Genetic control of dairy cow reproduction.
|
|
|
- Steven Marshall
- 5 years ago
- Views:
Transcription
1 Provided by the author(s) and University College Dublin Library in accordance with publisher policies. Please cite the published version when available. Title Genetic control of dairy cow reproduction Author(s) Moore, Stephen Publication date 2015 Publisher University College Dublin. School of Agriculture and Food Science Link to online version Item record/more information Downloaded T19:04:51Z The UCD community has made this article openly available. Please share how this access benefits you. Your story matters! Some rights reserved. For more information, please see the item record link above.
2 Genetic Control of Dairy Cow Reproduction A Thesis submitted to the National University of Ireland for the Degree of Doctor of Philosophy By Stephen Gerard Moore (B.Agr.Sc.) 1 Animal and Bioscience Research Department, Animal and Grassland Research and Innovation Centre, Teagasc Moorepark, Fermoy, Co. Cork, Ireland Head of Programme: Dr. Pat Dillon 2 School of Agriculture and Food Science, College of Agriculture, Food Science and Veterinary Medicine, University College Dublin, Belfield, Dublin 4, Ireland Head of School: Professor Alex Evans Research Supervisors 1 Dr. S.T. Butler 2 Dr. T. Fair September 2014
3 Statement of Original Authorship I hereby certify that the submitted work is my own, was completed while registered as a candidate for the Degree of Doctor of Philosophy with the National University of Ireland, and I have not obtained a degree elsewhere on the basis of the research presented in this submitted work. Stephen Moore
4 Table of Contents Acknowledgements... vii Publications from this Thesis... viii Peer-reviewed journal publications... viii Book chapter... viii Refereed conference publications... viii Technical publications... x List of Tables... xi List of Figures... xiii Glossary of Terms... xiv Thesis Abstract... xv Introduction to this Thesis... 1 References... 3 Literature Review Importance of Fertility to Irish Dairy Production Global Trends in Dairy Cow Fertility Genetic Selection in Ireland Fertility in the Dairy Cow Postpartum Uterine Health and Resumption of Ovarian Cyclicity The Oestrous Cycle Follicular Environment and Oocyte Competence Corpus Luteum Development Circulating Progesterone Concentrations Uterine Environment and Embryo Development Embryo Loss Major Animal Factors Controlling the Reproductive Performance of Dairy Cows Metabolic Status Body Condition Score Genetic influence on Reproductive Performance Genetic Influence on Energy Status Genetic Influence on Detailed Reproductive Phenotypes Influence of Genomic Regions and Variants on Reproductive Performance Influence of Dairy Breeds on Reproductive Performance Influence of Holstein Strain on Reproductive Performance i
5 Influence of Genetic Merit for Fertility on Reproductive Performance Rationale for the Studies Undertaken References Genetic merit for fertility traits in Holstein cows: Transition period, uterine health and resumption of cyclicity Preface Abstract Introduction Materials and Methods Animal Model Feed and Management System Animal Measurements Hormone and Metabolite Analysis Data Handling Statistical Analysis Results Milk Production Energy Balance, DMI, BCS and BW Blood Metabolites and Metabolic Hormones Postpartum Uterine Health Postpartum Resumption of Cyclicity Discussion DMI and Energy Balance Energy Metabolites and Metabolic Hormones Body Condition Score Uterine Health Resumption of Cyclicity Conclusions References Genetic merit for fertility traits in Holstein cows: Factors affecting circulating progesterone concentrations Preface Abstract Introduction Materials and Methods Animal Model Study Feed and Management System ii
6 Animal Measurements Ovulation Synchronisation Blood Sampling Ovarian Ultrasonography P4 Clearance RNA Extraction and cdna Synthesis Primer Design and Reference Gene Selection Real Time-qPCR Study Feed and Management System Ovulation Synchronisation Blood Sampling Ovarian Ultrasonography Blood Sample Analysis Ultrasound Image Analysis Data Handling Statistical Analysis Results Study Milk Production and Animal Characteristics Ovarian Characteristics and Reproductive Hormones P4 Metabolism Study Milk Production and Animal Characteristics Ovarian Characteristics and Reproductive Hormones Discussion Preovulatory Follicle Characteristics and Circulating E2 Concentrations Circulating P4 Concentrations Corpus Luteum Characteristics P4 Clearance Conclusions References Follicular fluid and serum metabolites in Holstein cows are predictive of genetic merit for fertility Preface Abstract iii
7 5.3 Introduction Materials and Methods Animal Model Feed and Management System Animal Measurements Ovulation Synchronisation Blood Sampling Ovarian Ultrasonography Ultrasound Image Analysis Follicular Fluid Sampling Reproductive Hormone Analysis Metabolite Extraction and Data Analysis Statistical Analysis Results General Characteristics of the Cows and the Largest Follicle Fatty Acid Profiles Composition of Follicular Fluid Composition of Serum Amino Acid Profiles Composition of Follicular Fluid Composition of Serum Discussion Characteristics of Fatty Acid Profiles Characteristics of Amino Acid Profiles Ability of Metabolites to Predict Fertility Genotype Conclusions References Differentially expressed genes in the endometrium and corpus luteum of Holstein cows selected for high and low fertility are enriched for sequence variants associated with fertility Preface Abstract Introduction Materials and Methods Lactating Holstein Cow Genetic Model of Fertility Ovulation Synchronization Tissue Biopsies RNA Extraction iv
8 6.4.5 cdna Library Preparation and Sequencing mrna Sequence Quality and Alignment Differential Analysis of Gene Expression Pathway Analysis of DEG Genome-Wide Association Studies using High Density Genotypes Concordance Analysis Genome-Wide Association using Whole Genome Sequence Results Gene Expression in Endometrium and Corpus Luteum of High and Low Fertility cows Concordance of Differentially Expressed Genes in Endometrium and Corpus Luteum with High-density Genome-wide Association Studies Sequence Variants Genome-wide Association Study for Fertility Discussion Concordance between Differentially Expressed Genes and Fertility Genome-wide Association Studies PGF2α-related QTL Regions Associated with Fertility Steroidogenesis-related QTL Regions Associated with Fertility mrna Processing-related QTL Regions and Sequence Variants Associated with Fertility Immune-related QTL Regions and Sequence Variants Associated with Fertility Energy Status-related QTL Region Associated with Fertility Conclusions References General Discussion Summary Chapter 2 Literature Review Chapter 3 - Genetic Merit for Fertility Traits in Holstein cows: Transition Period, Uterine Health and Resumption of Cyclicity Chapter 4 - Genetic Merit for Fertility Traits in Holstein cows: Factors Affecting Circulating Progesterone Concentrations Chapter 5 - Differences in Follicular Fluid and Serum Metabolites between Holstein Cows Selected for High and Low Fertility are Predictive of Fertility Genotype Chapter 6 - Differentially Expressed Genes in the Endometrium and Corpus Luteum of Holstein cows Selected for High and Low Fertility are Enriched for Sequence Variants Associated with Fertility Limitations Sample Size Endometrial Biopsy Association versus Causation Future Work Validation of Detailed Phenotypes v
9 7.8.2 Further Characterisation of Fert+ and Fert- cows Mechanisms Linking Metabolism with Fertility Overall Conclusions and Implications References vi
10 Acknowledgements I would like to acknowledge the opportunity presented to me by Teagasc to work on this project at the Animal and Grassland Research and Innovation Centre, Moorepark. I am grateful to all my colleagues, led by Dr. Pat Dillon, Head of Centre with whom I have had the privilege to work it. The commitment shown by all, to improving dairy production has been inspiring. To Dr. Stephen Butler, my boss, thank you for your knowledge, encouragement, advice and friendship. You have been a fantastic mentor. It has been a pleasure to work with you, and the repro team of Jonathon, Sean, Ian, Mary, Hazel, Francis, Shane, and the two visitors, Drs. Matt Lucy (Mizzou) and Paul Fricke (UW-Madision). I thoroughly enjoyed every step. Also, I greatly appreciate the contributions to this thesis, and the assistance and advice of Dr. Trudee Fair and Prof. Pat Lonergan (University College Dublin). Thanks to Drs. Jennie Pryce and Amanda Chamberlain, you were both incredibly welcoming during my three months in Melbourne. Along with Drs. Ben Hayes and Kath Kemper (DEPI), and Dr. Donagh Berry (Teagasc Moorepark), I thank each of you for excellent explanations of various topics related to Animal Breeding and Genetics, and for collaborating on this project. Technical assistance from Jimmy Quinn (Genexcel Irl. Ltd); Dr. John Browne (University College Dublin); Drs. Matt McCabe and Paul Cormican (Teagasc Grange), and Dr. Jos Tibbits (DEPI) is greatly appreciated. Thank you also to John Paul Murphy, Fergal Coughlan and the Moorepark farm staff for your assistance and good humour throughout. To my fellow mischief makers/debaters/pals Will, Vinny, Brian, PJ, Cathal, and Ian you, along with Mags, Ellen, Jess, Aine, Elodie and Hazel made the past few years go by so quickly. The many laughs with Amy, Eugene, the Grassland lads, Justine, Marion, Mary, Pat, Roberta, Sean and Yris won t be forgotten either! To Dee, Thank you for being a huge support and distraction! Life would be negative craic without each of you!! This thesis is dedicated to my Family. A huge thank you to Jer and Kate, for keeping me grounded, and to my parents, Stevie and Catherine, for your love, guidance and interest, and for encouraging and supporting my education. vii
11 Publications from this Thesis Peer-reviewed journal publications Moore, S. G., S. Scully, J. A. Browne, T. Fair and S. T. Butler Genetic merit for fertility traits in Holstein cows: V. Factors affecting circulating progesterone concentrations. J. Dairy Sci. 97: Moore, S. G., T. Fair, P. Lonergan and S. T. Butler Genetic merit for fertility traits in Holstein cows: IV. Transition period, uterine health and resumption of cyclicity. J. Dairy Sci. 97: McParland, E. Kennedy, E. Lewis, S. G. Moore, B. McCarthy, M. O Donovan and D. P. Berry Genetic parameters of dairy cow energy intake and body energy status predicted using mid-infrared spectrometry of milk. J. Dairy Sci. 98: McParland, S., E. Lewis, E. Kennedy, S. G. Moore, S. T. Butler, B. McCarthy, M. O Donovan, J. E. Pryce and D. P. Berry Mid-infrared spectrometry of milk as a predictor of feed intake and efficiency in lactating dairy cows. J. Dairy. Sci. 97: Book chapter Butler, S. T., S. B. Cummins, M. M. Herlihy, I. A. Hutchinson and S. G. Moore Optimizing productive and reproductive performance in the grazing cow. Reproduction in Domestic Ruminants VIII, Japan. Refereed conference publications Moore, S. G., M. McCabe, P. Cormican, T. Fair, P. Lonergan, A.J. Chamberlain, J.E. Pryce and S. T. Butler Effect of genetic merit for fertility traits on the transcriptome of the bovine corpus luteum on day 13 of the oestrous cycle. Reproduction in Domestic Ruminants VIII, Japan Moore, S. G., P. Lonergan, T. Fair, and S. T. Butler Invited talk: Physiology of cows with divergent genetic merit for fertility traits during the transition viii
12 period. Proceedings of the ADSA/ASAS/CSAS Joint Annual Meeting, Kansas City, Missouri, July Moore, S. G., M. McCabe, P. Cormican, T. Fair, P. Lonergan, A.J. Chamberlain, J.E. Pryce and S. T. Butler The effect of genetic merit for fertility traits on the transcriptome of the bovine endometrium on d 13 of the oestrous cycle. Proceedings of the International Cow Fertility Conference, Westport, Ireland, May Moore, S. G., M. McCabe, P. Cormican, T. Fair, P. Lonergan, A.J. Chamberlain, J.E. Pryce and S. T. Butler The effect of genetic merit for fertility traits on the transcriptome of the bovine endometrium on d 13 of the oestrous cycle. Proceedings of the Agricultural Research Forum, Tullamore, Ireland, March Moore, S. G., P. Lonergan, T. Fair, and S.T. Butler Physiology of cows with divergent genetic merit for fertility traits during the transition period. Proceedings of the 64 th Annual Meeting of the European Federation of Animal Science, Nantes, France, August Moore, S. G., A.C.O. Evans. P. Lonergan, T. Fair, and S. T. Butler The effect of genetic merit for fertility traits on dry matter intake, milk production and uterine health. Proceedings of the Agricultural Research Forum, Tullamore, Ireland, March Moore, S. G., A. O Gorman, L. Brennan, A.C.O. Evans. P. Lonergan, T. Fair, and S. T. Butler The effect of genetic merit for fertility traits on the follicular fluid metabolome. Proceedings of the Agricultural Research Forum, Tullamore, Ireland, March Moran, B., S.T. Butler, S.B. Cummins, S.G. Moore, D.E. MacHugh and C.J. Creevey Differential gene expression and alternative transcription in endometrial tissue of a lactating cow model of fertility. Proceedings of the Agricultural Research Forum, Tullamore, Ireland, March Moore, S. G., Scully, S., Crowe, M.A., Evans. A.C.O., Lonergan, P., Fair, T. and Butler, S.T Factors affecting plasma progesterone concentration in cows divergent in genetic merit for fertility traits. Proceedings of the 63 rd Annual Meeting of the European Federation of Animal Science, Bratislava, Slovakia, August Moore, S. G., S. Scully, M.A. Crowe, A.C.O. Evans. P. Lonergan, T. Fair, and S. T. Butler Examination of the factors responsible for differences in ix
13 circulating progesterone in lactating dairy cows with divergent genetic merit for fertility traits. Proceedings of the Agricultural Research Forum, Tullamore, Ireland, March Technical publications Stephen Moore and Stephen Butler Genetic merit for fertility traits affects uterine health. TResearch Volume 8: Number 4. Winter 2013 Stephen Moore and Stephen Butler The effect of genetic merit for fertility traits on the uterine health in dairy cows. Proceedings of Moorepark 13 Irish Dairying Harvesting the potential. 3 July x
14 List of Tables Table Title Page 2.1 Fertility performance of the Irish national dairy herd in Global Trends in Dairy Cow Fertility Structural characteristics of cells of dioestrus bovine corpus 14 luteum 2.4 Physiological mechanisms associated with the superior 23 reproductive performance of New Zealand strain Holstein- Friesian cows compared with North American strain Holstein- Friesian cows 2.5 Milk production and fertility differences between Fert+ and 23 Fert- cows during first lactation 3.1 The mean estimated breeding value (and SD) for both 49 genotypes based on their sire, maternal grandsire and maternal great grand-sire estimated breeding values 3.2 Ingredient and nutrient composition of the transition period diet The effect of genetic merit for fertility traits on daily milk 56 production variables during the first 35 weeks of lactation 3.4 The effect of genetic merit for fertility traits on mean BCS and 59 BW variables 3.5 The effect of genetic merit for fertility traits on mean vaginal 64 mucus score in Fert+ and Fert- cows until week eight of lactation 3.6 The effect of genetic merit for fertility traits on mean PMN 65 counts of the uterus and proportion of cows classified with endometritis on week 3 and 6 postpartum 4.1 The mean estimated breeding value (and SD) for both 83 genotypes based on their sire, maternal grandsire and maternal grand grand-sire estimated breeding values 4.2 Ingredient and nutrient composition of lactating cow diet Primer sequences used in real time quantitative PCR The effect of genetic merit for fertility traits on ovarian 96 characteristics and reproductive hormones during Study The effect of genetic merit for fertility traits on P4 half-life and 98 MCR 4.6 The effect of genetic merit for fertility traits on ovarian 103 characteristics during Study The mean estimated breeding value (and SD) for both 120 genotypes based on their sire, maternal grandsire and maternal grand grand-sire estimated breeding values 5.2 Ingredient and nutrient composition of non-lactating and 121 lactating cow diet 5.3 The effect of genetic merit for fertility traits on characteristics 128 of the largest follicle on day 7 of the oestrous cycle 5.4 The effect of genetic merit for fertility traits on the fatty acid 132 composition of follicular fluid 5.5 The effect of genetic merit for fertility traits on the fatty acid 135 composition of serum 5.6 The effect of genetic merit for fertility traits on the amino acid 138 composition of follicular fluid xi
15 5.7 The effect of genetic merit for fertility traits on the amino acid 140 composition of serum 5.8 Comparison of significant differences in follicular fluid and 142 serum fatty acid concentrations across five studies 5.9 Comparison of significant differences in follicular fluid and 144 serum amino acid concentrations across three studies 6.1 The mean estimated breeding value (and SD) for both 158 genotypes based on their sire, maternal grandsire and maternal grand grand-sire estimated breeding values 6.2 Processing of endometrium and corpus luteum RNA-seq data Endometrial genes determined to be differentially expressed 172 between Fert+ and Fert- cows on d 13 of the oestrous cycle 6.4 Corpus luteum genes determined to be differentially expressed 173 between Fert+ and Fert- cows on d 13 of the oestrous cycle 6.5 Categories of differentially expressed genes in the corpus 193 luteum between Fert+ and Fert- cows on day 13 of the oestrous cycle 6.6 Sequence variants associated with fertility in the Australian 195 dairy cattle population 6.7 QTL regions validated by both the Irish GWAS and Australian GWAS previously associated with female fertility traits 198 xii
16 List of Figures Figure Title Page 2.1 Schematic representation of pasture-based seasonal-calving 7 systems of milk production 2.2 Reproductive outcomes in British-Friesian versus Holstein-Friesian 10 cows 2.3 Sequence of reproductive events in the dairy cow Temporal profile of milk production and metabolic indicators 17 during the transition period 3.1 Mean daily milk yield and milk solids yield profiles of Fert+ and 57 Fert- cows during 35 weeks of lactation 3.2 Mean dry matter intake and calculated energy balance of Fert+ and 58 Fert- cows from weeks -2 to 5 relative to parturition 3.3 Mean body condition score and body weight from weeks -2 to relative to parturition 3.4 Mean circulating glucose, NEFA and BHBA concentrations in 62 Fert+ and Fert- cows from week -2 to 8 relative to parturition 3.5 Mean circulating IGF1 and insulin in Fert+ and Fert- cows from 63 week -2 to 8 relative to parturition 4.1 The effect of genetic merit for fertility traits on dry matter intake, 95 body weight and milk yield during the 4 weeks prior to completion of the study 4.2 The effect of genetic merit for fertility traits on circulating E2 and 97 P4 concentrations during Study The effect of genetic merit for fertility traits on progesterone (P4) 99 metabolism 4.4 The effect of genetic merit for fertility traits on reproductive 101 hormones, follicle diameter and CL characteristics during Study Box plots depicting circulating P4 concentrations from Study during d 5, 7, 10 and 13 of the oestrous cycle in Fert+ and Fertcows 5.1 ROC curves produced using (a) significant follicular fluid fatty 131 acids (b) significant serum fatty acids (c) significant follicular fluid amino acids and (d) significant serum amino acids 6.1 Gene body plots of RNA-sequenced libraries Manhattan plots for calving interval in Australian and Irish dairy cattle populations 171 xiii
17 Glossary of Terms AI BCS BHBA BW CI CIDR CL DIM DMI E2 Ebal EBI EBV GH GnRh IGF1 LH mrna MRP MSD MUFA NAHF NEFA P4 PGF2α PMN PUFA ROC RT-qPCR SFA TMR UFL Artificial Insemination Body Condition Score β-hydroxybutyrate Body Weight Confidence Interval Controlled Internal Drug Release Device Corpus Luteum Days in Milk Dry Matter Intake Oestradiol Energy Balance Economic Breeding Index Economic Breeding Value Growth Hormone Gonadotropin Releasing Hormone Insulin-like Growth Factor-1 Luteinising Hormone Messenger Ribonucleic acid Maternal Recognition of Pregnancy Mating Start Date Monounsaturated Fatty Acid North American Holstein Friesian Non-Esterified Fatty Acid Progesterone Prostaglandin F2α Polymorphonuclear Neutrophils Polyunsaturated Acid Receiver Operating Characteristic Real-time Quantitative Polymerase Chain Reaction Saturated Fatty Acid Total Mixed Ration Unité Fourragère Lait xiv
18 Thesis Abstract The decline in dairy cow reproductive performance compromised the productivity and profitability of dairy production worldwide. The phenotypic performance of lactating cows with similar proportions of Holstein genes, similar genetic merit for milk production traits, but either good (Fert+) or poor (Fert-) genetic merit for fertility traits managed in a standardised environment was compared. The objective of this study was to elucidate the physiological mechanisms contributing to suboptimal reproductive performance in lactating dairy cows. Fert+ cows had greater dry matter intake during the first five weeks postpartum, more favourable metabolic status during the transition period, better uterine health during early lactation, were more likely to have resumed cyclicity by week six postpartum, and had greater body condition score and milk production throughout lactation compared with Fert- cows. Preovulatory concentrations of oestradiol, corpus luteum volume and circulating concentrations of progesterone were greater in Fert+ cows compared with Fert- cows during the oestrous cycle. The metabolic clearance rate of progesterone was similar in both genotypes and differences in the hepatic mrna abundance of genes responsible for progesterone metabolism were minor. Small differences in the abundance of fatty acids and amino acids were detected between genotypes on day seven of the oestrous cycle. Greater abundance of n-6 and total polyunsaturated fatty acids and lesser abundance of saturated fatty acids was observed in the serum of Fert+ cow compared with Fert- cows on day seven of the oestrous cycle. Fatty acid differences in follicular fluid and serum and amino acid differences in follicular fluid were highly predictive of fertility genotype. Combination of transcriptome analysis of the endometrium and corpus luteum on day 13 of the oestrous cycle with genome-wide association studies and whole genome sequence data identified quantitative trait loci regions and putative causal mutations associated with genetic variation in dairy cow fertility. The endometrial expression profile suggested prolonged uterine inflammation, greater prostaglandin F 2α synthesis and secretion, and compromised energy status in Fert- cows. The luteal expression profile suggested reduced prostaglandin F 2α response, greater steroidogenesis and mrna processing in Fert+ cows. Collectively, the results highlight the importance of the uterine environment and ovarian activity for the phenotypic fertility differences between genotypes. The study has identified physiological mechanisms controlled by genetic merit for fertility traits that may support reproductive performance without antagonising milk production. This novel lactating cow genetic model of fertility represents a robust and valuable resource for on-going fertility research. xv
19 Chapter One Introduction to this Thesis The success of dairy production in pasture based systems is centred on achieving high milk solids production per cow and per hectare from a predominantly pasture-based diet. A compact calving season in spring is critical to maximising farm productivity and profitability (Shalloo, 2009). Fertility data indicates that reproductive performance has been suboptimal for many years but that substantial variation exists between herds ( Intensive international research has concluded that the causes of suboptimal reproductive performance in dairy herds are multifactorial (Lucy, 2001, Walsh et al., 2011), with genotype (Butler, 2013, Berry et al., 2014, Khatkar et al., 2014), nutritional status (Roche et al., 2011, Butler, 2014) and reproductive management (McDougall, 2006, Bisinotto et al., 2014), all contributing factors. In Ireland, greater milk production of the national herd was facilitated by the use of a selection index focused solely on the genetic improvement of milk production traits (Relative Breeding Index). Introgression of North American Holstein genetics into a primarily British Friesian population resulted in the proportion of Holstein genes in the dairy herd increasing from 8% in 1990 to 63% by 2001, a period that coincided with the decline in calving rate to first service from 55% to 44% (Evans et al., 2006). Since 2001, a more holistic approach has developed using a multi-trait selection index to identify the most profitable sires for Irish dairy production systems (Berry, 2007). This index is called the Economic Breeding Index (EBI), and weightings are currently placed on milk production (32.9%), fertility (34.9%), calving (9.2%), beef (8.6%), maintenance (7.2%), management (4.0%) and health (3.6%) ( The sequence of biological processes that must successfully occur in a timely manner from calving to the establishment of pregnancy, are the recovery of uterine health; resumption of ovarian cyclicity; oestrous behaviour and ovulation of a competent oocyte; fertilisation; corpus luteum development and progesterone secretion; embryo development; maternal recognition of pregnancy; and implantation (Lucy, 1
20 2001, Hansen, 2002). It has been well established that alterations to the metabolic environment in response to lactation influence these biological events (Garnsworthy et al., 2008, Wathes, 2012, Butler, 2014). Elucidating the specific mechanisms involved has received intense interest (Velazquez et al., 2008, Thatcher et al., 2010, Wathes et al., 2012, Butler, 2014, Lucy et al., 2014). Previous studies comparing animal models of high and low fertility have attempted to determine these interactions by manipulation of the diet (Sangsritavong et al., 2002, Cerri et al., 2009, Fouladi-Nashta et al., 2009); comparing lactation status (Bender et al., 2010, Maillo et al., 2012); varying genetic merit for milk production (high genetic merit vs. low genetic merit; (Snijders et al., 2000, Kennedy et al., 2003, Pollott and Coffey, 2008)); and varying Holstein ancestry (New Zealand Holstein-Friesian vs. North American Holstein-Friesian cows (Horan et al., 2005, Lucy et al., 2009, Walker et al., 2012, Meier et al., 2014). These studies greatly increased our understanding of interactions of metabolic status, nutritional status and genotype with dairy cow fertility, but were confounded by effects of diet, genetic merit for milk production, phenotypic milk production, and lactation status. To minimise the variation that may have existed in those studies, a unique lactating cow genetic model of fertility was established by Teagasc Moorepark. The herd consisted of two groups of Holstein cows with similar genetic merit for milk production traits, but with either good (Fert+) or poor (Fert-) genetic merit for calving interval. Confounding factors known to affect reproductive performance were similar for both genotypes. Large differences in phenotypic fertility performance between the genotypes were detected with practically similar milk production (Cummins et al., 2012a), indicating that a robust and valuable resource had been established to determine the physiological mechanisms responsible for suboptimal reproductive performance in lactating dairy cows. Subsequent studies (Cummins et al., 2012b, c) determined that the primary differences between genotypes were (i) greater BCS; (ii) greater circulating insulin and IGF1; (iii) stronger oestrus expression; (iv) fewer silent heats; (v) less ovulation failure after oestrus; and (vi) greater circulating P4 concentrations in Fert+ cows compared with Fert- cows. 2
21 References Bender, K., S. Walsh, A. C. O. Evans, T. Fair, and L. Brennan Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction 139(6): Berry, D. P., Laurence Shalloo, Andrew Cromie, Victor Olori, Roel Veerkamp, Pat Dollin, Peter Amer, Ross Evans, Francis Kearney, Brian Wickham The economic breeding index: a generation on. Berry, D. P., E. Wall, and J. E. Pryce Genetics and genomics of reproductive performance in dairy and beef cattle. animal 8(Supplements1): Bisinotto, R. S., E. S. Ribeiro, and J. E. P. Santos Synchronisation of ovulation for management of reproduction in dairy cows. animal 8(Supplements1): Butler, S. T Genetic control of reproduction in dairy cows. Reproduction, Fertility and Development 26(1):1-11. Butler, S. T Nutritional management to optimize fertility of dairy cows in pasture-based systems. animal 8(Supplements1): Cerri, R. L. A., S. O. Juchem, R. C. Chebel, H. M. Rutigliano, R. G. S. Bruno, K. N. Galvao, W. W. Thatcher, and J. E. P. Santos Effect of fat source differing in fatty acid profile on metabolic parameters, fertilization, and embryo quality in high-producing dairy cows. Journal of Dairy Science 92(4): Cummins, S. B., P. Lonergan, A. C. O. Evans, D. P. Berry, R. D. Evans, and S. T. Butler. 2012a. Genetic merit for fertility traits in Holstein cows: I. Production characteristics and reproductive efficiency in a pasture-based system. Journal of Dairy Science 95(3): Cummins, S. B., P. Lonergan, A. C. O. Evans, and S. T. Butler. 2012b. Genetic merit for fertility traits in Holstein cows: II. Ovarian follicular and corpus luteum dynamics, reproductive hormones, and estrus behavior. Journal of Dairy Science 95(7): Cummins, S. B., S. M. Waters, A. C. O. Evans, P. Lonergan, and S. T. Butler. 2012c. Genetic merit for fertility traits in Holstein cows: III. Hepatic expression of somatotropic axis genes during pregnancy and lactation. Journal of Dairy Science 95(7): Evans, R. D., P. Dillon, F. Buckley, D. P. Berry, M. Wallace, V. Ducrocq, and D. J. Garrick Trends in milk production, calving rate and survival of cows in 14 Irish dairy herds as a result of the introgression of Holstein-Friesian genes. Animal Science 82(04): Fouladi-Nashta, A. A., K. E. Wonnacott, C. G. Gutierrez, J. G. Gong, K. D. Sinclair, P. C. Garnsworthy, and R. Webb Oocyte quality in lactating dairy cows fed on high levels of n-3 and n-6 fatty acids. Reproduction 138(5): Garnsworthy, P., K. Sinclair, and R. Webb Integration of Physiological Mechanisms That Influence Fertility in Dairy Cows. animal 2: Hansen, P. J Embryonic mortality in cattle from the embryo's perspective. Journal of Animal Science 80(E-Suppl_2):E Horan, B., J. F. Mee, P. O Connor, M. Rath, and P. Dillon The effect of strain of Holstein-Friesian cow and feeding system on postpartum ovarian function, animal production and conception rate to first service. Theriogenology 63(3): Kennedy, J., P. Dillon, K. O'Sullivan, F. Buckley, and M. Rath The effect of genetic merit for milk production and concentrate feeding level on the reproductive performance of Holstein-Friesian cows in a grass-based system. Animal Science 76:
22 Khatkar, M. S., I. A. S. Randhawa, and H. W. Raadsma Meta-assembly of genomic regions and variants associated with female reproductive efficiency in cattle. Livestock Science 166(0): Lucy, M. C Reproductive Loss in High-Producing Dairy Cattle: Where Will It End? Journal of Dairy Science 84(6): Lucy, M. C., S. T. Butler, and H. A. Garverick Endocrine and metabolic mechanisms linking postpartum glucose with early embryonic and foetal development in dairy cows. animal 8(Supplements1): Lucy, M. C., G. A. Verkerk, B. E. Whyte, K. A. Macdonald, L. Burton, R. T. Cursons, J. R. Roche, and C. W. Holmes Somatotropic axis components and nutrient partitioning in genetically diverse dairy cows managed under different feed allowances in a pasture system. Journal of Dairy Science 92(2): Maillo, V., D. Rizos, U. Besenfelder, V. Havlicek, A. K. Kelly, M. Garrett, and P. Lonergan Influence of lactation on metabolic characteristics and embryo development in postpartum Holstein dairy cows. Journal of Dairy Science 95(7): McDougall, S Reproduction performance and management of dairy cattle. Journal of Reproduction and Development 52(1): Meier, S., M. D. Mitchell, C. G. Walker, J. R. Roche, and G. A. Verkerk Amino acid concentrations in uterine fluid during early pregnancy differ in fertile and subfertile dairy cow strains. Journal of Dairy Science 97(3): Pollott, G. E. and M. P. Coffey The Effect of Genetic Merit and Production System on Dairy Cow Fertility, Measured Using Progesterone Profiles and On- Farm Recording. Journal of Dairy Science 91(9): Roche, J. R., C. R. Burke, S. Meier, and C. G. Walker Nutrition reproduction interaction in pasture-based systems: is nutrition a factor in reproductive failure? Animal Production Science 51(12): Sangsritavong, S., D. K. Combs, R. Sartori, L. E. Armentano, and M. C. Wiltbank High Feed Intake Increases Liver Blood Flow and Metabolism of Progesterone and Estradiol-17β in Dairy Cattle. Journal of Dairy Science 85(11): Shalloo, L Pushing the barriers on milk costs/ outputs. Proceedings on the National Dairy Conference: Snijders, S. E. M., P. Dillon, D. O'Callaghan, and M. P. Boland Effect of genetic merit, milk yield, body condition and lactation number on in vitro oocyte development in dairy cows. Theriogenology 53(4): Thatcher, W., J. Santos, F. Silvestre, I. Kim, and C. Staples Perspective on Physiological/Endocrine and Nutritional Factors Influencing Fertility in Postpartum Dairy Cows. Reproduction in Domestic Animals 45:2-14. Velazquez, M. A., L. J. Spicer, and D. C. Wathes The role of endocrine insulinlike growth factor-i (IGF-I) in female bovine reproduction. Domestic Animal Endocrinology 35(4): Walker, C. G., M. D. Littlejohn, M. D. Mitchell, J. R. Roche, and S. Meier Endometrial gene expression during early pregnancy differs between fertile and subfertile dairy cow strains. Physiological Genomics 44(1): Walsh, S. W., E. J. Williams, and A. C. O. Evans A review of the causes of poor fertility in high milk producing dairy cows. Animal Reproduction Science 123(3 4): Wathes, D. C Mechanisms Linking Metabolic Status and Disease with Reproductive Outcome in the Dairy Cow. Reproduction in Domestic Animals 47:
23 Wathes, D. C., A. M. Clempson, and G. E. Pollott Associations between lipid metabolism and fertility in the dairy cow. Reproduction, Fertility and Development 25(1):
24 Chapter 2 Literature Review 6
25 2.1 Importance of Fertility to Irish Dairy Production With the abolition of European Union milk quotas in 2015, a 50% increase in national milk production by 2020 is anticipated (Department of Agriculture, 2010). This will be achieved by increasing the size of the national dairy cow herd and increasing milk production per cow. Dairy production in pasture based systems is centred on achieving high milk solids production per cow and per hectare from a predominantly pasturebased diet and supplementation with concentrate and/or conserved forages during pasture deficits. Due to the seasonal nature of grass growth, the herd calves during the three months of late winter/early spring, is bred during the three months of late spring/early summer and is dried-off in late lactation during mid-winter (Figure 2.1). Figure 2.1. Schematic representation of pasture-based seasonal-calving systems of milk production. Top panel: temporal patterns of pasture growth and herd feed demand. The objective is to match the timing of peak herd feed demand with peak pasture growth rates. Bottom panel: Seasonal pattern of calving, breeding and drying off. A compact calving pattern results in: (i) peak herd feed demand coinciding with peak pasture growth rate; (ii) most cows calved 42 days at mating start date; (iii) rapid pregnancy establishment at the start of the breeding period; (iv) most cows having a long lactation on a primarily pasture-based diet; (v) most cows having a dry period of eight to ten weeks. Figure courtesy of B. Horan, Teagasc Moorepark and adapted from Holmes et al. (2002). 7
26 A compact calving season, defined as 90% of the herd calving in a six week period (Butler, 2014), is critical to the success of pasture-based dairy production. It facilitates high levels of pasture utilisation and low levels of supplementary feeding through the use of highly fertile cows capable of high milk solids production (Shalloo, 2009). The mean calving date in Ireland is currently mid-march; however, improvement to mid-february (the optimal mean calving date) would result in economic gains for dairy producers (Shalloo et al., 2014) and processors (Geary et al., 2012). Achieving the six-week calving rate of 90% requires high fertility performance from replacement heifers and the lactating herd. The most recent fertility data for the national dairy herd indicates that a large improvement is required to achieve this target fertility performance, as demonstrated by the top 5% of herds (Table 2.1). Improving the current six week calving rate of 76% in heifers (Berry et al., 2013) and 56% in cows by 1% would increase net farm profitability by 3.51 per heifer and 9.26 per cow (Shalloo et al., 2014). Table 2.1. Fertility performance of the Irish national dairy herd in 2014 Key Performance Indicators Mean Target Top 5% Bottom 5% Calving interval (days) Six-week calving rate (%) Calves per cow per year Global Trends in Dairy Cow Fertility Dairy cow fertility is defined as the ability to establish a successful pregnancy if submitted for breeding at the correct time relative to ovulation (Darwash et al., 1997). Declines in fertility performance have been evident in both pasture-based and confinement production systems (Table 2.2), coincident with large increases in milk production and genetic selection solely for milk production traits. A recent survey of fertility trends in Holstein-Friesian cows from 16 countries suggests that the rate of fertility decline has eased since the previous decade ( ), and may have begun to improve (Pryce et al., 2014). Genetic correlations among milk, protein and fat yield with most fertility traits demonstrate a strong antagonistic relationship (Berry et al., 2014), yet, understanding the interaction between production and fertility has been difficult. Studies of phenotypic data have reported negative associations (Nebel and McGilliard, 1993), no association (Patton et al., 2007), or a positive association between milk production and fertility (Buckley et al., 2003), reflecting the importance of herd 8
27 management and animal health in understanding the interactions (Leblanc, 2010, Bello et al., 2012). Table 2.2. Global Trends in Dairy Cow Fertility Period Fertility trait Change Location 1951 to 1996 CRFS (%) 66 to 40 New York state s to 2000s CRFS (%) 65 to 55 New Zealand to 2004 Calving interval (days) 379 to 389 Ireland to 2005 Calving interval (months) 12.5 to 12.5 Norway to 2001 CRFS (%) 55 to 44 Ireland to 2006 CRFS (%) 54 to 45 United States to 2009 CRFS (%) 63 to 50 Australia 7 CRFS = Conception rate to first service 1 =Butler (1998) 2 =Burke and Fowler (2007) 3 =Berry et al. (2014) 4 =Refsdal (2007) 5 =Evans et al. (2006) 6 =Norman et al. (2009) 7 =Macmillan (2012) 2.3 Genetic Selection in Ireland The genetic composition of Irish dairy cattle has progressed from Dairy Shorthorn in the 1960s to dual-purpose British Friesian in the 1980s to the North American Holstein- Friesian (NAHF), which is presently the predominant dairy breed (Evans et al., 2006). In Ireland, use of NAHF genetics grew from < 10% in 1977 to ~ 80% in 1995 (Simm, 1998). The introgression of Holstein genetics resulted in the proportion of Holstein genetics in the dairy herd increasing from 8% in 1990 to 63% by 2001 (Evans et al., 2006). Similar trends were reported in New Zealand (Harris and Kolver, 2001). Exports of NAHF semen from the US increased from 13% in 1981 to 30% in 2006 (Funk, 2006). In Ireland, selection indices for dairy cattle have evolved in recent decades. Initially, a selection index focused solely on the genetic improvement of milk production traits (Relative Breeding Index) was utilised. During this time, sires generated for confinement TMR-based milk production systems were utilised to improve the genetic merit for milk production of the national dairy herd. This selection policy resulted in dairy cows that mobilised excessive amounts of adipose tissue in early lactation, and had suboptimal reproductive performance when managed in Irish pasturebased production systems (Buckley et al., 2000, Kennedy et al., 2003, Horan et al., 2004, Horan et al., 2005a, b, Dillon et al., 2006, McCarthy et al., 2007a, b). Diskin et al. (2011) attributed greater occurrence of early embryo loss as the primary contributor to the decline in reproductive performance (Figure 2.2). 9
28 In 2001, a more holistic approach was developed using a multi-trait selection index to identify the most profitable sires for Irish dairy production systems. This was termed the Economic Breeding Index (EBI), which currently includes 6 traits (weighting in parenthesis); milk production (32.9%), fertility (34.9%), calving (9.2%), beef (8.6%), maintenance (7.2%), management (4.0%) and health (3.6%) ( Figure 2.2. Reproductive outcomes in British-Friesian versus Holstein-Friesian cows (Diskin et al., 2011). 2.4 Fertility in the Dairy Cow The sequence of events for optimal reproductive performance of dairy cows is presented in Figure 2.3 and are discussed in this section. 10
29 Figure 2.3. Sequence of reproductive events in the dairy cow. Each event depends on the success of the preceding events. Numbers indicate optimum range (days after calving) to achieve an average calving interval of 365 days. Major temporal factors that influence success are: energy balance (EBal), which should start to increase early in lactation; insulin, which stimulates resumption of oestrous cycles, but may reduce oocyte quality; P4, which is low during anoestrus, high during luteal phases of cycles, and low during follicular phases of cycles; and PGF 2α which stimulates uterine involution and corpus luteum regression, but has to be suppressed for successful implantation and maintenance of pregnancy. Adapted from Garnsworthy et al. (2008) Postpartum Uterine Health and Resumption of Ovarian Cyclicity At calving, the uterus is invaded by bacteria in the environment, many of which are uniquely associated with either a metritic, endometritic or healthy uterine status (Santos and Bicalho, 2012). The presence of pathogenic bacteria induces an inflammatory response, characterised by the infiltration of neutrophils and macrophages, and the accumulation of uterine pus (Sheldon et al., 2014). This is a normal physiological response. Subsequent development of uterine disease depends on the type of bacteria involved and on the immune response of the cow, and is associated with reduced subsequent fertility % of dairy cows are classified as having endometritis by weeks four to six postpartum, with unfavourable consequences for fertility (Williams et al., 2005, McDougall et al., 2011). Reduced fertility could arise via mechanisms affecting the ovary (Williams et al., 2007, Williams et al., 2008), follicular environment (Green et al., 2011), endometrium and embryo (Hoelker et al., 2012, Ledgard et al., 2013). Coincident with the recovery of uterine health, resumption of ovarian cyclicity must also occur in a timely manner. After calving, follicles resume their wave-like growth pattern in response to increasing follicle stimulating hormone (FSH) concentration within the first week (Savio et al., 1990), but ovulation of a dominant follicle does not occur until luteinizing hormone (LH) secretion from the anterior pituitary reaches a frequency of one pulse per hour (Crowe, 2008). The first ovulation after calving is frequently not associated with oestrous behaviour, and is usually followed by a short oestrous cycle, oestrous behaviour and ovulation (Crowe, 2008). Factors associated with the resumption of cyclicity include the size of the dominant follicle (Austin et al., 1999) and insulin-like growth factor-1 (IGF1) bioavailability 11
30 (Canty et al., 2006). Nyman et al. (2014) reported that the interval from calving to commencement of luteal activity ranged from 27 to 34 days based on milk progesterone (P4) data. An earlier resumption of ovarian cyclicity is usually associated with greater fertility (Santos et al., 2009, Galvão et al., 2010), although Horan et al. (2005b) reported a negative association. In seasonal calving herds, problem cows with uterine infection and anoestrus are most common in the late calving cohort The Oestrous Cycle Cattle are non-seasonal, polyoestrous animals that experience oestrous cycles consisting of two to three waves of follicular growth during a period of 18 to 24 days (Forde et al., 2011b). Pregnancy rates tend to be greater in cows experiencing three versus two follicular waves (Townson et al., 2002). Each wave consists of three stages: selection, dominance and either atresia or ovulation. Selection involves the recruitment of a cohort of follicles following a surge in FSH that declines within four days of wave emergence. From this cohort of follicles, a single follicle achieves dominance by growing more quickly, achieves greater oestrogen synthetic capacity than other recruited follicles, acquires LH receptors and inhibits the growth of competitor follicles (Thatcher et al., 1996, Adams et al., 2008). In the absence of FSH, LH is essential for maintenance of dominant follicle steroidogenesis (Thatcher et al., 1996, Bao and Garverick, 1998, Roche et al., 1998). During periods of elevated circulating P4 concentrations, the dominant follicle is unable to ovulate and so undergoes atresia. Following removal of the source oestradiol (E2) and inhibin another FSH surge occurs, preceding the emergence of a new follicular wave. Regression of the corpus luteum (CL) occurs on either day 16 or 19 of a two wave or three wave oestrous cycle, respectively (Adams et al., 2008), and is instigated by increased pulsatility and amplitude of uterine prostaglandin F 2α (PGF 2α ) release. Once the threshold for E2 is reached, activation of oestrogen receptors in the brain induces oestrous behaviour. With circulating P4 concentrations at basal levels and peak circulating E2 concentrations, the surge centre of the hypothalamus is activated to release gonadotropin releasing hormone (GnRH), thereby facilitating a surge release of LH from the pituitary and ovulation of the dominant follicle (Senger, 1997). Synchronisation between oestrous behaviour and ovulation is regulated by oestrogen receptor-dependent transcription of GnRH and GnRH receptor genes (Woelders et al., 2014). At ovulation, the dominant follicle ruptures to release the oocyte in response to a surge release of LH. If insemination 12
31 occurs, subsequent pregnancy success is associated with ovulatory follicle size (Geary et al., 2013) Follicular Environment and Oocyte Competence Competent oocytes are capable of: (i) resuming meiosis; (ii) cleaving following fertilisation; (iii) developing to the blastocyst stage; and (iv) maintaining pregnancy to term in good health (Sirard et al., 2006). Even though fertilisation rates of up to 100% have been reported (Sartori et al., 2002b), a significant proportion of embryos degenerate before the blastocyst stage (reviewed by Sartori et al. (2010)). Compromised oocytes have been implicated as a contributor to low pregnancy rates in cattle, because greater fertility has been achieved following embryo transfer compared with AI (Vasconcelos et al., 2006, Demetrio et al., 2007). Bovine oocytes have a considerable lipid component and metabolise glucose, lactate, pyruvate, triglyceride and amino acids (Sturmey et al., 2009). Dramatic alterations in circulating metabolites that occur as the dairy cow progresses through the early lactation period are reflected in follicular fluid (Leroy et al., 2004). It has been hypothesized that the oocytes that ovulate during the breeding season have been exposed to the early lactation period of metabolic stress during follicle development, with negative consequences for fertility. Specifically, greater concentrations of non-esterified fatty acids (NEFA) and β-hydroxybutyrate (BHBA) have been implicated in compromised follicle steroidogenesis, oocyte development and embryo quality (Leroy et al., 2005, Vanholder et al., 2006, Van Hoeck et al., 2011); however, caution should be taken when interpreting in vitro studies. While dietary fat manipulation is reflected in the follicular environment, alterations to the lipid content of oocytes have not been detected in vivo (Sturmey et al., 2009). At the animal level, the major determinants of oocyte competence are puberty, parity status, genetic merit for milk production, BCS, dietary protein and heat stress (Hansen, 2002) Corpus Luteum Development After ovulation, the theca interna undergoes both hypertrophy and hyperplasia, progresses to the antral space and becomes dispersed amongst granulosa cells. Luteal tissue is composed of 4 cell types; large luteal cells (LLC) that develop from granulosa 13
32 cells, small luteal cells (SLC) that develop from theca cells, endothelial cells and fibroblast cells (Table 2.3). Table 2.3. Structural characteristics of cells of dioestrus bovine corpus luteum (adapted from Wiltbank (1994)). LLC (%) SLC (%) EC (%) FC (%) Tissue volume Cell number LLC = large luteal cells SLC = small luteal cells EC = endothelial cells FC = fibroblast cells Luteinizing hormone is the primary hormone responsible for CL maintenance and P4 synthesis. Luteinizing hormone receptors are present on both LLC and SLC, although LLC s, which are responsible for 80% of the P4 synthesised (Niswender et al., 1994), eventually become unresponsive to LH. In SLC s, LH stimulates P4 production via increased cholesterol esterase activity and cholesterol transport from the cytoplasm across the inner mitochondrial membrane, the major rate-limiting step in P4 steroidogenesis. As the CL develops, mrna abundance and activity of enzymes (P450 scc, 3β-HSD, cholesterol esterase) and receptors (LH and FSH) involved in P4 steroidogenesis increase in support of cholesterol transport (Niswender et al., 1994). Progesterone production is determined primarily by the volume of luteal tissue and the rate of blood flow, but their importance is dependent on the stage of the oestrous cycle (Juengel and Niswender, 1999). Reports on the relative importance of CL tissue volume and blood flow are currently conflicting. Mann (2009) reported no correlation between CL volume and circulating P4 concentrations once the CL had matured. Conversely, correlation analysis between ultrasound images and circulating P4 concentrations indicated that CL volume is the primary determinant (Bollwein et al., 2012) Circulating Progesterone Concentrations Progesterone is the essential hormone of pregnancy and has been implicated in the control of most reproductive events during the oestrous cycle (Lonergan, 2011, Wiltbank et al., 2014). Greater circulating P4 concentrations during growth of the preovulatory follicle (Xu et al., 1997, Herlihy et al., 2011, Colazo et al., 2013) and post ovulation (Stronge et al., 2005, McNeill et al., 2006, Larson et al., 2007) are associated with fertility improvements. Persistent follicles develop in low circulating P4 concentrations; these follicles ovulate ooctyes that have undergone premature 14
33 maturation and subsequent embryo development is compromised (Austin et al., 1999, Mihm et al., 1999). Conversely, high circulating P4 concentrations at the time of AI due to incomplete luteolysis are associated with compromised fertility (Martins et al., 2011). Insufficient P4 synthesis by the CL and greater metabolic clearance rate (MCR) by the liver are the principal mechanisms responsible for reduced circulating P4 concentrations during the luteal phase (Sangsritavong et al., 2002, Sartori et al., 2002a). The necessity for greater dry matter intake to meet the demands of rapidly increasing milk production is the primary reason for increased MCR by the liver (Sangsritavong et al., 2002, Reynolds et al., 2003, Rhinehart et al., 2009) Uterine Environment and Embryo Development In vitro culture of embryos to the blastocyst stage is possible, but genomic and phenotypic differences between in vivo versus in vitro derived embryos indicate compromised development (Rizos et al., 2002), even when pregnancies develop to term (Lazzari et al., 2002). Therefore it can be concluded that the uterine environment is more favourable than the in vitro environment. Transport proteins, ions, mitogens, cytokines, lymphokines, enzymes, hormones, growth factors, proteases and protease inhibitors, amino acids, glucose, fructose, and vitamins are the key components of histotroph essential for successful embryo development (Bazer et al., 2011). The stages involved in early embryo development include embryonic genome activation, morula development, hatching of the blastocyst and trophoblast elongation (Hansen, 2002, Peippo et al., 2011). The events leading to maternal recognition of pregnancy on days 15 to 18 after ovulation involve a complex communication process between the rapidly elongating embryo, CL and endometrium. These include: (i) reduced expression of the P4 receptor in the endometrium following exposure to P4; (ii) embryo production of interferon-τ to prevent the endometrial expression of oestrogen and oxytocin receptors, thereby preventing oxytocin-induced synthesis and release of PGF 2α from the endometrium; and (iii) CL maintenance and continued P4 production (Senger, 1997). The uterine environment anticipates establishing a pregnancy until at least the period of maternal recognition of pregnancy (Lonergan and Forde, 2014). Prior to maternal recognition of pregnancy, the primary factors influencing the endometrial transciptome are the stage of the oestrous cycle and circulating P4 concentrations (Forde et al., 2011a). Greater 15
34 postovulatory circulating P4 concentrations are associated with superior embryo development (Carter et al., 2008), presumably via alterations to the endometrial transcriptome and protein secretions (Forde et al., 2013). Alterations to the uterine environment imposed by lactation status or infection may influence fertility in dairy cows. Rizos et al. (2010) reported greater embryo recovery and greater blastocyst development when in vitro fertilised oocytes were cultured from day two to seven of the oestrous cycle in nulliparous heifers compared with lactating dairy cows. The expression profile of endometrial immune cells was reported to be anti-inflammatory in anticipation of pregnancy establishment (Oliveira et al., 2013) but the presence of neutrophils in the uterus may alter this expression profile, with possible consequences for pregnancy establishment (Hoelker et al., 2012) Embryo Loss Based on fertilisation rates of 90% and calving rates of 55%, Sreenan and Diskin (1986) calculated embryo loss to be about 40%. Since reported fertilisation rates seem to have remained reasonably static in recent decades (Sartori et al., 2010; Sreenan and Diskin, 1986), fertilisation problems do not appear to be a primary factor in the declining fertility, at least in temperate climates. In regions where dairy cows experience heat stress, fertilisation rates of only 55% have been reported during summer (Sartori et al., 2002). The period before maternal recognition of pregnancy is responsible for the majority of conceptus loss (Diskin and Sreenan, 1980; Dunne et al., 2000; Horan et al., 2004; López-Gatius et al., 2004; Silke et al., 2002). Embryo loss does occur prior to day 8 after artificial insemination, particularly in very high milk production cows when compared with heifers (Sartori et al., 2002), but the majority of embryo loss occurs between days 8 to 16 after artificial insemination. 2.5 Major Animal Factors Controlling the Reproductive Performance of Dairy Cows The causes of subfertility in dairy cow are multifactorial (Lucy, 2001, Walsh et al., 2011), with nutritional status (Roche et al., 2011, Butler, 2014), reproductive management (McDougall, 2006, Bisinotto et al., 2014) and genotype (Berry et al., 2014, Khatkar et al., 2014) all identified as contributing factors. The impact of improved reproductive management and increased selection intensity for fertility are now beginning to materialise. The decline in female fertility in the Holstein-Friesian breed 16
35 has either plateaued or in some populations begun to improve (Pryce et al., 2014). Major animal factors that affect the sequence of reproductive events presented in Figure 2.2 are discussed in this section Metabolic Status The transition period in cattle is described as the period from three weeks prepartum to three weeks postpartum, and is associated with the potential occurrence of multiple diseases that affect production, fertility, and health (Drackley, 1999). With the onset of lactation, the dairy cow experiences a marked shift in metabolism due to the uncoupling of the somatotropic axis. In early lactation, available glucose is prioritised in support of mammary milk synthesis by increasing hepatic gluconeogenesis and limiting lipogenesis in adipose tissue (Lucy, 2008). During this period, a hypoinsulinaemic environment prevails; hepatic expression of GHR mrna is suppressed, preventing binding of GH to its receptor, thereby avoiding the negative feedback mechanism that controls its synthesis (Lucy, 2008). Typically, the dairy cow enters a period of negative energy balance a few days before calving, reaches a nadir two weeks later, and returns to positive EBal by week ten postpartum (Butler, 2003). During negative EBal, adipose tissue is mobilised in support of milk production and BCS declines. The metabolic status during the postpartum period is characterised by alterations to DMI, milk production, hepatic mrna abundance of growth hormone receptor 1A (GHR 1A), and circulating concentrations of GH, insulin, IGF1, glucose, NEFA and BHBA (Figure 2.4). Figure 2.4. Temporal profile of milk production and metabolic indicators during the transition period. Adapted from Lucy et al. (2001) 17
36 The primary metabolites and metabolic hormones associated with superior fertility are insulin, IGF1 and glucose. Greater insulin concentrations support earlier recoupling of the somatotropic axis and up-regulation of hepatic mrna abundance of hepatic expression of GHR 1A and IGF1 (Butler et al., 2003). Insulin was greater in cows that ovulated the first wave dominant follicle (Butler et al., 2006). Numerous studies have reported positive associations between IGF1 and fertility (Taylor et al., 2004, Patton et al., 2007, Wathes, 2012). IGF1 acts on the ovary by promoting ovulation of the first postpartum dominant follicle, increases the mrna abundance of LH and FSH receptors (Lucy, 2000), amplifies FSH-stimulated E2 production (Bao and Garverick, 1998), and promotes P4 production in small luteal cells (Niswender et al., 1994). Energy balance during the first three weeks postpartum is correlated with the interval to ovulation (Canfield and Butler, 1990, Reist et al., 2003). Dry matter intake, the primary source of variation in EBal, is greater in cows that ovulate the first postpartum dominant follicle (Butler et al., 2006). Glucose is also a key metabolic regulator of fertility. Garverick et al. (2013) reported greater circulating glucose concentrations during the first week postpartum in cows that subsequently became pregnant to first service. Glucose may also be a key component of the early postpartum immune response to pathogenic bacteria as it is an important energy substrate for immune cells (Ingvartsen and Moyes, 2013). Glucose is also the principal metabolic fuel of the ovary (Rabiee et al., 1999). Circulating NEFA and BHBA concentrations during the transition period may not be suitable predictors of reproductive performance. While circulating NEFA concentrations were greater during the first two weeks postpartum in cows not pregnant to first service (Garverick et al., 2013), Burke et al. (2010) reported no differences in cows classified with or without endometritis and Chapinal et al. (2012) reported no differences between cows pregnant or not pregnant to first service Body Condition Score Positive effects of BCS on fertility have been reported in pasture and confinement dairy systems. Body condition score is a subjective assessment of adipose tissue reserves, and is evaluated on a scale of 1 to 5 in Ireland (Edmonson et al., 1989). Mobilisation of adipose tissue reserves results in a decline in BCS until about 50 to 100 days in milk 18
37 (Berry et al., 2006), which is then replenished during the remainder of lactation and the subsequent dry period. Low BCS at calving prolongs the post-partum anoestrous interval through delayed ovarian activity, infrequent LH pulses and development of gonadotropin-unresponsive follicles (Diskin et al., 2003). In a study involving over 6,000 cows in 74 Irish spring-calving herds, Buckley et al. (2003) reported positive associations between BCS at 60 and 100 days postpartum and 21-day submission rate and 6-week pregnancy rate, and between BCS at nadir and pregnancy rate to 1 st service. Roche et al. (2007) evaluated 2,600 lactation records from 900 New Zealand dairy cows, and reported a positive effect of BCS on resumption of cyclicity prior to mating start date and a negative effect of BCS loss from calving to nadir on 21-day submission rate. Santos et al. (2009) examined the records of 6,400 Holstein dairy cows from 4 dairy farms in a confinement production system. The proportion of animals that were cycling by 65 days postpartum and pregnancy rates per AI were greatest, intermediate and least when BCS loss from calving to AI was zero, < 1 unit and > 1 unit, respectively. Minimising the loss of BCS in early lactation is difficult, regardless of feeding level; however, greater levels of feeding do improve BCS gain (Roche et al., 2009) Genetic influence on Reproductive Performance The potential to improve fertility through genetic selection has been accepted internationally. This has been implemented through the inclusion of health and fertility traits in selection indices worldwide (Miglior et al., 2005). The rate of adoption of this approach, however, varied greatly among countries. The Nordic countries were the leaders in this regard, and incorporated health and fertility traits in their selection index since the 1970s. In comparison, Ireland and the USA only began to include nonproduction traits in their dairy selection indices in 2001 and 2003, respectively (Berry, 2007; Philipsson and Lindhé, 2003; VanRaden et al., 2004). More recently, crossbreeding of dairy breeds has been implemented to address declining fertility. A crossbreeding strategy allows the introduction of favourable genes from another breed that has been selected more strongly for traits of interest, removes inbreeding depression, and capitalises on hybrid vigour (Buckley et al., 2014). 19
38 Genetic Influence on Energy Status The increase in genetic merit for milk production is the primary factor responsible for increased phenotypic milk production and the decline in reproductive performance. Genetic correlation between milk production traits and reproductive traits are antagonistic (Berry et al., 2014). The primary physiological components of this antagonism are likely explained by alterations in (i) feed intake, (ii) energy balance, and (iii) circulating concentrations of energy metabolites, metabolic hormones and reproductive hormones, discussed previously (Veerkamp et al., 2003). This imbalance may have been widened due to the greater severity of negative EBal, as the genetic correlation between milk yield and DMI has been reported to be between 0.44 to 0.65 (Veerkamp et al., 2003). In the UK, cows with high genetic merit for milk production had delayed commencement of luteal activity and reduced oestrous expression compared with cows that had average genetic merit for milk production (Pollott and Coffey, 2008). In Ireland, cows with a high genetic merit for milk production had greater milk yield, reduced BCS, reduced in vitro blastocyst development (Snijders et al., 2000) and reduced conception rates (Snijders et al., 2001) compared with cows that had average genetic merit for milk production. Many reproductive traits are under moderate to strong genetic control (Berry et al., 2014). The lactation profile of BCS is also under moderate genetic control, with heritability estimates varying from 0.07 to 0.6 (Berry et al., 2008). Studies have indicated that significant variation in BCS exists both within and between breeds (Horan et al., 2005a, Friggens et al., 2007, Lucy et al., 2009, Prendiville et al., 2011a, Heins et al., 2012) Genetic Influence on Detailed Reproductive Phenotypes Additional efforts to increase the rate of genetic gain for female fertility include the measurement of more detailed reproductive phenotypes (Nyman et al., 2014, Carthy et al., 2014, Fitzgerald et al., 2014, Walsh et al., 2014) to reduce the unexplained variation in fertility, thereby improving the heritability estimates of female fertility from the current range of 0.02 to 0.04 (Berry et al., 2014). Antral follicle count (Walsh et al., 2014), the interval from calving to commencement of luteal activity (Nyman et al., 2014) and the interval from calving to the first oestrous activity period (Løvendahl and Chagunda, 2009) had moderate heritability estimates of 0.31, 0.18, and 0.18, respectively. Interestingly, antral follicle count and the interval from calving to commencement of luteal activity had unfavourable genetic relationships with milk production variables. These deep phenotypes may become useful in genetic evaluations 20
39 if sufficient records become available. The prospect of automated monitoring of animal health, ovarian activity and oestrus activity is quickly becoming a reality due to developments in in-line milk meters ( and activity monitors ( Influence of Genomic Regions and Variants on Reproductive Performance Genomic regions and variants affecting the reproductive performance of dairy cattle have been identified. A meta-analysis of genome-wide association studies and quantitative trait loci studies reported strong associations between regions of BTA1, 2, 3, 5, 6, 7, 9, 13, 16 and fertility (Khatkar et al., 2014). Genome-wide association studies have identified genomic regions associated with a range of fertility traits in dairy cattle (Pryce et al., 2010, Cole et al., 2011, Berry et al., 2012, Hoglund et al., 2014). Haplotypes that are embryonic lethal in the homozygous state have been identified on BTA1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 15, 18, 19, 20, 21, 24, 25, 26, 29 (VanRaden et al., 2011, Fritz et al., 2013, Sahana et al., 2013, Sonstegard et al., 2013, Daetwyler et al., 2014, McClure et al., 2014). The frequency of these embryonic lethal haplotypes may have increased due to greater selection intensity in dairy populations. The increase in inbreeding depression has had negative consequences for reproductive performance (VanRaden and Miller, 2006, McParland et al., 2007) Influence of Dairy Breeds on Reproductive Performance It became evident from the results of a number of breed comparison trials in Ireland and elsewhere that large variation existed in the reproductive performance of different breeds. Norwegian Red, F 1 Norwegian Red x Holstein-Friesian, Normande, F 1 Normande x Holstein-Friesian, Montbeliarde, F 1 Montbeliarde x Holstein-Friesian, and F 1 Holstein-Friesian x Jersey cows had greater BCS and superior reproductive performance compared with Holstein-Friesian cows (Walsh et al., 2008, Begley et al., 2009, Prendiville et al., 2011b). The superior reproductive performance of Norwegian Red cows compared with Holstein Friesian cows in Northern Ireland was most recently validated by Ferris et al. (2014). Over five lactations both breeds had similar milk solids production, but the Norwegian Red cows had greater CRFS and a greater proportion survived to the sixth lactation. Across the aforementioned studies, the Holstein-Friesian cows enrolled were primarily North-American genotypes, indicating their unsuitability for pasture-based milk production systems. Breed comparison studies in California 21
40 reported greater CRFS, increased survival, and fewer days open, but reduced milk production in Montbeliarde x Holstein and Scandinavian Red x Holstein cows compared with Holstein cows (Heins et al., 2006a, b). The genetic and physiological mechanisms contributing to the phenotypic fertility differences between breeds have not been well studied. Differences in BCS have been reported; however, differences in milk production may also have influenced the results. The influence of inbreeding and crossbreeding on embryo development has been evaluated. Lazzari et al. (2011) reported greater in vitro morula development, blastocyst development and in vivo ovoid embryo development on day six, seven and twelve of the oestrous cycle, respectively, in crossbred embryos compared with purebred or inbred embryos, presumably due to reduced levels of inbreeding depression Influence of Holstein Strain on Reproductive Performance It became evident from the results of a number of strain comparison farmlet trials in Ireland and New Zealand that large variation exists in the reproductive performance of different Holstein strains. Compared with the high production NAHF cows, high durability NAHF and New Zealand Holstein-Friesian cows had greater CRFS (Horan et al., 2005b), and were more profitable due to their superior reproductive performance (McCarthy et al., 2007c). The primary physiological differences between the strains are outlined in Table 2.4. The results of these studies indicate that cow genotype is critically important to the productivity and profitability of pasture-based milk production systems. 22
41 Table 2.4. Physiological mechanisms associated with the superior reproductive performance of New Zealand strain Holstein-Friesian cows compared with North American strain Holstein-Friesian cows. Greater BCS (Horan et al., 2005a, Roche et al., 2006, McCarthy et al., 2007b) Reduced BCS loss Greater BCS replenishment in late lactation Similar DMI per metabolic BW Patton et al. (2008) Similar or earlier commencement of luteal activity Harris and Kolver (2001); Horan et al. (2005b) Greater insulin responsiveness Patton et al. (2009) Greater circulating IGF1 Similar or greater hepatic IGF1 expression Greater endometrial expression of genes associated with: (i) immune tolerance to the embryo (ii) (iii) preventing luteolysis embryo support and development (Patton et al., 2008, Lucy et al., 2009) Lucy et al. (2009); McCarthy et al. (2009) Walker et al. (2012) Influence of Genetic Merit for Fertility on Reproductive Performance The differences in genotypic and phenotypic milk production, breeding goals and genetic diversity may have been confounding factors in the findings of the breed comparison and Holstein strain comparison studies outlined previously. To minimise the variation that may have existed in those studies, Teagasc Moorepark established a new herd consisting of two groups of Holstein cows with similar genetic merit for milk production traits, but with either good (Fert+) or poor (Fert-) genetic merit for calving interval. Animals were maintained as one herd, thus standardising all environmental factors that are known to affect reproductive performance. Large differences in phenotypic fertility performance between the genotypes were detected (Table 2.5) with practically similar milk production (Cummins et al., 2012a). Table 2.5. Milk production and fertility differences between Fert+ and Fert- cows during first lactation Variable Fert+ Fertn Milk yield (kg/day) Milk solids (kg/day) Calving to conception interval (days) Number of services per cow Number of services per pregnancy Pregnancy rate to first and second service (%) Six-week pregnancy rate (%) Adapted from Cummins et al. (2012a) 23
42 Subsequent studies examined the metabolic status and nutrient partitioning during lactation (Cummins et al., 2012a, c) and the characteristics of the oestrous cycle (Cummins et al., 2012b) to elucidate some of the physiological mechanisms contributing to the phenotypic fertility differences. The primary physiological differences between the genotypes were (i) greater BCS; (ii) greater circulating insulin and IGF1; (iii) stronger oestrous expression; (iv) fewer silent heats; (v) less ovulation failure after oestrous; and (vi) greater circulating P4 concentrations in Fert+ cows compared with Fert- cows. The study highlighted the large effects that genetic merit for fertility has on the physiological mechanisms influencing reproductive performance, without antagonising milk production. 2.6 Rationale for the Studies Undertaken Research was undertaken to continue the characterisation of a lactating cow genetic model of fertility to elucidate the physiological mechanisms contributing to suboptimal reproductive performance in lactating dairy cows. Two groups of Holstein cows with similar genetic merit for milk production traits, but with either good (Fert+) or poor (Fert-) genetic merit for calving interval were utilised. Animals were maintained as one herd, thus standardising all environmental factors that are known to affect reproductive performance (herd management, plane of nutrition, proportion of Holstein genes and genetic merit for milk production traits). The objectives of the research reported in this thesis are: 1) To monitor the transition period, uterine health and resumption of ovarian cyclicity in Fert+ and Fert- cows. 2) To examine the differences between Fert+ and Fert- cows in the factors that affect circulating progesterone concentrations during the oestrous cycle. 3) To identify potential differences between genotypes in the fatty acid and amino concentrations of follicular fluid and serum. 4) To compare the transcriptome of the endometrium and corpus luteum on day 13 of the oestrous cycle in Fert+ and Fert- cows and to identify genomic regions and variants potentially influencing reproductive performance. 5) To describe the results of the study and describe the implications from the work. 24
43 Chapter Three describes the phenotypic characterisation of the Fert+/Fert- herd during the transition and early lactation periods, with specific focus on monitoring dry matter intake, uterine health and resumption of ovarian cyclicity. Chapter Four quantifies preovulatory follicle and corpus luteum development, circulating steroid hormone concentrations and metabolic clearance rate of progesterone in Fert+ and Fert- cows. Chapter Five characterises the fatty and amino acid profiles in follicular fluid and serum in Fert+ and Fert- cows. Chapter Six compares the transcriptome of the endometrium and corpus luteum on day 13 of the oestrous cycle between Fert+ and Fert- cows, examines the concordance between differentially expressed genes and significant single nucleotide polymorphisms identified in fertility genome-wide association studies, and identifies genomic variants associated with fertility. Finally, Chapter Seven summarises the thesis, draws conclusions and implications from the work, describes limitations of the research, and identifies further areas for research. 25
44 2.7 References Adams, G. P., R. Jaiswal, J. Singh, and P. Malhi Progress in understanding ovarian follicular dynamics in cattle. Theriogenology 69(1): Austin, E. J., M. Mihm, M. P. Ryan, D. H. Williams, and J. F. Roche Effect of duration of dominance of the ovulatory follicle on onset of estrus and fertility in heifers. Journal of Animal Science 77(8): Bao, B. and H. A. Garverick Expression of steroidogenic enzyme and gonadotropin receptor genes in bovine follicles during ovarian follicular waves: a review. Journal of Animal Science 76(7): Bazer, F. W., G. Wu, G. A. Johnson, J. Kim, and G. Song Uterine Histotroph and Conceptus Development: Select Nutrients and Secreted Phosphoprotein 1 Affect MTOR Cell Signaling in Ewes. Biology of Reproduction. Begley, N., K. Pierce, and F. Buckley Milk production, udder health, body condition score and fertility performance of Holstein-Friesian, Norwegian Red and Norwegian Red Holstein-Friesian cows on Irish dairy farms. Pages in Breeding for robustness in cattle. Bello, N. M., J. S. Stevenson, and R. J. Tempelman Invited review: Milk production and reproductive performance: Modern interdisciplinary insights into an enduring axiom. Journal of Dairy Science 95(10): Berry, D. P., J. W. M. Bastiaansen, R. F. Veerkamp, S. Wijga, E. Wall, B. Berglund, and M. P. L. Calus Genome-wide associations for fertility traits in Holstein Friesian dairy cows using data from experimental research herds in four European countries. Animal 6(08): Berry, D. P., J. F. Kearney, K. Twomey, and R. D. Evans Genetics of reproductive performance in seasonal calving dairy cattle production systems. Irish Journal of Agricultural and Food Research 52(1):1-16. Berry, D. P., R. F. Veerkamp, and P. Dillon Phenotypic profiles for body weight, body condition score, energy intake, and energy balance across different parities and concentrate feeding levels. Livestock Science 104(1 2):1-12. Berry, D. P., E. Wall, and J. E. Pryce Genetics and genomics of reproductive performance in dairy and beef cattle. Animal 8(Supplements1): Bisinotto, R. S., E. S. Ribeiro, and J. E. P. Santos Synchronisation of ovulation for management of reproduction in dairy cows. Animal 8(Supplements1):
45 Bollwein, H., J. Lüttgenau, and K. Herzog Bovine luteal blood flow: basic mechanism and clinical relevance. Reproduction, Fertility and Development 25(1): Buckley, F., P. Dillon, M. Rath, and R. F. Veerkamp The Relationship Between Genetic Merit for Yield and Live Weight, Condition Score, and Energy Balance of Spring Calving Holstein Friesian Dairy Cows on Grass Based Systems of Milk Production. Journal of Dairy Science 83(8): Buckley, F., N. Lopez-Villalobos, and B. J. Heins Crossbreeding: implications for dairy cow fertility and survival. Animal 8(Supplements1): Buckley, F., K. O'Sullivan, J. F. Mee, R. D. Evans, and P. Dillon Relationships Among Milk Yield, Body Condition, Cow Weight, and Reproduction in Spring- Calved Holstein-Friesians. Journal of Dairy Science 86(7): Burke, C. R. and C. Fowler Fertility in New Zealand dairy herds: Industry situation and a way forward for improving on-farm reproductive performance. Date Accessed 18th June Burke, C. R., S. Meier, S. McDougall, C. Compton, M. Mitchell, and J. R. Roche Relationships between endometritis and metabolic state during the transition period in pasture-grazed dairy cows. Journal of Dairy Science 93(11): Butler, S., A. Marr, S. Pelton, R. Radcliff, M. Lucy, and W. Butler Insulin restores GH responsiveness during lactation-induced negative energy balance in dairy cattle: effects on expression of IGF-I and GH receptor 1A. Journal of Endocrinology 176(2): Butler, S. T Nutritional management to optimize fertility of dairy cows in pasture-based systems. Animal 8(Supplements1): Butler, S. T., S. H. Pelton, and W. R. Butler Energy Balance, Metabolic Status, and the First Postpartum Ovarian Follicle Wave in Cows Administered Propylene Glycol. Journal of Dairy Science 89(8): Butler, W. R Review: Effect of Protein Nutrition on Ovarian and Uterine Physiology in Dairy Cattle. Journal of Dairy Science 81(9): Butler, W. R Energy balance relationships with follicular development, ovulation and fertility in postpartum dairy cows. Livestock Production Science 83(2-3):
46 Canfield, R. W. and W. R. Butler Energy balance and pulsatile LH secretion in early postpartum dairy cattle. Domestic Animal Endocrinology 7(3): Canty, M. J., M. P. Boland, A. C. O. Evans, and M. A. Crowe Alterations in follicular IGFBP mrna expression and follicular fluid IGFBP concentrations during the first follicle wave in beef heifers. Animal Reproduction Science 93(3 4): Carter, F., N. Forde, P. Duffy, M. Wade, T. Fair, M. A. Crowe, A. C. O. Evans, D. A. Kenny, J. F. Roche, and P. Lonergan Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reproduction, Fertility and Development 20(3): Carthy, T. R., D. P. Berry, A. Fitzgerald, S. McParland, E. J. Williams, S. T. Butler, A. R. Cromie, and D. Ryan Risk factors associated with detailed reproductive phenotypes in dairy and beef cows. animal 8(05): Chapinal, N., M. E. Carson, S. J. LeBlanc, K. E. Leslie, S. Godden, M. Capel, J. E. P. Santos, M. W. Overton, and T. F. Duffield The association of serum metabolites in the transition period with milk production and early-lactation reproductive performance. Journal of Dairy Science 95(3): Colazo, M. G., A. Dourey, R. Rajamahendran, and D. J. Ambrose Progesterone supplementation before timed AI increased ovulation synchrony and pregnancy per AI, and supplementation after timed AI reduced pregnancy losses in lactating dairy cows. Theriogenology 79(5): Cole, J., G. Wiggans, L. Ma, T. Sonstegard, T. Lawlor, B. Crooker, C. Van Tassell, J. Yang, S. Wang, L. Matukumalli, and Y. Da Genome-wide association analysis of thirty one production, health, reproduction and body conformation traits in contemporary U.S. Holstein cows. BMC Genomics 12(1):408. Crowe, M. A Resumption of Ovarian Cyclicity in Post-partum Beef and Dairy Cows. Reproduction in Domestic Animals 43: Cummins, S. B., P. Lonergan, A. C. O. Evans, D. P. Berry, R. D. Evans, and S. T. Butler. 2012a. Genetic merit for fertility traits in Holstein cows: I. Production characteristics and reproductive efficiency in a pasture-based system. Journal of Dairy Science 95(3): Cummins, S. B., P. Lonergan, A. C. O. Evans, and S. T. Butler. 2012b. Genetic merit for fertility traits in Holstein cows: II. Ovarian follicular and corpus luteum 28
47 dynamics, reproductive hormones, and estrus behavior. Journal of Dairy Science 95(7): Cummins, S. B., S. M. Waters, A. C. O. Evans, P. Lonergan, and S. T. Butler. 2012c. Genetic merit for fertility traits in Holstein cows: III. Hepatic expression of somatotropic axis genes during pregnancy and lactation. Journal of Dairy Science 95(7): Daetwyler, H. D., A. Capitan, H. Pausch, P. Stothard, R. van Binsbergen, R. F. Brondum, X. Liao, A. Djari, S. C. Rodriguez, C. Grohs, D. Esquerre, O. Bouchez, M.-N. Rossignol, C. Klopp, D. Rocha, S. Fritz, A. Eggen, P. J. Bowman, D. Coote, A. J. Chamberlain, C. Anderson, C. P. VanTassell, I. Hulsegge, M. E. Goddard, B. Guldbrandtsen, M. S. Lund, R. F. Veerkamp, D. A. Boichard, R. Fries, and B. J. Hayes Whole-genome sequencing of 234 bulls facilitates mapping of monogenic and complex traits in cattle. Nature Genetics 46(8): Darwash, A. O., G. E. Lamming, and J. A. Woolliams The phenotypic association between the interval to post-partum ovulation and traditional measures of fertility in dairy cattle. Animal Science 65:9-16. Demetrio, D. G. B., R. M. Santos, C. G. B. Demetrio, and J. L. M. Vasconcelos Factors Affecting Conception Rates Following Artificial Insemination or Embryo Transfer in Lactating Holstein Cows. Journal of Dairy Science 90(11): Department of Agriculture, Fisheries and Food, Food Harvest Retrieved 4 March 2012, from pdf Dillon, P., D. P. Berry, R. D. Evans, F. Buckley, and B. Horan Consequences of genetic selection for increased milk production in European seasonal pasture based systems of milk production. Livestock Science 99(2-3): Diskin, M. G., D. R. Mackey, J. F. Roche, and J. M. Sreenan Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Animal Reproduction Science 78(3-4): Diskin, M. G., M. H. Parr, and D. G. Morris Embryo death in cattle: an update. Reproduction, Fertility and Development 24(1): Drackley, J. K Biology of Dairy Cows During the Transition Period: the Final Frontier? Journal of Dairy Science 82(11):
48 Edmonson, A. J., I. J. Lean, L. D. Weaver, T. Farver, and G. Webster A Body Condition Scoring Chart for Holstein Dairy Cows. Journal of Dairy Science 72(1): Evans, R. D., P. Dillon, F. Buckley, D. P. Berry, M. Wallace, V. Ducrocq, and D. J. Garrick Trends in milk production, calving rate and survival of cows in 14 Irish dairy herds as a result of the introgression of Holstein-Friesian genes. Animal Science 82(04): Ferris, C. P., D. C. Patterson, F. J. Gordon, S. Watson, and D. J. Kilpatrick Calving traits, milk production, body condition, fertility, and survival of Holstein-Friesian and Norwegian Red dairy cattle on commercial dairy farms over 5 lactations. Journal of Dairy Science 97(8): Fitzgerald, A. M., D. P. Berry, T. Carthy, A. R. Cromie, and D. P. Ryan Risk factors associated with multiple ovulation and twin birth rate in Irish dairy and beef cattle. Journal of Animal Science 92(3): Forde, N., M. E. Beltman, G. B. Duffy, P. Duffy, J. P. Mehta, P. O'Gaora, J. F. Roche, P. Lonergan, and M. A. Crowe. 2011a. Changes in the Endometrial Transcriptome During the Bovine Estrous Cycle: Effect of Low Circulating Progesterone and Consequences for Conceptus Elongation. Biology of Reproduction. Forde, N., M. E. Beltman, P. Lonergan, M. Diskin, J. F. Roche, and M. A. Crowe. 2011b. Oestrous cycles in Bos taurus cattle. Animal Reproduction Science 124(3 4): Forde, N., J. Mehta, P. McGettigan, S. Mamo, F. Bazer, T. Spencer, and P. Lonergan Alterations in expression of endometrial genes coding for proteins secreted into the uterine lumen during conceptus elongation in cattle. BMC Genomics 14(1):321. Friggens, N. C., P. Berg, P. Theilgaard, I. R. Korsgaard, K. L. Ingvartsen, P. Løvendahl, and J. Jensen Breed and Parity Effects on Energy Balance Profiles Through Lactation: Evidence of Genetically Driven Body Energy Change. Journal of Dairy Science 90(11): Fritz, S., A. Capitan, A. Djari, S. C. Rodriguez, A. Barbat, A. Baur, C. Grohs, B. Weiss, M. Boussaha, D. Esquerré, C. Klopp, D. Rocha, and D. Boichard Detection of Haplotypes Associated with Prenatal Death in Dairy Cattle and Identification of Deleterious Mutations in GART, SHBG and SLC37A2. Plos One 8(6):e
49 Funk, D. A Major Advances in Globalization and Consolidation of the Artificial Insemination Industry. Journal of Dairy Science 89(4): Galvão, K. N., M. Frajblat, W. R. Butler, S. B. Brittin, C. L. Guard, and R. O. Gilbert Effect of Early Postpartum Ovulation on Fertility in Dairy Cows. Reproduction in Domestic Animals 45(5):e207-e211. Garnsworthy, P., K. Sinclair, and R. Webb Integration of Physiological Mechanisms That Influence Fertility in Dairy Cows. animal 2: Garverick, H. A., M. N. Harris, R. Vogel-Bluel, J. D. Sampson, J. Bader, W. R. Lamberson, J. N. Spain, M. C. Lucy, and R. S. Youngquist Concentrations of nonesterified fatty acids and glucose in blood of periparturient dairy cows are indicative of pregnancy success at first insemination. Journal of Dairy Science 96(1): Geary, T. W., M. F. Smith, M. D. MacNeil, M. L. Day, G. A. Bridges, G. A. Perry, F. M. Abreu, J. A. Atkins, K. G. Pohler, E. M. Jinks, and C. A. Madsen Triennial Reproduction Symposium: Influence of follicular characteristics at ovulation on early embryonic survival. Journal of Animal Science 91(7): Geary, U., N. Lopez-Villalobos, D. J. Garrick, and L. Shalloo An analysis of the implications of a change to the seasonal milk supply profile in the Irish dairy industry utilizing a seasonal processing sector model. The Journal of Agricultural Science 150(03): Green, M. P., A. M. Ledgard, S. E. Beaumont, M. C. Berg, K. P. McNatty, A. J. Peterson, and P. J. Back Long-term alteration of follicular steroid concentrations in relation to subclinical endometritis in postpartum dairy cows. Journal of Animal Science 89(11): Hansen, P. J Embryonic mortality in cattle from the embryo's perspective. Journalof Animal Science. 80(E-Suppl_2):E Hansen, P. J., M. Drost, R. M. Rivera, F. F. Paula-Lopes, Y. M. Al-Katanani, C. E. Krininger Iii, and C. C. Chase Jr Adverse impact of heat stress on embryo production: causes and strategies for mitigation. Theriogenology 55(1): Harris, B. L. and E. S. Kolver Review of Holsteinization on Intensive Pastoral Dairy Farming in New Zealand. Journal of Dairy Science 84:E56-E61. Heins, B. J., L. B. Hansen, A. R. Hazel, A. J. Seykora, D. G. Johnson, and J. G. Linn Short communication: Jersey Holstein crossbreds compared with pure 31
50 Holsteins for body weight, body condition score, fertility, and survival during the first three lactations. Journal of Dairy Science 95(7): Heins, B. J., L. B. Hansen, and A. J. Seykora. 2006a. Fertility and Survival of Pure Holsteins Versus Crossbreds of Holstein with Normande, Montbeliarde, and Scandinavian Red. Journal of Dairy Science 89(12): Heins, B. J., L. B. Hansen, and A. J. Seykora. 2006b. Production of Pure Holsteins Versus Crossbreds of Holstein with Normande, Montbeliarde, and Scandinavian Red. Journal of Dairy Science 89(7): Herlihy, M. M., D. P. Berry, M. A. Crowe, M. G. Diskin, and S. T. Butler Evaluation of protocols to synchronize estrus and ovulation in seasonal calving pasture-based dairy production systems. Journal of Dairy Science 94(9): Hoelker, M., D. Salilew-Wondim, M. Drillich, G.-B. Christine, N. Ghanem, L. Goetze, D. Tesfaye, K. Schellander, and W. Heuwieser Transcriptional response of the bovine endometrium and embryo to endometrial polymorphonuclear neutrophil infiltration as an indicator of subclinical inflammation of the uterine environment. Reproduction, Fertility and Development 24(6): Hoglund, J., G. Sahana, B. Guldbrandtsen, and M. Lund Validation of associations for female fertility traits in Nordic Holstein, Nordic Red and Jersey dairy cattle. BMC Genetics 15(1):8. Holmes, C. W., G. F. Wilson, D. D. S. Mackenzie, D. S. Flux, I. M. Brookes, and A. W. F. Davey Milk production from pasture, 3rd edition. Butterworths of New Zealand, Wellington, New Zealand. Horan, B., P. Dillon, P. Faverdin, L. Delaby, F. Buckley, and M. Rath. 2005a. The Interaction of Strain of Holstein-Friesian Cows and Pasture-Based Feed Systems on Milk Yield, Body Weight, and Body Condition Score. Journal of Dairy Science 88(3): Horan, B., J. F. Mee, P. O Connor, M. Rath, and P. Dillon. 2005b. The effect of strain of Holstein-Friesian cow and feeding system on postpartum ovarian function, animal production and conception rate to first service. Theriogenology 63(3): Horan, B., J. F. Mee, M. Rath, P. O'Connor, and P. Dillon The effect of strain of Holstein-Friesian cow and feeding system on reproductive performance in seasonal-calving milk production systems. Animal Science 79:
51 Ingvartsen, K. L. and K. Moyes Nutrition, immune function and health of dairy cattle. animal 7(Supplements1): Juengel, J. L. and G. D. Niswender Molecular regulation of luteal progesterone synthesis in domestic ruminants. Journal of Reproduction and Fertility: Kennedy, J., P. Dillon, K. O'Sullivan, F. Buckley, and M. Rath The effect of genetic merit for milk production and concentrate feeding level on the reproductive performance of Holstein-Friesian cows in a grass-based system. Animal Science 76: Khatkar, M. S., I. A. S. Randhawa, and H. W. Raadsma Meta-assembly of genomic regions and variants associated with female reproductive efficiency in cattle. Livestock Science 166(0): Larson, S. F., W. R. Butler, and W. B. Currie Pregnancy rates in lactating dairy cattle following supplementation of progesterone after artificial insemination. Animal Reproduction Science 102(1-2): Lazzari, G., S. Colleoni, R. Duchi, A. Galli, F. D. Houghton, and C. Galli Embryonic genotype and inbreeding affect preimplantation development in cattle. Reproduction 141(5): Lazzari, G., C. Wrenzycki, D. Herrmann, R. Duchi, T. Kruip, H. Niemann, and C. Galli Cellular and Molecular Deviations in Bovine In Vitro-Produced Embryos Are Related to the Large Offspring Syndrome. Biology of Reproduction 67(3): Leblanc, S Assessing the Association of the Level of Milk Production with Reproductive Performance in Dairy Cattle. Journal of Reproduction and Development 56(S):S1-S7. Ledgard, A. M., G. A. Smolenski, H. Henderson, and R. S.-F. Lee Influence of pathogenic bacteria species present in the postpartum bovine uterus on proteome profiles. Reproduction, Fertility and Development:-. Leroy, J. L. M. R., T. Vanholder, J. R. Delanghe, G. Opsomer, A. Van Soom, P. E. J. Bols, J. Dewulf, and A. de Kruif Metabolic changes in follicular fluid of the dominant follicle in high-yielding dairy cows early post partum. Theriogenology 62(6): Leroy, J. L. M. R., T. Vanholder, B. Mateusen, A. Christophe, G. Opsomer, A. de Kruif, G. Genicot, and A. Van Soom Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 130(4):
52 Lonergan, P Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 76(9): Lonergan, P. and N. Forde Maternal-embryo interaction leading up to the initiation of implantation of pregnancy in cattle. animal 8(Supplements1): Løvendahl, P. and M. G. G. Chagunda Short communication: Genetic variation in estrus activity traits. Journal of Dairy Science 92(9): Lucy, M. C Regulation of Ovarian Follicular Growth by Somatotropin and Insulin-Like Growth Factors in Cattle1. Journal of Dairy Science 83(7): Lucy, M. C Reproductive Loss in High-Producing Dairy Cattle: Where Will It End? Journal of Dairy Science 84(6): Lucy, M. C Functional Differences in the Growth Hormone and Insulin-like Growth Factor Axis in Cattle and Pigs: Implications for Post-partum Nutrition and Reproduction. Reproduction in Domestic Animals 43: Lucy, M. C., H. Jiang, and Y. Kobayashi Changes in the Somatotrophic Axis Associated with the Initiation of Lactation. Journal of Dairy Science 84:E113- E119. Lucy, M. C., G. A. Verkerk, B. E. Whyte, K. A. Macdonald, L. Burton, R. T. Cursons, J. R. Roche, and C. W. Holmes Somatotropic axis components and nutrient partitioning in genetically diverse dairy cows managed under different feed allowances in a pasture system. Journal of Dairy Science 92(2): Macmillan, K. L The incalf Project: improving reproductive performance of cows in Australian dairy herds. Dairy Cow Fertility: Reproductive performance for efficient pasture-based systems:6-18. Maillo, V., D. Rizos, U. Besenfelder, V. Havlicek, A. K. Kelly, M. Garrett, and P. Lonergan Influence of lactation on metabolic characteristics and embryo development in postpartum Holstein dairy cows. Journal of Dairy Science 95(7): Mann, G. E Corpus luteum size and plasma progesterone concentration in cows. Animal Reproduction Science 115(1-4): Marei, W. F., D. C. Wathes, and A. A. Fouladi-Nashta Differential effects of linoleic and alpha-linolenic fatty acids on spatial and temporal mitochondrial distribution and activity in bovine oocytes. Reproduction, Fertility and Development 24(5):
53 Martins, J. P. N., R. K. Policelli, L. M. Neuder, W. Raphael, and J. R. Pursley Effects of cloprostenol sodium at final prostaglandin F2α of Ovsynch on complete luteolysis and pregnancy per artificial insemination in lactating dairy cows. Journal of Dairy Science 94(6): McCarthy, S., D. P. Berry, P. Dillon, M. Rath, and B. Horan. 2007a. Effect of strain of Holstein Friesian and feed system on calving performance, blood parameters and overall survival. Livestock Science 111(3): McCarthy, S., D. P. Berry, P. Dillon, M. Rath, and B. Horan. 2007b. Influence of Holstein-Friesian Strain and Feed System on Body Weight and Body Condition Score Lactation Profiles. Journal of Dairy Science 90(4): McCarthy, S., B. Horan, P. Dillon, P. O Connor, M. Rath, and L. Shalloo. 2007c. Economic Comparison of Divergent Strains of Holstein-Friesian Cows in Various Pasture-Based Production Systems. Journal of Dairy Science 90(3): McCarthy, S. D., S. T. Butler, J. Patton, M. Daly, D. G. Morris, D. A. Kenny, and S. M. Waters Differences in the expression of genes involved in the somatotropic axis in divergent strains of Holstein-Friesian dairy cows during early and mid lactation. Journal of Dairy Science 92(10): McClure, M. C., D. Bickhart, D. Null, P. VanRaden, L. Xu, G. Wiggans, G. Liu, S. Schroeder, J. Glasscock, J. Armstrong, J. B. Cole, C. P. Van Tassell, and T. S. Sonstegard Bovine Exome Sequence Analysis and Targeted SNP Genotyping of Recessive Fertility Defects BH1, HH2, and HH3 Reveal a Putative Causative Mutation in SMC2 for HH3. Plos One 9(3):e McDougall, S Reproduction performance and management of dairy cattle. Journal of Reproduction and Development 52(1): McDougall, S., H. Hussein, D. Aberdein, K. Buckle, J. Roche, C. Burke, M. Mitchell, and S. Meier Relationships between cytology, bacteriology and vaginal discharge scores and reproductive performance in dairy cattle. Theriogenology 76(2): McNeill, R. E., M. G. Diskin, J. M. Sreenan, and D. G. Morris Associations between milk progesterone concentration on different days and with embryo survival during the early luteal phase in dairy cows. Theriogenology 65(7):
54 McParland, S., J. F. Kearney, M. Rath, and D. P. Berry Inbreeding Effects on Milk Production, Calving Performance, Fertility, and Conformation in Irish Holstein-Friesians. Journal of Dairy Science 90(9): Mihm, M., N. Curran, P. Hyttel, P. G. Knight, M. P. Boland, and J. F. Roche Effect of dominant follicle persistence on follicular fluid oestradiol and inhibin and on oocyte maturation in heifers. Journal of Reprodion and Fertility 116(2): Nebel, R. L. and M. L. McGilliard Interactions of High Milk Yield and Reproductive Performance in Dairy Cows. Journal of Dairy Science 76(10): Niswender, G. D., J. L. Juengel, W. J. McGuire, C. J. Belfiore, and M. C. Wiltbank Luteal function: the estrous cycle and early pregnancy. Biology of Reproduction 50(2): Norman, H. D., J. R. Wright, S. M. Hubbard, R. H. Miller, and J. L. Hutchison Reproductive status of Holstein and Jersey cows in the United States. Journal of Dairy Science 92(7): Nyman, S., K. Johansson, D. J. de Koning, D. P. Berry, R. F. Veerkamp, E. Wall, and B. Berglund Genetic analysis of atypical progesterone profiles in Holstein-Friesian cows from experimental research herds. Journal of Dairy Science 97(11): Oliveira, L. J., N. Mansourri-Attia, A. G. Fahey, J. Browne, N. Forde, J. F. Roche, P. Lonergan, and T. Fair Characterization of the Th Profile of the Bovine Endometrium during the Oestrous Cycle and Early Pregnancy. Plos One 8(10):e Patton, J., D. A. Kenny, S. McNamara, J. F. Mee, F. P. O Mara, M. G. Diskin, and J. J. Murphy Relationships Among Milk Production, Energy Balance, Plasma Analytes, and Reproduction in Holstein-Friesian Cows. Journal of Dairy Science 90(2): Patton, J., J. J. Murphy, F. P. O Mara, and S. T. Butler Responses of North American and New Zealand strains of Holstein?Friesian dairy cattle to homeostatic challenges during early and mid-lactation. animal 3(02): Patton, J., J. J. Murphy, F. P. O?Mara, and S. T. Butler A comparison of energy balance and metabolic profiles of the New Zealand and North American strains of Holstein Friesian dairy cow. animal 2(06):
55 Peippo, J., Z. Machaty, and A. Peter Terminologies for the pre-attachment bovine embryo. Theriogenology 76(8): Pollott, G. E. and M. P. Coffey The Effect of Genetic Merit and Production System on Dairy Cow Fertility, Measured Using Progesterone Profiles and On- Farm Recording. Journal of Dairy Science 91(9): Prendiville, R., K. M. Pierce, L. Delaby, and F. Buckley. 2011a. Animal performance and production efficiencies of Holstein-Friesian, Jersey and Jersey Holstein- Friesian cows throughout lactation. Livestock Science 138(1 3): Prendiville, R., L. Shalloo, K. M. Pierce, and F. Buckley. 2011b. Comparative performance and economic appraisal of Holstein-Friesian, Jersey and Jersey Holstein-Friesian cows under seasonal pasture-based management. Irish Journal of Agricultural and Food Research 50(2): Pryce, J. E., S. Bolormaa, A. J. Chamberlain, P. J. Bowman, K. Savin, M. E. Goddard, and B. J. Hayes A validated genome-wide association study in 2 dairy cattle breeds for milk production and fertility traits using variable length haplotypes. Journal of Dairy Science 93(7): Pryce, J. E., R. Woolaston, D. P. Berry, E. Wall, M. Winters, R. Butler, and M. Shaffer World Trends in Dairy Cow Fertility. Proceedings, 10th World Congress of Genetics Applied to Livestock Production. Rabiee, A. R., I. J. Lean, J. M. Gooden, and B. G. Miller Relationships Among Metabolites Influencing Ovarian Function in the Dairy Cow. Journal of Dairy Science 82(1): Refsdal, A. O Reproductive performance of Norwegian cattle from 1985 to 2005: trends and seasonality. Acta Veterinaria Scandinavica 49:5-11. Reist, M., D. K. Erdin, D. von Euw, K. M. Tschümperlin, H. Leuenberger, H. M. Hammon, C. Morel, C. Philipona, Y. Zbinden, N. Künzi, and J. W. Blum Postpartum reproductive function: association with energy, metabolic and endocrine status in high yielding dairy cows. Theriogenology 59(8): Reynolds, C. K., P. C. Aikman, B. Lupoli, D. J. Humphries, and D. E. Beever Splanchnic Metabolism of Dairy Cows During the Transition From Late Gestation Through Early Lactation. Journal of Dairy Science 86(4): Rhinehart, J. D., M. J. Starbuck-Clemmer, J. A. Flores, R. A. Milvae, J. Yao, D. H. Poole, and E. K. Inskeep Low peripheral progesterone and late embryonic/early fetal loss in suckled beef and lactating dairy cows. Theriogenology 71(3):
56 Rizos, D., F. Carter, U. Besenfelder, V. Havlicek, and P. Lonergan Contribution of the female reproductive tract to low fertility in postpartum lactating dairy cows. Journal of Dairy Science 93(3): Rizos, D., F. Ward, P. Duffy, M. P. Boland, and P. Lonergan Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Molecular Reproduction and Development 61(2): Roche, J. F., M. Mihm, M. G. Diskin, and J. J. Ireland A Review of Regulation of Follicle Growth in Cattle. Journal of Animal Science 76(suppl 3): Roche, J. R., D. P. Berry, and E. S. Kolver Holstein-Friesian Strain and Feed Effects on Milk Production, Body Weight, and Body Condition Score Profiles in Grazing Dairy Cows. Journal of Dairy Science 89(9): Roche, J. R., C. R. Burke, S. Meier, and C. G. Walker Nutrition reproduction interaction in pasture-based systems: is nutrition a factor in reproductive failure? Animal Production Science 51(12): Roche, J. R., N. C. Friggens, J. K. Kay, M. W. Fisher, K. J. Stafford, and D. P. Berry Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. Journal of Dairy Science 92(12): Roche, J. R., K. A. Macdonald, C. R. Burke, J. M. Lee, and D. P. Berry Associations Among Body Condition Score, Body Weight, and Reproductive Performance in Seasonal-Calving Dairy Cattle. Journal of Dairy Science 90(1): Sahana, G., U. S. Nielsen, G. P. Aamand, M. S. Lund, and B. Guldbrandtsen Novel Harmful Recessive Haplotypes Identified for Fertility Traits in Nordic Holstein Cattle. Plos One 8(12):e Sangsritavong, S., D. K. Combs, R. Sartori, L. E. Armentano, and M. C. Wiltbank High Feed Intake Increases Liver Blood Flow and Metabolism of Progesterone and Estradiol-17β in Dairy Cattle. Journal of Dairy Science 85(11): Santos, J. E. P., H. M. Rutigliano, and M. F. S. Filho Risk factors for resumption of postpartum estrous cycles and embryonic survival in lactating dairy cows. Animal Reproduction Science 110(3-4): Santos, T. M. A. and R. C. Bicalho Diversity and Succession of Bacterial Communities in the Uterine Fluid of Postpartum Metritic, Endometritic and Healthy Dairy Cows. Plos One 7(12):e
57 Sartori, R., G. J. M. Rosa, and M. C. Wiltbank. 2002a. Ovarian Structures and Circulating Steroids in Heifers and Lactating Cows in Summer and Lactating and Dry Cows in Winter. Journal of Dairy Science 85(11): Sartori, R., R. Sartor-Bergfelt, S. A. Mertens, J. N. Guenther, J. J. Parrish, and M. C. Wiltbank. 2002b. Fertilization and Early Embryonic Development in Heifers and Lactating Cows in Summer and Lactating and Dry Cows in Winter. Journal of Dairy Science 85(11): Savio, J. D., M. P. Boland, N. Hynes, and J. F. Roche Resumption of follicular activity in the early post-partum period of dairy cows. Journal of Reproduction and Fertility 88(2): Senger, P. L Pathways to pregnancy and parturition. Current Conceptions, Inc., Pullman, Washington. Shalloo, L Pushing the barriers on milk costs/ outputs. Proceedings on the National Dairy Conference: Shalloo, L., A. Cromie, and N. McHugh Effect of fertility on the economics of pasture-based dairy systems. animal 8(Supplements1): Sheldon, I. M., J. G. Cronin, G. D. Healey, C. Gabler, W. Heuwieser, D. Streyl, J. J. Bromfield, A. Miyamoto, C. Fergani, and H. Dobson Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction 148(3):R41-R51. Sirard, M.-A., F. Richard, P. Blondin, and C. Robert Contribution of the oocyte to embryo quality. Theriogenology 65(1): Snijders, S. E. M., P. Dillon, D. O'Callaghan, and M. P. Boland Effect of genetic merit, milk yield, body condition and lactation number on in vitro oocyte development in dairy cows. Theriogenology 53(4): Snijders, S. E. M., P. G. Dillon, K. J. O Farrell, M. Diskin, A. R. G. Wylie, D. O Callaghan, M. Rath, and M. P. Boland Genetic merit for milk production and reproductive success in dairy cows. Animal Reproduction Science 65(1 2): Sonstegard, T. S., J. B. Cole, P. M. VanRaden, C. P. Van Tassell, D. J. Null, S. G. Schroeder, D. Bickhart, and M. C. McClure Identification of a Nonsense Mutation in CWC15 Associated with Decreased Reproductive Efficiency in Jersey Cattle. Plos One 8(1):e
58 Stronge, A. J. H., J. M. Sreenan, M. G. Diskin, J. F. Mee, D. A. Kenny, and D. G. Morris Post-insemination milk progesterone concentration and embryo survival in dairy cows. Theriogenology 64(5): Sturmey, R. G., A. Reis, H. J. Leese, and T. G. McEvoy Role of Fatty Acids in Energy Provision During Oocyte Maturation and Early Embryo Development. Reproduction in Domestic Animals 44: Taylor, V. J., Z. Cheng, P. G. A. Pushpakumara, D. C. Wathes, and D. E. Beever Relationships between the plasma concentrations of insulin-like growth factor-i in dairy cows and their fertility and milk yield. Veterinary Record 155(19): Thatcher, W., l. S. R. de, E. Schmitt, T. Diaz, L. Badinga, F. Simmen, C. Staples, and M. Drost Control and management of ovarian follicles in cattle to optimize fertility. Reproduction, Fertility and Development 8(2): Townson, D. H., P. C. Tsang, W. R. Butler, M. Frajblat, L. C. Griel, C. J. Johnson, R. A. Milvae, G. M. Niksic, and J. L. Pate Relationship of fertility to ovarian follicular waves before breeding in dairy cows. Journal of Animal Science 80(4): Van Hoeck, V., R. G. Sturmey, P. Bermejo-Alvarez, D. Rizos, A. Gutierrez-Adan, H. J. Leese, P. E. J. Bols, and J. L. M. R. Leroy Elevated Non-Esterified Fatty Acid Concentrations during Bovine Oocyte Maturation Compromise Early Embryo Physiology. Plos One 6(8):e Vanholder, T., J. Lmr Leroy, A. Van Soom, D. Maes, M. Coryn, T. Fiers, A. de Kruif, and G. Opsomer Effect of non-esterified fatty acids on bovine theca cell steroidogenesis and proliferation in vitro. Animal Reproduction Science 92(1-2): VanRaden, P. M. and R. H. Miller Effects of Nonadditive Genetic Interactions, Inbreeding, and Recessive Defects on Embryo and Fetal Loss by Seventy Days. Journal of Dairy Science 89(7): VanRaden, P. M., K. M. Olson, D. J. Null, and J. L. Hutchison Harmful recessive effects on fertility detected by absence of homozygous haplotypes. Journal of Dairy Science 94(12): Vasconcelos, J. L. M., D. G. B. Demétrio, R. M. Santos, J. R. Chiari, C. A. Rodrigues, and O. G. S. Filho Factors potentially affecting fertility of lactating dairy cow recipients. Theriogenology 65(1):
59 Veerkamp, R. F., B. Beerda, and T. van der Lende Effects of genetic selection for milk yield on energy balance, levels of hormones, and metabolites in lactating cattle, and possible links to reduced fertility. Livestock Production Science 83(2-3): Walker, C. G., M. D. Littlejohn, M. D. Mitchell, J. R. Roche, and S. Meier Endometrial gene expression during early pregnancy differs between fertile and subfertile dairy cow strains. Physiological Genomics 44(1): Walsh, S., F. Buckley, K. Pierce, N. Byrne, J. Patton, and P. Dillon Effects of Breed and Feeding System on Milk Production, Body Weight, Body Condition Score, Reproductive Performance, and Postpartum Ovarian Function. Journal of Dairy Science 91(11): Walsh, S. W., F. Mossa, S. T. Butler, D. P. Berry, D. Scheetz, F. Jimenez-Krassel, R. J. Tempelman, F. Carter, P. Lonergan, A. C. O. Evans, and J. J. Ireland Heritability and impact of environmental effects during pregnancy on antral follicle count in cattle. Journal of Dairy Science 97(7): Walsh, S. W., E. J. Williams, and A. C. O. Evans A review of the causes of poor fertility in high milk producing dairy cows. Animal Reproduction Science 123(3 4): Wathes, D. C Mechanisms Linking Metabolic Status and Disease with Reproductive Outcome in the Dairy Cow. Reproduction in Domestic Animals 47: Williams, E. J., D. P. Fischer, D. E. Noakes, G. C. W. England, A. Rycroft, H. Dobson, and I. M. Sheldon The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 68(4): Williams, E. J., D. P. Fischer, D. U. Pfeiffer, G. C. W. England, D. E. Noakes, H. Dobson, and I. M. Sheldon Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 63(1): Williams, E. J., K. Sibley, A. N. Miller, E. A. Lane, J. Fishwick, D. M. Nash, S. Herath, G. C. W. England, H. Dobson, and I. M. Sheldon ORIGINAL ARTICLE: The Effect of Escherichia coli Lipopolysaccharide and Tumour Necrosis Factor Alpha on Ovarian Function. American Journal Of Reproductive Immunology 60(5):
60 Wiltbank, M. C Cell types and hormonal mechanisms associated with mid-cycle corpus luteum function. Journal of Animal Science 72(7): Wiltbank, M. C., A. H. Souza, P. D. Carvalho, A. P. Cunha, J. O. Giordano, P. M. Fricke, G. M. Baez, and M. G. Diskin Physiological and practical effects of progesterone on reproduction in dairy cattle. animal 8(Supplements1): Woelders, H., T. van der Lende, A. Kommadath, M. F. W. te Pas, M. A. Smits, and L. M. T. E. Kaal Central genomic regulation of the expression of oestrous behaviour in dairy cows: a review. animal 8(05): Xu, Z. Z., L. J. Burton, and K. L. Macmillan Reproductive performance of lactating dairy cows following estrus synchronization regimens with PGF2α and progesterone. Theriogenology 47(3):
61 Chapter Three Genetic merit for fertility traits in Holstein cows: Transition period, uterine health and resumption of cyclicity 3.1 Preface At the time of thesis submission this chapter was published in Journal of Dairy Science (Accepted January 23, 2014; The full reference is: Moore, S.G., T. Fair, P. Lonergan, and S.T. Butler. Genetic merit for fertility traits in Holstein cows: IV. Transition period, uterine health and resumption of cyclicity. J. Dairy Sci. 2014, 97: Stephen Moore was the primary author and carried out the experimental work, statistical analysis and drafted the manuscript. Stephen Moore and Stephen Butler conceived, designed and coordinated the study. All authors interpreted the data and contributed to the manuscript. Formatting and reference style has been edited for consistency throughout the thesis. Figure and table captions have been assigned with a chapter prefix. Acknowledgements have been removed. All other aspects are consistent with the published manuscript. 43
62 3.2 Abstract The objective of this study was to monitor the dry matter intake (DMI), metabolic status, uterine health and resumption of cyclicity in cows with similar genetic merit for milk production traits but with either good (Fert+) or poor genetic merit (Fert-) for fertility traits. Twenty six cows were enrolled in the study, and data are reported for 15 Fert+ and 10 Fert- cows that completed the study. All cows received a total mixed ration diet during early lactation and were turned out to pasture in late spring. Dry matter intake was recorded daily from weeks -2 to 5 relative to parturition. Blood metabolites and metabolic hormones were measured from weeks -2 to 8 relative to parturition. Milk production, body condition score and body weight until week 35 of lactation are reported. To monitor uterine health, vaginal mucus was scored weekly on a scale of zero (no pus) to three ( 50% pus) from parturition to week 8 and uterine polymorphonuclear neutrophil count was measured at weeks 3 and 6 postpartum. Prepartum DMI was similar between genotypes, but during the postpartum period, Fert+ cows had significantly greater DMI than Fert- cows (19.7 vs kg DM/d). Energy balance at week 1 was significantly greater in Fert+ cows than Fert- cows (2.3 vs UFL/d). Fert+ cows had significantly greater daily milk solids production (1.9 vs. 1.7 kg/d) and tended to have greater daily milk yield (24.2 vs kg/d). Fert+ cows had significantly greater mean circulating insulin-like growth factor-1 (102.6 vs ng/ml) and tended to have greater mean circulating insulin (3.3 vs. 2.6 μiu/ml) compared with Fert- cows from weeks -2 to 8 relative to parturition. Mean circulating glucose (3.4 vs. 3.0 mmol/l) concentrations were significantly greater in Fert+ cows compared with Fert- cows from weeks -2 to 3 relative to parturition. Fert+ cows maintained significantly greater mean body condition score throughout lactation compared with Fert- cows (2.98 vs units). Fert+ cows had better uterine health compared with Fert- cows as evidenced by lower weekly vaginal mucus scores during weeks 2 to 6 postpartum, and based on uterine cytology a smaller proportion were classified as having endometritis at week 3 (0.42 vs. 0.78) and 6 (0.25 vs. 0.75). Also, a significantly greater proportion of Fert+ cows had resumed cyclicity by week 6 postpartum (0.86 vs. 0.20) compared with Fert- cows. Hence we report for the first time that genetic merit for fertility traits is associated with postpartum uterine health status. Superior uterine health and earlier resumption of cyclicity may be mediated through differences in DMI, energy balance, insulin, insulin-like growth factor-1 and body 44
63 condition score profiles. Importantly, phenotypic improvement in fertility traits was achieved without antagonising milk production. 45
64 3.3 Introduction The transition period in cattle is described as the period from 3 weeks pre-calving to 3 weeks post-calving, and is associated with the potential occurrence of a vast array of diseases that affect production, fertility and health (Drackley, 1999). During this period, the energy requirements of the foetus and the mammary gland increase at a greater rate than energy intake. Typically, dairy cows enter a period of negative energy balance (EBal) a few days before calving, reach nadir two weeks later and return to positive energy balance by week 10 (Butler, 2003). Some of the adverse effects of negative EBAL on fertility are mediated by delays in resumption of cyclicity (Butler et al., 2006). After parturition, circulating glucose is prioritised for the mammary gland instead of peripheral tissues and adipose tissue is mobilised, resulting in NEFA and glycerol release from adipose tissue, and a decline in body condition score (BCS). In the liver, NEFA can be (i) completely oxidised for energy; (ii) partially oxidised to form ketones; or (iii) esterified to form triglycerides, resulting in fatty liver (Ingvartsen, 2006). Concurrently, delayed recoupling of the somatotropic axis due to insufficient circulating insulin (Butler et al., 2003) increases the rate and duration of adipose tissue mobilisation. Peripartum concentrations of these metabolites have been shown to be different in cows that do or do not ovulate the follicle from the first postpartum follicular wave (Butler et al., 2006), and also in cows that do or do not become pregnant to first AI (Garverick et al., 2013). In addition, circulating concentrations of NEFA, BHBA and glucose have been incorporated into a model of "physiological imbalance" that is associated with the risk of disease during early lactation (Moyes et., 2013). Also, early resumption of ovarian cyclicity following parturition is a key factor in determining subsequent fertility (Darwash et al., 1997; Galvão et al., 2010). Positive effects of BCS on fertility have been reported in pasture and confinement dairy systems (Buckley et al., 2003; Roche et al., 2007; Santos et al., 2009). Attempts to minimise adipose tissue mobilisation during the early post-partum period through altered nutritional and management strategies have had limited success in pasture-based systems (Horan et al., 2005a; Roche et al., 2006). However, it has been shown that BCS is under strong genetic control and that it differs between breeds and between different strains within breed (Horan et al., 2005a, Friggens et al., 2007; Lucy et al., 2009; Prendiville et al., 2009; Cummins et al., 2012a; Heins et al., 2012). 46
65 It is generally accepted that all cows become exposed to bacteria after calving. The development of uterine disease depends on the type of bacteria involved and on the immune response of the cow, and is associated with reduced subsequent fertility (Sheldon et al., 2009). Clinical disease, lower dry matter intake (DMI), increased bacterial presence and increased NEFA and BHBA concentrations during the transition period have been associated with the incidence of endometritis between four and six weeks postpartum (LeBlanc, 2012). We have previously validated a lactating Holstein cow genetic model of fertility (Cummins et al., 2012a), and used this animal model to identify some of the effects of genetic merit for fertility traits on phenotypic measures of fertility (Cummins et al., 2012a, b, c). The aim of the current study was to determine the effect of genetic merit for fertility traits on DMI, energy balance, blood indicators of metabolic status during late gestation and the early lactation period, postpartum uterine health and the resumption of ovarian cyclicity. 47
66 3.4 Materials and Methods Animal Model A genetic model of fertility was established in Teagasc Moorepark to elucidate the mechanisms responsible for poor fertility in lactating Holstein dairy cows (Cummins et al., 2012a, b, c). Briefly, heifers with >75% Holstein genetics with extreme positive (i.e., poor fertility; Fert-) or negative (i.e., high fertility; Fert+) EBV for calving interval were selected from the national dairy cattle database. Within the Irish national herd, the selected heifers represented the top 25% in genetic merit for milk production. Fert- heifers represented the bottom 5% in genetic merit for calving interval, whereas Fert+ heifers represented the top 20% in genetic merit for calving interval. In subsequent years, herd replacements were generated by selecting suitable sires to maintain the difference in genetic merit for calving interval. The list was restricted to sires with >200 kg PTA for milk production, >0% PTA milk fat and protein concentration and >75% Holstein genetics. From this group, sires with >5 days PTA for calving interval were selected for mating with Fert- cows and sires with <-5 days PTA for calving interval were selected for mating with Fert+ cows. Twenty-six cows were enrolled in the current study and the EBV of the cows from both genotypes are summarized in Table 3.1. The parity structure of the Fert+ cows was 3, 4 and 8 cows in second, third and fourth lactation, respectively. The parity structure of the Fert- cows was 2, 3, and 6 cows in second, third and fourth lactation, respectively. Fert+ and Fert- cows were represented by 6 and 9 sires respectively. The maximum and minimum number of daughters from an individual sire for Fert+ and Fert- cows was 4 and 1; and 2 and 1, respectively Feed and Management System The experimental procedures involving animals on this study were licensed by the Department of Health, Ireland, in accordance with the Cruelty to Animals Act (Ireland 1876) and the European Community Directive 86/609/EEC. The study was undertaken at Teagasc Moorepark from January 2012 to December Cows were housed in a freestall barn during the dry period until 35 days post-partum. During the days approaching parturition, cows were moved to a straw-bedded calving shed. Dry matter intake was recorded daily using the Griffith Elder feeding system (Griffith Elder Ltd, Bury St Edmunds, Suffolk, UK). A prepartum diet of grass silage, concentrate ration, straw and dry cow mineral mix was fed ad libitum. Postpartum cows were fed a TMR 48
67 ad libitum plus 6 kg dairy concentrate per day at the a.m. and p.m. milkings. Feed refusals were removed every second day. Diet ingredients were sampled weekly and composited monthly for analysis. The ingredient and nutrient composition of the diets are outlined in Table 3.2. Cows were turned out to grass on March 26 th and managed as one herd in a rotational grazing system. Cows grazed a predominantly perennial ryegrass (Lolium perenne L.) sward with fresh pasture allocated daily. The mean daily herbage allowance was 14.5 ± 1.3 kg DM/cow/day which was supplemented with 3.2 ± 0.2 kg/cow/day of dairy concentrate fed at a.m. and p.m. milking. Table 3.1. The mean estimated breeding value 1 (and SD) for both genotypes based on their sire, maternal grandsire and maternal great grand-sire estimated breeding values Genotype 2 Item Fert+ Fert- No. of animals Holstein 94 (4.7) 95 (5.5) Milk (kg) 408 (154.0) 428 (143.4) Fat (kg) 21.2 (5.2) 18.2 (6.9) Fat (g/kg) 0.09 (0.08) 0.02 (0.1) Protein (kg) 17.4 (6.56) 18.0 (6.08) Protein (g/kg) 0.05 (0.06) 0.05 (0.07) Survival (%) 3.8 (0.82) -3.4 (1.12) Calving Interval (d) -6.4 (1.24) 8.2 (2.94) Sire calving interval (d) -9.3 (2.4) 12.3 (2.4) Maternal grandsire calving interval (d) -7.9 (3.34) 10.9 (4.5) 1 PTA were obtained from the Autumn 2012 official dairy evaluations published by the Irish Cattle Breeding Federation and multiplied by 2 to convert to EBV. Individual cow EBV were determined using the following formula: 0.5*sireEBV *MGSireEBV *MGGsireEBV 2 Fert+ = good-fertility cows; Fert- = poor-fertility cows Animal Measurements Cows were milked twice daily at 0800 and Milk yield was recorded at each milking using electronic milk meters (Dairymaster, Causeway, Co. Kerry, Ireland). Milk composition (fat, protein and lactose) was determined weekly from successive p.m. and a.m. samples by mid-infrared reflectance spectroscopy (FT6000 Milkscan instrument, Foss Electric, Hillerød, Denmark). Body weight and body condition score were recorded weekly from week 2 before calving to week 15 of lactation, and fortnightly thereafter. Body condition score was assessed using the 1 to 5 scale in 0.25 increments (Edmonson et al., 1989). Mean calving dates were February 11 (SD ± 15.9 d) and February 10 (SD ± 10.6 d) for Fert+ and Fert- cows, respectively. Energy balance was estimated as the difference between energy intake and the sum of energy 49
68 for maintenance and milk production, using the French net energy (NE) system (Jarrige, 1989). This system expresses energy units as unite fourragère lait (UFL) which is the NE content of 1 kg of air-dry standard barley for milk production. The energy required for maintenance and milk production were calculated using the equations developed by O' Mara (1997): Energy requirement for maintenance (UFL/day) = BW/100; Energy requirement for milk (UFL/kg of milk) = FC PC LC ; where FC = fat concentration (%), PC = protein concentration (%) and LC = lactose concentration (%). Blood samples were collected weekly for 2 weeks before expected date of calving, twice weekly during the first 4 weeks after calving and weekly thereafter until week 8 postpartum following the a.m. milking. Blood samples were collected via coccygeal venepuncture into vacutainers (Becton Dickinson, Plymouth, UK) containing lithium heparin, centrifuged at 2,000 x g for 15 min at 4 C, plasma decanted and stored at -20 C. Milk samples were collected during the a.m. milking 3 times per week (Monday, Wednesday and Friday) using electronic milk meters (Dairymaster, Causeway, Co. Kerry, Ireland). Milk samples were preserved (Lactab MarkIII, Thomson and Capper Ltd., Cheshire, UK) and stored at 4 C until progesterone (P4) analysis. Vaginal mucus was collected weekly after calving following the a.m. milking. The vulva and perineal area were sanitised with an antiseptic solution and dried with paper towels. A clean, lubricated, gloved hand was inserted through the vulva, and mucus was collected from the vagina into a 50 ml conical tube for inspection, and a character score was determined based on the criteria outlined by Williams et al. (2005): (0) clear and translucent mucus; (1) mucus containing flecks of white or off-white pus; (2) <50% white or off-white mucopurulent material.; or (3) 50% white or off-white mucopurulent material. 50
69 Table 3.2. Ingredient and nutrient composition of the transition period diet Dry cow diet (g/kg DM) Grass silage 760 Straw 140 Concentrate 100 Dry cow concentrate ingredients (g/kg as fed) Barley 250 Soya hulls 150 Rapeseed 150 Palm kernel meal 100 Milk solids 80 Sunflower meal 75 Citrus pulp 60 Dries distillers grain 60 Maize gluten 44 Minerals 1 25 Palm oil 6 Lactating cow diet (g/kg DM) Grass silage 340 Maize silage 310 Soyabean meal 110 Molasses 20 Parlour concentrate 230 Lactating cow concentrate ingredients (g/kg as fed) Barley 320 Soya hulls 205 Rapeseed 140 Field beans 100 Dried distillers grain 100 Molasses 60 Citrus pulp 50 Minerals 2 25 Nutrient composition of concentrate Dry cow Lactating cow DM (g/kg) Net energy (UFL/kg DM) Ash (g/kg DM) Crude Protein (g/kg DM) NDF (g/kg DM) Vitamin and mineral mix: g/kg of Na, 150 g/kg of Mg, mg/kg of cupric sulphate pentahydrate, 5556 mg/kg of zinc oxide, 198 mg/kg of cobaltous carbonate monohydrate, 110 mg/kg of sodium selenite, 1613 mg/kg of manganous oxide, 806 mg/kg of calcium iodate anhydrous, IU/kg of vitamin A, IU/kg of vitamin D, 500 mg/kg of vitamin E and 200 µg/kg of vitamin B g/kg of Ca, 100 g/kg of Na, 70 g/kg of P, 5 g/kg of Mg, 4500 mg/kg of Zn, 3000 mg/kg of Cu, 1500 mg/kg of Mn, 500 mg/kg of I, 400 mg/kg *Bioplex Cu, 400 mg/kg of *Bioplex Zn, 99 mg/kg of Co, 37 mg/kg of Se, IU/kg of vitamin A, IU/kg of vitamin D3, 1250 mg/kg of vitamin E and 200 mg/kg of vitamin B12. *Alltech Inc., Nicholasville, KY 51
70 Uterine cytology samples were collected on d 21 (SD ± 1 d) and d 42 (SD ± 2 d) postpartum. For each cow, uterine cytology samples were collected prior to vaginal mucus collection when both coincided. The vulva and perineal area were sanitised with antiseptic solution and dried with paper towels. An AI cannula (53 cm), enclosed by an embryo transfer (ET) plastic sheath (IMV Technologies, L Aigle, France), was placed in a plastic sleeve. The apparatus was guided through the cervix to the common body, and the plastic sleeve was pierced. The AI gun was removed, leaving the ET plastic sheath in situ. A sterile cytology brush, attached to copper wire (49 cm), was guided through the ET plastic sheath to the uterine body. An endometrial cytology sample was collected by one gentle rotation of the cytobrush against the uterine wall. The cytobrush was retracted into the ET plastic sheath and both were withdrawn from the uterus. The AI cannula was placed in disinfectant (Meddis, Medichem International Ltd, Sevenoaks, England) between uses. The cytobrush was smeared against glass microscope slides, in duplicate, allowed to air-dry and stained in the laboratory 24 h later. Smears were fixed to the slides with methanol. Slides were stained with separate eosin and thiazine reagents (Wescor Inc, South Logan, Utah, USA) by a slide stainer (Wescor Aerospray Automatic Slide Stainer, Wescor, Inc, South Logan, Utah, USA). The reagents were rinsed from the slide with an eosin rinse. Each slide was evaluated at 100x magnification by a single cytologist blind to the cow genotype. One hundred nucleated cells were counted, from which the percentage that were poylmorphonuclear neutrophils (PMN) was calculated Hormone and Metabolite Analysis Plasma samples collected at week -2, -1, 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7 and 8 relative to parturition (week 0) were analysed for concentrations of NEFA, BHBA and glucose by enzymatic colorimetry using an ABX Pentra 400 autoanalyser (ABX Mira, Montpellier, France; NEFA kit supplied by Wako Chemicals GmBH, Fuggerstraße 12, D Neuss, Germany; BHBA kit supplied by Randox Laboratories Limited, 55 Diamond Road, Crumlin, Co. Antrim, BT29 4QY, United Kingdom; Glucose kit supplied by Horiba ABX, Montpellier, France). Plasma samples collected at weeks -2, 0, 2, 4, 6 and 8 relative to parturition (0) were analysed for concentrations of insulin and insulin-like growth factor-1 (IGF1). Concentrations of insulin were determined by solidphase 125 I radioimmunoassay (Coat-A-Count Insulin, Diagnostic Products Corporation, Los Angeles, CA). Inter- and intra-assay coefficients of variation were 7.8% and 13.5%, respectively. Concentrations of IGF1 were determined by validated double antibody 52
71 radioimmunoassay, following ethanol:acetone:acetic acid extraction. Inter- and intraassay coefficients of variation were 2.7% and 12.1%, respectively. Both genotypes were equally represented in each assay and all samples for a cow were completed in a single assay. Milk P4 concentrations were measured using a competitive ELISA test (Ridgeway Science, Gloucester, UK). The inter- and intra-assay coefficients of variation were 7.8% and 9.7%, respectively and the sensitivity of the assay was 0.5 ng/ml Data Handling All data handling was performed using SAS (SAS Institute, 2006). Twenty-six cows were enrolled on this study. Records of one Fert- cow were removed from the statistical analysis due to ill-health. Data collected beyond lactation week 35 were excluded from the analysis because non-pregnant cows were culled before completing a full lactation. Daily measurements of milk, fat, protein and lactose yields were collapsed into average weekly yields. The milk fat to protein ratio was calculated by dividing the milk fat percent by the milk protein percent. Data were checked for normality. Suitable Box-Cox transformations were identified to normalise the distribution of lactose, MUN, BHBA, insulin, IGF1, NEFA and PMN count data. The distribution of somatic cell count data was normalised by natural logarithm transformation and reported as somatic cell score (SCS). Dry matter intake and energy balance data were available for 22 cows (12 Fert+ and 10 Fert) during the transition period. These data were analysed as 2 periods; prepartum (data for the final 3 weeks before calving date) and postpartum (data for the first 5 weeks after calving date). Linear interpolation was performed to calculate BW and BCS values for every week of the study. Blood metabolite and metabolic hormone data were analysed from 2 weeks prepartum to 8 weeks postpartum. In addition, glucose concentrations were also analysed from 2 weeks prepartum to 3 weeks postpartum. Cows were classified as having sub-clinical ketosis if BHBA concentrations were 1.2 and 2.9 mmol/l at least once during the first 3 weeks of lactation and were classified with clinical ketosis if BHBA concentrations were > 2.9 mmol/l at least once during the first 3 weeks of lactation (Oetzel, 2004). The timing of the BCS and BW nadir was identified as the earliest week during the first 15 weeks postpartum that the lowest value was recorded. Cows were classified as having endometritis at week 3 if PMN counts were greater than 18%, and at week 6 if PMN counts were greater than 10% (Sheldon et al., 2006). 53
72 3.4.6 Statistical Analysis All statistical analysis was performed using SAS (SAS Institute, 2006). Mixed model procedures were used to determine the effect of genotype on variables with repeated measures such as milk production, BW, BCS, DMI, energy balance, blood metabolite and metabolic hormone concentrations. A first-order auto regressive covariance structure was applied and cow nested within genotype was included as a random effect. Genotype, lactation week and their interaction were included as fixed effects. Parity, calving date and their interactions with genotype were included initially but removed if not significant (P > 0.1). The Tukey adjustment was included to correct for multiple comparison tests. Contrasts were written to compare glucose, insulin and IGF1 concentrations at individual time-points between genotypes. The effect of genotype on specific BCS variables (i.e., BCS at calving, postpartum BCS nadir, week of BCS nadir, BCS change from calving to nadir) and weekly vaginal mucus scores were analysed by one-way nonparametric test. Logistic regression was carried out using the GENMOD procedure to determine the effect of genetic merit for fertility traits on binary variables such as the proportion of animals classified as having endometritis, the proportion of animals to have resumed postpartum cyclicity and the proportion of animals classified with sub-clinical or clinical ketosis. Parity and calving date were included in the initial model but removed if not significant. Data were assumed to be binomially distributed and a logit link function was used in the model statement. The model tested the probability of a positive response and predicted probabilities were calculated from model solutions using the formula PP = (1 + e -(α+ßx) ), where α is the predicted intercept of the model, ß is the predicted regression coefficient(s), and x is the design matrix for the fixed effects. Odds ratios and confidence intervals were calculated as the exponent of model solutions. Fert+ cows were set as the reference group. Odds ratios are reported for Fert- cows relative to Fert+ cows. The interval from calving to achieving a vaginal mucus score of zero was determined by survival analysis using the LIFETEST procedure. In the analysis of postpartum interval to achieve a vaginal mucus score of zero, 2 cows were censored (1 Fert+ and 1 Fert-) at week 1 postpartum because they received intra-uterine antibiotic. 54
73 3.5 Results Milk Production The effect of genetic merit for fertility traits is summarised in Table 3.3. During the first 35 weeks, Fert+ cows tended to have greater mean daily milk yield (+ 1.9 kg/d; P = 0.08) and had greater (P = 0.05) mean daily milk solids production (1.89 ± 0.05 kg/d) than Fert- cows (1.74 ± 0.06 kg/d; Figure 3.1). Mean milk protein (+ 1.2 g/kg of milk) and lactose (+ 0.8 g/kg of milk) concentrations tended to be greater (both P = 0.1) in Fert+ cows compared with Fert- cows. Genotype had no effect on mean milk fat (P =0.98) or MUN (P = 0.6) concentrations, milk fat to protein ratio or SCS (both P = 0.4) Energy Balance, DMI, BCS and BW The effect of genetic merit for fertility traits on DMI and energy balance profiles from week 2 prepartum to week 5 postpartum are shown in Figure 3.2. Genotype had no effect on the mean prepartum DMI (14.8 vs kg DM/d for Fert+ and Fert- cows, respectively; P = 0.63) but Fert+ cows had greater mean postpartum DMI than Fert- cows (19.7 vs kg DM/d; P = 0.02). Neither prepartum (6.1 vs. 5.5 UFL/d for Fert+ and Fert- cows, respectively; P = 0.45) or postpartum (-0.3 vs UFL/d for Fert+ and Fert- cows, respectively, P = 0.37) energy balance was affected by genotype. However, energy balance at week 1 was greater in Fert+ than in Fert- cows (2.3 vs UFL/d, P = 0.02). Mean BCS and BW profiles from weeks -2 to 35 relative to parturition are shown in Figure 3.3. Mean BCS (2.98 vs units, P < ) and BW (578.5 vs kg, P = 0.05) were greater in Fert+ cows than in Fert- during the study period. Mean BCS at calving and the timing of BCS nadir were similar for both genotypes (Table 3.4) but the loss of body condition tended to be greater in Fert- cows than in Fert+ cows (additional 0.13 BCS loss; P = 0.1). At nadir, Fert+ cows had greater mean BCS than Fert- cows (P = 0.009). 55
74 Table 3.3. The effect of genetic merit for fertility traits on daily milk production variables during the first 35 weeks of lactation Genotype P-value Variable Fert+ Fert- SEM 1 Genotype Genotype x week Number of animal records Milk yield (kg/d) Protein (g/kg of milk) Fat (g/kg of milk) Lactose (g/kg of milk) 46.3 ( ) 45.5 ( ) MUN 3 (g/kg of milk) 31.9 ( ) 31.1 ( ) Milk solids 2 (kg/d) Fat to protein ratio SCS units ( ) 4.22 ( ) = pooled standard error 2 = sum of fat and protein yield 3 Data presented as LSM with 95% CI in parentheses 56
75 Milk solids (kg/d) Milk Yield (kg/d) Fert- Fert Week of lactation Figure 3.1. Mean daily milk yield and milk solids yield profiles of Fert+ and Fert- cows during 35 weeks of lactation. All values are LSM. Mean daily milk yield tended to be greater in Fert+ cows than Fert- cows (P = 0.08; SEM = 0.88). Mean daily milk solids yield (P = 0.05; SEM = 0.06) was greater in Fert+ cows compared with Fert- cows. 57
76 Energy balance (UFL/d) DMI (kg/d) Fert- Fert * Week relative to parturition Figure 3.2. Mean dry matter intake and calculated energy balance of Fert+ and Fertcows from weeks -2 to 5 relative to parturition. Prepartum dry matter intake was similar for both genotypes (P = 0.63; SEM = 0.75), but postpartum dry matter intake was greater in Fert+ cows compared with Fert- cows (P = 0.02; SEM = 0.79). Mean prepartum (P = 0.45; SEM = 0.54) and postpartum (P = 0.37; SEM = 0.71) energy balance were similar for both genotypes. Energy balance at week 1 was greater in Fert+ cows than Fert- cows (P = 0.02). * indicates P
77 Table 3.4. The effect of genetic merit for fertility traits on mean BCS and BW variables Genotype P-value Variable Fert+ Fert- SEM 1 Genotype Genotype x week Number of animal records Mean BW (kg) Mean BCS < BCS at calving BCS at nadir Week of BCS nadir BCS change from calving to nadir = pooled standard error 59
78 Body weight (kg) Body condition score (units) Fert- Fert Week relative to parturition Figure 3.3. Mean body condition score and body weight from weeks -2 to 35 relative to parturition. Fert+ cows maintained greater mean body condition score (P < 0.001; SEM 0.02) and body weight (P = 0.05; SEM = 11.09) than Fert- cows from weeks -2 to
79 3.5.3 Blood Metabolites and Metabolic Hormones The effect of genetic merit for fertility traits on circulating metabolites from two weeks prepartum to eight weeks postpartum is illustrated in Figure 3.4. Mean circulating glucose (P = 0.19), NEFA (P= 0.64) and BHBA (P = 0.92) concentrations during the period were similar in Fert+ and Fert- cows; however, mean circulating glucose concentrations were greater (P = 0.04) in Fert+ (3.40 mmol/l) cows than in Fert- (3.01 mmol/l) cows from weeks -2 to 3 relative to parturition. Four Fert- and four Fert+ cows were classified as having sub-clinical ketosis, but there was no effect of genotype (P = 0.49). Of these, one Fert+ and one Fert- cow were classified as having clinical ketosis, but there was no effect of genotype (P = 0.75). A genotype by week interaction existed for NEFA (P = 0.02; Figure 3.4). Mean circulating IGF1 and insulin concentrations for both genotypes are illustrated in Figure 3.5. Fert+ cows had greater mean IGF1 concentrations than Fert- cows (102.6 vs ng/ml, P = 0.001). There was a genotype by week interaction for mean IGF1 concentrations (P = ); the difference between genotypes decreased as time increased. Fert+ tended to have greater mean circulating insulin concentrations than Fert- cows (3.25 vs µiu/ml, P = 0.08). There was a tendency for a genotype by week interaction for mean insulin circulating concentrations (P = 0.04; Figure 3.5). 61
80 Plasma BHBA (mmol/l) Plasma NEFA (mmol/l) Plasma glucose (mmol/l) * * Fert- Fert+ Genotype: P P = = Genotype x week: P = 0.53 SEM: mmol/l Genotype: P P = 0.64 Genotype x week: P = % CI: (Fert+) (Fert-) Genotype: P P = 0.92 Genotype x week: P = % CI: (Fert+) (Fert-) Week relative to parturition Figure 3.4. Mean circulating glucose, NEFA and BHBA concentrations in Fert+ and Fert- cows from week -2 to 8 relative to parturition. Mean plasma glucose, NEFA and BHBA were similar in both genotypes. Values for NEFA and BHBA are backtransformed LSM with 95% CI. * indicates P indicates P
81 Plasma insulin (uiu/ml) Plasma IGF-I (ng/ml) 300 Fert- Fert Genotype: P = Genotype x week: P = < % CI: (Fert+) (Fert+) (Fert-) (Fert-) * * * * Genotype: P = 0.08 Genotype x week: P = % CI: (Fert+) (Fert-) (Fert-) * Week relative to parturition Figure 3.5. Mean circulating IGF1 and insulin in Fert+ and Fert- cows from week -2 to 8 relative to parturition. Plasma IGF1 was greater in Fert+ cows than in Fert- cows during the sampling period (P = 0.001) and the difference between genotypes decreased as time increased (P < ). Plasma insulin tended to be greater in Fert+ cows than in Fert- cows during the sampling period (P = 0.08) and there was a genotype by time interaction (P = 0.04). All values are back-transformed LSM with 95% CI. * indicates P indicates P
82 3.5.4 Postpartum Uterine Health The effect of genetic merit for fertility traits on postpartum vaginal mucus score is summarized in Table 3.5. Fert- cows had a greater vaginal mucus score than Fert+ cows on week 2 and 3 and tended to be greater on week 4 to 6. Survival analysis indicated that the postpartum interval required to achieve a vaginal mucus score of zero was not affected by genotype (P = 0.26). Table 3.5. The effect of genetic merit for fertility traits on mean vaginal mucus score in Fert+ and Fert- cows until week eight of lactation Genotype Week Fert+ (n) Fert- (n) P-value (15) 2.3 (10) (15) 2.5 (10) (14) 2.2 (10) (14) 1.1 (10) (14) 1.2 (10) (11) 1.2 (9) (8) 0.2 (6) (7) 0.0 (1) 0.74 On week 3 postpartum, there was no effect of genotype on the mean PMN counts (Table 3.6), but there was a tendency for Fert- cows to have increased odds of being classified as having endometritis (P = 0.09). At week 6 postpartum, Fert- cows had greater mean PMN counts (P = 0.04), and they had increased odds of being classified as having endometritis compared with Fert+ cows (P = 0.04) Postpartum Resumption of Cyclicity Two of 10 Fert- cows and 12 of 14 Fert+ cows had resumed cyclicity by week 6 after calving. Fert- cows had reduced odds of having resumed cyclicity by week 6 after calving compared with Fert+ cows (OR = 0.04; 95% CI = , P = 0.009). 64
83 Table 3.6. The effect of genetic merit for fertility traits on mean PMN counts of the uterus and proportion of cows classified with endometritis on week 3 and 6 postpartum Genotype PMN count (%) P-value OR (95% CI) PP (SE) P-value Week 3 Fert- (n = 9) ( ) (0.7, 34.3) 0.78 (0.83) 0.09 Fert+ (n = 12) ( ) (0.64) Week 6 Fert- (n = 8) ( ) (0.94, 86.52) 0.75 (0.88) 0.04 Fert+ (n = 8) 3.91 ( ) (0.70) OR = Odds ratio; PP = Predicted Probability 65
84 3.6 Discussion In dairy cattle, genetic selection in past decades focused solely on increasing milk production without regard to health and fertility traits, resulting in large increases in milk production and a decline in reproductive performance (Lucy, 2001; Pollott and Coffey, 2008). The two genotypes of cows enrolled on this study had similar genetic merit for milk production, but were divergent in genetic merit for fertility traits. Cows from both genotypes were exposed to the same management and nutrition, allowing the effect of genetic merit for fertility traits on phenotypic fertility measurements to be assessed. The results of this study indicate that it is possible to select animals with good genetic merit for fertility traits without compromising milk production. Fert+ cows had greater milk yield and milk solids production compared with Fert- cows. This is in agreement with the results of Cummins et al. (2012a) and validates the robustness of the animal model. There is disagreement between studies that have examined the relationship between milk production and reproductive performance. Conclusions have ranged from a negative association (Nebel and McGilliard, 1993), to no association (Patton et al., 2007), to a positive association (Buckley et al., 2003). It has also been suggested that factors such as herd management and animal health should be taken into account when investigating the interactions between production and fertility (Leblanc, 2010; Bello et al., 2012). In the current study, these concerns were avoided by managing both groups of cows as a single herd with similar nutrition and similar health protocols DMI and Energy Balance Dry matter intake and energy balance have previously been shown to be positively associated with fertility (Butler and Smith, 1989; Patton et al., 2007). In the current study, however, mean energy balance during the first 5 weeks of lactation was similar in Fert+ and Fert- cows. This is consistent with Patton et al. (2008), who reported no difference in early lactation energy balance between strains of Holstein Friesian from New Zealand (high fertility) and North America (low fertility). However, energy balance during week 1 of lactation was greater in Fert+ cows than Fert- cows. The greater DMI during the first 5 weeks of lactation in Fert+ cows compared with Fert- cows is an important finding from this study. Dry matter intake has been reported to account for the majority of the variation in energy balance during the early postpartum 66
85 period (Villa-Godoy et al., 1988; Patton et al., 2007) and is the main limiting factor to milk production (Roche et al., 2008). Feed allowance and type of feed, management, day length, weather, genetics, milk production, stage of production cycle and health status are factors that affect an animal s voluntary feed intake (Roche et al., 2008; Sepúlveda-Varas et al., 2012). Lower DMI in the first week postpartum is associated with increased incidence of sub-clinical ketosis (Goldhawk et al., 2009) and metritis (Huzzey et al., 2007). In the current study, mean circulating concentrations of NEFA and BHBA and the incidence of sub-clinical and clinical ketosis were not affected by genotype. There were, however, clear differences in uterine health between the two genotypes. Fert- cows had greater mean vaginal mucus scores from weeks 2 to 8, and a greater proportion were classified as having endometritis at week 6 compared with Fert+ cows. This suggests that lower postpartum DMI is a factor predisposing Fertcows to impaired uterine health. These data suggest an association between DMI and uterine health in Fert+ and Fert- cows that may support the results of Huzzey et al. (2007) Energy Metabolites and Metabolic Hormones Greater circulating glucose, insulin and IGF1 concentrations during the transition period indicate that Fert+ cows were in a more favourable metabolic status compared with Fert- cows. These results imply an earlier recoupling of the somatotropic axis in Fert+ cows, supporting the findings of Cummins et al. (2012a, c) that genetic merit for fertility traits affects the somatotropic axis. This is possibly due to greater circulating insulin, which has been reported to up-regulate hepatic expression of GHR 1A and IGF1 transcripts (Butler et al., 2003). Taylor et al. (2004) and Patton et al. (2007) have reported a positive association between circulating IGF1 and fertility in dairy cows, which is mediated through its positive effect on luteinising hormone secretion, follicle development, histotroph secretion and embryo development (Wathes, 2012). A positive association between circulating glucose during the early postpartum period and likelihood of conception at first service has recently been reported (Garverick et al., 2013). Circulating glucose concentrations during the immediate postpartum period may be a key indicator of a cow s adaptive ability to meet the glucose demands of rising milk production while minimising BCS loss, with longer term consequences for subsequent reproductive outcomes (i.e., uterine involution, immune function and uterine health, resumption of cyclicity, likelihood of conception). It is clear from the current study that genetic selection for fertility traits results in more favourable glucose status in 67
86 early lactation, and this may represent a key inherent difference between cows with good or poor genetic merit for fertility traits Body Condition Score With the onset of lactation, the dairy cow experiences a dramatic shift in metabolism due to the genetically controlled drive to support mammary milk synthesis. Consequently, a period of negative energy balance and adipose tissue mobilisation occurs, reflected by increased circulating NEFA concentrations (Drackley, 1999). Genetic selection for increased milk production potential has amplified the severity of adipose tissue mobilisation (McCarthy et al., 2007; Lucy et al., 2009). Body condition score is an assessment of adipose tissue reserves and can be used to detect temporal changes in energy balance status. Increased severity of adipose tissue mobilisation may be due to the widening gap between the energy required for greater milk production and the cow s voluntary DMI. Veerkamp et al. (2003) reported a genetic correlation of only between milk yield and DMI. In both pasture-based and TMR systems, however, attempts to minimise adipose tissue mobilisation during the early post-partum period through altered nutritional and management strategies have been largely unsuccessful (Horan et al., 2005a; Roche et al., 2006, Maltz et al., 2013), indicating that there is strong genetic control of the lactation profile of adipose tissue mobilisation. Berry et al. (2008) reported that heritability estimates for BCS ranged from 0.07 to 0.6. Positive effects of BCS on fertility have been reported in pasture and confinement dairy systems (Buckley et al., 2003; Roche et al., 2007; Santos et al., 2009). Similar findings have been reported in strain comparison studies using cows with New Zealand or North American ancestry (Horan et al., 2005a; Coleman et al., 2009). Holstein-Friesian cows of North American ancestry have lower BCS but greater milk yield compared with Holstein-Friesian cows of New Zealand ancestry on a pasturebased system (McCarthy et al., 2007; Horan et al., 2005a). These strains were divergent in genetic merit for both milk production traits (greater in North American strain) and fertility traits (greater in New Zealand strain). The current study reports greater BCS in Fert+ cows compared with Fert- cows, which have similar genetic merit for milk production traits but divergent genetic merit for fertility traits. Mean BCS loss from calving to nadir tended to be greater in Fert- cows than in Fert+ cows, even though milk production was greater for Fert+ cows and both groups had similar nutritional management. 68
87 The results of the current study are supported by the findings of Cummins et al. (2012a), and indicate that greater genetic merit for fertility traits supports a higher threshold BCS. Buckley et al. (2003) also reported a positive association between BCS and milk production. Together, these data suggest that the ability of Fert+ cows to maintain greater milk production and BCS profiles compared with Fert- cows is linked to greater postpartum DMI. Earlier recoupling of the somatotropic axis in Fert+ cows would facilitate the maintenance of greater BCS, while also maintaining greater milk production compared with Fert- cows (Cummins et al., 2012a, c) Uterine Health After calving, the uterus is exposed to microbial pathogens. The development and severity of uterine infection depends on the species of bacteria involved and their prevalence, and the immune response of the cow (Sheldon, 2004). Delayed clearance of uterine infection has serious consequences for reproductive performance (LeBlanc et al., 2002; Williams et al., 2005; McDougall et al., 2007; McDougall et al., 2011). The mechanisms responsible may include altered follicle development and function (Sheldon et al., 2002), delayed resumption of cyclicity (Galvão et al., 2010), altered follicle steroidogenesis (Green et al., 2011) and development of a smaller corpus luteum (Williams et al., 2007). Both genotypes were managed as one group in the same housing, and would theoretically have been exposed to the same bacteria during the peripartum period. Uterine health was assessed by scoring vaginal mucus weekly, which reflects the level of bacterial contamination (Williams et al., 2005), and by classifying cows as having endometritis based on the proportion of PMN in uterine cytology samples. At week 1, both genotypes had a similar vaginal mucus score. Thereafter, Fert- cows had greater vaginal mucus scores, indicating a slower clearance of bacterial contamination compared with Fert+ cows. Neutrophils enter the uterus from blood in response to chemokines, killing bacteria by phagocytosis (Sheldon, 2004). Fert- cows tended to have increased odds of being classified with endometritis based on PMN population at week 3 compared with Fert+ cows. By week 6, a similar proportion of Fert- cows were classified as having endometritis but Fert+ cows had made a substantial recovery. The results of this study clearly indicate that genetic merit for fertility traits affects postpartum uterine health, which may be a result of differences in the function of the immune system. Differences in the metabolic status are potential mediators of 69
88 immune function in dairy cows (Ingvartsen and Moyes, 2013). Hammon et al. (2006) reported impaired PMN function, lower DMI and greater NEFA and BHBA concentrations during the peripartum period in cows diagnosed with metritis or subclinical endometritis compared with healthy cows. Glucose, glutamine, NEFA and BHBA are major energy sources for immune cells (Ingvartsen and Moyes, 2013). While circulating NEFA and BHBA were similar between Fert+ and Fert- cows, DMI, energy balance at week 1 and circulating glucose concentrations were greater in Fert+ cows. This suggests that Fert+ cows had more glucose available in support of PMN function Resumption of Cyclicity Previous studies examining the resumption of ovarian cyclicity have reported positive (Galvão et al., 2010) and negative (Horan et al., 2005b) associations with fertility. Our results show that an early resumption of cyclicity is associated with genetic merit for good fertility which suggests that it is a positive fertility parameter. The interval from calving to resumption of cyclicity is dependent on the restitution of frequent luteinising hormone pulses from the anterior pituitary, which has been reported to be negatively associated with energy balance (Canfield and Butler, 1990). Greater energy balance at week 1, combined with greater concentrations of insulin and IGF1 may have supported earlier ovulation in Fert+ cows (Butler et al., 2006). 3.7 Conclusions Genetic merit for fertility traits had a significant effect on dry matter intake, metabolic status, uterine health and the resumption of postpartum cyclicity in cows with similar genetic merit for milk production and proportion of Holstein genetics that were exposed to the same management, nutrition and environment. Fert+ cows had greater DMI, energy balance at week 1, circulating insulin, IGF1 and glucose concentrations, maintained greater BCS, had superior uterine health and an earlier resumption of cyclicity, while also achieving greater milk production. These results may explain, at least in part, the differences in reproductive performance reported in this genetic model. 70
89 3.8 References Bello, N. M., J. S. Stevenson, and R. J. Tempelman Invited review: Milk production and reproductive performance: Modern interdisciplinary insights into an enduring axiom. Journal of Dairy Science 95(10): Berry, D. P., J. R. Roche, and M. P. Coffey Body condition score and fertility - more than just a feeling. Fertility in dairy cows: Bridging the gaps. M. D. Royal, N. C. Friggens, and R. F. Smith, ed. British Society of Animal Science, Cambridge University Press, Cambridge. UK. : Buckley, F., K. O'Sullivan, J. F. Mee, R. D. Evans, and P. Dillon Relationships Among Milk Yield, Body Condition, Cow Weight, and Reproduction in Spring- Calved Holstein-Friesians. Journal of Dairy Science 86(7): Butler, S. T., A. L. Marr, S. H. Pelton, R. P. Radcliff, M. C. Lucy, and W. R. Butler Insulin restores GH responsiveness during lactation-induced negative energy balance in dairy cattle: effects on expression of IGF1 and GH receptor 1A. Journal of Endocrinology 176(2): Butler, S. T., S. H. Pelton, and W. R. Butler Energy Balance, Metabolic Status, and the First Postpartum Ovarian Follicle Wave in Cows Administered Propylene Glycol. Journal of Dairy Science 89(8): Butler, W. R Energy balance relationships with follicular development, ovulation and fertility in postpartum dairy cows. Livestock Science 83(2-3): Butler, W. R. and R. D. Smith Interrelationships Between Energy Balance and Postpartum Reproductive Function in Dairy Cattle. Journal of Dairy Science 72(3): Canfield, R. W. and W. R. Butler Energy balance and pulsatile LH secretion in early postpartum dairy cattle. Domestic Animal Endocrinology 7(3): Coleman, J., K. M. Pierce, D. P. Berry, A. Brennan, and B. Horan The influence of genetic selection and feed system on the reproductive performance of springcalving dairy cows within future pasture-based production systems. Journal of Dairy Science 92(10): Cummins, S. B., P. Lonergan, A. C. O. Evans, D. P. Berry, R. D. Evans, and S. T. Butler. 2012a. Genetic merit for fertility traits in Holstein cows: I. Production characteristics and reproductive efficiency in a pasture-based system. Journal of Dairy Science 95(3):
90 Cummins, S. B., P. Lonergan, A. C. O. Evans, and S. T. Butler. 2012b. Genetic merit for fertility traits in Holstein cows: II. Ovarian follicular and corpus luteum dynamics, reproductive hormones, and estrus behavior. Journal of Dairy Science 95(7): Cummins, S. B., S. M. Waters, A. C. O. Evans, P. Lonergan, and S. T. Butler. 2012c. Genetic merit for fertility traits in Holstein cows: III. Hepatic expression of somatotropic axis genes during pregnancy and lactation. Journal of Dairy Science 95(7): Darwash, A. O., G. E. Lamming, and J. A. Woolliams The phenotypic association between the interval to post-partum ovulation and traditional measures of fertility in dairy cattle. Animal Science 65:9-16. Drackley, J. K Biology of Dairy Cows During the Transition Period: the Final Frontier? Journal of Dairy Science 82(11): Edmonson, A. J., I. J. Lean, L. D. Weaver, T. Farver, and G. Webster A Body Condition Scoring Chart for Holstein Dairy Cows. Journal of Dairy Science 72(1): Friggens, N. C., P. Berg, P. Theilgaard, I. R. Korsgaard, K. L. Ingvartsen, P. Løvendahl, and J. Jensen Breed and Parity Effects on Energy Balance Profiles Through Lactation: Evidence of Genetically Driven Body Energy Change. Journal of Dairy Science 90(11): Galvão, K. N., M. Frajblat, W. R. Butler, S. B. Brittin, C. L. Guard, and R. O. Gilbert Effect of Early Postpartum Ovulation on Fertility in Dairy Cows. Reprod. Domest. Anim. 45(5):e207-e211. Garverick, H. A., M. N. Harris, R. Vogel-Bluel, J. D. Sampson, J. Bader, W. R. Lamberson, J. N. Spain, M. C. Lucy, and R. S. Youngquist Concentrations of nonesterified fatty acids and glucose in blood of periparturient dairy cows are indicative of pregnancy success at first insemination. Journal of Dairy Science 96(1): Goldhawk, C., N. Chapinal, D. M. Veira, D. M. Weary, and M. A. G. von Keyserlingk Prepartum feeding behavior is an early indicator of subclinical ketosis. Journal of Dairy Science 92(10): Green, M. P., A. M. Ledgard, S. E. Beaumont, M. C. Berg, K. P. McNatty, A. J. Peterson, and P. J. Back Long-term alteration of follicular steroid concentrations in relation to subclinical endometritis in postpartum dairy cows. Jorunal of Animal Science 89(11):
91 Hammon, D. S., I. M. Evjen, T. R. Dhiman, J. P. Goff, and J. L. Walters Neutrophil function and energy status in Holstein cows with uterine health disorders. Veterinary Immunology and Immunopathology 113(1 2): Heins, B. J., L. B. Hansen, A. R. Hazel, A. J. Seykora, D. G. Johnson, and J. G. Linn Short communication: Jersey Holstein crossbreds compared with pure Holsteins for body weight, body condition score, fertility, and survival during the first three lactations. Journal of Dairy Science 95(7): Horan, B., P. Dillon, P. Faverdin, L. Delaby, F. Buckley, and M. Rath. 2005a. The interaction of strain of Holstein-Friesian cows and pasture-based feed systems on milk yield, body weight, and body condition score. Journal of Dairy Science 88(3): Horan, B., J. F. Mee, P. O Connor, M. Rath, and P. Dillon. 2005b. The effect of strain of Holstein-Friesian cow and feeding system on postpartum ovarian function, animal production and conception rate to first service. Theriogenology 63(3): Huzzey, J. M., D. M. Veira, D. M. Weary, and M. A. G. von Keyserlingk Prepartum Behavior and Dry Matter Intake Identify Dairy Cows at Risk for Metritis. Journal of Dairy Science 90(7): Ingvartsen, K. L Feeding- and management-related diseases in the transition cow: Physiological adaptations around calving and strategies to reduce feedingrelated diseases. Animal Feed Science and Technology 126(3 4): Ingvartsen, K. L. and K. Moyes Nutrition, immune function and health of dairy cattle. Animal 7(Supplements1): Jarrige, J In INRAtion v. 2.7: Microsoft computer program of ration formulation for ruminant livestock. Edited by J. Agabriel, P. Champciaux & C. Espinasse. Centre National d'études et de Ressources en Technologie Advancée, Dijon, France. Leblanc, S Assessing the Association of the Level of Milk Production with Reproductive Performance in Dairy Cattle. Journal of Reproduction and Development 56(S):S1-S7. LeBlanc, S. J Interactions of Metabolism, Inflammation, and Reproductive Tract Health in the Postpartum Period in Dairy Cattle. Reproduction in Domestic Animals 47: LeBlanc, S. J., T. F. Duffield, K. E. Leslie, K. G. Bateman, G. P. Keefe, J. S. Walton, and W. H. Johnson Defining and Diagnosing Postpartum Clinical 73
92 Endometritis and its Impact on Reproductive Performance in Dairy Cows. Journal of Dairy Science 85(9): Lucy, M. C Reproductive Loss in High-Producing Dairy Cattle: Where Will It End? Journal of Dairy Science 84(6): Lucy, M. C., G. A. Verkerk, B. E. Whyte, K. A. Macdonald, L. Burton, R. T. Cursons, J. R. Roche, and C. W. Holmes Somatotropic axis components and nutrient partitioning in genetically diverse dairy cows managed under different feed allowances in a pasture system Journal of Dairy Science 92(2): Maltz, E., L. F. Barbosa, P. Bueno, L. Scagion, K. Kaniyamattam, L. F. Greco, A. De Vries, and J. E. P. Santos Effect of feeding according to energy balance on performance, nutrient excretion, and feeding behaviour of early lactation dairy cows. Journal of Dairy Science 96(8): McCarthy, S., D. P. Berry, P. Dillon, M. Rath, and B. Horan Influence of Holstein-Friesian Strain and Feed System on Body Weight and Body Condition Score Lactation Profiles. Journal of Dairy Science 90(4): McDougall, S., H. Hussein, D. Aberdein, K. Buckle, J. Roche, C. Burke, M. Mitchell, and S. Meier Relationships between cytology, bacteriology and vaginal discharge scores and reproductive performance in dairy cattle. Theriogenology 76(2): McDougall, S., R. Macaulay, and C. Compton Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle. Animal Reproduction Science 99(1-2):9-23. Moyes, K. M., T. Larsen, and K. L. Ingvartsen Generation of an index for physiological imbalance and its use as a predictor of primary disease in dairy cows during early lactation. Journal of Dairy Science 96(4): Nebel, R. L. and M. L. McGilliard Interactions of High Milk Yield and Reproductive Performance in Dairy Cows. Journal of Dairy Science 76(10): O' Mara, F. P A net energy system for cattle and sheep. Belfield, Dublin 4, Ireland. Department of Animal Science and Production, Faculty of Agriculture, Universtity College Dublin. Oetzel, G. R Monitoring and testing dairy herds for metabolic disease. Veterinary Clinics of North America: Food Animal Practice 20(3): Patton, J., D. A. Kenny, S. McNamara, J. F. Mee, F. P. O Mara, M. G. Diskin, and J. J. Murphy Relationships Among Milk Production, Energy Balance, Plasma 74
93 Analytes, and Reproduction in Holstein-Friesian Cows. Journal of Dairy Science 90(2): Patton, J., J. J. Murphy, F. P. O Mara, and S. T. Butler A comparison of energy balance and metabolic profiles of the New Zealand and North American strains of Holstein Friesian dairy cow. Animal 2(06): Pollott, G. E. and M. P. Coffey The Effect of Genetic Merit and Production System on Dairy Cow Fertility, Measured Using Progesterone Profiles and On- Farm Recording. Journal of Dairy Science 91(9): Prendiville, R., K. M. Pierce, and F. Buckley An evaluation of production efficiencies among lactating Holstein-Friesian, Jersey, and Jersey Holstein- Friesian cows at pasture. Journal of Dairy Science 92(12): Roche, J. R., D. P. Berry, and E. S. Kolver Holstein-Friesian Strain and Feed Effects on Milk Production, Body Weight, and Body Condition Score Profiles in Grazing Dairy Cows. Journal of Dairy Science 89(9): Roche, J. R., D. Blache, J. K. Kay, D. R. Miller, A. J. Sheahan, and D. W. Miller Neuroendocrine and physiological regulation of intake with particular reference to domesticated ruminant animals. Nutrion Research Reviews 21(02): Roche, J. R., K. A. Macdonald, C. R. Burke, J. M. Lee, and D. P. Berry Associations Among Body Condition Score, Body Weight, and Reproductive Performance in Seasonal-Calving Dairy Cattle. Journal of Dairy Science 90(1): Santos, J. E. P., H. M. Rutigliano, and M. F. S. Filho Risk factors for resumption of postpartum estrous cycles and embryonic survival in lactating dairy cows. Animal Reproduction Science 110(3-4): SAS Institute SAS User s Guide: Statistics. SAS Institute Inc., Cary, NC. Sepúlveda-Varas, P., J. M. Huzzey, D. M. Weary, and M. A. G. Von Keyserlingk Behaviour of dairy cattle during the periparturient period and its relationship to illness after calving. Animal Production Science 53: Sheldon, I. M The postpartum uterus. Veterinary Clinics of North America: Food Animal Practice 20(3): Sheldon, I. M., J. Cronin, L. Goetze, G. Donofrio, and H.-J. Schuberth Defining Postpartum Uterine Disease and the Mechanisms of Infection and Immunity in the Female Reproductive Tract in Cattle. Biology of Reproduction 81(6):
94 Sheldon, I. M., G. S. Lewis, S. LeBlanc, and R. O. Gilbert Defining postpartum uterine disease in cattle. Theriogenology 65(8): Sheldon, I. M., D. E. Noakes, A. N. Rycroft, D. U. Pfeiffer, and H. Dobson Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 123(6): Taylor, V. J., Z. Cheng, P. G. A. Pushpakumara, D. C. Wathes, and D. E. Beever Relationships between the plasma concentrations of insulin-like growth factor-i in dairy cows and their fertility and milk yield. Veterinary Record 155(19): Veerkamp, R. F., B. Beerda, and T. van der Lende Effects of genetic selection for milk yield on energy balance, levels of hormones, and metabolites in lactating cattle, and possible links to reduced fertility. Livestock Science 83(2-3): Villa-Godoy, A., T. L. Hughes, R. S. Emery, L. T. Chapin and R. L. Fogwell Association between energy balance and luteal function in lactating dairy cows. Journal of Dairy Science 71(4): Wathes, D. C Mechanisms Linking Metabolic Status and Disease with Reproductive Outcome in the Dairy Cow. Reproduction in Domestic Animals 47: Williams, E. J., D. P. Fischer, D. E. Noakes, G. C. W. England, A. Rycroft, H. Dobson, and I. M. Sheldon The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 68(4):
95 Chapter Four Genetic merit for fertility traits in Holstein cows: Factors affecting circulating progesterone concentrations 4.1 Preface At the time of thesis submission this chapter was published in Journal of Dairy Science (Accepted May 11, 2014; The full reference is: Moore, S.G., S. Scully, J.A. Browne, T. Fair, and S.T. Butler. Genetic merit for fertility traits in Holstein cows: V. Factors affecting circulating progesterone concentrations. Journal of Dairy Science 2014, 97: Stephen Moore was the primary author and carried out the experimental work, statistical analysis and drafted the manuscript. Stephanie Scully carried out the transrectal ultrasonography with Doppler ultrasound and analysed the images. John Browne provided training on the techniques for RNA extraction, cdna synthesis and real timeqpcr. Trudee Fair and Stephen Butler conceived, designed and coordinated the study. All authors interpreted the data and contributed to the manuscript. Formatting and reference style has been edited for consistency throughout the thesis. Figure and table captions have been assigned with a chapter prefix. Acknowledgements have been removed. All other aspects are consistent with the published manuscript. 77
96 4.2 Abstract This study investigated the factors affecting circulating progesterone (P4) concentrations in cows with similar genetic merit for milk production traits, but with extremes of good (Fert+) or poor genetic merit for fertility traits (Fert-). Study 1: 28 cows were enrolled on an ovulation synchronisation protocol at 61±13 (± SD) days postpartum, and data are presented for 13 Fert+ and 9 Fert- cows that remained on the study. Progesterone concentrations were determined from d 0 to 9 (d 0 = oestrus), and on d 7 corpus luteum (CL) volume and blood flow area (BFA) were measured by B- mode and Doppler ultrasonography, respectively. Cows were administered prostaglandin F 2α (PGF 2α ) on d 7 p.m. and d 8 a.m. to regress the CL, and 2 CIDRs were inserted per vaginum on d 8 a.m. Liver biopsies were collected on d 9 and hepatic mrna abundance of genes involved in P4 catabolism was determined. On d 10, CIDRs were removed and frequent blood samples were collected to measure the rate of decline in circulating P4. Fert+ cows tended to have greater dry matter intake (DMI) compared with Fert- cows (+0.79 kg DM/d), but similar milk production (29.82 kg/d). After synchronized ovulation, the rate of increase in circulating P4 concentrations was greater in Fert+ cows compared with Fert- cows. There was no effect of genotype on CL volume, but BFA was 42% greater in Fert+ cows compared with Fert- cows. Fert- cows had greater mrna abundance of CYP3A compared with Fert+ cows, but mrna abundance of AKR1C1, AKR1C3, AKR1C4 and CYP2C were similar. The half-life and metabolic clearance rate (MCR) of P4 were similar in Fert+ cows and Fert- cows. Study 2: 23 cows were enrolled on an ovulation synchronisation protocol at 55±7 (± SD) days postpartum, and data are presented for 13 Fert+ and 8 Fert- cows that remained on the study. On d 4, 7, 10 and 13 (d 0 = oestrus), CL volume and BFA were measured as in Study 1. Progesterone concentrations were measured from d 1 to 13. Corpus luteum volume was 41% greater in Fert+ cows compared with Fert- cows but there was no effect of genotype on BFA. Mean circulating P4 concentrations were 79% greater in Fert+ cows compared with Fert- cows. Milk yield was similar in both genotypes. The results indicate that greater circulating P4 concentrations were primarily due to greater CL P4 synthetic capacity rather than differences in P4 clearance in this lactating cow genetic model of fertility. 78
97 4.3 Introduction Progesterone (P4) is an important regulator of events during the oestrous cycle and is essential for the maintenance of pregnancy. The well documented decline in fertility in dairy cows has been attributed to increased occurrence of embryo mortality, particularly during the period before maternal recognition of pregnancy (MRP; Diskin and Morris, 2008) which has been associated with inadequate circulating P4 concentrations during dioestrus of the oestrous cycle that preceded insemination and fertilisation (Lonergan, 2011). Indeed, several studies have reported an improvement in fertility performance when cows were placed on synchronisation protocols with supplemental P4 prior to ovulation (Xu et al., 1997; Herlihy et al., 2011; Colazo et al., 2013). Reduced circulating concentrations of P4 during preovulatory follicle development facilitates increased luteinising hormone pulsatility, greater incidence of multiple ovulations, lower likelihood of conception after AI and increased likelihood of embryo mortality between d 30 to 60 after AI (Cunha et al., 2008; Wiltbank et al., 2011). For successful MRP, the developing embryo must be capable of producing sufficient interferon tau. This is more likely to occur if there is a rapid increase in circulating P4 concentrations after fertilisation, which alters the endometrial transcriptome and protein secretions to promote development of a larger embryo (Forde et al., 2013). In support of this, studies have reported positive associations between milk P4 concentrations (Stronge et al., 2005; McNeill et al., 2006) or P4 supplementation (Larson et al., 2007) during the period prior to blastocyst hatching and subsequent pregnancy success. Circulating P4 concentrations during the oestrous cycle is a balance between the P4 synthetic capacity of the CL and the MCR by the liver. Factors affecting P4 secretion include the number of luteal cells, luteal cell steroidogenic capacity and ability to export P4 (Wiltbank, 1994). Per unit volume, the CL experiences the greatest blood flow of any endocrine organ, which is central to its function, particularly for the efficient uptake of cholesterol and for P4 secretion (Wiltbank, 1994). Mann (2009) reported a strong correlation between CL tissue mass and circulating P4 concentrations on d 5 of the oestrous cycle (R 2 = 0.64), but no correlation on d 8 (R 2 = 0.04) or d 16 (R 2 = 0.02), suggesting increased importance of luteal cell steroidogenic activity or CL blood flow as the CL matures. The factors that affect P4 MCR include liver blood flow and the activity of liver enzymes with P4 catabolic activity. Because liver blood flow is positively associated with DMI (Sangsritavong et al., 2002; Reynolds et al., 2003), 79
98 increased liver steroid clearance has been implicated as a potential disruptor of reproductive events, due to lower circulating concentrations of P4 and oestradiol (E2; Sangsritavong et al., 2002). In addition, Lemley et al. (2010) demonstrated that the liver expression of cytochrome P450 3A and 2C and aldo-keto reductase family 1, member C genes, which are involved in P4 inactivation, can be altered by dietary manipulation. Therefore, possible causes of low circulating P4 concentrations include inadequate CL P4 synthesis, increased liver blood flow, and greater liver P4 catabolic enzyme activity. Previously, we have reported positive effects of genetic merit for fertility traits on the reproductive performance of dairy cows (Cummins et al., 2012a), a phenotype associated with greater circulating P4 concentrations during the oestrous cycle (Cummins et al., 2012b). Therefore, two consecutive studies were carried out to identify the physiological mechanisms associated with greater circulating P4 concentrations in dairy cows with high genetic merit for fertility traits. 80
99 4.4 Materials and Methods Animal Model A genetic model of fertility was established in Teagasc Moorepark to elucidate the mechanisms responsible for poor fertility in lactating Holstein dairy cows (Cummins et al., 2012a,b,c). In autumn 2007 and 2008, nulliparous spring-calving heifers were identified from the national dairy cattle database (Irish Cattle Breeding Federation Bandon, Ireland). This population was limited to animals with EBV for milk production between +200 kg and +900 kg and >75% Holstein genetics. Within this population, heifers with extreme positive (i.e., poor fertility; Fert-) or negative (i.e., high fertility; Fert+) EBV for calving interval were selected. Only Fert- heifers from sires and maternal grand-sires with positive EBV for calving interval and Fert+ heifers from sires and maternal grand-sires with negative EBV for calving interval were selected. Nulliparous Fert- and nulliparous Fert+ heifers that passed the Moorepark Biosecurity Protocol were purchased and moved to the Moorepark Animal and Grassland Research and Innovation Centre in Fermoy, Co. Cork, Ireland. Within the Irish national herd, these heifers were representative of the top 25% in genetic merit for milk production. The Fert- heifers represented the bottom 5% in genetic merit for calving interval, whereas the Fert+ heifers represented the top 20% in genetic merit for calving interval. In subsequent years herd replacements were generated by selecting suitable sires to maintain the difference in genetic merit for calving interval. The list was restricted to sires with >200 kg PTA for milk production, >0% PTA milk fat and protein concentrations and >75% Holstein genetics. From this group, sires with >5 days PTA for calving interval were selected for mating with the Fert- cows and sires with <-5 days PTA for calving interval were selected for mating with the Fert+ cows. Twenty-eight and 23 cows were enrolled on Study 1 and 2, respectively, and the EBV s of the cows from both genotypes are summarized in Table 4.1. In Study 1, Fert+ and Fert- cows were represented by 5 and 11 sires respectively. The parity structure for the Fert+ cows was 4 and 11 cows in second and third lactation, respectively. The parity structure for the Fert- cows was 9 and 4 cows in second and third lactation, respectively. In Study 2, Fert+ and Fert- cows were represented by 6 and 9 sires respectively. Seventeen cows (9 Fert+ and 8 Fert-) were enrolled on both studies. The parity structure for the Fert+ cows was 3, 4 and 7 cows in second, third and fourth lactation, respectively. The parity 81
100 structure for the Fert- cows was 1, 3 and 6 cows in second third and fourth lactation, respectively. The experimental procedures involving animals on both studies were licensed by the Department of Health, Ireland, in accordance with the Cruelty to Animals Act (Ireland 1876) and the European Community Directive 86/609/EEC. 82
101 Table 4.1. The mean estimated breeding value 1 (and SD) for both genotypes based on their sire, maternal grandsire and maternal grand grand-sire estimated breeding values Study 1 Study 2 Genotype 2 Variable Fert+ Fert- Fert+ Fert- No. of animals Holstein 90.4 (7.2) 94.9 (7.0) 95 (4.7) 95 (5.8) Milk (kg) 363 (152) 434 (125) 417 (163) 445 (141) Fat (kg) 20 (6.2) 17 (5.7) 21 (5.5) 19 (7.1) Fat (g/kg) 1.0 (0.9) 0.6 (0.9) 0.7 (0.8) 0.2 (1.1) Protein (kg) 15 (5.7) 16.8 (4.9) 18 (7.0) 19 (5.8) Protein (g/kg) 0.33 (0.60) 0.35 (0.64) 0.58 (0.59) 0.59 (0.70) Survival (%) 3.62 (0.94) (2.04) 3.64 (0.83) (1.17) Calving Interval (d) (1.46) 8.05 (2.67) (1.34) 7.98 (2.99) Sire calving interval (d) -9.9 (1.9) 10.7 (3.7) -9.2 (2.6) 11.9 (3.5) Maternal grandsire calving interval (d) -6.0 (2.1) 12.7 (5.4) -8.2 (3.5) 11.2 (4.5) 1 PTA values were obtained from the Autumn 2012 official dairy evaluations published by the Irish Cattle Breeding Federation and multiplied by 2 to convert to EBV. Individual cow EBV were determined using the following formula: 0.5*sireEBV *MGsireEBV *MGGsireEBV 2 Fert+ = good-fertility cows; Fert- = poor-fertility cows 83
102 4.4.1 Study Feed and Management System The study was undertaken at Teagasc Moorepark from November 2010 to March Mean calving dates were November 2 (SD ± 37.1 d) and November 6 (SD ± 37.9 d) for the Fert+ and Fert- cows, respectively. Following parturition, cows were housed as one group in a freestall barn. Starting at d 40 (SD ± 15 d) post-partum, DMI was recorded daily using the Griffith Elder feeding system (Griffith Elder Ltd, Bury St Edmunds, Suffolk, UK). Cows were fed a total mixed ration ad libitum plus 5 kg lactating cow concentrate per day at the a.m. and p.m. milkings. Feed refusals were removed every second day. Diet ingredients were sampled weekly and composited monthly for analysis Animal Measurements Cows were milked twice daily at 8 a.m. and 4 p.m. Milk yield was recorded at each milking using electronic milk meters (Dairymaster, Causeway, Co. Kerry, Ireland). Milk composition (fat, protein and lactose) was determined weekly from successive a.m. and p.m. samples by mid-infrared reflectance spectroscopy (FT6000 Milkscan instrument, Foss Electric, Hillerød, Denmark). Body weight (BW) and body condition score (BCS) were recorded weekly. Body condition score was assessed using the 1 to 5 scale in 0.25 increments (Edmonson et al., 1989) Ovulation Synchronisation Cows were enrolled on an ovulation synchronisation protocol (CIDR_TAI) as previously described Herlihy et al., (2012). The mean days postpartum (± SD) when cows were enrolled in the protocol was 61 ± 15 (range: 37-78) and 60 ± 13 (range: 35-78) for the Fert+ and Fert- cows, respectively. On d -10, each cow was administered an i.m. injection of a gonadotropin-releasing hormone (GnRH) agonist containing 10 μg of buserelin (Receptal; Intervet Ireland, Dublin, Ireland), and a controlled internal drug release device containing 1.38 g of P4 (CIDR; Pfizer Ireland, Dublin, Ireland) was inserted per vaginum. On d -3, each cow was administered an i.m. injection of prostaglandin F 2α (PGF 2α ) containing 25 mg of dinoprost tromethamine (Lutalyse, Pfizer Ireland). On d -2, the CIDR device was removed and 36 h later, each cow was administered a second i.m. injection of GnRH agonist. 84
103 Blood Sampling Blood samples were collected once daily on d -3, -2, -1 (before a.m. milking) and twice daily on d 0, 1, 2, 3, 4, 5, 6, 7, 8 and 9 at 12 h intervals relative to synchronised oestrus (d 0) by coccygeal venepuncture into vacutainers containing lithium heparin (Becton Dickinson, Plymouth, UK), centrifuged at 2,000 x g for 15 min at 4 C; plasma was decanted and stored at -20 C Ovarian Ultrasonography Transrectal ultrasound examinations of both ovaries were carried out on d 0 and 7 relative to the synchronised oestrus. On d 0, follicles 5mm on the ipsilateral and contralateral ovaries were recorded. The clearest image of the largest follicle was frozen, and the cross-sectional height and width were measured using the internal calliper of the ultrasound machine (7.5 MHz transrectal transducer, Aloka SSD-900, Aloka Ltd., Tokyo, Japan). On d 7, the CL that developed from the ovulatory follicle was examined using a Voluson i ultrasound machine (GE Healthcare, Austria, Germany) equipped with a 12 Mhz linear array probe. Initially, CL were visualised in B-mode, and three images of each CL at its maximum diameter were captured and stored for further analysis. Ultrasound machine settings were then switched to power Doppler mode. The entire CL at its maximum size was fitted within a Doppler sample box and scanned for several seconds. Three images of each CL deemed representative of maximum blood flow with no flash artefacts were captured and stored for further analysis P4 Clearance Each cow was administered an i.m. injection of PGF 2α on d 7 p.m. and d 8 a.m. On d 8 a.m., two CIDRs, each containing 1.38 g of P4 were inserted per vaginum and an indwelling jugular catheter was inserted to facilitate frequent blood sampling. Cows were moved to an individual tie-stall barn. On d 9, a liver biopsy was collected from each cow. The biopsy site on the right flank was clipped, shaved, disinfected with Videne (Povidone-iodine, 7.5%; Ecolab, Leeds, UK) and methylated spirits and anaesthetised with Willcain (Procaine hydrochloride, 5.0%, Dechra Ltd, Shrewsbury, UK). A 1 cm incision was made through the skin between the 11 th and 12 th ribs and the biopsy tool was inserted to pierce the intercostal muscle and peritoneum. The liver was located and a 1 to 1.5 g sample was removed. The sample was washed in saline, blotted dry, snap frozen in liquid nitrogen and stored at -80 C. The incision site was sutured 85
104 and treated topically with Oxytetracycline spray (Duphacycline; Interchem Ireland, Naas, Ireland). Cows were administered antibiotic as a prophylactic (500 mg Ceftiofur Hydrochloride; Excenel RTU, Pfizer Animal Health). On d 10, frequent blood samples were collected via jugular catheter at -60, -45, -30, -15, 0, 15, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420, 540, and 660 min relative to removal of both CIDRs (0 minutes) to measure the half-life and MCR of P4. Catheter patency was maintained by flushing with 1 ml of heparinised sterile saline after each sample collection RNA Extraction and cdna Synthesis Total RNA was extracted from liver tissue using a standard Trizol-based method (Chomczynski and Sacchi, 1987). The tissue sample was weighed and 100 mg homogenised in 3 ml TRI Reagent (Sigma-Aldrich, Dublin) for 30 s at 6000 rpm using a Precellys 24 Dual bead-beater with ceramic beads (Bertin Technologies, Montignyle-Bretonneux). The homogenate was removed to sterile eppendorf tubes (Eppendorf, UK) and incubated at room temperature for 5 min.; 300 μl bromo-chloropropane (Sigma-Aldrich, Dublin) was added, samples were vortexed and incubated at room temperature for 3 min and then centrifuged at 12,000 x g for 10 min. at 4 C. The supernatant was removed to new sterile tubes. Isopropanol was added at 0.6 times the volume of supernatant and vortexed and centrifuged at 12,000 x g for 10 min. at 4 C to pellet the RNA. The supernatant was discarded and the pellet was washed twice in 99% ethanol (Sigma-Aldrich, Dublin) with centrifugation at 7,500 x g for 5 min. at 4 C. The RNA was resuspended in 35μL nuclease-free water (Sigma-Aldrich, Dublin). A kitbased protocol (RNeasy, Qiagen, UK) was used to clean the total RNA, removing the fraction below 200 bp as well as any DNA. RNA quality and concentration was determined using the Nanodrop ND-1000 (Nanodrop, Wilmington, Denver) and the Bioanalyser 2100 (Agilent Technologies, UK) using the RNA Nano chip. The 260/280 absorbance ratio ranged between 1.92 and 2.18 for all samples. The RNA integrity number and 28s:18s ratio ranged from 7.1 to 8.8 and 1.1 to 1.5, respectively. Subsequently, 500 ng of RNA was reverse transcribed to cdna using the High- Capacity cdna Reverse Transcription kit (Applied Biosystems, Foster city, CA, USA) per the manufacturer s instructions to synthesise cdna in a 20 µl reaction, diluted to 1 ng/μl of RNA equivalents with water and stored at -20 C. 86
105 Primer Design and Reference Gene Selection Primers were designed to span exon-exon junctions where possible (Table 4.3), and PCR product size was restricted to between 50 and 155 nucleotides, using NCBI/Primer-Blast; ( All primers were manufactured by Eurofins MWG (Ebersberg, Germany). The expression of β- Actin (ACTB), ribosomal protein L19 (RPL19), peptidylprolyl isomerase A (cyclophilin A) (PPIA) and mitogen-activated protein kinase 3 (MAPK3) were investigated as candidate reference genes on a subset of 12 samples that were representative of the two genotypes and their sires. The genorm application within the Biogazelle qbase plus software program ( Biogazelle, Ghent, Belgium) determined that PPIA and RPL19 combined were the most stably expressed reference genes, with an M value of The expected product sizes for all primers were confirmed by gel electrophoresis Real Time-qPCR All RT-qPCR reactions were performed in a 20.0 µl reaction using 5 μl (5 ng) cdna, 10 μl SYBR MasterMix (Bioline) and 300 nm of forward and reverse primers and) on clear 96-well plates (FrameStar). All samples were measured in duplicate. Primer efficiencies for each target were determined using a 1 in 4 serial dilution over 7 points and were shown to lie between 90% and 110%; non-template controls and minus RT controls were also included for each target. All reactions were run on a 7500 Real-Time PCR machine (Applied Biosystems) with the following cycling conditions: 50 C for 2 min, 95 C for 10 min, 40 cycles of 95 C for 15 sec, 60 C for 1 min, followed by 95 C for 15 sec, 60 C for 1 min and 95 C for 15 sec to create a dissociation curve, which was examined to ensure amplification was specific to the target gene. Relative gene expression values (CNRQs) were determined using the qbase software package Study Feed and Management System The objective of this study was to determine the temporal pattern of CL blood flow and circulating P4 concentrations during the oestrous cycle. The study was undertaken at Teagasc Moorepark from January 2012 to December Mean calving dates were February 17 (SD ± 19.7 d) and February 24 (SD ± 24.7 d) for the Fert+ and Fert- cows, 87
106 respectively. Following parturition, cows were housed in a freestall barn and were fed a total mix ration ad libitum plus 6 kg dairy concentrate per day at the a.m. and p.m. milkings. Diet ingredients were sampled weekly and composited monthly for analysis. The ingredient and nutrient composition of the diets are outlined in Table 4.2. Cows were turned out to grass on March 26 th and managed as one herd in a rotational grazing system. Cows grazed a predominantly perennial ryegrass (Lolium perenne L.) sward with fresh pasture allocated daily. The mean daily herbage allowance was 14.5 ± 1.3 kg DM/cow day -1, which was supplemented with 3.2 ± 0.2 kg/cow day -1 of lactating cow concentrate fed at a.m. and p.m. milking. Milk and BCS measurements were collected as described for Study Ovulation Synchronisation Cows were enrolled on the same ovulation synchronisation protocol used in Study 1. The mean days postpartum (± SD) when cows were enrolled on the protocol was 56 ± 5.7 (range: 45-67) and 54 ± 9.1 (range: 34-63) for the Fert+ and Fert- cows, respectively Blood Sampling Blood samples were collected once daily on d -3, -2, -1, 0, 1, 2, 3 (a.m. milking) and twice daily on d 4, 5, 6, 7, 8, 9, 10, 11, 12 (at 12 h intervals) and 13 (once, after a.m. milking) relative to synchronised oestrus (d 0) and processed as described in Study Ovarian Ultrasonography Transrectal ultrasound examinations of both ovaries were carried out on d 0, 4, 7, 10 and 13 using a Voluson i ultrasound machine equipped with a 12 Mhz linear array probe. Three images of follicles 15mm on d 0 and of the CL on d 4, 7, 10 and 13 were captured in B-mode and power Doppler mode as described in Study Blood Sample Analysis Circulating P4 concentrations were determined in samples collected from d 1 to 9 after synchronised oestrus and from -60 to 660 min relative to CIDR removal during Study 1, and in samples collected from d 1 to 13 after synchronised oestrus during Study 2 using a commercially available solid-phase radioimmunoassay (Coat-A-Count Progesterone, 88
107 Diagnostic Products Corp., Los Angeles, CA). The inter-and intra-assay CV s for Study1 were 17.2% and 11.1%, 10.0% and 8.3% and 9.4% and 6.9% for the low, Table 4.2. Ingredient and nutrient composition of lactating cow diet Lactating cow diet (% DM) Maize silage 36 Grass silage 33 Parlour concentrate 22 Soyabean meal 9 Lactating cow concentrate ingredients (% fresh) Wheat 25 Soyabean 15 Rapeseed extract 10 Sunflower seed extract 10 Palm kernel meal 10 Milk solids 5 Maize gluten 5 Citrus pulp 5 Soyabean hulls 5 Palm oil 4 Oat feed 4 Minerals 1 2 Nutrient composition of lactating cow concentrate DM (g/kg) 868 Net energy (UFL/kg DM) 0.92 Ash (g/kg DM)Crude protein (g/kg DM) 74 Crude protein (g/kg DM) 185 NDF (g/kg DM) Vitamin and Mineral mix: 229 g/kg of Ca, 100 g/kg of Na, 70 g/kg of P, 5 g/kg of Mg, 4500 mg/kg of Zn, 3000 mg/kg of Cu, 1500 mg/kg of Mn, 500 mg/kg of I, 400 mg/kg Bioplex* Cu, 400 mg/kg of Bioplex* Zn, 99 mg/kg of Co, 37 mg/kg of Se, IU/kg of vitamin A, IU/kg of vitamin D3, 1250 mg/kg of vitamin E and 200 mg/kg of vitamin B12. *Alltech Inc., Nicholasville, KY 89
108 Table 4.3. Primer sequences used in real time quantitative PCR Gene name Primer sequence 5 3 Product size Accession number AKR1C1 Forward: CCCAAAAGCACAAGCAGACCCCA 144 NM_ Reverse: GCCCTCTGCCCACAGTCTCACT AKR1C3 Forward: TTCTTGTTGGTGTCGGTCACCCT 99 NM_ Reverse: TCACCACCCCACAGAAGTAAAGAGT AKR1C4 Forward: GCTATTACTGGGTGTTGGTCACCCT 112 NM_ Reverse: ATCCTCTTCACAGCCCTAGAAGCA CYP2C19 Forward: TGACCTTGTCCCCAGCAGTATGC 83 NM_ Reverse: TGACTGTGCCCTTGGGAATGAGGT CYP3A5 Forward: GAATTGGCCACTCACCCTGATGTCC 88 NM_ Reverse: CATCATAGGTCGGAGGCGCCTTAT PPIA Forward: TCCATGGCAAATGCTGGCCCC 86 NM_ Reverse: ACGTGCTTGCCATCCAACCACT MAPK3 Forward: GACCCAACGGATGAGCCAG 123 NM_ Reverse: CACCCCAGGCTGGAAGC ACTB Forward: CGCCATGGATGATGATATTGC 66 NM_ Reverse: AAGCCGGCCTTGCACAT RPL19 Forward: GAAAGGCAGGCATATGGGTA 86 NM_ Reverse: TCATCCTCCTCATCCAGGTT AKR1C1 = aldo-keto reductase family 1, member C1; AKR1C3= aldo-keto reductase family 1, member C3; AKR1C4 = aldo-keto reductase family 1, member C4; CYP2C19 = cytochrome P450, family 2, subfamily C, polypeptide 19; CYP3A5 = cytochrome P450, family 3, subfamily A; PPIA = peptidylprolyl isomerase A (cyclophilin A); MAPK3 = mitogen-activated protein kinase 3; ACTB = β-actin; RPL19 = ribosomal protein L19 90
109 medium and high P4 pools respectively. The inter-and intra-assay CV s for Study 2 were 12.8% and 11.7%, 9.9% and 7.0% and 5.2% and 6.2% assay for the low, medium and high pools respectively. Circulating E2 concentrations were determined in samples collected on d -3, -2, -1 and 0 in the two studies by radioimmunoassay following extraction using E2 MAIA kits (Bio-Stat Diagnostic Systems Ltd. Stockport, Cheshire, UK). Peak circulating E2 concentration and day of peak were determined for each cow. The inter-and intra-assay CV s were 15.2% and 23.0%, 16.5% and 11.2% and 5.3% and 10.4% assay for the low, medium and high pools respectively. Circulating insulin and insulin-like growth factor 1 (IGF1) concentrations were determined in samples collected on d 0, 7 and 9 during Study 1 and d 0 and 13 during Study 2. Insulin concentrations were determined by solid-phase 125 I radioimmunoassay (Coat-A-Count Insulin, Diagnostic Products Corporation, Los Angeles, CA). Inter- and intra-assay coefficients of variation were 7.8% and 13.5%, respectively. Concentrations of IGF1 were determined using a validated double antibody radioimmunoassay, following ethanol:acetone:acetic acid extraction (Enright et al., 1989). Inter- and intra-assay coefficients of variation were 2.7% and 12.1%, respectively. Both genotypes were represented equally in each hormone assay, and all samples for each cow were completed within one assay Ultrasound Image Analysis The diameter of the follicle measured on d 0 in Study 1 was calculated as the mean of the cross-sectional height and width. The diameter of the follicle and CL scanned with the Volusan i ultrasound machine were calculated as the mean of 3 cross sectional diameters measured from each image. Corpus luteum volume was calculated using the formula: volume = 4/3 x π x (0.5 diameter) 3 and the volume of luteal cavities were calculated and subtracted if present. Luteal tissue area was calculated using the formula: area = π x (0.5 diameter) 2 and the area of luteal cavities were calculated and subtracted if present. Power Doppler images of CL and follicles were analysed using the computer programme Pixel Flux (Version 1.0, Chameleon Software, Leipzig, Germany) to quantify the area in cm 2 of coloured pixels within the CL or follicle, and which is considered a semi-quantitative measure of blood flow. Mean blood flow area of each structure was calculated using the three images. Corpus luteum relative blood flow area (relbfa) was calculated as blood flow area/luteal tissue area. 91
110 Data Handling Cows were deemed to have had a synchronised oestrus if the preovulatory follicle measured on d 0 resulted in CL formation and circulating P4 concentrations were < 0.7 ng/ml on d 1 and > 1.1 ng/ml on d 7. Twenty-eight cows were enrolled on Study 1. Records of six cows were not included in the statistical analysis (one Fert+ cow and two Fert- cows did not respond to the ovulation synchronisation protocol based on P4 profiles and one Fert+ cow and two Fert- cows were diagnosed as having uterine infection on d 7). Twenty-three cows were enrolled on Study 2. Records of 2 Fert- cows were not included in the statistical analysis because they did not respond to the ovulation synchronisation protocol based on P4 profiles. Daily measurements of milk yield were collapsed into average weekly yields. Milk production, DMI, BW and BCS data collected during the 4 week period before and during both studies are reported. Data were checked for normality. In Study 1, suitable Box-Cox transformations were identified to normalise the distribution of circulating E2 concentrations, P4 half-life and mrna abundance of AKR1C1 and AKR1C3. In Study 2, suitable Box-Cox transformations were identified to normalise the distribution of follicle diameter, BFA, CL volume, circulating E2 and P4 concentrations Statistical Analysis All statistical analysis was performed using SAS (SAS Institute, 2006), with the exception that R (R Core Team, 2013) was used to generate the box plots. Non-linear regression was used to calculate the parameter for rate of P4 decay for each cow by fitting parameter estimates from the first 60 min following removal of 2 CIDRs to the (c x t) following equation: f(t) = b * e where t = time, b = parameter for starting concentration of P4, c = parameter for rate of decay. Finally, P4 half-life and MCR were calculated using the following equations: Half-life (min) = ln(2)/c; MCR (%/min) = c x 100. Mixed model procedures were used to determine the effect of genotype on variables with repeated measures such as milk production, BW, BCS, DMI, reproductive hormone concentrations and CL measurements (Study 2). Fixed effects 92
111 included genotype, time (minute or day or week) and their interactions. Parity, calving date, block and their interactions with genotype were included initially but removed if not significant (P > 0.1). Cow nested within genotype was included as a random effect, time was included as a repeated effect and a first-order auto regressive covariance structure was applied. Mixed model procedures were used to determine the effect of genotype on variables without repeated measures. Genotype was included as a fixed effect. Parity, calving date, block and the interaction of parity with genotype were included initially but removed if not significant (P > 0.1). Cow nested within genotype was included as a random effect. The effect of genotype on day of peak circulating E2 concentrations was determined by one-way nonparametric test. Circulating P4 concentration data from d 3 to 7 of the oestrous cycle in both Study 1 and Study 2 were combined, and the rate of increase in circulating P4 concentrations for each genotype was determined by regressing circulating P4 concentration against day. 93
112 4.5 Results Study Milk Production and Animal Characteristics The effect of genetic merit for fertility traits on DMI, BW and milk yield is illustrated in Figure 4.1. Dry matter intake tended to be greater in Fert+ cows than Fert- cows (+0.79 kg DM/d, P = 0.06). Milk yield (mean: 29.8 kg/d, P = 0.98), milk solids yield (mean: 2.28 kg/d, P = 0.38), BCS (mean: 2.75 units, P = 0.37), circulating insulin (mean: 3.21 µiu/ml, P = 0.49) and IGF1 concentrations (mean: ng/ml, P = 0.13) were similar in both genotypes Ovarian Characteristics and Reproductive Hormones The effect of genetic merit for fertility traits on ovarian characteristics and reproductive hormones during Study 1 is summarized in Table 4.4. The diameter of the preovulatory follicle was 9% greater in Fert+ cows than Fert- cows (+1.44 mm, P = 0.04). There was no effect of genotype on CL volume (P = 0.12). The BFA of the CL was 42% greater in Fert+ cows compared with Fert- cows (+0.64 cm 2, P = 0.03). The relbfa of the CL was similar in both genotypes (P = 0.7). The profile of mean circulating E2 and P4 concentrations in Fert+ and Fertcows is illustrated in Figure 4.2. A genotype x day interaction for circulating E2 concentrations was observed (P = 0.01), which arose primarily because of a robust preovulatory increase in circulating E2 concentrations on day -1 in Fert+ cows that was not evident in Fert- cows. As a result, peak circulating E2 concentration was greater in Fert+ cows than in Fert- cows (+1.94 pg/ml, P = 0.007). Day of peak circulating E2 concentrations was similar in both genotypes (mean: d -1, P = 0.94). A genotype x day interaction for circulating P4 concentrations was observed (P = 0.03) because of a more rapid increase in Fert+ cows compared with Fert- cows. 94
113 Milk yield (kg/d) Body weight (kg) DMI (kg/d) Genotype: P = 0.06 Genotype x wk: P = 0.85 SEM: 0.28 kg Fert- Fert Genotype: P = 0.99 Genotype x wk: P = 0.70 SEM: kg Genotype: P = 0.98 Genotype x week: P = 0.17 SEM: 0.77 kg/d Week relative to sample week Figure 4.1. The effect of genetic merit for fertility traits on dry matter intake, body weight and milk yield during the 4 weeks prior to completion of the study. All values are LSM. Dry matter intake tended to be greater in Fert+ cows compared with Fertcows while body weight and milk yield were similar. indicates P
114 Table 4.4. The effect of genetic merit for fertility traits on ovarian characteristics and reproductive hormones during Study 1 Genotype Variable Fert+ Fert- SEM 1 Genotype Genotype x day Preovulatory follicle: Diameter (mm) Oestradiol (pg/ml): Day -3 to ( ) 1.40 ( ) Peak Corpus luteum: Volume (mm 3 ) BFA (cm 2 ) relbfa (%) BFI (cm/s) Progesterone (ng/ml): Day 1 to ( ) 0.73 ( ) = pooled standard error 2 = data presented as LSM with 95% CI in parentheses 96
115 Progesterone (ng/ml) Estradiol (pg/ml) Fert- Fert Day relative to oestrus Figure 4.2. The effect of genetic merit for fertility traits on circulating E2 and P4 concentrations during Study 1. All values are back-transformed LSM with 95% CI. Mean circulating E2 concentrations were similar in both genotypes but there was a genotype x day interaction (P = 0.01) because peak circulating E2 concentration was greater in Fert+ cows compared with Fert- cows (P = 0.007). Mean circulating P4 concentrations were similar in both genotypes but there was a genotype x day interaction (P = 0.03) because of a more rapid increase in Fert+ cows compared with Fert- cows. 97
116 P4 Metabolism The effect of genotype on hepatic mrna abundance of genes responsible for P4 catabolism is illustrated in Figure 4.3. The mrna abundance of CYP3A was greater in Fert- cows than Fert+ cows, P = 0.05) while the mrna abundance of AKR1C1, AKR1C3, AKR1C4 and CYP2C was similar in both genotypes (all P > 0.2). The profile of circulating P4 concentrations in Fert+ and Fert- cows during the P4 clearance study is illustrated in Figure 4.3. There was no effect of genotype on the half-life (P = 0.70) and MCR (P = 0.79) of P4 following removal of the two CIDRs (Table 4.5). Table 4.5. The effect of genetic merit for fertility traits on P4 half-life and MCR Genotype Variable Fert+ Fert- SEM P-value P4 MCR (%/min) P4 half-life (min) ( ) ( ) = pooled standard error 2 = data presented as LSM with 95% CI in parentheses 98
117 Progesterone (ng/ml) Relative mrna abundance (CNRQ) Fert- Fert+ AKR1C1 AKR1C3 AKR1C4 CYP2C CYP3A Fert- Fert+ * Time (min) relative to removal of 2 CIDRs Figure 4.3. The effect of genetic merit for fertility traits on progesterone (P4) metabolism. Top: the effect of genetic merit for fertility traits on hepatic mrna abundance of candidate genes involved in P4 metabolism. The mrna abundance of cytochrome P450, family 3, subfamily A (CYP3A) was greater in cows with poor genetic merit for fertility traits (Fert ) compared with cows with good genetic merit for fertility traits (Fert+) but the mrna abundance of aldo-keto reductase family 1, member C1 (AKR1C1), AKR1C3, AKR1C4, and cytochrome P450, family 2, subfamily C (CYP2C) was similar. Data are presented as LSM with 95% CI. Bottom: Circulating P4 concentrations in Fert+ and Fert cows from 60 min to 660 min relative to removal of 2 controlled internal drug release (CIDR) devices. CNRQ = calibrated, normalized relative quantity values. 99
118 4.5.2 Study Milk Production and Animal Characteristics Milk yield (mean: 31.7 kg/d, P = 0.81), milk solids yield (mean: 2.30 kg/d, P = 0.14), BW (mean: 540 kg, P = 0.16) and circulating insulin concentrations (3.23 µiu/ml, P = 0.28) were similar in both genotypes. Body condition score (2.88 vs units, P = ) and circulating IGF1 concentrations (128.3 ± 7.4 vs ± 9.4 ng/ml, P = 0.05) were greater in Fert+ cows compared with Fert- cows Ovarian Characteristics and Reproductive Hormones The effect of genetic merit for fertility traits on ovarian characteristics and reproductive hormones during Study 2 is summarized in Table 4.6. The diameter of the preovulatory follicle tended to be greater in Fert+ cows compared with Fert- cows (P = 0.09). Mean circulating E2 concentrations were 54% greater in Fert+ cows than in Fert- cows (+0.48 pg/ml, P = 0.02; Figure 4.4). Peak circulating E2 concentrations were 53% greater in Fert+ cows than in Fert- cows ( pg/ml, P = 0.01). Day of peak circulating E2 concentrations tended to be earlier in Fert- cows compared with Fert+ cows (P = 0.1). Corpus luteum volume tended to be 41% greater in Fert+ cows compared with Fert- cows ( mm 3, P = 0.06) but there was no effect of genotype on CL BFA (P = 0.45) or relbfa (P = 0.12). Mean circulating P4 concentrations were 79% greater in Fert+ cows than Fert- cows (+1.67 ng/ml, P < ; Figure 4.4 and Figure 4.5). A genotype x day interaction (P = ) arose because of a more rapid increase in circulating P4 concentrations in Fert+ cows after d 6. Simple linear regression analysis indicated that circulating P4 concentrations increased by 0.79 ng/ml day -1 and 0.52 ng/ml day -1 respectively. between d 3 and 7 of the oestrous cycle in Fert+ and Fert- cows, Regression equation for Fert+ cows (±SEM): P4 = (0.79±0.03)*day 2.12±0.18; R 2 = 0.71 Regression equation for Fert- cows (±SEM): P4 = (0.52±0.04)*day 1.26±0.21; R 2 =
119 101
120 Figure 4.4. The effect of genetic merit for fertility traits on reproductive hormones, follicle diameter and CL characteristics during Study 2. Values are back-transformed LSM with 95% CI. * indicates P indicates P 0.1. A: Follicle diameter tended to be greater in Fert+ cows compared with Fert- cows (P = 0.09). Mean circulating E2 concentrations were greater in Fert+ cows compared with Fert- cows (P = 0.02). There was a genotype x day interaction (P = 0.04) because peak circulating E2 concentrations were greater in Fert+ cows compared with Fert- cows (P = 0.01). B: There was no effect of genotype on CL BFA (P = 0.45). C: Mean circulating P4 concentrations were greater in Fert+ cows compared with Fert- cows (P < ) and there was a genotype x day interaction (P = ) because of a more rapid increase in Fert+ cows compared with Fert- cows. D: CL volume tended to be greater in Fert+ cows compared with Fert- cows (P = 0.06). 102
121 Table 4.6. The effect of genetic merit for fertility traits on ovarian characteristics during Study 2 Genotype P-value Variable Fert+ Fert- SEM 1 Genotype Genotype x day Follicle: Diameter (mm) ( ) ( ) BFA (cm 2 ) 0.20 ( ) 0.19 ( ) Oestradiol: Day -3 to 0 2 (pg/ml) 1.37 ( ) 0.89 ( ) Peak (pg/ml) Day of peak Corpus luteum: Volume (mm 3 ) ( ) ( ) BFA (cm 2 ) ( ) 1.24 ( ) relbfa (%) Progesterone: Day 1 to ( ) 2.11 ( ) - < = pooled standard error 2 = data presented as LSM with 95% CI in parentheses 103
122 Figure 4.5. Box plots depicting circulating P4 concentrations from Study 2 during d 5, 7, 10 and 13 of the oestrous cycle in Fert+ and Fert- cows. Circulating P4 concentrations were significantly greater in Fert+ cows compared with Fert- cows on d 5 (1.98 vs ng/ml, SEM = 0.21, P = 0.03), 7 (4.42 vs. 2.59, SEM = 0.28, P = ), 10 (7.01 vs. 3.66, SEM = 0.34, P < ) and 13 (7.96 vs. 4.63, SEM = 0.58, P = ) of the oestrous cycle 104
123 4.6 Discussion Fert+ cows had greater circulating P4 concentrations and greater CL volume compared with Fert- cows. The P4 synthetic capacity of the CL was the primary factor affecting circulating P4 concentrations since there was no effect of genotype on P4 clearance or on the factors that affect P4 clearance (i.e., milk production, DMI and hepatic mrna abundance of P4 catabolic genes). Volume was the primary factor affecting P4 synthetic capacity of the CL since there was no effect of genotype on CL BFA. Our results imply that greater preovulatory follicle diameter and greater peak E2 concentrations in Fert+ cows compared with Fert- cows may have been associated with subsequent CL volume and P4 synthetic capacity Preovulatory Follicle Characteristics and Circulating E2 Concentrations Our results indicate that preovulatory follicle diameter and preovulatory circulating E2 concentrations are important fertility traits. Fert+ cows had greater peak circulating E2 concentrations (d -1) compared with Fert- cows. This may be explained by greater preovulatory follicle diameter on the day of presumptive oestrus (significantly greater in Study 1, numerically greater in Study 2). Circulating E2 concentrations must reach the threshold required by the surge centre of the hypothalamus to release GnRH, thereby facilitating the surge release of luteinising hormone from the pituitary to initiate the cascade of events required for ovulation (Senger, 1997). Greater circulating preovulatory E2 concentrations have been reported in heifers compared with lactating cows (Sartori et al., 2004), and in dairy cows that subsequently became pregnant compared with dairy cows that did not (Lopes et al., 2007). Greater preovulatory follicle diameter has been reported in heifers (Siddiqui et al., 2009) and cows (Lopes et al., 2007) that subsequently became pregnant compared with those that did not. In addition, Stevenson et al., (2008) reported a positive correlation between the diameter of the ovulatory follicle at the time of CIDR removal and PGF 2α administration during a timed AI protocol and circulating P4 concentrations 9 d later in heifers Circulating P4 Concentrations The results of Study 1 and 2, combined with the results of Cummins et al., (2012b) provide strong evidence that superior genetic merit for fertility traits is associated with greater circulating P4 concentrations in lactating dairy cows. The P4 profiles from Study 1 and Study 2 indicate two clear characteristics. First, in keeping with our 105
124 previous findings (Cummins et al., 2012b), mean circulating P4 concentrations were greater in Fert+ cows compared with Fert- cows. Second, the interaction between genotype and time in both studies indicates that Fert+ cows have a more rapid postovulatory increase in circulating P4 concentrations compared with Fert- cows. Simple linear regression equations indicated that the rate of increase in P4 between days 3 and 7 was 52% greater in Fert+ cows compared with Fert- cows. While the difference was smaller than in the current study, Walker et al., (2012) reported greater circulating P4 concentrations in cows with New Zealand ancestry (good fertility) compared with cows with North American ancestry (poor fertility). It is clear that circulating P4 concentrations during the prebreeding and postbreeding periods are important fertility traits. These findings are supported by data from several studies that indicated supplemental P4 improved conception rates (Macmillan and Peterson, 1993; Cunha et al., 2008; Herlihy et al., 2011; Colazo et al., 2013) synchrony of ovulation (Herlihy et al., 2011; Colazo et al., 2013) and reduced pregnancy loss between d 30 to 60 (Cunha et al., 2008; Colazo et al., 2013; Herlihy et al., 2013). Consistent with these results, improved fertility in lactating dairy cows was achieved by the use of a CIDR between d 3.5 to 10 post breeding (Larson et al., 2007) and by human chorionic gonadotropin (hcg) on d 5 post breeding (Nascimento et al., 2013a). However, other studies have reported no improvement in pregnancy rates (Lonergan, 2011). The success of P4 supplementation, or administration of GnRH or hcg, may be dependent on the timing of the treatment and may only be beneficial in cows with low circulating P4 concentrations (Lonergan, 2011) as lower circulating P4 concentrations from d 6 to 8 has been associated with negative pregnancy status following diagnosis on d 29 post AI (Lopes et al., 2007). Greater circulating P4 concentrations in nulliparous Holstein heifers compared with lactating Holstein dairy cows is a likely mediator of superior fertility in heifers (Sartori et al., 2004; Wolfenson et al., 2004; Rizos et al., 2010). Nascimento et al., (2013b) evaluated the effectiveness of CIDR, hcg or CIDR plus hcg treatments from d 5 post oestrus at raising circulating P4 concentrations in lactating dairy cows. Only cows receiving the CIDR plus hcg treatment achieved circulating P4 concentrations similar to those in heifers. Circulating P4 concentrations on d 13 of the oestrous cycle in Fert+ cows in the current study were similar to cows treated with hcg, whereas Fertcows achieved circulating P4 concentrations similar to control cows that received no treatment in the study reported by Nascimento et al., (2013b). These results indicate the 106
125 important role of genetic selection for fertility traits in improving circulating P4 concentrations in lactating dairy cows. It would be interesting to examine the conception rate response if circulating P4 concentrations in Fert- cows was increased to be similar to Fert+ cows Corpus Luteum Characteristics Progesterone secretion is determined by the volume and blood flow of the CL, the metabolic rate of the luteal cells and stage of the oestrous cycle. We assessed CL function based on volume, BFA and relbfa using transrectal ultrasonography. While several authors reported that positive associations between CL size and circulating P4 concentrations depended on stage of the oestrous cycle (Mann, 2009; Lüttgenau et al., 2011; Rizos et al., 2012), Herzog et al., (2010) reported that CL BFA was a better indicator of CL function than size during d 7 to 14 of the oestrous cycle. During this period, circulating P4 concentrations and BFA doubled while CL area increased only by 25%. However, Lüttgenau et al., (2011) reported no correlation between BFA and circulating P4 concentrations during the mid-luteal phase in a subsequent study by the same group. As a result, Lüttgenau et al., (2011) suggested relbfa as a more relevant assessment of CL function by accounting for CL size. In a review of their studies, Bollwein et al., (2012) concluded that the correlation between BFA and circulating P4 concentrations reported by Herzog et al., (2010) was due to the fact that both measurements were closely associated with CL size. In Study 1, based on ultrasound measurements at a single time point on d 7 of the oestrous cycle, CL volume was numerically greater and BFA was significantly greater in Fert+ cows compared with Fert- cows. In Study 2, employing a more thorough assessment, based on ultrasound measures at 4 time points, mean CL volume tended to be greater in Fert+ cows compared with Fert- cows but BFA was not affected by genotype. relbfa was not affected by genotype in either study. Taken together, our results indicate that CL volume rather than blood flow is the major factor determining CL P4 synthetic capacity in agreement with Lüttgenau et al., (2011) P4 Clearance The removal of P4 from circulation is determined by the rate of blood flow through the liver and the activity of liver enzymes with P4 catabolic activity. Increased liver blood flow due to greater DMI (Sangsritavong et al., 2002; Reynolds et al., 2003) and the 107
126 associated increase in liver steroid clearance has been implicated as a potential disruptor of reproductive events in high producing dairy cows. Sangsritavong et al., (2002), Lemley et al., (2010) and Hutchinson et al., (2012) have reported that it is possible to alter P4 clearance through dietary manipulation. The major genes responsible for P4 catabolism in the liver belong to the cytochrome P450 family (Murray, 1991; Murray, 1992). These are mixed-function monooxygenases that inactivate P4 by hydroxylation to hydroxyprogesterone. In addition, the aldo-keto reductase family inactivate P4 by reduction to hydroxyprogesterone (Penning et al., 2000). While there was no effect of genotype on mrna abundance of AKR1C1, AKR1C3, AKR1C4 and CYP2C, Fert- cows had greater mrna abundance of CYP3A. Down-regulation of CYP3A or reduced activity of its enzyme in response to elevated circulating insulin has been previously reported (Lemley et al., 2008, 2009, 2010). There were no differences in circulating insulin concentrations between Fert+ and Fert- cows at the time of the liver biopsy in the current study. The lack of an effect of genotype on the MCR and half-life of P4 seems reasonable considering four of the five candidate genes were not differentially expressed. In addition, milk yield was similar between genotypes in both studies. While the Fert+ cows tended to have greater DMI compared with the Fert- cows, the difference equates to only 3.6%, whereas Sangsritavong et al., (2002) maintained a difference of 200% in DMI between treatment groups in their study. 4.7 Conclusions Insufficient circulating P4 concentrations have been implicated as a major cause of poor fertility in lactating dairy cows. The naturally occurring differences in circulating P4 concentrations between Fert+ and Fert- cows provided us with a unique opportunity to examine the factors that affect circulating P4 concentrations without the need to artificially manipulate their diet or hormone profiles. The results of our studies indicate: (i) Fert+ cows had greater circulating P4 concentrations during the oestrous cycle than Fert- cows; (ii) that these differences were due to greater CL volume rather than differences in P4 clearance; and (iii) genetic selection for high circulating P4 concentrations is possible without antagonising milk production. 108
127 4.8 References Bollwein, H., J. Lüttgenau, and K. Herzog Bovine luteal blood flow: basic mechanism and clinical relevance. Reproduction Fertility and Development 25(1): Colazo, M. G., A. Dourey, R. Rajamahendran, and D. J. Ambrose Progesterone supplementation before timed AI increased ovulation synchrony and pregnancy per AI, and supplementation after timed AI reduced pregnancy losses in lactating dairy cows. Theriogenology 79(5): Cummins, S. B., P. Lonergan, A. C. O. Evans, D. P. Berry, R. D. Evans, and S. T. Butler. 2012a. Genetic merit for fertility traits in Holstein cows: I. Production characteristics and reproductive efficiency in a pasture-based system. Journal of Dairy Science 95(3): Cummins, S. B., P. Lonergan, A. C. O. Evans, and S. T. Butler. 2012b. Genetic merit for fertility traits in Holstein cows: II. Ovarian follicular and corpus luteum dynamics, reproductive hormones, and estrus behavior. Journal of Dairy Science 95(7): Cummins, S. B., S. M. Waters, A. C. O. Evans, P. Lonergan, and S. T. Butler. 2012c. Genetic merit for fertility traits in Holstein cows: III. Hepatic expression of somatotropic axis genes during pregnancy and lactation. Journal of Dairy Science 95(7): Cunha, A. P., J. G. Guenther, M. J. Maroney, J. O. Giordano, A. B. Nascimento, S. Bas, H. Ayres, and M. C. Wiltbank Effects of high vs. low progesterone concentrations during Ovsynch on double ovulation rate and pregnancies per AI in high producing dairy cows. Journal of Dairy Science 91:(Suppl. 1), 246. [Abstract]. Diskin, M. and D. Morris Embryonic and Early Foetal Losses in Cattle and Other Ruminants. Reproduction in Domestic Animal. 43(Suppl. 2): Edmonson, A. J., I. J. Lean, L. D. Weaver, T. Farver, and G. Webster A Body Condition Scoring Chart for Holstein Dairy Cows. Journal of Dairy Science 72(1): Enright, W. J., L. T. Chapin, W. M. Moseley, S. A. Zinn, M. B. Kamdar, L. F. Krabill, and H. A. Tucker Effects of infusions of various doses of bovine growth hormone-releasing factor on blood hormones and metabolites in lactating Holstein cows. Journal of Endocrinology. 122(3):
128 Forde, N., J. Mehta, P. McGettigan, S. Mamo, F. Bazer, T. Spencer, and P. Lonergan Alterations in expression of endometrial genes coding for proteins secreted into the uterine lumen during conceptus elongation in cattle. BMC Genomics 14(1):321. Herlihy, M. M., D. P. Berry, M. A. Crowe, M. G. Diskin, and S. T. Butler Evaluation of protocols to synchronize estrus and ovulation in seasonal calving pasture-based dairy production systems. Journal of Dairy Science 94(9): Herlihy, M. M., M. A. Crowe, D. P. Berry, M. G. Diskin, and S. T. Butler Factors associated with fertility outcomes in cows treated with protocols to synchronize estrus and ovulation in seasonal-calving, pasture-based dairy production systems. Journal of Dairy Science 96(3): Herlihy, M. M., M. A. Crowe, M. G. Diskin, and S. T. Butler Effects of synchronization treatments on ovarian follicular dynamics, corpus luteum growth, and circulating steroid hormone concentrations in lactating dairy cows. Journal of Dairy Science 95(2): Herzog, K., M. Brockhan-Lüdemann, M. Kaske, N. Beindorff, V. Paul, H. Niemann, and H. Bollwein Luteal blood flow is a more appropriate indicator for luteal function during the bovine estrous cycle than luteal size. Theriogenology 73(5): Hutchinson, I. A., R. J. Dewhurst, A. C. O. Evans, P. Lonergan, and S. T. Butler Effect of grass dry matter intake and fat supplementation on progesterone metabolism in lactating dairy cows. Theriogenology 78(4): Larson, S. F., W. R. Butler, and W. B. Currie Pregnancy rates in lactating dairy cattle following supplementation of progesterone after artificial insemination. Animal Reproduction Science 102(1-2): Lemley, C. O., S. T. Butler, W. R. Butler, and M. E. Wilson Short Communication: Insulin Alters Hepatic Progesterone Catabolic Enzymes Cytochrome P450 2C and 3A in Dairy Cows. Journal of Dairy Science 91(2): Lemley, C. O., K. A. Vonnahme, L. R. Tager, K. M. Krause, and M. E. Wilson Diet-induced alterations in hepatic progesterone (P4) catabolic enzyme activity and P4 clearance rate in lactating dairy cows. Journal of Endocrinology 205(3):
129 Lemley, C. O., T. A. Wilmoth, L. R. Tager, K. M. Krause, and M. E. Wilson Effect of a high cornstarch diet on hepatic cytochrome P450 2C and 3A activity and progesterone half-life in dairy cows. Journal of Dairy Science 93(3): Lonergan, P Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 76(9): Lopes, A. S., S. T. Butler, R. O. Gilbert, and W. R. Butler Relationship of preovulatory follicle size, estradiol concentrations and season to pregnancy outcome in dairy cows. Animal Reproduction Science 99(1 2): Lüttgenau, J., S. E. Ulbrich, N. Beindorff, A. Honnens, K. Herzog, and H. Bollwein Plasma progesterone concentrations in the mid-luteal phase are dependent on luteal size, but independent of luteal blood flow and gene expression in lactating dairy cows. Animal Reproduction Science 125(1-4): Macmillan, K. L. and A. J. Peterson A new intravaginal progesterone releasing device for cattle (CIDR-B) for oestrous synchronisation, increasing pregnancy rates and the treatment of post-partum anoestrus. Animal Reproduction Science 33(1-4):1-25. Mann, G. E Corpus luteum size and plasma progesterone concentration in cows. Animal Reproduction Science 115(1-4): McNeill, R. E., M. G. Diskin, J. M. Sreenan, and D. G. Morris Associations between milk progesterone concentration on different days and with embryo survival during the early luteal phase in dairy cows. Theriogenology 65(7): Murray, M Microsomal cytochrome P450-dependent steroid metabolism in male sheep liver. Quantitative importance of 6β-hydroxylation and evidence for the involvement of a P450 from the IIIA subfamily in the pathway. Journal of Steroid Biochemistry and Molelcular Biology. 38(5): Murray, M Participation of a cytochrome P450 enzyme from the 2C subfamily in progesterone 21-hydroxylation in sheep liver. Journal of Steroid Biochemistry and Molelcular Biology. 43(6): Nascimento, A. B., R. W. Bender, A. H. Souza, H. Ayres, R. R. Araujo, J. N. Guenther, R. Sartori, and M. C. Wiltbank. 2013a. Effect of treatment with human chorionic gonadotropin on day 5 after timed artificial insemination on fertility of lactating dairy cows. Journal of Dairy Science 96(5):
130 Nascimento, A. B., A. H. Souza, J. N. Guenther, F. P. Costa, R. Sartori, and M. C. Wiltbank. 2013b. Effects of treatment with human chorionic gonadotrophin or intravaginal progesterone-releasing device after AI on circulating progesterone concentrations in lactating dairy cows. Reprod. Fertil. Dev. 25(5): Penning, T. M., M. E. Burczynski, J. M. Jez, C. F. Hung, H. K. Lin, H. Ma, M. Moore, N. Palackal, and K. Ratnam Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochemical Journal. 351(1): R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL Reynolds, C. K., P. C. Aikman, B. Lupoli, D. J. Humphries, and D. E. Beever Splanchnic Metabolism of Dairy Cows During the Transition From Late Gestation Through Early Lactation. Journal of Dairy Science 86(4): Rizos, D., F. Carter, U. Besenfelder, V. Havlicek, and P. Lonergan Contribution of the female reproductive tract to low fertility in postpartum lactating dairy cows. Journal of Dairy Science 93(3): Rizos, D., S. Scully, A. K. Kelly, A. D. Ealy, R. Moros, P. Duffy, A. Al Naib, N. Forde, and P. Lonergan Effects of human chorionic gonadotrophin administration on Day 5 after oestrus on corpus luteum characteristics, circulating progesterone and conceptus elongation in cattle. Reprod. Fertil. Dev. 24(3): Sangsritavong, S., D. K. Combs, R. Sartori, L. E. Armentano, and M. C. Wiltbank High Feed Intake Increases Liver Blood Flow and Metabolism of Progesterone and Estradiol-17β in Dairy Cattle. Journal of Dairy Science 85(11): Sartori, R., J. M. Haughian, R. D. Shaver, G. J. M. Rosa, and M. C. Wiltbank Comparison of Ovarian Function and Circulating Steroids in Estrous Cycles of Holstein Heifers and Lactating Cows. Journal of Dairy Science 87(4): SAS Institute SAS User s Guide: Statistics. SAS Institute Inc., Cary, NC. Senger, P. L Pathways to pregnancy and parturition. Current Conceptions, Inc., Pullman, Washington. Siddiqui, M. A. R., M. Almamun, and O. J. Ginther Blood flow in the wall of the preovulatory follicle and its relationship to pregnancy establishment in heifers. Animal Reproduction Science 113(1 4):
131 Stevenson, J. L., J. C. Dalton, J. E. P. Santos, R. Sartori, A. Ahmadzadeh, and R. C. Chebel Effect of Synchronization Protocols on Follicular Development and Estradiol and Progesterone Concentrations of Dairy Heifers. Journal of Dairy Science 91(8): Stronge, A. J. H., J. M. Sreenan, M. G. Diskin, J. F. Mee, D. A. Kenny, and D. G. Morris Post-insemination milk progesterone concentration and embryo survival in dairy cows. Theriogenology 64(5): Walker, C. G., M. D. Littlejohn, M. D. Mitchell, J. R. Roche, and S. Meier Endometrial gene expression during early pregnancy differs between fertile and subfertile dairy cow strains. Physiol. Genomics 44(1): Wiltbank, M. C Cell types and hormonal mechanisms associated with mid-cycle corpus luteum function. J. Anim. Sci. 72(7): Wiltbank, M. C., R. Sartori, M. M. Herlihy, J. L. M. Vasconcelos, A. B. Nascimento, A. H. Souza, H. Ayres, A. P. Cunha, A. Keskin, J. N. Guenther, and A. Gumen Managing the dominant follicle in lactating dairy cows. Theriogenology 76(9): Wolfenson, D., G. Inbar, Z. Roth, M. Kaim, A. Bloch, and R. Braw-Tal Follicular dynamics and concentrations of steroids and gonadotropins in lactating cows and nulliparous heifers. Theriogenology 62(6): Xu, Z. Z., L. J. Burton, and K. L. Macmillan Reproductive performance of lactating dairy cows following estrus synchronization regimens with PGF2α and progesterone. Theriogenology 47(3):
132 Chapter Five Follicular fluid and serum metabolites in Holstein cows are predictive of genetic merit for fertility 5.1 Preface At the time of thesis submission this chapter is in preparation for submission to a peerreviewed journal. Stephen Moore was the primary author and carried out the animal experiment, statistical analysis and drafted the manuscript. Aoife O Gorman and Lorraine Brennan performed the metabolite analysis and receiver operating characteristic curve analysis. Trudee Fair and Stephen Butler conceived, designed and coordinated the study. All authors interpreted the data and contributed to the manuscript. Formatting and reference style has been edited for consistency throughout the thesis. Figure and table captions have been assigned with a chapter prefix. Acknowledgements have been removed. 114
133 5.2 Abstract The objectives of this study were: (i) to characterize the fatty acid and amino acid profile of follicular fluid and serum on day 7 of the oestrous cycle in dairy cows with similar genetic merit for milk production but with extremes of good (Fert+) or poor (Fert-) genetic merit for fertility; and (ii) to identify potential biomarkers of fertility in dairy cows. Twenty-eight lactating dairy cows (15 Fert+, 13 Fert-) and seven nonlactating dairy cows (3 Fert+, 4 Fert-) were enrolled in an ovulation synchronization protocol. All cows were fed a total mixed ration diet during the study. On day 7 of the oestrous cycle (0 = oestrus), follicular fluid from the first wave dominant follicle was collected by transvaginal follicular aspiration and serum was collected by coccygeal venepuncture. Day 7 was selected to enable collection of a sufficient volume of follicular fluid. Metabolites were extracted from follicular fluid and serum, and analysed by gas chromatography mass spectrometry. Statistical analysis was performed on the data by mixed model procedures to identify metabolites significantly different between Fert+ and Fert- cows, and subsequently by receiver operating characteristic curve analysis to determine the predictive value of these metabolites. The five most abundant fatty acids in follicular fluid (linoleic acid, stearic acid, oleic acid, palmitic acid and α-linolenic acid) were not affected by genotype; however, alterations to the abundance of nine fatty acids (arachidic acid, heneicosanoic acid, myristic acid, behenic acid, myristoleic acid, heptadecenoic acid, cis-11-eicosanoic acid, nervonic acid and γ- linolenic acid) that collectively accounted for 2.4% of the total fatty acid content may reflect small but important differences to the follicular microenvironment. In serum, greater abundance of total polyunsaturated fatty acids and n-6 polyunsaturated fatty acids in Fert+ cows, and greater abundance of total saturated fatty acids in Fert- cows represented the most pronounced effect of genotype in the study. Follicular fluid concentrations of cysteine, leucine, ornithine, proline and tyrosine and serum concentrations of asparagine, creatinine, cysteine, methionine, proline and valine were affected by genotype. Receiver operating characteristic curve analysis indicated that the follicular fluid and serum fatty acids and follicular fluid amino acids that were significantly affected by genotype were potential candidates to successfully predict fertility genotype. The results indicated unique differences in the follicular fluid and serum metabolite profiles between Fert+ and Fert- cows that were none the less, highly predictive of fertility genotype. 115
134 5.3 Introduction Poor oocyte quality has been implicated as a contributor to low pregnancy rates in dairy cattle, as greater pregnancy rates have been achieved in lactating dairy cows following embryo transfer compared with artificial insemination in the animals (Putney et al., 1989, Ambrose et al., 1999, Vasconcelos et al., 2006). Rates of fertilization failure in dairy cows are reported to range from 0-45% (Sartori et al., 2002). Even when fertilization is achieved, however, poor oocyte competence is associated with compromised development of the subsequent embryo (Rahman et al., 2012, Demyda- Peyrás et al., 2013). Circulating concentrations of glucose and non-esterified fatty acids (NEFA) fluctuate during the transition from pregnancy to lactation; however, greater circulating glucose concentrations and lesser circulating NEFA concentrations during this time are associated with cows that became pregnant to first insemination (Garverick et al., 2013). Greater concentrations of NEFA and β-hydroxybutyrate (BHBA) in follicular fluid are associated with reduced steroidogenesis and proliferation of follicular thecal cells, and in vitro studies have demonstrated detrimental effects on oocyte quality (Leroy et al., 2005, Vanholder et al., 2006). In vitro maturation studies have reported that the predominant circulating NEFAs (oleic, palmitic and stearic acid) had a negative impact on oocyte meiotic and cytoplasmic maturation, fertilization, blastocyst formation and embryo quality (Van Hoeck et al., 2011). It is likely that this is due to the high lipid content (McKeegan and Sturmey, 2011) and high RNA and protein synthetic capacity of bovine oocytes during maturation (Tomek et al., 2002), which appear to be altered by exposure to these metabolites (Aardema et al., 2011, Van Hoeck et al., 2013). The relationship between fertility and amino acid profiles in serum and follicular fluid has received much less attention than fatty acid profiles. Amino acids are, however, of interest to dairy cow fertility because they are utilized by oocytes (Gu et al., 2015) and some, e.g. leucine and methionine, have been reported to affect oocyte competence (Rooke et al., 2009) and neutrophil function (Osorio et al., 2013), and cause alterations to the embryo transcriptome (Peñagaricano et al., 2013). Interestingly, oocyte developmental competence can also be predicted from the fatty acid and amino acid profiles of bovine follicular fluid (Matoba et al., 2014) or from the amino acid profile of in vitro maturation media (Hemmings et al., 2012). The 116
135 objectives of this paper were (i) to characterize the fatty acid and amino acid composition of follicular fluid and serum collected on d 7 of the oestrous cycle from dairy cows with similar genetic merit for milk production, but either good (Fert+) or poor (Fert-) genetic merit for fertility; and (ii) to identify potential biomarkers of fertility in dairy cows. The hypothesis was that differences in the follicular fluid and serum metabolite profile between Fert+ and Fert- cows were predictive of fertility genotype. 117
136 5.4 Materials and Methods Animal Model A lactating cow genetic model of fertility was established in Teagasc Moorepark to elucidate the mechanisms responsible for poor fertility in lactating Holstein dairy cows (Cummins et al., 2012). Briefly, heifers with >75% Holstein genetics with either extreme positive (i.e., poor fertility; Fert-) or negative (i.e., good fertility; Fert+) estimated breeding values (EBV) for calving interval were selected from the national dairy cattle database. Within the Irish national herd, the selected heifers were representative of the top 25% in genetic merit for milk production. Fert- heifers were representative of the bottom 5% in genetic merit for calving interval, whereas Fert+ heifers were representative of the top 20% in genetic merit for calving interval. Eighteen Fert+ cows (3 non-lactating and 15 lactating; n = 1, 6, 11 in parity 1, 2 and 3, respectively and 17 Fert- cows (4 non-lactating and 13 lactating; n = 2, 11 and 3 in parity 1, 2 and 3, respectively, were enrolled in the study. The EBV s of the cows from both genotypes are summarized in Table 1. Fert+ and Fert- cows were represented by 5 and 12 sires, respectively. The maximum and minimum number of daughters from an individual sire for Fert+ and Fert- cows was 6 and 2, and 2 and 1, respectively. The experimental procedures involving animals on both studies were licensed by the Department of Health, Ireland, in accordance with the Cruelty to Animals Act (Ireland 1876) and the European Community Directive 86/609/EEC Feed and Management System The study was undertaken at Teagasc Moorepark from November 2010 to March Lactating and non-lactating cows were enrolled in this study. The mean calving dates were November 2 (SD ± 37.1 days) and November 6 (SD ± 37.9 days) 2010 for the lactating Fert+ and Fert- cows, respectively. The parity structure for the Fert+ cows was 4 and 11 cows in second and third lactation, respectively. The parity structure for the Fert- cows was 9 and 4 cows in second and third lactation, respectively. Following parturition, lactating cows were housed as one group in a freestall barn and fed a total mixed ration ad libitum plus five kg lactating cow concentrate per day at the a.m. and p.m. milkings. Non-lactating cows that did not conceive during the previous breeding season were housed as one group in the same freestall barn and fed a total mixed ration 118
137 ad libitum. The mean calving dates were February 18 (SD ± 38) and April 5 (SD ± 100) 2009 for the non-lactating Fert+ and Fert- cows, respectively. The parity structure for the Fert+ cows was 1 and 2 cows in first and second lactation, respectively. The parity structure for the Fert- cows was 2 and 2 cows in first and second lactation, respectively. Feed refusals were removed every second day. Diet ingredients were sampled weekly and composited monthly for analysis (Table 5.2). 119
138 Table 5.1. The mean estimated breeding value 1 (and SD) for both genotypes based on their sire, maternal grandsire and maternal grand grand-sire estimated breeding values Genotype 2 Fert+ cows Fert- cows Variable Non-lactating Lactating Non-lactating Lactating No. of animals Holstein (%) 93.8 (3.2) 90.0 (7.2) 90.6 (9.4) 94.0 (13.5) Milk (kg) 279 (119) 363 (154) (120) 434 (125) Fat (kg) 15.8 (3.7) 20 (6.2) 16.3 (2.3) 16.3 (6.6) Fat (g/kg) 1.0 (1.4) 1.0 (0.8) -0.4 (0.6) 0.02 (1.0) Protein (kg) 12.3 (1.1) 15.1 (5.7) 19.1 (5.6) 16.1 (5.9) Protein (g/kg) 0.6 (0.6) 0.4 (0.6) 0.4 (1.0) 0.2 (0.8) Survival (%) 3.78 (0.76) 3.5 (0.96) (1.4) -3.1 (1.08) Calving Interval (d) -6.2 (1.3) -6.0 (1.5) 8.5 (2.04) 8.74 (2.9) Sire calving interval (d) -8.7 (2.9) (1.9) 8.7 (3.9) 10.8 (3.7) Maternal grandsire calving interval (d) -6.2 (0.5) -6.0 (2.1) 12.5 (3.2) 12.7 (5.4) 1 PTA values were obtained from the Autumn 2012 official dairy evaluations published by the Irish Cattle Breeding Federation and multiplied by 2 to convert to EBV. Individual cow EBV were determined using the following formula: 0.5*sireEBV *MGsireEBV *MGGsireEBV 2 Fert+ = good-fertility cows; Fert- = poor-fertility cows 120
139 Table 5.2. Ingredient and nutrient composition of non-lactating and lactating cow diet Non-lactating cow diet (% DM) Grass silage 76 Straw 14 Concentrate 10 Dry cow concentrate ingredients (% fresh) Barley 25 Soya hulls 15 Rapeseed 15 Distillers 6 Citrus pulp 6 Minerals 2.5 Sunflower meal 7.5 Palm kernel meal 10 Milk Solids 8 Maize gluten 4.4 Oil 0.6 Lactating cow diet (% DM) Maize silage 36 Grass silage 33 Parlour concentrate 22 Soyabean meal 9 Lactating cow concentrate ingredients (% fresh) Wheat 25 Soyabean 15 Rapeseed extract 10 Sunflower seed extract 10 Palm kernel meal 10 Milk solids 5 Maize gluten 5 Citrus pulp 5 Soyabean hulls 5 Palm oil 4 Oat feed 4 Minerals 1 2 Nutrient composition concentrate (DM basis) Non-lactating Lactating DM (g/kg) Net energy (UFL/kg DM) Ash (g/kg DM)Crude protein (g/kg DM) Crude protein (g/kg DM) NDF (g/kg DM) Vitamin and Mineral mix: 229 g/kg of Ca, 100 g/kg of Na, 70 g/kg of P, 5 g/kg of Mg, 4500 mg/kg of Zn, 3000 mg/kg of Cu, 1500 mg/kg of Mn, 500 mg/kg of I, 400 mg/kg Bioplex* Cu, 400 mg/kg of Bioplex* Zn, 99 mg/kg of Co, 37 mg/kg of Se, IU/kg of vitamin A, IU/kg of vitamin D3, 1250 mg/kg of vitamin E and 200 mg/kg of vitamin B12. *Alltech Inc., Nicholasville, KY 121
140 5.4.3 Animal Measurements Cows were milked twice daily at 0800 and 1600 hours. Milk yield was recorded at each milking using electronic milk meters (Dairymaster, Causeway, Co. Kerry, Ireland). Milk composition (fat, protein and lactose) was determined weekly from successive evening and morning samples by mid-infrared reflectance spectroscopy (FT6000 Milkscan instrument, Foss Electric, Hillerød, Denmark). Body weight and body condition score were recorded weekly. Body condition score was assessed using the 1 to 5 scale in 0.25 increments (Edmonson et al., 1989) Ovulation Synchronisation Cows were enrolled in an ovulation synchronization protocol as described previously (Herlihy et al., 2012). The mean days postpartum when cows were enrolled in the protocol was 61 ± 15 and 60 ± 13 for the Fert+ and Fert- lactating cows, respectively, and 632 ± 38 and 586 ± 100 for the Fert+ and Fert- non-lactating cows, respectively. On d 0 of the protocol, each cow was administered an intramuscular injection of a GnRH agonist containing 10 μg of buserelin (Receptal; Intervet Ireland, Dublin, Ireland), and a controlled internal drug release device containing 1.38 g of progesterone (P4; CIDR, Pfizer Ireland, Dublin, Ireland) was inserted per vaginum. On day 7, each cow was administered an intramuscular injection of PGF 2α containing 25 mg of dinoprost tromethamine (Lutalyse, Pfizer Ireland). On day 8, the CIDR device was removed and 36 h later, each cow was administered a second intramuscular injection of GnRH agonist Blood Sampling Blood samples were collected on day 7 relative to synchronized oestrus (day 0) by coccygeal venepuncture into serum vacutainers (Becton Dickinson, Plymouth, UK), kept at room temperature for 30 min and centrifuged at 2000 x g for 10 min at 4 C; serum was decanted and stored at -80 C Ovarian Ultrasonography Transrectal ultrasound examination of both ovaries was carried out on day 7 relative to the synchronized oestrus. The largest follicle was examined using a Voluson i ultrasound machine (GE Healthcare, Austria, Germany) equipped with a 12 MHz linear array probe. Initially, the follicle was visualized in B-mode, and three images at its 122
141 maximum diameter were captured and stored for further analysis. Ultrasound machine settings were then switched to power Doppler mode to measure follicle blood flow area. At its largest apparent size, the entire follicle was fitted within a Doppler sample box and scanned for several seconds. Three images deemed representative of maximum blood flow with no flash artefacts were captured and stored for further analysis Ultrasound Image Analysis The diameter of the follicle measured on day 7 was calculated as the mean of three cross sectional diameters measured from each image. Power Doppler images were analysed using the computer programme Pixel Flux (Version 1.0, Chameleon Software, Leipzig, Germany) to quantify the area in cm 2 of coloured pixels within the follicle, which is considered a semi-quantitative measure of blood flow. Mean blood flow area of each structure was calculated using the three images Follicular Fluid Sampling After ultrasonography of the largest follicle, follicular fluid was aspirated using an Esaote Pie medical scanner equipped with a multi-angle ovum pick-up probe (Pie Medical Equipment B.V., Maastricht, the Netherlands) as described by Bols et al. (1995). Cows were sedated with an intravenous injection of xylazine (1 mg/100 kg body weight) and caudal epidural anaesthesia was induced with 4 ml of 2% lidocaine. Faeces were evacuated from the rectum and the vulva and perineal area were sanitized with antiseptic solution and dried with paper towels. The probe was inserted into the vagina so that the transducer face was applied to the vaginal wall. The ovary with the largest follicle was manipulated against the vaginal wall and over the transducer face so that the follicle was visible on the monitor. A disposable 19 G hypodermic needle was guided through the vaginal wall to puncture the follicular wall. The follicular fluid was aspirated using a foot-operated suction pump at 44 mm Hg into a sterile 50 ml conical tube (Becton Dickinson, Plymouth, UK). The tube was immediately placed on ice and transported to the laboratory for processing. The collection tubing was washed with double-distilled H 2 O between each cow. In the laboratory, the aspirate was transferred to sterile 1.5 ml eppendorf tubes (Eppendorf, UK) and centrifuged at 3,500 g x 30 min at 4 C. Aliquots of 200 µl were transferred to 1.5 ml eppendorf tubes, snap frozen in liquid nitrogen and stored at -80 C. 123
142 5.4.9 Reproductive Hormone Analysis Circulating and follicular fluid P4 concentrations were determined in samples collected from d 1 to 7.5 after synchronized oestrus relative to CIDR removal using a commercially available solid-phase radioimmunoassay (Coat-A-Count Progesterone, Diagnostic Products Corp., Los Angeles, CA). The inter-and intra-assay CV s for plasma were 17.2% and 11.1%, 10.0% and 8.3% and 9.4% and 6.9% for the low, medium and high P4 pools, respectively. The intra-assay CV s for follicular fluid were 10.9% and 6.0% for the low and high P4 pools respectively. Follicular fluid E2 concentrations were determined in samples by radioimmunoassay following extraction using E2 MAIA kits (Bio-Stat Diagnostic Systems Ltd. Stockport, Cheshire, UK). The intra-assay CV was 20.0% and 7.8% for the low and high E2 pools respectively. Follicular fluid cholesterol concentrations were determined by enzymatic colorimetry using an RX imola clinical biochemistry analyser (Randox Laboratories, Crumlin, UK; cholesterol kit supplied by Randox Laboratories). Both genotypes were represented equally in each hormone assay, and all samples for an individual cow were completed within one assay Metabolite Extraction and Data Analysis Follicular fluid and serum samples were thawed on ice before analysis. For analysis of organic compounds, 200 µl of follicular fluid or serum was combined with 20 µl of nonadecanoic acid (C19:0) (1 mg/ml methanol) as an internal standard and extracted using a 1:2 mixture of chloroform:methanol based on the method of Bligh and Dyer (1959). Extracts were derived by methylation using methanolic BF 3 (BF 3 /MeOH, 14% w/v). Derivatives were re-suspended in 200 µl of hexane and 1 µl was injected into the GC/MS. The GC temperature was initially 70 C for 2 min, increased by 15 C/min to 190 C and held for 9 min, then increased by 5 C/min to 230 C and held for 13 min and finally raised to 320 C by 20 C/min and held for 10 min. Aqueous compounds were isolated using a methanol extraction (Jiye et al., 2008) following deproteinization with acetonitrile. Briefly, 200 µl of deproteinized sample was combined with 20 µl of 13 C myristic acid (Cambridge Isotopes, Andover, MA, USA) as an internal standard before extraction with 800 µl methanol. Extracts were dried and samples were methoximized using 60 µl of methoxamine hydrochloride (20 mg/ml in pyridine) for 17 hours at room temperature prior to silylation with 60 µl of N-methyl-N-(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane for 1 124
143 hour. Samples were diluted with 200 µl of hexane and analysed by gas chromatography mass spectrometry (GC/MS). The GC/MS system was comprised of an Agilent 7890A GC coupled with a 5975C MS. The GC temperature was initially 70 C for 2 min, increased by 5 C/min to 260 C, held for 41 min and finally raised to 320 C by 30 C/min and held for 3 min. After a solvent delay of 1 min full scan, mass spectra were recorded within a scan range of atomic mass units (amu) at 2 scans/second. Calibration was achieved by comparison of peak areas for amino acids and fatty acids with known reference standards (Amino acid standard A9906 and Supelco 37 component FAME mix, Sigma Aldrich, Ireland) using Agilent Chemstation (MSD E ) and by comparison of their mass spectra with those in the National Institute of Standards and Technology (NIST) Library 2.0 (2005). For quality control purposes, one aliquot from a pool of follicular fluid/serum was extracted and analysed in parallel with each batch of samples. Automatic peak detection was carried out with Agilent Chemstation MSD. Mass spectra deconvolution was performed with Automated Mass Spectral Deconvolution and Identification System (AMDIS, version 2.65). Peaks with a signal-to-noise (S/N) ratio lower than 30 were rejected, which is an acceptable level to avoid false positives as reported by Norli et al. (2010). To obtain accurate peak areas for the internal standard and specific peaks/compounds, one quant mass for each peak was specified as the target ion and three masses were selected as qualifier ions. Each data file was manually analysed for false positives/negatives in Agilent Chemstation. Fatty acids are reported as a percentage of the total fatty acid concentration. Amino acid concentrations are reported as µmol/ml. Total saturated fatty acid (SFA) fraction, total monounsaturated fatty acid (MUFA) fraction and total polyunsaturated fatty acid (PUFA) fraction, indices of desaturase enzyme activity in C16 fatty acids ( 9 - desat (16)) and in C18 fatty acids ( 9 -desat (18)), and elongase enzyme activity in chain lengthening from C16 to C18 fatty acids were calculated using previously published equations (Malau-Aduli et al., 1998) Statistical Analysis All data were statistically analysed using SAS (SAS Institute, 2006). Data were checked for normality. Appropriate Box-Cox transformations were identified to normalize the distribution of data where necessary. Mixed model procedures with genotype as a fixed 125
144 effect and cow nested within genotype as a random effect were used. Parity, calving date, block, lactation status (lactating or non-lactating), synchronization status (oestrous cycle synchronized or not synchronized), follicle status (oestrogenic or not oestrogenic; determined by follicular fluid E2:P4 ratio) and the interaction of parity with genotype were included as fixed effects initially, but removed if not significant (P > 0.1). Effects were deemed significant if P 0.05 and tending to be significant if P 0.1. The inclusion of lactating and non-lactating cows in the study allowed the effect of lactation status to be tested. Receiver operating characteristic (ROC) curve analysis was performed on the fatty acid and amino acid data, separately and in combination, using the ROCCET: ROC Curve Explorer & Tester software ( ROC curves are generally considered the method of choice for evaluating the performance of potential biomarkers (Xia et al., 2013). The classification performance (sensitivity and specificity) of the samples was assessed by the area under the curve (AUC) as follows: = excellent; = good; = fair; = poor; and = fail (Xia et al., 2013). 126
145 5.5 Results General Characteristics of the Cows and the Largest Follicle There was no effect of genotype on milk yield (mean = 29.8 kg/day; P = 0.9), milk solids yield (mean = 2.28 kg/day; P = 0.4), body weight (mean = 610 kg; P = 0.9) and body condition score (mean = 2.75 units; P = 0.4). Follicle diameter was greater in Fert- cows compared with Fert+ cows (P = 0.03), but there was no effect of genotype on follicle blood flow area (Table 5.3). Follicular fluid E2 concentrations tended to be greater in Fert+ cows (P = 0.09) compared with Fert- cows. Follicular fluid P4 concentrations, E2:P4 ratio and cholesterol concentrations were similar in both genotypes (all P > 0.6). It was not possible to determine the E2, P4 and cholesterol concentrations in all follicular fluid samples due to the initial volume of follicular fluid collected. 127
146 Table 5.3. The effect of genetic merit for fertility on characteristics of the largest follicle on day 7 of the oestrous cycle Genotype Variable Fert+ (n) Fert- (n) SEM 1 P-value Diameter (mm) (18) (16) Blood flow area (cm 2 ) 0.19 (17) 0.21 (16) Estradiol concentration (pg/ml) 3.83 (13) 2.95 (8) Progesterone concentration (ng/ml) 0.61 (12) 0.64 (6) Estradiol/progesterone ratio 6.80 (12) 6.09 (6) Cholesterol concentration (mmol/l) 1.39 (13) 1.32 (6) = pooled standard error 128
147 5.5.2 Fatty Acid Profiles Composition of Follicular Fluid A total of 27 fatty acids were identified in the follicular fluid (Table 5.4). The mean total fatty acid concentration in follicular fluid samples was 810 µg/ml and was similar in both genotypes (P = 0.3). Genetic merit for fertility affected four SFA (arachidic acid, heneicosanoic acid, myristic acid and behenic acid), four MUFA (myristoleic acid, heptadecenoic acid, cis-11- eicosanoic acid and nervonic acid) and one PUFA (γlinolenic acid), all of which were greater in Fert- cows compared with Fert+ cows. The seven fatty acids significantly affected by genotype accounted for 1.7% of the total fatty acid content. The five most abundant fatty acids (abundance in parentheses) were linoleic acid (43%), stearic acid (14%), oleic acid (12%), palmitic acid (11%) and α- linolenic acid (6%). Collectively, these fatty acids accounted for 86% of the total fatty acid content, but none were affected by genotype (all P > 0.1). Genetic merit for fertility had no effect on total SFA, total MUFA and total PUFA. ROC curve analysis using the fatty acids that were significantly different (P 0.05; Table 5.4) resulted in an AUC of 0.95 (Figure 5.1a) for discriminating between Fert+ and Fert- cows. This favourable AUC value indicates that these fatty acids had high predictive ability for fertility genotype. 129
148 130
149 Figure 5.1 (a-d): ROC curves produced using (a) significant follicular fluid fatty acids (AUC = 0.95; 95% CI = ) (b) significant serum fatty acids (AUC = 0.86; 95% CI = ) (c) significant follicular fluid amino acids (AUC = 0.85; 95% CI = ) and (d) significant serum amino acids (AUC = 0.72; 95% CI = ). ROC: receiver operating characteristic. AUC: area under the curve. CI: confidence interval 131
150 Table 5.4. The effect of genetic merit for fertility on the fatty acid composition of follicular fluid. Values are expressed as percentage of the total fatty acid concentration (%) 1. Genotype Variable Fert+ Fert- P-value n Myristoleic acid (C14:1) 0.03 ( ) 0.06 ( ) Myristic acid (C14:0) 0.56 ( ) 0.62 ( ) 0.07 Pentadecenoic acid (C15:1) 0.08 ( ) 0.1 ( ) NS Pentadecanoic acid (C15:0) 0.60 ( ) 0.60 ( ) NS Palmitoleic acid (C16:1) 1.85 ( ) 1.78 ( ) NS Palmitic acid (C16:0) ( ) ( ) NS Heptadecenoic acid (C17:1) 0.53 ( ) 0.67 ( ) 0.01 Heptadecanoic acid (C17:0) 0.72 ( ) 0.76 ( ) NS γ-linolenic acid (C18:3n6) 0.64 ( ) 1.06 ( ) Linoleic acid (C18:2n6) ( ) ( ) NS α-linolenic acid (C18:3n3) 6.25 ( ) 6.16 ( ) NS Oleic acid (C18:1n9c) ( ) ( ) NS Stearic acid (C18:0) ( ) ( ) NS Arachidonic acid (C20:4n6) 2.49 ( ) 2.55 ( ) NS Eicosapentaenoic acid (C20:5n3) 0.97 ( ) 1.10 ( ) NS Dihomo-γ-linolenic acid (C20:3n6) 2.11 ( ) 2.06 ( ) NS cis-eicosadienoic acid (C20:2) 0.76 ( ) 0.78 ( ) NS cis-11-eicosanoic acid (C20:1) 0.05 ( ) 0.13 ( ) < Eicosatrienoic acid (C20:3n3) 0.02 ( ) 0.01 ( ) NS Arachidic acid (C20:0) 0.06 ( ) 0.08 ( ) Heneicosanoic acid (C21:0) ( ) ( ) Docosahexaenoic acid (C22:6n3) 0.21 ( ) 0.21 ( ) NS Erucic acid (C22:1n9) 0.01 ( ) 0.02 ( ) NS Behenic acid (C22:0) 0.09 ( ) 0.11 ( ) 0.09 Tricosanoic acid (C23:0) 0.18 ( ) 0.17 ( ) NS Nervonic acid (C24:1) 0.08 ( ) 0.11 ( ) Lignoceric acid (24:0) 0.22 ( ) 0.21 ( ) NS 132
151 Genotype Variable Fert+ Fert- P-value Total SFA ( ) ( ) NS Total MUFA ( ) ( ) NS Total PUFA ( ) ( ) NS (n-3)pufa 7.21 ( ) 7.64 ( ) NS (n-6)pufa ( ) ( ) NS (n-6):(n-3)pufa ratio 7.10 ( ) 6.43 ( ) NS PUFA:SFA ratio 2.03 ( ) 2.04 ( ) NS 9 -desaturase (16) ( ) ( ) NS 9 -desaturase (18) ( ) ( ) NS Elongase ( ) ( ) NS SFA = saturated fatty acid; MUFA = monounsaturated fatty acid; PUFA = Polyunsaturated fatty acid; (n-3)pufa = (C18:3n3 + C20:5n3 + C20:3n3 + C22:6n3). (n-6)pufa = (C18:3n6 + C18:2n6 + C20:4n6 + C20:3n6) 9 -desaturase (16) = index of desaturase activity in C16 fatty acids 100 ( 9 -desaturase (18) = index of desaturase activity in C18 fatty acids 100 ( C16:1 ). C16:1+C16:0 C18:1n9 ). C18:1n9+C18:0 Elongase = index of elongase index activity in chain lengthening of C16-C18 fatty acids 100 ( C18:1n9+C18:0 ). (C16:1+C16:0+C18:1n9+C18:0) 1 = data presented as LSM with 95% CI in parentheses 133
152 Composition of Serum A total of 27 fatty acids were identified in serum (Table 5.5). The mean total fatty acid concentration in follicular fluid samples was 2757 µg/ml and was similar in both genotypes (P = 0.5). Genetic merit for fertility affected two SFA (palmitic acid and heneicosanoic acid), three MUFA (pentadecenoic acid, heptadecenoic acid and nervonic acid) and four PUFA (linoleic acid, γ-linolenic acid, dihomo-γ-linolenic acid and ciseicosadienoic acid). The abundance of palmitic acid and nervonic acid was greater in Fert- cows, but the abundance of the five other fatty acids was greater in Fert+ cows. The five most abundant fatty acids (abundance in parentheses) were linoleic acid (37%), oleic acid (21%), stearic acid (12%), palmitic acid (12%) and α-linolenic acid (5%), which collectively accounted for 89% of the total fatty acid content. The fatty acids that were significantly affected by genotype accounted for 49% of the total fatty acid content. The proportion of fatty acids classified as saturated was greater in Fert- cows compared with Fert+ cows (P = 0.03). The proportion of fatty acids classified as polyunsaturated was greater in Fert+ cows compared with Fert- cows (P = 0.01); this was due to a greater proportions of n-6 PUFA (P = 0.04) rather than n-3 PUFA. The PUFA:SFA ratio (P = 0.02) was greater in Fert+ cows compared with Fert- cows, as was the 9 -desaturase activity in C16 fatty acids (P = ). ROC curve analysis using the fatty acids that were significantly different (P 0.05; Table 5.5) resulted in an AUC of 0.86 (Figure 5.1b) for discriminating between Fert+ and Fert- cows. This favourable AUC value indicates that these fatty acids had high predictive ability for fertility genotype. 134
153 Table 5.5. The effect of genetic merit for fertility on the fatty acid composition of serum. Values are expressed as percentage of the total fatty acid concentration (%) 1. Genotype Variable Fert+ Fert- P-value n Myristoleic acid (C14:1) 0.14 ( ) 0.14 ( ) NS Myristic acid (C14:0) 1.59 ( ) 1.87 ( ) NS Pentadecenoic acid (C15:1) 0.11 ( ) 0.08 ( ) 0.02 Pentadecanoic acid (C15:0) 0.43 ( ) ) NS Palmitoleic acid (C16:1) 1.35 ( ) 1.17 ( ) NS Palmitic acid (C16:0) 9.94 ( ) ( ) 0.01 Heptadecenoic acid (C17:1) 0.59 ( ) 0.46 ( ) 0.09 Heptadecanoic acid (C17:0) 0.89 ( ) 1.02 ( ) NS γ-linolenic acid (C18:3n6) 0.51 ( ) 0.25 ( ) Linoleic acid (C18:2n6) ( ) ( ) 0.03 α- Linolenic acid (C18:3n3) 5.95 ( ) 4.57 ( ) NS Oleic acid (C18:1n9c) ( ) ( ) NS Stearic acid (C18:0) 9.57 ( ) ( ) NS Arachidonic acid (C20:4n6) 2.44 ( ) 2.85 ( ) NS Eicosapentaenoic acid (C20:5n3) 0.7 ( ) 0.54 ( ) NS Dihomo-γ-linolenic acid (C20:3n6) 1.99 ( ) ) 0.07 cis-eicosadienoic acid (C20:2) 0.48 ( ) 0.39 ( ) 0.10 cis-11-eicosanoic acid (C20:1) 0.12 ( ) 0.12 ( ) NS Eicosatrienoic acid (C20:3n3) 0.04 ( ) 0.04 ( ) NS Arachidic acid (C20:0) 0.13 ( ) 0.13 ( ) NS Heneicosanoic acid (C21:0) 0.01 ( ) ( ) Docosahexaenoic acid (C22:6n3) 0.08 ( ) 0.06 ( ) NS Erucic acid (C22:1n9) 0.17 ( ) 0.19 ( ) NS Behenic acid (C22:0) 0.21 ( ) 0.23 ( ) NS Tricosanoic acid (C23:0) 0.23 ( ) 0.27 ( ) NS Nervonic acid (C24:1) 0.09 ( ) 0.15 ( ) Lignoceric acid (24:0) 0.13 ( ) 0.14 ( ) NS 135
154 Genotype Variable Fert+ Fert- P-value Total SFA ( ) ( ) 0.03 Total MUFA ( ) ( ) NS Total PUFA ( ) ( ) 0.01 (n-3)pufa 6.47 ( ) 5.25 ( ) NS (n-6)pufa ( ) ( ) 0.04 (n-6):(n-3)pufa ratio 5.95 ( ) 6.69 ( ) NS PUFA:SFA ratio 3.02 ( ) 2.19 ( ) desaturase (16) ( ) 7.90 ( ) desaturase (18) ( ) ( ) NS Elongase ( ) ( ) NS SFA = saturated fatty acid; MUFA = monounsaturated fatty acid; PUFA = Polyunsaturated fatty acid; (n-3)pufa = (C18:3n3 + C20:5n3 + C20:3n3 + C22:6n3). (n-6)pufa = (C18:3n6 + C18:2n6 + C20:4n6 + C20:3n6) 9 -desaturase (16) = index of desaturase activity in C16 fatty acids 100 ( 9 -desaturase (18) = index of desaturase activity in C18 fatty acids 100 ( C16:1 ). C16:1+C16:0 C18:1n9 ). C18:1n9+C18:0 Elongase = index of elongase index activity in chain lengthening of C16-C18 fatty acids 100 ( C18:1n9+C18:0 ). (C16:1+C16:0+C18:1n9+C18:0) 1 = data presented as LSM with 95% CI in parentheses 136
155 5.5.3 Amino Acid Profiles Composition of Follicular Fluid A total of 18 amino acids were identified in follicular fluid (Table 5.6). Genetic merit for fertility affected the concentrations of five amino acids. The concentrations of cysteine, leucine and ornithine were greater whereas the concentrations of tyrosine were lower and the concentrations of proline tended to be lower in Fert+ cows compared with Fert- cows. The five most abundant amino acids (abundance in parentheses) were alanine (16%), proline (12%), threonine (12%), valine (11%) and glutamine (10%), which collectively accounted for 61% of the total amino acid content. ROC curve analysis using the amino acid metabolites that were significantly different (P 0.05; Table 5.6) resulted in an AUC of 0.85 (Figure 5.1c) for discriminating between Fert+ and Fert- cows. This favourable AUC value indicates that these amino acids had high predictive ability for fertility genotype. 137
156 Table 5.6. The effect of genetic merit for fertility on the amino acid composition of follicular fluid. Genotype Variable Fert+ Fert- P-value n Alanine 0.82 ( ) 0.88 ( ) NS Asparagine 0.07 ( ) 0.06 ( ) NS Aspartic acid 0.01 ( ) 0.03 ( ) NS Creatinine 0.05 ( ) 0.04 ( ) NS Cysteine 0.07 ( ) 0.04 ( ) 0.03 Glutamine 0.53 ( ) 0.58 ( ) NS Glycine 0.42 ( ) 0.36 ( ) NS Isoleucine 0.33 ( ) 0.22 ( ) NS Leucine 0.53 ( ) 0.22 ( ) Lysine 0.05 ( ) 0.07 ( ) NS Methionine 0.03 ( ) 0.04 ( ) NS Ornithine 0.08 ( ) 0.02 ( ) < Phenylalanine 0.27 ( ) 0.46 ( ) NS Proline 0.57 ( ) 0.80 ( ) 0.09 Serine 0.39 ( ) 0.37 ( ) NS Threonine 0.94 ( ) 0.79 ( ) NS Tyrosine 0.01 ( ) 0.04 ( ) Valine 0.73 ( ) 0.28 ( ) NS 1 = data presented as LSM (µmol/ml) with 95% CI in parentheses 138
157 Composition of Serum A total of 18 amino acids were identified in serum (Table 5.7). Genetic merit for fertility affected the concentrations of six amino acids: creatinine, cysteine, methionine, proline and valine were greater in Fert+ cows compared with Fert- cows and the concentrations of asparagine tended to be greater in Fert- cows compared with Fert+ cows. The five most abundant amino acids (abundance in parentheses) were alanine (15%), proline (14%), valine (12%), glycine (10%) and leucine (9%), which collectively accounted for 60% of the total amino acid content. ROC curve analysis using the amino acid metabolites that were significantly different (P 0.05; Table 5.7) resulted in a low AUC value of 0.72 (Figure 5.1d). This AUC value indicates that these amino acids had poor predictive ability for fertility genotype. 139
158 Table 5.7. The effect of genetic merit for fertility on the amino acid composition of serum. Values are reported as means (µmol/ml) 1. Genotype Variable Fert+ Fert- P-value n Alanine 0.64 ( ) 0.67 ( ) NS Asparagine 0.06 ( ) 0.09 ( ) 0.08 Aspartic acid 0.07 ( ) 0.05 ( ) NS Creatinine 0.05 ( ) 0.03 ( ) 0.02 Cysteine 0.05 ( ) 0.02 ( ) Glutamine 0.52 ( ) 0.50 ( ) NS Glycine 0.41 ( ) 0.31 ( ) NS Isoleucine 0.35 ( ) 0.30 ( ) NS Leucine 0.38 ( ) 0.28 ( ) NS Lysine 0.05 ( ) 0.05 ( ) NS Methionine 0.05 ( ) 0.04 ( ) 0.05 Ornithine 0.02 ( ) 0.01 ( ) NS Phenylalanine 0.12 ( ) 0.12 ( ) NS Proline 0.87 ( ) 0.65 ( ) 0.04 Serine 0.31 ( ) 0.27 ( ) NS Threonine 0.37 ( ) 0.29 ( ) NS Tyrosine 0.04 ( ) 0.03 ( ) NS Valine 0.46 ( ) 0.35 ( ) = data presented as LSM (µmol/ml) with 95% CI in parentheses 140
159 5.6 Discussion This study reports on the effect of genetic merit for fertility on the fatty acid and amino acid profile of follicular fluid and serum collected from lactating and non-lactating Holstein dairy cows on day 7 of a synchronized oestrous cycle. That lactation status did not affect the variables examined in this study indicates that the metabolite profile of follicular fluid and serum is not dependent on lactation status. The differences identified may represent potential biomarkers of fertility. Follicular fluid proportions of total SFA, total PUFA and total MUFA were not affected by genotype; however, alterations to seven low-abundance fatty acids may reflect small but important differences to the follicular microenvironment. In serum, greater proportions of total PUFA and n-6 PUFA in Fert+ cows and a greater proportion of total SFA in Fert- cows represented the most pronounced effect of genotype in the current study. In agreement with the findings of Bender et al. (2010) and Aardema et al. (2015) the fatty acid and amino acid profiles were unique to follicular fluid and to serum, suggesting local control of the follicular microenvironment. Of the fatty acids that were affected by genotype in both follicular fluid and serum, only nervonic acid had a similar trend in both matrices (i.e., greater in Fert- cows compared with Fert+ cows). Conversely, heptadecenoic acid, γ-linolenic acid and heneicosanoic acid had opposite trends in follicular fluid and serum (i.e., greater in serum and lesser in follicular fluid, or vice versa). Previous studies have identified potential metabolite biomarkers of fertility in follicular fluid or serum using various models to represent high and low fertility in cattle or humans (Zeron et al., 2001, Bender et al., 2010, Matoba et al., 2014). It needs to be acknowledged, however, that differences in the fertility models investigated, the diets being fed, and seasonal variation make direct comparisons between studies difficult. While the models used were very diverse, a trend emerges from the comparison across the studies. Of particular note is the increase in SFA in poorer fertility models (Tables 5.8 and 5.9). Our study is the first to report on a model where all animals were exposed to the same environment, with identical management, nutrition and health protocols. Hence, any differences identified were due solely to differences in genetic merit for fertility between Fert+ and Fert- cows. 141
160 Table 5.8. Comparison of significant differences in follicular fluid and serum fatty acid concentrations across five studies Fatty acid Current study Bender 1 Zeron 2 Matoba 3 O'Gorman 4 C14:1 FF 100% Nd nd nd C14:0 FF 11% FF 95% ; S 45% Nd S nd S nd C15:1 S 38% Nd S nd nd C16:1 FF 222% FF 213% ; S nd S nd nd C16:0 S 39% FF 73% FF 35% ; S nd FF 44% ; S na FF 38% ; S nd C17:1 FF 26% ; S 28% Nd S nd nd C18:3n6 FF 66% ; S 104% FF 130% ; S 119% S nd S nd S nd C18:2n6 S 20% FF 359% ; S 189% S nd S nd S nd C18:3n3 FF 101% ; S 67% S nd FF 77% ; S nd S nd C18:1n9 FF 141% S nd S nd S nd C18:0 FF 73% FF 272% ; S nd S nd FF 26% ; S nd C20:4n6 FF 63% ; S nd S nd FF 28% ; S nd C20:5n3 FF 350% ; S nd S nd S nd C20:3n6 S 28% FF 63% ; S 92% S nd S nd FF 35% ; S nd C20:2 S 23% FF 259% ; S 30% S nd S nd S nd C20:1 FF 160% FF 136% ; S 63% FF 400% ; S nd nd S nd C20:0 FF 33% Nd S nd FF 58% ; S nd C21:0 FF 50% ; S 50% Nd nd nd C22:6n3 FF 138% ; S 220% FF 350% ; S nd S nd FF 96% ;S nd C22:1n9 FF 112% Nd nd FF 67% ; S nd C22:0 FF 22% FF 132% Nd nd S nd C23:0 FF 140% ; S 823% Nd nd FF 50% ; S na C24:1 FF 38% ; S 66% Nd nd nd C24:0 Nd nd FF 103% ; S nd 142
161 Fatty acid Current study Bender 1 Zeron 2 Matoba 3 O'Gorman 4 Total SFA S 25% FF74% Nd FF 42% ; S nd FF 19% ; S nd Total MUFA FF 140% Nd S nd S nd Total PUFA S 12% FF215% ; S 123% Nd S nd FF 29% ; S nd (n-3)pufa FF 51% Nd S nd FF 81% ; S nd (n-6)pufa S 12% FF 287% ; S 161% Nd S nd S nd (n-6):(n-3) PUFA ratio FF 139% ; S116% Nd S nd FF 43% ; S nd 1 = Current study; Fert- cows relative to Fert+ cows 2 = (Bender et al., 2010); Lactating cows relative to non-lactating heifers 3 = (Zeron et al., 2001); Summer season relative to winter season 4 = (Matoba et al., 2014); Degenerate embryos relative to blastocyst embryos 5 = (O'Gorman et al., 2013); Non-cleaved embryos relative to cleaved embryos = P 0.1 SFA = saturated fatty acid; MUFA = monounsaturated fatty acid; PUFA = Polyunsaturated fatty acid; (n-3)pufa = (C18:3n3 + C20:5n3 + C20:3n3 + C22:6n3). (n-6)pufa = (C18:3n6 + C18:2n6 + C20:4n6 + C20:3n6) FF = follicular fluid S = serum nd = Follicular fluid or serum fatty acid concentrations not determined in study S nd = Serum fatty acid concentration not determined in study 143
162 Table 5.9. Comparison of significant differences in follicular fluid and serum amino acid concentrations across three studies Amino acid Current study 1 Bender 2 Matoba 3 Alanine FF 75% ; S nd FF 54% Asparagine S 50% nd nd Creatinine S 66% nd nd Cysteine FF 75% ; S 75% nd S nd Glycine FF 71% FF 83% Glutamate nd FF 73% Glutamine FF 110% Leucine FF 141% nd S nd Methionine S 20 nd S nd Ornithine FF 300% nd nd Proline FF 40% ; S 34% nd S nd Tyrosine FF 2700% nd S nd Valine S 31% nd S nd 1 = Current study; Fert- cows relative to Fert+ cows 2 = (Bender et al., 2010); Lactating cows relative to non-lactating heifers 3 = (Matoba et al., 2014); Degenerate embryos relative to blastocyst embryos FF = Follicular fluid nd = Follicular fluid or serum amino acid concentrations not determined in study S nd = Serum amino acid concentration not determined in study 144
163 5.6.1 Characteristics of Fatty Acid Profiles In follicular fluid, the proportion of myristic acid, behenic acid and γ-linolenic acid were greater or tended to be greater in Fert- cows compared with Fert+ cows. These fatty acids were previously associated with poor fertility by Bender et al. (2010). cis-11- eicosanoic acid was greater in Fert+ cows compared with Fert- cows and was greater in follicular fluid collected in winter compared with summer (Zeron et al., 2001). Greater follicular fluid proportions of total saturated fatty acid, palmitic acid and palmitoleic acid have previously been documented in low fertility models (Zeron et al., 2001, Bender et al., 2010, O'Gorman et al., 2013, Matoba et al., 2014), but these fatty acids and fatty acid categories were not different in the current study. Four serum fatty acids or fatty acid categories were significantly associated with fertility in the current study. These were also previously reported by Bender et al. (2010) (total PUFA, n-6 PUFA, γ-linolenic acid and linoleic acid) but the direction of the relationship was completely reversed. This is likely due to: (i) the fundamental differences in the design of the fertility models; and (ii) the variation in lactation status, parity, diet and dry matter intake that existed between the lactating cows and nonlactating heifers reported in Bender et al. (2010). There is some disagreement in the literature on the effect of supplementary linoleic acid on fertility. Fouladi-Nashta et al. (2009) reported that changes in dietary intake of fatty acids was reflected in plasma and milk of lactating dairy cows, but fatty acids in granulosa cells were not affected, concluding that the ovary has the ability to buffer against major fluctuations in circulating fatty acids. In response to dietary supplementation of cows with calcium salts of linoleic and trans-octadecenoic acid, Juchem et al. (2010) reported greater prostaglandin F 2α metabolite concentrations on day one postpartum in primiparous cows and greater pregnancy rates in primi- and multi-parous cows. Cerri et al. (2009) reported greater fertilization rates, greater embryo quality and greater numbers of accessory sperm. Bilby et al. (2006) reported that cows supplemented with calcium salts of palm and soybean oil enriched with linoleic acid had no effect on oocyte quality but preovulatory follicle diameter and subsequent corpus luteum volume were greater. In contrast, Marei et al. (2010) reported negative consequences on oocyte maturation in vitro. The serum proportions of pentadecenoic acid, heptadecenoic acid and heneicosanoic acid were greater in Fert+ cows compared with Fert- cows, but nervonic 145
164 acid proportions were greater in Fert- cows. These fatty acids are a mixture of SFA and MUFA. They are lowly-abundant fatty acids in serum, and also lowly abundant in the diet of grazing or silage-fed cows (Mohammed et al., 2010). The mechanism responsible for these differences between Fert+ and Fert- cows is unclear, as is their potential involvement in reproductive function. Serum proportions of palmitic acid and total SFA were greater in Fert- cows compared with Fert+ cows. Palmitic acid is a major component of the mobilized NEFA fraction that is associated with compromised oocyte health in lactating dairy cows (Leroy et al., 2005). In the current study, Fert+ cows tended to have greater dry matter intake compared with Fert- cows (Moore et al., 2014). Greater palmitic acid concentrations in Fert- cows may be explained by slower passage rate through the rumen, allowing greater opportunity for biohydrogenation of palmitoleic acid to palmitic acid (Loften et al., 2014) Characteristics of Amino Acid Profiles Follicular fluid concentrations of leucine and cysteine were greater in Fert+ cows compared with Fert- cows. Positive associations between leucine concentrations and fertility have been reported in relation to in vitro maturation of oocytes (Hemmings et al., 2012) and development of embryos cultured in vitro (Rooke et al., 2009). Follicular fluid concentrations of proline tended to be 40% greater and tyrosine was 300% greater in Fert- cows compared with Fert+ cows, respectively, but to our knowledge have not previously been associated with fertility. Serum concentrations of cysteine and methionine were greater in Fert+ cows compared with Fert- cows. Positive associations between cysteine and fertility have been reported in relation to sperm function (Sarıözkan et al., 2009) and development of embryos cultured in vitro (Lott et al., 2011, Nabenishi et al., 2012). Also, methionine has been linked with alterations to immune function. Supplementation of dairy cows with methionine during the transition period increased the phagocytosis capability of blood neutrophils at day 21 postpartum (Osorio et al., 2013). Supplementation of dairy cows with methionine from calving until around day 70 postpartum resulted in altered gene expression in embryos associated with early embryo development and innate and adaptive immune response (Peñagaricano et al., 2013). 146
165 5.6.3 Ability of Metabolites to Predict Fertility Genotype The results of the ROC curve analysis indicated that the follicular fluid fatty acids achieved the most accurate classification of fertility genotype. Nevertheless, serum fatty acids also achieved a high AUC, and may be sufficient for determination of fertility status; serum has the considerable benefit of being easier to obtain than follicular fluid. Metabolomics-based approaches have previously identified panels of candidate biomarkers for the authentication of different beef production systems (Osorio et al., 2012) or prediction of disease occurrence during the transition period of dairy cows (Hailemariam et al., 2014). The Fert+/Fert- animal model used in this study consisted of a small number of animals that were representative of extremes in genetic merit for fertility in the Irish national herd. Additional studies are required to independently validate the association between these metabolites with dairy cow fertility. 5.7 Conclusions Alterations to the fatty acid and amino acid metabolome in serum and follicular fluid reflect the physiological status of dairy cows, with favourable and unfavourable consequences for their health and fertility. We identified alterations to fatty acids and amino acids in follicular fluid and serum that may explain, at least in part, the differences in reproductive performance reported in this genetic model. These biomarkers were highly predictive of the fertility genotype of the dairy cows used in the study, but they must be validated in an independent population. 147
166 5.8 References Aardema, H., B. M. Gadella, C. H. A. van de Lest, J. F. H. M. Brouwers, T. A. E. Stout, B. A. J. Roelen, and P. L. A. M. Vos Free fatty acid levels in fluid of dominant follicles at the preferred insemination time in dairy cows are not affected by early postpartum fatty acid stress. Journal of Dairy Science 98(4): Aardema, H., P. L. A. M. Vos, F. Lolicato, B. A. J. Roelen, H. M. Knijn, A. B. Vaandrager, J. B. Helms, and B. M. Gadella Oleic Acid Prevents Detrimental Effects of Saturated Fatty Acids on Bovine Oocyte Developmental Competence. Biology of Reproduction 85(1): Ambrose, J. D., M. Drost, R. L. Monson, J. J. Rutledge, M. L. Leibfried-Rutledge, M. J. Thatcher, T. Kassa, M. Binelli, P. J. Hansen, P. J. Chenoweth, and W. W. Thatcher Efficacy of Timed Embryo Transfer with Fresh and Frozen In Vitro Produced Embryos to Increase Pregnancy Rates in Heat-Stressed Dairy Cattle. Journal of Dairy Science 82(11): Bender, K., S. Walsh, A. C. O. Evans, T. Fair, and L. Brennan Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction 139(6): Bilby, T. R., J. Block, B. C. do Amaral, O. Sa Filho, F. T. Silvestre, P. J. Hansen, C. R. Staples, and W. W. Thatcher Effects of Dietary Unsaturated Fatty Acids on Oocyte Quality and Follicular Development in Lactating Dairy Cows in Summer. Journal of Dairy Science 89(10): Bligh, E. G. and W. J. Dyer A Rapid Method of Total Lipid Extraction and Purification. Canadian Journal of Biochemistry and Physiology 37(8): Bols, P. E. J., J. M. M. Vandenheede, A. Van Soom, and A. de Kruif Transvaginal ovum pick-up (OPU) in the cow: A new disposable needle guidance system. Theriogenology 43(3): Cerri, R. L. A., S. O. Juchem, R. C. Chebel, H. M. Rutigliano, R. G. S. Bruno, K. N. Galvao, W. W. Thatcher, and J. E. P. Santos Effect of fat source differing in fatty acid profile on metabolic parameters, fertilization, and embryo quality in high-producing dairy cows. Journal of Dairy Science 92(4): Cummins, S. B., P. Lonergan, A. C. O. Evans, D. P. Berry, R. D. Evans, and S. T. Butler Genetic merit for fertility traits in Holstein cows: I. Production 148
167 characteristics and reproductive efficiency in a pasture-based system. Journal of Dairy Science 95(3): Demyda-Peyrás, S., J. Dorado, M. Hidalgo, J. Anter, L. De Luca, E. Genero, and M. Moreno-Millán Effects of oocyte quality, incubation time and maturation environment on the number of chromosomal abnormalities in IVF-derived early bovine embryos. Reproduction, Fertility and Development 25(7): Edmonson, A. J., I. J. Lean, L. D. Weaver, T. Farver, and G. Webster A Body Condition Scoring Chart for Holstein Dairy Cows. Journal of Dairy Science 72(1): Fouladi-Nashta, A. A., K. E. Wonnacott, C. G. Gutierrez, J. G. Gong, K. D. Sinclair, P. C. Garnsworthy, and R. Webb Oocyte quality in lactating dairy cows fed on high levels of n-3 and n-6 fatty acids. Reproduction 138(5): Garverick, H. A., M. N. Harris, R. Vogel-Bluel, J. D. Sampson, J. Bader, W. R. Lamberson, J. N. Spain, M. C. Lucy, and R. S. Youngquist Concentrations of nonesterified fatty acids and glucose in blood of periparturient dairy cows are indicative of pregnancy success at first insemination. Journal of Dairy Science 96(1): Gu, L., H. Liu, X. Gu, C. Boots, K. Moley, and Q. Wang Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell. Mol. Life Sci. 72(2): Hailemariam, D., R. Mandal, F. Saleem, S. M. Dunn, D. S. Wishart, and B. N. Ametaj Identification of predictive biomarkers of disease state in transition dairy cows. Journal of Dairy Science 97(5): Hemmings, K. E., H. J. Leese, and H. M. Picton Amino Acid Turnover by Bovine Oocytes Provides an Index of Oocyte Developmental Competence In Vitro. Biology of Reproduction 86(5):165, Herlihy, M. M., M. A. Crowe, M. G. Diskin, and S. T. Butler Effects of synchronization treatments on ovarian follicular dynamics, corpus luteum growth, and circulating steroid hormone concentrations in lactating dairy cows. Journal of Dairy Science 95(2): Juchem, S. O., R. L. A. Cerri, M. Villaseñor, K. N. Galvão, R. G. S. Bruno, H. M. Rutigliano, E. J. DePeters, F. T. Silvestre, W. W. Thatcher, and J. E. P. Santos Supplementation with Calcium Salts of Linoleic and trans-octadecenoic Acids Improves Fertility of Lactating Dairy Cows. Reproduction in Domestic Animals 45(1):
168 Leroy, J. L. M. R., T. Vanholder, B. Mateusen, A. Christophe, G. Opsomer, A. de Kruif, G. Genicot, and A. Van Soom Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 130(4): Loften, J.R., Linn, J.G., Drackley, J.K., Jenkins, T.C., Soderholm, C.G., and Kertz, A.F. (2014) Invited review: Palmitic and stearic acid metabolism in lactating dairy cows. Journal of Dairy Science 97(8), Lott, W. M., V. M. Anchamparuthy, M. L. McGilliard, I. K. Mullarky, and F. C. Gwazdauskas Influence of Cysteine in Conjunction with Growth Factors on the Development of In Vitro-Produced Bovine Embryos. Reproduction in Domestic Animals 46(4): Malau-Aduli, A. E., B. D. Siebert, C. D. Bottema, and W. S. Pitchford Breed comparison of the fatty acid composition of muscle phospholipids in Jersey and Limousin cattle. Journal of Animal Science 76(3): Marei, W. F., D. C. Wathes, and A. A. Fouladi-Nashta Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction 139(6): Matoba, S., K. Bender, A. G. Fahey, S. Mamo, L. Brennan, P. Lonergan, and T. Fair Predictive value of bovine follicular components as markers of oocyte developmental potential. Reproduction, Fertility and Development 26(2): McKeegan, P. J. and R. G. Sturmey The role of fatty acids in oocyte and early embryo development. Reproduction, Fertility and Development 24(1): Mohammed, R., J. J. Kennelly, J. K. G. Kramer, K. A. Beauchemin, C. S. Stanton, and J. J. Murphy Effect of grain type and processing method on rumen fermentation and milk rumenic acid production. Animal 4(08): Moore, S. G., S. Scully, J. A. Browne, T. Fair, and S. T. Butler Genetic merit for fertility traits in Holstein cows: V. Factors affecting circulating progesterone concentrations. Journal of Dairy Science 97(9): Nabenishi, H., H. Ohta, T. Nishimoto, T. Morita, K. Ashizawa, and Y. Tsuzuki The effects of cysteine addition during in vitro maturation on the developmental competence, ROS, GSH and apoptosis level of bovine oocytes exposed to heat stress. Zygote 20(03): O'Gorman, A., M. Wallace, E. Cottell, M. J. Gibney, F. M. McAuliffe, M. Wingfield, and L. Brennan Metabolic profiling of human follicular fluid identifies 150
169 potential biomarkers of oocyte developmental competence. Reproduction 146(4): Osorio, J. S., P. Ji, J. K. Drackley, D. Luchini, and J. J. Loor Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function. Journal of Dairy Science 96(10): Osorio, M. T., A. P. Moloney, L. Brennan, and F. J. Monahan Authentication of beef production systems using a metabolomic-based approach. animal 6(01): Peñagaricano, F., A. H. Souza, P. D. Carvalho, A. M. Driver, R. Gambra, J. Kropp, K. S. Hackbart, D. Luchini, R. D. Shaver, M. C. Wiltbank, and H. Khatib Effect of Maternal Methionine Supplementation on the Transcriptome of Bovine Preimplantation Embryos. Plos One 8(8):e Putney, D. J., M. Drost, and W. W. Thatcher Influence of summer heat stress on pregnancy rates of lactating dairy cattle following embryo transfer or artificial insemination. Theriogenology 31(4): Rahman, M. B., L. Vandaele, T. Rijsselaere, M. Zhandi, D. Maes, M. Shamsuddin, and A. Van Soom Oocyte quality determines bovine embryo development after fertilisation with hydrogen peroxide-stressed spermatozoa. Reproduction, Fertility and Development 24(4): Rooke, J. A., A. Ainslie, R. G. Watt, F. M. Alink, T. G. McEvoy, K. D. Sinclair, P. C. Garnsworthy, and R. Webb Dietary carbohydrates and amino acids influence oocyte quality in dairy heifers. Reproduction, Fertility and Development 21(3): Sarıözkan, S., M. N. Bucak, P. B. Tuncer, P. A. Ulutaş, and A. Bilgen The influence of cysteine and taurine on microscopic oxidative stress parameters and fertilizing ability of bull semen following cryopreservation. Cryobiology 58(2): Sartori, R., R. Sartor-Bergfelt, S. A. Mertens, J. N. Guenther, J. J. Parrish, and M. C. Wiltbank Fertilization and Early Embryonic Development in Heifers and Lactating Cows in Summer and Lactating and Dry Cows in Winter. Journal of dairy science 85(11): Tomek, W., H. Torner, and W. Kanitz Comparative Analysis of Protein Synthesis, Transcription and Cytoplasmic Polyadenylation of mrna during 151
170 Maturation of Bovine Oocytes in vitro. Reproduction in Domestic Animals 37(2): Van Hoeck, V., J. L. M. R. Leroy, M. Arias Alvarez, D. Rizos, A. Gutierrez-Adan, K. Schnorbusch, P. E. J. Bols, H. J. Leese, and R. G. Sturmey Oocyte developmental failure in response to elevated nonesterified fatty acid concentrations: mechanistic insights. Reproduction 145(1): Van Hoeck, V., R. G. Sturmey, P. Bermejo-Alvarez, D. Rizos, A. Gutierrez-Adan, H. J. Leese, P. E. J. Bols, and J. L. M. R. Leroy Elevated Non-Esterified Fatty Acid Concentrations during Bovine Oocyte Maturation Compromise Early Embryo Physiology. Plos One 6(8):e Vanholder, T., J. Lmr Leroy, A. Van Soom, D. Maes, M. Coryn, T. Fiers, A. de Kruif, and G. Opsomer Effect of non-esterified fatty acids on bovine theca cell steroidogenesis and proliferation in vitro. Animal Reproduction Science 92(1-2): Vasconcelos, J. L. M., D. G. B. Demétrio, R. M. Santos, J. R. Chiari, C. A. Rodrigues, and O. G. S. Filho Factors potentially affecting fertility of lactating dairy cow recipients. Theriogenology 65(1): Xia, J., D. I. Broadhurst, M. Wilson, and D. S. Wishart Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics 9(2): Zeron, Y., A. Ocheretny, O. Kedar, A. Borochov, D. Sklan, and A. Arav Seasonal changes in bovine fertility: relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction 121(3):
171 Chapter Six Differentially expressed genes in the endometrium and corpus luteum of Holstein cows selected for high and low fertility are enriched for sequence variants associated with fertility 6.1 Preface At the time of thesis submission this chapter was in preparation for submission to a peer-reviewed journal. Stephen Moore was the primary author and carried out the experimental work, differential expression analysis, concordance analysis and drafted the manuscript. Matt McCabe provided training in the techniques for RNA extraction and cdna library preparation. Paul Cormican aligned the mrna sequence data to UMD 3.1 build of the bovine genome. Donagh Berry performed the fertility genome-wide association study with the Irish dairy cattle population. Ben Hayes performed the imputation of whole genome sequence data. Kath Kemper performed the fertility genome-wide association studies with the Australian dairy cattle population. Stephen Moore, Stephen Butler Jennie Pryce, Ben Hayes, Amanda Chamberlain, Trudee Fair and Pat Lonergan conceived, designed and coordinated the study. All authors interpreted the data and contributed to the manuscript. 153
172 6.2 Abstract Despite the importance of fertility in many mammalian species, including humans and livestock, there has been little success dissecting the genetic basis of this trait, reflecting a low heritability. In dairy cattle, improved fertility is a key breeding goal and has a high economic value. The genetic basis of fertility in dairy cattle may also be more similar to humans than in other model species such as mice, given that both cattle and humans typically have one, or occasionally two or more, offspring per parturition. In this study we combine a unique genetic model of fertility (cattle which have been selected for high and low fertility and show substantial difference in fertility level), with gene expression data from these cattle, and genome-wide association study results from imputed whole genome sequence data in more than 20,000 cattle with fertility records, to identify quantitative trait loci (QTL) regions and sequence variants that are linked to, and in some cases may be responsible for, genetic variation in fertility. Our hypothesis was that genes differentially expressed in the endometrium and corpus luteum on day 13 of the oestrous cycle between cows with either good or poor genetic merit for fertility would be enriched for genetic variants associated with fertility in cattle. Three hundred and fifty-five QTL regions and 17 sequence variants (P < 10-5 ) primarily associated with prostaglandin F 2α, steroidogenesis, mrna-processing and immune-related processes were identified. Of the 355 QTL regions, 93 were validated by both Australian and Irish genome-wide association studies using high-density genotypes, with signals for fertility detected primarily on BTA18, 5, 7, 8 and 29. A number of plausible causative mutations were identified for follow up studies. These included one missense variant significantly associated with fertility in EIF4EBP3. The SIFT value for this variant of 0.01; indicate the amino acid substitution was predicted to affect its protein function. The results of this study enhance our understanding of (i) the contribution of the endometrium and corpus luteum transcriptome to phenotypic fertility differences; and (ii) the genetic architecture of fertility in a mammalian species with low fecundity. For dairy cattle, including these variants in predictions of genomic breeding values may improve the rate of genetic gain for this critical trait. 154
173 6.3 Introduction The genetic basis of variation in fertility between individuals is of great interest in mammals, particularly humans and livestock. While a number of studies have identified genetic variants affecting male fertility e.g. Kosova et al. (2012), Pausch et al. (2014), female fertility is more challenging to dissect as the trait has a low heritability and collection of phenotypes is difficult. One of the first GWAS for human female fertility was recently published (Aschebrook-Kilfoy et al., 2015). Dairy cattle are a potential model for dissecting the genetic basis of fertility in mammals that have one, or occasionally two or more, offspring per parturition, and gestation times of approximately nine months fertility phenotypes are routinely recorded in large volumes in several countries, and whole genome sequence data is available for the key ancestors of modern dairy cattle populations (Daetwyler et al., 2014). Dairy cattle fertility also has a high economic value in its own right, and there is evidence that fertility in dairy cattle has declined significantly in recent decades (Lucy, 2001, Walsh et al., 2011). The causes of this decline are multifactorial (Lucy, 2001, Walsh et al., 2011), and include negative pleiotropic effects with variants improving milk production (Kadri et al., 2014). More recently, greater selection intensity for fertility traits (Berry et al., 2014) and improved reproductive management (Bisinotto et al., 2014, Butler, 2014) has halted the downward trend in female fertility in the Holstein-Friesian breed, and in some populations fertility has improved (Pryce et al., 2014). More rapid improvement is necessary to return the fertility of the Holstein- Friesian to previous levels, and to improve the economic viability of dairy farming. The establishment and maintenance of pregnancy involves a complex interplay between the endometrium, the embryo and the corpus luteum (CL) (Robinson et al., 2008, Meidan, 2014). The endometrium, a mucosal membrane lining the lumen of the uterus, promotes embryo development via secretions in the histotroph (Forde et al., 2013, Forde et al., 2014a,b) and is also involved in the regulation of the oestrous cycle (Spencer et al., 2008). After ovulation, cellular reorganization and angiogenesis of the ovulatory follicle is essential to create a highly vascularized CL capable of producing a rapid rise in progesterone (P4) concentrations (Robinson et al., 2014). The endometrium and CL are obvious targets for gene expression studies to detect differentially expressed genes (DEG), for example between high and low fertility cattle (Robinson et al., 2010). Another method used to identify genomic variation involved in a trait is a genome-wide 155
174 association study (GWAS). A number of GWAS have been conducted for fertility traits in cattle (Huang et al., 2010, Pryce et al., 2010, Sahana et al., 2010, Cole et al., 2011, Berry et al., 2012, Hoglund et al., 2014), and in humans (Aschebrook-Kilfoy et al., 2015). In cattle, genomic variation associated with fertility traits was detected on BTA1, 5, 13, 16 and 18 (Khatkar et al., 2014), however to date, there has been little agreement between studies. This is partly due to the complex nature of fertility traits, but also due to insufficient power, inconsistencies in the fertility traits used and the high significance threshold required to avoid detecting false-positives (Khatkar et al., 2014). An alternative approach is to use prior information from candidate gene or functional pathway studies to focus on specific genomic regions that are likely to harbour variants directly affecting biological processes. The advantage of this approach is that less stringent significance thresholds can be applied than with a traditional GWAS, since the false discovery rate is reduced (Pimentel et al., 2011, Cochran et al., 2013, Raven et al., 2014). In this paper, we used global gene expression profiles from endometrium and CL and GWAS and imputed sequence data to identify variants associated with dairy cow fertility. Differentially expressed genes in the CL and endometrium that are important for phenotypic fertility were identified using a unique resource herd of cows with similar genetic merit for milk production traits, but either good (Fert+) or poor (Fert-) genetic merit for fertility (Cummins et al., 2012a, Butler, 2013). The results of this study enhance our understanding of (i) the contribution of the endometrium and CL transcriptome to phenotypic reproductive performance; (ii) the genetic architecture affecting fertility in a higher mammal that has a small number of offspring per parturition, and (iii) genetic variants that could be used to accelerate genomic selection for improved fertility in dairy cattle. 156
175 6.4 Materials and Methods Lactating Holstein Cow Genetic Model of Fertility A lactating cow genetic model of fertility was established in Teagasc Moorepark, Ireland to elucidate the mechanisms responsible for sub-optimal fertility in lactating Holstein dairy cows (Cummins et al., 2012a). Briefly, heifers of >75% Holstein ancestry with either extreme positive (i.e. poor fertility; Fert-), or negative (i.e. high fertility; Fert+) estimated breeding value (EBV) for calving interval were selected from the Irish national dairy cattle database. Genetic evaluations for calving interval are undertaken 3-times annually in a multi-trait genetic evaluation model that includes the first five parity records for calving interval and other reproductive traits. Within the Irish national herd, the selected heifers represented the top 25% in genetic merit for milk production. Fert- heifers represented the bottom 5% in genetic merit for calving interval, whereas Fert+ heifers represented the top 20% in genetic merit for calving interval. In subsequent years, herd replacements were generated by selecting suitable artificial insemination sires to maintain the difference in genetic merit for calving interval. The selection criteria for candidate sires were: >200 kg predicted transmitting ability (PTA) for milk production, positive PTA for milk fat and protein concentration, and possessed >75% Holstein genetic ancestry. Sires with >5 days (mean = 6.50, SD = 1.54) PTA for calving interval were selected for mating with Fert- cows and sires with <-5 days (mean = -5.47, SD = 1.12) PTA for calving interval were selected for mating with Fert+ cows. Fourteen cows were enrolled in an ovulation synchronization protocol, 8 Fert+ and 6 Fert-. The EBVs of the cows from both genotypes are summarized in Table 6.1. Fert+ and Fert- cows were sired by 5 and 6 sires, respectively. The experimental procedures involving animals were licensed by the Department of Health, Ireland, in accordance with the Cruelty to Animals Act (Ireland 1876) and the European Community Directive 86/609/EEC. The management of the Fert+ and Fert- cows has been described in detail elsewhere (Moore et al., 2014a). Mean calving dates were February 19 (SD ± 22.3 days) and February 20 (SD ± 16.8 days) for the Fert+ and Fertcows, respectively. 157
176 Table 6.1. The mean estimated breeding value 1 (and SD) for both genotypes based on their sire, maternal grandsire and maternal great grand-sire estimated breeding values Genotype Variable Fert+ Fert- No. of animals 8 6 Holstein percentage 95 (4.7) 95 (5.8) Milk (kg) 438 (176) 482 (158) Fat (kg) 22 (6.4) 17.2 (8.4) Fat (g/kg) 0.08 (0.1) (0.1) Protein (kg) 19.2 (7.3) 18.6 (7.2) Protein (g/kg) 0.08 (0.04) 0.04 (0.08) Survival (%) 3.4 (1.6) -3.6 (1.2) Calving Interval (d) -6.4 (1.6) 4.1 (1.4) Sire calving interval (d) -9.2 (1.6) 11.4 (3.8) Maternal grandsire calving interval (d) -8.6 (4.2) 11.8 (4.6) 1 PTA values were obtained from the Autumn 2012 official dairy evaluations published by the Irish Cattle Breeding Federation and multiplied by 2 to convert to EBV. Individual cow EBV were determined using the following formula: 0.5*sireEBV *MGsireEBV *MGGsireEBV 2 Fert+ = good-fertility cows; Fert- = poor-fertility cows 158
177 6.4.2 Ovulation Synchronization Cows were enrolled in an ovulation synchronization protocol (CIDR_TAI) as described by Herlihy et al. (2012). Mean days postpartum (± SD) when cows were enrolled in the protocol was 56 ± 5.4 (range: 47-63) and 56 ± 3.6 (range: 50-61) for the Fert+ and Fert- cows, respectively. On day -10 of the protocol, each cow was administered an intramuscular injection of a gonadotropin releasing hormone (GnRH) agonist containing 10 μg of buserelin (Receptal; Intervet Ireland, Dublin, Ireland), and a controlled internal drug release device containing 1.38 g of P4 (CIDR, Pfizer Ireland, Dublin, Ireland) was inserted per vaginum. On day -3, each cow was administered an intramuscular injection of prostaglandin F 2α (PGF 2α ) containing 25 mg of dinoprost tromethamine (Lutalyse, Pfizer Ireland). On day -2, the CIDR device was removed and 36 hours later, each cow was administered a second intramuscular injection of GnRH agonist Tissue Biopsies On day 13 of the oestrous cycle, endometrium and CL biopsies were collected from each cow as described by Chapwanya et al. (2009b) and Kot et al. (1999), respectively. Briefly, cows were sedated with intravenous xylazine (1 mg/100 kg body weight) and caudal epidural anaesthesia was induced using 4 ml of 2% lidocaine to prevent abdominal straining. The vulva and perineal area were sanitized with antiseptic solution and dried. The luteal biopsy was performed using a tissue biopsy needle (16 gauge, 48 cm, trocar tip. SABD T; US Biopsy, Franklin, Indiana, USA) placed in the needle guide of an ovum pick-up probe (7.5 MHz convex transducer, Esaote Pie Medical Equipment B.V., Maastricht, the Netherlands). The endometrial biopsy was collected from a site in the uterine horn ipsilateral to the CL with an endometrial biopsy tool (Kruuse, Langeskov, Denmark), approximately 3 cm past the uterine bifurcation. Tissue samples were rinsed with saline, blotted dry and trimmed of any connective or myometrial tissue, identified by visual examination. Biopsy samples were immediately snap frozen in liquid nitrogen and stored at -80 C RNA Extraction Samples from eight of the thirteen Fert+ cows and six of the ten Fert- cows were selected; these animals were clinically healthy, responded appropriately to the ovulation synchronization protocol based on circulating P4 concentrations and were representative of a suitable mix of sires. Total RNA was extracted from endometrial tissue using a Trizol-based method (Chomczynski and Sacchi, 1987). The mean (± SD) weight of the 159
178 endometrial tissue samples was 67 (± 33) mg. Each sample was homogenized in 3 ml TRI Reagent (Sigma-Aldrich, Dublin) in sterile glass scintillation vials for 30 seconds using a Polytron PT 10/35 GT homogenizer (Kinematica) at 30,000 rpm until all tissue was in suspension. Homogenate was incubated at room temperature for 5 min, aliquots of 1 ml were transferred to sterile Eppendorf tubes (Eppendorf, UK) and 100 μl Bromo-Chloropropane (Sigma, Dublin) was added. Samples were shaken vigorously for 15 seconds to mix and incubated at room temperature for 2 min. Next, samples were centrifuged at 12,000 x g for 15 min at 4 C; 500 μl of aqueous phase (containing RNA) was transferred to new Eppendorf tubes and 300 μl of isopropanol (Sigma-Aldrich, Dublin) was added. Tubes were inverted 10 times to mix and centrifuged at 12,000 x g for 10 min at 4 C. The supernatant was removed; the pellet was washed in 75% ethanol (Sigma-Aldrich, Dublin) and centrifuged at 12,000 x g for 5 min at 4 C. The supernatant was removed and the pellet allowed to air dry for 5 min. Each RNA pellet was resuspended in 20 μl of RNAse-free water (Sigma-Aldrich, Dublin) and pooled into one Eppendorf tube. RNA concentration and quality was evaluated Nanodrop ND-1000 (Nanodrop, Wilmington, Denver) and the RNA Nano 6000 chip on the Bioanalyser 2100 (Agilent Technologies, UK), respectively. Total RNA was purified using the RNeasy Plus Mini kit (Qiagen, Manchester, UK), removing RNAs < 200 nucleotides and any genomic DNA contamination. The 260/280 absorbance ratio, RNA integrity number and 28s:18s ratio of clean RNA ranged from 1.85 to 2.29, 7.0 to 9.4 and 1.2 to 2.2, respectively for endometrium samples. Clean RNA samples were stored at -80 C cdna Library Preparation and Sequencing mrna samples were converted to cdna libraries for sequencing following the protocol of the Illumina TruSeq RNA Sample Preparation Kit v2 (Illumina, San Diego, California). RNAseq libraries were amplified by 11 cycles of PCR. Library concentration was determined by Qubit (Invitrogen, UK) and quality was determined using DNA-1000 chips on a Bioanalyser 2100 (Agilent Technologies, UK). A randomblock design was used to reduce the risk of technical bias in the experimental design. Each sample was sequenced on a single lane over a total of two flow-cells on the Illumina HiSeq 2500 platform to generate 40 million 75 base paired-end reads, and FASTQ files were created using CASAVA v1.9 (Illumina Inc). 160
179 6.4.6 mrna Sequence Quality and Alignment FASTQC v was used to perform basic quality control checks on raw sequence data. Trim Galore ( was used to remove adaptor sequences and low quality bases from the 3 end of the sequence reads; reads less than 20 bases in length were then discarded. The remaining reads were aligned to the bovine genome (UMD3.1 assembly) (Zimin et al., 2009) using STAR v2.3.0 (Chen et al., 2009) allowing two mismatches to account for sequencing errors and single nucleotide polymorphisms (SNP). The variation in gene body coverage by the RNA-Seq reads from 5 to 3 ends was assessed using RSeQC (Wang et al., 2012). Only uniquely mapped reads were retained for downstream analysis. featurecounts (Liao et al., 2014) was used to assign uniquely aligned reads to Ensembl (v73) annotated exons. Reads mapping to multiple features or overlapping genes were discarded. Read counts for all samples were amalgamated into a single matrix for subsequent differential expression analysis Differential Analysis of Gene Expression Differential expression analysis of the endometrium and CL count data was performed separately using the Bioconductor software package edger (Robinson et al., 2010) with the R statistical programming language. Genes with <1 count per million in only six endometrial samples or five CL samples (the lowest level of replication) were removed from the dataset. Library size was normalized by the Trimmed Mean of M-values. The edger package assumes that RNA-seq data have a negative binomial distribution. A fixed effects model was fitted to the read counts (expressed as counts per million) for each gene with genotype (Fert+, Fert-), parity (2, 3, 4) and sample date (n=4) all included as fixed effects. DEG were identified based on the likelihood ratio test. P- values were adjusted using the Benjamini and Hochberg (1995) method with a false discovery rate of 0.05 to correct for multiple testing. Ensembl Biomart ( was used to search the UMD 3.1 database for descriptions of the DEG. Attempts were made to annotate genes described as uncharacterized proteins by analyzing their protein coding sequence with the NCBI Blast tool. A summary of the endometrium and CL RNA-seq data processing steps is shown in Table
180 6.4.8 Pathway Analysis of DEG Pathway analysis of the DEG was conducted by over-representation analysis using GOSeq (version ) in the R statistical programming language (R Development Core Team, 2014). GOSeq accounts for gene length bias. The KEGG database (release 71.0) was used to define pathways that contain significantly more DEG than would be expected by chance given the background set of all genes found expressed in the tissue (Kanehisa et al., 2014) Genome-Wide Association Studies using High Density Genotypes Illumina ( high-density (BovineHD) genotypes (777,962 SNP) were available for 719 Holstein-Friesian AI bulls from Ireland. Illumina Bovine HD genotypes were available for 1,620 Holstein bulls and cows and 125 Jersey bulls from Australia. The genotypes were edited using the genotype quality control processes described by Berry and Kearney (2011) for the Irish genotypes and Erbe et al. (2012) for the Australian genotypes. Following quality control, 630,337 and 616,350 segregating autosomal SNP remained in the Irish and Australian datasets, respectively. A further 4,682 AI bulls in Ireland and 16,794 bulls and cows in Australia (12,056 Holstein and 4,738 Jersey) had Illumina BovineSNP50 beadchip genotypes (54,001 SNP), after applying similar quality control edits as for the HD genotypes, all lower density genotypes were used to impute, within country, BovineHD genotypes using Beagle (Browning and Browning, 2007). Predicted transmitting ability values for calving interval for each of the Irish genotyped bulls was available from the December 2013 Irish genetic evaluations. Calving interval in Ireland is evaluated in a multi-trait model (with calving to first service interval, number of services and survival) treating calving interval in each of the first five parities as separate traits. The single PTA value per animal is the average of each of the individual parity PTAs. Predicted transmitting ability values were deregressed using the full pedigree as described by Purfield et al. (2014). Only animals with a PTA reliability >40% were retained for the GWAS. The final dataset for the Irish GWAS consisted of 2,660 sires. Calving interval trait deviations (for cows) and daughter trait deviations (for bulls) for the genotyped animals were available from the Australian Dairy Herd Improvement Scheme official April 2013 evaluation for the genotyped animals in 162
181 Australia. Trait deviations were calculated within breed and were corrected for fixed effects including contemporary group, age, and permanent environment, and heterosis. Daughter trait deviations were the average of trait deviations for a bull s daughters. The GWAS in both countries were undertaken separately in WOMBAT (Meyer, 2007) using a series of univariate animal linear mixed models, where each SNP was fitted, one at a time as a continuous fixed effect (i.e., number of copies of an allele) in the model. In the Australian GWAS, additional fixed effects for breed, gender and gender nested within breed were also included in the statistical model. The direct additive genetic effect of the animal was included as a random effect linked to the pedigree file. Phenotypes were weighted by a function of the information contributing to that phenotypic record as outlined by Garrick et al. (2009). Test-statistics were obtained for each SNP separately. 163
182 Table 6.2. Processing of endometrium and corpus luteum RNA-seq data Reads Endometrium Corpus luteum Original Read pairs (SD) 19,066,636 (2,652,478) 19,632,540 (3,667,891) Quality Filtered Read Pairs (SD) 18,937,717 (2,614,068) 19,503,505 (3,652,194) Uniquely Aligned Read Pairs (SD) 17,008,347 (2,571,379) 18,051,665 (3,463,308) Uniquely Mapped Read Pairs Overlapping Protein Coding Genes (SD) 12,449,396 (1,895,381) 13,980,981 (2,354,851) Genes in UMD 3.1 Total 24,616 24,616 Less than zero counts 5,006 5,185 Less than one count per million in n samples 1 5,665 6,343 Retained 13,945 13,088 1 Genes with less than one count per million in six endometrium samples or five corpus luteum samples were removed from the dataset. Six and five samples represents to lowest number of replicates per genotype in the endometrium and corpus luteum, respectively. 164
183 Concordance Analysis The genomic position of each DEG was identified using the Bos taurus genes (UMD3.1) dataset downloaded from Ensembl BioMart database ( A region 500 kilobases (kb) flanking either side of the centre of each DEG was calculated. The number of SNP in this one megabase (Mb) region included in the Irish and Australian GWAS was quantified and the number of these SNP expected to be associated with calving interval in the GWAS was calculated assuming a false discovery rate of The false discovery threshold was calculated as mp/n, where m is the total number of SNP tested, P is the P-value, and n is the number of variants that were actually significant. If the number of SNP associated with fertility in a one Mb region was greater than expected by chance, then the region was deemed to have been validated and is hereafter referred to as a QTL region. Concordance of DEG located on the X chromosome was not considered because SNP on the X chromosome were not included in the Australian and Irish GWAS Genome-Wide Association using Whole Genome Sequence Genotypes for variants of DEG in the CL and endometrium (including region 2 kb upstream and downstream of these genes) identified in 1000 bull genomes Run2.0 (Daetwyler et al., 2014) from 129 Holstein and 15 Jerseys, were imputed into the Australian data set of 16,794 bulls and cows with HD genotypes. Beagle (Browning and Browning, 2007) was used for imputation. The model fitted to the sequence variants was as described for the Australian GWAS. Subsequently, the number of significant SNP (P < 10-5 and P < 10-8 ) within each DEG, and 2 kb upstream and downstream of the gene start and stop position, was counted. The false discovery threshold was calculated for each significance threshold as mp/n, where m is the total number of variants tested per threshold, P is the P-value of the threshold, and n is the number of variants that were actually significant at that threshold. 165
184 6.5 Results Gene Expression in Endometrium and Corpus Luteum of High and Low Fertility cows RNA from the endometrium (biopsy size: mean = 66.9 mg; SD = 32.9 mg) of eight Fert+ and six Fert- cows, and from the CL (biopsy size: mean = 4.1 mg; SD = 2.7 mg) of seven Fert+ and five Fert- cows was extracted and massively parallel sequenced. Following stringent quality control, the number of filtered reads per cow was 19,066,636 and 19,632,540 read pairs were obtained from sequencing the endometrium and CL libraries, respectively. Of these, ~65% and ~71% of the endometrium and CL sequence reads, respectively, were uniquely mapped and overlapped with protein coding genes. Gene body plots of the libraries indicated no bias in read coverage from 5 to 3 ends (Figure 6.1). Of the 24,616 genes in the bovine genome, ~57% and ~51% (endometrium and CL respectively) had sufficient coverage for differential expression following the quality control. 166
185 Figure 6.1 Gene body plots of RNA-sequenced libraries 167
186 6.5.2 Concordance of Differentially Expressed Genes in Endometrium and Corpus Luteum with High-density Genome-wide Association Studies Nine endometrial genes and 560 CL genes were differentially expressed between Fert+ and Fert- cows (P 0.05; Tables 6.3 and 6.4). Three genes, leukocyte immunoglobulinlike receptor, subfamily A (with TM domain), member 6 (LILRA6*), SAA3* and feline leukaemia virus subgroup C cellular receptor family, member 2 (FLVCR2) were differentially expressed in both the endometrium and the CL, with the same direction of expression between genotypes in both tissues; hence, a total of 566 DEG were identified across the two tissues combined. Genome wide association studies for fertility with 630K genome wide SNP were conducted in two populations 1) 2,660 Holstein bulls with daughters in Ireland recorded for calving interval, and 2) 16,794 bulls and cows in Australia (12,056 Holstein cattle and 4,738 Jersey cattle). The fertility traits evaluated were calving interval, and in the case of bulls, the average of their daughters calving interval. Calving interval is the length in time in days from one calving to the next. A linear mixed model fitting SNP as a fixed effect and population structure from pedigree and breed was used (44) to evaluate the effect of each SNP on the fertility trait. The GWAS identified a number of genome regions with significant SNP (Figure 6.2). Next we investigated if the differentially expressed gene sets from the endometrium and CL identified above were enriched for significant SNP from the GWAS. The region 500 kb upstream and downstream of the gene centre was considered in this analysis. Excluding DEG on the X chromosome, 547 individual DEG were identified across the endometrium and CL datasets. Of the 547 DEG, 203 identified QTL regions validated by the Australian GWAS (37%; indicated with AU), 245 identified QTL regions validated by the Irish GWAS (45%; indicated with IE) and 355 identified QTL regions validated by either the Irish GWAS or the Australian GWAS (Figure 6.2, Table 6.3 and 6.4). Interestingly, 93 DEG identified QTL regions validated by both the Irish GWAS and the Australian GWAS (17%; indicated with *). Of these 93 DEG, ~54% were located on the following chromosomes: BTA18 (23%), 5 (9%), 7 (8%), 8 (8%) and 29 (6%). The endometrial expression profile identified: (i) more severe uterine inflammation in Fert- cows indicated by greater expression of serum amyloid A3 (SAA3*) and secreted phosphoprotein 1 (SPP1)); (ii) suboptimal energy status in Fertcows indicated by greater expression of protein kinase, AMP-activated, gamma 3 non- 168
187 catalytic subunit (PRKAG3 AU ); and (iii) greater PGF 2α synthesis and secretion in Fertcows indicated by greater expression of prostaglandin F synthetase II-like (PGFS2*) and ATP-binding cassette sub-family C member 4 (ABCC4 AU ) compared with Fert+ cows. The luteal expression profile identified greater PGF 2α response in Fert- cows compared with Fert+ cows indicated by lesser expression of two homologs of ADAMTS-like 5 (ADAMTSL5 IE ), ATPase, Ca++ transporting, cardiac muscle, fast twitch 1 (ATP2A1 AU ) and nuclear receptor subfamily 5, group A, member 1 (NR5A1) and greater expression of crystallin, alpha B (CRYAB), inhibin, beta A (INHBA*), interleukin 4 receptor (IL4R), serpin peptidase inhibitor, clade B (ovalbumin), member 2 (SERPINB2 IE ), thrombospondin 1 (THBS1 IE ) and tissue factor pathway inhibitor 2 (TFPI2 AU ). Reduced steroidogenesis in Fert- cows compared with Fert+ cows was indicated by lesser expression of NR5A1 and two homologs of StAR-related lipid transfer (START) domain containing 9 (STARD9 IE ) and greater expression of cytochrome P450, subfamily IIIA, polypeptide 4 (CYP3A4). Genes involved in the cytoskeleton, extracellular matrix (ECM), mrna replication, zinc finger motifs; the cell cycle, DNA repair and apoptosis were also differentially expressed between genotypes, with their expression primarily down regulated in Fert- cows (Table 6.5). KEGG pathway analysis of DEG in the CL revealed over-representation of genes involved in the spliceosome pathway (P-value = 0.06). 169
188 170
Bioenergetic factors affecting conception rate in Holstein Friesian cows
 Bioenergetic factors affecting conception rate in Holstein Friesian cows J. Patton, 2*, D. Kenny 2, J.F. Mee, F.P. O Mara 2 and J.J. Murphy Teagasc, Dairy Production Research Centre, Moorepark, Fermoy,
Bioenergetic factors affecting conception rate in Holstein Friesian cows J. Patton, 2*, D. Kenny 2, J.F. Mee, F.P. O Mara 2 and J.J. Murphy Teagasc, Dairy Production Research Centre, Moorepark, Fermoy,
are associated with low fertility in dairy cows
 J. Dairy Sci. 95 :2355 2361 http://dx.doi.org/ 10.3168/jds.2011-4325 American Dairy Science Association, 2012. Open access under CC BY-NC-ND license. are associated with low fertility in dairy cows F.
J. Dairy Sci. 95 :2355 2361 http://dx.doi.org/ 10.3168/jds.2011-4325 American Dairy Science Association, 2012. Open access under CC BY-NC-ND license. are associated with low fertility in dairy cows F.
Abnormal progesterone profiles as a sign of functional imbalance in the transition period.
 Abnormal progesterone profiles as a sign of functional imbalance in the transition period. John M. Christensen 1 & Christina Ahm Petersen 2 1 Lattec I/S, Slangerupgade 69, 3400 Hillerød, Denmark 2 Lattec
Abnormal progesterone profiles as a sign of functional imbalance in the transition period. John M. Christensen 1 & Christina Ahm Petersen 2 1 Lattec I/S, Slangerupgade 69, 3400 Hillerød, Denmark 2 Lattec
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Synchronization of Ovulation and Fixed-Time Insemination for Improvement of Conception Rate in Dairy Herds with Poor Estrus Detection Efficiency
 Journal of Reproduction and Development, Vol. 45, No. 1, 1999 Synchronization of Ovulation and Fixed-Time Insemination for Improvement of Conception Rate in Dairy Herds with Poor Estrus Detection Efficiency
Journal of Reproduction and Development, Vol. 45, No. 1, 1999 Synchronization of Ovulation and Fixed-Time Insemination for Improvement of Conception Rate in Dairy Herds with Poor Estrus Detection Efficiency
EFFECTS OF NEGATIVE ENERGY BALANCE ON REPRODUCTION IN DAIRY COWS
 EFFECTS OF NEGATIVE ENERGY BALANCE ON REPRODUCTION IN DAIRY COWS RENATE KNOP, H. CERNESCU Faculty of Veterinary Medicine Timisoara, Calea Aradului No. 119, 300645, Timisoara, Romania E-mail: renate.knop@uex-usambt.org
EFFECTS OF NEGATIVE ENERGY BALANCE ON REPRODUCTION IN DAIRY COWS RENATE KNOP, H. CERNESCU Faculty of Veterinary Medicine Timisoara, Calea Aradului No. 119, 300645, Timisoara, Romania E-mail: renate.knop@uex-usambt.org
Nutrient partitioning in dairy cattle. Matthew C. Lucy. Department of Animal Sciences, University of Missouri
 NUTRIENT PARTITIONING AND REPRODUCTIVE PERFORMANCE IN DAIRY COWS Matthew C. Lucy Department of Animal Sciences, University of Missouri Take Home Messages Blood growth hormone (GH) concentrations increase
NUTRIENT PARTITIONING AND REPRODUCTIVE PERFORMANCE IN DAIRY COWS Matthew C. Lucy Department of Animal Sciences, University of Missouri Take Home Messages Blood growth hormone (GH) concentrations increase
Proceedings, The Applied Reproductive Strategies in Beef Cattle Workshop, September 5-6, 2002, Manhattan, Kansas
 20 10 0 Proceedings, The Applied Reproductive Strategies in Beef Cattle Workshop, September 5-6, 2002, Manhattan, Kansas REVIEW OF FOLLICULAR GROWTH AND THE BOVINE ESTROUS CYCLE Milo C. Wiltbank Department
20 10 0 Proceedings, The Applied Reproductive Strategies in Beef Cattle Workshop, September 5-6, 2002, Manhattan, Kansas REVIEW OF FOLLICULAR GROWTH AND THE BOVINE ESTROUS CYCLE Milo C. Wiltbank Department
Transition, energy balance and reproduction
 Transition, energy balance and reproduction Do we all speak the same language? Jo Leroy DVM, PhD TALK 1: Energy metabolism and fertility: what is the link? Linking NEB with fertility TALK 2: How to set
Transition, energy balance and reproduction Do we all speak the same language? Jo Leroy DVM, PhD TALK 1: Energy metabolism and fertility: what is the link? Linking NEB with fertility TALK 2: How to set
THIS ARTICLE IS SPONSORED BY THE MINNESOTA DAIRY HEALTH CONFERENCE.
 THIS ARTICLE IS SPONSORED BY THE MINNESOTA DAIRY HEALTH CONFERENCE. ST. PAUL, MINNESOTA UNITED STATES OF MINNESOTA Reproductive Research in Jersey Cows Ricardo C. Chebel, DVM, MPVM Introduction A very
THIS ARTICLE IS SPONSORED BY THE MINNESOTA DAIRY HEALTH CONFERENCE. ST. PAUL, MINNESOTA UNITED STATES OF MINNESOTA Reproductive Research in Jersey Cows Ricardo C. Chebel, DVM, MPVM Introduction A very
early lactation cow health
 The role of energy balance in transition and early lactation cow health Dr School of Veterinary Medicine University College Dublin Agricultural Science Association Lyons Farm October 14 th 2015 Background
The role of energy balance in transition and early lactation cow health Dr School of Veterinary Medicine University College Dublin Agricultural Science Association Lyons Farm October 14 th 2015 Background
IGF-1.
 1006 2 *1 1 2 sisaas33@gmail.com.... IGF-1.. - -.. LH LH GnRH.. :.......(1).(2) in vitro 1007..(3) (6) (5) (4).. in vitro. (7)... ) 50. (9) (8) ( 10 (3). (10).(11)...(12).(13) IGF-1. IGF-1..(14).(16).(15)
1006 2 *1 1 2 sisaas33@gmail.com.... IGF-1.. - -.. LH LH GnRH.. :.......(1).(2) in vitro 1007..(3) (6) (5) (4).. in vitro. (7)... ) 50. (9) (8) ( 10 (3). (10).(11)...(12).(13) IGF-1. IGF-1..(14).(16).(15)
Manipulation of Ovarian Function for the Reproductive Management of Dairy Cows
 Veterinary Research Communications,28(2004) 111 119 2004 Kluwer Academic Publishers. Printed in the Netherlands Manipulation of Ovarian Function for the Reproductive Management of Dairy Cows W.W. Thatcher1*,
Veterinary Research Communications,28(2004) 111 119 2004 Kluwer Academic Publishers. Printed in the Netherlands Manipulation of Ovarian Function for the Reproductive Management of Dairy Cows W.W. Thatcher1*,
Advanced Non-Cycling Program. Health
 Advanced Non-Cycling Program Health Why Treat Non-Cycling Cows? Treating cows that have not been detected in oestrus ( non-cycling ) prior to the planned start of mating with DIB-Synch provides a return
Advanced Non-Cycling Program Health Why Treat Non-Cycling Cows? Treating cows that have not been detected in oestrus ( non-cycling ) prior to the planned start of mating with DIB-Synch provides a return
Dr. Julio Giordano. Ovulation. Follicle. Corpus Luteum. GnRH
 Dr. Julio Giordano Follicle Corpus Luteum LH FSH E2 Hypothalamic hormones Gonadotropin releasing hormone () Hormone Concentration CL LH (ng/ml) 12 10 8 6 4 2 LH Response Cows Treated with 28 h (22-36)
Dr. Julio Giordano Follicle Corpus Luteum LH FSH E2 Hypothalamic hormones Gonadotropin releasing hormone () Hormone Concentration CL LH (ng/ml) 12 10 8 6 4 2 LH Response Cows Treated with 28 h (22-36)
Concentrations of Luteinizing Hormone and Ovulatory Responses in Dairy Cows Before Timed Artificial Insemination
 Concentrations of Luteinizing Hormone and Ovulatory Responses in Dairy Cows Before Timed Artificial Insemination S. L. Pulley, D. H. Keisler, S. L. Hill, and J. S. Stevenson Summary The objective of this
Concentrations of Luteinizing Hormone and Ovulatory Responses in Dairy Cows Before Timed Artificial Insemination S. L. Pulley, D. H. Keisler, S. L. Hill, and J. S. Stevenson Summary The objective of this
Physiological Mechanisms Linking Reproduction to Nutrition in High-Producing Dairy Cows
 Physiological Mechanisms Linking Reproduction to Nutrition in High-Producing Dairy Cows Matthew C. Lucy Department of Animal Science University of Missouri, Columbia Introduction Dairy cattle are inseminated
Physiological Mechanisms Linking Reproduction to Nutrition in High-Producing Dairy Cows Matthew C. Lucy Department of Animal Science University of Missouri, Columbia Introduction Dairy cattle are inseminated
Ovarian follicular development in cattle
 Ovarian follicular development in cattle John P Kastelic Professor of Theriogenology Head, Department of Production Animal Health University of Calgary Calgary, Alberta, Canada Overview Prenatal development
Ovarian follicular development in cattle John P Kastelic Professor of Theriogenology Head, Department of Production Animal Health University of Calgary Calgary, Alberta, Canada Overview Prenatal development
The physiology of multifactorial problems limiting the establishment of pregnancy in dairy cattle
 CSIRO PUBLISHING Reproduction, Fertility and Development, 2012, 24, 233 237 http://dx.doi.org/10.1071/rd11912 The physiology of multifactorial problems limiting the establishment of pregnancy in dairy
CSIRO PUBLISHING Reproduction, Fertility and Development, 2012, 24, 233 237 http://dx.doi.org/10.1071/rd11912 The physiology of multifactorial problems limiting the establishment of pregnancy in dairy
Homeorhesis is orchestrated changes in metabolism of body tissue required to sustain a specific physiological status.
 Interaction Between Nutrition and Reproduction in Dairy Cows Amin Ahmadzadeh Animal and Veterinary Science Department University of Idaho Homeorhesis is orchestrated changes in metabolism of body tissue
Interaction Between Nutrition and Reproduction in Dairy Cows Amin Ahmadzadeh Animal and Veterinary Science Department University of Idaho Homeorhesis is orchestrated changes in metabolism of body tissue
Concentrations of luteinizing hormone and ovulatory responses in dairy cows before timed artificial insemination
 Kansas Agricultural Experiment Station Research Reports Volume 0 Issue Dairy Research (98-0) Article 8 0 Concentrations of luteinizing hormone and ovulatory responses in dairy cows before timed artificial
Kansas Agricultural Experiment Station Research Reports Volume 0 Issue Dairy Research (98-0) Article 8 0 Concentrations of luteinizing hormone and ovulatory responses in dairy cows before timed artificial
OVARIAN RESPONSES AND CONCEPTION RATES IN RESPONSE TO GnRH, hcg, AND PROGESTERONE 1
 Dairy Research 2006 OVARIAN RESPONSES AND CONCEPTION RATES IN RESPONSE TO GnRH, hcg, AND PROGESTERONE 1 J. S. Stevenson, M. A. Portaluppi, D. E. Tenhouse, A. Lloyd, D. R. Eborn, S. Kacuba 2 and J. M. DeJarnette
Dairy Research 2006 OVARIAN RESPONSES AND CONCEPTION RATES IN RESPONSE TO GnRH, hcg, AND PROGESTERONE 1 J. S. Stevenson, M. A. Portaluppi, D. E. Tenhouse, A. Lloyd, D. R. Eborn, S. Kacuba 2 and J. M. DeJarnette
Ovarian Dysfunction in Dairy Cows
 Ovarian Dysfunction in Dairy Cows James Ferguson University of Pennsylvania, School of Veterinary Medicine, Kennett Square, PA, USA Email: ferguson@upenn.edu Take Home Messages For this paper, lactating
Ovarian Dysfunction in Dairy Cows James Ferguson University of Pennsylvania, School of Veterinary Medicine, Kennett Square, PA, USA Email: ferguson@upenn.edu Take Home Messages For this paper, lactating
Transition cow physiology related to fertility in lactation. W. Ron Butler Cornell University
 Transition cow physiology related to fertility in lactation W. Ron Butler Cornell University Positive association between early ovulation postpartum and pregnancy during lactation 100 80 60 40 20 0 Non-Cyc
Transition cow physiology related to fertility in lactation W. Ron Butler Cornell University Positive association between early ovulation postpartum and pregnancy during lactation 100 80 60 40 20 0 Non-Cyc
TREATMENT OUTCOMES IN POSTPARTUM ANOESTRUS COWS GUIDED BY TRANSRECTAL ULTRASONOGRAPHY ABSTRACT
 Progress. Agric. 24(1 & 2): 93 100, 2013 ISSN 1017-8139 TREATMENT OUTCOMES IN POSTPARTUM ANOESTRUS COWS GUIDED BY TRANSRECTAL ULTRASONOGRAPHY M. R. Islam, N. S. Juyena 1 *, M. M. U. Bhuiyan, M. M. Rahman
Progress. Agric. 24(1 & 2): 93 100, 2013 ISSN 1017-8139 TREATMENT OUTCOMES IN POSTPARTUM ANOESTRUS COWS GUIDED BY TRANSRECTAL ULTRASONOGRAPHY M. R. Islam, N. S. Juyena 1 *, M. M. U. Bhuiyan, M. M. Rahman
Sow Reproduction and Seasonal Infertility. Darlington Pig Discussion Group 13 th March 2014 Richard Bull
 Sow Reproduction and Seasonal Infertility Darlington Pig Discussion Group 13 th March 2014 Richard Bull Richard Bull Taurus Concepts Ltd Sow Reproduction Endogenous Hormones Gland Hormone Function Hypothalamus
Sow Reproduction and Seasonal Infertility Darlington Pig Discussion Group 13 th March 2014 Richard Bull Richard Bull Taurus Concepts Ltd Sow Reproduction Endogenous Hormones Gland Hormone Function Hypothalamus
Prostaglandin F 2α. J. S. Stevenson, S. L. Pulley, and H. I. Mellieon, Jr.
 Prostaglandin F 2α and GnRH Administration Improved Progesterone tatus, Luteal Number, and Proportion of Ovular and Anovular Dairy Cows with Corpora Lutea efore a Timed Artificial Insemination Program
Prostaglandin F 2α and GnRH Administration Improved Progesterone tatus, Luteal Number, and Proportion of Ovular and Anovular Dairy Cows with Corpora Lutea efore a Timed Artificial Insemination Program
Factors Influencing Reproductive Efficiency
 Factors Influencing Reproductive Efficiency W.W. THATCHER Department of Animal Sciences, IFAS, University of Florida, Gainseville, FL 32611 Tel: 352-392-5590 Fax: 352-392-5595 thatcher@dds.ufl.edu F. MOREIRA
Factors Influencing Reproductive Efficiency W.W. THATCHER Department of Animal Sciences, IFAS, University of Florida, Gainseville, FL 32611 Tel: 352-392-5590 Fax: 352-392-5595 thatcher@dds.ufl.edu F. MOREIRA
Reproduktion. Martin Tang Sørensen
 Reproduktion Martin Tang Sørensen mso@agrsci.dk Formål Beskrive centrale elementer i reproduktionen Fremstille en model af disse elementer Data og observationer fra forskning og praksis vedr. den højtydende
Reproduktion Martin Tang Sørensen mso@agrsci.dk Formål Beskrive centrale elementer i reproduktionen Fremstille en model af disse elementer Data og observationer fra forskning og praksis vedr. den højtydende
Physiological Mechanisms Leading to Reproductive Decline in Dairy Cattle
 Physiological Mechanisms Leading to Reproductive Decline in Dairy Cattle Matthew C. Lucy Department of Animal Science University of Missouri, Columbia Introduction Dairy cattle are inseminated and pregnancy
Physiological Mechanisms Leading to Reproductive Decline in Dairy Cattle Matthew C. Lucy Department of Animal Science University of Missouri, Columbia Introduction Dairy cattle are inseminated and pregnancy
Fertility in Beef Cattle
 406-874-8215 tom.geary@ars.usda.gov Fertility in Beef Cattle Tom Geary Reproductive Physiologist Pregnancy Diagnosis Cow Fertility Breeding Season Vibrio Trich Stress Bull Fertility BVD Plant / Water Toxins
406-874-8215 tom.geary@ars.usda.gov Fertility in Beef Cattle Tom Geary Reproductive Physiologist Pregnancy Diagnosis Cow Fertility Breeding Season Vibrio Trich Stress Bull Fertility BVD Plant / Water Toxins
Ovarian Characteristics, Serum Hormone Concentrations, and Fertility in Lactating Dairy Cows in Response to Equine Chorionic Gonadotropin
 Ovarian Characteristics, Serum Hormone Concentrations, and Fertility in Lactating Dairy Cows in Response to quine Chorionic Gonadotropin S. L. Pulley, L. D. Wallace, H. I. Mellieon, and J. S. Stevenson
Ovarian Characteristics, Serum Hormone Concentrations, and Fertility in Lactating Dairy Cows in Response to quine Chorionic Gonadotropin S. L. Pulley, L. D. Wallace, H. I. Mellieon, and J. S. Stevenson
Why Cycle Control?" Manipulating Ovulation and Estrous Synchronization" Manipulating Ovulation" Cattle" Principle of PGF 2α Use"
 Why Cycle Control?" Manipulating Ovulation and Estrous Synchronization" John Parrish 1. Group females for parturition: " a) Decrease labor, calving period Reduce calving season" b) More uniform weaning
Why Cycle Control?" Manipulating Ovulation and Estrous Synchronization" John Parrish 1. Group females for parturition: " a) Decrease labor, calving period Reduce calving season" b) More uniform weaning
Abstracts for the KSAR and JSAR Joint Symposium. Fertility control in female domestic animals: From basic understanding to application
 Abstracts for the KSAR and JSAR Joint Symposium Fertility control in female domestic animals: From basic understanding to application Current Research Orientation in Livestock Reproduction in Korea Choong-Saeng
Abstracts for the KSAR and JSAR Joint Symposium Fertility control in female domestic animals: From basic understanding to application Current Research Orientation in Livestock Reproduction in Korea Choong-Saeng
The Why s, What s, and How s of Timed Artificial Insemination Programs
 Kansas Agricultural Experiment Station Research Reports Volume 1 Issue 8 Dairy Research Article 5 January 2015 The Why s, What s, and How s of Timed Artificial Insemination Programs J. Stevenson Kansas
Kansas Agricultural Experiment Station Research Reports Volume 1 Issue 8 Dairy Research Article 5 January 2015 The Why s, What s, and How s of Timed Artificial Insemination Programs J. Stevenson Kansas
Pathways to improved fertility
 Pathways to improved fertility Do we all speak the same language? Jo Leroy DVM, PhD Jo.leroy@uantwerpen.be In this talk Energy metabolism and fertility: focusing on what really matters Fertility the deep
Pathways to improved fertility Do we all speak the same language? Jo Leroy DVM, PhD Jo.leroy@uantwerpen.be In this talk Energy metabolism and fertility: focusing on what really matters Fertility the deep
A differential equation model to investigate the dynamics of the bovine estrous cycle
 A differential equation model to investigate the dynamics of the bovine estrous cycle H. M. T. Boer 1,2, C. Stötzel 3, S. Röblitz 3, H. Woelders 1 1 Animal Breeding and Genomics Centre, Wageningen UR Livestock
A differential equation model to investigate the dynamics of the bovine estrous cycle H. M. T. Boer 1,2, C. Stötzel 3, S. Röblitz 3, H. Woelders 1 1 Animal Breeding and Genomics Centre, Wageningen UR Livestock
Can Genomics of Dry Matter Intake in Transition Cows Improve Health and Fertility?
 Can Genomics of Dry Matter Intake in Transition Cows Improve Health and Fertility? W. Ron Butler Department of Animal Science Cornell University Periparturient transition period Cascade of metabolic and
Can Genomics of Dry Matter Intake in Transition Cows Improve Health and Fertility? W. Ron Butler Department of Animal Science Cornell University Periparturient transition period Cascade of metabolic and
Mechanisms Linking Postpartum Metabolism with Reproduction
 Mechanisms Linking Postpartum Metabolism with Reproduction Matthew C. Lucy Division of Animal Sciences, University of Missouri, Columbia, MO Email: Lucym@missouri.edu Take Home Messages The reproductive
Mechanisms Linking Postpartum Metabolism with Reproduction Matthew C. Lucy Division of Animal Sciences, University of Missouri, Columbia, MO Email: Lucym@missouri.edu Take Home Messages The reproductive
ANGUS B E E F B U L L E T I N / January 2001
 Synchronizing with GnRH by JACK WHITTIER & TOM GEARY What is GnRH and how does it work? A short lesson in endocrinology may help answer this question. GnRH is the abbreviation for gonadotropin-releasing
Synchronizing with GnRH by JACK WHITTIER & TOM GEARY What is GnRH and how does it work? A short lesson in endocrinology may help answer this question. GnRH is the abbreviation for gonadotropin-releasing
REPRODUCTION & GENETICS. Hormones
 REPRODUCTION & GENETICS Hormones http://www.youtube.com/watch?v=np0wfu_mgzo Objectives 2 Define what hormones are; Compare and contrast the male and female hormones; Explain what each hormone in the mail
REPRODUCTION & GENETICS Hormones http://www.youtube.com/watch?v=np0wfu_mgzo Objectives 2 Define what hormones are; Compare and contrast the male and female hormones; Explain what each hormone in the mail
Onset and Duration of Luteal Activity Postpartum and Their Effect on First Insemination Conception Rate in Lactating Dairy Cows
 FULL PAPER Theriogenology Onset and Duration of Luteal Activity Postpartum and Their Effect on First Insemination Conception Rate in Lactating Dairy Cows Abdelrahim HOMMEIDA 1), Toshihiko NAKAO 2) * and
FULL PAPER Theriogenology Onset and Duration of Luteal Activity Postpartum and Their Effect on First Insemination Conception Rate in Lactating Dairy Cows Abdelrahim HOMMEIDA 1), Toshihiko NAKAO 2) * and
Why Cycle Control? Manipulating Ovulation and Estrous Synchronization. Manipulating Ovulation. Cattle. Principle of PGF 2a Use
 Why Cycle Control? Manipulating and Estrous Synchronization John Parrish 1. Group females for parturition: a) Decrease labor, calving period Reduce calving season b) More uniform weaning weights. 2. Reduce
Why Cycle Control? Manipulating and Estrous Synchronization John Parrish 1. Group females for parturition: a) Decrease labor, calving period Reduce calving season b) More uniform weaning weights. 2. Reduce
Nutrition, Negative Energy Balance and Fertility in the Postpartum Dairy Cow
 Nutrition, Negative Energy Balance and Fertility in the Postpartum Dairy Cow Butler, W.R., Department of Animal Science, Cornell University, Ithaca, New York 14853-4801, USA Email: wrb2@cornell.edu ABSTRACT
Nutrition, Negative Energy Balance and Fertility in the Postpartum Dairy Cow Butler, W.R., Department of Animal Science, Cornell University, Ithaca, New York 14853-4801, USA Email: wrb2@cornell.edu ABSTRACT
Animal and Veterinary Science Department University of Idaho. REGULATION OF REPRODUCTION AVS 222 (Instructor: Dr. Amin Ahmadzadeh) Chapter 5
 Animal and Veterinary Science Department University of Idaho REGULATION OF REPRODUCTION AVS 222 (Instructor: Dr. Amin Ahmadzadeh) Chapter 5 I. DEFINITIONS A. Endocrine Gland B. Hormone Chemical messenger
Animal and Veterinary Science Department University of Idaho REGULATION OF REPRODUCTION AVS 222 (Instructor: Dr. Amin Ahmadzadeh) Chapter 5 I. DEFINITIONS A. Endocrine Gland B. Hormone Chemical messenger
REVIEW Possibility of Diagnosing Uterine Function in Cows
 JARQ 50 (2), 115-119 (2016) http://www.jircas.affrc.go.jp REVIEW Possibility of Diagnosing Uterine Function in Cows Kosuke IGA 1 *, Naoki TAKENOUCHI 2, Manabu SHIMIZU 1 and Yuji HIRAO 3 1 Livestock and
JARQ 50 (2), 115-119 (2016) http://www.jircas.affrc.go.jp REVIEW Possibility of Diagnosing Uterine Function in Cows Kosuke IGA 1 *, Naoki TAKENOUCHI 2, Manabu SHIMIZU 1 and Yuji HIRAO 3 1 Livestock and
M. Irfan-ur-Rehman Khan, M. A. Rana and N. Ahmad. Department of Theriogenology, University of Veterinary and Animal Sciences, Lahore, Pakistan
 82 ULTRASONIC MONITORING OF FOLLICLES AND CORPORA LUTEA DURING SYNCHRONIZATION IN SUMMER ANOESTROUS NILI RAVI BUFFALOES AND THEIR SUBSEQUENT SUPEROVULATORY RESPONSE M. Irfan-ur-Rehman Khan, M. A. Rana
82 ULTRASONIC MONITORING OF FOLLICLES AND CORPORA LUTEA DURING SYNCHRONIZATION IN SUMMER ANOESTROUS NILI RAVI BUFFALOES AND THEIR SUBSEQUENT SUPEROVULATORY RESPONSE M. Irfan-ur-Rehman Khan, M. A. Rana
Genetics and genomics of reproductive performance in dairy and beef cattle
 Animal (2014), 8:s1, pp 105 121 The Animal Consortium 2014 doi:10.1017/s1751731114000743 animal Genetics and genomics of reproductive performance in dairy and beef cattle D. P. Berry 1, E. Wall 2 and J.
Animal (2014), 8:s1, pp 105 121 The Animal Consortium 2014 doi:10.1017/s1751731114000743 animal Genetics and genomics of reproductive performance in dairy and beef cattle D. P. Berry 1, E. Wall 2 and J.
New Trends For Estrus Synchronization Using A Combination Of Gonadotropins, Prostaglandin And Estradiol Cypionate In Dairy Cows
 ISPUB.COM The Internet Journal of Veterinary Medicine Volume 3 Number 2 New Trends For Estrus Synchronization Using A Combination Of Gonadotropins, Prostaglandin And H Amer Citation H Amer. Estradiol Cypionate
ISPUB.COM The Internet Journal of Veterinary Medicine Volume 3 Number 2 New Trends For Estrus Synchronization Using A Combination Of Gonadotropins, Prostaglandin And H Amer Citation H Amer. Estradiol Cypionate
Using dietary crude protein to manipulate energy balance in early lactation dairy cows
 Using dietary crude protein to manipulate energy balance in early lactation dairy cows S.J. Whelan 1,3, F.J. Mulligan 2 B. Flynn 3, J.J. Callan 3 and K.M. Pierce 1 1 School of Agriculture and Food Science
Using dietary crude protein to manipulate energy balance in early lactation dairy cows S.J. Whelan 1,3, F.J. Mulligan 2 B. Flynn 3, J.J. Callan 3 and K.M. Pierce 1 1 School of Agriculture and Food Science
COMPARISON OF HCG VS GNRH EFFECTS IN DOUBLE OVSYNCH ON FIRST-SERVICE CONCEPTION RATES IN ANESTRUS DAIRY COWS
 TRADITION AND MODERNITY IN VETERINARY MEDICINE, 2018, vol. 3, No 1(4): 70 76 COMPARISON OF HCG VS GNRH EFFECTS IN DOUBLE OVSYNCH ON FIRST-SERVICE CONCEPTION RATES IN ANESTRUS DAIRY COWS Gundars Naglis
TRADITION AND MODERNITY IN VETERINARY MEDICINE, 2018, vol. 3, No 1(4): 70 76 COMPARISON OF HCG VS GNRH EFFECTS IN DOUBLE OVSYNCH ON FIRST-SERVICE CONCEPTION RATES IN ANESTRUS DAIRY COWS Gundars Naglis
Managing the dominant follicle in lactating dairy cows
 Available online at www.sciencedirect.com Theriogenology 76 (2011) 1568 1582 Advances in Bovine Reproduction and Embryo Technology Managing the dominant follicle in lactating dairy cows M.C. Wiltbank a,
Available online at www.sciencedirect.com Theriogenology 76 (2011) 1568 1582 Advances in Bovine Reproduction and Embryo Technology Managing the dominant follicle in lactating dairy cows M.C. Wiltbank a,
1950s 1 st calf from surgical ET Frozen semen LN 2
 1 Fertility and Reproduction Advances 1950s 1 st calf from surgical ET Frozen semen LN 2 Progestins used to synchronize estrus 2 Fertility and Reproduction Advances 1950s 1 st calf from surgical ET Frozen
1 Fertility and Reproduction Advances 1950s 1 st calf from surgical ET Frozen semen LN 2 Progestins used to synchronize estrus 2 Fertility and Reproduction Advances 1950s 1 st calf from surgical ET Frozen
MILK DEVELOPMENT COUNCIL
 MILK DEVELOPMENT COUNCIL Economic Feeding for High Fertility Project No. 99/T2/17 ADAS BRIDGETS RESEARCH CENTRE STUDY 99/T2/17 (XCAAX) Economic Feeding for High Fertility Report prepared by Miss Helen
MILK DEVELOPMENT COUNCIL Economic Feeding for High Fertility Project No. 99/T2/17 ADAS BRIDGETS RESEARCH CENTRE STUDY 99/T2/17 (XCAAX) Economic Feeding for High Fertility Report prepared by Miss Helen
Strategies for Resynchronization of Ovulation and Timed AI. Paul M. Fricke, Ph.D. Professor of Dairy Science, University of Wisconsin Madison
 Strategies for Resynchronization of Ovulation and Timed AI Paul M. Fricke, Ph.D. Professor of Dairy Science, University of Wisconsin Madison Introduction Many confinement-based dairy systems in the U.S.
Strategies for Resynchronization of Ovulation and Timed AI Paul M. Fricke, Ph.D. Professor of Dairy Science, University of Wisconsin Madison Introduction Many confinement-based dairy systems in the U.S.
Scientific Papers-Animal Science Series: Lucrări Ştiinţifice - Seria Zootehnie, vol. 70
 Scientific Papers-Animal Science Series: Lucrări Ştiinţifice - Seria Zootehnie, vol. 70 PRELIMINARY RESULTS REGARDING ESTRUS SYNCHRONIZATION IN POSTPARTUM DAIRY COWS WITH GnRH ANALOGUE, PRID INTRAVAGINAL
Scientific Papers-Animal Science Series: Lucrări Ştiinţifice - Seria Zootehnie, vol. 70 PRELIMINARY RESULTS REGARDING ESTRUS SYNCHRONIZATION IN POSTPARTUM DAIRY COWS WITH GnRH ANALOGUE, PRID INTRAVAGINAL
A Very Specific System
 Evaluating the advantages of block-calving Establishing the targets required to maintain a block-calving pattern Examining specific management requirements. Objective: To establish the key factors to maintain
Evaluating the advantages of block-calving Establishing the targets required to maintain a block-calving pattern Examining specific management requirements. Objective: To establish the key factors to maintain
Heat Stress in Dairy Cows - Reproductive Problems and Control Measures Samal, L. Odisha University of Agriculture & Technology, Bhubaneswar -India
 Page14 Heat Stress in Dairy Cows - Reproductive Problems and Control Measures Samal, L. Odisha University of Agriculture & Technology, Bhubaneswar -India Corresponding Author: lipismitasamal@gmail.com
Page14 Heat Stress in Dairy Cows - Reproductive Problems and Control Measures Samal, L. Odisha University of Agriculture & Technology, Bhubaneswar -India Corresponding Author: lipismitasamal@gmail.com
Investigation: The Human Menstrual Cycle Research Question: How do hormones control the menstrual cycle?
 Investigation: The Human Menstrual Cycle Research Question: How do hormones control the menstrual cycle? Introduction: The menstrual cycle (changes within the uterus) is an approximately 28-day cycle that
Investigation: The Human Menstrual Cycle Research Question: How do hormones control the menstrual cycle? Introduction: The menstrual cycle (changes within the uterus) is an approximately 28-day cycle that
5/6/2015. Milk Production per Cow in the US. Current World Records
 Genomic Selection for Improved Fertility of Dairy Cows with Emphasis on Cyclicity and Pregnancy P.J. Pinedo, J.E.P. Santos, W.W. Thatcher, K.N. Galvão, R.C. Bicalho, R.O. Gilbert, G. Schuenemann, G. Rosa,
Genomic Selection for Improved Fertility of Dairy Cows with Emphasis on Cyclicity and Pregnancy P.J. Pinedo, J.E.P. Santos, W.W. Thatcher, K.N. Galvão, R.C. Bicalho, R.O. Gilbert, G. Schuenemann, G. Rosa,
GONADOTROPHIN (LUTEINISING)- RELEASING HORMONE AND ANALOGUES (GnRH OR LHRH)
 GONADOTROPHIN (LUTEINISING)- RELEASING HORMONE AND ANALOGUES (GnRH OR LHRH) Naturally occurring hormone, produced by the hypothalamus and transferred to the anterior pituitary gland in the hypophyseal
GONADOTROPHIN (LUTEINISING)- RELEASING HORMONE AND ANALOGUES (GnRH OR LHRH) Naturally occurring hormone, produced by the hypothalamus and transferred to the anterior pituitary gland in the hypophyseal
9 Managing blockcalving
 9 Managing block-calving herds Objective: To establish the key factors to maintain a successful block-calving system. Challenge: Recognise the need for excellent fertility performance and management for
9 Managing block-calving herds Objective: To establish the key factors to maintain a successful block-calving system. Challenge: Recognise the need for excellent fertility performance and management for
Fixed-Time Artificial Insemination (TAI) in Suckled Beef Cows in Response to Equine Chorionic Gonadotropin (ecg)
 Fixed-Time Artificial Insemination (TAI) in Suckled Beef Cows in Response to Equine Chorionic Gonadotropin (ecg) Guilherme Marquezini 1, Vitor Mercadante 1, Logan Wallace 2, Stacey Pulley 2, KC Olson 2,
Fixed-Time Artificial Insemination (TAI) in Suckled Beef Cows in Response to Equine Chorionic Gonadotropin (ecg) Guilherme Marquezini 1, Vitor Mercadante 1, Logan Wallace 2, Stacey Pulley 2, KC Olson 2,
CASE 41. What is the pathophysiologic cause of her amenorrhea? Which cells in the ovary secrete estrogen?
 CASE 41 A 19-year-old woman presents to her gynecologist with complaints of not having had a period for 6 months. She reports having normal periods since menarche at age 12. She denies sexual activity,
CASE 41 A 19-year-old woman presents to her gynecologist with complaints of not having had a period for 6 months. She reports having normal periods since menarche at age 12. She denies sexual activity,
breeders really don t want to miss!!!
 Oestrus induction in the canine species: dream or reality? The bitch: a mono-oestrian species Most mammals In the bitch in seasons twice a year Restricted breeding Breed variations: periods breeders really
Oestrus induction in the canine species: dream or reality? The bitch: a mono-oestrian species Most mammals In the bitch in seasons twice a year Restricted breeding Breed variations: periods breeders really
Influence of large follicles on oestrus induction and ovulation after embryo collection in superovulated Japanese Black cows
 J. Reprod. Engineer. 2015; 17: 1 5. http://sreprod.jp/contents.htm = Original Article = Journal of REPRODUCTION ENGINEERING Influence of large follicles on oestrus induction and ovulation after embryo
J. Reprod. Engineer. 2015; 17: 1 5. http://sreprod.jp/contents.htm = Original Article = Journal of REPRODUCTION ENGINEERING Influence of large follicles on oestrus induction and ovulation after embryo
Factors affecting anestrus in dairy cows at the rural areas in Bangladesh
 Volume: 2, Issue: 1 Page: 22-34 2018 ISSN 2520-4750 (Online) & ISSN 2521-3040 (Print) International Journal of Science and Business Factors affecting anestrus in dairy cows at the rural areas in Bangladesh
Volume: 2, Issue: 1 Page: 22-34 2018 ISSN 2520-4750 (Online) & ISSN 2521-3040 (Print) International Journal of Science and Business Factors affecting anestrus in dairy cows at the rural areas in Bangladesh
Relationships of Negative Energy Balance with Fertility
 Relationships of Negative Energy Balance with Fertility W. Ronald Butler Department of Animal Science, Cornell University 203 Morrison Hall, Ithaca, NY 14853 Email: wrb2@cornell.edu 1. Take Home Message
Relationships of Negative Energy Balance with Fertility W. Ronald Butler Department of Animal Science, Cornell University 203 Morrison Hall, Ithaca, NY 14853 Email: wrb2@cornell.edu 1. Take Home Message
Benefits of OPU/IVF (IVP) in Dairy Cattle. M.V. Ramon Tosta Duarte Deforest WI - Reproduction Supervisor ST-Genetics
 Benefits of OPU/IVF (IVP) in Dairy Cattle M.V. Ramon Tosta Duarte Deforest WI - Reproduction Supervisor ST-Genetics What is OPU/IVF (IVP)? ARTs used at ST Genetics Donor Selection Donors Animal Welfare
Benefits of OPU/IVF (IVP) in Dairy Cattle M.V. Ramon Tosta Duarte Deforest WI - Reproduction Supervisor ST-Genetics What is OPU/IVF (IVP)? ARTs used at ST Genetics Donor Selection Donors Animal Welfare
Assessment of an Activity Monitoring System for Detection of Estrus and Timing of Artificial Insemination in Lactating Dairy Cows
 extension Assessment of an Activity Monitoring System for Detection of Estrus and Timing of Artificial Insemination in Lactating Dairy Cows articles.extension.org/pages/70309/assessment-of-an-activity-monitoring-system-for-detection-of-estrus-and-timing-of-artificial-in
extension Assessment of an Activity Monitoring System for Detection of Estrus and Timing of Artificial Insemination in Lactating Dairy Cows articles.extension.org/pages/70309/assessment-of-an-activity-monitoring-system-for-detection-of-estrus-and-timing-of-artificial-in
Nutritional and metabolic mechanisms. in the ovarian follicle
 Nutritional and metabolic mechanisms in the ovarian follicle Joëlle Dupont Team Leader : «Interaction Metabolism and Reproduction» Unit of Physiology of Reproduction and Behaviors UMR 6175 INRA/CNRS/Université
Nutritional and metabolic mechanisms in the ovarian follicle Joëlle Dupont Team Leader : «Interaction Metabolism and Reproduction» Unit of Physiology of Reproduction and Behaviors UMR 6175 INRA/CNRS/Université
The reproductive lifespan
 The reproductive lifespan Reproductive potential Ovarian cycles Pregnancy Lactation Male Female Puberty Menopause Age Menstruation is an external indicator of ovarian events controlled by the hypothalamicpituitary
The reproductive lifespan Reproductive potential Ovarian cycles Pregnancy Lactation Male Female Puberty Menopause Age Menstruation is an external indicator of ovarian events controlled by the hypothalamicpituitary
Fertility of a Single Service: Annual Cost of Early Embryonic Loss to U.S. Beef Industry. Nutrient Partitioning
 Fertility in Beef Cows Tom Geary Reproductive Physiologist Fertility of a Single Service: Beef Cattle Early Embryonic Loss 95 100 Percentage, % 80 Maternal Recognition of Pregnancy Failure - most loss
Fertility in Beef Cows Tom Geary Reproductive Physiologist Fertility of a Single Service: Beef Cattle Early Embryonic Loss 95 100 Percentage, % 80 Maternal Recognition of Pregnancy Failure - most loss
FAT SUPPLEMENTATION FOR BEEF CATTLE: EFFECT ON REPRODUCTIVE EFFICIENCY AND CALF GROWTH
 FAT SUPPLEMENTATION FOR BEEF CATTLE: EFFECT ON REPRODUCTIVE EFFICIENCY AND CALF GROWTH F. Anez-Osuna 1, 2, H.A. (Bart) Lardner 1, 2, G. Penner 2, P. Jefferson 1, J. Campbell 3, C. Fitzsimmon 4 and J. McKinnon
FAT SUPPLEMENTATION FOR BEEF CATTLE: EFFECT ON REPRODUCTIVE EFFICIENCY AND CALF GROWTH F. Anez-Osuna 1, 2, H.A. (Bart) Lardner 1, 2, G. Penner 2, P. Jefferson 1, J. Campbell 3, C. Fitzsimmon 4 and J. McKinnon
Five-day Resynch Programs in Dairy Cows Including Controlled Internal Drug Release at Two Stages Post- Artificial Insemination
 Five-day Resynch Programs in Dairy Cows Including Controlled Internal Drug Release at Two Stages Post- Artificial Insemination S. L. Pulley, S. L. Hill, and J. S. Stevenson Summary Two experiments were
Five-day Resynch Programs in Dairy Cows Including Controlled Internal Drug Release at Two Stages Post- Artificial Insemination S. L. Pulley, S. L. Hill, and J. S. Stevenson Summary Two experiments were
Female Reproductive System. Lesson 10
 Female Reproductive System Lesson 10 Learning Goals 1. What are the five hormones involved in the female reproductive system? 2. Understand the four phases of the menstrual cycle. Human Reproductive System
Female Reproductive System Lesson 10 Learning Goals 1. What are the five hormones involved in the female reproductive system? 2. Understand the four phases of the menstrual cycle. Human Reproductive System
Parturition to resumption of ovarian cyclicity: comparative aspects of beef and dairy cows
 Animal (2014), 8:s1, pp 40 53 The Animal Consortium 2014 doi:10.1017/s1751731114000251 animal Parturition to resumption of ovarian cyclicity: comparative aspects of beef and dairy cows M. A. Crowe 1, M.
Animal (2014), 8:s1, pp 40 53 The Animal Consortium 2014 doi:10.1017/s1751731114000251 animal Parturition to resumption of ovarian cyclicity: comparative aspects of beef and dairy cows M. A. Crowe 1, M.
Supplementary crude protein and phosphorus levels: effect on spring milk production in dairy cows Michael Reid 1,2
 Supplementary crude protein and phosphorus levels: effect on spring milk production in dairy cows Michael Reid 1,2 Dr M O Donovan 1, Prof C Elliot 2, Dr J Bailey 3, Dr C Watson 3, S Lalor 4 and Dr E Lewis
Supplementary crude protein and phosphorus levels: effect on spring milk production in dairy cows Michael Reid 1,2 Dr M O Donovan 1, Prof C Elliot 2, Dr J Bailey 3, Dr C Watson 3, S Lalor 4 and Dr E Lewis
Female Reproductive Physiology. Dr Raelia Lew CREI, FRANZCOG, PhD, MMed, MBBS Fertility Specialist, Melbourne IVF
 Female Reproductive Physiology Dr Raelia Lew CREI, FRANZCOG, PhD, MMed, MBBS Fertility Specialist, Melbourne IVF REFERENCE Lew, R, Natural History of ovarian function including assessment of ovarian reserve
Female Reproductive Physiology Dr Raelia Lew CREI, FRANZCOG, PhD, MMed, MBBS Fertility Specialist, Melbourne IVF REFERENCE Lew, R, Natural History of ovarian function including assessment of ovarian reserve
Nutritional management of energy balance in cows during early lactation
 Nutritional management of energy balance in cows during early lactation K.J. Shingfield 1 and J. Vilkki 2 MTT Agrifood Research Finland 1 Animal Production Research 2 Biotechnology and Food Research Overview
Nutritional management of energy balance in cows during early lactation K.J. Shingfield 1 and J. Vilkki 2 MTT Agrifood Research Finland 1 Animal Production Research 2 Biotechnology and Food Research Overview
Reproduction in Cattle
 Reproduction in Cattle Third Edition P.J.H. BALL BSc, PhD A.R. PETERS BA, DVetMed, PhD, FRCVS, FIBiol 2004 by P.J.H. Ball and A.R. Peters Editorial Offices: Blackwell Publishing Ltd, 9600 Garsington Road,
Reproduction in Cattle Third Edition P.J.H. BALL BSc, PhD A.R. PETERS BA, DVetMed, PhD, FRCVS, FIBiol 2004 by P.J.H. Ball and A.R. Peters Editorial Offices: Blackwell Publishing Ltd, 9600 Garsington Road,
Improving fertility to timed artificial insemination by manipulation of circulating progesterone concentrations in lactating dairy cattle
 CSIRO PUBLISHING Reproduction, Fertility and Development, 2012, 24, 238 243 http://dx.doi.org/10.1071/rd11913 Improving fertility to timed artificial insemination by manipulation of circulating progesterone
CSIRO PUBLISHING Reproduction, Fertility and Development, 2012, 24, 238 243 http://dx.doi.org/10.1071/rd11913 Improving fertility to timed artificial insemination by manipulation of circulating progesterone
Relationship between size of the ovulatory follicle and pregnancy success in beef heifers 1
 Published December 8, 2014 Relationship between size of the ovulatory follicle and pregnancy success in beef heifers 1 G. A. Perry,* 2 M. F. Smith, A. J. Roberts,* M. D. MacNeil,* and T. W. Geary* 3 *USDA-ARS,
Published December 8, 2014 Relationship between size of the ovulatory follicle and pregnancy success in beef heifers 1 G. A. Perry,* 2 M. F. Smith, A. J. Roberts,* M. D. MacNeil,* and T. W. Geary* 3 *USDA-ARS,
UNDERSTANDING EMBRYO-TRANSFER (ET) A GUIDE TO THE BENEFIT OF ET IN YOUR HERD
 UNDERSTANDING EMBRYO-TRANSFER (ET) A GUIDE TO THE BENEFIT OF ET IN YOUR HERD Embryo Transfer allows one superior cow to produce a greater number of calves than normal in her lifetime TABLE OF CONTENTS
UNDERSTANDING EMBRYO-TRANSFER (ET) A GUIDE TO THE BENEFIT OF ET IN YOUR HERD Embryo Transfer allows one superior cow to produce a greater number of calves than normal in her lifetime TABLE OF CONTENTS
Follicular Deviation and Acquisition of Ovulatory Capacity in Bovine Follicles 1
 BIOLOGY OF REPRODUCTION 65, 143 149 (21) Follicular Deviation and Acquisition of Ovulatory Capacity in Bovine Follicles 1 Roberto Sartori, 3,4 Paul M. Fricke, 2,3,4 João C.P. Ferreira, 6 O.J. Ginther,
BIOLOGY OF REPRODUCTION 65, 143 149 (21) Follicular Deviation and Acquisition of Ovulatory Capacity in Bovine Follicles 1 Roberto Sartori, 3,4 Paul M. Fricke, 2,3,4 João C.P. Ferreira, 6 O.J. Ginther,
Chapter 27 The Reproductive System. MDufilho
 Chapter 27 The Reproductive System 1 Figure 27.19 Events of oogenesis. Before birth Meiotic events 2n Oogonium (stem cell) Mitosis Follicle development in ovary Follicle cells Oocyte 2n Primary oocyte
Chapter 27 The Reproductive System 1 Figure 27.19 Events of oogenesis. Before birth Meiotic events 2n Oogonium (stem cell) Mitosis Follicle development in ovary Follicle cells Oocyte 2n Primary oocyte
Effect of reducing the period of follicle dominance in a timed artificial insemination protocol on reproduction of dairy cows
 J. Dairy Sci. 93 :2976 2988 doi: 10.3168/jds.2009-2870 American Dairy Science Association, 2010. Effect of reducing the period of follicle dominance in a timed artificial insemination protocol on reproduction
J. Dairy Sci. 93 :2976 2988 doi: 10.3168/jds.2009-2870 American Dairy Science Association, 2010. Effect of reducing the period of follicle dominance in a timed artificial insemination protocol on reproduction
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,700 108,500 1.7 M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,700 108,500 1.7 M Open access books available International authors and editors Downloads Our
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Reproductive Efficiency in Dairy Cattle
 Acta Scientiae Veterinariae, 2011. 39(Suppl 1): s183 - s202. ISSN 1679-9216 (Online) Use of Applied Repr eproduc ductiv tive Technolo echnologies (FTAI, FTET) ) to Improve the Reproductive Efficiency in
Acta Scientiae Veterinariae, 2011. 39(Suppl 1): s183 - s202. ISSN 1679-9216 (Online) Use of Applied Repr eproduc ductiv tive Technolo echnologies (FTAI, FTET) ) to Improve the Reproductive Efficiency in
Effects of modified FSH surges on follicle selection and codominance in heifers
 Anim. Reprod., v.2. n.1, p.28-40, Jan./March 2005 Effects of modified FSH surges on follicle selection and codominance in heifers T.J. Acosta 1,2, M.A. Beg 1, O.J. Ginther 1, 3 1 Department of Animal Health
Anim. Reprod., v.2. n.1, p.28-40, Jan./March 2005 Effects of modified FSH surges on follicle selection and codominance in heifers T.J. Acosta 1,2, M.A. Beg 1, O.J. Ginther 1, 3 1 Department of Animal Health
1. During the follicular phase of the ovarian cycle, the hypothalamus releases GnRH.
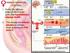 1. During the follicular phase of the ovarian cycle, the hypothalamus releases GnRH. 2. This causes the anterior pituitary to secrete small quantities of FSH and LH. 3. At this time, the follicles in the
1. During the follicular phase of the ovarian cycle, the hypothalamus releases GnRH. 2. This causes the anterior pituitary to secrete small quantities of FSH and LH. 3. At this time, the follicles in the
Basic Reproduction & Genetics. Steve Pritchard UNL Extension Educator Boone-Nance Counties
 Basic Reproduction & Genetics Steve Pritchard UNL Extension Educator Boone-Nance Counties Hormonal Regulation of the Estrous Cycle Several hormones regulate the estrous cycle Changes in the concentrations
Basic Reproduction & Genetics Steve Pritchard UNL Extension Educator Boone-Nance Counties Hormonal Regulation of the Estrous Cycle Several hormones regulate the estrous cycle Changes in the concentrations
Development of Fertility Programs for High Producing Dairy Cows
 Development of Fertility Programs for High Producing Dairy Cows Paul M. Fricke M. C. Wiltbank, P. D. Carvalho, and J. O. Giordano Theriogenology 44:915; 1995 GnRH PGF2 GnRH TAI 7 Days 48 h 16 h Ovsynch
Development of Fertility Programs for High Producing Dairy Cows Paul M. Fricke M. C. Wiltbank, P. D. Carvalho, and J. O. Giordano Theriogenology 44:915; 1995 GnRH PGF2 GnRH TAI 7 Days 48 h 16 h Ovsynch
ARM & HAMMER ANIMAL NUTRITION. Fast track for life.
 ARM & HAMMER ANIMAL NUTRITION Fast track for life. OVERVIEW New Name, Same Formula The ESSENTIOM TM Advantage Next Lap: Omega Health Pregnancy Wins. Diseases Loses. Get the Job Done Right Fast Track to
ARM & HAMMER ANIMAL NUTRITION Fast track for life. OVERVIEW New Name, Same Formula The ESSENTIOM TM Advantage Next Lap: Omega Health Pregnancy Wins. Diseases Loses. Get the Job Done Right Fast Track to
Update of Economic Breeding Index
 Update of Economic Breeding Index Donagh Berry, Siobhan Ring, Alan Twomey, Alan Hurley, Morgan O Sullivan, Sinead McParland Teagasc, Moorepark, Ireland Donagh.berry@teagasc.ie Relative emphasis Evolution
Update of Economic Breeding Index Donagh Berry, Siobhan Ring, Alan Twomey, Alan Hurley, Morgan O Sullivan, Sinead McParland Teagasc, Moorepark, Ireland Donagh.berry@teagasc.ie Relative emphasis Evolution
Ovarian follicular dynamics and superovulation in cattle
 Ovarian follicular dynamics and superovulation in cattle John P Kastelic Professor of Theriogenology Head, Department of Production Animal Health University of Calgary Calgary, Alberta, Canada Factors
Ovarian follicular dynamics and superovulation in cattle John P Kastelic Professor of Theriogenology Head, Department of Production Animal Health University of Calgary Calgary, Alberta, Canada Factors
Course: Animal Production. Instructor: Ms. Hutchinson. Objectives: After completing this unit of instruction, students will be able to:
 Course: Animal Production Unit Title: Hormones TEKS: 130.3 (C)(6)(A) Instructor: Ms. Hutchinson Objectives: After completing this unit of instruction, students will be able to: A. Define what hormones
Course: Animal Production Unit Title: Hormones TEKS: 130.3 (C)(6)(A) Instructor: Ms. Hutchinson Objectives: After completing this unit of instruction, students will be able to: A. Define what hormones
New Science New practices
 International Cow Fertility Conference New Science New practices 18-21 May 2014 Castlecourt Hotel, Westport, Mayo, Ireland Jointly hosted by the British Society of Animal Science (BSAS), Teagasc, University
International Cow Fertility Conference New Science New practices 18-21 May 2014 Castlecourt Hotel, Westport, Mayo, Ireland Jointly hosted by the British Society of Animal Science (BSAS), Teagasc, University
Before embarking on this, or other modules, candidates must fulfil the following criteria:
 Ref. No. Title: Category and Value: C-C.5 Bovine reproduction C - 10 Credits Notional Study Hours: 100 GENERAL GUIDANCE NOTES The following applies to all C modules. Before embarking on this, or other
Ref. No. Title: Category and Value: C-C.5 Bovine reproduction C - 10 Credits Notional Study Hours: 100 GENERAL GUIDANCE NOTES The following applies to all C modules. Before embarking on this, or other
