Anatomy and regulation of the central melanocortin system
|
|
|
- Dorcas Beatrix Bishop
- 6 years ago
- Views:
Transcription
1 FEEDING REGULATION AND OBESITY REVIEW Anatomy and regulation of the central melanocortin system Roger D Cone The central melanocortin system is perhaps the best-characterized neuronal pathway involved in the regulation of energy homeostasis. This collection of circuits is unique in having the capability of sensing signals from a staggering array of hormones, nutrients and afferent neural inputs. It is likely to be involved in integrating long-term adipostatic signals from leptin and insulin, primarily received by the hypothalamus, with acute signals regulating hunger and satiety, primarily received by the brainstem. The system is also unique from a regulatory point of view in that it is composed of fibers expressing both agonists and antagonists of melanocortin receptors. Given that the central melanocortin system is an active target for development of drugs for the treatment of obesity, diabetes and cachexia, it is important to understand the system in its full complexity, including the likelihood that the system also regulates the cardiovascular and reproductive systems. The gene encoding pro-opiomelanocortin (POMC) produces two different classes of peptides, melanocortins and β-endorphins, which have diverse functions as both hormones and neuropeptides. The melanocortin peptides, which include adrenocorticotropin and α-, β- and γ-melanocyte stimulating hormones (MSH), mediate their effects through a family of five related G protein coupled melanocortin receptors, MC1R through MC5R. In the periphery, POMC is expressed primarily in the pituitary, skin and hair follicle. Adrenocorticotropin, derived from pituitary POMC, acts to regulate adrenal glucocorticoid production through the MC2R or adrenocorticotropin receptor. Melanocortin peptides in skin and hair act in a paracrine fashion to regulate the relative production of the yellow-red and brown-black pigments through the MC1R or MSH receptor expressed at these sites. MC5R is also expressed in the periphery in a variety of exocrine glands, where it seems to be involved in regulating the secretion of exocrine gland products. In the brain, the mammalian central melanocortin system is defined as a collection of CNS circuits that include (i) neurons that express hypothalamic neuropeptide Y and agouti gene related protein (NPY/AgRP) or POMC and that originate in the arcuate nucleus, (ii) brainstem POMC neurons originating in the commissural nucleus of the solitary tract (NTS) and (iii) downstream targets of these POMC and AgRP neurons expressing the melanocortin-3 (MC3R) and melanocortin-4 receptors (MC4R). In the CNS, melanocortin peptides are agonists of the MC3R and MC4R, whereas AgRP is a high-affinity antagonist of both these receptors. The melanocortin system is unique in that many melanocortin receptor sites in the CNS receive projections from both agonist-expressing POMC Vollum Institute and the Center for the Study of Weight Regulation, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA. Correspondence should be addressed to R.D.C. (cone@ohsu.edu). Published online 26 April 2005; doi: /nn1455 fibers and antagonist-expressing AgRP fibers, whereas some seem to receive only agonist innervation 1. Another unique feature of the central melanocortin system is that it acts like a rheostat on energy storage 2 : there are gene dosage effects for the POMC and MC4R genes in which heterozygous loss of either gene produces intermediate phenotypes, a phenomenon unusual for G protein coupled receptor signaling systems. In the mouse, obesity results from many different lesions to the system, including ectopic expression of the agouti protein, another endogenous antagonist of the MC4R, in the CNS (ref. 3), deletion of either MC4R (ref. 2) or MC3R (ref. 4), deletion of the POMC gene 5 or transgenic overexpression of AgRP 6. The melanocortin obesity syndrome can occur in humans, as shown by an agouti mouse like syndrome in two families, resulting from null mutations in the gene encoding POMC 7. These patients have a rare syndrome that includes adrenocorticotropic hormone (ACTH) insufficiency, red hair and obesity, resulting from the lack of ACTH peptide in the serum, and a lack of α-msh in skin and brain, respectively. These data demonstrate that the central melanocortin circuitry subserves energy homeostasis in humans, as it does in the mouse. Heterozygous frameshift mutations in the human MC4R gene, associated with non-syndromic obesity, were subsequently identified in two separate families 8,9. Further studies now show that haploinsufficiency of the MC4R in humans is the most common monogenic cause of severe obesity, accounting for up to 5% of cases 10,11. The detailed clinical picture of the syndrome 12,13 is virtually identical to that reported for the mouse 2,14,15, with increased adipose mass, increased linear growth and lean mass, hyperinsulinemia greater than that seen in matched obese controls and severe hyperphagia. Although the severity of the obesity epidemic has focused attention on the need for better therapeutics and thus the role of the central melanocortin system in energy homeostasis, it is important to note that data also support a role of this system in cardiovascular and sexual function, as described in more detail below. NATURE NEUROSCIENCE VOLUME 8 NUMBER 5 MAY
2 Anatomy of the hypothalamic melanocortin system The major site of POMC expression in the CNS originates in neurons of the arcuate nucleus (Fig. 1). In mice there are approximately 3,000 such POMC-positive cells, most of which also express the anorectic peptide cocaine amphetamine related transcript (CART). The POMC- and CART-positive (POMC/CART) cell bodies are found throughout the rostrocaudal extent of the arcuate nucleus and periarcuate area of the hypothalamus Proceeding from rostral to caudal, arcuate POMC cells send a dense bundle of fibers ventral to the anterior commissure, to several nuclei in the septal region, including the bed nucleus of the stria terminalis and lateral septal nucleus, as well as to the nucleus accumbens in the caudate putamen. More caudally, fibers are seen projecting to the periventricular region of the thalamus and to the medial amygdala. Within the hypothalamus, the densest fibers project to the periventricular nucleus, the paraventricular nucleus (PVH) and the perifornical region, with some fibers seen in most hypothalamic regions. These hypothalamic POMC-expressing neurons (hereafter referred to as POMC neurons ) also send two descending sets of projections to the brainstem: one via the periaquaductal gray and dorsomedial tegmentum, which is thought to innervate the rostral NTS and lateral reticular nucleus (A5 C1 cell groups), and another, believed to be the predominant descending bundle, via the ventral tegmental area, innervating the rostral NTS, ventrolateral medulla (A1 cell group) nucleus ambiguous and spinal cord. Approximately half of the α-msh immunoreactivity in the brainstem is thought to derive from hypothalamic POMC neurons, and the other half derives from a smaller number (~300) of POMC neurons in the brainstem, described in more detail below 19,20. Although the POMC neurons of the arcuate nucleus are defined neurochemically by virtue of their expression of POMC and CART peptides, controversy continues to exist regarding neurotransmitters that may also be present in these cells. GABAergic currents have been identified resulting from the autologous synapses formed by cultured POMC neurons purified on the basis of fluorescence from transgenic mice expressing green fluorescent protein (GFP) under the control of the POMC promoter 21. Furthermore, approximately one-third of arcuate POMC neurons express the mrna for glutamic acid decarboxylase. However, glutamate transporter (EAT3) immunoreactivity is also expressed in some of these cells, implying that some POMC neurons may be glutamatergic 22. However, POMC neurons have not yet been shown to be functionally glutamatergic or GABAergic in a slice preparation. As the arcuate NPY/AgRP-expressing neurons (hereafter referred to as NPY/AgRP neurons ) express the potent MC3R and MC4R antagonist AgRP, they are also a critical component of the central melanocortin system. AgRP-immunoreactive fibers appear primarily in a subset of the same hypothalamic and septal brain regions containing dense POMC innervation, with the densest fibers found innervating the PVH, dorsomedial hypothalamus (DMH), posterior hypothalamus and septal regions around the anterior commissure 1,23. Within the rat hypothalamus, POMC fibers have a much wider distribution than AgRP: moderate fiber density is seen in almost every nucleus, with the possible exceptions of the ventromedial hypothalamus (VMH) and supraoptic nucleus. In the limited number of studies published, AgRP-immunoreactive fibers were also notably absent in the rat in additional divisions of the neuraxis receiving POMC fibers, such as the rostral portion of the brain stem, hippocampus, amygdala, corpus striatum and olfactory cortex tract. Ultimately, an understanding of the nature of the MC3R- and MC4R-expressing target cells is also essential to completing this anatomical picture of the central melanocortin system, but space limitations preclude discussion of this topic here. AgRP Adiposity signals: leptin Vagal afferent Satiety signals: CCK Figure 1 Schematic of the central melanocortin system. POMC neurons in the arcuate nucleus of the hypothalamus and the nucleus tractus solitarius of the brainstem are both adjacent to circumventricular organs and receive and integrate signals from adipostatic factors and satiety factors, respectively. Blue, nuclei containing POMC neurons; magenta, circumventricular organs adjacent to POMC neurons; yellow, a small sample of representative nuclei containing MC4R-positive neurons that may serve to integrate adipostatic and satiety signals; red arrows, representative POMC projections; blue arrows, representative AgRP projections; dashed arrows, secondary projections linking POMC neurons in hypothalamus and brainstem with common effector sites; AP, area postrema; ARC, arcuate nucleus; BST, bed nucleus of the stria terminalus; CEA, central nucleus of the amygdala; DMV, dorsal motor nucleus of the vagus; LH, lateral hypothalamic area; LPB, lateral parabrachial nucleus; ME, median eminence; NTS, nucleus tractus solitarius; PVN, paraventricular nucleus of the hypothalamus; RET, reticular nucleus. Figure modified from ref. 92. Hormonal regulation of the hypothalamic melanocortin system Situated between the third ventricle and the median eminence, in which the portal vascular system functions to transport neuroendocrine releasing factors to the anterior pituitary, the arcuate nucleus is uniquely positioned to sample factors in both blood and cerebrospinal fluid (Fig. 1).Though not classically viewed as such, the arcuate nucleus may sometimes function as a circumventricular organ, existing functionally outside the blood-brain barrier. Although the mechanisms involved are not clear, hypophysiotropic neurons as well as arcuate POMC and NPY neurons have been shown to send fibers into the median eminence. The median eminence may thus contribute to the sensing and response by POMC/CART and NPY/AgRP neurons to blood-born hormone and nutrient signals relevant to both energy homeostasis and hunger/satiety signaling (Fig. 2). For example, growth hormone is known to access arcuate neurons that express growth hormone releasing hormone (GHRH), acting as an autoinhibitory signal for GHRH release. Two methods have been instrumental in characterizing the regulation of these neurons. The first is the analysis of POMC/CART and AgRP/NPY gene expression or c-fos expression as a marker of neuronal activation. With the discovery of the adipostatic hormone leptin, attention focused on defining neural circuits responsible for mediating the leptin signal. POMC- and NPY neurons were identified as two neurochemically defined sites of expression of the leptin receptor in the CNS 24 and as sites where c-fos is activated by both peripheral and central administration of leptin 25. Even before studies on the effect of the leptin gene product on NPY, data suggested that aberrant regulation of orexigenic hypothalamic NPY was responsible for a component of the obesity syndrome in leptin-deficient mice 26. This hypothesis was confirmed when it was demonstrated that crossing leptin-deficient (Lep ob/ob ) mice with NPY-null (Npy / ) mice reduced the obesity 572 VOLUME 8 NUMBER 5 MAY 2005 NATURE NEUROSCIENCE
3 of the Lep ob/ob mice by approximately 50% (ref. 27). Fasting potently upregulates levels of AgRP and NPY mrna (5 10 ) and modestly decreases POMC mrna levels (20 50%), and leptin replacement to pre-fasting levels normalizes expression of these genes Before the discovery of leptin as the principal adipostatic hormone, extensive research on insulin demonstrated that this hormone has some adipostatic activity as well (for review, see ref. 31). Central administration of insulin reduces food intake and body weight, and notably, the arcuate nucleus is one of the prominent sites of insulin receptor expression. Like leptin, insulin upregulates arcuate NPY and AgRP expression and decreases arcuate POMC gene expression; it also activates ATPsensitive K + channels in what are likely to be arcuate NPY neurons 32. Although leptin and insulin activate different signal transduction pathways, both hormones seem to activate phosphatidylinositol 3 -kinase (PI3K) in the hypothalamus and seem to be dependent on PI3K for their anorexigenic activity. A mouse strain with a brain-specific insulin receptor knockout shows a mild obesity syndrome 33, arguing for a physiological role for central insulin action in energy homeostasis, and as described below, central insulin signaling also seems to be important for the regulation of glucose production by the liver 34. Estrogen is another hormone that is likely to regulate POMC neurons in a manner relevant to energy homeostasis. The arcuate nucleus and VMH are major sites of estrogen receptor expression. POMC neurons are a presynaptic target of estrogens 35 and provide synaptic input to GnRH-expressing neurons. Estrogens can also modulate the activity of G protein linked inwardly rectifying potassium channels (GIRKs) in POMC neurons 36. Direct evidence has not yet been obtained, however, to determine whether the anorexigenic actions of estrogen depend on arcuate POMC neurons or on other elements of the central melanocortin system. Electrophysiological recording using mouse hypothalamic slice preparations, in which the rare POMC/CART and NPY/AgRP cell types could be identified using either POMC-GFP or NPY-sapphire transgenes, has also provided a method for direct analyses of the regulation of these cell types. For example, one transgenic line was created using the hypothalamic POMC promoter driving expression of enhanced green fluorescent protein 37. Colocalization studies using an antibody Figure 2 Schematic of the melanocortin system within the arcuate nucleus of the hypothalamus. NPY/AgRP and POMC neurons within the arcuate nucleus form a coordinately regulated network because of dense NPY/ AgRP fibers projecting to POMC cell bodies. Some receptors for the large numbers of hormones and neuropeptides known to regulate the network are indicated. LepR, leptin receptor; µ-or, µ opioid receptor; Y2R, type 2 NPY receptor. In most cases, whether the receptors are presynaptic or postsynaptic is not known. Figure modified from ref. 37. against the POMC peptide β-endorphin showed that more than 99% of arcuate POMC neurons expressed detectable GFP. Whole-cell patchclamp recordings and a loose cell-attached patch method using this system were then developed to characterize the responsiveness of these neurons to leptin and other agents All POMC neurons seem to show spontaneous action potentials, and leptin was found to inhibit the release of GABA from NPY terminals synapsing onto POMC neurons (Fig. 2); in addition, immunoelectron microscopy demonstrated that many POMC cell bodies are contacted by terminals containing both GABA and NPY 37. In addition to characterization of leptin action, this preparation was also used to demonstrate that MC3R is an inhibitory autoreceptor on the POMC circuit 37. Notably, levels of gene expression in the central melanocortin system reflect metabolic state, as does the electrical activity of the NPY/AgRP neurons, and this property is maintained in hypothalamic slices used for electrophysiological studies 42. The spontaneous firing rate of the NPY/AgRP neuron is generally quite low (0.5 Hz) and is elevated threefold by fasting. The activation can be prevented by administration of leptin to the food-deprived animal. The best evidence of a role for the hypothalamic melanocortin system in a direct response to acute signals of satiety and hunger comes from data on ghrelin. Identified as an endogenous ligand for the growth hormone secretagogue receptor (GHSR) 43,44, ghrelin is an acylated 28- amino-acid peptide predominantly secreted by the stomach, regulated by ingestion of nutrients and having potent effects on appetite 47,48. Ghrelin levels are markedly reduced with meal ingestion in both rodents and humans but rebound to baseline before the next meal and increase after an overnight fast GHSRs have been found on arcuate NPY neurons 49, and pharmacological doses of ghrelin injected peripherally or into the hypothalamus activate c-fos solely in arcuate NPY neurons in rats 50. Such doses also stimulate food intake and obesity, in part by stimulating NPY and AgRP expression 51 53, thus antagonizing leptin s anorexic effect 53. On the cellular level, electrophysiological analyses suggest that ghrelin acts on the arcuate NPY/AgRP neurons to coordinately activate these orexigenic cells, while inhibiting the anorexigenic POMC cells by increasing GABA release onto them 38. Ghrelin can also stimulate appetite and food intake in humans when given in a supraphysiological dose, but whether physiological changes in ghrelin levels or ghrelin signaling affect human energy homeostasis remains unknown. Ghrelin s characteristics make it unique among the gut-derived signals. Unlike other enteropancreatic signals involved with energy homeostasis, ghrelin secretion is inhibited in response to meals, and instead of acting as a satiety signal (like CCK or PYY 3 36 ), ghrelin stimulates appetite, potentially through arcuate signaling. These properties strongly suggest that ghrelin is a candidate meal-initiating signal 47. Much work needs to be done to establish the physiological role of ghrelin in meal initiation and body weight regulation and to establish its mechanism or mechanisms of action. Considerable evidence indicates that the melanocortin system is central to ghrelin s effects on food intake. Stimulation of food intake by ghrelin administration is blocked by administration of NPY receptor antagonists 53 and reduced in Npy / mice. Administration of the melanocortin agonist MTII blocks further stimulation of weight gain by growth hormone releasing peptide-2, a synthetic GHSR agonist, in Npy / mice 54. Finally, peripheral administration of ghrelin activates c-fos expression only in arcuate NPY/AgRP neurons, not in other hypothalamic or brainstem sites 55, and ablation of the arcuate nucleus blocks the actions of ghrelin administration on feeding but not elevation of growth hormone 56. Despite activating c-fos only in arcuate NPY neurons, peripheral ghrelin may access the arcuate nucleus through vagal afferents. GHSR is expressed on vagal afferents, and ghrelin suppresses firing of vagal nerves. Furthermore, surgical or chemical vagotomy blocks stimulation of feeding and NATURE NEUROSCIENCE VOLUME 8 NUMBER 5 MAY
4 c-fos activation in the arcuate nucleus after peripheral but not central administration of ghrelin 57. How can these findings be assembled into a single model? Peripheral ghrelin may largely suppress neurons in brainstem satiety centers, thus explaining the lack of c-fos activation at these sites. The NTS sends dense catecholaminergic projections to the arcuate nucleus, so although a convergence of ascending vagal afferent information arrives at the arcuate nucleus from both the brainstem and other hypothalamic nuclei, it is possible that NTS neurons inhibited by ghrelin synapse directly with NPY arcuate neurons, thus explaining the absence of other hypothalamic neurons activated by peripheral ghrelin. Although the possibility is still controversial, there may be a novel set of ghrelin-positive neurons in the hypothalamus. These cells, identified by immunohistochemistry, make numerous connections with NPY fibers in the arcuate nucleus as well as with other neurons involved in energy homeostasis 38. Little data exists about the regulation of hypothalamic ghrelin or about how information from these neurons is integrated with information derived from peripheral ghrelin, which is also known to act on the NPY/POMC arcuate network. Peptide YY (PYY), a peptide related to NPY and pancreatic peptide, is released postprandially by endocrine cells in the ileum and colon 58. PYY is found in vivo in both a full-length 36-amino-acid and a 34-aminoacid form (PYY 3 36 ), in an approximately 2:1 to 1:1 molar ratio 59. PYY is a potent agonist of both Y1 and Y2 receptors, whereas PYY 3 36 is a Y2-specific agonist, with an approximately 1,000-fold greater affinity for the Y2 than for the Y1 receptor. Recently, PYY 3 36 was demonstrated to inhibit food intake acutely in rats in a Y2-dependent manner and to activate c-fos in a small number (10 15%) of arcuate POMC neurons 60,61. Initially, it was proposed that PYY 3 36 might access arcuate Y2 receptor sites, altering food intake by activating arcuate POMC neurons. However, PYY induced inhibition of food intake has been observed in both MC4R 60 and POMC 62 knockout mice, making this model less likely. Furthermore, peripheral administration of PYY 3 36 also activates c-fos in the brainstem and induces conditioned taste aversion, suggesting a mechanism of action independent of the central melanocortin system 63. Nutrients and the hypothalamic melanocortin system It has been proposed that the hypothalamus, in addition to responding to long-term adipostatic signals (leptin and insulin) and to acute satiety/hunger hormones (ghrelin) may also depend on a direct nutrient sensor to regulate food intake and energy balance. This concept is essentially an extension of the glucostatic hypothesis, which postulated that meal initiation may be regulated, in part, by central sensing of blood glucose levels 64. For example, intracerebroventricular admin istration of oleic acid, a long-chain fatty acid, inhibits food intake and glucose production 65. Furthermore, animals overfed a high-fat diet become resistant to the anorexigenic effects of oleic acid 66. Additionally, central inhibition of the rate-limiting enzyme of fatty acid oxidation, carnitine palmitoyltransferase-1, also decreases food intake and glucose production 67. These studies all involved intracerebroventricular administration, which allows access to both forebrain and hindbrain sites, so additional work will be needed to determine the sites where lipids or lipid oxidation might act as an energy sensor. Another cellular energy sensor, the enzyme AMP kinase (AMPK), has also been recently been demonstrated to be involved in energy homeostasis 68,69. Notably, the enzyme is not only a cellular energy sensor but is also inhibited in certain hypothalamic nuclei by leptin, insulin, melanocortin agonists, high glucose levels and refeeding. Furthermore, constitutively active AMPK blocks inhibition of feeding and weight loss induced by leptin. Although AMPK activity is inhibited in the arcuate nucleus and PVH by refeeding 69, exercise in rats seems not to alter the hypothalamic activity of the enzyme 70. The expression and activity of AMPK in POMC, AgRP or MC4R neurons has not yet been reported, although the data above suggest that AMPK levels in the PVH may be mediated by MC4R agonists. Thus far, one line of evidence shows directly that the melanocortin system might be a component of a hypothalamic nutrient sensor. POMC neurons express both Kir6.2 channel and sulfonylurea receptor- 1 channel subunits and thus are likely to have a functional ATP-sensitive K + channel, a mechanism for linking neuronal activity to levels of cellular energy stores. Furthermore, the firing rate of the cells seems to be responsive to glucose concentrations in the bath 41. Ultimately, formal proof of the physiological relevance of a nutrient sensor to hypothalamic control of energy homeostasis will require demonstrating several things: first, that hypothalamic systems such as the melanocortin system respond directly either to postprandial changes in blood nutrient levels or to chronic changes resulting from obesity or starvation; and second, that the degree of response is significant relative to responses to changes in leptin, ghrelin, insulin, glucocorticoids, CCK and other hormones that have evolved to communicate aspects of metabolic state to the CNS. Neural inputs to the hypothalamic melanocortin system It is also important to consider neural inputs to the arcuate and brainstem melanocortin-expressing neurons and inputs to MC3R and MC4R cells. It is likely that the melanocortin system interacts at one or more levels with a wide variety of other circuits involved in energy homeostasis, including those regulating motivated behaviors, circadian rhythm, reproductive function and olfaction. A fundamental aspect of these circuits is also the seemingly asymmetrical innervation of POMC neurons by a dense array of NPY/AgRP fibers derived from adjacent arcuate NPY/AgRP cells, as discussed above. As a consequence, the arcuate POMC and NPY/AgRP cell bodies constitute a functional unit in which neural inputs to NPY/AgRP cells may rapidly affect both NPY/AgRP and POMC neurons. Alternatively, it is possible to imagine inputs to arcuate POMC cells that do not alter the activity of the NPY/AgRP neurons. Neural inputs to brainstem POMC neurons remain largely uncharacterized, although as described below, these cells do receive direct inputs from vagal afferent nerves. In the hypothalamus, α-adrenergic and serotonergic inputs to the melanocortin system have been characterized, although neither system has been well-characterized neuroanatomically. For example, POMC neurons express 5-HT 2c receptors and can be activated by dexfenfluramine, and inhibition of food intake by this compound in the mouse is blocked by the melanocortin antagonist SHU9119 (ref. 39). Serotonergic inputs to POMC neurons may also be important for the actions of cytokines on the melanocortin circuits during cachexia. Both arcuate NPY/AgRP and POMC neurons also receive innervation from orexin neurons originating in the LHA 71, and tachykinin-immunoreactive fibers synapsing onto arcuate NPY/AgRP cells have been characterized as well. Anatomy and regulation of the brainstem melanocortin system The brainstem is classically understood as the center for detection of and response to hunger and satiety signals. An alternate route for satiety factors and nutrients to activate the hypothalamic POMC neurons would involve the known action of these factors at the brainstem, as sites of vagal afferent action and gut peptide action involve brainstem cell groups such as the NTS that send dense projections to mediobasal hypothalamic cell groups like the arcuate nucleus. The NTS is the primary site for innervation by vagal afferents from the gut (for review, see ref. 72). The afferent branches deriving from 574 VOLUME 8 NUMBER 5 MAY 2005 NATURE NEUROSCIENCE
5 different aspects of the gastrointestinal tract map viscerotopically along the NTS, from its rostral to its caudal aspect 73. Rostrally, the NTS is a bilaterally symmetrical nucleus that merges into a single medial body at its caudal extent, called the commissural NTS 74. Vagal afferents deriving input from the upper gastrointestinal tract respond to three basic stimuli: gastric and duodenal distension or contraction, chemical contents of the lumen and gut peptides and neurotransmitters released from the stomach and duodenum in response to nutrients 72. In the rat, vagal afferents responding to gastric and duodenal distension tend to map to the medial and commissural divisions of the NTS 75,76. CCK vagal afferents also map to the caudal NTS 77. The dorsal motor nucleus of the vagus, located just ventral to the NTS, is the primary site of motor efferents to the gut and is densely innervated by NTS fibers. Together, these cell groups form the dorsal vagal complex (DVC) and serve as the neuroanatomical substrate for the vago-vagal reflex. Several lines of evidence suggest an important role for melanocortin signaling within the brainstem in the satiety and vago-vagal reflexes. First, a discrete site of POMC expression in the brainstem (Fig. 3) has been identified in the commissural NTS 78,79. ACTH, α-msh and β- endorphin immunoreactivity have been identified in these cells 79, and deafferentation of the hypothalamic POMC-expressing fibers using knife cuts 19 or treatment with monosodium glutamate 20 indicate that the arcuate POMC fibers exclusively innervate forebrain, midbrain and periaquaductal gray matter. POMC NTS fibers are found in many sites in the caudal mesencephalon and spinal cord, with sites of dual innervation by hypothalamic and brainstem POMC fibers seen in the locus coeruleus, parabrachial nucleus, rostral NTS, dorsal motor nucleus of the vagus and lateral reticular nucleus. MC4R mrna is also expressed in multiple sites within the brainstem, with quite high levels within the DMV, NTS and parabrachial nucleus 80,81. Fourthventricular 82 and parenchymal DVC injections 83 of melanocortin agonists and antagonists produce effects on both food intake and weight gain comparable to those seen with lateral ventricular injections. These data imply a marked coordination between the brainstem and hypothalamic melanocortin systems. In addition to signals from gut distension, gut peptides stimulated by meal intake mediate satiety through centers in the brainstem. These signals then are thought to interact primarily with centers of longterm weight regulation through neural connections to the hypothalamus to regulate total daily intake by adjusting meal size, number of meals or both. Cholecystokinin is a good example of such a gut peptide. Produced by the gastrointestinal tract in response to meal ingestion, CCK s diverse actions include stimulation of pancreatic enzyme secretion and intestinal motility, inhibition of gastric motility and acute inhibition of feeding. Early experiments involving peripheral administration of CCK supported a role for increased CCK levels in the early termination of a meal 84. The finding that repeated injections of CCK lead to reduced meal size without a change in body weight, because of a compensatory increase in meal frequency, argued against CCK acting as a signal regulating long-term body weight 85. Studies using CCK receptor specific antagonists as well as surgical or chemical vagotomy have shown that the effects of CCK on satiety are specifically mediated by one type of CCK receptor, CCKAR, on afferent vagal nerves 86,87. This interaction between acute vagal input from CCKA receptors and body weight set point is primarily mediated by neural connections to the hypothalamus; this interaction is affected by insulin and leptin signals and inputs from the hindbrain, which receives input from vagal afferents. Indeed, central administration of insulin and leptin potentiates the satiety-inducing effects of peripherally administered CCK 88,89 Figure 3 Distribution of POMC neurons within the nucleus tractus solitarius of the brainstem. POMC expression defines a unique population of THnegative, GLP-1 negative cells within the medial nucleus of the NTS. AP, area postrema; Cb, cerebellum; CC, central canal; DMV, dorsal motor nucleus of the vagus; Sol, solitary tract; SolC, commissural nucleus of the solitary tract. and, with repeated injections, leads to a sustained weight loss greater than that resulting from injection of the agents separately 90. Although basomedial hypothalamic cell groups involved in leptin signaling are densely innervated by catecholaminergic neurons from the brainstem, a recent report demonstrates that norepinephrine is not required for CCK-induced reduction of feeding, as the dopamine β-hydroxylase knockout mouse is still responsive to CCK-induced satiety 91. However, the brainstem POMC neurons may be one of the noncatecholaminergic cell groups involved in transmitting CCK s satiety signal. Approximately 30% of the POMC NTS neurons are activated, as determined by induction of c-fos immunoreactivity, after intraperitoneal administration of a dose of CCK that initiates satiety 92. Furthermore, the central melanocortin system as a whole is clearly important for the satiety effect mediated by CCK, as Mc4r / mice are largely resistant to CCK-induced satiety, and fourth-ventricular administration of the melanocortin antagonist SHU9119 seems more potent than third-ventricular administration in blocking the actions of CCK 92. Only a very small fraction of cells activated in the brainstem by CCK-induced satiety are POMC NTS cells. Thus, whereas CCK-mediated satiety seems dependent on MC4R signaling, it is unlikely to be dependent on the POMC NTS cells. The regulation of POMC NTS cells by CCK may be the first report of regulation of these cells: feeding-induced satiety also produces upregulation of c-fos in 10 15% of these cells 92. Finally, the POMC NTS cells are also activated by leptin (K.L.J. Ellacott and R.D.C., personal communication). Approximately 50% of the cells showed induction of c-fos immunoreactivity after peripheral leptin treatment, and approximately 30% of all c-fos immunoreactive cells in the NTS after leptin treatment were POMC-GFP positive. Although α-msh and β-endorphin immunoreactivity were observed in the commissural NTS in early reports characterizing these cells in the rat, these peptides seem to be expressed at low levels in the brainstem, and achieving these results is technically challenging. The availability of POMC-GFP mouse strains has facilitated all the recent studies of the regulation and neurochemical identity of these cells, but a more detailed analysis of their POMC peptide content and regulation remains an important goal. Using POMC-GFP immunoreactivity as a marker, these cells have been demonstrated to be CART-negative, tyrosine hydroxylase (TH)-negative and glucagon-like peptide (GLP)- 1 negative NTS neurons 92. Thus, they represent a previously unknown class of NTS neurons regulated by both leptin and acute satiety signals. They have also been shown to be innervated by vagal afferents 93. NATURE NEUROSCIENCE VOLUME 8 NUMBER 5 MAY
6 Additional roles for the central melanocortin system The melanocortin system is probably the best-characterized CNS system involved in the regulation of energy homeostasis, and a number of animal experiments suggest potential therapeutic applications of melanocortin agonists and antagonists in the treatment of obesity and diabetes, as well as cachexia, respectively. With this in mind, it is important to remember that the POMC and NPY/AgRP neurons project to a wide array of brain nuclei, with MC4R alone being identified in over 100 sites 80. Not unexpectedly, administration of melanocortins has been shown to have physiological effects beyond the regulation of food intake and energy expenditure, including effects on blood pressure, heart rate, inflammation, natriuresis and erectile function. The actions of melanocortins on the cardiovascular system are complex and involve multiple receptors. Central or intracarotid administration of the MC3R-specific agonist γ-msh has pressor and cardioacceleratory effects, although the pressor activity of the peptide does not seem to require MC3R, implicating an uncharacterized receptor. Hypotensive and bradycardic responses to electrical stimulation of the arcuate nucleus can be blocked by administration of the melanocortin antagonist SHU9119 (ref. 94) within the DVC, suggesting a role for brainstem MC4R in cardiovascular control. The central melanocortin system also influences inflammation: administration of α-msh has potent anti-inflammatory effects that are dependent on intact autonomic outflow, perhaps related to the control of vascular permeability (for review, see ref. 95). The physiological relevance of these findings is not yet clear, although ACTH and α-msh have resuscitative effects in models of hemorrhagic shock. The melanocortin system also is involved in natriuresis 96. γ-msh stimulates natriuresis through kidney MC3R (ref. 97), and both γ-msh deficient and MC3R knockout mice develop salt-sensitive hypertension 98. In γ-msh deficient prohormone convertase-2 knockout mice, central infusion of γ-msh corrects the salt-sensitive hypertension, implicating central MC3R sites in this pathway as well. Central melanocortin receptors are also implicated in the control of sexual function, stimulating lordosis in female rats and erectile activity in male rodents as well as in humans 99. Both central and peripheral MC4R are implicated in erectile function, and MC3R is implicated in lordosis. Melanocortins have been tested in clinical trials for the treatment of erectile dysfunction 100. Conclusions The central melanocortin system is a fascinating collection of neural circuits that provides an ideal neuroanatomical substrate for the integration of long-term adipostatic signals from leptin with acute hunger and satiety signals from vagal afferent activity, gut satiety and hunger peptides, and nutrients. The positioning of POMC and NPY/AgRP cell bodies adjacent to the median eminence, and of brainstem POMC cell bodies adjacent to the area postrema, imply that the system may be sampling blood-borne hormone and nutrient concentrations. There are reciprocal projections from brainstem and hypothalamic sites of melanocortin cell bodies to integrative and motor centers with high densities of MC4R expression, such as the PVH and DMV. This argues that the system is ultimately important in integrating many afferent inputs with behavioral and autonomic responses that adjust energy intake and expenditure, thus maintaining energy homeostasis. Although the regulation of POMC and NPY/AgRP neurons has received considerable attention, understanding of the nature and regulation of MC3R and MC4R neurons remains in its infancy. For example, efferent pathways downstream of the melanocortin system have been only partially characterized. Likewise, few details exist concerning the mechanisms by which the melanocortin system stores information regarding levels of energy stores and integrates this information with output to affect levels of intake and expenditure. The central melanocortin system has potential as a site for therapeutic intervention for disorders such as obesity, diabetes and cachexia. However, to avoid potential side effects, it will also be important to learn more about the possible roles for these circuits in cardiovascular function, natriuresis, inflammation and sexual function. ACKNOWLEDGMENTS The author would like to thank the many students, postdoctoral fellows and collaborators who participated in the work from his laboratory discussed in this review. The author would also like to thank L. Vaskalis for creating the illustrations. This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. COMPETING FINANCIAL INTERESTS The authors declare competing financial interests (see the Nature Neuroscience website for details). Received 15 February; accepted 15 March 2005 Published online at 1. Haskell-Luevano, C. et al. Characterization of the neuroanatomical distribution of agouti-related protein (AGRP) immunoreactivity in the rhesus monkey and the rat. Endocrinology 140, (1999). 2. Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, (1997). 3. Yen, T.T., Gill, A.M., Frigeri, L.G., Barsh, G.S. & Wolff, G.L. Obesity, diabetes, and neoplasia in yellow A vy / mice: ectopic expression of the agouti gene. FASEB J. 8, (1994). 4. Butler, A.A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, (2000). 5. Yaswen, L., Diehl, N., Brennan, M.B. & Hochgeschwender, U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 5, (1999). 6. Ollmann, M.M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, (1997). 7. Krude, H. et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19, (1998). 8. Vaisse, C., Clement, K., Guy-Grand, B. & Froguel, P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat. Genet. 20, (1998). 9. Yeo, G.S.H. et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 20, (1998). 10. Farooqi, I.S. et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Invest. 106, (2000). 11. Vaisse, C. et al. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J. Clin. Invest. 106, (2000). 12. Farooqi, I.S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348, (2003). 13. Branson, R. et al. Binge eating as a major phenotype of melanocortin 4 receptor gene mutations. N. Engl. J. Med. 348, (2003). 14. Butler, A.A. et al. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat. Neurosci. 4, (2001). 15. Fan, W. et al. The central melanocortin system can directly regulate serum insulin levels. Endocrinology 141, (2000). 16. Watson, S.J., Akil, H., Richard, C.W. & Barchas, J.D. Evidence for two separate opiate peptide neuronal systems and the coexistence of β-lipotropin, β-endorphin, and ACTH immunoreactivities in the same hypothalamic neurons. Nature 275, (1978). 17. Jacobowitz, D.M. & O Donohue, T.L. α-melanocyte-stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc. Natl. Acad. Sci. USA 75, (1978). 18. Nilaver, G. et al. Adrenocorticotropin and beta-lipotropin in the hypothalamus. Localization in the same arcuate neurons by sequential immunocytochemical procedures. J. Cell Biol. 81, (1979). 19. Joseph, S.A. & Michael, G.J. Efferent ACTH-IR opiocortin projections from nucleus tractus solitarius: a hypothalamic deafferentation study. Peptides 9, (1988). 20. Pilcher, W.H. & Joseph, S.A. Differential sensitivity of hypothalamic and medullary opiocortin and tyrosine hydroxylase neurons to the neurotoxic effects of monosodium glutamate (MSG). Peptides 7, (1986). 21. Hentges, S.T. et al. GABA release from POMC neurons. J. Neurosci. 24, (2004). 22. Collin, M. et al., Plasma membrane and vesicular glutamate transporter mrnas/ proteins in hypothalamic neurons that regulate body weight. Eur. J. Neurosci. 18, (2003). 23. Broberger, C., Johansen, J., Johansson, C., Schalling, M. & Hokfelt, T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and 576 VOLUME 8 NUMBER 5 MAY 2005 NATURE NEUROSCIENCE
7 monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA 95, (1998). 24. Hakansson, M.L., Hulting, A.L. & Meister, B. Expression of leptin receptor mrna in the hypothalamic arcuate nucleus relationship with NPY neurones. Neuroreport 7, (1996). 25. Elmquist, J.K., Ahima, R.S., Maratos-Flier, E., Flier, J.S. & Saper, C.B. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 138, (1997). 26. Stephens, T. et al. The role of neuropeptide Y in the antiobesity action of the obesity gene product. Nature 377, (1995). 27. Erickson, J., Hollopeter, G. & Palmiter, J.D. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274, (1996). 28. Mizuno, T.M. & Mobbs, C.V. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 140, (1999). 29. Mizuno, T.M. et al. Hypothalamic pro-opiomelanocortin mrna is reduced by fasting and corrected in ob/ob and db/db mice, but is stimulated by leptin. Diabetes 47, (1998). 30. Schwartz, M.W. et al. Leptin increases hypothalamic pro-opiomelanocortin mrna expression in the rostral arcuate nucleus. Diabetes 46, (1997). 31. Niswender, K.D., Baskin, D.G. & Schwartz, M.W. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol. Metab. 15, (2004). 32. Spanswick, D., Smith, M.A., Mirshamsi, S., Routh, V.H. & Ashford, M.L. Insulin activates ATP-sensitive K + channels in hypothalamic neurons of lean, but not obese rats. Nat. Neurosci. 3, (2000). 33. Bruning, J.C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, (2000). 34. Obici, S., Zhang, B.B., Karkanias, G. & Rossetti, L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 8, (2002). 35. Blum, M., Roberts, J.L. & Wardlaw, S.L. Androgen regulation of proopiomelanocortin gene expression and peptide content in the basal hypothalamus. Endocrinology 124, (1989). 36. Kelly, M.J., Qiu, J. & Ronnekleiv, O.K. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann. NY Acad. Sci. 1007, 6 16 (2003). 37. Cowley, M.A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, (2001). 38. Cowley, M.A. et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, (2003). 39. Heisler, L.K. et al. Activation of central melanocortin pathways by fenfluramine. Science 297, (2002). 40. Roseberry, A.G., Liu, H., Jackson, A.C., Cai, X. & Friedman, J.M. Neuropeptide Y- mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron 41, (2004). 41. Ibrahim, N. et al. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 144, (2003). 42. Takahashi, K.A. & Cone, R.D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146, (2005). 43. Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, (1999). 44. Kojima, M., Hosoda, H., Matsuo, H. & Kangawa, K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol. Metab. 12, (2001). 45. Ariyasu, H. et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 86, (2001). 46. Tschop, M. et al. Post-prandial decrease of circulating human ghrelin levels. J. Endocrinol. Invest. 24, RC19 RC21 (2001). 47. Cummings, D.E. et al. A preprandial rise in plasma ghrelin levels suggest a role in meal initiation in humans. Diabetes 50, (2001). 48. Tschop, M., Smiley, D.L. & Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 407, (2000). 49. Willesen, M., Kristensen, P. & Romer, J. Co-localization of growth hormone secretagogue receptor and NPY mrna in the arcuate nucleus of the rat. Neuroendocrinology 70, (1999). 50. Hewson, A.K. & Dickson, S.L. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J. Neuroendocrinol. 12, (2000). 51. Kamegai, J. et al. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology 141, (2000). 52. Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, (2001). 53. Shintani, M. et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 50, (2001). 54. Tschop, M., Statnick, M.A., Suter, T.M. & Heiman, M.L. GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology 143, (2002). 55. Wang, L., Saint-Pierre, D.H. & Tache, Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci. Lett. 325, (2002). 56. Tamura, H. et al. Ghrelin stimulates GH but not food intake in arcuate nucleus ablated rats. Endocrinology 143, (2002). 57. Date, Y. et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123, (2002). 58. Adrian, T.E. et al. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89, (1985). 59. Grandt, D. et al. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1 36 and PYY Regul. Pept. 51, (1994). 60. Halatchev, I.G., Ellacott, K.L., Fan, W. & Cone, R.D. Peptide YY3 36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology 145, (2004). 61. Batterham, R.L. et al. Gut hormone PYY(3 36) physiologically inhibits food intake. Nature 418, (2002). 62. Challis, B.G. et al. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-yy(3 36). Proc. Natl. Acad. Sci. USA 101, (2004). 63. Halatchev, I.G. & Cone, R.D. Peripheral administration of PYY 3 36 produces conditioned taste aversion in mice. Cell Metab. 1, (2005). 64. Mayer, J. Regulation of energy intake and body weight: the glucostatic theory and the lipostatic hypothesis. Ann. NY Acad. Sci. 63, (1955). 65. Obici, S. et al. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51, (2002). 66. Morgan, K., Obici, S. & Rossetti, L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J. Biol. Chem. 279, (2004). 67. Obici, S., Feng, Z., Arduini, A., Conti, R. & Rossetti, L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat. Med. 9, (2003). 68. Andersson, U. et al. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 279, (2004). 69. Minokoshi, Y. et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, (2004). 70. Andersson, U. et al. Exercise in rats does not alter hypothalamic AMP-activated protein kinase activity. Biochem. Biophys. Res. Commun. 329, (2005). 71. Horvath, T.L., Diano, S. & van den Pol, A.N. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J. Neurosci. 19, (1999). 72. Schwartz, G.J. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition 16, (2000). 73. Altschuler, S.M., Bao, X.M., Bieger, D., Hopkins, D.A. & Miselis, R.R. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J. Comp. Neurol. 283, (1989). 74. Saper, C.B. Central autonomic system. in The Rat Nervous System (ed. Paxinos, G.) (Academic Press, San Diego, 1995). 75. Raybould, H.E., Gayton, R.J. & Dockray, G.J. CNS effects of circulating CCK8: involvement of brainstem neurones responding to gastric distension. Brain Res. 342, (1985). 76. Zhang, X., Fogel, R. & Renehan, W.E. Relationships between the morphology and function of gastric- and intestine-sensitive neurons in the nucleus of the solitary tract. J. Comp. Neurol. 363, (1995). 77. Rinaman, L., Verbalis, J.G., Stricker, E.M. & Hoffman, G.E. Distribution and neurochemical phenotypes of caudal medullary neurons activated to express cfos following peripheral administration of cholecystokinin. J. Comp. Neurol. 338, (1993). 78. Joseph, S.A., Pilcher, W.H. & Bennet-Clarke, C. Immunocytochemical localization of ACTH parikarya in nucleus tractus solitarius: evidence for a second opiocortin neuronal system. Neurosci. Lett. 38, (1983). 79. Palkovits, M., Mezey, E. & Eskay, R.L. Pro-opiomelanocortin-derived peptides (ACTH/ β-endorphin/α-msh) in brainstem baroreceptor areas of the rat. Brain Res. 436, (1987). 80. Mountjoy, K. et al. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 8, (1994). 81. Kishi, T. et al. Expression of melanocortin 4 receptor mrna in the central nervous system of the rat. J. Comp. Neurol. 457, (2003). 82. Grill, H.J., Ginsberg, A.B., Seeley, R.J. & Kaplan, J.M. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J. Neurosci. 18, (1998). 83. Williams, D.L., Kaplan, J.M. & Grill, H.J. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology 141, (2000). 84. Gibbs, J., Falasco, J.D. & McHugh, P.R. Cholecystokinin-decreased food intake in rhesus monkeys. Am. J. Physiol. 230, (1976). 85. Crawley, J.N. & Beinfeld, M.C. Rapid development of tolerance to the behavioural actions of cholecystokinin. Nature 302, (1983). 86. Smith, G.P., Jerome, C., Cushin, B.J., Eterno, R. & Simansky, K.J. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 213, (1981). 87. Dourish, C.T., Ruckert, A.C., Tattersall, F.D. & Iversen, S.D. Evidence that decreased NATURE NEUROSCIENCE VOLUME 8 NUMBER 5 MAY
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction. Laura Gunter
 Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
CNS Control of Food Intake. Adena Zadourian & Andrea Shelton
 CNS Control of Food Intake Adena Zadourian & Andrea Shelton Controlling Food Intake Energy Homeostasis (Change in body adiposity + compensatory changes in food intake) Background Information/Review Insulin
CNS Control of Food Intake Adena Zadourian & Andrea Shelton Controlling Food Intake Energy Homeostasis (Change in body adiposity + compensatory changes in food intake) Background Information/Review Insulin
Copyright 2017 The Guilford Press
 This is a chapter excerpt from Guilford Publications. Eating Disorders and Obesity: A Comprehensive Handbook, Third Edition. Edited by Kelly D. Brownell and B. Timothy Walsh. Copyright 2017. Purchase this
This is a chapter excerpt from Guilford Publications. Eating Disorders and Obesity: A Comprehensive Handbook, Third Edition. Edited by Kelly D. Brownell and B. Timothy Walsh. Copyright 2017. Purchase this
Internal Regulation II Energy
 Internal Regulation II Energy Reading: BCP Chapter 16 lookfordiagnosis.com Homeostasis Biologically, what is necessary for life is a coordinated set of chemical reactions. These reactions take place in
Internal Regulation II Energy Reading: BCP Chapter 16 lookfordiagnosis.com Homeostasis Biologically, what is necessary for life is a coordinated set of chemical reactions. These reactions take place in
Motivation 1 of 6. during the prandial state when the blood is filled
 Motivation 1 of 6 I. INTRODUCTION A. Motivation: a condition (usually internal) that initiates, activates, or maintains goal-directed behavior. B. Archery analogy 1. undrawn bow has no potential energy
Motivation 1 of 6 I. INTRODUCTION A. Motivation: a condition (usually internal) that initiates, activates, or maintains goal-directed behavior. B. Archery analogy 1. undrawn bow has no potential energy
Ingestive Behaviors 33. Introduction. Page 1. control and story lines. (a review of general endocrinology) Integration (or the basic reflex arc model)
 Ingestive Behaviors 33 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Ingestive Behaviors 33 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Chapter 12. Ingestive Behavior
 Chapter 12 Ingestive Behavior Drinking a. fluid compartments b. osmometric thirst c. volumetric thirst Eating a. energy sources b. starting a meal c. stopping a meal d. eating disordersd Drinking a. fluid
Chapter 12 Ingestive Behavior Drinking a. fluid compartments b. osmometric thirst c. volumetric thirst Eating a. energy sources b. starting a meal c. stopping a meal d. eating disordersd Drinking a. fluid
Chapter 1. General introduction. Part of this chapter is adapted from Adan et.al., Br.J.Pharmacol. 2006;149:815
 Chapter 1 General introduction Part of this chapter is adapted from Adan et.al., Br.J.Pharmacol. 2006;149:815 Chapter 1 B. Tiesjema, 2007 GENERAL INTRODUCTION NEURAL CIRCUITS INVOLVED IN ENERGY BALANCE
Chapter 1 General introduction Part of this chapter is adapted from Adan et.al., Br.J.Pharmacol. 2006;149:815 Chapter 1 B. Tiesjema, 2007 GENERAL INTRODUCTION NEURAL CIRCUITS INVOLVED IN ENERGY BALANCE
Ingestive Behaviors 21. Introduction. Page 1. control and story lines. (a review of general endocrinology) Integration (or the basic reflex arc model)
 Ingestive Behaviors 21 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Ingestive Behaviors 21 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Bi156 lecture 2, 1/6/12. Eating and weight regulation
 Bi156 lecture 2, 1/6/12 Eating and weight regulation Introduction: weight regulation in an affluent society In our society much effort and money is expended on regulation of weight. Failure to maintain
Bi156 lecture 2, 1/6/12 Eating and weight regulation Introduction: weight regulation in an affluent society In our society much effort and money is expended on regulation of weight. Failure to maintain
Gut hormones KHATTAB
 Gut hormones PROF:ABD ALHAFIZ HASSAN KHATTAB Gut as an endocrine gland The talk will cover the following : Historical background. Why this subject is chosen. Gastro-intestinal hormones and their function.
Gut hormones PROF:ABD ALHAFIZ HASSAN KHATTAB Gut as an endocrine gland The talk will cover the following : Historical background. Why this subject is chosen. Gastro-intestinal hormones and their function.
1 Neuroregulation of Appetite
 Chapter 1 / Neuroregulation of Appetite 3 1 Neuroregulation of Appetite Ofer Reizes, PhD, Stephen C. Benoit, PhD, and Deborah J. Clegg, PhD CONTENTS INTRODUCTION THE DUAL-CENTERS HYPOTHESIS CONTROL OF
Chapter 1 / Neuroregulation of Appetite 3 1 Neuroregulation of Appetite Ofer Reizes, PhD, Stephen C. Benoit, PhD, and Deborah J. Clegg, PhD CONTENTS INTRODUCTION THE DUAL-CENTERS HYPOTHESIS CONTROL OF
Motility Conference Ghrelin
 Motility Conference Ghrelin Emori Bizer, M.D. Division of Gastroenterology/Hepatology November 21, 2007 Ghrelin: Basics Hormone produced by the A-like A endocrine cells in the oxyntic mucosa (stomach body
Motility Conference Ghrelin Emori Bizer, M.D. Division of Gastroenterology/Hepatology November 21, 2007 Ghrelin: Basics Hormone produced by the A-like A endocrine cells in the oxyntic mucosa (stomach body
Hormonal Regulation of Food Intake
 Physiol Rev 85: 1131 1158, 2005; doi:10.1152/physrev.00015.2004. Hormonal Regulation of Food Intake SARAH STANLEY, KATIE WYNNE, BARBARA McGOWAN, AND STEPHEN BLOOM Endocrine Unit, Imperial College Faculty
Physiol Rev 85: 1131 1158, 2005; doi:10.1152/physrev.00015.2004. Hormonal Regulation of Food Intake SARAH STANLEY, KATIE WYNNE, BARBARA McGOWAN, AND STEPHEN BLOOM Endocrine Unit, Imperial College Faculty
Hypothalamus. Small, central, & essential.
 Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Figure 1: The leptin/melanocortin pathway Neuronal populations propagate the signaling of various molecules (leptin, insulin, ghrelin) to control
 Leptin Deficiency Introduction The leptin/melanocortin pathway plays a key role in the hypothalamic control of food intake. It is activated following the systemic release of the adipokine leptin (LEP)
Leptin Deficiency Introduction The leptin/melanocortin pathway plays a key role in the hypothalamic control of food intake. It is activated following the systemic release of the adipokine leptin (LEP)
! acts via the autonomic nervous system. ! maintaining body weight within tight limits. ! ventromedial (VMN) ! arcuate (ARC) ! neuropeptide Y (NPY)
 Brain Regulates energy homeostatis Glucose Sensing in the Brain Seminar 2012 Gareth Price! acts in response to circulating signals of nutrient states! acts via the autonomic nervous system Ensures a balance
Brain Regulates energy homeostatis Glucose Sensing in the Brain Seminar 2012 Gareth Price! acts in response to circulating signals of nutrient states! acts via the autonomic nervous system Ensures a balance
Ingestive Behavior: Feeding & Weight Regulation. Hypovolemic vs. Osmotic Thirst
 Ingestive Behavior: Feeding & Weight Regulation 1 Hypovolemic Thirst Receptors, CNS, Responses Salt Appetite Digestive components Glucose Homeostasis: Insulin & Glucagon Diabetes Mellitus 1 & 2 CNS Hypothalamic
Ingestive Behavior: Feeding & Weight Regulation 1 Hypovolemic Thirst Receptors, CNS, Responses Salt Appetite Digestive components Glucose Homeostasis: Insulin & Glucagon Diabetes Mellitus 1 & 2 CNS Hypothalamic
Central nervous system control of food intake
 insight review article Central nervous system control of food intake Michael W. Schwartz*, Stephen C. Woods, Daniel Porte Jr*, Randy J. Seeley & Denis G. Baskin* Departments of Medicine* and Biological
insight review article Central nervous system control of food intake Michael W. Schwartz*, Stephen C. Woods, Daniel Porte Jr*, Randy J. Seeley & Denis G. Baskin* Departments of Medicine* and Biological
LESSON 3.3 WORKBOOK. How do we decide when and how much to eat?
 Appetite The psychological desire to eat, driven by feelings of pleasure from the brain. Hunger The biological or physiological need to eat, caused by a release of hormones from the digestive tract. LESSON
Appetite The psychological desire to eat, driven by feelings of pleasure from the brain. Hunger The biological or physiological need to eat, caused by a release of hormones from the digestive tract. LESSON
Νευροφυσιολογία και Αισθήσεις
 Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 16 Κίνητρα Συμπεριφοράς ή Υποκίνηση (Motivation) Introduction Types of behavior Unconscious reflexes Voluntary
Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 16 Κίνητρα Συμπεριφοράς ή Υποκίνηση (Motivation) Introduction Types of behavior Unconscious reflexes Voluntary
Insulin-Leptin Interactions
 Insulin-Leptin Interactions Ahmed S., Al-Azzam N., Cao B. Karshaleva B., Sriram S., Vu K. If you understand a system, you can predict it. Agenda - Energy homeostasis Overview of leptin and insulin Signaling
Insulin-Leptin Interactions Ahmed S., Al-Azzam N., Cao B. Karshaleva B., Sriram S., Vu K. If you understand a system, you can predict it. Agenda - Energy homeostasis Overview of leptin and insulin Signaling
Neurobiology of food intake in health and disease
 Neurobiology of food intake in health and disease Gregory J. Morton, Thomas H. Meek and Michael W. Schwartz Abstract Under normal conditions, food intake and energy expenditure are balanced by a homeostatic
Neurobiology of food intake in health and disease Gregory J. Morton, Thomas H. Meek and Michael W. Schwartz Abstract Under normal conditions, food intake and energy expenditure are balanced by a homeostatic
Leptin Intro/Signaling. ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph
 Leptin Intro/Signaling ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph Overview Intro to Leptin Definition & Sources Physiology Bound vs. Free Receptors Signaling JAK/STAT MAPK PI3K ACC Experimental findings
Leptin Intro/Signaling ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph Overview Intro to Leptin Definition & Sources Physiology Bound vs. Free Receptors Signaling JAK/STAT MAPK PI3K ACC Experimental findings
Digestion: Endocrinology of Appetite
 Digestion: Endocrinology of Dr. Ritamarie Loscalzo Medical Disclaimer: The information in this presentation is not intended to replace a one on one relationship with a qualified health care professional
Digestion: Endocrinology of Dr. Ritamarie Loscalzo Medical Disclaimer: The information in this presentation is not intended to replace a one on one relationship with a qualified health care professional
FLASH CARDS. Kalat s Book Chapter 10 Alphabetical
 FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
Behavioral and Motivational mechanisms of Brain. Limbic system and the Hypothalamus
 Behavioral and Motivational mechanisms of Brain Limbic system and the Hypothalamus 1 General functions 1. Control of behavior 2. Control level of activities in different parts of brain 3. Motivational
Behavioral and Motivational mechanisms of Brain Limbic system and the Hypothalamus 1 General functions 1. Control of behavior 2. Control level of activities in different parts of brain 3. Motivational
BIOLOGY - CLUTCH CH.45 - ENDOCRINE SYSTEM.
 !! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
!! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
biological psychology, p. 40 The study of the nervous system, especially the brain. neuroscience, p. 40
 biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
PERSPECTIVE. The hardship of obesity: a soft-wired hypothalamus. Tamas L Horvath FEEDING REGULATION AND OBESITY
 FEEDING REGULATION AND OBESITY PERSPECTIVE The hardship of obesity: a soft-wired hypothalamus Tamas L Horvath Food intake and energy expenditure are determinants of metabolic phenotype and are regulated
FEEDING REGULATION AND OBESITY PERSPECTIVE The hardship of obesity: a soft-wired hypothalamus Tamas L Horvath Food intake and energy expenditure are determinants of metabolic phenotype and are regulated
processes in the central nervous system (CNS), affecting many of the during the course of ethanol treatment. Ethanol stimulates the release of
 INTRODUCTION INTRODUCTION Neuroscience research is essential for understanding the biological basis of ethanol-related brain alterations and for identifying the molecular targets for therapeutic compounds
INTRODUCTION INTRODUCTION Neuroscience research is essential for understanding the biological basis of ethanol-related brain alterations and for identifying the molecular targets for therapeutic compounds
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
Chapter 3. Structure and Function of the Nervous System. Copyright (c) Allyn and Bacon 2004
 Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
number Done by Corrected by Doctor
 number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
Neurotransmitter Systems I Identification and Distribution. Reading: BCP Chapter 6
 Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Central Nervous System Regulation of Food Intake
 Central Nervous System Regulation of Food Intake Michael W. Schwartz Introduction Some 50 years ago, Gordon Kennedy introduced the hypothesis that body fat stores are subject to homeostatic regulation
Central Nervous System Regulation of Food Intake Michael W. Schwartz Introduction Some 50 years ago, Gordon Kennedy introduced the hypothesis that body fat stores are subject to homeostatic regulation
Chapter 24 Cholesterol, Energy Balance and Body Temperature. 10/28/13 MDufilho
 Chapter 24 Cholesterol, Energy Balance and Body Temperature 10/28/13 MDufilho 1 Metabolic Role of the Liver Hepatocytes ~500 metabolic functions Process nearly every class of nutrient Play major role in
Chapter 24 Cholesterol, Energy Balance and Body Temperature 10/28/13 MDufilho 1 Metabolic Role of the Liver Hepatocytes ~500 metabolic functions Process nearly every class of nutrient Play major role in
Investigation of the role of nesfatin-1/nucb2 in the central nervous system. Ph.D. thesis Katalin Könczöl
 Investigation of the role of nesfatin-1/nucb2 in the central nervous system Ph.D. thesis Katalin Könczöl Semmelweis University János Szentágothai Doctoral School of Neurosciences Supervisor: Official reviewers:
Investigation of the role of nesfatin-1/nucb2 in the central nervous system Ph.D. thesis Katalin Könczöl Semmelweis University János Szentágothai Doctoral School of Neurosciences Supervisor: Official reviewers:
Chemical Control of Behavior and Brain 1 of 9
 Chemical Control of Behavior and Brain 1 of 9 I) INTRO A) Nervous system discussed so far 1) Specific 2) Fast B) Other systems extended in space and time 1) Nonspecific 2) Slow C) Three components that
Chemical Control of Behavior and Brain 1 of 9 I) INTRO A) Nervous system discussed so far 1) Specific 2) Fast B) Other systems extended in space and time 1) Nonspecific 2) Slow C) Three components that
An important function of the central nervous
 Perspectives in Diabetes Insulin Signaling in the Central Nervous System A Critical Role in Metabolic Homeostasis and Disease From C. elegans to Humans Daniel Porte, Jr., 1,2,3 Denis G. Baskin, 3 and Michael
Perspectives in Diabetes Insulin Signaling in the Central Nervous System A Critical Role in Metabolic Homeostasis and Disease From C. elegans to Humans Daniel Porte, Jr., 1,2,3 Denis G. Baskin, 3 and Michael
Energy flow in the organism
 I. Parameters of energy metabolism, basal metabolic rate, measurements. II. Control of food intake, hunger and satiety Péter Sántha, 12.02. 2017. Energy flow in the organism NUTRIENTS PHYSICAL WORK HEAT
I. Parameters of energy metabolism, basal metabolic rate, measurements. II. Control of food intake, hunger and satiety Péter Sántha, 12.02. 2017. Energy flow in the organism NUTRIENTS PHYSICAL WORK HEAT
Neurobiology of Addiction
 Neurobiology of Addiction Domenic A. Ciraulo, MD Director of Alcohol Pharmacotherapy Research Center for Addiction Medicine Department of Psychiatry Massachusetts General Hospital Disclosure Neither I
Neurobiology of Addiction Domenic A. Ciraulo, MD Director of Alcohol Pharmacotherapy Research Center for Addiction Medicine Department of Psychiatry Massachusetts General Hospital Disclosure Neither I
ENDOCRINOLOGY. Dr.AZZA SAJID ALKINANY 2 nd STAGE
 ENDOCRINOLOGY Dr.AZZA SAJID ALKINANY 2 nd STAGE THE RELATIONSHIP AMONG THE HYPOTHALMUS,POSTERIOR PITUITARY AND TARGET TISSUES. The posterior pituitary does not produce its own hormones, but stores and
ENDOCRINOLOGY Dr.AZZA SAJID ALKINANY 2 nd STAGE THE RELATIONSHIP AMONG THE HYPOTHALMUS,POSTERIOR PITUITARY AND TARGET TISSUES. The posterior pituitary does not produce its own hormones, but stores and
Energy Balance. Applied Human Metabolism VII. Energy Out. Factors that effect BMR/RMR 17/03/2016
 Energy Balance Applied Human Metabolism VII Weight Regulation The balance of energy taken in or leaving the body determines body mass Energy In = Energy Out Weight Maintenance Energy In < Energy Out Weight
Energy Balance Applied Human Metabolism VII Weight Regulation The balance of energy taken in or leaving the body determines body mass Energy In = Energy Out Weight Maintenance Energy In < Energy Out Weight
Homeostasis and Mechanisms of Weight Regulation
 Homeostasis and Mechanisms of Weight Regulation Purpose In this activity students will investigate how negative feedback mechanisms function to maintain homeostatic balance using a recently discovered
Homeostasis and Mechanisms of Weight Regulation Purpose In this activity students will investigate how negative feedback mechanisms function to maintain homeostatic balance using a recently discovered
Understanding obesity by local overexpression of neuropeptides: a viral vector based approach
 Understanding obesity by local overexpression of neuropeptides: a viral vector based approach Cover photo: Chris Timmers Cover design: Marije Tiesjema Print: Gildeprint, Enschede ISBN: 978-90-39344675
Understanding obesity by local overexpression of neuropeptides: a viral vector based approach Cover photo: Chris Timmers Cover design: Marije Tiesjema Print: Gildeprint, Enschede ISBN: 978-90-39344675
Supplementary Figure 1
 Supplementary Figure 1 Arcuate ChIEF-tdTomato neurons expressed TH These micrographs show that TH-Cre-ChIEF-tdTomato (magenta), expressed by AAV in a TH-Cre mouse, were immunostained with TH (green) in
Supplementary Figure 1 Arcuate ChIEF-tdTomato neurons expressed TH These micrographs show that TH-Cre-ChIEF-tdTomato (magenta), expressed by AAV in a TH-Cre mouse, were immunostained with TH (green) in
Hypothalamus. To learn how the brain regulates neuroendocrine secretions NTA Ch 14, pgs Key Figs: 14-3; 14-4,
 Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Ghrelin mediates stressinduced. behavior in mice. Chuang et al 2011 L3: Love, Lust, Labor
 Ghrelin mediates stressinduced food-reward behavior in mice Chuang et al 2011 L3: Love, Lust, Labor Agenda Introduction What is Ghrelin? Previous Models New model Methods Results Discussion Conclusion
Ghrelin mediates stressinduced food-reward behavior in mice Chuang et al 2011 L3: Love, Lust, Labor Agenda Introduction What is Ghrelin? Previous Models New model Methods Results Discussion Conclusion
THE ROLE OF DIETARY FAT IN HYPOTHALAMIC INSULIN AND LEPTIN RESISTANCE AND THE PATHOGENESIS OF OBESITY. Kelly Ann Posey.
 THE ROLE OF DIETARY FAT IN HYPOTHALAMIC INSULIN AND LEPTIN RESISTANCE AND THE PATHOGENESIS OF OBESITY By Kelly Ann Posey Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University
THE ROLE OF DIETARY FAT IN HYPOTHALAMIC INSULIN AND LEPTIN RESISTANCE AND THE PATHOGENESIS OF OBESITY By Kelly Ann Posey Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University
Neuroendocrinology an integrative approach
 Neuroendocrinology an integrative approach JP Advis DVM, Ph.D. Bartlett Hall, Animal ciences, Cook, (848) 932-9240, advis@aesop.rutgers.edu 06 Course website: rci.rutgers.edu/~advis Material to be covered:
Neuroendocrinology an integrative approach JP Advis DVM, Ph.D. Bartlett Hall, Animal ciences, Cook, (848) 932-9240, advis@aesop.rutgers.edu 06 Course website: rci.rutgers.edu/~advis Material to be covered:
BIOL 2458 A&P II CHAPTER 18 SI Both the system and the endocrine system affect all body cells.
 BIOL 2458 A&P II CHAPTER 18 SI 1 1. Both the system and the endocrine system affect all body cells. 2. Affect on target cells by the system is slow. Affect on target cells by the system is fast. INTERCELLULAR
BIOL 2458 A&P II CHAPTER 18 SI 1 1. Both the system and the endocrine system affect all body cells. 2. Affect on target cells by the system is slow. Affect on target cells by the system is fast. INTERCELLULAR
The central melanocortin system affects the hypothalamopituitary thyroid axis and may mediate the effect of leptin
 The central melanocortin system affects the hypothalamopituitary thyroid axis and may mediate the effect of leptin M.S. Kim, C.J. Small, S.A Stanley, D.G.A. Morgan, L.J. Seal, W.M. Kong, C.M.B. Edwards,
The central melanocortin system affects the hypothalamopituitary thyroid axis and may mediate the effect of leptin M.S. Kim, C.J. Small, S.A Stanley, D.G.A. Morgan, L.J. Seal, W.M. Kong, C.M.B. Edwards,
Lesson 14. The Nervous System. Introduction to Life Processes - SCI 102 1
 Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Hormones and Neurons
 Hormones and Neurons Appetite Regulation and Weight Control The Story of Leptin, Ghrelin, and PYY and the Treatment of Obesity Michael A. Bush, M.D. CA-AACE Annual Meeting Symposium May 5, 2017 1 phat
Hormones and Neurons Appetite Regulation and Weight Control The Story of Leptin, Ghrelin, and PYY and the Treatment of Obesity Michael A. Bush, M.D. CA-AACE Annual Meeting Symposium May 5, 2017 1 phat
REGULATION OF FOOD INTAKE AND NUTRITIONAL STATE
 REGULATION OF FOOD INTAKE AND NUTRITIONAL STATE INTAKE OUTPUT CENTER OF SATIETY ncl. ventromedialis in hypothalamus - CENTER OF HUNGER (permanently active) lateral hypothalamus (nucleus under fasciculus
REGULATION OF FOOD INTAKE AND NUTRITIONAL STATE INTAKE OUTPUT CENTER OF SATIETY ncl. ventromedialis in hypothalamus - CENTER OF HUNGER (permanently active) lateral hypothalamus (nucleus under fasciculus
The Emotional Nervous System
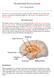 The Emotional Nervous System Dr. C. George Boeree Emotion involves the entire nervous system, of course. But there are two parts of the nervous system that are especially significant: The limbic system
The Emotional Nervous System Dr. C. George Boeree Emotion involves the entire nervous system, of course. But there are two parts of the nervous system that are especially significant: The limbic system
The melanocortin signaling pathway in the medial hypothalamus
 Starvation after AgRP neuron ablation is independent of melanocortin signaling Qi Wu, Maureen P. Howell, Michael A. Cowley, and Richard D. Palmiter Howard Hughes Medical Institute and Department of Biochemistry,
Starvation after AgRP neuron ablation is independent of melanocortin signaling Qi Wu, Maureen P. Howell, Michael A. Cowley, and Richard D. Palmiter Howard Hughes Medical Institute and Department of Biochemistry,
Orexin and Sleep. Team: A Little Bit of Leptin
 Orexin and Sleep Team: A Little Bit of Leptin Intro to Orexin 1997 -Scripps Research Institute gene expression in the hypothalamus Found gene clone 35 - expression limited to the lateral hypothalamus NTs
Orexin and Sleep Team: A Little Bit of Leptin Intro to Orexin 1997 -Scripps Research Institute gene expression in the hypothalamus Found gene clone 35 - expression limited to the lateral hypothalamus NTs
Brain regulation of food intake and appetite: molecules and networks
 Journal of Internal Medicine 2005; 258: 301 327 doi:10.1111/j.1365-2796.2005.01553.x REVIEW Brain regulation of food intake and appetite: molecules and networks C. BROBERGER From the Department of Neuroscience,
Journal of Internal Medicine 2005; 258: 301 327 doi:10.1111/j.1365-2796.2005.01553.x REVIEW Brain regulation of food intake and appetite: molecules and networks C. BROBERGER From the Department of Neuroscience,
Chapter 16: Endocrine System 1
 Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
Low ambient temperature lowers cholecystokinin and leptin plasma concentrations in adult men
 ISPUB.COM The Internet Journal of Gastroenterology Volume 7 Number 2 Low ambient temperature lowers cholecystokinin and leptin plasma concentrations in adult men M Pizon, P Tomasic, K Sztefko, Z Szafran
ISPUB.COM The Internet Journal of Gastroenterology Volume 7 Number 2 Low ambient temperature lowers cholecystokinin and leptin plasma concentrations in adult men M Pizon, P Tomasic, K Sztefko, Z Szafran
Insights from Rare Obesity Disorders
 Insights from Rare Obesity Disorders Ashley Shoemaker, MD, MSCI Ian M. Burr Division of Pediatric Endocrinology and Diabetes Disclosures Research funding: Zafgen, AstraZeneca, Rhythm Member, Zafgen Hypothalamic
Insights from Rare Obesity Disorders Ashley Shoemaker, MD, MSCI Ian M. Burr Division of Pediatric Endocrinology and Diabetes Disclosures Research funding: Zafgen, AstraZeneca, Rhythm Member, Zafgen Hypothalamic
Hypothalamus. lies below the hypothalamic sulcus. includes the following ventral surface structures:
 Hypothalamus I. Overview The Hypothalamus is a division of the diencephalon. lies within the floor and ventral part of the walls of the third ventricle, functions primarily in the maintenance of homeostasis.
Hypothalamus I. Overview The Hypothalamus is a division of the diencephalon. lies within the floor and ventral part of the walls of the third ventricle, functions primarily in the maintenance of homeostasis.
Nature Neuroscience: doi: /nn Supplementary Figure 1. Splenic atrophy and leucopenia caused by T3 SCI.
 Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
CHAPTER 48: NERVOUS SYSTEMS
 CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control
 NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
CHAPTER 13&14: The Central Nervous System. Anatomy of the CNS
 CHAPTER 13&14: The Central Nervous System Anatomy of the CNS in human consists of brain and spinal cord as stated earlier neurons have little support from their extracellular matrix and depend on glial
CHAPTER 13&14: The Central Nervous System Anatomy of the CNS in human consists of brain and spinal cord as stated earlier neurons have little support from their extracellular matrix and depend on glial
THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN. COGS 163 By: Pranav Singh Alexandra Villar
 THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN COGS 163 By: Pranav Singh Alexandra Villar INTRODUCTION Insulin is a hormone produced in the pancreas by the islets of Langerhans that regulates the
THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN COGS 163 By: Pranav Singh Alexandra Villar INTRODUCTION Insulin is a hormone produced in the pancreas by the islets of Langerhans that regulates the
Nature Neuroscience: doi: /nn Supplementary Figure 1. Visualization of AT1a-positive cells using AT1a lacz/+ mouse.
 Supplementary Figure 1 Visualization of AT1a-positive cells using AT1a lacz/+ mouse. (a f) Immunohistochemical detection of β-gal in the mouse brain. Coronal sections at the respective anteroposterior
Supplementary Figure 1 Visualization of AT1a-positive cells using AT1a lacz/+ mouse. (a f) Immunohistochemical detection of β-gal in the mouse brain. Coronal sections at the respective anteroposterior
Chapter 20 Endocrine System
 Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Gastric Artery Embolization for Weight Loss: Rationale
 Gastric Artery Embolization for Weight Loss: Rationale Gary Siskin, MD FSIR Professor and Chairman Department of Radiology Albany Medical Center Albany, New York Gary Siskin, M.D. Consultant/Advisory Board:
Gastric Artery Embolization for Weight Loss: Rationale Gary Siskin, MD FSIR Professor and Chairman Department of Radiology Albany Medical Center Albany, New York Gary Siskin, M.D. Consultant/Advisory Board:
Unit 3: The Biological Bases of Behaviour
 Unit 3: The Biological Bases of Behaviour Section 1: Communication in the Nervous System Section 2: Organization in the Nervous System Section 3: Researching the Brain Section 4: The Brain Section 5: Cerebral
Unit 3: The Biological Bases of Behaviour Section 1: Communication in the Nervous System Section 2: Organization in the Nervous System Section 3: Researching the Brain Section 4: The Brain Section 5: Cerebral
THE CENTRAL NERVOUS SYSTEM. The Brain & Spinal Cord
 THE CENTRAL NERVOUS SYSTEM The Brain & Spinal Cord Review: Nervous System Parallel Distributed Processing Composition of the CNS Nuclei: Clusters of neurons in the CNS ( neighborhoods ) Fiber Tracts/Pathways:
THE CENTRAL NERVOUS SYSTEM The Brain & Spinal Cord Review: Nervous System Parallel Distributed Processing Composition of the CNS Nuclei: Clusters of neurons in the CNS ( neighborhoods ) Fiber Tracts/Pathways:
Nervous System C H A P T E R 2
 Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Autonomic Nervous System and Hypothalamus
 Lu Chen LSA room 201 Phone: (510) 643-8163 Email: luchen@berkeley.edu Office hours: M,W,F, 10-11 am 1 Autonomic Nervous System and Hypothalamus Lu Chen, Ph.D. MCB, UC Berkeley 2 Hypothalamus Brain stem
Lu Chen LSA room 201 Phone: (510) 643-8163 Email: luchen@berkeley.edu Office hours: M,W,F, 10-11 am 1 Autonomic Nervous System and Hypothalamus Lu Chen, Ph.D. MCB, UC Berkeley 2 Hypothalamus Brain stem
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Is ghrelin a signal for the development of metabolic systems?
 Commentaries Is ghrelin a signal for the development of metabolic systems? Kevin L. Grove and Michael A. Cowley Division of Neuroscience, Oregon National Primate Research Center, Oregon Health & Science
Commentaries Is ghrelin a signal for the development of metabolic systems? Kevin L. Grove and Michael A. Cowley Division of Neuroscience, Oregon National Primate Research Center, Oregon Health & Science
Psychology in Your Life
 Sarah Grison Todd Heatherton Michael Gazzaniga Psychology in Your Life SECOND EDITION Chapter 2 The Role of Biology in Psychology 1 2016 W. W. Norton & Company, Inc. 2.1 How Do Our Nervous Systems Affect
Sarah Grison Todd Heatherton Michael Gazzaniga Psychology in Your Life SECOND EDITION Chapter 2 The Role of Biology in Psychology 1 2016 W. W. Norton & Company, Inc. 2.1 How Do Our Nervous Systems Affect
The role of leptin receptor signaling in feeding and neuroendocrine function
 Review TRENDS in Endocrinology and Metabolism Vol.14 No.10 December 2003 447 The role of leptin receptor signaling in feeding and neuroendocrine function Sarah H. Bates and Martin G. Myers Jr Research
Review TRENDS in Endocrinology and Metabolism Vol.14 No.10 December 2003 447 The role of leptin receptor signaling in feeding and neuroendocrine function Sarah H. Bates and Martin G. Myers Jr Research
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Axon Nerve impulse. Axoplasm Receptor. Axomembrane Stimuli. Schwann cell Effector. Myelin Cell body
 Nervous System Review 1. Explain a reflex arc. 2. Know the structure, function and location of a sensory neuron, interneuron, and motor neuron 3. What is (a) Neuron Axon Nerve impulse Axoplasm Receptor
Nervous System Review 1. Explain a reflex arc. 2. Know the structure, function and location of a sensory neuron, interneuron, and motor neuron 3. What is (a) Neuron Axon Nerve impulse Axoplasm Receptor
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane
 Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
Hormones. Prof. Dr. Volker Haucke Institut für Chemie-Biochemie Takustrasse 6
 Hormones Prof. Dr. Volker Haucke Institut für Chemie-Biochemie Takustrasse 6 Tel. 030-8385-6920 (Sekret.) 030-8385-6922 (direkt) e-mail: vhaucke@chemie.fu-berlin.de http://userpage.chemie.fu-berlin.de/biochemie/aghaucke/teaching.html
Hormones Prof. Dr. Volker Haucke Institut für Chemie-Biochemie Takustrasse 6 Tel. 030-8385-6920 (Sekret.) 030-8385-6922 (direkt) e-mail: vhaucke@chemie.fu-berlin.de http://userpage.chemie.fu-berlin.de/biochemie/aghaucke/teaching.html
Chapter 14 The Autonomic Nervous System Chapter Outline
 Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
NEUROSCIENCE. W1 Divisions of the nervous system PSYC1002 NOTES
 PSYC1002 NOTES NEUROSCIENCE W1 Divisions of the nervous system Nervous system: - CNS o Brain and spinal cord - Peripheral Nervous System o Sensory nerves o Motor nerves o Autonomic nervous system o Enteric
PSYC1002 NOTES NEUROSCIENCE W1 Divisions of the nervous system Nervous system: - CNS o Brain and spinal cord - Peripheral Nervous System o Sensory nerves o Motor nerves o Autonomic nervous system o Enteric
MOLECULAR BIOLOGY OF DRUG ADDICTION. Sylvane Desrivières, SGDP Centre
 1 MOLECULAR BIOLOGY OF DRUG ADDICTION Sylvane Desrivières, SGDP Centre Reward 2 Humans, as well as other organisms engage in behaviours that are rewarding The pleasurable feelings provide positive reinforcement
1 MOLECULAR BIOLOGY OF DRUG ADDICTION Sylvane Desrivières, SGDP Centre Reward 2 Humans, as well as other organisms engage in behaviours that are rewarding The pleasurable feelings provide positive reinforcement
Peripubertal, leptin-deficient ob/ob female mice were used in an investigation of
 ESSICK-BROOKSHIRE, ELIZABETH ANN, M.S. The Effects of Peripherally Administered 17-β Estradiol and BIBP3226, a NPY Y1 Receptor Antagonist, on Food Intake, Body Mass, Reproductive Development and Behavior
ESSICK-BROOKSHIRE, ELIZABETH ANN, M.S. The Effects of Peripherally Administered 17-β Estradiol and BIBP3226, a NPY Y1 Receptor Antagonist, on Food Intake, Body Mass, Reproductive Development and Behavior
Hormonal regulation of. Physiology Department Medical School, University of Sumatera Utara
 Hormonal regulation of nutrient metabolism Physiology Department Medical School, University of Sumatera Utara Homeostasis & Controls Successful compensation Homeostasis reestablished Failure to compensate
Hormonal regulation of nutrient metabolism Physiology Department Medical School, University of Sumatera Utara Homeostasis & Controls Successful compensation Homeostasis reestablished Failure to compensate
Brief Critical Reviews
 Brief Critical Reviews March 2003: 101 104 Ghrelin: Update 2003 Ghrelin is a recently described peptide hormone that is secreted by endocrine cells in the gastrointestinal tract. Although its initial discovery
Brief Critical Reviews March 2003: 101 104 Ghrelin: Update 2003 Ghrelin is a recently described peptide hormone that is secreted by endocrine cells in the gastrointestinal tract. Although its initial discovery
Chapter 6 Communication, Integration, and Homeostasis
 Chapter 6 Communication, Integration, and Homeostasis About This Chapter Cell-to-cell communication Signal pathways Novel signal molecules Modulation of signal pathways Homeostatic reflex pathways Cell-to-Cell
Chapter 6 Communication, Integration, and Homeostasis About This Chapter Cell-to-cell communication Signal pathways Novel signal molecules Modulation of signal pathways Homeostatic reflex pathways Cell-to-Cell
9.01 Introduction to Neuroscience Fall 2007
 MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
Chapter 17. Nervous System Nervous systems receive sensory input, interpret it, and send out appropriate commands. !
 Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Nervous and Endocrine System Exam Review
 Directions: Read each question and complete the statement using the multiple choice responses I. Nervous System 1. The interpretation of olfactory receptor information would fall under which general function
Directions: Read each question and complete the statement using the multiple choice responses I. Nervous System 1. The interpretation of olfactory receptor information would fall under which general function
Neurotransmitter Systems III Neurochemistry. Reading: BCP Chapter 6
 Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Human Anatomy. Autonomic Nervous System
 Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
