Title: Assessing Recommendations Related To Timeliness of Newborn Screening
|
|
|
- Sybil Skinner
- 5 years ago
- Views:
Transcription
1 Title: Assessing Recommendations Related To Timeliness of Newborn Screening Purpose: In January 2014, the Secretary s Discretionary Advisory Committee on Heritable Diseases on Newborns and Children (Committee) tasked the Laboratory Standards and Procedures Subcommittee to review current policies and practices relating to timeliness of newborn screening (NBS) in the United States. Background: In 2006 the report, Newborn Screening: Toward a Uniform Screening Panel and System made four recommendations on the timeliness of newborn screening. The Laboratory Standards and Procedures Subcommittee (Subcommittee) was tasked to (1) outline the NBS system, (2) investigate existing gaps and barriers in NBS systems, (3) identify best practices for achieving these goals, (4) develop a list of time-critical conditions that require urgent follow-up, (5) review the four recommendations in light of new technologies and (6) suggest revisions, if needed. The Association of Public Health Laboratories (APHL) in conjunction with the Laboratory Standards and Procedures Subcommittee developed this survey to identify the current practices, gaps, and barriers related to NBS timeliness in state NBS programs. Stakeholder input and results from this survey will be compiled into a report for the Laboratory Standards and Procedures Subcommittee to present to the Committee. All results will be presented in aggregate format and no individual or state will be identified. (Note: The Subcommittee recognized that there were inconsistencies in the 2006 report on the metrics used to define timeliness and will be addressing this in their report. For the purposes of this survey, birth is used as the reference point.)
2 SECTION 1: COMMUNICATION BETWEEN THE STATE NEWBORN SCREENING (NBS) PROGRAM AND BIRTHING FACILITIES 1. Does your NBS program provide feedback to individual birthing facility (ies) on the following? Please check all that apply. o Complete essential information (information the state NBS program needs to track a newborn, etc.) o NBS specimen collection time o NBS specimen transit time (from birthing facility to NBS laboratory) o Unsatisfactory NBS specimens o Other- please specify: I don t know [please go to question 3] No monitoring is done [please go to question 3] 2. How often is feedback about the above topic(s) provided to the birthing facility(ies)? o Monthly o Quarterly o Annually o Other- please specify: 3. Does your state provide technical assistance and/or training to birthing facility(ies)? o Yes [please go to question 3a] o No [please go to question 4] o I don t know [please go to question 4] 3a. How often is technical assistance and/or training provided to the birthing facility(ies)? o Monthly o Quarterly o Annually o Upon request/ other- please specify:
3 SECTION 2: STATE NBS PROGRAMS AND THE FOUR RECOMMENDATIONS RELATED TO TIMELINESS OF NEWBORN SCREENING Recommendation 1: Initial newborn screening (NBS) specimens should be collected at 24 to 48 hours of life. 4. Does your NBS program have a mechanism to ensure that all newborns in your state are screened? o Yes [please go to question 4a] o No [please go to question 4b] o I don t know [please go to question 5] 4a. Please describe your state s mechanism: 4b. What are the barriers that prevent your NBS program form ensuring each newborn is screened? 5. What percent of initial specimens are collected at hours of life? % of initial specimens are collected at hours of life My program does not collect this information 6. What percent of initial NBS specimens are collected at less than 24 hours of life? % of initial specimens are collected at less than24 hours of life My program does not collect this information
4 7. Please indicate the degree of the factors listed below have on your NBS Program's ability to meet Recommendation 1 (Initial newborn screening (NBS) specimens should be collected at 24 to 48 hours of life)? High turnover of staff responsible for dried blood spot collection at birthing facilities Compliance with collection from premature/sick infants (CLSI and/or Program standards) guidelines Lack of education of nurse managers/quality assurance staff at birthing facilities Lack of education of personnel who collect the specimens at birthing facilities Major Moderate Minor No Lack of feedback provided to birthing facilities on initial specimen collection performance Premature births Release of newborn prior to 24 hours of life Timing of practices in routine newborn care (nursing protocols) Transfer of newborn before specimen is collected 7a. Please describe any other gaps or barriers regarding your NBS program s ability to meet Recommendation What is your NBS program currently doing to ensure the collection of the initial newborn screening at hours of life? 9. What are the 3 most critical things that can be done to ensure initial specimens are collected in this timeframe within your state?
5 Recommendation 2: Newborn screening specimens should be received at the Laboratory within 24 hours of collection. 10. What percent of NBS specimens are received at your laboratory within 24 hours of collection? % of NBS specimens are received at the laboratory within 24 hours of collection My program does not collect this information 11. Please indicate the degree of the factors listed below have on your NBS Program's ability to meet Recommendation 2 (NBS specimens should be received at the laboratory within 24 hours of collection)? All NBS performed out of state Batching of specimens by birthing centers Geographic distance from birthing facility to NBS laboratory Inability to collect data on the date and time of sample send out time at that birthing facility Inability to collect data on the date and time of specimen receipt in the NBS laboratory Laboratory not accessioning specimens on the weekends or holidays Lack of dedicated courier system Lack of education of send-out personnel to send samples daily Lack of feedback provided to birthing hospitals on performance around timeliness of sending specimens to the laboratory Operating hours of courier system Operating hours of laboratory Weather delays Major Moderate Minor No
6 11a. Please describe any other gaps or barriers regarding your NBS program s ability to meet Recommendation What is your NBS program currently doing to ensure that all NBS specimens are received at the laboratory within 24 hours of collection? 13. What are the 3 most critical things that can be done to ensure all NBS specimens are received at the laboratory in this timeframe within your state? Recommendation 3: Newborn screen results for time-critical conditions should be available within 5 days of life. 14. Does your NBS program differentiate conditions based on how time-critical they are? o Yes [please go to questions 15 thru 20] o No [please go to question 21] 15. What conditions are considered time-critical in your state? Please check all that apply. o 3-Hydroxy-3-methylglutaric aciduria (HMG) o Argininosuccinic aciduria (ASA) o B-Ketothiolase (BKT) o Carnitine acylcarnitine translocase deficiency (CACT) o Carnitine palmitoyltransferase type 1 deficiency (CPT1A) o Carnitine palmitoyltransferase type II deficiency (CPT2) o Citrullinemia type II (CIT II) o Citrullinemia, type I (CIT) o Classic galactosemia (GALT) o Congenital adrenal hyperplasia(cah) o Glutaric aciduria type 1 (GA1) o Isovaleric acidemia (IVA) o Long chain L-3-hydroxyacyl-coA dehydrogenase deficiency (LCHAD) o Maple syrup urine disease (MSUD) o Medium chain acyl-coa-dehydrogenase deficiency (MCAD) o Methylmalonic acidemia ( methylmalonyl CoA mutase) (MUT) o Methylmalonic acidemia with hyperhomocysteinemia cobalamin C,D (CblC,D) o Propionic acidemia (PROP) o Trifunctional protein deficiency (TFP) o Very long chain acyl-coa dehydrogenase deficiency (VLCAD) o Other- please specify:
7 16. What percent of NBS results for time-critical conditions are available within 5 days of life? % of NBS results for time-critical conditions are available within 5 days of life? My program does not collect this information 17. What percent of NBS presumptive positive results for time-critical conditions are available within 5 days of life? % of NBS presumptive positive results for time-critical conditions are available within 5 days of life? My program does not collect this information 18. Please indicate the degree of the factors listed below have on your NBS Program's ability to meet Recommendation 3 (Newborn screen results for timecritical conditions should be available within 5 days of life)? All NBS performed out of state Contracts negotiated by state not program Current system not allowing for parsing of specific conditions Home births not reported (delayed screening) Laboratory not accessioning specimens on weekends or holidays Lack of consensus or state guidelines on which conditions are characterized as time-critical Operating hours of courier system Operating hours of NBS laboratory Second tier testing performed Specimen receipt time falls outside of the recommended time frame Staff turnover within the NBS program Major Moderate Minor No 18a. Please describe any other gaps or barriers regarding your NBS program s ability to meet Recommendation 3?
8 19. What is your NBS program currently doing to ensure that NBS results for time-critical conditions are available within 5 days of life? 20. What are the 3 most critical things that can be done to ensure NBS results for timecritical conditions are available within 5 days of life within your state? 21. What is your NBS program s target timeframe for availability of all positive NBS results? Recommendation 4: All NBS results should be available within 5 days of collection. 22. What percent of NBS results are available within 5 days of collection? % of NBS results are available within 5 days of collection My program does not collect this information 23. Please indicate the degree of the factors listed below have on your NBS Program's ability to meet Recommendation 4 (All NBS results should be available within 5 days of collection)? Ability to implement change Major Moderate Minor No All NBS performed out of state Delays in the processes that lead up to release of NBS results (specimen collection, receipt at NBS laboratory, etc.) Nature of the test itself (e.g., Lysosomal storage disorders requiring incubation; retest algorithms) Operating hours of NBS laboratory Release of paper NBS results to submitters via US postal service Second tier testing performed Technical issues and lack of timely support for NBS laboratory activities 23a. Please describe any other gaps or barriers regarding your NBS program s ability to meet Recommendation 4?
9 24. What are the 3 most critical things that can be done to ensure all NBS results for timecritical conditions are available within 5 days of collection within your state? 25. What is your NBS program currently doing to ensure that NBS results are available within 5 days of collection? Section 3: New technology/ new tests and their on timeliness of NBS The next set of questions assess whether new technology or adding new tests (e.g., testing for lysosomal storage disorders (LSDs), severe combined immunodeficiency (SCID)) in the last 8-10 years has ed the ability of the newborn screening system to meet the timeliness goals. Please answer the following questions for your NBS program. 26. Has the use of new technology or adding new tests in your NBS program improved and/or hindered your ability to perform timely newborn screenings (e.g., return of critical results within 5 days of life and/or return of all results within 5 days of sample receipt in the laboratory)? Please check all that apply. Improved [please go to question 26a] Hindered [please go to question 26b] Neither [please go to question 27] 26a. Please describe the improvements from the use of new technology or the addition of new tests in your NBS program. 26b. Please describe the hindrance from the use of new technology or the addition of new tests in your NBS program. 27. Does your laboratory perform 2nd tier molecular testing on the following disorders? Please check all that apply. o Cystic fibrosis o Galactosemia o Medium-chain acyl-coa dehydrogenase deficiency o Sickle cell disease o Very long-chain acyl-coa dehydrogenase deficiency o Other, please specify: Lab does not perform 2nd tier molecular testing [please go to question 29]
10 28. By how much does 2nd tier molecular testing delay notification of your positive NBS results? 1 day 2 days More than 2 days Does not affect notification Cystic fibrosis Galactosemia Medium-chain acyl-coa dehydrogenase deficiency Sickle cell disease Very long-chain acyl-coa dehydrogenase deficiency Other, please specify 28a. Please provide any comments you have regarding your choices from the previous question. 29. Does your laboratory perform 2nd tier tandem mass spectrometry (MS/MS)? o Yes [please go to question 30] o No [end survey] 30. Please list the disorders for which your laboratory performs 2 nd tier MS/MS? Disorder #1 [please go to question 31] Disorder #2 Disorder #3 Disorder #4 o I don t know [end survey] 31. By how much does 2 nd tier MS/MS testing delay notification of your positive NBS results? 1 day 2 days More than 2 days Does not affect notification Disorder #1 Disorder #2 Disorder #3 Disorder #4 31a. Please provide any comments you have regarding your choices from the previous question Thank you for completing the survey!
TIMELINESS OF NEWBORN SCREENING: RECOMMENDATIONS FROM ADVISORY COMMITTEE ON HERITABLE DISORDERS IN NEWBORNS AND CHILDREN
 TIMELINESS OF NEWBORN SCREENING: RECOMMENDATIONS FROM ADVISORY COMMITTEE ON HERITABLE DISORDERS IN NEWBORNS AND CHILDREN Susan Tanksley, PhD May 19, 2015 TIMELINESS - BACKGROUND In order to effectively
TIMELINESS OF NEWBORN SCREENING: RECOMMENDATIONS FROM ADVISORY COMMITTEE ON HERITABLE DISORDERS IN NEWBORNS AND CHILDREN Susan Tanksley, PhD May 19, 2015 TIMELINESS - BACKGROUND In order to effectively
Overview of Newborn Screening, Potential Uses of Residual Dried Blood Spots, and Protection of Privacy
 Overview of Newborn Screening, Potential Uses of Residual Dried Blood Spots, and Protection of Privacy Alan R. Fleischman, M.D. Senior Vice President and Medical Director Chair, Federal Advisory Committee,
Overview of Newborn Screening, Potential Uses of Residual Dried Blood Spots, and Protection of Privacy Alan R. Fleischman, M.D. Senior Vice President and Medical Director Chair, Federal Advisory Committee,
Newborn Screening: Focus on Treatment
 Newborn Screening: Focus on Treatment Alan R. Fleischman, M.D. Senior Vice President and Medical Director March of Dimes National Conference of State Legislatures July 21, 2008 Newborn Screening: A Public
Newborn Screening: Focus on Treatment Alan R. Fleischman, M.D. Senior Vice President and Medical Director March of Dimes National Conference of State Legislatures July 21, 2008 Newborn Screening: A Public
For Your Baby s Health Department of Health
 Newborn Screening For Your Baby s Health Department of Health Why is my baby tested? To help make sure your baby will be as healthy as possible. The blood test provides important information about your
Newborn Screening For Your Baby s Health Department of Health Why is my baby tested? To help make sure your baby will be as healthy as possible. The blood test provides important information about your
Positive Newborn Screens: What do you do next?
 Positive Newborn Screens: What do you do next? James B. Gibson, MD, Ph.D. Biochemical Geneticist at Specially for Children Clinical Associate Professor of Pediatrics UTHSCSA Carla R. Scott, MD Pediatric
Positive Newborn Screens: What do you do next? James B. Gibson, MD, Ph.D. Biochemical Geneticist at Specially for Children Clinical Associate Professor of Pediatrics UTHSCSA Carla R. Scott, MD Pediatric
Newborn Screening in Washington State Saving lives with a simple blood spot
 Newborn Screening in Washington State Saving lives with a simple blood spot Ashleigh Ragsdale, MPH Gauri Gupta, MScPH Objectives Newborn Screening Overview Process and Law Completing Collection Cards Video
Newborn Screening in Washington State Saving lives with a simple blood spot Ashleigh Ragsdale, MPH Gauri Gupta, MScPH Objectives Newborn Screening Overview Process and Law Completing Collection Cards Video
(f) "Birthing center" means any facility that is licensed by the Georgia Department of Community Health as a birthing center;
 Ga. Comp. R. & Regs. r. 511-5-5-.02 Definitions Rule 511-5-5-.02. Definitions (a) "Abnormal test result" is a test result from blood testing or physiologic monitoring that is outside the screening limits
Ga. Comp. R. & Regs. r. 511-5-5-.02 Definitions Rule 511-5-5-.02. Definitions (a) "Abnormal test result" is a test result from blood testing or physiologic monitoring that is outside the screening limits
The Compelling Benefits of Routine 2 nd NBS: A Fifteen-Year Review in Washington State. Saving lives with a simple blood spot
 The Compelling Benefits of Routine 2 nd NBS: A Fifteen-Year Review in Washington State Caroline T. Nucup-Villaruz, MD Primary Author NBS Consultant - Disorder FU Santosh Shaunak Co-Author & Presenter Laboratory
The Compelling Benefits of Routine 2 nd NBS: A Fifteen-Year Review in Washington State Caroline T. Nucup-Villaruz, MD Primary Author NBS Consultant - Disorder FU Santosh Shaunak Co-Author & Presenter Laboratory
NEWBORN SCREENING. in Massachusetts: Answers for You and Your Baby. University of Massachusetts Medical School
 University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Answers for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Answers for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
[R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE PLANTATIONS DEPARTMENT OF HEALTH. February October October 1992 (E) September 1995
![[R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE PLANTATIONS DEPARTMENT OF HEALTH. February October October 1992 (E) September 1995 [R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE PLANTATIONS DEPARTMENT OF HEALTH. February October October 1992 (E) September 1995](/thumbs/95/122505027.jpg) RULES AND REGULATIONS PERTAINING TO THE NEWBORN METABOLIC, ENDOCRINE, AND HEMOGLOBINOPATHY SCREENING PROGRAM AND THE NEWBORN HEARING LOSS SCREENING PROGRAM [R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE
RULES AND REGULATIONS PERTAINING TO THE NEWBORN METABOLIC, ENDOCRINE, AND HEMOGLOBINOPATHY SCREENING PROGRAM AND THE NEWBORN HEARING LOSS SCREENING PROGRAM [R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE
Newborn Metabolic Screening Programme Annual Report
 Newborn Metabolic Screening Programme Annual Report January to December 2015 Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland District Health Board.
Newborn Metabolic Screening Programme Annual Report January to December 2015 Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland District Health Board.
MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES
 NEWBORN SCREENING Protecting your newborn MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES [ 1 ] NEWBORN SCREENING [ 2 ] FREQUENTLY ASKED QUESTIONS [ 4 ] DISORDERS INCLUDED IN NEWBORN SCREENING [ 12 ]
NEWBORN SCREENING Protecting your newborn MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES [ 1 ] NEWBORN SCREENING [ 2 ] FREQUENTLY ASKED QUESTIONS [ 4 ] DISORDERS INCLUDED IN NEWBORN SCREENING [ 12 ]
NEWBORN SCREENING. health.mo.gov/newbornscreening MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES
 NEWBORN SCREENING health.mo.gov/newbornscreening MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES Table of Contents NEWBORN SCREENING...1 FREQUENTLY ASKED QUESTIONS...2 DISORDERS INCLUDED IN NEWBORN SCREENING...4
NEWBORN SCREENING health.mo.gov/newbornscreening MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES Table of Contents NEWBORN SCREENING...1 FREQUENTLY ASKED QUESTIONS...2 DISORDERS INCLUDED IN NEWBORN SCREENING...4
NEWBORN SCREENING. in Massachusetts: Information for You and Your Baby. University of Massachusetts Medical School
 University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Information for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Information for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015
 Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015 Metabolic birth defects can cause physical problems, mental retardation and, in some cases, death. It is
Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015 Metabolic birth defects can cause physical problems, mental retardation and, in some cases, death. It is
Newborn bloodspot testing
 Policy HUMAN GENETICS SOCIETY OF AUSTRALASIA ARBN. 076 130 937 (Incorporated Under the Associations Incorporation Act) The liability of members is limited RACP, 145 Macquarie Street, Sydney NSW 2000, Australia
Policy HUMAN GENETICS SOCIETY OF AUSTRALASIA ARBN. 076 130 937 (Incorporated Under the Associations Incorporation Act) The liability of members is limited RACP, 145 Macquarie Street, Sydney NSW 2000, Australia
Further expansion of the neonatal screening panel in the Netherlands
 Further expansion of the neonatal screening panel in the Netherlands J.Gerard Loeber APHL-NBSGT, St.Louis (MO), USA 290216 Population Area Newborns 6.01 million 0.35:1 16.8 million 180,693 sq km 4.3:1
Further expansion of the neonatal screening panel in the Netherlands J.Gerard Loeber APHL-NBSGT, St.Louis (MO), USA 290216 Population Area Newborns 6.01 million 0.35:1 16.8 million 180,693 sq km 4.3:1
Newborn Screening in Manitoba. Information for Health Care Providers
 Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
Newborn Metabolic Screening Programme. Annual Report
 Newborn Metabolic Screening Programme Annual Report January to December 2016 1 Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland District Health Board.
Newborn Metabolic Screening Programme Annual Report January to December 2016 1 Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland District Health Board.
2015 Annual Report for New Mexico s Newborn Screening (NBS) Program
 2015 Annual Report for New Mexico s Newborn Screening (NBS) Program Sawyer-A Family s Story Inside this Edition A Family Story......1 Why Screen....4 When to Screen...4 What is NBS....5 Screened Conditions..5
2015 Annual Report for New Mexico s Newborn Screening (NBS) Program Sawyer-A Family s Story Inside this Edition A Family Story......1 Why Screen....4 When to Screen...4 What is NBS....5 Screened Conditions..5
Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis)
 Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis) 1) Argininosuccinic acidemia (ASA) a) Incidence: ~1 in 70,000 b) Deficiency in an enzyme of
Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis) 1) Argininosuccinic acidemia (ASA) a) Incidence: ~1 in 70,000 b) Deficiency in an enzyme of
Newborn Screening: What s New?
 Newborn Screening: What s New? Patricia M. Jones, PhD (Department of Pathology, University of Texas Southwestern Medical Center and Children s Medical Center of Dallas, Dallas, TX) DOI: 10.1309/LMOOXWVPSM5FC3B6
Newborn Screening: What s New? Patricia M. Jones, PhD (Department of Pathology, University of Texas Southwestern Medical Center and Children s Medical Center of Dallas, Dallas, TX) DOI: 10.1309/LMOOXWVPSM5FC3B6
Newborn Metabolic Screening Programme
 Newborn Metabolic Screening Programme Annual Report January to December 2017 Released 2018 health.govt.nz Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland
Newborn Metabolic Screening Programme Annual Report January to December 2017 Released 2018 health.govt.nz Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland
NEWBORN METABOLIC SCREEN, MINNESOTA
 Lab Dept: Test Name: Chemistry NEWBORN METABOLIC SCREEN, MINNESOTA General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: PKUN Newborn Screen for Hyopothyroidism, Phenylketonuria (PKU),
Lab Dept: Test Name: Chemistry NEWBORN METABOLIC SCREEN, MINNESOTA General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: PKUN Newborn Screen for Hyopothyroidism, Phenylketonuria (PKU),
Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism
 Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism Patricia Jones, PhD DABCC FACB UT Southwestern Medical Center Children s Medical Center Dallas, Texas Learning Objectives Justify
Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism Patricia Jones, PhD DABCC FACB UT Southwestern Medical Center Children s Medical Center Dallas, Texas Learning Objectives Justify
Considerations in Choosing Screening Conditions: One (US) Approach
 22 Plenary Considerations in Choosing Screening Conditions: One (US) Approach Bradford L Therrell Jr, 1,2 MS, PhD Abstract The lack of a national policy on newborn screening (NBS) in the United States
22 Plenary Considerations in Choosing Screening Conditions: One (US) Approach Bradford L Therrell Jr, 1,2 MS, PhD Abstract The lack of a national policy on newborn screening (NBS) in the United States
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 12/19/17 SECTION: MEDICINE LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 MEDICAL FOODS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
MEDICAL FOODS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
Annual Report Calendar Year 2015
 Annual Report Calendar Year 2015 Page 1 of 19 Contents 1. SAMPLE VOLUMES IN 2015...3 1.1 SCREENING SAMPLES...3 1.1.1 INFANTS SCREENED...3 1.1.2 DECLINED/DEFERRED TESTING...4 1.1.3 MISSED SCREENS...5 1.2
Annual Report Calendar Year 2015 Page 1 of 19 Contents 1. SAMPLE VOLUMES IN 2015...3 1.1 SCREENING SAMPLES...3 1.1.1 INFANTS SCREENED...3 1.1.2 DECLINED/DEFERRED TESTING...4 1.1.3 MISSED SCREENS...5 1.2
Annual Report Calendar Year 2014
 Annual Report Calendar Year 2014 1 Contents 1. SAMPLE VOLUMES IN 2014...4 1.1 SCREENING SAMPLES...4 1.1.1 INFANTS SCREENED...4 1.1.2 DECLINED/DEFERRED TESTING...5 1.1.3 MISSED SCREENS...6 1.2 NON-SCREENING
Annual Report Calendar Year 2014 1 Contents 1. SAMPLE VOLUMES IN 2014...4 1.1 SCREENING SAMPLES...4 1.1.1 INFANTS SCREENED...4 1.1.2 DECLINED/DEFERRED TESTING...5 1.1.3 MISSED SCREENS...6 1.2 NON-SCREENING
Beyond the case for NBS in South Africa. Chris Vorster 28/05/2016
 Beyond the case for NBS in South Africa Chris Vorster 28/05/2016 The case for NBS in SA Economic justification Cost Utility analysis = Cost/QALY GDP/Capita Immediate implementation (WHO) Political justification
Beyond the case for NBS in South Africa Chris Vorster 28/05/2016 The case for NBS in SA Economic justification Cost Utility analysis = Cost/QALY GDP/Capita Immediate implementation (WHO) Political justification
Most common is the congenital adrenogenital syndrome (AGS) or congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency.
 Newborn Screening Examination parameters: TSH-neonatal (hypothyreosis), 17-OH progesterone (AGS), galactose (galactosemia), galactose-uridyl transferase (galacto semia), biotinidase (biotinidase ), phenylalanine
Newborn Screening Examination parameters: TSH-neonatal (hypothyreosis), 17-OH progesterone (AGS), galactose (galactosemia), galactose-uridyl transferase (galacto semia), biotinidase (biotinidase ), phenylalanine
A Guide for Prenatal Educators
 A Guide for Prenatal Educators Why Teach Newborn Screening This booklet is designed to make it easy for you, a prenatal educator, to effectively inform expectant parents about newborn screening. All of
A Guide for Prenatal Educators Why Teach Newborn Screening This booklet is designed to make it easy for you, a prenatal educator, to effectively inform expectant parents about newborn screening. All of
HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up
 HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up Dr. Josephine Chong Clinical Professional Consultant Centre of Inborn Errors
HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up Dr. Josephine Chong Clinical Professional Consultant Centre of Inborn Errors
Attachment 1. Newborn Screening Program Description
 Attachment 1 Newborn Screening Program Description The core mission of Texas Newborn Screening Program is to save children s lives through the early detection of life-threatening disorders. 34 Newborn
Attachment 1 Newborn Screening Program Description The core mission of Texas Newborn Screening Program is to save children s lives through the early detection of life-threatening disorders. 34 Newborn
Inborn Errors of Metabolism From Neonatal Screening to Metabolic Pathways
 19 May 2015 Hospital Authority Convention 2015 Corporate Scholarship Presentation II Paediatric Services Inborn Errors of Metabolism From Neonatal Screening to Metabolic Pathways Dr Grace Poon Associate
19 May 2015 Hospital Authority Convention 2015 Corporate Scholarship Presentation II Paediatric Services Inborn Errors of Metabolism From Neonatal Screening to Metabolic Pathways Dr Grace Poon Associate
Saving Lives Through Newborn Screening: Making the Most of a Simple Blood Test
 Saving Lives Through Newborn Screening: Making the Most of a Simple Blood Test February 26, 2018 John D. Thompson, PhD, MPH, MPA Director - NBS Program Washington State Public Health Laboratories What
Saving Lives Through Newborn Screening: Making the Most of a Simple Blood Test February 26, 2018 John D. Thompson, PhD, MPH, MPA Director - NBS Program Washington State Public Health Laboratories What
2016 Annual Report for New Mexico s Newborn Screening (NBS) Program
 2016 Annual Report for New Mexico s Newborn Screening (NBS) Program Felipe - A Family s Story Inside this Edi on A Family Story..... 1 Why Screen.... 3 When to Screen... 3 What is NBS... 4 Screened Condi
2016 Annual Report for New Mexico s Newborn Screening (NBS) Program Felipe - A Family s Story Inside this Edi on A Family Story..... 1 Why Screen.... 3 When to Screen... 3 What is NBS... 4 Screened Condi
Annual Report to the Newborn Screening Ontario Advisory Council Calendar Year 2017
 Annual Report to the Newborn Screening Ontario Advisory Council Calendar Year 2017 415 Smyth Road, Ottawa Ontario K1H 8M8 Phone: 6137383222 1877NBS8330 Fax: 6137380853 Contents 1. SAMPLE VOLUMES IN 2017...4
Annual Report to the Newborn Screening Ontario Advisory Council Calendar Year 2017 415 Smyth Road, Ottawa Ontario K1H 8M8 Phone: 6137383222 1877NBS8330 Fax: 6137380853 Contents 1. SAMPLE VOLUMES IN 2017...4
How MS/MS Revolutionized Newborn Screening
 How MS/MS Revolutionized Newborn Screening David S Millington, PhD Medical Research Professor of Pediatrics Director, Biochemical Genetics Laboratory Duke University Medical Center NEWBORN SCREENING IN
How MS/MS Revolutionized Newborn Screening David S Millington, PhD Medical Research Professor of Pediatrics Director, Biochemical Genetics Laboratory Duke University Medical Center NEWBORN SCREENING IN
What s new in newborn screening?
 PERSPECTIVE What s new in newborn screening? Bradford L Therrell Jr 1,2, Colleen Buechner 1,2, Michele A Lloyd-Puryear 3,4, Peter C van Dyck 3,5 & Marie Y Mann 3,6 Author for correspondence 1 National
PERSPECTIVE What s new in newborn screening? Bradford L Therrell Jr 1,2, Colleen Buechner 1,2, Michele A Lloyd-Puryear 3,4, Peter C van Dyck 3,5 & Marie Y Mann 3,6 Author for correspondence 1 National
Arizona Newborn Screening Program
 ARIZONA DEPARTMENT OF HEALTH SERVICES BUREAU OF STATE LABORATORY SERVICES Arizona Newborn Screening Program GUIDELINES DIVISION OF PUBLIC HEALTH SERVICES AUGUST 2010 with revisions (January 2011) Table
ARIZONA DEPARTMENT OF HEALTH SERVICES BUREAU OF STATE LABORATORY SERVICES Arizona Newborn Screening Program GUIDELINES DIVISION OF PUBLIC HEALTH SERVICES AUGUST 2010 with revisions (January 2011) Table
Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry: Experience of the Pilot Study in Andhra Pradesh, India
 Indian J Pediatr (August 2011) 78(8):953 960 DOI 10.1007/s12098-011-0398-9 ORIGINAL ARTICLE Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry: Experience of the Pilot Study
Indian J Pediatr (August 2011) 78(8):953 960 DOI 10.1007/s12098-011-0398-9 ORIGINAL ARTICLE Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry: Experience of the Pilot Study
Washington State Newborn Screening
 Washington State Newborn Screening SAVING LIVES WITH A SIMPLE BLOOD SPOT WA State DOH 1 Washington State Numbers 1 2 173,000 specimens 90,000 newborns 2-screen state NBS 17 lab staff 5 disorder FU staff
Washington State Newborn Screening SAVING LIVES WITH A SIMPLE BLOOD SPOT WA State DOH 1 Washington State Numbers 1 2 173,000 specimens 90,000 newborns 2-screen state NBS 17 lab staff 5 disorder FU staff
For the Ongoing Management of Babies under 1 year old.
 Pathway Author: Nicy Turney, Senior Nurse Professional Lead, Health Visiting 19.06.17 For the Ongoing Management of Babies under 1 year old. Check Health Records (CHR) to undertake daily checks to identify:
Pathway Author: Nicy Turney, Senior Nurse Professional Lead, Health Visiting 19.06.17 For the Ongoing Management of Babies under 1 year old. Check Health Records (CHR) to undertake daily checks to identify:
Newborn screening for additional metabolic diseases using second-tier strategies Juergen G. Okun, PhD
 Newborn screening for additional metabolic diseases using second-tier strategies Juergen G. Okun, PhD 5th EFLM-UEMS European Joint Congress in Laboratory Medicine 10-13 October 2018 in Antalya, Turkey
Newborn screening for additional metabolic diseases using second-tier strategies Juergen G. Okun, PhD 5th EFLM-UEMS European Joint Congress in Laboratory Medicine 10-13 October 2018 in Antalya, Turkey
Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol
 Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Version 7 Effective Date: 15 th February 2016
Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Version 7 Effective Date: 15 th February 2016
IHE Quality, Research, and Public Health (QRPH) White Paper Newborn Screening
 Integrating the Healthcare Enterprise 5 IHE Quality, Research, and Public Health (QRPH) White Paper 2009-2010 10 Newborn Screening Version 0.3 15 Date: September 1, 2009 Main Editor: Anna Orlova Contributors:
Integrating the Healthcare Enterprise 5 IHE Quality, Research, and Public Health (QRPH) White Paper 2009-2010 10 Newborn Screening Version 0.3 15 Date: September 1, 2009 Main Editor: Anna Orlova Contributors:
Newborn Screening: Blood Spot Disorders
 Newborn Screening: Blood Spot Disorders Arizona s Newborn Screening Program Program Overview Panel of Disorders Disorder Descriptions Program Components Hospitals ADHS Lab ADHS Follow-up ADHS Billing Medical
Newborn Screening: Blood Spot Disorders Arizona s Newborn Screening Program Program Overview Panel of Disorders Disorder Descriptions Program Components Hospitals ADHS Lab ADHS Follow-up ADHS Billing Medical
Proposal for EXPANDED NEWBORN SCREENING
 Proposal for EXPANDED NEWBORN SCREENING Tel:+971-4-4503875 Fax:+971-4-4503874 DuBiotech, P.O.Box 212671Dubai, UAE. Email: info@easternbiotech.com www.easternbiotech.com Winner of Dubai SME100 Ranked #19
Proposal for EXPANDED NEWBORN SCREENING Tel:+971-4-4503875 Fax:+971-4-4503874 DuBiotech, P.O.Box 212671Dubai, UAE. Email: info@easternbiotech.com www.easternbiotech.com Winner of Dubai SME100 Ranked #19
ERNDIM QA Scheme for qualitative blood spot acylcarnitine analysis. Annual Report 2010
 UniversitätsKlinikum Heidelberg Universitätsklinik für Kinder- und Jugendmedizin Stoffwechselzentrum Heidelberg Stoffwechsellabor Im Neuenheimer Feld 430 69120 Heidelberg To Stoffwechselzentrum Heidelberg
UniversitätsKlinikum Heidelberg Universitätsklinik für Kinder- und Jugendmedizin Stoffwechselzentrum Heidelberg Stoffwechsellabor Im Neuenheimer Feld 430 69120 Heidelberg To Stoffwechselzentrum Heidelberg
Newborn Screening in Japan: Restructuring for the New Era
 Plenary 13 Newborn Screening in Japan: Restructuring for the New Era Seiji Yamaguchi, 1 MD Abstract Nationwide neonatal mass screening for inherited metabolic diseases has started in Japan since 1977.
Plenary 13 Newborn Screening in Japan: Restructuring for the New Era Seiji Yamaguchi, 1 MD Abstract Nationwide neonatal mass screening for inherited metabolic diseases has started in Japan since 1977.
Short and Long Term Stability of 3-Hydroxy acylcarnitines Enriched Dried Blood Spots Stored at Various Temperatures and Humidities
 Short and Long Term Stability of 3-Hydroxy acylcarnitines Enriched Dried Blood Spots Stored at Various Temperatures and Humidities Timothy Lim Biochemical Mass Spectrometry Laboratory Newborn Screening
Short and Long Term Stability of 3-Hydroxy acylcarnitines Enriched Dried Blood Spots Stored at Various Temperatures and Humidities Timothy Lim Biochemical Mass Spectrometry Laboratory Newborn Screening
State of the Art of Rare Disease - Activities in EU Member States and Other European Countries. Portugal Report
 State of the Art of Rare Disease - Activities in EU Member States and Other European Countries Definition of a Rare Disease Portugal Report Portugal has adopted the European Commission definition of a
State of the Art of Rare Disease - Activities in EU Member States and Other European Countries Definition of a Rare Disease Portugal Report Portugal has adopted the European Commission definition of a
A Brief History of Newborn Screening
 A Brief History of Newborn Screening The first 50 years. Ken Pass I didn t do this alone Thanks to: Amy Hoffman Alex Kemper Kathy Harris Piero Rinaldo and others 1902 Liv Dag Asbjorn Follin Pediatrics
A Brief History of Newborn Screening The first 50 years. Ken Pass I didn t do this alone Thanks to: Amy Hoffman Alex Kemper Kathy Harris Piero Rinaldo and others 1902 Liv Dag Asbjorn Follin Pediatrics
Screening Newborns for Congenital Disorders
 Screening Newborns for Congenital Disorders Gary L. Hoffman, BS; Ronald H. Laessig, PhD ABSTRACT The Newborn Screening Laboratory at the Wisconsin State Laboratory of Hygiene (WSLH) tests all newborn babies
Screening Newborns for Congenital Disorders Gary L. Hoffman, BS; Ronald H. Laessig, PhD ABSTRACT The Newborn Screening Laboratory at the Wisconsin State Laboratory of Hygiene (WSLH) tests all newborn babies
Sequencing in Newborn Screening Introduction and Background
 Sequencing in Newborn Screening Introduction and Background Suzanne Cordovado, PhD Newborn Screening and Molecular Biology Branch Division of Laboratory Sciences Centers for Disease Control and Prevention
Sequencing in Newborn Screening Introduction and Background Suzanne Cordovado, PhD Newborn Screening and Molecular Biology Branch Division of Laboratory Sciences Centers for Disease Control and Prevention
Inborn Errors of Metabolism. Metabolic Pathway. Digestion and Fasting. How is Expanded Newborn Screening Different? MS/MS. The body is a factory.
 Inborn Errors of Metabolism The body is a factory. Inborn errors of metabolism are rare genetic disorders in which the body cannot properly turn food into energy. The disorders are usually caused by defects
Inborn Errors of Metabolism The body is a factory. Inborn errors of metabolism are rare genetic disorders in which the body cannot properly turn food into energy. The disorders are usually caused by defects
Inborn Errors of Metabolism (IEM)
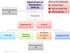 Clinical Presentation Inborn Errors of Metabolism (IEM) Click on the following: - Clinical Pearl - link to movie clip - link to picture Investigations Blood Work Urine No Acidosis NH 4 + Metabolic Acidosis
Clinical Presentation Inborn Errors of Metabolism (IEM) Click on the following: - Clinical Pearl - link to movie clip - link to picture Investigations Blood Work Urine No Acidosis NH 4 + Metabolic Acidosis
Alvaro Serrano Russi MD University of Iowa Hospitals and Clinics
 Retrospective Analysis of the Region 4 Post Analytical Tool and Confirmatory Testing for Long Chain Fatty Acid Oxidation Disorders Screened in the State of Iowa Alvaro Serrano Russi MD University of Iowa
Retrospective Analysis of the Region 4 Post Analytical Tool and Confirmatory Testing for Long Chain Fatty Acid Oxidation Disorders Screened in the State of Iowa Alvaro Serrano Russi MD University of Iowa
The Changing Moral Focus of Newborn Screening: An Ethical Analysis by the President s Council on Bioethics
 The Changing Moral Focus of Newborn Screening: An Ethical Analysis by the President s Council on Bioethics Appendix Newborn Screening: An International Survey By Joseph A. Raho, Research Analyst In addition
The Changing Moral Focus of Newborn Screening: An Ethical Analysis by the President s Council on Bioethics Appendix Newborn Screening: An International Survey By Joseph A. Raho, Research Analyst In addition
Mississippi Newborn Screening Report
 Mississippi Newborn Screening Report 2011-2016 Introduction The primary goal of the Newborn Screening Program is to screen every infant born in the state and refer infants with abnormal results to appropriate
Mississippi Newborn Screening Report 2011-2016 Introduction The primary goal of the Newborn Screening Program is to screen every infant born in the state and refer infants with abnormal results to appropriate
Diagnose a broad range of metabolic disorders with a single test, Global MAPS
 PEDIATRIC Assessing or diagnosing a metabolic disorder commonly requires several tests. Global Metabolomic Assisted Pathway Screen, commonly known as Global MAPS, is a unifying test for analyzing hundreds
PEDIATRIC Assessing or diagnosing a metabolic disorder commonly requires several tests. Global Metabolomic Assisted Pathway Screen, commonly known as Global MAPS, is a unifying test for analyzing hundreds
Metabolic Changes in ASD. Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius
 Metabolic Changes in ASD Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius 12 patients 3 Autism: Ages 3/3/3.7 3 PDD: Ages 3/3/6 3 Asperger: Ages 6/7/15.1 3 Speech delay and Sensory Problems (SHL):
Metabolic Changes in ASD Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius 12 patients 3 Autism: Ages 3/3/3.7 3 PDD: Ages 3/3/6 3 Asperger: Ages 6/7/15.1 3 Speech delay and Sensory Problems (SHL):
GUIDE TO NEWBORN SCREENING PROGRAMME
 \ GUIDE TO NEWBORN SCREENING PROGRAMME 1 MEDILAB PROFILE MEDILAB, the leading independent provider of Clinical Laboratory Diagnostic Services in Cyprus, was established in 1980 by Mr. C. Pavlides and has
\ GUIDE TO NEWBORN SCREENING PROGRAMME 1 MEDILAB PROFILE MEDILAB, the leading independent provider of Clinical Laboratory Diagnostic Services in Cyprus, was established in 1980 by Mr. C. Pavlides and has
Utility of Microarrays in Molecular Genetics
 Utility of Microarrays in Molecular Genetics Madhuri Hegde, Ph.D., FACMG Associate Professor Senior Director Department of Human Genetics Emory Genetics Laboratory Emory University School of Medicine Atlanta,
Utility of Microarrays in Molecular Genetics Madhuri Hegde, Ph.D., FACMG Associate Professor Senior Director Department of Human Genetics Emory Genetics Laboratory Emory University School of Medicine Atlanta,
Post mortem investigation of Inherited Metabolic Disease - the last opportunity for a diagnosis -
 Post mortem investigation of Inherited Metabolic Disease - the last opportunity for a diagnosis - Dr Simon Olpin Lead Clinical Scientist in Inherited Metabolic Disease Sheffield Children s Hospital SIDS/SUDI
Post mortem investigation of Inherited Metabolic Disease - the last opportunity for a diagnosis - Dr Simon Olpin Lead Clinical Scientist in Inherited Metabolic Disease Sheffield Children s Hospital SIDS/SUDI
Health and Wellness for all Arizonans. azdhs.gov
 To identify newborns with certain, rare disorders and assist families of affected infants so that they receive appropriate and timely treatment to prevent or delay serious medical problems. To identify
To identify newborns with certain, rare disorders and assist families of affected infants so that they receive appropriate and timely treatment to prevent or delay serious medical problems. To identify
Newborn Bloodspot Screening Information for parents
 Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
QA/QC 2 Strategies to Reduce False Positives and False Negatives (Part 2)
 QA/QC 2 Strategies to Reduce False Positives and False Negatives (Part 2) Tuesday, Oct. 28 10:30am-12:00pm Moderators Patricia Hunt, Texas Department of State Health Services and Bob Currier, California
QA/QC 2 Strategies to Reduce False Positives and False Negatives (Part 2) Tuesday, Oct. 28 10:30am-12:00pm Moderators Patricia Hunt, Texas Department of State Health Services and Bob Currier, California
NEONATAL SCREENING FOR INBORN ERRORS OF METABOLISM OUR EXPERIENCE AT CABRI, GULF MEDICAL UNIVERSITY
 GMJ GULF MEDICAL JOURNAL ORAL PROCEEDINGS NEONATAL SCREENING FOR INBORN ERRORS OF METABOLISM OUR EXPERIENCE AT CABRI, GULF MEDICAL UNIVERSITY I.A. Shaafie 1 *, A.D. Vijay Raju 2, P.K. Menon 3 1Division
GMJ GULF MEDICAL JOURNAL ORAL PROCEEDINGS NEONATAL SCREENING FOR INBORN ERRORS OF METABOLISM OUR EXPERIENCE AT CABRI, GULF MEDICAL UNIVERSITY I.A. Shaafie 1 *, A.D. Vijay Raju 2, P.K. Menon 3 1Division
Newborn Bloodspot Screening Information for parents
 Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Assessing the follow-up of BART hemoglobin reported by the Wisconsin Newborn Screening Laboratory
 Assessing the follow-up of BART hemoglobin reported by the Wisconsin Newborn Screening Laboratory Bao Yang MPH Candidate Background The Wisconsin Newborn Screening (NBS) Laboratory screens 70,000 babies
Assessing the follow-up of BART hemoglobin reported by the Wisconsin Newborn Screening Laboratory Bao Yang MPH Candidate Background The Wisconsin Newborn Screening (NBS) Laboratory screens 70,000 babies
ERNDIM QA Scheme for Qualitative Blood Spot Acylcarnitine Analysis. Annual Report 2011
 UniversitätsKlinikum Heidelberg University Children s Hospital Metabolic Laboratory Im Neuenheimer Feld 430 69120 Heidelberg To University Children s Hospital Angelika-Lautenschläger-Klinik Department
UniversitätsKlinikum Heidelberg University Children s Hospital Metabolic Laboratory Im Neuenheimer Feld 430 69120 Heidelberg To University Children s Hospital Angelika-Lautenschläger-Klinik Department
Joshua Hellmann Foundation - Newborn Metabolic Screening Program
 Joshua Hellmann Foundation - Newborn Metabolic Screening Program Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Last update: 11 June 2013 Target inborn errors of metabolism (IEM)
Joshua Hellmann Foundation - Newborn Metabolic Screening Program Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Last update: 11 June 2013 Target inborn errors of metabolism (IEM)
Selective newborn screening of amino acid, fatty acid and organic acid disorders in the Kingdom of Bahrain.
 Selective newborn screening of amino acid, fatty acid and organic acid disorders in the Kingdom of Bahrain. Jamal Golbahar PhD Associate Professor of Molecular Medicine, Department of Molecular Medicine,
Selective newborn screening of amino acid, fatty acid and organic acid disorders in the Kingdom of Bahrain. Jamal Golbahar PhD Associate Professor of Molecular Medicine, Department of Molecular Medicine,
Newborn Screening Manual
 Newborn Screening Manual A GUIDE FOR NEWBORN CARE PROVIDERS N ONATAL Newborn Screening Manual: A guide for newborn care providers This manual was created by Newborn Screening Ontario (NSO) as a comprehensive
Newborn Screening Manual A GUIDE FOR NEWBORN CARE PROVIDERS N ONATAL Newborn Screening Manual: A guide for newborn care providers This manual was created by Newborn Screening Ontario (NSO) as a comprehensive
Assessing Mothers Knowledge of the Wisconsin Newborn Screening Program. Sara Wolfgram UW Master of Public Health Symposium August 11, 2006
 Assessing Mothers Knowledge of the Wisconsin Newborn Screening Program Sara Wolfgram UW Master of Public Health Symposium August 11, 2006 Background Wisconsin Newborn Screening (NBS): Began in 1965 Heel
Assessing Mothers Knowledge of the Wisconsin Newborn Screening Program Sara Wolfgram UW Master of Public Health Symposium August 11, 2006 Background Wisconsin Newborn Screening (NBS): Began in 1965 Heel
Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet
 Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet Website:www.tipn.org.tw Telephone:(02)85962050 Ext. 401-403 Service line:(02)85962065 Fax:(02)85962067
Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet Website:www.tipn.org.tw Telephone:(02)85962050 Ext. 401-403 Service line:(02)85962065 Fax:(02)85962067
Executive summary. Criteria for inclusion in the screening programme
 Executive summary Health Council of the Netherlands. Neonatal screening: new recommendations. The Hague: Health Council of the Netherlands, 2015; publication no. 2015/08 Shortly after birth, almost every
Executive summary Health Council of the Netherlands. Neonatal screening: new recommendations. The Hague: Health Council of the Netherlands, 2015; publication no. 2015/08 Shortly after birth, almost every
Newborn Metabolic Screening Programme. Annual Report
 Newborn Metabolic Screening Programme Annual Report January to December 2014 Copyright The copyright owner of this publication is the Ministry of Health, which is part of the New Zealand Crown. The Ministry
Newborn Metabolic Screening Programme Annual Report January to December 2014 Copyright The copyright owner of this publication is the Ministry of Health, which is part of the New Zealand Crown. The Ministry
Short Term Follow Up Tandem Mass Spectrometry Workshop Agenda
 Short Term Follow Up Tandem Mass Spectrometry Workshop Agenda June 11-15, 2018 APHL Headquarters 8515 Georgia Ave Silver Spring, MD 20910 This meeting is sponsored by the Association of Public Health Laboratories
Short Term Follow Up Tandem Mass Spectrometry Workshop Agenda June 11-15, 2018 APHL Headquarters 8515 Georgia Ave Silver Spring, MD 20910 This meeting is sponsored by the Association of Public Health Laboratories
2017 Newborn Screening and Genetic Testing Symposium September 10, 2017
 Removing Short-chain Acyl-CoA Dehydrogenase (SCAD) Deficiency and Isobutyryl-CoA Dehydrogenase Deficiency (IBD) from the Newborn Screening Panel: Michigan s Experience 2017 Newborn Screening and Genetic
Removing Short-chain Acyl-CoA Dehydrogenase (SCAD) Deficiency and Isobutyryl-CoA Dehydrogenase Deficiency (IBD) from the Newborn Screening Panel: Michigan s Experience 2017 Newborn Screening and Genetic
Green Amber Red Assurance Level
 A re-audit of the turnaround time of samples for the Sickle Cell and Thalassaemia Newborn Screening Programme, in the Manchester Newborn Screening Laboratory Clinical Audit Report May 2014 Clinical Audit
A re-audit of the turnaround time of samples for the Sickle Cell and Thalassaemia Newborn Screening Programme, in the Manchester Newborn Screening Laboratory Clinical Audit Report May 2014 Clinical Audit
MS/MS APPROACHES TO CLINICAL TESTING FOR INBORN ERRORS OF METABOLISM
 MS/MS APPROACHES TO CLINICAL TESTING FOR INBORN ERRORS OF METABOLISM Tina M. Cowan, PhD Director, Clinical Biochemical Genetics Laboratory Stanford University Medical Center Presented at the 2011 Stanford
MS/MS APPROACHES TO CLINICAL TESTING FOR INBORN ERRORS OF METABOLISM Tina M. Cowan, PhD Director, Clinical Biochemical Genetics Laboratory Stanford University Medical Center Presented at the 2011 Stanford
Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol
 Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Last update: 14 February 2014 (version 6) Joshua
Joshua Hellmann Foundation Newborn Metabolic Screening Program Clinical Protocol Centre of Inborn Errors of Metabolism The Chinese University of Hong Kong Last update: 14 February 2014 (version 6) Joshua
e-learning Fatty Acid Oxidation Defects Camilla Reed and Dr Simon Olpin Sheffield Children s Hospital
 e-learning Fatty Acid Oxidation Defects Camilla Reed and Dr Simon Olpin Sheffield Children s Hospital Fatty Acids Fatty acids are a major source of energy and body fat is an energy dense material. They
e-learning Fatty Acid Oxidation Defects Camilla Reed and Dr Simon Olpin Sheffield Children s Hospital Fatty Acids Fatty acids are a major source of energy and body fat is an energy dense material. They
Pilot Study of Newborn Screening (NBS) for Inborn Errors of Metabolism (IEM) in Collaboration with Department of Health and Hospital Authority
 HA Convention 2017 Service Enhancement Presentation Healthcare Advances, Research and Innovations Pilot Study of Newborn Screening (NBS) for Inborn Errors of Metabolism (IEM) in Collaboration with Department
HA Convention 2017 Service Enhancement Presentation Healthcare Advances, Research and Innovations Pilot Study of Newborn Screening (NBS) for Inborn Errors of Metabolism (IEM) in Collaboration with Department
Newborn Screening. Helping babies start life healthy. Minnesota Newborn Screening Program
 Newborn Screening Helping babies start life healthy Minnesota Newborn Screening Program What is newborn screening? Newborn screening is a set of three tests that check babies for serious, rare conditions.
Newborn Screening Helping babies start life healthy Minnesota Newborn Screening Program What is newborn screening? Newborn screening is a set of three tests that check babies for serious, rare conditions.
BROADENING YOUR PATIENT S OPTIONS FOR GENETIC CARRIER SCREENING.
 BROADENING YOUR PATIENT S OPTIONS FOR GENETIC CARRIER SCREENING. The Inheritest SM Carrier Screen provides relevant genetic screening for many inherited diseases found throughout the pan-ethnic US population.
BROADENING YOUR PATIENT S OPTIONS FOR GENETIC CARRIER SCREENING. The Inheritest SM Carrier Screen provides relevant genetic screening for many inherited diseases found throughout the pan-ethnic US population.
Cost-Effectiveness of Expanded Newborn Screening in Texas
 Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jval Cost-Effectiveness of Expanded Newborn Screening in Texas Simrandeep K. Tiwana, MBA, PhD 1,2, *,, Karen L. Rascati,
Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jval Cost-Effectiveness of Expanded Newborn Screening in Texas Simrandeep K. Tiwana, MBA, PhD 1,2, *,, Karen L. Rascati,
So Much More Than The PKU Test
 Newborn Metabolic Screening So Much More Than The PKU Test Sarah Viall, MSN, PPCNP BC Newborn Screening Program Coordinator Division of Genetics & Metabolism Conflicts of Interest I have no conflicts of
Newborn Metabolic Screening So Much More Than The PKU Test Sarah Viall, MSN, PPCNP BC Newborn Screening Program Coordinator Division of Genetics & Metabolism Conflicts of Interest I have no conflicts of
Curr Pediatr Res 2017; 21 (4):
 Curr Pediatr Res 2017; 21 (4): 665-673 ISSN 0971-9032 www.currentpediatrics.com The frequency of inherited metabolic and endocrine disorders in the eastern and north-western Jawf provinces of : Four years
Curr Pediatr Res 2017; 21 (4): 665-673 ISSN 0971-9032 www.currentpediatrics.com The frequency of inherited metabolic and endocrine disorders in the eastern and north-western Jawf provinces of : Four years
Slide 1: Newborn Bloodspot Screening for Medium-chain Acyl-CoA Dehydrogenase Deficiency (MCADD)
 Slide 1: Newborn Bloodspot Screening for Medium-chain Acyl-CoA Dehydrogenase Deficiency (MCADD) The information has been produced with thanks to the NHS Newborn Blood Spot Screening Programme. 1 Slide
Slide 1: Newborn Bloodspot Screening for Medium-chain Acyl-CoA Dehydrogenase Deficiency (MCADD) The information has been produced with thanks to the NHS Newborn Blood Spot Screening Programme. 1 Slide
Medium-chain acyl-coa dehydrogenase (MCAD) deficiency and Expanded Newborn Screening in Japan
 Medium-chain acyl-coa dehydrogenase (MCAD) deficiency and Expanded Newborn Screening in Japan Seiji YAMAGUCHI, MD Department of Pediatrics, Shimane University (co-authors) Jamiyan Purevsuren, Yuki Hasegawa,
Medium-chain acyl-coa dehydrogenase (MCAD) deficiency and Expanded Newborn Screening in Japan Seiji YAMAGUCHI, MD Department of Pediatrics, Shimane University (co-authors) Jamiyan Purevsuren, Yuki Hasegawa,
The Many Lives of the Newborn Screening Dried Blood Spot (the LIFE CYCLE of a Blood Spot)
 The Many Lives of the Newborn Screening Dried Blood Spot (the LIFE CYCLE of a Blood Spot) Piero Rinaldo, MD, PhD Professor of Laboratory Medicine T. Denny Sanford Professor of Pediatrics Mayo Clinic College
The Many Lives of the Newborn Screening Dried Blood Spot (the LIFE CYCLE of a Blood Spot) Piero Rinaldo, MD, PhD Professor of Laboratory Medicine T. Denny Sanford Professor of Pediatrics Mayo Clinic College
VLCAD At a Glance. Before VLCAD was included on the newborn screening test, three types of VLCAD were recognized by the severity of the condition:
 VLCAD At a Glance VLCAD is one of several *Fatty Acid Oxidation Disorders (FAOD) in which there is an inability to break down certain fats, caused by an enzyme deficiency. This results in a decreased ability
VLCAD At a Glance VLCAD is one of several *Fatty Acid Oxidation Disorders (FAOD) in which there is an inability to break down certain fats, caused by an enzyme deficiency. This results in a decreased ability
Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme
 Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme Charles Turner Laboratory Guy s Hospital (Evelina Childrens Hospital, St Thomas
Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme Charles Turner Laboratory Guy s Hospital (Evelina Childrens Hospital, St Thomas
Mississippi Newborn Screening Guide for Healthcare Providers
 Mississippi Newborn Screening Guide for Healthcare Providers Mississippi State Department of Health Office of Child & Adolescent Health Bureau of Genetic Services October 2015 TABLE OF CONTENTS Introduction...
Mississippi Newborn Screening Guide for Healthcare Providers Mississippi State Department of Health Office of Child & Adolescent Health Bureau of Genetic Services October 2015 TABLE OF CONTENTS Introduction...
Newborn Metabolic Screening Programme. Annual Report
 Newborn Metabolic Screening Programme Annual Report January to December 2011 Copyright The copyright owner of this publication is the Ministry of Health, which is part of the New Zealand Crown. The Ministry
Newborn Metabolic Screening Programme Annual Report January to December 2011 Copyright The copyright owner of this publication is the Ministry of Health, which is part of the New Zealand Crown. The Ministry
