Ethel S. Siris 1 and G. David Roodman 2
|
|
|
- Mariah Eileen Ryan
- 5 years ago
- Views:
Transcription
1 Chapter 59. Paget s Disease of Bone Ethel S. Siris 1 and G. David Roodman 2 1 Department of Medicine, Columbia University College of Physicians and Surgeons, New York, New York; and 2 Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania INTRODUCTION Paget s disease of bone is a localized disorder of bone remodeling. The process is initiated by increases in osteoclastmediated bone resorption, with subsequent compensatory increases in new bone formation, resulting in a disorganized mosaic of woven and lamellar bone at affected skeletal sites. This structural change produces bone that is expanded in size, less compact, more vascular, and more susceptible to deformity or fracture than is normal bone. (1) Clinical signs and symptoms will vary from one patient to the next depending on the number and location of affected skeletal sites, as well as on the degree and extent of the abnormal bone turnover. It is believed that most patients are asymptomatic, but a substantial minority may experience a variety of symptoms, including bone pain, secondary arthritic problems, bone deformity, excessive warmth over bone from hypervascularity, and a variety of neurological complications caused in most instances by compression of neural tissues adjacent to pagetic bone. ETIOLOGY Although Paget s disease is the second most common bone disease after osteoporosis, little is known about its pathogenesis why it is highly localized, the potential role paramyxoviral infection might play, the basis for the unusual geographic distribution, and the contribution of a genetic component to the disease process. It is abundantly clear that there is a strong genetic predisposition involved in the pathophysiology of Paget s disease. Paget s disease occurs commonly in families and can be transmitted vertically between generations in an affected family. In patients with Paget s disease described in several clinical series, 15 30% have positive family histories of the disorder. (2) An extensive study of relatives of 35 patients with Paget s disease in Madrid revealed that 40% of the patients had at least one first-degree relative affected with the disease. (3) Other studies have confirmed an autosomal dominant pattern of inheritance for Paget s disease. (4) Familial aggregation studies in a United States population (5) suggest that the risk of a firstdegree relative of a pagetic subject developing the condition is seven times greater than is the risk for someone who does not have an affected relative. Several genetic loci have been linked to familial Paget s disease. Cody et al. (6) described a predisposition locus on chromosome 18q in a large family with Paget s disease, and other groups (7,8) have identified different predisposition loci on chromosome 18, and on chromosome 6. No specific genes have been identified at these loci, and none are located on chromosome 13, which contains the RANKL gene. In a Japanese family with atypical Paget s disease, a mutation in the RANK gene has been reported, (9) but this mutation is not found in the overwhelming majority of patients with familial Paget s disease. (10) Laurin et al. (11) in Quebec have mapped a mutation in a gene on 5q35-QTER, which encodes a ubiquitin binding protein, sequestasome-1 (SQSTM14/p62 ZIP ), as a candidate gene for Dr. Siris is a consultant for Merck, Procter & Gamble, and Novartis, but not in the field of Paget s disease or its treatment. Dr. Roodman is a consultant for Scios, Merck, and Novartis. 320 Paget s disease because of its association with the NF- B signaling pathway. This mutation results in a proline to leucine substitution at amino acid 392 of the protein that was not found in 291 controls. The mutation was detected in 11 of 24 French- Canadian families with Paget s disease and 18 unrelated Paget s disease patients. Sequestasome-1 acts as an anchor protein and plays an important role in the NF- B signaling pathway. It binds either TNF receptor associated factor (TRAF)-6 in the interleukin (IL)-1 or receptor-interacting protein (RIP)-1 in the TNF signaling pathway to activate NF- B. However, the p62 ZIP mutation does not completely explain the pagetic phenotype. There is large phenotypic variability in patients with Paget s disease associated with the mutation in p62 ZIP. For example, one individual who is 77 years- old and carried this p62 ZIP mutation had no signs of the disease. (12) Moreover, homozygotes and heterozygotes seem to be similarly affected, suggesting that other genetic and/or environmental factors such as a common viral infection may contribute to the variability and the severity of Paget s disease of bone. (12) Hocking et al. (8) studied a large group of patients with familial Paget s disease and found a mutation in the p62 ZIP gene in 19% of the patients. A second mutation in p62 ZIP in which a T insertion that introduces a stop code in position 396 was also found in 6% of the families, and a third mutation affecting a splice donor site in intron A was found in 2% of the families. Thus, 30% of patients with familial Paget s disease have mutations in the p62 ZIP gene. These mutations are associated with a variable clinical phenotype, including no evidence of Paget s disease in at least one or two individuals, and they cannot explain the highly localized nature of the disease. Ethnic and geographic clustering of Paget s disease also has been described, with the intriguing observation that the disorder is quite common in some parts of the world but relatively rare in others. Clinical observations indicate that the disease is most common in Europe, North America, Australia, and New Zealand. Studies surveying radiologists have computed prevalence rates in hospitalized patients 55 years in several European cities and found the highest percentages in England (4.6%) and France (2.4%), with other Western European countries reporting slightly lower prevalences (e.g., % in Ireland, 1.3% in Spain and West Germany, and 0.5% in Italy and Greece). (13) There is a remarkable focus of Paget s disease in Lancashire, England, where % of people 55 years in several Lancashire towns had radiographs revealing Paget s disease. (14) Prevalence rates seem to decrease from north to south in Europe, except for the finding that Norway and Sweden have a particularly low rate (0.3%). (13) Few data are available from Eastern Europe, but Russian colleagues indicate that Paget s disease is not uncommon in that country. The disorder is seen in Australia and New Zealand at rates of 3 4%. (15) Paget s disease is distinctly rare in Asia, particularly in China, India, and Malaysia, although occasional cases of Indians living in the United States have been documented. Similar radiographic studies have described a prevalence of % in several areas of sub-saharan Africa. (15) In Israel, the disease is seen predominantly in Jews (16) but was recently found to exist in Israeli Arabs as well. (17) In Argentina, the disease seems to be restricted to an area surrounding Buenos Aires and predominantly occurs in patients descended from European immi-
2 PAGET S DISEASE OF BONE / 321 grants. (18) It is estimated, based on very few studies, that 2 3% of people 55 years living in the United States have Paget s disease. It is believed that most Americans with Paget s disease are white and of Anglo-Saxon or European descent. The disorder is described in blacks, and most clinical series from hospitals in major American cities report having black patients. (2,19) Some recent studies have remarked on an apparent decline in the frequency and severity of Paget s disease in both New Zealand and Great Britain. (20,21) The basis for this decline is unknown, but the changes are too rapid to be explained by a genetic cause and cannot be explained by migration patterns of persons with a predisposition to Paget s disease. For 30 years, studies have suggested that Paget s disease may result from a chronic paramyxoviral infection. This is based on ultrastructural studies by Rebel et al., (22) who showed that nuclear and, less commonly, cytoplasmic inclusions that were similar to nucleocapsids from paramyxoviruses were present in osteoclasts from Paget s disease patients. Mills et al. (23) also reported that the measles virus nucleocapsid antigen was present in osteoclasts from patients with Paget s disease, but not from patients with other bone diseases. In some specimens, both measles virus and respiratory syncytial virus nucleocapsid proteins were shown by immunocytochemistry on serial sections. Similarly, Basle et al. (24) have also shown the presence of measles virus nucleocapsid protein in patients with Paget s disease, but also found other paramyxoviral proteins as well. Recently, Friedrichs et al. (25) have reported the full sequence of the measles virus nucleocapsid protein isolated from a patient with Paget s disease as well as 700 bp of measles virus nucleocapsid protein sequence from three other patients. In contrast, Gordon et al., (26) using in situ hybridization studies, examined specimens from English patients with Paget s disease and found canine distemper virus nucleocapsid protein in 11 of 25 patients. Mee et al., (27) using highly sensitive in situ PCR techniques, found that osteoclasts from 12 of 12 English patients with Paget s disease expressed canine distemper virus nucleocapsid transcripts. Kurihara et al. (28) have provided in vitro evidence for a possible pathophysiologic role for measles virus in the abnormal osteoclast activity in Paget s disease. They transfected the measles virus nucleocapsid gene into normal human osteoclast precursors and showed that the osteoclasts that formed expressed many of the abnormal characteristics of pagetic osteoclasts. However, other workers have been unable to confirm the presence of measles virus or canine distemper virus in pagetic osteoclasts, (29) so that the role of a chronic paramyxoviral infection in Paget s disease remains controversial. Recently, Kurihara et al. targeted the measles virus nucleocapsid gene to cells in the osteoclast lineage in transgenic mice, and 40% of these mice developed localized bone lesions that are similar to lesions seen in patients with Paget s disease. However, these results do not show a cause and effect relationship between measles virus and Paget s disease. (30) Among the many questions that need to be explained to understand a putative viral etiology of Paget s disease are as follows. (1) Because paramyxoviral infections such as measles virus occur worldwide, why does Paget s disease have a very restricted geographic distribution? (2) How does the virus persist in osteoclasts in patients who are immunocompetent for such long periods of time, because measles virus infections generally occur in children rather than adults, and Paget s disease is usually diagnosed in elderly patients over the age of 55? The presence of an acquired or inherited genetic component to explain Paget s disease has its limitations as well. It is very difficult to explain the variable phenotypic presentation of patients with familial Paget s disease, especially that some of these patients who carry the mutated gene do not have Paget s disease although they are 70 years of age. Furthermore, it is very difficult to explain how a mutation of a specific gene expressed in bone results in a highly focal disease such as Paget s disease. More likely, environmental factors and genetic factors are both required for patients to develop Paget s disease. PATHOLOGY Histopathologic Findings in Paget s Disease The initiating lesion in Paget s disease is an increase in bone resorption. This occurs in association with an abnormality in the osteoclasts found at affected sites. Pagetic osteoclasts are more numerous than normal and contain substantially more nuclei than do normal osteoclasts, with up to 100 nuclei per cell noted by some investigators. In response to the increase in bone resorption, numerous osteoblasts are recruited to pagetic sites where active and rapid new bone formation occurs. It is generally believed that the osteoblasts are intrinsically normal, (31) but this has not been proven conclusively. In the earliest phases of Paget s disease, increased osteoclastic bone resorption dominates, a picture appreciated radiographically by an advancing lytic wedge or blade-of-grass lesion in a long bone or by osteoporosis circumscripta, as seen in the skull. At the level of the bone biopsy, the structurally abnormal osteoclasts are abundant. After this, there is a combination of increased resorption and relatively tightly coupled new-bone formation, produced by the large numbers of osteoblasts present at these sites. During this phase, and presumably because of the accelerated nature of the process, the new bone that is made is abnormal. Newly deposited collagen fibers are laid down in a haphazard rather than a linear fashion, creating more primitive woven bone. The woven-bone pattern is not specific for Paget s disease, but it does reflect a high rate of bone turnover. The end product is the so-called mosaic pattern of woven bone plus irregular sections of lamellar bone linked in a disorganized way by numerous cement lines representing the extent of previous areas of bone resorption. The bone marrow becomes infiltrated by excessive fibrous connective tissue and by an increased number of blood vessels, explaining the hypervascular state of the bone. Bone matrix at pagetic sites is usually normally mineralized, and tetracycline labeling shows increased calcification rates. It is not unusual, however, to find areas of pagetic biopsy specimens in which widened osteoid seams are apparent, perhaps reflecting inadequate calcium/phosphorus products in localized areas where rapid bone turnover heightens mineral demands. In time, the hypercellularity at a locus of affected bone may diminish, leaving the end product of a sclerotic, pagetic mosaic without evidence of active bone turnover. This is so-called burned out Paget s disease. Typically, all phases of the pagetic process can be seen at the same time at different sites in a particular subject. Scanning electron microscopy affords an excellent view of the chaotic architectural changes that occur in pagetic bone and provides the visual imagery that makes comprehensible the loss of structural integrity. Figure 1 compares the appearances of normal and of pagetic bone using this technique. Figure 2 show the mosaic pattern of disorganized bone in Paget s disease in most of the field, contrasted with a normal pattern of new bone deposition after restoration of normal turnover with bisphosphonate therapy.
3 322 / CHAPTER 59 FIG. 1. Scanning electron micrographs with sections of normal bone (left) and pagetic bone (right). Both samples were taken from the iliac crest. The normal bone shows the trabecular plates and marrow spaces to be well preserved, whereas the pagetic bone has completely lost this architectural appearance. Extensive pitting of the pagetic bone is apparent, caused by dramatically increased osteoclastic bone resorption. [Photographs courtesy of Dr. David Dempster. The first photograph is reprinted from J Bone Miner Res :15 21 with permission of the American Society for Bone and Mineral Research. The second photograph is reproduced from Siris ES, Canfield RE 1995 Paget s disease of bone. In: Becker KL (ed.) Principles and Practice of Endocrinology and Metabolism. 2nd ed. JB Lippincott, Philadelphia, PA, USA, pp , with permission.] Biochemical Parameters of Paget s Disease Increases in the urinary excretion of biomarkers of bone resorption such as collagen cross-links and associated peptides (e.g., N-telopeptide of type 1 collagen [NTX], C-telopeptide of type 1 collagen [CTX], deoxypyridinoline [DPD]) (32) reflect the primary lesion in Paget s disease, the increase in bone resorption. Increases in osteoblastic activity are associated with elevated levels of serum alkaline phosphatase. In untreated patients, the values of these two markers rise in proportion to each other, offering a reflection of the preserved coupling between resorption and formation. From the clinical perspective, the degree of elevation of these indices offers an approximation of the extent or severity of the abnormal bone turnover, with higher levels reflecting a more active, ongoing localized metabolic process. Interestingly, the patients with the highest alkaline phosphatase elevations (e.g., 10 times the upper limit of normal) typically have involvement of the skull as at least one site of the disorder. Active monostotic disease (other than skull) may have lower biochemical values than polyostotic disease. Lower values (e.g., less than three times the upper limit of normal) may reflect a lesser extent of involvement (i.e., fewer sites on bone scans or radiographs) or a lesser degree of increased bone turnover at affected skeletal sites. However, mild elevations in a patient with highly localized disease (e.g., the proximal tibia) may be associated with symptoms and clear progression of disease at the affected site over time. Indeed, a so-called normal alkaline phosphatase (e.g., a value a slightly less than the upper limit of normal for the assay) may not truly be normal for the pagetic patient. Today many would argue that to be confident that the value is normal (and the disease quiescent), a result in the middle of the normal range is required. In addition to offering some estimate of the degree of increased bone turnover, measurement of a bone resorption marker and serum alkaline phosphatase is useful in observing the disorder over time and especially for monitoring the effects of treatment. Potent bisphosphonates are capable of normalizing the biochemical markers (i.e., producing a remission of the bone remodeling abnormality) in a majority of patients and bringing the markers to near normal in most others so that the monitoring role has heightened importance in assessing treatment effects. A urinary resorption marker such as the N- or C-telopeptide of collagen may become normal in days to a few weeks after bisphosphonate therapy is initiated. It is often most practical and least expensive, however, to monitor serum alkaline phosphatase as the sole biochemical endpoint, with a baseline measure and subsequent follow-up tests at intervals appropriate for the therapy used. If a patient has concomitant elevations of liver enzymes, a measurement of bone-specific alkaline phosphatase can be especially helpful. Serum osteocalcin, however, is not a useful measurement in Paget s disease. Serum calcium levels are typically normal in Paget s disease, but they may become elevated in two special situations. First, if a patient with active, usually extensive Paget s disease is immobilized, the loss of the weight-bearing stimulus to new bone formation may transiently uncouple resorption and accretion, so that increasing hypercalciuria and hypercalcemia may occur. This is a relatively infrequent occurrence. Alternatively, when hypercalcemia is discovered in an otherwise healthy, ambulatory patient with Paget s disease, coexistent primary hyperparathyroidism may be the cause. Inasmuch as increased levels of PTH can drive the intrinsic pagetic remodeling abnormality to even higher levels of activity, correction of primary hyperparathyroidism in such cases is indicated. Several investigators have commented on the 15 20% prevalence of secondary hyperparathyroidism (associated with normal levels of serum calcium) in Paget s disease, typically seen in patients with very high levels of serum alkaline phosphatase. (33,34) The increase in PTH is believed to reflect the need to increase calcium availability to bone during phases of very active pagetic bone formation, particularly in subjects in whom dietary intake of calcium is inadequate. Secondary hyperparathyroidism and transient decreases in serum calcium also can occur in some patients being treated with potent bisphosphonates such as pamidronate, alendronate, risedronate, or zoledronic acid. This results from the effective and rapid suppression of bone resorption in the setting of ongoing new-bone formation. (35) Later, as restoration of coupling occurs with time, PTH levels fall. The problem can be largely avoided by being certain that such patients are and remain replete in both calcium and vitamin D. Elevations in serum uric acid and serum citrate have been described in Paget s disease and are of unclear clinical significance. (1) Gout has been noted in this disorder, but it is uncertain whether it is more common in pagetic patients than in nonpagetic subjects. Hypercalciuria may occur in some patients with Paget s disease, presumably because of the in- FIG. 2. Iliac crest bone with Paget s disease under polarized light. This patient had been treated with potent bisphosphonate therapy. Older bone is present in a pattern of woven bone (W), but new bone deposition after suppression of increased pagetic turnover shows a normal pattern of bone deposition (arrows).
4 PAGET S DISEASE OF BONE / 323 creased bone resorption, and kidney stones are occasionally found as a consequence of this abnormality.(1) Clinical Features of Paget s Disease Paget s disease affects both men and women, with most series describing a slight male predominance. It is rarely observed to occur in individuals 25 years of age, it is thought to develop as a clinical entity after the age of 40 in most instances, and it is most commonly diagnosed in people over the age of 50. In a survey of 800 selected patients in the United States, 600 of whom had symptoms, the average age at diagnosis was 58 years.(36) It seems likely that many patients have the disorder for a period of time before any diagnosis is made, especially because it is often an incidental finding. It is important to emphasize the localized nature of Paget s disease. It may be monostotic, affecting only a single bone or portion of a bone (Fig. 3), or may be polyostotic, involving two or more bones. Sites of disease are often asymmetric. A patient might have a pagetic right femur with a normal left, involvement of only one-half the pelvis, or involvement of several noncontiguous vertebral bodies. Clinical observation suggests that in most instances, sites affected with Paget s disease when the diagnosis is made are the only ones that will show pagetic change over time. Although progression of disease within a given bone may occur (Fig. 4), the sudden appearance of new sites of involvement years after the initial diagnosis is uncommon. This information can be very reassuring for patients who often worry about extension of the disorder to new areas of the skeleton as they age. The most common sites of involvement include the pelvis, femur, spine, skull, and tibia. The bones of the upper extremity, as well as the clavicles, scapulae, ribs, and facial bones, are less commonly involved, and the hands and feet are only rarely affected. It is generally believed that most patients with Paget s disease are asymptomatic and that the disorder is most often diagnosed when an elevated serum alkaline phosphatase is noted on routine screening or when a radiograph taken for an unrelated problem reveals typical skeletal changes. The development of symptoms or complications of Paget s disease is influenced by the particular areas of involvement, the interrelationship between affected bone and adjacent structures, the extent of metabolic activity, and presence or absence of disease progression within an affected site. Signs and Symptoms Bone pain from a site of pagetic involvement, experienced either at rest or with motion, is probably the most common symptom. The direct cause of the pain may be difficult to characterize and requires careful evaluation. Pagetic bone associated with a high turnover state has an increased vascularity, leading to a sensation of warmth of the skin overlying bone (e.g., skull or tibia) that some patients perceive as an unpleasant sensation. Small transverse lucencies along the expanded cortices of involved weight-bearing bones or advancing, lytic, blade-of-grass lesions sometimes cause pain. It is postulated that microfractures frequently occur in pagetic bone and can cause discomfort for a period of days to weeks. A bowing deformity of the femur or tibia can lead to pain for several possible reasons. A bowed limb is typically shortened, resulting in specific gait abnormalities that can lead to abnormal mechanical stresses. Clinically severe secondary arthritis can occur at joints adjacent to pagetic bone (e.g., the hip, knee, or ankle). The secondary gait problems also may lead to arthritic changes on the contralateral nonpagetic side, particularly at the hip. Back pain in pagetic patients is another difficult symptom to FIG. 3. Radiograph of a humerus showing typical pagetic change in the distal half, with cortical thickening, expansion, and mixed areas of lucency and sclerosis, contrasted with normal bone in the proximal half. assess. Nonspecific aches and pains may emanate from enlarged pagetic vertebrae in some instances; vertebral compression fractures also may be seen at pagetic sites. In the lumbar area, spinal stenosis with neural impingement may arise, producing radicular pain and possibly motor impairment. Degenerative changes in the spine may accompany pagetic changes,
5 324 / CHAPTER 59 and it is useful for the clinician to determine which symptoms arise as a consequence of the pagetic process and which result from degenerative disease of nonpagetic vertebrae. Kyphosis may occur, or there may be a forward tilt of the upper back, particularly when a compression fracture or spinal stenosis is present. Treatment options will differ, depending on the cause of the symptoms. When Paget s disease affects the thoracic spine, there may rarely be syndromes of direct spinal cord compression with motor and sensory changes. Several cases of apparent direct cord compression with loss of neural function have now been documented to have resulted from a vascular steal syndrome, whereby hypervascular pagetic bone steals blood from the neural tissue.(37) Paget s disease of the skull, shown radiographically in Fig. 5, may be asymptomatic, but common complaints in up to one third of patients with skull involvement may include an increase in head size with or without frontal bossing or deformity, or headache, sometimes described as a band-like tightening around the head. Hearing loss may occur as a result of isolated or combined conductive or neurosensory abnormalities; recent data suggest cochlear damage from pagetic involvement of the temporal bone with loss of bone density in the cochlear capsule is an important component.(38) Cranial nerve palsies (such as in nerves II, VI, and VII) occur rarely. With extensive skull involvement, a softening of the base of the skull may produce platybasia, or flattening, with the development of basilar invagination, so that the odontoid process begins to extend upward as the skull sinks downward on it. This feature can be appreciated by various radiographic measures including skull radiographs and CT or MRI scans. Although many patients with severe skull changes may have radiographic evidence of basilar invagination, a relatively small number develop a very serious complication, such as direct brainstem compression or an obstructive hydrocephalus and increased intracranial pressure caused by blockage of cerebrospinal fluid flow. Pagetic involvement of the facial bones may cause facial deformity, dental problems, and, rarely, narrowing of the airway. Mechanical changes of these types may lead to a nasal intonation when the patient is speaking. FIG. 4. This series of radiographs of a pagetic tibia show progression of pagetic change and bowing deformity in an untreated patient. This individual s Paget s disease was limited to the tibia and was associated with a serum alkaline phosphatase level that was generally only mildly elevated to about twice the upper limit of normal. Note the distal progression of cortical thickening with time, as well as the worsening of the bowing deformity. (Reprinted from Siris ES, Feldman F 1997 Clinical vignette: Natural history of untreated Paget s disease of the tibia. J Bone Miner Res 12: , with permission of the American Society for Bone and Mineral Research.) FIG. 5. Typical cotton-wool appearance of an enlarged pagetic skull with marked osteoblastic change. The patient had an increase in head size and deafness. Fracture through pagetic bone is an occasional and serious complication. These fractures may be either traumatic or pathological, particularly involving long bones with active areas of advancing lytic disease; the most common involve the femoral shaft or subtrochanteric area.(39) The increased vascularity of actively remodeling pagetic bone (i.e., with a moderately increased serum alkaline phosphatase) may lead to substantial blood loss in the presence of fractures caused by trauma. Fractures also may occur in the presence of areas of malignant degeneration, a rare complication of Paget s disease. Far more common are the small fissure fractures along the convex surfaces of bowed lower extremities, which may be asymptomatic, stable, and persistent for years, but sometimes a more extensive transverse lucent area extends medially from the cortex and may lead to a clinical fracture with time. As described later, there are data indicating that blade-of-grass lytic areas as well as these larger transverse fractures may respond to antipagetic treatment and heal. These types of lesions warrant radiographic follow-up over time. Conversely, the smaller fissure fractures typically do not change with treatment and, in the absence of new pain, rarely require extensive radiographic monitoring. In most cases, fracture through pagetic bone heals normally, although some groups have reported as high as a 10% rate of nonunion. Neoplastic degeneration of pagetic bone is a relatively rare event, occurring with an incidence of 1%. This abnormality has a grave prognosis, typically manifesting itself as new pain at a pagetic site. The most common site of sarcomatous change seems to be the pelvis, with the femur and humerus next in frequency.(40) Typically these lesions are destructive. The majority of the tumors are classified as osteogenic sarcomas, although both fibrosarcomas and chondrosarcomas are also seen. Current treatment regimens emphasize maximal resection of tumor mass and chemotherapy and sometimes radiotherapy. Unfortunately, in these typically elderly patients, death from massive local extension of disease or from pulmonary metastases occurs in the majority of cases in 1 3 years. Benign giant-cell tumors also may occur in bone affected by Paget s disease. These lesions may present as localized masses at the affected site. Radiographic evaluation may disclose lytic changes. Biopsy reveals clusters of large osteoclast-like cells,
6 PAGET S DISEASE OF BONE / 325 which some authors believe represent reparative granulomas. (41) These tumors may show a remarkable sensitivity to glucocorticoids, so in many instances, the mass will shrink or even disappear after treatment with prednisone or dexamethasone. (42) DIAGNOSIS When Paget s disease is suspected, the diagnostic evaluation should include a careful medical history and physical examination. The possibility of a positive family history and a symptom history should be ascertained. Gout, pseudogout, and arthritis are all possible complications of Paget s disease. Rarely patients with underlying intrinsic heart disease may develop congestive heart failure in the presence of severe Paget s disease. There are also reports suggesting that patients may have an increased incidence of calcific aortic disease. (43) Angioid streaks are seen on funduscopic examination of the eye in some patients with polyostotic Paget s disease. The physical examination also should note the presence or absence of warmth, tenderness, or bone deformity in the skull, spine, pelvis, and extremities, as well as evidence of loss of range of motion at major joints or leg length discrepancy. Laboratory tests include measurement of serum alkaline phosphatase and in some cases a urinary marker of bone resorption, as described earlier. Radiographic studies (bone scans and conventional radiographs) complete the initial evaluation. Bone biopsy is not usually indicated, as the characteristic radiographic and laboratory findings are diagnostic in most instances. Bone scans are the most sensitive means of identifying pagetic sites and are most useful for this purpose. Scans are nonspecific, however, and also can be positive in nonpagetic areas that have degenerative changes or, more ominously, may reflect metastatic disease. Plain radiographs of bones noted to be positive on the bone scan provide the most specific information, because the changes noted on the radiograph are usually characteristic to the point of being pathognomonic. Examples of these are shown in Figs Enlargement or expansion of bone, cortical thickening, coarsening of trabecular markings, and typical lytic and sclerotic changes may be found. Radiographs also provide data on the status of the joints adjacent to involved sites, identify fissure fractures, indicate the degree to which lytic or sclerotic lesions predominate, and show the presence or absence of deformity or fracture. Repeated scans or radiographs are usually unnecessary in observing patients over time, unless new symptoms develop or current symptoms become significantly worse. The possibility of an impending fracture or, rarely, of sarcomatous change should be borne in mind in these situations. Although imaging studies such as CT or MRI scans are not usually required in routine cases, a CT scan may be helpful in the assessment of a fracture where radiographs are not sufficient, and MRI scans are quite useful in assessing the possibility of sarcoma, giant cell tumor, or metastatic disease at a site of Paget s disease, in which case discovery of an accompanying soft tissue mass aids in diagnosis. The characteristic X-ray and clinical features of Paget s disease usually eliminate problems with differential diagnosis. However, an older patient may occasionally present with severe bone pain, elevations of the serum alkaline phosphatase and urinary N-telopeptide or deoxypyridinoline, a positive bone scan, and less-than-characteristic radiographic areas of lytic or blastic change. Here the possibility of metastatic disease to bone or some other form of metabolic bone disease (e.g., osteomalacia with secondary hyperparathyroidism) must be considered. Old radiographs and laboratory tests are very helpful in this setting, because normal studies a year earlier would make a diagnosis of Paget s disease less likely. A similar dilemma occurs when someone with known and established Paget s disease develops multiple painful new sites; here, too, the likelihood of metastatic disease must be carefully considered, and bone biopsy for a tissue diagnosis may be indicated. TREATMENT Antipagetic Therapy Specific antipagetic therapy consists of those agents capable of suppressing the activity of pagetic osteoclasts. Currently approved agents available by prescription in the United States include six bisphosphonate compounds: orally administered etidronate, tiludronate, alendronate, and risedronate; intravenously administered pamidronate and zoledronic acid; and parenterally administered synthetic salmon calcitonin. Each of these is discussed later. Between the mid-1970s, when treatments became available for the first time, and the mid-1990s, the mainstays of therapy were calcitonin and etidronate. However, these agents should generally be replaced as the first lines of therapy by the newer bisphosphonates, pamidronate, alendronate, risedronate and zoledronic acid, all progressively more potent than either etidronate or calcitonin, offering the potential for greater disease suppression and frank remission (i.e., normalization of pagetic indices) for prolonged periods. In addition to the newer bisphosphonates mentioned earlier, clodronate, more potent than etidronate and available in several other countries, has been shown to be effective in Paget s disease. (44) Olpadronate, neridronate, and ibandronate have significant activity in Paget s disease. (45 47) Gallium nitrate, approved in the United States for the treatment of cancer hypercalcemia, has been studied for efficacy in Paget s disease. (48) Other symptomatic treatments for Paget s disease, including analgesics, antiinflammatory drugs, use of orthotics or canes, and selected orthopedic and neurosurgical interventions, have important roles in management in many patients. Two logical indications for treatment of Paget s disease are to relieve symptoms and to prevent future complications. It has been clearly shown that suppression of the pagetic process by any of the available agents can effectively ameliorate certain symptoms in the majority of patients. Symptoms such as bone aches or pain (probably the most common complaints of Paget s disease), excessive warmth over bone, headache caused by skull involvement, low -back pain secondary to pagetic vertebral changes, and some syndromes of neural compression (e.g., radiculopathy and some examples of slowly progressive brainstem or spinal cord compression) are the most likely to be relieved. Pain caused by a secondary arthritis from pagetic bone involving the spine, hip, knee, ankle, or shoulder may or may not respond to antipagetic treatment. Filling in of osteolytic blade-of-grass lesions in weight-bearing bones has been reported in some treated cases with either calcitonin or bisphosphonates. On the other hand, a bowed extremity or other bone deformity will not change after treatment, and clinical experience indicates that deafness is unlikely to improve, although limited studies suggest that progression of hearing loss may be slowed (49) or even, in one case with pamidronate, reversed. (50) A second indication for treatment is to prevent the development of late complications in those patients deemed to be at risk, based on their sites of involvement and evidence of active disease, as shown by elevated levels of bone turnover markers. Admittedly, it has not been proved that suppression of pagetic bone turnover will prevent future complications. However, as
7 326 / CHAPTER 59 shown in Fig. 2, there is a restoration of normal patterns of new bone deposition in biopsy specimens after suppression of pagetic activity. It is also clear that active, untreated disease can continue to undergo a persistent degree of abnormal bone turnover for many years, with the possibility of severe bone deformity over time, as shown in Fig. 4. Indeed, substantial (e.g., 50%) but incomplete suppression of elevated indices of bone turnover with older therapies has been associated with disease progression (51) ; with bisphosphonates such as pamidronate, alendronate, risedronate, and zoledronic acid, however, indices become normal after treatment for extended periods in the majority of patients and approach normal in most of the rest. Thus, in the view of some investigators, the presence of asymptomatic but active disease (i.e., a serum alkaline phosphatase above normal) at sites where the potential for later problems or complications exists (e.g., weight-bearing bones, areas near major joints, vertebral bodies, extensively involved skull) is an indication for treatment. (52) The need for treatment in this setting may be particularly valid in patients who are younger, for whom many years of coexistence with the disorder is likely. However, even in the elderly, one can justify treatment if a degree of bone deformity is present that might create serious problems in the next few years. Others argue that the evidence does not yet support such use, because it has not been shown in clinical trials that disease suppression reduces progression of deformity. (53) Although controlled studies are not available to prove effectiveness in this situation, the use of a potent bisphosphonate before elective surgery on pagetic bone also is recommended. (54) The goal here is to reduce the hypervascularity associated with moderately active disease (e.g., a 3-fold or more elevation in serum alkaline phosphatase) to reduce the amount of blood loss at operation. Recently, recommendations for the management of Paget s disease have been published as guideline or management documents by consensus panels in the United States, (52) United Kingdom, (53) and Canada, (55) and the reader is referred to these thoughtful reviews. Bisphosphonates The discussion that follows will consider this class of drugs in their ascending order of potency and to some extent in terms of their historical development. It should be emphasized that while any of these medications might be chosen in a specific case, the agents that are considered to be first line at the time of this writing are pamidronate, alendronate, risedronate, and zoledronic acid. Etidronate. Etidronate was the first bisphosphonate to have been used clinically in the United States for Paget s disease (56,57) and was one of the two mainstays of therapy (with salmon calcitonin) for nearly 20 years. It is the least potent of the currently available bisphosphonate drugs. Etidronate is commercially available as Didronel (Procter & Gamble, Mason, OH, USA) in a 200- or 400-mg tablet. Although only a small percentage of the administered dose is absorbed, 5 mg/ kg/day will provide a 50% lowering of biochemical indices and a reduction in symptoms in the majority of patients. All bisphosphonates have the capacity to impair mineralization of newly forming bone if high enough doses are used. The dose of etidronate is limited by the fact that the doses that most effectively reduce the increased bone resorption can also impair mineralization, compelling the use of lower doses given for no longer than 6 months at a time. Thus, the recommended regimen for the agent is 5 mg/kg/day (i.e., 400 mg in most patients, taken with a small amount of water midway in a 4-h fast any time of day) for a 6-month period, followed by at least 6 months of no treatment. Etidronate is contraindicated in the presence of advancing lytic changes in a weight-bearing bone. Over several years of repeated 6-months-on, 6 months-off cycles, long-term benefit with maintenance of lower levels of pagetic biochemical activity has been observed in many patients, although others have become resistant to it after repeated courses. A failure to adhere to a cyclic low-dose regimen as described can induce bone pain and, occasionally, fracture caused by focal osteomalacia secondary to mineralization problems from excessive etidronate. However, careful cyclic management has been well tolerated by the majority of patients. Occasionally mild transient diarrhea may occur with etidronate, but this does not usually require more than a day or two of withholding the agent, after which it maybe taken again. More severe new pain in patients taking etidronate warrants stopping the drug and evaluating the patient before continuing therapy to be certain that lytic disease or impending fracture (particularly in a weight-bearing extremity) has not been exacerbated. In summary, etidronate is generally well tolerated and relatively easy to administer, but it affords a less robust suppression of turnover than the newer bisphosphonates and has the associated risk of mineralization problems with over use. Tiludronate. Tiludronate is about 10 times more potent than etidronate, and its use at effective doses is not associated with mineralization problems. Approved by the Food and Drug Administration (FDA) for Paget s disease in 1997, it is available as Skelid (Sanofi-Aventis, Bridgewater, NJ, USA) in a 200-mg tablet. The recommended dosage is 400 mg daily for 3 months with a 3-month post-treatment observation period, after which the serum alkaline phosphatase is likely to have reached its nadir. This approach led to a normal serum alkaline phosphatase at the 6-month point in 24 35% of moderately affected subjects in clinical trials. (58,59) It is generally well tolerated, with a minority of patients experiencing mild upper gastrointestinal upset. It seems to offer the benefits of etidronate without the risk of mineralization problems in patients for whom this might be a concern (e.g., those with lytic disease in lower extremities) and one half the total number of days of pills in a treatment course. As with etidronate, 400 mg of tiludronate should be taken with some water (in this case, 6 8 oz) at least 2 h away from food, and the patient should not lie down for the next 30 minutes. Patients also need to be calcium and vitamin D replete, but calcium supplements, like food, should not be taken within 2hofthetiludronate dose. Clinical experience with tiludronate is still relatively limited, with few data regarding duration of efficacy. It is a reasonable alternative to etidronate in patients with mild disease because it is well tolerated and requires only 3 months of active treatment. Patients who respond should have serum alkaline phosphatase measured at 3- to 4-month intervals and can be retreated when indices increase above normal or above a nadir level by 25% or more. Pamidronate. Pamidronate is in the range of 100 times more potent than etidronate. With its availability in the mid-1990s, a new philosophy of and approach to management became available to the clinician. The greater potency of pamidronate (and also of the other newer agents, alendronate, risedronate, and zoledronic acid) allows a majority of patients to experience a normalization of pagetic indices rather than only partial suppression, as is seen with calcitonin, etidronate, and (in most cases) tiludronate. Second, the effects may be longer lasting, so a limited course of treatment may provide up to a year or more
8 PAGET S DISEASE OF BONE / 327 of disease suppression. Third, all of the potent newer bisphosphonates have a much more favorable ratio of inhibition of bone resorption to inhibition of mineralization, so the threat of focal osteomalacia should be markedly reduced if not eliminated. With pamidronate there is an opportunity to individualize the dosing regimen to the needs of the specific patient, and there really is no single best dose. Indeed, the literature is replete with numerous approaches, (60 62) all of which seem to be effective. The package insert for pamidronate, available as Aredia (Novartis, East Hanover, NJ, USA), recommends three daily infusions of 30 mg each, over a period of 4 h each time, in 500 ml of normal saline or 5% dextrose in water. In clinical practice, experience has shown that this is probably not the best mode of administration. This dose is probably not high enough to achieve normalization of indices for many patients, and three daily infusions are highly impractical in most settings. Clinical experience indicates that patients with relatively mild disease may have a substantial reduction of alkaline phosphatase to normal or near normal with a single 60- to 90-mg infusion given over a 2-h period in ml of 5% dextrose in water. Patients with more moderate to severe disease (e.g., serum alkaline phosphatase levels more than three to four times normal) may require multiple infusions of mg infused as described and given on a once weekly or biweekly basis, primarily based on physician and patient convenience. Two to four 60-mg doses or three 90-mg doses may suffice in moderate disease (e.g., serum alkaline phosphatase in the range of four to five times normal). Total doses in the range of mg may be required in some severe cases (serum alkaline phosphatase times normal), given over a number of weeks. Suppression of urinary markers can often be noted within a few days after an infusion, but the serum alkaline phosphatase may take up to 2 3 months to reach its nadir. For moderate to severe cases, giving three to four 60-mg doses and then reassessing at 3 months with the possibility of more treatment is a reasonable approach. A successful course of therapy can result in 1 year or more of continued disease suppression with markers of turnover at normal or near-normal levels. Side effects may include a low-grade fever and flu-like symptoms in the first 24 h after the first ever infusion (decreasing in likelihood with repeated dosing) and the possibility of mild and transient hypocalcemia, hypophosphatemia, and lymphopenia. Venous irritation may arise, especially if an insufficient volume of fluid is used or if the fluid extravasates. It is desirable to provide oral calcium supplements at a dose of 500 mg, two or three times daily, and vitamin D 3, IU daily, to prevent or ameliorate a reduction in serum calcium and concomitant rise in PTH. Overall, pamidronate offers the opportunity to titrate the dosage as required in the individual patient, with the possibility of normalization or near normalization of biochemical indices and the potential for substantial and prolonged reduction in disease progression in many patients. In our view, it is most useful in patients with mild disease, in whom a single infusion may afford long-term benefit in a very cost-effective manner, and in severe and refractory cases, in which delivery of drug by vein bypasses problems with oral absorption. It is also a drug of choice for individuals who experience esophageal symptoms from alendronate or risedronate. The need for outpatient intravenous administration of multiple doses may be expensive and inconvenient in some cases. However, the rapid onset of symptomatic improvement and overall potency of the agent make it an obvious choice for cases with neurologic compression syndromes, for severe and painful lytic disease with or without impending fracture, and as a pretreatment of active Paget s disease before elective surgery to shrink the hypervascularity of the pagetic bone and decrease the amount of bleeding at operation. There has been one report of asymptomatic mineralization abnormalities with dosing in the usual clinical range, (63) but this is not the general experience. Recently, there has been a report of patients developing secondary resistance to pamidronate after repeated use. (64) Alendronate. Approved by the FDA for the treatment of Paget s disease in the fall of 1995, alendronate, sold as Fosamax (Merck, West Point, PA, USA), is an orally administered aminobisphosphonate that is 700 times more potent than etidronate and is not associated with mineralization problems at therapeutically effective doses. In a study of 89 patients with moderate to severe disease who received 6 months of either alendronate, 40 mg daily, or etidronate, 400 mg/day, alendronate led to a normalized serum alkaline phosphatase in 63% of subjects compared with 17% for etidronate; overall, alendronate led to a mean fall in alkaline phosphatase of 79% compared with 44% with etidronate. (65) Alendronate seemed to be as well tolerated as etidronate in this study, although symptoms of upper gastrointestinal discomfort or nausea, or the less common but more serious complication of esophageal ulceration, should be watched for at the 40-mg dose. Biopsy specimens from patients treated with alendronate revealed normal patterns of deposition of new bone (65,66) and radiologic improvement. (66) The recommended dose is 40 mg daily for 6 months to be taken on arising in the morning after an overnight fast with 8 oz of tap water. The patient is instructed not to take anything else orally (except more water) and not to lie down for at least 30 minutes after the dose. It is important with alendronate, as with pamidronate and tiludronate, that patients be replete in vitamin D and have a daily calcium intake of 1 to 1.5 g to avoid hypocalcemia early in the treatment course. Biochemical remissions may persist for months after a single course. Retreatment guidelines are incomplete as follow-up data are few, but many physicians have given repeat 6-month courses of alendronate once indices rise above normal with good success in re-establishing complete or partial biochemical remission. Risedronate. More than 1000 times more potent than etidronate, risedronate was approved by the FDA for use in Paget s disease in Risedronate is available as Actonel (Procter & Gamble). Studies with risedronate have described the efficacy of a 30-mg dose given for 2 (67) or 3 (68) months to patients with moderately active disease. These short courses of therapy led to a nearly 80% reduction in serum alkaline phosphatase and normalization of indices of bone turnover in 50 70% of patients. Thus, 30 mg can be given daily for 2 months, with a follow-up measurement of serum alkaline phosphatase 1 month later; if the value is not yet normal or near normal, a third or fourth month could be offered with a good likelihood of normalcy or near normalcy of indices thereafter, with a prolonged period of disease suppression similar to that achieved with pamidronate or alendronate. Once again, adequacy of calcium and vitamin D intake is important to avoid hypocalcemia. The 30-mg risedronate dose is taken with 8 oz of water on arising in the morning after an overnight fast, with no other oral intake (except water) and no lying down for 30 minutes after the dose. In the clinical trials, the main side effects were mild upper gastrointestinal upset in 15% of patients; symptoms of esophageal irritation were not common in these clinical trials with the 30-mg dose but should be kept in mind by the physician until more data are available. A few cases of iritis were seen, something also reported rarely with pamidronate.
9 328 / CHAPTER 59 Zoledronic Acid. The newest of the available drugs for Paget s disease, this agent is expected to be approved for this indication by the FDA in More potent than the previously described bisphosphonates, it is given at a dose of 5 mg, administered as a single 15-minute intravenous infusion. Unlike pamidronate, which has been available for many years and has been administered in a variety of ways with respect to dose and dosing interval with success, the data on zoledronic acid for Paget s disease come primarily from a clinical trial comparing a single dose of 5 mg of zoledronic acid with 2 months of oral risedronate, 30 mg/day, in a group of 357 patients with a mean baseline serum alkaline phosphatase about four times greater than normal. (69) Six months after initiation of treatment, 89% of patients treated with zoledronic acid and 58% of those receiving risedronate had a normal serum alkaline phosphatase. The main side effects seen with zoledronic acid included a flu-like syndrome in 9.6% of patients with myalgia, fever, headache, rigors, and nausea each in about 7% of subjects and bone pain in 5% within the first 2 3 days after treatment, a finding similar to that seen with pamidronate. Eight patients who received zoledronic acid also had hypocalcemia that was mildly symptomatic in only two, neither of whom was taking the prescribed calcium or vitamin D. One hundred thirteen of the original 182 patients assigned to zoledronic acid were followed without further treatment after the end of the 6-month study; and at a mean of 190 days later (i.e., about 1 year after treatment), all but one still maintained a therapeutic response, defined as either a normal serum alkaline phosphatase or a reduction of at least 75% from baseline in the alkaline phosphatase excess (difference between the measured level and the mid-point of the reference range). Further studies will be needed to determine the typical durations of biochemical remission in clinical practice. Osteonecrosis of the jaw has recently been described as a complication typically following dental extractions in patients receiving relatively high doses of potent bisphosphonates such as zoledronic acid and pamidronate given primarily for management of bone metastases. A few patients with Paget s disease have also been reported to have had this complication, but in some instances, these individuals received what seemed to be excessive amounts of bisphosphonate (e.g., 5 years of 40 mg/day of alendronate in one case, 18 months of monthly pamidronate 90 mg in another). (70) Calcitonin The polypeptide hormone, salmon calcitonin, is available therapeutically as a synthetic formulation for parenteral administration. It, like human and other calcitonins, was first shown to be efficacious in Paget s disease 30 years ago. (71,72) At present, the formulation approved for use in Paget s disease in the United States, sold as Miacalcin (Novartis), must be injected subcutaneously or intramuscularly. A nasal spray formulation of salmon calcitonin, approved by the FDA for use in postmenopausal osteoporosis, is available, although not specifically approved for treatment of Paget s disease. The usual starting dose is 100 U (0.5 ml; the drug is available in a 2-ml vial), generally self-injected subcutaneously, initially on a daily basis. Symptomatic benefit may be apparent in a few weeks, and the biochemical benefit (typically about a 50% reduction from baseline in serum alkaline phosphatase) is usually seen after 3 6 months of treatment. After this period, many clinicians reduce the dose to U every other day or three times weekly. Often, a dose of 50 U three times weekly after the first few months of therapy will maintain the achieved benefit. Patients with moderate to severe disease may require indefinite treatment to maintain a 50% reduction in the biochemical indices and symptomatic relief, but milder or monostotic disease may allow discontinuation of treatment for prolonged periods. Escape from the efficacy of salmon calcitonin may sometimes occur after a variable period of benefit. In some cases, this may be caused by a postulated downregulation of receptors, but in other instances, it may be a consequence of the development of neutralizing antibodies to the salmon polypeptide. (73) The main side effects of parenteral salmon calcitonin include, in a minority of patients, the development of nausea or queasiness, with or without flushing of the skin of the face and ears. These annoying side effects may last from a few minutes to several hours after each injection, although many patients can avoid them by experimenting with taking the agent at bedtime, with food, without food, and so on. Although these side effects are unpleasant, they do not seem to be serious or harmful, and most patients develop a tolerance to them. In summary, however, it is apparent that the newer bisphosphonates offer both greater effectiveness and ease of use, suggesting that this agent will be used in the future primarily by patients who do not tolerate oral or intravenous bisphosphonate therapy. Intranasal calcitonin is available as Miacalcin Nasal Spray. It seems to have a lower incidence of the side effects described earlier. The optimal dose in Paget s disease with the present formulation is not known, but anecdotal evidence suggests that, in occasional patients with mild disease, the 200-U single spray dose given daily may lower biochemical indices and relieve mild symptoms, such as increased warmth in a pagetic tibia. Other Therapies Analgesics, nonsteroidal anti-inflammatory agents, and Cox-2 inhibitors may be tried empirically with or without antipagetic therapy to relieve pain. Pagetic arthritis (i.e., osteoarthritis caused by deformed pagetic bone at a joint space) may cause periods of pain that are often helped by these agents. Surgery on pagetic bone (54) may be necessary in the setting of established or impending fracture. Elective joint replacement, more complex with Paget s disease than with typical osteoarthritis, is often very successful in relieving refractory pain. Rarely, osteotomy is performed to alter a bowing deformity in the tibia. Neurosurgical intervention is sometimes required in cases of spinal cord compression, spinal stenosis, or basilar invagination with neural compromise. Although medical management may be beneficial and adequate in some instances, all cases of serious neurological compromise require immediate neurological and neurosurgical consultation to allow the appropriate plan of management to be developed. As improved therapies emerge, long-term suppression of pagetic activity may have a preventive role in Paget s disease and, possibly, may obviate the need for surgical management in many cases. REFERENCES 1. Kanis JA 1998 Pathophysiology and Treatment of Paget s Disease of Bone. 2nd ed. Martin Dunitz, London, UK. 2. Siris ES, Canfield RE, Jacobs TP 1980 Paget s disease of bone. Bull NY Acad Med 56: Morales-Piga AA, Rey-Rey JS, Corres-Gonzalez J, Garcia-Sagredo IM, Lopez-Abente G 1995 Frequency and characteristics of familial aggregation of Paget s disease of bone. J Bone Miner Res 10: McKusick VA 1972 Heritable disorders of connective tissue, 5th ed. CV Mosby, St. Louis, MO, USA, pp Siris ES, Ottman R, Flaster E, Kelsey JL 1991 Familial aggregation of Paget s disease of bone. J Bone Miner Res 6: Cody JD, Singer FR, Roodman GD, Otterund B, Lewis TB, Leppert M
10 PAGET S DISEASE OF BONE / Genetic linkage of Paget disease of bone to chromosome 18q. Am J Hum Genet 61: Good DA, Busfield F, Fletcher BH, Duffy DL, Kesting JB, Andersen J, Shaw JT 2002 Linkage of Paget disease of bone to a novel region on human chromosome 18q23. Am J Hum Genet 70: Hocking LJ, Herbert CA, Nicholls RK, Williams F, Bennett ST, Cundy T, Nicholson GC, Wuyts W, Van Hul W, Ralston SH 2001 Genomewide search in familial Paget disease of bone shows evidence of genetic heterogeneity with candidate loci on chromosomes 2q36, 10p13, and 5q35. Am J Hum Genet 69: Sparks AB, Peterson SN, Bell C, Loftus BJ, Hocking L, Cahill DP, Frassica FJ, Streeten EA, Levine MA, Fraser CM, Adams MD, Broder S, Venter JC, Kinzler KW, Vogelstein B, Ralston SH 2001 Mutation screening of the TNFRSF11A gene encoding receptor activator of NF kappa B (RANK) in familial and sporadic Paget s disease of bone and osteosarcoma. Calcif Tissue Int 68: Hocking L, Slee F, Haslam SI, Cundy T, Nicholson G, van Hul W, Ralston SH 2000 Familial Paget s disease of bone: Patterns of inheritance and frequency of linkage to chromosome 18q. Bone 26: Laurin N, Brown JP, Morissette J, Raymond V 2002 Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet : Laurin N, Morissette J, Raymond V, Brown JP 2002 Large phenotypic variability of Paget disease of bone caused by the P392L sequestasome 1/p62 mutation. J Bone Miner Res 17:S1;S Barker DJ 1984 The epidemiology of Paget s disease of bone. Br Med Bull 40: Barker DJP, Chamberlain AT, Guyer PH, Gardner MJ 1980 Paget s disease of bone: The Lancashire focus. BMJ 280: Barry HC 1969 Paget s Disease of Bone. E&SLivingstone, Edinburgh, Scotland. 16. Dolev E, Samuel R, Foldes J, Brickman M, Assia A, Liberman U 1994 Some epidemiological aspects of Paget s disease in Israel. Semin Arthritis Rheum 23: Lowenthal MN, Alkalay D, Abu Rabbia Y, Liel Y 1995 Paget s disease of bone in Negev Bedouin: Report of two cases. Isr J Med Sci 31: Mautalen C, Pumarino H, Blanco MC, Gonzalez D, Ghiringhelli G, Fromm G 1994 Paget s disease: The South American experience. Semin Arthritis Rheum 23: Guyer PH, Chamberlain AT 1980 Paget s disease of bone in two American cities. BMJ 280: Cundy T, McAnulty K, Wattie D, Gamble G, Rutland M, Ibbertson HK 1997 Evidence for secular changes in Paget s disease. Bone 20: Cooper C, Schafheutle K, Dennison E, Kellingray S, Guyer P, Barker D 1999 The epidemiology of Paget s disease in Britain: Is the prevalence decreasing?. J Bone Miner Res 14: Rebel A, Malkani K, Basle M, Bregeon C 1997 Is Paget s disease of bone a viral infection? Calcif Tissue Res 22(Suppl): Mills BG, Singer FR, Weiner LP, Suffin SC, Stabile E, Holst P 1984 Evidence for both respiratory syncytial virus and measles virus antigens in the osteoclasts of patients with Paget s disease of bone. Clin Orthop 183: Basle M, Rebel A, Pouplard A, Kouyoumdjian S, Filmon R, Loepatezour A 1979 Demonstration by immunofluorescence and immunoperoxidase of an antigen of the measles type in the osteoclasts of Paget s disease of bone. Bull Assoc Anat 63: Friedrichs WE, Reddy SV, Bruder JM, Cundy T, Cornish J, Singer FR, Roodman GD 2002 Sequence analysis of measles virus nucleocapsid transcripts in patients with Paget s disease. J Bone Miner Res 17: Gordon MT, Mee AP, Sharpe PT 1994 Paramyxoviruses in Paget s disease. Semin Arthritis Rheum 23: Mee AP, Dixon JA, Hoyland JA, Davies M, Selby PL, Mawer EB 1998 Detection of canine distemper virus in 100% of Paget s disease samples by in situ-reverse transcriptase-polymerase chain reaction. Bone 23: Kurihara N, Reddy SV, Menaa C, Anderson D, Roodman GD 2000 Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest : Ooi CG, Walsh CA, Gallagher JA, Fraser WD 2000 Absence of measles virus and canine distemper virus transcripts in long-term bone marrow cultures from patients with Paget s disease of bone. Bone 27: Kunihara N, Zhou H, Reddy SV, Garcia-Palacios V, Subler MA, Dempster DW, Windle JJ, Roodman GD 2006 Expression of measles virus nucleocapsid protein in osteoclasts indures Paget s disease like bone lesions in mice. J Bone Miner Res 21: Rebel A, Basle M, Pouplard A, Malkani K. Filmon R, Lepatezour A 1980 Bone tissue in Paget s disease of bone: Ultrastructure and immunocytology. Arthritis Rheum 23: Calvo MS, Eyre DR, Gundberg CR 1996 Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev 17: Meunier PJ, Coindre JM, Edouard CM, Arlot ME 1980 Bone histomorphometry in Paget s disease: Quantitative and dynamic analysis of pagetic and non-pagetic bone tissue. Arthritis Rheum 23: Siris ES, Clemens TP, McMahon D, Gordon AG, Jacobs TP, Canfield RE 1989 Parathyroid function in Paget s disease of bone. J Bone Miner Res 4: Siris ES, Canfield RE The parathyroids and Paget s disease of bone. In: Bilezikian J, Levine M, Marcus R (eds.) The Parathyroids. Raven Press, New York, NY, USA, pp Siris ES 1991 Indications for medical treatment of Paget s disease of bone. In: Singer FR, Wallach S (eds.) Paget s disease of bone: Clinical assessment: Present and future therapy. Elsevier, New York, NY, USA, pp Herzberg L, Bayliss E 1980 Spinal cord syndrome due to noncompressive Paget s disease of bone: A spinal artery steal phenomenon reversible with calcitonin. Lancet 2: Monsell EM 2004 The mechanism of hearing loss in Paget s disease of bone. Laryngoscope 114: Barry HC 1980 Orthopedic aspects of Paget s disease of bone. Arthritis Rheum 23: Wick MR, Siegal GP, Unni KK, McLeod RA, Greditzer HB 1981 Sarcomas of bone complicating osteitis deformans (Paget s disease). Am J Surg PathoI 5: Upchurch KS, Simon LS, Schiller AL, Rosenthal DI, Campion EW, Krane SM 1983 Giant cell reparative granulomas of Paget s disease of bone: A unique clinical entity. Ann Intern Med 98: Jacobs TP, Michelsen J, Polay J, D Adamo AC, Canfield RE 1979 Giant cell tumor in Paget s disease of bone: Familial and geographic clustering. Cancer 44: Strickenberger SA, Schulman SP, Hutchins GM 1987 Association of Paget s disease of bone with calcific aortic valve disease. Am J Med 82: Delmas PD,Chapuy MC, VignonE, Charon S, Briancon D, Alexandre C, Edouard C, Meunier PJ 1982 Long-term effects of dichloromethylene diphosphonate in Paget s disease of bone. J Clin Endocrinol Metab 54: Gonzalez DC, Mautalen CA 1999 Short term therapy with oral olpadronate in active Paget s disease of bone. J Bone Miner Res 14: Adami S, Bevilacqua M, Broggini M, Filliponi P, Ortolani S, Palummeri E, Uliveri F, Nannipieri F, Braga V 2002 Short term intravenous therapy with neridronate in Paget s disease. Clin Exp Rheumatol 20: Woitge HW, Oberwittler H, Heichel S, Grauer A, Ziegler R, Seibel MJ 2000 Short- and long-term effects of ibandroante treatment on bone turnover in Paget disease of bone. Clin Chem 46: Bockman RS, Wilhelm F, Siris E, Singer F, Chausner A, Bitton R, Kotler J, Bosco BJ, Eyre DR, Levenson D 1995 A multi-center, prospective trial of gallium nitrate in patients with advanced Paget s disease of bone. J Clin Endocrinol Metab 80: EI-Sammaa M, Linthicum FH, House HP, House JW 1986 Calcitonin as treatment for hearing loss in Paget s disease. Am J Otol 7: Murdin L, Yeoh LH 2005 Hearing loss treated with pamidronate. J R Soc Med 98: Meunier PI, Vignot E 1995 Therapeutic strategy in Paget s disease of bone. Bone 17:489S 49IS. 52. Lyles KW, Siris ES, Singer FR, Meunier PJ 2001 A clinical approach to the diagnosis and management of Paget s disease of bone. J Bone Miner Res 16: Selby PL, Davie MWJ, Ralston SH, Stone MD 2002 Guidelines on the management of Paget s disease of bone. Bone 31: Kaplan FS 1999 Surgical management of Paget s disease. J Bone Miner Res 14:S2; Drake WM, Kendler DL, Brown JP 2001 Consensus statement on the modern therapy of Paget s disease of bone from a Western Osteoporosis Alliance Symposium. Clin Ther 23: Altman RD, Johnston CC, Khairi MRA, Wellman H, Serafini AN, Sankey RR 1973 Influence of disodium etidronate on clinical and laboratory manifestations of Paget s disease of bone (osteitis deformans). N Engl J Med 289: Canfield R, Rosner W, Skinner J, McWhorter J, Resnick L, Feldman F, Kammerman S, Ryan K, Kunigonis M, Bohne W 1977 Diphosphonate therapy of Paget s disease of bone. J Clin Endocrinol Metab 44: Roux C, Gennari C, Farrerons J, Devogelaer JP, Mulder H, Kruse HP, Picot C, Titeux L, Reginster JY, Dougados M 1995 Comparative pro-
11 330 / CHAPTER 60 spective, double-blind, multi-center study of the efficacy of tiludronate and etidronate in the treatment of Paget s disease of bone. Arthritis Rheum 38: McClung MR, Tou CPK, Goldstein NH, Picot C 1995 Tiludronate therapy for Paget s disease of bone. Bone 17:493S 496S. 60. Siris ES 1994 Perspectives: A practical guide to the use of pamidronate in the treatment of Paget s disease. J Bone Miner Res 9: Harinck HI, Papapoulos SE, Blanksrna HJ, Moolenaar AJ, Vermeij P, Bijvoet OL 1987 Paget s disease of bone: Early and late responses to three different modes of treatment with aminohydroxypropylidene bisphosphonate (APD). BMJ 295: Trombetti A, Arlot M, Thevenon J, Uebelhart B, Meunier PJ 1999 Effects of multiple intravenous pamidronate courses in Paget s disease of bone. Rev Rhum Engl Ed 66: Adamson BB, Gallacher SJ, Byars J, Ralston SH, Boyle IT, Boyce BF 1993 Mineralisation defects with pamidronate therapy for Paget s disease. Lancet 342: Gutteridge DH, Ward LC, Stewart GO, Retallack RW, Will RK, Prince RL, Criddle A, Bhagat CI, Stuckey BG, Price RI, Kent GN, Faulkner DL, Geelhoed E, Gan SK, Vasikaran S 1999 Paget s disease: Acquired resistance to one aminobisphosphonate with retained response to another. J Bone Miner Res 14:S2; Siris E, Weinstein RS, Altman R, Conte JM, Favus M, Lombardi A, Lyles K, McIlwain H, Murphy WA Jr., Reda C, Rude R, Seton M, Tiegs R, Thompson D, Tucci JR, Yates AJ, Zimering M 1996 Comparative study of alendronate vs. etidronate for the treatment of Paget s disease of bone. J Clin Endocrinol Metab 81: Reid IR, Nicholson GC, Weinstein RS, Hosking DJ, Cundy T, Kotowicz MA, Murphy WA Jr., Yeap S, Dufresne S, Lombardi A, Musliner TA, Thompson DE, Yates AJ 1996 Biochemical and radiologic improvement in Paget s disease of bone treated with alendronate: A randomized, placebo-controlled trial. Am J Med 171: Miller PD, Adachi JD, Brown JP, Khairi RA, Lang R, Licata AA, McClung MR, Ryan WG, Singer FR, Siris ES, Tenenhouse A, Wallach S, Bekker PJ, Axelrod DW 1997 Risedronate vs. etidronate: Durable remission with only two months of 30 mg risedronate. J Bone Miner Res 12:S Siris ES, Chines AA, Altman RD, Brown JP, Johnson CC Jr., Lang R, McClung MR, Mallette LE, Miller PD, Ryan WG, Singer FR, Tucci JR, Eusebio RA, Bekker PJ 1998 Risedronate in the treatment of Paget s disease: An open-label, multicenter study. J Bone Miner Res 13: Reid IR, Miller P, Lyles K, Fraser W, Brown J, Saidi Y, Mesenbrink P, Su G, Pak J, Zelenakas K, Luchi M, Richardson P, Hosking D 2005 A single infusion of zoledronic acid improves remission rates in Paget s disease: A randomized controlled comparison with risedronate. N Engl J Med 353: Carter G, Goss AN, Doecke C 2005 Bisphosphonates and avascular necrosis of the jaw: A possible association Med J Aust 182: Woodhouse NJY, Bordier P, Fisher M, Joplin GF, Reiner M, Kalu DN, Foster GV, MacIntyre I 1971 Human calcitonin in the treatment of Paget s bone disease. Lancet 1: DeRose J, Singer F, Avramides A, Flores A, Dziadiw R, Baker RK, Wallach S 1974 Response of Paget s disease to porcine and salmon calcitonins: Effects of long term treatment. Am J Med 56: Singer FR, Ginger K 1991 Resistance to calcitonin. In: Singer FR, Wallach S (eds.) Paget s disease of bone: Clinical assessment, present and future therapy. Elsevier, New York, New York, USA, pp Chapter 60. Nutritional and Drug-Induced Rickets and Osteomalacia John M. Pettifor MRC Mineral Metabolism Research Unit and the Department of Paediatrics, Chris Hani Baragwanath Hospital and the University of Witwatersrand, Johannesburg, South Africa The author has reported no conflicts of interest. INTRODUCTION Once a scourge for people living in cities of northern Europe, North America, and China, nutritional rickets and osteomalacia were considered by the latter half of the 20th century to have been all but eradicated from a number of countries except in a few at-risk groups such as preterm infants, the elderly, and the infirm. This was made possible through health education, the availability of vitamin D supplements, and the fortification of foods such as milk and margarine. However, over the past three decades, attention has been drawn to an apparent resurgence of the problem not only in the United States and the United Kingdom but also in a number of developing countries. Rickets is a disorder associated with a failure of or delay in the mineralization of endochondral new bone formation at the growth plates, whereas osteomalacia is characterized by a failure of mineralization of newly formed osteoid at sites of bone turnover or periosteal and endosteal apposition. Thus, in children, whose growth plates (physes) are not closed, rickets and osteomalacia are found together, whereas in adults with fused growth plates, only osteomalacia will be noted. Although distinguishing between the two conditions might seem arbitrary, it is possible that the two conditions (endochondral calcification and osteoid mineralization) might respond differently to treatment as has been suggested in children with X-linked hypophosphatemic rickets. The causes of rickets and osteomalacia are numerous, and many of the causes will be discussed in this and other chapters. In this chapter, nutritional and drug-induced causes will be described. NUTRITIONAL CAUSES OF RICKETS/ OSTEOMALACIA Nutritional rickets/osteomalacia may be caused by deficiencies of vitamin D, calcium, or phosphorus. While each of these causes will be considered separately, deficiencies of each may contribute to a greater or lesser degree to the disease in an individual patient. Vitamin D Deficiency Although considered to be a nutrient, vitamin D is found in only small quantities in the majority of natural foods, and these supplies are generally insufficient to meet the vitamin D requirements of humans. Vitamin D sufficiency is maintained in most populations through its formation in the skin from 7-dehydrocholesterol under the influence of UV-B irradiation from sunlight. In situations of impaired dermal synthesis, as may occur in countries of extreme latitude (e.g., in northern Europe, northern China, and northern North America), because of extensive clothing coverage of the skin (in the Middle
12 NUTRITIONAL AND DRUG-INDUCED RICKETS AND OSTEOMALACIA / 331 Eastern region), (1) in darkly pigmented persons living in countries with limited UV-B irradiation (immigrants to Europe and blacks), in the elderly (because of decreased substrate being available for dermal synthesis of vitamin D), or because of the use of sunscreens, food fortification (infant milk formulas in most countries, cow s milk in the United States, and margarine) or vitamin D supplementation may be necessary to maintain an adequate vitamin D status. Despite vitamin D fortification of foods in some countries and the recommendation of vitamin D supplementation in at-risk populations (particularly breastfed infants, the elderly, and the infirm), vitamin D deficiency rickets/osteomalacia continues to be public health problem in a number of countries: in the United States, increasing numbers of reports are highlighting rickets in infants of black parents, especially in those who have been breastfed for prolonged periods. (2) It is possible that in these toddlers low dietary calcium intakes during the weaning period may exacerbate the problem. In the United Kingdom and other northern European countries, Asian and immigrant populations (from Turkey and African countries) seem to be particularly at risk, where the disease affects not only breastfed infants but also occurs in adolescents and women. In the Middle East, a high prevalence of rickets and osteomalacia has been described in Muslim women and their infants. (3) In Tibet and Mongolia, clinical rickets has been described in 60% of young infants. (4) Other groups particularly at risk are those individuals living in areas of poor UV-B irradiation, who are vegan or vegetarian, or who have other extreme dietary restrictions such as macrobiotic diets. With the improvement in health care in many developed countries, life expectancy has increased dramatically, resulting in large numbers of elderly and infirm, often living in retirement villages or old-age homes, where there are limited opportunities of receiving adequate sunlight exposure. In such situations, vitamin D deficiency may develop insidiously, resulting in impaired muscle function, bone pain, and an increased risk of fractures. Pathogenesis. Vitamin D, or more specifically the active metabolite 1,25(OH) 2 D, is essential for maintaining normocalcemia through ensuring adequate intestinal calcium absorption. Vitamin D deficiency also reduces intestinal phosphate absorption but not to the same extent as it affects calcium absorption. Inadequate intestinal calcium absorption, particularly in the growing child, leads to a fall in blood ionized calcium concentrations and secondary hyperparathyroidism. Low 1,25(OH) 2 D levels may contribute to secondary hyperparathyroidism through the reduction of the suppressive effects of 1,25(OH) 2 D on PTH gene transcription. Secondary hyperparathyroidism has a number of effects, which produces the typical biochemical changes seen in vitamin D deficiency. Through its actions on the kidney, urinary calcium excretion is decreased, and renal tubular phosphate loss is increased, reducing the tubular reabsorption of phosphate (TRP) and tubular maximum for phosphate (TmP/GFR). As a consequence of the increased phosphate loss, serum phosphate levels are typically reduced, despite an increase in phosphate release from bone. Increased bone resorption, which occurs through the indirect effect of PTH to increase both osteoclast numbers and activity, leads to osteopenia. Although 1,25(OH) 2 D has effects on osteoblast differentiation and osteoclast precursors, it is thought that the major osseous features of vitamin D deficiency rickets and osteomalacia are as a result of the effects of hypocalcemia and hypophosphatemia on bone mineralization and of the effects of secondary hyperparathyroidism on bone turnover. Clinical Features. The clinical features of rickets are more rapid in onset and generally of a more severe nature than those of osteomalacia, which may take several years to manifest. Although the features of rickets characteristically manifest as deformities of the skeletal system, other organ systems, such as the muscular and immune systems, are involved as well. In the young infant, symptoms and signs of hypocalcemia (tetany, apneic episodes, stridor, or convulsions) may be the only features. The skeletal features in the infant and young child include skull abnormalities, such as a delay in closure of the anterior fontanelle, softening of the skull bones leading to craniotabes, and parietal and frontal bossing giving the hot-cross bun appearance. Chest deformities include enlargement of the costochondral junctions leading to the rachitic rosary, indrawing of the lower ribs at the sites of attachment of the diaphragm resulting in the Harrison s sulcus, and narrowing of the lateral diameter of the chest (violin case deformity). Softening of the ribs and enlargement of the costochondral junctions in association with muscular hypotonia and an inability to clear secretions may lead to severe respiratory distress and an increase in the severity and frequency of lower respiratory tract infections. (5) Weight bearing leads to deformities of the long bones and the characteristic enlargement of the growth plates, especially palpable at the wrist and knee. Deformities of the long bones depend on the stresses placed on the bones, such that in the young infant, bowing of the distal radius and ulna and anterior bowing of the tibia may occur. In the toddler, exaggeration of the normal bow legs (varus deformity) is frequent, whereas in the older child, knock-knees (valgus deformity) or mixed valgus and varus deformities of the legs (windswept deformity) may occur. (6) Long-standing rickets may result in deformities of the pelvis, with narrowing of the pelvic outlet and resultant increased risk of obstructed labor in females later in life. Minimal trauma fractures of the long bones may occur, particularly in the young infant and in the elderly with osteomalacia. Bone pain is often a feature in severe rickets/ osteomalacia, which in the older patient may be confused with arthritis. Delay in the eruption of permanent dentition and enamel hypoplasia of both primary and secondary teeth may occur, depending on the age of the child when rickets occurred, because it must occur at the time of tooth and enamel formation. Muscular hypotonia may be pronounced, resulting in a delay in gross motor milestones, whereas in older children and adults with osteomalacia, proximal muscle weakness may result in difficulties in getting out of chairs and climbing stairs. A waddling gait may also be present. Osteomalacia associated with muscle weakness and gait instability in the elderly may be responsible for an increase in the propensity to fall, thus increasing fracture risk over and above the risk associated with increased bone fragility. Although in vitro studies clearly show a role for 1,25(OH) 2 D in immune modulation, (7) there are few studies that have documented impaired immunity in subjects with vitamin D deficiency. (8) Laboratory Investigations. The typical biochemical features of vitamin D deficiency rickets/osteomalacia are hypocalcemia, hypophosphatemia, and elevated PTH and alkaline phosphatase values. Although serum calcium and phosphorus values may occasionally be within the normal reference range for age before treatment, once treatment has commenced, values tend to rise. Serum 25-hydroxyvitamin D [25(OH)D] levels are typically lower than normal, with the majority of patients having values 5 ng/ml (12.5 nm). These low levels of 25(OH)D are a hallmark of vitamin D deficiency and help to differentiate the latter from other causes of rickets/ osteomalacia. The measurement of serum 1,25(OH) 2 D levels is
13 332 / CHAPTER 60 of osteopenia, which may be confused with osteoporosis. Only rarely may Looser zones be noted to suggest the diagnosis of osteomalacia. Thus, unlike the typical picture of rickets in children, osteomalacia in adults is difficult to diagnose radiographically and requires biochemical, and often, histological confirmation.(10) FIG. 1. X-ray of the wrist of an infant with vitamin D deficiency rickets. Note the widened growth plate, the fraying and splaying of the distal metaphyses of the radius and ulna, the poor developed ulna epiphysis, and the coarsening and sparseness of the metaphyseal trabeculae. not particularly helpful in differentiating the various causes of nutritional rickets/osteomalacia because values may be low, normal, or elevated. Markers of bone turnover reflect the increased activity associated with secondary hyperparathyroidism. However, serum osteocalcin concentrations are typically within the normal range, despite elevated alkaline phosphatase values.(9) Urine calcium excretion is typically low, whereas the TRP and TmP/GFR are decreased. A generalized aminoaciduria and/or bicarbonaturia is also present. Radiological Findings. The radiographic changes associated with rickets can develop very rapidly, which is different from those of osteomalacia, which may take years to become radiographically apparent. The classical features of rickets occur at the growth plates of long bones and are best seen at the distal end of the radius and ulna or at the tibial and femoral growth plates around the knee. The features at the growth plate include widening of the physis with fraying, cupping, and splaying of the metaphyses (Fig. 1) and underdevelopment of the epiphysis. The earliest sign at the wrist is considered to be a loss of the clear demarcation between the growth plate and the metaphysis, with loss of the provisional zone of calcification. The diaphyses of the long bones appear osteopenic and may show thinning of the cortices with periosteal new bone formation. Looser zones, which manifest as short radiolucent lines through the cortex perpendicular to the shaft, are typical of osteomalacia, although they may be seen rarely in other metabolic bone diseases. They are most frequently noted in the medial cortices of the femurs and in the pelvis and ribs. Because of secondary hyperparathyroidism, the trabecular pattern of the metaphyses becomes coarse and sparse, and subperiosteal erosions may be noted along the phalanges. In adults with osteomalacia, the most common feature is that Treatment. The mainstay of treatment is the provision of vitamin D (D2 or D3) to correct the vitamin deficiency. In infants and young children, vitamin D drops, ,000 IU/day ( g) for 1 2 months, effectively raise 25(OH)D levels to normal and correct serum calcium, phosphorus, and PTH values usually within 2 3 weeks, although the elevated alkaline phosphatase values and radiological abnormalities take longer to return to normal. An early radiographic sign of healing is the appearance of the provisional zone of calcification at the boundary between the physis and metaphysis, which appears as a well-defined sclerotic line. Older children and adults may be treated on a similar regimen, but compliance is probably better if a weekly dose of vitamin D 50,000 IU (1.25 mg) is taken orally for several months. In some countries, a single oral or intramuscular dose of vitamin D 600,000 IU (15 mg) is preferred. There is no indication for or advantage in using one of the vitamin D metabolites, 25(OH)D or 1,25-(OH)2D, or the vitamin D analog, 1 -hydroxycholecalciferol, for the treatment of simple nutritional vitamin D deficiency. However, the metabolites may be more effective in the management of vitamin D deficiency resulting from intestinal malabsorption syndromes.(11) In this situation, UV irradiation of the skin may also be effective. Calcium supplements (50 mg Ca/kg/day orally) may also be recommended in the initial stages of management, especially if dietary calcium intakes are poor, and advice on ensuring a dietary calcium intake near the adequate intake or recommended daily allowance (RDA) for age should be given. Initially symptomatic hypocalcemia may require parenteral calcium administration to correct the symptoms (1 2 mg Ca/kg, IV, as 10% calcium gluconate). Prevention. Despite the availability of cheap and effective means of preventing vitamin D deficiency, it remains a public health problem in a number of countries in specific age or cultural groups. It is these groups that need to be targeted with educational messages, and possibly, vitamin D supplementation. Table 1 lists the recommended dietary intakes for vitamin D in the United Kingdom and North America. It should be noted that, in both countries, higher intakes are recommended in the elderly to compensate for possible decreased sun exposure, decreased dermal synthesis of vitamin D, and decreased responsiveness of the small intestine to vitamin D. In the TABLE 1. RECOMMENDED VITAMIN D INTAKES IN AND NORTH AMERICA Age 0 6 months 7 months to 3 years 4 50 years 50 years Pregnancy and lactation UK recommended nutrient intake (g/day)(54) * 10 (61 years) 10 THE UNITED KINGDOM USA/Canadian adequate intake (g/day)(16) (51 70 years) 15 (71 years) 5 * The United Kingdom believes that healthy ambulatory persons should be able to obtain their vitamin D requirement from sunlight exposure.
14 NUTRITIONAL AND DRUG-INDUCED RICKETS AND OSTEOMALACIA / 333 United Kingdom, infants 6 months of age also have higher dietary recommendations because it is assumed that sunlight exposure is limited and that exclusively breastfed infants receive little vitamin D from breast milk. (12) The United Kingdom also recommends that all breastfed infants receive vitamin D supplements, whereas the American Academy of Pediatrics suggests that only at-risk infants should be considered for supplementation. In countries such as in the Middle East and Turkey or in communities such as Asians in the United Kingdom or blacks, where vitamin D deficiency is prevalent among mothers, it makes sense to supplement the mother during pregnancy to provide the breastfed newborn with stores of vitamin D and to reduce the incidence of neonatal hypocalcemia. (13) Although a daily supplement of 400 IU (10 g) vitamin D is highly effective in preventing rickets in infants and young children, in situations where compliance may be a problem, intermittent high doses of vitamin D (100,000 IU [2.5 mg] every 3 months) have been shown to be successful. (14) Vitamin D supplementation should also be considered in the infirm and elderly because normal food sources and the limited sun exposure are generally insufficient to meet the vitamin D requirements of these groups. Over the last decade, considerable controversy has existed as to what might be considered optimal vitamin D status, particularly in the elderly. Evidence suggests that circulating 25(OH)D concentrations 75 nm (30 ng/ml) might be necessary to optimize BMD and muscle function and reduce falls and fractures. (15) To obtain such 25(OH)D concentrations, vitamin D intakes of IU/day might be needed; however, these are considerably higher than the adequate intake recommended by the Institute of Medicine. (16) Dietary Calcium Deficiency Over the past 20 or so years, growing interest has been shown in the role of low dietary calcium intakes in the pathogenesis of rickets in children. In the 1970s, a few isolated reports appeared of sick infants on highly modified lowcalcium diets developing rickets, which responded to calcium supplements. (17) More recently, studies from South Africa, (18) Nigeria, (19) India, (20) and Bangladesh (21) have suggested that dietary calcium deficiency may be a major cause of rickets in children outside the infant age group living in developing countries. Characteristically, the children live in tropical or subtropical climates and are thus exposed to adequate amounts of sunshine. They consume a diet that is very low in calcium (estimated at 200 mg calcium daily or 20% of the RDA) and is relatively monotonous, containing almost no dairy products but is high in phytates and oxalates. Pathogenesis. It is suggested that the low dietary calcium intake, often exacerbated by a diet high in oxalate and phytate content that impairs intestinal calcium absorption, is unable to meet the demands of the growing skeleton. This results in a fall in serum ionized calcium concentration, which in turn stimulates secondary hyperparathyroidism and a cascade of biochemical changes similar to those described in vitamin D deficiency (Fig. 2). Dietary calcium deficiency may also affect vitamin D metabolism through stimulation of 1,25(OH) 2 D production, which itself, in turn, leads to an increase in the catabolism of 25(OH)D. In situations of relative vitamin D insufficiency, this increased catabolism of 25(OH)D may precipitate vitamin D deficiency. Such increased catabolism of 25(OH)D has been proposed as the pathogenesis of the high prevalence of rickets in the Asian community in the United Kingdom. (22) There is some evidence that a similar mechanism may be important in the pathogenesis of rickets in black infants and toddlers. (23) It is important to point out that isolated dietary calcium deficiency and vitamin D deficiency are at the two FIG. 2. The pathogenesis of rickets/osteomalacia caused by dietary calcium deficiency. ends of the pole as far as the pathogenesis of nutritional rickets is concerned; in between these two poles are combinations of the two causes, such that vitamin D sufficiency may be converted to vitamin D deficiency by low dietary calcium intakes, and vitamin D insufficiency may precipitate dietary calcium deficiency by preventing optimal calcium absorption. (24) It is likely that many cases of nutritional rickets have a combination of these two deficiencies to varying degrees in their pathogenesis. Clinical Presentation. Although calcium deficiency rickets has been described in young infants, these are the exception, because the majority of young infants are fed breast-milk or milk formulas, which are good calcium sources. Calcium deficiency rickets typically occurs after the weaning period, when calcium intakes fall as a result of the low calcium content of traditional diets of many communities in developing countries. In Nigeria, the mean age of presentation is around 4 years, although the onset of symptoms may have occurred several years earlier. (25) In South Africa, the mean age of presentation is older, around 8 years. The typical presentation is that of progressive lower limb deformities (bow legs, knock-knees, or windswept deformities; Fig. 3). The other features of rickets tend to be less pronounced than in vitamin D deficiency, although rachitic rosaries and enlarged wrists are useful clinical signs. (26) In the Nigerian study, delayed motor milestones were noted by the parents. (25) In the South African children, one of the striking differences between vitamin D deficiency and dietary calcium deficiency was noted to be the absence of muscle weakness in the calcium deficiency group. (27) Laboratory Investigations and Radiological Features. The biochemical features of dietary calcium deficiency are very similar to those of vitamin D deficiency (i.e., hypocalcemia, variable hypophosphatemia, and elevated alkaline phosphatase and PTH values). However, the distinguishing features relate to differences in vitamin D status. In typical dietary calcium deficiency, serum 25(OH)D values are within the normal range ( 10 ng/ml [ 25 nm]) and 1,25(OH) 2 D values are elevated, as would be expected in response to hypocalcemia. The radiographic features of dietary calcium deficiency rick-
15 334 / CHAPTER 60 ment of dietary calcium deficiency rickets.(19) However, in communities in which it is unclear whether vitamin D insufficiency is playing a role or not, it is prudent to add vitamin D supplements to the regimen. Prevention. In developing countries, where dietary calcium deficiency is prevalent, prevention of the disease by increase in the consumption of calcium-rich foods may be problematic, because dairy products are often scarce and too expensive for the affected communities. However, the use of locally available foods, such as ground dried fish with its bones, or the addition of limestone to the food, might be beneficial. Phosphate Deficiency Isolated nutritional phosphate deficiency is an uncommon cause of rickets/osteomalacia because phosphorus is ubiquitous in foods and the usual dietary intake of phosphate meets the recommended dietary allowances in most situations. However, such mineral deficiency may occur in the breastfed, very low birth weight (VLBW) infant,(28) during prolonged parenteral nutrition in sick patients,(29) and in patients on prolonged antacid therapy.(30) It should be noted that low serum phosphate levels may be a major contributing factor to the pathogenesis of rickets/osteomalacia caused by vitamin D deficiency. However, in that situation, phosphate depletion is dependent on secondary hyperparathyroidism that arises in response to impaired intestinal calcium absorption. FIG. 3. A girl from rural South Africa suffering from dietary calcium deficiency rickets with windswept deformities of the legs. ets are similar to those described for vitamin D deficiency with growth plate changes, osteomalacia, and features of secondary hyperparathyroidism (Fig. 4). Treatment. A number of studies have documented the effectiveness of oral calcium supplements (1000 mg/day for 6 months) without supplemental need for vitamin D in the treat- FIG. 4. X-rays of the knee of a child with dietary calcium deficiency. The changes are similar to those described in vitamin D deficiency, with widening of the growth plates and splaying and fraying of the metaphyses.
16 NUTRITIONAL AND DRUG-INDUCED RICKETS AND OSTEOMALACIA / 335 calcium and phosphorus in total parenteral nutrition (TPN) solutions. These problems have been largely overcome in the newer parenteral nutrition solutions. Treatment. Although the pathogenesis of metabolic bone disease in very low birth weight infants is multifactorial, the major factor seems to be an inadequate intake of phosphate. Soy-based formulas should be avoided, because their phosphorus content is generally less readily available, and if VLBW infants are fed breast milk, breast-milk fortifiers, which increase the Ca and Pi content of the intake (among other nutrients) should be provided until the infant is ready for discharge from hospital. An oral Pi intake of 120 mg/kg/day should be achieved. An adequate vitamin D intake of 200 IU/kg/day should also be ensured. (31) Metabolic bone disease as a consequence of TPN should be treated by providing adequate amounts of Ca and Pi in the intravenous solutions. Furthermore, vitamin D sufficiency should also be maintained. FIG. 5. The pathogenesis of the biochemical abnormalities in phosphate deficiency rickets/osteomalacia. Pathogenesis. Phosphate deficiency induced by either dietary insufficiency or reduced intestinal absorption results in a fall in serum phosphate levels in association with increased renal reabsorption of phosphate (increased TRP or TmP/GFR). Hypophosphatemia stimulates renal 1 -hydroxylase with a consequent increase in serum 1,25(OH) 2 D levels, which lead to increased bone resorption, intestinal calcium absorption, and hypercalciuria (Fig. 5). Unlike vitamin D deficiency, PTH levels and vitamin D status are normal in phosphate depletion syndromes. Hypophosphatemia results in impaired bone mineralization and the development of rickets and osteomalacia. Clinical Presentation and Laboratory Investigations. Metabolic bone disease is a common problem in very low birth weight preterm infants. It typically manifests between 6 and 12 weeks postnatally, and has been estimated to occur radiologically in 50% of infants with birth weights 1000 g, and in 20% of those weighing 1500 g. The disease is most common in those infants who are breast-milk or soy-formula fed or in those who have had prolonged illnesses requiring periods of parenteral nutrition, corticosteroid, or diuretic administration. The clinical manifestations vary from a picture of mildly undermineralized bones to severe rickets with multiple fractures and deformities, which, if involving the ribs, may be severe enough to lead to respiratory distress. The biochemical changes associated with the development of the disease include a rise in alkaline phosphatase levels and the development of hypophosphatemia ( 1.8 mm). It is difficult to give a cut-off value for alkaline phosphatase because different assays are used by various laboratories, but a value 7-fold that of the adult normal is highly suggestive of rickets in the preterm infant. Urine phosphate excretion is reduced while urine calcium excretion is increased. The clinical picture and biochemical abnormalities in prolonged parenteral nutrition are very similar to those described above. The reasons for the development of metabolic bone disease relate to the difficulty in providing adequate amounts of DRUG-INDUCED RICKETS/OSTEOMALACIA The causes of drug-induced rickets/osteomalacia may be divided into three large groups: those that primarily result in hypocalcemia, those that primarily cause hypophosphatemia, and those that have a direct effect on the mineralization process. Drugs or medications may cause rickets/osteomalacia through each of the three mechanisms described above (Table 2). Drugs Resulting in Hypocalcemia Drugs that inhibit the production or intestinal absorption of vitamin D or interfere with its metabolism or action on its target organs may all produce hypocalcemia and consequent rickets/osteomalacia. Sunscreens. With the increasing concern about the adverse effects of sunlight and particularly UV irradiation on the skin among health professionals and the general public, sunscreen use has become more prevalent. The extensive use of these products may impair vitamin D 3 formation in the skin, resulting in vitamin D deficiency rickets/osteomalacia, particularly in those subjects whose vitamin D status might normally be marginal. Recently rickets in children has been reported to be as a consequence of the use of sunscreens. (32) TABLE 2. CAUSES OF DRUG-INDUCED RICKETS/OSTEOMALACIA Drugs resulting in hypocalcemia Inhibitors of vitamin D formation or intestinal absorption Sunscreens Cholestyramine Increased catabolism of vitamin D or its metabolites Anticonvulsants Drugs resulting in hypophosphatemia Inhibitors of intestinal phosphate absorption Aluminum containing antacids Impaired renal phosphate reabsorption Cadmium Ifosfamide Saccharated ferric oxide Direct impairment of mineralization Parenteral aluminum Fluoride Etidronate
17 336 / CHAPTER 60 Cholestyramine. Similarly, impairment of vitamin D absorption from the gastrointestinal tract may also lead to osteomalacia as has been reported with the prolonged use of cholestyramine, an anion exchange resin used to bind bile salts in the gut in post-ileectomy diarrhea. (33) Anticonvulsants. A number of drugs has been described to interfere with the normal metabolism of vitamin D to 25(OH)D and 1,25(OH) 2 D, leading to alterations in calcium homeostasis and rickets/osteomalacia by increasing the catabolism of vitamin D and its metabolites through inducing hepatic cytochrome P450 enzymes. These include a number of anticonvulsants (phenobarbitone, phenytoin, and carbamazepine). (34) Phenytoin has been shown to have direct effects on decreasing calcium absorption and increasing bone resorption as well. The development of rickets/osteomalacia is more common in those subjects on anticonvulsant therapy who have limited exposure to sunlight such as those who are institutionalized (35) or handicapped, where the prevalence has been reported to be as high as 60%. Severe convulsions or spastic cerebral palsy may result in frequent long-bone fractures in such situations. Typically, the biochemical abnormalities include hypocalcemia, low 25(OH)D levels, and elevated alkaline phosphatase and PTH values. It is unclear what the prevalence of anticonvulsant-associated bone disease is in ambulatory patients, because some studies have shown little effect of anticonvulsants on calcium homeostasis. (36) The disturbances in calcium homeostasis can be prevented or rapidly corrected by the use of vitamin D supplements (up to 4000 IU [100 g] per day), although alkaline phosphatase levels may remain elevated, especially in phenobarbitone-treated subjects through induction of hepatic enzymes. Drugs Resulting in Hypophosphatemia Drugs may induce hypophosphatemia by either impairing phosphate absorption from the gastrointestinal tract or by increasing phosphate loss from the kidney. Inhibitors of Intestinal Phosphate Absorption. As has been discussed earlier in this chapter, phosphate deficiency through an inadequate dietary intake is very unlikely to occur because phosphate is found ubiquitously in the diet. An important exception to this is found in the breast-fed VLBW infant, because breast milk contains inadequate phosphate, and possibly calcium, to meet the demands of the very rapidly growing infant during the first several months of life. Inadequate intestinal phosphate absorption may occur through the inhibition of its absorption through the long-term use of aluminum containing antacids, which bind phosphate, thereby making it unavailable for absorption. The milk-alkali syndrome is characterized by clinical features of hypophosphatemia (muscle weakness and rickets/osteomalacia) and by hypercalcemia, elevated alkaline phosphatase levels, normal PTH values, and elevated 1,25(OH) 2 D levels. Typically, such patients have a history of consuming large quantities of aluminum-containing antacids, often with the consumption of milk to manage chronic dyspeptic symptoms. Aluminum given parenterally over a prolonged period of time also causes osteomalacia, but the mechanism is thought to be different from that of oral aluminum administration. Phosphate depletion as a result of antacid ingestion can be effectively treated by removing the aluminum-containing antacids. Provision of oral Pi supplements may also be considered until the bone disease responds (clinical improvement in bone pain and muscle weakness, elevation of serum phosphorus values, and a fall in alkaline phosphatase concentrations). Impaired Renal Phosphate Reabsorption. A number of heavy metals, such as cadmium, (37) and drugs, such as ifosfamide (38,39) and saccharated ferric oxide, (40) have been implicated in causing rickets/osteomalacia through damage to renal tubular function. Cadmium. Cadmium exposure leads to Itai-Itai disease, which was first described in Japan. (41) The pathogenesis of the osteoporosis and osteomalacia in the disease is not completely elucidated, but cadmium exposure has been shown to lead to permanent damage to glomerular and tubular function, leading to low molecular proteinuria with the excretion of 2 - microglobulin, phosphaturia, and progressive renal failure. Hypophosphatemia and reduced levels of 1,25(OH) 2 D are characteristic of the disease. Whether cadmium s direct effects on osteoclast and osteoblast function are important in the pathogenesis of the disease are unclear. Ifosfamide. Ifosfamide, a chemotherapeutic agent used in the treatment of solid tumors, has been reported to produce a renal Fanconi syndrome and hypophosphatemic rickets/osteomalacia, particularly in children, although the complication has also been noted in adults. (31,32) Saccharated Ferric Oxide. Prolonged intravenous use of saccharated ferric oxide (SFO) for the treatment of iron deficiency anemia causes reversible proximal renal tubular damage, hypophosphatemia, and depressed 1,25(OH) 2 D levels, leading to osteomalacia. (33) SFO is also thought to inhibit mineralization directly. Cessation of therapy and the use of oral phosphate supplements correct the biochemical and histological abnormalities. Impairment of Mineralization A number of agents are known to produce rickets/ osteomalacia through direct inhibition of mineralization at the mineralization front in bone and in the growth plate cartilage. These include aluminum, (42) fluoride, (43) and etidronate, a first generation bisphosphonate. (44) Aluminum. The mechanism by which parenterally acquired aluminum causes rickets/osteomalacia is different from that associated with the oral use of aluminum containing antacids in subjects with normal renal function. The two major conditions, in which aluminum accumulation may occur, are TPN and hemodialysis. In both situations, the aluminum is acquired through contamination of the TPN or hemodialysis fluids. Aluminum may also accumulate through the use of aluminum phosphate binders in patients with impaired renal function. Aluminum inhibits PTH release and 1 -hydroxylase activity. Furthermore, it seems to have direct effects on bone, inhibiting osteoblastic activity and preventing mineralization of preformed osteoid. The net result of all these effects is the production of an adynamic bone disease or a low bone turnover osteomalacia/rickets. (45) The severity of the bone disease correlates with the extent of stainable aluminum at the bone surfaces. With the increased awareness of the importance of aluminum contamination as a cause of bone disease in patients receiving TPN or hemodialysis, regulations have been introduced to reduce the contamination. (42) This has been assisted by the removal of casein hydrolysates as the protein source in TPN solutions. Furthermore, aluminum-based phosphate binding agents have been replaced by the use of calcium-based oral medications, or other resins, in renal failure patients. Alumi-
18 NUTRITIONAL AND DRUG-INDUCED RICKETS AND OSTEOMALACIA / 337 num bone disease may respond to the use of deferoxamine, a chelating agent. Fluoride. The attention of researchers in the developed world has been focused on the toxic effects of fluoride on bone since the introduction of oral fluoride as an experimental drug for the treatment of osteoporosis. (46) With the development of newer effective drugs for the treatment of osteoporosis, fluoride therapy has fallen into disrepute. However, in a number of developing countries, such as India, South Africa, and Kenya, which have areas of endemic fluorosis, the harmful effects of the excessive ingestion of fluoride have been known for many years. (47) Fluoride is incorporated in the newly formed hydroxyapatite crystal at the mineralization front, where it stabilizes the crystal and prevents its dissolution; furthermore, it stimulates osteoblastic activity but also inhibits mineralization. The mineralization defect is aggravated by low dietary calcium intakes. Although there is good evidence that fluoride therapy increases vertebral BMD, there is no good evidence that it reduces fracture rates in osteoporosis. (46) Endemic fluorosis, after the long-term ingestion of water with fluoride contents of between 3 and 16 ppm, is associated with the insidious onset of generalized bone pain, stiffness, rigidity, and limitation of movement at the spine and the development of crippling deformities. (48) In children, endemic genu valgum and clinical features of rickets have been described. (49,50) Radiographically, osteosclerosis and irregular osteophyte formation are noted in the spine, with calcification of the intervertebral ligaments. The pelvis also shows osteosclerotic changes with calcification of the sacrotuberous and sacroiliac ligaments. Similarly, interosseous membrane calcification is noted in the forearms and lower limbs. In children, features of rickets at the growth plate (51) and osteomalacia with Looser zones may be noted. Histologically, widened osteoid seams, mineralization defects, poorly mineralized new bone formation, and features of increased bone turnover have been described together with areas of hypermineralization. Brown staining of the teeth is a characteristic feature in those subjects who were exposed to excessive fluoride intakes during the period of primary and secondary tooth formation. Etidronate. The bisphosphonates have become established therapeutic agents in the management of osteoporosis, Paget s disease, and hypercalcemia of malignancy, among other generalized bone conditions. However, they are all analogs of pyrophosphate, an endogenous inhibitor of mineralization. Etidronate, the first bisphosphonate to be approved for clinical use, has been shown to induce impaired mineralization at high doses (20 mg/kg). (52) At lower doses, the incidence of osteomalacia was reduced markedly, but was still evident in biopsy specimens. The newer generations of bisphosphonates do not seem to have the same side effects and have been used extensively in the management of osteoporosis without evidence of impairing mineralization. REFERENCES 1. Andiran N, Yordam N, Ozon A 2002 Risk factors for vitamin D deficiency in breast-fed newborns and their mothers. Nutrition 18: Kreiter SR, Schwartz RP, Kirkman HN, Jr., Charlton PA, Calikoglu AS, Davenport ML 2000 Nutritional rickets in African American breast-fed infants. J Pediatr 137: Sedrani SH 1986 Are Saudis at risk of developing vitamin D deficiency?. Saudi Med J 7: Harris NS, Crawford PB, Yangzom Y, Pinzo L, Gyaltsen P, Hudes M 2001 Nutritional and health status of Tibetan children living at high altitudes. N Engl J Med 344: Muhe L, Luiseged S, Mason KE, Simoes EAF 1997 Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 349: Oginni LM, Badru OS, Sharp CA, Davie MW, Worsfold M 2004 Knee Angles and Rickets in Nigerian Children. J Pediatr Orthop 24: DeLuca HF, Cantorna MT 2001 Vitamin D: Its role and uses in immunology. FASEB J 15: Lorente F, Fontan G, Jara P, Casas C, Garcia-Rodriguez MC, Ojeda JA 1976 Defective neutrophil motility in hypovitaminosis D rickets. Acta Paediatr Scand 65: Daniels ED, Pettifor JM, Moodley GP 2000 Serum osteocalcin has limited usefulness as a diagnostic marker for rickets. Eur J Pediatr 159: Parfitt AM 1997 Vitamin D and the pathogenesis of rickets and osteomalacia. In: Feldman D, Glorieux FH, Pike JW (eds.) Vitamin D. Academic Press, San Diego, CA, USA, pp Basha B, Rao DS, Han ZH, Parfitt AM 2000 Osteomalacia due to vitamin D depletion: A neglected consequence of intestinal malabsorption. Am J Med 108: Specker BL, Tsang RC, Hollis BW 1985 Effect of race and diet on human-milk vitamin D and 25-hydroxyvitiamin D. Am J Dis Child 139: Andiran N, Yordam N, Ozon A 2002 Risk factors for vitamin D deficiency in breast-fed newborns and their mothers. Nutrition 18: Zeghoud F, Ben-Mekhbi H, Djeghri N, Garabedian M 1994 Vitamin D prophylaxis during infancy: Comparison of the long- term effects of three intermittent doses (15, 5, or 2.5 mg) on 25- hydroxyvitamin D concentrations. Am J Clin Nutr 60: Dawson-Hughes B 2005 The role of vitamin D in fracture prevention. Available online at BoneKEy Osteovision 2: Standing Committee on the Scientific Evaluation of Dietary Reference Intakes IoM 1997 Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press, Washington, DC, USA. 17. Kooh SW, Fraser D, Reilly BJ, Hamilton JR, Gall D, Bell L 1977 Rickets due to calcium deficiency. N Engl J Med 297: Marie PJ, Pettifor JM, Ross FP, Glorieux FH 1982 Histological osteomalacia due to dietary calcium deficiency in children. N Engl J Med 307: Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei CO, Reading JC, Chan GM 1999 A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. N Engl J Med 341: Balasubramanian K, Rajeswari J, Gulab, Govil YC, Agarwal AK, Kumar A, Bhatia V 2003 Varying role of vitamin D deficiency in the etiology of rickets in young children vs. adolescents in northern India. J Trop Pediatr 49: Fischer PR, Rahman A, Cimma JP, Kyaw-Myint TO, Kabir AR, Talukder K, Hassan N, Manaster BJ, Staab DB, Duxbury JM, Welch RM, Meisner CA, Haque S, Combs GF Jr 1999 Nutritional rickets without vitamin D deficiency in Bangladesh. J Trop Pediatr 45: Clements MR 1989 The problem of rickets in UK Asians. J Hum Nutr Diet 2: DeLucia MC, Mitnick ME, Carpenter TO 2003 Nutritional rickets with normal circulating 25-hydroxyvitamin D: A call for reexamining the role of dietary calcium intake in North American infants. J Clin Endocrinol Metab 88: Pettifor JM 1994 Privational rickets: A modern perspective. J Roy Soc Med 87: Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei C, Chan GM 2000 Case-control study of factors associated with nutritional rickets in Nigerian children. J Pediatr 137: Thacher TD, Fischer PR, Pettifor JM 2002 The usefulness of clinical features to identify active rickets. Ann Trop Paediatr 22: Pettifor JM 1991 Dietary calcium deficiency. In: Glorieux FH (ed.) Rickets, vol. 21. Raven Press, New York, NY, USA, pp Backstrom MC, Kuusela AL, Maki R 1996 Metabolic bone disease of prematurity. Ann Med 28: Klein GL, Chesney RW 1986 Metabolic bone disease associated with total parenteral nutrition. In: Lebenthal E (ed.) Total Parenteral Nutrition: Indication, Utilization, Complications, and Pathophysiological Considerations, 1st ed. Raven Press, New York, NY, USA, pp Pivnick EK, Kerr NC, Kaufman RA, Jones DP, Chesney RW 1995 Rickets secondary to phosphate depletion. A sequela of antacid use in infancy. Clin Pediatr (Phila) 34: Backstrom MC, Maki R, Kuusela AL, Sievanen H, Koivisto AM, Ikonen RS, Kouri T, Maki M 1999 Randomised controlled trial of vitamin D supplementation on bone density and biochemical indices in preterm infants. Arch Dis Child Fetal Neonatal Educ 80:F161 F Zlotkin S 1999 Vitamin D concentrations in Asian children living in
19 338 / CHAPTER 61 England. Limited vitamin D intake and use of sunscreens may lead to rickets. BMJ 318: Compston JE, Horton LW 1978 Oral 25-hydroxyvitamin D 3 in the treatment of osteomalacia associated with ileal resection and cholestyramine therapy. Gastroenterology 74: Hahn TJ, Hendin BA, Scharp CR, Haddad JG 1972 Effect of chronic anticonvulsant therapy on serum 25-hydroxycalciferol levels in adults. N Engl J Med 287: Bischof F, Basu D, Pettifor JM 2002 Pathological long-bone fractures in residents with cerebral palsy in a long-term care facility in South Africa. Dev Med Child Neurol 44: Ala-Houhala M, Korpela R, Koivikko M, Koskinen T, Koskinen M, Koivula T 1986 Long-term anticonvulsant therapy and vitamin D metabolism in ambulatory pubertal children. Neuropediatrics 17: Berglund M, Akesson A, Bjellerup P, Vahter M 2000 Metal-bone interactions. Toxicol Lett : Kintzel PE 2001 Anticancer drug-induced kidney disorders. Drug Saf 24: Garcia AA 1995 Ifosfamide-induced Fanconi syndrome. Ann Pharmacother 29: Sato K, Shiraki M 1998 Saccharated ferric oxide-induced osteomalacia in Japan: Iron-induced osteopathy due to nephropathy. Endocr J 45: Jarup L 2002 Cadmium overload and toxicity. Nephrol Dial Transplant 17: Klein GL 1995 Aluminum in parenteral solutions revisited again. Am J Clin Nutr 61: Kleerekoper M 1996 Fluoride and the skeleton. Crit Rev Clin Lab Sci 33: Silverman SL, Hurvitz EA, Nelson VS, Chiodo A 1994 Rachitic syndrome after disodium etidronate therapy in an adolescent. Arch Phys Med Rehabil 75: Tannirandorn P, Epstein S 2000 Drug-induced bone loss. Osteoporos Int 11: Haguenauer D, Welch V, Shea B, Tugwell P, Adachi JD, Wells G 2000 Fluoride for the treatment of postmenopausal osteoporotic fractures: A meta- analysis. Osteoporos Int 11: Mithal A, Trivedi N, Gupta SK, Kumar S, Gupta RK 1993 Radiological spectrum of endemic fluorosis: Relationship with calcium intake. Skeletal Radiol 22: Teotia SPS, Teotia M 1984 Endemic fluorosis in India: A challenging national health problem. J Assoc Physicians India 32: Krishnamachari KAVR, Sivakumar B 1976 Endemic genu valgum: A new dimension to the fluorosis in India. Fluoride 9: Pettifor JM, Schnitzler CM, Ross FP, Moodley GP 1989 Endemic skeletal fluorosis in children: Hypocalcemia and the presence of renal resistance to parathyroid hormone. Bone Miner 7: Khandare AL, Harikumar R, Sivakumar B 2005 Severe bone deformities in young children from vitamin D deficiency and fluorosis in Bihar-India. Calcif Tissue Int 76: Silverman SL, Hurvitz EA, Nelson VS, Chiodo A 1994 Rachitic syndrome after disodium etidronate therapy in an adolescent. Arch Phys Med Rehabil 75: Department of Health 1998 Nutrition and Bone Health, 49th ed. The Stationary Office, London, UK. Chapter 61. Rickets Caused by Impaired Vitamin D Activation and Hormone Resistance: Pseudovitamin D Deficiency Rickets and Hereditary Vitamin D Resistant Rickets Marie B. Demay Endocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts INTRODUCTION The clinical observation that rickets and osteomalacia could be cured with vitamin D repletion or sunlight led to the identification of a subset of affected individuals who were resistant to this therapeutic intervention. The skeletal abnormalities in patients who did not respond to this conventional therapy often resolved with pharmacologic doses of vitamin D or 25- hydroxyvitamin D. Subsequent clinical and basic studies showed that these patients could largely be subdivided into two categories: those in whom treatment with physiological doses of 1,25-dihydroxyvitamin D effected a cure and those in whom it did not. Characterization of biochemical and subsequently genetic abnormalities led to the identification of two rare autosomal recessive disorders: pseudovitamin D deficiency rickets (PDDR; OMIM ) and hereditary vitamin D resistant rickets (HVDRR; OMIM ), previously known as VDDR types I and II. PSEUDOVITAMIN D DEFICIENCY RICKETS The identification of 1,25-dihydroxyvitamin D as the active metabolite of vitamin D led to studies that revealed a subset of The author has reported no conflicts of interest. patients with inherited rickets in whom treatment with this hormone effected a cure. Based on the biochemical parameters and the clinical response of these patients, it was postulated that this autosomal recessively inherited disorder was a consequence of impaired production of 1,25-dihydroxyvitamin D. (1,2) Studies in placental cells isolated from an affected individual confirmed an absence of 25-hydroxyvitamin D 1 -hydroxylase activity, (3) pointing directly to impaired enzyme activity as the cause of the disorder. The cloning of the 25-hydroxyvitamin D 1 -hydroxylase gene (4 6) led to the identification of inactivating mutations in affected individuals, (7 9) thus confirming the hypothesis that had been proposed based on previous clinical and basic studies. Individuals affected by this disorder usually present in the first year of life with rickets and osteomalacia and may also have hypocalcemic seizures. Although studies in rodent models have shown that intestinal calcium absorption is largely 1,25- dihydroxyvitamin D independent the first 2 weeks of life, gradually being replaced by a 1,25-dihydroxvitamin D dependent active transport mechanism, (10) it is not know whether an analogous transition occurs in humans that could account for the onset of the clinical disorder after the first 3 months of life. As a consequence of hypocalcemia, patients have secondary hyperparathyroidism that leads to the development of hypophosphatemia because of an increase in renal
20 PDDR AND HVDRR / 339 TABLE 1. CHARACTERISTICS OF PDDR AND HDVRR Disorder Gene mutated Calcium Phosphorus PTH 25(OH)D 1,25(OH) 2 D Alopecia PDDR 1 -hydroxylase Low Low High Normal Low/undetectable Absent HVDRR VDR Low Low High Normal High Variable phosphate clearance. In addition to renal phosphate losses, amino aciduria may be observed, presumably because of secondary hyperparathyroidism. There is an increase in serum alkaline phosphatase levels, and radiological examination is notable for evidence of rickets. Unlike patients with nutritional rickets, affected individuals have normal circulating levels of 25-hydroxyvitamin D. However, levels of 1,25-dihydroxyvitamin D are undetectable or markedly reduced (Table 1). Bone biopsy specimens show the presence of osteomalacia and a decrease in mineral apposition in untreated individuals. (11) However, treatment with 1,25-dihydroxyvitamin D and calcium supplementation effectively cures the disorder by bypassing the enzymatic defect. Before the availability of 1 hydroxylated vitamin D metabolites, patients were treated with pharmacologic amounts of vitamin D or 25-hydroxyvitamin D at doses that were 100-fold those required to treat patients with nutritional vitamin D deficiency. The availability of 1,25- dihydroxyvitamin D led to the observation that restoration of normal serum levels of this hormone effected a cure. (11 13) In fact, treatment with 1 -hydroxylated vitamin D metabolites and calcium leads to an increase in serum calcium levels within 24 h and radiographic healing of rickets within 2 3 months. (12) Although treatment of this disorder is uniformly effective, lifelong therapy is required, because discontinuation of therapy results in impaired intestinal calcium absorption, followed by clinical evidence of vitamin D deficiency within a few days. Therapeutic compliance and efficacy should be monitored by measurements of serum calcium, phosphorus, immunoreactive PTH (ipth), and alkaline phosphatase. Although there may be an increase in alkaline phosphatase levels in the early stages of treatment, associated with healing of osteomalacic lesions, levels should return to normal within 6 12 weeks of therapy. Adequate calcium intake should be insured because studies in affected humans as well as in a mouse model of this disorder show that the skeletal abnormalities are a direct consequence of impaired intestinal calcium absorption. (14) Therapy should be adjusted to maintain serum calcium and ipth levels within the normal range and to avoid hypercalciuria, because the latter may lead to nephrolithiasis. In growing children, monitoring should be performed every 6 8 weeks until a therapeutic response has been observed. Thereafter, monitoring should be performed at least every 6 months so that the therapeutic regimen can be adjusted to accommodate the mineral ion needs of a growing skeleton. Because the requirements of the adult skeleton are less variable, monitoring should be performed every 6 12 months once skeletal maturity has been achieved. More frequent monitoring, in both children and adults, is required during intercurrent illnesses, such as gastroenteritis, which can lead to a decrease in calcium intake or impaired intestinal calcium and 1,25-dihydroxyvitamin D absorption. Similarly, more frequent monitoring is required during pregnancy, and doses of 1,25-dihydroxyvitamin D often need to be increased by % to meet the needs of the fetus without further compromising the maternal skeleton. (13) Treatment of PDDR is effective, and if instituted early, can prevent the skeletal and dental abnormalities that are a consequence of the disordered mineral ion homeostasis in these patients with 25-hydroxyvitamin D 1 -hydroxylase mutations. Institution of treatment after the development of rickets leads to the cure of rickets and osteomalacia but is not uniformly associated with prevention of enamel hypoplasia in the dentition that develops postnatally. (15) Thus, although lifelong therapy is required, early clinical recognition permits institution of a safe and effective therapeutic regimen for this disorder. HEREDITARY VITAMIN D RESISTANT RICKETS The observation that a group of patients with congenital rickets was not responsive to treatment with physiological doses of 1,25-dihydroxyvitamin D permitted the identification of a subset of rachitic patients with markedly elevated levels of this hormone, in whom resistance to the biological effects of this hormone were suspected. (16) Unlike fibroblasts isolated from normal individuals, which, when treated with 1,25- dihydroxyvitamin D, showed a decrease in proliferation and an increase in 24-hydroxylation of vitamin D metabolites, cells from affected individuals were not responsive to 1,25- dihydroxyvitamin D. (17 19) This impaired biological responsiveness to 1,25-dihydroxyvitamin D was associated with defective hormone binding or impaired nuclear uptake of radiolabeled 1,25-dihydroxyvitamin D. (20 22) Cloning of the vitamin D receptor (VDR) enabled studies directed at confirming that mutation of the vitamin D receptor was the molecular basis for this disorder. (23 26) The VDR is a member of the nuclear receptor superfamily. (27) These nuclear receptors were originally thought to be ligand-dependent transcription factors, but studies directed at examining the in vitro and in vivo effects of these receptors have revealed a number of ligand-independent actions. Unlike the closely related thyroid and retinoid receptors, to date only one nuclear VDR isoform has been identified. (23) Analogous to other members of the nuclear receptor superfamily, the VDR has a DNA-binding domain comprised of two zinc fingers, a ligand binding domain, a domain required for heterodimerization with the retinoid-x receptor, and a transactivation domain that includes regions required for recruitment of nuclear receptor co-modulators. (27) Mutations in each of these functional domains have been found in affected patients. (28,29) CLINICAL PRESENTATION Like PDDR, HVDRR is an autosomal recessive disorder and is often associated with parental consanguinity. (28) The parents, who are obligate heterozygotes, are phenotypically normal. Affected individuals usually present in infancy with rickets and osteomalacia, accompanied by symptoms of hypocalcemia, including tetany and seizures. The clinical presentation is similar to that of patients with 25-hydroxyvitamin D 1 hydroxylase mutations, with the exception that affected individuals in some kindreds with VDR mutations develop progressive alopecia beginning the first year of life, progressing to alopecia totalis. It was postulated that the development of alopecia was associated with a more severe clinical phenotype (30) and, in fact, all mutations in the DNA binding domain reported to date, are associated with alopecia. (28) However, some affected individuals with alopecia have been reported to have spontaneous remissions of their disordered mineral ion
21 340 / CHAPTER 61 homeostasis, (31) suggesting either that they may have less severe hormone resistance or that other compensatory mechanisms are brought into play. Radiological examination of affected individuals is analogous to that observed in patients with PDDR, including widened, radiolucent metaphyses characteristic of rickets and evidence of osteomalacia, including the presence of pseudofractures. Dental abnormalities, including enamel hypoplasia and oligodontia may also be seen. (32) Metabolic abnormalities include hypocalcemia, secondary hyperparathyroidism, and hypophosphatemia caused by a PTH-dependent increase in urinary phosphate clearance. The development of secondary hyperparathyroidism may be associated with aminoaciduria and osteitis fibrosa cystica. An increase in serum alkaline phosphatase levels is also observed. Recent studies in mice with targeted ablation of the VDR, (33) as well as in other hypophosphatemic mouse models, (34) point to hypophosphatemia as the etiological basis for the growth plate expansion. These studies show that hypophosphatemia is associated with impaired apoptosis of hypertrophic chondrocytes and that phosphate ions induce apoptosis of these cells in a differentiation-dependent fashion by activating the caspase 9 dependent mitochondrial apoptotic pathway. (33) Thus, normalizing serum phosphorus levels may be required to prevent rickets and to achieve normal growth. The single parameter that distinguishes patients with HVDRR from PDDR is the presence of elevated levels of 1,25-dihydroxyvitamin D (Table 1). The dramatic increase in hormone levels is thought to reflect induction of the renal 25-hydroxyvitamin D 1 -hydroxylase gene by the high PTH levels, as well as impaired inactivation of 1,25-dihydroxyvitamin D, because expression of the major enzyme involved in its inactivation, the vitamin D 24-hydroxylase, requires the receptor-dependent effects of 1,25-dihydroxyvitamin D for its activation. (35) Affected individuals have a dramatic impairment in intestinal calcium absorption, (36) despite markedly elevated serum levels of 1,25-dihydroxyvitamin D. Unlike patients affected by PDDR, in whom a uniformly successful treatment response is observed, treatment of patients with HVDRR often presents a challenge. In approximately one half of the patients impaired, receptor function is present rather than absent. In these individuals, pharmacologic doses of vitamin D or 1,25- dihydroxyvitamin D can overcome the resistance syndrome, ameliorating intestinal calcium absorption, and consequently, the clinical phenotype. (36) In other patients, this regimen is ineffective. In this subset of severely affected patients, skeletal manifestations can be treated by intravenous infusion with calcium, thus bypassing the defect in intestinal calcium absorption. The observation that these infusions can cure rickets and osteomalacia, as well as normalize the mineral apposition rate, (37) suggests that the receptor-dependent actions of 1,25- dihydroxyvitamin D are not essential for normal growth and skeletal homeostasis. In support of this hypothesis is the observation that prevention of abnormal mineral ion homeostasis in mice lacking functional VDRs results in a radiologically and histomorphometrically normal skeleton with normal biomechanical properties. (38) Chronic therapy for this disorder is usually required. The mainstay of therapy is combined treatment with calcium and pharmacologic doses vitamin D metabolites. Although rare, sporadic remissions have been described in affected individuals during late adolescence, after the high mineral ion demands of the growing skeleton have abated. In children, the efficacy of the therapeutic regimen should be reassessed every 4 6 weeks by evaluation of serum calcium and phosphorus levels. Immunoreactive PTH levels and alkaline phosphatase levels should be measured every 2 3 months, because an increase in PTH levels will antedate the decrease in serum calcium and allow therapeutic intervention in a more timely fashion. In patients in whom calcium infusions are required, these infusions should be continued until calcium, phosphorus, PTH, and alkaline phosphatase levels are normalized. Infusions should be resumed when these parameters become abnormal. In patients on chronic oral therapy, more frequent monitoring is required during intercurrent gastrointestinal illnesses or other situations where calcium intake or absorption may be compromised. Patients with HVDRR are fertile; however, modification of the therapeutic regimen is usually required in the second and third trimesters because of the increased calcium requirements associated with pregnancy. The increased maternal calcium need continues through the period of lactation. While appropriate treatment, whether oral or intravenous, ameliorates the skeletal consequences of VDR mutations, it does not affect the development of alopecia. (39) Studies in a mouse model of HVDRR showed that VDR expression in the keratinocyte component of the hair follicle is both necessary (40) and sufficient to prevent alopecia. (41) Interestingly, the actions of the VDR required to maintain normal hair are ligandindependent. (42) Clarifying the molecular actions of the VDR in this unique model system will undoubtedly elucidate novel molecular interactions of this nuclear receptor. CONCLUSION Investigations directed at elucidating the biochemical and molecular basis for these two autosomal recessive disorders provided considerable insight into the regulation of hormone synthesis and the molecular basis of hormone action. They also enabled the development of animal models to further elucidate novel actions of 1,25-dihydroxyvitamin D and its receptor. Analogous to other patients affected by hereditary disorders, genetic counseling should be offered to affected individuals and their parents. While the therapeutic regimen for patients affected by PDDR is effective, treatment of HVDRR still presents a challenge. Although parenteral calcium infusions have been successful in treating the skeletal sequelae of this disorder, a novel therapeutic regimen that would overcome the defect in intestinal calcium absorption would facilitate longterm compliance. In a similar fashion, characterization of the downstream effects of the VDR in the epidermal keratinocyte may enable the development of a therapeutic regimen directed at preventing alopecia in affected patients. REFERENCES 1. Fraser D, Kooh SW, Kind HP, Holick MF, Tanaka Y, DeLuca HF 1973 Pathogenesis of hereditary vitamin-d-dependent rickets: An inborn error of vitamin D metabolism involving defective conversion of 25- hydroxyvitamin D to 1,25-dihydroxyvitamin D. N Engl J Med 289: Drezner MK, Feinglos MN 1977 Osteomalacia due to 1,25- dihydroxycholecalciferol deficiency. J Clin Invest 60: Glorieux FH, Arabian A, Delvin EE 1995 Pseudo-vitamin D deficiency: Absence of 25-hydroxyvitamin D 1 -hydroxylase activity in human placenta decidual cells. J Clin Endocrinol Metab 80: St-Arnaud R, Messerliann S, Moir JM, Omdahl JL, Glorieux FH 1997 The 25-hydroxyvitamin D 1-alpha hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J Bone Miner Res 12: Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, Portale AA 1997 Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol 11: Shinki T, Shimada H, Wakino S, Anazawa H, Hayashi M, Saruta T, DeLuca HF, Suda T 1997 Cloning and expression of rat 25- hydroxyvitamin D3 1-alpha-hydroxylase cdna. Proc Natl Acad Sci USA 94:
22 PDDR AND HVDRR / Kitanaka S TK, Murayama A, Sato T, Okumura K, Nogami M, Hasegawa Y, Niimi H, Yanagisawa J, Tanaka T, Kato S 1998 Inactivating mutations in the 25-hydroxyvitamin D 1 alpha hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N Engl J Med 338: Wang JT LC, Burridge SM, Fu GK, Labuda M, Portale AA, Miller WL 1998 Genetics of vitamin D 1alpha-hydroxylase deficiency in 17 families. Am J Hum Genet 63: Yoshida T MT, Tenenhouse HS, Goodyer P, Shinki T, Suda T, Wakino S, Hayashi M, Saruta T 1998 Two novel 1alpha-hydroxylase mutations in French-Canadians with vitamin D dependency rickets type I1. Kidney Int 54: Dostal LA, Toverud SU 1984 Effect of vitamin D 3 on duodenal calcium absorption in vivo during early development. Am J Physiol 246:G528 G534, Delvin EE, Glorieux FH, Marie PJ, Pettifor JM 1981 Vitamin D dependency: Replacement therapy with calcitriol. Pediatrics 99: Reade TM, Scriver CR, Glorieux FH, Nogrady B, Delvin E, Poirier R, Holick MF, DeLuca HF 1975 Response to crystalline 1 -hydroxyvitamin D 3 in vitamin D dependency. Pediatr Res 9: Glorieux FH 1990 Calcitriol treatment in vitamin D-dependent and vitamin D-resistant rickets. Metabolism 39: Dardenne O, Prud homme J, Glorieux FH, St-Arnaud R 2004 Rescue of the phenotype of CYP27B1 (1alpha-hydroxylase)-deficient mice. J Steroid Biochem Mol Biol 89 90: Arnaud C, Maijer R, Reade T, Scriver CR, Whelan DT 1970 Vitamin D dependency: An inherited postnatal syndrome with secondary hyperparathyroidism. Pediatrics 46: Brooks MH, Bell NH, Love L, Stern PH, Orfei E, Queener SF, Hamstra AJ, DeLuca HF 1978 Vitamin D-dependent rickets Type II: Resistance of target organs to 1,25-dihydroxyvitamin D. N Engl J Med 298: Clemens TL, Adams JS, Horiuchi N, Gilchrest BA, Cho H, Tsuchiya Y, Matsuo N, Suda T, Holick MF 1983 Interaction of 1,25- dihydroxyvitamin-d 3 with keratinocytes and fibroblasts from skin of normal subjects and a subject with vitamin-d-dependent rickets, type II: A mode of action of 1,25-dihydroxyvitamin D 3. J Clin Endocrinol Metab 56: Griffin JE, Zerwekh JE 1983 Impaired stimulation of 25-hydroxyvitamin D-24-hydroxylase in fibroblasts from a patient with vitamin D-dependent rickets, type II: A form of receptor-positive resistance to 1,25- dihydroxyvitamin D 3. J Clin Invest 72: Gamblin GT, Liberman UA, Ell C, Downs RW Jr, DeGrange DA, Marx SJ 1985 Vitamin D-dependent rickets type II: Defective induction of 25-hydroxyvitamin D 3-24-hydroxylase by 1,25-dihydroxyvitamin D in cultured skin fibroblasts. J Clin Invest 75: Feldman D, Chen T, Cone C, Hirst M, Shani S, Benderli A, Hochberg Z 1982 Vitamin D resistant rickets with alopecia: Cultured skin fibroblasts exhibit defective cytoplasmic receptors and unresponsiveness to 1,25(OH) 2 D 3. J Clin Endocrinol Metab 55: Eil C, Liberman UA, Rosen JF, Marx SJ 1981 A cellular defect in hereditary viramin-d-depenedent rickets type II: Defective nuclear uptake of 1,25-dihydroxyvitamin D in cultured skin fibroblasts. N Engl J Med 304: Eil C, Liberman UA, Marx SJ 1986 The molecular basis for resistance to 1,25-dihydroxyvitamin D: Studies in cells cultured from patients with hereditary hypocalcemic 1,25(OH) 2 D 3 -resistant rickets. Adv Exp Med Biol 196: Hughes MR, Malloy PJ, Kieback DG, Kesterson RA, Pike JW, Feldman D, O Malley BW 1988 Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science 242: Hughes MR, Malloy PJ, O Malley BW, Pike JW, Feldmann D 1991 Genetic defects of the 1,25-dhydroxyvitamin D 3 receptor. J Recept Res 11: Malloy PJ, Hochberg Z, Pike JW, Feldman D 1989 Abnormal binding of vitamin D receptors to deoxyribonucleic acid in a kindred with vitamin D-dependent rickets, type II. J Clin Endocrinol Metab 68: Malloy PJ, Hochberg Z, Tiosano D, Pike JW, Hughes MR, Feldman D 1990 The molecular basis of hereditary 1,25-dihydroxyvitamin D 3 resistant rickets in seven related families. J Clin Invest 86: Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW 1998 The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J Bone Miner Res 13: Malloy PJ, Pike JW, Feldman D 1999 The vitamin D receptor and the syndrome of hereditary 1,25- dihydroxyvitamin D-resistant rickets. Endocr Rev 20: Malloy PJ, Xu R, Peng L, Clark PA, Feldman D 2002 A novel mutation in helix 12 of the vitamin D receptor impairs coactivator interaction and causes hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Mol Endocrinol 16: Marx SJ, Bliziotes MM, Nanes M 1986 Analysis of the relation between alopecia and resistance to 1,25-dihydroxyvitamin D. Clin Endocrinol (Oxf) 25: Takeda E, Yokota I, Kawakami I, Hashimoto T, Kuroda Y, Arase S 1989 Two siblings with vitamin-d-dependent rickets type II: No recurrence of rickets for 14 years after cessation of therapy. Eur J Pediatr 149: Bell NH 1980 Vitamin D-dependent rickets type II. Calcif Tissue Int 31: Sabbagh Y, Carpenter TO, Demay M 2005 Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA 102: Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD 2003 Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest 111: Kerry D, Dwivedi P, Hahn C, Morris H, Omdahl J, May B 1996 Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D 24-hydroxylase (CYP24) promoter. J Biol Chem 22: Tsuchiya Y, Matsuo N, Cho H, Kumagai M, Yasaka A, Suda T, Orimo H, Shiraki M 1980 An unusual form of vitamin D-dependent rickets in a child: Alopecia and marked end-organ hyposensitivity to biologically active vitamin D. J Clin Endocrinol Metab 51: Balsan S, Garabedian M, Larchet M, Gorski A-M, Cournot G, Tau C, Bourdeau A, Silve C, Ricour C 1986 Long-term nocturnal calcium infusions can cure rickets and promote normal mineralization in hereditary resistance to 1,25-dihydroxyvitamin D. J Clin Invest 77: Amling M, Priemel M HT, Chapin K, Rueger JM, Baron R, Demay MB 1999 Rescue of the skeletal phenotype of vitamin D receptor ablated mice in the setting of normal mineral ion homeostasis: Formal histomorphometric and biomechanical analyses. Endocrinology 140: Al-Aqeel A, Ozand P, Sobki S, Sewairi W, Marx S 1993 The combined use of intravenous and oral calcium for the treatment of vitamin D dependent rickets type II (VDDRII). Clin Endocrinol (Oxf) 39: Sakai Y, Kishimoto J, Demay M 2001 Metabolic and cellular analysis of alopecia in vitamin D receptor knockout mice. J Clin Invest 107: Chen C, Sakai Y, Demay M 2001 Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology 142: Skorija K, Cox M, Sisk JM, Dowd DR, MacDonald PN, Thompson CC, Demay MB 2005 Ligand-independent actions of the vitamin D receptor maintain hair follicle homeostasis. Mol Endocrinol 19:
23 Chapter 62. Hypophosphatemic Vitamin D Resistant Rickets Uri S. Alon Bone and Mineral Disorders Clinic, Section of Pediatric Nephrology, Children s Mercy Hospital, University of Missouri-Kansas City, School of Medicine, Kansas City, Missouri INTRODUCTION In the face of normal kidney function, all cases of rickets, other than a few with stage 1 vitamin D deficient rickets, are characterized by hypophosphatemia. (1,2) Differentiation between primary hypophosphatemia and secondary, which is caused by hyperparathyroidism, can be easily achieved by measuring serum PTH concentration. (2) In cases of abnormalities in calcium homeostasis, and more frequently vitamin D metabolism, serum PTH is elevated, causing increased tubular loses of phosphate. Treatment of these cases is aimed at correcting the abnormalities in calcium and vitamin D metabolism and requires no phosphate supplementation. On the other hand, rickets caused by a primary abnormality in phosphate metabolism is characterized by the presence of normal serum PTH concentration. Its etiology, whether renal or extrarenal, can be determined by analyzing urine phosphate excretion in the face of normal dietary intake of the mineral. If elevated, the pathophysiology resides within the proximal renal tubule; if low, an extrarenal source should be investigated. If the renal tubule is to be implicated, either a primary genetic etiology or a secondary tubular damage (such as that after exposure to certain nephrotoxic agents) is the likely source of phosphate loss. The most common genetic form of hypophosphatemic rickets is the one transmitted as an X-linked dominant trait (XLH), described first by Albright and named by him hypophosphatemic vitamin D resistant rickets. (3) Clinical and radiological features of all types of rickets are similar, but bone histology varies depending on the presence or absence of hyperparathyroidism. (4) In primary hypophosphatemic rickets and osteomalacia, histology shows accumulation of undermineralized osteoid with no evidence of increased osteoclast activity and excessive bone resorption. Consequently, bone mass is not always decreased. A DXA study in treated children and adolescents showed decreased BMD in the appendicular skeleton and increased BMD in the lumbar spine. (5) The author has reported no conflicts of interest. 342 GENETICS AND PATHOPHYSIOLOGY Originally an abnormality at the sodium-dependent phosphate transporter (NaPi-2A) at the tubular brush border membrane was disclosed, but it became clear that this was not the primary source of the pathophysiology in XLH. (6) Further studies to define the genetic and molecular basis of the disease have led to the identification of PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) located at Xp This gene encodes a membranebound endopeptidase primarily expressed in osteoblasts, osteocytes, odontoblasts, muscle, lung and ovary. Deletions affecting phex, the murine homolog of PHEX, were identified in the Hyp and Gy mice, the rodent analogs of human XLH. (7) Other mice strains with point mutations in phex also show tubular phosphate wasting, hypophosphatemia, and rickets. (7) More than 180 PHEX mutations have been identified (http: in numerous XLH families and individual cases, but some patients show no such abnormalities. (8) No phenotype genotype correlation was established between the PHEX gene and disease pattern. (8,9) Furthermore, overexpression of the human PHEX gene did not fully correct abnormalities in the Hyp mouse. (7) Both humans and the rodent mice model show insufficient production of 1,25-dihydroxyvitamin D in the face of low blood phosphate concentration (which normally stimulates increased production of the active vitamin D metabolite). Indeed, in the human autosomal recessive disease of hypophosphatemic rickets and hypercalciuria, patients exhibit high serum concentration of calcitriol, increased intestinal calcium absorption, and consequently, hypercalciuria and development of nephrocalcinosis and nephrolithiasis. Treatment of this disease, its genetic basis yet unknown, requires only oral phosphate supplementation. (10) In XLH, no cause and effect relationship has been established between the tubular phosphate leak and decreased calcitriol production. The observation of development of hyperphosphaturia and hypophosphatemia in an XLH patient with end-stage kidney disease who received a kidney transplant from a healthy donor, (11) as well as studies on Hyp mice using parabiosis (12) and cross-renal transplantation, (13) provided evidence that the hyperphosphaturia in this disease is not caused by a primary defect in the kidney, but is caused by a circulating factor. Based on findings in patients with tumor-induced osteomalacia in whom a phosphaturic factor was extracted from the tumor, this factor was originally named phosphatonin. (14 16) Multiple studies in recent years showed the presence of several phosphaturic agents, but at this point, abnormalities in fibroblast growth factor-23 (FGF-23) are at the center of attention. (17 20) Besides inhibiting tubular phosphate reabsorption, FGF-23 also inhibits 1,25-vitamin D production and stimulates 24,25- vitamin D production. (21) In Hyp mice, FGF-23 mrna expression is increased in the bones, and circulating FGF-23 concentration is high. (19) Similarly, circulating levels of FGF-23 are elevated in many but not all XLH patients. (22) Although originally believed that it is the lack of PHEX-dependent endopeptidase that is causing the high FGF-23 level, some dispute this association. (21,23) Contrary to familial tumoral calcinosis where recessive mutations in FGF-23 were identified, (24,25) thus far, no functional FGF-23 mutations have been detected in XLH patients. (8) CLINICAL MANIFESTATIONS During the first year of life, before a child starts to walk, findings are minimal or nonexistent. In infants, diagnosis is established almost exclusively in those in whom XLH is suspected based on their family history. Once the child starts to walk, progressive deformities of the lower extremities and decrease in growth rate become evident and alert parents and health professionals. In later childhood, if still untreated, dental complications appear as well, because of poorly mineralized dentin, enlarged pulp chambers, and root canals, causing multiple periradicular abscesses in caries-free teeth that result in early teeth decay in the young adult. (26) In fact, in some adult carriers, history of dental abscesses might be the only clinical
24 FAMILIAL HYPOPHOSPHATEMIC RICKETS / 343 finding. In other female carriers, the only finding might be asymptomatic hypophosphatemia, making the latter the marker for this disease. Other manifestations in the adult patient may include involvement of the spine and ligaments and hearing impairment. (27,28) Although an earlier study suggested that hearing impairment may indicate the possibility of a variant of the disease equivalent to the situation in the Gy mouse, (29) more recent studies showed that hearing impairment is rarely seen in children with XLH but more commonly seen in adults and therefore may be part of the natural history of the disease. (28) It is possible that both the spinal nerve involvement and the neurosensory hearing loss are the result of bony changes. It is yet unknown whether these skeletal changes are the results of the disease itself of a complication of its treatment. (27) Besides the radiographic findings of rickets, diagnosis is based on the biochemical findings of hypophosphatemia, hyperphosphaturia, elevated serum alkaline phosphatase activity with normal serum creatinine, calcium, PTH, 25-hydroxyvitamin D, and inappropriately low 1,25-dihydroxyvitamin D concentrations. TREATMENT Because of our current inability to correct the tubular phosphate losses, the only way to correct hypophosphatemia is by providing the patients with large quantities of oral phosphate preparations. Each dose has a very short lifetime, so it is recommended to provide four to five doses a day. The recommended starting dose is 40 mg/kg/day, which can be adjusted up to a maximum of 100 mg/kg/day, not to exceed 3.0 g of elemental phosphate per day. Treatment with phosphate alone will result in mild hypocalcemia triggering increased secretion of PTH. This will further lower the tubular threshold for phosphate, (30) resulting in a vicious cycle that will require additional intake of phosphate and consequently further increase in PTH secretion. To offset this effect of phosphate administration, patients are concomitantly treated by supraphysiologic doses of 1,25-dihydroxyvitamin D. The recommended starting dose is 25 ng/kg/day, with a maximum of 70 ng/kg/day, divided to two doses. Because of tendency for nocturnal hyperparathyroidism, if calcitriol is divided unevenly, the higher dose should be given at night. (31) With the combined treatment, one can expect healing of the rickets, and in some patients, spontaneous improvement in leg deformities or good response to braces, minimizing the need for corrective orthopedic surgery. Biochemically, one should aim at keeping serum phosphate at the lower range of normal for age, with which alkaline phosphatase activity will decrease to normal or close to normal and bone radiographs will show healing of the rickets. Some patients will show improved growth rates, especially when treatment is started early. (32) In those not showing sufficient gain in height, growth hormone (GH) therapy might be considered. Although some researchers reported improved height with GH therapy, (33) others point to a possible increase in the discrepancy between body trunk and lower limbs growth. (34,35) Histologically, the phosphate and vitamin D combination was shown to improve and in some cases heal the mineralization defect on the trabecular surface. (4) Because of the fact that bone turnover is lower and epiphyseal plates are fused, the role of treatment of adults is still not fully clear. It seems, however, that symptomatic adults with osteomalacia do benefit from treatment (36) ; it is as yet unknown, however, whether this treatment should be by 1,25-dihydroxyvitamin D alone or in combination with phosphate. (4) A single study showed the beneficial effect of 24,25- dihydroxyvitamin D, (37) but as of now, this treatment has not become standard of care for XLH. Whereas treatment with dipyridamole was found to be effective in adult patients with TABLE 1. PREPARATIONS FOR TREATMENT OF XLII Calcitriol Oral solution Rocaltrol 1 g/ml Capsules Rocaltrol: 0.25 g, 0.5 g Phosphate Powder Neutra-Phos: phosphorus 250 mg (8 mmol), potassium 278 mg (7.125 meq), sodium 164 mg (7.125 meq) Neutra-Phos-K: phosphorus 250 mg (8 mmol), potassium 556 mg (14.25 meq) (sodium free) Oral solution Fleet Phospho-Soda: phosphate 4 mmol, sodium 4.82 meq/ml (equivalent to monobasic sodium phosphate monohydrate 2.4 g and dibasic sodium phosphate heptahydrate 0.9 g per 5 ml) Tablets K-Phos M/F: phosphorus mg (4 mmol), potassium 44.5 mg (1.1 meq), sodium 67 mg (2.9 meq) K-Phos Neutral: phosphorus 250 mg (8 mmol), potassium 45 mg (1.1 meq), sodium 298 mg (13 meq) K-Phos No. 2: phosphorus 250 mg (8 mmol), potassium 88 mg (2.3 meq), sodium 134 mg (5.8 meq) K-Phos Original: phosphorus 114 mg (3.7 mmol), potassium 144 mg (3.7 meq) (sodium free) Uro-KP-Neutral: phosphorus 258 mg (8 mmol), potassium 49.4 mg (1.27 meq), sodium mg (10.9 meq) tubular phosphate leak, (38) it was reported to be ineffective in XLH patients. (39) A recent study in the Hyp mouse showed the potential of indomethacin in reducing hyperphosphaturia and improving serum phosphate concentration without lowering glomerular filtration rate. (40) The application of this novel finding in humans awaits further research. Future treatment modalities might be aimed at lowering FGF-23 levels or blocking its activity. COMPLICATIONS OF TREATMENT Phosphate supplementation is usually well tolerated and can be provided in the form of liquid preparation to young children and as a tablet to older children and adults. At times, high doses of the phosphate can result in diarrhea. In such cases, it is recommended to stop treatment for a few days, start at a lower dose, and increase it gradually. It is also seems more reasonable to use K-phosphate preparations rather than Na-K-phosphate ones (Table 1). A high load of sodium may enhance calciuria with its potential bone and kidney complications, whereas potassium is known to have an anticalciuric effect and may augment urinary citrate. (41) Furthermore, sodium administration causes volume expansion that enhances phosphaturia, and finally, acidic K-phospate preparations (K-Phos Original; Beach Pharmaceuticals, Tampa, FL, USA) may lower urine ph, desirable in decreasing the risk of calcium phosphate precipitation in the kidneys. (42) Before the introduction of 1,25- dihydroxyvitamin D, patients were treated with large amounts of vitamin D, which often resulted in severe complications, including hypercalcemia, hypercalciuria, and kidney damage. On the other hand, insufficient dosing of vitamin D resulted in the development of secondary and at times tertiary hyperparathyroidism. The introduction of 1,25-dihydroxyvitamin D simplified treatment by allowing better dose adjustment. (27) The two most common complications of therapy are nephrocalcinosis and hyperparathyroidism. (27) Nephrocalcinosis was reported in up to % of treated patients. (8,43,44) In the
25 344 / CHAPTER 62 majority of patients, nephrocalcinosis is asymptomatic and does not tend to progress, but in some instances, it was reported to be associated with tubular dysfunction. Kidney biopsy specimens in XLH patients with nephrocalcinosis, as well as in Hyp mice treated with high doses of phosphate, showed the calcifications to be composed of calcium phosphate. (45) It is therefore reasonable to prefer the use of phosphate preparations that maintain the urine acidity, thus decreasing the risk of deposition of calcium phosphate precipitates in the kidney. Thiazide diuretics have been shown to arrest the progression of nephrocalcinosis. (46) Recently, nephrocalcinosis was reported to resolve in a few teenagers treated with thiazides/amiloride diuretics. (47) The combination thiazides/amiloride not only reduces calciuria and may reverse nephrocalcinosis, but it might also improve bone mineralization. (48) Hyperparathyroidism is detected in 20 25% of treated patients, and in some of them, may lead to hypertension and renal failure. (8,49) Although an abnormality in the PHEX chromosome in the parathyroid glands was reported in one patient, (50) and a few infant patients may present with mild hyperparathyroidism, in the vast majority of patients, hyperparathyroidism is believed to be caused by the repetitive hypocalcemic effect of phosphate administration. If detected early, secondary hyperparathyroidism can be reversed by medical means (decreased phosphate and increased vitamin D doses). Under some circumstances, all treatment has to be stopped for 4 6 weeks to normalize serum PTH concentration before treatment can be resumed. In some patients, secondary hyperparathyroidism may progress to autonomous, hypercalcemic, tertiary hyperparathyroidism, which requires parathyroidectomy. (51,52) In cases in which a subtotal parathyroidectomy was conducted, there might be a need to proceed to a total parathyroidectomy if the remaining gland develops hyperplasia by itself. The introduction of calcimimetic agents may assist in the prevention and treatment of hyperparathyroidism in this disorder. CONCLUSION As more and more evidence accumulates concerning the pathophysiology of hypophosphatemic vitamin D resistant rickets, hope exists for new modes of treatment, in particular, preventing excess FGF-23 activity on bone and kidney. Until this futuristic treatment is available, current management continues with phosphate and vitamin D preparations. However, this treatment should be monitored carefully and routinely for possible side effects. In particular, routine measurements of kidney functions, serum PTH level, and urine calcium/ creatinine ratio should be done every 3 4 months, combined with annual ultrasounds. Use of Ca-sparing diuretics seems to be beneficial in preventing renal complications, and calcimimetic agents may have a role in preventing and treating secondary hyperparathyroidism. ACKNOWLEDGMENTS This study was supported by the Sam and Helen Kaplan Research Fund in Pediatric Nephrology. I thank Regina Johnson for excellent administrative assistance. REFERENCES 1. Srivastava T, Alon US 2002 Stage I vitamin D-deficiency rickets mimicking pseudohypoparathyroidism type II. Clin Pediatr 41: Pattargarn A, Alon US 2001 Antacid-induced rickets in infancy. Clin Pediatr 40: Albright F, Butlet AM, Bloomberg E 1937 Rickets resistant to vitamin D therapy. Am J Dis Child 54: Glorieux FH 2003 Hypophospatemic vitamin D-resistant rickets. In Favus MJ (ed.) Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 5th ed. American Society for Bone and Mineral Research, Washington, DC, USA, pp Shore RM, Langman CB, Poznansk AK 2000 Lumbar and radial bone mineral density in children and adolescents with X-linked hypophosphatemia: Evaluation with dual X-ray. Skeletal Radiol 29: Tenenhouse HS, Scriver CR, McInnes RR, Clorieux FH 1978 Renal handling of phosphate in vivo and in vitro by the X-linked hypophosphatemic male mouse (Hyp/Y): Evidence for a defect in the brush border membrane. Kidney Int 14: Erben R, Dagmar M, Wever K, Jonsson K, Juppner H, Lanske B 2005 Overexpression of human PHEX under the -actin promoter does not fully rescue the hype mouse phenotype. J Bone Miner Res 20: Cho HY, Lee BH, Kang JH, Ha IS, Cheong HI, Choi, Y 2005 A clinical and molecular genetic study of hypophosphatemic rickets in children. Pediatr Res 58: Holm IA, Nelson AE, Robinson BG, Mason RS, Marsh DJ, Cowell CT, Carpenter TO 2001 Mutational analysis and genotype-phenotype correlation of the PHEX gene in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab 86: Jones A, Tzenova J, Frappier D, Crumley M, Roslin N, Kos C, Tieder M, Langman C, Proesmans W, Carpenter T, Rice A, Anderson D, Morgan K, Fujiwara T, Tenehouse H 2001 Hereditary hypophosphatemic rickets with hypercalciuria is not caused by mutations in the Na/Pi cotransporter NPT2 gene. J Am Soc Nephrol 12: Morgan JM, Hawley WL, Chenoweth AI, Retan JW, Diethelm AG 1974 Renal transplantation in hypophosphatemia with vitamin D-resistant rickets. Arch Intern Med 134: Meyer RA, Meyer MH, Gray RW 1989 Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia mice. J Bone Miner Res 4: Nesbitt T, Coffman TM, Drezner MK 1992 Cross-transplantation of kidneys in normal and Hyp mice: Evidence that the Hyp phenotype is unrelated to an intrinsic renal defect. J Clin Invest 89: Cai, Q, Hodgson ST, Kao PC, Lennon VA, Klee GG, Zinmiester AR, Kumar Rajiv 1994 Inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med 330: Schiavi SC, Kumar R 2004 The phosphatonin pathway: New insights in phosphate homeostasis. Kidney Int 65: Ward LM, Rauch F, White KE, Filler G, Matzinger MA, Letts M, Travers R, Econs MJ, Glorieux FH 2004 Resolution of severe, adolescent-onset hyphosphatemic rickets following resection of an FGF-23-producing tumour of the distal ulna. Bone 34: Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR 2004 MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone 34: Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Tasasaki T, Fukumoto S, Yamashita T 2004 FGF-23 transgenic mice demonstarate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun 314: Shimada T, MIzutani S, Muto T, Yoneya T, Hino R, Takeda S. Takeuchi Y, Fujita T, Fukumoto S, Yamishita T 2001 Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Pro Natl Acad Sci USA 98: Carpenter TO, Ellis BK, Insogna KL, Philbrick WM, Sterpka J, Shimkets R 2005 Fibroblast growth factor 7: An inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab 90: Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T 2004 FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljuggren O, Oba K, Yang IM, Mijauchi A, Econs MJ, Lavigne J, Juppner H 2003 Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348: Benet-Pages A, Lorenz-Depiereux B, Zischka H, White KE, Econs MJ, Strom TM 2004 FGF23 is processed by proprotein convertases but not by PHEX. Bone 35: Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, White KE 2005 A novel recessive mutation in fibroblast growth factor causes familial tumoral calcinosis. J Clin Endrocrinol Metab 90: Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B 2005 An FGF23
26 TUMOR-INDUCED OSTEOMALACIA / 345 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet 14: Pereira CM, de Andrade CR, Vargas PA, Coletta RD, de Almeida OP, Lopes MA 2004 Dental alterations associated with X-linked hypophasphatemic rickets. J Endod 30: Wilson DW, Alon U 1993 Renal hypophosphatemia. In: Alon U, Chan JCM (eds.) Phosphate in Pediatric Health and Disease. CRC Press, Boca Raton, FL, USA, pp Fishman G, Miller-Hansen D, Jacobsen C, Singhal VK, Alon US 2004 Hearing impairment in familial X-linked hypophosphatemic rickets. Eur J Pediatr 163: Boneh A, Reade TM, Scriver CR, Rishikof E 1987 Audiometric evidence for two forms of X-linked hypophosphatemia in humans, apparent counterparts of Hyp and Gy mutations in mouse. Am J Med Genet 27: Alon U, Chan JCM 1984 Effects of PTH and 1,25 dihydroxyvitamin D3 on tubular handling of phosphate in hypophosphatemic rickets. J Clin Endocrinol Metab 58: Carpenter TO, Mitnick MA, Ellison A, Smith C, Insogna KL 1994 Nocturnal hyperparathyroidism: A frequent feature of X-linked hypophosphatemia. J Clin Endocrinol Metab 78: Makitie O, Doria A, Kooh SW, Cole WG, Daneman A, Sochett E 2003 Early treatment improves growth and biochemical and radiographic outcome in X-linked hypophosphatemic rickets. J Clin Endrocrinol Metab 88: Baroncelli GI, Bertelloni S, Ceccarelli C, Saggese G 2001 Effect of growth hormone treatment on final height, phosphate metabolism, and bone mineral density in children with X-linked hypophosphatemic rickets. J Pediatr 138: Haffner D, Nissel R, Wuhl E, Mehls O 2004 Effects of growth hormone treatment on body proportions and final height among small children with X-linked hypophosphatemic rickets. Pediatr 11:e593 e Reusz GS, Miltényi G, Stubnya G, Szabó A, Horváth C, Byrd DJ, Péter F, Tulassay T 1997 X-linked hypophosphatemia: Effects of treatment with recombinant human growth hormone. Pediatr Nephrol 11: Sullivan W, Carpenter T, Glorieux FH, Travers R, Insogna K 1992 A prospective trial of phosphate and 1,25-dihydroxyvitamin D3 therapy in symptomatic adults with X-linked hypophasphatemic rickets. J Clin Endocrinol Metab 75: Carpenter TO, Keller M, Schwartz D, Mitnick M, Smith C, Ellison A, Carey D, Comite F, Horst R, Travers R, Glorieux FH, Gundberg CM, Poole AR, Insogna KL ,25-Dihydroxyvitamin D supplementation corrects hyperparathyroidism and improves skeletal abnormalities in X-linked hypophosphatemic rickets a clinical research center study. J Clin Endocrinol Metab 81: Prié D, Blanchet FB, Essig M, Jourdain J, Friedlander G 1998 Dipyridamole decreases renal phosphate leak and augments serum phosphorus in patients with low renal phosphate threshold. J Am Soc Nephrol 9: Seikaly MG, Quigley R, Baum M 2000 Effect of dipyridamole on serum and urinary phosphate in x-linked hypophosphatemia. Pediatr Nephrol 15: Baum M, Loleh S, Seikaly M, Dwarakanath V, Quigley R 2003 Correction of proximal tubule phosphate transport defect in hyp mice in vivo and in vitro with indomethacin. Proc Natl Acad Sci USA 100: Osorio AV and Alon US 1997 The relationship between urinary calcium, sodium and potassium excretion and the role of potassium in treating idiopathic hypercalciuria. Pediatrics 100: Alon US, Moore W 2002 Effect of long-term treatment with acid phosphate on the development of nephrocalcinosis in familial hypophosphatemic rickets. Pediatric Academic Societies Annual Meeting, Baltimore, MD, USA, May 5 8, Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M 1991 Effects of therapy in X-lined hypophosphatemic rickets. N Engl J Med 325: Nehgme R, Fahey JT, Smith C, Carpenter TO 1997 Cardiovascular abnormalities in patients with X-linked hypophosphatemia. J Clin Endocrinol Metab 82: Alon U, Donaldson DL, Hellerstein S, Warady BA, Harris DJ 1992 Metabolic and histologic investigation of the nature of nephrocalcinosis in children with hypophosphatemic rickets and the Hyp mouse. J Pediatr 120: Seikaly MG, Baum M 2001 Thiazide diuretics arrest the progression of nephrocalcinosis in children with X-linked hypophosphatemia. Pediatrics 106:e6 e Auron A, Alon US 2005 Resolution of medullary nephrocalcinosis in children with metabolic bone disorders. Pediatr Nephrol 20: Alon U, Chan JCM 1985 The effects of hydrochlorothiazide and amiloride in renal hypophosphatemic rickets. Pediatrics 75: Alon US, Monzavi R, Lilien M, Rasoulpour M, Geffner ME, Yadin O 2003 Hypertension in hypophosphatemic rickets role of secondary hyperparathyroidism. Pediatr Nephrol 18: Blydt-Hansen TD, Tenenhouse HS, Goodyer P 1999 PHEX expression in parathyroid gland and parathyroid hormone dysregulation in X-linked hypophasphatemia. Pediatr Nephrol 13: Chan JCM, Young RB, Alon U, Mamunes P 1983 Hypercalcemia in children with disorders of calcium and phosphate metabolism during long-term treatment with 1,25 dihydroxyvitamin D3. Pediatrics 72: Savio RM, Gosnell JE, Posen S, Reeve TS, Delbridge LW 2004 Parathyroidectomy for tertiary hyperparathyroidism associated with X-linked dominant hypophosphatemic rickets. Arch Surg 139: Chapter 63. Tumor-Induced Osteomalacia Suzanne M. Jan de Beur Department of Medicine, The Johns Hopkins University School of Medicine, Baltimore, Maryland INTRODUCTION Tumor-induced osteomalacia (TIO), or oncogenic osteomalacia, is an acquired, paraneoplastic syndrome of renal phosphate wasting that resembles genetic forms of hypophosphatemic rickets. Since the initial observation by McCrance, (1) clinical and experimental studies implicate the humoral factor(s) that tumors produce in the profound biochemical and skeletal alterations that characterize TIO. TIO is a rare disorder; however, progress in understanding its pathogenesis is contributing to our understanding of hypophosphatemic disorders and of normal phosphate homeostatic mechanisms. Dr. Jan de Beur has a consultant agreement with Genzyme. CLINICAL AND BIOCHEMICAL MANIFESTATIONS Although the preponderance of patients with TIO is observed in adults (usually diagnosed in the sixth decade), this syndrome may present at any age. In the clinical setting, these patients report long-standing, progressive muscle and bone pain, weakness, and fatigue that often predate the fractures that complicate TIO. Children with TIO display rachitic features including gait disturbances, growth retardation, and skeletal deformities. The occult nature of TIO delays its recognition, and the average time from onset of symptoms to a correct diagnosis often exceeds 2.5 years. (2) Once the syndrome is recognized, an average of 5 years elapses from the time of diagnosis to the identification of the underlying tumor. (3) Until the underlying tumor is identified, other renal phosphate-wasting syndromes must be considered. There-
27 346 / CHAPTER 63 FIG. 1. Radiographic and histological features of TIO. (A) Octreotide scan showing small mesenchymal tumor in the head of the humerus (arrowhead). (B) Hemangiopericytoma with numerous pericytes and vascular channels (H&E stain). Original magnification, 100. (C) Bone biopsy with Goldner stain. Excessive osteoid or unmineralized bone matrix composed mainly of collagen stains pink (black). Mineralized bone stains blue (gray). Normal bone usually has a very thin, barely visible layer of osteoid. The presence of excessive osteoid is indicative of osteomalacia. This bone biopsy show severe osteomalacia. Original magnification, 20.(46) fore, it is important to note that, in patients with TIO, a family history of hypophosphatemia and bone disorders is absent. A rapid onset and severe clinical course may help differentiate TIO from X linked hypophosphatemic rickets (XLH). Identification of previously normal serum phosphorus level in an adult patient supports the diagnosis of TIO, although in rare instances patients with autosomal dominant hypophosphatemic rickets (ADHR) can present as adults. In situations when inherited hypophosphatemic rickets must be excluded, genetic testing for mutations in the PHEX gene and FGF-23 gene is indicated. In the management of presumptive TIO, clinical diligence, serial physical examination, and appropriate imaging are required to successfully detect the underlying tumor. One of the major obstacles to diagnosing TIO is that serum phosphorus measurements are no longer included in the standard comprehensive metabolic panel. Thus, hypophosphatemia is not identified unless it is ordered specifically by the clinician. The biochemical hallmarks of TIO are low serum concentrations of phosphorus, phosphaturia secondary to reduced proximal renal tubular phosphate reabsorption, and frankly low or inappropriate normal levels of serum calcitriol [1,25(OH)2D] that are expected to be elevated in the face of hypophosphatemia. The degree of hypophosphatemia is usually profound and can range from 0.7 to 2.4 mg/dl.(2) However, serum calcium and 25-hydroxyvitamin D levels are invariably normal, and serum concentrations of intact PTH are only occasionally elevated. Alkaline phosphatase is typically elevated and derived primarily from bone. A more global proximal tubular defect that results in glucosuria, and amino aciduria may accompany the phosphaturia. Bone histomorphometry reveals severe osteomalacia with clear evidence of a mineralization defect with increased mineralization lag time and excessive osteoid (Fig. 1). The dual defect of renal phosphate wasting in concert with impaired calcitriol synthesis results in poor bone mineralization and fractures.(2,4) If untreated, severe osteomalacia leads to fractures of the long bones as well as vertebra and ribs with resultant chest wall deformity and respiratory compromise. DIAGNOSTIC EVALUATION Laboratory Studies When evaluating a patient with suspected TIO, the work-up includes fasting serum phosphorus, a chemistry panel with serum calcium, alkaline phosphatase, and creatinine, an intact PTH level, and serum 1,25(OH)2D. In addition, a fasting 2-h urine phosphorus, creatinine, calcium, amino acids, and glucose are measured. As an indication of renal tubular phosphate clearance, the maximum tubular reabsorption of phosphate factored for glomerular filtration rate (TmP/GFR) is calculated. In the setting of renal phosphate wasting, the TmP/GFR is lower than expected for the degree of hypophosphatemia. In some instances when confirmation of the diagnosis is warranted, a tetracycline double-labeled, iliac crest bone biopsy is obtained for bone histomorphometric studies. Imaging General. Plain radiographs exhibit characteristics of osteomalacia including generalized osteopenia, pseudofractures, and coarsened trabeculae. Radiographs of children with TIO show widened epiphyses and other features of rickets. Diffuse skeletal uptake, referred to as a superscan, and focal uptake at sites of fractures are characteristic features on 99Technecium bone scintigraphy. In general, plain films show features of osteomalacia; however, it is impossible to differentiate the underlying etiology of the osteomalacia with these modalities. Tumor Localization. Detection and localization of the culprit tumor in TIO is imperative because complete surgical resection is curative. However, the mesenchymal tumors that cause this syndrome are often small, slow growing, and frequently situated in unusual anatomical sites; therefore, conventional imaging techniques often fail to localize them. Because in vitro studies show that many mesenchymal tumors express somatostatin receptors (SSTR),(5) 111In-pentetreotide scintigraphy (octreotide scan), a scanning technique that uses a radiolabeled somatostatin analog, has been used to successfully detect and localize these tumors in some patients with TIO (Fig. 1).(3,6) The mesenchymal tumors that express SSTR are not limited to those associated with TIO; thus, careful biochemical confirmation of the syndrome is necessary before embarking on exhaustive imaging efforts.(7) Successful tumor localization has been reported in a few patients with other imaging techniques such as whole body MRI(8) and positron emission tomography.(9) In a single instance, venous sampling for fibroblast growth factor
28 TUMOR-INDUCED OSTEOMALACIA / 347 (FGF)-23 was used to confirm an identified mass was the causative tumor in a patient with TIO. (10) With conventional imaging such as magnetic resonance scanning or CT, special attention directed to craniofacial locations and the extremities is indicated because these are more common locations for tumors in TIO, although tumors have been found distributed through out the body. DIFFERENTIAL DIAGNOSIS Osteomalacia in adults and rickets in children can result from a variety of conditions including abnormal vitamin D metabolism (which in itself has a long differential diagnosis, abnormal bone matrix, enzyme defects, inhibitors of mineralization, calcium and phosphorus deficiency, and renal phosphate wasting. TIO is a syndrome of impaired renal phosphorus reabsorption; therefore, the discussion will focus on differentiating TIO from other renal phosphate-wasting disorders. In contrast to more common forms of osteomalacia that share clinical features with TIO, patients with TIO have normal serum calcium, normal serum 25-hydroxyvitamin D, normal intact PTH, low 1,25(OH) 2 D, and inappropriately elevated urine phosphorus. With the appropriate battery of tests, TIO is readily distinguishable from most common forms of osteomalacia. However, TIO is biochemically indistinguishable from several inherited forms of hypophosphatemic rickets: X linked hypophosphatemic rickets (XLH) and autosomal dominant hypophosphatemic rickets (ADHR). (11) Because patients with XLH and ADHR exhibit a variable age of onset, it is critical to take a careful family history in patients with hypophosphatemia. The clinical consequences of TIO are typically present for many years before the causal tumor is identified; this further obscures the clinical distinction between TIO and inherited forms of hypophosphatemic, vitamin D resistant rickets. In contrast to XLH, patients with TIO exhibit symptoms of weakness, pain, and fractures that are more severe and disabling. Stress and insufficiency fractures are more typical of TIO, whereas lower extremity deformity and short stature are characteristic of XLH and ADHR. Serum FGF-23 levels are generally elevated in patients with TIO but are also elevated in patients with ADHR and some patients with XLH. Furthermore, normal serum FGF-23 levels do not eliminate the diagnosis of TIO. (11,12) The diagnosis of TIO is dependent on the identification of the culprit tumor and remission of the syndrome after complete tumor resection. Genetic testing of the PHEX and FGF-23 genes, which are defective in XLH and ADHR, respectively, is commercially available and may be indicated when a definitive diagnosis is necessary. (13) Another inherited renal phosphate-wasting syndrome, hereditary hypophosphatemic rickets with hypercalciuria (HHRH), is clinically similar to TIO, with bone pain, osteomalacia, and muscle weakness as prominent features; however, the distinction is easily made with biochemical testing. (14) Both syndromes are characterized by hypophosphatemia owing to decreased renal phosphorus reabsorption; however, patients with HHRH exhibit elevated levels of calcitriol and hypercalciuria that distinguish it from TIO, XLH, and ADHR. Recently, the molecular basis of this syndrome has been identified as biallelic mutations in SLC34A3, the gene that encodes the NaPiIIc sodium phosphate transporter. (15,16) A new hypophosphatemic syndrome was described in two patients with hypophosphatemia secondary to renal phosphate wasting and osteopenia or nephrolithiasis. This was caused by heterozygous, dominant-negative, mutations in the renal type IIa sodium-phosphate co-transporter gene (NPT-2). The prominent symptoms of bone pain and muscle weakness seen in TIO are absent in those with NPT-2 mutations. Furthermore, the presence of hypercalciuria and elevated calcitriol make these patients easily distinguishable from patients with TIO. (17) TUMORS The mesenchymal tumors that are associated with TIO are characteristically slow-growing, complex, polymorphous neoplasms that have been subdivided into four groups based on their histological features: (1) phosphaturic mesenchymal tumor, mixed connective tissue type (PMTMCT); (2) osteoblastoma-like tumors; (3) ossifying fibrous-like tumors; and (4) nonossifying fibrous-like tumors. (18) The PMTMCT subtype is the most common and comprises 70 80% of the mesenchymal tumors associated with TIO. (18,19) Characterized by an admixture of spindle cells, osteoclast-like giant cells, prominent blood vessels, cartilage-like matrix, and metaplastic bone, these tumors occur equally in soft tissue and bone. Although typically benign, malignant variants of PMTMCT have been described. FGF-23 message is abundantly expressed in these tumors, (20 22) and FGF-23 protein is detectable by immunoblot and immunohistochemistry. (23) In one series, 17 of 21 PMT- MCT tumors had detectable FGF-23 protein expression. (19) The granular cytoplasm within the spindle cells exhibits the most consistent staining and seems to be the source of FGF-23. These mesenchymal tumors are small, indolent and remotely located. Although found in a variety of anatomical locations, including the long bones, the nasopharynx, the sinuses, and the groin, (3) these tumors are most commonly located in the extremities and appendicular skeleton. (19) While tumor localization is frequently a prolonged and arduous task, once detected, the anatomical inaccessibility of the tumors make complete resection difficult. Successful tumor detection requires careful physical examination, diligent follow-up, and periodic imaging. Although TIO is typically caused by benign mesenchymal tumors, the syndrome has also been associated with a variety carcinomas, neurofibromatosis, linear nevus syndrome, and fibrous dysplasia of bone. Because the multiplicity of lesions and resultant inability to completely resect the entire tumor burden, demonstration of biochemical and radiographic improvement with surgery has been lacking save for a few cases. PATHOPHYSIOLOGY Dual Defect: Renal Phosphate Wasting and Abnormal Vitamin D Metabolism The basic pathophysiology of TIO is hypophosphatemia secondary to inhibition of renal phosphate reabsorption compounded by a vitamin D synthetic defect that blunts the compensatory rise in calcitriol in response to hypophosphatemia. Profound hypophosphatemia results in muscle pain and weakness, osteomalacia, and fractures. Experimental evidence suggests that the biochemical and skeletal defects in TIO are caused by a humoral factor (or factors), coined phosphatonin, produced by mesenchymal tumors. Tumor extracts can inhibit phosphate transport in vitro, (23) produce phosphaturia and hypophosphatemia in vivo, (24) and inhibit renal 25- hydroxyvitamin D-1- -hydroxylase activity in cultured kidney cells. (25) Furthermore, complete surgical resection of tumor tissue results in normalization of serum phosphate and calcitriol, reversal of renal phosphate loss, and eventually, remineralization of bone. (2,3) Identifying Phosphatonin : FGF-23 Although several groups have reported tumor cultures or tumor extracts inhibit phosphate transport, slow growth of
29 348 / CHAPTER 63 cultured tumor cells and the frequent loss of phosphateinhibitory activity in culture hampered the identification of the phosphaturic substance produced by these tumors. By examining highly expressed genes in TIO tumors, (21,26,27) several candidate genes for the phosphaturic substance(s) produced by these tumors have been identified. Included among these genes is FGF-23, a novel FGF, which was contemporaneously identified by positional cloning as the defective gene in ADHR. (28) In contrast to the low expression in normal tissues, (28) FGF-23 is highly expressed in TIO tumors. (21,28) Conditioned media and purified FGF-23 can inhibit phosphate transport in opossum kidney cells (OK), a model of renal proximal tubular epithelium. (20,29) When injected into mice, FGF-23 reduces serum phosphate and increases fractional excretion of phosphorus. (27,30) Mice chronically exposed to FGF-23 become hypophosphatemic with increased renal phosphate clearance, show reduced bone mineralization, and have reduced expression of renal 25-hydroxyvitamin D-1- hydroxylase with decreased circulating levels of calcitriol. (27) The biochemical and skeletal abnormalities of transgenic mice that overexpress FGF-23 mimic human TIO. (31,32) Conversely, FGF-23 deficient mice exhibit growth retardation and early death, with biochemical abnormalities that include hyperphosphatemia, elevated calcitriol, and hypercalcemia. (33,34) Circulating FGF-23 is detectable in human serum. (11,12) Individuals with TIO exhibit elevated serum levels of FGF-23 that plummet after complete tumor resection. However, some individuals with TIO have normal or only mildly elevated levels underscoring the heterogeneous composition of phosphatonin. FGF-23 is also central in the pathogenesis of ADHR. Missense mutations in one of two arginine residues at positions 176 or 179 have been identified in affected members of four unrelated ADHR families. (28) This clustering of missense mutations suggests that they are activating mutations. Furthermore, the mutated arginine residues, located in the consensus proprotein convertase cleavage RXXR motif, prevent the degradation of FGF-23 and thus may result in prolonged or enhanced FGF-23 action. (20,31,35 37) Amassing evidence suggests that FGF-23 may be key in the pathogenesis of XLH. XLH is caused by mutations in the PHEX gene, (38) which encodes an M13 metalloprotease. Speculation about the function of PHEX paired with data that implicate both an intrinsic osteoblast defect and a humoral factor in the pathogenesis of XLH led to the hypothesis that the substrate for PHEX is the humoral factor responsible for TIO. The endogenous substrate for PHEX remains unknown, and it remains unclear if PHEX modifies FGF-23 directly (20,39) or indirectly. (40) It is clear that FGF-23 plays a central role in several disorders of renal phosphate wasting (Fig. 2). In TIO, tumors ectopically produce FGF-23 that inhibits renal tubular reabsorption of phosphate and downregulates the 25- hydroxyvitamin D-1- -hydroxylase, resulting in hypophosphatemia and osteomalacia. In ADHR, FGF-23 bears mutations that enhance it biological activity and render it resistant to inactivation by proteolytic cleavage with resultant renal phosphate wasting, hypophosphatemia, bone deformity, and rickets. In XLH, mutated PHEX directly or indirectly leads to accumulation of FGF-23 that exerts its phosphaturic activity at the renal proximal tubule. Other Phosphatonin Candidates Secreted Frizzled Related Protein 4 (sfrp4). Gene expression profiles of mesenchymal tumors associated with TIO (21) showed several genes that encoded secreted proteins that were highly and differentially expressed. This analysis revealed a second phosphatonin candidate, sfrp4. FRPs are a class of molecules that inhibit Wnt signaling by acting as a decoy receptor. Wnt signaling is important in development especially of the skeleton and kidney. Recently, two disorders of bone mass accrual have been linked to activation or inhibition of Wnt signaling through it co-receptor, LRP5. Thus, it is possible that modulation of Wnt signaling may be important in regulating determinants of bone mass including some aspects of mineral ion homeostasis. Several lines of evidence suggest sfrp4 has phosphaturic properties. sfrp4 inhibits phosphate transport in cultured renal epithelial cells, it reduces fractional excretion of phosphorus when infused into mice and rats, and with longer-term exposure, sfrp4 produces hypophosphatemia with blunting of the compensatory increase in 25- hydroxyvitamin D-1- -hydroxylase expression. (41) Matrix Extracellular Phosphoglycoprotein. Matrix extracellular phosphoglycoprotein (MEPE)/osteoregulin is a recently identified secreted protein that displays structural features of an extracellular matrix protein and is highly expressed in bone marrow and differentiated osteoblasts. (26) Three independent investigators showed that MEPE is highly expressed in mesenchymal tumors associated with TIO. (20,26,27) MEPE-deficient mice exhibit increased BMD caused by enhanced bone mineralization, (42) whereas overexpression of MEPE in TIO is associated with impaired bone mineralization, suggesting that MEPE is an important negative regulator of bone mineralization. MEPE, when given intraperitoneally to mice, produced renal phosphate wasting and hypophosphatemia (43) ; furthermore, MEPE inhibits phosphate transport in cultured human renal epithelial cells. (43) Intriguing data are emerging that MEPE may be a substrate for PHEX (20) or modify PHEX function by a nonproteolytic interaction. (44) FGF-7. Recently, Carpenter et al. (45) showed that FGF-7 is overexpressed in tumors associated with TIO and that it had phosphaturic activity in cultured cells. TREATMENT The definitive treatment for TIO is complete tumor resection. This results in rapid correction of the biochemical perturbations and remineralization of bone. However, even after the diagnosis of TIO is made, the tumor often remains obscure or incompletely resected. In the case of malignant tumors associated with TIO, such as prostate cancer, complete resection may not be possible. Therefore, many times, medical management of this disorder is necessary. The current practice is to treat TIO with phosphorus supplementation in combination with calcitriol. The phosphorus supplementation serves to replace ongoing renal phosphorus loss and the calcitriol supplements replace insufficient renal production of 1,25-dihydroxyvitamin D and enhance renal and gastrointestinal phosphorus reabsorption. Generally, patients are treated with phosphorus (2 g/day), in divided doses, and calcitriol (1 3 g/day). (2) In some cases, administration of calcitriol alone may improve the biochemical abnormalities seen in TIO and heal the osteomalacia. Therapy and dosing should be tailored to improve symptoms, maintain fasting phosphorus in the low normal range, normalize alkaline phosphatase, and control secondary hyperparathyroidism, without inducing hypercalcemia or hypercalciuria. With appropriate treatment, muscle and bone pain will improve, and healing of the osteomalacia will ensue. Monitoring for therapeutic complications of high doses of calcitriol and phosphorus is important to prevent unintended
30 TUMOR-INDUCED OSTEOMALACIA / 349 FIG. 2. Mechanisms of FGF-23 excess in renal phosphate-wasting syndromes. In TIO, FGF-23 and other phosphatonins ectopically produced by a mesenchymal tumor lead to excess circulating FGF-23 levels. In ADHR, FGF-23 excess results from mutations in the FGF-23 gene that render the protein resistant to cleavage and inactivation. In XLH, the mechanism of FGF-23 excess is more speculative; mutations in the PHEX endopeptidase (presumably located on osteoblasts or osteocytes) are thought to either directly or indirectly result in FGF-23 excess by interfering with processing and inactivation of FGF-23. (46) hypercalcemia, nephrocalcinosis, and nephrolithiasis. Although parathyroid autonomy has been reported in only a few cases of TIO, the true incidence is likely higher with prolonged treatment with phosphorus (alone or in combination with vitamin D) because it stimulates parathyroid function that can eventually lead to autonomy. To assess safety and efficacy of therapy, monitoring of serum and urine calcium, renal function, and parathyroid status is recommended at least every 3 months. Octreotide in vitro and in vivo has been shown to inhibit secretion of hormones by many neuroendocrine tumors. The expression of SSTRs that bind octreotide by some mesenchymal tumors provided the rationale for a therapeutic trial of octreotide in several patients with TIO and residual tumor. In one case, treatment with subcutaneous octreotide g three times a day resulted in correction of hypophosphatemia, improvement in phosphaturia, and reduction in alkaline phosphatase. (7) However, in two other patients, despite 8 weeks of treatment with subcutaneous octreotide up to 200 g three times daily, serum levels of phosphorus and calcitriol failed to increase, and the tubular reabsorption of phosphate remained depressed. (4) Given the limited and mixed experience with octreotide treatment in TIO, this therapy should be reserved for the most severe cases that are refractory to current medical therapy. As we understand the pathophysiology of this disorder more fully, specific therapies directed at attenuating the effect of excess FGF-23 and the other humoral factors elaborated in TIO no doubt will be developed. REFERENCES 1. McCrance RA 1947 Osteomalacia with Looser s nodes (milkman s syndrome) due to a raised resistance to vitamin D acquired about the age of 15 years. Q J Med 16: Drezner MK 1999 Tumor-induced osteomalacia. In: Favus MJ (ed.) Primer on Metabolic Bone Diseases and Disorders of Mineral Metabolism, 4th ed. Lippincott-Raven, Philadelphia, PA, USA, pp Jan de Beur SM, Streeten EA, Civelek AC, McCarthy EF, Uribe L, Watts N, Marx S, Sharon M, Levine MA 2002 Localization of mesenchymal tumors causing oncogenic osteomalacia with somatostatin receptor imaging. Lancet 359:
31 350 / CHAPTER Kumar R 2000 Tumor-induced osteomalacia and the regulation of phosphate homeostasis. Bone 27: Reubi JC, Waser B, Laissue JA, Gebbers JO 1996 Somatostatin and vasoactive intestinal peptide receptors in human mesenchymal tumors: in vitro identification. Cancer Res 56: Seufert J, Ebert K, Muller J, Eulert J, Hendrich C, Werner E, Schuuze N, Schulz G, Kenn W, Richtmann H, Palitzsch KD, Jakob F 2001 Octreotide therapy for tumor-induced osteomalacia. N Engl J Med 345: Jan de Beur SM, Levine MA 2002 Molecular pathogenesis of hypophosphatemic rickets. J Clin Endocrinol Metab 87: Avila NA, Skarulis M, Rubino DM, Doppman JL 1996 Oncogenic osteomalacia: Lesion detection by MR skeletal survey. Am J Roentgenol 167: Dupond JL, Mahammedi H, Prie D, Collin F, Gil H, Blagosklonov O, Ricbourg B, Meaux-Ruault N, Kantelip B 2005 Oncogenic osteomalacia: Diagnostic importance of fibroblast growth factor 23 and F-18 fluorodeoxyglucose PET/CT SCAN for the diagnosis and follow-up in one case. Bone 36: Takeuchi Y, Suzuki H, Ogura S, Imai R, Yamazaki Y, Yamashita T, Miyamoto Y, Okazaki H, Nakamura K, Nakahara K, Fukumoto S, Fujita T 2004 Venous sampling for fibroblast growth factor-23 confirms preoperative diagnosis of tumor-induced osteomalacia. J Clin Endocrinol Metab 89: Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S 2002 Increased circulatory level of biologically active full-length FGF23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87: Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H 2003 Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348: Gene Dx Gene testing for XLH (PHEX) and ADHR (FGF23) are commercially available. Available online at and www-.medichecks.com. Accessed March 28, Teider M, Modai D, Samuel R 1985 Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med 312: Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM 2006 Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodiumphosphate cotransporter gene SLC34A3. Am J Hum Genet 78: Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu- Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H 2006 LC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78: Prie D, Huart V, Bakouh N, Planelles G, Dellis O, Gerard B, Hulin P, Benque-Blanchet F, Silve C, Grandchamp B, Friedlander G 2002 Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med 347: Weidner N, Santa CD 1987 Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer 59: Folpe AL, Fanburg-Smith JC, Weiss SW, the Phosphaturic Mesenchymal Tumor Study Group 2003 Most phosphaturic mesenchymal tumors are a single entity: An analysis of 31 cases. Mod Pathol 16:12A. 20. Bowe A, Finnegan R, Jan de Beur SM, Vassiliadis J, Cho J, Levine MA, Kumar R, Schiavi SC 2001 FGF23 Inhibits phosphate transport in vitro and is a substrate for the PHEX endopeptidase. Biochem Biophys Res Commun 284: Jan de Beur SM, Finnegan RB, Vassiliadis J, Cook B, Barberio D, Estes S, Manavalon P, Petroziello J, Madden S, Cho JY, Kumar R, Levine MA, Schiavi SC 2002 Tumors associated with oncogenic osteomalacia express markers of bone and mineral metabolism. J Bone Miner Res 17: White KE, Jonsson KB, Carn G, Hampson G, Spector TD, Mannstadt M, Lorenz-Depiereux B, Miyauchi A, Yang IM, Ljunggren O, Meitinger T, Strom TM, Juppner H, Econs MJ 2001 The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab 86: Cai Q, Hodgson SF, Kao PC, Lennon VA, Klee GG, Zinsmiester AR, Kumar R 1994 Brief report: Inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med 330: Popovtzer MM 1981 Tumor-induced hypophosphatemic osteomalacia (TIO): Evidence for a phosphaturic cyclic AMP-independent action of tumor extract. Clin Res 29:418A. 25. Miyauchi A, Fukase M, Tsutsumi M, Fujita T 1998 Hemangiopericytomainduced osteomalacia: Tumor transplantation in nude mice causes hypophosphatemia and tumor extracts inhibit renal 25-hydroxyvitamin D-1- hydroxylase activity. J Clin Endocrinol Metab 67: Rowe PS, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL 2000 MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics 67: Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T 2001 Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98: The ADHR Consortium 2000 Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF 23. Nat Genet 26: Yamashita T, Konishi M, Miyake A, Inui Ki, Itoh N 2002 Fibroblast Growth Factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem 27: Shimada T, Muto T, Urakawa, Yoneya I, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T 2002 Mutant FGF23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophophatemia in vivo. Endocrinology 143: Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T 2004 FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commum 314: Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB 2004 Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(i) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145: Sitara D, Razzzaque M, Hesse M, Yoganathan S, Taguchi T, Erben R, Juppner H, Lanske B 2004 Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23: Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T 2004 Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ 2001 Autosomal dominant hypophosphatemic rickets mutations stabilize FGF23. Kidney Int 60: Bai XY, Miao D, Goltzman D, Karaplis AC 2003 The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem 278: Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N 2003 Human fibroblast growth factor-23 mutants suppress Na -dependent phosphate co-transport activity and 1alpha, 25- dihydroxyvitamin D3 production. J Biol Chem 278: The HYP Consortium 1995 A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 11: Campos M, Couture C, Hirata IY, Juliano MA, Loisel TP, Crine P, Juliano L, Boileau G, Carmona AK 2003 Human recombinant PHEX has a strict S1 specificity for acidic residues and cleaves peptides derived from FGF23 and MEPE. Biochem J. 373: Guo R, Lui S, Spurney RF, Quarles LD 2001 Analysis of recombinant Phex: An endopeptidase in search of a substrate. Am J Physiol Endocrinol Metab 281:E837 E Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, Jan de Beur SM, Schiavi SC, Kumar R 2003 Frizzled Related Protein 4 is a potent phosphaturic agent: Properties of a novel phosphatonin-like substance. J Clin Invest 112: Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA 2003 Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem 278: Rowe PS, Kumagai Y, Gutierrez G, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR 2004 MEPE
32 HYPOPHOSPHATASIA / 351 has the properties of an osteoblastic phosphatonin and minihibin. Bone 34: Rowe PS, Garrett IR, Schwartz PM, Carnes DL, Lafer EM, Mundy GR, Gutierrez GE 2005 Surface Plasmon resonance confirms that MEPE binds to PHEX via the MEPE-ASARM motif: A model for impaired mineralization in X linked rickets (HYP). Bone 36: Carpenter TO, Ellis BK, Insogna KL, Philbrick WM, Sterpka J, Shimkets R 2004 Fibroblast growth factor 7: An inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab 90: Jan de Beur SM 2005 Tumor-induced osteomalacia. JAMA 294: Chapter 64. Hypophosphatasia Michael P. Whyte Division of Bone and Mineral Diseases, Washington University School of Medicine at Barnes-Jewish Hospital and Center for Metabolic Bone Disease and Molecular Research, Shriners Hospitals for Children, St. Louis, Missouri INTRODUCTION Hypophosphatasia (OMIM , , ) is a rare, heritable type of rickets or osteomalacia that occurs in all races (although especially uncommon in blacks). The incidence for the severe forms is 1 per 100,000 births; mild forms are more prevalent. (1,2) Approximately 300 cases have been reported. This inborn error of metabolism is characterized biochemically by subnormal activity of the tissue-nonspecific (bone/liver/ kidney) isoenzyme of alkaline phosphatase (TNSALP). Activities of the tissue-specific intestinal, placental, and germ-cell ALP isoenzymes are not diminished. (3) Although there is considerable overlap in severity among them, four principal clinical forms of hypophosphatasia are reported, depending on the age at which skeletal lesions are discovered: perinatal, infantile, childhood, and adult. When dental manifestations alone are present, the condition is called odontohypophosphatasia. (4) Generally, the earlier the onset of skeletal problems, the more severe the clinical course. (1,2) The author has reported no conflicts of interest. CLINICAL PRESENTATION Although some TNSALP is normally present in all tissues, hypophosphatasia affects predominantly the skeleton and teeth. Severity of clinical expression is, however, remarkably variable (e.g., death may occur in utero or mild symptoms may go undiagnosed in adults). (1,2) Perinatal hypophosphatasia manifests during gestation. Pregnancies may be complicated by polyhydramnios. Typically, extreme skeletal hypomineralization causes caput membranaceum and short, deformed limbs apparent at birth. Rarely, an unusual bony spur protrudes from a major long bone. (5) Most affected newborns survive only briefly while suffering increasing respiratory compromise and sometimes unexplained fever, anemia (perhaps from encroachment on the marrow space by excessive osteoid), failure to gain weight, irritability, periodic apnea with cyanosis and bradycardia, intracranial hemorrhage, and pyridoxine-dependent seizures. Survival is very rare. (1,2) Infantile hypophosphatasia becomes clinically apparent before 6 months of age. Developmental milestones often seem normal until poor feeding, inadequate weight gain, hypotonia, and wide fontanels are noted. (6) Rachitic deformities then manifest. Hypercalcemia and hypercalciuria can cause recurrent vomiting, nephrocalcinosis and, occasionally, renal compromise. Despite widely open fontanels (actually hypomineralized areas of calvarium), functional craniosynostosis can occur. Raised intracranial pressure may be associated with bulging of the anterior fontanel, proptosis, and papilledema. Mild hypertelorism and brachycephaly can appear. A flail chest predisposes to pneumonia. During the months after diagnosis, there may be spontaneous improvement or progressive skeletal deterioration. About 50% of patients die within 1 year. (6) Prognosis seems to improve if there is survival beyond infancy. (1,2) Childhood hypophosphatasia varies greatly in severity. Premature loss of deciduous teeth ( 5 years of age) from hypoplasia or aplasia of dental cementum is a major clinical hallmark. Odontohypophosphatasia is diagnosed when radiographs show no evidence of skeletal disease. The lower incisors are typically lost first, but in severe cases the entire dentition can be affected. Exfoliation occurs without root resorption; teeth slide out intact from sockets. Dental radiographs often show enlarged pulp chambers and root canals forming shell teeth. The prognosis for the permanent dentition is more favorable. When rickets is present, delayed walking with a waddling gait, short stature, and a dolichocephalic skull with frontal bossing are often apparent. Static myopathy is a poorly understood complication. Childhood hypophosphatasia may improve spontaneously during puberty, but recurrence of skeletal symptoms is likely during adult life. (1,2) Adult hypophosphatasia usually presents during middle age, often with painful and poorly healing, recurrent, metatarsal stress fractures. (7) Pain in the thighs or hips can reflect femoral pseudofractures. About 50% of patients give histories consistent with rickets and/or premature loss of deciduous teeth during childhood. (7) Chondrocalcinosis occurs frequently, and calcium pyrophosphate dihydrate crystal deposition disease and calcific periarthritis trouble some patients. (8) Femoral pseudofractures generally mend after intramedullary rodding. (9) LABORATORY FINDINGS Hypophosphatasia is diagnosed from a consistent clinical history and physical findings, radiographic or histopathological evidence of rickets or osteomalacia, and the presence of low serum ALP activity (hypophosphatasemia). (1) Diagnosticians must appreciate changes in the normal range for serum ALP activity with age and understand that rarely other conditions (including severe cases of osteogenesis imperfecta and cleidocranial dysplasia) and treatments can cause hypophosphatasemia. (1) Rickets/osteomalacia in hypophosphatasia is distinctly unusual because serum levels of calcium and inorganic phosphate (Pi) are not reduced. In fact, hypercalcemia and hypercalciuria
33 352 / CHAPTER 64 occur frequently in perinatal and infantile hypophosphatasia, apparently because of dyssynergy between gut absorption of calcium and defective skeletal growth and mineralization (severely affected patients may also show progressive skeletal demineralization).(6) Affected children and adults have serum Pi levels that are above mean levels for age-matched controls, and 50% are hyperphosphatemic. Enhanced renal reclamation of Pi (increased transport maximum for phosphate [TmP]/ glomerular filtration rate [GFR]) accounts for this abnormality.(1) In serum, vitamin D metabolite concentrations are typically normal.(1) PTH levels may be suppressed. At least three phosphocompounds accumulate endogenously in hypophosphatasia(1,3): phosphoethanolamine (PEA), inorganic pyrophosphate (PPi), and pyridoxal 5 -phosphate (PLP). Demonstration of phosphoethanolaminuria supports the diagnosis but is not specific because PEA can be modestly increased in a variety of other disorders, and normal levels can occur in mild cases. Assay of PPi in plasma and urine is a research technique. If vitamin B6 supplements are not taken, an elevated plasma level of PLP seems to be the most sensitive and specific test for hypophosphatasia among these markers. In general, the lower the serum level of ALP activity for age and the greater the plasma PLP level, the more severe the clinical manifestations.(1,3) RADIOLOGIC FINDINGS Perinatal hypophosphatasia manifests pathognomonic features.(10) In extreme cases, the skeleton may be so poorly calcified that only the base of the skull is visualized. In less remarkable patients, the calvarium may be ossified at central portions of individual membranous bones and give the illusion that the sutures are open and widely separated. Marked skeletal undermineralization occurs with severe rachitic changes. Segments of the spinal column may appear missing. Fractures are also common. Infantile hypophosphatasia causes characteristic but less severe changes.(10) Abrupt transition from relatively normal appearing diaphyses to hypomineralized metaphyses can suggest a sudden metabolic deterioration. Worsening rickets with progressive skeletal demineralization and fracture heralds a lethal outcome. Skeletal scintigraphy may identify closed sutures that appear widened radiographically. Childhood hypophosphatasia often features characteristic tongues of radiolucency that project from rachitic growth plates into metaphyses (Fig. 1). True, premature fusion of cranial sutures can cause a beaten-copper appearance of the skull. Adult hypophosphatasia is associated with osteopenia, metatarsal stress fractures, chondrocalcinosis, and proximal femoral pseudofractures. FIG. 1. The metaphysis of the proximal tibia of this 10-year-old boy with mild childhood hypophosphatasia shows a subtle but characteristic tongue of radiolucency (arrows). Note, however, that his rickets does not manifest with widening of the growth plate. patients usually have low or low-normal serum ALP activity and sometimes mildly elevated plasma PLP levels and modest phosphoethanolaminuria. Challenge with vitamin B6 (pyridoxine) orally is followed by a distinctly abnormal increment in plasma PLP levels in patients, and in some carriers.(1) The mode of inheritance for the milder forms of hypophosphatasia (odontohypophosphatasia, childhood hypophosphatasia, and adult-onset disease) can be autosomal dominant or recessive.(1,11,12) HISTOPATHOLOGIC FINDINGS BIOCHEMICAL GENETIC DEFECT Nondecalcified sections of bone reveal histological features of rickets or osteomalacia (without secondary hyperparathyroidism) in all clinical forms of hypophosphatasia except odontohypophosphatasia.(1) However, biochemical or histochemical detection of low ALP activity in osseous tissue distinguishes hypophosphatasia from other disorders. Open cranial sutures are actually uncalcified osteoid. Dental histopathology shows aplasia or hypoplasia of cementum.(4) Enlarged pulp chambers indicate impaired dentinogenesis. Changes vary from tooth to tooth. In keeping with an inborn error of metabolism that selectively compromises the TNSALP isoenzyme, autopsy studies of perinatal and infantile hypophosphatasia show profound deficiency of ALP activity in bone, liver, and kidney, but not in the intestine or placenta. More than 170 different mutations have been identified worldwide in the TNSALP gene.(11 14) INHERITANCE Perinatal and infantile hypophosphatasia are inherited as autosomal recessive traits. Parents of these severely affected PATHOGENESIS Studies of vitamin B6 metabolism in hypophosphatasia indicate that TNSALP regulates the extracellular concentration of a variety of phosphocompounds.(3) Accumulation of PPi, an inhibitor of hydroxyapatite crystal formation and growth, is increasingly incriminated in the impaired skeletal mineralization.(2,3,15,16) The TNSALP knockout mouse model, which re-
34 HYPOPHOSPHATASIA / 353 capitulates infantile hypophosphatasia, (17) is helping to clarify the physiological role of TNSALP. TREATMENT There is no established medical therapy for hypophosphatasia. Marrow cell transplantation seemed to rescue and improve a patient with the infantile form. (6) Dietary Pi restriction to correct hyperphosphatemia and thereby reduce inhibition of TNSALP by Pi is being tested in milder cases. (18) A preliminary report suggests that teriparatide might stimulate TNSALP biosynthesis by osteoblasts and heal fractures. (19) Unless there is documented deficiency, it seems important to avoid traditional treatments for rickets or osteomalacia (e.g., vitamin D sterols and mineral supplementation) because circulating levels of calcium, Pi, 25(OH)D, and 1,25(OH) 2 D are usually not reduced. (1) Furthermore, traditional regimens may exacerbate any predisposition to hypercalcemia or hypercalciuria. The hypercalcemia of perinatal or infantile hypophosphatasia may respond to restriction of dietary calcium and to salmon calcitonin and/or glucocorticoid therapy. (20) Fractures in children and adults usually mend; however, healing may be delayed, including after osteotomy. Placement of load-sharing intramedullary rods, rather than load-sparing plates, seems best for the acute or prophylactic treatment of fractures and pseudofractures in adults. (9) Expert dental care is important. Dentures may be necessary even for pediatric patients. PRENATAL DIAGNOSIS Perinatal hypophosphatasia can be detected in utero. Combined use of serial sonography (with attention to the limbs as well as to the skull) and radiologic study of the fetus have been successful in the second trimester. (21) First-trimester diagnosis is now based on DNA. (21) Importantly, however, some cases of childhood hypophosphatasia may manifest bowing in utero that does not reflect a lethal skeletal dysplasia and corrects postnatally. (5) REFERENCES 1. Whyte MP 2001 Hypophosphatasia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B (eds.) The Metabolic and Molecular Bases of Inherited Disease, 8th ed. McGraw-Hill, New York, NY, USA, pp Caswell AM, Whyte MP, Russell RG 1992 Hypophosphatasia and the extracellular metabolism of inorganic pyrophosphate: Clinical and laboratory aspects. Crit Rev Clin Lab Sci 28: Whyte MP 2002 Hypophosphatasia: Nature s window on alkaline phosphatase function in man. In: Bilezikian J, Raisz L, Rodan G (eds.) Principles of Bone Biology, 2nd ed. Academic Press, San Diego, CA, USA, pp Van den Bos T, Handoko G, Niehof A, Ryan LM, Coburn SP, Whyte MP, Beertsen W 2005 Cementum and dentin in hypophosphatasia. J Dent Res 84: Pauli RM, Modaff P, Sipes SL, Whyte MP 1999 Mild hypophosphatasia mimicking severe osteogenesis imperfecta in utero: Bent but not broken. Am J Med Genet 86: Whyte MP, Kurtzberg J, McAlister WH, Mumm S, Podgornik MN, Coburn SP, Ryan LM, Miller CR, Gottesman GS, Smith AK, Douville J, Waters-Pick B, Armstrong RD, Martin PL 2003 Marrow cell transplantation for infantile hypophosphatasia. J Bone Miner Res 18: Weinstein RS, Whyte MP 1981 Heterogeneity of adult hypophosphatasia: Report of severe and mild cases. Arch Intern Med 141: Chuck AJ, Pattrick MG, Hamilton E, Wilson R, Doherty M 1989 Crystal deposition in hypophosphatasia: A reappraisal. Ann Rheum Dis 48: Coe JD, Murphy WA, Whyte MP 1986 Management of femoral fractures and pseudofractures in adult hypophosphatasia. J Bone Joint Surg Am 68: Shohat M, Rimoin DL, Gerber HE, Lachman RS 1991 Perinatal hypophosphatasia: Clinical, radiologic, and morphologic findings. Pediatr Radiol 21: Henthorn PS, Raducha M, Fedde KN, Lafferty MA, Whyte MP 1992 Different missense mutations at the tissue-nonspecific alkaline phosphatase gene locus in autosomal recessively inherited forms of mild and severe hypophosphatasia. Proc Natl Acad Sci USA 89: Mornet E 2000 Hypophosphatasia: The mutations in the tissuenonspecific alkaline phosphatase gene. Hum Mutat 15: Mornet E 2005 Tissue nonspecific alkaline phosphatase gene mutations database. Available online at Accessed May 13, Brun-Heath I, Taillandier A, Serre JL, Mornet E 2005 Characterization of 11 novel mutations in the tissue non-specific alkaline phosphatase gene responsible for hypophosphatasia and genotype-phenotype correlations. Mol Genet Metab 84: Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL 2002 Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA 99: Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millan JL 2004 Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: An integrated model of the pathogenesis of mineralization disorders. Am J Pathol 164: Fedde KN, Blair L, Silverstein J, Coburn SP, Ryan LM, Weinstein RS, Waymire K, Narisawa S, Millan JL, MacGregor GR, Whyte MP 1999 Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res 14: Wenkert D, Podgornik MN, Coburn SP, Ryan LM, Mumm S, Whyte MP 2002 Dietary phosphate restriction therapy for hypophosphatasia: Preliminary observations. J Bone Miner Res 17:S Deal C, Whyte MP 2005 Adult hypophosphatasia treated with teriparatide. J Bone Miner Res 20:S Barcia JP, Strife CF, Langman CB 1997 Infantile hypophosphatasia: Treatment options to control hypercalcemia, hypercalciuria, and chronic bone demineralization. J Pediatr 130: Henthorn PS, Whyte MP 1995 Infantile hypophosphatasia: Successful prenatal assessment by testing for tissue-nonspecific alkaline phosphatase gene mutations. Prenat Diagn 15:
35 Chapter 65. Fanconi Syndrome and Renal Tubular Acidosis Peter J. Tebben, 1 Leslie F. Thomas, 2 and Rajiv Kumar 3 1 Department of Internal Medicine, Division of Endocrinology, Diabetes, Metabolism, and Nutrition, Mayo Clinic, Rochester, Minnesota; 2 Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota; and 3 Mayo Proteomic Research Center, Department of Internal Medicine, Department of Biochemistry and Molecular Biology, Division of Nephrology, Division of Endocrinology, Diabetes, Metabolism, and Nutrition, Mayo Clinic, Rochester, Minnesota DEFINITION AND PRESENTATION Fanconi syndrome is a disorder of renal proximal tubules. The principle characteristics include decreased reabsorption of phosphorus, glucose, and amino acids. These findings are often accompanied by metabolic acidosis secondary to proximal tubular bicarbonate wasting (type II RTA). Although impaired handling of potassium, calcium, uric acid, sodium, water, and low-molecular-weight proteins have also been described, (1 5) these abnormalities are not necessary to establish the diagnosis. Laboratory findings include hypophosphatemia, hyperphosphaturia, and a low tubular maximum for inorganic phosphate; glycosuria with a normal plasma glucose concentration; generalized aminoaciduria; hypobicarbonatemia, excessive bicarbonate excretion in the urine and a low tubular maximum for bicarbonate; and elevated serum alkaline phosphatase, normal serum calcium, normal PTH, normal serum 25-hydroxyvitamin D [25(OH)D 3 ], and inappropriately low or normal serum 1,25-dihydroxyvitamin D [1,25(OH) 2 D 3 ] concentrations. Radiological studies reveal changes consistent with osteomalacia or rickets. Children with Fanconi syndrome present clinically with growth failure and rickets. Lower extremity deformities including genu varus or valgum may be evident on exam. Radiographs show widened physeal plates and flared metaphyses. Fanconi syndrome has been associated with many diseases (Table 1), including the lysosomal storage disease, cystinosis, which is the most common inherited cause in the pediatric population. Adults present with osteomalacia, which manifests as bone pain, proximal muscle weakness, and spontaneous fractures. Pseudofractures can be seen on X-ray in various locations including the proximal femur. Multiple myeloma is the most common cause of Fanconi syndrome in adults. Showing the biochemical abnormalities noted above; hypophosphatemia and a reduced tubular maximum for phosphate, glycosuria with a normal plasma glucose concentration, generalized aminoaciduria, and hypobicarbonatemia can establish the diagnosis. Because some patients will have serum phosphorus values that fall within the low normal range, it is useful to calculate the fractional excretion of phosphorus that will be elevated in Fanconi syndrome. PHYSIOLOGY OF SOLUTE TRANSPORT IN THE PROXIMAL TUBULE The transport of filtered solute across the proximal tubular membrane requires multiple specialized transport proteins that are present in the luminal brush border. Reabsorption of inorganic phosphate (Pi) occurs primarily in the proximal tubule by a sodium phosphate co-transporter system (NaPi-2). (6) The NaPi-2 co-transporter is located in the brush border of renal tubule epithelial cells and is influenced principally by dietary phosphate intake and PTH. (7) However, there are additional TABLE 1. DISORDERS ASSOCIATED WITH FANCONI SYNDROME Acquired Multiple myeloma Lymphoma Light chain nephropathy Amyloidosis Sjögrens syndrome Nephrotic syndrome Renal transplantation Balkan nephropathy Paroxysmal nocturnal hemoglobinuria Vitamin D deficiency Interstitial nephritis/uveitis syndrome Renal vein thrombosis Drugs Outdated tetracycline Methyl-3-chromone 6-Mercaptopurine Gentamicin Valproic acid Streptozocin Isophthalanilide Ifosphamide Cephalothin Heavy metals Lead Cadmium Mercury Uranium Platinum Copper Bismuth Other Parquat Lysol Toluene inhalation Heritable Cystinosis Lowe syndrome Hereditary fructose intolerance Tyrosinemia Galactosemia Glycogen storage disease Wilson s disease Cytochrome oxidase deficiency Subacute necrotizing encephalomyelopathy Alport syndrome Leigh s syndrome Idiopathic (AD, AR, XLR) Fanconi-Bickel syndrome Dent s disease GRACILE syndrome Rod-cone dystrophy, sensorineural deafness, and renal dysfunction Pearson syndrome The authors have reported no conflicts of interest. 354
36 FANCONI SYNDROME AND RENAL TUBULAR ACIDOSIS / 355 factors that influence phosphate transport in the proximal renal tubule including vitamin D, fibroblast growth factor 23 (FGF23), and secreted frizzled related protein 4 (sfrp4). (8,9) Vitamin D enhances renal phosphate reabsorption, whereas PTH, FGF23, and sfrp4 decrease renal phosphate reclamation. (10) Amino acids are almost entirely reabsorbed in the proximal tubule by a variety of sodium-dependent transporters. (11) Transporters for acidic, basic, and neutral amino acids have been identified as well as carriers that are specific to single amino acids. (11) In Fanconi syndrome, all amino acids are lost in excess in the urine; however, urinary amino acid losses do not seem to be of clinical consequence. Glucose normally is reabsorbed with great efficiency by the proximal tubule. This is accomplished by sodium-dependent secondary active transport. Two separate transporters have been identified in the apical membrane: a high-capacity low-affinity transporter (SGLT2) in the S1 segment and a low-capacity high affinity transporter (SGLT1) in the S3 segment. (11,12) Glucose is transported out of the epithelial cell through the basolateral membrane by the sodium-glucose transporter GLUT2. Under normal conditions, very little glucose passes from the proximal tubule into the final urine. Glycosuria may contribute to the polyuria and polydipsia often seen in Fanconi syndrome but is otherwise clinically insignificant. MECHANISMS OF ACIDIFICATION OF THE URINE Acidification of the urine is accomplished by both the removal of base from, and the addition of acid to, the glomerular filtrate. Removal of base involves a process by which virtually all filtered HCO 3 is reabsorbed. Addition of acid involves the combination of secreted H with filtered weak acids that are not completely reabsorbed and with NH 3 that is produced in the kidney. The two main mechanisms used by the kidney to maintain acid base balance include proximal tubule HCO 3 reabsorption and distal tubule H excretion. (13,14) Under physiologic conditions, 80 90% of filtered HCO 3 is reabsorbed at the level of the proximal tubule through a carbonic anhydrasedependent mechanism. (15) H is secreted into the tubular lumen, principally through a Na -H antiport, and combines with filtered HCO 3.H 2 O and CO 2 are subsequently formed and passively diffuse across the luminal membrane into the cell. Intracellular H and HCO 3 are generated. The H is recycled back into the lumen, and HCO 3 is transported across the basolateral membrane through a Na -3(HCO 3 ) cotransporter. Concentration gradients that favor the action of apical Na transporters, such as the Na H antiport that secretes H into the tubular lumen, and intracellular carbonic anhydrase that generates HCO3, are maintained by the basolateral Na K ATPase and Na -3(HCO 3 ) cotransporter. (16,17) The majority of the remaining filtered HCO 3 (10 20%) is reabsorbed distally through similar mechanisms. In the distal tubule, H secretion takes place through a H - ATPase. HCO 3 generated within the cells of the collecting tubule is directed into the systemic circulation through a basolateral membrane Cl -HCO 3 co-transporter. In addition, NH 3 generation by the kidney facilitates the secretion of H. (18) Renal tubular acidosis results from a failure to reabsorb bicarbonate in the proximal tubule or a failure to generate or secrete H in the distal tubule. Type II renal tubular acidosis (RTA), which is often associated with Fanconi syndrome, results from excessive loss of bicarbonate from the proximal tubule. The urine ph in type II RTA can be variable in contrast to type I RTA, in which it is consistently 5.5. This is because of the ability of the proximal tubule to reabsorb the filtered load of HCO 3 once the serum level falls below a given threshold. If untreated, the serum HCO 3 will usually remain 12 mm, and the urine ph may fall below 5.5. Shortly after HCO 3 is administered for treatment or for diagnostic purposes, the transport maximum for HCO 3 will be exceeded, and the urine ph will rise significantly. The presence of type II RTA can be confirmed by measuring serum HCO 3 and urine ph after an intravenous or oral load of bicarbonate. A urine ph 6.5 with a serum HCO 3 of 22 mm or less will establish the diagnosis. (19) In type I RTA, the distal tubule is unable to excrete hydrogen ion appropriately. As a result, the serum bicarbonate level can drop to 10 mm, and the urine ph is 5.5. Inappropriately maintaining a urine ph 5.5 after ammonium chloride administration (100 mg/kg orally) is consistent with the diagnosis of type I RTA. Hypokalemia and nephrolithiasis are common findings, and these characteristics can be used to help distinguish type I from type II RTA. Type I RTA can occur in Fanconi syndrome if there is damage to the distal tubule as well as the proximal tubule by the causal agent (e.g., drug or metal). Hypoaldosteronism with associated hyperkalemia and a nonanion gap metabolic acidosis is known as type IV RTA. In this condition, the action of aldosterone on the Na reabsorbing cells of the collecting tubule is diminished. The normal reabsorption of Na, thought to contribute to luminal electronegativity and a favorable K secretory gradient, does not occur and leads to persistent hyperkalemia. (20) The precise mechanism by which the mild nonanion gap metabolic acidosis occurs is not completely understood, although diminished NH 4 production plays a key role. Laboratory values consistent with type IV RTA include a plasma HCO 3 concentration 17 mm and a urine ph usually 5.5. Type IV RTA is not commonly associated with Fanconi syndrome. PATHOGENESIS OF HYPOPHOSPHATEMIA AND BONE DISEASE IN FANCONI SYNDROME Renal proximal tubule epithelial cells have a high metabolic requirement. Because so many filtered solutes are affected in Fanconi syndrome, a generalized cellular toxicity seems more likely to account for the syndrome than multiple independent transport defects. Fanconi syndrome probably results from disrupted mitochondrial ATP production and/or Na /K -ATPase activity. (21 24) Either of these mechanisms could lead to a diminished sodium electrochemical gradient that drives the majority of solute transport across the luminal membrane. It is likely that multiple toxic proteins, drugs, insoluble metabolic products, or metals alter proximal tubule cell function in a global manner by diverse pathways. The end result is reduced solute reabsorption. High fractional excretion of phosphate found in the setting of hypophosphatemia is a hallmark of Fanconi syndrome. Several mechanisms are likely responsible including those involving vitamin D, PTH, and the NaPi-2 co-transporter. Along with dietary intake, the extracellular pool of phosphorus is regulated by 1,25(OH) 2 D 3 and PTH. (9,25) 1,25(OH) 2 D 3 increases phosphate absorption in the intestine and phosphate reabsorption in the kidney. (26,27) 25(OH)D 3 conversion to the more active metabolite, 1,25(OH) 2 D 3, can be stimulated by PTH alone or by a low serum Pi through a PTH-independent mechanism. (28) However, the expected elevation of 1,25(OH) 2 D 3 concentration in the face of hypophosphatemia is not seen in Fanconi syndrome. The result is a relative or absolute vitamin D deficiency. The 25(OH) 2 D 3 1 -hydroxylase is a multicomponent mitochondrial enzyme located in the renal cortex, the activity of which is reduced in an experimental model of Fanconi syndrome. (29) Significant hepatic damage or reduced renal mass associated with many of the diseases listed in Table
37 356 / CHAPTER 65 TABLE 2. SERUM CALCIUM, PHOSPHORUS, PTH, 25(OH)D, AND 1,25(OH) 2 D 3 CONCENTRATIONS AND URINE SOLUTE CONCENTRATIONS IN VARIOUS HYPOPHOSPHATEMIC CONDITIONS Condition spi sca spth s25(oh)d s1,25(oh) 2 D U Pi FE Pi U Ca FE Ca U HCO3 FE HCO3 U Glu FE Glu U AA Fanconi syndrome D N N or I N D or N I I N N I I I I I Nutritional vitamin D D D I D V I I D D N or I N or I N N N or I deficiency, malabsorption Impaired intestinal Pi D N N N I D D I I N N N N N absorption (use of binders) X-linked D N N N DorN I I N N N N N N N hypophosphatemic rickets (XLH) Autosomal dominant D N N N DorN I I N N N N N N N hypophosphatemic rickets (ADHR) Tumor-induced D N N N DorN I I N N N N N N N osteomalacia Vitamin D dependent Dor D I N D I I D D NorI NorI N N NorI rickets (type 1) low N Vitamin D dependent D D I N I I I D D N or I N or I N N N or I rickets (type 2) Primary Dor I I N N or I I I I D N or I N or I N N N or I hyperparathyroidism low N Humoral hypercalcemia Dor I D or N D or N I I I D N or I N or I N N N or I of malignancy low N N Hereditary hypophosphatemic rickets with hypercalciuria D N D or N N I I I I I N N N N N D, decreased; I, increased; N, normal; V, variable. 1 may also account for the vitamin D deficiency. However, vitamin D deficiency has been described in Fanconi syndrome patients without significant liver disease and with normal or minimally reduced glomerular filtration rates. (30,31) This would suggest that additional, poorly understood, mechanisms are contributing to the relatively low 1,25(OH) 2 D 3 concentration. PTH, which is elevated in some patients with Fanconi syndrome, acts to reduce the number of NaPi-2 co-transporters in the luminal membrane, thereby reducing the tubular transport maximum for Pi, resulting in renal Pi loss. In addition, a maleic acid induced model of Fanconi syndrome in rats causes downregulation of the NaPi-2 co-transporter, (32) offering another possible explanation for the phosphaturia and hypophosphatemia seen in human Fanconi syndrome. Regardless of the mechanism of increased Pi losses in the urine, the diminished serum phosphorus will lead to poor bone mineralization. Inorganic phosphate plays a vital role in bone formation and is normally incorporated into the bone matrix produced by osteoblasts. This involves a complex process by which hydroxyapatite crystals [Ca 5 (PO 4 ) 3 OH] are formed within the matrix. (33) When Pi is not available in sufficient quantity, rickets/osteomalacia will result with thickened osteoid seams that are readily apparent on bone biopsy. (30) The bone disease in Fanconi syndrome could be worsened by the presence of acidosis. Renal tubular acidosis is commonly seen in patients with Fanconi syndrome. It is generally a type II RTA, although defects in H ion excretion may also be present. The pathogenesis of bone disease in chronic metabolic acidosis is multifactorial. As previously discussed, Fanconi syndrome manifests as rickets in children and as osteomalacia in adults. It is assumed that phosphaturia and hypophosphatemia play a major role in the development of rickets/osteomalacia as seen in the other disorders such as X-linked hypophosphatemic rickets (XLH), oncogenic osteomalacia (OOM), and autosomal dominant hypophosphatemic rickets (ADHR). The bone disease in these phosphaturic disorders is well established but is not associated with metabolic acidosis. Acidosis can cause bone disease independent of phosphate wasting. Bone serves as a large reservoir of buffer for excess H. (34) Acute and chronic acidosis induce demineralization of bone and increased urinary losses of calcium. (34) Patients with RTA have lower BMD and increased osteoid volume compared with reference values. (35,36) Osteoblast-like cells cultured in an acidic environment show an increased response to PTH and increased mrna for the PTH/ PTH-related peptide (PTHrP) receptor. (37) This would presumably increase bone turnover in favor of resorption. Cultured mouse calvaria exposed to an acidic environment show increased osteoclastic and decreased osteoblastic activity as determined by measurements of collagen synthesis, alkaline phosphatase activity, and -glucuronidase activity compared with controls. (34,38) Impaired conversion of 25(OH)D 3 in the rat kidney exposed to a low ph show that vitamin D metabolism is impaired in an acidic environment. (39) Low 1,25(OH) 2 D 3 concentrations could also be caused by impaired delivery of substrate [25(OH)D 3 ] to the renal tubule epithelial cell if transport regulatory proteins are affected by acidosis or the underlying disorder causing Fanconi syndrome. (40,41) There are many pathways by which chronic acidosis seem to be inhibiting bone formation and enhancing demineralization.
38 FANCONI SYNDROME AND RENAL TUBULAR ACIDOSIS / 357 DIFFERENTIAL DIAGNOSIS Other disorders mimicking Fanconi syndrome are listed in Table 2. Hypophosphatemia is seen in several metabolic bone disorders that should be distinguished from Fanconi syndrome. This can usually be accomplished by determining serum phosphorus, calcium, PTH, and vitamin D (both metabolites) concentrations, as well as urine studies to measure excretion rates of several filtered solutes. Historical clues, such as inheritance patterns in familial disease, may also be useful in narrowing the differential diagnosis. TREATMENT Treatment of Fanconi syndrome induced bone disease should be based on its underlying cause. If the associated disease can be treated or the offending agent removed (Table 1), Fanconi syndrome may resolve, and the metabolic bone disease remit. If the underlying cause of Fanconi syndrome cannot be identified or corrected, treatment with phosphorus, vitamin D, and/or bicarbonate may be necessary. The relative contribution of disordered phosphorus, vitamin D, and bicarbonate metabolism may vary in individual patients. Phosphate and calcium replacement have been reported to improve osteomalacia and rickets in Fanconi syndrome. (36,42 45) Large doses of phosphate may be required for healing of the rickets or osteomalacia and is available in several preparations (Table 3). Frequent doses throughout the day (four to five times) are preferred over larger, less frequent doses. This will minimize wide fluctuations in serum phosphorus that may lead to secondary hyperparathyroidism and undesirable gastrointestinal symptoms. Vitamin D replacement with vitamin D 2 or D 3 have been successfully used in the treatment of Fanconi syndrome. (30,36,42,46) Because many diseases associated with Fanconi syndrome are also characterized by renal failure, the relative potency and duration of toxicity of the specific form of vitamin D replacement should be taken into account. Ergocalciferol and calcitriol are readily available forms of vitamin D. Calcitriol is several-fold more potent than ergocalciferol; however, the duration of toxicity is considerably shorter. Alkali therapy alone can also improve RTA-associated osteomalacia. (47) Oral bicarbonate in doses of meq/kg/day are typically required to correct the acidosis caused by a proximal RTA with or without Fanconi syndrome. Bicarbonate replacement should be distributed during the day and not given as a single daily dose. Distal RTA bicarbonate requirements are considerably less (1 2 meq/kg/day in divided doses). However, children with distal RTA may require significantly higher doses to achieve normal growth rates. (48) Many forms of alkali TABLE 3. ORAL PHOSPHATE REPLACEMENT Phosphorus (mg) Content per dose Sodium (meq) Potassium (meq) K-Phos (tablet) Original Neutral MF # Neutra-Phos (powder) Neutra-Phos K (powder) Uro-KP-Neutral (tablet) Fleet phospho-soda (liquid) 128/ml 4.8/ml 0 Joulie s solution (liquid) 30.4/ml 0.76/ml 0 replacement are available (Table 4) and can be tailored to the individual patient needs. In type I RTA, it is often necessary to replace potassium before initiating alkali replacement, because correction of the acidosis will worsen hypokalemia. Treatment of type IV RTA includes a low potassium diet, alkali replacement, and occasionally a loop diuretic. If long-term treatment with phosphorus and vitamin D are necessary, close monitoring of serum biochemistries and renal function is imperative. Secondary hyperparathyroidism is frequently seen in patients treated chronically with phosphorus and vitamin D therapy for various hypophosphatemic disorders. (49,50) Several reports of tertiary hyperparathyroidism requiring surgical intervention have also been published. (50 52) Periodic renal ultrasound may help identify patients at risk for significant renal impairment associated with treatment by identifying nephrocalcinosis (53,54) before obvious changes in serum creatinine or glomerular filtration rate are noted. REFERENCES TABLE 4. ORAL BICARBONATE REPLACEMENT Potassium citrate (Urocit-K) 540 mg tablet 5 meq per tablet 1080 mg tablet 10 meq per tablet Potassium citrate citric acid Oral solution (Polycitra-K) 2 meq/1 ml Crystals (Polycitra-K) 30 meq per packet Sodium citrate citric acid (Bicitra or 1 meq/1 ml Shohl s solution) Potassium citrate sodium citrate citric 2 meq/1 ml acid (Polycitra) Sodium bicarbonate 325 mg tablet 3.87 meq per tablet 650 mg tablet 7.74 meq per tablet Baking soda 60 meq/teaspoon 1. Sebastian A, McSherry E, Morris RC Jr 1971 On the mechanism of renal potassium wasting in renal tubular acidosis associated with the Fanconi syndrome (type 2 RTA). J Clin Invest 50: Rodriguez Soriano J, Houston IB, Boichis H, Edelmann CM Jr 1968 Calcium and phosphorus metabolism in the fanconi syndrome. J Clin Endocrinol Metab 28: Rodriquez-Soriano J, Vallo A, Castillo G, Oliveros R 1980 Renal handling of water and sodium in children with proximal and distal renal tabular acidosis. Nephron 25: Houston IB, Boichis H, Edelmann CM Jr 1968 Fanconi syndrome with renal sodium wasting and metabolic alkalosis. Am J Med 44: Dillard MG, Pesce AJ, Pollak VE, Boreisha I 1971 Proteinuria and renal protein clearances in patients with renal tubular disorders. J Lab Clin Med 78: Biber J, Custer M, Magagnin S, Hayes G, Werner A, Lotscher M, Kaissling B, Murer H 1996 Renal Na/Pi-cotransporters. Kidney Int 49: Murer H, Lotscher M, Kaissling B, Levi M, Kempson SA, Biber J 1996 Renal brush border membrane Na/Pi-cotransport: Molecular aspects in PTH-dependent and dietary regulation. Kidney Int 49: Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, Jan De Beur SM, Schiavi SC, Kumar R 2003 Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest 112: Kumar R 2002 New insights into phosphate homeostasis: Fibroblast growth factor 23 and frizzled-related protein-4 are phosphaturic factors derived from tumors associated with osteomalacia. Curr Opin Nephrol Hypertens 11: Schiavi SC, Kumar R 2004 The phosphatonin pathway: New insights in phosphate homeostasis. Kidney Int 65: Moe OW, Berry CA, Rector FCJ 2000 Renal transport of glucose, amino acids, sodium, chloride, and water. In: Brenner BM, Rector FC (eds.) The Kidney, 6th ed., vol. 1. Saunders, Philadelphia, PA, USA, pp
39 358 / CHAPTER Kanai Y, Lee WS, You G, Brown D, Hediger MA 1994 The human kidney low affinity Na /glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 93: Kurtzman NA 2000 Renal tubular acidosis syndromes. South Med J 93: Gluck SL, Iyori M, Holliday LS, Kostrominova T, Lee BS 1996 Distal urinary acidification from Homer Smith to the present. Kidney Int 49: Rector FCJ, Carter NW, Seldin DW 1965 The mechanism of bicarbonate reabsorption in the proximal and distal tubules of the kidney. J Clin Invest 44: Preisig PA, Ives HE, Cragoe EJ, Jr., Alpern RJ, Rector FC Jr 1987 Role of the Na /H antiporter in rat proximal tubule bicarbonate absorption. J Clin Invest 80: Soleimani M, Aronson PS 1989 Ionic mechanism of Na -HCO3- cotransport in rabbit renal basolateral membrane vesicles. J Biol Chem 264: Hamm LL, Simon EE 1987 Roles and mechanisms of urinary buffer excretion. Am J Physiol 253:F595 F Gluck SL 1998 Acid-base. Lancet 352: Rodriguez Soriano J 2002 Renal tubular acidosis: The clinical entity. J Am Soc Nephrol 13: Guan S, el-dahr S, Dipp S, Batuman V 1999 Inhibition of Na-K-ATPase activity and gene expression by a myeloma light chain in proximal tubule cells. J Invest Med 47: Batuman V, Guan S, O Donovan R, Puschett JB 1994 Effect of myeloma light chains on phosphate and glucose transport in renal proximal tubule cells. Ren Physiol Biochem 17: Coor C, Salmon RF, Quigley R, Marver D, Baum M 1991 Role of adenosine triphosphate (ATP) and NaK ATPase in the inhibition of proximal tubule transport with intracellular cystine loading. J Clin Invest 87: Castano E, Marzabal P, Casado FJ, Felipe A, Pastor-Anglada M 1997 Na,K( )-ATPase expression in maleic-acid-induced Fanconi syndrome in rats. Clin Sci (Lond) 92: Berndt T, Knox FG 1992 Renal regulation of phosphate excretion. In: Seldin DW, Giebisch G (eds.) The Kidney: Physiology and Pathophysiology, 2 ed. Raven Press, New York, NY, USA, pp Steele TH, Engle JE, Tanaka Y, Lorenc RS, Dudgeon KL, DeLuca HF 1975 Phosphatemic action of 1,25-dihydroxyvitamin D3. Am J Physiol 229: Tanaka Y, Deluca HF 1974 Role of 1,25-dihydroxyvitamin D3 in maintaining serum phosphorus and curing rickets. Proc Natl Acad Sci USA 71: Tanaka Y, Deluca HF 1973 The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys 154: Brewer ED, Tsai HC, Szeto KS, Morris RC Jr 1977 Maleic acid-induced impaired conversion of 25(OH)D3 to 1,25(OH)2D3: Implications for Fanconi s syndrome. Kidney Int 12: Clarke BL, Wynne AG, Wilson DM, Fitzpatrick LA 1995 Osteomalacia associated with adult Fanconi s syndrome: Clinical and diagnostic features. Clin Endocrinol (Oxf) 43: Colussi G, De Ferrari ME, Surian M, Malberti F, Rombola G, Pontoriero G, Galvanini G, Minetti L 1985 Vitamin D metabolites and osteomalacia in the human Fanconi syndrome. Proc Eur Dial Transplant Assoc Eur Ren Assoc 21: Haviv YS, Wald H, Levi M, Dranitzki-Elhalel M, Popovtzer MM 2001 Late-onset downregulation of NaPi-2 in experimental Fanconi syndrome. Pediatr Nephrol 16: Neuman WF 1980 Bone material and calcification mechanisms. In: Urist MR (ed.) Fundamental and Clinical Bone Physiology. Lippincott, Philadelphia, PA, USA, pp Bushinsky DA, Frick KK 2000 The effects of acid on bone. Curr Opin Nephrol Hypertens 9: Domrongkitchaiporn S, Pongsakul C, Stitchantrakul W, Sirikulchayanonta V, Ongphiphadhanakul B, Radinahamed P, Karnsombut P, Kunkitti N, Ruang-raksa C, Rajatanavin R 2001 Bone mineral density and histology in distal renal tubular acidosis. Kidney Int 59: Dalmak S, Erek E, Serdengecti K, Okar I, Ulku U, Basaran M 1996 A case study of adult-onset hypophosphatemic osteomalacia with idiopathic fanconi syndrome. Nephron 72: Disthabanchong S, Martin KJ, McConkey CL, Gonzalez EA 2002 Metabolic acidosis up-regulates PTH/PTHrP receptors in UMR osteoblast-like cells. Kidney Int 62: Krieger NS, Sessler NE, Bushinsky DA 1992 Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Physiol 262:F442 F Kawashima H, Kraut JA, Kurokawa K 1982 Metabolic acidosis suppresses 25-hydroxyvitamin in D3 1alpha-hydroxylase in the rat kidney. Distinct site and mechanism of action. J Clin Invest 70: Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI 2001 Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci USA 98: Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE 1999 An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96: Zeier M, Ritz E 2000 The bedridden osteomalacic patient with Fanconi syndrome in pre-terminal renal failure. Nephrol Dialysis Transplant 15: Long WS, Seashore MR, Siegel NJ, Bia MJ 1990 Idiopathic Fanconi syndrome with progressive renal failure: A case report and discussion. Yale J Biol Med 63: Smith R, Lindenbaum RH, Walton RJ 1976 Hypophosphataemic osteomalacia and Fanconi syndrome of adult onset with dominant inheritance. Possible relationship with diabetes mellitus. Qtly J Med 45: Harrison NA, Bateman JM, Ledingham JG, Smith R 1991 Renal failure in adult onset hypophosphatemic osteomalacia with Fanconi syndrome: A family study and review of the literature. Clin Nephrol 35: Lambert J, Lips P 1989 Adult hypophosphataemic osteomalacia with Fanconi syndrome presenting in a patient with neurofibromatosis. Netherlands J Med 35: Wrong OM, Richards P 1972 Treatment of osteomalacia of renal tubular acidosis by sodium bicarbonate alone. Lancet 2: McSherry E, Morris RC Jr 1978 Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J Clin Invest 61: Makitie O, Kooh SW, Sochett E 2003 Prolonged high-dose phosphate treatment: A risk factor for tertiary hyperparathyroidism in X-linked hypophosphatemic rickets. Clin Endocrinol (Oxf) 58: Alon U, Newsome H Jr, Chan JC 1984 Hyperparathyroidism in patients with X-linked dominant hypophosphatemic rickets application of the calcium infusion test as an indicator for parathyroidectomy. Int J Pediatr Nephrol 5: Rivkees SA, el-hajj-fuleihan G, Brown EM, Crawford JD 1992 Tertiary hyperparathyroidism during high phosphate therapy of familial hypophosphatemic rickets. J Clin Endocrinol Metab 75: Savio RM, Gosnell JE, Posen S, Reeve TS, Delbridge LW 2004 Parathyroidectomy for tertiary hyperparathyroidism associated with X-linked dominant hypophosphatemic rickets. Arch Surg 139: Goodyer PR, Kronick JB, Jequier S, Reade TM, Scriver CR 1987 Nephrocalcinosis and its relationship to treatment of hereditary rickets. J Pediatr 111: Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M 1991 Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med 325:
40 Chapter 66. Renal Osteodystrophy Kevin J. Martin, Ziyad Al-Aly, and Esther A. Gonzalez Division of Nephrology, Saint Louis University School of Medicine, St. Louis, Missouri INTRODUCTION Renal osteodystrophy is the skeletal component of a variety of abnormalities of bone and mineral metabolism that may occur as a complication of chronic kidney disease. These abnormalities may include abnormal values for serum calcium, phosphorus, PTH, and other bone markers, as well as extraskeletal calcification, and can be associated with bone pain, abnormal bone structure, and an increased incidence of fracture. The abnormalities in bone may vary widely from situations where bone turnover may be abnormally high as a manifestation of the effects of elevated levels of PTH on bone, and are manifested histologically by osteitis fibrosa, to the opposite end of the spectrum, where bone turnover may be abnormally low, which is often termed adynamic bone. Either extreme may be accompanied by abnormal mineralization of bone, which can be manifested as osteomalacia. The patterns of skeleton abnormality commonly occur in combination and give rise to a term known as mixed renal osteodystrophy, which is usually associated with some signs of the secondary hyperparathyroidism associated with mineralization defects. In the setting of chronic kidney disease, the skeleton may also be affected by other processes, such as the accumulation of -2 microglobulin, and in addition, systemic therapy for kidney disease, such as corticosteroid therapy, or coincidental bone abnormalities from postmenopausal or other forms of osteoporosis, may also be present. Much has been learned about the pathogenesis of these abnormalities in recent years, and the understanding of these factors has lead to therapeutic strategies of minimize both the skeletal and extraskeletal pathology. PATHOGENESIS OF RENAL OSTEODYSTROPHY High Turnover Renal Osteodystrophy High turnover renal osteodystrophy is the result of the effects of high levels of PTH on bone. Hyperplasia of the parathyroid glands and high levels of PTH are well known to occur early in the course of chronic kidney disease. (1 3) The development of hyperparathyroidism in this setting is the result of a variety of factors, which are shown in Fig. 1, and include retention of phosphorus as kidney function declines, reductions in the levels of calcitriol in serum, a number of intrinsic abnormalities in the parathyroid gland, disorders of parathyroid cell growth, abnormal control of serum calcium, and skeletal resistance to the calcemic actions of PTH. (4) These abnormalities are all closely interrelated and one or more may predominate in any given patient at different levels of renal insufficiency. The importance of phosphate retention on the pathogenesis of secondary hyperparathyroidism has been shown by several studies over many years. (5,6) This has been accompanied by observations that dietary phosphate reduction in proportion to the decreased glomerular filtration rate (GFR) in experimental renal disease is associated with prevention of the development of hyperparathyroidism. While this observation is not in doubt and has been confirmed in many experimental systems, as well as in patients, the mechanism by which phosphorus affects the Dr. Martin has had grant support from and served as a consultant for Abbott, Amgen, and Shire. All other authors have reported no conflicts of interest. development of hyperparathyroidism continues to generate some controversy. The original hypothesis was that phosphorus retention with decreasing kidney function lead to decreases in ionized calcium, which would trigger a compensatory increase with PTH. (7) Subsequent studies, however, have shown that hypocalcemia is not necessary for hyperparathyroidism to occur, (8) and therefore, other mechanisms must be involved. Because an additional consequence of phosphate retention is limitation of the production of calcitriol, it is possible that reductions in the levels of calcitriol allow hyperparathyroidism to occur and progress. Observations in experimental animals and humans have confirmed that there is a slow decline in the levels of calcitriol that begins early in the course of chronic kidney disease, and therefore, this is also a plausible hypothesis. (9) Further studies have shown that phosphorus may directly affect the parathyroid gland by increasing PTH secretion and parathyroid growth, and conversely, prevention of phosphate retention is associated with prevention of these abnormalities in the parathyroid gland. (10 12) The effect of phosphorus may well be directly on the parathyroid gland, but could also act through the calcitriol mechanism, because changes in the levels of serum phosphorus could alter the production of calcitriol by the kidney. The precise mechanism by which phosphorus could affect the parathyroid gland is not well understood at the present time. It has been shown, however, that phosphate retention or high phosphorus diets are associated with increased levels of PTH mrna in parathyroid, and this effect seems to be caused by an effect on the stability of PTH mrna. (13) The precise mechanism of this effect continues to be studied, and studies have shown that phosphate retention may be associated with alterations in proteins that are involved in regulating the degradation of PTH mrna within the parathyroid. (14) A potential signaling mechanism has been suggested by the demonstration that high extracellular phosphate concentrations may alter parathyroid function by reducing the production of arachadonic acid by parathyroid tissue. (15,16) Phosphorus also seems to play an important role in the regulation of parathyroid growth. Thus, a high phosphorus diet has been shown to increase parathyroid hyperplasia. Conversely, a diet low in phosphorus is effective in preventing increased parathyroid growth in the setting of chronic kidney disease. A low phosphorus diet has been associated with upregulation of the cell cycle regulator, p21, which may play a role in the prevention of parathyroid hyperplasia. (17) A high phosphorus diet seems to be mediated by increases in the expression of TGF-, which would work through the EGF receptor in stimulating parathyroid growth. (18) The mechanism by which phosphorus affects the parathyroid gland is not understood at the present time, and the search continues for a putative phosphate sensor that may trigger the cellular changes, but this has not been shown at the present time. Because calcitriol is an important regulator of parathyroid function, it is also important to consider that phosphorus retention may affect parathyroid function by decreasing the production of calcitriol. In addition, recent data have suggested that phosphate retention may be associated with an increase in the levels of fibroblast growth factor (FGF)-23, which has also been shown to be a potent inhibitor of 1- -hydroxylase activity, and accordingly, this mechanism could serve to limit the 359
41 360 / CHAPTER 66 FIG. 1. Summary of factors that contribute to the secondary hyperparathyroidism of chronic renal failure. PTG, parathyroid glands; VDR, vitamin D receptor. production of calcitriol by the kidney in the setting of chronic kidney disease. (19) Other factors associated with chronic kidney disease, such as acidosis, may also impact on the ability of the diseased kidney to increase calcitriol production. In recent years, attention has been focused on an additional mechanism that could potentially limit the ability of the kidney to produce calcitriol. These data suggest that decreased delivery of the precursor, 25-hydroxyvitamin D, which circulates while bound to a vitamin D binding protein, could impact on the ability of the kidney to produce calcitriol. (20) This 25- hydroxy-d-bound vitamin D binding protein is filtered at the glomerulus and is taken up in the proximal tubule by a mechanism that involves megalin, which seems to be required for the internalization of the 25-hydroxyvitamin D binding protein into the cell and is the rate-limiting step for the production of calcitriol by the 1- -hydroxylase enzyme. This mechanism, obviously then, in the course of chronic kidney disease, may limit the ability of the kidney to produce increased calcitriol levels, even in circumstances where PTH levels are high. In addition to deceases in the production of calcitriol, the setting of chronic kidney disease may also be associated with resistance to the peripheral actions of calcitriol, and it has been suggested that uremic serum contains factors that seem to decrease the ability of the vitamin D receptor to interact with the vitamin D responsive element on DNA. (21) Further studies have suggested that this may be caused by reductions in retinoid X receptor (RXR), the essential binding partner for the vitamin D receptor, and its interaction with the vitamin D response element (VDRE) on DNA. (22) The principal regulator of PTH secretion is serum calcium, acting on the calcium-sensing receptor on the parathyroid. In the setting of chronic kidney disease, it has been shown that there seems to be reduced expression of the calcium-sensing receptor as the parathyroid glands enlarge and undergo hyperplasia. (23,24) The consequences of the reductions in the calciumsensing receptor could lead to abnormal calcium-regulated PTH secretion. An important issue as to whether parathyroid hyperplasia causes a reduction in expression of calcium-sensing receptor, or alternatively, whether the reduced calcium sensing receptor facilitated parathyroid growth, has been studied in experimental animals, with the results showing that the parathyroid proliferation seems to precede the reductions in the levels of the calciumsensing receptor. (25) In some patients with severe hyperparathyroidism, an abnormal setpoint for calcium has been shown, which would consequently lead to abnormal calcium-regulated PTH secretion. The issue of parathyroid setpoint in uremia has been investigated by a number of studies, and it seems that the setpoint may be altered by multiple factors, including the baseline level of serum calcium, the rate of change of serum calcium, the magnitude of parathyroid hyperplasia, and polymorphisms of the calcium-sensing receptor gene. (26 30) Thus, with these multiple factors involved, heterogeneity of results is to be expected, and indeed, this has been found in clinical studies. Calcitriol also has many effects on the parathyroid gland, which may contribute to many of the abnormalities. The effects of calcitriol on parathyroid function are summarized in Fig. 2. While these factors may play a role in the pathogenesis of the parathyroid abnormalities, these actions of calcitriol can also be exploited as a therapeutic agent, which is discussed below. It has also been recognized that as a consequence of parathyroid growth, not only is there a decrease in the expression of the calcium sensing receptor, but there are also decreases in the expression of the vitamin D receptor as the parathyroid glands undergo proliferation. (31 33) Studies have shown that as parathyroid hyperplasia progresses, the glands take on a nodular appearance, and some of these nodules may represent monoclonal expansions of parathyroid cells. (34) Thus, similar to studies with the calcium-sensing receptor, the loss of vitamin D receptor expression may be a consequence of parathyroid growth. However, both pathways can be exploited therapeutically. Thus, evidence has been presented to show that administration of active vitamin D sterols can attenuate parathyroid cell growth in experimental models of uremia, and similarly, administration of calcimimetic agents has also been shown to suppress and prevent further parathyroid hyperplasia in experimental models of kidney disease. An additional contributor to hyperparathyroidism in the setting of kidney disease may be related to the phenomenon of skeletal resistance to the calcemic actions of PTH, which were described many years ago. In essence, it was observed that the administration of PTH resulted in a lesser increase in serum calcium in patients or experimental animals with kidney disease. (35) Studies of the mechanisms involved have uncovered many potential contributors, such as phosphorus retention, decreased levels of calcitriol, downregulation of the PTH receptor, and potentially, PTH peptides, with truncations in their N terminus, a topic which is discussed in more detail below. Pathogenesis of Low Turnover Renal Osteodystrophy As mentioned above, low bone turnover can be seen in association with kidney disease and is characterized by extremely low rates of bone formation, and in some cases, with the severe mineralization defect of osteomalacia, which is manifested by an increased volume of unmineralized bone
42 RENAL OSTEODYSTROPHY / 361 FIG. 2. Summary of direct and indirect effects of calcitriol administration to control PTH secretion in patients with chronic renal failure. Direct effects refer to changes in parathyroid cell synthesis and secretion of PTH, and indirect effects are actions of calcitriol on intestinal calcium absorption and skeletal response to PTH. matrix. It now seems that the majority of cases of osteomalacia in the setting of chronic kidney disease have been caused by accumulation of aluminum at the mineralization front, and because the discontinuation of the use of aluminum-based phosphate binders, osteomalacia in the setting of chronic kidney disease is virtually nonexistent. However, a substantial number of patients have extremely low bone turnover not associated with osteomalacia, which is termed adynamic bone. This is found with increasing frequency in patients on dialysis and seems to be especially prevalent among patients on peritoneal dialysis. The pathogenesis of adynamic bone is complex and includes a large number of factors, which are shown in Fig. 3. Many of these factors have in common that they serve to decrease the ambient levels of PTH, and thus induce a relative degree of hypoparathyroidism, which may be associated with decreasing bone formation rate. (36) The multiple factors involved show the complexity of the problem, but suggest that some therapeutic interventions for high bone turnover may facilitate the development of abnormally low bone turnover, such as the use of high levels of calcium-based phosphate binders, an elevated calcium intake, excess use of active vitamin D sterols, the presence of increasing age or diabetes, and a variety of other factors, which can all contribute. It is also possible that some of the disturbances in a variety of growth factors and cytokines could directly impact on bone formation rate. Other Factors That May Play a Role in Renal Bone Disease In addition to the broad histological types of skeletal abnormality outlined above, there are other associated metabolic abnormalities with chronic kidney disease that may adversely impact the skeleton. An important factor might be the presence of metabolic acidosis, which can affect the bone by liberation of bone mineral as the hydrogen ions are buffered by bone carbonate. Evidence is also being presented that acidosis can facilitate cell mediated bone resorption and therefore may alter the biological effects of PTH or vitamin D on bone. (37 39) The expression of the PTH receptor may also be affected by acidosis, as shown in osteoblast-like cells in vitro. (40) Acidosis can also affect the RANKL osteoprotogerin (OPG) system in a direction favoring bone resorption, and this, together with disturbances of other cytokines, such as interleukin-6 (IL-6) or IL-1, may contribute to the bony abnormalities associated with chronic kidney disease. (41) It is important to remember that the skeleton may also be affected because of other systemic factors, such as reductions in sex hormones, which could contribute to an osteoporotic component of renal osteodystrophy in various patient groups. Similarly, the use of corticosteroids for treatment of the preexisting kidney disease may also introduce a component of corticosteroid-induced osteoporosis that could complicate the manifestations of renal osteodystrophy. Clinical Manifestations Symptomatic bone disease in adult patients with advanced kidney disease is relatively unusual, and symptoms are only likely to appear late in the course. Children with renal osteodystrophy may manifest linear growth failure, deformities of the extremities, slipped epiphyses and fractures, and often at a relatively preserved GFR. In both adults and children, most FIG. 3. Factors that contribute to relative hypoparathyroidism and decreased bone formation rate (BFR) in patients with chronic renal failure. Note that improvement in calcium intake and absorption or increased dialysate calcium may contribute to the relative hypoparathyroidism through an increase in serum calcium.
What is Osteoporosis?
 What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
Overview. Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases. People Centred Positive Compassion Excellence
 Overview Osteoporosis and Metabolic Bone Disease Dr Chandini Rao Consultant Rheumatologist Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases Bone Biology Osteoporosis Increased bone remodelling
Overview Osteoporosis and Metabolic Bone Disease Dr Chandini Rao Consultant Rheumatologist Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases Bone Biology Osteoporosis Increased bone remodelling
Knee pain An uncommon cause
 Knee pain An uncommon cause Dr.Nihal Gunatilake - Consultant Rheumatologist - CSTH Dr.Dinesha Sudusinghe - Registrar Medicine Case history Mrs.J, 57 years P/C B/L knee pain for 2 years H/P/C Apparently
Knee pain An uncommon cause Dr.Nihal Gunatilake - Consultant Rheumatologist - CSTH Dr.Dinesha Sudusinghe - Registrar Medicine Case history Mrs.J, 57 years P/C B/L knee pain for 2 years H/P/C Apparently
Bone Metastases. Sukanda Denjanta, M.Sc., BCOP Pharmacy Department, Chiangrai Prachanukroh Hospital
 Bone Metastases Sukanda Denjanta, M.Sc., BCOP Pharmacy Department, Chiangrai Prachanukroh Hospital 1 Outline Pathophysiology Signs & Symptoms Diagnosis Treatment Spinal Cord Compression 2 General Information
Bone Metastases Sukanda Denjanta, M.Sc., BCOP Pharmacy Department, Chiangrai Prachanukroh Hospital 1 Outline Pathophysiology Signs & Symptoms Diagnosis Treatment Spinal Cord Compression 2 General Information
Paget s Disease of Bone
 Paget s Disease of Bone Copyright Copyright 2019 American 2019 American Associa7on Associa7on of Clinical of Clinical Endocrinologists Endocrinologists 1 A Common Bone Disorder Paget s disease of bone
Paget s Disease of Bone Copyright Copyright 2019 American 2019 American Associa7on Associa7on of Clinical of Clinical Endocrinologists Endocrinologists 1 A Common Bone Disorder Paget s disease of bone
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP
 Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Anabolic Therapy With Teriparatide Indications Beyond Osteoporosis
 Anabolic Therapy With Teriparatide Indications Beyond Osteoporosis Andreas Panagopoulos MD, PhD Upper Limb & Sports Medicine Orthopaedic Surgeon Assistant Professor, University of Patras Outline Teriparatide
Anabolic Therapy With Teriparatide Indications Beyond Osteoporosis Andreas Panagopoulos MD, PhD Upper Limb & Sports Medicine Orthopaedic Surgeon Assistant Professor, University of Patras Outline Teriparatide
Monostotic Paget s Disease: A Case Report
 Chin J Radiol 2002; 27: 117-121 117 CASE REPORT Monostotic Paget s Disease: A Case Report CHI-CHEN HOU 1 CHI WEI LO 2 JINN-MING CHANG 1 CHING-CHERNG TZENG 3 Department of Diagnostic Radiology 1, Orthopedics
Chin J Radiol 2002; 27: 117-121 117 CASE REPORT Monostotic Paget s Disease: A Case Report CHI-CHEN HOU 1 CHI WEI LO 2 JINN-MING CHANG 1 CHING-CHERNG TZENG 3 Department of Diagnostic Radiology 1, Orthopedics
Clinician s Guide to Prevention and Treatment of Osteoporosis
 Clinician s Guide to Prevention and Treatment of Osteoporosis Published: 15 August 2014 committee of the National Osteoporosis Foundation (NOF) Tipawan khiemsontia,md outline Basic pathophysiology screening
Clinician s Guide to Prevention and Treatment of Osteoporosis Published: 15 August 2014 committee of the National Osteoporosis Foundation (NOF) Tipawan khiemsontia,md outline Basic pathophysiology screening
Awaisheh. Mousa Al-Abbadi. Abdullah Alaraj. 1 Page
 f #3 Awaisheh Abdullah Alaraj Mousa Al-Abbadi 1 Page *This sheet was written from Section 1 s lecture, in the first 10 mins the Dr. repeated all the previous material relating to osteoporosis from the
f #3 Awaisheh Abdullah Alaraj Mousa Al-Abbadi 1 Page *This sheet was written from Section 1 s lecture, in the first 10 mins the Dr. repeated all the previous material relating to osteoporosis from the
Paget s Disease THE FACTS. Revised: April 2013 Review: April 2015 Version: 3. Diana Wilkinson Healthcare & Education Officer Paget s Association
 Paget s Disease THE FACTS Diana Wilkinson Healthcare & Education Officer Paget s Association Revised: April 2013 Review: April 2015 Version: 3 Contents Introduction 2 What is Paget s disease? 3 What causes
Paget s Disease THE FACTS Diana Wilkinson Healthcare & Education Officer Paget s Association Revised: April 2013 Review: April 2015 Version: 3 Contents Introduction 2 What is Paget s disease? 3 What causes
Sponsor / Company: sanofi-aventis and Proctor & Gamble Drug substance(s): Risedronate (HMR4003)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
Bone metastases of solid tumors Diagnosis and management by
 Bone metastases of solid tumors Diagnosis and management by Dr/RASHA M Abd el Motagaly oncology consultant Nasser institute adult oncology unit 3/27/2010 1 Goals 1- Know the multitude of problem of bone
Bone metastases of solid tumors Diagnosis and management by Dr/RASHA M Abd el Motagaly oncology consultant Nasser institute adult oncology unit 3/27/2010 1 Goals 1- Know the multitude of problem of bone
Primary bone tumors > metastases from other sites Primary bone tumors widely range -from benign to malignant. Classified according to the normal cell
 Primary bone tumors > metastases from other sites Primary bone tumors widely range -from benign to malignant. Classified according to the normal cell counterpart and line of differentiation. Among the
Primary bone tumors > metastases from other sites Primary bone tumors widely range -from benign to malignant. Classified according to the normal cell counterpart and line of differentiation. Among the
DISEASES WITH ABNORMAL MATRIX
 DISEASES WITH ABNORMAL MATRIX MSK-1 FOR 2 ND YEAR MEDICAL STUDENTS Dr. Nisreen Abu Shahin CONGENITAL DISEASES WITH ABNORMAL MATRIX OSTEOGENESIS IMPERFECTA (OI): also known as "brittle bone disease" a group
DISEASES WITH ABNORMAL MATRIX MSK-1 FOR 2 ND YEAR MEDICAL STUDENTS Dr. Nisreen Abu Shahin CONGENITAL DISEASES WITH ABNORMAL MATRIX OSTEOGENESIS IMPERFECTA (OI): also known as "brittle bone disease" a group
Managing Bone Pain in Metastatic Disease. Rachel Schacht PA-C Medical Oncology and Hematology Associates Presented on 11/2/2018
 Managing Bone Pain in Metastatic Disease Rachel Schacht PA-C Medical Oncology and Hematology Associates Presented on 11/2/2018 None Disclosures Managing Bone Pain in Metastatic Disease This lecture will
Managing Bone Pain in Metastatic Disease Rachel Schacht PA-C Medical Oncology and Hematology Associates Presented on 11/2/2018 None Disclosures Managing Bone Pain in Metastatic Disease This lecture will
Patients should receive supplemental calcium and vitamin D, if dietary intake is inadequate (see PRECAUTIONS).
 PRODUCT CIRCULAR Tablets Once Weekly 70 mg I. THERAPEUTIC CLASS Bisphosphonates are synthetic analogs of pyrophosphate that bind to the hydroxyapatite found in bone. is a bisphosphonate that acts as a
PRODUCT CIRCULAR Tablets Once Weekly 70 mg I. THERAPEUTIC CLASS Bisphosphonates are synthetic analogs of pyrophosphate that bind to the hydroxyapatite found in bone. is a bisphosphonate that acts as a
The Bare Bones of Osteoporosis. Wendy Rosenthal, PharmD
 The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
Case 4 Generalised bone pain
 Case 4 Generalised bone pain C A 34- year- old woman presented complaining of multifocal pain in her chest and legs. The pain was intermittent, was aggravated by weight bearing. Initially was alleviated
Case 4 Generalised bone pain C A 34- year- old woman presented complaining of multifocal pain in her chest and legs. The pain was intermittent, was aggravated by weight bearing. Initially was alleviated
Osteoporosis. When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of.
 Osteoporosis When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of. Osteoblasts by definition are those cells present in the bone and are involved
Osteoporosis When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of. Osteoblasts by definition are those cells present in the bone and are involved
1/28/2017. Orthopedic Implications Of Osteoporosis Introduction. Orthopedic Implications Of Osteoporosis Introduction
 The Orthopedic Implications Of Prolonged In Osteoporosis Donald A. Wiss MD Director Of Orthopedic Trauma Cedars Sinai Medical Center Orthopedic Implications Of Osteoporosis Introduction Osteoporosis Most
The Orthopedic Implications Of Prolonged In Osteoporosis Donald A. Wiss MD Director Of Orthopedic Trauma Cedars Sinai Medical Center Orthopedic Implications Of Osteoporosis Introduction Osteoporosis Most
What Lung Cancer Patients Need to Know About Bone Health. A Publication of The Bone and Cancer Foundation
 What Lung Cancer Patients Need to Know About Bone Health A Publication of The Bone and Cancer Foundation Contents THIS PUBLICATION PROVIDES IMPORTANT INFORMATION ABOUT THE RELATIONSHIP BETWEEN LUNG CANCER
What Lung Cancer Patients Need to Know About Bone Health A Publication of The Bone and Cancer Foundation Contents THIS PUBLICATION PROVIDES IMPORTANT INFORMATION ABOUT THE RELATIONSHIP BETWEEN LUNG CANCER
Download slides:
 Download slides: https://www.tinyurl.com/m67zcnn https://tinyurl.com/kazchbn OSTEOPOROSIS REVIEW AND UPDATE Boca Raton Regional Hospital Internal Medicine Conference 2017 Benjamin Wang, M.D., FRCPC Division
Download slides: https://www.tinyurl.com/m67zcnn https://tinyurl.com/kazchbn OSTEOPOROSIS REVIEW AND UPDATE Boca Raton Regional Hospital Internal Medicine Conference 2017 Benjamin Wang, M.D., FRCPC Division
Hths 2231 Laboratory 13 Alterations in Musculoskeletal
 Watch Movie: Osteoporosis Answer the movie questions on the worksheet. Complete activities 1-4. Activity #1: Click on the website link in activity 1 to review the structure and function of bone. Activity
Watch Movie: Osteoporosis Answer the movie questions on the worksheet. Complete activities 1-4. Activity #1: Click on the website link in activity 1 to review the structure and function of bone. Activity
Osteoporosis - New Guidelines. Michelle Glass B.Sc. (Pharm) June 15, 2011
 Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss. A Publication of The Bone and Cancer Foundation
 Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss A Publication of The Bone and Cancer Foundation Contents This publication includes important information about
Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss A Publication of The Bone and Cancer Foundation Contents This publication includes important information about
Chapter 39: Exercise prescription in those with osteoporosis
 Chapter 39: Exercise prescription in those with osteoporosis American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise testing and prescription (6th ed.). New York:
Chapter 39: Exercise prescription in those with osteoporosis American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise testing and prescription (6th ed.). New York:
Pediatric metabolic bone diseases
 Pediatric metabolic bone diseases Classification and overview of clinical and radiological findings M. Mearadji International Foundation for Pediatric Imaging Aid www.ifpia.com Introduction Metabolic bone
Pediatric metabolic bone diseases Classification and overview of clinical and radiological findings M. Mearadji International Foundation for Pediatric Imaging Aid www.ifpia.com Introduction Metabolic bone
Talib A. Najjar, DMD, MDS, PhD Professor Oral & Maxillofacial Surgery Rutgers University
 Talib A. Najjar, DMD, MDS, PhD Professor Oral & Maxillofacial Surgery Rutgers University 1 Biochemistry Interaction with Oral & Systemic Diseases Periodontal disease Jaw Bone Necrosis due to Bisphosphonate
Talib A. Najjar, DMD, MDS, PhD Professor Oral & Maxillofacial Surgery Rutgers University 1 Biochemistry Interaction with Oral & Systemic Diseases Periodontal disease Jaw Bone Necrosis due to Bisphosphonate
Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss. A Publication of The Bone and Cancer Foundation
 Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss A Publication of The Bone and Cancer Foundation Contents This publication includes important information about
Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss A Publication of The Bone and Cancer Foundation Contents This publication includes important information about
AACE/ACE Osteoporosis Treatment Decision Tool
 AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
Aredia. For more info on Aredia, please see this link from MedlinePlus:
 Aredia Mr. Reeve, in his appearance on the Larry King Show on September 23, 2002 (rebroadcast 2/23/03 and several more times since) said the following: The way you prevent osteoporosis is by bearing weight,
Aredia Mr. Reeve, in his appearance on the Larry King Show on September 23, 2002 (rebroadcast 2/23/03 and several more times since) said the following: The way you prevent osteoporosis is by bearing weight,
Osteoporosis. Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective. Old Definition of Osteoporosis
 Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS
 New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS Wednesday, December 1, 2010 1:00 p.m. to 2:00 p.m. ET 1. I m 55 years old. I ve been taking Fosavance
New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS Wednesday, December 1, 2010 1:00 p.m. to 2:00 p.m. ET 1. I m 55 years old. I ve been taking Fosavance
Oklahoma Spine & Brain Institute is Proud to Introduce Michael Thambuswamy, MD, MBA
 Oklahoma Spine & Brain Institute is Proud to Introduce Michael Thambuswamy, MD, MBA Michael Thambuswamy, M.D., is from the Tulsa area where he graduated, with honors, from Jenks High School. He completed
Oklahoma Spine & Brain Institute is Proud to Introduce Michael Thambuswamy, MD, MBA Michael Thambuswamy, M.D., is from the Tulsa area where he graduated, with honors, from Jenks High School. He completed
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Disclosures. Diagnostic Challenges in Osteoporosis: Whom To Treat 9/25/2014
 Disclosures Diagnostic Challenges in Osteoporosis: Whom To Treat Ethel S. Siris, MD Columbia University Medical Center New York, NY Consultant on scientific issues for: AgNovos Amgen Eli Lilly Merck Novartis
Disclosures Diagnostic Challenges in Osteoporosis: Whom To Treat Ethel S. Siris, MD Columbia University Medical Center New York, NY Consultant on scientific issues for: AgNovos Amgen Eli Lilly Merck Novartis
Bisphosphonates in the Management of. Myeloma Bone Disease
 Bisphosphonates in the Management of Myeloma Bone Disease James R. Berenson, MD Medical & Scientific Director Institute for Myeloma & Bone Cancer Research Los Angeles, CA Myeloma Bone Disease Myeloma cells
Bisphosphonates in the Management of Myeloma Bone Disease James R. Berenson, MD Medical & Scientific Director Institute for Myeloma & Bone Cancer Research Los Angeles, CA Myeloma Bone Disease Myeloma cells
Osteoporosis and Lupus. Andrew Ruthberg, MD University Rheumatologists
 Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
Bisphosphonates. Making intelligent drug choices
 Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
nogg Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK
 nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
PROTOCOLS. Osteogenesis imperfecta. Principal investigator. Co-investigators. Background
 Osteogenesis imperfecta Principal investigator Leanne M. Ward, MD, FRCPC, paediatric endocrinologist Division of Endocrinology and Metabolism, Children s Hospital of Eastern Ontario, 401 Smyth Rd., Ottawa
Osteogenesis imperfecta Principal investigator Leanne M. Ward, MD, FRCPC, paediatric endocrinologist Division of Endocrinology and Metabolism, Children s Hospital of Eastern Ontario, 401 Smyth Rd., Ottawa
Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab 120mg for Bone Metastases
 ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
Osteoporosis. Overview
 v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
Horizon Scanning Technology Briefing. Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal. National Horizon Scanning Centre
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Epidemiology of Low back pain
 Low Back Pain Definition Pain felt in your lower back may come from the spine, muscles, nerves, or other structures in that region. It may also radiate from other areas like the mid or upper back, a inguinal
Low Back Pain Definition Pain felt in your lower back may come from the spine, muscles, nerves, or other structures in that region. It may also radiate from other areas like the mid or upper back, a inguinal
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr Zoledronic Acid A (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when Zoledronic Acid A was
PART III: CONSUMER INFORMATION Pr Zoledronic Acid A (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when Zoledronic Acid A was
HYPERCALCEMIA. Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences
 HYPERCALCEMIA Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences ESSENTIALS OF DIAGNOSIS Serum calcium level > 10.5 mg/dl Serum ionized
HYPERCALCEMIA Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences ESSENTIALS OF DIAGNOSIS Serum calcium level > 10.5 mg/dl Serum ionized
Paget's bone disease : A series of 40 cases
 Paget's bone disease : A series of 40 cases Poster No.: C-1835 Congress: ECR 2014 Type: Scientific Exhibit Authors: H. Tayari 1, Y. Hentati 2, F. Frikha 1, K. Chermi 1, I. Kobbi 1, E. Keywords: DOI: Daoud
Paget's bone disease : A series of 40 cases Poster No.: C-1835 Congress: ECR 2014 Type: Scientific Exhibit Authors: H. Tayari 1, Y. Hentati 2, F. Frikha 1, K. Chermi 1, I. Kobbi 1, E. Keywords: DOI: Daoud
Functions of the Skeletal System. Chapter 6: Osseous Tissue and Bone Structure. Classification of Bones. Bone Shapes
 Chapter 6: Osseous Tissue and Bone Structure Functions of the Skeletal System 1. Support 2. Storage of minerals (calcium) 3. Storage of lipids (yellow marrow) 4. Blood cell production (red marrow) 5. Protection
Chapter 6: Osseous Tissue and Bone Structure Functions of the Skeletal System 1. Support 2. Storage of minerals (calcium) 3. Storage of lipids (yellow marrow) 4. Blood cell production (red marrow) 5. Protection
The Role of Radiotherapy in Metastatic Breast Cancer. Shilpen Patel MD, FACRO Associate Professor Departments of Radiation Oncology and Global Health
 The Role of Radiotherapy in Metastatic Breast Cancer Shilpen Patel MD, FACRO Associate Professor Departments of Radiation Oncology and Global Health Indications for Palliative Pain Control Radiation Bone
The Role of Radiotherapy in Metastatic Breast Cancer Shilpen Patel MD, FACRO Associate Professor Departments of Radiation Oncology and Global Health Indications for Palliative Pain Control Radiation Bone
DENOSUMAB (PROLIA & XGEVA )
 DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
MARK D. MURPHEY MD, FACR. Physician-in-Chief, AIRP. Chief, Musculoskeletal Imaging
 ALPHABET SOUP AND CYSTIC LESIONS OF THE BONE MARK D. MURPHEY MD, FACR Physician-in-Chief, AIRP Chief, Musculoskeletal Imaging ALPHABET SOUP AND CYSTIC LESIONS OF THE BONE Giant cell tumor (GCT) Unicameral
ALPHABET SOUP AND CYSTIC LESIONS OF THE BONE MARK D. MURPHEY MD, FACR Physician-in-Chief, AIRP Chief, Musculoskeletal Imaging ALPHABET SOUP AND CYSTIC LESIONS OF THE BONE Giant cell tumor (GCT) Unicameral
SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over 3 Weeks. A SEPARATE WORKSHEET WILL BE PROVIDED.
 BIO 211; Anatomy and Physiology I REFERENCE: CHAPTER 07 1 Dr. Lawrence Altman Naugatuck Valley Community College LECTURE TOPICS OUTLINE SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over
BIO 211; Anatomy and Physiology I REFERENCE: CHAPTER 07 1 Dr. Lawrence Altman Naugatuck Valley Community College LECTURE TOPICS OUTLINE SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over
Atypical Femoral Fractures With Long Term Bisphosphonates Use
 Atypical Femoral Fractures With Long Term Bisphosphonates Use There has been an increase in the number of atypical femoral fractures in pa4ents on long term bisphosphonate therapy (e.g Fosamax) Accordingly
Atypical Femoral Fractures With Long Term Bisphosphonates Use There has been an increase in the number of atypical femoral fractures in pa4ents on long term bisphosphonate therapy (e.g Fosamax) Accordingly
A Patient s Guide to Dropped Head Syndrome
 A Patient s Guide to Dropped Head Syndrome 228 West Main, Suite C Missoula, MT 59802 Phone: info@spineuniversity.com DISCLAIMER: The information in this booklet is compiled from a variety of sources. It
A Patient s Guide to Dropped Head Syndrome 228 West Main, Suite C Missoula, MT 59802 Phone: info@spineuniversity.com DISCLAIMER: The information in this booklet is compiled from a variety of sources. It
GLOSSARY. This glossary includes definitions for patients who have cancer with bone involvement. New definitions will be added periodically.
 GLOSSARY This glossary includes definitions for patients who have cancer with bone involvement. New definitions will be added periodically. For more in-depth information, please refer to the Bone and Cancer
GLOSSARY This glossary includes definitions for patients who have cancer with bone involvement. New definitions will be added periodically. For more in-depth information, please refer to the Bone and Cancer
Kristen M. Nebel, DO PENN/ LGHP Geriatrics. Temple Family Medicine Review
 Kristen M. Nebel, DO PENN/ LGHP Geriatrics 10/3/17 Temple Family Medicine Review OBJECTIVES Define Revised 2017 American College of Physician Recommendations Screening, Prevention and Treatment Application
Kristen M. Nebel, DO PENN/ LGHP Geriatrics 10/3/17 Temple Family Medicine Review OBJECTIVES Define Revised 2017 American College of Physician Recommendations Screening, Prevention and Treatment Application
PART III: CONSUMER INFORMATION
 PART III: CONSUMER INFORMATION Pr ACLASTA (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when ACLASTA was approved for sale
PART III: CONSUMER INFORMATION Pr ACLASTA (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when ACLASTA was approved for sale
Common Prescription mg/ day mg/ day mg/day
 Table 19.1. Medical Management of Burning Mouth Syndrome Medications Examples of Agents Dosage Common Prescription Tricyclic antidepressants Amitryptyline (Elavil ) 10 150 mg/ day 10 mg at bedtime; increase
Table 19.1. Medical Management of Burning Mouth Syndrome Medications Examples of Agents Dosage Common Prescription Tricyclic antidepressants Amitryptyline (Elavil ) 10 150 mg/ day 10 mg at bedtime; increase
Rama Nada. - Mousa Al-Abbadi. 1 P a g e
 - 1 - Rama Nada - - Mousa Al-Abbadi 1 P a g e Bones, Joints and Soft tissue tumors Before we start: the first 8 minutes was recalling to Dr.Mousa s duties, go over them in the slides. Wherever you see
- 1 - Rama Nada - - Mousa Al-Abbadi 1 P a g e Bones, Joints and Soft tissue tumors Before we start: the first 8 minutes was recalling to Dr.Mousa s duties, go over them in the slides. Wherever you see
Management of the complications of myeloma and side-effects of treatment Christine Morris Clinical Nurse Specialist in Myeloma Royal Derby Hospital
 Management of the complications of myeloma and side-effects of treatment Christine Morris Clinical Nurse Specialist in Myeloma Royal Derby Hospital Common problems in myeloma Myeloma-related complications/symptoms
Management of the complications of myeloma and side-effects of treatment Christine Morris Clinical Nurse Specialist in Myeloma Royal Derby Hospital Common problems in myeloma Myeloma-related complications/symptoms
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr Zoledronic Acid A (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when Zoledronic Acid A was
PART III: CONSUMER INFORMATION Pr Zoledronic Acid A (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when Zoledronic Acid A was
Osteoporosis. Treatment of a Silently Developing Disease
 Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Osteoporosis in Men Wendy Rosenthal PharmD. This program has been brought to you by PharmCon
 Osteoporosis in Men Wendy Rosenthal PharmD This program has been brought to you by PharmCon Osteoporosis in Men Speaker: Dr. Wendy Rosenthal, President of MedOutcomes, will be the presenter for this webcast.
Osteoporosis in Men Wendy Rosenthal PharmD This program has been brought to you by PharmCon Osteoporosis in Men Speaker: Dr. Wendy Rosenthal, President of MedOutcomes, will be the presenter for this webcast.
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS The following questions are representative of questions that patients and family members ask when they visit the Bone and Cancer Foundation website or contact the Foundation
FREQUENTLY ASKED QUESTIONS The following questions are representative of questions that patients and family members ask when they visit the Bone and Cancer Foundation website or contact the Foundation
Dorset Pathway for the use of bisphosphonates for post-menopausal women with breast cancer. Summary
 Summary Appendix 1: Selection of patients suitable for adjuvant bisphosphonates* Appendix 2 Adjuvant Bisphosphonates for Early Breast Cancer Information for patients This leaflet is intended to support
Summary Appendix 1: Selection of patients suitable for adjuvant bisphosphonates* Appendix 2 Adjuvant Bisphosphonates for Early Breast Cancer Information for patients This leaflet is intended to support
PATIENT INFORMATION BROCHURE
 PATIENT INFORMATION BROCHURE PATIENT INFORMATION BROCHURE This brochure is designed to help you make an informed decision about your surgery. Please read this entire document carefully. Keep this document
PATIENT INFORMATION BROCHURE PATIENT INFORMATION BROCHURE This brochure is designed to help you make an informed decision about your surgery. Please read this entire document carefully. Keep this document
NATIONAL COALITION FOR OSTEOPOROSIS AND RELATED BONE DISEASES
 NATIONAL COALITION FOR OSTEOPOROSIS AND RELATED BONE DISEASES Fact Sheet FY 2007 What is the Mission of the Bone Coalition? The National Coalition for Osteoporosis and Related Bone Diseases is dedicated
NATIONAL COALITION FOR OSTEOPOROSIS AND RELATED BONE DISEASES Fact Sheet FY 2007 What is the Mission of the Bone Coalition? The National Coalition for Osteoporosis and Related Bone Diseases is dedicated
Guideline for the investigation and management of osteoporosis. for hospitals and General Practice
 Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
fractures. Malignant transformation such as osteosarcoma is less common (0.3%). 4 Tere is a trend towards more axial involvement in older people.
 Osteology 31 Paget s disease Paget s disease of bone becomes increasingly more common with age with a prevalence of 10% at the age of 70 years. Te diagnosis may be missed unless the clinician is vigilant,
Osteology 31 Paget s disease Paget s disease of bone becomes increasingly more common with age with a prevalence of 10% at the age of 70 years. Te diagnosis may be missed unless the clinician is vigilant,
Scottish Medicines Consortium
 Scottish Medicines Consortium zoledronic acid 5mg/100ml solution for infusion (Aclasta) No. (317/06) Novartis 8 September 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Scottish Medicines Consortium zoledronic acid 5mg/100ml solution for infusion (Aclasta) No. (317/06) Novartis 8 September 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Plain Film CT. Principal Modality (2): Case Report # [] Date accepted: 15 March 2014
![Plain Film CT. Principal Modality (2): Case Report # [] Date accepted: 15 March 2014 Plain Film CT. Principal Modality (2): Case Report # [] Date accepted: 15 March 2014](/thumbs/89/100531147.jpg) Radiological Category: Musculoskeletal Principal Modality (1): Principal Modality (2): Plain Film CT Case Report # [] Submitted by: Dr. Jason E. Lally, M.D. Faculty reviewer: Dr. Naga Ramesh Chinapuvvula,
Radiological Category: Musculoskeletal Principal Modality (1): Principal Modality (2): Plain Film CT Case Report # [] Submitted by: Dr. Jason E. Lally, M.D. Faculty reviewer: Dr. Naga Ramesh Chinapuvvula,
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 15 December 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: 345 098-5) DIDRONEL 400 mg, tablets B/14 (CIP code: 333
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: 345 098-5) DIDRONEL 400 mg, tablets B/14 (CIP code: 333
Southern Derbyshire Shared Care Pathology Guidelines. Primary Hyperparathyroidism
 Southern Derbyshire Shared Care Pathology Guidelines Primary Hyperparathyroidism Please use this Guideline in Conjunction with the Hypercalcaemia Guideline Definition Driven by hyperfunction of one or
Southern Derbyshire Shared Care Pathology Guidelines Primary Hyperparathyroidism Please use this Guideline in Conjunction with the Hypercalcaemia Guideline Definition Driven by hyperfunction of one or
ROTATOR CUFF INJURIES / IMPINGEMENT SYNDROME
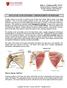 ROTATOR CUFF INJURIES / IMPINGEMENT SYNDROME Shoulder injuries are common in patients across all ages, from young, athletic people to the aging population. Two of the most common problems occur in the
ROTATOR CUFF INJURIES / IMPINGEMENT SYNDROME Shoulder injuries are common in patients across all ages, from young, athletic people to the aging population. Two of the most common problems occur in the
Osteoporosis Update. Greg Summers Consultant Rheumatologist
 Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Patient Information Brochure
 Patient Information Brochure Patient Information Brochure This brochure is designed to help you make an informed decision about your surgery. Please read this entire document carefully. Keep this document
Patient Information Brochure Patient Information Brochure This brochure is designed to help you make an informed decision about your surgery. Please read this entire document carefully. Keep this document
DISEASES AND DISORDERS
 DISEASES AND DISORDERS 9. 53 10. Rheumatoid arthritis 59 11. Spondyloarthropathies 69 12. Connective tissue diseases 77 13. Osteoporosis and metabolic bone disease 95 14. Crystal arthropathies 103 15.
DISEASES AND DISORDERS 9. 53 10. Rheumatoid arthritis 59 11. Spondyloarthropathies 69 12. Connective tissue diseases 77 13. Osteoporosis and metabolic bone disease 95 14. Crystal arthropathies 103 15.
New Osteoporosis Guidelines: What you and your health provider need to know QUESTION & ANSWER
 The first of the newsletters on these Qs and As should include a refresher on the Virtual Forums what they are, how they work, etc. The fact is that less than 5% of COPN members tune in for any given Forum.
The first of the newsletters on these Qs and As should include a refresher on the Virtual Forums what they are, how they work, etc. The fact is that less than 5% of COPN members tune in for any given Forum.
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women
 S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
SpongeBone Menopants*
 SpongeBone Menopants* Adam Fershko, MD, FACP Kettering Health Network *Postmenopausal Osteoporosis Objectives O Epidemiology O Clinical significance O Pathophysiology O Screening and Diagnosis O Treatment
SpongeBone Menopants* Adam Fershko, MD, FACP Kettering Health Network *Postmenopausal Osteoporosis Objectives O Epidemiology O Clinical significance O Pathophysiology O Screening and Diagnosis O Treatment
Surgical and orthotic possibilities of bone tumour pain management
 Surgical and orthotic possibilities of bone tumour pain management Chaloupka, R., Grosman, R., Repko, M., Tichý, V. Ortopedická klinika FN Brno-Bohunice Ortopedická klinika, FN Brno, Jihlavská 20, 625
Surgical and orthotic possibilities of bone tumour pain management Chaloupka, R., Grosman, R., Repko, M., Tichý, V. Ortopedická klinika FN Brno-Bohunice Ortopedická klinika, FN Brno, Jihlavská 20, 625
NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT
 NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF OSTEOPOROSIS: OVERVIEW Definitions Risk factors
NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF OSTEOPOROSIS: OVERVIEW Definitions Risk factors
Oral Alendronate Vs. Three-Monthly Iv Ibandronate In The Treatment Of Postmenopausal Osteoporosis
 Oral Alendronate Vs. Three-Monthly Iv Ibandronate In The Treatment Of Postmenopausal Osteoporosis Miriam Silverberg A. Study Purpose and Rationale More than 70% of fractures in people after the age of
Oral Alendronate Vs. Three-Monthly Iv Ibandronate In The Treatment Of Postmenopausal Osteoporosis Miriam Silverberg A. Study Purpose and Rationale More than 70% of fractures in people after the age of
TREATMENT OF OSTEOPOROSIS
 TREATMENT OF OSTEOPOROSIS Summary Prevention is the key issue in the management of osteoporosis. HRT is the agent of choice for prevention of postmenopausal osteoporosis. Bisphosphonates and Calcitonin
TREATMENT OF OSTEOPOROSIS Summary Prevention is the key issue in the management of osteoporosis. HRT is the agent of choice for prevention of postmenopausal osteoporosis. Bisphosphonates and Calcitonin
Current Management of Metastatic Bone Disease
 Current Management of Metastatic Bone Disease Evaluation and Medical Management Dr. Sara Rask Head, Medical Oncology Simcoe Muskoka Regional Cancer Centre www.rvh.on.ca Objectives 1. Outline an initial
Current Management of Metastatic Bone Disease Evaluation and Medical Management Dr. Sara Rask Head, Medical Oncology Simcoe Muskoka Regional Cancer Centre www.rvh.on.ca Objectives 1. Outline an initial
Zerlinda (MRP DK/H/2265/001)
 Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Summary of the risk management plan by product
 Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Suspecting Tumors, or Could it be cancer?
 Suspecting Tumors, or Could it be cancer? Donna E. Reece, M.D. Princess Margaret Cancer Centre University Health Network Toronto, ON CANADA 07 February 2018 Background Low back pain is common However,
Suspecting Tumors, or Could it be cancer? Donna E. Reece, M.D. Princess Margaret Cancer Centre University Health Network Toronto, ON CANADA 07 February 2018 Background Low back pain is common However,
Fig Articular cartilage. Epiphysis. Red bone marrow Epiphyseal line. Marrow cavity. Yellow bone marrow. Periosteum. Nutrient foramen Diaphysis
 Fig. 7.1 Articular cartilage Epiphysis Red bone marrow Epiphyseal line Marrow cavity Yellow bone marrow Nutrient foramen Diaphysis Site of endosteum Compact bone Spongy bone Epiphyseal line Epiphysis Articular
Fig. 7.1 Articular cartilage Epiphysis Red bone marrow Epiphyseal line Marrow cavity Yellow bone marrow Nutrient foramen Diaphysis Site of endosteum Compact bone Spongy bone Epiphyseal line Epiphysis Articular
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
New Dual-energy X-ray Absorptiometry Machines (idxa) and Vertebral Fracture Assessment
 Case 1 New Dual-energy X-ray Absorptiometry Machines (idxa) and Vertebral Fracture Assessment (VFA) History and Examination Your wealthy friend who is a banker brings his 62-year-old mother to your office
Case 1 New Dual-energy X-ray Absorptiometry Machines (idxa) and Vertebral Fracture Assessment (VFA) History and Examination Your wealthy friend who is a banker brings his 62-year-old mother to your office
The Radiology Assistant : Bone tumor - ill defined osteolytic tumors and tumor-like lesions
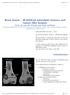 Bone tumor - ill defined osteolytic tumors and tumor-like lesions Henk Jan van der Woude and Robin Smithuis Radiology department of the Onze Lieve Vrouwe Gasthuis, Amsterdam and the Rijnland hospital,
Bone tumor - ill defined osteolytic tumors and tumor-like lesions Henk Jan van der Woude and Robin Smithuis Radiology department of the Onze Lieve Vrouwe Gasthuis, Amsterdam and the Rijnland hospital,
Page 1
 Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
HIGH LEVEL - Science
 Learning Outcomes HIGH LEVEL - Science Describe the structure and function of the back and spine (8a) Outline the functional anatomy and physiology of the spinal cord and peripheral nerves (8a) Describe
Learning Outcomes HIGH LEVEL - Science Describe the structure and function of the back and spine (8a) Outline the functional anatomy and physiology of the spinal cord and peripheral nerves (8a) Describe
CASE STUDY: PRO-DENSE Injectable Regenerative Graft Used to Backfill a Bone Cavity Following Resection of a Giant Cell Tumor
 : PRO-DENSE Used to Backfill a Bone Cavity Following Resection of a Giant Cell Tumor Contributed by: Matthew J. Seidel, MD* Lauren A. Schwartz, NP Scottsdale, AZ *Dr. Seidel is a paid consultant for Wright
: PRO-DENSE Used to Backfill a Bone Cavity Following Resection of a Giant Cell Tumor Contributed by: Matthew J. Seidel, MD* Lauren A. Schwartz, NP Scottsdale, AZ *Dr. Seidel is a paid consultant for Wright
Pathologic Fracture of the Femur in Brown Tumor Induced in Parathyroid Carcinoma: A Case Report
 CASE REPORT Hip Pelvis 28(3): 173-177, 2016 http://dx.doi.org/10.5371/hp.2016.28.3.173 Print ISSN 2287-3260 Online ISSN 2287-3279 Pathologic Fracture of the Femur in Brown Tumor Induced in Parathyroid
CASE REPORT Hip Pelvis 28(3): 173-177, 2016 http://dx.doi.org/10.5371/hp.2016.28.3.173 Print ISSN 2287-3260 Online ISSN 2287-3279 Pathologic Fracture of the Femur in Brown Tumor Induced in Parathyroid
