PRODUCT MONOGRAPH. (Pyridostigmine Bromide Tablets) Antimyasthenic - Cholinergic
|
|
|
- Eleanor Charles
- 5 years ago
- Views:
Transcription
1 1 PRODUCT MONOGRAPH Pr MESTINON Tablets, 60 mg Pr MESTINON -SR (Slow-Release) Tablets, 180 mg (Pyridostigmine Bromide Tablets) Antimyasthenic - Cholinergic Valeant Canada LP Prepared: September 28, St-Elzear Blvd W Laval, Revised: September 9, 2014 Quebec H7L 4A8, Canada Control#:
2 2 PRODUCT MONOGRAPH Pr MESTINON Tablets, 60 mg Pr MESTINON -SR (Slow-Release) Tablets, 180 mg (Pyridostigmine Bromide Tablets) Antimyasthenic - Cholinergic ACTIONS: Pyridostigmine is a cholinergic agent which acts primarily by the inhibition of cholinesterase. It enhances cholinergic action by facilitating the transmission of impulses across neuromuscular junctions. It also has a direct cholinomimetic effect on skeletal muscle and possibly on autonomic ganglion cells and neurons of the central nervous system. Because of its quaternary ammonium structure, moderate doses of pyridostigmine do not cross the blood-brain barrier to produce CNS effects. Extremely high doses, however, produce CNS stimulation followed by CNS depression, in addition to a depolarizing neuromuscular blockade. Pyridostigmine is an analog of neostigmine. However, it differs from neostigmine in certain clinically significant respects; for example, pyridostigmine is more effectively absorbed from the alimentary tract than is neostigmine; with equipotent doses, pyridostigmine has a slower onset and longer duration of action, and produces fewer gastrointestinal side effects than neostigmine. After oral administration, Mestinon
3 3 generally has an onset of action of 20 minutes and a duration of action of approximately 6 hours; as for Mestinon -SR, it has an onset of action of 30 to 60 minutes and a duration of action of 6 to 12 hours. INDICATIONS: Mestinon and Mestinon -SR are indicated for the symptomatic treatment of myasthenia gravis. In acute myasthenic crises where difficulty in breathing and swallowing is present, the parenteral form should be used. The patient can be transferred to the oral form as soon as it can be tolerated. CONTRAINDICATIONS: Mestinon and Mestinon -SR are contraindicated in patients with known hypersensitivity to anticholinesterase agents. Because of the presence of the bromide ion, they should not be used in patients with a prior history of reaction to bromides. They are also contraindicated in patients with peritonitis or mechanical obstruction of the intestinal or urinary tract. WARNINGS: Mestinon and Mestinon -SR should be used with caution in patients with epilepsy, bronchial asthma, bradycardia, recent coronary occlusion, vagotonia, hyperthyroidism, cardiac arrhythmias or peptic ulcer. Large oral doses of the drug should be avoided in patients with megacolon or decreased gastrointestinal motility. In these patients, the drug may accumulate and result in toxicity when gastrointestinal motility is restored.
4 4 PRECAUTIONS: General: Although failure of patients to show clinical improvement may reflect underdosage, it can also be indicative of overdosage. It is important to differentiate between myasthenic crisis and cholinergic crisis caused by overdosage of Mestinon or Mestinon -SR. Both conditions result in extreme muscle weakness but require radically different treatment. (See Overdosage Section) Information for Patients: Complete restoration of muscle strength is rare in myasthenia gravis, and patients should be cautioned not to increase their dose, in an attempt to relieve their symptoms, without consulting their physician. The patient should be encouraged to keep a daily record of his or her condition to assist the physician in determining an optimal therapeutic regimen. Drug Interactions: Atropine antagonizes the muscarinic effects of pyridostigmine and this interaction may be utilized to counteract the effects of pyridostigmine. (See Overdosage Section) Pyridostigmine bromide does not antagonize, and in fact may prolong the phase I block of depolarizing muscle relaxants such as succinylcholine or decamethonium. Certain antibiotics, especially neomycin, streptomycin and kanamycin, have a mild but definite nondepolarizing blocking action which may accentuate neuromuscular block. These antibiotics should be used in the myasthenic patient only where definitely
5 5 indicated, and then careful adjustment should be made of adjunctive anticholinesterase dosage. Local and some general anesthetics, antiarrhythmic agents and other drugs that interfere with neuromuscular transmission should be used cautiously, if at all, in patients with myasthenia gravis; the dose of pyridostigmine bromide may have to be increased accordingly. In severe myasthenia gravis, neostigmine has been used in combination with pyridostigmine to provide the benefits of short and long-term activity; because of the possibility of reduced intestinal motility and increased toxicity, this combination should be used only under strict medical supervision. Carcinogenesis, Mutagenesis and Impairment of Fertility: Carcinogenicity and mutagenicity studies have not been performed with pyridostigmine bromide. A fertility and general reproductive performance study was performed in rats at dosages of 15 and 40 mg/kg/day. There were no adverse effects on pregnancy rate, average number of implantation sites, average number of embryos per dam, percent resorptions, duration of gestation, litter size, pup viability or pup growth. Pregnancy: Teratogenic effects: Pregnancy category B Reproductive studies have been performed in rats at dosages up to 40 mg/kg/day (2 times the maximum recommended human dose; 4.6 times the average recommended
6 6 dose). These studies have revealed no evidence of impaired fertility or harm to the fetus due to pyridostigmine bromide. There are, however, no adequate and well controlled studies in pregnant women. However, pyridostigmine bromide, like other cholinesterase inhibitors, contains a quaternary ammonium and, therefore, would be expected to cross the placenta only to a limited extent. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Non-teratogenic effects: Of newborn infants whose mothers have received anticholinesterase drugs for treatment of myasthenia gravis, 10 to 20 percent were observed to have transient muscular weakness. Nursing Mothers: It is not known whether pyridostigmine bromide is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions from pyridostigmine in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Pediatric Use: See Dosage and Administration Section.
7 7 ADVERSE REACTIONS: Side effects are generally due to an exaggeration of pharmacological effects of which increased salivation and fasciculation are the most common. Abdominal cramps and diarrhea may also occur. The following additional adverse reactions have been reported following the use of Mestinon and Mestinon -SR: Respiratory: Gastrointestinal: Musculoskeletal: Dermatologic: Miscellaneous: Increased bronchial secretions. Nausea, vomiting, increased peristalsis. Muscle cramps. Urticaria, rash. Miosis, diaphoresis, weakness, allergic reactions. SYMPTOMS AND TREATMENT OF OVERDOSAGE: As is true of all anticholinesterase agents, overdosage of Mestinon or Mestinon -SR can cause cholinergic crisis, which is characterized by increasing muscle weakness and which, through involvement of the muscles of respiration, may result in death. Myasthenic crisis, due to an increase in the severity of the disease, is also accompanied by extreme muscle weakness, and thus may be difficult to distinguish from cholinergic crisis on a symptomatic basis. However, such differentiation is extremely important, because increases in the dose of pyridostigmine bromide or other drugs in this class in the presence of cholinergic crisis or of a refractory or "insensitive" state could have grave consequences. The two types of crises may be differentiated by the use of Tensilon (edrophonium chloride) as well as by clinical judgment.
8 8 Treatment of the two conditions differs radically. Whereas the presence of myasthenic crisis requires more intensive anticholinesterase therapy, cholinergic crisis calls for the prompt withdrawal of all drugs of this type. The immediate use of atropine in cholinergic crisis is also recommended. A syringe containing 1 mg of atropine sulfate should be immediately available to be given in aliquots intravenously to counteract severe cholinergic reactions. Atropine also may be used to abolish or minimize gastrointestinal side effects or other muscarinic reactions; but such use, by masking signs of overdosage, can lead to inadvertent induction of cholinergic crisis. DOSAGE AND ADMINISTRATION: The dosage, route and frequency of administration depend on the requirements and clinical response of the patients. The dosage schedule should be adjusted for each patient and changed as the need arises. Dosage requirements in patients with myasthenia gravis may vary from day to day, according to remissions and exacerbations of the disease and the physical and emotional stress suffered by the patient. Larger portions of the label daily dose may be given at times when the patient is more prone to fatigue (afternoon, mealtimes, etc.). In the initial treatment of myasthenia gravis, oral Mestinon and Mestinon -SR should be started at a dosage smaller than that required to produce maximum strength, and daily dosage gradually increased at intervals of 48 hours or more. Changes in oral dosage may take several days to show results. When a further increase in dosage produces no corresponding increase in muscle strength, dosage should be reduced to
9 9 the previous level so that the patient receives the smallest dose necessary to produce maximum strength. Note: For information on a diagnostic test for myasthenia gravis, and for the evaluation and stabilization of anticholinesterase therapy, see Product Monograph on Tensilon (edrophonium chloride injection). The immediate effect of a Mestinon -SR 180 mg tablet is about equal to that of a 60 mg conventional tablet; however, the duration of drug action, although varying in individual patients, averages 2 ½ times that of a 60 mg dose. One to three 180 mg tablets, once or twice daily (180 mg to 1.08 g a day), will usually be sufficient to control symptoms; however, the needs of individual patients may vary markedly from this average. For optimal control, it may be necessary to use conventional tablets or syrup in conjunction with Mestinon -SR therapy. Mestinon -SR tablets are particularly useful for bedtime administration in patients who are very weak upon awakening. Mestinon and Mestinon -SR tablets should be swallowed whole. Do not crush. However, in certain cases, Mestinon and Mestinon -SR tablets, can be cut in half; but Mestinon -SR tablets should not be crushed or quartered since this would destroy too much of the sustained release matrix. Due to the slow-release mechanism of the tablet, the matrix may pass through the intestinal system intact. However, it should be noted that the medicinal ingredient has
10 10 been released through the gastro-intestinal tract over an 8 to 12 hour passing time and only the matrix is rejected. HOW SUPPLIED: Mestinon, white, flat compressed tablet, cross-scored on one side and embossed MESTINON 60 - V on the other contains 60 mg of pyridostigmine bromide. Nonmedicinal ingredients: lactose, silicone dioxide and stearic acid. Energy: 4.6 kj (1.1 kcal). Gluten-, paraben-, sodium-, sulfite- and tartrazine-free. Bottles of 100. Mestinon -SR capsule-shaped, flattened on two sides with a single score on one face, light straw coloured tablets, embossed MES V 180, each containing 180 mg pyridostigmine bromide. Nonmedicinal ingredients: calcium phosphate, carnauba wax, isopropyl alcohol and magnesium stearate. Energy: 2.3 kj (0.5 kcal). Gluten-, lactose-, paraben-, sodium-, sulfite-, and tartrazine-free. Bottles of 30. Store in a dry place between 15 and 30 C (59 and 86 F), in a well-closed container with the desiccant enclosed. Note: Because of the hygroscopic nature of the Mestinon and Mestinon -SR tablets, mottling may occur. This does not affect their efficacy.
11 11 CHEMISTRY AND PHARMACOLOGY: Chemical Name: 3-Hydroxy-1-methylpyridinium bromide dimethylcarbamate Structural Formula: CH 3 N O Br O N CH 3 CH 3 Molecular Formula: C 9 H 13 BrN 2 O 2 Molecular Weight: Daltons Description: Pyridostigmine Bromide is a hygroscopic, white or practically white, crystalline powder having an agreeable, characteristic odour. It is freely soluble in water, in alcohol, and in chloroform; slightly soluble in solvent hexane; and practically insoluble in ether. Pharmacology: Pyridostigmine inhibits the hydrolysis of acetylcholine by competing with acetylcholine for attachment to acetylcholinesterase at sites of cholinergic transmission. The pyridostigmine-enzyme complex is hydrolysed at a much slower rate than the acetylcholine-enzyme complex, resulting in accumulation of acetylcholine at cholinergic synapses with prolonged and exaggerated effects. The generalized cholinergic responses produced by pyridostigmine include miosis, increased tonus of intestinal and skeletal musculature, constriction of bronchi and ureters, bradycardia, and stimulation of secretion by salivary and sweat glands.
12 12 Following oral administration of Mestinon -SR, the mean peak pyridostigmine plasma concentration is reached by 2 hours. The remainder of the dose is released over 8-12 hours. When administered concomitantly with food, the time to reach mean peak plasma concentration can be increased to about 3 hours; however, the extent of absorption of pyridostigmine is not affected. Pyridostigmine does not bind to plasma protein. The drug has been reported to cross the placenta and, after large oral doses, to decrease fetal plasma cholinesterase activity. Animals administered radioactive-labelled pyridostigmine orally showed presence of radioactivity in most tissues except brain, fat, thymus, and intestinal wall. In a comparative pharmacokinetic study, determined in 10 healthy subjects given pyridostigmine bromide (4 mg i.v. over 30 minutes, and 60 mg orally), the oral availability as measured by the AUC ratio was 11.5% to 18.9% ( =14.3%). The mean t ½ of the plasma level decline after oral dosing was 200 minutes, twice as long as the terminal elimination t ½ after intravenous infusion (97 minutes), indicating that absorption may proceed at a slower rate than elimination. Pyridostigmine not only undergoes hydrolysis by cholinesterases but is also metabolized by microsomal enzymes in the liver. The major metabolite of pyridostigmine is 3-hydroxy-N-methylpyridinium. Patients with severe myasthenia gravis appear to metabolize and excrete the drug faster than patients having a milder form of the disease. Pyridostigmine and its metabolites are excreted in the urine by tubular secretion. Approximately 10% of the administered dose is excreted in the urine
13 13 as intact drug in 24 hours, but a considerable individual variation in urinary excretion patterns has been shown by patients with myasthenia gravis. TOXICITY: The acute toxicity of pyridostigmine bromide in mice and in rats is summarized in the following table: ACUTE TOXICITY ANIMAL ROUTE LD 50 S.E. SYMPTOMS Mice i.v mg/kg Tremors, Straub reaction, exophthalmia Mice i.m mg/kg Tremors, exophthalmia, salivation (watery), lacrimation, respiratory failure Rats Rats i.v. i.m mg/kg mg/kg Tremors, piloerection, lacrimation, bloody tears, respiratory failure Salivation, tremors, lacrimation, bloody tears The LD 50 for pyridostigmine in rats fed by the oral route is reported to be 86 mg/kg body weight. An assessment of the morphological changes in rats caused by pyridostigmine has shown that respiratory failure occurred within 1 hour and resulted in death in four of the six animals that received a single oral dose of 80 mg/kg body weight. No
14 14 spontaneous death occurred in animals given a single dose of 20 to 40 mg of Pyridostigmine per kg of body weight, although these doses caused a marked reduction of the acetylcholinesterase activity in the whole blood and in the erythrocytes. The histological findings in the skeletal muscles of these animals revealed, particularly in the diaphragm, unequivocal, severe lesions within 24 hours, characterized by disseminated single fibre necrosis or necrosis of grouped fibres associated with infiltrates of polymorphonuclear neutrophilic granulocytes, lymphoid and histiocytic cells. The staining of the motor endplates in areas where necrosis occurred revealed marked changes of the nerve endings: their ramifications were mostly rarefied, shortened, and plump.
15 15 REFERENCES: 1. American Hospital Formulary Service, Washington, D.C., The American Society of Hospital Pharmacists, Aquilonius S.M., et al. Pharmacokinetics and oral bioavailability of pyridostigmine in man. Eur J Clin Pharmacol 1980;18: Kornfeld P. et al. Metabolism of 14 C labeled pyridostigmine in myasthenia gravis. Neurology 1970; 20: Chan K. et al. The isolation and determination of neostigmine, pyridostigmine and their metabolites in human biological fluids. J Pharmacol Methods 1978; 1: Kornfeld P. et al. Studies in myasthenia gravis: pyridostigmine-c 14 metabolism after thymectomy. Neurology 1975; 25: Somani S.M. et al. Pyridostigmine metabolism in man. Clin Pharmacol Therap 1972; 13: Hoar R.M. and Woo D. Reproduction studies of pyridostigmine bromide in rats. RCR N-22291, February 11, Adamsons Jr. K., and Joelson I. The effects of pharmacologic agents upon the fetus and newborn. Am J Obst and Gynecol 1966; 96: McNall P.G., and Jafarnia M. Management of myasthenia gravis in the obstetrical patient. Am J Obst and Gynecol 1965; 92: Breyer-Pfatt U., et al. Pyridostigmine kinetics in heathy subjects and patients with myasthenia gravis. Clin Pharmacol Therap 1985; 37: Fromherz K., and Pellmont B. Pharmakologische Wirkungen des MESTINON "Roche" (Dimethylcarbaminsaureester des-1-methyl-3-oxypyridinium-bromid; Pyridotigmin bromid). Schweiz Med Wschr 1953; 49:
16 Gebbers J-O, et al. Acute toxicity of pyridostigmine in rats: histological findings. Arch Toxicol 1986; 58:
PROSTIGMIN (neostigmine bromide)
 PROSTIGMIN (neostigmine bromide) TABLETS DESCRIPTION: Prostigmin (neostigmine bromide), an anticholinesterase agent, is available for oral administration in 15 mg tablets. Each tablet also contains gelatin,
PROSTIGMIN (neostigmine bromide) TABLETS DESCRIPTION: Prostigmin (neostigmine bromide), an anticholinesterase agent, is available for oral administration in 15 mg tablets. Each tablet also contains gelatin,
MESTINON NAME OF THE MEDICINE. Description: Pharmacology: Pyridostigmine Bromide. CAS number:
 MESTINON NAME OF THE MEDICINE Pyridostigmine Bromide. C9H13BrN2O2 CAS number: 101-26-8 Description: Mestinon contains, as active substance, pyridostigmine bromide, chemically described as the dimethylcarbamic
MESTINON NAME OF THE MEDICINE Pyridostigmine Bromide. C9H13BrN2O2 CAS number: 101-26-8 Description: Mestinon contains, as active substance, pyridostigmine bromide, chemically described as the dimethylcarbamic
NEW ZEALAND DATA SHEET. Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution containing 2.5 mg/ml Neostigmine methylsulphate.
 NEW ZEALAND DATA SHEET NAME OF MEDICINE NEOSTIGMINE METHYLSULPHATE INJECTION B.P. Solution for injection 2.5 mg/ml. PRESENTATIONS Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution
NEW ZEALAND DATA SHEET NAME OF MEDICINE NEOSTIGMINE METHYLSULPHATE INJECTION B.P. Solution for injection 2.5 mg/ml. PRESENTATIONS Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution
DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related to acetylcholine.
 BETHANECHOL CHLORIDE TABLETS, USP 5 mg, 10 mg, 25 mg and 50 mg Rx only DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related
BETHANECHOL CHLORIDE TABLETS, USP 5 mg, 10 mg, 25 mg and 50 mg Rx only DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 AUSTRALIAN PRODUCT INFORMATION NEOSTIGMINE INJECTION BP (NEOSTIGMINE METHYLSULFATE) 1 NAME OF THE MEDICINE Neostigmine methylsulfate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml sterile solution of
AUSTRALIAN PRODUCT INFORMATION NEOSTIGMINE INJECTION BP (NEOSTIGMINE METHYLSULFATE) 1 NAME OF THE MEDICINE Neostigmine methylsulfate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml sterile solution of
PRODUCT MONOGRAPH. Edrophonium Chloride Injection, USP. 10 mg/ml. Nondepolarizing Neuromuscular Antagonist
 PRODUCT MONOGRAPH Pr TENSILON Edrophonium Chloride Injection, USP 10 mg/ml Nondepolarizing Neuromuscular Antagonist Valeant Canada LP 2150 St-Elzear Blvd., West Laval, Quebec H7L 4A8 Canada Date of Preparation:
PRODUCT MONOGRAPH Pr TENSILON Edrophonium Chloride Injection, USP 10 mg/ml Nondepolarizing Neuromuscular Antagonist Valeant Canada LP 2150 St-Elzear Blvd., West Laval, Quebec H7L 4A8 Canada Date of Preparation:
CLINICAL PHARMACOLOGY
 robaxin / robaxin -7 50 (methocarbamol tablets, USP) Rx Only DESCRIPT ION robaxin /robaxin -750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant
robaxin / robaxin -7 50 (methocarbamol tablets, USP) Rx Only DESCRIPT ION robaxin /robaxin -750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant
IPRAVENT Respules/Respirator solution (Ipratropium bromide)
 Published on: 19 Sep 2014 IPRAVENT Respules/Respirator solution (Ipratropium bromide) Composition IPRAVENT Respules Each 2 ml contains: Ipratropium Bromide BP equivalent to Ipratropium Bromide (anhydrous)
Published on: 19 Sep 2014 IPRAVENT Respules/Respirator solution (Ipratropium bromide) Composition IPRAVENT Respules Each 2 ml contains: Ipratropium Bromide BP equivalent to Ipratropium Bromide (anhydrous)
Autonomic Pharmacology: Cholinergic agonists
 Autonomic Pharmacology: Cholinergic agonists Öner Süzer www.onersuzer.com osuzer@istanbul.edu.tr Last update: 23.01.2013 1 Acetylcholine Acetylcholine (ACh), the naturally occurring neurotransmitter for
Autonomic Pharmacology: Cholinergic agonists Öner Süzer www.onersuzer.com osuzer@istanbul.edu.tr Last update: 23.01.2013 1 Acetylcholine Acetylcholine (ACh), the naturally occurring neurotransmitter for
PRESCRIBING INFORMATION PRODUCT MONOGRAPH MANDELAMINE*
 PRESCRIBING INFORMATION PRODUCT MONOGRAPH MANDELAMINE* (Methenamine Mandelate Tablets USP) 500 mg Urinary Antibacterial DATE OF PREPARATION : 16-Aug-2005 8250 Décarie Blvd, suite 110 Montréal, QC Canada,
PRESCRIBING INFORMATION PRODUCT MONOGRAPH MANDELAMINE* (Methenamine Mandelate Tablets USP) 500 mg Urinary Antibacterial DATE OF PREPARATION : 16-Aug-2005 8250 Décarie Blvd, suite 110 Montréal, QC Canada,
Cholinergic receptors( cholinoceptors ) are two families muscarinic and nicotinic depending on their affinities to cholinomimetic agents(agents that
 Cholinergic drugs Cholinergic drugs Are drugs act on receptors that are activated by acetylcholine(ach) which is the neurotransmitter of the parasympathetic nervous system. ACH is synthesized in the cholinergic
Cholinergic drugs Cholinergic drugs Are drugs act on receptors that are activated by acetylcholine(ach) which is the neurotransmitter of the parasympathetic nervous system. ACH is synthesized in the cholinergic
SUCRALFATE TABLETS, USP
 1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
למידע נוסף ניתן לעיין בעלון לרופא באתר משרד הבריאות או ליצור קשר טלפוני בטלפון
 חברת פרמה מדיס שמחה לבשר על תחילת שיווקו של תכשיר חדש. שם התכשיר: NEOSTIGMINE - HAMELN 2.5 MG/ML INJECTION צורת מינון: SOLUTION FOR INJECTION דרך מתן: I.M, I.V, S.C הרכב )חומר פעיל(: NEOSTIGMINE METHYLSULFATE
חברת פרמה מדיס שמחה לבשר על תחילת שיווקו של תכשיר חדש. שם התכשיר: NEOSTIGMINE - HAMELN 2.5 MG/ML INJECTION צורת מינון: SOLUTION FOR INJECTION דרך מתן: I.M, I.V, S.C הרכב )חומר פעיל(: NEOSTIGMINE METHYLSULFATE
PRESCRIBING INFORMATION mg primaquine phosphate equivalent to 15 mg primaquine base. Antimalarial
 PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
LESSON ASSIGNMENT. LESSON OBJECTIVES 9-1. Given a group of statements, select the statement that best describes the term cholinergic agent.
 LESSON ASSIGNMENT LESSON 9 Cholinergic Agents. TEXT ASSIGNMENT Paragraphs 9-1 through 9-6. LESSON OBJECTIVES 9-1. Given a group of statements, select the statement that best describes the term cholinergic
LESSON ASSIGNMENT LESSON 9 Cholinergic Agents. TEXT ASSIGNMENT Paragraphs 9-1 through 9-6. LESSON OBJECTIVES 9-1. Given a group of statements, select the statement that best describes the term cholinergic
Summary of Product Characteristics
 Translation from Latvian Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT Neiromidin 20 mg tablets Neiromidin 5 mg/ml solution for injection Neiromidin 15 mg/ml solution for injection
Translation from Latvian Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT Neiromidin 20 mg tablets Neiromidin 5 mg/ml solution for injection Neiromidin 15 mg/ml solution for injection
Immodium / loprarmide
 Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
SUCRALFATE TABLETS, USP
 1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
MOVICOL Junior Powder for Solution (macrogol 3350)
 MOVICOL Junior Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Product Description: Each sachet of MOVICOL Junior contains: Macrogol 3350 Sodium chloride Sodium bicarbonate Potassium chloride
MOVICOL Junior Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Product Description: Each sachet of MOVICOL Junior contains: Macrogol 3350 Sodium chloride Sodium bicarbonate Potassium chloride
Drugs Affecting the Autonomic Nervous System-2 Cholinergic agents
 Drugs Affecting the Autonomic Nervous System-2 Cholinergic agents Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Cholinergic agents, Cholinomimetic drugs,
Drugs Affecting the Autonomic Nervous System-2 Cholinergic agents Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Cholinergic agents, Cholinomimetic drugs,
Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml
 New Zealand Datasheet Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml Solution for injection Glycopyrronium bromide 0.5 mg/ml & Neostigmine metilsulfate 2.5 mg/ml Presentation is
New Zealand Datasheet Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml Solution for injection Glycopyrronium bromide 0.5 mg/ml & Neostigmine metilsulfate 2.5 mg/ml Presentation is
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
MOVICOL Junior Chocolate Flavour Powder for Solution (macrogol 3350)
 MOVICOL Junior Chocolate Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Chocolate Flavour Product Description: Each sachet of MOVICOL Junior Chocolate contains: Macrogol 3350
MOVICOL Junior Chocolate Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Chocolate Flavour Product Description: Each sachet of MOVICOL Junior Chocolate contains: Macrogol 3350
MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350)
 MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350) NAME OF THE MEDICINE: MOVICOL Liquid Orange Flavour, Concentrate for Oral Solution. DESCRIPTION: A clear colourless solution.
MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350) NAME OF THE MEDICINE: MOVICOL Liquid Orange Flavour, Concentrate for Oral Solution. DESCRIPTION: A clear colourless solution.
Lorazepam Tablets, USP
 Lorazepam Tablets, USP DESCRIPTION: Lorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H -1,4-benzodiazepin-2-one: Cl H N N O Cl OH It is a white
Lorazepam Tablets, USP DESCRIPTION: Lorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H -1,4-benzodiazepin-2-one: Cl H N N O Cl OH It is a white
PRODUCT INFORMATION. Colistin Link. Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial
 PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
PHENOXYBENZAMINE HYDROCHLORIDE Capsules USP
 PHENOXYBENZAMINE HYDROCHLORIDE Capsules USP Rx only DESCRIPTION Each Phenoxybenzamine Hydrochloride Capsule USP, with red cap and body, is imprinted with "54 036", and contains 10 mg of phenoxybenzamine
PHENOXYBENZAMINE HYDROCHLORIDE Capsules USP Rx only DESCRIPTION Each Phenoxybenzamine Hydrochloride Capsule USP, with red cap and body, is imprinted with "54 036", and contains 10 mg of phenoxybenzamine
DESCRIPTION: Each tablet for oral administration contains:
 HYOSCYAMINE SULFATE 0.125 MG TABLETS Rx Only 1 2 3 4 DESCRIPTION: Each tablet for oral administration contains: I-Hyoscyamine sulfate... 0.125 mg Inactive ingredients include: green dye, lactose monohydrate,
HYOSCYAMINE SULFATE 0.125 MG TABLETS Rx Only 1 2 3 4 DESCRIPTION: Each tablet for oral administration contains: I-Hyoscyamine sulfate... 0.125 mg Inactive ingredients include: green dye, lactose monohydrate,
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets
 PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker
 PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350)
 MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Lemon-Lime Flavour Product Description: Each sachet of MOVICOL Lemon-Lime contains: Macrogol 3350 Sodium chloride Sodium
MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Lemon-Lime Flavour Product Description: Each sachet of MOVICOL Lemon-Lime contains: Macrogol 3350 Sodium chloride Sodium
ROSOBAC-1GM / ROSOBAC-FORT
 ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
AUSTRALIAN PRODUCT INFORMATION APO-MEBEVERINE. Mebeverine hydrochloride
 1 NAME OF THE MEDICINE Mebeverine hydrochloride AUSTRALIAN PRODUCT INFORMATION APO-MEBEVERINE Mebeverine hydrochloride 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each APO-MEBEVERINE tablet contains mebeverine
1 NAME OF THE MEDICINE Mebeverine hydrochloride AUSTRALIAN PRODUCT INFORMATION APO-MEBEVERINE Mebeverine hydrochloride 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each APO-MEBEVERINE tablet contains mebeverine
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
Cholinergic antagonists
 Cholinergic antagonists Cholinergic antagonists: They are also called anticholinergic drugs or cholinergic blockers, this group include: 1.Antimuscarinic agents ( atropine, ipratropium, scopolamine) 2.
Cholinergic antagonists Cholinergic antagonists: They are also called anticholinergic drugs or cholinergic blockers, this group include: 1.Antimuscarinic agents ( atropine, ipratropium, scopolamine) 2.
New Zealand Data Sheet
 New Zealand Data Sheet ATROPT EYE DROPS Atropine Sulfate Presentation ATROPT EYE DROPS contains atropine Sulfate (1%) in a sterile aqueous base. A clear, or almost clear, slightly viscous, colourless liquid
New Zealand Data Sheet ATROPT EYE DROPS Atropine Sulfate Presentation ATROPT EYE DROPS contains atropine Sulfate (1%) in a sterile aqueous base. A clear, or almost clear, slightly viscous, colourless liquid
Leucovorin Calcium Tablets USP, 5 mg, 10 mg, 15 mg, and 25 mg
 Leucovorin Calcium Tablets USP, 5 mg, 10 mg, 15 mg, and 25 mg Rx only DESCRIPTION Leucovorin Calcium Tablets USP contain either 5 mg, 10 mg, 15 mg or 25 mg leucovorin as the calcium salt of N-[4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]
Leucovorin Calcium Tablets USP, 5 mg, 10 mg, 15 mg, and 25 mg Rx only DESCRIPTION Leucovorin Calcium Tablets USP contain either 5 mg, 10 mg, 15 mg or 25 mg leucovorin as the calcium salt of N-[4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]
PRODUCT MONOGRAPH ZONALON CREAM 5% GENERIC NAME DOXEPIN HYDROCHLORIDE CREAM 5% THERAPEUTIC CLASSIFICATION TOPICAL ANTIPRURITIC
 PRODUCT MONOGRAPH ZONALON CREAM 5% GENERIC NAME DOXEPIN HYDROCHLORIDE CREAM 5% THERAPEUTIC CLASSIFICATION TOPICAL ANTIPRURITIC Valeant Canada LP. Date of Preparation: Montreal, Quebec March 19, 2013 H4R
PRODUCT MONOGRAPH ZONALON CREAM 5% GENERIC NAME DOXEPIN HYDROCHLORIDE CREAM 5% THERAPEUTIC CLASSIFICATION TOPICAL ANTIPRURITIC Valeant Canada LP. Date of Preparation: Montreal, Quebec March 19, 2013 H4R
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
MOVICOL HALF PI December MOVICOL-Half. Powder for Solution (macrogol 3350) Potassium 5.4 mmol/l. Bicarbonate 17 mmol/l
 MOVICOL -Half Powder for Solution (macrogol 3350) Product Name: Product Description: MOVICOL-Half Each sachet of MOVICOL-Half contains: Macrogol 3350 6.563 g Sodium chloride 175.4 mg Sodium bicarbonate
MOVICOL -Half Powder for Solution (macrogol 3350) Product Name: Product Description: MOVICOL-Half Each sachet of MOVICOL-Half contains: Macrogol 3350 6.563 g Sodium chloride 175.4 mg Sodium bicarbonate
Ganglionic Blockers. Ganglion- blocking agents competitively block the action of
 Ganglionic Blockers Ganglion- blocking agents competitively block the action of acetylcholine and similar agonists at nicotinic (Nn) receptors of both parasympathetic and sympathetic autonomic ganglia.
Ganglionic Blockers Ganglion- blocking agents competitively block the action of acetylcholine and similar agonists at nicotinic (Nn) receptors of both parasympathetic and sympathetic autonomic ganglia.
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
Active ingredients: Metoclopramide Hydrochloride mg Equivalent to metoclopramide hydrochloride anhydrous mg
 Name Primperan 10 mg / 2 ml Metoclopramide hydrochloride anhydrous Solution for I.M. or I.V. injection (Ampoules) Composition Each 2 ml ampoule contains: Active ingredients: Metoclopramide Hydrochloride.
Name Primperan 10 mg / 2 ml Metoclopramide hydrochloride anhydrous Solution for I.M. or I.V. injection (Ampoules) Composition Each 2 ml ampoule contains: Active ingredients: Metoclopramide Hydrochloride.
CLINICAL PHARMACOLOGY
 Rev. Mar. 2017 DESCRIPTION Each five-sided dye free MOTOFEN tablet contains: 1 mg of difenoxin (equivalent to 1.09 mg of difenoxin hydrochloride) and 0.025 mg of atropine sulfate (equivalent to 0.01 mg
Rev. Mar. 2017 DESCRIPTION Each five-sided dye free MOTOFEN tablet contains: 1 mg of difenoxin (equivalent to 1.09 mg of difenoxin hydrochloride) and 0.025 mg of atropine sulfate (equivalent to 0.01 mg
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION Bacitracin USP (Bacitracin for Injection USP) For Topical or Intramuscular Use in Solution 50 000 IU Sterile Powder Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada Highway
PRESCRIBING INFORMATION Bacitracin USP (Bacitracin for Injection USP) For Topical or Intramuscular Use in Solution 50 000 IU Sterile Powder Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada Highway
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup.
 Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
is for submucosal injection only ie. local administration only (see
 HAEMOROL TM Phenol BP 5%w/v Presentation HAEMOROL TM is a clear, yellowish, viscous solution containing 5% w/v of Phenol BP in almond oil. Uses Actions Phenol is an antiseptic and disinfectant. It is also
HAEMOROL TM Phenol BP 5%w/v Presentation HAEMOROL TM is a clear, yellowish, viscous solution containing 5% w/v of Phenol BP in almond oil. Uses Actions Phenol is an antiseptic and disinfectant. It is also
Neuromuscular Junction
 Muscle Relaxants Neuromuscular Junction Cholinergic antagonists Neuromuscular-blocking agents (mostly nicotinic antagonists): interfere with transmission of efferent impulses to skeletal muscles. These
Muscle Relaxants Neuromuscular Junction Cholinergic antagonists Neuromuscular-blocking agents (mostly nicotinic antagonists): interfere with transmission of efferent impulses to skeletal muscles. These
Metopirone. (Metyrapone Capsules) 250 mg
 Metopirone (Metyrapone Capsules) 250 mg Diagnostic Test of Pituitary Adrenocorticotropic Function Rx only Prescribing Information DESCRIPTION Metopirone (metyrapone Capsules) 250 mg is an inhibitor of
Metopirone (Metyrapone Capsules) 250 mg Diagnostic Test of Pituitary Adrenocorticotropic Function Rx only Prescribing Information DESCRIPTION Metopirone (metyrapone Capsules) 250 mg is an inhibitor of
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
 AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
![PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate](/thumbs/81/82665370.jpg) NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
PRODUCT MONOGRAPH THEOLAIR. (theophylline anhydrous) Theolair Liquid 80 mg/15 ml. Bronchodilator. Date of Revision: February 13 th, 2013
 PRODUCT MONOGRAPH THEOLAIR (theophylline anhydrous) Theolair Liquid 80 mg/15 ml Bronchodilator Manufactured by: Valeant Canada LP 2150 St-Elzear Blvd., West Laval, Quebec H7L 4A8 Canada Date of Revision:
PRODUCT MONOGRAPH THEOLAIR (theophylline anhydrous) Theolair Liquid 80 mg/15 ml Bronchodilator Manufactured by: Valeant Canada LP 2150 St-Elzear Blvd., West Laval, Quebec H7L 4A8 Canada Date of Revision:
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
Strength: 60 mg Pack Size: 30/90/100/500 Tablets per bottle Revision No.: 00
 EMERGENCY OVERVIEW Each Pyridostigmine Bromide Tablets intended for oral administration contains Pyridostigmine Bromide and excipients generally considered to be non- toxic and non-hazardous in small quantities
EMERGENCY OVERVIEW Each Pyridostigmine Bromide Tablets intended for oral administration contains Pyridostigmine Bromide and excipients generally considered to be non- toxic and non-hazardous in small quantities
SUMMARY OF PRODUCT CHARACTERISTICS. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
PRESCRIBING INFORMATION Including Patient Medication Information. Colistimethate for Injection USP (colistimethate sodium)
 PRESCRIBING INFORMATION Including Patient Medication Information Pr Colistimethate for Injection USP (colistimethate sodium) Powder for Solution (equivalent to 150 mg colistin base) Antibiotic SteriMax
PRESCRIBING INFORMATION Including Patient Medication Information Pr Colistimethate for Injection USP (colistimethate sodium) Powder for Solution (equivalent to 150 mg colistin base) Antibiotic SteriMax
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
Core Safety Profile. Pharmaceutical form(s)/strength: Solution for Injection CZ/H/PSUR/0005/002 Date of FAR:
 Core Safety Profile Active substance: Rocuronium bromide Pharmaceutical form(s)/strength: Solution for Injection P-RMS: CZ/H/PSUR/0005/002 Date of FAR: 31.05.2012 4.3 Contraindications Hypersensitivity
Core Safety Profile Active substance: Rocuronium bromide Pharmaceutical form(s)/strength: Solution for Injection P-RMS: CZ/H/PSUR/0005/002 Date of FAR: 31.05.2012 4.3 Contraindications Hypersensitivity
2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Macrovic Junior powder for oral solution contains the following active ingredients:
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Macrovic Junior powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Macrovic Junior powder for oral solution
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Macrovic Junior powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Macrovic Junior powder for oral solution
NERVOUS SYSTEM NERVOUS SYSTEM. Somatic nervous system. Brain Spinal Cord Autonomic nervous system. Sympathetic nervous system
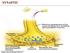 SYNAPTIC NERVOUS SYSTEM NERVOUS SYSTEM CENTRAL NERVOUS SYSTEM PERIPHERAL NERVOUS SYSTEM Brain Spinal Cord Autonomic nervous system Somatic nervous system Sympathetic nervous system Parasympathetic nervous
SYNAPTIC NERVOUS SYSTEM NERVOUS SYSTEM CENTRAL NERVOUS SYSTEM PERIPHERAL NERVOUS SYSTEM Brain Spinal Cord Autonomic nervous system Somatic nervous system Sympathetic nervous system Parasympathetic nervous
PRODUCT INFORMATION Panadeine EXTRA
 PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP
 PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PRODUCT INFORMATION LOFENOXAL
 PRODUCT INFORMATION LOFENOXAL Diphenoxylate hydrochloride 2.5 mg and Atropine sulfate 25 microgram Name of the medicines Diphenoxylate Hydrochloride: C30H32N2O2, HCl M. W. = 489.1 CAS registry no.: 3810-80-8
PRODUCT INFORMATION LOFENOXAL Diphenoxylate hydrochloride 2.5 mg and Atropine sulfate 25 microgram Name of the medicines Diphenoxylate Hydrochloride: C30H32N2O2, HCl M. W. = 489.1 CAS registry no.: 3810-80-8
50% Concentrated Injection
 NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
3 DOSAGE FORMS AND STRENGTHS
 PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
PARACOD Tablets (Paracetamol + Codeine phosphate)
 Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Compound Macrogol 13.72 g powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Compound Macrogol 13.72 g
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Compound Macrogol 13.72 g powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Compound Macrogol 13.72 g
PRESCRIBING INFORMATION. BaciJect. Bacitracin for injection U.S.P. Powder for Solution. 50,000 Units/Vial. Antibiotic
 PRESCRIBING INFORMATION BaciJect Bacitracin for injection U.S.P. Powder for Solution 50,000 Units/Vial Antibiotic SteriMax Inc. 2770 Portland Drive, Oakville, ON L6H 6R4 Date of Revision: July 30, 2018
PRESCRIBING INFORMATION BaciJect Bacitracin for injection U.S.P. Powder for Solution 50,000 Units/Vial Antibiotic SteriMax Inc. 2770 Portland Drive, Oakville, ON L6H 6R4 Date of Revision: July 30, 2018
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT {To be completed nationally} 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 mg tablets: each tablet contains 1 mg granisetron (as hydrochloride).
Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT {To be completed nationally} 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 mg tablets: each tablet contains 1 mg granisetron (as hydrochloride).
Chapter 18 Neuromuscular Blocking Agents Study Guide and Application Exercise
 Chapter 18 Neuromuscular Blocking Agents Study Guide and Application Exercise 1. Read chapter 2. Review objectives (p.305) 3. Review key terms and definitions (p.305) Add: Cholinesterase inhibitor Vagal
Chapter 18 Neuromuscular Blocking Agents Study Guide and Application Exercise 1. Read chapter 2. Review objectives (p.305) 3. Review key terms and definitions (p.305) Add: Cholinesterase inhibitor Vagal
PRODUCT INFORMATION. SUDAFED Sinus 12 Hour Relief Tablets
 PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
SUCRALFATE ISELPIN 1 g Tablet
 1.0 PHARMACOLOGIC CATEGORY CYTOPROTECTOR 2.0 DESCRIPTION SUCRALFATE ISELPIN 1 g Tablet Sucralfate is a white, amorphous powder which is soluble in strong acids and in alkalis but practically insoluble
1.0 PHARMACOLOGIC CATEGORY CYTOPROTECTOR 2.0 DESCRIPTION SUCRALFATE ISELPIN 1 g Tablet Sucralfate is a white, amorphous powder which is soluble in strong acids and in alkalis but practically insoluble
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT WICK Chesty Cough Syrup 200 mg/15 ml 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 15 ml syrup contains 200 mg guaifenesin. Each ml syrup
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT WICK Chesty Cough Syrup 200 mg/15 ml 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 15 ml syrup contains 200 mg guaifenesin. Each ml syrup
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fexofenadine hydrochloride 180 mg film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 180mg
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fexofenadine hydrochloride 180 mg film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 180mg
PHARMACEUTICAL INFORMATION AZILSARTAN
 AZEARLY Tablets Each Tablet Contains Azilsartan 20/40/80 mg PHARMACEUTICAL INFORMATION AZILSARTAN Generic name: Azilsartan Chemical name: 2-Ethoxy-1-{[2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-4-biphenylyl]methyl}-
AZEARLY Tablets Each Tablet Contains Azilsartan 20/40/80 mg PHARMACEUTICAL INFORMATION AZILSARTAN Generic name: Azilsartan Chemical name: 2-Ethoxy-1-{[2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-4-biphenylyl]methyl}-
Nervous System. Peripheral Nervous System ( PNS ) Central Nervous System ( CNS ) Somatic. Autonomic ( ANS ) Enteric.
 NERVOUS SYSTEM Nervous System Peripheral Nervous System ( PNS ) Central Nervous System ( CNS ) Efferent (Motor) Afferent (Sensor) Autonomic ( ANS ) Somatic Parasympathetic Sympathetic Enteric Autonomic
NERVOUS SYSTEM Nervous System Peripheral Nervous System ( PNS ) Central Nervous System ( CNS ) Efferent (Motor) Afferent (Sensor) Autonomic ( ANS ) Somatic Parasympathetic Sympathetic Enteric Autonomic
Abiraterone Acetate is an antiandrogen used in the treatment of Castration-Resistant Prostate Cancer(CRPC).
 For the use only of an Oncologist or a Hospital or a Laboratory ABIRATERONE ACETATE TABLETS Zabiteron-250 COMPOSITION Abiraterone Acetate Tablets 250mg Each uncoated tablets contains: Abiraterone Acetate
For the use only of an Oncologist or a Hospital or a Laboratory ABIRATERONE ACETATE TABLETS Zabiteron-250 COMPOSITION Abiraterone Acetate Tablets 250mg Each uncoated tablets contains: Abiraterone Acetate
Package Insert. Elkar
 Package Insert Elkar Product Summary 1. Name of the medicinal product Elkar 500 mg tablets 2. Qualitative and quantitative composition Each tablet contains levocarnitine 500 mg. 3. Pharmaceutical form
Package Insert Elkar Product Summary 1. Name of the medicinal product Elkar 500 mg tablets 2. Qualitative and quantitative composition Each tablet contains levocarnitine 500 mg. 3. Pharmaceutical form
Has little therapeutic value. Has multiple actions. Has short t ½ Activates muscarinic & nicotinic receptors. 10/17/2017 2
 Has little therapeutic value. Has multiple actions. Has short t ½ Activates muscarinic & nicotinic receptors. 10/17/2017 2 Muscarinic stimulation: On the CVS: -ve chronotropic & inotropic effects. Decrease
Has little therapeutic value. Has multiple actions. Has short t ½ Activates muscarinic & nicotinic receptors. 10/17/2017 2 Muscarinic stimulation: On the CVS: -ve chronotropic & inotropic effects. Decrease
SANDOMIGRAN (pizotifen malate)
 SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
Didrex CIII brand of benzphetamine hydrochloride tablets
 Didrex CIII brand of benzphetamine hydrochloride tablets DESCRIPTION DIDREX Tablets contain the anorectic agent benzphetamine hydrochloride. Benzphetamine hydrochloride is a white crystalline powder readily
Didrex CIII brand of benzphetamine hydrochloride tablets DESCRIPTION DIDREX Tablets contain the anorectic agent benzphetamine hydrochloride. Benzphetamine hydrochloride is a white crystalline powder readily
Package Insert. D-Bright
 Package Insert D-Bright Product Summary 1. Name of the medicinal product D-Bright 2. Qualitative and quantitative composition Each ml contains Cholecalciferol (Vitamin D3) IP 400 IU in a flavoured syrupy
Package Insert D-Bright Product Summary 1. Name of the medicinal product D-Bright 2. Qualitative and quantitative composition Each ml contains Cholecalciferol (Vitamin D3) IP 400 IU in a flavoured syrupy
NEUROMUSCULAR BLOCKING AGENTS
 NEUROMUSCULAR BLOCKING AGENTS Edward JN Ishac, Ph.D. Associate Professor, Pharmacology and Toxicology Smith 742, 828-2127, Email: eishac@vcu.edu Learning Objectives: 1. Understand the physiology of the
NEUROMUSCULAR BLOCKING AGENTS Edward JN Ishac, Ph.D. Associate Professor, Pharmacology and Toxicology Smith 742, 828-2127, Email: eishac@vcu.edu Learning Objectives: 1. Understand the physiology of the
Neosynephrine. Name of the Medicine
 Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
1 INDICATIONS AND USAGE. 1.1 Limitation of Use FULL PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TRIFERIC safely and effectively. See full prescribing information for TRIFERIC. TRIFERIC (ferric
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TRIFERIC safely and effectively. See full prescribing information for TRIFERIC. TRIFERIC (ferric
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
Pharmacology Autonomic Nervous System Lecture10
 Pharmacology Autonomic Nervous System Lecture10 Note : Most of the time in this lecture, the doctor was only reading from the slides, so I m going to write only the extra information he mentioned So Please
Pharmacology Autonomic Nervous System Lecture10 Note : Most of the time in this lecture, the doctor was only reading from the slides, so I m going to write only the extra information he mentioned So Please
3 PHARMACEUTICAL FORM Coated tablet Round, white to off-white, sugar coated tablets, plain on both sides.
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Mebeverine hydrochloride 135 mg coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each coated tablet contains 135 mg of mebeverine
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Mebeverine hydrochloride 135 mg coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each coated tablet contains 135 mg of mebeverine
PRESCRIBING INFORMATION Including Patient Medication Information USP
 PRESCRIBING INFORMATION Including Patient Medication Information Pr COLISTIMETHATE FOR INJECTION USP Sterile Colistimethate sodium Equivalent to 150 mg colistin base Parenteral Powder for Solution Antibiotic
PRESCRIBING INFORMATION Including Patient Medication Information Pr COLISTIMETHATE FOR INJECTION USP Sterile Colistimethate sodium Equivalent to 150 mg colistin base Parenteral Powder for Solution Antibiotic
AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1
 9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER
 PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER NAME OF MEDICINE Sodium Cromoglycate for Oral Inhalation. C 23 H 14 Na 2 O 11 Mol Wt. 512.3 CAS No. [15826-37-6] Sodium cromoglycate
PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER NAME OF MEDICINE Sodium Cromoglycate for Oral Inhalation. C 23 H 14 Na 2 O 11 Mol Wt. 512.3 CAS No. [15826-37-6] Sodium cromoglycate
PRODUCT INFORMATION. Ammonium chloride is an expectorant that has an irritant effect on mucous membranes.
 PRODUCT INFORMATION BENADRYL Original Oral Liquid (New Formula) Name of the Medicine Diphenhydramine hydrochloride Ammonium chloride The chemical name for diphenhydramine hydrochloride is 2-(diphenylmethoxy)-N,Ndimethylethanamine
PRODUCT INFORMATION BENADRYL Original Oral Liquid (New Formula) Name of the Medicine Diphenhydramine hydrochloride Ammonium chloride The chemical name for diphenhydramine hydrochloride is 2-(diphenylmethoxy)-N,Ndimethylethanamine
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION
 Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
IPRAVENT Rotacaps (Ipratropium bromide)
 Published on: 10 Jul 2014 IPRAVENT Rotacaps (Ipratropium bromide) Composition IPRAVENT Rotacaps Each capsule contains: Ipratropium Bromide BP, equivalent to Ipratropium Bromide (anhydrous) 40 mcg Excipient..q.s.
Published on: 10 Jul 2014 IPRAVENT Rotacaps (Ipratropium bromide) Composition IPRAVENT Rotacaps Each capsule contains: Ipratropium Bromide BP, equivalent to Ipratropium Bromide (anhydrous) 40 mcg Excipient..q.s.
PACKAGE INSERT CALCIUM GLUCONATE INJECTION, USP 10% Electrolyte Replenisher mosmol/ml 680 mosmol/l Ca meq/ml ph
 CALCIUM GLUCONATE INJECTION, USP 10% Electrolyte Replenisher 0.68 mosmol/ml 680 mosmol/l Ca ++ 0.465 meq/ml ph 6.0 8.2 DESCRIPTION Calcium Gluconate Injection, USP is a sterile, nonpyrogenic supersaturated
CALCIUM GLUCONATE INJECTION, USP 10% Electrolyte Replenisher 0.68 mosmol/ml 680 mosmol/l Ca ++ 0.465 meq/ml ph 6.0 8.2 DESCRIPTION Calcium Gluconate Injection, USP is a sterile, nonpyrogenic supersaturated
Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS:
 0BCore Safety Profile Active substance: Betaxolol eyedrops Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS: HU/H/PSUR/0010/002 Date of FAR: 20.03.2013 4.2 Posology
0BCore Safety Profile Active substance: Betaxolol eyedrops Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS: HU/H/PSUR/0010/002 Date of FAR: 20.03.2013 4.2 Posology
