Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur. Lecture -02 Amino Acids II
|
|
|
- Elaine Perry
- 5 years ago
- Views:
Transcription
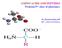
1 Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48) As we saw last time, the amino acids consider or have rather, an asymmetric carbon atom to them. To this carbon atom is attached an amino group and a carboxylic group. And we have the different side chains; we found out that we have twenty common amino acids, with twenty side chains, twenty odd side chains, which differ greatly in the property that they have and we have associated with them a three-letter code and one letter code. If we go to this amino acid, this side chain corresponds to the amino acid, Glutamine. The three-letter code for Glutamine is GLN and the one letter code is Q. It is something that you have to remember regarding the amino acids. Now, if you notice in the structure that we have here, that is called as stick structure, in the stick structure, we do not see the hydrogens. The nitrogens are always marked in blue, the oxygen atoms are marked in red. The carbon atoms are marked in green, that is for the asymmetric carbon and the others are marked in grey. Do you see that clearly? Now this is universal. Nitrogen atoms are always in blue, acidic or rather oxygen atoms are always in red and carbon atoms are in grey. Now, the
2 next thing that we looked at was the joining of the two amino acids. The joining of the two amino acids gave raise to what is called the peptide bond. Now, when we look at the peptide bond, we see that, we have associated the carboxylic group of the previous amino acid, with the amino group of the next amino acid. So, this R (left side R in the peptide bond) group corresponds to the first amino acid of this particular dipeptide and this R (right side R in the peptide bond) group corresponds to the second amino acid of the dipeptide, which means that in a polypeptide chain. You always have the first amino acid begin with an amino terminus. It is never written any other way. You always have the first amino acid in a protein, in a polypeptide chain, as the amino terminus. It always is an NH2 terminus. And the carboxylic terminus is always the end of the protein chain. (Refer Slide Time: 03:48) Now, if we go to the next Slide, we just have the one that I showed you earlier, where we have the elimination of water to form the peptide bond. And in this case, we have a tripeptide and from the R groups, we can identify the peptide. Now you understand that, as we have a protein chain or polypeptide chain, this is going to get longer and longer. Now, as it gets longer, we have here in the peptide bonds, Glycine because it has two hydrogens. We have Alanine because it has just the methyl group attached to it and this is Cysteine because it has SH group attached to it. (Refer Slide Time: 04:32)
3 And we found out also, that this Cysteine amino acid can form what is called a disulfide linkage. So what we saw in the previous Slide was the formation of a peptide bond. How is the peptide bond formed again? It is formed by the elimination of water between two amino acids. So, we have the carboxylic group of one amino acid, lose its OH, with the H of the amino group of the next amino acid. And that is a strong covalent bond that is formed. The only other covalent bond that is formed in proteins is this disulfide linkage that is formed by the linking of two Cysteine residues that may be following the protein chain, to form what is called the disulfide linkage that is the S-S linkage. This S-S linkage, that is shown in the above slide. Now, I mentioned about this experiment, where the whole protein chain was unfolded and it was found that exactly the same disulfide linkages were formed. I will just go through that experiment once. (Refer Slide Time: 05:49)
4 Now, when we look at a protein structure, which we will be doing in the other classes to come, this is what is called a native structure, an active native structure. What does an active structure mean? It means that, this protein or this enzyme has its function intact. So when we are looking at this active enzyme, we know that its enzymatic activity is to the fullest, because it is a folded protein, it is in its native structure. Now, if you add beta mercaptoethanol to this, beta mercaptoethonal will reduce the S-S linkages, to form SH. So, your cysteine S-S disulfide bond is now, a cysteine residue over again. So now, we have added beta mercaptoethonal, the structure of which is given below, just the BME that is written here. Then we add urea. What urea does, is we learn again later, is it unfolds the protein. Now, what do you mean by unfolding the protein? It means that all the interactions that occur in the folded protein, which we will be studying as we go along or understanding protein structures, the whole thing is unfolded to form a long polypeptide chain. (Refer Slide Time: 07:31)
5 So, that is what it is going to look like now. Why, because we have the native structure, we had four S-S linkages. Now, what happened to those four S-S linkages? We reduced the S-S linkages to form SH. Then what we did is, after we form SH, we added urea. Once you add urea, what happens is it unfolds the protein completely. So, now we have this long chain, with eight SH bonds to it. So, what is this? It is the denatured, inactive, random coil. What is the random coil mean? It means, it is not folded any more. It is just like any necklace or to speak with beads on it. It is not folded. So, now what happens is, the SH is now a free to link with any other SH. You have eight SHs. So, number one could link with number five or it could link with number six or with seven. So now, when we look at this structure, we believe that the SHs could practically or possibly link with any others. (Refer Slide Time: 08:47)
6 Because, there are many such conformations possible. But now, what actually happens is, we remove the beta mercaptoethonal. In the removal of BMA, what we are ensuring is that, these SH linkages or rather the S-S linkages can now reform. Because, we are now, instead of reducing it, we are oxidizing it. So, if we look at these SH linkages that can form, we understand that since there are many confirmations possible, it might form any one. Then what we do is, we remove urea. So, what I am doing? I am allowing the formation of S-S linkages; I am allowing the protein to refold. In doing so, it is found that it forms the exact same structure that was there before. What is this structure? It is exactly just one conformation. And what is this there? It is the native structure of the enzyme that is fully active, with the four disulfide bonds, correct to what was there in the original protein. If you just think of the whole thing, it is extremely fascinating, considering that this protein has hundred and twenty-four amino acids linked together. It has a hundred and twenty-four amino acids linked together and it had eight SH linkages, which could have bonded with any other one. I allow the protein to unfold. So, when I have this unfolded structure, it could have essentially formed any or folded into any conformation, but that is not happened. It always forms this one conformation that is the native structure of the fully active protein, with the exact four disulfide bonds, correctly linked with one another. And that is what extremely intriguing about protein folding. And it is still not understood to date. Nobody understands how a protein folds into the same structure, each and every time. (Refer Slide Time: 11:28)
7 Now, these are some modified amino acids, which we just need to know the names of, considering that these amino acids are present in proteins, to some extent. The names are Hydroxyproline, What is different here is, there is specific OH attached to this. Can you see the OH attached to the Hydroxyproline? That is one other amino acid, not among the common amino acids, that you observe in proteins. Another one, the second one is gamma carboxyglutamate. What is glutamate? Glutamate is, when you have the single carboxylic acid group here. If you have another carboxylic attached to it, it becomes gamma carboxyglutamate. And this is the structure, which you see in some proteins. Another one is phosphoserine, linked to the O. What is serine? Serine is, now you recognize this as part of the polypeptide chain. How is this part of the polypeptide chain? That is just kept here and I will show you how this is part of the polypeptide chain. If you look at this region (showing the phosphoserine), what do we see here? This is the NH of which amino acid? The serine, the phosphoserine in this case, and what is this (showing C=O), the part of? It is carboxylic part of this phosphoserine (()) (13:15). So what is happening next? Here, if I link these two together, the covalent bond of the peptide one bond would be this, linked to the NH of the next amino acid. (Interacting with the students) So, what group is this (showing the lower part of phosphoserine)? Your R group. Now, you can see again. If you look at the carboxyglutamate, what do I have here? I have part of the polypeptide chain. And
8 you always notice, what is the one that you have on the left, the nitrogen, the amino part of the carboxylic acid always. Because, that is the way you read the protein. Because now, you are not always going to get structures, you are going to get letters, one after the other. So, what do you have to do? You have to read the sequence of the protein. When you read the sequence of the protein, then you have to recognize the symbols and these are redundant. What is the information that you need, you only need to know, what R is there? That s all you need to know. Because, if I tell you that I have glycine, alanine, valine. If you know the structures, you can draw the polypeptide chain. Because, you know how they are linked to one another. You know that the NH2 has to be on the glycine side. The carboxylic part has to be on the valiant side. And the Alanine would be intermediate like one of these. So that is the way, we consider all the amino acids. All the information you actually need, is the series of letters. The alphabet, the amino acid alphabet that comprises proteins or polypeptide chains. So what are the ones, the modified ones that we could have, there are all these, but these are the common ones. We can have hydroxyproline, in which are, hydroxyl is attached here, we can have carboxyglutamate, where the carboxy is attached here, we can have phosphoserine, where the phospho is attached here, the phosphate grouped to the inside of OH. we can have phosphor tyrosine, tyrosine remember is one of the aromatic amino acid that we studied in the last class. What are the other two? They are phenylalanine and tryptophan. So what do we have here, instead of the OH that we would have for tyrosine, we have the phosphate attached to it. So, it is now phosphor tyrosine. Now usually, when the polypeptide chain is formed, this is not a common occurrence. All these happen, all the modified amino acids that you see, occurred after the protein is found. So these are usually as I have mentioned, written here, these are modifications that actually occur after the protein is synthesized and what we call them are post-translational modifications. Because, the process of translation is how you get from RNA to protein. What is the central dogma of biology? It is DNA to RNA to protein. The process of DNA going to RNA is known as transcription.
9 The process of RNA going to protein is known as translation. So after the translation, it means you have RNA, translating to the protein. So, you have the synthesized protein. Now, if you have what is called the post-translational modification, you would have these specific modifications, that I have shown on the Slide that occur in the already synthesized protein. (Refer Slide Time: 17:41) Now, what we see on this Slide are grouping of amino acids. We mentioned some groupings last time, when I showed you the individual structures of the amino acids. But these are extremely important in the properties of the proteins, in the catalyst of the enzymes and the catalytic properties of the proteins, in their functional properties, in their surface properties, of the proteins. The grouping of these amino acids is very important. Now, if you go to different sites or even different books, there are certain differences in the groupings. But that does not really matter because what we will be looking at is, we will be looking at the closed groupings, meaning that we will be looking at Aspartic acid and Glutamic acid. These are the acidic amino acids. We look at Arginine, Lysine and Histidine. Now this is where, there is one change at times, where the Histidine at times occurs in the positive set and at times occurs in the uncharged polar set. We will see why that occurs, in a few Slides from now. So the ones that definitely negative are Aspartic acid and Glutamic acid. The ones that are definitely positive are Arginine and Lysine. We also see Histidine in this group, in this particular grouping of amino acids. Now, what does this mean, if we have a negative amino acid or we have a positive amino acid,
10 these amino acids, as I mentioned earlier are likely to be on the surface of the protein. Why? Because, they are going to interact with the solvent. You know that when we are speaking about the solvent, the protein is where? It is in a solution of physiological PH that is, say you have protein in your body, it is in the blood, the blood is primarily a lot of water in there, so what kind of solution is it, it is a polar solvent. So, all of the ones that we are looking in here, would favorably interact with the polar solvent. If you look at the second set that we have here, these are uncharged polar. So, where do these charges come from, they come from the specific side chains that they have. And if you have a knowledge of the side chains you know exactly, what they are. Then, we have the Arginine and Lysine. In the uncharged polar set, you see how we have the changes, these are the three letter codes and these are the one letter codes. Then we have the nonpolar amino acids. These nonpolar amino acids would also have called hydrophobic amino acids, would tend to be away from the solvent. Why? Because, they have no favorable atoms, no favorable interactions with the solvent. Why is that, because all the side chains mostly contain carbon and hydrogen. So, what do we have here? We have Alanine, we have Glycine, we have Valine, we have Leucine, Isoleucine, Proline, Phenylalanine, Methionine, Tryptophan and Cysteine. Last three of these have hetero atoms in them. Methionine has a Sulphur. Tryptophan has a nitrogen and Cysteine also has a Sulphur. But, since they have a predominant amount of carbon and hydrogen to them, they are put in the nonpolar group. So, where would these favorably be? Unless they have specific reasons to be on the surface, they would rather be breading in the protein. What we mean by breading in the protein is we study later when we look at protein structures. (Refer Slide Time: 22:14)
11 Now, we have to consider specific properties of amino acids. What are these properties? One thing that we looked at is, we have looked at the size and the shape. Shape means, what do I mean by shape or size, size means molecular mass. The smallest one is Glycine. It has the hydrogen atom in that. What is the next smallest one? Alanine, why? Because it has the methyl group. Then gradually we can go there, what is the largest and bulkiest one we could have, Tryptophan is the bulkiest one and the longest is Arginine, Lysine. Arginine is the longest in and we could have Lysine. So, these are the long chain amino acids that you could have. So the size and the shape of the amino acid is dependent on what? On the R group. Because the rest of it, it is the same. What is the rest of it? We have the isometric carbon atom that is referred to as the alpha carbon atom, then we have the amino group, the carboxylic group and a hydrogen that is common to all amino acids. Then, it is only the size and the shape of the R group that differs. What else? The charge. We just saw in the previous Slide, how we have the groupings. So, we can have positive amino acids. What are the positive amino acids? Lysine and Arginine. What are the negative amino acids? Aspartic acid and Glutamic acid. The next could be the polar amino acids. The ones, now what is the difference between the charged ones and the polar ones? The polar ones, preferably some of them being small like Serine, can occur in the center part of the proteins. They will form hydrogen bonds. Because, they have an OH to them, like Serine has an OH,
12 so it can preferably form a hydrogen bond. Threonine has an OH, that can also form a hydrogen bond. What about the amide ones? What do we have? We have two amines. What are they? Asparagine and Glutamine. So, we could have sets of hydrogen bonds formed with them as well. So, we could have them being polar, forming hydrogen bonds, but uncharged. That is what the differences. Then we have, probably the most important property of amino acids, the hydrophobicity. That is extremely important because it determines the protein folding nature. That is what is, we will study a lot of this hydrophobicity as we go along. Another one is aromaticity. We find three amino acids that are aromatic in nature, Phenylalanine, Tryptophan and Tyrosine. And as I mentioned before, in the last class that it is these three aromatic amino acids that contribute to the absorbents of proteins. The optical density or the UV absorbents that you see of proteins is solely due to the aromatic amino acid residues. And this we monitor at two hundred and eighty nanometers. That the UV absorbents of proteins is usually monitored at two eighty nanometers and the absorbents of the optical density that is absorbed is solely due to the presence at to eighty nanometers of the aromatic amino acids, Phenylalanine, Tyrosine and Tryptophan. Then, we have the confirmation that is usually determined by the side chain. What you mean by the confirmation of the amino acid? The side chain, when I have a long side chain, it has single bonds in it. It is free to rotate in three-dimensional space. So, I am going to have confirmation, associated with the property of the amino acid. If I have an Alanine, I have just the methyl group. So, that will not as such have any confirmation because all of it will look identical. But, if we have a long side chain like Glycine or Arginine, we have a lot of single bonds in there. What can happen to those single bonds? We can rotate. As they rotate, see I have the Lysine rotate. Then what is going to happen? The NH2 group that forms the end of the Lysine group, will what? that will also rotate? That would also change and as it changes, it is going to change the confirmation of its side chain.
13 This we will study, the propensity to adopt a particular conformation. This comes, when we will between the secondary structure of proteins, which we will be doing in detail later on. And the relative position in proteins we can say from the properties that we already know. What are these? One is the hydrophobicity. From the hydrophobicity, I can straight away say that, say for example of Phenylalanine or Leucine or Isoleucine, is expected to be in the central part of the protein. Lysine and Arginine are an acidic amino acid, like Aspartic acid or Glutamic acid, is expected to be where? On the surface of the protein. (Refer Slide Time: 27:57) Now, this is very important, Histidine. Remember I mentioned two Slides ago, why Histidine is sometimes considered to be a positively charged amino acid and sometimes it is not. The reason is, all of you know what pka means. We will be studying this in great detail, because this is extremely important for protein structure. Now the side chain that you have here, is the side chain for the Histidine. Now if you notice, what Histidine can do is, it can accept a proton and as it accepts the proton, the side chain gets protonated. So what has happened here? What is this? This is my asymmetric alpha carbon atom. The nomenclature of this is such, that if this is the alpha carbon atom. Since the carboxylic acid part and the amino part is common to all amino acids, this carboxylic carbon is always referred to as just C.
14 This C alpha means that the side chain is connected to this carbon atom. Then we have this CH2. What comes after alpha? Beta. So, this is the C beta. There is a specific nomenclature, which we will study as we do protein structure later on. But for now, it will (()) (29:57) say that two C alpha is attached C beta of the side chain. So, Alanine will just have the C beta. The next one in this case would be, what would this be? This would be a C gamma. Then we would have two deltas, a C delta and an N delta and so on and so forth. So, just by the nomenclature alone, you can actually identify which amino acid it belongs to. Why? Because the odd chains are different for each case. So, an Alanine is just going to have a single C beta. What about Valine? It is going to have the C alpha. Every amino acid has a C alpha. What about Valine? It is going to have a C beta and two C gammas, a C gamma one and a C gamma two. So, let us get back to the understanding of the protein donor and protein acceptor capabilities of the Histidine side chain. The pka of six means, that when there is an equilibrium at a certain PH, where it can either accept the proton or it can donate the proton. Now, apart from what is here, which we will be studying in a moment, the ionization of amino acids. At this particular point, I can protonate this nitrogen to form NH plus. It can also deprotonate to form this pack. So this is an equilibrium. Now this equilibrium occurs at a PH close or rather between 6 and 7. Now if you look at what the physiological PH is, that is the PH of blood, the PH of which all the enzymatic reactions in our body are taking place, that is at a PH of 7.4. The physiological PH is 7.4. Now, what happens to Histidine is that, because of this protonation and de-protonation that can occur. Histidine forms a large a number of the catalytic sites of proteins. Just because, it can accept and donate a proton, close to the physiological PH, which is not the case with the other amino acids. If you are too basic, your pka is going to be too high. The PH of the body does not reach that amount of protonation or de-protonation. Because the physiological PH of our blood, where all the reactions that are taking place in our body, is close to the pka value of the Histidine side chain. This forms a component in many enzymatic reactions, in many catalytic sites of proteins. So, what are we talking about? We are talking about basically, the ionization of amino acids. So,
15 what do I mean by ionization of amino acid. Initially, if I am at a very low PH, what is going to happen to the carboxylic group? It is going to remain as COOH. What is going to happen to the amine group? It is going to remain as NH3 plus. Because I have larger amount of protons in solution. That is what we are going to exactly see here. (Refer Slide Time: 34:19) So, at the PH of one, when the PH is very low, I have COOH. I also have NH3 plus. We all understand that. Why? Because I have an acidic solution, which has sufficient amount of protons, to protonate all the side chains or all the amino groups. We are not talking about the side chain right now. But, since the amino acid itself has a carboxylic group and amine group, we can consider the protonation of those two sides alone. So, what do we have here? We have act, a PH of one. We have our carboxylic acid protonated. We have our amine group protonated. There is no other place, where a proton can be accepted here. As you increase the PH or rather, if you consider a titration, say you are adding base, so what is going to happen, the base is going to abstract the protons from the amino acid, in the order or in the ease of its abstraction. So, what is the first one that is going to get lost? It is the carboxylic acid one. So, at a PH of seven, I have lost this. So, I am no longer C double bond OOH. I am now COO-. But, the amine group does not lose its proton at this PH. I keep on increasing the PH or rather, I keep on adding more base. At a point, I will lose the proton that is attached to the amine group. So, if I do an ordinary titration of the amino acid, I will get how many inflection points? Two. Why? One corresponding to the loss of the carboxylic acid H, the other corresponding to the amine H plus.
16 So this is where, I get one set, what is this pka? This is pka two. What does that mean? It means, as I close on and reach the PH close to two, I am going to lose this proton. This pka is ten. So, after I reach a PH of ten, will I lose this proton? So at a PH of twelve, I am now at this position, where I have lost all the protons possible (())(37:32). Is that clear? So initially what did I have? I had everything protonated at a low PH. As I go on, what do I have? I have a form. What is the charge on this form? It is a Zwitterionic form. It has a positive charge to it and a negative charge to it. So it is effectively charges zero. That is Zwitterionic form of the amino acid. Every amino acid is going to have this form. Now, what becomes fascinating is, suppose my R group also has a charge to it. So, where is it going to become interesting? When I have acidic group or an amine group attached to it, so this will come into the picture, when I have Aspartic acid or Glutamic acid, Lysine and Arginine and the Histidine for the pka six. (Refer Slide Time: 38:39) If I consider, the ionization of the amino acid, I have an equilibrium dissociation constant associated with it. This is true for all acids. What is the equation that we have here? We have [HA], dissociating into [H+] and [A-] and what is this Ka associated with it? It is this equilibrium here, where I have [H+], [A-] and [HA]. Now, you all studied about buffers, where we are looking at what is called as Henderson-Hasselbalch equation.
17 What is that equation? You basically rearrange this equation, rearrange the expression rather and we write it in such a manner that we get the [H+] on the left hand side. And, I know that the negative logarithm of hydrogen ion concentration corresponds to the PH. So what do I have here? I have the ph of the solution equal to the pka, right, what is the pka? It corresponds to this equilibrium + log [A-]/[HA]. What is this? This is the base form. This is the acid form. Rather the salt form or the acid form. That is the way, we write the Henderson- Hasselbalch equation and if the concentration of these two are equal, it means, I have equal amount of un-dissociated and dissociated forms. Then, this is going to have a ratio of one, making the logarithm 0 and my ph double bond pka. So, what are we considering here, we are considering our amino acids as 1HA form. And we consider the dissociation from [HA] to [H+] and [A-]. And what am I getting from there? I am getting a ph double bond pka, when my [A-] double bond [HA]. (Refer Slide Time: 41:24) Now, this is what happens when we titrate an amino acid. The titration of an amino acid is going to show a diagram like this, which you will understand very clearly. First of all, we consider these as Ampholytes, why? Because they have both an acidic and basic group attached to it. That is true for all amino acids. You have an acidic group, which is the carboxylic acid group. You have a basic group, which is the amino group. So, these are called Ampholytes.
18 Now, as the base is added, what is here along the X axis, we have number of OHequivalence added. So, basically what you are doing? You are adding NaOH to your amino acid solution. Initially, if we make the ph very low, what does my amino acid look like? All of its protonated. So, I have COOH and NH3+. Now, what am I doing? I am abstracting H+. How? By adding OH-. So, if I add OH-, I abstract protons so there is a dissociation immediately. As soon as, I hit upon this ph, what happens? What do I have at this ph? From the previous Slide, I have equal amount of this species, which is my A- and this species. Now, I want you to understand this very clearly, because you have to remember that as soon as you start OHaddition, this H+ starts getting abstracted. So, as soon as I start the first drop of OH-, does not bring you to the pka, but it starts abstracting H+. So, I come to a point, as I keep adding my OH-, I come to a point, where this is equal to this. What is that point? That is when my ph is equal to my pka. So, the pk1 value as it is called, is What does this mean? It means at this point, I have equal amount of the carboxylic acid anion here and the COO- and COOH. Now, what I am doing? Now, in its deterionic form (()) (44:50), I keep on adding OH-. As I add OH- further now, what is going to happen? At some point, I am going to lose the amine proton. The NH3+ has to lose its proton, when I cross its pk2, then after I met this level, say. So, I am at pk1, where I have equal amount as I keep on adding OH- now, I have more amount of A- than HA. Now, I keep on going with the titration, then, when I come to this point, what do I have? I have equal amounts of this form, which is now my A- and my HA, which is this. So, this is my H as this is my acid, which has this additional H+ added to it. As I add OH-, I abstract this proton at a particular ph, the pk2 will correspond to a ph, where I have equal amounts of this species and this species. So, now, if I keep on adding OH-, I will just be increasing the ph, because, I now have just OH- in solution and this. This would be the titration of Glycine. You would have two inflexion points, the first corresponding to the carboxylic acid proton, the second to the amine proton. But, now the fun comes, when we do not have H2 here.
19 Suppose, I have come thing else, suppose I have Glutamate, what does Glutamate have now? It has an additional COOH to it. (Refer Slide Time: 47:10) So, what do I have at low ph now? At low ph, look at this species, this is our side chain. This is the carboxylic acid part of the amino acid; this is my NH3+. At this point, all of them are protonated. Why? Because my ph is very low. So, at my low ph, that is at this region here, all of these are protonated. I am now adding OH-. What is going to happen? I will start abstracting the proton or rather the group that will most easily lose its proton will start the equilibration. That will be this proton. The proton that belong to the carboxylic acid of the amino acid not of the side chain will be lost first. So, what is the species that is being formed? It is this. This is still protonated; this is still protonated. Now, I am continuing with the titration. I still have how many more protons that I could lose? Two. One belonging to the COOH of the side chain and one the NH3 protons. As I go on further with my OH- addition, my titration, the next easiest one to be removed is the side chain one. Now, I keep on going. Now, what is going to happen? As I am now going to lose the last proton that is left, that is here, the amine group. So, the three pk values that I now have correspond to the, what does it correspond to? We have 3 protons that could be lost. (Refer Slide Time: 50:01)
20 So, we have the first one lost, which is the carboxylic acid group attached to the C alpha of the amino acid. Not the side chain, because, why do we lose that first? Because, that is the most acidic proton. Then, we go on to the next one, which is the next most acidic proton the side chain one. So, we have the side chain one that we are going to lose. Then, we lose the amine one. What do I have in my curve here? I have a pk1 value, I have a pkr value. What is the pkr? R is the side chain, so it corresponds to the side chain. Then, we have pk2 value, corresponding to the amine. Let us do Histidine now. When we see the Histidine pkr, remember, we showed the pk of 6, where we had the changes or where it could accept a proton or donate a proton. So, that is exactly what is happening here. What do we have? We have now at low ph, what do we have? All the possible sides protonated, so we have the side chain protonated, we have the amine group of amino acid protonated, we have the COOH protonated. As we increase the OHadditions, then what is going to happen? Is I am going to lose this proton? So, as I lose this proton, what is the next one that I am going to lose? The one belonging to the side chain. The last one that I am going to lose is going to be the one belonging to the most basic group, which is the amine group. Again, I have my profile or my titration curve rather look like this. So, I have a pk1, corresponding to the COOH, I have my pkr corresponding to the side chain and I have my pk2 corresponding to the amine group.
21 (Refer Slide Time: 52:47) So, what do we have here? For the Glycine, I have two pk values. What is this form? It is this zwitterionic form. So, at a particular point, if I go back, Ok, what do I have here? Here I have a pkr value, but here I have something called a pi value. That is the isoelectric point. What do you mean by an isoelectric point? An isoelectric point is the point at which you would have the zwitterionic form of this amino acids and the charge would be zero. Is the charge zero at this point? It is. So, what is my pi value, it is the sum of the pk of the carboxylic group, the pk of the amine group by 2. What happens if I have the glutamide? When is the charge zero for the glutamic acid? The charge is zero for the glutamic acid at what point? When it is between these two. This pk2 for the amine, this is pkr, this is pka. So, it first loses this one. So, in between these two, my charge is what? Zero. So, what is my pi value? divided by 2. The net charge of the solution is always zero. It depends on the ph of the solution it is. If you have a ph corresponding to the pi, then the charge on the amino acids is zero. When we have Lysine, what does Lysine have? Lysine has an additional NH3+. When you do a titration curve or if you study a titration curve of Lysine, it is going to behave differently. And what do we have? We have the pi now, at a point, where Lysine is uncharged. That is between the pk of amino group and the pk of the side chain this time. So, what is the pi of Lysine? It is very high. (Refer Slide Time: 55:56)
22 To summarize what we have is we have the Ionizations of the amino acids. These are the regions, low ph regions corresponding to aspartic. glutamic acid, the intermediate regions to Histidine, then we have Lysine and finally we have Arginine. These Lysine and Arginine are the two basic Amino acid, aspartic acid and glutamic acids are two acidic amino acids, histidine acts as the proton acceptor and proton donor, depending on what ph you are at. We shall stop here for today and so, the discussion we had today was on understanding the properties of the amino acids, their size, their charge, their polarity and the titrations of the amino acids, which is extremely important when we consider the ionization that could occur based on how or what the ph of the solution is. Thank you.
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Metabolic Classification of the Amino Acids
 Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay
 Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
2018/9/24 AMINO ACIDS. Important. 436 Notes Original slides. 438 notes Extra information
 2018/9/24 AMINO ACIDS Important. 436 Notes Original slides. 438 notes Extra information Objectives: What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino
2018/9/24 AMINO ACIDS Important. 436 Notes Original slides. 438 notes Extra information Objectives: What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino
Amino acids. Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester,
 Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
BIOB111 - Tutorial activity for Session 14
 BIOB111 - Tutorial activity for Session 14 General topics for week 7 Session 14 Amino acids and proteins Students review the concepts learnt and answer the selected questions from the textbook. General
BIOB111 - Tutorial activity for Session 14 General topics for week 7 Session 14 Amino acids and proteins Students review the concepts learnt and answer the selected questions from the textbook. General
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Tala Saleh. Tamer Barakat. Diala Abu-Hassan
 13 Tala Saleh Tamer Barakat Diala Abu-Hassan Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome
13 Tala Saleh Tamer Barakat Diala Abu-Hassan Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome
CHAPTER 3 Amino Acids, Peptides, Proteins
 CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Introduction to proteins and protein structure
 Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
3. AMINO ACID AND PEPTIDES
 3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
BIO 311C Spring Lecture 15 Friday 26 Feb. 1
 BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
استاذ الكيمياءالحيوية
 قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Amino Acids. Lecture 4: Margaret A. Daugherty. Fall Swiss-prot database: How many proteins? From where?
 Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
CHM333 LECTURE 6: 1/25/12 SPRING 2012 Professor Christine Hrycyna AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID:
 AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
MITOCW MIT7_01SCF11_track13_300k.mp4
 MITOCW MIT7_01SCF11_track13_300k.mp4 HAZEL SIVE: All right. Let's move on to the second topic of our discussion today, which we will start today and then continue on Friday. And this is a discussion of
MITOCW MIT7_01SCF11_track13_300k.mp4 HAZEL SIVE: All right. Let's move on to the second topic of our discussion today, which we will start today and then continue on Friday. And this is a discussion of
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Biochemistry 210 Chapter 22
 Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
So where were we? But what does the order mean? OK, so what's a protein? 4/1/11
 So where were we? We know that DNA is responsible for heredity Chromosomes are long pieces of DNA DNA turned out to be the transforming principle We know that DNA is shaped like a long double helix, with
So where were we? We know that DNA is responsible for heredity Chromosomes are long pieces of DNA DNA turned out to be the transforming principle We know that DNA is shaped like a long double helix, with
Midterm 1 Last, First
 Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Human Biochemistry Option B
 Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
THE UNIVERSITY OF MANITOBA. DATE: Oct. 22, 2002 Midterm EXAMINATION. PAPER NO.: PAGE NO.: 1of 6 DEPARTMENT & COURSE NO.: 2.277/60.
 PAPER NO.: PAGE NO.: 1of 6 GENERAL INSTRUCTIONS You must mark the answer sheet with pencil (not pen). Put your name and enter your student number on the answer sheet. The examination consists of multiple
PAPER NO.: PAGE NO.: 1of 6 GENERAL INSTRUCTIONS You must mark the answer sheet with pencil (not pen). Put your name and enter your student number on the answer sheet. The examination consists of multiple
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
BCH302 [Practical] 1
![BCH302 [Practical] 1 BCH302 [Practical] 1](/thumbs/78/76808179.jpg) BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
Section 1 Proteins and Proteomics
 Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
Structure of -amino acids. Stereoisomers of -amino acids. All amino acids in proteins are L-amino acids, except for glycine, which is achiral.
 amino acids Any of a large number of compounds found in living cells that contain carbon, oxygen, hydrogen, and nitrogen, and join together to form proteins. Amino acids contain a basic amino group (NH
amino acids Any of a large number of compounds found in living cells that contain carbon, oxygen, hydrogen, and nitrogen, and join together to form proteins. Amino acids contain a basic amino group (NH
1.4. Lipids - Advanced
 1.4. Lipids - Advanced www.ck12.org In humans, triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of excess starches and other
1.4. Lipids - Advanced www.ck12.org In humans, triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of excess starches and other
Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds.
 Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
I) Choose the best answer: 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine.
 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
Organic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins
 rganic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins No. 1 of 10 1. Which amino acid does not contain a chiral center? (A) Serine (B) Proline (C) Alanine (D) Phenylalanine (E) Glycine
rganic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins No. 1 of 10 1. Which amino acid does not contain a chiral center? (A) Serine (B) Proline (C) Alanine (D) Phenylalanine (E) Glycine
Biomolecules Amino Acids & Protein Chemistry
 Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Introduction. Basic Structural Principles PDB
 BCHS 6229 Protein Structure and Function Lecture 1 (October 11, 2011) Introduction Basic Structural Principles PDB 1 Overview Main Goals: Carry out a rapid review of the essentials of protein structure
BCHS 6229 Protein Structure and Function Lecture 1 (October 11, 2011) Introduction Basic Structural Principles PDB 1 Overview Main Goals: Carry out a rapid review of the essentials of protein structure
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
PROTEIN. By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM
 PROTEIN By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM OBJECTIVES OF THE LECTURE By the end of this lecture, student can: Define
PROTEIN By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM OBJECTIVES OF THE LECTURE By the end of this lecture, student can: Define
If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out.
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
PEPTIDES and PROTEINS
 PEPTIDES and PROTEINS - Learned basic chemistry of amino acids structure and charges - Chemical nature/charges of amino acids is CRUCIAL to the structure and function of proteins - Amino acids can assemble
PEPTIDES and PROTEINS - Learned basic chemistry of amino acids structure and charges - Chemical nature/charges of amino acids is CRUCIAL to the structure and function of proteins - Amino acids can assemble
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
The Structure and Function of Macromolecules
 The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
Amino Acids االحماض األمينية
 Amino Acids االحماض األمينية Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Cell Structure Amino Acids The study of
Amino Acids االحماض األمينية Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Cell Structure Amino Acids The study of
Protein Folding LARP
 Protein Folding LARP Version: 1.0 Release: April 2018 Amplyus 2018 minipcr TM Protein Folding LARP (Live Action Role Play) Summary Materials In this activity, students will role play to make a folded protein
Protein Folding LARP Version: 1.0 Release: April 2018 Amplyus 2018 minipcr TM Protein Folding LARP (Live Action Role Play) Summary Materials In this activity, students will role play to make a folded protein
The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5
 Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Activities for the α-helix / β-sheet Construction Kit
 Activities for the α-helix / β-sheet Construction Kit The primary sequence of a protein, composed of amino acids, determines the organization of the sequence into the secondary structure. There are two
Activities for the α-helix / β-sheet Construction Kit The primary sequence of a protein, composed of amino acids, determines the organization of the sequence into the secondary structure. There are two
MITOCW watch?v=xms9dyhqhi0
 MITOCW watch?v=xms9dyhqhi0 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality, educational resources for free.
MITOCW watch?v=xms9dyhqhi0 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality, educational resources for free.
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AP Bio. Protiens Chapter 5 1
 Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
Maha AbuAjamieh. Tamara Wahbeh. Mamoon Ahram
 12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
AMINO ACIDS. Qualitative Tests
 AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
A Chemical Look at Proteins: Workhorses of the Cell
 A Chemical Look at Proteins: Workhorses of the Cell A A Life ciences 1a Lecture otes et 4 pring 2006 Prof. Daniel Kahne Life requires chemistry 2 amino acid monomer and it is proteins that make the chemistry
A Chemical Look at Proteins: Workhorses of the Cell A A Life ciences 1a Lecture otes et 4 pring 2006 Prof. Daniel Kahne Life requires chemistry 2 amino acid monomer and it is proteins that make the chemistry
AMINO ACIDS NON-ESSENTIAL ESSENTIAL
 Edith Frederika Introduction A major component of food is PROTEIN The protein ingested as part of our diet are not the same protein required by the body Only 40 to 50 gr of protein is required by a normal
Edith Frederika Introduction A major component of food is PROTEIN The protein ingested as part of our diet are not the same protein required by the body Only 40 to 50 gr of protein is required by a normal
Chapter 2 Biosynthesis of Enzymes
 Chapter 2 Biosynthesis of Enzymes 2.1 Basic Enzyme Chemistry 2.1.1 Amino Acids An amino acid is a molecule that has the following formula: The central carbon atom covalently bonded by amino, carboxyl,
Chapter 2 Biosynthesis of Enzymes 2.1 Basic Enzyme Chemistry 2.1.1 Amino Acids An amino acid is a molecule that has the following formula: The central carbon atom covalently bonded by amino, carboxyl,
CHY2026: General Biochemistry. Unit 4:Amino Acid Chemistry
 CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
Protiens and Amino Acids 2
 Protiens and Amino Acids 2 By Alaa J. Mahrath, Biochemistry Department / College of Medicine/ Babylon University 2 Amino Acid Stereoisomers Protiens and Amino Acids All the a-amino acids except for glycine
Protiens and Amino Acids 2 By Alaa J. Mahrath, Biochemistry Department / College of Medicine/ Babylon University 2 Amino Acid Stereoisomers Protiens and Amino Acids All the a-amino acids except for glycine
Model 2: Aldohexoses, aldopentoses, ketohexoses and ketopentoses
 Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Towards a New Paradigm in Scientific Notation Patterns of Periodicity among Proteinogenic Amino Acids [Abridged Version]
![Towards a New Paradigm in Scientific Notation Patterns of Periodicity among Proteinogenic Amino Acids [Abridged Version] Towards a New Paradigm in Scientific Notation Patterns of Periodicity among Proteinogenic Amino Acids [Abridged Version]](/thumbs/84/90818314.jpg) Earth/matriX: SCIENCE TODAY Towards a New Paradigm in Scientific Notation Patterns of Periodicity among Proteinogenic Amino Acids [Abridged Version] By Charles William Johnson Earth/matriX Editions P.O.
Earth/matriX: SCIENCE TODAY Towards a New Paradigm in Scientific Notation Patterns of Periodicity among Proteinogenic Amino Acids [Abridged Version] By Charles William Johnson Earth/matriX Editions P.O.
PAPER No. : 16, Bioorganic and biophysical chemistry MODULE No. : 22, Mechanism of enzyme catalyst reaction (I) Chymotrypsin
 Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Charges on amino acids and proteins. ph 1. ph 7. Acidic side chains: glutamate and aspartate
 harges on amino acids and proteins Acidic side chains: glutamate and aspartate A A- + + + - + Basic side chains: arginine, lysine & histidine Glycine @ p 1 B+ B + + + The amino group, pka 9.6 3 N+ The
harges on amino acids and proteins Acidic side chains: glutamate and aspartate A A- + + + - + Basic side chains: arginine, lysine & histidine Glycine @ p 1 B+ B + + + The amino group, pka 9.6 3 N+ The
Chapter 20 and GHW#10 Questions. Proteins
 Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Protein Investigator. Protein Investigator - 3
 Protein Investigator Objectives To learn more about the interactions that govern protein structure. To test hypotheses regarding protein structure and function. To design proteins with specific shapes.
Protein Investigator Objectives To learn more about the interactions that govern protein structure. To test hypotheses regarding protein structure and function. To design proteins with specific shapes.
Distribution of the amino acids in Nature Frequency in proteins (%)
 Distribution of the amino acids in ature Amino Acid Frequency in proteins (%) Leucine Alanine Glycine erine Valine Glutamic acid Threonine Arginine Lysine Aspartic acid Isoleucine Proline Asparagine Glutamine
Distribution of the amino acids in ature Amino Acid Frequency in proteins (%) Leucine Alanine Glycine erine Valine Glutamic acid Threonine Arginine Lysine Aspartic acid Isoleucine Proline Asparagine Glutamine
! Proteins are involved functionally in almost everything: " Receptor Proteins - Respond to external stimuli. " Storage Proteins - Storing amino acids
 Proteins Most structurally & functionally diverse group! Proteins are involved functionally in almost everything: Proteins Multi-purpose molecules 2007-2008 Enzymatic proteins - Speed up chemical reactions!
Proteins Most structurally & functionally diverse group! Proteins are involved functionally in almost everything: Proteins Multi-purpose molecules 2007-2008 Enzymatic proteins - Speed up chemical reactions!
Sheet #1 Dr. Mamoun Ahram 01/07/2014. Amino Acids
 Amino Acids Dr s office in physiology department in floor #1 Office hours: every day after 1 *How to study biochemistry for Dr. Mamoun s material: Slides will be sent before the lecture, try to read it
Amino Acids Dr s office in physiology department in floor #1 Office hours: every day after 1 *How to study biochemistry for Dr. Mamoun s material: Slides will be sent before the lecture, try to read it
Q1: Circle the best correct answer: (15 marks)
 Q1: Circle the best correct answer: (15 marks) 1. Which one of the following incorrectly pairs an amino acid with a valid chemical characteristic a. Glycine, is chiral b. Tyrosine and tryptophan; at neutral
Q1: Circle the best correct answer: (15 marks) 1. Which one of the following incorrectly pairs an amino acid with a valid chemical characteristic a. Glycine, is chiral b. Tyrosine and tryptophan; at neutral
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site. Still having trouble understanding the material? Check
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site. Still having trouble understanding the material? Check
This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is worth 2 points.
 MBB 407/511 Molecular Biology and Biochemistry First Examination - October 1, 2002 Name Social Security Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is
MBB 407/511 Molecular Biology and Biochemistry First Examination - October 1, 2002 Name Social Security Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is
M1 - Renal, Fall 2007
 University of Michigan Deep Blue deepblue.lib.umich.edu 2007-09 M1 - Renal, Fall 2007 Lyons, R.; Burney, R. Lyons, R., Burney, R. (2008, August 07). Renal. Retrieved from Open.Michigan - Educational Resources
University of Michigan Deep Blue deepblue.lib.umich.edu 2007-09 M1 - Renal, Fall 2007 Lyons, R.; Burney, R. Lyons, R., Burney, R. (2008, August 07). Renal. Retrieved from Open.Michigan - Educational Resources
Lipids: diverse group of hydrophobic molecules
 Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
CHAPTER 21: Amino Acids, Proteins, & Enzymes. General, Organic, & Biological Chemistry Janice Gorzynski Smith
 CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination # 5: Section Five May 7, Name: (print)
 Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Dr. Nafith Abu Tarboush
 11 Dr. Nafith Abu Tarboush July 3 rd 2013 Marah Dannoun Continuation of amino acids applications in life other than tryptophan and tyrosine: 1. Glutamate (glutamic acid): it s modified to give monosodium
11 Dr. Nafith Abu Tarboush July 3 rd 2013 Marah Dannoun Continuation of amino acids applications in life other than tryptophan and tyrosine: 1. Glutamate (glutamic acid): it s modified to give monosodium
Content Lecture 1 Lecture 2 - Amino acid Peptide and Polypeptide Lecture 3 Lecture 4
 Good Morning Class! Content Lecture 1 - Basic knowledge for Biochemistry - Cell: Structure and Function - Water Lecture 2 - Amino acid Peptide and Polypeptide Lecture 3 - Protein Structure and Function
Good Morning Class! Content Lecture 1 - Basic knowledge for Biochemistry - Cell: Structure and Function - Water Lecture 2 - Amino acid Peptide and Polypeptide Lecture 3 - Protein Structure and Function
Raghad Abu Jebbeh. Amani Nofal. Mamoon Ahram
 ... 14 Raghad Abu Jebbeh Amani Nofal Mamoon Ahram This sheet includes part of lec.13 + lec.14. Amino acid peptide protein Terminology: 1- Residue: a subunit that is a part of a large molecule. 2- Dipeptide:
... 14 Raghad Abu Jebbeh Amani Nofal Mamoon Ahram This sheet includes part of lec.13 + lec.14. Amino acid peptide protein Terminology: 1- Residue: a subunit that is a part of a large molecule. 2- Dipeptide:
Short polymer. Dehydration removes a water molecule, forming a new bond. Longer polymer (a) Dehydration reaction in the synthesis of a polymer
 HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
