Intestinal mucosal barrier function in health and disease. Turner, JR Nature Rev. Immunology 9: 799 (2009)
|
|
|
- Preston Doyle
- 6 years ago
- Views:
Transcription
1 Chapter 9 O-GalNAc Glycans - Essentials of Glycobiology 2 nd edition Intestinal mucosal barrier function in health and disease. Turner, JR Nature Rev. Immunology 9: 799 (2009) From Mucins to Mucus Thornton DJ and Sheehan JK, Proc Am Thorac Soc., 1:54 (2004) Mucin structure, aggregation, physiological functions and biomedical applications Bansil R. and Turner BS Curr Opin in Colloid & Interface Sci, 11:164 (2006) Mucins in the mucosal barrier to infection Linden et al, Nature Immunology 1: 183 (2008) The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Paszek et al, Nature 511: 319 (2014)
2 Chapter 9 O-GalNAc Glycans Essentials of Glycobiology 2 nd edition
3 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; Chapter 9O-GalNAc Glycans Inka Brockhausen, Harry Schachter, and Pamela Stanley. Correction O-glycosylation is a common covalent modification of serine and threonine residues of mammalian glycoproteins. This chapter describes the structures, biosynthesis, and functions of glycoproteins that are often termed mucins. In mucins, O-glycans are covalently α-linked via an N-acetylgalactosamine (GalNAc) moiety to the -OH of serine or threonine by an O-glycosidic bond, and the structures are named mucin O-glycans or O-GalNAc glycans. The focus of this chapter is on mucins and mucin-like glycoproteins that are heavily O-glycosylated, although glycoproteins that carry only one or a few O- GalNAc glycans are also briefly discussed. There are also several types of nonmucin O-glycans, including α-linked O-fucose, β-linked O-xylose, α-linked O-mannose, β-linked O-GlcNAc (N-acetylglucosamine), α- or β-linked O-galactose, and α- or β-linked O-glucose glycans (discussed in Chapters 12 and 16 18). In this chapter, however, the term O-glycan refers to mucin O-glycans, unless otherwise specified. Mucin glycoproteins are ubiquitous in mucous secretions on cell surfaces and in body fluids. DISCOVERY AND BACKGROUND In 1865, E. Eichwald, a Russian physician working in Germany, provided the first chemical evidence that mucins are proteins conjugated to carbohydrate. He also showed that mucins are widely distributed in the animal body. In 1877, F. Hoppe-Seyler discovered that mucins produced by the epithelial cells of mucous membranes and salivary glands contain an acidic carbohydrate, identified many years later to be sialic acid. Mucins are present at many epithelial surfaces of the body, including the gastrointestinal, genitourinary, and respiratory tracts, where they shield the epithelial surfaces against physical and chemical damage and protect against infection by pathogens. Mucin O-glycans begin with an α-linked N- acetylgalactosamine residue linked to serine or threonine. The N-acetylgalactosamine may be extended with sugars including galactose, N-acetylglucosamine, fucose, or sialic acid, but not mannose, glucose, or xylose residues. There are four common O-GalNAc glycan core structures, designated cores 1 through 4 and an additional four designated cores 5 though 8 (Table 9.1). Mucin O-glycans can be branched, and many sugars or groups of sugars on mucin O-glycans are antigenic (Table 9.1). Important modifications of mucin O-glycans include O-acetylation of sialic acid and O-sulfation of galactose and N- acetylglucosamine. Thus, mucin O-glycans are often very heterogeneous, with hundreds of different chains being present in some mucins. Numerous chemical, enzymatic, and spectroscopic methods are applied to analyze the sugar composition and the linkages among sugars of mucin O-glycans. MUCIN GLYCOPROTEINS Mucins are heavily O-glycosylated glycoproteins found in mucous secretions and as transmembrane glycoproteins of the cell surface with the glycan exposed to the external environment. The mucins in mucous secretions can be large and polymeric (gel-forming mucins) or smaller and monomeric (soluble file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 1/12
4 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf mucins). Many epithelial cells produce mucin, but gel-forming mucins are produced primarily in the goblet or mucous cells of the tracheobronchial, gastrointestinal, and genitourinary tracts. In goblet cells, mucins are stored intracellularly in mucin granules from which they can be quickly secreted upon external stimuli. The hallmark of mucins is the presence of repeated peptide stretches called variable number of tandem repeat (VNTR) regions that are rich in serine or threonine O-glycan acceptor sites and have an abundance of clustered mucin O-glycans that may comprise 80% of the molecule by weight. The tandem repeats are usually rich in proline residues that appear to facilitate O-GalNAc glycosylation. Mucins may have hundreds of O-GalNAc glycans attached to serine or threonine residues in the VNTR regions. The clustering of O-GalNAc glycans causes mucin glycoproteins to adopt an extended bottle brush conformation (Figure 9.1). About 20 different mucin genes have been cloned, and they are expressed in a tissue-specific fashion. For example, different mucin genes are expressed in different regions of the gastrointestinal tract, suggesting that they serve specific functions. The first mucin gene to be cloned encoded a transmembrane mucin termed MUC1. Mucins that span the plasma membrane are known to be involved in signal transduction, to mediate cell cell adhesion, or to have an antiadhesive function. Cell-surface mucins contain an extracellular domain with a central VNTR region that carries O-GalNAc glycan chains, a single transmembrane domain, and a small cytoplasmic tail at the carboxyl terminus. The mucin genes, their transcripts, the resulting mucin proteins, and the attached O-GalNAc glycans all exhibit extreme variability. Different mucins vary in the number and composition of the peptide repeats in their VNTR regions. Within the same mucin, the repeats usually vary in their amino acid sequences. It has not yet been possible to determine exactly which of the many different O-GalNAc glycan structures isolated from a purified mucin preparation are attached to specific serine or threonine residues in the tandem repeats. However, in some less densely glycosylated glycoproteins, specific O-GalNAc glycosylation sites have been identified. The structures of O-glycans at these defined sites are usually heterogeneous and any mucin includes a range of glycoforms. One of the exceptions is the Antarctic fish antifreeze glycoprotein, which carries only Galβ1-3GalNAc- at threonine residues of alanine-alaninethreonine repeat sequences. Some mucins contain a hydrophobic transmembrane domain that serves to insert the molecule into the cell membrane. Secreted mucins have cysteine-rich regions and cystine knots that are responsible for their polymerization and the formation of extremely large molecules of several million daltons. Mucins may also have protein domains similar to motifs present in other glycoproteins. For example, D domains found in mucins are also found in von Willebrand factor, a glycoprotein important in blood clotting. The D domain may regulate mucin polymerization (Figure 9.1). Mucin-like glycoproteins such as GlyCAM-1, CD34, and PSGL-1 are less densely O-glycosylated membrane-associated glycoproteins that mediate cell cell adhesion. Most glycoproteins with O-GalNAc glycans carry several O-glycans, although some, such as interleukin-2 and erythropoietin, carry a single O-GalNAc glycan. The expression of mucin genes is regulated by a large number of cytokines and growth factors, differentiation factors, and bacterial products. The purification of gel-forming mucins is achieved by density-gradient centrifugation. Purified mucins have distinct viscoelastic properties that contribute to the high viscosity of mucous secretions. They are hydrophilic and contain charges that attract water and salts. Bacteria, viruses, and other microbes are trapped by mucins and sometimes adhere to specific O-GalNAc glycans that serve as receptors (see Tables 34.1 and 34.2). The ciliary action of the mucous membrane epithelium aids in the removal of microbes and small particles. file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 2/12
5 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf Mucins hydrate and protect the underlying epithelial cells, but they have also been shown to have roles in fertilization, blastocyst implantation, and the immune response. Cell-surface MUC1 and mucin-like glycoproteins have roles in cell adhesion (see Chapter 31). A number of diseases are associated with abnormal mucin gene expression and abnormal mucin carbohydrate structures and properties. These include cancer, inflammatory bowel disease, lung disease, and cystic fibrosis. The expression of underglycosylated MUC1 is often increased in individuals with cancer (see Chapters 43 and 44). O-GalNAc GLYCAN STRUCTURES The simplest mucin O-glycan is a single N-acetylgalactosamine residue linked to serine or threonine. Named the Tn antigen, this glycan is often antigenic. The most common O-GalNAc glycan is Galβ1-3GalNAc-, and it is found in many glycoproteins and mucins (Table 9.1, Figure 9.2). It is termed a core 1 O-GalNAc glycan because it forms the core of many longer, more complex structures. It is antigenic and is also named the T antigen. Both Tn and T antigens may be modified by sialic acid to form sialylated-tn or -T antigens, respectively. Another common core structure contains a branching N-acetylglucosamine attached to core 1 and is termed core 2 (Table 9.1, Figure 9.2). Core 2 O-GalNAc glycans are found in both glycoproteins and mucins from a variety of cells and tissues. Linear core 3 and branched core 4 O-GalNAc glycans (Table 9.1, Figure 9.2) have been found only in secreted mucins of certain mucin-secreting tissues, such as bronchi, colon, and salivary glands. Core structures 5 8 have an extremely restricted occurrence. Mucins with core 5 have been reported in human meconium and intestinal adenocarcinoma tissue, whereas core 6 structures are found in human intestinal mucin and ovarian cyst mucin. Core 8 has been reported in human respiratory mucin. Bovine submaxillary mucin was shown to contain O-GalNAc glycans with relatively short chains, including those containing a core 7 structure (Table 9.1). All of the core structures can be sialylated. However, only cores 1 4 and core 6 have been shown to occur as extended, complex O-glycans that carry antigens such as the ABO and Lewis blood group determinants, the linear i antigen (Galβ1-4GlcNAcβ1-3Gal-), and the GlcNAcβ1-6 branched I antigens (Table 9.1). Both type 1 (based on the Galβ1-3GlcNAc sequence) and type 2 (based on the Galβ1-4GlcNAc sequence) extensions can be repeated several times in poly-n-acetyllactosamine units, which provide a scaffold for the attachment of additional sugars or functional groups. The terminal structures of O-GalNAc glycans may contain fucose, galactose, N-acetylglucosamine, and sialic acid in α-linkages, N- acetylgalac-tosamine in both α- and β-linkages, and sulfate. Many of these terminal sugar structures are antigenic or represent recognition sites for lectins. In particular, the sialylated and sulfated Lewis antigens are ligands for selectins (see Chapter 31). Poly-N-acetyllactosamine units and terminal structures may also be found on N-glycans and glycolipids (see Chapter 13). ISOLATION, PURIFICATION, AND ANALYSIS OF MUCIN O- GLYCANS All O-GalNAc glycans may be released from the peptide by an alkali treatment called β-elimination. The N-acetylgalactosamine residue attached to serine or threonine is reduced to N-acetylgalactosaminitol by borohydride as it is released. Conversion to N-acetylgalactosaminitol prevents the rapid alkali-catalyzed degradation of the released O-GalNAc glycan by a peeling reaction. Glycoproteins that contain both N- and O-glycans will retain their N-glycans and specifically lose O-glycans when subjected to β- elimination. However, harsh alkali treatment will also disrupt peptide bonds and degrade the mucin. Unsubstituted N-acetylgalactosamine residues may also be released from serine or threonine by a specific N-acetylgalactosaminidase, and unsubstituted Galβ1-3GalNAc (core 1) can be released with an enzyme file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 3/12
6 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf termed O-glycanase. For core 1 O-GalNAc glycans substituted with sialic acids, the sialic acid must first be removed by treatment with sialidase or mild acid before O-glycanase treatment will be successful. No enzymes are known to release larger O-glycans from the peptide, in contrast to the availability of several enzymes that release N-glycans from asparagine (see Chapter 8). Released O-GalNAc glycans may be separated into different species by chromatographic methods such as gel filtration, anion-exchange chromatography, and high-pressure liquid chromatography. Chemical derivatization of purified reduced O-glycans helps in the analysis of sugar composition and the determination of linkages between sugars by gas chromatography and mass spectrometry (see Chapter 47). With improvements in the sensitivity of mass spectrometry, it is now possible to analyze small amounts and identify structures on the basis of their mass in complex mixtures. Structural analysis by nuclear magnetic resonance (NMR) spectroscopy is more precise, but requires more material and highly purified compounds. The NMR spectra of hundreds of O-glycans have been published and are used as references to identify known and new O-glycan structures. Other tools to analyze the structures of O- GalNAc glycans include specific exoglycosidases that remove terminal sugars, antibodies to N- acetylgalactosamine (anti-tn) and core 1 (anti-t), and lectins such as peanut agglutinin (which binds to core 1) and Helix pomatia agglutinin (which binds to terminal N-acetylgalactosamine) (see Chapter 45). O-GalNAc glycans in a specific cell line or tissue may be predicted based on the presence of active glycosyltransferases required for their synthesis. BIOSYNTHESIS OF O-GalNAc GLYCANS Polypeptide-N-Acetylgalactosaminyltransferases The first step of mucin O-glycosylation is the transfer of N-acetylgalactosamine from UDP-GalNAc to serine or threonine residues, which is catalyzed by a polypeptide-n-acetyl-galactosaminyltransferase (ppgalnact). There are at least 21 polypeptide-n-acetylgalactosaminetransferases (ppgalnact-1 to -21) that differ in their amino acid sequences and are encoded by different genes. Homologs of ppgalnact genes are expressed in all eukaryotic organisms and a high degree of sequence identity exists between mouse and human ppgalnacts. Immunohistochemistry studies have demonstrated that some ppgalnacts localize to the cis-golgi in submaxillary glands (see Chapter 3). However, more recent studies suggest that in human cervical cancer cells, several enzymes of this family are located throughout the Golgi. The subcellular localization of ppgalnacts and other glycosyltransferases involved in O-glycosylation has a critical role in determining the range of O-glycans synthesized by a cell. Thus, a ppgalnact localized to a late compartment of the Golgi will act just before the secretion of a glycoprotein and is likely to produce a number of incomplete shorter O-GalNAc glycans, a factor that will contribute to the glycan heterogeneity of a mucin. ppgalnact expression levels vary considerably between cell types and mammalian tissues. All ppgalnacts bind UDP-GalNAc (the donor of N-acetylgalactosamine), but they often differ in the protein substrates to which they transfer N-acetylgalactosamine. Such differences allow ppgalnacts to be distinguished. Many ppgalnacts do not efficiently transfer N-acetylgalactosamine to serine, but only to threonine in peptide acceptors used in in vitro assays. Nevertheless, the VNTR regions of mucins appear to be fully O-glycosylated. Many ppgalnacts appear to have a hierarchical relationship with one another, such that one enzyme cannot attach an N-acetylgalactosamine until an adjacent serine or threonine is glycosylated by a different ppgalnact. Thus, coexpression in the same cell of ppgalnacts with complementary, partly overlapping acceptor substrate specificities probably ensures efficient O- GalNAc glycosylation. file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 4/12
7 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf Although defined amino acid sequons that accept N-acetylgalactosamine have not been identified, certain amino acids are preferred in the substrate. Proline residues near the site of N-acetylgalactosamine addition are usually favorable to mucin O-glycosylation, whereas charged amino acids may interfere with ppgalnact activity. It is possible that the role of proline is to expose serine or threonine residues in a β- turn conformation, leading to more efficient O-glycosylation. Databases are available that estimate the likelihood of O-glycosylation at specific sites based on known sequences around O-glycosylation sites of several hundreds of glycoproteins (e.g., OGLYCBASE and NetOGlyc). The domain structure of ppgalnacts is that of a type II membrane protein, typical of all Golgi glycosyltransferases (see Chapter 3). In addition, a distinct lectin-like domain has been discovered at the carboxyl terminus of several ppgalnacts. The lectin domain is used for binding to N- acetylgalactosamine residues that have already been added to the glycoprotein. In vitro studies using O- glycosylated peptides as acceptor substrates have shown that further addition of N-acetylgalactosamine is strongly influenced by the presence of existing N-acetylgalactosamine and larger O-glycans in the acceptor substrate, suggesting a molecular mechanism for the hierarchical relationships of ppgalnacts. Purification of ppgalnacts has been achieved using mucin peptide and nucleotide sugar derivatives as affinity reagents. The recent elucidation of the crystal structure of ppgalnact-1 showed that the catalytic site of the enzyme is spatially separated from the lectin-binding site, and that there is a nucleotide sugarand Mn ++ -binding groove containing an aspartate-x-histidine motif. The unique structure of ppgalnact- 1 suggests that this transferase can accommodate a number of different glycosylated acceptor substrates. Synthesis of O-GalNAc Glycan Core Structures The transfer of the first sugar from UDP-GalNAc directly to serine or threonine in a protein initiates the biosynthesis of all O-GalNAc glycans. Subsequently, with the addition of the next sugar, different mucin O-glycan core structures are synthesized (Figure 9.2). In contrast to the initial reactions of N- glycosylation and O-mannosylation, no lipid-linked intermediates are involved in O-GalNAc glycan biosynthesis, and no glycosidases appear to be involved in the processing of O-GalNAc glycans within the Golgi. The O-glycans of mucins produced in a specific tissue predict the enzyme activities that are present in that tissue. The glycosyltransferases that are involved solely in the assembly of mucin O-GalNAc glycans are listed in Table 9.2. The many other enzymes that may be involved also contribute to the synthesis of N-glycans and glycolipids. The first sugar (N-acetylgalactosamine) added to the protein creates the Tn antigen (GalNAc-Ser/Thr), which is uncommon in normal mucins, but is often found in mucins derived from tumors. This suggests that the extension of O-GalNAc glycans beyond the first sugar is blocked in some cancer cells. Another common cancer-associated structure found in mucins is the sialyl-tn antigen, which contains a sialic acid residue linked to C-6 of N-acetylgalactosamine (Table 9.1). O-Acetylation of the sialic acid residue masks the recognition of sialyl-tn by anti-sialyl-tn antibodies. No other sugars are known to be added to the sialyl-tn antigen. There are eight O-GalNAc glycan core structures (Table 9.1), most of which may be further substituted by other sugars. N-Acetylgalactosamine is converted to core 1 (Galβ1-3GalNAc-) by a core 1 β1-3 galactosyltransferase termed T synthase or C1GalT-1 (Figure 9.3). This activity is present in most cell types. However, to be exported from the endoplasmic reticulum in vertebrates, T synthase requires a specific molecular chaperone called Cosmc. Cosmc is an ER protein that appears to bind specifically to T synthase and ensures its full activity in the Golgi. Lack of core 1 synthesis can be due to either defective T synthase or the absence of functional Cosmc chaperone. The result is high expression of Tn and sialyl- Tn antigens. Immunoglobulin A nephropathy in humans is associated with low expression of Cosmc, and a mutation in the Cosmc gene gives rise to a condition called the Tn syndrome. file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 5/12
8 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf In many serum glycoproteins and mucins, the T antigen is substituted by sialic acid at C-3 of galactose and at C-6 of N-acetylgalactosamine (Figure 9.3). These substitutions add a negative charge to the O- GalNAc glycan. They also prevent other modifications of core 1. The cell surfaces of many leukemia and tumor cells contain large numbers of sialylated core 1 O-GalNAc glycans. On rare occasions, core 1 remains unsubstituted, leaving the T antigen exposed, for example, in cancer and inflammatory bowel disease. In these cases, it is likely that there is an abnormality in either the sialylation of core 1 or further extension and branching of core 1. Core 2 mucin O-glycans are branched core 1 structures that are produced in many tissues, including the intestinal mucosa. The synthesis of core 2 O-GalNAc glycans is regulated during activation of lymphocytes, cytokine stimulation, and embryonic development. Leukemia and cancer cells and other diseased tissues have abnormal amounts of core 2 O-GalNAc glycans. The synthesis of core 2 O-GalNAc glycans has been correlated with tumor progression. Because of their branched nature, core 2 O-glycans can block the exposure of mucin peptide epitopes. The enzyme responsible for core 2 synthesis is core 2 β1-6 N-acetylglucosaminyltransferase or C2GnT (Figure 9.3). At least three genes encode this subfamily (C2GnT-1 to -3) of a larger family of β1-6 N-acetylglucosaminyltransferases that catalyze the synthesis of GlcNAcβ1-6-linked branches. There are two major types of core 2 β1-6 N- acetylglucosaminyltransferases. The L type (leukocyte type, C2GnT-1 and -3) synthesizes only the core 2 structure, whereas the M type (mucin type, C2GnT-2) is also involved in the synthesis of core 4 and other GlcNAcβ1-6-linked branches. The L enzyme is active in many tissues and cell types, but the M enzyme is found only in mucin-secreting cell types. The expression and activity of both the L and M enzymes are altered in certain tumors. The synthesis of core 3 O-GalNAc glycans appears to be restricted mostly to mucous epithelia from the gastrointestinal and respiratory tracts and the salivary glands. The enzyme responsible is core 3 β1-3 N- acetylglucosaminetransferase (C3GnT; Figure 9.4). Although in vitro assays of this enzyme suggest that it is relatively inefficient, the enzyme must act efficiently in vivo in colonic goblet or mucous cells because secreted colonic mucins mainly carry core 3 and few core 1, core 2, or core 4 O-GalNAc glycans. The activity of C3GnT is especially low in colonic tumors and virtually absent from tumor cells in culture. The synthesis of core 4 by the M-type β1-6 N-acetylglucosaminetransferase (C2GnT-2; see above) requires the prior synthesis of a core 3 O-GalNAc glycan (Figure 9.4). The glycosyltransferase synthesizing core 5 must exist in colonic tissues and in colonic adenocarcinoma because these tissues produce mucin with core 5. The enzymes synthesizing cores 5 to 8 remain to be characterized. Synthesis of Complex O-GalNAc Glycans The elongation of the galactose residue of core 1 and core 2 O-GalNAc glycans is catalyzed by a β1-3 N- acetylglucosaminyltransferase (Table 9.2) that is specific for O-GalNAc glycans. These O-glycans can also be extended by N-acetylglucosaminyltransferases and galactosyltransferases to form repeated GlcNAcβ1-3Galβ1-4 (poly-n-acetyllactosamine) sequences that represent the little i antigen. Less common elongation reactions are the formation of GalNAcβ1-4GlcNAc- (LacdiNAc) and Galβ1-3GlcNAc- sequences. Linear poly-n-acetyllactosamine units can be branched by members of the β1-6 N- acetylglucosaminyltransferase family, resulting in the large I antigen. Some of these branching reactions are common to other O- and N-glycans and glycolipids (see Chapter 13). The ABO and other glycan-based blood groups as well as sialic acids, fucose, and sulfate are common terminal structures in mucins as in other glycoconjugates. The families of glycosyltransferases that catalyze the addition of these terminal structures are described in more detail in Chapters 13 and 14. In contrast to N-glycans, O-GalNAc glycans do not have NeuAcα2-6Gal linkages, although the NeuAcα2-6GalNAc moiety is common, for example, in the sialyl-tn antigen and in elongated core 1 and 3 structures. Thus, in most mammalian mucin-producing cells, α2-6 sialyltransferases act on N- file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 6/12
9 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf acetylgalactosamine, and α2-3 sialyltransferases act on galactose. Some of the sialyltransferases and sulfotransferases prefer O-GalNAc glycans as their substrate (Table 9.2), but many of these enzymes have an overlapping specificity and also act on N-glycan structures as acceptor substrates. Control of O-GalNAc Glycan Synthesis The acceptor specificities of glycosyltransferases and sulfotransferases are the main factors determining the structures of O-GalNAc glycans found in mucins, and these specificities restrict the high number of theoretically possible O-glycans to only a few hundred. The specificities also direct the pathways that are feasible. For example, the sialylated core 1 structure NeuAcα2-6(Galβ1-3)GalNAc- can be synthesized only by adding a sialic acid residue to core 1, but not by adding a galactose residue to the sialyl-tn antigen, because the sialic acid prevents the action of core 1 β1-3 galactosyltransferase. The disialylated core 1 structure NeuAcα2-3Galβ1-3(NeuAcα2-6)GalNAc- is synthesized by the addition of the α2-3-linked sialic acid first to core 1, followed by the addition of the α2-6-linked sialic acid to N- acetylgalactosamine. These enzymes can therefore recognize at least two sugar residues and are often (but not always) blocked by the presence of sialic acid. Expression of sialyltransferases is consequently often associated with shorter O-GalNAc glycans. When a core 1 O-GalNAc glycan is sialylated to form NeuAcα2-3Galβ1-3GalNAc-, core 2 cannot be synthesized because the L-type core 2 β1-6 N- acetylglucosaminyltransferase requires an unsubstituted core 1 structure as the acceptor substrate. Detailed specificity studies suggest that the L-type core 2 β1-6 N-acetylglucosaminyltransferase recognizes unsubstituted C-4 and C-6 hydroxyl groups of both the galactose and N-acetylgalactosamine residues of a core 1 O-GalNAc glycan. Another important control factor is that the relative activities of the glycosyltransferases determine the relative amounts of O-GalNAc glycans in mucins. Because of partially overlapping localization in the cisand medial-golgi compartments of breast cancer cells, core 2 β1-6 N-acetylglucosaminyltransferase (C2GnT-1) competes with α2-3 sialyltransferase for the common core 1 substrate (Figure 9.3). Therefore, the relative activities of these two enzymes determine the nature of the O-GalNAc glycans synthesized and the antigenic properties of the cell surface. Depending on which activity predominates, the cellsurface mucin MUC1 may be highly sialylated and its O-glycans relatively small or its O-glycans may be large and more complex due to the presence of extended, branched core 2 O-GalNAc glycans. In MUC1 from breast cancer cells, the presence of these sialylated small O-glycans leads to the exposure of underlying mucin peptide epitopes. In vitro studies have shown that the activities of transferases are controlled by many factors such as metal ions and membrane components. Only two activities involved in O-glycan biosynthesis have been found to be regulated by the presence of specific binding proteins. The first is β1-4 galactosyltransferase 1 (β4galt-1), which can bind to α-lactalbumin in mammary gland to change its preferred acceptor substrate from N-acetylglucosamine to glucose, thereby forming lactose. The second enzyme is core 1 β1-3 galactosyltransferase (C1GalT-1), which requires the coexpression of the molecular chaperone Cosmc. The first step of O-GalNAc glycosylation is clearly regulated by the amino acid sequence of the acceptor substrate. Although individual ppgalnacts may prefer certain sites in the acceptor substrate, a combination of enzymes expressed in the same cell compartment ensures efficient mucin O- glycosylation. Several enzymes of the O-glycosylation pathway recognize their substrates in the context of the underlying peptide. The sequence of the peptide moiety of the acceptor substrate may direct the synthesis of cores 1, 2, and 3 as well as the extension of N-acetylglucosamine-terminating core structures by galactose (Figures 9.3 and 9.4). It appears that glycosyltransferases are arranged in a diffuse assembly line within the Golgi compartments. This intracellular localization of enzymes allows them to act on mucin substrates in a file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 7/12
10 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf specific sequence. Thus, early-acting enzymes synthesize the substrates for the next reactions. Because several enzymes can often use the same substrate, and there is extensive overlap of both localization and specificities, large numbers of different O-glycan structures are synthesized, leading to the heterogeneity seen in mucins. The concentrations of nucleotide sugar and sulfate-donor substrates within the Golgi and the rate of substrate transport throughout the Golgi are additional important control factors (see Chapter 4). The sensitive and complex balance of these various factors appears to be altered upon cell differentiation, cytokine stimulation, and in disease states. FUNCTIONS OF O-GalNAc GLYCANS O-GalNAc glycosylation is probably an essential process because all mammalian cell types studied to date express ppgalnacts. However, when the ppgalnact-1 gene was deleted in mice the animals appeared to be unaffected, possibly due to the fact that another ppgalnact replaces the function of ppgalnact-1. In the secreted mucins of the respiratory, gastrointestinal, and genitourinary tracts, as well as those of the eyes, the O-GalNAc glycans of mucous glycoproteins are essential for their ability to hydrate and protect the underlying epithelium. Mucins also trap bacteria via specific receptor sites within the O-glycans of the mucin. Some sugar residues or their modifications can mask underlying antigens or receptors. For example, O-acetyl groups on the sialic acid residue of the sialyl-tn antigen prevent recognition by anti-sialyl-tn antibodies. Gut bacteria enzymatically remove this blocking group. Bacteria can cleave sulfate with sulfatases or terminal sugars with glycosidases. Because the O-glycans are hydrophilic and usually negatively charged, they promote binding of water and salts and are major contributors to the viscosity and adhesiveness of mucus, which forms a physical barrier between lumen and epithelium. The removal of microbes and particles trapped in mucus is an important physiological process. However, in diseases such as cystic fibrosis, the abnormally high viscosity of the mucus leads to obstruction and life-threatening tissue malfunction. O-GalNAc glycans, especially in the highly glycosylated mucins, have a significant effect on the conformation of the attached protein. Depending on the size and bulkiness of O-glycans, underlying peptide epitopes can be variably recognized by antibodies. O-glycosylation of mucins provides almost complete protection from protease degradation, and it is possible that the sparse O-glycosylation of some secreted glycoproteins, such as the single O-GalNAc glycan on interleukin-2, has a similar protective role. Cell lines defective in specific O-glycosylation pathways can be used as models to study the role of O- glycosylation (see Chapter 46). For example, due to a defect in UDP-Glc-4-epimerase that reversibly converts UDP-GlcNAc to UDP-GalNAc, the ldld cell line does not produce GalNAc-Ser/Thr linkages unless N-acetylgalactosamine is added to the cell medium. The addition of galactose as well as N- acetylgalactosamine results in complete O-GalNAc glycans. This model has been used to demonstrate that O-glycans of cell-surface receptors may regulate receptor stability and expression levels. O-GalNAc glycans change during lymphocyte activation and are abnormal in leukemic cells where an increase in core 2 and a decrease in core 1 O-glycans are often seen. The ligands for selectin-mediated interactions between endothelial cells and leukocytes are commonly based upon sialyl Lewis x epitopes attached to core 2 O-GalNAc glycans. This type of selectin glycan interaction is important for the attachment of leukocytes to the capillary endothelium during homing of lymphocytes or the extravasation of leukocytes during the inflammatory response (see Chapter 31). Removal of core 2 O-GalNAc glycans by eliminating the C2GnT-1 gene in mice results in a severe deficiency in the immune system, particularly in the selectin-binding capability of leukocytes (see Chapter 50). Cancer cells often express sialyl Lewis x epitopes and may thus use the selectin-binding properties of their file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 8/12
11 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf cell surface as a mechanism to invade tissues. The critical role of O-glycans in this process has been studied in cell lines treated with the O-glycan extension inhibitor benzyl-α-galnac (see Chapter 50). This compound acts as a competitive substrate for the synthesis of core 1, core 2, core 3, and core 4 O- glycans in cells and thus causes a reduction in the synthesis of complex O-GalNAc glycans. As a consequence, mucins carry a higher number of unmodified N-acetylgalactosamine residues and shorter O- GalNAc glycans. Inhibitor-treated cancer cells lose the ability to bind to E-selectin and endothelial cells in vitro. Reproductive tissues produce mucins and O-glycosylated glycoproteins that may have important roles in fertilization. Specific terminal O-glycan structures have been shown to form the ligands for sperm-egg interactions in several species (see Chapter 25). The changes of O-glycans commonly observed in diseases can be due to the actions of cytokines or growth factors that affect cell growth, differentiation, and cell death and alter the expression of glycosyltransferase genes. Although these glycosylation changes may be considered a side effect of a pathological condition, they can also significantly contribute to the ultimate pathology and the course of disease. In cancer, the biosynthesis of O-GalNAc glycans is often abnormal due to either decreased or increased expression and activities of specific glycosyltransferases. An altered cell-surface glycocalyx may then affect the biology and survival of the cancer cell. FURTHER READING 1. Tabak LA. In defense of the oral cavity: Structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol. 1995;57: [PubMed: ] 2. van Klinken BJW, Dekker J, Büller HA, Einerhand AWC. Mucin gene structure and expression: Protection vs. adhesion. Am J Physiol. 1995;269:G [PubMed: ] 3. Hansen JE, Lund O, Nielsen JO, Brunak S. O-GLYCBASE: A revised database of O-glycosylated proteins. Nucleic Acids Res. 1996;24: [PMC free article: PMC145605] [PubMed: ] 4. Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta. 1999;1473: [PubMed: ] 5. Fukuda M. Roles of mucin-type O-glycans in cell adhesion. Biochim. Biophys. Acta. 2002;1573: [PubMed: ] 6. Brockhausen I. Sulphotransferases acting on mucin-type oligosaccharides. Biochem Soc Trans. 2003;31: [PubMed: ] 7. Lowe JB, Marth J. A genetic approach to mammalian glycan function. Annu Rev Biochem. 2003;72: [PubMed: ] 8. ten Hagen KG, Fritz TA, Tabak LA. All in the family: The UDP-GalNAc:polypeptide N- acetylgalactosaminyltransferases. Glycobiology. 2003;13:1R 16R. [PubMed: ] 9. Hollingsworth MA, Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer. 2004;4: [PubMed: ] 10. Robbe C, Capon C, Coddeville B, Michalski JC. Structural diversity and specific distribution of O- glycans in normal human mucins along the intestinal tract. Biochem J. 2004;384: [PMC free article: PMC ] [PubMed: ] 11. Wandall HH, Irazoqui F, Tarp MA, Bennett EP, Mandel U, Takeuchi H, Kato K, Irimura T, Suryanarayanan G, Hollingsworth MA, Clausen H. The lectin domains of polypeptide GalNActransferases exhibit carbohydrate-binding specificity for GalNAc: Lectin binding to GalNAcglycopeptide substrates is required for high density GalNAc-O-glycosylation. Glycobiology. 2007;17: [PubMed: ] 12. Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 9/12
12 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf Physiol. 2008;70: [PubMed: ] Figures FIGURE 9.1. A simplified model of a large secreted mucin. FIGURE 9.1 A simplified model of a large secreted mucin. The VNTR (variable number of tandem repeat) region rich in serine, threonine, and proline is highly O-glycosylated and the peptide assumes an extended bottle brush conformation. Hundreds of O-GalNAc glycans with many different structures may be attached to serine or threonine residues in the VNTR domains. The cysteine-rich regions at the ends of the molecules are involved in disulfide bond formation to form large polymers of several million daltons. D domains have similarity to von Willebrand factor and are also involved in polymerization. FIGURE 9.2. Complex O-GalNAc glycans with different core structures. FIGURE 9.2 Complex O-GalNAc glycans with different core structures. Representative examples of complex O- GalNAc glycans with extended core 1, 2, or 4 structures from human respiratory mucins and an O- GalNAc glycan with an extended core 3 structure from human colonic mucins. All four core structures (in boxes) can be extended, branched, and terminated by fucose in various linkages, sialic acid in α2-3 linkage, or blood group antigenic determinants (Table 9.1). Core structures 1 and 3 may also carry sialic acid α2-6-linked to the core N-acetylgalactosamine. Symbol Key: FIGURE 9.2. Complex O-GalNAc glycans with different core structures. FIGURE 9.3. Biosynthesis of core 1 and 2 O-GalNAc glycans. FIGURE 9.3 Correction Biosynthesis of core 1 and 2 O-GalNAc glycans. Shown is the biosynthesis of some extended core 1 and core 2 O-GalNAc glycans. The linkage of N-acetylgalactosamine to serine or threonine to form the Tn antigen, catalyzed by polypeptide-n-acetylgalactosaminyltransferases (ppgalnacts), is the basis for all core structures. Core 1 β1-3 galactosyltransferase (C1GalT-1) synthesizes core 1 (T antigen) and requires a specific molecular chaperone, Cosmc. Core 1 may be substituted with sialic acid or N- acetylglucosamine (as shown) or by fucose or sulfate (not shown). Substitution of core 1 by a β1-3-linked N-acetylglucosamine prevents the synthesis of core 2 by core 2 β1-6 N-acetylglucosaminyltransferase (C2GnT), an enzyme that is highly specific for unmodified core 1 as a substrate. Red cross marks blocked pathway. In breast cancer cells, C2GnT and α2-3 sialyltransferase (ST3Gal I) compete for the common core 1 substrate. If sialyltransferase activity is high, chains will be mainly short with sialylated core 1 structures; if C2GnT activity is high, chains will be complex and large. The Galβ1-3 residue of the core 1 O-GalNAc glycan and both N-acetylglucosamine and galactose branches of the core 2 O-GalNAc glycan may be extended and carry terminal blood group and Lewis antigens (see Table 9.1). file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 10/12
13 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf Symbol Key: FIGURE 9.3. Biosynthesis of core 1 and 2 O-GalNAc glycans. FIGURE 9.4. Biosynthesis of core 3 and core 4 O-GalNAc glycans. FIGURE 9.4 Biosynthesis of core 3 and core 4 O-GalNAc glycans. N-Acetylgalactosamine is substituted by a β1-3 N- acetylglucosamine residue to form core 3. This reaction is catalyzed by core 3 β1-3 N-acetylglucosaminyltransferase (C3GnT), which is active in colonic mucosa. Core 3 may be substituted with sialic acid in α2-6 linkage to N-acetylgalactosamine or with galactose in β1-4 linkage to N- acetylglucosamine. These substitutions prevent the synthesis of core 4 by core 2/4 β1-6 N- acetylglucosaminyltransferase (C2GnT-2). Red crosses mark blocked pathways. Unmodified core 3 can be branched by C2GnT-2 to form core 4. Both core 3 and core 4 O-GalNAc glycans can be extended to complex chains carrying determinants similar to those of core 1 and core 2 O-GalNAc glycans. Symbol Key: FIGURE 9.4. Biosynthesis of core 3 and core 4 O-GalNAc glycans. Tables TABLE 9.1 Structures of O-glycan cores and antigenic epitopes found in mucins O-Glycan Core Tn antigen Sialyl-Tn antigen Core 1 or T antigen Core 2 Core 3 Core 4 Core 5 Core 6 Core 7 Core 8 Epitope Blood groups O, H Blood group A Blood group B Linear B Blood group i Blood group I Blood group Sd(a), Cad Structure GalNAcαSer/Thr Siaα2-6GalNAcαSer/Thr Galβ1-3GalNAcαSer/Thr GlcNAcβ1-6(Galβ1-3)GalNAcαSer/Thr GlcNAcβ1-3GalNAcαSer/Thr GlcNAcβ1-6(GlcNAcβ1-3)GalNAcαSer/Thr GalNAcα1-3GalNAcαSer/Thr GlcNAcβ1-6GalNAcαSer/Thr GalNAcα1-6GalNAcαSer/Thr Galα1-3GalNAcαSer/Thr Fucα1-2Gal- GalNAcα1-3(Fucα1-2)Gal- Galα1-3(Fucα1-2)Gal- Galα1-3Gal- Galβ1-4GlcNAcβ1-3Gal- Galβ1-4GlcNAcβ1-6(Galβ1-4GlcNAcβ1-3)Gal- GalNAcβ1-4(Siaα2-3)Gal- file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 11/12
14 4/14/2016 O-GalNAc Glycans - Essentials of Glycobiology - NCBI Bookshelf Blood group Lewis a Galβ1-3(Fucα1-4)GlcNAc- Blood group Lewis x Galβ1-4(Fucα1-3)GlcNAc- Blood group sialyl-lewis x Siaα2-3Galβ1-4(Fucα1-3)GlcNAc- Blood group Lewis y Fucα1-2Galβ1-4(Fucα1-3)GlcNAc- TABLE 9.2 Glycosyltransferases specific for mucin O-GalNAc glycans Enzyme Short form Polypeptide N-acetylgalactosaminyltransferase ppgalnact-1 to -24 Core 1 β1-3 galactosyltransferase C1GalT-1 or T synthase Core 2 β1-6 N-acetylglucosaminyltransferase C2GnT-1, C2GnT-3 Core 3 β1-3 N-acetylglucosaminyltransferase C3GnT-1 Core 2/4 β1-6 N-acetylglucosaminyltransferase C2GnT-2 Elongation β1-3 N-acetylglucosaminyltransferase elongation β3gnt-1 to -8 Core 1 α2-3 sialyltransferase ST3Gal I, ST3Gal IV α2-6 sialyltransferase ST6GalNAc I, II, III or IV Core 1 3-O-sulfotransferase Gal3ST4 Secretor gene α1-2 fucosyltransferase FucT-I, FucT-II Copyright 2009, The Consortium of Glycobiology Editors, La Jolla, California. Bookshelf ID: NBK1896PMID: Contents < PrevNext > file:///users/tgilstrap/desktop/readings/april%2021-kamil%20/chapter%209_2nd%20edition.html 12/12
15 Intestinal mucosal barrier function in health and disease. Turner, JR Nature Rev. Immunology 9: 799 (2009)
16 REVIEWS Intestinal mucosal barrier function in health and disease Jerrold R. Turner Abstract Mucosal surfaces are lined by epithelial cells. These cells establish a barrier between sometimes hostile external environments and the internal milieu. However, mucosae are also responsible for nutrient absorption and waste secretion, which require a selectively permeable barrier. These functions place the mucosal epithelium at the centre of interactions between the mucosal immune system and luminal contents, including dietary antigens and microbial products. Recent advances have uncovered mechanisms by which the intestinal mucosal barrier is regulated in response to physiological and immunological stimuli. Here I discuss these discoveries along with evidence that this regulation shapes mucosal immune responses in the gut and, when dysfunctional, may contribute to disease. Mucosa-associated lymphoid tissue (MALT). The collections of B cells, T cells, plasma cells, macrophages and other antigen-presenting cells found in the mucosal linings of organs including the gastrointestinal tract, lungs, salivary glands and conjuctiva. Tight junction Also known as the zonula occludens, this is a site of close apposition of adjacent epithelial cell membranes kiss points that create a barrier against the free diffusion of water and solutes. Department of Pathology, The University of Chicago, 5841 South Maryland, MC 1089, Chicago, Illinois 60637, USA. jturner@bsd.uchicago.edu doi: /nri2653 Complex multicellular organisms interface with their external environments at multiple sites, including mucosae of the airways, oral cavity, digestive tract and genitourinary tract, and the skin. Although the skin is the most visible site of interface, the combined area of the mucosal surfaces is much greater than that of the skin. Mucosae are also the primary sites at which the mucosa-associated lymphoid tissue (MALT) is exposed to and interacts with the external environment. The magnitude of these interactions is greatest in the gastrointestinal tract, which is the largest mucosal surface and is also in continuous contact with dietary antigens and diverse microorganisms. Thus, mucosal surfaces, particularly in the intestines, are crucial sites of innate and adaptive immune regulation. A central mediator of interactions between MALT and the external environment, including the intestinal lumen, is the epithelium that covers the mucosa. Epithelial cells establish and maintain the barrier, which in the skin is a tight, although not impermeant, seal. By contrast, most mucosal epithelial cells form leaky barriers. This is necessary to support universal functions that include fluid exchange, which occurs at nearly all mucosal surfaces, as well as essential tissue-specific functions. Thus, the precise permeability characteristics of each barrier type differ based on the functions supported. For example, the ion-selective properties of the barrier vary considerably along the length of a nephron to promote or restrict transport of specific ions in each segment 1. Similarly, the ability of tight junctions to discriminate between and restrict passage of solutes based on size (a characteristic known as size selectivity) varies with location in the intestine, as permeability to larger solutes decreases from the crypt to the villus 2. In addition to these fixed differences, mucosal permeability of many tissues is adaptable and may be regulated in response to extracellular stimuli, such as nutrients, cytokines and bacteria. Recent advances have uncovered some of the mechanisms by which physiological and immunological stimuli affect cellular and extracellular components of the intestinal barrier. In this article I review our current understanding of the mechanisms that regulate intestinal barrier integrity, and discuss the hypothesis that the mucosal barrier can shape pro-inflammatory and immunoregulatory responses in the context of homeostasis and disease. Although they may affect barrier function, dendritic cells (DCs), which extend dendritic processes across the tight junctions in the distal small intestine 3,4, and intraepithelial lymphocytes are not discussed here. In addition, the functions of M cells, which are specialized epithelial cells that deliver antigens directly to intra epithelial lymphocytes and to subepithelial lymphoid tissues by transepithelial vesicular transport from the gut lumen, are reviewed elsewhere 5. Finally, detailed analyses of the interactions between epithelial cells and luminal microorganisms, as well as the intricacies of mucosal immune regulation, have also been recently reviewed elsewhere 6,7. This Review therefore focuses on the roles of the epithelial cell barrier in health and disease, with particular emphasis on the junctional complexes between intestinal epithelial cells, which have a crucial role in barrier regulation. nature REvIEwS Immunology volume 9 november Macmillan Publishers Limited. All rights reserved
17 REVIEWS Mucins A family of heavily glycosylated proteins that are secreted as large aggregates by mucous epithelial cells. Unstirred layer A thin layer of fluid at epithelial cell surfaces that is separated from the mixing forces created by luminal flow and, in the intestine, peristalsis. Coeliac disease A chronic inflammatory condition of the upper small intestine in humans that is caused by immunological hypersensitivity to the α-gliadin component of wheat gluten. It can cause severe villous atrophy, which can lead to malabsorption and malnutrition if gluten-containing foods are not removed from the diet. Anatomy of mucosal barriers Extracellular components of the barrier. Most mucosal surfaces are covered by a hydrated gel formed by mucins (FIG. 1a). Mucins are secreted by specialized epithelial cells, such as gastric foveolar mucous cells and intestinal goblet cells, and create a barrier that prevents large particles, including most bacteria, from directly contacting the epithelial cell layer 8. The importance of mucus gel hydration is shown by cystic fibrosis, in which the production of hyperviscous mucus contributes to pulmonary, pancreatic and intestinal disease 9. Defective mucus production has also been reported in various immunemediated diseases, and spontaneous colitis develops in mice that lack specific mucin genes 10. Although small molecules pass through the heavily glycosylated mucus layer with relative ease, bulk fluid flow is limited and thereby contributes to the development of an unstirred layer of fluid at the epithelial cell surface. As the unstirred layer is protected from convective mixing forces, the diffusion of ions and small solutes is slowed. In the stomach, this property of the unstirred layer works with epithelial cell bicarbonate secretion to maintain a zone of relative alkalinity at the mucosal surface 11. The unstirred layer of the small intestine slows nutrient absorption by reducing the rate at which nutrients reach the transporting protein-rich microvillus brush border, but may also contribute to absorption by limiting the extent to which small nutrients released by the activities of brush border digestive enzymes are lost by diffusion into the lumen. unstirred layer defects have not been linked to specific diseases. However, it is interesting to note that increased unstirred layer thickness has been reported in coeliac disease 12, in which it may contribute to nutrient malabsorption. Cellular components of the mucosal barrier. The primary responsibility for mucosal barrier function resides with the epithelial cell plasma membrane, which is impermeable to most hydrophilic solutes in the absence of specific transporters. Accordingly, direct epithelial cell damage, such as that induced by mucosal irritants or cytotoxic agents, including some drugs used for cancer chemotherapy, results in a marked loss of barrier function. However, in the presence of an intact epithelial cell layer, the paracellular pathway between cells must be sealed. This function is mediated by the apical junctional complex, which is composed of the tight junction and subjacent adherens junction (FIG. 1b). Both tight and a Apical junctional complex b Microvilli Epithelial cells Mucus Basement membrane Goblet cell Unstirred layer Intraepithelial lymphocyte Claudin Occludin E-cadherin ZO1 MLCK F-actin Myosin α-catenin 1 Tight junction Adherens junction β-catenin Plasma cell Lamina propria lymphocyte Desmoglein Desmocollin Keratin Desmosome Desmoplakin Figure 1 Anatomy of the mucosal barrier. a The human intestinal mucosa is composed of a simple layer of columnar epithelial cells, as well as the underlying lamina propria and muscular mucosa. Goblet cells, which synthesize and release mucin, as well as other differentiated epithelial cell types, are present. The unstirred layer, which cannot be seen histologically, is located immediately above the epithelial cells. The tight junction, a component of the apical junctional complex, seals the paracellular space between epithelial cells. Intraepithelial lymphocytes are located above the basement membrane, but are subjacent to the tight junction. The lamina propria is located beneath the basement membrane and contains immune cells, including macrophages, dendritic cells, plasma cells, lamina propria lymphocytes and, in some cases, neutrophils. b An electron micrograph and corresponding line drawing of the junctional complex of an intestinal epithelial cell. Just below the base of the microvilli, the plasma membranes of adjacent cells seem to fuse at the tight junction, where claudins, zonula occludens 1 (ZO1), occludin and F actin interact. E cadherin, α catenin 1, β catenin, catenin δ1 (also known as p120 catenin; not shown) and F actin interact to form the adherens junction. Myosin light chain kinase (MLCK) is associated with the perijunctional actomyosin ring. Desmosomes, which are located beneath the apical junctional complex, are formed by interactions between desmoglein, desmocollin, desmoplakin and keratin filaments. 800 november 2009 volume Macmillan Publishers Limited. All rights reserved
18 REVIEWS Paracellular pathway The route of transepithelial transport that involves passive movement through the space between adjacent cells. Adherens junction Also known as the zonula adherens, this junction is immediately subjacent to the tight junction and requires the activity of lineage-specific Ca 2+ -dependent adhesion proteins, termed cadherins. Desmosome An adhesive junction that connects adjacent epithelial cells. These junctions are composed of multiple protein subunits and are the points where keratin filaments attach to the plasma membrane. Claudin From the Latin claudere, meaning to close, members of this family of transmembrane proteins are variably expressed by specific epithelial cell types and thereby contribute to the unique barrier properties of different tissues. Occludin The first transmembrane tight junction protein identified. The function of occludin remains controversial but it is likely to have roles in barrier regulation and tumour suppression. It also serves as a cofactor in hepatitis C virus entry. Zonula occludens 1 A peripheral membrane, or plaque, protein containing multiple protein interaction domains that, along with the related protein zonula occludens 2, is required for tight junction assembly. Transcellular pathway The route of transepithelial transport that involves active or passive movement across cell membranes, usually as a result of the action of specific transport channels. Transepithelial transport The sum of transport through the transcellular and paracellular pathways. adherens junctions are supported by a dense perijunctional ring of actin and myosin that, as discussed below, can regulate barrier function. As implied by the name, the adherens junctions, along with desmosomes, provide the strong adhesive bonds that maintain cellular proximity and are also a site of intercellular communication. Loss of adherens junctions results in disruption of cell cell and cell matrix contacts, ineffective epithelial cell polarization and differentiation, and premature apoptosis 13. Adherens junctions are composed of cadherins, a family of transmembrane proteins that form strong, homotypic interactions with molecules on adjacent cells. The cytoplasmic tail of the epithelial cadherin, E-cadherin (also known as cadherin-1), interacts directly with catenin δ1 (also known as p120 catenin) and β-catenin. In turn, β-catenin binds to α-catenin 1, which regulates local actin assembly and contributes to development of the perijunctional actomyosin ring. Adherens junctions are required for assembly of the tight junction, which seals the paracellular space. Tight junctions are multi-protein complexes composed of transmembrane proteins, peripheral membrane (scaffolding) proteins and regulatory molecules that include kinases. The most important of the transmembrane proteins are members of the claudin family, which define several aspects of tight junction permeability, as discussed below. Claudins are expressed in a tissue-specific manner, and mutation or deletion of individual family members can have profound effects on organ function. The role of occludin, a transmembrane tight junction protein that interacts directly with claudins and actin, is less well understood. Peripheral membrane proteins, such as zonula occludens 1 (Zo1) and Zo2, are crucial to tight junction assembly and maintenance, partly owing to the fact that these proteins include multiple domains for interaction with other proteins, including claudins, occludin and actin. The tight junction limits solute flux along the paracellular pathway, which is typically more permeable than the transcellular pathway. The tight junction is, therefore, the rate-limiting step in transepithelial transport and the principal determinant of mucosal permeability. Thus, it is important to understand the specific barrier properties of the tight junction, which can be defined in terms of size selectivity and charge selectivity. At least two routes allow transport across the tight junction, and emerging data suggest that the relative contributions of these types of paracellular transport may be regulated independently 2,14,15. one route, the leak pathway, allows paracellular transport of large solutes, including limited flux of proteins and bacterial lipopolysaccharides 14,15. Although the size at which particles are excluded from the leak pathway has not been precisely defined, it is clear that materials as large as whole bacteria cannot pass. As might be expected for a route that allows large solutes to cross, the leak pathway does not show charge selectivity. Flux across the leak pathway may be increased by cytokines, including interferon-γ (IFnγ) in vitro and tumour necrosis factor (TnF) in vitro and in vivo A second pathway is characterized by small pores that are thought to be defined by tight junction-associated claudin proteins, which are also primary determinants of charge selectivity These pores have a radius that excludes molecules larger than 4 Å 14,15. Expression of specific claudins varies between organs and even within different regions of a single organ and, as detailed below, can be modified by external stimuli, such as cytokines. Thus, tight junctions show both size selectivity and charge selectivity, and these properties may be regulated individually or jointly by physiological or pathophysiological stimuli. Interdependence of transport routes Transepithelial transport requires a selectively permeable barrier. The vectorial nature of transcellular transport, which is required for effective absorption and secretion, generates a transepithelial concentration gradient. Transepithelial transport would, therefore, be ineffective without a tight junction barrier, as diffusion would allow equalization of concentrations on both sides of the epithelium. Thus, active transcellular transport depends on the presence of an intact tight junction barrier. The selective permeability of the tight junction barrier also allows transepithelial gradients to drive passive paracellular transport of ions and water 17,20. For example, claudin-16, which is necessary for tight junction cation selectivity, is mutated in the human disease familial hypomagnesaemia with hypercalciuria and nephro calcinosis 20. Paracellular absorption of Mg 2+ and Ca 2+ in the thick ascending limb of Henle requires a cation-selective tight junction barrier, the absence of which results in urinary loss of Mg 2+ and Ca 2+ (ReF. 20). Similarly, apical na + H + -exchange protein 3 (nhe3) is required for transcellular na + absorption in the renal tubule and intestine, and also contributes to the transepithelial na + gradient that drives paracellular water absorption (BOX 1). The tight junction barrier to paracellular na + efflux prevents dissipation of the gradient established by nhe3-mediated transcellular transport. Tight junction barrier regulation modifies absorption. In addition to providing the driving force for paracellular transport, transcellular transport can activate intracellular signalling events that regulate the tight junction barrier. The best studied physiological example of this is the increased intestinal paracellular permeability that is induced by apical na + nutrient co-transport 21. This allows passive paracellular absorption of nutrients and water to amplify transcellular nutrient absorption, particularly when high luminal nutrient concentrations exceed the capacity of apical na + nutrient co-transporters 22. ultrastructural analyses of intestinal epithelial cells during na + nutrient co-transport revealed condensation of the perijunctional actomyosin ring, suggesting that cytoskeletal contraction could be involved in this physiological process 21. Subsequent studies confirmed this hypothesis and showed that the Ca 2+ calmodulin-dependent serine threonine protein kinase myosin light chain kinase (MLCK) is essential for na + nutrient co-transport-induced tight junction regulation 23,24. Moreover, MLCK activation alone is sufficient to increase tight junction perme ability, both in vitro and in vivo 25,26. The mechanisms that mediate this nature REvIEwS Immunology volume 9 november Macmillan Publishers Limited. All rights reserved
19 REVIEWS Box 1 Coordination of transcellular and paracellular transport The signal transduction pathway that enhances paracellular permeability following initiation of Na + glucose co-transport has been characterized and includes activation of mitogen-activated protein kinase cascades, trafficking of Na + H + -exchange protein 3 (NHE3) to the apical membrane and myosin light chain kinase (MLCK) activation 23 24,107. Increased NHE3 activity at the apical membrane enhances Na + absorption, which (along with the Na + absorbed as a result of Na + glucose a Normal homeostasis b Barrier disruption only co-transport) increases the transcellular Na + gradient and promotes paracellular water absorption (see the figure, part a). The MLCK-dependent increase in tight junction permeability enhances paracellular absorption of water and small solutes, such as glucose, that are concentrated in the unstirred layer. This might contribute to the observation that rehydration after infectious diarrhoea, or even exercise, is more effective when oral rehydration solutions contain Na + and carbohydrates 108 (see the figure, part b). By contrast, tumour necrosis factor activates both protein kinase Cα (PKCα) and MLCK to cause diarrhoea. NHE3 inhibition, mediated by PKCα, reduces the transcellular Na + gradient that normally drives water absorption (see the figure, part c). This synergizes with MLCKdependent increases in tight junction permeability to allow water to flow into the lumen, thereby causing large-volume diarrhoea 17 (see the figure, part d). No diarrhoea Na + c Na + malabsorption only Mild diarrhoea Na + H 2 O Na + H 2 O Na + No diarrhoea d Na + malabsorption and barrier disruption Copious diarrhoea H 2 O Na + Na + H 2 O Na + H 2 O Na + Thick ascending limb of Henle The portion of the nephron just proximal to the distal tubule. This is a site of active Na +, K + and Cl reabsorption, which generates an electrochemical gradient that drives paracellular reabsorption of Mg 2+ and Ca 2+. Myosin light chain kinase The Ca 2+ calmodulindependent kinase that phosphorylates myosin II regulatory light chain at serine 19 and threonine 18 to activate myosin ATPase. MLCK-dependent tight junction regulation have been studied in detail owing to their contributions to nutrient and water absorption under normal physiological conditions and to the diarrhoea associated with acute barrier loss (BOX 1). Barrier regulation by immune stimuli The ability of cytokines, such as TnF and IFnγ, to regulate the function of the tight junction barrier was first described 20 years ago 27. Since then, increased tight junction protein transcription, vesicular removal of proteins from the tight junction, tight junction protein degradation, kinase activation and cytoskeletal modulation have all been proposed to mediate cytokine-induced loss of tight junction barrier function. Although extensive apoptosis of epithelial cells may also cause barrier loss, the relevance of single-cell apoptosis to barrier dysfunction remains controversial owing to differing results in diverse experimental systems. Tight junction regulation through the cytoskeleton. TnF and IFnγ modify tight junction barrier function in intestinal 27,28, renal 29, pulmonary 30 and salivary gland 31 epithelia as well as between endothelial cells 32. The effects of TnF on barrier integrity have been best studied in the gut, where this cytokine has a central role in many diseases associated with intestinal epith elial barrier dysfunction, including inflammatory bowel disease 33, intestinal ischaemia 28,34 and graft-versushost disease 35. For example, although the effect of therapy with TnF-specific antibodies may be largely due to the overall reduction in inflammation, it is notable that this treatment corrects barrier dysfunction in patients with Crohn s disease 36. Increased mucosal TnF production may also contribute to increased intestinal permeability and susceptibility to colitis in mice with defective mucin biosynthesis 10,37. MLCK has been shown to have a central role in TnFinduced epithelial and endothelial barrier dysregulation, both in vitro and in vivo 16, Similar to na + nutrient co-transport, TnF-induced MLCK activation seems to increase paracellular flux through the leak pathway 16 17,23. This MLCK activation occurs as a result of increased enzymatic activity and increased MLCK transcription and translation, both in vitro and in vivo 16,40,42. Similarly, MLCK expression and activity are increased in intestinal epithelial cells of patients with inflammatory bowel disease 43. The degree to which MLCK expression and activity are increased correlates with local disease activity, suggesting that these processes may be regulated by local cytokine signalling in these patients 43. MLCK is also a fundamental intermediate in barrier dysfunction induced by the TnF family member LIGHT (also known as TnFSF14) 17,44, interleukin-1β (IL-1β) 45, enteropathogenic Escherichia coli infection 39, Helicobacter pylori infection 46, giardiasis 47, lipopolysaccharide 48,49 and the ethanol metabolite acetaldehyde 50. Thus, MLCK activation can be viewed as a common final pathway of acute tight junction regulation in response to a broad range of immune and infectious stimuli. 802 november 2009 volume Macmillan Publishers Limited. All rights reserved
20 REVIEWS a c Figure 2 Differential effects of cytokines on tight junction structure and function. a Occludin is normally concentrated at the tight junction (arrow) in jejunal villus epithelium. The perijunctional actomyosin ring (red) and nuclei (blue) are shown for reference. b Exogenous tumour necrosis factor increases myosin light chain kinase activity, which causes perijunctional myosin II regulatory light chain phosphorylation and triggers occludin (green) endocytosis (arrow). This increases flux across the tight junction leak pathway and enhances paracellular permeability to large solutes. c Claudin 2 expression (green) is limited to crypt epithelial cells; it is not expressed by epithelial cells at the mucosal surface in the normal colon. F actin (red) and nuclei (blue) are shown for reference. d Interleukin 13 can stimulate claudin 2 expression (green) in surface epithelial cells (arrows). This increases flux across small tight junction pores, thereby enhancing paracellular cation permeability. Caveolae Specialized flask-shaped invaginations of the plasma membrane that contain the protein caveolin-1 and cholesterol. These proteins mediate uptake of some extracellular materials and are involved in cell signalling. b d Although it is clear that MLCK phosphorylates myosin II regulatory light chain (MLC) within the perijunctional actomyosin ring to activate myosin ATPase activity, the subsequent molecular events that cause increased permeability are poorly defined. However, recent work suggests that Zo1, which interacts directly with actin, occludin, claudins and other proteins, may be an essential effector of perijunctional actomyosin ring-mediated tight junction regulation 25,51,52. Endocytic removal of the transmembrane protein occludin from the tight junction is also common in actomyosin-dependent, cytokine-mediated tight junction regulation 38,53 (FIG. 2). In vitro studies of tight junction regulation induced by LIGHT suggest that occludin endocytosis occurs via caveolae and that inhibition of this process can prevent loss of barrier integrity despite MLCK activation 44. Caveolar endocytosis of occludin has also been associated with loss of epithelial and endothelial tight junction barrier function in response to actin disruption 54 and chemokine signalling 55, respectively, in vitro. However, other mechanisms of occludin removal may also be involved as in vitro studies have shown that IFnγ-induced occludin internalization is mediated by myosin ATPase-dependent macropinocytosis 53,56, and occludin cleavage may even modify the barrier to enhance transepithelial migration of inflammatory cells in the lung 57. nevertheless, further work is necessary to define the contributions of occludin and endocytosis to in vivo tight junction regulation. This is particularly important as, despite numerous in vitro studies demonstrating a role for occludin in tight junction function, intestinal barrier function is intact in occludin-deficient mice 58. Future analyses of the response of occludin-deficient mice to stress, with particular reference to intestinal tight junction function, as well as studies of potential compensatory changes in other, perhaps not yet discovered, proteins that allow these mice to avoid intestinal disease will be of great interest. Although MLCK activation is clearly important, it is not the only means of cytoskeletal tight junction regulation. other mediators include myosin ATPase 51, the activity of which is regulated by MLC phosphorylation ; members of the Rho kinase family 41,51,53,61, which can both phosphorylate MLC directly 59 and inhibit MLC phosphatase 62 ; and AMP-activated protein kinase 52, which is activated during stress and can also directly phosphorylate MLC 63. Moreover, Rho kinases and AMP-activated protein kinase each have diverse effects that are separate from myosin function, and it is likely that at least some of these contribute to tight junction regulation. Tight junction permeability and claudin expression. As discussed above, regulation of perijunctional actomyosin provides a means of rapidly and reversibly regulating the paracellular leak pathway. By contrast, synthesis and trafficking of claudin proteins provides a means of regulating tight junction pores over longer periods. The perijunctional actomyosin ring does not seem to be directly involved in this mechanism of barrier regulation, which is consistent with the fact that claudins do not interact with actin directly. Expression of specific claudin proteins changes during development, differentiation and disease and in response to stressors including cytokines in intestinal 64, renal 65 and alveolar 66 epithelial cells. In addition to affecting barrier function, altered patterns of claudin expression may have other consequences. For example, claudin proteins have been associated with control of cell and organ growth. It may be, therefore, that some changes in claudin protein expression enhance cell proliferation and regeneration, as might be necessary to compensate for cell loss in colitis. This may explain the increased claudin-1 expression by intestinal epithelial cells of patients with inflammatory bowel disease 67. However, claudin-1 has also been shown to enhance neoplastic transformation, tumour growth and metastasis in experimental models 68. Thus, changes in claudin expression may have undesired consequences. one common change in claudin expression that directly affects barrier function is the increased claudin-2 expression by intestinal epithelial cells in animal models of colitis (FIG. 2) and patients with inflammatory bowel disease 64. Consistent with this, IL-13 and IL-17, which are increased in the mucosa of patients with colitis 64,69, reduce barrier function and increase claudin-2 expression in cultured intestinal epithelium monolayers 64,70. In vitro studies have shown that claudin-2 expression increases the number of pores that allow paracellular flux of cations, predominantly na +, and small molecules with radii less than 4 Å 14,71, and recent analyses have provided new insight into the structure of these pores 72. However, it is not clear whether increased claudin-2 expression contributes to disease progression or, as proposed above for claudin-1, is an adaptive nature REvIEwS Immunology volume 9 november Macmillan Publishers Limited. All rights reserved
21 REVIEWS Table 1 Barrier defects associated with intestinal disease Disease or model Crohn s disease Cause of barrier defect Unknown, associated with a frameshift insertion at nucleotide 3020 of NOD2 Timing of barrier defect Before clinical onset and before clinical relapse Effects on tight junction proteins and cytoskeleton Claudin 2 upregulation, MLCK activation and occludin downregulation Ulcerative colitis Unknown Not well studied Claudin 2 upregulation, MLCK activation and occludin downregulation IL 10 deficient mice Immune signalling (IL 10 deficiency) Role of commensal microorganisms Antibiotics can be helpful in maintaining remission Refs 43,64,109 Not defined 43,64,109 Before clinical onset Not defined Eradication of microorganisms prevents disease Coeliac disease Not well studied Not well studied Not defined No demonstrated role 110,111 Systemic T cell activation CD4 + CD45RB hi T cell adoptive transfer model of colitis MLCK activation Cytokine release and epithelial cell damage Associated with acute diarrhoea With clinical onset MLCK activation and occludin endocytosis MLCK activation, claudin 2 upregulation and occludin endocytosis SAMP1/yit mice Unknown Before clinical onset Claudin 2 upregulation and occludin downregulation DSS induced colitis Epithelial cell damage After DSS treatment but before clinical onset Not defined 76,97 Not defined 38 Eradication of microorganisms reduces severity Not defined 77 Eradication of microorganisms exacerbates disease TNBS induced colitis Immune signalling Not well studied Claudin 18 upregulation Probiotics can reduce disease severity Mucin 2 deficient mice MDR1A deficent mice JAM A deficient mice Clostridium difficile induced colitis EPEC infection Graft versus host disease Not well studied Not well studied Not defined Not defined 10,37 Unknown, follows increased CCL2 production After immune cell activation Reduced occludin phosphorylation Increased epithelial cell response to LPS precedes disease ,116 JAM A deficiency Not well studied JAM A deficiency Not defined 89,90 Actomyosin disruption and glucosylation of Rho proteins Type III secretion (of bacterial proteins) Associated with elevated TNF production With release of toxin and disease onset After infection Loss of ZO1 and ZO2 MLCK activation and occludin endocytosis Antibiotics predispose to disease 117 Not defined 118 After clinical onset Not defined Eradication of microorganisms limits disease CCL2, CC chemokine ligand 2; DSS, dextran sulphate sodium; EPEC, enteropathogenic Escherichia coli; IL 10, interleukin 10; JAM A, junctional adhesion molecule A; LPS, lipopolysaccharide; MDR1A, multidrug resistance protein 1a; MLCK, myosin light chain kinase; NOD, nucleotide binding oligomerization domain; TNF, tumour necrosis factor; TNBS, trinitrobenzene sulphonic acid; ZO, zonula occludens. 35 SAMP1/yit mice An outbred mouse strain that spontaneously develops a chronic intestinal inflammation similar to human Crohn s disease. response that promotes homeostasis. nevertheless, the consistency of intestinal epithelial claudin-2 upregulation in disease suggests that this should be a subject of further study. Barrier function and immunity Barrier loss is associated with disease risk. A large body of circumstantial evidence suggests that intestinal barrier dysfunction is associated with the pathogenesis of Crohn s disease. This includes the observation that a subset of first-degree relatives of patients with Crohn s disease has increased intestinal permeability despite being completely healthy 73. Interestingly, genetic analyses have linked increased intestinal permeability in these healthy relatives to the Crohn s disease-associated frameshift insertion at nucleotide 3020 of the gene encoding the cytoplasmic sensor nucleotide-binding oligomerization domain-containing 2 (NOD2) 74 (TABLe 1), and the same NOD2 mutation has been associated with decreased IL-10 production by peripheral blood mononuclear cells 75. Thus, one explanation for the increased intestinal permeability observed in some of these healthy relatives is that they have subclinical mucosal immune activation, perhaps with increased TnF production, that leads to barrier dysfunction without overt disease. This hypothesis would also explain why IL-10-deficient and SAMP1/yit mice, which develop spontaneous colitis and enteritis, respectively, show increased intestinal permeability before disease onset 76,77. The suggestion that permeability is merely a sensitive indicator of mucosal 804 november 2009 volume Macmillan Publishers Limited. All rights reserved
22 REVIEWS CD4 + CD45RB hi T cell adoptive transfer colitis model A well-characterized model of chronic colitis induced by transfer of CD4 + CD45RB hi (naive) T cells from healthy wild-type mice into immunodeficient syngeneic recipients. immune activation would also explain the observation that, during clinical remission, increased intestinal permeability is a predictor of relapse in patients with Crohn s disease 78. However, barrier dysfunction must also be regarded as a potential contributor to disease progression. The classic experiment showing a role for epithelial cell function in maintaining mucosal immune homeostasis used chimeric mice in which dominant-negative cadherin was expressed in some intestinal epithelial stem cells, resulting in disruption of the adherens junctions 13. This led to profound epithelial cell defects that included incomplete polarization, brush border and actin cytoskeletal disruption, accelerated crypt villus migration and premature apoptosis 13. Moreover, the mucus layer overlying epithelial cells expressing dominant-negative cadherin was disrupted and numerous adherent bacteria were present at these sites 13. Patchy, transmural enteritis developed in these mice and was limited to regions where epithelial cells expressed dominant-negative cadherin 79. In addition, epithelial cell dysplasia was occasionally present in these areas 79. This study has often been cited as evidence that tight junction barrier loss is sufficient to cause inflammatory bowel disease and, although tight junctions were not specifically examined, they were likely to be defective. However, although this important study shows that apical junctional complex disruption causes enteritis, the broad range of epithelial cell defects induced makes it impossible to draw conclusions regarding the specific contributions of the tight junction to homeostasis or disease. Similarly, although dextran-sulphate sodium (DSS)-induced colitis is associated with increased intestinal permeability, this is the result of widespread epithelial cell damage 80 rather than targeted dysfunction of the tight junction barrier. Altered sensitivity of genetically modified mice to DSS must therefore be viewed in the context of epithelial cell injury and repair and cannot be interpreted as a function of disrupted tight junction permeability alone. Isolated barrier loss is insufficient to cause disease. one study has reported an in vivo model of barrier dysfunction induced by direct activation of endogenous tight junction regulatory mechanisms 26. Although no overt developmental defects or disease developed in mice in which intestinal epithelial cells transgenically expressed constitutively active MLCK, increased intestinal tight junction permeability was observed 26. This increase in paracellular permeability was quantitatively similar to that induced by activation of na + glucose co-transport and was not an indiscriminate loss of barrier function 26, as occurs with exposure to DSS or dominant-negative cadherin expression. In addition, MLCK inhibition normalized MLC phosphorylation in epithelial cells and paracellular permeability 26, suggesting that compensatory changes in pathways that regulate MLC phosphorylation or barrier function were not induced. Moreover, growth of these mice was normal, and intestinal morphology, enterocyte structure, tight junction and adherens junction organization, actomyosin and brush border architecture, and epithelial proliferation, migration and apoptosis were all similar to that observed in wild-type mice 26. This suggests that the effects of constitutively active MLCK expression in these mice were specific to its effect on the tight junction. Thus, consistent with the presence of barrier dysfunction in healthy relatives of patients with Crohn s disease, these mice provide evidence to suggest that increased tight junction permeability in the absence of more extensive epithelial cell dysfunction is insufficient to cause intestinal disease. Further analysis of mice expressing constitutively active MLCK did, however, provide evidence of mucosal immune cell activation, including increased numbers of lamina propria T cells, enhanced mucosal IFnγ, TnF and IL-10 transcription and repositioning of CD11c + DCs to the superficial lamina propria 26. Thus, despite being insufficient to cause disease, chronic increases in intestinal permeability as a result of continuous activation of a physiological pathway of tight junction regulation does lead to mucosal immune cell activation. Because a subset of healthy first-degree relatives of patients with Crohn s disease will ultimately develop the disease, and because a similar fraction of these healthy relatives have increased intestinal permeability, it has been suggested that tight junction barrier dysfunction is one factor that contributes to the development of inflammatory bowel disease 84. This hypothesis is consistent with a case report documenting increased intestinal permeability 8 years prior to disease onset in a healthy relative of a patient with Crohn s disease 85. However, given that the subject of the case report had two firstdegree relatives with Crohn s disease and was, therefore, at increased risk of developing the disease regardless of intestinal permeability measures, this report does not provide evidence that barrier dysfunction itself is related to disease onset. The relationship between barrier dysfunction and disease has been assessed using mice with a constitutively active MLCK transgene, which have increases in permeability that are quantitatively and qualitatively similar to those in healthy relatives of patients with Crohn s disease. Recombination-activating gene 1-knockout mice (which lack mature B and T cells) expressing constitutively active MLCK, as well as littermates that did not express constitutively active MLCK, received CD4 + CD45RB hi naive T cells from wild-type mice (the CD4 + CD45RB hi T cell adoptive transfer colitis model). Both groups of mice developed colitis. However, the disease developed more rapidly and was more severe, in clinical, biochemical and histological terms, in the mice that expressed constitutively active MLCK 26. Although one could argue that this accelerated disease progression was due to effects of constitutively active MLCK expression beyond tight junction regulation, other epithelial cell defects were not apparent in these mice (see above), perhaps because constitutively active MLCK targets an endogenous signalling pathway. Thus, targeted increases in intestinal epithelial tight junction permeability through constitutive activation of the pathophysiologically relevant MLCK pathway are sufficient to accelerate the onset and enhance the severity of immune-mediated colitis. nature REvIEwS Immunology volume 9 november Macmillan Publishers Limited. All rights reserved
23 REVIEWS Latency-associated peptide A small peptide derived from the N-terminal region of the TGFβ precursor protein; it can modulate TGFβ signalling. Conversely, the data above also suggest that inhibition of MLCK, which is sufficient to reverse acute TnFinduced barrier loss 38, may have therapeutic benefit in immune-mediated colitis. This notion is supported by a preliminary in vivo report using mice with a knockout of non-muscle MLCK 86. However, as non-muscle MLCK has many functions, including an essential role in neutrophil transendothelial migration 87, the results of this report may reflect changes beyond tight junction barrier preservation. Another recent study showed that a peptide capable of enhancing small-intestinal barrier function reduced the extent of mucosal inflammation in IL-10-deficient mice 88. However, this result must be interpreted with caution, as the peptide used (which is thought to antagonize the putative extracellular pathway signalling molecule zonulin) activates an undefined regulatory pathway and the overall physiology of peptide-treated mice has not been examined in detail. Thus, although the finding is intriguing, further investigation is necessary to determine whether barrier preservation, by MLCK inhibition or other means, can prevent or reverse intestinal disease. Two studies have recently reported that junctional adhesion molecule-a (JAM-A)-deficient mice have reduced intestinal barrier function and increased rates of intestinal epithelial cell proliferation and apoptosis 89,90. These mice also have intestinal mucosal immune cell activation as defined by increased numbers of neutrophils in the intestinal mucosa 89,90. In addition, one study reported an increase in the number of distal-colonic lymphoid aggregates in JAM-A-deficient mice 90, although neither lymphoid aggregates nor enhanced mucosal cytokine expression was detected in a subsequent analysis 89. However, as JAM-A is widely expressed by epithelial cells, endothelial cells, platelets, antigen-presenting cells, circulating neutrophils, monocytes, lymphocytes and platelets, and as the mice studied were not epithelial cell-specific knockouts, it is not clear whether the observed effects were due to altered tight junction integrity, changes in epithelial cell shape 91, an increased rate of epithelial cell apoptosis 90 or trapping of neutrophils within mucosal vessels owing to the loss of endothelial cell-expressed JAM-A. nevertheless, it is intriguing that JAM-A-deficient mice showed increased sensitivity to DSS-induced colitis 89,90. By contrast, the sensitivity of mice with an endothelium-specific JAM-A deficiency to DSS was similar to that of wild-type control mice, indicating that the response of mice with universal JAM-A deficiency was not solely due to loss of endothelial JAM-A 89. unfortunately, the use of mice with a universal JAM-A deficiency and the presence of other epithelial cell abnormalities, particularly increased epithelial cell turnover, in the absence of exogenous stimuli limits interpretation of these studies. Even so, data from JAM-A-deficient mice do support the hypothesis that mucosal barrier loss can enhance the severity of colitis. overall, data from both human subjects and mouse experimental models show that defects in tight junction barrier function are insufficient to cause disease. However, several lines of evidence suggest that increased paracellular permeability can increase mucosal immune activity, enhance disease progression and severity and, possibly, be a risk factor for development of disease. Finally, although much more investigation is needed, early reports indicate that restoration of tight junction barrier function may be effective, either alone or in combination with other agents, in preventing disease in at-risk individuals or maintaining remission in patients with inflammatory bowel disease. Barrier loss activates immunoregulatory processes. why is tight junction barrier dysfunction alone insufficient to cause disease? Endoscopic mucosal resection, which removes the epithelium and mucosa completely and therefore causes barrier loss far greater than that caused by targeted tight junction dysfunction, is insufficient to cause chronic disease. Therefore, immuno regulatory mechanisms must be induced by the host following barrier loss to prevent inappropriate inflammatory responses, as mucosal damage is a daily occurrence in the gastrointestinal tract. Moreover, these immunoregulatory mechanisms must be robust because, even in patients with inflammatory bowel disease, endoscopic mucosal resection is insufficient to initiate a relapse to active disease. The increased transcription of IL-10 in the intestinal mucosa of mice with a constitutively active MLCK transgene may provide a clue to which immuno regulatory processes are triggered by barrier dysfunction. In addition, responses to transient barrier loss have been used to explore mucosal immunoregulation 92. In this model, intrarectal administration of ethanol caused transient epithelial cell damage, mucosal erosion and barrier loss. The induction of barrier loss was followed by an increase in the numbers of IFnγ- and IL-10-producing lamina propria mononuclear cells and lamina propria CD4 + CD25 + T cells that express latency-associated peptide (LAP) on their surface 92. Remarkably, such ethanol administration conferred protection from subsequent trinitrobenzene sulphonic acid (TnBS)-induced colitis, and this protection required the presence of LAP + T cells 92. The induction of these LAP + T cells was shown to depend on CD11c + DCs, Toll-like receptor 2 signalling and a normal luminal microorganism population 92. Although further study is needed to understand the mechanisms of LAP + T cell induction by transient mucosal damage, it is interesting that in different experimental systems increased intestinal epithelial cell tight junction permeability increased the number of CD11c + DCs in the superficial lamina propria 86, and that interactions with intestinal epithelial cells enhance the ability of bone marrow-derived CD11c + DCs to induce the differentiation of regulatory T cells 93. Together, these observations support the hypothesis that the interactions between CD11c + DCs and luminal materials are regulated by tight junction permeability and are also central to mucosal immune homeostasis. other data also support important roles for luminal material, particularly microorganisms and their products, in mucosal immune regulation 94. For example, antibiotics can be helpful in the management of Crohn s disease 95. Although the mechanisms by which antibiotics 806 november 2009 volume Macmillan Publishers Limited. All rights reserved
24 REVIEWS Epithelial cell Lamina propria LAP Claudin Occludin TGFβ Retinoic acid T Reg cell Claudin-2 IL-13 T H 2 cell Na + Small solutes Na + Small solutes TLR IL-10 and TGFβ Bacterial products and dietary antigens Antigen APC T cell MHC TCR Actomyosin ring MLCK IFNγ TNF T H 1 cell Endosome Figure 3 The epithelium and tight junction as integrators of mucosal homeostasis. Minor barrier defects allow bacterial products and dietary antigens to cross the epithelium and enter the lamina propria. This can lead to disease or homeostasis. If the foreign materials are taken up by antigen presenting cells (APCs), such as dendritic cells, that direct the differentiation of T helper 1 (T H 1) or T H 2 cells, disease can develop. In this process, APCs and T H 1 cells can release tumour necrosis factor (TNF) and interferon γ (IFNγ), which signal to epithelial cells to increase flux across the tight junction leak pathway, thereby allowing further leakage of bacterial products and dietary antigens from the lumen into the lamina propria and amplifying the cycle of inflammation. This may, ultimately, culminate in established disease. Alternatively, interleukin 13 (IL 13) released by T H 2 cells increases flux across small cation selective pores, potentially contributing to ongoing disease. Conversely, homeostasis may dominate if APCs promote regulatory T (T Reg ) cell differentiation, which can be enhanced by epithelial cell derived transforming growth factor β (TGFβ) and retinoic acid. The T Reg cells display latency associated peptide (LAP) on their surfaces and may secrete IL 10 and TGFβ to prevent disease. MLCK, myosin light chain kinase; TLR, Toll like receptor; TCR, T cell receptor. promote maintenance of remission in patients with Crohn s disease are not defined, they may be related to the observation that luminal microorganisms are required in experimental models of inflammatory bowel disease that include IL-10-deficient mice 96,97 and adoptive transfer colitis. Microorganisms are also necessary for the development of increased intestinal permeability before the onset of overt disease in IL-10-deficient mice 76. Although this observation is not entirely understood, one interpretation is that the limited flux of microbial products that normally occurs across intact intestinal epithelial tight junctions is sufficient to trigger mucosal immune activation in IL-10-deficient mice. This mucosal immune activation could, in turn, result in the release of cytokines that cause increased intestinal permeability and accelerate disease progression (FIG. 3). However, in addition to immune cells, epithelial cells can respond to foreign materials. Lipopolysaccharide, for example, enhances paracellular permeability in pulmonary and intestinal epithelium 48,49. Conversely, epithelial Toll-like receptor 2 activation may restore barrier function 98. It will therefore be of interest to define the microbial products, dietary components and other factors that influence mucosal immune status following alterations in intestinal epithelial tight junction permeability. Tight junctions integrate mucosal homeostasis one interpretation of the available data is that the tight junction barrier integrates the relationship between luminal material and mucosal immune function (FIG. 3). In most individuals, this is a healthy relationship in which regulated increases in tight junction permeability or transient epithelial cell damage trigger the release of pro-inflammatory cytokines, such as TnF and IFnγ, as well as immunoregulatory responses. Immunoregulatory responses may include DC conditioning by epithelial cell-derived transforming growth factor-β (TGFβ) and retinoic acid, both of which can enhance regulatory T cell differentiation 99,100. This precarious balance between proinflammatory and immunoregulatory responses can fail if there are exaggerated responses to pro-inflammatory cytokines, as may be associated with mutations in the endoplasmic reticulum stress response transcription factor X-box-binding protein 1 (XBP1) (ReF. 101), insufficient IL-10 production (as in IL-10-deficient mice and, possibly, in patients with IL10 promoter polymorphisms 102 ) or inadequate immune tolerance to luminal antigens and microbial products 94 (potentially because of NOD2 mutations in patients with inflammatory bowel disease ). As a result, mucosal immune cell activation may proceed unchecked and the release of cytokines, including TnF and IL-13, may enhance barrier loss that, in turn, allows further leakage of luminal material and perpetuates the pro-inflammatory cycle. This model highlights the roles of a susceptible host and defective epithelial cell barrier function as key components of the pathogenesis of intestinal inflammatory disease and explains the crucial role of the epithelial barrier in moulding mucosal immune responses. Conclusions Significant progress has been made in understanding the processes by which physiological and pathophysiological stimuli, including cytokines, regulate the tight junction. Early reports suggest that restoration of tight junction barrier function may have benefit 38,86. However, the mechanisms of tight junction regulation will have to be defined in greater detail if they are to be viable pharmacological targets. Conversely, recent data have emphasized the presence of immune mechanisms that maintain mucosal homeostasis despite barrier dysfunction, and some data suggest that the epithelium orchestrates these immunoregulatory events through direct interactions with innate immune cells. Future elucidation of the processes that integrate mucosal barrier function, or dysfunction, and immune regulation to prevent or perpetuate disease may lead to novel therapeutic approaches for diseases associated with increased mucosal permeability. nature REvIEwS Immunology volume 9 november Macmillan Publishers Limited. All rights reserved
25 REVIEWS 1. Kiuchi-Saishin, Y. et al. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J. Am. Soc. Nephrol. 13, (2002). 2. Fihn, B. M., Sjoqvist, A. & Jodal, M. Permeability of the rat small intestinal epithelium along the villus crypt axis: effects of glucose transport. Gastroenterology 119, (2000). 3. Chieppa, M., Rescigno, M., Huang, A. Y. & Germain, R. N. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 203, (2006). 4. Niess, J. H. et al. CX 3 CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, (2005). 5. Neutra, M. R. & Kozlowski, P. A. Mucosal vaccines: the promise and the challenge. Nature Rev. Immunol. 6, (2006). 6. Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nature Rev. Immunol. 8, (2008). 7. Coombes, J. L. & Powrie, F. Dendritic cells in intestinal immune regulation. Nature Rev. Immunol. 8, (2008). 8. Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, (2008). 9. Pilewski, J. M. & Frizzell, R. A. Role of CFTR in airway disease. Physiol. Rev. 79, S215 S255 (1999). 10. Heazlewood, C. K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54 (2008). 11. Baumgartner, H. K. & Montrose, M. H. Regulated alkali secretion acts in tandem with unstirred layers to regulate mouse gastric surface ph. Gastroenterology 126, (2004). 12. Strocchi, A. et al. Measurements of the jejunal unstirred layer in normal subjects and patients with celiac disease. Am. J. Physiol. 270, G487 G491 (1996). 13. Hermiston, M. L. & Gordon, J. I. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J. Cell Biol. 129, (1995). 14. Van Itallie, C. M. et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J. Cell Sci. 121, (2008). This detailed analysis of tight junction size selectivity is the first study to show a role for claudins in the paracellular flux of uncharged solutes. 15. Watson, C. J., Hoare, C. J., Garrod, D. R., Carlson, G. L. & Warhurst, G. Interferon-γ selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J. Cell Sci. 118, (2005). 16. Wang, F. et al. Interferon-γ and tumour necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by upregulating myosin light chain kinase expression. Am. J. Pathol. 166, (2005). 17. Clayburgh, D. R., Musch, M. W., Leitges, M., Fu, Y. X. & Turner, J. R. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J. Clin. Invest. 116, (2006). 18. Amasheh, S. et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 115, (2002). 19. Colegio, O. R., Van Itallie, C., Rahner, C. & Anderson, J. M. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol. Cell Physiol. 284, C1346 C1354 (2003). 20. Simon, D. B. et al. Paracellin-1, a renal tight junction protein required for paracellular Mg 2+ resorption. Science 285, (1999). This is the first description of a human disease caused by tight junction defects. 21. Madara, J. L. & Pappenheimer, J. R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J. Membr. Biol. 100, (1987). 22. Meddings, J. B. & Westergaard, H. Intestinal glucose transport using perfused rat jejunum in vivo: model analysis and derivation of corrected kinetic constants. Clin. Sci. (Lond.) 76, (1989). 23. Turner, J. R. et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 273, C1378 C1385 (1997). This is the first report of MLCK-dependent physiological regulation of tight junction permeability. 24. Berglund, J. J., Riegler, M., Zolotarevsky, Y., Wenzl, E. & Turner, J. R. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na + glucose cotransport. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G1487 G1493 (2001). 25. Shen, L. et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 119, (2006). 26. Su, L. et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136, (2009). This is the first in vivo analysis of physiologically relevant barrier dysfunction and consequent immunoregulation. 27. Madara, J. L. & Stafford, J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Invest. 83, (1989). 28. Taylor, C. T., Dzus, A. L. & Colgan, S. P. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumour necrosis factorα. Gastroenterology 114, (1998). 29. Mullin, J. M., Laughlin, K. V., Marano, C. W., Russo, L. M. & Soler, A. P. Modulation of tumour necrosis factor-induced increase in renal (LLC-PK1) transepithelial permeability. Am. J. Physiol. 263, F915 F924 (1992). 30. Mazzon, E. & Cuzzocrea, S. Role of TNF-α in lung tight junction alteration in mouse model of acute lung inflammation. Respir. Res. 8, 75 (2007). 31. Baker, O. J. et al. Proinflammatory cytokines tumour necrosis factor-α and interferon-γ alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am. J. Physiol. Cell Physiol. 295, C1191 C1201 (2008). 32. Tiruppathi, C., Naqvi, T., Sandoval, R., Mehta, D. & Malik, A. B. Synergistic effects of tumour necrosis factor-α and thrombin in increasing endothelial permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L958 L968 (2001). 33. Baert, F. J. et al. Tumour necrosis factor α antibody (infliximab) therapy profoundly downregulates the inflammation in Crohn s ileocolitis. Gastroenterology 116, (1999). 34. Tamion, F. et al. Gut ischemia and mesenteric synthesis of inflammatory cytokines after hemorrhagic or endotoxic shock. Am. J. Physiol. 273, G314 G321 (1997). 35. Brown, G. R. et al. Tumour necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology 116, (1999). 36. Suenaert, P. et al. Anti-tumour necrosis factor treatment restores the gut barrier in Crohn s disease. Am. J. Gastroenterol. 97, (2002). 37. Van der Sluis, M. et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, (2006). 38. Clayburgh, D. R. et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J. Clin. Invest. 115, (2005). This is the first in vivo demonstration of the role of MLCK in TNF-induced tight junction regulation. 39. Zolotarevsky, Y. et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123, (2002). This is the first report of the role of MLCK in TNF-induced tight junction regulation. 40. Ma, T. Y., Boivin, M. A., Ye, D., Pedram, A. & Said, H. M. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin lightchain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G422 G430 (2005). 41. McKenzie, J. A. & Ridley, A. J. Roles of Rho/ROCK and MLCK in TNF-α-induced changes in endothelial morphology and permeability. J. Cell. Physiol. 213, (2007). 42. Graham, W. V. et al. Tumour necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signalling events. Characterization of the human long myosin light chain kinase promoter. J. Biol. Chem. 281, (2006). 43. Blair, S. A., Kane, S. V., Clayburgh, D. R. & Turner, J. R. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab. Invest. 86, (2006). 44. Schwarz, B. T. et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology 132, (2007). 45. Al-Sadi, R., Ye, D., Dokladny, K. & Ma, T. Y. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 180, (2008). 46. Wroblewski, L. E. et al. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 136, (2009). 47. Scott, K. G., Meddings, J. B., Kirk, D. R., Lees-Miller, S. P. & Buret, A. G. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology 123, (2002). 48. Moriez, R. et al. Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am. J. Pathol. 167, (2005). 49. Eutamene, H. et al. LPS-induced lung inflammation is linked to increased epithelial permeability: role of MLCK. Eur. Respir. J. 25, (2005). 50. Ferrier, L. et al. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am. J. Pathol. 168, (2006). 51. Van Itallie, C. M., Fanning, A. S., Bridges, A. & Anderson, J. M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 20, (2009). 52. Scharl, M., Paul, G., Barrett, K. E. & McCole, D. F. Adenosine monophosphate activated protein kinase mediates the interferon-γ-induced decrease in intestinal epithelial barrier function. J. Biol. Chem. 284, (2009). 53. Utech, M. et al. Mechanism of IFN-γ-induced endocytosis of tight junction proteins: Myosin IIdependent vacuolarization of the apical plasma membrane. Mol. Biol. Cell 16, (2005). 54. Shen, L. & Turner, J. R. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol. Biol. Cell 16, (2005). 55. Stamatovic, S. M., Keep, R. F., Wang, M. M., Jankovic, I. & Andjelkovic, A. V. Caveolae-mediated internalization of occludin and claudin-5 during CCL2- induced tight junction remodeling in brain endothelial cells. J. Biol. Chem. 284, (2009). 56. Bruewer, M. et al. Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 19, (2005). 57. Chun, J. & Prince, A. TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe 5, (2009). 58. Saitou, M. et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11, (2000). 59. Amano, M. et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, (1996). 60. Higashihara, M., Takahata, K. & Kurokawa, K. Effect of phosphorylation of myosin light chain by myosin light chain kinase and protein kinase C on conformational change and ATPase activities of human platelet myosin. Blood 78, (1991). 61. Jou, T. S., Schneeberger, E. E. & James Nelson, W. Structural and functional regulation of tight junctions by rhoa and rac1 small GTPases. J. Cell Biol. 142, (1998). 62. Kimura, K. et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, (1996). 63. Lee, J. H. et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447, (2007). 64. Heller, F. et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129, (2005). Here, claudin-2 upregulation in inflammatory bowel disease is shown for the first time. 65. Balkovetz, D. F., Chumley, P. & Amlal, H. Downregulation of claudin-2 expression in renal epithelial cells by metabolic acidosis. Am. J. Physiol. Renal Physiol. 297, F604 F611 (2009). 808 november 2009 volume Macmillan Publishers Limited. All rights reserved
26 REVIEWS 66. Wray, C. et al. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L219 L227 (2009). 67. Weber, C. R., Nalle, S. C., Tretiakova, M., Rubin, D. T. & Turner, J. R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Invest. 88, (2008). 68. Dhawan, P. et al. Claudin-1 regulates cellular transformation and metastatic behaviour in colon cancer. J. Clin. Invest. 115, (2005). 69. Fujino, S. et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52, (2003). 70. Kinugasa, T., Sakaguchi, T., Gu, X. & Reinecker, H. C. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 118, (2000). 71. Yu, A. S. et al. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J. Gen. Physiol. 133, (2009). 72. Angelow, S. & Yu, A. S. Structure-function studies of claudin extracellular domains by cysteine-scanning mutagenesis. J. Biol. Chem. 284, (2009). 73. Hollander, D. et al. Increased intestinal permeability in patients with Crohn s disease and their relatives. A possible etiologic factor. Ann. Intern. Med. 105, (1986). This is the first demonstration of increased intestinal permeability in healthy relatives of patients with Crohn s disease. 74. Buhner, S. et al. Genetic basis for increased intestinal permeability in families with Crohn s disease: role of CARD insC mutation? Gut 55, (2006). 75. Noguchi, E., Homma, Y., Kang, X., Netea, M. G. & Ma, X. A Crohn s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnrnp-a1. Nature Immunol. 10, (2009). 76. Madsen, K. L. et al. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm. Bowel Dis. 5, (1999). 77. Olson, T. S. et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J. Exp. Med. 203, (2006). 78. Wyatt, J., Vogelsang, H., Hubl, W., Waldhoer, T. & Lochs, H. Intestinal permeability and the prediction of relapse in Crohn s disease. Lancet 341, (1993). 79. Hermiston, M. L. & Gordon, J. I. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 270, (1995). The first demonstration that genetic insults in epithelial cells can cause experimental inflammatory bowel disease. 80. Yan, Y. et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One 4, e6073 (2009). 81. Williams, K. L. et al. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology 120, (2001). 82. Brown, S. L. et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J. Clin. Invest. 117, (2007). 83. Fukata, M. et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signalling: Role in proliferation and apoptosis in the intestine. Gastroenterology 131, (2006). 84. Hollander, D. Crohn s disease a permeability disorder of the tight junction? Gut 29, (1988). 85. Irvine, E. J. & Marshall, J. K. Increased intestinal permeability precedes the onset of Crohn s disease in a subject with familial risk. Gastroenterology 119, (2000). 86. Su, L., Nalle, S. C., Sullivan, E. A., Fu, Y.-X. & Turner, J. R. Genetic ablation of myosin light chain kinase limits epithelial barrier dysfunction and attenuates experimental inflammatory bowel disease. Gastroenterology Abstr.136, A81 (2009). 87. Xu, J. et al. Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating β2 integrins. Nature Immunol. 9, (2008). 88. Arrieta, M. C., Madsen, K., Doyle, J. & Meddings, J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 58, (2009). 89. Vetrano, S. et al. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology 135, (2008). 90. Laukoetter, M. G. et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 204, (2007). 91. Kang, L. I. et al. Deletion of JAM-A causes morphological defects in the corneal epithelium. Int. J. Biochem. Cell Biol. 39, (2007). 92. Boirivant, M. et al. A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology 135, (2008). This is the first detailed analysis of the effects of transient epithelial cell damage on immunoregulation in the gut. 93. Iliev, I. D., Mileti, E., Matteoli, G., Chieppa, M. & Rescigno, M. Intestinal epithelial cells promote colitisprotective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. (2009). This study shows that epithelial cells can direct DCs to facilitate regulatory T cell differentiation. 94. Sartor, R. B. Microbial influences in inflammatory bowel diseases. Gastroenterology 134, (2008). 95. Ewaschuk, J. B., Tejpar, Q. Z., Soo, I., Madsen, K. & Fedorak, R. N. The role of antibiotic and probiotic therapies in current and future management of inflammatory bowel disease. Curr. Gastroenterol. Rep. 8, (2006). 96. Aranda, R. et al. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4 +, CD45RB high T cells to SCID recipients. J. Immunol. 158, (1997). 97. Sellon, R. K. et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66, (1998). 98. Cario, E. Barrier-protective function of intestinal epithelial Toll-like receptor 2. Mucosal Immunol. 1, S62 S66 (2008). 99. Mucida, D. et al. Reciprocal T H 17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, (2007) Coombes, J. L. et al. A functionally specialized population of mucosal CD103 + DCs induces Foxp3 + regulatory T cells via a TGF-β and retinoic aciddependent mechanism. J. Exp. Med. 204, (2007) Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, (2008) Fisher, S. A. et al. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn s disease. Nature Genet. 40, (2008) Watanabe, T. et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J. Clin. Invest. 118, (2008) Yang, Z. et al. NOD2 transgenic mice exhibit enhanced MDP-mediated downregulation of TLR2 responses and resistance to colitis induction. Gastroenterology 133, (2007) Hedl, M., Li, J., Cho, J. H. & Abraham, C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc. Natl Acad. Sci. USA 104, (2007) Kraus, T. A., Cheifetz, A., Toy, L., Meddings, J. B. & Mayer, L. Evidence for a genetic defect in oral tolerance induction in inflammatory bowel disease. Inflamm. Bowel Dis. 12, (2006) Shiue, H., Musch, M. W., Wang, Y., Chang, E. B. & Turner, J. R. Akt2 phosphorylates ezrin to trigger NHE3 translocation and activation. J. Biol. Chem. 280, (2005) Hirschhorn, N. et al. Decrease in net stool output in cholera during intestinal perfusion with glucosecontaining solutions. N. Engl. J. Med. 279, (1968) Zeissig, S. et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn s disease. Gut 56, (2007) Duerksen, D. R., Wilhelm-Boyles, C. & Parry, D. M. Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig. Dis. Sci. 50, (2005) Pearson, A. D., Eastham, E. J., Laker, M. F., Craft, A. W. & Nelson, R. Intestinal permeability in children with Crohn s disease and coeliac disease. Br. Med. J. (Clin. Res. Ed) 285, (1982) Wang, F. et al. IFN-γ-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 131, (2006) Powrie, F. et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RB hi CD4 + T cells. Immunity 1, (1994) Stepankova, R. et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RB high CD4 + T cells. Inflamm. Bowel Dis. 13, (2007) Collett, A. et al. Early molecular and functional changes in colonic epithelium that precede increased gut permeability during colitis development in mdr1a / mice. Inflamm. Bowel Dis. 14, (2008) Resta-Lenert, S., Smitham, J. & Barrett, K. E. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a / mice. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G153 G162 (2005) Nusrat, A. et al. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immun. 69, (2001) McNamara, B. P. et al. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Invest. 107, (2001). Acknowledgements I am indebted to current and past members of my laboratory for discussions and investigations that contributed to this article. Thanks also to A. M. Marchiando and C. R. Weber for the images used in figures 1 and 2. Work in my laboratory is currently supported by the US National Institutes of Health (DK061931, DK068271, DK67887 and HL091889), the University of Chicago Cancer Center (CA14599), the University of Chicago Digestive Disease Research Core Center ( D K ), t h e U S D e p a r t m e n t o f D e f e n s e (W81XWH ), the Broad Medical Research Program (IBD-0272) and the Chicago Biomedical Consortium. I apologize to colleagues whose work and publications could not be referenced owing to space constraints. DATABASES UniProtKB: α catenin 1 β catenin catenin δ1 claudin 1 claudin 2 claudin 16 E cadherin IFNγ IL 1β IL 10 JAM A LAP LIGHT MLCK NHE3 TNF XBP1 ZO1 ZO2 FURTHER INFORMATION Jerrold R. Turner s homepage: uchicago.edu/faculty/jturner.html All links ARE ACTIvE In ThE online pdf nature REvIEwS Immunology volume 9 november Macmillan Publishers Limited. All rights reserved
27 From Mucins to Mucus. Thornton DJ and Sheehan JK, Proc Am Thorac Soc., 1:54 (2004)
28 From Mucins to Mucus Toward a More Coherent Understanding of This Essential Barrier David J. Thornton and John K. Sheehan Wellcome Trust Centre for Cell-Matrix Research, School of Biological Sciences, University of Manchester, Manchester, United Kingdom; and Department of Biophysics and Biochemistry, Cystic Fibrosis Research Center, University of North Carolina, Chapel Hill, North Carolina Mucus is essential for protection of the airways; however, in chronic airway disease mucus hypersecretion is an important factor in morbidity and mortality. The properties of the mucus gel are dictated in large part by the oligomeric mucins and, over the past decade, we have gained a better understanding of the molecular nature of these complex O-linked glycoproteins. We know now that MUC5AC mucins, as well as different glycoforms of the MUC5B mucin, are the predominant gel-forming glycoproteins in airways mucus. Furthermore, the amount, molecular size, and morphology of these glycoproteins can be altered in disease. From more recent data, it has become clear that oligomeric mucins alone do not constitute mucus, and other mucin and nonmucin components must be important contributors to mucus organization and hence airways defense. Therefore, the challenge over the coming decade will be to investigate how the oligomeric mucins are organized to yield functional mucus. Such studies will provide a clearer perception of airways mucosal protection and may highlight specific components as potential targets for therapeutic strategies for the treatment of hypersecretory disease. Keywords: mucus; sputum; MUC5AC; MUC2; MUC5B The respiratory mucus gel performs a number of essential functions that collectively lead to the protection of the airways. This highly hydrated gel, in conjunction with ciliated epithelial cells, forms the mucociliary escalator, which, along with cough, is essential for the maintenance of sterile and unobstructed airways. By contrast, overproduction of mucus with altered rheologic properties is an important factor in the morbidity and mortality of chronic airways disease (e.g., asthma, cystic fibrosis [CF], and chronic obstructive pulmonary disease [COPD]) (1 3). As a result of the change in physical properties of the gel, mucociliary clearance is impaired or abolished with the attendant problems of poor gas exchange and/or inflammation and/or bacterial colonization. Thus, production of mucus with the correct properties for airways protection is paramount for health. A variety of factors have been found to trigger mucus hypersecretion, including proinflammatory cytokines (e.g., interleukin-4, interleukin-13, epidermal growth factor, and tumor necrosis factor- ), ATP, bacterial exoproducts, and host proteases (4 9), but the mechanisms underlying mucus biogenesis and secretion, and its molecular composition and supramolecular organization, are still obscure. The major gel-forming components of the airways mucus gel are the oligomeric mucins, and the hypothesis that drives our research is that these glycoproteins (Received in original form June 19, 2003; accepted in final form September 30, 2003) Supported by the Wellcome Trust and the National Asthma Campaign. Correspondence and requests for reprints should be addressed to David J. Thornton, B.Sc., Ph.D., Wellcome Trust Centre for Cell-Matrix Research, School of Biological Sciences, University of Manchester, Stopford Building, Manchester, M13 9PT, UK. Dave.Thornton@man.ac.uk Proc Am Thorac Soc Vol 1. pp 54 61, 2004 DOI: /pats Internet address: play the vital role in determining the physical properties of this essential barrier in health and disease. However, it should also be pointed out, especially in pathologic conditions, that other molecules such as proteoglycans and DNA may contribute to the physical properties of airways mucus. Over the last decade, great strides have been made in identifying and characterizing the major oligomeric mucins present in airways sputa. However, there have been no studies to date that relate the ensemble of their physical properties (i.e., length, charge density, macromolecular architecture) to a specific set of rheologic parameters for a mucus gel, never mind its optimization to a specific function, such as ciliary transport. In this article, we have been given the task of reviewing our own contributions to this area. We have also suggested further avenues of investigation that will be necessary to gain a better understanding of the biology and pathobiology of this essential barrier. MUCINS WHAT ARE THEY? Mucins are currently defined as high-m r glycoproteins that contain at least one and sometimes multiple protein domains that are sites of extensive O-glycan attachment (mucin-like domains). Thus, the composition of these glycoproteins is dominated by carbohydrate, which can total in some cases as much as 80% of the weight of the molecule. There appear to be two major types of mucin, one thought to be monomeric and, primarily, but not exclusively, located at the cell surface, and the other oligomeric. This latter type is secreted and thought to be responsible for the rheologic properties of mucus. The mucin-like domains are enriched in the amino acids serine and threonine, which are the sites for the attachment to the linkage sugar N-acetylgalactosamine. In addition to N-acetylgalactosamine, mucin oligosaccharides may also contain N-acetylglucosamine, galactose, fucose, sialic acids, and sulfate. The oligosaccharides have remarkable structural diversity and all of their specific roles remain to be fully understood. However, a consequence of their attachment to the polypeptide is to cause it to stiffen, resulting in a large expansion of the volume domain of the molecule, which gives mucins their characteristic space-filling, and thus gel-making, properties (10). Furthermore, it has long been known that certain bacteria bind specific oligosaccharide ligands (for a review see [11]). It is likely that the primary function of the oligosaccharide diversity is to enhance the possibility that bacteria bind to mucus, thus facilitating their removal by mucociliary transport. Moreover, by providing competing receptors for cell-surface glycoconjugates, mucins may trap bacteria and make them less successful in their attempts to colonize the epithelium. Thus, the array of oligosaccharides expressed on the mucins of an individual may play a key role in governing the susceptibility to infection. The studies we shall summarize have been concerned solely with the gel-forming mucins. From our studies in collaboration with Ingemar Carlstedt in the early 1990s, it was clear that the gelforming mucins in airways mucus consisted of a heterogeneous mixture of glycoproteins that was physically similar and extremely large (12 16). The mucus gel could be almost completely solubilized by noncovalent bond breaking agents (e.g., 6M guanidi-
29 Thornton and Sheehan: From Mucins to Mucus 55 Figure 1. Electron micrographs of (A) an intact oligomeric mucin, along with a generic model for oligomeric mucin organization and (B) reduced mucin subunits. (A) Transmission electron micrograph of a respiratory mucin preparation showing a single oligomeric mucin chain. Mucins were isolated from sputum by solubilization with 6 M guanidinium chloride (GuCl) followed by purification by two stages of cesium chloride (CsCl) isopynic density gradient centrifugation, first in 4 M GuCl/CsCl and then in 0.2 M GuCl/CsCl (12). Also shown is a cartoon of an expanded view of a section of the mucin chain highlighting the intermolecular, end-to-end, disulfide (S-S)-mediated assembly of the mucin subunits (monomers). The polypeptide consists of alternating stretches of dense O-linked glycosylation (mucin-like domains) and folded, intramolecular disulfide bond stabilized regions (depicted as knots). A single monomer spans the two S-S linkages. (B) Transmission electron micrograph of reduced mucin subunits generated after treatment of oligomeric mucins (prepared as in A) with a reducing agent (e.g., 10 mm dithiothreitol). The reduced subunits are approximately 600 nm in length. Also shown is a cartoon of the reduced mucin subunit highlighting the unfolding of the intramolecular disulfide stabilized domains situated at the ends and interspersed between the mucin-like domains. SH represents reduced cysteine residues. nium chloride), a process usually achieved overnight but in some cases taking days. Thus, entanglement of the long mucin chains was considered to be the primary mechanism for gel formation and, as a consequence, the size, concentration, and chemical nature of the mucins are important factors for determining the properties of the gel. Physical characterization of the mucins isolated from sputum using light scattering and electron microscopy showed them to be polydisperse in both mass (2 40 md) and size ( m in length). Furthermore, these studies demonstrated that the component mucin monomers or subunits (2 3 md) are assembled linearly and held together by disulfide bonds (17). Figure 1 shows electron micrographs of oligomeric respiratory mucins before and after treatment with a reducing agent, along with the model proposed for their structure. It is important to note that for these studies the mucins were isolated and purified in highly chaotropic solvents (e.g., 4 6 M guanidinium chloride) to preserve their primary structure. Thus, the observed architecture may not reflect their native conformation in mucus. A more realistic view of their native conformation will require characterization of the mucins after isolation using nonchaotropic agents. WHICH MUCINS ARE IMPORTANT IN THE AIRWAYS AND WHAT ARE THEY LIKE? It is now apparent that these oligomeric glycoproteins are members of a larger family of mucins. Of the 13 mucin genes (MUC) currently identified, only 4 (MUC2, MUC5AC, MUC5B, and MUC6) contain the cysteine-rich motifs in their C- and N-terminal domains necessary for oligomerization (18 21). Three of these genes, MUC2, MUC5AC, and MUC5B, are expressed in the airways. A number of important studies on airways mucins have concentrated on messenger RNA expression, in particular focusing on MUC2 and MUC5AC, and highlight specific diseaserelated factors that upregulate these two mucins (4 9, 22 24). However, the fact that these macromolecules are synthesized and then stored awaiting the appropriate stimulus for secretion implies that messenger RNA studies may be misleading for the amount of mucin in mucus. This is borne out by biochemical data, which have demonstrated that the products of the MUC5AC and MUC5B genes are predominant in sputum (25 29) and that the MUC2 mucin is barely detectable (see subsequent text). Thus, there seems to be a discrepancy between MUC2 messenger RNA levels and the occurrence of the mature MUC2 mucin in sputum. This may be due to the apparent insolubility of this glycoprotein, as has been reported for MUC2 isolated from small-intestinal mucus (30), causing it to be retained in the airways. While our data do not rule this out, in the single study we have performed, where the mucus was physically removed from an asthmatic lung postmortem, there was still little evidence for this mucin (3, 31). Matching the polypeptides of the different mucins present in mucus to their respective genes has been a long process, which was eventually achieved by fractionation of the mucins (after reduction of their disulfide bonds) by anion exchange chromatography and agarose gel electrophoresis, and then by a combination of biochemical analyses (i.e., amino acid composition, peptide sequencing, and peptide fingerprinting) as well as reactivity with mucin-specific antisera (for more details see Figure 2). The analysis of multiple sputum samples revealed that the two major mucin gene products, MUC5AC and MUC5B have different chemical properties. The MUC5AC mucin has a more homogeneous charge distribution than MUC5B, which occurs in differently charged forms (26). In most samples analyzed, two different MUC5B forms (glycoforms) are found, and while their charge density is not identical between individuals it is apparent that a high and low charge form occurs in most cases (Figure 3). These glycoforms also exhibit different buoyant densities and, as a result, can be partially separated by isopycnic density centrifugation where there is a direct correlation between their charge and buoyant densities (17). As far as we can tell, each glycoform is found in separate oligomeric mucins and is not part of a heterooligomer. This separation, coupled with the distinctive pattern of glycosylation, at least at the level of charge density, associated with each mucin species, may reflect their different cellular origins (see Where Are Mucins Synthesized?). Studies performed on the intact MUC5AC and MUC5B mucins suggest that these mucins have different macromolecular characteristics. Sedimentation studies have indicated that both are polydisperse in size, and this may arise from the oligomeriza-
30 56 PROCEEDINGS OF THE AMERICAN THORACIC SOCIETY VOL Figure 2. Identifying the major oligomeric mucins in sputum. Sputum is a mixture of oligomeric mucins (MUC2, MUC5AC, and MUC5B), which are polydisperse in size and share similar biochemical and biophysical properties. Therefore, purification of the individual mucin species is not possible with intact mucins but can be achieved by working with preparations of reduced mucins. (A) A typical anion exchange chromatogram of a reduced and alkylated respiratory mucin preparation. In this case, the separation matrix was a Mono Q HR 5/5 column, which was eluted with a linear salt gradient of lithium perchlorate containing 6 M urea (dashed line). The mucin distribution (solid line) was monitored with the periodic acid Schiff s (PAS) reagent that detects the glycan component of the molecule. In this example, the mucin distribution was pooled into three populations with increasing charge density (I, II, and III), which were further purified by rerunning on the Mono Q column. Fractions from the rerun were pooled on the basis of their electrophoretic mobility on a 1% agarose gel and their reactivity with a number of lectins (data not shown) to yield 3 distinct populations. (B) The 3 resultant pools were subjected to 1% agarose gel electrophoresis and a Western blot of the agarose gel was probed with an antiserum that recognizes oligomeric mucins (64). Other Western blots (not shown) were probed with antisera specific for MUC2, MUC5AC, and MUC5B mucins (25, 26, 44) and the bands that were reactive with these probes are labeled. Two of the bands were reactive with the MUC5B antiserum, and these correspond to populations I and III, which had very different charge densities as assessed by anion exchange chromatography (see Fig. 2A). Therefore, these bands are labeled as high- and low-charge variants of MUC5B. The third band corresponding to the intermediate charge species (population II) on the Mono Q column was reactive with the MUC5AC antiserum. None of the bands were stained with the MUC2 antiserum. The identification of mucins was not based solely on antisera reactivity but also relied on chemical analyses. For example, (C) shows the MALDI-TOF MS peptide fingerprint after trypsin treatment of a reduced and alkylated MUC5B mucin preparation. The four major peptides highlighted (peptides 1 4) were purified by reverse-phase chromatography on a C18 column and their sequence determined by N-terminal sequencing (26). Their sequences and locations within the MUC5B polypeptide are shown in the boxed schematic diagram. This represents the central portion of the MUC5B apoprotein, showing the cysteine-rich domains (cys1 7, black boxes) and the R and R-end domains (hatched boxes) corresponding to the glycosylated mucin-like domains (adapted from [20]). The four major peptides are located in some of the seven cys-domains, which share a similar, repetitive sequence. The 62 amino acid sequence shown is found in three of these domains (cys 4, 5, and 6) and the 2 peptides with masses 1,132.5 D (peptide 2) and 1,687.7 D (peptide 3) are also found in cys 7. The multiple occurrences of these four peptides explain their dominance of the MUC5B tryptic fingerprint. Amino acid composition can also be used to identify mucins. Shown in (D) are the predicted contents (calculated from published deduced amino acid sequences) of serine, threonine, and proline in the mucin-like domains of the MUC2, MUC5AC, and MUC5B mucins. This analysis highlights the uniqueness of the MUC2 mucin in this regard. Also shown are the experimentally derived values for these three amino acids generated from amino acid analysis of the highly glycosylated fragments of the mucin (corresponding to the mucin-like domains) obtained after trypsin digestion of population I (MUC5B) and population II (MUC5AC) from the Mono Q column. These data confirm the absence of significant amounts of MUC2 in a typical mucin preparation. For greater experimental detail concerning the separation and characterization of the different mucin populations see Reference (65). tion of a variable number of their constituent monomers and/or as a consequence of specific proteolytic processing and/or degradation. Moreover, these studies showed that the MUC5AC mucins exhibited a lower average sedimentation rate than the MUC5B glycoprotein, which was taken to indicate that MUC5AC was smaller than MUC5B (27, 32). However, this is probably not the case, as we have shown that MUC5AC is in fact highly oligomerized and the apparent difference in size may be explained by a
31 Thornton and Sheehan: From Mucins to Mucus 57 Figure 3. Agarose gel electrophoresis of reduced sputum samples. Sputa, NaCl-induced from healthy airways (lanes 1, 6 and 7) and spontaneous secretions from diseased airways, were solubilized in 6 M GuCl and then dialyzed into 6 M urea. The samples were reduced with 10 mm dithiothreitol, treated with 25 mm iodoacetamide to alkylate-free thiol groups and then subjected to electrophoresis on a 0.7% agarose gel (for more details see Reference [65]). After electrophoresis, gels were transferred to nitrocellulose and probed with antisera specific for (A) MUC5AC and (B) MUC5B mucins (section from the data presented in Reference [39]). These blots were also stained with an antiserum directed against MUC2, but no bands were visualized (data not shown). The results show the greater variation in electrophoretic migration of the reduced MUC5B mucins compared with MUC5AC; furthermore, with the exception of one sample (lane 6), all the others exhibit two different MUC5B charge forms. These data also demonstrate that there is a larger variation in electrophoretic mobility between low-charge populations than there is between highly charged forms. difference in physical characteristics of these two mucins (33). The MUC5AC polymer has a low mass per unit length, suggesting small oligosaccharides, and adopts a very stiff extended conformation in solution, whereas the MUC5B mucin appears to adopt a more compact structure. Further evidence that these two mucins have different macromolecular features is indicated by their behavior on agarose gel electrophoresis. Unreduced MUC5AC preparations exhibit a ladder-like banding pattern and oligomeric species containing 16 monomers can be discerned (33). By contrast, unreduced MUC5B mucins barely enter the gel (Thornton and coworkers, unpublished observation). It is clear that MUC5AC and MUC5B are the predominant gel-forming mucins in the airways and that they have distinct chemical and physical features. What effect these differences between the mucins have on the gel properties has yet to be determined. Moreover, at an even more fundamental level, we do not know whether MUC5AC and MUC5B mucins are blended together to form the mucus gel or if they form the basis of separate gels. The latter has been suggested in gastric mucus, where laminated layers containing different mucins have been reported (34). WHERE ARE MUCINS SYNTHESIZED? Immunolocalization studies on normal airways, using mucinspecific antisera, have shown that MUC5AC mucins are produced predominantly by goblet cells in the surface epithelium. MUC5B mucins on the other hand, originate mainly, but not exclusively, from the mucous cells of the submucosal glands (17, 27, 32), and this distribution is consistent with messenger RNA expression as shown by in situ hybridization (35). While antisera to MUC5AC and MUC5B have been used to localize their respective cellular sources, we do not have, and may never have, probes to the polypeptide that are specific for each of the glycoforms of the MUC5B mucin. Thus, we do not know if they have different sites of synthesis. We have previously shown in salivary mucin preparations that the highly charged variant of MUC5B was reactive with a monoclonal antibody F2 that is specific for the carbohydrate epitope, the sulfo-lewis a antigen (SO 3-3Gal 1 3GlcNAc) (36). This antibody has been shown to stain a subset of glandular mucous cells in normal human trachea (27). Whereas this suggests different cellular locations for the MUC5B variants, immunolocalization data from carbohydrate-directed probes are not conclusive owing to potential cross-reactivity with other glycoproteins. Thus, polypeptide probes would be desirable and might allow us to conclusively pinpoint their cellular sources; however, unless we can find differences in their polypeptide moieties, this will not be possible. It is interesting to speculate that the spatial separation of mucin production in the normal airways (i.e., MUC5AC on the surface and MUC5B in the glands), coupled to the fact that the two sites are controlled by different secretory mechanisms, may allow for fine-tuning of the mucus composition to modulate mucus properties depending on the challenge in the airways. On the basis of the immunolocalization and expression data, it seemed logical at that time to believe that the mucin composition of mucus would inform us on the contribution of the various cellular sources to the secretion and, therefore, might throw up possible cellular targets for therapies aimed at modulating mucin, and hence mucus production in hypersecretory diseases. However, in hypersecretory disease, this marked spatial separation of synthesis does not seem to hold. Such diseases are associated with significant hyperplasia and metaplasia of mucin secreting cells, and it has been shown that MUC5AC production can also occur in glandular mucous cells, while some MUC5B and MUC2 synthesis can be detected in surface goblet cells (37, 38). Whether these mucins can be coexpressed in the same cell or arise from different cells has not yet been determined. Because we do not know the relative change in expression of the mucins at the different locations, we cannot be certain that their occurrence is not a valid indicator of the major source of their production. Thus, there is an obvious need for further, more in-depth studies on mucin expression in disease. ARE AIRWAYS MUCINS ALTERED IN DISEASE? In a series of studies, we have shown that the gel-forming mucins can be changed in amount, type, and size in airways disease (3, 12, 13, 31). For example, in the viscid mucus obstructing the airways of an individual who died in status asthmaticus, there were changes in all three parameters. There was an approximately seven-fold increase in mucin concentration compared with healthy secretions. Furthermore, we demonstrated that the only mucin type responsible for the aberrant physical properties of this gel was a low-charge glycoform of the MUC5B mucin with unusual structural features and extreme size (3, 31). The clinical outcome in this case demonstrated the dramatic consequences of alterations in the mucin component of mucus and highlighted the need to determine the molecular profile of mucins in sputum. Therefore, in order to gain a more detailed picture of mucin type (gene product and glycoform) and quantity in normal and pathologic respiratory mucus, we developed a quantitative Western blotting assay to measure the levels of MUC2, MUC5AC,
32 58 PROCEEDINGS OF THE AMERICAN THORACIC SOCIETY VOL and the different glycoforms of the MUC5B mucins directly from sputum (39). Data from 44 samples (15 NaCl-induced normal, 10 asthma, 10 CF, and 9 COPD) showed, as our previous nonquantitative studies had suggested, that MUC5AC and MUC5B are the major gel-forming mucins in sputum. By contrast, the MUC2 mucin was present in only trace amounts, confirming our earlier observations that MUC2 is only a minor component in sputum. Moreover, this study showed that airways mucus is not a single substance and is comprised of variable amounts of MUC5AC and MUC5B glycoproteins. Somewhat surprisingly, the variation in content was particularly marked in the normal samples. A key observation to come from this work was that, compared with secretions from normal subjects and individuals with asthma, there was more MUC5B in CF and COPD sputa and, most notably, there was a significant increase in the amount of the low charge form of the MUC5B mucin in the diseased, possibly infected sputa. This might suggest a link between infection/inflammation and MUC5B production. The functional consequences of mucus with different mucin compositions, and in particular of the different charge forms of the MUC5B mucin, are at present completely obscure. We would suggest the general hypothesis that MUC5AC, being primarily the product of goblet cells, may have the major mechanical function of facilitating ciliary clearance of mucus, whereas MUC5B emanating from the glands may form the basis of a gel, the primary role of which is to help with the clearance of specific pathogens or other irritants. The abnormal mucin composition of the gel in CF and COPD airways may provide an insight into the altered physical nature of these secretions and ultimately suggest potential cellular targets for therapy. These observations again highlight the need for more studies on mucin expression in disease, and in particular on MUC5B expression, which has so far been largely ignored. Furthermore, these findings reemphasize the need to develop reliable probes for the MUC5B mucin glycoforms to establish their cellular origin. GEL-FORMING MUCINS PRODUCED BY AIRWAYS CELLS IN AIR LIQUID INTERFACE CULTURE Studies of human airways secretions have been, and will continue to be, essential to highlight changes associated with disease. However, these investigations are fraught with difficulty related to mucus collection, as well as possible changes in the mucins being disguised by nonspecific postsecretion processing. Furthermore, the effects of extraneous environmental influences are difficult to control and, for ethical reasons, it is impossible to perform in vivo studies of mucin/mucus biogenesis and secretion. Thus, to progress our understanding of both normal and diseased airways, there is an urgent need for in vitro systems that mimic in vivo mucus production. For this purpose a number of laboratories have developed airways epithelial cell cultures (40 43). The normal human tracheobronchial air liquid interface cultures instigated by Nettesheim and colleagues are of particular interest (40). When cultured in the presence of retinoic acid, normal human tracheobronchial cells grow and differentiate into a mucociliary epithelium. They secrete functional mucus onto their apical surface (40) that is clearly capable of ciliary transport. This culture system appears to mimic in vivo airways mucin production in that all three oligomeric airways mucins, MUC5AC, MUC5B, and MUC2 are expressed. Furthermore, their basal levels of expression can be modulated by physiologically relevant regulators (e.g., thyroid hormone and epidermal growth factor) (44 46). We have investigated the apical secretions from such cultures and have shown that MUC5AC and MUC5B are the predominant mucins stored and secreted by normal human tracheobronchial cells (passage 2). Moreover, these mucins have similar macromolecular properties to their counterparts in human airways (44). However, while the MUC5AC mucin exhibits a similar charge profile to MUC5AC in vivo, by contrast, the MUC5B mucin produced is more homogeneous, with evidence of only a single (perhaps high-charge) glycoform. Thus, the mucin phenotype and hence the resultant properties of the secretions may not be a truly accurate reflection of in vivo mucus. Nonetheless, such culture systems provide a powerful and manipulable experimental system and seem set to form the basis of ever widening studies that should revolutionize our understanding of the biology and pathobiology of mucus over the next few years. For example, work from Boucher and collaborators using cultures derived from cells with the CF defect has shown that the dynamics of the attached mucus layer are altered and, in these compared with normal cultures, mucus transport is reduced or even abolished (41). AIRWAYS MUCUS GEL FORMATION OLIGOMERIC MUCINS ARE NOT THE WHOLE STORY! While size and concentration of the mucins are key factors controlling gel formation, concentrated solutions of mucins alone do not reproduce all the physical properties of the gel (47). This is emphasized in our recent findings where we have shown, using confocal fluorescence recovery after photobleaching, that the network properties of mucus cannot be recapitulated by concentrated mucin solutions even at higher than in vivo mucin concentration (48). Unlike some other methods for looking at mucus properties, confocal fluorescence recovery after photobleaching is nondestructive and provides a powerful approach to investigating the properties of macromolecules in concentrated solution, including complex mixtures, such as in mucus, under equilibrium conditions, and in the absence of concentration gradients and flow and shear forces (48, 49). We have used it to determine lateral tracer diffusion of fluorescent molecular probes (i.e., bovine serum albumin and mucins) and of rigid polystyrene microspheres of different size in mucus at physiological concentrations. It is important to note that the tracer diffusion measurements alone are not a measure of gel formation, which involves polymer crosslinks and entanglement and occurs in concentrated solutions of all flexible polymers. However, they provide a quantitative comparison of the network caused by gel-formation, or entanglement, that impedes the free diffusion of other macromolecules in a concentration-dependent way. Our initial confocal fluorescence recovery after photobleaching analysis of MUC5B (extracted and purified in the presence of 4 6 M guanidinium chloride) showed that in concentrated solutions its properties resulted from polymer entanglement with no evidence of protein protein self-association or glycan-mediated interactions (48). A direct comparison of concentrated guanidinium chloride-purified MUC5B mucin solutions with the native saliva from which it was prepared revealed that the MUC5B mucin did not replicate the properties of the parent mucus. At similar MUC5B concentrations to those found in saliva, the guanidinium chloride purified mucin was approximately 20 times more permeable, suggesting that there was a higher order structure in the native mucus, which may involve other components in the secretion (48). Further analyses have implicated calcium as a key regulator of MUC5B mucin supramolecular organization in salivary mucus (50). This study demonstrated a reversible, calcium-dependent interaction between MUC5B mucins that was sensitive to denaturation by chaotropic solvents (e.g., 6 M guanidinium chloride), treatment with reducing agents and the proteinase trypsin (50). This study also highlighted the need for alternative, nondenaturing solvents to disassemble mucus gels for investigation of their functions. The fact that gel-forming mucins alone do not constitute mu-
33 Thornton and Sheehan: From Mucins to Mucus 59 cus may not be totally surprising because it is a complex mixture of ions, mucins, glycoproteins, proteins, and lipids and, like the mucins, the total amount of these other components is increased in hypersecretory disease (51 54). Some of these components contribute to airways protection by targeting pathogens (e.g., secretory IgA, proline-rich proteins, defensins, lysozyme, and transferrin), and others may act to modulate the organization and hence the properties of the gel. For example, in the gastrointestinal tract the mitogenic/reparative trefoil peptides are intimately associated with mucus and have been suggested to bind to oligomeric mucins and increase mucus viscosity (55). Work from our laboratory has demonstrated that a large glycoprotein, gp-340, is found in complexes with MUC5B in airways mucus (56). It is interesting to speculate on the consequences of an interaction of gp-340 with the mucin network. Gp-340 clearly has an important antibacterial role in mucus, either via its direct binding to bacteria (57) or by its association with the bacterialbinding collectin surfactant protein-d (58). Thus, binding of gp-340, and likely other protective factors, to the gel network increases the functionality of the mucus by facilitating clearance of sequestered bacteria from the respiratory tract via the mucociliary escalator or cough. It is clear from these two examples that nonmucin glycoproteins and proteins play key roles in mucosal protection and mucus organization; but while there have been many studies on the molecular components of mucus, we do not yet know their full complement or which of these are associated with the mucins and what function(s) they perform. These other components may be over- or under-expressed in disease conditions, which would have an effect on the hosts ability to prevent colonization or the rheologic properties of mucus may be compromised with attendant problems. Thus proteomics has a key role to play in identifying the different molecular species impacting on mucus structure and function. A final but no less important factor that will have a major influence on the properties of airways mucus is the availability of water on the epithelial surface. Currently, the relationship between mucin hydration and mucus properties is not well understood. We know that oligomeric mucins are preassembled into large oligomers within the cell, where they are stored within granules in a largely dehydrated form. However, after secretion the requirements of a specific mucin to hydrate are unknown but are likely affected by a variety of factors, including their size, charge density, and organization. Furthermore, their hydration will be intimately coupled with the ionic composition and water availability of the environment into which they are secreted. This will in turn be affected by the other biomolecules already present in that environment. Aberrant mucin hydration may be of particular importance in the airways of patients with CF. In CF, the competition for water on the airways epithelium appears to be more severe, and the water imbalance in the epithelial fluid (41, 59, 60) may well have a strong negative impact on mucin unpackaging and hydration. This may explain the abnormal properties of the mucus, which are a feature of this disease. OTHER AIRWAYS MUCINS While this article has focused on the oligomeric mucins, these are not the only mucins expressed in the airways. Other members of the mucin family (MUC1, MUC4, MUC7, MUC11, and MUC13) are expressed and these mucins, with the exception of MUC7, are thought primarily, though not exclusively, to occur in transmembrane forms, which can arise in mucus due to shedding from the cell surface (61 63). However, in the case of MUC1 and MUC4, there are alternatively spliced forms that are secreted (61 63). There is little biochemical characterization of these mucins, and their amounts in mucus are unknown. Furthermore, their role in mucus has yet to be defined and there is clearly an urgent need to understand their function in mucus. Conclusions As far as the oligomeric mucins are concerned, an extensive array of mucin-specific probes are available, the methodologies necessary for their separation and characterization have been developed, and the air liquid cultures offer a more physiologically relevant in vitro system for their study. Thus, we are well placed to perform new studies of the cell and structural biology of the mucins and mucus, which is essential for a more coherent picture of how this essential barrier functions to protect the airways. Finally, there are currently few effective therapies to alleviate mucus hypersecretion, and these studies may highlight specific components as potential targets for future therapeutic strategies for the treatment of hypersecretory conditions. Acknowledgment : The authors acknowledge the substantial contributions to the research reviewed here by Ingemar Carlstedt (University of Lund, Sweden), Paul Richardson (St. Georges Hospital, London, UK), Paul Nettesheim, Tom Gray, and Peter Koo (NIEHS, U.S.A.), and Sara Kirkham, Marj Howard, Nagma Khan, David Knight, Bertrand Raynal, and Tim Hardingham (University of Manchester, UK). References 1. Phillips GJ, James SL, Lethem MI. Rheological properties and hydration of airway mucus. In Rogers DF and Lethem MI, editors. Airway mucus: basic mechanisms and clinical perspectives. Basel: Birkhauser Verlag; pp Puchelle E, Zahm JM, de Bentzmann S, Gaillard D. In Rogers DF and Lethem MI, editors. Airway mucus: basic mechanisms and clinical perspectives. Basel: Birkhauser Verlag; pp Sheehan JK, Richardson PS, Fung DCK, Howard M, Thornton DJ. Analysis of respiratory mucus glycoproteins in asthma: a detailed study from a patient who died in status asthmaticus. Am J Respir Cell Mol Biol 1995;13: Levine SJ, Larivee P, Logun C, Angus CW, Ognibene FP, Shelhamer JH. Tumor necrosis factor- induces mucin hypersecretion and MUC2 gene expression in airway epithelial cells. Am J Respir Cell Mol Biol 1995;12: Li D, Gallup M, Fan N, Szymkowski DE, Basbaum CB. Cloning of the amino-terminal 5 -flanking region of the human MUC5AC mucin gene and transcriptional up-regulation by bacterial exoproducts. J Biol Chem 1998;273: Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases MUC5AC mrna and protein expression in respiratory epithelial cells. Am J Physiol 1999;276:L835 L Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103: Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases MUC5AC mrna and protein expression in respiratory epithelial cells. Am J Physiol 1999;276:L835 L Nadel J Role of epidermal growth factor receptor cascade in airway hypersecretion and proposal for novel therapy. In Salathe M, editor. Cilia and mucus. New York: Marcel Dekker; pp Jentoft N. Why are proteins O-glycosylated. Trends Biochem Sci 1990; 15: Scharfman A, Lamblin G, Roussel P. Interactions between human respiratory mucins and pathogens. Biochem Soc Trans 1995;23: Thornton DJ, Davies JR, Kraayenbrink M, Richardson PS, Sheehan JK, Carlstedt I. Mucus glycoproteins from normal human tracheobronchial secretions. Biochem J 1990;265: Thornton DJ, Sheehan JK, Lindgren H, Carlstedt I. Mucus glycoproteins from cystic fibrotic sputum: macromolecular properties and structural architecture. Biochem J 1991;276: Thornton DJ, Sheehan JK, Carlstedt I. Heterogeneity of mucus glycoproteins from cystic fibrotic sputum. Biochem J 1991;276: Thornton DJ, Devine PL, Hanski C, Howard M, Sheehan JK. Identification of two major populations of mucins in respiratory secretions. Am J Respir Crit Care Med 1994;150:
34 60 PROCEEDINGS OF THE AMERICAN THORACIC SOCIETY VOL Sheehan JK, Thornton DJ, Somerville M, Carlstedt I. The structure and heterogeneity of respiratory mucus glycoproteins. Am Rev Respir Dis 1991;144:S4 S Thornton DJ, Davies JR, Carlstedt I, Sheehan JK. Structure and biochemistry of human respiratory mucins. In Rogers DF and Lethem MI, editors. Airway mucus: basic mechanisms and clinical perspectives. Basel: Birkhauser Verlag; pp Gum JR, Hicks JW, Toribara MW, Siddiki B, Kim YS. Molecular cloning of the human intestinal mucin (MUC2) cdna: identification of the amino terminus and the overall sequence similarity to prepro-von Willebrand factor. J Biol Chem 1994;269: Van de Bovenkamp JHB, Hau CM, Strous GJAM, Buller HA, Dekker J, Einerhand AWC. Molecular cloning of human gastric mucin MUC5AC reveals reserved cysteine-rich D-domains and a putative leucine zipper motif. Biochem Biophys Res Commun 1998;245: Desseyn J-L, Buisine MP, Porchet N, Aubert JP, Laine A. Genomic organization of the human mucin gene MUC5B cdna and genomic sequences upstream of the large central exon. J Biol Chem 1998;273: Toribara NW, Ho SB, Gum E, Gum JR, Lau P, Kim YS. The carboxylterminal sequence of the human secretory mucin, MUC6: analysis of the primary amino acid sequence. J Biol Chem 1997;272: Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci USA 1999;96: Takeyama K, Jung B, Shim JJ, Burgel PR, Pick TD, Ueki IF, Protin U, Kroschel P, Nadel JA. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2001;280:L165 L Shim JJ, Dabbagh K, Ueki IF, Pick TD, Burgel PR, Takeyama K, Tam DCW, Nadel JA. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am J Physiol Lung Cell Mol Physiol 2001;280:L134 L Thornton DJ, Carlstedt I, Howard M, Devine PL, Price MR, Sheehan JK. Respiratory mucins: identification of core proteins and glycoforms. Biochem J 1996;316: Thornton DJ, Howard M, Khan N, Sheehan JK. Identification of two glycoforms of the MUC5B mucin in human respiratory mucus. J Biol Chem 1997;272: Wickström C, Davies JR, Eriksen GV, Veerman ECI, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J 1998;334: Hovenberg HW, Davies JR, Herrmann A, Linden CJ, Carlstedt I. MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconj J 1996;13: Davies JR, Svitacheva N, Lannefors L, Kornfalt R, Carlstedt I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis secretions. Biochem J 1999;344: Herrmann A, Davies JR, Lindell G, Martensson S, Packer NH, Swallow DM, Carlstedt I. Studies on the insoluble glycoprotein complex from human colon: identification of reduction-insensitive MUC2 oligomers and C-terminal cleavage. J Biol Chem 1999;274: Sheehan JK, Howard M, Richardson PS, Longwill T, Thornton DJ. Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug. Biochem J 1999;338: Hovenberg HW, Davies JR, Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from goblet cells. Biochem J 1996;318: Sheehan JK, Brazeau C, Kutay S, Pigeon H, Kirkham S, Howard M, Thornton DJ. Physical characterization of the MUC5AC mucin: a highly oligomeric glycoprotein whether isolated from cell culture or in vivo from respiratory mucous secretions. Biochem J 2000;347: Ota H, Katasuyama T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J 1992;24: Copin MC, Devisme L, Buisine MP, Marquette CH, Wurtz A, Aubert JP, Gosselin B, Porchet N. From normal respiratory mucosa to epidermoid carcinoma: expression of human mucin genes. Int J Cancer 2000;86: Thornton DJ, Khan N, Mehrotra R, Howard M, Veerman E, Packer NH, Sheehan JK. Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology 1999;9: Davies JR, Carlstedt I. Respiratory tract mucins. In Salathe M, editor. Cilia and mucus. New York: Marcel Dekker; pp Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt I, Nicholson AG, Chung KF. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir Med 2002;96: Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J 2002;361: Gray T, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucocilliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 1996;14: Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998;95: Berger JT, Voynow JA, Peters KW, Rose MC. Respiratory carcinoma cell lines, MUC genes and glycoconjugates. Am J Respir Cell Mol Biol 1999;20: Rose MC, Piazza FM, Chen YA, Alimam MZ, Bautista MV, Letwin N, Rajput B. Model systems for investigating mucin gene expression in airway diseases. J Aerosol Med 2000;13: Thornton DJ, Gray T, Nettesheim P, Howard M, Koo JS, Sheehan JK. Characterisation of the MUC5AC and MUC5B mucins synthesised by cultured normal human tracheobronchial epithelial cells. Am J Physiol 2000;278:L1118 L Gray T, Nettesheim P, Basbaum C, Koo JS. Regulation of mucin gene expression in human tracheobronchial epithelial cells by thyroid hormone. Biochem J 2001;353: Gray T, Koo PS, Nettesheim P. Regulation of mucous differentiation and mucin gene expression in the tracheobronchial epithelium. Toxicology 2001;160: Sellers A, Allen A. Gastrointestinal mucus rheology. In Chantler E and Ratcliffe NA, editors. Mucus and related topics. Cambridge: The Company of Biologists Limited; pp Raynal BD, Hardingham TE, Thornton DJ, Sheehan JK. Concentrated solutions of salivary MUC5B mucin do not replicate the gel-forming properties of saliva. Biochem J 2002;362: Gribbon P, Hardingham TE. Macromolecular diffusion of biological polymers measured by confocal fluorescence recovery after photobleaching. Biophys J 1998;75: Raynal B, Hardingham TE, Sheehan JK, Thornton DJ. Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J Biol Chem 2003;278: Lopez-Vidriero MT, Das I, Reid LM. Airway secretion: source, biochemical and rheological properties. In Brain JD, Proctor DF, Reid LM, editors. Respiratory defence mechanisms. New York: Marshall-Dekker; pp Lopez-Vidriero MT, Reid LM. Chemical markers of mucous and serum glycoproteins and their relation to viscosity in mucoid and purulent sputum from various hypersecretory diseases. Am Rev Respir Dis 1978; 117: Boat TF, Cheng PW, Leigh MW. Biochemistry of mucus. In Takishima T and Shimura S, editors. Airway secretion. New York: Marcel Dekker; pp Widdicombe JG. Airway surface liquid: concepts and measurements. In Rogers DF and Lethem MI, editors. Airway mucus: basic mechanisms and clinical perspectives. Basel: Birkhauser Verlag; pp Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Invest 2002;32: Thornton DJ, Davies JR, Kirkham S, Gautrey A, Khan N, Richardson PS, Sheehan JK. Identification of a nonmucin glycoprotein (gp-340) from a purified respiratory mucin preparation: evidence for an association involving the MUC5B mucin. Glycobiology 2001;11: Prakobphol A, Xu F, Hoang VM, Larsson T, Bergstrom J, Johansson I, Frangsmyr L, Holmskov U, Leffler H, Nilsson C, et al. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J Biol Chem 2000; 275: Holmskov U, Lawson P, Teisner B, Tornoe I, Willis AC, Morgan C, Kock C, Reid BM. Isolation and characterisation of a new member of the scavenger receptor superfamily, glycoprotein-340 (gp-340), as a lung surfactant protein-d binding molecule. J Biol Chem 1997;272: Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J Biol Chem 2002;277:
35 Thornton and Sheehan: From Mucins to Mucus Ballard ST, Trout L, Mehta A, Inglis SK. Liquid secretion inhibitors reduce mucociliary transport in glandular airways. Am J Physiol Lung Cell Mol Physiol 2002;283:L329 L Moniaux N, Escande F, Batra SK, Porchet N, Laine A, Aubert JP. Alternative splicing generates a family of putative secreted and membrane-associated MUC4 mucins. Eur J Biochem 2000;267: Williams SJ, Wreschner DH, Tran M, Eyre HJ, Sutherland GR, McGuckin MA. MUC13, a Novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem 2001;276: Sharma P, Dudus L, Nielsen PA, Clausen H, Yankaskas JR, Hollingsworth MA, Engelhardt JF. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am J Respir Cell Mol Biol 1998;19: Sheehan JK, Boot-Handford RP, Chantler E, Carlstedt I, Thornton DJ. Evidence for shared epitopes within the naked protein domains of human mucus glycoproteins. Biochem J 1991;274: Thornton DJ, Khan N, Sheehan JK. Identification and separation of mucins and their glycoforms. In Corfield AP, editor. Mucin methods and protocols; methods in molecular biology series. Totowa, NJ: Humana; pp
36 Mucin structure, aggregation, physiological functions and biomedical applications. Bansil R. and Turner BS Curr Opin in Colloid & Interface Sci, 11:164 (2006)
37 Current Opinion in Colloid & Interface Science 11 (2006) Mucin structure, aggregation, physiological functions and biomedical applications Rama Bansil a, *, Bradley S. Turner b,c,1 a Department of Physics and Center for Polymer Studies, Boston University, Boston, MA 02215, United States b Molecular Biology, Cell Biology and Biochemistry, Boston University, Boston, MA 02215, United States c Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02115, United States Available online 18 January 2006 Abstract An understanding of the basic structure, viscoelastic properties and interactions of mucin glycoproteins is of considerable interest to food science because of the important protective role that these macromolecules play in gastric physiology. The polymeric/colloidal behavior of mucins is complicated due to their large size (2 50 MDa) and complex structure with domains involving hydrophilic/hydrophobic, hydrogen bonds and electrostatic interactions, and their propensity to aggregate and form complexes with other polymers. These properties are also of direct relevance to the numerous diseases involving mucins, and to the problems of uptake of nutrients and delivering drugs through the mucus barrier. In this review we describe the current state of understanding of the relevant properties, with an emphasis on recent research. The findings described in this review are of direct relevance to the uptake of nutrients in digestion. D 2005 Published by Elsevier Ltd. Keywords: Mucin; Aggregation; Gelation; Gastric mucus; Mucoadhesion; Glycoprotein; Biorheology 1. Introduction Mucus is a complex viscous adherent secretion synthesized by specialized goblet cells in the columnar epithelium that lines all of the organs that are exposed to the external environment. This includes the respiratory tract, the gastrointestinal tract, the reproductive tract, and the oculo-rhino-otolaryngeal tracts. It serves many functions in those locations, among which are lubrication for the passage of objects, maintenance of a hydrated layer over the epithelium, a barrier to pathogens and noxious substances and as a permeable gel layer for the exchange of gases and nutrients with the underlying epithelium [1,2]. In addition to its protective functions, mucus is also involved in many disease processes. Mucus also is the first barrier with which nutrients and enteric drugs must interact and diffuse through, in order to be absorbed and gain access to the circulatory system and their target end organs. * Corresponding author. Tel.: addresses: rb@buphy.bu.edu (R. Bansil), bturner@caregroup.harvard.edu (B.S. Turner). 1 Tel.: Molecular biology and biochemical structure of mucins 2.1. Composition of mucus Mucus is composed primarily of water ( 95%), but also contains salts, lipids such as fatty acids, phospholipids and cholesterol [1], proteins which serve a defensive purpose such as lysozyme, immunoglobulins, defensins, growth factors and trefoil factors. However, the main component that is responsible for its viscous and elastic gel-like properties is the glycoprotein mucin Mucins Mucins are large, extracellular glycoproteins with molecular weights ranging from 0.5 to 20 MDa. Both membrane bound mucins, and secreted mucins [3&] share many common features. They are both highly glycosylated consisting of 80% carbohydrates primarily N-acetylgalactosamine, N-acetylglucosamine, fucose, galactose, and sialic acid (N-acetylneuraminic acid) and traces of mannose and sulfate. The oligosaccharide chains consisting of 5 15 monomers, exhibit moderate branching and are attached to the protein core by /$ - see front matter D 2005 Published by Elsevier Ltd. doi: /j.cocis
38 R. Bansil, B.S. Turner / Current Opinion in Colloid & Interface Science 11 (2006) O-glycosidic bonds to the hydroxyl side chains of serine and threonines and arranged in a bottle brush configuration about the protein core. The protein core, making up the remaining 20% of the molecular mass ( kda), is arranged into distinct regions. First, a central glycosylated region, which is comprised of a large number of tandem repeats that are rich in serine, threonine and proline (STP repeats), which can make up greater than 60% of the amino acids. Second, located at the amino and carboxy terminals, and sometimes interspersed between the STP-repeats, are regions with an amino acid composition more representative of globular proteins, relatively little O-glycosylation and a few N-glycosylation sites [4] and a high proportion of cysteine (> 10%). These cysteine rich regions contain domains that possess sequence similarity to von Willebrand factor (vwf) C and D domains, and C-terminal cystine knot domains [5&,6,7], and have been shown to be involved in dimerization via disulfide bond formation, and subsequent polymerization of the dimers to form multimers [8] (Fig. 1) Mucin genes Currently, approximately 19 mucin (designated MUC) genes have been identified cloned and partially sequenced in the human, and homologs to many of them have been identified in the mouse and rat [5&]. Only three MUC (MUC1, MUC2 and MUC5B) genes have been totally sequenced due to the large size of the central tandem repeats, which are difficult to accurately assemble. Many of the smaller membrane bound mucins are not considered true mucins since they only share the STP repeats and glycosylation with other mucins [3&]. Of the remaining true mucins (MUC1 7), the secreted mucins (MUC 2, 5AC, 5B and 6) are located in a cluster within 500 kb on the short arm of chromosome 11 (11p15) [9]. It is thought that they represent homologs that diverged from a common ancestor gene on chromosome 11 [10]. The STP-repeat sequences for each MUC gene are unique to each species, whereas the cys-rich regions share a large degree of similarity [5&]. Different collections of mucin genes are expressed in different tissues. Such repeats and super repeats are relatively uncommon in normal proteins. 3. Physical properties of mucin in dilute solution Mucin has been difficult to characterize, owing to its large molecular weight, polydispersity and high degree of glycosylation. Earlier biophysical studies, reviewed by Bansil et al. [11&] and Harding [12&] primarily using light scattering methods showed that mucin was a somewhat stiffened random coil with a radius of gyration around 100 nm. NMR studies of MUC1 [13,14&] revealed very little alpha helix, a small amount of beta and mostly random coil. The large size of mucin has, on the other hand, turned out to be of great advantage in imaging the molecule directly. Earlier transmission electron micrographic studies by Fiebrig et al. [15] revealed long fibers approximately 400 nm long in pig gastric mucin (PGM). More recent Atomic Force Microscopy (AFM) studies of ocular mucin [16&] show individual fibers with a broad distribution of contour lengths. While most of the fibers are between nm long, the tail of the distribution extends to 1500 nm. They also estimated a persistence length of about 36 nm from these images, which confirms the extended nature of the polymer. In another AFM study of ocular mucin, Brayshaw et al. [17] demonstrated the multimeric nature of mucin, by observing in situ depolymerization on treatment with DTT (dithiothreitol). Longer fibers, up to 2 Am in length, were observed in PGM by Deacon et al. [18]. McMaster et al. [19] examined ocular mucin using tapping Fig. 1. (a) A schematic drawing of the pig gastric mucin (PGM) monomer consisting of glycosylated regions flanked by regions with relatively little glycosylation. (b) The symbols indicate the different domains in the sketch in (a). (This representation is based in part on Figs. 1 and 2 of Dekker et al. [3 & ]). At the bottom of the figure we show (c) a dimer formed by two monomeric subunits linked via disulfide bonds in the non-glycosylated regions and in (d) dimers that are further disulfide linked to form higher multimers. This gives rise to the high molecular weight and polydispersity of secretory mucins. Polymers>16-mers have been described in MUC5AC from human airway secretions by Sheehan et al. [8]. (The bottom part of the figure is adapted from Fig. 8 of Ref. [8].) Reprinted with permission from [21].
39 166 R. Bansil, B.S. Turner / Current Opinion in Colloid & Interface Science 11 (2006) mode AFM under a buffer and observed regular variations in height along the length of the fiber which they interpret as glycosylated regions of the mucin molecules. Round et al. [20] correlated the conformations with differing amounts of glycosylation by imaging different fractions obtained on a CsCl gradient. However the sugar chains are too mobile in their solvated state to be seen fully extended in an AFM image, which requires a high degree of immobilization on the substrate, the AFM tip might have penetrated the brush without sensing it. AFM images of PGM which was about 50% deglycosylated showed that the deglycosylated portions re-folded forming compact globular structures [21]. Thus the sugars are important for maintaining the extended conformation of mucin. The conformation of mucin also depends on factors such as ph and ionic strength. For example, dynamic light scattering (DLS) studies [22] have shown that as the ph is lowered in dilute solutions (< 5 mg/ml) PGM undergoes a conformational change from an isotropic random coil (with end to end length of 390 nm and persistence length of 8 10 nm) at ph 7 to an anisotropic extended random coil (with end-to-end length of 490 nm, persistence length 43 nm) at ph 2. This is paralleled by an increase in the sedimentation coefficient from 11S at ph 7 to greater than 31S at ph 3 [23]. In a recent paper the conformation of commercially available PGM from Sigma in dilute solution examined by viscosity and circular dichroism measurements reveals similar changes as function of ph which are attributed to the unfolding of hydrophobic domains at low ph [24]. These authors also measured the zeta (1) potential of mucin and found that its isoelectric point lies between 2 and 3, suggesting that the charge on the molecule varies as ph is lowered. 4. Colloidal properties of mucin 4.1. Aggregation and gelation Mucins exhibit a tendency to aggregate and form gels. Taylor et al. [23] used rheological techniques to investigate the structure and formation of the pig gastric mucus gel and showed that both transient and non-transient interactions are responsible in maintaining the gel matrix. Purified mucin also has been shown to form gels. Human tracheobronchial mucin forms a gel below 30 -C at concentrations above 14 mg/ml [25]. Gelation was also observed in canine submaxillary mucin in the chaotropic (denaturing) solvent guanidine HCl [26]. These authors suggest that since non-covalent interactions are destabilized by guanidine HCl, gelation in these high molecular weight fractions involves the interpenetration of the carbohydrate side chains [26]. Our laboratory has focused on gelation of gastric mucin at low ph, which has implications for the physiologically relevant question of how the stomach is prevented from being digested by the acidic gastric juice it secretes. Bhaskar et al. [27] showed a reversible increase in viscosity of aqueous solutions of pig gastric mucin (PGM) at ph 2. DLS studies [22] show that at concentrations above 10 mg/ml gelation occurs below ph 4. At low phs we also observed an increase in the hydrophobicity of the protein core of PGM as indicated by its increased binding of the fluorescent hydrophobic dye 1-anilinonaphthyl-8-sulfonic acid (ANS). Atomic force microscopy images confirm that while PGM exists as single molecules at ph 6 (about 400 nm in length), it forms aggregates below ph 4 [21,28]. The viscosity increase and gelation are not observed if PGM is broken into its subunits (by treating with enzymes such as pronase or disulfide reduction with DTT) or salt concentration is increased. Commercial preparations of PGM available from Sigma chemicals (which include protease treatment during purification) also do not gel [28] Model for ph induced gelation of PGM This accumulated data showing a sharp increase in the observed changes at ph 4 and the lack of gelation in PGM which is broken into the subunits has led us to suggest that PGM gelation involves interactions of side chains of amino acids with pks around 4, such as Asp or Glu from the nonglycosylated regions of the molecule. At neutral ph of 6 7 these non-glycosylated Cys-rich regions of PGM are in conformations with hydrophobic domains hidden in folds of stabilized by salt bridges between negatively charged carboxylates and positively charged amino groups. At acidic ph 2 the carboxylates of the salt bridges are protonated, breaking the salt bridges and allowing the unfolding and exposure of hydrophobic regions of protein (Fig. 2). Circular dichroism studies reported by Lee et al. [24] on pig gastric mucin from Sigma also confirm the conformational transition at ph 4 and below. The hydrophobic domains on adjacent molecules are then able to associate acting as the crosslinks of a gel. Hydrophobic interactions among protein domains were also shown to be involved in aggregation of human tracheobronchial mucin [25]. The gelation of mucin is a complex problem, involving the interplay of electrostatic and hydrophobic interactions, somewhat reminiscent of the association of multiblock copolymers in solvents that have differential solubility to the component blocks. The entanglement of the sugar side chains further contributes to the high viscosity of mucin solutions (Fig. 2) Liquid crystalline properties of mucin Mucin glycoprotein has also been shown to exhibit liquid crystalline order. Viney, et al. [30] had shown that slug mucin forms nematic liquid crystals. A detailed study of the temperature and concentration dependence of the liquid crystalline behavior of commercially available mucin from Sigma was reported by Davis et al. [31] who suggest that the interactions of interdigitated sugar side chains are responsible for the nematic behavior. Waigh et al. [32] confirmed these observations by neutron scattering, comparing Sigma mucin at high concentrations in the presence and absence of an external magnetic field to produce orientation. Purified PGM, on the other hand, did not show this liquid crystalline orientation, suggesting that the Sigma mucin, which consists of a single mucin monomer, is more rigid and easier to orient than the multimeric form of native PGM in which the non-glycosylated
40 R. Bansil, B.S. Turner / Current Opinion in Colloid & Interface Science 11 (2006) Fig. 2. A model showing how the breaking of electrostatic interactions around ph 4 and below can produce a conformational change in PGM from a random coiltoa rod (or stiff worm-like chain) and lead to gelation at high concentrations at low ph by exposing hydrophobic regions which were folded and thus sequestered in the interior at neutral ph. [Reproduced from Ref. [29], PhD dissertation of Xingxiang Cao, Boston University, 1997, with permission of the author]. portions serve as flexible links between the rigid, glycosylated, monomeric domains Adhesive properties of mucin The well know tendency of other substances to adhere to mucin, known as mucoadhesivity, is not surprising given that this glycoprotein exhibits electrostatic, hydrophobic, and H- bonding interactions [33]. By the same token the molecule can be anti-adhesive, for example to negatively charged species. Thus mucin coated AFM tips adhered to mica in presence of divalent cations [34] but did not adhere to mica coated with mucin, presumably due to the polyelectrolytic charge repulsion. Bovine submaxillary mucin from Sigma could be adsorbed to hydrophobic polystyrene surface rendering it hydrophilic [35]. Mucin lipid interactions are well known, reflecting the presence of hydrophobic domains [36]. In this context, it is worth noting that since mucin is negatively charged it binds to positive ions. For example, Chromium III binding has been shown to cause significant conformational changes and it can lead to aggregation of mucin [37] Diffusion of macromolecules and particles and fluid flow through mucin In view of the protective function of mucus studying the diffusion of other molecules through it is of considerable physiological interest. It is also an important factor in the design of drugs which have to diffuse through the mucus layer, or kept from entering it. While many small molecules diffuse readily through mucus, the diffusion of larger particles depends both on size and muco-adhesive interactions. The diffusion of latex particles and lipid vesicles in purified gall bladder mucin has been examined using DLS [38] and fluorescence recovery after photobleaching [39] methods. Olmsted et al. [40] showed that while many small viruses ( nm) could diffuse through cervical mucus, others such as the Herpes Simplex virus got stuck to the mucus. Celli et al. [41] have exploited the movement of latex particles through mucin solutions to measure its rheological properties as a function of ph using microscope based DLS. Finally, it is worth noting that the transport of water and other fluids through mucus and purified mucin solutions is a very interesting problem in fluid dynamics. Due to its high viscosity, water and other low viscosity fluids injected under a pressure gradient across an unstirred mucin solution do not simply diffuse, but are transported by a viscous fingering mechanism [42,43]. This transport of HCl through channels produced by the viscous fingering mechanism has also been demonstrated in vivo in rats [44]. The mucus in the immediate vicinity of the surface of the acid channel will gel, thereby confining the acid in a tube. Similarly the mucus layer on the luminal surface of the stomach will gel as the acid is emptied into the lumen. The negatively charged mucin gel prevents back diffusion of H + via the mechanism of Donnan equilibrium.
41 168 R. Bansil, B.S. Turner / Current Opinion in Colloid & Interface Science 11 (2006) Biomedical function and applications 5.1. Mucus and disease The physical state of the mucus, change in the concentration of secreted mucin, and the strong dependence of its physicochemical properties on environmental factors such as ionic strength and ph play an important role in many diseases. For example, besides serving as a barrier to bacteria, many bacteria reside within the mucus and possess specific adhesins that specifically bind to it [45]. This includes pathogenic strains of Pseudomonas, Streptococcus and Pneumococcus. Helicobacter pylori particularly resides in the mucus layer of the stomach, and is a common cause of ulcers [46]. Some parasitic organisms also produce their own layers of mucus to evade the immune system [47]. Second, overproduction of mucus is involved in cystic fibrosis [48&], bronchitis, asthma [49] and in middle ear infections, and mucus gels serve as the matrix in which gallstones are nucleated and grow [50]. Thirdly, mucus underproduction is present in dry eye syndromes [51&] and in some forms of ulcer disease. Finally, mucus expression and composition is altered in cancers of epithelial origin [52&&] Mucus and pharmacology Mucus is the first barrier with which nutrients and enteric drugs must interact and diffuse through, in order to be absorbed and gain access to the circulatory system and their target end organs. There is great interest in methods to optimize these socalled muco-adhesive interactions for improved drug delivery. Various molecular interactions have been exploited to enhance mucoadhesion, including, polyelectrolytic interactions (chitosans, poly-acrylic acid, etc.) hydrogen bonds (hydrogels), [33] and disulfide binding (thiomers) [53]. Efforts are underway to design ph sensitive drug carriers such as gels which will not release the drug in the acidic environment of the stomach but will do so in the basic environment of the intestine and colon. In this context, we emphasize recent studies [54] which show that the resting stomach has a ph close to 4 which drops to 2 during active acid secretion. Mucin can be used as a high molecular weight additive to improve the adherence of artificial tear drops in treating dry eye syndrome [55]. Efforts to develop nanoparticles for mucosal DNA vaccines and gene therapy are also being considered [56]. The abnormality of the sugars in cell membrane bound mucins from cancerous cells has also been targeted as a potential for cancer vaccine development. Excellent reviews of oral mucosal drug delivery are collected in Ref. [57&]. 6. Conclusions We have reviewed recent, as well as some pertinent older literature describing the biophysical properties of mucin in solution and its properties of interest in colloid science. The picture that emerges is that of a macromolecule with a complex organization into domains with different structures. From the polymeric viewpoint mucin can be considered as a multiblock copolymer with alternating polyelectrolytic domains having a grafted sugar brush, connected by flexible regions with less glycosylation and the tendency to form multimers. The molecule has both hydrophobic and hydrophilic regions with the ability to form H-bonds, and electrostatic interactions. This wide range of interactions causes it to aggregate gel and form mucoadhesive interactions with other substances. These physical properties are of direct relevance to the physiological functions of mucus in normal and diseased states. An understanding these properties is of considerable current interest because of the many biomedical applications. [Note: Topics that are not covered in this review such as mucin glycosylation [58], signal transduction [59], and secretion [60], can be found in the indicated references.] Acknowledgements The authors would like to thank Dr. Nezam H. Afdhal, Dr. K. Ramakrishnan Bhaskar, Dr. XingXiang Cao, Prof. Bernard Chasan, and Dr. Zhenning Hong for our long-standing collaboration on studies of mucin. Our research on mucin has been supported by grants from NIH (P.I. Dr. N.H. Afdhal) and NSF (P.I. R.B.). References and recommended readings [1] Allen A. Structure and function of gastrointestinal mucus. In: Johnson L, editor. Physiology of the gastroenterology tract, 1st edn. New York, NY Raven Press; p [2] Neutra M, Forstner J. Gastrointestinal mucus: synthesis, secretion, and function. In: Johnson L, editor. Physiology of the gastrointestinal tract, 2nd edn. New York, NY Raven Press; Chapter 34. [3 & ] Dekker J, Rossen J, Büller H, Einerhand A. The MUC family: an obituary. Trends Biochem Sci 2002;27(3): Review article discussing the controversies concerning the defining characteristics of the MUC family. Contains clear schematic illustrations of the two major groups of mucins and their structural domains. [4] Bell S, Xu G, Khatri I, Wang R, Rahman S, Forstner J. N-linked oligosaccharides play a role in disulphide-dependent dimerization of intestinal mucin Muc2. Biochem J 2003;373(Pt 3): [5 & ] Perez-Vilar J, Hill R. The structure and assembly of secreted mucins. J Biol Chem 1999;274(45): Review article which contains numerous sequence alignments between the various homologous regions of the different mucin genes. [6] Turner B, Bhaskar K, Hadzopoulou-Cladaras M, LaMont J. Cysteine-rich regions of pig gastric mucin contain von Willebrand factor and cystine knot domains at the carboxyl terminal. Biochim Biophys Acta 1999; 1447(1): [7] Bell S, Xu G, Forstner J. Role of the cystine-knot motif at the C-terminus of rat mucin protein Muc2 in dimer formation and secretion. Biochem J 2001;357(Pt1): [8] Sheehan J, Kirkham S, Howard M, Woodman P, Kutay S, Brazeau C, et al. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J Biol Chem 2004;279(15): [9] Pigny P, Guyonnet-Duperat V, Hill A, Pratt W, Galiegue-Zouitina S, D Hooge M, et al. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics 1996; 38(3): & Of special interest. && Of outstanding interest.
42 R. Bansil, B.S. Turner / Current Opinion in Colloid & Interface Science 11 (2006) [10] Desseyn J, Aubert J, Porchet N, Laine A. Evolution of the large secreted gel-forming mucins. Mol Biol Evol 2000;17(8): [11 & ] Bansil R, Stanley E, LaMont J. Mucin biophysics. Annu Rev Physiol 1995;57: This review describes the various biophysical methods that have been used to elucidate mucin structure and function. [12 & ] Harding SE. The macrostructure of mucus glycoproteins in solution. Adv Carbohydr Chem Biochem 1989;47: Excellent review of the solution properties of mucins. [13] Fontenot J, Tjandra N, Bu D, Ho C, Montelaro R, Finn O. Biophysical characterization of one-, two-, and three-tandem repeats of human mucin (muc-1) protein core. Cancer Res 1993;53(22): [14 & ] Otvos L, Cudic M. Conformation of glycopeptides. Mini Rev Med Chem 2003;3(7): A review article that describes the biophysical and computational techniques that have been employed to determine the structures of the glycosylated regions of mucins and other glycoproteins. [15] Fiebrig I, Harding S, Rowe A, Hyman S, Davis S. Transmission electron microscopy studies on pig gastric mucin and its interactions with chitosan. Carbohydr Polym 1995;28(3): [16 & ] Round A, Berry M, McMaster T, Stoll S, Gowers D, Corfield A, et al. Heterogeneity and persistence length in human ocular mucins. Biophys J 2002;83(3): Research article which uses atomic force microscopy in order to obtain a measure of the sizes and size distributions of mucin molecules. [17] Brayshaw D, Berry M, McMaster T. Reducing a polymer to its subunits as an aid to molecular mapping. Nanotechnology 2004;15(11): [18] Deacon M, McGurk S, Roberts C, Williams P, Tendler S, Davies M, et al. Atomic force microscopy of gastric mucin and chitosan mucoadhesive systems. Biochem J 2000;348(Pt 3): [19] McMaster T, Berry M, Corfield A, Miles M. Atomic force microscopy of the submolecular architecture of hydrated ocular mucins. Biophys J 1999;77(1): [20] Round A, Berry M, McMaster T, Corfield A, Miles M. Glycopolymer charge density determines conformation in human ocular mucin gene products: an atomic force microscope study. J Struct Biol 2004;145(3): [21] Hong Z, Chasan B, Bansil R, Turner B, Bhaskar K, Afdhal N. Atomic force microscopy reveals aggregation of gastric mucin at low ph. Biomacromolecules 2005;6(6): [22] Cao X, Bansil R, Bhaskar K, Turner B, LaMont J, Niu N, et al. phdependent conformational change of gastric mucin leads to sol gel transition. Biophys J 1999;76(3): [23] Taylor C, Allen A, Dettmar P, Pearson J. The gel matrix of gastric mucus is maintained by a complex interplay of transient and nontransient associations. Biomacromolecules 2003;4(4): [24] Lee S, Müller M, Rezwan K, Spencer N. Porcine Gastric Mucin (PGM) at the Water/Poly(dimethylsiloxane) (PDMS) Interface: influence of ph and ionic strength on its conformation, adsorption, and aqueous lubrication properties. Langmuir 2005;21(18): [25] Bromberg L, Barr D. Self-association of mucin. Biomacromolecules 2000;1(3): [26] McCullagh C, Gupta R, Jamieson A, Blackwell J. Gelation of fractionated canine submaxillary mucin in a chaotropic solvent. Int J Biol Macromol 1996;18(4): [27] Bhaskar K, Gong D, Bansil R, Pajevic S, Hamilton J, Turner B, et al. Profound increase in viscosity and aggregation of pig gastric mucin at low ph. Am J Physiol 261(5 Pt 1):G [28] Koèevar-Nared J, Kristl J, Šmid-Korbar J. Comparative rheological investigation of crude gastric mucin and natural gastric mucus. Biomaterials 1997;18(9): [29] Cao X. Aggregation and gelation of mucin and its interactions with lipid vesicles. Ph. D Dissertation, Boston University, Boston MA [30] Viney C, Huber A, Verdugo P. Liquid-crystalline order in mucus. Macromolecules 1993;26(4): [31] Davies J, Viney C. Water-mucin phases: conditions for mucus liquid crystallinity. Thermochim Acta 1998;315(1): [32] Waigh T, Papagiannopoulos A, Voice A, Bansil R, Unwin A, Dewhurst C, et al. Entanglement coupling in porcine stomach mucin. Langmuir 2002;18(19): [33] Harding S, Davis S, Deacon M, Fiebrig I. Biopolymer mucoadhesives. Biotechnol Genet Eng Rev 1999;16: [34] Berry M, McMaster T, Corfield A, Miles M. Exploring the molecular adhesion of ocular mucins. Biomacromolecules 2001;2(2): [35] Shi L, Caldwell K. Mucin adsorption to hydrophobic surfaces. J Colloid Interface Sci 2000;224(2): [36] Rogunova M, Blackwell J, Jamieson A, Pasumar-Thy M, Gerken T. Effects of lipids on the structure and rheology of gels formed by canine submaxillary mucin. Biorheology 1997;34(4 5): [37] Shrivastava H, Nair B. Structural modification and aggregation of mucin by chromium (III) complexes. J Biomol Struct Dyn 2003;20(4): [38] Cao X, Bansil R, Gantz D, Moore EW, Niu N, Afdhal N. Diffusion behavior of lipid vesicles in entangled polymer solutions. Biophys J 1997; 73(4): [39] Afdhal NH, Cao X, Bansil R, Hong Z, Thompson C, Brown B, et al. Interaction of mucin with cholesterol enriched vesicles: role of mucin structural domains. Biomacromolecules 2004;5(2): [40] Olmsted S, Padgett J, Yudin A, Whaley K, Moench T, Cone R. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J 2001;81(4): [41] Celli J, Gregor B, Turner B, Afdhal N, Bansil R, Erramilli S. Viscoelastic properties and dynamics of porcine gastric mucin. Biomacromolecules 2005;6(3): [42] Bhaskar K, Garik P, Turner B, Bradley J, Bansil R, Stanley H, et al. Viscous fingering of HCl through gastric mucin. Nature 1992;360(6403): [43] Fujita T, Ohara S, Sugaya T, Saigenji K, Hotta K. Effects of rabbit gastrointestinal mucins and dextran on hydrochloride diffusion in vitro. Comp Biochem Physiol B 2000;126(3): [44] Johansson M, Synnerstad I, Holm L. Acid transport through channels in the mucous layer of rat stomach. Gastroenterology 2000;119: [45] Scharfman A, Lamblin G, Roussel P. Interactions between human respiratory mucins and pathogens. Biochem Soc Trans 1995;23(4): [46] Slomiany B, Slomiany A. Role of mucus in gastric mucosal protection. J Physiol Pharmacol 1991;42(2): [47] Jain M, Karan D, Batra S, Varshney G. Mucins in protozoan parasites. Front Biosci 2001;6:D [48] Boucher R. New concepts of the pathogenesis of cystic fibrosis lung & disease. Eur Respir J 2004;23(1): Review article of the role of many factors such as ions, bacterial infection, fluid dynamics and their relationship to and effect on mucus in cystic fibrosis. [49] Rose M. Mucins: structure, function and role in pulmonary diseases. Am J Physiol 1992;263(4 Pt 1):L [50] Smith B, LaMont J: The Pathogenesis of Gallstones. Hospital Practice (12): 93 99, [51] Argüeso P, Gipson I. Epithelial mucins of the ocular surface: structure, & biosynthesis and function. Exp Eye Res 2001;73(3): Review of the basic elements of mucin structure and regulation with special reference to the surface of the eye in the normal and disease states. [52] Hollingsworth M, Swanson B. Mucins in cancer: protection and control && of the cell surface. Nat Rev Cancer 2004;4(1): An excellent comprehensive review of both secreted and membrane mucins, their possible functions in signal transduction. It also compares the roles of mucins in normal and cancerous states and also contains very informative illustrations. [53] Leitner V, Walker G, Bernkop-Schnurch A. Thiolated polymers: evidence for the formation of disulphide bonds with mucus glycoproteins. Eur J Pharm Biopharm 2003;56(2): [54] Baumgartner H, Montrose M. Regulated alkali secretion acts in tandem with unstirred layers to regulate mouse gastric surface ph. Gastroenterology 2004;126(3): [55] Khanvilkar K, Donovan M, Flanagan D. Drug transfer through mucus. Adv Drug Deliv Rev 2001;48:
43 170 R. Bansil, B.S. Turner / Current Opinion in Colloid & Interface Science 11 (2006) [56] Dawson M, Krauland E, Wirtz D, Hanes J. Transport of polymeric nanoparticle gene carriers in gastric mucus. Biotechnol Prog 2004;20(3): [57 & ] Rathbone MJ, editor. Oral mucosal drug delivery. Marcel Dekker Inc; This book has excellent contributions by many authors and provides a comprehensive review of the issues related to mucus in oral drug delivery. [58] Van den Steen P, Rudd P, Dwek R, Opdenakker G. Concepts and priciples of O-linked glycosylation. Crit Rev Biochem Mol Biol 1998; 33(3): [59] Basbaum C, Lemjabbar H, Longphre M, Li D, Gensch E, McNamara N. Control of mucin transcription by diverse injury-induced signaling pathways. Am J Respir Crit Care Med 1999;160: [60] Verdugo P. Goblet cells secretion and mucogenesis. Annu Rev Physiol 1990;52:
44 Mucins in the mucosal barrier to infection. Linden et al, Nature Immunology 1: 183 (2008)
45 nature publishing group REVIEW Mucins in the mucosal barrier to infection SK L ind en 1, P Sutton 2, NG Karlss on 3, V Koroli k 4 and MA McGu ck in 1 The mucosal tissues of the gastrointestinal, respiratory, reproductive, and urinary tracts, and the surface of the eye present an enormous surface area to the exterior environment. All of these tissues are covered with resident microbial flora, which vary considerably in composition and complexity. Mucosal tissues represent the site of infection or route of access for the majority of viruses, bacteria, yeast, protozoa, and multicellular parasites that cause human disease. Mucin glycoproteins are secreted in large quantities by mucosal epithelia, and cell surface mucins are a prominent feature of the apical glycocalyx of all mucosal epithelia. In this review, we highlight the central role played by mucins in accommodating the resident commensal flora and limiting infectious disease, interplay between underlying innate and adaptive immunity and mucins, and the strategies used by successful mucosal pathogens to subvert or avoid the mucin barrier, with a particular focus on bacteria. MUCINS AN INTEGRAL PART OF THE MUCOSAL BARRIER Mucosal epithelial tissues have evolved multiple mechanisms of defense in response to their vulnerability to microbial attack due to their exposure to the external environment. The mucosal epithelial cells form a contiguous lining that acts as a barrier between the moist exterior environment and the remainder of the host. In addition, these cells, both constitutively and in response to microbes, together with underlying leukocytes, secrete many defensive compounds into the mucosal fluid, including mucins, antibodies, defensins, protegrins, collectins, cathlecidins, lysozyme, histatins, and nitric oxide. 1 3 Together, these different defensive compounds form a physical barrier and have direct antimicrobial activity, and the ability to opsonize microbes to aid clearance. Mucin glycoproteins, however, can fulfill all of these roles individually. Mucosal pathogens, almost by definition, have evolved mechanisms to subvert these mucosal defensive measures. The first barrier the pathogen encounters is the highly hydrated mucus gel that covers the mucosal surface and protects the epithelial cells against chemical, enzymatic, microbial, and mechanical insult. Mucosal surfaces are coated with a layer of viscous mucus ranging in thickness from 10 μ m in the eye 4 and trachea 5 t o 300 μ m in the stomach and 700 μ m in the intestine. 6 8 This mucus layer is not static but moves to clear trapped material. In the gastrointestinal tract, the outer mucus layer is continually removed by movement of the luminal contents, whereas in the respiratory tract cilia drive its movement. Mucin glycoproteins produced by mucus-producing cells in the epithelium or submucosal glands are the major macromolecular constituent of mucus and are responsible for the viscous properties of the mucus gel. In addition to forming a relatively impervious gel, which acts as a lubricant, a physical barrier, and a trap for microbes, mucus provides a matrix for a rich array of antimicrobial molecules. Underneath the mucus layer, the cells present a dense forest of highly diverse glycoproteins and glycolipids, which form the glycocalyx. Membrane-anchored cell-surface mucin glycoproteins are a major constituent of the glycocalyx in all mucosal tissues. The glycocalyx is highly variable from tissue to tissue; for example, the glycocalyx of human intestinal microvilli tips is thick ( nm) in comparison with the glycocalyx of the lateral microvilli surface (30 60 nm). 9,10 The oligosaccharide moieties of the molecules forming the glycocalyx and the mucus layer are highly diverse, and the average turnover time of the human jejunal glycocalyx is 6 12 h. 11 Consequently, both the secreted and adherent mucosal barriers are constantly renewed and could potentially be rapidly adjusted to changes in the environment, for example, in response to microbial infection. MUCIN BIOSYNTHESIS AND STRUCTURE The tremendous energy investment by mucosal tissues in the production of mucins in the basal state, but particularly in response to infection, is testimony to the importance of these 1 Mucosal Diseases Program, Mater Medical Research Institute and The University of Queensland, Level 3 Aubigny Place, Mater Hospitals, South Brisbane, Queensland, Australia. 2 Centre for Animal Biotechnology, School of Veterinary Science, University of Melbourne, Melbourne, Victoria, Australia. 3 Centre for BioAnalytical Sciences, Department of Chemistry, National University of Ireland, Galway, Ireland. 4 Institute for Glycomics, Griffith University, Gold Coast, Queensland, Australia. Correspondence: MA McGuckin ( mmcguckin@mmri.mater.org.au ) Received 27 November 2007; accepted 16 January 2008; published online 5 March doi: /mi MucosalImmunology VOLUME 1 NUMBER 3 MAY
46 REVIEW glycoproteins. Epithelial mucins are a heterogenous family of large complex glycoproteins containing a dense array of O -linked carbohydrates typically comprising over 70 % of their mass. These glycans are concentrated in large peptide domains of repeating amino-acid sequences rich in serine and threonine. The size and number of repeats vary between the mucins, and in many of the genes there are genetic polymorphisms in the number of repeats (variable number of tandem repeats or VNTR polymorphisms), which means the size of individual mucins can differ substantially between individuals. Each mucin is thought to form a filamentous protein carrying typically 100s of complex oligosaccharide structures, 12 giving the mucin a bottle-brush appearance. To date, at least 16 human mucins have been included in the family, and the expression profile of mucins varies between tissues with the gastrointestinal tract showing the highest and most diverse expression (see Table 1 ). Mucins can be divided into three distinct subfamilies: (a) secreted gel-forming mucins, (b) cell-surface mucins, and (c) secreted non-gel-forming mucins ( Table 1 ). Gel-forming mucins, which are the major constituent of mucus and confer its viscoelastic properties, are encoded by a cluster of four highly related genes on chromosome and a similar gene on chromosome Gel-forming mucins contain N- and C-terminal cysteine-rich domains that are both involved in homo-oligomerization mediated by inter-molecular disulfide bonds. 17,18 The current model for mucin oligomerization is that dimerization occurs rapidly during biosynthesis in the endoplasmic reticulum preceding or concomitant with N -glycosylation but before O -glycosylation in the Golgi apparatus, which in turn is followed by multimerization of dimers. 19 Oligomerization is likely to produce either extended filamentous structures or, more probably, web-like molecular structures likely to be critical to the rheological properties of the mucus gel The extended conformation caused by dense glycosylation enables the molecules to occupy large volumes, with the secreted oligomeric mucins occupying volumes equivalent to those of small bacteria. 25 The secreted non-oligomerizing mucins include the MUC7 salivary mucin, which can self-aggregate but is not thought to contribute significantly to mucus properties, and the MUC8 respiratory mucin, which has not been fully characterized to date. There are 11 known genes encoding cell-surface mucins expressed by a wide diversity of mucosal tissues, with substantial redundancy in many tissues (see Table 1 ). Cell-surface mucins are present on the apical membrane of all mucosal epithelial cells and contain large extracellular VNTR domains predicted to form rigid elongated structures. Together with their high expression, this indicates that these molecules are likely to be a prominent, probably dominating, constituent of the glycocalyx and may provide a barrier that limits access of other cells and large molecules to the cell surface. During synthesis, most cell-surface mucins appear to be cleaved into two subunits in a region known as an SEA module, which is often flanked by epidermal growth factor-like domains. 26 Structural studies of the MUC1 SEA module suggest that the cleavage occurs via autoproteolysis and that the two subunits remain non-covalently associated throughout biosynthesis. 27 However, importantly, the Table 1 Tissue distribution of mucins Mucin Distribution References Secreted gel forming MUC2 MUC5AC MUC5B MUC6 MUC19 Small intestine, colon, respiratory tract, eye, middle ear epithelium Respiratory tract, stomach, cervix, eye, middle ear epithelium Respiratory tract, salivary glands, cervix, gallbladder, seminal fluid, middle ear epithelium Stomach, duodenum, gallbladder, pancreas, seminal fluid, cervix, middle ear epithelium Sublingual gland, submandibular gland, respiratory tract, eye, middle ear epithelium Secreted non-gel forming (monomeric) MUC7 Cell surface MUC1 MUC3A/B MUC4 MUC12 MUC13 MUC15 MUC16 MUC17 MUC20 Salivary glands, respiratory tract, middle ear epithelium Stomach, breast, gallbladder, cervix, pancreas, respiratory tract, duodenum, colon, kidney, eye, B cells, T cells, dendritic cells, middle ear epithelium Small intestine, colon, gall bladder, duodenum, middle ear epithelium Respiratory tract, colon, stomach, cervix, eye, middle ear epithelium Colon, small intestine, stomach, pancreas, lung, kidney, prostate, uterus Colon, small intestine, trachea, kidney, appendix, stomach, middle ear epithelium spleen, thymus, prostate, testis, ovary, small intestine, colon, peripheral blood leukocyte, bone marrow, lymph node, tonsil, breast, fetal liver, lungs, middle ear epithelium Peritoneal mesothelium, reproductive tract, respiratory tract, eye, middle ear epithelium Small intestine, colon, duodenum, stomach, middle ear epithelium Kidney, placenta, colon, lung, prostate, liver, middle ear epithelium ,236, ,243, ,235, ,249, , ,243, 257, ,255, ,262 35,235, , , , ,269 extracellular α -subunit can be shed from the cell surface either mediated via a second distinct cleavage event 28,29 or p e r h ap s via physical shear forces separating the two domains at the original cleavage site as suggested by Macao et al. 27 Mut at i on 184 VOLUME 1 NUMBER 3 MAY
47 REVIEW of the cleavage site inhibits MUC1 shedding in transfected mammary and respiratory epithelial cells without affecting cell surface expression, indicating the importance of the initial cleavage in shedding. 30 Furthermore, most cell-surface mucin genes appear to undergo alternative splicing and also encode directly secreted isoforms lacking transmembrane and cytoplasmic domains These isoforms are stored in subapical granules and in goblet cell thecae and are secreted both constitutively and following stimuli. Consequently, due to shedding and direct secretion, cell-surface mucins can also be seen as components of secreted mucus. 35 MUC1 is the most extensively studied membrane-associated mucin and is the most ubiquitously expressed across all mucosal tissues. MUC1 has been estimated to be nm in length (depending on the number of tandem repeats), suggesting it will tower above other molecules attached to the plasma membrane. 36 MUC1 associated with the cell surface is constantly internalized (0.9% of the surface fraction min 1 ) and recycled. 37 Internalization occurs by clathrin-mediated endocytosis, and alterations in O- glycan structure stimulate endocytosis and intracellular accumulation. 38 During recycling, sialic acid is added to the premature form of MUC1. 37 Complete sialylation requires several rounds of recycling, one cycle taking approximately 2.5 h. 37 Pulse-chase experiments indicate that the half-life of MUC1 in the plasma membrane is h, 37,39 suggesting that the average MUC1 molecule recycles up to 10 times before release. 37 Recycling rates vary between cell lines and possibly environmental conditions and have not been measured in non-transformed cells, making it very difficult to extrapolate to the real rate of recycling and cell-surface half-life in vivo. T he cytoplasmic tail appears to interact with the cytoskeleton and secondary signaling molecules, whereas the extracellular domains of MUC1 and other cell-surface mucins interact with extracellular matrix components and other cells The cytoplasmic domains of the cell surface mucins are complex, often contain known phosphorylation motifs, and are highly conserved across species, suggesting important intracellular functions. The best-studied mucin in this regard is MUC1, which has been explored mainly in terms of its role in cancer cell biology rather than in mucosal defense. We and others have shown phosphorylation of the MUC1 cytoplasmic domain 41,48 52 as well as molecular association with β -catenin, 41,51 linking MUC1 with the Wnt pathway, which is involved in epithelial growth, migration, and wound repair. More recently, it has been shown that the cytoplasmic domain can be cleaved and that the cleaved domain translocates to mitochondria and, together with the p53 transcription factor, to the nucleus, where it modulates the cell cycle and protects against the apoptotic response to genotoxic stress. 53,54 Some pathogenic bacteria produce genotoxins, and thus this protective effect, first identified in cancer cells, may have evolved as part of the natural epithelial defense against microbial genotoxins. We have recently shown that in vitro MUC1 protects p53-expressing epithelial cells from the effects of cytolethal distending toxin, a genotoxin produced by Campylobacter jejuni. 55,56 In vivo, C. jejuni more densely colonized the stomachs of Muc1 / mice, but this effect was not seen in isogenic mutants lacking cytolethal distending toxin, indicating that Muc1 lowers gastric colonization at least in part via inhibiting the activity of cytolethal distending toxin. 55 Many of the other cell-surface mucins also contain potential phosphorylation sites and cleavage motifs in the immediate intracellular region of their cytoplasmic domains and may be similarly cleaved. Importantly, there is also evidence that interaction with bacteria can induce phosphorylation of MUC1 in vitro. 57 Signaling by the cytoplasmic domains of cell-surface mucins is complex and much remains to be elucidated about their mode of action. However, the evidence to date suggests that these domains are involved in cellular programs regulating growth and apoptosis in mucosal cells perhaps in response to microbes and / or their toxins. MUCIN GLYCOSYLATION The carbohydrate structures present on mucosal surfaces vary according to cell lineage, tissue location, and developmental stage. Evidence is emerging that mucin glycosylation can alter in response to mucosal infection and inflammation, and this may be an important mechanism for unfavorably changing the niche occupied by mucosal pathogens. The extensive O- glycosylation of the mucins protects them from proteolytic enzymes and induces a relatively extended conformation. 25 The oligosaccharides on secreted mucins are clustered into heavily glycosylated domains (typically 600 1,200 amino acids long) separated by shorter nonglycosylated regions. 25 The O- linked glycans contain 1 20 residues, which occur both as linear and branched structures (see Table 2 ). The carbohydrate chain is initiated with an N- acetylgalactosamine (GalNAc) linked to serine or threonine and is elongated by the formation of the so-called core structures followed by the backbone region (type-1 and type-2 chains). The chains are typically terminated by fucose (Fuc), galactose (Gal), GalNAc, or sialic acid residues in the peripheral region, forming histo-blood-group antigens such as A, B, H, Lewis-a (Le a ), Lewis-b (Le b ), Lewis-x (Le x ), Lewis-y (Le y ), as well as sialyl-le a and sialyl-le x structures. Sulfation of Gal and N- acetylglucosamine (GlcNAc) residues causes further diversification. In addition to the O -linked glycans, mucins contain a smaller number of N -linked oligosaccharides, which have been implicated in folding, oligomerization (MUC2), or surface localization (MUC17) The carbohydrate structures present on mucins are determined by the expression of specific glycosyl transferases. Thus, mucin glycosylation is governed by genetics (due to polymorphisms in these enzymes), tissue-specific enzyme expression, and host and environmental factors influencing transferase expression. As an example of the impact of host genotype, the H type-1 structure is made by the secretor (Se) gene product, and the majority (80% of Caucasians, all South American Indians and Orientals) carry this structure and are thus referred to as s e c re tors. 61 Individuals may also express the Lewis (Le) gene (90% of the Caucasian population) and, provided that they are also secretors, will modify H type-1 into the Le b st r u c tu re. 61,62 If they are nonsecretors, type-1 chains without its blood group antigen H will be turned into Le a structures. 61,62 The third MucosalImmunology VOLUME 1 NUMBER 3 MAY
48 REVIEW Table 2 Common O -linked oligosaccharide structures on mucins Nomenclature Core type Core 1 Structure -Gal β 1-3GalNAc α 1-Ser/Thr Core 2 -Gal β 1-3(-GlcNAc β 1-6)GalNAc α 1- Ser/Thr Core 3 -GlcNAc β 1-3GalNAc α 1-Ser/Thr Core 4 -GlcNAc β 1-3(GlcNAc β 1-6)GalNAc α 1- Ser/Thr N-Acetyllactosamine elongation type Type 1 -Gal β 1-3GlcNAc β 1- Type 2 -Gal β 1-4GlcNAc β 1- Branching i-antigen -Gal β 1-4GlcNAc β 1-3Gal β 1- (unbranched) I-antigen -Gal β 1-4GlcNAc β 1-3(-Gal β 1-4GlcNAc β 1-6)Gal β 1- (branched) Terminal structures Blood group H Fuc α 1-2Gal β 1- Blood group A Fuc α 1-2(GalNAc α 1-3)Gal β 1- Blood group B Fuc α 1-2(Gal α 1-3)Gal β 1- Terminal structures (Type 1 based) Lewis a (Le a ) Gal β 1-3(Fuc α 1-4)GlcNAc β 1- Lewis b (Le b ) Fuc α 1-2Gal β 1-3(Fuc α 1-4)GlcNAc β 1- (includes H) Sialyl-Le a NeuAc( α 2-3)Gal β 1-3(Fuc α 1-4) GlcNAc β 1- Terminal structures (Type 2 based) Lewis x (Le x ) Gal β 1-4(Fuc α 1-3)GlcNAc β 1- Lewis y (Le y ) Fuc α 1-2Gal β 1-4(Fuc α 1-3)GlcNAc β 1- (includes H) Sialyl-Le x NeuAc α 2-3 Gal β 1-4(Fuc α 1-3) GlcNAc β 1- Sulfation 3 Sulfation HSO 3-3Gal β 1-6 Sulfation HSO 3-6GlcNAc β 1- Examples of combined epitopes H-type 1 Fuc α 1-2Gal β 1-3GlcNAc β 1- Sialylated type 2 NeuAc α 2-3Gal β 1-4GlcNAc β 1- Se phenotype with simultaneous expression of Le a a n d L e b antigens has been described as the weak-secretor (Se w ) phenotype. 63,64 The dual expression of Le a / L e b is a cons e quence of an enzymatically weak Se-transferase in combination with an intact Le-transferase. 63,64 The terminal structures of mucin oligosaccharides are highly heterogeneous and vary between / within species and between and even within tissues. The array of oligosaccharide structures on individual mucin molecules is also somewhat determined by stochastic events as the mucin protein moves through the Golgi apparatus. 65 This structural diversity may allow the mammalian host to cope with diverse and rapidly changing pathogens, as reflected by the observation that susceptibility to specific pathogens differs between people with different histo-blood groups, 66 as exemplified by the observations that individual Se phenotype may determine the ratio of infection as well as the course and severity of urinary tract infections, Norwalk virus induced acute gastroenteritis and Helicobacter pylori -induced gastric diseases There is also a strong correlation between distinct adhesive properties of H. pylori endemic in specific human populations and the mucin blood group carbohydrate structures expressed by these populations. 70 These differences in the external barrier to infection can be equated with the diversity in underlying innate and adaptive immunity (e.g., polymorphisms in MHC, cytokines), which is thought to have evolved for the same reasons. REGULATION AND MODULATION OF THE MUCIN BARRIER The gel-forming mucins are produced by cells in the epithelial surface and / or by glands located in the submucosal connective tissues, and secretion occurs via both constitutive and regulated pathways. 71 Both gel-forming and cell-surface mucins show constitutive and inducible gene expression in mucosal epithelial cells. The promoters of the MUC genes have generally not been fully characterized, although partial promoter characterization is available for human MUC1, MUC2, MUC3, 82 MUC4, MUC5AC, 79,86 and MUC5B. 87 Differential regulation of individual mucin genes is evident between different mucosal tissues and throughout differing regions of the larger epithelial tracts. For example, differing promoter regions are involved in the differential regulation of constitutive MUC2 expression in the small and large intestines. 78 Expression of cell-surface and gel-forming mucins can be upregulated by inflammatory cytokines such as interleukin (IL)-1 β, IL-4, IL-6, IL-9, IL-13, interferons, tumor necrosis factor- α, n it r i c oxide, and other uncharacterized inflammatory factors. 82, Responsiveness to these cytokines provides a link between mucins, innate mucosal immunity, and mucosal inflammatory responses. Neutrophils can also stimulate increases in production of both gel-forming and cell-surface mucins by mucosal epithelial cells via neutrophil elastase Microbi a l products can stimulate increased production of mucins by mucosal epithelial cells. 103, In fact, there is evidence that adherence of probiotic bacteria upregulates cell-surface mucin expression in vitro, 121,122 perhaps representing an important part of the mechanism by which probiotic bacteria limit infection by pathogens. In contrast, the lipopolysaccharide of the pathogen H. pylori decreases mucin synthesis in gastric epithelial cells in vitro via activation of cpla-2, 123 representing a mechanism by which a pathogen can favorably modulate the mucus barrier. 186 VOLUME 1 NUMBER 3 MAY
49 REVIEW The constitutive pathway continuously secretes sufficient mucin to maintain the mucus layer, whereas the regulated pathway affords a massive discharge as a response to environmental and / or (patho)physiological stimuli, including cholinergic stimuli, inflammatory cytokines, prostaglandins, lipopolysaccharide, bile salts, nucleotides, nitric oxide, vasoactive intestinal peptide, and neutrophil elastase. 93,95,103,113, St i mu l ate d mucin release can occur immediately and is accompanied by hydration, resulting in approximately a hundredfold expansion in volume of the secretory granule contents. 132,133 Shedding of the large extracellular α -subunits of cell-surface mucins from the cell surface and release of secreted splice variants of cellsurface mucins are less well understood. The protease ADAM17 (also known as TACE) has been shown to trigger shedding of MUC1 in endometrial cells in response to tumor necrosis factorα, 28,134 and the matrix metalloproteinase-1 also appears to be an effective MUC1 sheddase. 29 In addition to regulation of their synthesis and release, mucins are regulated in terms of their glycosylation. Altering mucin carbohydrates may block mechanisms that pathogens use to subvert the mucin barrier. Tumor necrosis factor- α alters sialylation of mucins produced by a tracheal cell line 135 and expression of both fucosyltransferases and α -2,3-sialyltransferases by normal bronchial mucosal explants. 136 In respiratory epithelial cells, the Th2 cytokines IL-4 and IL-13 increase expression of core 2 β -1,6- N -acetylglucosaminyltransferase, which forms β -1,6- branched structures, including core 2, core 4, and blood group I antigen. 137 In addition, glycosylation changes occur during infection / inflammation, for example, in individuals with cystic fibrosis or chronic bronchitis, 138 a s we l l a s H. pylori -infected individuals. 69,139 The inflammation-associated mucin sialylation shown in patients with H. pylori infection returns to the normal pattern following successful bacterial clearance with antibiotics. 140 In rhesus monkeys that share strong similarities in mucin glycosylation and the natural history of H. pylori infection with humans, 141 H. pylori infection induces time-dependent changes of mucosal glycosylation that alter the H. pylori a d he - sion targets. 69 Such fine-tuned kinetics of host glycosylation dynamically modulate host bacterial interactions, appearing to balance the impact of infection and thereby may determine the severity of disease. 69 Another example of dynamic changes in mucins occurs following infection of rats with the intestinal parasite Nippostrongylus brasiliensis ; infection induces increased production and several alterations in the glycosylation of intestinal Muc2 gel-forming mucin, one of which coincides with expulsion of the parasite These alterations in glycosylation appear to be driven partly by CD4 + T cells, as CD4 but not CD8 depletion blocks the increase in mucin production, change in glycosylation and worm expulsion, 148 and also by T-cell-independent mechanisms. 149 Such changes in mucin glycosylation need to be considered as a component of innate and adaptive immune responses to mucosal infection. MICROBIAL ADHERENCE TO THE EPITHELIUM To colonize mucosal surfaces and invade the host, microbes typically must first penetrate the secreted mucus barrier and then either attach to the apical surface of epithelial cells decorated with the cell-surface mucins or release toxins that disrupt epithelial integrity. Bacterial adhesion to host cells can be mediated by hydrophobic interactions, cation bridging (i.e., divalent cations counteracting the repulsion of the negatively charged surfaces of bacteria and host) and receptor ligand binding. One of the most extensively studied mechanisms of bacterial adhesion is via lectins and their corresponding glycosylated receptors. Binding is usually of low affinity, but clustering of adhesins and receptors results in multivalent binding. Fimbriae (or pili), outer membrane proteins, and cell wall components (e.g., lipopolysaccharide) may all function as adhesins. Adhesion can affect the bacteria by stimulation / inhibition of growth as well as induction of other adhesive structures and proteins required for invasion such as secretion systems. On the other hand, effects of adhesion on host cells can include altered morphology, fluid loss, induction of cytokine release, upregulation of adhesion molecules, and apoptosis. 150 Many bacterial adhesins bind oligosaccharides present on mucins. Whether bacterial mucin binding events favor the bacteria or the host is a key question. In reality, for some organisms, this may be a truly commensal relationship with benefits for both the bacteria (by facilitating retention in a favorable niche and even by providing mucin oligosaccharides for metabolism) and the host (by retaining bacteria in the outer areas of the mucus barrier where they cannot harm the underlying epithelium and also limiting the niche available for pathogenic bacteria). Numerous interactions between microorganisms and mucins and / or mucin-type carbohydrates have been demonstrated (see Table 3 ). Bacteria may have multiple adhesins with different carbohydrate specificities, and modulation of surface receptor density, kinetic parameters, or topographical distributions of these receptors on cell membranes regulate adhesion. As an example, H. pylori binds to mucin oligosaccharides via at least four adhesins, which differ substantially with anatomical site along the oro-gastric infection route, mucin type, ph, and gastric disease status. 139, Thus, for H. pylori, binding to mucins can have differing consequences during colonization of the oral-to-gastric niches and during long-term infection. MUCINS AS DECOYS FOR MICROBIAL ADHESINS Mucus hypersecretion ensuing from infection is testament to the role of mucus as a component of host defense. Although mucins are the major macromolecular constituent of mucus and are largely responsible for formation of the mucus gel, the precise nature of their role in host defense has not been well demonstrated empirically. Formation of the mucus gel is important in itself, as it provides a biophysical barrier as well as a matrix supporting the retention of a host of antimicrobial molecules. However, the secreted mucins themselves are likely to function as decoys for adhesins that have been evolved by pathogens to engage the cell surface, as the mucins express many of the oligosaccharide structures found on the cell surface and are constitutively produced in large amounts, constantly washing the mucosal surfaces ( Figure 1 ). Some mucins are effective viral agglutinating agents and exogenously applied mucins MucosalImmunology VOLUME 1 NUMBER 3 MAY
50 REVIEW Table 3 Characterized interactions between mucins and microbes Tissue derived mucins Mucin Carbohydrate Microbe References Respiratory mucins MUC1 Sialic acids P. aeruginosa, Haemophilus influenzae, S. aureus, influenza viruses Salivary mucins MUC5B MUC7 (DMBT1-Muclin) Sulfated Le a Sialic acids, Sialyl Le x, Le b P. aeruginosa, H. pylori, Streptococcus sanguis, Streptococcus gordonii, Actinobacillus actinomycetemcomitans, Streptococcus spp., Candida albicans 163,181, Gastric mucins MUC5AC MUC1 A, B, H, Le b H. pylori 139,151,176,281,282 Intestinal mucins MUC2 Enterotoxigenic Escherichia coli, Enteropathogenic E. coli, Salmonella typhimurium, Shigella boydii, Shigella sonnei, Campylobacter upsaliensis, Yersinia enterolitica, C. albicans, reoviruses 162, In most studies, only the tissue origin of the mucin has been determined. Which mucins and carbohydrates are responsible for the binding was only determined for a small proportion of the interactions. The mucin and carbohydrate columns thus do not indicate that all microbes listed interact via these specifi c structures, but merely that these have been shown to bind to some of the bacteria. are effective inhibitors of viral infection in in vitro-cultured cells. 154,155 Streptococcus pyogenes, Tritrichomonas foetus, Tritrichomonas mobilensis, influenza viruses, reoviruses, adenoviruses, enteroviruses, and coronaviruses bind to sialic acids, which are present both at the epithelial surface and on mucins Despite the accepted dogma that secreted mucins limit infection, there are few empirical in vivo data demonstrating their importance. The only secreted mucin for which genetically deficient animals are available is the intestinal mucin, Muc2. Muc2 / mice develop spontaneous inflammation, presumably due to the absence of the major component of intestinal mucus, leading to increased exposure to the normal intestinal microbial flora. 166,167 As yet, there are no reports of controlled infection experiments in these mice. Further models of secreted mucin deficiency are required to comprehensively determine the importance of secreted mucins in preventing and clearing mucosal infection. Many pathogens require direct binding to, or penetration of, mucosal epithelial cells to cause pathology. The widest diversity of cell-surface mucin expression is in the mucosal tissues most at risk of infection, such as the gastrointestinal tract, respiratory tract, and eye; notably, nine of the ten cell-surface mucins are expressed in the large intestine, which is the most microbe-rich mucosal environment. Importantly, their ability to be shed from the cell surface has led us to hypothesize that one of the main functions of cell-surface mucins is to act as releasable decoy ligands for microbes attempting to anchor themselves to the glycocalyx. Cell-surface mucins initiate intracellular signaling in response to bacteria, suggesting that they have both a barrier and reporting function on the apical surface of all mucosal epithelial cells. 57 However, until recently, much of the evidence had been circumstantial or restricted to in vitro an a ly s i s. For example, upregulation of MUC3 expression in colonic cells has been correlated with decreased binding of enteropathogenic E. coli. 121,122 In vitro studies have shown that expression of MUC1 by transfection inhibits reovirus and adenovirus infection of MDCK cells by up to tenfold. 168,169 Milk can limit bacterial and viral infections of the gastrointestinal tract and this has been attributed in part to the presence of large amounts of cell-surface mucins, chiefly MUC1 and MUC15, in the milkfat globule membrane Muc1 / mice were reported to display chronic infection and inflammation of the reproductive tract, reducing fertility rates. In this latter study, only normal endogenous bacteria were isolated, suggesting that these species become opportunistic pathogens in the absence of Muc In addition, Muc1 / mice were reported to have a high frequency of eye inflammation / infection involving Corynebacteria, Staphylococci and Streptococci, 174 a lt houg h t his could not be duplicated in a different mouse background held in alternative housing conditions. 175 We recently demonstrated that the intestinal pathogen C. jejuni binds to fucosylated mucin oligosaccharides. Controlled infection experiments demonstrated rapid transit of C. jejuni across the gastrointestinal barrier and greater intestinal pathology in Muc1 / mice. 55 Bone marrow transplantation studies demonstrated that the increased susceptibility was due to loss of Muc1 on epithelium rather than on leukocytes (which can also express Muc1). Loss of Muc1 had no discernable effects on the abundance or constituency of the intestinal microbial flora. Muc1 appears to prevent C. jejuni infection both by protecting cells from the effects of the cytolethal distending toxin (see above) and by acting as a releasable decoy. 55 We have also demonstrated that even though H. pylori can bind Muc1, that primary murine gastric epithelial cells expressing Muc1 bind fewer H. pylori t h an Muc1 / cells. 176 This paradoxical result is explained by Muc1 acting as a releasable decoy, i.e., the bacteria bind Muc1 expressed on epithelial cells, which is then shed by the host. Due to the absence of this decoy molecule, Muc1 / mice develop an approximately fivefold greater colonization density of H. pylori from the first days following infection that is maintained for at least 2 months. Consequently, Muc1 / mice develop severe gastritis not found in wild-type mice. 176 Heterozygous mice that have a lower level of gastric Muc1 188 VOLUME 1 NUMBER 3 MAY
51 REVIEW Figure 1 Diagrammatic representation of mucins in the mucosal barrier to infection. ( a ) The normal mucosa is covered with a continuously replenished thick mucus layer retaining host-defensive molecules. Commensal and environmental microbes may live in the outer mucus layer but the layer ensures that contact of microbes with epithelial cells is rare. ( b ) Early in infection, many pathogens actively disrupt the mucus layer and thereby gain access to the epithelial cell surface. In addition, this alters the environment for commensal and environmental microbes and opportunistic pathogenesis may occur. ( c ) Pathogens that break the secreted mucus barrier reach the apical membrane surface, which is decorated with a dense network of large cell-surface mucins. Pathogens bind the cell-surface mucins via lectin interactions and the mucin extracellular domains are shed as releasable decoy molecules. Consequent to contact with microbes and shedding of the extracellular domain, signal transduction by the cytoplasmic domains of the cell-surface mucins modulates cellular response to the presence of microbes. ( d ) In response to infection, there are alterations in mucins that are driven directly by epithelial cells and in response to signals from underlying innate and adaptive immunity. These alterations include goblet cell hyperplasia and increased mucin secretion and altered mucin glycosylation (depicted by the color change) affecting microbial adhesion and the ability of microbes to degrade mucus. These changes in mucins work in concert with other arms of immunity to clear the infection. protein expression show intermediate colonization densities, which suggests that polymorphisms in MUC1 or genes that regulate its expression could underlie susceptibility to H. pyloriinduced pathology in human populations. In fact, in humans, individuals with short MUC1 alleles (encoding smaller extracellular mucin domains) have a higher propensity to develop gastritis following H. pylori infection This may be indicative of lower efficacy of smaller MUC1 extracellular mucin domains allowing increased access of bacteria to the epithelial surface, or these alleles may be surrogate markers of polymorphisms influencing the level of gastric MUC1 expression. Recently, a similar protective role has been demonstrated for the extremely large MUC16 cell-surface mucin in human corneal epithelial cells. Greater binding of Staphlylococcus aureus occurs to in vitro - cultured corneal cells when MUC16 is depleted by RNAi. 180 Paradoxically, our demonstrations of Muc1-limiting infection in the gastrointestinal tract are opposite to that found in a model of respiratory Pseudomonas aeruginosa infection in the lung in which Muc1 / mice have an increased clearance of bacteria and a reduced inflammatory response to infection. 181 MUC1 binds the P. aeruginosa flagellin, 182,183 but intriguingly appears to inhibit flagellin-stimulated TLR5-mediated activation of NF- κ B by an as yet unclear mechanism requiring the MUC1 cytoplasmic domain. 181,184 Whereas an infection-promoting role of a molecule highly expressed on the apical surface of a broad array of mucosal epithelia appears counterintuitive, an anti-inflammatory role for MUC1 in some tissues is consistent with evolutionary adaptations to clear infection by local defense without potentially damaging inflammation, where possible. Further investigations are required with a broad array of pathogens in multiple tissues to clearly delineate the participation of the family of cell-surface mucins in mucosal defense. OTHER PROTECTIVE ROLES OF MUCINS Mucins have direct and indirect roles in defense from infection distinct from their ability to form a physical barrier and act as adhesion decoys. Not only do mucin oligosaccharides bind microbes, but also, in some cases, they either have direct antimicrobial activity or carry other antimicrobial molecules. A mucin oligosaccharide, α -1-4-linked N -acetyl-glucosamine, which is expressed by some gastric mucins, has been shown to directly interfere with synthesis of H. pylori cell wall components. 185 H. pylori must live within gastric mucus to remain protected from luminal gastric ph and prevent expulsion into the intestine. The antimicrobial mucin oligosaccharide probably acts to limit H. pylori expansion within gastric mucus. The nonoligomerizing secreted salivary mucin MUC7 has inherent direct candidacidal activity via a histatin domain at its N-terminus. 186,187 In addition, there is evidence for direct binding of antimicrobial molecules such as histatins and statherin by mucins that would help retain the antimicrobial molecules in the correct mucosal microenvironment where they can best protect the host. For example, MUC7 binds statherin and histatin-1, 188 and the other major mucin in saliva, MUC5B, binds histatin-1, -3, and -5 and statherin. 189 S e c r e t o r y Ig A (siga) is secreted via mucosal epithelial cells and needs to be MucosalImmunology VOLUME 1 NUMBER 3 MAY
52 REVIEW retained in the immediate mucosal environment to maximize exclusion of pathogens. siga is retained at high concentrations in mucus where it can efficiently trap the progress of pathogens, although the mechanism(s) for retaining siga in mucus are not well understood. Interestingly, secretory component, which is tightly bound to the Fc region of dimeric-iga to form siga, carries oligosaccharide structures similar to those on mucins. 190 In the absence of secretory component carbohydrates, siga fails to associate with mucus and fails to prevent infection in a murine respiratory bacterial infection model, substantiating both the physiological importance of siga mucin interactions and the importance of secretory component carbohydrates in maintaining this interaction. 191 It is also tempting to speculate that the poly-anionic mucins bind the poly-cationic antimicrobial defensin peptides that are co-secreted into mucus. Interactions of mucins with other secreted antimicrobial molecules has not been fully explored largely due to difficulties in extracting and purifying mucins in the absence of denaturing agents likely to disrupt such interactions. The cell-surface mucins are an integral component of the glycocalyx where they are likely to interact with proteoglycans and other molecules that could retain host defense molecules in a molecular complex covering the apical cell surface. 192,193 Therefore, other mucins and mucin oligosaccharides may yet prove to have direct and indirect antimicrobial activity. Regardless of whether antimicrobial molecules are retained in mucus by direct binding with mucins or by the biophysical properties of mucus, if mucin synthesis is aberrant or secreted mucins are degraded, the antimicrobial molecules will have impaired efficacy. SUBVERSION OF THE MUCIN BARRIER BY MUCOSAL PATHOGENS Perhaps the best evidence for the importance of the mucin barrier to infection is the wide variety of strategies used by microbes to subvert or avoid this barrier. Mucin barrier subversion strategies used by microbes include the production of enzymes capable of degrading mucin core proteins and mucin carbohydrates, and effective motility through mucus gels. Motility is important for bacterial mucosal pathogens to facilitate breaking through the physical mucus barrier. In fact, a vast proportion of mucosal bacterial pathogens are flagellated. 194,195 H. pylori t h at h av e dysfunctional flagella have a greatly reduced ability to infect. 196 H. pylori uses motility for initial colonization and to attain robust infection. In conjunction with motility, degradative enzymes such as glycosulfatases, sialidases, sialate O -acetylesterases, N -acetyl neuraminate lyases and mucinases are produced by a broad range of bacterial pathogens to destabilize the mucus gel and remove mucin decoy carbohydrates for adhesins The protozoan parasite Entamoeba histolytica cleaves the MUC2 mucin, which is the major structural component of the intestinal mucus, and this cleavage is predicted to depolymerize the MUC2 polymers. 202 The size of the polymer is important for the formation of entangled gels and the viscous properties of mucus; consequently, cleavage of the mucin polymer will effectively result in a local disintegration of mucus. 203 There is evidence that these degradative enzymes are critical for microbial pathogenesis. For example, the Vibrio cholerae Hap A, which has both mucinolytic and cytotoxic activity, is induced by mucin and required for translocation through mucin-containing gels. 204 The widespread and critically required expression of neuraminidases by a wide variety of sialic acid-binding mucosal viruses underlines the importance of elimination of mucin carbohydrates for their pathogenicity. 160 Lipopolysaccharide from H. pylori decreases mucin synthesis, 123 and the mucin carbohydrate-binding adhesins BabA and SabA undergo phase variation and change expression during infection, 153,205 w h i ch may allow them to evade this host defense mechanism. AVOIDANCE OF THE MUCIN BARRIER BY MUCOSAL PATHOGENS Another strategy commonly used by mucosal pathogens is to avoid the mucin barrier. Intestinal M cells, specifically designed to capture and present microbes to the underlying lymphoid tissue, can be regarded as a hole in the mucin barrier. The dome epithelium in which they lie lacks goblet cells, and therefore does not produce gel-forming mucins, and their apical cell surface has only sparse microvilli and an apparently thin glycocalyx. 206,207 Although no studies have measured the expression of individual cell-surface mucins in M cells, there appear to be differences in the glycocalyx mucins between M cells and adjacent intestinal mucosal epithelial cells. In some species, M cells can be identified by their pattern of lectin binding to specific cell-surface carbohydrates that differ with other mucosal epithelial cells. 208,209 Consequently, even though M cells constitute only a very small percentage of mucosal epithelial cells, they are the major point of attachment and / or entry used by a large number of mucosal pathogens including bacteria (e.g., S. typhimurium, Shigella flexneri, Yersinia enterocolitica, and V. cholerae ), viruses (e.g., reovirus, HIV-1, and polio virus) and parasites (e.g., Cryptosporidia). 206,210,211 Another strategy used by pathogens to avoid the cell-surface mucin barrier, once mucus is penetrated or M cells are invaded, is to disrupt the tight junctions between adjacent mucosal epithelial cells thereby exposing the vulnerable lateral membranes not protected by the glycocalyx. Such examples include S. flexneri, 212 enteropathogenic E. coli, 213 Porphyromonas gingivalis 214 and H. pylori. 215 MODELS TO INVESTIGATE INTERACTIONS BETWEEN MICROBES AND MUCINS Numerous models, including cancer cell-lines, organ cultures of gastric biopsies and whole animals have been used to investigate mucin microbe interactions. Although they express orthologs to most human mucins, the most commonly used laboratory animals such as rats and mice have differing glycosylation of some of their mucins. In fact, it is tempting to speculate that differences in mucin glycosylation between mammalian species may underlie some of the differences in infectivity / pathogenicity for individual microbial pathogens. Murine knockout models are only available for Muc1, 216 Muc2, 166 a n d Muc13 (M.A. McGuckin, unpublished data), and there are also mutants with aberrant Muc2 assembly. 217 Thus, there is a need for more models, as mouse knockouts, although limited by the slightly 190 VOLUME 1 NUMBER 3 MAY
53 REVIEW different glycosylation, still represent an important way to collect information of the in vivo function of mucins in infection. Because human pathogens commonly have adhesins for human carbohydrate structures, it is important to select appropriate models for individual pathogens. For example, the effects of H. pylori infection on the mouse are mild, and gastric cancer is not induced even after long-term exposure without other stimuli or genetic defects, although the mouse may develop chronic atrophic gastritis. 218,219 H. pylori can colonize the guinea pig and the Mongolian gerbil and cause a severe inflammatory response but does not induce cancer in the absence of exogenous chemical carcinogens. 220 These small animal models are useful to study some aspects of H. pylori infection and have the advantage of being relatively cheap. In contrast, rhesus monkeys naturally have persistent H. pylori infection leading to loss of mucus, gastritis, gastric ulcers and even cancer In addition, the anatomy and physiology of the GI tract of the rhesus monkey, as well as the expression of mucins and mucin glycosylation are very similar to that in human. 141 However, this model is expensive, the monkeys can have preexisting natural infection, and primate research has a higher level of ethical considerations. In vitro microbial mammalian cocultures are used extensively to elucidate the mechanisms by which microbes adhere, invade, and signal to the host, and to examine ensuing mammalian cell responses. These complex interactions are reliant on appropriate gene expression and cellular functioning of both the microbial and mammalian cells. It is therefore critical that appropriate microbial and mammalian cells are used and that the environment created experimentally is as similar to the human mucosal environment as possible. Human cell lines commonly used for in vitro infection studies have a highly variable expression of mucins and mucin glycosylation, and generally have very low production and secretion of gel-forming mucins. 225 Investigators using these models need to be aware of these limitations and consider them in interpreting their data. Additional important issues to consider are choice of cell line and, depending on the type of bacteria, oxygen tension. 225,226 With respect to appropriate mucin production, primary human tracheobronchial epithelial cells cultured in an air liquid interface represent the most physiological cell cultures in which infection studies can currently be undertaken. 227 Ex vivo -cultured tissue explants provide another potential avenue for exploring microbial mucin interactions in vitro CONCLUSIONS The personal repertoire of expression of mucin core proteins and their glycans, mucin allele length, and transient changes in mucin expression and glycosylation in response to infection or stress, as well as variations in environmental conditions may all affect microbial interaction with host mucins and the pathogenic consequences of microbial colonization. Rather than a static barrier, mucins should be considered as a dynamic responsive component of the mucosal barrier that interacts with and responds to other elements of innate and adaptive immunity. Difficulties in working with these complex glycoproteins and the paucity of physiological experimental systems need to be overcome if we are to fully understand the roles of mucins in host defe ns e f rom infe c t ion. DISCLOSURE The authors declared no conflict of interest Society for Mucosal Immunology REFERENCES 1. Kagnoff, M. F. & Eckmann, L. Epithelial cells as sensors for microbial infection. J. Clin. Invest. 100, 6 10 ( 1997 ). 2. Lu, J., Teh, C., Kishore, U. & Reid, K. B. Collectins and fi colins: sugar pattern recognition molecules of the mammalian innate immune system. Biochim. Biophys. Acta 1572, ( 2002 ). 3. Raj, P. A. & Dentino, A. R. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol. Lett. 206, 9 18 ( 2002 ). 4. Prydal, J. I., Muir, M. G. & Dilly, P. N. Comparison of tear fi lm thickness in three species determined by the glass fibre method and confocal microscopy. Eye 7, ( 1993 ). 5. Mercer RR ML, R. & Crapo, J. D. Mucous lining layers in human and rat airways. Ann. Rev. Resp. Dis. 145, 355 ( 1992 ). 6. Strugala, V., Allen, A., Dettmar, P. W. & Pearson, J. P. Colonic mucin: methods of measuring mucus thickness. Proc. Nutr. Soc. 62, ( 2003 ). 7. Jordan, N., Newton, J., Pearson, J. & Allen, A. A novel method for the visualization of the in situ mucus layer in rat and man. Clin. Sci. (London) 95, ( 1998 ). 8. Atuma, C., Strugala, V., Allen, A. & Holm, L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G922 G929 ( 2001 ). 9. Soler, M. et al. Adhesion-related glycocalyx study: quantitative approach with imaging-spectrum in the energy filtering transmission electron microscope (EFTEM). FEBS Lett. 429, ( 1998 ). 10. Ito, S. Structure and function of the glycocalyx. Fed. Proc. 28, ( 1969 ). 11. Madara, J. & Trier, J. Functional morphology of the mucosa of the small intestine. In Physiology of the Gastrointestinal Tract (Johnson L.R., ed) ( Raven press, 1987 ). 12. Klein, A. et al. Isolation and structural characterization of novel sialylated oligosaccharide-alditols from respiratory-mucus glycoproteins of a patient suffering from bronchiectasis. Eur. J. Biochem. 211, ( 1993 ). 13. Pigny, P. et al. Human mucin genes assigned to 11p15.5: identifi cation and organization of a cluster of genes. Genomics 38, ( 1996 ). 14. Desseyn, J. L., Aubert, J. P., Porchet, N. & Laine, A. Evolution of the large secreted gel-forming mucins. Mol. Biol. Evol. 17, ( 2000 ). 15. Desseyn, J. L. et al. Evolutionary history of the 11p15 human mucin gene family. J. Mol. Evol. 46, ( 1998 ). 16. Chen, Y. et al. Genome-wide search and identifi cation of a novel gel-forming Mucin MUC19/Muc19 in glandular tissues. Am. J. Respir. Cell Mol. Biol. 30, ( 2003 ). 17. Gum, J. R., Hicks, J. W., Toribara, N. W., Siddiki, B. & Kim, Y. S. Molecular cloning of human intestinal mucin (MUC2) cdna. Identifi cation of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 269, ( 1994 ). 18. Perez-Vilar, J., Eckhardt, A. E., DeLuca, A. & Hill, R. L. Porcine submaxillary mucin forms disulfi de-linked multimers through its amino-terminal d-domains. J. Biol. Chem. 273, ( 1998 ). 19. Asker, N., Baeckstrom, D., Axelsson, M. A. B., Carlstedt, I. & Hansson, G. C. The human MUC2 mucin apoprotein appears to dimerize before O -glycosylation and shares epitopes with the insoluble mucin of rat small intestine. Biochem. J. 308, ( 1995 ). 20. Perez-Vilar, J. & Hill, R. L. The structure and assembly of secreted mucins. J. Biol. Chem. 274, ( 1999 ). 21. Lidell, M. E. et al. The recombinant C-terminus of the human MUC2 mucin forms dimers in Chinese-hamster ovary cells and heterodimers with full-length MUC2 in LS 174 T cells. Biochem. J. 372, ( 2003 ). 22. Godl, K. et al. The N terminus of the MUC2 mucin forms trimers that are held together within a trypsin-resistant core fragment. J. Biol. Chem. 277, ( 2002 ). 23. Sheehan, J. K. et al. Physical characterization of the MUC5AC mucin: a highly oligomeric glycoprotein whether isolated from cell culture or in vivo MucosalImmunology VOLUME 1 NUMBER 3 MAY
54 REVIEW from respiratory mucous secretions. Biochem. J. 347 (Part 1), ( 2000 ). 24. Sheehan, J. K., Howard, M., Richardson, P. S., Longwill, T. & Thornton, D. J. Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug. Biochem. J. 338, ( 1999 ). 25. Jentoft, N. Why are proteins O -glycosylated? Trends Biochem. Sci. 15, ( 1990 ). 26. Wreschner, D. H. et al. Generation of ligand-receptor alliances by SEA module-mediated cleavage of membrane-associated mucin proteins. Protein Sci. 11, ( 2002 ). 27. Macao, B., Johansson, D. G., Hansson, G. C. & Hard, T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat. Struct. Mol. Biol. 13, ( 2006 ). 28. Thathiah, A., Blobel, C. P. & Carson, D. D. Tumor necrosis factor-alpha converting enzyme/adam 17 mediates MUC1 shedding. J. Biol. Chem. 278, ( 2003 ). 29. Thathiah, A. & Carson, D. D. MT1-MMP mediates MUC1 shedding independently of TACE/ADAM17. Biochem. J. 382 (Part 1), ( 2004 ). 30. Lillehoj, E. P., Han, F. & Kim, K. C. Mutagenesis of a Gly-Ser cleavage site in MUC1 inhibits ectodomain shedding. Biochem. Biophys. Res. Commun. 307, ( 2003 ). 31. Zrihan-Licht, S. et al. Characterization and molecular cloning of a novel MUC1 protein devoid of tandem repeats expressed in human breast cancer tissue. Eur. J. Biochem. 224, ( 1994 ). 32. Williams, S. J. et al. Two novel mucin genes downregulated in colorectal cancer identifi ed by differential display. Cancer Res. 59, ( 1999 ). 33. Williams, S. J., Munster, D. J., Quin, R. J., Gotley, D. C. & McGuckin, M. A. The MUC3 gene encodes a transmembrane mucin and is alternatively spliced. Biochem. Biophys. Res. Commun. 261, ( 1999 ). 34. Choudhury, A. et al. Alternate splicing at the 3 -end of the human pancreatic tumor-associated mucin MUC4 cdna. Teratog. Carcinog. Mutagen 21, ( 2001 ). 35. Williams, S. J. et al. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J. Biol. Chem. 276, ( 2001 ). 36. Hilkens, J., Ligtenberg, M. J., Vos, H. L. & Litvinov, S. V. Cell membraneassociated mucins and their adhesion-modulating property. Trends Biochem. Sci. 17, ( 1992 ). 37. Litvinov, S. V. & Hilkens, J. The epithelial sialomucin, episialin, is sialylated during recycling. J. Biol. Chem. 268, ( 1993 ). 38. Altschuler, Y. et al. Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state. Mol. Biol. Cell 11, ( 2000 ). 39. Thingstad, T., Vos, H. L. & Hilkens, J. Biosynthesis and shedding of epiglycanin: a mucin-type glycoprotein of the mouse TA3Ha mammary carcinoma cell. Biochem. J. 353, ( 2001 ). 40. Pandey, P., Kharbanda, S. & Kufe, D. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 55, ( 1995 ). 41. Li, Y., Kuwahara, H., Ren, J., Wen, G. & Kufe, D. The c-src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3 beta and beta-catenin. J. Biol. Chem. 276, ( 2001 ). 42. Li, Q., Ren, J. & Kufe, D. Interaction of human MUC1 and beta-catenin is regulated by Lck and ZAP-70 in activated Jurkat T cells. Biochem. Biophys. Res. Commun. 315, ( 2004 ). 43. Regimbald, L. H. et al. The breast mucin MUC1 as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 56, ( 1996 ). 44. Nath, D. et al. Macrophage-tumour cell interactions: identifi cation of muc1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology 98, ( 1999 ). 45. Gubbels, J. A. et al. Mesothelin-MUC16 binding is a high affi nity, N -glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 5, 50 ( 2006 ). 46. Ciborowski, P. & Finn, O. J. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro : potential role in metastasis. Clin. Exp. Metastasis 19, ( 2002 ). 47. Komatsu, M., Carraway, C. A. C., Fregien, N. L. & Carraway, K. L. Reversible disruption of cell-matrix and cell cell interactions by overexpression of sialomucin complex. J. Biol. Chem. 272, ( 1997 ). 48. Li, Y. Q., Bharti, A., Chen, D. S., Gong, J. L. & Kufe, D. Interaction of glycogen synthase kinase 3 β with the DF3/MUC1 carcinoma-associated antigen and β -catenin. Mol. Cell Biol. 18, ( 1998 ). 49. Quin, R. J. & McGuckin, M. A. Phosphorylation of MUC1 correlates with changes in cell cell adhesion. Int. J. Cancer 87, ( 2000 ). 50. Li, Y. et al. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-src and beta-catenin. J. Biol. Chem. 276, ( 2001 ). 51. Ren, J., Li, Y. & Kufe, D. Protein kinase C delta regulates function of the DF3/MUC1 carcinoma antigen in beta-catenin signaling. J. Biol. Chem. 277, ( 2002 ). 52. Li, Y., Liu, D., Chen, D., Kharbanda, S. & Kufe, D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene 22, ( 2003 ). 53. Ren, J. et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell 5, ( 2004 ). 54. Wei, X., Xu, H. & Kufe, D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell 7, ( 2005 ). 55. McAuley, J. L. et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 117, ( 2007 ). 56. Lara-Tejero, M. & Galan, J. E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290, ( 2000 ). 57. Lillehoj, E. P., Kim, H., Chun, E. Y. & Kim, K. C. Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am. J. Physiol. Lung Cell Mol. Physiol. 287, L809 L815 ( 2004 ). 58. McCool, D. J., Okada, Y., Forstner, J. F. & Forstner, G. G. Roles of calreticulin and calnexin during mucin synthesis in LS180 and HT29/A1 human colonic adenocarcinoma cells. Biochem. J. 341 (Part 3), ( 1999 ). 59. Ho, J. J. et al. N -glycosylation is required for the surface localization of MUC17 mucin. Int. J. Oncol. 23, ( 2003 ). 60. Kui Wong, N. et al. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J. Biol. Chem. 278, ( 2003 ). 61. Oriol, R. ABO, Hh, Lewis, and secretion serology, genetics, and tissue distribution. In Blood Cell Biochemistry, Vol 6 (Cartron, J., Rouger, P., eds) ( Plenum press, New York, 1995 ). 62. Oriol, R., Le Pendu, J. & Mollicone, R. Genetics of ABO, H, Lewis, X and related antigens. Vox Sang 51, ( 1986 ). 63. Henry, S., Oriol, R. & Samuelsson, B. Lewis histo-blood group system and associated secretory phenotypes. Vox Sang 69, ( 1995 ). 64. Yu, L. C. et al. Polymorphism and distribution of the secretor alpha(1,2)- fucosyltransferase gene in various Taiwanese populations. Transfusion 41, ( 2001 ). 65. Gerken, T. A. Kinetic modeling confi rms the biosynthesis of mucin core 1 (beta-gal(1-3) alpha-galnac- O -Ser/Thr) O -glycan structures are modulated by neighboring glycosylation effects. Biochemistry 43, ( 2004 ). 66. Marionneau, S. et al. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 83, ( 2001 ). 67. Marionneau, S., Airaud, F., Bovin, N. V., Le Pendu, J. & Ruvoen-Clouet, N. Infl uence of the combined ABO FUT2, and FUT3 polymorphism on susceptibility to Norwalk virus attachment. J. Infect. Dis. 192, ( 2005 ). 68. Lomberg, H., Jodal, U., Leffl er, H., De Man, P. & Svanborg, C. Blood group non-secretors have an increased infl ammatory response to urinary tract infection. Scand. J. Infect. Dis. 24, ( 1992 ). 69. Linden, S. K. et al. Glycan secretor phenotypes modulate mucosal innate immunity and affect H. pylori infection. PLoS Pathog. 4, e2 ( 2008 ). 70. Aspholm-Hurtig, M. et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science 305, ( 2004 ). 71. Rogers, D. F. Airway goblet cells: responsive and adaptable front-line defenders. Eur. Respir. J. 7, ( 1994 ). 192 VOLUME 1 NUMBER 3 MAY
55 REVIEW 72. Kovarik, A., Peat, N., Wilson, D., Gendler, S. J. & Taylor-Papadimitriou, J. Analysis of the tissue-specifi c promoter of the MUC1 gene. J. Biol. Chem. 268, ( 1993 ). 73. Abe, M. & Kufe, D. Transcriptional regulation of DF3 gene expression in human MCF-7 breast carcinoma cells. J. Cell Physiol. 143, ( 1990 ). 74. Zaretsky, J. Z. et al. Analysis of the promoter of the MUC1 gene overexpressed in breast cancer. FEBS Lett. 461, ( 1999 ). 75. Velcich, A., Palumbo, L., Selleri, L., Evans, G. & Augenlicht, L. Organization and regulatory aspects of the human intestinal mucin gene (MUC2) locus. J. Biol. Chem. 272, ( 1997 ). 76. Gum, J. R., Hicks, J. W. & Kim, Y. S. Identifi cation and characterization of the MUC2 (human intestinal mucin) gene 5 -fl anking region: promoter activity in cultured cells. Biochem. J. 325, ( 1997 ). 77. Nogami, H. et al. Sp1 protein contributes to airway specifi c rat Muc2 mucin gene transcription. Gene 198, ( 1997 ). 78. Gum, J. R. Jr et al. Goblet cell-specifi c expression mediated by the MUC2 mucin gene promoter in the intestine of transgenic mice. Am. J. Physiol. 276, G666 G676 ( 1999 ). 79. Perrais, M., Pigny, P., Copin, M. C., Aubert, J. P. & Van Seuningen, I. Induction of MUC2 and MUC5AC mucin by factors of the epidermal growth factor family is mediated by EGF-R/Ras/Raf/MAPK signaling cascade and Sp1. J. Biol. Chem. 19, 19 ( 2002 ). 80. Yamamoto, H., Bai, Y. Q. & Yuasa, Y. Homeodomain protein CDX2 regulates goblet-specifi c MUC2 gene expression. Biochem. Biophys. Res. Commun. 300, ( 2003 ). 81. van der Sluis, M. et al. The murine Muc2 mucin gene is transcriptionally regulated by the zinc-fi nger GATA-4 transcription factor in intestinal cells. Biochem. Biophys. Res. Commun. 325, ( 2004 ). 82. Shekels, L. L. & Ho, S. B. Characterization of the mouse Muc3 membrane bound intestinal mucin 5 coding and promoter regions: regulation by infl ammatory cytokines. Biochim. Biophys. Acta 1627, ( 2003 ). 83. Perez, A., Barco, R., Fernandez, I., Price-Schiavi, S. A. & Carraway, K. L. PEA3 transactivates the Muc4/Sialomucin complex promoter in mammary epithelial and tumor cells. J. Biol. Chem. 278, ( 2003 ). 84. Fauquette, V. et al. Transcription factor AP-2alpha represses both the Mucin MUC4 expression and pancreatic cancer cell proliferation. Carcinogenesis 28, ( 2007 ). 85. Perrais, M. et al. Characterization of human mucin gene MUC4 promoter: importance of growth factors and proinfl ammatory cytokines for its regulation in pancreatic cancer cells. J. Biol. Chem. 276, ( 2001 ). 86. Kim, S. W. et al. Regulation of mucin gene expression by CREB via a nonclassical RA signaling pathway. Mol. Cell Biol. 27, ( 2007 ). 87. Van Seuningen, I., Perrais, M., Pigny, P., Porchet, N. & Aubert, J. P. Sequence of the 5 -fl anking region and promoter activity of the human mucin gene MUC5B in different phenotypes of colon cancer cells. Biochem. J. 348, ( 2000 ). 88. Shirotani, K., Taylor-Papadimitriou, J., Gendler, S. J. & Irimura, T. Transcriptional regulation of the MUC1 mucin gene in colon carcinoma cells by a soluble factor identifi cation of a regulatory element. J. Biol. Chem. 269, ( 1994 ). 89. Vos, H. L., van der Valk, S. W., Prinsenberg, T., Mooi, W. J. & Hilkens, J. IL6-induction of the MUC1/episialin promoter in T47D cells. In Proceedings of 4th International Workshop on Carcinoma-Associated Mucins 30 ( 1996 ). 90. Temann, U. A. et al. A novel role for murine IL-4 in vivo : induction of MUC5ac gene expression and mucin hypersecretion. Am. J. Respir. Cell Mol. Biol. 16, ( 1997 ). 91. Dabbagh, K. et al. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 162, ( 1999 ). 92. Longphre, M. et al. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J. Clin. Invest. 104, ( 1999 ). 93. Smirnova, M. G., Birchall, J. P. & Pearson, J. P. TNF-alpha in the regulation of MUC5AC secretion: some aspects of cytokine-induced mucin hypersecretion on the in vitro model. Cytokine 12, ( 2000 ). 94. Kim, Y. D. et al. Regulation of IL-1beta-mediated MUC2 gene in NCI-H292 human airway epithelial cells. Biochem. Biophys. Res. Commun. 274, ( 2000 ). 95. Enss, M. L. et al. Proinfl ammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Infl amm. Res. 49, ( 2000 ). 96. Louahed, J. et al. Interleukin-9 upregulates mucus expression in the airways. Am. J. Respir. Cell Mol. Biol. 22, ( 2000 ). 97. Shim, J. J. et al. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am. J. Physiol. Lung Cell Mol. Physiol. 280, L134 L140 ( 2001 ). 98. Gaemers, I. C., Vos, H. L., Volders, H. H., van der Valk, S. W. & Hilkens, J. A stat-responsive element in the promoter of the episialin/muc1 gene is involved in its overexpression in carcinoma cells. J. Biol. Chem. 276, ( 2001 ). 99. Smirnova, M. G., Kiselev, S. L., Birchall, J. P. & Pearson, J. P. Up-regulation of mucin secretion in HT29-MTX cells by the pro-infl ammatory cytokines tumor necrosis factor-alpha and interleukin-6. Eur. Cytokine Netw. 12, ( 2001 ) Whittaker, L. et al. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am. J. Respir. Cell Mol. Biol. 27, ( 2002 ) Seong, J. K. et al. Upregulation of MUC8 and downregulation of MUC5AC by infl ammatory mediators in human nasal polyps and cultured nasal epithelium. Acta Otolaryngol. 122, ( 2002 ) Kim, Y. D. et al. Interleukin-1beta induces MUC2 gene expression and mucin secretion via activation of PKC-MEK/ERK, and PI3K in human airway epithelial cells. J. Korean Med. Sci. 17, ( 2002 ) Smirnova, M. G., Guo, L., Birchall, J. P. & Pearson, J. P. LPS up-regulates mucin and cytokine mrna expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol. 221, ( 2003 ) Iwashita, J. et al. mrna of MUC2 is stimulated by IL-4, IL-13 or TNF-alpha through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol. Cell Biol. 81, ( 2003 ) Song, K. S. et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-msk1-creb activation in human airway epithelial cells. J. Biol. Chem. 278, ( 2003 ) Li, S., Intini, G. & Bobek, L. A. Modulation of MUC7 mucin expression by exogenous factors in airway cells in vitro and in vivo. Am. J. Respir. Cell Mol. Biol. 35, ( 2006 ) Damera, G., Xia, B. & Sachdev, G. P. IL-4 induced MUC4 enhancement in respiratory epithelial cells in vitro is mediated through JAK-3 selective signaling. Respir. Res. 7, 39 ( 2006 ) Koga, T. et al. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: Its signaling pathway and biological implication. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L693 L701 ( 2007 ) Andrianifahanana, M. et al. IFN-gamma-induced expression of MUC4 in pancreatic cancer cells is mediated by STAT-1 upregulation: a novel mechanism for IFN-gamma response. Oncogene 26, ( 2007 ) Song, J. S. et al. Nitric oxide induces MUC5AC mucin in respiratory epithelial cells through PKC and ERK dependent pathways. Respir. Res. 8, 28 ( 2007 ) Fischer, B. M. & Voynow, J. A. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am. J. Respir. Cell Mol. Biol. 26, ( 2002 ) Fischer, B. M. et al. Neutrophil elastase increases MUC4 expression in normal human bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 284, L671 L679 ( 2003 ) Voynow, J. A. et al. Neutrophil elastase increases MUC5AC mrna and protein expression in respiratory epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 20, ( 1999 ) Lundgren, J. D., Rieves, R. D., Mullol, J., Logun, C. & Shelhamer, J. H. The effect of neutrophil protenase enzymes on the release of mucus from feline and human airway cultures. Resp. Med. 88, ( 1994 ) Dwyer, T. M. & Farley, J. M. Human neutrophil elastase releases two pools of mucinlike glycoconjugate from tracheal submucosal gland cells. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L675 L682 ( 2000 ) Kuwahara, I. et al. Neutrophil elastase stimulates MUC1 gene expression through increased Sp1 binding to the MUC1 promoter. Am. J. Physiol. Lung Cell Mol. Physiol. 289, L355 L362 ( 2005 ) Yanagihara, K., Seki, M. & Cheng, P. W. Lipopolysaccharide induces mucus cell metaplasia in mouse lung. Am. J. Respir. Cell Mol. Biol. 24, ( 2001 ). MucosalImmunology VOLUME 1 NUMBER 3 MAY
56 REVIEW 118. Lemjabbar, H. & Basbaum, C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8, ( 2002 ) Dohrman, A. et al. Mucin gene (MUC2 and MUC5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim. Biophys. Acta 1406, ( 1998 ) Caballero-Franco, C., Keller, K., De Simone, C. & Chadee, K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G315 G322 ( 2007 ) Mack, D. R., Michail, S., Wei, S., McDougall, L. & Hollingsworth, M. A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276, G941 G950 ( 1999 ) Mack, D. R., Ahrne, S., Hyde, L., Wei, S. & Hollingsworth, M. A. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52, ( 2003 ) Slomiany, B. L. & Slomiany, A. Cytosolic phospholipase A2 activation in Helicobacter pylori lipopolysaccharide-induced interference with gastric mucin synthesis. IUBMB Life 58, ( 2006 ) Kim, K. C., Lee, B. C., Pou, S. & Ciccolella, D. Effects of activation of polymorphonuclear leukocytes on airway goblet cell mucin release in a co-culture system. Infl amm. Res. 52, ( 2003 ) Fischer, B. M., Krunkosky, T. M., Wright, D. T., Dolanokeefe, M. & Adler, K. B. Tumor necrosis factor-alpha (TNF-alpha) stimulates mucin secretion and gene expression in airway epithelium in vitro. Chest 107 (3 Suppl), 133S 135S ( 1995 ) Hollande, E., Fanjul, M., Claret, S., Forguelafi tte, M. E. & Bara, J. Effects of VIP on the regulation of mucin secretion in cultured human pancreatic cancer cells (CAPAN-1). In vitro Cell Dev. Biol. 31, ( 1995 ) Kishioka, C., Okamoto, K., Kim, J. & Rubin, B. K. Regulation of secretion from mucous and serous cells in the excised ferret trachea. Respir. Physiol. 126, ( 2001 ) Klinkspoor, J. H. et al. Mucin secretion by the human colon cell line LS174T is regulated by bile salts. Glycobiology 9, ( 1999 ) Jarry, A., Vallette, G., Branka, J. E. & Laboisse, C. Direct secretory effect of interleukin-1 via type I receptors in human colonic mucous epithelial cells (HT29-C1.16E). Gut 38, ( 1996 ) Kim, K. C., Park, H. R., Shin, C. Y., Akiyama, T. & Ko, K. H. Nucleotideinduced mucin release from primary hamster tracheal surface epithelial cells involves the p-2u purinoceptor. Eur. Respir. J. 9, ( 1996 ) Gottke, M. & Chadee, K. Exogenous nitric oxide stimulates mucin secretion from LS174T colonic adenocarcinoma cells. Infl amm. Res. 45, ( 1996 ) Tam, P. Y. & Verdugo, P. Control of mucus hydration as a Donnan equilibrium process. Nature 292, ( 1981 ) Verdugo, P. Mucin exocytosis. Am. Rev. Respir. Dis. 144, S33 S37 ( 1991 ) Thathiah, A. et al. TNF-alpha stimulates MUC1 synthesis and Ectodomain release in a human uterine epithelial cell line. Endocrinology 145, ( 2004 ) Delmotte, P. et al. Tumor necrosis factor alpha increases the expression of glycosyltransferases and sulfotransferases responsible for the biosynthesis of sialylated and/or sulfated Lewis x epitopes in the human bronchial mucosa. J. Biol. Chem. 277, ( 2002 ) Delmotte, P. et al. Infl uence of TNFalpha on the sialylation of mucins produced by a transformed cell line MM-39 derived from human tracheal gland cells. Glycoconj. J. 18, ( 2001 ) Beum, P. V., Basma, H., Bastola, D. R. & Cheng, P. W. Mucin biosynthesis: upregulation of core 2 beta 1,6 N -acetylglucosaminyltransferase by retinoic acid and Th2 cytokines in a human airway epithelial cell line. Am. J. Physiol. Lung Cell Mol. Physiol. 288, L116 L124 ( 2005 ) Schulz, B. L. et al. Glycosylation of sputum mucins is altered in cystic fi brosis patients. Glycobiology 17, ( 2007 ) Linden, S. K., Wickstrom, C., Lindell, G. & Carlstedt, I. Four modes of adhesion are used during Helicobacter pylori binding to human mucins in the oral and gastric niches. Helicobacter (in press) ( 2008 ) Ota, H. et al. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 433, ( 1998 ) Lind é n, S., Bor é n, T., Dubois, A. & Carlstedt, I. Rhesus monkey gastric mucins and their Helicobacter pylori binding properties. Biochem. J. 379, 1 11 ( 2004 ) Koninkx, J. F., Mirck, M. H., Hendriks, H. G., Mouwen, J. M. & van Dijk, J. E. Nippostrongylus brasiliensis : histochemical changes in the composition of mucins in goblet cells during infection in rats. Exp. Parasitol. 65, ( 1988 ) Yamauchi, J. et al. Altered expression of goblet cell- and mucin glycosylation-related genes in the intestinal epithelium during infection with the nematode Nippostrongylus brasiliensis in rat. APMIS 114, ( 2006 ) Oinuma, T., Abe, T., Nawa, Y., Kawano, J. & Suganuma, T. Glycoconjugates in rat small intestinal mucosa during infection with the intestinal nematode Nippostrongylus brasiliensis. Adv. Exp. Med. Biol. 371B, ( 1995 ) Holmen, J. M., Olson, F. J., Karlsson, H. & Hansson, G. C. Two glycosylation alterations of mouse intestinal mucins due to infection caused by the parasite Nippostrongylus brasiliensis. Glycoconj. J. 19, ( 2002 ) Karlsson, N. G. et al. Identifi cation of transient glycosylation alterations of sialylated mucin oligosaccharides during infection by the rat intestinal parasite Nippostrongylus brasiliensis. Biochem. J. 350, ( 2000 ) Olson, F. J. et al. Blood group A glycosyltransferase occurring as alleles with high sequence difference is transiently induced during a Nippostrongylus brasiliensis parasite infection. J. Biol. Chem. 277, ( 2002 ) Khan, W. I., Abe, T., Ishikawa, N., Nawa, Y. & Yoshimura, K. Reduced amount of intestinal mucus by treatment with anti-cd4 antibody interferes with the spontaneous cure of Nippostrongylus brasiliensis - infection in mice. Parasite Immunol. 17, ( 1995 ) Kawai, Y. et al. T cell-dependent and -independent expression of intestinal epithelial cell-related molecules in rats infected with the nematode Nippostrongylus brasiliensis. APMIS 115, ( 2007 ) Salyers, A. & Whitt, D. Bacterial Pathogenesis: A Molecular Approach 2nd edn ( ASM Press, Washington, DC, 1994 ) Linden, S., Mahdavi, J., Hedenbro, J., Boren, T. & Carlstedt, I. Effects of ph on Helicobacter pylori binding to human gastric mucins: identifi cation of binding to non-muc5ac mucins. Biochem. J. 384, ( 2004 ) Teneberg, S. et al. Carbohydrate binding specifi city of the neutrophilactivating protein of Helicobacter pylori. J. Biol. Chem. 272, ( 1997 ) Mahdavi, J. et al. Helicobacter pylori SabA adhesin in persistent infection and chronic infl ammation. Science 297, ( 2002 ) Chen, C. C., Baylor, M. & Bass, D. M. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology 105, ( 1993 ) Yolken, R. H., Ojeh, C., Khatri, I. A., Sajjan, U. & Forstner, J. F. Intestinal mucins inhibit rotavirus replication in an oligosaccharide-dependent manner. J. Infect. Dis. 169, ( 1994 ) Hytonen, J., Haataja, S. & Finne, J. Streptococcus pyogenes glycoprotein-binding strepadhesin activity is mediated by a surfaceassociated carbohydrate-degrading enzyme, Pullulanase. Infect. Immun. 71, ( 2003 ) Schwegmann, C., Zimmer, G., Yoshino, T., Enss, M. & Herrler, G. Comparison of the sialic acid binding activity of transmissible gastroenteritis coronavirus and E. coli K99. Virus Res. 75, ( 2001 ) Bisaillon, M., Senechal, S., Bernier, L. & Lemay, G. A glycosyl hydrolase activity of mammalian reovirus sigma1 protein can contribute to viral infection through a mucus layer. J. Mol. Biol. 286, ( 1999 ) Helander, A. et al. The viral sigma1 protein and glycoconjugates containing alpha2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J. Virol. 77, ( 2003 ) Matrosovich, M. & Klenk, H. D. Natural and synthetic sialic acidcontaining inhibitors of infl uenza virus receptor binding. Rev. Med. Virol. 13, ( 2003 ) Alexander, D. A. & Dimock, K. Sialic acid functions in enterovirus 70 binding and infection. J. Virol. 76, ( 2002 ) Willoughby, R. E. Rotaviruses preferentially bind O -linked sialylglycoconjugates and sialomucins. Glycobiology 3, ( 1993 ) Couceiro, J. N., Paulson, J. C. & Baum, L. G. Infl uenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specifi city. Virus Res. 29, ( 1993 ). 194 VOLUME 1 NUMBER 3 MAY
57 REVIEW 164. Babal, P. & Russell, L. C. Sialic acid-specifi c lectin-mediated adhesion of Tritrichomonas foetus and Tritrichomonas mobilensis. J. Parasitol. 85, ( 1999 ) Ryan, P. A., Pancholi, V. & Fischetti, V. A. Group A streptococci bind to mucin and human pharyngeal cells through sialic acid-containing receptors. Infect. Immun. 69, ( 2001 ) Velcich, A. et al. Colorectal cancer in mice genetically defi cient in the mucin Muc2. Science 295, ( 2002 ) Van der Sluis, M. et al. Muc2-defi cient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, ( 2006 ) Walters, R. W., Pilewski, J. M., Chiorini, J. A. & Zabner, J. Secreted and transmembrane mucins inhibit gene transfer with AAV4 more effi ciently than AAV5. J. Biol. Chem. 277, ( 2002 ) Arcasoy, S. M. et al. MUC1 and other sialoglycoconjugates inhibit adenovirus mediated gene transfer to epithelial cells. Am. J. Respir. Cell Mol. Biol. 17, ( 1997 ) Peterson, J. A., Patton, S. & Hamosh, M. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol. Neonate 74, ( 1998 ) Yolken, R. H. et al. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J. Clin. Invest. 90, ( 1992 ) Dekker, J. & Tytgat, K. M. Binding of rotavirus by a 46 kd milkglycoprotein may prevent gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 17, ( 1993 ) DeSouza, M. M. et al. MUC1/episialin: a critical barrier in the female reproductive tract. J. Reprod. Immunol. 45, ( 1999 ) Kardon, R. et al. Bacterial conjunctivitis in Muc1 null mice. Invest. Ophthalmol. Vis. Sci. 40, ( 1999 ) Danjo, Y., Hazlett, L. D. & Gipson, I. K. C57BL/6 mice lacking Muc1 show no ocular surface phenotype. Invest. Ophthalmol. Vis. Sci. 41, ( 2000 ) McGuckin, M. A. et al. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 133, ( 2007 ) Vinall, L. E. et al. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology 123, ( 2002 ) Carvalho, F. et al. MUC1 gene polymorphism and gastric cancer an epidemiological study. Glycoconj. J. 14, ( 1997 ) Silva, F. et al. MUC1 polymorphism confers increased risk for intestinal metaplasia in a Colombian population with chronic gastritis. Eur. J. Hum. Genet. 11, ( 2003 ) Blalock, T. D. et al. Functions of MUC16 in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 48, ( 2007 ) Lu, W. et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J. Immunol. 176, ( 2006 ) Lillehoj, E. P. et al. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell Mol. Physiol. 280, L181 L187 ( 2001 ) Lillehoj, E. P., Kim, B. T. & Kim, K. C. Identifi cation of Pseudomonas aeruginosa fl agellin as an adhesin for Muc1 mucin. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L751 L756 ( 2002 ) Kato, K., Lu, W., Kai, H. & Kim, K. C. Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of TLR5 signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L686 L692 ( 2007 ) Kawakubo, M. et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305, ( 2004 ) Gururaja, T. L. et al. Candidacidal activity prompted by N -terminus histatin-like domain of human salivary mucin (MUC7). Biochim. Biophys. Acta Prot. Struct. Mol. Enzymol. 1431, ( 1999 ) Bobek, L. A. & Situ, H. MUC7 20-Mer: investigation of antimicrobial activity, secondary structure, and possible mechanism of antifungal action. Antimicrob. Agents Chemother. 47, ( 2003 ) Bruno, L. S. et al. Two-hybrid analysis of human salivary mucin MUC7 interactions. Biochim. Biophys. Acta 1746, ( 2005 ) Iontcheva, I., Oppenheim, F. G., Offner, G. D. & Troxler, R. F. Molecular mapping of statherin- and histatin-binding domains in human salivary mucin MG1 (MUC5B) by the yeast two-hybrid system. J. Dent. Res. 79, ( 2000 ) Royle, L. et al. Secretory IgA N - and O -glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem. 278, ( 2003 ) Phalipon, A. et al. Secretory component: a new role in secretory IgAmediated immune exclusion in vivo. Immunity 17, ( 2002 ) Salathe, M., Forteza, R. & Conner, G. E. Post-secretory fate of host defence components in mucus. Novartis Found Symp. 248, ( 2002 ) Forteza, R. et al. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J. 15, ( 2001 ) Josenhans, C. & Suerbaum, S. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291, ( 2002 ) Moens, S. & Vanderleyden, J. Functions of bacterial fl agella. Crit. Rev. Microbiol. 22, ( 1996 ) Ottemann, K. M. & Lowenthal, A. C. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect. Immun. 70, ( 2002 ) Corfi eld, A. P., Wagner, S. A., Clamp, J. R., Kriaris, M. S. & Hoskins, L. C. Mucin degradation in the human colon: production of sialidase, sialate O -acetylesterase, N -acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect. Immun. 60, ( 1992 ) Haider, K. et al. Production of mucinase and neuraminidase and binding of Shigella to intestinal mucin. J. Diarrhoeal Dis. Res. 11, ( 1993 ) Corfi eld, A. P. et al. The roles of enteric bacterial sialidase, sialate O -acetyl esterase and glycosulfatase in the degradation of human colonic mucin. Glycoconj. J. 10, ( 1993 ) Homer, K. A., Whiley, R. A. & Beighton, D. Production of specifi c glycosidase activities by Streptococcus intermedius strain UNS35 grown in the presence of mucin. J. Med. Microbiol. 41, ( 1994 ) Roberton, A. M. & Wright, D. P. Bacterial glycosulphatases and sulphomucin degradation. Canad. J. Gastroenterol. 11, ( 1997 ) Lidell, M. E., Moncada, D. M., Chadee, K. & Hansson, G. C. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc. Natl. Acad. Sci. USA 103, ( 2006 ) Allen, A., Flemstrom, G., Garner, A. & Kivilaakso, E. Gastroduodenal mucosal protection. Physiol. Rev. 73, ( 1993 ) Silva, A. J., Pham, K. & Benitez, J. A. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149, ( 2003 ) Solnick, J. V., Hansen, L. M., Salama, N. R., Boonjakuakul, J. K. & Syvanen, M. Modifi cation of Helicobacter pylori outer membrane protein expression during experimental infection of rhesus macaques. Proc. Natl. Acad. Sci. USA 101, ( 2004 ) Siebers, A. & Finlay, B. B. M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 4, ( 1996 ) Neutra, M. R., Mantis, N. J., Frey, A. & Giannasca, P. J. The composition and function of M cell apical membranes: implications for microbial pathogenesis. Semin. Immunol. 11, ( 1999 ) Lelouard, H., Reggio, H., Mangeat, P., Neutra, M. & Montcourrier, P. Mucin-related epitopes distinguish M cells and enterocytes in rabbit appendix and Peyer s patches. Infect. Immun. 67, ( 1999 ) Lelouard, H. et al. Glycocalyx on rabbit intestinal M cells displays carbohydrate epitopes from Muc2. Infect. Immun. 69, ( 2001 ) Vazquez-Torres, A. & Fang, F. C. Cellular routes of invasion by enteropathogens. Curr. Opin. Microbiol. 3, ( 2000 ) Jones, B., Pascopella, L. & Falkow, S. Entry of microbes into the host: using M cells to break the mucosal barrier. Curr. Opin. Immunol. 7, ( 1995 ) Sakaguchi, T., Kohler, H., Gu, X., McCormick, B. A. & Reinecker, H. C. Shigella fl exneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol. 4, ( 2002 ) Goosney, D. L., Gruenheid, S. & Finlay, B. B. Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu. Rev. Cell Dev. Biol. 16, ( 2000 ) Katz, J., Sambandam, V., Wu, J. H., Michalek, S. M. & Balkovetz, D. F. Characterization of Porphyromonas gingivalis -induced degradation of MucosalImmunology VOLUME 1 NUMBER 3 MAY
58 REVIEW epithelial cell junctional complexes. Infect. Immun. 68, ( 2000 ) Amieva, M. R. et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300, ( 2003 ) Spicer, A. P., Rowse, G. J., Lidner, T. K. & Gendler, S. J. Delayed mammary tumor progression in Muc-1 null mice. J. Biol. Chem. 270, ( 1995 ) Heazlewood, C. K. et al. Aberrant mucin assembly in mice causes ER stress and spontaneous infl ammation resembling ulcerative colitis. PLoS Med. 5, e54 ( 2008 ) Sjunnesson, H., Sturegard, E., Grubb, A., Willen, R. & Wadstrom, T. Comparative study of Helicobacter pylori infection in guinea pigs and mice elevation of acute-phase protein C3 in infected guinea pigs. FEMS Immunol. Med. Microbiol. 30, ( 2001 ) Kim, D. H. et al. Long-term evaluation of mice model infected with Helicobacter pylori : focus on gastric pathology including gastric cancer. Aliment Pharmacol. Ther. 18 (Suppl 1), ( 2003 ) Sturegard, E. et al. Severe gastritis in guinea-pigs infected with Helicobacter pylori. J. Med. Microbiol. 47, ( 1998 ) Dubois, A. et al. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori : implications for human disease. Infect. Immun. 64, ( 1996 ) Dubois, A. et al. Host specifi city of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology 116, ( 1999 ) Dubois, A. et al. Seroepizootiology of Helicobacter pylori gastric infection in nonhuman primates housed in social environments. J. Clin. Microbiol. 33, ( 1995 ) Dubois, A. et al. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology 106, ( 1994 ) Linden, S. K., Driessen, K. M. & McGuckin, M. A. Improved in vitro model systems for gastrointestinal infection by choice of cell line, ph, microaerobic conditions and optimization of culture conditions. Helicobacter 12, ( 2007 ) Cottet, S., Corthesy-Theulaz, I., Spertini, F. & Corthesy, B. Microaerophilic conditions permit to mimic in vitro events occurring during in vivo Helicobacter pylori infection and to identify Rho/Rasassociated proteins in cellular signaling. J. Biol. Chem. 277, ( 2002 ) Thornton, D. J. et al. Characterization of mucins from cultured normal human tracheobronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L1118 L1128 ( 2000 ) Smoot, D. T. et al. Development of a human stomach explant organ culture system to study the pathogenesis of Helicobacter pylori. Digestion 46, ( 1990 ) Olfat, F. O., Naslund, E., Freedman, J., Boren, T. & Engstrand, L. Cultured human gastric explants: a model for studies of bacteria-host interaction during conditions of experimental Helicobacter pylori infection. J. Infect. Dis. 186, ( 2002 ) Haque, A. et al. Early interactions of Salmonella enterica serovar with human small intestinal epithelial explants. Gut 53, ( 2004 ) Gum, J. R. et al. Molecular cloning of human intestinal mucin cdnas. Sequence analysis and evidence for genetic polymorphisms. J. Biol. Chem. 264, ( 1989 ) Ogata, S., Uehara, H., Chen, A. & Itzkowitz, S. H. Mucin gene expression in colonic tissues and cell lines. Cancer Res. 52, ( 1992 ) Dohrman, A. et al. Distribution of lysozyme and mucin (MUC2 and MUC3) mrna in human bronchus. Exp. Lung Res. 20, ( 1994 ) Berry, M., Ellingham, R. B. & Corfi eld, A. P. Human preocular mucins refl ect changes in surface physiology. Br. J. Ophthalmol. 88, ( 2004 ) Kerschner, J. E. Mucin gene expression in human middle ear epithelium. Laryngoscope 117, ( 2007 ) Porchet, N. et al. Human mucin genes: genomic organization and expression of MUC4, MUC5AC and MUC5B. Biochem. Soc. Trans. 23, ( 1995 ) Inatomi, T. et al. Expression of secretory mucin genes by human conjunctival epithelia. Invest. Ophthalmol. Vis. Sci. 37, ( 1996 ) Gipson, I. K. et al. Mucin genes expressed by human female reproductive tract epithelia. Biol. Reprod. 56, ( 1997 ) Spurr-Michaud, S., Argueso, P. & Gipson, I. Assay of mucins in human tear fl uid. Exp. Eye Res. 84, ( 2007 ) Gipson, I. K. et al. The Amount of MUC5B mucin in cervical mucus peaks at midcycle. J. Clin. Endocrinol. Metab. 86, ( 2001 ) Escande, F., Porchet, N., Aubert, J. P. & Buisine, M. P. The mouse Muc5b mucin gene: cdna and genomic structures, chromosomal localization and expression. Biochem. J. 363, ( 2002 ) Davies, J. R. et al. Respiratory tract mucins: structure and expression patterns. Novartis Found Symp. 248, ( 2002 ) Vandenhaute, B. et al. Mucin gene expression in biliary epithelial cells. J. Hepatol. 27, ( 1997 ) Russo, C. L. et al. Mucin gene expression in human male urogenital tract epithelia. Hum. Reprod. 21, ( 2006 ) Toribara, N. W. et al. Human gastric mucin. Identifi cation of a unique species by expression cloning. J. Biol. Chem. 268, ( 1993 ) Debolos, C., Garrido, M. & Real, F. X. MUC6 apomucin shows a distinct normal tissue distribution that correlates with lewis antigen expression in the human stomach. Gastroenterology 109, ( 1995 ) Bartman, A. E. et al. The MUC6 secretory mucin gene is expressed in a wide variety of epithelial tissues. J. Pathol. 186, ( 1998 ) Yu, D. F. et al. MUC19 expression in human ocular surface and lacrimal gland and its alteration in Sjogren syndrome patients. Exp. Eye Res. 86, ( 2007 ) Bobek, L. A., Tsai, H., Biesbrock, A. R. & Levine, M. J. Molecular cloning, sequence, and specifi city of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). J. Biol. Chem. 268, ( 1993 ) Sharma, P. et al. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am. J. Respir. Cell Mol. Biol. 19, ( 1998 ) Pinkus, G. S. & Kurtin, P. J. Epithelial membrane antigen a diagnostic discriminant in surgical pathology: immunohistochemical profi le in epithelial, mesenchymal, and hematopoietic neoplasms using paraffi n sections and monoclonal antibodies. Hum. Pathol. 16, ( 1985 ) Swallow, D. M. et al. The human tumour-associated epithelial mucins are coded by an expressed hypervariable gene locus PUM. Nature 328, ( 1987 ) Wreschner, D. H. et al. Human epithelial tumor antigen cdna sequences. Differential splicing may generate multiple protein forms. Eur. J. Biochem. 189, ( 1990 ) Ho, S. B. et al. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 55, ( 1995 ) Gipson, I. K. & Inatomi, T. Mucin genes expressed by the ocular surface epithelium. Prog. Retinal Eye Res. 16, ( 1997 ) Wykes, M. et al. MUC1 epithelial mucin (CD227) is expressed by activated dendritic cells. J. Leukoc. Biol. 72, ( 2002 ) Ho, S. B. et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 53, ( 1993 ) Pratt, W. S. et al. Multiple transcripts of MUC3: evidence for two genes, MUC3A and MUC3B. Biochem. Biophys. Res. Commun. 275, ( 2000 ) Porchet, N. et al. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cdna containing tandemly repeated sequences of 48 base pairs. Biochem. Biophys. Res. Commun. 175, ( 1991 ) Moniaux, N. et al. Alternative splicing generates a family of putative secreted and membrane-associated MUC4 mucins. Eur. J. Biochem. 267, ( 2000 ) Gipson, I. K. et al. MUC4 and MUC5b transcripts are the prevalent mucin messenger ribonucleic acids of the human endocervix. Biol. Reprod. 60, ( 1999 ) Packer, L. M., Williams, S. J., Callaghan, S., Gotley, D. C. & McGuckin, M. A. Expression of the cell surface mucin gene family in adenocarcinomas. Int. J. Oncol. 25, ( 2004 ) Pallesen, L. T., Berglund, L., Rasmussen, L. K., Petersen, T. E. & Rasmussen, J. T. Isolation and characterization of MUC15, a novel cell membrane-associated mucin. Eur. J. Biochem. 269, ( 2002 ) Yin, B. W. & Lloyd, K. O. Molecular cloning of the CA125 ovarian cancer antigen: identifi cation as a new mucin, MUC16. J. Biol. Chem. 276, ( 2001 ). 196 VOLUME 1 NUMBER 3 MAY
59 REVIEW 265. Hori, Y., Spurr-Michaud, S., Russo, C. L., Argueso, P. & Gipson, I. K. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest. Ophthalmol. Vis. Sci. 45, ( 2004 ) Andersch-Bjorkman, Y., Thomsson, K. A., Holmen Larsson, J. M., Ekerhovd, E. & Hansson, G. C. Large scale identifi cation of proteins, mucins, and their O -glycosylation in the endocervical mucus during the menstrual cycle. Mol. Cell Proteomics 6, ( 2007 ) Gipson, I. K. et al. MUC16 is lost from the uterodome (pinopode) surface of the receptive human endometrium: in vitro evidence that MUC16 is a barrier to trophoblast adherence. Biol. Reprod. 78, ( 2008 ) Gum, J. R. Jr, Crawley, S. C., Hicks, J. W., Szymkowski, D. E. & Kim, Y. S. MUC17, a novel membrane-tethered mucin. Biochem. Biophys. Res. Commun. 291, ( 2002 ) Higuchi, T. et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J. Biol. Chem. 279, ( 2004 ) Kubiet, M., Ramphal, R., Weber, A. & Smith, A. Pilus-mediated adherence of Haemophilus infl uenzae to human respiratory mucins. Infect. Immun. 68, ( 2000 ) Scharfman, A. et al. Adhesion of Pseudomonas aeruginosa to respiratory mucins and expression of mucin-binding proteins are increased by limiting iron during growth. Infect. Immun. 64, ( 1996 ) Shuter, J., Hatcher, V. B. & Lowy, F. D. Staphylococcus aureus binding to human nasal mucin. Infect. Immun. 64, ( 1996 ) Reddy, M. S. Binding of the pili of Pseudomonas aeruginosa to a low-molecular-weight mucin and neutral cystatin of human submandibular-sublingual saliva. Curr. Microbiol. 37, ( 1998 ) Hoffman, M. P. & Haidaris, C. G. Analysis of Candida albicans adhesion to salivary mucin. Infect. Immun. 61, ( 1993 ) Veerman, E. C. et al. Sulfated glycans on oral mucin as receptors for Helicobacter pylori. Glycobiology 7, ( 1997 ) Murray, P. A., Prakobphol, A., Lee, T., Hoover, C. I. & Fisher, S. J. Adherence of oral streptococci to salivary glycoproteins. Infect. Immun. 60, ( 1992 ) Prakobphol, A. et al. Separate oligosaccharide determinants mediate interactions of the low-molecular-weight salivary mucin with neutrophils and bacteria. Biochem. 38, ( 1999 ) Liu, B. et al. The recombinant N-terminal region of human salivary mucin MG2 (MUC7) contains a binding domain for oral Streptococci and exhibits candidacidal activity. Biochem. J. 345, ( 2000 ) Liu, B. et al. Interaction of human salivary mucin MG2, its recombinant N-terminal region and a synthetic peptide with Actinobacillus actinomycetemcomitans. J. Periodontal. Res. 37, ( 2002 ) Bosch, J. A. et al. Stress as a determinant of saliva-mediated adherence and coadherence of oral and nonoral microorganisms. Psychosom. Med. 65, ( 2003 ) Hirmo, S., Artursson, E., Puu, G., Wadstrom, T. & Nilsson, B. Helicobacter pylori interactions with human gastric mucin studied with a resonant mirror biosensor. J. Microbiol. Methods 37, ( 1999 ) Linden, S. et al. Strain- and blood group-dependent binding of Helicobacter pylori to human gastric MUC5AC glycoforms. Gastroenterology 123, ( 2002 ) Sun, R., Anderson, T. J., Erickson, A. K., Nelson, E. A. & Francis, D. H. Inhibition of adhesion of Escherichia coli k88ac fi mbria to its receptor, intestinal mucin-type glycoproteins, by a monoclonal antibody directed against a variable domain of the fi mbria. Infect. Immun. 68, ( 2000 ) Mack, D. R. & Blainnelson, P. L. Disparate in vitro inhibition of adhesion of enteropathogenic Escherichia coli rdec-1 by mucins isolated from various regions of the intestinal tract. Pediatr. Res. 37, ( 1995 ) Rajkumar, R., Devaraj, H. & Niranjali, S. Binding of Shigella to rat and human intestinal mucin. Mol. Cell Biochem. 178, ( 1998 ) Vimal, D. B., Khullar, M., Gupta, S. & Ganguly, N. K. Intestinal mucins: the binding sites for Salmonella typhimurium. Mol. Cell Biochem. 204, ( 2000 ) Jin, L. Z. & Zhao, X. Intestinal receptors for adhesive fi mbriae of enterotoxigenic Escherichia coli (ETEC) K88 in swine a review. Appl. Microbiol. Biotechnol. 54, ( 2000 ) Sylvester, F. A., Philpott, D., Gold, B., Lastovica, A. & Forstner, J. F. Adherence to lipids and intestinal mucin by a recently recognized human pathogen, Campylobacter upsaliensis. Infect. Immun. 64, ( 1996 ) Mantle, M. & Husar, S. D. Binding of Yersinia enterocolitica to purifi ed, native small intestinal mucins from rabbits and humans involves interactions with the mucin carbohydrate moiety. Infect. Immun. 62, ( 1994 ) de Repentigny, L., Aumont, F., Bernard, K. & Belhumeur, P. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect. Immun. 68, ( 2000 ). MucosalImmunology VOLUME 1 NUMBER 3 MAY
60 Article: The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Paszek et al, Nature 511: 319 (2014)
61 ARTICLE doi: /nature13535 The cancer glycocalyx mechanically primes integrin-mediated growth and survival Matthew J. Paszek 1,2,3,4, Christopher C. DuFort 1,2, Olivier Rossier 5,6, Russell Bainer 1,2, Janna K. Mouw 1, Kamil Godula 7,8 {, Jason E. Hudak 7, Jonathon N. Lakins 1, Amanda C. Wijekoon 1,2, Luke Cassereau 1,2, Matthew G. Rubashkin 1,2, Mark J. Magbanua 9,10, Kurt S. Thorn 11, Michael W. Davidson 12, Hope S. Rugo 9,10, John W. Park 9,10, Daniel A. Hammer 13, Grégory Giannone 5,6, Carolyn R. Bertozzi 7,14,15 & Valerie M. Weaver 1,2,9,16 Malignancy is associated with altered expression of glycans and glycoproteins that contribute to the cellular glycocalyx. We constructed a glycoprotein expression signature, which revealed that metastatic tumours upregulate expression of bulky glycoproteins. A computational model predicted that these glycoproteins would influence transmembrane receptor spatial organization and function. We tested this prediction by investigating whether bulky glycoproteins in the glycocalyx promote a tumour phenotype in human cells by increasing integrin adhesion and signalling. Our data revealed that a bulky glycocalyx facilitates integrin clustering by funnelling active integrins into adhesions and altering integrin state by applying tension to matrix-bound integrins, independent of actomyosin contractility. Expression of large tumourassociated glycoproteins in non-transformed mammary cells promoted focal adhesion assembly and facilitated integrindependent growth factor signalling to support cell growth and survival. Clinical studies revealed that large glycoproteins are abundantly expressed on circulating tumour cells from patients with advanced disease. Thus, a bulky glycocalyx is a feature of tumour cells that could foster metastasis by mechanically enhancing cell-surface receptor function. The composition of cell surface glycans and glycoproteins changes markedly and in tandem with cell fate transitions occurring in embryogenesis, tissue development, stem-cell differentiation and diseases such as cancer 1 3. Nevertheless, our understanding of the biochemical functions of glycans fails to explain fully why broad changes in glycosylation and glycoprotein expression are critical to cell fate specification and in what ways are they linked to disease. It is currently unclear whether changes in glycan and glycoprotein expression reflect a global and more general mechanism that directs cell and tissue behaviour. From a materials perspective, glycan and glycoprotein expression dictates the bulk physical properties of the glycocalyx the exterior cell surface layer across which information flows from the microenvironment to signal transduction pathways originating at the plasma membrane. Although the biophysical functions of the glycocalyx are largely untested, computational models predict that bulky glycoproteins can promote transmembrane receptor organization, including the clustering of integrins at adhesion sites 4. These models suggest that glycocalyxmediated integrin clustering would promote the assembly of mature adhesion complexes and collaborate to enhance growth factor signalling 5 phenotypes that are associated with cancer 6,7. We demonstrate that a global modulation of the physical properties of the glycocalyx alters integrin organization and function, and present evidence for how the glycocalyx can be co-opted in malignancy to support tumour cell growth and survival. Regulation of integrin assembly by bulky glycoproteins To determine whether glycocalyx bulk contributes to a cancer phenotype, we used gene expression microarray data to relate metastasis to expression of genes for which protein products contribute to the glycocalyx. The likely contribution of gene product to glycocalyx bulk was estimated based on the protein s extracellular domain structure and predicted number of glycosylation sites (Extended Data Fig. 1). Using these estimates we obtained evidence for upregulation of transcripts encoding bulky glycoproteins and some classes of glycosyltransferases, which catalyse the glycosylation of cell surface proteins, in primary tumours of patients with distant metastases relative to those with localized tumour growth (P for bulky transmembrane proteins, Kolmogorov Smirnov test; Fig. 1a and Extended Data Fig. 1). To understand whether bulky glycoproteins could promote tumour aggression by regulating integrin adhesions, we developed an integrated biochemical and mechanical model that incorporates integrins, the extracellular matrix (ECM), the cell membrane and the glycocalyx (Extended Data Fig. 2). The model revealed that the kinetic rates of integrin ECM interactions are tightly coupled to the distances between receptor ligand pairs and, thus, the physical constraints imposed by the glycocalyx. In the presence of bulky glycoproteins, the model predicted that integrin ECM binding is most favourable at sites of pre-existing adhesive contact, where the membrane and ECM substrate are in closest proximity (Fig. 1b). Elsewhere, bulky glycoproteins sterically restrict efficient 1 Department of Surgery and Center for Bioengineering and Tissue Regeneration, University of California, San Francisco, California 94143, USA. 2 Bay Area Physical Sciences-Oncology Program, University of California, Berkeley, California 94720, USA. 3 School of Chemical and Biomolecular Engineering, Cornell University, Ithaca, New York 14853, USA. 4 Laboratory for Atomic and Solid State Physics and Kavli Institute at Cornell for Nanoscale Science, Cornell University, Ithaca, New York 14853, USA. 5 Interdisciplinary Institute for Neuroscience, University of Bordeaux, UMR 5297, F Bordeaux, France. 6 CNRS, Interdisciplinary Institute for Neuroscience, University of Bordeaux, UMR 5297, F Bordeaux, France. 7 Department of Chemistry, University of California, Berkeley, California 94720, USA. 8 The Molecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA. 9 Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, California 94143, USA. 10 Division of Hematology/Oncology, University of California, San Francisco, California 94143, USA. 11 Department of Biochemistry and Biophysics, University of California, San Francisco, California 94158, USA. 12 National High Magnetic Field Laboratory and Department of Biological Science, The Florida State University, Tallahassee, Florida 32310, USA. 13 Departments of Chemical and Biomolecular Engineering and Bioengineering, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA. 14 Department of Molecular Biology, University of California, Berkeley, California 94720, USA. 15 Howard Hughes Medical Institute, University of California, Berkeley, California 94720, USA. 16 Departments of Anatomy and Bioengineering and Therapeutic Sciences and Eli and Edythe Broad Center for Regeneration Medicine and Stem Cell Research, University of California, San Francisco, California 94143, USA. {Present address: Department of Chemistry and Biochemistry, University of California, San Diego, California 92093, USA Macmillan Publishers Limited. All rights reserved 00 MONTH 2014 VOL 000 NATURE 1
62 RESEARCH ARTICLE a One-sided P e Short (3 nm) Long (80 nm) All genes Upregulation with metastasis Mimetic Membrane Bulky Vinculin b Relative bond rate Merge Computational model Glycocalyx thickness (nm) Distance from adhesion (nm) f Short (3 nm) c Mean: 75 nm Glyoprotein Slow Integrin d Extracellular matrix Figure 1 The cancer glycocalyx drives integrin clustering. a, Violin plots showing increased expression of genes encoding bulky transmembrane proteins in primary tumours of patients with distant metastases relative to those with local invasion. White dots and thick black lines indicate the median and interquartile range of the P value distribution of all transcripts within each class: all genes, all membrane proteins (Mem.), and bulky transmembrane proteins (Bulky). b, Computed relative rate of integrin ECM ligand bond formation as a function of distance from a pre-existing adhesion cluster. c, Model of proposed glycocalyx-mediated integrin clustering. Shorter distances between integrin ligand pairs result in faster kinetic rates of binding. Fast Kinetic trap Long (80 nm) Mean: 94 nm Slow Membrane height (nm) g Adhesion rate (pn s 1 ) d Lipid tail None Mimetic *** Glycoprotein mimetics HO OH O HO AcHN O N HO OH O HO AcHN O N 3 nm 80 nm h Adhesion area (μm 2 ) HO OH AcHN O OH N O Mimetic *** None 3 nm 30 nm 80 nm d, Cartoon showing structure of glycoprotein mimetics with lipid insertion domain. e, Fluorescence micrographs of MEC adhesion complexes (vinculin mcherry) and glycomimetics of the indicated length (scale bar, 3 mm). f, SAIM images of DiI-labelled ventral plasma membrane topography in MECs incorporated with glycomimetics (scale bar, 2.5 mm). g, Rate of integrin substrate adhesion measured using single cell force spectroscopy in MECs with incorporated glycomimetics. h, Quantification of the total adhesion complex area per cell in MECs with incorporated glycomimetics. All results are the mean 6 s.e.m. of three separate experiments. Statistical significance is given by *P, 0.05; **P, 0.01; ***P, integrin matrix engagement (Fig. 1b) by increasing the gap between the plasma membrane and ECM. Thus, the model predicted that whereas bulky glycoproteins reduce the overall integrin-binding rate, they enhance, rather than impede, integrin clustering and focal adhesion assembly by generating a physically based kinetic trap (Fig. 1c). To test experimentally whether bulky glycoproteins could drive integrin clustering and focal adhesion assembly, we generated a series of synthetic mucin glycoprotein mimetics of increasing length that rapidly intercalate into the plasma membrane and project perpendicularly to the cell surface 8,9. These glycopolymers consisted of a long-chain polymer backbone, pendant glycan chains that mimic the structures of natural mucin O-glycans, a phospholipid for membrane insertion, and a fluorophore for imaging (Fig. 1d and Extended Data Fig. 3a c). We found that large glycopolymers with lengths of 80 nm, significantly longer than the reported integrin length of 20 nm 10, are consistently excluded from sites of integrin adhesion on the surface of non-malignant mammary epithelial cells (MECs; Fig. 1e). Shorter polymers with lengths of 3 or 30 nm were not excluded (Fig. 1e). Because the mimetics possessed minimal biochemical interactivity with cell surface proteins (Extended Data Fig. 3d), the data suggest a physical interplay between bulky glycoproteins and integrin receptors. To determine how the largest polymer mimetics influence the nanoscale spatial features of the cell ECM interface, we measured the topography of the ventral cell membrane using scanning angle interference microscopy (SAIM), a fluorescence-based microscopy technique that enables imaging with 5 10-nm axial resolution and diffraction-limited (,250 nm) lateral resolution 11. Polymers designed to mimic large native glycoproteins (,80 nm) expanded the membrane ECM gap by 19 nm (Fig. 1f). Consistent with computational predictions, the large glycoprotein mimetics reduced the overall rate of integrin bond formation, but significantly enhanced clustering of integrins into focal adhesions (Fig. 1g, h). Shorter glycoprotein mimetics (3 and 30 nm) did not have an impact on integrin clustering, even when incorporated at higher surface densities (Fig. 1h). We next asked whether cancer-associated glycoproteins could similarly influence the spatial distribution of integrins and the assembly of focal adhesions. On the basis of our large-scale gene expression analysis, we determined that the transmembrane mucin glycoprotein, MUC1, which has a highly glycosylated ectodomain that projects out up to 200 nm from the cell surface 12, was upregulated in metastatic tumours (nominal P via one-sided t-test). In agreement with our analysis, we measured high levels of MUC1 on the surface of several breast cancer cell lines, as well as v-src and HRAS-transformed MECs (Fig. 2a). To assess the impact of MUC1 on focal adhesion assembly, we expressed MUC1 on the surface of non-malignant MECs, to levels comparable to those of transformed MECs and breast cancer lines. MUC1 expression induced striking membrane topographical features, which included regions of high curvature, and a significant expansion of the cell membrane ECM gap (Fig. 2b, c and Extended Data Fig. 4a). Expression of an ectodomain-truncated MUC1 construct did not significantly change the gap compared to control MECs (Fig. 2c and Extended Data Fig. 4a). Our model predicted that the membrane topographies we observed in MUC1-expresing cells would facilitate integrin clustering through the kinetic trap. In agreement with these predictions, expression of full-length MUC1 significantly enhanced the size of adhesion clusters and the total adhesion area per cell (Fig. 2d, e and Extended Data Fig. 5a). The adhesion assembly phenotype did not require the MUC1 cytoplasmic tail, which mediates MUC1 s biochemical activity (Fig. 2e) 13, or direct interactions between MUC1 and fibronectin (Extended Data Fig. 5b). Together, these results are consistent with a physically based mechanism of integrin clustering. To gain additional insight into the coupled dynamics between integrins and MUC1, we conducted time-lapse imaging of fluorescently labelled MUC1 and the adhesion plaque protein vinculin. We observed that MUC1 and integrin adhesions spatially segregate on the cell surface in a temporally correlated manner (Fig. 2d, Extended Data Fig. 5c and Supplementary Movie 1), suggesting a physical communication between these molecules. Further evidence for a physical interplay between 2 NATURE VOL MONTH Macmillan Publishers Limited. All rights reserved
63 ARTICLE RESEARCH a MUC1 Normalized counts c Membrane height (nm) e Adhesion area (μm 2 ) A-Cont. 10A-v-Src 10A-HRAS Intensity (AU) ** *** MCF7 T47D ** Control +MUC1(ΔTR) +MUC1 Control +MUC1(ΔTR) +MUC1(ΔCT) d +MUC1 +MUC1(ΔTR) b Control +MUC1 50 MUC1 Membrane 74 Membrane height (nm) Vinculin Membrane (ROI) MUC1/Vinc. (ROI) f MUC1 tracks/paxillin Tracks/paxillin (ROI) MUC1 and integrins was obtained in mouse embryonic fibroblasts (MEFs) using single-particle tracking photo-activation localization microscopy (sptpalm 14,15 ) to track MUC1 diffusive trajectories. We noted that whereas MUC1 was mobile in the plasma membrane, it rarely crossed into integrin adhesion zones (Fig. 2f and Extended Data Fig. 6a). We next tested our model s prediction that MUC1 would favour integrin clustering by physically impeding integrin ECM binding outside of adhesive contacts. We recorded the trajectories of individual b 3 integrin molecules using sptpalm to determine the location and fraction of mobile (confined and freely diffusive) and matrix-bound, immobilized Figure 2 The bulky cancer-associated glycoprotein MUC1 drives integrin clustering. a, Cartoon of MUC1 and quantification of MUC1 cell-surface levels on control (10A-Cont.), transformed (10A-v-Src, 10A-HRAS) and tumour (MCF7, T47D) cells. b, Topographical SAIM images of representative mcherry CAAX-labelled ventral plasma membranes in control and MUC1 GFP-expressing (1MUC1) MECs (Scale bar, 5 mm; region of interest (ROI) scale bar, 2 mm). c, Quantification of mean plasma membrane height in control MECs and those ectopically expressing ectodomain-truncated MUC1 GFP (1MUC1(DTR)) and wild-type MUC1 GFP (1MUC1). Results are the mean 6 s.e.m. of at least 15 cell measurements in duplicate experiments. d, Fluorescence micrographs of MUC1(DTR) or wild-type MUC1 expressed in MECs and their focal adhesions labelled with vinculin mcherry (scale bar, 3 mm; ROI scale bar, 1.5 mm). e, Quantification of the total adhesion complex area per cell in control non-malignant MECs (control) and those ectopically expressing MUC1(DTR), wild-type MUC1, or cytoplasmic-tail-deleted MUC1 (1MUC1(DCT)). Results are the mean 6 s.e.m. of three separate experiments. f, Left panel: trajectories of individual meos2-tagged MUC1 proteins recorded at 50 Hz using sptpalm (green) and focal adhesions visualized with paxillin GFP (red) in MEFs (scale bar, 3 mm). Right panel: the ROI from the left panel with individual MUC1 tracks displayed in multiple colours (scale bar, 1 mm). Statistical significance is given by *P, 0.05; **P, 0.01; ***P, integrin 15. Analysis of b 3 integrin trajectories after manganese activation in MEFs revealed a significant increase in the total level of immobilized integrin at the plasma membrane, both inside and outside adhesive contacts (Fig. 3a and Extended Data Fig. 6b e). By contrast, the immobilized b 3 integrin in MEFs expressing high MUC1 was restricted to sites of adhesion (Fig. 3a c and Extended Data Fig. 6e). These results are consistent with single-cell force spectroscopy measurements, which indicated that MUC1 expression reduces the net rate of integrin ECM bond formation (Extended Data Fig. 5d). Mucin expression did not have a significant impact on the free diffusion of integrins (Extended Data Fig. 6b d). Importantly, we observed that integrins frequently diffused across the mucin adhesive zone boundary and could immobilize rapidly once in the adhesive zone (Fig. 3d, Extended Data Fig. 6f, g and Supplementary Movie 2). Together, our results indicate that large glycoproteins act as physical steric barriers that impede integrin immobilization and thus funnel integrins into adhesive contacts. Bulky glycoproteins exert force on integrin bonds Integrins switch between activity states by undergoing a conformational change that is facilitated by tensile force 16,17. Given the order of magnitude difference in the size of MUC1 (,200 nm 12 ) as compared to integrins (,20 nm 10 ), and the close proximity of these molecules within the cell ECM interface, we hypothesized that large glycoproteins, such as MUC1, modify integrin structure and function by applying force to matrix-bound receptors. Abiding by Newton s third law, if large glycoproteins exert a tensile force on integrins, then we should detect a reciprocal strain on the glycoproteins. Consistent with this hypothesis, mucins imaged with SAIM appeared compressed or mechanically bent near integrin adhesive contacts (Fig. 4a and Extended Data Fig. 4a). Furthermore, single-cell force spectroscopy revealed that MECs expressing high levels of exogenous MUC1 required higher compressive force application at the ECM substrate interface to promote integrin-mediated adhesion when compared to control MECs (Fig. 4b). To test further whether integrin adhesions strain bulky transmembrane glycoproteins, we generated a genetically encoded construct conceptually similar to a strain gauge, consisting of a cysteine-free cyan and yellow fluorescent protein pair (CFP and YFP) separated by an elastic linker 18, which we inserted into the ectodomain of full-length and truncated MUC1 proteins (Fig. 4c and Extended Data Fig. 4b). Fluorescence resonance energy transfer (FRET) served as the readout of distance between the CFP and YFP pair and, thus, functioned as a reporter of molecular strain. When the full-length reporter was expressed in MECs, we observed high FRET efficiencies in the cell substrate interface (Fig. 4d, e and Extended Data Fig. 7). FRET efficiency was significantly lower in MECs expressing the ectodomain-truncated construct, indicative of lower molecular strain (Fig. 4d, e). The highest FRET efficiencies correlated spatially with sites of adhesive contact, consistent with integrin adhesions straining bulky transmembrane glycoproteins and glycoproteins exerting a reciprocal restoring force on integrins (Fig. 4d and Extended Data Fig. 7e, f). We next examined whether the bulky glycoprotein MUC1 could induce conformational changes that would activate integrins independent of the contractile cytoskeleton. We used a bi-functional crosslinker that can specifically link extracellular fibronectin and bound a 5 b 1 integrins that are in a tension-dependent conformation 17. Inhibition of actomyosin contractility, using the myosin inhibitor blebbistatin or the Rho kinase inhibitor Y-27632, abrogated most of the fibronectin crosslinked integrins in MECs expressing empty vector (Fig. 4f and Extended Data Fig. 8a). By contrast, MUC1-expressing MECs formed tensioned bonds with the ECM substrate, even when cells were pre-treated with contractility inhibitors before plating (Fig. 4f and Extended Data Fig. 8a). Of note, the myosin-independent integrin clusters observed in the MUC1- expressing MECs recruited activated cytoplasmic adaptors typically associated with mature adhesion structures and nucleated actin (Extended Data Fig. 8b). These results suggest that large, cancer-associated glycoproteins not only facilitate integrin clustering but also physically alter 2014 Macmillan Publishers Limited. All rights reserved 00 MONTH 2014 VOL 000 NATURE 3
64 RESEARCH ARTICLE a No Mn 2+ Mn2+ activation Control +MUC1 Paxillin/β 3 integrin Paxillin/β 3 tracks (ROI) MUC1/β 3 integrin MUC1/β 3 tracks (ROI) Figure 3 Bulky glycoproteins spatially regulate immobilization of activated integrins. a, Left panels: fluorescence micrographs displaying paxillin GFP-labelled focal adhesions in control cells or MUC1-rich regions in MUC1 GFP-expressing MEFs, and positions of individual meos2-fused b3 integrins. Cells were treated without or with Mn 21 to activate integrins (scale bar, 3 mm). Right panels: magnified area of interest showing fluorescence micrographs of focal adhesions visualized with paxillin GFP in control MEFs or MUC1 in MUC1 GFP-expressing MEFs, and individual b3 integrin trajectories recorded with sptpalm. Single molecule trajectories are colourcoded to indicate immobile and mobile (confined and freely diffusive) b 3 integrin state and do so, at least in part, independently of cytoskeletal tension. Bulky glycoproteins promote growth and survival Tumour metastasis is a multi-step process that depends on the efficient dissemination of primary cancer cells and their subsequent colonization Mobile (free) Mobile (confined) Immobile b Control - outside adh. contacts 25 Imm. Mobile 20 β 3 15 β 3 + Mn Occurence (%) c Outside adhesive contacts (%) Cont. Mn 2+ + Mn 2+ *** +MUC1 Mn 2+ + Mn 2+ +MUC1 - inside MUC1-rich areas 25 Imm. Mobile 20 β 3 15 β 3 + Mn log[d (μm 2 s 1 )] at distant metastatic sites 19. Thus, the ability to survive, particularly within unfavourable microenvironments and under minimally adhesive conditions, is a prerequisite for efficient tumour cell metastasis 19. Given their ability to promote integrin adhesion assembly, we hypothesized that bulky glycoproteins could facilitate metastasis by promoting focal adhesion signalling to enhance tumour cell growth and survival. Free Confined Immobile + MUC1: inside adh. contacts 15 Imm. Mobile β 3 10 β 3 + Mn MUC1-rich Adhesion zone integrins (scale bar, 1 mm). b, Distribution of b 3 integrin diffusion coefficients recorded before or after Mn 21 treatment in control MEFs outside of adhesive contacts (left), MUC1-transfected MEFs inside MUC1-rich areas (middle), and MUC1-transfected MEFs outside MUC1-rich areas, including adhesive contacts (right). c, Fraction of immobilized, confined and freely diffusive b 3 integrins outside of adhesive contacts in control MEFs (Ctrl) and MUC1- transfected cells (MUC1) before and after Mn 21 treatment. Results are the mean 6 s.e.m. Statistical significance is given by *P, 0.05; **P, 0.01; ***P, d, Fluorescence micrograph of MUC1 GFP and an illustrative single integrin trajectory in MEFs treated with Mn 21 (scale bar, 1 mm). d Free Immobile a GFP Mem. MUC1 fluorescence Vinculin (ROI) MUC1 height (ROI) Height (nm) b Adhesion rate (pn s 1 ) Ctrl +MUC1 c 527 nm FRET CFP YFP Substrate ,000 Compressive force (pn) 440 nm d +MUC1(ΔTR) sensor FRET Vinc. (ROI) FRET (ROI) +MUC1 sensor FRET Vinc. (ROI) FRET (ROI) FRET efficiency (%) e Normalized counts (no.) 1.0 MUC1(ΔTR) 0.8 MUC FRET efficiency (%) f Tensed integrin area (μm 2 ) Ctrl *** ** DMSO Blebb. Y27632 *** ** +MUC1 DMSO Blebb. Y27632 Figure 4 Integrins are mechanically loaded and re-enforced by bulky glycoproteins. a, GFP-fluorescence and topographic SAIM images of MUC1 GFP (scale bars, 3 mm) and the corresponding focal adhesions visualized with vinculin mcherry. b, Adhesion rate versus force of contact between cell and substrate (compressive force) measured with single-cell force spectroscopy for control and MUC1-expressing MECs. Results are the mean 6 s.e.m. of at least 10 cell measurements per point. c, Schematic of FRETbased MUC1 compressive strain gauge. d, FRET efficiency maps of ectodomain-truncated (1MUC1(DTR) sensor) and wild-type (1MUC1 sensor) strain gauges measured at the ventral cell surface of MECs and the corresponding vinculin mcherry-labelled focal adhesions (scale bar, 8 mm; ROI scale bar, 1 mm). e, Histogram of observed FRET efficiencies of wild-type MUC1 and MUC1(DTR) strain gauges. f, Quantification of fibronectincrosslinked a5 integrin in control and MUC1-expressing normal MECs treated with solvent alone (DMSO), myosin-ii inhibitor (blebbistatin; 50 mm), or Rho kinase inhibitor (Y-27632; 10 mm) for 1 h followed by detergent-extraction to reveal the fibronectin bound integrin that is under mechanical tension. Results are the mean 6 s.e.m. of three separate experiments. Statistical significance is given by *P, 0.05; **P, 0.01; ***P, NATURE VOL MONTH Macmillan Publishers Limited. All rights reserved
65 ARTICLE RESEARCH Consistent with this notion, analysis of human data sets revealed that patients with aggressive breast cancers that presented with circulating tumour cells (CTCs) express disproportionately high amounts of bulky glycoproteins and have altered glycosyltransferase expression profiles (Fig. 5a and Extended Data Fig. 1d, e). Furthermore, analysis of genes expressed within CTCs isolated from a cohort of breast cancer patients with metastatic disease confirmed that several predicted bulky glycoproteins could be detected in these patient samples (Fig. 5b). We next examined whether a bulky glycocalyx could promote growth and survival of non-malignant MECs. Using our glycoprotein mimetics, we observed that untreated MECs or MECs incorporated with short (3 nm) or medium (30 nm) length mimetics were not viable h after they were plated on highly compliant hydrogel substrates that mimic the stiffness of soft sites of colonization, like lung or brain (Young s modulus, E Pa; Fig. 5c). By contrast, MECs incorporated with long glycoprotein mimetics (80 nm) remained viable (Fig. 5c). Analysis of gene expression profiles and immunofluorescence analysis of freshly isolated CTCs in our human metastatic breast cancer cohort revealed that MUC1 could be detected in most of the samples examined (Fig. 5b). Similar to results with the synthetic mimetics, we observed that ectopic expression of either full-length or a tailless, signalling-defective MUC1 in non-malignant MECs permitted their growth and survival even when plated as single cells on compliant hydrogels (Fig. 5d and Extended Data Fig. 9a). We noted that the CTCs in our cohort also expressed high levels of CD44, a receptor that binds and retains bulky hyaluronic acid (HA) glycan structures on the cell surface (Extended Data Fig. 10a) 20. Similar to our observations with MUC1 and bulky glycoprotein mimetics, we observed that HA and integrins exhibit an anti-correlated spatial distribution on the surface of transformed MECs (Extended Data Fig. 10b). Inhibition of HA synthesis or HA cell-surface retention significantly reduced the growth of transformed MECs on compliant hydrogels, raising the possibility that bulky cell-surface constituents, in addition to MUC1, could similarly promote tumour aggression (Extended Data Fig. 10b). However, unlike the experiments with tailless MUC1 or the glycoprotein mimetics, which lack signalling capability, we cannot exclude that HA-induced growth and survival phenotypes are not also, at least in part, induced through HA s direct biochemical signalling activity 20,21. We next addressed whether a bulky glycocalyx promotes MEC growth and survival by regulating focal adhesion assembly and crosstalk with growth factor signalling pathways 5,7. We found that pharmacological inhibition of kinases linked to growth factor signalling, including phosphoinositide 3-kianse (PI(3)K), mitogen-activated kinase, and Src kinase, each independently inhibited the growth and survival of MUC1-expressing MECs on highly compliant substrates (Fig. 5e). We also noted that the MUC1 growth and survival phenotype requires integrin engagement and integrin signalling through focal adhesion kinase (FAK), which mediates crosstalk between integrin and growth factor signalling pathways (Fig. 5f and Extended Data Fig. 9b) 5,6. Non-malignant MECs expressing the MUC1 ectodomain, but not control MECs, assembled distinct focal adhesion structures with activated Y397-phosphorylated FAK on compliant substrates (Extended Data Fig. 8c). Furthermore, MECs expressing wild-type or tailless, signalling defective MUC1, and plated on the compliant substrates showed enhanced Y118-phosphorylated paxillin, ERK and AKT activation in response to epidermal growth factor stimulation (Fig. 5g and Extended Data Fig. 8d). This response was attenuated by FAK inhibition (Fig. 5g, h and Extended Data Fig. 8d). a One-sided P f Proliferation (AU) Upregulated in CTCs All genes Membrane Bulky Soft ECM, +MUC1(ΔCT) FAK Inhib. DMSO Day b MUC1 CD44 EPCAM MEGF9 NOTCH1 SDC1 Muc5B L1CAM CHL1 MUC4 MUC16 g perk Total ERK Unstim. Control Human CTCs ERK activation Low High +MUC1(ΔCT) +MUC1(ΔCT) MUC1/Nuc. + : 91± 1.4% +MUC1(ΔCT) +FAKi Stim. Unstim. Stim. Unstim. Stim. Figure 5 Bulky glycoproteins promote cell survival and are expressed in CTCs. a, Violin plots showing that genes encoding bulky transmembrane proteins are more highly expressed in primary human tumours in patients with circulating tumour cells (CTCs). White dots and thick black lines indicate the median and interquartile range of the P-value distribution of transcripts of all cellular genes (all genes), all transmembrane proteins (membrane), and bulky transmembrane proteins (bulky). b, Heat map quantifying gene expression of bulky glycoproteins in CTCs isolated from 18 breast cancer patients (x axis; left), and representative immunofluorescence micrograph of MUC1 detected on human patient CTCs (right; scale bar, 5 mm). Quantification of the percentage of CTCs with detectable MUC1 is shown. c, Cell death in control non-malignant MECs and those with incorporated glycomimetics quantified 24 h after plating on a soft (140 Pa) fibronectin-conjugated hydrogel substrate. d, Cell death (left graph) and growth (right graph) of control MECs and those expressing cytoplasmic-tail-deleted MUC1 (1MUC1(DCT)) Cell death (%) h pakt (fold increase) c Ctrl Soft ECM Soft Stiff *** None 3 nm 30 nm 80 nm +MUC1(ΔCT) Soft Stiff ECM stiffness d Cell death (%) Control +MUC1(ΔCT) i Spatial control ECM Soft ECM Cells per colony (no.) Growth and survival *** Control +MUC1(ΔCT) Adhesion plaque e Cells per colony (no.) Soft ECM, +MUC1(ΔCT) *** *** *** Kinetic trap Integrin DMSO PI(3)K In. MEK In. Src In. Mucin Reciprocal force quantified 48 h after plating on a soft hydrogel. e, Quantification of the number of vehicle (DMSO), PI(3)K inhibitor, MEK inhibitor, or Src inhibitor-treated control and MUC1(DCT)-expressing MECs per colony 48 h after plating on a soft hydrogel. f, Proliferation of solvent (DMSO) or FAK-inhibitor-treated MUC1(DCT)-expressing MECs quantified at the indicated day after plating on soft hydrogels. g, Representative western blots showing phosphorylated and total ERK in control and MUC1(DCT)-expressing MECs plated on soft hydrogels unstimulated or stimulated with EGF. Cells were treated with solvent (control, 1MUC1(DCT)) or FAK inhibitor (1MUC1(DCT) 1 FAKi) before stimulation. h, Bar graphs showing quantification of immunoblots probed for activated AKT in control and MUC1(DCT)-expressing non-malignant MECs 24 h after plating on soft versus stiff hydrogels. i, Model summarizing biophysical regulation of integrin-dependent growth and survival by bulky glycoproteins. In all bar graphs, results are the mean 6 s.e.m. of at least 2 3 separate experiments (*P, 0.05; **P, 0.01; ***P, 0.001) Macmillan Publishers Limited. All rights reserved 00 MONTH 2014 VOL 000 NATURE 5
66 RESEARCH ARTICLE Together, these findings indicate that a bulky glycocalyx can promote tumour aggression by enhancing integrin-dependent growth and survival (Fig. 5i). Discussion We present evidence to support a new paradigm for the biological function of cell surface glycans and glycoproteins. Independent of, and in addition to, their biochemical properties, we demonstrate how bulky constituents of the glycocalyx can physically influence receptor organization and activity. Although the current investigation focuses on integrins, a bulky glycocalyx could, in principle, regulate any transmembrane receptor that interacts with a tethered ligand. Candidate systems include neurological and immunological synapses 22,cell celladhesions 23, and juxtacrine signalling complexes composed of receptors, like ephrin 24. Membrane topographical features imprinted by large glycoproteins could also directly influence plasma membrane lipid organization, protein sorting and endocytosis 25,26. The diversity of these processes suggests that the physiological relevance of the glycocalyx may be broad. For example, bulky glycoproteins and glycan structures, such as neuroligins, neurexins and polysialic acid, have a crucial role in neuronal development, maintenance and plasticity 27,28. Thus, it is plausible that the glycocalyx has a prominent role in orchestrating multiple biological processes occurring at the plasma membrane. Our observations provide a tractable explanation for why large glycan structures and glycoproteins, like HA and mucins, as well as regulatory enzymes, are so frequently elevated in many solid tumours 13,20. Indeed, the growth and survival advantages afforded by these molecules may preferentially select for cancer cells with a prominent glycocalyx and favour tumour cell dissemination and metastasis. Mechanical perturbations to cell and tissue structure have a causal role in tumour development and progression 29,30, and we now implicate the glycocalyx s importance in the metastatic mechano-phenotype. Our results suggest that the glycocalyx and its molecular constituents are attractive targets for therapeutic interventions aimed at normalizing transmembrane receptor signalling. METHODS SUMMARY Complete descriptions of the bioinformatics pipeline, computational model and expression constructs are presented in Supplementary Notes 1, 2 and 3, respectively. Compliant hydrogels were fabricated from soft polyacrylamide (E Pa) functionalized with fibronectin 31 and plated with single cells for all hydrogel experiments. FRET measurements were conducted in living cells on a spinning disk confocal (photobleaching FRET) or confocal (lifetime imaging) microscope 32 (see also Supplementary Note 4). SptPALM 15,SAIM 11, single cell force spectroscopy 33, integrin crosslinking 17, and fibronectin fibrillogenesis 34 measurements and assays were conducted as previously described. Glycoprotein mimetics were synthesized and characterized as described in Supplementary Note 6 and subsequently incubated with suspended cells (2 mm for 1 h) to incorporate onto the cell surface immediately before experimentation. For gene expression analysis of CTCs, 20 pools of CTCs were isolated from the blood of 18 metastatic breast cancer patients and quantified by qpcr 35. Immunofluorescence of CTCs was conducted on samples isolated from three patients 35. Online Content Methods, along with any additional Extended Data display items and Source Data, are available in the online version of the paper; references unique to these sections appear only in the online paper. Received 3 August 2013; accepted 23 May Published online 25 June Lanctot, P. M., Gage, F. H. & Varki, A. P. The glycans of stem cells. Curr. Opin. Chem. Biol. 11, (2007). 2. Haltiwanger, R. S. & Lowe, J. B. Role of glycosylation in development. Annu. Rev. Biochem. 73, (2004). 3. Varki, A., Kannagi, R. & Toole, B. P. in Essentials Glycobiol. (Varki, A. et al.) (Cold Spring Harbor Laboratory Press, 2009). 4. Paszek, M. J., Boettiger, D., Weaver, V. M. & Hammer, D. A. Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLOS Comput. Biol. 5, e (2009). 5. Walker, J. L., Fournier, A. K. & Assoian, R. K. Regulation of growth factor signaling and cell cycle progression by cell adhesion and adhesion-dependent changes in cellular tension. Cytokine Growth Factor Rev. 16, (2005). 6. Desgrosellier, J. S. & Cheresh, D. A. Integrins in cancer: biological implications and therapeutic opportunities. Nature Rev. Cancer 10, 9 22 (2010). 7. Shibue, T. & Weinberg, R. A. Integrin b1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl Acad. Sci. USA 106, (2009). 8. Godula, K. et al. Control of the molecular orientation of membrane-anchored biomimetic glycopolymers. J. Am. Chem. Soc. 131, (2009). 9. Godula, K., Rabuka, D., Nam, K. T. & Bertozzi, C. R. Synthesis and microcontact printing of dual end-functionalized mucin-like glycopolymers for microarray applications. Angew. Chem. Int. Edn Engl. 48, (2009). 10. Eng, E. T., Smagghe, B. J., Walz, T. & Springer, T. A. Intact aiibb3 integrin is extended after activation as measured by solution X-ray scattering and electron microscopy. J. Biol. Chem. 286, (2011). 11. Paszek, M. J. et al. Scanning angle interference microscopy reveals cell dynamics at the nanoscale. Nature Methods 9, (2012). 12. Hattrup, C. L. & Gendler, S. J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 70, (2008). 13. Kufe, D. W. Mucins in cancer: function, prognosis and therapy. Nature Rev. Cancer 9, (2009). 14. Manley, S. et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nature Methods 5, (2008). 15. Rossier, O. et al. Integrins b1 and b3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nature Cell Biol. 14, (2012). 16. Chen, W., Lou, J., Evans, E. A. & Zhu, C. Observing force-regulated conformational changes and ligand dissociation from a single integrin on cells. J. Cell Biol. 199, (2012). 17. Friedland, J. C., Lee, M. H. & Boettiger, D. Mechanically activated integrin switch controls alpha5beta1 function. Science 323, (2009). 18. Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, (2010). 19. Nguyen, D. X., Bos, P. D. & Massagué, J. Metastasis: from dissemination to organspecific colonization. Nature Rev. Cancer 9, (2009). 20. Toole, B. P. Hyaluronan: from extracellular glue to pericellular cue. Nature Rev. Cancer 4, (2004). 21. Bono, P., Rubin, K., Higgins, J. M. & Hynes, R. O. Layilin, a novel integral membrane protein, is a hyaluronan receptor. Mol. Biol. Cell 12, (2001). 22. Dustin, M. L. Signaling at neuro/immune synapses. J. Clin. Invest. 122, (2012). 23. Coombs, D., Dembo, M., Wofsy, C. & Goldstein, B. Equilibrium thermodynamics of cell-cell adhesion mediated by multiple ligand-receptor pairs. Biophys. J. 86, (2004). 24. Salaita, K. et al. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science 327, (2010). 25. Groves, J. T. Bending mechanics and molecular organization in biological membranes. Annu. Rev. Phys. Chem. 58, (2007). 26. Lundmark, R. & Carlsson, S. R. Driving membrane curvature in clathrin-dependent and clathrin-independent endocytosis. Semin. Cell Dev. Biol. 21, (2010). 27. Comoletti, D. et al. Synaptic arrangement of the neuroligin/b-neurexin complex revealed by X-ray and neutron scattering. Structure 15, (2007). 28. Hildebrandt, H., Mühlenhoff, M., Weinhold, B. & Gerardy-Schahn, R. Dissecting polysialic acid and NCAM functions in brain development. J. Neurochem. 103 (Suppl. 1), (2007). 29. Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, (2009). 30. Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, (2005). 31. Lakins, J. N., Chin, A. R. & Weaver, V. M. Exploring the link between human embryonic stem cell organization and fate using tension-calibrated extracellular matrix functionalized polyacrylamide gels. Methods Mol. Biol. 916, (2012). 32. Bruns, N., Pustelny, K., Bergeron, L. M., Whitehead, T. A. & Clark, D. S. Mechanical nanosensor based on FRET within a thermosome: damage-reporting polymeric materials. Angew. Chem. Int. Ed. 48, (2009). 33. Friedrichs, J., Helenius, J. & Muller, D. J. Quantifying cellular adhesion to extracellular matrix components by single-cell force spectroscopy. Nature Protocols 5, (2010). 34. Pankov, R. & Momchilova, A. Fluorescent labeling techniques for investigation of fibronectin fibrillogenesis (labeling fibronectin fibrillogenesis). Methods Mol. Biol. 522, (2009). 35. Magbanua, M. J. M. et al. Genomic profiling of isolated circulating tumor cells from metastatic breast cancer patients. Cancer Res. 73, (2013). Supplementary Information is available in the online version of the paper. Acknowledgements We thank S. Gendler, J. Goedhart and M. McMahon for cdnas, as indicated in the Methods section. We thank A. Walker for bioinformatics support, L. Hauranieh for assistance in CTC analysis, H. Aaron for assistance with FLIM, J. B. Sibarita and M. Lagardère for support in sptpalm analysis, B. Hoffman in design of the FRET sensor, and T. Wittmann in assistance with pbfret measurements. Image acquisition was partly performed at the Nikon Imaging Center and Biological Imaging Development Center at UCSF and the Berkeley Molecular Imaging Center. This work was supported by the Kavli Institute and UCSF Program for Biomedical Breakthrough postdoctoral fellowships to M.J.P.; DoD NDSEG Fellowship to M.G.R.; NIH GM59907 to C.R.B.; NIH Pathway to Independence Award K99 EB to K.G.; French 6 NATURE VOL MONTH Macmillan Publishers Limited. All rights reserved
67 ARTICLE RESEARCH Ministry of Research, CNRS, ANR grant Nanomotility, INSERM, Fondation ARC pour la Recherche sur le Cancer, France BioImaging ANR-10-INBS-04-01, and Conseil Régional Aquitaine to O.R. and G.G.; NIH AI A1 to D.A.H.; The Breast Cancer Research Foundation to M.J.M., H.S.R. and J.W.P.; NIH 2R01GM to C.R.B. and V.M.W.; and BCRP DOD Era of Hope Scholar Expansion grant BC122990, and NIH NCI grants U54CA , U54CA , 1U01 ES , and CA A1 to V.M.W. Author Contributions V.M.W. andm.j.p. conceived the project. V.M.W., M.J.P. and C.R.B. provided project management. M.J.P., A.C.W., M.W.D. and J.N.L. designed and constructed expression constructs. C.C.D. and M.J.P conducted single cell force spectroscopy measurements. M.J.P. and K.S.T. designed and implemented the interference microscope. M.J.P., L.C. and M.G.R. performed fluorescence, FRET and interferometric imaging and M.J.P. wrote the accompanying analysis software. O.R. and G.G. conducted sptpalm experiments and analysed the results. V.M.W., R.B. and M.J.P. designed the bioinformatics pipeline and R.B. implemented the pipeline and performed the large-scalegene expression analysis. J.K.M., A.C.W. andm.j.p. fabricated and conducted experiments on compliant hydrogels. M.J.M., H.S.R. and J.W.P. isolated CTCs and measured CTC gene and protein expression. K.G., J.E.H. and C.R.B. designed, synthesized and characterized the glycoprotein mimetics. M.J.P and D.A.H. constructed and implemented the computational model. M.J.P. and V.M.W wrote the manuscript with input from all authors. Author Information Reprints and permissions information is available at The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper. Correspondence and requests for materials should be addressed to V.M.W Macmillan Publishers Limited. All rights reserved 00 MONTH 2014 VOL 000 NATURE 7
68 RESEARCH ARTICLE METHODS Bioinformatics. To estimate protein-level contributions to extracellular membrane bulkiness, we used TMHMM to identify extracellular domains within each isoform sequence (RefSeq v47) and counted the number of putative extracellular glycosylation sites predicted by NetOGlyc 3.1 and search of N-glycosylation motifs. Genewise enrichment of mrna upregulation among bulky proteins inclinical data (GEO accessions GSE12276 and GSE31364) was tested by permuting P values quantifying evidence for upregulation in the appropriate samples. Variance in mrna upregulation explained by membrane bulkiness was estimated by regressing the negative logtransformed P values on the square root of the combined N- and O-glycosylation sites and comparing the residuals with the intercept model. Additional details of the analysis and models are provided in Supplementary Note 1. Computational model. A mechanical model of the cell ECM interface was constructed as described previously 4. A summary of the model is described in Supplementary Note 2 and parameters are detailed in Supplementary Table 1. Antibodies and reagents. Antibodies used include: mouse monoclonal antibody (mab) vinculin (MAB674; Millipore), mouse mab talin (8d4; Sigma), rat mab b 1 - integrin (AIIBII), rabbit mab paxillin (Y113; Abcam), rabbit mab FAK py397 (141-9; Invitrogen), rabbit polyclonal antibody (pab) a 5 -integrin (AB1928; Millipore), mouse mab MUC1 (HMPV; BD Pharminigen), hamster mab MUC1 (CT2; Thermo Scientific), rabbit mab Src Family py416 (D49G4; Cell Signaling), mouse mab FAK (77; BD Transduction Laboratories), rabbit pab paxillin py118 (2541; Cell Signaling), rabbit mab pan-akt (C67E7; Cell Signaling), rabbit pab AKT ps473 (9271; Cell Signaling); rabbit mab ERK1/2 pt202/pt204 (197G2; Cell Signaling); rabbit pab ERK1/2 (9102; Cell Signaling); rabbit mab Gapdh (14C10; Cell Signaling); Alexa 488 and Alexa 568 conjugated goat anti-mouse and anti-rabbit mabs (Invitrogen); FITC conjugated anti-hamster mabs; Cy5-conjugatedgoat antimouse and rabbit mabs (Jackson); and HRP conjugated anti-rabbit and anti-mouse mabs. Chemical inhibitors used in these studies include ROCK inhibitor Y (Cayman Chemical), myosin-ii inhibitor (-)-blebbistatin (CaymanChemical), FAK inhibitor FAK inhibitor 14 (Tocris), MEK inhibitor U0126 (Cell Signaling), PI(3)K inhibitor Wortmannin (Cell Signaling), Src inhibitor Src I1 (Sigma), and DiI (Molecular Probes). Cell culture conditions. All cells were maintained at 37 uc and 5% CO 2. MCF10A human MECs(ATCC)were cultured in DMEM F12 (Invitrogen) supplemented with 5% donor horse serum (Invitrogen), 20 ng ml 21 epidermal growth factor (Peprotech), 10 mgml 21 insulin (Sigma), 0.5 mgml 21 hydrocortisone (Sigma), 0.1 mgml 21 cholera toxin (Sigma), and 100 units ml 21 penicillin/streptomycin. MCF7 and T47D breast tumour lines (ATCC) were grown in DMEM supplemented with 10% fetal bovine serum (Hyclone) and 100 units ml 21 penicillin/streptomycin. 293T cells (ATCC) were maintained in DMEM supplemented with 10% donor horse serum, 2 mm L-glutamine, and penicillin/streptomycin. Mouse embryonic fibroblasts (MEFs) were cultured in DMEM with 10% fetal bovine serum. Cell lines were tested routinely for mycoplasma contamination. For transient gene expression in MECs, constructs in pcdna3.1 or Clonetech-style vectors were nucleofected with Kit V (Lonza) using program T h before experimentation. Transient transfection in MEFs was conducted 48 h before experimentation using Fugene 6 (Roche) or nucleofection. For stable cell lines harbouring tetracycline inducible transgenes, expression was induced with 0.2 ng ml 21 doxycycline 24 h before experimentation. The conditional v-src oestrogen receptor fusion (v-src ER) wasactivated with 1 mm 4-hydroxytamoxifen 48 h before experimentation to achieve transformation. For perk, py118 paxillin, and pakt studies, cells were plated on fibronectin-conjugated polyacrylamide hydrogels, serum-starved overnight, and stimulated with 20 ng ml 21 EGF before collecting protein lysates. Data are reported as the fold increase of phosphorylated protein relative to total protein, following EGF stimulation. Preparation of cellular substrates. Glass and silicon substrates were prepared by glutaraldehyde activation followed by conjugation with 10 mgml 21 (glass) or 20 mgml 21 (silicon) fibronectin as described 11. Compliant polyacrylamide hydrogel substrates (soft: 2.5% acrylamide, 0.03% Bis-acrylamide; stiff: 10% acrylamide, 0.5% Bisacrylamide) were prepared as previously described with one modification: functionalization with succinimidyl ester was with 0.01% N6, 0.01% Bis-acrylamide, 0.025% Irgacure 2959, and 0.002% Di(trimethylolpropane) tetraacrylate (Sigma) 31. Following functionalization with succinimidyl ester, hydrogels were conjugated overnight with 20 mgml 21 fibronectin at 4 uc and rinsed twice with PBS and DMEM before cell plating. Generation of expression constructs. A description of cdna constructs and their construction is provided in Supplementary Note 3. Generation of stable cell lines. Stable transgene expression was achieved through retroviral or lentiviral transduction as previously described 11,30. Flow cytometry and cell sorting. Cell surface MUC1 was labelled directly with FITC-conjugated mab MUC1 (clone HMPV). Cytometry and sorting were conducted on a FACSAria II (BD Biosciences). Immunofluorescence and imaging. Cells were fixed and labelled as previously described and imaged at random on a Zeiss LSM 510 microscope system with a 100X Plan Apochromat NA 1.4 objective and 488 nm Argon, 543 nm HeNe, and 633 nm HeNe excitation lines 30. Live epithelial cell imaging and FRET. Normal growth media was exchanged for a similar formulation lacking phenol red and supplemented with 15 mm HEPES buffer, ph 7.4. Cells were imaged on a Ti-E Perfect Focus System (Nikon) equipped with a CSU-X1 spinning disk confocal unit; 454 nm, 488 nm, 515 nm and 561 nm lasers; an Apo TIRF 60X NA 1.49 objective; electronic shutters; a charged-coupled device camera (Clara; Andor) and controlled by NIS-Elements software (Nikon). For measurement of FRET efficiency, theacceptor photobleaching method pbfret was implemented with live cells on the spinning disk confocal. Cyan fluorescent protein (CFP) was first imaged with 454 nm excitation and a 480/20 emission filter, yellow fluorescent protein (YFP) was subsequently bleachedusing a 100 mw 515 nm laser for 10 s, and CFP was imaged again following bleaching of YFP. Microscope Z-focus was maintained during image acquisition using the Perfect Focus System. Background images were constructed by imaging 10 unique cell-free regions on the coverslip and averaging the intensity at each pixel. The FRET efficiency was calculated on a pixel-by-pixel basis according to: FRET efficiency (%) 5 1{ I pre{b pre 100% I post {B post where I pre is the CFP intensity before bleaching YFP, B pre is the CFP-channel background intensity before bleaching YFP, I post is the CFP intensity after bleaching YFP, and B post is the CFP-channel background intensity after bleaching YFP. Appropriate controls were implemented to account for inadvertent CFP photobleaching, incomplete YFP photobleaching, and intermolecular FRET (see Supplementary Note 4). Time-domain fluorescence lifetime imaging microscopy (FLIM) for additional FRET sensor characterization was implemented with an inverted Zeiss LSM510 Axiovert 200Mmicroscope with a Plan NeoFLUAR 40X/1.3 NA DIC oil-immersion objective lens, equipped with a TCSPC controller (SPC-830 card; Becker & Hickl, Berlin, Germany) as described previously 32. CFP was excited with 440 nm light generated by frequency doubling of 880 nm pulses from a mode-locked Ti:sapphire laser (Mai-Tai, Spectra-Physics, fs pulse width, 80 MHz repetition rate, and Frequency Doubler and Pulse Selector, Spectra-Physics, Model 3980). The emission light was passedthrough a NFT 440beamsplitter, directedtothe fibre-out port of the confocal scan-head, filtered with a480bp40 filter (ChromaTechnology, Rockingham, VT) and detected by a PMC-100 photomultiplier (Becker & Hickl). The pinhole was set togive anoptical slice of,4.0 mm. Images of pixels were averaged over,120 s. Data analysis toproduce anintensity image and a FLIM image was done offline using the pixel-based fitting software SPCImage (Becker & Hickl), assuming double exponential decay during the first 8.5 ns of the 12.5 ns interval between laser pulses. Images were scaled to pixels and no binning was used. Lifetime distributions were calculated for a masked portion of the FLIM image, generated with a triangle algorithm threshold of the photo count intensity image. Scanning angle interference microscopy. Cells were plated overnight on reflective silicon substrates, fixed or roofed to remove the dorsal membrane (for MUC1 GFP imaging) and then fixed, and imaged randomly as previously described, scanning the incident angle of excitation light from 0u to 42u with a one-degree sampling rate 11. Z-positions were localized with custom algorithms previously described and available on request 11. Single particle tracking photo-activation localization microscopy (sptpalm). sptpalm experiments were performed and analysed as previously described 15. Briefly, live MEFs were imaged at 37 uc in a Ludin chamber on a Ti Perfect Focus System equipped with a Plan Apo 100X NA 1.45 objective, and an electron multiplying charge-coupled device (Evolve; Photometrics). For photo-activation localization, cells expressing meos2-tagged constructs were activated using a 405 nm laser (Omicron) and the photo-activated fluorophores were excited simultaneously with a 561 nm laser (Cobolt Jive). The powers of the activation and excitation lasers were adjusted to keep the number of activated molecules constant and well separated. GFP fusions of paxillin or MUC1 were imaged in between each sptpalm sequence by imaging the GFP signal above the unconverted meos2 background. The acquisition was driven by Metamorph software (Molecular Devices) in streaming mode at 50 Hz. For tracking, single-molecules were localized and tracked over time using a combination of wavelet segmentation and simulated annealing algorithms. Trajectories lasting at least 20 frames were selected for further quantification, including calculation of immobile, confined and free-diffusing fraction (see Supplementary Note 5) 15. Preparation of glycopolymer-coated cell surfaces. Mucin mimetic glycopolymers with lipid insertion domains were synthesized and characterized as described in Supplementary Note 6. For incorporation into the plasma membrane, cells were 2014 Macmillan Publishers Limited. All rights reserved
69 ARTICLE RESEARCH suspended in DMEM and incubated with 2 mm glycopolymer for 1 h. Cells were pelleted by centrifugation and re-suspended in growth media to remove unincorporated polymer. Quantification of adhesion complexes. Images of adhesions in fixed, immunolabelled cells or cells expressingpaxillin mcherrywererandomlyacquired, smoothed with a median filter, and background subtracted (12 pixel diameter) in ImageJ. Adhesion sizes and the number of adhesions per cell were subsequently quantified in ImageJ with the Analyze Particles tool. Integrin crosslinking assay. Cells were incubated in suspension with inhibitor (Y or Blebbistatin) orcontrol solvent for 1 h beforeplating onglass substrates. Integrin was crosslinked to fibronectin with 1 mm 3,39-dithiobis(sulfosuccinimidylpropionate) (Pierce Chemical) and cells were extracted with SDS buffer as previously described 17. Crosslinked a5 integrin was immuno-labelled and imaged at random with a Plan Apo VC 60X objective on a Nikon TE2000 epi-fluorescence microscope equipped with a charged-coupled device camera (HQ2; Photometrics). Single cell force spectroscopy. Measurements were performed on an Asylum MFP- 3D-BIO atomic force microscope as previously described 33. Briefly, cells were attached to a streptavidin-coated, tipless cantileverusing biotinylated jacalin (MUC1-expressing cells) or concanavalin A (all other cells) and pressed against the adhesive substrate with a calibrated force and duration before measuring the force required to detach the cell from the substrate. All measurements were conducted on fibronectin- or BSA-coated glass slides at room temperature. The relative rate of adhesion was calculated as the slope of a linear fit of cellular detachment force against contact time. Assessment of fibronectin fibrillogenesis. Human recombinant fibronectin was labelled with N-hydroxysuccinimide Alexa568 (Invitrogen) according to manufacturer s protocol and dialysed extensively in PBS. Conversion of soluble, fluorescently labelled fibronectin from the growth media into insoluble fibrils was imaged according to published protocol 34. Briefly, MCF10A complete growth media was prepared with donor horse serum that was depleted of fibronectin using gelatin Sepharose 4B (GE Healthcare). MCF10A cells were plated in the depleted media on fibronectin-conjugated glass coverslips and incubated the next day in 10 mgml 21 labelled fibronectin for one hour. Cells were quickly rinsed in PBS, fixed in 4% paraformaldehyde, and imaged at random on a spinning disk confocal. Isolation and gene expression profiling of CTCs. Twenty CTC samples were isolatedfrom the blood of 18 metastatic breast cancer patients as previously described 35. Briefly, whole blood was subjected to EpCAM-based immunomagnetic enrichment followed by fluorescence-activated cell sorting of CTCs defined as nucleated, EpCAM-positive, CD45-negative cells. CTCs were sorted directly onto lysis buffer (Taqman PreAmp Cells-to-Ct kit, Life Technologies). cdna of target genes were pre-amplified (14 cycles) and measured via qpcr analysis. The mean Ct for ACTB and GAPDH was used for normalization to calculate relative gene expression (DCt). Studies involving CTCs were approved by the UCSF Committee on Human Research. Samples were obtained with IRB approved consent from all patients. Immunofluorescence labelling of CTCs. CTC samples were isolated from the blood of three metastatic breast cancer patients as described for gene expression profiling. Isolated CTCs were mounted and fixed on poly-l-lysine-coated slides and labelled with FITC-conjugated MUC1 mab (Clone HPMV). As a control, purified white blood cells from the same patients were prepared similarly, and their immunofluorescence was compared to CTC samples. Statistics. Statistical significance of experimental data sets was determined by Student s t-test after confirming that the data met appropriate assumptions (normality, homogenous variance and independent sampling). Statistical analyses of microarray gene expression data sets are described in detail in Supplementary Note 1. All public microarray data were downloaded from the NCBI Gene Expression Omnibus website and analysed using custom R scripts (all Perl, PHP and R scripts used in this work are available on request) Macmillan Publishers Limited. All rights reserved
70 RESEARCH ARTICLE Extended Data Figure 1 Large-scale gene expression analysis reveals increased expression of genes encoding bulky glycoproteins and glycanmodifying enzymes in primary tumours of patients with disseminated disease. a, Bioinformatics pipeline to estimate the extracellular bulkiness of a protein from its corresponding amino acid sequence. For each isoform sequence, the transmembrane and extramembrane domains were identified using a hidden Markov model (TMHMM). A combination of motif searches and neural network prediction then identified likely N- and O-glycosylation sites within each sequence. Isoform-level bulkiness estimates were generated by summing the number of predicted N- and O-glycosylation sites located within the extramembrane regions of the isoform. b, Heat map depicting the pairwise spearman correlation coefficients calculated by comparing all per-gene estimates of the total number of extra-membrane amino acids (AAoutside), N-glycosylation sites (Nglyc), O-glycosylation sites (Oglyc), and the overall bulkiness measure (total sites; for example, the sum of extra-membrane N- and O- glycosylation sites). Correlation coefficients relating the corresponding gene-wise measures are listed in the corresponding cells and depicted on a colour scale, where white corresponds to perfect correlation (rho 5 1), and the dendrograms indicate the overall relationship between the parameters, estimated by Euclidean distance. High correlation coefficients indicate that gene-wise estimates of the compared parameters are similarly ranked (for example, genes with high values of X also tend to have high values of Y). The data indicate that the number of extracellular N-glycosylation sites and O-glycosylation sites identified within a gene are only weakly correlated, and neither dominates the total number of sites estimated per gene. c, Violin plots contrasting the distributions of gene-wise one-sided P values (y axis) quantifying evidence for transcriptional upregulation of glycosidases and glycosyltransferases, and subsets of glycosyltransferases (sialyltransferases and N-acetylgalactosaminyltransferases) with the full distribution. White dots and thick black lines indicate the median and interquartile range of the genewise P-value distribution among category members, and the width of the violin along the y axis indicates the density of the corresponding values. P values are derived from comparisons of expression levels in primary tumours of patients with or without distant metastases using a t-test. Indicated P values were estimated using a one-sided Kolmogorov Smirnov test. d, Violin plots quantifying transcriptional upregulation of glycan-modifying enzymes in primary tumours of patients presenting with circulating tumour cells compared to tumours without detectable circulating tumour cells. e, Table of bulky glycoproteins and potential bulk-adding glycosyltransferases whose expression is upregulated in tumours that present with circulating tumour cells Macmillan Publishers Limited. All rights reserved
71 ARTICLE RESEARCH Extended Data Figure 2 Computational model of the cell ECM interface. Schematic of an integrated model that describes how the physical properties of the glycocalyx influence integrin ECM interactions. The cell surface is modelled as a three-dimensional elastic plate; the ECM as a rigid substrate underneath the cell surface; and the glycocalyx as a repulsive potential between the plate and substrate. To compute stress strain behaviour, the model is discretized using the three-dimensional lattice spring method, the cross-section of which is depicted above. Integrins are tethered to the cell surface and their distance-dependent binding to the ECM substrate is calculated according to the Bell model. To calculate integrin-binding rate as a function of lateral distance from an adhesion cluster, an adhesion cluster is first constructed by assembling a bond structure. The rates for additional integrin ECM bonds then are computed at various distances from the cluster Macmillan Publishers Limited. All rights reserved
72 RESEARCH ARTICLE Extended Data Figure 3 Synthesis and characterization of glycoprotein mimetics. a, Scheme for synthesis of lipid-terminated mucin mimetics labelled with Alexa Fluor 488 (AF488). b, Reagents and yields for the synthesis of polymers 3a c. c, Characteristics of polymers 6a c based on 1H NMR spectra. Glycoprotien mimetics were engineered to have minimal biochemical interactivity with cell surface lectins. d, Flow cytometry results quantifying incorporation of polymer on the surface of mammary epithelial cells (left) and binding with recombinant Alexa568-labelled galectin-3 with or without competitive inhibitor, b-lactose (right). Although a weak affinity between galectin-3 and the pendant N-acetylgalactosimes has previously been reported, the results suggest that incorporation of polymer does not significantly change the affinity of the cell surface for lectins Macmillan Publishers Limited. All rights reserved
73 ARTICLE RESEARCH Extended Data Figure 4 MUC1 expression constructs. a, Schematic of MUC1 expression constructs. Full-length MUC1 consists of a large ectodomain with 42 mucin-type tandem repeats, a transmembrane domain, and short cytoplasmic tail. The tandem repeats and cytoplasmic tail are deleted in MUC1(DTR) and MUC1(DCT), respectively. For fluorescent protein fusions, memerald (GFP) and meos2 are fused to the C terminus of full-length MUC1 or MUC1(DCT). b, Schematic of MUC1 strain sensor and control constructs. Cysteine-free mturqoiuse2 (CFP), Venus (YFP), or a FRET module consisting of the fluorescent proteins separated by an elastic linker (8 repeats of GPGGA) are inserted into the MUC1 ectodomain adjacent to the MUC1 tandem repeats. The mucin tandem repeats are deleted in ectodomain-truncated variants (DTR) Macmillan Publishers Limited. All rights reserved
74 RESEARCH ARTICLE Extended Data Figure 5 MUC1-mediated adhesion formation. a, Quantification of the average number of large adhesions, greater than 1 mm 2, per area of cell in control epithelial cells (Control) and those ectopically expressing ectodomain-truncated MUC1 (1MUC1(DTR)), wild-type MUC1 (1MUC1), or cytoplasmic-tail-deleted MUC1 (1MUC1(DCT)). Results are the mean 6 s.e.m. of three separate experiments. b, Fluorescence micrographs showing immuno-labelled MUC1 and fluorescently labelled fibronectin fibrils in control and MUC1-expressing epithelial cells. Soluble, labelled fibronectin in the growth media was deposited by cells at sites of cell matrix adhesion. Binding of soluble fibronectin to MUC1 was not detected. Scale bar, 10 mm. c, Time lapse images of MUC1 YFP and vinculin mcherry, showing the dynamics of adhesion assembly (Vinc.) and MUC1 patterning (MUC1). Scale bar, 1 mm. d, Rate of adhesion measured with single cell force spectroscopy of control (Cont.), a 5 integrin-blocked (anti-a 5 ), and MUC1- expressing cells (1MUC1) to fibronectin-coated surfaces and control cells to BSA-coated surfaces (BSA). Results are the mean 6 s.e.m. of at least 15 cell measurements. Statistical significance is given by *P, 0.05; **P, 0.01; ***P, Macmillan Publishers Limited. All rights reserved
75 ARTICLE RESEARCH Extended Data Figure 6 b 3 integrin mobility in MUC1-expressing cells. a, Molecular diffusivity and adhesion enrichment measured with sptpalm in mouse embryonic fibroblasts (MEFs). Adhesion enrichment is reported as the ratio of the number of molecules detected inside focal adhesions per unit area to the number of molecules detected outside focal adhesions per unit area. b, Mean diffusion coefficients measured for freely diffusive b 3 integrin tracks outside of adhesive contacts in control (Cont.) and MUC1-transfected (1MUC1) MEFs with and without Mn 21 to activate b 3. c, Mean diffusion coefficients measured for confined b 3 integrin tracks outside of adhesive contacts in MEFs with and without Mn 21. d, Mean radius of confinement measured for confined b 3 integrin tracks outside of adhesive contacts in MEFs with and without Mn 21. e, Fraction of immobilized (Imm.), confined (Conf.), and freely diffusive (Free) b 3 integrins inside of adhesive contacts in control and MUC1-transfected MEFs with and without Mn 21 treatment. f, From left to right, panels show GFP-tagged wild-type MUC1 (red) and positions of individual b 3 integrins (green) in MEFs without Mn 21 treatment (left panel) and individual integrin trajectories recorded with sptpalm within MUC1-rich regions, outside MUC1-rich regions, and that cross MUC1 boundaries (scale bar, 2 mm). The ratio of integrins crossing out versus crossing in the MUC1 boundaries per cell is close to one ( , n 5 9 cells, 4,145 trajectories) showing that the flux of free diffusing integrins crossing in or out the mucin region is the same. g, From left to right, panels show integrin trajectories within an arbitrary region drawn in a MUC1-rich area (dashed white circles), outside of the circled region, and that cross the circled region (scale bar, 2 mm). The ratio of integrins crossing the MUC1-rich boundaries versus the fictive boundaries per cell is close to one ( , n 5 9 cells, 9,321 trajectories), showing that the MUC1 adhesive zone boundary does not affect the diffusive crossing of integrins. For all bar graphs, results are the mean 6 s.e.m Macmillan Publishers Limited. All rights reserved
76 RESEARCH ARTICLE Extended Data Figure 7 MUC1 strain gauge. a, Western blot of indicated construct expressed in HEK 293T cells and probed with anti-gfp family antibody, or full-length MUC1 construct expressed in HEK 293T cells and probed with an antibody against the MUC1 tandem repeats. b, Pseudocoloured images showing similar FRET efficiencies measured by the photobleaching FRET method for mammary epithelial cells (MECs) expressing low (Low) and high (High) levels of the sensor construct. Scale bar, 5 mm. c, Plot showing the level of CFP bleaching per CFP imaging cycle in MECs. d, Control images showing minimal intermolecular FRET in MECs expressing similar levels of both MUC1 CFP and MUC1 YFP. e, Micrographs showing the emitted photons from CFP and their fluorescence lifetimes in MECs expressing ectodomain-truncated (MUC1(DTR) sensor) or full-length MUC1 strain sensors (MUC1 sensor). Shorter lifetimes are indicative of higher energy transfer between the CFP donor and YFP acceptor, and thus closer spatial proximity of the donor and acceptor (scale bar, 10 mm). f, Representative profile of CFP lifetimes and emitted photons of the full-length MUC1 sensor along the red line in panel e. Pixels 0 and 40 correspond to the base and tip of the arrow, respectively. A drop in fluorescence lifetime (Lifetime) is often observed before the drop in MUC1 molecular density (Photons) as an adhesive zone is approached Macmillan Publishers Limited. All rights reserved
77 ARTICLE RESEARCH Extended Data Figure 8 Tension-dependent integrin activation and focal adhesion assembly in MUC1-expressing cells. a, Fluorescence micrographs of fibronectin-crosslinked a5 integrin in control and MUC1-expressing mammary epithelial cells (MECs) treated with solvent alone (DMSO), myosin- II inhibitor (blebbistatin; 50 mm), or Rho kinase inhibitor (Y-27632; 10 mm) for 1 h and detergent-extracted following crosslinking. Only fibronectin-bound integrins under mechanical tension are crosslinked and visualized following detergent extraction (scale bar, 15 mm). b, Fluorescence micrographs showing formation of myosin-independent adhesion complexes in MUC1-expressing MECs. Cells were pre-treated for 1 h and plated for 2 h in 50 mm blebbistatin (scale bar, 10 mm). c, Fluorescence micrographs of paxillin mcherry and immuno-labelled activated FAK (py397) in control and MUC1(DCT) expressing MECs plated on compliant fibronectin-conjugated hydrogels (E Pa; scale bar, 3 mm; ROI scale bar, 0.5 mm). d, Western blots showing phosphorylation of paxillin (py118) in control and MUC1-expressing MECs on compliant substrates (E Pa) following overnight serum starvation and stimulation with EGF. MUC1-expressing cells treated with a pharmacological inhibitor of focal adhesion kinase (1FAKi) for 1 h before EGF stimulation did not exhibit robust paxillin phosphorylation Macmillan Publishers Limited. All rights reserved
78 RESEARCH ARTICLE Extended Data Figure 9 Cell proliferation on soft ECM. a, Fluorescence micrographs showing DAPI-stained nuclei of control and MUC1(DCT)- expressing MECs after 24 h of plating on soft, fibronectin-conjugated hydrogels (E Pa; scale bar, 250 mm). The majority of cells plated as single cells, indicating that multi-cell colonies that formed at later time points were largely attributed to cell proliferation. b, Quantification of cell proliferation of MUC1(DCT)-expressing epithelial cells on soft hydrogels conjugated with bovine serum albumin (BSA) or fibronectin (Fn). Cells plated similarly on BSA and Fn hydrogels, but cell proliferation was significantly enhanced on Fn hydrogels. Results are the mean 6 s.e.m with statistical significance given by *P, 0.05; **P, 0.01; ***P, Macmillan Publishers Limited. All rights reserved
79 ARTICLE RESEARCH Extended Data Figure 10 Hyaluronic acid production by tumour cells promotes cellular growth. a, Quantification of hyaluronic acid (HA) cell surface levels on control (10A-Cont.), transformed (10A-v-Src, 10A-HRAS) and malignant (MCF7, T47D) mammary epithelial cells (MECs). b, Fluorescence micrographs of HA and immuno-labelled paxillin on the v-src transformed MECs (scale bars, 3 mm). c, Quantification of the number of v-src-transformed MECs per colony 48 h after plating on soft polyacrylamide gels (fibronectin-conjugated) and treated with vehicle (DMSO), hyaluronic acid synthesis inhibitor 4-methylumbelliferone (14MU; 0.3 mm), or competitive inhibitor HA oligonucleotides (1Oligo; 12-mer average oligonucleotide size; 100 mg ml 21 ). Results are the mean 6 s.e.m with statistical significance is given by *P, 0.05; **P, 0.01; ***P, Macmillan Publishers Limited. All rights reserved
What sort of Science is Glycoscience? (Introductory lecture)
 Glycosciences: Glycobiology & Glycochemistry e-learning course What sort of Science is Glycoscience? (Introductory lecture) Paula Videira Faculdade de Ciências Médicas Nova University, Lisbon Portugal
Glycosciences: Glycobiology & Glycochemistry e-learning course What sort of Science is Glycoscience? (Introductory lecture) Paula Videira Faculdade de Ciências Médicas Nova University, Lisbon Portugal
Significance and Functions of Carbohydrates. Bacterial Cell Walls
 Biochemistry 462a - Carbohydrate Function Reading - Chapter 9 Practice problems - Chapter 9: 2, 4a, 4b, 6, 9, 10, 13, 14, 15, 16a, 17; Carbohydrate extra problems Significance and Functions of Carbohydrates
Biochemistry 462a - Carbohydrate Function Reading - Chapter 9 Practice problems - Chapter 9: 2, 4a, 4b, 6, 9, 10, 13, 14, 15, 16a, 17; Carbohydrate extra problems Significance and Functions of Carbohydrates
Biosynthesis of N and O Glycans
 TechNote #TNGL101 Biosynthesis of N and O Glycans These suggestions and data are based on information we believe to be reliable. They are offered in good faith, but without guarantee, as conditions and
TechNote #TNGL101 Biosynthesis of N and O Glycans These suggestions and data are based on information we believe to be reliable. They are offered in good faith, but without guarantee, as conditions and
The addition of sugar moiety determines the blood group
 The addition of sugar moiety determines the blood group Sugars attached to glycoproteins and glycolipids on the surfaces of red blood cells determine the blood group termed A, B, and O. The A and B antigens
The addition of sugar moiety determines the blood group Sugars attached to glycoproteins and glycolipids on the surfaces of red blood cells determine the blood group termed A, B, and O. The A and B antigens
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Biochemistry: A Short Course
 Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 10 Carbohydrates 2013 W. H. Freeman and Company Chapter 10 Outline Monosaccharides are aldehydes or ketones that contain two or
Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 10 Carbohydrates 2013 W. H. Freeman and Company Chapter 10 Outline Monosaccharides are aldehydes or ketones that contain two or
Chapter 11. Learning objectives: Structure and function of monosaccharides, polysaccharide, glycoproteins lectins.
 Chapter 11 Learning objectives: Structure and function of monosaccharides, polysaccharide, glycoproteins lectins. Carbohydrates Fuels Structural components Coating of cells Part of extracellular matrix
Chapter 11 Learning objectives: Structure and function of monosaccharides, polysaccharide, glycoproteins lectins. Carbohydrates Fuels Structural components Coating of cells Part of extracellular matrix
Molecular and Cellular Basis of Immune Protection of Mucosal Surfaces
 Molecular and Cellular Basis of Immune Protection of Mucosal Surfaces Department of Biologic & Materials Sciences School of Dentistry University of Michigan Ann Arbor, Michigan 48109-1078 1 Image quality
Molecular and Cellular Basis of Immune Protection of Mucosal Surfaces Department of Biologic & Materials Sciences School of Dentistry University of Michigan Ann Arbor, Michigan 48109-1078 1 Image quality
Glycosaminoglycans: Anionic polysaccharide chains made of repeating disaccharide units
 Glycosaminoglycans: Anionic polysaccharide chains made of repeating disaccharide units Glycosaminoglycans present on the animal cell surface and in the extracellular matrix. Glycoseaminoglycans (mucopolysaccharides)
Glycosaminoglycans: Anionic polysaccharide chains made of repeating disaccharide units Glycosaminoglycans present on the animal cell surface and in the extracellular matrix. Glycoseaminoglycans (mucopolysaccharides)
Tissues. tissue = many cells w/ same structure and function. cell shape aids its function tissue shape aids its function
 Tissues tissue = many cells w/ same structure and function cell shape aids its function tissue shape aids its function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings
Tissues tissue = many cells w/ same structure and function cell shape aids its function tissue shape aids its function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Glycoproteins and Mucins. B.Sopko
 Glycoproteins and Mucins B.Sopko Content Glycoproteins: Structures and Linkages Interconversions and activation of dietary sugars Other pathways of sugar nucleotide metabolism Biosynthesis of oligosaccharides
Glycoproteins and Mucins B.Sopko Content Glycoproteins: Structures and Linkages Interconversions and activation of dietary sugars Other pathways of sugar nucleotide metabolism Biosynthesis of oligosaccharides
Histology = the study of tissues. Tissue = a complex of cells that have a common function
 { EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
{ EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
Glycosaminoglycans, Proteoglycans, and Glycoproteins
 Glycosaminoglycans, Proteoglycans, and Glycoproteins Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy I. OVERVIEW OF GLYCOSAMINOGLYCANS
Glycosaminoglycans, Proteoglycans, and Glycoproteins Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy I. OVERVIEW OF GLYCOSAMINOGLYCANS
Rama Abbady. Odai Bani-Monia. Diala Abu-Hassan
 5 Rama Abbady Odai Bani-Monia Diala Abu-Hassan Lipid Rafts Lipid rafts are aggregates (accumulations) of sphingolipids. They re semisolid clusters (10-200 nm) of cholesterol and sphingolipids (sphingomyelin
5 Rama Abbady Odai Bani-Monia Diala Abu-Hassan Lipid Rafts Lipid rafts are aggregates (accumulations) of sphingolipids. They re semisolid clusters (10-200 nm) of cholesterol and sphingolipids (sphingomyelin
4. If a GT uses a one-step mechanism it is retaining or inverting? 5. Name: Gal GlcNAc Neu5Gc Xyl GlcA
 Primer 1. The 4-epimer of Glucose once it is 2-N-acetylated is called? 2. If the SNFG shape of a monosaccharide is a diamond it is an? 3. Which of the 11 common mammalian monosaccharides is: a) a pentose?
Primer 1. The 4-epimer of Glucose once it is 2-N-acetylated is called? 2. If the SNFG shape of a monosaccharide is a diamond it is an? 3. Which of the 11 common mammalian monosaccharides is: a) a pentose?
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
189,311, , ,561, ,639, ,679, Ch13; , Carbohydrates
 Lecture 31 (12/8/17) Reading: Ch7; 258-267 Ch10; 371-373 Problems: Ch7 (text); 26,27,28 Ch7 (study-guide: applying); 2,5 Ch7 (study-guide: facts); 6 NEXT (LAST!) Reading: Chs4,6,8,10,14,16,17,18; 128-129,
Lecture 31 (12/8/17) Reading: Ch7; 258-267 Ch10; 371-373 Problems: Ch7 (text); 26,27,28 Ch7 (study-guide: applying); 2,5 Ch7 (study-guide: facts); 6 NEXT (LAST!) Reading: Chs4,6,8,10,14,16,17,18; 128-129,
BIOSYNTHESIS OF CANCER-RELATED CARBOHYDRATE ANTIGENS. Fabio Dall Olio Department of Experimental Pathology University of Bologna, Italy
 BIOSYNTHESIS OF CANCER-RELATED CARBOHYDRATE ANTIGENS Fabio Dall Olio Department of Experimental Pathology University of Bologna, Italy TOPICS OF THE LECTURE 1. Structure and function of some representative
BIOSYNTHESIS OF CANCER-RELATED CARBOHYDRATE ANTIGENS Fabio Dall Olio Department of Experimental Pathology University of Bologna, Italy TOPICS OF THE LECTURE 1. Structure and function of some representative
The Interaction of the Gut Microbiota with the Mucus Barrier in Health and Disease in Human
 Review The Interaction of the Gut Microbiota with the Mucus Barrier in Health and Disease in Human Anthony P. Corfield Mucin Research Group, School of Clinical Sciences, Bristol Royal Infirmary, Level
Review The Interaction of the Gut Microbiota with the Mucus Barrier in Health and Disease in Human Anthony P. Corfield Mucin Research Group, School of Clinical Sciences, Bristol Royal Infirmary, Level
Abdullah zurayqat. Bahaa Najjar. Mamoun Ahram
 9 Abdullah zurayqat Bahaa Najjar Mamoun Ahram Polysaccharides Polysaccharides Definition and Structure [Greek poly = many; sacchar = sugar] are complex carbohydrates, composed of 10 to up to several thousand
9 Abdullah zurayqat Bahaa Najjar Mamoun Ahram Polysaccharides Polysaccharides Definition and Structure [Greek poly = many; sacchar = sugar] are complex carbohydrates, composed of 10 to up to several thousand
A. Lipids: Water-Insoluble Molecules
 Biological Substances found in Living Tissues Lecture Series 3 Macromolecules: Their Structure and Function A. Lipids: Water-Insoluble Lipids can form large biological molecules, but these aggregations
Biological Substances found in Living Tissues Lecture Series 3 Macromolecules: Their Structure and Function A. Lipids: Water-Insoluble Lipids can form large biological molecules, but these aggregations
Summary of Endomembrane-system
 Summary of Endomembrane-system 1. Endomembrane System: The structural and functional relationship organelles including ER,Golgi complex, lysosome, endosomes, secretory vesicles. 2. Membrane-bound structures
Summary of Endomembrane-system 1. Endomembrane System: The structural and functional relationship organelles including ER,Golgi complex, lysosome, endosomes, secretory vesicles. 2. Membrane-bound structures
Epithelial Tissue. Functions include: 1. Protection 4. Absorption 2. Secretion 5. Filtration 3. Sensory reception
 Tissues There are 4 primary tissue types in the human body: 1. Epithelial (covering/lining) 2. Connective (support) 3. Muscle (movement) 4. Nervous (control) Epithelium Epithelial Tissue Covers the surface
Tissues There are 4 primary tissue types in the human body: 1. Epithelial (covering/lining) 2. Connective (support) 3. Muscle (movement) 4. Nervous (control) Epithelium Epithelial Tissue Covers the surface
LECTURE 12: MUCOSAL IMMUNITY GUT STRUCTURE
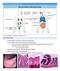 LECTURE 12: MUCOSAL IMMUNITY GUT STRUCTURE - Small intestine in humans is around 3-4 metres long - Internal surface of the small intestines are lined by villi o Villi are composed of absorptive cells (epithelial/enterocytes)
LECTURE 12: MUCOSAL IMMUNITY GUT STRUCTURE - Small intestine in humans is around 3-4 metres long - Internal surface of the small intestines are lined by villi o Villi are composed of absorptive cells (epithelial/enterocytes)
An aldose contains an aldehyde functionality A ketose contains a ketone functionality
 RCT Chapter 7 Aldoses and Ketoses; Representative monosaccharides. (a)two trioses, an aldose and a ketose. The carbonyl group in each is shaded. An aldose contains an aldehyde functionality A ketose contains
RCT Chapter 7 Aldoses and Ketoses; Representative monosaccharides. (a)two trioses, an aldose and a ketose. The carbonyl group in each is shaded. An aldose contains an aldehyde functionality A ketose contains
CELLS. Cells. Basic unit of life (except virus)
 Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Introduction. Biochemistry: It is the chemistry of living things (matters).
 Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
The recruitment of leukocytes and plasma proteins from the blood to sites of infection and tissue injury is called inflammation
 The migration of a particular type of leukocyte into a restricted type of tissue, or a tissue with an ongoing infection or injury, is often called leukocyte homing, and the general process of leukocyte
The migration of a particular type of leukocyte into a restricted type of tissue, or a tissue with an ongoing infection or injury, is often called leukocyte homing, and the general process of leukocyte
Chapter 3, Part A (Pages 37-45): Leukocyte Migration into Tissues
 Allergy and Immunology Review Corner: Chapter 3, Part A (pages 37-45) of Cellular and Molecular Immunology (Seventh Edition), by Abul K. Abbas, Andrew H. Lichtman and Shiv Pillai. Chapter 3, Part A (Pages
Allergy and Immunology Review Corner: Chapter 3, Part A (pages 37-45) of Cellular and Molecular Immunology (Seventh Edition), by Abul K. Abbas, Andrew H. Lichtman and Shiv Pillai. Chapter 3, Part A (Pages
Most mammalian cells are located in tissues where they are surrounded by a complex extracellular matrix (ECM) often referred to as connective tissue.
 GLYCOSAMINOGLYCANS Most mammalian cells are located in tissues where they are surrounded by a complex extracellular matrix (ECM) often referred to as connective tissue. The ECM contains three major classes
GLYCOSAMINOGLYCANS Most mammalian cells are located in tissues where they are surrounded by a complex extracellular matrix (ECM) often referred to as connective tissue. The ECM contains three major classes
Structures Common to Different Glycans
 NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor
CHAPTER 05 Histology: EPITHELIUM
 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
TRANSPORT PROCESSES. 1b. moving proteins into membranes and organelles
 1b. moving proteins into membranes and organelles SLIDE 1 A typical mammalian cell contains up to 10,000 different kinds of proteins. The vast majority of these proteins are synthesized by cytosolic ribosomes,
1b. moving proteins into membranes and organelles SLIDE 1 A typical mammalian cell contains up to 10,000 different kinds of proteins. The vast majority of these proteins are synthesized by cytosolic ribosomes,
TISSUES TYPES. CHAPTER 05 Histology: EPITHELIUM BIO 211: ANATOMY & PHYSIOLOGY I. HISTOLOGY = the study of tissues
 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
Tissues. Definition. A group of similar cells and their intercellular substances specialized to perform a specific function.
 Chapter 4 - Tissues Tissues Definition A group of similar cells and their intercellular substances specialized to perform a specific function. Tissues Epithelial covers exposed surfaces, lines internal
Chapter 4 - Tissues Tissues Definition A group of similar cells and their intercellular substances specialized to perform a specific function. Tissues Epithelial covers exposed surfaces, lines internal
Tissues. tissue = many cells w/ same structure and function. cell shape aids function tissue shape aids function. Histology = study of tissues
 Tissues tissue = many cells w/ same structure and function cell shape aids function tissue shape aids function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings Connective
Tissues tissue = many cells w/ same structure and function cell shape aids function tissue shape aids function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings Connective
Respiratory Pharmacology: Treatment of Cystic Fibrosis
 Respiratory Pharmacology: Treatment of Cystic Fibrosis Dr. Tillie-Louise Hackett Department of Anesthesiology, Pharmacology and Therapeutics University of British Columbia Associate Head, Centre of Heart
Respiratory Pharmacology: Treatment of Cystic Fibrosis Dr. Tillie-Louise Hackett Department of Anesthesiology, Pharmacology and Therapeutics University of British Columbia Associate Head, Centre of Heart
General information. Cell mediated immunity. 455 LSA, Tuesday 11 to noon. Anytime after class.
 General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
Chapt. 10 Cell Biology and Biochemistry. The cell: Student Learning Outcomes: Describe basic features of typical human cell
 Chapt. 10 Cell Biology and Biochemistry Cell Chapt. 10 Cell Biology and Biochemistry The cell: Lipid bilayer membrane Student Learning Outcomes: Describe basic features of typical human cell Integral transport
Chapt. 10 Cell Biology and Biochemistry Cell Chapt. 10 Cell Biology and Biochemistry The cell: Lipid bilayer membrane Student Learning Outcomes: Describe basic features of typical human cell Integral transport
Cell Membranes. Dr. Diala Abu-Hassan School of Medicine Cell and Molecular Biology
 Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Chemical Composition of the Cell. B. Balen
 Chemical Composition of the Cell B. Balen Table 2-2 Molecular Biology of the Cell ( Garland Science 2008) 1. Water the most abundant substance in the cell! Where did it come from? several hypothesis: -
Chemical Composition of the Cell B. Balen Table 2-2 Molecular Biology of the Cell ( Garland Science 2008) 1. Water the most abundant substance in the cell! Where did it come from? several hypothesis: -
 SUMMARY Coeliac disease is a common food intolerance in Western populations, in which it has a prevalence of about 1%. In early infancy, when the transition is made to a gluten-containing diet (particularly
SUMMARY Coeliac disease is a common food intolerance in Western populations, in which it has a prevalence of about 1%. In early infancy, when the transition is made to a gluten-containing diet (particularly
General Structure of Digestive Tract
 Dr. Nabil Khouri General Structure of Digestive Tract Common Characteristics: Hollow tube composed of a lumen whose diameter varies. Surrounded by a wall made up of 4 principal layers: Mucosa Epithelial
Dr. Nabil Khouri General Structure of Digestive Tract Common Characteristics: Hollow tube composed of a lumen whose diameter varies. Surrounded by a wall made up of 4 principal layers: Mucosa Epithelial
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Content. Course (LV 21246): Fr. 12:00-14:00 16 Lectures
 Content Course (LV 21246): Fr. 12:00-14:00 16 Lectures Content 1: Overview Occurrence of carbohydrates in nature Glycopolymers and glycoconjugates Carbohydrates in animals Carbohydrates in bacteria and
Content Course (LV 21246): Fr. 12:00-14:00 16 Lectures Content 1: Overview Occurrence of carbohydrates in nature Glycopolymers and glycoconjugates Carbohydrates in animals Carbohydrates in bacteria and
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
2- Minimum toxic concentration (MTC): The drug concentration needed to just produce a toxic effect.
 BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
Digestive System 7/15/2015. Outline Digestive System. Digestive System
 Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins
 Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Tissues Review 4 type
 Tissues Review 4 type Tissues Definition: a group of closely associated cells that perform related functions and are similar in structure Between cells: nonliving extracellular material Four basic types
Tissues Review 4 type Tissues Definition: a group of closely associated cells that perform related functions and are similar in structure Between cells: nonliving extracellular material Four basic types
Chapter 6. Antigen Presentation to T lymphocytes
 Chapter 6 Antigen Presentation to T lymphocytes Generation of T-cell Receptor Ligands T cells only recognize Ags displayed on cell surfaces These Ags may be derived from pathogens that replicate within
Chapter 6 Antigen Presentation to T lymphocytes Generation of T-cell Receptor Ligands T cells only recognize Ags displayed on cell surfaces These Ags may be derived from pathogens that replicate within
Tissue: The Living Fabric: Part A
 PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 4 Tissue: The Living Fabric: Part A Tissues Groups of cells similar in structure and function Types of tissues Epithelial
PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 4 Tissue: The Living Fabric: Part A Tissues Groups of cells similar in structure and function Types of tissues Epithelial
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
TECHNICAL BULLETIN. R 2 GlcNAcβ1 4GlcNAcβ1 Asn
 GlycoProfile II Enzymatic In-Solution N-Deglycosylation Kit Product Code PP0201 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description Glycosylation is one of the most common posttranslational
GlycoProfile II Enzymatic In-Solution N-Deglycosylation Kit Product Code PP0201 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description Glycosylation is one of the most common posttranslational
BIOL 158: BIOLOGICAL CHEMISTRY II
 BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 1: Membranes Lecturer: Christopher Larbie, PhD Introduction Introduction Cells and Organelles have membranes Membranes contain lipids, proteins and polysaccharides
BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 1: Membranes Lecturer: Christopher Larbie, PhD Introduction Introduction Cells and Organelles have membranes Membranes contain lipids, proteins and polysaccharides
Exam 3 Fall 2015 Dr. Stone 8:00. V max = k cat x E t. ΔG = -RT lnk eq K m + [S]
![Exam 3 Fall 2015 Dr. Stone 8:00. V max = k cat x E t. ΔG = -RT lnk eq K m + [S] Exam 3 Fall 2015 Dr. Stone 8:00. V max = k cat x E t. ΔG = -RT lnk eq K m + [S]](/thumbs/75/71985573.jpg) Exam 3 Fall 2015 Dr. Stone 8:00 Name There are 106 possible points (6 bonus points) on this exam. There are 8 pages. v o = V max x [S] k cat = kt e - ΔG /RT V max = k cat x E t ΔG = -RT lnk eq K m + [S]
Exam 3 Fall 2015 Dr. Stone 8:00 Name There are 106 possible points (6 bonus points) on this exam. There are 8 pages. v o = V max x [S] k cat = kt e - ΔG /RT V max = k cat x E t ΔG = -RT lnk eq K m + [S]
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Glycosylation in the control of selectin counter-receptor structure and function
 John B. Lowe Glycosylation in the control of selectin counter-receptor structure and function Author s address John B. Lowe Howard Hughes Medical Institute, Department of Pathology, University of Michigan
John B. Lowe Glycosylation in the control of selectin counter-receptor structure and function Author s address John B. Lowe Howard Hughes Medical Institute, Department of Pathology, University of Michigan
Dr. Heba Kalbouneh. Dr. Heba Kalbouneh. Dr. Heba Kalbouneh
 Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Basement membrane: What is the basement membrane? - It is a layer of ECM separating the epithelial cells from the underlying connective tissue Basement
Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Basement membrane: What is the basement membrane? - It is a layer of ECM separating the epithelial cells from the underlying connective tissue Basement
(b) Stomach s function 1. Dilution of food materials 2. Acidification of food (absorption of dietary Fe in small intestine) 3. Partial chemical digest
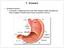 (1) General features a) Stomach is widened portion of gut-tube: between tubular and spherical; Note arranged of smooth muscle tissue in muscularis externa. 1 (b) Stomach s function 1. Dilution of food
(1) General features a) Stomach is widened portion of gut-tube: between tubular and spherical; Note arranged of smooth muscle tissue in muscularis externa. 1 (b) Stomach s function 1. Dilution of food
The Cell Membrane (Ch. 7)
 The Cell Membrane (Ch. 7) Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those structure
The Cell Membrane (Ch. 7) Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those structure
What location in the gastrointestinal (GI) tract has tight, or impermeable, junctions between the epithelial cells?
 CASE 32 A 17-year-old boy presents to his primary care physician with complaints of diarrhea for the last 2 days. The patient states that he just returned to the United States after visiting relatives
CASE 32 A 17-year-old boy presents to his primary care physician with complaints of diarrhea for the last 2 days. The patient states that he just returned to the United States after visiting relatives
Anatomy PHL 212. Dr. Dina A. A. Hassan. -
 Anatomy PHL 212 Dr. Dina A. A. Hassan Associate Professor College of Pharmacy (Female Section) Sattam Bin Abdulaziz University Al kharj / Kingdom of Saudi Arabia Email :- da.hassan@psau.edu.sa 1 Anatomy
Anatomy PHL 212 Dr. Dina A. A. Hassan Associate Professor College of Pharmacy (Female Section) Sattam Bin Abdulaziz University Al kharj / Kingdom of Saudi Arabia Email :- da.hassan@psau.edu.sa 1 Anatomy
11/25/2017. THE IMMUNE SYSTEM Chapter 43 IMMUNITY INNATE IMMUNITY EXAMPLE IN INSECTS BARRIER DEFENSES INNATE IMMUNITY OF VERTEBRATES
 THE IMMUNE SYSTEM Chapter 43 IMMUNITY INNATE IMMUNITY EXAMPLE IN INSECTS Exoskeleton made of chitin forms the first barrier to pathogens Digestive system is protected by a chitin-based barrier and lysozyme,
THE IMMUNE SYSTEM Chapter 43 IMMUNITY INNATE IMMUNITY EXAMPLE IN INSECTS Exoskeleton made of chitin forms the first barrier to pathogens Digestive system is protected by a chitin-based barrier and lysozyme,
Plasma (cell) membrane
 Plasma Membrane 1. Chemical composition of the plasmalemma 2. Models of cell membrane structure 3. Structure and functions of membrane proteins carriers (transporters) channels receptors 4. Cell coat (glycocalyx)
Plasma Membrane 1. Chemical composition of the plasmalemma 2. Models of cell membrane structure 3. Structure and functions of membrane proteins carriers (transporters) channels receptors 4. Cell coat (glycocalyx)
Epithelial Lecture Test Questions
 Epithelial Lecture Test Questions 1. Which of the following free surfaces lack(s) epithelia: a. lung alveoli (air sacs) b. hard palate c. joint cavities d. abdominal cavity e. salivary gland ducts 2. Which
Epithelial Lecture Test Questions 1. Which of the following free surfaces lack(s) epithelia: a. lung alveoli (air sacs) b. hard palate c. joint cavities d. abdominal cavity e. salivary gland ducts 2. Which
Name: Multiple choice questions. Pick the BEST answer (2 pts ea)
 Exam 1 202 Oct. 5, 1999 Multiple choice questions. Pick the BEST answer (2 pts ea) 1. The lipids of a red blood cell membrane are all a. phospholipids b. amphipathic c. glycolipids d. unsaturated 2. The
Exam 1 202 Oct. 5, 1999 Multiple choice questions. Pick the BEST answer (2 pts ea) 1. The lipids of a red blood cell membrane are all a. phospholipids b. amphipathic c. glycolipids d. unsaturated 2. The
The gallbladder. Bile secretion:
 The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
Biology of Sialyl Glycans Part 1: Overview of sialic acids in glycans. Joseph Lau
 Biology of Sialyl Glycans Part 1: Overview of sialic acids in glycans Joseph Lau Synopsis Sialicacid modifications on glycans:biology, synthesis, and function Not comprehensive review, but select aspects
Biology of Sialyl Glycans Part 1: Overview of sialic acids in glycans Joseph Lau Synopsis Sialicacid modifications on glycans:biology, synthesis, and function Not comprehensive review, but select aspects
A look at macromolecules (Text pages 38-54) What is the typical chemical composition of a cell? (Source of figures to right: Madigan et al.
 A look at macromolecules (Text pages 38-54) What is the typical chemical composition of a cell? (Source of figures to right: Madigan et al. 2002 Chemical Bonds Ionic Electron-negativity differences cause
A look at macromolecules (Text pages 38-54) What is the typical chemical composition of a cell? (Source of figures to right: Madigan et al. 2002 Chemical Bonds Ionic Electron-negativity differences cause
2. Epithelial Tissues Dr. Manal Othman
 Biology-232 GENERAL HISTOLOGY 2. Epithelial Tissues Dr. Manal Othman Anatomy Department CMMS, AGU HISTOLOGY: w Study of the structure and function of tissues and organs at the microscopic levels. w Tissues
Biology-232 GENERAL HISTOLOGY 2. Epithelial Tissues Dr. Manal Othman Anatomy Department CMMS, AGU HISTOLOGY: w Study of the structure and function of tissues and organs at the microscopic levels. w Tissues
Review Questions: Janeway s Immunobiology 8th Edition by Kenneth Murphy
 Review Questions: Janeway s Immunobiology 8th Edition by Kenneth Murphy Chapter 11 (pages 429-460): Dynamics of Adaptive Immunity prepared by Kelly von Elten, Walter Reed National Military Medical Center,
Review Questions: Janeway s Immunobiology 8th Edition by Kenneth Murphy Chapter 11 (pages 429-460): Dynamics of Adaptive Immunity prepared by Kelly von Elten, Walter Reed National Military Medical Center,
Overview of the immune system
 Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
Glycoprotein Deglycosylation Kit Cat. No
 Visit our interactive pathways at /pathways User Protocol 362280 Rev. 23 February 2006 RFH Page 1 of 5 Glycoprotein Deglycosylation Kit Cat. No. 362280 Note that this user protocol is not lot-specific
Visit our interactive pathways at /pathways User Protocol 362280 Rev. 23 February 2006 RFH Page 1 of 5 Glycoprotein Deglycosylation Kit Cat. No. 362280 Note that this user protocol is not lot-specific
HLA and antigen presentation. Department of Immunology Charles University, 2nd Medical School University Hospital Motol
 HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
Immunology - Lecture 2 Adaptive Immune System 1
 Immunology - Lecture 2 Adaptive Immune System 1 Book chapters: Molecules of the Adaptive Immunity 6 Adaptive Cells and Organs 7 Generation of Immune Diversity Lymphocyte Antigen Receptors - 8 CD markers
Immunology - Lecture 2 Adaptive Immune System 1 Book chapters: Molecules of the Adaptive Immunity 6 Adaptive Cells and Organs 7 Generation of Immune Diversity Lymphocyte Antigen Receptors - 8 CD markers
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Sheet #5 Dr. Mamoun Ahram 8/7/2014
 P a g e 1 Protein Structure Quick revision - Levels of protein structure: primary, secondary, tertiary & quaternary. - Primary structure is the sequence of amino acids residues. It determines the other
P a g e 1 Protein Structure Quick revision - Levels of protein structure: primary, secondary, tertiary & quaternary. - Primary structure is the sequence of amino acids residues. It determines the other
Protein sorting (endoplasmic reticulum) Dr. Diala Abu-Hsasan School of Medicine
 Protein sorting (endoplasmic reticulum) Dr. Diala Abu-Hsasan School of Medicine dr.abuhassand@gmail.com An overview of cellular components Endoplasmic reticulum (ER) It is a network of membrane-enclosed
Protein sorting (endoplasmic reticulum) Dr. Diala Abu-Hsasan School of Medicine dr.abuhassand@gmail.com An overview of cellular components Endoplasmic reticulum (ER) It is a network of membrane-enclosed
AP Biology Cells: Chapters 4 & 5
 AP Biology Cells: Chapters 4 & 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The was the first unifying principle of biology. a. spontaneous generation
AP Biology Cells: Chapters 4 & 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The was the first unifying principle of biology. a. spontaneous generation
Dr Mahmood S Choudhery, PhD, Postdoc (USA) Assistant Professor Tissue Engineering and Regenerative Medicine King Edward Medical University/Mayo
 Integration of Cells into Tissues Dr Mahmood S Choudhery, PhD, Postdoc (USA) Assistant Professor Tissue Engineering and Regenerative Medicine King Edward Medical University/Mayo Hospital Lahore 1. How
Integration of Cells into Tissues Dr Mahmood S Choudhery, PhD, Postdoc (USA) Assistant Professor Tissue Engineering and Regenerative Medicine King Edward Medical University/Mayo Hospital Lahore 1. How
189,311, , ,561, ,639, ,679, Ch13; , Carbohydrates. Oligosaccharides: Determination of Sequence
 Lecture (2//7) Reading: Chs4,6,8,0,4,6,7,8; 28-29, 89,,77-80,555-557,56,62-622,69,662-66,679, 69-694 Ch; 497-50, 507-54 Problems: Ch (text); 5,6,9,0,22,24 Ch7 (study-guide: applying); 4 Ch7 (study-guide:
Lecture (2//7) Reading: Chs4,6,8,0,4,6,7,8; 28-29, 89,,77-80,555-557,56,62-622,69,662-66,679, 69-694 Ch; 497-50, 507-54 Problems: Ch (text); 5,6,9,0,22,24 Ch7 (study-guide: applying); 4 Ch7 (study-guide:
4. ABSORPTION. Transport mechanisms. Absorption ABSORPTION MECHANISMS. Active transport. Active transport uses metabolic energy
 4. ABSORPTION ABSORPTION MECHANISMS Once the digestive process is completed, the nutrients have to be transferred across the digestive tract epithelium into the intracellular space and eventually into
4. ABSORPTION ABSORPTION MECHANISMS Once the digestive process is completed, the nutrients have to be transferred across the digestive tract epithelium into the intracellular space and eventually into
Mucosal immunity Reddy April Deveshni Reddy Allergy Meeting 13 April 2012
 Deveshni Reddy Allergy Meeting 13 April First recorded by Hippocrates over 2000 years ago. 1921: Prausnitz and Kustner demonstrated that substance responsible for Kustner s fish allergy was present in
Deveshni Reddy Allergy Meeting 13 April First recorded by Hippocrates over 2000 years ago. 1921: Prausnitz and Kustner demonstrated that substance responsible for Kustner s fish allergy was present in
Bio & 241 A&P Unit 1 / Lecture 3
 Bio & 241 A&P Unit 1 / Lecture 3 Tissues All body tissues arise from three fundamental embryonic tissues. Endoderm: forms epithelial tissues lining internal organs such as the GI tract Mesoderm: connective
Bio & 241 A&P Unit 1 / Lecture 3 Tissues All body tissues arise from three fundamental embryonic tissues. Endoderm: forms epithelial tissues lining internal organs such as the GI tract Mesoderm: connective
Histology Notes -Part 1: Epithelial Tissues
 Introduction Group of cells w/ similar structure & function = TISSUE Four Basic Tissue Types 1. Epithelial-covers 2. Connective-supports 3. Muscular*-produces movement (will discuss in the muscular system
Introduction Group of cells w/ similar structure & function = TISSUE Four Basic Tissue Types 1. Epithelial-covers 2. Connective-supports 3. Muscular*-produces movement (will discuss in the muscular system
Molecular Organization of the Cell Membrane
 Molecular Organization of the Cell Membrane A walk from molecules to a functional biostructure Cell Membrane Definition An ultrastructure separating connecting the cell to the environment 1 Coarse chemical
Molecular Organization of the Cell Membrane A walk from molecules to a functional biostructure Cell Membrane Definition An ultrastructure separating connecting the cell to the environment 1 Coarse chemical
Review of Biochemistry
 Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
Membrane Structure and Membrane Transport of Small Molecules. Assist. Prof. Pinar Tulay Faculty of Medicine
 Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
Membrane transport. Pharmacy Dr. Szilvia Barkó
 Membrane transport Pharmacy 04.10.2017 Dr. Szilvia Barkó Cell Membranes Cell Membrane Functions Protection Communication Import and and export of molecules Movement of the cell General Structure A lipid
Membrane transport Pharmacy 04.10.2017 Dr. Szilvia Barkó Cell Membranes Cell Membrane Functions Protection Communication Import and and export of molecules Movement of the cell General Structure A lipid
Carbohydrates. Learning Objective
 , one of the four major classes of biomolecules, are aldehyde or ketone compounds with multiple hydroxyl groups. They function as energy stores, metabolic intermediates and important fuels for the body.
, one of the four major classes of biomolecules, are aldehyde or ketone compounds with multiple hydroxyl groups. They function as energy stores, metabolic intermediates and important fuels for the body.
The Adaptive Immune Response: T lymphocytes and Their Functional Types *
 OpenStax-CNX module: m46560 1 The Adaptive Immune Response: T lymphocytes and Their Functional Types * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m46560 1 The Adaptive Immune Response: T lymphocytes and Their Functional Types * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Mucosal Immunology Sophomore Dental and Optometry Microbiology Section I: Immunology. Robin Lorenz
 Mucosal Immunology Sophomore Dental and Optometry Microbiology Section I: Immunology Robin Lorenz rlorenz@uab.edu Why do we Need to Understand How the Mucosal Immune System Works? The mucosa is the major
Mucosal Immunology Sophomore Dental and Optometry Microbiology Section I: Immunology Robin Lorenz rlorenz@uab.edu Why do we Need to Understand How the Mucosal Immune System Works? The mucosa is the major
Student name ID # 2. (4 pts) What is the terminal electron acceptor in respiration? In photosynthesis?
 1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? 2. (4 pts) What is the terminal electron acceptor
1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? 2. (4 pts) What is the terminal electron acceptor
Small intestine. Small intestine
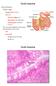 General features Tubular organ longest part; 5-6 m most of chemical digestion absorption of nutrients reabsorption of H2O occurs. Two structural features; maximize the lumenal surface area villi microvilli
General features Tubular organ longest part; 5-6 m most of chemical digestion absorption of nutrients reabsorption of H2O occurs. Two structural features; maximize the lumenal surface area villi microvilli
Cells & Tissues. Chapter 3
 Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Biological Sciences 4087 Exam I 9/20/11
 Name: Biological Sciences 4087 Exam I 9/20/11 Total: 100 points Be sure to include units where appropriate. Show all calculations. There are 5 pages and 11 questions. 1.(20pts)A. If ph = 4.6, [H + ] =
Name: Biological Sciences 4087 Exam I 9/20/11 Total: 100 points Be sure to include units where appropriate. Show all calculations. There are 5 pages and 11 questions. 1.(20pts)A. If ph = 4.6, [H + ] =
Chemical Biology of Protein O-Glycosylation
 University of Colorado, Boulder CU Scholar Chemistry & Biochemistry Graduate Theses & Dissertations Chemistry & Biochemistry Spring 1-1-2016 Chemical Biology of Protein O-Glycosylation Patrick Chaffey
University of Colorado, Boulder CU Scholar Chemistry & Biochemistry Graduate Theses & Dissertations Chemistry & Biochemistry Spring 1-1-2016 Chemical Biology of Protein O-Glycosylation Patrick Chaffey
