Battalion TM Universal Spacer System
|
|
|
- Lucy Higgins
- 6 years ago
- Views:
Transcription
1 Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual patient anatomy. The implants are manufactured from PEEK Optima LT1 with/without titanium coated endplates and tantalum markers. All materials are surgical grade conforming to ASTM F2026 (PEEK), ASTM F1580 (titanium coating), and ASTM F560 (tantalum). Use with supplemental fixation systems from Alphatec Spine such as: Zodiac Polyaxial Spinal Fixation System, Arsenal Spinal Fixation System, Illico MIS Posterior Fixation System, Illico FS Facet Fixation System, and BridgePoint Spinous Process Fixation System. INDICATIONS: The Battalion System is indicated for spinal fusion procedures in skeletally mature patients at one or two contiguous levels in the thoracolumbar spine. Thoracic: T1-T2 to T11-T12, or at the thoracolumbar junction (T12-L1), following discectomy for the treatment of a symptomatic degenerative disc disease (DDD), including thoracic disc herniation (myelopathy and/or radiculopathy with or without axial pain). The lateral approach is limited to levels T5-6 to T11-T12. Lumbar: L1-L2 to L5-S1, for the treatment of degenerative disc disease (DDD) with up to Grade I spondylolisthesis or retrolisthesis at the involved level(s). DDD is defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies. The Battalion System is intended for use on patients who have had at least six months of nonoperative treatment. The Battalion System is intended for use with autograft or allograft (eg. Allogenic bone graft composed of cancellous and/or corticocancellous bone graft) and supplemental fixation systems that are cleared by FDA for use in the thoracic and lumbar spine. CONTRAINDICATIONS: The Battalion System is contraindicated for: 1. Patients with bone resorption related disease (e.g. osteopenia), bone and/or joint disease, or deficient soft tissue at the wound site. 2. Patients with infection, inflammation, fever, tumors, elevated white blood count, obesity, pregnancy, mental illness and other medical conditions, which would prohibit beneficial surgical outcome. 3. Patients with allergy or intolerance to PEEK, titanium, or tantalum 4. Patients resistant to following post-operative restrictions on movement especially in athletic and occupational activities. 5. Patients with prior fusion at the level(s) to be treated. 6. Spinal surgery cases that do not require bone grafting and/or spinal fusion. 7. Reuse or multiple uses of the implant. INS-078C Page 1 of 7
2 WARNINGS: 1. The implants and Single-Use instruments are provided sterile a. Do not re-sterilize implants b. Do not use implants if package is opened or damaged or if expiration date has passed. 2. Components of this system should not be used with components from other systems or manufacturers. 3. Do not comingle dissimilar materials (e.g., titanium and stainless steel) within the same construct. 4. All instruments except the Single-Use Instruments are provided non-sterile and must be cleaned and sterilized prior to surgery. See CLEANING and STERILIZATION sections in this IFU. Sterile single use instruments are disposable devices, designed for single use and should not be reused or reprocessed. Reprocessing of single use instruments may lead to instrument damage and possible improper function. 5. Implants are single use devices. Do not reuse. While an implant may appear undamaged, it may have small defects or internal stress patterns that could lead to fatigue failure. In addition, the removed implant has not been designed or validated for the decontamination of microorganisms. Reuse of this product could lead to cross-infection and/or material degradation as a result of the decontamination process. 6. These implants are used only to provide internal fixation, in conjunction with graft and supplemental fixation, during the bone fusion process. A successful result may not be achieved in every instance. The benefit of spinal fusions utilizing any vertebral body replacement has not been adequately established in patients with stable spines. 7. Potential risks identified with the use of these fusion devices, which may require additional surgery, include device component failure, loss of fixation, pseudoarthrosis (i.e. non-union), fracture of the vertebra, neurological injury, and/or vascular or visceral injury. 8. Risk factors that may affect successful surgical outcomes include: alcohol abuse, obesity, patients with poor bone, muscle and/or nerve quality. Patients who use tobacco or nicotine products should be advised of the consequences that an increased incidence of non-union has been reported with patients who use tobacco or nicotine products. PRECAUTIONS: 1. Implantation should be performed only by experienced spinal surgeons with specific training in the use of this device because this is a technically demanding procedure presenting a risk of serious injury to the patient. 2. These implants have not been evaluated for safety and compatibility in the Magnetic Resonance (MR) environment. It has not been tested for heating or migration in the MR environment. The safety of the Battalion System in the MR environment is unknown. Scanning a patient who has this device may result in patient injury. 3. Installation and positional adjustment of implants must only be done with special equipment and instruments specific to these devices. They must not be used with other instrumentation unless specifically recommended by Alphatec Spine Inc., because the combination with other instrumentation may be incompatible. 4. Based on dynamic testing results, the physician/surgeon should consider the levels of implantation, patient weight, patient activity level, other patient conditions, etc., which may impact the performance of this system 5. Patients with previous spinal surgery at the level(s) to be treated may have different clinical outcomes compared to those without previous surgery. INS-078C Page 2 of 7
3 POSSIBLE ADVERSE EFFECTS: Possible adverse effects include: 1. Initial or delayed loosening, bending, dislocation and/or breakage of device components. 2. Physiological reaction to implant devices due to foreign body intolerance including inflammation, local tissue reaction, seroma, and possible tumor formation. 3. Loss of desired spinal curvature, spinal correction and/or a gain or loss in height. 4. Infection and/or hemorrhaging. 5. Non-union and/or pseudarthrosis. 6. Neurological disorder, pain and/or abnormal sensations caused by improper placement of the device, and/or instruments. 7. Subsidence of the device into the vertebral body. 8. Revision surgery. 9. Death. PREOPERATIVE MANAGEMENT: 1. Only patients meeting the criteria listed in the indications for the use section should be selected. 2. Surgeons should have a complete understanding of the surgical technique, system indications, contraindications, warnings and precautions, safety information, as well as functions and limitations of the implants and instruments. 3. Careful preoperative planning should include implantation strategy and a verification of required inventory for the case. 4. The condition of all implants and instruments should be checked prior to use. Damaged and/or worn implants and instruments should not be used. INTRAOPERATIVE MANAGEMENT: 1. The surgical technique manual should be followed carefully. 2. To prevent possible nerve damage and associated disorders, extreme caution should be taken to avoid the spinal cord and nerve roots at all times. Fluoroscopy should be employed where vision is obstructed. 3. Bone graft must be placed in the area to be fused and graft material must extend from the upper to the lower vertebrae being fused POSTOPERATIVE MANAGEMENT: Postoperative management by the surgeon is essential. This includes instructing, warning, and monitoring the compliance of the patient. 1. Patient should be informed regarding the purpose and limitations of the implanted devices. 2. The surgeon should instruct the patient regarding the amount and time frame after surgery of any weight bearing activity. The increased risk of bending, dislocation, and/or breakage of the implanted devices, as well as an undesired surgical result are consequences of any type of early or excessive weight bearing, vibratory motion, falls, jolts or other movements preventing proper healing and/or fusion development. 3. Implanted devices should be revised or removed if bent, dislocated or broken. 4. Immobilization should be considered in order to prevent bending, dislocation, or breakage of the implanted device in case of delayed, malunion, or nonunion of bone. Immobilization should continue until a complete bone fusion mass has developed and been confirmed. 5. Postoperative patients should be instructed not to use tobacco or nicotine products, INS-078C Page 3 of 7
4 consume alcohol, or use non-steroidal anti-inflammatory drugs and aspirin, as determined by the surgeon. Complete postoperative management to maintain the desired result should also follow implant surgery. Instrument Preparation: Cleaning, inspection, lubrication, and sterilization must be performed by hospital personnel trained in the general procedures involving contaminant removal. Instruments, containers, and trays must be thoroughly cleaned prior to sterilization and lubrication of instruments. All instrument hinged, rotating, and articulating parts must be lubricated prior to sterilization with a water soluble and sterilizable lubricant intended for surgical instruments (Hinge-Free for example). Cleaning of Instruments, Container, and Trays: All solutions for cleaning must be prepared per the manufacturer s instructions. Instruments provided in a set, must be removed from the set for cleaning. Instrument trays, containers, and lids must be thoroughly cleaned separately until visually clean. Ensure instruments are in the fully extended, open position throughout cleaning. Disconnect Quick Connect handles from the shafted instruments prior to cleaning. Pay special attention during cleaning to complex instruments, such as those with, cannulas, hinges, retractable features, mated surfaces, and textured surface finishes. Use of water with high mineral content should be avoided. Manual Cleaning Steps for Instruments (Required) Battalion Inserter: Disassemble the Axial Straight and Curved Inserters per step a) to disassemble into two components prior to cleaning; disassemble Offset Straight Inserters into its 3 components per steps a) and b) prior to cleaning: a) Press the circular button (marked for cleaning ) on the sleeve subassembly, slide the sleeve away from the handle and completely over the shaft. b) Remove the Shaft Subassembly from the Handle Subassembly by pressing the gold rectangular button; grasp the knurled impact cap at the back; simultaneously twist and pull the cap away from the handle until the shaft releases; pull shaft all the way through the handle. LLIF Inserter: Disassemble the LLIF Angled Inserters into five main components prior to cleaning; disassemble the LLIF Straight Inserter into its four main components, disregard step b: a) Press the circular button on the sleeve subassembly, slide the sleeve away from the handle and completely over the shaft. b) Remove the inserter tip from the sleeve. c) Remove the shaft from the Driver Subassembly by pressing the angled button on the side of the button housing and pulling on the shaft until it detaches from the Driver Subassembly. INS-078C Page 4 of 7
5 d) Remove the Handle Subassembly from the Driver Subassembly by pressing the rectangular button on the driver subassembly and pulling on the handle until it detaches from the Driver Subassembly. LLIF Retractor: Disassemble the LLIF Retractor into its six main components prior to cleaning: a) Remove the blades from the frame arms by pulling back the golden lever located on the head of each frame arm, and pulling up on the blades. b) For cleaning, position the retractor body in the closed position by pushing up on the golden lever ring, and pulling the retractor handles apart. c) Remove the handles from the retractor by pressing down on the golden buttons, and pulling them out of the retractor body. Step 1 Step 2 Step 3 Step 4 Step 5 Step 6 Step 7 Rinse devices in ambient temperature tap water to remove excess soil. Submerge instrument in enzyme solution such as Polystica 2X Enzymatic or equivalent. Actuate the instrument while it is submerged and soak for a minimum of 10 minutes. Actuate and scrub the instrument using a soft bristled brush to brush any lumens for a minimum of 2 minutes. If needed, actuate at several locations to access all surfaces. Use of a syringe (minimum of 50 ml) or water jet is recommended for hard to reach areas and repeat 3 times. Rinse instruments in Deionized / Reverse Osmosis water for a minimum of 1 minute. Submerge and actuate instruments in a cleaning solution such as Prolystica 2X Alkaline (ph 11.2) or equivalent, and sonicate for a minimum of 10 minutes. Thoroughly rinse instruments with Deionized / Reverse Osmosis water to remove all detergent residues. Dry instruments with clean, lint free cloth or filtered compressed air. Automatic Washer Cleaning Steps for Instruments Important - Manual Cleaning Steps 1 and 2 are required before performing the Automated Washer / Disinfector Cycle Steps. Step 1 Step 2 Step 3 Step 4 Follow steps 1 and 2 of the Manual Cleaning Steps for Instruments. Thoroughly rinse instruments in ambient temperature tap water to remove detergent residuals. Place instruments in fully extended open position into washer and process through a standard washer/disinfector instrument cycle. PreWash, cold tap water, for a minimum of 2 minutes. INS-078C Page 5 of 7
6 Step 5 Step 6 Step 7 Step 8 Step 9 Enzyme wash (such as Prolystica 2X enzymatic or equivalent), hot tap water, for a minimum of 1 minute. Detergent wash (such as Prolystica 2X Alkaline (ph11.2) or equivalent), Hot tap water (66 C/150 F minimum), for a minimum of 2 minutes. Rinse 2x, hot tap water, for a minimum of 15 seconds. Purified Water rinse, Hot (66 C/150 F minimum), for a minimum of 10 seconds. Hot Air Dry, (115 C/239 F minimum), for a minimum of 10 minutes. INSPECTION: Inspect each instrument, container, and tray to ensure that all visible contamination has been removed. If contamination is noted, repeat the cleaning/disinfection process. Check the action of moving parts (e.g. hinges, box-locks, connectors, sliding parts, etc.) to ensure smooth operation throughout the intended range of motion. Check instruments with long slender features (particularly rotating instruments) for distortion. Drill bits, reamers, rasps and other cutting instruments should be inspected after processing with alkaline detergents. Inspect instruments for any other damage, wear and/or corrosion. STERILIZATION / RESTERILIZATION OF INSTRUMENTS: All instruments are provided non-sterile and must be cleaned and sterilized before use. Instruments must be sterilized using the appropriate cycle parameters in the tables below. Alphatec perforated trays have been validated to achieve sterility using FDA cleared sterilization accessories (container and filters). FDA cleared reusable or paper filters should be used to achieve and maintain sterility after processing. Alphatec perforated container/tray configurations have also been validated to achieve sterility using FDA cleared sterilization wrap. Perforated container/tray configurations must be double wrapped in sterilization wrap to allow steam to penetrate and make direct contract with all surfaces. Do not stack trays during sterilization. Table 1 Sterilization Parameters Method Cycle Type Minimum Temperature Minimum Exposure Time Steam Pre-vacuum 270 F (132 C) 4 minutes Minimum Drying Time 30 minutes (followed by a 15 minutes cool down period)* * Battalion Lateral Instruments INS-078C Page 6 of 7
7 RETURNING INSTRUMENTS TO ALPHATEC SPINE: All used products returning to Alphatec Spine must undergo all steps of cleaning, inspection, and terminal sterilization before being returned to Alphatec Spine. Documentation of decontamination should be included. COMPLAINT HANDLING / REPORTING: All product complaints relating to safety, efficacy or performance of the product should be reported immediately to Alphatec Spine by telephone, fax, , or letter, per contact information below. All complaints should be accompanied by name, part number, and lot numbers. The person formulating the complaint should provide their name, address, and as many details as possible. You may contact Customer Service directly at customerservice@alphatecspine.com For Surgical Technique Guides or additional information regarding the products, please contact Alphatec Spine, Inc. Customer Service directly at customerservice@alphatecspine.com Caution: Federal law (USA) restricts these instruments to sale by or on the order of a physician. For a listing of Symbols and Explanations, see alphatecspine.com/eifu Alphatec Spine, Inc El Camino Real Carlsbad, CA USA Ph: (760) Ph: (800) Fax: (800) alphatecspine.com INS-078C Page 7 of 7
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
XYcor Expandable Spinal Spacer System
 XYcor Expandable Spinal Spacer System GENERAL INFORMATION: The XYcor Expandable Spinal Spacer System (XYcor System) is an intervertebral body fixation system consisting of implants with various widths,
XYcor Expandable Spinal Spacer System GENERAL INFORMATION: The XYcor Expandable Spinal Spacer System (XYcor System) is an intervertebral body fixation system consisting of implants with various widths,
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
Alamo T Transforaminal Lumbar Interbody System Surgical Technique
 Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Alamo C. Cervical Interbody System Surgical Technique. An Alliance Partners Company
 Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
SURGICAL TECHNIQUE GUIDE TRESTLE. Anterior Cervical Plating System
 SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
Cervical Spacer System surgical technique
 Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF)
 Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
EXACTECH SPINE. Operative Technique. Cervical Spacer System. Surgeon focused. Patient driven. TM
 EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
Zavation Z-Link Cervical
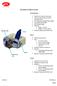 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Posterior Lumbar Interbody Fusion System
 Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Surgical Technique. Apache Anterior Lumbar Interbody Fusion
 Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
CROSS -FUSE P E E K V B R / I B F SYST E M
 S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
Royal Oak Cervical Plate System
 Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
product overview Implant heights range from 8mm-20mm in 2mm increments, with two lordocic angle options of 6 and 12.
 ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
Apache Cervical Interbody Fusion Device. Surgical Technique. Page of 13. LC-005 Rev F
 LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
TABLE OF CONTENTS. Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview. Implants 3. Instruments 5. Surgical Technique 10
 Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
PILLAR AL. Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) PEEK Spacer System OPERATIVE TECHNIQUE
 PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
Surgical Technique. Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion
 Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
VTI INTERLINK PEDICLE SCREW SYSTEM
 VTI INTERLINK PEDICLE SCREW SYSTEM SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. DEVICE DESCRIPTION The VTI InterLink Pedicle Screw System is comprised of polyaxial pedicle screws in various diameters
VTI INTERLINK PEDICLE SCREW SYSTEM SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. DEVICE DESCRIPTION The VTI InterLink Pedicle Screw System is comprised of polyaxial pedicle screws in various diameters
ACP. Anterior Cervical Plate System SURGICAL TECHNIQUE
 ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. Oblique Lumbar Interbody Fusion
 SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System
 X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
SURGICAL TECHNIQUE GUIDE SOLANAS. Posterior Cervico-Thoracic Fixation System Adjustable Bridge System
 SURGICAL TECHNIQUE GUIDE SOLANAS Posterior Cervico-Thoracic Fixation System Adjustable Bridge System 2 SURGICAL TECHNIQUE GUIDE Preface SOLANAS Posterior Cervico-Thoracic Fixation System is designed to
SURGICAL TECHNIQUE GUIDE SOLANAS Posterior Cervico-Thoracic Fixation System Adjustable Bridge System 2 SURGICAL TECHNIQUE GUIDE Preface SOLANAS Posterior Cervico-Thoracic Fixation System is designed to
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
OPERATIVE TECHNIQUE PTC PEEK FORZA. spacer system
 OPERATIVE TECHNIQUE PTC PEEK FORZA spacer system TABLE OF CONTENTS Introduction 1 Operative Technique 2 Instruments 12 FORZA PEEK Part Numbers 20 FORZA PTC Part Numbers 22 Modular Implant Inserter 24 Disassembly
OPERATIVE TECHNIQUE PTC PEEK FORZA spacer system TABLE OF CONTENTS Introduction 1 Operative Technique 2 Instruments 12 FORZA PEEK Part Numbers 20 FORZA PTC Part Numbers 22 Modular Implant Inserter 24 Disassembly
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Thoracolumbar Solutions. Zyston Curve. Interbody Spacer System. Surgical Technique Guide
 Thoracolumbar Solutions Zyston Curve Interbody Spacer System Surgical Technique Guide 2 Zyston Curve Interbody Spacer System Surgical Technique Guide The Zyston Curve Interbody System is designed to optimize
Thoracolumbar Solutions Zyston Curve Interbody Spacer System Surgical Technique Guide 2 Zyston Curve Interbody Spacer System Surgical Technique Guide The Zyston Curve Interbody System is designed to optimize
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
TiLock 2 Spinal System. Surgical Technique
 TiLock 2 Spinal System Surgical Technique Table of Contents Page Preoperative Planning 4 Pedicle Preparation 5 Probe 5 Tap Pedicle 6 Screw Options 7 Screw Insertion 8 Aligning the Windows 9 Rod Insertion
TiLock 2 Spinal System Surgical Technique Table of Contents Page Preoperative Planning 4 Pedicle Preparation 5 Probe 5 Tap Pedicle 6 Screw Options 7 Screw Insertion 8 Aligning the Windows 9 Rod Insertion
Cervical Screw System
 Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
EFSPINE CERVICAL COMBINED SET DISC PROTHESIS ORGANIZER BOX
 EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
Veyron -C Anterior Cervical System Surgical Technique
 Veyron -C Anterior Cervical System Surgical Technique 2 Veyron-C Anterior Cervical System Surgical Technique Veyron-C Anterior Cervical System Surgical Technique Description, Indications & Contraindications...3
Veyron -C Anterior Cervical System Surgical Technique 2 Veyron-C Anterior Cervical System Surgical Technique Veyron-C Anterior Cervical System Surgical Technique Description, Indications & Contraindications...3
Solitaire Anterior Spinal System
 Surgical Technique Solitaire Anterior Spinal System Independent Stabilization for the Anterior Column Available in Titanium and Contents Introduction... Page 1 Design Features... Page 2 Instruments...
Surgical Technique Solitaire Anterior Spinal System Independent Stabilization for the Anterior Column Available in Titanium and Contents Introduction... Page 1 Design Features... Page 2 Instruments...
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Y o u r Id e a s En g i n e e r e d t o Li f e
 ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
QIN4432TRICREV01. IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage. Version 1 1 STERILE PRODUCT
 IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
Zimmer Facet Screw System Surgical Technique
 Zimmer Facet Screw System Surgical Technique 2 Zimmer Facet Screw System Surgical Technique Zimmer Facet Screw System Surgical Technique Description, Indications & Contraindications...3 Surgical Technique...4
Zimmer Facet Screw System Surgical Technique 2 Zimmer Facet Screw System Surgical Technique Zimmer Facet Screw System Surgical Technique Description, Indications & Contraindications...3 Surgical Technique...4
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system.
 T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
InFix. Anterior Lumbar Device. Surgical Technique Guide
 InFix Anterior Lumbar Device Surgical Technique Guide 2 InFix Anterior Lumbar Device Surgical Technique Guide InFix Anterior Lumbar System s modular design is intended to restore lordosis, disc height
InFix Anterior Lumbar Device Surgical Technique Guide 2 InFix Anterior Lumbar Device Surgical Technique Guide InFix Anterior Lumbar System s modular design is intended to restore lordosis, disc height
BONE GRAFT WASHER _Rev A IMPORTANT INFORMATION ON THE BONE GRAFT WASHER
 0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
SURGICAL TECHNIQUE GUIDE
 SURGICAL TECHNIQUE GUIDE Table of Contents Indications and Cage Description... 3 Instruments... 4-5 Surgical Technique... 6-16 Instructions for Use... 17-23 Content, clearances, approvals, specifications,
SURGICAL TECHNIQUE GUIDE Table of Contents Indications and Cage Description... 3 Instruments... 4-5 Surgical Technique... 6-16 Instructions for Use... 17-23 Content, clearances, approvals, specifications,
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL
 ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL Contents: Introduction Indications Contraindications Warnings Precautions Implant Overview Instruments Overview Surgical Technique
ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL Contents: Introduction Indications Contraindications Warnings Precautions Implant Overview Instruments Overview Surgical Technique
Surgical Technique SUSTAIN -O. Radiolucent Spacer
 Surgical Technique SUSTAIN -O Radiolucent Spacer At Globus, we move with a sense of urgency to deliver innovations that improve the quality of life for patients with spinal disorders. We are inspired by
Surgical Technique SUSTAIN -O Radiolucent Spacer At Globus, we move with a sense of urgency to deliver innovations that improve the quality of life for patients with spinal disorders. We are inspired by
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Thunderbolt. surgical technique. MIS Pedicle Screw System. Where Nimble and Secure Intersect
 Thunderbolt TM MIS Pedicle Screw System Where Nimble and Secure Intersect surgical technique i www.choicespine.com System Features Dovetail set screw: Minimizes head splay and cross-threading Secure connection
Thunderbolt TM MIS Pedicle Screw System Where Nimble and Secure Intersect surgical technique i www.choicespine.com System Features Dovetail set screw: Minimizes head splay and cross-threading Secure connection
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
VECTRA-T SURGICAL TECHNIQUE. The Translational Anterior Cervical Palate System. This publication is not intended for distribution in the USA.
 VECTRA-T The Translational Anterior Cervical Palate System This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control This description alone does not provide
VECTRA-T The Translational Anterior Cervical Palate System This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control This description alone does not provide
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine.
 Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
HydraLok. Operative Technique. Polyaxial Pedicle Screw System
 HydraLok Operative Technique Polyaxial Pedicle Screw System Table of Contents Introduction...1 OPERATIVE TECHNIQUE OVERVIEW...2 DETAILED OPERATIVE TECHNIQUE...4 LOCATE AND PREPARE THE PEDICLE...4 PROBE
HydraLok Operative Technique Polyaxial Pedicle Screw System Table of Contents Introduction...1 OPERATIVE TECHNIQUE OVERVIEW...2 DETAILED OPERATIVE TECHNIQUE...4 LOCATE AND PREPARE THE PEDICLE...4 PROBE
VTI INTERFUSE T SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. 1/20
 VTI INTERFUSE T SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. 1/20 CONTENTS InterFuse T Product Description Indications for Use X-Ray Marker Locations Product Specifications Instrument Set 3 4 5 STEP
VTI INTERFUSE T SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. 1/20 CONTENTS InterFuse T Product Description Indications for Use X-Ray Marker Locations Product Specifications Instrument Set 3 4 5 STEP
SURGICAL TECHNIQUE MANUAL. InterFuse T
 1 CONTENTS InterFuse T Product Description 3 Indications for Use 3 X-Ray Marker Locations 4 Product Specifications 4 Instrument Set 5 Step 1 Preoperative Planning 8 Patient Positioning 8 Step 2 Disc Removal
1 CONTENTS InterFuse T Product Description 3 Indications for Use 3 X-Ray Marker Locations 4 Product Specifications 4 Instrument Set 5 Step 1 Preoperative Planning 8 Patient Positioning 8 Step 2 Disc Removal
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
