Spermatogenesis, sperm output and seminal quality of
|
|
|
- Barbra Booker
- 5 years ago
- Views:
Transcription
1 Spermatogenesis, sperm output and seminal quality of Holstein bulls electroejaculated after administration of oxytocin W. E. Berndtson and G. Igboeli Department of Animal and Nutritional Sciences, University of New Hampshire, Durham, NH 03824, U.S.A. Summary. The 12- to 24-month-old Holstein bulls were electroejaculated twice on each of 3 days per week throughout the study. After a 2-week stabilization period and subsequent 2-week pre-treatment period, 7 bulls were given 50 i.u. oxytocin via the jugular vein 10 min before each first ejaculate for 10 weeks. The 7 control bulls were handled identically but did not receive oxytocin. All bulls were castrated at the end of the study. Oxytocin was without effect on spermatogenesis (P > 0\m=.\10).Oxytocin did not alter the total number of spermatozoa harvested per collection day (P > 0\m=.\10),but increased the number of spermatozoa in first ejaculates by an average of 34\m=.\2%(P < 0\m=.\025).Oxytocin did not affect sperm quality (P > 0\m=.\10) as judged by the motility of spermatozoa in fresh semen or by the motility or percentage of spermatozoa with intact acrosomes in thawed semen. It is concluded that 50 i.u. oxytocin enhanced sperm output in first ejaculates of electroejaculated bulls without altering daily sperm production or seminal quality. Keywords: bull; spermatogenesis; sperm output; oxytocin; semen Introduction Sexual preparation by 'false mounting' or active restraint of beef or dairy bulls typically results in about a 50% increase in the number of spermatozoa in first ejaculates taken with an artificial vagina (Hale & Almquist, 1960; Almquist, 1973). Sexual preparation also induces the release of oxytocin (Sharma & Hays, 1973) which, if injected before ejaculation into an artificial vagina, increases the sperm output of rams, bulls and rabbits (Kihlstrom & Melin, 1963; Knight & Lindsay, 1970; Knight, 1974a; Schefels & ElAzab, 1975; Voglmayr, 1975; Agmo et al, 1978). Presumably, oxytocin stimulates smooth muscle contractility (Knight, 1972, 1974b), thereby enhancing sperm transport within the extragonadal ducts before and/or during ejaculation. The collection of semen with an artificial vagina is generally preferred to collection by electro ejaculation. However, electroejaculation has proved especially useful for the harvesting of semen from bulls for whom collection with an artificial vagina is precluded by inadequate libido or physical disabilities. Since electroejaculation is usually performed without sexual preparation pro cedures known to elicit oxytocin secretion, it seemed possible that exogenous oxytocin might enhance sperm output if given before electroejaculation. However, to be useful, oxytocin would not only need to enhance sperm output, but would need to be without adverse effects on spermato genesis or seminal quality. The effect of chronically-administered oxytocin on bovine spermato genesis and seminal quality is unknown. The objective of the present study was therefore to determine the effect of oxytocin, given before the electroejaculation of bulls maintained on an Present address: Department of Animal Science, University of Nigeria, Nsukka, Nigeria.
2 intensive seminal collection schedule, on spermatogenesis, sperm output and seminal quality of Holstein bulls. Materials and Methods General The 12- to 24-month-old Holstein bulls were housed indoors and restrained by individual neck chains. Each bull received approximately 3-4 kg of commercial dairy ration per day and grass hay ad libitum. Measurements of body weight and scrotal circumference were taken on Days 28, 14,0, + 14, +28, +42, +56 and +68, where Day 0 represents the first day of treatment. All 14 bulls were electroejaculated twice on each Monday, Wednesday and Friday for 14 weeks. An interval of 10 min was maintained between the collection of first and second ejaculates, while the order in which individual bulls were presented for collection of semen was randomized on any given day. The first 2 weeks of the experiment served to accustom bulls to this procedure and allowed the stabilization of extragonadal sperm reserves and sperm output (Amann & Almquist, 1961b). Data for seminal characteristics during this period were not utilized for statistical analyses. The next 2 weeks constituted a pretreatment period, which provided baseline data for the seminal characteristics of each bull. At the completion of the pretreatment period, individual bulls were placed into one of 7 groups of 2 bulls each based primarily on the similarity of sperm output. Scrotal circumference, body weight and motility of spermatozoa in fresh semen served as additional criteria for pairing, when necessary. Assignment of individual bulls to control or treated groups was conducted randomly from within each pair. Beginning on Day 0 (the first day of the treatment period) and for the remainder of the 10-week treatment period, each treated bull was given 50 i.u. oxytocin via the jugular vein 10 min before the collection of each first ejaculate. Oxytocin was provided in an aqueous solution (20 i.u./ml) containing chlorobutanol (0-5%) as a preservative and acetic acid to adjust ph. Control bulls were handled similarly and were subjected to jugular venepuncture, but did not receive oxytocin. All bulls were castrated on Day +68. Evaluation of seminal characteristics and quality. Insofar as possible, the pre-sperm and sperm-rich fractions of each electroejacuiate were harvested separately. Immediately upon collection, both fractions were assessed for volume and sperm concentration. The latter was determined spectrophotometrically (Foote et al, 1978), except when samples were too dilute or had been contaminated with urine or other foreign matter. The sperm concentration of such samples was determined haemocytometrically. Each fresh ejaculate was also evaluated for the percentage of pro gressively motile spermatozoa. This measurement was taken on the sperm-rich fraction except when this sample was contaminated or otherwise inappropriate. The resulting data were used to calculate the number of spermatozoa (total and motile) per ejaculate or per day. On Days 5, +9, +23, +37, +51 and +65, semen was processed by conventional methods for freezing in 0-5-ml French plastic straws (DeAbreu et al, 1979). First and second ejaculates were pooled for freezing within bulls (exclusive of contaminated samples). Samples from each date were stored until the completion of the experiment, when two straws per sample were removed and thawed by immersion in 35 C water for 12 sec (DeAbreu et al, 1979). The post-thaw motility of spermatozoa from each straw was assessed by each of two independent observers. Thawing and preparation of coded samples for evaluation was by a third individual. Subsequently, each thawed sample was incubated at 37 C for 2 h, fixed by addition of a glutaraldehyde solution (Johnson et al, 1976) and used to determine the percentage of spermatozoa with intact acrosomes (Saacke & Marshall, 1968). Acrosomal integrity was assessed by duplicate determinations on each sample by each of two independent evaluators without knowledge of the sample identity. All observations of post-thaw motility and acrosomal integrity for any given ejaculate were pooled within and over observers for statistical analysis. Post-castration procedures. Immediately upon castration, each testis was weighed, the tunica albugínea was removed and weighed, and the weight of the testicular parenchyma was calculated as the difference. One slice of tissue approximately midway between the dorsal and ventral pole was placed in Zenker-formol fixative for 24 h, washed in running tap water for 24 h and transferred to 70% ethanol (Berndtson & Desjardins, 1974). Subsequently, portions of this tissue were dehydrated, embedded in paraffin wax, sectioned at 5 µ, stained with periodic acid-schiff s reagent and haematoxylin and used for quantitative histometric evaluations of spermatogenesis. Additional portions of unfixed testicular parenchyma, from the middle and dorsal and ventral poles of each testis, were pooled, placed in a pre-weighed vial and stored at 25 C for use in establishing testicular spermatid reserves. Each epididymis was trimmed of extraneous tissue and divided into proximal and distal segments at the mid-corpus region. Each segment was weighed, sealed in a plastic bag and stored at 25 C until used to quantify epididymal sperm reserves. Quantification of spermatogenesis from extirpated tissues. Testicular spermatid reserves were quantified by enumeration of elongated spermatids in homogenates of testicular parenchyma (Amann & Almquist, 1961a). Daily sperm production (DSP) was calculated utilizing a time divisor of 5-32 days, which equalled the number of days of sperm production represented by homogenization-resistant elongated spermatids (Amann et al, 1974). Counts were performed in duplicate by each of two technicians. The average coefficients of variability between technicians for the DSP/g and DSP/testis of the 28 testes were 12-6 and 12-7%, respectively. Data for the DSP of each testis were pooled over technician and the means were used for statistical analysis. Spermatogenesis was quantified histometrically via the direct enumeration of the nuclei of type A spermatogonia, preleptotene primary spermatocytes, pachytene primary spermatocytes and round step 8 spermatids and of Sertoli
3 cell nuclei with a visible nucleolus in cross-sections of Stage VIII seminiferous tubules, as defined by Berndtson & Desjardins (1974). For each testis, the aforementioned nuclei were counted in each of 10 seminiferous tubular crosssections by each of two evaluators (20 tubules per testis). The resulting crude counts were converted to true counts by application of Abercrombie's formula, whereby true count = crude count section thickness/ (section thick ness + nuclear or nucleolar diameter) (when measurements are in µ : Abercrombie, 1946; Berndtson, 1977) and for tubular shrinkage by application of a Sertoli cell correction factor (Lino, 1971; Berndtson, 1977). For the Abercrombie correction, each observer independently measured 5 nuclei of each germ cell type and 5 Sertoli cell nucleoli for each testis with an ocular micrometer, and the correction was applied on a within-testis, within-observer basis. Data were pooled over evaluators for statistical analysis. In a separate study (Berndtson et al, 1987), the average coefficients of variation between duplicate estimates of the number of germ cells per tubular cross-section, for which each estimate was based upon counts for a total of only 10 tubular cross-sections per testis (i.e. for 5 rather than 10 cross-sections per each observer), were as follows: type A spermatogonia, 9-8%; preleptotene primary spermatocytes, 4-8%; pachytene primary spermatocytes, 4-8%; and step 8 spermatids, 40% Statistical analyses. Because inherent differences in some of these measures among bulls before treatment would probably influence the results, data were handled as follows. When data sets involved repeated measurements over time (body weight, scrotal circumference, sperm motility, volume and sperm concentration of the pre-sperm and sperm-rich fractions of each ejaculate and total spermatozoa per ejaculate or per day), analyses of variance were conducted via the repeated measures design (SAS Institute, 1985) for which each seminal collection day represented a level of the time factor, with data adjusted for pre-treatment differences. The adjustment consisted of subtracting the pre-treatment mean for each measure on an individual-bull basis from each value and then addition of the grand mean for the experiment to the resulting difference (Berndtson et al, 1979). Analyses were also conducted with unadjusted data. Although the results were generally similar, all levels of statistical significance which follow are based on analyses with adjusted data, but unadjusted means are presented. Data obtained from tissues removed at castration (testicular and epididymal weights, daily sperm production (DSP) per gram of parenchyma or per testis and numbers of germ cells per seminiferous tubular cross-section) were subjected to analyses of variance as a randomized complete block design, with pair as a factor (utilizing the pairing, based on pre-treatment data, from which assignment to treatment or control groups was conducted). Results Body weight and scrotal circumference Oxytocin was without effect (P > 010) on body weight (Table 1). Bulls gained weight throughout the experiment ( < 0005). The rate of gain over time, which averaged about 0-7 kg per day, was unaffected (P > 010) by oxytocin administration. Table 1. Mean + s.e. body weight and scrotal circumference of control and oxytocin-treated bulls Body wt (kg) Scrotal circ. (cm) Day Control Oxytocin Control Oxytocin ± ± ± ± ± Values were similar (P > OTO) for control and oxytocin-treated bulls. Oxytocin also was without effect (P > 010) on scrotal circumference (Table 1). Scrotal circumference did not change significantly during the experiment for bulls of either treatment group (P > 0 10). Since scrotal circumference is related to testicular weight, it was not surprising
4 that oxytocin also was without effect on the weight of reproductive organs removed at castration (Table 2, > 010). Seminal characteristics and sperm output The volume of pre-sperm and sperm-rich fractions of first and second ejaculates was unaffected by the administration of oxytocin (Table 3, > OTO). For each of these variables there were differences associated with collection day (P < 0-005). However, the variations appeared to be of a sporadic nature and, as for all seminal characteristics (i.e. seminal volume, sperm concentration and motility), treatment time (i.e. collection day) interactions were non-significant (P > 010). Similarly, oxytocin did not alter the sperm concentration of the pre-sperm or sperm-rich fractions of first ejaculates (P > 005) or of the pre-sperm fraction of second ejaculates (Table 3, > 010). However, oxytocin reduced the sperm concentration within the sperm-rich fraction of second ejaculates (Table 3, < 001). The influence of oxytocin on the sperm concentration of second ejaculates was consistent with an effect of this hormone on sperm output. The total number of spermatozoa harvested per collec tion day was similar for control and oxytocin-treated bulls (P > 010, Table 3). Sperm output increased over time ( < 0005), but the increase was similar (P > 010) for bulls of both groups. For example, the number of spermatozoa harvested per collection day averaged 4-52 and for control and oxytocin-treated bulls respectively during the 2-week pretreatment period, while corresponding means for the last 2 weeks of the experiment were 5-61 and , respectively. However, the administration of oxytocin caused a marked increase (P < 0025) in the number of spermatozoa in first ejaculates and a corresponding decrease (P < 0025) in the number within second ejaculates (Table 3). Of the total spermatozoa harvested per collection day, 47-3, 41-3, 430, 45-5, 410 and 45-6% were obtained in the first ejaculates of control bulls during the pretreatment period and during each successive 2-week period of the study. In contrast, the corresponding percentages for oxytocin-treated bulls averaged 33-2, 56-9, 541, 620, 58-3 and 59-2, respectively. Although it is not clear why the relative sperm output in first and second ejaculates of bulls in the two groups differed during the pretreatment period, the percentage of the total sperma tozoa present within first ejaculates quite consistently averaged about 43-3% in controls and 58T % in oxytocin-treated bulls during the treatment period. This corresponds to a 34-2% increase in the sperm output of first ejaculates due to oxytocin (i.e. 58T/ ). Spermatogenesis The sperm output of bulls maintained on a regular 6-times/week seminal collection schedule approaches sperm production (Amann et al, 1974). Therefore, the similarity of sperm output throughout the present experiment was indicative of an absence of an effect of oxytocin on sperma togenesis. Further direct evidence of this is presented in Table 4. Despite differences among pairs of bulls (P < 005), oxytocin was without effect (P > 0-10) on the numbers of type A spermatogonia, preleptotene primary spermatocytes, pachytene primary spermatocytes or round step 8 spermatids per Stage VIII seminiferous tubular cross-section (Table 4). In addition, oxytocin was without effect (P > 010) on daily sperm production per gram of testicular parenchyma, per testis or per bull, as judged by the numbers of homogenization-resistant elongated spermatids. Epididymal sperm reserves Since oxytocin did not alter rates of sperm production or total daily sperm output, it was not surprising that oxytocin was without effect on the epididymal sperm reserves within tissues removed at castration on Day +68, 1 day after the last ejaculation (Table 5). Neither the total paired epididymal sperm reserves nor the distribution of spermatozoa within the proximal or distal epididymal segments were influenced by oxytocin administration (P > 010).
5 Table 2. Mean ( + s.e.) weight (g) of reproductive organs from control and oxytocin-treated bulls at castration Organ Control Oxytocin Paired testes Testicular parenchyma Paired epididymides Proximal epididymis Distal epididymis ± ± Organ weights were similar (P > 0-10) for control and oxytocin-treated bulls. Table 3. Seminal characteristics (means ± s.e.) of control and oxytocin-treated bulls during the pretreatment* and treatment! periods Seminal characteristics Pretreatment periodi Pre-sperm vol. (ml) Pre-sperm cone. ( 10"~6) Sperm-rich vol. (ml) Sperm-rich cone. ( 10~6) Treatment periodi Pre-sperm vol. (ml) Pre-sperm cone. ( IO-6) Sperm-rich vol. (ml) Sperm-rich cone. ( 10"6) Total spermatozoa/ejaculate ( 10 9) Control Oxytocin-treated 1st ejaculate 2nd ejaculate 1st ejaculate 2nd ejaculate 3-9 ± ± ± ± 0-2a 75-8 ± 8-6a 5-3 ± 0-2a " 2-14 ± ± d 0-31d 0-4 ld 0-26d 0-27" 3-7 ± ± ± " ll-lb " ± 17-7" 2-38 ± 3-07 ± ± f 0-38f 0-42f 0-12f 0-45f ± ± a a a a ± ± " 0-39e 0-38» 0-43e 0-28e» t ^Standard errors among days. "Means for first ejaculates were similar (P > 005) for control and oxytocin-treated bulls. bcmeans for second ejaculates that do not bear a similar superscript differ (P < 001). de,fgmeans for first (de) or second (fb) ejaculates that do not bear a similar superscript differ ( < 25) ± b ± 8-4" 7-2 ± 0-3" C 3-28 ± ± s 018s 017e Seminal quality The percentage of progressively motile spermatozoa in fresh ejaculates is shown in Table 6. Oxytocin was without effect (P > 010) on the motility of spermatozoa in first and second ejacu lates. Also, oxytocin did not influence the percentage of progressively motile spermatozoa or the percentage of spermatozoa with intact acrosomes in frozen-thawed semen (Table 1, > 0-10). It was therefore concluded that oxcytocin did not influence sperm quality.
6 Table 4. Spermatogenesis in control and oxytocin-treated bulls as judged by numbers of germ cells per stage VIII seminiferous tubular cross-section and via determination of daily sperm production (DSP) from numbers of homogenization-resistant elongated spermatids (means + s.e.) Treatment Control Oxytocin Type A spermatogonia Preleptotene spermatocytes Pachytene spermatocytes Step 8 spermatids DSP/g(xlO"6) Left testis Right testis Mean DSP/paired testes ( IO"9) DSP of left testis DSP of right testis Germ celisitubular cross-section ± ± Daily sperm production ± ± ± ± ± Germ cell numbers and DSP were similar (P > OTO) for control and oxytocintreated bulls. Table 5. Epididymal sperm reserves (x 10 9) of control and oxytocin-treated bulls Control Oxytocin Paired proximal epididymides Left proximal Right proximal Paired distal epididymides Left distal Right distal Total paired epididymides ± ± ± IT Values are mean + s.e. All values for control and oxytocin-treated bulls are similar (P>0T0). Discussion The present investigation was designed to permit an evaluation of the effects of oxytocin, given before collection of first ejaculates, on spermatogenesis, sperm output and seminal quality of electroejaculated bulls. The frequency of electroejaculation (6 times/week) was adopted for two reasons. First, at such a frequency sperm output approaches sperm production. Thus, sperm out put was available as one of three criteria for assessment of sperm production rates. In addition, since there is little reason to exceed such a frequency for bulls maintained for use in artificial insemination programmes, the oxytocin regimen used herein would probably constitute a maximal
7 Table 6. Mean + s.e. percentage of progressively motile sper matozoa in ejaculates from control and oxytocin-treated bulls Time period (weeks) -2& 1& 3& 5& 7& 9& st ejaculate Control 2nd ejaculate 1st ejaculate Oxytocin 2nd ejaculate 58-8 ± ± ± ± ± ± ± ± IT 611 ± ± ± ± ± All values for control and oxytocin-treated bulls are similar (P > 010). Table 7. Mean + s.e. post-thaw motility and acrosomal integrity of spermatozoa from control and oxytocin-treated bulls Motility (%) Intact acrosomes (%) Day Control Oxytocin Control Oxytocin ± ± ± ± ± ± ± ± ± ± ± ± 2-4 All values are similar (P > 010) for control and oxytocin-treated bulls. dosage for the potential purpose of enhancing sperm output. The present results (Tables 2-4) constitute exceptionally strong evidence that oxytocin was without effect on spermatogenesis when given at the dosage and frequency employed herein. However, it was apparent a priori that, if oxytocin enhanced sperm output, it could not sustain a prolonged increase in total daily sperm output, at such a frequency of ejaculation, unless spermatogenesis was enhanced. Therefore, if oxytocin enhanced sperm output during ejaculation without altering sperm production, one of two responses might be detected: (1) a short-term increase in sperm output during the early phase of the treatment period, caused by a lowering of epididymal sperm reserves. Such a response was not noted (Tables 3 and 5); and (2) more spermatozoa might be harvested in first ejaculates with a corresponding reduction in the number for second ejaculates. The latter could be anticipated because of the reduced number of epididymal spermatozoa available for ejaculation upon increased removal during the first ejaculate, and because of the relatively short half-life of the oxytocin response. It was clear that oxytocin did enhance sperm output of electroejaculated bulls (Table 3) and that the 34-2% increase in first ejaculates was roughly comparable to the increase noted due to sexual preparation of bulls from which semen was collected with an artificial vagina (Hale & Almquist, 1960; Almquist, 1973). It is not known whether sperm output might be enhanced further by the administration of oxytocin before both ejaculates. However, in the absence of an effect on sperm production, it would
8 seem that the use of oxytocin to enhance sperm output would be most advantageous for bulls electroejaculated less frequently and/or when the collection of only a single ejaculate was planned. By administering oxytocin, it might be possible to reduce the frequency of electroejaculation for bulls with certain physical disabilities, thereby reducing stress to the bull and the labour associated with collection and subsequent processing of semen, without compromising the number of inseminates available over time. Ejaculates harvested by electroejaculation typically have a greater volume and lower sperm concentration than those obtained with an artificial vagina (Ball, 1974). The technique of the individual performing the electroejaculation can also influence these factors. Although the primary reason for recording seminal volume and sperm concentration was to permit calculation of sperm output, the former also provide some insight into the potential effects of oxytocin on accessory sex gland function. The absence of an effect of oxytocin on the volume of the pre-sperm or sperm-rich fractions of the first or second ejaculates (P > 0-10) is consistent with the likely absence of an effect of oxytocin on the accessory sex glands of the bull. At the same time, oxytocin altered the number of spermatozoa in first and second ejaculates despite the general absence of effects on seminal volumes or sperm concentrations. The latter are quite variable and tend to change inversely in relation to one another. Accordingly, we suspect that the product of these two measures (i.e. sperm output) was less variable and/or was influenced to a greater extent by oxytocin than was either of these individual components, thus revealing statistically significant treatment effects on total sperm output. Because most semen is frozen for use in artificial insemination programmes, the effects of oxytocin on post-thaw seminal quality were examined. The semen was frozen by a scheduled protocol and some samples were processed which would have been regarded as unacceptable for processing under most commercial artificial insemination programmes. For such reasons, the present results with frozen semen should be viewed as constituting a preliminary assessment only. While the results are not extensive, they provide no reason to suspect that oxytocin impaired the ability of spermatozoa to withstand freezing or thawing. This is consistent with the acceptability for freezing of semen obtained by collection methods known to elicit endogenous oxytocin secretion (Sharma et al, 1972). In contrast to these results, the motility of spermatozoa in fresh ejaculates was assessed by evaluation of approximately 1000 ejaculates during the 2-week pre-treatment and 10-week treatment periods. Given the size of this sample, the absence of an effect of oxytocin on motility (P > 0T 0) constitutes very strong evidence that oxytocin was without effect on this criterion. From the evidence presented herein, it is concluded that oxytocin did not alter the quality of semen obtained via electroejaculation of young dairy bulls. Scientific contribution No from the New Hampshire Agricultural Experiment Station. Supported in part by grants-in-aid from Eastern Artificial Insemination Cooperative, Inc. (Ithaca, New York), Select Sires (Plain City, Ohio), and American Breeders Service (DeForest, Wisconsin). We thank Clayton Penniman (Office of Biometrics) for statistical advice and Wendy Harding, David Anderson and Christopher Jacques for technical assistance. Abercrombie, M. (1946) Estimation of nuclear popu lation from microtome sections. Anat. Ree. 94, Agmo,., Andersson, R. & Johansson, C. (1978) Effect of oxytocin on sperm numbers in spontaneous rat ejaculates. Biol. Reprod. 18, Almquist. J.O. (1973) Effects of sexual preparation on sperm output, semen characteristics and sexual activity of beef bulls with a comparison to dairy bulls. J. Anim. Sci. 36, References Amann, R.P. & Almquist, J.O. (1961a) Reproductive capacity of dairy bulls. I. Technique for direct measurement of gonadal and extragonadal sperm reserves. J. Dairy Sci. 44, Amann, R.P. & Almquist, J.O. (1961b) Reproductive capacity of dairy bulls. V. Detection of testicular deficiencies and requirements for experimentally evaluating testis function from semen characteristics. J. Dairy Sci. 44, Amann, R.P., Kavanaugh, J.F., Griel, L.C., Jr, &
9 Voglmayr, J.K. (1974) Sperm production of Holstein bulls determined from testicular spermatid reserves, after cannulation of rete testis or vas deferens, and by daily ejaculation. J. Dairy Sci. 57, Ball, L. (1974) Electroejaculation of bulls. Proc. 5th N.A.A.B. Tech. Confi, A.I. & Reprod. pp Berndtson, W.E. (1977) Methods for quantifying mam malian spermatogenesis: a review. J. Anim. Sci. 44, Berndtson, W.E. & Desjardins, C. (1974) The cycle of the seminiferous epithelium and spermatogenesis in the bovine testis. Am. J. Anat. 140, Berndtson, W.E., Chenoweth, P.J., Seidel, G.E., Jr, Pickett, B.W. & Olar, T.T. (1979) Influence of pros taglandin F2a on spermatogenesis, spermatozoal out put, seminal quality, testosterone levels and libido of yearling beef bulls. J. Anim. Sci. 49, Berndtson, W.E., Igboeli, G. & Parker, W.G. (1987) The numbers of Sertoli cells in mature Holstein bulls and their relationship to quantitative aspects of sperma togenesis. Biol. Reprod. 37, DeAbreu, R.M., Berndtson, W.E., Smith, R.L. & Pickett, B.W. (1979) Effect of post-thaw warming on viability of bovine spermatozoa thawed at different rates in French straws. J. Dairy Sci. 62, Foote, R.H., Arrióla, J. & Wall, R.J. (1978) Principles and procedures for photometric measurement of sperm cell concentration. Proc. 7th N.A.A.B. Tech. Confi., A.I. & Reprod. pp Hale, E.B. & Almquist, J.O. (I960) Relation of sexual behavior to germ cell output in farm animals. J. Dairy Sci. (April Suppl.) 43, Johnson, L., Berndtson, W.E. & Pickett, B.W. (1976) An improved method for evaluating acrosomes of bovine spermatozoa. J. Anim. Sci. 42, Kihlstrom, J.E. & Melin, P. (1963) The influence of oxytocin upon some seminal characteristics in the rabbit. Acta physiol. Scand. 59, Knight, T.W. (1972) In vivo effects of oxytocin on the contractile activity of the cannulated epididymis and vas deferens in rams. J. Reprod. Fert. 28, 141, Abstr. Knight, T.W. (1974a) The effect of oxytocin and adrena line on the semen output of rams. J. Reprod. Fert. 39, Knight, T.W. (1974b) A qualitative study of factors affecting the contractions of the epididymis and ductus deferens of the ram. J. Reprod. Fert. 40, Knight, T.W. & Lindsay, D.R. (1970) Short- and longterm effects of oxytocin on quality and quantity of semen from rams. J. Reprod. Fert. 21, Lino, B.F. (1971) Cell count correction factors for the quantitative histological analysis of the germinal epithelium of the ram. Anat. Ree. 170, Saacke, R.G. & Marshall, CE. (1968) Observations on the acrosomal cap of fixed and unfixed bovine sper matozoa. J. Reprod. Fert. 16, SAS Institute Inc. (1985) SAS User's Guide: Statistics, Version 5 Edition pp SAS, Cary. Schefels, W. & ElAzab, A. (1975) The effect of oxytocin on ejaculate composition in the bull. Theriogenology 4, 134, Abstr. Sharma, O.P. & Hays, R.L. (1973) Release of an oxytocic substance following genital stimulation in bulls. J. Reprod. Fert. 35, Sharma, S.C, Fitzpatrick, R.J. & Ward, W.R. (1972) Coital-induced release of oxytocin in the ram. J. Reprod. Fert. 31, , Abstr. Voglmayr, J.K. (1975) Output of spermatozoa and fluid by the testis of the ram and its response to oxytocin. J. Reprod. Fert. 43, Recieved 10 April 1987
of the rabbit testis were obtained.
 FERTILITY AND STERILITY Copyright 1970 by The Williams & Wilkins Co. Vol. 21, No.9, September 1970 Printed in U.S.A. THE MALE RABBIT. IV. QUANTITATIVE TESTICULAR HISTOLOGY AND COMPARISONS BETWEEN DAILY
FERTILITY AND STERILITY Copyright 1970 by The Williams & Wilkins Co. Vol. 21, No.9, September 1970 Printed in U.S.A. THE MALE RABBIT. IV. QUANTITATIVE TESTICULAR HISTOLOGY AND COMPARISONS BETWEEN DAILY
EFFECT OF THAWING RATE AND POST-THAW TEMPERATURE ON MOTILITY AND ACROSOMAL MAINTENANCE IN BOVINE SEMEN FROZEN IN PLASTIC STRAWS l,2
 EFFECT OF THAWING RATE AND POST-THAW TEMPERATURE ON MOTILITY AND ACROSOMAL MAINTENANCE IN BOVINE SEMEN FROZEN IN PLASTIC STRAWS l,2 P. L. Senger, W. C. Becker and J. K. Hillers Washington State University
EFFECT OF THAWING RATE AND POST-THAW TEMPERATURE ON MOTILITY AND ACROSOMAL MAINTENANCE IN BOVINE SEMEN FROZEN IN PLASTIC STRAWS l,2 P. L. Senger, W. C. Becker and J. K. Hillers Washington State University
Male Reproductive System
 Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System
 Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
(Received 6th October 1972)
 EFFECT OF TESTOSTERONE POLYDIMETHYL- SILOXANE IMPLANTS UPON SPERM PRODUCTION, LIBIDO AND ACCESSORY SEX ORGAN FUNCTION IN RABBITS L. L. EWING, L. G. STRATTON and C. DESJARDINS Department of Physiological
EFFECT OF TESTOSTERONE POLYDIMETHYL- SILOXANE IMPLANTS UPON SPERM PRODUCTION, LIBIDO AND ACCESSORY SEX ORGAN FUNCTION IN RABBITS L. L. EWING, L. G. STRATTON and C. DESJARDINS Department of Physiological
2 Sperm Production and its Harvest
 2 Sperm Production and its Harvest William E. Berndtson* University of New Hampshire, Durham, New Hampshire, USA Introduction Spermatogenesis requires ~60 days in most mammals. It encompasses a series
2 Sperm Production and its Harvest William E. Berndtson* University of New Hampshire, Durham, New Hampshire, USA Introduction Spermatogenesis requires ~60 days in most mammals. It encompasses a series
Daily sperm production and epididymal sperm
 Daily sperm production and epididymal sperm pubertal and adult rats G. W. Robb, R. P. Amann and G. J. Killian reserves of Dairy Breeding Research Center, The Pennsylvania State University, University Park,
Daily sperm production and epididymal sperm pubertal and adult rats G. W. Robb, R. P. Amann and G. J. Killian reserves of Dairy Breeding Research Center, The Pennsylvania State University, University Park,
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
Histology of Male Reproductive system (1)
 Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
The Use of Rabbits in Male Reproductive Toxicology
 Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS
 Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
University Park, Pennsylvania 16802, U.S.A
 A MODIFIED TECHNIQUE FOR CANNULATING THE RETE TESTIS OF THE BULL J. K. VOGLMAYR, J. F. KAVANAUGH, L. C. GRIEL, Jr and R. P. AMANN Dairy Breeding Research Center, Department of Dairy Science, and Department
A MODIFIED TECHNIQUE FOR CANNULATING THE RETE TESTIS OF THE BULL J. K. VOGLMAYR, J. F. KAVANAUGH, L. C. GRIEL, Jr and R. P. AMANN Dairy Breeding Research Center, Department of Dairy Science, and Department
Testicular growth, hormone concentrations, seminal characteristics and sexual behaviour in stallions
 Testicular growth, hormone concentrations, seminal characteristics and sexual behaviour in stallions J. Naden, R. P. Amann and E. L. Squires Animal Reproduction Laboratory, Colorado State University, Fort
Testicular growth, hormone concentrations, seminal characteristics and sexual behaviour in stallions J. Naden, R. P. Amann and E. L. Squires Animal Reproduction Laboratory, Colorado State University, Fort
Reproductive Effects of Feeding Gossypol and Vitamin E to Bulls
 Reproductive Effects of Feeding Gossypol and Vitamin E to Bulls J. Velasquez-Pereira P.J. Chenoweth L.R. McDowell C.A. Risco C.A. Staples D. Prichard F.G. Martin M.C. Calhoun S.N. Williams N.S. Wilkinson
Reproductive Effects of Feeding Gossypol and Vitamin E to Bulls J. Velasquez-Pereira P.J. Chenoweth L.R. McDowell C.A. Risco C.A. Staples D. Prichard F.G. Martin M.C. Calhoun S.N. Williams N.S. Wilkinson
The Reproductive System
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
The Reproductive System
 16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
THE MALE RABBIT. VII. STUDIES ON RESORPTION OF PH} THYMIDINE.LABELED SPERMATOZOA IN THE EPIDIDYMIS*
 Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE MALE RABBIT. VII. STUDIES ON RESORPTION OF PH} THYMIDINE.LABELED SPERMATOZOA IN THE
Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE MALE RABBIT. VII. STUDIES ON RESORPTION OF PH} THYMIDINE.LABELED SPERMATOZOA IN THE
The Reproductive System
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
Cornell University, Ithaca, New York
 T TESTICLAR GROWTH AND RELATED SPERM OTPT IN DAIRY BLLS 1.2 J. HAI-IN, 3 R. H. FOOTE AND G. E. SEIDEL, JR. Cornell niversity, Ithaca, New York HE demand for sperm from outstanding sires has increased with
T TESTICLAR GROWTH AND RELATED SPERM OTPT IN DAIRY BLLS 1.2 J. HAI-IN, 3 R. H. FOOTE AND G. E. SEIDEL, JR. Cornell niversity, Ithaca, New York HE demand for sperm from outstanding sires has increased with
describe the parts and function of semen and the glands that contribute to it
 You need to be able to: describe spermatogenesis (How is sperm made?) describe the anatomy of a sperm describe the parts and function of semen and the glands that contribute to it How is sperm made? Spermatogenesis
You need to be able to: describe spermatogenesis (How is sperm made?) describe the anatomy of a sperm describe the parts and function of semen and the glands that contribute to it How is sperm made? Spermatogenesis
The Male Reproductive System
 The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
Animal Science 434. Semen Collection. Effect of Age on Sperm Output. Age When Semen Can Be Collected. Text: Ch. 10 and 11. Sexual Behavior (cont.
 Animal Science 434 Age When Semen Can Be Collected Sexual Behavior (cont.) B. Applied Reproductive Behavior of the Male: Semen Collection and Processing Text: Ch. 10 and 11 Bull Boar Ram Stallion Dog 12
Animal Science 434 Age When Semen Can Be Collected Sexual Behavior (cont.) B. Applied Reproductive Behavior of the Male: Semen Collection and Processing Text: Ch. 10 and 11 Bull Boar Ram Stallion Dog 12
Primary sex organs (gonads): testes and ovaries. Accessory reproductive organs: ducts, glands, and external genitalia
 Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
The Effect of Low Ambient Temperatures on Sperm Production, Epididymal Sperm Reserves, and Semen Characteristics of Roars
 BIOLOGY OF REPRODUCTION 2, 23-28 (197) The Effect of Ambient Temperatures on Sperm Production, Epididymal Sperm Reserves, and Semen Characteristics of Roars ERNEST E. SWIERSTRA Research Station, Canada
BIOLOGY OF REPRODUCTION 2, 23-28 (197) The Effect of Ambient Temperatures on Sperm Production, Epididymal Sperm Reserves, and Semen Characteristics of Roars ERNEST E. SWIERSTRA Research Station, Canada
Male Reproductive System. Dr Maan Al-Abbasi PhD, MSc, MBChB, MD
 Male Reproductive System Dr Maan Al-Abbasi PhD, MSc, MBChB, MD Learning Objectives 1. Describe the General Anatomy of the Male Reproductive System 2. Identify the structures that are related to the prostate.
Male Reproductive System Dr Maan Al-Abbasi PhD, MSc, MBChB, MD Learning Objectives 1. Describe the General Anatomy of the Male Reproductive System 2. Identify the structures that are related to the prostate.
Management and health of the bull s genital tract
 Pathway of sperm through the male genital system Management and health of the bull s genital tract Presented by Dr. Don Monke Select Sires, Inc. Plain City, Ohio February 17 To accurately evaluate a bull,
Pathway of sperm through the male genital system Management and health of the bull s genital tract Presented by Dr. Don Monke Select Sires, Inc. Plain City, Ohio February 17 To accurately evaluate a bull,
Animal Science 434" Semen Collection" Effect of Age on Sperm Output" Age When Semen Can Be Collected" Text: Ch. 10 and 11"
 Animal Science 434" Age When Semen Can Be Collected" Lecture 15b: Sexual Behavior (cont.) B. Applied Reproductive Behavior of the Male: Semen Collection and Processing Text: Ch. 10 and 11" "Bull "Boar
Animal Science 434" Age When Semen Can Be Collected" Lecture 15b: Sexual Behavior (cont.) B. Applied Reproductive Behavior of the Male: Semen Collection and Processing Text: Ch. 10 and 11" "Bull "Boar
Effects of Label Dose Permethrin Use in Yearling Angus Beef Bulls on Reproductive Function and Testicular Histopathology
 AS 66 ASL R2958 205 Effects of Label Dose Permethrin Use in Yearling Angus Beef Bulls on Reproductive Function and Testicular Histopathology Tyler M. Dohlman Iowa State University, tdohlman@iastate.edu
AS 66 ASL R2958 205 Effects of Label Dose Permethrin Use in Yearling Angus Beef Bulls on Reproductive Function and Testicular Histopathology Tyler M. Dohlman Iowa State University, tdohlman@iastate.edu
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Male Anatomy. testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics
 Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
(Haltmeyer & Eik-Neis, 1969; Katongole, Naftolin & Short, 1971). rutting behaviour and a rise in the male hierarchy (Lincoln, Youngson &
 THE RELATIONSHIP BETWEEN SOCIAL STATUS AND REPRODUCTIVE ACTIVITY IN MALE IMPALA, AEPYCEROS MELAMPUS P. S. BRAMLEY and W. B. NEAVES Departments of Animal Physiology and Anatomy, University of Nairobi, P.O.
THE RELATIONSHIP BETWEEN SOCIAL STATUS AND REPRODUCTIVE ACTIVITY IN MALE IMPALA, AEPYCEROS MELAMPUS P. S. BRAMLEY and W. B. NEAVES Departments of Animal Physiology and Anatomy, University of Nairobi, P.O.
Adapted from Preg. & Part., Senger
 MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
Reproductive System Purpose General Structures Male Structures Functions Female Anatomy Structures Functions Clinical Applications
 The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
Anatomy and Physiology of Farm Animals. Lecture Six. Anatomy and Physiology of Male Reproductive System II
 Anatomy and Physiology of Farm Animals Lecture Six Anatomy and Physiology of Male Reproductive System II 1 The male reproductive system Two testes Scrotum Spermatic cords Accessory glands Penis Prepuce
Anatomy and Physiology of Farm Animals Lecture Six Anatomy and Physiology of Male Reproductive System II 1 The male reproductive system Two testes Scrotum Spermatic cords Accessory glands Penis Prepuce
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE
 ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
submitted in partial fulfillment of the
 EFFECT OF SEMEN THAW METHOD ON PREGNANCY RATES IN HOLSTEIN HEIFERS by MARY KAY SCHMIDT B.S., University of Wisconsin-River Falls, 1976 A MASTER'S THESIS submitted in partial fulfillment of the requirements
EFFECT OF SEMEN THAW METHOD ON PREGNANCY RATES IN HOLSTEIN HEIFERS by MARY KAY SCHMIDT B.S., University of Wisconsin-River Falls, 1976 A MASTER'S THESIS submitted in partial fulfillment of the requirements
Medical School Histology Basics Male Reproductive System. VIBS 289 lab
 Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Pathology of Male Reproductive System 1
 Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM
 LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
Male Reproductive Physiology
 Male Reproductive Physiology Overview Anatomy Function Endocrine and spermatogenesis Testis epididymus,vas deferens,seminal vesicles and prostate Hypothalamic pituitary testicular axis Hormones of the
Male Reproductive Physiology Overview Anatomy Function Endocrine and spermatogenesis Testis epididymus,vas deferens,seminal vesicles and prostate Hypothalamic pituitary testicular axis Hormones of the
The spermatogenesis CHARACTERISTICS OF THE SPERMATOZOON 26/04/2017. Reproductive Biotechnologies Andrology I. Prof. Alberto Contri
 Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Male Reproduction Organs. 1. Testes 2. Epididymis 3. Vas deferens 4. Urethra 5. Penis 6. Prostate 7. Seminal vesicles 8. Bulbourethral glands
 Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM 1. The male reproductive system is made up of the following structures, EXCEPT: a. prostate; b. testicle; c. spermatic ducts; d. vestibular bulbs; e. seminal vesicles. 2.The testicle:
MALE REPRODUCTIVE SYSTEM 1. The male reproductive system is made up of the following structures, EXCEPT: a. prostate; b. testicle; c. spermatic ducts; d. vestibular bulbs; e. seminal vesicles. 2.The testicle:
The Male Reproductive System
 The Male Reproductive System The male reproductive system Testes Genital ducts Accessory sex glands: seminal vesicles prostate bulbourethral glands External genitalia: penis Structure of the Testis Tunica
The Male Reproductive System The male reproductive system Testes Genital ducts Accessory sex glands: seminal vesicles prostate bulbourethral glands External genitalia: penis Structure of the Testis Tunica
Short communication Testis developments and puberty in the male Tokara (Japanese native) goat
 Animal Reproduction Science 64 (2000) 127 131 Short communication Testis developments and puberty in the male Tokara (Japanese native) goat S. Nishimura a,, K. Okano b, K. Yasukouchi c, T. Gotoh a, S.
Animal Reproduction Science 64 (2000) 127 131 Short communication Testis developments and puberty in the male Tokara (Japanese native) goat S. Nishimura a,, K. Okano b, K. Yasukouchi c, T. Gotoh a, S.
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER)
 THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
Male reproduction. Cross section of Human Testis ผศ.ดร.พญ.ส ว ฒณ ค ปต ว ฒ ภาคว ชาสร รว ทยา คณะแพทยศาสตร ศ ร ราชพยาบาล 1. Aims
 Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Spermiogram and sperm reserves in hybrid Bos indicus \m=x\
 Spermiogram and sperm reserves in hybrid Bos indicus \m=x\ Bos taurus bulls after scrotal insulation S. Wildeus and K. W. Entwistle Department of Tropical Veterinary Science, James Cook University, Townsville,
Spermiogram and sperm reserves in hybrid Bos indicus \m=x\ Bos taurus bulls after scrotal insulation S. Wildeus and K. W. Entwistle Department of Tropical Veterinary Science, James Cook University, Townsville,
Breeding Soundness Evaluation (BSE) of Bulls
 Breeding Soundness Evaluation (BSE) of Bulls Beef Bull Evaluation Reproductive merit economically > important than growth and carcass quality Evaluate Before purchase Before each breeding season If infertility
Breeding Soundness Evaluation (BSE) of Bulls Beef Bull Evaluation Reproductive merit economically > important than growth and carcass quality Evaluate Before purchase Before each breeding season If infertility
Male Reproductive System Dr. Gary Mumaugh
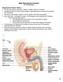 Male Reproductive System Dr. Gary Mumaugh Reproductive System Basics Primary sex organs (gonads) testes in males, ovaries in females Gonads produce sex cells called gametes (gametes means spouses) and
Male Reproductive System Dr. Gary Mumaugh Reproductive System Basics Primary sex organs (gonads) testes in males, ovaries in females Gonads produce sex cells called gametes (gametes means spouses) and
Semen-induced ovulation in the bactrian camel (Camelus bactrianus)
 Semen-induced ovulation in the bactrian camel (Camelus bactrianus) B. X. Chen, Z. X. Yuen and G. W. Pan Department of Veterinary Medicine, Gansu Agricultural University, Wuwei, Gansu and *Haixi Institute
Semen-induced ovulation in the bactrian camel (Camelus bactrianus) B. X. Chen, Z. X. Yuen and G. W. Pan Department of Veterinary Medicine, Gansu Agricultural University, Wuwei, Gansu and *Haixi Institute
Understanding spermatogenesis is central to probing
 Journal of Andrology, Vol. 29, No. 5, September/October 2008 Copyright E American Society of Andrology The Cycle of the Seminiferous Epithelium in Humans: A Need to Revisit? Review RUPERT P. AMANN From
Journal of Andrology, Vol. 29, No. 5, September/October 2008 Copyright E American Society of Andrology The Cycle of the Seminiferous Epithelium in Humans: A Need to Revisit? Review RUPERT P. AMANN From
STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM
 Unit 7A STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM LEARNING OBJECTIVES 1. Learn the structures of the male reproductive system. 2. Learn the functions of the male reproductive system. 3. Learn
Unit 7A STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM LEARNING OBJECTIVES 1. Learn the structures of the male reproductive system. 2. Learn the functions of the male reproductive system. 3. Learn
Efferent Ducts and Epididymis
 increase) the secretion of each of the androgen regulated proteins. Regulation of spermatogenesis is therefore an extremely complex cascade of cell-cell interactions with the Leydig cells supporting germ
increase) the secretion of each of the androgen regulated proteins. Regulation of spermatogenesis is therefore an extremely complex cascade of cell-cell interactions with the Leydig cells supporting germ
Basic histology 5/4/2015
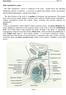 Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
Sweat glands of the scrotum of the bull
 s of the scrotum of the bull N. B. Blazquez, G. J. Mallard and S. R. Wedd Department of Animal Husbandry, University of Bristol, School of Veterinary Science, Langford House, Langford, Bristol BS18 7DU,
s of the scrotum of the bull N. B. Blazquez, G. J. Mallard and S. R. Wedd Department of Animal Husbandry, University of Bristol, School of Veterinary Science, Langford House, Langford, Bristol BS18 7DU,
Chapter 26: Reproductive Systems. Male 11/29/2015. Male reproductive system is composed of... BIO 218 Fall Gonads (testes)
 Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Reproductive Endocrinology. Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007
 Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Effect of straw size and thawing time on quality of cryopreserved buffalo (Bubalus bubalis) semen
 Vol. 11, No. 1 49 SHORT COMMUNICATION Effect of straw size and thawing time on quality of cryopreserved buffalo (Bubalus bubalis) semen Muhammad S Ansari, Bushra A. Rakha, Syed M. H. Andrabi, Shamim Akhter
Vol. 11, No. 1 49 SHORT COMMUNICATION Effect of straw size and thawing time on quality of cryopreserved buffalo (Bubalus bubalis) semen Muhammad S Ansari, Bushra A. Rakha, Syed M. H. Andrabi, Shamim Akhter
Male Reproductive System
 21-1 21-2 Reproductive System Male Reproductive System Genital Tract In males the testes, held outside the body in the scrotum (optimum temp of about 35 0 C), produce sperm. Sperm mature in coiled tubes
21-1 21-2 Reproductive System Male Reproductive System Genital Tract In males the testes, held outside the body in the scrotum (optimum temp of about 35 0 C), produce sperm. Sperm mature in coiled tubes
12/3/12. Managing Bull Development to Optimize Fertility Rearing bulls for fertility
 Managing Bull Development to Optimize Fertility Rearing bulls for fertility Effect of post weaning nutrition (after normal calf hood nutrition) - testis size - age at puberty - semen quality Effect of
Managing Bull Development to Optimize Fertility Rearing bulls for fertility Effect of post weaning nutrition (after normal calf hood nutrition) - testis size - age at puberty - semen quality Effect of
EFFECT OF MONTH AND STALLION ON SEMINAL CHARACTERISTICS AND SEXUAL BEHAVIOR 1, 2
 E EFFECT OF MONTH AND STALLION ON SEMINAL CHARACTERISTICS AND SEXUAL BEHAVIOR 1, 2 B. W. PICKETT, 3 L. C. FAULKNER 3 AND T. M. SUTHERLAND 4 Colorado State University, Fort Collim XTENSIVE studies have
E EFFECT OF MONTH AND STALLION ON SEMINAL CHARACTERISTICS AND SEXUAL BEHAVIOR 1, 2 B. W. PICKETT, 3 L. C. FAULKNER 3 AND T. M. SUTHERLAND 4 Colorado State University, Fort Collim XTENSIVE studies have
Semen evaluation in domestic animals I
 Reproductive Biotechnologies Andrology I I Semen evaluation in domestic animals I Prof. Alberto Contri Different aims 1. Diagnosis 2. Handling and preservation Related to the male - Breeding soundness
Reproductive Biotechnologies Andrology I I Semen evaluation in domestic animals I Prof. Alberto Contri Different aims 1. Diagnosis 2. Handling and preservation Related to the male - Breeding soundness
Small Ruminant Reproductive Management Workshop
 Small Ruminant Reproductive Management Workshop Animal Nutrition and Physiology Center, North Dakota State University Sponsors: American Sheep and Goat Center, North Dakota State University, University
Small Ruminant Reproductive Management Workshop Animal Nutrition and Physiology Center, North Dakota State University Sponsors: American Sheep and Goat Center, North Dakota State University, University
FIGURE The tunica albuginea is a connective tissue capsule forming the outer part of each testis.
 Testicular Histology (see p. 1034 in text) FIGURE 28.3 1. The tunica albuginea is a connective tissue capsule forming the outer part of each testis. 2. Septa are extensions of the tunica albuginea that
Testicular Histology (see p. 1034 in text) FIGURE 28.3 1. The tunica albuginea is a connective tissue capsule forming the outer part of each testis. 2. Septa are extensions of the tunica albuginea that
Examining Breeding Soundness of Beef Bulls
 Examining Breeding Soundness of Beef Bulls A herd bull that will serve a higher percentage of cows during a limited breeding season is essential to a successful cow-calf operation. In many of these operations,
Examining Breeding Soundness of Beef Bulls A herd bull that will serve a higher percentage of cows during a limited breeding season is essential to a successful cow-calf operation. In many of these operations,
Environmental effects and repeatability estimates for sperm production and semen quality of Holstein bulls
 Open Access 971 Original study Environmental effects and repeatability estimates for sperm production and semen quality of Holstein bulls Ismaïl Boujenane and Khouloud Boussaq Department of Animal Production
Open Access 971 Original study Environmental effects and repeatability estimates for sperm production and semen quality of Holstein bulls Ismaïl Boujenane and Khouloud Boussaq Department of Animal Production
Preservation of Liquid Boar Semen: Effect of Genotype, Boar and Sperm Parameters on Motility and Acrosome Integrity
 VETERINARY RESEARCH INTERNATIONAL Journal homepage: www.jakraya.com/journal/vri ORIGINAL ARTICLE Preservation of Liquid Boar Semen: Effect of Genotype, Boar and Sperm Parameters on Motility and Acrosome
VETERINARY RESEARCH INTERNATIONAL Journal homepage: www.jakraya.com/journal/vri ORIGINAL ARTICLE Preservation of Liquid Boar Semen: Effect of Genotype, Boar and Sperm Parameters on Motility and Acrosome
Male Reproductive Structures I. Overview A. Main functions: 1. Produce a haploid male gamete (sperm) 2. Deposit sperm in the female so fertilization
 Male Reproductive Structures I. Overview A. Main functions: 1. Produce a haploid male gamete (sperm) 2. Deposit sperm in the female so fertilization may occur! A. Scrotum 1. Muscular pouch that holds the
Male Reproductive Structures I. Overview A. Main functions: 1. Produce a haploid male gamete (sperm) 2. Deposit sperm in the female so fertilization may occur! A. Scrotum 1. Muscular pouch that holds the
IN normal male fowls, four developmental stages of spermatogenetic activity
 Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
REPRODUCCIÓN. La idea fija. Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
Chapter 28: REPRODUCTIVE SYSTEM: MALE
 Chapter 28: REPRODUCTIVE SYSTEM: MALE I. FUNCTIONAL ANATOMY (Fig. 28.1) A. Testes: glands which produce male gametes, as well as glands producing testosterone 2. Seminiferous tubules (Fig.28.3; 28.5) a.
Chapter 28: REPRODUCTIVE SYSTEM: MALE I. FUNCTIONAL ANATOMY (Fig. 28.1) A. Testes: glands which produce male gametes, as well as glands producing testosterone 2. Seminiferous tubules (Fig.28.3; 28.5) a.
18 Urinary system. 19 Male reproductive system. Female reproductive system. Blok 11: Genital and Urinary Tract Diseases
 Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Initiation and Development of Semen Production in the Guinea Pig
 Initiation and Development of Semen Production in the Guinea Pig M. FREUND, Ph.D. SEXUAL DEVELOPMENT and maturation of male animals may be studied by the use of a number of technics, such as the sacrifice
Initiation and Development of Semen Production in the Guinea Pig M. FREUND, Ph.D. SEXUAL DEVELOPMENT and maturation of male animals may be studied by the use of a number of technics, such as the sacrifice
Bull BreedingSoundness Evaluation
 Bull BreedingSoundness Evaluation Scott Norman (BVSc, PhD, DipACT, GCEd) Registered Specialist in Veterinary Reproduction Associate Professor in Theriogenology, School of Animal and Veterinary Sciences
Bull BreedingSoundness Evaluation Scott Norman (BVSc, PhD, DipACT, GCEd) Registered Specialist in Veterinary Reproduction Associate Professor in Theriogenology, School of Animal and Veterinary Sciences
Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature
 REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
EFFECT OF AGE ON TESTICULAR GROWTH AND CONSISTENCY OF HOLSTEIN AND ANGUS BULLS 1
 EFFECT OF AGE ON TESTCULAR GROWTH AND CONSSTENCY OF HOLSTEN AND ANGUS BULLS 1 G. H. Coulter 2, L. L. Larson 3 and R. H. Foote Cornell University 4, thaca, New York 14853 SUARY A total of 5,99 scrotal circumference
EFFECT OF AGE ON TESTCULAR GROWTH AND CONSSTENCY OF HOLSTEN AND ANGUS BULLS 1 G. H. Coulter 2, L. L. Larson 3 and R. H. Foote Cornell University 4, thaca, New York 14853 SUARY A total of 5,99 scrotal circumference
- production of two types of gametes -- fused at fertilization to form zygote
 Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
IB 140 Midterm #1 PRACTICE EXAM (lecture topics 1-5)
 IB 140 Midterm #1 PRACTICE EXAM (lecture topics 1-5) For all the questions on this exam, the correct answer is the single best answer that is available in the answer key. 1) Which of the following is NOT
IB 140 Midterm #1 PRACTICE EXAM (lecture topics 1-5) For all the questions on this exam, the correct answer is the single best answer that is available in the answer key. 1) Which of the following is NOT
Male Reproductive System. Anatomy
 Male Reproductive System Medical Terminology Chapter Seven HIT # 141 Anatomy Testis or testicle = main male sex organs, paired, oval shaped, enclosed in a sac called the scrotum. Seminiferous tubules =
Male Reproductive System Medical Terminology Chapter Seven HIT # 141 Anatomy Testis or testicle = main male sex organs, paired, oval shaped, enclosed in a sac called the scrotum. Seminiferous tubules =
The Reproductive System. Presenter: Dr. Jim Hurrell
 The Reproductive System Presenter: Dr. Jim Hurrell A Warm Welcome from My Faculty TEAM and Me!!! 2 The Pledge of Allegiance 3 Veterinary Technician/NURSE Oath 4 5 Come Hang Out with Us in Our Awesome Facebook
The Reproductive System Presenter: Dr. Jim Hurrell A Warm Welcome from My Faculty TEAM and Me!!! 2 The Pledge of Allegiance 3 Veterinary Technician/NURSE Oath 4 5 Come Hang Out with Us in Our Awesome Facebook
Microscope Requirements
 SEMEN EVALUATION EQUIPMENT Microscope Requirements Good quality lenses Phase-contrast preferred for % progressive motility evaluations Objectives 10X, 20X*, 40X*, 100X, minimum Heated stage preferred *Preferably
SEMEN EVALUATION EQUIPMENT Microscope Requirements Good quality lenses Phase-contrast preferred for % progressive motility evaluations Objectives 10X, 20X*, 40X*, 100X, minimum Heated stage preferred *Preferably
Study Guide Answer Key Reproductive System
 Biology 12 Human Biology Textbook: BC Biology 12 Study Guide Answer Key Reproductive System 1. Distinguish between a gamete and a gonad using specific examples from the male and female systems. Gonads
Biology 12 Human Biology Textbook: BC Biology 12 Study Guide Answer Key Reproductive System 1. Distinguish between a gamete and a gonad using specific examples from the male and female systems. Gonads
Animal Science 434. Sperm Head. Sperm From Different Species. Sperm Structure. Epididymis, Ejaculation and Semen. Head Acrosome Neck Middle Piece
 Sperm Structure Head Acrosome Neck Middle Piece Animal Science 434 Annulus Principal Piece Epididymis, Ejaculation and Semen End Piece Sperm From Different Species Sperm Head (Equatorial Segment) Nucleus
Sperm Structure Head Acrosome Neck Middle Piece Animal Science 434 Annulus Principal Piece Epididymis, Ejaculation and Semen End Piece Sperm From Different Species Sperm Head (Equatorial Segment) Nucleus
I nfluence of Semen on the Motility of the Uterus in the Guinea Pig
 I nfluence of Semen on the Motility of the Uterus in the Guinea Pig In-Vitro Studies M. FREUND, PH.D., AND ALBERT M. LEFKOVITS, A.B. AN UNANSWERED QUESTION lo on the physiology of reproduction is: "Are
I nfluence of Semen on the Motility of the Uterus in the Guinea Pig In-Vitro Studies M. FREUND, PH.D., AND ALBERT M. LEFKOVITS, A.B. AN UNANSWERED QUESTION lo on the physiology of reproduction is: "Are
relatively unpredictable environmental factors. PATTERNS OF CHANGE IN THE REPRODUCTIVE ORGANS OF THE MALE POCKET GOPHER, GEOMYS PINETIS
 PATTERNS OF CHANGE IN THE REPRODUCTIVE ORGANS OF THE MALE POCKET GOPHER, GEOMYS PINETIS KATHERINE CARTER EWEL Department of Zoology, University offlorida, Gainesville, Florida {Received 6th April 1971,
PATTERNS OF CHANGE IN THE REPRODUCTIVE ORGANS OF THE MALE POCKET GOPHER, GEOMYS PINETIS KATHERINE CARTER EWEL Department of Zoology, University offlorida, Gainesville, Florida {Received 6th April 1971,
Outline. Male Reproductive System Testes and Sperm Hormonal Regulation
 Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
VETERINARY CONSIDERATIONS MALE BREEDING SOUNDNESS EXAMS AND
 VETERINARY CONSIDERATIONS MALE BREEDING SOUNDNESS EXAMS AND VENEREAL DISEASES IN BULLS Bret McNabb, DVM, MPVM, DACT University of California, Davis School of Veterinary Medicine Physical Examination A
VETERINARY CONSIDERATIONS MALE BREEDING SOUNDNESS EXAMS AND VENEREAL DISEASES IN BULLS Bret McNabb, DVM, MPVM, DACT University of California, Davis School of Veterinary Medicine Physical Examination A
Effect of dietary selenium and vitamin E on spermatogenic development in boars 1,2
 Effect of dietary selenium and vitamin E on spermatogenic development in boars 1,2 J. Marin-Guzman 3, D. C. Mahan 4, and J. L. Pate 5 The Ohio State University and The Ohio Agricultural Research and Development
Effect of dietary selenium and vitamin E on spermatogenic development in boars 1,2 J. Marin-Guzman 3, D. C. Mahan 4, and J. L. Pate 5 The Ohio State University and The Ohio Agricultural Research and Development
ADVERSE EFFECTS OF VASECTOMY: SPERM GRANULOMA OF EPIDIDYMIDES V. P. DIXIT
 ADVERSE EFFECTS OF VASECTOMY: SPERM GRANULOMA OF EPIDIDYMIDES V. P. DIXIT Reproduct ion Physiology Section, Department of Zoology, University of Rajasthan, Jaipur-302004 Summary: Rats and mice were vasectomized
ADVERSE EFFECTS OF VASECTOMY: SPERM GRANULOMA OF EPIDIDYMIDES V. P. DIXIT Reproduct ion Physiology Section, Department of Zoology, University of Rajasthan, Jaipur-302004 Summary: Rats and mice were vasectomized
Determination of Daily Sperm Production (DSP) in Rabbit (Oryctolagus cuniculus) Bucks using Testicular Parameters
 Greener Journal of Agricultural Sciences ISSN: 2276-7770; ICV: 6.15 Vol. 5 (4), pp. 141-148, July 2015 Copyright 2017, the copyright of this article is retained by the author(s) http://gjournals.org/gjas
Greener Journal of Agricultural Sciences ISSN: 2276-7770; ICV: 6.15 Vol. 5 (4), pp. 141-148, July 2015 Copyright 2017, the copyright of this article is retained by the author(s) http://gjournals.org/gjas
Male Reproductive System
 Male Reproductive System The male reproductive system consists of a number of sex organs that are part of the reproductive process. The following sections describe the function of each part of the male
Male Reproductive System The male reproductive system consists of a number of sex organs that are part of the reproductive process. The following sections describe the function of each part of the male
Solving the Heat Stress Problem
 Breeding Herd Education Series 2012-2013 Timely, relevant & convenient learning Thank you for participating in SowBridge 2012-13. To start this presentation, advance one slide by pressing enter or the
Breeding Herd Education Series 2012-2013 Timely, relevant & convenient learning Thank you for participating in SowBridge 2012-13. To start this presentation, advance one slide by pressing enter or the
Sperm storage capacity and total protein concentration in the testes of bucks in the native tropical environment
 Vol. 9(7), pp. 154-158, July 2017 DOI: 10.5897/JVMAH2017.0582 Article Number: 947F3B164974 ISSN 2141-2529 Copyright 2017 Author(s) retain the copyright of this article http://www.academicjournals.org/jvmah
Vol. 9(7), pp. 154-158, July 2017 DOI: 10.5897/JVMAH2017.0582 Article Number: 947F3B164974 ISSN 2141-2529 Copyright 2017 Author(s) retain the copyright of this article http://www.academicjournals.org/jvmah
MALE REPRODUCTIVE SYSTEM
 1 MALE REPRODUCTIVE SYSTEM SCPA 602 Anatomical Basis for Pathological Study Updated: 20.09.2018 Lect. Nisamanee Charoenchon, PhD nisamanee.cha@mahidol.ac.th Department of Pathobiology, Mahidol University
1 MALE REPRODUCTIVE SYSTEM SCPA 602 Anatomical Basis for Pathological Study Updated: 20.09.2018 Lect. Nisamanee Charoenchon, PhD nisamanee.cha@mahidol.ac.th Department of Pathobiology, Mahidol University
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Proper steps for bull semen dilution and freezing. IMV Technologies France
 Proper steps for bull semen dilution and freezing IMV Technologies France Introduction Since Polge reported the first successful cryopreservation of spermatozoa in 1949, spermatozoa from many mammalian
Proper steps for bull semen dilution and freezing IMV Technologies France Introduction Since Polge reported the first successful cryopreservation of spermatozoa in 1949, spermatozoa from many mammalian
