DEPLETION OF GLUTATHIONE IN THE KIDNEY AND THE RENAL DISPOSITION OF ADMINISTERED INORGANIC MERCURY
|
|
|
- Lillian Stone
- 5 years ago
- Views:
Transcription
1 /97/ $02.00/0 DRUG METABOLISM AND DISPOSITION Vol. 25, No. 4 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A. DEPLETION OF GLUTATHIONE IN THE KIDNEY AND THE RENAL DISPOSITION OF ADMINISTERED INORGANIC MERCURY RUDOLFS K. ZALUPS AND LAWRENCE H. LASH Division of Basic Medical Sciences (R.K.Z.), Mercer University School of Medicine; and Department of Pharmacology (L.H.L.), Wayne State University School of Medicine (Received November 26, 1996; accepted January 24, 1997) ABSTRACT: The primary aim of the present study was to evaluate the effects of different means of depleting glutathione (GSH) in the kidneys and liver on the renal and hepatic accumulation and disposition of a nontoxic dose of inorganic mercury. Renal and hepatic disposition of mercury were evaluated 1 hr after the intravenous administration of a 0.5 mol/kg dose of mercuric chloride in control rats and rats pretreated with acivicin, buthionine sulfoximine (BSO), or diethylmaleate (DEM). Pretreatment with acivicin or DEM caused significant decreases in the net renal accumulation of mercury during the first hour after the injection of mercuric chloride. The primary effects of these two pretreatments occurred in the renal cortex, although pretreatment with DEM also caused significant decreases in the concentration of mercury in the outer stripe of the outer medulla. Despite the fact that pretreatment with BSO caused a reduction in the renal content of GSH, comparable with that caused by DEM, pretreatment with BSO had no significant effect on the renal disposition of mercury. Pretreatment with acivicin, BSO, or DEM also caused significant decreases in measurable reduced GSH, with BSO and DEM having the most pronounced effects. Injection of the nontoxic dose of mercuric chloride after pretreatment with acivicin resulted in slightly, but significantly, decreased hepatic content of mercury. Interestingly, pretreatment with BSO or DEM actually caused significant increases in the hepatic content of mercury 1 hr after the injection of mercuric chloride. We postulate that this effect was due to a diminished ability of hepatocytes to export mercuric conjugates of GSH out into either the bile or blood. The results of this study indicate that depletion of renal GSH by conjugation reactions between GSH and DEM leads to an acute reduction in the renal accumulation of inorganic mercury. However, the results also indicate that depletion of renal levels of GSH by inhibition of GSH synthesis does not affect acutely the ability of the kidneys to accumulate inorganic mercury. Thus, it seems that factors in addition to intracellular GSH status play an important role in the renal accumulation/retention of inorganic mercury. It has been postulated by numerous investigators that modification, particularly depletion, of intracellular concentrations of GSH 1 in the kidneys has a significant influence on the renal disposition of inorganic mercury. Unfortunately, controversy arose because of differences in findings obtained from studies directed at testing this hypothesis. Depending on the mode by which renal GSH status had been modified and the dose of mercury administered, varied responses from decreased (1 3) to increased (4) renal accumulation of administered inorganic mercury have been reported in animals in which the renal concentration of GSH had been decreased. In an attempt to shed some light on this controversy, the present study was designed to evaluate and compare, in rats, the effects of three different methods commonly used to deplete the renal concentrations of GSH on the renal disposition of inorganic mercury after the administration of a nontoxic dose of mercuric chloride. The primary aim of the present study was to test the hypothesis that mode of depletion of renal GSH is an important determinant in the renal disposition of inorganic mercury. This study was supported in part by grants from the National Institutes of Health (ES05157 and DK40725). 1 Abbreviations used are: GSH, glutathione; BSO, buthionine sulfoximine; DEM, diethylmaleate; GSSG, glutathione disulfide. Send reprint requests to: Dr. Rudolfs K. Zalups, Division of Basic Medical Sciences, Mercer University School of Medicine, 1550 College Street, Macon, GA A critical difference between this study and previous investigations on the effects of alterations in GSH status on the disposition of inorganic mercury in tissues and organs (particularly in the kidney) is that we used a nontoxic dose of inorganic mercury, whereas toxic doses were used in the previous studies (1 3). Hence, complications from the pathophysiological effects of toxic doses of inorganic mercury should not confound the results from the present study. Our result showed that, depending on the method by which intracellular concentrations of GSH are decreased (i.e. inhibition of GSH turnover with acivicin, inhibition of synthesis of GSH with BSO, or conjugation of GSH with DEM), there are differences in the renal and hepatic contents of inorganic mercury 1 hr after the administration of the nonnephrotoxic dose of mercuric chloride. Materials and Methods Animals and Groups. Forty male Sprague Dawley rats were used in the present study. They were purchased from Harlan Sprague-Dawley (Indianapolis, IN) at a weight of g. After 3 4 days of acclimation, the animals were separated into groups of five. There were four main types of groups used in the present study. One type served as a control, one type was pretreated with acivicin (to inhibit the enzyme -glutamyltransferase), one type was pretreated with BSO to deplete renal and hepatic GSH, and the last type was pretreated with DEM as a second method to deplete renal and hepatic GSH. Two sets of each type of group were used. One set was used for determining renal and hepatic GSH status at the time that a nontoxic dose of mercuric chloride was administered. The other set was used to evaluate the effect of altering GSH status on the disposition of the injected mercury.
2 GSH STATUS AND RENAL DISPOSITION OF MERCURY 517 During all stages of the present study, the animals were allowed water and a commercial laboratory diet for rats ad libitum. Pretreatments. As mentioned previously, acivicin was administered to inhibit the activity of the enzyme -glutamyltransferase in the kidneys and liver. The injection protocol used in the animals pretreated with acivicin is a slight modification of the one established by Scott and Curthoys (5), which results in the inhibition of 97% of the activity of -glutamyltransferase in the kidneys. First, the animals received a 10 mg/kg intraperitoneal dose of acivicin in 2.0 ml/kg normal saline [0.9% (w/v) aqueous sodium chloride]. Ninety minutes later, the animals received a second 10 mg/kg dose of acivicin that was administered into the left femoral vein (while they were anesthetized lightly with ether). Sixty minutes after the second dose, the animals either received a nontoxic 0.5 mol/kg intravenous dose of mercuric chloride or were anesthetized with a 50 mg/kg dose of sodium pentobarbital (intraperitoneally) to obtain samples of kidneys and liver for determination of GSH status. BSO and DEM were chosen to deplete GSH in the kidneys and liver because depletion could be attained by different mechanisms. Depletion of GSH after pretreatment with BSO is accomplished by inhibition of -glutamylcysteine synthetase (6), which is the rate-limiting enzyme involved in the intracellular synthesis of GSH. Pretreatment with DEM results in depletion of GSH by formation of DEM-GSH conjugates. The injection protocols used to pretreat rats with BSO or DEM were slight modifications of ones described previously by Baggett and Berndt (1). In brief, rats pretreated with BSO were given a 2.0 mmol/kg dose iv in 4.0 ml/kg normal saline. Rats that were pretreated with DEM received a 3.37 mmol/kg dose ip in 2.0 ml/kg corn oil. Two hours after pretreatment with either BSO or DEM, the animals either received the nontoxic 0.5 mol/kg intravenous dose of mercuric chloride or were anesthetized with a 50 mg/kg dose of sodium pentobarbital (intraperitoneally) to obtain samples of kidneys and liver for determination of GSH status. Twenty-four hours before experimentation, the BSO- and DEM-pretreated rats received a priming dose of DEM intraperitoneally. On examination, after the administration of acivicin, BSO, or DEM, the gross structural characteristics of the kidneys seemed normal, even 1 hr after the administration of the 0.5 mol/kg dose of mercuric chloride. This suggests that no toxic effects occurred as a result of the pretreatments used during the periods studied. Evaluation of GSH Status in Samples of Kidneys and Liver. As stated previously, one set of control, acivicin-pretreated, BSO-pretreated, and DEMpretreated rats were anesthetized with sodium pentobarbital immediately after their respective pretreatment. Once anesthesia had been induced, the kidneys and liver were removed, cleared of fat and connective tissue, and weighed quickly. Subsequently, a half kidney and a sample of renal cortex and outer stripe of the outer medulla were obtained from the left kidney and a 1-g sample of liver was obtained. Immediately after each sample of tissue was obtained from each animal, it was placed quickly into a cold (4 C) solution containing 1 mm bathophenanthroline disulfonic acid in 10% (v/v) perchloric acid. Bathophenanthroline was added to inhibit autooxidation during processing of the samples. The samples of one half kidney and liver were placed in 10 ml of the solution, whereas the other renal samples were placed in 1.5 ml of the solution. All of the samples were homogenized in a glass/teflon homogenizer with a variable speed motor. Homogenates were centrifuged at 4 C at 1,250g for 10 min. The supernatants (perchloric acid extracts) were used for subsequent analyses. Concentrations of GSH and cysteine were measured as S-carboxymethyl and N-dinitrophenyl derivatives, and GSSG was measured as the N,N-bisdinitrophenyl derivative using HPLC. Separations were achieved with a Bondapak amine 10- m cartridge (8 mm 10 cm) (Waters Associates, Milford, MA) with a Waters model 600E multisolvent delivery system using a methanol-acetate mobile phase and gradient elution. Derivatives were detected at 365 nm on a Waters model 490 detector and were quantified with respect to standards using a Waters model 745 data module. Concentrations of GSH, GSSG, and cysteine are expressed in mole units per gram of wet weight of tissue. Injection of Inorganic Mercury. The other set of control, acivicin-pretreated, BSO-pretreated, and DEM-pretreated rats (not used in the assessment of GSH status) was administered a 0.5 mol/kg dose of mercuric chloride into the femoral vein after pretreatment. Radioactive inorganic mercury in the form of mercuric chloride ( 203 HgCl 2 ; Buffalo Materials Corp., Buffalo, NY) was added to the injection solution containing nonradioactive mercury. The injection solution was designed to deliver 0.5 mol Hg 2 /kg and 4 Ci 203 Hg 2 /kg in 2.0 ml normal saline (0.2 ml injection volume/100 g body weight). Acquisition of Tissues and Determination of the Content of Mercury in the Tissues. One hour after the injection of the mercuric chloride, animals were anesthetized with a 50 mg/kg dose of sodium pentobarbital (intraperitoneally). Once the animals were anesthetized, two 1-ml samples of whole blood were obtained from the inferior vena cava. One milliliter of whole blood was placed and sealed in a preweighed mm, round-bottom, gammacounting tube. The other 1.0 ml of whole blood was centrifuged at 10,000g to separate the cellular fraction of blood from the plasma. Both plasma and cellular fractions were placed individually, and sealed, in gamma-counting tubes. After the blood had been obtained, the kidneys and liver were removed, cleared of fat and connective tissue, and weighed quickly. Each of the two kidneys was cut along the transverse plane. One half of each kidney was placed and sealed in a preweighed gamma-counting tube. A 3-mm section of kidney was sliced away from the midregion of the remaining half of the left kidney, and samples of cortex, outer and inner stripes of the outer medulla, and outer and inner stripes of the inner medulla were obtained. A 1-g sample of liver was also obtained. The amount of radioactivity in the samples of tissues and injection solution (standards) was determined by counting the samples in a 1282 Compugamma CS deep-well gamma spectrometer that is equipped with a 3-inch sodium iodide crystal (Wallac, Gaithersburg, MD) and operates at a counting efficiency of 50% for 203 Hg 2. The content of mercury in the samples was calculated by dividing the activity of 203 Hg 2 (dpm) in the sample by the specific activity of 203 Hg 2 (dpm/nmol) in the injection solution. Concentrations of mercury in the tissues are expressed as percentage of the administered dose per gram of tissue, and the content of mercury in organs is expressed simply as a percentage of the administered dose. The total volume of blood in rats was estimated to be 6% of body weight. The 1-hr time point after the administration of mercuric chloride was chosen for measurements of the disposition of inorganic mercury, because the kinetics for the uptake of mercury in the kidney and liver are greatest during this period. During this initial hour after the injection of a nontoxic dose of mercuric chloride, 80 85% of the total renal burden of mercury that is present 24 hr after mercuric chloride is taken up by the kidney. Moreover, the 1-hr time point was chosen, because we wanted to evaluate the effects of depletion of GSH on the disposition of mercury at a time when it was most likely that GSH status would not have recovered from the changes induced by the administration of acivicin, BSO, or DEM. Statistical Analyses. Statistical differences between means, of any parameter measured, for the four groups of rats studied were assessed using a one-way analysis of variance followed by Tukey s multiple comparison procedure. Data expressed as a percentage of total were first normalized using the arcsine transformation before performing any parametric statistical procedure. The arcsine transformation takes the arcsine of the square root of the decimal fraction of the percentage score. The level of significance for any of the statistical procedures used was chosen a priori to be p Results Concentration of GSH in Renal Tissues. Weights of the animals in which GSH status was evaluated, and the weights of their kidneys and liver are presented in table 1. Decreased concentrations of GSH in the left kidney were detected in all rats that were pretreated with acivicin, BSO, or DEM (fig. 1A). However, the greatest decreases occurred in the rats that were pretreated with BSO or DEM. In these animals, the concentration of GSH in the left kidney was 54 66% lower than that in the control rats. No significant difference in the renal concentration of mercury was detected between the rats pretreated with BSO and the rats pretreated with DEM. All differences mentioned in this section are statistically significant unless stated otherwise. Just as was the case at the level of the whole kidney, pretreatment with acivicin, BSO, or DEM caused significant decreases in the concentration of GSH in the renal cortex. However, the effect of acivicin was slightly more prominent in the renal cortex than at the
3 518 ZALUPS AND LASH TABLE 1 Concentration of GSSG and cysteine (CYS) in the kidneys and liver of rats treated with compounds that alter renal and hepatic GSH status [CYS] in Liver [CYS] in Renal OS/OM [CYS] in Renal Cortex [CYS] in Kidney [GSSG] in Liver [GSSG] in Renal OS/OM [GSSG] in Renal Cortex [GSSG] in Kidney Weight of Liver Weight of Right Kidney Weight of Left Kidney Animal Body Weight Group g g g g mol/g mol/g mol/g mol/g mol/g mol/g mol/g mol/g Control N Acivicin N * * * * * BSO N ** ** * ** * DEM N *** *** * **** * Values represent mean SE for five rats. OS/OM, outer stripe of outer medulla. Consult with Materials and Methods for details of injections and treatments. * Significantly different (p 0.05) from the corresponding mean for the control group. ** Significantly different (p 0.05) from the corresponding means for the control group and the group treated with acivicin. *** Significantly different (p 0.05) from the corresponding means for the control group, the group treated with acivicin, and the group treated with both BSO. **** Significantly different (p 0.05) from the corresponding means for the group treated with acivicin and the group treated with BSO. FIG. 1.Concentration of GSH in renal tissues (A) from groups of control rats and rats pretreated with acivicin, BSO, or DEM, and concentration of mercury in renal tissue (B) from groups of control rats and rats pretreated with acivicin, BSO, or DEM 1 hr after the intravenous injection of a 0.5 mol/kg dose of mercuric chloride. See Materials and Methods for details of pretreatments and handling of tissues. Values are mean SE. * Significantly different (p 0.05) from the corresponding mean for the control group. ** Significantly different (p 0.05) from the means for the corresponding groups of control rats and rats pretreated with acivicin. *** Significantly different (p 0.05) from the means for the corresponding groups of control rats, rats pretreated with acivicin, and rats pretreated with BSO. level of the whole kidney. In the rats pretreated with acivicin, the mean renal concentration of GSH was 45% lower than that in control rats. In addition, the mean concentration of GSH in the renal cortex of the rats pretreated with either BSO or DEM was 64% lower than that in the control rats. A slightly different pattern of effect was detected in the samples of the outer stripe of the outer medulla. Unlike in the other renal zones, pretreatment with acivicin actually caused an increase in the concentration of GSH. The mean concentration of GSH in the rats pretreated with acivicin was 38% greater than that in the control rats. In the rats pretreated with either BSO or DEM, the mean concentration of GSH in the renal outer stripe of the outer medulla was between 65 77% lower than that in control rats. Concentrations of Mercury in Renal Tissues. Body weights and the weights of the kidneys and liver in the animals that received the 0.5 mol/kg dose of mercuric chloride are presented in table 2. Pretreatment with either acivicin or DEM, but not with BSO, resulted in decreased concentrations of mercury at the level of the whole kidney 1 hr after the intravenous administration of a nontoxic
4 GSH STATUS AND RENAL DISPOSITION OF MERCURY 519 Group TABLE 2 Concentration of injected mercury in renal zones and blood of rats treated with compounds that alter renal and hepatic GSH status Animal Body Weight Weight of Left Kidney Weight of Right Kidney Weight of Liver [Hg 2 ] in IS/OM [Hg 2 ] in Inner Medulla [Hg 2 ] in Blood g g g g % dose/g % dose/g % dose/g Control N Acivicin N BSO N DEM N Values represent mean SE for five rats. IS/OM, inner stripe of outer medulla. Consult with Materials and Methods for details of injections and treatments. All animals received an intravenous 0.5 mol/kg dose of mercuric chloride containing 4 Ci/kg 203 Hg 2 after being pretreated with 1 of the 4 protocols used. The disposition of inorganic mercury in the renal tissues and blood was evaluated 1 hr after injection of mercuric chloride. 0.5 mol/kg dose of mercuric chloride (fig. 1B). Pretreatment with either acivicin or DEM caused the renal concentration of mercury to be 46 48% less than that in the control rats or the rats pretreated with BSO. A pattern of accumulation of mercury, similar to that found at the level of the of whole kidney, was detected in the samples of renal cortex. Pretreatment with acivicin or DEM, but not BSO, resulted in decreased concentrations of mercury in the renal cortex. The renal cortical concentrations of mercury in the rats pretreated with acivicin or DEM were 49 59% lower than those in the control rats. Interestingly, pretreatment with BSO caused an approximate 43% increase, pretreatment with DEM caused an approximate 38% decrease, and pretreatment with acivicin had no effect on the concentration of mercury in the outer stripe of the outer medulla relative to that in control rats. The effects of the three pretreatments on the disposition of mercury in the inner stripe of the outer medulla and inner medulla are presented in table 2. Very little mercury generally accumulates in these two zones, which is confirmed by the present data. There did seem to be somewhat greater concentrations of mercury in both of these renal zones after any of the pretreatments. However, the differences between the means are not statistically significant at p Concentration of GSH and Mercury in the Liver. Significant effects on hepatic concentrations of GSH were detected in rats pretreated with acivicin, BSO, or DEM (fig. 2A). All three forms of pretreatment caused decreases in the hepatic concentration of GSH. However, the greatest effects were detected in the rats pretreated with BSO or DEM. The mean hepatic concentration of GSH was 61% lower in the rats pretreated with BSO and 91% lower in the rats pretreated with DEM than in the control rats. Mixed effects of the three forms of pretreatment on the hepatic concentration of mercury were detected 1 hr after the intravenous injection of the 0.5 mol/kg dose of mercuric chloride (fig. 2B). In the rats pretreated with acivicin, the hepatic concentration of mercury was 19% less than that in the control rats. By contrast, the hepatic concentration of mercury increased in the rats pretreated with either BSO or DEM. In fact, the concentration of mercury was 51% greater in the rats pretreated with BSO and 76% greater in the rats pretreated with DEM than in the control rats. Concentrations of GSSG in Renal and Hepatic Tissues. Concentrations of GSSG in the kidney decreased (relative to that in control rats) in the rats pretreated with acivicin or BSO and increased in the rats pretreated with DEM (table 1). Only in the rats pretreated with BSO was there a significant effect on the concentration of GSSG in the renal cortex. Pretreatment with BSO caused the concentration of GSSG to decrease in this zone. By contrast, only in rats pretreated with DEM was there a significant effect on the concentration of GSSG in the renal outer stripe of the outer medulla. Pretreatment with DEM caused the concentration of GSSG to increase in this zone. In rats pretreated with BSO or DEM, significant changes in the concentration of GSSG in the liver were detected (table 1). These pretreatments caused the mean hepatic concentration of GSSG to decrease. Concentrations of Cysteine in Renal and Hepatic Tissues. Rats pretreated with acivicin or BSO had lower concentrations of cysteine in their kidneys than control rats (table 1). Decreased concentrations of cysteine, relative to control values, were also detected in the renal cortex of the rats pretreated with acivicin or DEM and in the renal outer stripe of the outer medulla of the rats that were administered any of the three pretreatments. Although the concentrations of cysteine tended to be lower in rats pretreated with acivicin, BSO, or DEM, the differences in the means for the hepatic concentration of cysteine between the four groups of animals were found not to be statistically significant (table 1). Total Content of GSH in the Total Renal Mass and Liver. The content of GSH in the total renal mass of the rats pretreated with acivicin was 25% lower than that in corresponding control rats (fig. 3A). Rats pretreated with acivicin also had 31% less GSH in their liver relative to that in control rats (fig. 3C). In the rats pretreated with either BSO or DEM, the content of GSH in the total renal mass was 60 68% lower than that in corresponding control rats and 46 58% lower than in the corresponding rats treated with acivicin. In addition, the content of GSH in the liver of the rats pretreated with BSO was 75% and 64% lower than that in control rats and the rats pretreated with acivicin, respectively. Moreover, the content of GSH in the liver of the rats treated with DEM was 91%, 86%, and 63% lower than that in control rats, the rats pretreated with acivicin, and the rats pretreated with BSO, respectively. Total Content of Mercury in the Total Renal Mass and Liver. Significant effects on the content of mercury in the total renal mass 1 hr after the injection of the 0.5 mol/kg dose of mercuric chloride were detected only in rats pretreated with acivicin or DEM (fig. 3B). The content of mercury in the total renal mass was 46% lower in the rats pretreated with acivicin and 51% lower in the rats pretreated with DEM than in the control rats. Pretreatment with acivicin caused the total content of mercury in the liver to decrease, whereas pretreatment with either BSO or DEM caused the content of mercury in the liver to increase (fig. 3D). The
5 520 ZALUPS AND LASH FIG. 2.Concentration of GSH in the liver (A) of groups of control rats and rats pretreated with acivicin, BSO, or DEM, and concentration of mercury in the liver (B) of groups of control rats and rats pretreated with acivicin, BSO, or DEM 1 hr after the intravenous injection of a 0.5 mol/kg dose of mercuric chloride. See Materials and Methods for details of pretreatments and handling of tissues. Values are mean SE. * Significantly different (p 0.05) from the corresponding mean for the control group. ** Significantly different (p 0.05) from the means for the corresponding groups of control rats and rats pretreated with acivicin. *** Significantly different (p 0.05) from the means for the corresponding groups of control rats, rats pretreated with acivicin, and rats pretreated with BSO. mean hepatic content of mercury in the rats pretreated with acivicin was 22% lower than that in the control rats. By contrast, the mean hepatic content of mercury in the rats pretreated with either BSO or DEM was 24% greater than that in the control rats. Disposition of Mercury in the Blood. The influence of each pretreatment used on the concentration of mercury in the blood is presented in table 2. No statistically significant differences among the means for the concentration of mercury in blood were detected. More mercury was present in the blood of the rats pretreated with acivicin or DEM, but not BSO, than was in the blood of the control FIG. 3.Content of GSH in the total renal mass (A) and liver (C) of groups of control rats and rats pretreated with acivicin, BSO, or DEM, and the content of mercury in the total renal mass (B) and liver (D) of groups of control rats and rats pretreated with acivicin, BSO, or DEM 1 hr after the intravenous injection of a 0.5 mol/kg dose of mercuric chloride. See Materials and Methods for details of pretreatments and handling of tissues. Values are mean SE. * Significantly different (p 0.05) from the corresponding mean for the control group. ** Significantly different (p 0.05) from the means for the corresponding groups of control rats and rats pretreated with acivicin. *** Significantly different (p 0.05) from the means for the corresponding groups of control rats, rats pretreated with acivicin, and rats pretreated with BSO. Significantly different (p 0.05) from the means for the corresponding groups of control rats and rats pretreated with BSO. rats (fig. 4). In addition, of the mercury in the blood, more was present in the plasma in the rats pretreated with DEM (but not acivicin or BSO) than in the control rats. Discussion In the present study, pretreatment of rats with acivicin, BSO, and DEM caused significant changes in renal GSH status, particularly in the cortex and outer stripe of the outer medulla. All three pretreatments led to a precipitous reduction in the overall renal concentration of GSH. Pretreatment with BSO and DEM was most effective in causing reductions in the overall renal concentration of GSH. Interestingly, pretreatment with acivicin caused a significant depletion of GSH in the renal cortex, but led to a significant increase in the concentration of GSH in the renal outer stripe of the outer medulla. The fall in the concentration of GSH in the cortex would seem to be
6 GSH STATUS AND RENAL DISPOSITION OF MERCURY 521 FIG. 4.Content of mercury in the blood (A) and the amount of mercury in blood present in the plasma (B) of groups of control rats and rats pretreated with acivicin, BSO, or DEM 1 hr after the intravenous injection of a 0.5 mol/kg dose of mercuric chloride. See Materials and Methods for details of pretreatments and handling of tissues. Values are mean SE. * Significantly different (p 0.05) from the corresponding mean for the control group. *** Significantly different (p 0.05) from the means for the corresponding groups of control rats, rats pretreated with acivicin, and rats pretreated with BSO. Significantly different (p 0.05) from the means for the corresponding groups of control rats and rats pretreated with BSO. consistent with the actions of acivicin. Because the compound significantly inhibits the actions of the -glutamyltransferase lining the brush-border membrane of proximal tubules (5), a significant fraction of filtered and secreted GSH in the proximal tubular lumen is not enzymatically broken down to cysteine, which is normally reabsorbed very rapidly and efficiently by the sodium-dependent neutral amino acid transport system. By reducing the pool of reabsorbable cysteine, which serves as the rate-limiting substrate involved in the biosynthesis of GSH, intracellular depletion of GSH occurs. Consistent with this notion are the findings from the present study indicating that the concentrations of cysteine in the cortex were reduced significantly after pretreatment with acivicin. The concentration of cysteine in the renal outer stripe of the outer medulla was also decreased significantly after pretreatment with acivicin, and yet the concentration of GSH in this zone of the kidney increased. It is not exactly clear as to what is responsible for this heterogeneous response. However, one possibility is that as the intraluminal concentration of GSH becomes sufficiently high enough in the terminal segments of the proximal tubule, as a result of the diminished enzymatic degradation of the tripeptide, an unmasking of a reabsorptive mechanism involving the uptake of GSH as an intact tripeptide occurs. Although it is unlikely that any luminal reabsorption of GSH, as an intact tripeptide, occurs in vivo under normal homeostatic conditions, uptake of GSH in terminal segments of the proximal tubule during near complete inhibition of the -glutamyltransferase cannot be ruled out at present. Studies on isolated proximal tubular cells from the rat (7, 8) have characterized sodiumdependent uptake and cellular accumulation of intact GSH. However, this uptake seems to occur mainly at the basolateral membrane (9). It should also be mentioned that there is evidence for dipeptide transport across the luminal membrane. In a recent study by Barfuss et al. (10), it was shown that the dipeptide glycylsarcosine, which is resistant to dipeptidase activity, can be transported across the luminal membrane of proximal tubular epithelial cells in an intact form. When a nontoxic dose of mercuric chloride had been administered after pretreatment with acivicin, an approximate 50% reduction in accumulation of mercury resulted during the first hour after injection of mercury. The decreased renal accumulation of mercury was due almost exclusively to diminished uptake and/or accumulation of mercury in the renal cortex, inasmuch as the concentration of mercury in the outer stripe of the outer medulla, as well as the other zones, was not affected significantly by pretreatment with acivicin. Diminished uptake of mercury after pretreatment with acivicin is also consistent with findings from other studies (2, 11 14). These findings indicate that pretreatment with acivicin causes the renal uptake of mercury to decrease significantly, and causes the urinary excretion of mercury and GSH to increase in rats and mice. In addition, the findings from these studies provide substantial evidence that the primary luminal mechanism involved in the uptake of mercury along proximal tubular epithelial cells is dependent on the activity of the -glutamyltransferase on the brush-border membrane. Significant depletion of renal levels of GSH (by 60 68%) by pretreatment with DEM, but not BSO, also caused a near 50% reduction in the renal content of mercury by the end of the first hour after the injection of the 0.5 mol/kg dose of mercuric chloride. This depletion was due to decreased uptake and/or accumulation of mercury in both the cortex and outer stripe of the outer medulla. Pretreatment with BSO had no effect on concentration of mercury at the level of the whole kidney or in the renal cortex, despite the fact the level of depletion of renal GSH was similar to that attained with the use of DEM. It did, however, cause a slight, but significant increase in the concentration of mercury in the outer stripe of the outer medulla. The findings from the present study have rather significant implications with respect to the effects of depletion of renal GSH and the renal disposition of administered inorganic mercury. First of all, they indicate that, when renal levels of GSH are depleted by a mechanism involving conjugation, there is a decrease in the net accumulation of mercury shortly after exposure to nontoxic levels of inorganic mercury. In reality, the term depletion should be used loosely under these circumstances, inasmuch as the total intracellular number of molecules of GSH is probably not affected significantly, but rather, the free sulfhydryl group on the cysteinyl residue of a significant number of molecules of GSH is oxidized in a conjugation reaction with DEM. On the other hand, the present findings indicate that when intracellular concentrations of GSH are depleted in the renal cortex and outer stripe of the outer medulla by inhibiting the synthesis of new GSH with BSO, the net renal accumulation of mercury is not affected significantly during the first hour after exposure to nontoxic levels of inorganic mercury. It is unclear at present as to why the apparent net renal cortical accumulation of mercury remains unchanged during the inhibition of synthesis of new GSH. One partial explanation is that cysteinyl residues arising from the intraluminal degradation of filtered GSH, or preexisting GSH that was secreted into the lumen, help maintain temporarily free thiol status in the proximal tubular epithelial cells after they are reabsorbed. This hypothesis is supported to a limited degree by the data on the concentration of cysteine in the renal cortex, which indicate that there was not a statistically significant change in the concentration of cysteine in the renal cortex after pretreatment with BSO. The use of a nonnephrotoxic dose of mercuric chloride in the present study thus allowed us to examine effects of altered thiol status on the renal disposition of inorganic mercury without the complications of any apparent diminution of renal function. In addition to low molecular weight thiols, such as GSH and cysteine, protein-sulfhydryls located on the plasma membrane and the intracellular compartment are also important ligands for inorganic
7 522 ZALUPS AND LASH mercury. In fact, protein-sulfhydryl groups likely account for a significant portion of the total intracellular ligands for mercuric ions. Because protein-sulfhydryls are present at substantially higher amounts than low molecular weight thiols, the changes in the intracellular concentrations of GSH and cysteine that were produced by the pretreatments are unlikely to cause changes in their amounts during the 1-hr time frame for these experiments. Hence, variations in the renal and hepatic contents of inorganic mercury caused by the pretreatments should be due to changes in the concentrations of low molecular weight thiols and not to compensatory changes in the amount of protein-sulfhydryls. Effects of depletion of renal GSH by DEM and BSO on the renal disposition of administered mercury have been studied by several groups of investigators. However, varied results have been reported due to one factor or another. For example, Johnson (3) demonstrated that depletion of nonprotein sulfhydryls resulted in diminished renal uptake of inorganic mercury after the administration of a highly nephrotoxic 15 mg/kg dose of mercuric chloride. Baggett and Berndt (1) also reported that depletion of renal levels of nonprotein-sulfhydryls by pretreatment with DEM alone or in combination with BSO caused decreases in the renal accumulation of inorganic mercury during the initial hours after the administration of a nephrotoxic 4.0 mg/kg dose of mercuric chloride. Depletion of nonprotein-sulfhydryl groups in the kidney by pretreatment with DEM has also been reported to cause a reduction in the renal accumulation of mercury during the initial 2 hr after the administration of methyl mercury (15). The previously mentioned findings are consistent with those from the present study. By contrast, Girardi and Elias (4) reported in a more recent study increased net renal accumulation of mercury in rats given a nephrotoxic 5.0 mg/kg dose of mercuric chloride when renal levels of GSH were depleted by 25% after pretreatment with DEM. In another recent study, depletion of renal GSH by pretreatment with BSO caused the net renal accumulation of mercury to decrease in mice after exposure to mercury vapor (16). One of the confounding factors that significantly affects the ability to interpret the data from some of these previous studies is that nephrotoxic doses of inorganic mercury were used. When toxic doses of mercury are used, it becomes extremely difficult to dissect out the influence of the toxic effects of mercury from the effects of the actual depletion of GSH on the renal disposition of both mercury and GSH. It should be emphasized that the present study is apparently the first to examine the effects of acivicin, DEM, or BSO on the renal disposition of inorganic mercury in rats injected with a nontoxic dose of mercuric chloride. In addition to having effects on renal GSH status, all three pretreatments used in the present study caused the concentration and content of GSH in the liver to decrease. By far, pretreatment with DEM and then BSO were most effective in depleting hepatic levels of GSH. Pretreatment with acivicin caused a slight but significant decrease in the net accumulation of mercury. However, pretreatment with either BSO or DEM increased substantially the net hepatic accumulation of mercury. The mechanism by which acivicin causes decreased hepatic accumulation of mercury may be similar to the one responsible for decreased renal uptake and accumulation of mercury. Because the -glutamyltransferase lines the canalicular portion of the plasma membrane of hepatocytes, it is likely that prevention of the degradation of GSH to which inorganic mercury is bound, would most likely prevent the entry of the mercuric ion into the hepatocyte, much like what occurs in the luminal compartment of the proximal tubule. It is less clear as to what mechanism is responsible for the enhanced hepatic accumulation of mercury in light of depletion of GSH. One possibility is that GSH plays a role as a ligand to conjugate with mercuric ions within hepatocytes and that the formation of a mercuric- GSH conjugate is important in the extrusion or outward movement of mercury from the hepatocyte across the cannalicular and/or sinusoidal membrane. Depletion of GSH in hepatocytes would then diminish the amount of mercuric ions that could be conjugated with GSH, and thus, could result in increased hepatocellular accumulation/retention of mercury. This hypothesis is supported, in part, by data linking the hepatocellular secretion of mercury and GSH into the bile (17, 18). In conclusion, this study has demonstrated that depletion of intracellular GSH can alter significantly the accumulation of a nontoxic dose of inorganic mercury in the kidneys, liver, and blood. The mode by which GSH depletion was induced, however, played an important role on the influence exerted in the disposition of mercury. We suggest that whereas GSH or cysteine status by themselves have the potential to influence the uptake and accumulation of mercury, particularly in the kidneys, there are additional factors that affect the renal and hepatic handling of mercury. A critical difference between this study and some previous investigations (on the effects of GSH depletion on the transport and disposition of mercury) is that we used a nontoxic dose of inorganic mercury, whereas others have used moderately to highly nephrotoxic doses. We believe that this is the first study in which a nontoxic dose of inorganic mercury was used in the evaluation of GSH status on the renal and hepatic disposition of inorganic mercury. References 1. J. M. Baggett and W. O. Berndt: The effect of depletion of nonprotein sulfhydryls by diethyl maleate plus buthionine sulfoximine on renal uptake of mercury in the rat. Toxicol. Appl. Pharmacol. 83, (1986). 2. W. O. Berndt, J. M. Baggett, A. Blacker, and M. Houser: Renal glutathione and mercury uptake by kidney. Fundam. Appl. Toxicol. 5, (1985). 3. D. R. Johnson: Role of renal cortical sulfhydryl groups in development of mercury-induced renal toxicity. J. Toxicol. Environ. Health 9, (1982). 4. G. Girardi and M. M. Elias: Effectiveness of N-acetylcysteine in protecting against mercuric chloride-induced nephrotoxicity. Toxicology 67, (1991). 5. R. D. Scott and N. P. Curthoys: Renal clearance of glutathione measured in rats pretreated with inhibitors of glutathione metabolism. Am. J. Physiol. 252, F877 F882 (1987). 6. O. W. Griffith and A. Meister: Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-N-butyl homocysteine sulfoximine). J. Biol. Chem. 254, (1979). 7. T. M. Hagan, T. Y. Aw, and D. P. Jones: Glutathione uptake and protection against oxidative injury in isolated kidney cells. Kidney Int. 34, (1986). 8. T. M. Visarius, D. A. Putt, J. M. Schare, D. M. Pegouske, and L. H. Lash: Pathways of glutathione metabolism and transport in isolated proximal tubular cells from rat kidney. Biochem. Pharmacol. 52, (1996). 9. L. H. Lash and D. P. Jones: Renal glutathione transport: characteristics of the sodium-dependent system in the basal-lateral membrane. J. Biol. Chem. 259, (1984). 10. D. W. Barfuss, V. Ganapathy, and F. H. Liebach: Evidence for active dipeptide transport in isolated proximal straight tubules. Am. J. Physiol. 255, F177 F188 (1988). 11. J. deceaurriz, J. P. Payan, G. Morel, and M. T. Brondeau: Role of extracellular glutathione and -glutamyltranspeptidase in the disposition and kidney toxicity of inorganic mercury in rats. J. Appl. Toxicol. 14, (1994).
8 GSH STATUS AND RENAL DISPOSITION OF MERCURY T. Tanaka, A. Naganuma, and N. Imura: Role of -glutamyltranspeptidase in renal uptake and toxicity of inorganic mercury in mice. Toxicology 60, (1990). 13. T. Tanaka-Kagawa, A. Naganuma, and N. Imura: Tubular secretion and reabsorption of mercury compounds in mouse kidney. J. Pharmacol. Exp. Ther. 264, (1993). 14. R. K. Zalups: Organic anion transport and action of -glutamyl transpeptidase in kidney linked mechanistically to renal tubular uptake of inorganic mercury. Toxicol. Appl. Pharmacol. 132, (1995). 15. R. J. Richardson and S. D. Murphy: Effect of glutathione depletion on tissue deposition of methylmercury in rats. Toxicol. Appl. Pharmacol. 31, (1975). 16. C.-Y. Kim, C. Watanabe, and H. Satoh: Effects of buthionine sulfoximine (BSO) on mercury distribution after Hg exposure. Toxicology 98, (1995). 17. N. Ballatori and T. W. Clarkson: Biliary transport of glutathione and methylmercury. Am. J. Physiol. 244, G435 G441 (1983). 18. N. Ballatori and T. W. Clarkson: Dependence of biliary secretion of inorganic mercury on the biliary transport of glutathione. Biochem. Pharmacol. 33, (1984).
Rudolfs K. Zalups,* Delon W. Barfuss, and Lawrence H. Lash
 Toxicology and Applied Pharmacology 154, 135 144 (1999) Article ID taap.1998.8562, available online at http://www.idealibrary.com on Disposition of Inorganic Mercury Following Biliary Obstruction and Chemically
Toxicology and Applied Pharmacology 154, 135 144 (1999) Article ID taap.1998.8562, available online at http://www.idealibrary.com on Disposition of Inorganic Mercury Following Biliary Obstruction and Chemically
Pretreatment with p-aminohippurate inhibits the renal uptake and accumulation of injected inorganic mercury in the rat
 ELSEVIER Toxicology 103 (1995) 23-35 Pretreatment with p-aminohippurate inhibits the renal uptake and accumulation of injected inorganic mercury in the rat Rudolfs K. Zalups* a, Delon W. Barfussb Division
ELSEVIER Toxicology 103 (1995) 23-35 Pretreatment with p-aminohippurate inhibits the renal uptake and accumulation of injected inorganic mercury in the rat Rudolfs K. Zalups* a, Delon W. Barfussb Division
Molecular Interactions with Mercury in the Kidney
 0031-6997/113/5201-0113$03.00/0 PHARMACOLOGICAL REVIEWS Vol. 52, No. 1 Copyright 20113 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A. Molecular Interactions with
0031-6997/113/5201-0113$03.00/0 PHARMACOLOGICAL REVIEWS Vol. 52, No. 1 Copyright 20113 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A. Molecular Interactions with
RUDOLFS K. ZALUPS, LISA D. PARKS, VERNON T. CANNON, and DELON W. BARFUSS
 0026-895X/98/020353-11$3.00/0 Copyright by The American Society for Pharmacology and Experimental Therapeutics All rights of reproduction in any form reserved. MOLECULAR PHARMACOLOGY, 54:353 363 (1998).
0026-895X/98/020353-11$3.00/0 Copyright by The American Society for Pharmacology and Experimental Therapeutics All rights of reproduction in any form reserved. MOLECULAR PHARMACOLOGY, 54:353 363 (1998).
75% Nephrectomy and the Disposition of Inorganic Mercury In DMSA-Treated Rats Lacking Functional MRP2
 JPET Fast This Forward. article has not Published been copyedited on and December formatted. The 23, final 2009 version as may DOI:10.1124/jpet.109.163774 differ from this version. JPET #163774 75% Nephrectomy
JPET Fast This Forward. article has not Published been copyedited on and December formatted. The 23, final 2009 version as may DOI:10.1124/jpet.109.163774 differ from this version. JPET #163774 75% Nephrectomy
Relationships between the Renal Handling of DMPS and DMSA and the Renal Handling of Mercury
 pubs.acs.org/crt Relationships between the Renal Handling of DMPS and DMSA and the Renal Handling of Mercury Rudolfs K. Zalups* and Christy C. Bridges Division of Basic Medical Sciences, 1550 College Street,
pubs.acs.org/crt Relationships between the Renal Handling of DMPS and DMSA and the Renal Handling of Mercury Rudolfs K. Zalups* and Christy C. Bridges Division of Basic Medical Sciences, 1550 College Street,
Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats In Vivo
 Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats In Vivo BERNHARD H. LAUTERBURG, GEORGE B. CORCORAN, and JERRY R. MITCHELL, Baylor College of
Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats In Vivo BERNHARD H. LAUTERBURG, GEORGE B. CORCORAN, and JERRY R. MITCHELL, Baylor College of
Molecular and ionic mimicry and the transport of toxic metals
 Toxicology and Applied Pharmacology 204 (2005) 274 308 Review Molecular and ionic mimicry and the transport of toxic metals Christy C. Bridges, Rudolfs K. Zalups* Division of Basic Medical Sciences, Mercer
Toxicology and Applied Pharmacology 204 (2005) 274 308 Review Molecular and ionic mimicry and the transport of toxic metals Christy C. Bridges, Rudolfs K. Zalups* Division of Basic Medical Sciences, Mercer
Temporal Changes in Metallothionein Gene Transcription in Rat Kidney and Liver: Relationship to Content of Mercury and Metallothionein Protein 1
 0022-3565/00/2951-0074$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 295, No. 1 Copyright 2000 by The American Society for Pharmacology and Experimental Therapeutics 2604/849232
0022-3565/00/2951-0074$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 295, No. 1 Copyright 2000 by The American Society for Pharmacology and Experimental Therapeutics 2604/849232
Online publication date: 25 June 2010
 This article was downloaded by: [informa internal users] On: 28 July 2010 Access details: Access Details: [subscription number 755239602] Publisher Taylor & Francis Informa Ltd Registered in England and
This article was downloaded by: [informa internal users] On: 28 July 2010 Access details: Access Details: [subscription number 755239602] Publisher Taylor & Francis Informa Ltd Registered in England and
MRP2 and the DMPS- and DMSA-Mediated Elimination of Mercury in TR and Control Rats Exposed to Thiol S-Conjugates of Inorganic Mercury
 TOXICOLOGICAL SCIENCES 105(1), 211 220 (2008) doi:10.1093/toxsci/kfn107 Advance Access publication May 28, 2008 MRP2 and the DMPS- and DMSA-Mediated Elimination of Mercury in TR and Control Rats Exposed
TOXICOLOGICAL SCIENCES 105(1), 211 220 (2008) doi:10.1093/toxsci/kfn107 Advance Access publication May 28, 2008 MRP2 and the DMPS- and DMSA-Mediated Elimination of Mercury in TR and Control Rats Exposed
Amino Acid Transporters Involved in Luminal Transport of Mercuric Conjugates of Cysteine in Rabbit Proximal Tubule
 0022-3565/01/2982-780 789$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 298, No. 2 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 3568/916924
0022-3565/01/2982-780 789$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 298, No. 2 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 3568/916924
Differences in the Fate of Methylmercury between Mice with and without Hair
 450 Journal of Health Science, 52(4) 450 454 (2006) Differences in the Fate of Methylmercury between Mice with and without Hair Tatsumi Adachi*, a, b and Takashi Kuwana a, c a Department of Basic Medical
450 Journal of Health Science, 52(4) 450 454 (2006) Differences in the Fate of Methylmercury between Mice with and without Hair Tatsumi Adachi*, a, b and Takashi Kuwana a, c a Department of Basic Medical
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):
 Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 A schematic representation of the most relevant
Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 A schematic representation of the most relevant
Heterogeneity of glutathione synthesis and secretion in the proximal tubule of the rabbit
 Heterogeneity of glutathione synthesis and secretion in the proximal tubule of the rabbit LISA D. PARKS, 1 RUDOLFS K. ZALUPS, 2 AND DELON W. BARFUSS 1 1 Biology Department, Georgia State University, Atlanta
Heterogeneity of glutathione synthesis and secretion in the proximal tubule of the rabbit LISA D. PARKS, 1 RUDOLFS K. ZALUPS, 2 AND DELON W. BARFUSS 1 1 Biology Department, Georgia State University, Atlanta
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Effect of orally administered glutathione on glutathione levels in some organs of rats: role of specific transporters
 British Journal of Nutrition (1997), 78, 293-300 293 Effect of orally administered glutathione on glutathione levels in some organs of rats: role of specific transporters BY FABIO FAVILLI, PATRIZIA MARRACCINI,
British Journal of Nutrition (1997), 78, 293-300 293 Effect of orally administered glutathione on glutathione levels in some organs of rats: role of specific transporters BY FABIO FAVILLI, PATRIZIA MARRACCINI,
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Autometallographic Localization of Inorganic Mercury in the Kidneys of Rats: Effect of Unilateral Nephrectomy and Compensatory Renal Growth
 EXPERIMENTAL AND MOLECULAR PATHOLOGY 54, 10-21 (191) Autometallographic Localization of Inorganic Mercury in the Kidneys of Rats: Effect of Unilateral Nephrectomy and Compensatory Renal Growth RUDOLFS
EXPERIMENTAL AND MOLECULAR PATHOLOGY 54, 10-21 (191) Autometallographic Localization of Inorganic Mercury in the Kidneys of Rats: Effect of Unilateral Nephrectomy and Compensatory Renal Growth RUDOLFS
April 08, biology 2201 ch 11.3 excretion.notebook. Biology The Excretory System. Apr 13 9:14 PM EXCRETORY SYSTEM.
 Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
Christy C. Bridges, Lucy Joshee, and Rudolfs K. Zalups. Division of Basic Medical Sciences, Mercer University School of Medicine, Macon, Georgia
 0022-3565/08/3241-383 390$20.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 324, No. 1 Copyright 2008 by The American Society for Pharmacology and Experimental Therapeutics 130708/3288879
0022-3565/08/3241-383 390$20.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 324, No. 1 Copyright 2008 by The American Society for Pharmacology and Experimental Therapeutics 130708/3288879
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Homocysteine and the Renal Epithelial Transport and Toxicity of Inorganic Mercury: Role of Basolateral Transporter Organic Anion Transporter 1
 J Am Soc Nephrol 15: 2023 2031, 2004 Homocysteine and the Renal Epithelial Transport and Toxicity of Inorganic Mercury: Role of Basolateral Transporter Organic Anion Transporter 1 RUDOLFS K. ZALUPS and
J Am Soc Nephrol 15: 2023 2031, 2004 Homocysteine and the Renal Epithelial Transport and Toxicity of Inorganic Mercury: Role of Basolateral Transporter Organic Anion Transporter 1 RUDOLFS K. ZALUPS and
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
Enhanced Renal Toxicity by Inorganic Mercury in Metallothionein-Null Mice
 0022-3565/97/2833-1529$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 283, No. 3 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/97/2833-1529$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 283, No. 3 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Collin College. BIOL Anatomy & Physiology. Urinary System. Summary of Glomerular Filtrate
 Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Acids and Bases their definitions and meanings
 Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
PARTS OF THE URINARY SYSTEM
 EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
Lithium-induced Tubular Dysfunction. Jun Ki Park 11/30/10
 Lithium-induced Tubular Dysfunction Jun Ki Park 11/30/10 Use of Lithium Mid 19 th century: treatment of gout Late 19 th century: used for psychiatric disorders Early 20 th century: sodium substitute to
Lithium-induced Tubular Dysfunction Jun Ki Park 11/30/10 Use of Lithium Mid 19 th century: treatment of gout Late 19 th century: used for psychiatric disorders Early 20 th century: sodium substitute to
Figure 26.1 An Introduction to the Urinary System
 Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
III. TOXICOKINETICS. Studies relevant to the toxicokinetics of inorganic chloramines are severely
 III. TOXICOKINETICS Introduction Studies relevant to the toxicokinetics of inorganic chloramines are severely limited. However, studies done with various chlorinated amino compounds (including organic
III. TOXICOKINETICS Introduction Studies relevant to the toxicokinetics of inorganic chloramines are severely limited. However, studies done with various chlorinated amino compounds (including organic
The Importance and Regulation of Hepatic Glutathione
 THE YALE JOURNAL OF BIOLOGY AND MEDICINE 54 (1981), 497-502 The Importance and Regulation of Hepatic Glutathione NEIL KAPLOWITZ, M.D. Gastroenterology, Medical and Research Services, Wadsworth Veterans
THE YALE JOURNAL OF BIOLOGY AND MEDICINE 54 (1981), 497-502 The Importance and Regulation of Hepatic Glutathione NEIL KAPLOWITZ, M.D. Gastroenterology, Medical and Research Services, Wadsworth Veterans
Unit 2b: EXCRETION OF DRUGS. Ms.M.Gayathri Mpharm (PhD) Department of Pharmaceutics Krishna Teja Pharmacy college Subject code: 15R00603 (BPPK)
 Unit 2b: EXCRETION OF DRUGS By Ms.M.Gayathri Mpharm (PhD) Department of Pharmaceutics Krishna Teja Pharmacy college Subject code: 15R00603 (BPPK) Excretion, along with metabolism and tissue redistribution,
Unit 2b: EXCRETION OF DRUGS By Ms.M.Gayathri Mpharm (PhD) Department of Pharmaceutics Krishna Teja Pharmacy college Subject code: 15R00603 (BPPK) Excretion, along with metabolism and tissue redistribution,
Introduction. Acids, Bases and ph; a review
 0 P a g e Introduction In this sheet, we discuss acidbase balance in our body and the role of kidneys in its establishment. Arrangement of topics is different from that of the lecture, to assure consistency
0 P a g e Introduction In this sheet, we discuss acidbase balance in our body and the role of kidneys in its establishment. Arrangement of topics is different from that of the lecture, to assure consistency
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Interrelationship between Angiotensin Catecholamines. Tatsuo SATO, M.D., Masaru MAEBASHI, M.D., Koji GOTO, M.D., and Kaoru YOSHINAGA, M.D.
 Interrelationship between Angiotensin and Catecholamines Tatsuo SATO, M.D., Masaru MAEBASHI, M.D., Koji GOTO, M.D., and Kaoru YOSHINAGA, M.D. SUMMARY Urinary catecholamines were measured with an attempt
Interrelationship between Angiotensin and Catecholamines Tatsuo SATO, M.D., Masaru MAEBASHI, M.D., Koji GOTO, M.D., and Kaoru YOSHINAGA, M.D. SUMMARY Urinary catecholamines were measured with an attempt
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
28/04/2013 LEARNING OUTCOME C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS STUDENT ACHIEVEMENT INDICATORS URINARY SYSTEM & EXCRETION
 LEARNING OUTCOME C13 Analyse the functional interrelationships of the structures of the urinary system Learning Outcome C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS Students who have fully met this
LEARNING OUTCOME C13 Analyse the functional interrelationships of the structures of the urinary system Learning Outcome C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS Students who have fully met this
rabbit, 45 min for dog) and more slowly for dehydrocholic acid (25- decrease, questioning the mechanism by which bile acids increase bile
 J. Physiol. (1972), 224, pp. 259-269 259 With 6 text-ftgure8 Printed in Great Britain SPECIES DIFFERENCES IN THE CHOLERETIC RESPONSE TO BILE SALTS BY CURTIS D. KLAASSEN From the Clinical Pharmacology and
J. Physiol. (1972), 224, pp. 259-269 259 With 6 text-ftgure8 Printed in Great Britain SPECIES DIFFERENCES IN THE CHOLERETIC RESPONSE TO BILE SALTS BY CURTIS D. KLAASSEN From the Clinical Pharmacology and
INTRODUCTION
 CHAPTER I 37 3.1.0.0. INTRODUCTION Molecular oxygen is an essential nutrient for higher forms of life. In addition to its normal physiological reactions, oxygen and hs partially reduced forms, ROS oxidize
CHAPTER I 37 3.1.0.0. INTRODUCTION Molecular oxygen is an essential nutrient for higher forms of life. In addition to its normal physiological reactions, oxygen and hs partially reduced forms, ROS oxidize
NORMAL POTASSIUM DISTRIBUTION AND BALANCE
 NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
Chapter 13 The Urinary System
 Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Synthesis and Transport of Glutathione by Cultured Human Retinal Pigment Epithelial Cells
 Synthesis and Transport of Glutathione by Cultured Human Retinal Pigment Epithelial Cells P. Carl Davidson* Paul Sternberg, Jr., Dean P. Jones,*\ and Robyn L. Reed* Purpose. To characterize synthesis and
Synthesis and Transport of Glutathione by Cultured Human Retinal Pigment Epithelial Cells P. Carl Davidson* Paul Sternberg, Jr., Dean P. Jones,*\ and Robyn L. Reed* Purpose. To characterize synthesis and
Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog
 Tohoku J. exp. Med., 1966, 88, 361-366 Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog Sennosuke Ohukuzi Deparment of Physiology (Prof. T. Suzuki),
Tohoku J. exp. Med., 1966, 88, 361-366 Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog Sennosuke Ohukuzi Deparment of Physiology (Prof. T. Suzuki),
NIH Public Access Author Manuscript Kidney Int. Author manuscript; available in PMC 2013 November 01.
 NIH Public Access Author Manuscript Published in final edited form as: Kidney Int. 2013 May ; 83(5): 779 782. doi:10.1038/ki.2012.468. Need to quickly excrete K +? Turn off NCC Alicia A. McDonough 1 and
NIH Public Access Author Manuscript Published in final edited form as: Kidney Int. 2013 May ; 83(5): 779 782. doi:10.1038/ki.2012.468. Need to quickly excrete K +? Turn off NCC Alicia A. McDonough 1 and
A&P 2 CANALE T H E U R I N A R Y S Y S T E M
 A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Thiol redox systems. Chao Sun (CCK KI) Ting Jia (RBC UNL) Saeed Eshtad (MBB KI) Weishu Fan (RBC UNL)
 Thiol redox systems Chao Sun (CCK KI) Ting Jia (RBC UNL) Saeed Eshtad (MBB KI) Weishu Fan (RBC UNL) Questions What is the function of thioredoxin? Questions What is the function of thioredoxin? How many
Thiol redox systems Chao Sun (CCK KI) Ting Jia (RBC UNL) Saeed Eshtad (MBB KI) Weishu Fan (RBC UNL) Questions What is the function of thioredoxin? Questions What is the function of thioredoxin? How many
EXCRETORY SYSTEM E. F. G. H.
 XRTORY SYSTM 1. Label the following parts of the nephron in the diagram below:..... F. G. H. I. J. K. L. 2. Identify the following as either True or False: There is a greater osmotic concentration in the
XRTORY SYSTM 1. Label the following parts of the nephron in the diagram below:..... F. G. H. I. J. K. L. 2. Identify the following as either True or False: There is a greater osmotic concentration in the
TITLE PAGE. Transport of N-Acetylcysteine S-Conjugates of Methylmercury in. MDCK Cells Transfected Stably with hoat1
 JPET This Fast article Forward. has not been Published copyedited and on formatted. May 20, The 2005 final as version DOI:10.1124/jpet.105.086645 may differ from this version. TITLE PAGE Transport of N-Acetylcysteine
JPET This Fast article Forward. has not been Published copyedited and on formatted. May 20, The 2005 final as version DOI:10.1124/jpet.105.086645 may differ from this version. TITLE PAGE Transport of N-Acetylcysteine
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
WHY DO WE NEED AN EXCRETORY SYSTEM? Function: To eliminate waste To maintain water and salt balance To maintain blood pressure
 EXCRETORY SYSTEM WHY DO WE NEED AN EXCRETORY SYSTEM? Function: To eliminate waste To maintain water and salt balance To maintain blood pressure These wastes include: Carbon dioxide Mostly through breathing
EXCRETORY SYSTEM WHY DO WE NEED AN EXCRETORY SYSTEM? Function: To eliminate waste To maintain water and salt balance To maintain blood pressure These wastes include: Carbon dioxide Mostly through breathing
Lund, 1948), the effect of which was to produce glomerular lesions without. relationship between increased protein loads and the tubular reabsorption
 544 J. Phy8iol. (1961), 156, pp. 544-554 With 5 text-ftgure8 Printed in Great Britain TUBULAR REABSORPTION OF PROTEIN IN RATS WITH EXPERIMENTAL PROTEINURIA BY D. MENDEL* From the Department of Physiology,
544 J. Phy8iol. (1961), 156, pp. 544-554 With 5 text-ftgure8 Printed in Great Britain TUBULAR REABSORPTION OF PROTEIN IN RATS WITH EXPERIMENTAL PROTEINURIA BY D. MENDEL* From the Department of Physiology,
Urinary System BIO 250. Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat. Routes of Waste Elimination
 Urinary System BIO 250 Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat Routes of Waste Elimination Skin: Variable amounts of heat, salts, and water; small amounts of urea and
Urinary System BIO 250 Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat Routes of Waste Elimination Skin: Variable amounts of heat, salts, and water; small amounts of urea and
Mechanism of Detoxification
 Mechanism of Detoxification Prof.Dr. Hedef Dhafir El-Yassin 1 Objectives: 1. To list the detoxification pathways 2. To describe detoxification pathways and phases in the liver, 2 3 4 o Xenobiotics are
Mechanism of Detoxification Prof.Dr. Hedef Dhafir El-Yassin 1 Objectives: 1. To list the detoxification pathways 2. To describe detoxification pathways and phases in the liver, 2 3 4 o Xenobiotics are
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
clearing activity is produced and destroyed in the rat. Both the
 THE SITES AT WHICH PLASMA CLEARING ACTIVITY IS PRODUCED AND DESTROYED IN THE RAT. By G. H. JEFFRIES. From the Sir William Dunn School of Pathology, Oxford. (Received for publication 25th June 1954.) CLEARING
THE SITES AT WHICH PLASMA CLEARING ACTIVITY IS PRODUCED AND DESTROYED IN THE RAT. By G. H. JEFFRIES. From the Sir William Dunn School of Pathology, Oxford. (Received for publication 25th June 1954.) CLEARING
Acid Base Balance. Professor Dr. Raid M. H. Al-Salih. Clinical Chemistry Professor Dr. Raid M. H. Al-Salih
 Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Excretory System. Biology 2201
 Excretory System Biology 2201 Excretory System How does the excretory system maintain homeostasis? It regulates: Body heat Water-salt concentrations Acid-base concentrations Metabolite concentrations ORGANS
Excretory System Biology 2201 Excretory System How does the excretory system maintain homeostasis? It regulates: Body heat Water-salt concentrations Acid-base concentrations Metabolite concentrations ORGANS
Excretory System. Excretory System
 Excretory System Biology 2201 Excretory System How does the excretory system maintain homeostasis? It regulates: Body heat Water-salt concentrations Acid-base concentrations Metabolite concentrations 1
Excretory System Biology 2201 Excretory System How does the excretory system maintain homeostasis? It regulates: Body heat Water-salt concentrations Acid-base concentrations Metabolite concentrations 1
corn oil. The controls received an equivalent
 Effect of 2,3,7,8-Tetrachlorodibenzo-p-dioxin on the Biliary Excretion of Indocyanine Green in Rat by Slang W1. Hwang* Chlorinated dibenzodioxins have been found as contaminants of various technical chlorinated
Effect of 2,3,7,8-Tetrachlorodibenzo-p-dioxin on the Biliary Excretion of Indocyanine Green in Rat by Slang W1. Hwang* Chlorinated dibenzodioxins have been found as contaminants of various technical chlorinated
After studying this lecture, you should be able to...
 Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
The Weight of the Evidence: The Role of Chelation in the Treatment of Arsenic and Mercury Poisoning
 The Weight of the Evidence: The Role of Chelation in the Treatment of Arsenic and Mercury Poisoning Michael J. Kosnett, MD, MPH Associate Clinical Professor Division of Clinical Pharmacology & Toxicology
The Weight of the Evidence: The Role of Chelation in the Treatment of Arsenic and Mercury Poisoning Michael J. Kosnett, MD, MPH Associate Clinical Professor Division of Clinical Pharmacology & Toxicology
CHAPTER 6 FUNCTIONAL PROPERTIES OF PROTEIN HYDROLYSATES
 68 CHAPTER 6 FUNCTIONAL PROPERTIES OF PROTEIN HYDROLYSATES 6.1 INTRODUCTION Functional properties can be defined as the overall physicochemical properties of proteins in food systems during processing,
68 CHAPTER 6 FUNCTIONAL PROPERTIES OF PROTEIN HYDROLYSATES 6.1 INTRODUCTION Functional properties can be defined as the overall physicochemical properties of proteins in food systems during processing,
Renal Regulation of Sodium and Volume. Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM
 Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
UNIT 3 Conditions supporting life
 Biology Form 4 Page 32 Ms. R. Buttigieg UNIT 3 Conditions supporting life In this unit we shall be seeing how an important condition that supports life is the ability of the organism to maintain a constant
Biology Form 4 Page 32 Ms. R. Buttigieg UNIT 3 Conditions supporting life In this unit we shall be seeing how an important condition that supports life is the ability of the organism to maintain a constant
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
12/7/10. Excretory System. The basic function of the excretory system is to regulate the volume and composition of body fluids by:
 Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
ACTIVE TRANSPORT OF SALICYLATE BY RAT JEJUNUM
 Quarterly Journal of Experimental Physiology (1981) 66, 91-98 91 Printed in Great Britain ACTIVE TRANSPORT OF SALICYLATE BY RAT JEJUNUM R. B. FISHER University Laboratory of Physiology, Oxford (RECEIVED
Quarterly Journal of Experimental Physiology (1981) 66, 91-98 91 Printed in Great Britain ACTIVE TRANSPORT OF SALICYLATE BY RAT JEJUNUM R. B. FISHER University Laboratory of Physiology, Oxford (RECEIVED
Renal Pharmacology. Diuretics: Carbonic Anhydrase Inhibitors Thiazides Loop Diuretics Potassium-sparing Diuretics BIMM118
 Diuretics: Carbonic Anhydrase Inhibitors Thiazides Loop Diuretics Potassium-sparing Diuretics Renal Pharmacology Kidneys: Represent 0.5% of total body weight, but receive ~25% of the total arterial blood
Diuretics: Carbonic Anhydrase Inhibitors Thiazides Loop Diuretics Potassium-sparing Diuretics Renal Pharmacology Kidneys: Represent 0.5% of total body weight, but receive ~25% of the total arterial blood
Renal Physiology - Lectures
 Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Excretion and Water Balance
 Excretion and Water Balance In the body, water is found in three areas, or compartments: Plasma, the liquid portion of the blood without the blood cells, makes up about 7 percent of body fluid. The intercellular
Excretion and Water Balance In the body, water is found in three areas, or compartments: Plasma, the liquid portion of the blood without the blood cells, makes up about 7 percent of body fluid. The intercellular
What is excretion? Excretion is the removal of metabolic waste from the body.
 Excretion What is excretion? Excretion is the removal of metabolic waste from the body. Excretion in Plants Plants produce very little waste products. Plants lose oxygen and water vapour through the stomata.
Excretion What is excretion? Excretion is the removal of metabolic waste from the body. Excretion in Plants Plants produce very little waste products. Plants lose oxygen and water vapour through the stomata.
Sunday, July 17, 2011 URINARY SYSTEM
 URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
Urinary System and Excretion. Bio105 Lecture 20 Chapter 16
 Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Non-protein nitrogenous substances (NPN)
 Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Salt and Water Balance and Nitrogen Excretion
 Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Review Article. Glutathione as an antioxidant in inorganic mercury induced nephrotoxicity
 Review Article Glutathione as an antioxidant in inorganic mercury induced nephrotoxicity Jan at 1,2, Ali A 2, Haq QMR 1 1 Department of Bioscience, Jamia Millia Islamia, New Delhi, 2 Department of Biotechnology,
Review Article Glutathione as an antioxidant in inorganic mercury induced nephrotoxicity Jan at 1,2, Ali A 2, Haq QMR 1 1 Department of Bioscience, Jamia Millia Islamia, New Delhi, 2 Department of Biotechnology,
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
One-Compartment Open Model: Intravenous Bolus Administration:
 One-Compartment Open Model: Intravenous Bolus Administration: Introduction The most common and most desirable route of drug administration is orally by mouth using tablets, capsules, or oral solutions.
One-Compartment Open Model: Intravenous Bolus Administration: Introduction The most common and most desirable route of drug administration is orally by mouth using tablets, capsules, or oral solutions.
Excretion of Drugs. Prof. Hanan Hagar Pharmacology Unit Medical College
 Excretion of Drugs Prof. Hanan Hagar Pharmacology Unit Medical College Excretion of Drugs By the end of this lecture, students should be able to! Identify main and minor routes of excretion including renal
Excretion of Drugs Prof. Hanan Hagar Pharmacology Unit Medical College Excretion of Drugs By the end of this lecture, students should be able to! Identify main and minor routes of excretion including renal
Urinary System. Chapter 17 7/19/11. Introduction
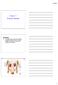 7/19/11 Chapter 17 Urinary System Introduction A. The urinary system consists of two kidneys that filter the blood, two ureters, a urinary bladder, and a urethra to convey waste substances to the outside.
7/19/11 Chapter 17 Urinary System Introduction A. The urinary system consists of two kidneys that filter the blood, two ureters, a urinary bladder, and a urethra to convey waste substances to the outside.
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Outline Urinary System. Urinary System and Excretion. Urine. Urinary System. I. Function II. Organs of the urinary system
 Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
RENAL FUNCTION An Overview
 RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
Chapter 44. Osmoregulation and Excretion
 Chapter 44 Osmoregulation and Excretion Overview: A Balancing Act Physiological systems of animals operate in a fluid environment Relative concentrations of water and solutes must be maintained within
Chapter 44 Osmoregulation and Excretion Overview: A Balancing Act Physiological systems of animals operate in a fluid environment Relative concentrations of water and solutes must be maintained within
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
1. The Fibrous Capsule covers the outside of the kidney. It is made of fat and fibers.
 Slide 2 The kidney has a number of functions. First is the excretion of toxic metabolic waste through urine production. The kidneys filter blood plasma and as a result of filtering blood, the kidneys help
Slide 2 The kidney has a number of functions. First is the excretion of toxic metabolic waste through urine production. The kidneys filter blood plasma and as a result of filtering blood, the kidneys help
