Amino Acid Transporters Involved in Luminal Transport of Mercuric Conjugates of Cysteine in Rabbit Proximal Tubule
|
|
|
- Harold Dixon
- 5 years ago
- Views:
Transcription
1 /01/ $3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 298, No. 2 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 3568/ JPET 298: , 2001 Printed in U.S.A. Amino Acid Transporters Involved in Luminal Transport of Mercuric Conjugates of Cysteine in Rabbit Proximal Tubule VERNON T. CANNON, RUDOLFS K. ZALUPS, and DELON W. BARFUSS Biology Department, Georgia State University, Atlanta, Georgia (V.T.C., D.W.B.); and Division of Basic Medical Sciences, Mercer University School of Medicine, Macon, Georgia (R.K.Z.) Received November 20, 2000; accepted April 17, 2001 This paper is available online at ABSTRACT The primary aim of the present study was to test the hypothesis that amino acid transport systems are involved in absorptive transport of dicysteinylmercury (cysteine-hg-cysteine). Luminal disappearance flux [J D, fmol min 1 (mm tubular length) 1 ] of inorganic mercury (Hg 2 ), in the form of dicysteinylmercury, was measured in isolated perfused S 2 segments with various amino acids or amino acid analogs in the luminal compartment under one of two conditions, in the presence or absence of Na. The control perfusion fluid contained 20 M dicysteinylmercury. Replacing Na in both the bathing and perfusing solutions with N-methyl-D-glucamine reduced the J D of Hg 2 by about 40%. Nine amino acids and two amino acid analogs were coperfused individually (at millimolar concentrations) with dicysteinylmercury. The amino acids and amino acid analogs that had the greatest effect on the J D of Hg 2 were L-cystine, L-serine, L- histidine, L-tryptophan, and 2-( )-endoamino-bicycloheptane-2-carboxylic acid. The greatest reduction (76%) in the total J D of Hg 2 occurred when L-cystine was coperfused with dicysteinylmercury in the presence of Na. Overall, the current findings indicate that Hg 2 is transported from the lumen into proximal tubular epithelial cells via amino acid transporters that recognize dicysteinylmercury. In addition, the data indicate that multiple amino acid transporters are involved in the luminal uptake of dicysteinylmercury, including the Na -dependent low-affinity L-cystine, B 0, and ASC systems and the Na -independent L-system. Furthermore, the transport data obtained when L-cystine was added to the luminal fluid indicate strongly that dicysteinylmercury is likely transported as a molecular homolog of L-cystine. Recent findings from whole animal (Zalups and Barfuss, 1996), luminal membrane-vesicle (Zalups and Lash, 1997), and isolated-perfused-tubule experiments (Cannon et all, 2000) indicate that the primary luminal mechanism by which inorganic mercuric ions gain access to the cytoplasm of proximal tubular epithelial cells is by being transported as a mercuric conjugate of L-cysteine. Based on the bonding characteristics of mercuric ions (Hughes, 1957; Rabenstein, 1989; Ballatori, 1991; Zalups and Lash, 1994) and the thermodynamic stability of mercuric conjugates of sulfhydryl-containing molecules in aqueous solution (Rabenstein, 1989), it has been postulated that the primary mercuric conjugate of L- cysteine that is transported at the luminal plasma membrane is dicysteinylmercury (cysteine-hg-cysteine). Since the mercuric conjugate dicysteinylmercury is structurally similar to the amino acid L-cystine (cysteine-cysteine), we had hypothesized previously that one or more of the amino acid This study was supported by grants from the National Institutes of Environmental Health Sciences (ES to R.K.Z. and D.W.B. and ES to R.K.Z.). Vernon Cannon was supported by a graduate student minority supplement to Grant ES05157 awarded by the National Institutes of Environmental Health Sciences. transport systems that transports L-cystine also transports dicysteinylmercury. Recent findings from isolated perfused tubule experiments of Cannon et al. (2000) strongly support this hypothesis. Since the majority of amino acid transport systems are not highly specific for any one particular amino acid, it is reasonable to postulate that mercuric conjugates of L-cysteine may also be transported by amino acid transport mechanisms that do not transport L-cystine. These include both Na -dependent and Na -independent transport systems. Some of the better described Na -dependent transporters include system-a, system-asc, system-b 0, and system-b 0,. System A is found in various organs and is involved in the transport of most dipolar amino acids (Hammerman and Sacktor, 1977). Amino acids or amino acid analogs transported by system-a include L-glycine, L-proline, and -methylamino-isobutyric acid (MeAIB) (Mircheff et al., 1982; Tate et al., 1989). Consequently, these compounds can be used as competitive inhibitors of this transporter. System-ASC is also found in various organs and is known to transport L- serine, L-alanine, and L-cysteine, all of which can serve as effective competitive inhibitors. In addition, system-asc has ABBREVIATIONS: MeAIB, -methylamino-isobutyric acid; BCH, 2-( )-endoamino-bicycloheptane-2-carboxylic acid; J D, luminal disappearance flux, fmol min 1 (mm tubular length) 1 ; APM, artificial perfusion medium. 780
2 Renal Transport of Dicysteinylmercury 781 been implicated in the transport of cationic or protonated anionic amino acids (Hammerman and Sacktor, 1977; Kragh- Hansen and Sheikh, 1984). System-B 0 is similar to system- ASC in its location and transport specificity, except it has a higher affinity for the larger neutral amino acids than system-asc. System-B 0,, which has been localized in oocytes, blastocysts, and the luminal plasma membrane of enterocytes and renal proximal tubular epithelial cells, is involved in the transport of a wide range of amino acids, including the amino acids, L-alanine, L-valine, L-tryptophan, and L-lysine (Boerner et al., 1986). There are at least two primary Na -independent amino acid transport systems reported to be present in the basolateral (Pineda et al., 1999) and luminal membranes of the proximal tubule, respectively. These are system-l and system-b 0,. System-L is located in a variety of organ systems and is involved in the transport of amino acids with large bulky hydrophobic side chains. Examples include L-phenylalanine, L-tryptophan, and the bicyclic amino acid analog 2-( )-endoamino-bicycloheptane-2-carboxylic acid (BCH) (Hammerman and Sacktor, 1977; Deves and Boyd, 1998). System-b 0, also transports a wide variety of amino acids. Although system-b 0, is similar to system-b 0,, it appears to discriminate against amino acids having less extensively branched R-groups attached to the -carbon. This system has a high affinity for neutral and cationic amino acids, and has been shown to transport L-lysine and L-cystine (Boerner et al., 1986). The primary objective of the present study was to test the hypothesis that known amino acid transport mechanisms are involved in the absorptive transport of dicysteinylmercury in pars recta (S 2 ) segments of the rabbit proximal tubule. The strategy used in this study was to coperfuse isolated S 2 segments through the lumen with dicysteinylmercury in the presence or absence of significantly higher concentrations of various amino acids or analogs that are transported by Na - dependent and Na -independent amino acid transport systems. This allowed us to determine whether these compounds inhibit the luminal disappearance flux (J D )ofhg 2 in the form of dicysteinylmercury. It was assumed that significant reductions in the J D of Hg 2 induced by the presence of an amino acid or amino acid analog would provide evidence implicating a role of specific amino acid transport systems in the lumen-to-cell and/or cell-to-bath transport of dicysteinylmercury in proximal tubular epithelial cells. Materials and Methods Hypothesis and Experimental Design The primary hypothesis tested in the current study is that the mercuric conjugate dicysteinylmercury is transported across the luminal membrane into the epithelial cells lining S 2 segments of the rabbit proximal tubule via one or more amino acid transport systems. To test this hypothesis, S 2 segments of the rabbit proximal tubule were perfused with a modified Ringer s solution [artificial perfusion medium (APM)] containing a 4:1 ratio of L-cysteine to Hg 2 to ensure the formation of the mercuric conjugate dicysteinylmercury (at a concentration of 20 M). The luminal disappearance flux (J D )ofhg 2, presumably in the form of dicysteinylmercury, was measured with and without purported inhibitory substrates of various amino acid transporters in the perfusing medium. To ensure that the potential inhibitor (amino acid or amino acid analog) was at a great enough concentration to compete with dicysteinylmercury for the transport site, a 50 to 250:1 ratio of inhibitor to dicysteinylmercury was maintained. Perfusion was carried out under two conditions, in the presence or complete absence of Na in the perfusing and bathing solutions. This allowed us to assess the role of Na - dependent versus Na -independent transporters in the uptake of the putative substrate dicysteinylmercury. Of necessity, we assumed that a decrease in J D of Hg 2 from the luminal fluid that occurred in the presence an amino acid or amino acid analog in the luminal fluid was the result of competitive inhibition. It is not possible to perfuse segments of the proximal tubule with a great enough range of concentrations of dicysteinylmercury to establish a change of J max or K m, which is required to delineate between competitive and noncompetitive inhibition. This limitation is the result of the low specific activity of 203 Hg 2 and the fact that higher concentrations (above 100 M) of dicysteinylmercury induce toxic effects in the perfused tubule. Consequently, we have assumed that any inhibition is competitive unless other investigators have reported noncompetitive inhibition by the particular amino acid or amino acid analog, as is the case for L-lysine. Note: Notwithstanding the fact that mercuric ions can bind to various nucleophilic groups, the affinity constant for bonding to thiolate anions is on the order of to 10 20, whereas the affinity constants for bonding to oxygen- or nitrogen-containing ligands, such as carbonyl groups or amino groups, are about 10 orders of magnitude lower (Hughes, 1957). When mercuric ions and at least a 2-fold greater concentration of small thiol-containing molecules are present in aqueous solution, one can predict with high probability [based on the 13 C NMR findings of Rabenstein (1989)] that each mercuric ion will form a thermodynamically stable linear II coordinate covalent bond with the thiol group of two molecules of the respective thiolcontaining compound. Thus, addition of a 4-fold higher concentration of cysteine (relative to the concentration of inorganic mercury) ensures the formation of thermodynamically stable linear II coordinate covalent complexes between each mercuric ion and two molecules of cysteine. Moreover, the bonding characteristics of mercuric ions (Hughes, 1957; Rabenstein, 1989) predict that they would remain bonded to the thiol group of cysteine, even in the presence of low millimolar concentrations of other nonthiol-containing amino acids or amino acid analogs, which provide a substantial pool of nucleophilic functional groups for mercuric ions to bind. Assessment of Role of Na -Dependent Amino Acid Transporters System-A. To determine whether system-a is capable of transporting dicysteinylmercury into proximal tubular cells across the luminal membrane, 3 mm L-proline or L-MeAIB were coperfused individually with 20 M Hg 2 and 80 M L-cysteine. System-ASC and B 0. The potential role of system-asc and/or system-b 0 in the luminal uptake of dicysteinylmercury was assessed in experiments where 3 mm L-serine was coperfused with 20 M Hg 2 and 80 M L-cysteine System-B 0,. Proximal tubular segments were perfused with 3 mm L-tryptophan, L-lysine, or L-valine with 20 M Hg 2 and 80 M L-cysteine to determine whether system-b 0, is capable of transporting dicysteinylmercury. Cystine Transporters. Assessment of a specific putative, lowaffinity Na -dependent L-cystine amino acid transport system in the luminal disappearance of dicysteinylmercury was done by coperfusing 1 mm L-cystine with 20 M Hg 2 and 80 M L-cysteine. Effect of D-Enantiomer of Cystine. To determine whether the luminal transport of dicysteinylmercury is stereospecific, S 2 segments were perfused through the lumen with 20 M Hg 2,80 M L-cysteine, and 1 mm D-cystine. Additional control experiments were carried out in tubules perfused through the lumen with 20 MHg 2, 80 M L-cysteine with or without 1 mm L-cystine. These experiments were carried at the end of all the other transport experiments. Consequently, additional control data were needed to maintain internal consistency.
3 782 Cannon et al. Assessment of Role of Na -Independent Amino Acid Transporters In these experiments, Na in both perfusing and bathing solutions was replaced with N-methyl-D-glucamine. System-L. The potential role of system-l in the luminal disappearance flux of dicysteinylmercury was assessed by coperfusing 20 M Hg 2 and 80 M L-cysteine with 3 mm L-histidine, 3 mm L- tryptophan, 3 mm cycloleucine, 5 mm L-phenylalanine, or 5 mm BCH. System-b 0,. Coperfusion of 20 M Hg 2 and 80 M L-cysteine with either 3 mm L-lysine or L-cystine was carried out to assess the potential role of system-b 0, in transporting dicysteinylmercury from the luminal fluid into the epithelial cells lining the pars recta of the proximal tubule. Animals. Female, New Zealand White, specific pathogen-free rabbits (Myrtle s Rabbitry, Inc. Farm, Thompson Station, TN) were used in the present study. Prior to experimentation, the rabbits were maintained on regular rabbit chow and given water ad libitum. All experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Composition of Perfusing and Bathing Solutions. In all experiments, the perfusing and bathing solutions consisted of simple electrolyte solutions. The perfusing solution (APM) contained the following: 140 mm Na, 140 mm Cl,5mMK, 2.5 mm Ca 2, 1.2 mm Mg 2, 1.2 mm SO 2 4, 2 mm HPO 2 4 /H 2 PO 4,1mMD-glucose, and 0.5 mm glutamine. ph was adjusted to 7.4 with 1 M NaOH. To evaluate cytotoxicity of inorganic mercury, we placed the vital dye FD&C green No.3 (809 Da) in the perfusate at a concentration of 250 nm. Final osmolality was adjusted to 290 mosmol kg 1 H 2 O, with doubly distilled and deionized water. L-[ 3 H]glucose (50 mci ml 1, 58.8 mci mg 1 ) (34 M) was used as a volume marker in all experiments and was added to the perfusing solution only. The concentration of Hg 2 (see note below) in the perfusing solution was 20 M in all experiments. All perfusing solutions containing Hg 2 also contained radioactive mercuric ions ( 203 Hg 2, 33.6 mci mg 1 ). When transport was studied in the presence of Na, the control perfusing solution was the artificial perfusing medium to which L-proline (3.0 mm), L-valine (3.0 mm), MeAIB (5.0 mm), BCH (5 mm), L-serine (3 mm), L-tryptophan (3 mm), L-phenylalanine (5.0 mm), or L-lysine (3.0 mm) was added. In all experiments, the bathing solution was the Na -containing APM without the vital dye added. In experiments where transport was studied under Na -independent conditions, Na in both the perfusing and bathing solutions was replaced with corresponding amounts of N-methyl-D-glucamine. Dissection Solution. The tubular dissection solution was a sucrose/phosphate buffer: 125 mm sucrose, 13.3 mm anhydrous monosodium dihydrogen phosphate, and 56 mm anhydrous disodium monohydrogen phosphate. The ph was adjusted to 7.4 with NaOH or HCl. The osmolarity was adjusted to 290 mosm/kg of water by adding water or NaCl. Chemicals. All other chemicals were obtained from Sigma (St. Louis, MO), unless otherwise noted. The isotope 203 Hg 2, in the form of mercuric chloride, was obtained from Buffalo Materials Corporation (Buffalo, NY). L-[ 3 H]Glucose (14.6 Ci mmol 1, 1 mci ml 1 ) was obtained from PerkinElmer Instruments (Shelton, CT). Obtaining, Identifying, and Perfusing S 2 Segments of Proximal Tubule. The methods used for obtaining, identifying, and perfusing each tubule on the day of experimentation were the same as those we have described previously (Zalups et al., 1991; Zalups and Barfuss, 1996). Collecting Samples. To measure the J D [fmol min 1 (mm tubule length) 1 ]ofhg 2 from the luminal compartment, three samples (collectates) of luminal fluid exiting a perfused tubular segment were collected from each perfused tubule. The time required to fill the constant volume pipette ( 60 nl) was used to calculate the collection rate (nl min 1 ). Each collectate sample was added to 8 ml of scintillation fluid (Opti-Fluor; Packard, Meriden, CT). To measure the rate of lumen-to-bath leak [fmol min 1 (mm tubule length) 1 ] of the volume marker L-[ 3 H]glucose, the aspirated bathing solution from the flow-through bath (0.3 ml) was collected at the rate of 0.25 ml min 1 into 20-ml scintillation vials at 5-min intervals. To each vial, 8 ml of scintillation fluid (Opti-Fluor; Packard) was added. The collectate and bathing fluid samples were then counted in a Beckman 5800 scintillation counter to quantify of the amount 3 H and 203 Hg present in each sample using standard isotopic methods. Assessment of Cellular and Tubular Pathology. During each experiment, the perfused tubule was observed microscopically during the entire perfusion process to detect any pathology. Typical pathological changes detected in S 2 segments of the proximal tubule exposed to inorganic mercury include cellular swelling, cytoplasmic vacuolization, shedding of brush-border membrane (blebbing) of the apical plasma membrane, and cellular uptake of the vital dye FD&C green. Calculations. The calculations used to determine rates of luminal disappearance flux (J D )ofhg 2 and lumen-to-bath leak of the volume maker (L-[ 3 H]glucose) are the same as those described previously (Zalups and Barfuss, 1996). Statistical Analysis. A minimum of four tubules was perfused under each experimental condition. Moreover, data for each parameter assessed were obtained from tubular segments isolated from at least two animals. In each perfused tubule, three or more measurements of the J D of Hg 2 were averaged. The mean values for J D from each tubule were used to compute the overall mean and standard error under each experimental condition. Data were first subjected to the Kolmogorov-Smirnov test for normality and then the Levene s test of homogeneity of variance. If both tests were not statistically significant (at P 0.05) a one-way analysis of variance and Tukey s honestly significant difference post hoc test was performed with a significance level set at P If a set of data failed the normality test or the test for homogeneity of variance, the nonparametric Kruskal-Wallis analysis of variance by ranks, followed by a Mann- Whitney U test analysis was performed with the level of significance set at P Results Effect of Various Amino Acids on Na -Dependent Transport of Hg 2 Control data for the experiments designed to assess inhibition of the J D of dicysteinylmercury under Na -dependent conditions were obtained from tubular segments perfused with the APM containing 20 M Hg 2 and 80 M L-cysteine. During 30 min of perfusion, the J D of Hg 2 averaged approximately 102 fmol min 1 (mm tubular length) 1 (Fig. 1). To assess whether amino acids transported by systems-a and/or B 0, are involved in the luminal transport of dicysteinylmercury, 3 mm L-proline, 3 mm L-valine, or 5 mm MeAIB was added to the control perfusate. The presence of each of these amino acids in the perfusate did not have a significant effect on the J D of Hg 2 (Fig. 1). Additionally, L-proline and L-valine had no effect on the concentration of Hg 2 in the collectate, while the presence of MeAIB in the perfusate caused a 250% increase in the concentration of Hg 2 in the collectate (Table 1). The mean J D of Hg 2 (in the form of dicysteinylmercury) was reduced markedly when either 3 mm L-lysine or 3 mm L-serine was present in the control perfusion solution (Fig. 2). More specifically, addition of 3 mm L-lysine or 3 mm L-serine to the perfusate caused an approximate 52 or 53% decrease in the J D of Hg 2, respectively. The concentration of Hg 2 in
4 Renal Transport of Dicysteinylmercury 783 Fig. 1. Luminal disappearance flux (J D )ofhg 2 in isolated S 2 segments of the rabbit proximal tubule perfused with 20 M Hg 2 and 80 M L-cysteine, with or without 3.0 mm L-proline, 3.0 mm L-valine, or 5.0 mm MeAIB in the perfusing solution. All tubules were perfused and bathed with solutions containing 140 mm Na. Each column represents the mean S.E. obtained from at least four perfused tubular S 2 segments., significantly different (P 0.05) from the mean value obtained from the control tubules perfused with 20 M Hg 2 and 80 M cysteine without any additional amino acids or amino acid analogs in the perfusing solution. TABLE 1 Concentration of Hg 2 in collectate, leak rate of L-[ 3 H]glucose, and visual toxicity data from S 2 segments of the rabbit proximal tubule perfused with 20 M Cys-Hg-Cys (20 M Hg 2 and 80 M L-cysteine) alone (control) or with various potential competitive inhibitors in the presence of 140 mm Na Perfusion and bathing solutions contained 140 mm Na. Values are mean S.E. for at least four tubules. These data supplement the data shown in Figs Group Collectate Concentration of Hg 2 M Intercellular Leak of L-[ 3 H]glucose fmol min 1 mm 1 Toxic Effects Control None L-Valine (3 mm) None L-Proline (3 mm) None MeAIB (3 mm) * None BCH (5 mm) * None L-Lysine (3 mm) * None L-Serine (3 mm) * None Cycloleucine (3 mm) * None L-Tryptophan (3 mm) * None L-Phenylalanine (5 mm) * None L-Cystine (1 mm) * None * Significantly different (P 0.05) from the mean value obtained from the control tubules. Significantly different (P 0.05) from the mean value obtained from tubules not perfused with Hg 2. The level of leak in control tubules is fmol min 1 (mm tubule length 1 ) (Barfuss and Schafer, 1981). the samples of collectate increased by 290 and 345%, respectively, when L-lysine or L-serine were coperfused with dicysteinylmercury (Table 1). These particular experiments were designed to determine whether the amino acids transported by systems-asc, B 0,B 0,, and/or b 0, are involved with the luminal transport of dicysteinylmercury. Relative to control values, significant decreases in the J D of Hg 2 were detected in S 2 segments perfused through the lumen with 5 mm BCH (38% decrease), 5 mm cycloleucine (57% decrease), 3 mm L-tryptophan (62% decrease), or 5 mm L-phenylalanine (65% decrease) (Fig. 3). Among these conditions evaluated, the J D of Hg 2 was lowest in the tubules perfused with L-phenylalanine and was highest in tubules perfused with BCH. The percentage of change in the concentration of Hg 2 in the samples of collectate increased by 240, 330, 360, and 470%, respectively, when BCH, cycloleucine, L-tryptophan, or L-phenylalanine was present in the perfusate (Table 1). The effect of these amino acids on the J D of Hg 2 was examined to determine whether the amino acids transported System-L participates in the absorptive transport of dicysteinylmercury. Among the experiments in which both perfusing and bathing solutions contained 140 mm Na, the greatest effect on the J D of Hg 2 was detected between the group of tubules perfused with 1 mm L-cystine and the group of control tubules (Fig. 4). The J D of Hg 2 in the tubules perfused with 1 mm L-cystine averaged approximately 25 fmol min 1 (mm tubular length) 1, which is 76% lower than the average J D of Hg 2 in the control tubules. In addition, the concentration of Hg 2 in the samples of collectate from the tubules perfused with 1 mm L-cystine was 470% greater than that in the samples of collectate from the control tubules (Table 1). These experiments were designed to determine whether similarity in molecular homology between dicysteinylmercury and the amino acid cystine plays an important role in the transport of dicysteinylmercury by transportproteins involved in proximal tubular absorption of cystine. To determine whether the effect on L-cystine on the luminal uptake of dicysteinylmercury is stereospecific, the effect of D- cystine on the luminal uptake of dicysteinylmercury was examined. With 140 mm Na present in the luminal and basolateral fluid compartments, coperfusing S 2 segments with 20 MHg 2 and 80 M L-cysteine with 1 mm D-cystine did not result in a significant reduction in the J D of Hg 2 (in the form of dicysteinylmercury). The transport data obtained from the separate set of control tubules (matched for these experiments) confirmed that 1 mm L-cystine inhibited the J D of dicysteinylmercury to a
5 784 Cannon et al. Fig. 2. Luminal disappearance flux (J D )ofhg 2 in isolated S 2 segments of the rabbit proximal tubule perfused with 20 M Hg 2 and 80 M L-cysteine, with or without 3.0 mm L-lysine or 3.0 mm L-serine in the perfusing solution. All tubules were perfused and bathed with solutions containing 140 mm Na. Each column represents the mean S.E. obtained from at least four perfused tubular S 2 segments., significantly different (P 0.05) from the mean value obtained from the control tubules perfused with 20 M Hg 2 and 80 M L-cysteine without any additional amino acids or amino acid analogs in the perfusing solution. greater extent than any other amino acid (by 85% in these additional tubules). Thus, two independent series of experiments indicate that L-cystine is a very effective inhibitor of the luminal uptake of inorganic mercury (in the form of dicysteinylmercury) in proximal tubular segments. Cellular Toxicity with Na Being Present in Both Perfusing and Bathing Solutions. In all of the aforementioned experiments, no visual evidence of acute cellular toxicity was detected. However, lumen-to-bath leak of L-[ 3 H]glucose was slightly but significantly greater (2 3 times) in four groups (control, MeAIB, BCH, and L-phenylalanine) than that in normal tubules not perfused with Hg 2 [ 2.5 fmol min 1 (mm tubule length) 1 ] (Barfuss and Schafer, 1981). By contrast, the presence of L-valine, L-proline, L-lysine, L-serine, cycloleucine, L-tryptophan, or L-cystine in the luminal compartment had no effect on the rate of leak of L-[ 3 H]glucose (Table 1). Effect of Various Amino Acids on Na -Independent Transport of Hg 2 Replacement of Na in both the perfusing and bathing solutions with N-methyl-D-glucamine caused a significant reduction (40%) in J D of Hg 2 (Fig. 5). The concentration of Fig. 3. Luminal disappearance flux (J D )ofhg 2 in isolated S 2 segments of the rabbit proximal tubule perfused with 20 M Hg 2 and 80 M L-cysteine, with or without 5.0 mm BCH, 5.0 mm cycloleucine, 3.0 mm L-tryptophan, or 3.0 mm L-phenylalanine in the perfusing solution. All tubules were perfused and bathed with solutions containing 140 mm Na. Each column represents the mean S.E. obtained from at least four perfused tubular S 2 segments., significantly different (P 0.05) from the mean value obtained from the control tubules perfused with 20 M Hg 2 and 80 M cysteine without any additional amino acids or amino acid analogs in the perfusing solution.
6 Renal Transport of Dicysteinylmercury 785 Fig. 4. Luminal disappearance flux (J D )ofhg 2 in isolated S 2 segments of the rabbit proximal tubule perfused with 20 M Hg 2 and 80 M L-cysteine, with or without 1.0 mm L-cystine in the perfusing solution. All tubules were perfused and bathed with solutions containing 140 mm Na. Each column represents the mean S.E. obtained from at least four perfused tubular S 2 segments., significantly different (P 0.05) from the mean value obtained from the control tubules perfused with 20 M Hg 2 and 80 M L-cysteine without any additional amino acids or amino acid analogs in the perfusing solution. Hg 2 in the samples of collectate increased by 260%, compared with the concentration of Hg 2 in the samples of collectate from the tubules perfused in the presence of Na (control group in Table 1). Effects of L-Lysine. After Na had been replaced with N-methyl-D-glucamine, in both the perfusing and bathing solutions, no significant difference could be detected in the J D of Hg 2 between S 2 segments perfused through the lumen with 3.0 mm L-lysine and the corresponding group of control S 2 segments (Fig. 6). The concentration of Hg 2 in the samples of collectate from these two groups was also not significantly different. These experiments were preformed to assess the extent the amino acid transport systems L, y, and b 0, play in the luminal transport of dicysteinylmercury. In contrast to the lack of effect of L-lysine on the J D of Hg 2, addition of BCH (5 mm), L-tryptophan (3 mm), or L-histidine (3 mm) to a Na -free perfusate caused additional significant reductions in the J D of Hg 2 (Fig. 6). More precisely, when BCH, L-tryptophan or L-histidine was present in the Na -free perfusate containing 20 M Hg 2 and 80 M L-cysteine, the J D of Hg 2 was 38, 68, and 77% lower, respectively, than the J D value obtained in the corresponding control tubules perfused under Na -free conditions. The concentration of Hg 2 in the samples of collectate increased by 37, 87, or 93%, respectively, when BCH, L-tryptophan, or L-histidine was present in the perfusate (Table 2). These experiments were preformed to determine whether the amino acid transport system-l plays a role in the luminal transport of dicysteinylmercury. Cellular Toxicity in Na -Free Conditions. Much like when 140 mm Na was present in the perfusing and bathing solutions, no visible signs of toxicity were observed during the experiments in which L-lysine, BCH, L-tryptophan, or L-histidine was used as a competitive inhibitor in the presence of 140 mm N-methyl-D-glucamine. There were, however, significant increases in the lumen-to-bath flux of the leak indicator (L-[ 3 H]glucose) with the use of some of the amino acids. The rate of leak was not elevated significantly when L-lysine, BCH, or L-tryptophan was present in the perfusate containing N-methyl-D-glucamine, when compared with that detected in the Na -free control experiments. Only when L-histidine was present in the perfusate, was the rate of leak of L-[ 3 H]glucose increased ( 3 times). Fig. 5. Effect of replacing Na with N-methyl-D-glucamine, in both bathing and perfusing solutions, on the luminal disappearance flux (J D )of Hg 2 in isolated S 2 segments of the rabbit proximal tubule perfused with 20 M Hg 2 and 80 M L-cysteine. Each column represents the mean S.E. obtained from at least four perfused tubular S 2 segments., significantly different (P 0.05) from the mean value obtained from the control tubules perfused with 20 M Hg 2 and 80 M L-cysteine with Na - containing perfusion and bathing solutions.
7 786 Cannon et al. Fig. 6. Luminal disappearance flux (J D )ofhg 2 in isolated S 2 segments of the rabbit proximal tubule perfused with 20 M Hg 2 and 80 M L-cysteine, with or without 3.0 mm lysine, 5.0 mm BCH, 3.0 mm L-tryptophan, or 3.0 mm L-histidine in the perfusing solution under Na -free conditions. All tubules were perfused and bathed with solutions containing 140 mm N- methyl-d-glucamine. Each column represents the mean S.E. obtained from at least four perfused tubular S 2 segments., significantly different (P 0.05) from the mean value obtained from the control tubules perfused with 20 M Hg 2 and 80 M L-cysteine without any additional amino acids or amino acid analogs in the perfusing solution. Discussion Several lines of in vivo and in vitro evidence indicate that the luminal absorptive transport of Hg 2 in the renal proximal tubule is linked to the activity of -glutamyltransferase and cysteinylglycinase (Berndt et al., 1985; Tanaka et al., 1990; Tanaka-Kagawa et al., 1993; deceaurriz et al., 1994; Zalups, 1995; Cannon et al., 2000). In the kidney, these enzymes are found almost exclusively in the luminal plasma membrane of proximal tubular epithelial cells. Consequently, the actual luminal uptake of Hg 2 in the proximal tubule appears to involve the transport of some cleavage product(s) formed by the actions of these enzymes. Because the activities of both -glutamyltransferase and cysteinylglycinase are very high in the luminal compartment of proximal tubular segments, the most likely primary species of Hg 2 transported at the luminal membrane would appear to be a mercuric conjugate of L-cysteine (dicysteinylmercury). Substantive in vivo and in vitro data support the hypothesis that dicysteinylmercury (cysteine-hg-cysteine) is one of the primary forms of Hg 2 taken up by proximal tubular epithelial cells (Zalups and Barfuss, 1996; Zalups and Lash, 1997). By far, the most convincing evidence supporting the luminal transport of a mercuric conjugate of L-cysteine comes from the isolated perfused tubule studies of Cannon et al. (2000). These investigators demonstrated in isolated perfused S 2 proximal tubular segments that the rates of luminal uptake (luminal disappearance flux, J D )ofhg 2 in the form of dicysteinylmercury were approximately 2-fold greater than the rates of luminal uptake of Hg 2 in the form of mercuric conjugates of either glutathione or cysteinylglycine. They also demonstrated that addition of millimolar concentrations of L-lysine or cycloleucine to a perfusate containing 20 M Hg 2 and 80 M L-cysteine (the same as in the present study) caused an approximate 50% reduction in the net rate of absorptive transport of Hg 2. Collectively, these findings led the investigators to postulate that luminal uptake of Hg 2, presumably in the form of dicysteinylmercury, occurs through one or more transporters shared by L-cystine and the dibasic amino acid L-lysine. Consistent with the isolated perfused tubule-findings of Cannon et al. (2000) are the results of the Richardson et al. (1975). These researchers demonstrated that the uptake of Hg 2 in renal cortical slices was reduced when the slices were exposed to mercuric conjugates of L-cysteine and an excess of the amino acid L-histidine or L-lysine. In addition, Wei et al. (1999) have demonstrated in isolated fragments of the rabbit proximal tubule that proximal tubular cells may sequester several mercuric conjugates, including mercuric conjugates of cysteine, by a neutral amino acid transport mechanism. It should be kept in mind, however, that uptake TABLE 2 Concentration of Hg 2 in collectate, leak rate of L-[ 3 H]glucose, and visual toxicity data from S 2 segments of the rabbit proximal tubule perfused with 20 M Cys-Hg-Cys (20 M Hg 2 and 80 M L-cysteine) alone (control) or with various potential competitive inhibitors in the presence of 140 mm N-methyl-D-glucamine In all groups the perfusion and bathing solutions were Na -free APM. Values are mean S.E. for at least four tubules. These data supplement the data shown in Figs. 5 and 6. Group Collectate Concentration of Hg 2 M Intercellular Leak of L-[ 3 H]glucose fmol min 1 mm 1 Toxic Effects Control None L-Lysine (3 mm) None BCH (5 mm) * None L-Tryptophan (3 mm) * None L-Histidine (3 mm) * None * Significantly different (P 0.05) from the mean value obtained from the control tubules. Significantly different (P 0.05) from the mean value obtained from the tubules not perfused with Hg 2. The level of leak in these control tubules was fmol min 1 (mm tubule length) 1 (Barfuss and Schafer, 1981).
8 Renal Transport of Dicysteinylmercury 787 in tissue slices and nonperfused isolated tubular fragments probably results primarily via transport mechanisms located on the basolateral membrane. Other than these findings, there has been a paucity of data dealing with potential mechanisms involved in the renal tubular uptake and transport of Hg 2 in the kidney. This deficit served as the primary impetus for the present study. The experiments in the present study were designed specifically to investigate the potential role of some of the better characterized amino acid transport systems in the disappearance of the mercuric conjugate dicysteinylmercury from the luminal fluid of the S 2 segment of the rabbit proximal tubule. The Na -dependent amino acid transporters targeted were systems A, ASC, B 0, and B 0,, while the Na -independent amino acid transporters targeted were systems L and b 0,. Na -Dependent Transport of Dicysteinylmercury. According to our present findings, system-a does not appear to be involved significantly in the luminal uptake of dicysteinylmercury in the proximal tubule. This conclusion is based on the findings that the luminal uptake of 20 M dicysteinylmercury was not inhibited significantly by millimolar concentrations of L-proline or MeAIB, which are established substrates for the system-a amino acid transporter (Fig. 1) (Hammerman and Sacktor, 1977). The flux data from the present study also indicate that the Na -dependent, cationic amino acid transporter, system B 0,, which has been localized in the brush-border membrane of proximal tubular epithelial cells (Boerner et al., 1986), does not play a significant role in the luminal uptake of dicysteinylmercury in pars recta segments of the proximal tubule. Evidence supporting this assertion comes mainly from the experiments in which addition of L-valine to the perfusing medium did not affect significantly the luminal J D of Hg 2 (Fig. 1). It was surprising that L-valine did not have an effect, while L-lysine had profound effects, on the luminal absorptive transport of dicysteinylmercury (Fig. 2). This is particularly surprising because both of these amino acids are known to be competitive inhibitors of system-b 0, (Boerner et al., 1986). These and other data from the present study suggest that the inhibitory effects of L-lysine on the J D of Hg 2 (as dicysteinylmercury) are most likely due to inhibition of system-asc, rather than system-b 0,. This effect of L-lysine on the uptake of dicysteinylmercury may be due actually to a noncompetitive inhibitory effect on the ASC amino acid transport system. It is thought that the positive charge on L-lysine occupies the Na -binding site on the ASC transporter. This argument has been used to explain the effects of L-lysine on transport processes in erythrocytes (Young et al., 1988). It appears that the Na -dependent luminal transport of dicysteinylmercury most likely involves, at least in part, a neutral amino acid transport system like system-asc and/or B 0. The classical ASC and B 0 transporter systems have been implicated in the transport of the amino acid L-serine (Kragh- Hansen and Sheikh, 1984). Support for our hypothesis that system-asc and/or B 0 is involved in the luminal transport of dicysteinylmercury comes from the experiments where L- serine effectively inhibited approximately 53% of the J D of Hg 2 (as dicysteinylmercury) (Fig. 2). This decrease in J D is also reflected in the large increase in the collectate concentration of Hg 2 that resulted from inhibition of uptake of Hg 2 at the luminal membrane (Table 1). In addition, BCH (a substrate for the Na -dependent B 0 system) inhibited the J D of Hg 2 by about 40 fmol min 1 (mm tubular length) 1 (Fig. 3), while it decreased the J D of Hg 2 by only 20 fmol min 1 (mm tubular length) 1 (Fig. 6) under Na -free conditions, which implicates system B 0 in the transport of dicysteinylmercury. Dicysteinylmercury may also be transported by a low-affinity transporter that is specific for L-cystine only (Gunther and Silbernagl, 1981). Addition of L-cystine to the perfusate had the most profound effects on the J D of dicysteinylmercury, resulting in a 76% (to 85%) reduction in the rate of uptake (Fig. 4). By marked contrast, addition of the D-enantiomer of cystine to the perfusion medium did not result in an inhibitory effect on the luminal absorptive transport of dicysteinylmercury. These findings indicate strongly that dicysteinylmercury and L-cystine share a common, stereospecific transporter. These findings also support the hypothesis that dicysteinylmercury enters into proximal tubular cells across the luminal plasma membrane as a molecular homolog of cystine. Na -Independent Transport of Dicysteinylmercury. Findings from the current study show clearly that the luminal transport of dicysteinylmercury uses both Na -dependent and Na -independent transport mechanisms to absorb this mercuric conjugate from the luminal fluid. Primary support for this conclusion comes from the data showing that an approximate 40% reduction in the J D of Hg 2 occurs in S 2 segments when Na in both perfusing and bathing solutions is replaced with N-methyl-D-glucamine (Fig. 5). The Na -independent cationic amino acid transport system-b 0, was shown not to be involved significantly in the luminal uptake of dicysteinylmercury in the current study. Interestingly, this system is known to transport L-lysine (Boerner et al., 1986). With the addition of 3 mm L-lysine to the perfusion medium, there was no change in the luminal transport of dicysteinylmercury under Na -free conditions (Fig. 6). This lack of effect of L-lysine suggests that systemb 0, is not involved in the luminal uptake of dicysteinylmercury. Data from the present study do, however, indicate strongly that the Na -independent system-l contributes substantially to the luminal disappearance of dicysteinylmercury in isolated S 2 segments of the proximal tubule. It has been demonstrated that system-l transports neutral amino acids and amino acid analogs with bulky hydrophobic side chains, such as L-phenylalanine, cycloleucine, L-tryptophan, L-histidine, and BCH (Hammerman and Sacktor, 1977; Deves and Boyd, 1998). BCH, L-tryptophan, L-phenylalanine, and cycloleucine inhibited individually the luminal disappearance of dicysteinylmercury as indicated by the reduced luminal uptake and increased concentration of Hg 2 in the collectate when Na was present in the perfusion and bathing solutions (Fig. 3; Table 1). By inference, cycloleucine is transported by system-l because of the bulky hydrophobic side chain on this molecule. Indeed, L-phenylalanine and cycloleucine have been shown to exhibit mutual inhibition (Gunther and Silbernagl, 1981). BCH and L-tryptophan were also able to reduce significantly the J D of dicysteinylmercury when Na was absent from the perfusate (Fig. 6; Table 2). Significant reductions in the luminal uptake of dicysteinylmercury also occurred under Na -dependent conditions when cycloleucine was present in the perfusing solution (Fig.
9 788 Cannon et al. 3). Collectively, these data support the hypothesis that the Na -independent transport of dicysteinylmercury involves an L -type neutral amino acid transporter. The site of this inhibition is probably located at the basolateral membrane for the cell-to-bath exit of dicysteinylmercury, since system-l is thought to be located only at this site (Pineda et al., 1999). Potential Nonspecific Effects. One might be led to speculate that some of the decreases in J D detected with the use of millimolar concentrations of amino acids could be due, in part, to competitive binding of mercuric ions to the nucleophilic functional groups (such as carboxyl, amide, and amine groups) present on the amino acids. However, this is very unlikely based on the tremendously strong and thermodynamically stable bond formed between mercuric ions and the sulfur atom of the thiol group of cysteine. To put some perspective on this issue, the reported affinity (constant) for mercuric ions bonding to a thiolate anion is on the order of times greater than that for mercury bonding to any other nucleophilic group (Hughes, 1957). The lack of inhibition in the luminal uptake of dicysteinylmercury when L-proline, L-valine, or D-cystine was present in the perfusion fluid leads one to conclude that competitive binding to nonthiol-containing nucleophiles is not a major contributing factor responsible for the reductions in the luminal uptake of dicysteinylmercury detected with the use of certain amino acids and analogs. The data obtained with the L- and D-enantiomers of cystine also support this conclusion. In fact, these data support the hypothesis that the luminal disappearance of dicysteinylmercury is due to absorptive transport at the site(s) of stereospecific amino acid transporters. Another potential nonspecific effect that could potentially affect transport of mercuric conjugates relates to the influence of the removal of Na from the perfusing and bathing solutions in the experiments designed to assess Na -independent transport. Indeed, replacement of Na with N-methyl-D-glucamine could have altered intracellular levels of both H and Ca 2 via Na exchangers (Geigel et al., 1989; Frindt and Windhager, 1990; Lyu et al., 1991), which could have an effect on transport parameters. Only additional investigations can provide substantive information on the potential effects of altered intracellular ph and/or Ca 2 on the luminal absorptive transport of dicysteinylmercury in the presence and/or absence of various amino acids. One point is certain, however, and that is, if there were significant alterations in the intracellular milieu induced by the removal of Na, they were not reflected pathologically, since the morphology of the tubular epithelial cells appeared normal throughout the perfusion process. No evidence of cellular swelling, luminal membrane blebbing, uptake of the vital dye FD&C green, and/or cellular vacuolization was detected in the tubules perfused under Na -free conditions. Moreover, intercellular leak in the tubules was generally within the normal range (Table 2). Therefore, we interpret any decrease in absorptive transport associated with Na -free conditions as an indication of a specific Na -dependent absorptive transport process. The primary conclusions from this study are that the mercuric conjugate dicysteinylmercury is transported from the luminal fluid of S 2 segments of the proximal tubule by both Na -dependent and Na -independent amino acid transport systems and that these transport systems recognize dicysteinylmercury as a molecular homolog of L-cystine (cysteine-cysteine) or as an independent molecular structure. Moreover, our data indicate that the transport of dicysteinylmercury is stereospecific. The Na -dependent transport of dicysteinylmercury appears to involve the specific low-affinity L-cystine transporter, system-b 0, and system-asc. The L - type amino acid transporter(s) appear to account for most of the Na -independent transport of dicysteinylmercury from the luminal fluid. References Ballatori N (1991) Mechanisms of metal transport across liver cell plasma membrane. Drug Metab Rev 23: Barfuss DW and Schafer JA (1981) Differences in active and passive transport of glucose alone the proximal nephron. Am J Physiol 240: Berndt WO, Bagget JM, Blacker A and Houser M (1985) Renal glutathione and mercury uptake by kidney. Fundam Appl Toxicol 5: Boerner PM, Evans-Layng HSU and Saier JRMH (1986) Polarity of neutral amino acid transport and characterization of a broad specificity transport activity in kidney epithelial cell line, MDCK. J Biol Chem 261: Cannon VT, Barfuss DW and Zalups RK (2000) Molecular homology and the luminal transport of Hg 2 in the renal proximal tubule. J Am Soc Nephrol 11: deceaurriz J, Payan JP, Morel P and Brondeau MT (1994) Role of extracellular glutathione and -glutamyltranspeptidase in the disposition and kidney toxicity of inorganic mercury in rats. J Appl Toxicol 14: Deves R and Boyd CAR (1998) Transporters of cationic amino acids in animal cells, discovery, structure and function. Physiol Rev 78: Frindt G and Windhager EE (1990) Ca 2 -dependent inhibition of sodium transport in rabbit cortical collecting tubules. Am J Physiol 258:F568 F582. Geigel J, Giebisch G and Boron WF (1989) Basolateral sodium-coupled acid-base transport mechanisms of the rabbit proximal tubule. Am J Physiol 257:F790 F797. Gunther R and Silbernagl S (1981) Renal handling of L-histidine studied by continuous microperfusion and free flow micropuncture in the rat. Pfluegers Arch 389: Hammerman MR and Sacktor B (1977) Transport of amino acids in renal brush border vesicles. Uptake of L-proline. J Biol Chem 25: Hughes WL (1957) A physicochemical rationale for the biological activity of mercury and its compounds. Ann NY Acad Sci 65: Kragh-Hansen U and Sheikh MI (1984) Serine uptake by luminal and basolateral membrane vesicles from rabbit kidney. J Physiol (Lond) 354: Lyu RM, Smith L and Smith JB (1991) Sodium-calcium exchange in renal epithelial cells: dependence on cell sodium and completive inhibition by magnesium. J Membr Biol 124: Mircheff AK, Kippen I, Hirayama B and Wright EM (1982) Delineation of sodiumstimulated amino acid transport pathways in rabbit kidney brush border vesicles. J Membr Biol 64: Pineda M, Fernandez E, Torrents D, Estevez R, Lopez C, Camps M, Lloberas J, Zorzano A and Palacin M (1999) Identification of a membrane protein, LAT-2, that Co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity of small and large zwitterionic amino acids. J Biol Chem 274: Rabenstein DL (1989) Metal complexes of glutathione and their biological significance, in Glutathione: Chemical, Biochemical and Medical Aspects: Coenzymes and Cofactors (Dolphin D, Avramovic O and Poulson R eds) vol 3, pp , John Wiley & Sons, New York. Richardson RJ, Wilder AC and Murphy SD (1975) Uptake of mercury and mercuryamino acid complexes by rat renal cortex slices. Proc Soc Exp Biol Med 150: Tate SS, Urade R, Getchell TV and Udenfriend S (1989) Expression of the mammalian Na -independent L system amino acid transport in Xenopus laevis oocytes. Arch Biochem Biophys 275: Tanaka T, Naganuma A and Nobumasa I (1990) Role of -glutamyltranspeptidase in renal uptake and toxicity of inorganic mercury in mice. Toxicology 60: Tanaka-Kagawa T, Naganuma A and Imura N (1993) Tubular secretion and reabsorption of mercury compounds in mouse kidney. J Pharmacol Exp Ther 264: Wei H, Qiu L, Divine KK, Ashbaugh MD, McIntyre LC Jr, Fernando Q and Gandolfi AJ (1999) Toxicity and transport if three synthesized mercury-thiolcomplexes in isolated rabbit renal proximal tubule suspensions. Drug Chem Toxicol 22: Young JD, Mason DK and Fincham DA (1988) Topological similarities between harmaline inhibit sites on Na -dependent amino acid transport system ASC in human erythrocytes and Na -independent asc in horse erythrocytes. J Biol Chem 263: Zalups RK (1995) Organic anion transport and action of -glutamyltranspeptidase in kidney linked mechanistically to renal tubular uptake of inorganic mercury. Toxicol Appl Pharmacol 132:
RUDOLFS K. ZALUPS, LISA D. PARKS, VERNON T. CANNON, and DELON W. BARFUSS
 0026-895X/98/020353-11$3.00/0 Copyright by The American Society for Pharmacology and Experimental Therapeutics All rights of reproduction in any form reserved. MOLECULAR PHARMACOLOGY, 54:353 363 (1998).
0026-895X/98/020353-11$3.00/0 Copyright by The American Society for Pharmacology and Experimental Therapeutics All rights of reproduction in any form reserved. MOLECULAR PHARMACOLOGY, 54:353 363 (1998).
Molecular Interactions with Mercury in the Kidney
 0031-6997/113/5201-0113$03.00/0 PHARMACOLOGICAL REVIEWS Vol. 52, No. 1 Copyright 20113 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A. Molecular Interactions with
0031-6997/113/5201-0113$03.00/0 PHARMACOLOGICAL REVIEWS Vol. 52, No. 1 Copyright 20113 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A. Molecular Interactions with
DEPLETION OF GLUTATHIONE IN THE KIDNEY AND THE RENAL DISPOSITION OF ADMINISTERED INORGANIC MERCURY
 0090-9556/97/2504-0516 523$02.00/0 DRUG METABOLISM AND DISPOSITION Vol. 25, No. 4 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A. DEPLETION OF GLUTATHIONE
0090-9556/97/2504-0516 523$02.00/0 DRUG METABOLISM AND DISPOSITION Vol. 25, No. 4 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A. DEPLETION OF GLUTATHIONE
Rudolfs K. Zalups,* Delon W. Barfuss, and Lawrence H. Lash
 Toxicology and Applied Pharmacology 154, 135 144 (1999) Article ID taap.1998.8562, available online at http://www.idealibrary.com on Disposition of Inorganic Mercury Following Biliary Obstruction and Chemically
Toxicology and Applied Pharmacology 154, 135 144 (1999) Article ID taap.1998.8562, available online at http://www.idealibrary.com on Disposition of Inorganic Mercury Following Biliary Obstruction and Chemically
Relationships between the Renal Handling of DMPS and DMSA and the Renal Handling of Mercury
 pubs.acs.org/crt Relationships between the Renal Handling of DMPS and DMSA and the Renal Handling of Mercury Rudolfs K. Zalups* and Christy C. Bridges Division of Basic Medical Sciences, 1550 College Street,
pubs.acs.org/crt Relationships between the Renal Handling of DMPS and DMSA and the Renal Handling of Mercury Rudolfs K. Zalups* and Christy C. Bridges Division of Basic Medical Sciences, 1550 College Street,
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Molecular and ionic mimicry and the transport of toxic metals
 Toxicology and Applied Pharmacology 204 (2005) 274 308 Review Molecular and ionic mimicry and the transport of toxic metals Christy C. Bridges, Rudolfs K. Zalups* Division of Basic Medical Sciences, Mercer
Toxicology and Applied Pharmacology 204 (2005) 274 308 Review Molecular and ionic mimicry and the transport of toxic metals Christy C. Bridges, Rudolfs K. Zalups* Division of Basic Medical Sciences, Mercer
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Homocysteine and the Renal Epithelial Transport and Toxicity of Inorganic Mercury: Role of Basolateral Transporter Organic Anion Transporter 1
 J Am Soc Nephrol 15: 2023 2031, 2004 Homocysteine and the Renal Epithelial Transport and Toxicity of Inorganic Mercury: Role of Basolateral Transporter Organic Anion Transporter 1 RUDOLFS K. ZALUPS and
J Am Soc Nephrol 15: 2023 2031, 2004 Homocysteine and the Renal Epithelial Transport and Toxicity of Inorganic Mercury: Role of Basolateral Transporter Organic Anion Transporter 1 RUDOLFS K. ZALUPS and
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
P-aminohippurate Accumulation in Kidney Cortex Slices: Stimulation by Dicarboxylates, Amino Acids and Their Oxoanalogues
 Physiol. Res. 40:339-344, 1991 P-aminohippurate Accumulation in Kidney Cortex Slices: Stimulation by Dicarboxylates, Amino Acids and Their Oxoanalogues R. DZIJRIK, M. GERYKOVA, V. SPUSTOVA Center of Clinical
Physiol. Res. 40:339-344, 1991 P-aminohippurate Accumulation in Kidney Cortex Slices: Stimulation by Dicarboxylates, Amino Acids and Their Oxoanalogues R. DZIJRIK, M. GERYKOVA, V. SPUSTOVA Center of Clinical
Acids and Bases their definitions and meanings
 Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
Factors Affecting Inorganic Mercury Transport and Toxicity in the Isolated Perfused Proximal Tubule1
 actors Affecting Inorganic Mercury Transport and Toxicity in the Isolated Perfused Proximal Tubule1 Rudolfs K. Zalups,2 Mary K. Robinson, and Delon W. Barfuss R.K. Zalups, Division of Basic Medical Sciences,
actors Affecting Inorganic Mercury Transport and Toxicity in the Isolated Perfused Proximal Tubule1 Rudolfs K. Zalups,2 Mary K. Robinson, and Delon W. Barfuss R.K. Zalups, Division of Basic Medical Sciences,
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Heterogeneity of glutathione synthesis and secretion in the proximal tubule of the rabbit
 Heterogeneity of glutathione synthesis and secretion in the proximal tubule of the rabbit LISA D. PARKS, 1 RUDOLFS K. ZALUPS, 2 AND DELON W. BARFUSS 1 1 Biology Department, Georgia State University, Atlanta
Heterogeneity of glutathione synthesis and secretion in the proximal tubule of the rabbit LISA D. PARKS, 1 RUDOLFS K. ZALUPS, 2 AND DELON W. BARFUSS 1 1 Biology Department, Georgia State University, Atlanta
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
Inorganic Mercury Transport in the Proximal Tubule of the Rabbit. Delon W. Barfuss,1 Mary K. Robinson, and Rudolfs K. Zalups
 norganic Mercury Transport in the Proximal Tubule of the Rabbit Delon W. Barfuss,1 Mary K. Robinson, and Rudolfs K. Zalups D.W. Barfuss, Biology Department, Georgia State University, Atlanta, GA M.K. Robinson,
norganic Mercury Transport in the Proximal Tubule of the Rabbit Delon W. Barfuss,1 Mary K. Robinson, and Rudolfs K. Zalups D.W. Barfuss, Biology Department, Georgia State University, Atlanta, GA M.K. Robinson,
Pretreatment with p-aminohippurate inhibits the renal uptake and accumulation of injected inorganic mercury in the rat
 ELSEVIER Toxicology 103 (1995) 23-35 Pretreatment with p-aminohippurate inhibits the renal uptake and accumulation of injected inorganic mercury in the rat Rudolfs K. Zalups* a, Delon W. Barfussb Division
ELSEVIER Toxicology 103 (1995) 23-35 Pretreatment with p-aminohippurate inhibits the renal uptake and accumulation of injected inorganic mercury in the rat Rudolfs K. Zalups* a, Delon W. Barfussb Division
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Amino Acids االحماض األمينية
 Amino Acids االحماض األمينية Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Cell Structure Amino Acids The study of
Amino Acids االحماض األمينية Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Cell Structure Amino Acids The study of
Online publication date: 25 June 2010
 This article was downloaded by: [informa internal users] On: 28 July 2010 Access details: Access Details: [subscription number 755239602] Publisher Taylor & Francis Informa Ltd Registered in England and
This article was downloaded by: [informa internal users] On: 28 July 2010 Access details: Access Details: [subscription number 755239602] Publisher Taylor & Francis Informa Ltd Registered in England and
Metabolic Classification of the Amino Acids
 Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
TITLE PAGE. Transport of N-Acetylcysteine S-Conjugates of Methylmercury in. MDCK Cells Transfected Stably with hoat1
 JPET This Fast article Forward. has not been Published copyedited and on formatted. May 20, The 2005 final as version DOI:10.1124/jpet.105.086645 may differ from this version. TITLE PAGE Transport of N-Acetylcysteine
JPET This Fast article Forward. has not been Published copyedited and on formatted. May 20, The 2005 final as version DOI:10.1124/jpet.105.086645 may differ from this version. TITLE PAGE Transport of N-Acetylcysteine
Introduction. Acids, Bases and ph; a review
 0 P a g e Introduction In this sheet, we discuss acidbase balance in our body and the role of kidneys in its establishment. Arrangement of topics is different from that of the lecture, to assure consistency
0 P a g e Introduction In this sheet, we discuss acidbase balance in our body and the role of kidneys in its establishment. Arrangement of topics is different from that of the lecture, to assure consistency
3. AMINO ACID AND PEPTIDES
 3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
Membrane Transport. Anatomy 36 Unit 1
 Membrane Transport Anatomy 36 Unit 1 Membrane Transport Cell membranes are selectively permeable Some solutes can freely diffuse across the membrane Some solutes have to be selectively moved across the
Membrane Transport Anatomy 36 Unit 1 Membrane Transport Cell membranes are selectively permeable Some solutes can freely diffuse across the membrane Some solutes have to be selectively moved across the
THE UNIVERSITY OF MANITOBA. DATE: Oct. 22, 2002 Midterm EXAMINATION. PAPER NO.: PAGE NO.: 1of 6 DEPARTMENT & COURSE NO.: 2.277/60.
 PAPER NO.: PAGE NO.: 1of 6 GENERAL INSTRUCTIONS You must mark the answer sheet with pencil (not pen). Put your name and enter your student number on the answer sheet. The examination consists of multiple
PAPER NO.: PAGE NO.: 1of 6 GENERAL INSTRUCTIONS You must mark the answer sheet with pencil (not pen). Put your name and enter your student number on the answer sheet. The examination consists of multiple
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay
 Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Chymotrypsin Lecture. Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin
 Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
Amino acids. Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester,
 Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
1.4. Lipids - Advanced
 1.4. Lipids - Advanced www.ck12.org In humans, triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of excess starches and other
1.4. Lipids - Advanced www.ck12.org In humans, triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of excess starches and other
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
االمتحان النهائي لعام 1122
 االمتحان النهائي لعام 1122 Amino Acids : 1- which of the following amino acid is unlikely to be found in an alpha-helix due to its cyclic structure : -phenylalanine -tryptophan -proline -lysine 2- : assuming
االمتحان النهائي لعام 1122 Amino Acids : 1- which of the following amino acid is unlikely to be found in an alpha-helix due to its cyclic structure : -phenylalanine -tryptophan -proline -lysine 2- : assuming
Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion
 Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion OLGA H. BROKL AND WILLIAM H. DANTZLER Department of Physiology, College of Medicine, University of Arizona, Tucson,
Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion OLGA H. BROKL AND WILLIAM H. DANTZLER Department of Physiology, College of Medicine, University of Arizona, Tucson,
Maha AbuAjamieh. Tamara Wahbeh. Mamoon Ahram
 12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Q1: the overall folding of a single protein subunit is called : -tertiary structure -primary structure -secondary structure -quaternary structure -all of the above Q2 : disulfide
بسم هللا الرحمن الرحيم Q1: the overall folding of a single protein subunit is called : -tertiary structure -primary structure -secondary structure -quaternary structure -all of the above Q2 : disulfide
(5) 1. List five unusual properties of water resulting from its hydrogen bonded structure
 BCH 4053 June 1, 2001 Points HOUR TEST 1 NAME (5) 1. List five unusual properties of water resulting from its hydrogen bonded structure. Page Points 1 2 3 4 5 Total (5) 2. Draw a diagram to show how water
BCH 4053 June 1, 2001 Points HOUR TEST 1 NAME (5) 1. List five unusual properties of water resulting from its hydrogen bonded structure. Page Points 1 2 3 4 5 Total (5) 2. Draw a diagram to show how water
CHM333 LECTURE 6: 1/25/12 SPRING 2012 Professor Christine Hrycyna AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID:
 AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Section 1 Proteins and Proteomics
 Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
AMINO ACIDS. Qualitative Tests
 AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds.
 Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
Midterm 1 Last, First
 Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
v. reitschlagerova Renal Amino Acid Excretion and Aging H. NADVORNlKOVA, O. SCHUCK, V. TEPLAN, D. TOMKOVA,
 Physiol. Res. 40:87-94, 1991 Renal Amino Acid Excretion and Aging H. NADVORNlKOVA, O. SCHUCK, V. TEPLAN, D. TOMKOVA, v. reitschlagerova Institute for Clinical and Experimental Medicine, Prague Received
Physiol. Res. 40:87-94, 1991 Renal Amino Acid Excretion and Aging H. NADVORNlKOVA, O. SCHUCK, V. TEPLAN, D. TOMKOVA, v. reitschlagerova Institute for Clinical and Experimental Medicine, Prague Received
Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE
 Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE I. RELATED TOPICS Integumentary system Cerebrospinal fluid Aqueous humor Digestive juices Feces Capillary dynamics Lymph circulation Edema Osmosis
Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE I. RELATED TOPICS Integumentary system Cerebrospinal fluid Aqueous humor Digestive juices Feces Capillary dynamics Lymph circulation Edema Osmosis
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Collin College. BIOL Anatomy & Physiology. Urinary System. Summary of Glomerular Filtrate
 Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chapter 11: Enzyme Catalysis
 Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
UNIVERSITY OF GUELPH CHEM 4540 ENZYMOLOGY Winter 2005 Quiz #2: March 24, 2005, 11:30 12:50 Instructor: Prof R. Merrill ANSWERS
 UNIVERSITY F GUELPH CHEM 4540 ENZYMLGY Winter 2005 Quiz #2: March 24, 2005, 11:30 12:50 Instructor: Prof R. Merrill ANSWERS Instructions: Time allowed = 80 minutes. Total marks = 30. This quiz represents
UNIVERSITY F GUELPH CHEM 4540 ENZYMLGY Winter 2005 Quiz #2: March 24, 2005, 11:30 12:50 Instructor: Prof R. Merrill ANSWERS Instructions: Time allowed = 80 minutes. Total marks = 30. This quiz represents
11/05/1431. Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate
 Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Chemistry 20 Chapter 14 Proteins
 Chapter 14 Proteins Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and connective tissue
Chapter 14 Proteins Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and connective tissue
EH1008 Biomolecules. Inorganic & Organic Chemistry. Water. Lecture 2: Inorganic and organic chemistry.
 EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
CHAPTER 3 Amino Acids, Peptides, Proteins
 CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
Amino Acids. Lecture 4: Margaret A. Daugherty. Fall Swiss-prot database: How many proteins? From where?
 Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
BCH302 [Practical] 1
![BCH302 [Practical] 1 BCH302 [Practical] 1](/thumbs/78/76808179.jpg) BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
BIOLOGY 103 Spring 2001 MIDTERM LAB SECTION
 BIOLOGY 103 Spring 2001 MIDTERM NAME KEY LAB SECTION ID# (last four digits of SS#) STUDENT PLEASE READ. Do not put yourself at a disadvantage by revealing the content of this exam to your classmates. Your
BIOLOGY 103 Spring 2001 MIDTERM NAME KEY LAB SECTION ID# (last four digits of SS#) STUDENT PLEASE READ. Do not put yourself at a disadvantage by revealing the content of this exam to your classmates. Your
Organic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins
 rganic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins No. 1 of 10 1. Which amino acid does not contain a chiral center? (A) Serine (B) Proline (C) Alanine (D) Phenylalanine (E) Glycine
rganic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins No. 1 of 10 1. Which amino acid does not contain a chiral center? (A) Serine (B) Proline (C) Alanine (D) Phenylalanine (E) Glycine
PAPER No. : 16, Bioorganic and biophysical chemistry MODULE No. : 22, Mechanism of enzyme catalyst reaction (I) Chymotrypsin
 Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Acid - base equilibrium
 Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
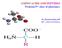 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
Acid-Base Balance 11/18/2011. Regulation of Potassium Balance. Regulation of Potassium Balance. Regulatory Site: Cortical Collecting Ducts.
 Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Diuretics having the quality of exciting excessive excretion of urine. OED. Inhibitors of Sodium Reabsorption Saluretics not Aquaretics
 Diuretics having the quality of exciting excessive excretion of urine. OED Inhibitors of Sodium Reabsorption Saluretics not Aquaretics 1 Sodium Absorption Na Entry into the Cell down an electrochemical
Diuretics having the quality of exciting excessive excretion of urine. OED Inhibitors of Sodium Reabsorption Saluretics not Aquaretics 1 Sodium Absorption Na Entry into the Cell down an electrochemical
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Enzyme Catalytic Mechanisms. Dr. Kevin Ahern
 Enzyme Catalytic Mechanisms Dr. Kevin Ahern Cleave Peptide Bonds Specificity of Cutting Common Active Site Composition/Structure Mechanistically Well Studied Chymotrypsin Chymotrypsin Catalysis H2O Chymotrypsin
Enzyme Catalytic Mechanisms Dr. Kevin Ahern Cleave Peptide Bonds Specificity of Cutting Common Active Site Composition/Structure Mechanistically Well Studied Chymotrypsin Chymotrypsin Catalysis H2O Chymotrypsin
QUALITATIVE ANALYSIS OF AMINO ACIDS AND PROTEINS
 QUALITATIVE ANALYSIS OF AMINO ACIDS AND PROTEINS Amino acids are molecules containing an amine group, a carboxylic acid group and a side chain that varies between different amino acids. Amino acids of
QUALITATIVE ANALYSIS OF AMINO ACIDS AND PROTEINS Amino acids are molecules containing an amine group, a carboxylic acid group and a side chain that varies between different amino acids. Amino acids of
Renal physiology D.HAMMOUDI.MD
 Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
Protein Folding LARP
 Protein Folding LARP Version: 1.0 Release: April 2018 Amplyus 2018 minipcr TM Protein Folding LARP (Live Action Role Play) Summary Materials In this activity, students will role play to make a folded protein
Protein Folding LARP Version: 1.0 Release: April 2018 Amplyus 2018 minipcr TM Protein Folding LARP (Live Action Role Play) Summary Materials In this activity, students will role play to make a folded protein
If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out.
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Renal physiology V. Regulation of acid-base balance. Dr Alida Koorts BMS
 Renal physiology V Regulation of acidbase balance Dr Alida Koorts BMS 712 012 319 2921 akoorts@medic.up.ac.za Hydrogen ions (H + ): Concentration and origin Concentration in arterial blood, resting: [H
Renal physiology V Regulation of acidbase balance Dr Alida Koorts BMS 712 012 319 2921 akoorts@medic.up.ac.za Hydrogen ions (H + ): Concentration and origin Concentration in arterial blood, resting: [H
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
MBB 694:407, 115:511. Please use BLOCK CAPITAL letters like this --- A, B, C, D, E. Not lowercase!
 MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
The ADME properties of most drugs strongly depends on the ability of the drug to pass through membranes via simple diffusion.
 1 MEDCHEM 562 Kent Kunze Lecture 1 Physicochemical Properties and Drug Disposition The ADME properties of most drugs strongly depends on the ability of the drug to pass through membranes via simple diffusion.
1 MEDCHEM 562 Kent Kunze Lecture 1 Physicochemical Properties and Drug Disposition The ADME properties of most drugs strongly depends on the ability of the drug to pass through membranes via simple diffusion.
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Functions of Proximal Convoluted Tubules
 1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
150 mm HCO How Does the Pancreas Do It? Clues from Computer Modelling of the Duct Cell
 JOP. J. Pancreas (Online) 2001; 2(4 Suppl):198202. 150 mm How Does the Pancreas Do It? Clues from Computer Modelling of the Duct Cell Yoshiro Sohma 1, Michael A Gray 2, Yusuke Imai 1, Barry E Argent 2
JOP. J. Pancreas (Online) 2001; 2(4 Suppl):198202. 150 mm How Does the Pancreas Do It? Clues from Computer Modelling of the Duct Cell Yoshiro Sohma 1, Michael A Gray 2, Yusuke Imai 1, Barry E Argent 2
Principles of Renal Physiology. 4th Edition
 Principles of Renal Physiology 4th Edition Principles of Renal Physiology 4th Edition Chris Lote Professor of Experimental Nephrology, University of Birmingham, UK SPRINGER SCIENCE+BUSINESS MEDIA, B.V.
Principles of Renal Physiology 4th Edition Principles of Renal Physiology 4th Edition Chris Lote Professor of Experimental Nephrology, University of Birmingham, UK SPRINGER SCIENCE+BUSINESS MEDIA, B.V.
Carbon and the Molecular Diversity of Life. Chapter 4
 Carbon and the Molecular Diversity of Life Chapter 4 CARBON Carbon is has ability to form large and complex, molecules Aspirin molecular formula? A triglyceride Organic chemistry is study of carbon compounds
Carbon and the Molecular Diversity of Life Chapter 4 CARBON Carbon is has ability to form large and complex, molecules Aspirin molecular formula? A triglyceride Organic chemistry is study of carbon compounds
Transport across the cell membrane
 Transport across the cell membrane Learning objectives Body compartments ECF and ICF Constituents Lipid Bilayer: Barrier to water and water-soluble substances ions glucose H 2 O urea CO 2 O 2 N 2 halothane
Transport across the cell membrane Learning objectives Body compartments ECF and ICF Constituents Lipid Bilayer: Barrier to water and water-soluble substances ions glucose H 2 O urea CO 2 O 2 N 2 halothane
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Glycolysis. Cellular Respiration
 Glucose is the preferred carbohydrate of cells. In solution, it can change from a linear chain to a ring. Energy is stored in the bonds of the carbohydrates. Breaking these bonds releases that energy.
Glucose is the preferred carbohydrate of cells. In solution, it can change from a linear chain to a ring. Energy is stored in the bonds of the carbohydrates. Breaking these bonds releases that energy.
