Lauren E. Cipriano, BSc, BA, 1 C. Anthony Rupar, PhD, 2 Gregory S. Zaric, PhD 1. Introduction
|
|
|
- Bruce Phillips
- 5 years ago
- Views:
Transcription
1 Blackwell Publishing IncMalden, USAVHEValue in Health Blackwell Publishing Original ArticleCost-Effectiveness of Newborn ScreeningCipriano et al. Volume 10 Number VALUE IN HEALTH The Cost-Effectiveness of Expanding Newborn Screening for up to 21 Inherited Metabolic Disorders Using Tandem Mass Spectrometry: Results from a Decision-Analytic Model Lauren E. Cipriano, BSc, BA, 1 C. Anthony Rupar, PhD, 2 Gregory S. Zaric, PhD 1 1 Richard Ivey School of Business, University of Western Ontario, London, ON, Canada; 2 Child and Parent Resource Institute, Children s Health Research Foundation, and Departments of Biochemistry and Paediatrics, University of Western Ontario, London, ON, Canada ABSTRACT Objectives: In 2005, in Ontario, Canada, newborns were only screened for phenylketonuria (PKU) and hypothyroidism. Tandem mass spectrometry (MS/MS) has since been implemented as a new screening technology because it can screen for PKU and many other diseases simultaneously. We estimated the cost-effectiveness of using this technology to expand the Ontario newborn screening program to screen for each disease independently and for hypothetical bundles of up to 21 metabolic diseases. Methods: We constructed a decision-analytic model to estimate the incremental costs and life-years of survival that can be gained by screening or changing screening technologies. Costs and health benefits were estimated for a cohort of babies born in Ontario in 1 year. Secondary sources and expert opinion were used to estimate the test characteristics, disease prevalence, treatment effectiveness, disease progression rates, and mortality. The London Health Sciences Centre Case Costing Initiative, the Ontario Health Insurance Plan Schedule, and the Ontario Drug Benefits plan formulary were used to estimate costs. Results: Changing screening technologies, from the Guthrie test to MS/MS, for PKU detection had an incremental cost of $5,500,000 per life-year (LY) gained. We identified no diseases for which the incremental cost of screening for just that disease was less than $100,000 per LY gained. The incremental costs of screening ranged from $222,000 (HMG-CoA lyase deficiency) to $142,500,000 (glutaric acidemia type II) per LY gained. Screening for a bundle of diseases including PKU and the 14 most cost-effective diseases to screen for cost less than $70,000 per LY gained, and the incremental costeffectiveness of adding each of the 14 diseases to the bundle was less than $100,000 per LY gained. The incremental cost of adding the 15th most cost-effective disease was $309,400 per LY gained. Conclusions: Early diagnosis and treatment of metabolic disease is important to reduce disease severity and delay or prevent the onset of the disease. Screening at birth reduces the morbidity, mortality, and social burden associated with the irreversible effects of disease on the population. Our analysis suggests that the cost-efficiencies gained by using MS/MS to screen for bundles of diseases rather than just one disease are sufficient to warrant consideration of an expanded screening program. It is, however, not costeffective to screen for all diseases that can be screened for using this technology. Keywords: cost-effectiveness, infant mortality, metabolic disease, newborn screening, policy. Introduction Screening newborns for metabolic disease began with the screening of phenylketonuria (PKU) in the 1960s, but technological advancements in many diagnostic areas have led to the expansion of newborn screening to include other inborn errors of metabolism (IEMs), hemoglobinopathies, hormone deficiencies, enzyme deficiencies, and hearing impairment. The use of tandem mass spectrometry (MS/MS) in newborn screening programs enables screening for 30 or more Address correspondence to: Gregory S. Zaric, Richard Ivey School of Business, 1151 Richmond Street, N. University of Western Ontario London, ON N6A 3K7, Canada. gzaric@ivey.uwo.ca /j x metabolic disorders in a single analysis through the differentiation and quantitation of multiple molecules within a single specimen [1]. Elevation of one biochemical marker may indicate that an infant has one of several diseases, whereas the elevation of a combination of markers may lead physicians to a specific diagnosis more quickly. At birth, most infants born with an IEM do not have symptoms, although infants with some disorders can present acutely within the first few days of life. Typically, these early presentations include metabolic acidosis, hyperammonemia, hypoglycemia, or seizures. Children with an IEM may present later in life with physical or developmental delay, or metabolic crises. Many IEMs can be treated by restrictive diets and medications to greatly reduce and prevent mortality 2007, International Society for Pharmacoeconomics and Outcomes Research (ISPOR) /07/
2 84 and morbidity, including mental retardation, severe neurological disease, and physical deformity [2 9]. Early diagnosis and treatment generally improves outcomes. Screening programs are often designed to screen all infants for selected IEMs and identify those children that require further investigation to obtain a definitive diagnosis so that appropriate treatment can begin before clinical symptoms develop. Although each individual IEM is rare, summing the incidences of the 21 metabolic diseases discussed in this article indicates that these diseases collectively affect approximately 1 in every 3000 babies born in Ontario. A dried blood spot (DBS) is collected from every newborn in Ontario. Between 1965 and 2006 the DBS was tested for abnormally high levels of phenylalanine using the Guthrie bacterial inhibition assay [10], and a positive PKU diagnosis was confirmed by a quantitative amino acid test on a second blood sample. Babies with other diseases were diagnosed after clinical presentation. Diagnosis of an IEM can be challenging because the disorders are individually rare, symptoms are often similar to those observed in acquired diseases, and the metabolic decompensations are often Cipriano et al. associated with intercurrent illnesses. Treatment begins once a diagnosis is made; however, irreversible damage may have already occurred. The American College of Medical Genetics recommends universal screening for 29 diseases, including 17 diseases that can be screened for using MS/MS [11]. Nevertheless, determining which diseases are appropriate for inclusion in screening programs is controversial, involving both scientific and political judgments [12 16]. In 2005, screening programs across Canada varied widely, ranging from 2 to 29 diseases, with only Ontario and New Brunswick screening for two diseases [17]. In 2000, two-thirds of US states screened for six or fewer diseases [18], whereas in 2005, every state screened for more than eight diseases, and two-thirds of states screened for more than 30 diseases [19]. Model-based estimates for the cost-effectiveness of screening are available for PKU [20 22], mediumchain acyl-coa dehydrogenase deficiency (MCADD) [22 25], and some other IEMs [7,22,25,26] primarily for the US and UK populations. This article presents a decision-analytic model (Fig. 1) to evaluate the Figure 1 Schematic diagram of decision model. The small square represents the decision to implement a policy of newborn screening that includes a specific disease, or to continue the status quo of not screening newborns for the disease. In the case of phenylketonuria (PKU), the small square represents the decision to change the screening technology to mass spectrometry, or to continue with the current technology; therefore, in the case of PKU, the No Screening branch is replaced with a Screening branch. Circles represent chance events. The model divides babies into two groups based on whether they have or do not have the disease. In the expanded newborn screening strategy, all babies are initially tested for the disease; those who test positive have their original sample retested. If a baby has two positive test results, their parents are contacted, confirmatory testing is initiated, and prospective treatment begins. This model assumes that the confirmatory testing has a sensitivity and a specificity of 100%. Babies with the disease may have a severe or mild disease course, early or late onset, or may never become affected by the disease. These diseasespecific variations were modeled by dividing infants with the disease into Categories 1 and 2, and in some cases, category 3.
3 Cost-Effectiveness of Newborn Screening 85 incremental costs, health benefits, and incremental cost-effectiveness ratio for expanding the newborn screening program in Ontario using MS/MS, to change screening technologies for PKU, and to potentially include 20 additional diseases that can be tested for at birth. Methods All costs are presented in 2004 Canadian dollars. Costs in other currencies were converted at the average international exchange rate for August Costs from other periods in time were adjusted using the Canadian Health Care Price Index [27]. All costs and benefits were discounted at 3%. Important assumptions in this analysis are summarized in Table 1. Incidence and Severity Patients with each disease are divided by disease severity, age of onset, or responsiveness to treatment. We defined category 1 as the neonatal, classical, severe, or early-onset forms of the diseases. We defined category 2 as the later-onset, chronic, or milder forms of the diseases. Some diseases have such mild variations that they would not necessarily be detected or treated without newborn screening; we defined these as category 3 (Table 2 and Supplementary Table S1). In patients, the symptoms and outcomes associated with each disease variation are not mutually exclusive, but for the purposes of this analysis, only three distinct categories were modeled. For diseases with more than three recognized variations, variations were grouped based on patient outcome. Sensitivity, Specificity, and Positive Predictive Rate We assumed that all positive MS/MS test results are repeated on the original sample to confirm the result, which increases the positive predictive rate of screening. Nevertheless, because the repeated MS/MS test does not occur on an independently taken blood spot, the possibility of incorrect indication due to biological variation, poor sample collection, and sample contamination does not change. To account for this, the specificity of the repeated MS/MS test was reduced. We assumed that the average positive predictive rate of an expanded Ontario newborn screening program would be 20%, similar to the California Newborn Screening trial (a pilot program) [28]. In addition, the positive predictive rates of tyrosinemia and MCADD have been reported as 2% [29] and 87% [28], respectively. Using these known positive predictive rates and holding the sensitivity of the test constant, we estimated the specificity of the second MS/ MS test. There are diseases, such as MCADD, where the screening test may identify patients in whom the disease may never develop symptomatically. In this analysis, we segregated these patients into category 3 and assumed that although they would never develop the disease they are treated. Health Benefits Mortality rates were estimated from the 2000 World Health Organization Life Table for Canada [30]. Information regarding incremental mortality for persons with metabolic diseases were collected from the literature where available and when not available was estimated by experts (Table 2). Health benefits are expressed in terms of changes in life-years (LYs) of survival associated with a given screening program. Start-Up Costs Based on manufacturer-estimated MS/MS throughput of approximately 50,000 samples per year (Waters Corporation, Mississauga, Ontario, Canada), estimated machine downtime for maintenance, and approximately 130,000 births per year [31], we estimated that Ontario would require three mass spectrometers. Sample quotations were acquired from PerkinElmer, Inc. (Woodbridge, Ontario, Canada) and Waters Corporation for all the capital equipment required to start an MS/MS facility. The total cost depended on the company, as well as the brand of MS/ MS, autosampler, and gas generation system selected. Training costs, computers and analysis software, and 5 years of full-service maintenance are included in Table 1 Summary of key assumptions Screening can be carried out on any subset of the 21 diseases considered in this analysis. All diseases are grouped into three levels of severity: (1) neonatal, classical, severe, or early onset forms of the disease; (2) later-onset, chronic, or milder forms of the disease; and (3) mild variations that would not be detected or treated without newborn screening. Patients in the first two categories would eventually be diagnosed clinically. Patients in the third category have the same life expectancy at birth as members of the general population. All initially positive screening results are confirmed with a second tandem mass spectrometry (MS/MS) analysis before the patient is contacted. Positive results from MS/MS testing are confirmed with other technologies before a final diagnosis is made. While waiting for confirmation, all patients receive 3 months of treatment, three appointments with a metabolic specialist, one appointment with a social worker, and one appointment with a genetic counselor. Patients receiving dietary therapy also receive three appointments with a registered dietitian. Start-up and operating costs were estimated using quotations provided by two manufacturers of MS/MS equipment. Hospital-related costs were obtained from case-costing at the London Health Sciences Center, London, Ontario. All costs are expressed in 2004 Canadian dollars. Future costs and benefits are discounted at 3%.
4 86 Cipriano et al. Table 2 Disease incidence and proportion of patients affected with specific disease course and life expectancy (LE) Disease Incidence % of patients Category 1 Category 2 Category 3 Average LE (years)* Average LE (years)* Early diagnosis Clinical diagnosis % of patients Early diagnosis Clinical diagnosis % of patients Fatty acid β-oxidation disorders Carnitine transporter defect 1:120,667 [29] 50 [33] [33] Carnitine palmitoyl transferase I deficiency >1:250,000 [1] Carnitine/acylcarnitine translocase deficiency 1:362,000 [29] 86 [34] Carnitine palmitoyl transferase II deficiency (CPTII) >1:250,000 [1] 20* * Very long-chain acyl-coa dehydrogenase deficiency 1:120,667 [29] 36 [35] 25 # 12 # 64 [35] Long-chain hydroxyl acyl-coa dehydrogenase deficiency (LCHADD) >1:250,000 [1] Medium-chain acyl-coa dehydrogenase deficiency 1:15,400 [36] 70 [1,7] [1,7] Glutaric acidemia type II >1:250,000 [1] months Organic acidemias Hydroxymethylglutaryl (HMG)-CoA lyase deficiency >1:250,000 [1] 80 [7] [7] 3-methylcrotonyl-CoA carboxylase deficiency 1:50,000 [1] 80 [7] [7] Glutaric acidemia type I 1:50,000 [37]** 76 [38] [38,39] [7,38,40] Isovaleric acidemia 1:362,000 [29] 50 [41] [41] Methylmalonic acidemia 1:48,000 [42] 83 [43] [43] Propionic acidemia 1:50,000 [1] Urea cycle disorders Arginemia (arginase deficiency) 1:363,000 [44] 60 [45] [45] Arginosuccinic aciduria (arginosuccinate lyase deficiency) (ASA) 1:70,000 [29,44] 60 [45] [45] Citrullinemia (arginosuccinate synthase deficiency) 1:57,000 [44] Amino acidemias Tyrosinemia type I (hepatorenal tyrosinemia) (TYR) 1:100,000 [46] 77 [7,25] [7,25] Homocystinuria (HCYS) 1:250,000 [47] 43 [2] [2] Maple syrup urine disease 1:82,900 [36] 84 [9,48] 35 [9,48] [9,48] Phenylketonuria and variants 1:11,700 [49,50] [49] *Expert opinion combined with literature reports of natural history cited as sources for Incremental Mortality in Supplementary Table S1. When incidence was stated as >1:250,000 in the literature, we used 1:250,000 in the base case. Assume to be the same as CPT II. Patients included in category 1 (86%): patients indicated as having a severe phenotype, patients with unclassified phenotype but having died within year 0, and 20 known neonatal sibling deaths (undiagnosed). [34] Category 1 (36%) includes 9 infants who presented before day 2, and half (7) of the unexplained sibling deaths; category 2 (64%) includes 14 infants who presented between 2 and 8 months, 5 who presented later, half (7) of the unexplained sibling deaths and 2 presymptomatically treated patients who never clinically presented. [35] # Assume to be the same as LCHADD. **Carrier frequency in the aboriginal populations of Island Lake, Manitoba and Ojibway-Cree dialect-speaking Aboriginals of north-western Ontario estimated to be 1:17, incidence of 1:235. [37] Assume to be the same as ASA. Incidence of TYR in the Saguenay-lac St Jean region in Quebec is 1:1846 and the incidence in Quebec is 1:16,786. [46] Incidence of HCYS in the Pennsylvania Mennonite population is approximately 1:760. [2,47]
5 Cost-Effectiveness of Newborn Screening 87 these estimates, with total costs before financing of $19,650, $78,325, and $412,500, respectively. We assumed that the capital equipment would have a 5- year life, with no salvage value. The annual cost, assuming that the capital costs would be financed for 5 years at 6.5% annual interest [32] and that payments would be equally distributed over the 5 years, ranged from $337,326 to $490,379 (Table 3). Confirmatory Testing When both the initial and repeated MS/MS test results are positive, the family is contacted so that treatment can begin immediately and the MS/MS test result can be confirmed using a different diagnostic technology on an independently taken blood sample. Some confirmatory tests are readily available in Ontario and the Table 3 Base case values and costs of the screening program, health care, hospitalization, education, and social services. All costs are presented in 2004 Canadian dollars Parameter Base case Reference Participation rate 98% [51] Guthrie screening program Bacterial inhibition assay sensitivity 98.50% [49] Bacterial inhibition assay specificity 99.95% [49] Cost per sample (averaged estimates of 70,000 and 100,000 samples per year), $0.66 * including a sample repeat rate of 2% Cost of clinical diagnosis $4,389 Mass spectrometry screening program Sensitivity (%) Fatty acid β-oxidation disorders 100 [25,52] Organic acidemias 100 [25] Urea cycle disorders (not including CPS and OTC deficiencies) [29] Amino acidemias (except B 6 -responsive HCYS) 100 [7,53] B 6 -responsive HCYS 38.7 [2] Specificity (%) Fatty acid β-oxidation disorders [29] Organic acidemias [25,29] Urea cycle disorders [29] Amino acidemias [25,53] Costs ($) Annual equipment cost 490,380 See text Staff expense per sample, two estimates were averaged for MS/MS operation in 0.85 the Ontario provincial laboratory Reagent and consumables per sample Average per capita health care costs ($) Average health care costs (>1 year) 5,211 [54] Average health care costs (1 4 years) 800 [54] Average health care costs (5 14 years) 693 [54] Average health care costs (15 19 years) 1,089 [54] Average health care costs (20 44 years) 1,825 [54] Average health care costs (45 64 years) 4,244 [54] Average health care costs (65 79 years) 7,736 [54] Average health care costs (80+ years) 14,417 [54] Hospitalizations ($) Emergency room visit 1,167 See text Inpatient hospitalization, patient under 10 years 2,055 See text Inpatient hospitalization, patient over 10 years 1,765 See text Additional health care services ($) Initial call to schedule appointment (15-minute phone call with registered nurse) 7.03 [55] Initial specialist consult (all presumptive positives) [56] Subsequent pediatrician and specialist appointments (per appointment) [56] Registered dietitian appointments (per 60 minute appointment) [57] Initial genetic counseling appointment (in the year of diagnosis) [56] Follow-up genetic counseling appointment (3 years after initial diagnosis) [56] Maintenance diagnostics ($) Quantitative amino acid analysis 108 [58] Urine organic acids 108 [58] Abdominal ultrasound 339 [56,57] Nondietary treatments ($) Hemodialysis initial acute 1, [56,57] Hemodialysis chronic or repeat [56,57] Liver transplant 67, [56,57] Kidney transplant 32, [56,57]
6 88 Cipriano et al. Table 3 continued Parameter Base case Reference Pharmaceutical treatments($) (approximate annual cost) Arginine (660 mg/kg/day) 41,063 Estimated Ascorbic acid (100 mg/day) 30 [57] Betaine (6 g/day) 73 Estimated Carbamazepine (200 mg/day) 90 [59] Cobalamin (1.5 mg/day) 1,700 [59] Cysteine (6 g/day) 5,724 [57] Glycine (375 mg/kg/day) 685 Estimated L-carnitine (100 mg/kg/day) 7,565 [57] MCT (120 ml/day) 4,380 [59] Metronidazole (250 mg/day) 155 [59] NTBC (1 mg/kg/day) 91,250 Estimated Pyridoxine (300 mg/day) 82 [59] Riboflavin (200 mg/day) 58 Estimated Sodium-phenylbutyrate (500 mg/kg/day) 71,237 Estimated Thiamine (500 mg/day) 3,980 [57] Dietary treatments ($) Elemental formula (year 0) 4,000 [60] Elemental formula (1 8 years) 5,000 [60] Elemental formula (8 16 years) 6,000 [60] Elemental formula (over 16 years) 5,000 [60] Low protein foods (1 8 years) 2,000 [60] Low protein foods (>8 years) 3,500 [60] Education and social services ($) Standard education (5 18 years) 7,500 [61] Additional classroom support 18,000 [62] Advanced classroom support 27,000 [62] Living in an assisted-living facility 18,000 See text Living in an institutional facility 36,000 See text Social worker appointments (per 60 minute appointment) [57] Discount rate for costs and benefits 3% *Described as requiring approximately 0.15 full-time-equivalent (FTE) technician, 0.25 FTE technician II, and 0.15 FTE head technologist. (N. Lepage et al., unpublished) N. Lepage et al., unpublished. We assumed that one hospital stay and two emergency visits with laboratory tests included would be required to clinically diagnose a patient with an inborn error of metabolism. PerkinElmer (Woodbridge, Ontario, Canada) sells a kit that combines all the reagents required for detection of amino acids and acylcarnitines, standards, and derivatizing agent for $7.86 per clinical test. Waters Corporation (Mississauga, Ontario, Canada) did not have a price at the time of our analysis. Approximate annual cost for a 25-kg individual using an average treatment regimen. CPS, carbamoyl phosphate synthetase; HCYS, homocystinuria; MCT, medium chain triglycerideoil; NTBC, 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione; OTC, ornithine transcarbamylase. costs of those tests were estimated using the Ministry of Health Schedule of Laboratory Fees [58]. Rare metabolic enzyme assays are, however, often performed outside Canada because they are otherwise unavailable. We assumed that these tests would continue to be performed outside of Canada. Once an IEM is suspected through clinical presentation, confirmatory testing is also required. Nevertheless, the cost of that testing is not incurred until the year of clinical diagnosis. In addition, other costs associated with clinical diagnosis are also incurred in the year of diagnosis. We estimated these costs to be $4389 (the value of one hospital stay and two emergency room visits with laboratory tests). Based on personal experience, we assumed that, on average, confirmation test results would be available within 3 months. During this period the patient and/or patient s family (depending on the age of the patient at diagnosis) would receive three 60-minute appointments with a metabolic specialist, one 60-minute appointment with a social worker, and one 2-hour appointment with a genetic counselor. For diseases that require nutritional counseling, the patient and/or patient s family would also have three 60-minute appointments with a registered dietitian before confirmation test results were available. In the sensitivity analysis, we evaluated the effect of reduced quality of life that parents may experience during this uncertain 3-month period, ranging from 0.97 to 0.99 annualized [24]. Treatment Once the patient and/or patient s family receives confirmation of diagnosis, we assumed that the patient would receive treatment and blood tests for therapeutic monitoring and indications of disease/treatment complications for the remainder of his/her life. We also assumed that the patient and/or patient s family would receive a second appointment with a genetic counselor 3 years after the initial diagnosis. Patients and their families would have regular appointments with metabolic specialists, dietitians, and social workers. The regularity of these appointments varies with age, severity of disease, ability to adhere to treatment, and responsiveness to treatment (Supplementary Table S2).
7 Cost-Effectiveness of Newborn Screening 89 Hospitalization We estimated the total cost of hospitalization, length of stay, and emergency room visits using the cost of treating patients with metabolic diseases, acidosis, and alkalosis at London Health Sciences Centre (LHSC) [57]. We pooled the costing data for all the International Classification of Diseases, 10th Edition (ICD- 10) codes included (ICD-10 codes defining primary or secondary diagnosis: E , E71.3, E , E72.5, E72.8, E87.2, E87.3), and then divided them by type of hospitalization: inpatient, 493 cases; and emergency visits, 34 visits. The average cost of an emergency department visit was $974. Inpatient hospitalizations were further divided by age: less than 10 years, 45 cases; and more than 10 years, 448 cases. The average lengths of stay were 12 and 16 days, respectively. The average cost of an inpatient stay was $1952 per day for patients less than 10 years of age and $1675 per day for patients more than 10 years of age. The average daily cost of all inpatient hospitalizations for all reasons at LHSC was $1314 [57]. Physician costs are billed directly to the Ministry of Health and Long-Term Care (MOHLTC) and are not included in the cost of hospital care. Using the Ontario MOHLTC s Schedule of Benefits for Physician Services [56], we estimated the cost of physician services to bring the final cost of an emergency department visit to $1167, and of inpatient hospitalizations to $2055 and $1765 per day. Social Services and Education We assumed that all families of infants who received a diagnosis of an IEM receive 1 hour with a social worker in the year of diagnosis and 1 hour in the subsequent year. One study indicates that parents of infants diagnosed via a screening program are better able to cope with the challenges of the disease than parents whose children are diagnosed clinically [63]. Therefore, in the base case we assumed that the 9% reported in that study to have difficulty coping despite early diagnosis and the 29% reported to have difficulty coping after a child has been clinically diagnosed with a metabolic disease [63] would continue to receive 1 hour of social work services per year until they are 18 years of age. In the sensitivity analysis, we tested a range of other assumptions, including that social work use is equal at the low rate (9% of patients each year), equal at the high rate (29% of patients each year), and eliminated for all groups. For all disease states and outcomes, all school-age children attend public school and, unless otherwise noted, do not require special assistance. Information regarding intelligence level and social and physical development were acquired from secondary sources and, when not available, expert opinion (Supplementary Table S2). Patients described as moderately disabled were assumed to require additional classroom support between ages 5 and 18 years, estimated to cost $18,000 per year [62], and were assumed to live in an assisted-living facility from age 18 until 75, at a cost of $18,000 per year. We assumed that patients described as severely disabled required advanced classroom support for more than 80% of the school day between ages 5 and 18 years, costing $27,000 per year [62], and that they live in an institutional facility from age 18 until 75, costing $36,000 per year. Although the copayment structure in Ontario varies, depending on the patient s ability to pay, we estimated this to be the total cost based on similarly styled, not-for-profit US facilities. Screening Program Strategies Two program strategies were assessed: each disease was assessed independently, and diseases were assessed in bundles. Policymakers may choose to screen for any one individual disease and so when assessed individually, the incremental cost of each disease included the entire capital cost of investing in the MS/MS technology, the variable cost of screening, and the cost of program maintenance. One benefit of MS/MS, however, is that several diseases can be screened for at one time, enabling policymakers to design a screening program without incurring additional capital costs when additional diseases are considered. Diseases were bundled into groups based on their individual cost-effectiveness and sufficient detail is provided such that the costeffectiveness of bundles not presented can be determined. Results Screening by MS/MS for PKU Only In the base case, changing technologies for PKU screening costs an additional $2.26 million each year and has an incremental cost-effectiveness ratio (ICER) of $5,492,114 per LY gained (Table 4). Every 6 years of screening using MS/MS would prevent one false negative (0.17 fewer false negatives per year) and LY will be gained by each birth cohort. Screening by MS/MS for Other IEMs The cost-effectiveness of screening for each disease independently ranged from $221,719 to $142,462,687 per LY gained (Fig. 2a, Table 4). The four most cost-effective diseases to screen for (HMG-CoA lyase deficiency, MCADD, methylmalonic acidemia, and 3-methylcrotonyl-CoA carboxylase deficiency), assuming each is screened for independently, have incremental costs ranging from $221,719 to $266,991 per LY gained. Among all the diseases assessed, these four diseases do not have the lowest incremental costs, but they do have the largest incremental LY gains. Screening for MCADD alone has an ICER of $253,161 per LY gained. This is higher than others
8 90 Cipriano et al. Table 4 Incremental cost-effectiveness of each disease evaluated independently and a breakdown of incremental costs, savings, and life-years (LYs) gained per patient screened Disease Incremental cost ($) (including start-up*) Incremental cost ($) (excluding start-up) Incremental LYs gained ($) ( 10 5 ) ICER ($) (including start-up) ICER ($) (excluding start-up) Fatty acid β-oxidation disorders Carnitine transporter defect ,039 42,340 Carnitine palmitoyl transferase I deficiency ,192,814 92,814 Carnitine/acylcarnitine translocase deficiency ,137,500 93,750 Carnitine palmitoyl transferase II deficiency ,243,125 95,000 Very long-chain acyl-coa dehydrogenase deficiency ,269 37,037 Long-chain hydroxyl acyl-coa dehydrogenase deficiency ,063,830 86,702 Medium-chain acyl-coa dehydrogenase deficiency ,161 62,798 Glutaric acidemia type II ,462,687 5,373,134 Organic acidemias HMG-CoA lyase deficiency ,719 13,914 3-methylcrotonyl-CoA carboxylase deficiency ,991 68,182 Glutaric acidemia type I ,623 48,071 Isovaleric acidemia ,529,600 60,000 Methylmalonic acidemia ,927 13,482 Propionic acidemia ,276 27,885 Urea cycle disorders Arginemia ,979,167 2,448,333 Arginosuccinic aciduria ,035,275 1,440,777 Citrullinemia ,512,810 1,753,719 Amino acidemias Tyrosinemia type I , ,409 Homocystinuria ,270, ,200 Maple syrup urine disease ,135 15,426 Phenylketonuria and variants ,492, ,839 *Program start-up and base operation costs (whether screening for one or more diseases) is $18.37 per infant. For additional detail, please see Supplementary Table S3. i.e., first row: 3.59 = = The incremental cost-effectiveness ratio (ICER) describes the incremental cost required to acquire the benefit of 1 additional LY. It is calculated by dividing the total incremental cost by the incremental LYs gained. have reported; however, those studies have only evaluated the incremental cost of adding MCADD to an existing screening panel [7,23,34]. The incremental cost of adding MCADD to a screening panel estimated here ($62,798 per LY gained, Table 5) is consistent with another reported estimate of $41,862 per quality adjusted life-year (QALY) gained [23]. Other groups have made different assumptions about the duration of carnitine supplementation; if we make similar assumptions and stop carnitine treatment in all MCADD patients at age 18 [23] or age 5 [7], the ICER of screening for MCADD becomes cost saving, consistent with estimates ranging from $300 per LY gained to cost saving [7,24,25]. Glutaric acidemia type II (GAII) is the least costeffective disease to screen for under base case assumptions. The total incremental cost of screening for just GAII is $2.5 million, but each infant who receives a diagnosis of GAII only gains LY. This results in an ICER of $142.5 million per LY gained. Screening for Combinations of Diseases Diseases were ranked in order of their incremental cost-effectiveness, so that the most cost-effective screening program, including the greatest number of diseases, could be identified. The order and the costeffectiveness of adding additional diseases to a screening program are presented in Table 5. Screening for PKU and three diseases will result in five infants each year who will be diagnosed and treated earlier and who will gain a total of 30 LYs. Screening for PKU and 14 diseases will result in 92 LYs gained across the 27 infants who will receive an early diagnosis each year. Screening for all 21 diseases will result in 32 early diagnoses each year and 107 LYs gained across the cohort (Fig. 2b). When we consider the marginal cost-effectiveness of adding diseases to a screening program in which PKU screening has absorbed all the fixed costs, adding the third disease (maple syrup urine disease) costs $15,426 per LY gained. Adding the seventh disease (glutaric acidemia type I) to the bundle costs $48,071 per LY gained and adding the 14th disease (carnitine/ acylcarnitine translocase deficiency) costs $95,000 per LY gained. Although the average cost-effectiveness for PKU and 15 diseases is less than $100,000 per LY gained, the marginal cost-effectiveness of adding the 15th disease (tyrosinemia type I) is $309,409 per LY gained. The least cost-effective IEMs to screen for are those that affect the urea cycle (arginemia, arginosuccinic aciduria, and citrullinemia). This is largely due to a
9 Cost-Effectiveness of Newborn Screening 91 (A) $9,000,000 Figure 2 Cost-effectiveness plane. (A) Incremental costs and effectiveness for each disease if screened for independently. Each square represents a screening program that screens for only one disease and includes the full cost of the screening technology, maintenance, and technician time. (B) Incremental costs and effectiveness for bundles of diseases. Each square represents a screening program that screens for phenylketonuria (PKU) and subsequent diseases reading left to right (as shown in Table 5 from top to bottom). Incremental costs and life-years gained are expressed per cohort of 130,000 babies. ARG, arginemia (arginase deficiency); ASA, arginosuccinic aciduria; CAT, carnitine/ acylcarnitine translocase deficiency; CIT, citrullinemia (arginosuccinate synthase deficiency); CPT1, carnitine palmitoyl transferase I deficiency; CPT2, carnitine palmitoyl transferase II deficiency; CTD, carnitine transporter defect; GA1, glutaric acidemia type I; GA2, glutaric acidemia type II; HCYS, homocystinuria; HMG, HMG-CoA lyase deficiency; IVA, isovaleric acidemia; MCADD, medium-chain acyl-coa dehydrogenase deficiency; MMA, methylmalonic acidemia; MS/MS, tandem mass spectrometry; MSUD, maple syrup urine disease; PA, proprionic acidemia; VLCADD, very long-chain acyl- CoA dehydrogenase deficiency; LCHADD, longchain hydroxyl acyl-coa dehydrogenase deficiency; TYR1, tyrosinemia type I; 3-MCC, 3- methylcrotonyl-coa carboxylase deficiency. Incremental Costs Associated with Screening using MS/MS per cohort of 130,000 babies (B) Incremental Costs Associated with Screening using MS/MS per cohort of 130,000 babies $8,000,000 $7,000,000 $6,000,000 $5,000,000 $4,000,000 $1,000,000 $0 ARG CIT $3,000,000 CPT2 CAT HCYS LCHADD GA2 IVA CPT1 $2,000,000 PKU $25,000,000 $20,000,000 $15,000,000 $10,000,000 $5,000,000 ASA CTD TYR1 VLCADD MSUD PA GA1 MMA 3-MCC MCADD HMG Life-Years Gained per Cohort of 130,000 PKU + CTD + MMA + HMG + MSUD+ PA + VLCADD + CAT + CPT2 + CPT1 + IVA + 3-MCC + MCADD + LCHADD + GA1 + CIT + ASA + HCYS + TYR1 + GA2 + ARG $ Life-Years Gained per Cohort of 130,000 low number of LYs gained combined with a high incremental cost associated with treating patients with sodium-phenylbutyrate. Urea cycle disorders frequently result in death before a clinical diagnosis is made. Although early diagnosis leads to enhanced survival in some cases, it also results in an expensive treatment regimen. Combined screening for the urea cycle disorders would cost $17.5 million and only detect 4.5 affected infants each year. Infants detected with urea cycle disorders in one cohort will gain 8.7 LYs. The ICER for screening for the three urea cycle disorders included in this model is $2.0 million. Carbamoyl phosphate synthetase deficiency and ornithine transcarbamylase deficiency are not reliably detected by MS/MS and so were not included in this analysis. We varied each model parameter in a one-way sensitivity analysis (Table 6 and Supplementary Table S4). Birth volume variation across the year may produce times when three mass spectrometers cannot supply the timely results needed; however, substantial sensitivity analysis on the cost of MS/MS shows that if a fourth ($24.00 per sample) or even fifth ($27.00 per sample) instrument is required, the average costeffectiveness of a screening program including PKU and 14 diseases is less than $85,000 per LY gained. The model is most sensitive to MS/MS specificity. The ICER of screening for PKU and 14 diseases is $173,100 per LY gained when the specificity of the MS/MS test is reduced by just 0.05% from the base case. Four pessimistic scenarios were evaluated in which both the sensitivity and specificity of MS/MS were reduced in combination with increased costs and reduced life expectancy. The incremental cost of screening for PKU and 14 diseases increases to $149,700 when all costs are increased by 50% and the life expectancy of early diagnosed patients are reduced
10 92 Cipriano et al. Table 5 Incremental costs, savings, life-years gained, and cost-effectiveness of adding each disease to a screening strategy in the base case scenario per patient screened Screening programs Disease added Incremental cost of adding the disease to the bundle ($) Incremental cost of bundle ($) Life-years gained adding the disease to the bundle ( 10 4 )* Life-years gained for the bundle ( 10 4 ) ICER of adding the disease to the bundle ($) ICER of the bundle ($) PKU Phenylketonuria ,492,114 5,492,114 PKU Methylmalonic acidemia , ,746 PKU HMG-CoA lyase deficiency , ,104 PKU Maple syrup urine disease ,426 91,545 PKU Propionic acidemia ,885 77,693 PKU Very long-chain acyl-coa dehydrogenase deficiency ,037 72,370 PKU Carnitine transporter defect ,340 69,424 PKU Glutaric acidemia type I ,071 66,102 PKU Isovaleric acidemia ,000 65,931 PKU Medium-chain acyl-coa dehydrogenase deficiency ,798 65,373 PKU methylcrotonyl-CoA carboxylase deficiency ,182 65,782 PKU Long-chain hydroxyl acyl-coa dehydrogenase deficiency ,702 66,384 PKU Carnitine palmitoyl transferase I deficiency ,814 67,043 PKU Carnitine palmitoyl transferase II deficiency ,750 67,726 PKU Carnitine/acylcarnitine translocase deficiency ,000 68,346 PKU Tyrosinemia type I ,409 83,045 PKU Homocystinuria ,200 85,098 PKU Arginosuccinic aciduria ,440, ,351 PKU Citrullinemia ,753, ,564 PKU Arginemia ,448, ,550 PKU Glutaric acidemia type II ,373, ,389 *i.e., first row: = = ICER, incremental cost-effectiveness ratio. by 25%. Reductions in specificity have a significant impact on cost-effectiveness estimates in the pessimistic scenarios. Discussion Changing screening technologies for PKU alone is not cost-effective by currently accepted standards [64]. Nevertheless, a screening program including PKU and 14 diseases would have an incremental cost of less than $70,000 per LY gained. Each disease being screened for would have an incremental cost of less than $100,000 per LY gained and the program would identify approximately 27 babies born with a treatable metabolic disease in Ontario each year. Some diseases will continue to have high mortality rates despite the expansion of newborn screening programs. Proper identification of the cause of death, however, will permit parents to seek genetic counseling. Although many factors contribute to parental decision-making, Read found that 56% of parents who have a child with an inherited metabolic disease wanted prenatal diagnosis in a future pregnancy, 10% wanted to selectively terminate a future affected pregnancy, and 41% were already taking measures to prevent future affected pregnancies [65]. These numbers may vary across religious and cultural groups [66] as well as with the severity of disease observed in the first child [67,68]. Accurate information may, however, aid future decision-making. Screening has been found to be cost-effective by other research groups [7,20,23,24,69], although the estimated incremental cost per LY gained varies widely. Estimates for the addition of MCADD to an existing screening program range from cost saving to $41,862 per QALY [7,23 25]. One estimate of adding a series of fatty acid oxidation disorders and organic acidemias to a screening panel suggests a cost of $15,252 per QALY [23]. Pollitt et al. estimated the cost-effectiveness of screening for more than a dozen individual IEMs as well as other diseases that can be screened for at birth [7]. In contrast to our study, Pollitt et al. found several diseases, such as methylmalonic acidemia and propionic acidemia, to be more costeffective than we did. Severe forms of these diseases, however, were excluded from their analysis because they were deemed untreatable. Others have stopped short of presenting a detailed economic analysis of each IEM because they have found screening for MCADD alone to be costeffective enough to justify the adoption of MS/MS screening [23]. In contrast, we believe that the variation in MS/MS sensitivity and specificity to identify other diseases, different costs of treatment, and different potential benefits from early intervention indicate that the addition of each disease requires independent evaluation. Having already justified investment in MS/ MS technology does not indicate that it is economical to use MS/MS for every potential use. This article indicates that, in fact, having already invested in MS/MS,
11 Cost-Effectiveness of Newborn Screening 93 Table 6 Summary of sensitivity results* Parameter Base case Range tested Marginal cost-effectiveness ratio ($ per LY gained) PKU + 3 PKU + 7 PKU + 14 Average cost effectiveness ratio for PKU + 14 diseases Base case result for reference $15,426 $48,071 $95,000 $68,346 Incidence: See Table % of $36,000 $12,200 $61,700 $44,400 $164,900 $79,300 $109,900 $54,400 Base Case Mass spectrometry screening program: All disease sensitivity See Table 3 Less 10% $29,700 $64,500 $897,800 $221,100 All disease specificity See Table 3 Less 1% $352,700 $572,000 $2595,600 $668,346 Costs (total test cost per infant) $18.37 $10 $50 $15,426 $15,700 $48,071 $48,000 $93,900 $95,100 $56,300 $113,500 Potential legal costs: If every false negative was $0 $0 $5,000,000 $15,426 $48,071 $95,000 $63,300 reimbursed some value in year 10 If every clinical diagnosis was $0 $0 $500,000 Cost saving Cost saving $9,100 Cost saving reimbursed for lack of earlier detection some value in year 10 Life expectancy: See Table 2 All reduced $13,700 $66,200 $369,100 $112,800 25% All increased $21,100 $34,700 $73,200 $46,800 25% All + 20 years (max 78 years) $20,600 $33,600 $58,600 $41,300 Quality of life for parents during uncertain diagnosis period (applies to all IEM cases and 1 LY 0.99 QALY $16,900 $49,300 $134,400 $73,500 false positives for 3 months) (annualized) 1 LY 0.97 QALY $20,700 $70,100 $694,400 $104,000 (annualized) Discount rate 3% 0 9% $16,000 $31,400 $43,300 $82,000 $78,000 $255,000 $51,400 $130,800 Pessimistic scenarios: 1. Reduced sensitivity (10%) and $407,600 $539,000 $2,610,600 $770,100 reduced specificity (1%) for all diseases $2,610, All costs increased (50%) $7,400 $23,600 $48,300 $46, All costs increased (50%) and $20,400 $98,900 $552,600 $149,700 reduced life expectancy (25%) 4. Reduced sensitivity (10%), reduced specificity (1%), increased costs (50%), and reduced life expectancy (25%) $751,500 $1,687,700 $13,268,800 $2,042,500 *Please see Supplementary Table S4 for additional information. IEM, inborn error of metabolism; PKU, phenylketonuria; QALY, quality-adjusted life-year. screening for arginosuccinate lyase deficiency, citrullinemia, arginemia, and GAII are not cost-effective even when the costs and benefits are averaged across all diseases in the screening program. This model includes a broad societal perspective, including not only the costs of screening and initial assessment, but also the downstream costs of counseling, education, and long-term tertiary care. This model also assumes that all patients would treat their disease for life; however, most patients on restricted diets relax the restrictions in young adulthood [70]. In Ontario, the current provincially insured benefits for these patients end at age 18 years. Prohibitive cost has also been identified as one barrier to access to treatment with respect to maternal PKU [71]. Restricted diet for life is the only way to prevent neurological deterioration from classical PKU [72 74] and maternal PKU [75]. The incremental cost-effectiveness of dietary treatment for life versus ceasing treatment in young adulthood has not been evaluated. Our analysis assesses outcomes in LYs gained because there is insufficient information in the literature to generate quality of life adjustments for all the diseases and disease states discussed here. Additionally, there are several unresolved problems using QALY measurements in infants and children [76 82]. Using QALYs instead of LYs gained may overestimate the cost-effectiveness of screening when the quality of life in the additional years is low. Conversely, using QALYs instead of LYs gained may result in an underestimation of the cost-effectiveness when the number of additional years gained is low but the quality of life in all years is improved. The latter is likely the case with MCADD, for example. Given the lack of peer-reviewed long-term data in this area [83], the reported challenges of generating credible and legitimate quality of life estimates for infants and children [76 82], and evidence that quality of life assessments do not substantially alter the cost-effectiveness of interventions [84], we decided to
12 94 mediate this limitation through extensive sensitivity analysis. This study relied on the costing information from LHSC, a large academic hospital. Costs provided did not include physician costs, and a fee for service compensation method was assumed. These costs and this compensation scheme may not be representative of other hospitals. Nevertheless, LHSC does have significant experience treating patients with metabolic disease as it is the tertiary care center responsible for patients with metabolic disease from a large geographic region. This study does not consider the potential need for additional investments in infrastructure or human resource training outside of the MS/MS laboratory. The large volume of potential positives that will result from the MS/MS screen may overwhelm the current IEM specialists and treatment support structures. Coordination of communication, follow-up evaluation, and treatment support services may need additional management and investment. There is strong support for the idea that screening would result in longer, healthier patient lives [5 9,25,26,33,37,40,41,47,48,85 97]. The data in this article, however, relied on several studies with a small number of cases and on expert opinion. The literature on disease incidence, life expectancy, and dependence on social services by these populations is not robust. Robust data is unattainable in an unscreened population and only good program management will eventually reveal the outcomes of screening. Our analysis suggests that in order for an MS/MS screening program to be cost-effective several diseases must be screened for in a single bundle. It is, however, not cost-effective to include all possible diseases in that bundle. The incremental cost of screening per LY gained can be optimized by screening for PKU and nine diseases, including methylmalonic acidemia, HMG-CoA lyase deficiency, maple syrup urine disease, propionic acidemia, very long-chain acyl-coa dehydrogenase deficiency, carnitine transporter defect, glutaric acidemia type I, isovaleric acidemia, and MCADD. Using a threshold of $100,000 per LY gained, we find moderate evidence to support the adoption of screening for PKU and 14 additional diseases [64]. Therefore, consistent with more than half of North American jurisdictions that have already initiate MS/MS screening including British Columbia, Saskatchewan, and Nova Scotia, there is evidence to support Ontario s adoption of expanded newborn screening. Source of financial support: This study was funded by the Natural Science and Engineering Research Council of Canada. Acknowledgments: The authors thank Suzanne Ratko and Kathy Corley, London Health Sciences Centre, for their Cipriano et al. assistance in describing patient experiences and care, and Dr. Charles Botz, London Health Sciences Centre, for his assistance in estimating costs. References 1 Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem 2003;49: Mudd SH, Skovby F, Levy HL, et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet 1985;37: Sigauke E, Rakheja D, Kitson K, Bennett MJ. Carnitine palmitoyltransferase II deficiency: a clinical, biochemical, and molecular review. Lab Invest 2003;83: Jones PM, Bennett MJ. The changing face of newborn screening: diagnosis of inborn errors of metabolism by tandem mass spectrometry. Clin Chim Acta 2002;324: Melnyk AR, Matalon R, Henry BW, et al. Prospective management of a child with neonatal citrullinemia. J Pediatr 1993;122: Sander J, Janzen N, Sander S, et al. Neonatal screening for citrullinaemia. Eur J Pediatr 2003;162: Pollitt RJ, Green A, McCabe CJ, et al. Neonatal screening for inborn errors of metabolism: cost, yield and outcome. Health Technol Assess 1997;1: Hoffmann GF, Athanassopoulos S, Burlina AB, et al. Clinical course, early diagnosis, treatment, and prevention of disease in glutaryl-coa dehydrogenase deficiency. Neuropediatrics 1996;27: Chuang DT, Shih VE. Chapter 87: Maple syrup urine disease (Branched-chain ketoaciduria). In: Scriver CR, ed., The Metabolic and Molecular Bases of Inherited Disease (8th ed.). New York: McGraw-Hill, Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963;32: American College of Medical Genetics/American Society of Human Genetics Test and Technology Transfer Committee Working Group. Tandem mass spectrometry in newborn screening. Genet Med 2000;2: Cunningham G. The science and politics of screening newborns. N Engl J Med 2002;346: Howell R. The high price of false positives. Mol Genet Metab 2006;87: Rhead W, Irons M. The call from the newborn screening laboratory: frustration in the afternoon. Pediatr Clin North Am 2004;51: Ross L. Screening for conditions that do not meet the Wilson and Jungner criteria: the case of Duchenne muscular dystrophy. Am J Med Gen A 2006;140: Wong B. Universal neonatal hearing screening: to screen or not to screen. Hong Kong Med J 2004;10:4. 17 Hanley WB. Newborn screening in Canada Are we out of step? Paediatr Child Health 2005;10:203 7.
HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up
 HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up Dr. Josephine Chong Clinical Professional Consultant Centre of Inborn Errors
HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up Dr. Josephine Chong Clinical Professional Consultant Centre of Inborn Errors
Positive Newborn Screens: What do you do next?
 Positive Newborn Screens: What do you do next? James B. Gibson, MD, Ph.D. Biochemical Geneticist at Specially for Children Clinical Associate Professor of Pediatrics UTHSCSA Carla R. Scott, MD Pediatric
Positive Newborn Screens: What do you do next? James B. Gibson, MD, Ph.D. Biochemical Geneticist at Specially for Children Clinical Associate Professor of Pediatrics UTHSCSA Carla R. Scott, MD Pediatric
For Your Baby s Health Department of Health
 Newborn Screening For Your Baby s Health Department of Health Why is my baby tested? To help make sure your baby will be as healthy as possible. The blood test provides important information about your
Newborn Screening For Your Baby s Health Department of Health Why is my baby tested? To help make sure your baby will be as healthy as possible. The blood test provides important information about your
Overview of Newborn Screening, Potential Uses of Residual Dried Blood Spots, and Protection of Privacy
 Overview of Newborn Screening, Potential Uses of Residual Dried Blood Spots, and Protection of Privacy Alan R. Fleischman, M.D. Senior Vice President and Medical Director Chair, Federal Advisory Committee,
Overview of Newborn Screening, Potential Uses of Residual Dried Blood Spots, and Protection of Privacy Alan R. Fleischman, M.D. Senior Vice President and Medical Director Chair, Federal Advisory Committee,
A Guide for Prenatal Educators
 A Guide for Prenatal Educators Why Teach Newborn Screening This booklet is designed to make it easy for you, a prenatal educator, to effectively inform expectant parents about newborn screening. All of
A Guide for Prenatal Educators Why Teach Newborn Screening This booklet is designed to make it easy for you, a prenatal educator, to effectively inform expectant parents about newborn screening. All of
Comprehensive cost-utility analysis of newborn screening strategies Carroll A E, Downs S M
 Comprehensive cost-utility analysis of newborn screening strategies Carroll A E, Downs S M Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion on NHS
Comprehensive cost-utility analysis of newborn screening strategies Carroll A E, Downs S M Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion on NHS
Newborn Screening in Manitoba. Information for Health Care Providers
 Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
Newborn Screening: Blood Spot Disorders
 Newborn Screening: Blood Spot Disorders Arizona s Newborn Screening Program Program Overview Panel of Disorders Disorder Descriptions Program Components Hospitals ADHS Lab ADHS Follow-up ADHS Billing Medical
Newborn Screening: Blood Spot Disorders Arizona s Newborn Screening Program Program Overview Panel of Disorders Disorder Descriptions Program Components Hospitals ADHS Lab ADHS Follow-up ADHS Billing Medical
Newborn Screening: Focus on Treatment
 Newborn Screening: Focus on Treatment Alan R. Fleischman, M.D. Senior Vice President and Medical Director March of Dimes National Conference of State Legislatures July 21, 2008 Newborn Screening: A Public
Newborn Screening: Focus on Treatment Alan R. Fleischman, M.D. Senior Vice President and Medical Director March of Dimes National Conference of State Legislatures July 21, 2008 Newborn Screening: A Public
Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015
 Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015 Metabolic birth defects can cause physical problems, mental retardation and, in some cases, death. It is
Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015 Metabolic birth defects can cause physical problems, mental retardation and, in some cases, death. It is
Inborn Errors of Metabolism. Metabolic Pathway. Digestion and Fasting. How is Expanded Newborn Screening Different? MS/MS. The body is a factory.
 Inborn Errors of Metabolism The body is a factory. Inborn errors of metabolism are rare genetic disorders in which the body cannot properly turn food into energy. The disorders are usually caused by defects
Inborn Errors of Metabolism The body is a factory. Inborn errors of metabolism are rare genetic disorders in which the body cannot properly turn food into energy. The disorders are usually caused by defects
[R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE PLANTATIONS DEPARTMENT OF HEALTH. February October October 1992 (E) September 1995
![[R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE PLANTATIONS DEPARTMENT OF HEALTH. February October October 1992 (E) September 1995 [R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE PLANTATIONS DEPARTMENT OF HEALTH. February October October 1992 (E) September 1995](/thumbs/95/122505027.jpg) RULES AND REGULATIONS PERTAINING TO THE NEWBORN METABOLIC, ENDOCRINE, AND HEMOGLOBINOPATHY SCREENING PROGRAM AND THE NEWBORN HEARING LOSS SCREENING PROGRAM [R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE
RULES AND REGULATIONS PERTAINING TO THE NEWBORN METABOLIC, ENDOCRINE, AND HEMOGLOBINOPATHY SCREENING PROGRAM AND THE NEWBORN HEARING LOSS SCREENING PROGRAM [R23-13-MET/HRG] STATE OF RHODE ISLAND AND PROVIDENCE
Cost-Effectiveness of Expanded Newborn Screening in Texas
 Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jval Cost-Effectiveness of Expanded Newborn Screening in Texas Simrandeep K. Tiwana, MBA, PhD 1,2, *,, Karen L. Rascati,
Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jval Cost-Effectiveness of Expanded Newborn Screening in Texas Simrandeep K. Tiwana, MBA, PhD 1,2, *,, Karen L. Rascati,
Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis)
 Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis) 1) Argininosuccinic acidemia (ASA) a) Incidence: ~1 in 70,000 b) Deficiency in an enzyme of
Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis) 1) Argininosuccinic acidemia (ASA) a) Incidence: ~1 in 70,000 b) Deficiency in an enzyme of
NEWBORN METABOLIC SCREEN, MINNESOTA
 Lab Dept: Test Name: Chemistry NEWBORN METABOLIC SCREEN, MINNESOTA General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: PKUN Newborn Screen for Hyopothyroidism, Phenylketonuria (PKU),
Lab Dept: Test Name: Chemistry NEWBORN METABOLIC SCREEN, MINNESOTA General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: PKUN Newborn Screen for Hyopothyroidism, Phenylketonuria (PKU),
Further expansion of the neonatal screening panel in the Netherlands
 Further expansion of the neonatal screening panel in the Netherlands J.Gerard Loeber APHL-NBSGT, St.Louis (MO), USA 290216 Population Area Newborns 6.01 million 0.35:1 16.8 million 180,693 sq km 4.3:1
Further expansion of the neonatal screening panel in the Netherlands J.Gerard Loeber APHL-NBSGT, St.Louis (MO), USA 290216 Population Area Newborns 6.01 million 0.35:1 16.8 million 180,693 sq km 4.3:1
The spectrum and outcome of the. neonates with inborn errors of. metabolism at a tertiary care hospital
 The spectrum and outcome of the neonates with inborn errors of metabolism at a tertiary care hospital Dr. Sevim Ünal Neonatology Division, Ankara Children s Hematology Oncology Research Hospital, Ankara,
The spectrum and outcome of the neonates with inborn errors of metabolism at a tertiary care hospital Dr. Sevim Ünal Neonatology Division, Ankara Children s Hematology Oncology Research Hospital, Ankara,
INBORN ERRORS OF METABOLISM (IEM) IAP UG Teaching slides
 INBORN ERRORS OF METABOLISM (IEM) 1 OBJECTIVES What are IEMs? Categories When to suspect? History and clinical pointers Metabolic presentation Differential diagnosis Emergency and long term management
INBORN ERRORS OF METABOLISM (IEM) 1 OBJECTIVES What are IEMs? Categories When to suspect? History and clinical pointers Metabolic presentation Differential diagnosis Emergency and long term management
Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism
 Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism Patricia Jones, PhD DABCC FACB UT Southwestern Medical Center Children s Medical Center Dallas, Texas Learning Objectives Justify
Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism Patricia Jones, PhD DABCC FACB UT Southwestern Medical Center Children s Medical Center Dallas, Texas Learning Objectives Justify
Title: Assessing Recommendations Related To Timeliness of Newborn Screening
 Title: Assessing Recommendations Related To Timeliness of Newborn Screening Purpose: In January 2014, the Secretary s Discretionary Advisory Committee on Heritable Diseases on Newborns and Children (Committee)
Title: Assessing Recommendations Related To Timeliness of Newborn Screening Purpose: In January 2014, the Secretary s Discretionary Advisory Committee on Heritable Diseases on Newborns and Children (Committee)
Economics of tandem mass spectrometry screening of neonatal inherited disorders Pandor A, Eastham J, Chilcott J, Paisley S, Beverley C
 Economics of tandem mass spectrometry screening of neonatal inherited disorders Pandor A, Eastham J, Chilcott J, Paisley S, Beverley C Record Status This is a critical abstract of an economic evaluation
Economics of tandem mass spectrometry screening of neonatal inherited disorders Pandor A, Eastham J, Chilcott J, Paisley S, Beverley C Record Status This is a critical abstract of an economic evaluation
Attachment 1. Newborn Screening Program Description
 Attachment 1 Newborn Screening Program Description The core mission of Texas Newborn Screening Program is to save children s lives through the early detection of life-threatening disorders. 34 Newborn
Attachment 1 Newborn Screening Program Description The core mission of Texas Newborn Screening Program is to save children s lives through the early detection of life-threatening disorders. 34 Newborn
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 12/19/17 SECTION: MEDICINE LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 MEDICAL FOODS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
MEDICAL FOODS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
Economic evaluation of tandem mass spectrometry screening in California Feuchtbaum L, Cunningham G
 Economic evaluation of tandem mass spectrometry screening in California Feuchtbaum L, Cunningham G Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion
Economic evaluation of tandem mass spectrometry screening in California Feuchtbaum L, Cunningham G Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion
Newborn Bloodspot Screening Information for parents
 Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
TIMELINESS OF NEWBORN SCREENING: RECOMMENDATIONS FROM ADVISORY COMMITTEE ON HERITABLE DISORDERS IN NEWBORNS AND CHILDREN
 TIMELINESS OF NEWBORN SCREENING: RECOMMENDATIONS FROM ADVISORY COMMITTEE ON HERITABLE DISORDERS IN NEWBORNS AND CHILDREN Susan Tanksley, PhD May 19, 2015 TIMELINESS - BACKGROUND In order to effectively
TIMELINESS OF NEWBORN SCREENING: RECOMMENDATIONS FROM ADVISORY COMMITTEE ON HERITABLE DISORDERS IN NEWBORNS AND CHILDREN Susan Tanksley, PhD May 19, 2015 TIMELINESS - BACKGROUND In order to effectively
Selective newborn screening of amino acid, fatty acid and organic acid disorders in the Kingdom of Bahrain.
 Selective newborn screening of amino acid, fatty acid and organic acid disorders in the Kingdom of Bahrain. Jamal Golbahar PhD Associate Professor of Molecular Medicine, Department of Molecular Medicine,
Selective newborn screening of amino acid, fatty acid and organic acid disorders in the Kingdom of Bahrain. Jamal Golbahar PhD Associate Professor of Molecular Medicine, Department of Molecular Medicine,
Newborn Bloodspot Screening Information for parents
 Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Neonatal Screening of Inborn Errors of Metabolism Using Tandem Mass Spectrometry
 Ontario Health Technology Assessment Series 2003; Vol. 3, No. 3 Neonatal Screening of Inborn Errors of Metabolism Using Tandem Mass Spectrometry An Evidence-Based Analysis May 2003 \ Medical Advisory Secretariat
Ontario Health Technology Assessment Series 2003; Vol. 3, No. 3 Neonatal Screening of Inborn Errors of Metabolism Using Tandem Mass Spectrometry An Evidence-Based Analysis May 2003 \ Medical Advisory Secretariat
Newborn bloodspot testing
 Policy HUMAN GENETICS SOCIETY OF AUSTRALASIA ARBN. 076 130 937 (Incorporated Under the Associations Incorporation Act) The liability of members is limited RACP, 145 Macquarie Street, Sydney NSW 2000, Australia
Policy HUMAN GENETICS SOCIETY OF AUSTRALASIA ARBN. 076 130 937 (Incorporated Under the Associations Incorporation Act) The liability of members is limited RACP, 145 Macquarie Street, Sydney NSW 2000, Australia
NEWBORN SCREENING. in Massachusetts: Answers for You and Your Baby. University of Massachusetts Medical School
 University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Answers for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Answers for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
Medical Foods for Inborn Errors of Metabolism
 Medical Foods for Inborn Errors of Metabolism Policy Number: Original Effective Date: MM.02.014 02/18/2000 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 08/23/2013 Section: Medicine Place(s)
Medical Foods for Inborn Errors of Metabolism Policy Number: Original Effective Date: MM.02.014 02/18/2000 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 08/23/2013 Section: Medicine Place(s)
NEWBORN SCREENING IN JAPAN
 NEWBORN SCREENING IN JAPAN Kikurnaro Aoki KagawaNutrition University, Saitama, Japan Abstract. In the 19701s, the government began to take steps for the treatment of congenital diseases. Mass newborn screening
NEWBORN SCREENING IN JAPAN Kikurnaro Aoki KagawaNutrition University, Saitama, Japan Abstract. In the 19701s, the government began to take steps for the treatment of congenital diseases. Mass newborn screening
Newborn Screening: What s New?
 Newborn Screening: What s New? Patricia M. Jones, PhD (Department of Pathology, University of Texas Southwestern Medical Center and Children s Medical Center of Dallas, Dallas, TX) DOI: 10.1309/LMOOXWVPSM5FC3B6
Newborn Screening: What s New? Patricia M. Jones, PhD (Department of Pathology, University of Texas Southwestern Medical Center and Children s Medical Center of Dallas, Dallas, TX) DOI: 10.1309/LMOOXWVPSM5FC3B6
Organic Acid Disorders
 Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Screening Newborns for Inborn Errors of Metabolism by Tandem Mass Spectrometry
 The new england journal of medicine original article Screening Newborns for Inborn Errors of Metabolism by Tandem Mass Spectrometry Bridget Wilcken, M.B., Ch.B., Veronica Wiley, Ph.D., Judith Hammond,
The new england journal of medicine original article Screening Newborns for Inborn Errors of Metabolism by Tandem Mass Spectrometry Bridget Wilcken, M.B., Ch.B., Veronica Wiley, Ph.D., Judith Hammond,
(f) "Birthing center" means any facility that is licensed by the Georgia Department of Community Health as a birthing center;
 Ga. Comp. R. & Regs. r. 511-5-5-.02 Definitions Rule 511-5-5-.02. Definitions (a) "Abnormal test result" is a test result from blood testing or physiologic monitoring that is outside the screening limits
Ga. Comp. R. & Regs. r. 511-5-5-.02 Definitions Rule 511-5-5-.02. Definitions (a) "Abnormal test result" is a test result from blood testing or physiologic monitoring that is outside the screening limits
Inborn Errors of Metabolism From Neonatal Screening to Metabolic Pathways
 19 May 2015 Hospital Authority Convention 2015 Corporate Scholarship Presentation II Paediatric Services Inborn Errors of Metabolism From Neonatal Screening to Metabolic Pathways Dr Grace Poon Associate
19 May 2015 Hospital Authority Convention 2015 Corporate Scholarship Presentation II Paediatric Services Inborn Errors of Metabolism From Neonatal Screening to Metabolic Pathways Dr Grace Poon Associate
Organic Acid Disorders
 Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
So Much More Than The PKU Test
 Newborn Metabolic Screening So Much More Than The PKU Test Sarah Viall, MSN, PPCNP BC Newborn Screening Program Coordinator Division of Genetics & Metabolism Conflicts of Interest I have no conflicts of
Newborn Metabolic Screening So Much More Than The PKU Test Sarah Viall, MSN, PPCNP BC Newborn Screening Program Coordinator Division of Genetics & Metabolism Conflicts of Interest I have no conflicts of
Newborn Screening in Washington State Saving lives with a simple blood spot
 Newborn Screening in Washington State Saving lives with a simple blood spot Ashleigh Ragsdale, MPH Gauri Gupta, MScPH Objectives Newborn Screening Overview Process and Law Completing Collection Cards Video
Newborn Screening in Washington State Saving lives with a simple blood spot Ashleigh Ragsdale, MPH Gauri Gupta, MScPH Objectives Newborn Screening Overview Process and Law Completing Collection Cards Video
The Compelling Benefits of Routine 2 nd NBS: A Fifteen-Year Review in Washington State. Saving lives with a simple blood spot
 The Compelling Benefits of Routine 2 nd NBS: A Fifteen-Year Review in Washington State Caroline T. Nucup-Villaruz, MD Primary Author NBS Consultant - Disorder FU Santosh Shaunak Co-Author & Presenter Laboratory
The Compelling Benefits of Routine 2 nd NBS: A Fifteen-Year Review in Washington State Caroline T. Nucup-Villaruz, MD Primary Author NBS Consultant - Disorder FU Santosh Shaunak Co-Author & Presenter Laboratory
Organic Acid Disorders
 Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Metabolic Changes in ASD. Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius
 Metabolic Changes in ASD Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius 12 patients 3 Autism: Ages 3/3/3.7 3 PDD: Ages 3/3/6 3 Asperger: Ages 6/7/15.1 3 Speech delay and Sensory Problems (SHL):
Metabolic Changes in ASD Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius 12 patients 3 Autism: Ages 3/3/3.7 3 PDD: Ages 3/3/6 3 Asperger: Ages 6/7/15.1 3 Speech delay and Sensory Problems (SHL):
Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry: Experience of the Pilot Study in Andhra Pradesh, India
 Indian J Pediatr (August 2011) 78(8):953 960 DOI 10.1007/s12098-011-0398-9 ORIGINAL ARTICLE Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry: Experience of the Pilot Study
Indian J Pediatr (August 2011) 78(8):953 960 DOI 10.1007/s12098-011-0398-9 ORIGINAL ARTICLE Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry: Experience of the Pilot Study
Most common is the congenital adrenogenital syndrome (AGS) or congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency.
 Newborn Screening Examination parameters: TSH-neonatal (hypothyreosis), 17-OH progesterone (AGS), galactose (galactosemia), galactose-uridyl transferase (galacto semia), biotinidase (biotinidase ), phenylalanine
Newborn Screening Examination parameters: TSH-neonatal (hypothyreosis), 17-OH progesterone (AGS), galactose (galactosemia), galactose-uridyl transferase (galacto semia), biotinidase (biotinidase ), phenylalanine
MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES
 NEWBORN SCREENING Protecting your newborn MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES [ 1 ] NEWBORN SCREENING [ 2 ] FREQUENTLY ASKED QUESTIONS [ 4 ] DISORDERS INCLUDED IN NEWBORN SCREENING [ 12 ]
NEWBORN SCREENING Protecting your newborn MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES [ 1 ] NEWBORN SCREENING [ 2 ] FREQUENTLY ASKED QUESTIONS [ 4 ] DISORDERS INCLUDED IN NEWBORN SCREENING [ 12 ]
Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme
 Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme Charles Turner Laboratory Guy s Hospital (Evelina Childrens Hospital, St Thomas
Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme Charles Turner Laboratory Guy s Hospital (Evelina Childrens Hospital, St Thomas
Understanding metabolic disease
 Understanding metabolic disease Let s build a restaurant Chris Hendriksz Birmingham Children s Hospital 2006 The Task Let s build a restaurant! 2 Partners join to draw the plan. How can we change the
Understanding metabolic disease Let s build a restaurant Chris Hendriksz Birmingham Children s Hospital 2006 The Task Let s build a restaurant! 2 Partners join to draw the plan. How can we change the
How MS/MS Revolutionized Newborn Screening
 How MS/MS Revolutionized Newborn Screening David S Millington, PhD Medical Research Professor of Pediatrics Director, Biochemical Genetics Laboratory Duke University Medical Center NEWBORN SCREENING IN
How MS/MS Revolutionized Newborn Screening David S Millington, PhD Medical Research Professor of Pediatrics Director, Biochemical Genetics Laboratory Duke University Medical Center NEWBORN SCREENING IN
Metabolic Disorders. Chapter Thomson - Wadsworth
 Metabolic Disorders Chapter 28 1 Metabolic Disorders Inborn errors of metabolism group of diseases that affect a wide variety of metabolic processes; defective processing or transport of amino acids, fatty
Metabolic Disorders Chapter 28 1 Metabolic Disorders Inborn errors of metabolism group of diseases that affect a wide variety of metabolic processes; defective processing or transport of amino acids, fatty
Information for health professionals
 Introduction of a new screening test for newborn babies in Wales Newborn bloodspot screening for Medium chain acyl-coa dehydrogenase deficiency (MCADD) Newborn bloodspot screening for MCADD is being introduced
Introduction of a new screening test for newborn babies in Wales Newborn bloodspot screening for Medium chain acyl-coa dehydrogenase deficiency (MCADD) Newborn bloodspot screening for MCADD is being introduced
Fatty Acid Oxidation Disorders Organic Acid Disorders
 Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial,
Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial,
Pilot Study of Newborn Screening (NBS) for Inborn Errors of Metabolism (IEM) in Collaboration with Department of Health and Hospital Authority
 HA Convention 2017 Service Enhancement Presentation Healthcare Advances, Research and Innovations Pilot Study of Newborn Screening (NBS) for Inborn Errors of Metabolism (IEM) in Collaboration with Department
HA Convention 2017 Service Enhancement Presentation Healthcare Advances, Research and Innovations Pilot Study of Newborn Screening (NBS) for Inborn Errors of Metabolism (IEM) in Collaboration with Department
SUMMARY THE RELEVANCE OF THE NEONATAL URINE SCREENING FOR INBORN ERRORS OF METABOLISM PERFORMED IN QUÉBEC. Introduction
 SUMMARY THE RELEVANCE OF THE NEONATAL URINE SCREENING FOR INBORN ERRORS OF METABOLISM PERFORMED IN QUÉBEC Introduction Screening newborns for a number of hereditary diseases is common practice throughout
SUMMARY THE RELEVANCE OF THE NEONATAL URINE SCREENING FOR INBORN ERRORS OF METABOLISM PERFORMED IN QUÉBEC Introduction Screening newborns for a number of hereditary diseases is common practice throughout
Fatty Acid Oxidation Disorders
 Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social
Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social
Amino Acid Disorders. Disorder name: Tyrosinemia, type 1. What is tyrosinemia 1? Genetic Fact Sheets for Parents
 Genetic Fact Sheets for Parents Amino Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Amino Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
GUIDE TO NEWBORN SCREENING PROGRAMME
 \ GUIDE TO NEWBORN SCREENING PROGRAMME 1 MEDILAB PROFILE MEDILAB, the leading independent provider of Clinical Laboratory Diagnostic Services in Cyprus, was established in 1980 by Mr. C. Pavlides and has
\ GUIDE TO NEWBORN SCREENING PROGRAMME 1 MEDILAB PROFILE MEDILAB, the leading independent provider of Clinical Laboratory Diagnostic Services in Cyprus, was established in 1980 by Mr. C. Pavlides and has
NEWBORN SCREENING. in Massachusetts: Information for You and Your Baby. University of Massachusetts Medical School
 University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Information for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
University of Massachusetts Medical School NEWBORN SCREENING in Massachusetts: Information for You and Your Baby New England Newborn Screening Program Biotech 4, 2nd Floor UMass Medical School 377 Plantation
Inborn errors of metabolism (IEMs) represent a
 PEDIATRICS Nov 2003 VOL. 112 NO. 5 Newborn Screening by Tandem Mass Spectrometry for Medium-Chain Acyl-CoA Dehydrogenase Deficiency: A Cost-Effectiveness Analysis Laura N. Venditti, MSc*; Charles P. Venditti,
PEDIATRICS Nov 2003 VOL. 112 NO. 5 Newborn Screening by Tandem Mass Spectrometry for Medium-Chain Acyl-CoA Dehydrogenase Deficiency: A Cost-Effectiveness Analysis Laura N. Venditti, MSc*; Charles P. Venditti,
Beyond the case for NBS in South Africa. Chris Vorster 28/05/2016
 Beyond the case for NBS in South Africa Chris Vorster 28/05/2016 The case for NBS in SA Economic justification Cost Utility analysis = Cost/QALY GDP/Capita Immediate implementation (WHO) Political justification
Beyond the case for NBS in South Africa Chris Vorster 28/05/2016 The case for NBS in SA Economic justification Cost Utility analysis = Cost/QALY GDP/Capita Immediate implementation (WHO) Political justification
NEWBORN SCREENING. health.mo.gov/newbornscreening MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES
 NEWBORN SCREENING health.mo.gov/newbornscreening MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES Table of Contents NEWBORN SCREENING...1 FREQUENTLY ASKED QUESTIONS...2 DISORDERS INCLUDED IN NEWBORN SCREENING...4
NEWBORN SCREENING health.mo.gov/newbornscreening MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES Table of Contents NEWBORN SCREENING...1 FREQUENTLY ASKED QUESTIONS...2 DISORDERS INCLUDED IN NEWBORN SCREENING...4
Inborn Errors of Metabolism (IEM)
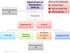 Clinical Presentation Inborn Errors of Metabolism (IEM) Click on the following: - Clinical Pearl - link to movie clip - link to picture Investigations Blood Work Urine No Acidosis NH 4 + Metabolic Acidosis
Clinical Presentation Inborn Errors of Metabolism (IEM) Click on the following: - Clinical Pearl - link to movie clip - link to picture Investigations Blood Work Urine No Acidosis NH 4 + Metabolic Acidosis
2017 Newborn Screening and Genetic Testing Symposium September 10, 2017
 Removing Short-chain Acyl-CoA Dehydrogenase (SCAD) Deficiency and Isobutyryl-CoA Dehydrogenase Deficiency (IBD) from the Newborn Screening Panel: Michigan s Experience 2017 Newborn Screening and Genetic
Removing Short-chain Acyl-CoA Dehydrogenase (SCAD) Deficiency and Isobutyryl-CoA Dehydrogenase Deficiency (IBD) from the Newborn Screening Panel: Michigan s Experience 2017 Newborn Screening and Genetic
Newborn Bloodspot Screening (NBS) Training for Health Visitors. December 2017
 Newborn Bloodspot Screening (NBS) Training for Health Visitors www.newbornbloodspotscreening.wales.nhs.uk December 2017 Aims To enable you to gain a clear understanding of the following: Aim and rationale
Newborn Bloodspot Screening (NBS) Training for Health Visitors www.newbornbloodspotscreening.wales.nhs.uk December 2017 Aims To enable you to gain a clear understanding of the following: Aim and rationale
For the Ongoing Management of Babies under 1 year old.
 Pathway Author: Nicy Turney, Senior Nurse Professional Lead, Health Visiting 19.06.17 For the Ongoing Management of Babies under 1 year old. Check Health Records (CHR) to undertake daily checks to identify:
Pathway Author: Nicy Turney, Senior Nurse Professional Lead, Health Visiting 19.06.17 For the Ongoing Management of Babies under 1 year old. Check Health Records (CHR) to undertake daily checks to identify:
Considerations in Choosing Screening Conditions: One (US) Approach
 22 Plenary Considerations in Choosing Screening Conditions: One (US) Approach Bradford L Therrell Jr, 1,2 MS, PhD Abstract The lack of a national policy on newborn screening (NBS) in the United States
22 Plenary Considerations in Choosing Screening Conditions: One (US) Approach Bradford L Therrell Jr, 1,2 MS, PhD Abstract The lack of a national policy on newborn screening (NBS) in the United States
Guideline for the diagnosis and management of isovaleryl-coa-dehydrogenase deficiency (isovaleric acidemia) - a systematic review -
 Guideline for the diagnosis and management of isovaleryl-coa-dehydrogenase deficiency (isovaleric acidemia) - a systematic review - Guideline development group International interdisciplinary guideline
Guideline for the diagnosis and management of isovaleryl-coa-dehydrogenase deficiency (isovaleric acidemia) - a systematic review - Guideline development group International interdisciplinary guideline
Slide 1: Newborn Bloodspot Screening for Medium-chain Acyl-CoA Dehydrogenase Deficiency (MCADD)
 Slide 1: Newborn Bloodspot Screening for Medium-chain Acyl-CoA Dehydrogenase Deficiency (MCADD) The information has been produced with thanks to the NHS Newborn Blood Spot Screening Programme. 1 Slide
Slide 1: Newborn Bloodspot Screening for Medium-chain Acyl-CoA Dehydrogenase Deficiency (MCADD) The information has been produced with thanks to the NHS Newborn Blood Spot Screening Programme. 1 Slide
Health and Wellness for all Arizonans. azdhs.gov
 To identify newborns with certain, rare disorders and assist families of affected infants so that they receive appropriate and timely treatment to prevent or delay serious medical problems. To identify
To identify newborns with certain, rare disorders and assist families of affected infants so that they receive appropriate and timely treatment to prevent or delay serious medical problems. To identify
Saudi Population & Genetic Diseases
 CHALLENGES TO TREATMENT OF INHERITED METABOLIC DISORDES: THE SAUDI EXPEREINCE Zuhair N. Al-Hassnan, MD Dept. of Medical Genetics, King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia
CHALLENGES TO TREATMENT OF INHERITED METABOLIC DISORDES: THE SAUDI EXPEREINCE Zuhair N. Al-Hassnan, MD Dept. of Medical Genetics, King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia
Mary Seeterlin, PhD Michigan Department of Community Health. Prevent Disease Promote Wellness Improve Quality of Life
 Mary Seeterlin, PhD Michigan Department of Community Health Prevent Disease Promote Wellness Improve Quality of Life CPTII - Three Clinical Phenotypes Lethal Neonatal Most severe form Symptoms begin within
Mary Seeterlin, PhD Michigan Department of Community Health Prevent Disease Promote Wellness Improve Quality of Life CPTII - Three Clinical Phenotypes Lethal Neonatal Most severe form Symptoms begin within
Organic Acid Disorders
 Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Fatty Acid Oxidation Disorders
 Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social
Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social
Carnitine palmitoyl transferase 2 deficiency (CPT2) is a rare inherited disorder that occurs when
 CPT2 Deficiency Carnitine palmitoyl transferase 2 deficiency (CPT2) is a rare inherited disorder that occurs when the last step in the entry of fats into sac-like bodies called mitochondria is blocked.
CPT2 Deficiency Carnitine palmitoyl transferase 2 deficiency (CPT2) is a rare inherited disorder that occurs when the last step in the entry of fats into sac-like bodies called mitochondria is blocked.
Amino Acid Disorders. What is ASAL deficiency? Genetic Fact Sheets for Parents
 Genetic Fact Sheets for Parents Amino Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Amino Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Newborn Screening in Japan: Restructuring for the New Era
 Plenary 13 Newborn Screening in Japan: Restructuring for the New Era Seiji Yamaguchi, 1 MD Abstract Nationwide neonatal mass screening for inherited metabolic diseases has started in Japan since 1977.
Plenary 13 Newborn Screening in Japan: Restructuring for the New Era Seiji Yamaguchi, 1 MD Abstract Nationwide neonatal mass screening for inherited metabolic diseases has started in Japan since 1977.
UREA CYCLE DISORDERS - The What, Why, How and When
 UREA CYCLE DISORDERS - The What, Why, How and When George A. Diaz, MD, PhD Program for Inherited Metabolic Diseases Department of Genetics and Genomic Sciences Department of Pediatrics Icahn School of
UREA CYCLE DISORDERS - The What, Why, How and When George A. Diaz, MD, PhD Program for Inherited Metabolic Diseases Department of Genetics and Genomic Sciences Department of Pediatrics Icahn School of
Screening for Phenylketonuria: A Literature Update for the U.S. Preventive Services Task Force
 Screening for Phenylketonuria: A Literature Update for the U.S. Preventive Services Task Force Prepared by: Iris Mabry-Hernandez, MD, MPH Tracy Wolff, MD, MPH Kathy Green, MD, MPH Corresponding Author:
Screening for Phenylketonuria: A Literature Update for the U.S. Preventive Services Task Force Prepared by: Iris Mabry-Hernandez, MD, MPH Tracy Wolff, MD, MPH Kathy Green, MD, MPH Corresponding Author:
Introduction to Organic Acidemias. Hilary Vernon, MD PhD Assistant Professor of Genetic Medicine Johns Hopkins University 7.25.
 Introduction to Organic Acidemias Hilary Vernon, MD PhD Assistant Professor of Genetic Medicine Johns Hopkins University 7.25.2014 A Brief Historical Overview Garrod, Archibald E. 1902. The Incidence of
Introduction to Organic Acidemias Hilary Vernon, MD PhD Assistant Professor of Genetic Medicine Johns Hopkins University 7.25.2014 A Brief Historical Overview Garrod, Archibald E. 1902. The Incidence of
Clinical Management of Organic Acidemias and OAA Natural History Registry. Kim Chapman MD PhD Children s National Rare Disease Institute
 Clinical Management of Organic Acidemias and OAA Natural History Registry Kim Chapman MD PhD Children s National Rare Disease Institute Disclosure Nothing to disclose concerning this lecture Organic acid?
Clinical Management of Organic Acidemias and OAA Natural History Registry Kim Chapman MD PhD Children s National Rare Disease Institute Disclosure Nothing to disclose concerning this lecture Organic acid?
Screening Newborns for Congenital Disorders
 Screening Newborns for Congenital Disorders Gary L. Hoffman, BS; Ronald H. Laessig, PhD ABSTRACT The Newborn Screening Laboratory at the Wisconsin State Laboratory of Hygiene (WSLH) tests all newborn babies
Screening Newborns for Congenital Disorders Gary L. Hoffman, BS; Ronald H. Laessig, PhD ABSTRACT The Newborn Screening Laboratory at the Wisconsin State Laboratory of Hygiene (WSLH) tests all newborn babies
Inborn Errors of Metabolism Clinical Approach to Diagnosis and Treatment
 Case Scenario: 15 year old girl presented in ED with aggressive behaviour and hallucinations. No associated fever, vomiting, seizures or developmental concerns Previously well Inborn Errors of Metabolism
Case Scenario: 15 year old girl presented in ED with aggressive behaviour and hallucinations. No associated fever, vomiting, seizures or developmental concerns Previously well Inborn Errors of Metabolism
UK NATIONAL METABOLIC BIOCHEMISTRY NETWORK GUIDELINES FOR THE INVESTIGATION OF HYPERAMMONAEMIA
 UK NATIONAL METABOLIC BIOCHEMISTRY NETWORK GUIDELINES FOR THE INVESTIGATION OF HYPERAMMONAEMIA Hyperammonaemia results from defective catabolism of amino acids to urea. Recognition and treatment of hyperammonaemia,
UK NATIONAL METABOLIC BIOCHEMISTRY NETWORK GUIDELINES FOR THE INVESTIGATION OF HYPERAMMONAEMIA Hyperammonaemia results from defective catabolism of amino acids to urea. Recognition and treatment of hyperammonaemia,
LOW CITRULLINE AS A MARKER FOR THE PROXIMAL UREA CYCLE DEFECTS EXPERIENCE OF THE NEW ENGLAND NEWBORN SCREENING PROGRAM
 LOW CITRULLINE AS A MARKER FOR THE PROXIMAL UREA CYCLE DEFECTS EXPERIENCE OF THE NEW ENGLAND NEWBORN SCREENING PROGRAM Inderneel Sahai, MD, FACMG Newborn Screening and Genetic Testing Symposium Oct 2014
LOW CITRULLINE AS A MARKER FOR THE PROXIMAL UREA CYCLE DEFECTS EXPERIENCE OF THE NEW ENGLAND NEWBORN SCREENING PROGRAM Inderneel Sahai, MD, FACMG Newborn Screening and Genetic Testing Symposium Oct 2014
Carbamoyl Phosphate Synthetase (code 50470) Notice of Assessment
 Carbamoyl Phosphate Synthetase (code 50470) Notice of Assessment June 2013 DISCLAIMER: This document was originally drafted in French by the Institut national d'excellence en santé et en services sociaux
Carbamoyl Phosphate Synthetase (code 50470) Notice of Assessment June 2013 DISCLAIMER: This document was originally drafted in French by the Institut national d'excellence en santé et en services sociaux
Executive summary. Criteria for inclusion in the screening programme
 Executive summary Health Council of the Netherlands. Neonatal screening: new recommendations. The Hague: Health Council of the Netherlands, 2015; publication no. 2015/08 Shortly after birth, almost every
Executive summary Health Council of the Netherlands. Neonatal screening: new recommendations. The Hague: Health Council of the Netherlands, 2015; publication no. 2015/08 Shortly after birth, almost every
Newborn Screening: Past, Present and the Future
 The Hong Kong College of Pathologists, Incorporated in Hong Kong with Limited Liability Volume 11, Issue 2 August 2016 Editorial note: In this topical update, Dr Chloe Mak reviews the history and development
The Hong Kong College of Pathologists, Incorporated in Hong Kong with Limited Liability Volume 11, Issue 2 August 2016 Editorial note: In this topical update, Dr Chloe Mak reviews the history and development
Disorder name: Argininemia / Arginase deficiency Acronym: ARG 1 deficiency
 Genetic Fact Sheets for Parents Amino Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Amino Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
2015 Annual Report for New Mexico s Newborn Screening (NBS) Program
 2015 Annual Report for New Mexico s Newborn Screening (NBS) Program Sawyer-A Family s Story Inside this Edition A Family Story......1 Why Screen....4 When to Screen...4 What is NBS....5 Screened Conditions..5
2015 Annual Report for New Mexico s Newborn Screening (NBS) Program Sawyer-A Family s Story Inside this Edition A Family Story......1 Why Screen....4 When to Screen...4 What is NBS....5 Screened Conditions..5
Saving Lives Through Newborn Screening: Making the Most of a Simple Blood Test
 Saving Lives Through Newborn Screening: Making the Most of a Simple Blood Test February 26, 2018 John D. Thompson, PhD, MPH, MPA Director - NBS Program Washington State Public Health Laboratories What
Saving Lives Through Newborn Screening: Making the Most of a Simple Blood Test February 26, 2018 John D. Thompson, PhD, MPH, MPA Director - NBS Program Washington State Public Health Laboratories What
Coverage Guidelines: Oral Formula: Rhode Island Products
 Coverage Guidelines: Oral Formula: Rhode Island Products Effective: August 9, 2017 Clinical Documentation and Prior Authorization Required Applies to: Coverage Guideline, No Prior Authorization Tufts Health
Coverage Guidelines: Oral Formula: Rhode Island Products Effective: August 9, 2017 Clinical Documentation and Prior Authorization Required Applies to: Coverage Guideline, No Prior Authorization Tufts Health
Newborn Metabolic Screening Programme Annual Report
 Newborn Metabolic Screening Programme Annual Report January to December 2015 Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland District Health Board.
Newborn Metabolic Screening Programme Annual Report January to December 2015 Disclaimer This publication reports on information provided to the Ministry of Health by the Auckland District Health Board.
Xiaolei Xie, Marta Kozak. Thermo Fisher Scientific, 355 River Oaks Parkway, San Jose, CA Introduction
 Comparison of Non-derivatization and Derivatization Tandem Mass Spectrometry Methods for Analysis of Amino Acids, Acylcarnitines, and Succinylacetone in Dried Blood Spots Xiaolei Xie, Marta Kozak Thermo
Comparison of Non-derivatization and Derivatization Tandem Mass Spectrometry Methods for Analysis of Amino Acids, Acylcarnitines, and Succinylacetone in Dried Blood Spots Xiaolei Xie, Marta Kozak Thermo
Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet
 Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet Website:www.tipn.org.tw Telephone:(02)85962050 Ext. 401-403 Service line:(02)85962065 Fax:(02)85962067
Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet Website:www.tipn.org.tw Telephone:(02)85962050 Ext. 401-403 Service line:(02)85962065 Fax:(02)85962067
CYSTIC FIBROSIS. The condition:
 CYSTIC FIBROSIS Both antenatal and neonatal screening for CF have been considered. Antenatal screening aims to identify fetuses affected by CF so that parents can be offered an informed choice as to whether
CYSTIC FIBROSIS Both antenatal and neonatal screening for CF have been considered. Antenatal screening aims to identify fetuses affected by CF so that parents can be offered an informed choice as to whether
QA/QC 2 Strategies to Reduce False Positives and False Negatives (Part 2)
 QA/QC 2 Strategies to Reduce False Positives and False Negatives (Part 2) Tuesday, Oct. 28 10:30am-12:00pm Moderators Patricia Hunt, Texas Department of State Health Services and Bob Currier, California
QA/QC 2 Strategies to Reduce False Positives and False Negatives (Part 2) Tuesday, Oct. 28 10:30am-12:00pm Moderators Patricia Hunt, Texas Department of State Health Services and Bob Currier, California
e-learning Fatty Acid Oxidation Defects Camilla Reed and Dr Simon Olpin Sheffield Children s Hospital
 e-learning Fatty Acid Oxidation Defects Camilla Reed and Dr Simon Olpin Sheffield Children s Hospital Fatty Acids Fatty acids are a major source of energy and body fat is an energy dense material. They
e-learning Fatty Acid Oxidation Defects Camilla Reed and Dr Simon Olpin Sheffield Children s Hospital Fatty Acids Fatty acids are a major source of energy and body fat is an energy dense material. They
Newborn screening with tandem mass spectrometry:
 Newborn screening with tandem mass spectrometry: Examining its cost-effectiveness in the Wisconsin Newborn Screening Panel Ralph P. Insinga, BA, Ronald H. Laessig, PhD, and Gary L. Hoffman, BA Objective:
Newborn screening with tandem mass spectrometry: Examining its cost-effectiveness in the Wisconsin Newborn Screening Panel Ralph P. Insinga, BA, Ronald H. Laessig, PhD, and Gary L. Hoffman, BA Objective:
Community Genetics. Hanan Hamamy Department of Genetic Medicine & Development Geneva University Hospital
 Community Genetics Hanan Hamamy Department of Genetic Medicine & Development Geneva University Hospital Training Course in Sexual and Reproductive Health Research Geneva 2011 Definition of Community Genetics
Community Genetics Hanan Hamamy Department of Genetic Medicine & Development Geneva University Hospital Training Course in Sexual and Reproductive Health Research Geneva 2011 Definition of Community Genetics
