ABSTRACT. The objective of this study was to assess the effectiveness of a Nycodenz gradient
|
|
|
- Prosper Holland
- 5 years ago
- Views:
Transcription
1 ABSTRACT MILLER, STEPHANIE RENEE. Assessment of nycodenz gradient on enrichment and culture of perinatal porcine spermatogonial stem cells. (Under the direction of Robert M. Petters). The objective of this study was to assess the effectiveness of a Nycodenz gradient enrichment method to enrich a dissociated single cell suspension of porcine testicular cells for spermatogonia, and to observe the separated fractions from the gradient over a 14-day culture period for cell viability and number of spermatogonia in culture. Two germ cell specific genes, VASA and DAZL, were utilized for detection of spermatogonia using immunohistochemistry. The control group included cultures generated from the enzymatic digestion of porcine testes prior to the enrichment protocol for each replicate. The NycoDenz gradient consistently separated the isolated cell suspension into three distinct layers and a pellet, all of which were assessed for spermatogonial enrichment. Testis cells were isolated and seeded in culture on day 0. Cell viability and percent of spermatogonia was assessed on day 0, 7, and 14 of culture. Viability was determined using trypan blue exclusion assay and quantified using a hemocytometer. Spermatogonia were morphologically identified as round, plump cells with a large amount of cytoplasm. Visualization of spermatogonia was facilitated by immunostaining with DAZL and VASA polyclonal antibodies and cells exhibiting morphological characteristics in addition to bright, concentrated fluorescence were counted as spermatogonia.
2 ASSESSMENT OF NYCODENZ GRADIENT ON ENRICHMENT AND CULTURE OF PERINATAL PORCINE SPERMATOGONIAL STEM CELLS By STEPHANIE RENEE MILLER A thesis submitted to the Graduate Faculty of North Carolina State University in partial fulfillment of the requirements for the Degree of Master of Science ANIMAL SCIENCE Raleigh 2006 APPROVED BY: Chair of Advisory Committee Dr. Robert Petters Dr. William Flowers Dr. Paul Mozdziak Dr. Jim Petitte
3 BIOGRAPHY Stephanie Miller was born on November 17, 1981 in Bedford, Pennsylvania to Patrick and Patricia Miller. Along with her younger sister and older brother, she attended grade school at St. Thomas the Apostle Catholic School. Upon graduation from Bedford High School in May of 1999, the author chose to pursue her undergraduate degree in Animal Science at North Carolina State University. In August of 1999, the author left her family in Bedford and moved to Raleigh, North Carolina to be the first in the family to complete a bachelor s degree in May Soon after graduation, she began a graduate program in May 2003 at North Carolina State University and is pursuing her Master of Science in Animal Science. ii
4 ACKNOWLEDGEMENTS I would like to extend a special thank you to Dr. Robert Petters. I appreciate him accepting me as his graduate student and his knowledge, guidance, and patience have helped me to achieve my goals and to grow as an individual. Dr. Petters has taught me persistence, independence, and resourcefulness; all characteristics that will help me succeed in with future endeavors. I would also like to extend my appreciation to my committee members, Dr. William Flowers, Dr. Paul Mozdziak, and Dr. Jim Petitte, for their guidance and support. My entire committee has shown me how individuals from various disciplines can work together to achieve a common goal. I would like to express a very special thanks to Mr. Jeff Sommer and Dr. Jose Estrada for their daily technical advice, support, and assistance. I also would like to thank Bruce Collins, Curtis Powell, Ellen Fritz, and Stephen Beasley for their knowledge and collaboration. I would like to thank my family and friends for their encouragement, friendship, and support throughout my time in graduate school: Mom, Dad, Jenny, Aunt Kathy, Meredith Johnson, and Nicola Wrench. My dear friend Melissa, I would not have been able to get through without your encouragement and support. I also would like to extend my appreciation to my Tang Soo Do instructor Master Ken Marsh: your patience, teaching, and constant guidance pushed me to improve, regardless if I wanted to or not. To my closest friend Ben, thank you for everything you have done. I will never be able to express how much I have appreciated your encouragement, assistance, patience, and love. iii
5 TABLE OF CONTENTS LIST OF TABLES vii LIST OF FIGURES viii INTRODUCTION AND REVIEW OF THE LITERATURE SPERMATOGENESIS IN VIVO..1 Early development of testicular cells Maturation from fetal to puberty....2 Process of Spermatogenesis...5 ISOLATION OF SPERMATOGONIA...14 Flow Cytometry...14 Magnetic Activated Cell Sorting Velocity Sedimentation Percoll Separation Nycodenz Gradient.. 26 IDENTIFICATION OF SPERMATOGONIA Deleted in Azoospermia-like (DAZL) VASA...37 IN VITRO SPERMATOGONIA CULTURE.45 Development of Spermatogonia Porcine Spermatogonia Cell Culture...52 Cell Behavior in Culture GENE TRANSFER IN PORCINE SPERMATOGONIA...58 Types of Gene Transfer...58 iv
6 Transduction of Germ Cells Transplantation of Germ Cells STATEMENT OF THE PROBLEM MATERIALS AND METHODS Experimental Design Reagents and Media Isolation of Porcine Testis Cells.. 71 Enrichment of Spermatogonia Viability of Spermatogonia Cultures...74 Assessment of Spermatogonia Number in Culture.. 74 Spermatogonia in Suspension.. 75 Spermatogonia Attached to Culture Dish Statistical Analysis RESULTS Effect of Enrichment Gradient on Cell Viability in culture Effect of Enrichment Gradient on Number of Spermatogonia in Culture...85 Effect of Enrichment Gradient on Number of Spermatogonia Attached to Plate Effect of Enrichment Gradient on Number of Spermatogonia in Suspension 92 Effect of Enrichment Gradient on Total Number of Spermatogonia in culture Identification of Cell Types in Gradient Layers DISCUSSION v
7 LITERATURE CITED APPENDICES Appendix A. SAS Code Appendix B. Sample Digital Images vi
8 LIST OF TABLES Table 1. Porcine testicular development Table 2. Significance of layer, day, replicate, and layer by day effects on percent viability Table 3. Cell viability for each layer and day Table 4. Significance of layer, day, replicate, and layer by day effects on percent spermatogonia Table 5. Percent of spermatogonia in attached cell population for each layer and day...89 Table 6. Percent of spermatogonia in suspension cell population for each layer and day...96 Table 7. Percent of spermatogonia in total cell population for each layer and day vii
9 LIST OF FIGURES Figure 1. Spermatogenesis in vivo...12 Figure 2. Diagram of spermatogenesis...13 Figure 3. Structure of Nycodenz...29 Figure 4. Cell cycle and its restriction points...57 Figure 5. Discontinuous Nycodenz gradient prior to centrifugation...78 Figure 6. Discontinuous Nycodenz gradient after centrifugation...78 Figure 7. Layer effect on viability for all days...81 Figure 8. Day effect on viability for all layers...81 Figure 9. Replicate effect on viability for all layers and days...82 Figure 10. Cell viability of each layer on day 0 of culture...83 Figure 11. Cell viability of each layer on day 7 of culture...83 Figure 12. Cell Viability of each layer on day 14 of culture...84 Figure 13. Layer effect on percent of spermatogonia in attached cell population for all days...88 Figure 14. Day effect on percent of spermatogonia in attached cell population for all layers...89 Figure 15. Percent of spermatogonia in attached cell population in gradient layers on day 0 of culture...90 Figure 16. Percent of spermatogonia in attached cell population in gradient layers on day 7 of culture...90 Figure 17. Percent of spermatogonia in attached cell population in gradient layers on day 14 of culture...91 Figure 18. Layer effect on percent of spermatogonia in suspension for all days...95 viii
10 Figure 19. Day effect on percent of spermatogonia in suspension for all layers...95 Figure 20. Percent of spermatogonia in suspension in gradient layers on day 0 of culture...96 Figure 21. Percent of spermatogonia in suspension in gradient layers on day 7 of culture...97 Figure 22. Percent of spermatogonia in suspension in gradient layers on day 14 of culture...97 Figure 23. Layer effect on total percent of spermatogonia for all days Figure 24. Overall day effect on total percent of spermatogonia in culture Figure 25. Percent of total spermatogonia on day 0 of culture Figure 26. Percent of total spermatogonia on day 7 of culture Figure 27. Percent of total spermatogonia on day 14 of culture ix
11 INTRODUCTION AND REVIEW OF LITERATURE This review will cover the topics of in vivo spermatogenesis, the techniques used for isolation of spermatogonia, the maintenance and detection of spermatogenic cells in culture, and gene transfer in spermatogonia. The primary focus will be on porcine spermatogonia; however, the mouse and other species will be discussed. SPERMATOGENESIS IN VIVO EARLY DEVELOPMENT OF TESTICULAR CELLS Primordial germ cells in the early embryo originate from the proximal epiblast. Prior to sexual differentiation in the developing embryo, primordial germ cells migrate from the base of the allantois and populate the sexually indifferent genital ridge at about 21 days of gestation in the pig (Pelliniemi, 1975). The primordial germ cells then populate the indifferent gonads of the developing fetus and become gonocytes. As development continues, gonadal expression of the sex determining gene, Sry, causes pre-sertoli cells to differentiate at day 11.5 post coitum (Tilmann and Capel, 2002). Directly after differentiation of the pre-sertoli cells, the indifferent gonad becomes a differentiated testis between days 27 and 56 of gestation in pigs. Other cells differentiate in the testis including endothelial cells, Leydig cells, fibroblasts, and peritubular myoid cells (Franca et al., 2005). As the gonads of the genetically male fetus develop, structural changes occur including the differentiation of the Sertoli and Leydig cells and the formation of the seminiferous cords. Seminiferous cord formation is a result of morphological interactions between Sertoli cell precursors, germ cells, and peritubular myoid cells (Franca et al., 2005). During this early stage, anti-mullerian hormone (AMH) is secreted by Sertoli cells, which is 1
12 responsible for suppressing the development of the female reproductive tract (Capel, 2000). The fetal Leydig cells produce the steroid hormone testosterone, which is responsible for the developmental regulation of secondary sex characteristics. Follicle stimulating hormone (FSH) is responsible for postnatal proliferation of Sertoli cells and thyroid hormone (T3) has a role in the transition from mitotic to a non-mitotic state, which occurs in Sertoli cells before puberty (Capel, 2000). In this period of fetal development, the proper level of androgens and estrogens is important for normal male development (Franca et al., 2005). MATURATION FROM FETAL STAGE TO PUBERTY Sertoli cells have many specialized functions in the porcine testis, including providing support for the differentiating germinal cells within the seminiferous epithelium, phagocytosis of cytoplasmic bodies shed from the late spermatids at spermiation, release of mature spermatozoa into the seminiferous lumen of the testis, and providing nutrition to the developing germ cells. Together with adjacent cells, Sertoli cells provide a protected environment for the developing germ cells by forming a blood-testis barrier that compartmentalizes the seminiferous epithelium via tight junctions into a luminal and adluminal compartment. The junctions between neighboring Sertoli cells isolate the germ cells in the adluminal compartment, which is inaccessible to macromolecules from the bloodstream that carry nutritive value, causing the nutrition of the germ cells to be dependent on the Sertoli cells. Sertoli cells provide the germ cells with pyruvate and lactate as energy sources, as well as iron and other vitamins necessary for survival (Russell and Griswold, 1993). 2
13 The total number of Sertoli cells established in the testis during development dictates the adult size of the testis and the sperm production in the adult animal (Franca et al., 2000). The number of Sertoli cells present in the testis is important because each cell can only support a limited number of developing germ cells. The pattern of Sertoli cell proliferation in pigs after birth is quite different from that found in various rodent species, perhaps due to the delayed onset of puberty. In pigs, there are two periods of mitotic Sertoli cell proliferation. The first phase takes place after birth up to 1 month of age where the Sertoli cell population increases several fold. The second phase of postnatal proliferation occurs during the third and fourth month of development and augments the cell population by 80%. After four months of age, the Sertoli cell population slightly decreases until seven to sixteen months of age were the population stabilizes and cell division ceases. After the onset of puberty, no Sertoli cell death is normally observed in the adult porcine testis (Franca et al., 2000). During testicular development, the plasma FSH levels are highest during Sertoli cell proliferation. FSH is important for mitotic activity of postnatal Sertoli cells, maturation of Sertoli cells, and initiation of the first wave of spermatogenesis (Franca et al., 2000). The majority of the intertubular space in the porcine testis is occupied by Leydig cells, which are found in clusters within the testis. Leydig cells are responsible for the biosynthesis and secretion of testosterone under the control of leutinizing hormone (LH) and (FSH) from the pituitary (Capel, 2000). Leydig cell development in pigs exists in three phases: a transient phase during the early fetal period, a transient phase during the perinatal period, and the final phase from 3 months of age through puberty and into adulthood. In pigs, Leydig cell volume and the 3
14 number of LH receptors change during the different periods of development (Van Straaten and Wensing, 1978). The first phase of Leydig cell development begins immediately after gonad differentiation where the population of mature cells increases and a high level of testosterone is produced. It is during this first period of Leydig cell development that the accessory sex organs develop (Van Straaten and Wensing, 1978). Leydig cells develop in the second phase during the 5-week period surrounding the time of birth. In this period before and after birth, a large number of differentiated cells emerge with a strong steroidgenic capability (Van Straaten and Wensing, 1978). The third phase of Leydig cell proliferation occurs 13 weeks after birth and is important for the maintenance of accessory sexual glands and spermatogenesis, both of which are androgen dependent. During stage three of Leydig cell proliferation is when the population of cells characteristic of the adult testis is formed (Van Straaten and Wensing, 1978). Throughout male development, germ cells undergo two phases including the developmental phase and the differentiation phase. During the developmental phase, the germ cells initially develop in the embryo and eventually become primordial germ cells and migrate to colonize the developing gonads in the fetus. After the primordial germ cells colonize the gonad, they undergo sexual differentiation. The differentiation phase of male germ cells includes the differentiation of early spermatogonia to spermatozoa (Desjardins and Ewing, 1993). At birth the number of porcine germ cells in the seminiferous tubule is very low, but dramatically increases between two and fourfold at monthly intervals from 4 to 5 months of age and then stabilizes at about 7 months of age. During testicular development in the pig, a 4
15 correlation was observed between the number of germ cells in the seminiferous cord and testicular weight (Franca et al., 2000). After birth germ cells proliferate continuously, but an apparent decrease in the density of germ cells is observed, likely due to the increased proliferation of other testicular cells. The growth rate of the germ cells has been estimated to be half the rate of developing Sertoli cells. The germ cells are undergoing mitosis during this time, and apoptosis was rarely observed. This pattern of proliferation is in contrast to the proliferation of germ cells in rodent species, where germ cells are quiescent or undergo massive degeneration after birth (Gondos and Byskov, 1981). As puberty approaches in pigs, the number of Sertoli cells, Leydig cells, and germ cells stabilizes (Franca et al., 2000). PROCESS OF SPERMATOGENESIS Spermatogenesis is a highly regulated process that takes place in the seminiferous tubules of the testis and includes all transformation steps necessary for germ cells to develop into differentiated spermatozoa (Figure 1). Spermatogenesis encompasses three distinct processes throughout development including spermatocytogenesis in the basal compartment when the A 1 spermatogonia undergo many developmental steps to become type B spermatogonia, meiosis in the adluminal compartment of the seminiferous tubules which gives rise to spermatids, and spermiogenesis also in the adluminal compartment that includes transformation of spermatids into the fully developed and differentiated spermatozoa (Figure 2),(Senger, 1997). The time of development of the male germ cells though spermatogenesis varies between species, and average times of porcine testicular development is summarized in table 1. 5
16 Spermatocytogenesis starts with the most immature germ cells called spermatogonia. A spermatogonium is the name used to describe a relatively unspecialized diploid germ cell in the seminiferous epithelium that has the ability to undergo mitosis and ultimately yield primary spermatocytes. The several types of spermatogonia are classified on the amount of heterochromatin present in the nucleus. Presence of heterochromatin is indicative of a more differentiated cell; therefore, the most primitive cell is the type A spermatogonium. Intermediate spermatogonia are more developed than type A spermatogonia, and type B spermatogonia are the most differentiated (DeRooij and Russell, 2000). Many types of type A spermatogonia exist, the most primitive of which is the A-single (A s ) which has also been referred to as A-isolated or stem cell spermatogonia (DeRooij and Russell, 2000). The A s cell is functionally defined as able to give rise to other stem cells in addition to initiating the development of cells that are committed to form differentiated cells. Along with the functional definition of A s spermatogonia, morphologically the cells are the only type of spermatogonia without the presence of intercellular bridges. The formation of intercellular bridges plays an important role in spermatogenesis in the boar. The bridges between cells allow for sharing of gene products and synchronization of cellular activities allowing for synchronized development (DeRooij and Russell, 2000). In the beginning of spermatocytogenesis, the A s spermatogonia are in close proximity to the basement membrane at the periphery of the seminiferous tubule. The route of development from stem cell to a cell committed to mitosis is still a topic for debate. There are two possible fates of a spermatogonial stem cell, one of which is another stem cell and the other is a cell committed to differentiation. Currently two theories describe this method of development using either symmetrical division or asymmetrical division. The 6
17 asymmetrical division theory describes the spermatogonial stem cell dividing to yield a spermatogonial stem cell and another single spermatogonial cell that will again divide to yield a pair of spermatogonia (A pr ). The daughter cell that is a stem cell will divide and produce two new stem cells. The symmetrical division theory supports the idea that the fate of the daughter cells could depend on a mechanism that regulates the formation of an intercellular bridge. The stem cell can divide to either form a pair of spermatogonia (A pr ) or two separate stem cells. Although some reports of asymmetrical division are present in the literature, the theory of symmetrical divisions is the predominate theory (DeRooij and Russell, 2000). About half of the resulting daughter cells stay connected together by an intercellular bridge and become A-paired (A pr ) spermatogonia. The other half of the daughter cells migrates apart and goes through self-renewing divisions to maintain the stem cell population in the testis. A pr spermatogonia are committed to the differentiation pathway and develop to form chains of A-aligned (A al ) spermatogonia consisting of 4, 8, or 16 cells (Goldberg, 2000). A s, A pr, and A al spermatogonia are all considered undifferentiated spermatogonia, although these cells are very similar to the A spermatogonia referred to as differentiating. In fact, A pr and A al spermatogonia are differentiated in that they are irreversibly committed to developing towards differentiated spermatogonia (DeRooij and Russell, 2000). A s, A pr, and A al spermatogonia all divide at random during the cycle of the seminiferous epithelium. The A al spermatogonia will develop into the first generation of differentiating calls called A 1 spermatogonia (Goldberg, 2000). This step in development is a transition from the cycling of undifferentiated spermatogonia to rigid mitotic divisions of type A spermatogonia. The transition to A 1 spermatogonia does not include a mitotic 7
18 division, but instead a transformation occurs in the G 0 /G 1 phase of the cell cycle to change the A al spermatogonia to A 1 spermatogonia (DeRooij and Russell, 2000). The spermatogonial germ cells then undergo mitotic divisions while staying connected by intercellular bridges and migrate toward the lumen of the seminiferous tubule. At the beginning of the rigid mitotic divisions, the spermatogonia are specialized diploid germ cells with a chromosomal content of 2n and are termed A 1 spermatogonia. A 1 spermatogonia mitotically divide to yield two A 2 spermatogonia. The A 2 spermatogonia enter mitosis again to form A 3 spermatogonia, and A 4 spermatogonia are formed after an additional mitotic division. The process of A-spermatogonia mitotically progressing to A 4 spermatogonia is continued indefinitely and is supplied with A 1 spermatogonia by a pool of precursor stem cells. The next step of spermatocytogenesis involves the A 4 spermatogonia mitotically dividing to form intermediate spermatogonia, which in turn divide to form B- spermatogonia. Type B-spermatogonia then divide mitotically, forming primary spermatocytes that have a DNA content of 4N, which begin development in the adluminal compartment of the seminiferous tubule (Senger, 1997). After the development of spermatogonia into primary spermatocytes, the meiosis stage of spermatogenesis begins. Meiosis occurs in two steps in the adluminal compartment of the seminiferous tubule and is necessary to reduce the gamete chromosome number to the haploid state of 1n. The primary spermatocytes enter prophase I of the first meiotic division which consists of five stages: preleptotene, leptotene, zygotene, pachytene and diplotene. During the preleptotene phase, DNA is replicated and segments of homologous chromosomes are exchanged to contribute to the genetic diversity of the secondary spermatocytes that form after meiosis is complete. Upon completion of prophase I of 8
19 meiosis, the primary spermatocytes progress through the other stages of meiosis: metaphase I, anaphase I, and telophase I to become secondary spermatocytes with a DNA content of 2C. The developmental phases of meiosis II are similar to that of meiosis I to form haploid spermatids carrying a DNA content of only 1N (Senger, 1997). The final step of spermatogenesis is spermiogenesis, beginning with the haploid spermatids and includes four phases that involve physical changes from spherical spermatids to differentiated spermatozoa capable of fertilization. The first phase of spermiogenesis is the Golgi phase where the acrosome and the beginning of the axoneme are developed. During the cap phase, the acrosome flattens to form a cap over the anterior portion of the nucleus consisting of an outer acrosomal membrane and an inner acrosomal membrane. It is also in this phase where the axoneme begins to elongate from the nucleus of the cell. The acrosomal phase involves the spreading of the acrosome to cover the anterior portion of the nucleus and the cytoplasm and nucleus begin to elongate. At this time the spermatids become embedded in the Sertoli cells with the developing tails protruding into the lumen of the seminiferous tubule. The final stage of spermiogenesis is the maturation phase where the mitochondria assemble and dense fibers form around the flagellum. After all steps of spermiogenesis have been completed, spermiation or the release of spermatozoa occurs from the Sertoli cells into the lumen of the seminiferous tubule (Senger, 1997). The process of spermatogenesis occurs in cycles in the seminiferous epithelium, and involves progression through a series of stages at one segment along the seminiferous tubule. The cycle of the seminiferous epithelium of the boar can be divided into eight stages, each of which is composed of a typical cellular association. If a cross section of the seminiferous epithelium is examined, usually one stage of the cycle is represented. The duration of the 9
20 cycle varies by species and is about 8.3 days in the boar. The complete process of spermatogenesis from type A-spermatogonia to differentiated spermatozoa requires 39 days in the boar. During this time period, developing cells in a specific area of the seminiferous tubule complete about 4.5 cycles of the seminiferous epithelium (Senger, 1997). Stage I of the spermatogenic cycle begins with an absence of spermatozoa in the lumen of the seminiferous tubule to the onset of spermatid elongation and lasts for 1.1 days. The basement membrane is lined with Sertoli cells and type A spermatogonia. Also present in this stage are primary spermatocytes in preleptotene and leptotene and older primary spermatocytes in pachytene along with spermatids with spherical nuclei. In stage II, the basement membrane is lined with Sertoli cells, type A spermatogonia and also present are zygotene and pachytene primary spermatocytes. Stage II spans the process of spermatid nuclei elongation from beginning to end and lasts for 1.4 days. The III stage of the seminiferous epithelium in the boar lasts 0.4 days, starts from the end of spermatid elongation and includes up to the beginning of the meiotic division of the primary spermatocytes. The basement membrane is lined with the same cells as in the previous stages, and two generations of primary spermatocytes are present. Stage IV spans the time frame between the beginning of the first meiotic division and the end of the second meiosis which takes 1.2 days. The basement membrane is now lined with Sertoli cells and type A spermatogonia. In the early part of stage four, the old primary spermatocytes undergo meiosis I to yield secondary spermatocytes which are only present in this stage because they quickly progress through meiosis II and form spherical spermatids. The end of the second meiosis up to when nuclei of spermatids take on a dusty appearance indicating the presence of heterochromatin in the nucleus, encompasses the 0.8 day long stage V of the cycle of the 10
21 seminiferous epithelium. The basement membrane in stage V is lined with Sertoli cells and type A spermatogonia. The primary spermatocytes in this stage enter into pachytene where the nuclei will augment and migrate away from the basement membrane. As the spermatogonia become more differentiated, they will contain more heterochromatin in the nucleus, which is a characteristic of more differentiated spermatogonia. Stage VI starts when the spermatid nuclei appear dusty and ends when all spermatozoa move toward the lumen and have left the Sertoli cells, a period of 1.6 days. Type B and type A spermatogonia are present near the end of this stage. Stage VII lasts for 1.0 days and includes the beginning of spermatozoa movement to the lumen up to the end of the migration. At this time, the basement membrane is lined with Sertoli cells and spermatogonia. Either at the end of stage seven or early in stage eight, type B spermatogonia mitotically divide to form preleptotene primary spermatocytes. The final stage, stage VIII, of the cycle of the seminiferous epithelium in the boar takes 0.8 days and spans the time period of when the spermatozoa line the lumen up until their complete disappearance from the lumen. Some B spermatogonia may be present along with type A spermatogonia in the final stage. In addition, primary spermatocytes are present in the preleptotene and in pachytene stages of stage eight (Swierstra, 1968). The process of spermatogenesis is a unique, highly complex, and highly efficient method of generating gametes that deliver genes to subsequent generations. Spermatogenesis encompasses the development of cells throughout spermatocytogenesis, meiosis, and spermiogenesis to yield mature spermatozoa capable of fertilization and producing young. Understanding the process of spermatogenesis will allow for utilization of cells at specific 11
22 times of development for manipulation in vitro and subsequent generation of transgenic animals. Figure 1. Spermatogenesis in vivo. Desjardins and Ewing,
23 Figure 2. Diagram of spermatogenesis. Adapted from Senger, month Undifferentiated type A spermatogonia, Leydig, Sertoli 2 months Undifferentiated type A spermatogonia, 1º Spermatocytes, Leydig, Sertoli days Formation of the Blood Testis Barrier 4 months 2º Spermatocytes, Leydig, Sertoli 6 months Spermatozoa, Leydig, Sertoli Birth Undifferentiated type A spermatogonia, Leydig, Sertoli Table 1. Porcine testicular development. Adapted from Cole and Foxcroft,
24 ISOLATION OF SPERMATOGONIA FLOW CYTOMETRY Flow cytometry is a method of sorting cells that utilizes automated equipment to measure and separate cells in a suspension. The equipment used is similar to a microscope equipped with a flow cell, various lasers, an amplification system, and a computer to convert and analyze the measurements. One type of flow cytometry is FACS, or fluorescentactivated cell sorting. FACS uses various characteristics of cells including light scattering or fluorescence to sort cells. FACS is a very accurate separation method if the characteristics of the cells to be separated are known. Some major drawbacks to using FACS include the large number of cells initially needed to perform FACS, the loss of cells during the sorting procedure, and the expense of purchasing the equipment. Despite these issues, FACS is used in many areas of research, including in the field of reproduction. Fluorescent-activated cell sorting has been used for isolation of different spermatogonia cell types in different species and to enrich for a specific type of spermatogonia. FACS has been used to separate spermatogonia in mice (Lassalle et al., 1999; Janca et al., 1986; Petit et al., 1995; Lo et al., 2005; Mays-Hoopes et al., 1995; Shinohara et al., 2000), rats (Malkov and Don, 1998; Iwanami et al., 2006), bulls (Izadyar et al., 2002a; Izadyar et al., 2002b), humans (Levek-Motola et al., 2005), and monkeys (Moudgal et al., 1997) but no report was found in the literature isolating porcine spermatogonia with FACS. Mouse testis cells have been separated by Shinohara s research group using two parameters, cell size and intercellular complexity to enrich for spermatogonia stem cells (Shinohara et al., 2000). This separation was achieved by analysis of light scattering patterns 14
25 of observed cells into four fractions. Forward light scatter pattern allowed separation of cells by size, and side light scatter indicated cell shape and intercellular complexity. Unfortunately, the most enriched fraction was not statistically different from the control when wild-type animals were used. The lack of difference between the control and experimental group was attributed to two factors (Shinohara et al., 2000). The labeling and sorting procedure could have reduced the number of target cells in the experimental group when compared to the untreated controls, as was reported 1979 (Hunt, 1979). Also the testis cell population may have been too complex to sort using the specified parameters (Shinohara et al., 2000). FACS was used in another study to isolate round spermatids from the murine testis (Lassalle et al., 1999). The procedure utilized a Percoll gradient separation prior to the FACS and resulted in a 2-fold enrichment of round spermatids. The identification of isolated cells as round spermatids was confirmed by reverse transcriptase-polymerase chain reaction analysis for two genes specifically transcribed from the haploid genome, PRM2 and SP-10. The viability of enriched spermatids was reported as 99%, demonstrating that FACS can be successful at enriching differentiated male germ cells (Lassalle et al., 1999). Recently a study was done using FACS to study cryptorchid mouse testes to enrich for spermatogonial stem cells (Lo et al., 2005). Cells were stained with a rhodamine fluorescent dye and sorted according to fluorescent intensity. The quiescent nature of stem cells produces a low fluorescence with this dye, and allowed for a 17- to 20- fold enrichment of the murine spermatogonial stem cells. No information on the viability of separated stem cells was available (Lo et al., 2005). 15
26 It is evident from the literature that FACS can be a valuable tool for the separation and enrichment of various cells; however, some problems exist when working with spermatogonial cells. Populations of testis cells have been separated using FACS, but the success of spermatogonial stem cell enrichment with high viability is not common since viability statistics were not consistently reported after separation. When using normal murine testis tissue, only round spermatids were successfully enriched using FACS in conjunction with a pre-enrichment gradient (Lassalle et al., 1999). The enrichment of spermatogonial stem cells was successful using FACS, but only with the additional enrichment of using a cryptorchid animal model (Lo et al., 2005). Cell viability after separation is an important factor to consider when evaluating the effectiveness of FACS enrichment. Some studies reported viability of the most enriched portion of cells, but no viability statistics were reported before separation to show FACS effect on viability. The cost of FACS equipment, volume of cells necessary for separation, questionable effects on viability, and overall effectiveness of FACS for enrichment of spermatogonial stem cells places doubt on FACS as the optimal method for enrichment of porcine spermatogonial stem cells. MAGNETIC ACTIVATED CELL SORTING The sorting of cells using magnetism is based on the use of metallic beads and strong magnetic separators. The particles used are small, 50nm beads called Microbeads that are paramagnetic particles composed of iron oxide and polysaccharide. The microbeads are coupled to various specific antibodies that bind to the targeted cells contained in the cell suspension. A strong magnetic field is used to retain the cells bound to the antibody 16
27 conjugated to the microbead. Upon removal of magnets, an enriched cell population is formed. The microbeads can be used for positive selection or depletion within the original heterogeneous cell suspension. In 30 minutes, the magnetic activated cell separation (MACS) can be performed and yield a high purity of the cells of interest (MiltenyiBiotec, 2006). The microbeads are biodegradable, and it is suggested that they have no known effect on subsequent experiments, although no supporting literature was cited. The effects of the degradation of the beads in culture is not known, although a method of separating the beads from the cells exists requiring a longer separation time and exposure of the cells to more reagents. The effect of reagents used to separate the beads from the target cell population is also not known. Some drawbacks to using magnetic activated cell sorting include the cost of separation apparatus, questionable effects on viability, and the low amount of cells recovered from the isolation procedure. Separation of cells using magnetic beads has been used in many types of research including the study of germ cells. The isolation of murine spermatogonial stem cells has been facilitated by magnetic bead separation (Giuili et al., 2002; Kubota et al., 2004; Van Der Wee et al., 2001; Hofmann et al., 2005b; Shinohara and Brinster, 2000), while enrichment of monkey and hamster spermatogonial cells has also been attempted (Schonfeldt et al., 1999). To date no studies using magnetic separation with porcine testicular cells have been reported. In general, when spermatogonial stem cells have been separated, a high degree of cell purity was obtained, but with a low cell yield. Furthermore, for MACS to be performed, a specific antibody must be available for the target cell population, which can be a problem when dealing with spermatogonial stem cells. 17
28 In 1999, magnetic cell sorting was used to enrich spermatogonia from mouse, hamster, and monkey testicular tissue (Schonfeldt et al., 1999). The authors used microbeads that recognized the c-kit receptor to enrich the testicular cell suspension for spermatogonia. Enrichment of 23% to 26% was reported, with the average viability of cells from various species ranging from 81% to 91%. Although the results seem successful in enriching for spermatogonia stem cells, the authors used c-kit receptor as the method for identifying spermatogonia, which is expressed in several different cell types within the testis (Schonfeldt et al., 1999). Magnetic activated cell separation was used to isolate mouse type A spermatogonia to study spermatogonia proliferation in culture (Van Der Wee et al., 2001). The isolated population of spermatogonia was identified using antibodies that recognize the c-kit receptor. The enriched cell population was reported to contain 95% spermatogonial stem cells; however, the effectiveness of this method is questionable considering that c-kit receptor is expressed in several cell types in the testis and no viability was reported. In addition, the number of cells recovered from the magnetic separation was 10-fold lower when compared with the velocity sedimentation isolation technique (Van Der Wee et al., 2001). In a later study, testicular tissue isolated from transgenic mice at various ages was used for isolation of spermatogonial stem cells (Kubtoa et al., 2004). Magnetic microbeads conjugated to Thy-1 antibody were used to isolate cells from wild-type adult, cryptorchid adult, pup, and neonate testes. The highest enrichment of spermatogonial stem cells was 30- fold, which was isolated from the cryptorchid adult mice. No viability was reported for the separated cell population after the isolation procedure (Kubtoa et al., 2004). 18
29 In a recent study, the testicular tissue of 6-day old mice was used to isolate a subset of type A spermatogonia using magnetic activated cell separation (Hofmann et al., 2005b). The magnetic beads were conjugated to the antibody GFRα-1 for isolation of spermatogonial stem cells, and purity of the target population was reported to be almost 100%. Before magnetic isolation, the testicular cell suspension was subject to a velocity sedimentation technique that yielded a 90% enrichment of spermatogonia. The very high purity of the cell population is attributed to the effects of both enrichment techniques, and not only the magnetic separation. Although high purity was obtained using magnetic isolation and velocity sedimentation, a low number of cells resulted from using this technique (Hofmann et al., 2005b). It is evident that separation of spermatogonial stem cells with a high purity can be achieved using magnetic activated cell separation techniques (Hofmann et al., 2005b). Despite the effectiveness of enriching suspensions of testicular cells for spermatogonial stem cells, many issues should be addressed before using MACS as the enrichment method of choice. A specific antibody must be available to separate the target cell population from the cell suspension, which can be a problem when dealing with spermatogonial stem cells. The cost of magnetic separation is not as great as FACS, but the magnet apparatus and microbeads specific to each experiment must be purchased. After reviewing previous experiments done using this technique on testicular tissue, the effect on viability and on subsequent experiments is still questionable since viability was not often reported. One problem stated by researchers that have utilized this technique is that a low number of cells are recovered after separation. Magnetic cell sorting has been used primarily on rodent species and in one report on monkey tissue, but not with porcine tissue. In consideration of 19
30 the high cell purity recovered from the use of magnetic beads with abovementioned concerns, magnetic activated cell sorting may be an effective, but not optimal, method of enriching spermatogonial stem cells. VELOCITY SEDIMENTATION Velocity sedimentation is a method of separating cells based on the cells rate of sedimentation. This method is useful in separating cells with different diameters, or cells with similar diameters, but different densities. Sedimentation at unit gravity is a simple technique that is particularly useful separation method when cell suspensions contain a diversity in diameter and or density (Richter, 1975). The use of density gradient centrifugation in which cells are spun to their equilibrium positions in a gradient of bovine serum albumin dates back to the early 1960s. This method separates cells on the basis of sedimentation velocity. Briefly, a layer of cells is placed on top of a gradient composed of various concentrations of bovine serum albumin diluted in phosphate buffered saline. The entire gradient is bottom-loaded, allowing the layer of cells to be loaded first and remain on top of all other layers. The cells start in a band at the top of the gradient and sediment down through the various density layers by the force of gravity. The cells are separated in the various density layers primarily on the basis of size (Miller and Phillips, 1968). The use of a continuous density gradient using bovine serum albumin can be quite successful separating cells of different size and density from an initially heterogeneous population of cells in suspension. This method of separation has been used in the mouse, rat, and porcine model with good results, although some problems exist. The preparation for 20
31 velocity sedimentation is tedious and time consuming, requiring 45 minutes to load the density gradient. After the gradient is prepared, 4 additional hours are needed to sediment the cells. The total time necessary for separation can have detrimental effects on cell viability either immediately upon separation or during a long culture period after separation. In addition, serum temperature must be monitored as aggregates will form in the cell suspension if it is allowed to warm or mix with a medium of a different temperature (Miller and Phillips, 1968). Separation of spermatogonial cells by sedimentation velocity has been used for a variety of applications including the identification of murine testis cells (Bellve et al., 1977; Romrell et al., 1976), the study of genes involved in rodent spermatogenesis (Gold et al., 1983; Yu et al., 2003; Guo et al., 2004; Dym et al., 1995;), and the evaluation of culture conditions on porcine spermatogonia (Dirami et al., 1999). Adult mouse spermatogenic cells were separated by sedimentation velocity in a linear bovine serum albumin gradient generated by combining various concentrations of 4% and 2% serum (Romrell et al., 1976). Although various cell populations were separated, no spermatogonia were recovered from the gradient. The lack of recovery of the spermatogonia was attributed to either the enzymatic treatment prior to the sedimentation gradient, or the low percentage of spermatogonia contained in the original cell suspension. Of the recovered cell types, a viability of 98% was reported immediately after sedimentation separation (Romrell et al., 1979). An improvement on the previous study was done using prepuberal mice testicular tissue (Bellve et al., 1977). The same method of sedimentation was used as by Romrell and associates (Romrell et al., 1979), but with a longer sedimentation time. This improvement allowed for the recovery of primitive type A spermatogonia, type A and B spermatogonia, 21
32 preleptotene, leptotene, zygotene, pachytene spermatocytes and Sertoli cells. The cells in similar layers were pooled and the percentage of each cell type was determined for individual fractions. Identification of germ cells was accomplished by comparison of isolated cells to the morphological data generated from a concurrent study of mouse postnatal development. One fraction contained 90% primitive type A spermatogonia while another fraction contained 91% type A spermatogonia. After sedimentation viability was reported as 96% (Bellve et al., 1977). Sedimentation velocity using a bovine serum gradient was used to separate type A spermatogonia from porcine testes (Dirami et al., 1999). Similar gradient layers were combined and the purity of type A spermatogonia was 80-85% as detected using c-kit receptor rabbit anti-mouse antibody. Viability was monitored every 24 hours, and dropped to less than 20% by 48 hours of culture. The amount of viable cells was reported as undetectable by 120 hours of culture (Dirami et al., 1999). Recently, velocity sedimentation was used to isolate different spermatogenic cell types from male mice (Guo et al., 2004). Different ages of mice were used for the isolation of specific cell types, and cell suspensions were subjected to a 3-hour sedimentation period. Cell purity of each collected fraction was assessed on morphological characteristics that were not specified. Fractions containing similar cell types were pooled. The purity of cell populations from the gradient was reported as 94% for type A spermatogonia and 90% for type B spermatogonia. No information on cell viability after velocity sedimentation was reported (Guo et al., 2004). The method of velocity sedimentation has been used to enrich spermatogonia in various species with over 90% purity in some cases, although some concerns exist. A 22
33 technical issue to consider with this isolation method is the time involved to load the gradient and for sedimentation to occur; the procedure can take five hours for loading and enriching the cell suspension with the gradient. In addition to the time that it takes for velocity sedimentation, the effects of the time elapsed on viability must be taken into account. In experiments mentioned, the high viability reported was immediately after separation. In the one experiment that measured viability of separated cells in culture over time, the number of viable cells decreased dramatically by 120 hours of culture (Dirami et al., 1999). Another technical problem is the handling of the serum that is involved with making the gradient. The serum will cause cells to aggregate if the cell suspension is allowed to warm or if it is mixed with a medium of a different temperature. The major expense of velocity sedimentation is the cost of the large amount of serum required to separate the cells, which is much less than the cost of equipment needed for MACS or FACS. Although serum is a readily available substance, making velocity sedimentation a viable option for most labs, if a defined culture medium is desired for subsequent culture of the cells, this technique must be altered. When the result of velocity sedimentation is examined, it is difficult to determine what the success rate is because of the method of identifying the purity of the population recovered from the gradient. In one experiment, the morphology was examined with criteria not mentioned (Guo et al., 2004); another used the antibody c-kit for identification (Dirami et al., 1999) which is still debated in the literature. One experiment used a concurrent study of spermatogonial development that reported 90% purity of primitive type A spermatogonia (Bellve et al., 1977), suggesting that velocity sedimentation can be used to get high purity of spermatogonia with high viability immediately following the isolation of cells. When 23
34 considering all current information, velocity sedimentation can be a successful, but not optimal, method for the isolation of spermatogonial stem cells. PERCOLL SEPARATION Percoll is a gradient medium comprised of polyvinylpyrrolidine-treated colloidal silica that has low viscosity and osmolality, two features that are ideal for the separation of various types of cells (Sigma, 1998). Although Percoll has ideal separation features, it is not as diverse in application as other gradient media because of its limitations on use and storage. Percoll must be diluted with balanced salt solutions or a gel will form. Additionally, the standard use of Percoll must be modified to make separation of subcellular components possible. Autoclaving prior to dilution must be brief and at a low temperature with no exposure to air or solid will form at the solution-air interface. Percoll is relatively inexpensive, can be stored frozen for six months, and must be mixed well after thawing (Sigma, 1998). An important consideration when using Percoll to separate cells is the toxic effect it can have on cells. In 1981, the toxicity of Percoll was assessed after separation of rat epidermal cells. The viability of separated cells was variable, with the percentage of viable cells ranging from 30% to as high as 95% in different layers (Brysk et al., 1981). The average viability of separated cells was around 60%, indicating that Percoll may have a toxic effect on the cells. Percoll has been used to separate adult and prepuberal types of spermatogonia cells from porcine (Marret and Durand, 2000), bovine (Izadyar et al., 2002a; Izadyar et al., 2002b), and murine (Morena et al., 1996; van Pelt et al., 1996; Koh et al., 2004; Bucci et al., 24
35 1986) animal models. Marret and Durand separated testicular cells from 3-week old Meishan pigs with Percoll and yielded a population of cells that were at least 78% enriched for spermatogonia. Spermatogonia were identified by their absence of vimentin labeling, a protein that is solely expressed by somatic cells. The cells that did not stain with vimentin were identified as germline cells, and were counted as spermatogonia although no spermatogonia specific identification techniques were used. Cell viability was reported as higher than 80% in the enriched populations (Marret and Durand, 2000). Cells isolated from 6-month old bovine testes were separated using Percoll and the population of type A spermatogonia was reported as 73% pure (Izadyar et al., 2002a). Type A spermatogonia were identified by the presence of Dolichos biflorus agglutinin (DBA), a lectin with expression in the bull testis restricted to the germ line (Ertl and Wrobel, 1992). Viability was measured throughout the enzymatic digestion and the percentage of viable cells dropped after exposure to Percoll to 80% (Izadyar et al., 2002a). In one experiment utilizing the vitamin A-deficient rat model, testes were subjected to a Percoll gradient for enrichment of type A spermatogonia (van Pelt et al., 1996). A preplating enrichment protocol was employed prior to the Percoll gradient, which resulted in an enriched cell population containing 76-80% of type A spermatogonia. Identification of type A spermatogonia was accomplished by morphology assessment, and cells were counted using a hemocytometer. Immediately after enrichment, viability was 95-99%, and after three days the cell population remained 95% viable (van Pelt et al., 1996). It is clear that Percoll separation can be a useful tool for isolating spermatogonial cells from various species (Ertl and Wrobel, 1992; Izadyar et al., 2002a; Marret and Durand, 2000; van Pelt et al., 1996). Percoll features ideal low viscosity and osmolality for separating 25
36 cells, but has limitations of use concerning ph range, solubility, and difficulty of sterilization. Percoll is available at a low cost, but has a short shelf life in the laboratory (Sigma, 1998). The toxicity of Percoll is variable, with cell viability ranging from 30% to 95% after separation (Brysk et al., 1981; van Pelt et al., 1996). The benefit of this time efficient and inexpensive separation technique when compared to the limitation of use, variable toxicity to cells, and average effectiveness makes Percoll separation a viable, but not ideal, choice for separation of spermatogonial cells. NYCODENZ GRADIENT The nonionic iodinated gradient medium Nycodenz was first reported for use in 1982 (Rickwood et al., 1982). Nycodenz has a systematic name of 5-(N-2,3- dihydorxypropylacetamido) 2,4,6 triiodo-n,n bis(2,3 dihydroxypropyl)- isophthalamide (Figure 3) and is commercially available under the trade name HistoDenz. This gradient medium features low osmolality and viscosity in addition to high density, allowing it to effectively separate a range of sizes from macromolecules to viable cells. Nycodenz is water soluble, making it easily manipulated in the laboratory. Nycodenz is a stable compound, with a shelf-life of five years (Sigma, 2004). Nycodenz can be filter sterilized and remains stable when exposed to heat; therefore, providing a means of sterilizing the gradient medium prior to fractionation of live cells for culture. Mammalian cells do not seem to metabolize Nycodenz (Bertoni and Alexander, 1981). An additional marked benefit of using Nycodenz is the ease with which it can be removed from cell samples. Nycodenz is significantly lower in toxicity compared with other gradient media and 26
37 allows for the retention of normal cellular morphology upon exposure to the gradient medium (Mutzel et al., 1980; Aakhus et al., 1980). The use of Nycodenz is not as common as other separation techniques found in the literature. Nycodenz has been used for the separation of different cells from various species including murine (Mayanagi et al., 2003) and avian (Zhao and Kuwana, 2003) primordial germ cells in addition to human blood cells (Boyum, 1976). To date, there are no reports of using Nycodenz to separate spermatogonia cells in any species, including the pig. Nycodenz was successfully used to yield a highly enriched population of primordial germ cells for the preparation of anti-mouse primordial germ cell antibody (Mayangi et al., 2003). Gradient solutions were layered from high to low density with a cell suspension layered on top and centrifuged for 15 minutes. Cells were recovered from bands formed after centrifugation, and the highest percentage of PGCs in a band of cells was 95%. Multiple experiments were completed using Nycodenz to enrich for PGCs and the viability of separated cells was consistently more than 80% (Mayangi et al., 2003). A method of purification for avian circulating primordial germ cells was developed using Nycodenz gradient centrifugation (Zhao and Kuwana, 2003). A gradient was generated using different densities of Nycodenz, and the cell suspension was layered on top the gradient and centrifuged for 20 minutes. The highest purity of primordial germ cells collected was 93.5%. No viability statistics were reported (Zhao and Kuwana, 2003). Nycodenz gradient centrifugation for the separation of cells has been used primarily for the separation of primordial germ cells up to this point in time. Despite the sparse reports of Nycodenz use, it has great potential to be an optimal method of separating cells. Nycodenz features a low osmolality and viscosity with a high density allowing it to separate 27
38 a wide range of cell types (Sigma, 2004). When compared to other methods, Nycodenz separation has a time advantage over flow cytometry and velocity sedimentation by only requiring 20 minutes to separate cells. The quicker separation time could allow more time for cell manipulation in procedures after separation, or could decrease the number of cells that die during manipulation due to long experimental procedures. An obvious, but important advantage of Nycodenz separation over Percoll separation is that it can be easily autoclaved and filter sterilized. Nycodenz is also more stable in terms of shelf life than other gradient separation methods, is not cytotoxic, and is easily removed from the separated cells. Nycodenz separation, unlike MACS separation, does not use antibodies therefore allowing purification while maintaining cell surface antigens. The reported purity of the target cell population in both experiments using Nycodenz separation was above 90%, and viability of the cell population was reported as above 80%. Although Nycodenz separation has not been previously used to separate porcine spermatogonial cells, it possesses features that appear superior to alternative separation methods. The quick, inexpensive, non-toxic, sterile separation with Nycodenz will effectively separate cells while allowing experimentation downstream of separation by leaving cell surface antigens free. Nycodenz separation can be performed without costly equipment and features an easy method of separation without the limitations on temperature, ph, and stability that are hindrances when using the alternative separation methods. The advantages over alternative separation methods in consideration with the positive results from experiments using this separation method provide evidence for Nycodenz to have potential as the method of choice for the separation of porcine spermatogonial cells. 28
39 Figure 3. Structure of Nycodenz. (Sigma, 2006). 29
40 IDENTIFICATION OF SPERMATOGONIA The need to identify the population of spermatogonial stem cells within the porcine testis is essential for manipulation of these cells in vitro. Identification of spermatogonial stem cells based on morphology alone is not possible, necessitating the use of molecular markers for identification. Two specific markers will be discussed, DAZL and VASA, because of their consistent expression specific to the germline across various species (Jiao et al., 2002; Rilianawati et al., 2003; Xu et al., 2001; Fujiwara et al., 1994; Tanaka et al., 2000; Lee et al., 2005; Clark et al., 2004; Noce et al., 2001). The present study will use DAZL and VASA as markers of porcine spermatogonial stem cells will serve as an effective identification tool in animals ranging from 3 to 8 days old. Although VASA and DAZL have been detected in germ cells more differentiated than the spermatogonial stem cells (Xu et al., 2001; Lin et al., 2002; Saunders et al., 2003; Yen et al., 2004; Renolds and Cooke, 2005; Reijo et al., 2000; Collier et al., 2005; Clark et al., 2004; Ghabrial and Schupbach, 1999; Raz, 2000; Castrillon et al., 2000; Tsunekawa et al., 2000; Lee et al., 2005; Dyce et al., 2006), the only cells present in the porcine testis at 3-8 days old include Leydig cells, Sertoli cells, and spermatogonia (Franca et al., 2000; Van Straaten and Wensing, 1978). VASA and DAZL are not expressed in any cell type other than the spermatogonial stem cells in pigs of this age, allowing for effective identification of the spermatogonial stem cells within the testis population. 30
41 DAZL The Deleted in Azoospermia (DAZ) family of genes is made up of DAZ, and its two homologs DAZ-like (DAZL) and BOULE (Becherini et al., 2004). Evolutionary the oldest gene in the family is BOULE, while by way of a gene duplication event, DAZL is considered to have arisen from BOULE. Phylogenetic studies indicate that the Y-chromosomal DAZ gene is the youngest member of the family and arose from the transposition, repeat amplification, and pruning of the DAZL gene (Reynolds and Cooke, 2005; Lin et al., 2002). The DAZ gene is present on the Y chromosome in only humans, apes, and Old World Monkeys (Jiao et al., 2002). All other mammals, including these species, contain the DAZL gene which is 80% homologous to DAZ (Jiao et al., 2002). Both DAZL and BOULE exist as autosomal single copy genes and encode for proteins with one RNA recognition motif (Reynolds and Cooke, 2005). Four DAZ genes are located on the Y chromosome and encode proteins with a series of 8-24 DAZ repeats. The protein product of Dazl is similar to Daz with the exception of containing only one DAZ repeat (Xu et al., 2001). The DAZ gene family encodes proteins that bind RNA and contain a ribonucleoprotein (RNP)-motif type of binding domain at their amino terminus (Jiao et al., 2002). The Daz family of proteins binds to several mrnas in the 5 or 3 untranslated region in vivo and in vitro and is believed to be involved in the post-transcriptional regulation of mrna expression (Becherini et al., 2004). The Daz proteins have been shown to be associated with protein synthesis machinery and to interact with proteins that are involved in mrna transport, localization, or regulation of mrna translocation of several genes including Tpx-1, Pam, GRSF1, Trf2, and Cappß1 (Jiao et al., 2002). 31
42 Although many studies have attempted to elucidate the RNA binding activity to determine Dazl function, the process remains unclear (Venables et al., 2001). A multiple number of studies have been conducted to determine the RNA and protein expression patterns for the DAZ family genes in many animal models (Reynolds and Cooke, 2005). DAZ genes are considered to control meiotic components at the translational level (Reynolds and Cooke, 2005). Recently, it has been suggested that Dazl proteins stimulate translation of target mrnas by recruiting poly (A)-binding proteins to enhance initiation of translation, and that the translation stimulation ability for Dazl proteins has been conserved in the family (Collier et al., 2005). All Daz protein family members are exclusively expressed in germ cells (Jiao et al., 2002; Rilianawati et al., 2003; Xu et al., 2001). The RNA-binding proteins that are encoded by the DAZ genes act as master controllers of meiosis; therefore, the DAZ genes are essential for gametogenesis because of their role in regulating the translation of specific mrnas (Reynolds and Cooke, 2005). Translation is strictly regulated in gametogenesis because transcription stops early in gamete formation and the mrnas produced must be stored if their protein product is needed later in the process (Reynolds and Cooke, 2005). Although the DAZ gene family is not required for viability of an organism, loss of any member of the gene family results in disruption of haploid gamete production and loss of the germline (Reynolds and Cooke, 2005). Function of the Daz proteins is not clearly defined, but it is believed that the proteins are not functionally redundant (Collier et al., 2005). Dazl has been shown experimentally to interact with a protein required to maintain the proliferative state of stem cells and to prevent differentiation, suggesting Dazl may have a role in maintaining stem cell populations 32
43 (Reynolds and Cooke, 2005). The study of gametogenesis and the role of DAZ genes in male development has revealed that the proteins may function at many points throughout male germ cell development. The proteins were found to be involved during the early establishment of the population of spermatogonial stem cells and during meiosis. Evidence of this early role of DAZ genes is the presence of Daz proteins in the gonocytes of fetal testes prior to the process of spermatogenesis and meiosis (Reijo et al., 2000). The DAZL gene has been studied in many model organisms in an attempt to determine its role in development. In all cases Dazl proteins are germ cell specific; consequently, they are often used as molecular markers for germ cells (Reijo et al., 2000; Rilianawati et al., 2003). Creating DAZL knock-out organisms across the animal kingdom has helped elucidate various roles in germ cell differentiation; however, the null phenotypes and expression patterns differ in different species (Xu et al., 2001; Venebles et al., 2001). In Drosophila, the disruption of the DAZL gene results in male infertility (Reijo et al., 2000). More specifically, a block in G2/M of meiosis I occurs in the Drosophila knock-out organism (Venables et al., 2001). Mice that have a Dazl deficiency lack formation of spermatozoa and the only germ cells present are spermatogonia, rendering the mice infertile (Schrans-Stassen et al., 2001). The absence of DAZL in mice is reported to cause a cease in germ-cell mitotic divisions in both sexes (Ruggiu et al., 1997). The most advanced germ cells present in the testes of murine DAZL knockouts included a small amount of leptotene spermatocytes in conjunction with no synaptonemal complexes being formed, suggesting that Dazl is essential for germ cells to progress to the zygotene stage of spermatogenesis (Rilianawati et al., 2003). It is apparent that a deficiency of Dazl in various organisms has differing effects on male germ cell formation. It is likely that DAZL s protein product functions at numerous steps of 33
44 spermatogenic development, but that the dearth of the protein becomes problematic at various steps of spermatogenesis in different animal phyla or species. In the adult human, the Dazl proteins are exclusively expressed in the germ cells with localization in the cytoplasm and nucleus of spermatogonia and in the cytoplasm of spermatocytes undergoing meiosis (Xu et al., 2001). Other studies have revealed that Daz is expressed in many cellular compartments throughout male germ cell development; Daz proteins are present in nuclei and cytoplasm in fetal gonocytes and spermatogonia (Lin et al., 2002). During meiosis of the germ cells, the proteins are relocated in the cytoplasm of spermatocytes (Lin et al., 2002). In the mouse, Dazl is expressed from embryonic day 12.5 onward, and it is found in spermatogonia and primary spermatocytes in the adult male (Saunders et al., 2003). Dazl and Daz are both present in primordial germ cells within fetal gonads, with localization in the nuclei of the cells (Yen, 2004). The Dazl protein is detected the cytoplasm of pachytene spermatocytes and in the nuclei of spermatogonia in adult mouse and human testes (Yen, 2004). In adult DAZL knock out animals undergoing spermatogenesis, few cells progress beyond the A-aligned stage to the A1 transition, and germ cell numbers are reduced before leptotene (Rilianawati et al., 2003). An explanation of these observations is that the germ cell numbers are reduced because Dazl functions in mitosis or in commitment to meiosis which provides a loss of committed pre-meiotic cells. One reason that few cells progress beyond the A-aligned stage of spermatogenesis is that the testicular structure is severely disrupted due to the absence of mature germ cells (Rilianawati et al., 2003). 34
45 In DAZL knock-out mice, the only germ cells present in the seminiferous epithelium were A spermatogonia. Three percent of the germ cells developed further and quickly underwent apoptosis. It has been suggested that that protein encoded by DAZL is directly involved in the spermatogenesis transition from A-aligned to A1 stage (Schrans-Stassen et al., 2001). In mice, the Dazl protein has been detected in spermatogonia, gonocytes, primordial germ cells, and in all stages of spermatogenesis up to haploid spermatids (Reynolds and Cooke, 2005). The Dazl protein was found to be cytoplasmic and expressed strongest in pachytene spermatocytes (Reynolds and Cooke, 2005). Daz proteins in the human and Dazl proteins in the mouse are both present in the cytoplasm and nucleus of adult spermatogonia and fetal gonocytes, but are found to be only cytoplasmic in cells that have entered meiosis (Reynolds and Cooke, 2005). Immunohistochemical studies have been used as a tool for determining where the DAZL gene product is localized in the cell to clarify its function. Immunocytochemistry detected the presence of Dazl proteins in the nucleus and cytoplasm of germ cells. Previously it was shown that Dazl protein is found in the nucleus and cytoplasm of mouse fetal gonocytes, but is present only in the nucleus of the spermatogonia (Hofmann, et al., 2005a). Rodent and human testis sections were examined with antibodies specific for Daz, Dazl, and Boule and all were shown to be exclusively expressed in the germline (Rilianawati et al., 2003). Using immunohistochemistry, it was found that the proteins of the Daz family are mostly nuclear during the premeiotic steps of germ cell development, which may indicate a function of the Daz proteins involves RNA storage or processing in the nucleus. It appears 35
46 that the proteins move to the cytoplasm at the time of meiosis (Reijo et al., 2000). In the mouse, Dazl is localized in the nuclei and cytoplasm of fetal gonocytes; however, in spermatogonial cells, Dazl is present in the nuclei and later migrates to the cytoplasm during meiosis (Collier et al., 2005). In human adults, hybridization studies indicated that Dazl transcripts are primarily found in spermatogonia and primary spermatocytes (Reijo et al., 2000). In conclusion, the Deleted in Azoospermia Like gene homolog has been found to be exclusively expressed in germ cells, and is required for their development and differentiation (Jiao et al., 2002). The protein encoded by the DAZL gene has been found to stimulate translation of specific mrnas which are involved in the early establishment of spermatogonial stem cells and meiosis (Reynolds and Cooke, 2005; Reijo et al., 2000). The protein has been detected in the nucleus before meiosis and found to function in the cytoplasm during meiosis (Reijo et al., 2000; Collier et al., 2005). This cell-specific expression can be useful when identifying spermatogonial stem cells in vitro, and has been used as a molecular marker for spermatogonial stem cells from various species (Hofmann et al., 2005a; Rilianawati et al., 2003). The use of DAZL as a marker for porcine spermatogonia is not as well researched as it is in other species; however, the use of DAZL to detect porcine spermatogonia should be effective considering the conservation of expression across species. 36
47 VASA The VASA gene locus encodes a protein product that is the best characterized member of the Dead-box family of proteins (Tanka et al., 2000). The Dead-box family includes 30 proteins produced in organisms ranging from bacteria to humans (Pause and Sonenberg, 1992). Some more prominent Dead-box proteins include p68, a nuclear protein found in the nucleus of mammalian dividing cells, and eif-4a which is an eukaryotic initiation factor (Lee et al., 2005). Based on the helicase activity observed in vitro, the Deadbox family is believed to be a group of RNA helicases (Pause and Sonenberg, 1992). Eight highly conserved motifs are found in each member of the Dead-box family, one of which is the Dead region that codes for Asp- Glu-Ala-Asp, which spells DEAD when the single letter abbreviation of the amino acid sequence is used. The eight conserved motifs are essential for RNA binding and unwinding, and ATP binding and hydrolysis; however, the specific function of these proteins is not known (Pause and Sonenberg, 1992). All Dead-box proteins possess an ATP-dependent RNA helicase that is postulated to post-transcriptionally regulate genes (Pause and Sonenberg, 1992). It has been suggested that the genes in the family, including VASA, use energy from ATP hydrolysis to unwind the secondary structure of the mrna upstream of the initiation codon, which facilitates the attachment of the 40S ribosome necessary for translation initiation (Wassarman and Steitz, 1991). The Dead-box family of proteins has an important role in cell growth, RNA splicing, spermatogenesis, and germ cell formation (Lee et al., 2005). Vasa s specific role as an RNA helicase may be involved in regulating germ cell proliferation (Tanka et al., 2000). The VASA gene was originally characterized in Drosophila melanogaster, and the 7kb coding region of the gene is divided into seven exons separated by six introns (Hay et al., 1988). The VASA gene sequence contains five repeats of the heptad sequence F/S-R-G-G- 37
48 E/Q-G-G near the amino terminus and six helicase motifs including the Dead region characteristic of all Dead-box family members. In addition to the conserved motifs found in most helicases, the carboxyl terminus of Vasa contains many negatively charged amino acid residues which is a common feature of nucleic acid binding proteins and further supports the proposed function of Vasa to regulate translation (Hay et al., 1988). Embryonic stem cells have been used to study the expression of Vasa throughout development. Clark and colleagues reported that mature sperm in vivo could be produced by transplanting Vasa-expressing embryonic stem cells into recipient testes (Clark et al., 2004). The expression of three genes was studied in cultured embryonic stem cells, and based on their gene expression, three populations were distinguished (Hubner et al., 2003). One group of cells that resembled premigratory germ cells was positive for Oct 3/4 and C-kit expression, but negative for Vasa expression. Another group of cells that resembled postmigratory germ cells such as PGCs and gonocytes was positive for Oct 3/4 and Vasa expression, but negative for C-kit expression. The third population of cells was negative for Oct 3/4 and C-kit, but did express Vasa and resembled postmigratory cells about to enter prophase 1 of meiosis (Hubner et al., 2003). This data suggests that Vasa is a marker for postmigratory germ cells, and that probing for Vasa expression during the differentiation of embryonic stem cells will allow for the identification of more mature stages of germ cell development (Clark et al., 2004). As mentioned earlier, the VASA gene encodes an ATP-dependent RNA helicase that has been well studied in Drosophila melanogaster (Lee et al., 2005). In addition to observation in the fruit fly, genes homologous to VASA have been identified on the basis of structural conservation in germ cells of other species including fish, frogs, mice, rats, 38
49 humans, chickens, and pigs (Lee et al., 2005). VASA homolog genes are specifically expressed in the germ line and are used as molecular probes to define the stage of germ cell development in each species. In mammals, the Vasa protein is cytoplasmically expressed in the developing germ cells, and is present in both male and female germ cells (Fujiwara et al., 1994; Tanaka et al., 2000). Within the germ cell, the polar granule structure is known to contain germ cell-specific translational machinery and it has been found and demonstrated that this is where localization of the protein product of Vasa occurs and functions as a translational regulator (Lee et al., 2005; Noce et al., 2001). Vasa protein is thought to regulate translation by binding target mrnas involved in the establishment of germ cells and consequentially controlling their onset of translation (Styhler et al., 1998; Noce et al., 2001). In addition to the necessary protein product of VASA, the time of expression seems to be important as it relates to gene function. The time of mammalian VASA gene expression is during late migration as germ cells enter the gonads and is similar to the time of VASA s Drosophila zygotic transcription, which may suggest that an evolutionally conserved critical step of germ cell differentiation is correlated to Vasa expression (Clark et al., 2004; Noce et al., 2001). This expression profile is observed across mammals, indicating that protein expression of the VASA homolog could serve as a valuable marker to specify an important step in the fate determination of germ cells (Fujiwara et al., 1994). Since its first characterization in the Drosophila melanogaster genome, other homologs of the VASA gene have been identified in many species. Based on sequence similarity, germ-cell specific expression, and localization, the mouse VASA homolog (MVH), pig VASA homolog (PVH), chicken VASA homolog (CVH), and teleost fish VASA 39
50 homolog (VAS) were identified (Lee et al., 2005; Toyooka et al., 2000; Fujiwara et al., 1994). The VASA gene was originally discovered as a maternal-effect gene in the fruit fly required for proper germ cell specification and formation of abdominal segments, and it is known that VASA RNA is expressed in the early embryonic and in germline stem cells (Liang et al., 1994; Lasko and Ashburner, 1998). The protein of the VASA gene is expressed in germ cells upon formation and throughout embryonic development. Protein localization is found in the polar granules, nuclear bodies, and cytoplasmic masses within the embryo. As the male germ cells develop, the protein is expressed up until early stages of spermatogenesis. The function of Vasa in germ cell development is further elucidated by studies of VASA mutants. Schupbach and colleagues (Ghabrial and Schupbach, 1999) observed that when Drosophila embryos inherit mutant VASA RNA from the mother, they fail to form germ cells. A gene homologous to VASA was detected in the mouse (MVH), and has been characterized by multiple researchers (Castrillon et al., 2000; Kotaja et al., 2006; Tanaka et al., 2000). The Mvh protein is expressed in germ cells upon arrival to the genital ridge at embryonic day 9.5, which is estimated to be 3 days after germ cell determination (Tanaka et al., 2000). As the PGCs enter the genital ridge, they undergo cell division. PGCs stop dividing by 13.4 dpc and those cells in the male enter mitotic arrest in the G1 stage of the cell cycle. PGC differentiation and proliferation is believed to be controlled by surrounding somatic cells within the gonad at the time when Vasa is expressed, suggesting that expression could be dependent on the cellular interaction of the PGCs with the surrounding somatic cells 40
51 (Tanaka et al., 2000). The Vasa protein is required in PGCs and throughout stages in spermatogenesis before the pachytene spermatocyte stage. The requirement of the Vasa protein up to the pachytene spermatocyte stage could demonstrate the need for the gene product of VASA for differentiation of germ cells into gametes (Raz, 2000). Expression in males occurs throughout spermatogonial development up to the round spermatid stage. After meiosis of the germ cells, the Mvh protein becomes localized as a large granule near the nucleus and can be detected up to the spermatid nucleus elongation stage (Raz, 2000). In male mice VASA mutants, defects in primordial germ cell proliferation and differentiation are present, along with a complete depletion of postmeiotic germ cells due to premeiotic germ cells not completing the meiotic process and instead undergoing apoptosis. The absence of postmeiotic germ cells suggests that the function of Mvh is essential for the development and differentiation of male germ cells rather than for their survival and has little effect on germ-line precursor cells (Tanaka et al., 2000). A homolog to the VASA gene has been found in humans and is also referred to as VASA (Castrillon et al., 2000). VASA RNA is expressed in both the fetus and adult, with male and female expression gonad specific. The protein product of the VASA gene is specifically expressed in germ cells from primordial germ cells through development to spermatids in males and to oocytes in females. In spermatocytes, the protein has been localized with granular staining, although no protein localization has been determined in adult oocytes (Castrillon et al., 2000). The Vasa protein was present in spermatid, spermatocytes, and spermatogonia which exhibited variable staining that may correspond to type A and B spermatogonia. Neither Sertoli cells nor spermatozoa stained for the presence 41
52 of Vasa, and there was no staining observed in sections of the epididymis containing spermatozoa (Castrillon et al., 2000). Kobayashi and colleagues studied the expression of the teleost fish VASA homolog (VAS) (Kobayashi et al., 2000). Zygotic expression of vas was restricted to the germ line and was found only in germ cells up to 3 days after hatching. Expression of VAS in the testes of the fish was reported as strong in spermatogonia with a decrease in expression in early primary spermatocytes. VAS RNA signals were observed in male germ cells at stages from spermatogonia to early primary spermatocytes; however, no VAS RNA was detected in diplotene primary spermatocytes, secondary spermatocytes or in any spermatogenic cells more advanced in development (Kobayashi et al., 2000). A homolog of VASA was detected in chicken that demonstrated germline-specific expression (Tsunekawa et al., 2000). Cells that expressed the chicken Vasa homolog (Cvh) were first detected starting from the first cleavage of fertilized eggs during embryogenesis. The Cvh protein was detected specifically in the cytoplasm of primordial germ cells with no detection of the protein observed in any non-germ cell type throughout development. Cvh expression in the male was detected exclusively in the testis, with expression present in developing spermatogonia cells through mature spermatocytes. Anti-Cvh staining was used to detect localization of the protein in the cytoplasm of germ cells; however, no staining was detected in somatic Leydig and Sertoli cells. Anti-Cvh staining was detected in cell stages from spermatogonia to round spermatids, but not during or after the elongated spermatid stage of spermatogenesis development. Adult testis sections from quail, turtle, and snake were prepared and stained with the anti-cvh to determine if cross-reactivity in various species would occur. Testicular germ cells were specifically stained in a similar manner in 42
53 these species as observed in the chicken testis. This similar staining suggests the presence of VASA homologs with common sequences in other birds and reptiles (Tsunekawa et al., 2000). The presence of a homolog to VASA has been identified in the pig (Lee et al., 2005; Dyce et al., 2005; Toyooka et al., 2000), but it has not been well characterized. The porcine VASA homolog (PVH) was cloned to investigate expression within the germ cells of pigs (Lee et al., 2005). It was found that the PVH was 91% homologous to the human VASA, and 85% homologous to the mouse VASA gene. The Pvh protein was found to be present in the oocytes and spermatocytes of adult and fetal gonads, and that it was present exclusively in fate-committed germline cells from 24 days post coitum onward (Lee et al., 2005; Dyce et al., 2006). In the fetal testis of pigs, the Pvh protein was observed in cells located around the seminiferous tubules. In the adult, the protein was detected within the spermatogonia and weakly in spermatocytes. Additionally, the Pvh protein was detected in porcine PGCs, but not in embryonic germ cells (Lee et al., 2005). Toyooka and colleagues detected positive signals for Vasa in the cytoplasm of porcine spermatogenic germ cells (Toyooka et al., 2000). The VASA gene is conserved in all vertebrate and invertebrate species, and the expression of VASA homologs is present exclusively in male and female germ cells throughout development. The staining patterns in cytoplasm or granules observed in various species my reflect interspecies differences in protein localization, although Vasa protein expression restricted to the germ cell lineage is conserved throughout phylogeny (Castrillon et al., 2000). The conservation of Vasa protein expression will facilitate the differentiation of 43
54 spermatogonial stem cells from other cells in culture, specifically porcine spermatogonial stem cells to evaluate enrichment. 44
55 IN VITRO SPEMATOGONIA CULTURE DEVELOPMENT OF SPERMATOGONIA CULTURE The culture techniques used for spermatogonia stem cell culture are quite different today than when Champy first cultured male germ cells in 1920 (Champy, 1920 as reviewed by Staub, 2001). Champy utilized small pieces of testis from adult male rabbits cultured in blood plasma to observe development in vitro. Although the differentiating germ cells were observed to die within 2 days, he reported that some somatic and undifferentiated germ cells could survive for a week in culture. Champy was able to observe cell mitosis during the short lifespan of the cultures, which was the first report of male germ cells entering mitotic prophase in vitro (Champy, 1920 as reviewed by Staub, 2001). As culture techniques for male germ cells advanced, the lifetime of cells in culture increased. In 1937, Michailow reported a 70-day life span for undifferentiated germ cells isolated from seminiferous tubules of immature rabbits (Michailow, 1937 as reviewed by Staub, 2001). The culture of male germ cells became of great interest as more researchers made advances to prolong the lifetime of in vitro cultures. Trowell developed a method to maintain various mature organs in synthetic medium with a goal for the organs to retain normal histological appearance for a week in vitro in 1959 (Trowell 1959). Unsuccessful culture attempts led to the study of the atmosphere in which the cultures were incubated. Trowell concluded that the testis was sensitive to oxidative stress after observation of evidence of oxygen poisoning. Although Trowell succeeded in increasing survival time of seminiferous tubules in culture by changing the gas phase of culture to contain 5%CO 2, no living cells were observed after 6 days of culture (Trowell, 1959). 45
56 In the 1960 s Steinberger and colleagues began studying the culture techniques of male germ cells to develop an in vitro culture system enabling long-term survival of testicular fragments to facilitate the study of male germ cell differentiation in vitro (Steinberger and Dixon, 1959; Steinberger et al., 1964a; Steinberger et al., 1964b; Steinberger and Steinberger, 1965; Steinberger, 1967). In 1964, Steinberger et al. utilized testicular tissue from 14-day-old rats for studying various culture conditions and found that Eagle s medium supplemented with sodium pyruvate, several amino acids, and 10% calf serum was most effective. The Sertoli cells and tubular structure of the testicular fragments were maintained for 6 months when incubated in an atmosphere of 5%CO 2 and 95% air, at 31 C and cultured at ph of 7.0 ± 0.2. Although the Sertoli cells were able to survive in the optimum culture conditions, the ability of the culture conditions to maintain the germinal cells was unsuccessful (Steinberger et al., 1964a). Years later, an interesting discovery was made in 1979 by Aizawa and Nishimune using adult cryptorchid mice testicular tissue. While using tritiated thymidine to study spermatogonia mitosis in vitro, they discovered that the presence of serum in the culture media is necessary for the early differentiation processes of spermatogenesis of type A spermatogonia (Aizawa and Nishimune, 1979). These results were in contrast with the earlier conclusions of the Steinberger team that the differentiation of germ cells could be achieved in defined medium without serum (Steinberger and Steinberger, 1966; Steinberger and Steinberger, 1967). Aizawa and Nishimune suggested that the Steinberger team was mistaking type B spermatogonia for type A spermatogonia in culture; the two types of spermatogonia are difficult to distinguish, and type B spermatogonia differentiation has been shown to be serum independent in vitro (Aizawa and Nishimune, 1979). 46
57 In 1983, researchers were interested in the coculture of rat germ cells with somatic Sertoli cells (Tres and Kierszenbaum, 1983). Tres and Kierszenbaum reported that germ cells were only able to differentiate when in close contact with Sertoli cells in defined medium. The authors also noted that for cultures to be successful, the cell associations found between somatic and germ cells in vivo must be maintained in vitro (Tres and Kierszenbaum, 1983). Nagao reported positive results from a new method of culturing male germ cells (Nagao, 1989). Before 1989, it was believed that culturing germ cells with somatic cells was required for successful cultures (Tres and Kierszenbaum, 1983). Nagao used prepuberal rats and subjected the testicular cells to a series of enzymatic treatments including collagenase and trypsin prior to seeding the cultures. The dispersed testicular cells were cultured for 2 weeks, and it was found that the addition of epinephrine and norepinephrine to the culture medium increased cell viability (Nagao, 1989). A functional assay for unequivocal identification of spermatogonial stem cells utilizing the transplantation of stem cells to generate colonies in vivo (Brinster and Zimmermann, 1994) was developed to allow researchers to study various culture conditions for spermatogonial stem cells and quantify the number of spermatogonial stem cells by transplantation into a sterile recipient animal. Upon transplantation only stem cells were able to generate colonies in vivo, allowing the quantification of stem cells. Despite the assay s setbacks including length of time of quantification after transplantation and the variability of transplantation technique on colonization, the assay would also allow for spermatogonial stem cells to be genetically manipulated in vitro and pass the integrated gene through the germline into the progeny of the recipient (Brinster and Zimmermann, 1994). 47
58 Brinster s research team was interested in the manipulation of the stem cells in vitro and reported successful cryopreservation of mouse spermatogonial stem cells in 1996 (Avarbock et al., 1996). Before this attempt, limited success was documented with freezing male germ cells, with some success using immature rat testis pieces. Brinster s team used a standard procedure for freezing somatic cells to cryopreserve murine testis cell suspensions. The frozen suspensions were stored for up to 156 days and transplanted into busulfan-treated recipients (Avarbock et al., 1996). Cells from the donor mice carried the lacz transgene that allowed for visualization of the stem cell progeny after transplantation. Transplanted cells were able to initiate spermatogenesis and mature spermatozoa in the recipient testes (Avarbock et al., 1996). Later in 1996, the Brinster team reported successfully generating rat spermatogenesis in a mouse testis (Clouthier et al., 1996). Donor cells were isolated from fertile rats carrying the MT-lacZ transgene, which was expressed in germ cells and Sertoli cells to facilitate the identification of the stem cell progeny after transplantation. Recipient mice were immunodeficient and sterilized by busulfan treatment to eliminate endogenous germ cells. After the transplantation of rat germ cells into the seminiferous tubules of recipient mice, testes were analyzed at various time points to reveal the rat testes cells had populated the tubules and that mature forms of rat spermatozoa were able to be identified (Clouthier et al., 1996). To investigate culture length for spermatogonial stem cells, Nagano and Brinster used testis cells from various ages of mice and cultured them with or without feeder cells for up to 111 days before transplantation into donor mice (Nagano and Brinster, 1998). Cells from both young and old donor mice were able to establish spermatogenesis in recipient 48
59 seminiferous tubules after transplantation. Brinster s group suggested that stem cell division occurred in culture, based on the observation that the population of stem cells in culture after a period of incubation was larger than the population of spermatogonial stem cells found in a newly seeded culture (Nagano and Brinster, 1998). Despite all the advances in the culture of spermatogonial stem cells, little was known about the biochemical or morphological characteristics of the cells. Studying the cells proved difficult as 1 in 5000 testis cells in the mouse is a stem cell. Additionally, in vitro observation of spermatogonial stem cells was difficult because there was no detectable morphological difference between stem cells and early-differentiated spermatogonial cells. In 2000, Brinster s research group reported using a somewhat complicated method for selecting mouse spermatogonial stem cells from a population of testis cells (Shinohara et al., 2000). Utilizing fluorescence-activated cell sorting combined with expression of cell surface markers, Shinohara isolated spermatogonial stem cells from cryptorchid mice. It was reported that the resulting population of cells was enriched 166-fold for spermatogonial stem cells and expressed no c-kit, had high expression of α6-integrin, low expression of αvintegrin, and exhibited low side scatter which indicates a low amount of intercellular complexity. The cell population resulting from the selection was then transplanted into the seminiferous tubules of a sterile recipient for quantification as described earlier (Shinohara et al., 2000; Brinster and Zimmermann, 1994). One year later, Brinster s research group produced transgenic mice using retroviral transduction of spermatogonial stem cells (Nagano et al., 2001). Transgenic progeny were produced from in vitro transfection of spermatogonial stem cell cultures with a retrovirus vector carrying the lacz reporter gene driven by the Pgk-1 promoter. The researchers 49
60 reported that stable integration and expression of the transgene was observed in 2-20% of transfected cells, while 4.5% of the progeny of transplantation recipients are transgenic (Nagano et al., 2001). The first spermatogonial cell line was generated in 2002 by cells isolated from 6-dayold mice (Feng et al., 2002). Isolated cells were transfected with a retrovirus vector carrying a gene that would cause the over expression of telomerase activity and subsequent immortalization. Immortalized cells were found to express many markers of spermatogonial stem cells, but no transplantation assay was used to unequivocally define the cells as stem cells. When the immortalized cells were influenced by addition of stem cell factor to the culture medium, the cells were able to differentiate into spermatocytes and round spermatids (Feng et al., 2002). To better understand the regulation of proliferation or differentiation in spermatogonial stem cells, Brinster s research team subjected spermatogonial stem cells in vitro to various treatments and then transplanted the cultured cells into the seminiferous tubules of a sterile recipient to study the effect of in vitro microenvironment on survival of spermatogonial stem cells in a 7-day culture system (Nagano et al., 2003). Treatments included various feeder cells, growth and inhibitory factors, various transforming growth factors, incubation temperatures, and medium composition. Nagano hypothesized that improvement of spermatogonial stem cell maintenance in vitro can be achieved by suppressing germ cell differentiation and subsequentially the cascade of events leading up to differentiation (Nagano et al., 2003). Another long-term culture experiment performed by Kanatsu-Shinohara et al in 2003 demonstrated that spermatogonial proliferation in vitro can be accomplished by adding 50
61 a combination of growth factors to medium containing 1% fetal bovine serum and utilizing mouse embryonic feeder cells. Shinohara s group utilized gonocytes from neonatal mice and cultured them in medium containing leukemia factor, basic fibroblast growth factor, glial cell line-derived neutrophic factor (GDNF), and epidermal growth factor. Spermatogonial stem cell proliferation was reported over a 5-month period, and the cultured cells were able to produce spermatogenesis after transplantation to the seminiferous tubules of infertile recipients. Mature sperm resulting from the transplantation was normal as it was able to fertilize eggs and produce normal offspring (Kanatsu-Shinohara et al., 2003). In 2004 Hamara and colleagues studied spermatogonial stem cells culture conditions and found that spermatogonial stem cells cultured on STO feeder cells lost stem cell activity in vitro while cells cultured on mouse Sertoli cell line-1(msc-1) feeder cells maintained their stem cell activity and were able to colonize recipient seminiferous tubules after transplantation (Hamra et al., 2004). Hamra and colleagues were also able to define transcripts that correlate strongly with spermatogonial stem cell activity and transcripts that change near the time of loss of stem cell activity in culture (Hamra et al., 2004). Spermatogonial stem cells were cultured for over 2 years and expansion of the cultured cells was fold (Kanatsu-Shinohara et al., 2005). Throughout the 2 years in culture, the growth rate was constant and cells maintained a normal euploid karyotype. Fertile offspring was produced after transplantation and subsequent spermatogenesis of spermatogonial stem cells which were cultured for 2 years. During the long culture period, the proliferative potential of the spermatogonial stem cells was studied by observing telomere length. Telomeres were found to shorten after time in culture, indicating that despite the long 51
62 proliferation period observed in culture, spermatogonial stem cells have a limited proliferation potential (Kanatsu-Shinohara et al., 2005). As research in the area of male germ cell culture continues, the characteristics of biologically active spermatogonial stem cells will become more clear, and optimal culture conditions will allow for efficient genetic modification of the cells for production of transgenic animals. Culture techniques and genetic manipulation strategies will be expanded to include other domestic animal species for an endless amount of possible applications. PORCINE SPERMATOGONIA CELL CULTURE The first report of isolation of porcine spermatogonia for culture was in 1999 (Dirami et al., 1999). In this study, type A spermatogonia were isolated from Yorkshire prepubertal pigs that were 80 days of age. The authors used sedimentation velocity at unity gravity to separate type A spermatogonia from the testis cell suspension and identified type A spermatogonia using c-kit expression. No information was available on c-kit expression in the pig, but the authors identified the presence of c-kit within separated cells and determined the cells were type A spermatogonia (Dirami et al., 1999). Dirami and colleagues cultured purified type A porcine spermatogonia cells for 120 hours in either DMEM/F12 medium without any supplementation or in potassium-rich medium derived by the simplex optimization method (KSOM). Cell viability was assessed in 24-hour intervals by MTT colorimetric assay, which utilizes the change of a yellow substrate to blue to identify live cells (Mosmann, 1983). After 48 hours of culture, less than 20% of the cells in DMEM/F12 without supplementation were viable, but 40-50% of the spermatogonia cultured in KSOM were viable. These results indicated that KSOM medium 52
63 alone could support the survival of porcine spermatogonia longer than could DMEM/F12 medium without supplementation. An additional experiment studied the effect of stem cell factor (SCF) and granulocyte macrophage-colony stimulating factor (GM-CSF) on the survival of the porcine spermatogonia cultured in KSOM medium. It was found that SCF may promote the survival of the porcine type A spermatogonia (Dirami et al., 1999). In 2000, Marret and Durand studied the effects of germ cell purification, fetal calf serum, and extracellular matrix on the culture of porcine spermatogonia (Marret and Durand, 2000). Testes were used from 3 week old Meishan pigs and tissue was subject to an enzymatic treatment to generate a single cell suspension. Dissociated cells were subject to a Percoll discontinuous density gradient to separate the spermatogonia from the cell suspension. Cells were harvested and the amount of spermatogonia was determined indirectly using vimentin immunostaining, which stains only somatic cells (Marret and Durand, 2000). Cells were incubated in DMEM/F12 medium with or without 5% fetal calf serum supplementation for 9 days. Spermatogonia viability was determined on selected days of culture using trypan blue exclusion. The number of viable cells in both culture media decreased with time; however, the viability of cells with serum supplementation was always significantly higher (Marret and Durand, 2000). The viability of purified spermatogonia dropped quickly in culture as 80% of the germ cells were lost over the 9 day culture period (Marret and Durand, 2000). Although the same trend in viability occurred in the cultures with or with out a Sertoli cell feeder layer, the decrease in viability was more pronounced in cultures on a Sertoli cell feeder layer. The viability found in this experiment was higher than reported in the previous work by Dirami and colleagues (Dirami et al., 1999), which could be due to a different method used to assess 53
64 viability and the different in vitro environment of the cells. Marret and colleagues supplemented the basal media with various molecules including hormones and growth factors that promote cell life. The percentage of spermatogonia that incorporated BrdU was measured in this experiment, which indicates if the cells enter the S phase of the cell cycle. No spermatogonia incorporated BrdU in either culture condition, which may be an indication of the need of germ cells to have interactions with somatic cells for entry into the S phase of the cell cycle (Marret and Durand, 2000). BrdU was incorporated when spermatogonia were co-cultured with fragments of seminiferous tubules. Marret and Durand also found that the uptake of BrdU was similar between the two treatment groups of culture with fetal calf serum or culture on an extracellular matrix (Marret and Durand, 2000). Another observation during the experiment was that the seminiferous tubule fragment co-culture was only able to maintain the number of spermatogonia in culture in the presence of fetal calf serum, suggesting that fetal calf serum promotes spermatogonia survival (Marret and Durand, 2000). These studies on the culture of porcine spermatogonia are important and essential for progress in this research area. Isolation of the spermatogonial stem cells was accomplished using a non-linear Percoll gradient in conjunction with sedimentation velocity using a BSA gradient. Based on the method used to identify the spermatogonial stem cells, both methods provided good results. The identification of spermatogonia was attempted with the use of c- kit receptor in conjunction with immunohistochemistry, but no evidence was available on the expression of c-kit in porcine stem cells (Dirami et al., 1999). An alternative technique to identify spermatogonia utilized vimentin staining, which stains only somatic cells, leaving the germ line cells unstained and unable to be identified (Marret and Durand, 2000). The methods used in both studies are not optimal; accurate identification of porcine 54
65 spermatogonial stem cells requires detection of spermatogonial stem cell specific markers. Identifying spermatogonial stem cells with specific markers will allow for accurate assessment of these purification and culture techniques in addition to the development of improved purification methods. In both experiments, viability of cells in culture decreased with time, but the kinetics of the decrease was dependent on the medium used. Viability was consistently higher when fetal calf serum was supplemented in the media, and it was found that SCF may promote spermatogonia survival, either added separately to a basal medium, or added because of its presence in fetal calf serum (Marret and Durand, 2000). Another important observation from these studies is the behavior of spermatogonia in culture. It was found that purified spermatogonia were not entering the S phase of the cell cycle, as indicated by no BrdU incorporation into the cells (Marret and Durand, 2000). BrdU incorporation does occur at a low percentage when purified cells are co-cultured with tubule fragments or in the presence of serum, indicating that either tight intercellular communications or an element present in serum is necessary for cells to enter the S phase and proliferate in vitro. More research is needed before the optimum method of culture for porcine spermatogonial cells is defined. Choice of culture environment currently depends on the needs and goals of the experiment. Utilization of serum in culture has been shown to increase viability and perhaps stimulate spermatogonial cell division (Marret and Durand, 2000); however, the components of serum remain undefined. Culturing porcine spermatogonia on a feeder layer or tubule matrix has variable results, but can stimulate in vitro cell division (Marret and Durand, 2000). In the present study, addition of serum to the 55
66 culture medium was used because of its positive affect on cell viability (Marret and Durand, 2000). The porcine spermatogonial cells in this study were not cultured on a feeder layer to allow for measurements of viability and cell number to be easily taken and because no clear advantage of cell co-cultures compared to the culture of cells with serum supplementation was concluded in previous studies. In the present study, a relatively simple environment was chosen for the culture of porcine spermatogonial cells to lay groundwork for future experiments and to allow the procedures to be easily reproduced in the laboratory. CELL BEHAVIOR IN CULTURE Based on environmental conditions, cells will exhibit different behaviors in culture including proliferation, differentiation, and apoptosis. What the cells are doing in culture is a good indication of the effectiveness of the culture system; therefore, a basic understanding of how cells grow and divide is needed. The process of the cell cycle allows cells to multiply via cell division. The cell cycle has four phases, including M phase, G1 phase, S phase, and G2 phase (Figure 4). M phase is also known as mitosis, which is when the condensed chromatin within the cell segregates into each daughter cell. The other three phases of the cell cycle are collectively referred to as interphase, the first of which is Gap 1 phase, or G1, where progression toward DNA synthesis occurs. There are checkpoints in the G1 phase that will determine if the cell will exit the cell cycle reversibly and go into G0, exit the cell cycle permanently to differentiate, or continue dividing. The S phase occurs after the G1 phase and it is during this phase where the DNA replication occurs. The final stage of interphase is the G2 phase, which is when the cell prepares for another cell division cycle and reentry into mitosis. Checkpoints occur after 56
67 the end of the S phase and the G2 phase, which regulate progression through the cycle by allowing DNA repair if needed, or by initiating entry into apoptosis (Freshney, 2000). The cell cycle is regulated by different factors, many of which are not clearly understood. Cells successfully progressing through the cell cycle are proliferating, and proliferation in culture can be affected by environmental conditions. If cells are seeded at a light density, the cells will have free edges and the capability to spread, which permits entry into the cell cycle. When cells either reach a high density because of proliferation, or are seeded at a high density, the cell to cell contact inhibits cell proliferation and cells will exit the cell cycle to either a quiescent state of G0, terminally differentiate, or undergo apoptosis (Freshney, 2000). Cells need energy to progress through the cell cycle, which is generated by the process of glycolysis. Media used for cell culture usually contain glucose which is used as a carbon source for energy-producing glycolysis. In cell culture, glycolysis occurs in anaerobic conditions, since the cells are normally submerged in medium. During culture, the citric acid cycle remains active in the cell and amino acids in the medium can also be used as a carbon source to generate energy (Freshney, 2000). Restriction Points Figure 4. The cell cycle with restriction checkpoints. Checkpoints determine if the cell will go forward with division or stop dividing. Adapted from Freshney,
68 GENE TRANSFER IN PORCINE SPERMATOGONIA TYPES OF GENE TRANSFER Many methods exist for transferring genetic information into cells, with some of the most useful systems integrating information directly into the germ line. Microinjection, electroporation, calcium phosphate precipitation, lipofection, and viral vectors all have been used to transfer genetic information into cells. Here, methods using virus vectors will be discussed as they have proven to be most effective gene transfer vehicles in spermatogonial stem cells. Retroviruses are a group of viruses that have the ability to convert viral genetic information from an RNA to a DNA form during infection of animal cells. Retroviruses are spherical structures that contain two RNA molecules and at least nine different types of protein (Drlica, 1992). Retroviruses are divided into two categories, simple and complex, which are distinguishable by genome organization. Simple retroviruses encompass the Murine leukemia-related viral group (MuLV), among others. Complex retroviruses include Human T-cell leukemia-bovine leukemia viral group, Spumaviruses, and Lentiviruses (Coffin et al., 1997). In addition to the obvious difference between simple and complex, simple retroviruses can only infect actively dividing cells. All retroviruses have similar overall structure consisting of three major coding domains: gag, pol, and env. Gag directs the synthesis of structural proteins, pol encodes viral enzymes such as integrase and reverse transcriptase, and env is the source of structural envelope proteins that facilitate attachment and fusion of virus with the target cell surface (Trono, 2002). The first stage of infection is the entry of the virus into the cell. The reverse transcriptase, a type of DNA polymerase, replicates a DNA copy from the viral RNA 58
69 genome. The new viral DNA is then inserted into the target cell s chromosome such that mrna can be synthesized. The integrated gene is flanked on both sides with long terminal repeats (LTRs) that are thought to be involved in the binding of the integrase protein, which facilitates the insertion of the new viral DNA into the chromosome. The mrna derived from the viral insert can be translated and made into the protein (Drlica et al., 1992). Vectors for gene therapy are either replication competent or replication defective. Replication defective vectors generally have a deletion of protein encoding sequences that are replaced by the transgene of interest. In addition, changes in the LTRs upstream or downstream of the transgene can be introduced to prevent the virus from replicating. Replication competent vectors can productively infect target cells, although replication defective vectors are the preferred type to prevent the spread of the virus (Knipe and Howley, 2001). The major disadvantage of retroviruses is that they are unable to efficiently infect non-dividing cells (Trono, 2002). This shortcoming led to investigation of alternative methods of gene delivery, including Lentiviruses, which also belong to the Retroviridae family. One of the most studied lentiviruses is the Human Immunodeficiency Virus (HIV). Two types of the HIV lentivirus exist, HIV-1 and HIV-2, with the main difference being that HIV-1 infects non-human primates, and HIV-2 is a human virus (Buschschacher, 2003). Lentiviruses are complex retroviruses that have the ability to infect non-dividing, differentiated mammalian cells. Another difference between lentiviruses and simple retroviruses is that in addition to three major coding domains: gag, pol, and env, lentiviruses contain the auxiliary genes (vpr, vif, vpu, nef) and regulatory genes (tat and rev) that have important functions during viral life cycle. Tat is a protein that regulates lentiviral gene 59
70 expression at the transcriptional level, while rev is a protein that regulates lentiviral gene expression at the post transcriptional level. Tat functions by binding to a cis-acting element located within the LTR to upregulate the activity of the promoter to enhance viral transcription and gene expression. Rev recognizes a cis-acting element located in the mid portion of the env gene to regulate viral gene expression after transcription (Trono, 2002). The mechanism that lentiviruses use to transduce terminally differentiated and nondividing cells is not exactly known. The preintegration nucleoprotein complex (PIC) is recognized by the nuclear import machinery of the target cells and is imported through the nucleopore by active transport. HIV-1 has many nucleophilic determinants that allow the entry of the preintegration nucleoprotein complex into the cell. Three of these proteins have been identified in HIV-1: Gag-encoded matrix protein, integrase, and vpr. The gag-encoded matrix protein has two nuclear localization signal motifs, and if both are inactivated, the virus is defective in nuclear transport (Knipe and Howley, 2001). Integrase proteins of lentiviruses contain signals that direct the protein to the nucleus, although there is no evidence that this signal promotes nuclear import. It has been suggested that integrase has some homologous sequences to the nuclear localization signal motifs, although inactivation causes exclusion of integrase, normal reverse transcription (Trono, 2002). TRANSDUCTION OF GERM CELLS In 1994, Brinster and colleagues reported that spermatogonial stem cells could be transduced by a retroviral vector during the transplantation of cells to a recipient mouse testis. The transduction occurred at a low frequency, but spermatogenesis was re-established 60
71 and maintained for 6 months after transplantation (Brinster and Zimmermann, 1994; Brinster and Avarbock, 1994). In 2000, a lacz reporter gene was delivered with a retrovirus into male germ line stem cells and was expressed for more than 6 months after transplantation into the mouse testis recipient (Nagano et al., 2000). It was found that periodic infections using neonatal cells produced the highest transfection efficiency of 1.8%, which is attributed to an enriched stem cell population in the neonate, and/or better stem cell proliferation (Nagano et al., 2000). Mouse pup and adult spermatogonial stem cells were transfected using a retroviral vector, with an efficiency of 20% for pup cells and 2% for adult cells from cryptorchid organisms (Nagano et al., 2001a). Transfected cells were transplanted to recipient testes, and the transgene was found to be transmitted to 4.5% of progeny, with subsequent generations also expressing the transgene (Nagano et al., 2001a). These findings demonstrated that male germ-line stem cells could be easily transduced with a retrovirus and that the vector could be integrated stably and passed to future generations (Nagano et al., 2001a). A previously used retroviral vector carrying the lacz reporter gene was used to transfect rat spermatogonial stem cells (Orwig et al., 2002). The transduction efficiency was lower than that in the previous mouse studies (Brinster and Zimmermann, 1994; Brinster and Avarbock, 1994; Nagano et al., 2000; Nagano et al., 2001a), in fact, transduction to mouse pup spermatogonial stem cells was 3.6-fold better than in the rat pup cells. The reason that the low efficiency occurred is unclear, but postulated that the rat cell cycle status in vitro was slower and yielded lower integration of the virus vector. No transgenic progeny resulted from the experiment, although it was demonstrated that rat pup spermatogonial stem cells could be transduced, although at a lower efficiency than in the mouse. It became evident that 61
72 enrichment of the stem cell population, improved culture conditions to encourage proliferation and survival, increase in virus titer, and alternative vectors could improve spermatogonial stem cell transduction in the rat and in other species (Orwig et al., 2002). In 2002, Nagano and colleagues utilized two types of lentiviruses to transfect adult cryptorchid and pup spermatogonial stem cells in mice (Nagano et al., 2002). The transplantation of transfected stem cells resulted in spermatogenic colonies present in 100% of the mice pups and 28.6% of the adult mice. The transduction efficiency was found to be 4.6-fold higher in pup cells, which can be explained by the 1-fold increase in pup stem cells progressing through the cell cycle in vitro when compared to the adult. Although lentiviruses can infect cells not dividing, the transduction is less efficient in cells arrested in the G 0 stage of the cell cycle (Nagano et al., 2002). Success of the mouse experiments using lentiviral transduction led to the investigation of lentiviral transduction in rat spermatogonial stem cells. Rat spermatogonial stem cells were demonstrated to retain stem cell characteristics and can efficiently transmit inserted genes through the germ line after 4 days in culture prior to transplantation (Herma et al., 2002). Live young were generated from this experiment, and 30% of the pups carried the transduced gene. Furthermore, the integrations were stable and could be transferred to the F2 generation at a 50% frequency (Herma et al., 2002). Taken all together, the experiments investigating gene transfer to spermatogonial stem cells reveal that cells from an immature animal are more readily transfected regardless of species, and that both retroviral and lentiviral vectors have great success delivering the foreign gene to the cells. Transplantation success of these transfected germ cells is essential for the efficient production of transgenic animals. 62
73 TRANSPLANTATION OF GERM CELLS Transplantation of testis cells was first accomplished in the mouse in 1994, with a goal of developing a functional assay to unequivocally define the spermatogonial stem cell (Brinster and Zimmermann, 1994; Brinster and Avarbock, 1994). Spermatogonial stem cell transplantation in mice has since been accomplished by many researchers with positive results, in addition to the transplantation of spermatogonial stem cells of other species into mice (Nagano et al., 1998; Ohbo et al., 2003). The procedure for germ cell transplantation involves harvesting testis tissue from a fertile male, digesting the tissue to generate a single cell suspension that includes spermatogonial stem cells, and delivering the cell suspension via injection or catheterization to the seminiferous tubules of a recipient, where colonies of donor-derived spermatogenesis will be established (Brinster, 2002). Once transplanted, the donor cells must migrate through the seminiferous epithelium to the appropriate location for survival. The donor cells must then proliferate and differentiate in the recipient seminiferous epithelium to produce spermatogonia (Nagano et al., 2001b). Spermatogenesis after germ cell transplantation has been reported, despite the exposure to enzymes for cell dissociation and manipulation that occurred throughout the transplantation procedure (Brinster and Zimmermann, 1994). The transplantation of the spermatogonial stem cells is important because they are the only cells in the postnatal animal that self renew in addition to contributing genes to future generations, and in this respect are simultaneously essential and unique for species continuation (Nagano et al., 2001b). It has been found that spermatogonial stem cells are more efficient than gonocytes in repopulating and establishing spermatogenesis in the recipient (Brinster and Avarbock, 1994). 63
74 In the initial study, spermatogonial stem cells were isolated from testes of mice that carried a transgene for the Escherichia coli β-galactosidase gene. The testis cells were injected into seminiferous tubules of infertile recipient mice, and the cells were able to colonize and repopulate the testes. Progeny and mature spermatozoa were produced as a result of stem cell transplantation to the recipient testis, and 38 of 104 testes used showed colonization (Brinster and Avarbock, 1994). The success of the transplantation into non-fertile recipients is encouraging, especially since during the transfer, the donor cells can be subject to genetic manipulation to produce transgenic spermatogonia and consequentially transgenic offspring by fertilization of selected female gametes in vitro or in vivo (Dobrinski et al., 2001; Nagano et al., 2001a). Spermatogenesis occurred after maintenance of mouse spermatogonial stem cells in culture before transplantation into recipient mice, indicating that spermatogonial stem cells have the potential to be manipulated in vitro and remain functional upon transplantation. It is not surprising that spermatogonial stem cells can survive under various in vitro conditions as it is commonly known that the spermatogonial stem cell is resistant in vivo and is the last of the germ cells to be destroyed by detrimental environmental factors or trauma to the testis area (Nagano et al., 1998). Methods to enrich for spermatogonial stem cells prior to genetic manipulation and transplantation could increase the efficiency of this novel approach to the generation of transgenic animals. The efficiency of this method to produce transgenic animals could be high since one transformed spermatogonial stem cell is theoretically able to form 4,096 spermatids (Brinster and Zimmermann, 1994). The technique of transplanting testis cells from one male to another is an important step to the novel approach to produce transgenic animals (Brinster and Zimmermann, 1994; Brinster and Avarbock, 1994). 64
75 After the initial study in mice, the transplantation of spermatogonial stem cells into testes was expanded to larger animal species recipients including the monkey, bovine, rat, and human (Schlatt et al., 1999). The initial transplantation method developed in mice involved injection of the spermatogonial stem cells into the superficial seminiferous tubules, but the transfer of the cells by microinjection or cannulation was difficult and inefficient in the larger species (Schlatt et al., 1999). The difficulty led to the development of an injection technique into the rete testes using ultrasonography to guide the injection needle. Schlatt s group used intact adult male cynomolgus monkeys as injection recipients. The monkeys were pretreated with a gonadotrophin releasing hormone to reduce the testis volume; however, no technique to reduce endogenous spermatogenic activity was performed. Injection to the rete testis allowed a large volume to be transplanted into the testis and avoided the need for open surgery. Very few transplanted germ cells were detected by BrdU staining in the seminiferous tubule 4 weeks after the transfer. Only a small percentage of transplanted cells could be detected with BrdU, since only the cells in the S-phase during the culture before transplantation would have incorporated the BrdU. Despite the inefficient labeling, the presence of BrdU-labeled cells at the base of the seminiferous tubules underlines the success of the modified transplantation technique for large species (Schlatt et al., 1999). The development of a transplantation technique for large animals allowed for the first germ cell transplantation in pigs (Honaramooz et al., 2002). This important event is one of the many steps needed to generate transgenic farm animals through manipulation of the male germ line. Fluorescent-labeled porcine testis cells were transplanted into recipient prepubertal pig testes and all attempts were successful but one, which was attributed to 65
76 inaccurate catheter placement. Transplanted donor cells were detected in 50% of the recipient seminiferous tubules for up to 4 weeks after the procedure (Honaramooz et al., 2002). As mentioned, prepubertal pigs were used as recipients, which allowed for the reduction of the number of endogenous germ cells without administering treatment that could be detrimental to the spermatogonial environment in vivo (Honaramooz et al., 2002). The transplantation of donor cells to the prepubertal pig meets less resistance when compared to the adult animal, and the donor cells did not need to migrate through multiple layers of developing spermatogenic cells to colonize the basement membrane. Because of the age of the animals, donor-derived spermatogenesis was neither observed nor expected; however, the observation of position and maintenance of transplanted cells indicates that porcine donor testis cells survive in and could colonize the recipient prepubertal pig testis (Honaramooz et al., 2002). The development of transgenic pigs is interesting for many reasons including the potential to provide organs for xenotransplantation to humans, to generate useful drugs in vivo, and to use as models for human disease. To date, microinjection of zygotes or somatic cell nuclear transfer techniques have been used to generate the majority of transgenic pigs. High expense and low efficiency make these techniques difficult; additionally the developmental abnormalities associated with success of these techniques makes a more desirable method to generate transgenic animals necessary. An alternative approach to generate transgenic livestock could be the transplantation of spermatogonial stem cells that have been transduced with a desired gene (Honaramooz et al., 2002). Development of transplantation techniques for large species (Schlatt et al., 1999), culture of porcine 66
77 spermatogonial stem cells (Dirami et al., 1999; Marret and Durand, 2000), and transfection of spermatogonial stem cells will all provide the means necessary for generation of transgenic pigs by manipulation of male germ-line cells. 67
78 STATEMENT OF THE PROBLEM The objectives described in this thesis were to assess the effectiveness of a Nycodenz gradient enrichment method to enrich a dissociated single cell suspension of porcine testicular cells for spermatogonia, and to observe the separated fractions from the gradient over a 14-day culture period for cell viability and number of spermatogonia in culture. Two germ-cell specific genes, DAZL and VASA, were utilized for identification of spermatogonia using immunohistochemistry. Spermatogonial stem cell isolation and enrichment are well characterized in the murine models, but are in the early developmental stages when using porcine tissue. Evaluation of a novel enrichment protocol will allow a larger population of spermatogonial stem cells to be manipulated in vitro for use in gene transfer. The use of such biotechnologies in the porcine model are also in the early developmental stages, therefore maintenance of spermatogonial stem cells for an extended period of time in vitro may be beneficial. The development of such techniques in the porcine model will provide novel ways of producing transgenic pigs to be used for models of human disease, xenotransplantation of organs to humans, and bioreactors of drugs for human use. The benefits to be gained through such research are monumental, therefore necessitating the development of isolation, enrichment, identification, and culture techniques in the porcine model system. 68
79 MATERIALS AND METHODS EXPERIMENTAL DESIGN The effectiveness of a NycoDenz gradient method to enrich a dissociated single cell suspension of porcine testicular cells for spermatogonia and to observe the separated fractions from the gradient over a 14-day culture period for cell viability and number of spermatogonia in culture was evaluated. Two germ-cell specific genes, DAZL and VASA, were utilized for identification of spermatogonia using immunohistochemistry. Cultures generated from the enzymatic digestion of porcine testes prior to the enrichment protocol for each of 10 experimental replicates were used as control. The NycoDenz gradient consistently separated the isolated cell suspension into four distinct layers. Both separated and control cultures were conducted in identical hextuplicates to facilitate assessment of cell viability and spermatogonia number on three days with minimal disruption to the culture environment. On days that cell viability and spermatogonia number were assessed, a whole culture was used for each parameter to ensure a sufficient amount of cells was included in the sample. Testis cells were isolated and seeded in culture on day 0. Spermatogonia were morphologically defined as round, plump cells with a large amount of cytoplasm, as is consistent with prior observation of spermatogonia cultures in the literature. Visualization of spermatogonia was facilitated by immunostaining with DAZL and VASA polyclonal antibodies and cells exhibiting morphological characteristics in addition to bright, concentrated fluorescence were counted as spermatogonia. 69
80 REAGENTS AND MEDIA Dulbecco s Phosphate-Buffered Saline (DPBS-D1408, Lot #72K2449) without calcium chloride and magnesium chloride, type IV collagenase (C-5138, Lot #035K8633), HistoDenz (D2158, Lot #074K1298), trypsin-edta solution with 5.0g porcine trypsin and 2g EDTA, sterile-filtered, cell culture tested (T4174, Lot #82K2354), and trypan blue solution, 0.4% (T8154, Lot #123K2404) were purchased from Sigma Chemical Company (St. Louis, MO). Phosphate buffered saline with 11.9mM phosphates, 137mM sodium chloride, and 2.7mM potassium chloride (Lot # ), paraformaldehyde powder (Lot #906117), and methanol (Lot #020378) were purchased from Fisher Scientific (Hampton, NH). Alexa Fluor 488 chicken anti-rabbit IgG (H+L) (A21441, Lot #99E2-1), and propidium iodide 2.4mM solution in DMSO (L7011, Lot #0281-2) were purchased from Molecular Probes (Carlsbad, CA). Novolsan solution (Lot #062793) was purchased from Fort Dodge Laboratories, Inc. Kanamycin monosulfate (Lot # ) was purchased from Mediatech Inc. (Herndon, VA). Rabbit polyclonal antibodies for VASA and DAZL were a kind gift from Dr. J. Petitte (NC State University, NC). Dulbecco s Modified Eagle s Medium-high glucose with 4500 mg/l glucose, L- glutamine, and sodium pyruvate, without sodium bicarbonate, cell culture tested (DMEM- D7777, Lot #093K83131), sodium bicarbonate, cell culture tested (S5761, Lot #023K0133), fetal bovine serum, sterile-filtered, heat-inactivated, and cell culture tested (FBS-F4135, Lot #035K8420), and antibiotic/antimycotic suspension, stabilized, sterile-filtered, cell culture tested (A5955, Lot #114K23901) were purchased from Sigma Chemical Company (St. Louis, MO). 70
81 DMEM basal medium was supplemented with 1.50g/L of sodium bicarbonate, 10% FBS, and 1% antibiotic/antimycotic to form complete medium, which was used for all cultures. The complete medium was filter sterilized and stored at 4ºC until needed, with a storage time of no longer than 7 days. All cultures were incubated at 37ºC in an atmosphere of 5% CO 2 in air with 100% humidity. ISOLATION OF PORCINE TESTIS CELLS Prepubertal Landrace-Yorkshire cross pigs (2-8 days old; Swine Educational Unit NC State University, Raleigh, NC) are castrated by the industry standard method as a part of routine husbandry by the farm personnel. All animal procedures performed at the Swine Educational Unit were approved by North Carolina State University. Testes, as a byproduct of castration, were collected on site in sterile conical tubes and transported to the laboratory on ice. The parietal tunica vaginalis along with a portion of the epididymus was removed, leaving the tail of the epididymus and whole testis. Partially dissected testes were rinsed in DPBS with kanamycin for 10 minutes. Kanamycin solution was decanted and testes were washed in a 30% Novolsan solution in DPBS for 10 minutes. Novolsan solution was decanted and testes were washed three times in DPBS. Testes were dissected in a sterile environment by removing the tunica albugina, mediastinum, and remaining epididymus. Testicular parenchyma was transferred to a sterile conical tube for enzymatic digestion; an average of 2.0g of testicular parenchyma was digested for each replicate. Ten experimental replicates were conducted, each including a separate enzymatic digestion of testicular tissue, Nycodenz gradient separation, collection of layers, and seeding of identical cultures, as described previously. 71
82 Collagenase solution was prepared using 20mg of type IV collagenase (Sigma, C- 5138, Lot #035K8633), 10ml of DPBS, and.1ml antibiotic/antimycotic. Collagenase solution was sterile filtered through a 0.22µm syringe filter. Testicular parenchyma was incubated in collagenase solution at 37ºC with 200rpm of agitation for 3 hours to generate a suspension. The cell suspension was centrifuged at 210g for 5 minutes, and the supernatant was removed. The pellet was resuspended in 10ml of DPBS and centrifuged at 1000rpm for 5 minutes. Supernatant was removed and the pellet was resuspended in 5ml of DPBS. The washed cell suspension was filtered through a pour-through 70µm nylon filter (Fisher Scientific), and the suspension was brought up to a volume of 10ml with DPBS. A portion of the cell suspension was used to plate down a control group to an average density of 2.85 x 10 7 cells/ml and remaining suspension was stored on ice until used for spermatogonia enrichment procedure. ENRICHMENT OF SPERMATOGONIA HistoDenz, (Sigma Chemical Company trade name for Nycodenz) was used to generate a discontinuous density gradient. Nycodenz powder was dissolved in DPBS and diluted to four final concentrations of g/ml, g/ml, g/ml, and 1.084g/ml. Aliquots of each concentration were stored frozen for all replicates of the experiment. The maximum storage of frozen aliquots was 4 weeks. The Nycodenz concentrations were chosen based on results from the purification of mouse PGCs with Nycodenz (Mayanagi et al., 2003), and on an evaluation of various Nycodenz concentrations to form 4 distinct cell populations upon centrifugation (data not shown). A discontinuous Nycodenz gradient column was prepared by layering 1ml of each Nycodenz concentration from lowest density 72
83 to highest density using a sterile pasture pipette without disturbing the previous layers. Gradients were formed in 5ml polystyrene round bottom test tubes (Cat #14-595A) purchased from Fisher Scientific (Hampton, NH). Nycodenz solutions and cell suspensions were kept on ice while the discontinuous gradient was prepared. Four gradient columns were used for each experimental replicate, with 1ml of cell suspension layered on top of each gradient (Figure 5). Gradient columns were centrifuged at 300g for 15 minutes at 4ºC and rotors were allowed to decelerate without using the brake. After centrifugation, gradient columns consistently displayed 4 separated cell populations (Figure 6). Each layer was collected with a sterile pipette and centrifuged at 500g for 8 minutes. Supernatant was removed and the pellet was resuspended in 0.5ml of warmed complete medium. Collected layers from all 4 gradient columns were pooled with similar layers and cells were mixed thoroughly. The cell suspension was divided equally in 0.5ml portions and inoculated into six culture wells. Three cultures were seeded in 15mm polystyrene Nunclon treated culture dishes (Cat # ) purchased from Fisher Scientific (Hampton, NH). Three other cultures were seeded in 16mm polystyrene vacuum gas plasma treated culture dishes (Cat # ) purchased from Fisher Scientific (Hampton, NH). The creation of six identical cultures allowed one culture to be used for each assessment of viability and number of spermatogonia on three different days of the experiment. Cell density at initiation of culture was on average 5.74x10 6 cells/ml for layer 1, 1.112x10 7 cells/ml for layer 2, 9.77x10 6 cells/ml for layer 3, and 5.69x10 7 cells/ml for layer 4. Cultures were incubated at 37ºC in an atmosphere of 5% CO 2 in air with 100% humidity. 73
84 VIABILITY OF SPERMATOGONIA CULTURES Viability was assessed in cultures on day 0, 7, and 14 of incubation with day 0 assessment occurring after 5 hours of incubation. Viability assessment was accomplished by removing spent medium from cultures and washing adherent cells twice with DPBS. Spent medium and washes were combined, centrifuged at 335g for 5 minutes and supernatant was removed. Cultures were exposed to warm trypsin EDTA for 10 minutes at 37ºC until cells were no longer adherent to the culture dish. Complete media was used to resuspend the pellet and provide trypsin inactivation. Trypsin and cells were removed from the culture dish, combined with cells removed with washes, and mixed well. Cell viability was assessed via trypan blue exclusion assay (Freshney, 2000), which identifies live cells by their exclusion of the blue dye. 0.25ml of cell suspension was diluted 1:1 in trypan blue stain and loaded into each side of a hemocytometer for assessment of viability (Freshney, 2000). An upright light microscope was used to count the number of live and dead cells in five of the 25 squares in the middle of the hemocytometer slide on each side. The values were averaged, and the percent of viable cells in each sample was determined. ASSESSMENT OF SPERMATOGONIA NUMBER IN CULTURE Number of spermatogonia in culture was assessed on day 0, 7, and 14 of incubation with day 0 assessment occurring after 5 hours of incubation. Culture plates were removed from incubation and spent medium was removed and saved. Cells in suspension were prepared and assessed separately from attached cells (see below). 74
85 SPERMATOGONIA IN SUSPENSION Cells attached to dishes were washed twice with cold PBS and washes were added to spent medium. The cell suspension generated from washes and culture medium was centrifuged for 3 minutes at 335g. Supernatant was removed and the pellet was resuspended in cold PBS. The cells were washed three times with PBS, each time centrifuging cells, removing supernatant, and resuspending in PBS. The cells were centrifuged again, supernatant was removed, and cells were resuspended in 0.4% paraformaldehyde (PFA) for incubation at 4ºC overnight. The cells were washed 5 times with PBS, each time centrifuging cells, removing supernatant, and resuspending in PBS. A blocking solution was prepared with PBS supplemented with 10% FBS and was added to cells and incubated for 15 minutes at room temperature. Cell suspensions were centrifuged for 3 minutes at 335g and the supernatant was removed. Rabbit polyclonal primary antibodies VASA and DAZL were diluted 1:50 in blocking solution and incubated in cell suspensions overnight at room temperature. Cell suspensions were centrifuged for 3 minutes at 335g and the supernatant was removed. Chicken-anti rabbit secondary antibody conjugated to a fluorescent dye (Alexa Fluor 488, Molecular Probes) was diluted 1:200 in blocking solution and added to the cell suspension. Cells were incubated for 2 hours at room temperature in the dark. Cells were then washed twice for 10 minutes each in PBS by removing supernatant after centrifugation, resuspending the cells in PBS, and allowing them to incubate for 10 minutes. Propidium iodide was added to each suspension at a 1:2000 dilution. A wet mount slide was prepared for each cell suspension, and cells were observed and photographed with a Leitz Fluovert inverted microscope equipped with epifluorescence and a Diagnostic Instruments, Inc. Spot RT color camera. The microscope was also 75
86 equipped with 10x and 20x phase objectives and 2.5x, 20x, and 40x bright field objectives. The epifluorescent lamp on the microscope was used with a fluorescent cube filter I3, which is used to excite the fluorescent dyes fluorescein or green fluorescent protein that have an emission spectra in the 488 range. Ten images from different areas of each slide were saved and analyzed by AlphaEase FC Stand Alone image analysis software for quantification of spermatogonia in culture (Alpha Innotech, 2003). The total number of cells and the number of spermatogonia was recorded from each image and observations from the 10 images were combined to determine an average percentage of spermatogonia in suspension. SPERMATOGONIA ATTACHED TO CULTURE DISH Cells attached to culture dishes were washed twice with cold PBS and fixed in 0.4% paraformaldehyde (PFA) for 3 minutes at 4ºC. Cells were incubated for another 10 minutes at room temperature. The PFA was removed and cells were washed three times for 5 minutes each in PBS. Methanol was added to the cell culture and incubated for 6 minutes at -20ºC. PBS was used to briefly wash cells 5 times. A blocking solution was prepared with PBS supplemented with 10% FBS and incubated with the cells for 15 minutes at room temperature. Rabbit polyclonal primary antibodies VASA and DAZL were diluted 1:50 in blocking solution and incubated in cell suspensions overnight at room temperature. Chickenanti rabbit secondary antibody conjugated to a fluorescent dye (Alexa Fluor 488, Molecular Probes) was diluted 1:200 in blocking solution and added to the cell cultures. Cells were incubated for 2 hours at room temperature in the dark. Cells were then washed twice for 10 minutes each in PBS. 5.0ul of Propidium iodide was diluted in 1ml of PBS for a final dilution of 1:200 and the solution was added to each culture. Cultures were observed and 76
87 photographed with an inverted microscope equipped with epifluorescence. Ten digital images from different areas of the culture dish from each culture were saved and analyzed by AlphaEase FC Stand Alone image analysis software for quantification of spermatogonia in culture (Alpha Innotech, 2003). The total number of cells and the number of spermatogonia were recorded from each image and observations from the 10 images were combined for an average percentage of spermatogonia in the culture. STATISTICAL ANALYSIS Percentages of cell viability and spermatogonia in culture were initially analyzed as raw data. The raw data were not normally distributed; therefore, the percentages were transformed using an arcsine transformation (Ott and Longnecker, 2001; Snedecor and Cochran, 1998). The transformed data were analyzed with Statistical Analysis Software (SAS) version using the analysis of variance procedures for general linear models (Cary, N.C.). A probability of p < 0.05 was considered statistically significant. All data are reported as sample least squares means ± standard error prior to transformation and the significance differences were generated from an analysis of the transformed data. 77
88 Cell Suspension g/ml g/ml g/ml g/ml Figure 5. Discontinuous Nycodenz gradient prior to centrifugation. Interface 1 Interface 2 Interface 3 Pellet Figure 6. Discontinuous Nycodenz gradient after centrifugation. 78
89 RESULTS EFFECT OF NYCODENZ GRADIENT ON CELL VIABILITY IN CULTURE The viability of cells studied was found to be affected by the population of cells in culture and the amount of time spent in incubation. The data collected from viability assays indicated that significant differences in viability occurred across individual layers and days of culture, between replicates, and among layers considered by day of culture (Table 2). Viability values were an average of ten replicates. When all days of the study were combined, the pellet of the enrichment gradient contained a significantly higher proportion of viable cells than all other layers, including the control group (P < 0.05). Layers 1 and 2 of NycoDenz gradients contained a significantly lower proportion of viable cells when compared with control and other layers (P < 0.001). The percentage of viable cells in the control group was statistically similar to the viability percentage found in layer 3 (P > 0.5) (Figure 7). Cell viability decreased as the number of days in culture increased. The viability of cells in culture on day 0 and 7 are similar (P > 0.10); however, cell viability significantly decreased by day 14 (P < ) (Figure 8). The effect of replicate on cell viability was significant (P < ). Specifically, replicate 3 was statistically different from seven out of nine other replicates. Replicate 4 was similar to replicate 3 (P > 0.5), but was different from three of the other nine replicates (Figure 9). The effect of time in culture on cell viability within layer was examined and found to be significant (P < 0.005). Time in culture was assessed on day 0, 7, and 14. Values were reported as average percentages from the observation of ten replicates (Table 3). 79
90 On day 0 of culture, the percentage of viable cells in the control group was similar to the percent of viable cells in layer 3 and in the pellet (P > 0.10). Layers 1 and 2 were similar in the percentage of viable cells (P > 0.10), and were lower in viability than the pellet and the control group (P < ) (Figure 10). The viability of cells was also measured on day 7 of culture. Cell viability in the control group was similar to the viability of each layer (P > 0.10). The pellet contained a significantly higher percentage of viable cells when compared to the viability in layers 1 and 2 (P < 0.001) (Figure 11). Viability was measured on day 14 of the study and a significant difference in viability was found between layer 1 and the pellet of the NycoDenz gradient. The cell viability in the pellet was higher than the viability from layer 1 (P < 0.05). The viability of the control group did not differ from the viability of the individual layers (P > 0.10) (Figure 12). 80
91 Table 2. Significance of layer, day, replicate, and layer by day effects on percent viability. Effect was considered significant if p < Layer Effect Day Effect Rep Effect Layer x Day Effect Cell Viability of Culture P < P < P < P < 0.05 Layer Effect on Viability Percent of Cells Viable a b a c c Control Layer 1 Layer 2 Layer 3 Pellet Figure 7. Layer effect on viability for all days. With all days combined, the pellet contained statistically more viable cells than any other group. Layers 1 and 2 contained the lowest amount of viable cells. Values reported are the mean ± SEM. Bars lacking a common letter differ, P < Day Effect on Viability 100 Percent of Cells Viable a a b 0 Day 0 Day 7 Day 14 Figure 8. Day effect on viability for all layers. With all layers combined, the amount of viable cells in culture decreased with time in culture. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
92 Replicate Effect on Viability Percent of Cells Viable a a ac a ab ab ac ac b bc Experimental Replicate Figure 9. Replicate effect on viability for all layers and days. Replicate 3 is different than 7 other replicates. Replicate 4 is similar to three, but different than 3 other replicates. Values reported are the mean ± SEM. Bars lacking a common letter differ, P < Table 3. Cell viability for each layer and day. Values reported are the mean ± SEM. Layer Day Mean (%) + Std Error ± Control ± ± ± Layer ± ± ± Layer ± ± ± Layer ± ± ± Pellet ± ±
93 Day 0 Viability Percent of Cells Viable ab cd d ac b 0 Control Layer 1 Layer 2 Layer 3 Pellet Figure 10. Cell viability of each layer on day 0 of culture. The pellet contained the highest amount of viable cells, which was similar to the control group, but significantly different from all other groups. Layer 2 contained the lowest amount of viable cells, which was similar to the amount in layer 1, but significantly lower than all other groups. Values reported are the mean ± SEM. Bars lacking a common letter differ, P < Day 7 Viability Percent of Cells Viable ab a a ab b 0 Control Layer 1 Layer 2 Layer 3 Pellet Figure 11. Cell viability of each layer on day 7 of culture. The pellet contained the highest amount of viable cells, but was only significantly different than layers 1 and 2. The control was similar to all layers. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
94 Day 14 Viability Percent of Cells Viable a ab ab ab b Control Layer 1 Layer 2 Layer 3 Layer 4 Figure 12. Cell viability of each layer on day 14 of culture. The pellet contained the highest amount of viable cells, but was only significantly different than layer 1. The control was similar to all layers. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
95 EFFECT OF ENRICHMENT GRADIENT ON NUMBER OF SPERMATOGONIA IN CULTURE The spermatogonia in culture was assessed in three ways: as a percentage of spermatogonia within the cell population in suspension, as a percentage of spermatogonia within the cell population attached to the culture dish, and as the total percentage of spermatogonia with both cell populations combined. Percentages of spermatogonia values reported were an average of five replicates. The data collected indicated that significant differences in the percentage of spermatogonia contained in a cell population occurred across individual layers (P < ) and day (P < 0.05) of culture. The effect of replicate was not significant in the number of spermatogonia attached to the plate, nor was it significant in the cell population contained in suspension (P > 0.10). Replicate only became a significant effect when both cell populations were combined as a total count of spermatogonia in culture (P < 0.05). No significance was found in the layers considered by day data for neither measure of spermatogonia individually in suspension or attached nor for the total spermatogonia population with suspension and attached to dish combined (P > 0.10) (Table 4). EFFECT OF ENRICHMENT GRADIENT ON NUMBER OF SPERMATOGONIA ATTACHED TO PLATE The percent of spermatogonia attached to the plate was one of three measurements taken to determine the effectiveness of the NycoDenz gradient on enriching the cell population for spermatogonia. The effect of treatment was found to be significant, with the cell population in the pellet of the gradient containing the lowest percentage of spermatogonia. The percentage of spermatogonia in the pellet was similar to the percentage 85
96 contained in layer 3 (P > 0.10), but was statistically lower than the percentages in the control and layers 1 and 2 (P < 0.001). Layer 2 of the enrichment gradient contained the highest percentage of spermatogonia, which was significantly higher than layer 3 and the pellet (P < ), but was similar to the percentages found in control and layer 1 (P > 0.10) (Figure 13). The percent of spermatogonia attached to the plate was affected by length of time spent in culture. On day 0, the percent of spermatogonia attached to the plate was the lowest (P < 0.05). The percent of spermatogonia attached to the plate on days 7 and 14 were similar (P > 0.90), and were as abovementioned, statistically different from the percent of spermatogonia found on day 0 (P < 0.05) (Figure 14). The effect of replicate on the percent of spermatogonia attached to the plate was negligible. No significant difference was found between any replicates (P > 0.1). The effect that separated layers considered by day had on the percentage of spermatogonia attached to the plate was examined for days 0, 7, and 14 of the experiment. Although the overall layer by day data was not statistically significant (P > 0.10), the data was analyzed to determine the effect that each day had on the percentage of spermatogonia in the population of cells within each treatment. Values were reported as average percentages from the observation of five replicates. The mean percentage and standard error of each layer on each day is contained in table 5. On day 0 of culture, a significant difference was found in the number of spermatogonia attached to the plate. The cell population contained in layer 2 of the enrichment gradient contained a significantly higher percentage of spermatogonia when compared to the spermatogonia percentage contained in the pellet (P < 0.05); however, none 86
97 of the separated layers were found to be significantly different from the control group (P > 0.05) (Figure 15). After analysis of the individual layers considered by day effect on day 7, no statistical differences were found (P > 0.05) (Figure 16). A significant difference was found in the number of spermatogonia attached to the plate on day 14 of culture. Similar to the results found on day 0, the cell population in layer 2 of the enrichment gradient contained a significantly higher percentage of spermatogonia when compared to the spermatogonia percentage found in the pellet (P < 0.01). Even with this statistical difference, none of the individual layers were found to be significantly different from the control group (P > 0.10) (Figure 17). 87
98 Table 4. Significance of layer, day, replicate, and layer by day effects on percent spermatogonia Layer Effect Day Effect Rep Effect Layer x Day Effect Spermatogonia Attached P < P < 0.05 P > 0.10 P > 0.10 Spermatogonia in Suspension P < P < 0.05 P > 0.10 P > 0.10 Spermatogonia Total in Culture P < P < 0.05 P < 0.05 P > 0.10 Layer Effect of Attached Spermatogonia Spermatogonia Percentage c bc bc ab a Control Layer 1 Layer 2 Layer 3 Pellet Figure 13. Layer effect on percent of spermatogonia in attached cell population for all days. Layer 2 contained the highest percent of spermatogonia, but was only significantly different than layer 3 and the pellet. The pellet contained the lowest percentage of spermatogonia, significantly lower than control and layers 1 and 2. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
99 Day Effect on Attached Spermatogonia Spermatogonia Percentage b b a Day 0 Day 7 Day 14 Figure 14. Day effect on percent of spermatogonia in attached cell population for all layers. With all layers combined, the percent of spermatogonia was higher on day 7 and 14 when compared with the percent of spermatogonia on day 0. Values reported are the mean ± SEM. Bars lacking a common letter differ, P < Table 5. Percent of spermatogonia in attached cell population for each layer and day. Values reported are the mean ± SEM. Layer Day Mean + Std Error ± Control ± ± ± Layer ± ± ± Layer ± ± ± Layer ± ± ± Pellet ± ± *Values are reported as percent of spermatogonia attached to culture dish 89
100 Day 0 Spermatogonia Attached Spermatogonia Percentage a ab b ab ab Control Layer 1 Layer 2 Layer 3 Pellet Figure 15. Percent of spermatogonia in attached cell population in gradient layers on day 0 of culture. Layer 2 contained the highest percentage of spermatogonia, but was only significantly different than the pellet. Control was similar to all layers. Values reported are the mean ± SEM. Bars lacking a common letter differ, P < Day 7 Spermatogonia Attached Spermatogonia Percentage a a a a a Control Layer 1 Layer 2 Layer 3 Pellet Figure 16. Percent of spermatogonia in attached cell population in gradient layers on day 7 of culture. All groups were similar. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
101 Day 14 Spermatogonia Attached Spermatogonia Percentage a ab ab b ab Control Layer 1 Layer 2 Layer 3 Pellet Figure 17. Percent of spermatogonia in attached cell population in gradient layers on day 14 of culture. Layer 2 contained the highest percentage of spermatogonia, but was only different from the pellet. Control was similar to all layers. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
102 EFFECT OF ENRICHMENT GRADIENT ON NUMBER OF SPERMATOGONIA IN SUSPENSION The percent of spermatogonia in suspension was the second measurement taken to determine the effectiveness of the gradient on enriching the cell population for spermatogonia. The effect of layer was found to be significant, specifically, that the percent of spermatogonia in suspension in layer 2 was statistically higher than the percent of spermatogonia in all other layers, including the control group (P < 0.01). In addition, the percent of spermatogonia in the cell population of the pellet was statistically lower than all other layers (P < 0.001). The control group was statistically similar to only one of the individual layers, which was layer 1 (P > 0.10). The percent of spermatogonia in the cell population in layer 3 was significantly higher than the percentage found in the pellet (P < 0.01), but significantly lower than the percentage of spermatogonia found in layer 2 (P < ). The percent of spermatogonia found in layer 3 was also significantly lower that that of the percentage found in the control group (P < 0.05) (Figure 18). The day of culture was also found to have a significant effect on the percent of spermatogonia attached to the plate. On day 0 of the experiment, the cultures contained a significantly lower percentage of spermatogonia in suspension when compared to the percentage of spermatogonia in cultures on day 14 (P < 0.05). There was no difference in the percentage of spermatogonia in cultures when day 7 was compared with days 0 or 14 of culture (P > 0.05) (Figure 19). The effect of replicate on the percent of spermatogonia in suspension was negligible. No significant difference was found between any replicates (P > 0.10). 92
103 The effect that layers considered by day on the percentage of spermatogonia in suspension was examined for days 0, 7, and 14 of culture. Although the overall layer considered by day data were not statistically significant (P > 0.10), the data were analyzed to determine the effect that each day had on the percentage of spermatogonia in the population of cells within each treatment. Values were reported as average percentages from the observation of five replicates. The mean percentage and standard error of each layer for each day is contained in table 6. On day 0 of culture, one significant difference was found in the number of spermatogonia in suspension when the pellet was compared to the control and all other individual layers. The cell population contained in the pellet was significantly lower in the percentage of spermatogonia than all other layers, including the control (P < 0.005). The control was statistically similar to all other individual layers (P > 0.10) (Figure 20). One statistical difference was found in the percent of spermatogonia on day 7 when layer 2 was compared with the pellet. The population of cells contained in layer 2 of the enrichment gradient had the highest percent of spermatogonia and was statistically higher than the percent of spermatogonia found in the group with the lowest percentage, the pellet (P < ). Despite this difference, the control was statistically similar to all individual layers (P > 0.05) (Figure 21). On day 14 of culture, a few statistical differences existed in the number of spermatogonia in suspension. The population of cells contained in the control was statistically higher in percent of spermatogonia than the cell population contained in both layer 3 and the pellet (P < 0.05). The percent of spermatogonia in the cell population of layer 1 was similar to that found in the cell population of the control group and layers 2 and 3, but 93
104 statistically higher than the percent of spermatogonia found in the pellet (P < 0.05). Layer 2 contained the highest percentage of spermatogonia, but was only significantly different than spermatogonia percent found in layer 3 and the pellet (P < ) (Figure 22). 94
105 Layer Effect on Spermatogonia in Suspension Spermatogonia Percentage a c cd d b Control Layer 1 Layer 2 Layer 3 Pellet Figure 18. Layer effect on percent of spermatogonia in suspension for all days. Layer 2 contained the highest percentage of spermatogonia. The pellet contained the lowest percentage of spermatogonia. Control was only similar to layer 1. Values reported are the mean ± SEM. Bars lacking a common letter differ, P < Day Effect of Spermatogonia in Suspension Spermatogonia Percentage ab b a Day 0 Day 7 Day 14 Figure 19. Day effect on percent of spermatogonia in suspension for all layers. With all layers combined, the percent of spermatogonia in suspension was highest on day 14 and lowest on day 0. The percent of spermatogonia on day 7 was similar to both other days. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
106 Table 6. Percent of spermatogonia in suspension cell population for each layer and day. Values reported are the mean ± SEM. Layer Day Mean ± Std Error ± Control ± ± ± Layer ± ± ± Layer ± ± ± Layer ± ± ± Pellet ± ± Day 0 Spermatogonia in Suspension Spermatogonia Percentage a a a a Control Layer 1 Layer 2 Layer 3 Pellet Figure 20. Percent of spermatogonia in suspension in gradient layers on day 0 of culture. The pellet contained the lowest percentage of spermatogonia. Control was similar to layers 1, 2, and 3. Values reported are the mean ± SEM. Bars lacking a common letter differ, P < b 96
107 Day 7 Spermatogonia in Suspension Spermatogonia Percentage a ab ab ab b Control Layer 1 Layer 2 Layer 3 Pellet Figure 21. Percent of spermatogonia in suspension in gradient layers on day 7 of culture. Layer 2 contained the highest percentage of spermatogonia, but was only significantly different from the pellet. Control was similar to all layers. Values reported are the mean ± SEM. Bars lacking a common letter differ, P < Day 14 Spermatogonia in Suspension Spermatogonia Percentage a a ac cb b Control Layer 1 Layer 2 Layer 3 Pellet Figure 22. Percent of spermatogonia in suspension in gradient layers on day 14 of culture. Layer 2 contained the highest percentage of spermatogonia, but was only significantly different from layer 3 and the pellet. The pellet contained the lowest percentage of spermatogonia, significantly lower than control and layers 1 and 2. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
108 EFFECT OF ENRICHMENT GRADIENT ON TOTAL NUMBER OF SPERMATOGONIA IN CULTURE The third measurement taken to determine the effectiveness of the gradient on enriching the cell population for spermatogonia was the total percent of spermatogonia in culture. This measurement allowed for the combination of both previous measurements to determine the enrichment of spermatogonia in the entire culture. The effect of layer was found to be significant in that the percentage of spermatogonia in the control group was similar to the percentage of spermatogonia found in layer 1, but was statistically different from all other separated layers. Layer 2 contained the highest percentage of spermatogonia and was statistically higher than the percentage of spermatogonia all other groups (P < 0.001). Layer 3 contained a higher percentage of spermatogonia than the pellet (P < 0.01), and a lower percentage of spermatogonia than the control and layers 1 and 2 (P < 0.01). The pellet contained the lowest percentage of spermatogonia (P < 0.01) (Figure 23). The effect of day on the total percent of spermatogonia in culture was significant because on day 14 of the experiment, the percent of spermatogonia in culture was statistically higher than on day 0 (P < 0.05). The total percent of spermatogonia in cultures on day 7 was similar to the percentages found on days 0 and 14 (P > 0.10) (Figure 24). The effect of replicate was significant on the total percent of spermatogonia in culture. Replicate 4 of the experiment was different from replicate 5 (P < 0.05); however, both replicates in question were not statistically different from the other replicates (P > 0.05). The effect that layer considered by day had on the total percent of spermatogonia in culture was examined for days 0, 7, and 14 of culture. Although the overall layer by day data were not statistically significant (P > 0.10), the data were analyzed to determine any patterns 98
109 that the effect of each day had on the total percentage of spermatogonia within each separated layer. Values were reported as average percentages from the observation of five replicates. The mean percentage and standard error of each separated layer for each day is contained in table 7. One day 0 of culture, the percentage of spermatogonia found in the pellet of the enrichment gradient was significantly lower than the percentage of spermatogonia found in the control and layers 1 and 2 (P < 0.01). The percentage of spermatogonia found in layer 2 of the gradient was numerically the highest, but only significantly different from the percentages in layer 3 and the pellet (P < 0.001) (Figure 25). Significant differences in the total percent of spermatogonia were present between separated layers on day 7 of the experiment. The total percentage of spermatogonia contained in the pellet was again the lowest, and significantly lower than the percent of spermatogonia in the control group and layer 2 (P < 0.05). The highest percentage of spermatogonia was found in layer 2 of the enrichment gradient, and was significantly higher than the percent of spermatogonia found in layer 3 and the pellet (P < 0.05), but similar to the percentage found in the control group and layer 1 of the gradient (P > 0.10) (Figure 26). Significant differences found in the total percent of spermatogonia on day 14 of the experiment were similar to the results of day 0 and day 7. The total percent of spermatogonia present in the pellet was statistically lower than percent of spermatogonia contained in the control and layers 1 and 2 (P < 0.01). The highest percentage of total spermatogonia in culture was again found in layer 2, which was significantly higher than the spermatogonia percentages in layer 3 and the pellet (P < 0.001), but similar to the percentages found in the control group and layer 1 (P > 0.10) (Figure 27). 99
110 Layer Effect on Total Spermatogonia Spermatogonia Percentage c a a d b Control Layer 1 Layer 2 Layer 3 Pellet Figure 23. Layer effect on total percent of spermatogonia for all days. Layer 2 contained the highest percent of total spermatogonia. The pellet contained the lowest percent of total spermatogonia. Control was only similar to layer 1. Values reported are the mean ± SEM. Bars lacking a common letter differ, P < Day Effect on Total Spermatogonia Spermatogonia Percentage b ab a Day 0 Day 7 Day 14 Figure 24. Overall day effect on total percent of spermatogonia in culture. For all layers combined, the total percent of spermatogonia in culture increased with time. The highest percentage of spermatogonia was found on day 14, which was significantly different than day 0. Day 7 was similar to both other days. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
111 Table 7. Percent of spermatogonia in total cell population for each layer and day. Values reported are the mean ± SEM. Layer Day Mean + Std Error ± Control ± ± ± Layer ± ± ± Layer ± ± ± Layer ± ± ± Pellet ± ± Day 0 Total Spermatogonia in Culture Spermatogonia Percentage a ac ac bc b Control Layer 1 Layer 2 Layer 3 Pellet Figure 25. Percent of total spermatogonia on day 0 of culture. Layer 2 contained the highest percentage of total spermatogonia, but was only significantly different than layer 3 and the pellet. The pellet contained the lowest percentage of total spermatogonia, which was significantly lower than control and layers 1 and 2. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
112 Spermatogonia Percentage Day 7 Total Spermatogonia in Culture a ac ac bc b Control Layer 1 Layer 2 Layer 3 Pellet Figure 26. Percent of total spermatogonia on day 7 of culture. Layer 2 contained the highest percent of total spermatogonia on day 7, but was only significantly different than layer 3 and the pellet. The lowest percent of total spermatogonia was found in the pellet, which was significantly lower than control and layers 1 and 2. Values reported are the mean ± SEM. Bars lacking a common letter differ, P < Day 14 Total Spermatogonia in Culture Spermatogonia Percentage a ac ac bc b Control Layer 1 Layer 2 Layer 3 Pellet Figure 27. Percent of total spermatogonia on day 14 of culture. Layer 2 contained the highest percent of total spermatogonia, but was only significantly higher than layer 3 and the pellet. The pellet contained the lowest percent of spermatogonia, which was significantly different than control and layers 1 and 2. Values reported are the mean ± SEM. Bars lacking a common letter differ, P <
113 IDENTIFICATION OF CELL TYPES IN GRADIENT LAYERS The testis of a piglet before maturity contains various types of cells even though spermatogenesis has not begun. Testicular cells isolated from 2-8 day old boars were utilized in this study, and cells present in boars this age include undifferentiated type A spermatogonia, Leydig cells, Sertoli cells, connective tissue, and vascular tissue. The cells were subject to enzymatic digestion and separated based on density using a discontinuous Nycodenz gradient. The resulting cell populations appeared fairly homogenous with respect to morphology, and upon examination the cell types that made up each layer were assessed. In the perinatal porcine testis, germ cells have been described to have a round to oval shape with a centrally located nucleus. The concentration of cytoplasmic organelles in these germ cells is considered sparse when compared to other cells in the testis. Leydig cells in the perinatal porcine testis make up a majority of the testicular volume and are characterized by a high amount of intercellular complexity, specifically endoplasmic reticulum which aids in the production of androgens (Cole and Foxcroft, 1982). Sertoli cells are also present in the porcine testis at this time, and exhibit increased mitotic activity during the first month of development after birth. Sertoli cells are small in young animals (Russell and Griswold, 1993), and contain some intercellular complexity, typically more than what is observed in germ cells (Cole and Foxcroft, 1982). Based on morphological descriptions, the location of cells in the density gradient, and the percentage of cells identified as spermatogonia, the specific type of cell contained in each layer can be deduced. The cells observed in layer 1 would have been the largest, as they failed to travel through the gradient solution that was 1.028g/ml. The percentage of cells identified as spermatogonia in this layer was typically higher than what was found in layer 3 103
114 and the pellet, but lower than what was found in layer 2. Cells in this layer had various morphologies; some were small and round, and some were large and oval shaped. This information leads to the conclusion that the cells in layer one were Leydig cells and spermatogonia. The spermatogonia were identified by the antibody staining, and the morphology of the other cells in the layer matches the description offered by Cole and Foxcroft (1982) that Leydig cells are dense and full of endoplasmic reticulum. Layer 2 contained the highest number of spermatogonia; however, other cells existed in this layer. Some larger, round cells were present that were similar to the Leydig cells identified in layer 1. In addition smaller, round cells were present in this layer that were morphologically similar to Sertoli cells (Cole and Foxcroft, 1982). The cell population in layer 3 contained a combination of cell types. Many cells were observed in this layer, and some cells appeared similar to the erythrocytes found in the pellet. Other cells were larger than the erythrocytes and round shaped. Although they were rare, spermatogonia were also identified in this layer. Based on the morphology observed and the number of cells present, the majority of cells in this layer are identified as Sertoli cells, which is consistent with prior descriptions of round size and the high amount of mitotic activity associated with Sertoli cells (Cole and Foxcroft, 1982; Russell and Griswold, 1993). Also found in this layer were erythrocytes and germ cells as previously described. The pellet contained the highest amount of cells, and all the cells were small, round, and exhibited the characteristic dimple of erythrocytes when in culture. Additionally this cell population appeared red in color during the culture period and after separation while all other cell layers appeared white. 104
115 DISCUSSION In this study, the effectiveness of a NycoDenz gradient method to enrich dissociated porcine testicular cells for spermatogonia was assessed. Additionally, the separated fractions generated from the separation gradient were observed over a 14-day culture period for cell viability and percentage of spermatogonia in culture. Spermatogonia were identified using morphology and immunohistochemical detection of two germ-cell specific genes, DAZL and VASA. Fractionated cells were seeded into culture and monitored for viability via trypan blue exclusion assay (Freshney, 2000) on day 0, 7, and 14 of culture. The percentage of viable cells was affected by isolated layer, day of culture, and layer considered by each day of the culture period. Viability was first examined with all days of the experiment combined to determine what the viability of each group was overall. The pellet contained a population of cells that was 90.94% viable, which was the highest percentage of viable cells found in all layers, including the control. The viability of layers 1 and 2 was found to be 63.75% and 65.89%, respectively, which was significantly lower than all other groups. Cell viability was next examined with all layers combined to determine how the overall effect of time in culture influenced cell viability. It was found that the amount of viable cells in culture decreased with the length of time. The overall viability found on day 0 and 7 were similar (79.98% and 79.94%), but viability had dropped to 69.16% by day 14. Finally, cell viability was examined in each layer on each day. On the first day of culture, day 0, the viability of layer 1 and 2 (64.72% and 59.08%) were significantly lower than the 93.83% viability of the control group. The other layers had a viability similar to the control. The viability of layers on day 7 105
116 and 14 were similar, with the control similar to all other groups. On both days, the viability found in the pellet was the highest, being significantly higher than layer 1. Upon data analysis, it was found that viability data taken from some replicates varied significantly from other replicates. More specifically, the viability data from replicate three was lower than all other replicates, and was statistically different from seven other replicates. In addition, the viability found in replicate 4 was lower than eight other replicates, and was statistically different from three replicates. The lower viability found in these two replicates could be attributed to a longer exposure to the enzyme trypsin, which is used to detach the cells from the culture dish during the viability assay. Long exposure of cells to trypsin has a negative effect on viability. It is feasible that another explanation of the decreased viability is that the cells were harvested from pigs that were less healthy than others used in the experiment. Both replicates 3 and 4 used cells from piglets that came from the same litter, and this litter was not utilized for any other replicates. The number of spermatogonia in culture was detected using antibodies against two germ-cell specific genes, VASA and DAZL. To quantify the amount of spermatogonia in each culture, the cells in suspension were fixed separately from the cells attached to the plate. The reason for this is twofold; quantification of cells attached to the plate and suspension slides is more accurate when cells are not overlapping. Also because the ideal culture conditions of porcine spermatogonia are not known, observing where spermatogonia prefer to live in culture will allow for more information to be gained concerning the optimal conditions of spermatogonia culture conditions. The total number of spermatogonia was measured on day 0, 7, and 14 of culture using a digital camera mounted to a microscope equipped with epifluorescence. Ten digital images 106
117 from each portion of the culture (suspension and cells attached) were analyzed with AlphaEase FC Stand Alone image analysis software for quantification of spermatogonia in culture (Alpha Innotech, 2003). Sample images of labeled cells in culture and in suspension can be found in appendix B. The amount of total spermatogonia in culture was found to be affected by individual layer. Layer 2 of the enrichment gradient was found to contain the highest percentage of total spermatogonia, 26.40%, which was significantly higher than the percentage of spermatogonia found in any other group. The cells in the pellet were 1.22% spermatogonia, which was the lowest group, significantly lower than any other group. The percent of spermatogonia in the control group (14.97%) was similar to the percentage of spermatogonia found in layer 1 (13.72%). The total percentage of spermatogonia in layer 2 was enriched almost 2-fold over the control group. The percentage of total spermatogonia was affected by day of culture, and it was found that the total percentage of spermatogonia in culture on day 14 was significantly higher than the total percentage of spermatogonia on day 0 of culture. A higher percentage of spermatogonia on day 14 is an interesting observation; for many years it was believed that spermatogonia did not divide in culture and instead remained quiescent (Marret and Durand, 2000). The statistically higher percentage of total spermatogonia on day 14 suggests that the spermatogonia underwent cell division during the culture period, however care should be taken before reaching this conclusion as spermatogonia division is rarely observed and is slow, which makes it unlikely that the spermatogonia divided during the 14-day culture period. The total percentage of spermatogonia was examined for each layer on each day, and the percentage of total spermatogonia in the pellet was consistently significantly lower than the spermatogonia percentage found in the control for each day data was collected. Also, the 107
118 percentage of total spermatogonia in layer 2 was consistently the highest percentage of spermatogonia of any other group. The percent of spermatogonia in culture was further examined by analyzing the percentage of spermatogonia found in the culture suspension separately from the percentage of spermatogonia found in the cell population attached to the culture dish. The percent of spermatogonia attached was affected by layer, with layer 3 and the pellet containing 4.88% and 1.11%, respectively, which was significantly lower than the percent of spermatogonia in the control group. The percent of spermatogonia found in layer 2 was 23.15%, which was the highest percentage and was nearly 2-fold the amount of spermatogonia found in the control group (12.87%). The percent of attached spermatogonia followed the trend of total spermatogonia when the data was analyzed by each day. With all separated layers combined, the percent of spermatogonia attached was higher on day 7 and 14 when compared with the percent of spermatogonia on day 0. Finally, the percent of spermatogonia attached to the culture dish was examined in each layer on each day. The percent of spermatogonia attached on each day of the control was similar to all the other individual layers. Despite this similarity, trends in the percent of spermatogonia were observed over the course of the culture period. The percent of spermatogonia in layer 2 was consistently the highest percentage found in all groups, and the amount of spermatogonia in the pellet was consistently the lowest. The percentage of spermatogonia found in the cell culture suspension was then analyzed. The percent of spermatogonia in suspension was affected by layer, specifically that the percent of spermatogonia found in layer 2 was 36.28%, significantly higher than the percentage of spermatogonia found in control (12.87%), and higher than the percentage of 108
119 spermatogonia found attached to the culture dish. The percentage of spermatogonia found in the pellet was 2.51%, which was significantly lower than the percentage of spermatogonia found in the control group, but was higher than the percentage of spermatogonia found in the pellet of the cells attached to the culture dish. The percentage of spermatogonia in suspension was found to increase with time in culture, consistent with the observations for spermatogonia attached to the culture dish. The percent of spermatogonia in suspension was analyzed for each layer on each day, and observations on both day 0 and 7 were similar in that the percentage of spermatogonia in the control were alike the percentage of spermatogonia found in every other group. On day 14, the percent of spermatogonia found in layer 3 and the pellet was significantly lower than the control. The trend observed in spermatogonia attached that layer 2 consistently possessed the highest percentage of spermatogonia and the pellet consistently possessed the lowest percentage of spermatogonia was seen in suspension population as well. The viability of cells in culture decreased with time in culture, but the percent of spermatogonia increased with time in culture. The observed dynamic of viability and percent of spermatogonia can lead to several conclusions. The viability data included both somatic and germ cells in the culture, and either all cell types decreased in viability at the same rate or the somatic cells were not as robust as the germ cells. The former hypothesis would lead to the conclusion that germ cells underwent cell mitosis during the culture period, which is possible but not widely reported in the literature. The latter hypothesis is more likely, that a majority of the cells that died over the course of the culture period were somatic cells. It has been reported that spermatogonial stem cells are the most robust cells of the testis, which would support the hypothesis that the somatic cells died more quickly in culture than the 109
120 germ cells. Because the viability and number of spermatogonia were measured as percents of the entire cell population, the decreased number of somatic cells during the later days in culture would make the percentage of spermatogonia increase, which is what was observed during the culture period. An inverse relationship between the percentage of spermatogonia and cell viability was found for some treatments. The viability of the pellet was consistently the highest of all groups; however, the percent of spermatogonia in the pellet was consistently the lowest of all groups. A similar relationship was found in layer 2, which consistently contained the highest percentage of spermatogonia but contained one of the lowest amounts of viable cells. The lower viability of the more enriched layer is unexpected, but some explanations can be offered. One possibility is that although the stem cells are the most robust of the testicular cells, they depend on the other cells in the testis for survival. It has been reported that the interactions of germ cells with somatic cells is important for entry into the S phase of the cell cycle (Marret and Durand, 2000), and perhaps this relationship also facilitates spermatogonia survival. This hypothesis is further supported by previous reports in the literature that successfully cultured porcine spermatogonia with the coculture of supporting cells or seminiferous tubule fragments (Marret and Durand, 2000). Viability in the co-cultures was higher than when spermatogonia was cultured alone, which was also demonstrated in this study as the control group had a higher viability than the spermatogonia enriched layer. Marret and Durand also observed a large drop in viability of spermatogonia over the period of culture that was not observed in this experiment, the viability of cells in the enriched layer remained low throughout the experiment. 110
121 The data generated from this study shows that porcine spermatogonia can be separated using a discontinuous NycoDenz gradient method. This method is simple and short while having a minimal effect on cell viability. More research in this area is necessary to improve the separation technique, as the enrichment of spermatogonia was not as high as expected. This could be improved by separating the cells twice with a gradient to increase the enrichment of spermatogonia, or adding an additional step of pre-plating the cell suspension prior to separation. These techniques may produce a more enriched population of spermatogonial stem cells, but could have adverse effects on cell viability. In this study, testis tissue was obtained as a byproduct of castration and was from boars no more than 8 days old. More research should be done on pigs of various ages, as germ cells proliferate dramatically in pigs from 4 to 5 months of age (Franca et al., 2000). Using testis tissue from young piglets allowed for the availability of large amount of cells to be separated and for effective identification of spermatogonia because germ cell differentiation had not yet occurred; however, the number of germ cells near birth is somewhat low (Franca et al., 2000). The lower than expected enrichment may have been attributed to the low number of spermatogonia present in the testis tissue used. 111
122 LITERATURE CITED Aakhus, T., S. Sommerfelt, H. Stormorken, and K. Dahlstrom Tolerance and excretion of iohexol after intravenous injection in healthy volunteers. Acta Radiologica Supplementium 362: Affara, N The role of the Y chromosome in male infertility. Expert Reviews in Molecular Medicine, Aizawa, S., and Y. Nishimune In-vitro differentiation of type A spermatogonia in mouse cryptorchid testis. Journal of Reproduction and Fertility 56: Avarbock, M., C. Brinster, and R. Brinster Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nature Medicine 2(6): Becherini, L., E. Guarducci, S. Degl'Innocenti, M. Rotondi, G. Forti, and C. Krausz DAZL polymorphisms and susceptibility to spermatogenic failure: An example of remarkable ethnic differences. International Journal of Andrology 27: Bellve, A., J. Cavicchia, C. Millette, D. O'Brien, Y. Bhatnagar, and M. Dym Spermatogenic cells of the prepuberal mouse. The Journal of Cell Biology 74: Bertoni, J., and G. Alexander Studies on the mechanism of toxicity of metrizamide-- competitive inhibition of yeast hexokinase. Biochemical Pharmacology 30: Boyum, A Isolation of lymphocytes, granulocytes, and macrophages. Scandavian Journal of Immunology Supplement 5: Brinster, R Germline stem cell transplantation and transgenesis. Science 296: Brinster, R., and M. Avarbock Germline transmission of donor haplotype following spermatogonial transplantation. Proceedings of the National Academy of Science 91: Brinster, R., and J. Zimmermann Spermatogenesis following male germ-cell transplantation. Proceedings of the National Academy of Science 91: Brysk, M., J. Snider, and E. Smith Separation of newborn rat epidermal cells on discontinuous isokinetic gradients of Percoll. Journal of Investigative Dermatology 77: Bucci, L., W. Brock, T. Johnson, and M. Meistrich Isolation and biochemical studies of enriched populations of spermatogonia and early primary spermatocytes from rat testes. Biology of Reproduction 34:
123 Buchschacher, G Lentiviral vector systems for gene transfer. Eurekah.com, Georgetown. Capel, B The battle of the sexes. Mechanisms of Development 92: Castrillon, D., B. Quade, T. Wang, C. Quigley, and C. Crum The human VASA gene is specifically expressed in the germ cell lineage. Proceedings of the National Academy of Science 97: Clark, A., M. Bodnar, M. Fox, R. Rodriquez, M. Abeyta, M. Firpo, and R. Pera Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Human Molecular Genetics 13: Clouthier, D., M. Avarbock, S. Maika, R. Hammer, and R. Brinster Rat spermatogenesis in mouse testis. Nature 381: Coffin, J., S. Hughes, and H. Varmus Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. Cole, D., and G. Foxcroft Control of pig reproduction. Butterworth Scientific, Boston Collier, B., B. Gorgoni, C. Loveridge, H. Cooke, and N. Gray The DAZL family proteins are PABP-binding proteins that regulate translocation in germ cells. European Molecular Biology Organization 24: De Rooij, D., and L. Russell All you wanted to know about spermatogonia but were afraid to ask. Journal of Andrology 21: Desjardins, C., and Ewing, L Cell and molecular biology of the testis. Oxford University Press, New York Dirami, G., N. Ravindranath, V. Pursel, and M. Dym Effects of stem cell factor and granulocyte macrophage-colony stimulating factor on survival of porcine type A spermatogonia cultured in KSOM. Biology of Reproduction 61: Dobrinski, I., M. Avarbock, and R. Brinster Germ cell transplantation from large domestic animals into mouse testes. Molecular Reproduction and Development 57: Drlica, K Understanding DNA and gene cloning: A guide for the curious. John Wiley & Sons, Inc., New York. Dyce, P., L Wen, and J. Li In vitro germline potential of stem cells derived from fetal porcine skin. Nature Cell Biology 8(4):
124 Dym, M., M. Jia, G. Dirami, J. Price, S. Rabin, I. Mocchetti, and N. Ravindranath Expression of c-kit receptor and its autophosphorylation in immature rat type A spermatogonia. Biology of Reproduction 52: Ertl, C., and K. Wrobel Distribution of sugar residues in the bovine testis during postnatal ontogenesis demonstrated with lectin-horseradish peroxidase conjugates. Histochemistry 97(2): Feng, L., Y. Chen, L. Dettin, R. Pera, J. Herr, E. Goldberg, M. Dym Generation and in vitro differentiation of a spermatogonial cell line. Science 297(5580): Franca, L., G. Avelar, and F. Almedia Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology 63: Franca, L., V. Silva, H. Chiarini-Garcia, S. Garcia, and L. Deblujuk Cell proliferation and hormonal changes during postnatal development of the testis in the pig. Biology of Reproduction 63: Freshney, R. I Culture of animal cells: A manual of basic technique. 4 ed. Wiley-Liss, Inc., New York Fujiwara, Y., T. Komiya, H. Kawabata, M. Sato, H. Fujimoto, M. Furusawa, and T. Noce Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression of germ cell lineage. Proceedings of the National Academy of Science 91: Ghabrial, A., and T. Schupbach Activation of a meiotic checkpoint regulates translation of Gurken during Drosophila oogenesis. Nature Cell Biology 1: Giuili, G., A. Tomljenovic, N. Labrecque, M. Oulad-Abdelghani, M. Rassoulzadegan, and F. Cuzin Murine spermatogonial stem cells: Targeted transgene expression and purification in an active state. European Molecular Biology Organization Reports 3: Gold, B., L. Stern, F. Bradley, and N. Hecht Gene expression during mammalian spermatogenesis: Evidence for stage-specific differences in mrna populations. The Journal of Experimental Zoology 225: Goldberg, E The testis from stem cell to sperm function. Springer, New York Gondos B, A. Byskov Germ cell kinetics in the neonatal rabbit testis. Cell Tissue Research 215:
125 Guo, R., Z. Yu, J. Guan, Y. Ge, J. Ma, S. Li, S. Wang, S. Xue, and D. Han Stagespecific and tissue-specific expression characteristics of differentially expressed genes during mouse spermatogenesis. Molecular Reproduction and Development 67: Hamra, F., N. Schultz, K. Chapman, D. Grellhesl, J. Cronkhite, R. Hammer, and D. Garbers Defining the spermatogonial stem cell. Developmental Biology 269: Hay, B., L. Jan, and Y. Jan A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell 55: Herma, F., J. Gatlin, K. Chapman, D. Grellhesl, J. Garcia, R. Hammer, and D. Garbers Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proceedings of the National Academy of Science 99: Hofmann, M., L. Braydich-Stolle, L. Dettin, E. Johnson, and M. Dym. 2005a. Immortalization of mouse germ line stem cells. Stem Cells 23: Hofmann, M., L. Braydich-Stolle, and M. Dym. 2005b. Isolation of male germ-line stem cells; influence of GDNF. Developmental Biology 279: Honaramooz, A., S. Megee, and I. Dobrinski Germ cell transplantation in pigs. Biology of Reproduction 66: Hubner, K., G. Fuhrmann, L. Christenson, J. Kehler, R. Reinbold, R. Fuente, J. Wood, J. Strass III, M. Boiani, and H. Scholer Derivation of oocytes from mouse embryonic stem cells. Science 300: Hunt, S The presence of Thy-1 on the surface of rat lymphoid stem cells and colonyforming units. European Journal of Immunology 9: Iwanami, Y., T. Kobayashi, M. Kato, M. Hirabayashi, and S. Hochi Characteristics of rat round spermatids differentiated from spermatogonial cells during co-culture with Sertoli cells, assessed by flow cytometry, microinsemination, and RT-PCR. Theriogenology 65: Izadyar, F., J. Matthihs-Rijsenbilt, K. Ouden, L. Creemers, H. Woelders, and D. De Rooij. 2002b. Development of a cryopreservation protocol for type A spermatogonia. Journal of Andrology 23: Izadyar, F., G. Spierenberg, L. Creemers, K. Ouden, and D. De Rooij. 2002a. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction 124:
126 Janca, F., L. Jost, and D. Evenson Mouse testicular and sperm cell development characterized from birth to adulthood by dual parameter flow cytometry. Biology of Reproduction 34: Jiao, X., P. Trifillis, and M. Kiledjian Identification of target messenger RNA substrates for the murine deleted in azoospermia-like RNA-binding protein. Biology of Reproduction 66: Kanatsu-Shinohara, M., N. Ogonuk, K. Inoue, H. Miki, A. Ogura, S. Toyokun, and T. Shinohara Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biology of Reproduction 69: Kanatsu-Shinohara, M., N. Ogonuki, T. Iwano, J. Lee, Y. Kazuki, K. Inoue, H. Miki, M. Takehashi, S. Toyokuni, Y. Shinkai, M. Oshimura, F. Ishino, A. Ogura, and T. Shinohara Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development 132(18): Knipe, D., and P. Howley Fields virology. Lippincott Williams & Wilkins, Philadelphia. Kobayashi, T., H. Kajiura-Kobayashi, and Y. Nagahama Differential expression of vasa homologue gene in the germ cells during oogenesis and spermatogenesis in a teleost fish, tilapia, Oreochromis niloticus. Mechanisms of Development 99: Koh, K., M. Komiyama, Y. Toyama, T. Adachi, and C. Mori Percoll fractionation of adult mouse spermatogonia improves germ cell transplantation. Asian Journal of Andrology 6: Kotaja, N., S. Bhattacharyya, L. Jaskiewicz, S. Kimmins, M. Parvinen, W. Filipowicz, and P. Sassone-Corsi The chromatoid body of male germ cells: Similarity with processing bodies and presence of Dicer and microrna pathway components. Proceedings of the National Academy of Science 103: Kubota, H., M. Avarbock, and R. Brinster Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biology of Reproduction 71: Lasko, P., and M. Ashburner The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4a. Nature 335: Lassalle, B., A. Ziyyat, J. Testart, C. Finaz, and A. Lefevre Flow cytometric method to isolate round spermatids from mouse testis. Human Reproduction 14: Lee, G., H. Kim, S. Lee, M. Kang, D. Kim, C. Lee, S. Kang, B. Lee, and W. Hwang Characterization of pig Vasa homolog gene and specific expression in germ cell lineage. Molecular Reproduction and Development 72:
127 Levek-Motola, N., Y. Soffer, L. Shochat, A. Raziel, L. Lewin, and R. Golan Flow cytometry of human semen: A preliminary study of a non-invasive method for the detection of spermatogenic defects. Human Reproduction 20: Liang, L., W. Diehl-Jones, and P. Lasko Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development 120: Lin, Y., C. Chen, H. Sun, S. Tsai, J. Lin, and P. Kuo Presence of DAZL transcript and protein in mature human spermatozoa. Fertility and Sterility 77: Lo, K., V. Brugh III, M. Parker, and D. Lamb Isolation and enrichment of murine spermatogonial stem cells using rhodamine 123 mitochondrial dye. Biology of Reproduction 72: Malkov, M., Y. Fisher, and J. Don Developmental schedule of the postnatal rat testis determined by flow cytometry. Biology of Reproduction 59: Marret, C., and P. Durand Culture of porcine spermatogonia: Effects of purification of the germ cells, extracellular matrix, and fetal calf serum on their survival and multiplication. Reproduction Nutrition Development 40: Mayanagi, T., R. Kurosawa, K. Ohnuma, A. Ueyama, K. Ito, and J. Takahashi Purification of mouse primordial germ cells by Nycodenz. Reproduction 125: Mays-Hoopes, L., J. Bolen, A. Riggs, and J. Singer-Sam Preparation of spermatogonia, spermatocytes, and round spermatids for analysis of gene expression using fluorescence-activated cell sorting. Biology of Reproduction 53: Miller, R., and R. Phillips Separation of cells by velocity sedimentation. Journal of Cell Physiology 73: MiltenyiBiotec MACS technology flyer. pdf.php?mm_id=680&session_id=a24ce232525a9106ee50b9c67c16424a&ip= p Morena, A., C. Boitani, M. Pesce, M. Felici, and M. Stefanini Isolation of highly purified type A spermatogonia from prepubertal rat testis. Journal of Andrology 17: Mosmann, T Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65:
128 Moudgal, N., M. Sairam, H. Krishnamurthy, S. Sridhar, H. Krishnamurthy, and H. Khan Immunization of male bonnet monkeys (M.radiata) with a recombinant FSH receptor preparation affects testicular function and fertility. Endocrinology 138: Mutzel, W., H. Siefert, and U. Speck Biochemical-pharmacologic properties of iohexol. Acta Radiologica Supplementium 362: Nagano, M., M., Avarbock, E., Leondia, C., Brinster, and R., Brinster Culture of mouse spermatogonial stem cells. Tissue and Cell 30: Nagano, M., C. Brinster, K. Orwig, B. Ryu, M. Avarbock, and R. Brinster. 2001a. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proceedings of the National Academy of Science 98: Nagano, M., and R. Brinster Spermatogonial transplantation and reconstitution of donor cell spermatogenesis in recipient mice. Acta Pathologica, Microbiologica et Immunologica Scandinavica 106: Nagano, M., J. McCarrey, and R. Brinster. 2001b. Primate spermatogonial stem cells colonize mouse testes. Biology of Reproduction 64: Nagano, M., B. Ryu, C. Brinster, M. Avarbock, and R. Brinster Maintenance of mouse male germ line stem cells in vitro. Biology of Reproduction 68(6): Nagano, M., T. Shinohara, M. Avarbock, and R. Brinster Retrovirus-mediated gene delivery into male germ line stem cells. Federation of European Biochemical Societies 475: Nagano, M., D. Watson, B. Ryu, J. Wolfe, and R. Brinster Lentiviral vector transduction of male germ line stem cells in mice. Federation of European Biochemical Societies 524: Nagao, Y. Viability of meiotic prophase spermatocytes of rats is facilitated in primary culture of dispersed testicular cells on collagen gel by supplementing epinephrine or norepinephrine: evidence that meiotic prophase spermatocytes complete meiotic divisions in vitro In Vitro Cellular Developmental Biology 25: Noce, T., S. Okamoto-Ito, and N. Tsunekawa Vasa homolog genes in mammalian germ cell development. Cell Structure and Function 26: Ohbo, K., S. Yoshida, M. Ohmura, O. Ohneda, T. Ogawa, H. Tsuchiya, T. Kuwana, J. Kehler, K. Abe, H. Scholer, and T. Suda Identification and characterization of stem cells in prepubertal spermatogenesis in mice. Developmental Biology 258:
129 Orwig, K., M. Avarbock, and R. Brinster Retrovirus-mediated modification of male germline stem cells in rats. Biology of Reproduction 67: Ott, R., and M. Longnecker An introduction to statistical methods and data analysis. Wadsworth Group, United States Pause, A., and N. Sonenberg Mutational analysis of a DEAD box RNA helicase: The mammalian translation initiation factor eif-4a. The EMBO Journal 11: Pelliniemi, L Ultrastructure of the early ovary and testis in pig embryos. American Journal of Anatomy 144: Petit, J., M. Ratinuad, E. Cordelli, M. Spano, and R. Julien Mouse testis cell sorting according to DNA and mitochondrial changes during spermatogenesis. Cytometry 19: Raz, E The function and regulation of vasa-like genes in germ-cell development. Genome Biology 1(3): Reijo, R., D. Dorfman, R. Slee, A. Renshaw, K. Loughlin, H. Cooke, and D. Page DAZ family proteins exist throughout male germ cell development and transit from nucleus to cytoplasm at meiosis in humans and mice. Biology of Reproduction 63: Reynolds, N., and H.J. Cooke Role of the DAZ genes in male fertility. Reproductive BioMedicine Online 10: Richter, G Problems connected with the separation of different kinds of cells. In: M. Epstein (ed.) International review of experimental pathology No. 14. p Academic Press, Inc, New York. Rickwood, D., T. Ford, and J. Graham Nycodenz: A new nonionic iodinated gradient medium. Analytical Biochemistry 123: Rilianawati, R., M. Taggart, and H. Cooke Spermatogenesis in testes of Dazl null mice after transplantation of wild-type germ cells. Reproduction 126: Romrell, L., A. Bellve, and D. Fawcett Separation of mouse spermatogenic cells by sedimentation velocity. Developmental Biology 49: Ruggiu, M., R. Speed, M. Taggart, S. McKay, F. Kilanowski, P. Saunders, J. Dorin, and H. Cooke The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 389: Russell, L., and M. Griswold The Sertoli cell. Cache River Press, Clearwater
130 Saunders, P., J. Turner, M. Ruggiu, M. Taggart, P. Burgoyne, D. Elliott and H. Cooke Absence of mdazl produces a final block on germ cell development at meiosis. Reproduction 126: Schlatt, S., G. Rosiepen, G. Weinbauer, C. Rolf, R. Brook, and E. Nieschlag Germ cell transfer into rat, bovine, monkey, and human testes. Human Reproduction 14: Schonfeldt, V., H. Krishnamurthy, L. Foppiani, and S. Schlatt Magnetic cell sorting is a fast and effective method of enriching viable spermatogonia from Djungarian hamster, mouse, and Marmoset monkey. Biology of Reproduction 61: Schrans-Stassen, B., P. Saunders, H. Cooke, and D. de Rooij Nature of the spermatogenic arrest in Dazl -/- mice. Biology of Reproduction 65: Senger, P Pathways to pregnancy and partuition. Current Conceptions, Inc., Moscow, ID Shinohara, T., and R. Brinster Enrichment and transplantation of spermatogonial stem cells. International Journal of Andrology Supplement 23: Shinohara, T., K. Orwig, M. Avarbock, and R. Brinster Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proceedings of the National Academy of Science 97: Sigma HistoDenz product information. 20information%20sheet/d2158pis.pdf. p 1-2. Sigma Percoll product information. 20information%20sheet/p1644pis.pdf. p 1-4. Snedecor, G. W., and W. CochranW Statistical Methods, eighth ed. Iowa State University Press, Ames, IA. Staub, C A century of research on mammalian male germ cell meiotic differentiation in vitro. Journal of Andrology 22(6): Steinberger, A Maintenance of adult human testicular tissue in culture. Anatomical Record 157: Steinberger, A., and E. Steinberger Differentiation of rat seminiferous epithelium in organ culture. Journal of Reproduction and Fertility 9: Steinberger, A., and E. Steinberger Stimulatory effect of vitamins and glutamine on the differentiation of germ cells in rat testes organ culture grown in chemically defined media. Experimental Cell Research 44(2-3):
131 Steinberger, A., and E. Steinberger Factors affecting spermatogenesis in organ cultures of mammalian testes. Journal of Reproduction and Fertility Supplement 2: Steinberger, A., E. Steinberger, and W. Perloff. 1964a. Mammalian testes in organ culture. Experimental Cell Research 36: Steinberger, E., A. Steinberger, and W. Perloff. 1964b. Initiation of spermatogenesis in Vitro. Endocrinology 74: Steinberger, E., and W. Dixon Some observations on the effect of heat on the testicular germinal epithelium. Fertility and Sterility 10(6): Styhler, S., A. Nakamura, A. Swan, B. Suter, and P. Lasko vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development 125: Swierstra, E Cytology and duration of the cycle of the seminiferous epithelium of the boar; duration of spermatozoan transit through the epididymis. Anatomical Record 161: Tanaka, S., Y. Toyooka, R. Akasu, Y. Katoh-Fukui, Y. Nakahara, R. Suzuki, M. Yokoyama, and T. Noce The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes and Development 14: Tilmann, C., and B. Capel Cellular and molecular pathways regulating mammalian sex determination. Recent Progress in Hormone Research 57: Toyooka, Y., N. Tsunekawa, Y. Takahashi, Y. Matsui, M. Satoh, and T. Noce Expression and intercellular localization of mouse Vasa-homologue protein during germ cell development. Mechanisms of Development 93: Tres, L., and A. Kierszenbaum Viability of rat spermatogenic cells in vitro is facilitated by their coculture with Sertoli cells in serum-free hormone-supplemented medium. Proceedings of the National Academy of Sciences, USA 80: Trono, D Lentiviral vectors. Springer, New York. Trowell, O The culture of mature organs in a synthetic medium. Experimental Cell Research 16: Tsunekawa, N., M. Naito, Y. Sakai, T. Nishida, and T. Noce Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 127:
132 Van Der Wee, K., E. Johnson, G. Dirami, M. Dym, and M. Hormann Immunomagentic isolation and long-term culture of mouse type A spermatogonia. Journal of Andrology 22: Van Pelt, A., A. Morena, F. van Dissel-Emiliani, C. Boitani, I. Gaemers, D. de Rooij, and M. Stefanini Isolation of the synchronized A spermatogonia from adult A- deficient rat testes. Biology of Reproduction 55: Van Straaten, H., and C. Wensing Leydig cell development in the testis of the pig. Biology of Reproduction 18: Venables, J., M. Riggiu, and H. Cooke The RNA-binding specificity of the mouse Dazl protein. Nucleic Acids Research 29: Wassarman, D., and J. Steitz Alive with DEAD proteins. Nature 349: Weiss, M., M. Vigier, D. Hue, M. Perrard-Sapori, C. Marret, O. Avallet, and P. Durand Pre- and postmeiotic expression of male germ cell-specific genes throughout 2- week cocultures of rat germinal and Sertoli cells. Biology of Reproduction 57: Xu, E., F. Moore, and R. Pera A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proceedings of the National Academy of Science 98: Yen, P Putative biological functions of the DAZ family. International Journal of Andrology 27: Yu, Z., R. Guo, Y. Ge, J. Ma, J. Guan, S. Li, X. Sun, S. Xue, and D. Han Gene expression profiles in different stages of mouse spermatogenic cells during spermatogenesis. Biology of Reproduction 69: Zhao, D., and T. Kuwana Purification of avian circulating primordial germ cells by Nycodenz density gradient centrifugation. British Poultry Science 44:
133 APPENDICES 123
134 Transformation of percentage data Input rep trt day via spe1 spe2 spe3; Avia=ARSIN(SQRT(via/100)); Aspe1= ARSIN(SQRT(spe1/100)); Aspe2= ARSIN(SQRT(spe2/100)); Aspe3= ARSIN(SQRT(spe3/100)); Run; APPENDIX A: SAS CODE Proc GLM; Class trt rep day; Model Avia Aspe1 Aspe2 Aspe3=trt day rep trt*day rep(trt); Test H=trt e=rep(trt); LSmeans trt day rep trt*day/pdiff adjust=tukey; Proc GLM; Class trt rep day; Model Avia Aspe1 Aspe2 Aspe3=trt day rep trt*day rep(trt); Means trt day rep trt*day/lines LSD; Run; 124
135 APPENDIX B: SAMPLE DIGITAL IMAGES The images included in this appendix are a sample of the images used for cell quantification. Cells were fixed and processed as previously described, and the digital images were captured using the inverted microscope equipped with epifluorescence and a Diagnostic Instruments, Inc. Spot RT color camera. Two different fluorescent dyes were used to stain the cells (fluorescein and Propidium iodide), and the camera software was programmed to capture images after exposing the green fluorescence from fluorescein and the red fluorescence from Propidium iodide. The cells attached to the dish (images 1-6, 9-14, 17-22, 25-30, 33-38) had an exposure to green light for 7 seconds, followed by an exposure to red light for 8 seconds. The cells in suspension were fixed to slides as opposed to dishes and required a different exposure. The cells in suspension (images 7, 8, 15, 16, 23, 24, 31, 32, 39, 40) had a exposure to green light for 10 seconds and then to red light for 9 seconds. 125
136 1. Control cells attached on day Control cells attached on day
137 3. Control cells attached on day Control cells attached on day
138 5. Control cells attached on day Control cells attached on day
139 7. Control cells in suspension on day Control cells in suspension on day
140 9. Layer 1 cells attached on day Layer 1 cells attached on day
141 11. Layer 1 cells attached on day Layer 1 cells attached on day
142 13. Layer 1 cells attached on day Layer 1 cells attached on day
143 15. Layer 1 cells in suspension on day Layer 1 cells in suspension on day
144 17. Layer 2 cells attached on day Layer 2 cells attached on day
145 19. Layer 2 cells attached on day Layer 2 cells attached on day
146 21. Layer 2 cells attached on day Layer 2 cells attached on day
147 23. Layer 2 cells in suspension on day Layer 2 cells in suspension on day
148 25. Layer 3 cells attached on day Layer 3 cells attached on day
149 27. Layer 3 cells attached on day Layer 3 cells attached on day
150 29. Layer 3 cells attached on day Layer 3 cells attached on day
151 31. Layer 3 cells in suspension on day Layer 3 cells in suspension on day
152 33. Layer 4 cells attached on day Layer 4 cells attached on day
153 35. Layer 4 cells attached on day Layer 4 cells attached on day
154 37. Layer 4 cells attached on day Layer 4 cells attached on day
155 39. Layer 4 cells in suspension on day Layer 4 cells in suspension on day
Adapted from Preg. & Part., Senger
 MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
The spermatogenesis CHARACTERISTICS OF THE SPERMATOZOON 26/04/2017. Reproductive Biotechnologies Andrology I. Prof. Alberto Contri
 Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Histology of Male Reproductive system (1)
 Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Cell Divisions. The autosomes represent the whole body. * Male Sex Chromosomes: XY * Female Sex Chromosomes: XX
 Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Production of Fertile Sperm. Animal Science 434. Hormonal Regulation of the Testis. hormonal regulation of the testis
 roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
5 15/3/2012. Malik Al-Momani
 5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
Male Reproductive System
 Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Embryology 3. Spermatogenesis:
 Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Testicular stem cells
 Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Lesson 1. Quiz (short) Cell cycle Chromosomes Mitosis phases
 Lesson 1 Quiz (short) Cell cycle Chromosomes Mitosis phases 2 Cell division is needed for Growth (Mitosis) Repair (Mitosis) Reproduction (Meiosis) 3 Mitosis consists of 4 phases (division of the nuclear
Lesson 1 Quiz (short) Cell cycle Chromosomes Mitosis phases 2 Cell division is needed for Growth (Mitosis) Repair (Mitosis) Reproduction (Meiosis) 3 Mitosis consists of 4 phases (division of the nuclear
Male Reproductive System
 Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Animal Development. Lecture 3. Germ Cells and Sex
 Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Chapter 22 The Reproductive System (I)
 Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
The Cell Life Cycle. S DNA replication, INTERPHASE. G 2 Protein. G 1 Normal THE CELL CYCLE. Indefinite period. synthesis. of histones.
 Mitosis & Meiosis The Cell Life Cycle INTERPHASE G 1 Normal cell functions plus cell growth, duplication of organelles, protein synthesis S DNA replication, synthesis of histones THE CELL CYCLE M G 2 Protein
Mitosis & Meiosis The Cell Life Cycle INTERPHASE G 1 Normal cell functions plus cell growth, duplication of organelles, protein synthesis S DNA replication, synthesis of histones THE CELL CYCLE M G 2 Protein
Male Anatomy. testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics
 Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
- production of two types of gametes -- fused at fertilization to form zygote
 Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
Male Reproduction Organs. 1. Testes 2. Epididymis 3. Vas deferens 4. Urethra 5. Penis 6. Prostate 7. Seminal vesicles 8. Bulbourethral glands
 Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Gametogenesis. To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis. Introduction
 Gametogenesis To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell
Gametogenesis To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM
 LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
BIOL 2402 Reproductive Systems
 Collin College Dr. Chris Doumen BIOL 2402 Reproductive Systems 1 Reproductive System Most systems between males and females in the human body are similar in structure. The exception of course are the organs
Collin College Dr. Chris Doumen BIOL 2402 Reproductive Systems 1 Reproductive System Most systems between males and females in the human body are similar in structure. The exception of course are the organs
BIOH122 Session 26 Gametogenesis. Introduction. 1. a. Define gametogenesis. b. What cells are gametes?
 BIOH122 Session 26 Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell division processes that occur during the cell cycle? d. Define the cell cycle.
BIOH122 Session 26 Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell division processes that occur during the cell cycle? d. Define the cell cycle.
Male reproduction. Cross section of Human Testis ผศ.ดร.พญ.ส ว ฒณ ค ปต ว ฒ ภาคว ชาสร รว ทยา คณะแพทยศาสตร ศ ร ราชพยาบาล 1. Aims
 Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Physiology of Male Reproductive System
 Physiology of Male Reproductive System the anterior pituitary gland serves as the primary control of reproductive function at puberty Ant Pituitary secretes FSH & large amounts of LH (ICSH) FSH & LH cause
Physiology of Male Reproductive System the anterior pituitary gland serves as the primary control of reproductive function at puberty Ant Pituitary secretes FSH & large amounts of LH (ICSH) FSH & LH cause
Mohammad Sha ban. Basheq Jehad. Hamzah Nakhleh
 11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
The Male Reproductive System
 The Male Reproductive System The male reproductive system Testes Genital ducts Accessory sex glands: seminal vesicles prostate bulbourethral glands External genitalia: penis Structure of the Testis Tunica
The Male Reproductive System The male reproductive system Testes Genital ducts Accessory sex glands: seminal vesicles prostate bulbourethral glands External genitalia: penis Structure of the Testis Tunica
The Male Reproductive System
 The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
Gametogenesis. Dr Corinne de Vantéry Arrighi Dr Hervé Lucas
 WHO Collaborating Center for Research in Human Reproduction Clinic for Infertility and Gynecological Endocrinology University Hospital, Geneva, Switzerland Gametogenesis Dr Corinne de Vantéry Arrighi Dr
WHO Collaborating Center for Research in Human Reproduction Clinic for Infertility and Gynecological Endocrinology University Hospital, Geneva, Switzerland Gametogenesis Dr Corinne de Vantéry Arrighi Dr
Physiologic Anatomy of the Male Sexual Organs
 Reproductive and Hormonal Functions of the Male The reproductive functions of the male can be divided into three major subdivisions: (1) spermatogenesis, which means simply the formation of sperm; (2)
Reproductive and Hormonal Functions of the Male The reproductive functions of the male can be divided into three major subdivisions: (1) spermatogenesis, which means simply the formation of sperm; (2)
Meiosis & Sexual Reproduction. AP Biology
 Meiosis & Sexual Reproduction 2007-2008 Cell division / Asexual reproduction Mitosis produce cells with same information identical daughter cells exact copies clones same amount of DNA same number of chromosomes
Meiosis & Sexual Reproduction 2007-2008 Cell division / Asexual reproduction Mitosis produce cells with same information identical daughter cells exact copies clones same amount of DNA same number of chromosomes
Seminiferous Tubules
 Testes The testes are compound tubular glands that lie within a scrotal sac, suspended from the body by a spermatic cord. The testes are dual organs that act as exocrine glands producing a holocrine secretion,
Testes The testes are compound tubular glands that lie within a scrotal sac, suspended from the body by a spermatic cord. The testes are dual organs that act as exocrine glands producing a holocrine secretion,
Unit 4: Cell Division Guided Notes
 Unit 4: Cell Division Guided Notes 1 Chromosomes are structures that contain material When Eukaryotes are not dividing, DNA and Proteins are in a mass called: When the cell divides, it condenses and becomes
Unit 4: Cell Division Guided Notes 1 Chromosomes are structures that contain material When Eukaryotes are not dividing, DNA and Proteins are in a mass called: When the cell divides, it condenses and becomes
Spermatogenesis: An Overview
 Spermatogenesis: An Overview 2 Rakesh Sharma and Ashok Agarwal Abstract The purpose of this chapter is to provide a comprehensive overview of spermatogenesis and the various steps involved in the development
Spermatogenesis: An Overview 2 Rakesh Sharma and Ashok Agarwal Abstract The purpose of this chapter is to provide a comprehensive overview of spermatogenesis and the various steps involved in the development
Medical School Histology Basics Male Reproductive System. VIBS 289 lab
 Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Reproductive System Purpose General Structures Male Structures Functions Female Anatomy Structures Functions Clinical Applications
 The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
Basic histology 5/4/2015
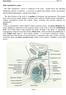 Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
Primary sex organs (gonads): testes and ovaries. Accessory reproductive organs: ducts, glands, and external genitalia
 Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
DAX1, testes development role 7, 8 DFFRY, spermatogenesis role 49 DMRT genes, male sex differentiation role 15
 Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Reproductive Endocrinology. Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007
 Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature
 REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
 MOCK TEST I (HUMAN REPRODUCTION) 1. In human transfer of sperms into female genital tract is called as 1) Fertilization 2) Implantation 3) Insemination 4) Gestation 2. In male human scrotum maintains the
MOCK TEST I (HUMAN REPRODUCTION) 1. In human transfer of sperms into female genital tract is called as 1) Fertilization 2) Implantation 3) Insemination 4) Gestation 2. In male human scrotum maintains the
ANATOMY. lecture # : 2 1 Date : Lecturer : Maher Hadidi
 ANATOMY lecture # : 2 1 Date : Lecturer : Maher Hadidi Spermatogenesis Transformation of Spermatogonia into mature sperm that begins at puberty into old age. Provide motility of the sperm...-c:tail to
ANATOMY lecture # : 2 1 Date : Lecturer : Maher Hadidi Spermatogenesis Transformation of Spermatogonia into mature sperm that begins at puberty into old age. Provide motility of the sperm...-c:tail to
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following hormones controls the release of anterior pituitary gonadotropins? A) LH
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following hormones controls the release of anterior pituitary gonadotropins? A) LH
All You Wanted to Know About Spermatogonia but Were Afraid to Ask
 All You Wanted to Know About Spermatogonia but Were Afraid to Ask Review DIRK G. DE ROOIJ* AND LONNIE D. RUSSELL From the *Department of Cell Biology, University Medical Center Utrecht, Utrecht, The Netherlands;
All You Wanted to Know About Spermatogonia but Were Afraid to Ask Review DIRK G. DE ROOIJ* AND LONNIE D. RUSSELL From the *Department of Cell Biology, University Medical Center Utrecht, Utrecht, The Netherlands;
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER)
 THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
HANDOUT # 1 GAMETOGENESIS
 Gametogenesis 1 HANDOUT # 1 GAMETOGENESIS Anatomy Department R.A. FADEL Gametogenesis 2 بسم هللا الرحمن الرحيم ت هأ م وى }46 } ا نل و و ا زل و ج و ن و و و و و ن ه و أ ل ه ث وى }45{ إ ول ن أ و ة م ن ان
Gametogenesis 1 HANDOUT # 1 GAMETOGENESIS Anatomy Department R.A. FADEL Gametogenesis 2 بسم هللا الرحمن الرحيم ت هأ م وى }46 } ا نل و و ا زل و ج و ن و و و و و ن ه و أ ل ه ث وى }45{ إ ول ن أ و ة م ن ان
11. SEXUAL DIFFERENTIATION. Germinal cells, gonocytes. Indifferent stage INDIFFERENT STAGE
 11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
SPERMATOGENESIS IN VITRO
 SPERMATOGENESIS IN VITRO INDUCTION OF PROLIFERATION, MEIOSIS AND DIFFERENTIATION Mário Sousa Lab Cell Biology Institute of Biomedical Sciences (ICBAS) University of Porto msousa@icbas.up.pt Spermatogonia
SPERMATOGENESIS IN VITRO INDUCTION OF PROLIFERATION, MEIOSIS AND DIFFERENTIATION Mário Sousa Lab Cell Biology Institute of Biomedical Sciences (ICBAS) University of Porto msousa@icbas.up.pt Spermatogonia
Sperm production. Sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production. Sperm production. Controlling sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Hormones of brain-testicular axis
 (Hormone Function) Hormones of brain-testicular axis anterior pituitary drives changes during puberty controlled by GnRH from hypothalamus begins to secrete FSH, LH LH targets interstitial endocrinocytes
(Hormone Function) Hormones of brain-testicular axis anterior pituitary drives changes during puberty controlled by GnRH from hypothalamus begins to secrete FSH, LH LH targets interstitial endocrinocytes
Chapter 2. Mitosis and Meiosis
 Chapter 2. Mitosis and Meiosis Chromosome Theory of Heredity What structures within cells correspond to genes? The development of genetics took a major step forward by accepting the notion that the genes
Chapter 2. Mitosis and Meiosis Chromosome Theory of Heredity What structures within cells correspond to genes? The development of genetics took a major step forward by accepting the notion that the genes
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE
 ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
Gametogenesis. Omne vivum ex ovo All living things come from eggs.
 Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
The Reproductive System
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
The Use of Rabbits in Male Reproductive Toxicology
 Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
General Embryology. School of Medicine Department of Anatomy and Histology School of medicine The University of Jordan
 General Embryology 2019 School of Medicine Department of Anatomy and Histology School of medicine The University of Jordan https://www.facebook.com/dramjad-shatarat What is embryology? Is the science that
General Embryology 2019 School of Medicine Department of Anatomy and Histology School of medicine The University of Jordan https://www.facebook.com/dramjad-shatarat What is embryology? Is the science that
Biology 4361 Developmental Biology. October 11, Multiple choice (one point each)
 Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
Functions of male Reproductive System: produce gametes deliver gametes protect and support gametes
 Functions of male Reproductive System: produce gametes deliver gametes protect and support gametes Spermatogenesis occurs in the testes after puberty. From the testes they are deposited into the epididymas
Functions of male Reproductive System: produce gametes deliver gametes protect and support gametes Spermatogenesis occurs in the testes after puberty. From the testes they are deposited into the epididymas
Female and Male Gametogenesis
 19 2 Female and Male Gametogenesis Nina Desai, Jennifer Ludgin, Rakesh Sharma, Raj Kumar Anirudh, and Ashok Agarwal 2.1 Introduction 21 2.2 Structure of the Ovary 21 2.3 Overview of Oogenesis 22 2.3.1
19 2 Female and Male Gametogenesis Nina Desai, Jennifer Ludgin, Rakesh Sharma, Raj Kumar Anirudh, and Ashok Agarwal 2.1 Introduction 21 2.2 Structure of the Ovary 21 2.3 Overview of Oogenesis 22 2.3.1
Meiosis. Oh, and a little bit of mitosis
 Meiosis Oh, and a little bit of mitosis Haploid Cells- The sex cells (egg and sperm) only contain half of the genetic diversity that diploid cells do. For humans this would mean 23 single chromosomes.
Meiosis Oh, and a little bit of mitosis Haploid Cells- The sex cells (egg and sperm) only contain half of the genetic diversity that diploid cells do. For humans this would mean 23 single chromosomes.
Biology of Reproduction- Zool 346 Exam 2
 Biology of Reproduction- Zool 346 Exam 2 ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER. Some critical words are boldfaced. This exam is 7 pages long.
Biology of Reproduction- Zool 346 Exam 2 ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER. Some critical words are boldfaced. This exam is 7 pages long.
Morphogenesis of the residual body of the mouse testis
 93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
Cycle of the Seminiferous Epithelium of the Guinea Pig
 Cycle of the Seminiferous Epithelium of the Guinea Pig A Method for Identification of the Stages Yves Clermont, Ph.D. IN THE GUINEA PIG, the cells of the seminiferous epithelium are arranged in definite
Cycle of the Seminiferous Epithelium of the Guinea Pig A Method for Identification of the Stages Yves Clermont, Ph.D. IN THE GUINEA PIG, the cells of the seminiferous epithelium are arranged in definite
Biology of Reproduction-Biol 326
 Biology of Reproduction-Biol 326 READ ALL INSTRUCTIONS CAREFULLY. ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER REGARDLESS OF WHAT YOU MARK ON THE
Biology of Reproduction-Biol 326 READ ALL INSTRUCTIONS CAREFULLY. ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER REGARDLESS OF WHAT YOU MARK ON THE
Unit 15 ~ Learning Guide
 Unit 15 ~ Learning Guide Name: INSTRUCTIONS Complete the following notes and questions as you work through the related lessons. You are required to have this package completed BEFORE you write your unit
Unit 15 ~ Learning Guide Name: INSTRUCTIONS Complete the following notes and questions as you work through the related lessons. You are required to have this package completed BEFORE you write your unit
Outline. Male Reproductive System Testes and Sperm Hormonal Regulation
 Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Male Reproductive Physiology
 Male Reproductive Physiology Overview Anatomy Function Endocrine and spermatogenesis Testis epididymus,vas deferens,seminal vesicles and prostate Hypothalamic pituitary testicular axis Hormones of the
Male Reproductive Physiology Overview Anatomy Function Endocrine and spermatogenesis Testis epididymus,vas deferens,seminal vesicles and prostate Hypothalamic pituitary testicular axis Hormones of the
Reproductive physiology. About this Chapter. Case introduction. The brain directs reproduction 2010/6/29. The Male Reproductive System
 Section Ⅻ Reproductive physiology Ming-jie Wang E-Mail: mjwang@shmu.edu.cn About this Chapter The reproductive organs and how they work the major endocrine functions of sexual glands actions of sex hormones
Section Ⅻ Reproductive physiology Ming-jie Wang E-Mail: mjwang@shmu.edu.cn About this Chapter The reproductive organs and how they work the major endocrine functions of sexual glands actions of sex hormones
Pathology of Male Reproductive System 1
 Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
Chromosomes & Cell Division
 Chromosomes & Cell Division Cell Division The growth and splitting of cells into two new, identical cells called daughter cells. Mitosis Meiosis DNA replicates Parent cell Chromosomes separate Cell division
Chromosomes & Cell Division Cell Division The growth and splitting of cells into two new, identical cells called daughter cells. Mitosis Meiosis DNA replicates Parent cell Chromosomes separate Cell division
Chapter 28: REPRODUCTIVE SYSTEM: MALE
 Chapter 28: REPRODUCTIVE SYSTEM: MALE I. FUNCTIONAL ANATOMY (Fig. 28.1) A. Testes: glands which produce male gametes, as well as glands producing testosterone 2. Seminiferous tubules (Fig.28.3; 28.5) a.
Chapter 28: REPRODUCTIVE SYSTEM: MALE I. FUNCTIONAL ANATOMY (Fig. 28.1) A. Testes: glands which produce male gametes, as well as glands producing testosterone 2. Seminiferous tubules (Fig.28.3; 28.5) a.
MALE REPRODUCTIVE SYSTEM
 1 MALE REPRODUCTIVE SYSTEM SCPA 602 Anatomical Basis for Pathological Study Updated: 20.09.2018 Lect. Nisamanee Charoenchon, PhD nisamanee.cha@mahidol.ac.th Department of Pathobiology, Mahidol University
1 MALE REPRODUCTIVE SYSTEM SCPA 602 Anatomical Basis for Pathological Study Updated: 20.09.2018 Lect. Nisamanee Charoenchon, PhD nisamanee.cha@mahidol.ac.th Department of Pathobiology, Mahidol University
Spermatogenesis in Man
 Spermatogenesis in Man I. Nuclear Morphology During Spermatogenesis in Man BRUNETTO CHIARELLI, PH.D., ARTHUR FALEK, PH.D., KAREN J. BACK, B.S., and C. THOMAS COWART, M.D. THE SEQUENCE of transformations
Spermatogenesis in Man I. Nuclear Morphology During Spermatogenesis in Man BRUNETTO CHIARELLI, PH.D., ARTHUR FALEK, PH.D., KAREN J. BACK, B.S., and C. THOMAS COWART, M.D. THE SEQUENCE of transformations
SUPPLEMENTAL INFORMATION FOR. PAX7 expression defines germline stem cells in the adult testis
 SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
The Reproductive System
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM
 Unit 7A STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM LEARNING OBJECTIVES 1. Learn the structures of the male reproductive system. 2. Learn the functions of the male reproductive system. 3. Learn
Unit 7A STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM LEARNING OBJECTIVES 1. Learn the structures of the male reproductive system. 2. Learn the functions of the male reproductive system. 3. Learn
10.7 The Reproductive Hormones
 10.7 The Reproductive Hormones December 10, 2013. Website survey?? QUESTION: Who is more complicated: men or women? The Female Reproductive System ovaries: produce gametes (eggs) produce estrogen (steroid
10.7 The Reproductive Hormones December 10, 2013. Website survey?? QUESTION: Who is more complicated: men or women? The Female Reproductive System ovaries: produce gametes (eggs) produce estrogen (steroid
The Reproductive System
 16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
 a Control IgG Intestine c Testis b Thymus 1 3 2 S S 2 1 3 4 4 Figure S1 The wild-type mouse (C57BL/6J) organs (intestine, thymus and testis) were frozen in liquid nitrogen and sectioned at 5 µm on a cryostat.
a Control IgG Intestine c Testis b Thymus 1 3 2 S S 2 1 3 4 4 Figure S1 The wild-type mouse (C57BL/6J) organs (intestine, thymus and testis) were frozen in liquid nitrogen and sectioned at 5 µm on a cryostat.
Chapter 4 The Chromosome Theory of Inheritance
 Chapter 4 The Chromosome Theory of Inheritance 4-1 Sections to study 4.1 Chromosomes: The carriers of genes 4.2 Mitosis: Cell division that preserves chromosome number 4.3 Meiosis: Cell division that halve
Chapter 4 The Chromosome Theory of Inheritance 4-1 Sections to study 4.1 Chromosomes: The carriers of genes 4.2 Mitosis: Cell division that preserves chromosome number 4.3 Meiosis: Cell division that halve
Chapter 26: Reproductive Systems. Male 11/29/2015. Male reproductive system is composed of... BIO 218 Fall Gonads (testes)
 Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
2. Which of the following factors does not contribute to ion selectivity?
 General Biology Summer 2014 Exam II Sample Answers 1. Which of the following is TRUE about a neuron at rest? A. The cytosol is positive relative to the outside B. Na+ concentrations are higher inside C.
General Biology Summer 2014 Exam II Sample Answers 1. Which of the following is TRUE about a neuron at rest? A. The cytosol is positive relative to the outside B. Na+ concentrations are higher inside C.
Reproductive Toxicology
 Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
Chromosomes and Cell Cycle
 Chromosomes and Cell Cycle Cell Basics There are trillions of cells in your body Cells are microscopic Cells have DNA inside a structure called the nucleus The nucleus is enclosed by a structure called
Chromosomes and Cell Cycle Cell Basics There are trillions of cells in your body Cells are microscopic Cells have DNA inside a structure called the nucleus The nucleus is enclosed by a structure called
Histology of Male Reproductive System
 Histology of Male Reproductive System Lecture Objectives Describe the histological features of the male reproductive system Male Reproductive System The male structures of reproduction include the: testes,
Histology of Male Reproductive System Lecture Objectives Describe the histological features of the male reproductive system Male Reproductive System The male structures of reproduction include the: testes,
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
Study Guide. Biology 3101B. Science. Reproduction and Development. Adult Basic Education. Biology 2101A Biology 2101C Biology 3101A.
 Adult Basic Education Science Reproduction and Development Prerequisites: Biology 2101A Biology 2101C Biology 3101A Credit Value: 1 Text: Biology. Bullard, Chetty, et al; McGraw-Hill Ryerson, 2003 Biology
Adult Basic Education Science Reproduction and Development Prerequisites: Biology 2101A Biology 2101C Biology 3101A Credit Value: 1 Text: Biology. Bullard, Chetty, et al; McGraw-Hill Ryerson, 2003 Biology
Unit 5: Cell Cycle, Mitosis, Meiosis & Drug Influence Influence on Nervous System
 Unit 5: Cell Cycle, Mitosis, Meiosis & Drug Influence Influence on Nervous System 1. Which of the following is NOT related to a cell s surface area to volume ratio? a. Cell size b. Number of nuclei c.
Unit 5: Cell Cycle, Mitosis, Meiosis & Drug Influence Influence on Nervous System 1. Which of the following is NOT related to a cell s surface area to volume ratio? a. Cell size b. Number of nuclei c.
Unit 2: Reproduction and Development. The Cell Cycle
 PAGE : 1 The Cell Cycle Cell Cycle: A continuous series of cell growth and division for a cell. All cells go through a cell cycle of some sort. The cell cycle consists of two stages. a. Growth Phase Diagram
PAGE : 1 The Cell Cycle Cell Cycle: A continuous series of cell growth and division for a cell. All cells go through a cell cycle of some sort. The cell cycle consists of two stages. a. Growth Phase Diagram
Chapter1 Introduction
 Chapter1 Introduction Male subfertility is a very significant global problem. Epidemiological data show that approximately 1-in-7 couples are classed as subfertile [1]. Sperm dysfunction is the single
Chapter1 Introduction Male subfertility is a very significant global problem. Epidemiological data show that approximately 1-in-7 couples are classed as subfertile [1]. Sperm dysfunction is the single
IB 140 Midterm #1 PRACTICE EXAM (lecture topics 1-5)
 IB 140 Midterm #1 PRACTICE EXAM (lecture topics 1-5) For all the questions on this exam, the correct answer is the single best answer that is available in the answer key. 1) Which of the following is NOT
IB 140 Midterm #1 PRACTICE EXAM (lecture topics 1-5) For all the questions on this exam, the correct answer is the single best answer that is available in the answer key. 1) Which of the following is NOT
Animal Science 434! Tonic and Preovulatory Surge of GnRH! Tonic and Preovulatory Surge of GnRH! Lecture 11: The Follicular Phase of the Estrous Cycle!
 Tonic and Preovulatory Surge of GnRH! Animal Science 434! Lecture 11: The Follicular Phase of the Estrous Cycle!! (-)! Hypothalamus! GnRH! Estradiol! (-)! Tonic and Preovulatory Surge of GnRH! Anterior!
Tonic and Preovulatory Surge of GnRH! Animal Science 434! Lecture 11: The Follicular Phase of the Estrous Cycle!! (-)! Hypothalamus! GnRH! Estradiol! (-)! Tonic and Preovulatory Surge of GnRH! Anterior!
5.1. KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. 68 Reinforcement Unit 2 Resource Book
 5.1 THE CELL CYCLE KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. Cells have a regular pattern of growth, DNA duplication, and division that is called the cell cycle.
5.1 THE CELL CYCLE KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. Cells have a regular pattern of growth, DNA duplication, and division that is called the cell cycle.
Cell Division. The Process of Cell Division Section Section 10.2: The Process of Cell Division 12/8/2010
 The Process of Cell Division Section 10.2 Biology B Section 10.2: The Process of Cell Division The student will investigate and understand common mechanisms of inheritance and protein synthesis. Key concepts
The Process of Cell Division Section 10.2 Biology B Section 10.2: The Process of Cell Division The student will investigate and understand common mechanisms of inheritance and protein synthesis. Key concepts
INVESTIGATING THE ROLE OF VASCULAR ENDOTHELIAL GROWTH FACTOR IN BOVINE TESTIS DEVELOPMENT KYLE CODY CAIRES
 INVESTIGATING THE ROLE OF VASCULAR ENDOTHELIAL GROWTH FACTOR IN BOVINE TESTIS DEVELOPMENT By KYLE CODY CAIRES A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE
INVESTIGATING THE ROLE OF VASCULAR ENDOTHELIAL GROWTH FACTOR IN BOVINE TESTIS DEVELOPMENT By KYLE CODY CAIRES A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE
