ECAT FOUNDATION REPORT
|
|
|
- Ashlyn Gilbert
- 5 years ago
- Views:
Transcription
1 ECAT FOUNDATION External quality Control for Assays and Tests With a focus on Thrombosis and Haemostasis REPORT SURVEY 218-M2 Labcode ECAT Foundation
2 Ec AT Exitheranafol cqusaloityn CThornotmrobl foosrisaas nsadyfis aenino d Tests is FOUNDATION Page 3 of 18 Sample No Sample Details Prior Use Unit Expiry Date Homogeneity Number of Participants Number of Responders Screening Plasma positive for (LA Ratio approx. 1.6) Prior Use: None Units: Ratio 11-March % Homogeneity Parameter LA Ratio Response Rate 86% Assay Normal Borderline Prolonged No Classification Your result Screening 1 Screening 2 Screening 3 APTT daptt 16 dpt drvvt Prolonged KOT 5 Other 1 1 PT 2 5 SCT 1 4 Ratio n assigned CV (%) range panel 1 value z-score panel 2 z-score panel 3 z-score APTT I.L. APTT SP I.L. HemosIL SynthAsil I.L. MixCon Siemens Actin ES Siemens Actin FSL Siemens Pathromtin SL Stago/Roche PTT Stago/Roche PTT LA Stago/Roche Staclot LA Trinity Biotech Automated APTT Trinity Biotech TriniClot APTT-HS daptt Stago/Roche PTT LA dpt drvvt American Diagnostics DVVtest I.L. HemosIL drvvt screen I.L. LAC screen Precision Biologic LA check Siemens LA1 screen Stago / Roche DRVVT screen Tcoag TriniClot Lupus Screen Technoclone LA Screen PT SCT Haematex SACT Reagent I.L. SCT screen ei 4
3 ECAT r FOUNDATION Ex.temal quality Control for Assays and Tests With a focus on Thrombosis and Haemostasis Page 5 of 18 Mixing Sample No Sample Details Prior Use Unit Expiry Date Homogeneity Number of Participants Number of Responders Plasma positive for (LA Ratio approx. 1.6) Prior Use: None Units: Ratio 11-March A Homogeneity Parameter LA Ratio Response Rate 61% Assay Normal Borderline Prolonged No Classification APTT daptt 3 16 dpt dr\nt KCT 4 Other 1 2 PNP 1 PT 2 SCT 5 2 Your result Mixing 1 Mixing 2 Mixing 3 Ratio assigned CV range panel 1 z-score panel 2 z-score panel 3 z-score value APTT I.L. APTT SP I.L. HemosIL SynthAsil I.L. MixCon Siemens Actin FSL Siemens Pathromtin SL Stago/Roche PTT Stago/Roche PTT LA Trinity Biotech Automated APTT Trinity Biotech TriniClot APTT-HS daptt Stago/Roche PTT LA dpt drvvt I.L. HemosIL drvvt screen I.L. LAC screen Precision Biologic LA check Siemens LA1 screen Stago / Roche DRVVT screen Technoclone LA Screen SCT Haematex SACT Reagent I.L. SCT screen Je,9,"?
4 T External quality Control for Assays and Tests '13 A With a focus on Thrombosis and Haemostasis 44k EC FOUNDATION Page 7 of 18 Sample No Sample Details Prior Use Unit Expiry Date Homogeneity Number of Participants Number of Responders Confirmation Plasma positive for (LA Ratio approx. 1.6) Prior Use: None Units: Ratio 11-March % Homogeneity Parameter LA Ratio Response Rate 85% Assay Positive Borderline Negative Na Classification Your result Confirm 1 Confirm 2 Confirm 3 APTT daptt 16 1 dpt 4 7 drvvt LA Positive Other 1 1 PNP 9 2 PT 1 SCT 95 2 Ratio n assigned CV (%) range value panel 1 z-score panel 2 z-score panel 3 z-score APTT I I.L. Hemosil SynthAFax I.L. MixCon Siemens Actin FS Stago/Roche Staclot LA daptt dpt drvvt American Diagnostics DVVconfirm I.L. HemosIL drvvt confirm I.L. LAC confirm Precision Biologic LA sure Siemens LA2 confirmation Stago/Roche DRVVT Confirm Tcoag TriniClot Lupus confirm Technoclone LA Confirm SCT I.L. SCT confirm c.. 4'
5 Extemal quality Control for Assays and Tests With a focus on Thrombosis and Haemostasis 4, EC AT FOUNDATION Page 9 of 18 Ratio Screening/Confirmation Sample No Sample Details Plasma positive for (LA Ratio approx. 1.6) Prior Use Prior Use: None Unit Units: Ratio Expiry Date 11-March-22 Homogeneity 2.2 % Homogeneity Parameter Number of Participants 598 Number of Responders 53 Response Rate LA Ratio 88.63% Ratio Screening/Confirmation n assigned CV (%) range panel 1 z-score panel 2 z-score panel 3 z-score value APTT daptt dpt drvvt SCT , Assays b' 12- o "Cro cb ce N.. 1 co C 4-- r - co N LC) h - CO N 41 gro) CD d * V A Other PdRVVT APTT SCT
6 External quality Control for Assays and Tests Ec AT With a focus on Thrombosis and Haemostasis FOUNDATION Page 11 of 18 Sample No Sample Details Prior Use Unit Expiry Date Homogeneity Number of Participants Number of Responders AntiCardiolipin Antibodies IgG Plasma positive for (LA Ratio approx. 1.6) Prior Use: None Units: GPL, U/mL, CU/mL 11-March % Homogeneity Parameter LA Ratio Response Rate 36% Classification Negative Borderline Low Positive Medium Positive High Positive No Conclusion Total IgG assigned CV (%) range Your result z-score value Total Group - GPL Aeskulisa Diagnotic GmbH Bio-rad Biorad Bioplex INOVA Quanta Lite Orgentec (Alegria) Orgentec (Elisa) Reaads Thermo Scientific EliA U/mL Euroimmun CU/mL I.L. Acustar / INOVA Quanta Flash GPL r ZO az. rellehlgill ) co N. P. ( (c. g :4 - '4 773,1 N cri cci ci cci cci (Ni N NNN A BOther Ongentec (Alegria) Thermo Scientific EliA INOVA Quanta Lite
7 .ikecat.," FOUNDATION External quality Control for Assays and Tests With a focus on Thrombosis and Haemostasis Page 13 of 18 Sample No Sample Details Prior Use Unit Expiry Date Homogeneity Number of Participants Number of Responders AntiCardiolipin Antibodies IgM Plasma positive for (LA Ratio approx. 1.6) Prior Use: None Units: MPL, U/mL, CU/mL 11-March % Homogeneity Parameter LA Ratio Response Rate 32% Classification Negative Borderline Low Positive Medium Positive High Positive No Conclusion Total IgM assigned CV (%) range Your result z-score value Total Group MPL Aeskulisa Diagnotic GmbH Bio-rad INOVA Quanta Lite Orgentec (Alegna) Orgentec (Elisa) Reaads Thermo Scientific EliA U/mL CU/mL I.L. Acustar / INOVA Quanta Flash _ MPL il 6 co In Tilerel 4- en_, cb (,) dr, V r - ce co nr t..- CD Cs1 N. ty) CO e CNJ ci c,i ei LO N: co d ei e: «5 r-: oi ä CN 1111 Ot h e r morgentec (Alegria) B Therme Scientific EliA INOVA Quanta Ute A /3?
8 T External "wr FOUNDATION quality Control for Assays and Tests With a focus on Thrombosis and Haemostasis Page 15 of 18 Sample No Sample Details Prior Use Unit Expiry Date Homogeneity Number of Participants Number of Responders ß2-Glycoprotein I Antibodies IgG Plasma positive for (LA Ratio approx. 1.6) Prior Use: None Units: U, U/mL, CU/mL 11-March % Homogeneity Parameter LA Ratio Response Rate 31% Classification Negative Borderline Low Positive Medium Positive High Positive No Conclusion Total IgG assigned CV (%) range Your result z-score value Ratio U Aeskulisa Diagnotic GmbH Bio-rad INOVA Quanta Lite Orgentec (Alegria) Orgentec (Elisa) Reaads Thermo Scientific EliA U/mL Euroimmun CU/mL I.L. Acustar / INOVA Quanta Flash O Other 6erloolemfecei e co v cr) cn cn ce cn c» 5 OD CO OD CO co CD c.; CO CO d d C l Aeskulisa The rmo Scientitic EliA C 1 A Orgentec (Alegria) INOVA Quanta Ute
9 Ec A FOUNDATION External quality Control for Assays and Tests VVith a focus on Thrombosis and Haemostasis Page 17 of 18 Sample No Sample Details Prior Use Unit Expiry Date Homogeneity Number of Participants Number of Responders ß2-Glycoprotein 1 Antibodies 1gM Plasma positive for (LA Ratio approx. 1.6) Prior Use: None Units: U, U/mL, CU/mL 11-March % Homogeneity Parameter LA Ratio Response Rate 26% Classification Negative Borderline Low Positive Medium Positive High Positive No Conclusion Total IgM assigned CV (%) value Your result z-score Ratio U Aeskulisa Diagnotic GmbH Bio-rad INOVA Quanta Lite Orgentec (Alegria) Orgentec (Elisa) Thermo Scientific EliA U/mL CU/mL I.L. Acustar / INOVA Quanta Flash , e c71 v 'I' CO cr CO r- cs4 CD d d ei ei ei A Other 111 wthermo Scientitic EliA je (2>
10 ov iec A FOUNDATION T External quality Control for Assays and Tests VVith a focus on Thrombosis and Haemostasis Page 18 of 18 For any method used for the measurement of this parameter with a CV 5 7.3% the contribution of the homogeneity in this reported CV will be to large and therefore Z-scores should be interpreted with caution. See for further details page 2 of Survey Manual 218 (T17/21217PM). Several participants reported a result below the detection limit (< [value]). These results were excluded in the statistical evaluation.
11 External quality Control for Assays and Tests VVith a focus on Thrombosis and Haemostasis -1 EC AT FOUNDATION Page 16 of 18 For any method used for the measurement of this parameter with a CV 5 7.3% the contribution of the homogeneity in this reported CV will be to large and therefore Z-scores should be interpreted with caution. See for further details page 2 of Survey Manual 218 (T17/21217PM). Several participants reported a result below the detection limit (< [value]) or above the measurement range (> [value]). These results were excluded in the statistical evaluation.
12 3,ECAT External quality Control for Assays and Tests VIAth a focus on Thrombosis and Haemostasis ' FOUNDATION Page 14 of 18 For any method used for the measurement of this parameter with a CV % the contribution of the homogeneity in this reported CV will be to large and therefore Z-scores should be interpreted with caution. See for further details page 2 of Survey Manual 218 (T17/21217PM). One participant (labcode ) reported a result as ratio. This result was excluded in the statistical evaluation. Several participants reported a result below the detection limit (< [value]). These results were excluded in the statistical evaluation.
13 EC AT FOUN DAT ION Ex.temal quality Control for Assays and Tests VVith a focus on Thrombosis and Haemostasis Page 12 of 18 For any method used for the measurement of this parameter with a CV 5 7.3% the contribution of the homogeneity in this reported CV will be to large and therefore Z-scores should be interpreted with caution. See for further details page 2 of Survey Manual 218 (T17/21217PM). One participant (labcode ) reported a result as ratio. This result was excluded in the statistical evaluation. Several participants reported a result below the detection limit (< [value]), while one participant reported a result above the measurement range (> [value]). These results were excluded in the statistical evaluation.
14 AT FOUNDATION External quality Control for Assays and Tests VVIth a focus on Thrombosis and Haemostasis Page 1 of 18 Final Conclusion Final Conclusion Negative Borderline Positive No Conclusion Your Result Total Positive In total 471 participants gave a final conclusion. Fifty-nine participants (approx. 11%) gave no final conclusion. The sample used in this survey was plasma from a patient diagnosed with (LA Ratio = approx. 1.6). No other types of inhibitor were present. Of the participants who gave a final conclusion, approximately 91% classified the sample as positive. Thus, the vast majority of the participants correctly classified this sample as positive. Several participants stated that there is an indication that this sample is positive for lupus anticoagulant but in real clinical practice this should be confirmed in a new sample after 12 weeks. Some participants indicated that the presence of direct oral anticoagulants and/or an increased CRP level should be excluded. A few other participants indicated that a decreased level of some coagulation factors could be possible too.
15 c A T External quality Control for Assays and Tests With a focus on Thrombosis and Haemostasis le FOUNDATION Page 8 of 18 Assays g ce co co äddäää zother wdrvvt BAPTT SCT dpt For any method used for the measurement of this parameter with a CV 5 7.3% the contribution of the homogeneity in this reported CV will be to large and therefore Z-scores should be interpreted with caution. See for further details page 2 of Survey Manual 218 (T17/21217PM). The vast majority of the performed confirmation tests (approx. 93%) were classified as borderline or positive.
16 FOUNDATION External quality Control for Assays and Tests VVith a focus on Thrombosis and Haemostasis Page 6 of 18 Assays 21 APTT 7 DRVVT cb e V 5IIIIII!! chc& ci, 4r D C71 co Other drvvt APTT SCT daptt c<4 c\i c i A 2-1C, I I 1 IM II I II N N N cb N- CO 3 N CO Ct LC-3 CO N- CO 3 C3 d * V A BOther Siemens Pathromtin Siemens Actin FSL MOther I.L. APTT SP BStago/Roche PTT LA In Stago/Roche PTT I.L. drvv7 screen alt HemosIL SynthAsi 2-1- CO 3 CD N e e L() LO Siemens Stago/Roche A For any method used for the measurement of this parameter with a CV 5 7.3% the contribution of the homogeneity in this reported CV will be to large and therefore Z-scores should be interpreted with caution. See for further details page 2 of Survey Manual 218 (T17/21217PM). The vast majority of the performed mixing tests (approx. 84%) were classified as borderline or prolonged.
17 ilk EC A T FOUNDATION External quality Control for Assays and Tests VVith a focus on Thrombosis and Haemostasis Page 4 of 18 Assays APTT DRVVT = (..) Ot h e r SC T CO e c 6, e CO ( N co N-.7 CO co cr, d (Ni CN c MdRVVT ZdAPTT APTT, !ITT a) CO e Co co cr> e V (NCNCNCN A Li)7TAID B Other ZISiernens Pathromtin 8 M Siemens Actin FSL ZIOther INSiemens I.L. I.L. APTT SP BStago/Roche PTT LA al St a g o/roche PTT Prec Biol StagofRoche 31.L. drvvt screen B I.L. tiemosil SynthAsi For any method used for the measurement of this parameter with a CV 5 7.3% the contribution of the homogeneity in this reported CV will be to!arge and therefore Z-scores should be interpreted with caution. See for further details page 2 of Survey Manual 218 (T17/21217PM). The vast majority of performed screening tests (94%) were classified as prolonged.
18 EC A le FOUNDATION External quality Control for Assays and Tests With a focus on Thrombosis and Haemostasis Page 2 of 18 Date of lssue : Survey : 218-M2 Report : Note: In the Survey Manual 218 detailed Information is given regarding the ECAT external quality assessment programme, including the statistical evaluation and explanation of the report. This Survey Manual 218 should be considered as an integral part of this survey report. Please notice the information regarding the homogeneity of samples used and the between-laboratory variation on page 21 of the Survey Manual. General Information Complaints Any complaints regarding this survey report should be reported to the ECAT before September 14th, 218. Complaints received after this date will not be taken into consideration. P.Meijer Executive Director Note: A printed version of the actual Survey Manual is provided to all participants once a year. This manual can also be downloaded from the member section of the ECAT website. ECAT Foundation Director: Dr. P. Meijer ECAT Office P.O. Box AC Voorschoten, The Netherlands phone +31() ; fax + 31() info@ecat.n1 Website: VAT number: NL Registration number with the Chamber of Commerce (KvK) Gouda : General terms of delivery are applicable to all our services. All rights reserved. No part of this report may be reproduced, stored in a retrieval system, or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior permission from the ECAT Foundation. Appendices are an integrel part of the total report.
ECAT FOUNDATION REPORT
 ECAT External quality Control for Assays and Tests With a focus on Thrombosis and Haemostasis REPORT SURVEY 2015-1 lupus Anticoagulant labeode 9907152 Copyright @ 2015 ECAT Foundation FOUNDATlON External
ECAT External quality Control for Assays and Tests With a focus on Thrombosis and Haemostasis REPORT SURVEY 2015-1 lupus Anticoagulant labeode 9907152 Copyright @ 2015 ECAT Foundation FOUNDATlON External
ECAT FOUNDATION REPORT
 ECAT External quality Control for Assays and Tests REPORT SURVEY 017-M Main Labcode 990715 Copyright 017 ECAT Foundation NECAT f r External quality Control for Assays and Tests Page 3 of 15 OVERVIEW Z-SCORES
ECAT External quality Control for Assays and Tests REPORT SURVEY 017-M Main Labcode 990715 Copyright 017 ECAT Foundation NECAT f r External quality Control for Assays and Tests Page 3 of 15 OVERVIEW Z-SCORES
Worldwide Report Coagulation Lot Exp 31 Oct 2018
 April 0 Worldwide Report Coagulation Lot 780 Exp Oct 08 Activated Partial Thromboplastin Time (APTT) Diagnostica Stago C.K. Prest Seconds 0.7 0.89.8 9 0.7 0.89.8 9 8.8..7 8.8..7 9.90 0.97.0 9.90 0.97.0
April 0 Worldwide Report Coagulation Lot 780 Exp Oct 08 Activated Partial Thromboplastin Time (APTT) Diagnostica Stago C.K. Prest Seconds 0.7 0.89.8 9 0.7 0.89.8 9 8.8..7 8.8..7 9.90 0.97.0 9.90 0.97.0
Insights on the Quality of Coagulation Testing
 Insights on the Quality of Coagulation Testing Piet Meijer ECAT Foundation The Netherlands No conflicts of interest HAEMOSTATIC BALANCE BLEEDING HAEMOSTATIC BALANCE THROMBOSIS HAEMOSTATIC BALANCE Thrombosis
Insights on the Quality of Coagulation Testing Piet Meijer ECAT Foundation The Netherlands No conflicts of interest HAEMOSTATIC BALANCE BLEEDING HAEMOSTATIC BALANCE THROMBOSIS HAEMOSTATIC BALANCE Thrombosis
Mohammadreza Tabatabaei IBTO COAG LAB
 Tests for the Evaluation of Lupus Anticoagulants t Mohammadreza Tabatabaei MSc Hematology blood bank MSc Hematology blood bank IBTO COAG LAB Lupus Anticoagulants General Background Lupus anticoagulants
Tests for the Evaluation of Lupus Anticoagulants t Mohammadreza Tabatabaei MSc Hematology blood bank MSc Hematology blood bank IBTO COAG LAB Lupus Anticoagulants General Background Lupus anticoagulants
Factor VIII Inhibitor Testing: Nijmegen Modifications, Experience and Recommendations
 Factor VIII Inhibitor Testing: Nijmegen Modifications, Experience and Recommendations Bert Verbruggen Radboud University Nijmegen Medical Centre The Netherlands 2-12-2008 DISCLOSURE None 2-12-2008 2 ECAT
Factor VIII Inhibitor Testing: Nijmegen Modifications, Experience and Recommendations Bert Verbruggen Radboud University Nijmegen Medical Centre The Netherlands 2-12-2008 DISCLOSURE None 2-12-2008 2 ECAT
Lupus Anticoagulant Testing Performance and Practices by North American Clinical Laboratories
 Coagulation and Transfusion Medicine / Lupus Anticoagulant Testing Lupus Anticoagulant Testing Performance and Practices by North American Clinical Laboratories Francine R. Dembitzer, MD, 1 Marlies R.
Coagulation and Transfusion Medicine / Lupus Anticoagulant Testing Lupus Anticoagulant Testing Performance and Practices by North American Clinical Laboratories Francine R. Dembitzer, MD, 1 Marlies R.
Antiphospholipid Syndrome
 Antiphospholipid Syndrome EliA Cardiolipin and EliA β2-glycoprotein I Fully Automated Testing for Antiphospholipid Syndrome (APS) Testing for APS according to classification criteria determination of anti-β2-glycoprotein
Antiphospholipid Syndrome EliA Cardiolipin and EliA β2-glycoprotein I Fully Automated Testing for Antiphospholipid Syndrome (APS) Testing for APS according to classification criteria determination of anti-β2-glycoprotein
Sang Hyuk Park, 1,2 Seongsoo Jang, 3 Chan-Jeoung Park, 3 and Hyun-Sook Chi Introduction
 BioMed Research International Volume 2016, Article ID 2641526, 6 pages http://dx.doi.org/10.1155/2016/2641526 Research Article Clinical Application of Revised Laboratory Classification Criteria for Antiphospholipid
BioMed Research International Volume 2016, Article ID 2641526, 6 pages http://dx.doi.org/10.1155/2016/2641526 Research Article Clinical Application of Revised Laboratory Classification Criteria for Antiphospholipid
MEASUREMENT OF ANTICOAGULANT EFFECTS OF NEW ORAL ANTICOAGULANTS PRACTICAL ASPECTS
 MEASUREMENT OF ANTICOAGULANT EFFECTS OF NEW ORAL ANTICOAGULANTS PRACTICAL ASPECTS Désirée Coen Herak Department of Laboratory Diagnostics University Hospital Centre Zagreb WARFARIN ERA Warfarin monopoly
MEASUREMENT OF ANTICOAGULANT EFFECTS OF NEW ORAL ANTICOAGULANTS PRACTICAL ASPECTS Désirée Coen Herak Department of Laboratory Diagnostics University Hospital Centre Zagreb WARFARIN ERA Warfarin monopoly
Beyond Clotting Time Clot Waveform Analysis (CWA)
 Beyond Clotting Time Clot Waveform Analysis (CWA) DR CHUEN WEN TAN CONSULTANT DEPARTMENT OF HAEMATOLOGY SINGAPORE GENERAL HOSPITAL FELLOW ANZAC RESEARCH INSTITUTE HAEMATOLOGY, CONCORD HOSPITAL Outline
Beyond Clotting Time Clot Waveform Analysis (CWA) DR CHUEN WEN TAN CONSULTANT DEPARTMENT OF HAEMATOLOGY SINGAPORE GENERAL HOSPITAL FELLOW ANZAC RESEARCH INSTITUTE HAEMATOLOGY, CONCORD HOSPITAL Outline
Determination of APTT factor sensitivity the misguiding guideline
 International Journal of Laboratory Hematology ORIGINAL ARTICLE The Official journal of the International Society for Laboratory Hematology INTERNATIONAL JOURNAL OF LABORATORY HEMATOLOGY Determination
International Journal of Laboratory Hematology ORIGINAL ARTICLE The Official journal of the International Society for Laboratory Hematology INTERNATIONAL JOURNAL OF LABORATORY HEMATOLOGY Determination
Antiphospholipid antibodies in patients with venous thrombosis at Kenyatta National Hospital
 Research article Department of Human Pathology, School of Medicine, University of Nairobi, P. O. Box 19676 00202, Nairobi, Kenya Corresponding author: Dr KA Barasa, Department of Human Pathology, School
Research article Department of Human Pathology, School of Medicine, University of Nairobi, P. O. Box 19676 00202, Nairobi, Kenya Corresponding author: Dr KA Barasa, Department of Human Pathology, School
D-DIMER EXERCISE
 ECAT FOUNDATION EXTERNAL QUALITY ASSESSMENT PROGRAMME IN HAEMOSTASIS AND THROMBOSIS D-DIMER EXERCISE 25-2 LABORATORY CODE: 12 ECAT Foundation P.O. Box 2215 231 CE Leiden The Netherlands E-Mail: P.Meijer@ecat.nl
ECAT FOUNDATION EXTERNAL QUALITY ASSESSMENT PROGRAMME IN HAEMOSTASIS AND THROMBOSIS D-DIMER EXERCISE 25-2 LABORATORY CODE: 12 ECAT Foundation P.O. Box 2215 231 CE Leiden The Netherlands E-Mail: P.Meijer@ecat.nl
A Percent Correction Formula for Evaluation of Mixing Studies
 Coagulation and Transfusion Medicine / A PERCENT CORRECTION FORMULA FOR EVALUATION OF MIXING STUDIES A Percent Correction Formula for Evaluation of Mixing Studies Sheng-hsiung Chang, MD, Veronica Tillema,
Coagulation and Transfusion Medicine / A PERCENT CORRECTION FORMULA FOR EVALUATION OF MIXING STUDIES A Percent Correction Formula for Evaluation of Mixing Studies Sheng-hsiung Chang, MD, Veronica Tillema,
Andexanet Alfa: Reversal agent for FXa Inhibitors
 Andexanet Alfa: Reversal agent for FXa Inhibitors Grace Gilmore, Prof Ross Baker, Dr Jim Tiao, Dr Quintin Hughes, Dr Jasmine Tay, Scott McGregor WA Centre for Thrombosis and Haemostasis, Murdoch University,
Andexanet Alfa: Reversal agent for FXa Inhibitors Grace Gilmore, Prof Ross Baker, Dr Jim Tiao, Dr Quintin Hughes, Dr Jasmine Tay, Scott McGregor WA Centre for Thrombosis and Haemostasis, Murdoch University,
HEMOSTASIS Quality Time saving Economy Practicability Quality assurance Precision
 Quality Time saving Economy Practicability Quality assurance Precision exclusive distributor of for France, Netherlands, Belgium and Luxembourg List of frozen reagents In 1992, Precision BioLogic began
Quality Time saving Economy Practicability Quality assurance Precision exclusive distributor of for France, Netherlands, Belgium and Luxembourg List of frozen reagents In 1992, Precision BioLogic began
THROMBOSIS PRODUCT HIGHLIGHTS
 PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS has extensive experience and know-how in the Anti-Phospholipid Syndrome (APS) field and offers the most comprehensive panel of anti-phospholipid tests, employing highly
PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS has extensive experience and know-how in the Anti-Phospholipid Syndrome (APS) field and offers the most comprehensive panel of anti-phospholipid tests, employing highly
College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) 2009 GH2-B (fresh pooled samples) * = NGSP certified at the time of the survey
 College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) The American Diabetes Association (ADA) recommends that laboratories use only HbA1c assay methods that have been NGSP certified and
College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) The American Diabetes Association (ADA) recommends that laboratories use only HbA1c assay methods that have been NGSP certified and
Generate Knowledge Lupus Anticoagulant Testing Made Simple
 Generate Knowledge Lupus Anticoagulant Testing Made Simple Paul Riley, PhD, MBA Learning objectives Describe antiphospholipid syndrome (aps) and role of the lupus anticoagulant (LA) in thrombosis Present
Generate Knowledge Lupus Anticoagulant Testing Made Simple Paul Riley, PhD, MBA Learning objectives Describe antiphospholipid syndrome (aps) and role of the lupus anticoagulant (LA) in thrombosis Present
Interference of C-reactive protein with clotting times
 Clin Chem Lab Med 24; aop Letter to the Editor Katrien M.J. Devreese *, Charlotte J. Verfaillie, Frank De Bisschop and Joris R. Delanghe Interference of C-reactive protein with clotting times DOI.55/cclm-24-96
Clin Chem Lab Med 24; aop Letter to the Editor Katrien M.J. Devreese *, Charlotte J. Verfaillie, Frank De Bisschop and Joris R. Delanghe Interference of C-reactive protein with clotting times DOI.55/cclm-24-96
Point of Care Testing in Coagulation
 Point of Care Testing in Coagulation Dr Steve Kitchen Scientific Director,UK NEQAS Blood Coagulation Head Scientist Coagulation Laboratory, Royal Hallamshire Hospital Sheffield UK POCT in Haemostasis Patient
Point of Care Testing in Coagulation Dr Steve Kitchen Scientific Director,UK NEQAS Blood Coagulation Head Scientist Coagulation Laboratory, Royal Hallamshire Hospital Sheffield UK POCT in Haemostasis Patient
Commentary by R. Little, Ph.D., NGSP Network Coordinator for the NGSP Steering Committee
 College of American Pathologists (CAP) GH5 Survey Data: (updated 8/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the NGSP.
College of American Pathologists (CAP) GH5 Survey Data: (updated 8/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the NGSP.
Lupus Anticoagulants (LA), Antiphospholipid (APL) Antibodies & APL Syndrome: Review & Update. Antiphospholipid. Antiphospholipid
 , Antiphospholipid (APL) Antibodies & APL Syndrome: Review & Update William L. Nichols, MD Mayo Clinic College of Medicine Rochester, Minnesota USA Disclosures & Objectives (Nichols) Disclosures Relevant
, Antiphospholipid (APL) Antibodies & APL Syndrome: Review & Update William L. Nichols, MD Mayo Clinic College of Medicine Rochester, Minnesota USA Disclosures & Objectives (Nichols) Disclosures Relevant
Commentary by R. Little, Ph.D., NGSP Network Coordinator for the NGSP Steering Committee
 College of American Pathologists (CAP) GH5 Survey Data: (updated 12/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the
College of American Pathologists (CAP) GH5 Survey Data: (updated 12/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the
Interactive Case Vignette: DOACs (Direct Oral Anti-Coagulants) vs. Warfarin for APLA (Anti-Phospholipid Antibody Syndrome)
 Interactive Case Vignette: DOACs (Direct Oral Anti-Coagulants) vs. Warfarin for APLA (Anti-Phospholipid Antibody Syndrome) Ara Metjian, MD Duke Debates Benign Hematologic Highlights April 22, 2017 Disclaimer
Interactive Case Vignette: DOACs (Direct Oral Anti-Coagulants) vs. Warfarin for APLA (Anti-Phospholipid Antibody Syndrome) Ara Metjian, MD Duke Debates Benign Hematologic Highlights April 22, 2017 Disclaimer
LOGO. Department of Clinical Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University & Thai Society of Clinical Pathology
 LOGO Improvement of Coagulation Lab Practice in Thailand via Thailand NEQAS for Blood Coagulation : 4-year Experience Nisarat Opartkiattikul MD,PhD Panutsaya Tientadakul, MD. Department of Clinical Pathology,
LOGO Improvement of Coagulation Lab Practice in Thailand via Thailand NEQAS for Blood Coagulation : 4-year Experience Nisarat Opartkiattikul MD,PhD Panutsaya Tientadakul, MD. Department of Clinical Pathology,
Cardiovascular risk factors are major determinants of thrombotic risk in patients with the lupus anticoagulant
 Posch et al. BMC Medicine (2017) 15:54 DOI 10.1186/s12916-017-0807-7 RESEARCH ARTICLE Open Access Cardiovascular risk factors are major determinants of thrombotic risk in patients with the lupus anticoagulant
Posch et al. BMC Medicine (2017) 15:54 DOI 10.1186/s12916-017-0807-7 RESEARCH ARTICLE Open Access Cardiovascular risk factors are major determinants of thrombotic risk in patients with the lupus anticoagulant
Comparison of Three Methods for Measuring Factor VIII Levels in Plasma
 Coagulation and Transfusion Medicine / THREE FACTOR VIII ASSAYS Comparison of Three Methods for Measuring Factor VIII Levels in Plasma Wayne L. Chandler, MD, Chris Ferrell, MT(ASCP), Joo Lee, MT(ASCP),
Coagulation and Transfusion Medicine / THREE FACTOR VIII ASSAYS Comparison of Three Methods for Measuring Factor VIII Levels in Plasma Wayne L. Chandler, MD, Chris Ferrell, MT(ASCP), Joo Lee, MT(ASCP),
Hemostasis Test Validation, Performance and Reference Intervals
 Hemostasis Test Validation, Performance and Reference Intervals Richard A. Marlar, Ph.D. Pathology and Laboratory Medicine Oklahoma City VA Medical Center University of Oklahoma Health Sciences Center
Hemostasis Test Validation, Performance and Reference Intervals Richard A. Marlar, Ph.D. Pathology and Laboratory Medicine Oklahoma City VA Medical Center University of Oklahoma Health Sciences Center
Warfarin Does Not Interfere with Lupus Anticoagulant Detection by Dilute Russell s Viper Venom Time
 Clin. Lab. 2009;55:XXX-XXX Copyright ORIGINAL ARTICLE Warfarin Does Not Interfere with Lupus Anticoagulant Detection by Dilute Russell s Viper Venom Time HORATIU OLTEANU 2, KATHARINE. A. DOWNES 1, JIGAR
Clin. Lab. 2009;55:XXX-XXX Copyright ORIGINAL ARTICLE Warfarin Does Not Interfere with Lupus Anticoagulant Detection by Dilute Russell s Viper Venom Time HORATIU OLTEANU 2, KATHARINE. A. DOWNES 1, JIGAR
Recommendations for Celiac Disease Testing. IgA & ttg IgA
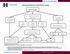 Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Presented by Marcelo Cardona, MT(ASCP) Johns Hopkins University
 Presented by Marcelo Cardona, MT(ASCP) Johns Hopkins University Alert or critical values represent those assay results that require prompt, rapid clinical attention to avert significant study-participant
Presented by Marcelo Cardona, MT(ASCP) Johns Hopkins University Alert or critical values represent those assay results that require prompt, rapid clinical attention to avert significant study-participant
Accepted: 12 November 2018
 Received: 2 August 2018 Revised: 12 November 2018 Accepted: 12 November 2018 DOI: 10.1111/hae.13656 ORIGINAL ARTICLE Laboratory science Accurate measurement of extended half life and unmodified factor
Received: 2 August 2018 Revised: 12 November 2018 Accepted: 12 November 2018 DOI: 10.1111/hae.13656 ORIGINAL ARTICLE Laboratory science Accurate measurement of extended half life and unmodified factor
WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT. Product: Enzygnost HBsAg 6.0 WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT Product: Enzygnost HBsAg 6.0 WHO reference number: PQDx 0173-064-00 Enzygnost HBsAg 6.0 with product codes OPFM03, OPFM05, OPFM07(Q)
WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT Product: Enzygnost HBsAg 6.0 WHO reference number: PQDx 0173-064-00 Enzygnost HBsAg 6.0 with product codes OPFM03, OPFM05, OPFM07(Q)
Use of PSI on CS instruments
 Johan Claes, Version 01, 1 st September 2016 User day hemostasis 2016 Use of PSI on CS instruments Restricted Siemens Healthcare GmbH, 2016 Hemostasis Lab Challenges Preanalytical problems in Haemostasis
Johan Claes, Version 01, 1 st September 2016 User day hemostasis 2016 Use of PSI on CS instruments Restricted Siemens Healthcare GmbH, 2016 Hemostasis Lab Challenges Preanalytical problems in Haemostasis
EARLY ONLINE RELEASE
 EARLY ONLINE RELEASE Note: This article was posted on the Archives Web site as an Early Online Release. Early Online Release articles have been peer reviewed, copyedited, and reviewed by the authors. Additional
EARLY ONLINE RELEASE Note: This article was posted on the Archives Web site as an Early Online Release. Early Online Release articles have been peer reviewed, copyedited, and reviewed by the authors. Additional
M E D I C A L L A B O R A T O R Y
 M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 7 Immunology 2017 MLE-M1 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 7 Immunology 2017 MLE-M1 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
ACTICLOT dpt REF 824. IVD C i 2 l. Dilute Prothrombin Time Test for the Determination of Lupus Anticoagulants (LA) (CPT Code No.
 Sekisui Diagnostics, LLC 500 West Avenue, Stamford, CT 06902 M Tel. (203) 602-7777 Fax (203) 602-2221 INTENDED USE The ACTICLOT dpt is intended for the qualitative determination of Lupus Anticoagulants
Sekisui Diagnostics, LLC 500 West Avenue, Stamford, CT 06902 M Tel. (203) 602-7777 Fax (203) 602-2221 INTENDED USE The ACTICLOT dpt is intended for the qualitative determination of Lupus Anticoagulants
Individual Lab Report Ci-Trol Nov,2016. Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC Your Lab
 Individual Lab Report Ci-Trol Nov,2016 ST VINCENT MEDICAL CENTER LABORATORY(LAB# 7300 ) 2131 WEST THIRD STREET LOS ANGELES CA USA 90057 Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC SYSMEX
Individual Lab Report Ci-Trol Nov,2016 ST VINCENT MEDICAL CENTER LABORATORY(LAB# 7300 ) 2131 WEST THIRD STREET LOS ANGELES CA USA 90057 Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC SYSMEX
TECHNICAL SERIES. Lupus Anticoagulants: Basic Concepts and Laboratory Diagnosiss. ...Setting trends TULIP DIAGNOSTICS (P) LTD.
 For the use of Registered Medical Practioners and Laboratories only TECHNICAL SERIES Lupus Anticoagulants: Basic Concepts and Laboratory Diagnosiss TULIP DIAGNOSTICS (P) LTD. Gitanjali, Tulip Block, Dr.
For the use of Registered Medical Practioners and Laboratories only TECHNICAL SERIES Lupus Anticoagulants: Basic Concepts and Laboratory Diagnosiss TULIP DIAGNOSTICS (P) LTD. Gitanjali, Tulip Block, Dr.
The first and only fully-automated, multiplexed solution for Measles, Mumps, Rubella and Varicella-zoster virus antibody testing
 Bio-Rad Laboratories BioPlex 2200 System BioPlex 2200 MMRV IgG Kit The first and only fully-automated, multiplexed solution for Measles, Mumps, Rubella and Varicella-zoster virus antibody testing Bio-Rad
Bio-Rad Laboratories BioPlex 2200 System BioPlex 2200 MMRV IgG Kit The first and only fully-automated, multiplexed solution for Measles, Mumps, Rubella and Varicella-zoster virus antibody testing Bio-Rad
Design Verification. Form: /5 Rev. 007 valid of
 Design Verification g 1 Determination of Diagnostic Sensitivity and Diagnostic Specificity... 2 Crossreactivity... 3 Potentially interfering substances... 3 2 Imprecision... 4 3 Standardisation... 4 4
Design Verification g 1 Determination of Diagnostic Sensitivity and Diagnostic Specificity... 2 Crossreactivity... 3 Potentially interfering substances... 3 2 Imprecision... 4 3 Standardisation... 4 4
New Anticoagulants: Laboratory Monitoring?? Dr Steve Kitchen Sheffield Haemostasis and Thrombosis centre UK
 New Anticoagulants: Laboratory Monitoring?? Dr Steve Kitchen Sheffield Haemostasis and Thrombosis centre UK Ideal Anticoagulant (Hirsh et al 2004) High efficacy to safety ratio Predictable dose response
New Anticoagulants: Laboratory Monitoring?? Dr Steve Kitchen Sheffield Haemostasis and Thrombosis centre UK Ideal Anticoagulant (Hirsh et al 2004) High efficacy to safety ratio Predictable dose response
M E D I C A L L A B O R A T O R Y
 M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 6 Immunology 2016 MLE-M3 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 6 Immunology 2016 MLE-M3 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
Third Party Controls for Your Dade Behring Systems
 Bio-Rad Laboratories Q U A L I T Y C O N T R O L Third Party Controls for Your Dade Behring Systems The Complete QC Solution from Bio-Rad Laboratories Bio-Rad has the control you need for your Dade Behring
Bio-Rad Laboratories Q U A L I T Y C O N T R O L Third Party Controls for Your Dade Behring Systems The Complete QC Solution from Bio-Rad Laboratories Bio-Rad has the control you need for your Dade Behring
ISO INTERNATIONAL STANDARD. Steel products Employer's qualification system for non-destructive testing (NDT) personnel
 INTERNATIONAL STANDARD ISO 11484 Second edition 2009-02-15 Steel products Employer's qualification system for non-destructive testing (NDT) personnel Produits en acier Système de qualification, par l'employeur,
INTERNATIONAL STANDARD ISO 11484 Second edition 2009-02-15 Steel products Employer's qualification system for non-destructive testing (NDT) personnel Produits en acier Système de qualification, par l'employeur,
IMMUNOWELL CARDIOLIPIN ANTIBODY (IGM) TEST
 IMMUNOWELL CARDIOLIPIN ANTIBODY (IGM) TEST Product No. 3100 For In Vitro Diagnostic Use SEE CALIBRATION VALUES, TABLE 2, PAGE 3 INTENDED USE The ImmunoWELL Cardiolipin Antibody (IgM) Test is an enzyme
IMMUNOWELL CARDIOLIPIN ANTIBODY (IGM) TEST Product No. 3100 For In Vitro Diagnostic Use SEE CALIBRATION VALUES, TABLE 2, PAGE 3 INTENDED USE The ImmunoWELL Cardiolipin Antibody (IgM) Test is an enzyme
Optimal Utilization of Thrombophilia Testing
 Optimal Utilization of Thrombophilia Testing Rajiv K. Pruthi, MBBS Special Coagulation Laboratory & Comprehensive Hemophilia Center Division of Hematology/Internal Medicine Dept of Laboratory Medicine
Optimal Utilization of Thrombophilia Testing Rajiv K. Pruthi, MBBS Special Coagulation Laboratory & Comprehensive Hemophilia Center Division of Hematology/Internal Medicine Dept of Laboratory Medicine
cobas c 501 analyzer and cobas c 311 analyzer Within Run Imprecision Guidelines
 cobas c 501 analyzer and cobas c 311 analyzer General Information How to use these guidelines Unless otherwise indicated, the data presented is the same for both the cobas c 501 analyzer and the cobas
cobas c 501 analyzer and cobas c 311 analyzer General Information How to use these guidelines Unless otherwise indicated, the data presented is the same for both the cobas c 501 analyzer and the cobas
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Haematology & Blood Transfusion Contact: Dr Fergus Jack Poole Hospital Tel: +44 (0) 1202 442 497 Longfleet Road E-Mail: fergus.jack@poole.nhs.uk
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Haematology & Blood Transfusion Contact: Dr Fergus Jack Poole Hospital Tel: +44 (0) 1202 442 497 Longfleet Road E-Mail: fergus.jack@poole.nhs.uk
Use of PSI on CS instruments
 Johan Claes, Version 01, 1 st September 2016 User day hemostasis 2016 Use of PSI on CS instruments Restricted Siemens Healthcare GmbH, 2016 Hemostasis Lab Challenges Preanalytical problems in Haemostasis
Johan Claes, Version 01, 1 st September 2016 User day hemostasis 2016 Use of PSI on CS instruments Restricted Siemens Healthcare GmbH, 2016 Hemostasis Lab Challenges Preanalytical problems in Haemostasis
VIDRL & WHO Measles IgM Proficiency Panel Final Report Prepared by Jennie Leydon, Michaela Riddell & Dr Mike Catton Director VIDRL
 VIDRL & WHO Measles IgM Proficiency Panel 00702 Final Report Prepared by Jennie Leydon, Michaela Riddell & Dr Mike Catton Director VIDRL Table of Contents Tables and Figures... 3 Abbreviations... 4 Introduction...
VIDRL & WHO Measles IgM Proficiency Panel 00702 Final Report Prepared by Jennie Leydon, Michaela Riddell & Dr Mike Catton Director VIDRL Table of Contents Tables and Figures... 3 Abbreviations... 4 Introduction...
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Spire Portsmouth Hospital Bartons Road Havant PO9 5NP United Kingdom Contact: Natalie Peck E-Mail: natalie.peck@spirehealthcare.com Website:
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Spire Portsmouth Hospital Bartons Road Havant PO9 5NP United Kingdom Contact: Natalie Peck E-Mail: natalie.peck@spirehealthcare.com Website:
AN INTERESTING CASE OF RESPIRATORY DISTRESS. Dr.V.Akila Devi DNB PG Southern Railway Headquarters Hospital Chennai
 AN INTERESTING CASE OF RESPIRATORY DISTRESS Dr.V.Akila Devi DNB PG Southern Railway Headquarters Hospital Chennai 11 month old female infant 1 st born to parents of NC marriage referred from Kolkatta H/O:
AN INTERESTING CASE OF RESPIRATORY DISTRESS Dr.V.Akila Devi DNB PG Southern Railway Headquarters Hospital Chennai 11 month old female infant 1 st born to parents of NC marriage referred from Kolkatta H/O:
ISO INTERNATIONAL STANDARD. Steel products Employer's qualification system for non-destructive testing (NDT) personnel
 INTERNATIONAL STANDARD ISO 11484 Second edition 2009-02-15 Steel products Employer's qualification system for non-destructive testing (NDT) personnel Produits en acier Système de qualification, par l'employeur,
INTERNATIONAL STANDARD ISO 11484 Second edition 2009-02-15 Steel products Employer's qualification system for non-destructive testing (NDT) personnel Produits en acier Système de qualification, par l'employeur,
Keywords: anticoagulants, blood coagulation, blood coagulation tests, coagulation, dabigatran, drug monitoring. Introduction
 Journal of Thrombosis and Haemostasis, : 49 50 DOI: 0./jth.08 ORIGINAL ARTICLE Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based
Journal of Thrombosis and Haemostasis, : 49 50 DOI: 0./jth.08 ORIGINAL ARTICLE Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based
Know for sure! Your Power for Health. PapilloCheck and PapilloCheck high-risk HPV-Genotyping: The Clear Edge in Early Detection of Cervical Cancer.
 Your Power for Health Laboratory Information hr-hpv DNA-Chip Know for sure! PapilloCheck and PapilloCheck high-risk HPV-Genotyping: The Clear Edge in Early Detection of Cervical Cancer. PapilloCheck and
Your Power for Health Laboratory Information hr-hpv DNA-Chip Know for sure! PapilloCheck and PapilloCheck high-risk HPV-Genotyping: The Clear Edge in Early Detection of Cervical Cancer. PapilloCheck and
Comparison of Samples Obtained From 3.2% Sodium Citrate Glass and Two 3.2% Sodium Citrate Plastic Blood Collection Tubes Used in Coagulation Testing
 Coagulation and Transfusion Medicine / GLASS VS PLASTIC IN HEMOSTASIS TESTING Comparison of Samples Obtained From 3.2% Sodium Citrate Glass and Two 3.2% Sodium Citrate Plastic Blood Collection Tubes Used
Coagulation and Transfusion Medicine / GLASS VS PLASTIC IN HEMOSTASIS TESTING Comparison of Samples Obtained From 3.2% Sodium Citrate Glass and Two 3.2% Sodium Citrate Plastic Blood Collection Tubes Used
Chlamydia trachomatis
 Chlamydia trachomatis 2012 EQA Programme A Final Report QAB004101 (CTDNA12A) Dr Anton M van Loon Scientific Experts on behalf of QCMD Report authorised by the QCMD Executive in May 2012 A UKAS accredited
Chlamydia trachomatis 2012 EQA Programme A Final Report QAB004101 (CTDNA12A) Dr Anton M van Loon Scientific Experts on behalf of QCMD Report authorised by the QCMD Executive in May 2012 A UKAS accredited
Research Article Seizures in Primary Antiphospholipid Syndrome: The Relevance of Smoking to Stroke
 Hindawi Publishing Corporation Clinical and Developmental Immunology Volume 2012, Article ID 981519, 7 pages doi:10.1155/2012/981519 Research Article Seizures in Primary Antiphospholipid Syndrome: The
Hindawi Publishing Corporation Clinical and Developmental Immunology Volume 2012, Article ID 981519, 7 pages doi:10.1155/2012/981519 Research Article Seizures in Primary Antiphospholipid Syndrome: The
Improved Distinction of Factor V Wild-Type and Factor V Leiden Using a Novel Prothrombin-Based Activated Protein C Resistance Assay
 Coagulation and Transfusion Medicine / A NOVEL PROTHROMBIN-BASED APC-R ASSAY Improved Distinction of Factor V Wild-Type and Factor V Leiden Using a Novel Prothrombin-Based Activated Protein C Resistance
Coagulation and Transfusion Medicine / A NOVEL PROTHROMBIN-BASED APC-R ASSAY Improved Distinction of Factor V Wild-Type and Factor V Leiden Using a Novel Prothrombin-Based Activated Protein C Resistance
6.5 Enteral Nutrition: Other Formulas: ß Hydroxyl Methyl Butyrate (HMB) May 2015
 6.5 Enteral Nutrition: Other Formulas: ß Hydroxyl Methyl Butyrate () May 2015 There were no new randomized controlled trials since the 2013 update and hence there are no changes to the following summary
6.5 Enteral Nutrition: Other Formulas: ß Hydroxyl Methyl Butyrate () May 2015 There were no new randomized controlled trials since the 2013 update and hence there are no changes to the following summary
HOVON 141 CLL. Version 3, 25JUL2018. Table Required investigations at entry, during treatment and during follow up.
 Table 10.2.1 Required investigations at entry, during treatment and during follow up At entry 1 each induction cycle cycle 2 Cycle 3 day 8 and 15 Cycle 4 day1 and 6 cycle 9 day 15 of cycle 15 9 cycle 15:
Table 10.2.1 Required investigations at entry, during treatment and during follow up At entry 1 each induction cycle cycle 2 Cycle 3 day 8 and 15 Cycle 4 day1 and 6 cycle 9 day 15 of cycle 15 9 cycle 15:
Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis.
 Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Bossuyt X, Cohen Tervaert JW, Arimura Y, Blockmans D, Flores-Suárez LF, Guillevin
Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Bossuyt X, Cohen Tervaert JW, Arimura Y, Blockmans D, Flores-Suárez LF, Guillevin
Immunohematology. IH-QC Modular System. Select. Combine. Control.
 Immunohematology IH-QC Modular System Select. Combine. Control. IH-QC Modular System Select. Combine. Control. Transfusion guidelines recommend regular checking of test materials, test methods, local working
Immunohematology IH-QC Modular System Select. Combine. Control. IH-QC Modular System Select. Combine. Control. Transfusion guidelines recommend regular checking of test materials, test methods, local working
Anticardiolipin Antibodies in Patients With Type 2 Diabetes Mellitus
 CM&R Rapid Release. Published online ahead of print February 26, 2009 as doi:10.3121/cmr.2008.828 Original Research Anticardiolipin Antibodies in Patients With Type 2 Diabetes Mellitus José María Calvo-Romero,
CM&R Rapid Release. Published online ahead of print February 26, 2009 as doi:10.3121/cmr.2008.828 Original Research Anticardiolipin Antibodies in Patients With Type 2 Diabetes Mellitus José María Calvo-Romero,
ESIM 2014 Clinical Case Presentation Israel. Ben-Sasson Maayan Bnei-Zion medical center Haifa
 ESIM 2014 Clinical Case Presentation Israel Ben-Sasson Maayan Bnei-Zion medical center Haifa Presentation A 24 YO male,a ping-pong player, presented to the ER with acute onset of right upper extremity
ESIM 2014 Clinical Case Presentation Israel Ben-Sasson Maayan Bnei-Zion medical center Haifa Presentation A 24 YO male,a ping-pong player, presented to the ER with acute onset of right upper extremity
IL-6 VALIDATIONS. Several suppliers reagents correlate well with R&D Systems HS ELISA, the goldstandard assay normally used in our laboratory.
 IL-6 VALIDATIONS Several suppliers reagents correlate well with R&D Systems HS ELISA, the goldstandard assay normally used in our laboratory. Assays are not always harmonized with respect to standardization.
IL-6 VALIDATIONS Several suppliers reagents correlate well with R&D Systems HS ELISA, the goldstandard assay normally used in our laboratory. Assays are not always harmonized with respect to standardization.
Lupus anticoagulant: performance of the tests as recommended by the latest ISTH guidelines
 Journal of Thrombosis and Haemostasis, 9: 1776 1783 DOI: 10.1111/j.1538-7836.2011.04420.x ORIGINAL ARTICLE Lupus anticoagulant: performance of the tests as recommended by the latest ISTH guidelines J.
Journal of Thrombosis and Haemostasis, 9: 1776 1783 DOI: 10.1111/j.1538-7836.2011.04420.x ORIGINAL ARTICLE Lupus anticoagulant: performance of the tests as recommended by the latest ISTH guidelines J.
10/24/2013. Heparin-Induced Thrombocytopenia (HIT) Anticoagulation Management in ECMO Therapy:
 Anticoagulation Management in ECMO Therapy: Heparin-Induced (HIT) Michael H. Creer, MD Professor of Pathology Director, Clinical Laboratories, Medical Co- Director, Hematopathology and Chief, Division
Anticoagulation Management in ECMO Therapy: Heparin-Induced (HIT) Michael H. Creer, MD Professor of Pathology Director, Clinical Laboratories, Medical Co- Director, Hematopathology and Chief, Division
Quantitative Measurement of Cardiac Biomarkers
 Cardiac Biomarker Analyzer Quantitative Measurement of Cardiac Biomarkers Troponin I, Myoglobin, CK-MB, D-Dimer, NTproBNP, hscrp Results in 17 minutes Direct measurement from whole blood samples 6 parallel
Cardiac Biomarker Analyzer Quantitative Measurement of Cardiac Biomarkers Troponin I, Myoglobin, CK-MB, D-Dimer, NTproBNP, hscrp Results in 17 minutes Direct measurement from whole blood samples 6 parallel
Il laboratorio: verso una migliore definizione dei test
 Il laboratorio: verso una migliore definizione dei test Armando Tripodi Angelo Bianchi Bonomi Hemophilia and Thrombosis Center Dept. of Clinical Sciences and Community Health University of Milano Laboratory
Il laboratorio: verso una migliore definizione dei test Armando Tripodi Angelo Bianchi Bonomi Hemophilia and Thrombosis Center Dept. of Clinical Sciences and Community Health University of Milano Laboratory
M E D I C A L L A B O R A T O R Y
 M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 7 Immunology 2017 MLE-M3 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 7 Immunology 2017 MLE-M3 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Phospholipids as a Predisposing Factor of Recurrent Miscarriage in Sudanese Women Jevara Mohamed
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Phospholipids as a Predisposing Factor of Recurrent Miscarriage in Sudanese Women Jevara Mohamed
Internal Quality Control in the Haemostasis laboratory. Dr Steve Kitchen Sheffield Haemophilia and Thrombosis centre & UK NEQAS Blood Coagulation
 Internal Quality Control in the Haemostasis laboratory Dr Steve Kitchen Sheffield Haemophilia and Thrombosis centre & UK NEQAS Blood Coagulation Why do we need Quality control? Philadelphia Enquirer Aug
Internal Quality Control in the Haemostasis laboratory Dr Steve Kitchen Sheffield Haemophilia and Thrombosis centre & UK NEQAS Blood Coagulation Why do we need Quality control? Philadelphia Enquirer Aug
Medical Laboratory Accreditation Programme
 Client Client Number 121 Address PO Box 6064, Dunedin North, Dunedin, 9059 Plunket House, 472 George Street, North Dunedin, Dunedin, 9016 Telephone 03 477-6981 Fax 03 477-9160 Authorised Representative
Client Client Number 121 Address PO Box 6064, Dunedin North, Dunedin, 9059 Plunket House, 472 George Street, North Dunedin, Dunedin, 9016 Telephone 03 477-6981 Fax 03 477-9160 Authorised Representative
Instructions for use. Histamine Release
 Instructions for use Histamine Release Supplementary kit for the determination of the release of histamine from heparinized whole blood (this kit has to be used in combination with the Histamine ELISA,
Instructions for use Histamine Release Supplementary kit for the determination of the release of histamine from heparinized whole blood (this kit has to be used in combination with the Histamine ELISA,
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS. Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring
 MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
Using the Variability of INRs to indicate the Risk of an Event in DAWN AC
 Predicting Clinical Events Using the Variability of INRs to indicate the Risk of an Event in DAWN AC Syd Stewart, Managing Director, 4S DAWN Clinical Software Introduction It is widely agreed that neither
Predicting Clinical Events Using the Variability of INRs to indicate the Risk of an Event in DAWN AC Syd Stewart, Managing Director, 4S DAWN Clinical Software Introduction It is widely agreed that neither
Bowel Cancer Screening for Scotland
 Vision 3 Bowel Cancer Screening for Scotland (BoSS) Copyright INPS Ltd 2014 The Bread Factory, 1A Broughton Street, Battersea, London, SW8 3QJ T: +44 (0) 207 501700 F:+44 (0) 207 5017100 W: www.inps.co.uk
Vision 3 Bowel Cancer Screening for Scotland (BoSS) Copyright INPS Ltd 2014 The Bread Factory, 1A Broughton Street, Battersea, London, SW8 3QJ T: +44 (0) 207 501700 F:+44 (0) 207 5017100 W: www.inps.co.uk
B19 Virus EQA Programme Final Report QAV (B19DNA14)
 B19 Virus 2014 EQA Programme Final Report QAV034116 (B19DNA14) Prof. Hubert GM Niesters Scientific Expert on behalf of QCMD Report authorised by the QCMD Executive in July 2014 A UKAS accredited proficiency
B19 Virus 2014 EQA Programme Final Report QAV034116 (B19DNA14) Prof. Hubert GM Niesters Scientific Expert on behalf of QCMD Report authorised by the QCMD Executive in July 2014 A UKAS accredited proficiency
Chlamydia trachomatis
 Chlamydia trachomatis 2012 EQA Programme A Final Report QAB004101 (CTDNA12A) Version 2 Dr Anton M van Loon Scientific Experts on behalf of QCMD Report authorised by the QCMD Executive in May 2012 A UKAS
Chlamydia trachomatis 2012 EQA Programme A Final Report QAB004101 (CTDNA12A) Version 2 Dr Anton M van Loon Scientific Experts on behalf of QCMD Report authorised by the QCMD Executive in May 2012 A UKAS
Bio-Rad Laboratories. Cardiac Assessment Controls. Value Assigned for Clinical Laboratory and Point of Care Test Systems
 Bio-Rad Laboratories Cardiac Assessment Controls Cardiac Assessment Controls Value Assigned for Clinical Laboratory and Point of Care Test Systems Solving the Challenges of Troponin I Testing for the Central
Bio-Rad Laboratories Cardiac Assessment Controls Cardiac Assessment Controls Value Assigned for Clinical Laboratory and Point of Care Test Systems Solving the Challenges of Troponin I Testing for the Central
M E D I C A L L A B O R A T O R Y
 M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 1 Total Commitment to Education and Service Provided by ACP, Inc. Immunology MLE M1 Table of Contents 2011 Evaluation Criteria...
M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 1 Total Commitment to Education and Service Provided by ACP, Inc. Immunology MLE M1 Table of Contents 2011 Evaluation Criteria...
The Taipan snake venom time: a new test for lupus anticoagulant
 J Clin Pathol 1994;47:497-51 The Taipan snake venom time: a new test for lupus anticoagulant 497 Department of Haematology, University College London Medical School, London WClE 6HX M Rooney T McNally
J Clin Pathol 1994;47:497-51 The Taipan snake venom time: a new test for lupus anticoagulant 497 Department of Haematology, University College London Medical School, London WClE 6HX M Rooney T McNally
HAEMATOLOGY. Test Name Status Result Unit Reference Interval HbA1c (Glycated Haemoglobin) 5.4 % Method : HPLC Sample Type : Whole Blood EDTA
 Institution Report Date 25-Dec-2016 05:59 PM HAEMATOLOGY HbA1c (Glycated Haemoglobin) 5.4 % 4.5-6.0 Method : HPLC Sample Type : Whole Blood EDTA REMARKS In vitro quantitative determination of HbA1c in
Institution Report Date 25-Dec-2016 05:59 PM HAEMATOLOGY HbA1c (Glycated Haemoglobin) 5.4 % 4.5-6.0 Method : HPLC Sample Type : Whole Blood EDTA REMARKS In vitro quantitative determination of HbA1c in
HEMATOLOGY AND COAGULATION ANALYTIC PROTOCOLS
 1 of 6 Policy #: 800 (PLH-800-13) Initiated Date: 12/4/2002 Reviewed Date: 8/1/2016 Subject: HEMATOLOGY AND COAGULATION ANALYTIC PROTOCOLS Approved by: Laboratory Director, Jerry Barker (electronic signature)
1 of 6 Policy #: 800 (PLH-800-13) Initiated Date: 12/4/2002 Reviewed Date: 8/1/2016 Subject: HEMATOLOGY AND COAGULATION ANALYTIC PROTOCOLS Approved by: Laboratory Director, Jerry Barker (electronic signature)
Plasma Levels of Protein Z and Antiphospholipid Protein Antibodies in Patients with Ischemic Stroke
 Mohamed Elkhateeb et al. Plasma Levels of Protein Z and Antiphospholipid Protein Antibodies in Patients with Ischemic Stroke Mohamed Elkhateeb 1, Ibrahim Elmenshawy 1, Hanan Azzam 2, Tarek Selim 2, Nashwa
Mohamed Elkhateeb et al. Plasma Levels of Protein Z and Antiphospholipid Protein Antibodies in Patients with Ischemic Stroke Mohamed Elkhateeb 1, Ibrahim Elmenshawy 1, Hanan Azzam 2, Tarek Selim 2, Nashwa
Lupus anticoagulant-hypoprothrombinemia syndrome: report of two cases and review of the literature
 (2015) 24, 736 745 http://lup.sagepub.com CONCISE REPORT anticoagulant-hypoprothrombinemia syndrome: report of two cases and review of the literature SMN Mulliez 1, F De Keyser 2, C Verbist 2, A Vantilborgh
(2015) 24, 736 745 http://lup.sagepub.com CONCISE REPORT anticoagulant-hypoprothrombinemia syndrome: report of two cases and review of the literature SMN Mulliez 1, F De Keyser 2, C Verbist 2, A Vantilborgh
Newer Anti-Anginal Agents and Anticoagulants
 Newer Anti-Anginal Agents and Anticoagulants Satish Gadi, MD FACC FSCAI Interventional Cardiologist, Cardiovascular Institute of the South (CIS) Baton Rouge Clinical Assistant Professor, Tulane University
Newer Anti-Anginal Agents and Anticoagulants Satish Gadi, MD FACC FSCAI Interventional Cardiologist, Cardiovascular Institute of the South (CIS) Baton Rouge Clinical Assistant Professor, Tulane University
Product and price list for reference materials and services from QSE GmbH
 Product and price list for reference materials and services from QSE GmbH Valid from: January 01, 2018 Seite Products for checking inhibitor analysis 2 Products for checking analysis of somatic cells 2
Product and price list for reference materials and services from QSE GmbH Valid from: January 01, 2018 Seite Products for checking inhibitor analysis 2 Products for checking analysis of somatic cells 2
Evaluation of antiphospholipid antibodies testing for the diagnosis of antiphospholipid syndrome
 Original Article Evaluation of antiphospholipid antibodies testing for the diagnosis of antiphospholipid syndrome Paula Gonçalves Perches¹, Daniela Pezzutti Domingues¹, Andréia Latanza Gomes², Augusta
Original Article Evaluation of antiphospholipid antibodies testing for the diagnosis of antiphospholipid syndrome Paula Gonçalves Perches¹, Daniela Pezzutti Domingues¹, Andréia Latanza Gomes², Augusta
Date 29 th March 2018 Our Ref FA_Anticoag/MGPG/Mar18 Enquiries to Frances Adamson Extension Direct Line tadamson(anhs.
 NHS Grampian Westholme Woodend Hospital Queens Road ABERDEEN AB15 6LS NHS Grampian Date 29 th March 2018 Our Ref FA_Anticoag/MGPG/Mar18 Enquiries to Frances Adamson Extension 56689 Direct Line 01224 556689
NHS Grampian Westholme Woodend Hospital Queens Road ABERDEEN AB15 6LS NHS Grampian Date 29 th March 2018 Our Ref FA_Anticoag/MGPG/Mar18 Enquiries to Frances Adamson Extension 56689 Direct Line 01224 556689
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Spire Portsmouth Hospital Bartons Road Havant PO9 5NP United Kingdom Contact: Natalie Peck E-Mail: natalie.peck@spirehealthcare.com Website:
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Spire Portsmouth Hospital Bartons Road Havant PO9 5NP United Kingdom Contact: Natalie Peck E-Mail: natalie.peck@spirehealthcare.com Website:
ORIGINAL PAPERS. Marek Cieśla 1, B D, Ewa Wypasek 1, 2, B D 1, 2, A, E, F. Abstract
 ORIGINAL PAPERS Adv Clin Exp Med 2014, 23, 5, 729 733 ISSN 1899 5276 Copyright by Wroclaw Medical University Marek Cieśla 1, B D, Ewa Wypasek 1, 2, B D 1, 2, A, E, F, Anetta Undas IgA Antiphospholipid
ORIGINAL PAPERS Adv Clin Exp Med 2014, 23, 5, 729 733 ISSN 1899 5276 Copyright by Wroclaw Medical University Marek Cieśla 1, B D, Ewa Wypasek 1, 2, B D 1, 2, A, E, F, Anetta Undas IgA Antiphospholipid
T he antiphospholipid syndrome (APS) is a thrombophilic
 1639 EXTENDED REPORT ntiphospholipid antibody tests: spreading the net M L ertolaccini, S Gomez, J F P Pareja, Theodoridou, G Sanna, G R V Hughes, M Khamashta... See end of article for authors affiliations...
1639 EXTENDED REPORT ntiphospholipid antibody tests: spreading the net M L ertolaccini, S Gomez, J F P Pareja, Theodoridou, G Sanna, G R V Hughes, M Khamashta... See end of article for authors affiliations...
ISO IDF 177 INTERNATIONAL STANDARD. Dried skimmed milk Determination of vitamin D content using high-performance liquid chromatography
 INTERNATIONAL STANDARD ISO 14892 IDF 177 First edition 2002-02-01 Dried skimmed milk Determination of vitamin D content using high-performance liquid chromatography Lait écrémé sec Détermination de la
INTERNATIONAL STANDARD ISO 14892 IDF 177 First edition 2002-02-01 Dried skimmed milk Determination of vitamin D content using high-performance liquid chromatography Lait écrémé sec Détermination de la
Urgent Field Corrective Action
 Siemens Healthcare Diagnostics Products GmbH N Latex CDT Urgent Field Corrective Action PP-18-002.A.OUS May 2018 Negative bias of %CDT values Our records indicate that your facility may have received the
Siemens Healthcare Diagnostics Products GmbH N Latex CDT Urgent Field Corrective Action PP-18-002.A.OUS May 2018 Negative bias of %CDT values Our records indicate that your facility may have received the
Toxoplasma gondii IgM (Toxo IgM)
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
