High-Flow Nasal Cannulae in Very Preterm Infants after Extubation
|
|
|
- Anne Cameron
- 6 years ago
- Views:
Transcription
1 original article High-Flow Nasal Cannulae in Very Preterm Infants after Extubation Brett J. Manley, M.B., B.S., Louise S. Owen, M.D., Lex W. Doyle, M.D., Chad C. Andersen, M.B., B.S., David W. Cartwright, M.B., B.S., Margo A. Pritchard, Ph.D., Susan M. Donath, M.A., and Peter G. Davis, M.D. A bs tr ac t Background The use of high-flow nasal cannulae is an increasingly popular alternative to nasal continuous positive airway pressure (CPAP) for noninvasive respiratory support of very preterm infants (gestational age, <32 weeks) after extubation. However, data on the efficacy or safety of such cannulae in this population are lacking. Methods In this multicenter, randomized, noninferiority trial, we assigned 303 very preterm infants to receive treatment with either high-flow nasal cannulae (5 to 6 liters per minute) or nasal CPAP (7 cm of water) after extubation. The primary outcome was treatment failure within 7 days. Noninferiority was determined by calculating the absolute difference in the risk of the primary outcome; the margin of noninferiority was 20 percentage points. Infants in whom treatment with high-flow nasal cannulae failed could be treated with nasal CPAP; infants in whom nasal CPAP failed were reintubated. Results The use of high-flow nasal cannulae was noninferior to the use of nasal CPAP, with treatment failure occurring in 52 of 152 infants (34.2%) in the nasal-cannulae group and in 39 of 151 infants (25.8%) in the CPAP group (risk difference, 8.4 percentage points; 95% confidence interval, 1.9 to 18.7). Almost half the infants in whom treatment with high-flow nasal cannulae failed were successfully treated with CPAP without reintubation. The incidence of nasal trauma was significantly lower in the nasal-cannulae group than in the CPAP group (P = 0.01), but there were no significant differences in rates of serious adverse events or other complications. From the Newborn Research Centre, Royal Women s Hospital (B.J.M., L.S.O., L.W.D., P.G.D.), the Departments of Obstetrics and Gynaecology (B.J.M., L.S.O., L.W.D., P.G.D.) and Paediatrics (L.W.D., S.M.D.), University of Melbourne, and the Critical Care and Neurosciences Theme (B.J.M., L.S.O., L.W.D., P.G.D.) and Clinical Epidemiology and Biostatistics Unit (S.M.D.), Murdoch Children s Research Institute, Melbourne, VIC, the Department of Neonatal Medicine, Women s and Children s Hospital, Adelaide, SA (C.C.A.), and Women s and Newborn Services, Royal Brisbane and Women s Hospital (D.W.C., M.A.P.), the Department of Paediatrics and Child Health, University of Queensland (D.W.C.), and the University of Queensland Centre for Clinical Research (M.A.P.), Brisbane, QLD all in Australia. Address reprint requests to Dr. Manley at the Newborn Research Centre, Level 7, Royal Women s Hospital, 20 Flemington Rd., Parkville, VIC 3052, Australia, or at brett.manley@thewomens.org.au. N Engl J Med 2013;369: DOI: /NEJMoa Copyright 2013 Massachusetts Medical Society. Conclusions Although the result for the primary outcome was close to the margin of noninferiority, the efficacy of high-flow nasal cannulae was similar to that of CPAP as respiratory support for very preterm infants after extubation. (Funded by the National Health and Medical Research Council; Australian New Zealand Clinical Trials Network number, ACTRN ) n engl j med 369;15 nejm.org october 10,
2 In the United States, approximately 75,000 infants were classified as very preterm (gestational age, <32 weeks) in Very preterm infants have substantially higher mortality and morbidity than term infants, partly because they are more prone to respiratory failure and often require mechanical ventilation through an endotracheal tube after birth. Once they recover from their acute breathing problems, the best way to achieve successful extubation from mechanical ventilation is controversial. Nasal continuous positive airway pressure (CPAP) is known to be superior to no positive-pressure support 2 and is the current standard of care for noninvasive respiratory support of very preterm infants. The use of high-flow nasal cannulae is an alternative means of providing noninvasive respiratory support to very preterm infants. Such cannulae deliver heated and humidified gas at flow rates of more than 1 liter per minute through small binasal prongs. Because high-flow nasal cannulae have a simpler interface with the infant and smaller prongs than nasal CPAP, the cannulae are perceived as easier to use, more comfortable for the infant, and advantageous for mother infant bonding. 3,4 The use of high-flow nasal cannulae is increasingly popular for noninvasive respiratory support in neonatal intensive care units around the world. 3,5 In 2008, the devices were being used in up to two thirds of academic neonatal units in the United States. 6 High-flow nasal cannulae are used to prevent extubation failure in preterm infants, with prescribed flow rates typically ranging from 2 to 8 liters per minute. 3,7,8 However, there is little evidence regarding the risks and benefits of this new technique. 9,10 Several mechanisms of action of high-flow nasal cannulae in preterm infants have been described, 11 but the contribution of each is undefined. Furthermore, concern has been raised that the generation of unregulated distending pressure from high-flow nasal cannulae might injure the lungs. 12,13 We hypothesized that high-flow nasal cannulae are an appropriate alternative to nasal CPAP, even if the devices do not provide superior respiratory support. We performed a multicenter, randomized, noninferiority trial of high-flow nasal cannulae, as compared with nasal CPAP, as respiratory support after extubation in preterm infants born at a gestational age of less than 32 weeks. Me thods Study Design and Oversight From May 31, 2010, to July 3, 2012, we recruited infants in three Australian neonatal intensive care units at the Royal Women s Hospital, Melbourne; Women s and Children s Hospital, Adelaide; and the Royal Brisbane and Women s Hospital, Brisbane. The trial was designed and conducted by the authors. The human research ethics committee at each center approved the trial. The study was funded by the participating neonatal units and by the National Health and Medical Research Council. All authors vouch for the accuracy and completeness of the data and the fidelity of the study to the protocol, which is available with the full text of this article at NEJM.org. There was no commercial support for this study. Patients Infants were eligible if they were born at a gestational age of less than 32 weeks, were receiving mechanical ventilation through an endotracheal tube, and were scheduled to undergo extubation for the first time to noninvasive respiratory support. Infants were ineligible if their gestational age was more than 36 weeks at the time of extubation, if they were participating in a concurrent study that prohibited inclusion, if they had a known major congenital anomaly that might affect breathing, or if maximal intensive care was not being provided. All parents or guardians provided written informed consent. Randomization A computer-generated block-randomization sequence with random block sizes was used. All infants were stratified according to gestational age (<26 weeks vs. 26 weeks) and study center. Infants who were part of multiple births underwent individual randomization. Clinicians opened consecutively numbered, sealed, opaque envelopes immediately before extubation to determine the study-group assignment. In the case of an unplanned extubation in an infant for whom consent had already been obtained, randomization occurred if the treating physician decided to provide noninvasive respiratory support. If the infant required resuscitation and immediate reintubation, randomization did not occur, and the infant remained eligible for inclusion with the next planned extubation n engl j med 369;15 nejm.org october 10, 2013
3 Nasal Cannulae in Very Preterm Infants after Extubation Study Intervention Infants received oral or intravenous caffeine (either a loading dose of 20 mg per kilogram of body weight or a maintenance dose of 5 to 10 mg per kilogram) in the 24 hours before extubation. The treating physician made the decision to extubate an infant before randomization. Infants received their assigned treatment immediately after extubation. Treatment was considered to have failed if an infant who was receiving maximal respiratory support with the assigned treatment met one or more of the following four criteria for failure within 7 days after extubation: a fraction of inspired oxygen of 0.2 or more above the baseline value before extubation that was required to maintain a peripheral oxygen saturation of 88 to 92%; a ph of less than 7.2 and a partial pressure of carbon dioxide of more than 60 mm Hg on an arterial or free-flowing capillary blood gas sample; more than one apneic episode requiring intermittent positive-pressure ventilation within a 24-hour period or six or more apneic episodes requiring stimulation within 6 consecutive hours; or an urgent need for reintubation and mechanical ventilation, as determined by the treating physician. Infants in the nasal-cannulae group were treated with the Optiflow device, which included the MR850 humidifier and binasal infant cannulae (Fisher & Paykel Healthcare). Infants were fitted with prongs that maintained a leak at the nose, with the aim of occluding approximately half the nares. The device includes a pressurerelief valve that limits circuit pressure to 45 cm of water. The starting flow rate was based on the size of the prongs used, with 5 liters per minute for premature or neonatal prongs or 6 liters per minute for infant, intermediate infant, or pediatric prongs. Flow rates were altered at the physician s discretion in a stepwise fashion, with mandated limits between 2 liters per minute and the maximum recommended for the prong size: 6 liters per minute for premature and neonatal prongs, 7 liters per minute for infant or intermediate infant prongs, and 8 liters per minute for pediatric prongs. For infants who were weaned to 2 liters per minute and who had a fraction of inspired oxygen of less than 0.3 for more than 24 hours, treatment with the high-flow nasal cannulae could be stopped, although such cessation of therapy was not mandatory, and earlier cessation was ordered at the discretion of the treating team if the fraction of inspired oxygen was less than 0.3. Infants in whom treatment with high-flow nasal cannulae failed were treated with nasal CPAP (7 cm of water), with the same pressure limits as those used in the CPAP group. If the CPAP treatment failed under the maximal pressure during the first 7 days after extubation, infants were reintubated. Infants in the CPAP group were started on a pressure of 7 cm of water with either binasal midline prongs (Fisher & Paykel Healthcare) or subnasal prongs (Hudson RCI), depending on the practice in the individual unit. Nasal CPAP was generated with the use of a mechanical ventilator or an underwater bubble system. Pressures were altered at the physician s discretion, within limits of 5 to 8 cm of water during the first 7 days after extubation. Nonsynchronized nasal intermittent positive-pressure ventilation could be used at the time of extubation or at any later time during the admission, with a maximum inflating pressure of 25 cm of water and a maximum rate of 40 inflations per minute. Once infants were weaned to a nasal CPAP of 5 cm of water with a fraction of inspired oxygen of less than 0.3 for more than 24 hours, nasal CPAP could be discontinued. Infants in whom CPAP treatment failed at a maximal pressure of 8 cm of water (with or without nonsynchronized nasal intermittent positive-pressure ventilation) were reintubated. Infants in the CPAP group were not permitted to receive treatment with high-flow nasal cannulae at any time during their admission. Study Outcomes The primary outcome was treatment failure within 7 days (168 hours) after extubation. Prespecified secondary outcomes included reintubation during the primary-outcome period, death before hospital discharge, a requirement for supplemental oxygen at a gestational age of 36 weeks, pneumothorax after trial entry, total days of any respiratory support after trial entry, duration of oxygen supplementation after trial entry, and length of hospital admission. Nursing and medical staff members, who were aware of studygroup assignments, regularly assessed the nasal skin and septum for trauma caused by pressure from the binasal prongs. Data included the incidence of any nasal trauma, its cause, and the need for change of treatment because of nasal trauma. n engl j med 369;15 nejm.org october 10,
4 1099 Infants born at <32 wk of gestational age were screened for eligibility 622 Were ineligible 530 Did not undergo ventilation 30 Died before extubation 25 Were excluded 15 Had major congenital anomaly 8 Were included in another extubation study 2 Had gestational age 36 wk 14 Were extubated to no support 13 Received palliative care 10 Were extubated at another hospital 477 Were eligible 174 Did not undergo randomization 77 Were not approached 34 Had consent declined 28 Had medical reasons 19 Were unable to have consent provided for social reasons 10 Had unplanned extubation 6 Had consent provided but did not undergo randomization 3 Died before randomization 2 Had consent withdrawn 1 Missed randomization 303 Underwent randomization 152 Were assigned to high-flow nasal cannulae 151 Were assigned to nasal CPAP 152 Were included in primary analysis 151 Were included in primary analysis Figure 1. Enrollment and Outcomes. Serious adverse events were predefined as pneumothorax occurring while the infants received the assigned treatment and death. These events were reported to the principal investigator and the relevant ethics committee as they occurred. Data were collected until death or the first discharge home from the hospital. Statistical Analysis We estimated that extubation would have a failure rate of 25% for very preterm infants on the basis of a review of 2 years of data from the lead center (the Royal Women s Hospital, Melbourne), where high-flow nasal cannulae had not been used previously. We prespecified the margin of noninferiority for high-flow nasal cannulae as 20 percentage points above the failure rate for nasal CPAP. Since little guidance was available for such a comparison, the choice of margin was somewhat arbitrary. Our rationale was that many clinicians already strongly prefer the use of highflow nasal cannulae, and the devices are in widespread use because of their perceived benefits over nasal CPAP. We allowed the use of rescue nasal CPAP for infants in whom treatment with highflow nasal cannulae failed. This procedure reflected 1428 n engl j med 369;15 nejm.org october 10, 2013
5 Nasal Cannulae in Very Preterm Infants after Extubation Table 1. Characteristics of the Infants and Their Mothers.* Characteristic Nasal-Cannulae Group (N = 152) CPAP Group (N = 151) Mothers White race no. (%) 127 (83.6) 120 (79.5) Primigravida no. (%) 61 (40.1) 59 (39.1) Exposure to antenatal glucocorticoids no. (%) 142 (93.4) 143 (94.7) Cesarean section no. (%) 101 (66.4) 101 (66.9) Infants Gestational age No. of wk 27.7± ±1.9 <26 wk no. (%) 32 (21.1) 31 (20.5) Birth weight g 1041± ±327 Male sex no. (%) 89 (58.6) 72 (47.7) Multiple birth no. (%) 49 (32.2) 52 (34.4) Intubated in the delivery room no. (%) 102 (67.1) 91 (60.3) Median Apgar score at 5 min (IQR) 7 (6 8) 8 (6 8) Surfactant treatment no. (%) 141 (92.8) 144 (95.4) Caffeine treatment before extubation no. (%) 151 (99.3) 148 (98.0) Median postnatal age at extubation (IQR) hr 43.2 ( ) 38.5 ( ) Median duration of mechanical ventilation before 36 ( ) 36 (20 93) extubation (IQR) hr ph before extubation 7.33± ±0.06 Partial pressure of carbon dioxide before extubation 44.2± ±9.0 mm Hg Fraction of inspired oxygen before extubation 0.23± ±0.04 * Plus minus values are means ±SD. There were no significant differences between the two groups. CPAP denotes continuous positive airway pressure, and IQR interquartile range. Race was reported by the investigators. The Apgar score was not known for three infants. Blood gas results were from capillary (heel-prick) or arterial blood samples. clinical practice around the world at centers where both treatments are commonly available. The primary outcome was short-term treatment efficacy, rather than death or disability, for which a lower noninferiority margin might be recommended. 14 Noninferiority was determined by calculating the absolute risk difference and 95% confidence interval for the primary outcome. For treatment with high-flow nasal cannulae to be noninferior, the upper limit of the 95% confidence interval had to be below 20 percentage points, and the lower limit of the 95% confidence interval had to be below zero. 14 We determined that a sample of 300 infants was required to show noninferiority for high-flow nasal cannulae with a power of 87%. All analyses were performed on an intentionto-treat basis, and infants remained in their assigned group for all outcomes. For the primary outcome and dichotomous secondary outcomes, we calculated the difference between the groups using risk differences and 95% confidence intervals. We used chi-square tests to compare secondary dichotomous outcomes and the appropriate parametric test (Student s t-test) or nonparametric test (Wilcoxon rank-sum test) to compare continuous outcomes. An independent data and safety monitoring committee performed two interim analyses, when the primary outcome was known for 100 infants and for 200 infants, and on the basis of each analysis recommended that the study continue unaltered. All analyses were performed with the use of Stata/IC software, version 12.0 (StataCorp). n engl j med 369;15 nejm.org october 10,
6 Table 2. Reasons for Treatment Failure, Reintubation, and Other Secondary Outcomes. Outcome Nasal-Cannulae Group (N = 152) CPAP Group (N = 151) Risk Difference (95% CI)* P Value percentage points Reason for treatment failure no./ total no. (%) Apnea 32/52 (61.5) 25/39 (64.1) 2.6 ( 22.6 to 17.5) 0.80 Increase in fraction of inspired 21/52 (40.4) 20/39 (51.3) 10.9 ( 31.5 to 9.7) 0.30 oxygen Respiratory acidosis 6/52 (11.5) 2/39 (5.1) 6.4 ( 4.7 to 17.5) 0.29 Urgent need for intubation 2/52 (3.8) 4/39 (10.3) 6.4 ( 17.3 to 4.5) 0.22 Reintubation within 7 days after 27 (17.8) 38 (25.2) 7.4 ( 16.6 to 1.8) 0.12 extubation no. (%) Median no. of days of respiratory support 34 (7 to 55) 38 (11 to 57) NA 0.44 after trial entry (IQR) Median no. of days of oxygen therapy 38 (0 to 78) 49 (8 to 83) NA 0.15 after trial entry (IQR) Median weight gain during first 7 days 20 ( 42 to 79.5) 10 ( 54 to 75) NA 0.39 after extubation (IQR) g Median discharge weight (IQR) g 2886 (2515 to 3290) 2950 (2500 to 3524) NA 0.53 Median no. of days in a tertiary care center (IQR) 65 (43 to 91) 64 (45 to 104) NA 0.56 Median no. of days in any hospital (IQR) 79 (63 to 105) 84 (65 to 106) NA 0.38 * Positive values favor the CPAP group, and negative values favor the nasal-cannulae group. Treatment may have failed for more than one reason. In one infant in the CPAP group, treatment failed because a set pressure of more than 8 cm of water was prescribed during the primary-outcome period. One infant had a measured ph of exactly 7.20, and one infant had a measured ph of Respiratory support included the use of high-flow nasal cannulae, nasal CPAP, nonsynchronized nasal intermittent positive-pressure ventilation, and mechanical ventilation through an endotracheal tube. Infants who died before discharge were excluded. This category includes only hospital days, even though some infants received oxygen therapy after being discharged. R esult s Study Patients A total of 303 infants underwent randomization (152 to the nasal-cannulae group and 151 to the CPAP group) during the study period (Fig. 1). The demographic and clinical characteristics of the mothers and infants in the two groups were similar (Table 1). Primary Outcome The use of high-flow nasal cannulae was found to be noninferior to the use of nasal CPAP by our definition, with treatment failure occurring in 52 of 152 infants (34.2%) in the nasal-cannulae group and 39 of 151 infants (25.8%) in the CPAP group (risk difference, 8.4 percentage points; 95% confidence interval, 1.9 to 18.7). Treatment failure stratified according to gestational-age subgroup is provided in Table S1 in the Supplementary Appendix, available at NEJM.org. The most common reason for treatment failure in the two study groups was apnea (Table 2), with no significant between-group difference in the reason for failure. Failure was most likely to occur during the first day after extubation in the two groups (Fig. 2). Reintubation during the First 7 Days after Extubation Of the 52 infants in whom treatment with highflow nasal cannulae failed during the first 7 days after extubation, 25 (48%) were successfully treated with nasal CPAP or nonsynchronized nasal intermittent positive-pressure ventilation without reintubation. Thus, only 17.8% of infants in the nasal-cannulae group were reintubated, as compared with 25.2% of those in the CPAP group (P = 0.12) (Table 2). In the latter group, 3 infants received nonsynchronized nasal intermittent positive-pressure ventilation immediately after extubation, and 36 infants (23.8%) received non n engl j med 369;15 nejm.org october 10, 2013
7 Nasal Cannulae in Very Preterm Infants after Extubation synchronized nasal intermittent positive-pressure ventilation during the first 7 days after extubation; of these infants, 22 were reintubated after treatment failure. Other Secondary Outcomes and Adverse Events There were no significant between-group differences in rates of other secondary outcomes (Table 2) or in rates of death or other serious adverse events (Table 3). The pneumothorax rate after trial entry was low in the two study groups. In the nasal-cannulae group, infants had a significantly lower incidence of nasal trauma than those in the CPAP group (39.5% vs. 54.3%, P = 0.01), and fewer infants required a change in therapy because of nasal trauma (P = 0.001). No infant in either group required surgical correction for nasal trauma. Almost half the cases of nasal trauma in the nasal-cannulae group were diagnosed while the infants were receiving other types of noninvasive respiratory support. If the diagnosis of nasal trauma was limited to cases that were diagnosed during the assigned treatment, the between-group difference was greater, with a rate of 19.1% in the nasal-cannulae group versus 53.0% in the CPAP group (P<0.001). Discussion On the basis of our prespecified definition of noninferiority, the use of high-flow nasal cannulae was noninferior to the use of nasal CPAP as respiratory support for very preterm infants after extubation, with a between-group difference of 8.4 percentage points that favored CPAP. When treatment with high-flow nasal cannulae failed, about half the infants were successfully treated with CPAP without reintubation, resulting in a nonsignificant between-group difference in the rate of reintubation within 7 days after extubation. Among infants in the nasal-cannulae group, there was no increase in rates of death or complications and there was a decrease in the rate of nasal trauma, as compared with the CPAP group. The use of high-flow nasal cannulae has been widely adopted without evidence of safety and efficacy. The results of two small, randomized trials of these devices, as compared with nasal CPAP, after extubation have been reported previously. Both these trials were conducted in a single center and had a superiority design. In the study by Campbell et al., infants with a birth weight of less than 1250 g were randomly assigned to Percentage of Infants without Treatment Failure No. at Risk High-flow nasal cannulae Nasal CPAP Nasal CPAP High-flow nasal cannulae No. of Days after Extubation Figure 2. Very Preterm Infants without Treatment Failure after Extubation. Shown are the percentages of very preterm infants (gestational age, <32 weeks) in whom treatment with either high-flow nasal cannulae or nasal continuous positive airway pressure (CPAP) did not fail after extubation during the primary outcome period (7 days). Treatment failure occurred in 52 of 152 infants (34.2%) in the nasal-cannulae group and 39 of 151 infants (25.8%) in the CPAP group (risk difference, 8.4 percentage points) one of the two treatments after extubation. Significantly more infants in the nasal-cannulae group required reintubation within 7 days, probably because of the reduced flow rates (<2 liters per minute) that were used. In the study by Collins et al., 16 which involved 132 very preterm infants, the investigators used a different high-flow nasal-cannulae device (Vapotherm) and an increased flow rate (8 liters per minute) after extubation. They found no significant between-group difference in the primary outcome of treatment failure within 7 days and no significant difference in reintubation rates, although the authors acknowledged that the study was underpowered for these outcomes. Nasal-trauma scores were lower in the nasal-cannulae group than in the CPAP group, which is consistent with our findings. In our multicenter trial, 63 of the infants had a gestational age of less than 26 weeks, but the study was not powered to evaluate the efficacy or safety of high-flow nasal cannulae in this extremely preterm subgroup. The treatment-failure rate was very high among these infants, regardless of the assigned treatment, and the risk difference was 20 percentage points in favor of nasal CPAP (see the Supplementary Appendix). Given this finding, we think that clinicians should be n engl j med 369;15 nejm.org october 10,
8 Table 3. Adverse Events. Event Nasal-Cannulae Group (N = 152) CPAP Group (N = 151) Risk Difference (95% CI)* P Value no. (%) percentage points Death before discharge 5 (3.3) 6 (4.0) 0.7 ( 4.9 to 3.5) 0.75 Oxygen supplementation at gestational age of 36 wk Glucocorticoid treatment for lung disease after trial entry Pneumothorax 47 (30.9) 52 (34.4) 3.5 ( 14.1 to 7.0) (17.8) 30 (19.9) 2.1 ( 10.9 to 6.7) 0.64 Anytime after trial entry 1 (0.7) 4 (2.6) 2.0 ( 4.9 to 0.9) 0.17 During assigned treatment 0 1 (0.7) 0.6 ( 1.9 to 0.6) 0.32 Discharged home with oxygen 21 (13.8) 15 (9.9) 3.9 ( 3.4 to 11.2) 0.30 Proven sepsis after trial entry 26 (17.1) 30 (19.9) 2.8 ( 11.5 to 6.0) 0.54 Patent ductus arteriosus treated with medication or surgery 64 (42.1) 64 (42.4) 0.3 ( 11.4 to 10.8) 0.96 Necrotizing enterocolitis stage 2 or 3 3 (2.0) 7 (4.6) 2.7 ( 6.7 to 1.4) 0.20 Isolated intestinal perforation 1 (0.7) 2 (1.3) 0.7 ( 2.9 to 1.6) 0.56 Laser surgery for retinopathy of prematurity 8 (5.3) 8 (5.3) 0.0 ( 5.1 to 5.0) 0.99 Intraventricular hemorrhage grade 3 or 4 3 (2.0) 8 (5.3) 3.3 ( 7.5 to 0.9) 0.12 Cystic periventricular leukomalacia 5 (3.3) 3 (2.0) 1.3 ( 2.3 to 4.9) 0.48 Nasal trauma Any documented 60 (39.5) 82 (54.3) 14.8 ( 25.9 to 3.7) 0.01 Leading to change of treatment 8 (5.3) 27 (17.9) 12.6 ( 19.7 to 5.5) Caused by the assigned treatment 29 (19.1) 80 (53.0) 33.9 ( 44.0 to 23.8) <0.001 * Positive values favor the CPAP group, and negative values favor the nasal-cannulae group. This event was prespecified as a serious adverse event. Infants who died before hospital discharge were excluded. cautious before using high-flow nasal cannulae as first-line respiratory support in extremely preterm infants after extubation. The risk difference for treatment failure among infants with a gestational age of 26 weeks or more was 5 percentage points. The results of our trial pertain only to infants who have been extubated and should not be extrapolated to the use of high-flow nasal cannulae as primary respiratory support after birth. We used the Fisher & Paykel high-flow nasal-cannulae system in our trial, but there is no evidence that any one commercially available system is superior to another. 17 The flow rates for the nasal cannulae that we used have been shown to result in distending pressures in vivo that are similar to, or slightly lower than, commonly set nasal CPAP pressures Since it was not possible to blind the intervention, we used prespecified, objective criteria for treatment failure as the primary outcome to minimize bias. We allowed the use of rescue nasal CPAP in the nasal-cannulae group only after treatment failure. We acknowledge that this may have influenced some of the secondary outcomes in the nasalcannulae group. Because the use of nasal CPAP prevented the reintubation of almost half the infants in whom treatment with nasal cannulae had failed, it seems reasonable to use high-flow nasal cannulae initially after extubation if both therapies are available. In our trial, nonsynchronized nasal intermittent positive-pressure ventilation could be used in infants receiving nasal CPAP. If the use of nonsynchronized nasal intermittent positive n engl j med 369;15 nejm.org october 10, 2013
9 Nasal Cannulae in Very Preterm Infants after Extubation pressure ventilation had not been permitted until nasal CPAP had failed, the risk difference for the primary outcome might have been smaller. When appraising the result of a noninferiority trial, both the risk difference and its 95% confidence interval are considered. Although our best estimation of the risk difference was 8.4 percentage points, we acknowledge that the upper limit of the 95% confidence interval was close to the margin of noninferiority (20 percentage points) and that slightly different failure rates in either group could have altered our conclusions. Further research on the use of high-flow nasal cannulae in preterm and term newborn infants is required. Since such cannulae are used with increasing frequency in the nontertiary care setting, randomized trials of high-flow nasal cannulae as primary respiratory support from birth will be important. We conclude that treatment with high-flow nasal cannulae was noninferior to the use of nasal CPAP as respiratory support after extubation in very preterm infants. Since our trial was underpowered to show noninferiority in infants with a gestational age of less than 26 weeks, the use of high-flow nasal cannulae as first-line respiratory support after extubation in this extremely preterm group requires caution. Supported by a program grant (606789) and a Centre for Clinical Research Excellence grant (546519) from the National Health and Medical Research Council. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. We thank the patients families and the staff members who cared for them at the three participating centers, and research nurses Connie Wong, Donna Hovey, Louise Goodchild, and Ros Lontis for their assistance in recruitment and data collection. References 1. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for National vital statistics reports. Vol. 61. No. 5. Hyattsville, MD: National Center for Health Statistics, (DHHS publication no. (PHS) ) 2. Davis P, Henderson-Smart D. Postextubation prophylactic nasal continuous positive airway pressure in preterm infants: systematic review and meta-analysis. J Paediatr Child Health 1999;35: Hough JL, Shearman AD, Jardine LA, Davies MW. Humidified high flow nasal cannulae: current practice in Australasian nurseries, a survey. J Paediatr Child Health 2012;48: Manley BJ, Owen L, Doyle LW, Davis PG. High-flow nasal cannulae and nasal continuous positive airway pressure use in non-tertiary special care nurseries in Australia and New Zealand. J Paediatr Child Health 2012;48: Nath P, Ponnusamy V, Willis K, Bissett L, Clarke P. Current practices of high and low flow oxygen therapy and humidification in UK neonatal units. Pediatr Int 2010;52: Hochwald O, Osiovich H. The use of high flow nasal cannulae in neonatal intensive care units: is clinical practice consistent with the evidence? J Neonat Perinat Med 2010;3: Holleman-Duray D, Kaupie D, Weiss MG. Heated humidified high-flow nasal cannula: use and a neonatal early extubation protocol. J Perinatol 2007;27: Shoemaker MT, Pierce MR, Yoder BA, DiGeronimo RJ. High flow nasal cannula versus nasal CPAP for neonatal respiratory disease: a retrospective study. J Perinatol 2007;27: Wilkinson D, Andersen C, O Donnell CP, De Paoli AG. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev 2011; 5:CD Manley BJ, Dold SK, Davis PG, Roehr CC. High-flow nasal cannulae for respiratory support of preterm infants: a review of the evidence. Neonatology 2012;102: Shaffer TH, Alapati D, Greenspan JS, Wolfson MR. Neonatal non-invasive respiratory support: physiological implications. Pediatr Pulmonol 2012;47: Finer NN. Nasal cannula use in the preterm infant: oxygen or pressure? Pediatrics 2005;116: Finer NN, Mannino FL. High-flow nasal cannula: a kinder, gentler CPAP? J Pediatr 2009;154: Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 2006;295: [Erratum, JAMA 2006;296:1842.] 15. Campbell DM, Shah PS, Shah V, Kelly EN. Nasal continuous positive airway pressure from high flow cannula versus Infant Flow for preterm infants. J Perinatol 2006; 26: Collins CL, Holberton JR, Barfield C, Davis PG. A randomized controlled trial to compare heated humidified high-flow nasal cannulae with nasal continuous positive airway pressure postextubation in premature infants. J Pediatr 2012;162(5): 949.e1-954.e Miller SM, Dowd SA. High-flow nasal cannula and extubation success in the premature infant: a comparison of two modalities. J Perinatol 2010;30: Spence KL, Murphy D, Kilian C, Mc- Gonigle R, Kilani RA. High-flow nasal cannula as a device to provide continuous positive airway pressure in infants. J Perinatol 2007;27: Kubicka ZJ, Limauro J, Darnall RA. Heated, humidified high-flow nasal cannula therapy: yet another way to deliver continuous positive airway pressure? Pediatrics 2008;121: Wilkinson DJ, Andersen CC, Smith K, Holberton J. Pharyngeal pressure with high-flow nasal cannulae in premature infants. J Perinatol 2008;28:42-7. Copyright 2013 Massachusetts Medical Society icmje recommendations The International Committee of Medical Journal Editors (ICMJE) has revised and renamed its Uniform Requirements. The new ICMJE Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals are available at n engl j med 369;15 nejm.org october 10,
Nasal High-Flow Therapy for Primary Respiratory Support in Preterm Infants
 The new england journal of medicine Original Article Nasal High-Flow Therapy for Primary Respiratory Support in Preterm Infants Calum T. Roberts, M.B., Ch.B., Louise S. Owen, M.D., Brett J. Manley, Ph.D.,
The new england journal of medicine Original Article Nasal High-Flow Therapy for Primary Respiratory Support in Preterm Infants Calum T. Roberts, M.B., Ch.B., Louise S. Owen, M.D., Brett J. Manley, Ph.D.,
Name and title of the investigators responsible for conducting the research: Dr Anna Lavizzari, Dr Mariarosa Colnaghi
 Protocol title: Heated, Humidified High-Flow Nasal Cannula vs Nasal CPAP for Respiratory Distress Syndrome of Prematurity. Protocol identifying number: Clinical Trials.gov NCT02570217 Name and title of
Protocol title: Heated, Humidified High-Flow Nasal Cannula vs Nasal CPAP for Respiratory Distress Syndrome of Prematurity. Protocol identifying number: Clinical Trials.gov NCT02570217 Name and title of
Randomised controlled trial of weaning strategies for preterm infants on nasal continuous positive airway pressure
 Tang et al. BMC Pediatrics (2015) 15:147 DOI 10.1186/s12887-015-0462-0 RESEARCH ARTICLE Open Access Randomised controlled trial of weaning strategies for preterm infants on nasal continuous positive airway
Tang et al. BMC Pediatrics (2015) 15:147 DOI 10.1186/s12887-015-0462-0 RESEARCH ARTICLE Open Access Randomised controlled trial of weaning strategies for preterm infants on nasal continuous positive airway
CPAP failure in preterm infants: incidence, predictors and consequences
 CPAP failure in preterm infants: incidence, predictors and consequences SUPPLEMENTAL TEXT METHODS Study setting The Royal Hobart Hospital has an 11-bed combined Neonatal and Paediatric Intensive Care Unit
CPAP failure in preterm infants: incidence, predictors and consequences SUPPLEMENTAL TEXT METHODS Study setting The Royal Hobart Hospital has an 11-bed combined Neonatal and Paediatric Intensive Care Unit
Non-invasive ventilatory strategies, such as
 R E S E A R C H P A P E R Heated Humidified High Flow Nasal Cannula versus Nasal Continuous Positive Airway Pressure as Primary Mode of Respiratory Support for Respiratory Distress in Preterm Infants DEEPARAJ
R E S E A R C H P A P E R Heated Humidified High Flow Nasal Cannula versus Nasal Continuous Positive Airway Pressure as Primary Mode of Respiratory Support for Respiratory Distress in Preterm Infants DEEPARAJ
Kugelman A, Riskin A, Said W, Shoris I, Mor F, Bader D.
 Heated, Humidified High-Flow Nasal Cannula (HHHFNC) vs. Nasal Intermittent Positive Pressure Ventilation (NIPPV) for the Primary Treatment of RDS, A Randomized, Controlled, Prospective, Pilot Study Kugelman
Heated, Humidified High-Flow Nasal Cannula (HHHFNC) vs. Nasal Intermittent Positive Pressure Ventilation (NIPPV) for the Primary Treatment of RDS, A Randomized, Controlled, Prospective, Pilot Study Kugelman
ROLE OF PRESSURE IN HIGH FLOW THERAPY
 ROLE OF PRESSURE IN HIGH FLOW THERAPY Thomas L. Miller, PhD, MEd Director, Clinical Research and Education Vapotherm, Inc. Research Assistant Professor of Pediatrics Jefferson Medical College This information
ROLE OF PRESSURE IN HIGH FLOW THERAPY Thomas L. Miller, PhD, MEd Director, Clinical Research and Education Vapotherm, Inc. Research Assistant Professor of Pediatrics Jefferson Medical College This information
Clinical Guideline: Heated Humidified High Flow Nasal Cannula (HHHFNC) Guideline
 EOE Neonatal ODN Clinical Guideline: Heated Humidified High Flow Nasal Cannula (HHHFNC) Guideline Authors: Dr Eliana Panayiotou and Dr Bharat Vakharia For use in: EoE Neonatal Units Guidance specific to
EOE Neonatal ODN Clinical Guideline: Heated Humidified High Flow Nasal Cannula (HHHFNC) Guideline Authors: Dr Eliana Panayiotou and Dr Bharat Vakharia For use in: EoE Neonatal Units Guidance specific to
5/3/2012. Goals and Objectives HFNC. High-Flow Oxygen Therapy: Real Benefit or Just a Fad?
 High-Flow Oxygen Therapy: Real Benefit or Just a Fad? Timothy R. Myers MBA, RRT-NPS Director, Women s & Children s Respiratory Care & Procedural Services and Pediatric Heart Center Rainbow Babies & Children
High-Flow Oxygen Therapy: Real Benefit or Just a Fad? Timothy R. Myers MBA, RRT-NPS Director, Women s & Children s Respiratory Care & Procedural Services and Pediatric Heart Center Rainbow Babies & Children
This is a pre-copyedited, author-produced PDF of an article accepted for publication in Journal of Neonatal Nursing following peer review.
 This is a pre-copyedited, author-produced PDF of an article accepted for publication in Journal of Neonatal Nursing following peer review. The version of record [Journal of Neonatal Nursing (February 2013)
This is a pre-copyedited, author-produced PDF of an article accepted for publication in Journal of Neonatal Nursing following peer review. The version of record [Journal of Neonatal Nursing (February 2013)
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION
 CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION Method of maintaining low pressure distension of lungs during inspiration and expiration when infant breathing spontaneously Benefits Improves oxygenation
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION Method of maintaining low pressure distension of lungs during inspiration and expiration when infant breathing spontaneously Benefits Improves oxygenation
ORIGINAL ARTICLE. DD Woodhead, DK Lambert, JM Clark and RD Christensen. Intermountain Healthcare, McKay-Dee Hospital, Ogden, UT, USA
 ORIGINAL ARTICLE Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, crossover trial DD Woodhead, DK Lambert,
ORIGINAL ARTICLE Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, crossover trial DD Woodhead, DK Lambert,
Neonatal Resuscitation Using a Nasal Cannula: A Single-Center Experience
 Original Article Neonatal Resuscitation Using a Nasal Cannula: A Single-Center Experience Pedro Paz, MD, MPH 1 Rangasamy Ramanathan, MD 1,2 Richard Hernandez, RCP 2 Manoj Biniwale, MD 1 1 Division of Neonatal
Original Article Neonatal Resuscitation Using a Nasal Cannula: A Single-Center Experience Pedro Paz, MD, MPH 1 Rangasamy Ramanathan, MD 1,2 Richard Hernandez, RCP 2 Manoj Biniwale, MD 1 1 Division of Neonatal
COMPARISON OF THE EFFICIENCY OF CAFFEINE VERSUS AMINOPHYLLINE FOR THE TREATMENT OF APNOEA OF PREMATURITY
 CASE STUDIES COMPARISON OF THE EFFICIENCY OF CAFFEINE VERSUS AMINOPHYLLINE FOR THE TREATMENT OF APNOEA OF PREMATURITY Gabriela Ildiko Zonda 1, Andreea Avasiloaiei 1, Mihaela Moscalu 2, Maria Stamatin 1
CASE STUDIES COMPARISON OF THE EFFICIENCY OF CAFFEINE VERSUS AMINOPHYLLINE FOR THE TREATMENT OF APNOEA OF PREMATURITY Gabriela Ildiko Zonda 1, Andreea Avasiloaiei 1, Mihaela Moscalu 2, Maria Stamatin 1
High-flow nasal cannula use in a paediatric intensive care unit over 3 years
 High-flow nasal cannula use in a paediatric intensive care unit over 3 years Tracey I Wraight and Subodh S Ganu Respiratory illness and/or distress is the commonest reason for non-elective paediatric intensive
High-flow nasal cannula use in a paediatric intensive care unit over 3 years Tracey I Wraight and Subodh S Ganu Respiratory illness and/or distress is the commonest reason for non-elective paediatric intensive
Noah Hillman M.D. IPOKRaTES Conference Guadalajaira, Mexico August 23, 2018
 Postnatal Steroids Use for Bronchopulmonary Dysplasia in 2018 + = Noah Hillman M.D. IPOKRaTES Conference Guadalajaira, Mexico August 23, 2018 AAP Policy Statement - 2002 This statement is intended for
Postnatal Steroids Use for Bronchopulmonary Dysplasia in 2018 + = Noah Hillman M.D. IPOKRaTES Conference Guadalajaira, Mexico August 23, 2018 AAP Policy Statement - 2002 This statement is intended for
Advantages and disadvantages of different nasal CPAP systems in newborns
 Intensive Care Med (2004) 30:926 930 DOI 10.1007/s00134-004-2267-8 N E O N A T A L A N D P A E D I A T R I C I N T E N S I V E C A R E V. Buettiker M. I. Hug O. Baenziger C. Meyer B. Frey Advantages and
Intensive Care Med (2004) 30:926 930 DOI 10.1007/s00134-004-2267-8 N E O N A T A L A N D P A E D I A T R I C I N T E N S I V E C A R E V. Buettiker M. I. Hug O. Baenziger C. Meyer B. Frey Advantages and
Nasal CPAP in Neonatology: We Can Do Better
 Nasal CPAP in Neonatology: We Can Do Better COI Disclosure I do not have any conflict of interest, nor will I be discussing any off-label product use. This class has no commercial support or sponsorship,
Nasal CPAP in Neonatology: We Can Do Better COI Disclosure I do not have any conflict of interest, nor will I be discussing any off-label product use. This class has no commercial support or sponsorship,
Facilitating EndotracheaL Intubation by Laryngoscopy technique and Apneic Oxygenation Within the Intensive Care Unit (FELLOW)
 Facilitating EndotracheaL Intubation by Laryngoscopy technique and Apneic Oxygenation Within the Intensive Data Analysis Plan: Apneic Oxygenation vs. No Apneic Oxygenation Background Critically ill patients
Facilitating EndotracheaL Intubation by Laryngoscopy technique and Apneic Oxygenation Within the Intensive Data Analysis Plan: Apneic Oxygenation vs. No Apneic Oxygenation Background Critically ill patients
Bubble CPAP for Respiratory Distress Syndrome in Preterm Infants
 R E S E A R C H P A P E R Bubble CPAP for Respiratory Distress Syndrome in Preterm Infants JAGDISH KOTI*, SRINIVAS MURKI, PRAMOD GADDAM, ANUPAMA REDDY AND M DASARADHA RAMI REDDY From Fernandez Hospital
R E S E A R C H P A P E R Bubble CPAP for Respiratory Distress Syndrome in Preterm Infants JAGDISH KOTI*, SRINIVAS MURKI, PRAMOD GADDAM, ANUPAMA REDDY AND M DASARADHA RAMI REDDY From Fernandez Hospital
Hyaline membrane disease. By : Dr. Ch Sarishma Peadiatric Pg
 Hyaline membrane disease By : Dr. Ch Sarishma Peadiatric Pg Also called Respiratory distress syndrome. It occurs primarily in premature infants; its incidence is inversely related to gestational age and
Hyaline membrane disease By : Dr. Ch Sarishma Peadiatric Pg Also called Respiratory distress syndrome. It occurs primarily in premature infants; its incidence is inversely related to gestational age and
Admission/Discharge Form for Infants Born in Please DO NOT mail or fax this form to the CPQCC Data Center. This form is for internal use ONLY.
 Selection Criteria Admission/Discharge Form for Infants Born in 2016 To be eligible, you MUST answer YES to at least one of the possible criteria (A-C) A. 401 1500 grams o Yes B. GA range 22 0/7 31 6/7
Selection Criteria Admission/Discharge Form for Infants Born in 2016 To be eligible, you MUST answer YES to at least one of the possible criteria (A-C) A. 401 1500 grams o Yes B. GA range 22 0/7 31 6/7
Prophylactic Aminophylline for Prevention of Apnea at Higher-Risk Preterm Neonates
 Iran Red Crescent Med J. 2014 August; 16(8): e12559. Published online 2014 August 5. DOI: 10.5812/ircmj.12559 Research Article Prophylactic Aminophylline for Prevention of Apnea at Higher-Risk Preterm
Iran Red Crescent Med J. 2014 August; 16(8): e12559. Published online 2014 August 5. DOI: 10.5812/ircmj.12559 Research Article Prophylactic Aminophylline for Prevention of Apnea at Higher-Risk Preterm
ROLE OF EARLY POSTNATAL DEXAMETHASONE IN RESPIRATORY DISTRESS SYNDROME
 INDIAN PEDIATRICS VOLUME 35-FEBRUAKY 1998 ROLE OF EARLY POSTNATAL DEXAMETHASONE IN RESPIRATORY DISTRESS SYNDROME Kanya Mukhopadhyay, Praveen Kumar and Anil Narang From the Division of Neonatology, Department
INDIAN PEDIATRICS VOLUME 35-FEBRUAKY 1998 ROLE OF EARLY POSTNATAL DEXAMETHASONE IN RESPIRATORY DISTRESS SYNDROME Kanya Mukhopadhyay, Praveen Kumar and Anil Narang From the Division of Neonatology, Department
ADMISSION/DISCHARGE FORM FOR INFANTS BORN IN 2019 DO NOT mail or fax this form to the CPQCC Data Center. This form is for internal use ONLY.
 1 Any eligible inborn infant who dies in the delivery room or at any other location in your hospital within 12 hours after birth and prior to admission to the NICU is defined as a "Delivery Room Death."
1 Any eligible inborn infant who dies in the delivery room or at any other location in your hospital within 12 hours after birth and prior to admission to the NICU is defined as a "Delivery Room Death."
AEROSURF Phase 2 Program Update Investor Conference Call
 AEROSURF Phase 2 Program Update Investor Conference Call November 12, 2015 Forward Looking Statement To the extent that statements in this presentation are not strictly historical, including statements
AEROSURF Phase 2 Program Update Investor Conference Call November 12, 2015 Forward Looking Statement To the extent that statements in this presentation are not strictly historical, including statements
Nasal versus oral intubation for mechanical ventilation of newborn infants (Review)
 Nasal versus oral intubation for mechanical ventilation of newborn infants (Review) Spence K, Barr P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published
Nasal versus oral intubation for mechanical ventilation of newborn infants (Review) Spence K, Barr P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published
NEONATAL NEWS Here s Some More Good Poop
 NEONATALNEWS Here ssomemoregoodpoop WINTEREDITION2010 THISNEWSLETTERISPUBLISHEDPERIODICALLYBYTHENEONATOLOGISTSOF ASSOCIATESINNEWBORNMEDICINETOCONVEYNEWANDUPDATEDPOLICIES ANDGUIDELINESANDPROVIDEGENERALEDUCATIONTONICUCARETAKERSAT
NEONATALNEWS Here ssomemoregoodpoop WINTEREDITION2010 THISNEWSLETTERISPUBLISHEDPERIODICALLYBYTHENEONATOLOGISTSOF ASSOCIATESINNEWBORNMEDICINETOCONVEYNEWANDUPDATEDPOLICIES ANDGUIDELINESANDPROVIDEGENERALEDUCATIONTONICUCARETAKERSAT
Ge Zheng, 1,2 Xiao-qiu Huang, 2 Hui-hui Zhao, 2 Guo-Xing Jin, 2 and Bin Wang Introduction. 2. Methods
 Hindawi Canadian Respiratory Journal Volume 2017, Article ID 3782401, 6 pages https://doi.org/10.1155/2017/3782401 Clinical Study The Effect of the Treatment with Heated Humidified High-Flow Nasal Cannula
Hindawi Canadian Respiratory Journal Volume 2017, Article ID 3782401, 6 pages https://doi.org/10.1155/2017/3782401 Clinical Study The Effect of the Treatment with Heated Humidified High-Flow Nasal Cannula
OmniOx (HFT 500) Jan Prepared by MEKICS. OmniOx-HFT500 Jan ver1.1 1
 OmniOx (HFT 500) Jan. 2013. Prepared by MEKICS OmniOx-HFT500 Jan. 2013 ver1.1 1 0. Index 1. Introduce of OmniOx (HTF500) 2. Specification (HFT, CPAP+) 3. The World Best Features 2 OmniOx-HFT500 Jan. 2013
OmniOx (HFT 500) Jan. 2013. Prepared by MEKICS OmniOx-HFT500 Jan. 2013 ver1.1 1 0. Index 1. Introduce of OmniOx (HTF500) 2. Specification (HFT, CPAP+) 3. The World Best Features 2 OmniOx-HFT500 Jan. 2013
Early nasal intermittent positive pressure ventilation(nippv) versus early nasal continuous positive airway pressure(ncpap) for preterm
 Cochrane Database of Systematic Reviews Early nasal intermittent positive pressure ventilation(nippv) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants(review) LemyreB,LaughonM,BoseC,DavisPG
Cochrane Database of Systematic Reviews Early nasal intermittent positive pressure ventilation(nippv) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants(review) LemyreB,LaughonM,BoseC,DavisPG
Surfactant Administration
 Approved by: Surfactant Administration Gail Cameron Senior Director Operations, Maternal, Neonatal & Child Health Programs Dr. Paul Byrne Medical Director, Neonatology Neonatal Policy & Procedures Manual
Approved by: Surfactant Administration Gail Cameron Senior Director Operations, Maternal, Neonatal & Child Health Programs Dr. Paul Byrne Medical Director, Neonatology Neonatal Policy & Procedures Manual
Von Reuss and CPAP, Disclosures CPAP. Noninvasive respiratory therapieswhy bother? Noninvasive respiratory therapies- types
 Noninvasive respiratory therapiesby a nose? NEO- The Conference for Neonatology February 21, 2014 Disclosures I have no relevant financial relationships to disclose or conflicts of interest to release.
Noninvasive respiratory therapiesby a nose? NEO- The Conference for Neonatology February 21, 2014 Disclosures I have no relevant financial relationships to disclose or conflicts of interest to release.
STOP ROP The STOP-ROP Multicenter Study Group: Pediatrics 105:295, 2000 Progression to Threshold Conventional Sat 89-94% STOP ROP
 Hrs TcPO2 > 80 nnhg (weeks 1 4) OXYGEN TARGETS: HOW GOOD ARE WE IN ACHIEVING THEM Oxygen Dependency GA wks Eduardo Bancalari MD University of Miami Miller School of Medicine Jackson Memorial Medical Center
Hrs TcPO2 > 80 nnhg (weeks 1 4) OXYGEN TARGETS: HOW GOOD ARE WE IN ACHIEVING THEM Oxygen Dependency GA wks Eduardo Bancalari MD University of Miami Miller School of Medicine Jackson Memorial Medical Center
Exclusion Criteria 1. Operator or supervisor feels specific intra- procedural laryngoscopy device will be required.
 FELLOW Study Data Analysis Plan Direct Laryngoscopy vs Video Laryngoscopy Background Respiratory failure requiring endotracheal intubation occurs in as many as 40% of critically ill patients. Procedural
FELLOW Study Data Analysis Plan Direct Laryngoscopy vs Video Laryngoscopy Background Respiratory failure requiring endotracheal intubation occurs in as many as 40% of critically ill patients. Procedural
A Trial Comparing Noninvasive Ventilation Strategies in Preterm Infants
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article A Trial Comparing Noninvasive Ventilation Strategies in Preterm Infants Haresh Kirpalani, B.M., M.Sc., David Millar, M.B., Brigitte
T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article A Trial Comparing Noninvasive Ventilation Strategies in Preterm Infants Haresh Kirpalani, B.M., M.Sc., David Millar, M.B., Brigitte
Home-Made Continuous Positive Airways Pressure Device may Reduce Mortality in Neonates with Respiratory Distress in Low-Resource Setting
 JOURNAL OF TROPICAL PEDIATRICS, VOL. 60, NO. 5, 2014 Home-Made Continuous Positive Airways Pressure Device may Reduce Mortality in Neonates with Respiratory Distress in Low-Resource Setting by Subhashchandra
JOURNAL OF TROPICAL PEDIATRICS, VOL. 60, NO. 5, 2014 Home-Made Continuous Positive Airways Pressure Device may Reduce Mortality in Neonates with Respiratory Distress in Low-Resource Setting by Subhashchandra
Provide guidelines for the management of mechanical ventilation in infants <34 weeks gestation.
 Page 1 of 5 PURPOSE: Provide guidelines for the management of mechanical ventilation in infants
Page 1 of 5 PURPOSE: Provide guidelines for the management of mechanical ventilation in infants
Newborn Life Support. NLS guidance.
 Kelly Harvey, ANNP NWNODN, previously Wythenshawe Hospital has shared this presentation with the understanding that it is for personal use following your attendance at the 8th Annual Senior Neonatal Nursing
Kelly Harvey, ANNP NWNODN, previously Wythenshawe Hospital has shared this presentation with the understanding that it is for personal use following your attendance at the 8th Annual Senior Neonatal Nursing
Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants(review)
 Cochrane Database of Systematic Reviews Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants (Review) SubramaniamP,HoJJ,DavisPG Subramaniam
Cochrane Database of Systematic Reviews Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants (Review) SubramaniamP,HoJJ,DavisPG Subramaniam
Non Invasive Ventilation In Preterm Infants. Manuel Sanchez Luna Hospital General Universitario Gregorio Marañón Complutense University Madrid
 Non Invasive Ventilation In Preterm Infants Manuel Sanchez Luna Hospital General Universitario Gregorio Marañón Complutense University Madrid Summary Noninvasive ventilation begings in the delivery room
Non Invasive Ventilation In Preterm Infants Manuel Sanchez Luna Hospital General Universitario Gregorio Marañón Complutense University Madrid Summary Noninvasive ventilation begings in the delivery room
Acute Paediatric Respiratory Pathway
 Guideline for the use of high Flow Nasal Cannula Oxygen Therapy (Optiflow or Airvo) in Children with Bronchiolitis or an acute respiratory illness Introduction: High flow nasal cannula (HFNC) oxygen enables
Guideline for the use of high Flow Nasal Cannula Oxygen Therapy (Optiflow or Airvo) in Children with Bronchiolitis or an acute respiratory illness Introduction: High flow nasal cannula (HFNC) oxygen enables
Uses 1,2,3 : Labeled: Prevention of respiratory distress syndrome in premature infants
 Brand Name: Surfaxin Generic Name: lucinactant Manufacturer 1 : Discovery Laboratories, Inc. Drug Class 2,3 : Synthetic lung surfactant Uses 1,2,3 : Labeled: Prevention of respiratory distress syndrome
Brand Name: Surfaxin Generic Name: lucinactant Manufacturer 1 : Discovery Laboratories, Inc. Drug Class 2,3 : Synthetic lung surfactant Uses 1,2,3 : Labeled: Prevention of respiratory distress syndrome
Short-Term Outcome Of Different Treatment Modalities Of Patent Ductus Arteriosus In Preterm Infants. Five Years Experiences In Qatar
 ISPUB.COM The Internet Journal of Cardiovascular Research Volume 7 Number 2 Short-Term Outcome Of Different Treatment Modalities Of Patent Ductus Arteriosus In Preterm Infants. Five Years Experiences In
ISPUB.COM The Internet Journal of Cardiovascular Research Volume 7 Number 2 Short-Term Outcome Of Different Treatment Modalities Of Patent Ductus Arteriosus In Preterm Infants. Five Years Experiences In
Hazards and Benefits of Postnatal Steroids. David J. Burchfield, MD Professor and Chief, Neonatology University of Florida
 Hazards and Benefits of Postnatal Steroids David J. Burchfield, MD Professor and Chief, Neonatology University of Florida Disclosures I have no financial affiliations or relationships to disclose. I will
Hazards and Benefits of Postnatal Steroids David J. Burchfield, MD Professor and Chief, Neonatology University of Florida Disclosures I have no financial affiliations or relationships to disclose. I will
heated humidified high-flow nasal cannula therapy in children F A Hutchings, 1 T N Hilliard, 1 P J Davis 2 Review
 1 Department of Paediatric Respiratory Medicine, Bristol Royal Hospital for Children, Bristol, UK 2 Department of Paediatric Intensive Care, Bristol Royal Hospital for Children, Bristol, UK Correspondence
1 Department of Paediatric Respiratory Medicine, Bristol Royal Hospital for Children, Bristol, UK 2 Department of Paediatric Intensive Care, Bristol Royal Hospital for Children, Bristol, UK Correspondence
Usefulness of DuoPAP in the treatment of very low birth weight preterm infants with neonatal respiratory distress syndrome
 European Review for Medical and Pharmacological Sciences 2015; 19: 573-577 Usefulness of DuoPAP in the treatment of very low birth weight preterm infants with neonatal respiratory distress syndrome B.
European Review for Medical and Pharmacological Sciences 2015; 19: 573-577 Usefulness of DuoPAP in the treatment of very low birth weight preterm infants with neonatal respiratory distress syndrome B.
Cover Page. The handle holds various files of this Leiden University dissertation
 Cover Page The handle http://hdl.handle.net/1887/46692 holds various files of this Leiden University dissertation Author: Zanten, Henriëtte van Title: Oxygen titration and compliance with targeting oxygen
Cover Page The handle http://hdl.handle.net/1887/46692 holds various files of this Leiden University dissertation Author: Zanten, Henriëtte van Title: Oxygen titration and compliance with targeting oxygen
SARASOTA MEMORIAL HOSPITAL DEPARTMENT POLICY
 PS1006 SARASOTA MEMORIAL HOSPITAL DEPARTMENT POLICY TITLE: NON-INVASIVE VENTILATION FOR THE Job Title of Reviewer: EFFECTIVE DATE: REVISED DATE: Director, Respiratory Care Services 126.685 (neo) 3/26/15
PS1006 SARASOTA MEMORIAL HOSPITAL DEPARTMENT POLICY TITLE: NON-INVASIVE VENTILATION FOR THE Job Title of Reviewer: EFFECTIVE DATE: REVISED DATE: Director, Respiratory Care Services 126.685 (neo) 3/26/15
Practical Application of CPAP
 CHAPTER 3 Practical Application of CPAP Dr. Srinivas Murki Neonatologist Fernadez Hospital, Hyderabad. A.P. Practical Application of CPAP Continuous positive airway pressure (CPAP) applied to premature
CHAPTER 3 Practical Application of CPAP Dr. Srinivas Murki Neonatologist Fernadez Hospital, Hyderabad. A.P. Practical Application of CPAP Continuous positive airway pressure (CPAP) applied to premature
GS3. Understanding How to Use Statistics to Evaluate an Article. Session Summary. Session Objectives. References. Session Outline
 GS3 Understanding How to Use Statistics to Evaluate an Article Reese H. Clark, MD Director of Research Pediatrix Medical Group Neonatologist Greenville Memorial Hospital, Greenville, SC The speaker has
GS3 Understanding How to Use Statistics to Evaluate an Article Reese H. Clark, MD Director of Research Pediatrix Medical Group Neonatologist Greenville Memorial Hospital, Greenville, SC The speaker has
Objectives. Apnea Definition and Pitfalls. Pathophysiology of Apnea. Apnea of Prematurity and hypoxemia episodes 5/18/2015
 Apnea of Prematurity and hypoxemia episodes Deepak Jain MD Care of Sick Newborn Conference May 2015 Objectives Differentiating between apnea and hypoxemia episodes. Pathophysiology Diagnosis of apnea and
Apnea of Prematurity and hypoxemia episodes Deepak Jain MD Care of Sick Newborn Conference May 2015 Objectives Differentiating between apnea and hypoxemia episodes. Pathophysiology Diagnosis of apnea and
O) or to bi-level NCPAP (group B; n=20, lower CPAP level=4.5 cm H 2
 1 NICU, Children s Hospital, Via Castelvetro, Milan, Italy 2 Laboratory, V.Buzzi Children s Hospital ICP, Milan, Italy 3 Department of Statistics, Catholic University, Milan, Italy Correspondence to Gianluca
1 NICU, Children s Hospital, Via Castelvetro, Milan, Italy 2 Laboratory, V.Buzzi Children s Hospital ICP, Milan, Italy 3 Department of Statistics, Catholic University, Milan, Italy Correspondence to Gianluca
Original Policy Date
 MP 8.01.17 Inhaled Nitric Oxide Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return to Medical Policy Index
MP 8.01.17 Inhaled Nitric Oxide Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return to Medical Policy Index
SWISS SOCIETY OF NEONATOLOGY. Spontaneous intestinal perforation or necrotizing enterocolitis?
 SWISS SOCIETY OF NEONATOLOGY Spontaneous intestinal perforation or necrotizing enterocolitis? June 2004 2 Stocker M, Berger TM, Neonatal and Pediatric Intensive Care Unit, Children s Hospital of Lucerne,
SWISS SOCIETY OF NEONATOLOGY Spontaneous intestinal perforation or necrotizing enterocolitis? June 2004 2 Stocker M, Berger TM, Neonatal and Pediatric Intensive Care Unit, Children s Hospital of Lucerne,
Benefits of Caffeine Citrate: Neurodevelopmental Outcomes of ELBW Infants
 St. Catherine University SOPHIA Master of Arts in Nursing Theses Nursing 12-2011 Benefits of Caffeine Citrate: Neurodevelopmental Outcomes of ELBW Infants Teri Johnson St. Catherine University Follow this
St. Catherine University SOPHIA Master of Arts in Nursing Theses Nursing 12-2011 Benefits of Caffeine Citrate: Neurodevelopmental Outcomes of ELBW Infants Teri Johnson St. Catherine University Follow this
** SURFACTANT THERAPY**
 ** SURFACTANT THERAPY** Full Title of Guideline: Surfactant Therapy Author (include email and role): Stephen Wardle (V4) Reviewed by Dushyant Batra Consultant Neonatologist Division & Speciality: Division:
** SURFACTANT THERAPY** Full Title of Guideline: Surfactant Therapy Author (include email and role): Stephen Wardle (V4) Reviewed by Dushyant Batra Consultant Neonatologist Division & Speciality: Division:
NON INVASIVE LIFE SAVERS. Non Invasive Ventilation (NIV)
 Table 1. NIV: Mechanisms Of Action Decreases work of breathing Increases functional residual capacity Recruits collapsed alveoli Improves respiratory gas exchange Reverses hypoventilation Maintains upper
Table 1. NIV: Mechanisms Of Action Decreases work of breathing Increases functional residual capacity Recruits collapsed alveoli Improves respiratory gas exchange Reverses hypoventilation Maintains upper
Neonatal Life Support Provider (NLSP) Certification Preparatory Materials
 Neonatal Life Support Provider (NLSP) Certification Preparatory Materials NEONATAL LIFE SUPPORT PROVIDER (NRP) CERTIFICATION TABLE OF CONTENTS NEONATAL FLOW ALGORITHM.2 INTRODUCTION 3 ANTICIPATION OF RESUSCITATION
Neonatal Life Support Provider (NLSP) Certification Preparatory Materials NEONATAL LIFE SUPPORT PROVIDER (NRP) CERTIFICATION TABLE OF CONTENTS NEONATAL FLOW ALGORITHM.2 INTRODUCTION 3 ANTICIPATION OF RESUSCITATION
ARTIFICIAL INTELLIGENCE FOR PREDICTION OF SEPSIS IN VERY LOW BIRTH WEIGHT INFANTS
 ARTIFICIAL INTELLIGENCE FOR PREDICTION OF SEPSIS IN VERY LOW BIRTH WEIGHT INFANTS Markus Leskinen MD PhD, Neonatologist Children s Hospital, University of Helsinki and Helsinki University Hospital The
ARTIFICIAL INTELLIGENCE FOR PREDICTION OF SEPSIS IN VERY LOW BIRTH WEIGHT INFANTS Markus Leskinen MD PhD, Neonatologist Children s Hospital, University of Helsinki and Helsinki University Hospital The
Systems differ in their ability to deliver optimal humidification
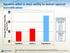 Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Disclosure COULD AUTOMATED CONTROL OF OXYGEN LEVELS IMPROVE SURVIVAL AND REDUCE NEC? Oxygen Dependency
 COULD AUTOMATED CONTROL OF OXYGEN LEVELS IMPROVE SURVIVAL AND REDUCE NEC? Eduardo Bancalari MD University of Miami Miller School of Medicine Jackson Memorial Medical Center Sydney 206 Disclosure The University
COULD AUTOMATED CONTROL OF OXYGEN LEVELS IMPROVE SURVIVAL AND REDUCE NEC? Eduardo Bancalari MD University of Miami Miller School of Medicine Jackson Memorial Medical Center Sydney 206 Disclosure The University
Critical Appraisal Practicum. Fabio Di Bello Medical Implementation Manager
 Critical Appraisal Practicum Fabio Di Bello Medical Implementation Manager fdibello@ebsco.com What we ll talk about today: DynaMed process for appraising randomized trials and writing evidence summaries
Critical Appraisal Practicum Fabio Di Bello Medical Implementation Manager fdibello@ebsco.com What we ll talk about today: DynaMed process for appraising randomized trials and writing evidence summaries
With wider acceptance of the early use of
 Weaning of nasal CPAP in preterm infants: who, when and how? a systematic review of literature Shaili Amatya, Deepa Rastogi, Alok Bhutada, Shantanu Rastogi New York, USA Background: There is increased
Weaning of nasal CPAP in preterm infants: who, when and how? a systematic review of literature Shaili Amatya, Deepa Rastogi, Alok Bhutada, Shantanu Rastogi New York, USA Background: There is increased
Title Neonatal and Paediatric High-Flow Nasal Cannula Oxygen Therapy Guideline. Department Paediatrics / Neonates Date Issued
 Document Control Title Author Neonatal and Paediatric High-Flow Nasal Cannula Oxygen Therapy Guideline Author s job title Directorate Medical Version Department Paediatrics / Neonates Date Issued Status
Document Control Title Author Neonatal and Paediatric High-Flow Nasal Cannula Oxygen Therapy Guideline Author s job title Directorate Medical Version Department Paediatrics / Neonates Date Issued Status
Serious Air Leak Syndrome Complicating High-Flow Nasal Cannula Therapy: A Report of 3 Cases
 CASE REPORT Serious Air Leak Syndrome Complicating High-Flow Nasal Cannula Therapy: A Report of 3 Cases AUTHORS: Satyanarayan Hegde, MD a and Parthak Prodhan, MBBS b a Division of Pediatric Pulmonary Medicine,
CASE REPORT Serious Air Leak Syndrome Complicating High-Flow Nasal Cannula Therapy: A Report of 3 Cases AUTHORS: Satyanarayan Hegde, MD a and Parthak Prodhan, MBBS b a Division of Pediatric Pulmonary Medicine,
Simulation 3: Post-term Baby in Labor and Delivery
 Simulation 3: Post-term Baby in Labor and Delivery Opening Scenario (Links to Section 1) You are an evening-shift respiratory therapist in a large hospital with a level III neonatal unit. You are paged
Simulation 3: Post-term Baby in Labor and Delivery Opening Scenario (Links to Section 1) You are an evening-shift respiratory therapist in a large hospital with a level III neonatal unit. You are paged
Minimizing Lung Damage During Respiratory Support
 Minimizing Lung Damage During Respiratory Support University of Miami Jackson Memorial Medical Center Care of the Sick Newborn 15 Eduardo Bancalari MD University of Miami Miller School of Medicine Jackson
Minimizing Lung Damage During Respiratory Support University of Miami Jackson Memorial Medical Center Care of the Sick Newborn 15 Eduardo Bancalari MD University of Miami Miller School of Medicine Jackson
Pneumothorax in preterm babies has been associated with an increased risk of intraventricular hemorrhage (IVH)
 Identification of Pneumothorax in Very Preterm Infants Risha Bhatia, MB, MA, MRCPCH, Peter G. Davis, MD, FRACP, Lex W. Doyle, MD, FRACP, Connie Wong, RN, and Colin J. Morley, MD, FRACP, FRCPCH Objective
Identification of Pneumothorax in Very Preterm Infants Risha Bhatia, MB, MA, MRCPCH, Peter G. Davis, MD, FRACP, Lex W. Doyle, MD, FRACP, Connie Wong, RN, and Colin J. Morley, MD, FRACP, FRCPCH Objective
High Flow Nasal Cannula Oxygen HFNC. Dr I S Kalla Department of Pulmonology University of the Witwatersrand
 786 High Flow Nasal Cannula Oxygen HFNC Dr I S Kalla Department of Pulmonology University of the Witwatersrand Disclaimer I was a scep@c un@l I used it Now I am a firm believer HFNC The Fisher and Paykel
786 High Flow Nasal Cannula Oxygen HFNC Dr I S Kalla Department of Pulmonology University of the Witwatersrand Disclaimer I was a scep@c un@l I used it Now I am a firm believer HFNC The Fisher and Paykel
Continuous distending pressure for respiratory distress in preterm infants(review)
 Cochrane Database of Systematic Reviews Continuous distending pressure for respiratory distress in preterm infants(review) HoJJ,SubramaniamP,DavisPG Ho JJ, Subramaniam P, Davis PG.. Cochrane Database of
Cochrane Database of Systematic Reviews Continuous distending pressure for respiratory distress in preterm infants(review) HoJJ,SubramaniamP,DavisPG Ho JJ, Subramaniam P, Davis PG.. Cochrane Database of
Neonatal Respiratory Physiotherapy. Nicky Hawkes Advanced Respiratory Physiotherapist Oct 2011
 Neonatal Respiratory Physiotherapy Nicky Hawkes Advanced Respiratory Physiotherapist Oct 2011 Respiratory Physiotherapy?..Not just percussion! Assessment of baby s respiratory status and deciding what
Neonatal Respiratory Physiotherapy Nicky Hawkes Advanced Respiratory Physiotherapist Oct 2011 Respiratory Physiotherapy?..Not just percussion! Assessment of baby s respiratory status and deciding what
Research Article Timing of Caffeine Therapy and Neonatal Outcomes in Preterm Infants: A Retrospective Study
 International Pediatrics Volume 2016, Article ID 9478204, 6 pages http://dx.doi.org/10.1155/2016/9478204 Research Article Timing of Caffeine Therapy and Neonatal Outcomes in Preterm Infants: A Retrospective
International Pediatrics Volume 2016, Article ID 9478204, 6 pages http://dx.doi.org/10.1155/2016/9478204 Research Article Timing of Caffeine Therapy and Neonatal Outcomes in Preterm Infants: A Retrospective
9/15/2017. Disclosures. Heated High Flow Nasal Cannula: Hot Air or Optimal Noninvasive Support? Objectives. Aerogen Pharma
 Heated High Flow Nasal Cannula: Hot Air or Optimal Noninvasive Support? Rob DiBlasi RRT-NPS, FAARC Program Manager Research/QI, Respiratory Therapy Principle Investigator, Seattle Children s Research Institute
Heated High Flow Nasal Cannula: Hot Air or Optimal Noninvasive Support? Rob DiBlasi RRT-NPS, FAARC Program Manager Research/QI, Respiratory Therapy Principle Investigator, Seattle Children s Research Institute
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE
 Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
Received: Jun 15, 2013; Accepted: Nov 08, 2013; First Online Available: Jan 24, 2014
 Original Article Iran J Pediatr Feb 2014; Vol 24 (No 1), Pp: 57-63 Management of Neonatal Respiratory Distress Syndrome Employing ACoRN Respiratory Sequence Protocol versus Early Nasal Continuous Positive
Original Article Iran J Pediatr Feb 2014; Vol 24 (No 1), Pp: 57-63 Management of Neonatal Respiratory Distress Syndrome Employing ACoRN Respiratory Sequence Protocol versus Early Nasal Continuous Positive
CHEST PHYSIOTHERAPY IN NICU PURPOSE POLICY STATEMENTS SITE APPLICABILITY PRACTICE LEVEL/COMPETENCIES. The role of chest physiotherapy in the NICU
 PURPOSE The role of chest physiotherapy in the NICU POLICY STATEMENTS In principle chest physiotherapy should be limited to those infants considered most likely to benefit with significant respiratory
PURPOSE The role of chest physiotherapy in the NICU POLICY STATEMENTS In principle chest physiotherapy should be limited to those infants considered most likely to benefit with significant respiratory
MEDICAL POLICY I. POLICY POLICY TITLE POLICY NUMBER INHALED NITRIC OXIDE MP-4.021
 Original Issue Date (Created): August 23, 2002 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Inhaled nitric oxide may be considered medically necessary as
Original Issue Date (Created): August 23, 2002 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Inhaled nitric oxide may be considered medically necessary as
Patent Ductus Arteriosus: Philosophy or Pathology?
 Patent Ductus Arteriosus: Philosophy or Pathology? Disclosure Ray Sato, MD is a speaker for Prolacta Biosciences, Inc. This presentation will discuss off-label uses of acetaminophen and ibuprofen. RAY
Patent Ductus Arteriosus: Philosophy or Pathology? Disclosure Ray Sato, MD is a speaker for Prolacta Biosciences, Inc. This presentation will discuss off-label uses of acetaminophen and ibuprofen. RAY
Neonatal Resuscitation in What is new? How did we get here? Steven Ringer MD PhD Harvard Medical School May 25, 2011
 Neonatal Resuscitation in 2011- What is new? How did we get here? Steven Ringer MD PhD Harvard Medical School May 25, 2011 Conflicts I have no actual or potential conflict of interest in relation to this
Neonatal Resuscitation in 2011- What is new? How did we get here? Steven Ringer MD PhD Harvard Medical School May 25, 2011 Conflicts I have no actual or potential conflict of interest in relation to this
Recommendations for Hospital Quality Measures in 2011:
 Pediatric Measures: Recommendations for Hospital Quality Measures in 2011: Based on the input of a group of healthcare stakeholders, the following new hospital measures are recommended: 1) Home Management
Pediatric Measures: Recommendations for Hospital Quality Measures in 2011: Based on the input of a group of healthcare stakeholders, the following new hospital measures are recommended: 1) Home Management
By: Armend Lokku Supervisor: Dr. Lucia Mirea. Maternal-Infant Care Research Center, Mount Sinai Hospital
 By: Armend Lokku Supervisor: Dr. Lucia Mirea Maternal-Infant Care Research Center, Mount Sinai Hospital Background My practicum placement was at the Maternal-Infant Care Research Center (MiCare) at Mount
By: Armend Lokku Supervisor: Dr. Lucia Mirea Maternal-Infant Care Research Center, Mount Sinai Hospital Background My practicum placement was at the Maternal-Infant Care Research Center (MiCare) at Mount
Evaluation of a Nasal Cannula in Noninvasive Ventilation Using a Lung Simulator
 Evaluation of a Nasal in Noninvasive Ventilation Using a Lung Simulator Narayan P Iyer MD and Robert Chatburn MHHS RRT-NPS FAARC BACKGROUND: Nasal noninvasive ventilation (NIV) is a common form of noninvasive
Evaluation of a Nasal in Noninvasive Ventilation Using a Lung Simulator Narayan P Iyer MD and Robert Chatburn MHHS RRT-NPS FAARC BACKGROUND: Nasal noninvasive ventilation (NIV) is a common form of noninvasive
5 Million neonatal deaths each year worldwide. 20% caused by neonatal asphyxia. Improvement of the outcome of 1 million newborns every year
 1 5 Million neonatal deaths each year worldwide 20% caused by neonatal asphyxia Improvement of the outcome of 1 million newborns every year International Liaison Committee on Resuscitation (ILCOR) American
1 5 Million neonatal deaths each year worldwide 20% caused by neonatal asphyxia Improvement of the outcome of 1 million newborns every year International Liaison Committee on Resuscitation (ILCOR) American
Steven Ringer MD PhD April 5, 2011
 Steven Ringer MD PhD April 5, 2011 Disclaimer Mead Johnson sponsors programs such as this to give healthcare professionals access to scientific and educational information provided by experts. The presenter
Steven Ringer MD PhD April 5, 2011 Disclaimer Mead Johnson sponsors programs such as this to give healthcare professionals access to scientific and educational information provided by experts. The presenter
SWISS SOCIETY OF NEONATOLOGY. Neonatal gastric perforation
 SWISS SOCIETY OF NEONATOLOGY Neonatal gastric perforation September 2002 2 Zankl A, Stähelin J, Roth K, Boudny P and Zeilinger G, Children s Hospital of Aarau (ZA, SJ, RK, ZG) and Institute of Pathology
SWISS SOCIETY OF NEONATOLOGY Neonatal gastric perforation September 2002 2 Zankl A, Stähelin J, Roth K, Boudny P and Zeilinger G, Children s Hospital of Aarau (ZA, SJ, RK, ZG) and Institute of Pathology
Evaluating the Effect of Flow and Interface Type on Pressures Delivered With Bubble CPAP in a Simulated Model
 Evaluating the Effect of Flow and Interface Type on Pressures Delivered With Bubble CPAP in a Simulated Model Stephanie A Bailes RRT-NPS NREMT-B, Kimberly S Firestone MSc RRT, Diane K Dunn RRT-NPS, Neil
Evaluating the Effect of Flow and Interface Type on Pressures Delivered With Bubble CPAP in a Simulated Model Stephanie A Bailes RRT-NPS NREMT-B, Kimberly S Firestone MSc RRT, Diane K Dunn RRT-NPS, Neil
1. Can you convince me that one should use CPAP and avoid mechanical ventilation?
 CHAPTER 12 FAQ's Dr Rhishikesh Thakre Neonatologist Aurangabad, Mahrashtra Following are bedside tips one learns with experience. So as to guide the newcomer who should not repeat the same mistakes, the
CHAPTER 12 FAQ's Dr Rhishikesh Thakre Neonatologist Aurangabad, Mahrashtra Following are bedside tips one learns with experience. So as to guide the newcomer who should not repeat the same mistakes, the
Nottingham Children s Hospital
 High Flow Nasal Cannula Therapy Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Guide line for the use of HFNCT (High Flow Nasal Cannula Therapy) Contact Name
High Flow Nasal Cannula Therapy Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Guide line for the use of HFNCT (High Flow Nasal Cannula Therapy) Contact Name
Lung Wit and Wisdom. Understanding Oxygenation and Ventilation in the Neonate. Jennifer Habert, BHS-RT, RRT-NPS, C-NPT Willow Creek Women s Hospital
 Lung Wit and Wisdom Understanding Oxygenation and Ventilation in the Neonate Jennifer Habert, BHS-RT, RRT-NPS, C-NPT Willow Creek Women s Hospital Objectives To review acid base balance and ABG interpretation
Lung Wit and Wisdom Understanding Oxygenation and Ventilation in the Neonate Jennifer Habert, BHS-RT, RRT-NPS, C-NPT Willow Creek Women s Hospital Objectives To review acid base balance and ABG interpretation
Yorkshire & Humber Neonatal ODN (South) Clinical Guideline
 Yorkshire & Humber Neonatal ODN (South) Clinical Guideline Title: Ventilation Author: Dr Cath Smith updated September 2017, written by Dr Elizabeth Pilling May 2011 Date written: May 2011 Review date:
Yorkshire & Humber Neonatal ODN (South) Clinical Guideline Title: Ventilation Author: Dr Cath Smith updated September 2017, written by Dr Elizabeth Pilling May 2011 Date written: May 2011 Review date:
INFANTS WITH birth weights less
 CLINICAL SCIENCES of Retinopathy of Prematurity Michael X. Repka, MD; Earl A. Palmer, MD; Betty Tung, MS; for the Cryotherapy for Retinopathy of Prematurity Cooperative Group Objective: To report the timing
CLINICAL SCIENCES of Retinopathy of Prematurity Michael X. Repka, MD; Earl A. Palmer, MD; Betty Tung, MS; for the Cryotherapy for Retinopathy of Prematurity Cooperative Group Objective: To report the timing
SAMPLE. V.12.1 Special Report: Very Low Birthweight Neonates. I. Introduction
 I. Introduction V.12.1 Special Report: Very Low Birthweight Neonates The delivery of a very low birth weight infant continues to present many challenges to families and health care providers in spite of
I. Introduction V.12.1 Special Report: Very Low Birthweight Neonates The delivery of a very low birth weight infant continues to present many challenges to families and health care providers in spite of
GE Healthcare. Non Invasive Ventilation (NIV) For the Engström Ventilator. Relief, Relax, Recovery
 GE Healthcare Non Invasive Ventilation (NIV) For the Engström Ventilator Relief, Relax, Recovery COPD is currently the fourth leading cause of death in the world, and further increases in the prevalence
GE Healthcare Non Invasive Ventilation (NIV) For the Engström Ventilator Relief, Relax, Recovery COPD is currently the fourth leading cause of death in the world, and further increases in the prevalence
TRAINING NEONATOLOGY SILVANA PARIS
 TRAINING ON NEONATOLOGY SILVANA PARIS RESUSCITATION IN DELIVERY ROOM INTRODUCTION THE GLOBAL RESUSCITATION BURDEN IN NEWBORN 136 MILL NEWBORN BABIES EACH YEAR (WHO WORLD REPORT) 5-8 MILL NEWBORN INFANTS
TRAINING ON NEONATOLOGY SILVANA PARIS RESUSCITATION IN DELIVERY ROOM INTRODUCTION THE GLOBAL RESUSCITATION BURDEN IN NEWBORN 136 MILL NEWBORN BABIES EACH YEAR (WHO WORLD REPORT) 5-8 MILL NEWBORN INFANTS
SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE
 SARASOTA MEMORIAL HOSPITAL TITLE: NURSING PROCEDURE Management, Monitoring & Documentation of a Clinically Significant Cardiopulmonary Event (CSCPE) (NUR47) DATE: REVIEWED: PAGES: 9/09 9/17 1 of 6 PS1094
SARASOTA MEMORIAL HOSPITAL TITLE: NURSING PROCEDURE Management, Monitoring & Documentation of a Clinically Significant Cardiopulmonary Event (CSCPE) (NUR47) DATE: REVIEWED: PAGES: 9/09 9/17 1 of 6 PS1094
Research in high flow therapy: Mechanisms of action. Kevin Dysart, Thomas L. Miller, Marla R. Wolfson, Thomas H. Shaffer
 Research in high flow therapy: Mechanisms of action Kevin Dysart, Thomas L. Miller, Marla R. Wolfson, Thomas H. Shaffer 64666_03_Mech0fAction.indd 1 Respiratory Medicine (2009) xx, 1e6 ARTICLE IN PRESS
Research in high flow therapy: Mechanisms of action Kevin Dysart, Thomas L. Miller, Marla R. Wolfson, Thomas H. Shaffer 64666_03_Mech0fAction.indd 1 Respiratory Medicine (2009) xx, 1e6 ARTICLE IN PRESS
POLICY. Number: Title: APPLICATION OF NON INVASIVE VENTILATION FOR ACUTE RESPIRATORY FAILURE. Authorization
 POLICY Number: 7311-60-024 Title: APPLICATION OF NON INVASIVE VENTILATION FOR ACUTE RESPIRATORY FAILURE Authorization [ ] President and CEO [ x ] Vice President, Finance and Corporate Services Source:
POLICY Number: 7311-60-024 Title: APPLICATION OF NON INVASIVE VENTILATION FOR ACUTE RESPIRATORY FAILURE Authorization [ ] President and CEO [ x ] Vice President, Finance and Corporate Services Source:
For personal use only
 Fisher & Paykel Healthcare Inspired and world-leading healthcare solutions DEUTSCHE BANK CRAIGS NZ COMPANIES DAY Sydney, 8 March 2016 1 Investment Highlights o A leader in respiratory and OSA treatment
Fisher & Paykel Healthcare Inspired and world-leading healthcare solutions DEUTSCHE BANK CRAIGS NZ COMPANIES DAY Sydney, 8 March 2016 1 Investment Highlights o A leader in respiratory and OSA treatment
