Understanding spermatogenesis is central to probing
|
|
|
- Tabitha Anthony
- 5 years ago
- Views:
Transcription
1 Journal of Andrology, Vol. 29, No. 5, September/October 2008 Copyright E American Society of Andrology The Cycle of the Seminiferous Epithelium in Humans: A Need to Revisit? Review RUPERT P. AMANN From the Animal Reproduction and Biotechnology Laboratory, Colorado State University, Fort Collins, Colorado. ABSTRACT: Understanding the dynamics of spermatogenesis is central to clinical andrology or to probing environmental effects on human testes. This review considers what is known about renewal and proliferation of spermatogonia, how germ cells are organized in cellular associations constituting the cycle of the seminiferous epithelium, relative frequencies of cellular associations, durations of the cycle of the seminiferous epithelium and spermatogenesis, and measurement of daily sperm production. Daily sperm production (DSP) per testis tends to decline with advancing age. Regardless of age, there is substantial loss of potential sperm from degeneration of spermatocytes, but not spermatids. DSP per gram testis parenchyma or DSP per testis cannot be predicted on the basis of testis size or age of a man. The review shows why our 1960s data base is neither robust nor precise and suggests how deficiencies might be rectified. New cellular associations should be defined, with none representing.15% of the cycle of the seminiferous epithelium. Then determine when A pale -spermatogonia become committed to proliferate or how many mitotic divisions occur thereafter. Restudy the duration of spermatogenesis because the accepted value might be in error by,6 days. Restudying human spermatogenesis will benefit clinicians, toxicologists, and epidemiologists probing testis function by direct evaluations or indirectly via evaluations of quantity and quality of sperm ejaculated. It also will benefit scientists interested in renewal and proliferation of spermatogonia, or a spermatogonium as a prototype stem cell. J Androl 2008;29: Understanding spermatogenesis is central to probing interrelations between environmental factors and testis function or in deciding whether a deficiency in number or quality of sperm ejaculated, or both, contributes to a couple s difficulty in initiating pregnancy. For.110 years, histologists have described spermatogenesis in terms of the progression of changes in individual germ cells and their grouping in cellular associations or stages. Herein, the former term is used, because it is more descriptive. For those competent in French or German, early papers still provide fascinating reading (see citations in Roosen-Runge, 1962; Clermont, 1967). Modern study of human spermatogenesis was pioneered by Rosen-Runge (1952, 1956), Clermont and Leblond (1955), Clermont (1963), and Heller and Clermont (1963, 1964). From the perspective of a clinician or subfertile patient couple, current descriptions of cellular associations, cycle of the seminiferous epithelium, and duration of spermatogenesis might be adequate. However, from the perspective of a scientist interested in renewal and proliferation or differentiation of spermatogonia or of a Correspondence to: Rupert P. Amann, 909 Centre Ave, No. 123, Fort Collins, CO ( rpalra62@comcast.net). Received for publication December 1, 2007; accepted for publication May 18, DOI: /jandrol spermatogonium as a prototype stem cell, current information is imprecise. Perhaps most important, epidemiologists increasingly are probing possible linkages between fetal or adult exposure to a putative or known chemical and aberrations in testis function and seminal characteristics. Such endeavors require robust information on the kinetics and biology of spermatogenesis. Hence, it is appropriate to ask whether current knowledge is adequate. This review is restricted to spermatogenesis in normal adults. Prenatal differentiation of primordial germ cells to stem cells and their normal progressive development into gonocytes, undifferentiated A-spermatogonia, and, finally, differentiated A-spermatogonia is a separate subject. However, both normal and aberrant transformation of primordial germ cells can occur. Clark (2007) provides an excellent introduction to the link between germ cells and testicular cancer. Rajpert-De Meyts (2006), Olesen et al (2007), and Veeramachaneni (2008) summarize the linkage among environmental pollutants, changes in spermatogenesis, and testicular cancer. With this paper, I have 4 goals: 1) provide a brief overview of spermatogenesis, 2) review what is known about the cycle of the seminiferous epithelium, 3) review measurement of daily sperm production and data on quantitative features of spermatogenesis, and 4) discuss what information is needed and why. This required a 469
2 470 Journal of Andrology N September ÙOctober 2008 careful review of primary papers and the consideration of deficiencies in certain papers. Overview of Spermatogenesis Spermatogenesis occurs in the seminiferous epithelium, which consists of Sertoli cells (somatic cells) and several types of germ cells (Roosen-Runge, 1952; Clermont, 1963, 1966a,b, 1972; Heller and Clermont, 1964; Courot et al, 1970; de Kretser and Kerr, 1994). Roosen-Runge (1962) reviews the derivation of terms used to describe spermatogenesis. In humans, the germ cells are best termed (Roosen-Runge and Barlow, 1953; Clermont, 1972; Aponte et al, 2005; Ehmcke and Schlatt, 2006; Ehmcke et al, 2006): progenitor A dark -spermatogonia; progenitor A pale -spermatogonia; committed A pale -spermatogonia; B-spermatogonia; preleptotene, leptotene, zygotene, pachytene, and diplotene spermatocytes; secondary spermatocytes; spermatids; and spermatozoa. (Sometimes preleptotene spermatocytes were termed young primary spermatocytes. ) The progenitor A dark -spermatogonia apparently are stem or reserve spermatogonia on the basis of location and infrequent division, but with an unknown stimulus, they can produce progenitor A pale -spermatogonia. Progenitor and committed A pale -spermatogonia look alike, but the latter divide to produce B-spermatogonia. Spermatogenesis is the totality of 4 sequential processes (McLachlan et al, 2002; Schlegel and Hardy, 2002; Yoshida et al, 2007): 1. Spermatogonial proliferation and differentiation involve renewal of progenitor A dark - and A pale -spermatogonia, division of committed A pale -spermatogonia to form B-spermatogonia, and their division to form preleptotene spermatocytes. 2. Meiosis of spermatocytes includes the last synthesis of DNA in preleptotene spermatocytes and 2 meiotic divisions to form spermatids. 3. Spermiogenesis is transformation of a spherical spermatid to a spermlike mature spermatid. 4. Spermiation is breaking the structures and bonds anchoring a mature spermatid to a Sertoli cell, so the spermatozoon is released into the tubule lumen and can be washed out of the seminiferous tubule. These processes are interdependent, although each has distinct demands for regulatory molecules from nearby Sertoli, peritubular, and Leydig cells, as well as the vascular network. In humans, the interval between commitment of an A pale -spermatogonium to proliferate and spermiation of resulting spermatozoa averages 74 days (Heller and Clermont, 1964). Regardless of species, there are overarching features of spermatogenesis: 1. For adults of a species, germ cell development follows a rigid timescale, with minor biological variation. 2. At small circumscribed locations within each seminiferous tubule and at relatively infrequent intervals for that location (7 16 days, depending on species), spermatogonia (A pale -spermatogonia in humans) simultaneously become committed and enter active spermatogenesis. This leads to clusters of developing cells evolving synchronously from a spermatogonium to many spermatozoa. 3. Germ cells developing from a committed spermatogonium are connected by intercellular bridges, which provide cytoplasmic continuity among members of a cohort of cells, except where cell death eliminates a connecting cell. 4. Because the duration of spermatogenesis is much longer than the interval between commitment of spermatogonia at a specific site in a tubule (,74 days vs,16 days in humans), cohorts of germ cells are layered, with the youngest near the basement membrane. These layers sometimes are termed generations. Intermingling of cohorts of germ cells is programmed, because Sertoli cells systematically move maturing spermatids toward the basal area and, several days later, back toward the tubule lumen. 5. Collectively, the 4 5 cohorts of germ cells theoretically developing concurrently at a circumscribed location in a tubule are termed a cellular association; 6 are used for humans. 6. Because spermatogenesis is a dynamic process and members of each cohort are developing synchronously (separated in humans by,16 days), appearance of the cellular association at a given point in a tubule is changing repetitively, much like frames in a movie with a loop format. 7. Death of germ cells reduces the potential number of spermatozoa and is a feature of all testes. 8. Collectively, from multiple points within the seminiferous tubules of a typical human testis, sperm are released each and every minute. Spermatogenesis in humans is different from the process in bulls, mice, rabbits, rats, stallions, or other common mammals. Obvious differences include the 3- dimensional (3D) organization of the seminiferous epithelium and low number of sperm produced daily per gram of testis. The pattern of spermatogonial renewal and proliferation also is different. The difference in sperm
3 Amann N Human Spermatogenesis 471 Figure 1. The cycle of the seminiferous epithelium. Examination of cross sections of human testes reveal 6 cellular associations (Clermont, 1963; designated I VI on the x axis). The types of germ cells that should be present in each cellular association are illustrated in the 6 columns (upward from basement membrane to tubule lumen) as generations of progressively more differentiated cells. Relative duration (%) of each cellular association, on the basis of frequency of occurrence in specially prepared biopsy specimens from 4 men, also is shown; coefficients of variation ranged from 18% for cellular associations I and V to 58% for association III. Drawing based on Clermont (1963), with detail on spermatid development from Heller and Clermont (1964). Note that Heller et al (1969:figure 2) depict formation of A pale - and B-spermatogonia in cellular association V and preleptotene spermatocytes in cellular association III. Reinterpretations (Ehmcke and Schlatt, 2006; Ehmcke et al, 2006) of Clermont s (1966a,b) data led to conclusions that A dark -spermatogonia indeed are stem cells, to allow repopulation of a testis, and that A pale -spermatogonia might undergo an unreported division (designated by?) near the start of cell association II. If it is established that A pale - spermatogonia produced in cell association II (designated?) are the first cells committed to differentiate, as suggested by Ehmcke and Schlatt (2006), then the duration of spermatogenesis would be close to 4.2 cycles or,68 days. production per gram of testis parenchyma is important to clinicians and clinical epidemiologists. Variation among human testes is much greater than that among individual mice, rabbits, or rats used by most experimentalists (Berndtson, 2008). Readers should consult excellent reviews on human spermatogenesis, or comparative aspects of spermatogenesis, such as Ortavant (1959), Clermont (1963, 1966b, 1972), Heller and Clermont (1964), Courot et al (1970), Holstein and Roosen-Runge (1981), Johnson et al (1992, 2000), de Kretser and Kerr (1994), de Rooij and Russell (2000), Schlegel and Hardy (2002), and Ehmcke and Schlatt (2006). Unraveling the puzzle of spermatogonial renewal, differentiation, and proliferation in humans requires the distinction of subtle changes in spermatogonia and spermatids or other germ cells and also precise information on cellular associations in which a given cell type is found, how cellular associations transition in the cycle of the seminiferous epithelium, frequency of occurrence (%) or relative duration of each cellular association, and duration (days) of 1 cycle of the seminiferous epithelium. This same information is necessary to calculate a time divisor to use when quantifying sperm production. Most knowledge about the cycle of the seminiferous epithelium is based on testis biopsies from groups of 3, 4, 8, or 11 prisoners who volunteered for research and were stated to be healthy but were otherwise not described. By today s standards, a foundation based on 3 or 4 individuals would be inadequate. What Is Known About the Cycle of the Seminiferous Epithelium? Germ cells develop in consistent groupings termed cellular associations. In humans, 6 cellular associations are used (Figure 1), designated with Roman numerals. Two to 4 cellular associations usually are evident in an essentially round cross section through a human seminiferous tubule (Roosen-Runge, 1952; Clermont, 1963). Cellular associations can be defined in several ways, but
4 472 Journal of Andrology N September ÙOctober 2008 inevitably, this requires recognition of specific cell types. However, appearance of germ cells is dependent on methods of fixation, embedment, staining, and visualization. Therefore, I include procedures with descriptions of what is seen. Newer methods reveal distinguishing features better than those used 40 years ago. Descriptions of germ cells in stained slides are in general agreement (Roosen-Runge and Barlow, 1953; Roosen-Runge, 1962; Clermont, 1963, 1966a,b; Heller and Clermont, 1964; Rowley and Heller, 1971; Holstein and Roosen-Runge, 1981; Hocherau de Reviers et al, 1984). However, the pattern for proliferation of spermatogonia is uncertain, and there are multiple schemes for classification of human spermatids. Because these elements are used to define cellular associations, they are considered first. Pattern of Spermatogonial Proliferation Clermont (1966a) described A dark -, A pale -, and B-spermatogonia in sections from biopsies fixed in Zenker-formal and stained with hematoxylin and eosin (H&E) but commented (Clermont 1966b) that some did not look like either A- or B-spermatogonia. He mapped and counted spermatogonial nuclei and mitotic plates in different cellular associations (3 5 biopsies, depending on cell type). There was approximately 1 A pale -spermatogonium per A dark -spermatogonium, and A pale -spermatogonia usually were in pairs located near the basement membrane (Clermont 1966a). Calculated ratios of A pale - spermatogonia:b-spermatogonia:preleptotene spermatocytes were 1:2:4 (Clermont 1966b). The pattern for division of A pale -spermatogonia is uncertain. Apparently, some A pale -spermatogonia proliferate to produce more A pale -spermatogonia, whereas others produce B-spermatogonia. Under the model favored by Clermont (1966b), and most frequently cited, each member of a pair of committed A pale - spermatogonia undergoes only 1 division, to provide 2 B-spermatogonia (Clermont, 1967, 1972; Ehmcke et al, 2006). An alternative model was proposed and favored by Ehmcke and Schlatt (2006); 1 member of a pair of committed A pale -spermatogonia divides to provide a new pair of committed A pale -spermatogonia, whereas the other member of the original pair undergoes a division to provide 2 daughter A pale -spermatogonia (? in Figure 1) which, in turn, divide to produce B-spermatogonia. With either model, 8 preleptotene spermatocytes are produced per original pair of A pale -spermatogonia. When B-spermatogonia are formed and divide is also uncertain. Clermont (1963, 1966b) concluded that they were formed at the transition between cellular associations VI and I, were obvious in cellular associations I and II, and divided only when forming preleptotene spermatocytes in late association II or early association III. This is depicted in Figure 1 herein. However, Clermont (1966a,b) was inconsistent because in figures 3 and 5 of these papers, B-spermatogonia were depicted only in cellular associations I and II and occasionally early III, whereas figure 10 in both papers clearly shows B-spermatogonia throughout cellular association VI and none in association III. Heller et al (1969) and Rowley and Heller (1971) also concluded that B-spermatogonia were formed sometime during cellular association V and remained through cellular association II. The above interpretations of spermatogonial proliferation in humans are different from that of Roosen- Runge (1952, 1956, 1962) or Roosen-Runge and Barlow (1953). On the basis of nuclear size or length of the metaphase plate, they concluded that spermatogonia underwent 7 divisions, the last yielding preleptotene spermatocytes. Although a range in nuclear diameter was reported, they were unable to distinguish A- vs B- spermatogonia. Despite extensive counts of germ cells and Sertoli cells in 100 biopsies from normal men, Rowley and Heller (1971) expressed uncertainty concerning when mitotic divisions occurred and the pattern of spermatogonial proliferation. Although modeling spermatogonial proliferation was not their goal, Johnson et al (1987) estimated numbers of germ cells in 30 testes (testes fixed by perfusion with glutaraldehyde,15 hours after the sudden death of men in good health; toluidine blue stained Epon sections) from measures of volume density and nuclear diameter. On the basis of Clermont (1966b), Johnson et al (1987) assumed that B-spermatogonia were present throughout cellular associations VI, I, and II and divided only once to produce 2 preleptotene spermatocytes. They found,11 preleptotene/leptotene spermatocytes per B-spermatogonium, rather than the expected 2:1 ratio. Johnson et al (1987) eliminated the problem by rationalization considered unsatisfactory by this reviewer. Classification of Spermatids Pioneering periodic acid Schiff (PAS; Orths fixative was best) staining of testis tissue, Clermont and Leblond (1955) described how human spermatids develop through a series of 12 steps (Figure 2, column A). The first 7 steps are characterized by subtle changes in acrosome morphology, in that the nucleus remains spherical. During steps 8 12, the nucleus gradually changes shape and shrinks as nucleoprotein condenses; changes in the acrosome continue. However, Clermont (1963) abandoned PAS stain for human testes in favor of Zenker-formal fixation and H&E staining. Zenker-formal preserved details of nuclei in spermatogonia and spermatocytes better than Orths fixative, but gave inconsistent preservation of early acrosomal structures revealed by PAS stain. Use of H&E-stained sections (Zenker-formal fixation) forced a different scheme to describe changes in spermatids because the acrosome provided little information. On the
5 Amann N Human Spermatogenesis 473 basis primarily of nuclear characteristics (shape, size, intensity of staining), Clermont (1963) described 4 types of spermatids (S a, S b, S c, and S d ) in H&E sections. Subsequently, this was expanded to 6 types (S a,s b1,s b2, S c,s d1,ands d2 ) via better distinction of nuclear staining/ elongation and sequestration of cytoplasm (Figure 2, column B; Heller and Clermont, 1964; Clemont, 1966b) or even 7 types (distinguishing S a1 and S a2 spermatids; Heller et al, 1969). The 6-type classification of Heller and Clermont (1964) is most commonly used. More detail within spermatids can be resolved when plastic sections are viewed by a light or electron microscope (Figure 2, columns C E). With light microscopy, bright-field, phase contrast, or differential interference contrast optics each has advantages and limitations (Johnson et al, 1981, 1992; Johnson 1994). With an electron microscope, de Kretser (1969) was able to link changes in fine structure of spermatids with Clermont s (1966b) descriptions for spermatids in H&Estained sections; 6 steps were described (column C in Figure 2). Holstein (1976) defined and illustrated 8 steps in spermatid development. These illustrations were retained, and finer discrimination of spermatids with spherical nuclei was added, in the superbly illustrated Holstein and Roosen-Runge (1981; some are reproduced in column D in Figure 2). Examination of Figure 2 reveals that the steps in spermatid development described on the basis of PAS or H&E staining (columns A and B, respectively) are not uniformly distributed over the 24 days (left y axis) required for spermiogenesis. Rather, the distinguishing changes in the acrosomic system are clustered on days 6 10 (column A, steps 4 8). Obvious nuclear changes are clustered on days 9 16 and around day 19. Both de Kretser (1969) and Johnson et al (1992) intentionally fit their observations (columns C and E) to the 6-step classification of Heller and Clermont (1964). In contrast, Holstein (1976) and Holstein and Roosen-Runge (1981) developed their own classification scheme for spermatids and described 8 major steps plus 5 subtle substeps (column D). Looking ahead, use of glutaraldehyde fixation, plastic sections, and toluidine blue stain (column E) could enable discernment of subtle changes in both nuclear and acrosome development with a light microscope, not detectable with H&E staining of paraffin sections (column B). Cellular Associations A classification scheme of cellular associations is essential to understanding spermatogenesis because it allows description and quantitation of the seminiferous epithelium and reveals the cycle of the seminiferous epithelium. Any scheme is arbitrary (Roosen-Runge, 1962). Descriptions and illustrations for cellular associations in a human testis presented by Clermont (1963) are widely cited, although enhancement via consideration of 6 rather than 4 types of spermatids (Heller and Clermont, 1964) often is overlooked. Defining descriptions of cellular associations were based on H&E-stained paraffin sections prepared from Zenker-formal fixed tissue (Clermont 1963; Heller and Clermont, 1964). Spermatids were described from nuclear characteristics as S a,s b1,s b2,s c,s d1, and S d2. A key to discriminating cellular associations is that cellular associations I and II contain 2 generations of spermatids (S a and S d1 or S d2 ) whereas cellular associations III V contain 2 generations of primary spermatocytes (preleptotene, leptotene, or zygotene plus pachytene or diplotene; Figure 1). Chromosomal characteristics during meiosis of spermatocytes, location of spermatids, and shape of spermatid nuclei also help. Cellular association VI is defined by presence of metaphase plates of the first or second meiotic divisions, secondary spermatocytes, or both. Examination of Figure 2 using the right y axis provides understanding of the uniformity of spermatids within a cellular association (association I) or variety of subtly different forms (associations III and IV). The start of cellular association I is evidenced by newly formed spermatids, each with a spherical nucleus. Presence of B-spermatogonia also adds discrimination for associations I and II, although sometimes they are not seen in 1-mm plastic sections (Johnson et al, 1987). A dark - and A pale -spermatogonia usually are present in all cellular associations (Clermont, 1963). As in all aspects of biology, there are grey areas, wherein some groups of cells are transitioning to fit the next description. Furthermore, in human testes, there are atypical cellular associations because of missing spermatogonia, spermatocytes, or spermatids, and also from intermingling of associations (Roosen-Runge, 1952; Clermont, 1963; de Kretser, 1969; Johnson et al, 1987). It is likely that the chaotic nature of the human seminiferous epithelium has been overemphasized, in that,0.1% of cellular associations are atypical in men with high sperm production (Schulze, 1982; Chaturvedi and Johnson, 1993). However, men with low daily sperm production per gram often are missing a generation or generations of germ cells (Johnson et al, 1992). With the use of perfusion fixation and staining of plastic sections with toluidine blue, it is likely that cellular associations could be discerned routinely in human seminiferous tubules. This is evidenced in Johnson et al (1992:figure 1; note that they allocated uniform intervals to the 6 cellular associations), although they followed Clermont s classification. Also phase contrast or Nomarski differential interference contrast optics would allow resolution of subtle differences in cell morphology not detectable with the
6 474 Journal of Andrology N September ÙOctober 2008 Figure 2. Relationship of steps in human spermiogenesis as presented in: (A) Clermont and Leblond (1955:238, with permissions from Wiley- Liss); (B) Heller and Clermont (1964:548, Elsevier); (C) de Kretser (1969:479, Springer-Verlag); (D) Holstein and Roosen-Runge (1981:figures 35 and 41, Grosse Verlag); and (E) Johnson et al (1992:1093, Society for the Study of Reproduction). Array of their illustrations along the timeline (left y axis) is approximate and was based on interpretation of the publication. Duration of spermiogenesis based on Heller and Clermont (1964). Relative durations of cellular associations (right y axis) are from Clermont (1963). Note that Heller et al (1969:figure 2;
7 Amann N Human Spermatogenesis 475 bright-field optics available around Images of each of Clermont s 6 cellular associations, as viewed by Nomarski optics, are in Johnson (1994). Descriptions of the 6 cellular associations by Johnson et al (1992, 2001) are very helpful, but no attempt was made to expand and redefine the classification scheme described by Heller and Clermont (1964). Relative Duration of Cellular Associations The relative duration of each of the 6 cellular associations (x axis in Figure 1, right y axis in Figure 2) is calculated from frequency of occurrence of a given cellular association in cross sections through seminiferous tubules. Because several cellular associations are found in a cross section of human seminiferous tubule, Clermont (1963) traced limits of each cellular association within a cross section and cut out the tracings. Total weight of tracings for a given cellular association was expressed relative to total weight for all tracings for that testis. Relative durations of the 6 cellular associations were 30%, 20%, 6%, 8%, 31%, and 5%, respectively (Clermont, 1963). However, these means were based on only tubule cross sections for 1 biopsy from each of 4 individuals. With Clermont s (1963) data, it was calculated that 95% confidence intervals (CIs) for the relative frequencies of cellular associations I VI encompassed 21% 38%, 13% 26%, 0.5% 12%, 3% 12%, 22% 40%, and 3% 7%, respectively. Data on relative frequency of cellular association reported by Heller et al (1969) or Rowley and Heller (1971) usually are overlooked. Heller et al (1969) examined testis biopsies from 44 healthy men (normal semen and hormonal profiles). They identified and counted germ cells in 30 randomly selected cellular associations per biopsy, and calculated relative occurrence of each cellular association. That data set was enlarged to 100 biopsies in Rowley and Heller (1971), who reported mean frequencies of 23%, 17%, 17%, 13%, 23%, and 7% for cellular associations I VI, respectively. These values seem different from Clermont s (1963) values of 30%, 20%, 6%, 8%, 31%, and 5%, respectively, but except for associations III and IV, they fall just within the 95% CIs I calculated for Clermont s values. These papers do not mention checking for within-sample repeatability of observations. For these reasons, the relative durations of cellular associations in the cycle of the human seminiferous epithelium should be considered as imprecise values. Spatial Organization of Cellular Associations The 3D space occupied by a given cellular association is small. Hence, each cross section viewed microscopically contains several cellular associations (Figure 3; Clermont, 1963; Heller and Clermont, 1964; Kerr and de Kretser, 1981; Johnson, 1994; Johnson et al, 1996). This might be because pairs or clusters of progenitor spermatogonia occupy a smaller area along the basement membrane than in rodents or domesticated animals and might provide committed A-spermatogonia less synchronously. Historically, organization of cellular associations along the length of a human seminiferous tubule was considered as without pattern (Roosen-Runge, 1952; Clermont, 1963), and clearly different from the sequential juxtaposition of cellular associations in substantial 3D space found in common mammals (eg, rat; de Kretser and Kerr, 1994). However, Schulz (1982) photographed sections of plastic-embedded human testes stained with toluidine blue and made 2D reconstructions (19 tubules in 3 testes; mm lengths). These micrographs were used to plot Cartesian coordinates for primary spermatocytes (Schulze and Rehder, 1984), relative to the geometric center of a tubule segment, and apply computer modeling (Schulze et al, 1986). They concluded that at a given point in time, development of spermatocytes was progressively more advanced, in a spiral with decreasing radius, down the length of a tubule and that spermatids displayed a similar pattern in a separate spiral. In their opinion, typically there was an orderly sequence of cellular associations, I VI (occasionally with a reversal of order), with an oblique orientation. This was depicted (Schulze and Rehder, 1984) as intertwined helical bands of sequentially organized cellular associations. This concept was challenged by Johnson (1994), who made 3D maps of cellular associations, rather than primary spermatocytes, on the basis of successive 22-mm plastic sections (7 tubules from 6 men; mm lengths). He did not find a complete sequence of 6 contiguous cellular associations along the length of a tubule, although 2 or 3 consecutive abutting cellular associations were found. Further study (Johnson et al, 1996; 8 tubules from 6 men, possibly same testes as earlier paper) involved plotting limits and geometric centers of each cellular association, computerized reconstructions, and hypothesis testing. Actual sequences, random number sequences (same total cellular associations as for actual sequences), and ideal sequences (I VI, repeatedly) were compared with the use of 2 paradigms. For actual sequences, 2 3 consecutive r with H&E stain like column B) depict S a1 -spermatids in cellular association I and S a2 -spermatids in cellular association II but do not describe how to distinguish them. TEM indicates transmission electron microscopy. Illustrations were extracted from the referenced publications, touched-up, and regrouped.
8 476 Journal of Andrology N September ÙOctober 2008 Figure 3. Diagrams of cellular associations in a portion of an actual human seminiferous tubule (A D) or a hypothetical seminiferous tubule from an animal such as bull, mouse, rabbit, or rat (E). In these views, cellular associations are keyed, and dotted lines depict the area occupied by germ cells developing from 1 committed spermatogonium. In surface views, it is evident that in man (A), a cellular association usually occupies a smaller area on the basement membrane than in an animal (E). In humans, typically there are 2 4 cellular associations in an essentially round cross section through a tubule (C), whereas in many common mammals, 1 cellular association per cross section is typical (not shown). A longitudinal section through this human seminiferous tubule (B) reveals how a cross section such as D (solid line in B) might reveal atypical cellular associations, with elements of cellular associations V and VI (left aspect) or II and I (right aspect), viewing from basement membrane to lumen. Hence, in the left aspect, leptotene and pachytene spermatocytes are atypically associated with rather advanced S c -spermatids. In the right aspect, B-spermatogonia are associated with pachytene spermatocytes, S a -spermatids less advanced than expected for cellular association II, and S d1 -spermatids rather than S d2 -spermatids. Compare this situation with cells normally found in cellular associations V, VI, II, and I, as shown in Figure 1. Panels A D are based on a detailed reconstruction shown in Figure 5 of Johnson (1994) but are drawn as surface and sectional views rather than a laid open tubule. cellular associations represented,16% of all cellular associations, but 4 6 consecutively represented only,8% of all cellular associations; the majority of abutting cellular associations were nonsequential. Importantly, incidences of 2 3 or 4 6 sequential cellular associations were not significantly different for actual sequences and random number sequences. Johnson et al (1996) discussed in detail why their results might have differed from those of Schulze and Rehder (1984). Johnson et al (1996) concluded that the arrangement of cellular associations in a human seminiferous tubules at any point in time reflected random occurrences, presumably around puberty. Once initiated at a site, the commitment process apparently is remembered and replicated every,16 days at that site (or driven by an unknown signal). Duration of Spermatogenesis A time frame for human spermatogenesis was established by intratesticular injection of 3 H-thymidine to label DNA during synthesis, last in preleptotene spermatocytes. Progression of those cells or labeled daughter cells was studied in autoradiograms of specially prepared sections from 11 biopsies taken 4 46 days later (Heller and Clermont, 1963, 1964). Figure 4 illustrates the biology underlying this study, and the relationship between 1 cycle of the seminiferous epithelium and the total process of spermatogenesis (requires approximately 4.6 cycles). Heller and Clermont (1963, 1964) concluded that 1 cycle of the seminiferous epithelium required,16 days and the duration of spermatogenesis was,74 days. This classic study has not been replicated. Use of a nonradioactive tracer (eg, Misell et al, 2006) does not eliminate the need for biopsies. The concept of cellular associations assumes that all cells of one type are similar in age and genetically programmed changes. For humans, however, individual cellular associations encompass,1 to,5 days. Hence, during a given cellular association, multiple events occur within each of the 4 5 different types of germ cells as each evolves, or even divides, between the beginning and end of a cellular association. Heller and Clermont (1964) reported that neither norethandrolone (depressing spermatogenesis) nor human chorionic gonadotropin affected the time frame for development of germ cells (1 individual per treatment). Animal data summarized by Courot et al (1970) support the contention that altered hormonal status, imposed or natural in seasonal breeders, does not affect the duration of the cycle of the seminiferous epithelium. With rabbits, imposition of planned ejaculation or sexual rest near the time of 3 H-thymidine injection did not affect the interval until mature spermatids underwent spermiation (Amann, 1972), but emission/ejaculation hasten transit of sperm through the epididymis. However, there is no study in which individual males were ejaculated frequently or sexually rested for.3 mo and then given 3 H-thymidine
9 Amann N Human Spermatogenesis 477 Figure 4. Spermatogenesis in humans. (A) Likely pattern of spermatogonial development (renewal of progenitor Apale-spermatogonia and proliferation of committed Apale-spermatogonia), which is the first subprocess within spermatogenesis. Two types of spermatogonia usually are described (Heller and Clermont, 1964; Clermont 1966a) namely, Adark- and Apale-spermatogonia. Adark-spermatogonia are considered stem cells (Clermont 1966a), constituting a regenerative reserve (left; Aponte et al, 2005). The pattern depicted is based on Clermont s (1966a,b) observations as interpreted by Ehmcke and Schlatt (2006). Apale-spermatogonia renew their population (center) and also form Apale-spermatogonia committed to differentiation (right) and formation of B-spermatogonia. (B) Depiction of the 4.6 cycles of the seminiferous epithelium required for a committed Apale-spermatogonium (designated start in lower left, day 0) to produce a group of spermatozoa completing spermiation,74 days later (designated end in upper right; day 74) and rapidly carried into the efferent ducts by fluid flow. When 3H-thymidine was injected intratesticularly, 1 hour later, the most advanced cells incorporating the label were preleptotene spermatocytes in cellular association III (blue asterisk), although these cells are formed late in cellular association II. As shown, these cells were,24 days or one-third the way through spermatogenesis; less advanced cells synthesizing DNA also were labeled (blue asterisks in B- and Apale-spermatogonia). Subsequent advancement of labeled cells was followed by autoradiography of biopsy specimens (Heller and Clermont, 1964; Heller et al, 1964). There is imprecision associated with the measurements plus slight variation within and among individuals in the duration of spermatogenesis. The 95% confidence interval (CI) was estimated on the basis of data in Heller and Clermont (1964) showing 5% 10% variation in the duration of 1 cycle of the seminiferous epithelium or interval between injection of 3H-thymidine and spermiation. Epididymal transit time was estimated from data on daily sperm production (DSP) and numbers of sperm within regions of the epididymis (Amann and Howards, 1980; Amann, 1981; Johnson and Varner, 1988) because estimates based on appearance of labeled sperm in the epididymis or ejaculated semen have a greater error (Amann, 1972). Efficiency of sperm production (DSP/g) and frequency of emission do not affect the duration of spermatogenesis (see text), but DSP has a substantial effect on the transit time of sperm through the human epididymis (Johnson and Varner, 1988). Color figure available online at to allow tracking development of germ cells while the same ejaculation frequency continued. Nevertheless, the conclusion of Ortavant (1959:43) when spermatogenesis is disturbed, a certain number of [germ] cells degenerate, but those that continue their development until the end [of spermatogenesis] do so with the same speed remains generally correct. In humans, the duration of spermatogenesis probably does not change. Testis descent is completed years before initial commitment of Apale-spermatogonia to form B-spermatogonia. This is in contrast to hamsters, mice, and rats. In these animals, spermatogenesis is initiated shortly after birth and before testes are positioned in the scrotum; the interval from initial
10 478 Journal of Andrology N September ÙOctober 2008 commitment of spermatogonia through formation of preleptotene spermatocytes is accelerated for the first days after birth, and the process then slows to adult timing (van Haaster and de Rooij, 1993). The temporary acceleration of spermatogenesis might be due to elevated temperature in nonscrotal testes. Measurement of Daily Sperm Production Overview Study of success in spermatogenesis demands measurement of 2 general features: quantity of sperm produced and quality of each spermatozoon in a subsample representative of the total population recently produced. Qualitative success of spermatogenesis is largely independent of number of sperm produced. Quality of many sperm can be reduced without a detectable change in number produced (Veeramachaneni et al, 2001, 2007, 2008). Importantly, quality of ejaculated sperm reflects extra-testicular events as well as spermatogenesis. Daily sperm production (DSP) is the number of sperm produced per day by a testis or the 2 testes of an individual (Amann, 1970b). DSP is the quantitative measure for success in spermatogenesis. Number of sperm produced daily per gram of testicular parenchyma (DSP/g), reflects the efficiency of sperm production and is useful in certain comparisons. Prolonged or severe reduction, or both, or failure, in production of committed A pale -spermatogonia is one cause of reduced DSP. The other is an unusually high rate of germ cell death by apoptosis, or other causes, at specific points in spermatogenesis. Either or both problems could detectably reduce DSP per gram testis and overall DSP. With extensive loss of germ cells, both DSP per gram testis and testis weight might diminish. However, even complete elimination of germ cells in 1 tubule (ie, Sertoli cell only tubule) might not noticeably reduce DSP per gram or DSP per testis. There is no evidence that sexual arousal or ejaculation frequency changes testis weight or DSP per gram on the basis of extensive data for bulls, rabbits, and rams (Amann, 1962, 1970b, 1981; Amann et al, 1974; Almquist, 1982). Understanding how DSP might be estimated via noninvasive methods in an epidemiologic study is helped by knowing how DSP is measured. DSP can be estimated on the basis of daily sperm output (DSO), provided certain conditions are met (Amann, 1970b, 1981; Amann and Chapman, in preparation). DSP can be measured by morphometric analysis of fixed tissues (Amann and Almquist, 1962; Kennelly and Foote, 1964; Swierstra, 1966) or by enumeration of specific germ cells in homogenates of testicular parenchyma (Ortavant, 1958; Amann and Almquist, 1961). A time divisor is necessary to convert total number of cells of a certain type present at sampling to number produced per day. The time divisor is calculated from the relative durations (%) of each cellular association, the duration (days) of 1 cycle of the seminiferous epithelium, and knowledge of the cellular associations in which the cells counted are found. For several reasons, uncertainty in the time divisor is greater for humans than for bulls, rabbits, rats, etc. A separate section is devoted to appropriate time divisors for humans. Morphometric Approach The combination of fixative, embedment, and staining can facilitate identification of germ cells or classification of cellular associations. Glutaraldehyde, osmium, Epon, and toluidine blue (Johnson et al, 1981) or Bouins, methacrylate, PAS, and hematoxylin (Zhengwei et al, 1998) are good, but formalin followed by paraffin should be avoided. Perfusion via the testicular artery is preferable to immersion fixation, because fewer artifacts are introduced and shrinkage of tissue is minimal. Morphometric approaches to measure DSP are based on 1 or more small samples of fixed tissue. They are very tedious but allow separate tabulations of B-spermatogonia and several types of primary spermatocytes, several types of spermatids, or both, each within its cellular association. DSP can be calculated from the theoretical yield of sperm from each of several immature germ cell types and compared with the yield of mature spermatids. Principles of analysis and sources of error were detailed in Amann (1970a,b). Johnson et al (1980a, 1981, 1983) applied this approach to quantification of human spermatogenesis. Sampling was systematic and results likely differ little from what might have been obtained had a full dissector method (Gundersen and Jensen, 1985) been used. Zhengwei et al (1998) applied a dissector approach to stereologic analysis of human testis biopsies and estimated numbers of B-spermatogonia, pachytene spermatocytes, and S a or S d spermatids per Sertoli cell nucleus (they cautioned that extrapolations to testis totals would be suspect). Johnson et al (1984c) and Zhengwei et al (1998) both reported,2.5 S a -spermatids per Sertoli cell, whereas Rowley and Heller (1971) reported,1.6. Shrinkage of tissue during processing can profoundly affect the calculated number of germ cells per testis and, hence, the value for DSP (Amann, 1970b). The extent of shrinkage depends on size of the tissue piece, fixative, and embedment process. With immersion-fixed tissue embedded in paraffin, final volume ranges between,0.90 and 0.47 of the fresh value (Amann, 1970a,b; Zhengwei et al, 1990). Shrinkage should be measured for each tissue piece. With perfusion-fixed tissue embedded in plastic, shrinkage might be negligible.
11 Amann N Human Spermatogenesis 479 Johnson et al (1981, 1983) concluded that a correction for tissue shrinkage was not necessary for glutaraldehyde, perfusion-fixed human testis tissue embedded in plastic. They found that means for diameter of spherical spermatid nuclei (data for 10 testes), seminiferous tubule diameter (data for 3 testes), and proportion of interstitial tissue were not significantly different in unfixed and plastic-embedded tissues. These measures were made with better than 2% precision. The issue of tissue shrinkage might be avoided if desired contrasts can be obtained as a ratio of number of germ cells of a stipulated type (eg, S a -spermatid nucleus) per Sertoli cell nucleolus (conspicuous and only 1 per nucleus). This approach does not provide DSP per gram testis or DSP per testis but might detect whether a suspected or imposed agent altered efficiency of sperm production. Indeed, number of spermatids per Sertoli cell has greater sensitivity or power than measurement of DSP per gram (Berndtson, 2008). Rowley and Heller (1971) found that, for 35 normal males, the amongindividual coefficient of variation (CV) for mean number of Sertoli cells per essentially round tubule cross section was 18%, but the CV for S a -spermatids per Sertoli cell was,35%. Variation in number of S a - spermatids per Sertoli cell is greater for year-old men than for year-old men (CVs of 48% and 27%, respectively; calculated from Johnson et al, 1984c). Homogenization Approach Spermatids become extremely resistant to physical destruction (homogenization of unfixed tissue) at a certain point in spermiogenesis (Ortavant, 1958), although condensation of nuclear material is more variable and less complete in human spermatids than in those from other species (Johnson et al, 1980a). Regardless, homogenization-resistant spermatids can be enumerated in an appropriate suspension of unfixed testis with the use of phase contrast microscopy (Amann and Almquist, 1961; Amann and Lambiase, 1969; Amann, 1970b; Amann and Howards, 1980; Johnson et al, 1980a). If glutaraldehyde-fixed tissue is used, nuclei of most testicular cells resist homogenization and can be identified and enumerated with the use of Nomarski optics in the microscope (Johnson et al, 1981). Comparison data suggest that nuclei of few if any germ cells of a given type, or Sertoli and Leydig cells, are destroyed; they can be identified and enumerated (Johnson et al, 1981, 1998). Use of fixed tissue should be the first choice because more cell types can be enumerated in homogenates of fixed tissue than unfixed tissue. Also, it is likely that immersion of small pieces of tissue (eg, biopsy) in glutaraldehyde would be appropriate to provide samples for homogenization measurement of DSP and also to process for morphometric study or subjective histopathologic examination. With unfixed tissue, a pair of crucial questions arise: When during spermiogenesis do spermatid nuclei become resistant to the homogenization procedure actually used (buffer, specific homogenizer container and blade configuration, rpm, and volume processed)? Is this a square-wave transition from nonresistant immature forms to youngest resistant form plus all the more mature cells? With fixed tissue, one might wonder whether 100% of the nuclei of interest survived and were detected in the homogenate. In either case, the lifespan of the germ cells counted must be estimated accurately. Time Divisor Total number of spermatids (or other cell type) enumerated in any of these approaches to measuring DSP represents several days production of sperm. A time divisor is needed to convert number of enumerated cells per unit volume, or mass, to number of these cells produced each day (details in Amann, 1970b; Amann and Howards, 1980; Johnson et al, 1980a). It is assumed all cells of interest develop on an identical time frame. This is unlikely. Also, values for the relative and absolute durations of each cellular association and when cells are formed are imprecise. Documenting correctness of any time divisor is difficult, and neither approach described next has been used with humans. One approach is a labeling study. Rabbit testes were taken at known intervals after injection of 3 H-thymidine; homogenization-resistant spermatids were counted; and autoradiograms of testis sections, testis homogenates, or both were evaluated for labeled cells (Orgebin-Crist, 1968; Amann and Lambiase, 1969). Both concluded that spermatids in cellular associations V VIII were resistant. However, reported time divisors differed by.50% because of laboratory differences in values for the relative frequency of these cellular associations (49% vs 37%) and measured duration of 1 cycle of the seminiferous epithelium (11.3 vs 10.4 days). The other approach is seeking congruence between calculated DSP and actual number of sperm leaving a testis. For bulls, initially it was assumed that homogenization-resistant spermatids represented 3.27 days production (Amann and Almquist, 1962). Subsequently, unilateral placement of an indwelling catheter into the rete testis, proximal ductus deferens, or both, with quantitative collection and enumeration of sperm passing through the catheter over a number of days, enabled comparisons with presurgical sperm output in semen collected daily (divided by 2 to represent 1 testis) and DSP measured using the homogenization procedure (Amann et al, 1974). On a within-bull basis (n 5 10), numbers of sperm obtained daily by the first 3 methods did not differ significantly. In a separate comparison (n
12 480 Journal of Andrology N September ÙOctober ), numbers of sperm obtained via a ductus deferens catheter or ejaculated semen did not differ from DSP, provided the time divisor was revised upward from 3.27 to 5.32 days. Hence, it was concluded that spermatids in cellular associations IV and V, as well as VI VIII, were resistant to homogenization of unfixed tissue. (Cellular associations are numbered differently in studies by Amann than in Figures 1 4 herein [see Hochereau et al, 1984:figure 2.2].) Amann et al (1974, 1976) suggested comparable revisions in time divisors for homogenization-resistant spermatids in unfixed tissue from other species. Revised values were: boar, 6.19; bull, 5.32; rhesus monkey, 4.37; rabbit, 5.35; ram, 4.99; Wistar rat, 6.10; and stallion, 6.00 days. For unfixed human testes, Amann and Howards (1980) considered that S d1 and S d2 spermatids (cellular associations I and II; see Figure 2, column B) were resistant to homogenization, and a time divisor of 7.9 days was used. In retrospect, had they studied de Kretser (1969), they might have excluded most or all S d1 spermatids. Indeed, Johnson et al (1980a) concluded that nuclei of S d1 spermatids were not resistant to homogenization. They also concluded that values of DSP per gram, measured by counting spermatids in homogenates of fixed tissue, were similar to those obtained with morphometric procedures (putative gold standard ). Johnson et al (1981) then counted homogenization-resistant spermatids in 10 unfixed testes and separately counted S a + S b,s c, and S d1 + S d2 spermatids in homogenates of 10 contralateral fixed testes. To bring congruence in values of DSP per gram, they recommended a time divisor of 2.9 days for homogenizationresistant spermatids in unfixed human testes processed by their procedure. Collectively, these reports support a conclusion that the best time divisor for calculation of DSP from spermatids enumerated in homogenates (using Johnson s procedure) of unfixed human testis tissue would be 2.9 days, on the assumption that 0% of all S d1 spermatids and,90% of all S d2 spermatids remain to be counted. For fixed testes, the best approach is to collectively count S d1 + S d2 spermatids and use a time divisor of 7.9 days (Johnson et al, 1984a). If S a + S b spermatids are enumerated in homogenates of fixed human testis, a time divisor of 8.9 days is appropriate (Johnson et al, 1981). Values for DSP in Humans A man s testicular size, testis volume, or age do not allow meaningful prediction of his DSP or DSP per gram of testis parenchyma. Testis weight or volume accounts for,14% of the variation in DSP per testis, with effects of age removed (Johnson et al, 1984b). Among individuals (n 5 132), the CVs for DSP per gram and DSP per testis were.50%. Hence, estimated testis volume should not be used to predict Figure 5. Daily sperm production (DSP) in humans. (A) DSP tends to be significantly lower in older men than in younger men (minimum and maximum values shown, together with 25th percentile, median, and 75th percentile). (B) There is substantial variation among men in efficiency of sperm production (DSP/g parenchyma) of their right testis, but DSP per gram tends to be lower in older men (r 5.33; slope X). However, age accounts for only 11% of variation in DSP per gram for the right testis or 8% considering either testis. Not shown is that testis weight is independent of age and that DSP per gram is independent of testis weight (Johnson et al, 1984b). Modified and redrawn from Johnson et al (1984b). DSP and has little utility when allocating individuals to study groups. When men in a study of DSP are grouped by age, the mean or modal value for the younger group usually is significantly greater than that for the older group (Figure 5A; Johnson et al, 1984a; Johnson et al, 1986). This is not because testes of older men are smaller than those of younger men; on average, they are not (but see discussion in Johnson et al, 1984b). Rather, there is a small, but significant (P,.01), decline in DSP per gram with advancing age (Figure 5B). Nevertheless, age accounts for only,8% of the variation of DSP per testis (Johnson et al, 1984b). (Note: men studied by Johnson s group experienced sudden death; testes were promptly removed and processed, usually by perfusion to clear blood followed by glutaraldehyde fixative.) Also, Johnson et al (1984b) concluded that around 33uN latitude of Texas, any summer vs winter difference in mean DSP per gram was undetectable. Clinical andrologists recognize the benefit from examination of 2 biopsies from a patient (McLachlan et al, 2007), when biopsy is considered necessary. However, Johnson et al (1980b) opined measurement
13 Amann N Human Spermatogenesis 481 Table. Summary of literature on DSP per man, DSP per gram testis parenchyma, and paired testes weight a Age Range, yr, or Race DSP/2 Testes 95% CI, millions DSP/g Parenchyma 95% CI, millions No. of men Weight 2 Testes Parenchyma 95% CI, g Cells Time Enumerated Divisor, d Reference Homogenization of unfixed testes S d1 + S d2 7.9 Amann and Howards (1980) S d2 2.9 Johnson et al (1984b) S d S d2 2.9 Johnson and Varner (1988) Homogenization of fixed tissues na S a + S b 8.9 Johnson et al (1981) na As above S d1 + S d S a + S b 8.9 Johnson et al (1984a) S a + S b S a + S b 8.9 Johnson et al (1986) S a + S b na na S a + S b 8.9 Johnson et al (1987) na na S a + S b 8.9 Morphometric analyses (toluidine blue-stained Epon sections) na Spherical 8.9 Johnson et al (1980a) Spherical 8.9 Johnson et al (1981) Na na Spherical 8.9 Johnson et al (1990) Average for fixed homogenates & morphometric data Chinese Spherical 8.9 Johnson et al (1998) Hispanic Spherical 8.9 White Spherical 8.9 Abbreviations: CI, confidence interval; DSP, daily sperm production. a In some cases, approximate values for 95% CIs were calculated from graphic presentations of means and standard errors. Three early reports were excluded because data were combined in Johnson et al (1984b). It is possible that some individuals were included in more than 1 listed study. of DSP per gram (after homogenization of fixed tissue) with a portion of a single biopsy would correctly reflect that individual s DSP pooled across both testes. This conclusion is tenuous. Only 1 testis from each of 12 men was biopsied, and values for DSP per gram in the biopsy were correlated and compared with the mean value for 6 samples (top, middle, lower thirds of both testes) for that individual rather than the value for the corresponding third of the biopsied testis. Furthermore, the important question is whether the 2 values for an individual are comparable, not whether there is a relationship between them or whether a t test finds no significant difference between means pooled across both testes vs the value from 1 biopsy. To compare 2 measures of the same thing (eg, DSP/g), standard regression/correlation or t test approaches are not ideal for reasons discussed by Bland and Altman (1986), Glantz (2002), and Dallal (2007). Johnson et al (1980a,b, 1984a) concluded that mean DSP per gram is not significantly different among the top, middle, or lower thirds of a testis or between the 2 testes of an individual. Johnson et al (1980a) reported a CV for DSP per gram testis of,15%, apparently representing variation among thirds of a testis averaged across 20 testes. Later, values for the 3 portions of testes from 10 young men (20 48 years) and 10 older men (50 85 years) were subjected to 1-way analysis of variance, separately for left and right testes and each age group. Statistical analysis of their data (Johnson et al, 1984a:table 1) showed that DSP per gram in a given third of a testis is not consistently high or low for all individuals but did not address the question of whether there is substantial variation, or concordance, among the 3 portions of a given testis. This could be answered by the intraclass correlation coefficient or multiple paired comparisons by the Bland-Altman procedure (Dallal 2007). If raw data from Johnson et al (1980a,b, 1984a) are available, appropriate statistical analysis should remove uncertainty if DSP per gram usually is concordant among thirds of a testis, adequately represented by a single biopsy, and usually concordant between testes of an individual. Publications on DSP in humans are summarized in the Table. For younger men,,20 40 or 50 years of age, the 95% CIs for weight of the parenchyma in paired testes (g), DSP per gram (10 6 /day), and DSP per man (10 6 ) tended to be near 28 40, , and For men over 50 years of age, the respective 95% CIs tended
14 482 Journal of Andrology N September ÙOctober 2008 to be near 25 35, , and Although original publications did not state that data for DSP per gram or per testis were not normally distributed, it is possible that use of a transformed endpoint (eg, log DSP/g) might reduce the standard error and, hence, width of the back-transformed 95% CI. In males of a species with a substantial infantile phase, including humans, bulls, and rabbits but not mice or rats, there is a period of time as spermatogenesis gets up to speed (Courot et al, 1970). The change in DSP per gram between puberty (sperm first produced) and sexual maturity (maximum DSP achieved by an individual) has not been studied systematically for humans. Available data for humans (Amann, 1981:table A1) show that DSP per gram is low in young men as the process starts up. Means were 0.4, 3.5, and, DSP/g parenchyma for men 16 18, 19 20, or years old, respectively (means for 6, 10, and 40 testes). It is not known why testes in certain men produce far fewer sperm each day than those in other individuals. In older men, but not younger men,,40% of preleptotene/ leptotene spermatocytes degenerate before they become pachytene spermatocytes. However, across men of all ages, a deficiency in number of pachytene spermatocytes per gram testis parenchyma accounted for only,50% of the variation in DSP per gram (Johnson et al, 1983, 1987, 2000). In addition, and regardless of age, 30% 40% of pachytene/diplotene spermatocytes fail to produce spherical spermatids; apparently the problem is in meiosis-2. Approximately 70% of the variation in DSP per gram is associated with degeneration of germ cells during late meiosis. Virtually all spherical spermatids complete spermiogenesis. Extensive degeneration of primary or secondary spermatocytes is consistent with the observation (Johnson et al, 1992) that, in testes with a low DSP per gram, at least 1 generation of germ cells was missing in,50% of cellular associations examined. Such missing generations were far less common in testes with high DSP per gram. Also, men with a low DSP per gram tended to have fewer cellular associations per cross section of seminiferous tubule (Chaturvedi and Johnson, 1993). Johnson et al (2000) discounted the concept that degeneration of germ cells was a mechanism to eliminate cells with abnormal chromosomes because the magnitude of degeneration was relatively constant, and they speculated that it might be a mechanism to adjust number of germ cells to a number sustainable by available Sertoli cells. Obviously, sperm production is intimately dependent on Sertoli cells. Men years old have,30% fewer Sertoli cells per gram testis parenchyma than men years old, and,40% fewer Sertoli cells per testis (Johnson et al, 1984c, 1986). Nevertheless, numbers of all germ cells or spherical spermatids per Sertoli cell were similar for both age groups. Across all 71 men, the correlation between number of Sertoli cells and DSP was These results confirmed earlier studies. However, DSP per man is independent of number of Leydig cells (Neaves et al, 1987), probably because steroidogenic capacity is more dependent on volume of smooth endoplasmic reticulum than cell number. Indeed, total volume of Leydig cell smooth endoplasmic reticulum was correlated with DSP per testis (r 5.80; n 5 10 men; Johnson et al, 1990). Planning Studies Where Change in DSP Is an Endpoint Planning any study should include use of the among-individual CV for the measure(s) of interest, to estimate number of individuals needed per group to detect a difference of a given magnitude among 2 or more groups. From the preceding discussion, it is obvious that CVs for DSP are likely to be large. Berndtson (2008) calculated among-individual CVs for DSP per gram and DSP per testis in publications for humans; his paper includes information for several species. For testis homogenates, Berndtson s values for among-individual CVs in DSP per gram averaged 48% and 89%, for 7 groups of younger men and 4 groups of older men. For DSP per testis, respective CVs were approximately 58% and 104% (5 and 2 groups). For DSP data based on morphometric analysis, only data for younger men were summarized (Berndtson, 2008) and for both DSP per gram and DSP per testis the among-individual CVs averaged 46%. These values far exceed imprecision in values for a given testis because within-individual CVs are,15%, as documented in several of Johnson s publications. Assumptions needed to use information on CVs to estimate optimal sample size for a study were discussed by Berndtson (1991) and are considered on numerous web sites. For an anticipated magnitude of difference between mean values for a measured attribute, there are tradeoffs between probability or effect of making the wrong conclusion and sample size. Taking the CVs presented above and entering them in table 1 in Berndtson (1991) suggests that an 80% probability of detecting a 30% difference in DSP per testis (or individual) would require at least 63 young men per group, but if one wished to have a 90% probability of detecting a 20% difference, at least 235 young men per group would be needed. For older men, corresponding values are approximately 190 and 540, respectively. If DSP per gram is the endpoint, fewer individuals would be needed. Increasing precision of individual measurements would not decrease these numbers because the crucial feature is interindividual variation. Studies of human DSP per gram or DSP per testis rarely have used
15 Amann N Human Spermatogenesis individuals per group, so a conclusion of no significant difference might result from a type II error. What Knowledge Is Needed About the Cycle of the Seminiferous Epithelium? Cellular Associations There are several problems. First, the cycle has been defined in terms of only 6 cellular associations, of which 3 collectively account for 81% of the cycle (Figure 1). Early on, Heller and Clermont (1964) noted the need for discernment of more cellular associations, but no one has reported success or failure in this task. To better understand human spermatogenesis, it is important to develop a scheme involving $10 cellular associations, each representing,15% of the duration of a cycle of the seminiferous epithelium (ie,,2.4 days). This might be accomplished by use of phase contrast (Johnson et al, 1981) or Normaski optics (Johnson, 1994; Johnson et al, 2001), glutaraldehyde fixation and plastic sections stained with toluidine blue (Johnson et al, 1992), or novel immunochemical stains. Images published by Johnson and colleagues suggest that identification of distinct but gradually different steps in spermiogenesis could fill voids in column E of Figure 2 and allow definition of perhaps 10 cellular associations. Recall that Johnson s group intentionally fit identifications of germ cells to fit cellular associations described by Clermont (1963). Second, the primary report for the relative frequency of cellular associations (Clermont, 1963), which provides the accepted basis for calculations of both relative and absolute timing of spermatogenesis, was based on a total of 277 tubule cross sections representing 4 individuals. Rather than trying to resolve any difference between values reported by Clermont (1963) and Rowley and Heller (1971), a new classification scheme for cellular associations should be established and their relative frequencies then established on the basis of a robust data set. Properly fixed tissues necessary for this task might be available from Dr Larry Johnson, Department of Veterinary Anatomy and Public Health, Texas A&M University, College Station, Texas. Repeatability of scoring cellular associations, or estimating their relative frequencies, has never been reported for human testes. This gap should be filled, although for other species, it is evident that scoring can be repeatable. Coefficient of repeatability for triplicate data sets (each based on independent scoring of 800 cross sections through seminiferous tubules per testis) for 8 rabbit testes was.82 (Amann, 1970a). However, in comparing results in Swierstra and Foote (1963), Orgebin-Crist (1968), and Amann and Lambiase (1969), it seems that relative frequencies might differ among observers or laboratories. Number of tubule cross sections or cellular associations scored per testis affects repeatability or precision, as does the technician. Information in Clermont (1963) is insufficient to estimate sampling needs for good precision, but it is likely that 3 17 groups of cellular associations III and VI were encountered in his observations of tubules in a given testis. This is too few. Heller et al (1969) and Rowley and Heller (1971) did not address repeatability of observations for percentage of each cellular association in a given biopsy. They arbitrarily evaluated 30 essentially round cross sections for a given biopsy; probably, they found.12 groupings per biopsy only for cellular associations I and V. Third, concurrent with the above, precision and accuracy of available measurements of the time base underlying the cycle of the seminiferous epithelium might be improved. This is considered in the duration of spermatogenesis section. Spermatogonia The pattern and timing of spermatogonial renewal and proliferation to yield primary spermatocytes, and how spermatogonial differentiation is regulated, remain unknown. No model for spermatogonial renewal and proliferation proposed during the last 40 years was based on new data. All used data in Clermont (1966a) for cellular associations, when spermatogonial mitosis occurred, and ratios between cell types. The original, favored pattern proposed by Clermont (1966a,b) was largely unchallenged until recently (Aponte et al, 2005; Ehmcke and Schlatt, 2006; Ehmcke et al, 2006). The pattern of spermatogonial renewal and proliferation depicted in Figure 4 is a plausible model. Because cellular associations when a spermatogonium becomes committed to differentiation or division to form primary spermatocytes are uncertain, the duration of spermatogenesis also is uncertain. Unanswered questions include: When are different spermatogonia formed? When is a spermatogonium committed to differentiation? Are spermatogonial divisions synchronous. Does a given event always occur in the same cellular association? When and why do many spermatogonia die before producing preleptotene spermatocytes? Study of human spermatogonia would benefit from application of time lapse photography, with confocal or differential interference contrast microscopy to view cultured whole-mount tubules. This would enable linking changes in cell location, cell numbers, and mitotic metaphase plates within the framework of redefined cellular associations. Optical sectioning and imaging should allow 3D study of living human seminiferous tubules. This is not fantasy because the approach has been used with mouse testes (Yoshida et al, 2007). Alternatively, in vitro labeling of DNA in spermatogonia early during short-term culture of intact
16 484 Journal of Andrology N September ÙOctober 2008 Figure 6. Differences within a pair of testes, and among 25 rabbits, in the interval from intravenous injection of 3 H-thymidine to spermiation of labeled sperm. Rabbits were either sexually rested (gray bars) or ejaculated 16/day (black bars). The top of a bar represents location of the advancing front of labeled spermatids or spermatozoa in both testes and epididymides of individual rabbits as determined by autoradiography. The interval from 3 H-thymidine injection to release of labeled sperm ranged from 30.5 to 32.5 days. For 12 of 12 epididymides examined on day 33.0, labeled sperm were in the efferent ducts in 1, caput in 6, and corpus in 5 (not shown). Apparently the duration of 1 cycle of the seminiferous epithelium can differ slightly within an individual. Modified and redrawn from Amann (1972). seminiferous tubules, with appropriate subsequent processing and detection of labeled cells and cellular association in sections, could help establish when A- or B-spermatogonia are getting ready to divide. Duration of Spermatogenesis Understanding available data. The duration of spermatogenesis is,74 days in humans (Heller and Clermont, 1964; Heller et al, 1969). Heller and Clermont (1964:570) stated The whole spermatogenesis which extends over the duration of 4.6 cycles thus consumes approximately 74 days, and discussed why 4.6 cycles is correct rather than some other value. Authors citing a value of 64 days as the duration of spermatogenesis (eg, de Kretser and Kerr, 1994; Schlegel and Hardy, 2002) ignore the fact that spermatogenesis includes proliferation of spermatogonia (Heller and Clermont, 1963; Courot et al, 1970). As evident in Figure 4, an interval of 9 10 days needs to be added to the time from 3 H-thymidine injection to spermiation. Unfortunately, in a frequently cited review, Clermont (1972:table 2) gave a value of 64 days as duration of spermatogenesis in humans, although he discussed in detail the need to include spermatogonial events leading up to preleptotene spermatocytes within the duration of spermatogenesis and gave an approximation of this time interval. It is too common for authors to state that spermatogenesis includes proliferation of committed spermatogonia through spermiation, yet state that the entire spermatogenic process requires 64 days. Until a better value is available, the duration of human spermatogenesis should be cited as,74 days with attribution to Heller and Clermont (1964). Methodology. Duration of spermatogenesis in humans was measured by injection of 3 H-thymidine, followed by testis biopsies to follow progression of labeled germ cells and detection of labeled cells by autoradiography (Heller and Clermont, 1963, 1964). There is no alternative to detecting label in testis biopsies, because detection of labeled sperm in ejaculated semen cannot provide a precise value. To avoid 3 H-thymidine or 5-bromo-29-deoxyuridine, Misell et al (2006) had subjects drink 2 H 2 O 2 36 per day for 14 days and monitored arrival of 2 H-DNA in sperm obtained by masturbation (after 2 days of abstinence) at,2-week intervals. They correctly noted that the duration of epididymal transit should be subtracted from the interval to appearance of labeled sperm in semen but did not do this themselves! In any case, their approach was fatally flawed. Apparently, the authors failed to recognize that it was impossible to accurately anticipate when the most advanced labeled germ cells, or subsequent populations of labeled cells, were liberated from the seminiferous epithelium. (For the same reason, an early estimate of epididymal transit time [Rowley et al, 1970] is invalid.) This problem was discussed in Amann (1972) and is discussed in the next section. The paradigm used by Misell et al (2006) also precluded accurate timing of when labeled sperm first were available for ejaculation or first were ejaculated because semen was obtained only every 2 weeks. Furthermore, their calculations failed to recognize that spermatogenesis includes proliferation of spermatogonia (Heller and Clermont, 1963; Courot et al, 1970; Clermont, 1972). With a modern approach, a new scheme for classification of cellular associations, and informed consent of subjects, a new study of the duration of
The spermatogenesis CHARACTERISTICS OF THE SPERMATOZOON 26/04/2017. Reproductive Biotechnologies Andrology I. Prof. Alberto Contri
 Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
The Use of Rabbits in Male Reproductive Toxicology
 Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
Identification of the spermatogenic stages in living seminiferous tubules of man
 Identification of the spermatogenic stages in living seminiferous tubules of man V. Nikkanen, K.-O. S\l=o"\derstr\l=o"\m and M. Parvinen Department of Obstetrics and Gynecology, Turku University Central
Identification of the spermatogenic stages in living seminiferous tubules of man V. Nikkanen, K.-O. S\l=o"\derstr\l=o"\m and M. Parvinen Department of Obstetrics and Gynecology, Turku University Central
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Histology of Male Reproductive system (1)
 Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Adapted from Preg. & Part., Senger
 MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
Cycle of the Seminiferous Epithelium of the Guinea Pig
 Cycle of the Seminiferous Epithelium of the Guinea Pig A Method for Identification of the Stages Yves Clermont, Ph.D. IN THE GUINEA PIG, the cells of the seminiferous epithelium are arranged in definite
Cycle of the Seminiferous Epithelium of the Guinea Pig A Method for Identification of the Stages Yves Clermont, Ph.D. IN THE GUINEA PIG, the cells of the seminiferous epithelium are arranged in definite
Cell Divisions. The autosomes represent the whole body. * Male Sex Chromosomes: XY * Female Sex Chromosomes: XX
 Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Male Reproductive System
 Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM
 LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
Production of Fertile Sperm. Animal Science 434. Hormonal Regulation of the Testis. hormonal regulation of the testis
 roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
of the rabbit testis were obtained.
 FERTILITY AND STERILITY Copyright 1970 by The Williams & Wilkins Co. Vol. 21, No.9, September 1970 Printed in U.S.A. THE MALE RABBIT. IV. QUANTITATIVE TESTICULAR HISTOLOGY AND COMPARISONS BETWEEN DAILY
FERTILITY AND STERILITY Copyright 1970 by The Williams & Wilkins Co. Vol. 21, No.9, September 1970 Printed in U.S.A. THE MALE RABBIT. IV. QUANTITATIVE TESTICULAR HISTOLOGY AND COMPARISONS BETWEEN DAILY
5 15/3/2012. Malik Al-Momani
 5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
Male reproduction. Cross section of Human Testis ผศ.ดร.พญ.ส ว ฒณ ค ปต ว ฒ ภาคว ชาสร รว ทยา คณะแพทยศาสตร ศ ร ราชพยาบาล 1. Aims
 Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Germ Cell Degeneration During Postprophase of Meiosis and Serum Concentrations of Gonadotropins in Young Adult and Older Adult Men
 BIOLOGY OF REPRODUCTION 31, 779-784 (1984) Germ Cell Degeneration During Postprophase of Meiosis and Serum Concentrations of Gonadotropins in Young Adult and Older Adult Men LARRY JOHNSON,2 CHARLES S.
BIOLOGY OF REPRODUCTION 31, 779-784 (1984) Germ Cell Degeneration During Postprophase of Meiosis and Serum Concentrations of Gonadotropins in Young Adult and Older Adult Men LARRY JOHNSON,2 CHARLES S.
Male Reproductive System
 Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Embryology 3. Spermatogenesis:
 Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Spermatogenesis in Man
 Spermatogenesis in Man I. Nuclear Morphology During Spermatogenesis in Man BRUNETTO CHIARELLI, PH.D., ARTHUR FALEK, PH.D., KAREN J. BACK, B.S., and C. THOMAS COWART, M.D. THE SEQUENCE of transformations
Spermatogenesis in Man I. Nuclear Morphology During Spermatogenesis in Man BRUNETTO CHIARELLI, PH.D., ARTHUR FALEK, PH.D., KAREN J. BACK, B.S., and C. THOMAS COWART, M.D. THE SEQUENCE of transformations
Influence of three different histological methods on the morphology and morphometrical data in human testis
 Histol Histopathol (2017) 32: 27-34 http://www.hh.um.es Histology and Histopathology From Cell Biology to Tissue Engineering Influence of three different histological methods on the morphology and morphometrical
Histol Histopathol (2017) 32: 27-34 http://www.hh.um.es Histology and Histopathology From Cell Biology to Tissue Engineering Influence of three different histological methods on the morphology and morphometrical
IN normal male fowls, four developmental stages of spermatogenetic activity
 Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
TIMING OF SPERMATOGENESIS IN FOUR NONHUMAN PRIMATE SPECIES*
 FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No.5, May 1973 Printed in U.S.A. TIMING OF SPERMATOGENESIS IN FOUR NONHUMAN PRIMATE SPECIES* ARNOLD B. BARR, M.D. Department
FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No.5, May 1973 Printed in U.S.A. TIMING OF SPERMATOGENESIS IN FOUR NONHUMAN PRIMATE SPECIES* ARNOLD B. BARR, M.D. Department
2 Sperm Production and its Harvest
 2 Sperm Production and its Harvest William E. Berndtson* University of New Hampshire, Durham, New Hampshire, USA Introduction Spermatogenesis requires ~60 days in most mammals. It encompasses a series
2 Sperm Production and its Harvest William E. Berndtson* University of New Hampshire, Durham, New Hampshire, USA Introduction Spermatogenesis requires ~60 days in most mammals. It encompasses a series
Morphogenesis of the residual body of the mouse testis
 93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER)
 THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
Testicular stem cells
 Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Medical School Histology Basics Male Reproductive System. VIBS 289 lab
 Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Primary sex organs (gonads): testes and ovaries. Accessory reproductive organs: ducts, glands, and external genitalia
 Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL CRYPTORCHIDISM: A PRELIMINARY REPORT*
 FERTILITY AND STERILITY Copyright 1970 by the Williams & Wilkins Co. Vol. 21, No. 11, November 1970 Printed in U.S.A. A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL
FERTILITY AND STERILITY Copyright 1970 by the Williams & Wilkins Co. Vol. 21, No. 11, November 1970 Printed in U.S.A. A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL
Animal Development. Lecture 3. Germ Cells and Sex
 Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Nature Genetics: doi: /ng Supplementary Figure 1. Assessment of sample purity and quality.
 Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM
 Unit 7A STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM LEARNING OBJECTIVES 1. Learn the structures of the male reproductive system. 2. Learn the functions of the male reproductive system. 3. Learn
Unit 7A STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM LEARNING OBJECTIVES 1. Learn the structures of the male reproductive system. 2. Learn the functions of the male reproductive system. 3. Learn
Male Anatomy. testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics
 Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Chapter 28: REPRODUCTIVE SYSTEM: MALE
 Chapter 28: REPRODUCTIVE SYSTEM: MALE I. FUNCTIONAL ANATOMY (Fig. 28.1) A. Testes: glands which produce male gametes, as well as glands producing testosterone 2. Seminiferous tubules (Fig.28.3; 28.5) a.
Chapter 28: REPRODUCTIVE SYSTEM: MALE I. FUNCTIONAL ANATOMY (Fig. 28.1) A. Testes: glands which produce male gametes, as well as glands producing testosterone 2. Seminiferous tubules (Fig.28.3; 28.5) a.
Quantitative differences between variants of
 Quantitative differences between variants of A spermatogonia in man R. Paniagua, M. Nistal, P. Amat, M. C. Rodr\l=i'\guez,and J. R. Alonso "Department of Cytology and Histology, Faculty ofbiology, University
Quantitative differences between variants of A spermatogonia in man R. Paniagua, M. Nistal, P. Amat, M. C. Rodr\l=i'\guez,and J. R. Alonso "Department of Cytology and Histology, Faculty ofbiology, University
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
The Male Reproductive System
 The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
Spermatogenesis, sperm output and seminal quality of
 Spermatogenesis, sperm output and seminal quality of Holstein bulls electroejaculated after administration of oxytocin W. E. Berndtson and G. Igboeli Department of Animal and Nutritional Sciences, University
Spermatogenesis, sperm output and seminal quality of Holstein bulls electroejaculated after administration of oxytocin W. E. Berndtson and G. Igboeli Department of Animal and Nutritional Sciences, University
Reproductive Toxicology
 Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
Chapter1 Introduction
 Chapter1 Introduction Male subfertility is a very significant global problem. Epidemiological data show that approximately 1-in-7 couples are classed as subfertile [1]. Sperm dysfunction is the single
Chapter1 Introduction Male subfertility is a very significant global problem. Epidemiological data show that approximately 1-in-7 couples are classed as subfertile [1]. Sperm dysfunction is the single
Physiologic Anatomy of the Male Sexual Organs
 Reproductive and Hormonal Functions of the Male The reproductive functions of the male can be divided into three major subdivisions: (1) spermatogenesis, which means simply the formation of sperm; (2)
Reproductive and Hormonal Functions of the Male The reproductive functions of the male can be divided into three major subdivisions: (1) spermatogenesis, which means simply the formation of sperm; (2)
Infertility is not an uncommon problem in Western
 Review Article A Practical Approach to Testicular Biopsy Interpretation for Male Infertility Lisa A. Cerilli, MD; Wayne Kuang, MD; David Rogers, MD Infertility is not an uncommon problem in Western societies
Review Article A Practical Approach to Testicular Biopsy Interpretation for Male Infertility Lisa A. Cerilli, MD; Wayne Kuang, MD; David Rogers, MD Infertility is not an uncommon problem in Western societies
describe the parts and function of semen and the glands that contribute to it
 You need to be able to: describe spermatogenesis (How is sperm made?) describe the anatomy of a sperm describe the parts and function of semen and the glands that contribute to it How is sperm made? Spermatogenesis
You need to be able to: describe spermatogenesis (How is sperm made?) describe the anatomy of a sperm describe the parts and function of semen and the glands that contribute to it How is sperm made? Spermatogenesis
The Reproductive System
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
SUPPLEMENTAL INFORMATION FOR. PAX7 expression defines germline stem cells in the adult testis
 SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
Male Reproduction Organs. 1. Testes 2. Epididymis 3. Vas deferens 4. Urethra 5. Penis 6. Prostate 7. Seminal vesicles 8. Bulbourethral glands
 Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Gametogenesis. Omne vivum ex ovo All living things come from eggs.
 Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
Chapter 26: Reproductive Systems. Male 11/29/2015. Male reproductive system is composed of... BIO 218 Fall Gonads (testes)
 Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Mohammad Sha ban. Basheq Jehad. Hamzah Nakhleh
 11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE
 ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
Basic histology 5/4/2015
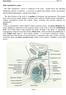 Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
ABSTRACT. The objective of this study was to assess the effectiveness of a Nycodenz gradient
 ABSTRACT MILLER, STEPHANIE RENEE. Assessment of nycodenz gradient on enrichment and culture of perinatal porcine spermatogonial stem cells. (Under the direction of Robert M. Petters). The objective of
ABSTRACT MILLER, STEPHANIE RENEE. Assessment of nycodenz gradient on enrichment and culture of perinatal porcine spermatogonial stem cells. (Under the direction of Robert M. Petters). The objective of
18 Urinary system. 19 Male reproductive system. Female reproductive system. Blok 11: Genital and Urinary Tract Diseases
 Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature
 REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
Hormones of brain-testicular axis
 (Hormone Function) Hormones of brain-testicular axis anterior pituitary drives changes during puberty controlled by GnRH from hypothalamus begins to secrete FSH, LH LH targets interstitial endocrinocytes
(Hormone Function) Hormones of brain-testicular axis anterior pituitary drives changes during puberty controlled by GnRH from hypothalamus begins to secrete FSH, LH LH targets interstitial endocrinocytes
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
Reproductive Endocrinology. Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007
 Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Semen evaluation in domestic animals I
 Reproductive Biotechnologies Andrology I I Semen evaluation in domestic animals I Prof. Alberto Contri Different aims 1. Diagnosis 2. Handling and preservation Related to the male - Breeding soundness
Reproductive Biotechnologies Andrology I I Semen evaluation in domestic animals I Prof. Alberto Contri Different aims 1. Diagnosis 2. Handling and preservation Related to the male - Breeding soundness
Pathology of Male Reproductive System 1
 Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
THE ROLE OF TESTICULAR GERM CELL APOPTOSIS DURING EQUINE SPERMATOGENESIS. A Dissertation NOAH LELAND HENINGER III
 THE ROLE OF TESTICULAR GERM CELL APOPTOSIS DURING EQUINE SPERMATOGENESIS A Dissertation by NOAH LELAND HENINGER III Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment
THE ROLE OF TESTICULAR GERM CELL APOPTOSIS DURING EQUINE SPERMATOGENESIS A Dissertation by NOAH LELAND HENINGER III Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment
Microscope Requirements
 SEMEN EVALUATION EQUIPMENT Microscope Requirements Good quality lenses Phase-contrast preferred for % progressive motility evaluations Objectives 10X, 20X*, 40X*, 100X, minimum Heated stage preferred *Preferably
SEMEN EVALUATION EQUIPMENT Microscope Requirements Good quality lenses Phase-contrast preferred for % progressive motility evaluations Objectives 10X, 20X*, 40X*, 100X, minimum Heated stage preferred *Preferably
Unit 15 ~ Learning Guide
 Unit 15 ~ Learning Guide Name: INSTRUCTIONS Complete the following notes and questions as you work through the related lessons. You are required to have this package completed BEFORE you write your unit
Unit 15 ~ Learning Guide Name: INSTRUCTIONS Complete the following notes and questions as you work through the related lessons. You are required to have this package completed BEFORE you write your unit
THE MALE RABBIT. VII. STUDIES ON RESORPTION OF PH} THYMIDINE.LABELED SPERMATOZOA IN THE EPIDIDYMIS*
 Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE MALE RABBIT. VII. STUDIES ON RESORPTION OF PH} THYMIDINE.LABELED SPERMATOZOA IN THE
Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE MALE RABBIT. VII. STUDIES ON RESORPTION OF PH} THYMIDINE.LABELED SPERMATOZOA IN THE
All You Wanted to Know About Spermatogonia but Were Afraid to Ask
 All You Wanted to Know About Spermatogonia but Were Afraid to Ask Review DIRK G. DE ROOIJ* AND LONNIE D. RUSSELL From the *Department of Cell Biology, University Medical Center Utrecht, Utrecht, The Netherlands;
All You Wanted to Know About Spermatogonia but Were Afraid to Ask Review DIRK G. DE ROOIJ* AND LONNIE D. RUSSELL From the *Department of Cell Biology, University Medical Center Utrecht, Utrecht, The Netherlands;
Reproductive System Purpose General Structures Male Structures Functions Female Anatomy Structures Functions Clinical Applications
 The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
Spermatogenesis: An Overview
 Spermatogenesis: An Overview 2 Rakesh Sharma and Ashok Agarwal Abstract The purpose of this chapter is to provide a comprehensive overview of spermatogenesis and the various steps involved in the development
Spermatogenesis: An Overview 2 Rakesh Sharma and Ashok Agarwal Abstract The purpose of this chapter is to provide a comprehensive overview of spermatogenesis and the various steps involved in the development
FIGURE The tunica albuginea is a connective tissue capsule forming the outer part of each testis.
 Testicular Histology (see p. 1034 in text) FIGURE 28.3 1. The tunica albuginea is a connective tissue capsule forming the outer part of each testis. 2. Septa are extensions of the tunica albuginea that
Testicular Histology (see p. 1034 in text) FIGURE 28.3 1. The tunica albuginea is a connective tissue capsule forming the outer part of each testis. 2. Septa are extensions of the tunica albuginea that
The Male Reproductive System
 The Male Reproductive System The male reproductive system Testes Genital ducts Accessory sex glands: seminal vesicles prostate bulbourethral glands External genitalia: penis Structure of the Testis Tunica
The Male Reproductive System The male reproductive system Testes Genital ducts Accessory sex glands: seminal vesicles prostate bulbourethral glands External genitalia: penis Structure of the Testis Tunica
Male Reproductive Physiology
 Male Reproductive Physiology Overview Anatomy Function Endocrine and spermatogenesis Testis epididymus,vas deferens,seminal vesicles and prostate Hypothalamic pituitary testicular axis Hormones of the
Male Reproductive Physiology Overview Anatomy Function Endocrine and spermatogenesis Testis epididymus,vas deferens,seminal vesicles and prostate Hypothalamic pituitary testicular axis Hormones of the
Lesson 1. Quiz (short) Cell cycle Chromosomes Mitosis phases
 Lesson 1 Quiz (short) Cell cycle Chromosomes Mitosis phases 2 Cell division is needed for Growth (Mitosis) Repair (Mitosis) Reproduction (Meiosis) 3 Mitosis consists of 4 phases (division of the nuclear
Lesson 1 Quiz (short) Cell cycle Chromosomes Mitosis phases 2 Cell division is needed for Growth (Mitosis) Repair (Mitosis) Reproduction (Meiosis) 3 Mitosis consists of 4 phases (division of the nuclear
MALE REPRODUCTIVE SYSTEM
 1 MALE REPRODUCTIVE SYSTEM SCPA 602 Anatomical Basis for Pathological Study Updated: 20.09.2018 Lect. Nisamanee Charoenchon, PhD nisamanee.cha@mahidol.ac.th Department of Pathobiology, Mahidol University
1 MALE REPRODUCTIVE SYSTEM SCPA 602 Anatomical Basis for Pathological Study Updated: 20.09.2018 Lect. Nisamanee Charoenchon, PhD nisamanee.cha@mahidol.ac.th Department of Pathobiology, Mahidol University
Computer Simulation of the Rodent Spermatogonial Stem Cell Niche 1
 BIOLOGY OF REPRODUCTION (2013) 88(5):131, 1 11 Published online before print 27 March 2013. DOI 10.1095/biolreprod.113.108639 Computer Simulation of the Rodent Spermatogonial Stem Cell Niche 1 Dirk G.
BIOLOGY OF REPRODUCTION (2013) 88(5):131, 1 11 Published online before print 27 March 2013. DOI 10.1095/biolreprod.113.108639 Computer Simulation of the Rodent Spermatogonial Stem Cell Niche 1 Dirk G.
Sperm production. Sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production. Sperm production. Controlling sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
BIOL 2402 Reproductive Systems
 Collin College Dr. Chris Doumen BIOL 2402 Reproductive Systems 1 Reproductive System Most systems between males and females in the human body are similar in structure. The exception of course are the organs
Collin College Dr. Chris Doumen BIOL 2402 Reproductive Systems 1 Reproductive System Most systems between males and females in the human body are similar in structure. The exception of course are the organs
ABNORMAL SPERMATOGENESIS IN XYY MALES: A REPORT ON 4 CASES ASCERTAINED THROUGH A POPULATION STUDY*
 FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No.5, May 1973 Printed in U.S.A. ABNORMAL SPERMATOGENESIS IN XYY MALES: A REPORT ON 4 CASES ASCERTAINED THROUGH A POPULATION
FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No.5, May 1973 Printed in U.S.A. ABNORMAL SPERMATOGENESIS IN XYY MALES: A REPORT ON 4 CASES ASCERTAINED THROUGH A POPULATION
Histology of Male Reproductive System
 Histology of Male Reproductive System Lecture Objectives Describe the histological features of the male reproductive system Male Reproductive System The male structures of reproduction include the: testes,
Histology of Male Reproductive System Lecture Objectives Describe the histological features of the male reproductive system Male Reproductive System The male structures of reproduction include the: testes,
HANDOUT # 1 GAMETOGENESIS
 Gametogenesis 1 HANDOUT # 1 GAMETOGENESIS Anatomy Department R.A. FADEL Gametogenesis 2 بسم هللا الرحمن الرحيم ت هأ م وى }46 } ا نل و و ا زل و ج و ن و و و و و ن ه و أ ل ه ث وى }45{ إ ول ن أ و ة م ن ان
Gametogenesis 1 HANDOUT # 1 GAMETOGENESIS Anatomy Department R.A. FADEL Gametogenesis 2 بسم هللا الرحمن الرحيم ت هأ م وى }46 } ا نل و و ا زل و ج و ن و و و و و ن ه و أ ل ه ث وى }45{ إ ول ن أ و ة م ن ان
THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS
 Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
 MOCK TEST I (HUMAN REPRODUCTION) 1. In human transfer of sperms into female genital tract is called as 1) Fertilization 2) Implantation 3) Insemination 4) Gestation 2. In male human scrotum maintains the
MOCK TEST I (HUMAN REPRODUCTION) 1. In human transfer of sperms into female genital tract is called as 1) Fertilization 2) Implantation 3) Insemination 4) Gestation 2. In male human scrotum maintains the
Meiosis & Sexual Reproduction. AP Biology
 Meiosis & Sexual Reproduction 2007-2008 Cell division / Asexual reproduction Mitosis produce cells with same information identical daughter cells exact copies clones same amount of DNA same number of chromosomes
Meiosis & Sexual Reproduction 2007-2008 Cell division / Asexual reproduction Mitosis produce cells with same information identical daughter cells exact copies clones same amount of DNA same number of chromosomes
Outline. Male Reproductive System Testes and Sperm Hormonal Regulation
 Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Quantitative studies on spermatogenesis in buffalo (Bubalus bubalis)
 Quantitative studies on spermatogenesis in buffalo (Bubalus bubalis) G. S. Bilaspuri, S. S. Guraya To cite this version: G. S. Bilaspuri, S. S. Guraya. Quantitative studies on spermatogenesis in buffalo
Quantitative studies on spermatogenesis in buffalo (Bubalus bubalis) G. S. Bilaspuri, S. S. Guraya To cite this version: G. S. Bilaspuri, S. S. Guraya. Quantitative studies on spermatogenesis in buffalo
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
The Reproductive System
 16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
For more information about how to cite these materials visit
 Author: A. Kent Christensen, Ph.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Author: A. Kent Christensen, Ph.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Seminiferous Tubules
 Testes The testes are compound tubular glands that lie within a scrotal sac, suspended from the body by a spermatic cord. The testes are dual organs that act as exocrine glands producing a holocrine secretion,
Testes The testes are compound tubular glands that lie within a scrotal sac, suspended from the body by a spermatic cord. The testes are dual organs that act as exocrine glands producing a holocrine secretion,
ANATOMY. lecture # : 2 1 Date : Lecturer : Maher Hadidi
 ANATOMY lecture # : 2 1 Date : Lecturer : Maher Hadidi Spermatogenesis Transformation of Spermatogonia into mature sperm that begins at puberty into old age. Provide motility of the sperm...-c:tail to
ANATOMY lecture # : 2 1 Date : Lecturer : Maher Hadidi Spermatogenesis Transformation of Spermatogonia into mature sperm that begins at puberty into old age. Provide motility of the sperm...-c:tail to
SPERMATOGENESIS IN VITRO
 SPERMATOGENESIS IN VITRO INDUCTION OF PROLIFERATION, MEIOSIS AND DIFFERENTIATION Mário Sousa Lab Cell Biology Institute of Biomedical Sciences (ICBAS) University of Porto msousa@icbas.up.pt Spermatogonia
SPERMATOGENESIS IN VITRO INDUCTION OF PROLIFERATION, MEIOSIS AND DIFFERENTIATION Mário Sousa Lab Cell Biology Institute of Biomedical Sciences (ICBAS) University of Porto msousa@icbas.up.pt Spermatogonia
The Reproductive System
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
Sami Ventelä, 1,2 Hiroshi Ohta, 3 Martti Parvinen, 2 and Yoshitake Nishimune 3
 BIOLOGY OF REPRODUCTION 66, 1422 1429 (2002) Development of the Stages of the Cycle in Mouse Seminiferous Epithelium after Transplantation of Green Fluorescent Protein-Labeled Spermatogonial Stem Cells
BIOLOGY OF REPRODUCTION 66, 1422 1429 (2002) Development of the Stages of the Cycle in Mouse Seminiferous Epithelium after Transplantation of Green Fluorescent Protein-Labeled Spermatogonial Stem Cells
Chapter 22 The Reproductive System (I)
 Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Morphometric Studies on Rat Seminiferous Tubules
 THE AMERCAN JOURNAL OF ANATOMY 165:1-25 (1982) Morphometric Studies on Rat Seminiferous Tubules TUNG-YANG NG AND A. KENT CHRSTENSEN Department of Anatomy and Cell Biology, The University of Michigan Medical
THE AMERCAN JOURNAL OF ANATOMY 165:1-25 (1982) Morphometric Studies on Rat Seminiferous Tubules TUNG-YANG NG AND A. KENT CHRSTENSEN Department of Anatomy and Cell Biology, The University of Michigan Medical
Review: Spermatogenesis in the bull
 Animal (2018), 12:S1, pp s27 s35 The Animal Consortium 2018. This is an Open Access article, distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives licence (http://creativecommons.org/licenses/by-nc-nd/4.0/),
Animal (2018), 12:S1, pp s27 s35 The Animal Consortium 2018. This is an Open Access article, distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives licence (http://creativecommons.org/licenses/by-nc-nd/4.0/),
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM 1. The male reproductive system is made up of the following structures, EXCEPT: a. prostate; b. testicle; c. spermatic ducts; d. vestibular bulbs; e. seminal vesicles. 2.The testicle:
MALE REPRODUCTIVE SYSTEM 1. The male reproductive system is made up of the following structures, EXCEPT: a. prostate; b. testicle; c. spermatic ducts; d. vestibular bulbs; e. seminal vesicles. 2.The testicle:
Animal Reproduction. Overview of Male Reproduction. # lectures for cumulative test # 01 book 01
 Animal Reproduction JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (732) 932-9240, advis@aesop.rutgers.edu 04 Course website: rci.rutgers.edu/~advis Material to be covered: About lecture Meetings
Animal Reproduction JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (732) 932-9240, advis@aesop.rutgers.edu 04 Course website: rci.rutgers.edu/~advis Material to be covered: About lecture Meetings
Cytological Studies on Human Spermatogenic and Sustentacular (Sertoli) Cells
 Cytological Studies on Human Spermatogenic and Sustentacular (Sertoli) Cells By Setsuko Ogata Department of Anatomy, Tokyo Women's Medical College, Shinjuku, Tokyo, Japan (Director : Prof. Dr. Kura Kubota)
Cytological Studies on Human Spermatogenic and Sustentacular (Sertoli) Cells By Setsuko Ogata Department of Anatomy, Tokyo Women's Medical College, Shinjuku, Tokyo, Japan (Director : Prof. Dr. Kura Kubota)
Spermatogenesis Following Experimental Testicular Ischemia
 Spermatogenesis Following Experimental Testicular Ischemia Frank Hinman, Jr, MD, and Gilbert I Smith, MD REGENERATION of the spermatogenic elements of the testis after depression by testosterone and by
Spermatogenesis Following Experimental Testicular Ischemia Frank Hinman, Jr, MD, and Gilbert I Smith, MD REGENERATION of the spermatogenic elements of the testis after depression by testosterone and by
Lab #9: Kidney: Gross Anatomy & Histology
 Name Date Lab #9: Kidney: Gross Anatomy & Histology Lab #10: Male Reproductive System: Human Models & Histology Lab #11: Female Reproductive System: Human Models & Histology Stuff to Know Dr. L. Bacha
Name Date Lab #9: Kidney: Gross Anatomy & Histology Lab #10: Male Reproductive System: Human Models & Histology Lab #11: Female Reproductive System: Human Models & Histology Stuff to Know Dr. L. Bacha
