(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
|
|
|
- Tamsyn Lauren Holt
- 5 years ago
- Views:
Transcription
1 US A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/ A1 KO et al. (43) Pub. Date: Oct. 13, 2016 (54) ADSORBENT FOR REDUCING UREMIC A6IR 9/00 ( ) TOXINS IN VIVO A6II 45/06 ( ) (52) U.S. Cl. (71) Applicant: BIO-MEDICAL CARBON CPC... A61K 33/44 ( ); A61K 9/0053 TECHNOLOGY CO., LTD., Taichung ( ); A61K 45/06 ( ); B01.J City (TW) 20/205 ( ); B01J 20/ ( ); B01J 20/28061 ( ); B01J 20/ Jui-Hsiang LIN, TAICHUNG CITY (TW); Wei-Shan HSU, TAICHUNG (57) ABSTRACT CITY (TW); Wan-Yu CHUNG, TAICHUNG CITY (TW) An adsorbent for reducing urem1c tox1ns 1n V1VO includes polyacrylonitrile-based activated carbon fibers having the (21) Appl. No.: 14/683,501 following properties: a) an average diameter of 5 um to 30 um; b) a BET specific surface area of more than 390 m/g: (22) Filed: Apr. 10, 2015 c) a total acidic group content of larger than 1.2 med/g or a total basic group content of larger than 1 med/g. Because the Publication Classification adsorbent of the present disclosure has a higher adsorption capacity for precursors of uremic toxins than for the normal (51) Int. Cl. enzyme protein in intestinal tract, the adsorbent of the A6 IK 33/44 ( ) present disclosure can effectively prevent the deterioration BOI 20/28 ( ) of renal function, and thus can be used as a therapeutic agent BOI 20/20 ( ) and a preventive for kidney disease.
2 Patent Application Publication Oct. 13, 2016 Sheet 1 of 4 US 2016/ A1 FIG. a 43 indole Olipase oup E comparative exampie example 2 example 3 example 4 example 5 example FIG 2
3 Patent Application Publication Oct. 13, 2016 Sheet 2 of 4 US 2016/ A1 3 g..} : ---, 3 Y. 3. \ y re: X. --Nep g e N 3 : NX & : N: -who, Nep-ACF R 3. i S. 6 f 8 9. week(s) offeeding g e Y o m-w. s -H Nep - s -- Nep ACF &S week(s) of feeding FIG. 4
4 Patent Application Publication Oct. 13, 2016 Sheet 3 of 4 US 2016/ A1 enoe W. -ho. Neg Atf C, week(s) of feeding FIG. S
5 Patent Application Publication Oct. 13, 2016 Sheet 4 of 4 US 2016/ A1 FIG. 7
6 US 2016/ A1 Oct. 13, 2016 ADSORBENT FOR REDUCING UREMC TOXINS IN VIVO BACKGROUND OF THE INVENTION Field of the Invention 0002 The present disclosure relates generally to an orally administered substance for treating or preventing kidney disease and more particularly, to an adsorbent capable of reducing uremic toxins in Vivo, which is used to prevent the deterioration of renal function or to reduce the deterioration rate of renal function Description of the Related Art The metabolic wastes excreted from kidney are known as uremic toxins. The concentration of the uremic toxin of the serum is an index of renal function. One of the uremic toxins, indoxyl Sulfate, is regarded as one of the major reasons for the deterioration of chronic kidney dis ease. Generally speaking, tryptophan obtained from food is metabolized into indole by intestinal bacteria, and then the indole is metabolized into indoxyl sulfate in the liver, which in turn is excreted from kidney. When the renal function deteriorates, indoxyl Sulfate and other uremic toxins, such as creatinine, blood urea nitrogen, etc., accumulates in the body, leading to kidney failure eventually. For a kidney failure patient, kidney transplantation or dialysis treatment is necessary to Sustain the patient s life. However, kidney transplantation is not always easy to carry out, and dialysis treatment tends to cause a complication Such as thrombosis. Therefore, if the amount of uremic toxins accumulated in the body at the initial stage of deterioration of renal function can be minimized, the deterioration rate of renal function can be reduced, thereby avoiding the need of dialysis treatment When fed into intestine, an excellent adsorbent for reducing uremic toxins preferably has a high adsorption capacity for precursors of uremic toxins such as indole, but a low adsorption capacity for enzyme protein Such as lipase presented in intestinal tract. An adsorbent having Such selectivity can thus reduce the amount of uremic toxins accumulated in the body and can maintain the normal function of gastro-intestinal tract. A conventional adsorbent has a poor adsorption capacity for precursors of uremic toxins and has no selectivity, thus it can neither effectively reduce the deterioration of renal function nor maintain the normal function of gastro-intestinal tract. SUMMARY OF THE INVENTION It is an objective of the present disclosure to provide an adsorbent for reducing uremic toxins in vivo, which is capable of effectively removing precursors of uremic toxins in gastro-intestinal tract under the condition that the enzyme protein in intestinal tract may not be affected, reducing the rate of accumulation of uremic toxins in vivo, reducing the deterioration rate of renal function, and avoiding the need of dialysis treatment To attain the above-mentioned objective, the pres ent disclosure provides an adsorbent, which comprises poly acrylonitrile-based activated carbon fibers having the fol lowing properties: a) an average diameter of 5-30 jum; b) a BET specific surface area of more than 390 m/g; and c) a total acidic group content of larger than 1.2 med/g or a total basic group content of larger than 1 med/g In comparison with the conventional adsorbent, the adsorbent of the present disclosure has a higher adsorption capacity for precursors of uremic toxins than for enzyme protein in intestinal tract; it can effectively reduce the amount of uremic toxins accumulated in the body while maintaining the normal function of the gastro-intestinal tract; it can be used as a therapeutic agent for kidney disease to reduce the deterioration rate of renal function or can be used as a preventive for kidney disease to avoid the need of dialysis treatment. BRIEF DESCRIPTION OF THE DRAWINGS 0009 FIG. 1 is a scanning electron microscopic (SEM) image of polyacrylonitrile-based activated carbon fibers used in an example 1 of the present disclosure FIG. 2 is a diagram showing the adsorption per centages of indole and lipase in vitro of examples 1 to 5 of the present disclosure and a comparative example FIG. 3 is a diagram of an in vivo test showing the concentration changes of indoxyl Sulfate in serums of a rat in a normal group (WT), a rat in a control group (Nep), and a rat in an experimental group (Nep-ACF) FIG. 4 is a diagram of an in vivo test showing the concentration changes of blood urea nitrogen in serums of the rat in the normal group (WT), the rat in the control group (Nep), and the rat in the experimental group (Nep-ACF) FIG. 5 is a diagram of an in vivo test showing the concentration changes of creatinine in serums of the rat in the normal group (WT), the rat in the control group (Nep), and the rat in the experimental group (Nep-ACF) FIG. 6 is a stained slice image of a kidney of the rat in the normal group FIG. 7 is a stained slice image of a kidney of the rat in the control group FIG. 8 is a stained slice image of a kidney of the rat in the experimental group that is fed with the adsorbent according to example 1 of the present disclosure. DETAILED DESCRIPTION OF EMBODIMENTS The technical features and the effect of the present disclosure will become more fully understood from the detailed description and embodiments given herein below and the properties mentioned in each embodiment are deter mined by the following methods Average diameter and average length were deter mined by a scanning electron microscope (S-4800, from Hitachi company, Japan) BET specific surface area and percentages of micropores (diameter of less than 2 nm), mesopores (diam eter of between 2 nm and 50 nm), and macropores (diameter of larger than 50 nm) were determined by a high-resolution specific surface area analyzer (ASAP2020, from Micromer itics company, America) Density was determined by a true density measure ment method including the steps of vacuumizing a closed space in which an adsorbent is placed, removing the water vapor filled in the pores of the adsorbent and then refilling nitrogen gas, and calculating the amount of the refilled nitrogen gas to determine the true Volume and weight of the adsorbent to obtain the true density Determination of total acidic group content was carried out as follows. 1 g of adsorbent was added into 50 ml of 0.05Naqueous sodium hydroxide (NaOH), the mix ture was shaken for 48 hours at room temperature. After filtration, the filtrate was titrated to neutral with 0.05N
7 US 2016/ A1 Oct. 13, 2016 aqueous hydrogen chloride (HCl). The total acidic group content (per gram of sample, med/g) can be calculated from the amount of HCl used for titration. Determination of total basic group content was carried out as follows. 1 g of adsorbent was added into 50 ml of 0.05Naqueous HCl, the mixture was shaken for 24 hours at room temperature. After filtration, the filtrate was titrated to neutral with 0.05N aqueous NaOH. The total basic group content (per gram of sample, med/g) can be calculated from the amount of NaOH used for titration Adsorption test of indole and lipase in vitro was carried out in a simulated Solution simulating intestinal tract condition g of adsorbent was added into 10 ml of the simulated solution containing 100 ppm indole, 100 ppm lipase, and 5 wt % bile acid. After reacting for three hours at 37 C., the residual concentration of the indole and lipase in the simulated Solution was measured. The adsorption percentage was calculated based on the following equation: residual concentration adsorption percentage (%) = X 100 original concentration 0023 Regarding evaluation of food safety, BALB/c mice were fed with 5 wt % of the adsorbent of the present disclosure relative to the total weight of the feed each day for 30 days, and then the survival rate, behavior and color of feces of the mice were monitored to evaluate the food safety Adsorption test of precursors of uremic toxins in vivo was carried out as follows. Three male Sprague Dawley rats, weighing between 200 g and 250 g, were divided into a normal group (WT), a control group (Nep) and an experimental group (Nep-ACF). The rat in the normal group was not subjected to kidney excision and was fed with normal feed; the rat in the control group was subjected to 5/6 kidney excision and was fed with normal feed; and the rat in the experimental group was subjected to 5/6 kidney excision and was fed with the feed containing 5 wt % of the adsorbent of the present disclosure. These rats were fed for 10 weeks and the concentrations of indoxyl Sulfate, blood urea nitrogen, and creatinine in serums were measured every week After the adsorption test of precursors of uremic toxins in vivo was completed, pathological diagnosis of kidney tissue was conducted by obtaining the kidney tissue slices of these rats, which were stained by hematoxylin and eosin stain (H&E stain). The severity grading scheme of pathological diagnosis of kidney tissue was based on the method recited in Toxicologic Pathology, vol. 30, no. 1, pp 93-96, 2002, Shackelford et al. In the aforesaid method, a specific range of the kidney tissue was determined and graded, by the extent of the renal tubular degeneration or regeneration, into Grade 1 (minimal, less than 1%); Grade 2 (slight, 1 to 25%); Grade 3 (moderate, 26 to 50%); Grade 4 (moderately severe, 51 75%); and Grade 5 (severe, 76 to 100%). Example An adsorbent for reducing uremic toxins according to example 1 is a capsule in which polyacrylonitrile-based activated carbon fibers are encapsulated. The polyacryloni trile-based activated carbon fibers were prepared by treating an oxidized polyacrylonitrile-based carbon fiber material with carbon dioxide gas containing water vapor at a tem perature of 1000 C. for 5 minutes and grinding the carbon fiber material thus treated. In this example, the oxidized polyacrylonitrile-based carbon fiber material is formed by oxidizing a polyacrylonitrile-based carbon fiber cloth (Panex R 30, from Zoltek Companies, Inc.) which contains 90 wt % of polyacrylonitrile and 10 wt % of Rayon or petroleum pitch. The polyacrylonitrile-based activated car bon fibers thus prepared have an average diameter of 7.6 um; a BET specific surface area of 964 m/g; a density of 2.13 g/m; a percentage of micropores of 22%, a percentage of mesopores of 78%, and a percentage of macropores of 0%; an average length of um; a total acidic group of meq/g; and a total basic group of 1.30 meq/g. FIG. 1 is the SEM image showing the structure of the polyacrylo nitrile-based activated carbon fibers used in example FIG. 2 is the diagram showing the test result of the adsorption in vitro. As shown in FIG. 2, the adsorbent of example 1 has an adsorption percentage of indole of 89.6% and an adsorption percentage of lipase of 2.33%. Based on the result that the survival rate and behavior of the mice are normal and the colors of feces of the mice are dark, it is deduced that the adsorbent is excreted from gastro-intestinal tract of mice after digestion, and thus the adsorbent has good food safety FIG. 3 is the diagram showing the test result of the adsorption of indoxyl sulfate in vivo. As shown in FIG. 3, the concentration of indoxyl sulfate in serum of the rat in Nep-ACF (1.55 ng/ml) is lower than that of the rat in Nep (2.6 ng/ml) after a week. During ten weeks, all of the test results show that the concentrations of indoxyl sulfate in serum of the rat in Nep-ACF are lower than those of the rat in Nep. Therefore, it is proved that the adsorbent of the present disclosure can effectively reduce the amount of uremic toxins accumulated in the body of rat with deterio rated renal function FIG. 4 is the diagram showing the test result of the adsorption of blood urea nitrogen in vivo. As shown in FIG. 4, the concentration of blood urea nitrogen in serum of the rat in Nep-ACF (36 mg/dl) is lower than that of the rat in Nep (38 mg/dl) after a week. After ten weeks, the concen tration of blood urea nitrogen in serum of the rat in Nep ACF (30 mg/dl) is slightly higher than that of the rat in WT (22 mg/dl) of about 8 mg/dl, whereas the concentration of blood urea nitrogen in serum of the rat in Nep (56 mg/dl) is higher than that of the rat in WT of about 34 mg/dl. That is, the accumulated concentration of blood urea nitrogen in serum of the rat in Nep-ACF is only 24% of that of the rat in Nep. Therefore, it is proved that the adsorbent of the present disclosure can effectively reduce the amount of blood urea nitrogen accumulated in the body of rat with deteriorated renal function FIG. 5 is the diagram showing the test result of the adsorption of creatinine in vivo. As shown in FIG. 5, the concentration of creatinine in serum of the rat in Nep-ACF (0.6 mg/dl) is lower than that of the rat in Nep (0.75 mg/dl) after a week. After ten weeks, the concentration of creatinine in serum of the rat in Nep-ACF (0.61 mg/dl) is slightly higher than that of the rat in WT (0.36 mg/dl) of about 0.25 mg/dl, whereas the concentration of creatinine in serum of the rat in Nep (0.86 mg/dl) is higher than that of the rat in WT of about 0.5 mg/dl. That is, the accumulated concen tration of creatinine in serum of the rat in Nep-ACF is only 50% of that of the rat in Nep. Therefore, it is proved that the
8 US 2016/ A1 Oct. 13, 2016 adsorbent of the present disclosure can effectively reduce the amount of creatinine accumulated in the body of rat with deteriorated renal function FIGS. 6-8 show the severity of kidney tissue dam age of the rats. As shown in FIG. 6, the rat in WT has normal glomerulus 20 and renal tubular 22. FIG. 7 shows that the rat in Nep has degenerated or regenerated renal tubular 24 and has the severity of Grade 3. FIG. 8 shows that the rat in Nep-ACF has degenerated or regenerated renal tubular 24 and has the severity of Grade 2. In comparison with FIGS. 6-8, it is proved that the severity of kidney damage of the rat with deteriorated renal function can be decreased after the rat is fed with the adsorbent of the present disclosure for ten weeks In conclusion, the adsorbent of example 1 has a higher adsorption capacity for precursors of uremic toxins than for enzyme protein in intestinal tract, has a good food safety, can effectively reduce the concentration of the uremic toxins in Vivo, and can reduce the severity of kidney damage. Accordingly, the adsorbent of example 1 can decrease the deterioration rate of renal function while main taining the normal function of gastro-intestinal tract to avoid the need of dialysis treatment. Comparative Example A commercially available food-grade activated bamboo charcoal powder is used as an adsorbent of the comparative example. The activated bamboo charcoal pow der has an irregular shape; an average particle diameter of 2.9:1.4 um; a BET specific surface area of 329 m/g; and a percentage of micropores of 15.7%, a percentage of mes opores of 83.5%, and a percentage of macropores of 0.7%. As shown in FIG. 2, the adsorbent of the comparative example has an adsorption percentage of indole of 17.1% and an adsorption percentage of lipase of 23.25%, that is the adsorbent of the comparative example has a higher adsorp tion capacity for enzyme protein in intestinal tract than for precursors of uremic toxins. Accordingly, the adsorbent of the comparative example cannot effectively reduce the amount of uremic toxins accumulated in the body and may disturb the normal function of gastro-intestinal tract Because the adsorbent of example 1 has a higher adsorption capacity for precursors of uremic toxins and a lower adsorption capacity for enzyme protein in intestinal tract than the adsorbent of the comparative example, the adsorbent of example 1 can reduce the deterioration rate of renal function while maintaining the normal function of the gastro-intestinal tract. In addition to example 1, the follow ing examples 2 to 5 have similar effect. Example An adsorbent according to example 2 is a capsule in which polyacrylonitrile-based activated carbon fibers are encapsulated. The polyacrylonitrile-based activated carbon fibers were prepared by oxidizing the polyacrylonitrile based carbon fiber cloth (Panex R 30, from Zoltek Compa nies, Inc.); treating the oxidized polyacrylonitrile-based car bon fiber cloth with the carbon dioxide gas containing water vapor at a temperature of 900 C. for 20 minutes; and grinding the carbon fiber thus treated. The polyacrylonitrile based activated carbon fibers have an average diameter of 9.3 um; a BET specific surface area of 398 m/g: a density of g/m; a percentage of micropores of 18%, a percentage of mesopores of 82%, and a percentage of macropores of 0%; an average length of um; a total acidic group of med/g; and a total basic group of 0.9 med/g. The test result of the adsorption in vitro is shown in FIG. 2, in which the adsorbent of example 2 has an adsorp tion percentage of indole of 75.3% and an adsorption percentage of lipase of 6.3%. It can be seen, from the aforesaid result, that the adsorbent of example 2 has a higher adsorption capacity for precursors of uremic toxins than for enzyme protein in intestinal tract. Example An adsorbent according to example 3 is a capsule in which polyacrylonitrile-based activated carbon fibers are encapsulated. The polyacrylonitrile-based activated carbon fibers were prepared by oxidizing the polyacrylonitrile based carbon fiber cloth (Panex R 30, from Zoltek Compa nies, Inc.); treating the oxidized polyacrylonitrile-based car bon fiber cloth with the carbon dioxide gas containing water vapor at a temperature of 900 C. for 40 minutes; and grinding the carbon fiber thus treated. The polyacrylonitrile based activated carbon fibers have an average diameter of 8.6 um; a BET specific surface area of 92.1 m/g; a density of g/m; a percentage of micropores of 21%, a percentage of mesopores of 79%, and a percentage of macropores of 0%; an average length of 21.9t1.4 um; a total acidic group of med/g; and a total basic group of 1.26 med/g. The test result of the adsorption in vitro is shown in FIG. 2, in which the adsorbent of example 3 has an adsorp tion percentage of indole of 84.7% and an adsorption percentage of lipase of 4.4%. It can be seen, from the aforesaid result, that the adsorbent of example 3 has a higher adsorption capacity for precursors of uremic toxins than for enzyme protein in intestinal tract. Example An adsorbent according to example 4 is a capsule in which polyacrylonitrile-based activated carbon fibers are encapsulated. The polyacrylonitrile-based activated carbon fibers were prepared by oxidizing the polyacrylonitrile based carbon fiber cloth (Panex R 30, from Zoltek Compa nies, Inc.); treating the oxidized polyacrylonitrile-based car bon fiber cloth with the carbon dioxide gas containing water vapor at a temperature of 1000 C. for 20 minutes; and grinding the carbon fiber thus treated. The polyacrylonitrile based activated carbon fibers have an average diameter of 6 um; a BET specific surface area of 1244 m/g; a density of g/m; a percentage of micropores of 18%, a percent age of mesopores of 82%, and a percentage of macropores of 0%; an average length of um; a total acidic group of med/g; and a total basic group of med/g. The test result of the adsorption in vitro is shown in FIG. 2, in which the adsorbent of example 4 has an adsorp tion percentage of indole of 84.3% and an adsorption percentage of lipase of 8.9%. It can be seen, from the aforesaid result, that the adsorbent of example 4 has a higher adsorption capacity for precursors of uremic toxins than for enzyme protein in intestinal tract. Example An adsorbent according to example 5 is a capsule in which polyacrylonitrile-based activated carbon fibers are encapsulated. The polyacrylonitrile-based activated carbon
9 US 2016/ A1 Oct. 13, 2016 fibers were prepared by oxidizing the polyacrylonitrile based carbon fiber cloth (Panex R 30, from Zoltek Compa nies, Inc.); treating the oxidized polyacrylonitrile-based car bon fiber cloth with the carbon dioxide gas containing water vapor at a temperature of 1000 C. for 40 minutes; and grinding the carbon fiber thus treated. The polyacrylonitrile based activated carbon fibers have an average diameter of 5.6 um; a BET specific surface area of 1494 m/g; a density of g/m; a percentage of micropores of 19%, a percentage of mesopores of 81%, and a percentage of macropores of 0%; an average length of um; a total acidic group of 1.7 med/g; and a total basic group of med/g. The test result of the adsorption in vitro is shown in FIG. 2, in which the adsorbent of example 5 has an adsorp tion percentage of indole of 81.2% and an adsorption percentage of lipase of 24.5%. It can be seen, from the aforesaid result, that the adsorbent of example 5 has a higher adsorption capacity for precursors of uremic toxins than for enzyme protein in intestinal tract As described above, the adsorbent which com prises the polyacrylonitrile-based activated carbon fibers having an average diameter of 5-30 um, a BET specific surface area of more than 390 m?g, a total acidic group content of larger than 1.2 med/g or a total basic group content of larger than 1 med/g, can have a higher adsorption capacity for precursors of uremic toxins than for enzyme protein in intestinal tract. As such, the adsorbent can reduce the deterioration rate of renal function while maintaining the normal function of gastro-intestinal tract to avoid dialysis treatment. Preferably, the polyacrylonitrile-based activated carbon fibers have the average diameter of 5-10 um. Pref erably, the polyacrylonitrile-based activated carbon fibers have an average length of more than 20 Lum. Preferably, the polyacrylonitrile-based activated carbon fibers have the BET specific surface area in a range of 900 m/g to 1500 m?g. Preferably, the polyacrylonitrile-based activated car bon fibers have the total acidic group content in a range of 1.2 med/g to 1.7 med/g or the total basic group content in a range of 1 med/g to 1.7 med/g; and more preferably, the polyacrylonitrile-based activated carbon fibers have the total acidic group content in a range of meq/g to 1.7 med/g or the total basic group content in a range of 1.26 med/g to meq/g The polyacrylonitrile-based activated carbon fibers contained in the adsorbent of the present disclosure can be prepared by oxidizing the polyacrylonitrile-based carbon fiber cloth (Panex R 30, from Zoltek Companies, Inc.); treating the oxidized polyacrylonitrile-based carbon fiber cloth with the carbon dioxide gas containing water vapor at the temperature in a range of 700 C. to 1000 C. for 1 to 60 minutes; and grinding the carbon fiber thus treated. In the present disclosure, there is no specific limit on the polyacry lonitrile-based carbon fiber cloth, that is a carbon fiber cloth which may or may not contain Rayon or petroleum pitch can be used to prepare the carbon fibers of the present disclosure The adsorbent of the present disclosure may further comprise a plurality of activated particles such as silver, gold, aluminum, lead, zinc, copper or titanium dioxide particles attached to surfaces of the polyacrylonitrile-based activated carbon fibers, such that the adsorbent may have the ability to reduce the bacteria in intestinal tract and thereby further reducing the concentration of indoxyl sulfate in serum of rat. The activated particles may preferably have a diameter in a range of 1 nm to 500 um It should be understood that the above detailed description and specific examples are given by way of illustration only and are not limitative of the present disclo sure. Numerous variations and modifications within the spirit of the present disclosure are intended to be included within the scope of the appended claims. What is claimed is: 1. An adsorbent for reducing uremic toxins in vivo, comprising: polyacrylonitrile-based activated carbon fibers having the following properties: (a) an average diameter ranging from 5 um to 30 um; (b) a BET specific surface area of more than 390 m/g: and (c) a total acidic group content of larger than 1.2 med/g or a total basic group content of larger than 1 med/g. 2. The adsorbent for reducing uremic toxins in Vivo as activated carbon fibers have the total acidic group content ranging from 1.2 med/g to 1.7 med/g or the total basic group content ranging from 1 med/g to 1.7 med/g. 3. The adsorbent for reducing uremic toxins in vivo as activated carbon fibers have the total acidic group content ranging from med/g to 1.7 med/g or the total basic group content ranging from 1.26 meq/g to med/g. 4. The adsorbent for reducing uremic toxins in vivo as activated carbon fibers have an average length of more than 20 um. 5. The adsorbent for reducing uremic toxins in vivo as activated carbon fibers are prepared by treating an oxidized polyacrylonitrile-based carbon fiber material with a carbon dioxide gas containing water vapor at a temperature ranging from 700 C. to 1000 C. for 1 to 60 minutes. 6. The adsorbent for reducing uremic toxins in vivo as activated carbon fibers are prepared by oxidizing a poly acrylonitrile-based carbon fiber cloth which contains 90 wt % of polyacrylonitrile and 10 wt % of Rayon or petroleum pitch, and treating the oxidized polyacrylonitrile-based car bon fiber cloth with carbon dioxide gas containing water vapor at a temperature ranging from 700 C. to 1000 C. for 1 to 60 minutes. 7. The adsorbent for reducing uremic toxins in vivo as activated carbon fibers have the average diameter of 5 um to 10 um. 8. The adsorbent for reducing uremic toxins in vivo as activated carbon fibers have the BET specific surface area ranging from 900 m/g to 1500 m/g. 9. The adsorbent for reducing uremic toxins in vivo as claimed in claim 1, further comprising a plurality of acti vated particles attached to surfaces of the polyacrylonitrile based activated carbon fibers; wherein the plurality of acti vated particles are selected from the group consisting of silver particles, gold particles, aluminum particles, lead particles, Zinc particles, copper particles and titanium diox ide particles.
10 US 2016/ A1 Oct. 13, The adsorbent for reducing uremic toxins in vivo as claimed in claim 9, wherein the plurality of activated par ticles have a diameter ranging from 1 nm to 500 um. k k k k k
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 2009.02451 05A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0245105 A1 HO (43) Pub. Date: Oct. 1, 2009 (54) METHOD FOR NETWORK TRANSMISSION (30) Foreign Application
(19) United States US 2009.02451 05A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0245105 A1 HO (43) Pub. Date: Oct. 1, 2009 (54) METHOD FOR NETWORK TRANSMISSION (30) Foreign Application
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 2006O115435A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0115435 A1 Wilkens (43) Pub. Date: (54) DENTAL TOOTHPASTE, SOLUTION, TABLET AND SYSTEM WITH PLAOUE COLOR INDICATOR
(19) United States US 2006O115435A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0115435 A1 Wilkens (43) Pub. Date: (54) DENTAL TOOTHPASTE, SOLUTION, TABLET AND SYSTEM WITH PLAOUE COLOR INDICATOR
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 2008.0092779A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0092779 A1 Wang et al. (43) Pub. Date: Apr. 24, 2008 (54) ULTRAVIOLET ABSORBER FORMULATION (30) Foreign Application
US 2008.0092779A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0092779 A1 Wang et al. (43) Pub. Date: Apr. 24, 2008 (54) ULTRAVIOLET ABSORBER FORMULATION (30) Foreign Application
( 12 ) Patent Application Publication ( 10 ) Pub. No. : US 2017 / A1 ( 52 ) U. S. CI.
 TAN TANITIKAN UIT IN DE ON A MATATANI MATATU US 20170304494A1 ( 19 ) United States ( 12 ) Patent Application Publication ( 10 ) Pub. No. : US 2017 / 0304494 A1 Maldonado et al. ( 43 ) Pub. Date : Oct.
TAN TANITIKAN UIT IN DE ON A MATATANI MATATU US 20170304494A1 ( 19 ) United States ( 12 ) Patent Application Publication ( 10 ) Pub. No. : US 2017 / 0304494 A1 Maldonado et al. ( 43 ) Pub. Date : Oct.
EXCRETION QUESTIONS. Use the following information to answer the next two questions.
 EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States US 201701 65099A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0165099 A1 Buddharaju (43) Pub. Date: Jun. 15, 2017 (54) CONDOM CATHETER (52) U.S. Cl. CPC... A61F 5/453
(19) United States US 201701 65099A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0165099 A1 Buddharaju (43) Pub. Date: Jun. 15, 2017 (54) CONDOM CATHETER (52) U.S. Cl. CPC... A61F 5/453
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1. (51) Int. Cl. s 8
 (19) United States US 2007.0049567A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0049567 A1 Wiley (43) Pub. Date: Mar. 1, 2007 (54) HORMONE REPLACEMENT COMPOSITION AND METHOD (76) Inventor:
(19) United States US 2007.0049567A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0049567 A1 Wiley (43) Pub. Date: Mar. 1, 2007 (54) HORMONE REPLACEMENT COMPOSITION AND METHOD (76) Inventor:
(12) United States Patent
 USOO951 0624B2 (12) United States Patent Li et al. (10) Patent No.: (45) Date of Patent: US 9,510,624 B2 Dec. 6, 2016 (54) DISPOSABLE ELECTRONIC CIGARETTE (75) Inventors: Yonghai Li, Shenzhen (CN); Zhongli
USOO951 0624B2 (12) United States Patent Li et al. (10) Patent No.: (45) Date of Patent: US 9,510,624 B2 Dec. 6, 2016 (54) DISPOSABLE ELECTRONIC CIGARETTE (75) Inventors: Yonghai Li, Shenzhen (CN); Zhongli
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 US 20070087996A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0087996 A1 Hagiwara (43) Pub. Date: (54) METHOD OF EXTRACTING FUCOIDAN (52) U.S. Cl.... 514/54; 536/54 (76)
US 20070087996A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0087996 A1 Hagiwara (43) Pub. Date: (54) METHOD OF EXTRACTING FUCOIDAN (52) U.S. Cl.... 514/54; 536/54 (76)
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150230484A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0230484 A1 Döring (43) Pub. Date: (54) LACTOSE-FREE MILK PRODUCTS (52) U.S. Cl. CPC... A23C 9/1206 (2013.01)
(19) United States US 20150230484A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0230484 A1 Döring (43) Pub. Date: (54) LACTOSE-FREE MILK PRODUCTS (52) U.S. Cl. CPC... A23C 9/1206 (2013.01)
ADSORPTION AND DESORPTION OF METAL IONS BY SYSTEMS BASED ON CELLULOSE DERIVATIVES THAT CONTAIN AMINO ACID RESIDUES"
 (41) Vol. 41, No.6 (1985) T-235 (Received May 24, 1984) ADSORPTION AND DESORPTION OF METAL IONS BY SYSTEMS BASED ON CELLULOSE DERIVATIVES THAT CONTAIN AMINO ACID RESIDUES" By Toshihiko Sato, Shigenori
(41) Vol. 41, No.6 (1985) T-235 (Received May 24, 1984) ADSORPTION AND DESORPTION OF METAL IONS BY SYSTEMS BASED ON CELLULOSE DERIVATIVES THAT CONTAIN AMINO ACID RESIDUES" By Toshihiko Sato, Shigenori
EXCRETION IN HUMANS 31 JULY 2013
 EXCRETION IN HUMANS 31 JULY 2013 Lesson Description In this lesson we: Discuss organs of excretion Look at the structure of the urinary system Look at the structure and functioning of the kidney Discuss
EXCRETION IN HUMANS 31 JULY 2013 Lesson Description In this lesson we: Discuss organs of excretion Look at the structure of the urinary system Look at the structure and functioning of the kidney Discuss
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 2016O1 OO694A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0100694 A1 PELLETER (43) Pub. Date: Apr. 14, 2016 (54) KNEE PILLOW Publication Classification (71) Applicant:
US 2016O1 OO694A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0100694 A1 PELLETER (43) Pub. Date: Apr. 14, 2016 (54) KNEE PILLOW Publication Classification (71) Applicant:
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 20160135857A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0135857 A1 Marrero, SR. (43) Pub. Date: May 19, 2016 (54) CURVEDTIBIOTALARFUSION NAIL AND Publication Classification
US 20160135857A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0135857 A1 Marrero, SR. (43) Pub. Date: May 19, 2016 (54) CURVEDTIBIOTALARFUSION NAIL AND Publication Classification
Feedstuffs Analysis G-22-1 PROTEIN
 Feedstuffs Analysis G-22-1 PROTEIN PRINCIPLE SCOPE Many modifications of the Kjeldahl method have been accepted for the estimation of protein in organic materials. It comprises sample oxidation and conversion
Feedstuffs Analysis G-22-1 PROTEIN PRINCIPLE SCOPE Many modifications of the Kjeldahl method have been accepted for the estimation of protein in organic materials. It comprises sample oxidation and conversion
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0193911A1 Ketsela et al. US 2006O193911A1 (43) Pub. Date: (54) (75) (73) (21) (22) (60) CONTROLLED RELEASE VENLAFAXNE FORMULATIONS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0193911A1 Ketsela et al. US 2006O193911A1 (43) Pub. Date: (54) (75) (73) (21) (22) (60) CONTROLLED RELEASE VENLAFAXNE FORMULATIONS
...d. EEEEEEE/EE/EE. (12) Patent Application Publication (10) Pub. No.: US 2008/ A1. (19) United States
 (19) United States US 200802684.85A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0268485 A1 Guarino et al. (43) Pub. Date: Oct. 30, 2008 (54) ICONIC DISPLAY OF MARKERS FOR A METER (76) Inventors:
(19) United States US 200802684.85A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0268485 A1 Guarino et al. (43) Pub. Date: Oct. 30, 2008 (54) ICONIC DISPLAY OF MARKERS FOR A METER (76) Inventors:
SDIVISION. Attorney Docket No Date: 14 May 2008
 SDIVISION DEPARfMENT OF THE NAVY NAVAL UNDERSEA WARFARE CENTER NEWPORT OFFICE OF COUNSEL PHONE: (401) 832-3653 FAX: (401) 832-4432 NEWPORT DSN: 432-3653 Attorney Docket No. 95764 Date: 14 May 2008 The
SDIVISION DEPARfMENT OF THE NAVY NAVAL UNDERSEA WARFARE CENTER NEWPORT OFFICE OF COUNSEL PHONE: (401) 832-3653 FAX: (401) 832-4432 NEWPORT DSN: 432-3653 Attorney Docket No. 95764 Date: 14 May 2008 The
Kidneys and Homeostasis
 16 The Urinary System The Urinary System OUTLINE: Eliminating Waste Components of the Urinary System Kidneys and Homeostasis Urination Urinary Tract Infections Eliminating Waste Excretion Elimination of
16 The Urinary System The Urinary System OUTLINE: Eliminating Waste Components of the Urinary System Kidneys and Homeostasis Urination Urinary Tract Infections Eliminating Waste Excretion Elimination of
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0039210 A1 Yates et al. US 20090039210A1 (43) Pub. Date: Feb. 12, 2009 (54) (76) (21) (22) (60) CPAP HOSE SUPPORT SYSTEM Inventors:
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0039210 A1 Yates et al. US 20090039210A1 (43) Pub. Date: Feb. 12, 2009 (54) (76) (21) (22) (60) CPAP HOSE SUPPORT SYSTEM Inventors:
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0113348 A1 Douskey et al. US 2015O113348A1 (43) Pub. Date: Apr. 23, 2015 (54) (71) (72) (73) (21) (22) IMPLEMENTING MISR COMPRESSION
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0113348 A1 Douskey et al. US 2015O113348A1 (43) Pub. Date: Apr. 23, 2015 (54) (71) (72) (73) (21) (22) IMPLEMENTING MISR COMPRESSION
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0166282 A1 Martinez et al. US 20140166282A1 (43) Pub. Date: (54) (71) (72) (73) (21) (22) (60) FUNCTIONALIZED HYDROGEN SULFIDE
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0166282 A1 Martinez et al. US 20140166282A1 (43) Pub. Date: (54) (71) (72) (73) (21) (22) (60) FUNCTIONALIZED HYDROGEN SULFIDE
(12) United States Patent (10) Patent No.: US 6,691,578 B1
 USOO6691578B1 (12) United States Patent (10) Patent No.: US 6,691,578 B1 Puskas (45) Date of Patent: Feb. 17, 2004 (54) MEASUREMENT SYSTEMS FOR 4,710,233 A 12/1987 Hohmann et al.... 205/701 ULTRASOUND
USOO6691578B1 (12) United States Patent (10) Patent No.: US 6,691,578 B1 Puskas (45) Date of Patent: Feb. 17, 2004 (54) MEASUREMENT SYSTEMS FOR 4,710,233 A 12/1987 Hohmann et al.... 205/701 ULTRASOUND
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 US 20070050.058A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0050058 A1 Zuziak et al. (43) Pub. Date: Mar. 1, 2007 (54) PLACEMAT FOR CALCULATING AND Related U.S. Application
US 20070050.058A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0050058 A1 Zuziak et al. (43) Pub. Date: Mar. 1, 2007 (54) PLACEMAT FOR CALCULATING AND Related U.S. Application
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0274397 A1 Weimann et al. US 2011027.4397A1 (43) Pub. Date: Nov. 10, 2011 (54) (75) (73) (21) (22) TIGHT-BUFFERED OPTICAL FIBER
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0274397 A1 Weimann et al. US 2011027.4397A1 (43) Pub. Date: Nov. 10, 2011 (54) (75) (73) (21) (22) TIGHT-BUFFERED OPTICAL FIBER
PHYSICAL PROPERTIES AND DETECTION OF NORMAL CONSTITUENTS OF URINE
 PHYSICAL PROPERTIES AND DETECTION OF NORMAL CONSTITUENTS OF URINE - OBJECTIVES: 1- The simple examination of urine. 2- To detect some of the normal organic constituents of urine. 3- To detect some of the
PHYSICAL PROPERTIES AND DETECTION OF NORMAL CONSTITUENTS OF URINE - OBJECTIVES: 1- The simple examination of urine. 2- To detect some of the normal organic constituents of urine. 3- To detect some of the
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 US 2003O130096A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0130096 A1 LaCroce (43) Pub. Date: Jul. 10, 2003 (54) BARBELL WITH PLURAL HAND GRIPPING Publication Classification
US 2003O130096A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0130096 A1 LaCroce (43) Pub. Date: Jul. 10, 2003 (54) BARBELL WITH PLURAL HAND GRIPPING Publication Classification
60 Provisional application No. 60/030,168, Nov. 13, administration to plants. The fertilizer additives include
 USOO59970A United States Patent (19) 11 Patent Number: 5,997,0 Dean () Date of Patent: Dec. 7, 1999 54 FERTILIZER COMPOSITIONS INCLUDING FOREIGN PATENT DOCUMENTS CHELATED METALIONS 1433 8/1980 Germany...
USOO59970A United States Patent (19) 11 Patent Number: 5,997,0 Dean () Date of Patent: Dec. 7, 1999 54 FERTILIZER COMPOSITIONS INCLUDING FOREIGN PATENT DOCUMENTS CHELATED METALIONS 1433 8/1980 Germany...
IIII. United States Patent (19) Nolan et al. 11 Patent Number: 5,776,150 45) Date of Patent: Jul. 7, 1998
 United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
United States Patent (19) Groesch et al.
 United States Patent (19) Groesch et al. 54 DUMMY FOR CAR CRASH TESTING 75) Inventors: Lothar Groesch; Gabriel Netzer, both of Stuttgart; Lothar Kassing, Nufringen, all of Fed. Rep. of Germany 73) Assignee:
United States Patent (19) Groesch et al. 54 DUMMY FOR CAR CRASH TESTING 75) Inventors: Lothar Groesch; Gabriel Netzer, both of Stuttgart; Lothar Kassing, Nufringen, all of Fed. Rep. of Germany 73) Assignee:
United States Patent (19) Ashmead et al.
 United States Patent (19) Ashmead et al. 54 (75) 73) ZINCAMINO ACID CHELATES HAVING LGANDS COMPRISED OF GLYCINE AND A SULFUR-CONTAINING AMINO ACIDS Inventors: Stephen D. Ashmead; David C. Wheelwright,
United States Patent (19) Ashmead et al. 54 (75) 73) ZINCAMINO ACID CHELATES HAVING LGANDS COMPRISED OF GLYCINE AND A SULFUR-CONTAINING AMINO ACIDS Inventors: Stephen D. Ashmead; David C. Wheelwright,
IIII IIHill. United States Patent (19) Shelley et al. 11 Patent Number: 5,505, Date of Patent: Apr. 9, 1996
 United States Patent (19) Shelley et al. IIII IIHill USOO961A 11 Patent Number: Date of Patent: Apr. 9, 1996 (54) 75) 73) 21 22 63 51 (52) 58) 56 GELATIN CAPSULES CONTAINING A HIGHILY CONCENTRATED ACETAMNOPHEN
United States Patent (19) Shelley et al. IIII IIHill USOO961A 11 Patent Number: Date of Patent: Apr. 9, 1996 (54) 75) 73) 21 22 63 51 (52) 58) 56 GELATIN CAPSULES CONTAINING A HIGHILY CONCENTRATED ACETAMNOPHEN
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 2014001 1160A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0011160 A1 JOrneus et al. (43) Pub. Date: Jan. 9, 2014 (54) (71) (72) (73) (21) (22) (30) ABUTMENT SYSTEMAND
(19) United States US 2014001 1160A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0011160 A1 JOrneus et al. (43) Pub. Date: Jan. 9, 2014 (54) (71) (72) (73) (21) (22) (30) ABUTMENT SYSTEMAND
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O130094A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0130094A1 Graham (43) Pub. Date: Jun. 16, 2005 (54) ORTHODONTIC ACCESSORY ARCH BAR (52) U.S. Cl.... 433/20
(19) United States US 2005O130094A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0130094A1 Graham (43) Pub. Date: Jun. 16, 2005 (54) ORTHODONTIC ACCESSORY ARCH BAR (52) U.S. Cl.... 433/20
Engineering the Growth of TiO 2 Nanotube Arrays on Flexible Carbon Fibre Sheets
 Engineering the Growth of TiO 2 Nanotube Arrays on Flexible Carbon Fibre Sheets Peng Chen, a Li Gu, b Xiudong Xue, a Mingjuan Li a and Xuebo Cao* a a Key Lab of Organic Synthesis of Jiangsu Province and
Engineering the Growth of TiO 2 Nanotube Arrays on Flexible Carbon Fibre Sheets Peng Chen, a Li Gu, b Xiudong Xue, a Mingjuan Li a and Xuebo Cao* a a Key Lab of Organic Synthesis of Jiangsu Province and
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 20090305855A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0305855A1 Terre (43) Pub. Date: Dec. 10, 2009 (54) ABDOMINAL EXERCISE MACHINE (57) ABSTRACT An abdominal exercise
(19) United States US 20090305855A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0305855A1 Terre (43) Pub. Date: Dec. 10, 2009 (54) ABDOMINAL EXERCISE MACHINE (57) ABSTRACT An abdominal exercise
US A United States Patent (19) 11 Patent Number: 5,635,209 Groenewoud et al. 45 Date of Patent: Jun. 3, 1997
 US005635209A United States Patent (19) 11 Patent Number: Groenewoud et al. 45 Date of Patent: Jun. 3, 1997 (54) STABILIZED COMPOSITION OF LEVOTHYROXNE SODIUM MEDICATION 4,585,652 4,818,531 4/1986 Miller
US005635209A United States Patent (19) 11 Patent Number: Groenewoud et al. 45 Date of Patent: Jun. 3, 1997 (54) STABILIZED COMPOSITION OF LEVOTHYROXNE SODIUM MEDICATION 4,585,652 4,818,531 4/1986 Miller
(12) United States Patent Clapp et al. US 7,206,386 B2 Apr. 17, 2007 US B2. (45) Date of Patent:
 US007206386B2 (12) United States Patent Clapp et al. (io) Patent No.: (45) Date of Patent: US 7,206,386 B2 Apr. 17, 2007 (54) METHOD AND SYSTEM FOR ELECTRONIC COMMUNICATION WITH THE HEARING IMPAIRED (75)
US007206386B2 (12) United States Patent Clapp et al. (io) Patent No.: (45) Date of Patent: US 7,206,386 B2 Apr. 17, 2007 (54) METHOD AND SYSTEM FOR ELECTRONIC COMMUNICATION WITH THE HEARING IMPAIRED (75)
EFFECT OF AN ALUMINUM SUPPLEMENT ON NUTRIENT DIGESTIBILITY AND MINERAL METABOLISM IN THOROUGHBRED HORSES
 K.A. Roose et al. 119 EFFECT OF AN ALUMINUM SUPPLEMENT ON NUTRIENT DIGESTIBILITY AND MINERAL METABOLISM IN THOROUGHBRED HORSES K. A. ROOSE, K. E. HOEKSTRA, J. D. PAGAN, R. J. GEOR Kentucky Equine Research,
K.A. Roose et al. 119 EFFECT OF AN ALUMINUM SUPPLEMENT ON NUTRIENT DIGESTIBILITY AND MINERAL METABOLISM IN THOROUGHBRED HORSES K. A. ROOSE, K. E. HOEKSTRA, J. D. PAGAN, R. J. GEOR Kentucky Equine Research,
30.1 Organization of the Human Body
 30.1 Organization of the Human Body Lesson Objectives Describe how the human body is organized. Explain homeostasis. Lesson Summary Organization of the Body The levels of organization in a multicellular
30.1 Organization of the Human Body Lesson Objectives Describe how the human body is organized. Explain homeostasis. Lesson Summary Organization of the Body The levels of organization in a multicellular
The Urinary System. Lab Exercise 38. Objectives. Introduction
 Lab Exercise The Urinary System Objectives - Be able to identify the structures of the urinary system and give their function - Be able to recognize the gross anatomy of the kidney - Identify the components
Lab Exercise The Urinary System Objectives - Be able to identify the structures of the urinary system and give their function - Be able to recognize the gross anatomy of the kidney - Identify the components
RENAL FUNCTION An Overview
 RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 US 200700.94767A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0094767 A1 Liu (43) Pub. Date: (54) POWDER FREE VINYL NITRILE Publication Classification CO-POLYMER GLOVES
US 200700.94767A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0094767 A1 Liu (43) Pub. Date: (54) POWDER FREE VINYL NITRILE Publication Classification CO-POLYMER GLOVES
The Urinary System. BIOLOGY OF HUMANS Concepts, Applications, and Issues. Judith Goodenough Betty McGuire
 BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 16 The Urinary System Lecture Presentation Anne Gasc Hawaii Pacific University and University of Hawaii
BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 16 The Urinary System Lecture Presentation Anne Gasc Hawaii Pacific University and University of Hawaii
Estimation of Serum Urea. BCH472 [Practical] 1
![Estimation of Serum Urea. BCH472 [Practical] 1 Estimation of Serum Urea. BCH472 [Practical] 1](/thumbs/86/93847865.jpg) Estimation of Serum Urea BCH472 [Practical] 1 -Urea: Urea is the highest non-protein nitrogen compound in the blood. Urea is the major excretory product of protein metabolism. It is formed by urea cycle
Estimation of Serum Urea BCH472 [Practical] 1 -Urea: Urea is the highest non-protein nitrogen compound in the blood. Urea is the major excretory product of protein metabolism. It is formed by urea cycle
1 ADJUSTMENT THRESHOLD-N. United States Patent (19) Donehoo et al. 5,660,184. Aug. 26, 1997 PATIENT'S HEARTBEAT PACEMAKEREVENT QRS DETECTION THRESHOLD
 United States Patent (19) Donehoo et al. US00566O184A 11 Patent Number: 45 Date of Patent: 5,660,184 Aug. 26, 1997 54 PACEMAKER PULSE DETECTION AND ARTIFACT REJECTION 75 Inventors: Robert F. Donehoo, Lutz;
United States Patent (19) Donehoo et al. US00566O184A 11 Patent Number: 45 Date of Patent: 5,660,184 Aug. 26, 1997 54 PACEMAKER PULSE DETECTION AND ARTIFACT REJECTION 75 Inventors: Robert F. Donehoo, Lutz;
(12) United States Patent (10) Patent No.: US 6,334,451 B1
 USOO6334451B1 (12) United States Patent (10) Patent o.: US 6,334,451 B1 Yang (45) Date of Patent: Jan. 1, 2002 (54) TOOTHBRUSH WITH FILLIG OF 1,340,043 A 5/1920 Grace... 132/311 TOOTHPASTE 1,451,941. A
USOO6334451B1 (12) United States Patent (10) Patent o.: US 6,334,451 B1 Yang (45) Date of Patent: Jan. 1, 2002 (54) TOOTHBRUSH WITH FILLIG OF 1,340,043 A 5/1920 Grace... 132/311 TOOTHPASTE 1,451,941. A
1) Autotrophic nutrition in plants 2) Nutrition in Human Beings. 3) transportation in human being 4) Excretion in human being
 1 KENDRIYA VIDYALAYA VSN NAGPUR HOLIDAY HOMEWORK Class X A and X B (SCIENCE) Learn Chapter 1, 6 And 10 Worksheet on Chapter 1 and Chapter 6 Prepare presentation on 1) Autotrophic nutrition in plants 2)
1 KENDRIYA VIDYALAYA VSN NAGPUR HOLIDAY HOMEWORK Class X A and X B (SCIENCE) Learn Chapter 1, 6 And 10 Worksheet on Chapter 1 and Chapter 6 Prepare presentation on 1) Autotrophic nutrition in plants 2)
Estimation of Serum Urea
 Estimation of Serum Urea 1 -Urea: Urea is the highest non-protein nitrogen compound in the blood. Urea is the major excretory product of protein metabolism. It is formed by urea cycle in the liver from
Estimation of Serum Urea 1 -Urea: Urea is the highest non-protein nitrogen compound in the blood. Urea is the major excretory product of protein metabolism. It is formed by urea cycle in the liver from
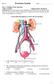
Use the following diagram to answer the next question. 1. In the diagram above, pressure filtration occurs in a. W b. X c. Y d. Z
 Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0042561 A1 HALL et al. US 201700.42561A1 (43) Pub. Date: Feb. 16, 2017 (54) (71) (72) (21) (22) (63) SKN GRAFT DEVICES AND
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0042561 A1 HALL et al. US 201700.42561A1 (43) Pub. Date: Feb. 16, 2017 (54) (71) (72) (21) (22) (63) SKN GRAFT DEVICES AND
United States Patent (19)
 United States Patent (19) Tsuchiya et al. (54) ODINE ADSORBENT 75 Inventors: Hiroyuki Tsuchiya; Kiyomi Funabashi; Makoto Kikuchi, all of Hitachi, Japan 73 Assignee: Hitachi, Ltd., Tokyo, Japan 21 Appl.
United States Patent (19) Tsuchiya et al. (54) ODINE ADSORBENT 75 Inventors: Hiroyuki Tsuchiya; Kiyomi Funabashi; Makoto Kikuchi, all of Hitachi, Japan 73 Assignee: Hitachi, Ltd., Tokyo, Japan 21 Appl.
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States US 2017005 1231A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0051231 A1 Mancosky (43) Pub. Date: (54) METHOD OF EXTRACTING CBD, THC, AND OTHER COMPOUNDS FROM CANNABS
(19) United States US 2017005 1231A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0051231 A1 Mancosky (43) Pub. Date: (54) METHOD OF EXTRACTING CBD, THC, AND OTHER COMPOUNDS FROM CANNABS
(12) United States Patent
 USOO7022364B1 (12) United States Patent De Meuter et al. (10) Patent No.: (45) Date of Patent: Apr. 4, 2006 (54) SUGAR-FREE HARD COATINGS PREPARED FROM LIQUID MIXTURES OF ERYTHRTOL AND SORBITOL (75) Inventors:
USOO7022364B1 (12) United States Patent De Meuter et al. (10) Patent No.: (45) Date of Patent: Apr. 4, 2006 (54) SUGAR-FREE HARD COATINGS PREPARED FROM LIQUID MIXTURES OF ERYTHRTOL AND SORBITOL (75) Inventors:
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090287233A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0287233 A1 Huculak (43) Pub. Date: Nov. 19, 2009 (54) SMALL GAUGE MECHANICAL TISSUE Publication Classification
US 20090287233A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0287233 A1 Huculak (43) Pub. Date: Nov. 19, 2009 (54) SMALL GAUGE MECHANICAL TISSUE Publication Classification
(12) United States Patent (10) Patent No.: US 7,109,035 B2
 US00790B2 (12) United States Patent () Patent No.: US 7,9,0 B2 Haddad () Date of Patent: Sep. 19, 2006 (54) SYNTHETIC URINE AND METHOD OF 4,590,800 A 5/1986 Shimoda... T3/449 MAKING SAME 4,714,564 A *
US00790B2 (12) United States Patent () Patent No.: US 7,9,0 B2 Haddad () Date of Patent: Sep. 19, 2006 (54) SYNTHETIC URINE AND METHOD OF 4,590,800 A 5/1986 Shimoda... T3/449 MAKING SAME 4,714,564 A *
Renal Disease and PK/PD. Anjay Rastogi MD PhD Division of Nephrology
 Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
United States Patent (19) James
 United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
(12) United States Patent
 (12) United States Patent Hotta et al. USOO686 1039B1 (10) Patent No.: () Date of Patent: Mar. 1, 2005 (54) METHOD FOR PURIFICATION OF PHOSPHORIC ACID HIGH PURITY POLYPHOSPHORIC ACID (75) Inventors: Kiyoshi
(12) United States Patent Hotta et al. USOO686 1039B1 (10) Patent No.: () Date of Patent: Mar. 1, 2005 (54) METHOD FOR PURIFICATION OF PHOSPHORIC ACID HIGH PURITY POLYPHOSPHORIC ACID (75) Inventors: Kiyoshi
(12) United States Patent
 (12) United States Patent USOO9682046B2 () Patent No.: Nishiwaki et al. () Date of Patent: Jun. 20, 2017 (54) ADSORBENTS FOR ORAL (56) References Cited ADMINISTRATION U.S. PATENT DOCUMENTS (71) Applicant:
(12) United States Patent USOO9682046B2 () Patent No.: Nishiwaki et al. () Date of Patent: Jun. 20, 2017 (54) ADSORBENTS FOR ORAL (56) References Cited ADMINISTRATION U.S. PATENT DOCUMENTS (71) Applicant:
UNIT 3 Conditions supporting life
 Biology Form 4 Page 32 Ms. R. Buttigieg UNIT 3 Conditions supporting life In this unit we shall be seeing how an important condition that supports life is the ability of the organism to maintain a constant
Biology Form 4 Page 32 Ms. R. Buttigieg UNIT 3 Conditions supporting life In this unit we shall be seeing how an important condition that supports life is the ability of the organism to maintain a constant
Excretory Lecture Test Questions Set 1
 Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Chapter 12. Excretion and the Interaction of Systems
 Chapter 12 Excretion and the Interaction of Systems 1 2 Goals for This Chapter 1. Identify the main structures and functions of the human excretory system 2. Explain the function of the nephron 3. Describe
Chapter 12 Excretion and the Interaction of Systems 1 2 Goals for This Chapter 1. Identify the main structures and functions of the human excretory system 2. Explain the function of the nephron 3. Describe
Nephrology - the study of the kidney. Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system
 Urinary System Nephrology - the study of the kidney Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system Functions of the Urinary System 1. Regulation
Urinary System Nephrology - the study of the kidney Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system Functions of the Urinary System 1. Regulation
Standardization of a Base, Mass Percent of an Acid
 Experiment 7 Standardization of a Base, Mass Percent of an Acid Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
Experiment 7 Standardization of a Base, Mass Percent of an Acid Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Mechanochemical Dry Conversion of Zinc Oxide to Zeolitic Imidazolate Framework
 Mechanochemical Dry Conversion of Zinc Oxide to Zeolitic Imidazolate Framework Shunsuke Tanaka, *a,b Koji Kida, a Takuya Nagaoka, a Takehiro Ota a and Yoshikazu Miyake a,b a Department of Chemical, Energy
Mechanochemical Dry Conversion of Zinc Oxide to Zeolitic Imidazolate Framework Shunsuke Tanaka, *a,b Koji Kida, a Takuya Nagaoka, a Takehiro Ota a and Yoshikazu Miyake a,b a Department of Chemical, Energy
Ulllted States Patent [19] [11] Patent Number: 5,997,600
![Ulllted States Patent [19] [11] Patent Number: 5,997,600 Ulllted States Patent [19] [11] Patent Number: 5,997,600](/thumbs/86/93631727.jpg) US005997600A Ulllted States Patent [19] [11] Patent Number: 5,997,600 Dean [45] Date of Patent: Dec. 7, 1999 [54] FERTILIZER COMPOSITIONS INCLUDING FOREIGN PATENT DOCUMENTS CHELATED METAL IONS 143365 8/1980
US005997600A Ulllted States Patent [19] [11] Patent Number: 5,997,600 Dean [45] Date of Patent: Dec. 7, 1999 [54] FERTILIZER COMPOSITIONS INCLUDING FOREIGN PATENT DOCUMENTS CHELATED METAL IONS 143365 8/1980
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 20080082047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0082047 A1 Harmon (43) Pub. Date: Apr. 3, 2008 (54) VEIN HOLDER (52) U.S. Cl.... 604/115 (76) Inventor: Stoney
US 20080082047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0082047 A1 Harmon (43) Pub. Date: Apr. 3, 2008 (54) VEIN HOLDER (52) U.S. Cl.... 604/115 (76) Inventor: Stoney
b. S --r 10. re-20. (12) Patent Application Publication (10) Pub. No.: US 2014/ A1. (19) United States. (43) Pub. Date: Aug.
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0227326 A1 Cagle et al. US 20140227326A1 (43) Pub. Date: (54) (71) (72) (73) (21) (22) (63) (60) METHOD OF DELVERING NASAL
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0227326 A1 Cagle et al. US 20140227326A1 (43) Pub. Date: (54) (71) (72) (73) (21) (22) (63) (60) METHOD OF DELVERING NASAL
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
IHIH IIII. United States Patent (19) 11 Patent Number: 5,417, Date of Patent: May 23, Kawai et al.
 United States Patent (19) Kawai et al. 54 METHOD FOR EXTRACTING AMARGIN LINE FOR DESIGNING AN ARTIFICIAL CROWN 75) Inventors: Masaharu Kawai, Kanagawa; Katsuya Miyoshi, Tokyo; Masami Baba, Saitama, all
United States Patent (19) Kawai et al. 54 METHOD FOR EXTRACTING AMARGIN LINE FOR DESIGNING AN ARTIFICIAL CROWN 75) Inventors: Masaharu Kawai, Kanagawa; Katsuya Miyoshi, Tokyo; Masami Baba, Saitama, all
Chapter: Interactions of Human Systems
 Table of Contents Chapter: Interactions of Human Systems Section 1: The Human Organism Section 2: How Your Body Works The Human Organism Organization in the Human Body Although your body is not made of
Table of Contents Chapter: Interactions of Human Systems Section 1: The Human Organism Section 2: How Your Body Works The Human Organism Organization in the Human Body Although your body is not made of
(12) Patent Application Publication (10) Pub. No.: US 2001/ A1
 US 2001 0026788A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2001/0026788A1 Piskorz (43) Pub. Date: (54) METHOD OF PRODUCING A NICOTINE (30) Foreign Application Priority Data
US 2001 0026788A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2001/0026788A1 Piskorz (43) Pub. Date: (54) METHOD OF PRODUCING A NICOTINE (30) Foreign Application Priority Data
( 12 ) United States Patent
 ( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
You should wear eye protection throughout this practical. Ammonia is corrosive and dangerous to the environment.
 Practical 3 - N (d)(m)urine Analysis Evaluating and reporting on observations This practical focuses on Recording data, drawing conclusions and evaluation. You will also be developing other assessed skills
Practical 3 - N (d)(m)urine Analysis Evaluating and reporting on observations This practical focuses on Recording data, drawing conclusions and evaluation. You will also be developing other assessed skills
(12) United States Patent
 (12) United States Patent Yanagawa et al. USOO6462231B1 (10) Patent No.: () Date of Patent: Oct. 8, 2002 (54) METHOD OF PRODUCING CARBOXYLIC ACID AND ALCOHOL (75) Inventors: Masatoshi Yanagawa, Niigata-ken
(12) United States Patent Yanagawa et al. USOO6462231B1 (10) Patent No.: () Date of Patent: Oct. 8, 2002 (54) METHOD OF PRODUCING CARBOXYLIC ACID AND ALCOHOL (75) Inventors: Masatoshi Yanagawa, Niigata-ken
Experiment 7, Analysis of KHP by titration with NaOH Wright College, Department of Physical Science and Engineering
 Name Date Experiment 7, Analysis of KHP by titration with NaOH Wright College, Department of Physical Science and Engineering In this experiment, you will determine the amount (percent) of potassium hydrogen
Name Date Experiment 7, Analysis of KHP by titration with NaOH Wright College, Department of Physical Science and Engineering In this experiment, you will determine the amount (percent) of potassium hydrogen
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 20080040891A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0040891 A1 Tyler (43) Pub. Date: Feb. 21, 2008 (54) EXERCISE EQUIPMENT HANDLE GRIPS Publication Classification
(19) United States US 20080040891A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0040891 A1 Tyler (43) Pub. Date: Feb. 21, 2008 (54) EXERCISE EQUIPMENT HANDLE GRIPS Publication Classification
(12) United States Patent
 (12) United States Patent USO0971 7274B2 (10) Patent No.: US 9,717.274 B2 Daehne et al. (45) Date of Patent: Aug. 1, 2017 (54) SMOKE-FREE CIGARETTE, CIGAR OR (52) U.S. Cl. PIPE CPC... A24F 47/008 (2013.01);
(12) United States Patent USO0971 7274B2 (10) Patent No.: US 9,717.274 B2 Daehne et al. (45) Date of Patent: Aug. 1, 2017 (54) SMOKE-FREE CIGARETTE, CIGAR OR (52) U.S. Cl. PIPE CPC... A24F 47/008 (2013.01);
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 20060051474A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0051474 A1 Furlong et al. (43) Pub. Date: (54) (76) (21) (22) (60) PROTEIN-ENRICHED NUT BUTTER COMPOSITIONS
(19) United States US 20060051474A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0051474 A1 Furlong et al. (43) Pub. Date: (54) (76) (21) (22) (60) PROTEIN-ENRICHED NUT BUTTER COMPOSITIONS
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0254.184 A1 Horlacher et al. US 200802541 84A1 (43) Pub. Date: (54) (76) (21) (22) (30) POWDER CONTAINING POLYUNSATURATED FATTY
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0254.184 A1 Horlacher et al. US 200802541 84A1 (43) Pub. Date: (54) (76) (21) (22) (30) POWDER CONTAINING POLYUNSATURATED FATTY
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150273 183A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0273183 A1 Foley et al. (43) Pub. Date: Oct. 1, 2015 (54) INTERMITTENTURINARY CATHETER ASSEMBLY AND ANADAPTER
(19) United States US 20150273 183A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0273183 A1 Foley et al. (43) Pub. Date: Oct. 1, 2015 (54) INTERMITTENTURINARY CATHETER ASSEMBLY AND ANADAPTER
Excretory System Workbook
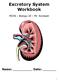 Excretory System Workbook MCHS Biology 20 Mr. Korotash Name: Date: 1 Study the diagram above. Name the structures and indicate their functions by completing the following table: Structure 1. Function 2.
Excretory System Workbook MCHS Biology 20 Mr. Korotash Name: Date: 1 Study the diagram above. Name the structures and indicate their functions by completing the following table: Structure 1. Function 2.
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0002979 A1 Guha US 2011 0002979A1 (43) Pub. Date: Jan. 6, 2011 (54) STYRENE MALEICANHYDRIDE BASED FORMULATION FORMALE CONTRACEPTION
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0002979 A1 Guha US 2011 0002979A1 (43) Pub. Date: Jan. 6, 2011 (54) STYRENE MALEICANHYDRIDE BASED FORMULATION FORMALE CONTRACEPTION
SECTION (2): HAZARDOUS INGREDIENTS Range % (by weight) SECTION (3): PHYSICAL DATA (Typical values. May vary from sample to sample)
 MATERIAL SAFETY DATA SHEET MANUFACTURER: MAXIM CHEMICAL INTERNATIONAL LTD. 1305 HALIFAX STREET, REGINA, SK S4R 1T9 PHONE: (306) 347-0444 SECTION (1): PRODUCT IDENTIFICATION AND USE PRODUCT NAME: OXALIC
MATERIAL SAFETY DATA SHEET MANUFACTURER: MAXIM CHEMICAL INTERNATIONAL LTD. 1305 HALIFAX STREET, REGINA, SK S4R 1T9 PHONE: (306) 347-0444 SECTION (1): PRODUCT IDENTIFICATION AND USE PRODUCT NAME: OXALIC
(12) United States Patent (10) Patent No.: US 9,039,574 B2
 US0090395.74B2 (12) United States Patent (10) Patent No.: US 9,039,574 B2 Wilson (45) Date of Patent: May 26, 2015 (54) EXERCISE RING USPC... 482/24, 44 50, 79, 91, 92, 121-128, 482/131, 139 (76) Inventor:
US0090395.74B2 (12) United States Patent (10) Patent No.: US 9,039,574 B2 Wilson (45) Date of Patent: May 26, 2015 (54) EXERCISE RING USPC... 482/24, 44 50, 79, 91, 92, 121-128, 482/131, 139 (76) Inventor:
USOO A United States Patent (19) 11 Patent Number: 5,504,055 Hsu 45 Date of Patent: Apr. 2, 1996
 IIII USOO5A United States Patent (19) 11 Patent Number: Hsu Date of Patent: Apr. 2, 1996 54 METAL AMINO ACID CHELATE 4,436,547 3/1984 Sampson... 71/76 4,8,716 5/1989 Ashmead...... 4f72 75 Inventor: Hsinhung
IIII USOO5A United States Patent (19) 11 Patent Number: Hsu Date of Patent: Apr. 2, 1996 54 METAL AMINO ACID CHELATE 4,436,547 3/1984 Sampson... 71/76 4,8,716 5/1989 Ashmead...... 4f72 75 Inventor: Hsinhung
(12) United States Patent (10) Patent No.: US 8.426,654 B2
 USOO8426654B2 (12) United States Patent () Patent No.: US 8.426,654 B2 B0ensch et al. (45) Date of Patent: Apr. 23, 2013 (54) METHOD FOR THE PRODUCTION OF E. bi A 3.28. push tal eoka et al. FATTY ALCOHOLS
USOO8426654B2 (12) United States Patent () Patent No.: US 8.426,654 B2 B0ensch et al. (45) Date of Patent: Apr. 23, 2013 (54) METHOD FOR THE PRODUCTION OF E. bi A 3.28. push tal eoka et al. FATTY ALCOHOLS
BCH 447. Estimation of Serum Urea
 BCH 447 Estimation of Serum Urea 1 Objective: Estimation of Blood urea nitrogen (BUN) in serum sample. 2 -Urea: Urea is the highest non-protein nitrogen compound in the blood. Urea is the major excretory
BCH 447 Estimation of Serum Urea 1 Objective: Estimation of Blood urea nitrogen (BUN) in serum sample. 2 -Urea: Urea is the highest non-protein nitrogen compound in the blood. Urea is the major excretory
NATIONAL KIDNEY MONTH
 NATIONAL KIDNEY MONTH According to the WebMD website, kidneys have several specific roles: Maintain your body s balance of water and concentration of minerals, such as sodium, potassium, magnesium and
NATIONAL KIDNEY MONTH According to the WebMD website, kidneys have several specific roles: Maintain your body s balance of water and concentration of minerals, such as sodium, potassium, magnesium and
DEPARTMENT OF THE NAVY NAVAL UNDERSEA WARFARE CENTER DIVISION NEWPORT
 DEPARTMENT OF THE NAVY NAVAL UNDERSEA WARFARE CENTER DIVISION NEWPORT JTO OFFICE OF COUNSEL (PATENTS) 1176 HOWELL STREET BUILDING 112T, CODE OOOC * NEWPORT, RHODE ISLAND 02841-1708 PHONE: 401 832-4736
DEPARTMENT OF THE NAVY NAVAL UNDERSEA WARFARE CENTER DIVISION NEWPORT JTO OFFICE OF COUNSEL (PATENTS) 1176 HOWELL STREET BUILDING 112T, CODE OOOC * NEWPORT, RHODE ISLAND 02841-1708 PHONE: 401 832-4736
(12) United States Patent
 US009 138575B2 (12) United States Patent Osypka (54) (71) BALLOON CATHETER Applicant: Peter Osypka Stiftung, Grenzach-Wyhlen (DE) (72) Inventor: Peter Osypka, Grenzach-Wyhlen (DE) (73) Assignee: Peter
US009 138575B2 (12) United States Patent Osypka (54) (71) BALLOON CATHETER Applicant: Peter Osypka Stiftung, Grenzach-Wyhlen (DE) (72) Inventor: Peter Osypka, Grenzach-Wyhlen (DE) (73) Assignee: Peter
The Excretory System
 The Excretory System The excretory system The excretory system includes the skin, lungs and kidneys which all release metabolic wastes from the body. The kidneys, skin and the lungs are the principle organs
The Excretory System The excretory system The excretory system includes the skin, lungs and kidneys which all release metabolic wastes from the body. The kidneys, skin and the lungs are the principle organs
Sepetka et al. (45) Date of Patent: May 3, ) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson.
 United States Patent (19) 11 USOO5308342A Patent Number: Sepetka et al. (45) Date of Patent: May 3, 1994 54) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson. o 4,775,371 10/1988 Mueller, Jr.... 604/280
United States Patent (19) 11 USOO5308342A Patent Number: Sepetka et al. (45) Date of Patent: May 3, 1994 54) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson. o 4,775,371 10/1988 Mueller, Jr.... 604/280
Product Safety Assessment VenPure Borohydride Products
 Product Safety Assessment VenPure Borohydride Products Product Safety Assessment documents are available at: www.dow.com/productsafety/finder/. Select a Topic: Names Product Overview Manufacture of Product
Product Safety Assessment VenPure Borohydride Products Product Safety Assessment documents are available at: www.dow.com/productsafety/finder/. Select a Topic: Names Product Overview Manufacture of Product
(12) United States Patent (10) Patent No.: US 6,276,248 B1. Cranna (45) Date of Patent: Aug. 21, 2001
 USOO6276248B1 (12) United States Patent (10) Patent No.: Cranna () Date of Patent: Aug. 21, 2001 (54) BAND SAW BLADE HAVING REDUCED 5,331,876 * 7/1994 Hayden, Sr.... 83/851 NOISE AND UNIFORM TOOTH LOADING
USOO6276248B1 (12) United States Patent (10) Patent No.: Cranna () Date of Patent: Aug. 21, 2001 (54) BAND SAW BLADE HAVING REDUCED 5,331,876 * 7/1994 Hayden, Sr.... 83/851 NOISE AND UNIFORM TOOTH LOADING
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 20160376526A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0376526 A1 Smith (43) Pub. Date: (54) POTASSIUM SOAPS THAT CAN BE A61O 19/10 (2006.01) THICKENED WITH CHLORIDE
(19) United States US 20160376526A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0376526 A1 Smith (43) Pub. Date: (54) POTASSIUM SOAPS THAT CAN BE A61O 19/10 (2006.01) THICKENED WITH CHLORIDE
Piersch (45) Date of Patent: Jun. 29, 1993
 United States Patent (19) 11) USOO5222902A Patent Number: 5,222,902 Piersch (45) Date of Patent: Jun. 29, 1993 54 INTERLOCKING BLOCKS 3,890,738 6/1975 Bassani... 44.6/1 3,900,985 8/1975 Yoen... 44.6/124
United States Patent (19) 11) USOO5222902A Patent Number: 5,222,902 Piersch (45) Date of Patent: Jun. 29, 1993 54 INTERLOCKING BLOCKS 3,890,738 6/1975 Bassani... 44.6/1 3,900,985 8/1975 Yoen... 44.6/124
(12) United States Patent (10) Patent No.: US 6,365,596 B1
 USOO63696B1 (12) United States Patent (10) Patent No.: US 6,365,596 B1 Valenti (45) Date of Patent: Apr. 2, 2002 (54) ORAL PHARMACEUTICAL COMPOSITIONS (51) Int. Cl."... A61K 31/44 CONTAINING BUPRENORPHIN
USOO63696B1 (12) United States Patent (10) Patent No.: US 6,365,596 B1 Valenti (45) Date of Patent: Apr. 2, 2002 (54) ORAL PHARMACEUTICAL COMPOSITIONS (51) Int. Cl."... A61K 31/44 CONTAINING BUPRENORPHIN
