INVESTOR PRESENTATION
|
|
|
- Dwayne Morrison
- 5 years ago
- Views:
Transcription
1 INVESTOR PRESENTATION APRIL
2 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. CERTAIN OF THE INFORMATION CONTAINED HEREIN CONCERNING ECONOMIC TRENDS AND PERFORMANCE IS BASED UPON OR DERIVED FROM INFORMATION PROVIDED BY THIRD-PARTY CONSULTANTS AND OTHER INDUSTRY SOURCES. WHILE GENFIT BELIEVES THAT SUCH INFORMATION IS ACCURATE AND THAT THE SOURCES FROM WHICH IT HAS BEEN OBTAINED ARE RELIABLE, GENFIT HAS NOT INDEPENDENTLY VERIFIED THE ASSUMPTIONS ON WHICH PROJECTIONS OF FUTURE TRENDS AND PERFORMANCE ARE BASED. IT MAKES NO GUARANTEE, EXPRESS OR IMPLIED, AS TO THE ACCURACY AND COMPLETENESS OF SUCH INFORMATION. THIS PRESENTATION CONTAINS CERTAIN FORWARD-LOOKING STATEMENTS. ALTHOUGH GENFIT BELIEVES ITS EXPECTATIONS REFLECTED IN SUCH FORWARD-LOOKING STATEMENTS ARE BASED ON REASONABLE ASSUMPTIONS, THESE FORWARD-LOOKING STATEMENTS ARE SUBJECT TO NUMEROUS RISKS AND UNCERTAINTIES, WHICH COULD CAUSE ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE EXPRESSED IN, OR IMPLIED OR PROJECTED BY, THE FORWARD-LOOKING INFORMATION AND STATEMENTS. THESE RISKS AND UNCERTAINTIES INCLUDE AMONG OTHER THINGS, THE UNCERTAINTIES INHERENT IN RESEARCH AND DEVELOPMENT, IN PARTICULAR IN ELAFIBRANOR, PBC AND RELATED TO BIOMARKERS, PROGRESSION OF, RESULTS OF CLINICAL DATA FROM, THE RESOLVE-IT TRIALS, REVIEW AND APPROVALS BY REGULATORY AUTHORITIES, SUCH AS THE FDA OR THE EMA, INCLUDING REGARDING ELAFIBRANOR, PBC AND BIOMARKERS, THE SUCCESS OF ANY IN-LICENSING STRATEGIES, AND GENERALLY GENFIT S CONTINUED ABILITY TO RAISE CAPITAL TO FUND ITS DEVELOPMENT, AS WELL AS THOSE DISCUSSED OR IDENTIFIED IN THE PUBLIC FILINGS WITH THE AMF MADE BY GENFIT, INCLUDING THOSE LISTED UNDER SECTION 7 MAIN RISKS AND UNCERTAINTIES OF THE COMPANY S HALF YEAR 2016 BUSINESS AND FINANCIAL REPORT WHICH IS AVAILABLE ON GENFIT S WEBSITE ( ) AND ON THE WEBSITE OF THE AUTORITE DES MARCHES FINANCIERS ( THE INFORMATION, OPINIONS AND FORWARD LOOKING INFORMATION CONTAINED IN THIS PRESENTATION SPEAK ONLY AS OF THE DATE OF THIS DOCUMENT, UNLESS OTHERWISE INDICATED HEREIN. OTHER THAN AS REQUIRED BY APPLICABLE LAW, GENFIT IS NOT UNDER ANY, AND UNDERTAKES NO OBLIGATION TO, UPDATE OR KEEP CURRENT THE INFORMATION CONTAINED IN THIS PRESENTATION AND ANY OPINIONS EXPRESSED THEREIN ARE SUBJECT TO CHANGE WITHOUT NOTICE. NOTHING IN THE PRESENTATION CONSTITUTES AN OFFER OF SECURITIES FOR SALE IN THE UNITED STATES OF AMERICA, CANADA, JAPAN, THE UNITED KINGDOM, AUSTRALIA OR ANY OTHER JURISDICTION WHERE IT IS UNLAWFUL TO DO SO. 2
3 GENFIT: Introduction Public company focused on metabolic diseases & associated complications, including liver related disorders World-leading expert in nuclear receptor based drug discovery Founded in 1999 (Lille, Paris, France Cambridge, US) 130 employees Since 2006, Euronext Paris compartment B (GNFT) Market capitalization of ~ 1bn Lead program in NASH: Nonalcoholic Steatohepatitis Actively enrolling Phase 3 with elafibranor: First-in-class molecule, fast-track designation, accelerated approval Subpart H (FDA) and Conditional approval (EMA) Biomarker program in NASH/NAFLD* Rich pipeline addressing other key unmet needs: PBC, fibrosis, auto-immune diseases *: Nonalcoholic Fatty Liver Disease 3
4 GENFIT Pipeline: Targeting Unmet Needs in Liver and Autoimmune Diseases NASH Programs PHASE 2 Programs OTHER RESEARCH Programs PROGRAM OBJECTIVE DETAILS PROGRAM MATURITY TIMELINE (E) ELAFIBRANOR in NASH 1 st LINE TREATMENT PEDIATRIC, ANCILLARY, etc. COMBO THERAPY BIOMARKERS DIAGNOSTIC mirnas ELAFIBRANOR in PBC FIBROSIS, CIRRHOSIS FIBROSIS, CIRRHOSIS AUTO-IMMUNE DISEASES TREATMENT REPURPOSING MARKETED DRUG HIT-to-LEAD (TGFTX4) (TGFTX1) PPAR α/δ agonist (First-in-class) PPAR α/δ agonist (First-in-class) PPAR α/δ agonist and other MoAs PPARα/δ agonist Nitazoxanide Undisclosed RORγT Inverse agonist PHASE 3 PHASE 2 PHASE 2 PHASE 2 End of enrollment Q PK/PD H Undisclosed Start development H st patient Q IND filing Phase 2a H Preclinical regulatory Q Pre-IND studies On-going (psoriasis) 4
5 NASH: Underlying Cause of Progressive Fibrosis that Results from Necroinflammation, Leads to Cirrhosis and HCC Leading cause of liver disease in developed countries; ~20mn in US suffer from NASH & advanced fibrosis Cardiovascular events are the leading cause of death in NASH Multifaceted disease Matteoni, Gastro 1999 Adams, Gastro 2005 Ekstedt, Hepatol 2006 Ong, J Hepatol 2008 Dunn, AJG 2008 Sorderberg, Hepatol 2010 Targher, NEJM 2010 Williams, Gastro 2011 Chalasani, Gastro 2012 Torres, Clin Gastro Hepatol 2012 Wree, Nat. Rev Gastroenterol Hepatol 2013 Rinella, JAMA 2015 Bazick, Diabetes Care
6 NASH and Hepatocyte Ballooning are Relevant Targets to Prevent Long Term Outcomes* NASH Ballooning n= 619 n= 619 NASH and hepatocyte ballooning identified as histological features correlated with clinical outcomes and mortality Strongly supports clinical development and use of elafibranor *: Angulo et al., Gastroenterology 2015 Aug; 149 (2): e10. doi: /j.gastro Epub 2015 Apr 29. 6
7 NASH and Fibrosis: Two Simple Analogies to Understand the Disease Paradigm Underlying Cause = Primary Target For Pharmaceutical Drugs Measure of Progression Clinical Outcome HIV HCV Abacavir, dolutegravir, etravirine, rilpivirine, etc. Sofosbuvir, simeprevir, dasabuvir, etc. FIBROSIS CIRRHOSIS NASH Elafibranor, first drug that targets NASH resolution CIRRHOSIS F4 Arun Paris NASH Symposium
8 The Rising Prevalence of NASH is a Major Health Concern Waitlist Registrations for Liver Transplantation New HCC Cases US Wong, 2015 Reported HCC cases were derived from reported liver cancer cases SEER database 8
9 PROFILE OF ELAFIBRANOR & CLINICAL RESULTS 9
10 Elafibranor, First-in-class, has Pluripotent Activities: PPARa and d Regulate Multiple Pathways Essential in NASH PPARa and d Regulate Multiple Pathways Essential in NASH i Fibrogenesis (TGFβ1, αsma, Col1α1) i Oxidative stress (CAT, SOD) i Inflammation(MCP-1, IL-6, TNFα) i steatosis (h lipid utilization) i Oxidative stress (CAT, SOD) i ALT, GGT, ALP h Hepatic hemodynamics LIVER DYSFUNCTION FIBROSIS CVD RISK i Atherogenic lipid profile i Endothelial dysf. (ET-1, RGS5, Nox) i Vessel Ox stress (CAT, GPx1, HO1) i Vessel inflam (ICAM1, MCP1) h Triglyceride clearance (APOC3) i VLDL-APOB & ildl-apob i sd-ldl cholesterol level h HDL cholesterol level (APOA1/A2) h NEFA utilization (ACOX, CPT1, EHHADH) i NEFA level (lipolysis, β-oxidation) LIPID METABOLISM PPARα/δ GLUCOSE HOMEOSTASIS INFLAMMATION i NF-κB, i TLRs i TNFα, IL-1β i IL-6, CRP, SAA, HG, fibrinogen i Kupffer cell activation (BCL6) h Insulin sensitivity (Fgf21) i Hepatic glucose output (PEPCK, FAS, ACC, PDG, G6PDH) i insulin 10
11 Rationale for Treating NASH With Elafibranor, a Dual PPARα/δ Agonist With Multiple Activities Cariou et al. 2011, Diabetes Care, 34(9): Cariou et al. 2013, Diabetes Care, 34(9): Staels et al. 2013, Hepatology, 58(6):
12 Elafibranor in NASH: GOLDEN-505 Phase 2 Results¹ GOLDEN-505 Phase 2 Results* Elafibranor is safe, well tolerated and significantly improves cardiometabolic risk profile Elafibranor meets the re-defined Resolution of NASH without worsening of fibrosis endpoint, recommended as primary endpoint for pivotal trials in NASH: Ballooning = 0; Inflammation = 0 (or 1); No worsening of fibrosis (1 stage) Results on histology after 12 months Elafibranor 120mg vs placebo on NASH resolution Elafibranor 120mg Responders vs Non responders on Fibrosis (ITT) ¹: Ratziu et al., Gastroenterology 2016; 150: ; Elafibranor 120mg, n= 94; Elafibranor 80mg, n=93; placebo, n= 92; NASH Resolution at 12 months. 12
13 Mean change versus baseline GOLDEN-505 Phase 2 Results* Elafibranor Responders have Significant Histological Responses 1 Elafibranor 120mg Responders vs Elafibranor 120mg Non-Responders 0,5 0-0,5-1 -1,5 # # ### -2-2,5-3 -3,5 ### ### #: p<0.05 ###: p<0.001 Patients who resolved their NASH* showed significant reduction in liver fibrosis while non-responders did not show any change from baseline NAS Steatosis Ballooning Inflammation Fibrosis NASH components Elafibranor 120 mg Responders Elafibranor 120 mg Non responders *: Ratziu et al., Gastroenterology 2016; 150: Analysis with the re-defined and currently recommended NASH resolution endpoint for pivotal trials in NASH 13
14 % patients Changes in Hepatocyte Ballooning and Lobular Inflammation Drive Fibrosis Response % patients % patients Ballooning Inflammation Steatosis Fibrosis Improvers Fibrosis Worseners Fibrosis Improvers Fibrosis Worseners Fibrosis Improvers Fibrosis Worseners N=7 N=78 N=129 N= N=9 N=72 N=138 N=18 p= p< Change in ballooning score Change in lobular inflammation score N=4 N=49 N=128 N=56 NS Change in steatosis score In GOLDEN-505 after only 1 year: Changes in hepatocyte ballooning and lobular inflammation are associated with fibrosis response (% of patient who improved or worsened their fibrosis score) No association between change in steatosis score and fibrosis response P-value derived from Khi2 after grouping of extreme with low counts 14
15 Activity Index Correlates With Change in Fibrosis Score and Response: NASH Resolution Induces Fibrosis Improvement % patients Activity Index = Ballooning Score + Inflammation Score fibrosis score Fibrosis Improvers Fibrosis Worseners Mean±SE 1,5 70 N=7 N=39 N=77 N=82 N=27 N= p< Change in activity Index (Ballooning + Inflammation) 1 R² = 0,946 0,5 Activity Index ,5-1 -1,5 In GOLDEN-505 after only 1 year: Changes in in Activity Index is highly correlated with changes in fibrosis P-value derived from Khi2 after grouping of extreme with low counts 15
16 Effect size vs placebo (Absolute change) Elafibranor has Efficacy on Composites Scores, Liver and Inflammatory Markers Effect size vs placebo (U/L) Effect size vs placebo (g/l or log- HsCRP) Elafibranor 120mg vs Placebo on Composite Scores Elafibranor 120mg vs Placebo on Liver Markers Elafibranor 120mg vs Placebo on Inflammatory Markers FIBROTEST *** STEATOTEST *** NAFLD-Fibrosis score p= ,1-0, ** *** *** ALT AST GGT ALP -0,3-0,4 p=0.09 *** ** Fibrinogen Haptoglobin HsCRP (log) The effect size vs placebo was calculated and expressed as LSMean±Standard Error. ** : p< *** : p<
17 CVD Risk in NASH Patients is Under High Scrutiny by Regulatory Agencies and Experts Alike FDA/AASLD recommendation (Hepatology 2015): NASH is associated with type II diabetes, increased cardiovascular risk and cancer-related mortality (74-76). For this reason, it seems important to monitor LDL- and HDL-cholesterol, triglycerides, and diabetes control (e.g. hemoglobin A 1c) in phase 2b and 3 NASH trials. It is imperative that any drug developed for NASH be at least neutral from a cardiovascular risk perspective and ideally also reduce cardiovascular risks. Elafibranor has beneficial effects on top of standard of care (statins, anti-diabetic drugs, etc.) 17
18 Cardiovascular Diseases, Leading Cause of Death in NASH Patients Angulo et al., Gastroenterology 2015 Aug; 149 (2): e10. doi: /j.gastro Epub 2015 Apr 29 18
19 Effect size vs placebo (Absoute change - mmol/l) Elafibranor Improves CV Risk Factors: TG, Cholesterol, LDL-C, Remnant-C, HDL-C 0,2 Elafibranor 120mg vs Placebo on Lipid Markers *** 0,1 0-0,1-0,2-0,3-0,4 *** ** -0,5-0,6-0,7-0,8 *** *** *** TG Total-Chol Non-HDL-Chol LDL-Chol Remnant-Chol VLDL-Chol HDL-Chol The effect size vs placebo was calculated and expressed as LSMean±Standard Error. ** : p<0.01 *** : p<
20 Effect size vs placebo (% of baseline) Elafibranor Improves Glucose Homeostasis / Insulin Sensitivity in Type 2 Diabetic NASH Patients Effect size vs placebo (%) Elafibranor 120mg vs Placebo Elafibranor 120mg vs Placebo 0 0-0, ,1-20 ## -0,2-0,3-30 # -0, , # ## # -0,6-0,7-0,8 # HbA1c GFT # FPG Insulin HOMA-IR FFA Fructosamine C-peptide Significant decrease in HbA1C vs placebo The effect size vs placebo was calculated and expressed as LSMean±Standard Error. # : p<0.05 ## : p<
21 Elafibranor Improves NASH by Acting on All Cell Types Involved in NASH Parenchymal Cells KC Elafibranor EC HSC PPRE FAO decreases Liver fat NFkB AP-1 decreases Inflammation Fibrosis increases Insulin Sensitivity Elafibranor decreases Fibrosis Elafibranor improves Vaso-reactivity E T -1 Elafibranor decreases Inflammation 21
22 Elafibranor has Very Good Safety and Tolerability Profiles, Essential for a Long-term Treatment Very good tolerance confirmed after one year of treatment in Phase 2b No Death and no Major Cardiovascular Events (MACE) No effect on body weight No signal on cancer No meaningful change in safety markers and hematology No signal for pruritus Elafibranor 80mg Elafibranor 120mg Placebo Total Adverse event N=93 N=89 N=92 N=274 Nausea Vomiting Diarrhoea Headache Fatigue/asthenia Creatinine increase Abdominal pain Myalgia Decreased appetite Rash Pruritus
23 Elafibranor in NASH: RESOLVE-IT Phase 3 Design Details and Timing > 250 centers (worldwide) ~ 1000 patients ANTICIPATED MARKET AUTHORIZATION SUBPART H (FDA) CONDITIONAL APPROVAL (EMA) ~ 2000 patients FIRST TREATMENT PERIOD 18 MONTHS EXTENSION PERIOD Placebo Elafibranor 120mg Placebo Elafibranor 120mg 2:1 2:1 TRIAL INITIATION Q Study population: patients at risk of progression to clinical events NASH with a NAS 4 Fibrosis stage F2 and F3 (F1 + cardiometabolic risk) End of enrollment first ~1000 patients for Subpart H: ~Q WEEK INTERIM ANALYSIS Histological primary endpoint NASH RESOLUTION WITHOUT WORSENING OF FIBROSIS (central reading for all biopsies): Ballooning = 0 Inflammation = 0 (or 1) Without worsening fibrosis (1 stage) Histological key secondary endpoint improvement of histological fibrosis (to be considered as an additional labeling claim) END OF STUDY Prevention of NASH associated clinical events, including cirrhosis resulting from progressive fibrosis Read-out ~2000 patients : based on occurrence of a pre-defined number of events Read-out first ~1000 patients: ~Q
24 GENFIT: Addressing Key Unmet Needs in NASH TREATMENT Elafibranor currently evaluated in RESOLVE-IT Phase 3 trial Resolution of histological NASH Safe and well tolerated for long term treatment Beneficial cardiometabolic profile for global management of NASH patients DIAGNOSIS Biomarker program to unlock market potential Liver biopsy, the standard for diagnosis of NASH is invasive and costly NASH remains significantly under-diagnosed and in need of non-invasive diagnostics as alternative to liver biopsy GENFIT is amongst the leaders developing non-invasive blood based biomarkers for identification of NASH patients for treatment 24
25 GENFIT s Biomarker Program Exploiting in-house expertise in mirnas, biostatistics and unique database in NASH Goal: Identification of NASH patients who should receive treatment Healthy NAFLD CIRRHOSIS No NASH NASH NO TO BE TREATED Steatosis 1 Ballooning 1 Inflammation 1 NAS 4 F2 or higher Patients at risk to be treated are defined according to FDA/EMA/KOL s consensus on the histological scoring Discovery: Highly predictive 5 biomarker algorithm derived by two independent approaches Clinical data: Phase 2 GOLDEN-505 Cohort Biostatistical analysis mir-200a mir-34a A2M HbA1c P3NP 25
26 GENFIT s Proprietary Algorithms More Predictive Than Existing Scores to Identify NASH Patients for Treatment ¹, ² Identification of NASH patients with NAS 4 with F2 or F3 fibrosis Important for the patient: non-invasive diagnosis for treatment Important for the physician: facilitated diagnosis of patients to treat Important for the NASH market: to meet full potential GENFIT is developing proprietary biomarker algorithms to identify, diagnose and follow-up patients Innovation with bioinformatics and mirnas FDA position on NASH diagnosis there is an urgent unmet need to develop biomarkers that facilitate the diagnosis, identification of populations at risk, assessment of disease progression or regression, and/or response to treatment. Page 1401 ¹ Sanyal. AJ et al. 2016, Hepatology, Volume 64- Number 1 (SUPPL): 327A. ² Sanyal et al., EASL ILC 2016: A new method including the quantification of circulating mirnas allows the efficient identification of NASH patients at risk who should be treated 26
27 The NASH Education Program TM A GENFIT initiative The NASH The Education NASH Program TM A GENFIT initiative
28 The NASH Education Program TM A GENFIT initiative NASH is the result of modern lifestyles, and is closely related to the triple epidemic of: Obesity Diabetes Pre-diabetes The NASH Education Program TM is a public health initiative aimed at producing and disseminating essential medical knowledge, towards a targeted audience All physician specialties within the medical community that will be in charge of NASH patients, as soon as diagnostic and therapeutic solutions are made available Patients and their families who also have to understand causes, mechanisms, and consequences of the disease, in order to fully appreciate the importance of an early diagnosis and the usefulness of a treatment well-suited to their condition. The NASH Education Program TM is an endowment fund created by GENFIT to design, finance and manage relevant disease awareness activities in the NASH space Contact us and find out more about The Nash Education Program TM The NASH Education Program TM A GENFIT initiative 28
GENFIT OVERVIEW. September 2016
 2016.09 GENFIT OVERVIEW September 2016 1 Disclaimer Important information and forward looking statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. THE INFORMATION
2016.09 GENFIT OVERVIEW September 2016 1 Disclaimer Important information and forward looking statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. THE INFORMATION
INVESTOR PRESENTATION. November 16 th, 2015
 INVESTOR PRESENTATION November 16 th, 2015 1 Disclaimer Important information and forward looking statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE SINCE APRIL
INVESTOR PRESENTATION November 16 th, 2015 1 Disclaimer Important information and forward looking statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE SINCE APRIL
INVESTOR PRESENTATION
 2017.10.03 INVESTOR PRESENTATION OCTOBER 2017 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. CERTAIN
2017.10.03 INVESTOR PRESENTATION OCTOBER 2017 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. CERTAIN
GOLDEN-505 STUDY EASL INVESTOR EVENT. Vienna April 24, 2015
 GOLDEN-505 STUDY EASL INVESTOR EVENT Vienna April 24, 2015 1 Disclaimer Important Information and Forward Looking Statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE
GOLDEN-505 STUDY EASL INVESTOR EVENT Vienna April 24, 2015 1 Disclaimer Important Information and Forward Looking Statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE
INVESTOR PRESENTATION
 SEPTEMBER 2018 INVESTOR PRESENTATION September 2018 I. PIPELINE II. INTRODUCTION TO NASH III. GENFIT ASSETS IN NASH IV. BEYOND NASH V. MOVING FORWARD VI. CORPORATE HIGHLIGHTS 1 Disclaimer Important Information
SEPTEMBER 2018 INVESTOR PRESENTATION September 2018 I. PIPELINE II. INTRODUCTION TO NASH III. GENFIT ASSETS IN NASH IV. BEYOND NASH V. MOVING FORWARD VI. CORPORATE HIGHLIGHTS 1 Disclaimer Important Information
CORPORATE PRESENTATION
 NOVEMBER 2018 CORPORATE PRESENTATION November 2018 I. CORPORATE HIGHLIGHTS II. LEADERSHIP IN NASH & PBC APPENDIX 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS
NOVEMBER 2018 CORPORATE PRESENTATION November 2018 I. CORPORATE HIGHLIGHTS II. LEADERSHIP IN NASH & PBC APPENDIX 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS
GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019
 GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019 KOL event to be held ahead of expected data release with elafibranor in PBC by end of 2018 (Phase
GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019 KOL event to be held ahead of expected data release with elafibranor in PBC by end of 2018 (Phase
Jan vp24.i. GENFIT Overview. January 2014
 Jan 2014. vp24.i GENFIT Overview January 2014 1 Disclaimer Forward Looking Statement GENFIT IS A PUBLIC COMPANY LISTED ON NYSE EURONEXT (ALTERNEXT PARIS) STOCK EXCHANGE SINCE 2006. THIS DOCUMENT DOES NOT
Jan 2014. vp24.i GENFIT Overview January 2014 1 Disclaimer Forward Looking Statement GENFIT IS A PUBLIC COMPANY LISTED ON NYSE EURONEXT (ALTERNEXT PARIS) STOCK EXCHANGE SINCE 2006. THIS DOCUMENT DOES NOT
Evercore ISI Presentation- Madrigal
 Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
GENFIT Overview Focus on GFT505
 Apr 2014. v32fi GENFIT Overview Focus on GFT505 April 2014 1 Disclaimer Important Information and Forward Looking Statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE
Apr 2014. v32fi GENFIT Overview Focus on GFT505 April 2014 1 Disclaimer Important Information and Forward Looking Statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE
Company Overview. September 2018 NASDAQ: MDGL
 Company Overview September 2018 NASDAQ: MDGL 1 Forward Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our future financial or business
Company Overview September 2018 NASDAQ: MDGL 1 Forward Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our future financial or business
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical
 Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Madrigal s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial
 Madrigal s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial -- Statistically significantly more patients treated with
Madrigal s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial -- Statistically significantly more patients treated with
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014
 Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st NAFLD/NASH : an expanding burden on liver health
 First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
Update on Non-Alcoholic Fatty Liver Disease. Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI
 Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Forward-looking Statements
 NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
Improving the Lives of Patients with Liver Diseases
 Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
At Least 1 in 5 Patients in Your Practice Have Fatty Liver
 At Least 1 in 5 Patients in Your Practice Have Fatty Liver What Can You Tell Your Patients Magnus McLeod MD FRCPC Assistant Professor Dalhousie University 30-NOV-2017 NAFLD Non-Alcoholic Fatty Liver Disease
At Least 1 in 5 Patients in Your Practice Have Fatty Liver What Can You Tell Your Patients Magnus McLeod MD FRCPC Assistant Professor Dalhousie University 30-NOV-2017 NAFLD Non-Alcoholic Fatty Liver Disease
NON-ALCOHOLIC FATTY LIVER DISEASE:
 NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
FDA Introductory Remarks Stephanie O. Omokaro, MD
 FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
Forward-looking Statements
 Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding
Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding
Study 2 ( ) Pivotal Phase 3 Study Top-Line Results. October 29, 2018
 Study 2 (1002-047) Pivotal Phase 3 Study Top-Line Results October 29, 2018 Safe Harbor Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements
Study 2 (1002-047) Pivotal Phase 3 Study Top-Line Results October 29, 2018 Safe Harbor Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements
NONALCOHOLIC FATTY LIVER DISEASE. Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD. April 13, 2012
 NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD Simple Steatosis Fatty hepatocytes Intracellular fat
NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD Simple Steatosis Fatty hepatocytes Intracellular fat
Update on Nonalcoholic Fatty Liver Disease. Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic
 Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
NAFLD & NASH. Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology
 NAFLD & NASH Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology Indiana University School of Medicine ACG Midwest Regional Course,
NAFLD & NASH Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology Indiana University School of Medicine ACG Midwest Regional Course,
An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications
 REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic
 Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
Jefferies Healthcare Conference. June 25, 2008
 Jefferies Healthcare Conference June 25, 2008 Safe Harbor Statement Except for the historical information set forth herein, the matters set forth in this presentation,including without limitation statements
Jefferies Healthcare Conference June 25, 2008 Safe Harbor Statement Except for the historical information set forth herein, the matters set forth in this presentation,including without limitation statements
Corporate Update. NASDAQ: GALT April 9, 2018
 Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
NAFLD/NASH. Definitions. Pathology NASH. Vicki Shah PA-C, MMS Rush University Hepatology
 NAFLD/NASH Vicki Shah PA-C, MMS Rush University Hepatology Definitions NAFLD Evidence of hepatic steatosis by histology (5%) or imaging No causes for secondary fat accumulation EtOH, Drugs, hereditary
NAFLD/NASH Vicki Shah PA-C, MMS Rush University Hepatology Definitions NAFLD Evidence of hepatic steatosis by histology (5%) or imaging No causes for secondary fat accumulation EtOH, Drugs, hereditary
NAFLD: US GUIDELINES. US Guidelines for NAFLD
 NAFLD: US GUIDELINES Arun J Sanyal M.D. Charles Caravati Professor of Medicine Virginia Commonwealth University School of Medicine US Guidelines for NAFLD Represents consensus amongst AGA, AASLD and ACG
NAFLD: US GUIDELINES Arun J Sanyal M.D. Charles Caravati Professor of Medicine Virginia Commonwealth University School of Medicine US Guidelines for NAFLD Represents consensus amongst AGA, AASLD and ACG
-- Single Global Phase 3 Trial Expected to Begin in First Half of
 Catabasis Pharmaceuticals Reports Edasalonexent Preserved Muscle Function and Substantially Slowed Duchenne Muscular Dystrophy Disease Progression Through More Than One Year of Treatment -- Consistent
Catabasis Pharmaceuticals Reports Edasalonexent Preserved Muscle Function and Substantially Slowed Duchenne Muscular Dystrophy Disease Progression Through More Than One Year of Treatment -- Consistent
NAFLD: evidence-based management. Curso de residentes AEEH Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain
 NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
ENCORE-PH Top-line Results
 ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease.
 Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
PREVALENCE OF NAFLD & NASH
 - - PREVALENCE OF & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology 2011; 140:124-31) Dallas Heart Study Prevalence Numbers (Browning et al., Hepatology 2004;40:1387-95)
- - PREVALENCE OF & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology 2011; 140:124-31) Dallas Heart Study Prevalence Numbers (Browning et al., Hepatology 2004;40:1387-95)
NONALCOHOLIC FATTY LIVER DISEASE
 NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD, MSc Hepatology and Liver Transplantation University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Terminology Pathogenesis
NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD, MSc Hepatology and Liver Transplantation University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Terminology Pathogenesis
The Lipid Metabolism Company. Corporate Presentation March 2018
 The Lipid Metabolism Company Corporate Presentation March 2018 Disclaimer This document has been prepared by Cerenis Therapeutics (the "Company") and is for information purposes only. The information and
The Lipid Metabolism Company Corporate Presentation March 2018 Disclaimer This document has been prepared by Cerenis Therapeutics (the "Company") and is for information purposes only. The information and
Q4 Report Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO
 Q4 Report 2018 Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation has been prepared by Calliditas Therapeutics AB
Q4 Report 2018 Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation has been prepared by Calliditas Therapeutics AB
Anti-IL-33 (ANB020) Program
 Anti-IL-33 (ANB020) Program Phase 2a Peanut Allergy Clinical Trial Interim Data Update March 26 th 2018 NASDAQ: ANAB Safe Harbor Statement This presentation and the accompanying oral presentation contain
Anti-IL-33 (ANB020) Program Phase 2a Peanut Allergy Clinical Trial Interim Data Update March 26 th 2018 NASDAQ: ANAB Safe Harbor Statement This presentation and the accompanying oral presentation contain
NASH UPDATE ON DIAGNOSTICS AND THERAPY. Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine
 NASH UPDATE ON DIAGNOSTICS AND THERAPY Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine Conflicts of interest Salaried employee: of VCU Member of Board: McGuire VA Research Institute,
NASH UPDATE ON DIAGNOSTICS AND THERAPY Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine Conflicts of interest Salaried employee: of VCU Member of Board: McGuire VA Research Institute,
Fatty Liver Disease A growing epidemic
 Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Internal Medicine Grand Rounds University of Texas Southwestern Medical Center October 5, 2018
 Internal Medicine Grand Rounds University of Texas Southwestern Medical Center October 5, 2018 Nonalcoholic Fatty Liver Disease (NAFLD) Turns 38-What Have We Learned? Jay D. Horton, M.D. This is to acknowledge
Internal Medicine Grand Rounds University of Texas Southwestern Medical Center October 5, 2018 Nonalcoholic Fatty Liver Disease (NAFLD) Turns 38-What Have We Learned? Jay D. Horton, M.D. This is to acknowledge
METABOLIC SYNDROME AND HCV: FROM HCV
 METABOLIC SYNDROME AND HCV: FROM THEORY TO PRACTICE HCV Steatosis Insulin resistance Arun J Sanyal M.D. Chairman, Div. of Gastroenterology, Hepatology and Nutrition Virginia Commonwealth University Richmond,
METABOLIC SYNDROME AND HCV: FROM THEORY TO PRACTICE HCV Steatosis Insulin resistance Arun J Sanyal M.D. Chairman, Div. of Gastroenterology, Hepatology and Nutrition Virginia Commonwealth University Richmond,
EASL International Liver Congress Paris, France 14 April 2018
 NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
Seladelpar Interim Data Phase 2 Low Dose Study in PBC. July 17, 2017
 Seladelpar Interim Data Phase 2 Low Dose Study in PBC July 17, 2017 Seladelpar Phase 2 Low Dose Study in PBC Potential for superior efficacy and better tolerability 39% (5 mg) and 45% (10 mg) reductions
Seladelpar Interim Data Phase 2 Low Dose Study in PBC July 17, 2017 Seladelpar Phase 2 Low Dose Study in PBC Potential for superior efficacy and better tolerability 39% (5 mg) and 45% (10 mg) reductions
Fatty liver disease: What do we know?
 Fatty liver disease: What do we know? Prof. Dr. Claus Niederau Katholische Kliniken Oberhausen ggmbh St. Josef-Hospital Academic Teaching Hospital University of Duisburg-Essen NAFLD Non-Alcoholic Fatty
Fatty liver disease: What do we know? Prof. Dr. Claus Niederau Katholische Kliniken Oberhausen ggmbh St. Josef-Hospital Academic Teaching Hospital University of Duisburg-Essen NAFLD Non-Alcoholic Fatty
-- Edasalonexent Substantially Slowed Duchenne Muscular Dystrophy Disease Progression through 36 Weeks --
 Catabasis Pharmaceuticals Reports Positive Results from Open-Label Extension of Phase 2 MoveDMD Trial Evaluating Edasalonexent in Duchenne Muscular Dystrophy and Plans to Initiate Phase 3 Clinical Trial
Catabasis Pharmaceuticals Reports Positive Results from Open-Label Extension of Phase 2 MoveDMD Trial Evaluating Edasalonexent in Duchenne Muscular Dystrophy and Plans to Initiate Phase 3 Clinical Trial
GR-MD-02 for Indication of NASH Cirrhosis: NASH-CX Clinical Trial Results
 GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
The Skinny On Non Alcoholic Fatty Liver Disease
 The Skinny On Non Alcoholic Fatty Liver Disease UCSF Advances in Internal Medicine Monika Sarkar, MD, MAS UCSF Division of GI/Hepatology June 24th, 2015 Non Alcoholic Fatty Liver Disease: Outline Pathogenesis
The Skinny On Non Alcoholic Fatty Liver Disease UCSF Advances in Internal Medicine Monika Sarkar, MD, MAS UCSF Division of GI/Hepatology June 24th, 2015 Non Alcoholic Fatty Liver Disease: Outline Pathogenesis
NASH : Diagnosis and investigation. VII Workshop international, Curitiba, Brazil 29/08/2014
 NASH : Diagnosis and investigation VII Workshop international, Curitiba, Brazil 29/08/2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France Usual diagnostic circumstances
NASH : Diagnosis and investigation VII Workshop international, Curitiba, Brazil 29/08/2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France Usual diagnostic circumstances
Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016.
 Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016. Outline Definition NAFLD and NASH Magnitude of the problem
Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016. Outline Definition NAFLD and NASH Magnitude of the problem
Creating a Leading Global HBV Therapeutics Company. ARB-1467 Update Call December 12, 2016
 Creating a Leading Global HBV Therapeutics Company ARB-1467 Update Call December 12, 2016 NASDAQ: ABUS www.arbutusbio.com Forward Looking Statements This presentation contains forward-looking statements
Creating a Leading Global HBV Therapeutics Company ARB-1467 Update Call December 12, 2016 NASDAQ: ABUS www.arbutusbio.com Forward Looking Statements This presentation contains forward-looking statements
Paris Hepatology Conference PROGNOSIS OF NASH
 Paris Hepatology Conference PROGNOSIS OF NASH Palais des Congrès Monday 15th January 2018 14:45-15:00 PHC 2018 - www.aphc.info Driven to care Disclosures Advisory committees Speaking and teaching Research
Paris Hepatology Conference PROGNOSIS OF NASH Palais des Congrès Monday 15th January 2018 14:45-15:00 PHC 2018 - www.aphc.info Driven to care Disclosures Advisory committees Speaking and teaching Research
NAFLD 2017 Identifying and managing disease while waiting for a cure
 NAFLD 2017 Identifying and managing disease while waiting for a cure A. Sidney Barritt IV, MD, MSCR Associate Professor of Medicine UNC Liver Center High Impact Hepatology 4 November 2017 Disclosures I
NAFLD 2017 Identifying and managing disease while waiting for a cure A. Sidney Barritt IV, MD, MSCR Associate Professor of Medicine UNC Liver Center High Impact Hepatology 4 November 2017 Disclosures I
Fatty Liver Disease. Mark Thursz. Imperial College
 Fatty Liver Disease Mark Thursz Imperial College Non-Alcoholic Fatty Liver Disease UK adult obesity (BMI>30) 1980: 6% [M], 8% [F]. 1997: 17% [M], 20% [F]. By 2004, 23.6% of men and 23.8% of women were
Fatty Liver Disease Mark Thursz Imperial College Non-Alcoholic Fatty Liver Disease UK adult obesity (BMI>30) 1980: 6% [M], 8% [F]. 1997: 17% [M], 20% [F]. By 2004, 23.6% of men and 23.8% of women were
EASL-EASD-EASO Clinical Practice Guidelines for the management and treatment of NAFLD. Francesco Negro University of Geneva - Switzerland
 EASL-EASD-EASO Clinical Practice Guidelines for the management and treatment of NAFLD Francesco Negro University of Geneva - Switzerland EASL EASD - EASO G Marchesini CP Day J-F Dufour A Canbay V Nobili
EASL-EASD-EASO Clinical Practice Guidelines for the management and treatment of NAFLD Francesco Negro University of Geneva - Switzerland EASL EASD - EASO G Marchesini CP Day J-F Dufour A Canbay V Nobili
Improving Access to Quality Medical Care Webinar Series
 Improving Access to Quality Medical Care Webinar Series Presented by The Arizona Telemedicine Program and the Southwest Telehealth Resource Center 2015 UA Board of Regents Welcome AZ, UT, CO, NM & NV FLEX
Improving Access to Quality Medical Care Webinar Series Presented by The Arizona Telemedicine Program and the Southwest Telehealth Resource Center 2015 UA Board of Regents Welcome AZ, UT, CO, NM & NV FLEX
Non-Alcoholic Fatty Liver Disease
 Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
Ocaliva (obeticholic acid tablets)
 Ocaliva (obeticholic acid tablets) Policy Number: 5.01.619 Last Review: 11/2018 Origination: 11/2016 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Ocaliva (obeticholic acid tablets) Policy Number: 5.01.619 Last Review: 11/2018 Origination: 11/2016 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Update On Diabetic Dyslipidemia: Who Should Be Treated With A Fibrate After ACCORD-LIPID?
 Update On Diabetic Dyslipidemia: Who Should Be Treated With A Fibrate After ACCORD-LIPID? Karen Aspry, MD, MS, ABCL, FACC Assistant Clinical Professor of Medicine Warren Alpert Medical School of Brown
Update On Diabetic Dyslipidemia: Who Should Be Treated With A Fibrate After ACCORD-LIPID? Karen Aspry, MD, MS, ABCL, FACC Assistant Clinical Professor of Medicine Warren Alpert Medical School of Brown
NONALCOHOLIC STEATOHEPATITIS (NASH) - OPPORTUNITY ANALYSIS AND FORECASTS TO EVENT-DRIVEN UPDATE
 REFERENCE CODE GDHC034POA PUBLICAT ION DATE MARCH 2014 NONALCOHOLIC STEATOHEPATITIS (NASH) - - EVENT-DRIVEN UPDATE Executive Summary NASH: Key Metrics in Six Major Pharmaceutical Markets 2012 Epidemiology
REFERENCE CODE GDHC034POA PUBLICAT ION DATE MARCH 2014 NONALCOHOLIC STEATOHEPATITIS (NASH) - - EVENT-DRIVEN UPDATE Executive Summary NASH: Key Metrics in Six Major Pharmaceutical Markets 2012 Epidemiology
tage Percent Total & over Total & over Men Women Men Women
 Paul Angulo, MD, FACG, AGAF Professor of Medicine, Section Chief of Hepatology Division i i of Digestive i Diseases and Nutrition i University of Kentucky Medical Center Lexington, KY Paul Angulo, MD University
Paul Angulo, MD, FACG, AGAF Professor of Medicine, Section Chief of Hepatology Division i i of Digestive i Diseases and Nutrition i University of Kentucky Medical Center Lexington, KY Paul Angulo, MD University
Oral Testosterone (T) Non Alcoholic Steatohepatitis (NASH)
 Oral Testosterone (T) Non Alcoholic Steatohepatitis (NASH) 1 LPCN 1144: Well Positioned for Success Unique Mechanism of Action with Compelling Clinical Signal Targeting Full Spectrum of NASH Pathogenesis
Oral Testosterone (T) Non Alcoholic Steatohepatitis (NASH) 1 LPCN 1144: Well Positioned for Success Unique Mechanism of Action with Compelling Clinical Signal Targeting Full Spectrum of NASH Pathogenesis
NYSE AMER: MTNB. MAT9001 OVERVIEW. September 2018
 NYSE AMER: MTNB www.matinasbiopharma.com MAT9001 OVERVIEW September 2018 1 Forward Looking Statement This presentation contains "forward-looking statements" within the meaning of the Private Securities
NYSE AMER: MTNB www.matinasbiopharma.com MAT9001 OVERVIEW September 2018 1 Forward Looking Statement This presentation contains "forward-looking statements" within the meaning of the Private Securities
The EMA reflection paper on chronic liver disease and its implications for drug development in NASH
 The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
Madrigal Pharmaceuticals, Inc.
 Madrigal Pharmaceuticals, Inc. NASDAQ: MDGL 1 Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our future financial or business
Madrigal Pharmaceuticals, Inc. NASDAQ: MDGL 1 Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our future financial or business
Overview of the Clinical Trial Data on Non-alcoholic Steatohepatitis (NASH)
 Overview of the Clinical Trial Data on Non-alcoholic Steatohepatitis (NASH) Brent A. Neuschwander-Tetri, MD, FACP, FACG, AGAF, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology
Overview of the Clinical Trial Data on Non-alcoholic Steatohepatitis (NASH) Brent A. Neuschwander-Tetri, MD, FACP, FACG, AGAF, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms
 SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
Investor presentation. Bioshares Biotech Summit July 2017
 Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Diseases to Watch. Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic
 Diseases to Watch Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic steatohepatitis (NASH) - Prevalence and Symptoms - Risk Factors and Potential treatments - Target identification for NASH Robert
Diseases to Watch Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic steatohepatitis (NASH) - Prevalence and Symptoms - Risk Factors and Potential treatments - Target identification for NASH Robert
Edasalonexent (CAT-1004) Program
 Edasalonexent (CAT-1004) Program Oral small molecule designed to inhibit NF-κB for the treatment of Duchenne muscular dystrophy Joanne M. Donovan, MD, PhD Chief Medical Officer, Catabasis Pharmaceuticals
Edasalonexent (CAT-1004) Program Oral small molecule designed to inhibit NF-κB for the treatment of Duchenne muscular dystrophy Joanne M. Donovan, MD, PhD Chief Medical Officer, Catabasis Pharmaceuticals
Calliditas Therapeutics Q2 Report Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO
 Calliditas Therapeutics Q2 Report 2018 Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation may contain certain
Calliditas Therapeutics Q2 Report 2018 Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation may contain certain
AASLD Immune tolerant phase HBV NAFLD diagnostic HCC
 AASLD 2016 Immune tolerant phase HBV NAFLD diagnostic HCC Immune tolerant 3 Modified from Chan HLY and Wong VWS. Hepatitis B. In Zakim and Boyers s Hepatology 2012 2015 AMERICAN ASSOCIATION FOR THE S1T6UDY
AASLD 2016 Immune tolerant phase HBV NAFLD diagnostic HCC Immune tolerant 3 Modified from Chan HLY and Wong VWS. Hepatitis B. In Zakim and Boyers s Hepatology 2012 2015 AMERICAN ASSOCIATION FOR THE S1T6UDY
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH?
 WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
Yun-Jung Choi, Jiangao Song, Jeff D. Johnson, Charles McWherter. NASH-TAG Conference Park City, Utah January 4, 2019
 Combination Therapy of Seladelpar and Liraglutide Attenuates Obesity, Hepatic Steatosis and Fibrosis in a Diet-induced and Biopsy-confirmed Mouse Model of NASH Yun-Jung Choi, Jiangao Song, Jeff D. Johnson,
Combination Therapy of Seladelpar and Liraglutide Attenuates Obesity, Hepatic Steatosis and Fibrosis in a Diet-induced and Biopsy-confirmed Mouse Model of NASH Yun-Jung Choi, Jiangao Song, Jeff D. Johnson,
Laboratory analysis of the obese child recommendations and discussion. MacKenzi Hillard May 4, 2011
 Laboratory analysis of the obese child recommendations and discussion MacKenzi Hillard May 4, 2011 aka: What to do with Fasting Labs The Obesity Epidemic The prevalence of obesity in adolescents has tripled
Laboratory analysis of the obese child recommendations and discussion MacKenzi Hillard May 4, 2011 aka: What to do with Fasting Labs The Obesity Epidemic The prevalence of obesity in adolescents has tripled
Defining the gold standard in biomarker validation for NASH
 Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Metabolic defects underlying dyslipidemia in abdominal obesity
 Metabolic defects underlying dyslipidemia in abdominal obesity Professor Marja-Riitta Taskinen Department of Medicine, Division of Cardiology Helsinki University Hospital, Finland Disclosures: Honorariums/
Metabolic defects underlying dyslipidemia in abdominal obesity Professor Marja-Riitta Taskinen Department of Medicine, Division of Cardiology Helsinki University Hospital, Finland Disclosures: Honorariums/
A COMPANY INSPIRED BY REGENERATION
 OUR FOCUS A COMPANY INSPIRED BY REGENERATION MEET ALLY, a teacher, mother and motorcycle enthusiast with primary biliary cholangitis*(pbc), a rare and potentially fatal non-viral liver disease. OUR FOCUS
OUR FOCUS A COMPANY INSPIRED BY REGENERATION MEET ALLY, a teacher, mother and motorcycle enthusiast with primary biliary cholangitis*(pbc), a rare and potentially fatal non-viral liver disease. OUR FOCUS
NASH Bench to Bedside
 NASH Bench to Bedside October 2006 Anna Mae Diehl, M.D. Gastroenterology Division Duke University NonAlcoholic Fatty Liver Disease Common ~1/4-1/3 1/3 US adults Outcome highly variable Course indolent
NASH Bench to Bedside October 2006 Anna Mae Diehl, M.D. Gastroenterology Division Duke University NonAlcoholic Fatty Liver Disease Common ~1/4-1/3 1/3 US adults Outcome highly variable Course indolent
Non-Alcoholic Steatohepatitis (NASH): What the Gastroenterologist Should Know
 Non-Alcoholic Steatohepatitis (NASH): What the Gastroenterologist Should Know Naga P. Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology
Non-Alcoholic Steatohepatitis (NASH): What the Gastroenterologist Should Know Naga P. Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology
NASH Regulatory Landscape. Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH
 NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
Zafgen PWS Clinical Trial Program Overview. November 16, 2014
 Zafgen PWS Clinical Trial Program Overview November 16, 2014 2 Disclaimers Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information.
Zafgen PWS Clinical Trial Program Overview November 16, 2014 2 Disclaimers Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information.
Investor Overview. March 2019
 Investor Overview March 2019 Forward-Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words such as may, might, will,
Investor Overview March 2019 Forward-Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words such as may, might, will,
Fat, ballooning, plasma cells and a +ANA. Yikes! USCAP 2016 Evening Specialty Conference Cynthia Guy
 Fat, ballooning, plasma cells and a +ANA. Yikes! USCAP 2016 Evening Specialty Conference Cynthia Guy Goals Share an interesting case Important because it highlights a common problem that we re likely to
Fat, ballooning, plasma cells and a +ANA. Yikes! USCAP 2016 Evening Specialty Conference Cynthia Guy Goals Share an interesting case Important because it highlights a common problem that we re likely to
Sanofi Announces Results of ORIGIN, the World s Longest and Largest Randomised Clinical Trial in Insulin in Pre- and Early Diabetes
 PRESS RELEASE Sanofi Announces Results of ORIGIN, the World s Longest and Largest Randomised Clinical Trial in Insulin in Pre- and Early Diabetes Dublin, Ireland (15 June 2012) Sanofi presented results
PRESS RELEASE Sanofi Announces Results of ORIGIN, the World s Longest and Largest Randomised Clinical Trial in Insulin in Pre- and Early Diabetes Dublin, Ireland (15 June 2012) Sanofi presented results
Liver Forum Cirrhosis Working Group Arun J. Sanyal
 Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
What to do about the high ALT picked up at the annual review. Dr Michael Yee Consultant in Diabetes and Endocrinology
 What to do about the high ALT picked up at the annual review Dr Michael Yee Consultant in Diabetes and Endocrinology Mrs DC HPC PMH Type 2 Diabetes (decades) Regular retinal screening No foot complications/neuropathy
What to do about the high ALT picked up at the annual review Dr Michael Yee Consultant in Diabetes and Endocrinology Mrs DC HPC PMH Type 2 Diabetes (decades) Regular retinal screening No foot complications/neuropathy
What is NAFLD?.NASH? Presenter Disclosure Information. Learning Objectives. Case 1: Rob. Questions Pertinent to Rob
 Presenter Disclosure Information 5 6pm Nonalcoholic Fatty Liver Disease (NAFLD): Another Obesity-Related Epidemic SPEAKER Elliot Tapper, MD The following relationships exist related to this presentation:
Presenter Disclosure Information 5 6pm Nonalcoholic Fatty Liver Disease (NAFLD): Another Obesity-Related Epidemic SPEAKER Elliot Tapper, MD The following relationships exist related to this presentation:
Liver biopsy as the gold standard for diagnosis. Pierre BEDOSSA
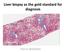 Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease
 REVIEW In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease Ting-Ting Chan, M.R.C.P., and Vincent Wai-Sun Wong, M.D. Nonalcoholic fatty liver disease (NAFLD) affects 15% to 40% of the general
REVIEW In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease Ting-Ting Chan, M.R.C.P., and Vincent Wai-Sun Wong, M.D. Nonalcoholic fatty liver disease (NAFLD) affects 15% to 40% of the general
Investigating general liver disease/transaminitis
 BHIVA Autumn Conference London 14 October 2016 Investigating general liver disease/transaminitis Emmanuel A. Tsochatzis Senior Clinical Lecturer and Consultant Hepatologist Institute for Liver and Digestive
BHIVA Autumn Conference London 14 October 2016 Investigating general liver disease/transaminitis Emmanuel A. Tsochatzis Senior Clinical Lecturer and Consultant Hepatologist Institute for Liver and Digestive
PLEO-CMT Top-line Results. Presentation October 16, 2018
 PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
Rodman & Renshaw 17 th Annual Global Investment Conference
 Rodman & Renshaw 17 th Annual Global Investment Conference September 10, 2015 NASDAQ: GALT www.galectintherapeutics.com 2015 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains,
Rodman & Renshaw 17 th Annual Global Investment Conference September 10, 2015 NASDAQ: GALT www.galectintherapeutics.com 2015 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains,
Metabolic Syndrome. Bill Roberts, M.D., Ph.D. Professor of Pathology University of Utah
 Metabolic Syndrome Bill Roberts, M.D., Ph.D. Professor of Pathology University of Utah Objectives Be able to outline the pathophysiology of the metabolic syndrome Be able to list diagnostic criteria for
Metabolic Syndrome Bill Roberts, M.D., Ph.D. Professor of Pathology University of Utah Objectives Be able to outline the pathophysiology of the metabolic syndrome Be able to list diagnostic criteria for
Tesamorelin Clinical Data Overview Jean-Claude Mamputu, PhD Senior Medical Advisor, Theratechnologies
 Tesamorelin Clinical Data Overview Jean-Claude Mamputu, PhD Senior Medical Advisor, Theratechnologies Copyright 2016. All Rights Reserved. Property of Theratechnologies Inc. Mechanism of Action of Tesamorelin
Tesamorelin Clinical Data Overview Jean-Claude Mamputu, PhD Senior Medical Advisor, Theratechnologies Copyright 2016. All Rights Reserved. Property of Theratechnologies Inc. Mechanism of Action of Tesamorelin
