Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry: Experience of the Pilot Study in Andhra Pradesh, India
|
|
|
- Job Hunter
- 5 years ago
- Views:
Transcription
1 Indian J Pediatr (August 2011) 78(8): DOI /s ORIGINAL ARTICLE Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry: Experience of the Pilot Study in Andhra Pradesh, India Inderneel Sahai & Thomas Zytkowicz & Srimannarayna Rao Kotthuri & Anantha Lakshmi Kotthuri & Roger B. Eaton & Radha Rama Devi Akella Received: 24 September 2010 / Accepted: 18 February 2011 / Published online: 17 March 2011 # Dr. K C Chaudhuri Foundation 2011 Abstract Objective To estimate the prevalence of the Inborn Errors of Metabolism (IEM), evaluate biomarker distributions and determine benefits of screening for the inborn errors of metabolism in Andhra Pradesh, India, using Tandem Mass Spectrometry (MS/MS). Methods The 4,946 newborns born during the period in four major Government Maternity Hospitals in a rural district in Andhra Pradesh, India, were screened at an established newborn screening laboratory in the US using their previously established norms. Results Forty-seven neonates had out-of-range results (5 high probability; 28 low probability; 14 indeterminate). Two infants with disorders (carnitine uptake disorder and isovaleric aciduria) identified by screening are currently doing well. One infant with presumed glutaric aciduria type II, was deceased at the time of reporting. Another infant, with glutaric aciduria type I, became symptomatic and died at the age of 1 year despite early detection and treatment. A comparison of the concentrations of biomarkers among babies born in India and those born in Massachusetts, US, was also undertaken and significant differences were noted. I. Sahai (*) : T. Zytkowicz : R. B. Eaton New England Newborn Screening Program, University of Massachusetts Medical School, 305 South Street, Jamaica Plain, Boston, MA 02130, USA Inderneel.sahai@umassmed.edu S. Rao Kotthuri : A. Lakshmi Kotthuri Konaseema Specialities, Amalapuram, E.G District, Andhra Pradesh, India R. R. D. Akella Centre for DNA Finger Printing and Diagnostics, Hyderabad, India Conclusions A high prevalence of disorders was observed, but to estimate the true extent of the IEM in India larger studies are required. This study also illustrates challenges encountered in disease management highlighting the importance of considering the access to confirmatory testing and continuing clinical care before implementing any largescale NBS for conditions with resource-intensive health needs such as the IEM detected by MS/MS. Keywords Newborn screening. Tandem mass spectrometry. Inborn errors of metabolism. Population based study Introduction Newborn screening provides an opportunity for identification of, and consequently early intervention in, presymptomatic infants with serious inborn disorders to prevent or mitigate adverse outcomes associated with these conditions. Screening is accomplished, primarily, by analysis of biomarkers in a micro-sample of blood collected from newborns using a spectrum of analytic methodologies. Currently, it is feasible to screen for more than sixty conditions in various categories (Endocrine, Inborn Errors of Metabolism, Hemoglobinopathies, Infectious and Immunological) and this number is only expected to increase over time with advancements in technology [1, 2]. The panel of disorders screened for, and the cohort of newborns screened, varies substantially inthedifferentpartsofthe world [3]. In many nations newborn screening is performed as standard practice on all babies born, while in others newborn screening is not feasible, or is available to only a subset of neonates.
2 954 Indian J Pediatr (August 2011) 78(8): Although efforts are underway to implement universal newborn screening, for at least a few common disorders, at present newborn screening is not performed on all newborns in India [4, 5]. Previous pilot studies conducted in India have shown a high prevalence of treatable disorders [6]. Chromatographic (TLC and HPLC), electrophoretic (cellulose acetate and agarose) and ELISA based assays were employed in the screening process and thus the metabolic disorders detected were limited. One of the more recent technologies introduced into newborn screening is tandem mass spectrometry (MS/MS). MS/MS allows simultaneous, rapid and accurate detection of multiple amino acids and acylcarnitines, and hence has expanded the spectrum of metabolic disorders identifiable by screening [7 10]. The authors report their experience of the first pilot study conducted in India to screen for inborn errors of metabolism using this methodology. Material and Methods Cohort The 4,946 newborns born during the period in four major Government Maternity Hospitals in a rural district in Andhra Pradesh, India, were screened. Heel prick capillary blood collected on S&S 903 filter paper was transported to the New England Newborn Screening Program (NENSP) via courier. The screening laboratory checked the appropriateness of samples for analysis. Unsatisfactory specimens were rejected and re-sampling was done for those rejected. The majority of technically satisfactory samples (60%) were obtained between day of life (DOL) 1 3 and while the newborns were still in the hospital. The remaining specimens (40%) were collected when the neonates presented to the hospital clinic for a routine check. Consent for inclusion in the study was obtained in accordance with the guidelines provided by the Indian council of Medical Research. Analysis The laboratory procedures, as previously reported [11], involved extraction of biomarkers from the dried blood spots into a methanol solution with stable isotope-labeled internal standards, and derivatization for analysis by MS/MS. Table 1 shows the disorders screened for and the primary markers measured for screening. The laboratory s previously established cutoffs, based on expected ranges in babies born in New England, were utilized and no separate cut-offs were established for specimens from babies in India. Specimens with biomarker values that were above New England Newborn Screening Program (NENSP) cut-offs were retested. Reporting and Follow-Up of Out-of-range Results All out-of-range results were sorted into one of the three distinct categories: Indeterminate, Low Probability and High Probability as per protocols in use by NENSP [Sahai I, Eaton RB. Customized FACT SHEETS : Category-Based Information Provided When Reporting Positive Results. Poster Newborn Screening and Genetic Testing Symposium, San Antonio, TX. 3 6 November2008]. The criteria for assigning positive results into one of these categories were developed after a retrospective analysis of archived results from babies screened by NENSP. The biochemical parameters used in defining the criteria include both magnitude of the deviation of the primary marker from the population mean and the biochemical profiles. To quantify biochemical profiles, NENSP uses Discriminator Indices that essentially are mathematical combinations of several characteristic analytes [Sahai I, Zytkovicz T, Grady T, et al. Improving Interpretation of Positive Newborn Screening Results: A metabolomic approach for analysis of MS/MS data in developing Predictive Indices. Poster 2007 Newborn Screening and Genetic Testing Symposium, Minneapolis, MN May 2007]. Results were communicated to the Principal Investigator (PI) in India along with the subcategory-based recommendations. For High Probability results, an expedient evaluation and confirmatory work-up was recommended. For the Low Probability results, additional intervention was suggested, but it varied based on the analyte involved. For markers whose deviations were likely to increase in affected neonates, but are associated with disorders that could present critically within days of birth (e.g. Cit & ASA), an immediate clinical evaluation and some testing (e.g. ammonia) was recommended by the PI. However confirmatory testing was recommended only if a repeat specimen also tested abnormal. For markers that may decrease over time even among affected neonates (e.g. C8, C5-DC & C14: 1), confirmatory testing was recommended in addition to a repeat screen. For Indeterminate results, a repeat filter paper screen was requested and confirmatory tests suggested only if the repeat specimen also tested abnormal. Evaluation and confirmatory testing was performed at the metabolic clinic in the DNA Diagnostic and Fingerprinting Laboratory in Hyderabad. Results Demographic Data Of the 4,946 initial specimens received, 4,870 specimens (Males 2,620; Females 2,238; No gender data on 12) were
3 Indian J Pediatr (August 2011) 78(8): Table 1 Primary markers analysed using tandem mass spectrometry during the study period, and the disorders screened for by these markers Primary marker/s a Disorder (Acronyn; OMIM catalogue number) Acylcarnitines Free Carnitine (C0) Carnitine palmitoyltransferase deficiency, type-ia (CPT-I A; ) Free Carnitine (C0)- Low Carnitine uptake disorder (CUD; ) Propionylcarnitine (C3) Propionic acidemia (PA; ) Methylmalonic aciduria, MUT type (MMA-MUT; ) Methylmalonic aciduria, cbl A type (Cbl A; ) Methylmalonic aciduria, cbl B type (Cbl B; ) Methylmalonic aciduria and Homocystinuria, cbl C type (Cbl C; ) Methylmalonic aciduria and Homocystinuria, cbl D type (Cbl D; ) Multiple carboxylase deficiency (MCD; ) Malonylcarnitine (C3DC) Malonyl-CoA decarboxylase deficiency (MAL;248360) Butyrylcarnitine (C4) Short-chain acyl-coa dehydrogenase deficiency (SCAD; ) Isobutyryl-CoA dehydrogenase deficiency (IBG; ) Ethylmalonic encephalopathy (EE; ) Hydroxybutyrylcarnitine (C4OH) 3-@Hydroxyacyl-CoA dehydrogenase deficiency (HAD; ) Tiglylcarnitine (C5:1) Beta-ketothiolase deficiency ( BKT; ) Isovalerylcarnitine (C5) Isovaleric acidemia (IVA; ) 2-Methylbutyryl-CoA dehydrogenase deficiency (2MBG; ) Hydroxyvalerylcarnitine (C5OH) 3-Methylcrotonyl-CoA carboxylase deficiency (3MCC; , ) 3-Methylglutaconic aciduria (3MGA; ) 2-Methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency (2M3HBA; ) Glutarylcarnitine (C5-DC) Glutaric aciduria, type I (GA-I; ) 3-Methylglutarylcarnitine (C6-DC) HMG-CoA lyase deficiency (HMG; ) Octanoylcarnitine (C8) Medium-chain acyl-coa dehydrogenase deficiency (MCAD; ) Decadienoylcarnitine (C10:2) 2,4-Dienoyl-CoA reductase deficiency (DE-RED; ) Tetradecadienoylcarnitine (C14:2) &/or Very long-chain acyl-coa dehydrogenase deficiency (VLCAD; ) Tetradeceneoylcarnitine (C14:1) Hydroxypalmitoylcarnitine (C16) &/or Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHAD; ) Hydroxyoleoylcarnitine (C18:1OH) Trifunctional protein deficiency (TFP; ) Palmitoylcarnitine (C16 ) &/or Carnitine palmitoyltransferase deficiency (CPT-II; & ) Oleoylcarnitine (C18:1) Carnitine-acylcarnitine translocase Deficiency (CACT; ) Elevation of Multiple Acylcarnitines (C4; C5-DC; C8) Glutaric Aciduria, type II (GA-II; ) Amino acids Arginine (Arg) Argininemia (ARG; ) Argininosuccinic acid (ASA) Argininosuccinic aciduria ( ASA; ) Citrulline (Cit) Citrullinemia, type I (CTLN-I; ) Citrullinemia, type II (CTLN-II; ) Citrulline (Cit) -Low Ornithine transcarbamylase deficiency (OTC; ) Carbamoylphosphate synthetase deficiency (CPS; ) N-acetylglutamate synthase deficiency (NAGS; ) Leucine (Leu) &/or Maple syrup urine disease ( MSUD; ) Valine (Val) Methionine (Met) Homocystinuria (HCY; ) Hypermethioninemia (MET; ) Ornithine (Orn) Hyperornithinemia Hyperammonemia Homocitrullinuria Syndrome (HHH; ) Phenylalanine (Phe) Phenylketonuria (PKU; ) Benign hyperphenylalaninemia (H-PHE; ) Biopterin Defects (BIOPT-BS; BIOPT REG)
4 956 Indian J Pediatr (August 2011) 78(8): Table 1 (continued) Primary marker/s a Tyrosine (Tyr) Disorder (Acronyn; OMIM catalogue number) Tyrosinemia, type I (276700; TYRI) Tyrosinemia, type II (276600; TYR II) Tyrosinemia, type III (276710; TYR III) a Unless noted otherwise, high primary marker concentrations constitute a positive screen technically satisfactory for testing. The distribution of age when specimens were collected is shown in Fig. 1; approximately 60% of the samples were collected within h of birth. In contrast, of the 134,539 initial technically satisfactory specimens received from babies born in Massachusetts (MA) in the same period, 94% were collected within h of birth. Also notable in this study was that only 2% of specimens from India were from babies weighing less than 1.5 kg or in the NICU. In comparison 7% of babies born in MA weighed less than 1.5 kg or were in the NICU when the initial specimen was collected. Distribution of Markers Table 2 shows the means, median, 1 st and 99 th percentile of the distribution of the primary biomarkers analysed by MS/ MS in neonates born in India. Also shown for comparison are the parameters of the same biomarkers in the babies born in MA. In view of the significant differences in the age at specimen collection and in the number of VLBW/ NICU babies in the two populations, only specimens collected between h from non-nicu babies weighing more than 1.5 kg were included for the comparison. Comparison was also restricted to specimens tested on the same tandem mass spectrometer (n=29,413 from MA and n=2,852 from India). Comparison of the concentrations of biomarkers in the two populations using T-test analysis was performed and p-values are shown in the last column. 70% 60% 50% 40% 30% 20% 10% 0% DOL < 1 DOL 1-3 DOL 3-7 DOL 7-30 DOL > 30 No Data * DOL: Day of life Fig. 1 Age at specimen collection Statistically significant differences were noted in the concentrations of most biomarkers, with the amino acids (except Leucine) and acylcarnitines (except free carnitine, C16 and C18:1) being higher in the babies born in Massachusetts as compared to babies born in India. Out-of range Results Of the 4,896 babies whose screening specimen was collected within 30 days of life, 47 had out-of-range results based on previously established NENSP cutoffs. The marker abnormality, category breakdown and final outcomes are shown in Table 3. Confirmatory Testing Plasma amino acids and urinary organic acid analyses were performed on neonates with out-of-range markers, where required, at the metabolic clinic of the DNA Diagnostic and Fingerprinting Center, Hyderabad. However, in view of the difficulty and expense involved in getting certain confirmatory studies such as plasma acylcarnitine analysis on babies included in the study, these studies were not performed. Instead, the authors relied on acylcarnitines measured on repeat dried blood spots for decision making and monitoring. In most cases molecular analyses were not undertaken. Clinical History and Outcomes of Cases Case I-Carnitine Uptake Deficiency (CUD) This male baby was the first-born child of a consanguineous marriage (parents were first cousins). He was noted to have low free carnitine (C0), and other markers in a profile consistent with CUD on newborn screening. At his initial clinical evaluation on DOL 8, he was clinically asymptomatic and his EKG was normal. Repeat testing revealed persistence of low free carnitine and he was started on oral carnitine 100 mg/kg body weight. At 6-months of age, carnitine supplementation was discontinued, but this resulted in a drop in his free carnitine to significantly low levels (3.6 μm). Carnitine supplementation was restarted, and carnitine level has remained in the normal range since. He is being followed periodically and at his last evaluation the
5 Indian J Pediatr (August 2011) 78(8): Table 2 A comparison of the primary marker parameters in specimens from babies born in India with those born in Massachusetts Analyte Specimens from India (n=2,852) Specimens from Massachusetts (n=29,413) T-test Median Mean 1st %ile 99th %ile Median Mean 1st %ile 99th %ile p C C C3DC C C4OH C5: C C5OH C5DC C6DC C C10: C14: C14: C C18: C16OH C18:OH Arg ASA Cit Leu Val Met Orn Phe Tyr Only specimens collected within h from babies not in the NICU, weighing more than 1,500 g and tested on the same tandem mass spectrometer are included in the analysis Higher median value shown in bold developmental milestones were normal and an EKG was normal. A second child was born to his parents last year, and does not appear to be affected based on a normal newborn screen. Case II- Glutaric Aciduria Type II (GA-II) This female child was born to a consanguineous couple (parents were first cousins). Significant elevations of multiple acylcarnitines (C4, C8, C5DC, C14), in a pattern consistent with GA-II, were noted in the screening specimen collected on DOL 3. Although the family was contacted immediately after the results became available, the baby was already deceased at the time. According to the parents the neonate had died suddenly on day of life 4. No confirmatory testing could be performed, however family was provided genetic counseling based on the presumptive diagnosis of GAII. Case III-Glutaric Aciduria-I (GAI) This male neonate was the first-born child of non-consanguineous parents. He was born at 36-wks gestation with a birth weight of 1.7 kg. His newborn screening revealed an elevation of C5DC in a pattern consistent with GAI. At his initial evaluation he was asymptomatic, active and feeding well. Urinary organic acid analysis revealed elevated glutaric acid and 3- hydroxyglutaric acid, confirming the diagnosis of GAI. In addition to the above metabolities, increased excretion of 2- ketoglutaric acid and succinic acid was noted. Treatment with carnitine and riboflavin and a low protein diet, with special emphasis on avoidance of foods high in lysine and tryptophan, was initiated. Since the specialized medical formula to provide the recommended daily allowances for the essential amino acids was not available at an affordable cost, the family opted not to continue with this. Over the
6 958 Indian J Pediatr (August 2011) 78(8): Table 3 Marker abnormality, category breakdown and final outcomes of all positive screens Out-of-range analyte Final outcome Category: high probability (n=5) LEU (n=1) Transient. Plasma amino acids normal. C5 (n=1) IVA. Thriving and developing normally at age 3 years LOW C0 (n=1) CUD. Thriving and developing normally at age 2-1/ 2 years C5 DC (n=1) GA-I. Died at age 1-yr despite treatment Multiple Markers (n=1) Metabolic profile consistent with GA-II. Deceased at time of contact. Category: low probability (n=28) LOW CIT (n=1) Transient. Plasma amino acid normal. C0 (n=1) C3 (n=1) C5OH (n=2) C8 (n=2) 985A>G mutation not present. Transient. Resolved on repeat testing. C10:2 (n=1) C16 &/OR C18:1 (n=5) 4/5 All asymptomatic. C14:1 (n=1) Transient. Resolved on repeat testing. However, followed as presumptive VLCAD till molecular analysis can be arranged. Asymptomatic. Multiple Markers (n=14). With at least 3 among LEU, C5, C8, C12, C14:1 & C14 above their cut-off, and the remaining in high range of normal 11/14 All asymptomatic. 3/14 lost to follow-up. Category: indeterminate (n=14) TYR (n=5) 4 No succinylacetone elevation 1 baby had expired at time of contact. PHE (n=3) 2 1 baby had expired at time of contact. LEU (n=2) 2 ORN (n=1) LOW CIT (n=1) PHE & LEU (n=2) 1 1 baby had expired at time of contact. course of time the infant developed developmental delay with myoclonic seizures and failure to thrive, and expired at 1 year of age. Case IV-Isovaleric Aciduria (IVA) One baby in the cohort was the third child of a couple whose two previous children had died of metabolic acidosis. This male baby was born at 34-wks weighing 1000 g. In view of the low birth weight and family history, he was managed in the NICU and was started on a low protein formula on DOL 3. The screening specimen, collected on DOL 2, revealed a significant elevation of C5 in a pattern consistent with IVA. Urinary organic acid analysis using GC-MS confirmed the diagnosis of IVA. His neonatal period was notable for a few episodes of diarrhea and severe anemia requiring blood transfusion. He has remained on a low protein diet and carnitine, and currently at the age of 3-years, his cognitive and motor development is normal. Discussion On comparing the demographic data of all the babies screened from India with those of babies born in Massachusetts, significant differences were noted in the percentage of babies who were noted to be in Intensive/Special Care Units (ICU) or had birth weights under 1.5 kg (2% in
7 Indian J Pediatr (August 2011) 78(8): India; 7% in MA), and in the age at specimen collection. The authors, therefore, restricted each population to specimens collected at h of age from non-nicu babies weighing over 1.5 kg, and analyzed on the same instrument, for further comparisons. It is interesting to note that even with these restrictions the concentrations of the biomarkers differed significantly among the normal babies born in India as compared to the babies born in Massachusetts. A similar difference was not noted among babies born in Massachusetts when compared to babies born in other New England states also tested by NENSP. In order to explain the differences, the authors compared other factors that may have contributed to the differences. Table 4 shows the demographic data of the two populations (after restriction to non-nicu babies of similar age and weight as described previously). One notable difference was the percentage of babies on breast milk (99% in India; 66% in MA). The authors feel that variations in norms related to feeding and maternal diet, possibly in addition to some founder effects affecting the metabolic pathways, are responsible for the observed differences in marker concentrations. The observed variations in biomarker concentrations among the populations raise the question for a need for population-specific screening cut-offs, but larger studies would be required to confirm that this is needed or justified to distinguish affected babies from normals. Careful analysis of the profiles using ratios and indices may be able to compensate for such differences. The spread of all positive screens into the three categories was substantially different among babies from India with those from MA. Of the 48 positive screens among babies born in India, 30% were in the Indeterminate Category and 60% were in the Low Probability Category. On the other hand, the category-based distribution pattern was reversed among babies born in MA, with 60% of screens falling in the Indeterminate Category and 30% in the Low Probability Category. This difference was primarily due to the number of babies with an elevation of multiple markers, with at least 3 among LEU, C5, C8, C12, C14:1, C14 and C16 above their individual cut-offs, and the remaining in the high range of normal. Of the 4, 896 initial specimens from India 14 were noted to have this profile, while of more than 100,000 specimens from MA tested during the same period only 2 were noted to have this pattern. An elevation of several markers in the pattern above is seen in neonates with Glutaric Aciduria-II; however all cases of GA-II detected previously by NENSP have had much higher concentrations (and fell in the High Probability category). When a much larger cohort of neonates from MA was analyzed previously, those babies with a similar elevation of multiple markers in the Low Probability category, and who had extensive diagnostic testing were all concluded to be false-positives; in these cases a maternal riboflavin deficiency or a transient mitochondrial dysfunction was suggested. The elevations in the 14 babies from India in the study resolved on retesting but an extensive diagnostic work-up was not performed. Although it is possible that some of the 14 neonates with this profile had GA-II, it is unlikely. Notably several markers (Leucine, C5 and C16) in this pattern of multi marker elevation have higher overall mean/median concentrations in specimens from babies from India. This difference in the median concentrations is likely related to the large number of neonates with the abovementioned specific pattern of out-of-range results, but the cause remains unclear. A higher prevalence of maternal Table 4 Comparison of demographic data for specimens utilized for marker concentration analysis India (n=2,852) Massachusetts (n=29,413) Weight distribution kg kg 288 1, kg 951 5, kg 1,268 12,688 > 3.5 kg 41 9,909 No Data Feeding status Breast fed ,349 Formula fed 3 6,856 Formula and breast fed 0 3,588 No data 2,511 4,620 Gender Male 1,509 15,003 Female 1,337 14,406 No data 6 4
8 960 Indian J Pediatr (August 2011) 78(8): riboflavin deficiency in the Indian population is a possibility but data were not available to confirm this speculation. To the best of authors knowledge, this was the first unselected population-based newborn screening study conducted for disorders using MS/MS in a region in India. More recently, the state of Goa has begun to screen all newborns born in a Goa Government healthcare facility for several conditions. The authors observed a high prevalence of inborn errors of metabolism to the extent of one in every two thousand newborns in the region. This pilot underscores the necessity of performing larger population-based newborn screening studies to estimate the true extent of the inborn errors of metabolism in India. It also illustrates some challenges that are likely to be encountered when screening for these disorders is introduced into the general population. The authors experienced significant difficulties in obtaining some confirmatory studies for infants who screened positive, due to lack of accessibility to many of the families to diagnostic-quality laboratory analyses. Procuring the specialized diets, the mainstay of treatment, for individuals confirmed to have a disorder was another arduous task because these rare formulas are not manufactured in India and have to be imported from outside the country at high expense. Benefits of screening can only be realized if infants identified by screening receive the necessary follow-up intervention and life-long therapy [12 14]. Hence it will be imperative to consider the availability of, and access to, confirmatory testing and continuing clinical management before implementing any large-scale population-based newborn screening for conditions with resource-intensive health needs, such as the inborn errors of metabolism detected using MS/MS. Acknowledgements The authors are grateful to the CDFD Director for authorizing the study and the Department of Biotechnology, Government of India, for financial support. Conflict of Interest None. Role of Funding Source of India. References Department of Biotechnology, Government 1. Wilcken B. Recent advances in newborn screening. J Inherit Metab Dis. 2007;30: Sahai I, Marsden D. Newborn Screening. Crit Rev in Cli Lab Sci. 2009;46: Pollitt RJ. International perspectives on newborn screening. J Inherit Metab Dis. 2006;29: Miller FA. The complex promise of newborn screening. Indian J Med Ethics. 2009;6: Kapoor S, Kabra M. Newborn screening in India current perspectives. Indian Pediatr. 2010;47: Rama Devi AR, Naushad SM. Newborn screening in India. Indian J Pediatr. 2004;71: Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13: Chace D, Naylor E. Expansion of newborn screening programs using automated tandem mass spectrometry. Ment Retard Dev Disabil Res Rev. 1999;5: Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003;49: Rinaldo P, Tortorelli S, Matern D. Recent developments and new applications of tandem mass spectrometry in newborn screening. Curr Opin Pediatr. 2004;16: Zytkovicz TH, Fitzgerald EF, Marsden D, et al. Tandem mass spectrometric analysis for amino, organic, and fatty acid disorders in newborn dried blood spots: a two-year summary from the New England Newborn Screening Program. Clin Chem. 2001;47: Wilcken B. Mini-symposium: newborn screening for inborn errors of metabolism clinical effectiveness. Inherit Metab Dis. 2006;29: Padilla C, Krotoski D, Therrell Jr B. Newborn screening progress in developing countries-overcoming internal barriers. Semin Perinatol. 2010;34: Wilcken B. Improving child health newborn screening for all? Ann Acad Med Singapore. 2008;37:3.
For Your Baby s Health Department of Health
 Newborn Screening For Your Baby s Health Department of Health Why is my baby tested? To help make sure your baby will be as healthy as possible. The blood test provides important information about your
Newborn Screening For Your Baby s Health Department of Health Why is my baby tested? To help make sure your baby will be as healthy as possible. The blood test provides important information about your
NEWBORN SCREENING. in Massachusetts: Answers for You and Your Baby. University of Massachusetts Medical School
 University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Answers for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Answers for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up
 HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up Dr. Josephine Chong Clinical Professional Consultant Centre of Inborn Errors
HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up Dr. Josephine Chong Clinical Professional Consultant Centre of Inborn Errors
Positive Newborn Screens: What do you do next?
 Positive Newborn Screens: What do you do next? James B. Gibson, MD, Ph.D. Biochemical Geneticist at Specially for Children Clinical Associate Professor of Pediatrics UTHSCSA Carla R. Scott, MD Pediatric
Positive Newborn Screens: What do you do next? James B. Gibson, MD, Ph.D. Biochemical Geneticist at Specially for Children Clinical Associate Professor of Pediatrics UTHSCSA Carla R. Scott, MD Pediatric
Newborn Screening: What s New?
 Newborn Screening: What s New? Patricia M. Jones, PhD (Department of Pathology, University of Texas Southwestern Medical Center and Children s Medical Center of Dallas, Dallas, TX) DOI: 10.1309/LMOOXWVPSM5FC3B6
Newborn Screening: What s New? Patricia M. Jones, PhD (Department of Pathology, University of Texas Southwestern Medical Center and Children s Medical Center of Dallas, Dallas, TX) DOI: 10.1309/LMOOXWVPSM5FC3B6
Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015
 Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015 Metabolic birth defects can cause physical problems, mental retardation and, in some cases, death. It is
Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015 Metabolic birth defects can cause physical problems, mental retardation and, in some cases, death. It is
NEWBORN SCREENING. in Massachusetts: Information for You and Your Baby. University of Massachusetts Medical School
 University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Information for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Information for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
Overview of Newborn Screening, Potential Uses of Residual Dried Blood Spots, and Protection of Privacy
 Overview of Newborn Screening, Potential Uses of Residual Dried Blood Spots, and Protection of Privacy Alan R. Fleischman, M.D. Senior Vice President and Medical Director Chair, Federal Advisory Committee,
Overview of Newborn Screening, Potential Uses of Residual Dried Blood Spots, and Protection of Privacy Alan R. Fleischman, M.D. Senior Vice President and Medical Director Chair, Federal Advisory Committee,
Xiaolei Xie, Marta Kozak. Thermo Fisher Scientific, 355 River Oaks Parkway, San Jose, CA Introduction
 Comparison of Non-derivatization and Derivatization Tandem Mass Spectrometry Methods for Analysis of Amino Acids, Acylcarnitines, and Succinylacetone in Dried Blood Spots Xiaolei Xie, Marta Kozak Thermo
Comparison of Non-derivatization and Derivatization Tandem Mass Spectrometry Methods for Analysis of Amino Acids, Acylcarnitines, and Succinylacetone in Dried Blood Spots Xiaolei Xie, Marta Kozak Thermo
MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES
 NEWBORN SCREENING Protecting your newborn MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES [ 1 ] NEWBORN SCREENING [ 2 ] FREQUENTLY ASKED QUESTIONS [ 4 ] DISORDERS INCLUDED IN NEWBORN SCREENING [ 12 ]
NEWBORN SCREENING Protecting your newborn MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES [ 1 ] NEWBORN SCREENING [ 2 ] FREQUENTLY ASKED QUESTIONS [ 4 ] DISORDERS INCLUDED IN NEWBORN SCREENING [ 12 ]
LOW CITRULLINE AS A MARKER FOR THE PROXIMAL UREA CYCLE DEFECTS EXPERIENCE OF THE NEW ENGLAND NEWBORN SCREENING PROGRAM
 LOW CITRULLINE AS A MARKER FOR THE PROXIMAL UREA CYCLE DEFECTS EXPERIENCE OF THE NEW ENGLAND NEWBORN SCREENING PROGRAM Inderneel Sahai, MD, FACMG Newborn Screening and Genetic Testing Symposium Oct 2014
LOW CITRULLINE AS A MARKER FOR THE PROXIMAL UREA CYCLE DEFECTS EXPERIENCE OF THE NEW ENGLAND NEWBORN SCREENING PROGRAM Inderneel Sahai, MD, FACMG Newborn Screening and Genetic Testing Symposium Oct 2014
Newborn Screening in Manitoba. Information for Health Care Providers
 Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis)
 Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis) 1) Argininosuccinic acidemia (ASA) a) Incidence: ~1 in 70,000 b) Deficiency in an enzyme of
Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis) 1) Argininosuccinic acidemia (ASA) a) Incidence: ~1 in 70,000 b) Deficiency in an enzyme of
Newborn Screening: Focus on Treatment
 Newborn Screening: Focus on Treatment Alan R. Fleischman, M.D. Senior Vice President and Medical Director March of Dimes National Conference of State Legislatures July 21, 2008 Newborn Screening: A Public
Newborn Screening: Focus on Treatment Alan R. Fleischman, M.D. Senior Vice President and Medical Director March of Dimes National Conference of State Legislatures July 21, 2008 Newborn Screening: A Public
NEWBORN SCREENING. health.mo.gov/newbornscreening MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES
 NEWBORN SCREENING health.mo.gov/newbornscreening MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES Table of Contents NEWBORN SCREENING...1 FREQUENTLY ASKED QUESTIONS...2 DISORDERS INCLUDED IN NEWBORN SCREENING...4
NEWBORN SCREENING health.mo.gov/newbornscreening MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES Table of Contents NEWBORN SCREENING...1 FREQUENTLY ASKED QUESTIONS...2 DISORDERS INCLUDED IN NEWBORN SCREENING...4
Title: Assessing Recommendations Related To Timeliness of Newborn Screening
 Title: Assessing Recommendations Related To Timeliness of Newborn Screening Purpose: In January 2014, the Secretary s Discretionary Advisory Committee on Heritable Diseases on Newborns and Children (Committee)
Title: Assessing Recommendations Related To Timeliness of Newborn Screening Purpose: In January 2014, the Secretary s Discretionary Advisory Committee on Heritable Diseases on Newborns and Children (Committee)
Selective newborn screening of amino acid, fatty acid and organic acid disorders in the Kingdom of Bahrain.
 Selective newborn screening of amino acid, fatty acid and organic acid disorders in the Kingdom of Bahrain. Jamal Golbahar PhD Associate Professor of Molecular Medicine, Department of Molecular Medicine,
Selective newborn screening of amino acid, fatty acid and organic acid disorders in the Kingdom of Bahrain. Jamal Golbahar PhD Associate Professor of Molecular Medicine, Department of Molecular Medicine,
Use of a Tandem Mass Spectrometry Research Method for the Analysis of Amino Acids and Acylcarnitines in Dried Blood Spots
 Use of a Tandem Mass Spectrometry Research Method for the Analysis of Amino Acids and Acylcarnitines in Dried Blood Spots Xiaolei Xie and Marta Kozak Thermo Fisher Scientific, San Jose, CA, USA Application
Use of a Tandem Mass Spectrometry Research Method for the Analysis of Amino Acids and Acylcarnitines in Dried Blood Spots Xiaolei Xie and Marta Kozak Thermo Fisher Scientific, San Jose, CA, USA Application
Short and Long Term Stability of 3-Hydroxy acylcarnitines Enriched Dried Blood Spots Stored at Various Temperatures and Humidities
 Short and Long Term Stability of 3-Hydroxy acylcarnitines Enriched Dried Blood Spots Stored at Various Temperatures and Humidities Timothy Lim Biochemical Mass Spectrometry Laboratory Newborn Screening
Short and Long Term Stability of 3-Hydroxy acylcarnitines Enriched Dried Blood Spots Stored at Various Temperatures and Humidities Timothy Lim Biochemical Mass Spectrometry Laboratory Newborn Screening
A Guide for Prenatal Educators
 A Guide for Prenatal Educators Why Teach Newborn Screening This booklet is designed to make it easy for you, a prenatal educator, to effectively inform expectant parents about newborn screening. All of
A Guide for Prenatal Educators Why Teach Newborn Screening This booklet is designed to make it easy for you, a prenatal educator, to effectively inform expectant parents about newborn screening. All of
Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism
 Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism Patricia Jones, PhD DABCC FACB UT Southwestern Medical Center Children s Medical Center Dallas, Texas Learning Objectives Justify
Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism Patricia Jones, PhD DABCC FACB UT Southwestern Medical Center Children s Medical Center Dallas, Texas Learning Objectives Justify
TIMELINESS OF NEWBORN SCREENING: RECOMMENDATIONS FROM ADVISORY COMMITTEE ON HERITABLE DISORDERS IN NEWBORNS AND CHILDREN
 TIMELINESS OF NEWBORN SCREENING: RECOMMENDATIONS FROM ADVISORY COMMITTEE ON HERITABLE DISORDERS IN NEWBORNS AND CHILDREN Susan Tanksley, PhD May 19, 2015 TIMELINESS - BACKGROUND In order to effectively
TIMELINESS OF NEWBORN SCREENING: RECOMMENDATIONS FROM ADVISORY COMMITTEE ON HERITABLE DISORDERS IN NEWBORNS AND CHILDREN Susan Tanksley, PhD May 19, 2015 TIMELINESS - BACKGROUND In order to effectively
Inborn Errors of Metabolism. Metabolic Pathway. Digestion and Fasting. How is Expanded Newborn Screening Different? MS/MS. The body is a factory.
 Inborn Errors of Metabolism The body is a factory. Inborn errors of metabolism are rare genetic disorders in which the body cannot properly turn food into energy. The disorders are usually caused by defects
Inborn Errors of Metabolism The body is a factory. Inborn errors of metabolism are rare genetic disorders in which the body cannot properly turn food into energy. The disorders are usually caused by defects
and Genetics Clinical Chemistry 47: (2001) Molecular Diagnostics
 Clinical Chemistry 47:11 1945 1955 (2001) Molecular Diagnostics and Genetics Tandem Mass Spectrometric Analysis for Amino, Organic, and Fatty Acid Disorders in Newborn Dried Blood Spots: A Two-Year Summary
Clinical Chemistry 47:11 1945 1955 (2001) Molecular Diagnostics and Genetics Tandem Mass Spectrometric Analysis for Amino, Organic, and Fatty Acid Disorders in Newborn Dried Blood Spots: A Two-Year Summary
Most common is the congenital adrenogenital syndrome (AGS) or congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency.
 Newborn Screening Examination parameters: TSH-neonatal (hypothyreosis), 17-OH progesterone (AGS), galactose (galactosemia), galactose-uridyl transferase (galacto semia), biotinidase (biotinidase ), phenylalanine
Newborn Screening Examination parameters: TSH-neonatal (hypothyreosis), 17-OH progesterone (AGS), galactose (galactosemia), galactose-uridyl transferase (galacto semia), biotinidase (biotinidase ), phenylalanine
2015 Annual Report for New Mexico s Newborn Screening (NBS) Program
 2015 Annual Report for New Mexico s Newborn Screening (NBS) Program Sawyer-A Family s Story Inside this Edition A Family Story......1 Why Screen....4 When to Screen...4 What is NBS....5 Screened Conditions..5
2015 Annual Report for New Mexico s Newborn Screening (NBS) Program Sawyer-A Family s Story Inside this Edition A Family Story......1 Why Screen....4 When to Screen...4 What is NBS....5 Screened Conditions..5
(f) "Birthing center" means any facility that is licensed by the Georgia Department of Community Health as a birthing center;
 Ga. Comp. R. & Regs. r. 511-5-5-.02 Definitions Rule 511-5-5-.02. Definitions (a) "Abnormal test result" is a test result from blood testing or physiologic monitoring that is outside the screening limits
Ga. Comp. R. & Regs. r. 511-5-5-.02 Definitions Rule 511-5-5-.02. Definitions (a) "Abnormal test result" is a test result from blood testing or physiologic monitoring that is outside the screening limits
Proposal for EXPANDED NEWBORN SCREENING
 Proposal for EXPANDED NEWBORN SCREENING Tel:+971-4-4503875 Fax:+971-4-4503874 DuBiotech, P.O.Box 212671Dubai, UAE. Email: info@easternbiotech.com www.easternbiotech.com Winner of Dubai SME100 Ranked #19
Proposal for EXPANDED NEWBORN SCREENING Tel:+971-4-4503875 Fax:+971-4-4503874 DuBiotech, P.O.Box 212671Dubai, UAE. Email: info@easternbiotech.com www.easternbiotech.com Winner of Dubai SME100 Ranked #19
Further expansion of the neonatal screening panel in the Netherlands
 Further expansion of the neonatal screening panel in the Netherlands J.Gerard Loeber APHL-NBSGT, St.Louis (MO), USA 290216 Population Area Newborns 6.01 million 0.35:1 16.8 million 180,693 sq km 4.3:1
Further expansion of the neonatal screening panel in the Netherlands J.Gerard Loeber APHL-NBSGT, St.Louis (MO), USA 290216 Population Area Newborns 6.01 million 0.35:1 16.8 million 180,693 sq km 4.3:1
GUIDE TO NEWBORN SCREENING PROGRAMME
 \ GUIDE TO NEWBORN SCREENING PROGRAMME 1 MEDILAB PROFILE MEDILAB, the leading independent provider of Clinical Laboratory Diagnostic Services in Cyprus, was established in 1980 by Mr. C. Pavlides and has
\ GUIDE TO NEWBORN SCREENING PROGRAMME 1 MEDILAB PROFILE MEDILAB, the leading independent provider of Clinical Laboratory Diagnostic Services in Cyprus, was established in 1980 by Mr. C. Pavlides and has
How MS/MS Revolutionized Newborn Screening
 How MS/MS Revolutionized Newborn Screening David S Millington, PhD Medical Research Professor of Pediatrics Director, Biochemical Genetics Laboratory Duke University Medical Center NEWBORN SCREENING IN
How MS/MS Revolutionized Newborn Screening David S Millington, PhD Medical Research Professor of Pediatrics Director, Biochemical Genetics Laboratory Duke University Medical Center NEWBORN SCREENING IN
[R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE PLANTATIONS DEPARTMENT OF HEALTH. February October October 1992 (E) September 1995
![[R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE PLANTATIONS DEPARTMENT OF HEALTH. February October October 1992 (E) September 1995 [R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE PLANTATIONS DEPARTMENT OF HEALTH. February October October 1992 (E) September 1995](/thumbs/95/122505027.jpg) RULES AND REGULATIONS PERTAINING TO THE NEWBORN METABOLIC, ENDOCRINE, AND HEMOGLOBINOPATHY SCREENING PROGRAM AND THE NEWBORN HEARING LOSS SCREENING PROGRAM [R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE
RULES AND REGULATIONS PERTAINING TO THE NEWBORN METABOLIC, ENDOCRINE, AND HEMOGLOBINOPATHY SCREENING PROGRAM AND THE NEWBORN HEARING LOSS SCREENING PROGRAM [R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE
Beyond the case for NBS in South Africa. Chris Vorster 28/05/2016
 Beyond the case for NBS in South Africa Chris Vorster 28/05/2016 The case for NBS in SA Economic justification Cost Utility analysis = Cost/QALY GDP/Capita Immediate implementation (WHO) Political justification
Beyond the case for NBS in South Africa Chris Vorster 28/05/2016 The case for NBS in SA Economic justification Cost Utility analysis = Cost/QALY GDP/Capita Immediate implementation (WHO) Political justification
Diagnose a broad range of metabolic disorders with a single test, Global MAPS
 PEDIATRIC Assessing or diagnosing a metabolic disorder commonly requires several tests. Global Metabolomic Assisted Pathway Screen, commonly known as Global MAPS, is a unifying test for analyzing hundreds
PEDIATRIC Assessing or diagnosing a metabolic disorder commonly requires several tests. Global Metabolomic Assisted Pathway Screen, commonly known as Global MAPS, is a unifying test for analyzing hundreds
Tandem Mass Neonatal Screening in Taiwan Report from One Center
 ORIGINAL ARTICLE Tandem Mass Neonatal Screening in Taiwan Report from One Center Hsiang-Po Huang, 1,2 Kai-Lin Chu, 1 Yin-Hsiu Chien, 1,3 Ming-Lee Wei, 3 Shu-Tzu Wu, 3 Shiao-Fang Wang, 3 Wuh-Liang Hwu 1,3
ORIGINAL ARTICLE Tandem Mass Neonatal Screening in Taiwan Report from One Center Hsiang-Po Huang, 1,2 Kai-Lin Chu, 1 Yin-Hsiu Chien, 1,3 Ming-Lee Wei, 3 Shu-Tzu Wu, 3 Shiao-Fang Wang, 3 Wuh-Liang Hwu 1,3
NEWBORN METABOLIC SCREEN, MINNESOTA
 Lab Dept: Test Name: Chemistry NEWBORN METABOLIC SCREEN, MINNESOTA General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: PKUN Newborn Screen for Hyopothyroidism, Phenylketonuria (PKU),
Lab Dept: Test Name: Chemistry NEWBORN METABOLIC SCREEN, MINNESOTA General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: PKUN Newborn Screen for Hyopothyroidism, Phenylketonuria (PKU),
Screening Newborns for Inborn Errors of Metabolism by Tandem Mass Spectrometry
 The new england journal of medicine original article Screening Newborns for Inborn Errors of Metabolism by Tandem Mass Spectrometry Bridget Wilcken, M.B., Ch.B., Veronica Wiley, Ph.D., Judith Hammond,
The new england journal of medicine original article Screening Newborns for Inborn Errors of Metabolism by Tandem Mass Spectrometry Bridget Wilcken, M.B., Ch.B., Veronica Wiley, Ph.D., Judith Hammond,
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 12/19/17 SECTION: MEDICINE LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 MEDICAL FOODS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
MEDICAL FOODS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
Newborn Screening in Washington State Saving lives with a simple blood spot
 Newborn Screening in Washington State Saving lives with a simple blood spot Ashleigh Ragsdale, MPH Gauri Gupta, MScPH Objectives Newborn Screening Overview Process and Law Completing Collection Cards Video
Newborn Screening in Washington State Saving lives with a simple blood spot Ashleigh Ragsdale, MPH Gauri Gupta, MScPH Objectives Newborn Screening Overview Process and Law Completing Collection Cards Video
Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol
 Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Last update: 14 February 2014 (version 6) Joshua
Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Last update: 14 February 2014 (version 6) Joshua
The Compelling Benefits of Routine 2 nd NBS: A Fifteen-Year Review in Washington State. Saving lives with a simple blood spot
 The Compelling Benefits of Routine 2 nd NBS: A Fifteen-Year Review in Washington State Caroline T. Nucup-Villaruz, MD Primary Author NBS Consultant - Disorder FU Santosh Shaunak Co-Author & Presenter Laboratory
The Compelling Benefits of Routine 2 nd NBS: A Fifteen-Year Review in Washington State Caroline T. Nucup-Villaruz, MD Primary Author NBS Consultant - Disorder FU Santosh Shaunak Co-Author & Presenter Laboratory
Lauren E. Cipriano, BSc, BA, 1 C. Anthony Rupar, PhD, 2 Gregory S. Zaric, PhD 1. Introduction
 Blackwell Publishing IncMalden, USAVHEValue in Health1098-30152006 Blackwell Publishing20071028397Original ArticleCost-Effectiveness of Newborn ScreeningCipriano et al. Volume 10 Number 2 2007 VALUE IN
Blackwell Publishing IncMalden, USAVHEValue in Health1098-30152006 Blackwell Publishing20071028397Original ArticleCost-Effectiveness of Newborn ScreeningCipriano et al. Volume 10 Number 2 2007 VALUE IN
Newborn Screening in Japan: Restructuring for the New Era
 Plenary 13 Newborn Screening in Japan: Restructuring for the New Era Seiji Yamaguchi, 1 MD Abstract Nationwide neonatal mass screening for inherited metabolic diseases has started in Japan since 1977.
Plenary 13 Newborn Screening in Japan: Restructuring for the New Era Seiji Yamaguchi, 1 MD Abstract Nationwide neonatal mass screening for inherited metabolic diseases has started in Japan since 1977.
Newborn Screening: Blood Spot Disorders
 Newborn Screening: Blood Spot Disorders Arizona s Newborn Screening Program Program Overview Panel of Disorders Disorder Descriptions Program Components Hospitals ADHS Lab ADHS Follow-up ADHS Billing Medical
Newborn Screening: Blood Spot Disorders Arizona s Newborn Screening Program Program Overview Panel of Disorders Disorder Descriptions Program Components Hospitals ADHS Lab ADHS Follow-up ADHS Billing Medical
Inborn Errors of Metabolism From Neonatal Screening to Metabolic Pathways
 19 May 2015 Hospital Authority Convention 2015 Corporate Scholarship Presentation II Paediatric Services Inborn Errors of Metabolism From Neonatal Screening to Metabolic Pathways Dr Grace Poon Associate
19 May 2015 Hospital Authority Convention 2015 Corporate Scholarship Presentation II Paediatric Services Inborn Errors of Metabolism From Neonatal Screening to Metabolic Pathways Dr Grace Poon Associate
The spectrum and outcome of the. neonates with inborn errors of. metabolism at a tertiary care hospital
 The spectrum and outcome of the neonates with inborn errors of metabolism at a tertiary care hospital Dr. Sevim Ünal Neonatology Division, Ankara Children s Hematology Oncology Research Hospital, Ankara,
The spectrum and outcome of the neonates with inborn errors of metabolism at a tertiary care hospital Dr. Sevim Ünal Neonatology Division, Ankara Children s Hematology Oncology Research Hospital, Ankara,
Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme
 Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme Charles Turner Laboratory Guy s Hospital (Evelina Childrens Hospital, St Thomas
Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme Charles Turner Laboratory Guy s Hospital (Evelina Childrens Hospital, St Thomas
Considerations in Choosing Screening Conditions: One (US) Approach
 22 Plenary Considerations in Choosing Screening Conditions: One (US) Approach Bradford L Therrell Jr, 1,2 MS, PhD Abstract The lack of a national policy on newborn screening (NBS) in the United States
22 Plenary Considerations in Choosing Screening Conditions: One (US) Approach Bradford L Therrell Jr, 1,2 MS, PhD Abstract The lack of a national policy on newborn screening (NBS) in the United States
Joshua Hellmann Foundation - Newborn Metabolic Screening Program
 Joshua Hellmann Foundation - Newborn Metabolic Screening Program Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Last update: 11 June 2013 Target inborn errors of metabolism (IEM)
Joshua Hellmann Foundation - Newborn Metabolic Screening Program Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Last update: 11 June 2013 Target inborn errors of metabolism (IEM)
INBORN ERRORS OF METABOLISM (IEM) IAP UG Teaching slides
 INBORN ERRORS OF METABOLISM (IEM) 1 OBJECTIVES What are IEMs? Categories When to suspect? History and clinical pointers Metabolic presentation Differential diagnosis Emergency and long term management
INBORN ERRORS OF METABOLISM (IEM) 1 OBJECTIVES What are IEMs? Categories When to suspect? History and clinical pointers Metabolic presentation Differential diagnosis Emergency and long term management
Medical Foods for Inborn Errors of Metabolism
 Medical Foods for Inborn Errors of Metabolism Policy Number: Original Effective Date: MM.02.014 02/18/2000 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 08/23/2013 Section: Medicine Place(s)
Medical Foods for Inborn Errors of Metabolism Policy Number: Original Effective Date: MM.02.014 02/18/2000 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 08/23/2013 Section: Medicine Place(s)
Health and Wellness for all Arizonans. azdhs.gov
 To identify newborns with certain, rare disorders and assist families of affected infants so that they receive appropriate and timely treatment to prevent or delay serious medical problems. To identify
To identify newborns with certain, rare disorders and assist families of affected infants so that they receive appropriate and timely treatment to prevent or delay serious medical problems. To identify
So Much More Than The PKU Test
 Newborn Metabolic Screening So Much More Than The PKU Test Sarah Viall, MSN, PPCNP BC Newborn Screening Program Coordinator Division of Genetics & Metabolism Conflicts of Interest I have no conflicts of
Newborn Metabolic Screening So Much More Than The PKU Test Sarah Viall, MSN, PPCNP BC Newborn Screening Program Coordinator Division of Genetics & Metabolism Conflicts of Interest I have no conflicts of
The Changing Moral Focus of Newborn Screening: An Ethical Analysis by the President s Council on Bioethics
 The Changing Moral Focus of Newborn Screening: An Ethical Analysis by the President s Council on Bioethics Appendix Newborn Screening: An International Survey By Joseph A. Raho, Research Analyst In addition
The Changing Moral Focus of Newborn Screening: An Ethical Analysis by the President s Council on Bioethics Appendix Newborn Screening: An International Survey By Joseph A. Raho, Research Analyst In addition
Inborn Errors of Metabolism (IEM)
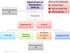 Clinical Presentation Inborn Errors of Metabolism (IEM) Click on the following: - Clinical Pearl - link to movie clip - link to picture Investigations Blood Work Urine No Acidosis NH 4 + Metabolic Acidosis
Clinical Presentation Inborn Errors of Metabolism (IEM) Click on the following: - Clinical Pearl - link to movie clip - link to picture Investigations Blood Work Urine No Acidosis NH 4 + Metabolic Acidosis
Inborn Errors of Metabolism Clinical Approach to Diagnosis and Treatment
 Case Scenario: 15 year old girl presented in ED with aggressive behaviour and hallucinations. No associated fever, vomiting, seizures or developmental concerns Previously well Inborn Errors of Metabolism
Case Scenario: 15 year old girl presented in ED with aggressive behaviour and hallucinations. No associated fever, vomiting, seizures or developmental concerns Previously well Inborn Errors of Metabolism
Newborn Screening by Tandem
 Newborn Screening by Tandem Mass Spectrometry in Texas Patricia Hunt Manager, Newborn Metabolic Screening Group Biochemistry and Genetics Branch Texas Department of State Health Services May 30, 2012 Texas
Newborn Screening by Tandem Mass Spectrometry in Texas Patricia Hunt Manager, Newborn Metabolic Screening Group Biochemistry and Genetics Branch Texas Department of State Health Services May 30, 2012 Texas
Post mortem investigation of Inherited Metabolic Disease - the last opportunity for a diagnosis -
 Post mortem investigation of Inherited Metabolic Disease - the last opportunity for a diagnosis - Dr Simon Olpin Lead Clinical Scientist in Inherited Metabolic Disease Sheffield Children s Hospital SIDS/SUDI
Post mortem investigation of Inherited Metabolic Disease - the last opportunity for a diagnosis - Dr Simon Olpin Lead Clinical Scientist in Inherited Metabolic Disease Sheffield Children s Hospital SIDS/SUDI
Attachment 1. Newborn Screening Program Description
 Attachment 1 Newborn Screening Program Description The core mission of Texas Newborn Screening Program is to save children s lives through the early detection of life-threatening disorders. 34 Newborn
Attachment 1 Newborn Screening Program Description The core mission of Texas Newborn Screening Program is to save children s lives through the early detection of life-threatening disorders. 34 Newborn
MS/MS APPROACHES TO CLINICAL TESTING FOR INBORN ERRORS OF METABOLISM
 MS/MS APPROACHES TO CLINICAL TESTING FOR INBORN ERRORS OF METABOLISM Tina M. Cowan, PhD Director, Clinical Biochemical Genetics Laboratory Stanford University Medical Center Presented at the 2011 Stanford
MS/MS APPROACHES TO CLINICAL TESTING FOR INBORN ERRORS OF METABOLISM Tina M. Cowan, PhD Director, Clinical Biochemical Genetics Laboratory Stanford University Medical Center Presented at the 2011 Stanford
Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol
 Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Version 7 Effective Date: 15 th February 2016
Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Version 7 Effective Date: 15 th February 2016
Metabolic Changes in ASD. Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius
 Metabolic Changes in ASD Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius 12 patients 3 Autism: Ages 3/3/3.7 3 PDD: Ages 3/3/6 3 Asperger: Ages 6/7/15.1 3 Speech delay and Sensory Problems (SHL):
Metabolic Changes in ASD Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius 12 patients 3 Autism: Ages 3/3/3.7 3 PDD: Ages 3/3/6 3 Asperger: Ages 6/7/15.1 3 Speech delay and Sensory Problems (SHL):
Alvaro Serrano Russi MD University of Iowa Hospitals and Clinics
 Retrospective Analysis of the Region 4 Post Analytical Tool and Confirmatory Testing for Long Chain Fatty Acid Oxidation Disorders Screened in the State of Iowa Alvaro Serrano Russi MD University of Iowa
Retrospective Analysis of the Region 4 Post Analytical Tool and Confirmatory Testing for Long Chain Fatty Acid Oxidation Disorders Screened in the State of Iowa Alvaro Serrano Russi MD University of Iowa
ACYLCARNITINES, QUANTITATIVE, PLASMA
 Lab Dept: Test Name: Chemistry ACYLCARNITINES, QUANTITATIVE, PLASMA General Information Lab Order Codes: ACYP Synonyms: CPT Codes: Test Includes: Glycine conjugates plasma 82017 Acylcarnitines, quantitative,
Lab Dept: Test Name: Chemistry ACYLCARNITINES, QUANTITATIVE, PLASMA General Information Lab Order Codes: ACYP Synonyms: CPT Codes: Test Includes: Glycine conjugates plasma 82017 Acylcarnitines, quantitative,
Introduction to Organic Acidemias. Hilary Vernon, MD PhD Assistant Professor of Genetic Medicine Johns Hopkins University 7.25.
 Introduction to Organic Acidemias Hilary Vernon, MD PhD Assistant Professor of Genetic Medicine Johns Hopkins University 7.25.2014 A Brief Historical Overview Garrod, Archibald E. 1902. The Incidence of
Introduction to Organic Acidemias Hilary Vernon, MD PhD Assistant Professor of Genetic Medicine Johns Hopkins University 7.25.2014 A Brief Historical Overview Garrod, Archibald E. 1902. The Incidence of
Urea Cycle Disorders and Hyperammonemia: Diagnosable Treatable Screenable
 Urea Cycle Disorders and Hyperammonemia: Diagnosable Treatable Screenable Marshall L. Summar, M.D. Chief, Division of Genetics and Metabolism Children s National Medical Center Washington, DC, USA Disclosure
Urea Cycle Disorders and Hyperammonemia: Diagnosable Treatable Screenable Marshall L. Summar, M.D. Chief, Division of Genetics and Metabolism Children s National Medical Center Washington, DC, USA Disclosure
Newborn Metabolic Screening Programme Annual Report
 Newborn Metabolic Screening Programme Annual Report January to December 2015 Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland District Health Board.
Newborn Metabolic Screening Programme Annual Report January to December 2015 Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland District Health Board.
Lab Guide 2019 Metabolic Section Lab Guide
 Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
QA/QC 2 Strategies to Reduce False Positives and False Negatives (Part 2)
 QA/QC 2 Strategies to Reduce False Positives and False Negatives (Part 2) Tuesday, Oct. 28 10:30am-12:00pm Moderators Patricia Hunt, Texas Department of State Health Services and Bob Currier, California
QA/QC 2 Strategies to Reduce False Positives and False Negatives (Part 2) Tuesday, Oct. 28 10:30am-12:00pm Moderators Patricia Hunt, Texas Department of State Health Services and Bob Currier, California
Metabolomic Analysis for Newborn Screening and Diagnosis of Metabolic Disorders
 Metabolomic Analysis for Newborn Screening and Diagnosis of Metabolic Disorders Mass Spectrometry and Separation Sciences for Laboratory Medicine Chicago October 1-2, 2015 Michael J. Bennett PhD, FRCPath,
Metabolomic Analysis for Newborn Screening and Diagnosis of Metabolic Disorders Mass Spectrometry and Separation Sciences for Laboratory Medicine Chicago October 1-2, 2015 Michael J. Bennett PhD, FRCPath,
A Brief History of Newborn Screening
 A Brief History of Newborn Screening The first 50 years. Ken Pass I didn t do this alone Thanks to: Amy Hoffman Alex Kemper Kathy Harris Piero Rinaldo and others 1902 Liv Dag Asbjorn Follin Pediatrics
A Brief History of Newborn Screening The first 50 years. Ken Pass I didn t do this alone Thanks to: Amy Hoffman Alex Kemper Kathy Harris Piero Rinaldo and others 1902 Liv Dag Asbjorn Follin Pediatrics
M-N. Neisseria gonorrhoeae. Neopterin, Serum. Analytik
 Neisseria gonorrhoeae Background: Neisseria gonorrhoeae is a gram negative oxidase positive bacteria producing a lipooligosaccharide endotoxin which contains lipid A without long repeating sugar side chains.
Neisseria gonorrhoeae Background: Neisseria gonorrhoeae is a gram negative oxidase positive bacteria producing a lipooligosaccharide endotoxin which contains lipid A without long repeating sugar side chains.
Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet
 Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet Website:www.tipn.org.tw Telephone:(02)85962050 Ext. 401-403 Service line:(02)85962065 Fax:(02)85962067
Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet Website:www.tipn.org.tw Telephone:(02)85962050 Ext. 401-403 Service line:(02)85962065 Fax:(02)85962067
Annual Report Calendar Year 2015
 Annual Report Calendar Year 2015 Page 1 of 19 Contents 1. SAMPLE VOLUMES IN 2015...3 1.1 SCREENING SAMPLES...3 1.1.1 INFANTS SCREENED...3 1.1.2 DECLINED/DEFERRED TESTING...4 1.1.3 MISSED SCREENS...5 1.2
Annual Report Calendar Year 2015 Page 1 of 19 Contents 1. SAMPLE VOLUMES IN 2015...3 1.1 SCREENING SAMPLES...3 1.1.1 INFANTS SCREENED...3 1.1.2 DECLINED/DEFERRED TESTING...4 1.1.3 MISSED SCREENS...5 1.2
UREA CYCLE DISORDERS - The What, Why, How and When
 UREA CYCLE DISORDERS - The What, Why, How and When George A. Diaz, MD, PhD Program for Inherited Metabolic Diseases Department of Genetics and Genomic Sciences Department of Pediatrics Icahn School of
UREA CYCLE DISORDERS - The What, Why, How and When George A. Diaz, MD, PhD Program for Inherited Metabolic Diseases Department of Genetics and Genomic Sciences Department of Pediatrics Icahn School of
Screening Newborns for Congenital Disorders
 Screening Newborns for Congenital Disorders Gary L. Hoffman, BS; Ronald H. Laessig, PhD ABSTRACT The Newborn Screening Laboratory at the Wisconsin State Laboratory of Hygiene (WSLH) tests all newborn babies
Screening Newborns for Congenital Disorders Gary L. Hoffman, BS; Ronald H. Laessig, PhD ABSTRACT The Newborn Screening Laboratory at the Wisconsin State Laboratory of Hygiene (WSLH) tests all newborn babies
THE ED APPROACH OF THE CHILD WITH SUSPECTED METABOLIC DISEASE
 THE ED APPROACH OF THE CHILD WITH SUSPECTED METABOLIC DISEASE Dr. Nadeem Qureshi M.D, FAAP, FCCM Associate Professor Pediatrics School of Medicine. St Louis University Attending Physician Pediatric Emergency
THE ED APPROACH OF THE CHILD WITH SUSPECTED METABOLIC DISEASE Dr. Nadeem Qureshi M.D, FAAP, FCCM Associate Professor Pediatrics School of Medicine. St Louis University Attending Physician Pediatric Emergency
Newborn Bloodspot Screening Information for parents
 Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Newborn Bloodspot Screening Information for parents
 Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
NEONATAL SCREENING FOR INBORN ERRORS OF METABOLISM OUR EXPERIENCE AT CABRI, GULF MEDICAL UNIVERSITY
 GMJ GULF MEDICAL JOURNAL ORAL PROCEEDINGS NEONATAL SCREENING FOR INBORN ERRORS OF METABOLISM OUR EXPERIENCE AT CABRI, GULF MEDICAL UNIVERSITY I.A. Shaafie 1 *, A.D. Vijay Raju 2, P.K. Menon 3 1Division
GMJ GULF MEDICAL JOURNAL ORAL PROCEEDINGS NEONATAL SCREENING FOR INBORN ERRORS OF METABOLISM OUR EXPERIENCE AT CABRI, GULF MEDICAL UNIVERSITY I.A. Shaafie 1 *, A.D. Vijay Raju 2, P.K. Menon 3 1Division
Slide 1: Newborn Bloodspot Screening for Medium-chain Acyl-CoA Dehydrogenase Deficiency (MCADD)
 Slide 1: Newborn Bloodspot Screening for Medium-chain Acyl-CoA Dehydrogenase Deficiency (MCADD) The information has been produced with thanks to the NHS Newborn Blood Spot Screening Programme. 1 Slide
Slide 1: Newborn Bloodspot Screening for Medium-chain Acyl-CoA Dehydrogenase Deficiency (MCADD) The information has been produced with thanks to the NHS Newborn Blood Spot Screening Programme. 1 Slide
Pilot Study of Newborn Screening (NBS) for Inborn Errors of Metabolism (IEM) in Collaboration with Department of Health and Hospital Authority
 HA Convention 2017 Service Enhancement Presentation Healthcare Advances, Research and Innovations Pilot Study of Newborn Screening (NBS) for Inborn Errors of Metabolism (IEM) in Collaboration with Department
HA Convention 2017 Service Enhancement Presentation Healthcare Advances, Research and Innovations Pilot Study of Newborn Screening (NBS) for Inborn Errors of Metabolism (IEM) in Collaboration with Department
Medium-chain acyl-coa dehydrogenase (MCAD) deficiency and Expanded Newborn Screening in Japan
 Medium-chain acyl-coa dehydrogenase (MCAD) deficiency and Expanded Newborn Screening in Japan Seiji YAMAGUCHI, MD Department of Pediatrics, Shimane University (co-authors) Jamiyan Purevsuren, Yuki Hasegawa,
Medium-chain acyl-coa dehydrogenase (MCAD) deficiency and Expanded Newborn Screening in Japan Seiji YAMAGUCHI, MD Department of Pediatrics, Shimane University (co-authors) Jamiyan Purevsuren, Yuki Hasegawa,
Coverage Guidelines: Oral Formula: Rhode Island Products
 Coverage Guidelines: Oral Formula: Rhode Island Products Effective: August 9, 2017 Clinical Documentation and Prior Authorization Required Applies to: Coverage Guideline, No Prior Authorization Tufts Health
Coverage Guidelines: Oral Formula: Rhode Island Products Effective: August 9, 2017 Clinical Documentation and Prior Authorization Required Applies to: Coverage Guideline, No Prior Authorization Tufts Health
ENHANCING PRECISION MEDICINE THROUGH CLINICAL MASS SPECTROMETRY PLATFORM. Dobrin Svinarov Alexander Hospital Medical University of Sofia, Bulgaria
 ENHANCING PRECISION MEDICINE THROUGH CLINICAL MASS SPECTROMETRY PLATFORM Dobrin Svinarov Alexander Hospital Medical University of Sofia, Bulgaria SYNOPSIS Technological transfer in laboratory medicine
ENHANCING PRECISION MEDICINE THROUGH CLINICAL MASS SPECTROMETRY PLATFORM Dobrin Svinarov Alexander Hospital Medical University of Sofia, Bulgaria SYNOPSIS Technological transfer in laboratory medicine
Critical Newborn Screens in Double Heterozygotes of Inborn Errors of Metabolism A Clinical Report and Recommendations
 International Journal of Neonatal Screening Case Report Critical Newborn Screens in Double Heterozygotes of Inborn Errors of Metabolism A Clinical Report and Recommendations Katherine G. Langley 1,2, Elizabeth
International Journal of Neonatal Screening Case Report Critical Newborn Screens in Double Heterozygotes of Inborn Errors of Metabolism A Clinical Report and Recommendations Katherine G. Langley 1,2, Elizabeth
Why 2002? Looking Back (2002) Looking Back. What Was I Doing in 2002? News You can Use (U.S. News & World Report - January 17, 2000)
 What have I been doing since 22? Milestones of a personal journey and memorable encounters along the metabolic path Why 22? What Was I Doing in 22? Piero Rinaldo, MD, PhD T. Denny Sanford Professor of
What have I been doing since 22? Milestones of a personal journey and memorable encounters along the metabolic path Why 22? What Was I Doing in 22? Piero Rinaldo, MD, PhD T. Denny Sanford Professor of
Newborn Metabolic Screening Programme. Annual Report
 Newborn Metabolic Screening Programme Annual Report January to December 2016 1 Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland District Health Board.
Newborn Metabolic Screening Programme Annual Report January to December 2016 1 Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland District Health Board.
Mary Seeterlin, PhD Michigan Department of Community Health. Prevent Disease Promote Wellness Improve Quality of Life
 Mary Seeterlin, PhD Michigan Department of Community Health Prevent Disease Promote Wellness Improve Quality of Life CPTII - Three Clinical Phenotypes Lethal Neonatal Most severe form Symptoms begin within
Mary Seeterlin, PhD Michigan Department of Community Health Prevent Disease Promote Wellness Improve Quality of Life CPTII - Three Clinical Phenotypes Lethal Neonatal Most severe form Symptoms begin within
Clinical Management of Organic Acidemias and OAA Natural History Registry. Kim Chapman MD PhD Children s National Rare Disease Institute
 Clinical Management of Organic Acidemias and OAA Natural History Registry Kim Chapman MD PhD Children s National Rare Disease Institute Disclosure Nothing to disclose concerning this lecture Organic acid?
Clinical Management of Organic Acidemias and OAA Natural History Registry Kim Chapman MD PhD Children s National Rare Disease Institute Disclosure Nothing to disclose concerning this lecture Organic acid?
TRANSAMINATION AND UREA CYCLE
 TRANSAMINATION AND UREA CYCLE USMAN SUMO FRIEND TAMBUNAN ARLI ADITYA PARIKESIT SEPTIANA BIOINFORMATICS GROUP DEPARTEMENT OF CHEMISTRY FACULTY OF MATHEMATIC AND SCIENCE UNIVERSITY OF INDONESIA What is transamination?
TRANSAMINATION AND UREA CYCLE USMAN SUMO FRIEND TAMBUNAN ARLI ADITYA PARIKESIT SEPTIANA BIOINFORMATICS GROUP DEPARTEMENT OF CHEMISTRY FACULTY OF MATHEMATIC AND SCIENCE UNIVERSITY OF INDONESIA What is transamination?
Endocrinology and Metabolism: Long-Term Stability of Amino Acids and Acylcarnitines in Dried Blood Spots
 Papers in Press. Published February 1, 2007 as doi:10.1373/clinchem.2006.076679 The latest version is at http://www.clinchem.org/cgi/doi/10.1373/clinchem.2006.076679 Clinical Chemistry 53:4 000 000 (2007)
Papers in Press. Published February 1, 2007 as doi:10.1373/clinchem.2006.076679 The latest version is at http://www.clinchem.org/cgi/doi/10.1373/clinchem.2006.076679 Clinical Chemistry 53:4 000 000 (2007)
A rough guide to Acylcarnitines
 A rough guide to Acylcarnitines Roy Talbot & Nigel Manning Roy.Talbot@sch.nhs.uk Dept. of Clinical Chemistry, Sheffield Children s Hospital Menu Acylcarnitines Basic Tandem MS theory SCADD MCADD LCHADD
A rough guide to Acylcarnitines Roy Talbot & Nigel Manning Roy.Talbot@sch.nhs.uk Dept. of Clinical Chemistry, Sheffield Children s Hospital Menu Acylcarnitines Basic Tandem MS theory SCADD MCADD LCHADD
Neonatal Screening using Shimadzu LCMS- 8040
 SEG- 2050 Application Note for The Analytical Scientist 11-13 Neonatal Screening using Shimadzu LCMS- 8040 Introduction The development of electrospray tandem mass spectrometry (LCMS/MS) in recent years
SEG- 2050 Application Note for The Analytical Scientist 11-13 Neonatal Screening using Shimadzu LCMS- 8040 Introduction The development of electrospray tandem mass spectrometry (LCMS/MS) in recent years
Newborn bloodspot testing
 Policy HUMAN GENETICS SOCIETY OF AUSTRALASIA ARBN. 076 130 937 (Incorporated Under the Associations Incorporation Act) The liability of members is limited RACP, 145 Macquarie Street, Sydney NSW 2000, Australia
Policy HUMAN GENETICS SOCIETY OF AUSTRALASIA ARBN. 076 130 937 (Incorporated Under the Associations Incorporation Act) The liability of members is limited RACP, 145 Macquarie Street, Sydney NSW 2000, Australia
Guideline for the diagnosis and management of isovaleryl-coa-dehydrogenase deficiency (isovaleric acidemia) - a systematic review -
 Guideline for the diagnosis and management of isovaleryl-coa-dehydrogenase deficiency (isovaleric acidemia) - a systematic review - Guideline development group International interdisciplinary guideline
Guideline for the diagnosis and management of isovaleryl-coa-dehydrogenase deficiency (isovaleric acidemia) - a systematic review - Guideline development group International interdisciplinary guideline
IHE Quality, Research, and Public Health (QRPH) White Paper Newborn Screening
 Integrating the Healthcare Enterprise 5 IHE Quality, Research, and Public Health (QRPH) White Paper 2009-2010 10 Newborn Screening Version 0.3 15 Date: September 1, 2009 Main Editor: Anna Orlova Contributors:
Integrating the Healthcare Enterprise 5 IHE Quality, Research, and Public Health (QRPH) White Paper 2009-2010 10 Newborn Screening Version 0.3 15 Date: September 1, 2009 Main Editor: Anna Orlova Contributors:
Past, present and future of newborn screening in Chile
 DOI 10.1007/s10545-010-9165-8 RESEARCH REPORT Past, present and future of newborn screening in Chile V. Cornejo & E. Raimann & J. F. Cabello & A. Valiente & C. Becerra & M. Opazo & M. Colombo Received:
DOI 10.1007/s10545-010-9165-8 RESEARCH REPORT Past, present and future of newborn screening in Chile V. Cornejo & E. Raimann & J. F. Cabello & A. Valiente & C. Becerra & M. Opazo & M. Colombo Received:
2016 Annual Report for New Mexico s Newborn Screening (NBS) Program
 2016 Annual Report for New Mexico s Newborn Screening (NBS) Program Felipe - A Family s Story Inside this Edi on A Family Story..... 1 Why Screen.... 3 When to Screen... 3 What is NBS... 4 Screened Condi
2016 Annual Report for New Mexico s Newborn Screening (NBS) Program Felipe - A Family s Story Inside this Edi on A Family Story..... 1 Why Screen.... 3 When to Screen... 3 What is NBS... 4 Screened Condi
