Assessing the Bioavailability of Synthetic Methionine and Lysine from Different Sources in Rainbow Trout (Oncorhynchus mykiss).
|
|
|
- Arthur Hill
- 5 years ago
- Views:
Transcription
1 Assessing the Bioavailability of Synthetic Methionine and Lysine from Different Sources in Rainbow Trout (Oncorhynchus mykiss). By Christopher David Powell A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Master of Science in Animal and Poultry Science Guelph, Ontario, Canada Christopher D. Powell, August, 2014 i
2 ABSTRACT ASSESSING THE BIOAVAILABILITY OF SYNTHETIC METHIONINE AND LYSINE FROM DIFFERENT SOURCES IN RAINBOW TROUT (ONCORHYNCHUS MYKISS). Christopher David Powell University of Guelph, 2014 Advisor: Professor D.P Bureau Relative bioavailability of L-methionine and a hydroxy methionine analogue (MHA-Ca) were compared to the commercially prevalent DL-methionine in a 12-week growth trial. A separate 12-week trial investigated relative bioavailability between L-lysine HCL and L-lysine sulphate. Basal diets were formulated to be deficient in methionine or lysine and were supplemented with increasing equimolar levels of methionine or lysine from three sources of methionine or two sources of lysine. Using a linear slope-ratio assay, bioavailability of L-methionine and DL-methionine were determined to be similar (p>0.10). Differences in bioavailability between DL-methionine and MHA-Ca were observed (p<0.05), with MHA-Ca being 69, 60 and 73% as bioavailable as DL-methionine based upon weight gain, growth rate (thermal-unit growth coefficient) and retained nitrogen response parameters respectively. L-lysine HCL and L-lysine sulphate were effective sources of lysine with no significant differences in bioavailability (p>0.10). In conclusion, differences in bioavailability exist between sources of synthetic methionine, but not lysine. ii
3 Acknowledgements I would first like to thank my advisor Dr. Dominique P. Bureau for his guidance and the wealth of knowledge that he has provided for me during my time in his lab. His ability to keep in communication even while on the opposite side of the world is actually quite astounding. Dr. Bureau s ability to provide feedback yet encourage independent work along with forcing me outside my comfort zone I feel has not only improved my ability as a researcher but also as a person. Thank you, Dom. Thank you to my advisory committee, Dr. John Cant and Dr. Cornelis F. M. de Lange for the help and ideas that you have provided me throughout the process. Thank you John in particular for your help in regards to my statistical analysis and coding. Kees I appreciate all your questions and ideas that you put forward during our meetings. I would also like to thank Dr. Andreas Lemme and Dr. Claudia Silva from Evonik Industries AG for spearheading this effort. I would also like to thank Dr. Margaret Quinton for her help in developing the initial statistical models used in my research. Our meetings and my analysis of your code helped me understand the practical application of statistics. I would also like to thank Jamie Hooft and Owen Skipper-Horton for all their help in teaching me the finer points of the statistical software SAS. I would like to thank all of my lab mates, there are too many to mention, but the lab really has developed into a second family for me. I would especially like to thank a former lab mate of mine Dr. M.A. Kabir Chowdhury for all his help through my project; your help was pivotal to my success. All the undergraduate volunteers who helped me run my trials, your work was much appreciated and much needed thank you. I would like to thank Evonik Industries AG (Hanau, Germany) for funding this research along with OMAFRA and the University of Guelph for provided me with a very generous scholarship. iii
4 Finally I would like to thank those who are the most important in my life, my family and friends and all those I love. In particular I would like to thank my parents David and Judith for their support, both emotionally and financially throughout my Master s and academic career as a whole. iv
5 Table of Contents CHAPTER 1 GENERAL INTRODUCTION Objectives... 3 CHAPTER 2 LITERATURE REVIEW Amino Acids: Chemistry and Structure Methionine Biochemistry & Metabolism Methionine Requirements Lysine Biochemistry & Metabolism Lysine Requirements Strategies for Meeting Essential Amino Acid Requirements Introduction to Synthetic Amino Acids Estimating Bioavailability of Essential Amino Acids Conclusions and perspectives CHAPTER 3 ASSESSING THE BIOAVAILABILITY OF L-METHIONINE AND A HYDROXY METHIONINE ANALOGUE (MHA-Ca) COMPARED TO DL-METHIONINE IN RAINBOW TROUT (ONCORHYNCHUS MYKISS) Abstract Introduction Methods Results Discussion v
6 3.5 Conclusion CHAPTER 4 - ASSESSING THE BIOAVAILABILITY OF L-LYSINE SULPHATE COMPARED TO L-LYSINE HCL IN RAINBOW TROUT (ONCORHYNCHUS MYKISS) Abstract Introduction Methods Results Discussion Conclusion CHAPTER 5 GENERAL DISCUSSION BIBLIOGRAPHY vi
7 List of tables Table 2.1 Essential and non-essential amino acids... 6 Table 2.2 Biological compounds and amino acid precursors in animals Table 2.3 Essential amino acid requirements of rainbow trout (dry matter basis) Table 2.4 Amino acid profiles of common animal feed ingredients Table 2.5 Methionine + cysteine and methionine requirements of various finfish species Table 2.6 Estimates of methionine requirement of rainbow trout Table 2.7 Estimated and recommended lysine requirement for various fish species Table 3.1 Ingredient composition of experimental diets Table 3.2 Experimental design methionine inclusion from various synthetic sources Table 3.3 Analyzed essential amino acid content of experiential diets Table 3.4 Proximate composition of experimental diets Table 3.5 Formulated and analyzed levels of methionine and MHA in experimental diets Table 3.6 Performance of rainbow trout in response to being fed increasing equimolar levels of methionine from different sources over a 12 week experimental period Table 3.7 Proximate composition of whole carcass of rainbow trout in response to being fed increasing equimolar levels of methionine from various sources for 12 weeks, expressed on a wet weight basis. 655 Table 3.8 Retained nitrogen (RN), recovered energy (RE), nitrogen retention efficiency (NRE), and energy retention efficiency (ERE) of rainbow trout in response to being fed increasing equimolar levels of methionine from various sources for 12 weeks Table 3.9 Relative bioavailability of L-Met and MHA-Ca compared to the standard DL-Met based on weight gain, growth rate and retained nitrogen values Table 4.1 Ingredient composition of experimental diets Table 4.2 Experimental design- addition of L-lysine from supplemental L-lysine sources vii
8 Table 4.3 Analysed essential amino acid composition of experimental diets (% dry matter) Table 4.4 Proximate composition of experimental diets (dry matter basis) Table 4.5 Formulated and analyzed total lysine and free (supplemental) lysine of experimental diets (dry matter basis) Table 4.6 Performance of rainbow trout in response to being fed diets containing increasing equimolar levels of L-lysine from two supplemental sources Table 4.7 Proximate composition of whole carcasses of rainbow trout in response to being fed diets containing increasing equimolar levels of L-lysine from two supplemental sources, on a wet weight basis Table 4.8 Retained nitrogen (RN), recovered energy (RE), nitrogen retention efficiency (NRE), and energy retention efficiency (ERE) of rainbow trout in response to being fed diets containing increasing equimolar levels of L-lysine from two supplemental sources Table 4.9 Relative bioavailability of L-lysine sulphate compared to the standard L-lysine HCL based on weight gain, TGC and retained nitrogen values viii
9 List of figures Figure 2.1 Metabolic pathways of sulphur amino acids Figure 2.2 Degradation of lysine to acetyl CoA with enzymes: 1 L-amino acid oxidase; 2, specific aminotransferase Figure 2.3 Increasing crude protein content of a diet as a strategy for meeting essential amino acid requirements Figure 2.4 Use of multiple protein sources as a strategy for meeting essential amino acid requirements Figure 2.5 Use of synthetic amino acids to supplement a deficient diet Figure 2.6 Four commonly used assays for determining bioavailability of a nutrient Figure 2.7 Determining nutrient bioavailability using a slope-ratio assay RBV=X S/X T..42 Figure 3.1 Growth curves of rainbow trout in response to being fed experimental diets containing graded equimolar levels of methionine from three synthetic sources Figure 3.2 Weight gain of rainbow trout in response to being fed diets containing increasing equimolar levels of methionine from DL-Met ( ), L-Met ( ), and MHA-Ca ( ) Figure 3.3 Thermal-unit growth coefficient (TGC) of rainbow trout in response to being fed diets containing increasing equimolar levels of methionine from DL-Met ( ), L-Met ( ), and MHA-Ca ( ). 622 Figure 3.4 Retained Nitrogen (g/fish) content of rainbow trout in response to being fed diets containing increasing equimolar levels methionine from DL-Met ( ), L-Met ( ), and MHA-Ca ( ) Figure 3.5 Weight gain of rainbow trout in response to being fed increasing equimolar levels of methionine from DL-Met ( ), L-Met ( ), and MHA-Ca ( ) Figure 3.6 Thermal-unit growth coefficient (TGC) of rainbow trout in response to being fed diets containing increasing equimolar levels of methionine from DL-Met ( ), L-Met ( ), and MHA-Ca ( ). 688 ix
10 Figure 3.7 Retained nitrogen (g/fish) of rainbow trout in response to being fed diets containing increasing equimolar levels of DL-Met ( ), L-Met ( ), and MHA-Ca ( ) Figure 4.1 Growth curves of rainbow trout in response to being fed experimental diets containing increasing equimolar levels of L-lysine from two supplemental sources Figure 4.2 Weight gain of rainbow trout in response to being fed diets containing increasing equimolar levels of lysine from L-lys sulphate ( ) and L-lys HCL ( ) Figure 4.3 Thermal-unit growth coefficient of rainbow trout in response to being fed diets containing increasing equimolar levels lysine from L-lys sulphate ( ) and L-lys HCL ( ) Figure 4.4 Retained nitrogen content (g/fish) of rainbow trout in response to being fed diets containing increasing equimolar levels of lysine from L-lys sulphate ( ) and L-Lys HCL ( ) Figure 4.5 Weight gain of rainbow trout in response to being fed diets containing increasing equimolar levels of L-lysine from L-lys sulphate ( ) and L-lys HCL ( ) Figure 4.6 Thermal-unit growth coefficient (TGC) of rainbow trout in response to being fed diets containing increasing levels of L-lysine from L-lys Sulphate ( ) and L-lys HCL ( ) Figure 4.7 Retained nitrogen (g/fish) of rainbow trout in response to being fed diets containing increasing equimolar levels of L-lysine from L-lys sulphate ( ) and L-lys HCL ( ) x
11 CHAPTER 1 GENERAL INTRODUCTION The ability to successfully feed diets containing high levels of plant protein ingredients to carnivorous salmonids is due to the improved understanding of their nutritional requirements and the greater characterization of available nutrients in feed ingredients. Digestibility and amino acid profiles of cost-effective protein sources are of particular interest, as well as, the essential amino acid requirements of the animal. In comparison to good quality fish meal, most plant proteins have a poorer amino acid profile and are often deficient in one or several essential amino acids. In particular methionine and lysine are often found in low levels in common plant protein ingredients including soybean meal, corn gluten meal, wheat gluten and most cereal grains (NRC, 2011). Therefore, diets containing high levels of these plant proteins can frequently contain levels of methionine and lysine that are below the requirement of the animal. Besides their importance in protein synthesis, lysine and methionine play key biochemical and structural roles in animals, both methionine and lysine are precursors to important molecules including carnitine which is responsible for the transportation of fatty acids to the mitochondria in order to generate metabolic energy. Methionine is a methyl donor and a precursor to many important biological molecules used in a diverse array of metabolic reactions. Animals fed diets that are deficient in methionine result in decreased animal growth. High incidence of cataracts and spinal deformities may arise in fish fed methionine deficient diets (Poston et al., 1977; Keembiyehetty and Gatlin, 1993; Walton et al., 1982, Rumsey et al., 1983; Cowey et al., 1992). Lysine plays an important structural role in all types of proteins including transmembrane proteins and various connective tissues, with depressed growth and health issues, such as fin erosion, arising when fish are fed lysine deficient diets (Di Lullo et al., 2002, Ketola, 1983, Walton et al., 1984, 1986). Estimates of methionine and lysine requirements for 1
12 rainbow trout on a dry matter basis are 0.7% and 2.4% of the diet, respectively, in order to achieve maximum growth (NRC, 2011). Crystalline amino acids have been used in the animal feed industry for over 50 years allowing for the wider use of economical feed ingredients and greater flexibility in feed formulations while ensuring amino acid requirements are met. Unlike protein-bound amino acids, crystalline amino acids are not part of a polypeptide chain and are commonly referred to as free amino acids. Crystalline amino acids may be referred to as synthetic or supplemental amino acids in reference to their production technique. As methionine and lysine are most commonly the first and second most limiting essential amino acids a variety of commercially produced synthetic sources of these amino acids exist. Commercial synthetic methionine sources include the widely used DL-methionine along with the hydroxy analogue of methionine (MHA), both products of chemical synthesis. Other sources of synthetic methionine exist, such as L-methionine which is used primarily for laboratory purposes as commercial production techniques are inefficient. Instead of being products of chemical synthesis, supplemental sources of lysine including L-lysine HCL and L-lysine sulphate are produced through bacterial fermentation. Various sources of both methionine and lysine are commercially available, as differences between sources exist, their relative ability to provide a metabolically active, utilizable form of methionine or lysine is of interest. Bioavailability is defined as the extent to which an ingested nutrient is digested and absorbed in a form that can be utilized by the animal (Batterham, 1992; Lewis and Bayley, 1995). Bioavailability of synthetic amino acids between sources can be compared in order to understand the relative ability of these sources to supply a particular amino acid in an utilizable form. One method that allows for the direct and practical assessment of bioavailability of individual amino acids is the slope-ratio assay which has been used extensively in farmed animals (Lewis and Bailey, 1995). 2
13 In a slope-ratio assay, a basal diet is formulated to be deficient in the nutrient under investigation yet meet all other known nutritional requirements of the animal. The basal diet is then supplemented with increasing levels of the test nutrient from different test sources. Animal response, weight gain, growth rate etc. are then regressed against nutrient supplementation level and slopes of the lines are compared against a reference nutrient source in order to determine relative bioavailability. Slope-ratio assays have successfully been applied to determine relative bioavailability of amino acids in protein-bound ingredients as well as between crystalline or supplemental sources of essential amino acids (Boebel and Baker, 1982; Wang et al., 2007, Yi et al., 2006; Smiricky-Tjardes et al., 2004; Batterham et al., 1979, Li et al., 2009; El-Haroun and Bureau, 2007; Rodehutscord et al., 2000). 1.1 Objectives The objective of this study was to investigate the relative bioavailability between three sources of synthetic methionine (DL-methionine, L-methionine and MHA-Ca) and two sources of synthetic lysine (L-lysine sulphate and L-lysine HCL) in two separate growth trials using a slope-ratio assay methodology. 3
14 CHAPTER 2 LITERATURE REVIEW 2.1 Amino Acids: Chemistry and Structure Proteins are organic compounds found in all living organisms and serve crucial structural and metabolic roles. At the molecular level proteins are simply chains of bound amino acids folded into a particular structure. These chains consist of amino acids bound to one another through amide bounds which bind the α-amino group of one amino acid to the carboxyl group of another (Brody, 1999). Chains of amino acid monomers are classified by the number of amino acids bound in the chain; dipeptides consist of two bound amino acids, while polypeptides contain a continuous linear non-branching chain of amino acid monomers (Brody, 1999). The general structure of amino acid follows the H 2NCHRCOOH template whereby R represents a side chain which distinguishes amino acids from one another. Individual amino acid molecules contain both an amine and carboxyl group along with the R side chain whose properties influence the size, shape and electrical charge of a specific amino acid. With the exception of proline and hydroxyproline all amino acids found in living organisms are considered α-amino acids meaning the primary amino group and the carboxyl group are attached to the same (α) carbon (Lloyd et al., 1978). With the exception of glycine, the α-carbon of an amino acid is bound to four different groups; a carboxyl group, an amino group, an R side chain and a hydrogen atom. The four different groups that are bound to the α-carbon are able to exist in two different spatial arrangements that are non-superimposable mirror images of each other. The two asymmetric spatial arrangements are commonly referred to as L or D isomers of an α-amino acid, with L isomers arranged with the amino group to the left of the α-carbon and hydrogen atom to the right while the opposite is true for D isomers. With few exceptions, amino acids are naturally found in the L-configuration which is commonly referred to as an L-isomer. The amino acids 4
15 that make up proteins are solely L-isomers as cells specifically synthesize the L isomer of an amino acid due to the asymmetry of enzyme activity sites which causes the reactions that they catalyze to be stereospecific (Nelson & Cox, 2000). Twenty primary amino acids are used by cells during protein biosynthesis, and are commonly referred to the twenty standard amino acids of proteins. Each of these twenty amino acids contain a unique R group, however properties of R groups may be similar between individual amino acids allowing their classification into five categories. The five categories are: nonpolar aliphatic R groups, aromatic R groups, polar uncharged R groups, positively charge basic R groups and negatively charged acidic R groups. The properties of these R groups influence the structural role that these amino acids have in proteins, with the nonpolar aliphatic and aromatic R groups being hydrophobic and stabilizing proteins through hydrophobic interactions, while polar uncharged R groups are hydrophilic. The charged R groups are also highly hydrophilic and due to their charges influence protein folding and structure. The standard twenty amino acids can be separated into two categories: Essential amino acids (EAAs) and non-essential amino acids (NEAAs) (Table 2.1). Both essential and non-essential amino acids are equally important for protein synthesis, the differences between the two is based on the ability of the body to synthesis, or synthesize in sufficient amounts, a particular amino acid. Non-essential amino acids can be synthetized from precursors in the body of the organism through transamination reactions whereby amino groups from either an amino acid or a simple amine are transferred to a suitable carbon source creating a non-essential amino acid. Essential amino acids, although capable of transamination reactions, either cannot be synthetized in the body or cannot be synthetized in the body at a sufficient rate as to meet the physiological needs for this amino acid by the organism (Nelson & Cox, 2000). Cysteine and tyrosine are considered semi-essential or conditionally essential as they can be synthesized from the essential amino acids methionine and phenylalanine respectively. 5
16 Table 2.1 Essential and non-essential amino acids Essential amino acid Non-essential amino acid Tryptophan Cysteine 1 Valine Tyrosine 1 Phenylalanine Arginine Lysine Leucine Methionine Isoleucine Threonine Histidine Proline Glutamate Glutamine Glycine Serine Alanine Aspartate Aspartic Acid 1 Conditionally essential Adapted from D Mello (2003a) 6
17 2.1.1 Amino Acid Metabolism and Catabolism Both dietary amino acids and amino acids which result from the endogenous breakdown of body proteins enter the metabolic pool and depending on the current metabolic demands on the animal, may be used for protein synthesis and/or as precursors for other biological molecules (Kaushik & Seiliez, 2010). Protein synthesis is the formation of polypeptides and finally proteins through successive attachment of amino acids according to highly specific template. The ability to bio-synthesis proteins depends on the configuration of the amino acid whereby D-isomers must undergo a metabolic racemization to the L-isomer form before they are able to be incorporated into a protein (Friedman & Gumbmann 1984). Protein deposition in an animal occurs when the rate of protein synthesis is greater than the rate protein degradation, in the majority of fish and shrimp species protein synthesis and associated body protein deposition accounts for 25-55% of total amino acids consumed (NRC 2011). Protein deposition has been recognised to be the driving force behind weight gain as a strong correlation between protein deposition, water deposition and live weight gain exist (Dumas et al., 2007). Along with their role in protein synthesis amino acids may serve as precursors for many biological molecules such as enzymes, hormones, neurotransmitters and many other metabolic intermediates. Table 2.2 displays various important biological compounds their amino acid precursors and the physiological function of these compounds. Besides their role in anabolism, amino acids may also follow catabolic pathways which are dependent on the current metabolic demands of the animal. When dietary energy intake is limiting protein deposition, EAA may be partitioned away from protein synthesis and towards catabolism to meet a certain metabolic need, commonly referred to as preferential catabolism (Moughan, 2003). However, when the energy supply of a diet is not limiting protein synthesis or amino acid intake is below an animal s requirement for maximum protein deposition, inevitable amino acids catabolism will still 7
18 occur through active catabolic pathways (Moughan, 1995). Finally, amino acids that are in excess of the cumulative amino acid demands of protein synthesis, maintenance requirements, and losses due to inevitable and preferential catabolism, will result in the catabolism of these excess amino acids (NRC, 2011). 8
19 Table 2.2 Biological compounds and amino acid precursors in animals. Biological compound Amino acid precursor Physiological function Purines and pyrimidines Glycine and aspartic acid Constituents of nucleotides and nucleic acids Creatine Glycine and arginine Energy storage as creatine phosphate in muscle Glcoholic and taurocholic acids Glycine and cysteine Bile acids, aid in fat digestion and absorption Thyroxine, epinephrine, and Tyrosine Hormones norepinephrine Ethanolamine and choline Serine Constituents of phospholipids Histamine Histidine A vasodepressor Serotonin Tryptophan Transmission of nerve impulses Porphyrins Glycine Constituent of hemoglobin and cytochromes Niacin Tryptophan Vitamin Melanin Tyrosine Pigment of hair, skin, and eyes Methyl groups Methionine DNA methylation Glutathione Cysteine Important antioxidant Carnitine Lysine and methionine Transportation of fatty acids Adapted from Lloyd (1978). 9
20 2.1.2 Essential Amino Acid Requirements In animal-nutrition, two techniques were traditionally used to determine if a specific amino acid is considered an essential amino acid; isotopic labelling studies or feeding trials, with feeding trials being the more popular technique (Wilson, 1989). Through the systematic deletion of certain amino acids in the diet and analysing the corresponding performance of the fish, essential amino acids can be differentiated from non-essential amino acids for a particular species of fish (Wilson, 1989; Cowey 1994). Using this technique, Nose et al. (1974) tested the essentially of 18 amino acids for common carp and found that by excluding any 10 out of the 18 amino acids resulted in significant growth reduction. These amino acids were arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine which are the same 10 essential amino acids (Table 2.3) required by most animals (Ketola, 1982; Wilson, 1989; NRC, 1993). Along with the 10 essential amino acids, both tyrosine and cysteine are considered semi/conditional essential as they can be synthesized from the essential amino acids, phenylalanine and methionine respectively. Although the 10 essential amino acid do not differ between animals, the specific requirements of these 10 essential amino acids vary between both species and life stage. Initial studies on specific essential amino acid requirements for fish species starting in the 1950 s whereby essential amino acid requirements for Chinook salmon were first experimentally determined using a feeding trial (Halver, 1957). Currently well over 200 papers have been published estimating the dietary requirement of essential amino acids for fish and shrimp species, including channel catfish, common carp, Nile tilapia, Pacific salmon and rainbow trout along with many others (NRC, 2011 ). Table 2.3 contains minimal essential amino acid requirement in order to achieve maximum performance as percentage of diet on a dry-matter basis for rainbow trout. It is important to consider that these requirements are based off experimental data under optimal conditions using highly digestible purified feed ingredients. Modern animal feeds contain various protein sources, with each ingredient having a different amino acid profile 10
21 as seen in table 2.4. Modern feeds are being formulated to include higher levels of plant protein ingredients in partial substitution of animal proteins. Most cereal grains and plant proteins contain low levels of lysine or methionine. Consequently, these two amino acids are commonly the first and second most limiting essential amino acids in diets containing high levels of plant proteins. 11
22 Table 2.3 Essential amino acid requirements of rainbow trout (dry matter basis). Amino acid Amino acid requirement % of diet Arginine 1.5 Histidine 0.8 Isoleucine 1.1 Leucine 1.5 Lysine 2.4 Methionine 0.7 Methionine + cysteine 1.1 Phenylalanine 0.9 Phenylalanine + tyrosine 1.8 Threonine 1.1 Tryptophan 0.3 Valine 1.2 NRC (2011) Table 2.4 Amino acid profiles of common animal feed ingredients (% as-fed basis). Ingredient DM (%) Arg His Ile Leu Lys Met Cys Phe Tyr Thr Trp Val Fish meal Blood meal, sprdry Meat and Bone meal Poultry byproduct meal Gelatin Soybean meal Canola meal Corn gluten meal Wheat middlings Adapted from NRC (2011) 12
23 2.2 Methionine Biochemistry & Metabolism Of the 20 amino acids that make up the primary structure of proteins, two amino acids, methionine and cysteine, contain a sulphur atom. Methionine is an essential α-amino acid with the chemical formula HO 2CCH(NH 2)CH 2CH 2SCH 3 and is non-polar. While cysteine is considered a semiessential amino acid as it can be synthesized from serine requiring a trans-sulphuration reaction with methionine. Cysteine contains a highly reactive thiol side chain resulting in it having an important role in the structure and therefore function of many proteins and enzymes (NRC 2011). Besides its role in protein synthesis, L-methionine, can also be converted into S-adenosylmethionine and then S- adenosylhomocysteine releasing a methyl group in the process (Lewis, 2003). This methyl group can then be used in several metabolic processes, including DNA methylation and synthesis of carnitine from lysine, adrenaline from noradrenaline, and creatine from guanidine acetate (Simon, 1999). The conversion of methionine into cysteine, and the associated intermediate compounds including S-adenosylmethionine (SAM), is well understood and major intermediate compounds in this metabolic conversion (figure 2.1). Methionine can be activated by ATP to S-adenosylmethionine (SAM), facilitated by co-enzyme methionine adenosyltransferase. S-adenosylmethionine is an important methyl donor, and is second only to ATP in number of enzymes that require it (Brosnan and Brosnan, 2006). Once S-adenosylmethionine donates its methyl group to an appropriate acceptor it forms S- adenosylhomocysteine (SAH). SAH undergoes hydrolysis by SAH hydrolase forming homocysteine. The culmination of these reactions are referred to as transmethylation. Homocysteine is an important intermediate as it can be converted back to methionine following one of two remethylation pathways or homocysteine may follow the transsulfuration pathway resulting in the formation of the semi-essential amino acid cysteine. To regenerate methionine from homocysteine, a methyl group must be transferred back to homocysteine via a remethylation pathway (Brosnan and Brosnan, 2006). In these pathways, a 13
24 methyl group from either betaine or 5-methly-THF is donated to homocysteine resulting in the formation of methionine. The culmination of the transmethylation and remethylation pathways is referred to the methionine cycle. The methionine cycle does not involve the catabolism of methionine, this occurs in the transsulfuration pathway. During the transsulfuration pathway homocysteine is converted first to cystathionine then to cysteine by cystathionine b-synthase (CBS) and cystathionine g-lyase (CGL) respectively (Brosnan and Brosnan, 2006). The transsulfuration pathway, unlike the methionine cycle, is an irreversible reaction resulting in the ability of cysteine to be synthesized from methionine but the inability of methionine to be synthesized from cysteine. 14
25 Figure 2.1 Metabolic pathways of sulphur amino acids. Brosnan and Brosnan (2006) 15
26 Since methionine is an α-amino acid two isometric forms, L-methionine and D-Methionine, exist. L-methionine is the naturally occurring isomer of methionine, while D isomers may be formed through acid, base or heat damage to L-isomers (Friedman and Gumbmann, 1984). Additionally, certain invertebrates and bacteria have the ability to biosynthesize D-methionine, while chemical synthesis of methionine results in an isometric mixture of D and L isomers (Gomes and Kumar, 2005). L-methionine is the biologically active form of methionine, therefore D-methionine must undergo a metabolic transformation to L-methionine before being used for protein synthesis or other metabolic processes. The conversion of D-methionine to L-methionine is a two-step conversion; D-methionine is first oxidized by D-amino oxidase resulting in an α-keto organic acid (2-keto-4-(methylthiol)butyric acid). The resulting α-keto organic acid is then aminated by the transfer of an amino group from glutamate resulting in L- methionine (Lewis, 2003). Just as D-methionine is required to be converted to the L-isomer before becoming biologically active, other synthetic methionine products such as the hydroxy analog of methionine, 2-hydroxy-4-(methylthio)butanoic acid (HMB or OH-Met), must undergo metabolic conversions. The chemical synthesis of HMB results in racemic mix of D and L isomers. D-HMB and L- HMB require different enzymes, a dehydrogenase and oxidase respectively, in order to convert the isometric analogues of methionine to an α-keto acid (2-keto-4-(methylthiol)butyric acid) (Baker 2006). Once converted the α-keto acid follows the same metabolic pathway as the conversion of D-methionine to L-methionine. With the exception of primates, most animals including fish and crustacea species are able to synthesise D-amino oxidase allowing for the conversion of D-methionine to L-methionine (Lewis, 2003; NRC 2011). 16
27 2.3 Methionine Requirements Methionine and cysteine are both sulphur-containing amino acids and are commonly referred collectively as total sulphur amino acid (TSAA) in respect to the requirement of an animal for these two amino acids. Cysteine is considered a non-essential amino acid as it can be synthetized from the essential amino acid methionine. Therefore, when a diet does not meet the requirement of an organism for cysteine, dietary methionine can be converted to cysteine. Similarly if level of dietary cysteine is sufficient to meet the cysteine requirement of an animal, lower levels of methionine may be included in the diet in comparison to a cysteine free diet as methionine will not be converted to cysteine (Baker, 2006). The ability of cysteine to spare methionine is supported by research conducted by Walton et al. (1982) whereby the methionine requirement of rainbow trout decreased when dietary cysteine content increased from 0% to 2%. In agreement with Walton et al. (1982), the sparing effect of cysteine methionine in rainbow trout has also been reported by Rumsey et al. (1983) and Cowey et al. (1992). Unlike the ability of methionine to fulfil or partially fulfil the requirement of cysteine, cysteine is not able to fulfill the methionine requirement of an organism. This is a result of the cysathionine synthase reaction being one directional preventing cysteine being converted back to homocysteine which through the methionine cycle can be converted to methionine (Brosnan and Brosnan, 2006). The ability of methionine to be converted to cysteine and help meet the cysteine requirement of an animal causes difficulties in estimating methionine requirements. Therefore, it common to see requirements for methionine and cysteine expressed as either Met+Cys or simply TSAA. Depending on the study and experimental conditions estimates of TSAA requirements of rainbow trout range from 0.8% to 1.1% of the diet on a dry matter basis, with TSAA requirement of 1.1% recommended by the NRC (Rumsey et al., 1983; Cowey et al., 1992; NRC, 2011). TSAA of common fresh and marine fish according to the NRC (2011) can be found in table 2.5. As methionine can be readily converted to 17
28 cysteine the inclusion of cysteine in a diet has a sparing effect for methionine. It has been estimated that cysteine can replace methionine at an efficiency of 40-60% in order meet the TSAA of many fish species (Wilson, 2002). Cysteine replacement value of methionine vary between species with 60% replacement for channel catfish (Harding et al. 1977), 44% for blue tilapia (Liou, 1989), 42% for rainbow trout (Kim et al., 1992) and 40% for both red drum and hybrid striped bass (Moon and Gatlin, 1991; Griffin et al., 1992). However, there is a limit to the extent in which cysteine can spare methionine, studies by Kim et al. (1992) found a breaking point at 0.3% dietary cysteine, whereby any additional amounts of cysteine supplementation did not result in further sparing of methionine. Common feed ingredients contain relatively high levels of cysteine resulting in most practical fish feeds meeting cysteine requirements, subsequently it is often more practical to focus on meeting the methionine requirement of an animal. In order to accurately estimate methionine requirement, diets must contain excess cysteine in order to prevent the conversion of methionine to cysteine and the resulting overestimation of methionine requirement. Estimates of methionine requirement for rainbow trout can be found in table 2.6 with estimates of methionine requirement range from 0.5% to 0.9% of the diet on a dry matter basis (Rodehutscord et al., 1995). Differences in estimates can be attributed to many factors including the model used to determine requirement, life stage the fish etc. Based on the available literature, the NRC (2011) recommends a dietary methionine requirement of 0.7% on a drymatter basis for rainbow trout. Although it may be easier to estimate and meet the total sulphur amino acid requirements of a fish it is recommended to formulate diets to meet the methionine and cysteine requirements of a fish separately, or alternatively meet the methionine and methionine + cysteine requirements in order to properly meet their nutritional requirements (Rodehutscord et al., 1995). 18
29 Table 2.5 Methionine + cysteine and methionine requirements of various finfish species. Species Methionine + cysteine (%) Methionine (%) Atlantic Salmon Common carp Tilapia Channel catfish Hybrid striped bass Rainbow trout Asian sea bass Cobia Red drum Yellowtail NRC (2011) Table 2.6 Estimates of methionine requirement of rainbow trout. Requirement (% diet) Reference 0.8 Cowey et al., Kim et al., Rodehutscord et al., Rumsey et al.,
30 Like all amino acids required for proteins synthesis, if methionine is deficient the rate of protein synthesis will be negatively affected resulting in poor growth and a reduced feed efficiency rate. Diets that are deficient in an essential amino acids, including methionine, result in decreased feed intake which is a concern in any animal production system. Although the mechanism behind this depression in feed intake is not completely understood several studies have tried to describe the underlying biochemical mechanisms. Harper and Rogers (1965) hypothesised that drastic difference in amino acid patterns between muscle and blood plasma invoke an appetite-regulating response from the animal as a result of being fed an unbalanced diet. Besides its effect on growth and feed intake methionine deficiency can also cause physiological changes to the organism. This includes the formation of disulfide bonds in the lens of the eye resulting in the opacity of the lens and referred to at a cataract, this condition is evident in many fish species including; rainbow trout (Oncorhynchus mykiss), Atlantic salmon (Salmo salar), lake trout (Salvelinus namaycush) and hybrid sea bass (Morone chrysops x M. saxatilis) (Poston et al., 1977; Keembiyehetty and Gatlin, 1993; Walton et al., 1982, Rumsey et al., 1983; Cowey et al., 1992). Rate of formation and severity of the cataract depends on the extent in which a diet is deficient in methionine, with highly deficient diets resulting in cataract formation within 3 weeks and mass mortalities occurring after 4 weeks in hybrid striped sea bass (Keembiyehetty and Gatlin, 1993). Cataracts are detrimental to fish vision and result in reduced feed intake which further reduces growth. In terms of the formation of cataracts, cysteine is a precursor of glutathione, which plays a role in preventing oxidative damage to the protein bound thiol groups in the lens of the eye (Ferrer et al., 1990; Cowey et al., 1992). Therefore, fish fed diets that do not meet the methionine or cysteine requirement of an animal may not be able protect against oxidation of methionine in the lens or be able to replace the oxidized methionine molecules resulting in the formation of disulfide bonds between molecules. The formation of these disulfide bonds leads to the insolubility of leans proteins resulting in a cloudy or opaque lens which is referred to as a cataract (Simmons et al., 1999). 20
31 Like diets deficient in methionine, diets that contain methionine in great excess of requirement of that animal may also result in depressed growth. Methionine is the most toxic of the amino acids that are required for protein synthesis, with reduced growth being seen in mammals as a result of being fed diets containing methionine in excess of two to three times the requirement level of the animal (Edmonds and Baker, 1987; Baker 2006). Similar results have been found in rainbow trout whereby fish fed diets containing % total methionine resulted in decreased growth (Poppi et al., 2011). Methionine toxicity is thought to be caused by the accumulation of S-adenosylmethionine (SAM), a metabolite of methionine, in the liver (Regina et al., 1993). Methionine toxicity can be alleviated through the addition of either supplemental glycine or serine. The addition of serine encourages the removal of SAM through the transulfuration pathway (Figure 2.1), while glycine facilities the catabolism of SAM (Baker, 2006). The accumulation of SAM in the liver causes visible histological damage resulting in hepatic dysfunction (Regina et al., 1993). Although high levels of methionine can be toxic to an animal it is not a common concern when formulating feeds. This is due to the relatively low methionine content of feed ingredients and the fact that in order to see adverse toxic effects on the animal the level of methionine in the diet would have to be higher than any practical feed formulation would contain. 2.4 Lysine Biochemistry & Metabolism Lysine is an essential α-amino acid with the chemical formula HO 2CCH(NH 3)(CH 2) 4NH 2. Lysine contains a positively charged ε-amino group, a primary amine, resulting in lysine commonly forming hydrogen bonds and having important structural roles in proteins. Lysine plays an important tertiary structural role in transmembrane proteins, the positively charged hydrophilic R group of lysine encourages contact with water, while the hydrophobic nature of the rest of the molecule encourages contact with lipid molecules in the transmembrane layer (Buxbaum, 2007). Along with its structural role in transmembrane proteins lysine can also be found abundantly in body proteins of animals. In 21
32 particular lysine and lysine metabolites are found in collagen, the main structural protein in various connective tissues, and the most abundant protein in mammals (Di Lullo et al., 2002). The stability of the collagen tissue is due to the cross-linking of aldehydes, either lysine or hydroxylysine derived aldehydes, between collagen fibres. Lysyl oxidase is the enzyme that is responsible for converting the amine side chain of lysine and hydroxylysine, a post-translational hydroxyl modification of lysine, into these crosslinking stabilizing aldehydes (Eyre et al., 1984). Beside the role that lysine plays in protein structure and protein synthesis, lysine also follows various important catabolic pathways. Lysine is ketogenic, meaning during catabolism of the carbon skeleton of the amino acid a ketone is produced in the form of Acetyl CoA. The degradation of lysine into Acetly CoA is a multi-step process facilitated by L-amino acid oxidase and specific aminotransferase enzymes (D Mello, 2003a). The main intermediates and associated enzymes of the degradation of lysine to Acetyl CoA can be found in figure 2.2. Acetly CoA can then enter the citric acid cycle which is the final common energy producing pathway for carbohydrates and lipids along with the carbon skeleton of amino acids. 22
33 Figure 2.2 Degradation of lysine to acetyl CoA with enzymes: 1 L-amino acid oxidase; 2, specific aminotransferase. D Mello (2003a) 23
34 As lysine contains an α-carbon two isomers, L-lysine and D-lysine, occur. As all amino acids must be in the biologically active L-configuration to be utilized by an organism D-isomers must undergo oxidative deamination to an α-keto acid analogue then undergo L-specific re-amination by an amino acid specific aminotransferase. However, no aminotransferases exist for lysine rendering D-lysine nutritionally inactive (D Mello, 2003a). Additionally, the ε-amino group of lysine is reactive in nature which causes lysine to be susceptible to both heat damage and non-enzymatic glycolyzation, resulting in the formation of Maillard reaction products (Moughan and Rutherfurd, 1996). During Maillard reactions, the carbonyl group of glucose and the ε-amino group of lysine react to form fructosyllysine (ε- DFL) (Moughan et al., 1996). Fructosyllysine undergoes acid hydrolysis to form pyridosine, regenerated lysine and furosine. However, once a feedstuff undergoes a Maillard reaction irreversible damage occurs to lysine resulting in decreased availability (Moughan and Rutherfurd, 1996; Moughan et al., 1996; Carpenter, 1960). The reactive ε-amino group of lysine and the inability of the body to transform D- lysine to L-lysine results in lysine commonly being the first limiting essential amino acid in feeds especially those formulated with high level of cereal grains or ingredients exposed to high temperature conditions. Additionally, conventional methods of amino acid analysis are unable to distinguish between reactive lysine and mallard products, leading to the overestimation of biologically available lysine in feeds that have being processed in high temperature and pressure conditions. 2.5 Lysine Requirements Lysine is an essential amino acid and therefore the requirement for this amino acid must be fulfilled through dietary intake. As lysine is bound as part of proteins the crude protein level of an ingredient will have an effect on the lysine content of that ingredient. For example, high quality fish meal containing 72% crude protein contains higher levels of lysine then wheat middlings which only contains 17% crude protein, 7.3% vs. 0.7% on a DM basis (NRC, 2011). However, lysine content also 24
35 varies between protein sources, even when comparing ingredients with similar levels of crude protein. Anchovy fish meal and corn gluten meal contain comparable levels of crude protein, 65% and 64% respectively, while the lysine content of fish meal (5.1%) is substantially higher than in corn gluten meal (1.1%). Additionally since lysine is susceptible to heat damage, due to the reactive nature of the ε-amino group, ingredients processed under high temperatures will also contain low levels of lysine. Since lysine is commonly the first limiting essential amino acid in diets containing high levels of plant proteins or processed under harsh conditions, extensive research has been on conducted on the estimated lysine requirement of most farmed animals, including many commercially important fish species (table 2.6). Based on these estimates, the NRC estimated the lysine requirement of rainbow trout to be about 2.4% (DM basis) (NRC, 2011). Estimates of lysine requirements of other commonly cultured fish species can also be found in table 2.7. Estimates of amino acid requirements are conducted under laboratory conditions, using semi-purified or purified feed ingredients with close to 100% digestibility. 25
36 Table 2.7 Estimates of lysine requirement for various fish species. Species Estimated lys Reference requirement (% of diet) Atlantic salmon 2.0% Anderson et al. (1993) 2.4% NRC (2011) Nile tilapia 1.4 % Santiago and Lovell (1988) % Furuya et al. (2004) 1.44% Furuya et al. (2006) 1.6% NRC (2011) Rainbow trout 1.9% Walton et al. (1984) % Wang et al. (2010) 1.9% Walton et al. (1986) 1.3% Kim et al. (1992) % Rodehutscord et al. (1997) % Cheng et al. (2003) % Encarnacao et al. (2004) 2.4% NRC (2011) Besides the negative impact that lysine deficiency has on growth performances of the fish (growth, feed efficiency), lysine deficiencies also result in damages to fish tissues. Along with reduced 26
37 performance, rainbow trout fed diets highly deficient in lysine resulted in a high degree of caudal fin erosion along with mortality rates of roughly 50% (Ketola, 1983). Similarly, Walton et al. (1984, 1986) reported fin erosion in rainbow trout fed lysine deficient diets but mortality rates of these fish were not significantly impacted. Assuming equal sanitary conditions and thus equal level of waterborne pathogens between studies, the differences in mortality rates may be attributed to degree of lysine deficiency between studies. Ketola (1983) fed highly deficient diets containing 0.7% lysine (DM basis) while Walton et al. (1984,1986) fed diets containing either 1.0% and 1.9% lysine (DM basis). Regardless, it is apparent that lysine deficiency has a large impact on both fish growth and health and highly deficient diets can result in high mortality rates. Amino acid antagonisms are structurally similar amino acids that have deleterious interactions with one another (D Mello, 2003b). An antagonism relationship exists between lysine and arginine and this relationship is credited for depressed growth in various animals when fed diets containing excess lysine or arginine (Jones, 1961; D Mello and Lewis 1970). Since lysine and arginine are structurally similar they are transported by the same amino acid carrier and competitive inhibition between these two amino acids exist (Kaushik and Fauconneau, 1984). Therefore, when an animal is fed a diet containing high levels of lysine while still meeting the arginine requirement, the animal may display symptoms of arginine deficiency. This is due to competition for shared amino acid carriers, rate of arginine absorption and transportation could be affected. The lysine-arginine antagonism is found in some mammals but is prevalent in avian species as they lack the ability to synthesize arginine (D Mello, 2003b). Studies conducted on fish species including channel catfish, European sea bass, and Japanese flounder did not display an effect of feeding excess lysine on growth or plasma arginine levels in these species (Robinson et al., 1981; Tibaldi et al., 1994; Alam et al., 2002). 27
38 2.6 Strategies for Meeting Essential Amino Acid Requirements Like in other animal production industries feed is a leading cost of production and in aquaculture accounts for between 50-60% of total production costs (Sinha et al., 2011). Therefore meeting the nutritional requirements of the fish in a cost-efficient manner is a primary concern in enabling aquaculture to be an economically sustainable industry. Common ingredients in fish feed formulations include plant and animal proteins along with by-products such as fish meal, poultry byproduct meal, meat and bone meal, soybean meal, soy protein concentrates and corn gluten meal. Byproduct meals along with plant proteins are commonly included in fish feed formulations as they are economical and shown to have relatively high nutritive value to fish (Tacon et al., 2006). However, many of these ingredients, especially certain plant proteins, contain low levels of certain essential amino acids. Feeds which include high levels of these ingredients may result in diets that are deficient in certain essential amino acids. Therefore, in cases whereby a diet is deficient in essential amino acids there are three general feed formulation strategies used to help meet the essential amino acid requirement of an animal: 1) Increase total protein content of the diet, 2) combine various protein sources with complementary amino acid profiles, 3) supplementation with crystalline (synthetic) amino acids (NRC, 2011). The simplest approach to help meet the essential amino acid requirement of an animal when a formulated diet is deficient in one or more essential amino acids is to further increase the protein level of that diet. As amino acids are bound in proteins by further increasing the protein level of the diet the quantity of amino acids in the diet also increases. In cases whereby a diet is slightly deficient in certain essential amino acids increasing the amount of protein can result in amino acid levels meeting the requirement of the animal as seen in figure 2.3. However, there are limitations to this approach as increasing the inclusion of a protein source that is highly deficient in a particular essential amino acid 28
39 may result in a diet with a very high crude protein content, yet still deficient in that particular essential amino acid. This is common in swine nutrition whereby corn and soybean based diets with high inclusion rates can still result in a lysine deficient diet (Smiricky-Tjardes et al., 2004). Along with the limitations associated with this technique, proteins often represent the most expensive ingredient in feeds. Therefore, the strategy of increasing crude protein level of a diet in order to meet the essential amino acid requirement of an animal may result in a feed that is overly expensive, in addition to the possibility of depressing feed intake of the animal which is associated with being fed a diet with an imbalanced amino acid profile. Furthermore, water quality issues arise when increasing crude protein content of the diet as greater proportions of nitrogen may be expelled as waste into the water. Subsequently other formulation strategies may be more economically feasible and promote better growth. Each source of protein has unique amino acid profiles with some protein sources containing low levels of some essential amino acids while another protein source may contain high levels of that particular essential amino acid. For example, soybean meal contains 2.2% lysine while blood meal contains 8.2% lysine. The basis of the second formulation strategy is to use multiple sources of protein with complementary amino acid profiles in order to meet all essential amino acid requirement of an animal. Figure 2.4 provides a visual representation of how a single protein source A is not able to provide sufficient lysine or arginine in order to meet the requirement of these amino acids for an animal but contains an excess of methionine. Protein source B contains low levels of methionine but contains high amounts of lysine and arginine, both these source of protein contain low levels of at least one essential amino acid, but when combined all essential amino acid requirements are met. The use of multiple protein sources is a common practise in aquaculture as decreasing reliance on fish meal had led to the use of multiple economical plant and by-product protein sources in feed formulations (Tacon and Metain, 2008). However limitations in this feed formulation technique exist. Concerns regarding high 29
40 levels of plant protein inclusion exist due to their high fibre and indigestible carbohydrate content and presence of anti-nutritional factors (Dabrowski et al., 1989; Refstie et al., 2000). Furthermore an increasingly wide variety of ingredients are being included in the formulation of compound fish feeds but many aren t properly characterized (Gatlin et al., 2007; Hardy, 2010; Sinha et al., 2011; Tacon and Metian, 2008). Ongoing research is being conducted to properly understand the nutritive value of these ingredients in the context of fish nutrition (Bureau, 2008; Gatlin et al., 2007). A final formulation strategy is the use of crystalline amino acids to supplement the amino acid profile of a diet. As seen in figure 2.5 if a diet is deficient in certain amino acids, for example lysine and arginine, synthetic lysine and arginine can be added in precise calculated amounts to the deficient diet in order to meet the requirement of the animal for these amino acid. Crystalline amino acids have been in use in the animal feed industry for over 50 years with the benefit of allowing greater flexibility in diet formulation. Economical ingredients can be used at higher inclusion rates as deficiencies in the amino acid profile of the resulting diet can be balanced by the addition of corresponding crystalline amino acids. Therefore, crystalline amino acids are becoming a key component in cost-effective diets. 30
41 Figure 2.3 Increasing crude protein content of a diet as a strategy for meeting essential amino acid requirements. NRC (2011) Figure 2.4 Use of multiple protein sources as a strategy for meeting essential amino acid requirements. NRC (2011) 31
42 Figure 2.5 Use of synthetic amino acids to supplement a deficient diet. NRC (2011) 32
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
number Done by Corrected by Doctor Dr.Diala
 number 32 Done by Mousa Salah Corrected by Bahaa Najjar Doctor Dr.Diala 1 P a g e In the last lecture we talked about the common processes between all amino acids which are: transamination, deamination,
number 32 Done by Mousa Salah Corrected by Bahaa Najjar Doctor Dr.Diala 1 P a g e In the last lecture we talked about the common processes between all amino acids which are: transamination, deamination,
Classification of amino acids: -
 Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
AMINO ACIDS NON-ESSENTIAL ESSENTIAL
 Edith Frederika Introduction A major component of food is PROTEIN The protein ingested as part of our diet are not the same protein required by the body Only 40 to 50 gr of protein is required by a normal
Edith Frederika Introduction A major component of food is PROTEIN The protein ingested as part of our diet are not the same protein required by the body Only 40 to 50 gr of protein is required by a normal
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Catabolism of Carbon skeletons of Amino acids. Amino acid metabolism
 Catabolism of Carbon skeletons of Amino acids Amino acid metabolism Carbon skeleton Carbon Skeleton a carbon skeleton is the internal structure of organic molecules. Carbon Arrangements The arrangement
Catabolism of Carbon skeletons of Amino acids Amino acid metabolism Carbon skeleton Carbon Skeleton a carbon skeleton is the internal structure of organic molecules. Carbon Arrangements The arrangement
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
استاذ الكيمياءالحيوية
 قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Amino acid metabolism
 Amino acid metabolism The important reaction commonly employed in the breakdown of an amino acid is always the removal of its -amino group. The product ammonia is excreted after conversion to urea or other
Amino acid metabolism The important reaction commonly employed in the breakdown of an amino acid is always the removal of its -amino group. The product ammonia is excreted after conversion to urea or other
AMINO ACID METABOLISM. Sri Widia A Jusman Dept. of Biochemistry & Molecular Biology FMUI
 AMINO ACID METABOLISM Sri Widia A Jusman Dept. of Biochemistry & Molecular Biology FMUI Amino acids derived from dietary protein absorbed from intestine through blood taken up by tissues used for biosynthesis
AMINO ACID METABOLISM Sri Widia A Jusman Dept. of Biochemistry & Molecular Biology FMUI Amino acids derived from dietary protein absorbed from intestine through blood taken up by tissues used for biosynthesis
Nitrogen Metabolism. Overview
 Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Amino acid Catabolism
 Enzymatic digestion of dietary proteins in gastrointestinal-tract. Amino acid Catabolism Amino acids: 1. There are 20 different amino acid, they are monomeric constituents of proteins 2. They act as precursors
Enzymatic digestion of dietary proteins in gastrointestinal-tract. Amino acid Catabolism Amino acids: 1. There are 20 different amino acid, they are monomeric constituents of proteins 2. They act as precursors
Amino acids. Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester,
 Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Metabolism of amino acids. Vladimíra Kvasnicová
 Metabolism of amino acids Vladimíra Kvasnicová Classification of proteinogenic AAs -metabolic point of view 1) biosynthesis in a human body nonessential (are synthesized) essential (must be present in
Metabolism of amino acids Vladimíra Kvasnicová Classification of proteinogenic AAs -metabolic point of view 1) biosynthesis in a human body nonessential (are synthesized) essential (must be present in
CHM333 LECTURE 6: 1/25/12 SPRING 2012 Professor Christine Hrycyna AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID:
 AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
Biomolecules Amino Acids & Protein Chemistry
 Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Short polymer. Dehydration removes a water molecule, forming a new bond. Longer polymer (a) Dehydration reaction in the synthesis of a polymer
 HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
Lecture 10 - Protein Turnover and Amino Acid Catabolism
 Lecture 10 - Protein Turnover and Amino Acid Catabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction 2 Proteins are degraded into amino acids. Protein
Lecture 10 - Protein Turnover and Amino Acid Catabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction 2 Proteins are degraded into amino acids. Protein
The Structure and Function of Macromolecules
 The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
Amino Acid Metabolism
 Amino Acid Metabolism Last Week Most of the Animal Kingdom = Lazy - Most higher organisms in the animal kindom don t bother to make all of the amino acids. - Instead, we eat things that make the essential
Amino Acid Metabolism Last Week Most of the Animal Kingdom = Lazy - Most higher organisms in the animal kindom don t bother to make all of the amino acids. - Instead, we eat things that make the essential
Nitrogen Metabolism. Pratt and Cornely Chapter 18
 Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5
 Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Nitrogen Metabolism. Overview
 Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out.
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
AMINO ACID METABOLISM
 AMINO ACID METABOLISM Synthesis of Urea in Liver The series of reactions that form urea is known as the Urea Cycle or the Krebs-Henseleit Cycle. The urea cycle operates only to eliminate excess nitrogen.
AMINO ACID METABOLISM Synthesis of Urea in Liver The series of reactions that form urea is known as the Urea Cycle or the Krebs-Henseleit Cycle. The urea cycle operates only to eliminate excess nitrogen.
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
(65 pts.) 27. (10 pts.) 28. (15 pts.) 29. (10 pts.) TOTAL (100 points) Moorpark College Chemistry 11 Spring Instructor: Professor Gopal
 Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Lipids: diverse group of hydrophobic molecules
 Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Amino acid metabolism II
 Amino acid metabolism II Fates of amino acid carbon skeleton degradation to common intermediates pyruvate, intermediates of citric acid cycle, acetyl-coa Glucogenic AA precursors of glucose - degradation
Amino acid metabolism II Fates of amino acid carbon skeleton degradation to common intermediates pyruvate, intermediates of citric acid cycle, acetyl-coa Glucogenic AA precursors of glucose - degradation
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay
 Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Four Classes of Biological Macromolecules. Biological Macromolecules. Lipids
 Biological Macromolecules Much larger than other par4cles found in cells Made up of smaller subunits Found in all cells Great diversity of func4ons Four Classes of Biological Macromolecules Lipids Polysaccharides
Biological Macromolecules Much larger than other par4cles found in cells Made up of smaller subunits Found in all cells Great diversity of func4ons Four Classes of Biological Macromolecules Lipids Polysaccharides
Biochemistry: A Short Course
 Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 30 Amino Acid Degradation and the Urea Cycle 2013 W. H. Freeman and Company Chapter 30 Outline Amino acids are obtained from the
Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 30 Amino Acid Degradation and the Urea Cycle 2013 W. H. Freeman and Company Chapter 30 Outline Amino acids are obtained from the
Chapter 5: Structure and Function of Macromolecules AP Biology 2011
 Chapter 5: Structure and Function of Macromolecules AP Biology 2011 1 Macromolecules Fig. 5.1 Carbohydrates Lipids Proteins Nucleic Acids Polymer - large molecule consisting of many similar building blocks
Chapter 5: Structure and Function of Macromolecules AP Biology 2011 1 Macromolecules Fig. 5.1 Carbohydrates Lipids Proteins Nucleic Acids Polymer - large molecule consisting of many similar building blocks
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
UNIT 2 Amino acids and Proteins
 UNIT 2 Amino acids and Proteins Significance of Proteins 1. Keep the cells and tissues growing, renewing and mending 2. Take part in some kinds of important physiological activities 3. Oxidation and supply
UNIT 2 Amino acids and Proteins Significance of Proteins 1. Keep the cells and tissues growing, renewing and mending 2. Take part in some kinds of important physiological activities 3. Oxidation and supply
Sustainable Fish Diets for the 21st Century using Soybean Protein. Paul B. Brown, Purdue University, West Lafayette, Indiana, USA
 Sustainable Fish Diets for the 21st Century using Soybean Protein Paul B. Brown, Purdue University, West Lafayette, Indiana, USA pb@purdue.edu *Introduction *If you want to grow an animal, you must provide
Sustainable Fish Diets for the 21st Century using Soybean Protein Paul B. Brown, Purdue University, West Lafayette, Indiana, USA pb@purdue.edu *Introduction *If you want to grow an animal, you must provide
Impact of Dietary Crude Protein, Synthetic Amino Acid and Keto Acid Formulation on Nitrogen Excretion
 International Journal of Poultry Science (8): 49-46, 04 ISSN 68-856 Asian Network for Scientific Information, 04 Impact of Dietary Crude Protein, Synthetic Amino Acid and Keto Acid Formulation on Nitrogen
International Journal of Poultry Science (8): 49-46, 04 ISSN 68-856 Asian Network for Scientific Information, 04 Impact of Dietary Crude Protein, Synthetic Amino Acid and Keto Acid Formulation on Nitrogen
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Glycolysis. Cellular Respiration
 Glucose is the preferred carbohydrate of cells. In solution, it can change from a linear chain to a ring. Energy is stored in the bonds of the carbohydrates. Breaking these bonds releases that energy.
Glucose is the preferred carbohydrate of cells. In solution, it can change from a linear chain to a ring. Energy is stored in the bonds of the carbohydrates. Breaking these bonds releases that energy.
Metabolic Classification of the Amino Acids
 Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
1.4. Lipids - Advanced
 1.4. Lipids - Advanced www.ck12.org In humans, triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of excess starches and other
1.4. Lipids - Advanced www.ck12.org In humans, triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of excess starches and other
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
CHAPTER 21: Amino Acids, Proteins, & Enzymes. General, Organic, & Biological Chemistry Janice Gorzynski Smith
 CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
Biochemistry: A Short Course
 Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 31 Amino Acid Synthesis 2013 W. H. Freeman and Company Chapter 31 Outline Although the atmosphere is approximately 80% nitrogen,
Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 31 Amino Acid Synthesis 2013 W. H. Freeman and Company Chapter 31 Outline Although the atmosphere is approximately 80% nitrogen,
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Lecture 11 - Biosynthesis of Amino Acids
 Lecture 11 - Biosynthesis of Amino Acids Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction Biosynthetic pathways for amino acids, nucleotides and lipids
Lecture 11 - Biosynthesis of Amino Acids Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction Biosynthetic pathways for amino acids, nucleotides and lipids
Amino Acid Oxidation and the Urea Cycle
 Amino Acid Oxidation and the Urea Cycle Amino Acids: Final class of biomolecules whose oxidation contributes significantly to the generation of energy Undergo oxidation in three metabolic circumstances
Amino Acid Oxidation and the Urea Cycle Amino Acids: Final class of biomolecules whose oxidation contributes significantly to the generation of energy Undergo oxidation in three metabolic circumstances
Macromolecules Structure and Function
 Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
PROTEIN METABOLISM: SPECIFIC WAYS OF AMINO ACIDS CATABOLISM AND SYNTHESIS
 PROTEIN METABOLISM: SPECIFIC WAYS OF AMINO ACIDS CATABOLISM AND SYNTHESIS SPECIFIC WAYS OF AMINO ACID CATABOLISM After removing of amino group the carbon skeletons of amino acids are transformed into metabolic
PROTEIN METABOLISM: SPECIFIC WAYS OF AMINO ACIDS CATABOLISM AND SYNTHESIS SPECIFIC WAYS OF AMINO ACID CATABOLISM After removing of amino group the carbon skeletons of amino acids are transformed into metabolic
THE ROLE OF RENDERED PRODUCTS IN AQUACULTURE FEEDS Dr. Jesse Trushenski
 THE ROLE OF RENDERED PRODUCTS IN AQUACULTURE FEEDS Dr. Jesse Trushenski Center for Fisheries Aquaculture & Aquatic Sciences Southern Illinois University Carbondale Carbondale, Illinois USA saluski@siu.edu
THE ROLE OF RENDERED PRODUCTS IN AQUACULTURE FEEDS Dr. Jesse Trushenski Center for Fisheries Aquaculture & Aquatic Sciences Southern Illinois University Carbondale Carbondale, Illinois USA saluski@siu.edu
I) Choose the best answer: 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine.
 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination # 5: Section Five May 7, Name: (print)
 Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
PROTEIN. By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM
 PROTEIN By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM OBJECTIVES OF THE LECTURE By the end of this lecture, student can: Define
PROTEIN By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM OBJECTIVES OF THE LECTURE By the end of this lecture, student can: Define
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
(30 pts.) 16. (24 pts.) 17. (20 pts.) 18. (16 pts.) 19. (5 pts.) 20. (5 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
Amino acid metabolism I
 Amino acid metabolism I Jana Novotná Department of the Medical Chemistry and Clinical Biochemistry The 2nd Faculty of Medicine, Charles Univ. Metabolic relationship of amino acids DIETARY PROTEINS GLYCOLYSIS
Amino acid metabolism I Jana Novotná Department of the Medical Chemistry and Clinical Biochemistry The 2nd Faculty of Medicine, Charles Univ. Metabolic relationship of amino acids DIETARY PROTEINS GLYCOLYSIS
AP Bio. Protiens Chapter 5 1
 Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
Human Biochemistry Option B
 Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
OPPORTUNITIES FOR RENDERED PRODUCTS IN AQUACULTURE Advancing science & industry through partnership
 OPPORTUNITIES FOR RENDERED PRODUCTS IN AQUACULTURE Advancing science & industry through partnership Jesse T. Trushenski CENTER FOR FISHERIES, AQUACULTURE, & AQUATIC SCIENCES THE WORLD IS HUNGRY CGIAR CCAFS
OPPORTUNITIES FOR RENDERED PRODUCTS IN AQUACULTURE Advancing science & industry through partnership Jesse T. Trushenski CENTER FOR FISHERIES, AQUACULTURE, & AQUATIC SCIENCES THE WORLD IS HUNGRY CGIAR CCAFS
Molecules of Life. Chapter 22. Great Idea: A cell s major parts are constructed from a few simple molecular building blocks 1
 Molecules of Life Chapter 22 Great Idea: A cell s major parts are constructed from a few simple molecular building blocks 1 Chapter Outline Organic Molecules Organic Chemistry Proteins: The Workhorses
Molecules of Life Chapter 22 Great Idea: A cell s major parts are constructed from a few simple molecular building blocks 1 Chapter Outline Organic Molecules Organic Chemistry Proteins: The Workhorses
Proteins and Amino Acids. Benjamin Caballero, MD, PhD Johns Hopkins University
 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Cells. Variation and Function of Cells
 Cells Variation and Function of Cells Plasma Membrane= the skin of a cell, it protects and nourishes the cell while communicating with other cells at the same time. Lipid means fat and they are hydrophobic
Cells Variation and Function of Cells Plasma Membrane= the skin of a cell, it protects and nourishes the cell while communicating with other cells at the same time. Lipid means fat and they are hydrophobic
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
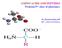 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
Amino Acids. Amino Acids. Fundamentals. While their name implies that amino acids are compounds that contain an NH. 3 and CO NH 3
 Fundamentals While their name implies that amino acids are compounds that contain an 2 group and a 2 group, these groups are actually present as 3 and 2 respectively. They are classified as α, β, γ, etc..
Fundamentals While their name implies that amino acids are compounds that contain an 2 group and a 2 group, these groups are actually present as 3 and 2 respectively. They are classified as α, β, γ, etc..
9/16/15. Properties of Water. Benefits of Water. More properties of water
 Properties of Water Solid/Liquid Density Water is densest at 4⁰C Ice floats Allows life under the ice Hydrogen bond Ice Hydrogen bonds are stable Liquid water Hydrogen bonds break and re-form Benefits
Properties of Water Solid/Liquid Density Water is densest at 4⁰C Ice floats Allows life under the ice Hydrogen bond Ice Hydrogen bonds are stable Liquid water Hydrogen bonds break and re-form Benefits
Amino acids. Ing. Petrová Jaroslava. Workshop on Official Controls of Feed AGR 46230, , Ankara. Turkey ÚKZÚZ - NRL RO Praha 1
 Amino acids Ing. Petrová Jaroslava Workshop on Official Controls of Feed AGR 46230, 6. 7. 12. 2011, Ankara. Turkey 6.12.2011 ÚKZÚZ - NRL RO Praha 1 Content of this presentation 1. Function of amino acids
Amino acids Ing. Petrová Jaroslava Workshop on Official Controls of Feed AGR 46230, 6. 7. 12. 2011, Ankara. Turkey 6.12.2011 ÚKZÚZ - NRL RO Praha 1 Content of this presentation 1. Function of amino acids
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
Bio 366: Biological Chemistry II Test #1, 100 points (7 pages)
 Bio 366: Biological Chemistry II Test #1, 100 points (7 pages) READ THIS: Take a numbered test and sit in the seat with that number on it. Remove the numbered sticker from the desk, and stick it on the
Bio 366: Biological Chemistry II Test #1, 100 points (7 pages) READ THIS: Take a numbered test and sit in the seat with that number on it. Remove the numbered sticker from the desk, and stick it on the
FISH NUTRITION 101 Feeds & Feeding Strategies for Aquaculture Dr. Jesse Trushenski
 FISH NUTRITION 101 Feeds & Feeding Strategies for Aquaculture Dr. Jesse Trushenski Center for Fisheries Aquaculture & Aquatic Sciences Southern Illinois University Carbondale Carbondale, Illinois USA saluski@siu.edu
FISH NUTRITION 101 Feeds & Feeding Strategies for Aquaculture Dr. Jesse Trushenski Center for Fisheries Aquaculture & Aquatic Sciences Southern Illinois University Carbondale Carbondale, Illinois USA saluski@siu.edu
The building blocks of life.
 The building blocks of life. The 4 Major Organic Biomolecules The large molecules (biomolecules OR polymers) are formed when smaller building blocks (monomers) bond covalently. via anabolism Small molecules
The building blocks of life. The 4 Major Organic Biomolecules The large molecules (biomolecules OR polymers) are formed when smaller building blocks (monomers) bond covalently. via anabolism Small molecules
Maha AbuAjamieh. Tamara Wahbeh. Mamoon Ahram
 12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds.
 Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
Lecture 17: Nitrogen metabolism 1. Urea cycle detoxification of NH 3 2. Amino acid degradation
 Lecture 17: Nitrogen metabolism 1. Urea cycle detoxification of NH 3 2. Amino acid degradation Reference material Biochemistry 4 th edition, Mathews, Van Holde, Appling, Anthony Cahill. Pearson ISBN:978
Lecture 17: Nitrogen metabolism 1. Urea cycle detoxification of NH 3 2. Amino acid degradation Reference material Biochemistry 4 th edition, Mathews, Van Holde, Appling, Anthony Cahill. Pearson ISBN:978
Name. The following exam contains 44 questions, valued at 2.6 points/question. 2. Which of the following is not a principal use of proteins?
 Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
CHAPTER 3. Carbon & the Molecular Diversity of Life
 CHAPTER 3 Carbon & the Molecular Diversity of Life Carbon: The Organic Element Compounds that are synthesized by cells and contain carbon are organic So what is inorganic? Why are carbon compounds so prevalent?
CHAPTER 3 Carbon & the Molecular Diversity of Life Carbon: The Organic Element Compounds that are synthesized by cells and contain carbon are organic So what is inorganic? Why are carbon compounds so prevalent?
Adverse Effects of Amino Acids
 Adverse Effects of Amino Acids AA is assumed that any surplus ingested by animals is disposed of without adverse effects. The ruminant has endowed a detoxification mechanisms by microbial metabolism of
Adverse Effects of Amino Acids AA is assumed that any surplus ingested by animals is disposed of without adverse effects. The ruminant has endowed a detoxification mechanisms by microbial metabolism of
Biochemistry 2 Recita0on Amino Acid Metabolism
 Biochemistry 2 Recita0on Amino Acid Metabolism 04-20- 2015 Glutamine and Glutamate as key entry points for NH 4 + Amino acid catabolism Glutamine synthetase enables toxic NH 4 + to combine with glutamate
Biochemistry 2 Recita0on Amino Acid Metabolism 04-20- 2015 Glutamine and Glutamate as key entry points for NH 4 + Amino acid catabolism Glutamine synthetase enables toxic NH 4 + to combine with glutamate
Chapter 20 and GHW#10 Questions. Proteins
 Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
