spermatogonial transplantation (testis/tem cels/spermaoneis/transec mice/fertty)
|
|
|
- Calvin Singleton
- 6 years ago
- Views:
Transcription
1 Proc. Natl. Acad. Sci. USA Vol. 91, pp , November 1994 Developmental Biology Germline transmission of donor haplotype following spermatogonial transplantation (testis/tem cels/spermaoneis/transec mice/fertty) RALPH L. BRINSTER* AND MARY R. AVARBOCK Laboratory of Reproductive Physiology, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA Contributed by Ralph L. Brinster, August 18, 1994 ABSTRACT Spermatogenesis is a complex, highly organized, very efficient process that is based upon the capacity of stem cell spermatogonia simultaneously to undergo selfrenewal and to provide progeny that differentiate Into mature spermatozoa. We report here that testis-derived cells transplanted Into the testis of an Infertile mouse will colonize seminiferous tubules and Initiate spermatogenesis in >70% of recipients. Testis-derived cells from newborn mice were less effective In colonizing recipient testes than cells from 5- to 15- or 21- to 28-day-old mice. Increasing the number of Sertoll cells in the donor cell population did not increase the efficiency of colonization. Unmodified embryonic stem cells were not able to substitute for testis-derived cells in colonizing testes but instead formed tumors in syngeneic as well as nonsyngeneic hosts. Finally, with recipients that maintained endogenous spermatogenesis, testis cell transpantatlon yielded mice in which up to 80% ofprogeny were sired by donor-derived spermatozoa. The technique of spermatogonial cell transplantation should provide a means to generate gelne modfications in a variety of species following development of spermatogonial culture techniques and should have additional applications in biology, medicine, and agriculture. Mature spermatozoa represent the product of a long developmental process that begins with differentiation of male primordial germ cells thought to arise from embryonic ectoderm (epiblast) in the 7-day-old mouse embryo (1, 2). During migration to the genital ridge, the number of primordial germ cells increases from about 100 on fetal day 7 to -25,000 on fetal day 13.5 (3, 4). Soon after arriving in the genital ridge, male germ cells stop dividing and remain quiescent within the seminiferous tubules of the fetal testis until several days after birth. During this period they are referred to as gonocytes (5, 6). Following birth the gonocytes divide and differentiate into spermatogonia, which then begin the complicated process of spermatogenesis. The spermatogonia, which form the basal layers within the seminiferous tubule, have been classified into three principal types: stem cell spermatogonia, proliferative spermatogonia, and differentiating spermatogonia (5, 7). The first two types appear to undergo self-renewal, and the proliferative spermatogonia simultaneously provide a population of differentiating spermatogonia, which are irreversibly committed to undertake the steps resulting in production of mature spermatozoa. During this process, spermatogonia undergo extensive replication, and most of the increase in cell number that characterizes spermatogenesis occurs at this time (7). Ultimately the spermatogonia form primary spermatocytes, which initiate the meiotic process and produce round spermatids. Round spermatids, in turn, undergo spermiogenesis, yielding the morphologically and functionally distinctive mature spermatozoa. Throughout The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" in accordance with 18 U.S.C solely to indicate this fact this process, differentiating cells are supported by Sertoli cells and form highly ordered, characteristic cellular associations within the seminiferous tubule (6-8). In the mouse, the time from spermatogonial differentiation to production of mature spermatozoa is 35 days, with roughly one-third of the time in spermatogonial mitosis, one-third in meiosis, and one-third in spermiogenesis (5-7). Considering the high efficiency ofsperm production (on the order of 107 spermatozoa produced daily in the rat per gram of testicular tissue; ref. 9) and the fact that this process is active from puberty to old age, spermatogenesis is truly a remarkable phenomenon. This process is based on the stem cell spermatogonia. These cells undergo self-renewal throughout life, and because of their ability to transmit genes from one generation to the next they represent the only replicating potentially totipotent population of cells in the adult body and may in this sense be thought of as immortal (1). To study the function of these cells during spermatogenesis and to establish a system to exploit their unique ability to transmit genes to successive generations (10), we have developed a technique to transfer cells from donor testes into an infertile recipient testis. We report here that spermatogonia appear more efficient than gonocytes in repopulating a recipient testis and that progeny result from transplanted donor spermatogonia. MATERIALS AND METHODS Cell Preparation. Male germ cells were isolated from mice of three different ages: between embryonic day 18 (E18) and postnatal day 2 (P2), between P5 and P15; and between P21 and P28. The basic procedures used have been described (11). Several modifications were introduced to increase the number and improve the handling of isolated cells. In brief, testes were collected (the larger numbers from the youngest animals), the tunica was removed, and the exposed tubules were subjected to collagenase (1 mg/ml) treatment followed by trypsin (0.25%) digestion. The released cells were centrifuged at 600 x g at 16'C for 5 min. After centrifugation, the supernatant was removed and the cells were suspended in injection medium. The composition ofthe medium was based on a formula originally developed for mouse eggs (12) and subsequently modified for use with primordial germ cells (13). These cell types have a requirement or preference for pyruvate and lactate as energy substrates, and male germ cells may share this characteristic because of their origin from primordial germ cells. The composition of the injection medium was 132 mm NaCl/7.8 mm Na2HPO4/2.6 mm KCl/1.1 mm KH2PO4/0.1 mm EDTA/0.25 mm pyruvate/3 mm lactate/1 mm glutamine/ 5.5 mm glucose/0.5% bovine serum albumin with DNase I Abbreviations: En, embryonic day n; Pn, postnatal day n; W/W, homozygous and heterozygous W mice; X-Gal, 5-bromo-4-chloro 3-indolyl P-D-galactoside. *To whom reprint requests should be addressed.
2 11304 Developmental Biology: Brinster and Avarbock (200 pg/ml) at ph 7.4. The cells were resuspended in 1.6 ml of the injection medium, and aliquots (400 IlI) were transferred to microcentrifuge tubes on ice. Cell concentration values for different-age testes were determined on samples of cells collected in an identical manner on days when injections were not performed. There was variation in cell concentration from day to day resulting from the number of testes available and the age of the donor males. An attempt was made to maximize the number of cells available in order to inject a concentrated solution, since the number of stem cells was unknown but thought to be small. One tube containing 400,l4 was used to inject the testes of a single mouse. Before the cell suspension was introduced into the injection pipette, 50.d of filtered (4%) trypan blue was added to the tube and the suspension was agitated. The dye allowed visualization of solution flow in the seminiferous tubule and was used to determine the number of dead cells in the injection suspension, which was generally <5%. It was not possible to measure the volume of cell suspension that entered the tubules, but the percent of surface tubules that filled with solution was recorded for each recipient testis. Sertoli cells were isolated and maintained as described (14). They were added to the germ cells just before injection into the tubules. Embryonic stem cells (AB-1) were a gift from Alan Bradley and were maintained as described (15, 16). Transplantation Procedure. Two separate protocols were used to transfer donor cells into recipient mice. In protocol 1, donor cells were isolated from the testes of C57BL/6 mice that were homozygous for a dominant mutant that resulted in tan belly hair. These cells were transferred into testes of compound heterozygous or homozygous mutant W mice (designated W/W), which do not show spermatogenesis (reviewed in ref. 17; R.L.B., unpublished data). In protocol 2, donor cells were isolated from mice (designated ZFlacZ) that were heterozygous for an Escherichia coli f3galactosidase transgene (lacz), which allowed round spermatids to be stained blue following incubation with substrate (18). Donor cells from these mice were injected into testes of (C57BL/6 x SJL)F1 males that had been treated with busul- Proc. NatL Acad Sci. USA 91 (1994) fan (40 mg/kg, intraperitoneally) at 4-6 weeks of age. This treatment destroys spermatogenic stem cells (19). Cell Injection. To minimize the number of injection sites and to increase the efficiency of injecting concentrated cell suspensions, a glass pipette with a 1-mm outside diameter and a 40-pum tip was secured in a micropipette holder [WPI Instruments (Waltham, MA) catalogue no. MPH6S]. The tip of a 1-ml plastic syringe was then inserted into the other end of the micropipette holder, and the syringe was cut at the 0.4-ml mark. The cell solution containing the dye was deposited in the syringe barrel, which was then screwed onto the metal end of an Eppendorf capillary holder attached by tubing to a pressure injector (Eppendorf model 5242). To expose the tubules, only one to three small incisions (1-3 mm) were made in the tunica per testis. With these modifications, an average of 80% of the surface tubules could be filled with concentrated cell suspension (Table 1). Analysis of Recipient Testes. To allow donor cells to undergo at least one cycle of spermatogenesis, recipient males were maintained a minimum of 50 days following injection before sacrifice. In protocol 1, the testes were fixed and 5-pam microscopic sections cut and stained with hemotoxylin and eosin. The number of tubule cross sections showing any stages of spermatogenesis was recorded. In protocol 2, the testes were incubated with 5-bromo-4-chloro-3-indolyl 1-Dgalactoside (X-Gal), and the number of tubules that stained blue recorded, up to a maximum of 12; beyond this, it was difficult to identify individual tubules because of their tortuous course, and these testes were recorded as 12+. For some mice in protocol 2, microscopic sections were cut and stained with neutral fast red. The number of tubule cross sections that stained blue generally was greater than the number of tubules visibly blue on gross examination. Progeny of recipient males (protocol 2 only) were analyzed either by incubating testes with X-Gal or by assaying for the presence ofthe transgene (18). RESULTS Donor cells were collected from mouse testes at three representative developmental stages. The first, between E18 Table 1. Spermatogonial stem cell transplantation into recipient testes Concentration of Donor No. of No. of testes Donor cell donor cells,* cell testes Percent surface Time to with donor No. of tubules with age, days no. x 10-6 straint injected area coveredt analysis, days cellsi (%) donor cells"i E18-P [10-51] C57BL/ ± 20 [30-100] 81 ± 20 [54-130] 6 (13) 3.5 ± 2.3 [1-7] ZFlacZ [40-100] 81 ± 14 [60-110] 18 (45) 3.2 ± 2.5 [1-9] P ± 18 [44-108] C57BL/ ± 19 [30-100] [70-300] 41 (87) 14.5 ± 16.2 [1-70] ZFlacZ ± 15 [50-100] 87 ± 37 [60-197] 49 (100) 8.1 ± 4.0 [1-12+] P ± 32 [45-123] C57BL/ ± 7 [75-95] 99 ± 43 [70-180] 7 (64) 16.7 ± 6.4 [9-26] ZFlacZ ± 15 [40-100] 80 ± 25 [60-155] 52 (100) 5.1 ± 3.4 [1-12+] Values are mean ± SD; range is in brackets. *No. of determinations of cell concentration was six for E18-P2, nine for P5-15, and six for P tc57bl/6 testis cells were transferred to the seminiferous tubules of homozygous or compound heterozygous WIW mice that lack spermatogenesis (protocol 1). ZFlacZ testis cells from mice of C57BL/6 x SJL genotype were transferred to the seminiferous tubules of (C57BL/6 x SJL)F1 hybrid mice in which endogenous spermatogenesis had been destroyed by busulfan treatment (protocol 2). ZFlacZ mice were heterozygous for a transgene (-3.5-kb cems177/lacz), and the transgenic lines were designated TgN(cl771acZ)226Bri and TgN(cl771acZ)227Bri (18). VPercent of the surface seminiferous tubules in the recipient testis filled by the injected cell suspension. No. of days from injection of donor cells to analysis of the recipient testis for presence of spermatogenesis. This represents the time available for donor cell proliferation. leffects of donor cell age and donor cell strain (C57BL/6 vs. ZFlacZ) were both significant by analysis of variance (0.05 > P > 0.01). "No. of tubules with spermatogenesis in those recipient testes that had evidence of donor cell colonization. In C57BL/6 donor cell recipients, the tubule cross sections with evidence of spermatogenesis were counted. In ZFlacZ donor cell recipients, the individual tubules stained blue were counted up to a maximum of 12. Tubules in testes with >12 stained could not be accurately counted. Therefore, in this column the values for C57BL/6 cells are not directly comparable to those for ZFlacZ cells. The difference between the C57BL/6 values for the E18-P2 age group and the other two groups was significant (P < 0.001). The difference between the ZFlacZ values for the E18-P2 age group and the P5-15 age group was significant (P < 0.001), and the difference between the E18-P2 age group and the P21-28 age group was also significant (0.02 > P > 0.01) (analyses by t test). The ZFlacZ statistical comparisons underestimate the true difference, because in testes with 12+ tubules staining, the exact number of tubules with spermatogenesis was too great to count accurately.
3 Developmental Biology: Brinster and Avarbock and P2, provided primarily gonocytes and mitotically active Sertoli cells. The second, between P5 and P15, provided germ cells and Sertoli cells in transition between the perinatal and adult stages. The third, between P21 and P28, provided quiescent Sertoli cells and a germ cell distribution approaching that of mature testes. The average time from cell transplantation to analysis was =90 days, with a range of days (Table 1). This represented 2-10 spermatogenic cycles. In total, 245 testes were injected with donor cells, of which 173 (71%o) showed evidence that transplanted cells had survived, colonized recipient seminiferous tubules, and initiated spermatogenesis (Table 1). Cells isolated from perinatal testes (E18 to P2) colonized 24 of 86 injected testes, with an average of 3 to 4 tubules (out of visible in a cross section) showing evidence of donor cell spermatogenesis. Donor cells isolated from P5 to P15 and P21 to P28 displayed a greater ability to colonize recipient testes: 90 of 96 and 59 of 63 testes, respectively, showed evidence of donor cell spermatogenesis. Donor cell age and strain (protocol 1 vs. protocol 2) both had a significant effect on the number of testes colonized (Table 1). The number of tubules supporting spermatogenesis for P5-15 and P21-28 cells was also significantly greater than for the perinatal cells. Although the concentration of donor cells injected was lower for suspensions from perinatal animals, reflecting the small size of donor testes, the percent of surface tubules filled with cell suspension (=80%) and the percent live cells, as indicated by trypan blue, were similar for all three donor-stage cell preparations. After colonization of recipient seminiferous tubules, donor cells generally established a morphological pattern representative of normal spermatogenesis. For protocol 1, all stages of sperm cell differentiation could be identified in microscopic cross sections of the seminiferous tubules. None of these stages was seen in control testes not injected with cells, because there is no endogenous spermatogenesis in W mice or in tubules of injected testes not populated with donor cells. Likewise, ZFlacZ donor cells (protocol 2) established normal spermatogenesis in busulfan-treated recipients with typical sperm cell associations and morphology of the tubules, and mature spermatozoa were produced (Fig. 1A). The testes of mice injected with busulfan but not receiving donor cells do not generally have tubules with spermatogenesis (ref. 19; R.L.B., unpublished data). In mice receiving a lower dose of busulfan (e.g., when there was leakage at the time of injection), some tubules could be repopulated by surviving endogenous stem cells. However, these tubules would be distinct, since round spermatids and subsequent stages derived from endogenous stem cells do not stain blue with X-Gal. The success of the transferred donor cells in colonizing the recipient testis varied, and some testes from ZFlacZ mice demonstrated up to 100 cross sections containing various stages of spermatogenesis. In testes with the most extensive colonization, by either C57BL/6 or ZFlacZ donor cells, mature spermatozoa were transported to the epididymis (Fig. 1B). No sperm were seen in epididymal tubules when recipient testes were not colonized by donor cells. As anticipated from the observation of spermatozoa in the epididymides of some experimental mice, spermatozoa could also be identified in the ejaculate of these recipient males. Initially, the number of spermatoza was insufficient to result in progeny (by normal mating). An approach to facilitate production of offspring from donor-derived spermatozoa would be to provide carrier spermatozoa. As a consequence of occasional busulfan leakage at the time of injection or variability of individual animal susceptibility, a few experimental recipients may acquire fertility following tubule repopulation by endogenous stem cells. Ifdonor cells have also become established, a fraction of tubules with spermatogenesis will stain blue (Fig. 2), and the ejaculate will contain Proc. Nad. Acad. Sci. USA 91 (1994) FIG. 1. Spermatogenesis in the testis ofa recipient male following transfer of ZFlacZ donor cells. (A) Testis from (C57BL/6 x SJL)F1 busulfan-treated male 6 months after donor cell transfer. Note active spermatogenesis and mature spermatozoa. Round spermatids and more mature stages stain blue. When stain is intense, immature stages also appear blue. (B) Tubules from epididymis of the mouse with testis shown ina. Many mature spermatozoa appear as red dots in the lumen of the tubules. Background blue color in the lumen is the result of f-galactosidase activity arising from the cytoplasm of immature sperm cells. Mature spermatozoa do not stain for a-galactosidase because they lack sufficient cytoplasm. (X-Gal followed by neutral fast red; A, x125; B, x55.) spermatozoa derived from endogenous and transplanted stem cells. Half the male progeny that develop from eggs fertilized with spermatozoa of ZFlacZ donor stem cell origin should have testes that stain blue with X-Gal, because transferred ZFlacZ cells are heterozygous for the transgene. Testes from progeny of a recipient in which spermatogenesis occurred from endogenous and transplanted stem cells are shown in Fig. 3; however, only 1 of 122 male offspring carried the transgene. To specifically generate recipients that would reestablish endogenous spermatogenesis, protocol 2 was used; 10 males were treated with busulfan at 30 mg/kg, and their testes were injected with donor cells from P5-15 or P21-28 mice. Testes of 7 recipients were examined days after injection. All were enlarged relative to animals treated with busulfan at 40 mg/kg, indicating a significant level of spermatogenesis. The testes contained 8.2 ± 4.0 (mean ± SD) blue-staining tubules. Of the 3 remaining males, 1 that received P5-15 cells is still infertile, 1 that received P21-28 cells has sired 6 of 39 transgenic progeny, and the third, which received P5-15 cells, has sired 6 of 15 transgenic progeny. Offspring were born beginning :8 months after donor cell injection. With this approach, up to 80%6o of the progeny of the male with the most successful colonization are from donor-derived spermatozoa, half of which carry the transgene. Although injected cell suspensions contained donor Sertoli cells, several experiments were performed in which Sertoli cells (10W-107) isolated as described were added to the donor cell suspension to determine whether this would enhance repopulation. With protocol 1 and donor cells from P5-15 mice, 10 of 12 recipient testes demonstrated spermatogenesis, with 13.0 ± 12.7 (mean + SD) tubule cross sections colonized per testis. With protocol 2 and the same age donor cells, 24 of25 testes had stained tubules with 9.0 ± 3.7 tubules stained per testis. The average time of analysis of the recipients after donor cell injection was 112 days. In general, the degree of colonization with and without additional Sertoli cells was similar (compare with Table 1). Embryonic stem cells are closely related or identical to embryonal carcinoma cells, which arise from male primordial
4 11306 Developmental Biology: Brinster and Avarbock Proc. Nad. Acad. Sci. USA 91 (1994).y,..; go FIG. 3. Testes from progeny of the male described in Fig. 2. The testis on the left stained blue with X-Gal, indicating the presence of the transgene in all cells. The testis on the right is from another offspring and is not blue because it does not contain the transgene. The seminiferous tubules of the parent male displayed a mosaic pattern of staining resulting from donor stem cell and endogenous stem cell areas of spermatogenesis (see Fig. 2). Progeny was -10 weeks old, born 11 months after donor cell transfer. (Bar = 0.25 cm.).:i4 injection of AB-1 cells (2 x 106 cells) produced tumors in 8 W/W54 mice in 30 days but not in 7 WIW mice in days, indicating that the WIW mice were resistant to tumor formation from these cells by this route of administration. FIG. 2. Testis of busulfan-treated recipient male injected with ZFlacZ donor cells 14 months previously. (A) Gross appearance. The testis is -80%o of normal size, because an inadequate busulfan effect permitted the reinitiation of spermatogenesis from endogenous stem cells (unstained tubules). Blue tubules indicate spermatogenesis from ZFlacZ donor spermatogonial stem cells. The testis has been partially transected longitudinally to allow for penetration offixative and stain (X-Gal). (Bar = 1 mm.) (B) Microscopic appearance. Blue tubule cross sections represent spermatogenesis from donor stem cells. Red tubule cross sections reflect spermatogenesis from endogenous stem cells. The ratio of blue to red tubules across several complete testis cross sections in this area was -1:12. Other areas of the testis have fewer blue tubules. (X-Gal followed by neutral fast red; x30.) germ cells (1). Both types of stem cells, as well as primordial germ cells, will colonize blastocysts and differentiate into all cell types of the body, including male germ cells (1, 15, 20). Therefore, the ability of embryonic stem cells to become spermatogonia was examined by injecting embryonic stem cells (AB-1; ref. 15) into the seminiferous tubules of WIWand W/W54 mice. The WS4 allele is carried on the 129/SvCP genetic background, from which AB-1 cells arise, and therefore the W/W5 cross would be immunologically tolerant to AB-1 cells. The W/W mice are of C57BL/6 genotype and would not be tolerant to AB-1 cells. Cell suspensions (15-60 x 106 cells per ml) were injected (0.4 ml per mouse) into the tubules of 10 W/W54 recipients to determine whether any spermatogenesis would develop. All of the mice died or had large testicular and/or abdominal tumors within 30 days of injection. In similar experiments, 9 W/W mice were injected with AB-1 cells. One of these animals died, and the remainder were examined between 24 and 130 days after injection. All but one of these animals developed tumors of the testes and/or in the abdominal cavity. Regardless of whether the testes were examined soon after injection (30 days) or at a later time (130 days) there was no sign of spermatogenesis, even in tubules not affected by tumorigenesis. Subcutaneous DISCUSSION The studies described above demonstrate that stem cells can be harvested from donor testes, maintained in vitro, transferred to a recipient testis, establish normal spermatogenesis, and produce functional spermatozoa that fertilize eggs and result in offspring. The production of young from the transplanted spermatogonial stem cells and the high percentage of recipient spermatozoa arising from the transplanted cells indicate that colonization of the seminiferous tubules can be very effective. Future efforts should increase the percentage of recipient males with high-level colonization and thereby increase the usefulness of spermatogonial transplantation. In these studies, donor cell populations were mixed, containing Sertoli cells and various stages of differentiating sperm cells that varied with the age of the donor testes (5, 6, 11). Among this diverse group of cell types, only cells with stem cell potential could initiate spermatogenesis. Spermatocytes, meiotic stages, and spermatids are not capable of self-renewal. Thus, even if donor cells representing later stages of spermatogonia established an interaction with recipient Sertoli cells and continued to differentiate, they would become fully mature and be shed into the lumen by 35 days after transfer (6, 7). Spermatogenic elements visible in recipient testes after this time would necessarily have arisen from donor cells with stem cell potential. The age of the testes from which donor cells were isolated had an influence on the efficiency of colonization ofrecipient testes. At birth the germ cells are gonocytes, but during the first week of life these cells differentiate into spermatogonia, which divide and begin to differentiate (5, 6). Thereafter, the spermatogonial cell population expands and differentiating stages of spermatogenesis appear successively. In prepubertal mice this first wave of spermatogenesis is synchronized, with spermatocytes appearing at about day 10, spermatids at about day 20, and mature spermatozoa at about day 35 (6, 11). Sertoli cells also undergo changes during this period. They continue to divide until between P10 and P14, after which they become mitotically quiescent (8). Thus, by recovering donor cells at birth, P5-15, and P21-28, three different mixed cell populations were tested for stem cell potential. Surprisingly, the results suggest that gonocytes, despite their primitive stage of development, were less efficient as donor cells
5 Developmental Biology: Brinster and Avarbock than older populations of cells containing spermatogonia. However, it has not been determined whether the stem cell populations in the three developmental stages tested have a differential sensitivity to the harvesting protocol employed. Although the number ofdead cells as indicated by trypan blue staining was similar for the three cell preparations, differential killing of small populations of stem cells may not have been detectable. Also, stem cells are likely to make up a different fraction of the total cell population at each stage (5-7). These factors may also account for the lower efficiency ofc57bl/6 donor cells in colonizing recipients. However, an additional element in this effect could be an inferior ability of the tubules in W recipients to support spermatogenesis. Spermatogenesis was morphologically normal in many tubules examined (7), indicating effective colonization of seminiferous tubules. The donor cell population contained both germ cells and Sertoli cells; therefore, it could not be determined whether the interacting Sertoli-spermatogonial units that established a spermatogenic colony and repopulated the tubule were composed of donor germ cells interacting with endogenous Sertoli cells, with transferred Sertoli cells, or both. However, when additional Sertoli cells were coinjected with germ cells they did not have a dramatic effect on colonization efficiency. This suggests that Sertoli cells were not a limiting factor and may indicate that endogenous Sertoli cells were adequate to support recolonization. Because of the close relationship of embryonic stem cells, embryonal carcinoma cells, and primordial germ cells, including their ability to differentiate into many tissue types (1, 15, 20), we examined the effect of the seminiferous tubule environment on their differentiation pathway. Under these experimental conditions, and in contrast to the blastocyst, the tubule environment was not able to control the growth and differentiation pathway of AB-1 cells. In vitro manipulation of embryonic stem cells may be required before this cell type can be directed to differentiate appropriately within the tubule microenvironment (10). The most striking result of these experiments was production of offspring from donor cell-derived spermatozoa. The ability to achieve an effective level of seminiferous tubule colonization and to produce efficiently donor cell-derived progeny will facilitate a wide range of experimental approaches using spermatogonial transplantation. The opportunity now exists to develop a culture system that allows expansion of spermatogonial stem cells and selection of specifically modified clones of individual cells; spermatogonial culture could not be developed without a technique to test the biological potential of cell populations isolated. A variety of agents (e.g., growth factors) can also be examined to ascertain their ability to modify the function of donor cells and recipient tubules. An important extension ofspermatogonial transplantation will be repopulation of testes of immunotolerant mice with donor cells from other species. In a properly prepared recipient, xenogeneic donor cell-derived spermatozoa may be matured along with endogenous spermatozoa in the epididymis, and the egg of the foreign species would provide the specificity of spermatozoal selection during in vitro or in vivo fertilization. The chicken will be an important species with which to examine the feasibility of this Proc. Natl. Acad Sci. USA 91 (1994) approach, since even testicular spermatozoa placed in the chicken oviduct are capable of fertilizing the egg (21). Production of offspring from spermatogonia transplanted to a recipient testis establishes a method to study and utilize these remarkable stem cells. Since spermatogonia constitute the only self-renewing population of cells in the adult body capable of contributing to succeeding generations, the ability to manipulate these cells in vitro before transfer to a recipient will represent a challenge and an opportunity to scientists interested in spermatogenesis, infertility, embryo development, and germline modifications. We thank our colleagues for many helpful discussions regarding the experiments and manuscript. We are grateful to Efren Leonida, Jr., for excellent technical assistance. We also thank D. Clouthierfor help in analyses, J. Zalles for histological slide preparation, J. Hayden for assistance with photographs, and C. Pope for manuscript preparation. Financial support for the work was from National Institutes of Health Grant HD23657 to R.L.B. 1. McLaren, A. (1992) Nature (London) 359, Matsui, Y., Zsebo, K. & Hogan, B. L. M. (1992) Cell 70, Mintz, B. & Russell, E. S. (1957) J. Exp. Zool. 134, Tam, P. P. L. & Snow, M. H. L. (1981) J. Embryol. Exp. Morphol. 64, Bellvd, A. R. (1979) in Oxford Reviews of Reproductive Biology, ed. Finn, C. A. (Clarendon, Oxford), Vol. 1, pp de Kretser, D. M. & Kerr, J. B. (1988) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. (Raven, New York), pp Russell, L. D., Ettlin, R. A., Hikim, A. P. & Clegg, E. D. (1990) in Histological and Histopathological Evaluation of the Testis (Cache River Press, Clearwater, FL), pp Gondos, B. & Berndston, W. E. (1993) in The Sertoli Cell, eds. Russell, L. D. & Griswold, M. D. (Cache River Press, Clearwater, FL), pp Wing, T. Y. & Christensen, A. K. (1982) Am. J. Anat. 165, Brinster, R. L. (1993) Int. J. Dev. Biol. 37, Bellvd, A. R., Cavicchia, J. C., Millette, C. F., O'Brien, D. A., Bhatnagar, Y. M. & Dym, M. (1977) J. Cell Biol. 74, Brinster, R. L. (1%5) J. Reprod. Fertil. 10, Brinster, R. L. & Harstad, H. (1977) Erp. Cell Res. 109, Karl, A. F. & Griswold, M. D. (1990) Methods Enzymol. 190, Bradley, A., Hasty, P., Davis, A. & Ramirez-Solis, R. (1992) BiolTechnology 10, Robertson, E. J. (1987) in Teratocarcinomas and Embryonic Stem Cells: A Practical Approach, ed. Robertson, E. J. (IRL, Oxford), pp Silvers, W. K. (1979) The Coat Colors of Mice: A Model for Mammalian Gene Action and Interaction (Springer, New York), pp Zambrowicz, B. P., Zimmermann, J. W., Harendza, C. J., Simpson, E. M., Page, D. C., Brinster, R. L. & Palmiter, R. D. (1994) Development (Cambridge, U.K.) 120, Bucci, L. R. & Meistrich, M. L. (1987) Mutat. Res. 176, Stewart, C. L., Gadi, I. & Bhatt, H. (1994) Dev. Biol. 161, Jones, R. C. & Lin, M. (1993) in Oxford Reviews in Reproduction Biology, ed. Milligan, D. R. (Oxford Univ., Oxford), Vol. 15, pp
Spermatogenesis following male germ-cell transplantation (spermatogonia/stem cd/testes/ nsc mice)
 Proc. Nati. Acad. Sci. USA Vol. 91, pp. 11298-11302, November 1994 Developmental Biology Spermatogenesis following male germ-cell transplantation (spermatogonia/stem cd/testes/ nsc mice) RALPH L. BRINSTER*
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 11298-11302, November 1994 Developmental Biology Spermatogenesis following male germ-cell transplantation (spermatogonia/stem cd/testes/ nsc mice) RALPH L. BRINSTER*
Pattern and Kinetics of Mouse Donor Spermatogonial Stem Cell Colonization in Recipient Testes 1
 BIOLOGY OF REPRODUCTION 60, 1429 1436 (1999) Pattern and Kinetics of Mouse Donor Spermatogonial Stem Cell Colonization in Recipient Testes 1 Makoto Nagano, Mary R. Avarbock, and Ralph L. Brinster 2 School
BIOLOGY OF REPRODUCTION 60, 1429 1436 (1999) Pattern and Kinetics of Mouse Donor Spermatogonial Stem Cell Colonization in Recipient Testes 1 Makoto Nagano, Mary R. Avarbock, and Ralph L. Brinster 2 School
5 15/3/2012. Malik Al-Momani
 5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Sami Ventelä, 1,2 Hiroshi Ohta, 3 Martti Parvinen, 2 and Yoshitake Nishimune 3
 BIOLOGY OF REPRODUCTION 66, 1422 1429 (2002) Development of the Stages of the Cycle in Mouse Seminiferous Epithelium after Transplantation of Green Fluorescent Protein-Labeled Spermatogonial Stem Cells
BIOLOGY OF REPRODUCTION 66, 1422 1429 (2002) Development of the Stages of the Cycle in Mouse Seminiferous Epithelium after Transplantation of Green Fluorescent Protein-Labeled Spermatogonial Stem Cells
Testicular stem cells
 Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Histology of Male Reproductive system (1)
 Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Germ cells and germ cell transplantation
 Int. J. Dev. Biol. 42: 855-860 (1998) EGF, epithelium and Germ cells and germ cell transplantation 855 Germ cells and germ cell transplantation ANNE MCLAREN* Wellcome/CRC Institute of Cancer and Developmental
Int. J. Dev. Biol. 42: 855-860 (1998) EGF, epithelium and Germ cells and germ cell transplantation 855 Germ cells and germ cell transplantation ANNE MCLAREN* Wellcome/CRC Institute of Cancer and Developmental
The spermatogenesis CHARACTERISTICS OF THE SPERMATOZOON 26/04/2017. Reproductive Biotechnologies Andrology I. Prof. Alberto Contri
 Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Production of Fertile Sperm. Animal Science 434. Hormonal Regulation of the Testis. hormonal regulation of the testis
 roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
Juvenile Spermatogonial Depletion (jsd ) Mutant Seminiferous Tubules Are Capable of Supporting Transplanted Spermatogenesis 1
 BIOLOGY OF REPRODUCTION 63, 1185 1191 (2000) Juvenile Spermatogonial Depletion (jsd ) Mutant Seminiferous Tubules Are Capable of Supporting Transplanted Spermatogenesis 1 H.L. Boettger-Tong, 2,3 D.S. Johnston,
BIOLOGY OF REPRODUCTION 63, 1185 1191 (2000) Juvenile Spermatogonial Depletion (jsd ) Mutant Seminiferous Tubules Are Capable of Supporting Transplanted Spermatogenesis 1 H.L. Boettger-Tong, 2,3 D.S. Johnston,
Germ Cell Transplantation in Fish
 Larvi 2009 Germ Cell Transplantation in Fish Goro Yoshizaki (Tokyo University of Marine Science and Technology, SORST/JST) Tuna Mackerel Body weight; 300 kg 300 g Body length; 3 m 30 cm Scombridae family
Larvi 2009 Germ Cell Transplantation in Fish Goro Yoshizaki (Tokyo University of Marine Science and Technology, SORST/JST) Tuna Mackerel Body weight; 300 kg 300 g Body length; 3 m 30 cm Scombridae family
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Microinsemination (Intracytoplasmic Sperm Injection) Microinsemination schedule. 1. Preparation of mediums
 Microinsemination (Intracytoplasmic Sperm Injection) Masumi Hirabayashi Section of Mammalian Transgenesis, Center for Genetic Analysis of Behavior, National Institute for Physiological Sciences, National
Microinsemination (Intracytoplasmic Sperm Injection) Masumi Hirabayashi Section of Mammalian Transgenesis, Center for Genetic Analysis of Behavior, National Institute for Physiological Sciences, National
SUPPLEMENTAL INFORMATION FOR. PAX7 expression defines germline stem cells in the adult testis
 SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
Adapted from Preg. & Part., Senger
 MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
Biology 4361 Developmental Biology. October 11, Multiple choice (one point each)
 Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
Cell Divisions. The autosomes represent the whole body. * Male Sex Chromosomes: XY * Female Sex Chromosomes: XX
 Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Assessment of Morphological and Functional Changes in the Mouse Testis and Epididymal Sperms Following Busulfan Treatment
 Iranian Biomedical Journal 11 (1): 15-22 (January 2007) Assessment of Morphological and Functional Changes in the Mouse Testis and Epididymal Sperms Following Busulfan Treatment Sayed Hadi Anjamrooz 1,
Iranian Biomedical Journal 11 (1): 15-22 (January 2007) Assessment of Morphological and Functional Changes in the Mouse Testis and Epididymal Sperms Following Busulfan Treatment Sayed Hadi Anjamrooz 1,
Outline. Male Reproductive System Testes and Sperm Hormonal Regulation
 Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM
 LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
Male Reproductive System
 Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Introduction. K. Zohni 1, X. Zhang 1, S.L. Tan 1, P. Chan 2, and M.C. Nagano 1, * ORIGINAL ARTICLE Andrology
 Human Reproduction, Vol.27, No.1 pp. 44 53, 2012 Advanced Access publication on November 14, 2011 doi:10.1093/humrep/der357 ORIGINAL ARTICLE Andrology The efficiency of male fertility restoration is dependent
Human Reproduction, Vol.27, No.1 pp. 44 53, 2012 Advanced Access publication on November 14, 2011 doi:10.1093/humrep/der357 ORIGINAL ARTICLE Andrology The efficiency of male fertility restoration is dependent
Reproductive Toxicology
 Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
The Use of Rabbits in Male Reproductive Toxicology
 Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
Male Reproductive System
 Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Meiosis & Sexual Reproduction. AP Biology
 Meiosis & Sexual Reproduction 2007-2008 Cell division / Asexual reproduction Mitosis produce cells with same information identical daughter cells exact copies clones same amount of DNA same number of chromosomes
Meiosis & Sexual Reproduction 2007-2008 Cell division / Asexual reproduction Mitosis produce cells with same information identical daughter cells exact copies clones same amount of DNA same number of chromosomes
Mohammad Sha ban. Basheq Jehad. Hamzah Nakhleh
 11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos
 /. Embryol. exp. Morph. Vol. 64, pp. 133-147, 1981 133 Printed in Great Britain Company of Biologists Limited 1981 Proliferation and migration of primordial germ cells during compensatory growth in mouse
/. Embryol. exp. Morph. Vol. 64, pp. 133-147, 1981 133 Printed in Great Britain Company of Biologists Limited 1981 Proliferation and migration of primordial germ cells during compensatory growth in mouse
Male reproduction. Cross section of Human Testis ผศ.ดร.พญ.ส ว ฒณ ค ปต ว ฒ ภาคว ชาสร รว ทยา คณะแพทยศาสตร ศ ร ราชพยาบาล 1. Aims
 Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM
 Unit 7A STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM LEARNING OBJECTIVES 1. Learn the structures of the male reproductive system. 2. Learn the functions of the male reproductive system. 3. Learn
Unit 7A STRUCTURE AND FUNCTION OF THE MALE REPRODUCTIVE SYSTEM LEARNING OBJECTIVES 1. Learn the structures of the male reproductive system. 2. Learn the functions of the male reproductive system. 3. Learn
Gametogenesis. Omne vivum ex ovo All living things come from eggs.
 Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
Chapter 26: Reproductive Systems. Male 11/29/2015. Male reproductive system is composed of... BIO 218 Fall Gonads (testes)
 Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
ABSTRACT. The objective of this study was to assess the effectiveness of a Nycodenz gradient
 ABSTRACT MILLER, STEPHANIE RENEE. Assessment of nycodenz gradient on enrichment and culture of perinatal porcine spermatogonial stem cells. (Under the direction of Robert M. Petters). The objective of
ABSTRACT MILLER, STEPHANIE RENEE. Assessment of nycodenz gradient on enrichment and culture of perinatal porcine spermatogonial stem cells. (Under the direction of Robert M. Petters). The objective of
Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature
 REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTIVE BIOTECHNOLOGY IN SWINE
 REPRODUCTIVE BIOTECHNOLOGY IN SWINE References * Animal breeding and infertility by M. J. Meredith * Controlled reproduction in pigs by I. Gordon * Reproduction in farm animals by E.S.E. Hafez * Progress
REPRODUCTIVE BIOTECHNOLOGY IN SWINE References * Animal breeding and infertility by M. J. Meredith * Controlled reproduction in pigs by I. Gordon * Reproduction in farm animals by E.S.E. Hafez * Progress
Biology 4361 Developmental Biology Exam 1 ID#: October 11, 2005
 Biology 4361 Developmental Biology Name: Key Exam 1 ID#: October 11, 2005 Multiple choice (one point each) 1. Primordial germ cells a. are immortal b. produce polar bodies c. are haploid d. are somatic
Biology 4361 Developmental Biology Name: Key Exam 1 ID#: October 11, 2005 Multiple choice (one point each) 1. Primordial germ cells a. are immortal b. produce polar bodies c. are haploid d. are somatic
Sperm production. Sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production. Sperm production. Controlling sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
IN normal male fowls, four developmental stages of spermatogenetic activity
 Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
Embryology 3. Spermatogenesis:
 Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Physiology of Male Reproductive System
 Physiology of Male Reproductive System the anterior pituitary gland serves as the primary control of reproductive function at puberty Ant Pituitary secretes FSH & large amounts of LH (ICSH) FSH & LH cause
Physiology of Male Reproductive System the anterior pituitary gland serves as the primary control of reproductive function at puberty Ant Pituitary secretes FSH & large amounts of LH (ICSH) FSH & LH cause
THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS
 Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Nonrandom contribution of left and right testes to germline transmission from mouse spermatogonial stem cells
 Biology of Reproduction, 2017, 97(6), 902 910 doi:10.1093/biolre/iox141 Research Article Advance Access Publication Date: 9 November 2017 Research Article Nonrandom contribution of left and right testes
Biology of Reproduction, 2017, 97(6), 902 910 doi:10.1093/biolre/iox141 Research Article Advance Access Publication Date: 9 November 2017 Research Article Nonrandom contribution of left and right testes
Supplemental Figure 1. (A) The localization of Cre DNA recombinase in the testis of Cyp19a1-Cre mice was detected by immunohistchemical analyses
 Supplemental Figure 1. (A) The localization of Cre DNA recombinase in the testis of Cyp19a1-Cre mice was detected by immunohistchemical analyses using an anti-cre antibody; testes at 1 week (left panel),
Supplemental Figure 1. (A) The localization of Cre DNA recombinase in the testis of Cyp19a1-Cre mice was detected by immunohistchemical analyses using an anti-cre antibody; testes at 1 week (left panel),
Spermatogonial stem cells: What does the future hold?
 F, V & V IN OBGYN, 2011, 3 (1): 36-40 Viewpoint Spermatogonial stem cells: What does the future hold? H. TOURNAYE, E. GOOSSENS Research unit Biology of the Testis; Department of Embryology and Genetics;
F, V & V IN OBGYN, 2011, 3 (1): 36-40 Viewpoint Spermatogonial stem cells: What does the future hold? H. TOURNAYE, E. GOOSSENS Research unit Biology of the Testis; Department of Embryology and Genetics;
SPERMATOGONIAL STEM CELL TRANSFER TO A MULE. Emily Smith
 SPERMATOGONIAL STEM CELL TRANSFER TO A MULE Emily Smith A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Horse Science Equine Physiology Middle Tennessee
SPERMATOGONIAL STEM CELL TRANSFER TO A MULE Emily Smith A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Horse Science Equine Physiology Middle Tennessee
ICSI with sperm derived from cultured testes (Nature 2011) Establishment of rabbit ips cells (J Biol Chem 2010)
 Efficient production of offspring in wild-derived strains of mice (Biol Reprod in press) (Presented by Keiji Mochida) Birth of offspring from ectopically transplanted PGCs (Biol Reprod 211) ICSI with sperm
Efficient production of offspring in wild-derived strains of mice (Biol Reprod in press) (Presented by Keiji Mochida) Birth of offspring from ectopically transplanted PGCs (Biol Reprod 211) ICSI with sperm
Morphogenesis of the residual body of the mouse testis
 93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
The use of Y-chromosome-specific repeated DNA sequences in the analysis of testis development in an XX/XY mouse
 Development 101 Supplement. 143 149 (1987) Printed in Great Britain The Company of Biologists Limited 1987 143 The use of Y-chromosome-specific repeated DNA sequences in the analysis of testis development
Development 101 Supplement. 143 149 (1987) Printed in Great Britain The Company of Biologists Limited 1987 143 The use of Y-chromosome-specific repeated DNA sequences in the analysis of testis development
Gametogenesis. To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis. Introduction
 Gametogenesis To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell
Gametogenesis To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell
HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY
 FERTILItY AND STI!RILITY Copyright 1974 The American Fertility Society Vol. 25, No.8, August 1974 PTillted in U.S.AI HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY FLETCHER C. DERRICK,
FERTILItY AND STI!RILITY Copyright 1974 The American Fertility Society Vol. 25, No.8, August 1974 PTillted in U.S.AI HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY FLETCHER C. DERRICK,
Rejuvenation of Gamete Cells; Past, Present and Future
 Rejuvenation of Gamete Cells; Past, Present and Future Denny Sakkas PhD Scientific Director, Boston IVF Waltham, MA, USA Conflict of Interest I have no conflict of interest related to this presentation.
Rejuvenation of Gamete Cells; Past, Present and Future Denny Sakkas PhD Scientific Director, Boston IVF Waltham, MA, USA Conflict of Interest I have no conflict of interest related to this presentation.
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER)
 THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
BIOH122 Session 26 Gametogenesis. Introduction. 1. a. Define gametogenesis. b. What cells are gametes?
 BIOH122 Session 26 Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell division processes that occur during the cell cycle? d. Define the cell cycle.
BIOH122 Session 26 Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell division processes that occur during the cell cycle? d. Define the cell cycle.
All You Wanted to Know About Spermatogonia but Were Afraid to Ask
 All You Wanted to Know About Spermatogonia but Were Afraid to Ask Review DIRK G. DE ROOIJ* AND LONNIE D. RUSSELL From the *Department of Cell Biology, University Medical Center Utrecht, Utrecht, The Netherlands;
All You Wanted to Know About Spermatogonia but Were Afraid to Ask Review DIRK G. DE ROOIJ* AND LONNIE D. RUSSELL From the *Department of Cell Biology, University Medical Center Utrecht, Utrecht, The Netherlands;
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following hormones controls the release of anterior pituitary gonadotropins? A) LH
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following hormones controls the release of anterior pituitary gonadotropins? A) LH
Physiologic Anatomy of the Male Sexual Organs
 Reproductive and Hormonal Functions of the Male The reproductive functions of the male can be divided into three major subdivisions: (1) spermatogenesis, which means simply the formation of sperm; (2)
Reproductive and Hormonal Functions of the Male The reproductive functions of the male can be divided into three major subdivisions: (1) spermatogenesis, which means simply the formation of sperm; (2)
Sterility in mutant (t Lx jt Ly } male mice
 /. Embryol. exp. Morph. Vol. 59, pp. 39-47, 1980 39 Printed in Great Britain Company of Biologists Limited 1980 Sterility in mutant (t Lx jt Ly } male mice II. A morphological study of spermatozoa By MARY
/. Embryol. exp. Morph. Vol. 59, pp. 39-47, 1980 39 Printed in Great Britain Company of Biologists Limited 1980 Sterility in mutant (t Lx jt Ly } male mice II. A morphological study of spermatozoa By MARY
Summary. Mouse eggs were fertilized in vitro, in the presence and
 THE R\l=O^\LEOF CUMULUS CELLS AND THE ZONA PELLUCIDA IN FERTILIZATION OF MOUSE EGGS IN VITRO A. PAVLOK and ANNE McLAREN Czechoslovak Academy of Sciences, Laboratory of Animal Genetics, Libechov, Czechoslovakia,
THE R\l=O^\LEOF CUMULUS CELLS AND THE ZONA PELLUCIDA IN FERTILIZATION OF MOUSE EGGS IN VITRO A. PAVLOK and ANNE McLAREN Czechoslovak Academy of Sciences, Laboratory of Animal Genetics, Libechov, Czechoslovakia,
CARD HyperOva (Superovulation Reagent for mouse)
 Product manual (Superovulation Reagent for mouse) Cat. No. KYD-010-EX -X5 Size: 5 1 ML Origin Serum of goat, Horse-derived villus gonatropin. Composition 1. Inhibin antiserum (Goat). 2. Equine chorionic
Product manual (Superovulation Reagent for mouse) Cat. No. KYD-010-EX -X5 Size: 5 1 ML Origin Serum of goat, Horse-derived villus gonatropin. Composition 1. Inhibin antiserum (Goat). 2. Equine chorionic
Computer Simulation of the Rodent Spermatogonial Stem Cell Niche 1
 BIOLOGY OF REPRODUCTION (2013) 88(5):131, 1 11 Published online before print 27 March 2013. DOI 10.1095/biolreprod.113.108639 Computer Simulation of the Rodent Spermatogonial Stem Cell Niche 1 Dirk G.
BIOLOGY OF REPRODUCTION (2013) 88(5):131, 1 11 Published online before print 27 March 2013. DOI 10.1095/biolreprod.113.108639 Computer Simulation of the Rodent Spermatogonial Stem Cell Niche 1 Dirk G.
Animal Development. Lecture 3. Germ Cells and Sex
 Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Reproductive System Purpose General Structures Male Structures Functions Female Anatomy Structures Functions Clinical Applications
 The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
Inhibition of Testicular Retinoic Acid Biosynthesis for Male Contraception. John K. Amory MD, MPH University of Washington October 30th, 2011
 Inhibition of Testicular Retinoic Acid Biosynthesis for Male Contraception John K. Amory MD, MPH University of Washington October 30th, 2011 Bisdichloroacetyldiamines (BDADs) Shown to reversibly inhibit
Inhibition of Testicular Retinoic Acid Biosynthesis for Male Contraception John K. Amory MD, MPH University of Washington October 30th, 2011 Bisdichloroacetyldiamines (BDADs) Shown to reversibly inhibit
Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish
 Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish Tomoyuki Okutsu*, Kensuke Suzuki*, Yutaka Takeuchi*, Toshio Takeuchi*, and Goro Yoshizaki*
Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish Tomoyuki Okutsu*, Kensuke Suzuki*, Yutaka Takeuchi*, Toshio Takeuchi*, and Goro Yoshizaki*
Knockout TM SR : ; ; ; : R ; R : A : X(2013) , ,, B. , (Knockout TM
 33 1 Vol.33 No.1 013 1 Dec. 013 Reproduction & Contraception doi: 10.7669/j.issn.03-37X.013.1.0804 E-mail: randc_journal@163.com Knockout TM SR ; ; ; 400014 : FBS Knockout TM SRKSR : FBS KSR HE TUNEL RT-PCR
33 1 Vol.33 No.1 013 1 Dec. 013 Reproduction & Contraception doi: 10.7669/j.issn.03-37X.013.1.0804 E-mail: randc_journal@163.com Knockout TM SR ; ; ; 400014 : FBS Knockout TM SRKSR : FBS KSR HE TUNEL RT-PCR
7.013 Problem Set 5 FRIDAY April 2nd, 2004
 MIT Department of Biology 7.013: Introductory Biology - Spring 2004 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Problem Set 5 FRIDAY April 2nd, 2004 Problem sets
MIT Department of Biology 7.013: Introductory Biology - Spring 2004 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Problem Set 5 FRIDAY April 2nd, 2004 Problem sets
DAX1, testes development role 7, 8 DFFRY, spermatogenesis role 49 DMRT genes, male sex differentiation role 15
 Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Induction of spermatogenic synchrony by retinoic acid in neonatal mice
 EDITOR'S Letter to CORNER the Editor Spermatogenesis 3:1, e23180; January/February/March 2013 2013; 2013 Landes Bioscience EDITOR'S CORNER Induction of spermatogenic synchrony by retinoic acid in neonatal
EDITOR'S Letter to CORNER the Editor Spermatogenesis 3:1, e23180; January/February/March 2013 2013; 2013 Landes Bioscience EDITOR'S CORNER Induction of spermatogenic synchrony by retinoic acid in neonatal
Chapter 14 The Reproductive System
 Biology 12 Name: Reproductive System Per: Date: Chapter 14 The Reproductive System Complete using BC Biology 12, page 436-467 14. 1 Male Reproductive System pages 440-443 1. Distinguish between gametes
Biology 12 Name: Reproductive System Per: Date: Chapter 14 The Reproductive System Complete using BC Biology 12, page 436-467 14. 1 Male Reproductive System pages 440-443 1. Distinguish between gametes
The Reproductive System
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
Chapter 22 The Reproductive System (I)
 Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
The site of action of the ichthyosis locus (ic) in the mouse, as determined by dermal-epidermal recombinations
 /. Embryol. exp. Morph. Vol. 32, 3, pp. 715-721, 1974 715 Printed in Great Britain The site of action of the ichthyosis locus (ic) in the mouse, as determined by dermal-epidermal recombinations BY MARGARET
/. Embryol. exp. Morph. Vol. 32, 3, pp. 715-721, 1974 715 Printed in Great Britain The site of action of the ichthyosis locus (ic) in the mouse, as determined by dermal-epidermal recombinations BY MARGARET
Chapter 46 ~ Animal Reproduction
 Chapter 46 ~ Animal Reproduction Overview Asexual (one parent) fission (parent separation) budding (corals) fragmentation & regeneration (inverts) parthenogenesis Sexual (fusion of haploid gametes) gametes
Chapter 46 ~ Animal Reproduction Overview Asexual (one parent) fission (parent separation) budding (corals) fragmentation & regeneration (inverts) parthenogenesis Sexual (fusion of haploid gametes) gametes
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Male Anatomy. testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics
 Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Chapter 36 Active Reading Guide Reproduction and Development
 Name: AP Biology Mr. Croft Chapter 36 Active Reading Guide Reproduction and Development Section 1 1. Distinguish between sexual reproduction and asexual reproduction. 2. Which form of reproduction: a.
Name: AP Biology Mr. Croft Chapter 36 Active Reading Guide Reproduction and Development Section 1 1. Distinguish between sexual reproduction and asexual reproduction. 2. Which form of reproduction: a.
A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL CRYPTORCHIDISM: A PRELIMINARY REPORT*
 FERTILITY AND STERILITY Copyright 1970 by the Williams & Wilkins Co. Vol. 21, No. 11, November 1970 Printed in U.S.A. A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL
FERTILITY AND STERILITY Copyright 1970 by the Williams & Wilkins Co. Vol. 21, No. 11, November 1970 Printed in U.S.A. A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL
Supplementary Materials and Methods
 Supplementary Materials and Methods Whole Mount X-Gal Staining Whole tissues were collected, rinsed with PBS and fixed with 4% PFA. Tissues were then rinsed in rinse buffer (100 mm Sodium Phosphate ph
Supplementary Materials and Methods Whole Mount X-Gal Staining Whole tissues were collected, rinsed with PBS and fixed with 4% PFA. Tissues were then rinsed in rinse buffer (100 mm Sodium Phosphate ph
Interlitter variation in progeny of chimaeric male mice
 Interlitter variation in progeny of chimaeric male mice Mia Buehr and Anne McLaren M.R.C. Mammalian Development Unit, University College London, Stephenson Way, London NW HE, U.K. Summary. Three chimaeric
Interlitter variation in progeny of chimaeric male mice Mia Buehr and Anne McLaren M.R.C. Mammalian Development Unit, University College London, Stephenson Way, London NW HE, U.K. Summary. Three chimaeric
Male Reproduction Organs. 1. Testes 2. Epididymis 3. Vas deferens 4. Urethra 5. Penis 6. Prostate 7. Seminal vesicles 8. Bulbourethral glands
 Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
The key role of vitamin A in spermatogenesis
 Review series The key role of vitamin A in spermatogenesis Cathryn A. Hogarth and Michael D. Griswold School of Molecular Biosciences, Washington State University, Pullman. Spermatogenesis in adult mammals
Review series The key role of vitamin A in spermatogenesis Cathryn A. Hogarth and Michael D. Griswold School of Molecular Biosciences, Washington State University, Pullman. Spermatogenesis in adult mammals
a. the tail disappears b. they become spermatids c. they undergo capacitation d. they have been stored in the uterus for several days
 (2 points each) Multiple Choice. Read each question thoroughly before answering. From the choices available, choose the answer that is the most correct. Place all answers on the accompanying answer sheet.
(2 points each) Multiple Choice. Read each question thoroughly before answering. From the choices available, choose the answer that is the most correct. Place all answers on the accompanying answer sheet.
ANATOMY. lecture # : 2 1 Date : Lecturer : Maher Hadidi
 ANATOMY lecture # : 2 1 Date : Lecturer : Maher Hadidi Spermatogenesis Transformation of Spermatogonia into mature sperm that begins at puberty into old age. Provide motility of the sperm...-c:tail to
ANATOMY lecture # : 2 1 Date : Lecturer : Maher Hadidi Spermatogenesis Transformation of Spermatogonia into mature sperm that begins at puberty into old age. Provide motility of the sperm...-c:tail to
Long-term survival of human spermatogonial stem cells in mouse testes
 FERTILITY AND STERILITY VOL. 78, NO. 6, DECEMBER 2002 Copyright 2002 American Society for Reproductive Medicine Published by Elsevier Science Inc. Printed on acid-free paper in U.S.A. Long-term survival
FERTILITY AND STERILITY VOL. 78, NO. 6, DECEMBER 2002 Copyright 2002 American Society for Reproductive Medicine Published by Elsevier Science Inc. Printed on acid-free paper in U.S.A. Long-term survival
REPRODUCCIÓN. La idea fija. Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
DRB666 Applied Developmental and Reproductive Biology (Spring 2013)
 DRB666 Applied Developmental and Reproductive Biology (Spring 2013) Director: 651 Ilalo Street, BSB163-3 e-mail: yyamazak@hawaii.edu Phone: (808) 692-1416 Instructors (e-mail): Steve Ward Yusuke Marikawa
DRB666 Applied Developmental and Reproductive Biology (Spring 2013) Director: 651 Ilalo Street, BSB163-3 e-mail: yyamazak@hawaii.edu Phone: (808) 692-1416 Instructors (e-mail): Steve Ward Yusuke Marikawa
Medical School Histology Basics Male Reproductive System. VIBS 289 lab
 Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Mouse sperm extraction:
 Mouse sperm extraction: This method of extraction is used for acrosome reaction assays, immunocytochemistry and biochemical assays. Collect two cauda epidydimus from one male, cut them 5 times and place
Mouse sperm extraction: This method of extraction is used for acrosome reaction assays, immunocytochemistry and biochemical assays. Collect two cauda epidydimus from one male, cut them 5 times and place
Web Activity: Simulation Structures of the Female Reproductive System
 differentiate. The epididymis is a coiled tube found along the outer edge of the testis where the sperm mature. 3. Testosterone is a male sex hormone produced in the interstitial cells of the testes. It
differentiate. The epididymis is a coiled tube found along the outer edge of the testis where the sperm mature. 3. Testosterone is a male sex hormone produced in the interstitial cells of the testes. It
The effects of cancer treatment on male infertility
 The effects of cancer treatment on male infertility Kirsi Jahnukainen Children s Hospital, Helsinki Karolinska Institutet, Stockholm Department of Pediatrics 21.11.2017 1 Disclosure statement I declare
The effects of cancer treatment on male infertility Kirsi Jahnukainen Children s Hospital, Helsinki Karolinska Institutet, Stockholm Department of Pediatrics 21.11.2017 1 Disclosure statement I declare
Strategic delivery: Setting standards Increasing and. Details: Output: Demonstrating efficiency. informing choice.
 Strategic delivery: Setting standards Increasing and informing choice Demonstrating efficiency economy and value Details: Meeting Scientific and Clinical Advances Advisory Committee Agenda item 6 Paper
Strategic delivery: Setting standards Increasing and informing choice Demonstrating efficiency economy and value Details: Meeting Scientific and Clinical Advances Advisory Committee Agenda item 6 Paper
Bi-potent Gonads. Sex Determination
 יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
Basic histology 5/4/2015
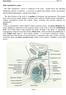 Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
What are the main functions of the male reproductive system? 1. Produce sperm 2. Deposit sperm into the female 3. Provide a pathway for the removal
 What are the main functions of the male reproductive system? 1. Produce sperm 2. Deposit sperm into the female 3. Provide a pathway for the removal of urine Where is sperm produced? -In the 2 testes What
What are the main functions of the male reproductive system? 1. Produce sperm 2. Deposit sperm into the female 3. Provide a pathway for the removal of urine Where is sperm produced? -In the 2 testes What
Correcting the Genetically Determined Sterility of * Male Mice
 Correcting the Genetically Determined Sterility of * Male Mice by GIORGIO VENERONI and ANGELO BIANCHI 1 Centro di Genetica, Pavia WITH ONE PLATE INTRODUCTION IN the house-mouse the factor W y (viable allele
Correcting the Genetically Determined Sterility of * Male Mice by GIORGIO VENERONI and ANGELO BIANCHI 1 Centro di Genetica, Pavia WITH ONE PLATE INTRODUCTION IN the house-mouse the factor W y (viable allele
