Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH
|
|
|
- Marvin Hamilton
- 5 years ago
- Views:
Transcription
1 Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH 2nd Annual NASH Summit Europe October 23-24, 2018 Frankfort, Germany Peter G. Traber, MD Partner, Alacrita Consulting Alacrita Consulting Inc 303 Wyman St., Suite 325 Waltham, MA Alacrita Consulting Ltd London BioScience Innovation Centre 2 Royal College Street, London NW1 0NH Alacrita Consulting AG Artherstrasse Zug, Switzerland
2 Disclosures Presenter was previously full time employee of Galectin Therapeutics (CEO and CMO until June 2018), but currently owns no equity in company. Page 1
3 Chronic Liver Disease, Cirrhosis and its Progression Chronic Liver Disease Liver Fibrosis Compensated Cirrhosis Decompensated Cirrhosis NASH Variceal Bleeding Viral Hepatitis Ascites Alcohol Encephalopathy Other Jaundice/Liver Failure Hepatocellular Carcinoma Page 2
4 Fibrosis Stage Progression Associated with NASH Stage 1 Stage 2 Stage 3 Stage 4/Cirrhosis NASH and Fibrosis Stage Survival Free of Liver Transplantation Based on Fibrosis Stage 1 Approximately one-third of patients with NASH will advance to Stage 3/4 fibrosis 2 An estimated 40% of NASH patients in the U.S. have a fibrosis stage of F2 or higher 3 NASH with advanced fibrosis carries the greatest risk of all-cause and liver-related mortality 2,4,5 1 Graphic taken from ICPT presentation May 2018 which re-graphs data from Angulo, et al. Gastroenterology 2015;149: Caldwell, et al. Dig Dis 2010;28: Estes, et al. Hepatology 2018;67: Dulai, et al. Hepatology 2017;65: Hagstrom, et al. J Hepatology 2017;67: Page 3
5 Percent Collagen in NASH Liver Biopsies per Stage of Fibrosis Liver Biopsy Sirius Red Morphometry by Fibrosis Stage 1 Collagen Accumulation in NASH 1 Data from Goodman and Harrison The distribution of fibrosis in NASH is important in staging as well as the amount of collagen While there is an increase in the median percent collagen from stage 0 to 3, there is a great deal of overlap of values. In stage 4, or cirrhosis, there is a marked increase in the median amount of collagen and a very broad range. These and other published data show that progression of fibrosis after the development of cirrhosis is a critical element for development of complications of cirrhosis Better methods of quantifying fibrosis is required for early drug assessment Page 4
6 Portal Hypertension is a Major Driver of Decompensation Increased pressure in the portal circulation is initiated by increased intrahepatic resistance to blood flow though the liver Page 5
7 Multiple Contributors to Increased Intrahepatic Blood Flow Resistance in Cirrhosis Normal Liver Acinar Unit Distorted Architecture in Cirrhosis Structural Components Scar tissue Stellate cells Regenerative nodules Neoangiogenesis Micro thrombosis Non-Structural Components Nitric Oxide Endothelin Eiconsanoids CO/others Endothelial Dysfunction Page 6
8 Cirrhosis Complications Center Around Increased Portal Vein Blood Pressure Encephalopathy Shunting Hepatic Insufficiency Increased Resistance Portal Hypertension Splanchnic vasodilatation Effective Hypovolemia Neurohormonal Activation Increased Cardiac Output Na/H2O Retention Increased Flow Ascites Page 7
9 Survival Between Compensated and Decompensated Cirrhosis Percent Alive D Aminco et. Al., J Hepatol 2006;44:217 (Graphic borrowed from Dr. Guadalupe Garcia-Tso) Page 8
10 Towards a Better Understanding of NASH Fibrosis/Cirrhosis Natural History Page 9
11 Adjusted Overall Survival Without Transplantation According to Fibrosis Stage and CTP Multinational with patients recruited from tertiary treatment centers in Europe, Asia, Cuba, and Australia A total of 458 subjects were included, of which 159 (35%) and 299 (65%) had bridging fibrosis and cirrhosis, respectively. Most cirrhotic patients were CTP-A5 (74%) Overall mean follow-up period was 5.5 years (range, years) Vilar-Gomez, et. al., Gastroenterology 2018;155: Page 10
12 Data Summary Graphic Vilar-Gomez, et. al., Gastroenterology 2018;155: Page 11
13 Phase 2b Results: Simtuzumab in NASH Patients with Bridging Fibrosis Large, randomized controlled clinical trial (n=219) evaluating two doses of SIM after 48 and 96 weeks of therapy Statistically significant reduction in liver collagen on biopsy over the course of the study in placebo group No difference between placebo and treatment groups in mean change in hepatic collagen by morphometry (primary endpoint) Harrison, et. al., Gastroenterology 2018;155: Page 12
14 HALT-C Trial: Progression of Fibrosis in Chronic Viral Hepatitis C ~2 years Collagen content assessed by liver biopsy morphometry in patients with chronic viral hepatitis C In lower and mid-range starting collagen %, there was a significant increase over 2 years The progression of fibrosis differs between chronic viral hepatitis C and NASH Goodman, et. al., Hepatology 2009;50: Page 13
15 Phase 2b Results: Simtuzumab in NASH Patients with Bridging Fibrosis Modified Ishak Score (0-6) NASH-CRN Score (0-4) Changes in fibrosis score NASH-CRN score is currently required by regulatory agencies In placebo patients with median observation of 29 months, 23% had at least a one stage improvement in NASH-CRN score, and there was no difference in SIM groups 20% of patients progressed to cirrhosis over mean observation of 30 months, using histologic or clinical signs Harrison, et. al., Gastroenterology 2018;155: Page 14
16 Phase 2b Results: Simtuzumab in NASH Patients with Compensated Cirrhosis Large, randomized controlled clinical trial (n=258) evaluating two doses of SIM after 48 and 96 weeks of therapy 67% with clinically significant portal hypertension and 43% with esophageal varices Primary endpoint was change in HVPG at week 96 No difference between placebo and treatment groups 32.1% of placebo group had 20% reduction in HVPG Harrison, et. al., Gastroenterology 2018;155: Page 15
17 Phase 2b Results: Simtuzumab in NASH Patients with Compensated Cirrhosis Modified Ishak Score (0-6) NASH-CRN Score (0-4) Changes in fibrosis score NASH-CRN score is currently required by regulatory agencies In placebo patients with median observation of 29 months, 8% had at least a one stage improvement in NASH- CRN score, and there was no difference in SIM groups Harrison, et. al., Gastroenterology 2018;155: Page 16
18 Liver-Related Clinical Events in NASH Patients with Compensated Cirrhosis Median follow-up of 30.7 months (IQR ) Liver related clinical events occurred in 18%, 24%, and 15% of SIM200, SIM700, and placebo, respectively; no differences between the groups Harrison, et. al., Gastroenterology 2018;155: Page 17
19 The Critical Cirrhosis Transition: Endpoints for Pre-Cirrhotic NASH Stage 3 Fibrosis Stage 4: Cirrhosis Pre-cirrhotic NASH Endpoints Surrogates for Accelerated Approval (agreement with Agencies as part of Phase 3 clinical trials) Proportion of patients who achieve 1 stage improvement in fibrosis without worsening of NASH Proportion of patients who achieve NASH resolution without worsening of liver fibrosis Clinical Outcomes for Full Approval Reduced time to cirrhosis complications, including the progression to cirrhosis Page 18
20 The Critical Cirrhosis Transition: Endpoints for NASH Cirrhosis Stage 3 Fibrosis Stage 4: Cirrhosis NASH Cirrhosis Endpoints Surrogates for Accelerated Approval (agreement with Agencies as part of Phase 3 clinical trials) Proportion of patients who achieve 1 stage improvement in fibrosis without worsening of NASH Clinical Outcomes for Full Approval Reduced time to cirrhosis complications The following are potential endpoints as there are no final phase 3 protocols Reduction in HVPG (endpoints will need to define threshold and degree of reduction in specific populations TBD) Reduced time to development of esophageal varices in patients with no varices at baseline Reduced time to cirrhosis complications Reduced time to cirrhosis complications Page 19
21 Targets for NASH Therapies Targets and drugs in current clinical trials for NASH cirrhosis Inhibition of apoptosis pathway Ø Emricasan Ø Selonsertib Anti-fibrotic Simtuzumab (reported) GR-MD-02 Metabolic regulator Ø BMS (FGF-21) FXR agonist Ø Obeticholic Acid Konerman, et. al., J. Hepatology Page 20
22 Phase 2/3 Clinical Trials in NASH Cirrhosis Drug (Company/Partner) MOA/Route of Administration Phase Studies NASH Cirrhosis Trial Supportive pre-cirrhotic NASH Fibrosis Trial Next Expected Data (estimate) Selonsertib (Gilead) ASK-1 inhib./oral STELLAR-4: compensated cirrhosis STELLAR-3: NASH with F3 fibrosis ATLAS*: F3 and F4 patients Q Q Q Obeticholic acid (Intercept) FXR Agonist/oral 3 3 REVERSE: compensated cirrhosis REGENERATE: NASH with F2/F3 fib JUL 2020 H GR-MD-02 (GALT) Galectin-3 inhib./iv 3 Compensated cirrhosis w/o varices--phase 3 start not yet announced TBA Emricasan (CNAT/Novartis) Pan-caspase inhib./oral ENCORE-PH (severe portal HTN) ENCORE-LF (decompensated cirrhosis) ENCORE-NF (NASH fibrosis) Q H H BMS (BMS) PEG-FGF21/subcut 2 P2b multiple dose; compensated cirrhosis P2b multiple dose; stage 3 fibrosis JAN 2020 JAN 2020 * ATLAS study evaluates Selonsertib in combination with GS-0976 (ACC inhibitor) and GS-9674 (FXR agonist) Page 21
23 NASH Cirrhosis Clinical Trials Mapped to Patient Segment NASH Cirrhosis Trial Supportive pre-cirrhotic NASH Fibrosis Trial Stage 1 Stage 2 Stage 3 Stage 4/Cirrhosis CompensatedCirrhosis No Varices Varices Decompensated Cirrhosis Selonsertib Selonsertib with ACC inh & FXR ag P3: STELLAR 3 P2: ATLAS P3: STELLAR 4 Obeticholic acid P3: REGENERATE P3: REVERSE Emricasan P2: ENCORE-NF P2: ENCORE-PH P2: ENCORE-LF GR-MD-02 P3 ready: TBA BMS (FGF21) P2 P2 Page 22
24 GR-MD-02: Phase 2b NASH Cirrhosis Study Results (NASH-CX) Compensated Cirrhosis, No Varices (50% Total) 1 Compensated Cirrhosis (Total Patients 1 ) Disclosure: Presenter previously full time employee of GALT, buy currently owns no equity in company. Figures taken from publicly disclosed July 2018 corporate presentation Page 23
25 GR-MD-02: Phase 2b NASH Cirrhosis Study Results Compensated Cirrhosis, No Varices (50% Total) 1 NASH-CX Study Conclusions First clinical trial to show positive results in compensated cirrhosis without esophageal varices Clinically meaningful effect in reducing portal pressure in subgroup of patients Improvement in liver cell death Reduction in the development of new varices Drug was safe and well tolerated Following meeting with FDA in May 2018, determined to be Phase 3-ready Proceeding with plans for a phase 3 clinical trial program Disclosure: Presenter previously full time employee of GALT, but currently owns no equity in company. Figures and text taken from publicly disclosed July 2018 corporate presentation Page 24
26 Estimated Data Milestones for NASH Cirrhosis Trials* Cirrhosis trial Supportive pre-cirrhosis trial with same drug and/or combos Trial STELLAR-4 STELLAR-3 ATLAS REVERSE REGENERATE ENCORE-PH ENCORE-LF ENCORE-NF BMS BMS GR-MD-02 Trial Phase P3 P3 P2 P3 P3 P2 P2 P2 P2 P2 P3 ready [Final study design and start date not announced as of Sept. 6, 2018 presentation] * Based on clinicaltrial.gov postings plus company guidance when available; when a specific month was designated, the milestone is indicated over the ensuing one quarter Page 25
27 Summary Observations Relevant for NASH Cirrhosis Clinical Development The natural history in a clinical trial environment of NASH with advanced fibrosis and NASH cirrhosis the is emerging with evaluation of large clinical trials. Fibrosis may improve even in the absence of significant weight loss in the context of a clinical trial. Potential factors include dietary changes, reduced alcohol intake, or increased exercise. The incidence of cirrhosis complications is relatively low at ~20% over 2.5 years, with the most common being ascites and encephalopathy. Development of new varices and variceal hemorrhage had an incidence of only 2% and 3%, respectively. Therapies that target portal hypertension may have utility in reducing complications of cirrhosis Extensive data sets will be reported over next 18 months that will substantially clarify natural history and potential for therapeutic intervention in cirrhosis Non-invasive and functional testing that effectively predicts the development of cirrhosis and complications of cirrhosis are desperately needed in this field Page 26
28 Thank You! Peter G. Traber, MD Partner, Alacrita Consulting Alacrita Consulting Inc 303 Wyman St., Suite 325 Waltham, MA Alacrita Consulting Ltd London BioScience Innovation Centre 2 Royal College Street, London NW1 0NH Alacrita Consulting AG Artherstrasse Zug, Switzerland
29 ENCORE-PH: Emricasan in NASH Cirrhosis and Severe Portal Hypertension Phase 2 Study # patients Groups Inclusion/Exclusion Criteria Primary Endpoints 240 EMR 50 mg EMR 25 mg EMR 5 mg Placebo Inclusion Liver biopsy with NASH cirrhosis HVPG 12 mmhg Compensated or decompensated with 1 event Exclusion Severe decompensation Child-Pugh score 10 Mean change in HVPG [Week 24] In this patient population with HVPG 12 mmhg, changes in HVPG may be an acceptable surrogate endpoint Page 28
30 ENCORE-LF: Emricasan in Decompensated NASH Cirrhosis Phase 2 Study # patients Groups Inclusion/Exclusion Criteria Primary Endpoints 210 EMR 25 mg EMR 5 mg Placebo Inclusion Liver biopsy with NASH cirrhosis History of variceal hemorrhage or moderate ascites MELD 12 and 20 Albumin 12 g/dl Serum creatine 1.5 mg/dl Exclusion Severe decompensation Child-Pugh score 10 Event-free survival on composite clinical endpoint [final treatment; at least 48 weeks to a max of 120 weeks] Page 29
31 ENCORE-NF: Emricasan in NASH Fibrosis Phase 2 Study # patients Groups Inclusion/Exclusion Criteria Primary Endpoints 330 EMR 50 mg EMR 5 mg Placebo Inclusion Liver biopsy definitive NASH NAS 4 with 1 in each component Fibrosis stage 1, 2, or 3 Exclusion Severe decompensation Child-Pugh score 10 Proportion of patients with 1 stage improvement in fibrosis without worsening of NASH [week 72] Page 30
32 BMS (FGF-21) in Compensated NASH Cirrhosis Phase 2 Study # patients Groups Inclusion/Exclusion Criteria Primary Endpoints dose levels Placebo Inclusion Liver biopsy with NASH cirrhosis (Stage 4 by NASH-CRN class) Exclusion No history of decompensation No hepatocellular carcinoma Proportion of patients who achieve a 1 stage improvement in fibrosis without worsening of NASH [Week 48] Change in NASH-CRN fibrosis score [Week 48] Change in NAFLD Activity Score [Week 48] Page 31
33 BMS (FGF-21) in NASH with Bridging Fibrosis (stage 3) Phase 2 Study # patients Groups Inclusion/Exclusion Criteria Primary Endpoints dose levels Placebo Inclusion Liver biopsy with NASH with bridging fibrosis (Stage 3 by NASH CRN classification) NASH with a score of at least 1 for steatosis, lobular inflammation, and ballooning Exclusion No history of decompensation No hepatocellular carcinoma Proportion of patients who achieve a 1 stage improvement in fibrosis without worsening of NASH [week 24] Proportion of patients who achieve NASH improvement with no worsening of fibrosis [week 24] Change in NAFLD Activity Score [Week 24] Page 32
Clinical Trials & Endpoints in NASH Cirrhosis
 Clinical Trials & Endpoints in NASH Cirrhosis April 25, 2018 Peter G. Traber, MD CEO & CMO, Galectin Therapeutics 2018 Galectin Therapeutics NASDAQ: GALT For more information, see galectintherapeutics.com
Clinical Trials & Endpoints in NASH Cirrhosis April 25, 2018 Peter G. Traber, MD CEO & CMO, Galectin Therapeutics 2018 Galectin Therapeutics NASDAQ: GALT For more information, see galectintherapeutics.com
ENCORE-PH Top-line Results
 ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
GR-MD-02 for Indication of NASH Cirrhosis: NASH-CX Clinical Trial Results
 GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
Corporate Update. NASDAQ: GALT April 9, 2018
 Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
European. Young Hepatologists Workshop. Organized by : Quantification of fibrosis and cirrhosis outcomes
 supported by from Gilea Quantification of fibrosis and cirrhosis outcomes th 5 European 5 European Young Hepatologists Workshop Young Hepatologists Workshop August, 27-29. 2015, Moulin de Vernègues Vincenza
supported by from Gilea Quantification of fibrosis and cirrhosis outcomes th 5 European 5 European Young Hepatologists Workshop Young Hepatologists Workshop August, 27-29. 2015, Moulin de Vernègues Vincenza
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH
 Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
Forward-looking Statements
 NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
Emricasan (IDN-6556) administered orally for 28 days lowers portal pressure in patients with compensated cirrhosis and severe portal hypertension
 Emricasan (IDN-6556) administered orally for 28 days lowers portal pressure in patients with compensated cirrhosis and severe portal hypertension Guadalupe Garcia-Tsao, Michael Fuchs, Mitchell Shiffman,
Emricasan (IDN-6556) administered orally for 28 days lowers portal pressure in patients with compensated cirrhosis and severe portal hypertension Guadalupe Garcia-Tsao, Michael Fuchs, Mitchell Shiffman,
Life After SVR for Cirrhotic HCV
 Life After SVR for Cirrhotic HCV KIM NEWNHAM MN, NP CIRRHOSIS CARE CLINIC UNIVERSITY OF ALBERTA Objectives To review the benefits of HCV clearance in cirrhotic patients To review some of the emerging data
Life After SVR for Cirrhotic HCV KIM NEWNHAM MN, NP CIRRHOSIS CARE CLINIC UNIVERSITY OF ALBERTA Objectives To review the benefits of HCV clearance in cirrhotic patients To review some of the emerging data
Hepatology for the Nonhepatologist
 Hepatology for the Nonhepatologist Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Cincinnati, Ohio Learning
Hepatology for the Nonhepatologist Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Cincinnati, Ohio Learning
Non-alcoholic fatty liver disease: time to take note and manage. Philip Newsome Professor of Hepatology & Director of Centre for Liver Research
 Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
Cirrhosis and Portal Hypertension Gastroenterology Teaching Project American Gastroenterological Association
 CIRRHOSIS AND PORTAL HYPERTENSION Cirrhosis and Portal Hypertension Gastroenterology Teaching Project American Gastroenterological Association WHAT IS CIRRHOSIS? What is Cirrhosis? DEFINITION OF CIRRHOSIS
CIRRHOSIS AND PORTAL HYPERTENSION Cirrhosis and Portal Hypertension Gastroenterology Teaching Project American Gastroenterological Association WHAT IS CIRRHOSIS? What is Cirrhosis? DEFINITION OF CIRRHOSIS
Cirrhosis is different from Fibrosis
 Riunione Monotematica AISF 2016 «The Future of Liver Disease: Beyond HCV is there a Role for Hepatologist» Milan 13 th -14 th 2016 Cirrhosis is different from Fibrosis I have not disclosures to declare
Riunione Monotematica AISF 2016 «The Future of Liver Disease: Beyond HCV is there a Role for Hepatologist» Milan 13 th -14 th 2016 Cirrhosis is different from Fibrosis I have not disclosures to declare
GR-MD-02 for Indication of NASH Cirrhosis
 for Indication of NASH Cirrhosis NASH-C Clinical Trial Results San Francisco, CA January 8-, 208 NASDAQ: GALT www.galectintherapeutics.com Forward Looking Statements This presentation contains, in addition
for Indication of NASH Cirrhosis NASH-C Clinical Trial Results San Francisco, CA January 8-, 208 NASDAQ: GALT www.galectintherapeutics.com Forward Looking Statements This presentation contains, in addition
Forward-looking Statements
 Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding
Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding
An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications
 REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
Corporate Overview. September 6, Harold H. Shlevin, Ph.D. Chief Executive Officer. H.C. Wainwright Conference
 Corporate Overview September 6, 2018 Harold H. Shlevin, Ph.D. Chief Executive Officer H.C. Wainwright Conference NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains,
Corporate Overview September 6, 2018 Harold H. Shlevin, Ph.D. Chief Executive Officer H.C. Wainwright Conference NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains,
FDA Introductory Remarks Stephanie O. Omokaro, MD
 FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
NASH UPDATE ON DIAGNOSTICS AND THERAPY. Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine
 NASH UPDATE ON DIAGNOSTICS AND THERAPY Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine Conflicts of interest Salaried employee: of VCU Member of Board: McGuire VA Research Institute,
NASH UPDATE ON DIAGNOSTICS AND THERAPY Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine Conflicts of interest Salaried employee: of VCU Member of Board: McGuire VA Research Institute,
The EMA reflection paper on chronic liver disease and its implications for drug development in NASH
 The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
Fatty Liver Disease A growing epidemic
 Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
DISCLOSURES. This activity is jointly provided by Northwest Portland Area Indian Health Board and Cardea
 DISCLOSURES This activity is jointly provided by Northwest Portland Area Indian Health Board and Cardea Cardea Services is approved as a provider of continuing nursing education by Montana Nurses Association,
DISCLOSURES This activity is jointly provided by Northwest Portland Area Indian Health Board and Cardea Cardea Services is approved as a provider of continuing nursing education by Montana Nurses Association,
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st NAFLD/NASH : an expanding burden on liver health
 First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
KOL Symposium on Portal Hypertension
 KOL Symposium on Portal Hypertension Striving to improve human health September 27 I 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other
KOL Symposium on Portal Hypertension Striving to improve human health September 27 I 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other
HCV care after cure. This program is supported by educational grants from
 HCV care after cure This program is supported by educational grants from Raffaele Bruno,MD Department of Infectious Diseases, Hepatology Outpatients Unit University of Pavia Fondazione IRCCS Policlinico
HCV care after cure This program is supported by educational grants from Raffaele Bruno,MD Department of Infectious Diseases, Hepatology Outpatients Unit University of Pavia Fondazione IRCCS Policlinico
Rodman & Renshaw 17 th Annual Global Investment Conference
 Rodman & Renshaw 17 th Annual Global Investment Conference September 10, 2015 NASDAQ: GALT www.galectintherapeutics.com 2015 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains,
Rodman & Renshaw 17 th Annual Global Investment Conference September 10, 2015 NASDAQ: GALT www.galectintherapeutics.com 2015 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains,
Update on Nonalcoholic Fatty Liver Disease. Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic
 Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Forward-looking Statements
 NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
B C Outlines. Child-Pugh scores
 B C 2016-12-09 Outlines Child-Pugh scores CT MRI Fibroscan / ARFI Histologic Scoring Systems for Fibrosis Fibrosis METAVIR Ishak None 0 0 Portal fibrosis (some) 1 1 Portal fibrosis (most) 1 2 Bridging
B C 2016-12-09 Outlines Child-Pugh scores CT MRI Fibroscan / ARFI Histologic Scoring Systems for Fibrosis Fibrosis METAVIR Ishak None 0 0 Portal fibrosis (some) 1 1 Portal fibrosis (most) 1 2 Bridging
Corporate Presentation
 Corporate Presentation February 2, 2017 NASDAQ: GALT www.galectintherapeutics.com 2017 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Corporate Presentation February 2, 2017 NASDAQ: GALT www.galectintherapeutics.com 2017 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Diagnostic Procedures. Measurement of Hepatic venous pressure in management of cirrhosis. Clinician s opinion
 5 th AISF Post-Meeting Course Diagnostic and Therapeutic Invasive Procedures in Hepatology Rome, February 25 th Diagnostic Procedures Measurement of Hepatic venous pressure in management of cirrhosis Clinician
5 th AISF Post-Meeting Course Diagnostic and Therapeutic Invasive Procedures in Hepatology Rome, February 25 th Diagnostic Procedures Measurement of Hepatic venous pressure in management of cirrhosis Clinician
Corporate Presentation
 Corporate Presentation August 2016 NASDAQ: GALT www.galectintherapeutics.com 2016 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Corporate Presentation August 2016 NASDAQ: GALT www.galectintherapeutics.com 2016 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
New York State HCV Provider Webinar Series
 New York State HCV Provider Webinar Series Overview of Fibrosis-Staging, Child s Pugh, MELD Scores Paul J Gaglio, MD, FACP, AGAF, FAASLD Director: Hepatology Outreach Professor of Medicine (in Surgery)
New York State HCV Provider Webinar Series Overview of Fibrosis-Staging, Child s Pugh, MELD Scores Paul J Gaglio, MD, FACP, AGAF, FAASLD Director: Hepatology Outreach Professor of Medicine (in Surgery)
Hepatitis C: New Antivirals in the Liver Transplant Setting. Maria Carlota Londoño Liver Unit Hospital Clínic Barcelona
 Hepatitis C: New Antivirals in the Liver Transplant Setting Maria Carlota Londoño Liver Unit Hospital Clínic Barcelona Patient survival Hepatitis C and Liver Transplantation Years after transplantation
Hepatitis C: New Antivirals in the Liver Transplant Setting Maria Carlota Londoño Liver Unit Hospital Clínic Barcelona Patient survival Hepatitis C and Liver Transplantation Years after transplantation
Hepatitis Alert: Management of Patients With HCV Who Have Achieved SVR
 Hepatitis Alert: Management of Patients With HCV Who Have Achieved SVR This program is supported by educational grants from AbbVie, Gilead Sciences, and Merck About These Slides Please feel free to use,
Hepatitis Alert: Management of Patients With HCV Who Have Achieved SVR This program is supported by educational grants from AbbVie, Gilead Sciences, and Merck About These Slides Please feel free to use,
Liver Pathology and the Clinician in 2015: At the Crossroads. Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York
 Liver Pathology and the Clinician in 2015: At the Crossroads Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York DISCLOSURES Consultant for: Salix Merck Gilead BMS Synageva Research funding:
Liver Pathology and the Clinician in 2015: At the Crossroads Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York DISCLOSURES Consultant for: Salix Merck Gilead BMS Synageva Research funding:
Hepatitis C: How sick can we treat? Robert S. Brown, Jr., MD, MPH Vice Chair, Transitions of Care Interim Chief, Division of
 Hepatitis C: How sick can we treat? Robert S. Brown, Jr., MD, MPH Vice Chair, Transitions of Care Interim Chief, Division of Gastroenterology & Hepatology www.livermd.org HCV in advanced disease In principle
Hepatitis C: How sick can we treat? Robert S. Brown, Jr., MD, MPH Vice Chair, Transitions of Care Interim Chief, Division of Gastroenterology & Hepatology www.livermd.org HCV in advanced disease In principle
2 nd International Workshop on NASH Biomarkers, Washington DC, May 5-6, 2017
 Hepatic Proton Density Fat Fraction Correlates With Histologic Measures of Steatosis and Is Responsive to Changes in Those Measures in a Multi-center Nonalcoholic Steatohepatitis Clinical Trial Michael
Hepatic Proton Density Fat Fraction Correlates With Histologic Measures of Steatosis and Is Responsive to Changes in Those Measures in a Multi-center Nonalcoholic Steatohepatitis Clinical Trial Michael
Hepatology For The Nonhepatologist
 Hepatology For The Nonhepatologist Andrew Aronsohn, MD Associate Professor of Medicine University of Chicago Chicago, Illinois Learning Objectives After attending this presentation, learners will be able
Hepatology For The Nonhepatologist Andrew Aronsohn, MD Associate Professor of Medicine University of Chicago Chicago, Illinois Learning Objectives After attending this presentation, learners will be able
Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease
 Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease Scott Friedman, M.D. Fishberg Professor of Medicine Dean for Therapeutic Discovery Chief, Division of Liver Diseases Icahn School of
Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease Scott Friedman, M.D. Fishberg Professor of Medicine Dean for Therapeutic Discovery Chief, Division of Liver Diseases Icahn School of
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014
 Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Update on Non-Alcoholic Fatty Liver Disease. Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI
 Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
GR-MD-02: Phase III Galectin-3 Inhibitor
 GR-MD-02: Phase III Galectin-3 Inhibitor July 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking
GR-MD-02: Phase III Galectin-3 Inhibitor July 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking
Assessment of Liver Function: Implications for HCC Treatment
 Assessment of Liver Function: Implications for HCC Treatment A/P Dan Yock Young MBBS, PhD, MRCP, MMed. FAMS Chair, University Medicine Cluster. NUHS Head, Department of Medicine, National University of
Assessment of Liver Function: Implications for HCC Treatment A/P Dan Yock Young MBBS, PhD, MRCP, MMed. FAMS Chair, University Medicine Cluster. NUHS Head, Department of Medicine, National University of
Treating patients with end-stage liver disease: Are we ready? Dr. Mino R. Mitri, M.D., C.M., M.Ed., FRCPC
 Treating patients with end-stage liver disease: Are we ready? Dr. Mino R. Mitri, M.D., C.M., M.Ed., FRCPC mino.mitri@ubc.ca No Conflict of Interest 157 patients 157 patients 6 transplanted Criteria Liver
Treating patients with end-stage liver disease: Are we ready? Dr. Mino R. Mitri, M.D., C.M., M.Ed., FRCPC mino.mitri@ubc.ca No Conflict of Interest 157 patients 157 patients 6 transplanted Criteria Liver
Contraindications. Indications. Complications. Currently TIPS is considered second or third line therapy for:
 Contraindications Absolute Relative Primary prevention variceal bleeding HCC if centrally located Active congestive heart failure Obstruction all hepatic veins Thomas D. Boyer, M.D. University of Arizona
Contraindications Absolute Relative Primary prevention variceal bleeding HCC if centrally located Active congestive heart failure Obstruction all hepatic veins Thomas D. Boyer, M.D. University of Arizona
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical
 Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Study Design to Validate Biomarkers of Therapeutic Response in NASH Due to Cirrhosis
 Study Design to Validate Biomarkers of Therapeutic Response in NASH Due to Cirrhosis Detlef Schuppan Institute of Translational Immunology and Research Center for Immune Therapy, University Medical Center
Study Design to Validate Biomarkers of Therapeutic Response in NASH Due to Cirrhosis Detlef Schuppan Institute of Translational Immunology and Research Center for Immune Therapy, University Medical Center
Evercore ISI Presentation- Madrigal
 Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Intron A Hepatitis C. Intron A (interferon alfa-2b) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.01.05 Subject: Intron A Hepatitis C Page: 1 of 5 Last Review Date: November 30, 2018 Intron A Hepatitis
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.01.05 Subject: Intron A Hepatitis C Page: 1 of 5 Last Review Date: November 30, 2018 Intron A Hepatitis
Management of HepatoCellular Carcinoma
 9th Symposium GIC St Louis - 2010 Management of HepatoCellular Carcinoma Overview Pierre A. Clavien, MD, PhD Department of Surgery University Hospital Zurich Zurich, Switzerland Hepatocellular carcinoma
9th Symposium GIC St Louis - 2010 Management of HepatoCellular Carcinoma Overview Pierre A. Clavien, MD, PhD Department of Surgery University Hospital Zurich Zurich, Switzerland Hepatocellular carcinoma
CORPORATE PRESENTATION
 CORPORATE PRESENTATION NASDAQ: GALT www.galectintherapeutics.com 1 Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within the meaning
CORPORATE PRESENTATION NASDAQ: GALT www.galectintherapeutics.com 1 Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within the meaning
Overview of the Clinical Trial Data on Non-alcoholic Steatohepatitis (NASH)
 Overview of the Clinical Trial Data on Non-alcoholic Steatohepatitis (NASH) Brent A. Neuschwander-Tetri, MD, FACP, FACG, AGAF, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology
Overview of the Clinical Trial Data on Non-alcoholic Steatohepatitis (NASH) Brent A. Neuschwander-Tetri, MD, FACP, FACG, AGAF, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology
Module 1 Introduction of hepatitis
 Module 1 Introduction of hepatitis 1 Training Objectives At the end of the module, trainees will be able to ; Demonstrate improved knowledge of the global epidemiology of the viral hepatitis Understand
Module 1 Introduction of hepatitis 1 Training Objectives At the end of the module, trainees will be able to ; Demonstrate improved knowledge of the global epidemiology of the viral hepatitis Understand
Beta-blockers in cirrhosis: Cons
 Beta-blockers in cirrhosis: Cons Eric Trépo MD, PhD Dept. of Gastroenterology. Hepatopancreatology and Digestive Oncology. C.U.B. Hôpital Erasme. Université Libre de Bruxelles. Bruxelles. Belgium Laboratory
Beta-blockers in cirrhosis: Cons Eric Trépo MD, PhD Dept. of Gastroenterology. Hepatopancreatology and Digestive Oncology. C.U.B. Hôpital Erasme. Université Libre de Bruxelles. Bruxelles. Belgium Laboratory
NON-ALCOHOLIC FATTY LIVER DISEASE:
 NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
Massimo Puoti SC Malattie Infettive ASST GRANDE OSPEDALE METROPOLITANO NIGUARDA MILANO. Beyond HCV cure: reversibility of liver damage
 Massimo Puoti SC Malattie Infettive ASST GRANDE OSPEDALE METROPOLITANO NIGUARDA MILANO Beyond HCV cure: reversibility of liver damage Beyond HCV cure: reversibility of liver damage Progression of Liver
Massimo Puoti SC Malattie Infettive ASST GRANDE OSPEDALE METROPOLITANO NIGUARDA MILANO Beyond HCV cure: reversibility of liver damage Beyond HCV cure: reversibility of liver damage Progression of Liver
Antiviral treatment in HCV cirrhotic patients on waiting list
 Antiviral treatment in HCV cirrhotic patients on waiting list Krzysztof Tomasiewicz Department of Hepatology and Infectious Diseases Medical University of Lublin, Poland Disclosures Consultancy/Advisory
Antiviral treatment in HCV cirrhotic patients on waiting list Krzysztof Tomasiewicz Department of Hepatology and Infectious Diseases Medical University of Lublin, Poland Disclosures Consultancy/Advisory
EASL International Liver Congress Paris, France 14 April 2018
 NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
Faculty Disclosure. Objectives. Cirrhosis Management for the Family Physician 18/11/2014
 Cirrhosis Management for the Family Physician Mang Ma, MD, FRCP Professor University of Alberta Faculty: Mang Ma Faculty Disclosure Relationships with commercial interests: Advisory Board: Merck, Gilead
Cirrhosis Management for the Family Physician Mang Ma, MD, FRCP Professor University of Alberta Faculty: Mang Ma Faculty Disclosure Relationships with commercial interests: Advisory Board: Merck, Gilead
NAFLD: US GUIDELINES. US Guidelines for NAFLD
 NAFLD: US GUIDELINES Arun J Sanyal M.D. Charles Caravati Professor of Medicine Virginia Commonwealth University School of Medicine US Guidelines for NAFLD Represents consensus amongst AGA, AASLD and ACG
NAFLD: US GUIDELINES Arun J Sanyal M.D. Charles Caravati Professor of Medicine Virginia Commonwealth University School of Medicine US Guidelines for NAFLD Represents consensus amongst AGA, AASLD and ACG
Evidence-Base Management of Esophageal and Gastric Varices
 Evidence-Base Management of Esophageal and Gastric Varices Rino Alvani Gani Hepatobiliary Division Department of Internal Medicine Faculty of Medicine Universitas Indonesia Cipto Mangunkusumo National
Evidence-Base Management of Esophageal and Gastric Varices Rino Alvani Gani Hepatobiliary Division Department of Internal Medicine Faculty of Medicine Universitas Indonesia Cipto Mangunkusumo National
Supplemental Tables. Parasitic Schistosomiasis increase < 1. Genetic Hemochromatosis increase < 1. autoimmune Autoimmune hepatitis (AIH) increase < 1
 Supplemental Tables Supplemental Table 1 Various etiologies of liver cirrhosis and their association with liver stiffness and AST/ALT ratio Disease category Cause Example LS AST/ALT Inflammatory liver
Supplemental Tables Supplemental Table 1 Various etiologies of liver cirrhosis and their association with liver stiffness and AST/ALT ratio Disease category Cause Example LS AST/ALT Inflammatory liver
Management of Cirrhosis Related Complications
 Management of Cirrhosis Related Complications Ke-Qin Hu, MD, FAASLD Professor of Clinical Medicine Director of Hepatology University of California, Irvine Disclosure I have no disclosure related to this
Management of Cirrhosis Related Complications Ke-Qin Hu, MD, FAASLD Professor of Clinical Medicine Director of Hepatology University of California, Irvine Disclosure I have no disclosure related to this
Principals of Kinetic Measures of Liver Function
 Principals of Kinetic Measures of Liver Function Focus on the Dual Cholate Clearance Test (HepQuant SHUNT)* Gregory T. Everson, MD Professor of Medicine Director of Hepatology University of Colorado Denver
Principals of Kinetic Measures of Liver Function Focus on the Dual Cholate Clearance Test (HepQuant SHUNT)* Gregory T. Everson, MD Professor of Medicine Director of Hepatology University of Colorado Denver
Hepatocellular Carcinoma. Markus Heim Basel
 Hepatocellular Carcinoma Markus Heim Basel Outline 1. Epidemiology 2. Surveillance 3. (Diagnosis) 4. Staging 5. Treatment Epidemiology of HCC Worldwide, liver cancer is the sixth most common cancer (749
Hepatocellular Carcinoma Markus Heim Basel Outline 1. Epidemiology 2. Surveillance 3. (Diagnosis) 4. Staging 5. Treatment Epidemiology of HCC Worldwide, liver cancer is the sixth most common cancer (749
Initial approach to ascites
 Ascites: Filling and Draining the Water Balloon Common Pathogenesis in Refractory Ascites, Hyponatremia, and Cirrhosis intrahepatic resistance sinusoidal portal hypertension Splanchnic vasodilation (effective
Ascites: Filling and Draining the Water Balloon Common Pathogenesis in Refractory Ascites, Hyponatremia, and Cirrhosis intrahepatic resistance sinusoidal portal hypertension Splanchnic vasodilation (effective
MANAGEMENT OF LIVER CIRRHOSIS: PRACTICE ESSENTIALS AND PATIENT SELF-MANAGEMENT
 MANAGEMENT OF LIVER CIRRHOSIS: PRACTICE ESSENTIALS AND PATIENT SELF-MANAGEMENT Sherona Bau, ACNP The Pfleger Liver Institute 200 UCLA Medical Plaza, Suite 214 Los Angeles, CA 90095 September 30, 2017 I
MANAGEMENT OF LIVER CIRRHOSIS: PRACTICE ESSENTIALS AND PATIENT SELF-MANAGEMENT Sherona Bau, ACNP The Pfleger Liver Institute 200 UCLA Medical Plaza, Suite 214 Los Angeles, CA 90095 September 30, 2017 I
Learning Objectives. After attending this presentation, participants will be able to:
 Learning Objectives After attending this presentation, participants will be able to: Describe HCV in 2015 Describe how to diagnose advanced liver disease and cirrhosis Identify the clinical presentation
Learning Objectives After attending this presentation, participants will be able to: Describe HCV in 2015 Describe how to diagnose advanced liver disease and cirrhosis Identify the clinical presentation
Ascites Management. Atif Zaman, MD MPH Oregon Health & Science University Professor of Medicine Division of Gastroenterology and Hepatology
 Ascites Management Atif Zaman, MD MPH Oregon Health & Science University Professor of Medicine Division of Gastroenterology and Hepatology Disclosure 1. The speaker Atif Zaman, MD MPH have no relevant
Ascites Management Atif Zaman, MD MPH Oregon Health & Science University Professor of Medicine Division of Gastroenterology and Hepatology Disclosure 1. The speaker Atif Zaman, MD MPH have no relevant
Screening for Portal Hypertension in Cirrhosis
 Screening for Portal Hypertension in Cirrhosis MASSIMO PINZANI, MD, PhD, FRCP Sheila Sherlock Chair of Hepatology UCL Institute for Liver and Digestive Health Royal Free Hospital, London, UK m.pinzani@ucl.ac.uk
Screening for Portal Hypertension in Cirrhosis MASSIMO PINZANI, MD, PhD, FRCP Sheila Sherlock Chair of Hepatology UCL Institute for Liver and Digestive Health Royal Free Hospital, London, UK m.pinzani@ucl.ac.uk
DIFFERENTIAL BENEFITS OF DAAs IN DIFFERENT PATIENT POPULATIONS. IN PATIENTS ON A WAITING LIST FOR TRANSPLANTATION. THE CLINIC.
 DIFFERENTIAL BENEFITS OF DAAs IN DIFFERENT PATIENT POPULATIONS. IN PATIENTS ON A WAITING LIST FOR TRANSPLANTATION. THE CLINIC. Robert J. de Knegt, Erasmus MC, Rotterdam r.deknegt@erasmusmc.nl Disclosures
DIFFERENTIAL BENEFITS OF DAAs IN DIFFERENT PATIENT POPULATIONS. IN PATIENTS ON A WAITING LIST FOR TRANSPLANTATION. THE CLINIC. Robert J. de Knegt, Erasmus MC, Rotterdam r.deknegt@erasmusmc.nl Disclosures
NAFLD: evidence-based management. Curso de residentes AEEH Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain
 NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
Oral Testosterone (T) Non Alcoholic Steatohepatitis (NASH)
 Oral Testosterone (T) Non Alcoholic Steatohepatitis (NASH) 1 LPCN 1144: Well Positioned for Success Unique Mechanism of Action with Compelling Clinical Signal Targeting Full Spectrum of NASH Pathogenesis
Oral Testosterone (T) Non Alcoholic Steatohepatitis (NASH) 1 LPCN 1144: Well Positioned for Success Unique Mechanism of Action with Compelling Clinical Signal Targeting Full Spectrum of NASH Pathogenesis
«STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin
 «STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin OUTLINE NAFLD overview NAFLD and menarche NAFLD and pregnancy NAFLD and menopause Other metabolic
«STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin OUTLINE NAFLD overview NAFLD and menarche NAFLD and pregnancy NAFLD and menopause Other metabolic
Jong Young Choi, M.D.
 The Liver Week 2014 Jong Young Choi, M.D. Dept. of Internal Medicine The Catholic University of Korea, College of Medicine The clinical study for natural history of LC is not many. Most of them was done
The Liver Week 2014 Jong Young Choi, M.D. Dept. of Internal Medicine The Catholic University of Korea, College of Medicine The clinical study for natural history of LC is not many. Most of them was done
CIRROSI E IPERTENSIONE PORTALE NELLA DONNA
 Cagliari, 16 settembre 2017 CIRROSI E IPERTENSIONE PORTALE NELLA DONNA Vincenza Calvaruso, MD, PhD Ricercatore di Gastroenterologia Gastroenterologia & Epatologia, Di.Bi.M.I.S. Università degli Studi di
Cagliari, 16 settembre 2017 CIRROSI E IPERTENSIONE PORTALE NELLA DONNA Vincenza Calvaruso, MD, PhD Ricercatore di Gastroenterologia Gastroenterologia & Epatologia, Di.Bi.M.I.S. Università degli Studi di
Liver biopsy as the gold standard for diagnosis. Pierre BEDOSSA
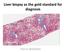 Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver failure &portal hypertension
 Liver failure &portal hypertension Objectives: by the end of this lecture each student should be able to : Diagnose liver failure (acute or chronic) List the causes of acute liver failure Diagnose and
Liver failure &portal hypertension Objectives: by the end of this lecture each student should be able to : Diagnose liver failure (acute or chronic) List the causes of acute liver failure Diagnose and
Variceal bleeding. Mainz,
 Variceal bleeding Mainz, 21.09.2008 Risk of complications 5 years 10 years Ascites 10 % 25 % HCC 10 % 25 % Bleeding < 5 % 5-10 % Enceph. < 5 % < 5 % Typical situation : Mortality 10 % to 40 % Sequence
Variceal bleeding Mainz, 21.09.2008 Risk of complications 5 years 10 years Ascites 10 % 25 % HCC 10 % 25 % Bleeding < 5 % 5-10 % Enceph. < 5 % < 5 % Typical situation : Mortality 10 % to 40 % Sequence
Controversies in Management of Portal Hypertension and Cirrhosis Complications in the Transplant Candidate
 Controversies in Management of Portal Hypertension and Cirrhosis Complications in the Transplant Candidate Patrick Northup, MD, FAASLD, FACG Medical Director, Liver Transplantation University of Virginia
Controversies in Management of Portal Hypertension and Cirrhosis Complications in the Transplant Candidate Patrick Northup, MD, FAASLD, FACG Medical Director, Liver Transplantation University of Virginia
Management of Chronic Liver Failure/Cirrhosis Complications in Hospitals. By: Dr. Kevin Dolehide
 Management of Chronic Liver Failure/Cirrhosis Complications in Hospitals By: Dr. Kevin Dolehide Overview DX Cirrhosis and Prognosis Compensated Decompensated Complications Of Cirrhosis Management Of Complications
Management of Chronic Liver Failure/Cirrhosis Complications in Hospitals By: Dr. Kevin Dolehide Overview DX Cirrhosis and Prognosis Compensated Decompensated Complications Of Cirrhosis Management Of Complications
Advances in percutaneous ablation and systemic therapies for hepatocellular carcinoma
 Advances in percutaneous ablation and systemic therapies for hepatocellular carcinoma Paris Hepatology Congress 2019 Pierre Nahon Service d Hépatologie Hôpital Jean Verdier Bondy Université Paris 13 INSERM
Advances in percutaneous ablation and systemic therapies for hepatocellular carcinoma Paris Hepatology Congress 2019 Pierre Nahon Service d Hépatologie Hôpital Jean Verdier Bondy Université Paris 13 INSERM
Histological subclassification of cirrhosis based on histological haemodynamic correlation
 Alimentary Pharmacology & Therapeutics Histological subclassification of cirrhosis based on histological haemodynamic correlation M. KUMAR*, P. SAKHUJA, A.KUMAR*,N.MANGLIK*,A.CHOUDHURY*,S.HISSAR*,A.RASTOGI
Alimentary Pharmacology & Therapeutics Histological subclassification of cirrhosis based on histological haemodynamic correlation M. KUMAR*, P. SAKHUJA, A.KUMAR*,N.MANGLIK*,A.CHOUDHURY*,S.HISSAR*,A.RASTOGI
The Impact of HBV Therapy on Fibrosis and Cirrhosis
 The Impact of HBV Therapy on Fibrosis and Cirrhosis Jordan J. Feld, MD, MPH Associate Professor of Medicine University of Toronto Hepatologist Toronto Centre for Liver Disease Sandra Rotman Centre for
The Impact of HBV Therapy on Fibrosis and Cirrhosis Jordan J. Feld, MD, MPH Associate Professor of Medicine University of Toronto Hepatologist Toronto Centre for Liver Disease Sandra Rotman Centre for
Regulatory update from Europe:
 Regulatory update from Europe: Interim endpoints in phase 3 NASH trials Elmer Schabel MD The previously proposed/presented co-primary evaluation of two composite endpoints for the interim has been accepted:
Regulatory update from Europe: Interim endpoints in phase 3 NASH trials Elmer Schabel MD The previously proposed/presented co-primary evaluation of two composite endpoints for the interim has been accepted:
Hepatitis C: Difficult-to-treat Patients 11th Paris Hepatology Conference 16th January 2018 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany
 Hepatitis C: Difficult-to-treat Patients 11th Paris Hepatology Conference 16th January 2018 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany PHC 2018 - www.aphc.info Disclosures Advisory boards:
Hepatitis C: Difficult-to-treat Patients 11th Paris Hepatology Conference 16th January 2018 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany PHC 2018 - www.aphc.info Disclosures Advisory boards:
Intron A (interferon alfa-2b) with ribavirin, (Moderiba, Rebetol, Ribasphere, RibaTab, ribavirin tablets/capsules - all strengths)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.01.06 Subject: Intron A Ribavirin Page: 1 of 6 Last Review Date: March 18, 2016 Intron A Ribavirin Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.01.06 Subject: Intron A Ribavirin Page: 1 of 6 Last Review Date: March 18, 2016 Intron A Ribavirin Description
Carvedilol or Propranolol in the Management of Portal Hypertension?
 Evidence Based Case Report Carvedilol or Propranolol in the Management of Portal Hypertension? Arranged by: dr. Saskia Aziza Nursyirwan RESIDENCY PROGRAM OF INTERNAL MEDICINE DEPARTMENT UNIVERSITY OF INDONESIA
Evidence Based Case Report Carvedilol or Propranolol in the Management of Portal Hypertension? Arranged by: dr. Saskia Aziza Nursyirwan RESIDENCY PROGRAM OF INTERNAL MEDICINE DEPARTMENT UNIVERSITY OF INDONESIA
The Yellow Patient. Dr Chiradeep Raychaudhuri, Consultant Hepatologist, Hull University Teaching Hospitals NHS Trust
 The Yellow Patient Dr Chiradeep Raychaudhuri, Consultant Hepatologist, Hull University Teaching Hospitals NHS Trust there s a yellow patient in bed 40. It s one of yours. Liver Cirrhosis Why.When.What.etc.
The Yellow Patient Dr Chiradeep Raychaudhuri, Consultant Hepatologist, Hull University Teaching Hospitals NHS Trust there s a yellow patient in bed 40. It s one of yours. Liver Cirrhosis Why.When.What.etc.
Hepatocytes produce. Proteins Clotting factors Hormones. Bile Flow
 R.J.Bailey MD Hepatocytes produce Proteins Clotting factors Hormones Bile Flow Trouble.. for the liver! Trouble for the Liver Liver Gall Bladder Common Alcohol Hep C Fatty Liver Cancer Drugs Viruses Uncommon
R.J.Bailey MD Hepatocytes produce Proteins Clotting factors Hormones Bile Flow Trouble.. for the liver! Trouble for the Liver Liver Gall Bladder Common Alcohol Hep C Fatty Liver Cancer Drugs Viruses Uncommon
CHAPTER 1. Alcoholic Liver Disease
 CHAPTER 1 Alcoholic Liver Disease Major Lesions of Alcoholic Liver Disease Alcoholic fatty liver - >90% of binge and chronic drinkers Alcoholic hepatitis precursor of cirrhosis Alcoholic cirrhosis end
CHAPTER 1 Alcoholic Liver Disease Major Lesions of Alcoholic Liver Disease Alcoholic fatty liver - >90% of binge and chronic drinkers Alcoholic hepatitis precursor of cirrhosis Alcoholic cirrhosis end
Management of Cirrhotic Complications Uncontrolled Ascites. Siwaporn Chainuvati, MD Siriraj Hospital Mahidol University
 Management of Cirrhotic Complications Uncontrolled Ascites Siwaporn Chainuvati, MD Siriraj Hospital Mahidol University Topic Definition, pathogenesis Current therapeutic options Experimental treatments
Management of Cirrhotic Complications Uncontrolled Ascites Siwaporn Chainuvati, MD Siriraj Hospital Mahidol University Topic Definition, pathogenesis Current therapeutic options Experimental treatments
Approved regimens for cirrhotic patients
 5th Workshop on HCV THERAPY ADVANCES New antivirals in clinical practice Approved regimens for cirrhotic patients Amsterdam, 4-5 december 2015 Disease burden in Spain 400000 350000 300000 F0 Peak cirrhosis
5th Workshop on HCV THERAPY ADVANCES New antivirals in clinical practice Approved regimens for cirrhotic patients Amsterdam, 4-5 december 2015 Disease burden in Spain 400000 350000 300000 F0 Peak cirrhosis
The impact of the treatment of HCV in developing Hepatocellular Carcinoma
 The impact of the treatment of HCV in developing Hepatocellular Carcinoma Paul Y Kwo, MD Professor of Medicine Medical Director, Liver Transplantation Gastroenterology/Hepatology Division Indiana University
The impact of the treatment of HCV in developing Hepatocellular Carcinoma Paul Y Kwo, MD Professor of Medicine Medical Director, Liver Transplantation Gastroenterology/Hepatology Division Indiana University
Defining the gold standard in biomarker validation for NASH
 Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Improving the Lives of Patients with Liver Diseases
 Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Paul Martin MD FACG. University of Miami
 Paul Martin MD FACG University of Miami 1 Liver cirrhosis of any cause Chronic C o c hepatitis epat t s B Risk increases with Male gender Age Diabetes Smoking ~5% increase in HCV-related HCC between 1991-28
Paul Martin MD FACG University of Miami 1 Liver cirrhosis of any cause Chronic C o c hepatitis epat t s B Risk increases with Male gender Age Diabetes Smoking ~5% increase in HCV-related HCC between 1991-28
Non-Alcoholic Fatty Liver Disease
 Non-Alcoholic Fatty Liver Disease Varun Saxena, MD MAS Gastroenterology and Hepatology Kaiser Permanente South San Francisco Assistant Clinical Professor University of California San Francisco January
Non-Alcoholic Fatty Liver Disease Varun Saxena, MD MAS Gastroenterology and Hepatology Kaiser Permanente South San Francisco Assistant Clinical Professor University of California San Francisco January
