metabolic mechanisms. in the ovarian follicle
 Nutritional and metabolic mechanisms in the ovarian follicle Joëlle Dupont Team Leader : «Interaction Metabolism and Reproduction» Unit of Physiology of Reproduction and Behaviors UMR 6175 INRA/CNRS/Université
Nutritional and metabolic mechanisms in the ovarian follicle Joëlle Dupont Team Leader : «Interaction Metabolism and Reproduction» Unit of Physiology of Reproduction and Behaviors UMR 6175 INRA/CNRS/Université
Sperm production. Sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production. Sperm production. Controlling sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
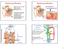 Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Oogenesis. Key Concepts. Female Reproductive Tract
 Oogenesis 1 Key Concepts Female Reproductive Tract Ovary Oogenesis Follicles Ovulation Corpus Luteum Molecular Activity Primordial Germ Cells (PGCs) 2 Female Reproductive Tract Ovary Oviduct Uterus Vagina
Oogenesis 1 Key Concepts Female Reproductive Tract Ovary Oogenesis Follicles Ovulation Corpus Luteum Molecular Activity Primordial Germ Cells (PGCs) 2 Female Reproductive Tract Ovary Oviduct Uterus Vagina
Endocrine secretion cells secrete substances into the extracellular fluid
 Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
CHARACTERISATION OF THE DEVELOPMENT AND HORMONAL REGULATION OF THE OVARIAN LYMPHATIC VASCULATURE
 CHARACTERISATION OF THE DEVELOPMENT AND HORMONAL REGULATION OF THE OVARIAN LYMPHATIC VASCULATURE Hannah Mary Brown Robinson Institute School of Paediatrics and Reproductive Health Research Centre for Reproductive
CHARACTERISATION OF THE DEVELOPMENT AND HORMONAL REGULATION OF THE OVARIAN LYMPHATIC VASCULATURE Hannah Mary Brown Robinson Institute School of Paediatrics and Reproductive Health Research Centre for Reproductive
Reproductive physiology. About this Chapter. Case introduction. The brain directs reproduction 2010/6/29. The Male Reproductive System
 Section Ⅻ Reproductive physiology Ming-jie Wang E-Mail: mjwang@shmu.edu.cn About this Chapter The reproductive organs and how they work the major endocrine functions of sexual glands actions of sex hormones
Section Ⅻ Reproductive physiology Ming-jie Wang E-Mail: mjwang@shmu.edu.cn About this Chapter The reproductive organs and how they work the major endocrine functions of sexual glands actions of sex hormones
Female Reproductive System. Justin D. Vidal
 Female Reproductive System Justin D. Vidal If you cannot identify the tissue, then it is probably part of the female reproductive system! Introduction The female reproductive system is constantly changing,
Female Reproductive System Justin D. Vidal If you cannot identify the tissue, then it is probably part of the female reproductive system! Introduction The female reproductive system is constantly changing,
Hormonal Control of Human Reproduction
 Hormonal Control of Human Reproduction Bởi: OpenStaxCollege The human male and female reproductive cycles are controlled by the interaction of hormones from the hypothalamus and anterior pituitary with
Hormonal Control of Human Reproduction Bởi: OpenStaxCollege The human male and female reproductive cycles are controlled by the interaction of hormones from the hypothalamus and anterior pituitary with
HCG mode of action versus GnRHa action for triggering of final oocyte maturation
 HCG mode of action versus GnRHa action for triggering of final oocyte maturation Nick Macklon Professor of Obstetrics and Gynaecology, University of Southampton A hammer to crack a nut hcg? How hard does
HCG mode of action versus GnRHa action for triggering of final oocyte maturation Nick Macklon Professor of Obstetrics and Gynaecology, University of Southampton A hammer to crack a nut hcg? How hard does
Milder is better? Advantages and disadvantages of "mild" ovarian stimulation for human in vitro fertilization
 Milder is better? Advantages and disadvantages of "mild" ovarian stimulation for human in vitro fertilization Revelli et al. Reproductive Biology and Endocrinology 2011, 9:25 Presenter: R2 孫怡虹 Background
Milder is better? Advantages and disadvantages of "mild" ovarian stimulation for human in vitro fertilization Revelli et al. Reproductive Biology and Endocrinology 2011, 9:25 Presenter: R2 孫怡虹 Background
Close to site of release (at synapse); binds to receptors in
 Chapter 18: The Endocrine System Chemical Messengers 1. Neural 2. Endocrine 3. Neuroendocrine 4. Paracrine 5. Autocrine Endocrine System --Endocrine and nervous systems work together --Endocrine vs. Nervous
Chapter 18: The Endocrine System Chemical Messengers 1. Neural 2. Endocrine 3. Neuroendocrine 4. Paracrine 5. Autocrine Endocrine System --Endocrine and nervous systems work together --Endocrine vs. Nervous
The menstrual cycle. François Pralong
 The menstrual cycle François Pralong Services d Endocrinologie, Diabétologie et Métabolisme, Hôpitaux Universitaires de Genève et Lausanne Centre des Maladies CardioVasculaires et Métaboliques, Lausanne
The menstrual cycle François Pralong Services d Endocrinologie, Diabétologie et Métabolisme, Hôpitaux Universitaires de Genève et Lausanne Centre des Maladies CardioVasculaires et Métaboliques, Lausanne
Hormones. BIT 230 Walsh Chapter 8
 Hormones BIT 230 Walsh Chapter 8 Hormones Regulatory molecules Affect all areas of metabolism Endocrine- hormones travel via the bloodstream to its target cell: true hormone Modern definition- any regulatory
Hormones BIT 230 Walsh Chapter 8 Hormones Regulatory molecules Affect all areas of metabolism Endocrine- hormones travel via the bloodstream to its target cell: true hormone Modern definition- any regulatory
Reproductive System (Hormone Function) Physiology Department Medical School, University of Sumatera Utara
 Reproductive System (Hormone Function) Physiology Department Medical School, University of Sumatera Utara 1 Endocrine Control: Three Levels of Integration Hormones of the hypothalamic-anterior pituitary
Reproductive System (Hormone Function) Physiology Department Medical School, University of Sumatera Utara 1 Endocrine Control: Three Levels of Integration Hormones of the hypothalamic-anterior pituitary
The Human Menstrual Cycle
 The Human Menstrual Cycle Name: The female human s menstrual cycle is broken into two phases: the Follicular Phase and the Luteal Phase. These two phases are separated by an event called ovulation. (1)
The Human Menstrual Cycle Name: The female human s menstrual cycle is broken into two phases: the Follicular Phase and the Luteal Phase. These two phases are separated by an event called ovulation. (1)
1. Be able to characterize the menstrual cycle from the perspective of the ovary a. Follicular phase b. Luteal phase
 Human Sexuality Exam II Review Material Gametogenesis: Oogenesis 1. Be able to characterize the menstrual cycle from the perspective of the ovary a. Follicular phase b. Luteal phase 2. Know the relative
Human Sexuality Exam II Review Material Gametogenesis: Oogenesis 1. Be able to characterize the menstrual cycle from the perspective of the ovary a. Follicular phase b. Luteal phase 2. Know the relative
Stage 4 - Ovarian Cancer Symptoms
 WELCOME Stage 4 - Ovarian Cancer Symptoms University of Baghdad College of Nursing Department of Basic Medical Sciences Overview of Anatomy and Physioloy II Second Year Students Asaad Ismail Ahmad,
WELCOME Stage 4 - Ovarian Cancer Symptoms University of Baghdad College of Nursing Department of Basic Medical Sciences Overview of Anatomy and Physioloy II Second Year Students Asaad Ismail Ahmad,
Menstrual Cycle. Last example of how a circle works. Course Outline. Topic #! Topic lecture! Silverthorn! Membranes (pre-requisite material)!!
 The goal of these lectures is to discuss how control system is formed and operates. For this, basic physiology associated with the control the menstrual cycle will be used. The sections for this lecture
The goal of these lectures is to discuss how control system is formed and operates. For this, basic physiology associated with the control the menstrual cycle will be used. The sections for this lecture
Male Reproduction Organs. 1. Testes 2. Epididymis 3. Vas deferens 4. Urethra 5. Penis 6. Prostate 7. Seminal vesicles 8. Bulbourethral glands
 Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Chapter 22 The Reproductive System (I)
 Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions
 CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions Yi Feng, Peng Cui, Xiaowei Lu, Brian Hsueh, Fredrik Möller Billig, Livia Zarnescu Yanez, Raju Tomer, Derek
CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions Yi Feng, Peng Cui, Xiaowei Lu, Brian Hsueh, Fredrik Möller Billig, Livia Zarnescu Yanez, Raju Tomer, Derek
ENDOCRINE CHARACTERISTICS OF ART CYCLES
 ENDOCRINE CHARACTERISTICS OF ART CYCLES DOÇ. DR. SEBİHA ÖZDEMİR ÖZKAN KOCAELI UNIVERSITY, SCHOOL OF MEDICINE, DEPARTMENT OF OBSTETRICS AND GYNECOLOGY, IVF UNIT 30.04.2014, ANTALYA INTRODUCTION The endocrine
ENDOCRINE CHARACTERISTICS OF ART CYCLES DOÇ. DR. SEBİHA ÖZDEMİR ÖZKAN KOCAELI UNIVERSITY, SCHOOL OF MEDICINE, DEPARTMENT OF OBSTETRICS AND GYNECOLOGY, IVF UNIT 30.04.2014, ANTALYA INTRODUCTION The endocrine
Reproductive Endocrinology
 Reproductive Endocrinology Reproductive Endocrinology Hypothalamic hormones Gonadotropin releasing hormone (GnRH) - stimulate release of FSH = follicle stimulating hormone LH = luteinizing hormone from
Reproductive Endocrinology Reproductive Endocrinology Hypothalamic hormones Gonadotropin releasing hormone (GnRH) - stimulate release of FSH = follicle stimulating hormone LH = luteinizing hormone from
Hormones of brain-testicular axis
 (Hormone Function) Hormones of brain-testicular axis anterior pituitary drives changes during puberty controlled by GnRH from hypothalamus begins to secrete FSH, LH LH targets interstitial endocrinocytes
(Hormone Function) Hormones of brain-testicular axis anterior pituitary drives changes during puberty controlled by GnRH from hypothalamus begins to secrete FSH, LH LH targets interstitial endocrinocytes
