Medical Policy. MP Placental and Umbilical Cord Blood as a Source of Stem Cells
|
|
|
- Godfrey Henderson
- 5 years ago
- Views:
Transcription
1 Medical Policy MP BCBSA Ref. Policy: Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies Hematopoietic Cell Transplantation for Non- Hodgkin Lymphomas Allogeneic Cell Transplantation for Myelodysplastic Syndromes and Myeloproliferative Neoplasms Allogeneic Hematopoietic Cell Transplantation for Genetic Diseases and Acquired Anemias Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Hematopoietic Cell Transplantation for Miscellaneous Solid Tumors in Adults Hematopoietic Cell Transplantation for Autoimmune Diseases Hematopoietic Cell Transplantation for Acute Myeloid Leukemia Hematopoietic Cell Transplantation for Central Nervous System Embryonal Tumors and Ependymoma Hematopoietic Cell Transplantation for Hodgkin Lymphoma Hematopoietic Cell Transplantation for Chronic Myelogenous Leukemia Hematopoietic Cell Transplantation as a Treatment of Acute Lymphoblastic Leukemia High-Dose Rate Temporary Prostate Brachytherapy Hematopoietic Cell Transplantation in the Treatment of Germ Cell Tumors Hematopoietic Cell Transplantation for Waldenström Macroglobulinemia DISCLAIMER Our medical policies are designed for informational purposes only and are not an authorization, explanation of benefits or a contract. Receipt of benefits is subject to satisfaction of all terms and conditions of the coverage. Medical technology is constantly changing, and we reserve the right to review and update our policies periodically. POLICY Transplantation of cord blood stem cells from related or unrelated donors may be considered medically necessary in patients with an appropriate indication for allogeneic stem cell transplant. Transplantation of cord blood stem cells from related or unrelated donors is considered investigational in all other situations.
2 Collection and storage of cord blood from a neonate may be considered medically necessary when an allogeneic transplant is imminent in an identified recipient with a diagnosis that is consistent with the possible need for allogeneic transplant. Prophylactic collection and storage of cord blood from a neonate may be considered not medically necessary when proposed for some unspecified future use as an autologous stem cell transplant in the original donor, or for some unspecified future use as an allogeneic stem cell transplant in a related or unrelated donor. POLICY GUIDELINES Refer to the evidence reviews for specific conditions and diseases that have patient selection criteria for which allogeneic stem cell transplantation may be considered medically necessary. BENEFIT APPLICATION Through the National Marrow Donor Program s Be the Match, eligible families within the United States can collect and store their neonate s cord blood unit free of charge. When the stored unit is transplanted, a fee is charged. A family is considered eligible if: or if: BACKGROUND the sibling of the neonate has been diagnosed with a disease treatable by a related cord blood transplant; the neonate does not have the same disease as the affected biological sibling (determined after birth); the affected sibling and the neonate have the same biologic parents; an affected biologic parent is enrolled in a clinical or research trial that would accept a haploidentical, related, allogeneic cord blood unit as a treatment option. BONE MARROW DISORDERS A variety of malignant diseases and nonmalignant bone marrow disorders are treated with myeloablative therapy followed by infusion of allogeneic stem and progenitor cells collected from immunologically compatible donors, either family members or an unrelated donor identified through a bone marrow donor bank. In some cases, a suitable donor is not found. Blood harvested from the umbilical cord and placenta shortly after delivery of neonates contains stem and progenitor cells capable of restoring hematopoietic function after myeloablation. This cord blood has been used as an alternative source of allogeneic stem cells. Cord blood is readily available and is thought to be antigenically naive, thus potentially minimizing the incidence of graft-versus-host disease and permitting the broader use of unrelated cord blood transplants. Unrelated donors are typically typed at low resolution for human leukocyte antigen A and B and at high resolution only for human leukocyte antigen DR; human leukocyte antigen matching at 4 of 6 loci is considered acceptable. Under this matching protocol, an acceptable donor can be identified for almost any patient. Several cord blood banks have been created in the United States and Europe. In addition to obtaining cord blood for specific related or unrelated patients, some cord blood banks collect and store neonate cord blood for some unspecified future use in the unlikely event that the child develops a condition that would require autologous transplantation. Also, some neonate cord blood is collected and stored for use by a sibling in whom an allogeneic transplant is anticipated due to a history of leukemia or other condition requiring an allogeneic transplant. Original Policy Date: November 1996 Page: 2
3 Standards and accreditation for cord blood banks are important for assisting transplant programs in knowing whether individual banks have quality control measures in place to address issues such as monitoring cell loss, change in potency, and prevention of product mix-up. 1 Two major organizations have created accreditation standards for cord blood banks in the US: the American Association of Blood Banks and the International NetCord Foundation/Foundation for the Accreditation of Cellular Therapy (NetCord/FACT). Both the AABB and the NetCord/FACT have developed and implemented a program of voluntary inspection and accreditation for cord blood banking. The AABB and the NetCord/FACT publish standards for cord blood banks that define the collection, testing, processing, storage, and release of cord blood products. 2 REGULATORY STATUS According to the U.S. Food and Drug Administration, cord blood stored for potential use by a patient unrelated to the donor meets the definitions of drug and biological products. As such, products must be licensed under a biologics license application or an investigational new drug application before use. Facilities that prepare cord blood units only for autologous and/or first- or second-degree relatives are required to register and list their products, adhere to Good Tissue Practices issued by the Food and Drug Administration, and use applicable processes for donor suitability determination. 3 RATIONALE This evidence review was created in November 1996 and has been updated regularly with searches of the MEDLINE database. The most recent literature update was performed through November 6, This review was informed by a 1996 and a 2001 TEC Assessment, which addressed the use of placental and umbilical cord blood in children and adults, respectively. 4,5 Evidence reviews assess the clinical evidence to determine whether the use of a technology improves the net health outcome. Broadly defined, health outcomes are length of life, quality of life, and ability to functionincluding benefits and harms. Every clinical condition has specific outcomes that are important to patients and to managing the course of that condition. Validated outcome measures are necessary to ascertain whether a condition improves or worsens; and whether the magnitude of that change is clinically significant. The net health outcome is a balance of benefits and harms. To assess whether the evidence is sufficient to draw conclusions about the net health outcome of a technology, 2 domains are examined: the relevance and the quality and credibility. To be relevant, studies must represent one or more intended clinical use of the technology in the intended population and compare an effective and appropriate alternative at a comparable intensity. For some conditions, the alternative will be supportive care or surveillance. The quality and credibility of the evidence depend on study design and conduct, minimizing bias and confounding that can generate incorrect findings. The randomized controlled trial (RCT) is preferred to assess efficacy; however, in some circumstances, nonrandomized studies may be adequate. RCTs are rarely large enough or long enough to capture less common adverse events and long-term effects. Other types of studies can be used for these purposes and to assess generalizability to broader clinical populations and settings of clinical practice. CORD BLOOD AS SOURCE OF STEM CELLS FOR STEM CELL TRANSPLANT Related Allogeneic Cord Blood Transplant The first cord blood transplant involved a child with Fanconi anemia; results were reported in Subsequently, other cord transplants have been performed in matched siblings. The results of these transplants have demonstrated that cord blood contains sufficient numbers of hematopoietic stem and progenitor cells to reconstitute pediatric patients. Lower incidences of acute and chronic graft-versus- Original Policy Date: November 1996 Page: 3
4 host disease (GVHD) have been observed when cord blood, compared with bone marrow, was used as the source of donor cells. 7 This led to the idea that cord blood could be banked and used as a source of unrelated donor cells, possibly without full human leukocyte antigen matching. 8 Unrelated Allogeneic Cord Blood Transplant The first prospective evaluation of unrelated cord blood transplant was the Cord Blood Transplantation study, published in The Cord Blood Transplantation study was designed to examine the safety of unrelated cord blood transplantation in infants, children, and adults. Two-year event-free survival was 55% in children with high-risk malignancies 9 and 78% in children with nonmalignant conditions. 10 Across all groups, the cumulative incidence of engraftment by day 42 was 80%. Engraftment and survival were adversely affected by lower cell doses, pre-transplant cytomegalovirus seropositivity in the recipient, non-european ancestry, and higher human leukocyte antigen mismatching. This slower engraftment led to longer hospitalizations and greater utilization of medical resources. 11 In the Cord Blood Transplantation study, outcomes in adults were inferior to the outcomes achieved in children. In 2012, Zhang et al published a meta-analysis of studies comparing unrelated donor cord blood transplantation with unrelated donor bone marrow transplantation in patients who had acute leukemia. 12 Reviewers identified 7 studies (total N=3389 patients). Pooled event rates of engraftment failure (n=5 studies) were 18% (127/694 patients) in the cord blood transplant group and 6% (57/951 patients) in bone marrow transplant groups. The rate of engraftment graft failure was significantly higher in cord blood transplant recipients (p<0.001). However, rates of acute GVHD were significantly lower in the cord blood transplant group. Pooled event rates of GVHD (n=7 studies) were 34% (397/1179 patients) in the cord blood group and 44% (953/2189 patients) in the bone marrow group (p<0.001). Relapse rates, reported in all studies, did not differ significantly between groups. Several survival outcomes, including overall survival (OS), leukemia-free survival, and non-relapse mortality, favored the bone marrow transplant group. Also, numerous retrospective and registry studies have generally found that unrelated cord blood transplantation is effective in both children and adults with hematologic malignancies and children with a variety of nonmalignant conditions For example, a 2014 study by Liu et al compared outcomes after unrelated donor cord blood transplantation with matched-sibling donor peripheral blood transplantation. 15 The study included patients ages 16 years or older who had hematologic malignancies. Seventy patients received unrelated cord blood, and 115 patients received human leukocyte antigenidentical peripheral blood stem cells, alone or in combination with bone marrow. Primary engraftment rates were similar in the 2 groups (97% in the cord blood group, 100% in the peripheral blood stem cell group). Rates for most outcomes, including grades III and IV acute GVHD and 3-year disease-free survival, were also similar between groups. However, the rate of chronic GVHD was lower in the unrelated-donor cord blood group. Specifically, limited or extensive chronic GVHD occurred in 12 (21%) of 58 evaluable patients in the cord blood group and in 46 (42%) of 109 evaluable patients in the peripheral blood stem cell group (p=0.005). In 2016, Mo et al reported on outcomes after umbilical cord blood and haploidentical hematopoietic cell transplantation in 129 children younger than 14 years old. 16 The 2-year probability of OS was 82% (95% confidence interval [CI], 72.2% to 91.8%) in the haploidentical hematopoietic cell transplantation group and 69.9% (95% CI, 58.0% to 81.2%) in the cord blood group. The difference in OS rates between groups was not statistically significant (p=0.07). The 2- year incidence of relapse was also similar in both groups: 16% (95% CI, 6.1% to 26.1%) in the haplo-hct group and 24.1% (95% CI, 12.5% to 37.5%) in the cord blood group (p=0.17). Also, transplantation of 2 umbilical cord blood units (or double-unit transplants) has been evaluated as a strategy to overcome cell dose limitations with 1 cord blood unit in older and heavier patients. Initial Original Policy Date: November 1996 Page: 4
5 experience at a university showed that using 2 units of cord blood for a single transplant in adults improved rates of engraftment and OS. 17 Although cell doses are higher with double-unit transplants, studies published to date have found that survival rates are similar to transplants using single-cord blood units, and there is some suggestion of higher rates of GVHD (see Tables 1 and 2). 18 Table 1. Summary of Key Trial Characteristics Interventions Author Countries Sites Dates Participants Active Comparator (Year) Wagner et al (2014) 18 1 Patients (age range, 1-21 y) who had high-risk acute leukemia, chronic myeloid leukemia, or myelodysplastic syndrome for whom there were 2 HLAmatched cord blood units available 2 units 1 unit HLA: human leukocyte antigen. Table 2. Summary of Key Trial Results (N=224) Study (Year) 1-Year OS 1-Year DFS Acute GVHD Chronic GVHD Wagner et al (2014) 18 Single unit (95% CI), % 73 (63 to 80) 70 (60 to 77) 13 (7 to 20) 30 (22 to 39) Double unit (95% CI), % 65 (56 to 74) 64 (54 to 72) 23 (15 to 31) 32 (23 to 40) p CI: confidence interval; DFS: disease-free survival; GVHD: graft-versus-host disease; OS: overall survival. Results of observational studies are similar to those of the Wagner RCT (see Tables 3 and 4). In a study by Scaradavou et al (2013), there was a significantly higher risk of acute GVHD (grade II-IV) in recipients of double-cord blood units treated during the first several years of observation. 19 In the later period ( ), rates of acute GVHD (grade II-IV) did not differ significantly between single and double units of cord blood. A 2017 analysis by Baron et al found no significant differences between single- and double- cord blood transplantation for relapse or non-relapse mortality, with a trend (p=0.08) toward a higher incidence of GVHD with double units. 20 Table 3. Summary of Key Observational Study Characteristics Treatment Author (Year) Study Type Dates Participants Arm 1 Arm 2 Follow- Up Scaradavou et al (2013) 19 Comparative cohort Single unit Double unit 2009 Baron et al (2017) 20 Registry Adults with first CBT for AML or ALL Single unit Double unit ALL: acute lymphocytic leukemia; AML: acute myeloid leukemia; CBT: cord blood transplantation. 2 y Original Policy Date: November 1996 Page: 5
6 Table 4. Summary of Key Observational Study Results Study (Year) Scaradavou et al (2013) 19 Single unit Double unit HR (95% CI) N Relapse Mortality Nonrelapse Mortality Acute GVHD (95% CI) (2.54 to 14.87) p < Baron et al (2017) 20 Single unit 17 28% 1.69 (0.68 to 4.18) 2 Double unit 36 36% 2 HR (95% CI) 0.9 (0.6 to 1.3) 0.8 (0.5 to 1.2) p CI: confidence interval; GVHD: graft-versus-host disease; HR: hazard ratio; OS: overall survival. Section Summary: Cord Blood as Source of Stem Cells for Stem Cell Transplant A number of observational studies and a meta-analysis of observational studies have compared outcomes after cord blood transplantation with stem cells from a different source. The meta-analysis found similar survival outcomes and lower GVHD after cord blood transplantation than bone marrow transplantation. Also, an RCT has compared single- and double-unit cord blood transplantation and found similar outcomes. PROPHYLACTIC COLLECTION AND STORAGE OF CORD BLOOD No studies have compared outcomes after prophylactic collection and storage of cord blood from a neonate for individuals who have an unspecified future need for transplant to standard care without cord blood collection and storage. Also, although blood banks are collecting and storing neonate cord blood for potential future use, data on the use of cord blood for autologous stem cell transplantation are limited. A 2017 position paper from the American Academy of Pediatrics noted that there is little evidence of the safety or effectiveness of autologous cord blood transplantation for treatment of malignant neoplasms. 21 Also, a 2009 survey of pediatric hematologists noted few transplants had been performed using cord blood stored in the absences of a known indication. 22 Section Summary: Prophylactic Collection and Storage of Cord Blood There is a lack of published evidence comparing outcomes after prophylactic collection and storage of cord blood from a neonate for individuals who have an unspecified future need for transplant with standard care without cord blood collection and storage. SUMMARY OF EVIDENCE For individuals who have an appropriate indication for allogeneic stem cell transplant who receive cord blood as a source of stem cells, the evidence includes a number of observational studies, a meta-analysis Original Policy Date: November 1996 Page: 6
7 of observational studies, and a randomized controlled trial comparing outcomes after single- or doublecord blood units. Relevant outcomes are overall survival, disease-specific survival, resource utilization, and treatment-related mortality. The meta-analysis of observational studies found similar survival outcomes and lower graft-versus-host disease after cord blood transplantation than bone marrow transplantation. In the randomized controlled trial, survival rates were similar after single- and doubleunit cord blood transplantation. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have an unspecified potential future need for stem cell transplant who receive prophylactic collection and storage of cord blood, the evidence includes no published studies. Relevant outcomes are overall survival, disease-specific survival, resource utilization, and treatment-related mortality. No evidence was identified on the safety or effectiveness of autologous cord blood transplantation from prophylactically stored cord blood for the treatment of malignant neoplasms. The evidence is insufficient to determine the effects of the technology on health outcomes. SUPPLEMENTAL INFORMATION PRACTICE GUIDELINES AND POSITION STATEMENTS American Academy of Pediatrics A position statement on cord blood banking for potential future transplantation was published by the American Academy of Pediatrics in The Academy recommended cord blood banking for public use, with a more limited role for private cord blood banking for families with a known fatal illness that could be rescued by cord blood transplant. U.K. Consensus Recommendations on Umbilical Cord Blood Transplantation In 2015, a consensus conference in the United Kingdom issued the following recommendation on umbilical cord blood transplantation 23 : We recommend that UCB [umbilical cord blood] be considered as an alternative source of HSC [hematopoietic stem cells] for transplantation for those patients without a suitably matched sibling or unrelated donor, defined as standard or clinical option transplants within the BSBMT [British Society of Blood and Marrow Transplantation] transplant indications tables. American College of Obstetricians and Gynecologists In 2015, the American College of Obstetricians and Gynecologists published an opinion on umbilical cord blood banking. 24 The statement discussed counseling patients on options for umbilical cord blood banking, as well as benefits and limitations of this practice. Relevant recommendations included the following: Umbilical cord blood collection should not compromise obstetric or neonatal care or alter routine practice for the timing of umbilical cord clamping. The current indications for cord blood transplant are limited to select genetic, hematologic, and malignant disorders. The routine storage of umbilical cord blood as biologic insurance against future disease is not recommended. American Society for Blood and Marrow Transplantation On behalf of the American Society for Blood and Marrow Transplantation, Ballen et al (2008) published recommendations related to the banking of umbilical cord blood 25 : Original Policy Date: November 1996 Page: 7
8 1. Public banking of cord blood is encouraged. 2. Storing cord blood for autologous (i.e., personal) use is not recommended. 3. Family member banking (collecting and storing cord blood for a family member) is recommended when there is a sibling with a disease that may be successfully treated with an allogeneic transplant. Family member banking on behalf of a parent with a disease that may be successfully treated with an allogeneic transplant is only recommended when there are shared HLA [human leukocyte]-antigens between the parents. U.S. PREVENTIVE SERVICES TASK FORCE RECOMMENDATIONS Not applicable. MEDICARE NATIONAL COVERAGE There is no national coverage determination. In the absence of a national coverage determination, coverage decisions are left to the discretion of local Medicare carriers. ONGOING AND UNPUBLISHED CLINICAL TRIALS Some currently unpublished trials that might influence this review are listed in Table 5. Table 5. Summary of Key Trials NCT No. Trial Name Ongoing NCT The Collection and Storage of Umbilical Cord Blood for Transplantation NCT Collection and Storage of Umbilical Cord Stem Cells for Treatment of Sickle Cell Disease NCT: national clinical trial. REFERENCES Planned Enrollment Completion Date 250,000 Jun ,999,999 none 1. Wall DA. Regulatory issues in cord blood banking and transplantation. Best Pract Res Clin Haematol. Jun 2010;23(2): PMID NetCord-FACT. International standards for cord blood collection banking and release of information accreditation manual. Sixth Edition Draft September; %20Draft pdf. Accessed December 20, Food and Drug Administration (FDA). Cord Blood Banking: Information for Consumers July 23; Accessed December 20, Blue Cross and Blue Shield Association Technology Evaluation Center (TEC). Placental and Umbilical Cord Blood as a Source of Stem Cells for Hematopoietic Support. TEC Assessments. 1996;Volume 11:Tab Blue Cross and Blue Shield Association Technology Evaluation Center (TEC). Transplanting Adult Patients with Hematopoietic Stem Cells from Placental and Umbilical Cord Blood TEC Assessments. 2001;Volume 16:Tab Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. Oct ;321(17): PMID Original Policy Date: November 1996 Page: 8
9 7. Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLAmismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graftversus-host disease. Blood. Aug ;88(3): PMID Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. May 1989;86(10): PMID Kurtzberg J, Cairo MS, Fraser JK, et al. Results of the Cord Blood Transplantation (COBLT) study unrelated donor banking program. Transfusion. Jun 2005;45(6): PMID Martin PL, Carter SL, Kernan NA, et al. Results of the Cord Blood Transplantation Study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant. Feb 2006;12(2): PMID Kurtzberg J. Update on umbilical cord blood transplantation. Curr Opin Pediatr. Feb 2009;21(1): PMID Zhang H, Chen J, Que W. Meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in acute leukemia patients. Biol Blood Marrow Transplant. Aug 2012;18(8): PMID Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. May ;97(10): PMID Kato K, Choi I, Wake A, et al. Treatment of patients with adult T cell leukemia/lymphoma with cord blood transplantation: a Japanese nationwide retrospective survey. Biol Blood Marrow Transplant. Dec 2014;20(12): PMID Liu HL, Sun ZM, Geng LQ, et al. Similar survival, but better quality of life after myeloablative transplantation using unrelated cord blood vs matched sibling donors in adults with hematologic malignancies. Bone Marrow Transplant. Aug 2014;49(8): PMID Mo XD, Tang BL, Zhang XH, et al. Comparison of outcomes after umbilical cord blood and unmanipulated haploidentical hematopoietic stem cell transplantation in children with high-risk acute lymphoblastic leukemia. Int J Cancer. Nov ;139(9): PMID Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. Feb ;105(3): PMID Wagner JE, Jr., Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. Oct ;371(18): PMID Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. Jan ;121(5): PMID Baron F, Ruggeri A, Beohou E, et al. Single- or double-unit UCBT following RIC in adults with AL: a report from Eurocord, the ALWP and the CTIWP of the EBMT. J Hematol Oncol. Jun ;10(1):128. PMID Shearer WT, Lubin BH, Cairo MS, et al. Cord Blood Banking for Potential Future Transplantation. Pediatrics. Nov 2017;140(5). PMID Thornley I, Eapen M, Sung L, et al. Private cord blood banking: experiences and views of pediatric hematopoietic cell transplantation physicians. Pediatrics. Mar 2009;123(3): PMID Hough R, Danby R, Russell N, et al. Recommendations for a standard UK approach to incorporating umbilical cord blood into clinical transplantation practice: an update on cord blood unit selection, Original Policy Date: November 1996 Page: 9
10 donor selection algorithms and conditioning protocols. Br J Haematol. Feb 2016;172(3): PMID Committee Opinion No. 648: Umbilical Cord Blood Banking. Obstet Gynecol. Dec 2015;126(6):e PMID Ballen KK, Barker JN, Stewart SK, et al. Collection and preservation of cord blood for personal use. Biol Blood Marrow Transplant. Mar 2008;14(3): PMID CODES Codes Number Description CPT No specific code HCPCS S2140 Cord blood harvesting for transplantation, allogeneic S2142 Cord blood derived stem-cell transplantation, allogeneic S2150 Bone marrow or blood-derived stem cells (peripheral or umbilical), allogeneic or autologous, harvesting, transplantation, and related complications; including: pheresis and cell preparation/storage; marrow ablative therapy; drugs; supplies; hospitalization with outpatient followup; medical/surgical, diagnostic, emergency, and rehabilitative services; and the number of days of pre- and post-transplant care in the global definition ICD-10-CM [See the diagnosis codes listed in the various policies relevant to allogeneic stem cell transplantation in the Therapy section of the MPRM (e.g., , , )] ICD-10-PCS 30243X0, 30243X1 Percutaneous transfusion, central vein, stem cells, cord blood, autologous or non-autologous, code list Type of Transplant Service Place of Service Inpatient POLICY HISTORY Date Action Description 09/11/14 Replace policy Policy updated with literature review through July 21, Policy statements unchanged. References 4, 16-17, and 23 added. 09/10/15 Replace policy Policy updated with literature review through August 10, 2015; reference 23 added. Policy statements unchanged. 01/14/16 Replace policy Policy updated with literature review through December 6, 2015; reference 27 added. Policy statements unchanged. 01/27/17 Replace policy Blue Cross of Idaho adopted changes as noted. Policy updated with literature review through November 9, 2016; references 17 and 23 added. Policy statements unchanged. 01/30/18 Replace policy Blue Cross of Idaho adopted changes as noted. Policy updated with literature review through November 6, 2017; references added; note 2 updated. Policy statements unchanged. Original Policy Date: November 1996 Page: 10
Placental and Umbilical Cord Blood as a Source of Stem Cells
 Placental and Umbilical Cord Blood as a Source of Stem Cells Policy Number: 7.01.50 Last Review: 12/2018 Origination: 12/2001 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Placental and Umbilical Cord Blood as a Source of Stem Cells Policy Number: 7.01.50 Last Review: 12/2018 Origination: 12/2001 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer
 Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: MM.07.014 Lines of Business: HMO; PPO Place of Service: Outpatient; Inpatient Current Effective Date: February 22, 2019 Original
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: MM.07.014 Lines of Business: HMO; PPO Place of Service: Outpatient; Inpatient Current Effective Date: February 22, 2019 Original
Related Policies None
 Medical Policy MP 7.01.13 BCBSA Ref. Policy: 7.01.13 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.13 BCBSA Ref. Policy: 7.01.13 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Haplo vs Cord vs URD Debate
 3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Autoimmune Diseases File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_autoimmune_diseases
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Autoimmune Diseases File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_autoimmune_diseases
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer
 Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: 8.01.23 Last Review: 1/2018 Origination: 9/2002 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: 8.01.23 Last Review: 1/2018 Origination: 9/2002 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Study for the improvement of umbilical cord blood sampling using a new trial apparatus
 bs_bs_banner doi:10.1111/jog.12179 J. Obstet. Gynaecol. Res. Vol. 40, No. 2: 405 409, February 2014 Study for the improvement of umbilical cord blood sampling using a new trial apparatus Naoki Masaoka
bs_bs_banner doi:10.1111/jog.12179 J. Obstet. Gynaecol. Res. Vol. 40, No. 2: 405 409, February 2014 Study for the improvement of umbilical cord blood sampling using a new trial apparatus Naoki Masaoka
The National Marrow Donor Program. Graft Sources for Hematopoietic Cell Transplantation. Simon Bostic, URD Transplant Recipient
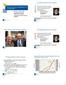 1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
Clinical Policy: Donor Lymphocyte Infusion
 Clinical Policy: Reference Number: PA.CP.MP.101 Effective Date: 01/18 Last Review Date: 11/16 Coding Implications Revision Log This policy describes the medical necessity requirements for a donor lymphocyte
Clinical Policy: Reference Number: PA.CP.MP.101 Effective Date: 01/18 Last Review Date: 11/16 Coding Implications Revision Log This policy describes the medical necessity requirements for a donor lymphocyte
Hematopoietic Stem Cell Transplantation for Epithelial Ovarian Cancer
 Hematopoietic Stem Cell Transplantation for Epithelial Ovarian Cancer Policy Number: Original Effective Date: MM.07.014 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 04/22/2016 Section:
Hematopoietic Stem Cell Transplantation for Epithelial Ovarian Cancer Policy Number: Original Effective Date: MM.07.014 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 04/22/2016 Section:
Clinical Policy: Donor Lymphocyte Infusion Reference Number: CP.MP.101 Last Review Date: 11/17
 Clinical Policy: Reference Number: CP.MP.101 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Clinical Policy: Reference Number: CP.MP.101 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Cord Blood Stem Cell Banking and Transplantation
 Cord Blood Stem Cell Banking and Transplantation JOHN W. ADAMSON New York Blood Center, New York, New York, USA Key Words. Cord blood Stem cells Cord blood banking Cord blood transplantation. Cord blood.stern
Cord Blood Stem Cell Banking and Transplantation JOHN W. ADAMSON New York Blood Center, New York, New York, USA Key Words. Cord blood Stem cells Cord blood banking Cord blood transplantation. Cord blood.stern
Clinical Use of Umbilical Cord Blood Hematopoietic Stem Cells
 Biology of Blood and Marrow Transplantation 12:34-41 (2006) 2006 American Society for Blood and Marrow Transplantation 1083-8791/06/1201-0107$32.00/0 doi:10.1016/j.bbmt.2005.09.006 Clinical Use of Umbilical
Biology of Blood and Marrow Transplantation 12:34-41 (2006) 2006 American Society for Blood and Marrow Transplantation 1083-8791/06/1201-0107$32.00/0 doi:10.1016/j.bbmt.2005.09.006 Clinical Use of Umbilical
Regence. Next Review: 08/2019 Last Review: 08/2018 Medicare Link(s) Revised: 10/01/2018 IMPORTANT REMINDER
 Regence Medicare Advantage Policy Manual Policy ID: M-TRA45 Stem Cell / Bone Marrow Transplants Published: 10/01/2018 Next Review: 08/2019 Last Review: 08/2018 Medicare Link(s) Revised: 10/01/2018 IMPORTANT
Regence Medicare Advantage Policy Manual Policy ID: M-TRA45 Stem Cell / Bone Marrow Transplants Published: 10/01/2018 Next Review: 08/2019 Last Review: 08/2018 Medicare Link(s) Revised: 10/01/2018 IMPORTANT
A Meta-Analysis of Unrelated Donor Umbilical Cord Blood Transplantation versus Unrelated Donor Bone Marrow Transplantation in Acute Leukemia Patients
 A Meta-Analysis of Unrelated Donor Umbilical Cord Blood Transplantation versus Unrelated Donor Bone Marrow Transplantation in Acute Leukemia Patients Haoran Zhang, Junmin Chen, Wenzhong Que Umbilical cord
A Meta-Analysis of Unrelated Donor Umbilical Cord Blood Transplantation versus Unrelated Donor Bone Marrow Transplantation in Acute Leukemia Patients Haoran Zhang, Junmin Chen, Wenzhong Que Umbilical cord
Bone Marrow Transplantation and the Potential Role of Iomab-B
 Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Cell Transplantation for Hodgkin Lymphoma File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_hodgkin_lymphoma
Corporate Medical Policy Hematopoietic Cell Transplantation for Hodgkin Lymphoma File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_hodgkin_lymphoma
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation in the Treatment of Germ File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_in_the_treatment_of_germ_cell_tumor
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation in the Treatment of Germ File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_in_the_treatment_of_germ_cell_tumor
Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017
 Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017 Allogeneic Transplant Recipients in the US, by Donor Type 9000
Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017 Allogeneic Transplant Recipients in the US, by Donor Type 9000
Donor Lymphocyte Infusion for Malignancies Treated with an Allogeneic Hematopoietic Stem-Cell Transplant
 Last Review Status/Date: September 2014 Page: 1 of 8 Malignancies Treated with an Allogeneic Description Donor lymphocyte infusion (DLI), also called donor leukocyte or buffy-coat infusion is a type of
Last Review Status/Date: September 2014 Page: 1 of 8 Malignancies Treated with an Allogeneic Description Donor lymphocyte infusion (DLI), also called donor leukocyte or buffy-coat infusion is a type of
Cord Blood Transplant. E. Gluckman Eurocord ESH-EBMT training course Vienna 2014
 Cord Blood Transplant E. Gluckman Eurocord ESH-EBMT training course Vienna 2014 Background Since 1988, umbilical cord blood (CB) has been successfully used to treat children and adults needing stem cell
Cord Blood Transplant E. Gluckman Eurocord ESH-EBMT training course Vienna 2014 Background Since 1988, umbilical cord blood (CB) has been successfully used to treat children and adults needing stem cell
NIH Public Access Author Manuscript Lancet Oncol. Author manuscript; available in PMC 2011 August 29.
 NIH Public Access Author Manuscript Published in final edited form as: Lancet Oncol. 2010 July ; 11(7): 653 660. doi:10.1016/s1470-2045(10)70127-3. Effect of Graft Source on Unrelated Donor Haemopoietic
NIH Public Access Author Manuscript Published in final edited form as: Lancet Oncol. 2010 July ; 11(7): 653 660. doi:10.1016/s1470-2045(10)70127-3. Effect of Graft Source on Unrelated Donor Haemopoietic
One-Unit versus Two-Unit Cord-Blood Transplantation for Hematologic Cancers
 The new england journal of medicine Original Article One-Unit versus Two-Unit Cord-Blood Transplantation for Hematologic Cancers John E. Wagner, Jr., M.D., Mary Eapen, M.B., B.S., Shelly Carter, D.Sc.,
The new england journal of medicine Original Article One-Unit versus Two-Unit Cord-Blood Transplantation for Hematologic Cancers John E. Wagner, Jr., M.D., Mary Eapen, M.B., B.S., Shelly Carter, D.Sc.,
Trends in Hematopoietic Cell Transplantation. AAMAC Patient Education Day Oct 2014
 Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Use of alternative donors in HSCT (Europe)
 Use of alternative donors in HSCT (Europe) Passweg JR, Baldomero H, Bader P et al (2015) Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors
Use of alternative donors in HSCT (Europe) Passweg JR, Baldomero H, Bader P et al (2015) Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors
What s new in Blood and Marrow Transplant? Saar Gill, MD PhD Jan 22, 2016
 What s new in Blood and Marrow Transplant? Saar Gill, MD PhD Jan 22, 2016 Division of Hematology-Oncology University of Pennsylvania Perelman School of Medicine 1 Who should be transplanted and how? Updates
What s new in Blood and Marrow Transplant? Saar Gill, MD PhD Jan 22, 2016 Division of Hematology-Oncology University of Pennsylvania Perelman School of Medicine 1 Who should be transplanted and how? Updates
Summary of Changes BMT CTN 1101 Version 7.0 to 8.0 Dated: January 18, Original text: Changed to: Rationale
 BMT CTN 1101 RIC ducb vs. Haplo Page 1 of 10 Date: January 20, 2017 Summary of Changes BMT CTN 1101 Version 7.0 to 8.0 Dated: January 18, 2017 A Multi-Center, Phase III, Randomized Trial of Reduced Intensity(RIC)
BMT CTN 1101 RIC ducb vs. Haplo Page 1 of 10 Date: January 20, 2017 Summary of Changes BMT CTN 1101 Version 7.0 to 8.0 Dated: January 18, 2017 A Multi-Center, Phase III, Randomized Trial of Reduced Intensity(RIC)
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): October 1, 2014 Most Recent Review Date (Revised): May 20, 2014 Effective Date: October 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Original Issue Date (Created): October 1, 2014 Most Recent Review Date (Revised): May 20, 2014 Effective Date: October 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Facet Arthroplasty. Policy Number: Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019
 Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Haploidentical Transplantation today: and the alternatives
 Haploidentical Transplantation today: and the alternatives Daniel Weisdorf MD University of Minnesota February, 2013 No matched sib: where to look? URD donor requires close HLA matching and 3-12 weeks
Haploidentical Transplantation today: and the alternatives Daniel Weisdorf MD University of Minnesota February, 2013 No matched sib: where to look? URD donor requires close HLA matching and 3-12 weeks
Medical Policy. MP Hematopoietic Cell Transplantation for Acute Myeloid Leukemia
 Medical Policy MP 8.01.26 BCBSA Ref. Policy: 8.01.26 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Therapy Related Policies 2.04.124 Genetic Testing for FLT3, NPM1, and CEBPA Variants in
Medical Policy MP 8.01.26 BCBSA Ref. Policy: 8.01.26 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Therapy Related Policies 2.04.124 Genetic Testing for FLT3, NPM1, and CEBPA Variants in
MP Ovarian and Internal Iliac Vein Embolization as a Treatment of Pelvic Congestion Syndrome
 Medical Policy MP 4.01.18 BCBSA Ref. Policy: 4.01.18 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: OB/GYN Reproduction Related Policies 4.01.11 Occlusion of Uterine Arteries Using Transcatheter
Medical Policy MP 4.01.18 BCBSA Ref. Policy: 4.01.18 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: OB/GYN Reproduction Related Policies 4.01.11 Occlusion of Uterine Arteries Using Transcatheter
Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer
 Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer Policy #: 401 Latest Review Date: November 2013 Category: Surgery Policy Grade: A Background/Definitions: As a general
Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer Policy #: 401 Latest Review Date: November 2013 Category: Surgery Policy Grade: A Background/Definitions: As a general
MP Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
 Medical Policy MP 8.01.15 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma BCBSA Ref. Policy: 8.01.15 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section:
Medical Policy MP 8.01.15 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma BCBSA Ref. Policy: 8.01.15 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section:
Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia
 Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia Policy Number: 8.01.32 Last Review: 7/2018 Origination: 7/2002 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia Policy Number: 8.01.32 Last Review: 7/2018 Origination: 7/2002 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Stem Cell Transplantation for Severe Aplastic Anemia
 Number of Transplants 10/24/2011 Stem Cell Transplantation for Severe Aplastic Anemia Claudio Anasetti, MD Professor of Oncology and Medicine Chair, Blood and Marrow Transplant Dpt Moffitt Cancer Center
Number of Transplants 10/24/2011 Stem Cell Transplantation for Severe Aplastic Anemia Claudio Anasetti, MD Professor of Oncology and Medicine Chair, Blood and Marrow Transplant Dpt Moffitt Cancer Center
What s a Transplant? What s not?
 What s a Transplant? What s not? How to report the difference? Daniel Weisdorf MD University of Minnesota Anti-cancer effects of BMT or PBSCT [HSCT] Kill the cancer Save the patient Restore immunocompetence
What s a Transplant? What s not? How to report the difference? Daniel Weisdorf MD University of Minnesota Anti-cancer effects of BMT or PBSCT [HSCT] Kill the cancer Save the patient Restore immunocompetence
Number: Policy *Please see amendment for Pennsylvania Medicaid at the end. Last Review 02/09/2017 Effective: 12/01/1997 Next Review: 02/08/2018
 1 of 15 03/31/2017 5:00 PM Number: 0190 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Aetna considers compatibility testing of prospective donors who are close family members
1 of 15 03/31/2017 5:00 PM Number: 0190 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Aetna considers compatibility testing of prospective donors who are close family members
MUD SCT. Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University
 MUD SCT Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University Outlines Optimal match criteria for unrelated adult donors Role of ATG in MUD-SCT Post-transplant
MUD SCT Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University Outlines Optimal match criteria for unrelated adult donors Role of ATG in MUD-SCT Post-transplant
Populations Interventions Comparators Outcomes Individuals: With primary amyloidosis
 Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
journal of medicine The new england Outcomes after Transplantation of Cord Blood or Bone Marrow from Unrelated Donors in Adults with Leukemia abstract
 The new england journal of medicine established in 1812 november 25, 2004 vol. 351 no. 22 Outcomes after Transplantation of Cord Blood or Bone Marrow from Unrelated Donors in Adults with Leukemia Mary
The new england journal of medicine established in 1812 november 25, 2004 vol. 351 no. 22 Outcomes after Transplantation of Cord Blood or Bone Marrow from Unrelated Donors in Adults with Leukemia Mary
KEY WORDS: Allogeneic, Hematopoietic cell transplantation, Graft-versus-host disease, Immunosuppressants, Cyclosporine, Tacrolimus
 A Retrospective Comparison of Tacrolimus versus Cyclosporine with Methotrexate for Immunosuppression after Allogeneic Hematopoietic Cell Transplantation with Mobilized Blood Cells Yoshihiro Inamoto, 1
A Retrospective Comparison of Tacrolimus versus Cyclosporine with Methotrexate for Immunosuppression after Allogeneic Hematopoietic Cell Transplantation with Mobilized Blood Cells Yoshihiro Inamoto, 1
Original article. Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, 2
 Original article http://dx.doi.org/1.3345/kjp.212.55.3.93 Korean J Pediatr 212;55(3):93-99 Hematopoietic stem cell transplantation in children with acute leukemia: similar outcomes in recipients of umbilical
Original article http://dx.doi.org/1.3345/kjp.212.55.3.93 Korean J Pediatr 212;55(3):93-99 Hematopoietic stem cell transplantation in children with acute leukemia: similar outcomes in recipients of umbilical
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: MM.07.011 Lines of Business: HMO; PPO Precertification: Required see section IV Current
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: MM.07.011 Lines of Business: HMO; PPO Precertification: Required see section IV Current
HEMATOPOIETIC CELL TRANSPLANTATION FOR EPITHELIAL OVARIAN CARCINOMA
 CARCINOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
CARCINOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA
 STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: Original Effective Date: MM.07.015 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 05/26/2017 Section: Transplants
Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: Original Effective Date: MM.07.015 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 05/26/2017 Section: Transplants
Medical Policy. MP Dynamic Spinal Visualization and Vertebral Motion Analysis
 Medical Policy BCBSA Ref. Policy: 6.01.46 Last Review: 09/19/2018 Effective Date: 12/15/2018 Section: Radiology Related Policies 6.01.48 Positional Magnetic Resonance Imaging 9.01.502 Experimental / Investigational
Medical Policy BCBSA Ref. Policy: 6.01.46 Last Review: 09/19/2018 Effective Date: 12/15/2018 Section: Radiology Related Policies 6.01.48 Positional Magnetic Resonance Imaging 9.01.502 Experimental / Investigational
HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA
 HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services,
HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services,
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
What are stem cells? A stem cell can differentiate into any one of 220 different specialised cells in the body STEM CELLS
 What are stem cells? Stem cells are often called MASTER CELLS, and form the foundation for your entire body as building blocks for the blood, immune system, tissue and organs. They can REPLICATE or REGENERATE
What are stem cells? Stem cells are often called MASTER CELLS, and form the foundation for your entire body as building blocks for the blood, immune system, tissue and organs. They can REPLICATE or REGENERATE
Review Article. Umbilical cord blood transplantation: the first 25 years and beyond. Introduction. Scientific basis of cord blood transplantation
 Review Article Umbilical cord blood transplantation: the first 25 years and beyond Karen K. Ballen, 1 Eliane Gluckman, 2 and Hal E. Broxmeyer 3 1 Massachusetts General Hospital Cancer Center, Boston, MA;
Review Article Umbilical cord blood transplantation: the first 25 years and beyond Karen K. Ballen, 1 Eliane Gluckman, 2 and Hal E. Broxmeyer 3 1 Massachusetts General Hospital Cancer Center, Boston, MA;
Transplantation - Challenges for the future. Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust
 Transplantation - Challenges for the future Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust Bone Marrow Transplantation Timeline, 1957-2006 Appelbaum F. N Engl J Med 2007;357:1472-1475
Transplantation - Challenges for the future Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust Bone Marrow Transplantation Timeline, 1957-2006 Appelbaum F. N Engl J Med 2007;357:1472-1475
Related Policies None
 Medical Policy MP 8.01.62 BCBSA Ref. Policy: 8.01.62 Last Review: 07/25/2018 Effective Date: 07/25/2018 Section: Therapy Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 8.01.62 BCBSA Ref. Policy: 8.01.62 Last Review: 07/25/2018 Effective Date: 07/25/2018 Section: Therapy Related Policies None DISCLAIMER Our medical policies are designed for informational
Allogeneic Hematopoietic Stem-Cell Transplantation for Myelodysplastic Syndromes and Myeloproliferative Neoplasms. Policy Specific Section:
 Medical Policy Allogeneic Hematopoietic Stem-Cell Transplantation for Myelodysplastic Syndromes and Myeloproliferative Type: Medical Necessity and Investigational / Experimental Policy Specific Section:
Medical Policy Allogeneic Hematopoietic Stem-Cell Transplantation for Myelodysplastic Syndromes and Myeloproliferative Type: Medical Necessity and Investigational / Experimental Policy Specific Section:
Stem cells. -Dr Dinesh Bhurani, MD, DM, FRCPA. Rajiv Gandhi Cancer Institute, Delhi, -Director, Department of Haematology and BMT
 Stem cells -Dr Dinesh Bhurani, MD, DM, FRCPA -Director, Department of Haematology and BMT Rajiv Gandhi Cancer Institute, Delhi, Flow of presentation Update on stem cell uses Haematopoietic stem cell transplantation
Stem cells -Dr Dinesh Bhurani, MD, DM, FRCPA -Director, Department of Haematology and BMT Rajiv Gandhi Cancer Institute, Delhi, Flow of presentation Update on stem cell uses Haematopoietic stem cell transplantation
Reduced-intensity Conditioning Transplantation
 Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
Telephone: ; Fax: ; E mail:
 MINUTES AND OVERVIEW PLAN CIBMTR WORKING COMMITTEE FOR GRAFT SOURCES & MANIPULATION Grapevine, TX Thursday, February 27, 2014, 2:45 4:45 pm Co Chair: Co Chair: Co Chair: Statisticians: Scientific Director:
MINUTES AND OVERVIEW PLAN CIBMTR WORKING COMMITTEE FOR GRAFT SOURCES & MANIPULATION Grapevine, TX Thursday, February 27, 2014, 2:45 4:45 pm Co Chair: Co Chair: Co Chair: Statisticians: Scientific Director:
Applications of Cord Blood Stem Cells
 Applications of Cord Blood Stem Cells over 360 families have used their banked cord blood in transplant or regenerative medicine research. 1 88 ViaCord has released over 360 units for transplant or infusion
Applications of Cord Blood Stem Cells over 360 families have used their banked cord blood in transplant or regenerative medicine research. 1 88 ViaCord has released over 360 units for transplant or infusion
SECOND ANNUAL INTERNATIONAL UMBILICAL CORD BLOOD SYMPOSIUM
 Biology of Blood and Marrow Transplantation 10:728-739 (2004) 2004 American Society for Blood and Marrow Transplantation 1083-8791/04/1010-0009$30.00/0 doi:10.1016/j.bbmt.2004.06.010 SECOND ANNUAL INTERNATIONAL
Biology of Blood and Marrow Transplantation 10:728-739 (2004) 2004 American Society for Blood and Marrow Transplantation 1083-8791/04/1010-0009$30.00/0 doi:10.1016/j.bbmt.2004.06.010 SECOND ANNUAL INTERNATIONAL
National Marrow Donor Program HLA-Matching Guidelines for Unrelated Marrow Transplants
 Biology of Blood and Marrow Transplantation 9:610-615 (2003) 2003 American Society for Blood and Marrow Transplantation 1083-8791/03/0910-0003$30.00/0 doi:10.1016/s1083-8791(03)00329-x National Marrow
Biology of Blood and Marrow Transplantation 9:610-615 (2003) 2003 American Society for Blood and Marrow Transplantation 1083-8791/03/0910-0003$30.00/0 doi:10.1016/s1083-8791(03)00329-x National Marrow
Umbilical cord blood transplantation for acute myeloid leukemia Anjali S. Advani a and Mary J. Laughlin b
 Umbilical cord blood transplantation for acute myeloid leukemia Anjali S. Advani a and Mary J. Laughlin b a The Cleveland Clinic Lerner College of Medicine, Department of Hematologic Oncology and Blood
Umbilical cord blood transplantation for acute myeloid leukemia Anjali S. Advani a and Mary J. Laughlin b a The Cleveland Clinic Lerner College of Medicine, Department of Hematologic Oncology and Blood
Surgical Treatment of Bilateral Gynecomastia
 Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
GVHD. URD unrelated donor
 1 GVHD T-B GVHD 1 RDrelated donor URDunrelated donor 2. Julie M. Vose and Steven Z. Pavletic. Haematopoietic Stem Cell Transplantation. In Goldman: Cecil Medicine, 23 rd ed.; chapter 184 (Saunders, An
1 GVHD T-B GVHD 1 RDrelated donor URDunrelated donor 2. Julie M. Vose and Steven Z. Pavletic. Haematopoietic Stem Cell Transplantation. In Goldman: Cecil Medicine, 23 rd ed.; chapter 184 (Saunders, An
ASBMT. Impact of the Direction of HLA Mismatch on Transplantation Outcomes in Single Unrelated Cord Blood Transplantation
 Biol Blood Marrow Transplant 19 (2013) 247e254 Impact of the Direction of HLA Mismatch on Transplantation Outcomes in Single Unrelated Cord Blood Transplantation ASBMT American Society for Blood and Marrow
Biol Blood Marrow Transplant 19 (2013) 247e254 Impact of the Direction of HLA Mismatch on Transplantation Outcomes in Single Unrelated Cord Blood Transplantation ASBMT American Society for Blood and Marrow
Cord-Blood Transplantation in Patients with Minimal Residual Disease
 The new england journal of medicine Original Article Cord-Blood Transplantation in Patients with Minimal Residual Disease Filippo Milano, M.D., Ph.D., Ted Gooley, Ph.D., Brent Wood, M.D., Ann Woolfrey,
The new england journal of medicine Original Article Cord-Blood Transplantation in Patients with Minimal Residual Disease Filippo Milano, M.D., Ph.D., Ted Gooley, Ph.D., Brent Wood, M.D., Ann Woolfrey,
An Introduction to Bone Marrow Transplant
 Introduction to Blood Cancers An Introduction to Bone Marrow Transplant Rushang Patel, MD, PhD, FACP Florida Hospital Medical Group S My RBC Plt Gran Polycythemia Vera Essential Thrombocythemia AML, CML,
Introduction to Blood Cancers An Introduction to Bone Marrow Transplant Rushang Patel, MD, PhD, FACP Florida Hospital Medical Group S My RBC Plt Gran Polycythemia Vera Essential Thrombocythemia AML, CML,
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 Last Review Status/Date: December 2016 Page: 1 of 6 Intraoperative Assessment of Surgical Description Breast-conserving surgery as part of the treatment of localized breast cancer is optimally achieved
Last Review Status/Date: December 2016 Page: 1 of 6 Intraoperative Assessment of Surgical Description Breast-conserving surgery as part of the treatment of localized breast cancer is optimally achieved
Hematopoietic Cell Transplantation for Breast Cancer
 Medical Policy Manual Transplant, Policy No. 45.29 Hematopoietic Cell Transplantation for Breast Cancer Next Review: January 2019 Last Review: February 2018 Effective: March 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Transplant, Policy No. 45.29 Hematopoietic Cell Transplantation for Breast Cancer Next Review: January 2019 Last Review: February 2018 Effective: March 1, 2018 IMPORTANT REMINDER
Introduction to Hematopoietic Stem Cell Transplantation
 Faculty Disclosures Introduction to Hematopoietic Stem Cell Transplantation Nothing to disclose Jeanne McCarthy-Kaiser, PharmD, BCOP Clinical Pharmacist, Autologous Stem Cell Transplant/Long- Term Follow-Up
Faculty Disclosures Introduction to Hematopoietic Stem Cell Transplantation Nothing to disclose Jeanne McCarthy-Kaiser, PharmD, BCOP Clinical Pharmacist, Autologous Stem Cell Transplant/Long- Term Follow-Up
Medical Benefit Effective Date: 07/01/12 Next Review Date: 05/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 05/11, 05/12
 Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic (80115) Medical Benefit Effective Date: 07/01/12 Next Review Date: 05/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 05/11, 05/12 The
Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic (80115) Medical Benefit Effective Date: 07/01/12 Next Review Date: 05/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 05/11, 05/12 The
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
Therapeutic Advances in Treatment of Aplastic Anemia. Seiji Kojima MD. PhD.
 Therapeutic Advances in Treatment of Aplastic Anemia Seiji Kojima MD. PhD. Department of Pediatrics Nagoya University Graduate School of Medicine Chairman of the Severe Aplastic Anemia Working Party Asia-Pacific
Therapeutic Advances in Treatment of Aplastic Anemia Seiji Kojima MD. PhD. Department of Pediatrics Nagoya University Graduate School of Medicine Chairman of the Severe Aplastic Anemia Working Party Asia-Pacific
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Are We There Yet? Gene Therapy and BMT as Curative Therapies in Sickle Cell. Ann Haight, MD 9 Sept 2017
 Are We There Yet? Gene Therapy and BMT as Curative Therapies in Sickle Cell Ann Haight, MD 9 Sept 2017 Spoiler alert Yes (we have a cure) And No Work to do! 2 Sickle Cell Treatment Options Supportive Care
Are We There Yet? Gene Therapy and BMT as Curative Therapies in Sickle Cell Ann Haight, MD 9 Sept 2017 Spoiler alert Yes (we have a cure) And No Work to do! 2 Sickle Cell Treatment Options Supportive Care
Blood and Marrow Transplant (BMT) for Sickle Cell Disease
 Blood and Marrow Transplant (BMT) for Sickle Cell Disease Rhiannon is now cured of sickle cell disease after BMT. Blood and marrow transplant (BMT) is a proven cure for sickle cell disease. This handbook
Blood and Marrow Transplant (BMT) for Sickle Cell Disease Rhiannon is now cured of sickle cell disease after BMT. Blood and marrow transplant (BMT) is a proven cure for sickle cell disease. This handbook
Reduced-Intensity Conditioning Stem Cell Transplantation: Comparison of Double Umbilical Cord Blood and Unrelated Donor Grafts
 Reduced-Intensity Conditioning Stem Cell Transplantation: Comparison of Double Umbilical Cord Blood and Unrelated Donor Grafts Yi-Bin Chen, 1 Julie Aldridge, 2 Haesook T. Kim, 2 Karen K. Ballen, 1 Corey
Reduced-Intensity Conditioning Stem Cell Transplantation: Comparison of Double Umbilical Cord Blood and Unrelated Donor Grafts Yi-Bin Chen, 1 Julie Aldridge, 2 Haesook T. Kim, 2 Karen K. Ballen, 1 Corey
5/9/2018. Bone marrow failure diseases (aplastic anemia) can be cured by providing a source of new marrow
 5/9/2018 or Stem Cell Harvest Where we are now, and What s Coming AA MDS International Foundation Indianapolis IN Luke Akard MD May 19, 2018 Infusion Transplant Conditioning Treatment 2-7 days STEM CELL
5/9/2018 or Stem Cell Harvest Where we are now, and What s Coming AA MDS International Foundation Indianapolis IN Luke Akard MD May 19, 2018 Infusion Transplant Conditioning Treatment 2-7 days STEM CELL
Medical Policy. MP Ingestible ph and Pressure Capsule
 Medical Policy BCBSA Ref. Policy: 2.01.81 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.20 Esophageal ph Monitoring 6.01.33 Wireless Capsule Endoscopy as a
Medical Policy BCBSA Ref. Policy: 2.01.81 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.20 Esophageal ph Monitoring 6.01.33 Wireless Capsule Endoscopy as a
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: Original Effective Date: MM.07.011 04/01/2008 Line(s) of Business: Current Effective Date:
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: Original Effective Date: MM.07.011 04/01/2008 Line(s) of Business: Current Effective Date:
NiCord Single Unit Expanded Umbilical Cord Blood Transplantation: Results of Phase I/II Trials
 NiCord Single Unit Expanded Umbilical Cord Blood Transplantation: Results of Phase I/II Trials Mitchell E. Horwitz, MD Duke University Medical Center Duke Cancer Institute Adult Umbilical Cord Blood Transplantation
NiCord Single Unit Expanded Umbilical Cord Blood Transplantation: Results of Phase I/II Trials Mitchell E. Horwitz, MD Duke University Medical Center Duke Cancer Institute Adult Umbilical Cord Blood Transplantation
Recommended Timing for Transplant Consultation
 REFERRAL GUIDELINES Recommended Timing for Transplant Consultation Published jointly by the National Marrow Donor Program /Be The Match and the American Society for Blood and Marrow Transplantation BeTheMatchClinical.org
REFERRAL GUIDELINES Recommended Timing for Transplant Consultation Published jointly by the National Marrow Donor Program /Be The Match and the American Society for Blood and Marrow Transplantation BeTheMatchClinical.org
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Policy Number: 8.01.15 Last Review: 6/2017 Origination: 12/2001 Next Review: 6/2018 Policy Blue Cross and
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Policy Number: 8.01.15 Last Review: 6/2017 Origination: 12/2001 Next Review: 6/2018 Policy Blue Cross and
HCT for Myelofibrosis
 Allogeneic HSCT for MDS and Myelofibrosis Sunil Abhyankar, MD Professor Medicine, Medical Director, Pheresis and Cell Processing University of Kansas Hospital BMT Program April 27 th, 213 HCT for Myelofibrosis
Allogeneic HSCT for MDS and Myelofibrosis Sunil Abhyankar, MD Professor Medicine, Medical Director, Pheresis and Cell Processing University of Kansas Hospital BMT Program April 27 th, 213 HCT for Myelofibrosis
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2018 Origination: 7/2013 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2018 Origination: 7/2013 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Related haploidentical donors versus matched unrelated donors
 Related haploidentical donors versus matched unrelated donors Bronwen Shaw, MD PhD Professor of Medicine, MCW Senior Scientific Director, CIBMTR Definition Matched Unrelated donor Refers to HLA matching
Related haploidentical donors versus matched unrelated donors Bronwen Shaw, MD PhD Professor of Medicine, MCW Senior Scientific Director, CIBMTR Definition Matched Unrelated donor Refers to HLA matching
Haematopoietic stem cell transplantation (SCT)
 HAEMATOPOIETIC TEM CELL TRANPLANTATION FOR CHILREN IN BELGIUM Marie-Françoise resse 1 & Yves Beguin 2 1 epartment of Paediatrics, ivision of Hemato-Oncology, UHOPL, University of Liege, Liège, Belgium;
HAEMATOPOIETIC TEM CELL TRANPLANTATION FOR CHILREN IN BELGIUM Marie-Françoise resse 1 & Yves Beguin 2 1 epartment of Paediatrics, ivision of Hemato-Oncology, UHOPL, University of Liege, Liège, Belgium;
Overview of Aplastic Anemia. Overview of Aplastic Anemia. Epidemiology of aplastic anemia. Normal hematopoiesis 10/6/2017
 Overview of Aplastic Anemia Overview of Aplastic Anemia Peter Westervelt, MD, PhD Professor of Medicine Chief, BMT/Leukemia Section Washington University School of Medicine Epidemiology Normal hematopoiesis
Overview of Aplastic Anemia Overview of Aplastic Anemia Peter Westervelt, MD, PhD Professor of Medicine Chief, BMT/Leukemia Section Washington University School of Medicine Epidemiology Normal hematopoiesis
COHEM Barcellona 2012 Hemoglobinopathies debate
 COHEM Barcellona 2012 Hemoglobinopathies debate September 8, 2012: h. 10:30-12:00 Hall: A Is it justified to perform BMT in hemoglobinopathies using unrelated and/or partially mismatched donors? HSCT indication
COHEM Barcellona 2012 Hemoglobinopathies debate September 8, 2012: h. 10:30-12:00 Hall: A Is it justified to perform BMT in hemoglobinopathies using unrelated and/or partially mismatched donors? HSCT indication
Umbilical Cord Blood Transplantation
 Umbilical Cord Blood Transplantation Current Results John E. Wagner, M.D. Blood and Marrow Transplant Program and Stem Cell Institute University of Minnesota Donor Choices Unrelated Marrow/PBSC Results
Umbilical Cord Blood Transplantation Current Results John E. Wagner, M.D. Blood and Marrow Transplant Program and Stem Cell Institute University of Minnesota Donor Choices Unrelated Marrow/PBSC Results
EBMT Complications and Quality of Life Working Party Educational Course
 EBMT Complications and Quality of Life Working Party Educational Course Organisers: R. Duarte, G. Basak 23-24 October 2014, Warsaw, Poland #EBMT2014 www.ebmt.org EBMT Complications and Quality of Life
EBMT Complications and Quality of Life Working Party Educational Course Organisers: R. Duarte, G. Basak 23-24 October 2014, Warsaw, Poland #EBMT2014 www.ebmt.org EBMT Complications and Quality of Life
Company Overview. January 2019
 Company Overview January 2019 1 Disclaimer This Presentation includes certain projections and forward-looking statements as of the date of this Presentation provided by Gamida Cell Ltd (the Company ).
Company Overview January 2019 1 Disclaimer This Presentation includes certain projections and forward-looking statements as of the date of this Presentation provided by Gamida Cell Ltd (the Company ).
REVIEW ARTICLE. Umbilical Cord Blood Transplantation: Where Do We Stand? ASBMT BB&MT. Raymond C. Wadlow, David L. Porter
 Biology of Blood and Marrow Transplantation 8:637-647 (2002) 2002 American Society for Blood and Marrow Transplantation ASBMT REVIEW ARTICLE Umbilical Cord Blood Transplantation: Where Do We Stand? Raymond
Biology of Blood and Marrow Transplantation 8:637-647 (2002) 2002 American Society for Blood and Marrow Transplantation ASBMT REVIEW ARTICLE Umbilical Cord Blood Transplantation: Where Do We Stand? Raymond
An Overview of Blood and Marrow Transplantation
 An Overview of Blood and Marrow Transplantation October 24, 2009 Stephen Couban Department of Medicine Dalhousie University Objectives What are the types of blood and marrow transplantation? Who may benefit
An Overview of Blood and Marrow Transplantation October 24, 2009 Stephen Couban Department of Medicine Dalhousie University Objectives What are the types of blood and marrow transplantation? Who may benefit
12 Dynamic Interactions between Hematopoietic Stem and Progenitor Cells and the Bone Marrow: Current Biology of Stem Cell Homing and Mobilization
 Table of Contents: PART I: Molecular and Cellular Basis of Hematology 1 Anatomy and Pathophysiology of the Gene 2 Genomic Approaches to Hematology 3 Regulation of Gene Expression, Transcription, Splicing,
Table of Contents: PART I: Molecular and Cellular Basis of Hematology 1 Anatomy and Pathophysiology of the Gene 2 Genomic Approaches to Hematology 3 Regulation of Gene Expression, Transcription, Splicing,
