Therapeutic Hypothermia: Treatment for Hypoxic-Ischemic Encephalopathy in the NICU
|
|
|
- Marlene Palmer
- 5 years ago
- Views:
Transcription
1 Therapeutic Hypothermia: Treatment for Hypoxic-Ischemic Encephalopathy in the NICU Denise M. Casey, RN, MS, CCRN, CPNP Nancy Tella, RN, BSN, CCRN Rachel Turesky, RN, BSN Michelle Labrecque, RN, MSN, CCRN Case Study Baby M was born limp, blue, and without respiratory effort at 38 weeks gestation to a 38-year-old, gravida 5, para 1, woman. Delivery was vaginal after a rapid progression of labor leaving no opportunity for a cesarean section. No other complications were noted during labor but a large surge at delivery, later diagnosed as uterine rupture, initially raised concerns about placental abruption. Apgar scores were 1, 2, and 4 at one, five, and ten minutes, respectively. She was resuscitated in the delivery room, intubated, and transferred in critical condition to the neonatal intensive care unit (NICU) at the birth hospital. Her initial cord ph was 6.7 and was slightly improved at 7.17 on arterial blood gas after resuscitation. Our NICU team was consulted because of her severe neurologic depression. The birth hospital was within walking distance of our tertiary care center and our neurologists went to evaluate her for the hypothermia protocol. Her neurologic exam was notable for dilated and unresponsive pupils, no spontaneous movements, and diminished reflexes and tone, consistent with moderate-to-severe encephalopathy. Seizure activity began at one hour of age and consisted of lip smacking, which was later confirmed by electroencephalogram (EEG). Enrollment criteria were met based on respiratory depression at birth requiring intubation and continued need for ventilation, concern for placental abruption, cord ph less than 7, and encephalopathy on exam and EEG. After stabilizing her airway and achieving central access to treat acidosis and seizures, the team prepared her for transfer to our NICU. At this point, the primary concern became her neurologic status. Baby M was admitted to our NICU for whole-body hypothermia at four hours of age with a diagnosis of hypoxic-ischemic encephalopathy (HIE). Therapeutic hypothermia using wholebody cooling was initiated within six hours of birth per protocol with the Cincinnati Subzero Blanketrol II. Shortly after admission, arterial access was established for hemodynamic monitoring and ease of lab drawing purposes. Additional anticonvulsants were given to control her seizures. Baby M s father had spent the night going back and forth between our NICU and the birth hospital because both his wife and newborn infant were quite sick. When he came in later that morning with Baby M s adult stepsiblings, explanations were given for each of the machines, her course of treatment up to that point, and our concerns about her neurologic status. Baby M s father tried to console his children despite his own fears and sadness. During the next several days, Baby M remained intubated for airway protection, was fluid restricted to prevent additional injury to her brain, and continued on anticonvulsants. Despite her traumatic delivery, Baby M remained stable from a cardiovascular standpoint without evidence of persistent pulmonary hypertension of the newborn (PPHN) or need for pressors. Her kidneys were affected by the hypoxic events at birth as evidenced by poor urine output and electrolyte disturbances requiring further fluid restriction and electrolyte boluses. Her liver enzymes were mildly elevated, but she showed no evidence of coagulopathy. She was hemodynamically stable after an airway was established. She completed the hypothermia protocol after 72 hours of cooling and began the rewarming process. EEG tracings obtained on day of life (DOL) 5 showed no evidence of seizure activity. Fosphenytoin was discontinued and she remained on phenobarbital alone. A magnetic resonance imaging (MRI) of the brain done on the same day showed minor changes in the occipital cortex, but no significant abnormalities. Disclosure The author discloses no relevant financial interest or affiliations with any commercial interests. Accepted for publication February Springer Publishing Company NOVEMBER/DECEMBER 2011, VOL. 30, NO
2 As she recovered and her sedation was lightened, she began to breathe and was extubated to room air on DOL 6. Intravenous fluids were liberalized and nasogastric feedings were initiated on DOL 7. Her neurologic exam continued to stabilize. Her pupils became reactive, suck and gag reflexes were present, and she began to move spontaneously. She was transferred back to her birth hospital on DOL 8 to continue to recuperate with her mother. At that point, she was tolerating full nasogastric feedings and was consistently interacting with her family. Additional MRI was obtained on DOL 12 and the subtle abnormalities seen earlier were no longer apparent. An EEG done the same day showed no evidence of seizure activity. She was discharged to home with her parents on DOL 15 on full oral feedings and off phenobarbital. She was followed in the neurology clinic every three to six months for the first 24 months of life. At the last scheduled visit, Baby M appeared to be growing well and meeting her developmental milestones with no apparent neurologic deficits. INTRODUCTION Hypoxic-ischemic encephalopathy (HIE) is defined as an interruption in the supply of oxygen (hypoxia) and/or blood flow (ischemia) going to the brain and body. This kind of interruption occurring either hours before birth or during labor and delivery can happen for several reasons such as compression of placenta, tearing of placenta from uterine wall, or compression of the cord. HIE occurs in 1/1,000 term live births and remains an important cause of mortality and neurodevelopmental deficits in infants. 1 Moderate encephalopathy carries a 10 percent risk of death and 30 percent risk of disability, whereas 60 percent of those patients with severe encephalopathy die and many, if not all who survive, have neurologic deficits. 2 The Sarnat and Sarnat system for neonatal encephalopathy guides the classification based on Stages 1 (mild), 2 (moderate), and 3 (severe). 3 The stages are classified based on the distinguishing features shown in Table 1. Until 2005, there was no other treatment for HIE other than conventional intensive care. HIE results from a lack of oxygen and blood supply that leads to metabolic acidosis, ischemia, and subsequently neurologic dysfunction. 4 Brain injury that ensues is characterized by TABLE 1 n Sarnat Staging of Encephalopathy Sarnat Stage 1 (Mild) Sarnat Stage 2 (Moderate) Sarnat Stage 3 (Severe) Hyperalert Lethargic Stuporous Normal tone Mild hypotonia Flaccid Overactive stretch reflexes Overactive stretch reflexes Decreased or absent stretch reflexes Weak suck Weak or absent suck Absent suck No seizures Common; focal or Uncommon multifocal Less than 24 hours 2 14 days Hours to weeks a biphasic process. The initial phase of hypoxic-ischemia results in a primary brain energy failure in which there are reductions in cerebral blood flow, oxygen, high- energy phosphorylated metabolites, and brain acidosis. 5,6 This phase is associated with intracellular derangements such as loss of membrane homeostasis, defective osmoregulation, and inhibition of protein synthesis. Loss of membrane homeostasis can lead to increases in intracellular calcium and osmotic dysregulation. 7 Elevated calcium levels then trigger many destructive pathways. 8 Approximately 6 12 hours after the initial insult, a secondary energy failure occurs leading to sustained brain injury. This phase typically is without brain acidosis. Secondary energy failure is a marker of the beginning of multiple pathways that lead to brain injury. The processes that take place within this phase are inflammation, apoptosis, oxidative injury, decrease growth factors, and protein synthesis. Because the cerebral energy state can be restored after the primary energy failure, it suggests there is a therapeutic window to implement interventions to avoid or diminish the secondary energy failure leading to brain injury. 6 During this biphasic process, the therapeutic window to implement interventions to reduce brain injury is during the 6 12 hour window prior to the secondary energy failure. A temperature reduction of 2 4 C decreases the rate of cell death and delays the cascade of metabolic changes that occur with hypoxia. Cerebral metabolism is reduced and hypothermia can delay secondary brain injury in HIE infants. 9 REVIEW OF EVIDENCE In 2005, two large clinical trials of 473 infants demonstrated that therapeutic hypothermia reduced the risk of death/disability in neonates with HIE. 1,2 The major difference between these two trials was whole-body cooling versus head cooling only. The Shankaran and associates trial looked at whole-body cooling with core temperature of 33.5 C by esophageal probe for 72 hours followed by a rewarming phase. There were 239 infants enrolled in this study and the inclusion criteria were infants $36 weeks, admitted less than six hours after birth, severe acidosis or perinatal complications, resuscitation at birth, and moderate-to-severe encephalopathy (clinical exam only). The neurodevelopmental outcomes were assessed at months of age and showed a reduced risk of death/ disability in infants with moderate-to-severe encephalopathy. 2 The Gluckman and associates trial was a head cooling study and core temperature was C by rectal probe for 72 hours followed by a rewarming phase. The sample size was 235 term infants. The inclusion criteria for this study were term infants with moderate-to-severe encephalopathy determined by clinical exam and an abnormal amplitude-integrated electroencephalogram (aeeg). The outcomes revealed that head cooling was not protective in a mixed population of infants with neonatal encephalopathy. The study did show there was improved survival without severe neurodevelopmental disability in infants with less severe aeeg changes. 1 An article published in 2009 by Azzopardi looked at neurodevelopmental outcomes in infants at least 36 weeks of age VOL. 30, NO. 6, NOVEMBER/DECEMBER
3 with perinatal asphyxia and found that infants in the cooled group had an increased rate of survival without neurologic abnormality. Among survivors, cooling resulted in reduced risks of cerebral palsy and improved developmental scores. 10 Based on the 2005 studies by Gluckman and associates and Shankara and colleagues, the National Institute of Child Health and Human Development (NICHD) held a workshop on hypothermia as a treatment for HIE. 1,2,11 Experts summarized the evidence on hypothermia and concluded that mild therapeutic hypothermia offered a potential for short-term benefits when used under strict protocols. In addition, they stated, therapeutic hypothermia is an evolving therapy and the long-term safety and efficacy are yet to be established. 11 They offered a framework for patient care with standardized protocols to continue to determine the safety and efficacy of this therapy while also suggesting hypothermia registries as a way to monitor, develop, refine, and optimize this new therapy. 11 One registry that has been developed in response to this suggestion is the Vermont Oxford Network Registry for Neonatal Encephalopathy. The Vermont Oxford Network was established in Hospitals enrolled in the Vermont Oxford Network are able to participate as long as they have internal review board (IRB) approval. The 4,464 records of neonatal encephalopathy have been received since the beginning of the registry. The 1,797 infants have received either selective head cooling or whole-body therapeutic hypothermia. After an evidence-based review, our NICU developed a therapeutic hypothermia protocol in The two large randomized clinical trials as well as the NICHD workshop recommendations formed the basis for our protocol. A team from the NICU including neonatology, neurology, and clinical nurse specialists all met to discuss the current evidence and, using the Gluckman and associates and Shankaran and colleagues trials as references, developed a total body hypothermia protocol for treatment of HIE. 1,2 The protocol included guidelines for cooling and maintenance as well as rewarming phases. As we continue to gain experience with this new treatment, the protocol will be updated to use evidence-based research as it becomes available and to maintain consistency with current practice. Our NICU has evaluated 59 patients for the therapeutic hypothermia protocol since initiation in Forty-two patients have started the therapy and 32 patients have completed the 72-hour cooling period. Ten patients have been taken off the protocol for the following reasons: six withdrawal of care (removing life support), three abnormal coagulation levels, and one for encephalopathy not caused by HIE. Twelve of these patients had EEG-confirmed seizures. We had a total of eight deaths. All surviving patients are followed by our neurology clinic on a regular basis to monitor for long-term outcomes. NURSING CARE HIGHLIGHTS OF A PATIENT UNDERGOING THERAPEUTIC HYPOTHERMIA The timing for therapeutic hypothermia is crucial, optimally it is initiated within six hours of birth. 2 To be considered for cooling infants with presumed HIE, must meet the following inclusion TABLE 2 n Whole-Body Cooling Inclusion Criteria Evidence of fetal distress as evidenced by one of the following: History of acute perinatal event (abruptio placentae, cord prolapse, variable or late decelerations) Biophysical profile,6/10 within 6 hours of birth Cord ph #7.0 or base deficit $16 meq/l and Evidence of neonatal distress as evidenced by at least one of the following: Apgar score #5 at 10 minutes Postnatal blood gas ph at,1 hour #7.0 or base deficit $16 meq/l Continued need for ventilation initiated at birth and continued for at least 10 minutes and Evidence of neonatal encephalopathy by physical exam (neurologist) and Abnormal cerebral function monitor (CFM) with minimum 20 minutes of recording Adapted from: Shankaran, S., Laptook, A. R., Ehrenkranz, R. A., Tyson, J. E., McDonald, S. A., Donovan, E. F.,... Jobe, A. H. (2005). Whole-body hypothermia for neonates with hypoxicischemic encephalopathy. The New England Journal of Medicine, 353(15), criteria: gestational age $36 weeks and birth weight.2,000 g; evidence of fetal distress; evidence of neonatal distress; evidence of neonatal encephalopathy; and abnormal cerebral function monitoring (CFM), which is a compressed three-lead EEG that provides information on global cerebral activity and is evaluated by the consulting neurology team (Table 2). Once the patient is determined to meet all of the inclusion criteria, the therapeutic hypothermia protocol is initiated. Prior to admission, the necessary equipment is set up including the cooling unit, cooling blanket, esophageal temperature probe, and infant warmer, with the heat off, but allowing skin temperature reading. Other necessary supplies are organized to ensure accuracy and readiness upon the infant s arrival. Protocol Initiation On admission, the infant is placed on a radiant warmer in manual mode with the heat o f f. The attending neonatologist, nurse practitioner, and the admitting nurse assess the infant, and a neurology consult is obtained at the bedside. In collaboration with neurology, the attending neonatologist confirms that the infant is a candidate for therapeutic hypothermia based on clinical exam, inclusion criteria, and a 20-minute aeeg. 1 The therapeutic hypothermia cooling procedure checklist and clinical practice guidelines are available at the bedside for reference and are used throughout the care of the patient (see Figure 1). An esophageal temperature probe is placed by the nurse to monitor core temperature, and the infant is placed directly on the cooling blanket. In the cooling and maintenance phase of therapy, the goal esophageal temperature is 33.5 C with a range of C. 2 Careful monitoring of the infant undergoing therapeutic hypothermia is a primary focus of nursing care once hypothermia is initiated. Each 372 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
4 FIGURE 1 n Cooling checklist. VOL. 30, NO. 6, NOVEMBER/DECEMBER
5 infant is monitored closely and for the purposes of this article, we have broken each of the monitoring categories out into systems to best describe the parameters individually. Systemic Effects Patients with moderate-to-severe HIE often appear sedated, pale, and hypotonic because of the brain injury they have sustained. They usually require ventilator support to maintain adequate oxygenation and ventilation. Peripheral perfusion is often poor, and the infant may require volume and inotropic support to maintain adequate blood pressure. Overall, tone is usually low with little to no spontaneous movement and, frequently, seizure activity is noted. Seizures are typically of early onset and occur in approximately percent of patients. 2,10 Neurologic Because of the potential for evolving injury, a neurologic exam is, at a minimum, performed hourly by the infant s nurse. Together with the medical team, those caring for the infant are constantly monitoring for any changes in neurologic status. In 374 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
6 these infants, neurologic assessment should include pupil checks; evaluation of level of consciousness and respiratory insufficiency; and evaluating for any evidence of seizures, signs of apnea/bradycardia, as well as signs of increased intracranial pressure. 12 CFM monitoring is continued throughout the 72-hour cooling period with readings evaluated by the neurology service because it can provide information on duration, intensity, and frequency of neonatal seizures, and a full 20-lead EEG and MRI are arranged at the discretion of the neurology service. An early MRI may be necessary for those patients with severe HIE to obtain additional information that may guide end-of-life discussions. The cooling blanket is not compatible with MRI. To prevent rewarming, the infant should remain on the cooling blanket for as long as possible, and the nurse caring for the patient should coordinate with radiology to minimize any wait time off the blanket. Sedation Infants undergoing hypothermia treatment in our NICU are typically sedated with a lower dose of fentanyl from the time of initiation of cooling until the rewarming process is complete. The goal of sedation during the cooling period is to optimize comfort and the efficacy of therapeutic hypothermia. Inadequate sedation may result in an increased metabolic rate as the infant attempts to warm himself or herself, therefore decreasing the effectiveness of the therapy. In our NICU, we use the State Behavioral Scale (SBS) to guide our sedation management with a goal of 21 to 22 during treatment. The fentanyl dosage is adjusted accordingly to maintain our target. Patients within this range are responsive to noxious stimuli and to a gentle touch or voice. 13 In reviewing the literature on HIE, there was only one article that mentioned using sedation with morphine or chloral hydrate if the infants appeared distressed. 10 Respiratory Infants receiving cooling are typically intubated, but some patients are able to be extubated during the cooling phase. It is important to note esophageal temperature on the requisition when arterial blood gases are sent to allow for temperaturecorrected results. 2 Hypothermia shifts the oxyhemoglobin curve and can result in a decreased oxygen delivery, but the metabolic rate is also lowered, decreasing oxygen consumption and carbon dioxide production. As a result of these reactions to cooling, ventilator settings may require frequent adjustment using the temperature-corrected arterial blood gas. 16 Cardiovascular The nurse closely monitors the infant s color and perfusion, and watches for bradycardia and arrhythmias. The arrhythmia most likely seen with therapeutic hypothermia is a sinus bradycardia. 10 This is a very frequent finding during therapeutic hypothermia and a normal physiologic response occurring with most hypothermia patients; the infant s perfusion is closely monitored and the infant is treated individually as needed. Because of the initial hypoxic insult, these infants typically require volume resuscitation and initiation of inotropic support. FIGURE 2 n Subcutaneous fat necrosis 1 and 2. Fluid and Electrolytes These patients are at risk for multiple electrolyte imbalances and do require frequent monitoring and correction based on laboratory values. Fluid restriction is anticipated with these patients to avoid fluid overload and thus cerebral edema. Maintaining sodium levels in the upper limits of normal also is important because these patients are at risk for cerebral edema. 14 Serum magnesium levels are also maintained in the upper limits of normal because of its potential neuroprotective effect. 15 This osmotherapy aids in improving intracranial elastance and compliance by causing water to flow from the brain s extracellular compartment into the vasculature, which decreases the intracranial volume. 14 Laboratory Monitoring Ideally, arterial and venous access is obtained prior to initiation of cooling to allow ease of lab draws, continuous blood pressure monitoring, and optimal medication administration. Baseline labs are obtained with follow-up monitoring based on clinical indication and protocol. Our protocol requires that labs be performed upon admission, and at 4, 8, 12, 24, 48, and 72 hours. VOL. 30, NO. 6, NOVEMBER/DECEMBER
7 FIGURE 3 n Rewarming checklist. 376 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
8 FIGURE 4 n Family info sheet. VOL. 30, NO. 6, NOVEMBER/DECEMBER
9 FIGURE 4 n Family info sheet. (continued) (continued) 378 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
10 Thoresen recommends using the same treatment principles for all asphyxiated infants: first, correct the hypovolemia, then use inotropes (dopamine or dobutamine), depending on echocardiogram and whether the cardiac contractility is normal or abnormal. 17 Hematology Coagulopathy may be induced by hypothermia, and a platelet count of greater than 100,000 should be maintained to compensate for decreased platelet function. Patients on this protocol may require transfusions of fresh frozen plasma, cryoprecipitate, and platelets. Infection Blood cultures are obtained. Antibiotics are continued for a minimum of 72 hours during the cooling period to provide prophylaxis in the setting of relative immune dysfunction. At the completion of 72 hours of cooling, antibiotics are discontinued if there are no obvious signs of infection. Skin Infants are repositioned every two hours, and a skin assessment is performed every four hours. Skin is monitored for color, perfusion, skin breakdown, or signs of subcutaneous fat necrosis. 2 4 Subcutaneous fat necrosis is characterized by induration, erythematous nodules and plaques over bony prominences such as back, arms, buttocks, thighs and cheeks of full term newborns (p. 257). 18 Subcutaneous fat necrosis is rare but patients with HIE are at risk for developing this condition because of hypoxia and induced hypothermia. Of the 42 infants we have treated in our NICU with whole-body cooling, two developed some form of subcutaneous fat necrosis. These infants are presented with areas of redness, ill-defined erythema, bruise-like appearance, and the skin looked taut or shiny (Figure 2). Rewarming At the completion of 72-hour cooling period, rewarming is initiated and takes place over a period of 10 hours. 1 The esophageal probe remains in place until the patient returns to standard care to allow for continuous monitoring through the rewarming process (Figure 3). During rewarming, the nurse should monitor for seizures and hypotension. 17 Following the cooling period, blood work is done every 24 hours during the rewarming process; particular attention is paid to serum potassium levels because hyperkalemia may occur during rewarming and as needed based on the infant s clinical status. Family Support Support for the infant s family begins in the delivery room and continues throughout the hospitalization. It is a very difficult time for families who are appropriately shocked by the events surrounding the delivery of their infant and are worried about their child s outcome. Once an infant is identified as a potential candidate for the hypothermia protocol, VOL. 30, NO. 6, NOVEMBER/DECEMBER
11 the attending neonatologist talks to the infant s parents to explain the treatment and potential risks, and obtains consent for treatment. A parent information guide to hypothermia treatment is also given to parents (Figure 4). In our NICU, parents have unlimited visitation 24 hours a day and are kept updated on their infant s condition and plan. An open environment of communication is maintained with parents and they are encouraged to ask questions as they arise. Family meetings are scheduled as needed and include parents, nursing, neonatology, neurology, and social work staff. Less formal updates also take place continually at the bedside. It is important for the team to maintain an understanding of the difficulties facing parents of a baby with HIE. Most of these parents have encountered unexpected circumstances around the delivery of their child. Many of their expectations about the birth of their baby have been shattered and they are understandably in crisis. The parents are likely worried that their infant may not survive, and as in Baby M s case, the mother of the baby may also be critically ill. Parents are often concerned about their baby s long-term neurologic outcome, and this can be an overwhelming concern for them. These feelings are compounded by the fact that they are unable to hold their baby, and they may be further frightened by the infant s pale and cold appearance. Providing explanations and discussing concerns can be helpful in reducing parental anxiety and in helping them to maintain some sense of control over a very stressful situation. Encouraging involvement in their infant s care when feasible will reinforce their role as parents and as members of the team. It is our goal and expectation to support both the baby and parents during this difficult and challenging time. CONCLUSION Hypothermia is an evolving new therapy that requires strict protocols to safely and effectively care for infants with HIE. The nursing care is rigorous for these infants and the monitoring is quite specific. The collaborative effort among all disciplines is essential for seamless delivery of care. Because this is a new therapy, there is a need for further research regarding long-term neurodevelopmental outcomes for infants having undergone this therapy. Further research needs to be undertaken on the sedation management of these infants because this has not been studied thoroughly in this infant population. We must also be cautious in our explanations to families regarding what this treatment may be capable of for their infant, balancing honesty and sensitivity. REFERENCES 1. Gluckman, P. D., Wyatt, J. S., Azzopardi, D., Ballard, R., Edwards, A. D., Ferriero, D. M.,... Gunn, A. J. (2005). Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet, 365(9460), Shankaran, S., Laptook, A. R., Ehrenkranz, R. A., Tyson, J. E., McDonald, S. A., Donovan, E. F.,... Jobe, A. H. (2005). Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England Journal of Medicine, 353(15), Sarnat, H. B., & Sarnat, M. S. (1976). Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of Neurology, 33(10), Verklan, M. T., & Walden, M. (Eds.). (2004). Neurologic disorders. In Core curriculum for neonatal intensive care nursing (3rd ed., pp ). St. Louis, MO: Elsevier Saunders. 5. Lorek, A., Takei, Y., Cady, E. B., Wyatt, J. S., Penrice, J., Edwards, A. D.,... Reynolds, E. O. R. (1994). Delayed ( secondary ) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: Continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatric Research, 36(6), Laptook, A. R. (2009). Use of therapeutic hypothermia for term infants with hypoxic-ischemia encephalopathy. Pediatric Clinics of North America, 56(3), Johnston, M. V., Trescher, W. H., Ishida, A., & Nakajima, W. (2001). Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatric Research, 49(6), Siesjö, B. K., & Bengtsson, F. (1989). Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: A unifying hypothesis. Journal of Cerebral Blood Flow and Metabolism, 9(2), Perlman, J. M. (2006). Intervention strategies for neonatal hypoxicischemic cerebral injury. Clinical Therapeutics, 28(9), Azzopardi, D. V., Strohm, B., Edwards, A. D., Dyet, L., Halliday, H. L., Juszczak, E.,... Brocklehurst, P. (2009). Moderate hypothermia to treat perinatal asphyxial encephalopathy. The New England Journal of Medicine, 361(14), Higgins, R. D., Raju, T. N., Perlman, J., Azzopardi, D. V., Blackmon, L. R., Clark, R. H.,... Wyatt, J. (2006). Hypothermia and perinatal asphyxia: Executive summary of the National Institute of Child Health and Human Development workshop. The Journal of Pediatrics, 148(2), Volpe, J. J. (2001). Hypoxic-ischemic encephalopathy: Clinical aspects. In Neurology of the Newborn (4th ed., pp ). Philadelphia, PA: W. B. Saunders. 13. Curley, M. A., Harris, S. K., Fraser, K. A., Johnson, R. A., & Arnold, J. H. (2006). State Behavioral Scale: A sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatric Critical Care Medicine, 7(2), Raslan, A., & Bhardwaj, A. (2007). Medical management of cerebral edema. Neurosurgical Focus, 22(5), E Crowther, C. A., Hiller, J. E., Doyle, L. W., & Haslam, R. R. (2003). Effect of magnesium sulfate given for neuroprotection before preterm birth: A randomized controlled trial. The Journal of the American Medical Association, 290(20), Polderman, K. H. (2004). Application of therapeutic hypothermia in the intensive care unit. Opportunities and pitfalls of a promising treatment modality Part 2: Practical aspects and side effects. Intensive Care Medicine, 30(5), Thoresen, M. (2008). Supportive care during neuroprotective hypothermia in the term newborn: Adverse effects and their prevention. Clinics in Perinatology, 35(4), Tran, J. T., & Sheth, A. P. (2003). Complications of subcutaneous fat necrosis of the newborn: A case report and review of the literature. Pediatric Dermatology, 20(3), For further information, please contact: Denise M. Casey, RN, MS, CCRN, CPNP denise.casey@childrens.harvard.edu 380 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
12 Neutral Head Positioning in Premature Infants for Intraventricular Hemorrhage Prevention: An Evidence-Based Review Sheila Malusky, DNP, RN, NNP-BC Ann Donze, MSN, RN, NNP-BC In the United States each year, approximately 57,000 infants are born prematurely. With the advancement of neonatal medicine during the past several decades, including improved methods of mechanical ventilation and the development of total parenteral nutrition (TPN) for neonates, even extremely low birth weight (ELBW) infants are now living longer and surviving. 1 Of these infants, percent of them will develop an intraventricular hemorrhage (IVH), 2 with the incidence being inversely proportional to gestational age. 3 The total financial cost that is estimated for these premature births is $26 billion or $51,600 for each individual premature birth. These costs include medical care, delivery costs, early intervention services, educational services, and lost family income. 4 Additionally, the average cost of an IVH adds another $53,600 to the cost of the initial hospitalization. 5 But the costs of IVH go far beyond the impact of the Disclosure The author discloses no relevant financial interest or affiliations with any commercial interests. Accepted for publication May injury in the individual and the financial burden to care for these babies. The impact can also be devastating to the family who has the responsibility of caring for a disabled child. The Abs t r a c t lifelong commitments to care for these individuals tax the family structure, family resiliency, and bring about the additional need for community support. There has been a multitude of research into the prevention of IVH in premature infants. 6 Some of these studies examine prenatal factors such as antenatal steroid use. 7 Other studies have focused on antenatal factors such as delivery room resuscitation methods. 8 Still other studies have focused on neonatal prevention methods such as pharmacologic interventions and neonatal care management methods. 9 One neonatal care management activity that has been examined in association with IVH prevention is infant head positioning. First studied in adult patients, cerebral blood flow changes in response to head position were also examined in neonates beginning in the 1980s The purpose of this article is to review current evidence on midline head positioning in the prevention of IVH. The goal With the advancement of neonatal medicine during the past several decades, premature and critically ill infants are living past the neonatal period and surviving. The survival of these infants at smaller birth weights and younger gestational ages puts them at an increased risk for intraventricular hemorrhages (IVHs). Although shifts in cerebral perfusion have been linked to the development of these brain bleeds, many seemingly benign care activities have been linked to changes in cerebral blood flow patterns, possibly contributing to IVHs. The purpose of this article is to evaluate the current evidence to determine if the practice of midline positioning for infants born less than 32 weeks gestation for possible IVH prevention is supported by the literature. Many of the researchers involved in these studies attributed the consequential venule leakage of blood to occlusion of the jugular venous drainage system following a turn in the position of the head. Additionally, the articles that examined the connection between the effects of head tilting on brain hemodynamics attributed changes on the infants potential inability to autoregulate cerebral blood flow adequately. Both of these findings were linked to the development of IVHs. Based on physiologic data and expert opinion, the authors found support in the literature and recommend implementing a plan of care that includes midline head positioning for premature infants. VOL. 30, NO. 6, NOVEMBER/DECEMBER Springer Publishing Company
13 of this review was to answer the clinical practice question: In infants born at,32 weeks gestation, does midline head positioning along with head of bed tilted upward for the first 72 hours of life, when compared with standard positioning, result in a lower incidence of IVH? ETIOLOGY AND PATHOPHYSIOLOGY OF INTRAVENTRICULAR HEMORRHAGE Although this potentially devastating medical condition can occur at any age, premature infants are at an increased risk because of their immature brain vasculature and also their inability to autoregulate shifts in cerebral perfusion, described as a pressure-passive circulatory state. 13,14 Of the bleeds that occur, 90 percent will develop during the first 72 hours of life, a time when these infants are in their most critically ill state. 3 Although extreme prematurity and illness have been associated with shifts in brain perfusion, many seemingly benign care activities and environmental factors have also been shown to cause changes in cerebral blood flow patterns. 2,13 16 Although there are many risk factors associated with the development of an IVH, some of which are noted in Table 1, one of the most prominent risks is prematurity. 3,17 This increased risk is caused by the presence of the germinal matrix, a network of delicate blood vessels within the premature brain that usually involutes between 32 weeks and term gestation. 3 Within this region, the capillary venule juncture is the originating site of these hemorrhages. 3 The fragile blood vessels within the germinal matrix are easily ruptured with any rapid changes in the levels of cerebral perfusion, which may lead to bleeding into the brain tissue or ventricles. 18 The structure of the venous system in this area of the brain can also lead to IVH because the system has a U-shaped vessel pattern prone to venous congestion near the germinal matrix, again causing vessel damage and bleeding. A cranial ultrasound view of a neonate without IVH can be seen in Figure 1, whereas Figure 2 shows a drawing of the U-shaped vascular anatomy. TABLE 1 n Associated Risk Factors for IVH Antenatal Risk Factors Prematurity Maternal infection Maternal inflammatory responses Maternal hypertention Maternal bleeding disorders Absent maternal steroid administration maternal diabetes Placental insertion disorders Oligohydramnios Maternal alcohol use Maternal smoking Poor prenatal care Infertility treatments Out-born delivery and neonatal transport Initial resuscitation efforts Asynchronous ventilatory support High continuous airway pressure Rapid fluid administration Rapid alteration in blood pressure Hypotension Hypocarbia or hypercarbia Pneumothorax Asphyxia Hypernatremia Hypoglycemia Thrombocytopenia Patent ductus arteriosis Seizure activity Routine NICU care: Tracheal suctioning, excessive handling, noxious stimulation, painful procedures, stress Any disease process or care activity associated with alteration in cerebral perfusion. A factor unique to this population is the inability of premature infants to autoregulate cerebral perfusion in response to physiologic and positional changes. 13 Autoregulation is the ability of the body to maintain a constant blood flow to the brain despite cerebral perfusion. 19 Inconsistencies in cerebral blood flow patterns have been observed during routine critical care of the premature infant. 16 Impaired autoregulation can be markedly pronounced in infants who are sick or extremely premature. 14,20 The mechanism of action that has been postulated is that during head rotation to the side, an occlusion or obstruction of the jugular venous venule drainage system could occur on the ipsilateral side of the head. This is followed by increased venous congestion in this area leading to vessel rupture. Figure 1 n Cranial ultrasound of a neonate without IVH. 382 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
14 Figure 2 n U-shaped vascular anatomy. Terminal vein Internal cerebral vein Medullary veins Vein of Golen Choroidal vein Thalamostriate vein By maintaining a neutral head position, it is theorized that venous obstruction could possibly be avoided, potentially preventing IVH caused by head position. INTRAVENTRICULAR HEMORRHAGE SEVERITY AND OUTCOMES Diagnosed by cranial ultrasound, an IVH can occur following serious illness of the infant or after no apparent insult at all. During the first four to five days of life, a time when premature infants are in their most critical state, 95 percent of all cases of IVH will develop. 21 Depending on the severity, some of these bleeds may be accompanied by an acute deterioration in clinical status, whereas some infants may show few symptoms until they reach school age. 22 Overall, Paige and Carney found the associated sequelae of IVH to range from minimally distinguishable effects (50 percent), to abnormal neurologic outcomes (20 30 percent), to an increase in incidence of mortality (10 30 percent). 22 Although the IVH has been broken down into classifications by grade, Volpe later developed an IVH labeling system based on a description of the neurologic pathology. 3,23,24 This was caused by some abnormalities that can occur that do not fit into the original classifications. Such cases could include isolated ventriculomegaly or instances of white matter injury that are not associated with IVH. 25 Volpe also advocated for the cessation of labeling white matter brain tissue hemorrhages, or parenchymal hemorrhages, Grade IV IVH. 3 This was caused by the origination of these bleeds, at times, occurring secondary to parenchymal infarction and not always being associated with bleeding within the ventricles. 25 The Papile IVH grading system also does not describe the site of origin for the IVH. 25 Most often, intracranial hemorrhages in premature infants originate in the germinal matrix, a highly vascular and fragile region in the premature infant s brain. Alternately, the choroid plexus can also be an origination site, although this is more common in term infants. 25,26 Regardless of site of origination or description of pathology, many bleeds are now being associated with the level of neurodevelopmental outcomes risk. 25 Low-grade risk bleeds are associated with Grade I and II hemorrhages. High-grade risk bleeds are associated with Grades III and IV hemorrhages. See Table 2 for a description of IVHs and statistics for very low birth weight (VLBW [,1,500 g]) infants. Because an IVH can be such a devastating event, there is a critical need to identify strategies to reduce IVH in this population. One proposed strategy is the use of midline positioning during the first 72 hours of life, a time when 90 percent of all IVH occur. 3 DEVELOPING THE CLINICAL PRACTICE QUESTION USING PICO FORMAT When completing an evidence-based review of the literature, developing a question that helps focus the literature search is the first step. PICO is a mnemonic term used to focus and describe each part of the clinical practice question: P is the population, I is the intervention, C is the comparison group, and O is the outcome. 33 The PICO question focuses on an intervention that is compared to the current standard of care. If there is evidence that an intervention may provide benefit without harm, an intervention may be implemented into practice. The PICO or clinical practice question that prompted this search was: Do infants born at 32 weeks gestation who are positioned with head in midline position and head of bed tilted upward for the first 72 hours of life have a lower incidence of IVH than infants who receive standard positioning? P 5 In infants born at 32 weeks gestation I 5 does midline head positioning along with the head of bed tilted upward for the first 72 hours of life C 5 compared with standard positioning O 5 result in a lower incidence of IVH LITERATURE SEARCH STRATEGIES A literature search using the keywords intracranial hemorrhages, cerebral ventricles, infant, and newborn was performed using Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Google Scholar. The search years were limited from 1980 to On the first search, 935 articles were found. The search was then limited to articles in English, human subjects, and infants from birth to 23 months of age. This search yielded 800 articles. The search was then altered to infant, premature, and cerebral ventricles and hemorrhage with the same limitations of English, human subjects, and infants from birth to 23 months of age. The reason that older research was admitted into this search is that this early time represents the initial study into IVH and premature infants. Many of the positioning studies were conducted during the 1980s VOL. 30, NO. 6, NOVEMBER/DECEMBER
15 and have not been repeated. The search then yielded 189 articles. When the term prevention was added to the search, 77 articles were found that most appropriately fit the clinical question. Following this, a search of the Cochrane Systematic Review Database was completed. Although many reviews on IVH prevention were present, the reviews focused on the medical management of IVH, such as medication administration. There was one review regarding developmental care and the prevention of morbidities, but no systematic review of nursing care activities or positioning was found. 34 Of the 77 articles reviewed, organization of the rest of the review consists of an evaluation of 11 articles. These articles most appropriately answered the review question regarding positioning the premature infant and IVH prevention. The other 66 articles that were discarded did not address research relating to positioning and IVH occurrence in the premature neonate. The following discussion synthesizes the evidence collected. Table 2 n Intraventricular Hemorrhages Papile s Classification by Severity Volpe s Classification: Description by Pathology Occurrence Rate Morbidity and Neurologic Outcomes Mortality Progression of Ventricular Dilatation Grade I: least severe mild IVH See Figure 3 Subependymal hemorrhage (SEH) also called germinal matrix hemorrhage (GMH) 25% 30% for Grades I II 27 Ten percent motor disability. This rate is comparable to premature infants without documented hemorrhage. 24 5% 3 4% 28 Grade II: considered mild-to-moderate IVH See Figure 4 Intraventricular hemorrhage (IVH) alternately called a SEH with progression into the lateral ventricles by,50% without dilatation. Most infants with Grade II IVH face the same neurologic outcomes associated with Grade I IVH, although the extent of bleed can lead to ventricular dilatation. 18 Patra et al. found significantly poorer neurodevelopmental outcomes in extremely low birth weight (ELBW) infants (,1,000 g) with Grades I II IVH at 20 months corrected age. This includes up to 15% of these ELBW infants with Grades I II IVH who develop cerebral palsy (CP) and 9% who develop deafness % 3 12% 28 Grade III: considered moderate-to-severe IVH IVH with ventricular dilatation SEH with progression into the lateral ventricles by.50% and/ or with dilatation of ventricles. 10% 12% for Grades III IV 29 Ventricular dilatation can result when blood blocks the cerebrospinal fluid pathway, leading to progressive ventricular dilatation and increased intracranial pressure. The morbidity related to posthemorrhagic hydrocephalus is significant, with up to 90% of these infants having some degree of neuromotor deficits and 25% with visual and auditory impairments. 30 In total, 76% of these infants will have pronounced disability and 56% have multiple impairments. 28 Fifty percent of these children will require some special education and enrichment programs % 3 74% 28 Grade IV: considered severe IVH See Figure 5 Intraparenchymal hemorrhage (IPH) hemorrhagic infarct into the white brain matter Generally unilateral with the prognosis most often associated with poor motor deficits as well as significant cognitive impairments. 24 Classified as the most severe IVH, many infants do not survive. 50% 3 71% 28 Periventricular leukomalacia (PVL) parenchymal, or white brain matter, necrosis often occurring following Grade IV IVH or a parenchymal infarct. Ten percent very low birth weight (VLBW) infants with PVL will develop CP with spastic diplegia and 50% will develop cognitive and behavioral deficits. 31, NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
16 Figure 3 n Ultrasound of Grade I IVH. Figure 4 n Ultrasound of Grade II IVH. Figure 5 n Ultrasound of Grade IV IVH. VOL. 30, NO. 6, NOVEMBER/DECEMBER
17 SYNTHESIS AND SUMMARY Details of all appraised studies can be found in the Appendix. The Five Strengths of Evidence 35 Type 1: strong evidence from at least one systematic review of multiple, well-designed randomized, controlled trials. Type 2: strong evidence from at least one properly designed randomized, controlled trial of appropriate size. Type 3: evidence from well-designed trials without randomization, single group pre post, cohort, time series, or matched case-control studies. Type 4: evidence from well-designed, nonexperimental studies from more than one center or research group. Type 5: opinions of respected authorities (based on clinical evidence), descriptive studies, or reports of expert committees. There was no meta-analysis or randomized controlled trials. The studies included nine predesigns and postdesigns, two repeated measures design, and one expert review panel report. One of the studies used both a premethodology and postmethodology as well as a repeated measure design, totaling 11 reviewed articles. 36 When synthesizing the evidence gathered regarding the positioning of premature infants and the potential effects of these positions on cerebral hemodynamics, the information gathered was analyzed following the PICO format. The purpose of this analysis was to determine whether the studies reviewed were homogenous. This is an important step in evaluating whether the study similarities lend to the pooling of evidence that may support a practice change. First, did all of the studies ask the same question? Not all of them. Although the articles were included in the review because of similar subject matter, the focus of some of these articles was not exactly homogenous. Eight of the articles studied changes in cerebral hemodynamic and two studied changes in intracranial pressure (ICP) in response to position changes. The final article gave expert opinion about infant positioning after benchmarking hospitals with low IVH rates. Next, the articles were examined to determine the homogeneity of the P, or population. Of the articles reviewed, nine studies examined preterm infants. A tenth article that examined full-term infants was also included because this study is often cited as a seminal article that examines changes in neonatal cerebral hemodynamic in response to position change. 11 Of the articles examined, there was a wide variation in gestational age, weight, postnatal age, and level of illness. These infants ranged from the most premature and critically ill infants to infants who were described by the authors as healthy premature infants. Because of the profoundly wide variation in levels of health and gestational age of the subjects, comparing outcomes for infants in this review was difficult. Additionally, none of these studies strictly examined the neonates during their first 72 hours of life, a time when most IVHs occur. 32 Next, the articles were examined to determine the homogeneity of the I, or interventions. The interventions reported for these studies centered on positioning or altering position of the newborn infant. Some of these articles focused on the effects of midline head positioning, whereas others looked at the effects of changes in the tilting position. Regardless of particular position change that each individual study examined, they all evaluated changes in cerebral hemodynamics or ICP in response to position change. Following the positioning interventions, an evaluation of cerebral blood flow or ICP was assessed. There were various instruments used to assess these measures. In the 10 articles with patient enrollment, 5 used near infrared spectroscopy (NIRS), 4 used ultrasound, and 2 used a transfontanel pressure transducer. The transfontanel pressure transducers were used in the first studies, followed by the use of ultrasound, and then NIRS methodology because technology has progressed. A brief description of the instrumentation used for the evaluations is listed in Table 3. The articles were then examined to determine the homogeneity of the O, or outcomes. When evaluating the outcomes reported in these studies, the groups could be separated into two divisions: those that evaluated the effects of head or body position changes and those that evaluated the effects of tilting. In those that evaluated head and body changes, several outcome measures were used and not all assessed the incidence of IVH, making the evaluation of the intervention difficult. In general, these articles demonstrated alterations in cerebral blood flow following position changes. One study found a significant decrease in tissue hemoglobin index and tissue oxygen index during head rotation in infants,26 weeks gestation. 37 A second study found a significant increase in cerebral blood volume (CBV) during 90-degree head rotation, which was pronounced in infants,1,200 g. 38 The third study found cerebral blood flow velocities were significantly higher in the TABLE 3 n Definition of Study Instruments Ultrasonography Trans-fontanel Pressure Transducer Near Infrared Spectroscopy (NIRS) A non-invasive radiological exam that uses a transducer to pass sound waves through soft tissue and fluid. A picture is produced when the returning echo bounces off internal structures and returns to the transducer. The resulting picture is formed when the data entered into the transducer is read by the ultrasound computer and is analyzed to produce real-time images. 44 A non-invasive device that measures intracranial pressure through a probe secured over the anterior fontanel. 10 A non-invasive neuro-imaging device that uses near-infrared light to evaluate real-time tissue oxygen and blood volume to interpret blood hemodynamics of the brain NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
18 supine position at one month of age during evaluation, and vertebral arterial flows in prone position were decreased. 39 A fourth study found jugular blood flow was decreased during 90-degree head rotation. 11 The three studies that evaluated ICP all found a significant decrease in ICP, with the head in midline position and the head of the bed elevated The second group of studies examined the effects of tilting. These studies evaluated CBV and found a significant increase with the head lowered in a dependent position. These studies also found significant alterations in CBV in response to tilting, especially in preterm or brain-injured infants. Another study evaluated for biphasic responses in cerebral blood flow velocities, demonstrating autoregulatory responses in preterm infants, and found significantly more reliable responses were elicited as gestational age increased but IVH outcome was not evaluated. 36 A complete description of the findings of these articles can be found in the Appendix. Although the final article was not a research study, information from such an article can still be valuable. This article by Carteaux and colleagues detailed the work of a multidisciplinary focus group that was formed to complete an evidence-based literature review, benchmarking activities, and expert committee review. The purpose of this evaluation was to identify potentially better practices (PBPs) that could lead to the reduction of IVH and periventricular leukomalacia (PVL) in VLBW and premature infants. 6 The group identified benchmark NICUs with the lowest incidence of IVH reported to the Vermont Oxford Network (VON). The VON, a consortium of more than 700 NICUs associated with the improvement of safety and quality of newborn care, formed a focus group to evaluate methods for reducing the incidence of IVHs in premature infants. 40 It then developed an NICU practice questionnaire for VON sites. Four sites with low incidence of intracranial hemorrhage were identified and used as a benchmark for clinical practice. Specific clinical practices at these sites were described through structured questionnaires and site visits. The information obtained from these sites was analyzed and used to help identify NICU practices that might be related to IVH prevention. A complete literature review was then completed on each of these NICU practices. Following the benchmarking and literature review, the group identified a final list of ten recommended practices for NICUs that were PBPs, which could help with the reduction of incidence of IVH. Use of midline positioning and bed elevation of 30 degrees was identified as PBPs. DISCUSSION The decision to make a practice change should be based on the grade, quality, and strength of the evidence after synthesizing all data. Of the 11 articles reviewed, 10 of them involved clinical trials. Of these, all were Type 3 evidence with a quasi-experimental, nonrandomized convenience sample design with five studies including a control group. All of the studies were small and no power analysis was commented upon. Many of the studies were older, and technology and medical practices may have changed. Finally, all nine studies that examined preterm infants would be considered stable neonates and may not reflect the group of patients who are at greatest risk for IVH, those who are severely premature during their first 72 hours of life. The final article, based on expert opinion, benchmarking, and review of the literature, reviewed multiple practices and their recommendations varied between level, depending on the strength of the evidence. 6 The level of evidence for the recommendations on head positioning by VON was IV and VI. 6 According to the Muir Gray schema of evidence that was used to rate the evidence, IV is defined as a welldesigned, nonexperimental study, and VI is defined as an evidence supported by casual theory of disease. 35 Although there are limitations to these 11 studies, they may still, however, provide some benefit to our patient population. Many investigators included discussion about how their study results could be interpreted. The expert opinion of many of the researchers attribute changes in cerebral oxygenation, increased CBV, and/or increased ICP to occlusion of the jugular venous drainage system following a turn in the position of the head. The proposed consequential backup of cerebral blood is an ongoing theme presented by many of the authors, although jugular blood flow was only analyzed in one study. 6,11,12,37 39 These authors then speculate that the risk of IVH was increased because of ruptures in the cerebral venous venule drainage system following blood accumulation secondary to the occlusion. Although jugular obstruction studies during head rotation have been documented in the adult population, results in the neonatal population are limited and similar results in this population are theorized. 11,38 The researchers concluded that venous obstruction caused by head position could be detrimental to these infants who are already at increased risk for IVH. A second observation that was discussed by the investigators was the connection between the effects of head tilting on brain hemodynamics. Of the four studies that examined tilting, the experts attributed the results, an increase in CBV and/or increased ICP, to the infants potential inability to autoregulate cerebral blood flow adequately. Because these findings were significantly increased in infants with PVL, brain injury, and those who were premature, the authors further speculated these findings may put these infants at a greater risk for developing IVH. All of the methods of measurement used throughout the studies showed a difference in cerebral hemodynamics when positioning was changed. These findings were present in the head rotation studies as well as the tilting ones. These differences were most marked in the ELBW infant. Present studies, unfortunately, only include outcome measures that were short-term and any changes in practice should include long-term outcome measurement. The investigators who completed the expert review 6 did discuss the lack of high-level evidence when evaluating infant positioning. 6 The decision to recommend the use of neutral head position was based on the potential benefits of this VOL. 30, NO. 6, NOVEMBER/DECEMBER
19 practice and the lack of harm. Based on these recommendations, many NICUs currently use these positioning practices. IMPLEMENTATION OF NEUTRAL HEAD POSITIONING The decision to implement midline/neutral head positioning and a 30-degree elevation in the HB in infants less than 32 weeks gestation for the first 72 hours of life can be recommended, at this point, based on physiologic data and the views of experts in the field. Furthermore, there have not been any adverse consequences identified when implementing these positioning changes. For units considering a change in neonatal positioning practices for potential IVH prevention such as the implementation of neutral head positioning, there are several important steps to facilitate change. These steps should only occur following critical appraisal of the evidence. Gather the Stakeholders The stakeholders incorporated in this practice change should include registered nurses who care for the infants and understand the fine nuances of caring for the infants. The physicians, advance practice nurses (APNs), and bedside staff nurses would be essential in identifying infants appropriate for this practice change and for ordering this care practice. Physical therapists would be needed to assist with positioning and obtaining positioning devices needed on an individual bases. Respiratory therapists would be important to help position infants in neutral head positioning while still receiving the necessary respiratory support. Specialized equipment may be needed to positioning these infants midline, especially if the infant is on an oscillating ventilator. Pharmacists input to maintain patient comfort may also be a necessity. Our unit has developed a multidisciplinary IVH prevention taskforce comprised of representation from all the mentioned stakeholders to evaluate the evidence and provide recommendations. Create a Detailed Action Plan The first steps to creating an action plan would be to use the stakeholders to discuss potential obstructions, plan nursing and medical team education, and plan parent education. An audit of current positioning practices can help the team assess the degree of change that is being proposed for the unit. This can help the team determine the amount of time this change may require and the amount of support needed to be successful with this change. Planning to assess outcome measures (IVH rates) prior to the practice change is important in evaluating if the practice change of midline positioning has made a difference in this patient population. Our unit has done this step in the IVH prevention taskforce. Assessing Environmental Readiness The stakeholders must evaluate and address the organization, environment, and whether it is supportive to this evidence-based practice (EBP) change at this time. The three areas to assess are organizational culture, organizational infrastructure, and organizational resources. 41 In assessing organizational culture, one would assess the values of the unit. Do the caregivers understand the importance of implementing evidence-based recommendations? Do the nurses and other caregivers understand the potential benefits and how to achieve neutral head positioning? Does the practice change support family-centered care, an important value to NICU caregivers? Does this practice change support developmental care practices, another important care value in NICUs? Have the team given input in identifying potential barriers to this practice change, including nursing barriers to care and equipment needs? In assessing organizational infrastructure, one would assess the organization s willingness to support evidence-based care practice changes. Does the unit have goals that state support of practices based on the most current evidence? 41 Has the organization made efforts to hire or train employees in the evidence-based process? The organizational resource assessment evaluates whether an organization is willing to support the man-hours needed to evaluate the evidence and implement these changes. Can the organization financially support the EBP process, which includes the evaluation, implementation, and assessment phase of this process? Is the organization willing to supply equipment and training time? If the organization cannot give full financial support for the practice change, are they willing to support further planning to identify creative alternates that support the evidence? Use Multiple Implementation Strategies When implementing a practice change, multiple implementation strategies can help ensure success. Does the education plan include multiple methods to reach the caregiving team, such as demonstrations of midline positioning and posters with pictures? Does the education presentations included multiple levels of medical knowledge, with the difference being parental education being easier to understand for the layperson and the medical education, including a more pathophysiologic approach to address the caregivers understanding of the rationale for change? When implementing these changes, are there multiple approaches to support the bedside nurses such as the team members available to address technical questions and nursing champions to encourage practice change use through exemplifying the practice? EVALUATING OUTCOMES Process Outcomes Once the practice change has occurred, a method to assess the practice change is essential. Is the practice implemented as designed? Can the caregiving team describe midline positioning and demonstrate this practice change correctly? Is midline positioning being performed routinely? Have the caregiving team identified further barriers to midline positioning as the practice occurs on a routine basis? A quality assurance plan to assess that the practice change is being 388 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
20 executed regularly and correctly is essential. This is important because it assesses whether a practice change is truly being carried out as planned. A method to evaluate this could be the formation of positioning super users to audit patient positioning and evaluate the need for further education based on their findings. The identification of barriers and development of solutions to barriers is essential in the success of the implementation of this change. Clinical Outcomes The final step in this process is an evaluation of the clinical outcomes. Has this change made an impact? How do the current IVH rates compare with the rates prior to the practice change, as well as the rates of others such as NICUs? Can we follow long-term outcomes such as incidence of cognitive, behavioral, and physical disabilities? Future Direction Although the cause of IVH in premature infants may be multifactorial and complicated, many investigators are currently searching for prevention methods. Some neonatal units have adopted IVH prevention bundles or multiple care practice changes that can potentially reduce the incidence of IVH. 42 Regardless of whether a unit is looking to make several practice changes or just one change at a time, there is little dispute that work toward a decrease in the incidence of IVH in premature infants should continue. Additionally, further research into neonatal positioning for IVH prevention should continue. Although the strength of evidence to support this practice change could be stronger, the decision to adopt this practice change should be evaluated and discussed in individual neonatal units. The adoption of the practice of midline positioning could still be recommended based on its potential benefits. With the increased survival of extremely premature infants, the incidence of IVH, a common neonatal morbidity, can be expected to rise proportionately. Although research into the prevention of this potentially devastating illness should continue, caregivers must continue to evaluate the literature in evaluation of their current practices. Because IVH can be so devastating to the infant, caregivers must strive to provide evidence-based care that can potentially prevent these occurrences. REFERENCES 1. Committee on Hospital Care, American Academy of Pediatrics. (2003). Family-centered care and the pediatrician s role. Pediatrics, 112(3 Pt. 1), McCrea, H. J., & Ment, L. R. (2008). The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clinics in Perinatology, 35(4), j.clp Volpe, J. J. (2008). Intracranial hemorrhage: Germinal matrix-intraventricular hemorrhage of the premature infant. In Neurology of the newborn (5th ed., pp ). Philadelphia, PA: Saunders Elsevier. 4. March of Dimes. (2009). About prematurity: The economic costs. Retrieved from 5. Russell, R. B., Green, N. S., Steiner, C. A., Meikle, S., Howse, J. L., Poschman, K.,... Petrini, J. R. (2007). Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics, 120(1), e1 e Carteaux, P., Cohen, H., Check, J., George, J., McKinley, P., Lewis, W.,... McConnell, C. (2003). Evaluation and development of potentially better practices for the prevention of brain hemorrhage and ischemic brain injury in very low birth weight infants. Pediatrics, 111(4 Pt. 2), e489 e Crowley, P. (2000). Prophylactic corticosteroids for preterm births. Cochrane Database of Systematic Reviews, (2), CD Kattwinkel, J., Niermeyer, S., Nadkarni, V., Tibballs, J., Phillips, B., Zideman, D.,... Osmond, M. (1999). Resuscitation of the newly born infant: An advisory statement from the Pediatric Working Group of the International Liaison Committee on Resuscitation. Resuscitation, 40(2), Vohr, B., & Ment, L. (1996). Intraventricular hemorrhage in the preterm infant. Early Human Development, 44(1), Emery, J. R., & Peabody, J. L. (1983). Head position affects intracranial pressure in newborn infants. The Journal of Pediatrics, 103(6), Cowan, F., & Thoresen, M. (1985). Changes in superior sagittal sinus blood velocities due to postural alterations and pressure on the head of the newborn infant. Pediatrics, 75(6), Goldberg, R. N., Joshi, A., Moscoso, P., & Castillo, T. (1983). The effect of head position on intracranial pressure in the neonate. Critical Care Medicine, 11(6), Owens, R. (2005). Intraventricular hemorrhage in the premature neonate. Neonatal Network, 24(3), Perlman, J. M. (2009). The relationship between systemic hemodynamic perturbations and periventricular-intraventricular hemorrhage A historical perspective. Seminars in Pediatric Neurology, 16(4), dx.doi.org/ /j.spen Ballabh, P. (2010). Intraventricular hemorrhage in premature infants: Mechanism of disease. Pediatric Research, 67(1), Limperopoulos, C., Gauvreau, K. K., O Leary, H., Moore, M., Bassan, H., Eichenwald, E. C.,... du Plessis, A. J. (2008). Cerebral hemodynamic changes during intensive care of preterm infants. Pediatrics, 122(5), e1006 e Vergani, P., Locatelli, A., Doria, V., Assi, F., Paterlini, G., Pezzullo, J. C., & Ghidini, A. (2004). Intraventricular hemorrhage and periventricular leukomalacia in preterm infants. Obstetrics and Gynecology, 104(2), Bloch, J. R. (2005). Antenatal events causing neonatal brain injury in premature infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 34(3), Kaiser, J. R., Gauss, C. H., & Williams, D. K. (2005). The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatric Research, 58(5), Wong, F. Y., Leung, T. S., Austin, T., Wilkinson, M., Meek, J. H., Wyatt, J. S., & Walker, A. M. (2008). Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics, 121(3), e604 e Linder, N., Haskin, O., Levit, O., Klinger, G., Prince, T., Naor, N.,... Sirota, L. (2003). Risk factors for intraventricular hemorrhage in very low birth weight premature infants: A retrospective case-control study. Pediatrics, 111(5 Pt. 1), e590 e Paige, P. L., & Carney, P. R. (2002). Neurological disorders. In G. B. Merenstein & S. L. Gardner (Eds.), Handbook of neonatal intensive care (5th ed., pp ). St. Louis, MO: Mosby. 23. Papile, L. A., Burstein, J., Burstein, R., & Koffler, H. (1978). Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. The Journal of Pediatrics, 92(4), Papile, L. A. (2002). Intracranial hemorrhage. In A. A. Fanaroff & R. J. Martin (Eds.), Neonatal-perinatal medicine: Diseases of the fetus and infant (7th ed., pp ). St. Louis, MO: Mosby. VOL. 30, NO. 6, NOVEMBER/DECEMBER
21 25. Vasileiadis, G. T. (2004). Grading intraventricular hemorrhage with no grades. Pediatrics, 113(4), Annibale, D. J. (2010). Periventricular hemorrhage-intraventricular hemorrhage. Medscape. Retrieved from article/ overview 27. Patra, K., Wilson-Costello, D., Taylor, H. G., Mercuri-Minich, N., & Hack, M. (2006). Grade I-II intraventricular hemorrhage in extremely low birth weight infants: Effects on neurodevelopment. The Journal of Pediatrics, 149(2), Murphy, B. P., Inder, T. E., Rooks, V., Taylor, G. A., Anderson, N. J., Mogridge, N.,... Volpe, J. J. (2002). Posthaemorrhagic ventricular dilatation in the premature infant: Natural history and predictors of outcome. Archives of Disease in Childhood. Fetal and Neonatal Edition, 87(1), F37 F Ment, L. R., Allen, W. C., Makuch, R. W., & Vohr, B. (2005). Grade 3 to 4 intraventricular hemorrhage and Bayley scores predict outcome. Pediatrics, 116(6), Chumas, P., Tyagi, A., & Livingston, J. (2001). Hydrocephalus what s new? Archives of Disease in Childhood. Fetal and Neonatal Edition, 85(3), F149 F Perlman, J. M. (1998). White matter injury in the preterm infant: An important determination of abnormal neurodevelopment outcome. Early Human Development, 53(2), Volpe, J. J. (2003). Cerebral white matter injury of the premature infantmore common than you think. Pediatrics, 112(1 Pt. 1), Melnyk, B. M., & Fineout-Overholt, E. (2005). Evidence-based practice in nursing and healthcare: A guide to best practice. Philadelphia, PA: Lippincott, Williams & Wilkins. 34. Symington, A., & Pinelli, J. (2003). Developmental care for promoting development and preventing morbidity in preterm infants. The Cochrane Library, (4). Retrieved from symington/symington.htm 35. Muir Gray, J. A. (1997). Evidence-based healthcare: How to make health policy and management decisions. London, United Kingdom: Churchill Livingstone. 36. Anthony, M. Y., Evans, D. H., & Levene, M. I. (1993). Neonatal cerebral blood flow velocity responses to changes in posture. Archives of Disease in Childhood, 69(3 Spec. No.), Ancora, G., Maranella, E., Aceti, A., Pierantoni, L., Grandi, S., Corvaglia, L., & Faldella, G. (2010). Effect of posture on brain hemodynamics in preterm newborns not mechanically ventilated. Neonatology, 97(3), Pellicer, A., Gayá, F., Madero, R., Quero, J., & Cabañas, F. (2002). Noninvasive continuous monitoring of the effects of head position on brain hemodynamics in ventilated infants. Pediatrics, 109(3), Eichler, F., Ipsiroglu, O., Arif, T., Popow, C., Heinzl, H., Urschitz, M., & Pollak, A. (2001). Position dependent changes of cerebral blood flow velocities in premature infants. European Journal of Pediatrics, 160(10), Vermont Oxford Network. (2008). What is the Vermont Oxford Network? In About Us. Retrieved from aspx?p5about/index.htm 41. Smith, J. R., & Donze, A. (2010). Assessing environmental readiness: First steps in developing an evidence-based practice implementation culture. The Journal of Perinatal & Neonatal Nursing, 24(1), Bedwell, S. M., Sekar, K. C., & Bright, B. C. (2010, May). Decrease in the incidence of intraventricular hemorrhages after the introduction of an IVH prevention bundle in the NICU. Presented at the Pediatric Academic Society Conference, Neonatal Neurology Platform, Vancouver, British Columbia, Canada. 43. Bozkurt, A., Rosen, A., Rosen, H., & Onaral, B. (2005). A portable near infrared spectroscopy system for bedside monitoring of newborn brain. Biomedical Engineering OnLine, 4(1), org/ / x U. S. Food and Drug Administration. (2008). Taking a close look at ultrasound. FDA Consumer Health Information. Retrieved from UCM pdf 45. Pichler, G., Urlesberger, B., Schmölzer, G., & Müller W. (2004). Effect of tilting on cerebral haemodynamics in preterm infants with periventricular leucencephalomalacia. Acta Paediatrica, 93(1), Pichler, G., van Boetzelar, M. C., Müller, W., & Urlesberger, B. (2001). Effect of tilting on cerebral hemodynamics in preterm and term infants. Biology of the Neonate, 80(3), Schrod, L., & Walter, J. (2002). Effect of head-up body tilt position on autonomic function and cerebral oxygenation in preterm infants. Biology of the Neonate, 81(4), About the Authors Sheila Malusky is a neonatal nurse practitioner with over 19 years of neonatal nursing care experience, currently working in the level III NICU at St. Louis Children s Hospital. Her interests include neonatal neurology and family-centered care. She would like to thank Dr. Lyla Lindholm at UMKC and St. Louis Children s Hospital NICU IVH Prevention Taskforce. Ms Malusky would especially like to thank Ms. Donze for her continuing mentorship and caring guidance. Ms. Malusky received her undergraduate degree from Maryville University in St. Louis, her graduate degree from Barnes-Jewish College of Nursing and Allied Health, and her doctoral degree from the University of Missouri, Kansas City. Ann Donze has over 34 years of experience in the NICU, with the past 15 years as a neonatal nurse practitioner. She has coordinated the neonatal nurse practitioner program at Barnes-Jewish College of Nursing and Allied Health. Ms. Donze currently cochairs the St. Louis Children s NICU research committee. Ms. Donze received her nursing diploma from Barnes School of Nursing, her undergraduate degree from Maryville University, and her graduate degree from Southern Illinois University-Edwardsville. For further information, please contact: Sheila Malusky, DNP, RN, NNP-BC sheilakm@bjc.org 390 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
22 appendix n Summary of Evidence 6,10 12,36 39,45 47 Article Citation Evidence Type Rating Strength/ Quality PICO Question Population Intervention Outcomes Study Limitations The effects of posture on brain hemodynamics in preterm newborns not mechanically ventilated Ancora et al., 2010 Effects of tilting on cerebral hemodynamics in preterm infants with periventricular leucencephalomalacia Pichler, Urlesberger, Schmolzer, & Muller, 2004 Quasi-experimental, non-randomized, convenience sample, withinsubject beforeand-after design with participants serving as their own controls Quasi-experimental, non-randomized convenience sample, with control group design. Level 2 Are there alterations in the brain homodynamics of preterm newborns following head and body position changes? The influence of gestational age, postnatal age, and nasal CPAP was also evaluated. Level 2 Following tilting bed up 20 degrees, are there any effects in the cerebral hemodynamics of preterm infants with or without PVL identified? Infants: 24 stable preterm infants. All with normal brain studies. Eleven on nasal CPAP. Mean GA: 27.5 weeks Mean Weight: 925 grams Mean Age: 10.3 days Infants: 35 stable preterm infants: Control group 25 infants with normal brain studies. Experimental group 10 infants with PVL. Mean GA: 30 weeks Mean Weight: 1225 grams Mean Age: 14 days Method: Infants were measured after placement in 6 different positions. Head: midline head position or head rotated 90 degrees to the side. Body: prone or supine. HOB: flat or elevated. Gestational age, CPAP and postnatal age were analyzed as independent variables. Study Instrument: Nearinfrared spectroscopy (NIRS). Biological Measures: Changes in tissue hemoglobin (hgb) index (nthi) and tissue oxygenation index (TOI) after posture variations. Method: Infants were measured before and after position changes by tilting bed up 20 degrees. Head and Body: Right lateral. HOB: From HOB flat to elevated. The 10 infants with PVL had 24 episodes of head tilted up 20 degrees for 30 minutes, and 19 episodes horizontal for 30 minutes. The 25 infants with PVL had 24 episodes of head tilted up 20 degrees for 30 minutes, and 23 episodes horizontal for 30 minutes. ANOVA was performed to evaluate tissue hemoglobin index (nthi) and tissue oxygenation index (TOI) in all positions. No significant changes in nthis or TOI for infants. 26 weeks gestation. nthi was significantly reduced in infant, 26 weeks during head rotation. nthi was in supine positions (both flat and at 30 degrees elevated) were significantly higher than supine position with head rotated to the side (p, 0.05). TOIs remained stable in all positions. CPAP and postnatal age were not significantly associatied with changes in nthi and TOI. Although both groups had significantly increased cerebral blood volume following a tilting downward maneuver, cerebral blood volume and cerebral hemoblobin oxygen index was significantly increased in infants with PVL compared to infants without PVL post tilting (p 0.01). Post tilting up, infants with PVL had a pronounced decrease in CBV and post tilting down had a pronounced increase in CBV. Small sample size with only 8 patients in the, 26 and, 27 weeks GA groups. Infants spent only 10 minutes in each position, which might not be long enough for full evaluation. No power analysis performed to determine number of subjects needed to reach statistical significance. Small sample size. Investigators noted that it was sometimes difficult to rule out artifacts. Infants in PVL group had significantly lower gestational age, birthweight, and weight. They had significantly higher PCA and chronological age. Only 30 minutes in each position and not all infants were able to complete the sequence. Study Instrument: NIRS No power analysis. (continued) VOL. 30, NO. 6, NOVEMBER/DECEMBER
23 appendix n Summary of Evidence (continued) Article Citation Evidence Type Rating Strength/ Quality PICO Question Population Intervention Outcomes Study Limitations Biological Measures: Measured Cerebral blood volume (CBV) and cerebral hemoglobin oxygen index (chbd). The analysis was completed using Student t-test for paired analysis and Mann- Whitney U-test using Statview software. Evaluation and development of potentially better practices for the prevention of brain hemorrhage and ischemic brain injury in very low birth weight infants. Carteaux et al., 2003 Evidence-based literature review, benchmarking, and expert committee review design Level 4 Expert Committee Report. Do they have a level for clinical practice guideline? This really fits that definition. Can an evaluation and development of potentially better practices for the prevention of brain hemorrhage and ischemic brain injury in very low birth weight infants be identified? Five Benchmark NICUs. Investigators also recorded EKG, pulse oximetry, capnography, and respiratory effort. Five NICUs who were VON members participated in a QI project to evaluate practices in benchmarked hospital s IVH prevention methods. They utilized benchmarking of practices in institutions with low incidence of IVH and PVL, systematic review of the literature, and expert consutation, the group then made recommendations. Ten potentially better practices were identified, including neutral head positioning and the use of developmental care strategies. Some of the PBPs were based on lower level evidence when no RCTs were available. Noninvasive continuous monitoring of the effects of head position on brain hemodynamics in ventilated infants. Pellicer, Gaya, Madero, Quero, & Cabanas, 2002 Quasi-experimental, non-randomized, convenience sample, withinsubject beforeand-after design with participants serving as their own controls. Level 2 Can the effects of head position on brain hemodynamics or changes in cerebral venous blood flow/ volume in ventilated infants be identified? Infants: 21 preterm infants. 13 on conventional ventilators, 8 on oscillators Mean GA: weeks Mean Weight: grams Mean Age: 5.8 days Method: Infants measured before and after being placed in multiple positions. Head: midline or head rotated 90 degrees to the side. Body: prone or supine. HOB: Flat or elevated 30 degrees. Infants measured every 10 minutes for 30 minutes in each position. Study Instrument: NIRS HUS was obtained after study to detect changes. Change in cerebral blood volume significantly increased with head turned 90 degrees. (p ). This change was most pronounced in infants, 1200 grams. There was also a significant change in cerebral blood flow relative to time spent in supine with head turned to side compared to head in midline (p ). There was no significant change in cerebral blood flow or any other physiologic variable: BP, oxygen saturation, PCO2. Small sample size. CBF measurements were unsuccessful in 9 infants. Tried to minimize bias by randomly assigning the starting position. Also all HUS and all NIR were read by the same investigator. Biological Measures: changes in cerebral blood volume ( CBV) and cerebral blood flow (CBF). (continued) 392 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
24 appendix n Summary of Evidence (continued) Article Citation Evidence Type Rating Strength/ Quality PICO Question Population Intervention Outcomes Study Limitations Effect of head-up body tilt position on autonomic function and cerebral oxygenation in preterm infants. Schrod & Walter, 2002 Position dependent changes of cerebral blood flow velocities in premature infants. Eichler et al., 2001 Quasi-experimental Level 2 In preterm infants, are there any negative effects of head elevated body tilt position (HETP) on systemic and cerebral oxygenation, circulation, and sympatheticvagal balance? Quasi-experimental, non-randomized convenience sample, nonequivocal control group beforeand-after design, and Repeatedmeasures design. Level 2 Can position dependent changes in cerebral blood flow velocities be identified in premature infants? Infants: 36 preterm infants Mean GA: weeks. Median GA 32.5 weeks. Mean Weight: grams. Median weight 1460 grams. Mean Age: 2 to 12 days of life. Infants: 23 stable preterm infants all with normal brain studies and none mechanically ventilated. Mean GA: 26.7 weeks Mean Weight: 1027 grams Mean Age: All infants were studies postnatal 3-5 days. Method: Preterm infants were measured after being placed in multiple head/body tilt positions. Head and Body: supine HOB: horizontal then elevated 30 degrees with each position lasting at least 20 minutes each. Infants were each measured 4 times in the various positions during study. Study Instrument: NIRs Biological Measure: Total cerebral hemoglobin content. Investigators also recorded EKG, Pulse oximetry, mean arterial pressure, and respiratory impedance curve. Method: Infants were measured before and after 4 position changes on 3 separate occasions: postnatal day 3-5, at one week, and at one month. Head: Centered when supine, turned to either side when supine. Body: prone or supine. HOB: Not discussed. Study Instrument: Ultrasound Biological Measures: Cerebral blood flow velocities of internal carotid artery, vertebral artery, and basilar artery. Continuous recordings revealed initial maximal fluctuations of total cerebral hemoglobin content (thb) up to 42% following HETP. After stabilization within several minutes, prolonged tilting did not result in any further significant changes of thb, heart rate, mean arterial pressure and oxygen saturation. Only preterm infants, or 51,500 grams showed a significant decrease of regional cerebral oxygen saturation (rso(2)) of about 2 5% from day 2 to 8, measured by pulseoxymetry. The study analysis was completed using SPSS statistic program. Nonparametric tests were applied. Cerebral blood flow velocities were significantly higher in the supine position at the one month of age evaluation. The researchers found a decrease in vertebral arterial flow in prone position, likely due to unilateral vessel compression. Birthweight and gestational age did not signfificantly influence cerebral blood flow. Small sample size. The investigators did not comment on direction of head (midline or rotated). Power analysis not completed. Small sample size. 6 infants lost to follow up at 10 days and an additonal infant lost to follow up at 1 month corrected age. Attempts to eliminate bias included the use of a second investigator blinded to infant positions. (continued) VOL. 30, NO. 6, NOVEMBER/DECEMBER
25 appendix n Summary of Evidence (continued) Article Citation Evidence Type Rating Strength/ Quality PICO Question Population Intervention Outcomes Study Limitations Effects of tilting on cerebral hemodynamics in preterm and term infants Pichler, Boetzelar, Muller, & Urlesberger, 2001 Quasi-experimental, non-randomized convenience sample, control group beforeand-after design. Level 2 Following tilting bed up 20 degrees, are there any effects in the cerebral hemodynamics of term and preterm infants identified? Thirty eight infants: 25 preterm, mean GA 33 weeks and mean weight 1899 grams. Term: 13 infants with mean GA 39 weeks and mean weight of 2969 grams. Method: Infants were measured before and after position changes by tilting bed up 20 degrees. Head and Body: Right lateral. HOB: From HOB flat to elevated. The preterm infants had 24 episodes of head tilted up 20 degrees for 30 minutes, and 23 episodes horizontal for 30 minutes. The term infants had 12 episodes of head tilted up 20 degrees for 30 minutes, and 10 episodes horizontal for 30 minutes. Study Instrument: NIRS Biological Measures: Measured Cerebral blood volume (CBV) and cerebral hemoglobin oxygen index (chbd). Although both groups had significantly increased cerebral blood volume following a tilting downward maneuver, cerebral blood volume (p 0.01) and cerebral hemoblobin oxygen index (p 0.001) were both significantly altered in preterm infants in both tilting up and tilting down maneuvers. There was also a correlation between postconceptional age and degree of change in cerebral blood volume (p 0.05). The analysis was completed using Student t-test for paired analysis and Mann-Whitney U-test using Statview software. Small sample size. Only 30 minutes in each position. No power analysis. Neonatal cerebral blood flow velocity responses to changes in posture Anthony, Evans, Levene, 1993 Quasi-experimental non randomized convenience sample using repeated measures design. Level 2 Crosssectional study using healthy full term infants as comparison group. In neonates, can cerebral blood flow changes, as demonstrated by uniphasic or biphasic responses, be elicited after changes in posture? Infants: 60 infants NICU Infants: 50, all with normal brain studies. Mean GA: 28 weeks Mean Weight: 1150 grams Mean Age: 1 week. Investigators also recorded EKG, Pulse oximetry, capnography, and respiratory effort. Methods: Infants measured before and after position changes by tilting. Head, Body, and HOB: In NICU infants, mattress position was altered for each testing period 20 degrees up and 20 degrees down from horizontal. Healthy infants were placed in a chair with a tiliting mechanism alternated between upright and lying back position. Study Instrument: Ultrasound Doppler probe was attached to each skin over temporal bone. Total of 501 episodes in 60 babies were monitored. Increasing percentage of biphasic responses in cerebral blood flow velocities as infant got older. Biphasic responses were considered seconday to autoregulatory responses. In the preterm infants, neither a high Pco2 nor a low MAP ( 25 mmhg) influenced the percentage of biphasic responses. Unclear if ICU infants were supine or prone and whether head was in midline. Concentrated only on tilting table. Focus of study was on autoregulation. Small study. (continued) 394 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
26 appendix n Summary of Evidence (continued) Article Citation Evidence Type Rating Strength/ Quality PICO Question Population Intervention Outcomes Study Limitations Healthy Term Infants: 10 Mean GA: 39 weeks Mean Weight: 3070 grams Mean Age: 1 week Biological Measures: Cerbral perfusion pressure was measure at each change and noted to be equivocal, no response, uniphasic (reflecting passive alteration in cerebral blood flow velocity), or biphasic (reflecting initial passive alteration, then active response to change in cerebral blood flow velocity). Unclear if investigators reading the CBVV. Changes in superior sagittal sinus blood velocities due to postural alterations and pressure on the head of the newborn infant. Cowen & Thoresen, 1985 Quasi-experimental, non-randomized convenience sample, control group beforeand-after design. Level 2 Are there changes in superior sagittal sinus blood velocities due to postural changes, jugular venous occlusion, and increased manual head pressure in the newborn? Infants: 18 term healthy newborns Mean GA: 39 weeks Mean Weight: 3683 grams Mean Age: 6 days Methods: Infants were measured after placement in 8 different positions. Infants were also measured while applying external head pressure and jugular venous occlusion. Head: midline head position, head rotated 90 degrees to the side, neck flexed and extended. Body: prone, supine, or lateral. HOB: flat throughout the study. Study Instrument: Ultrasound Biological Measures: Cerebral blood flow velocities (CBFV) of the superior sagittal sinus. Results revealed that 90 head turn occludes the jugular vein on the ipsilateral side and occlusion of venous flow on the contralateral side does not force blood drainage through the occlusion with subsequent increased cranial pressure changes. External head pressure also increases pressure and impedes venous drainage. Cerebral flood flow reduction: Supine Bilateral occlusion of right and left jugular 100% reduction in flow in all subjects Head turned 90 degrees to left Occlusion of ipsilateral jugular resulted in no change in flow in 25/26 subjects. Small sample size. Unclear regarding reliability of fontanometer used to accurately measure intracranial pressure. Investigators stated it was difficult to hold the hand held Doppler transducer in place during the study. Investigators were not blinded to infant position when interpreting results. Older study, but cited by many other studies. Occlusion of contralateral jugular resulted in 100% occlusion in 20/26 patients Increase in fontanel pressure correlated with decreased cerebral blood flow velocity. Correlation coefficient of (continued) VOL. 30, NO. 6, NOVEMBER/DECEMBER
27 appendix n Summary of Evidence (continued) Article Citation Evidence Type Rating Strength/ Quality PICO Question Population Intervention Outcomes Study Limitations Head pressure anterior posterior, lateral and occipital areas lead to variable results. Pressure on posterior fontanel always resulted in decreased cerebral blood flow velocity Head position affects intracranial pressure in newborn infants. Emery & Peabody, 1983 Quasi-experimental, non-randomized convenience sample, control group design. Level 2 In newborn infants, does head position affect intracranial pressure? Infants: 14 preterm infants 6 with a history of asphyxia and 8 without. Asphyxiated Infants: 6 Mean GA: 35 weeks Mean Age: 54 hours of life. Non- Asphyxiated Infants: 8 Mean GA: 36 weeks Mean Age: 43 hours of life. Method: Infants measured before and after 6 different position changes. Head: midline or 90 degrees head turn to right. Body: supine HOB: Flat, elevated 30 degrees or dependant 30 degrees. Study Instrument: Ultrasound and transfontanel pressure transducer. Measured at least 30 seconds in each position. Biological Measures: cerebral blood flow (CBf) and intracranial pressure (ICP). Statistical Analysis: Student paired t test using Bonferroni correction. Significant ICP in horizontal and dependant position when head turned to the right (p 0.001) for both groups. Significant increase in ICP with head midline and turned while in dependant position (p 0.001) for both groups. Significant decrease in ICP when head in midline and elevated (p 0.01) and even more decreased in asphyxiated infants (p 0.02). Small sample size Older study The effect of head position on intracranial pressure in the neonate was studied. Goldberg, Joshi, Moscoso, & Castillo, 1983 Quasi-experimental, non-randomized convenience sample design. Level 2 Are there any identified effects of head position on intracranial pressure in the neonate, as is found in the adult literature? Infants: 26 preterm infants. Mean GA: 33 weeks Mean Weight: 1497 grams Mean Age: 3 days, with a range of 1-10 days. Method: ICP measured every 1 minute for 10 minutes in each of 4 positions. A 4-minute stabilization period was given after every position change. Head: midline or turned to the right. Body: supine HOB: flat or elevated 30 degrees. Study Instrument: Ladd monitor to study intracranial pressure. The results suggest intracranial pressure was significantly lower in midline positions compared to positions with the head turned. The intracranial pressure was also significantly lower in patients whose head was elevated to 30 degrees. The position with the lowest measured intracranial pressure was head midline with head of bed elevated 30 degrees. Small sample size. Older study. Power not calculated. 10 minutes is a brief time period for study. To avoid bias, position order was randomly assigned. 396 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
28 Congenital Central Hypoventilation Syndrome and the PHOX2B Gene Mutation Tara L. Marion, RN, MSN, NNP-BC Wanda T. Bradshaw, BSN, MSN, PMC, DNP Congenital central hypoventilation syndrome (CCHS), a unique disorder of respiratory control, results from polyalanine repeat expansion mutations in the paired-like homeobox 2B (PHOX2B) gene in more than 90 percent of cases, and alternative PHOX2B mutations in remaining cases. It is a disorder in which affected individuals fail to breathe during sleep despite progressive hypercapnia and hypoxia. Diagnosis of CCHS is established by clinical findings and confirmatory molecular genetic testing. The diagnosis of CCHS depends on the documentation of hypoventilation during sleep in the absence of primary neuromuscular, lung, cardiac, or metabolic disease, or an identifiable brainstem lesion. Because many carriers of the disease CCHS are asymptomatic, it is difficult to determine the presence of the PHOX2B genetic mutation prior to birth. Most infants are diagnosed within the first 48 hours of life, after a dusky period during sleep. This is a critical time in the care of an infant with CCHS. Many times, a diagnosis is made after asphyxia has occurred and irreversible brain damage has been done. The purpose of this article is to provide an overview of CCHS and to discuss the implementation and value of genetic screening to assist in the diagnosis. All full-text English language articles from 1999 to 2010 were reviewed using CINAHL and PubMed. Accepted for publication March PATHOPHYSIOLOGY CCHS is a disorder of the central nervous system in which the automatic control of breathing is absent or impaired. Normally, the desire to breathe occurs with increasing carbon dioxide (CO 2 ) levels in the brain. In CCHS, a child s respiratory response to low blood oxygen (hypoxemia) or to Abs t r a c t CO 2 retention (hypercapnia) is typically delayed while they are awake, and absent, to varying degrees, during sleep, serious illness, and stress. Classic CCHS is characterized by adequate ventilation while the affected individual is awake, and by hypoventilation with normal respiratory rates and shallow breathing during sleep. More severely affected individuals hypoventilate when both awake and asleep. Both phenotypes present in the newborn period. 1 The autonomic nervous system controls breathing during quiet sleep. For this reason, ventilation is severely affected. Ventilation is better in active or rapid eye movement (REM) sleep when cortical input is at its greatest although still not normal. In the newborn period, many affected infants will not have the classically described sleep wakefulness differences; thus, they may appear to have chronic intermittent Congenital central hypoventilation syndrome (CCHS) is a rare syndrome of dysfunction of the autonomic nervous system characterized by a decreased response to hypercarbia. It is a disorder in which affected individuals fail to breathe during sleep despite progressive hypercapnia and hypoxia. Infants simply fall asleep and quit breathing. They are found by their parents or caregivers blue and lifeless. CCHS is an autosomal dominant disease. It has been linked with tumors of neural crest origin, segmental aganglionosis of the colon, and diffuse autonomic dysregulation but can occur alone. Discovery of the genetic link between the paired-like homeobox 2B (PHOX2B) genetic mutations and CCHS represents a breakthrough in the diagnosis of CCHS, association of mutated alleles with disease severity, and clues to the pathophysiology responsible for the disorder. Early genetic screening and intervention can provide the families of these infants with hope for achieving a normal life. Disclosure The author discloses no relevant financial interest or affiliations with any commercial interests. VOL. 30, NO. 6, NOVEMBER/DECEMBER Springer Publishing Company
29 duskiness, cyanosis, and measurable hypercapnia. 2 As oxygen saturations fall and carbon dioxide levels rise, affected infants demonstrate no increase in respiratory rate or effort and usually do not arouse or appear distressed. These children, if undetected or misdiagnosed, will present again at a later age with signs of right-sided heart failure and pulmonary hypertension from prolonged periods of hypoxia and hypercapnia. 2 Infants with CCHS typically present with apnea and respiratory arrest shortly after falling asleep. The primitive responses to hypoxia and hypercapnia that ordinarily stimulate respiratory drive in normal breathing are altered, and the infant fails to breathe effectively, or even struggle to do so. For this reason, patients with CCHS often do not display signs of respiratory distress, such as tachypnea, nasal flaring, or retractions. They do not struggle or gasp, they simply quit breathing. Without the aid of objective monitoring, hypoxia and hypercapnia are detected only at a late stage, after the onset of severe cyanosis and central nervous system depression. By this time, it can be too late, and irreversible neurologic damage may have been done. Children with CCHS often have physiologic and anatomic manifestations of a generalized autonomic nervous system dysfunction/ dysregulation (ANSD). Some may have altered development of neural crest-derived structures (i.e., Hirschsprung disease [HSCR]) and tumors of neural crest origin, including neuroblastoma, ganglioneuroma, and ganglioneuroblastoma. DIAGNOSING CONGENITAL CENTRAL HYPOVENTILATION SYNDROME AND IMPLICATIONS FOR CARE Clinical signs and symptoms and later performed molecular genetic testing confirm the diagnosis of CCHS. The diagnosis of CCHS depends on the documentation of hypoventilation during sleep in the absence of primary neuromuscular, lung, cardiac or metabolic disease, or an identifiable brainstem lesion. 3 Other reasons for hypoventilation during sleep, including infection, asphyxia, and trauma, must be delineated before the diagnosis of CCHS is made. The American Thoracic Society has issued a statement on the diagnosis of CCHS, and preparation of a revised statement is in process. 4 CCHS is diagnosed in individuals with the following: Hypoventilation with absent or negligible ventilatory sensitivity to hypercarbia and absent or variable ventilatory sensitivity to hypoxemia Generally adequate ventilation while awake, but hypoventilation with normal respiratory rate and shallow breathing (diminished tidal volume) during sleep, or Hypoventilation both while awake and asleep Absent perception of asphyxia (i.e., absent behavioral awareness of hypercarbia and hypoxemia) and absent arousal No evidence of primary neuromuscular, lung, or cardiac disease or identifiable brainstem lesion that might account for the constellation of symptoms The initial evaluation may include a detailed neurologic evaluation that may require a muscle biopsy, chest radiograph, fluoroscopy of the diaphragm, bronchoscopy, electrocardiogram, Holter recording, echocardiogram, and magnetic resonance imaging (MRI) of the brain and brainstem. Serum and urinary organic acids, amino acids, and carnitine levels should be obtained to rule out inborn errors of metabolism. Genetic testing is recommended for confirmatory diagnosis. CHARACTERISTIC FACIAL PHENOTYPES Todd and colleagues describe a characteristic facial phenotype in individuals with CCHS, which includes facies that are generally shorter and flatter, with significantly decreased upper-face height, excessive nasal tip protrusion, decreased nasolabial angle, short upper-lip height, and an inferior inflection of the lateral segment of vermillion border on the upper lip. 5 Using five variables to characterize facies (upper-lip height, biocular width, upper facial height, nasal tip protrusion, and the lip trait), Todd and colleagues found that 85.7 percent of individuals with CCHS and 82.2 percent of controls were correctly predicted. 5 These authors also found 86 percent of patients with CCHS could be identified by facial features (box shaped, generally shorter and flatter) and correctly distinguish them from 82 percent of controls. These authors also suggest that facial features may be distinctive in individuals with CCHS because the dorsal rhombencephalon and caudal midbrain give rise to neural crest tissue that develops into the facial structures, and the PHOX2B gene is expressed when these structures are developing in the embryo. 5 COMORBIDITIES AND CONGENITAL CENTRAL HYPOVENTILATION SYNDROME A case-control study found 89 percent of CCHS patients had cardiovascular symptoms (e.g., decreased heart rate variability, vasovagal syncope, cardiac dysrhythmias), 84 percent exhibited gastrointestinal autonomic symptoms (constipation, dysphagia, or gastroesophageal reflux), 82 percent demonstrated altered temperature regulation (lack of fever with infection), and 52 percent had altered pain perception as compared respectively with 5 percent, 5 percent, 0 percent, and 4 percent of the control subjects. Eighty-six percent had ophthalmologic abnormalities (sluggish or unreactive pupils, abnormal tearing, strabismus, anisocoria, miosis) versus 2 percent of the control group. 6 HSCR is characterized by the absence of intramural ganglion cells in the distal gut, which can result in bowel obstruction shortly after birth. A combination of CCHS and HSCR is a rare condition with variable severity. Both CCHS and HSCR are uncommon, and their co-occurrence may suggest a common etiology, probably involving a fault of neural crest development. 7 These two disorders are thought to be related within a class of diseases known as neurocristopathy. Neurocristopathies result from the malfunction of neural crest cells. 8 Examples of neurocristopathies include pheochromocytoma, neuroblastoma, medullary carcinoma of the thyroid, and carcinoid tumors NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
30 GENETIC TESTING AND CONGENITAL CENTRAL HYPOVENTILATION SYNDROME Prior to genetic testing and screening and discovery of the PHOX2B gene, CCHS was a diagnosis of exclusion, meaning all other causes of hypoventilation were ruled out, including X-linked myotubular myopathy, multiminicore disease, congenital myasthenic syndrome, altered airway or intrathoracic anatomy, diaphragmatic dysfunction, congenital cardiac disease, brainstem abnormality, and various metabolic diseases. This process was timely and costly and could have wasted valuable time in the management and treatment of the infant. PHOX2B mutations can now be detected by two methods: polymerase chain reaction (PCR) assay and sequencing microarray analysis. These tests are clinically available with the most cases being detected by PCR. Introduction of clinically available PHOX2B genetic testing allows for distinction between CCHS and other disorders in the differential diagnosis, such as severe prematurity, identifiable brainstem findings, asphyxia, infection, trauma, tumor, and infarction. 9,10 Because more than 90 percent of individuals with CCHS have a PHOX2B polyalanine expansion mutation and because PHOX2B polyalanine expansion testing (PCR) is a more sensitive test for detection of mosaicism, such testing should be performed first. Only if a PHOX2B polyalanine expansion mutation is not found in an individual with the CCHS phenotype should sequencing of the entire coding region and intron-exon boundaries of the PHOX2B gene be performed. 3 Polymerase Chain Reaction A PCR assay produces thousands to millions of copies of a small, specific DNA segment in a matter of hours. It is the first genetic test that should be carried out. A PCR should be performed on both the parents and the infant. It permits rapid detection of genetic aberrations. Each PCR assay is specific for one particular abnormality. In CCHS, the PCR assay is used to directly amplify and size the second polyalanine-coding triplet repeat sequence in exon 3 of the PHOX2B gene. This triplet repeat is expanded in most (about 90 percent) individuals with CCHS. The remaining individuals with CCHS will have mutations not detected by PCR. Those individuals with suspected CCHS and a negative PCR should have follow-up sequencing of the coding regions of the PHOX2B gene. It should be noted that 5 10 percent of CCHS patients inherited their PHOX2B mutation from a parent who has mosaicism or a lesser dose of the mutation, which explains why the parents are not affected with the CCHS phenotype. Because parents with PHOX2B mosaicism can pass the same PHOX2B mutation onto other children, it is necessary to test all parents of CCHS probands for mosaicism. The PCR assay PHOX2B Screening Test (and not sequencing) is the best available assay for identifying and quantifying mosaicism. Therefore, children suspected to have CCHS should ideally be tested by the PCR assay PHOX2B Screening Test, with follow-up sequencing if no mutation is found. All parents of children with identified polyalanine expansion mutations should be screened by the PCR assay PHOX2B Screening Test to determine mosaicism. 3 Sequence Analysis The process of sequence analysis evaluates the order of nucleotide bases, the structural units of both RNA and DNA. Three base pairs form a codon. The polyalanine repeat responsible for CCHS can be made up of any one of four codon combinations GCA, GCT, GCC, or GCG as each one encodes the amino acid alanine. The automated process of sequence analysis is used in cases of CCHS when the PCR is negative for the PHOX2B mutation. Sequence analysis of the entire coding region and intron-exon boundaries of PHOX2B can detect mutations in the 8 percent of individuals with the CCHS phenotype who do not have an expansion mutation. 3 LIMITATIONS OF THE SCIENCE Because many carriers of the disease CCHS are asymptomatic, it is difficult to determine the presence of the PHOX2B genetic mutation prior to birth. Most infants are diagnosed within the first 48 hours of life, after a dusky period following falling to sleep, and a possible anoxic event. Genetic testing for CCHS can be costly (U.S. $399). It is also important to note that results for a full gene analysis can take days, and specific mutation analysis days. This is a critical time in the care of an infant with CCHS. Prenatal diagnosis for pregnancies at increased risk is possible by analysis of DNA extracted from fetal cells obtained by amniocentesis usually performed at about weeks gestation or chorionic villus sampling (CVS) at about weeks gestation. The disease-causing allele of an affected family member must be identified before prenatal testing can be performed. CARE RECOMMENDATIONS FOR MEDICAL AND NURSING MANAGEMENT The treatment of CCHS is to ensure adequate ventilation for the patients who are unable to achieve adequate gas exchange during spontaneous breathing, or simply put, to breathe for them. It is important to note that simply providing supplemental oxygen to individuals with CCHS is not warranted. CCHS is a problem with ventilation. Thus, children with CCHS require home mechanical ventilation possibly for life. As children with CCHS do not usually have severe lung disease, they have many options for different techniques to provide mechanical assisted ventilatory support at home. 2 Infants are best ventilated with positive pressure ventilation (PPV) via tracheostomy and should have the tracheostomy placed soon after diagnosis. It is usually preferable to ventilate infants for 24 hours per day for some time, assuring that oxygenation and ventilation remain adequate to minimize possible damage to a developing brain. Passy-Muir (Irvine, California) one-way speaking valves and tracheostomy capping can be done while awake patients VOL. 30, NO. 6, NOVEMBER/DECEMBER
31 are older to allow for vocalization and use of the upper airway. Individuals with CCHS are not like other children on home mechanical ventilation. They must be managed with extreme vigilance because of their lack of objective or subjective responsivity to hypoxemia and hypercapnia. Transition to a home portable ventilator should be made in the hospital, and discharge planning for home is extensive. Arrangements for the home ventilator, a backup ventilator, hours per day of in-home nursing, supplemental oxygen, a pulse oximeter, and an end-tidal carbon dioxide monitor are required in the home prior to discharge. Responses to respiratory infections in children with CCHS differ from non-cchs ventilator-dependent children. Children with CCHS do not typically develop a fever, increase their respiratory rate or have dyspnea in response to pneumonia, or exhibit symptoms of severe hypoxia and/or hypercapnia. These limitations emphasize the importance of both objective pulse oximetry and end-tidal carbon dioxide monitoring, as well as highly skilled and consistent caretakers in the home. 2 All individuals with CCHS require assisted ventilation during sleep for life; thus, weaning these individuals off mechanical ventilation totally is not a realistic goal. For these children, ventilators are adjusted to provide end-tidal carbon dioxide values consistently between torr and oxygen saturation values greater than 95 percent. 2 Whereas it should be emphasized that children with CCHS are not candidates for weaning off mechanical-assisted ventilation while asleep, mobility and quality of life are maximized if the child can breathe unassisted for time while awake. Some CCHS children gradually develop the ability to breathe adequately during wakefulness. 9 Increased mobility and improved quality of life can be achieved by using diaphragm pacing. Diaphragm pacing entails surgical implantation of an electrode on the phrenic nerve, which is connected to a subcutaneous receiver. There is an external battery-operated transmitter and antenna placed on the skin over the receiver. The transmitter emits energy, similar to a radio transmission, which is converted into an electrical current by the receiver. This stimulates the phrenic nerve resulting in a diaphragmatic contraction. Settings on the transmitter include respiratory rate and electrical voltage and are adjusted to give enough tidal volume to allow for adequate oxygenation and ventilation. Therefore, diaphragm pacing is an attractive alternative mode of mechanically assisted ventilation for many patients with CCHS. 11 Diaphragm pacing is not typically recommended for the young child who requires only nighttime ventilatory support because the benefits do not outweigh the risks; however, for older adolescents and young adults, this could be an appropriate consideration. Education for parents and caretakers should include avoiding the use of alcohol and other impairing substances while caring for the child. Small toys and other items should be removed from within the child s reach because of their ability to occlude the tracheostomy. Education about their equipment and disease should begin as the child attains the ability to understand. CONCLUSION Since 2003 and the discovery of the PHOX2B gene, the treatment and genetic basis for CCHS has evolved new understanding and implications. Infants and children with CCHS are living healthier and less complicated lives because of the advances in care. With more genetic screening options being offered to families, and hopes of more to come, earlier detection of this life-altering disease is becoming a reality. Because CCHS is rare, it has not received research funding from private sources and must rely heavily on grants and donations from families. Although it is true that CCHS is a rare disease, the families that it affects are severely disrupted and rearranged. A national support group exists to assist families in dealing with this diagnosis and its implications. The CCHS Family Network works to arm families and individuals living with CCHS with information to make living with this diagnosis possible. Early detection of affected infants with CCHS is the single most important aspect of their care and management. The earlier intervention occurs, the better the outcome and prognosis of the child. Educating families about CCHS and providing them with resources in the community will arm them with a lifetime of knowledge and resourcefulness. REFERENCES 1. Weese-Mayer, D. E., Berry-Kravis, E. M., & Marazita, M. L. (2005). In pursuit (and discovery) of a genetic basis for congenital central hypoventilation syndrome. Respiratory Physiology & Neurobiology, 149(1 3), Chen, M. L., & Keens, T. G. (2004). Congenital central hypoventilation syndrome: Not just another rare disorder. Paediatric Respiratory Reviews, 5(3), Berry-Kravis, E. M., Zhou, L., Rand, C. M., & Weese-Mayer, D. E. (2006). Congenital central hypoventilation syndrome: PHOX2B mutations and phenotype. American Journal of Respiratory and Critical Care Medicine, 174(10), Weese-Mayer, D. E., Shannon, D. C., Keens, T. G., & Silvestri, J. M. (1999). American Thoracic Society Statement. Idiopathic congenital central hypoventilation syndrome. Diagnosis and management. American Journal of Respiratory and Critical Care Medicine, 160(1), pmid: Todd, E. S., Weinberg, S. M., Berry-Kravis, E. M., Silvestri, J. M., Kenny, A. S., Rand, C. M.,... Weese-Mayer, D. E. (2006). Facial phenotype in children and young adults with PHOX2B-determined congenital central hypoventilation syndrome: Quantitative pattern of dysmorphology. Pediatric Research, 59(1), /01.pdr d 6. Grigg-Damberger, M., & Wells, A. (2009). Central congenital hypoventilation syndrome: Changing face of a less mysterious but more complex genetic disorder. Seminars in Respiratory and Critical Care Medicine, 30(3), Ou-Yang, M. C., Yang, S. N., Hsu, Y. M., Ou-Yang, M. H., Haung, H. C., Lee, S. Y.,...Liu, C. A. (2007). Concomitant existence of total bowel aganglionosis and congenital central hypoventilation syndrome in a neonate with PHOX2B gene mutation. Journal of Pediatric Surgery, 42(2), e9 e Lai, D., & Schroer, B. (2008). Haddad syndrome: A case of an infant with central congenital hypoventilation syndrome and Hirschsprung disease. Journal of Child Neurology, 23(3), NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
32 9. Bachetti, T., Robbiano, A., Parodi, S., Matera, I., Merello, E., Capra, V.,... Ottonello, G. (2006). Brainstem anomalies in two patients affected by congenital central hypoventilation syndrome. American Journal of Respiratory and Critical Care Medicine, 174(6), doi.org/ /rccm cr 10. Bajaj, R., Smith, J., Trochet, D., Pitkin, J., Ouvrier, R., Graf, N.,... Kluckow, M. (2005). Congenital central hypoventilation syndrome and Hirschsprung s disease in an extremely preterm infant. Pediatrics. 115(6), e737 e Chen, M. L., Turkel, S. B., Jacobson, J. R., & Keens, T. G. (2006). Alcohol use in congenital central hypoventilation syndrome. Pediatric Pulmonology, 41(3), About the Author Tara L. Marion is a graduate of Duke University School of Nursing. She currently practices as a neonatal nurse practitioner with Brenner Children s Hospital, Wake Forest Baptist Health. She is the mother of Max, who is two and a half, and Georgia, who is four months. For further information, please contact: Tara L. Marion, RN, BSN, NNP-BC taramarion@hotmail.com VOL. 30, NO. 6, NOVEMBER/DECEMBER
33 .Lab Values. Neonatal alloimmune t h r o m b o c y t o p e n i a (NAIT) is a life-threatening disorder caused by fetomaternal platelet incompatibility analogous to that seen in rhesus (Rh) disease. In NAIT, maternal immunoglobulin G (IgG) antiplatelet antibodies cross the placenta, resulting in rapid destruction and removal of fetal platelets by the reticuloendothelial system. 1,2 Studies have shown that NAIT has an incidence of 1 of 1,000 live births, 3,4 with a mortality rate of percent 1 and the risk of long-term morbidities up to percent if intracranial hemorrhage (ICH) occurs. 3,5 This column will discuss the pathophysiology, differential diagnosis, morbidities, and treatment of NAIT and conclude with a relevant case study. PATHOPHYSIOLOGY Platelets are small cell fragments of very large bone marrow cells called megakaryocytes. 6 Platelets normally live for 10 days and then are removed by the spleen and liver. Neonatal thrombocytopenia historically is defined as a platelet count of less than 150, However, in recent studies, neonatal thrombocytopenia has been defined as a platelet count less than or equal to 123,000, 7 with severe neonatal thrombocytopenia as a platelet count less than 50,000. 7,8 NAIT subsequently occurs when the mother produces antiplatelet antibodies to the fetal platelets similar to what is seen with hemolytic disease of the newborn (HDN). Unlike HDN, though, NAIT can occur in the first pregnancy; in fact, 50 percent of all NAIT cases are seen in the first pregnancy. 1 This is because immunoglobulin G (IgG) is the only maternal antibody that crosses the normal placenta as early as weeks of gestation. At the same time, platelet antigens are seen in the fetus by 18 weeks of gestation, setting the stage for NAIT to develop. On the other hand, Rh sensitization occurs when there is a breech in the uterine wall, which most often occurs after delivery of the placenta. This is why with HDN, the first pregnancy is not affected. 2,9 11 For NAIT to develop, human platelet antigens (HPA, formally called PLA1, human platelet antigen 1a) must be absent in the mother but present in the father and subsequently present in the fetus. 12 Human platelet antigens (HPA-1a) are found in 98 percent of the Caucasian population and caused 75 percent of all NAIT cases. 13 The HPA-5b is second in frequency and is responsible for 16 Accepted for publication May Neonatal Alloimmune Thrombocytopenia: A Case Study Jodi Beachy, RNC, MSN, NNP Edi t o r Patricia Nash, MSN, NNP-BC percent of NAIT cases, 14 followed by HPA-5a, 15-a, and 15-b. 2 In Asians, HPA-4 is the predominant cause of NAIT. 3 HPAs are numbered from 1 to 24 in the order in which they were discovered. The letters a and b describe the frequency of occurrence with a being high and b being low. 9 HPA-1a, HPA-3a, and HPA-4a cause severe thrombocytopenia and HPA-5a and -5b are milder and rarely have intracranial hemorrhage (ICH). 3 CLINICAL PRESENTATION A newborn with NAIT will usually appear healthy. The mother will also be healthy and have a normal platelet count. The infant will often present with petechiae, bruising, excessive bleeding, and mucocutaneous purpura within the first few hours of life. When the newborn presents with these signs and symptoms, the platelet count, when checked, is typically less than 20, The platelet level may begin to drop early in pregnancy and may or may not self-correct. 3 Generally, though, a rapid postnatal drop in platelet levels is seen and caused in part by the increased exposure of other platelets from reticuloendothelial cells in the blood flow to the lungs. 2 Fourteen to twenty percent of newborns with NAIT will develop an ICH. 2,5 Fifty percent of ICH occur in utero. The presentation of ICH is variable ranging from no clinical signs to obvious signs, such as seizures, retinal hemorrhage, lethargy, tense fontanel, stupor, apnea, and bradycardia. 15 There is currently no routine prenatal screen for NAIT; therefore, the diagnosis is usually not made until after birth. 9 The mother s pregnancy is at high risk for NAIT if there is a history of a previous infant with NAIT or an infant with thrombocytopenia of unknown etiology. DIFFERENTIAL DIAGNOSIS The causes of newborn thrombocytopenia in the otherwise healthy newborn differ from thrombocytopenia seen in the sick newborn. The mother s pregnancy history and physical assessment can help determine a diagnosis. With NAIT, the mother usually experiences an uneventful pregnancy with normal platelet levels. 3 Alternately, thrombocytopenia may be seen in the neonate of women with a history of pregnancy-induced hypertension (PIH), drug use, or infection. 16 The most common cause of severe Disclosure The author discloses no relevant financial interest or affiliations with any commercial interests Springer Publishing Company NOVEMBER/DECEMBER 2011, VOL. 30, NO
34 thrombocytopenia in the well newborn is NAIT. 11 NAIT accounts for 20 percent of cases of thrombocytopenia in the healthy newborn. 5 The second most common cause, resulting in 10 percent of the cases of neonatal thrombocytopenia, is maternal idiopathic thrombocytopenia purpura (ITP). 17 Although only 10 percent of ITP mothers have infants with thrombocytopenia, it can be severe. 6,18 The differential diagnosis for healthy newborns with thrombocytopenia are presented in Table LABORATORY TESTING The infant s complete blood count (CBC) is generally normal other than the low platelet count; although if bleeding is severe, anemia may be present. The parents, not the infant, are screened for antigens. 20 NAIT is confirmed by identifying antiplatelet antibodies in the mother s blood as well as antigen incompatibility between the mother and the father. 12,20 The mother should be screened for HPA-1, HPA-3, and HPA-5, as well as for HPA-4, if the mother is Asian. 20 The father should also be screened. It is important to find the specific antigen to protect future pregnancies. 21 This is best done through a laboratory that has DNA testing and the ability to find rare antigens if needed. 21,22 In the early stages of neonatal thrombocytopenia, infection and bleeding disorders must be ruled out. However, most healthy newborns with severe thrombocytopenia have NAIT. 21 TREATMENT Platelet Transfusions Treatment of NAIT should begin without delay. 15 A platelet transfusion should be given if the infant s platelet count is less than 20,000 30,000 or less than 50,000 if the infant is bleeding or in critical condition. 9,23 In an emergent situation, the first course of action is to transfuse irradiated random donor platelets. HPA-1a negative donor platelets or washed and irradiated maternal platelets are ideal because the recovery is quicker, the effect is faster, and it lasts longer. These HPA-1a negative transfusions do not have the offending antigen, which is responsible for platelet destruction. However, the negative platelets are not always available when the infant is bleeding and needs immediate assistance. 2,3,9 Therefore, if random donor platelets are used that are HPA-1a positive, the infant will have a temporary improvement, but the platelet destruction will continue until the infant has cleared all antigens from the circulation. The target platelet level is 100,000 or greater. 22 A platelet infusion of 5 10 ml/kg should raise the platelet count by 50, ,000, 24 although most references recommend platelets of 10 ml/kg per transfusion. 25 Platelet transfusions are repeated until the level is greater than 100, It is especially important to maintain the platelet levels during the first 3 4 days of life to minimize the risk of ICH. 26 Once the platelet level has reached a normal level, it will remain there. Intravenous Immune Globulin Intravenous immune globulin (IVIG) is IgG in concentrated form. 27 IVIG is administered to decrease the production of and neutralize circulating antiplatelet antibodies. IVIG also works by blocking platelet Fc receptors and therefore decreasing destruction of platelets. 9,28 IVIG has been shown to reduce the frequency of ICH. 15 The effect of IVIG takes place in one to three days. 23,28 Typical dosage is mg/kg, but for infants with NAIT, dosages range from 400 to 1,000 mg/kg. IVIG is given over 2 6 hours and is compatible with dextrose 5% water (D 5 W), D 15 W, and total parenteral nutrition (TPN). 27 IVIG can be given up to two days in a row. 20 The infant s temperature, blood pressure, and respirations must be monitored closely with all transfusions. 29 When giving IVIG, in addition to monitoring the vital signs and intravenous site, also monitor the infant for the rare side effects of bronchospasm, renal failure, and laryngeal edema. 2,27 Fresh Frozen Plasma Generally, fresh frozen plasma (FFP) comes from whole blood and is frozen within 6 8 hours after collection. 25 FFP contains one international unit of clotting factors for every ml/kg 24 ; FFP also contains albumin and many other plasma proteins. FFP is most often used to prevent bleeding in the infant with severe thrombocytopenia of unknown origin. The recommended dose of FFP is ml/kg. 25 Cryoprecipitate Cryoprecipitate is thawed FFP, refrozen with plasma. The plasma contains high concentrations of clotting factors, especially Factors VII and VIII and fibrinogen. 29 Cryoprecipitate is used to treat infants with low fibrinogen clotting disorders and is not used once NAIT has been confirmed. SCREENING FOR INTRACRANIAL HEMORRHAGE Intracranial hemorrhage is the most devastating complication of NAIT, most of which occur in utero and can result in mortality or major morbidity. Consequently, all infants with a confirmed diagnosis or suspected NAIT should be screened by cranial ultrasound, computed tomography (CT) scan, or magnetic resonance imaging (MRI). 22 Cranial ultrasounds are usually the least expensive and fastest screen available for visualizing a hemorrhage. However, CT imaging and MRI can be more sensitive in finding some smaller intraventricular hemorrhages (IVHs) and subarachnoid bleeds. 30 Intracranial hemorrhage is found in 1 of 1,500 term newborns. Twenty-five percent are caused by NAIT, making it the most common cause of severe ICH. Parenchymal hemorrhage and IVH are the most common ICH seen in NAIT. 31 The connection between thrombocytopenia and ICH is most likely caused by the lack of protection of the vessel walls VOL. 30, NO. 6, NOVEMBER/DECEMBER
35 TABLE 1 n Differential Diagnosis for Well Newborn Presenting with Thrombocytopenia 1,2,6,18,19,20,21,22,26 Diagnosis Distinguishing Features (Maternal History Symptoms) Typical Platelet Count and Other Laboratory Exams Notes Maternal idiopathic thrombocytopenia purpura Maternal platelets low (though may be recovering) Infant platelets 50, ,000 Platelets drop during first days of life Autoimmune ICH 3% NAIT Maternal platelet levels normal Infant platelets under 20,000 Seen within first several hours of life ICH 14% Neonatal drug exposure Positive history Heparin Quinine Rare TARS (thrombocytopenia-absent radius syndrome) Severe thrombocytopenia from decreased production Absent radius ICH CAMT (Congenital amegakaryocytic thrombocytopenia) Severe thrombocytopenia Few megakaryocytes in bone marrow Rare Mimics NAIT Maternal drugs Quinidine Penicillin Dioxin Indomethacin Phenytoin Heparin Chromosomal abnormalities: Trisomy 18 Trisomy 13 Trisomy 21 Turner syndrome Clinical features of syndrome Less than 100,000 Low-percentage platelets 87% 54% 28% 31% Placental insufficiency IUGR and Maternal history, small infant Borderline low Recover by Day 10 PIH Wiskott-Aldrich syndrome Moderate thrombocytopenia Eczema and immunodeficiency Fanconi s anemia Congenital anomalies (muscular, microcephaly, and GU) Rare Cardiac anomalies Thrombosis Indwelling catheters or protein C deficiency Kasabach-Merritt syndrome Severe thrombocytopenia Prolonged PT and PTT Lesions on trunk Giant hemangiomas Key: GU 5 genitourinary; ICH 5 intracranial hemorrhage; IUGR 5 intrauterine growth restriction; NAIT 5 Neonatal alloimmune thrombocytopenia; PIH 5 pregnancy-induced hypertension; PT 5 prothrombin time; PTT 5 partial thromboplastin time. Note. Neonatal alloimmune thrombocytopenia; Maternal idiopathic thrombocytopenia purpura; Systemic lupus erythematosus; Giant hemangioma; Thrombosis (large renal vein thrombus); Neonatal drug exposure (heparin and quinine); Congenital thrombocytopenia bone marrow failure (thrombocytopenia-absent radius syndrome and congenital amegakaryocytic thrombocytopenia); Maternal pregnancy-induced hypertension; IUGR from placental insufficiency; Preeclampsia or chronic hypertension; Maternal drug exposure (heparin, penicillin, dioxin, antiseizure medications, and quinine); Congenital heart disease; Chromosome abnormalities (trisomies 21, 18, and 13 and Turner syndrome); Wiskott-Aldrich syndrome; Fanconi s anemia. 404 NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
36 normally provided by platelets. It is unknown whether the antiplatelet antibodies actually cause the platelets to be less effective in protecting the vessel walls. 31 Maternal antibodies begin to leave the infant s circulation at 48 hours of age. 32 Most courses of NAIT resolve by two weeks of age, with platelet levels usually normalizing completely by four weeks. 20,24 COMPLICATIONS AND LONG- TERM OUTCOMES Long-term outcomes are dependent on the severity of NAIT and the swiftness of treatment. 3 If there is no ICH, morbidity is low, although these infants are at a small risk for vision problems. 3,33 One-third of infants with NAIT and ICH will die. The rest are at risk for hydrocephaly, especially if the ICH occurs in utero; developmental delays, mental retardation, cerebral palsy, and seizures may also be seen. 2,33 MATERNAL FOLLOW-UP AND PREVENTION IN FUTURE PREGNANCIES Women who have had a newborn with NAIT must be taught the importance of early and regular prenatal care; 2 especially because all future pregnancies are at percent risk for severe fetal and neonatal thrombocytopenia, as the symptoms worsen with each subsequent pregnancy. 4,12 Close maternal follow-up with high-risk obstetrics is especially crucial if the first infant had an ICH. 34 Antenatal management focuses on minimizing the risk of ICH and currently includes treatment with IVIG and prednisone throughout the pregnancy to block maternal production of antibodies. 4,9,20 NEONATAL FOLLOW-UP Although NAIT is resolved by two weeks, the outpatient follow-up should include platelet levels for the rare but potential risk of the platelet levels dropping. Developmental and neurologic follow-up is also warranted if the infant experienced an ICH. Group B beta Streptococcus [GBBS] negative). Vacuum was used for nonreassuring fetal heart tones. The infant delivered in the vertex position with a loose nuchal cord. At delivery, she cried loudly and became pink quickly. Routine care was given, and the Apgar scores were 8 at one minute and 9 at five minutes. The infant stayed with the mother in the labor and delivery unit for the first hour of life. The one-hour assessment was normal except for bruising on the scalp. As the mother s blood type was O1, the cord blood was sent and noted to be A1 with a negative Coombs. By six hours of age, the infant was feeding poorly and was hypothermic. On assessment, she was pale pink, with decreased tone, irritability, and hyperresponsiveness to stimulation; her anterior and posterior fontanels were full. Bruising was noted over the entire scalp, with petechiae covering the entire chest, abdomen, arms, and legs. The infant had clear breath sounds, mild subcostal retractions with periodic breathing, and one episode of apnea; the rest of the exam was normal. The workup included a CBC with differential, prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (aptt), blood gas, fibrinogen, glucose level, blood culture, and CT scan of the brain. The CBC showed a left shift with a platelet count of 16,000; all other laboratory results were within an acceptable range. The CT scan showed a very large acute subdural hematoma on the right side of the cerebellum. The subdural hematoma extended along the falx (located between the cerebellar hemispheres) and the cerebellum was shifted to the left. The compressed cerebellum caused obstruction of the cisterns and increased the size of the temporal horns of the lateral ventricles, resulting in increased intracranial pressure (ICP) (Figure 1). The platelet count was repeated and dropped from 16,000 to 6,000 in one hour. Platelets were ordered but not available before the transport team arrived. FIGURE 1 n CAT scan shows intracranial hemorrhage. Blood from an acute bleed is seen as white once a clot is formed. CONCLUSION NAIT causes ICH and death in the otherwise healthy newborn. It is vital that immediate action is taken when NAIT occurs in the first days of life. With quick and proper treatment, the risks of death and long-term disabilities are diminished. NEONATAL ALLOIMMUNE THROMBOCYTOPENIA: A Case Study Baby girl A delivered vaginally at 41 weeks gestation, with vacuum assistance, to a 36-year-old, O1, Caucasian, primigravidous mother. The pregnancy was complicated by hypothyroidism, treated with Synthroid. All maternal serologies were negative (rubella immune, Venereal Disease Research Laboratory [VDRL] test negative, human immunodeficiency virus [HIV] negative, hepatitis B virus [HBV] negative, From Manco-Johnson, M., Rodden, D., & Collins, S. (2007). Newborn hematology. In G. Merenstein & S. Gardner (Eds.), Handbook of neonatal intensive care (6th ed., pp ). St. Louis, MO: Mosby. VOL. 30, NO. 6, NOVEMBER/DECEMBER
37 The infant was then transferred to a Level III NICU. With the results of the CT scan in hand, neurosurgery made an unsuccessful attempt to drain the blood at the bedside to decrease the ICP. The infant was therefore taken to the operating room for emergency surgery to remove the subdural clot. After surgery, the ICP dropped, and the ventricular size was normal on repeat CT scan. In the meantime, the mother was tested for antibodies. Her platelet count was normal, but her screen was positive for anti-hpa-1 antibodies, confirming the diagnosis of NAIT. During the first 36 hours of life, the infant received four transfusions of random donor platelets, one transfusion of packed red blood cells, one unit of FFP, and one dose of cryoprecipitate. The platelet count was followed every eight hours and showed improvement. By Day 5, the platelet count was 228,000, increasing to 346,000 by discharge on Day 7 of life. The infant was eating well and gaining weight by discharge. The platelet count remained stable at two weeks and one month of age. On the one-year follow-up, the infant is thriving and, most importantly, is developmentally appropriate. The infant had outpatient laboratory studies to check her platelet levels, which remained normal; she was also followed up in the developmental clinic. REFERENCES 1. Manco-Johnson, M., Rodden, D., & Collins, S. (2007). Newborn hematology. In G. Merenstein & S. Gardner (Eds.), Handbook of neonatal intensive care (6th ed., pp ). St. Louis, MO: Mosby. 2. Arnold, D. M., Smith, J. W., & Kelton, J. G. (2008). Diagnosis and management of neonatal alloimmune thrombocytopenia. Transfusion Medicine Reviews, 22(4), Kaplan, C. (2006). Alloimmune thrombocytopenia of the fetus and newborn. Blood Reviews, 16(1), Bussel, J. B., Berkowitz, R. L., Hung, C., Kolb, E. A., Wissert, M., Primiani, A.,... Macfarland, J. G. (2010). Intracranial hemorrhage in alloimmune thrombocytopenia: Stratified management to prevent recurrence in the subsequent affected fetus. American Journal of Obstetrics and Gynecology, 203(2), 135.e1 135.e Ghevaert, C., Campbell, K., Walton, J., Smith, G. A., Allen, D., Williamson, L. M.,... Ranasinghe, E. (2007). Management and outcome of 200 cases of fetomaternal alloimmune thrombocytopenia. Transfusion, 47(5), Jones, C. W. (2004). Platelet disorders. Newborn and Infant Nursing Reviews, 4(4), Wiedmeier, S. E., Henry, E., Sola-Visner, M. C., & Christensen, R. D. (2008). Platelet reference ranges for neonates, defined using data from over 47,000 patients in a multihospital healthcare system. Journal of Perinatology, 29(2), Roberts, I., & Murray, N. A. (2008). Neonatal thrombocytopenia. Seminars in Fetal & Neonatal Medicine, 13(4), Symington, A., & Paes B. (2010). Fetal and neonatal alloimmune thrombocytopenia: Harvesting the evidence to develop a clinical approach to management. American Journal of Perinatology, 28(2), Kaplan, C. (2003). Fetal and neonatal alloimmune thrombocytopenia. Orphanet Encyclopedia. Retrieved GB/uk-NAIT.pdf 11. Michelson, A. (2007). Platelets (2nd ed., pp ). Burlington, MA: Elsevier. 12. Cota, F., Zuppa, A. A., Luciano, R., Gallini, F., Savarese, I., Alighieri, G.,... Romagnoli, C. (2008). A severe case of intracranial hemorrhage due to alloimmune thrombocytopenia. The Journal of Maternal-Fetal & Neonatal Medicine, 21(11), Sola, M. C., Del Vecchio, A., & Rimsza, L. M. ( 2000). Evaluation and treatment of thrombocytopenia in the neonatal intensive care unit. Clinics in Perinatology, 27(3), van den Akker, E. S., & Oepkes, D. (2007). Fetal and neonatal alloimmune thrombocytopenia. Best Practice & Research Clinical Obstetrics and Gynaecology, 22(1), Blickstein, I., & Friedman, S. (2006). Fetal effects of autoimmune disease. In A. Fanaroff, R. Martin, & M. Walsh (Eds.) Fanaroff and Martin s neonatal-perinatal medicine, diseases of the fetus and infant (8th ed., pp ). Philadelphia, PA: Elsevier Mosby. 16. Cloherty, J. P., & Goorin, A. M. (2004). Thrombocytopenia. In J. P. Cloherty, E. C. Eichenwald, & A. R. Stark (Eds.), Manual of neonatal care (6th ed., pp ). Philadelphia, PA: Lippincott Williams & Wilkins. 17. Manco-Johnson, M., Rodden, D., & Collins, S. (2006). Newborn hematology. In G. Merenstein & S. Gardner (Eds.), Handbook of neonatal intensive care (6th ed., pp ). St. Louis, MO: Mosby. 18. Sola-Visner, M., Saxonhouse, M. A., & Brown, R. E. (2008). Neonatal thrombocytopenia: What we do and don t know. Early Human Development, 84(8), Luchtman-Jones, L., Schwarts, A., & Wilson, D. (2006). The blood and hematopoietic system. In R. J. Martin, A. A. Fanaroff, & M. C. Walsh (Eds.), Neonatal perinatal medicine, diseases of fetus and infant (8th ed., pp ). Philadelphia, PA: Elsevier Mosby. 20. Bussel, J. B. (2009). Diagnosis and management of the fetus and neonate with alloimmune thrombocytopenia. International Society of Thrombosis and Haemostasis, 7(1) Cloherty, J. P., & Goorin, A. M. (2008). Thrombocytopenia. In J. P. Cloherty, E. C. Eichenwald, & A. R. Stark (Eds.), Manual of neonatal care (6th ed., pp ). Philadelphia, PA: Wolters Kluwer, Lippincott Williams & Wilkins. 22. Bussel, J. B., & Sola-Visner, M. (2009). Current approaches to the evaluation and management of the fetus and neonate with immune thrombocytopenia. Seminars in Perinatology, 33(1), Bassler, D., Greinacher, A., Okascharoen C., Klenner, A., Ditomasso, J., Kiefel V.,... Paes, B. (2008). A systematic review and survey of the management of unexpected neonatal alloimmune thrombocytopenia. Transfusion Practice, 48(1), Bagwell, G. A. (2007). Hematologic system. In C. Kenner & J. W. Lott (Eds.), Comprehensive neonatal care, an interdisciplinary approach (pp ). Philadelphia, PA: Saunders Elsevier. 25. Poterjoy, B. S., & Josephson, C. D. (2009). Platelet, frozen plasma, cryoprecipitate: What is the clinical evidence for their use in the neonatal intensive care unit? Seminars in Perinatology. 33(1), Fernandes, C. J., Garcia-Prats, J. A., Mahoney, D. H., Jr., & Kim, M. S. (2009). Neonatal thrombocytopenia. UptoDate. Retrieved from Young, T., & Mangum, B (2009). Neofax (22nd ed.). Montvale, NJ: Thomson Reuters. 28. Leong, H., Stachnik, J., Bonk, M. E., & Matuszewski, K. A. (2008). Unlabeled uses of intravenous immune globulin. American Journal of Health-System Pharmacologists, 65(19), Watson, D., & Hearnshaw, K. (2010). Understanding blood groups and transfusion in nursing practice. Nursing Standard, 24(30), Gupta, S. N., Kechli, A. M., & Kanamalla, U. S. (2009). Intracranial hemorrhage in term newborns: Management and outcomes. Pediatric Neurology, 40(1), Bussel, J. B., & Primiani, A. (2007). Fetal and neonatal alloimmune thrombocytopenia: Progress and ongoing debates. Blood Reviews, 22(1), NOVEMBER/DECEMBER 2011, VOL. 30, NO. 6
38 32. Harkness, M. (2002). Neonatal alloimmune thrombocytopenia. British Journal of Midwifery, 10(2), Ward, M. J., Pauliny, J., Lipper, E. G., & Bussel, J. B. (2006). Longterm effects of fetal and neonatal alloimmune thrombocytopenia and its antenatal treatment on the medical and developmental outcomes of affected children. American Journal of Perinatology, 23(8), van den Akker, E. S., & Oepkes, D. (2008). Fetal and neonatal alloimmune thrombocytopenia. Best Practice & Research. Clinical Obstetrics & Gynaecology, 22(1), About the Author Jodi Beachy is a neonatal nurse practitioner in the Nationwide Children s Hospital Neonatal Special Care unit at Dublin Methodist Hospital Ohio Health. The author would like to thank Patricia Nash, RNC, MSN, NNP, for her editing expertise. For further information, please contact: Jodi Beachy, RNC, MSN, NNP beachyjodi@juno.com VOL. 30, NO. 6, NOVEMBER/DECEMBER
Hypothermia in Neonates with HIE TARA JENDZIO, DNP(C), RN, RNC-NIC
 Hypothermia in Neonates with HIE TARA JENDZIO, DNP(C), RN, RNC-NIC Objectives 1. Define Hypoxic-Ischemic Encephalopathy (HIE) 2. Identify the criteria used to determine if an infant qualifies for therapeutic
Hypothermia in Neonates with HIE TARA JENDZIO, DNP(C), RN, RNC-NIC Objectives 1. Define Hypoxic-Ischemic Encephalopathy (HIE) 2. Identify the criteria used to determine if an infant qualifies for therapeutic
Objectives. Birth Depression Management. Birth Depression Terms
 Objectives Birth Depression Management Regional Perinatal Outreach Program 2016 Understand the terms and the clinical characteristics of birth depression. Be familiar with the evidence behind therapeutic
Objectives Birth Depression Management Regional Perinatal Outreach Program 2016 Understand the terms and the clinical characteristics of birth depression. Be familiar with the evidence behind therapeutic
State of Florida Hypothermia Protocol. Michael D. Weiss, M.D. Associate Professor of Pediatrics Division of Neonatology
 State of Florida Hypothermia Protocol Michael D. Weiss, M.D. Associate Professor of Pediatrics Division of Neonatology I. Entry Criteria 1. Gestational Age greater than or equal to 35 weeks gestation
State of Florida Hypothermia Protocol Michael D. Weiss, M.D. Associate Professor of Pediatrics Division of Neonatology I. Entry Criteria 1. Gestational Age greater than or equal to 35 weeks gestation
Too Cool? Hypoxic Ischemic Encephalopathy and Therapeutic Hypothermia. Lauren Sacco DNP, ARNP, NNP-BC
 Too Cool? Hypoxic Ischemic Encephalopathy and Therapeutic Hypothermia Lauren Sacco DNP, ARNP, NNP-BC Pathophysiology of HIE Occurs in two energy failure phases: First phase happens during the initial insult
Too Cool? Hypoxic Ischemic Encephalopathy and Therapeutic Hypothermia Lauren Sacco DNP, ARNP, NNP-BC Pathophysiology of HIE Occurs in two energy failure phases: First phase happens during the initial insult
TREATMENT OF HYPOXIC ISCHEMIC ENCEPHALOPATHY WITH COOLING Children s Hospital & Research Center Oakland Guideline Revised by P.
 TREATMENT OF HYPOXIC ISCHEMIC ENCEPHALOPATHY WITH COOLING Children s Hospital & Research Center Oakland Guideline Revised 05-13-13 by P. Joe SCREENING FOR POTENTIAL COOLING PATIENTS Patients who are >
TREATMENT OF HYPOXIC ISCHEMIC ENCEPHALOPATHY WITH COOLING Children s Hospital & Research Center Oakland Guideline Revised 05-13-13 by P. Joe SCREENING FOR POTENTIAL COOLING PATIENTS Patients who are >
Running head: THERAPEUTIC HYPOTHERMIA AND TRANSPORT 1
 Running head: THERAPEUTIC HYPOTHERMIA AND TRANSPORT 1 Therapeutic Hypothermia for Neonatal Encephalopathy: Preparation for Transport to Cooling Center Teresa Z. Baker, DNP-S Annie L. Addison, FNP-S NURS
Running head: THERAPEUTIC HYPOTHERMIA AND TRANSPORT 1 Therapeutic Hypothermia for Neonatal Encephalopathy: Preparation for Transport to Cooling Center Teresa Z. Baker, DNP-S Annie L. Addison, FNP-S NURS
No social problems noted No past med hx Mother had spontaneous rupture of fetal membranes SB born on Needed to be resuscitated at birth
 No social problems noted No past med hx Mother had spontaneous rupture of fetal membranes SB born on 9-16-2011 Needed to be resuscitated at birth (included assisted vent) Had generalized edema and possible
No social problems noted No past med hx Mother had spontaneous rupture of fetal membranes SB born on 9-16-2011 Needed to be resuscitated at birth (included assisted vent) Had generalized edema and possible
State of Florida Systemic Supportive Care Guidelines. Michael D. Weiss, M.D. Associate Professor of Pediatrics Division of Neonatology
 State of Florida Systemic Supportive Care Guidelines Michael D. Weiss, M.D. Associate Professor of Pediatrics Division of Neonatology I. FEN 1. What intravenous fluids should be initiated upon admission
State of Florida Systemic Supportive Care Guidelines Michael D. Weiss, M.D. Associate Professor of Pediatrics Division of Neonatology I. FEN 1. What intravenous fluids should be initiated upon admission
NEONATAL HYPOXIC-ISCHAEMIC ENCEPHALOPATHY (HIE) & COOLING THERAPY
 Background NEONATAL HYPOXIC-ISCHAEMIC ENCEPHALOPATHY (HIE) & COOLING THERAPY A perinatal hypoxic-ischaemic insult may present with varying degrees of neonatal encephalopathy, neurological disorder and
Background NEONATAL HYPOXIC-ISCHAEMIC ENCEPHALOPATHY (HIE) & COOLING THERAPY A perinatal hypoxic-ischaemic insult may present with varying degrees of neonatal encephalopathy, neurological disorder and
This lecture will provide an overview of the neurologic exam of a neonate in the context of clinical cases.
 A11a Neuro Nuggets from the Trenches Michael D. Weiss, MD Associate Professor Department of Pediatrics, Division of Neonatology University of Florida, Gainesville, FL The speaker has signed a disclosure
A11a Neuro Nuggets from the Trenches Michael D. Weiss, MD Associate Professor Department of Pediatrics, Division of Neonatology University of Florida, Gainesville, FL The speaker has signed a disclosure
Too or Too Cold. Too Cold...Too Hot...Just Right. Temperature Control in Newborns. Temperature Balance in Newborns. Basics in the Delivery Room
 Too or Too Cold Neonatology Rediscovers Temperature Control Advances and Controversies in Clinical Pediatrics May 31, 2007 Terri A. Slagle Neonatology, CPMC Too Cold...Too Hot...Just Right Too Cold = Issues
Too or Too Cold Neonatology Rediscovers Temperature Control Advances and Controversies in Clinical Pediatrics May 31, 2007 Terri A. Slagle Neonatology, CPMC Too Cold...Too Hot...Just Right Too Cold = Issues
Surgical Options in Post Haemorrhagic Ventricular Dilation
 Surgical Options in Post Haemorrhagic Ventricular Dilation Benedetta Pettorini Consultant Paediatric Neurosurgeon Alder Hey Childrens Hospital Liverpool, UK Risk Factors for IVH 1. Prematurity: Occurs
Surgical Options in Post Haemorrhagic Ventricular Dilation Benedetta Pettorini Consultant Paediatric Neurosurgeon Alder Hey Childrens Hospital Liverpool, UK Risk Factors for IVH 1. Prematurity: Occurs
Guslihan Dasa Tjipta Division of Perinatology Department of Child Health Medical School University of Sumatera Utara
 Guslihan Dasa Tjipta Division of Perinatology Department of Child Health Medical School University of Sumatera Utara 1 Definition Perinatal asphyxia is a fetus/newborn, due to: is an insult to the Lack
Guslihan Dasa Tjipta Division of Perinatology Department of Child Health Medical School University of Sumatera Utara 1 Definition Perinatal asphyxia is a fetus/newborn, due to: is an insult to the Lack
Neonatal Therapeutic Hypothermia. A Wasunna Professor of Neonatal Medicine and Pediatrics School of Medicine, University of Nairobi
 Neonatal Therapeutic Hypothermia A Wasunna Professor of Neonatal Medicine and Pediatrics School of Medicine, University of Nairobi Definition of Perinatal Asphyxia *No agreed universal definition ACOG/AAP
Neonatal Therapeutic Hypothermia A Wasunna Professor of Neonatal Medicine and Pediatrics School of Medicine, University of Nairobi Definition of Perinatal Asphyxia *No agreed universal definition ACOG/AAP
Queen Charlotte Hospital
 Queen Charlotte Hospital Neuroprotection for neonatal encephalopathy Neonatal encephalopathy accounts for 1 million deaths worldwide and even greater numbers of disabled survivors In countries with
Queen Charlotte Hospital Neuroprotection for neonatal encephalopathy Neonatal encephalopathy accounts for 1 million deaths worldwide and even greater numbers of disabled survivors In countries with
Birth Asphyxia. Perinatal Depression. Birth Asphyxia. Risk Factors maternal. Risk Factors fetal. Risk Factors Intrapartum 2/12/2011
 Birth Asphyxia Perinatal Depression Sara Brown, ARNP Children s Hospital and Regional Medical Center May occur in utero, during labor/delivery or during the neonatal period Condition of impaired blood
Birth Asphyxia Perinatal Depression Sara Brown, ARNP Children s Hospital and Regional Medical Center May occur in utero, during labor/delivery or during the neonatal period Condition of impaired blood
Perinatal asphyxia: Pathophysiology and therapy
 Perinatal asphyxia: Pathophysiology and therapy Peter Davis Melbourne Australia With thanks to Dr Sue Jacobs Moderate or severe HIE Complicates ~1/1000 term live births: Mortality: >25% Major neurological
Perinatal asphyxia: Pathophysiology and therapy Peter Davis Melbourne Australia With thanks to Dr Sue Jacobs Moderate or severe HIE Complicates ~1/1000 term live births: Mortality: >25% Major neurological
Temperature Correction of Blood Gas Measurements during Therapeutic Hypothermia: Is it Time to Chill Out?
 Temperature Correction of Blood Gas Measurements during Therapeutic Hypothermia: Is it Time to Chill Out? Dr. Elizabeth Zorn Dr. Gwenyth Fischer Dr. Martha Lyon Disclosures (ML) Speaking Honoraria Radiometer
Temperature Correction of Blood Gas Measurements during Therapeutic Hypothermia: Is it Time to Chill Out? Dr. Elizabeth Zorn Dr. Gwenyth Fischer Dr. Martha Lyon Disclosures (ML) Speaking Honoraria Radiometer
COOLING FOR HYPOXIC ISCHEMIC ENCEPHALOPATHY
 COOLING FOR HYPOXIC ISCHEMIC ENCEPHALOPATHY Roger F. Soll H. Wallace Professor of Neonatology University of Vermont 19 th International Symposium on Neonatology Sao Paulo, Brazil DISCLOSURE Roger F. Soll
COOLING FOR HYPOXIC ISCHEMIC ENCEPHALOPATHY Roger F. Soll H. Wallace Professor of Neonatology University of Vermont 19 th International Symposium on Neonatology Sao Paulo, Brazil DISCLOSURE Roger F. Soll
Hypoxic-Ischemic Encephalopathy. TW de Witt University of Pretoria Department of Paediatrics Neonatology
 Hypoxic-Ischemic Encephalopathy TW de Witt University of Pretoria Department of Paediatrics Neonatology Background HIE remains a serious condition that causes significant mortality and longterm morbidity.
Hypoxic-Ischemic Encephalopathy TW de Witt University of Pretoria Department of Paediatrics Neonatology Background HIE remains a serious condition that causes significant mortality and longterm morbidity.
Wales Neonatal Network Guideline Guideline for the management of Infants with Moderate or Severe Perinatal Asphyxia requiring cooling.
 Guideline for the management of Infants with Moderate or Severe Perinatal Asphyxia requiring cooling. (Including flowchart for infants fulfilling A criteria or A and B criteria only) Perinatal asphyxia
Guideline for the management of Infants with Moderate or Severe Perinatal Asphyxia requiring cooling. (Including flowchart for infants fulfilling A criteria or A and B criteria only) Perinatal asphyxia
Case Presentations. Anamika B. Mukherjee, MD September 13, 2017
 Case Presentations Anamika B. Mukherjee, MD September 13, 2017 Nothing to disclose Disclosures Learning Objectives Use the CPQCC Toolkit for therapeutic hypothermia to apply the guidelines for screening
Case Presentations Anamika B. Mukherjee, MD September 13, 2017 Nothing to disclose Disclosures Learning Objectives Use the CPQCC Toolkit for therapeutic hypothermia to apply the guidelines for screening
Inclusion criteria for cooling: Babies should be assessed for 3 criteria: A, B and C. See Appendix 1 for a decision making flowchart.
 Guideline for the management of Infants with Moderate or Severe Perinatal Asphyxia requiring cooling. (Including flowchart for infants fulfilling A criteria or A and B criteria only) Perinatal asphyxia
Guideline for the management of Infants with Moderate or Severe Perinatal Asphyxia requiring cooling. (Including flowchart for infants fulfilling A criteria or A and B criteria only) Perinatal asphyxia
Disclosures. Objectives. Definition: HIE. HIE: Incidence. Impact 9/10/2018. Hypoxic Ischemic Encephalopathy in the Neonate
 Disclosures Hypoxic Ischemic Encephalopathy in the Neonate No relevant financial relationships or conflicts of interest to disclose Franscesca Miquel-Verges MD 2018 Review therapies currently under research
Disclosures Hypoxic Ischemic Encephalopathy in the Neonate No relevant financial relationships or conflicts of interest to disclose Franscesca Miquel-Verges MD 2018 Review therapies currently under research
TLC March 27, Shawn Hollinger-Neonatal Fellow CHEO
 TLC March 27, 2013 Presented/Prepared by: Shawn Hollinger, PGY5 Neonatal-Perinatal Medicine Resident - University of Ottawa With slides/images from Dr. Brigitte Lemyre Associate Professor of Pediatrics
TLC March 27, 2013 Presented/Prepared by: Shawn Hollinger, PGY5 Neonatal-Perinatal Medicine Resident - University of Ottawa With slides/images from Dr. Brigitte Lemyre Associate Professor of Pediatrics
Study of role of MRI brain in evaluation of hypoxic ischemic encephalopathy
 Original article: Study of role of MRI brain in evaluation of hypoxic ischemic encephalopathy *Dr Harshad Bhagat, ** Dr Ravindra Kawade, ***Dr Y.P.Sachdev *Junior Resident, Department Of Radiodiagnosis,
Original article: Study of role of MRI brain in evaluation of hypoxic ischemic encephalopathy *Dr Harshad Bhagat, ** Dr Ravindra Kawade, ***Dr Y.P.Sachdev *Junior Resident, Department Of Radiodiagnosis,
TOO COOL OR NOT TOO COOL- THERAPEUTIC HYPOTHERMIA IN THE ICU SCCM TX 2017 TED WU MD PEDIATRIC CRITICAL CARE UNIVERSITY OF TEXAS HEALTH SAN ANTONIO
 TOO COOL OR NOT TOO COOL- THERAPEUTIC HYPOTHERMIA IN THE ICU SCCM TX 2017 TED WU MD PEDIATRIC CRITICAL CARE UNIVERSITY OF TEXAS HEALTH SAN ANTONIO DISCLOSURE I have no relationships with commercial companies
TOO COOL OR NOT TOO COOL- THERAPEUTIC HYPOTHERMIA IN THE ICU SCCM TX 2017 TED WU MD PEDIATRIC CRITICAL CARE UNIVERSITY OF TEXAS HEALTH SAN ANTONIO DISCLOSURE I have no relationships with commercial companies
Neuro. Development. Judy Philbrook, NNP-BC. ! Primary neurulation! Prosencepahlic! Neuronal proliferation. ! 3-4 weeks! 2-3 months!
 Neuro Judy Philbrook, NNP-BC Microsoft clip art Development! Primary neurulation! Prosencepahlic! Neuronal proliferation! Neuronal migration! Organization! Myelination! 3-4 weeks! 2-3 months! 3-4 months!
Neuro Judy Philbrook, NNP-BC Microsoft clip art Development! Primary neurulation! Prosencepahlic! Neuronal proliferation! Neuronal migration! Organization! Myelination! 3-4 weeks! 2-3 months! 3-4 months!
Therapeutic Hypothermia for infants > 35 wks with moderate or severe Hypoxic Ischaemic Encephalopathy (HIE) Clinical Guideline Reference Number:
 This is an official Northern Trust policy and should not be edited in any way Therapeutic Hypothermia for infants > 35 wks with moderate or severe Hypoxic Ischaemic Encephalopathy (HIE) Clinical Guideline
This is an official Northern Trust policy and should not be edited in any way Therapeutic Hypothermia for infants > 35 wks with moderate or severe Hypoxic Ischaemic Encephalopathy (HIE) Clinical Guideline
TRAINING NEONATOLOGY SILVANA PARIS
 TRAINING ON NEONATOLOGY SILVANA PARIS RESUSCITATION IN DELIVERY ROOM INTRODUCTION THE GLOBAL RESUSCITATION BURDEN IN NEWBORN 136 MILL NEWBORN BABIES EACH YEAR (WHO WORLD REPORT) 5-8 MILL NEWBORN INFANTS
TRAINING ON NEONATOLOGY SILVANA PARIS RESUSCITATION IN DELIVERY ROOM INTRODUCTION THE GLOBAL RESUSCITATION BURDEN IN NEWBORN 136 MILL NEWBORN BABIES EACH YEAR (WHO WORLD REPORT) 5-8 MILL NEWBORN INFANTS
SWISS SOCIETY OF NEONATOLOGY. Severe apnea and bradycardia in a term infant
 SWISS SOCIETY OF NEONATOLOGY Severe apnea and bradycardia in a term infant October 2014 2 Walker JH, Arlettaz Mieth R, Däster C, Division of Neonatology, University Hospital Zurich, Switzerland Swiss Society
SWISS SOCIETY OF NEONATOLOGY Severe apnea and bradycardia in a term infant October 2014 2 Walker JH, Arlettaz Mieth R, Däster C, Division of Neonatology, University Hospital Zurich, Switzerland Swiss Society
Original article: Evaluation of hypoxic-ischaemic events in preterm neonates using trans cranial ultrasound
 Original article: Evaluation of hypoxic-ischaemic events in preterm neonates using trans cranial ultrasound Priyanka Upadhyay *, Ketki U Patil 1, Rajesh Kuber 2, Vilas Kulkarni 3, Amarjit Singh 4 * Chief
Original article: Evaluation of hypoxic-ischaemic events in preterm neonates using trans cranial ultrasound Priyanka Upadhyay *, Ketki U Patil 1, Rajesh Kuber 2, Vilas Kulkarni 3, Amarjit Singh 4 * Chief
ETIOLOGY AND PATHOGENESIS OF HYPOXIC-ISCHEMIC ENCEPHALOPATHY
 ETIOLOGY AND PATHOGENESIS OF HYPOXIC-ISCHEMIC ENCEPHALOPATHY HYPOXIC-ISCHEMIC ENCEPHALOPATHY Hypoxic-İschemic Encephalopathy Encephalopathy due to hypoxic-ischemic injury [Hypoxic-ischemic encephalopathy
ETIOLOGY AND PATHOGENESIS OF HYPOXIC-ISCHEMIC ENCEPHALOPATHY HYPOXIC-ISCHEMIC ENCEPHALOPATHY Hypoxic-İschemic Encephalopathy Encephalopathy due to hypoxic-ischemic injury [Hypoxic-ischemic encephalopathy
These signs should lead to the administration of high concentrations of
 Hypoxic-ischemic encephalopathy (HIE); (cont.) Clinical manifestations; *Intrauterine; growth restriction and increased vascular resistances may be the st manifestation of fetal hypoxia. *During labor;
Hypoxic-ischemic encephalopathy (HIE); (cont.) Clinical manifestations; *Intrauterine; growth restriction and increased vascular resistances may be the st manifestation of fetal hypoxia. *During labor;
Pathophysiology Review. Hypoxic-Ischemic Encephalopathy & Therapeutic Hypothermia. Objectives. What is Hypoxic-Ischemic Encephalopathy?
 Hypoxic-Ischemic Encephalopathy & Therapeutic Hypothermia Nancy Couto Nurse Practitioner, NICU London Health Sciences Centre, Children s Hospital nancy.couto@lhsc.on.ca 2014 12 17 Objectives Review Pathophysiology
Hypoxic-Ischemic Encephalopathy & Therapeutic Hypothermia Nancy Couto Nurse Practitioner, NICU London Health Sciences Centre, Children s Hospital nancy.couto@lhsc.on.ca 2014 12 17 Objectives Review Pathophysiology
Admission/Discharge Form for Infants Born in Please DO NOT mail or fax this form to the CPQCC Data Center. This form is for internal use ONLY.
 Selection Criteria Admission/Discharge Form for Infants Born in 2016 To be eligible, you MUST answer YES to at least one of the possible criteria (A-C) A. 401 1500 grams o Yes B. GA range 22 0/7 31 6/7
Selection Criteria Admission/Discharge Form for Infants Born in 2016 To be eligible, you MUST answer YES to at least one of the possible criteria (A-C) A. 401 1500 grams o Yes B. GA range 22 0/7 31 6/7
PedsCases Podcast Scripts
 PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Hypoxic Ischemic Encephalopathy. These podcasts are designed to give medical students an overview of key topics in pediatrics.
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Hypoxic Ischemic Encephalopathy. These podcasts are designed to give medical students an overview of key topics in pediatrics.
SWISS SOCIETY OF NEONATOLOGY. Spontaneous intestinal perforation or necrotizing enterocolitis?
 SWISS SOCIETY OF NEONATOLOGY Spontaneous intestinal perforation or necrotizing enterocolitis? June 2004 2 Stocker M, Berger TM, Neonatal and Pediatric Intensive Care Unit, Children s Hospital of Lucerne,
SWISS SOCIETY OF NEONATOLOGY Spontaneous intestinal perforation or necrotizing enterocolitis? June 2004 2 Stocker M, Berger TM, Neonatal and Pediatric Intensive Care Unit, Children s Hospital of Lucerne,
Therapeutic hypothermia for hypoxic ischemic encephalopathy using low-technology methods: A systematic review and meta-analysis
 Therapeutic hypothermia for hypoxic ischemic encephalopathy using low-technology methods: A systematic review and meta-analysis Rossouw G 1, Irlam J 2, Horn AR 1 1)Division of Neonatal Medicine, Department
Therapeutic hypothermia for hypoxic ischemic encephalopathy using low-technology methods: A systematic review and meta-analysis Rossouw G 1, Irlam J 2, Horn AR 1 1)Division of Neonatal Medicine, Department
Hypotension in the Neonate
 Neonatal Nursing Education Brief: Hypotension in the Neonate http://www.seattlechildrens.org/healthcare-professionals/education/continuing-medicalnursing-education/neonatal-nursing-education-briefs/ Neonatal
Neonatal Nursing Education Brief: Hypotension in the Neonate http://www.seattlechildrens.org/healthcare-professionals/education/continuing-medicalnursing-education/neonatal-nursing-education-briefs/ Neonatal
Review Article Current Controversies in Newer Therapies to Treat Birth Asphyxia
 International Pediatrics Volume 2011, Article ID 848413, 5 pages doi:10.1155/2011/848413 Review Article Current Controversies in Newer Therapies to Treat Birth Asphyxia Pia Wintermark Division of Newborn
International Pediatrics Volume 2011, Article ID 848413, 5 pages doi:10.1155/2011/848413 Review Article Current Controversies in Newer Therapies to Treat Birth Asphyxia Pia Wintermark Division of Newborn
NEONATOLOGY Healthy newborn. Neonatal sequelaes
 NEONATOLOGY Healthy newborn. Neonatal sequelaes Ágnes Harmath M.D. Ph.D. senior lecturer 11. November 2016. Tasks of the neonatologist Prenatal diagnosed condition Inform parents, preparation of necessary
NEONATOLOGY Healthy newborn. Neonatal sequelaes Ágnes Harmath M.D. Ph.D. senior lecturer 11. November 2016. Tasks of the neonatologist Prenatal diagnosed condition Inform parents, preparation of necessary
Natalia Gorelik 1, Ricardo Faingold 2, Alan Daneman 3, Monica Epelman 3,4. Original Article
 Original Article Intraventricular hemorrhage in term neonates with hypoxicischemic encephalopathy: a comparison study between neonates treated with and without hypothermia Natalia Gorelik 1, Ricardo Faingold
Original Article Intraventricular hemorrhage in term neonates with hypoxicischemic encephalopathy: a comparison study between neonates treated with and without hypothermia Natalia Gorelik 1, Ricardo Faingold
Running head: CASE STUDY NEONATAL HEAD COOLING 1
 Running head: CASE STUDY NEONATAL HEAD COOLING 1 Case Study 1: Head Cooling as Treatment for Neonatal Encephalopathy NURS 6035 Practicum I Teresa Z. Baker Texas Woman s University CASE STUDY NEONATAL HEAD
Running head: CASE STUDY NEONATAL HEAD COOLING 1 Case Study 1: Head Cooling as Treatment for Neonatal Encephalopathy NURS 6035 Practicum I Teresa Z. Baker Texas Woman s University CASE STUDY NEONATAL HEAD
Neonatal Seizure. Dr.Nawar Yahya. Presented by: Sarah Khalil Zeina Shamil Zainab Waleed Zainab Qahtan. Supervised by:
 Neonatal Seizure Supervised by: Dr.Nawar Yahya Presented by: Sarah Khalil Zeina Shamil Zainab Waleed Zainab Qahtan Objectives: What is neonatal seizure Etiology Clinical presentation Differential diagnosis
Neonatal Seizure Supervised by: Dr.Nawar Yahya Presented by: Sarah Khalil Zeina Shamil Zainab Waleed Zainab Qahtan Objectives: What is neonatal seizure Etiology Clinical presentation Differential diagnosis
Perinatal Depression. Lauren Sacco DNP, ARNP Seattle Children s
 Perinatal Depression Lauren Sacco DNP, ARNP Seattle Children s Birth Asphyxia May occur in utero, during labor/delivery or during the neonatal period Condition of impaired blood gas exchange that leads
Perinatal Depression Lauren Sacco DNP, ARNP Seattle Children s Birth Asphyxia May occur in utero, during labor/delivery or during the neonatal period Condition of impaired blood gas exchange that leads
8/20/12. Discuss the importance of thermoregulation in the neonate.
 Sharon Rush MSN NNP-BC Discuss the importance of thermoregulation in the neonate. To maintain correct body temperature range in order to: Reduce oxygen consumption Reduce calorie expenditure Maximize metabolic
Sharon Rush MSN NNP-BC Discuss the importance of thermoregulation in the neonate. To maintain correct body temperature range in order to: Reduce oxygen consumption Reduce calorie expenditure Maximize metabolic
Patent Ductus Arteriosus: Philosophy or Pathology?
 Patent Ductus Arteriosus: Philosophy or Pathology? Disclosure Ray Sato, MD is a speaker for Prolacta Biosciences, Inc. This presentation will discuss off-label uses of acetaminophen and ibuprofen. RAY
Patent Ductus Arteriosus: Philosophy or Pathology? Disclosure Ray Sato, MD is a speaker for Prolacta Biosciences, Inc. This presentation will discuss off-label uses of acetaminophen and ibuprofen. RAY
Kimberly M. Thornton, 1,2 Hongying Dai, 3 Seth Septer, 2,4 and Joshua E. Petrikin 1,2. 1. Introduction
 International Pediatrics, Article ID 643689, 7 pages http://dx.doi.org/10.1155/2014/643689 Research Article Effects of Whole Body Therapeutic Hypothermia on Gastrointestinal Morbidity and Feeding Tolerance
International Pediatrics, Article ID 643689, 7 pages http://dx.doi.org/10.1155/2014/643689 Research Article Effects of Whole Body Therapeutic Hypothermia on Gastrointestinal Morbidity and Feeding Tolerance
ADMISSION/DISCHARGE FORM FOR INFANTS BORN IN 2019 DO NOT mail or fax this form to the CPQCC Data Center. This form is for internal use ONLY.
 1 Any eligible inborn infant who dies in the delivery room or at any other location in your hospital within 12 hours after birth and prior to admission to the NICU is defined as a "Delivery Room Death."
1 Any eligible inborn infant who dies in the delivery room or at any other location in your hospital within 12 hours after birth and prior to admission to the NICU is defined as a "Delivery Room Death."
Neurological and Neuromuscular Disorders. Elizabeth Papp, RN, MSN, CNS
 Neurological and Neuromuscular Disorders Elizabeth Papp, RN, MSN, CNS June, 2018 Neuromuscular Birth Injuries: Overview Nerve damage caused by trauma during delivery Prolonged labor LGA CPD Abnormal presentation
Neurological and Neuromuscular Disorders Elizabeth Papp, RN, MSN, CNS June, 2018 Neuromuscular Birth Injuries: Overview Nerve damage caused by trauma during delivery Prolonged labor LGA CPD Abnormal presentation
Hummi Micro Draw Blood Transfer Device. An Important Addition to Your IVH Bundle
 Hummi Micro Draw Blood Transfer Device An Important Addition to Your IVH Bundle Hummi Micro Draw & Micro T Connector For Infec6on Control and IVH Risk Reduc6on The Next Genera6on System for Closed Micro
Hummi Micro Draw Blood Transfer Device An Important Addition to Your IVH Bundle Hummi Micro Draw & Micro T Connector For Infec6on Control and IVH Risk Reduc6on The Next Genera6on System for Closed Micro
Medical Follow-up of the High-Risk NICU Graduate
 Medical Follow-up of the High-Risk NICU Graduate Silvia Fajardo-Hiriart, M.D. Medical Director High-Risk Infant Follow-Up/Early Intervention Program University of Miami Miller School of Medicine Department
Medical Follow-up of the High-Risk NICU Graduate Silvia Fajardo-Hiriart, M.D. Medical Director High-Risk Infant Follow-Up/Early Intervention Program University of Miami Miller School of Medicine Department
Benefits of Caffeine Citrate: Neurodevelopmental Outcomes of ELBW Infants
 St. Catherine University SOPHIA Master of Arts in Nursing Theses Nursing 12-2011 Benefits of Caffeine Citrate: Neurodevelopmental Outcomes of ELBW Infants Teri Johnson St. Catherine University Follow this
St. Catherine University SOPHIA Master of Arts in Nursing Theses Nursing 12-2011 Benefits of Caffeine Citrate: Neurodevelopmental Outcomes of ELBW Infants Teri Johnson St. Catherine University Follow this
Insults to the Developing Brain & Effect on Neurodevelopmental Outcomes
 Insults to the Developing Brain & Effect on Neurodevelopmental Outcomes Ira Adams-Chapman, MD Assistant Professor of Pediatrics Director, Developmental Progress Clinic Emory University School of Medicine
Insults to the Developing Brain & Effect on Neurodevelopmental Outcomes Ira Adams-Chapman, MD Assistant Professor of Pediatrics Director, Developmental Progress Clinic Emory University School of Medicine
COOLING FOR NEONATAL HYPOXIC ISCHAEMIC ENCEPHALOPATHY (HIE) - GUIDELINE
 Background Objective Equipment Indications Contraindications When to initiate cooling in NPICU Procedure for therapeutic cooling NETS Transfer Issues Follow-up References Acknowledgements Related Documents
Background Objective Equipment Indications Contraindications When to initiate cooling in NPICU Procedure for therapeutic cooling NETS Transfer Issues Follow-up References Acknowledgements Related Documents
USE OF INHALED NITRIC OXIDE IN THE NICU East Bay Newborn Specialists Guideline Prepared by P Joe, G Dudell, A D Harlingue Revised 7/9/2014
 USE OF INHALED NITRIC OXIDE IN THE NICU East Bay Newborn Specialists Guideline Prepared by P Joe, G Dudell, A D Harlingue Revised 7/9/2014 ino for Late Preterm and Term Infants with Severe PPHN Background:
USE OF INHALED NITRIC OXIDE IN THE NICU East Bay Newborn Specialists Guideline Prepared by P Joe, G Dudell, A D Harlingue Revised 7/9/2014 ino for Late Preterm and Term Infants with Severe PPHN Background:
Intraventricular Hemorrhage and Periventricular Leukomalacia
 Intraventricular Hemorrhage and Periventricular Leukomalacia Intraventricular Hemorrhage Intraventricular hemorrhage (IVH) is bleeding inside the lateral ventricles. Bleeding frequently occurs in areas
Intraventricular Hemorrhage and Periventricular Leukomalacia Intraventricular Hemorrhage Intraventricular hemorrhage (IVH) is bleeding inside the lateral ventricles. Bleeding frequently occurs in areas
Hazards and Benefits of Postnatal Steroids. David J. Burchfield, MD Professor and Chief, Neonatology University of Florida
 Hazards and Benefits of Postnatal Steroids David J. Burchfield, MD Professor and Chief, Neonatology University of Florida Disclosures I have no financial affiliations or relationships to disclose. I will
Hazards and Benefits of Postnatal Steroids David J. Burchfield, MD Professor and Chief, Neonatology University of Florida Disclosures I have no financial affiliations or relationships to disclose. I will
An Overview of Bronchopulmonary Dysplasia and Chronic Lung Disease in Infancy
 An Overview of Bronchopulmonary Dysplasia and Chronic Lung Disease in Infancy Housekeeping: I have no financial disclosures Learning objectives: Develop an understanding of bronchopulmonary dysplasia (BPD)
An Overview of Bronchopulmonary Dysplasia and Chronic Lung Disease in Infancy Housekeeping: I have no financial disclosures Learning objectives: Develop an understanding of bronchopulmonary dysplasia (BPD)
The high risk neonate
 The high risk neonate Infant classification by gestational (postmenstrual) age Preterm. Less than 37 completed weeks (259 days). Term. Thirty-seven to 416/7 weeks (260-294 days). Post-term. Forty-two weeks
The high risk neonate Infant classification by gestational (postmenstrual) age Preterm. Less than 37 completed weeks (259 days). Term. Thirty-seven to 416/7 weeks (260-294 days). Post-term. Forty-two weeks
Infection. Risk factor for infection ACoRN alerting sign with * Clinical deterioration. Problem List. Respiratory. Cardiovascular
 The ACoRN Process Baby at risk Unwell Risk factors Post-resuscitation requiring stabilization Resuscitation Ineffective breathing Heart rate < 100 bpm Central cyanosis Support Infection Risk factor for
The ACoRN Process Baby at risk Unwell Risk factors Post-resuscitation requiring stabilization Resuscitation Ineffective breathing Heart rate < 100 bpm Central cyanosis Support Infection Risk factor for
NEONATAL CLINICAL PRACTICE GUIDELINE
 NEONATAL CLINICAL PRACTICE GUIDELINE Title: Brain Oxygen Monitoring in Newborns Using Near Infrared Spectroscopy (NIRS) Approval Date: Pages: June 2016 Approved by: Neonatal Patient Care Teams, HSC & SBH
NEONATAL CLINICAL PRACTICE GUIDELINE Title: Brain Oxygen Monitoring in Newborns Using Near Infrared Spectroscopy (NIRS) Approval Date: Pages: June 2016 Approved by: Neonatal Patient Care Teams, HSC & SBH
Neonatal Hypoxic-Ischemic Injury: Ultrasound and Dynamic Color Doppler Sonography perfusion of the Brain and Abdomen with pathologic correlation.
 Neonatal Hypoxic-Ischemic Injury: Ultrasound and Dynamic Color Doppler Sonography perfusion of the Brain and Abdomen with pathologic correlation. Ricardo Faingold,MD Montreal Children s Hospital Medical
Neonatal Hypoxic-Ischemic Injury: Ultrasound and Dynamic Color Doppler Sonography perfusion of the Brain and Abdomen with pathologic correlation. Ricardo Faingold,MD Montreal Children s Hospital Medical
Imaging the Premature Brain- New Knowledge
 Imaging the Premature Brain- New Knowledge Stein Magnus Aukland Haukeland University Hospital University of Bergen NORWAY No disclosure Imaging modalities O Skull X-ray O Computer Tomography O Cerebral
Imaging the Premature Brain- New Knowledge Stein Magnus Aukland Haukeland University Hospital University of Bergen NORWAY No disclosure Imaging modalities O Skull X-ray O Computer Tomography O Cerebral
Birth Asphyxia - Summary of the previous meeting and protocol overview
 Birth Asphyxia - Summary of the previous meeting and protocol overview Dr Ornella Lincetto, WHO Geneve Milano, 11June 2007 Vilka är Personality egenskaper med den astrologiska Tvillingarna? Objective of
Birth Asphyxia - Summary of the previous meeting and protocol overview Dr Ornella Lincetto, WHO Geneve Milano, 11June 2007 Vilka är Personality egenskaper med den astrologiska Tvillingarna? Objective of
Serum creatine kinase and lactic dehydrogenase levels as useful markers of immediate and long-term outcome of perinatal asphyxia
 Serum creatine kinase and lactic dehydrogenase levels as useful markers of immediate and long-term outcome of perinatal asphyxia D H Karunatilaka 1, G W D S Amaratunga 2, K D N I Perera 3, V Caldera 4
Serum creatine kinase and lactic dehydrogenase levels as useful markers of immediate and long-term outcome of perinatal asphyxia D H Karunatilaka 1, G W D S Amaratunga 2, K D N I Perera 3, V Caldera 4
10/13/2017. Newborn Care. Objectives. Cardiac Anatomy. Managing Transitional Physiology
 Newborn Care Managing Transitional Physiology Mary Coughlin MS, NNP, RNC-E President and Founder Caring Essentials Collaborative Boston, MA Objectives Upon completion of the learning session participants
Newborn Care Managing Transitional Physiology Mary Coughlin MS, NNP, RNC-E President and Founder Caring Essentials Collaborative Boston, MA Objectives Upon completion of the learning session participants
Initiation of passive cooling at referring centre is most predictive of achieving early therapeutic hypothermia in asphyxiated newborns
 Paediatrics & Child Health, 2017, 264 268 doi: 10.1093/pch/pxx062 Original Article Advance Access publication 23 May 2017 Original Article Initiation of passive cooling at referring centre is most predictive
Paediatrics & Child Health, 2017, 264 268 doi: 10.1093/pch/pxx062 Original Article Advance Access publication 23 May 2017 Original Article Initiation of passive cooling at referring centre is most predictive
Retrospectıve analysıs for newborn ınfants wıth hypoxıc-ıschemıc encephalopathy
 Basic Research Journal of Medicine and Clinical Sciences ISSN 2315-6864 Vol. 1(2) pp. 19-24 September 2012 Available online http//www.basicresearchjournals.org Copyright 2012 Basic Research Journal Full
Basic Research Journal of Medicine and Clinical Sciences ISSN 2315-6864 Vol. 1(2) pp. 19-24 September 2012 Available online http//www.basicresearchjournals.org Copyright 2012 Basic Research Journal Full
University of Bristol - Explore Bristol Research
 Wang, H., Cheng, Z., Liao, Y., Li, J., Weber, J., Thomas, A., & Faul, C. FJ. (2017). Conjugated Microporous Polycarbazole Networks as Precursors for Nitrogen Enriched Microporous Carbons for CO2 Storage
Wang, H., Cheng, Z., Liao, Y., Li, J., Weber, J., Thomas, A., & Faul, C. FJ. (2017). Conjugated Microporous Polycarbazole Networks as Precursors for Nitrogen Enriched Microporous Carbons for CO2 Storage
Ultrasound examination of the neonatal brain
 Ultrasound examination of the neonatal brain Guideline for the performance and reporting of neonatal and preterm brain ultrasound examination, by the Finnish Perinatology Society and the Paediatric Radiology
Ultrasound examination of the neonatal brain Guideline for the performance and reporting of neonatal and preterm brain ultrasound examination, by the Finnish Perinatology Society and the Paediatric Radiology
Pathophysiology and Cardiac Insights for Targeted Temperature Management in Emergency Medicine and Critical Care
 Pathophysiology and Cardiac Insights for Targeted Temperature Management in Emergency Medicine and Critical Care LINDSAY LEWIS BSN, RN, CCCC Faculty Disclosure I AM CURRENTLY EMPLOYED AS A CLINICAL MANAGER
Pathophysiology and Cardiac Insights for Targeted Temperature Management in Emergency Medicine and Critical Care LINDSAY LEWIS BSN, RN, CCCC Faculty Disclosure I AM CURRENTLY EMPLOYED AS A CLINICAL MANAGER
Post-Cardiac Arrest Syndrome. MICU Lecture Series
 Post-Cardiac Arrest Syndrome MICU Lecture Series Case 58 y/o female collapses at home, family attempts CPR, EMS arrives and notes VF, defibrillation x 3 with return of spontaneous circulation, brought
Post-Cardiac Arrest Syndrome MICU Lecture Series Case 58 y/o female collapses at home, family attempts CPR, EMS arrives and notes VF, defibrillation x 3 with return of spontaneous circulation, brought
PEDIATRIC BRAIN CARE
 PEDIATRIC BRAIN CARE The brain matters most! OVERVIEW OF NEURO ASSESSMENT 1. Overall responsiveness/activity 2. The eyes 3.? Increased ICP 4. Movements 5.? Seizures 6. Other OVERALL RESPONSIVENESS/ ACTIVITY
PEDIATRIC BRAIN CARE The brain matters most! OVERVIEW OF NEURO ASSESSMENT 1. Overall responsiveness/activity 2. The eyes 3.? Increased ICP 4. Movements 5.? Seizures 6. Other OVERALL RESPONSIVENESS/ ACTIVITY
Newborn Hypoxic Ischemic Brain Injury. Hisham Dahmoush, MBBCh FRCR Lucile Packard Children s Hospital at Stanford
 Newborn Hypoxic Ischemic Brain Injury Hisham Dahmoush, MBBCh FRCR Lucile Packard Children s Hospital at Stanford NO DISCLOSURES INTRODUCTION Neonatal hypoxic-ischemic encephalopathy (HIE) is a major cause
Newborn Hypoxic Ischemic Brain Injury Hisham Dahmoush, MBBCh FRCR Lucile Packard Children s Hospital at Stanford NO DISCLOSURES INTRODUCTION Neonatal hypoxic-ischemic encephalopathy (HIE) is a major cause
Addendum to the NRP Provider Textbook 6 th Edition Recommendations for specific modifications in the Canadian context
 Addendum to the NRP Provider Textbook 6 th Edition Recommendations for specific modifications in the Canadian context A subcommittee of the Canadian Neonatal Resuscitation Program (NRP) Steering Committee
Addendum to the NRP Provider Textbook 6 th Edition Recommendations for specific modifications in the Canadian context A subcommittee of the Canadian Neonatal Resuscitation Program (NRP) Steering Committee
NEONATAL SEIZURE. IAP UG Teaching slides
 NEONATAL SEIZURE 1 INTRODUCTION One of the important neonatal neurological emergencies requiring immediate medical care. Contribute to significant morbidity and mortality Incidence is around 0.5 to 0.8%
NEONATAL SEIZURE 1 INTRODUCTION One of the important neonatal neurological emergencies requiring immediate medical care. Contribute to significant morbidity and mortality Incidence is around 0.5 to 0.8%
The NeuroNICU From Concept to Clinical Service. MJ Harbert, MD Co-Director, NeuroNICU Service Rady Children s Hospital San Diego
 The NeuroNICU From Concept to Clinical Service MJ Harbert, MD Co-Director, NeuroNICU Service Rady Children s Hospital San Diego What is a NeuroNICU? Collaboration between Neonatology and Neurology Neonatal
The NeuroNICU From Concept to Clinical Service MJ Harbert, MD Co-Director, NeuroNICU Service Rady Children s Hospital San Diego What is a NeuroNICU? Collaboration between Neonatology and Neurology Neonatal
Fetal Heart Rate Monitoring Myths and Misperceptions s: Electronic Fetal Heart Rate Monitoring (EFM): Baseline Assumptions.
 Can FHR Monitoring Prevent Hypoxic-Ischemic Encephalopathy in the Newborn? Fetal Heart Rate Monitoring Myths and Misperceptions 1. Yes 2. No 72% Tekoa L. King CNM, MPH June 6, 2008 28% Yes No Objectives
Can FHR Monitoring Prevent Hypoxic-Ischemic Encephalopathy in the Newborn? Fetal Heart Rate Monitoring Myths and Misperceptions 1. Yes 2. No 72% Tekoa L. King CNM, MPH June 6, 2008 28% Yes No Objectives
Pediatric Neurointervention: Vein of Galen Malformations
 Pediatric Neurointervention: Vein of Galen Malformations Johanna T. Fifi, M.D. Assistant Professor of Neurology, Neurosurgery, and Radiology Icahn School of Medicine at Mount Sinai November 9 th, 2014
Pediatric Neurointervention: Vein of Galen Malformations Johanna T. Fifi, M.D. Assistant Professor of Neurology, Neurosurgery, and Radiology Icahn School of Medicine at Mount Sinai November 9 th, 2014
Hypothermia Presentation
 Hypothermia Presentation Thermoregulation Thermal regulation is a balance between heat production and heat loss. Despite marked changes in skin temperature, the body s homeostatic mechanisms are able to
Hypothermia Presentation Thermoregulation Thermal regulation is a balance between heat production and heat loss. Despite marked changes in skin temperature, the body s homeostatic mechanisms are able to
Total Body Cooling & Hypoxic Ischemic Encephalopathy in the Neonate Kaleidoscope 2017
 Total Body Cooling & Hypoxic Ischemic Encephalopathy in the Neonate Kaleidoscope 2017 LEIGH ANN CATES PHD, APRN, NNP -BC, RRT-NPS, CHSE N E O N ATA L N U R S E P R A C T I T I O N E R - T E X A S C H I
Total Body Cooling & Hypoxic Ischemic Encephalopathy in the Neonate Kaleidoscope 2017 LEIGH ANN CATES PHD, APRN, NNP -BC, RRT-NPS, CHSE N E O N ATA L N U R S E P R A C T I T I O N E R - T E X A S C H I
The Pharmacology of Hypotension: Vasopressor Choices for HIE patients. Keliana O Mara, PharmD August 4, 2018
 The Pharmacology of Hypotension: Vasopressor Choices for HIE patients Keliana O Mara, PharmD August 4, 2018 Objectives Review the pathophysiology of hypotension in neonates Discuss the role of vasopressors
The Pharmacology of Hypotension: Vasopressor Choices for HIE patients Keliana O Mara, PharmD August 4, 2018 Objectives Review the pathophysiology of hypotension in neonates Discuss the role of vasopressors
I mprovements in perinatal and neonatal care have contributed
 ORIGINAL ARTICLE Posthaemorrhagic ventricular in the premature infant: natural history and predictors of outcome B P Murphy, T E Inder, V Rooks, G A Taylor, N J Anderson, N Mogridge, L J Horwood, J J Volpe...
ORIGINAL ARTICLE Posthaemorrhagic ventricular in the premature infant: natural history and predictors of outcome B P Murphy, T E Inder, V Rooks, G A Taylor, N J Anderson, N Mogridge, L J Horwood, J J Volpe...
The Blue Baby. Network Stabilisation of the Term Infant Study Day 15 th March 2017 Joanna Behrsin
 The Blue Baby Network Stabilisation of the Term Infant Study Day 15 th March 2017 Joanna Behrsin Session Structure Definitions and assessment of cyanosis Causes of blue baby Structured approach to assessing
The Blue Baby Network Stabilisation of the Term Infant Study Day 15 th March 2017 Joanna Behrsin Session Structure Definitions and assessment of cyanosis Causes of blue baby Structured approach to assessing
SWISS SOCIETY OF NEONATOLOGY. Neonatal cerebral infarction
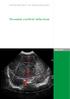 SWISS SOCIETY OF NEONATOLOGY Neonatal cerebral infarction May 2002 2 Mann C, Neonatal and Pediatric Intensive Care Unit, Landeskrankenhaus und Akademisches Lehrkrankenhaus Feldkirch, Austria Swiss Society
SWISS SOCIETY OF NEONATOLOGY Neonatal cerebral infarction May 2002 2 Mann C, Neonatal and Pediatric Intensive Care Unit, Landeskrankenhaus und Akademisches Lehrkrankenhaus Feldkirch, Austria Swiss Society
Surfactant Administration
 Approved by: Surfactant Administration Gail Cameron Senior Director Operations, Maternal, Neonatal & Child Health Programs Dr. Paul Byrne Medical Director, Neonatology Neonatal Policy & Procedures Manual
Approved by: Surfactant Administration Gail Cameron Senior Director Operations, Maternal, Neonatal & Child Health Programs Dr. Paul Byrne Medical Director, Neonatology Neonatal Policy & Procedures Manual
Noah Hillman M.D. IPOKRaTES Conference Guadalajaira, Mexico August 23, 2018
 Postnatal Steroids Use for Bronchopulmonary Dysplasia in 2018 + = Noah Hillman M.D. IPOKRaTES Conference Guadalajaira, Mexico August 23, 2018 AAP Policy Statement - 2002 This statement is intended for
Postnatal Steroids Use for Bronchopulmonary Dysplasia in 2018 + = Noah Hillman M.D. IPOKRaTES Conference Guadalajaira, Mexico August 23, 2018 AAP Policy Statement - 2002 This statement is intended for
GUIDELINE PHYSIOLOGY OF BIRTH ASPHYXIA
 GUIDELINE PHYSIOLOGY OF BIRTH ASPHYXIA The newborn is not an adult, nor a child. In people of all ages, death can occur from a failure of breathing and / or circulation. The interventions required to aid
GUIDELINE PHYSIOLOGY OF BIRTH ASPHYXIA The newborn is not an adult, nor a child. In people of all ages, death can occur from a failure of breathing and / or circulation. The interventions required to aid
Study of renal functions in neonatal asphyxia
 Original article: Study of renal functions in neonatal asphyxia *Dr. D.Y.Shrikhande, **Dr. Vivek Singh, **Dr. Amit Garg *Professor and Head, **Senior Resident Department of Pediatrics, Pravara Institute
Original article: Study of renal functions in neonatal asphyxia *Dr. D.Y.Shrikhande, **Dr. Vivek Singh, **Dr. Amit Garg *Professor and Head, **Senior Resident Department of Pediatrics, Pravara Institute
Hyaline membrane disease. By : Dr. Ch Sarishma Peadiatric Pg
 Hyaline membrane disease By : Dr. Ch Sarishma Peadiatric Pg Also called Respiratory distress syndrome. It occurs primarily in premature infants; its incidence is inversely related to gestational age and
Hyaline membrane disease By : Dr. Ch Sarishma Peadiatric Pg Also called Respiratory distress syndrome. It occurs primarily in premature infants; its incidence is inversely related to gestational age and
Difficulties at Birth: Long Term Developmental Outcomes
 Difficulties at Birth: Long Term Developmental Outcomes Alan D. Bedrick MD Division of Neonatology and Developmental Biology Department of Pediatrics University of Arizona Tucson, Arizona DISCLOSURE I
Difficulties at Birth: Long Term Developmental Outcomes Alan D. Bedrick MD Division of Neonatology and Developmental Biology Department of Pediatrics University of Arizona Tucson, Arizona DISCLOSURE I
Neonatal Resuscitation. Dustin Coyle, M.D. Anesthesiology
 Neonatal Resuscitation Dustin Coyle, M.D. Anesthesiology Recognize complications Maternal-fetal factors Maternal DM PIH Chronic HTN Previous stillbirth Rh sensitization Infection Substance abuse/certain
Neonatal Resuscitation Dustin Coyle, M.D. Anesthesiology Recognize complications Maternal-fetal factors Maternal DM PIH Chronic HTN Previous stillbirth Rh sensitization Infection Substance abuse/certain
Decrease in the incidence of Intraventricular hemorrhage (IVH) after the introduction of an IVH prevention bundle in the NICU
 Decrease in the incidence of Intraventricular hemorrhage (IVH) after the introduction of an IVH prevention bundle in the NICU Susan M Bedwell, MSN, Brianna Bright, MA and Kris C Sekar, MD. Neonatal Services,
Decrease in the incidence of Intraventricular hemorrhage (IVH) after the introduction of an IVH prevention bundle in the NICU Susan M Bedwell, MSN, Brianna Bright, MA and Kris C Sekar, MD. Neonatal Services,
Cover Page. The handle holds various files of this Leiden University dissertation
 Cover Page The handle http://hdl.handle.net/1887/22368 holds various files of this Leiden University dissertation Author: Lugt, Neeltje Margaretha van der Title: Neonatal pearls : safety and efficacy of
Cover Page The handle http://hdl.handle.net/1887/22368 holds various files of this Leiden University dissertation Author: Lugt, Neeltje Margaretha van der Title: Neonatal pearls : safety and efficacy of
INTRODUCTION The effect of CPAP works on lung mechanics to improve oxygenation (PaO 2
 2 Effects of CPAP INTRODUCTION The effect of CPAP works on lung mechanics to improve oxygenation (PaO 2 ). The effect on CO 2 is only secondary to the primary process of improvement in lung volume and
2 Effects of CPAP INTRODUCTION The effect of CPAP works on lung mechanics to improve oxygenation (PaO 2 ). The effect on CO 2 is only secondary to the primary process of improvement in lung volume and
1 Pediatric Advanced Life Support Science Update What s New for 2010? 3 CPR. 4 4 Steps of BLS Survey 5 CPR 6 CPR.
 1 Pediatric Advanced Life Support Science Update 2010 2 What s New for 2010? 3 CPR Take no longer than seconds for pulse check Rate at least on per minute (instead of around 100 per minute ) Depth change:
1 Pediatric Advanced Life Support Science Update 2010 2 What s New for 2010? 3 CPR Take no longer than seconds for pulse check Rate at least on per minute (instead of around 100 per minute ) Depth change:
Appendix 1. Causes of Neonatal Deaths. Interval between. Gestation at birth. birth and death. Allocation. (weeks +days ) Cause of death.
 Appendix 1. Causes of Neonatal Deaths Interval between Gestation at birth birth and death Allocation (weeks +days ) (days) Cause of death Amnioinfusion 25 +1/7 20 Respiratory and circulatory insufficiency
Appendix 1. Causes of Neonatal Deaths Interval between Gestation at birth birth and death Allocation (weeks +days ) (days) Cause of death Amnioinfusion 25 +1/7 20 Respiratory and circulatory insufficiency
Swiss neonatal network and Follow up Group
 Swiss neonatal network and Follow up Group March 2011 Barbara Brotschi and Cornelia Hagmann Hypoxic ischaemic encephalopathy Neonatal encephalopathy due to perinatal hypoxiaischaemia: clinically defined
Swiss neonatal network and Follow up Group March 2011 Barbara Brotschi and Cornelia Hagmann Hypoxic ischaemic encephalopathy Neonatal encephalopathy due to perinatal hypoxiaischaemia: clinically defined
