Survival of Legionella pneumophila in the Cold-Water Ciliate
|
|
|
- Alexina Gibbs
- 5 years ago
- Views:
Transcription
1 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Sept. 1991, p Vol. 57, No /91/ $2./ Copyright 1991, American Society for Microbiology Survival of Legionella pneumophila in the Cold-Water Ciliate Tetrahymena vorax HARRIETT E. SMITH-SOMERVILLE,* VIVIAN BUTZ HURYN, CATHERINE WALKER, AND ALVIN L. WINTERS Cell and Molecular Biology Group, Department of Biological Sciences, The University of Alabama, Tuscaloosa, Alabama Received 8 April 1991/Accepted 1 July 1991 The processing of phagosomes containing Legionella pneumophila and Escherichia coli were compared in Tetrahymena vorax, a hymenostome ciliated protozoan that prefers lower temperatures. L. pneumophila did not multiply in the ciliate when incubated at 2 to 22 C, but vacuoles containing L. pneumophila were retained in the cells for a substantially longer time than vacuoles with E. coli. Electron micrographs showed no evidence of degradation of L. pneumophila cells through 12 h, while E. coli cells in the process of being digested were observed in vacuoles 75 min after the addition of the bacterium. T. vorax ingested L. pneumophila normally, but by 1 to 15 min, the vacuolar membrane appeared denser than that surrounding nascent or newly formed phagosomes. In older vacuoles, electron-dense particles lined portions of the membrane. Acidification of the phagosomes indicated by the accumulation of neutral red was similar in T. vorax containing L. pneumophila or E. coli. This ciliate could provide a model for the analysis of virulence-associated intracellular events independent of the replication of L. pneumophila. Legionella pneumophila, a facultative intracellular pathogen with a permissible temperature range for growth of 25 to 42 C (32), has been isolated from aquatic environments including conventional cooling towers, streams, lakes, and potable water sources (6, 7, 11, 16, 21, 23). The ability of L. pneumophila to survive and reproduce in freshwater amoebae and Tetrahymena spp. (2, 3, 9, 1, 25, 31) has been suggested to provide protection for the bacterium from lower temperatures during the winter months (3). This relationship potentially could permit the existence and propagation of L. pneumophila in chilled water aquifer thermal energy storage systems in which aquifer water is intimately exposed to air by spray to chill the water from 17 to 22 C to 7 C or less for aquifer storage. Currently, only one aquifer thermal energy storage system is operating in the United States, and this prototype system has demonstrated the potential for notable energy savings in temperate regions (18). Previous investigators have examined the survival of L. pneumophila within protozoa at temperatures within the permissible range for growth of both bacteria and protozoa, including Tetrahymena pyriformis. To investigate the possible survival of L. pneumophila with protozoa active at lower temperatures, we selected T. vorax as a model host. This species has an optimum growth temperature of 2 C. Temperatures of -3 C are lethal. T. vorax is a hymenostome ciliate which in nature feeds by filtration and concentration of bacteria and other particulate material into food vacuoles (phagosomes) at the base of the cytopharynx of the oral apparatus. After separation from the cytopharynx, the phagosome enters a processing period during which the vacuole decreases in size and undergoes an initial acidification stage. This is followed by a digestive stage in which the vacuole contents are hydrolyzed by enzymes acquired by fusion with lysosomes. The final stage in the digestive cycle is the fusion of the vacuole with the cytoproct, a defined region of the cell surface, for release of * Corresponding author undigested material. In T. vorax, the timing of the stages of the digestive cycle has been determined by vacuole ph, acid phosphatase activity of isolated vacuoles, and initiation of the defecation period for vacuoles labeled with ferric oxide (29). Acidification begins shortly after detachment of the phagosome from the cytopharynx and is essentially complete by 3 min, whereas the digestive phase initiated by the fusion of lysosomes is entered between 3 and 6 min. The end of the processing period and the beginning of the defecation period occurs at 1 min. In this report, we compare the processing of vacuoles containing L. pneumophila with that of vacuoles with Escherichia coli, a bacterium normally digested by Tetrahymena spp. MATERIALS AND METHODS Cultures. L. pneumophila serogroup 1 (Philadelphia 1 strain) was obtained from James Barbaree (Centers for Disease Control, Atlanta, Ga.). Cryogenic cultures were inoculated onto buffered charcoal-yeast extract agar containing L-cysteine (.4 mg/liter) and incubated at 35 C under humidified air and 2.5% CO2 for 4 to 7 days. Bacteria were harvested by flooding the plate with sterile tap water, and the cell density was adjusted to a McFarland no. 5 standard. Samples were fixed in 2.5% (vol/vol) glutaraldehyde and 2% (wt/vol) paraformaldehyde in modified inorganic medium (27), and the optical density (A6) was measured. Dilutions of 1-6, 1-7, and 1-8 were plated in duplicate, and the cell density was determined as CFU. E. coli Y19 (Promega, Madison, Wis.) was inoculated into LB broth and incubated overnight. The bacteria were concentrated by centrifugation and suspended in distilled water. The optical density (A6) was adjusted to that of the L. pneumophila suspensions, or the cell density was adjusted to a McFarland no. 5 standard. Cultures of T. vorax (Kidder 1941) strain V2S were started by transferring 1 ml of a stationary-phase culture into 5 ml of sterile Loefer's medium (17) contained in a 5-ml Erlen-
2 VOL. 57, 1991 LEGIONELLA PNEUMOPHILA IN TETRAHYMENA VORAX 2743 meyer flask. The protozoa were grown for 2 days at 2 C. Cell density was determined with a Coulter Counter. Retention and growth of bacteria. Bacteria were stained for 1 min with 1 or 1,ug of 4',6-diamidino-2-phenylindole (DAPI) per ml, depending on the intensity of the fluorescence obtained with each preparation of the dye. The cells were pelleted, washed in inorganic medium, and suspended in inorganic medium to the initial volume. Bacterial plate counts were performed to confirm viability. In some experiments, T. vorax was washed three times in inorganic medium, suspended to the initial volume, and incubated for 1 h prior to the addition of the bacteria. The bacterial and T. vorax suspensions were combined at a bacteria/protozoa ratio of _14/1 and incubated at room temperature (2 to 22 C) for 5 min. Initially, different ratios of T. vorax and bacteria were mixed to observe the ingestion of bacteria during a 5-min pulse. The 14/1 ratio of bacteria to protozoa was necessary to produce a consistent number of protozoa containing vacuoles with bacteria. Lower bacterium/protozoan ratios yielded large numbers of protozoa with no vacuoles containing bacteria and a highly variable number of bacteria per vacuole in those protozoa with labeled vacuoles. At the end of the pulse, the suspension was centrifuged for 1 s at top speed in aniec clinical model centrifuge. The pellet was washed three times in inorganic medium, suspended in inorganic medium to the initial volume of the protozoan suspension, transferred to a 5-ml Erlenmeyer flask, and maintained at room temperature for the chase period of the experiment. For longer experiments, the pellet was suspended in Loefer's medium, and 2.5 ml were placed in 1 ml of Loefer's medium in a 125-ml Erlenmeyer flask. Samples were removed at intervals designated by the elapsed time following the addition of the bacteria and placed in an equal volume of 5% (vol/vol) glutaraldehyde and 4% (wt/vol) paraformaldehyde in inorganic medium. The number of vacuoles containing DAPI-stained bacteria was counted by using a Leitz Ortholux II microscope equipped with epifluorescence optics, and the mean number of vacuoles per 1 cells at each time was determined. For protozoa suspended in fresh Loefer's medium for the chase period, the mean was normalized to the initial density of the protozoa to adjust for cell division. Protozoa were photographed with Kodak T-Max 4 or Ektar 125 film by using a Nikon Optiphot microscope equipped with epifluorescence optics and a Microflex UFX-II photographic attachment. To estimate the number of CFU of L. pneumophila at each time, the procedure was repeated with unstained bacteria. Samples were removed at designated intervals, and dilutions of 1', 1-6, and1i-' were plated. Electron microscopy. T. vorax and unstained L. pneumophila, E. coli, or a suspension of equal volumes of both bacteria were combined and processed as described above. Aliquots were removed at the specified intervals and placed in an equal volume of either 5% (vol/vol) glutaraldehyde or 5% (vol/vol) glutaraldehyde and 4% (wt/vol) paraformaldehyde in inorganic medium. Samples were fixed in the primary fixative for 2 min, washed three times in inorganic medium, and postfixed in 1% osmium tetroxide in inorganic medium for 3 min. Following dehydration in a graded ethanol series, the cells were embedded in Spurr's lowviscosity resin (3). Sections were cut with a Sorvall MT 5 microtome, stained with uranyl acetate and lead citrate, and viewed with a Zeiss EM1OA electron microscope operated at 6kV. Acidification of vacuoles. T. vorax was washed and suspended in inorganic medium, ph 7., and incubated for 1 h. z LX cr_ U,) LUI D a A A A A TIME (min) FIG. 1. Representative semilogarithmic plots of the percent of vacuoles containing E. coli () or L. pneumophila (A) remaining in protozoa chased with inorganic medium for periods up to 36 min relative to the mean number of vacuoles with E. coli (3.6 vacuoles per cell) or with L. pneumophila (3.5 vacuoles per cell) present in cells after a 5-min pulse. Arrow indicates the end of the processing period and beginning of the defecation period (29). The ph of the medium was checked after the incubation period. The protozoa were pulsed with DAPI-stained bacteria for 5 min and chased in inorganic medium. At intervals during the chase period, the protozoa were stained with neutral red (5,ug/ml) for 1.5 min, rinsed with inorganic medium to remove the free base, and fixed in 1% (vol/vol) glutaraldehyde in inorganic medium. The samples were viewed and photographed immediately. A red color of a vacuole containing bacteria indicated that the vacuole was acidic. Neutral red diffuses rapidly across the membranes in the free-base form but becomes trapped in vesicles with acidic ph because of the reduced permeability of the protonated form (24). This technique permits qualitative identification of acidic compartments which appear red as a result of the accumulation of the protonated dye. RESULTS When either bacterium was supplied tot. vorax as its only food source, the mean number of vacuoles per cell containing fluorescent L. pneumophila remained essentially constant over a 6-h period, while the number of vacuoles containing the control bacterium E. coli began to decline between 6 and 7 min (Fig. 1), well before the beginning of the defecation period at 1 min (29). L. pneumophila also persisted in T. vorax supplemented with Loefer's medium to eliminate starvation effects, but the mean number of vacuoles with fluorescence characteristic of that after the 5-min pulse with L. pneumophila decreased gradually but significantly (P <.1) over 12 h (Fig. 2). Initially, few bacteria were present free in the medium, but after 2 to 3 h of the chase period, fluorescent spheres resembling vacuole contents were observed in the medium, and some ciliates with vacuoles containing only one to a few L. pneumophila cells in addition to the vacuoles formed during the 5-min pulse were noted. Some protozoa had individual bacteria throughout the cell; these protozoa often appeared to be dead or dying. By 24 h, few ciliates contained discrete heavily labeled vacuoles, but the number of viable cells of L. pneumophila (-17 CFU) remained constant over this time. L. pneumophila remained viable but did not grow on buffered charcoal-yeast extract agar medium at 2 to 22 C or
3 2744 SMITH-SOMERVILLE ET AL. APPL. ENVIRON. MICROBIOL. z z :2 LLJ 1 U) LJ 5 D T! E 3 (n Q 2 x Lō TIME (h) FIG. 2. Semilogarithmic plot of the percent of vacuoles containing L. pneumophila remaining in T. vorax placed in fresh Loefer's medium relative to the mean number of vacuoles with the bacterium (3.7 vacuoles per cell) present in cells after a 5-min pulse. The data (mean + SD for three populations) were normalized to the initial protozoan density. in Loefer's medium either at 2 to 22 C or at 35 C. The bacterium also did not multiply in T. vorax during the 12-h period at 2 to 22 C, nor did the number of CFU increase when the time was extended to 48 h (data not shown). T. vorax underwent cell division when resuspended in fresh Loefer's medium, but the increase in cell density was less for populations that had ingested L. pneumophila relative to controls without the bacterium (Fig. 3). Figures 4 and S illustrate vacuoles containing L. pneumophila and E. coli at different times after the addition of bacteria. These bacteria differ in appearance in electron micrographs, with L. pneumophila observed as substantially smaller than E. coli and less coccobacillary in shape. During ingestion, L. pneumophila was located in vacuoles formed at the cytopharynx of T. vorax (Fig. 4A). When long bacteria were ingested, they often caused the shape of the vacuole to deviate from the normal spherical shape (Fig. 4B). Electronlucent vesicles occurred near the membrane of phagosomes before and immediately after separation from the cytopharynx, while the vacuole is associated with the deep fiber (Fig. 4C). In some sections, these vesicles appeared to be fusing with the vacuolar membrane. Initially, the bacteria were dispersed within the vacuole (Fig. 4A through C and SA), but shortly after vacuole formation, both L. pneumophila and E. coli were compressed and close to the phagosomal membrane (Fig. 4B, 4D, and SB). While cells of E. coli were often in contact with each other and appressed to the membrane (Fig. SB), those of L. pneumophila were surrounded by an electron-lucent zone (Fig. 4D). By 75 min, lysosomes were adjacent to the membrane of some vacuoles containing E. coli, and many of the bacteria no longer showed the finely 15 F TIME (h) FIG. 3. Representative semilogarithmic plots of the cell density of T. vorax in the presence () and absence () of L. pneumophila when populations were washed and placed in fresh Loefer's medium. The difference in slopes is significant at the 95% confidence level. granular appearance of intact cells (Fig. 5C). The contents of these vacuoles frequently were separated from the membrane by a space. At 12 min, the vacuoles ranged from those with bacteria similar in appearance to bacteria in vacuoles at 75 min to vacuoles containing remnants of cell walls with few to no intact bacteria (Fig. SD through F). Vacuole fusion also was observed (Fig. SF). In contrast, L. pneumophila showed no signs of degradation through 12 h (Fig. 4E). The vacuoles were irregular in shape, with bacteria in invaginations. When L. pneumophila and E. coli were ingested together, both types of bacteria appeared to be digested during a processing time similar to that for E. coli alone (Fig. 4F). No vacuoles containing bacteria were observed in 6-h samples. The membrane of phagosomes in protozoa fixed immediately after a 5-min incubation with L. pneumophila was similar to that delimiting vacuoles in control cells containing E. coli throughout the digestive cycle (Fig. 4A through C, 5, and 6A); however, in samples washed and suspended before fixation (1 to 15 min after addition of the bacteria) and those at 3 min, most vacuoles appeared to be lined by a dense membrane (Fig. 4D and 6B). A few mitochondria were observed appressed to the membrane from this time through 12 h (Fig. 4D and E). Electron-dense particles similar to cytoplasmic ribosomes were present near the vacuole, but it is unclear whether these are aligned along the membrane in a manner characteristic of older vacuoles (Fig. 6C) until after 3 min. At later times, the membrane also lacked the density. Unlike the dense membrane which appears to border the entire surface of younger vacuoles, the electrondense particles do not completely line the membrane but are almost always present along the membrane adjacent to FIG. 4. Electron micrographs of sections through vacuoles in T. vorax containing L. pneumophila. Bar, 1 p.m. (A) Nascent vacuole in cell incubated with L. pneumophila for 5 min before fixation. (B) Section through two vacuoles formed during a 5-min incubation with bacteria before fixation. Note the shape of the vacuole containing a long bacterium. (C) Section through vacuole formed during a 5-min pulse with L. pneumophila. Note the electron-lucent vesicles around the phagosome (arrow) and the deep fiber (arrowhead), indicating that this is a newly formed vacuole near the oral apparatus. Inset shows a section through an electron-lucent vesicle fusing with a vacuole (bar,.1 p.m). (D) Section through a 3-min-old vacuole. Note clear spaces around bacteria, the dense appearance of the vacuole surface and the mitochondrion appressed to the membrane (arrow). (E) Section through a 12-h-old vacuole. Note presence of bacteria in invaginations (arrows) and the granular appearance along phagosomal membrane (arrowhead). Two mitochondria are appressed to the membrane (double arrows). (F) Section through 12-min-old vacuole containing both E. coli (large cells, arrow) and L. pneumophila (small cells, arrowhead). Both bacteria appear to be undergoing degradation.
4 2745 LEGIONELLA PNEUMOPHILA IN TETRAHYMENA VORAX VOL. 57, 1991
5 APPL. ENVIRON. MICROBIOL. SMITH-SOMERVILLE ET AL. 2746
6 VOL. 57, 1991 LEGIONELLA PNEUMOPHILA IN TETRAHYMENA VORAX 2747 FIG. 6. Sections through vacuoles illustrating appearance of vacuolar membrane. Bar,.1,.m. (A) Vacuole containing E. coli 75 min after addition of bacteria. Note appearance of vacuolar membrane (arrow). (B) Vacuole in protozoan fixed immediately following washing to remove L. pneumophli/a. The phagosomal membrane (arrow) appears dense. Electron-dense particles (arrowhead) occur near the membrane. (C) Vacuole containing L. pneiinophila 12 h after addition of bacteria. Electron-dense particles (arrowhead) line part of the membrane. bacteria. Vacuoles in control protozoa incubated with E. c oli showed no evidence of similar electron-dense particles around the membrane at any stage during the digestive cycle (Fig. 6A). Acidification was similar for vacuoles with L. pneirnophila and E. coli. At 3 min after the introduction of the bacteria, there was no detectable difference in the accumulation of neutral red by vacuoles containing either E. coli (Fig. 7A and B) or L. pneiu,nophila (Fig. 7C and D). Vacuoles with L. pneiumophila gave comparable results when chased with inorganic medium up to 9 min. DISCUSSION The results of this study show that while L. pnzeiumophila does not multiply in T. vorax at temperatures below its minimum growth temperature of 25 C (32), it is retained in phagosomes substantially longer than the normal processing period. While fluorescence of labeled E. coli began to disappear from vacuoles during the digestive stage and electron micrographs showed degradation of the bacterium, FIG. 7. Fluorescence (A and C) and bright-field (B and D) micrographs of T. lvorax treated with neutral red 3 min after addition of E. coli (A and B) or L. pneionophlila (C and D). The vacuoles containing DAPI-stained bacteria correspond to the neutral red-containing vacuoles, which appear dark in the bright-field micrographs. Bar. 1,um. vacuoles containing fluorescent L. pnelum)ophila did not decrease when the protozoa were maintained in stationary phase for 6 h. The mean number of fluorescent phagosomes did decline when T. vorax was returned to logarithmic growth by resuspending the cells in fresh Loefer's medium. This decrease does not appear to be due to digestion of the bacterium, since no evidence of degradation was seen in electron micrographs through 12 h and the number of viable L. pneirnoplil/a present in these samples remained constant. The decline may be due to defecation of the vacuoles without digestion of the contents in logarithmically growing cells. Alternatively, these protozoa may be more sensitive to virulent L. pnzeuitm2ophila than protozoa in stationary phase, which could result in the death of some protozoa, particularly those with a greater number of vacuoles containing bacteria. This could account not only for the continuous FIG. 5. Electron micrographs of sections through vacuoles in T. vorax containing E. co/i. Bar, 1,um. (A) Section through vacuole in protozoan fixed immediately following washing to remove bacteria. (B) Section through 3-min-old vacuole. Note bacteria appressed to each other and to the phagosomal membrane. (C) Section through 75-min-old vacuole. Some of the bacteria show evidence of degradation (arrow). Lysosomes (arrowhead) are located close to the membrane. The vacuole contents are separated from the membrane by a space. (D) Section through 12-min-old vacuole. The space between the membrane and contents is not present. Many of the bacteria are undergoing degradation. Vacuoles similar in appearance were also observed at 75 min. (E) Section through 12-min-old vacuole. Two intact bacteria remain. The vacuole contains remnants of digested E. coli. (F) Section of protozoan at 12 min. The two bacterial masses surrounded by a single membrane indicate that two vacuoles have fused.
7 2748 SMITH-SOMERVILLE ET AL. decline in the mean number of labeled vacuoles for T. vorax in logarithmic growth while that for protozoa in stationary phase remained constant, but also for the smaller increase in cell density of the protozoa in the presence of L. pneumophila than in control populations of protozoa without the bacterium, the presence of spheres with fluorescent bacteria in the medium (which may represent vacuole contents released upon cell death), and the inclusion of low numbers of bacteria in phagosomes after incubation in Loefer's medium for several hours possibly from ingestion of bacteria released into the medium. Weidenbach and Thompson (33) have reported that vacuoles are more fragile and difficult to isolate from Tetrahymena thermophila in logarithmic phase than from protozoa in stationary phase. It is interesting to note that a surprising number of macrostomal cells, a cell type into which T. vorax usually differentiates when starved and provided with an additional stimulus (4, 5, 15), were observed in samples removed during the later times of incubation (28), although the Loefer's medium should provide sufficient nutrients for growth throughout the period. The failure of T. vorax to digest L. pneumophila at a temperature unfavorable to multiplication of the bacterium differs from the results of Anand et al. (2) for Acanthamoeba palestinensis. They reported that although L. pneumophila survives and reproduces in vacuoles at 35 C, this protozoan digests the bacterium at 2 C. This may be due to the different concentrations of L. pneumophila present in the vacuoles of the two protozoa. Only one to several bacterial cells were contained in vacuoles of this protozoan, while T. vorax ingested a large number of L. pneumophila cells when the bacterium was provided to the protozoa at the cell density used in this study. When E. coli and L. pneumophila were ingested together, fewer cells of L. pneumophila were included in each vacuole, and the bacterium appeared to be degraded in a manner similar to that of E. coli. It is conceivable that decreased metabolism in L. pneumophila at the lower temperature necessitates the presence of a greater number of bacteria to block digestion. Studies on the interaction of L. pneumophila with human monocytes have indicated that viable, metabolically active bacteria are required to inhibit the phagocytic pathway (12-14, 19). Alternatively, E. coli or products of this bacterium could counteract the inhibition by L. pneumophila by interacting either with cells or products of L. pneumophila or with the membrane of the vacuole. Unlike human monocytes and amoebae which undergo phagocytosis by surrounding the particle with membrane derived from the plasmalemma, Tetrahymena spp. form vacuoles by the addition of membrane to the base of the cytopharynx (2, 26). Vacuole formation and ingestion of L. pneumophila occurred normally. Electron-lucent vesicles were adjacent to the phagosome before and immediately after separation from the cytopharynx, and in several instances they appeared to be fusing with the vacuole. These vesicles resemble the acidosomes of Paramecium spp., which are believed to be responsible for acidification of the phagosome (1) but which have not been observed in Tetrahymena spp. previously. Acidification of vacuoles indicated by the accumulation of neutral red was similar for vacuoles containing L. pneumophila and E. coli; however, this method only indicates acidic compartments within the cell and does not measure the actual ph. Horwitz and Maxfield (14) found that the ph of monocyte vacuoles with live L. pneumophila was -6.1, averaging.8 ph units higher than that for dead cells and.4 ph units above that for vacuoles containing E. coli. This ph is well above the minimum ph of 5.5 for multiplication of L. pneumophila (32). In a previous study, we found that the ph of vacuoles in T. vorax dropped to -6 within 1 min after formation and reached a minimum ph of 4.9 by 3 min (29). It is possible that although the vacuole with L. pneumophila begins the process of acidification, a change in the membrane or underlying region of the cytoplasm at around 1 to 15 min inhibits a further decrease in ph. Such a change could be induced by a product or products of L. pneumophila and could result in the difference in the appearance of the vacuole surface after this time. This alteration in the membrane or the presence of underlying electron-dense particles could block the fusion of lysosomes, as has been reported in monocytes (13). The modification in the membrane and underlying region of the vacuole precedes the time of lysosomal fusion, as determined by measurement of acid phosphatase activity in vacuoles of T. vorax isolated at different times in the processing period (29). Similar observations on the membrane of vacuoles containing L. pneumophila undergoing multiplication have been reported in T. pyriformis (8), Naegleria fowleri (22), and human monocytes (12). In monocytes, vacuoles with L. pneumophila often had one or more mitochondria apposed to the vacuolar membrane, a situation also observed in T. vorax; however, those in monocytes were also surrounded by smooth vesicles in the process of either fusing with or budding off from the vacuolar membrane to at least 4 h after infection. Similar vesicles were not present around the vacuoles in T. vorax. The behavior of L. pneumophila in the cold-water species T. vorax in the absence of bacterial growth is comparable to that described for other cells under conditions favorable for bacterial growth. This ciliate could provide a model in which to analyze the sequence of virulence-associated intracellular events independent of the replication of L. pneumophila. ACKNOWLEDGMENTS APPL. ENVIRON. MICROBIOL. We thank Catherine Shea for her helpful suggestions and her assistance with preparation of the graphs. This work was supported in part by the School of Mines and Energy Development, University of Alabama, and the U.S. Department of Energy through Pacific Northwest Laboratory via subcontract 5383-A-O. The Pacific Northwest Laboratory is operated for the U.S. Department of Energy by Battelle Memorial Institute. REFERENCES 1. Allen, R. D., and A. K. Fok Nonlysosomal vesicles (acidosomes) are involved in phagosome acidification in Paramecium. J. Cell Biol. 97: Anand, C. M., A. R. Skinner, A. Malic, and J. B. Kurtz Interaction of L. pneumophila and a free living amoeba (Acanthamoeba palestinensis). J. Hyg. 91: Barbaree, J. M., B. S. Fields, J. C. Feeley, G. W. Gorman, and W. T. Martin Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. AppI. Environ. Microbiol. 51: Buhse, H. E., Jr Microstome-macrostome transformation in Tetrahymena vorax strain V2 type S induced by a transforming principle, stomatin. J. Protozool. 14: Buhse, H. E., Jr., and L. Rasmussen A method for synchronization of cell division and macrostome formation in the ciliate Tetrahymena vorax. C.R. Trav. Lab. Carlsberg 4: Dondero, T. J., Jr., R. C. Rendtorff, G. F. Mallison, R. M. Weeks, J. S. Levy, E. W. Wong, and W. Schaffner An outbreak of Legionnaires' disease associated with a contaminated air-conditioning cooling tower. N. Engl. J. Med. 32:
8 VOL. 57, 1991 LEGIONELLA PNEUMOPHILA IN TETRAHYMENA VORAX England, A. C., III, D. W. Fraser, G. F. Mallison, D. C. Mackel, P. Skaliy, and G. W. Gorman Failure of Legionella pneumophila sensitivities to predict culture results from disinfectant-treated air-conditioning cooling towers. Appl. Environ. Microbiol. 43: Fields, B. S., J. M. Barbaree, E. B. Shotts, Jr., J. C. Feeley, W. E. Morrill, G. N. Sanden, and M. J. Dykstra Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect. Immun. 53: Fields, B. S., G. N. Sanden, J. M Barbaree, W. E. Morrill, R. W. Wadowsky, E. H. White, and J. C. Feeley Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr. Microbiol. 18: Fields, B. S., E. B. Shotts, Jr., J. C. Feeley, G. W. Gorman, and W. T. Martin Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl. Environ. Microbiol. 47: Fliermans, C. B., W. B. Cherry, L. H. Orrison, and L. Thacker Isolation of Legionella pneumophila from nonepidemicrelated aquatic habitats. Appi. Environ. Microbiol. 37: Horwitz, M. A Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158: Horwitz, M. A The Legionnaires' disease bacterium (Legionella pneumonpila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158: Horwitz, M. A., and F. R. Maxfield Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell Biol. 99: Kidder, G. W., D. M. Lilly, and C. L. Claff Growth studies on ciliates. IV. The influence of food on the structure and growth of Glaucoma vorax sp. nov. Biol. Bull. 78: Kurtz, J. B., C. J. R. Bartlett, U. A. Newton, R. A. White, and N. L. Jones Legionella pneumophila in cooling water systems: report of a survey of cooling towers in London and a pilot trial of selected biocides. J. Hyg. 88: Loefer, J. B., R. D. Owen, and E. Christensen Serological types among thirty-one strains of the ciliated protozoan, Tetrahymena pyriformis. J. Protozool. 5: Midkiff, K. C., Y. K. Song, and C. E. Brett (University of Alabama) Personal communication. 19. Mintz, C. S., J. Chen, and H. A. Shuman Isolation and characterization of auxotrophic mutants of Legionella pneumophila that fail to multiply in human monocytes. Infect. Immun. 56: Mislan, T. W., and H. E. Smith-Somerville Food vacuole morphology and membrane retrieval in the microstomal form of Tetrahymena vorax. J. Protozool. 33: Morris, G. K., C. M. Patton, J. C. Feeley, S. E. Johnson, G. W. Gorman, W. T. Martin, P. Skaily, G. F. Mallison, B. D. Politi, and D. C. Mackel Isolation of Legionnaires' disease bacterium from environmental samples. Ann. Intern. Med. 9: Newsome, A. L., R. L. Baker, R. D. Miller, and R. R. Arnold Interactions between Naegleria fowleri and Legionella pneumophila. Infect. Immun. 5: Orrison, L. H., W. B. Cherry, and D. Milan Isolation of Legionella pneumophila from cooling tower water by filtration. Appl. Environ. Microbiol. 41: Poole, B., and S. Ohkuma Effect of weak bases on the intralysosomal ph in mouse peritoneal macrophages. J. Cell Biol. 9: Rowbotham, T. J Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33: Sattler, C. A., and L. A. Staehelin Oral cavity of Tetrahymena pyriformis: a freeze-fracture and high-voltage electron microscopy study of the oral ribs, cytostome and forming food vacuole. J. Ultrastruct. Res. 66: Sherman, G. B., H. E. Buhse, Jr., and H. E. Smith A method for synchronous induction of food vacuoles in the macrostome form of Tetrahymena vorax. Trans. Am. Microsc. Soc. 1: Smith-Somerville, H. E. Unpublished data. 29. Smith-Somerville, H. E Correlation of digestive stage with changes in amphiphilic phagosomal proteins of Tetrahymena vorax. Eur. J. Cell Biol. 49: Spurr, A. R. 1%9. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26: Tyndall, R. L., and E. L. Domingue Cocultivation of Legionella pneumophila and free-living amoebae. Appl. Environ. Microbiol. 44: Wadowsky, R. M., R. Wolford, A. M. McNamara, and R. B. Yee Effect of temperature, ph, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl. Environ. Microbiol. 49: Weidenbach, A. L. S., and G. A. Thompson, Jr Studies on membrane formation in Tetrahymena pyriformis. VIII. On the origin of membranes surrounding food vacuoles. J. Protozool. 21:
Laboratory 10 Factors Controlling Phagocytosis in Tetrahymena,
 BIO354: Cell Biology Laboratory 1 Laboratory 1 Factors Controlling Phagocytosis in Tetrahymena, I. Introduction A characteristic feature of all eukaryotic cells is the ability to pinch off portions of
BIO354: Cell Biology Laboratory 1 Laboratory 1 Factors Controlling Phagocytosis in Tetrahymena, I. Introduction A characteristic feature of all eukaryotic cells is the ability to pinch off portions of
FORMATION OF A NOVEL PHAGOSOME BY THE LEGIONNAIRES' DISEASE BACTERIUM (LEGIONELLA
 Published Online: 1 October, 1983 Supp Info: http://doi.org/10.1084/jem.158.4.1319 Downloaded from jem.rupress.org on August 31, 2018 FORMATION OF A NOVEL PHAGOSOME BY THE LEGIONNAIRES' DISEASE BACTERIUM
Published Online: 1 October, 1983 Supp Info: http://doi.org/10.1084/jem.158.4.1319 Downloaded from jem.rupress.org on August 31, 2018 FORMATION OF A NOVEL PHAGOSOME BY THE LEGIONNAIRES' DISEASE BACTERIUM
Effect of Increasing Glucose Concentrations on the Growth Rate of Tetrahymena thermophila Gilbert Lee, Alexia Magarian, Vivian Ng, Sajjal Pirzada
 Effect of Increasing Glucose Concentrations on the Growth Rate of Tetrahymena thermophila Gilbert Lee, Alexia Magarian, Vivian Ng, Sajjal Pirzada Abstract The protozoan, Tetrahymena thermophila, is an
Effect of Increasing Glucose Concentrations on the Growth Rate of Tetrahymena thermophila Gilbert Lee, Alexia Magarian, Vivian Ng, Sajjal Pirzada Abstract The protozoan, Tetrahymena thermophila, is an
Susceptibility of Legionella pneumophila to Chlorine in Tap
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Nov. 1983, p. 1134-1139 0099-2240/83/111134-06$02.00/0 Copyright 1983, American Society for Microbiology Vol. 46, No. 5 Susceptibility of Legionella pneumophila
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Nov. 1983, p. 1134-1139 0099-2240/83/111134-06$02.00/0 Copyright 1983, American Society for Microbiology Vol. 46, No. 5 Susceptibility of Legionella pneumophila
Cooling Towers Received 1 May 1981/Accepted 24 August were not infected to have stayed in rooms that
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Jan. 1982, p. 24-244 0099-2240/82/010240-05$02.00/0 Vol. 43, No. 1 Failure of Legionella pneumophila Sensitivities to Predict Culture Results from Disinfectant-Treated
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Jan. 1982, p. 24-244 0099-2240/82/010240-05$02.00/0 Vol. 43, No. 1 Failure of Legionella pneumophila Sensitivities to Predict Culture Results from Disinfectant-Treated
Intercellular Matrix in Colonies of Candida
 JouRNAL OF BAcTEROLOGY, Sept. 1975, p. 1139-1143 Vol. 123, No. 3 Copyright 0 1975 American Society for Microbiology Printed in U.S.A. ntercellular Matrix in Colonies of Candida K. R. JOSH, J. B. GAVN,*
JouRNAL OF BAcTEROLOGY, Sept. 1975, p. 1139-1143 Vol. 123, No. 3 Copyright 0 1975 American Society for Microbiology Printed in U.S.A. ntercellular Matrix in Colonies of Candida K. R. JOSH, J. B. GAVN,*
Alteration in Bacterial Morphology by Optochin and Quinine Hydrochlorides1
 JOURNAL OF BACTERIOLOGY, Jan. 1969, p. 362-366 Copyright @ 1969 American Society for Microbiology Vol. 97, No. I Printed in U.S.A. Alteration in Bacterial Morphology by Optochin and Quinine Hydrochlorides1
JOURNAL OF BACTERIOLOGY, Jan. 1969, p. 362-366 Copyright @ 1969 American Society for Microbiology Vol. 97, No. I Printed in U.S.A. Alteration in Bacterial Morphology by Optochin and Quinine Hydrochlorides1
Effect of temperature on the growth rate of Tetrahymena thermophila. Rachael Callaghan, Petra Catsi, Pravnit Kooner and Nevrose Mangat
 Effect of temperature on the growth rate of Tetrahymena thermophila Rachael Callaghan, Petra Catsi, Pravnit Kooner and Nevrose Mangat Abstract In this experiment we studied the effect of temperature on
Effect of temperature on the growth rate of Tetrahymena thermophila Rachael Callaghan, Petra Catsi, Pravnit Kooner and Nevrose Mangat Abstract In this experiment we studied the effect of temperature on
Don t be salty! The effects of salinity on vacuole formation of Tetrahymena thermophila. Jullia Ayre, Florence Ng, David (SungJin) Suh
 The Expedition, UBC Ayre, Ng, Suh 1 Don t be salty! The effects of salinity on vacuole formation of Tetrahymena thermophila. Jullia Ayre, Florence Ng, David (SungJin) Suh Abstract The rate of vacuole formation
The Expedition, UBC Ayre, Ng, Suh 1 Don t be salty! The effects of salinity on vacuole formation of Tetrahymena thermophila. Jullia Ayre, Florence Ng, David (SungJin) Suh Abstract The rate of vacuole formation
Characterization of Amoebae Interactions with Four Non-Pneumophila Legionella Species
 Clemson University TigerPrints All Theses Theses 5-2016 Characterization of Amoebae Interactions with Four Non-Pneumophila Legionella Species Allison Margaret Palmer Clemson University, ampalme@g.clemson.edu
Clemson University TigerPrints All Theses Theses 5-2016 Characterization of Amoebae Interactions with Four Non-Pneumophila Legionella Species Allison Margaret Palmer Clemson University, ampalme@g.clemson.edu
The effect of zinc chloride on the formation of food vacuoles in Tetrahymena thermophila
 The effect of zinc chloride on the formation of food vacuoles in Tetrahymena thermophila Josie Francis, Gurtaj Mahil, Katarina Neuvonen and Alan Tsin ABSTRACT Heavy metals, such as zinc, are essential
The effect of zinc chloride on the formation of food vacuoles in Tetrahymena thermophila Josie Francis, Gurtaj Mahil, Katarina Neuvonen and Alan Tsin ABSTRACT Heavy metals, such as zinc, are essential
ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF BLUETONGUE VIRUS*
 Onderstepoort J. vet. Res. (1968), 35 (1), 139-150 Printed in the Repub. of S. Afr. by The Government Printer, Pretoria ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF BLUETONGUE VIRUS* G. LECATSAS, Veterinary
Onderstepoort J. vet. Res. (1968), 35 (1), 139-150 Printed in the Repub. of S. Afr. by The Government Printer, Pretoria ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF BLUETONGUE VIRUS* G. LECATSAS, Veterinary
Influence of Acanthamoeba castellanii on Intracellular Growth of Different Legionella Species in Human Monocytes
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Mar. 2000, p. 914 919 Vol. 66, No. 3 0099-2240/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Influence of Acanthamoeba castellanii
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Mar. 2000, p. 914 919 Vol. 66, No. 3 0099-2240/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Influence of Acanthamoeba castellanii
ELECTRON MICROSCOPIC STUDIES ON EQUINE ENCEPHALOSIS VIRUS
 Onderstepoort]. vet. Res. 40 (2), 53-58 (1973) ELECTRON MICROSCOPIC STUDIES ON EQUINE ENCEPHALOSIS VIRUS G. LECATSAS, B. J. ERASMUS and H. J. ELS, Veterinary Research Institute, Onderstepoort ABSTRACT
Onderstepoort]. vet. Res. 40 (2), 53-58 (1973) ELECTRON MICROSCOPIC STUDIES ON EQUINE ENCEPHALOSIS VIRUS G. LECATSAS, B. J. ERASMUS and H. J. ELS, Veterinary Research Institute, Onderstepoort ABSTRACT
Amino Acid Requirements for Legionella pneumophila Growth
 JOURNAL OF CLINICAL MICROBIOLOGY, May 1981, p. 865-869 0095-1137/81/050865-05$02.00/0 Vol. 13, No. 5 Amino Acid Requirements for Legionella pneumophila Growth MARTHA J. TESH AND RICHARD D. MILLER* Department
JOURNAL OF CLINICAL MICROBIOLOGY, May 1981, p. 865-869 0095-1137/81/050865-05$02.00/0 Vol. 13, No. 5 Amino Acid Requirements for Legionella pneumophila Growth MARTHA J. TESH AND RICHARD D. MILLER* Department
FINE STRUCTURE AND RNA SYNTHESIS OF TETRAHYMENA DURING CYTOCHALASIN B INHIBITION OF PHAGOCYTOSIS
 J. Cell Set. 27, 115-126 (1977) 115 Printed in Great Britain Company of Biologists Limited FINE STRUCTURE AND RNA SYNTHESIS OF TETRAHYMENA DURING CYTOCHALASIN B INHIBITION OF PHAGOCYTOSIS JYTTE R. NILSSON
J. Cell Set. 27, 115-126 (1977) 115 Printed in Great Britain Company of Biologists Limited FINE STRUCTURE AND RNA SYNTHESIS OF TETRAHYMENA DURING CYTOCHALASIN B INHIBITION OF PHAGOCYTOSIS JYTTE R. NILSSON
In vitro and Intracellular Activities of Peptide Deformylase. Inhibitor GSK against Legionella pneumophila Isolates
 AAC Accepts, published online ahead of print on 27 October 2014 Antimicrob. Agents Chemother. doi:10.1128/aac.04006-14 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 2 In vitro
AAC Accepts, published online ahead of print on 27 October 2014 Antimicrob. Agents Chemother. doi:10.1128/aac.04006-14 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 2 In vitro
The Effect of Temperature on Food Vacuole Formation in Tetrahymena thermophila
 The Effect of Temperature on Food Vacuole Formation in Tetrahymena thermophila Cindy Pham, Kattereya Kennedy, Nicola Popper, and Ravneet Ghotra Abstract Through the process of phagocytosis for food vacuole
The Effect of Temperature on Food Vacuole Formation in Tetrahymena thermophila Cindy Pham, Kattereya Kennedy, Nicola Popper, and Ravneet Ghotra Abstract Through the process of phagocytosis for food vacuole
psittaci by Silver-Methenamine Staining and
 JOURNAL OF BACTERIOLOGY, July 1972, p. 267-271 Copyright 1972 American Society for Microbiology Vol. 111, No. 1 Printed in U.S.A. Location of Polysaccharide on Chlamydia psittaci by Silver-Methenamine
JOURNAL OF BACTERIOLOGY, July 1972, p. 267-271 Copyright 1972 American Society for Microbiology Vol. 111, No. 1 Printed in U.S.A. Location of Polysaccharide on Chlamydia psittaci by Silver-Methenamine
From the Laboratory of Cellular Physiology and Immunology, The Rockefeller University, New York 10021
 Published Online: 1 February, 1981 Supp Info: http://doi.org/10.1084/jem.153.2.398 Downloaded from jem.rupress.org on April 7, 2018 INTERACTION OF THE LEGIONNAIRES' DISEASE BACTERIUM (LEGIONELLA PNEUMOPHILA)
Published Online: 1 February, 1981 Supp Info: http://doi.org/10.1084/jem.153.2.398 Downloaded from jem.rupress.org on April 7, 2018 INTERACTION OF THE LEGIONNAIRES' DISEASE BACTERIUM (LEGIONELLA PNEUMOPHILA)
EXPERIMENTAL SALMONELLOSIS
 EXPERIMENTAL SALMONELLOSIS INTRACELLULAR GROWTH OF Salmonella enteritidis INGESTED IN MONONUCLEAR PHAGOCYTES OF MICE, AND CELLULAR BASIS OF IMMUNITY SUSUMU MITSUHASHI, ICHIEI SATO, AND TOKUMITSU TANAKA
EXPERIMENTAL SALMONELLOSIS INTRACELLULAR GROWTH OF Salmonella enteritidis INGESTED IN MONONUCLEAR PHAGOCYTES OF MICE, AND CELLULAR BASIS OF IMMUNITY SUSUMU MITSUHASHI, ICHIEI SATO, AND TOKUMITSU TANAKA
Legionella. A detailed insight from our specialists. Overview Lifecycle How it manifests Strains and species Facts and statistics
 Legionella. A detailed insight from our specialists Overview Lifecycle How it manifests Strains and species Facts and statistics Laboratory process Sampling and analysis Illness and symptoms Interpreting
Legionella. A detailed insight from our specialists Overview Lifecycle How it manifests Strains and species Facts and statistics Laboratory process Sampling and analysis Illness and symptoms Interpreting
Instructions. Fuse-It-mRNA easy. Shipping and Storage. Overview. Kit Contents. Specifications. Note: Important Guidelines
 Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Microbiology of Atypical Pneumonia. Dr. Mohamed Medhat Ali
 Microbiology of Atypical Pneumonia Dr. Mohamed Medhat Ali Pneumonia P n e u m o n i a i s a n infection of the lungs that can be caused by viruses, bacteria, and fungi. Atypical! Pneumonia Symptoms. X-ray
Microbiology of Atypical Pneumonia Dr. Mohamed Medhat Ali Pneumonia P n e u m o n i a i s a n infection of the lungs that can be caused by viruses, bacteria, and fungi. Atypical! Pneumonia Symptoms. X-ray
Cell Division in a Species of Erwinia
 JOURNAL OF BACTERIOLOGY, Oct., 1965 Vol. 90, No. 4 Copyright 1965 American Society for Microbiology Printed in U.S.A. Cell Division in a Species of Erwinia IX. Electron Microscopy of Normally Dividing
JOURNAL OF BACTERIOLOGY, Oct., 1965 Vol. 90, No. 4 Copyright 1965 American Society for Microbiology Printed in U.S.A. Cell Division in a Species of Erwinia IX. Electron Microscopy of Normally Dividing
Storage of waters, underground, surface, sea and sewage, phenomenon is the more puzzling because the waters in their
 RELATION BETWEEN FOOD CONCENTRATION AND SURFACE FOR BACTERIAL GROWTH1 H. HEUKELEKIAN2 AND A. HELLER3 Agricultural Experiment Station, New Brunswick, New Jersey Received for publication April 19, 1940 Storage
RELATION BETWEEN FOOD CONCENTRATION AND SURFACE FOR BACTERIAL GROWTH1 H. HEUKELEKIAN2 AND A. HELLER3 Agricultural Experiment Station, New Brunswick, New Jersey Received for publication April 19, 1940 Storage
Factors Affecting the Virulence of Naegleria fowleri for Mice
 Proc. Helminthol. Soc. Wash. 47(1), 1980, p. 129-134 Factors Affecting the Virulence of Naegleria fowleri for Mice R. M. HAGGERTY AND D. T. JOHN Department of Microbiology, MCV Station, Box 678, Commonwealth
Proc. Helminthol. Soc. Wash. 47(1), 1980, p. 129-134 Factors Affecting the Virulence of Naegleria fowleri for Mice R. M. HAGGERTY AND D. T. JOHN Department of Microbiology, MCV Station, Box 678, Commonwealth
Possibility of Symbiosis between Some Gram-negative Bacteria and Legionella pneumophila.
 Possibility of Symbiosis between Some Gram-negative Bacteria and Legionella pneumophila 1 H. T. El Zanfaly, 2 H. Rüden and 2 K. Weist 1 Water Pollution Control Dept., National Research Center, Dokki, Cairo,
Possibility of Symbiosis between Some Gram-negative Bacteria and Legionella pneumophila 1 H. T. El Zanfaly, 2 H. Rüden and 2 K. Weist 1 Water Pollution Control Dept., National Research Center, Dokki, Cairo,
CytoPainter Lysosomal Staining Kit - Blue Fluorescence
 ab112135 CytoPainter Lysosomal Staining Kit - Blue Fluorescence Instructions for Use For staining Lysosomes in suspension and adherent cells by using our proprietary blue fluorescence probe This product
ab112135 CytoPainter Lysosomal Staining Kit - Blue Fluorescence Instructions for Use For staining Lysosomes in suspension and adherent cells by using our proprietary blue fluorescence probe This product
(From The Rockefeller Institute) Materials and Methods. Observations with the Electron Microscope
 ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
Lysosomes, Peroxisomes and Centrioles. Hüseyin Çağsın
 Lysosomes, Peroxisomes and Centrioles Hüseyin Çağsın Lysosomes Outline Endosomes Molecule transport to the lysosomes Endocytosis Exocytosis Autophagy Vacuoles Peroxisomes Centrioles Lysosomes Lysosomes
Lysosomes, Peroxisomes and Centrioles Hüseyin Çağsın Lysosomes Outline Endosomes Molecule transport to the lysosomes Endocytosis Exocytosis Autophagy Vacuoles Peroxisomes Centrioles Lysosomes Lysosomes
ENHANCEMENT OF THE GRANULATION OF ADRFNERGIC STORAGE VESICLES IN DRUG-FREE SOLUTION
 ENHANCEMENT OF THE GRANULATION OF ADRFNERGIC STORAGE VESICLES IN DRUG-FREE SOLUTION TAKASHI IWAYAMA and J. B. FURNESS. From the Department of Zoology, University of Melbourne, Victoria, Australia. Dr.
ENHANCEMENT OF THE GRANULATION OF ADRFNERGIC STORAGE VESICLES IN DRUG-FREE SOLUTION TAKASHI IWAYAMA and J. B. FURNESS. From the Department of Zoology, University of Melbourne, Victoria, Australia. Dr.
Temperature-Sensitive Mutants Isolated from Hamster and
 JOURNAL OF VIROLOGY, Nov. 1975, p. 1332-1336 Copyright i 1975 American Society for Microbiology Vol. 16, No. 5 Printed in U.S.A. Temperature-Sensitive Mutants Isolated from Hamster and Canine Cell Lines
JOURNAL OF VIROLOGY, Nov. 1975, p. 1332-1336 Copyright i 1975 American Society for Microbiology Vol. 16, No. 5 Printed in U.S.A. Temperature-Sensitive Mutants Isolated from Hamster and Canine Cell Lines
R. B. MARSHALL Department of Veterinary Pathology and Public Health, Massey University, Palmerston North, New Zealand
 THE ROUTE OF ENTRY OF LEPTOSPIRES INTO THE KIDNEY TUBULE R. B. MARSHALL Department of Veterinary Pathology and Public Health, Massey University, Palmerston North, New Zealand PLATES X and XI IT has been
THE ROUTE OF ENTRY OF LEPTOSPIRES INTO THE KIDNEY TUBULE R. B. MARSHALL Department of Veterinary Pathology and Public Health, Massey University, Palmerston North, New Zealand PLATES X and XI IT has been
Biol110L-Cell Biology Lab Spring Quarter 2012 Module 1-4 Friday April 13, 2012 (Start promptly; work fast; the protocols take ~4 h)
 Biol110L-Cell Biology Lab Spring Quarter 2012 Module 1-4 Friday April 13, 2012 (Start promptly; work fast; the protocols take ~4 h) A. Microscopic Examination of the Plasma Membrane and Its Properties
Biol110L-Cell Biology Lab Spring Quarter 2012 Module 1-4 Friday April 13, 2012 (Start promptly; work fast; the protocols take ~4 h) A. Microscopic Examination of the Plasma Membrane and Its Properties
Relationship of Ehrlichia canis-infected Mononuclear Cells to Blood Vessels of Lungs1
 INFECTION AND IMMUNITY, Sept. 1974, p. 590-596 Copyright 0 1974 American Society for Microbiology Vol. 10, No. 3 Printed in U.S.A. Relationship of Ehrlichia canis-infected Mononuclear Cells to Blood Vessels
INFECTION AND IMMUNITY, Sept. 1974, p. 590-596 Copyright 0 1974 American Society for Microbiology Vol. 10, No. 3 Printed in U.S.A. Relationship of Ehrlichia canis-infected Mononuclear Cells to Blood Vessels
Legionella pneumophila: an intracellular pathogen of phagocytes Prof. Craig Roy
 an intracellular pathogen of phagocytes Section of Microbial Pathogenesis, Yale University School of Medicine 1 Legionella pneumophila Gram-negative bacterium Facultative intracellular pathogen Protozoa
an intracellular pathogen of phagocytes Section of Microbial Pathogenesis, Yale University School of Medicine 1 Legionella pneumophila Gram-negative bacterium Facultative intracellular pathogen Protozoa
Survival of Aerobic and Anaerobic Bacteria in
 APPLIED MICROBIOLOGY, Mar. 1968, p. 445-449 Copyright 1968 American Society for Microbiology Vol. 16, No. 3 Printed in U.S.A. Survival of Aerobic and Anaerobic Bacteria in Chicken Meat During Freeze-Dehydration,
APPLIED MICROBIOLOGY, Mar. 1968, p. 445-449 Copyright 1968 American Society for Microbiology Vol. 16, No. 3 Printed in U.S.A. Survival of Aerobic and Anaerobic Bacteria in Chicken Meat During Freeze-Dehydration,
SUPPLEMENTARY MATERIAL. Sample preparation for light microscopy
 SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
Attachment of Two Myxoviruses to Ciliated Epithelial Cells
 ,1. gen. Virol. (I97O), 9, 77-88 77 Printed in Great Britain Attachment of Two Myxoviruses to Ciliated Epithelial Cells By R. R. DOURMASHKIN AND D. A. J. TYRRELL Clinical Research Centre Laboratories,
,1. gen. Virol. (I97O), 9, 77-88 77 Printed in Great Britain Attachment of Two Myxoviruses to Ciliated Epithelial Cells By R. R. DOURMASHKIN AND D. A. J. TYRRELL Clinical Research Centre Laboratories,
MYCOBACTERIA. Pulmonary T.B. (infect bird)
 MYCOBACTERIA SPP. Reservoir Clinical Manifestation Mycobacterium tuberculosis Human Pulmonary and dissem. T.B. M. lepra Human Leprosy M. bovis Human & cattle T.B. like infection M. avium Soil, water, birds,
MYCOBACTERIA SPP. Reservoir Clinical Manifestation Mycobacterium tuberculosis Human Pulmonary and dissem. T.B. M. lepra Human Leprosy M. bovis Human & cattle T.B. like infection M. avium Soil, water, birds,
A Compact and a Dispersed Form of the Golgi Apparatus
 A Compact and a Dispersed Form of the Golgi Apparatus of Fish Liver 1 D. James Morre and Carole A. Lembi Department of Botany and Plant Pathology Purdue University, Lafayette, Indiana 47907, and H. H.
A Compact and a Dispersed Form of the Golgi Apparatus of Fish Liver 1 D. James Morre and Carole A. Lembi Department of Botany and Plant Pathology Purdue University, Lafayette, Indiana 47907, and H. H.
ANSI/ASHRAE Standard Legionellosis: Risk Management for Building Water Systems
 ANSI/ASHRAE Standard 188-2015 Legionellosis: Risk Management for Building Water Systems Published June 26, 2015 Patricia T. Graef, P.E. Member SSPC-188 January, 2018 PURPOSE: Establish minimum legionellosis
ANSI/ASHRAE Standard 188-2015 Legionellosis: Risk Management for Building Water Systems Published June 26, 2015 Patricia T. Graef, P.E. Member SSPC-188 January, 2018 PURPOSE: Establish minimum legionellosis
Fact Sheet: Legionellosis March 2017
 Fact Sheet Fact Sheet: Legionellosis March 2017 Brought to you by the APSP Recreational Water Quality Committee (RWQC) I. INTRODUCTION Legionellosis is an infection caused by the bacterium Legionella.
Fact Sheet Fact Sheet: Legionellosis March 2017 Brought to you by the APSP Recreational Water Quality Committee (RWQC) I. INTRODUCTION Legionellosis is an infection caused by the bacterium Legionella.
Instructions. Fuse-It-Color. Overview. Specifications
 Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE
 TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE PAUL F. PARAKKAL. From the Department of Dermatology, Boston University School of Medicine, Boston, Massachusetts 02118 INTRODUCTION
TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE PAUL F. PARAKKAL. From the Department of Dermatology, Boston University School of Medicine, Boston, Massachusetts 02118 INTRODUCTION
Electron Microscopy of Small Cells: Mycoplasma hominis
 JOURNAL of BAcTRiowOY, Dc. 1969, p. 1402-1408 Copyright 0 1969 American Society for Microbiology Vol. 100, No. 3 Printed In U.S.A. NOTES Electron Microscopy of Small Cells: Mycoplasma hominis JACK MANILOFF
JOURNAL of BAcTRiowOY, Dc. 1969, p. 1402-1408 Copyright 0 1969 American Society for Microbiology Vol. 100, No. 3 Printed In U.S.A. NOTES Electron Microscopy of Small Cells: Mycoplasma hominis JACK MANILOFF
Recipes for Media and Solution Preparation SC-ura/Glucose Agar Dishes (20mL/dish, enough for 8 clones)
 Protocol: 300 ml Yeast culture preparation Equipment and Reagents needed: Autoclaved toothpicks Shaker Incubator set at 30 C Incubator set at 30 C 60 mm 2 sterile petri dishes Autoclaved glass test tubes
Protocol: 300 ml Yeast culture preparation Equipment and Reagents needed: Autoclaved toothpicks Shaker Incubator set at 30 C Incubator set at 30 C 60 mm 2 sterile petri dishes Autoclaved glass test tubes
MEASUREMENT OF THE DEGREE OF PROTECTION AFFORDED BY RESPIRATORY PROTECTIVE EQUIPMENT AGAINST MICROBIOLOGICAL AEROSOLS
 PII: S0003-^878(96)00082-8 Ann. occup. Hyg., Vol. 41, Supplement 1, pp. 636-640, 1997 British Occupational Hygiene Society Crown Copyright 1997 Published by Elsevier Science Ltd Printed in Great Britain
PII: S0003-^878(96)00082-8 Ann. occup. Hyg., Vol. 41, Supplement 1, pp. 636-640, 1997 British Occupational Hygiene Society Crown Copyright 1997 Published by Elsevier Science Ltd Printed in Great Britain
Biological Consulting Services
 Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Chapter 14. In Vitro Measurement of Phagocytosis and Killing of Cryptococcus neoformans by Macrophages. André Moraes Nicola and Arturo Casadevall
 Chapter 14 In Vitro Measurement of Phagocytosis and Killing of Cryptococcus neoformans by Macrophages André Moraes Nicola and Arturo Casadevall Abstract Macrophages are pivotal cells in immunity against
Chapter 14 In Vitro Measurement of Phagocytosis and Killing of Cryptococcus neoformans by Macrophages André Moraes Nicola and Arturo Casadevall Abstract Macrophages are pivotal cells in immunity against
Proliferation of Legionella pneumophila as an Intracellular Parasite
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Mar. 1984, p. 467-471 0099-2240/84/030467-05$02.00/0 Copyright C) 1984, Amerian Soiety for Mirobiology Vol. 47, No. 3 Proliferation of Legionella pneumophila as
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Mar. 1984, p. 467-471 0099-2240/84/030467-05$02.00/0 Copyright C) 1984, Amerian Soiety for Mirobiology Vol. 47, No. 3 Proliferation of Legionella pneumophila as
Acid phosphatase activity in the neutral red granules of mouse exocrine pancreas cells
 343 Acid phosphatase activity in the neutral red granules of mouse exocrine pancreas cells By JENNIFER M. BYRNE (From the Cytological Laboratory, Department of Zoology, University Museum, Oxford) With
343 Acid phosphatase activity in the neutral red granules of mouse exocrine pancreas cells By JENNIFER M. BYRNE (From the Cytological Laboratory, Department of Zoology, University Museum, Oxford) With
Legionellosis Water Management and Investigation Case Study
 Legionellosis Water Management and Investigation Case Study Michigan Department of Health and Human Services Bryce Spiker, MPH Legionellosis Epidemiologist Mike Wesenberg Environmental Health Specialist
Legionellosis Water Management and Investigation Case Study Michigan Department of Health and Human Services Bryce Spiker, MPH Legionellosis Epidemiologist Mike Wesenberg Environmental Health Specialist
MORPHOLOGIC CHARACTERISTICS OF THE CHEMICALLY INDUCED ACROSOME REACTION IN HUMAN SPERMATOZOA*
 FERTILITY AND STERILITY Copyright 1979 The American Fertility Society Vol. 32, No.1, July 1979 Printed in U.SA. MORPHOLOGIC CHARACTERISTICS OF THE CHEMICALLY INDUCED ACROSOME REACTION IN HUMAN SPERMATOZOA*
FERTILITY AND STERILITY Copyright 1979 The American Fertility Society Vol. 32, No.1, July 1979 Printed in U.SA. MORPHOLOGIC CHARACTERISTICS OF THE CHEMICALLY INDUCED ACROSOME REACTION IN HUMAN SPERMATOZOA*
What is Legionnaires' disease?
 Understanding Legionnaires disease: A Fact Sheet For Workers Organization(s): New York Committee for Occupational Safety and Health Other languages: Spanish Summary Statement: This NYCOSH fact sheet is
Understanding Legionnaires disease: A Fact Sheet For Workers Organization(s): New York Committee for Occupational Safety and Health Other languages: Spanish Summary Statement: This NYCOSH fact sheet is
10/13/11. Cell Theory. Cell Structure
 Cell Structure Grade 12 Biology Cell Theory All organisms are composed of one or more cells. Cells are the smallest living units of all living organisms. Cells arise only by division of a previously existing
Cell Structure Grade 12 Biology Cell Theory All organisms are composed of one or more cells. Cells are the smallest living units of all living organisms. Cells arise only by division of a previously existing
THE BACTERICIDAL PROPERTIES OF ULTRAVIOLET IRRADIATED LIPIDS OF THE SKIN
 THE BACTERICIDAL PROPERTIES OF ULTRAVIOLET IRRADIATED LIPIDS OF THE SKIN BY FRANKLIN A. STEVENS, M.D. (From the Department of Medicine, College of Physicians and Surgeons, Columbia University, and the
THE BACTERICIDAL PROPERTIES OF ULTRAVIOLET IRRADIATED LIPIDS OF THE SKIN BY FRANKLIN A. STEVENS, M.D. (From the Department of Medicine, College of Physicians and Surgeons, Columbia University, and the
The Structure of Viruses of the Newcastle Disease- Mumps-Influenza (Myxovirus) Group
 680 * VALENTINE, R. C. & ISAACS, A. (1957). J. gen. Microbiol. 16, 680-685 The Structure of Viruses of the Newcastle Disease- Mumps-Influenza (Myxovirus) Group BY R. C. VALENTINE AND A. IsAAcS National
680 * VALENTINE, R. C. & ISAACS, A. (1957). J. gen. Microbiol. 16, 680-685 The Structure of Viruses of the Newcastle Disease- Mumps-Influenza (Myxovirus) Group BY R. C. VALENTINE AND A. IsAAcS National
S. aureus NCTC 6571, E. coli NCTC (antibiotic
 ISO Sensitivity Test Agar Code: KM1204 A semi-defined nutritionally rich sensitivity medium. It is composed of specially selected peptones with a small amount of glucose, solidified with a very pure agar
ISO Sensitivity Test Agar Code: KM1204 A semi-defined nutritionally rich sensitivity medium. It is composed of specially selected peptones with a small amount of glucose, solidified with a very pure agar
Lucifer Yellow and fluorescein isothiocyanate uptake by cells of Morinda citrifolia in suspension cultures is not confined to the endocytotic pathway
 Lucifer Yellow and fluorescein isothiocyanate uptake by cells of Morinda citrifolia in suspension cultures is not confined to the endocytotic pathway D. O'DRISCOLL*, G. WILSON and M. W. STEER Botany Department,
Lucifer Yellow and fluorescein isothiocyanate uptake by cells of Morinda citrifolia in suspension cultures is not confined to the endocytotic pathway D. O'DRISCOLL*, G. WILSON and M. W. STEER Botany Department,
lactose-fermenting variants (reds). Appreciable lactose utilization variants. Hershey and Bronfenbrenner (1936) found the non-lactosefermenting
 THE LACTASE ACTIVITY OF ESCHERICHIA COLI- MUTABILE' CHARLES J. DEERE, ANNA DEAN DULANEY AND I. D. MICHELSON Department of Chemistry and Department of Bacteriology, University of Tennessee School of Biological
THE LACTASE ACTIVITY OF ESCHERICHIA COLI- MUTABILE' CHARLES J. DEERE, ANNA DEAN DULANEY AND I. D. MICHELSON Department of Chemistry and Department of Bacteriology, University of Tennessee School of Biological
(a) (i) Structures A and B are found in both the animal cell and the bacterial cell. B... (2)
 1 The diagrams show an animal cell and a bacterial cell. (a) (i) Structures A and B are found in both the animal cell and the bacterial cell. Use words from the box to name structures A and B. cell membrane
1 The diagrams show an animal cell and a bacterial cell. (a) (i) Structures A and B are found in both the animal cell and the bacterial cell. Use words from the box to name structures A and B. cell membrane
Development of ISO 11731:2017. Simon in t Veld Manager Microbiology Vitens Laboratorium Convenor ISO TC7/SC4/WG10 Legionella
 Development of ISO 11731:217 Simon in t Veld Manager Microbiology Vitens Laboratorium Convenor ISO TC7/SC4/WG1 Legionella Vitens Vitens Laboratory Content Standards ISO 11731:1998 ISO 11731-2:24 ISO 11731:217
Development of ISO 11731:217 Simon in t Veld Manager Microbiology Vitens Laboratorium Convenor ISO TC7/SC4/WG1 Legionella Vitens Vitens Laboratory Content Standards ISO 11731:1998 ISO 11731-2:24 ISO 11731:217
Analyzing the Effects of Fertilizer Concentration on the Rate of Food Vacuole Formation in Tetrahymena thermophila
 The Expedition, UBC Faragher, Seo, Vilchis, Wong 1 Abstract Analyzing the Effects of Fertilizer Concentration on the Rate of Food Vacuole Formation in Tetrahymena thermophila Annie Faragher, Joo Hwan Seo,
The Expedition, UBC Faragher, Seo, Vilchis, Wong 1 Abstract Analyzing the Effects of Fertilizer Concentration on the Rate of Food Vacuole Formation in Tetrahymena thermophila Annie Faragher, Joo Hwan Seo,
Mechanistic Studies of Pentamidine Analogs on Leishmania donovani Promastigotes
 Mechanistic Studies of Pentamidine Analogs on Leishmania donovani Promastigotes Undergraduate Honors Thesis The Ohio State University, College of Pharmacy Division of Medicinal Chemistry and Pharmacognosy
Mechanistic Studies of Pentamidine Analogs on Leishmania donovani Promastigotes Undergraduate Honors Thesis The Ohio State University, College of Pharmacy Division of Medicinal Chemistry and Pharmacognosy
9700 BIOLOGY. Mark schemes should be read in conjunction with the question paper and the Principal Examiner Report for Teachers.
 CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Advanced Subsidiary Level and GCE Advanced Level MARK SCHEME for the May/June 2013 series 9700 BIOLOGY 9700/53 Paper 5 (Planning, Analysis and Evaluation), maximum
CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Advanced Subsidiary Level and GCE Advanced Level MARK SCHEME for the May/June 2013 series 9700 BIOLOGY 9700/53 Paper 5 (Planning, Analysis and Evaluation), maximum
Lysosomes. Gr: lysis solution, soma body. Membrane bounded vesicles. Usually round ovoid or irregular electron dense bodies m.
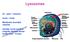 Lysosomes Gr: lysis solution, soma body Membrane bounded vesicles Usually round ovoid or irregular electron dense bodies 0.05 0.5 m. Lysosomes No. varies from a few to several hundred per cell, in different
Lysosomes Gr: lysis solution, soma body Membrane bounded vesicles Usually round ovoid or irregular electron dense bodies 0.05 0.5 m. Lysosomes No. varies from a few to several hundred per cell, in different
Large Surface Blebs on Escherichia coli Heated to Inactivating Temperatures
 JOURNAL OF BACTERIOLOGY, May 1973, p. 814-818 Copyright 0 1973 American Society for Microbiology Vol. 114, No. 2 Printed in U.S.A. Large Surface Blebs on Escherichia coli Heated to Inactivating Temperatures
JOURNAL OF BACTERIOLOGY, May 1973, p. 814-818 Copyright 0 1973 American Society for Microbiology Vol. 114, No. 2 Printed in U.S.A. Large Surface Blebs on Escherichia coli Heated to Inactivating Temperatures
Quantitative Assay of Paravaccinia Virus Based
 APPrU MICROBIOLOGY, JUly 1972, p. 138-142 Copyright 1972 American Society for Microbiology Vol. 24, No. 1 Printed in U.S.A. Quantitative Assay of Paravaccinia Virus Based on Enumeration of Inclusion-Containing
APPrU MICROBIOLOGY, JUly 1972, p. 138-142 Copyright 1972 American Society for Microbiology Vol. 24, No. 1 Printed in U.S.A. Quantitative Assay of Paravaccinia Virus Based on Enumeration of Inclusion-Containing
Legionnaires Disease Q&A (General) (Source: OSHA) (4/29/10)
 Legionnaires Disease Q&A (General) (Source: OSHA) (4/29/10) Q. What is Legionnaires Disease? A. Legionnaires disease is a common name for one of the several illnesses caused by Legionella bacteria. Legionnaires
Legionnaires Disease Q&A (General) (Source: OSHA) (4/29/10) Q. What is Legionnaires Disease? A. Legionnaires disease is a common name for one of the several illnesses caused by Legionella bacteria. Legionnaires
Endomembrane system, *Chloroplasts, *Mitochondria. *Learn these from text/connect1. Fertilization of a human cell
 Key Concepts: - Cells are the Basic Unit of Life Cell Theory, Surface to Volume - 2 Cell Types Prokaryotic, Eukaryotic - Cell Membrane Membrane Structure - Cell Organelles Endomembrane system, *Chloroplasts,
Key Concepts: - Cells are the Basic Unit of Life Cell Theory, Surface to Volume - 2 Cell Types Prokaryotic, Eukaryotic - Cell Membrane Membrane Structure - Cell Organelles Endomembrane system, *Chloroplasts,
Eucaryotic Cell Structure and Function
 Chapter 4 Eucaryotic Cell Structure and Function Eucaryotic and Procaryotic cells differ in the use of their cell membranes.! EC have membrane delimited nuclei! Play a role in the structure of many other
Chapter 4 Eucaryotic Cell Structure and Function Eucaryotic and Procaryotic cells differ in the use of their cell membranes.! EC have membrane delimited nuclei! Play a role in the structure of many other
N. O. Sanli-Yurudu A. Kimiran-Erdem. E. O. Arslan-Aydogdu Z. Zeybek S. Gurun. Keywords Legionella pneumophila Biocide Cooling tower
 Indian J Microbiol (Jan Mar 2012) 52(1):54 59 DOI 10.1007/s12088-011-0189-z ORIGINAL ARTICLE Efficacy of Colloidal Silver-Hydrogen Peroxide and 2-Bromo-2- nitroporopane-1,3-diol Compounds Against Different
Indian J Microbiol (Jan Mar 2012) 52(1):54 59 DOI 10.1007/s12088-011-0189-z ORIGINAL ARTICLE Efficacy of Colloidal Silver-Hydrogen Peroxide and 2-Bromo-2- nitroporopane-1,3-diol Compounds Against Different
GB Translated English of Chinese Standard: GB NATIONAL STANDARD OF THE
 Translated English of Chinese Standard: GB4789.30-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 4789.30-2016
Translated English of Chinese Standard: GB4789.30-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 4789.30-2016
RESEARCH ARTICLE. Lewis G. Tilney 1 *, Omar S. Harb 1, Patricia S. Connelly 1, Camenzind G. Robinson 2, and Craig R. Roy 2 SUMMARY
 RESEARCH ARTICLE 4637 How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane Lewis
RESEARCH ARTICLE 4637 How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane Lewis
SBI3U7 Cell Structure & Organelles. 2.2 Prokaryotic Cells 2.3 Eukaryotic Cells
 SBI3U7 Cell Structure & Organelles 2.2 Prokaryotic Cells 2.3 Eukaryotic Cells No nucleus Prokaryotic Cells No membrane bound organelles Has a nucleus Eukaryotic Cells Membrane bound organelles Unicellular
SBI3U7 Cell Structure & Organelles 2.2 Prokaryotic Cells 2.3 Eukaryotic Cells No nucleus Prokaryotic Cells No membrane bound organelles Has a nucleus Eukaryotic Cells Membrane bound organelles Unicellular
OCHROMONAS MALHAMENSIS
 THE INFLUENCE OF THE MODE OF NUTRITION ON THE DIGESTIVE SYSTEM OF OCHROMONAS MALHAMENSIS H. J. STOLTZE, N. S. T. LUI, O. R. ANDERSON, and O. A. ROELS From the Marine Biology Division of the Lamont-Doherty
THE INFLUENCE OF THE MODE OF NUTRITION ON THE DIGESTIVE SYSTEM OF OCHROMONAS MALHAMENSIS H. J. STOLTZE, N. S. T. LUI, O. R. ANDERSON, and O. A. ROELS From the Marine Biology Division of the Lamont-Doherty
Mt. San Antonio College Microbiology 22 Lab Schedule for Fall 2017 Tues/Thurs. Split Lab Sections ONLY
 Mt. San Antonio College Microbiology 22 Lab Schedule for Fall 2017 Tues/ Split Lab Sections ONLY Wk 1 Aug. 29 Orientation with Introductions & Safety Rules/Regulations Aug. 31 Orientation with Pathogen
Mt. San Antonio College Microbiology 22 Lab Schedule for Fall 2017 Tues/ Split Lab Sections ONLY Wk 1 Aug. 29 Orientation with Introductions & Safety Rules/Regulations Aug. 31 Orientation with Pathogen
What is the immune system? Types of Immunity. Pasteur and rabies vaccine. Historical Role of smallpox. Recognition Response
 Recognition Response Effector memory What is the immune system? Types of Immunity Innate Adaptive Anergy: : no response Harmful response: Autoimmunity Historical Role of smallpox Pasteur and rabies vaccine
Recognition Response Effector memory What is the immune system? Types of Immunity Innate Adaptive Anergy: : no response Harmful response: Autoimmunity Historical Role of smallpox Pasteur and rabies vaccine
Vitamin C and Ibuprofen Effects on Escherichia Coli. Timothy Leisenring Grade 11 Central Catholic High School
 Vitamin C and Ibuprofen Effects on Escherichia Coli Timothy Leisenring Grade 11 Central Catholic High School Rationale for Experiment Commonly, ingested materials are investigated for effects on human
Vitamin C and Ibuprofen Effects on Escherichia Coli Timothy Leisenring Grade 11 Central Catholic High School Rationale for Experiment Commonly, ingested materials are investigated for effects on human
Biology Multiple Choice, 2 pt each.
 Biology 3340 Spring 2007 Name Exam 1, Version A Write your name on both the exam booklet and the mark sense sheet. On the upper left corner of the mark sense sheet in the Key ID box, mark the version letter
Biology 3340 Spring 2007 Name Exam 1, Version A Write your name on both the exam booklet and the mark sense sheet. On the upper left corner of the mark sense sheet in the Key ID box, mark the version letter
Chapter 7: Membrane Structure & Function
 Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Chapter 7: Membrane Structure & Function. 1. Membrane Structure. What are Biological Membranes? 10/21/2015. Why phospholipids? 1. Membrane Structure
 Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
ESCHERICHIA COLI-MUTABILE1. antiseptics employed "activated" the lactase which was present, "activate" the lactase.
 ON THE "ACTIVATION" OF THE LACTASE OF ESCHERICHIA COLI-MUTABILE1 CHARLES J. DEERE Department of Chemistry, University of Tennessee School of Biological Sciences, Memphis Received for publication August
ON THE "ACTIVATION" OF THE LACTASE OF ESCHERICHIA COLI-MUTABILE1 CHARLES J. DEERE Department of Chemistry, University of Tennessee School of Biological Sciences, Memphis Received for publication August
Scanning Electron Microscopy of Thiobacilli
 Arch. Microbiol. 99, 323-329 (1974) 0 by Springer-Verlag 1974 Scanning Electron Microscopy of Thiobacilli Grown on Colloïdal Sulfur J. Baldensperger", L. J. Guarraia**, and W. J. Humphreys*** Department
Arch. Microbiol. 99, 323-329 (1974) 0 by Springer-Verlag 1974 Scanning Electron Microscopy of Thiobacilli Grown on Colloïdal Sulfur J. Baldensperger", L. J. Guarraia**, and W. J. Humphreys*** Department
Multiplication of Different Legionella Species in Mono Mac 6 Cells and in Acanthamoeba castellanii
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Apr. 1997, p. 1219 1224 Vol. 63, No. 4 0099-2240/97/$04.00 0 Copyright 1997, American Society for Microbiology Multiplication of Different Legionella Species in
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Apr. 1997, p. 1219 1224 Vol. 63, No. 4 0099-2240/97/$04.00 0 Copyright 1997, American Society for Microbiology Multiplication of Different Legionella Species in
Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well
 Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well 1. General Information... 1 2. Background... 1 3. Material and Equipment Required... 2 4. Experimental Procedure and Results...
Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well 1. General Information... 1 2. Background... 1 3. Material and Equipment Required... 2 4. Experimental Procedure and Results...
Morphological Changes and Pathology of Mouse Glomeruli Infected with a Streptococcal L-Form or Exposed to Lipoteichoic Acid
 INFECTION AND IMMUNITY, Dec. 1983, p. 1144-1151 Vol. 42, No. 3 0019-9567/83/121144-08$02.00/0 Copyright 1983, American Society for Microbiology Morphological Changes and Pathology of Mouse Glomeruli Infected
INFECTION AND IMMUNITY, Dec. 1983, p. 1144-1151 Vol. 42, No. 3 0019-9567/83/121144-08$02.00/0 Copyright 1983, American Society for Microbiology Morphological Changes and Pathology of Mouse Glomeruli Infected
A Tour of the Cell. reference: Chapter 6. Reference: Chapter 2
 A Tour of the Cell reference: Chapter 6 Reference: Chapter 2 Monkey Fibroblast Cells stained with fluorescent dyes to show the nucleus (blue) and cytoskeleton (yellow and red fibers), image courtesy of
A Tour of the Cell reference: Chapter 6 Reference: Chapter 2 Monkey Fibroblast Cells stained with fluorescent dyes to show the nucleus (blue) and cytoskeleton (yellow and red fibers), image courtesy of
THE EFFECTS OF ACIDITY UPON THE GROWTH OF PNEUMOCOCCUS IN CULTURE MEDIA CONTAINING PROTEINS
 THE EFFECTS OF ACIDITY UPON THE GROWTH OF PNEUMOCOCCUS IN CULTURE MEDIA CONTAINING PROTEINS BY WILLIAM H. KELLEY, M.D. (From the Department of Medicine of the Duke University School of Medicine, Durham,
THE EFFECTS OF ACIDITY UPON THE GROWTH OF PNEUMOCOCCUS IN CULTURE MEDIA CONTAINING PROTEINS BY WILLIAM H. KELLEY, M.D. (From the Department of Medicine of the Duke University School of Medicine, Durham,
Annexin V-Cy3 Apoptosis Detection Reagent
 ab14143 Annexin V-Cy3 Apoptosis Detection Reagent Instructions for Use For the rapid, sensitive and accurate measurement of apoptosis in various samples This product is for research use only and is not
ab14143 Annexin V-Cy3 Apoptosis Detection Reagent Instructions for Use For the rapid, sensitive and accurate measurement of apoptosis in various samples This product is for research use only and is not
Supplemental Data. Wang et al. (2013). Plant Cell /tpc
 Supplemental Data. Wang et al. (2013). Plant Cell 10.1105/tpc.112.108993 Supplemental Figure 1. 3-MA Treatment Reduces the Growth of Seedlings. Two-week-old Nicotiana benthamiana seedlings germinated on
Supplemental Data. Wang et al. (2013). Plant Cell 10.1105/tpc.112.108993 Supplemental Figure 1. 3-MA Treatment Reduces the Growth of Seedlings. Two-week-old Nicotiana benthamiana seedlings germinated on
Physiology of Parasites (512) Zoo 3(2+1) Ultrastructure of protozoa and its adaption for host cell invasion
 Physiology of Parasites (512) Zoo 3(2+1) Ultrastructure of protozoa and its adaption for host cell invasion 1 Introduction protozoa Many are important nutrient cyclers. Many are photoautotrophic & make
Physiology of Parasites (512) Zoo 3(2+1) Ultrastructure of protozoa and its adaption for host cell invasion 1 Introduction protozoa Many are important nutrient cyclers. Many are photoautotrophic & make
IN VITRO CELLULAR RESPONSES TO AUTOLOGOUS TUMOR EXTRACT DETECTED BY INHIBITION OF MACROPHAGE MIGRATION*1
 [Gann, 66, 167-174; April, 1975] IN VITRO CELLULAR RESPONSES TO AUTOLOGOUS TUMOR EXTRACT DETECTED BY INHIBITION OF MACROPHAGE MIGRATION*1 Tsuyoshi AKIYOSHI, Akira HATA, and Hideo TSUJI Department of Surgery,
[Gann, 66, 167-174; April, 1975] IN VITRO CELLULAR RESPONSES TO AUTOLOGOUS TUMOR EXTRACT DETECTED BY INHIBITION OF MACROPHAGE MIGRATION*1 Tsuyoshi AKIYOSHI, Akira HATA, and Hideo TSUJI Department of Surgery,
PMT. Contains ribosomes attached to the endoplasmic reticulum. Genetic material consists of linear chromosomes. Diameter of the cell is 1 µm
 1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
Electron Microscope Studies of HeLa Cells Infected with Herpes Virus
 244 STOKER, M. G. P., SMITH, K. M. & Ross, R. W. (1958). J. gen. Microbiol. 19,244-249 Electron Microscope Studies of HeLa Cells Infected with Herpes Virus BY M: G. P. STOKER, K. M. SMITH AND R. W. ROSS
244 STOKER, M. G. P., SMITH, K. M. & Ross, R. W. (1958). J. gen. Microbiol. 19,244-249 Electron Microscope Studies of HeLa Cells Infected with Herpes Virus BY M: G. P. STOKER, K. M. SMITH AND R. W. ROSS
CYTOTOXICITY TEST ON THE EFFECT OF MODULATED ELECTROMAGNETIC WAVE ON NON-PATHOGENIC E.COLI CULTURE
 CYTOTOXICITY TEST ON THE EFFECT OF MODULATED ELECTROMAGNETIC WAVE ON NON-PATHOGENIC E.COLI CULTURE Sandy F. Sanchez Jr., Greg Martin E. Teo, and Romeric F. Pobre Instrumentation Physics Laboratory, Physics
CYTOTOXICITY TEST ON THE EFFECT OF MODULATED ELECTROMAGNETIC WAVE ON NON-PATHOGENIC E.COLI CULTURE Sandy F. Sanchez Jr., Greg Martin E. Teo, and Romeric F. Pobre Instrumentation Physics Laboratory, Physics
7-AAD/CFSE Cell-Mediated Cytotoxicity Assay Kit
 7-AAD/CFSE Cell-Mediated Cytotoxicity Assay Kit Catalog Number KA1293 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of
7-AAD/CFSE Cell-Mediated Cytotoxicity Assay Kit Catalog Number KA1293 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of
