Phenotypic Rescue of a Peripheral Clock Genetic Defect via SCN Hierarchical Dominance
|
|
|
- Shauna Floyd
- 5 years ago
- Views:
Transcription
1 Cell, Vol. 110, , July 12, 2002, Copyright 2002 by Cell Press Phenotypic Rescue of a Peripheral Clock Genetic Defect via SCN Hierarchical Dominance Matthew P. Pando, 1,3 David Morse, 1,4 Nicolas Cermakian, 1,5 and Paolo Sassone-Corsi 1,2 1 Institute de Génétique et de Biologie Moléculaire et Cellulaire CNRS-INSERM-ULP 1 rue Laurent Fries Illkirch, Strasbourg France Summary The mammalian circadian system contains both cen- tral and peripheral oscillators. To understand the com- munication pathways between them, we have studied the rhythmic behavior of mouse embryo fibroblasts (MEFs) surgically implanted in mice of different genotypes. MEFs from Per1 / mice have a much shorter period in culture than do tissues in the intact animal. When implanted back into mice, however, the Per1 / MEF take on the rhythmic characteristics of the host. A functioning clock is required for oscillations in the target tissues, as arrhythmic clock c/c MEFs remain arrhythmic in implants. These results demonstrate that SCN hierarchical dominance can compensate for severe intrinsic genetic defects in peripheral clocks, but cannot induce rhythmicity in clock-defective tissues. Introduction factors, CLOCK and BMAL1 (Gekakis et al., 1998; Kume et al., 1999), both members of the bhlh-pas family (Hogenesch et al., 1998; King et al., 1997). The CLOCK:BMAL1 heterodimer binds to E box elements in the promoters of clock genes and clock-controlled genes (Gekakis et al., 1998; Jin et al., 1999; Ripperger et al., 2000; Travnickova- Bendova et al., 2002). CLOCK:BMAL1 drives the rhythmic transcription of both Period (Per) and Cryptochrome (Cry) genes, an event that initiates the expression of proteins involved in both positive and negative feedback loops (Gekakis et al., 1998; Shearman et al., 2000). The Cry gene products are inhibitors of CLOCK:BMAL1- mediated transcription through direct protein-protein interaction (Griffin et al., 1999; Kume et al., 1999) and are essential to the negative feedback loop (Griffin et al., 1999; Kume et al., 1999; Okamura et al., 1999; van der Horst et al., 1999; Vitaterna et al., 1999). The per2 gene product functions as a positive regulator of Bmal1 ex- pression (Oishi et al., 1998; Shearman et al., 2000). As BMAL1 is the likely limiting factor in CLOCK:BMAL1 heterodimer formation, its accumulation may drive the reinitiation of Per and Cry transcription. The decrease in Bmal1 expression in the SCN of Clock mutant and Per2 mutant mice (Shearman et al., 2000) may thus be due to the decreased PER2 levels in these mice. Peripheral tissues display oscillations in Per expres- sion similar to those of the SCN, but with a 3 9 hr phase delay (Cermakian et al., 2001; Oishi et al., 1998; Zylka et al., 1998), and these oscillators in culture dampen much faster than do SCN neurons (Abe et al., 2002; Allen et al., 2001; Balsalobre et al., 1998; Welsh et al., 1995; Yamazaki et al., 2000). Additionally, peripheral oscillations in Per2 are lost in SCN-lesioned animals (Sakamoto et al., 1998). These observations suggested that periph- eral clocks may require SCN-derived signals to drive or synchronize their oscillations. The control of peripheral clocks by the SCN is thought to occur through a combi- nation of neuronal and humoral signals (Allen et al., 2001; Balsalobre et al., 2000; Buijs and Kalsbeek, 2001; Le- Sauter and Silver, 1998; McNamara et al., 2001; Ueyama et al., 1999). While studies with serum-shocked MEFs from normal and Cry mutant mice indicate that several critical com- ponents constituting a peripheral clock are identical to those operating in the SCN (Balsalobre et al., 1998; Ya- gita et al., 2001, 2000), several observations suggest that significant differences exist. First, Bmal1 does not oscillate in the SCN of Clock mutant mice, but oscillates with reduced amplitude in the periphery (Oishi et al., 2000). This indicates that CLOCK is essential for Bmal1 The circadian system is responsible for regulating a wide variety of physiological and behavioral rhythms (Cer- makian and Sassone-Corsi, 2000; Dunlap, 1999; Pittendrigh, 1993; Reppert and Weaver, 2001; Young and Kay, 2001). Classically, the circadian system has been thought to be based on centralized clock structures. The discovery that both vertebrates and invertebrates have a widely dispersed circadian timing system challenged this view (Balsalobre et al., 1998; Giebultowicz et al., 2000; Plautz et al., 1997; Tosini and Menaker, 1996; Whitmore et al., 2000, 1998; Yamazaki et al., 2000). Indeed, many tissues contain intrinsic oscillators, and possibly every cell in a given tissue contains an autonomous clock. The mammalian central clock is located in the suprachiasmatic nucleus (SCN) within the anterior hypothalamus (Klein et al., 1991). The neurons of the SCN contain cell- autonomous, self-sustained circadian oscillators (Welsh et al., 1995), whose molecular basis involves several interconnected positive and negative transcriptionaltranslational feedback loops (Shearman et al., 2000). transcription only in the context of the SCN. Second, The central oscillator is driven by two transactivating two studies have implicated MOP4/NPA2, a homolog of CLOCK not expressed in the SCN in the regulation of 2 Correspondence: paolosc@igbmc.u-strasbg.fr vascular (McNamara et al., 2001) and forebrain clocks 3 Present address: ExonHit Therapeutics, 217 Perry Parkway, Build- (Reick et al., 2001). Thus, there may be tissue-specific ing 5, Gaithersburg, Maryland differences in the molecular composition of the circa- On leave from the Département des Sciences Biologiques, Unidian clock, and clock components that have subtle efversité de Montréal, 4101 Sherbrooke est, Montréal (QC) H1X 2B2, Canada. fects on central clock function may play a more promi- 5 Present address: Douglas Hospital Research Center, McGill University, 6875 LaSalle Blvd, Montreal (QC) H4H 1R3, Canada. nent role in the regulation of peripheral clocks and vice versa.
2 Cell 108 primary mouse embryo fibroblasts (MEF) from Per1 / Disruption of Per1 results in subtle phenotypic more severe in MEFs than in the SCN. While differences changes in circadian function (Bae et al., 2001; Cermakian in gene function and expression between SCN and pe- et al., 2001; Zheng et al., 2001). Per1 mutant mice ripheral clocks have not been extensively described, are able to entrain to standard light:dark (LD) cycles, mild divergences have been reported (Oishi et al., 2000). display a modest shortening of the free running circa- Here we have uncovered a profound difference, manifested dian period, a slight decrease in oscillator precision, by the dramatically shortened intrinsic period in and no significant alterations of clock gene expression the peripheral clocks of Per1 / mice. in the SCN. Interestingly, rhythmic expression of clock When MEFs from Clock c/c mutant mice were subjected genes in peripheral tissues appeared slightly delayed in to the same serum-shock treatment (Figure 1C), we consistently the Per1 mutant mice, suggesting a specific role for observed peaks of Per2 expression 28 and 56 Per1 in peripheral clocks (Cermakian et al., 2001). hr following the serum shock (Figure 1D). These data fit We show here that in drastic contrast to the phenotype poorly to a cosine curve (r 0.5) but are suggestive of of the Per1 null mice, Per1-deficient peripheral oscilla- a 28 hr period. This expression profile is reminiscent of tors placed in culture display an intrinsic period of only the extended activity period observed in heterozygous 20 hr. This implies that the function of PER1 is different Clock mutants and initial free-running rhythms for homozygous in SCN neurons and in peripheral tissues. We have exploited Clock mutants in constant darkness (DD; Vitain this difference to investigate the functional de- terna et al., 1994). However, if these data do reflect clock pendence of the peripheral clocks on the central pacemaker. function in these MEFs, this clock functions poorly, as We show that, under normal physiological judged from a comparison of the amplitude of the Per2 conditions, the SCN is able to rescue or compensate oscillations in the Per1 / and Clock c/c MEFs (Figure 1D). for genetic defects affecting the period of peripheral clocks. Using MEFs encapsulated in a collagen disk and Differential Induction of Per2 by Serum Shock implanted surgically into a host animal, we show that An interesting feature of these in vitro oscillations is the peripheral clocks are subordinated to the dominance clear difference in the kinetics of Per2 induction between exerted by the central clock and exhibit the characteris- the three MEF genotypes. To directly compare the varitics of the host SCN. ous MEFs, RNA levels for the first five time points of the wt, Per1 /, and Clock c/c samples were normalized to Results each other. The normalized RNAs were simultaneously processed and run next to each other on a single gel Unmasking a Short Period Peripheral Clock (Figure 1E). In wt MEFs, the abundance of Per2 tran- Phenotype in Per1 / Mice scripts increases gradually over the first 2 hr and is then We have recently reported that mice lacking the Per1 downregulated by 8 hr postinduction. In Per1 / MEFs, gene display an almost normal circadian phenotype, basal expression of Per2 (at time zero) is higher than in characterized by rhythms in locomotor activity approxiamplitude wt MEFs, induction is more rapid with a slightly higher mately an hour shorter than their wild-type (wt) littermates and downregulation occurs earlier (Figure 1F). (Cermakian et al., 2001). The only other disbasal This suggests that Per1 is involved in modulating both cernible phenotype in these Per1 mutant mice is a slight and signal-induced Per2 expression levels. Finally, phase delay in Per2 gene oscillation in peripheral tiscompared in the Clock c/c MEFs, Per2 activation is slightly delayed sues. This suggested that Per1 might play a specialized to what is seen in the wt MEFs, and the ampli- role in peripheral clocks. To test this, we established tude of the induction is significantly reduced (Figures 1E and 1F). This is similar to observations made in the embryos as a model for peripheral cell oscillators (Yagita SCN of Clock c/c mutants, where both Per1 and Per2 et al., 2001). For comparison, we also established MEFs transcripts are expressed at a reduced level (Jin et al., from wt embryos and Clock c/c mutant embryos. 1999) and their induction is blunted (Shearman and To determine if Per1 is an essential clock component Weaver, 1999). It also confirms that functional CLOCK of peripheral oscillators, we employed a slight variation protein is essential for proper Per2 transcriptional acti- of the serum-shock method described by Balsalobre et vation in peripheral oscillators. al. (1998) to initiate circadian gene transcript cycling in our MEFs. We observe a clear circadian oscillation of Hierarchical Dominance and Phenotypic Rescue Per2 gene expression upon serum shock of normal by the Central Clock MEFs (Figure 1A), as previously described for a variety To test if the central clock could compensate for the of cell lines (Balsalobre et al., 1998; McNamara et al., Per1 / defect in peripheral clocks, we had to reestab- 2001; Yagita et al., 2001). The average ( SD) of three lish a link between the SCN and the peripheral clocks experiments can be fit to a cosine with a period of 23.4 in the MEFs. We predicted that Per1 / MEFs in an 1.2 hr (r 0.77; Figure 1D). environment where they could receive and interpret Serum shock of Per1 / MEFs yielded a surprising SCN-controlled signals would show near normal circaresult (Figure 1B). Instead of the slightly shorter 23 hr dian oscillations of Per2 gene expression. To accomplish period predicted by the activity rhythms of Per1 / mice this, we established the implant procedure deperiod (Cermakian et al., 2001), the peripheral clocks within the picted schematically in Figure 2. Per1 / MEFs produced Per2 oscillations with a much The implantation procedure was first tested on shorter period. The period calculated by fitting of an Per1 / mice. Prior to implantation, the mice were average of three experiments to a cosine was 20 1 housed individually and entrained to a 12 hr light: 12 hr hr (r 0.73). Clearly, the effect of Per1 ablation is much dark (LD) cycle for at least two weeks. Mice were then
3 Communication between SCN and Peripheral Clocks 109 Figure 1. The Period of Per2 Oscillations in Serum-Shocked MEFs Is Genotype-Dependent Total RNA was isolated at the indicated time points from mouse embryo fibroblasts (MEFs) placed in serum free medium following a 2 hr exposure to 50% horse serum. (A C) RNase protection assay (RPA) of Per2 gene expression in serum-shocked (A) wild-type (WT) MEFs, (B) Per1 / MEFs, and (C) Clock c/c MEFs. (D) The Per2 oscillations were quantitated by densitometry and normalized to the highest value (100%). The mean and SD of three independent experiments is shown for all cell types with the data from WT or Per1 / MEFs fit to a cosine curve. (E) RPA of Per2 and -actin expression in WT, Per1 /, and Clock c/c MEFs following serum shock. RNA levels were equalized between all experiments to allow amplitude comparisons. (F) Per2 expression levels were quantified by densitometry and normalized to -actin. Shown are the average and standard deviation of three independent samplings for each time point. For all RPAs, 2 g E. coli trna (t) serves as a negative control reaction and the relative amounts of total RNA used for all samples are shown. placed in DD for three days and the Per1 / MEF-colla- what will be the period of that oscillation?; and (3) will gen disks were implanted on the fourth day in DD (between the resulting oscillation be in phase with host organs what corresponded to ZT1 and ZT3 of the previ- and tissues? Figure 3A shows RNase protection analysis ous LD cycle). At 4 hr intervals during days four and five of total RNA from wt host kidney and skeletal muscle, postimplantation, implants were recovered and skeletal and total RNA from Per1 / MEF-collagen implants recovered muscle and kidney samples were taken from each animal. from the same wt hosts. Kidney and skeletal Total RNA isolated from each tissue was then muscle display robust oscillations in Per2 expression probed to determine Per2 expression levels. with a period of approximately 24 hr in keeping with Three important questions could be addressed by this previous data (Cermakian et al., 2001). initial experiment: (1) will Per1 / MEFs, that had not Strikingly, the oscillation in Per2 expression in the received a serum shock, begin to oscillate when placed Per1 / MEF-collagen implant samples has a period and in a wt host animal?; (2) if they do begin to oscillate, phase equivalent to what is observed for the host tissues Figure 2. Schematic of the Implantation Procedure Following the production of MEFs, the cells are resuspended in a medium supplemented with collagen. After approximately 12 hr, a durable collagen matrix forms around the MEFs that confines them in a disk, or implant, with a diameter of approximately 1.5 cm. This collagen disk is then implanted subcutaneously on the back of a host mouse. After several days, implants and mouse tissues are collected for RNA purification and analysis.
4 Cell 110 Figure 3. Rescue of the Short Period Per1 / Peripheral Clock Phenotype In Vivo Collagen-embedded MEFs were prepared and implanted subcutaneously into mice. At each indicated time postimplantation skeletal muscle, kidney, and implant samples were harvested from a single mouse and total RNA was isolated. (A C) RPA of Per2 expression in (A) Per / host tissues and a Per1 / MEF-collagen implant, (B) Per / host tissues and a Per1 / MEF-collagen implant, and (C) Per / host tissues and a Clock c/c MEF-collagen implant. (D) Densitometric scans of the kidney and implant Per2 oscillations in (A) (blue curves) and (B) (red curves) were normalized to the highest value in the kidney (100%) and plotted as a function of time after implantation. trna (t) is used as a control, and the relative amounts of total RNA shown. (Figures 3A and 3D). We conclude that humoral signals the same wt tissues (compare Figures 1A and 1B). Finally, present in the host can indeed initiate and maintain an SCN lacking Per1 is still able to generate hupresent circadian oscillations in unstimulated MEFs and can moral signals capable of imposing a near normal periodicity thus compensate for the Per1 genetic defect in the implanted on a genetically defective peripheral clock. The peripheral clock. ability of the SCN to impart its periodicity on a peripheral A similar experiment was performed using Per1 / clock, which intrinsically would have a 20 hr period, is MEF-collagen implants introduced into Per1 / mice a striking example of hierarchical dominance within the (Figure 3B). Again, robust Per2 oscillations were observed mammalian circadian system. in the kidney and skeletal muscle of the Per1 / Lastly, the implantation experiment was varied by in- mice. The period of these oscillations is approximately troducing Clock c/c MEFs into a Per1 / host (Figure 3C). 24 hr, as expected from the mild circadian phenotype of Although the host tissues display the expected rhythmicity this mutation (Figure 3D). Importantly, Per2 expression of Per2 expression, there is no overt 24 hr periodthis again oscillates in the Per1 / MEF-collagen implants icity in the implant. Clearly, a robustly functioning clock with a periodicity and phase identical to that of the host. is essential if the implant is to exhibit rhythmic Per2 These results establish that Per1 has profoundly dif- expression. This in turn suggests that the Per2 rhythmicity ferent circadian functions depending on where it is expressed. observed in the Per1 / MEF implants is not simferent In the SCN of our Per1 / mouse model, it is clear ply driven but entrained by the SCN. Thus, the interaction that Per1 does not play a critical role in light-dependent between implant and host is more subtle than a signaling and entrainment. These Per1 / mice readily direct causal link between timing cues originating from entrain to an LD cycle and respond normally to phaseshifting a central pacemaker and their target. The timing cues light pulses given during the night phase (Cer- do exist but must be interpreted by the implants own makian et al., 2001). In addition, Per1 plays only a minor clock in order to act as a Zeitgeber. role in central clock function and timing, as evidenced We conclude from these observations that Per1 is by the almost normal locomotor rhythm of the Per1 / not an integral part of the core oscillatory machinery in mice. The peripheral tissues examined in the Per1 / peripheral clocks, because rhythmicity in gene expression mice thus display the same phase as that observed in is conserved in Per1 mutants (Figures 1B and 3)
5 Communication between SCN and Peripheral Clocks 111 (Cermakian et al., 2001). Furthermore, as the short pe- In contrast to their arrhythmicity in DD, Clock c/c mice riod phenotype caused by the deletion of Per1 in the maintained under LD conditions show clear activity periphery is revealed only when the tissue is deprived rhythms (Vitaterna et al., 1994). In agreement with these of signals from the SCN (compare Figures 1A and 3), observations, we find that the Per2 levels in Clock c/c Per1 may perhaps be responsible for intrinsic peripheral peripheral organs display a clear 24 hr periodicity (Figure clock accuracy or timing. 4D). However, there were two unusual features to these oscillations. First, while the Per2 transcript accumula- Phenotypic Inheritance by Peripheral Oscillators tion in these mice appeared similar in phase to wt mice, To explore the extent to which the central clock can the decrease in Per2 levels in Clock c/c mice does not impose its rhythmic period on peripheral oscillators, occur until the onset of the light period (Figure 4F). We Per1 / MEF-collagen implants were introduced into had originally predicted that the decrease in transcripmice heterozygous and homozygous for the Clock c mu- tional activity of the mutant CLOCK protein in vitro (Gektation. Per2 oscillations in the kidney, skeletal muscle, akis et al., 1998) might result in a delay in the phase of and Per1 / MEF-collagen implant samples collected Per2 accumulation, but our results suggest instead that from the Clock / mice are nearly identical to what was the Clock c mutation may affect the ability of PER or CRY seen in the Per1 / and Per1 / mice, as expected (com- to properly inhibit the transcriptional activity remaining pare Figures 3A and 4A). The period of Per2 oscillations in the mutant CLOCK in vivo. Second, given this Per2 in the Clock / kidney and implant obtained by curve rhythm in Clock c/c tissues in LD, the arrythmicity of fitting are hr (r 0.91) and hr Clock c/c MEFs in a wt host kept under DD was surprising (r 0.98), respectively (Figure 4E). (Figure 3C). This drastic difference underscores the im- Clock /c mice have free-running periods significantly portance of masking by light, which appears to affect longer than 24 hr (Vitaterna et al., 1994). We applied the the activity patterns of the animal through a pathway same implantation procedure described above to the independent of the SCN (Redlin and Mrosovsky, 1999). Clock /c mice, and predicted a longer period in Per2 Lastly, and even more interesting, the Per2 expression oscillation if the same central timer controlled locomotor pattern of wt MEF implants in these Clock c/c animals rhythms and rhythmic gene expression in peripheral tis- appears normal. In agreement with our previous results, sues. Indeed, the peak of Per2 expression is altered in the onset of Per2 accumulation in the implant mimics the kidney and skeletal muscle of Clock /c animals (Fig- that observed for other host tissues. However, in these ure 4B). Strikingly, the Per1 / MEF-collagen implants wt MEFs, the CLOCK protein is fully functional and the also display the long-period phenotype of the Clock /c decrease in Per2 is typical of wt animals. The masking mice (Figure 4B). Curve fitting shows that the Per2 effect of the LD cycle thus results in Per2 rhythms with rhythm has a similar period in the kidney ( hr, periods dependent on the Zeitgeber and waveforms der 0.92) and in the implant ( hr, r 0.63), both pendent on the genotypes of tissues. of which are longer than in the Clock / hosts (Figure 4E). Thus, not only the intrinsic 20 hr period of the Per2 Daytime Feeding Entrains MEF Clocks oscillation in Per1 / MEFs can be entrained by an SCN to a 24-Hour Rhythm with an approximately 24 hr period, it can also be en- LD cycles are able to entrain Per2 rhythms in peripheral trained to the even longer period of the Clock c heterozytissues even in mice whose clock is functioning poorly. gotes. These observations further support our con- Given that the masking effect of light can result in an tention that the SCN can mask genetic defects in effective restriction of feeding to the nocturnal phase, peripheral clocks and reinforce the idea of SCN domiand that restricted feeding can entrain oscillations in nance over the intrinsic rhythmic properties of peripheral oscillators. peripheral tissues, it is possible that the rhythmicity ob- After several days in constant darkness, Clock c/c mice served in the tissues of Clock c/c mice might have resulted display arrhythmicity in activity (Vitaterna et al., 1994) from feeding only during the dark phase of the cycle. and SCN neuronal firing (Herzog et al., 1998). Can cells To test directly the impact of restricted feeding protocapable of producing rhythmic oscillations do so in the cols on Per2 expression in the MEF implants, we pro- presence of an SCN that cannot? Clock c/c mice, which vided access to food only during the light phase of a have been maintained under DD for 12 days, have ranaffected by restricted feeding and maintained its normal LD cycle (Figure 5A). As expected, the SCN was not dom levels of Per2 expression in the kidney and skeletal muscle (Figure 4C). Intriguingly, there is some correlaas predicted (Damiola et al., 2000; Stokkan et al., 2001), phase with respect to the LD cycle (Figure 5B). Again tion between the expression levels of Per2 observed at certain time points, for example at 88 hr postimplantarespect the phase of Per2 expression in the liver is inverted with tion. It is possible that improperly regulated signals may to the normal phase under LD cycles (Figure still be originating from a randomly firing SCN, causing 5A). Liver is the tissue most susceptible to entrainment global fluctuations in Per2 expression in many peripheral by restricted feeding, but other tissues such as kidney tissues. We also observe that the Per1 / MEFs imgree. and muscle also show the phase reversal to some de- planted in a Clock c/c host exhibit the same arrhythmic The important feature of this experiment lies in phenotype displayed by the kidney and skeletal muscle. Per2 expression in the implant. Four days after implanta- Therefore, in the absence of directive signals from the tion in animals entrained by food restriction, Per1 / SCN, even cells that do contain a robust circadian oscilmately implants display a peak in Per2 expression at approxilator do not display a discernable rhythm. This experihost the same time as other peripheral tissues of the ment also indicates that the implantation procedure by (Figure 5A). The period of the implant is difficult to itself is not sufficient to initiate rhythmicity in an implant. determine, presumably due to the fact that feeding is a
6 Cell 112 Figure 4. The Rhythmic Phenotype of the Implant Is Dictated by Circadian Phenotype of the Host Collagen-embedded MEFs were prepared and implanted subcutaneously into mice. At each indicated time postimplantation, skeletal muscle, kidney, and implant samples were harvested from a single mouse and total RNA was isolated. (A D) RPA of Per2 expression in (A) Clock / host tissues and a Per1 / MEF-collagen implant, (B) Clock /c host tissues and a Per1 / MEFcollagen implant, (C) Clock c/c host tissues and a Per1 / MEF-collagen implant under DD, and (D) Clock c/c host tissues and a Per1 / MEFcollagen implant under LD. The light regime for each experiment is shown either by a black rectangle (constant darkness) or by alternating white and black rectangles (LD 12:12) immediately above the panels. (E) Densitometric scans of the kidney and implant Per2 oscillations in (A) (blue curves) and (B) (red curves) were normalized to the highest value in kidney (100%) and fit to a cosine curve. (F) Densitometric scans of the Per2 oscillations in the Per1 / MEF implant (green curve) fit to a cosine curve and the kidney (purple curve) of Clock c/c hosts maintained under LD conditions. trna (t) is used as a control and the relative amounts of total RNA shown. Implant Integrity A potential caveat to the results described above is the possibility that host cells could infiltrate the MEFcollagen implant, resulting in misleading results. How- ever, at the time points used for the experiments presented in this study, no gross infiltration of the implant by surrounding host tissues was observed by macroscopic examination. To completely rule out this potential prob- poor Zeitgeber for MEFs, as it is for the muscle. However, if the implant were not entrained by the feeding regime, it would be unlikely to display a prominent maximum at the same time as other tissues. Thus, uncoupling of the implant clock from the SCN did not unmask the phenotype observed in cell culture. Instead, food restriction to daytime actively entrains the clock intrinsic to the MEFs.
7 Communication between SCN and Peripheral Clocks 113 Figure 5. Entrainment of the Per2 Rhythm in the Implant by Daytime Restricted Feeding Collagen-embedded MEFs were prepared and implanted subcutaneously into mice. At each indicated time postimplantation, liver, skeletal muscle, kidney, and implant samples were harvested from a single mouse and total RNA was isolated. Brains were also harvested from the same mice and processed for in situ hybridization. Mice under a 12:12 LD cycle were entrained to daytime feeding for ten days prior to implantation and maintained under this schedule (shown schematically above A) for the duration of the experiment. (A) RPA of Per2 expression in WT host tissues and a Per1 / MEF-collagen implant. trna (t) is used as a control, and the relative amounts of total RNA shown. (B) In situ hybridization of serial sections from each brain with either a VIP or a Per1 probe at the indicated times postimplantation. lem, the fluorescent marker PKH26 was incorporated is likely to shed light on how these two types of oscillators into the cytoplasmic membrane of the MEFs prior to interact. There could even be subtle or significant implantation. This marker can be visualized using a fluo- differences between peripheral oscillators, which would rescence microscope following the retrieval of the MEFcollagen allow differential interpretation of signals originating implant from a host animal. from the SCN and the environment. Figure 6 shows sections of Per1 / MEF collagen implants We have demonstrated that striking differences exist that were placed subcutaneously into Per1 / between central and peripheral oscillators. Per2 oscilla- and Clock /c host animals. The implants were retrieved tions in Per1 / MEFs have a much shorter period than at a time corresponding to the final time point taken in in wt cells, indicating that Per1 has a drastically different each of the implantation experiments performed. Following function in central versus peripheral clocks. One possi- sectioning, samples were counterstained with ble explanation for this is that some functional redun- DAPI and analyzed. Skeletal muscle was harvested from dancy is present within the SCN and not within periphthe same animal as the implant for a negative control. eral clocks. In the SCN, Per1 appears to suppress Per2 MEFs are distinguished from host cells by the colocali- basal gene activity. In the absence of Per1, Per2 expreszation of red fluorescence with blue DAPI stained nuclei, sion peaks at a faster rate and with greater amplitude. as host cell nuclei do not exhibit any red fluorescence. Indeed, PER2 expression is increased in peripheral tis- The merged images show clear colocalization of red sues of Per1 null mice (Zheng et al., 2001). Because PKH26 staining with virtually all the DAPI stained nuclei PER2 positively regulates Bmal1, improperly regulated in the implant (Figure 6). Quantification of several fields Per2 activation may prematurely initiate the beginning from serial sections through implants showed that 98% of the subsequent cycle, thus giving rise to the short of the nuclei colocalized with PKH26 red fluorescence. period phenotype. It is likely that most if not all of the nuclei that did not The model of SCN control over physiology and behavcolocalize with red fluorescence were due to sectioning ior through neuronal and humoral outputs (Allen et al., and labeling variability. Therefore, we conclude that the 2001; Balsalobre et al., 2000; Buijs and Kalsbeek, 2001; observed Per2 expression results from the implanted LeSauter and Silver, 1998; McNamara et al., 2001; Ue- MEFs and not from contaminating host cells. yama et al., 1999) provided the framework for elucidating the mechanisms that regulate the interplay between the Discussion SCN and peripheral clocks. SCN-lesioned animals lose a variety of behavioral and physiological rhythms which The understanding of the physiological and functional can be partially restored with SCN transplants (Ralph et relationship between central and peripheral clocks is al., 1990; Silver et al., 1996). essential in circadian biology. Characterization of any By transplantation of MEFs we have revealed the differences between the SCN and peripheral oscillators dominance of the SCN over the activity of peripheral
8 Cell 114 Figure 6. MEF-Collagen Implants Maintain Their Integrity after 5 Days in the Host Per1 / MEFs were labeled with PKH26 prior to being incorporated into a collagen implant. Recovered implants and control skeletal muscle were sectioned, stained with DAPI, and examined with a fluorescence microscope. DAPI and PKH26 staining of Per1 / MEF-collagen implants harvested 5 days after implantation in a Per / host mouse (A) or in a Clock /c host mouse (B). No PKH26 staining is observed in skeletal muscle from the host. clocks. In the absence of external environmental cues, expect, is most susceptible. Our Per1 / MEF implants Per1 / MEFs take on a 24 hr period in wt hosts, a behave more similarly to muscle in this regard. Moreslightly longer period in Clock /c hosts, and an arrhyth- over, MEF implants acquire the same period of Per2 mic phenotype in Clock c/c hosts (Figure 4). The adoption oscillation as other peripheral tissues, i.e., 24 hr and not by the MEFs of the phenotypic characteristics of host 20 hr. Altogether, these data suggest that restricted SCNs illustrates the striking hierarchical dominance existing feeding does not simply uncouple peripheral oscillamals. between central and peripheral clocks in mam- tors from the SCN. This treatment instead acts through This strict hierarchy does not appear to operate an active mechanism, producing a bona fide signal that in Drosophila, as shown by maintenance of the phase can produce phase changes in some tissues. in transplanted excretory tubules into a host fly maintained It is important to emphasize that clock gene oscillation on an opposite cycle (Giebultowicz et al., 2000). in the periphery requires a functional oscillator. This can Additional evidence comes from studies on the olfactory be deduced from the lack of rhythmicity in Clock c/c MEFs response rhythms in the antennae, where local per defi- implanted in a wild-type host (Figure 3C). While SCNderived ciency results in arrhythmicity (Krishnan et al., 1999). signals can sustain oscillations in a heavily Environmental cycles seem to directly entrain peripheral damped peripheral oscillator, they are insufficient to clocks without the need of a dominant pacemaker induce oscillations in cells without intrinsic rhythmic ca- (Plautz et al., 1997). Similarly, peripheral tissues in zebra- pacity. How then can the rhythmicity in tissues of Clock c/c fish show independence from central clocks (Cermakian mice maintained under LD conditions be explained? An et al., 2000; Whitmore et al., 1998) and are light-respon- intriguing possibility is that light may be directly responsible sive (Whitmore et al., 2000). Peripheral circadian photoreception for neuronal of hormonal signals which act synersive has not been demonstrated in mammals, but gistically with SCN derived signals to force oscillations feeding schedule is a potent peripheral Zeitgeber (Damiola even in cells without oscillatory capability. et al., 2000; Stokkan et al., 2001). Three prominent pathways are directly or indirectly Under restricted feeding, the SCN can be uncoupled involved in the entrainment of central and peripheral from peripheral clocks, which become directly entrained clocks (Figure 7). Light entrainment, where photic stimuli to an environmental stimulus (Damiola et al., 2000; Stokkan are transmitted through the retina to the SCN, is essen- et al., 2001). We have placed implants into mice tial (Figure 7A). Once entrained to environmental light subjected to a daytime restricted feeding regime (Figure cycles, the SCN acts as a robust self-sustained oscillator 5). As noted previously, different tissues have different that resets or synchronizes peripheral clocks so that degrees of susceptibility to food. Liver, as one might their oscillations will be in phase with the environment
9 Communication between SCN and Peripheral Clocks 115 Figure 7. Schematic of Entrainment Mechanisms for Peripheral Oscillators in the Mouse Light, activity, and feeding schedule can all affect circadian rhythmicity of the SCN and/ or the peripheral tissues (A C). We have combined these pathways into a unified model (D), which also includes postulated influence of activity rhythms on feeding schedule and SCN-independent effects of light on peripheral tissues. (Brown and Schibler, 1999; Yamazaki et al., 2000). When activity and feeding rhythms results in a consequent isolated, peripheral oscillators lose overt rhythmicity entrainment of peripheral clocks. Alternatively, these after several cycles (Abe et al., 2002; Yamazaki et al., pathways may be acting on peripheral clocks in parallel 2000). However, entrainment of these peripheral clocks and could thus be either synergistic or competitive. depends on the presence of a functioning oscillator in However, our results with the Clock mutant argues these tissues (Figure 3C). against the latter possibility, as Clock c/c mice in LD dis- Other cues can affect the SCN. A feedback regulation play Per2 rhythms in peripheral organs. Moreover, a exists between the SCN and activity (Figure 7B). The comparison of the results obtained in these mutant mice central clock can be reset by arousal (Maywood et al., and with Clock c/c MEF implants in wt host suggests that 1999; Mrosovsky, 1996). In addition, the SCN regulates light may have an effect on the periphery through a behavioral rhythms through projections to other hypo- pathway circumventing the SCN. Recent observations thalamic nuclei (Abrahamson et al., 2001; Aston-Jones that suggest direct connections from the retina to hypo- et al., 2001). Recent data indicate that this may be thalamic nuclei regulating activity (Kramer et al., 2001) achieved, at least in part, through SCN-borne TGF and strengthen this view. Dissection of the entrainment path- signaling through EGF receptors in neurons of the sub- way into its various components is important to acquire paraventricular zone (Kramer et al., 2001). Activity a complete understanding of the mechanisms involved rhythms may also have direct or indirect effects on the in central and peripheral clock regulation. entrainment of peripheral clocks through, for example, What signals are generated by normal or mutant SCNs the timing of food absorption. that may regulate peripheral clocks? These may be involved Restricted food availability has been shown to act as in phase setting, driving peripheral oscillations, a Zeitgeber for peripheral clocks (Figure 7C; Damiola et inhibiting these pathways, or a combination of these. al., 2000; Krieger et al., 1977; Stokkan et al., 2001). Here, Glucocorticoids (Balsalobre et al., 2000; Le Minh et al., we demonstrate that this occurs through an active 2001) and nuclear hormones (McNamara et al., 2001) mechanism and not a simple uncoupling of peripheral have been implicated in regulating the phase of periph- clocks from the central pacemaker. Two possibilities eral clocks. In the present study, we have described a may explain how entrainment by food restriction can powerful and highly flexible implantation procedure that circumvent the otherwise strong link between the SCN may prove useful. It will allow a variety of combinations and the periphery. First, the SCN could affect peripheral of host and peripheral tissue genotypes to be tested in clocks primarily through the activity rhythms and food a straightforward and efficient manner. Instead of using intake; due to SCN function, nocturnal animals are more tissue-specific knockouts, one could use implant with active at night and thus eat mostly during this period. the appropriate signaling molecule mutated. In addition This nocturnal feeding entrains peripheral clocks through to flexibility, this approach allows the comparison, within a mechanism similar to the one involved in the daytime a single host animal, of clock gene expression in the restricted feeding experiments. In this case, daytime implant (a model for peripheral clocks) to that present restricted feeding would simply short circuit the SCN- in a variety of peripheral tissues. periphery connection, by imposing the time of food intake and therefore the signal for peripheral tissue clock Experimental Procedures resetting. A second way by which the SCN could influence peripheral clocks is through neuronal or humoral Cell Culture Mouse embryo fibroblast cultures were established by harvesting pathways linking the hypothalamus to the periphery in embryos at embryonic day 13. Head and liver removed, the reparallel to the activity-feeding pathway. This would maining embryonic tissue was passed five times through an 18- imply that the entrainment by food restriction is domi- gauge needle. The disrupted tissue was plated in DMEM 10% nant over other entrainment pathways, for at least some FCS (GibcoBRL) and cultured at 37 C in5%co 2. Cultures were tissues, as under these conditions the SCN and other passed 1:3 after reaching confluency and discarded after reaching oscillators appear uncoupled. passage 10. Serum shock was as described (Balsalobre et al., 1998) except These three pathways (Figure 7A 7C) can be intethat confluent MEFs were grown for three days in DMEM 1% FCS grated in a unified view that takes our results into ac- prior administration of DMEM 50% horse serum (GibcoBRL). count (Figure 7D). Light entrains SCN rhythms, and the SCN in turn synchronizes peripheral tissues, as time of In Situ Hybridization and RNase Protection Assays food administration and perhaps activity do. An interest- All riboprobes were generated using an in vitro transcription kit ing possibility is that the control exerted by the SCN over (Promega). In situ hybridization on frozen brain sections was as
10 Cell 116 described (Cermakian et al., 2001). Probes cover nt of Per1 ORF and nt of Genebank VIP sequence X RNase protection assays (RPA) were as described (Cermakian et al., 2001). References Abe, M., Herzog, E.D., Yamazaki, S., Straume, M., Tei, H., Sakaki, Y., Menaker, M., and Block, G.D. (2002). Circadian rhythms in isolated brain regions. J. Neurosci. 22, Formation of Collagen Implants Abrahamson, E.E., Leak, R.K., and Moore, R.Y. (2001). The suprachi- Collagen implants were formed as described (Palmer et al., 1989; asmatic nucleus projects to posterior hypothalamic arousal sys- St. Louis and Verma, 1988). Briefly, the listed components were tems. Neuroreport 12, combined in the following order: 1.5 ml of 2 DMEM (GibcoBRL), 1.5 ml of FCS (GibcoBRL), 1.5 ml of 3 mg/ml rat tail collagen (Sigma), Allen, G., Rappe, J., Earnest, D.J., and Cassone, V.M. (2001). Oscil- 250 l of 0.1 M NaOH, 150 l of 74 g/l KHCO 3, and 1.5 ml of MEFs lating on borrowed time: diffusible signals from immortalized supraresuspended in DMEM containing 10% FCS to a concentration of chiasmatic nucleus cells regulate circadian rhythmicity in cultured cells/ml. The mixture was then plated on 6 cm Petri dishes fibroblasts. J. Neurosci. 21, and incubated overnight at 37 C in5%co 2. Aston-Jones, G., Chen, S., Zhu, Y., and Oshinsky, M.L. (2001). A neural circuit for circadian regulation of arousal. Nat. Neurosci. 4, Subcutaneous Implantation and Tissue Harvesting Bae, K., Jin, X., Maywood, E.S., Hastings, M.H., Reppert, S.M., and Adult male mice were housed individually on a LD cycle for at least Weaver, D.R. (2001). Differential functions of mper1, mper2, and 2 weeks prior to implantation. For food entrainment, food availability mper3 in the SCN circadian clock. Neuron 30, was restricted to the daytime of a LD cycle for 10 days prior to implantation. Except for experiments in LD, after entrainment mice Balsalobre, A., Damiola, F., and Schibler, U. (1998). A serum shock were placed in DD for three days (9 days for the Clock c/c mice). On induces circadian gene expression in mammalian tissue culture the fourth day of DD, between ZT1 and ZT3 of the previous LD cells. Cell 93, cycle, mice were anesthetized and a small patch below the head was Balsalobre, A., Brown, S.A., Marcacci, L., Tronche, F., Kellendonk, shaved and sterilized. A small incision was made, a subcutaneous C., Reichardt, H.M., Schutz, G., and Schibler, U. (2000). Resetting pocket was opened using sterile scissors, and a MEF-collagen im- of circadian time in peripheral tissues by glucocorticoid signaling. plant rinsed three times in PBS was introduced into the pocket so Science 289, to rest on muscles of the upper back. The incision was sutured with Brown, S.A., and Schibler, U. (1999). The ins and outs of circadian stitches and mice returned to DD for three more days. timekeeping. Curr. Opin. Genet. Dev. 9, Starting at a time corresponding to ZT0 of the previous LD cycle, Buijs, R.M., and Kalsbeek, A. (2001). Hypothalamic integration of on the fourth day in DD postsurgery, the implant and various host central and peripheral clocks. Nat Rev Neurosci. 2, tissues (typically the kidney and skeletal muscle from the leg) were collected from one mouse. Tissues were collected from one mouse Cermakian, N., and Sassone-Corsi, P. (2000). Multilevel regulation every 4 hr through the end of the fifth day postsurgery. Dissections of the circadian clock. Nat. Rev. Mol. Cell. Biol. 1, were done under dim red light. Tissues were immediately frozen in Cermakian, N., Whitmore, D., Foulkes, N.S., and Sassone-Corsi, P. liquid nitrogen and stored at 80 C until processed for RNA. Obreveal (2000). Asynchronous oscillations of two zebrafish CLOCK partners served muscle and kidney oscillations in Per2 expression were consistent differential clock control and function. Proc. Natl. Acad. Sci. with previous data (Cermakian et al., 2001), demonstrating USA 97, that the surgical procedure had no direct phase-shifting effects. Cermakian, N., Monaco, L., Pando, M.P., Dierich, A., and Sassone- Corsi, P. (2001). Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO PKH26 Staining of MEFs J. 20, MEFs were labeled using the PKH26 red fluorescent cell linker kit (Sigma) following manufacturer s instructions. MEFs were allowed Damiola, F., Le Minh, N., Preitner, N., Kornmann, B., Fleury-Olela, to recover for 24 hr before being incorporated into collagen implants. F., and Schibler, U. (2000). Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the PKH26 labeled MEF-collagen implants were placed in three Per1 / suprachiasmatic nucleus. Genes Dev. 14, and three Clock /c mice. Implants and skeletal muscle were recovered at 120 hr postimplantation. Samples were immediately mounted in Dunlap, J.C. (1999). Molecular bases for circadian clocks. Cell 96, Cryomatrix embedding medium (Shandon). Ten m cryostat sections were rinsed in PBS, stained in 0.1 g/ml 4,6-diamidino-2-phenylindole Gekakis, N., Staknis, D., Nguyen, H.B., Davis, F.C., Wilsbacher, L.D., dihydrochloride (DAPI, Roche), rinsed 4 times in PBS, and mounted King, D.P., Takahashi, J.S., and Weitz, C.J. (1998). Role of the in Vectashield mounting medium (Vector Laboratories, Inc.). A DMLB CLOCK protein in the mammalian circadian mechanism. Science Leica microscope equipped with epifluorescence was used. 280, Giebultowicz, J.M., Stanewsky, R., Hall, J.C., and Hege, D.M. (2000). Acknowledgments Transplanted Drosophila excretory tubules maintain circadian clock cycling out of phase with the host. Curr. Biol. 10, We thank Estelle Heitz and Stéphanie Roux for their expert technical Griffin, E.A., Jr., Staknis, D., and Weitz, C.J. (1999). Light-indepenassistance, Anna Pinchak for Clock mouse genotyping, and all the dent role of CRY1 and CRY2 in the mammalian circadian clock. members of the Sassone-Corsi s laboratory for help, reagents, and Science 286, discussions. We are grateful to Joseph Takahashi and Martha Vita- Herzog, E.D., Takahashi, J.S., and Block, G.D. (1998). Clock controls terna (Northwestern University, Evanston, IL) for the Clock mutant circadian period in isolated suprachiasmatic nucleus neurons. Nat. mice, Tal Kafri for advice on collagen implants, Shirlee Tan for help Neurosci. 1, with mouse surgeries, and Till Roennenberg for advice on curve- Hogenesch, J.B., Gu, Y.Z., Jain, S., and Bradfield, C.A. (1998). The fitting programs. M.P.P. was supported by a Fondation de la Recher- basic-helix-loop-helix-pas orphan MOP3 forms transcriptionally che Médicale postdoctoral fellowship, D.M. by an INSERM Poste active complexes with circadian and hypoxia factors. Proc. Natl. Orange fellowship, and N.C. by a Canadian Institutes of Health Re- Acad. Sci. USA 95, search postdoctoral fellowship. Work in P.S.-C. s laboratory is sup- Jin, X., Shearman, L.P., Weaver, D.R., Zylka, M.J., de Vries, G.J., and ported by grants from CNRS, INSERM, CHUR, Human Frontier Sci- Reppert, S.M. (1999). A molecular mechanism regulating rhythmic ence Program, Organon Akzo/Nobel, Fondation pour la Recherche output from the suprachiasmatic circadian clock. Cell 96, Médicale, and Association pour la Recherche sur le Cancer. King, D.P., Zhao, Y., Sangoram, A.M., Wilsbacher, L.D., Tanaka, M., Antoch, M.P., Steeves, T.D., Vitaterna, M.H., Kornhauser, J.M., Received: November 2, 2001 Lowrey, P.L., et al. (1997). Positional cloning of the mouse circadian Revised: May 29, 2002 clock gene. Cell 89,
11 Communication between SCN and Peripheral Clocks 117 Klein, D., Moore, R.Y., and Reppert, S.M. (1991). Suprachiasmatic suprachiasmatic nucleus in the brain. J. Biol. Chem. 273, Nucleus: The Mind s Clock (New York: Oxford University Press) Kramer, A., Yang, F.C., Snodgrass, P., Li, X., Scammell, T.E., Davis, Shearman, L.P., and Weaver, D.R. (1999). Photic induction of period F.C., and Weitz, C.J. (2001). Regulation of daily locomotor activity gene expression is reduced in Clock mutant mice. Neuroreport 10, and sleep by hypothalamic EGF receptor signaling. Science 294, Shearman, L.P., Sriram, S., Weaver, D.R., Maywood, E.S., Chaves, Krieger, D.T., Hauser, H., and Krey, L.C. (1977). Suprachiasmatic I., Zheng, B., Kume, K., Lee, C.C., van der Horst, G.T., Hastings, nuclear lesions do not abolish food-shifted circadian adrenal and M.H., and Reppert, S.M. (2000). Interacting molecular loops in the temperature rhythmicity. Science 197, mammalian circadian clock. Science 288, Krishnan, B., Dryer, S.E., and Hardin, P.E. (1999). Circadian rhythms Silver, R., LeSauter, J., Tresco, P.A., and Lehman, M.N. (1996). A in olfactory responses of Drosophila melanogaster. Nature 400, diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382, Kume, K., Zylka, M.J., Sriram, S., Shearman, L.P., Weaver, D.R., Jin, X., Maywood, E.S., Hastings, M.H., and Reppert, S.M. (1999). mcry1 St. Louis, D., and Verma, I.M. (1988). An alternative approach to somatic cell gene therapy. Proc. Natl. Acad. Sci. USA 85, and mcry2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, Stokkan, K.A., Yamazaki, S., Tei, H., Sakaki, Y., and Menaker, M. (2001). Entrainment of the circadian clock in the liver by feeding. Le Minh, N., Damiola, F., Tronche, F., Schutz, G., and Schibler, U. Science 291, (2001). Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 20, Tosini, G., and Menaker, M. (1996). Circadian rhythms in cultured mammalian retina. Science 272, LeSauter, J., and Silver, R. (1998). Output signals of the SCN. Chro- Travnickova-Bendova, Z., Cermakian, N., Reppert, S.M., and Sasnobiol. Int. 15, sone-corsi, P. (2002). Bimodal regulation of mperiod promoters by Maywood, E.S., Mrosovsky, N., Field, M.D., and Hastings, M.H. CREB-dependent signaling and CLOCK:BMAL1 activity. Proc. Natl. (1999). Rapid down-regulation of mammalian period genes during Acad. Sci. USA 99, behavioral resetting of the circadian clock. Proc. Natl. Acad. Sci. Ueyama, T., Krout, K.E., Nguyen, X.V., Karpitskiy, V., Kollert, A., USA 96, Mettenleiter, T.C., and Loewy, A.D. (1999). Suprachiasmatic nucleus: McNamara, P., Seo, S., Rudic, R.D., Sehgal, A., Chakravarti, D., and a central autonomic clock. Nat. Neurosci. 2, FitzGerald, G.A. (2001). Regulation of clock and mop4 by nuclear van der Horst, G.T., Muijtjens, M., Kobayashi, K., Takano, R., Kanno, hormone receptors in the vasculature: a humoral mechanism to S., Takao, M., de Wit, J., Verkerk, A., Eker, A.P., van Leenen, D., et reset a peripheral clock. Cell 105, Mrosovsky, N. (1996). Locomotor activity and non-photic influences on circadian clocks. Biol. Rev. Camb. Philos. Soc. 71, al. (1999). Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, Vitaterna, M.H., King, D.P., Chang, A.M., Kornhauser, J.M., Lowrey, Oishi, K., Sakamoto, K., Okada, T., Nagase, T., and Ishida, N. (1998). P.L., McDonald, J.D., Dove, W.F., Pinto, L.H., Turek, F.W., and Taka- Antiphase circadian expression between BMAL1 and period homo- hashi, J.S. (1994). Mutagenesis and mapping of a mouse gene, logue mrna in the suprachiasmatic nucleus and peripheral tissues Clock, essential for circadian behavior. Science 264, of rats. Biochem. Biophys. Res. Commun. 253, Vitaterna, M.H., Selby, C.P., Todo, T., Niwa, H., Thompson, C., Oishi, K., Fukui, H., and Ishida, N. (2000). Rhythmic expression of Fruechte, E.M., Hitomi, K., Thresher, R.J., Ishikawa, T., Miyazaki, J., BMAL1 mrna is altered in Clock mutant mice: differential regulation et al. (1999). Differential regulation of mammalian period genes and in the suprachiasmatic nucleus and peripheral tissues. Biochem. circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl. Acad. Sci. USA 96, Biophys. Res. Commun. 268, Welsh, D.K., Logothetis, D.E., Meister, M., and Reppert, S.M. (1995). Okamura, H., Miyake, S., Sumi, Y., Yamaguchi, S., Yasui, A., Muijt- Individual neurons dissociated from rat suprachiasmatic nucleus jens, M., Hoeijmakers, J.H., and van der Horst, G.T. (1999). Photic express independently phased circadian firing rhythms. Neuron 14, induction of mper1 and mper2 in cry-deficient mice lacking a biolog ical clock. Science 286, Whitmore, D., Foulkes, N.S., Strahle, U., and Sassone-Corsi, P. Palmer, T.D., Thompson, A.R., and Miller, A.D. (1989). Production of (1998). Zebrafish Clock rhythmic expression reveals independent human factor IX in animals by genetically modified skin fibroblasts: peripheral circadian oscillators. Nat. Neurosci. 1, potential therapy for hemophilia B. Blood 73, Whitmore, D., Foulkes, N.S., and Sassone-Corsi, P. (2000). Light Pittendrigh, C.S. (1993). Temporal organization: reflections of a Dar- acts directly on organs and cells in culture to set the vertebrate winian clock-watcher. Annu. Rev. Physiol. 55, circadian clock. Nature 404, Plautz, J.D., Kaneko, M., Hall, J.C., and Kay, S.A. (1997). Independent Yagita, K., Yamaguchi, S., Tamanini, F., van Der Horst, G.T., Hoeijphotoreceptive circadian clocks throughout Drosophila. Science makers, J.H., Yasui, A., Loros, J.J., Dunlap, J.C., and Okamura, H. 278, (2000). Dimerization and nuclear entry of mper proteins in mammalian Ralph, M.R., Foster, R.G., Davis, F.C., and Menaker, M. (1990). Trans- cells. Genes Dev. 14, planted suprachiasmatic nucleus determines circadian period. Science 247, Redlin, U., and Mrosovsky, N. (1999). Masking by light in hamsters with SCN lesions. J. Comp. Physiol. [A] 184, Reick, M., Garcia, J.A., Dudley, C., and McKnight, S.L. (2001). NPAS2: an analog of clock operative in the mammalian forebrain. Science 293, Yagita, K., Tamanini, F., van Der Horst, G.T., and Okamura, H. (2001). Molecular mechanisms of the biological clock in cultured fibro- blasts. Science 292, Yamazaki, S., Numano, R., Abe, M., Hida, A., Takahashi, R., Ueda, M., Block, G.D., Sakaki, Y., Menaker, M., and Tei, H. (2000). Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, Young, M.W., and Kay, S.A. (2001). Time zones: a comparative genetics of circadian clocks. Nat Rev Genet. 2, Reppert, S.M., and Weaver, D.R. (2001). Molecular analysis of mam- malian circadian rhythms. Annu. Rev. Physiol. 63, Zheng, B., Albrecht, U., Kaasik, K., Sage, M., Lu, W., Vaishnav, Ripperger, J.A., Shearman, L.P., Reppert, S.M., and Schibler, U. S., Li, Q., Sun, Z.S., Eichele, G., Bradley, A., and Lee, C.C. (2001). (2000). CLOCK, an essential pacemaker component, controls ex- Nonredundant roles of the mper1 and mper2 genes in the mammapression of the circadian transcription factor DBP. Genes Dev. 14, lian circadian clock. Cell 105, Zylka, M.J., Shearman, L.P., Weaver, D.R., and Reppert, S.M. (1998). Sakamoto, K., Nagase, T., Fukui, H., Horikawa, K., Okada, T., Tanaka, H., Sato, K., Miyake, Y., Ohara, O., Kako, K., and Ishida, N. (1998). Multitissue circadian expression of rat period homolog outside of brain. Neuron 20, (rper2) mrna is governed by the mammalian circadian clock, the Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts
Advance in circadian rhythm genetics in mammals
 16 2 2004 4 Chinese Bulletin of Life Sciences Vol. 16, No. 2 Apr., 2004 1004-0374 (2004) 02-0104-05 1 100101 2 434025 9 24, Q41 A Advance in circadian rhythm genetics in mammals XU Zu-Yuan 1,2 (1 Beijing
16 2 2004 4 Chinese Bulletin of Life Sciences Vol. 16, No. 2 Apr., 2004 1004-0374 (2004) 02-0104-05 1 100101 2 434025 9 24, Q41 A Advance in circadian rhythm genetics in mammals XU Zu-Yuan 1,2 (1 Beijing
CIRCADIAN SIGNALING NETWORKS
 Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) FS 2014 P. Matthias and RG Clerc Roger G. Clerc 07.05.2014 CIRCADIAN SIGNALING NETWORKS Master pacemaker SCN «Slave clocks»
Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) FS 2014 P. Matthias and RG Clerc Roger G. Clerc 07.05.2014 CIRCADIAN SIGNALING NETWORKS Master pacemaker SCN «Slave clocks»
Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice
 Am J Physiol Regul Integr Comp Physiol 285: R57 R67, 2003. First published March 20, 2003; 10.1152/ajpregu.00023.2003. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice SiNae
Am J Physiol Regul Integr Comp Physiol 285: R57 R67, 2003. First published March 20, 2003; 10.1152/ajpregu.00023.2003. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice SiNae
Transcription Regulation And Gene Expression in Eukaryotes (Cycle G2 # )
 Transcription Regulation And Gene Expression in Eukaryotes (Cycle G2 #13709-01) CIRCADIAN SIGNALING NETWORKS RG. Clerc May 19. 2010 www.fmi.ch/training/teaching Circadian rythms : most physiological processes
Transcription Regulation And Gene Expression in Eukaryotes (Cycle G2 #13709-01) CIRCADIAN SIGNALING NETWORKS RG. Clerc May 19. 2010 www.fmi.ch/training/teaching Circadian rythms : most physiological processes
T. WU 1, Y. NI 1, F. ZHUGE 1, Z. FU 1. Introduction
 Physiol. Res. 59: 581-590, 2010 Resetting Process of Peripheral Circadian Gene Expression after the Combined Reversal of Feeding Schedule and Light/Dark Cycle Via a 24-h Light Period Transition in Rats
Physiol. Res. 59: 581-590, 2010 Resetting Process of Peripheral Circadian Gene Expression after the Combined Reversal of Feeding Schedule and Light/Dark Cycle Via a 24-h Light Period Transition in Rats
Biological Clocks. Lu Chen, Ph.D. MCB, UC Berkeley. Why Does Melatonin Now Outsell Vitamin C??
 Biological Clocks Lu Chen, Ph.D. MCB, UC Berkeley 1 Why Does Melatonin Now Outsell Vitamin C?? Wake / sleep complaints are extremely prevalent. Much melatonin is consumed in an attempt to overcome the
Biological Clocks Lu Chen, Ph.D. MCB, UC Berkeley 1 Why Does Melatonin Now Outsell Vitamin C?? Wake / sleep complaints are extremely prevalent. Much melatonin is consumed in an attempt to overcome the
Clicker Question. The Need to Decompose. Mechanism and Reduction: Decomposing Circadian Clocks
 Mechanism and Reduction: Decomposing Circadian Clocks Clicker Question On the Deductive-Nomological (DN) model of reduction, which of the following does not figure in providing the explanation (i.e., is
Mechanism and Reduction: Decomposing Circadian Clocks Clicker Question On the Deductive-Nomological (DN) model of reduction, which of the following does not figure in providing the explanation (i.e., is
Biological Clocks. Lu Chen, Ph.D. MCB, UC Berkeley. What is biological clock?
 Biological Clocks Lu Chen, Ph.D. MCB, UC Berkeley 1 What is biological clock? All eukaryotes and some prokaryotes display changes in gene activity, biochemistry, physiology, and behavior that wax and wane
Biological Clocks Lu Chen, Ph.D. MCB, UC Berkeley 1 What is biological clock? All eukaryotes and some prokaryotes display changes in gene activity, biochemistry, physiology, and behavior that wax and wane
The Success of Decomposition
 11/21/11 Mechanism and Levels of Organization: Recomposing and Situating Circadian Clocks The Success of Decomposition Moving beyond per, researchers in the 1990s and early 2000s identified many clock
11/21/11 Mechanism and Levels of Organization: Recomposing and Situating Circadian Clocks The Success of Decomposition Moving beyond per, researchers in the 1990s and early 2000s identified many clock
Time after time: inputs to and outputs from the mammalian circadian oscillators
 632 Review Time after time: inputs to and outputs from the mammalian circadian oscillators David Morse and Paolo Sassone-Corsi Oscillating levels of clock gene transcripts in the suprachiasmatic nucleus
632 Review Time after time: inputs to and outputs from the mammalian circadian oscillators David Morse and Paolo Sassone-Corsi Oscillating levels of clock gene transcripts in the suprachiasmatic nucleus
Environmental stimulus perception and control of circadian clocks Nicolas Cermakian* and Paolo Sassone-Corsi
 359 Environmental stimulus perception and control of circadian clocks Nicolas Cermakian* and aolo Sassone-Corsi Circadian rhythms are regulated by clocks located in specific structures of the central nervous
359 Environmental stimulus perception and control of circadian clocks Nicolas Cermakian* and aolo Sassone-Corsi Circadian rhythms are regulated by clocks located in specific structures of the central nervous
Sleep-Wake Cycle I Brain Rhythms. Reading: BCP Chapter 19
 Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
Molecular mechanism of cell-autonomous circadian gene. expression of Period2, a crucial regulator of the mammalian
 Molecular mechanism of cell-autonomous circadian gene expression of Period2, a crucial regulator of the mammalian circadian clock Makoto Akashi*, Tomoko Ichise*, Takayoshi Mamine and Toru Takumi* *Osaka
Molecular mechanism of cell-autonomous circadian gene expression of Period2, a crucial regulator of the mammalian circadian clock Makoto Akashi*, Tomoko Ichise*, Takayoshi Mamine and Toru Takumi* *Osaka
Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock
 RESEARCH COMMUNICATION Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock Makoto Akashi 1 and Eisuke Nishida 1,2 1 Department of Biophysics, Graduate School of Science
RESEARCH COMMUNICATION Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock Makoto Akashi 1 and Eisuke Nishida 1,2 1 Department of Biophysics, Graduate School of Science
Neurons and Hormones 1. How do animals perform the right behaviors at the right time? In the right context?
 Neurons and Hormones 1 How do animals perform the right behaviors at the right time? In the right context? Active at night only What if conflicting signals? Magnetic cues are always present But migrate
Neurons and Hormones 1 How do animals perform the right behaviors at the right time? In the right context? Active at night only What if conflicting signals? Magnetic cues are always present But migrate
Transcription Regulation And Gene Expression in Eukaryotes FS 2016 Graduate Course G2
 Transcription Regulation And Gene Expression in Eukaryotes FS 2016 Graduate Course G2 P. Matthias and RG Clerc Pharmazentrum Hörsaal 2 16h15-18h00 CIRCADIAN SIGNALING NETWORKS Master pacemaker SCN «slave
Transcription Regulation And Gene Expression in Eukaryotes FS 2016 Graduate Course G2 P. Matthias and RG Clerc Pharmazentrum Hörsaal 2 16h15-18h00 CIRCADIAN SIGNALING NETWORKS Master pacemaker SCN «slave
Peripheral Clocks: Keeping Up with the Master Clock
 Peripheral Clocks: Keeping Up with the Master Clock E. KOWALSKA AND S.A. BROWN University of Zurich, Institute for Pharmacology and Toxicology, 8057 Zurich, Switzerland Circadian clocks influence most
Peripheral Clocks: Keeping Up with the Master Clock E. KOWALSKA AND S.A. BROWN University of Zurich, Institute for Pharmacology and Toxicology, 8057 Zurich, Switzerland Circadian clocks influence most
Strong Resetting of the Mammalian Clock by Constant Light Followed by Constant Darkness
 The Journal of Neuroscience, November 12, 2008 28(46):11839 11847 11839 Behavioral/Systems/Cognitive Strong Resetting of the Mammalian Clock by Constant Light Followed by Constant Darkness Rongmin Chen,
The Journal of Neuroscience, November 12, 2008 28(46):11839 11847 11839 Behavioral/Systems/Cognitive Strong Resetting of the Mammalian Clock by Constant Light Followed by Constant Darkness Rongmin Chen,
All mammalian cells investigated to date seem to possess
 Clock Genes in the Heart Characterization and Attenuation With Hypertrophy Martin E. Young, Peter Razeghi, Heinrich Taegtmeyer Abstract We investigated whether the heart, like other mammalian organs, possesses
Clock Genes in the Heart Characterization and Attenuation With Hypertrophy Martin E. Young, Peter Razeghi, Heinrich Taegtmeyer Abstract We investigated whether the heart, like other mammalian organs, possesses
Stochastic simulations
 Circadian rhythms Stochastic simulations Circadian rhythms allow living organisms to live in phase with the alternance of day and night... Application to circadian clocks Didier Gonze Circadian rhythms
Circadian rhythms Stochastic simulations Circadian rhythms allow living organisms to live in phase with the alternance of day and night... Application to circadian clocks Didier Gonze Circadian rhythms
Circadian Rhythms in Physiology and Behavior. The Persistence of Memory, Salvador Dali, 1931
 Circadian Rhythms in Physiology and Behavior The Persistence of Memory, Salvador Dali, 1931 Homeostasis and Rhythms? Homeostasis (Bernard, 1878): All the vital mechanisms, however varied they may be, have
Circadian Rhythms in Physiology and Behavior The Persistence of Memory, Salvador Dali, 1931 Homeostasis and Rhythms? Homeostasis (Bernard, 1878): All the vital mechanisms, however varied they may be, have
Stochastic simulations
 Stochastic simulations Application to circadian clocks Didier Gonze Circadian rhythms Circadian rhythms allow living organisms to live in phase with the alternance of day and night... Circadian rhythms
Stochastic simulations Application to circadian clocks Didier Gonze Circadian rhythms Circadian rhythms allow living organisms to live in phase with the alternance of day and night... Circadian rhythms
CHAPTER12. Synthesis
 CHAPTER12 Synthesis 149 Chapter 12 The tau mutation and non-circadian rhythms Biological rhythms cover a wide range of frequencies, from milliseconds to years. In this thesis we have shown that an allele
CHAPTER12 Synthesis 149 Chapter 12 The tau mutation and non-circadian rhythms Biological rhythms cover a wide range of frequencies, from milliseconds to years. In this thesis we have shown that an allele
SUPPLEMENTARY INFORMATION
 Supplementary Table 2 Mouse circadian s and observed circadian and physiological phenotypes. Gene Circadian phenotype Ref. Associated physiological abnormality Ref. Bmal1/Mop3 (Arntl) Loss-of-circadian
Supplementary Table 2 Mouse circadian s and observed circadian and physiological phenotypes. Gene Circadian phenotype Ref. Associated physiological abnormality Ref. Bmal1/Mop3 (Arntl) Loss-of-circadian
The Suprachiasmatic Nucleus Entrains, But Does Not Sustain, Circadian Rhythmicity in the Olfactory Bulb
 The Journal of Neuroscience, January 21, 2004 24(3):615 619 615 Brief Communication The Suprachiasmatic Nucleus Entrains, But Does Not Sustain, Circadian Rhythmicity in the Olfactory Bulb Daniel Granados-Fuentes,
The Journal of Neuroscience, January 21, 2004 24(3):615 619 615 Brief Communication The Suprachiasmatic Nucleus Entrains, But Does Not Sustain, Circadian Rhythmicity in the Olfactory Bulb Daniel Granados-Fuentes,
Oscillation and Light Induction of timeless mrna in the Mammalian Circadian Clock
 The Journal of Neuroscience, 1999, Vol. 19 RC15 1of6 Oscillation and Light Induction of timeless mrna in the Mammalian Circadian Clock Shelley A. Tischkau, 1 Jeffrey A. Barnes, 1 Fang-Ju Lin, 2 Edith M.
The Journal of Neuroscience, 1999, Vol. 19 RC15 1of6 Oscillation and Light Induction of timeless mrna in the Mammalian Circadian Clock Shelley A. Tischkau, 1 Jeffrey A. Barnes, 1 Fang-Ju Lin, 2 Edith M.
Modeling Rhythms on Differents Levels: Cells, Tissues, and Organisms
 Modeling Rhythms on Differents Levels: Cells, Tissues, and Organisms Hanspeter Herzel Institute for Theoretical Biology (ITB) Charité and Humboldt University Berlin Molecular Chronobiology SCN-neuron nucleus
Modeling Rhythms on Differents Levels: Cells, Tissues, and Organisms Hanspeter Herzel Institute for Theoretical Biology (ITB) Charité and Humboldt University Berlin Molecular Chronobiology SCN-neuron nucleus
Molecular Signals of Mammalian Circadian Clock
 Kobe J. Med. Sci., Vol. 50, No. 4, pp. 101-109, 2004 Molecular Signals of Mammalian Circadian Clock JING ZHANG, XIN DONG, YOSHITO FUJIMOTO, and HITOSHI OKAMURA Division of Molecular Brain Science, Department
Kobe J. Med. Sci., Vol. 50, No. 4, pp. 101-109, 2004 Molecular Signals of Mammalian Circadian Clock JING ZHANG, XIN DONG, YOSHITO FUJIMOTO, and HITOSHI OKAMURA Division of Molecular Brain Science, Department
Functional central rhythmicity and light entrainment, but not liver and muscle rhythmicity, are Clock independent
 Am J Physiol Regul Integr Comp Physiol 291: R1172 R1180, 2006. First published May 18, 2006; doi:10.1152/ajpregu.00223.2006. Functional central rhythmicity and light entrainment, but not liver and muscle
Am J Physiol Regul Integr Comp Physiol 291: R1172 R1180, 2006. First published May 18, 2006; doi:10.1152/ajpregu.00223.2006. Functional central rhythmicity and light entrainment, but not liver and muscle
Intercellular Coupling Confers Robustness against Mutations in the SCN Circadian Clock Network
 Intercellular Coupling Confers Robustness against Mutations in the SCN Circadian Clock Network Andrew C. Liu, 1,2,11 David K. Welsh, 1,3,4,11 Caroline H. Ko, 6,7 Hien G. Tran, 1,2 Eric E. Zhang, 1,2 Aaron
Intercellular Coupling Confers Robustness against Mutations in the SCN Circadian Clock Network Andrew C. Liu, 1,2,11 David K. Welsh, 1,3,4,11 Caroline H. Ko, 6,7 Hien G. Tran, 1,2 Eric E. Zhang, 1,2 Aaron
Circadian Rhythm Disturbances: What Happens When Your Biological Clock Is In The Wrong Time Zone
 Circadian Rhythm Disturbances: What Happens When Your Biological Clock Is In The Wrong Time Zone Steven A. Thau MD Chief, Pulmonary, Sleep Department. Phelps Hospital, Northwell Health Internal Clock Examples
Circadian Rhythm Disturbances: What Happens When Your Biological Clock Is In The Wrong Time Zone Steven A. Thau MD Chief, Pulmonary, Sleep Department. Phelps Hospital, Northwell Health Internal Clock Examples
The dominant circadian pacemaker in the mammalian brain is
 The methamphetamine-sensitive circadian oscillator does not employ canonical clock genes Jennifer A. Mohawk, Matthew L. Baer, and Michael Menaker 1 Department of Biology, University of Virginia, Charlottesville
The methamphetamine-sensitive circadian oscillator does not employ canonical clock genes Jennifer A. Mohawk, Matthew L. Baer, and Michael Menaker 1 Department of Biology, University of Virginia, Charlottesville
Altered Entrainment and Feedback Loop Function Effected by a Mutant Period Protein
 The Journal of Neuroscience, February 1, 2000, 20(3):958 968 Altered Entrainment and Feedback Loop Function Effected by a Mutant Period Protein Peter Schotland, Melissa Hunter-Ensor, Todd Lawrence, and
The Journal of Neuroscience, February 1, 2000, 20(3):958 968 Altered Entrainment and Feedback Loop Function Effected by a Mutant Period Protein Peter Schotland, Melissa Hunter-Ensor, Todd Lawrence, and
Mechanisms of Behavioral Modulation
 Feb 19: Rhythms Mechanisms of Behavioral Modulation "Global" modulating mechanisms: act on diverse neural subsystems, changing threshold, selectivity, or strength of many responses EXAMPLES: hormones and
Feb 19: Rhythms Mechanisms of Behavioral Modulation "Global" modulating mechanisms: act on diverse neural subsystems, changing threshold, selectivity, or strength of many responses EXAMPLES: hormones and
PHYSIOLOGY AND MAINTENANCE Vol. V - Biological Rhythms - Tarja Porkka-Heiskanen, Jarmo T. Laitinen
 BIOLOGICAL RHYTHMS Tarja Porkka-Heiskanen, Institute of Biomedicine, University of Helsinki, Finland Jarmo T. Laitinen Department of Physiology, University of Kuopio, Finland Keywords: Light, melatonin,
BIOLOGICAL RHYTHMS Tarja Porkka-Heiskanen, Institute of Biomedicine, University of Helsinki, Finland Jarmo T. Laitinen Department of Physiology, University of Kuopio, Finland Keywords: Light, melatonin,
The Role of Sleep and Arousal in the Development of Obesity"
 The Role of Sleep and Arousal in the Development of Obesity" Dr. Fred W. Turek Center for Sleep & Circadian Biology Northwestern University Treatment and Prevention of Obesity: Approaches and Needs Copenhagen
The Role of Sleep and Arousal in the Development of Obesity" Dr. Fred W. Turek Center for Sleep & Circadian Biology Northwestern University Treatment and Prevention of Obesity: Approaches and Needs Copenhagen
mcry1 and mcry2 Are Essential Components of the Negative Limb of the Circadian Clock Feedback Loop
 Cell, Vol. 98, 193 205, July 23, 1999, Copyright 1999 by Cell Press mcry1 and mcry2 Are Essential Components of the Negative Limb of the Circadian Clock Feedback Loop Kazuhiko Kume,* # Mark J. Zylka,*
Cell, Vol. 98, 193 205, July 23, 1999, Copyright 1999 by Cell Press mcry1 and mcry2 Are Essential Components of the Negative Limb of the Circadian Clock Feedback Loop Kazuhiko Kume,* # Mark J. Zylka,*
The Drosophila melanogaster life cycle
 The Drosophila melanogaster life cycle Eclosion The first phenotype described in Drosophila as an endogenous rhythm (1954): Number of eclosed flies Hamblen et al., Gene3cs 1998 rhythm with a periodicity
The Drosophila melanogaster life cycle Eclosion The first phenotype described in Drosophila as an endogenous rhythm (1954): Number of eclosed flies Hamblen et al., Gene3cs 1998 rhythm with a periodicity
Phase-resetting Responses in Clock Null Mutant Mice
 Phase-resetting Responses in Clock Null Mutant Mice Vinhfield X. Ta Program in Biological Sciences Northwestern University Evanston, Illinois May 7, 2007 Joseph S. Takahashi Senior Thesis Advisor Jason
Phase-resetting Responses in Clock Null Mutant Mice Vinhfield X. Ta Program in Biological Sciences Northwestern University Evanston, Illinois May 7, 2007 Joseph S. Takahashi Senior Thesis Advisor Jason
Light and Glutamate-Induced Degradation of the Circadian Oscillating Protein BMAL1 during the Mammalian Clock Resetting
 The Journal of Neuroscience, October 15, 2000, 20(20):7525 7530 Light and Glutamate-Induced Degradation of the Circadian Oscillating Protein BMAL1 during the Mammalian Clock Resetting Teruya Tamaru, 1
The Journal of Neuroscience, October 15, 2000, 20(20):7525 7530 Light and Glutamate-Induced Degradation of the Circadian Oscillating Protein BMAL1 during the Mammalian Clock Resetting Teruya Tamaru, 1
Molecular Clocks in Mouse Skin
 See related commentary on pg ORIGINAL ARTICLE Molecular Clocks in Mouse Skin Miki Tanioka,,, Hiroyuki Yamada,, Masao Doi,, Hideki Bando, Yoshiaki Yamaguchi, Chikako Nishigori and Hitoshi Okamura, Clock
See related commentary on pg ORIGINAL ARTICLE Molecular Clocks in Mouse Skin Miki Tanioka,,, Hiroyuki Yamada,, Masao Doi,, Hideki Bando, Yoshiaki Yamaguchi, Chikako Nishigori and Hitoshi Okamura, Clock
Phase Shifts of Circadian Transcripts in Rat Suprachiasmatic Nucleus
 The Second International Symposium on Optimization and Systems Biology (OSB 08) Lijiang, China, October 31 November 3, 2008 Copyright 2008 ORSC & APORC, pp. 109 114 Phase Shifts of Circadian Transcripts
The Second International Symposium on Optimization and Systems Biology (OSB 08) Lijiang, China, October 31 November 3, 2008 Copyright 2008 ORSC & APORC, pp. 109 114 Phase Shifts of Circadian Transcripts
Molecular and Cellular Endocrinology
 Molecular and Cellular Endocrinology 349 (2012) 38 44 Contents lists available at ScienceDirect Molecular and Cellular Endocrinology journal homepage: www.elsevier.com/locate/mce Review The role of clock
Molecular and Cellular Endocrinology 349 (2012) 38 44 Contents lists available at ScienceDirect Molecular and Cellular Endocrinology journal homepage: www.elsevier.com/locate/mce Review The role of clock
Adrenergic regulation of clock gene expression in mouse liver
 Adrenergic regulation of clock gene expression in mouse liver Hideyuki Terazono*, Tatsushi Mutoh, Shun Yamaguchi, Masaki Kobayashi, Masashi Akiyama*, Rhyuta Udo*, Shigehiro Ohdo, Hitoshi Okamura, and Shigenobu
Adrenergic regulation of clock gene expression in mouse liver Hideyuki Terazono*, Tatsushi Mutoh, Shun Yamaguchi, Masaki Kobayashi, Masashi Akiyama*, Rhyuta Udo*, Shigehiro Ohdo, Hitoshi Okamura, and Shigenobu
Report. Circadian Clock Gene Bmal1 Is Not Essential; Functional Replacement with its Paralog, Bmal2
 Current Biology 20, 316 321, February 23, 2010 ª2010 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2009.12.034 Circadian Clock Gene Bmal1 Is Not Essential; Functional Replacement with its Paralog,
Current Biology 20, 316 321, February 23, 2010 ª2010 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2009.12.034 Circadian Clock Gene Bmal1 Is Not Essential; Functional Replacement with its Paralog,
In mammals, a circadian pacemaker located in the suprachiasmatic
 PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues Seung-Hee Yoo*, Shin Yamazaki, Phillip L. Lowrey*, Kazuhiro Shimomura*,
PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues Seung-Hee Yoo*, Shin Yamazaki, Phillip L. Lowrey*, Kazuhiro Shimomura*,
SERIES ARTICLE Circadian Rhythms
 Circadian Rhythms 3. Circadian Timing Systems: How are they Organized? Koustubh M Vaze and Vijay Kumar Sharma (left) Koustubh Vaze is a Research Associate in the Evolutionary and Organismal Biology Unit,
Circadian Rhythms 3. Circadian Timing Systems: How are they Organized? Koustubh M Vaze and Vijay Kumar Sharma (left) Koustubh Vaze is a Research Associate in the Evolutionary and Organismal Biology Unit,
Biological Rhythms. Today s lecture
 Biological Rhythms (a review of general endocrinology) 35 Neuroendocrine control: homeostatic responses and biological rhythms. A role for anticipation or feed-forward mechanisms or scheduled events. Biological
Biological Rhythms (a review of general endocrinology) 35 Neuroendocrine control: homeostatic responses and biological rhythms. A role for anticipation or feed-forward mechanisms or scheduled events. Biological
The Nobel Assembly at Karolinska Institutet has today decided to award. the 2017 Nobel Prize in Physiology or Medicine. jointly to
 The Nobel Assembly at Karolinska Institutet has today decided to award the 2017 Nobel Prize in Physiology or Medicine jointly to Jeffrey C. Hall, Michael Rosbash and Michael W. Young for their discoveries
The Nobel Assembly at Karolinska Institutet has today decided to award the 2017 Nobel Prize in Physiology or Medicine jointly to Jeffrey C. Hall, Michael Rosbash and Michael W. Young for their discoveries
RESEARCH ARTICLE Significant dissociation of expression patterns of the basic helix loop helix transcription factors Dec1 and Dec2 in rat kidney
 1257 The Journal of Experimental Biology 214, 1257-1263 2011. Published by The Company of Biologists Ltd doi:10.1242/jeb.052100 RESEARCH ARTICLE Significant dissociation of expression patterns of the basic
1257 The Journal of Experimental Biology 214, 1257-1263 2011. Published by The Company of Biologists Ltd doi:10.1242/jeb.052100 RESEARCH ARTICLE Significant dissociation of expression patterns of the basic
Daily injection of insulin attenuated impairment of liver circadian clock oscillation in the streptozotocin-treated diabetic mouse
 FEBS Letters 572 (2004) 206 210 FEBS 28689 Daily injection of insulin attenuated impairment of liver circadian clock oscillation in the streptozotocin-treated diabetic mouse Koji Kuriyama, Kei Sasahara,
FEBS Letters 572 (2004) 206 210 FEBS 28689 Daily injection of insulin attenuated impairment of liver circadian clock oscillation in the streptozotocin-treated diabetic mouse Koji Kuriyama, Kei Sasahara,
Food Intake Regulation & the Clock. Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD
 Food Intake Regulation & the Clock Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Circadian disruption affect multiple organ systems: The diagram provides examples of how circadian disruption
Food Intake Regulation & the Clock Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Circadian disruption affect multiple organ systems: The diagram provides examples of how circadian disruption
Supplementary Figure 1. Expression of the inducible tper2 is proportional to Dox/Tet concentration in Rosa-DTG/Per2 Per2-luc/wt MEFs.
 Supplementary Figure 1. Expression of the inducible tper2 is proportional to Dox/Tet concentration in Rosa-DTG/Per2 Per2-luc/wt MEFs. (a) Dose-responsive expression of tper2 by Dox. Note that there are
Supplementary Figure 1. Expression of the inducible tper2 is proportional to Dox/Tet concentration in Rosa-DTG/Per2 Per2-luc/wt MEFs. (a) Dose-responsive expression of tper2 by Dox. Note that there are
Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: Effects of transplanting the pacemaker
 University of Massachusetts Amherst ScholarWorks@UMass Amherst Biology Department Faculty Publication Series Biology 2006 Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral
University of Massachusetts Amherst ScholarWorks@UMass Amherst Biology Department Faculty Publication Series Biology 2006 Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral
Expression of Clock and Clock-Driven Genes in the Rat Suprachiasmatic Nucleus during Late Fetal and Early Postnatal Development
 Expression of Clock and Clock-Driven Genes in the Rat Suprachiasmatic Nucleus during Late Fetal and Early Postnatal Development Zuzana Kováčiková, 1 Martin Sládek, 1 Zdenka Bendová, Helena Illnerová, and
Expression of Clock and Clock-Driven Genes in the Rat Suprachiasmatic Nucleus during Late Fetal and Early Postnatal Development Zuzana Kováčiková, 1 Martin Sládek, 1 Zdenka Bendová, Helena Illnerová, and
The Time of Your Life
 The Time of Your Life By Paolo Sassone-Corsi, Ph.D. Editor s Note: The circadian rhythm the 24-hour cycle of the physiological processes of living beings is instrumental in determining the sleeping and
The Time of Your Life By Paolo Sassone-Corsi, Ph.D. Editor s Note: The circadian rhythm the 24-hour cycle of the physiological processes of living beings is instrumental in determining the sleeping and
An Abrupt Shift in the Day/Night Cycle Causes Desynchrony in the Mammalian Circadian Center
 The Journal of Neuroscience, July 9, 2003 23(14):6141 6151 6141 Behavioral/Systems/Cognitive An Abrupt Shift in the Day/Night Cycle Causes Desynchrony in the Mammalian Circadian Center Mamoru Nagano, 1
The Journal of Neuroscience, July 9, 2003 23(14):6141 6151 6141 Behavioral/Systems/Cognitive An Abrupt Shift in the Day/Night Cycle Causes Desynchrony in the Mammalian Circadian Center Mamoru Nagano, 1
Brain glucocorticoid receptors are necessary for the rhythmic expression of the. clock protein, PERIOD2, in the central extended amygdala in mice
 * Manuscript Click here to view linked References Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice 1 Segall,
* Manuscript Click here to view linked References Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice 1 Segall,
Biological rhythms. Types of biological rhythms
 Biological rhythms Types of biological rhythms 2/33 what do we call rhythm in a living organism? physiological events occurring at approximately regular times internally controlled rhythms: breathing,
Biological rhythms Types of biological rhythms 2/33 what do we call rhythm in a living organism? physiological events occurring at approximately regular times internally controlled rhythms: breathing,
LESSON 4.5 WORKBOOK How do circuits regulate their output?
 DEFINITIONS OF TERMS Homeostasis tendency to relatively stable equilibrium. Feed-forward inhibition control mechanism whereby the output of one pathway inhibits the activity of another pathway. Negative
DEFINITIONS OF TERMS Homeostasis tendency to relatively stable equilibrium. Feed-forward inhibition control mechanism whereby the output of one pathway inhibits the activity of another pathway. Negative
The Ticking CLOCK of HSV-2 Pathology Rebecca J. Bayliss 1 and Vincent Piguet 1,2,3
 The Ticking CLOCK of HSV-2 Pathology Rebecca J. Bayliss 1 and Vincent Piguet 1,2,3 1 Division of Infection and Immunity, Cardiff University School of Medicine, Cardiff, UK; 2 Division of Dermatology, Women
The Ticking CLOCK of HSV-2 Pathology Rebecca J. Bayliss 1 and Vincent Piguet 1,2,3 1 Division of Infection and Immunity, Cardiff University School of Medicine, Cardiff, UK; 2 Division of Dermatology, Women
Targeting of the attenuated diphtheria toxin (adta) into the melanopsin locus. a,
 doi: 1.138/nature6829 a DTA HSV- TK PGK-Neo Targeting construct b kb.85.65 L WT adta/+ adta/ adta Melanopsin (Opn 4) Genomic Locus 1 kb.4 Supplementary Figure 1: Targeting of the attenuated diphtheria
doi: 1.138/nature6829 a DTA HSV- TK PGK-Neo Targeting construct b kb.85.65 L WT adta/+ adta/ adta Melanopsin (Opn 4) Genomic Locus 1 kb.4 Supplementary Figure 1: Targeting of the attenuated diphtheria
Feeding Cues Alter Clock Gene Oscillations and Photic Responses in the Suprachiasmatic Nuclei of Mice Exposed to a Light/Dark Cycle
 1514 The Journal of Neuroscience, February 9, 2005 25(6):1514 1522 Behavioral/Systems/Cognitive Feeding Cues Alter Clock Gene Oscillations and Photic Responses in the Suprachiasmatic Nuclei of Mice Exposed
1514 The Journal of Neuroscience, February 9, 2005 25(6):1514 1522 Behavioral/Systems/Cognitive Feeding Cues Alter Clock Gene Oscillations and Photic Responses in the Suprachiasmatic Nuclei of Mice Exposed
All mammals exhibit an array of daily behavioral, physiological,
 Insight into molecular core clock mechanism of embryonic and early postnatal rat suprachiasmatic nucleus Martin Sládek, Alena Sumová*, Zuzana Kováčiková, Zdenka Bendová, Kristýna Laurinová, and Helena
Insight into molecular core clock mechanism of embryonic and early postnatal rat suprachiasmatic nucleus Martin Sládek, Alena Sumová*, Zuzana Kováčiková, Zdenka Bendová, Kristýna Laurinová, and Helena
TIMED RESTRICTED FEEDING RESTORES THE RHYTHMS OF EXPRESSION OF THE CLOCK PROTEIN, PER2, IN THE OVAL NUCLEUS OF
 * - Manuscript Click here to view linked References 1 1 1 1 1 0 1 0 TIMED RESTRICTED FEEDING RESTORES THE RHYTHMS OF EXPRESSION OF THE CLOCK PROTEIN, PER, IN THE OVAL NUCLEUS OF THE BED NUCLEUS OF THE
* - Manuscript Click here to view linked References 1 1 1 1 1 0 1 0 TIMED RESTRICTED FEEDING RESTORES THE RHYTHMS OF EXPRESSION OF THE CLOCK PROTEIN, PER, IN THE OVAL NUCLEUS OF THE BED NUCLEUS OF THE
Entrainment of the circadian clock in humans: mechanism and implications for sleep disorders.
 Impulse: The Premier Journal for Undergraduate Publications in the Neurosciences Entrainment of the circadian clock in humans: mechanism and implications for sleep disorders. David Metcalfe Warwick Medical
Impulse: The Premier Journal for Undergraduate Publications in the Neurosciences Entrainment of the circadian clock in humans: mechanism and implications for sleep disorders. David Metcalfe Warwick Medical
Behavioral implications of the dynamic regulation of microglia: the influence of a day versus a lifetime
 Behavioral implications of the dynamic regulation of microglia: the influence of a day versus a lifetime Laura K. Fonken Postdoctoral Fellow University of Colorado March 20, 2017 About me: Research Timeline
Behavioral implications of the dynamic regulation of microglia: the influence of a day versus a lifetime Laura K. Fonken Postdoctoral Fellow University of Colorado March 20, 2017 About me: Research Timeline
Drosophila Free-Running Rhythms Require Intercellular Communication
 Drosophila Free-Running Rhythms Require Intercellular Communication Ying Peng 1,2, Dan Stoleru 1,2, Joel D. Levine 1, Jeffrey C. Hall 1, Michael Rosbash 1,2* PLoS BIOLOGY 1 Department of Biology, Brandeis
Drosophila Free-Running Rhythms Require Intercellular Communication Ying Peng 1,2, Dan Stoleru 1,2, Joel D. Levine 1, Jeffrey C. Hall 1, Michael Rosbash 1,2* PLoS BIOLOGY 1 Department of Biology, Brandeis
The ins and outs of circadian timekeeping Steven A Brown* and Ueli Schibler
 588 The ins and outs of circadian timekeeping Steven A Brown* and Ueli Schibler Recent research in Drosophila and in mammals has generated fascinating new models for how circadian clocks in these organisms
588 The ins and outs of circadian timekeeping Steven A Brown* and Ueli Schibler Recent research in Drosophila and in mammals has generated fascinating new models for how circadian clocks in these organisms
Deletion of the Mammalian Circadian Clock Gene BMAL1/Mop3 Alters Baseline Sleep Architecture and the Response to Sleep Deprivation
 CIRCADIAN RHYTHMS Deletion of the Mammalian Circadian Clock Gene BMAL1/Mop3 Alters Baseline Sleep Architecture and the Response to Sleep Deprivation Aaron Laposky, PhD 1 ; Amy Easton, PhD 1 ; Christine
CIRCADIAN RHYTHMS Deletion of the Mammalian Circadian Clock Gene BMAL1/Mop3 Alters Baseline Sleep Architecture and the Response to Sleep Deprivation Aaron Laposky, PhD 1 ; Amy Easton, PhD 1 ; Christine
Circadian rhythm and Sleep. Radwan Banimustafa MD
 Circadian rhythm and Sleep Radwan Banimustafa MD Homeostasis Maintenance of equilibrium by active regulation of internal states: Cardiovascular function (blood pressure, heart rate) Body temperature Food
Circadian rhythm and Sleep Radwan Banimustafa MD Homeostasis Maintenance of equilibrium by active regulation of internal states: Cardiovascular function (blood pressure, heart rate) Body temperature Food
Review. The Network of Time: Understanding the Molecular Circadian System. Till Roenneberg and Martha Merrow
 Current Biology, Vol. 13, R198 R207, March 4, 2003, 2003 Elsevier Science Ltd. All rights reserved. PII S0960-9822(03)00124-6 The Network of Time: Understanding the Molecular Circadian System Review Till
Current Biology, Vol. 13, R198 R207, March 4, 2003, 2003 Elsevier Science Ltd. All rights reserved. PII S0960-9822(03)00124-6 The Network of Time: Understanding the Molecular Circadian System Review Till
Circadian Clocks: Setting Time By Food
 YOUNG INVESTIGATOR PERSPECTIVES Journal of Neuroendocrinology 19, 127 137 ª 2006 The Author. Journal Compilation ª 2006 Blackwell Publishing Ltd Circadian Clocks: Setting Time By Food J. Mendoza Institut
YOUNG INVESTIGATOR PERSPECTIVES Journal of Neuroendocrinology 19, 127 137 ª 2006 The Author. Journal Compilation ª 2006 Blackwell Publishing Ltd Circadian Clocks: Setting Time By Food J. Mendoza Institut
Eicosapentaenoic Acid Reverses the Oleic Acid-induced Reduction in Per1 mrna Expression in Cultured Rat Hepatocytes
 8 Ivyspring International Publisher Research Paper Journal of Biomedicine 2018; 3: 8-12. doi: 10.7150/jbm.23267 Eicosapentaenoic Acid Reverses the Oleic Acid-induced Reduction in Per1 mrna Expression in
8 Ivyspring International Publisher Research Paper Journal of Biomedicine 2018; 3: 8-12. doi: 10.7150/jbm.23267 Eicosapentaenoic Acid Reverses the Oleic Acid-induced Reduction in Per1 mrna Expression in
Supplementary Information for
 Supplementary Information for Involvement of urinary bladder Connexin43 and the circadian clock in the coordination of diurnal micturition rhythm Hiromitsu Negoro, 1,2 Akihiro Kanematsu, 1,3 Masao Doi,
Supplementary Information for Involvement of urinary bladder Connexin43 and the circadian clock in the coordination of diurnal micturition rhythm Hiromitsu Negoro, 1,2 Akihiro Kanematsu, 1,3 Masao Doi,
Sleep, Dreaming and Circadian Rhythms
 Sleep, Dreaming and Circadian Rhythms People typically sleep about 8 hours per day, and spend 16 hours awake. Most people sleep over 175,000 hours in their lifetime. The vast amount of time spent sleeping
Sleep, Dreaming and Circadian Rhythms People typically sleep about 8 hours per day, and spend 16 hours awake. Most people sleep over 175,000 hours in their lifetime. The vast amount of time spent sleeping
Circadian Rhythms in Isolated Brain Regions
 The Journal of Neuroscience, January 1, 2002, 22(1):350 356 Circadian Rhythms in Isolated Brain Regions Michikazu Abe, 1 * Erik D. Herzog, 1 * Shin Yamazaki, 1 Marty Straume, 1 Hajime Tei, 2 Yoshiyuki
The Journal of Neuroscience, January 1, 2002, 22(1):350 356 Circadian Rhythms in Isolated Brain Regions Michikazu Abe, 1 * Erik D. Herzog, 1 * Shin Yamazaki, 1 Marty Straume, 1 Hajime Tei, 2 Yoshiyuki
Dietmar Weinert Institute of Zoology, Martin-Luther-University Halle-Wittenberg, Halle, Germany
 Chronobiology International, 22(2): 179 205, (2005) Copyright # 2005 Taylor & Francis, Inc. ISSN 0742-0528 print/1525-6073 online DOI: 10.1081/CBI-200053473 REVIEW ONTOGENETIC DEVELOPMENT OF THE MAMMALIAN
Chronobiology International, 22(2): 179 205, (2005) Copyright # 2005 Taylor & Francis, Inc. ISSN 0742-0528 print/1525-6073 online DOI: 10.1081/CBI-200053473 REVIEW ONTOGENETIC DEVELOPMENT OF THE MAMMALIAN
Circadian Gene Expression in Individual Fibroblasts: Cell-Autonomous and Self-Sustained Oscillators Pass Time to Daughter Cells
 Cell, Vol. 119, 693 705, November 24, 2004, Copyright 2004 by Cell Press Circadian Gene Expression in Individual Fibroblasts: Cell-Autonomous and Self-Sustained Oscillators Pass Time to Daughter Cells
Cell, Vol. 119, 693 705, November 24, 2004, Copyright 2004 by Cell Press Circadian Gene Expression in Individual Fibroblasts: Cell-Autonomous and Self-Sustained Oscillators Pass Time to Daughter Cells
METABOLISM AND THE CONTROL OF CIRCADIAN RHYTHMS
 Annu. Rev. Biochem. 2002. 71:307 31 DOI: 10.1146/annurev.biochem.71.090501.142857 Copyright 2002 by Annual Reviews. All rights reserved First published as a Review in Advance on March 19, 2002 METABOLISM
Annu. Rev. Biochem. 2002. 71:307 31 DOI: 10.1146/annurev.biochem.71.090501.142857 Copyright 2002 by Annual Reviews. All rights reserved First published as a Review in Advance on March 19, 2002 METABOLISM
Na V 1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms
 Na V 1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms Sung Han a, Frank H. Yu b,1, Michael D. Schwartz c,2, Jonathan D. Linton a,d,
Na V 1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms Sung Han a, Frank H. Yu b,1, Michael D. Schwartz c,2, Jonathan D. Linton a,d,
PROMOTER ANALYSIS OF MAMMALIAN CLOCK CONTROLLED GENES
 65 PROMOTER ANALYSIS OF MAMMALIAN CLOCK CONTROLLED GENES KATARZYNA BOŻEK1 SZYMON M. KIE LBASA 2 k.bozek@biologie.hu-berlin.de kielbasa@molgen.mpg.de ACHIM KRAMER 3 HANSPETER HERZEL 1 achim.kramer@charite.de
65 PROMOTER ANALYSIS OF MAMMALIAN CLOCK CONTROLLED GENES KATARZYNA BOŻEK1 SZYMON M. KIE LBASA 2 k.bozek@biologie.hu-berlin.de kielbasa@molgen.mpg.de ACHIM KRAMER 3 HANSPETER HERZEL 1 achim.kramer@charite.de
Supplementary Figure 1 Validation of Per2 deletion in neuronal cells in N Per2 -/- mice. (a) Western blot from liver extracts of mice held under ad
 Supplementary Figure 1 Validation of Per2 deletion in neuronal cells in N Per2 -/- mice. (a) Western blot from liver extracts of mice held under ad libitum conditions detecting PER2 protein in brain and
Supplementary Figure 1 Validation of Per2 deletion in neuronal cells in N Per2 -/- mice. (a) Western blot from liver extracts of mice held under ad libitum conditions detecting PER2 protein in brain and
PROGRESS TOWARDS OBJECTIVES, SIGNIFICANT RESULTS, DEVIATIONS, REASONS FOR FAILING, CORRECTIVE ACTIONS
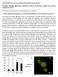 FINAL REPORT Grant Agreement PIEF-GA-2011-303197 Obesity and Light PROGRESS TOWARDS OBJECTIVES, SIGNIFICANT RESULTS, DEVIATIONS, REASONS FOR FAILING, CORRECTIVE ACTIONS For the total period (2 years) three
FINAL REPORT Grant Agreement PIEF-GA-2011-303197 Obesity and Light PROGRESS TOWARDS OBJECTIVES, SIGNIFICANT RESULTS, DEVIATIONS, REASONS FOR FAILING, CORRECTIVE ACTIONS For the total period (2 years) three
Scientific Background Discoveries of Molecular Mechanisms Controlling the Circadian Rhythm
 Scientific Background Discoveries of Molecular Mechanisms Controlling the Circadian Rhythm The 2017 Nobel Prize in Physiology or Medicine is awarded to Jeffrey C. Hall, Michael Rosbash and Michael W. Young
Scientific Background Discoveries of Molecular Mechanisms Controlling the Circadian Rhythm The 2017 Nobel Prize in Physiology or Medicine is awarded to Jeffrey C. Hall, Michael Rosbash and Michael W. Young
The changes in behavior that occur on a 24-hour basis to match the 24-hour changes in
 BASIC SCIENCE SEMINARS IN NEUROLOGY Current Understanding of the Circadian Clock and the Clinical Implications for Neurological Disorders Fred W. Turek, PhD; Christine Dugovic, PhD; Phyllis C. Zee, MD,
BASIC SCIENCE SEMINARS IN NEUROLOGY Current Understanding of the Circadian Clock and the Clinical Implications for Neurological Disorders Fred W. Turek, PhD; Christine Dugovic, PhD; Phyllis C. Zee, MD,
For animals to survive effectively, the timing of feedingrelated
 The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker Michihiro Mieda*, S. Clay Williams, James A. Richardson, Kohichi Tanaka*, and Masashi Yanagisawa *Laboratory of Molecular
The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker Michihiro Mieda*, S. Clay Williams, James A. Richardson, Kohichi Tanaka*, and Masashi Yanagisawa *Laboratory of Molecular
From the reveller to the lark
 In Focus: Chronobiology From the reveller to the lark The internal clock changes with age Prof. Dr. Anne Eckert, Neurobiological Laboratory for Brain Aging and Mental Health, Psychiatric University Clinics
In Focus: Chronobiology From the reveller to the lark The internal clock changes with age Prof. Dr. Anne Eckert, Neurobiological Laboratory for Brain Aging and Mental Health, Psychiatric University Clinics
Cellular DBP and E4BP4 proteins are critical for determining the period length of the circadian oscillator
 FEBS Letters 55 () 7 journal homepage: www.febsletters.org Cellular and proteins are critical for determining the period length of the circadian oscillator Daisuke Yamajuku,, Yasutaka Shibata, Masashi
FEBS Letters 55 () 7 journal homepage: www.febsletters.org Cellular and proteins are critical for determining the period length of the circadian oscillator Daisuke Yamajuku,, Yasutaka Shibata, Masashi
N.T.Kumar*,Thiago R,Sandro B,Vinicius R,Lisane V,Vanderlei P Versor Inovação,Santo André SP,Brazil. id* :
 General Theoretical Aspects of Cell Mechanics from Circadian Oscillator Point of View Based on Higher Order Logic(HOL) A Short Communication to Develop Informatics Framework Involving Bio-Chemical Concepts
General Theoretical Aspects of Cell Mechanics from Circadian Oscillator Point of View Based on Higher Order Logic(HOL) A Short Communication to Develop Informatics Framework Involving Bio-Chemical Concepts
Behavioral responses to combinations of timed light, food availability, and ultradian rhythms in the common vole (Microtus arvalis)
 Behavioral responses to combinations of timed light, food availability, and ultradian rhythms in the common vole (Microtus arvalis) Daan R. van der Veen 1,2, Dirk-Jan Saaltink 1,3, and Menno P. Gerkema
Behavioral responses to combinations of timed light, food availability, and ultradian rhythms in the common vole (Microtus arvalis) Daan R. van der Veen 1,2, Dirk-Jan Saaltink 1,3, and Menno P. Gerkema
Physiology of the Mammalian Circadian System Alan M. Rosenwasser Fred W. Turek
 29 Physiology of the Mammalian Circadian System Alan M. Rosenwasser Fred W. Turek ABSTRACT Our understanding of the physiology of the mammalian circadian system has increased enormously in just the past
29 Physiology of the Mammalian Circadian System Alan M. Rosenwasser Fred W. Turek ABSTRACT Our understanding of the physiology of the mammalian circadian system has increased enormously in just the past
Circadian Gene Expression in the Suprachiasmatic Nucleus
 Circadian Gene Expression in the Suprachiasmatic Nucleus 901 Circadian Gene Expression in the Suprachiasmatic Nucleus M U Gillette and S-H Tyan, University of Illinois at Urbana-Champaign, Urbana, IL,
Circadian Gene Expression in the Suprachiasmatic Nucleus 901 Circadian Gene Expression in the Suprachiasmatic Nucleus M U Gillette and S-H Tyan, University of Illinois at Urbana-Champaign, Urbana, IL,
TEMPORAL ORGANIZATION OF FEEDING IN SYRIAN HAMSTERS WITH A GENETICALLY ALTERED CIRCADIAN PERIOD
 CHRONOBIOLOGY INTERNATIONAL, 18(4), 657 664 (2001) TEMPORAL ORGANIZATION OF FEEDING IN SYRIAN HAMSTERS WITH A GENETICALLY ALTERED CIRCADIAN PERIOD Malgorzata Oklejewicz, 1, * Gerard J. F. Overkamp, 1 J.
CHRONOBIOLOGY INTERNATIONAL, 18(4), 657 664 (2001) TEMPORAL ORGANIZATION OF FEEDING IN SYRIAN HAMSTERS WITH A GENETICALLY ALTERED CIRCADIAN PERIOD Malgorzata Oklejewicz, 1, * Gerard J. F. Overkamp, 1 J.
The Human PER1 Gene is Inducible by Interleukin-6
 Journal of Molecular Neuroscience Copyright 2002 Humana Press Inc. All rights of any nature whatsoever reserved. ISSN0895-8696/02/18:105 110/$11.25 The Human PER1 Gene is Inducible by Interleukin-6 Dirk
Journal of Molecular Neuroscience Copyright 2002 Humana Press Inc. All rights of any nature whatsoever reserved. ISSN0895-8696/02/18:105 110/$11.25 The Human PER1 Gene is Inducible by Interleukin-6 Dirk
Make sure you remember the Key Concepts
 A2 Psychology Term 1 Module 4 Physiological Psychology Biological Rhythms, Sleep and Dreaming Area of Study: Biological Rhythms. Lesson 7 Getting you Thinking pg 403 Make sure you remember the Key Concepts
A2 Psychology Term 1 Module 4 Physiological Psychology Biological Rhythms, Sleep and Dreaming Area of Study: Biological Rhythms. Lesson 7 Getting you Thinking pg 403 Make sure you remember the Key Concepts
Welcome to Bi !
 Welcome to Bi156 2012! Professors: Paul Patterson (php@caltech.edu) Kai Zinn (zinnk@caltech.edu) TAs: Janna Nawroth Yanan Sui ysui@caltech.edu Student presentations Select a topic
Welcome to Bi156 2012! Professors: Paul Patterson (php@caltech.edu) Kai Zinn (zinnk@caltech.edu) TAs: Janna Nawroth Yanan Sui ysui@caltech.edu Student presentations Select a topic
Neurobiology of Circadian Rhythms
 ARC-IBRO ISN Joined Neuroscience School Behavioural Bioassays in Neuroscience: Brain and Behavior From Invertabrates To Small Mammals 4-14 December 2014 ICIPE, Nairobi KENYA Neurobiology of Circadian Rhythms
ARC-IBRO ISN Joined Neuroscience School Behavioural Bioassays in Neuroscience: Brain and Behavior From Invertabrates To Small Mammals 4-14 December 2014 ICIPE, Nairobi KENYA Neurobiology of Circadian Rhythms
Drosophila Photoreceptors Contain an Autonomous Circadian Oscillator That Can Function without period mrna Cycling
 The Journal of Neuroscience, January 15, 1998, 18(2):741 750 Drosophila Photoreceptors Contain an Autonomous Circadian Oscillator That Can Function without period mrna Cycling Yuzhong Cheng 1 and Paul
The Journal of Neuroscience, January 15, 1998, 18(2):741 750 Drosophila Photoreceptors Contain an Autonomous Circadian Oscillator That Can Function without period mrna Cycling Yuzhong Cheng 1 and Paul
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
