BCMB Chapter 3 (part1)
|
|
|
- Avis Ramsey
- 6 years ago
- Views:
Transcription
1 Chapter 3 (with parts of 4 and 5) Amino Acids and Primary Structures of Proteins BCMB Chapter 3 (part1) Diversity of protein function Complete definition of amino acids Memorize complete structure of 20 common amino acids!!! pka s of amino and carboxyl groups Amino acids with ionizable side groups Titration curve 1
2 Pink: make up ~ 97% mass of organisms Purple: five essential ions Light blue: most common trace elements Dark blue: less common trace elements PROTEIN STRUCTURE AND FUNCTION Mulder (1800s): Berzelius (1838): from Greek proteios "of first rank" Proteins recognize and many different types of molecules & most of the chemical necessary for life. 2
3 Examples of Protein Function Enzymatic catalysis: enzymes increase reaction rates by ; nearly all known enzymes are Transport & storage: many small molecules and ions are transported by ; examples: Coordinated motion: examples: myosin and actin (muscle movement) Examples of Protein Function Mechanical support: (tensile strength to skin and bones) Immune protection: Generation & transmission of nerve impulses: example: Control of growth and differentiation: (repressors, activators, transcription factors, hormones, regulation of translation) 3
4 WHAT ARE PROTEINS? Proteins are macromolecules made up of (20 amino acids) : consist of an, a, a and a distinctive bonded to a carbon atom. This carbon is called the because it is adjacent to the carboxyl group. BCMB Lecture 3 Diversity of protein function Complete definition of amino acids Memorize complete structure of 20 common amino acids!!! pka s of amino and carboxyal groups Amino acids with ionizable side groups Titration curves & pi 4
5 Amino acids in solution will be in a. The amino group and/or the carboxyl group will be charged depending upon the ph. The R group may also be charged. At neutral ph amino acids are predominantly Under normal cellular conditions amino acids are (dipolar ions): Amino group = -NH 3 + Carboxyl group = -COO - General Structure of the ionized from of an amino acid COOH COO - COO - NH 3 + C H R Fischer Projection? COO - : pka NH + : pka You MUST know this!!! 5
6 Two representations of an amino acid at neutral ph (a) Structure (b) Ball-and stick model (asymmetric) due to the tetrahedral array of 4 different groups around the -carbon (glycine is an exception). Thus all amino acids except glycine can exist as : two that are nonsuperimposable mirror images of each other. Enantiomers of amino acids are called D (right-handed) or L (left-handed) L and D refer to absolute configuration L-amino acids are the only constituents of 6
7 Mirror-image pairs of amino acids 20 different amino acids are found in proteins (side chains) that differ in size, shape, charge, hydrogen-bonding capacity & chemical reactivity 20 different amino acids found in proteins of all organisms from bacteria to humans The amino acid alphabet is at least years old The diversity of protein structure & function is due to the sequence and number of amino acids found in a protein ( ) It is essential that a biochemist commit to memory the structure of the 20 amino acids! Know this!! (structure, & 1 & 3 letter code) 7
8 Structures of the 20 Common Amino Acids You must learn the one and three letter abbreviations You must know the properties of their side chains (R groups) Classes: Aliphatic, Aromatic, Sulfur-containing, Alcohols, Bases, Acids and Amides You must be able to draw the structure of the 20 amino acids in their correct ionized form at any given ph Page 38 Page 40 Four amino acid structures -C not chiral 8
9 Stereoisomers of Isoleucine Isoleucine [I] (Ile) Aliphatic amino acid Page 38 Page 40 Ile has 2 chiral carbons, 4 possible stereoisomers *** (Ile) [I] Proline has a nitrogen in the aliphatic ring system Page 38 Page 40 Proline (Pro, P) - has a three carbon side chain bonded to the -amino nitrogen The heterocyclic pyrrolidine ring restricts the geometry of polypeptides 9
10 R Groups Side chains have aromatic groups Phenylalanine (Phe, F) - benzene ring (OD 260 nm) Tyrosine (Tyr, Y) - phenol ring (OD 280 nm) Tryptophan (Trp, W) - bicyclic indole group (OD 280 nm) OD at 280 nm is important for identifying proteins during protein purification. Aromatic amino acid structures Page 38 Page 40 pka = 10.5 benzene ring (OD 260 nm) phenol ring (OD 280 nm) indole group (OD 280 nm) 10
11 R Groups Methionine (Met, M) - (-CH 2 CH 2 SCH 3 ) Cysteine (Cys, C) - (-CH 2 SH) Two cysteine side chains can be cross-linked by forming a disulfide bridge (-CH 2 -S-S-CH 2 -) Disulfide bridges may stabilize the threedimensional structures of proteins Methionine and Cysteine Page 38 Page 40 Page 39 Page 41 pka =
12 Formation of cystine Two Cys side chains can be cross-linked by forming a disulfide bridge (-CH 2 -S-S-CH 2 -); disulfide bridges may stabilize 3D protein structure Oxidation: loss of an electron Side Chains with Serine (Ser, S) and Threonine (Thr, T) have uncharged polar side chains Page 39 Page 41 12
13 R Groups Histidine (His, H) - imidazole Lysine (Lys, K) - alkylamino group Arginine (Arg, R) - guanidino group Side chains are nitrogenous bases which are substantially positively charged at ph 7 (true for K & R) Page 40 Page 42 Structures of histidine, lysine and arginine pka = 6.0 pka = 10.5 pka = 12.5 imidazole alkylamino group guanidino group 13
14 R Groups and Derivatives Aspartate (Asp, D) and Glutamate (Glu, E) are dicarboxylic acids, and are negatively charged at ph 7 Asparagine (Asn, N) and Glutamine (Gln, Q) are uncharged but highly polar Structures of aspartate, glutamate, asparagine and glutamine Page 41 Page 43 pka = 3.9 pka = 4.1 dicarboxylic acids uncharged but highly polar 14
15 of Amino Acid Side Chains : the relative hydrophobicity of each amino acid The larger the, the greater the tendency of an amino acid to prefer a hydrophobic environment Hydropathy affects protein folding: *hydrophobic side chains tend to be in the *hydrophilic residues tend to be on the scale for amino acid residues (Free-energy change for transfer of an amino acid from interior of a lipid bilayer to water) The larger the hydropathy, the greater the tendency of an amino acid to prefer a hydrophobic environment Hydropathy: the relative hydrophobicity of each amino acid Amino acid Free-energy change for transfer (kjmol -1 ) Highly hydrophobic Isoleucine 3.1 Phenylalanine 2.5 Valine 2.3 Leucine 2.2 Methionine 1.1 Less hydrophobic Tryptophan 1.5* Alanine 1.0 Glycine 0.67 Cysteine 0.17 Tyrosine 0.08 Proline Threonine Serine -1.1 Highly hydrophilic Histidine -1.7 Glutamate -2.6 Asparagine -2.7 Glutamine -2.9 Apartate -3.0 Lysine -4.6 Arginine
16 BCMB Lecture 3 Diversity of protein function Complete definition of amino acids Memorize complete structure of 20 common amino acids!!! pka s of amino and carboxyal groups Amino acids with ionizable side groups Titration curves & pi Structures of the 20 Common Amino Acids You must learn the one and three letter abbreviations You must know the properties of their side chains (R groups) Classes: Aliphatic, Aromatic, Sulfurcontaining, Alcohols, Bases, Acids and Amides You must be able to draw the structure of the 20 amino acids in their correct ionized form at any given ph 16
17 Amino acids in solution will be in a charged state. The - and/or the - will be charged depending upon the ph. The may also be charged. At neutral ph amino acids are predominantly dipolar ions ( ). ( COOH COO - + H + ) (NH 3 + NH 2 +H + ) COO - NH 3 + C H R COO - : pka NH + : pka Note: ph dependance of the ionization state Figure 3.2, Page 37/39 17
18 Ionization of Amino Acids Ionizable groups in amino acids: (1) -carboxyl, (2) -amino, (3) some side chains Each ionizable group has a specific pk a AH B + H + For a solution ph below the pk a, the protonated form predominates (AH) For a solution ph above the pk a, the unprotonated (conjugate base) form predominates (B) Titration curves are used to determine pk a values pk 1 = 2.4 pk 2 = 9.9 pi Ala = isoelectric point (pi = ph when net charge is zero) Titration curve for alanine pi = ( ) / 2 =
19 pka values of amino acids All amino acids contain two ionizable groups: -carboxyl (pka ) and protonated -amino group (pka ) (Table 3.1, pg 41 gives some specific pka values) BUT FOR THIS COURSE use pka s from the notes. The R group of 7 amino acids are also ionizable. The pka of these R groups can range from 3.9 to You must memorize the pka s of the side chains of these seven amino acids! BCMB Lecture 3 Diversity of protein function Complete definition of amino acids Memorize complete structure of 20 common amino acids!!! pka s of amino and carboxyal groups Amino acids with ionizable side groups Titration curves & pi 19
20 α α pk a values of amino acid ionizable groups Table 3.1 pk a values of amino acid ionizable groups 20
21 (a) Titration curve of histidine pk 1 = 1.8 pk 2 = 6.0 pk 3 = 9.3 pi = ph at which net charge is zero To determine the pi: pick the pka above & below the point where the charge is zero and average those 2 pka s Ionization of Histidine Deprotonation of imidazolium ring pka=1.8 pka=9.3 21
22 (a) Titration curve of histidine pk 1 = 1.8 pk 2 = 6.0 pk 3 = 9.3 pi = ph at which net charge is zero pi = ( )/2 =7.65 To determine the pi: pick the pka above & below point where charge is zero and average those 2 pka s Ionization of Histidine Ionization of the protonated -carboxyl of glutamate 22
23 Deprotonation of the guanidinium group of Arg 23
24 BCMB 3100 Chapter 3 (part 2) Summary of amino acids Polypeptides: definition, structure, and direction Peptide bond Disulfide bonds Protein purification Methods to determine amino acid composition, cleavage of proteins, protein sequencing Diversity of proteins The 20 amino acid are divided into 7 groups : Gly, Ala, Val, Leu, Ile, Pro (G A V L I P) : Phe, Tyr, Trp (F Y W) : Met, Cys (M C) : Ser, Thr (S T) : His, Lys, Arg (H K R) : Asp, Glu (D E) : Asn, Gln (N Q) 24
25 Amino acids are linked by to form polypeptide chains carboxyl of one amino acid is joined to -amino group of another amino acid by a peptide bond (amide bond) with concomitant loss of H 2 O Many amino acids are joined by peptide bonds to form an polypeptide : amino acid unit in a polypeptide By convention the direction of a polypeptide is written starting with amino end H 2 O H 2 O H 2 O aa + aa + aa + aa NH 3+ -aa-aa-aa-aa-coo - Formation of a dipeptide H 2 O H O NH3 + C C O - R + H O NH3 + C C O - R H O H O NH3 + C C N C C O - R H R Figure
26 Most polypeptides contain between 50 and 2000 amino acids Average M.W. for an amino acid is 110 daltons so M.W. of most proteins is 5500 to daltons. (One dalton equals one atomic mass unit; kilodalton = 1000 daltons). Most proteins have M.W. of kd. Some proteins contain disulfide bonds that cross-link between cysteine residues by the oxidation of cysteine. Intracellular proteins often lack disulfides while extracellular proteins often have them. Peptide bond between two amino acids Formation of peptide bonds eliminates the ionizable -carboxyl and -amino groups of the free amino acids except for those at the amino and carboxy termini 26
27 Figure 4.2 Challenge of the Week to be given out on Work with your group of 4 people and find at least one example of a mutation in humans, or in industryrelevant plants, animals or microbes. Present, on a single, one-side, typed page, the amino acid mutated, the phenotype of the effect on the organism, the molecular reason that the mutation causes the effect(s), and the effect that this mutation has on/for humans. Hand in a single, one-sided, typed sheet of paper with ALL group members names (first and last name correctly spelled) as well as a single sentence behind each name describing their contribution to the answer. These will be collected ONLY at the Breakout Session on September 1. 27
28 Aspartame, an artificial sweetener Aspartame is a dipeptide methyl ester (aspartylphenylalanine methyl ester) About 200 times sweeter than table sugar Used in diet drinks Aspartame, an artificial sweetener Aspartame is a dipeptide methyl ester (aspartylphenylalanine methyl ester) About 200 times sweeter than table sugar Used in diet drinks 28
29 Cleaving, blocking disulfide bonds -mercaptoethanol See also Figure 4.4 Figure 4.5 Figure 4.5 Amino acid sequence of bovine insulin. This mature processed form of insulin exemplifies intra-peptide disulfide bonds (within the same chain) and inter-peptide disulfide bonds (between chains) 29
30 PROTEIN PURIFICATION General strategy Tissue disrupt crude fractionation selected fractionation Proteins can be separated by: : gel electrophoresis, gel filtration chromatography, dialysis, centrifugation : salting out : ion-exchange chromatography, isoelectric focusing : hydrophobic interaction chromatography, reverse phase chromatography : affinity chromatography, antibodies Purified proteins can be analyzed by: : Edman degradation, (proteolysis), Mass Spectrometry : X-ray crystallography, NMR : automated solid phase To purify large amounts of proteins one requires: 1. An for the protein (enzyme, antibody, etc.) 2. A to separate desired protein from "all" other proteins and to keep the protein "active during the process 3. Example strategy: Salting-out: the "specific" precipitation of a given protein at a specific high-salt concentration Ion exchange Gel-filtration Affinity chromatography Dialysis often used to remove salts: separation of protein from small molecules through a semipermeable membrane (cellulose) 30
31 Figure 5.3 Different types of chromatography (1) : proteins passed over a column filled with a hydrated porous beads made of a carbohydrate or polyacrylamide polymer (large molecules exit (elute) first) 31
32 Gel-filtration chromatography Fig 5.4 Different types of chromatography (2) : separation of proteins over a column filled with charged polymer beads (+ charged beads = anionexchange chromatography; - charged beads = cation exchange chromatography.) Positively charged proteins bind to beads of negative charge & vice versa. Bound proteins are eluted with salt. Non-charged proteins and proteins of similar charge to resin will elute first. 32
33 Fig 5.5 Ion-exchange chromatography: Different types of chromatography (3) : proteins are passed through a column of beads containing a covalently bound high affinity group for the protein of interest. Bound protein is eluted by free high affinity group. 33
34 Affinity chromatography: Fig 5.6 Different types of chromatography (4) of protein: hydrophobic interaction chromatography (HIC) and reversephase chromatography (RPC) are both based on interactions between hydrophobic patches on the surface of a protein and hydrophobicity of ligands (e.g. alkyl groups) covalently attached to a gel matrix. In RPC, proteins can bind very strongly to the gel and require non-polar solvents for their elution. In HIC protein binding is promoted by inclusion of salt in the solvent and elution of proteins is caused by decreasing or removing salt from the solvent. 34
35 Column Chromatography (a) Separation of a protein mixture (b) Detection of eluting protein peaks : movement of charged solutes through a gel in response to an electric field : chemically inert; polymerized acrylamide matrix of controlled pore size; allows separation of proteins based on mass and charge : (sodium dodecyl sulfate, page 73): anionic detergent used for polyacrylamide gel electrophoresis. It complexes with proteins (1 SDS/2 amino acids) denatured protein of negative charge proportional to protein mass. Note: reducing agents (mercaptoethanol, dithiothreitol) are also added to reduce disulfide bonds. Mobility of many proteins under these conditions is linearly proportional to the log of their mass. Smaller proteins migrate faster. 35
36 Polyacrylamide gel electrophoresis (PAGE): Fig 5.8 (a) SDS-PAGE Electrophoresis (b) Protein banding pattern after run Comassie Blue or Silver staining SDS 36
37 Proteins can also be separated by electrophoresis based on their native charge. (isoelectric point): ph at which net protein charge is zero : electrophoresis of proteins (w/o SDS) in a ph gradient to a position in the gel at which ph = pi. ph gradient formed by polyampholytes (small multi-charged polymers of many pis). Isoelectric focusing: Fig 5.10 Combined SDS-PAGE and Isoelectric focusing: 2D-PAGE Fig
38 Purification Table Fig 5.13 (see also Fig. 5.9) In western blotting or immunoblotting, proteins are separated in an SDS PAGE gel, transferred to a polymer, and then stained with a fluorescent antibody. 38
39 Fig 5.23 Amino Acid Composition (1) 1. Determine amino acid composition hydrolysis a. Peptide free amino acids 6 N HCl 100 C, 24 hr b. Amino acid composition of hydrolysates determined by automated cation-exchange chromatography (amino acid analyzer). Column contains solid granules of sulfonated polystyrene. Amino acids reacted with ninhydrin (yields colored product) & detected by O.D. 39
40 Acid-catalyzed hydrolysis of a peptide Problem: Asn Asp; Asn + Asp = Asx or B Gln Glu; Gln + Glu = Glx or Z Loose some: Ser, Thr, Tyr; Loose most Trp Amino Acid Composition (2) c. Another method for aa composition analysis is to treat protein hydrolysate with phenylisothiocyanate (PITC) at ph 9.0 to yield PITC-aa derivatives, separate by HPLC via hydrophobic attraction of aa side chains to hydrocarbon matrix of column and quantitate by OD 254 nm (due to PTC moiety). (The first pure protein analyzed for a.a. composition was -lactoglobulin. It took several years of work. Today amino acid analyzers allows composition analysis within 2-4 hours with samples as small as 1 pmole!!!) 40
41 Amino acid treated with PITC phenylisothiocyanate Chromatogram from HPLC-separated PTCamino acids Note: acid treatment converts Asn Asp; Gln Glu; Trp is destroyed, some loss of Ser, Thr, Tyr. B = (Asn Asp) + Asp; Z = (Gln Glu) + Glu 41
42 Fig Determine amino-terminal residue devised the first method to label N- terminal residue by reacting 2,4- dinitrophenylbenzene with -amino group yellow derivative. Subsequent hydrolysis (6N HCl) hydrolyzes away all other amino acids. N-terminal residue derivative identified by chromatography Today Dabsyl chloride ( colored derivative) or Dansyl chloride ( fluorescent derivative) are used. 42
43 3. Determination of amino acid sequence by Edman Degradation ( ) (1950) Edman Degradation is now done on sequenators (automated, liquid phase sequenator analysis by HPLC (one cycle 2 hr) Gas-phase sequenator: detection of pmole amounts (single SDS-PAGE band) Frederick Sanger determined the first complete sequence of a protein (insulin) in 1953 (51 amino acid long) Note: Amino acid sequence of small proteins and peptides is now commonly determined by mass spectrometry (e.g. electrospray MS, MALDI MS). Edman degradation procedure Phenylisothiocyanate (Edman reagent) ph = 9.0 Phenylthiocarbamoyl-peptide F 3 CCOOH 43
44 Edman degradation procedure (cont) Aqueous acid Polypeptide chain with n-1 amino acids Returned to alkaline conditions for reaction with additional phenylisothiocyanate in the next cycle of Edman degradation Edman degradation procedure (cont) Aqueous acid Polypeptide chain with n-1 amino acids Returned to alkaline conditions for reaction with additional phenylisothiocyanate in the next cycle of Edman degradation PTH- Submit remaining polypeptide to another round of Edman Degradation 44
45 Edman Degradation Summarized Fig 5.26 Protein Cleavage Edman degradation limited to polypeptides of 50 a.a. : Cyanogen bromide (CNBr): splits on carbonyl side of Met : : protease cleaves on carbonyl side of Arg and Lys : protease cleaves on carbonyl side of bulky hydrophobic and aromatic amino acids 45
46 Protein cleavage by BrCN 46
47 Cleavage, sequencing an oligopeptide See also Fig 5.27 must be removed from proteins by reduction & alkylation before sequencing. Reducing agent (iodoacetate) DTT ICH 2 COO- R-S-S-R R-SH HS-R R-S-CH 2 -COO- DNA recombinant technology DNA sequence of nascent protein Limitation of the amino acid sequence only from DNA code: it is only the sequence of the nascent protein. : direct polypeptide product of translation (no modification) 47
48 Cleaving, blocking disulfide bonds Protein amino acid sequences can be deduced from the sequence of nucleotides in the corresponding gene human & chimp Cyt c are identical Closely related species contain proteins with very similar amino acid sequences Differences reflect evolutionary change from a common ancestral protein sequence Phylogenetic tree for Cytochrome c 48
49 Polypeptide chain nomenclature Amino acid residues compose peptide chains Peptide chains are numbered from the N (amino) terminus to the C (carboxyl) terminus Example: (N) Gly-Arg-Phe-Ala-Lys (C) (or GRFAK) Formation of peptide bonds eliminates the ionizable -carboxyl and -amino groups of the free amino acids 49
BCMB 3100 Chapter 3 (part 1)
 BCMB 3100 Chapter 3 (part 1) Diversity of protein function Complete definition of amino acids Memorize complete structure of 20 common amino acids!!! pka s of amino and carboxyl groups Amino acids with
BCMB 3100 Chapter 3 (part 1) Diversity of protein function Complete definition of amino acids Memorize complete structure of 20 common amino acids!!! pka s of amino and carboxyl groups Amino acids with
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Biomolecules Amino Acids & Protein Chemistry
 Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
PROTEINS. Building blocks, structure and function. Aim: You will have a clear picture of protein construction and their general properties
 PROTEINS Building blocks, structure and function Aim: You will have a clear picture of protein construction and their general properties Reading materials: Compendium in Biochemistry, page 13-49. Microbiology,
PROTEINS Building blocks, structure and function Aim: You will have a clear picture of protein construction and their general properties Reading materials: Compendium in Biochemistry, page 13-49. Microbiology,
2. Which of the following amino acids is most likely to be found on the outer surface of a properly folded protein?
 Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
Metabolic Classification of the Amino Acids
 Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
استاذ الكيمياءالحيوية
 قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
Amino Acids. Lecture 4: Margaret A. Daugherty. Fall Swiss-prot database: How many proteins? From where?
 Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Amino Acids االحماض األمينية
 Amino Acids االحماض األمينية Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Cell Structure Amino Acids The study of
Amino Acids االحماض األمينية Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Cell Structure Amino Acids The study of
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
CHM333 LECTURE 6: 1/25/12 SPRING 2012 Professor Christine Hrycyna AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID:
 AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
Human Biochemistry Option B
 Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
3. AMINO ACID AND PEPTIDES
 3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
CHAPTER 21: Amino Acids, Proteins, & Enzymes. General, Organic, & Biological Chemistry Janice Gorzynski Smith
 CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5
 Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
AA s are the building blocks of proteins
 Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Maha AbuAjamieh. Tamara Wahbeh. Mamoon Ahram
 12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
CHAPTER 3 Amino Acids, Peptides, Proteins
 CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
Amino Acids. Amino Acids. Fundamentals. While their name implies that amino acids are compounds that contain an NH. 3 and CO NH 3
 Fundamentals While their name implies that amino acids are compounds that contain an 2 group and a 2 group, these groups are actually present as 3 and 2 respectively. They are classified as α, β, γ, etc..
Fundamentals While their name implies that amino acids are compounds that contain an 2 group and a 2 group, these groups are actually present as 3 and 2 respectively. They are classified as α, β, γ, etc..
Amino acids. Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester,
 Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
The Structure and Function of Macromolecules
 The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
Practice Problems 3. a. What is the name of the bond formed between two amino acids? Are these bonds free to rotate?
 Life Sciences 1a Practice Problems 3 1. Draw the oligopeptide for Ala-Phe-Gly-Thr-Asp. You do not need to indicate the stereochemistry of the sidechains. Denote with arrows the bonds formed between the
Life Sciences 1a Practice Problems 3 1. Draw the oligopeptide for Ala-Phe-Gly-Thr-Asp. You do not need to indicate the stereochemistry of the sidechains. Denote with arrows the bonds formed between the
Lipids: diverse group of hydrophobic molecules
 Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Proteins. Amino acids, structure and function. The Nobel Prize in Chemistry 2012 Robert J. Lefkowitz Brian K. Kobilka
 Proteins Amino acids, structure and function The Nobel Prize in Chemistry 2012 Robert J. Lefkowitz Brian K. Kobilka O O HO N N HN OH Ser65-Tyr66-Gly67 The Nobel prize in chemistry 2008 Osamu Shimomura,
Proteins Amino acids, structure and function The Nobel Prize in Chemistry 2012 Robert J. Lefkowitz Brian K. Kobilka O O HO N N HN OH Ser65-Tyr66-Gly67 The Nobel prize in chemistry 2008 Osamu Shimomura,
Chapter 3 Amino Acids, Peptides and Proteins
 Chapter 3 Amino Acids, Peptides and Proteins Amino Acids Amino acids can be formed from inorganic compounds under prebiotic conditions These compounds are the monomeric units that are joined to form polypeptides
Chapter 3 Amino Acids, Peptides and Proteins Amino Acids Amino acids can be formed from inorganic compounds under prebiotic conditions These compounds are the monomeric units that are joined to form polypeptides
Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
(30 pts.) 16. (24 pts.) 17. (20 pts.) 18. (16 pts.) 19. (5 pts.) 20. (5 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
BIO 311C Spring Lecture 15 Friday 26 Feb. 1
 BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
CHY2026: General Biochemistry. Unit 4:Amino Acid Chemistry
 CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
Lecture 4. Grouping Amino Acid 7/1/10. Proteins. Amino Acids. Where Are Proteins Located. Nonpolar Amino Acids
 Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination # 5: Section Five May 7, Name: (print)
 Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
BIOCHEMISTRY REVIEW. Overview of Biomolecules. Chapter 4 Protein Sequence
 BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 4 Protein Sequence 2 3 4 Are You Getting It?? A molecule of hemoglobin is compared with a molecule of lysozyme. Which characteristics do they share?
BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 4 Protein Sequence 2 3 4 Are You Getting It?? A molecule of hemoglobin is compared with a molecule of lysozyme. Which characteristics do they share?
Introduction to proteins and protein structure
 Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
BCHS 3304/ Exam I. September 26,
 Name: 5.5.# BCHS 3304/ Exam I September 26, 2002............... Instructions: 1. There are 13 pages to this exam. Count pages prior to beginning exam. You may use the back pages of the exam as scratch
Name: 5.5.# BCHS 3304/ Exam I September 26, 2002............... Instructions: 1. There are 13 pages to this exam. Count pages prior to beginning exam. You may use the back pages of the exam as scratch
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur. Lecture -02 Amino Acids II
 Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Midterm 1 Last, First
 Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Introduction. Basic Structural Principles PDB
 BCHS 6229 Protein Structure and Function Lecture 1 (October 11, 2011) Introduction Basic Structural Principles PDB 1 Overview Main Goals: Carry out a rapid review of the essentials of protein structure
BCHS 6229 Protein Structure and Function Lecture 1 (October 11, 2011) Introduction Basic Structural Principles PDB 1 Overview Main Goals: Carry out a rapid review of the essentials of protein structure
Short polymer. Dehydration removes a water molecule, forming a new bond. Longer polymer (a) Dehydration reaction in the synthesis of a polymer
 HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
LAB#23: Biochemical Evidence of Evolution Name: Period Date :
 LAB#23: Biochemical Evidence of Name: Period Date : Laboratory Experience #23 Bridge Worth 80 Lab Minutes If two organisms have similar portions of DNA (genes), these organisms will probably make similar
LAB#23: Biochemical Evidence of Name: Period Date : Laboratory Experience #23 Bridge Worth 80 Lab Minutes If two organisms have similar portions of DNA (genes), these organisms will probably make similar
If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out.
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Macromolecules Structure and Function
 Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
Multiple-Choice Questions Answer ALL 20 multiple-choice questions on the Scantron Card in PENCIL
 Multiple-Choice Questions Answer ALL 20 multiple-choice questions on the Scantron Card in PENCIL For Questions 1-10 choose ONE INCORRECT answer. 1. Which ONE of the following statements concerning the
Multiple-Choice Questions Answer ALL 20 multiple-choice questions on the Scantron Card in PENCIL For Questions 1-10 choose ONE INCORRECT answer. 1. Which ONE of the following statements concerning the
Amino Acids. Review I: Protein Structure. Amino Acids: Structures. Amino Acids (contd.) Rajan Munshi
 Review I: Protein Structure Rajan Munshi BBSI @ Pitt 2005 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2005 Amino Acids Building blocks of proteins 20 amino acids
Review I: Protein Structure Rajan Munshi BBSI @ Pitt 2005 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2005 Amino Acids Building blocks of proteins 20 amino acids
1. Describe the relationship of dietary protein and the health of major body systems.
 Food Explorations Lab I: The Building Blocks STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will be constructing animal and plant proteins using beads to represent the amino acids.
Food Explorations Lab I: The Building Blocks STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will be constructing animal and plant proteins using beads to represent the amino acids.
CHAPTER 3 Amino Acids, Peptides, Proteins
 CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
Date: EXERCISE 4. Figure 1. Amino acid structure.
 Student s name: Date: Points: Assistant s signature: Index numer: /6 EXERISE 4 AMIN AIDS AND PRTEINS. Amino acids are structural units (monomers) of proteins. There are 20 different amino acids coded for
Student s name: Date: Points: Assistant s signature: Index numer: /6 EXERISE 4 AMIN AIDS AND PRTEINS. Amino acids are structural units (monomers) of proteins. There are 20 different amino acids coded for
A Chemical Look at Proteins: Workhorses of the Cell
 A Chemical Look at Proteins: Workhorses of the Cell A A Life ciences 1a Lecture otes et 4 pring 2006 Prof. Daniel Kahne Life requires chemistry 2 amino acid monomer and it is proteins that make the chemistry
A Chemical Look at Proteins: Workhorses of the Cell A A Life ciences 1a Lecture otes et 4 pring 2006 Prof. Daniel Kahne Life requires chemistry 2 amino acid monomer and it is proteins that make the chemistry
Chapter 20 and GHW#10 Questions. Proteins
 Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
(65 pts.) 27. (10 pts.) 28. (15 pts.) 29. (10 pts.) TOTAL (100 points) Moorpark College Chemistry 11 Spring Instructor: Professor Gopal
 Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Chapter 21 Lecture Outline
 Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay
 Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination #5: Section Five December 7, Name: (print) Section:
 Moorpark College Chemistry 11 Fall 2011 Instructor: Professor Gopal Examination #5: Section Five December 7, 2011 Name: (print) Section: alkene < alkyne < amine < alcohol < ketone < aldehyde < amide
Moorpark College Chemistry 11 Fall 2011 Instructor: Professor Gopal Examination #5: Section Five December 7, 2011 Name: (print) Section: alkene < alkyne < amine < alcohol < ketone < aldehyde < amide
PAPER No. : 16, Bioorganic and biophysical chemistry MODULE No. : 22, Mechanism of enzyme catalyst reaction (I) Chymotrypsin
 Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Cahn - Ingold - Prelog system. Proteins: Evolution, and Analysis Lecture 7 9/15/2009. The Fischer Convention (1) G (2) (3)
 Chapter 4 (1) G Proteins: Evolution, and Analysis Lecture 7 9/15/2009 A V L I M P F W Chapter 4 (2) S (3) T N Q Y C K R H D E The Fischer Convention Absolute configuration about an asymmetric carbon related
Chapter 4 (1) G Proteins: Evolution, and Analysis Lecture 7 9/15/2009 A V L I M P F W Chapter 4 (2) S (3) T N Q Y C K R H D E The Fischer Convention Absolute configuration about an asymmetric carbon related
Four Classes of Biological Macromolecules. Biological Macromolecules. Lipids
 Biological Macromolecules Much larger than other par4cles found in cells Made up of smaller subunits Found in all cells Great diversity of func4ons Four Classes of Biological Macromolecules Lipids Polysaccharides
Biological Macromolecules Much larger than other par4cles found in cells Made up of smaller subunits Found in all cells Great diversity of func4ons Four Classes of Biological Macromolecules Lipids Polysaccharides
AP Bio. Protiens Chapter 5 1
 Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
INTRODUCTION TO BIOCHEMISTRY/POLYMERS. 3. With respect to amino acids, polypeptides, and proteins, know:
 INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
2. Which of the following is NOT true about carbohydrates
 Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
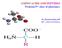 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
Name. The following exam contains 44 questions, valued at 2.6 points/question. 2. Which of the following is not a principal use of proteins?
 Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Protein Structure Klemens Wild, BZH,
 Protein Structure recommended books Proteins protein definition From gr. proteios (superior, erstrangig) 1836 JJ Berzelius Functions: structural, enzymes, muscle, transport immune system, Linear polymer
Protein Structure recommended books Proteins protein definition From gr. proteios (superior, erstrangig) 1836 JJ Berzelius Functions: structural, enzymes, muscle, transport immune system, Linear polymer
MBB 694:407, 115:511. Please use BLOCK CAPITAL letters like this --- A, B, C, D, E. Not lowercase!
 MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
The Basics: A general review of molecular biology:
 The Basics: A general review of molecular biology: DNA Transcription RNA Translation Proteins DNA (deoxy-ribonucleic acid) is the genetic material It is an informational super polymer -think of it as the
The Basics: A general review of molecular biology: DNA Transcription RNA Translation Proteins DNA (deoxy-ribonucleic acid) is the genetic material It is an informational super polymer -think of it as the
Tala Saleh. Tamer Barakat. Diala Abu-Hassan
 13 Tala Saleh Tamer Barakat Diala Abu-Hassan Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome
13 Tala Saleh Tamer Barakat Diala Abu-Hassan Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome
Proteins with similar functions often have αα s can act as both weak acids & weak bases similar αα sequences
 Chapter 5: Amino Acids, Peptides & Proteins Amino acids share common functional groups Amino, Carboxyl & H bonded to C Distinct chemistry of s result of side chains Proteins & polypeptides are polymers
Chapter 5: Amino Acids, Peptides & Proteins Amino acids share common functional groups Amino, Carboxyl & H bonded to C Distinct chemistry of s result of side chains Proteins & polypeptides are polymers
Methionine (Met or M)
 Fig. 5-17 Nonpolar Fig. 5-17a Nonpolar Glycine (Gly or G) Alanine (Ala or A) Valine (Val or V) Leucine (Leu or L) Isoleucine (Ile or I) Methionine (Met or M) Phenylalanine (Phe or F) Polar Trypotphan (Trp
Fig. 5-17 Nonpolar Fig. 5-17a Nonpolar Glycine (Gly or G) Alanine (Ala or A) Valine (Val or V) Leucine (Leu or L) Isoleucine (Ile or I) Methionine (Met or M) Phenylalanine (Phe or F) Polar Trypotphan (Trp
PROTEINS. Amino acids are the building blocks of proteins. Acid L-form * * Lecture 6 Macromolecules #2 O = N -C -C-O.
 Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
BCH302 [Practical] 1
![BCH302 [Practical] 1 BCH302 [Practical] 1](/thumbs/78/76808179.jpg) BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
