Xia 3 Spinal System. Ilios and revision surgical technique
|
|
|
- Paula Stokes
- 5 years ago
- Views:
Transcription
1 Xia 3 Spinal System
2 Table of contents Surgical technique Introduction... 3 Patient positioning Pelvic screw path preparation Pelvic screw placement... 7 Axial connection Parallel connection Final tightening Rod-to-rod connectors Offset connectors Xia 3 implant offerings Implant overview Instrument overview Trays Indications, contraindications, cautions and warnings
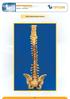
3 Introduction The evolution of the Xia 3 ilios and revision application is further evidence of the Stryker commitment to continue to listen to the global surgeon community and to provide implants and instruments that strive to offer optimal surgical solutions. The new ilios application was developed and built upon the success of the Xia Spinal System. The Ilios system provides a comprehensive offering of Xia long screws, closed head screws, biased-angle screws, offset connectors and rod-torod connectors as options for sacral-iliac fixation and revision applications. These implants are used for the treatment in long fusions to the sacrum in sagittal and/or coronal plane spinal deformities (scolosis, high-grade spondylolisthesis, flatback syndrome, etc.). Also, the system may be used in revision surgery (L5-S1 pseudoarthrosis) and in the treatment of sacral fractures. 3
4 Blocker Rods Angled Loading/ Side Loading RRC Monoaxial Screw D mm Polyaxial Screws D mm Top Loading/Side Loading RRC Parallel Closed RRC Long Offset Connector Open Head Revision RRC Closed Head Monoaxial Screw D mm Closed Head Polyaxial Screw D mm J-Hook Offset Connector Side Loading Long Offset Connector Large Diameter Polyaxial Screw D8.5, 9.5, 10.5mm Axial Revision RRC Screw Sacral Hook Medial Biased Polyaxial Screw D mm Biased Angle Screw D mm (Cephal Caudal Bias) Long Offset Connector Angled Monoaxial Screw D mm Closed Head Offset Connector 4
5 Patient positioning The patient is usually positioned prone on an appropriate spinal table. Care is taken to pad all bony prominences. The abdomen should not be compressed to help lower venous pressure (Fig. 1 and Fig. 2). Surgical levels may be verified clinically or radiographically. To ensure adequate exposure, the incision is made to extend just beyond the length of the intended fusion. Pre-operative planning defines the most appropriate implants, as well as the optimal location of insertion. Figure 1 Figure 2 Surgical access to the posterior superior iliac spine (PSIS) The lumbar spine is exposed first (Fig. 3). The lumbar spine exposure, surgical procedure (decompression, etc.) and instrumentation are completed prior to placement of iliac/pelvic screws. Iliac screw placement requires access to the hemipelvis, and specifically to the PSIS. The PSIS can be exposed using a separate longitudinal skin incision. Alternatively, the PSIS may be exposed through the same midline dorsal lumbar skin incision. The subcutaneous tissues are then raised laterally to expose the PSIS. The dissection proceeds above the dorsal lumbar fascia to expose the periosteum of the PSIS. The inner table is exposed to a depth of approximately 1.5cm to facilitate notching the crest and tunneling of the connector. This tunneling approach is straight forward and leaves the muscle attached, providing better coverage and a simpler closure (Fig. 4). Figure 3 Figure 4 ASIS Crest Iliac tubercle PSIS 5
6 Pelvic screw path preparation When the trajectory and depth are proven, measure the depth of the canal using the Xia Depth Gauge (884025). The starting point, which ideally is at the inferior pole of the PSIS, is prepared using either an osteotome or a rongeur (Fig. 5). The inferior pole of the PSIS may be preferable in most situations since it still allows iliac crest autograft to be harvested from the more cephaled portion of the hemipelvis. Furthermore, it still allows placement of an additional iliac screw from the more cephalad portion of the PSIS. Figure 5 When the iliac screw is placed at the inferior pole of the PSIS, it generally allows more room between the iliac screw and the S1 screw and avoids the hardware crowding associated with transverse connector placement and the S1 scew. Two iliac screws in each hemipelvis may be required in revision surgery, treatment of sacral fractures or treatment of sacropelvic instability. The iliac screw path is prepared with a Blunt Probe standing from the opposite side of the hemipelvis. The probe may be either Flat ( ) or Curved ( ) (Fig. 6). The ideal trajectory of the iliac screw is slightly above the sciatic notch and terminates in the quadrilateral plate of the pelvis. The trajectory offers superior osseous purchase. Figure 6 Adequate placement of the screw requires appropriate identification of the greater sciatic notch. The outer table of the hemipelvis can be exposed with subperiosteal dissection of the gluteal muscles. The sciatic notch may then be palpated digitally. The greater sciatic notch may also be visualized fluoroscopically and the tract prepared under fluoroscopic guidance. With more experience and understanding of the pelvic anatomy, the greater sciatic notch can be identified and/or palpated digitally without softtissue dissection of the outer table. The tract is then prepared using an anatomic probing technique. The integrity of the osseous tract is verified using a Pedicle Feeler ( ) to palpate the floor and all four bony walls (Fig. 7). Figure 7 6
7 Pelvic screw placement With the pelvic pathway prepared and proper screw length and diameter determined, the screw is prepared for insertion (Fig. 8). Verification of the appropriate osseous tract either by anatomic probing or tapping of the cancellous channel may or may not be necessary. The screw is then inserted into the hemipelvis in standard fashion. The top portion of the screw head should be at least flush or countersunk below the PSIS to avoid hardware prominence, which is considered the most common reason for pain (Fig. 9). Figure 8 Generally speaking, a mm diameter screw by 80mm long is used in most adult patients. Both the Xia 3 Polyaxial Screwdriver ( ) and Xia 3 Monoaxial Screwdriver ( ) provide a rigid connection between the screw and screwdriver (Fig. 10). Figure 9 Xia 3 also offers a new improved Iliac Screwdriver ( ) compatible with the following implants: T-Handle Closed Head Monoaxial Screw 48232DDLL Closed Head Polyaxial Screw 48237DDLL Round Handle T-Handle Ratchet Note: Handles are compatible with the monoaxial, polyaxial and iliac screwdrivers. Round Handle Ratchet Xia 3 Iliac Screwdriver Xia 3 Polyaxial Screwdriver Xia 3 Monoaxial Screwdriver
8 Axial connection The connection of the pelvic screw to the axial construct can either be direct (main construct rod captured by the iliac screw) or with a connecting implant (Fig. 10). Figure 10 Frequently on the convex side of the fractional lumbosacral curve, the axial rod can be easily contoured to continue from the S1 screw to the pelvic screw without the need of an additional connector. The preferable location of the offset connector is below the S1 screws as this provides stability at the distal foundation, augmenting the stability of the S1 SCR and immobilizing the lumbosacral junction (Fig. 11). Figure 11 8
9 The connection of the iliac screw to the longitudinal rod can be obtained with use of the Xia Offset Connectors ( , , , , , , , ) (Fig. 12). Xia Vitallium Rod Note: When using Xia Titanium implants, the surgeon may select a Xia Vitallium Rod. Vitallium Rod is recommended to be cut by the Table Top Rod Cutter or Table Top Rod Cutter Stand. Figure 12 Table Top Rod Cutter Table Top Rod Cutter Stand S The Xia 3 Inserter Tube can help align the Xia 3 Universal Tightener, 5mm and the Blocker with the implant (Fig. 13). Xia 3 Inserter Tube Note: The Xia 3 Universal Tightener is not to be used for final tightening. Figure 13 Xia 3 Universal Tightener, 5mm Note: The following connectors are compatible with a 6.0mm rod ONLY: Blocker Reference number Description Xia II Low Profile Closed Offset Connector Xia 3 Low Profile Long Closed Offset Connector Xia Rod-to-Rod Clamp Parallel Xia Rod-to-Rod Clamp Axial 9
10 Parallel connection The Xia Spinal System also offers a parallel connection from the main construct to the ilium. The Xia 3 Rod-to-Rod Connectors enable a surgeon to connect from the lumbar region to the ilium using a variety of options (Fig. 14): Xia II 0 Small Rod-to-Rod Connector Xia II 30 Small Rod-to-Rod Connector Xia II 0 Large Rod-to-Rod Connector Xia II 10 Large Rod-to-Rod Connector Xia 3 7mm Closed Parallel RRC Xia 3 11mm Closed Parallel RRC Xia 3 Angled Loading - Side Loading RRC Xia 3 Top Loading - Side Loading RRC Xia 3 12mm Parallel Revision Connector Open-Closed Xia 3 22mm Parallel Revision Connector Open-Closed These connectors can be used for iliac fixation in a revision surgery, on a stand alone basis or can be used in conjunction with an offset rod connection to place two screws in the ilium for supplemental iliac fixation. This offers surgeons added strength and flexibility in treating complicated spinal disorders in the lumbar and sacral regions. Figure 14 Note: The following connectors are compatible with a 6.0mm rod ONLY: Reference number Description Xia II Low Profile Closed Offset Connector The picture to the right shows an L2-S1 Revision case with iliac fixation implanted for stability. Then, because of junctional disease, the construct was extended up to T9 using various Xia 3 Rod-to-Rod Revision Connectors Xia 3 Low Profile Long Closed Offset Connector Xia Rod-to-Rod Clamp Parallel Xia Rod-to-Rod Clamp Axial 10
11 Final tightening The final tightening of the Xia Blocker is done by utilizing the Xia 3 Anti-Torque Key and the Torque Wrench or Audible Torque Wrench. The Torque Wrench indicates the optimum force which has to be applied to the implant for final tightening. Line up the two arrows to achieve this optimum torque of 12Nm. If using the Audible Torque Wrench, the blocker is completely tightened to 12Nm when the Audible Torque Wrench clicks once. Anti-Torque Key Torque Wrench Note: Do not overtighten. Note: The ES2 Torque Wrench or the MANTIS Redux Torque Wrench may also be used as an alternative to the Xia 3 Torque Wrench to final tighten the Xia 3 Blockers. Note: The ES2 Counter Torque Tube may also be used in conjunction with the Xia 3 Torque Wrench or Audible Torque Wrench. 12Nm Audible Torque T-Handle (Standard) G mm Hex Shaft (12Nm) GC Audible Torque Wrench Note: The Audible Torque Wrench consists of the 12Nm Audible Torque T-Handle (Standard) connected to the 5mm Hex Shaft (12Nm). ES2 Torque Wrench ES2 Counter Torque Tube
12 Rod-to-rod connectors Nine rod-to-rod connectors are offered with this set. All designs are available in stainless steel and titanium. All of these connectors use the Xia Blocker for final rod fixation. 1) Xia II 0 Small Rod-to-Rod Connector This open rod-to-rod connector accommodates parallel rods that are 12mm apart. Xia II 0 Small Rod-to-Rod Connector ) Xia II 30 Small Rod-to-Rod Connector This open rod-to-rod connector accommodates rods that are 12mm apart at angles of 30. Xia II 30 Small Rod-to-Rod Connector ) Xia II 0 Large Rod-to-Rod Connector This open rod-to-rod connector accommodates parallel rods that are 16mm apart. Xia II 0 Large Rod-to-Rod Connector ) Xia II 10 Large Rod-to-Rod Connector This open rod-to-rod connector accommodates rods that are 16mm apart at angles of 10. Xia II 10 Large Rod-to-Rod Connector Note: The following connectors are compatible with a 6.0mm rod ONLY: , , ,
13 5) Xia 3 Angled Loading-Side Loading RRC This angled open rod-to-rod connector accommodates rods that are 9.65mm apart and provides an easy connection for revision applications, with a 45 angle. Xia 3 Angled Loading-Side Loading RRC ) Xia 3 Top Loading-Side Loading RRC This top-loading/side-loading open connector provides an easy connection for revision applications, and accomodates rods that are 11mm apart. Xia 3 Top Loading-Side Loading RRC ) Xia 3 12mm Parallel Revision Connector Open-Closed This open/closed rod-to-rod connector accommodates rods that are 12mm (small) and 22mm (large) apart. Xia 3 12mm Parallel Revision Connector Open-Closed ) Xia 3 7mm Closed Parallel RRC This closed parallel rod-to-rod connector accommodates rods that are 7mm apart. Xia 3 7mm Closed Parallel RRC ) Xia 3 11mm Closed Parallel RRC This closed parallel rod-to-rod connector accommodates rods that are 11mm apart. Note: The following connectors are compatible with a 6.0mm rod ONLY: , , , Xia 3 11mm Closed Parallel RRC
14 Offset connectors Nine offset connector designs are offered in the ilios application. All designs are available in stainless steel and titanium. All of these connectors use a Xia Blocker for final rod fixation. 1) Xia II Long Offset Connector Neutral This connector is 80mm long and will be perpendicular to the rod when attached. This connector can be cut and bent for additional interoperative flexibility. Xia II Long Offset Connector Neutral ) Xia II Offset Connector Neutral This connector is 35mm long and will be perpendicular to the rod when attached. Xia II Offset Connector Neutral ) Xia 3 75 Offset Connector Bend This connector is 35mm long and has a 75 angle allowing better anatomical placement of the connector in the iliac region. Xia 3 75 Offset Connector Bend ) Xia II 105 Offset Connector Bend This connector is 35mm long and has a 105 angle allowing better anatomical placement of the connector in the iliac region. Xia II 105 Offset Connector Bend ) Xia 3 Long Offset Connector Open-Head This connector is 100mm long and will be perpendicular to the rod when attached. This connector can be cut and bent for additional interoperative flexibility. Xia 3 Long Offset Connector Open-Head Note: The following connectors are compatible with a 6.0mm rod ONLY: , , ,
15 Offset Connectors 6) Xia 3 Open-Side Loading Offset Connector This connector is 69mm long and will be perpendicular to the main rod construct. The open side allows or easy connection and flexibility. Xia 3 Open-Side Loading Offset Connector ) Xia 3 Small Closed Head Offset Connector This connector is 65mm long and offers a low-profile closedhead connection to the rod. Xia 3 Small Closed Head Offset Connector ) Xia 3 Low Profile Long Closed Offset Connector This connector is 100mm long and is similar to the Xia II closed offset connector, but in a longer length. Note: Short Xia II Connector. Xia 3 Low Profile Long Closed Offset Connector Xia II Short Offset Connector ) Xia 3 J Hook Offset Connector This connector is 100mm long and connects to the rod like the M.A.C. connectors, allowing for a simple connection to the rod. Xia 3 J Hook Offset Connector Note: The following connectors are compatible with a 6.0mm rod ONLY: , , ,
16 Implants Reference number Sterile reference number* Description N/A Xia II 0 Small Rod-to-Rod Connector N/A Xia II 30 Small Rod-to-Rod Connector N/A Xia II 0 Large Rod-to-Rod Connector N/A Xia II 10 Large Rod-to-Rod Connector S Xia 3 12mm Parallel Revision Connector Open-Closed S Xia 3 22mm Parallel Revision Connector Open-Closed S Xia 3 Angled Loading - Side Loading RRC S Xia 3 Top Loading - Side Loading RRC S Xia 3 7mm Closed Parallel RRC S Xia 3 11mm Closed Parallel RRC *Sterile implants are only available in certain markets outside the U.S. Please contact your Stryker sales representative for more information. Note: The following connectors are compatible with a 6.0mm rod ONLY: , , ,
17 Reference number Sterile reference number* Description N/A Xia II 75 Offset Connector Bend N/A Xia II Offset Connector Neutral N/A Xia II 105 Offset Connector Bend N/A Xia II Long Offset Connector Neutral S Xia 3 Low Profile Long Closed Offset Connector S Xia 3 Small Closed Head Offset Connector ** N/A Set Screw for Xia 3 Small Offset Connector Closed Head (M6 x 1) S Xia 3 Long Offset Connector Open-Head S Xia 3 J Hook Offset Connector S Xia 3 Open Side-Loading Offset Connector Revision implants Reference number Sterile reference number* Description S Xia 3 Axial Revision RRC N/A Xia 3 Sacral Hook *Sterile implants are only available in certain markets outside the U.S. Please contact your Stryker sales representative for more information. **These set screws come pre-packaged with these implants. This part need only be ordered as a replacement if lost or damaged. Note: The following connectors are compatible with a 6.0mm rod ONLY: , , ,
18 Reference number (20) (45) (20) (45) (20) (45) (25) (55) (25) (90) (25) (90) (60) (100) (60) (100) (20) (45) (20) (45) (20) (45) (25) (55) (25) (90) (25) (90) (60) (90) (60) (90) (30) (00) (30) (00) (30) (00) (30) (00) Description Xia 3 Ø4.0 x 20-45mm Biased Angle Polyaxial Screw Xia 3 Ø4.5 x 20-45mm Biased Angle Polyaxial Screw Xia 3 Ø5.0 x 20-45mm Biased Angle Polyaxial Screw Xia 3 Ø5.5 x 25-55mm Biased Angle Polyaxial Screw Xia 3 Ø6.5 x 25-90mm Biased Angle Polyaxial Screw Xia 3 Ø7.5 x 25-90mm Biased Angle Polyaxial Screw Xia 3 Ø8.5 x mm Biased Angle Polyaxial Screw Xia 3 Ø9.5 x mm Biased Angle Polyaxial Screw Xia 3 Ø4.0 x 20-45mm Medial Biased Angle Polyaxial Screw Xia 3 Ø4.5 x 20-45mm Medial Biased Angle Polyaxial Screw Xia 3 Ø5.0 x 20-45mm Medial Biased Angle Polyaxial Screw Xia 3 Ø5.5 x 25-55mm Medial Biased Angle Polyaxial Screw Xia 3 Ø6.5 x 25-90mm Medial Biased Angle Polyaxial Screw Xia 3 Ø7.5 x 25-90mm Medial Biased Angle Polyaxial Screw Xia 3 Ø8.5 x 60-90mm Medial Biased Angle Polyaxial Screw Xia 3 Ø9.5 x 60-90mm Medial Biased Angle Polyaxial Screw Xia 3 Ø6.5 x mm Closed Head Polyaxial Screw Xia 3 Ø7.5 x mm Closed Head Polyaxial Screw Xia 3 Ø8.5 x mm Closed Head Polyaxial Screw Xia 3 Ø9.5 x mm Closed Head Polyaxial Screw Set Screw for Xia 3 Closed Head Screw (M6 x 1) These set screws come pre-packaged with these implants. This part need only be ordered as a replacement if lost or damaged. 18
19 Reference number Sterile reference number* Description S Xia 3 Blocker (20) - (45) (20) - (45) (20) - (50) (25) - (55) (25) - (90) (25) - (90) (25) - (90) (25) - (90) (25) - (00) (40) - (00) (20) - (45)S (20) - (45)S N/A (25) - (55)S N/A (25) - (90)S N/A (25) - (90)S (25) - (00)S (40) - (00)S Xia 3 Ø4.0 x 20-45mm Monoaxial Screw Xia 3 Ø4.5 x 20-45mm Monoaxial Screw Xia 3 Ø5.0 x 20-50mm Monoaxial Screw Xia 3 Ø5.5 x 25-55mm Monoaxial Screw Xia 3 Ø6.0 x 25-90mm Monoaxial Screw Xia 3 Ø6.5 x 25-90mm Monoaxial Screw Xia 3 Ø7.0 x 25-90mm Monoaxial Screw Xia 3 Ø7.5 x 25-90mm Monoaxial Screw Xia 3 Ø8.5 x mm Monoaxial Screw Xia 3 Ø9.5 x mm Monoaxial Screw (20) - (45) (20) - (45) (20) - (50) (25) - (55) (25) - (90) (25) - (90) (25) - (90) (25) - (90) (25) - (00) (40) - (00) (40) - (00) (20) - (45)S (20) - (45)S N/A (25) - (55)S N/A (25) - (90)S N/A (25) - (90)S (25) - (00)S (40) - (00)S (40) - (00)S Xia 3 Ø4.0 x 20-45mm Polyaxial Screw Xia 3 Ø4.5 x 20-45mm Polyaxial Screw Xia 3 Ø5.0 x 20-50mm Polyaxial Screw Xia 3 Ø5.5 x 25-55mm Polyaxial Screw Xia 3 Ø6.0 x 25-90mm Polyaxial Screw Xia 3 Ø6.5 x 25-90mm Polyaxial Screw Xia 3 Ø7.0 x 25-90mm Polyaxial Screw Xia 3 Ø7.5 x 25-90mm Polyaxial Screw Xia 3 Ø8.5 x mm Polyaxial Screw Xia 3 Ø9.5 x mm Polyaxial Screw Xia 3 Ø10.5 x mm Polyaxial Screw *Sterile implants are only available in certain markets outside the U.S. Please contact your Stryker sales representative for more information. 19
20 Instruments Reference number Description Double-Ended 3.5 Setscrew Inserter Double Ended Pedicle Feeler Dual-Ended Universal Tightener Iliac Screwdriver One piece Iliac Screwdriver Two piece mm Modular Tap mm Modular Tap mm Modular Tap Modular Monodriver Shaft Pedicle Marker Inserter Pedicle Markers (Set of 6) Rod Gripper 20
21 Reference number Description Rod to Rod Connector Holder Self-Holding Polyaxial Screwdriver Shaft Soft Tissue Retractor Straight Blunt Probe SUK Derotator Clamp T-Handle, SUK Tube SUK Tube One-Piece SUK Tube Two-Piece S Table-Top Rod Cutter Stand Table-Top Rod Cutter L Tube Bender Left R Tube Bender Right 21
22 Reference number Description Rod Template (30) - (00) 3.0 Modular Tap 3.5 Modular Tap 4.0 Modular Tap 4.5 Modular Tap 5.0 Modular Tap 5.5 Modular Tap 6.5 Modular Tap 7.5 Modular Tap 8.5 Modular Tap 9.5 Modular Tap 10.5 Modular Tap Vise Grip Narrow Lamina Hook Preparer Cross Connector Inserter mm Hex Driver mm Hex Driver Cross Connector Measuring Device Rod Insertion Forceps Coronal Plane Bender Left Coronal Plane Bender Right S Ball Joint 22
23 Reference number Description Standard Hook Holder Lateral Hook Holder Straight Hook Holder T-Handle T-Handle, Ratchet Round Handle Round Handle, Ratchet Monoaxial Screwdriver (without handle) Shaft for Monoaxial Screwdriver Polyaxial Screwdriver (without handle) Shaft for Polyaxial Screwdriver Small Distractor Large Distractor 23
24 Reference number Description Small Compressor Large Compressor Pedicle Feeler - Stiff Pedicle Feeler - Medium Pedicle Feeler - Malleable Universal Tightener Inserter Tube French Bender One Handed Persuader Persuader Hex Drive T-Handle Rod Fork Rod Pusher Lamina Hook Preparer Curved Blunt Probe 24
25 Reference number Description Pedicle Hook Preparer Anti-Torque Key Xia 3 Torque Wrench ES2 Torque Wrench MANTIS Redux Torque Wrench G Nm Audible Torque T-Handle (Standard) G Nm Audible Torque T-Handle (Offset) GC mm Hex Shaft (12Nm) ES2 Counter Torque Tube Hook Impactor Monodriver Poly Adjustment Driver (without handle) Thoracic Pedicle Probe Rod Rotation Key Awl 25
26 Reference number Description S PA Screwdriver Sleeve, Sleeve for Screwdriver L In Situ Bender Left R In Situ Bender Right Ilios Tray Outlier Instrument Tray SUK Tray Outlier Implant Tray 26
27 Xia 3, Xia 4.5, and Xia Growth Rod Conversion Set STRYKER SPINE Spinal Fixation Systems NON-STERILE AND STERILE PRODUCT The STRYKER Spine Spinal Fixation Systems are made of devices for fixation of the non-cervical spine. They include smooth rods, screws, hooks, closure screws, connectors, and staples. The components are manufactured from either titanium material (Titanium alloy and CP Titanium), Stainless Steel or Cobalt-Chromium- Molybdenum Alloy. MATERIALS Xia 3 Spinal System Titanium Alloy: Ti6Al4V according to ISO and ASTM F-136: Screws, hooks, closure screws, connectors and rods. Pure Titanium: CP Ti grade 4 according to ISO and ASTM F-67: Rods Cobalt-Chromium-Molybdenum Alloy #1 according to ISO and ASTM F-1537: Rods. Xia 4.5 Spinal System Titanium Alloy: Ti6Al4V according to ISO and ASTM F-136: Screws, hooks, closure screws, connectors, rods, and staples. Cobalt-Chromium-Molybdenum Alloy #1 according to ISO and ASTM F-1537: Rods. Xia Growth Rod Conversion Set Titanium Alloy: Ti6Al4V according to ISO and ASTM F-136: Growth Rod Connectors Titanium and Stainless steel implants should not be mixed in a patient; otherwise corrosion may occur resulting in decreased mechanical resistance. Cobalt-Chromium-Molybdenum Alloy and Stainless steel implants should not be mixed in a patient; otherwise corrosion may occur resulting in decreased mechanical resistance. MATERIALS IDENTIFICATION Titanium: symbol T Stainless Steel: symbol S Cobalt-Chromium-Molybdenum: symbol C INDICATIONS Xia 3 Spinal System The Xia 3 Spinal System is intended for use in the non-cervical spine. When used as an anterior/anterolateral and posterior, non-cervical pedicle and non-pedicle fixation system, the Xia 3 Spinal System is intended to provide additional support during fusion using autograft or allograft in skeletally mature patients in the treatment of the following acute and chronic instabilities or deformities: Degenerative Disc Disease (as defined by back pain of discogenic origin with degeneration of the disc confirmed by patient history and radiographic studies) Spondylolisthesis Trauma (i.e. fracture of dislocation) Spinal stenosis Curvatures (i.e., scoliosis, kyphosis, and/or lordosis) Tumor Pseudarthrosis Failed previous fusion The 5.5 mm rods from the Stryker Spine Radius Spinal System and 6.0 mm Vitallium rods from the Xia Spinal System are intended to be used with the other components of the Xia 3 Spinal System. When used for posterior, non-cervical, pedicle screw fixation in pediatric patients, the Xia 3 Spinal System implants are indicated as an adjunct to fusion to treat progressive spinal deformities (i.e., scoliosis, kyphosis, or lordosis) including idiopathic scoliosis, neuromuscular scoliosis, and congenital scoliosis. Additionally, the Xia 3 Spinal System is intended to treat pediatric patients diagnosed with: spondylolisthesis/spondylolysis, fracture caused by tumor and/or trauma, pseudarthrosis, and/or failed previous fusion. This system is intended to be used with autograft and/ or allograft. Pediatric pedicle screw fixation is limited to a posterior approach. Xia 4.5 Spinal System The Xia 4.5 Spinal System is intended for anterior/anterolateral and posterior, non-cervical pedicle and non-pedicle fixation for the following indications: Degenerative Disc Disease (as defined by back pain of discogenic origin with degeneration of the disc confirmed by patient history and radiographic studies) Spondylolisthesis Trauma (i.e. fracture of dislocation) Spinal stenosis Curvatures (i.e., scoliosis, kyphosis, and/or lordosis) Tumor Pseudarthrosis Failed previous fusion The Stryker Spine DIAPASON Spinal System, Opus Spinal System, and Xia 4.5 Spinal System can be linked to the Xia 4.5 Spinal System via the rod-torod connector when used for the aforementioned indications in skeletally mature patients as an adjunct to fusion. Except for the staples, when used for posterior non-cervical pedicle screw fixation in pediatric patients, the Xia 4.5 Spinal System implants are indicated as an adjunct to fusion to treat progressive spinal deformities (i.e., scoliosis, kyphosis, or lordosis) including idiopathic scoliosis, neuromuscular scoliosis, and congenital scoliosis. Additionally, the Xia 4.5 Spinal System is intended to treat pediatric patients diagnosed with: spondylolisthesis/spondylolysis, fracture caused by tumor and/or trauma, pseudarthrosis, and/or failed previous fusion. This system is intended to be used with autograft and/ or allograft. Pediatric pedicle screw fixation is limited to a posterior approach. Xia Growth Rod Conversion Set The Xia Growth Rod Conversion Set is indicated in patients with potential for additional spinal growth under 10 years of age who require surgical treatment to obtain and maintain correction of severe, progressive, life-threatening, early-onset spinal deformities associated with thoracic insufficiency, including early-onset scoliosis. The Xia Growth Rod Conversion Set may be used with any cleared Xia 4.5 Spinal System rod construct. The Xia Growth Rod Conversion Set is not intended for use in conjunction with staples. CONTRAINDICATIONS Contraindications may be relative or absolute. The choice of a particular device must be carefully weighed against the patient s overall evaluation. 27
28 Circumstances listed below may reduce the chances of a successful outcome: Any abnormality present which affects the normal process of bone remodeling including, but not limited to, severe osteoporosis involving the spine, bone absorption, osteopenia, primary or metastatic tumors involving the spine, active infection at the site or certain metabolic disorders affecting osteogenesis. Insufficient quality or quantity of bone which would inhibit rigid device fixation. Previous history of infection. Excessive local inflammation. Open wounds. Obesity. An overweight or obese patient can produce loads on the spinal system which can lead to failure of the fixation of the device or to failure of the device itself. Patients having inadequate tissue coverage of the operative site. Pregnancy. A condition of senility, mental illness, or substance abuse. These conditions, among others, may cause the patient to ignore certain necessary limitations and precautions in the use of the implant, leading to failure or other complications. Foreign body sensitivity. Where material sensitivity is suspected, appropriate tests should be made prior to material selection or implantation. Other medical or surgical condition which would preclude the potential benefit of spinal implant surgery, such as the presence of tumors, congenital abnormalities, elevation of sedimentation rate unexplained by other diseases, elevation of white blood cell count (WBC), or marked left shift in the WBC differential count. ADDITIONAL CONTRAINDICATIONS FOR PEDIATRIC PATIENTS Any case where the implant components selected for use would be too large or too small to achieve a successful result. Any patient in which implant utilization would interfere with anatomical structures or expected physiological performance. Patients having inadequate tissue coverage of the operative site or inadequate bone stock or quality. These contraindications may be relative or absolute and must be taken into account by the physician when making his decision. The above list is not exhaustive. GENERAL CONDITIONS OF USE The implantation of pedicle screw spinal systems must be performed only by experienced spinal surgeons having undergone the necessary specific training in the use of such systems because this is a technically demanding procedure presenting a risk of serious injury to the patient. The information contained in the Package Insert is necessary but not sufficient for the use of these devices. This information is in no sense intended as a substitute for the professional judgment, skill and experience of the surgeon in careful patient selection, preoperative planning and device selection, knowledge of the anatomy and biomechanics of the spine, understanding of the materials and the mechanical characteristics of the implants used, training and skill in spinal surgery and the use of associated instruments for implantation, securing the patient s cooperation in following an appropriately defined post-operative management program and conducting scheduled post-operative follow-up examinations. INFORMATION FOR PATIENTS The surgeon must discuss all physical and psychological limitations inherent to the use of these devices with the patient. This includes the rehabilitation regimen, physical therapy, and wearing an appropriate orthosis as prescribed by the physician. Particular discussion should be directed to the issues of premature weight bearing, activity levels, and the necessity for periodic medical follow-up. The surgeon must warn the patient of the surgical risks and make aware of possible adverse effects. The surgeon must warn the patient that the devices cannot and do not replicate the flexibility, strength, reliability or durability of normal healthy bone, that the implants can break or become damaged as a result of strenuous activity or trauma, and that the devices may need to be replaced in the future. If the patient is involved in an occupation or activity which applies inordinate stress upon the implant (e.g., substantial walking, running, lifting, or muscle strain) the surgeon must advise the patient that resultant forces can cause failure of the devices. Patients who smoke have been shown to have an increased incidence of non-unions. Surgeons must advise patients of this fact and warn of the potential consequences. For diseased patients with degenerative disease, the progression of degenerative disease may be so advanced at the time of implantation that it may substantially decrease the expected useful life of the appliance. In such cases, orthopaedic devices may be considered only as a delaying technique or to provide temporary relief. INFECTION Transient bacteremia can occur in daily life. Dental manipulation, endoscopic examination and other minor surgical procedures have been associated with transient bacteremia. To help prevent infection at the implant site, it is advisable to use antibiotic prophylaxis before and after such procedures.. INSTRUMENTS Instruments are provided by STRYKER Spine and must be used to assure accurate implantation of the devices. While rare, intraoperative fracture or breakage of instruments can occur. Instruments which have experienced extensive use or extensive force are more susceptible to fracture depending on the operative precaution, number of procedures, disposal attention. Instruments must be examined for wear or damage prior to surgery. Surgeons must verify that the instruments are in good condition and operating order prior to each use during surgery REUSE Re-sterilization of implants provided sterile is strictly forbidden, regardless of the method that might be employed. Never reuse or re-implant spinal surgical implants. These could become contaminated resulting in infection. In addition, even though the device appears undamaged, it may have small defects which could compromise structural integrity reducing its service life and/or leading to patient injury. HANDLING Correct handling of the implant is extremely important. The operating surgeon should avoid notching or scratching the device. ALLERGY AND HYPERSENSITIVITY TO FOREIGN BODIES When hypersensitivity is suspected or proven, it is recommended that the tolerance of the skin to the materials that make up the implants be checked before they are implanted. 28
29 IMPLANT SELECTION AND USE The choice of proper shape, size and design of the implant for each patient is crucial to the success of the surgery. The surgeon is responsible for this choice which depends on each patient. Patients who are overweight may be responsible for additional stresses and strains on the device which can speed up metal fatigue and/or lead to deformation or failure of the implants. The size and shape of the bone structures determine the size, shape and type of the implants. Once implanted, the implants are subjected to stresses and strains. These repeated stresses on the implants should be taken into consideration by the surgeon at the time of the choice of the implant, during implantation as well as in the post-operative follow-up period. Indeed, the stresses and strains on the implants may cause metal fatigue or fracture or deformation of the implants, before the bone graft has become completely consolidated. This may result in further side effects or necessitate the early removal of the osteosynthesis device. Improper selection, placement, positioning and fixation of these devices may result in unusual stress conditions reducing the service life of the implant. Contouring or bending of rods or plates is recommended only if necessary according to the surgical technique of each system. Rods or plates should only be contoured with the proper contouring instruments. Incorrectly contoured rods/plates, or rods/plates which have been repeatedly or excessively contoured must not be implanted. The surgeon is to be thoroughly familiar with the surgical procedure, instruments and implant characteristics prior to performing surgery. Refer to the STRYKER Spine surgical protocols for additional procedural information. Periodic follow-up is recommended to monitor the position and state of the implants, as well as the condition of the adjoining bone. METAL COMPONENTS Some of the alloys utilized to produce orthopaedic implants contain metallic elements that may be carcinogenic in tissue cultures or intact organisms under unique circumstances. Questions have been raised in the scientific literature as to whether or not these alloys themselves may be carcinogenic in implant recipients. Studies conducted to evaluate this issue have not identified conclusive evidence of such phenomena. SYSTEM COMPATIBILITY While some degree of corrosion occurs on all implanted metal and alloys, contact of dissimilar metals may accelerate this corrosion process. The presence of corrosion may accelerate fatigue fracture of implants, and the amount of metal compounds released into the body system may also increase. Internal fixation devices, such as rods, hooks, screws, wires, etc., which come into contact with other metal objects, must be made from like or compatible metals. Because different manufacturers employ different materials, varying tolerances and manufacturing specifications, and differing design parameters, components of the system should not be used in conjunction with components from any other manufacturer s spinal system. Any such use will negate the responsibility of STRYKER Spine for the performance of the resulting mixed component implant. POSTOPERATIVE CARE Prior to adequate maturation of the fusion mass, implanted spinal instrumentation may need additional help to accommodate full load bearing. External support may be recommended by the physician from two to four months postoperatively or until x-rays or other procedures confirm adequate maturation of the fusion mass; external immobilization by bracing or casting may be employed. Surgeons must instruct patients regarding appropriate and restricted activities during consolidation and maturation for the fusion mass in order to prevent placing excessive stress on the implants which may lead to fixation or implant failure and accompanying clinical problems. Surgeons must instruct patients to report any unusual changes of the operative site to his/her physician. The physician should closely monitor the patient if a change at the site has been detected. ADVERSE EFFECTS While the expected life of spinal implant components is difficult to estimate, it is finite. These components are made of foreign materials which are placed within the body for the potential fusion of the spine and reduction of pain. However, due to the many biological, mechanical and physicochemical factors which affect these devices but cannot be evaluated in vivo, the components cannot be expected to indefinitely withstand the activity level and loads of normal healthy bone. Bending, disassembly or fracture of any or all implant components. Fatigue fracture of spinal fixation devices, including screws and rods, has occurred. Pain, discomfort, or abnormal sensations due to the presence of the device. Pressure on skin from components where inadequate tissue coverage exists over the implant, with the potential extrusion through the skin. Dural leak requiring surgical repair. Loss of proper spinal curvature, correction, height and/or reduction. Delayed Union or Nonunion: Internal fixation appliances are load sharing devices which are used to obtain alignment until normal healing occurs. In the event that healing is delayed, does not occur, or failure to immobilize the delayed/nonunion results, the implant will be subject to excessive and repeated stresses which can eventually cause loosening, bending or fatigue fracture. The degree or success of union, loads produced by weight bearing, and activity levels will, among other conditions, dictate the longevity of the implant. If a nonunion develops or if the implants loosen, bend or break, the device(s) should be revised or removed immediately before serious injury occurs. Loosening of spinal fixation implants can occur. Early mechanical loosening may result from inadequate initial fixation, latent infection, premature loading of the prosthesis, or trauma. Late loosening may result from trauma, infection, biological complications or mechanical problems, with the subsequent possibility of bone erosion, migration and/or pain. Peripheral neuropathies, nerve damage, heterotopic bone formation and neurovascular compromise, including paralysis, loss of bowel or bladder function, or foot-drop may occur. Serious complications may be associated with any spinal surgery. These complications include, but are not limited to: genitourinary disorders; gastrointestinal disorders; vascular disorders, including 29
30 thrombus; bronchopulmonary disorders, including emboli; bursitis, hemorrhage, myocardial infarction, infection, paralysis or death. Neurological, vascular, or soft tissue damage due directly to the unstable nature of the fracture, or to surgical trauma. Inappropriate or improper surgical placement of this device may cause distraction or stress shielding of the graft or fusion mass. This may contribute to failure of an adequate fusion mass to form. Decrease in bone density due to stress shielding. Intraoperative fissure, fracture, or perforation of the spine can occur due to implantation of the components. Postoperative fracture of bone graft, the intervertebral body, pedicle, and/ or sacrum above and/or below the level of surgery can occur due to trauma, the presence of defects, or poor bone stock. Adverse effects may necessitate reoperation or revision. The surgeon must warn the patient of these adverse effects as deemed necessary. ADDITIONAL ADVERSE EFFECTS FOR PEDIATRIC PATIENTS Inability to use pedicle screw fixation due to limitations (pedicle dimensions and/or distorted anatomy). Pedicle screw malpositioning, with or without neurological or vascular injury. Proximal or distal junctional kyphosis. Pancreatitis. Unintended fusion in Growth Rod patients Increased risk of post-operative infection and wound-healing issues in Growth Rod patients Increased risk of implant breakage in Growth Rod patients Implant prominence (symptomatic or asymptomatic). Pressure on the skin from component parts in patients with inadequate tissue coverage over the implant possibly causing skin penetration, irritation, fibrosis, necrosis, or pain. Post-operative change in spinal curvature, loss of correction, height, or reduction. Development of respiratory problems (e.g., pulmonary embolism, atelectasis, bronchitis, pneumonia, etc.) REMOVAL OF IMPLANTS These implants are temporary internal fixation devices designed to stabilize the operative site during the normal healing process. After healing occurs, these devices serve no functional purpose and can be removed. Removal may also be recommended in other cases, such as: Corrosion with a painful reaction Migration of the implant, with subsequent pain and/or neurological, articular or soft tissue lesions Pain or abnormal sensations due to the presence of the implants Infection or inflammatory reactions Reduction in bone density due to the different distribution of mechanical and physiological stresses and strains Failure or mobilization of the implant Standard ancillaries provided by STRYKER Spine can be used to remove the implants. Any decision by a physician to remove the internal fixation device must take into consideration such factors as the risk to the patient of the additional surgical procedure as well as the difficulty of removal. Removal of an unloosened spinal screw may require the use of special instruments to disrupt the interface at the implant surface. This technique may require practice in the laboratory before being attempted clinically. Implant removal should be followed by adequate postoperative management to avoid fracture or re-fracture. Removal of the implant after fracture healing is recommended. Metallic implants can loosen, bend, fracture, corrode, migrate, cause pain or stress shield bone. CAUTION Stryker Spine has not validated and does not recommend Flash Sterilization. For Product being used in the US, a sterilization wrap that is FDA cleared for the cycle parameters noted is required. PRE-OPERATIVE PRECAUTIONS Anyone using STRYKER Spine products can obtain a Surgical Technique brochure by requesting one from a distributor or from STRYKER Spine directly. Those using brochures published more than two years before the surgical intervention are advised to get an updated version. STRYKER Spine devices can only be used by doctors who are fully familiar with the surgical technique required and who have been trained to this end. The doctor operating must take care not to use the instruments to exert inappropriate stress on the spine or the implants and must scrupulously comply with any operating procedure described in the surgical technique provided by STRYKER Spine. For example, the forces exerted when repositioning an instrument in-situ must not be excessive as this is likely to cause injury to the patient. To reduce the risks of breakage, care must be taken not to distort the implants or nick, hit or score them with the instruments unless otherwise specified by the applicable STRYKER Spine Surgical Technique. Extreme care must be taken when the instruments are used near vital organs, nerves or vessels. CAUTION Federal law (U.S.A) restricts this device to sale by or on the order of a licensed physician. WARNING (U.S.A.) The safety and effectiveness of pedicle screw spinal systems have been established only for spinal conditions with significant mechanical instability or deformity requiring fusion with instrumentation. These conditions are significant mechanical instability or deformity of the thoracic, lumbar, and sacral spine secondary to spondylolisthesis (grades 3 and 4) of the L5-S1 vertebrae, degenerative spondylolisthesis with objective evidence of neurological impairment, fracture, dislocation, scoliosis, kyphosis, spinal tumor, and failed previous fusion (pseudoarthrosis). The safety and effectiveness of these devices for any other conditions are unknown. The Xia 3 Spinal System, Xia 4.5 Spinal System, and Xia Growth Rod Conversion Set have not been tested for heating or migration in the MR environment. ADDITIONAL WARNINGS FOR PEDIATRIC PATIENTS The safety and effectiveness of the Xia 3 Spinal System has not been established for use as part of a growing rod construct. This device is only intended to be used when definitive fusion is being performed at all instrumented levels. 30
31 Growth rod systems should only be used by surgeons who are experienced with pediatric posterior spine surgery procedures and have undergone hands-on training in both device implantation and adjustment. Only surgeons who are familiar with the implant components, instruments, procedure, clinical applications, biomechanics, adverse events, and risks associated with growth rod systems should use this device. A lack of adequate experience and/or training may lead to a higher incidence of adverse events, including neurologic complications. Growth rod constructs typically require repeated planned-lengthening procedures until a determination is made that the patient is ready for a final fusion procedure. Growth rod patients are more susceptible to post-operative infections and woundhealing issues, as well as the potential for implant breakage requiring unplanned surgical procedures. The physician should discuss these and all other potential complications with the patient and the patient s guardian. The use of pedicle screw fixation in the pediatric population may present additional risks when patients are of smaller stature or skeletally immature. Pediatric patients may have smaller spinal structures (pedicle diameter and length) that may preclude the use of pedicle screws or increase the risk of pedicle screw malpositioning and neurological or vascular injury. Patients not skeletally mature that undergo spinal fusion procedures may have reduced longitudinal spinal growth, or may be at risk for rotational spinal deformities (the crankshaft phenomenon ) due to continued differential growth of the anterior spine. Other adverse effects related to pedicle screw fixation, such as screw or rod bending, breakage, or loosening, may also occur in pediatric patients. Pediatric patients may be at increased risk for device related injury because of their small stature. PRECAUTIONS The implantation of pedicle screw spinal systems should be performed only by experienced spinal surgeons with specific training in the use of this pedicle screw spinal system because this is a technically demanding procedure presenting a risk of serious injury to the patient. Based on the fatigue testing results, the physician/surgeon should consider the levels of implantation, patient weight, patient activity level, other patient conditions, etc. which may impact on the performance of the system. While the final decision on implant removal is up to the surgeon and the patient, in most patients removal is indicated because the implants are not intended to transfer or support forces developed during normal activities. If the device is not removed following completion of its intended use, one or more of the following complications may occur: (1) corrosion, with localized tissue reaction or pain; (2) migration of implant position possibly resulting in injury; (3) risk of additional injury from postoperative trauma; (4) bending, loosening, and breaking which could make removal impractical or difficult; (5) pain, discomfort, or abnormal sensations due to the presence of the device, (6) possible increased risk of infection; (7) bone loss due to stress shielding; and (8) potential unknown or unexpected long term effects such as carcinogenesis. ADDITIONAL PRECAUTIONS FOR PEDIATRIC PATIENTS The implantation of pedicle screw spinal systems in pediatric patients should be performed only by experienced spinal surgeons with specific training in the use of this pedicle screw spinal system in pediatric patients because this is a technically demanding procedure presenting a risk of serious injury to the patient. Preoperative and operating procedures, including knowledge of surgical techniques, good reduction, and proper selection and placement of the implants are important considerations in the successful utilization of the system in pediatric patients. The selection of the proper size, shape, and design of the implant for each patient is crucial to the safe use of this device in pediatric patients. 31
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
Y o u r Id e a s En g i n e e r e d t o Li f e
 ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
QIN4432TRICREV01. IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage. Version 1 1 STERILE PRODUCT
 IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
STRYKER SPINE Spinal Fixation Systems XIA 3
 STRYKER SPINE Spinal Fixation Systems XIA 3 NON STERILE PRODUCT The STRYKER Spine Spinal Fixation Systems are made of devices for fixation of the non-cervical spine. They include smooth rods, screws, hooks,
STRYKER SPINE Spinal Fixation Systems XIA 3 NON STERILE PRODUCT The STRYKER Spine Spinal Fixation Systems are made of devices for fixation of the non-cervical spine. They include smooth rods, screws, hooks,
VTI INTERLINK PEDICLE SCREW SYSTEM
 VTI INTERLINK PEDICLE SCREW SYSTEM SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. DEVICE DESCRIPTION The VTI InterLink Pedicle Screw System is comprised of polyaxial pedicle screws in various diameters
VTI INTERLINK PEDICLE SCREW SYSTEM SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. DEVICE DESCRIPTION The VTI InterLink Pedicle Screw System is comprised of polyaxial pedicle screws in various diameters
SerratoTM. Surgical technique
 TM Surgical technique Table of contents Key design features.... 3 System overview.... 4 Surgical technique Patient positioning... 7 Preparing the pedicle.... 8 Screw insertion... 11 Rod contouring... 12
TM Surgical technique Table of contents Key design features.... 3 System overview.... 4 Surgical technique Patient positioning... 7 Preparing the pedicle.... 8 Screw insertion... 11 Rod contouring... 12
ISSYS LP Spinal Fixation Systems Package Insert
 ISSYS LP Spinal Fixation Systems Package Insert SPINAL ELEMENTS ISSYS LP Spinal Fixation System ISSYS LP Polyaxial Pedicle Screw System - ISSYS LP Monoaxial Screw System CAUTION: Federal law (U.S.A.) restricts
ISSYS LP Spinal Fixation Systems Package Insert SPINAL ELEMENTS ISSYS LP Spinal Fixation System ISSYS LP Polyaxial Pedicle Screw System - ISSYS LP Monoaxial Screw System CAUTION: Federal law (U.S.A.) restricts
operative technique Universal Application
 operative technique Universal Application Introduction Introduction Building upon the design rationale of the Xia Spinal System, the new Xia Spinal System represents the latest advancement in spinal implant
operative technique Universal Application Introduction Introduction Building upon the design rationale of the Xia Spinal System, the new Xia Spinal System represents the latest advancement in spinal implant
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
SECURIS SPINAL Fixation System Package Insert
 SECURIS SPINAL Fixation System Package Insert AMENDIA SECURIS SPINAL Fixation System CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE
SECURIS SPINAL Fixation System Package Insert AMENDIA SECURIS SPINAL Fixation System CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE
 Visit our website on www.biotech-medical.com The DLP - Dorso-Lumbar Polyaxial Screw System has been designed to address the pathologies of the thoracolumbar spine. The DLP System contains a wide range
Visit our website on www.biotech-medical.com The DLP - Dorso-Lumbar Polyaxial Screw System has been designed to address the pathologies of the thoracolumbar spine. The DLP System contains a wide range
Features & Benefits. 1) Wide Bone Graft Space. Anterior Margin 3mm Posterior Margin 1.5mm. Marker Margin
 ALIF Cage System LnK ALIF Cage System The LnK ALIF Cage can make solid inter-body fusion with optimized anatomical position. Broad bone graft area also makes perfect fusion. It can be used with additional
ALIF Cage System LnK ALIF Cage System The LnK ALIF Cage can make solid inter-body fusion with optimized anatomical position. Broad bone graft area also makes perfect fusion. It can be used with additional
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
TiLock 2 Spinal System. Surgical Technique
 TiLock 2 Spinal System Surgical Technique Table of Contents Page Preoperative Planning 4 Pedicle Preparation 5 Probe 5 Tap Pedicle 6 Screw Options 7 Screw Insertion 8 Aligning the Windows 9 Rod Insertion
TiLock 2 Spinal System Surgical Technique Table of Contents Page Preoperative Planning 4 Pedicle Preparation 5 Probe 5 Tap Pedicle 6 Screw Options 7 Screw Insertion 8 Aligning the Windows 9 Rod Insertion
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
Dymaxeon Spine System. Simple, Streamlined, Smart. Surgical Procedure
 Simple, Streamlined, Smart Surgical Procedure Introduction The Dymaxeon pedicle screw system offers the spinal surgeon an outstanding system for stabilization of spinal deformity, reduction of spondylolisthesis,
Simple, Streamlined, Smart Surgical Procedure Introduction The Dymaxeon pedicle screw system offers the spinal surgeon an outstanding system for stabilization of spinal deformity, reduction of spondylolisthesis,
HydraLok. Operative Technique. Polyaxial Pedicle Screw System
 HydraLok Operative Technique Polyaxial Pedicle Screw System Table of Contents Introduction...1 OPERATIVE TECHNIQUE OVERVIEW...2 DETAILED OPERATIVE TECHNIQUE...4 LOCATE AND PREPARE THE PEDICLE...4 PROBE
HydraLok Operative Technique Polyaxial Pedicle Screw System Table of Contents Introduction...1 OPERATIVE TECHNIQUE OVERVIEW...2 DETAILED OPERATIVE TECHNIQUE...4 LOCATE AND PREPARE THE PEDICLE...4 PROBE
ACP. Anterior Cervical Plate System SURGICAL TECHNIQUE
 ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
Cervical Screw System
 Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
PLIF/TPLIF Cage System
 PLIF/TPLIF Cage System LnK Lumbar Interbody Fusion Cage System LnK PLIF / T-PLIF Cage The LnK Interbody Fixation System implants are interbody fusion devices intended for use as an aid in spinal fixation.
PLIF/TPLIF Cage System LnK Lumbar Interbody Fusion Cage System LnK PLIF / T-PLIF Cage The LnK Interbody Fixation System implants are interbody fusion devices intended for use as an aid in spinal fixation.
EXCELLA ll. Spinal System
 EXCELLA ll Spinal System Excella II Spinal System INDICATIONS FOR USE The Innovasis Excella II Spinal System is intended for use in the non-cervical area of the spine. WARNING: The safety and effectiveness
EXCELLA ll Spinal System Excella II Spinal System INDICATIONS FOR USE The Innovasis Excella II Spinal System is intended for use in the non-cervical area of the spine. WARNING: The safety and effectiveness
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
Zimmer Facet Screw System Surgical Technique
 Zimmer Facet Screw System Surgical Technique 2 Zimmer Facet Screw System Surgical Technique Zimmer Facet Screw System Surgical Technique Description, Indications & Contraindications...3 Surgical Technique...4
Zimmer Facet Screw System Surgical Technique 2 Zimmer Facet Screw System Surgical Technique Zimmer Facet Screw System Surgical Technique Description, Indications & Contraindications...3 Surgical Technique...4
TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System
 TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System consists of rods (straight and curved), lock screws, and
TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System consists of rods (straight and curved), lock screws, and
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
SURGICAL TECHNIQUE GUIDE TRESTLE. Anterior Cervical Plating System
 SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
Alamo T Transforaminal Lumbar Interbody System Surgical Technique
 Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Alamo C. Cervical Interbody System Surgical Technique. An Alliance Partners Company
 Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
EXPEDIUM 5.5 Spine System Family Product Catalogue
 EXPEDIUM 5.5 Spine System Family Product Catalogue Including: EXPEDIUM EXPEDIUM Vertebral Body Derotation Universal Connector Set Cobalt Chromium Rods INTRODUCTION Contents EXPEDIUM Spine System is a comprehensive
EXPEDIUM 5.5 Spine System Family Product Catalogue Including: EXPEDIUM EXPEDIUM Vertebral Body Derotation Universal Connector Set Cobalt Chromium Rods INTRODUCTION Contents EXPEDIUM Spine System is a comprehensive
I. II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE
 I. GS1 II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE Introduction The Gold Standard Orthopaedics, LLC GS1 Spinal System designed in conjunction with Richard Holt, M.D. incorporates both strength and function
I. GS1 II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE Introduction The Gold Standard Orthopaedics, LLC GS1 Spinal System designed in conjunction with Richard Holt, M.D. incorporates both strength and function
Royal Oak Cervical Plate System
 Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
USS Variable Axis Screw (VAS) System. For posterior fixation of the lumbar spine.
 USS Variable Axis Screw (VAS) System. For posterior fixation of the lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction USS Variable Axis
USS Variable Axis Screw (VAS) System. For posterior fixation of the lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction USS Variable Axis
TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System
 Minimally Invasive Surgery (MIS) Pedicle Screw System Surgical Technique Guide 2 Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine Minimally Invasive Surgery (MIS) Pedicle Screw System
Minimally Invasive Surgery (MIS) Pedicle Screw System Surgical Technique Guide 2 Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine Minimally Invasive Surgery (MIS) Pedicle Screw System
X-spine Surgical Technique
 X-spine Surgical Technique The X90 Pedicle Screw System Revolutionary Design and Function This document is intended exclusively for experts in the field, particularly physicians, and is not intended for
X-spine Surgical Technique The X90 Pedicle Screw System Revolutionary Design and Function This document is intended exclusively for experts in the field, particularly physicians, and is not intended for
SURGICAL TECHNIQUE. SECURIS Pedicle Screw System for Minimally Invasive Surgery. 2 I SECURIS Pedicle Screw System
 Surgical Technique e Guide SECURIS Pedicle Screw System for Minimally Invasive Surgery Securis Pedicle Screw System has been engineered to provide temporary posterior stabilization of the thoracolumbar
Surgical Technique e Guide SECURIS Pedicle Screw System for Minimally Invasive Surgery Securis Pedicle Screw System has been engineered to provide temporary posterior stabilization of the thoracolumbar
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
A Fusion of Versatility, Performance and Ease.
 MATRIX Spine System. Degenerative and Deformity. System Guide A Fusion of Versatility, Performance and Ease. Instruments and implants approved by the AO Foundation The MATRIX Spine System is a universal
MATRIX Spine System. Degenerative and Deformity. System Guide A Fusion of Versatility, Performance and Ease. Instruments and implants approved by the AO Foundation The MATRIX Spine System is a universal
L8 Spine System SURGICAL TECHNIQUE. Add: No.1-8, Tianshan Road, Xinbei District, Changzhou, Jiangsu, China
 Add: No.-8, Tianshan Road, Xinbei District, Changzhou, Jiangsu, China 23022 Tel: 0086 59 8595556 Fax: 0086 59 859555 Http://www.kanghui.com Add: F25, Shanghai International Pharmaceutical Trad & Exhibition
Add: No.-8, Tianshan Road, Xinbei District, Changzhou, Jiangsu, China 23022 Tel: 0086 59 8595556 Fax: 0086 59 859555 Http://www.kanghui.com Add: F25, Shanghai International Pharmaceutical Trad & Exhibition
Cervical Spacer System surgical technique
 Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
SURGICAL TECHNIQUE GUIDE SOLANAS. Posterior Cervico-Thoracic Fixation System Adjustable Bridge System
 SURGICAL TECHNIQUE GUIDE SOLANAS Posterior Cervico-Thoracic Fixation System Adjustable Bridge System 2 SURGICAL TECHNIQUE GUIDE Preface SOLANAS Posterior Cervico-Thoracic Fixation System is designed to
SURGICAL TECHNIQUE GUIDE SOLANAS Posterior Cervico-Thoracic Fixation System Adjustable Bridge System 2 SURGICAL TECHNIQUE GUIDE Preface SOLANAS Posterior Cervico-Thoracic Fixation System is designed to
PATHWAY PLIF and PATHWAY TLIF. SPINAL ELEMENTS PATHWAY PLIF And PATHWAY TLIF PATHWAY
 PATHWAY PLIF and PATHWAY TLIF SPINAL ELEMENTS PATHWAY PLIF And PATHWAY TLIF PATHWAY CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE PRODUCT
PATHWAY PLIF and PATHWAY TLIF SPINAL ELEMENTS PATHWAY PLIF And PATHWAY TLIF PATHWAY CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE PRODUCT
Xia 4.5 Cortical Trajectory LITe. LIF Procedure. Surgical technique
 Xia 4.5 Cortical Trajectory LITe LIF Procedure Table of contents Case preparation... 3 System overview.... 5 Step 1: Exposure.... 6 Step 2: LITe midline retractor placement.... 6 Step 3: Starting point....
Xia 4.5 Cortical Trajectory LITe LIF Procedure Table of contents Case preparation... 3 System overview.... 5 Step 1: Exposure.... 6 Step 2: LITe midline retractor placement.... 6 Step 3: Starting point....
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
EXACTECH SPINE. Operative Technique. Cervical Spacer System. Surgeon focused. Patient driven. TM
 EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine.
 Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Thunderbolt. surgical technique. MIS Pedicle Screw System. Where Nimble and Secure Intersect
 Thunderbolt TM MIS Pedicle Screw System Where Nimble and Secure Intersect surgical technique i www.choicespine.com System Features Dovetail set screw: Minimizes head splay and cross-threading Secure connection
Thunderbolt TM MIS Pedicle Screw System Where Nimble and Secure Intersect surgical technique i www.choicespine.com System Features Dovetail set screw: Minimizes head splay and cross-threading Secure connection
Ref: Q400-09T1 EBI Spine. September 05/VS02. c/o BIOMET Spain Orthopaedics, S.L.
 Ref: Q400-09T1 EBI Spine. September 05/VS02 c/o BIOMET Spain Orthopaedics, S.L. www.ebimedical.com EBI Omega 21 TM LP Since its introduction in 1996, and with thousands of patients treated so far, the
Ref: Q400-09T1 EBI Spine. September 05/VS02 c/o BIOMET Spain Orthopaedics, S.L. www.ebimedical.com EBI Omega 21 TM LP Since its introduction in 1996, and with thousands of patients treated so far, the
Apache Cervical Interbody Fusion Device. Surgical Technique. Page of 13. LC-005 Rev F
 LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
Xia 3 Spinal System. AIS surgical technique
 Xia 3 Spinal System Table of contents Surgical technique Key design features.... 4 Patient positioning.... 8 Hook selection... 9 Hook insertion... 10 Preparing the pedicle.... 14 Screw insertion... 19
Xia 3 Spinal System Table of contents Surgical technique Key design features.... 4 Patient positioning.... 8 Hook selection... 9 Hook insertion... 10 Preparing the pedicle.... 14 Screw insertion... 19
Surgical Technique. Apache Anterior Lumbar Interbody Fusion
 Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
3 Operative Technique U.S. EDITION
 3 A N T E R I O R C E R V I C A L P L AT E S Y S T E M 3 Operative Technique U.S. EDITION Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE 3 OPERATIVE 12 INSTRUCTIONS FOR USE 16 PART NUMBERS Orthofix Spinal
3 A N T E R I O R C E R V I C A L P L AT E S Y S T E M 3 Operative Technique U.S. EDITION Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE 3 OPERATIVE 12 INSTRUCTIONS FOR USE 16 PART NUMBERS Orthofix Spinal
LITe. Plate System Anterior Surgical Technique
 LITe Plate System Table of Contents System Overview.... 3 Implant Overview.... 5 Step 1. Patient Positioning and Exposure... 7 Step 2. Endplate Preparation and Interbody Placement.... 8 Step 3. Implant
LITe Plate System Table of Contents System Overview.... 3 Implant Overview.... 5 Step 1. Patient Positioning and Exposure... 7 Step 2. Endplate Preparation and Interbody Placement.... 8 Step 3. Implant
Threshold Pedicular Fixation System Surgical Technique
 Threshold Pedicular Fixation System Surgical Technique Table of Contents Patient Preparation and Positioning... 2 Determining Incision Location... 3 Assembling the Cannulated Awl... 4 Guide Wire Placement...
Threshold Pedicular Fixation System Surgical Technique Table of Contents Patient Preparation and Positioning... 2 Determining Incision Location... 3 Assembling the Cannulated Awl... 4 Guide Wire Placement...
CSS Combined Spine System
 CSS Combined Spine System H- CSS Combined Spine System H- CSS Combined Spinal System : 800000 H-3 CSS Combined Spinal System : 800000 Ref.Number Description Pieces Ref.Number Description Pieces 00000 Open
CSS Combined Spine System H- CSS Combined Spine System H- CSS Combined Spinal System : 800000 H-3 CSS Combined Spinal System : 800000 Ref.Number Description Pieces Ref.Number Description Pieces 00000 Open
PATHWAY AVID SPINAL ELEMENTS PATHWAY AVID. CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician.
 PATHWAY AVID SPINAL ELEMENTS PATHWAY AVID CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE PRODUCT MUST BE STERILIZED PRIOR TO USE SINGLE
PATHWAY AVID SPINAL ELEMENTS PATHWAY AVID CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE PRODUCT MUST BE STERILIZED PRIOR TO USE SINGLE
IBD PEEK C. Anterior Cervical Spacer System. Surgical Technique
 IBD PEEK C Anterior Cervical Spacer System Surgical Technique Table of Contents Introduction.... 3 Step 1: Exposure... 3 Step 2: Discectomy... 4 Step 3: Endplate Preparation... 5 Step 4: Trialing... 6
IBD PEEK C Anterior Cervical Spacer System Surgical Technique Table of Contents Introduction.... 3 Step 1: Exposure... 3 Step 2: Discectomy... 4 Step 3: Endplate Preparation... 5 Step 4: Trialing... 6
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
TABLE OF CONTENTS. Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview. Implants 3. Instruments 5. Surgical Technique 10
 Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
ONE SYSTEM, MULTIPLE OPTIONS. Surgical Technique. Hip Knee Spine Navigation
 ONE SYSTEM, MULTIPLE OPTIONS Surgical Technique Hip Knee Spine Navigation MUST MINI Surgical Technique Hip Knee Spine Navigation INTRODUCTION The M.U.S.T. Mini posterior cervical screw system is a modular
ONE SYSTEM, MULTIPLE OPTIONS Surgical Technique Hip Knee Spine Navigation MUST MINI Surgical Technique Hip Knee Spine Navigation INTRODUCTION The M.U.S.T. Mini posterior cervical screw system is a modular
MANTIS Redux. Surgical Technique. LITe. True percutaneous screw and rod system Direct visualization Precise rod contouring allows multiple levels
 MANTIS Redux Surgical Technique True percutaneous screw and rod system Direct visualization Precise rod contouring allows multiple levels LITe Less Invasive Technologies MANTIS Redux Surgical Technique
MANTIS Redux Surgical Technique True percutaneous screw and rod system Direct visualization Precise rod contouring allows multiple levels LITe Less Invasive Technologies MANTIS Redux Surgical Technique
Surgical Technique. Guide and Ordering Information. Favored Angle Screw
 Surgical Technique Guide and Ordering Information Favored Angle Screw C O N T E N T S SURGICAL TECHNIQUE Introduction 1 Screwdriver Application and Screw Placement 2 Segmental Translation 3-7 Segmental
Surgical Technique Guide and Ordering Information Favored Angle Screw C O N T E N T S SURGICAL TECHNIQUE Introduction 1 Screwdriver Application and Screw Placement 2 Segmental Translation 3-7 Segmental
Surgical Technique FOR U.S. DOMESTIC USE ONLY
 Surgical Technique FOR U.S. DOMESTIC USE ONLY Polyaxial Screws HA Coated Polyaxial Screws Uniplanar Screws Reduction Screws Reduction Uniplanar Screws Modular Screws TABLE OF CONTENTS Reform Pedicle Screw
Surgical Technique FOR U.S. DOMESTIC USE ONLY Polyaxial Screws HA Coated Polyaxial Screws Uniplanar Screws Reduction Screws Reduction Uniplanar Screws Modular Screws TABLE OF CONTENTS Reform Pedicle Screw
PILLAR AL. Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) PEEK Spacer System OPERATIVE TECHNIQUE
 PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
Veyron -C Anterior Cervical System Surgical Technique
 Veyron -C Anterior Cervical System Surgical Technique 2 Veyron-C Anterior Cervical System Surgical Technique Veyron-C Anterior Cervical System Surgical Technique Description, Indications & Contraindications...3
Veyron -C Anterior Cervical System Surgical Technique 2 Veyron-C Anterior Cervical System Surgical Technique Veyron-C Anterior Cervical System Surgical Technique Description, Indications & Contraindications...3
Trio + Surgical Technique
 Trio + Surgical Technique Introduction Trio+ Surgical Technique The Trio+ Spinal System is a comprehensive system of implants and instruments used for the stabilization of the spine in the thoracic, lumbar,
Trio + Surgical Technique Introduction Trio+ Surgical Technique The Trio+ Spinal System is a comprehensive system of implants and instruments used for the stabilization of the spine in the thoracic, lumbar,
Spine. THOR Anterior Lumbar Plate Operative Technique
 Spine THOR Anterior Lumbar Plate Operative Technique Table of Contents THOR Anterior Lumbar Plate Surgical Technique Introduction 3 Implants 4 Instruments 5-6 Procedure 7-12 Removal of Implants 13 Indications
Spine THOR Anterior Lumbar Plate Operative Technique Table of Contents THOR Anterior Lumbar Plate Surgical Technique Introduction 3 Implants 4 Instruments 5-6 Procedure 7-12 Removal of Implants 13 Indications
Surgical Technique. Guide
 Surgical Technique Guide DESIGNING SURGEONS Darrel Brodke, M.D. University of Utah Medical Center Dept. of Orthopedic Surgery Salt Lake City, Utah Iain Kalfas, M.D., F.A.C.S The Cleveland Clinic Foundation
Surgical Technique Guide DESIGNING SURGEONS Darrel Brodke, M.D. University of Utah Medical Center Dept. of Orthopedic Surgery Salt Lake City, Utah Iain Kalfas, M.D., F.A.C.S The Cleveland Clinic Foundation
UniVise Spinous Process Fixation Plate System. Surgical Technique. Simple, One-Piece Design Anatomically Shaped Plates Optimized Radial Spike Pattern
 UniVise Spinous Process Fixation Plate System Simple, One-Piece Design Anatomically Shaped Plates Optimized Radial Spike Pattern Table of Contents Device Description... 3 UniVise Key Design Features...
UniVise Spinous Process Fixation Plate System Simple, One-Piece Design Anatomically Shaped Plates Optimized Radial Spike Pattern Table of Contents Device Description... 3 UniVise Key Design Features...
Technique Guide. Universal Spinal System (USS). Designed to achieve the goals of scoliosis surgery.
 Technique Guide Universal Spinal System (USS). Designed to achieve the goals of scoliosis surgery. Table of Contents Introduction Universal Spinal System 2 AO ASIF Principles of Internal Fixation 3 Indications
Technique Guide Universal Spinal System (USS). Designed to achieve the goals of scoliosis surgery. Table of Contents Introduction Universal Spinal System 2 AO ASIF Principles of Internal Fixation 3 Indications
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
MEDACTA UNCONSTRAINED SCREW TECHNOLOGY - REDUCTION SCREWS. Surgical Technique. Hip Knee Spine Navigation
 .U.S.T. MEDACTA UNCONSTRAINED SCREW TECHNOLOGY - REDUCTION SCREWS Hip Knee Spine Navigation M.U.S.T. Hip Knee Spine Navigation INTRODUCTION The Medacta Unconstrained Screw Technology [M.U.S.T.] Pedicle
.U.S.T. MEDACTA UNCONSTRAINED SCREW TECHNOLOGY - REDUCTION SCREWS Hip Knee Spine Navigation M.U.S.T. Hip Knee Spine Navigation INTRODUCTION The Medacta Unconstrained Screw Technology [M.U.S.T.] Pedicle
Surgical Technique. Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion
 Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
product overview Implant heights range from 8mm-20mm in 2mm increments, with two lordocic angle options of 6 and 12.
 ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Hinged Laminoplasty System surgical technique
 BlackbirdTM Hls Hinged Laminoplasty System surgical technique Blackbird TM Hls The ChoiceSpine Blackbird Hinged Laminoplasty System (HLS) design eliminates fitting plates through trial and error bending.
BlackbirdTM Hls Hinged Laminoplasty System surgical technique Blackbird TM Hls The ChoiceSpine Blackbird Hinged Laminoplasty System (HLS) design eliminates fitting plates through trial and error bending.
BONE GRAFT WASHER _Rev A IMPORTANT INFORMATION ON THE BONE GRAFT WASHER
 0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
Ascent. Surgical Technique. Breakthrough Thinking. Posterior Occipital Cervico-Thoracic (POCT) System. Germany U.S.A.
 Fusion Motion Preservation Biologics Surgical Technique Breakthrough Thinking Fusion I Motion Preservation I Biologics Posterior Occipital Cervico-Thoracic (POCT) System U.S.A. Corporate Headquarters Blackstone
Fusion Motion Preservation Biologics Surgical Technique Breakthrough Thinking Fusion I Motion Preservation I Biologics Posterior Occipital Cervico-Thoracic (POCT) System U.S.A. Corporate Headquarters Blackstone
ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL
 ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL Contents: Introduction Indications Contraindications Warnings Precautions Implant Overview Instruments Overview Surgical Technique
ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL Contents: Introduction Indications Contraindications Warnings Precautions Implant Overview Instruments Overview Surgical Technique
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Dual-Opening USS. For deformity correction.
 Dual-Opening USS. For deformity correction. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Dual-Opening USS 2 AO Principles 4 Indications 5 Preoperative
Dual-Opening USS. For deformity correction. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Dual-Opening USS 2 AO Principles 4 Indications 5 Preoperative
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
4.5 System. Surgical Technique. This publication is not intended for distribution in the USA.
 4.5 System Surgical Technique This publication is not intended for distribution in the USA. Contents EXPEDIUM 4.5 Spine System 2 Features and Benefits 3 Surgical Technique Extended Tandem Connector 4 Placement
4.5 System Surgical Technique This publication is not intended for distribution in the USA. Contents EXPEDIUM 4.5 Spine System 2 Features and Benefits 3 Surgical Technique Extended Tandem Connector 4 Placement
Asnis. Micro Cannulated screw system. Xpress operative technique
 Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
MATRIX Spine System Deformity. A posterior pedicle screw, hook, and rod fixation system.
 MATRIX Spine System Deformity. A posterior pedicle screw, hook, and rod fixation system. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction MATRIX Spine
MATRIX Spine System Deformity. A posterior pedicle screw, hook, and rod fixation system. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction MATRIX Spine
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
The Most Reliable Spinal Solution
 MFG : 09.02.20 REV : 1 4CIS VANE introduces you the reliable pedicle screw The Most Reliable Spinal Solution Non-slip threaded joint Ergonomic design Wedged trapezoid thread Superior locking mechanism
MFG : 09.02.20 REV : 1 4CIS VANE introduces you the reliable pedicle screw The Most Reliable Spinal Solution Non-slip threaded joint Ergonomic design Wedged trapezoid thread Superior locking mechanism
L-VARLOCK. Posterior Lumbar Cage with adjustable lordosis. S urgical T echnique
 L-VARLOCK Posterior Lumbar Cage with adjustable lordosis S urgical T echnique Introduction Designed and manufactured by KISCO International, L-VARLOCK cages are made of titanium alloy Ti 6AI 4V (standards
L-VARLOCK Posterior Lumbar Cage with adjustable lordosis S urgical T echnique Introduction Designed and manufactured by KISCO International, L-VARLOCK cages are made of titanium alloy Ti 6AI 4V (standards
USS II ILIO-SACRAL Modular System for Stable Fixation in the Sacrum and Illium
 USS II ILIO-SACRAL Modular System for Stable Fixation in the Sacrum and Illium Instruments and implants approved by the AO Foundation. This publication is not intended for distribution in the USA. TECHNIQUE
USS II ILIO-SACRAL Modular System for Stable Fixation in the Sacrum and Illium Instruments and implants approved by the AO Foundation. This publication is not intended for distribution in the USA. TECHNIQUE
USS Variable Axis Screw
 USS Variable Axis Screw Polyaxial side-opening pedicle screw Surgical technique Original Instruments and Implants of the Association for the Study of Internal Fixation AO/ASIF USS Variable Axis Screw
USS Variable Axis Screw Polyaxial side-opening pedicle screw Surgical technique Original Instruments and Implants of the Association for the Study of Internal Fixation AO/ASIF USS Variable Axis Screw
Sacropelvic Fixation. Ahmet Alanay M.D. Professor. Acıbadem Maslak Hospital Comprehensive Spine Center Istanbul TURKEY
 Sacropelvic Fixation Ahmet Alanay M.D. Professor Acıbadem Maslak Hospital Comprehensive Spine Center Istanbul TURKEY Conflict of Interest Grant Depuy & Synthes Definition Sacropelvic fixation Long spinal
Sacropelvic Fixation Ahmet Alanay M.D. Professor Acıbadem Maslak Hospital Comprehensive Spine Center Istanbul TURKEY Conflict of Interest Grant Depuy & Synthes Definition Sacropelvic fixation Long spinal
