Aminocoumarin antibiotics (Fig. 1) are very potent inhibitors
|
|
|
- Aldous Summers
- 5 years ago
- Views:
Transcription
1 CloQ, a prenyltransferase involved in clorobiocin biosynthesis Florence Pojer*, Emmanuel Wemakor*, Bernd Kammerer, Huawei Chen, Christopher T. Walsh, Shu-Ming Li*, and Lutz Heide* *Pharmazeutische Biologie, Auf der Morgenstelle 8, and Institut für Pharmakologie, Otfried-Müller-Strasse 10, Universität Tübingen, Tübingen, Germany; and Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA Contributed by Christopher T. Walsh, December 18, 2002 Ring A (3-dimethylallyl-4-hydroxybenzoic acid) is a structural moiety of the aminocoumarin antibiotics novobiocin and clorobiocin. In the present study, the prenyltransferase involved in the biosynthesis of this moiety was identified from the clorobiocin producer (Streptomyces roseochromogenes), overexpressed, and purified. It is a soluble, monomeric 35-kDa protein, encoded by the structural gene cloq. 4-Hydroxyphenylpyruvate and dimethylallyl diphosphate were identified as the substrates of this enzyme, with K m values determined as 25 and 35 M, respectively. A gene inactivation experiment confirmed that cloq is essential for ring A biosynthesis. Database searches did not reveal any similarity of CloQ to known prenyltransferases, and the enzyme did not contain the typical prenyl diphosphate binding site (N D)DXXD. In contrast to most of the known prenyltransferases, the enzymatic activity was not dependent on the presence of magnesium, and in contrast to the membrane-bound polyprenyltransferases involved in ubiquinone biosynthesis, CloQ did not accept 4-hydroxybenzoic acid as substrate. CloQ and the similar NovQ from the novobiocin producer seem to belong to a new class of prenyltransferases. Aminocoumarin antibiotics (Fig. 1) are very potent inhibitors of bacterial DNA gyrase (1). Novobiocin (Albamycin, Pharmacia Upjohn) is licensed as an antibiotic for human therapy. Novobiocin, clorobiocin, and coumermycin A 1 are formed by different Streptomyces strains (2, 3). The 3-prenylated 4-hydroxybenzoic acid (4HB) moiety of novobiocin (called ring A) has been shown to be derived from tyrosine and an isoprenoid precursor (4), but conflicting suggestions have been made for the prenylation substrate (5 8). 3-Prenylated 4HB moieties are known as intermediates in the biosynthesis of ubiquinones (9, 10) and shikonin (11), where they are formed from 4HB under catalysis of membrane-bound prenyltransferases. These enzymes contain the characteristic prenyl diphosphate binding site [(N D)DXXD] known from trans-prenyltransferases (12, 13). Surprisingly, cloning of the novobiocin and the clorobiocin biosynthetic gene clusters (14, 15) revealed neither a gene with sequence similarity to known prenyltransferases nor genes that could be assigned to 4HB biosynthesis, e.g., similar to the benzoate biosynthesis genes recently described in Streptomyces maritimus (16). A hypothesis for the formation of ring A was derived from studies on the biosynthesis of the aminocoumarin moiety (ring B) of novobiocin. Chen and Walsh (7) showed that the first two steps in ring B formation are the activation of L-tyrosine by NovH and the subsequent hydroxylation to -hydroxytyrosyl- NovH ( -OH-Tyr-S-NovH) under catalysis of the cytochrome P 450 enzyme NovI (Fig. 2). Alkali treatment of this product led to the formation of 4-hydroxybenzaldehyde (4HBAL), resulting from a retro-aldol cleavage of -OH-Tyr-S-NovH. It therefore seemed possible that ring A of novobiocin may also be derived from -OH-Tyr-S-NovH by a retro-aldol reaction, occurring either before or after prenylation of the aromatic nucleus (Fig. 2). Further support for this hypothesis came from the detection of the genes novr and clor in the biosynthetic gene clusters of novobiocin and clorobiocin, respectively, as their gene products Fig. 1. Structures of the aminocoumarin antibiotics. show similarity to aldolases, and the involvement of clor in ring A biosynthesis was proven by a gene inactivation experiment in the clorobiocin producer (15). The present study revealed, however, that ring A and ring B are formed by two distinct and independent pathways. CloQ was identified as a soluble aromatic prenyltransferase, which prenylates 4-hydroxyphenylpyruvate (4HPP) in clorobiocin biosynthesis. CloQ was found to be dissimilar to most prenyltransferases described so far and may indicate the existence of a new class of prenyltransferases. Materials and Methods Chemicals and Radiochemicals. [1-14 C]Dimethylallyl diphosphate (DMAPP) and L-[U- 14 C]tyrosine were obtained from Moravek Biochemicals (Brea, CA). -Hydroxy-L-tyrosine and unlabeled DMAPP were kindly provided by K.-H. van Pée (Institut für Biochemie, Dresden, Germany) and K. Yazaki (Wood Research Institute, Kyoto), respectively. 3-dimethylallyl (3DMA)-4HBAL was synthesized as described in ref. 17. Ring A of novobiocin (3-dimethylallyl-4-hydroxybenzoic acid) was prepared as described in ref. 18. Ring B of novobiocin (3-amino-4,7-dihydroxy- 8-methylcoumarin) was kindly provided by Pharmacia Upjohn. Bacterial Strains and Culture Conditions. Streptomyces roseochromogenes var. oscitans DS was kindly provided by Aventis. Wild-type and mutant strains of S. roseochromogenes were cultured as described in ref. 15. Escherichia coli strains used were XL1 Blue MRF (Stratagene) and BL21(DE3) (Novagen). DNA Manipulation. DNA manipulations were carried out as described in refs. 19 and 20. Southern blot analysis was performed Abbreviations: ring A, 3-dimethylallyl-4-hydroxybenzoic acid; ring B of novobiocin, 3- amino-4,7-dihydroxy-8-methylcoumarin; DMAPP, dimethylallyl diphosphate; 4HPP, 4-hydroxyphenylpyruvate; 4HB, 4-hydroxybenzoic acid; 4HBAL, 4-hydroxybenzaldehyde; 3DMA, 3-dimethylallyl; LC, liquid chromatography; ESI; electrospray ionization; CID, collision-induced dissociation mass spectroscopy; DMAT, dimethylallyltryptophan synthase. Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AF (CloQ) and AAF67510 (NovQ)]. To whom correspondence should be addressed. heide@uni-tuebingen.de PNAS March 4, 2003 vol. 100 no. 5 cgi doi pnas
2 BIOCHEMISTRY Fig. 2. Possible biosynthetic pathways to the prenylated 4HB moiety (ring A) of novobiocin and clorobiocin. on Hybond-N membranes (Amersham Pharmacia) with digoxigenin-labeled probes by using the DIG high prime DNA labeling and detection kit II (Roche Applied Science). Construction of In-Frame Deletion Mutants of S. roseochromogenes. For in-frame deletion of cloq in S. roseochromogenes, two fragments cloq-1 (727 bp) and cloq-2 (1,259 bp) were amplified. Primer pairs used were cloq-1 HindIII (5 -GTGCGC- GAAGCTTGGCCCCG-3 ) and cloq-1 PstI(5 -GAAGGCCT- GCAGGACGGT-3 ); and cloq-2 PstI (5 -CACGACCTG- CAGGCACTTCA-3 ) and cloq-2 BamHI (5 -ACCG- GGGGATCCCCTGCAA-3 ). Introduced restriction sites are underlined. The fragments were cloned into the corresponding sites of pbskt (21), containing a thiostrepton-resistance gene, resulting in pfp04. Transformation of S. roseochromogenes with pfp04 and selection for mutants resulting from single- and double-crossover recombination events were carried out as described in ref. 15. Chromosomal DNA from wild type and mutants was digested with BamHI and PvuII and hybridized with a probe containing a part of the clor gene immediately downstream of cloq. A single band of 1.5 kb was detected in the wild type, whereas a single band of 2.9 kb was detected in the cloq mutant, confirming the in-frame deletion of cloq. For the in-frame deletion of cloi in S. roseochromogenes, two fragments, denoted cloi-1 (1,249 bp) and cloi-2 (1,013 bp), were amplified. Primer pairs used were cloi-1 HindIII (5 -CGGCCAAGCTTGCCACGATG-3 ) and cloi-1 PstI (5 - GTACTCGGCCTGCAGAATCGG-3 ); and cloi-2 PstI (5 -GCATCCTGCAGGGGATGAGC-3 ) and cloi-2 XbaI (5 -GCCGGACTTCTAGATCCGTC-3 ). As for pfp04, the two fragments cloi-1 and cloi-2 were cloned into pbskt, resulting in pew02. After transformation, single- and subsequently doublecrossover mutants were obtained. Chromosomal DNA from wild type and mutants was digested with NcoI and hybridized with a 2-kb probe containing the cloi gene and the adjacent upstream and downstream region. A 2-kb band was detected in the wild type, whereas a 0.9-kb band was detected in cloi mutant, confirming the in-frame deletion of cloi. Feeding of the Mutants with Ring A, Ring B, and 3DMA-4HBAL. The cloi and cloq defective mutants were grown in 50 ml of preculture medium (15) supplemented with 1 mg of ring A or 1 mg of ring B of novobiocin. After 48 h, 5 ml of this preculture was inoculated into 50 ml of production medium (15), again supplemented with 1 mg of ring A or ring B. Another 1 mg of ring A or ring B was added after 2 days. After 7 days of cultivation, secondary metabolites were analyzed. The feeding of the cloq defective mutant with 3DMA-4HBAL was done in the same way. HPLC Analysis of Culture Extracts. Bacterial culture was acidified to ph 4 with HCl and extracted twice with an equal volume of ethyl acetate. The solvent was evaporated and the residue was resuspended in 1 ml of methanol. Metabolites were analyzed by HPLC with a Multosphere RP18-5 column (250 4 mm, 5 m) at a flow rate of 1 ml min. For analysis of clorobiocin and novclobiocin C102 production, a linear gradient of methanol (40 100%) in 1% aqueous formic acid was used, and elution was monitored at 340 nm. For the analysis of the accumulation of ring A by the cloi mutant, a linear gradient of methanol (50 80%) in 1% aqueous formic acid was used, and elution was monitored at 254 nm. The extracts were also analyzed by liquid chromatography (LC) using a Multosphere RP18-5 column (250 4 mm, 5 m) at a flow rate of 100 l min, coupled to an electrospray ionization (ESI) mass spectrometer TSQ Quantum (Thermo- Finnigan, San Jose, CA) in negative-ion mode under the addition of 10% ammonia (15 l min). A linear gradient of acetonitrile (30 100%) in 0.1% aqueous formic acid was used. The negative ion-esi collision-induced dissociation (CID) mass spectrum of clorobiocin was as follows: (m z) ([M H] ), 588, 507, 226. The negative ion-esi-cid mass spectrum of ring A was as follows: (m z) 205 ([M H] ), 161, 106. Pojer et al. PNAS March 4, 2003 vol. 100 no
3 Overexpression of Holo-NovH and NovI Proteins. The NovH construct phc10 and the NovI construct phc21 were described in ref. 7, and the phosphopantetheinyltransferase gene (sfp) cloned into psu20 was described in ref. 22. Holo-NovH was obtained by cotransformation of phc10 and sfp psu20 into E. coli BL21(DE3). Cells were grown in LB medium supplemented with 50 g ml kanamycin and 50 g ml chloramphenicol at 25 C toanod 600 of After cooling to 15 C, they were further cultured at this temperature to an OD 600 of before induction with 50 M isopropyl -Dthiogalactoside (IPTG). The cells were then allowed to grow for an additional 15 h at 15 C. Holo-NovH was subsequently purified as described in ref. 7. Expression of phc21 in E. coli BL21(DE3) and purification of NovI were carried out as described in ref. 7. Overexpression and Purification of CloQ Protein. For construction of the plasmid cloq-pgex4t1, cloq was amplified by using the primers cloq-nterm-bamhi (5 -GGAGGAAGTCGGATC- CGCTCTCCCGATAGATC-3 ) and cloq-cterm-xhoi (5 - AAGCCTCTCGAGTCGGGCACCTCCCATGGTC-3 ). Introduced restriction sites are underlined. The product was digested with BamHI and XhoI and ligated into pgex-4t-1 (Amersham Pharmacia) to give cloq-pgex4t1. E. coli BL21(DE3) cells were grown in 100 ml of LB medium supplemented with 50 g ml carbenicillin at 28 C until an OD 600 of 0.6 was reached. Isopropyl -D-thiogalactoside was added to a final concentration of 1 mm. After 5 h, the cells were harvested by centrifugation and broken by using a French press (SLM- Aminco, Urbana, IL). Cell debris was removed by centrifugation (30 min; 20,000 g). Purification of CloQ as a glutathione S-transferase (GST) fusion protein and subsequent cleavage of the GST tag by thrombin treatment were carried out according to the manufacturer s instructions (Amersham Pharmacia) using glutathione Sepharose 4B and the batch method. Protein Analysis. Standard protein techniques were used as described in refs. 23 and 24. The molecular weight of native CloQ was determined by gel filtration on a HiLoad Superdex 200 column that had been equilibrated with 50 mm Tris HCl buffer (ph 8.0) containing 150 mm NaCl. The column was calibrated with dextran blue 2000 (2,000 kda), aldolase (158 kda), albumin (67 kda), ovalbumin (43 kda), and ribonuclease A (13.7 kda) (Amersham Pharmacia). Incubation of Holo-NovH, NovI, and CloQ with L-[U- 14 C]Tyrosine. Holo-NovH was loaded with L-[U- 14 C]tyrosine at 24 C for 1.5 h in a reaction mixture (110 l) that contained 75 mm Tris HCl (ph 7.5), 5 mm MgCl 2, 2.5 M L-[U- 14 C]tyrosine (500 Ci mol; 1Ci 37 GBq), 3 mm ATP, 2 mm tris(2-carboxyethyl)phosphine, and 30 g of NovH. Subsequently, NADPH and spinach ferredoxin (Sigma) were added to a final concentration of 1.5 mm and 5 M, and 0.1 units of ferredoxin reductase (Sigma) and 25 g of NovI were added. Incubation was continued for 2hat 24 C. Then, 5 g of CloQ and 1 mm DMAPP were added, and incubation was continued for 1 h at 30 C before quenching with 500 l of 10% trichloroacetic acid. The precipitated proteins were pelleted by centrifugation, washed twice with 500 l of distilled water, and redissolved in 100 l of 0.1 M KOH. The tethered product was released from the carrier protein by incubating for 5 min at 60 C. The proteins were removed by acidifying the mixture with 5 l of 50% trifluoroacetic acid followed by centrifugation. Product formation was analyzed by HPLC with a Multosphere RP18-5 column (250 4 mm, 5 m) coupled to a radiodetector (Berthold, Bad Wildbad, Germany) at a flow rate of 1 ml min. A linear gradient of acetonitrile (0 100%) in 1% aqueous formic acid was used. A control incubation was carried out with denatured CloQ. Assay for 4HPP Dimethylallyltransferase Activity. For quantitative determination of enzyme activity, the reaction mixture (100 l) contained 75 mm Tris HCl (ph 7.5), 2.5 mm MgCl 2, 0.25 mm 4HPP, 0.5 mm DMAPP, and purified CloQ. After incubation for 10 min at 30 C, the reaction was stopped with 2 l of formic acid. Products were analyzed by HPLC at 285 nm as described above. The same conditions were used for incubation with L-tyrosine, -hydroxytyrosine, prephenic acid, 4HBA, and 4HBAL. For radioactivity assays, 19 M [1-14 C]DMAPP (3.4 GBq mol) was used instead of unlabeled DMAPP. Otherwise, the incubation was carried out and terminated as described above, with an incubation time of 60 min. The reaction mixture was extracted twice with 500 l of ethyl acetate. After evaporation, the residue was dissolved in 100 l of ethanol. For the conversion of 3DMA-4HPP to 3DMA-4HBAL, 50 l of the ethanolic solution was mixed with 50 l of 1 M NaOH and incubated at room temperature for 15 min. The reaction was stopped with 10 l of formic acid. The products were analyzed by HPLC as described above, using a radioactivity detector. Mass Spectroscopic Analysis of the Reaction Product of 4HPP Dimethylallyltransferase. The incubation mixture (500 l) contained 75 mm Tris HCl (ph 7.5), 2.5 mm MgCl 2, 0.1 mm 4HPP, 0.1 mm DMAPP, and 12.5 g of CloQ and was incubated at 30 C for 1 h. The reaction was stopped with 5 l of formic acid, and the mixture was extracted twice with 500 l of ethyl acetate. After evaporation, the residue was dissolved in 60 l of ethanol. A control incubation was carried out with denatured CloQ. Product formation was analyzed by liquid chromatography coupled to an ESI mass spectrometer as described above. The negative ion-esi-cid mass spectrum of 4HPP was as follows: m z 179 ([M H] ), 135, 107. The negative ion-esi-cid mass spectrum of 3DMA-4HPP was as follows: m z 247 ([M H] ), 203, 175. Analysis of Metal Content of CloQ. Zinc was determined by using a Unicam atom absorption spectrometer. Magnesium was determined spectrophotometrically by using the Roche Hitachi 902 clinical chemistry analyzer. Both the zinc and the magnesium contents were 0.1 mol per mol of CloQ. Results Inactivation of cloq. Sequence analysis of the gene clusters of novobiocin and clorobiocin (14, 15) did not reveal any genes with similarity to known prenyltransferases, nor genes containing the typical prenyl diphosphate binding site (N D)DXXD (12). The identification of candidates for prenyltransferase genes was facilitated, however, by a comparison of the biosynthetic gene clusters of novobiocin (14), clorobiocin (15), and coumermycin A 1 (25). The prenylated 4HB moiety (ring A) is present in novobiocin and clorobiocin but not in coumermycin A 1 (Fig. 1). Correspondingly, three genes were found to be present in the novobiocin and the clorobiocin clusters but absent in the coumermycin A 1 cluster: (i) the putative aldolase genes novr and clor, which were proven to be involved in ring A biosynthesis (15); (ii) novf and clof, with sequence similarity to prephenate dehydrogenase; and (iii) novq and cloq, which did not show any similarity to known genes in the database. If the prenyltransferases were contained within the clusters and were dissimilar to prenyltransferases described previously, novq and cloq would be candidate genes for these enzymes. A gene inactivation experiment was therefore carried out with cloq. To avoid polar effects on the genes downstream of cloq, especially on clor, an in-frame deletion of 810 bp within the coding sequence of cloq was created, and the correct genotype of the mutant was confirmed by Southern blotting (see Materials and Methods). HPLC analysis of the culture extracts showed that clorobiocin production was abolished in the cloq mutant (Fig cgi doi pnas Pojer et al.
4 Fig. 3. HPLC analysis of culture extracts of the cloq and cloi mutants. (A) Clorobiocin standard, wild type, cloq mutant, and cloq mutant fed with ring A. Detection was at 340 nm. The identity of clorobiocin ([M H] 695) was confirmed by LC-ESI-CID. Mass spectroscopic fragments are indicated. (B) Clorobiocin standard, wild type, and cloi mutant. Detection was at 340 nm. (C) Ring A standard, wild type, and cloi mutant. Detection was at 254 nm. The identity of ring A ([M H] 205) was confirmed by LC-ESI-CID. Mass spectroscopic fragments are indicated. 3A). However, on addition of ring A to the culture, the production of clorobiocin was restored to one-third of the wild-type level (Fig. 3A). This finding proved that cloq is involved in ring A biosynthesis. 3DMA-4HBAL is a possible intermediate of ring A biosynthesis (Fig. 2). Adding this compound to cloq mutant culture proved to be equally as effective in restoring clorobiocin production as the addition of ring A (data not shown). Expression and Purification of CloQ. CloQ was expressed in E. coli as a soluble GST fusion protein of 61.6 kda. After purification, GST was cleaved from CloQ by thrombin treatment and removed. This procedure resulted in apparently homogenous CloQ protein as judged by SDS PAGE (Fig. 4). The observed molecular mass corresponded to the calculated mass (35.6 kda). A protein yield of 6 mg of pure CloQ per liter of culture was obtained. Investigation of 4HB, 4HBAL, and -Hydroxytyrosyl-S-NovH ( -OH- Tyr-S-NovH) as Substrates of CloQ. 4HBAL can be formed from -OH-Tyr-S-NovH by retro-aldol cleavage (7). Subsequent prenylation and oxidation may lead to the formation of ring A of novobiocin or clorobiocin (Fig. 2). However, when the purified CloQ protein was incubated with 4HBAL, or with the corresponding acid 4HB, in the presence of DMAPP and Mg 2,no prenylated products were observed. Alternatively, prenylation of -OH-Tyr-S-NovH may precede the retro-aldol cleavage reaction in novobiocin and clorobiocin formation, rendering 3- Fig. 4. Purification of CloQ after overexpression as a fusion protein with GST. The SDS 12% polyacrylamide gel was stained with Coomassie brilliant blue. Lane 1, molecular mass standards; lane 2, total protein after isopropyl -Dthiogalactoside induction; lane 3, soluble protein after induction; lane 4, eluate after thrombin treatment. dimethylallyl- -hydroxytyrosine, in its enzyme-bound form, as an intermediate of ring A biosynthesis (Fig. 2). A natural product containing a 3-dimethylallyl- -hydroxytyrosyl residue has been identified previously in a fungus (26). We therefore prepared -OH-Tyr-S-NovH by incubation of L-[U- 14 C]tyrosine with NovH and NovI, as described by Chen and Walsh (7). When the resulting products were precipitated with trichloroacetic acid and cleaved with alkali, [ 14 C]4HBAL was detected by HPLC, representing the retro-aldol reaction product from -OH-Tyr- S-NovH. When CloQ, DMAPP, and Mg 2 were included in the enzyme incubation, however, no prenylated products were observed (Fig. 5A). Therefore, neither -OH-Tyr-S-NovH nor 4HB or 4HBAL was readily identified as substrates for CloQ. Inactivation of cloi: Proof for an Independent Pathway for Ring A Biosynthesis. The cytochrome P 450 enzyme CloI is responsible for the hydroxylation of Tyr-S-CloH to -OH-Tyr-S-CloH (Fig. 2). If the latter compound is indeed an intermediate in ring A biosynthesis, inactivation of cloi should lead to the abolishment of ring A formation. cloi was inactivated by in-frame deletion, and the genotype of the resulting mutant was confirmed by Southern blotting (see Materials and Methods). HPLC analysis of culture extracts of the cloi mutant showed abolishment of the clorobiocin production (Fig. 3B). This result was expected because CloI had been shown to catalyze an essential step in ring B biosynthesis (7). The same cultures were subsequently analyzed for the accumulation of ring A, using a different UV wavelength for detection (Fig. 3C). The wild type did not accumulate detectable amounts of this compound. However, ring A was clearly detected in the cloi mutant ( 3.5 mg liter of culture). This compound was identified mass spectroscopically, using LC-ESI-CID, by comparison to an authentic reference sample. Moreover, addition of ring B of novobiocin to the cloi mutant led to the formation of a clorobiocin analogue, termed novclobiocin C102, in which the chlorine atom of clorobiocin is replaced by a methyl group. This compound was identified mass spectroscopically, using negative LC-ESI-CID (m z 675 [M H], 488, 206). These experiments demonstrated that ring A can still be formed in the absence of CloI. Therefore, -OH-Tyr-S-CloH cannot be an intermediate in the biosynthesis of ring A of clorobiocin (Fig. 2). This finding prompted us to test additional compounds as substrates for CloQ. Identification of 4HPP as Substrate of CloQ. When CloQ was incubated with 4HPP and [1-14 C]DMAPP, the formation of a prenylation product was readily detected (Fig. 5B). This product was absent from control incubations with heat-denatured CloQ. BIOCHEMISTRY Pojer et al. PNAS March 4, 2003 vol. 100 no
5 Product formation showed a linear dependence on the amount of protein (up to 3 g per assay) and on the reaction time (up to 15 min). The reaction was strictly dependent on the presence of active CloQ, 4HPP, and DMAPP. The presence of Mg 2 enhanced prenyltransferase activity, with 2.5 mm being the most effective concentration. However, in the absence of divalent cations and in the presence of 5 mm EDTA, the enzyme retained 25% of its original activity. This finding is in contrast to the absolute requirement for divalent cations reported for most prenyltransferases (13). The addition of Ca 2 (2.5 mm) instead of Mg 2 resulted in 70% of the activity obtained with Mg 2.In contrast to many protein prenyltransferases that contain a tightly bound zinc atom (28), purified CloQ protein was found to contain neither zinc nor magnesium (see Materials and Methods). CloQ was found to be specific for the substrate 4HPP. No product formation was observed when L-tyrosine, 4HB, 4HBAL, or prephenic acid was used. Only with -hydroxy-l-tyrosine was formation of dimethylallyl- -hydroxytyrosine observed. The identity of the product was confirmed by LC-ESI-CID (m z: 264 [M H], 189, 134). The reaction velocity, however, was only 2% of that obtained with 4HPP. When DMAPP was replaced with isopentenyl diphosphate or geranyl diphosphate, no product formation was observed. The CloQ reaction apparently followed Michaelis Menten kinetics, and the K m values were determined as 25 M for 4HPP and 35 M for DMAPP. The maximum reaction velocity observed was 3,690 pkat mg 1, corresponding to a turnover number of 7.9 min 1. Fig. 5. Radioactive prenyltransferase assays with CloQ. The three incubation mixtures were analyzed by HPLC with radioactivity detection. The retention times of tyrosine, -OH-tyrosine, 4HBAL, 3DMA-4HBAL, and ring A were determined by using authentic reference samples. The incubation was repeated with nonradioactive substrates, and the product was analyzed mass spectroscopically by using LC- ESI-CID, resulting in the following ions: m z 247 ([M 1] ), 203 ([M 44] ), and 175 ([M 72] ). This is identical to the mass spectrum of a previously isolated sample of this compound, which had been identified by both mass spectroscopy and 1 H NMR (8). 4HPP is an unstable compound and decomposes to 4HBAL, especially in the presence of alkali (27). When the enzymatic prenylation products were treated with 0.5 M NaOH, a nearly complete conversion of 3DMA-4HPP to 3DMA-4HBAL was observed (Fig. 5C). The latter compound was identified in comparison with an authentic reference sample, synthesized according to ref. 17. Biochemical Properties and Kinetic Parameters of 4HPP Dimethylallyltransferase. The native molecular mass of CloQ was determined as 34.6 kda by using gel chromatography, showing that the protein was monomeric in solution. Moreover, the enzyme was soluble in the absence of detergents. Discussion In this study, cloq was identified as the structural gene for the prenyltransferase involved in the biosynthesis of the 3DMA-4HB moiety of clorobiocin (Fig. 1). 4HPP and DMAPP were identified as the substrates of CloQ. Inactivation of cloq led to abolishment of clorobiocin formation, which could be restored by addition of ring A. This observation showed that the reaction catalyzed by CloQ is an essential step of the biosynthesis of ring A of clorobiocin. Novobiocin, just as clorobiocin, contains a 3DMA-4HB moiety, and the novobiocin biosynthetic gene cluster contains the gene novq. The predicted gene product NovQ (323 aa) shows 84% identity on the amino acid level to CloQ (324 aa) (14, 15). Therefore, NovQ is likely to catalyze the prenyltransferase reaction in novobiocin biosynthesis. Surprisingly, database searches did not reveal any similarities between NovQ or CloQ and known prenyltransferases. The only match found for CloQ in a BLAST search was a hypothetical protein of Streptomyces coelicolor (E ). At present, very few sequences are available for aromatic prenyltransferases, i.e., enzymes catalyzing the formation of a carbon carbon bond between a prenyl group and an aromatic nucleus. Among the few aromatic prenyltransferases that have been cloned are those involved in the biosynthesis of ubiquinones (10), menaquinones (29), tocopherols (30), plastoquinones (31), and the prenyltransferase involved in formation of the plant secondary metabolite shikonin (11). All of these enzymes are integral membrane proteins, and their active centers include the prenyl diphosphate binding site (N D)DXXD similar to that of the trans-prenyltransferases (12, 13). In contrast, CloQ and NovQ do not show this motif and represent soluble enzymes, without membrane-spanning domains. The only other soluble aromatic prenyltransferase cloned so far is dimethylallyltryptophan synthase (DMAT), which is involved in ergot alkaloid biosynthesis in the fungus Claviceps (32 36). DMAT was found to be active in a metal-free buffer containing EDTA, in contrast to all previously known prenyltransferases (35, 36). It has been suggested that the reaction catalyzed by DMAT may not proceed via a carbonium ion (35), cgi doi pnas Pojer et al.
6 unlike the reaction of farnesyl-diphosphate synthase (37). In common with DMAT, CloQ was also active in the presence of EDTA, i.e., the reaction did not show an absolute requirement for divalent cations. Moreover, the K m values, the specific activity, and the turnover number determined for CloQ are similar to those reported for DMAT (34 36). However, the bacterial enzyme CloQ (35 kda) shows only low sequence similarity (20% identity, 33% similarity) to the 50-kDa protein deduced from the sequence of cpd1, suggested as the structural gene for the fungal enzyme DMAT (33). Nevertheless, CloQ, NovQ, and DMAT may belong to a previously unrecognized class of prenyltransferases that exist as soluble enzymes, do not contain the prenyl diphosphate binding motif (N D)DXXD, and are able to form carbon carbon bonds between isoprenoid and aromatic substrates. We thank Aventis for the generous gift of the S. roseochromogenes DS strain and for authentic clorobiocin. We are grateful to T. Luft for the synthesis of 3DMA-4HBAL; to S. Hildenbrand and R. Wahl, Tübingen University Hospital, for determinations of zinc and magnesium; and to D. Lawson (John Innes Foundation, Norwich, U.K.) for help in the preparation of the manuscript. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (to L.H. and S.-M.L.) and by the Fonds der Chemischen Industrie. 1. Gormley, N. A., Orphanides, G., Meyer, A., Cullis, P. M. & Maxwell, A. (1996) Biochemistry 35, Berger, J. & Batcho, A. D. (1978) J. Chromatogr. Lib. 15, Lanoot, B., Vancanneyt, M., Cleenwerck, I., Wang, L., Li, W., Liu, Z. & Swings, J. (2002) Int. J. Syst. Evol. Microbiol. 52, Li, S.-M., Hennig, S. & Heide, L. (1998) Tetrahedron Lett. 39, Kominek, L. A. (1972) Antimicrob. Agents Chemother. 1, Calvert, R. T., Spring, M. S. & Stoker, J. R. (1972) J. Pharm. Pharmacol. 24, Chen, H. & Walsh, C. T. (2001) Chem. Biol. 8, Steffensky, M., Li, S.-M., Vogler, B. & Heide, L. (1998) FEMS Microbiol. Lett. 161, Melzer, M. & Heide, L. (1994) Biochim. Biophys. Acta 1212, Meganathan, R. (2001) FEMS Microbiol. Lett. 203, Yazaki, K., Kunihisa, M., Fujisaki, T. & Sato, F. (2002) J. Biol. Chem. 277, Koyama, T., Tajima, M., Sano, H., Doi, T., Koike-Takeshita, A., Obata, S., Nishino, T. & Ogura, K. (1996) Biochemistry 35, Liang, P. H., Ko, T. P. & Wang, A. H. (2002) Eur. J. Biochem. 269, Steffensky, M., Mühlenweg, A., Wang, Z. X., Li, S.-M. & Heide, L. (2000) Antimicrob. Agents Chemother. 44, Pojer, F., Li, S.-M. & Heide, L. (2002) Microbiology 148, Hertweck, C. & Moore, B. S. (2000) Tetrahedron 56, Gluesenkamp, K. H. & Buechi, G. (1986) J. Org. Chem. 51, Kominek, L. A. & Meyer, H. F. (1975) Methods Enzymol. 43, Sambrook, J. & Russel, D. W. (2001) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), 3rd Ed. 20. Kieser, T., Bibb, M. J., Buttner, M. J., Chater, K. F. & Hopwood, D. A. (2000) Practical Streptomyces Genetics (John Innes Foundation, Norwich, U.K.). 21. Lombo, F., Siems, K., Brana, A. F., Mendez, C., Bindseil, K. & Salas, J. A. (1997) J. Bacteriol. 179, Bartolome, B., Jubete, Y., Martinez, E. & Cruz, F. (1991) Gene 102, Bradford, M. M. (1976) Anal. Biochem. 72, Laemmli, U. K. (1970) Nature 227, Wang, Z. X., Li, S.-M. & Heide, L. (2000) Antimicrob. Agents Chemother. 44, Barrow, C. J., Doleman, M. S., Bobko, M. A. & Cooper, R. (1994) J. Med. Chem. 37, Doy, C. H. (1960) Nature 186, Harris, C. M., Derdowski, A. M. & Poulter, C. D. (2002) Biochemistry 41, Suvarna, K., Stevenson, D., Meganathan, R. & Hudspeth, M. E. (1998) J. Bacteriol. 180, Schledz, M., Seidler, A., Beyer, P. & Neuhaus, G. (2001) FEBS Lett. 499, Collakova, E. & DellaPenna, D. (2001) Plant Physiol. 127, Tsai, H. F., Wang, H., Gebler, J. C., Poulter, C. D. & Schardl, C. L. (1995) Biochem. Biophys. Res. Commun. 216, Tudzynski, P., Holter, K., Correia, T., Arntz, C., Grammel, N. & Keller, U. (1999) Mol. Gen. Genet. 261, Lee, S. L., Floss, H. G. & Heinstein, P. (1976) Arch. Biochem. Biophys. 177, Cress, W. A., Chayet, L. T. & Rilling, H. C. (1981) J. Biol. Chem. 256, Gebler, J. C. & Poulter, C. D. (1992) Arch. Biochem. Biophys. 296, Poulter, C. D. & Rilling, H. C. (1976) Biochemistry 15, BIOCHEMISTRY Pojer et al. PNAS March 4, 2003 vol. 100 no
JBC Papers in Press. Published on May 30, 2003 as Manuscript M
 JBC Papers in Press. Published on May 30, 2003 as Manuscript M303190200 CloR, a bifunctional non-heme iron oxygenase involved in clorobiocin biosynthesis. Florence Pojer1, Rainer Kahlich2, Bernd Kammerer2,
JBC Papers in Press. Published on May 30, 2003 as Manuscript M303190200 CloR, a bifunctional non-heme iron oxygenase involved in clorobiocin biosynthesis. Florence Pojer1, Rainer Kahlich2, Bernd Kammerer2,
A soluble, magnesium-independent prenyltransferase catalyzes reverse and regular C-prenylations and O-prenylations of aromatic substrates
 A soluble, magnesium-independent prenyltransferase catalyzes reverse and regular C-prenylations and O-prenylations of aromatic substrates Yvonne Haagen a, Inge Unsöld a, Lucia Westrich a, Bertolt Gust
A soluble, magnesium-independent prenyltransferase catalyzes reverse and regular C-prenylations and O-prenylations of aromatic substrates Yvonne Haagen a, Inge Unsöld a, Lucia Westrich a, Bertolt Gust
Stabilization and enhanced reactivity of actinorhodin polyketide synthase minimal complex in polymer/nucleotide coacervate droplets
 Stabilization and enhanced reactivity of actinorhodin polyketide synthase minimal complex in polymer/nucleotide coacervate droplets John Crosby,* Tom Treadwell, Michelle Hammerton, Konstantinos Vasilakis,
Stabilization and enhanced reactivity of actinorhodin polyketide synthase minimal complex in polymer/nucleotide coacervate droplets John Crosby,* Tom Treadwell, Michelle Hammerton, Konstantinos Vasilakis,
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
Supporting Information for:
 Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Supporting information (protein purification, kinetic characterization, product isolation, and characterization by NMR and mass spectrometry):
 Supporting Information Mechanistic studies of a novel C-S lyase in ergothioneine biosynthesis: the involvement of a sulfenic acid intermediate Heng Song, 1 Wen Hu, 1,2 Nathchar Naowarojna, 1 Ampon Sae
Supporting Information Mechanistic studies of a novel C-S lyase in ergothioneine biosynthesis: the involvement of a sulfenic acid intermediate Heng Song, 1 Wen Hu, 1,2 Nathchar Naowarojna, 1 Ampon Sae
Manja Henze, Dorothee Merker and Lothar Elling. 1. Characteristics of the Recombinant β-glycosidase from Pyrococcus
 S1 of S17 Supplementary Materials: Microwave-Assisted Synthesis of Glycoconjugates by Transgalactosylation with Recombinant Thermostable β-glycosidase from Pyrococcus Manja Henze, Dorothee Merker and Lothar
S1 of S17 Supplementary Materials: Microwave-Assisted Synthesis of Glycoconjugates by Transgalactosylation with Recombinant Thermostable β-glycosidase from Pyrococcus Manja Henze, Dorothee Merker and Lothar
Luminescent platforms for monitoring changes in the solubility of amylin and huntingtin in living cells
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard. Product Number: AD0014
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
Organic and biochemical synthesis of monolignol biosynthetic pathway intermediates
 Jie Liu 2012-2-8 Organic and biochemical synthesis of monolignol biosynthetic pathway intermediates 1. Organic synthesis of 5-hydroxyferulic acid Malonic acid 3, 4-Dihydroxy-5-methoxy-benzaldehyde 0.1
Jie Liu 2012-2-8 Organic and biochemical synthesis of monolignol biosynthetic pathway intermediates 1. Organic synthesis of 5-hydroxyferulic acid Malonic acid 3, 4-Dihydroxy-5-methoxy-benzaldehyde 0.1
Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard. Product Number: AD0013
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard Product Number: AD0013 INTRODUCTION: Fluorescent isothiocyanato-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard Product Number: AD0013 INTRODUCTION: Fluorescent isothiocyanato-activated
Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin
 SUPPORTING INFORMATION Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin Anna R. Arnold, Andy Zhou, and Jacqueline K. Barton Division of Chemistry and Chemical Engineering, California
SUPPORTING INFORMATION Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin Anna R. Arnold, Andy Zhou, and Jacqueline K. Barton Division of Chemistry and Chemical Engineering, California
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2008
 Experimental Details Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Chemical Company and were used as received. 2-DOS and neamine were kindly provided by Dr. F. Huang. Paromamine
Experimental Details Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Chemical Company and were used as received. 2-DOS and neamine were kindly provided by Dr. F. Huang. Paromamine
Affinity Purification of Photosystem I from Chlamydomonas reinhardtii using a Polyhistidine Tag
 Affinity Purification of Photosystem I from Chlamydomonas reinhardtii using a Polyhistidine Tag Jonathan A. Brain Galina Gulis, Ph.D. 1 Kevin E. Redding, Ph.D. 2 Associate Professor of Chemistry Adjunct
Affinity Purification of Photosystem I from Chlamydomonas reinhardtii using a Polyhistidine Tag Jonathan A. Brain Galina Gulis, Ph.D. 1 Kevin E. Redding, Ph.D. 2 Associate Professor of Chemistry Adjunct
Supplementary Information
 Supplementary Information Structural basis of improved second generation 3-nitro-tyrosine trna synthetases Richard B. Cooley, Jessica L. Feldman, Camden M. Driggers, Taylor Bundy, Audrey L. Stokes, P.
Supplementary Information Structural basis of improved second generation 3-nitro-tyrosine trna synthetases Richard B. Cooley, Jessica L. Feldman, Camden M. Driggers, Taylor Bundy, Audrey L. Stokes, P.
SUPPLEMENTARY DATA. Materials and Methods
 SUPPLEMENTARY DATA Materials and Methods HPLC-UV of phospholipid classes and HETE isomer determination. Fractionation of platelet lipid classes was undertaken on a Spherisorb S5W 150 x 4.6 mm column (Waters
SUPPLEMENTARY DATA Materials and Methods HPLC-UV of phospholipid classes and HETE isomer determination. Fractionation of platelet lipid classes was undertaken on a Spherisorb S5W 150 x 4.6 mm column (Waters
Tivadar Orban, Beata Jastrzebska, Sayan Gupta, Benlian Wang, Masaru Miyagi, Mark R. Chance, and Krzysztof Palczewski
 Structure, Volume Supplemental Information Conformational Dynamics of Activation for the Pentameric Complex of Dimeric G Protein-Coupled Receptor and Heterotrimeric G Protein Tivadar Orban, Beata Jastrzebska,
Structure, Volume Supplemental Information Conformational Dynamics of Activation for the Pentameric Complex of Dimeric G Protein-Coupled Receptor and Heterotrimeric G Protein Tivadar Orban, Beata Jastrzebska,
LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade
 AD0017P-4 (en) 1 LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade INTRODUCTION Fluorescent isothiocyanato-activated (ITC-activated) Eu-W1024 chelate is optimized for labelling proteins
AD0017P-4 (en) 1 LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade INTRODUCTION Fluorescent isothiocyanato-activated (ITC-activated) Eu-W1024 chelate is optimized for labelling proteins
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Fig.S1 ESI-MS spectrum of reaction of ApA and THPTb after 16 h.
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Experiment Cleavage of dinucleotides Dinucleotides (ApA, CpC, GpG, UpU) were purchased from
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Experiment Cleavage of dinucleotides Dinucleotides (ApA, CpC, GpG, UpU) were purchased from
A biocatalytic hydrogenation of carboxylic acids
 Electronic Supplementary Information (ESI) for: A biocatalytic hydrogenation of carboxylic acids Yan Ni, Peter-Leon Hagedoorn,* Jian-He Xu, Isabel Arends, Frank Hollmann* 1. General Chemicals All the carboxylic
Electronic Supplementary Information (ESI) for: A biocatalytic hydrogenation of carboxylic acids Yan Ni, Peter-Leon Hagedoorn,* Jian-He Xu, Isabel Arends, Frank Hollmann* 1. General Chemicals All the carboxylic
Purification of Glucagon3 Interleukin-2 Fusion Protein Derived from E. coli
 Purification of Glucagon3 Interleukin-2 Fusion Protein Derived from E. coli Hye Soon Won Dept. of Chem. Eng. Chungnam National University INTRODUCTION Human interleukin-2(hil-2) - known as T Cell Growth
Purification of Glucagon3 Interleukin-2 Fusion Protein Derived from E. coli Hye Soon Won Dept. of Chem. Eng. Chungnam National University INTRODUCTION Human interleukin-2(hil-2) - known as T Cell Growth
Heparin Sodium ヘパリンナトリウム
 Heparin Sodium ヘパリンナトリウム Add the following next to Description: Identification Dissolve 1 mg each of Heparin Sodium and Heparin Sodium Reference Standard for physicochemical test in 1 ml of water, and
Heparin Sodium ヘパリンナトリウム Add the following next to Description: Identification Dissolve 1 mg each of Heparin Sodium and Heparin Sodium Reference Standard for physicochemical test in 1 ml of water, and
Prerequisites Protein purification techniques and protein analytical methods. Basic enzyme kinetics.
 Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
End of the Note Book
 End of the Note Book 16 September 2016 Colonies PCR Mix (25 µl total volume reaction): + clones - 12.5 µl DreamTaq PCR Mastermix 2X (ThermoScientific) - 2.5 µl 10X DreamTaq Green Buffer (ThermoScientific)
End of the Note Book 16 September 2016 Colonies PCR Mix (25 µl total volume reaction): + clones - 12.5 µl DreamTaq PCR Mastermix 2X (ThermoScientific) - 2.5 µl 10X DreamTaq Green Buffer (ThermoScientific)
VOL. 55 NO. 10, OCT THE JOURNAL OF ANTIBIOTICS pp
 VOL. 55 NO. 10, OCT. 2002 THE JOURNAL OF ANTIBIOTICS pp. 919-923 Detection of the Mevalonate Pathway in Streptomyces Species Using the 3-Hydroxy-3- methylglutaryl Coenzyme A Reductase Gene those with only
VOL. 55 NO. 10, OCT. 2002 THE JOURNAL OF ANTIBIOTICS pp. 919-923 Detection of the Mevalonate Pathway in Streptomyces Species Using the 3-Hydroxy-3- methylglutaryl Coenzyme A Reductase Gene those with only
Case 19 Purification of Rat Kidney Sphingosine Kinase
 Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
DELFIA Eu-DTPA ITC Chelate & Europium Standard
 AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
DELFIA Tb-DTPA ITC Chelate & Terbium Standard
 AD0035P-2 (en) 1 DELFIA Tb-DTPA ITC Chelate & AD0029 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-DTPA ITC Chelate is optimized for the terbium labelling of proteins and peptides for use
AD0035P-2 (en) 1 DELFIA Tb-DTPA ITC Chelate & AD0029 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-DTPA ITC Chelate is optimized for the terbium labelling of proteins and peptides for use
Key Words: Brassica oleraceae, glucosinolate, liquid chromatography mass spectrometry, FNH-I-003
 IDENTIFICATION OF MAJOR GLUCOSINOLATES IN BROCCOLI (Brassica oleracea var. italica) BY LIQUID CHROMATOGRAPHY MASS SPECTROMETRY (LC-MS) AND DETERMINATION OF ANTICANCER PROPERTIES OF BROCCOLI EXTRACTS Carlos
IDENTIFICATION OF MAJOR GLUCOSINOLATES IN BROCCOLI (Brassica oleracea var. italica) BY LIQUID CHROMATOGRAPHY MASS SPECTROMETRY (LC-MS) AND DETERMINATION OF ANTICANCER PROPERTIES OF BROCCOLI EXTRACTS Carlos
Student Number: To form the polar phase when adsorption chromatography was used.
 Name: Student Number: April 14, 2001, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Lab Section Final Examination Examiner: Dr. A. Scoot 1. Answer ALL questions in the space provided.. 2. The last page
Name: Student Number: April 14, 2001, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Lab Section Final Examination Examiner: Dr. A. Scoot 1. Answer ALL questions in the space provided.. 2. The last page
BIOL 158: BIOLOGICAL CHEMISTRY II
 BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 5: Vitamins and Coenzymes Lecturer: Christopher Larbie, PhD Introduction Cofactors bind to the active site and assist in the reaction mechanism Apoenzyme is an
BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 5: Vitamins and Coenzymes Lecturer: Christopher Larbie, PhD Introduction Cofactors bind to the active site and assist in the reaction mechanism Apoenzyme is an
DELFIA Tb-N1 DTA Chelate & Terbium Standard
 AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
Supplementary Figure 1. The proposed biosynthetic pathways for the A-ring transformation of the
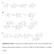 Supplementary Figure 1. The proposed biosynthetic pathways for the A-ring transformation of the kinamycin and lomaiviticin antibiotics. A, by Steven J. Gould; B, by Emily P. Balskus; C, by Bradley S. Moore.
Supplementary Figure 1. The proposed biosynthetic pathways for the A-ring transformation of the kinamycin and lomaiviticin antibiotics. A, by Steven J. Gould; B, by Emily P. Balskus; C, by Bradley S. Moore.
Identification & Confirmation of Structurally Related Degradation Products of Simvastatin
 Identification & Confirmation of Structurally Related Degradation Products of Simvastatin Power of QTRAP Systems for Identification and Confirmation of Degradation Products Dilip Reddy 1, Chandra Sekar
Identification & Confirmation of Structurally Related Degradation Products of Simvastatin Power of QTRAP Systems for Identification and Confirmation of Degradation Products Dilip Reddy 1, Chandra Sekar
Multiplex Protein Quantitation using itraq Reagents in a Gel-Based Workflow
 Multiplex Protein Quantitation using itraq Reagents in a Gel-Based Workflow Purpose Described herein is a workflow that combines the isobaric tagging reagents, itraq Reagents, with the separation power
Multiplex Protein Quantitation using itraq Reagents in a Gel-Based Workflow Purpose Described herein is a workflow that combines the isobaric tagging reagents, itraq Reagents, with the separation power
Electronic Supporting Information for. Mycobacterium tuberculosis H37Rv 3377c encodes the diterpene cyclase for producing the halimane skeleton
 Electronic Supporting Information for Mycobacterium tuberculosis 37Rv 3377c encodes the diterpene cyclase for producing the halimane skeleton Chiaki Nakano, a Tomoo Okamura, a Tsutomu Sato, a Tohru Dairi
Electronic Supporting Information for Mycobacterium tuberculosis 37Rv 3377c encodes the diterpene cyclase for producing the halimane skeleton Chiaki Nakano, a Tomoo Okamura, a Tsutomu Sato, a Tohru Dairi
Subsystem: Ubiquinone Biosynthesis Olga Zagnitko, Fellowship for Interpretation of Genomes
 Olga Zagnitko, Fellowship for Interpretation of Genomes Introduction Quinones are widely distributed in nature. They are best known as lipid-soluble components of membrane-bound electrontransport chains,
Olga Zagnitko, Fellowship for Interpretation of Genomes Introduction Quinones are widely distributed in nature. They are best known as lipid-soluble components of membrane-bound electrontransport chains,
Application Note Soy for Isoflavones by HPLC. Botanical Name: Glycine max L. Common Names: Parts of Plant Used: Beans.
 Application Note 0066 - Soy for Isoflavones by HPLC As published in The Handbook of Analytical Methods for Dietary Supplements Botanical Name: Glycine max L. Common Names: Soybean Parts of Plant Used:
Application Note 0066 - Soy for Isoflavones by HPLC As published in The Handbook of Analytical Methods for Dietary Supplements Botanical Name: Glycine max L. Common Names: Soybean Parts of Plant Used:
2010 Annual report. Principle Investigator: Dr. John W. Frost. Draths Corporation. ONR Award Number: N
 2010 Annual report Principle Investigator: Dr. John W. Frost Draths Corporation ONR Award Number: N000140710076 Award Title: Green Synthesis of Phloroglucinol: Exploiting Pseudomonas fluorescens and Scale-
2010 Annual report Principle Investigator: Dr. John W. Frost Draths Corporation ONR Award Number: N000140710076 Award Title: Green Synthesis of Phloroglucinol: Exploiting Pseudomonas fluorescens and Scale-
Recombinant Trypsin, Animal Origin Free
 Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Analytical Method for 2, 4, 5-T (Targeted to Agricultural, Animal and Fishery Products)
 Analytical Method for 2, 4, 5-T (Targeted to Agricultural, Animal and Fishery Products) The target compound to be determined is 2, 4, 5-T. 1. Instrument Liquid Chromatograph-tandem mass spectrometer (LC-MS/MS)
Analytical Method for 2, 4, 5-T (Targeted to Agricultural, Animal and Fishery Products) The target compound to be determined is 2, 4, 5-T. 1. Instrument Liquid Chromatograph-tandem mass spectrometer (LC-MS/MS)
Structural insights into the potential of 4-fluoroproline to modulate biophysical properties of proteins
 SUPPLEMENTARY INFORMATION Structural insights into the potential of 4-fluoroproline to modulate biophysical properties of proteins Bastian Holzberger, Samra Obeid, Wolfram Welte, Kay Diederichs, and Andreas
SUPPLEMENTARY INFORMATION Structural insights into the potential of 4-fluoroproline to modulate biophysical properties of proteins Bastian Holzberger, Samra Obeid, Wolfram Welte, Kay Diederichs, and Andreas
BIL 256 Cell and Molecular Biology Lab Spring, Tissue-Specific Isoenzymes
 BIL 256 Cell and Molecular Biology Lab Spring, 2007 Background Information Tissue-Specific Isoenzymes A. BIOCHEMISTRY The basic pattern of glucose oxidation is outlined in Figure 3-1. Glucose is split
BIL 256 Cell and Molecular Biology Lab Spring, 2007 Background Information Tissue-Specific Isoenzymes A. BIOCHEMISTRY The basic pattern of glucose oxidation is outlined in Figure 3-1. Glucose is split
FEBS 1138 January Paul R. Buckland and Bernard Rees Smith
 Volume 166, number 1 FEBS 1138 January 1984 A structural comparison receptors by of guinea pig thyroid and fat TSH photoaffinity labelling Paul R. Buckland and Bernard Rees Smith Endocrine Immunology Unit,
Volume 166, number 1 FEBS 1138 January 1984 A structural comparison receptors by of guinea pig thyroid and fat TSH photoaffinity labelling Paul R. Buckland and Bernard Rees Smith Endocrine Immunology Unit,
GtfA and GtfB are both required for protein O-glycosylation in Lactobacillus plantarum
 Supplemental information for: GtfA and GtfB are both required for protein O-glycosylation in Lactobacillus plantarum I-Chiao Lee, Iris I. van Swam, Satoru Tomita, Pierre Morsomme, Thomas Rolain, Pascal
Supplemental information for: GtfA and GtfB are both required for protein O-glycosylation in Lactobacillus plantarum I-Chiao Lee, Iris I. van Swam, Satoru Tomita, Pierre Morsomme, Thomas Rolain, Pascal
VaTx1 VaTx2 VaTx3. VaTx min Retention Time (min) Retention Time (min)
 a Absorbance (mau) 5 2 5 3 4 5 6 7 8 9 6 2 3 4 5 6 VaTx2 High Ca 2+ Low Ca 2+ b 38.2 min Absorbance (mau) 3 2 3 4 5 3 2 VaTx2 39.3 min 3 4 5 3 2 4. min 3 4 5 Supplementary Figure. Toxin Purification For
a Absorbance (mau) 5 2 5 3 4 5 6 7 8 9 6 2 3 4 5 6 VaTx2 High Ca 2+ Low Ca 2+ b 38.2 min Absorbance (mau) 3 2 3 4 5 3 2 VaTx2 39.3 min 3 4 5 3 2 4. min 3 4 5 Supplementary Figure. Toxin Purification For
Supplementary Material
 Supplementary Material Antimicrobial mechanism of theaflavins: They target 1-deoxy-D-xylulose 5-phosphate reductoisomerase, the key enzyme of the MEP terpenoid biosynthetic pathway Xian Hui, Qiao Yue,
Supplementary Material Antimicrobial mechanism of theaflavins: They target 1-deoxy-D-xylulose 5-phosphate reductoisomerase, the key enzyme of the MEP terpenoid biosynthetic pathway Xian Hui, Qiao Yue,
Chapter 3. Structure of Enzymes. Enzyme Engineering
 Chapter 3. Structure of Enzymes Enzyme Engineering 3.1 Introduction With purified protein, Determining M r of the protein Determining composition of amino acids and the primary structure Determining the
Chapter 3. Structure of Enzymes Enzyme Engineering 3.1 Introduction With purified protein, Determining M r of the protein Determining composition of amino acids and the primary structure Determining the
Unit 1, Section C.1. In which you will learn about: Solutions Electrolytes Saturation Solubility curves
 Unit 1, Section C.1 In which you will learn about: Solutions Electrolytes Saturation Solubility curves Some Definitions A solution is a homogeneous mixture of 2 or more substances in a single phase. One
Unit 1, Section C.1 In which you will learn about: Solutions Electrolytes Saturation Solubility curves Some Definitions A solution is a homogeneous mixture of 2 or more substances in a single phase. One
Identification of phanerosporic acid in birch degraded by Phanerochaete chrysosporium
 IRG/WP 03-10496 THE INTERNATIONAL RESEARCH GROUP ON WOOD PRESERVATION Section 1 Biology Identification of phanerosporic acid in birch degraded by Phanerochaete chrysosporium Michael D. Mozuch and Philip
IRG/WP 03-10496 THE INTERNATIONAL RESEARCH GROUP ON WOOD PRESERVATION Section 1 Biology Identification of phanerosporic acid in birch degraded by Phanerochaete chrysosporium Michael D. Mozuch and Philip
Designer Fentanyls Drugs that kill and how to detect them. Cyclopropylfentanyl
 Designer Fentanyls Drugs that kill and how to detect them Cyclopropylfentanyl Science for a safer world The in vitro metabolism of cyclopropylfentanyl Simon Hudson & Charlotte Cutler, Sport and Specialised
Designer Fentanyls Drugs that kill and how to detect them Cyclopropylfentanyl Science for a safer world The in vitro metabolism of cyclopropylfentanyl Simon Hudson & Charlotte Cutler, Sport and Specialised
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/ MCQs. Location : 102, 105, 106, 301, 302
 FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
The distribution of log 2 ratio (H/L) for quantified peptides. cleavage sites in each bin of log 2 ratio of quantified. peptides
 Journal: Nature Methods Article Title: Corresponding Author: Protein digestion priority is independent of their abundances Mingliang Ye and Hanfa Zou Supplementary Figure 1 Supplementary Figure 2 The distribution
Journal: Nature Methods Article Title: Corresponding Author: Protein digestion priority is independent of their abundances Mingliang Ye and Hanfa Zou Supplementary Figure 1 Supplementary Figure 2 The distribution
<Supplemental information>
 The Structural Basis of Endosomal Anchoring of KIF16B Kinesin Nichole R. Blatner, Michael I. Wilson, Cai Lei, Wanjin Hong, Diana Murray, Roger L. Williams, and Wonhwa Cho Protein
The Structural Basis of Endosomal Anchoring of KIF16B Kinesin Nichole R. Blatner, Michael I. Wilson, Cai Lei, Wanjin Hong, Diana Murray, Roger L. Williams, and Wonhwa Cho Protein
TKB1 Competent Cells. Instruction Manual. Research Use Only. Not for Use in Diagnostic Procedures. Catalog # Revision B
 TKB1 Competent Cells Instruction Manual Catalog #200134 Revision B Research Use Only. Not for Use in Diagnostic Procedures. 200134-12 LIMITED PRODUCT WARRANTY This warranty limits our liability to replacement
TKB1 Competent Cells Instruction Manual Catalog #200134 Revision B Research Use Only. Not for Use in Diagnostic Procedures. 200134-12 LIMITED PRODUCT WARRANTY This warranty limits our liability to replacement
Tenofovir disoproxil fumarate (Tenofoviri disoproxili fumaras)
 C 19 H 30 N 5 O 10 P. C 4 H 4 O 4 Relative molecular mass. 635.5. Chemical names. bis(1-methylethyl) 5-{[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-5-oxo-2,4,6,8-tetraoxa-5-λ 5 - phosphanonanedioate
C 19 H 30 N 5 O 10 P. C 4 H 4 O 4 Relative molecular mass. 635.5. Chemical names. bis(1-methylethyl) 5-{[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-5-oxo-2,4,6,8-tetraoxa-5-λ 5 - phosphanonanedioate
Carotenoid Oxygenases from Camellia sinensis, Osmanthus fragrans, and Prunus persica nucipersica
 Carotenoid Oxygenases from Camellia sinensis, Osmanthus fragrans, and Prunus persica nucipersica - Kinetics and Structure - Von der Fakultatfur Lebenswissenschaften der Technischen Universitat Carolo-Wilhelmina
Carotenoid Oxygenases from Camellia sinensis, Osmanthus fragrans, and Prunus persica nucipersica - Kinetics and Structure - Von der Fakultatfur Lebenswissenschaften der Technischen Universitat Carolo-Wilhelmina
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
Jagua (Genipin-Glycine) Blue (Tentative)
 0 out of 9 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 84th meeting 2017 Jagua (Genipin-Glycine) Blue (Tentative) This monograph was also
0 out of 9 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 84th meeting 2017 Jagua (Genipin-Glycine) Blue (Tentative) This monograph was also
Isolation, Separation, and Characterization of Organic Acids*
 In Dashek, William V., ed. Methods in plant biochemistry and molecular biology. Boca Raton, FL: CRC Press: pp. 107-113. Chapter 9.1997. Isolation, Separation, and Characterization of Organic Acids* William
In Dashek, William V., ed. Methods in plant biochemistry and molecular biology. Boca Raton, FL: CRC Press: pp. 107-113. Chapter 9.1997. Isolation, Separation, and Characterization of Organic Acids* William
Supporting Information
 Investigation of self-immolative linkers in the design of hydrogen peroxide metalloprotein inhibitors Jody L. Major Jourden, Kevin B. Daniel, and Seth M. Cohen* Department of Chemistry and Biochemistry,
Investigation of self-immolative linkers in the design of hydrogen peroxide metalloprotein inhibitors Jody L. Major Jourden, Kevin B. Daniel, and Seth M. Cohen* Department of Chemistry and Biochemistry,
Available online Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(12):519-523 Research Article ISSN : 0975-7384 CDEN(USA) : JCPRC5 Characterization of cyanidin 3-(6-acetylglucoside)-5-(3
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(12):519-523 Research Article ISSN : 0975-7384 CDEN(USA) : JCPRC5 Characterization of cyanidin 3-(6-acetylglucoside)-5-(3
Isolation and Purification of Organic Compounds Steam Distillation of Essential Oils
 Isolation and Purification of Organic Compounds Steam Distillation of Essential Oils Distillation relies on the fact that the substance with the greatest vapor pressure will be enriched in the vapor phase
Isolation and Purification of Organic Compounds Steam Distillation of Essential Oils Distillation relies on the fact that the substance with the greatest vapor pressure will be enriched in the vapor phase
ANALYTICAL SCIENCES OCTOBER 2018, VOL The Japan Society for Analytical Chemistry
 ANALYTICAL SCIENCES OCTOBER 2018, VOL. 34 1195 2018 The Japan Society for Analytical Chemistry Process for the Purification of cis-p-coumaric Acid by Cellulose Column Chromatography after the Treatment
ANALYTICAL SCIENCES OCTOBER 2018, VOL. 34 1195 2018 The Japan Society for Analytical Chemistry Process for the Purification of cis-p-coumaric Acid by Cellulose Column Chromatography after the Treatment
ph Switchable and Fluorescent Ratiometric Squarylium Indocyanine Dyes as Extremely Alkaline Sensors
 ph Switchable and Fluorescent Ratiometric Squarylium Indocyanine Dyes as Extremely Alkaline Sensors Jie Li, Chendong Ji, Wantai Yang, Meizhen Yin* State Key Laboratory of Chemical Resource Engineering,
ph Switchable and Fluorescent Ratiometric Squarylium Indocyanine Dyes as Extremely Alkaline Sensors Jie Li, Chendong Ji, Wantai Yang, Meizhen Yin* State Key Laboratory of Chemical Resource Engineering,
Supporting Information. 58 Pages. 6 Figures 4 Tables
 Light-Source-Dependent Effects of Main Water Constituents on Photodegradation of Phenicol Antibiotics: Mechanism and Kinetics Linke Ge, Jingwen Chen, * Xianliang Qiao, Jing Lin, Xiyun Cai Key Laboratory
Light-Source-Dependent Effects of Main Water Constituents on Photodegradation of Phenicol Antibiotics: Mechanism and Kinetics Linke Ge, Jingwen Chen, * Xianliang Qiao, Jing Lin, Xiyun Cai Key Laboratory
Supporting Information for. Boronic Acid Functionalized Aza-Bodipy (azabdpba) based Fluorescence Optodes for the. analysis of Glucose in Whole Blood
 Supporting Information for Boronic Acid Functionalized Aza-Bodipy (azabdpba) based Fluorescence Optodes for the analysis of Glucose in Whole Blood Yueling Liu, Jingwei Zhu, Yanmei Xu, Yu Qin*, Dechen Jiang*
Supporting Information for Boronic Acid Functionalized Aza-Bodipy (azabdpba) based Fluorescence Optodes for the analysis of Glucose in Whole Blood Yueling Liu, Jingwei Zhu, Yanmei Xu, Yu Qin*, Dechen Jiang*
Supporting Information
 Supporting Information For Heterologous production of thiostrepton A and biosynthetic engineering of thiostrepton analogs Chaoxuan Li, Feifei Zhang, and Wendy L. Kelly* Table of Contents Figure S1. PCR
Supporting Information For Heterologous production of thiostrepton A and biosynthetic engineering of thiostrepton analogs Chaoxuan Li, Feifei Zhang, and Wendy L. Kelly* Table of Contents Figure S1. PCR
SPRIN Protease Kit. Content Code Description Application. Covalently immobilised preparation of Subtilisin. Epoxy Acrylic Resin
 SPRIN Protease Kit SPRIN Protease Kit Normal (5 g each) Product code: SPKN SPRIN Protease Kit Large (20 g each) Product code: SPKL The kit contains four different covalently immobilised protease preparations,
SPRIN Protease Kit SPRIN Protease Kit Normal (5 g each) Product code: SPKN SPRIN Protease Kit Large (20 g each) Product code: SPKL The kit contains four different covalently immobilised protease preparations,
Naoya Takahashi, Keiya Hirota and Yoshitaka Saga* Supplementary material
 Supplementary material Facile transformation of the five-membered exocyclic E-ring in 13 2 -demethoxycarbonyl chlorophyll derivatives by molecular oxygen with titanium oxide in the dark Naoya Takahashi,
Supplementary material Facile transformation of the five-membered exocyclic E-ring in 13 2 -demethoxycarbonyl chlorophyll derivatives by molecular oxygen with titanium oxide in the dark Naoya Takahashi,
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/142604
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/142604
decarboxylation. Further work with the enzyme systems involved has shown
 THE BACTERIAL OXIDATION OF AROMATIC COMPOUNDS IV. STITDIES ON THE MECHANISM OF ENZYMATC DEGRADATION OF PROTOCATECHuiC ACID' R. Y. STANIER Department of Bacteriology, University of California, Berkeley,
THE BACTERIAL OXIDATION OF AROMATIC COMPOUNDS IV. STITDIES ON THE MECHANISM OF ENZYMATC DEGRADATION OF PROTOCATECHuiC ACID' R. Y. STANIER Department of Bacteriology, University of California, Berkeley,
Recent Studies of Phenylketonuria By: Jennifer Gastelum Dr. Koni Stone Copyright 2014
 Recent Studies of Phenylketonuria By: Jennifer Gastelum Dr. Koni Stone Copyright 2014 Phenylketonuria (PKU) is an autosomal recessive genetic disorder that causes an accumulation of toxic metabolites in
Recent Studies of Phenylketonuria By: Jennifer Gastelum Dr. Koni Stone Copyright 2014 Phenylketonuria (PKU) is an autosomal recessive genetic disorder that causes an accumulation of toxic metabolites in
Previous Class. Today. Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism. Protein Kinase Catalytic Properties
 Previous Class Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism Today Protein Kinase Catalytic Properties Protein Phosphorylation Phosphorylation: key protein modification
Previous Class Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism Today Protein Kinase Catalytic Properties Protein Phosphorylation Phosphorylation: key protein modification
Supporting Information
 Translation of DNA into Synthetic N-Acyloxazolidines Xiaoyu Li, Zev. J. Gartner, Brian N. Tse and David R. Liu* Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts
Translation of DNA into Synthetic N-Acyloxazolidines Xiaoyu Li, Zev. J. Gartner, Brian N. Tse and David R. Liu* Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts
Characterization of Disulfide Linkages in Proteins by 193 nm Ultraviolet Photodissociation (UVPD) Mass Spectrometry. Supporting Information
 Characterization of Disulfide Linkages in Proteins by 193 nm Ultraviolet Photodissociation (UVPD) Mass Spectrometry M. Montana Quick, Christopher M. Crittenden, Jake A. Rosenberg, and Jennifer S. Brodbelt
Characterization of Disulfide Linkages in Proteins by 193 nm Ultraviolet Photodissociation (UVPD) Mass Spectrometry M. Montana Quick, Christopher M. Crittenden, Jake A. Rosenberg, and Jennifer S. Brodbelt
Electronic Supplementary Information. Table of Contents
 Electronic Supplementary Information Examination of native chemical ligation using peptidyl prolyl thioester Takahiro Nakamura, Akira Shigenaga, Kohei Sato, Yusuke Tsuda, Ken Sakamoto, and Akira Otaka*
Electronic Supplementary Information Examination of native chemical ligation using peptidyl prolyl thioester Takahiro Nakamura, Akira Shigenaga, Kohei Sato, Yusuke Tsuda, Ken Sakamoto, and Akira Otaka*
Organic Chemistry Laboratory Fall Lecture 3 Gas Chromatography and Mass Spectrometry June
 344 Organic Chemistry Laboratory Fall 2013 Lecture 3 Gas Chromatography and Mass Spectrometry June 19 2013 Chromatography Chromatography separation of a mixture into individual components Paper, Column,
344 Organic Chemistry Laboratory Fall 2013 Lecture 3 Gas Chromatography and Mass Spectrometry June 19 2013 Chromatography Chromatography separation of a mixture into individual components Paper, Column,
SUPPLEMENTARY INFORMATION
 In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION DOI: 10.1038/NCHEM.2919 Direct observation of the influence of cardiolipin and antibiotics on lipid II binding to MurJ Jani
In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION DOI: 10.1038/NCHEM.2919 Direct observation of the influence of cardiolipin and antibiotics on lipid II binding to MurJ Jani
BabyBio IMAC columns DATA SHEET DS
 BabyBio IMAC columns DATA SHEET DS 45 655 010 BabyBio columns for Immobilized Metal Ion Affinity Chromatography (IMAC) are ready-to-use for quick and easy purification of polyhistidine-tagged (His-tagged)
BabyBio IMAC columns DATA SHEET DS 45 655 010 BabyBio columns for Immobilized Metal Ion Affinity Chromatography (IMAC) are ready-to-use for quick and easy purification of polyhistidine-tagged (His-tagged)
Dienes Derivatization MaxSpec Kit
 Dienes Derivatization MaxSpec Kit Item No. 601510 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Dienes Derivatization MaxSpec Kit Item No. 601510 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Sequential Extraction of Plant Metabolites
 ISSN: 2319-7706 Volume 4 Number 2 (2015) pp. 33-38 http://www.ijcmas.com Original Research Article Sequential Extraction of Plant Metabolites Shankar L. Laware* PG. Department of Botany, Fergusson College
ISSN: 2319-7706 Volume 4 Number 2 (2015) pp. 33-38 http://www.ijcmas.com Original Research Article Sequential Extraction of Plant Metabolites Shankar L. Laware* PG. Department of Botany, Fergusson College
Effect of Lincomycin and Clindamycin on Peptide
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Jan. 1975, p. 32-37 Copyright 0 1975 American Society for Microbiology Vol. 7, No. 1 Printed in U.S.A. Effect of Lincomycin and Clindamycin on Peptide Chain Initiation
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Jan. 1975, p. 32-37 Copyright 0 1975 American Society for Microbiology Vol. 7, No. 1 Printed in U.S.A. Effect of Lincomycin and Clindamycin on Peptide Chain Initiation
Supplementary Figure 1. Chemical structures of activity-based probes (ABPs) and of click reagents used in this study.
 Supplementary Figure 1. Chemical structures of activity-based probes (ABPs) and of click reagents used in this study. In this study, one fluorophosphonate (FP, 1), three nitrophenol phosphonate probes
Supplementary Figure 1. Chemical structures of activity-based probes (ABPs) and of click reagents used in this study. In this study, one fluorophosphonate (FP, 1), three nitrophenol phosphonate probes
ON THE NATURE OF THE TRANSALDOLASE-DIHYDROXYACETONE
 VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
Chapter PURIFICATION OF ALKALINE PROTEASES
 Chapter PURIFICATION OF ALKALINE PROTEASES E /xtracellular alkaline proteases produced by Bacillus sp. K 25 and bacillus pumilus K 242, were purified and the homogeneity was examined by electrophoresis.
Chapter PURIFICATION OF ALKALINE PROTEASES E /xtracellular alkaline proteases produced by Bacillus sp. K 25 and bacillus pumilus K 242, were purified and the homogeneity was examined by electrophoresis.
Overview of the Expressway Cell-Free Expression Systems. Expressway Mini Cell-Free Expression System
 Overview of the Expressway Cell-Free Expression Systems The Expressway Cell-Free Expression Systems use an efficient coupled transcription and translation reaction to produce up to milligram quantities
Overview of the Expressway Cell-Free Expression Systems The Expressway Cell-Free Expression Systems use an efficient coupled transcription and translation reaction to produce up to milligram quantities
The Different Folding Behavior of Insulin and Insulin-like Growth Factor 1 Is Mainly Controlled by Their B-Chain/Domain
 1556 Biochemistry 2002, 41, 1556-1567 The Different Folding Behavior of Insulin and Insulin-like Growth Factor 1 Is Mainly Controlled by Their B-Chain/Domain Zhan-Yun Guo, Lu Shen, and You-Min Feng* State
1556 Biochemistry 2002, 41, 1556-1567 The Different Folding Behavior of Insulin and Insulin-like Growth Factor 1 Is Mainly Controlled by Their B-Chain/Domain Zhan-Yun Guo, Lu Shen, and You-Min Feng* State
130327SCH4U_biochem April 09, 2013
 Option B: B1.1 ENERGY Human Biochemistry If more energy is taken in from food than is used up, weight gain will follow. Similarly if more energy is used than we supply our body with, weight loss will occur.
Option B: B1.1 ENERGY Human Biochemistry If more energy is taken in from food than is used up, weight gain will follow. Similarly if more energy is used than we supply our body with, weight loss will occur.
Double charge of 33kD peak A1 A2 B1 B2 M2+ M/z. ABRF Proteomics Research Group - Qualitative Proteomics Study Identifier Number 14146
 Abstract The 2008 ABRF Proteomics Research Group Study offers participants the chance to participate in an anonymous study to identify qualitative differences between two protein preparations. We used
Abstract The 2008 ABRF Proteomics Research Group Study offers participants the chance to participate in an anonymous study to identify qualitative differences between two protein preparations. We used
Supporting information
 Supporting information Figure legends Supplementary Table 1. Specific product ions obtained from fragmentation of lithium adducts in the positive ion mode comparing the different positional isomers of
Supporting information Figure legends Supplementary Table 1. Specific product ions obtained from fragmentation of lithium adducts in the positive ion mode comparing the different positional isomers of
SUPPLEMENTAL INFORMATION
 SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
GLYCATION OF PROTEINS IN ESCHERICHIA COLI: EFFECT OF NUTRIENT BROTH INGREDIENTS ON GLYCATION
 Industry GLYCATION OF PROTEINS IN ESCHERICHIA COLI: EFFECT OF NUTRIENT BROTH INGREDIENTS ON GLYCATION R. Dimitrova, R. Mironova, I. Ivanov Institute of Molecular biology, Bulgarian Academy of Sciences
Industry GLYCATION OF PROTEINS IN ESCHERICHIA COLI: EFFECT OF NUTRIENT BROTH INGREDIENTS ON GLYCATION R. Dimitrova, R. Mironova, I. Ivanov Institute of Molecular biology, Bulgarian Academy of Sciences
The Investigation of Factors Contributing to Immunosuppressant Drugs Response Variability in LC-MS/MS Analysis
 The Investigation of Factors Contributing to Immunosuppressant Drugs Variability in LC-MS/MS Analysis Joseph Herman, Dayana Argoti, and Sarah Fair Thermo Fisher Scientific, Franklin, MA, USA Overview Purpose:
The Investigation of Factors Contributing to Immunosuppressant Drugs Variability in LC-MS/MS Analysis Joseph Herman, Dayana Argoti, and Sarah Fair Thermo Fisher Scientific, Franklin, MA, USA Overview Purpose:
Thiol-Activated gem-dithiols: A New Class of Controllable. Hydrogen Sulfide (H 2 S) Donors
 Thiol-Activated gem-dithiols: A New Class of Controllable Hydrogen Sulfide (H 2 S) Donors Yu Zhao, Jianming Kang, Chung-Min Park, Powell E. Bagdon, Bo Peng, and Ming Xian * Department of Chemistry, Washington
Thiol-Activated gem-dithiols: A New Class of Controllable Hydrogen Sulfide (H 2 S) Donors Yu Zhao, Jianming Kang, Chung-Min Park, Powell E. Bagdon, Bo Peng, and Ming Xian * Department of Chemistry, Washington
MALDI-TOF. Introduction. Schematic and Theory of MALDI
 MALDI-TOF Proteins and peptides have been characterized by high pressure liquid chromatography (HPLC) or SDS PAGE by generating peptide maps. These peptide maps have been used as fingerprints of protein
MALDI-TOF Proteins and peptides have been characterized by high pressure liquid chromatography (HPLC) or SDS PAGE by generating peptide maps. These peptide maps have been used as fingerprints of protein
Figure 1 Original Advantages of biological reactions being catalyzed by enzymes:
 Enzyme basic concepts, Enzyme Regulation I III Carmen Sato Bigbee, Ph.D. Objectives: 1) To understand the bases of enzyme catalysis and the mechanisms of enzyme regulation. 2) To understand the role of
Enzyme basic concepts, Enzyme Regulation I III Carmen Sato Bigbee, Ph.D. Objectives: 1) To understand the bases of enzyme catalysis and the mechanisms of enzyme regulation. 2) To understand the role of
6. C-type cytochrome, soluble and membrane protein
 185 6. C-type cytochrome, soluble and membrane protein analysis of Rhodobacter sp SW2 and Rhodopseudomonas palustris TIE-1 ABSTRACT The ability to grown on Fe(II) is thought to be a primitive metabolism
185 6. C-type cytochrome, soluble and membrane protein analysis of Rhodobacter sp SW2 and Rhodopseudomonas palustris TIE-1 ABSTRACT The ability to grown on Fe(II) is thought to be a primitive metabolism
