INVESTOR PRESENTATION
|
|
|
- David Harrell
- 5 years ago
- Views:
Transcription
1 SEPTEMBER 2018 INVESTOR PRESENTATION September 2018 I. PIPELINE II. INTRODUCTION TO NASH III. GENFIT ASSETS IN NASH IV. BEYOND NASH V. MOVING FORWARD VI. CORPORATE HIGHLIGHTS 1
2 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. CERTAIN OF THE INFORMATION CONTAINED HEREIN CONCERNING ECONOMIC TRENDS AND PERFORMANCE IS BASED UPON OR DERIVED FROM INFORMATION PROVIDED BY THIRD-PARTY CONSULTANTS AND OTHER INDUSTRY SOURCES. WHILE GENFIT BELIEVES THAT SUCH INFORMATION IS ACCURATE AND THAT THE SOURCES FROM WHICH IT HAS BEEN OBTAINED ARE RELIABLE, GENFIT HAS NOT INDEPENDENTLY VERIFIED THE ASSUMPTIONS ON WHICH PROJECTIONS OF FUTURE TRENDS AND PERFORMANCE ARE BASED. IT MAKES NO GUARANTEE, EXPRESS OR IMPLIED, AS TO THE ACCURACY AND COMPLETENESS OF SUCH INFORMATION. THIS PRESENTATION CONTAINS CERTAIN FORWARD-LOOKING STATEMENTS. ALTHOUGH THE COMPANY BELIEVES ITS EXPECTATIONS ARE BASED ON REASONABLE ASSUMPTIONS, THESE FORWARD-LOOKING STATEMENTS ARE SUBJECT TO NUMEROUS RISKS AND UNCERTAINTIES, WHICH COULD CAUSE ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE EXPRESSED IN, OR IMPLIED OR PROJECTED BY, THE FORWARD-LOOKING STATEMENTS. THESE RISKS AND UNCERTAINTIES INCLUDE AMONG OTHER THINGS, THE UNCERTAINTIES INHERENT IN RESEARCH AND DEVELOPMENT, INCLUDING RELATED TO BIOMARKERS, PROGRESSION OF, AND RESULTS OF CLINICAL DATA FROM, THE RESOLVE-IT TRIAL, REVIEW AND APPROVALS BY REGULATORY AUTHORITIES, SUCH AS THE FDA OR THE EMA, REGARDING IN PARTICULAR, ELAFIBRANOR IN NASH AND PBC, AS WELL AS OTHER DRUG CANDIDATES IN OTHER INDICATIONS, AND BIOMARKERS, THE SUCCESS OF ANY INLICENSING STRATEGIES, THE COMPANY S CONTINUED ABILITY TO RAISE CAPITAL TO FUND ITS DEVELOPMENT, AS WELL AS THOSE DISCUSSED OR IDENTIFIED IN THE COMPANY S PUBLIC FILINGS WITH THE AMF, INCLUDING THOSE LISTED UNDER SECTION 4 MAIN RISKS AND UNCERTAINTIES OF THE COMPANY S 2017 REGISTRATION DOCUMENT REGISTERED WITH THE FRENCH AUTORITÉ DES MARCHÉS FINANCIERS ON APRIL 27, 2018 UNDER N R , WHICH IS AVAILABLE ON GENFIT S WEBSITE ( AND ON THE WEBSITE OF THE AMF ( OTHER THAN AS REQUIRED BY APPLICABLE LAW, THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO UPDATE OR REVISE ANY FORWARD-LOOKING INFORMATION OR STATEMENTS. THE INFORMATION, OPINIONS AND FORWARD LOOKING INFORMATION CONTAINED IN THIS PRESENTATION SPEAK ONLY AS OF THE DATE OF THIS DOCUMENT, UNLESS OTHERWISE INDICATED HEREIN. OTHER THAN AS REQUIRED BY APPLICABLE LAW, GENFIT IS NOT UNDER ANY, AND UNDERTAKES NO OBLIGATION TO, UPDATE OR KEEP CURRENT THE INFORMATION CONTAINED IN THIS PRESENTATION AND ANY OPINIONS EXPRESSED THEREIN ARE SUBJECT TO CHANGE WITHOUT NOTICE. NOTHING IN THE PRESENTATION CONSTITUTES AN OFFER OF SECURITIES FOR SALE IN THE UNITED STATES OF AMERICA, CANADA, JAPAN, THE UNITED KINGDOM, AUSTRALIA OR ANY OTHER JURISDICTION WHERE IT IS UNLAWFUL TO DO SO. 2
3 SEPTEMBER 2018 I. PIPELINE 3
4 GENFIT Pipeline Rich Pipeline, Targeting Unmet Needs in Liver & Autoimmune Diseases Targeting Unmet Needs in Liver and Autoimmune Diseases PROGRAM INDICATION TARGET DEVELOPMENT STAGE TIMELINE (E) ADULT NASH PPAR α/δ PHASE 3 Data readout End of 2019 Elafibranor PEDIATRIC NASH PPAR α/δ PHASE 2 Phase 2 Program In progress ADULT NASH (COMBO) PPAR α/δ + others Undisclosed Diagnostic NASH Composite score Elafibranor PBC PPARα/δ DEVELOPMENT PHASE 2 Regulatory approval 2020/21 Data readout End of 2018 Nitazoxanide FIBROSIS, CIRRHOSIS Stellate cell activation PHASE 2 Phase 2 start 2018 TGFTX4 FIBROSIS, CIRRHOSIS Undisclosed TGFTX1 AUTO-IMMUNE DISEASES RORγt Lead Optimization In progress Pre-IND* studies In progress (psoriasis) Pre-clinical Clinical * IND = Investigational New Drug 4
5 II. INTRODUCTION TO NASH Unmet needs Treatment paradigm 5
6 Three Major Unmet Needs in NASH: Considered Top Priority by Regulators and Healthcare Providers 1. TREATMENT Targeting the UNDERLYING CAUSE 2. DIAGNOSTIC Identifying PATIENTS 3. AWARENESS Optimizing STANDARD of CARE 6
7 NASH the Underlying Cause of Progressive Fibrosis Resulting from Necroinflammation Leads to Cirrhosis and HCC Leading cause of liver disease in developed countries; ~20mn in US suffer from NASH & advanced fibrosis Cardiovascular events are the leading cause of death in NASH Multifaceted disease Matteoni, Gastro 1999 Adams, Gastro 2005 Ekstedt, Hepatol 2006 Ong, J Hepatol 2008 Dunn, AJG 2008 Sorderberg, Hepatol 2010 Targher, NEJM 2010 Williams, Gastro 2011 Chalasani, Gastro 2012 Torres, Clin Gastro Hepatol 2012 Wree, Nat. Rev Gastroenterol Hepatol 2013 Rinella, JAMA 2015 Bazick, Diabetes Care
8 NASH and Ballooning are Correlated to Long Term Outcomes NASH Ballooning n= 619 n= 619 NASH and hepatocyte ballooning identified as histological features correlated with clinical outcomes and mortality *: Angulo et al., Gastroenterology 2015 Aug; 149 (2): e10. doi: /j.gastro Epub 2015 Apr 29. 8
9 % patients Ballooning and Inflammation (NASH Activity Index ) are Correlated with Fibrosis Reducing Ballooning & Inflammation correlates with Fibrosis improvement Worsening Ballooning & Inflammation correlates with Fibrosis worsening N=7 N=39 N=77 N=82 N=27 N=5 Fibrosis Improvers Fibrosis Worseners p< Reducing Ballooning & Inflammation Change in Activity Index (Ballooning + Inflammation) = NASH disease activity Worsening Ballooning & Inflammation GOLDEN505 dataset / P-value derived from Khi2 after grouping of extreme with low counts 9
10 NASH and Fibrosis: Two Simple Analogies to Understand the Disease Paradigm UNDERLYING CAUSE = Primary Target for Pharmaceutical Drugs Measure of Progression Clinical Outcome HIV HCV Abacavir, dolutegravir, etravirine, rilpivirine, etc. Sofosbuvir, simeprevir, dasabuvir, etc. FIBROSIS CIRRHOSIS NASH Elafibranor, first drug that targets NASH resolution CIRRHOSIS F4 Arun Paris NASH Symposium
11 III. GENFIT ASSETS IN NASH Elafibranor Phase 2 data: a competitive advantage Diagnostic and disease awareness programs 11
12 GENFIT s Approach to NASH: Comprehensive Clinical Management of NASH Patients 1. TREATMENT ELAFIBRANOR Phase 3 NASH resolution & progression to cirrhosis CVD benefit Safety High tolerability Backbone for combination therapy 12
13 Elafibranor, First-in-class, has Pluripotent Activities: PPARα/δ Regulate Multiple Pathways Essential in NASH PPARa and d Regulate Multiple Pathways Essential in NASH 13
14 GOLDEN-505 Elafibranor Phase in NASH 2 Results: A Solid Ground for RESOLVE-IT Phase 3 trial GOLDEN-505 Phase 2 Results* Elafibranor met the Resolution of NASH without worsening of fibrosis endpoint, recommended as primary endpoint for pivotal trials in NASH: Ballooning = 0; Inflammation = 0 (or 1); No worsening of fibrosis (1 stage) Elafibranor confirmed in NASH patients the improved cardiometabolic risk profile: Reduce LDLc, TGs Increase HDLc Improve insulin sensitivity Elafibranor was safe and well tolerated Data published in peer-review journal 14
15 Competitive Landscape on "NASH Resolution": Clinical Evidence from Phase 2 Supporting Phase 3 Designs Evidence from Phase 2 clinical trial supporting Phase 3 design with the approved registrational endpoint of "NASH Resolution without worsening of fibrosis" Duration Design Population Groups "NASH Resolution without worsening of fibrosis" p-value Elafibranor GENFIT 52 weeks R, DB, PBO NAS 4; F0-3 PBO 120mg 5% (1) 26% (1) 0.02 PHASE 3 Programs Ocaliva INTERCEPT GS-4997 GILEAD 72 weeks R, DB, PBO NAS 4; F0-3 PBO 25mg 6% (2) 20% (2) 0.03 N/A : no conclusive data on "NASH resolution" in Phase 2 no focus on "NASH resolution" in Phase 3 Cenicriviroc ALLERGAN N/A : no conclusive data on "NASH resolution" in Phase 2 no focus on "NASH resolution" in Phase 3 MGL-3196 MADRIGAL 36 weeks R, DB, PBO NAS 4; F0-3 PBO 80±20mg 6% (2,3) 27% (2, 3) 0.02 PHASE 2 Programs Aramchol GALMED 52 weeks R, DB, PBO NAS 4; F1-3 PBO 600mg 5% (2) 17% (2) > 0.05 NGM-282 NGM 12 weeks Open label NAS 4; F 1 3mg 11% (2,3,4) N/A (1) Ratziu et al, 2016 Gastroenterology (centers with randomization in all arms, to take into account the well known heterogeneity in the standard of care of NASH patients in different centers) (2) Source: Corporate publications/presentations. Data not published in peer-reviewed scientific journals (3) Results provided for NASH Resolution only, i.e. not for NASH Resolution without the worsening of fibrosis (4) No placebo arm 15
16 Effect size vs placebo (Absoute change - mmol/l) Effect size vs placebo (% of baseline) Effect size vs placebo (%) Elafibranor Phase in NASH 2 Trial: A Closer Look at Cardiometabolic Data GOLDEN-505 Phase 2 Results* Elafibranor 120mg vs Placebo on Lipid Markers Elafibranor 120mg vs Placebo on Glucose Homeostasis and Insulin Sensitivity in T2D NASH Patients 0,2 0,1 0-0,1-0,2-0,3-0,4-0,5-0,6-0,7-0,8 *** *** *** *** ** *** # # # ## ## # 0-0,1-0,2-0,3-0,4-0,5-0,6-0,7-0,8-0.46% # HbA1c -0,0 GFT ** : p<0.01 *** : p<0.001 # : p<0.05 ## : p<0.01 Significant decrease in HbA1c vs placebo 16
17 RESOLVE-IT Phase 3 Design: Details and Timing > 250 centers (worldwide) ~ 1000 patients ACCELERATED MARKET AUTHORIZATION SUBPART H (FDA) CONDITIONAL APPROVAL (EMA) ~ 2000 patients FIRST TREATMENT PERIOD 18 MONTHS Placebo Elafibranor 120mg 2:1 EXTENSION PERIOD Placebo Elafibranor 120mg 2:1 TRIAL INITIATION Q WEEK INTERIM ANALYSIS END OF STUDY Study population: patients at risk of progression to clinical events NASH with a NAS 4 Fibrosis stage F2 and F3 (F1 + cardiometabolic risk) End of enrollment first ~1000 patients for Subpart H: April 2018 Histological primary endpoint NASH RESOLUTION WITHOUT WORSENING OF FIBROSIS (central reading for all biopsies): Ballooning = 0 Inflammation = 0 (or 1) Without worsening fibrosis (1 stage) Histological key secondary endpoint improvement of histological fibrosis (to be considered as an additional labeling claim) Prevention of NASH associated clinical events, including cirrhosis resulting from progressive fibrosis Read-out ~2000 patients : based on occurrence of a pre-defined number of events Read-out first ~1000 patients: End
18 Elafibranor: Potential for First-Line Standalone Monotherapy, and also a Strong Foundation for Combination Therapy Favorable efficacy/safety/tolerability profile (phase 2b) Proactively identify the most effective combination therapies which: Reinforce elafibranor s demonstrated efficacy on NASH resolution Reinforce elafibranor s activity on fibrosis improvement ELAFIBRANOR (PPARa/d) DRUG X Example #1: Elafibranor + FXR Insulin sensitivity Plasma TGs, LDLc HDLc Steatosis Inflammation/Ox stress Hepatocyte damage NAS score Fibrosis (for patients resolving NASH) + Additionnal activity based on a complementary mechanism of action CSAA Example #2 : Elafibranor + NTZ CDAA/c control CDAA/c ELA 1mpk CDAA/c NTZ 100mpk CDAA/c ELA+NTZ 18
19 GENFIT s Approach to NASH: Comprehensive Clinical Management of NASH Patients 2. DIAGNOSTIC IVD BIOMARKER Non-invasive Easy-to-access Best performance vs existing scores Including mi-rna Entering Development phase 19
20 Diagnosis of NASH: a Key Unmet Need Recognized by the FDA BIOPSY IMAGING TECHNIQUES Imperfect "Gold Standard" Non-invasive, but limited alternative Current bottleneck Invasive ; discomfort Costly; Access Issues; Limited to specialized settings Limited to hepatologists and specialized settings Currently best prognostic test available Costly; Access Issues; Limited to specialized settings General tools, not accurate for assessing NASH Non invasive Good patient compliance Ideal situation there is an urgent unmet need to develop biomarkers that facilitate the diagnosis, identification of populations at risk, assessment of disease progression or regression, and/or response to treatment. Page 1401 BLOOD TEST Ideal for large scale adoption into the clinic and early detection Non invasive; Follows clinical workflow Cost-Effective: Accessible; No capital requirements Disease-specific diagnostic 20
21 GENFIT s Approach to Non-invasive IVD NASH Diagnostic Focus on a specific and relevant clinical question: HEALTHY NAFLD CIRRHOSIS NO NASH NASH NO TO BE TREATED Steatosis 1 Ballooning 1 Inflammation 1 NAS 4 F2 or higher Simple workflow, blood-based, and accurate test for routine clinical use Corporate Strategy for a global In Vitro Diagnostic NASH test FDA-approval (2020/2021) / CE-marking (2021) Global Partner(s): Assay kit development, manufacturing, and commercialization Intended use To identify patients who should be considered for therapeutic intervention 1 if suspected of NASH Monitoring disease evolution: Patients under treatment or patients at risk of progression (e.g. F1) Test should be used in conjunction with other clinical findings and/or laboratory parameters Expected market launch (US, EU): 2020/2021 (1) Patients with NAS Score >4 and fibrosis stage >2 as defined FDA/EMA/KOL s consensus on the histological scoring 21
22 NIS4 Training and Validation Receiver Operating Characteristic Analysis NIS4 combines circulating levels of mir-34a & A2M & HbA1c & YKL-40 (CHI3L1) Training set : GOLDEN (220 pts) Validation set: RESOLVE-IT-DIAG (467 pts) Optimization set: MERGED (687 pts) AUROC 0.81 Accuracy 79 % AUROC 0.81 Accuracy 72 % AUROC 0.82 Accuracy 75 % -Trained (logistic regression) and validated NIS4 in independent, multi-center cohorts of patients with suspected NASH who were prospectively screened for inclusion into interventional trials. - Diagnostic performances not influenced by: T2D status, obesity, ethnicities, gender, age 22
23 % population NIS4 Diagnostic Performance vs Other Scores ROC NIS4 vs Other Scores Distibution of Patients According to Fibrosis Stage and NIS4 Range Retest Retest and/or consider biopsy Consider intervention low medium low medium high high NIS4 risk category Figure 1. NIS4 outperforms existing blood-based scores to identify patients with active NASH and significant fibrosis (NAS>4/F 2). Figure 2. NIS4 scores above the high cut-off identified NAS 4/F 2 patients with an associated PPV >80%. Use of NIS4 would have led to the avoidance of >75% of liver biopsies in this patient population. LDT released by early 2019 FDA approval / CE-marking by 2020 with subsequent world-wide launch Intended population: adults suspected of having NASH (AASLD Practice Guidelines) 23
24 Our Vision of the Future Patient Journey with IVD Test (Adoption phase): Facilitate Initial Screening for Better Clinical Management 24
25 Our Vision of the Future Patient Journey with IVD Test (Post-adoption): Facilitate Initial Screening for Better Clinical Management 25
26 GENFIT s Approach to NASH: Comprehensive Clinical Management of NASH Patients 3. AWARENESS 26
27 The NASH Education Program TM : a GENFIT but Non-Partisan Initiative to Address a Key Unmet Need For BETTER CARE NASH (Non-Alcoholic SteatoHepatitis) PHYSICIANS Beyond hepatology PATIENTS Indidivuals at risk Families 1 2 Serious liver condition Unprecedented rise in prevalence Sub-Optimal PATIENT CARE 3 Yet still little known, because Silent, by nature Diagnostic = bottleneck No approved treatment yet UNACCEPTABLE situation The NASH Education Program TM 27
28 Bringing Together a Large and Diversified Coalition of Key Stakeholders Non- profit, patient advocacy groups, learned societies Benefactors 28
29 Ultimately Leading to a Large Success, on a Worldwide Scale 29
30 IV. BEYOND NASH Elafibranor addressing unmet needs in a rare disease: Primary Biliary Cholangitis (PBC) 30
31 Elafibranor Ideally Positioned to Address Important Unmet Needs in a Rare but Severe Chronic Liver Condition HIGH UNMET NEEDS Cholestatic chronic autoimmune disease Affecting intrahepatic bile ducts Rare but severe liver disease Prevalence in the general population : 0.05% Patient profile: women years old RATIONALE for ELAFIBRANOR Elafibranor consistently showed positive effects on ALP in all studied populations + Clinical evidence of beneficial effects induced by PPARα and PPARδ in PBC populations, supported by a well-identified mechanism of action Significant proportion of non/partial responders with current treatments in PBC patient population + Recent evidence showing the potential of PPARα to alleviate pruritus, a major symptom of PBC Major symptom in PBC is pruritus and is not addressed by current PBC therapies 31
32 Phase 2a Sudy with Elafibranor in PBC (Primary Biliary Cholangitis) 1 st Patient enrolled May WEEK ANALYSIS TREATMENT PERIOD 12 WEEKS Placebo Elafibranor 80mg Elafibranor 2:1 120mg Study population adult patients with PBC and inadequate response to ursodeoxycholic acid (UDCA) Primary endpoint effect of daily oral administration of elafibranor on serum alkaline phosphatase (ALP) End of enrollment 45 patients: July 2018 Read-out 45 patients: End 2018 Secondary endpoints include: ALP < 1.67 upper limit of normal (ULN) and total bilirubin within normal limit and > 15% decrease in ALP Paris, Toronto, UK PBC scores Pruritus and QoL (Quality of Life) Safety of elafibranor in a PBC population 32
33 V. MOVING FORWARD Market Access Commercialization 33
34 A Relevant Market Access Strategy: Considering Patient Needs and Pharmacoeconomics 4. MARKET ACCESS 34
35 Commercialization: Maximizing Sales Uptake while Transforming the Company Objective: transform GENFIT into a biopharma, with a mix revenue stream from: Direct sales of elafibranor Royalties from licensing deal Commercialization: Development of a global alliance with a large pharmaceutical company with a solid footprint in metabolic diseases Keep rights in some territories Keep some rights in some clinical settings Stand alone deal in Asia Organizational ramp-up: US/EU Market Access and Marketing teams 35
36 VI. CORPORATE HIGHLIGHTS Company profile Key takeaways Financial highlights 36
37 Company Profile (1/3) Overview Public company focused on metabolic diseases & associated complications, including liver related disorders World-leading expert in nuclear receptor based drug discovery Founded in 1999 (Lille, Paris, France Cambridge, US) 130 employees Since 2006, Euronext Paris compartment B (GNFT) Market capitalization of ~ 800M Lead program in NASH: Nonalcoholic Steatohepatitis Actively enrolling Phase 3 with elafibranor: First-in-class molecule, fast-track designation, accelerated approval Subpart H (FDA) and Conditional approval (EMA) Biomarker program in NASH/NAFLD* for non invasive and easy-to-access IVD (In-Vitro Diagnostic) Rich pipeline addressing other key unmet needs: PBC, fibrosis, auto-immune diseases *: Nonalcoholic Fatty Liver Disease **: In-Vitro Diagnotic 37
38 GENFIT Pipeline Company Profile (2/3): Rich Pipeline, Targeting Unmet Needs in Liver & Autoimmune Diseases Targeting Unmet Needs in Liver and Autoimmune Diseases PROGRAM INDICATION TARGET DEVELOPMENT STAGE TIMELINE (E) ADULT NASH PPAR α/δ PHASE 3 Data readout End of 2019 Elafibranor PEDIATRIC NASH PPAR α/δ PHASE 2 Phase 2 Program In progress ADULT NASH (COMBO) PPAR α/δ + others Undisclosed Diagnostic NASH Composite score Elafibranor PBC PPARα/δ DEVELOPMENT PHASE 2 Regulatory approval 2020/21 Data readout End of 2018 Nitazoxanide FIBROSIS, CIRRHOSIS Stellate cell activation PHASE 2 Phase 2 start 2018 TGFTX4 FIBROSIS, CIRRHOSIS Undisclosed TGFTX1 AUTO-IMMUNE DISEASES RORγt Lead Optimization In progress Pre-IND* studies In progress (psoriasis) Pre-clinical Clinical * IND = Investigational New Drug 38
39 Company Profile (3/3): A Comprehensive Strategy in NASH 1. TREATMENT Targeting the UNDERLYING CAUSE 2. DIAGNOSTIC Identifying PATIENTS 3. AWARENESS Optimizing STANDARD of CARE 39
40 Key takeaways 11. NASH: LARGE AND UNTAPPED MARKET OPPORTUNITY NASH is a therapeutic area with high unmet needs but no available treatment 22. GENFIT NASH PROGRAM: LEADING THE WAY Elafibranor First-in-class molecule World leader with one of the very few Phase 3 currently in progress Strong competitive profile based on efficacy + safety + tolerability + other benefits Diagnostic At the forefront of R&D for an easy-to-access solution 33. CATALYSTS : HIGH POTENTIAL VALUE Phase 2 data in PBC (rare disease) Phase 3 data in NASH (accelerated approval process) Regulatory and partnering/industrial milestones for NASH diagnostic Pediatric program in NASH Combination therapy in NASH 44. COMPANY PROFILE: STRONG NETWORK & EXPERTISE, RELEVANT FOOTPRINT Euro-American biotechnology company based in Lille, Paris and Boston, with a solid expertise in liver and metabolic diseases, and a strong network in NASH 40
41 Financial Highlights Cash position 255 million (Q1 2018) Last fund raisings -March 2016: private placement 50 million -October 2016: private placement 34 million -October 2016: rights issue 45 million -October 2017: convertible bonds 180 million Market cap -GNFT: 800M (Euronext) 41
42 END 42
CORPORATE PRESENTATION
 NOVEMBER 2018 CORPORATE PRESENTATION November 2018 I. CORPORATE HIGHLIGHTS II. LEADERSHIP IN NASH & PBC APPENDIX 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS
NOVEMBER 2018 CORPORATE PRESENTATION November 2018 I. CORPORATE HIGHLIGHTS II. LEADERSHIP IN NASH & PBC APPENDIX 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS
INVESTOR PRESENTATION
 2017.10.03 INVESTOR PRESENTATION OCTOBER 2017 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. CERTAIN
2017.10.03 INVESTOR PRESENTATION OCTOBER 2017 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. CERTAIN
INVESTOR PRESENTATION
 2017.04.24 INVESTOR PRESENTATION APRIL 2017 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. CERTAIN
2017.04.24 INVESTOR PRESENTATION APRIL 2017 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. CERTAIN
INVESTOR PRESENTATION. November 16 th, 2015
 INVESTOR PRESENTATION November 16 th, 2015 1 Disclaimer Important information and forward looking statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE SINCE APRIL
INVESTOR PRESENTATION November 16 th, 2015 1 Disclaimer Important information and forward looking statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE SINCE APRIL
GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019
 GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019 KOL event to be held ahead of expected data release with elafibranor in PBC by end of 2018 (Phase
GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019 KOL event to be held ahead of expected data release with elafibranor in PBC by end of 2018 (Phase
GENFIT OVERVIEW. September 2016
 2016.09 GENFIT OVERVIEW September 2016 1 Disclaimer Important information and forward looking statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. THE INFORMATION
2016.09 GENFIT OVERVIEW September 2016 1 Disclaimer Important information and forward looking statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. THE INFORMATION
Jan vp24.i. GENFIT Overview. January 2014
 Jan 2014. vp24.i GENFIT Overview January 2014 1 Disclaimer Forward Looking Statement GENFIT IS A PUBLIC COMPANY LISTED ON NYSE EURONEXT (ALTERNEXT PARIS) STOCK EXCHANGE SINCE 2006. THIS DOCUMENT DOES NOT
Jan 2014. vp24.i GENFIT Overview January 2014 1 Disclaimer Forward Looking Statement GENFIT IS A PUBLIC COMPANY LISTED ON NYSE EURONEXT (ALTERNEXT PARIS) STOCK EXCHANGE SINCE 2006. THIS DOCUMENT DOES NOT
Evercore ISI Presentation- Madrigal
 Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
GENFIT Overview Focus on GFT505
 Apr 2014. v32fi GENFIT Overview Focus on GFT505 April 2014 1 Disclaimer Important Information and Forward Looking Statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE
Apr 2014. v32fi GENFIT Overview Focus on GFT505 April 2014 1 Disclaimer Important Information and Forward Looking Statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE
GOLDEN-505 STUDY EASL INVESTOR EVENT. Vienna April 24, 2015
 GOLDEN-505 STUDY EASL INVESTOR EVENT Vienna April 24, 2015 1 Disclaimer Important Information and Forward Looking Statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE
GOLDEN-505 STUDY EASL INVESTOR EVENT Vienna April 24, 2015 1 Disclaimer Important Information and Forward Looking Statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE
Improving the Lives of Patients with Liver Diseases
 Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Ocaliva (obeticholic acid tablets)
 Ocaliva (obeticholic acid tablets) Policy Number: 5.01.619 Last Review: 11/2018 Origination: 11/2016 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Ocaliva (obeticholic acid tablets) Policy Number: 5.01.619 Last Review: 11/2018 Origination: 11/2016 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
NONALCOHOLIC STEATOHEPATITIS (NASH) - OPPORTUNITY ANALYSIS AND FORECASTS TO EVENT-DRIVEN UPDATE
 REFERENCE CODE GDHC034POA PUBLICAT ION DATE MARCH 2014 NONALCOHOLIC STEATOHEPATITIS (NASH) - - EVENT-DRIVEN UPDATE Executive Summary NASH: Key Metrics in Six Major Pharmaceutical Markets 2012 Epidemiology
REFERENCE CODE GDHC034POA PUBLICAT ION DATE MARCH 2014 NONALCOHOLIC STEATOHEPATITIS (NASH) - - EVENT-DRIVEN UPDATE Executive Summary NASH: Key Metrics in Six Major Pharmaceutical Markets 2012 Epidemiology
Intercept Pharmaceuticals Reports Second Quarter 2017 Financial Results and Provides Business Update
 July 31, 2017 Intercept Pharmaceuticals Reports Second Quarter 2017 Financial Results and Provides Business Update Worldwide net Ocaliva (obeticholic acid or OCA) 2Q 2017 sales of $30.4 million AESOP Phase
July 31, 2017 Intercept Pharmaceuticals Reports Second Quarter 2017 Financial Results and Provides Business Update Worldwide net Ocaliva (obeticholic acid or OCA) 2Q 2017 sales of $30.4 million AESOP Phase
Forward-looking Statements
 NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Ocaliva (obeticholic acid) Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ocaliva (obeticholic acid) Prime Therapeutics will review Prior Authorization
Ocaliva (obeticholic acid) Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ocaliva (obeticholic acid) Prime Therapeutics will review Prior Authorization
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014
 Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Creating a Leading Global HBV Therapeutics Company. ARB-1467 Update Call December 12, 2016
 Creating a Leading Global HBV Therapeutics Company ARB-1467 Update Call December 12, 2016 NASDAQ: ABUS www.arbutusbio.com Forward Looking Statements This presentation contains forward-looking statements
Creating a Leading Global HBV Therapeutics Company ARB-1467 Update Call December 12, 2016 NASDAQ: ABUS www.arbutusbio.com Forward Looking Statements This presentation contains forward-looking statements
NASH Regulatory Landscape. Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH
 NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
Idenix Pharmaceuticals Building a Leading Antiviral Franchise. Cowen & Company 27 th Annual Healthcare Conference March 13, 2007 Boston
 Idenix Pharmaceuticals Building a Leading Antiviral Franchise Cowen & Company 27 th Annual Healthcare Conference March 13, 2007 Boston Safe Harbor This presentation includes forward-looking statements
Idenix Pharmaceuticals Building a Leading Antiviral Franchise Cowen & Company 27 th Annual Healthcare Conference March 13, 2007 Boston Safe Harbor This presentation includes forward-looking statements
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical
 Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Calliditas Therapeutics Q2 Report Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO
 Calliditas Therapeutics Q2 Report 2018 Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation may contain certain
Calliditas Therapeutics Q2 Report 2018 Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation may contain certain
An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications
 REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
PLEO-CMT Top-line Results. Presentation October 16, 2018
 PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
NASH UPDATE ON DIAGNOSTICS AND THERAPY. Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine
 NASH UPDATE ON DIAGNOSTICS AND THERAPY Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine Conflicts of interest Salaried employee: of VCU Member of Board: McGuire VA Research Institute,
NASH UPDATE ON DIAGNOSTICS AND THERAPY Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine Conflicts of interest Salaried employee: of VCU Member of Board: McGuire VA Research Institute,
Corporate Presentation August 6, 2015
 Corporate Presentation August 6, 2015 Creating the Next Generation of CNS Drugs Forward-Looking Statement This presentation contains forward-looking statements. These statements relate to future events
Corporate Presentation August 6, 2015 Creating the Next Generation of CNS Drugs Forward-Looking Statement This presentation contains forward-looking statements. These statements relate to future events
Combining HS-110 and anti-pd-1 in NSCLC. September 1, 2015
 Combining HS-110 and anti-pd-1 in NSCLC September 1, 2015 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
Combining HS-110 and anti-pd-1 in NSCLC September 1, 2015 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
ARQ 087 Overview. FGFR Inhibitor. March 2017
 ARQ 087 Overview FGFR Inhibitor March 2017 Safe Harbor This presentation and other statements by ArQule contain forward-looking statements within the meaning of the Private Securities Litigation Reform
ARQ 087 Overview FGFR Inhibitor March 2017 Safe Harbor This presentation and other statements by ArQule contain forward-looking statements within the meaning of the Private Securities Litigation Reform
Madrigal s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial
 Madrigal s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial -- Statistically significantly more patients treated with
Madrigal s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial -- Statistically significantly more patients treated with
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st NAFLD/NASH : an expanding burden on liver health
 First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
A COMPANY INSPIRED BY REGENERATION
 OUR FOCUS A COMPANY INSPIRED BY REGENERATION MEET ALLY, a teacher, mother and motorcycle enthusiast with primary biliary cholangitis*(pbc), a rare and potentially fatal non-viral liver disease. OUR FOCUS
OUR FOCUS A COMPANY INSPIRED BY REGENERATION MEET ALLY, a teacher, mother and motorcycle enthusiast with primary biliary cholangitis*(pbc), a rare and potentially fatal non-viral liver disease. OUR FOCUS
Q4 Report Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO
 Q4 Report 2018 Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation has been prepared by Calliditas Therapeutics AB
Q4 Report 2018 Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation has been prepared by Calliditas Therapeutics AB
Update on Nonalcoholic Fatty Liver Disease. Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic
 Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
FDA Introductory Remarks Stephanie O. Omokaro, MD
 FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
Update on Non-Alcoholic Fatty Liver Disease. Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI
 Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Anti-IL-33 (ANB020) Program
 Anti-IL-33 (ANB020) Program Phase 2a Peanut Allergy Clinical Trial Interim Data Update March 26 th 2018 NASDAQ: ANAB Safe Harbor Statement This presentation and the accompanying oral presentation contain
Anti-IL-33 (ANB020) Program Phase 2a Peanut Allergy Clinical Trial Interim Data Update March 26 th 2018 NASDAQ: ANAB Safe Harbor Statement This presentation and the accompanying oral presentation contain
Rodman & Renshaw 17 th Annual Global Investment Conference
 Rodman & Renshaw 17 th Annual Global Investment Conference September 10, 2015 NASDAQ: GALT www.galectintherapeutics.com 2015 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains,
Rodman & Renshaw 17 th Annual Global Investment Conference September 10, 2015 NASDAQ: GALT www.galectintherapeutics.com 2015 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains,
ENCORE-PH Top-line Results
 ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer
 November 26, 2018 CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer VANCOUVER, Washington, Nov. 26, 2018 (GLOBE NEWSWIRE) -- CytoDyn Inc.
November 26, 2018 CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer VANCOUVER, Washington, Nov. 26, 2018 (GLOBE NEWSWIRE) -- CytoDyn Inc.
Corporate Presentation
 Corporate Presentation August 2016 NASDAQ: GALT www.galectintherapeutics.com 2016 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Corporate Presentation August 2016 NASDAQ: GALT www.galectintherapeutics.com 2016 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Liver biopsy as the gold standard for diagnosis. Pierre BEDOSSA
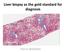 Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Ipsen announces CELESTIAL phase 3 pivotal trial results in the New England Journal of Medicine
 PRESS RELEASE Ipsen announces CELESTIAL phase 3 pivotal trial results in the New England Journal of Medicine Trial results formed the basis of regulatory filings in the U.S. and European Union for CABOMETYX
PRESS RELEASE Ipsen announces CELESTIAL phase 3 pivotal trial results in the New England Journal of Medicine Trial results formed the basis of regulatory filings in the U.S. and European Union for CABOMETYX
Investor Presentation
 Investor Presentation February 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities
Investor Presentation February 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities
Developing & Commercializing Targeted Small Molecule Drugs in Cancer
 Developing & Commercializing Targeted Small Molecule Drugs in Cancer SAFE HARBOR STATEMENT 2 Forward-looking statements made in the course of this presentation are made pursuant to the safe harbor provisions
Developing & Commercializing Targeted Small Molecule Drugs in Cancer SAFE HARBOR STATEMENT 2 Forward-looking statements made in the course of this presentation are made pursuant to the safe harbor provisions
EASL International Liver Congress Paris, France 14 April 2018
 NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
INVESTOR PRESENTATION
 INVESTOR PRESENTATION May 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities regulation.
INVESTOR PRESENTATION May 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities regulation.
Corporate Update. NASDAQ: GALT April 9, 2018
 Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
Jefferies 2016 Healthcare Conference. Reid Huber, PhD Chief Scientific Officer
 Jefferies 2016 Healthcare Conference Reid Huber, PhD Chief Scientific Officer June 8, 2016 Forward-looking Statements Except for the historical information set forth herein, the matters set forth in this
Jefferies 2016 Healthcare Conference Reid Huber, PhD Chief Scientific Officer June 8, 2016 Forward-looking Statements Except for the historical information set forth herein, the matters set forth in this
Company Overview. September 2018 NASDAQ: MDGL
 Company Overview September 2018 NASDAQ: MDGL 1 Forward Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our future financial or business
Company Overview September 2018 NASDAQ: MDGL 1 Forward Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our future financial or business
Santhera to Acquire Option from Idorsia for Exclusive Sub-License to First-in-class Dissociative Steroid Vamorolone. Webcast, 21 November 2018
 Santhera to Acquire Option from Idorsia for Exclusive Sub-License to First-in-class Dissociative Steroid Vamorolone Webcast, 21 November 2018 Disclaimer This presentation is not and under no circumstances
Santhera to Acquire Option from Idorsia for Exclusive Sub-License to First-in-class Dissociative Steroid Vamorolone Webcast, 21 November 2018 Disclaimer This presentation is not and under no circumstances
Overview of the Clinical Trial Data on Non-alcoholic Steatohepatitis (NASH)
 Overview of the Clinical Trial Data on Non-alcoholic Steatohepatitis (NASH) Brent A. Neuschwander-Tetri, MD, FACP, FACG, AGAF, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology
Overview of the Clinical Trial Data on Non-alcoholic Steatohepatitis (NASH) Brent A. Neuschwander-Tetri, MD, FACP, FACG, AGAF, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology
Jefferies Healthcare Conference. June 25, 2008
 Jefferies Healthcare Conference June 25, 2008 Safe Harbor Statement Except for the historical information set forth herein, the matters set forth in this presentation,including without limitation statements
Jefferies Healthcare Conference June 25, 2008 Safe Harbor Statement Except for the historical information set forth herein, the matters set forth in this presentation,including without limitation statements
Ipsen Acquisition of Clementia Pharmaceuticals. February 25, 2019
 Ipsen Acquisition of Clementia Pharmaceuticals February 25, 2019 Disclaimer & Safe Harbor This presentation includes only summary information and does not purport to be comprehensive. Forward-looking statements,
Ipsen Acquisition of Clementia Pharmaceuticals February 25, 2019 Disclaimer & Safe Harbor This presentation includes only summary information and does not purport to be comprehensive. Forward-looking statements,
Liver Forum Cirrhosis Working Group Arun J. Sanyal
 Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Eli Lilly and Company
 Eli Lilly and Company Strategic Diabetes Alliance with Boehringer Ingelheim January 11 th, 2011 Safe Harbor Provision This presentation contains forward-looking statements that are based on management's
Eli Lilly and Company Strategic Diabetes Alliance with Boehringer Ingelheim January 11 th, 2011 Safe Harbor Provision This presentation contains forward-looking statements that are based on management's
Corporate Presentation. June 2015
 Corporate Presentation June 2015 NASDAQ: RPTP Forward-Looking Statements This presentation contains forward-looking statements, as that term is defined in the Private Securities Litigation Reform Act of
Corporate Presentation June 2015 NASDAQ: RPTP Forward-Looking Statements This presentation contains forward-looking statements, as that term is defined in the Private Securities Litigation Reform Act of
Photocure ASA Executing the Strategy
 Photocure ASA Executing the Strategy NOVEMBER 2012 ERIK DAHL, CFO KATHLEEN DEARDORFF, COO Disclaimer The information included in this Presentation contains certain forward-looking statements that address
Photocure ASA Executing the Strategy NOVEMBER 2012 ERIK DAHL, CFO KATHLEEN DEARDORFF, COO Disclaimer The information included in this Presentation contains certain forward-looking statements that address
Global Coverage. Regional Coverage. Country Coverage Company Coverage
 Global Fatty Liver Disease Drugs Market By Type (ALD, NAFLD), By Stages of ALD and NAFLD, By Region, By Country: Opportunities and Forecast (2017-2022) By Type- ALD and NAFLD ALD Stages - Alcoholic Steatosis
Global Fatty Liver Disease Drugs Market By Type (ALD, NAFLD), By Stages of ALD and NAFLD, By Region, By Country: Opportunities and Forecast (2017-2022) By Type- ALD and NAFLD ALD Stages - Alcoholic Steatosis
PREVALENCE OF NAFLD & NASH
 - - PREVALENCE OF & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology 2011; 140:124-31) Dallas Heart Study Prevalence Numbers (Browning et al., Hepatology 2004;40:1387-95)
- - PREVALENCE OF & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology 2011; 140:124-31) Dallas Heart Study Prevalence Numbers (Browning et al., Hepatology 2004;40:1387-95)
Credit Suisse 27 th Annual Healthcare Conference
 CHANGING THE COURSE OF HUMAN HEALTH THROUGH BOLD PURSUITS IN SCIENCE Credit Suisse 27 th Annual Healthcare Conference November 14, 2018 Forward-Looking Statements and Adjusted Financial Information This
CHANGING THE COURSE OF HUMAN HEALTH THROUGH BOLD PURSUITS IN SCIENCE Credit Suisse 27 th Annual Healthcare Conference November 14, 2018 Forward-Looking Statements and Adjusted Financial Information This
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC
 Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Defining the gold standard in biomarker validation for NASH
 Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
World leader in navigated, non-invasive brain stimulation therapy and diagnosis
 World leader in navigated, non-invasive brain stimulation therapy and diagnosis Martin Jamieson CEO & Chairman of the Board Nexstim Plc Mikko Karvinen CFO Nexstim Plc Corporate Presentation, BioTrinity,
World leader in navigated, non-invasive brain stimulation therapy and diagnosis Martin Jamieson CEO & Chairman of the Board Nexstim Plc Mikko Karvinen CFO Nexstim Plc Corporate Presentation, BioTrinity,
Fat, ballooning, plasma cells and a +ANA. Yikes! USCAP 2016 Evening Specialty Conference Cynthia Guy
 Fat, ballooning, plasma cells and a +ANA. Yikes! USCAP 2016 Evening Specialty Conference Cynthia Guy Goals Share an interesting case Important because it highlights a common problem that we re likely to
Fat, ballooning, plasma cells and a +ANA. Yikes! USCAP 2016 Evening Specialty Conference Cynthia Guy Goals Share an interesting case Important because it highlights a common problem that we re likely to
MEETING PROSPECTUS. International Workshop on NASH Biomarkers
 International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
Photocure ASA Executing the Strategy
 Photocure ASA Executing the Strategy DECEMBER 6, 2012 KJETIL HESTDAL, CEO Disclaimer The information included in this Presentation contains certain forward-looking statements that address activities, events
Photocure ASA Executing the Strategy DECEMBER 6, 2012 KJETIL HESTDAL, CEO Disclaimer The information included in this Presentation contains certain forward-looking statements that address activities, events
NON-ALCOHOLIC FATTY LIVER DISEASE:
 NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
ArQule Jefferies Global Healthcare Conference June 2015
 ArQule Jefferies Global Healthcare Conference June 2015 1 ArQule Corporate Update Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning
ArQule Jefferies Global Healthcare Conference June 2015 1 ArQule Corporate Update Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning
July, ArQule, Inc.
 July, 2012 Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act with respect to clinical
July, 2012 Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act with respect to clinical
Oncology Therapeutics without Compromise APRIL 2011
 Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
Study 2 ( ) Pivotal Phase 3 Study Top-Line Results. October 29, 2018
 Study 2 (1002-047) Pivotal Phase 3 Study Top-Line Results October 29, 2018 Safe Harbor Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements
Study 2 (1002-047) Pivotal Phase 3 Study Top-Line Results October 29, 2018 Safe Harbor Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements
Pierre Legault CEO June 2, 2014
 April 2012 Pierre Legault CEO June 2, 2014 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
April 2012 Pierre Legault CEO June 2, 2014 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
Q1 Results 2018 Webcast presentation 26 April 2018
 Q1 Results 2018 Webcast presentation 26 April 2018 Disclaimer This presentation contains forward-looking statements, including (without limitation) statements concerning the progress of our clinical pipeline,
Q1 Results 2018 Webcast presentation 26 April 2018 Disclaimer This presentation contains forward-looking statements, including (without limitation) statements concerning the progress of our clinical pipeline,
Diagnostics for the early detection and prevention of colon cancer. Leerink Swann Global Health Care Conference February 2015
 Diagnostics for the early detection and prevention of colon cancer Leerink Swann Global Health Care Conference February 2015 Safe Harbor Statement Certain statements made in this news release contain forward-looking
Diagnostics for the early detection and prevention of colon cancer Leerink Swann Global Health Care Conference February 2015 Safe Harbor Statement Certain statements made in this news release contain forward-looking
Diagnosis and Management of PBC
 Diagnosis and Management of PBC Cynthia Levy, MD, FAASLD University of Miami Miller School of Medicine Miami, Florida 1 Primary Biliary Cholangitis (PBC) Chronic cholestatic liver disease Autoimmune in
Diagnosis and Management of PBC Cynthia Levy, MD, FAASLD University of Miami Miller School of Medicine Miami, Florida 1 Primary Biliary Cholangitis (PBC) Chronic cholestatic liver disease Autoimmune in
Fatty Liver Disease A growing epidemic
 Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Zafgen PWS Clinical Trial Program Overview. November 16, 2014
 Zafgen PWS Clinical Trial Program Overview November 16, 2014 2 Disclaimers Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information.
Zafgen PWS Clinical Trial Program Overview November 16, 2014 2 Disclaimers Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information.
AGM Presentation For the year to 30 September February 2016
 AGM Presentation For the year to 30 September 2015 25 February 2016 11 Forward Looking Statements This presentation includes forward-looking statements. These forward-looking statements involve known and
AGM Presentation For the year to 30 September 2015 25 February 2016 11 Forward Looking Statements This presentation includes forward-looking statements. These forward-looking statements involve known and
Annual Results 2017 & Business Update 13 April 2018
 Annual Results 2017 & Business Update 13 April 2018 1 Disclaimer These slides and the accompanying oral presentation contain forward-looking statements and information. Forward-looking statements are subject
Annual Results 2017 & Business Update 13 April 2018 1 Disclaimer These slides and the accompanying oral presentation contain forward-looking statements and information. Forward-looking statements are subject
Investor presentation. Bioshares Biotech Summit July 2017
 Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
PRESS RELEASE. Beaumont et al. Pancreas Journal 2012 ; 41(3) : / 5
 PRESS RELEASE Ipsen receives approval from European Commission for Xermelo (telotristat ethyl) for the treatment of carcinoid syndrome diarrhea in patients inadequately controlled by somatostatin analogue
PRESS RELEASE Ipsen receives approval from European Commission for Xermelo (telotristat ethyl) for the treatment of carcinoid syndrome diarrhea in patients inadequately controlled by somatostatin analogue
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017
 SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
Corporate Presentation June 2017
 Corporate Presentation June 2017 1 Safe Harbor & Disclaimer Statement This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995.
Corporate Presentation June 2017 1 Safe Harbor & Disclaimer Statement This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995.
Autoimmune and cholestatic liver diseases
 Autoimmune and cholestatic liver diseases Prof. Tom Hemming Karlsen Research Institute of Internal Medicine & Department of Transplantation Medicine University of Oslo & Oslo University Hospital, Norway
Autoimmune and cholestatic liver diseases Prof. Tom Hemming Karlsen Research Institute of Internal Medicine & Department of Transplantation Medicine University of Oslo & Oslo University Hospital, Norway
microrna Therapeutics Harnessing the power of micrornas to target multiple pathways of disease
 microrna Therapeutics Harnessing the power of micrornas to target multiple pathways of disease January 2018 Safe Harbor Statement Statements contained in this presentation regarding matters that are not
microrna Therapeutics Harnessing the power of micrornas to target multiple pathways of disease January 2018 Safe Harbor Statement Statements contained in this presentation regarding matters that are not
1 ST EUROPEAN. NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT.
 1 ST EUROPEAN NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT NEEDS ASSESSMENT NEEDS ASSESSMENT Non-alcoholic fatty liver disease (NAFLD) is an emerging liver disease affecting 25 % of
1 ST EUROPEAN NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT NEEDS ASSESSMENT NEEDS ASSESSMENT Non-alcoholic fatty liver disease (NAFLD) is an emerging liver disease affecting 25 % of
Diagnostics for the early detection and prevention of colorectal cancer.
 Diagnostics for the early detection and prevention of colorectal cancer. Company Presentation May 2013 Safe Harbor Statement Certain statements made in this presentation contain forward-looking statements
Diagnostics for the early detection and prevention of colorectal cancer. Company Presentation May 2013 Safe Harbor Statement Certain statements made in this presentation contain forward-looking statements
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH
 Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
33 rd Annual J.P. Morgan Healthcare Conference. January 2015
 33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update. August 2, 2018
 Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update August 2, 2018 FORWARD LOOKING STATEMENT This presentation includes forward-looking statements within the meaning of the Private Securities
Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update August 2, 2018 FORWARD LOOKING STATEMENT This presentation includes forward-looking statements within the meaning of the Private Securities
At Least 1 in 5 Patients in Your Practice Have Fatty Liver
 At Least 1 in 5 Patients in Your Practice Have Fatty Liver What Can You Tell Your Patients Magnus McLeod MD FRCPC Assistant Professor Dalhousie University 30-NOV-2017 NAFLD Non-Alcoholic Fatty Liver Disease
At Least 1 in 5 Patients in Your Practice Have Fatty Liver What Can You Tell Your Patients Magnus McLeod MD FRCPC Assistant Professor Dalhousie University 30-NOV-2017 NAFLD Non-Alcoholic Fatty Liver Disease
Corporate Presentation
 Corporate Presentation February 2, 2017 NASDAQ: GALT www.galectintherapeutics.com 2017 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Corporate Presentation February 2, 2017 NASDAQ: GALT www.galectintherapeutics.com 2017 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
ADAPTIMMUNE INVESTOR PRESENTATION. August 2016
 ADAPTIMMUNE INVESTOR PRESENTATION August 2016 DISCLAIMER This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
ADAPTIMMUNE INVESTOR PRESENTATION August 2016 DISCLAIMER This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
AESOP Overview and Inclusion/Exclusion Criteria Richard Pencek, PhD
 747-207 AESOP Overview and Inclusion/ Criteria Richard Pencek, PhD Sr Director, Clinical Research, Intercept Pharmaceuticals, Inc. 2 PSC Forum 2 AESOP: A Phase 2 Randomized, Placebo-Controlled Trial, Dose-Finding
747-207 AESOP Overview and Inclusion/ Criteria Richard Pencek, PhD Sr Director, Clinical Research, Intercept Pharmaceuticals, Inc. 2 PSC Forum 2 AESOP: A Phase 2 Randomized, Placebo-Controlled Trial, Dose-Finding
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic
 Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
The Lipid Metabolism Company. Corporate Presentation March 2018
 The Lipid Metabolism Company Corporate Presentation March 2018 Disclaimer This document has been prepared by Cerenis Therapeutics (the "Company") and is for information purposes only. The information and
The Lipid Metabolism Company Corporate Presentation March 2018 Disclaimer This document has been prepared by Cerenis Therapeutics (the "Company") and is for information purposes only. The information and
PBC treatment: the present and future. Maggie Bassendine Professor of Hepatology
 PBC treatment: the present and future Maggie Bassendine Professor of Hepatology Primary biliary cirrhosis 20-25yrs OLT/ Death Symptoms: Fatigue, itching, jaundice Autoimmune disease: Focal small bile duct
PBC treatment: the present and future Maggie Bassendine Professor of Hepatology Primary biliary cirrhosis 20-25yrs OLT/ Death Symptoms: Fatigue, itching, jaundice Autoimmune disease: Focal small bile duct
Developing Xanamem for Alzheimer s Dementia
 Developing Xanamem for Alzheimer s Dementia Dr. Bill Ketelbey CEO, Actinogen Medical Investor Presentation March 2015 Forward Statements This presentation has been prepared by Actinogen Limited. ( Actinogen
Developing Xanamem for Alzheimer s Dementia Dr. Bill Ketelbey CEO, Actinogen Medical Investor Presentation March 2015 Forward Statements This presentation has been prepared by Actinogen Limited. ( Actinogen
