Chapter 3 Amino Acids, Peptides and Proteins
|
|
|
- Ethelbert Dennis Shaw
- 6 years ago
- Views:
Transcription
1 Chapter 3 Amino Acids, Peptides and Proteins Amino Acids Amino acids can be formed from inorganic compounds under prebiotic conditions These compounds are the monomeric units that are joined to form polypeptides (aka. Proteins) Chapter 3 2 1
2 Amino Acids All proteins are built from a combination of the 20 amino acids H H R C COOH Amino NH 2 C α Structural Features ph R C COO - Carboxyl + NH 3 Amino acids are built around a central α-carbon atom Because an acid and a base are both present, an amino acid can form either a positive or negative ion In fact, at physiological ph, both the acid and the base are completely ionized, resulting in a compound with both positive and negative charge called a Zwitterion Chapter 3 3 Amino Acids Structural Features Four different substituents on the α-carbon atom means that the alpha carbon is a chiral carbon (Enantiomers!!) These chiral centers are optically active, meaning they rotate plane-polarized light Rotating light to the left Levorotatory (L) Rotating light to the right Dextrorotatory (D) To determine whether your amino acid (or sugar!) is D or L, you need to look at the fischer diagram with the carboxyl at the top and the R group on the bottom. If the amino group is to the LEFT then the amino acid is L! Chapter 3 4 2
3 Amino Acids Structural Features Asymmetry (D vs. L) and side chain differences result in great variety in the polypeptides formed The amino acid residues in proteins are exclusively L stereoisomers. There are other L-amino acids in living cells Some as biochemical intermediates Some with modified R-groups after synthesis D-amino acids have been found in only a small number of peptides: The peptides of bacterial cell walls and in antibiotic peptides. Overall, ~ 300 different amino acids occur in living organisms!! Gives nature a lot to work with when putting together proteins! Chapter 3 5 Amino Acids Classification of Amino Acids All amino acids have the same core structure, but vary in their side chains (the R group), which effects their physiochemical properties H R C COO - NH 3 + The variation in the R group allows the amino acids to be grouped based on the Polarity of their R group They fall into five distinct groupings as clusters of 7, 5, 3, 3, and 2: the aliphatics, polars, aromatics, basics, and acidics Hydrophobic Water fearing Non-polar side chains Hydrophilic Water Loving Polar, neutral or charged side chains Chapter 3 6 3
4 Amino Acids The Seven Aliphatics (Non-polar) These seven amino acids tend to cluster together within proteins, stabilizing structure through hydrophobic interactions. Chapter 3 7 Amino Acids The Five Uncharged (Polar) The R groups of these five amino acids are more soluble in water than those of the alphatic AAs due to the presence of hydroxyl, thiol and amide groups. Chapter 3 8 4
5 Amino Acids The Three Aromatics These three amino acids are relatively non-polar and all can participate in hydrophobic interactions. They all also absorb UV light due to their conjugation. The hydroxyl group of Tyr also allows this residue to form hydrogen bonds. Chapter 3 9 Amino Acids The Three Basics These three amino acids (along with the acidic AAs) are the most hydrophilic AAs and can participate in hydrogen bonding interactions as H-bond Donors. Chapter
6 Amino Acids The Two Acidics These two amino acids (along with the basic AAs) are the most hydrophilic AAs and can participate in hydrogen bonding interactions as H-bond acceptors. Chapter 3 11 Amino Acids Other ways to Group the AAs Which and how many have hydroxyl groups? contain sulfur? contain 5- or 6-membered rings? have pure hydrocarbon side chains? have nitrogen in their side chains? Which is biggest? smallest? most acidic? most basic? Chapter
7 Amino Acids Chapter 3 13 Amino Acids Classification of Amino Acids Chapter
8 Amino Acids Essential Vocabulary for CHE 421 You will be required to learn the structure, name, abbreviation, pk a values (average ones for the carboxyl and aminol; specific value for ionizable R groups!) and unique characteristics of each of these 20 amino acids. You will need command of these structures to be successful in the rest of this course Chapter 3 15 Amino Acids Unusual L-Amino Acids Found in Proteins In addition to the 20 standard AAs, proteins can (and do!) contain residues formed from the modification of common residues already incorporated into a polypeptide. Selenocysteine is a rare AA that is introduced into the protein as the modified version rather than modified once in place. Fig. 3-8a Sometimes referred to as the 21 st Amino Acid Chapter
9 Amino Acids Unusual L-Amino Acids Found Elsewhere Not all AAs are found in polypeptides. Many are found in metabolic and synthetic pathways. In what synthetic (ornithine) and degradation (citrulline) pathways do you think that ornithine and citrulline show up as intermediates? Fig. 3-8a Chapter 3 17 Amino Acids The Amino Acid as Zwitterion An AA has at least one acidic (carboxyl) and one basic (amino) group If both are charged at the same time, the AA is a zwitterion The R-group may also contribute charge Y, C, K, H, R, D and E Due to these multiple ionizable groups, a titration curve for an AA will have a minimum of two inflection points What happens to the structure above and below the pk a values for the amino and carboxyl groups? What form would be present at physiological ph, 7.4? Chapter
10 Amino Acids Titration of an Amino Acid Where does the zwitterion form occur? If the pk a of the carboxyl group of acetic acid is 4.76, why is the pk 1 of glycine s carboxyl group so much lower, at 2.34? How would the titration curve of Histidine vary? Chapter 3 19 Amino Acids Titration of a Charged Amino Acid Why is the pk 1 of Histidine s carboxyl group 1.82 compared to 2.34 for Glycine? How would the titration curve of Lysine vary? Chapter
11 Amino Acids Titration of an Amino Acid Local Chemistry Affects the Charge Properties of Amino Acids pk 1 lies between ph pk 2 lies between ph Chapter 3 21 Amino Acids Isoelectric Point (pi) The characteristic ph at which the net electric charge of the amino acid is zero is the isoelectric point (pi) For amino acids with a non-ionizable R group, this value is equal to the average of the two pk a values: pi = ½ (pk 1 + pk 2 ) At ph values above the pi what is the overall charge on the amino acid? What about below the pi? Why would this be good info to know? Chapter
12 Amino Acids Titration of a Charged Amino Acid What about the pi for AAs with an ionizable R group? Where s the Zwitterion? Chapter 3 23 Amino Acids Titration of a Charged Amino Acid Isoelectric Points for Amino Acids with Ionizable R-groups If an amino acid has two amino groups, or two carboxyl groups, the pi is equal to the average of the values of the two like groups. Example: L-Histidine pk 1 = 1.82, pk 2 = 6.00, pk 3 = 9.17 Therefore: pi = ( )/2 = 7.59 Why does this rule of thumb work? Chapter
13 Amino Acids Can You Estimate the Number of Amino Acids in a Protein? The answer is yes! First, you need to know that the weighted average MW of the 20 standard amino acids is 128. Next, you take the molecular weight of the protein and divide it by 110 (WHY? What happened to the other 18?) e.g. a protein of 55 kd = MW 55,000 is comprised of about 500 amino acids Chapter 3 25 Amino Acids Functional Groups in Macromolecules Can Provide Buffering Capacity Chapter
14 Amino Acids But Enzyme Catalysis is Also ph-sensitive Why might this be? Think about it Chapter 3 27 Amino Acids Let s See Who Learned Their AAs this Weekend Amino Acid Quiz Chapter
15 Peptides and Proteins Proteins Result from Peptide Bond Formation (a Condensation Reaction) Poor leaving group The peptide bond is formed through a nucleophilic displacement that takes place in the ribosome during translation. Good nucleophile G = + 21 kj/mole (very unfavorable so why are proteins so stable?) Peptide bond Chapter 3 29 Peptides and Proteins Can You Estimate the Isoelectric Point of a Protein? Review the rule of thumb for calculating the pi s of amino acids. How might this rule apply to peptides and proteins? Remember that polypeptides contain only one free α- COOH and α-nh 2 group, at opposite ends of the chain The R groups of some of the AAs in the polypeptide will be ionizable and contribute to the pi of the protein. Remember, the pk a values for both the termini and the R groups are effected by the chemical environment, so their use in this type of calculation will give you an estimated pi only (better to determine in the lab!) Chapter
16 Peptides and Proteins A Pentapeptide What is the AA sequence? What is the approximate pi? Chapter 3 31 Peptides and Proteins AA Composition of Proteins Each protein has a characteristic mix of AAs in its sequence. No two proteins are identical in this makeup. Some AAs appear frequently and other not so much. Chapter
17 Working with Proteins pi and AA Composition Amino Acid # of Positively Charged Residues 20 3 Chapter 3 33 pi Ala Arg Asn Asp Cys Gln Glu Gly His Ile Leu Lys Met Phe Pro Ser Thr Trp Tyr Val # of Negatively Charged Residues Lysozyme # in PP (%) 12 (9.3%) 3 (2.3%) 11 (8.5%) 5 (3.9%) 8 (6.2%) 5 (3.9%) 2 (1.6%) 12 (9.3%) 0 (0.0%) 5 (3.9%) 9 (7.0%) 17 (13.2%) 1 (0.8%) 2 (1.6%) 2 (1.6%) 9 (7.0%) 8 (6.2%) 5 (3.9%) 5 (3.9%) 8 (6.2%) 7 Pepsin A % in PP 16 (4.8%) 3 (0.9%) 15 (4.5%) 22 (6.7%) 6 (1.8%) 15 (4.5%) 14 (4.2%) 34 (10.3%) 1 (0.3%) 30 (9.1%) 21 (6.4%) 0 (0.0%) 3 (0.9%) 15 (4.5%) 15 (4.5%) 49 (4.8%) 26 (7.9%) 5 (1.5%) 17 (5.2%) 23 (7.0%) 36 Peptides and Proteins Multimeric Proteins Average AA molecular weight is 110. We can use this value to estimate the number of AAs in a protein if we know the MW of that protein. Multisubunit proteins consist of two or more separate polypeptide chains. If at least two of these chains are identical, the protein is oligomeric and each chain is a protomer Chapter
18 Peptides and Proteins Conjugated Proteins In addition to AAs, many proteins contain permenantly associated chemical groups called prosthetic groups These groups are often involved in the catalytic activity of enzymes or in structural maintainence Conjugated proteins are classified on the basis of the attached prosthetic group. Proteins can also contain more than one of these groups Chapter 3 35 Peptides and Proteins Protein Structure Beyond the AA Sequence CHP 3.4 CHP 4 Chapter
19 Working with Proteins Separation and Purification of Proteins A pure sample of protein is vital to determining its structure and function Proteins can be separated by exploiting their differences which are determined by their structure and amino acid sequence Solubility Ammonium Sulfate Precipitation Crystallization Charge Size Specific Binding Ion Exchange Chromatography Isoelectric Focusing Gel Filtration Chromatography SDS-PAGE Electrophoresis, Dialysis Affinity Chromatography Special Properties Heat Denaturation Engineered Properties Chapter 3 37 Working with Proteins Separation and Purification of Proteins Cell Lysis Soluble Fraction Centrifugation to remove cell debris/insoluble components Removal of nucleic acids (Optional) Liquid chromatography Protein Fraction Crude fractionation by precipitation Purified protein Enriched protein fraction Liquid Chapter 3 chromatography 38 19
20 Working with Proteins Column Chromatography In any type of column chromatography, you have the mobile phase and the stationary phase There are two types of stationary phases: Non-adsorptive: No interaction between the solute and the SP Adsorptive: Chemical interaction between the solute and the SP. You must alter your MP to elute the solute. As with all chromatography, you must equilibrate you column with MP prior to loading and eluting your solute! Chapter 3 39 Working with Proteins Column Chromatography Ion Exchange (IEC) Proteins differ in the number of charged amino acids (Asp, Glu, His, Arg, and Lys) found in their AA sequence. Therefore, they will vary in their overall charge at various ph values and the ph value at which their net charge is zero (their isoelectric point, pi) In IEC, the SP contains charged groups: Anion exchange (+++) / Cation exchange (- --) Strong and weak ion exchangers are available depending on your protein Sample eluted with increasing salt or change in ph Isoelectric point of protein generally needs to be known or several pilot runs need to be performed Chapter
21 Working with Proteins Column Chromatography Size Exclusion (SEC) A protein s size and shape affect its ability to move through a matrix of of porous beads or a porous membrane Proteins elute in decreasing order of size The SP is a non-absorptive & porous matrix SP with varying pore sizes are available Some uses include: Separating contaminating solutes and Protein fractionation Chapter 3 41 Working with Proteins Column Chromatography Affinity (Aff) Proteins can naturally or artificially contain special binding properties: Receptor-ligand interactions Protein-protein interactions Samples eluted by various means dependent on the binding interaction The SP contains specific ligands dependent on the binding property being exploited: Dyes Metals Substrates and cofactors Aff has a very high selectivity that often yields pure or nearly pure protein in minimal steps. Chapter
22 Working with Proteins Electrophoresis Electrophoresis is a method of molecule separation that exploits the size and charge of the components Here, the application of an electrical field to macromolecules in solution will cause them to migrate to either the: + anode (negatively charged molecules) cathode (positively charged molecules) Gels typically used as a support matrix act as molecular sieves through which molecules may or may not be able to pass based on their size: Agarose (DNA) and Polyacrylamide (Proteins) Chapter 3 43 Working with Proteins Electrophoresis SDS (sodium dodecyl sulfate) binds to proteins via hydrophobic interactions Usually about 1 SDS per 2 AAs When SDS added in excess: proteins coated with SDS, thus masking charge of protein and imparting overall negative charge proteins are denatured, meaning organizational structure of molecule is disrupted To completely denature protein, add a reducing agent such as β-mercaptoethanol (BME), dithiothreitol (DTT) and/or heat O Na + - O S O (CH 2 ) 11 CH 3 SDS-PAGE O Sodium dodecyl sulfate (SDS) Chapter
23 Working with Proteins Electrophoresis Determination of MW Chapter 3 45 Working with Proteins Isoelectric Focusing Procedure used to determine the pi of a protein Here, a ph gradient is established by allowing a mixture of low MW organic acids and bases distribute themselves in an electric field generated across a gel. When a protein mixture is added, the protein components migrate until they reach a ph that matches their pi value. Let s try Cytochrome c Peroxidase It contains 295 AA s pi / Molecular Mass Calculator Chapter
24 Working with Proteins 2-D Electrophoresis 2-D electrophoresis combines SDS-PAGE and IE into one experiment. This allows for the separation of proteins based on both MW and pi. Let s us separate proteins that have the same pi but varied MW and proteins that have the same MW but varied pi. Chapter 3 47 Enzyme Assay Working with Proteins Once we purify out a protein or even during the purification itself, how do we know we are focused in on our protein of interest? How do we know we have not thrown it out with our last step? For enzymes, we can use the reaction that it catalyzes to monitor the presence and amount of the enzyme throughout the purification To monitor this reaction, we need to design an assay that allows us to observe the catalytic effect of our enzyme. To develop an enzyme assay we must know the following: The overall equation for the catalyzed reaction An analytical procedure for determining the disappearance of substrate or appearance of product for the reaction Whether the enzyme requires co-factors such as metals or coenzymes for activity The dependence of the enzyme activity on substrate concentration The optimum ph for activity A temperature zone in which the enzyme is stable and has high activity Chapter
25 Working with Proteins Enzyme Activity One unit (1 U) of enzyme activity is defined as the amount of enzyme causing the transformation of 1.0 µmol of substrate per minute at 25 C. Activity is the total units of enzyme in a solution Specific Activity is the number of enzyme units per milligram of total protein. Activity Specific Activity Chapter 3 49 Covalent Structure of Proteins Protein Sequence The AA sequence of a protein represents very important information in determining its structure and function A human produces to proteins, each of which has a unique structure and sequence The AA sequence is important to the 3-D structure of the protein, which in turn, is important to the function of the protein. By knowing the AA sequence, we can make predictions about the 3-D structure, and the function of the protein. In addition, by comparing AA sequences for the same protein across species and within species, we can identify evolutionary changes and genetic defects which may cause disease Chapter
26 Covalent Structure of Proteins Protein Sequencing AA Composition Analysis In some cases, you may want to know the number of each type of amino acid within a polypeptide. The AA composition can be determined by completely hydrolyzing a protein under acidic conditions then analyzing the AAs using chromatography (usually via a detectable tag) Different amino acids in a peptide hydrolysate can be separated by ion-exchange chromatography on a sulfonated polystyrene resin (such as Dowex-50) Figure 4.18 from Biochemistry, 5 th Ed., Berg et al. Buffers (such as sodium citrate) of increasing ph are used to elute the amino acids from the column. The amount of each amino acid present is determined from the absorbance. Aspartate, which has an acidic side chain, is first to emerge, whereas arginine, which has a basic side chain, is the last. In this case, the original peptide is revealed to be composed of one aspartate, one alanine, one phenylalanine, one arginine, and two glycine residues Chapter 3 51 Covalent Structure of Proteins Protein Sequencing Various procedures are available for determination of a protein s amino acid sequence. The Sanger Method The Edman Degradation Method Regardless of the technique used, the basic approach for sequencing proteins is similar: Separate the mature protein into individual polypeptide strands (if needed) Use at least two different chemical and/or enzymatic methods to break the polypeptide into two sets of smaller peptide segments Determine the sequence of each segment Reconstruct the overall sequence by ordering overlapping segments from the two sets Repeat the fragmentation step without breaking intra-strand connections to identify any Cys residues involved in disulfide bridges Chapter
27 Covalent Structure of Proteins Protein Sequencing Preliminary Steps The complete AA sequence of a protein includes the sequence of each of its subunits (if any), so these subunits must be identified and isolated Disulfide bonds within the protein structure must also be broken top separate and fully lineraize polypeptide chains Disulfide bonds can be reductively cleaved by treating them with 2-betamercaptoethanol (BME) or another mercaptan (contains a SH group) H N H C O C H N H C O C CH 2 S S BME + 2 HSCH 2 CH 2 OH CH 2 SH + SH + SCH 2 CH 2 OH SCH 2 CH 2 OH H N CH 2 O C H C CH 2 O C C H The resulting free sulfhydral groups are then alkylated, usually by treatment with iodoacetate, to prevent reformation of the disulfide bond through O 2 oxidation H 2 H 2 CYS C SH + ICH 2 COO - CYS C S CH 2 COO - + HI Chapter 3 53 H N Covalent Structure of Proteins Protein Sequencing Preliminary Steps Once the disulfide bonds are disrupted, the subunits are allowed to separate (if present) and the polypeptide(s) are linearized Now, N-terminal analysis can reveal the number of different subunits. Frederick Sanger developed the reagent 1-fluoro-2,4-dinitrobenzene (FDNB) for this purpose Other reagents include Dansyl chloride and Dabsyl chloride All of these reagents react with primary amines (the N-terminal amino group) For this procedure, the reagent is added to the protein solution resulting in a labeling of all amino terminii. The labeled polypeptide(s) are then hydrolyzed into individual AAs and the label is identified chromatographically. H 3C N CH 3 Dansyl Chloride SO 2Cl H 3C N N N SO 2Cl Chapter 3 H 3C 54 Dabsyl Chloride 27
28 Covalent Structure of Proteins Protein Sequencing Edman Degradation If the polypeptide is small ( 50 AAs or less) you can determine the sequence using repeated cycles of Edman Degradation Here, Phenylisothiocyanate (PITC) reacts with the N-terminal amino group under alkaline conditions to form a phenylthiocarbamoyl (PTC) adduct. The peptide bond adjacent to this labeled terminus is cleaved under acidic conditions (Thifluoroacetic acid) This treatment cleaves only the peptide bond of interest! This derivative is then extracted in organic solvent and identified Chapter 3 55 Figure 4.21 from Biochemistry, 5 th Ed., Berg et al. Covalent Structure of Proteins Protein Sequencing Polypeptide Cleavage If the polypeptide is larger than ~ 50 AAs, it must be cleaved, either chemically or enzymatically, into specific fragments that are small enough to be sequenced using Edman Degradation. Endopeptidases are enzymes that catalyze the hydrolysis of internal peptide bonds These enzymes are in the enzyme family of proteases and have side chain requirements for the residues flanking the target peptide bond (aka the scissle bond) These fragments are then separated using chromatography or electrophoresis Figure 4.24 from Biochemistry, 5 th Ed., Berg et al. Chapter
29 Covalent Structure of Proteins Protein Sequencing Polypeptide Cleavage H 3 N NMTQGRCKPVNTFVHEPLVDVQNVCFK COO - You must cleave the above peptide into smaller fragments. Which of the proteases listed would yield the most fragments? How many? Which would yield the least fragments? How many? Chapter 3 57 Covalent Structure of Proteins Protein Sequencing Ordering Peptide Fragments Now that each fragment has been sequenced, the order of these fragments must be determined so that the entire polypeptide sequence can be established The AA sequence of the fragments formed from two separate cleavage methods are examined to identify overlapping sequences. Overlapping peptides can be identified and put in order Earlier identification of the Amino terminus can be used to identify the amino terminus fragment Chapter
30 Covalent Structure of Proteins Protein Sequencing Ordering Peptide Fragments You wish to determine the sequence of a short polypeptide. Cleavage with trypsin yields three smaller peptide: L E G Y N R Q A F V K Cleavage with chymotrypsin yields three peptides: Q A F N R L E V K G Y What is the sequence of this polypeptide? Chapter 3 59 Covalent Structure of Proteins Protein Sequencing Locating Disulfide Bonds If the primary sequence includes disulfide bonds, their location is also of interest To determine the location of the disulfide bonds: Take a new sample of the protein and separate the polypeptides but DO NOT reduce the disulfide bridges Subject this strand to the same chemical or enzymatic cleavage technique as before and separate the fragments using electrophoresis Compare this pattern to that of the original sample If there is one disulfide bridge in your polypeptide, what would you expect the difference to be between the two samples? How would you identify the location of the disulfide bridge? Chapter
31 Covalent Structure of Proteins Protein Sequencing Locating Disulfide Bonds Treatment of a polypeptide with 2-mercaptoethanol yields two polypeptides: A V C R T G C K N F L Y K C F R H T K C S Treatment of the intact polypeptide with trypsin yields fragments with the following AA composition A, R, C 2, S, V R, C 2, G, K, T, F N, L, F H, K, T K, T What are the positions of the disulfide bond(s)? Chapter 3 61 Covalent Structure of Proteins Protein Sequencing Chapter
32 Protein Sequence and Evolution Protein Sequencing and Mass Spectrometry Mass spectrometry has only recently become a tool of the biochemist This technique accurately measures the mass-to-charge ratio for ions in the gas phase New ionization techniques have allowed for the formation of macromolecule ions without degradation of the compound Matrix-assisted laser desorption/ionization (MALDI) Electrospray ionization (ESI) MS can be used to determined the molecular mass of a protein and to detect changes in this mass due to the presence of cofactors, metal ions, covalent modification due to reactions, etc. Tandem MS (MS/MS) can also be used to sequence short stretches of polypeptide. Chapter 3 63 Covalent Structure of Proteins Protein Synthesis Peptides are potentially useful as pharmacological agents making their production commercially profitable There are three ways to produce a peptide: Purification from the biological source Genetic engineering Direct chemical synthesis R. Bruce Merrifield developed an innovative technique for peptide synthesis by attaching a growing polypeptide to an insoluble support and blocking the growing end Chapter
33 Protein Sequence and Evolution Protein Sequence and Evolution As stated before, the function of a protein depends on its 3-D structure, which, in turn, depends on its AA sequence The field of Molecular Evolution uses nucleotide and peptide sequences to explore evolution Basically, if two organisms are closely related, the sequences of their genes and proteins should be similar As organisms diverge evolutionarily, their sequences will also diverge We now have the computer power to store, translate and compare thousands of sequences, both peptide and nucleotide! Chapter Protein Sequence and Evolution Databases Containing Gene and Protein Info After a protein s AA sequence has been determined, the information is usually deposited into a public database Several of these databases are available via the Internet and can be used to determine valuable information regarding proteins, nucleic acids and evolution Data Banks Containing Protein Sequences Swiss-Prot: Protein Info Resource: Protein Research Foundation: UniProt: Data Banks Containing Gene Sequences GenBank: European Bioinfor Institute: Chapter
34 Protein Sequence and Evolution Protein Sequence and Evolution There are some issues with this type of comparison, in that it is not as straight forward as just lining things up and calling it a match First, the AA residues required for function will usually be conserved across species while those residues of less importance may vary over time Second, there can be a rare transfer of a gene or group of genes from one species to another (lateral gene transfer). This may result in a false positive when running the sequence comparison. Can anyone think of an example of this lateral gene transfer? The study of molecular evolution generally focuses on families of closely related proteins, usually groups with functions that are essential to the viability of the organisms When comparing peptide sequences, you can find homologs, which can be either paralogs (in the same species) or orthologs (in different species). Chapter 3 67 Protein Sequence and Evolution Protein Sequence and Evolution A Blosum62 table (blocks substitution matrix complied from alignment that showed 62% identity) shows the scores assigned to AA substitutions within an alignment These substitutions can be conservative or nonconservative Remember that gaps in the sequence alignments incur penalties as well Chapter
35 Protein Sequence and Evolution Protein Sequence and Evolution In site directed mutagenesis experiments, Gly is often successfully substituted for Val, but Val can rarely substitute for Gly. Why? Below is a list of the first 10 residues of the B helix in Myoglobin from different species: Position Human Chicken Alligator Turtle Tuna Carp 1 D D K D D D 2 I I L L Y F 3 P A P S T E 4 G G E A T G 5 H H H H M T Which positions appear unable to tolerate substitutions? Which can tolerate conservative substitutions? Which are highly variable? Chapter G G G G G G 7 Q H H Q G G 8 E E E E L E 9 V V V V V V 10 L L I I L L Protein Sequence and Evolution Protein Sequence and Evolution Tree based on sequence divergence in the protein GroEL in bacteria The free end points of lines are external nodes and represent an extant species The points where two lines come together are internal nodes and represent extinct ancestor species The lengths of the lines connecting the nodes are proportional to the number of amino acid substitutions separating one species from another Chapter
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
2. Which of the following amino acids is most likely to be found on the outer surface of a properly folded protein?
 Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
BCMB Chapter 3 (part1)
 Chapter 3 (with parts of 4 and 5) Amino Acids and Primary Structures of Proteins BCMB 3100 - Chapter 3 (part1) Diversity of protein function Complete definition of amino acids Memorize complete structure
Chapter 3 (with parts of 4 and 5) Amino Acids and Primary Structures of Proteins BCMB 3100 - Chapter 3 (part1) Diversity of protein function Complete definition of amino acids Memorize complete structure
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
BCMB 3100 Chapter 3 (part 1)
 BCMB 3100 Chapter 3 (part 1) Diversity of protein function Complete definition of amino acids Memorize complete structure of 20 common amino acids!!! pka s of amino and carboxyl groups Amino acids with
BCMB 3100 Chapter 3 (part 1) Diversity of protein function Complete definition of amino acids Memorize complete structure of 20 common amino acids!!! pka s of amino and carboxyl groups Amino acids with
Cahn - Ingold - Prelog system. Proteins: Evolution, and Analysis Lecture 7 9/15/2009. The Fischer Convention (1) G (2) (3)
 Chapter 4 (1) G Proteins: Evolution, and Analysis Lecture 7 9/15/2009 A V L I M P F W Chapter 4 (2) S (3) T N Q Y C K R H D E The Fischer Convention Absolute configuration about an asymmetric carbon related
Chapter 4 (1) G Proteins: Evolution, and Analysis Lecture 7 9/15/2009 A V L I M P F W Chapter 4 (2) S (3) T N Q Y C K R H D E The Fischer Convention Absolute configuration about an asymmetric carbon related
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
AA s are the building blocks of proteins
 Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Amino Acids. Lecture 4: Margaret A. Daugherty. Fall Swiss-prot database: How many proteins? From where?
 Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
PROTEINS. Building blocks, structure and function. Aim: You will have a clear picture of protein construction and their general properties
 PROTEINS Building blocks, structure and function Aim: You will have a clear picture of protein construction and their general properties Reading materials: Compendium in Biochemistry, page 13-49. Microbiology,
PROTEINS Building blocks, structure and function Aim: You will have a clear picture of protein construction and their general properties Reading materials: Compendium in Biochemistry, page 13-49. Microbiology,
CHAPTER 21: Amino Acids, Proteins, & Enzymes. General, Organic, & Biological Chemistry Janice Gorzynski Smith
 CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
CHAPTER 3 Amino Acids, Peptides, Proteins
 CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
BIOCHEMISTRY REVIEW. Overview of Biomolecules. Chapter 4 Protein Sequence
 BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 4 Protein Sequence 2 3 4 Are You Getting It?? A molecule of hemoglobin is compared with a molecule of lysozyme. Which characteristics do they share?
BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 4 Protein Sequence 2 3 4 Are You Getting It?? A molecule of hemoglobin is compared with a molecule of lysozyme. Which characteristics do they share?
CHAPTER 3 Amino Acids, Peptides, Proteins
 CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Human Biochemistry Option B
 Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
AMINO ACIDS. Qualitative Tests
 AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
Proteins. Amino acids, structure and function. The Nobel Prize in Chemistry 2012 Robert J. Lefkowitz Brian K. Kobilka
 Proteins Amino acids, structure and function The Nobel Prize in Chemistry 2012 Robert J. Lefkowitz Brian K. Kobilka O O HO N N HN OH Ser65-Tyr66-Gly67 The Nobel prize in chemistry 2008 Osamu Shimomura,
Proteins Amino acids, structure and function The Nobel Prize in Chemistry 2012 Robert J. Lefkowitz Brian K. Kobilka O O HO N N HN OH Ser65-Tyr66-Gly67 The Nobel prize in chemistry 2008 Osamu Shimomura,
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is worth 2 points.
 MBB 407/511 Molecular Biology and Biochemistry First Examination - October 1, 2002 Name Social Security Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is
MBB 407/511 Molecular Biology and Biochemistry First Examination - October 1, 2002 Name Social Security Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Biomolecules Amino Acids & Protein Chemistry
 Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
BIO 311C Spring Lecture 15 Friday 26 Feb. 1
 BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur. Lecture -02 Amino Acids II
 Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Proteins with similar functions often have αα s can act as both weak acids & weak bases similar αα sequences
 Chapter 5: Amino Acids, Peptides & Proteins Amino acids share common functional groups Amino, Carboxyl & H bonded to C Distinct chemistry of s result of side chains Proteins & polypeptides are polymers
Chapter 5: Amino Acids, Peptides & Proteins Amino acids share common functional groups Amino, Carboxyl & H bonded to C Distinct chemistry of s result of side chains Proteins & polypeptides are polymers
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
The Structure and Function of Macromolecules
 The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
130327SCH4U_biochem April 09, 2013
 Option B: B1.1 ENERGY Human Biochemistry If more energy is taken in from food than is used up, weight gain will follow. Similarly if more energy is used than we supply our body with, weight loss will occur.
Option B: B1.1 ENERGY Human Biochemistry If more energy is taken in from food than is used up, weight gain will follow. Similarly if more energy is used than we supply our body with, weight loss will occur.
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Review of Biochemistry
 Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
BCH302 [Practical] 1
![BCH302 [Practical] 1 BCH302 [Practical] 1](/thumbs/78/76808179.jpg) BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
Chapter 3. Structure of Enzymes. Enzyme Engineering
 Chapter 3. Structure of Enzymes Enzyme Engineering 3.1 Introduction With purified protein, Determining M r of the protein Determining composition of amino acids and the primary structure Determining the
Chapter 3. Structure of Enzymes Enzyme Engineering 3.1 Introduction With purified protein, Determining M r of the protein Determining composition of amino acids and the primary structure Determining the
CHM333 LECTURE 6: 1/25/12 SPRING 2012 Professor Christine Hrycyna AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID:
 AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
3. AMINO ACID AND PEPTIDES
 3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
PAPER No. : 16, Bioorganic and biophysical chemistry MODULE No. : 22, Mechanism of enzyme catalyst reaction (I) Chymotrypsin
 Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Short polymer. Dehydration removes a water molecule, forming a new bond. Longer polymer (a) Dehydration reaction in the synthesis of a polymer
 HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
Chemistry 20 Chapter 14 Proteins
 Chapter 14 Proteins Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and connective tissue
Chapter 14 Proteins Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and connective tissue
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
(65 pts.) 27. (10 pts.) 28. (15 pts.) 29. (10 pts.) TOTAL (100 points) Moorpark College Chemistry 11 Spring Instructor: Professor Gopal
 Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Amino Acids. Review I: Protein Structure. Amino Acids: Structures. Amino Acids (contd.) Rajan Munshi
 Review I: Protein Structure Rajan Munshi BBSI @ Pitt 2005 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2005 Amino Acids Building blocks of proteins 20 amino acids
Review I: Protein Structure Rajan Munshi BBSI @ Pitt 2005 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2005 Amino Acids Building blocks of proteins 20 amino acids
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Biochemistry 210 Chapter 22
 Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Chapter 21 Lecture Outline
 Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Metabolic Classification of the Amino Acids
 Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Multiple-Choice Questions Answer ALL 20 multiple-choice questions on the Scantron Card in PENCIL
 Multiple-Choice Questions Answer ALL 20 multiple-choice questions on the Scantron Card in PENCIL For Questions 1-10 choose ONE INCORRECT answer. 1. Which ONE of the following statements concerning the
Multiple-Choice Questions Answer ALL 20 multiple-choice questions on the Scantron Card in PENCIL For Questions 1-10 choose ONE INCORRECT answer. 1. Which ONE of the following statements concerning the
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
Chymotrypsin Lecture. Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin
 Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
paper and beads don t fall off. Then, place the beads in the following order on the pipe cleaner:
 Beady Pipe Cleaner Proteins Background: Proteins are the molecules that carry out most of the cell s dayto-day functions. While the DNA in the nucleus is "the boss" and controls the activities of the cell,
Beady Pipe Cleaner Proteins Background: Proteins are the molecules that carry out most of the cell s dayto-day functions. While the DNA in the nucleus is "the boss" and controls the activities of the cell,
The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5
 Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Levels of Protein Structure:
 Levels of Protein Structure: PRIMARY STRUCTURE (1 ) - Defined, non-random sequence of amino acids along the peptide backbone o Described in two ways: Amino acid composition Amino acid sequence M-L-D-G-C-G
Levels of Protein Structure: PRIMARY STRUCTURE (1 ) - Defined, non-random sequence of amino acids along the peptide backbone o Described in two ways: Amino acid composition Amino acid sequence M-L-D-G-C-G
The Basics: A general review of molecular biology:
 The Basics: A general review of molecular biology: DNA Transcription RNA Translation Proteins DNA (deoxy-ribonucleic acid) is the genetic material It is an informational super polymer -think of it as the
The Basics: A general review of molecular biology: DNA Transcription RNA Translation Proteins DNA (deoxy-ribonucleic acid) is the genetic material It is an informational super polymer -think of it as the
Introduction to proteins and protein structure
 Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Arginine side chain interactions and the role of arginine as a mobile charge carrier in voltage sensitive ion channels. Supplementary Information
 Arginine side chain interactions and the role of arginine as a mobile charge carrier in voltage sensitive ion channels Craig T. Armstrong, Philip E. Mason, J. L. Ross Anderson and Christopher E. Dempsey
Arginine side chain interactions and the role of arginine as a mobile charge carrier in voltage sensitive ion channels Craig T. Armstrong, Philip E. Mason, J. L. Ross Anderson and Christopher E. Dempsey
Organic Chemistry 3540
 rganic Chemistry 3540 December 8, 2004 (8 Pages, 13 Parts) ame 1. (8%) Many organic compounds found in living systems are complex molecules which can be characterized, in part, by simply listing the chemical
rganic Chemistry 3540 December 8, 2004 (8 Pages, 13 Parts) ame 1. (8%) Many organic compounds found in living systems are complex molecules which can be characterized, in part, by simply listing the chemical
(30 pts.) 16. (24 pts.) 17. (20 pts.) 18. (16 pts.) 19. (5 pts.) 20. (5 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Chemical Mechanism of Enzymes
 Chemical Mechanism of Enzymes Enzyme Engineering 5.2 Definition of the mechanism 1. The sequence from substrate(s) to product(s) : Reaction steps 2. The rates at which the complex are interconverted 3.
Chemical Mechanism of Enzymes Enzyme Engineering 5.2 Definition of the mechanism 1. The sequence from substrate(s) to product(s) : Reaction steps 2. The rates at which the complex are interconverted 3.
Bioinformatics for molecular biology
 Bioinformatics for molecular biology Structural bioinformatics tools, predictors, and 3D modeling Structural Biology Review Dr Research Scientist Department of Microbiology, Oslo University Hospital -
Bioinformatics for molecular biology Structural bioinformatics tools, predictors, and 3D modeling Structural Biology Review Dr Research Scientist Department of Microbiology, Oslo University Hospital -
Amino acids. Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester,
 Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination # 5: Section Five May 7, Name: (print)
 Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Previous Class. Today. Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism. Protein Kinase Catalytic Properties
 Previous Class Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism Today Protein Kinase Catalytic Properties Protein Phosphorylation Phosphorylation: key protein modification
Previous Class Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism Today Protein Kinase Catalytic Properties Protein Phosphorylation Phosphorylation: key protein modification
Chapter 3. Protein Structure and Function
 Chapter 3 Protein Structure and Function Broad functional classes So Proteins have structure and function... Fine! -Why do we care to know more???? Understanding functional architechture gives us POWER
Chapter 3 Protein Structure and Function Broad functional classes So Proteins have structure and function... Fine! -Why do we care to know more???? Understanding functional architechture gives us POWER
INTRODUCTION TO BIOCHEMISTRY/POLYMERS. 3. With respect to amino acids, polypeptides, and proteins, know:
 INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
استاذ الكيمياءالحيوية
 قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
Lecture 4. Grouping Amino Acid 7/1/10. Proteins. Amino Acids. Where Are Proteins Located. Nonpolar Amino Acids
 Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
AP Bio. Protiens Chapter 5 1
 Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
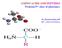 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
CHY2026: General Biochemistry. Unit 4:Amino Acid Chemistry
 CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
Chapter 11: Enzyme Catalysis
 Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
BCH Graduate Survey of Biochemistry
 BCH 5045 Graduate Survey of Biochemistry Instructor: Charles Guy Producer: Ron Thomas Director: Glen Graham Lecture 7 Slide sets available at: http://hort.ifas.ufl.edu/teach/guyweb/bch5045/index.html David
BCH 5045 Graduate Survey of Biochemistry Instructor: Charles Guy Producer: Ron Thomas Director: Glen Graham Lecture 7 Slide sets available at: http://hort.ifas.ufl.edu/teach/guyweb/bch5045/index.html David
Introduction to Biochemistry
 Life is Organized in Increasing Levels of Complexity Introduction to Biochemistry atom simple molecule What is the chemical makeup of living things? macromolecule organ organ system organism organelle
Life is Organized in Increasing Levels of Complexity Introduction to Biochemistry atom simple molecule What is the chemical makeup of living things? macromolecule organ organ system organism organelle
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Organic Molecules: Proteins
 Organic Molecules: Proteins Proteins Most structurally & functionally diverse group Function: involved in almost everything enzymes (pepsin, DNA polymerase) structure (keratin, collagen) carriers & transport
Organic Molecules: Proteins Proteins Most structurally & functionally diverse group Function: involved in almost everything enzymes (pepsin, DNA polymerase) structure (keratin, collagen) carriers & transport
PROTEINS. Amino acids are the building blocks of proteins. Acid L-form * * Lecture 6 Macromolecules #2 O = N -C -C-O.
 Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
UNIT 2 Amino acids and Proteins
 UNIT 2 Amino acids and Proteins Significance of Proteins 1. Keep the cells and tissues growing, renewing and mending 2. Take part in some kinds of important physiological activities 3. Oxidation and supply
UNIT 2 Amino acids and Proteins Significance of Proteins 1. Keep the cells and tissues growing, renewing and mending 2. Take part in some kinds of important physiological activities 3. Oxidation and supply
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Lipids: diverse group of hydrophobic molecules
 Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
BCHS 3304/ Exam I. September 26,
 Name: 5.5.# BCHS 3304/ Exam I September 26, 2002............... Instructions: 1. There are 13 pages to this exam. Count pages prior to beginning exam. You may use the back pages of the exam as scratch
Name: 5.5.# BCHS 3304/ Exam I September 26, 2002............... Instructions: 1. There are 13 pages to this exam. Count pages prior to beginning exam. You may use the back pages of the exam as scratch
Midterm 1 Last, First
 Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Page 1 of 5 Biochemistry I Fall 2017 Practice for Exam2 Dr. Stone Name
 Page 1 of 5 Biochemistry I Fall 2017 Practice for Exam2 Dr. Stone ame o answers will be provided. ere are some constants and equations that may be useful: K a = [+][A-]/[A] p = pka + log [A-]/[A] K a for
Page 1 of 5 Biochemistry I Fall 2017 Practice for Exam2 Dr. Stone ame o answers will be provided. ere are some constants and equations that may be useful: K a = [+][A-]/[A] p = pka + log [A-]/[A] K a for
From Atoms to Cells: Fundamental Building Blocks. Models of atoms. A chemical connection
 From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
Practice Problems 3. a. What is the name of the bond formed between two amino acids? Are these bonds free to rotate?
 Life Sciences 1a Practice Problems 3 1. Draw the oligopeptide for Ala-Phe-Gly-Thr-Asp. You do not need to indicate the stereochemistry of the sidechains. Denote with arrows the bonds formed between the
Life Sciences 1a Practice Problems 3 1. Draw the oligopeptide for Ala-Phe-Gly-Thr-Asp. You do not need to indicate the stereochemistry of the sidechains. Denote with arrows the bonds formed between the
Catalysis & specificity: Proteins at work
 Catalysis & specificity: Proteins at work Introduction Having spent some time looking at the elements of structure of proteins and DNA, as well as their ability to form intermolecular interactions, it
Catalysis & specificity: Proteins at work Introduction Having spent some time looking at the elements of structure of proteins and DNA, as well as their ability to form intermolecular interactions, it
Model 2: Aldohexoses, aldopentoses, ketohexoses and ketopentoses
 Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Macromolecules Structure and Function
 Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
