International Journal of Basic & Clinical Pharmacology. Profile of cutaneous adverse drug reactions of carbamazepine
|
|
|
- Amberlynn Lester
- 5 years ago
- Views:
Transcription
1 Print ISSN: Online ISSN: IJBCP International Journal of Basic & Clinical Pharmacology DOI: Original Research Article Profile of cutaneous adverse drug reactions of carbamazepine Meenakshi B. 1, Nirmala Devi P. 2 *, Radha M. 3, Abdul Rahuman M. B. 4, Shantaraman K. 5 1 Department of Pharmacology, 2 Department of Dermatology, 3 Department of Neurology, 4 Department of Psychiatry, 5 Department of Pathology, Tirunelveli Medical College, Tirunelveli, Tamil Nadu, India Received: 30 September 2017 Revised: 10 October 2017 Accepted: 27 October 2017 *Correspondence to: Dr. Nirmala Devi P., nirmaladevipalanivel@ gmail.com Copyright: the author(s), publisher and licensee Medip Academy. This is an openaccess article distributed under the terms of the Creative Commons Attribution Non- Commercial License, which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Carbamazepine, a commonly used antiepileptic drug is known to produce adverse effects including dangerous reactions like cutaneous hypersensitivity reactions such as drug induced hypersensitivity syndrome (DHS), Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The FDA released a warning that serious and potentially fatal skin reactions may occur after carbamazepine in patients positive for the HLA-B*1502 allele which occurs almost exclusively in patients with ancestry across broad areas of Asia, including South Asian Indians. This study profiles the cutaneous adverse drug reactions reported in this institute over a period of 3 years. Methods: This retrospective study was conducted at Tirunelveli Medical College Hospital by analysing patient case records based on adverse drug reactions reported between August 2014 and July 2017 in the adverse drug reaction monitoring centre. The age, gender, diagnosis, type of cutaneous ADR, duration of treatment, seriousness of reaction and outcome were recorded and analysed. Results: Among the total 25 reactions 36% were benign and 64% were severe reactions. According to Pharmaco vigilance Program of India 80% of the reactions were serious and 20% non serious. The commonest benign skin reaction was maculopapular eruption. SJS and TEN were the two very serious reactions which affected 8 patients totally. Exfoliative dermatitis was reported in 7 patients and Drug Hypersensitivity Syndrome (DHS) in one patient. Conclusions: Severe cutaneous reactions occur after carbamazepine and prevention of ADR requires prediction of predisposition which requires special studies of HLA or genomic assessment. These are the issues of interest for future research. Keywords: Carbamazepine, Cutaneous adverse drug reactions, DHS, SJS, TEN INTRODUCTION Carbamazepine, one of the commonly prescribed antiepileptic drugs is also prescribed for other diseases like neuralgia, schizophrenia and bipolar illness and is known to produce drowsiness, vertigo, ataxia, diplopia, blurred vision, serious haematological toxicities like aplastic anaemia, agranulocytois and hypersensitivity reactions including dangerous skin reactions, eosinophilia, lymphadenopathy and splenomegaly. 1 Carbamezepine is associated with more serious, immune mediated adverse drug reactions (ADR) including cutaneous hypersensitivity reactions such as drug induced hypersensitivity syndrome (DHS), Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). 2 SJS and TEN are severe cutaneous reactions with potential for elevated morbidity and mortality that require immediate intervention and timely management. 3 On 12 th December 2007, the FDA released a warning to health professionals and patients that serious and potentially fatal skin reactions may occur with the administration of carbamazepine in patients positive for the HLA-B*1502 allele This allele occurs almost exclusively in patients with ancestry across broad areas of Asia, including South Asian Indians. 4 International Journal of Basic & Clinical Pharmacology December 2017 Vol 6 Issue 12 Page 2876
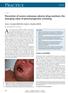
2 This tertiary care teaching hospital in south Tamilnadu runs an adverse drug reaction monitoring centre since June 2014 under Pharmacovigilance Programme of India and from here ADRs are reported to the National Coordinating Centre. Cutaneous reactions are regularly reported by the Department of Dermatology and various other departments to the adverse drug reactions monitoring centre. Many Cutaneous reactions after Carbamazepine have been reported from the departments of Neurology, Psychiatry and Dermatology. This study has been taken up to profile the cutaneous adverse drug reactions to Carbamazepine reported in this institute over a period of 3 years. METHODS This study was conducted at a tertiary care teaching hospital in South Tamilnadu as a retrospective analysis of patient case records in the departments of Dermatology based on adverse drug reactions reported in adverse reactions monitoring centre between August 2014 and July 2017 (3years) in the adverse drug reaction monitoring centre. Both benign and severe cutaneous reactions reported after taking Carbamazepine were included for the study. Cutaneous reactions due to other drugs (other than carbamazepine) and other adverse drug reactions after carbamazepine (other than cutaneous reactions) were excluded. According to Pharmacovigilance Programme, a reaction is said to be serious if it results in any one of the following events like death, life threatening, hospitalization or prolongation of hospitalization, disability, congenital anomaly or required intervention to prevent permanent impairment / damage. 5 The age, gender, diagnosis, type of cutaneous ADR, duration of treatment, seriousness of reaction and outcome were recorded in a format. The data were analysed for quantitative and descriptive statistics. RESULTS Records analysed show 25 cutaneous adverse drug reactions due to Carbamazepine reported during the study period of 3 years. 80% of the reactions were classified as serious and 20% as non serious (Table 2). Table 1: Age and gender distribution in patients reported with CADR after Carbamazepine. Demographic details n (% ) 1 Age range in years 1-10 years 1 (4%) years 1 (4%) years 12 (48%) years 4 (16%) years 1 (4%) years 2 (8%) >60 years 4 (16%) 2 Gender Male 12 (48%) Female 13 (52%) Table 2: The indication of Carbamazepine, type of Cutaneous reaction and seriousness of reaction. Indication of carbamazepine and details of reaction n (% ) 1 Indication of prescription Seizure 20 (80%) Neuralgia 2 (8%) Bipolar disorder 2 (8%) Cervical spondylotic myelopathy 1 (4%) 2 Type of cutaneous reaction Benign Reactions 9 (36%) Urticaria 2 (22.2%) Maculo papular eruption 6 (66.7%) Pruritus 1 (11.1%) Severe 16 (64%) Exfoliative dermatitis 7 (43.8%) DHS 1 (6.2%) TEN 7 (43.8%) SJS 1 (6.2%) 3 Seriousness of ADR Serious 20 (80%) Non serious 5 (20%) Of these 25 patients, 12 patients were between the age of 21 and 30 years, 4 patients were above 60 years and 1 patient was below 10 years. In the study group of 25 patients, 12 were male and 13 were female (Table 1). Of these patients 20 (80%) were prescribed carbamazepine for seizure disorders, 2 for neuralgia, 2 for bipolar illness and 1for cervical spondylotic myelopathy. Among the total 25 ADRs reported, 9 (36%) were classified as benign and 16 (64%) as severe reactions. The protocol of Pharmaco vigilance Program of India was applied to describe the seriousness of the reactions and out of 25reactions reported Figure 1: A patient with toxic epidermal necrolyis. International Journal of Basic & Clinical Pharmacology December 2017 Vol 6 Issue 12 Page 2877
3 Steven Johnson syndrome and toxic epidermal necrolysis were the two very serious complications (Figure 1 and Figure 4) in the group with 8 patients affected totally. Exfoliative dermatitis was another severe reaction reported in 7 patients and Drug Hypersensitivity Syndrome (DHS/TEN) in one patient (Figure 2 and Figure 3). reaction is the most common. Most drugs produce variable types of reactions, while some have a singular pattern. Cutaneous adverse reactions to drugs occur in up to 8% of the global general population and in 2-3% of hospitalized patients. Cutaneous ADR is reported in 3% of individuals receiving anticonvulsant drugs. 6 A paradoxical fact is that many drug reactions mimic dermatoses like exanthematous reactions, urticaria, photosensitive eruptions, fixed drug eruptions, erythema multiforme etc. Drug induced cutaneous ADRs are broadly classified as benign or severe. Reactions like exanthem, pruritus, eczema, urticaria, lichenoid drug eruptions, fixed drug eruptions, erythema nodosum and acneiform eruptions are classified as benign, while acute exanthematous pustulosis, drug reactions with eosinophilia and systemic symptoms (DRESS), drug induced exfoliative dermatitis, Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are classified as severe. 7,8 Figure 2: Patient with DHS/ toxic epidermal necrolyis. Figure 3: Jaundice in the patient with DHS/ TEN. DISCUSSION Figure 4: TEN in a patient with SLE. Adverse drug reaction is an undesirable response evoked by a medicinal substance of which cutaneous adverse drug Out of 25 cutaneous ADRs reported after taking Carbamazepine, 64% were severe reactions and 36% were benign reactions. Maculopapular or morbillifor m eruptions are exanthematous eruptions that typically start on the trunk and spread peripherally in a systematic fashion. Among the benign reactions in this study, maculopapular eruptions (66.7%) were the most common followed by urticaria (22.2%) and simple pruritus. This is in consonance with Knowles SR et al, who reported that the common cutaneous adverse reactions to medications included generalized morbilliform rashes or maculopapular eruptions (50-95%) and urticaria (5-22%). 9 Out of 25 patients, 2 had urticaria and these patients required withdrawal of the drug. Pruritus presented with eruptions within 1 week of initiation of the drug and resolved within 7-14 days of the withdrawal. Studies have shown that the drug specific T cells play a major role in these cutaneous reactions. 10 Among the 16 patients who were diagnosed with severe cutaneous reactions 43.8% of the patients had exfoliative dermatitis or toxic epidermal necrolysis each, while one of the patients had SJS and another had drug hypersensitivity syndrome (DHS). Generalized exfoliative dermatitis (GED) is a severe reaction to carbamezepine characterized by erythema and scaling affecting more than 90% of the body surface associated with increased epidermal turnover, decreased transit time and increased mitotic activity. The exact underlying mechanisms, through which these immunological pathways drive, are not clear while a complex interaction of cytokines, chemokines and adhesion molecules is hypothesized. There are no specific genetic markers for drug induced GED and withdrawal of the drug is the treatment while topical/systemic steroids may be indicated. 8 All 7 patients in this study were admitted, treated and discharged in good condition. Drug induced hypersensitivity syndrome (DHS) is sometimes called Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). This is characterized by International Journal of Basic & Clinical Pharmacology December 2017 Vol 6 Issue 12 Page 2878
4 drug induced rash accompanied by multi organ involvement, eosinophilia (absolute Eosinophil count >500), lymphocytosis and elevated liver function tests. DHS begins days after starting the drug. Low grade fever and pharyngitis may precede the eruptions. The eruptions are morbilliform initially and associated with oedema of face and neck. The eruptions begin on the trunk and face and spread centrifugally. 11 A 21 year old male was admitted with both skin and mucosal lesions and jaundice (Figure 2). While eliciting history it was revealed that he developed rashes 10 days after taking carbamazepine for his seizure disorder. The drug was withdrawn. His serum bilirubin, SGOT, SGPT and total WBCs were markedly raised. This could be a DHS/TEN overlap syndrome. The patient was referred to higher centre and the outcome is not known. The presence of the HLA-A3101 allele was associated with Carbamazepine induced hypersensitivity reactions among subjects of Northern European ancestry. 12 Toxic epidermal necrolysis and Stevens Johnson syndrome are acute life threatening mucocutaneous reactions characterized by extensive necrosis and detachment of the epidermis. 13 These reactions are reported to be associated with keratinocyte apoptosis at the level of the FAS receptor and its ligand. It is also hypothesized SJS/TEN are related to perforin released from lymphocytes, in low concentration that activates apoptosis and causes skin epidermis necrosis. 14 In SJS/TEN influenza like symptoms often precede the eruptions by a few days. Initially the skin lesions are usually macular and appear on the face spreading rapidly usually within 4 days. This is followed by desquamation or may form atypical targets with the purpuric centres that coalesce to form bullae and then slough. The skin lesions are inflammatory. In SJS always 2 or more mucosal surfaces are eroded and the oral mucosa and the conjunctiva are the most commonly affected. The patient may have photophobia, difficulty in swallowing, rectal erosions, painful urination and cough due to the mucosal involvement of the respective system. If it involves more than 10% of the skin surface it is SJS/TEN overlap and if more than 30% of the skin surface is involved it is TEN. 13 These two terms describe phenotypes within a severity spectrum and both the names are used to describe the syndrome like SJS/TEN. 8 Incidence of TEN is per million person years and 1.2 to 6 per million person years for SJS. More than 100 medications are known to cause SJS/TEN. The incidence of SJS/TEN in patients taking Carbamazepine is 14 per 1, 00, Latent period between the drug initiation and onset of SJS/TEN is about 7-10 days but it may range from 5 to 28 days. 8 In this study the latent period varied from 2 weeks to 9 weeks. According to a study conducted in Germany more than 90% of SJS and TEN cases occurred in the first 63 days of AED use. 16 Out of 25 total reported ADRs approximately one third of the reactions were SJS/TEN (Figure 1). All those patients required hospitalisation for 2 to 6 weeks and they were treated with systemic steroids and antibiotics. There was no mortality. Among the 8 patients with SJS or TEN only one patient developed symblepharon and was surgically managed by the ophtholmologist. One patient diagnosed with TEN had increased total count, elevated liver enzymes and histopathological examination confirmed TEN. That patient (Figure 3) was a known case of Systemic Lupus Erythematosus (SLE) with neuropsychiatric symptoms and seizure for which carbamazepine had been prescribed. She developed TEN 2 weeks after commencing the drug and she had elevated liver enzymes which might be due to the disease or drug. Chung et al, proved that there is a strong association in Han Chinese between a genetic marker, the human leukocyte antigen HLA-B*1502, and Stevens-Johnson syndrome induced by carbamazepine. 17 The relatively high incidence of HLA-B*1502 in many Asian populations has resulted in the FDA's decision to recommend testing for all Asians. Several reports have implicated carbamazepine as among the most common causes for SJS/TEN in Indians too. 18,19 According to the Pharmaco vigilance programme an ADR is said to be serious if it resulted in death, life threatening events, hospitalization or prolongation of hospitalization, disability, congenital-anomaly or required intervention to prevent permanent impairment / damage. Among the 25 CADRs reported after CBZ, 80% were labelled serious and life threatening and required hospitalization and acute intervention. This study could not reveal the incidence as the exact number of patients who were prescribed carbamazepine during the study period is not known. CONCLUSION Carbamazepine is a common drug used for certain types of epilepsy like the Complex Partial Seizures. It is found to be effective for specific types of epilepsy and hence finding alternate drugs is difficult, even with the advent of newer AED s. Carbamazepine is also used for other indications like neuralgia, bipolar disorders etc. Adverse drug reactions to carbamazepine are common and are usually benign in character, but severe reaction like SJS/TEN are reported. These reactions could lead to high morbidity and mortality. Hence early identification of the ADR is essential to save life. Prevention of ADR due to Carbamazepine requires prediction of predisposition which requires special studies of HLA or genomic assessment. These are the issues of interest for future research. ACKNOWLEDGEMENTS Authors would like to acknowledge the Pharmacovigilance Programme of India (PvPI), National Coordinating Center, Indian Pharmacopea Commission, Ghaziabad, UP for its kind support for the Adverse drug Monitoring Centre (AMC) and The Department of Health Research (DHR), Govt of India for its kind support for further studies through the Multi Disciplinary Research Unit, Tirunelveli Medical College, Tirunelveli. International Journal of Basic & Clinical Pharmacology December 2017 Vol 6 Issue 12 Page 2879
5 Funding: No funding sources Conflict of interest: None declared Ethical approval: The study was approved by the Institutional Ethics Committee REFERENCES 1. James O. McNamara. Pharmacotherapy of epilepsies Goodman & Gillman s The Pharmacological basis of Therapeutics 12 th Edition; Laurence Brunton McGraw Hill; 2011: Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Regi SCAR study group. Comprehensive survival analysis of a cohort of patients with StevensJohnson syndrome and toxic epidermal necrolysis. J Investig Dermatol. 2013;133: [PubMed] 3. Garcia JB, Ferro LS, Carvalho AB, da Rocha RM, de Souza LM, James W. Patterson Cutaneous drug reactions. Patterson Weedon s skin Pathology. 4 th Edition; Information for Healthcare Professionals: Dangerous or Even Fatal Skin Reactions - Carbamazepine (marketed as Carbatrol, Equetro, Tegretol, and generics) Available at: rugsafetyinformationforpatientsandproviders/ucm htm 5. Safety of Medicines. A guide to detecting and reporting adverse drug reactions. Why health professionals need to take action. Available at: 1/WH O_EDM_QSM_ pdf 6. Elias A, Madhusoodanan S, Pudukkadan D, Antony JT. Angioedema and maculopapular eruptions associated with carbamazepine administration. CNS Spectr May;11(5): Michael R. Ardern-Jone, Haur YL. Benign Cutaneous Adverse Reactions to drugs. Rook s Text book of Dermatology Volume 4 Editors. Christopher Griffiths, Jonathan Barker, Tanya Bleiker, Robert Chalmers, Daniel Creamer. 9 th Edition; 2010: Walsh S, Haur YL, Creamer D. Severe Cutaneous Adverse Reactions to Drugs. Rook s Text book of Dermatology Volume 4 Editors. Christopher Griffiths, Jonathan Barker, Tanya Bleiker, Robert Chalmers, Daniel Creamer. 9 th Edition; 2010: Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: Incidence, prevention and management. Drug Saf. 1999;21: Shear NH, Sandra R. Knowles. Cutaneous reactions to drugs. Fitzpatrick s Dermatology in General Medicine Lowell A. Goldsmith, Stephen I. Katz, Barbara A. Gilchrest, Army S. Paller, David J. Leffell, Klaus Wolff. 8 th Edition. 2008;1: James WD, Berger TG, Elston DM. Contact Dermatitis and drug eruptions Andrews Disease of the skin. Clinical Dermatology 12 th Edition; 2016: Mark McCormack. HLA-A3101 and Carbamazepin e- Induced Hypersensitivity Reactions in Europeans. N Engl J Med Mar 24;364(12): Valeyrie-Allanore L, Jean-Claude R. Chapter 40 Epidermal necrolysis and Toxic epidermal necrolysis. Fitzpatrick s Dermatology in General Medicine. Lowell A. Goldsmith, Stephen I. Katz, Barbara A. Gilchret, Amy S. Paller, David J. Leffell, Klaus Wolff 8 th Edition; 2012: Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin, Science Oct 16;282(5388): James WD, Berger TG, Elston DM, Neuhaus IM. Bullous Reactions: Stevens Johnson Syndrome and Toxic Epidermal Necrolysis. Andrews Diseases of the skin. Clinical Dermatology 12 th Edition; 2016: Mockenhaupt M, Messenheimer J, Tennis P, Schlingmann J. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics. Neurology Apr 12;64(7): Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens- Johnson syndrome. Nature Apr 1;428(6982): Mehta TY, Prajapati LM, Mittal B, Joshi CG, Sheth JJ, Patel DB, et al. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereol Leprol Nov-Dec;75(6): Chang CC, Too CL, Murad S, Hussein SH. Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int J Dermatol Feb;50(2): Cite this article as: Meenakshi B, Nirmala Devi P, Radha M, Rahuman AMB, Shantaraman K. Profile of cutaneous adverse drug reactions of carbamazepine. Int J Basic Clin Pharmacol 2017;6: International Journal of Basic & Clinical Pharmacology December 2017 Vol 6 Issue 12 Page 2880
Dilantin (phenytoin) ROBERT A. SCHWARTZ
 Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT
 OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
allergy Asia Pacific Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from
 pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
Prevalence and pattern of adverse cutaneous drug reactions presenting to a tertiary care hospital
 International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review
 Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Skin Manifestations of Drug Reactions
 Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Drug Allergy A Guide to Diagnosis and Management
 Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive Patients
 Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW
 SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
Emergency Dermatology Dr Melissa Barkham
 Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Cutaneous Drug Reactions
 Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India
 Study Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India David Pudukadan, Devinder Mohan Thappa Department of Dermatology and STD, Jawaharlal
Study Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India David Pudukadan, Devinder Mohan Thappa Department of Dermatology and STD, Jawaharlal
Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course
 Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients
 Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
Address for correspondence: Dr. Patel Raksha M. R-3, Doctor s Quarters, Jail Road, Vadodara- 01, Gujarat, India.
 Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Herbal and homeopathic products, often considered natural and non-toxic, can also cause adverse drug reactions.
 Idiosyncratic and potentially serious cutaneous adverse drug reactions (CADRs), although relatively rare, account for significant morbidity and mortality. RANNAKOE J LEHLOENYA, BSc, MB ChB, FCDerm (SA)
Idiosyncratic and potentially serious cutaneous adverse drug reactions (CADRs), although relatively rare, account for significant morbidity and mortality. RANNAKOE J LEHLOENYA, BSc, MB ChB, FCDerm (SA)
CASE REPORT ATYPICAL BULLOUS PYODERMA GANGRENOSUM WITH EARLY LESIONS MIMICKING CHICKEN POX
 ATYPICAL BULLOUS PYODERMA GANGRENOSUM WITH EARLY LESIONS MIMICKING CHICKEN POX Ramesh M 1, Kavya Raju Nayak 2, M.G. Gopal 3, Sharath Kumar B.C 4, Nandini A.S 5 HOW TO CITE THIS ARTICLE: Ramesh M, Kavya
ATYPICAL BULLOUS PYODERMA GANGRENOSUM WITH EARLY LESIONS MIMICKING CHICKEN POX Ramesh M 1, Kavya Raju Nayak 2, M.G. Gopal 3, Sharath Kumar B.C 4, Nandini A.S 5 HOW TO CITE THIS ARTICLE: Ramesh M, Kavya
Pemphigus in younger age group in Bangladeshi population
 ORIGINAL ARTICLE in younger age group in Bangladeshi population Abdul Wahab 1, MD, Lubna Khondker 1, MD, Jamal Uddin 1, MD, Ishrat Bhuiyan 2, MD Shirajul Islam Khan 3, MD, Zafrul Islam 1, MD, Rahmat Ali
ORIGINAL ARTICLE in younger age group in Bangladeshi population Abdul Wahab 1, MD, Lubna Khondker 1, MD, Jamal Uddin 1, MD, Ishrat Bhuiyan 2, MD Shirajul Islam Khan 3, MD, Zafrul Islam 1, MD, Rahmat Ali
Cutaneous drug reactions
 Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
PedsCases Podcast Scripts
 PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome
 Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review
 Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Emergency Dermatology. Emergency Dermatology
 Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
New product information wording Extracts from PRAC recommendations on signals
 20 July 2017 EMA/PRAC/406976/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 3-6 July 2017 PRAC The
20 July 2017 EMA/PRAC/406976/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 3-6 July 2017 PRAC The
Drug eruptions and hepatic involvement: a study
 International Journal of Research in Dermatology http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183819 Drug eruptions and hepatic involvement:
International Journal of Research in Dermatology http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183819 Drug eruptions and hepatic involvement:
Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening
 CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS
 PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
Adverse drug reactions
 Medications William Smith Adverse drug reactions Allergy? Side-effect? Intolerance? Background Adverse drug reactions (ADRs) vary from life-threatening anaphylaxis to minor common side-effects. Objective
Medications William Smith Adverse drug reactions Allergy? Side-effect? Intolerance? Background Adverse drug reactions (ADRs) vary from life-threatening anaphylaxis to minor common side-effects. Objective
Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis: a case report
 Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Syndrome de Lyell Approche diagnostique. seminaires iris. Veronique del Marmol Alexandre Chamoun Service de Dermatologie Hôpital Erasme.
 Syndrome de Lyell Approche diagnostique Veronique del Marmol Alexandre Chamoun Service de Dermatologie Hôpital Erasme Serge Jennes Hôpital Militaire Rash benign Pustulose exanthematique Aigue et généralisée
Syndrome de Lyell Approche diagnostique Veronique del Marmol Alexandre Chamoun Service de Dermatologie Hôpital Erasme Serge Jennes Hôpital Militaire Rash benign Pustulose exanthematique Aigue et généralisée
Bugs and Drugs: What s New in Hypersensitivity Reactions?
 Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Mechanisms of Drug Hypersensitivity Reactions
 18/5/213 Mechanisms of Drug Hypersensitivity Reactions Munir Pirmohamed NHS Chair of Pharmacogenetics Department of Molecular and Clinical Pharmacology Institute of Translational Medicine University of
18/5/213 Mechanisms of Drug Hypersensitivity Reactions Munir Pirmohamed NHS Chair of Pharmacogenetics Department of Molecular and Clinical Pharmacology Institute of Translational Medicine University of
University Journal of Pre and Para Clinical Sciences
 ISSN 2455 2879 Volume 2 Issue 4 2016 CASE REPORT- A RARE CASE OF DAPSONE HYPERSENSITIVITY SYNDROME VINOTH KUMAR CHITTARANJAN Department of Pharmacology, MADRAS MEDICAL COLLEGE AND GOVERNMENT GENERAL HOSPITAL
ISSN 2455 2879 Volume 2 Issue 4 2016 CASE REPORT- A RARE CASE OF DAPSONE HYPERSENSITIVITY SYNDROME VINOTH KUMAR CHITTARANJAN Department of Pharmacology, MADRAS MEDICAL COLLEGE AND GOVERNMENT GENERAL HOSPITAL
An Evidenced-Based Approach to the Adult with a Morbilliform Eruption
 Relatively An Evidenced-Based Approach to the Adult with a Morbilliform Eruption Ben Kaffenberger, MD Assistant Professor, Dermatology Director, Inpatient Dermatology Consult Service Ohio State University
Relatively An Evidenced-Based Approach to the Adult with a Morbilliform Eruption Ben Kaffenberger, MD Assistant Professor, Dermatology Director, Inpatient Dermatology Consult Service Ohio State University
Adverse Drug Reactions (ADRs) Outline
 Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
Pediatric Dermatology
 Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review
 140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
PEER REVIEW HISTORY ARTICLE DETAILS VERSION 1 - REVIEW
 PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
Cutaneous drug reactions to antiepileptic drugs and relation with HLA alleles in the Turkish population
 O R I G I N A L A R T I C L E Eur Ann Allergy Clin Immunol Vol 50, N 1, 36-41, 2018 S. Büyüköztürk 1, Ç. Kekik 2, A.Z. Gökyiğit 3, F.İ. Tezer Filik 4i, G. Karakaya 4, S. Saygı 4i, A.B. Dursun 5, S. Kirbaş
O R I G I N A L A R T I C L E Eur Ann Allergy Clin Immunol Vol 50, N 1, 36-41, 2018 S. Büyüköztürk 1, Ç. Kekik 2, A.Z. Gökyiğit 3, F.İ. Tezer Filik 4i, G. Karakaya 4, S. Saygı 4i, A.B. Dursun 5, S. Kirbaş
FDA Approves Carnexiv (carbamazepine) injection as Intravenous Short-Term Replacement Therapy for Certain Seizure Types
 FOR IMMEDIATE RELEASE FDA Approves Carnexiv (carbamazepine) injection as Intravenous Short-Term Replacement Therapy for Certain Seizure Types Carnexiv is the first FDA-approved intravenous carbamazepine
FOR IMMEDIATE RELEASE FDA Approves Carnexiv (carbamazepine) injection as Intravenous Short-Term Replacement Therapy for Certain Seizure Types Carnexiv is the first FDA-approved intravenous carbamazepine
Health economics and drug safety
 Health economics and drug safety Professor Dyfrig Hughes FFRPS FBPhS FLSW Centre for Health Economics & Medicines Evaluation Bangor University, Wales @HughesDyfrig ADEs that are not reactions to a medicine
Health economics and drug safety Professor Dyfrig Hughes FFRPS FBPhS FLSW Centre for Health Economics & Medicines Evaluation Bangor University, Wales @HughesDyfrig ADEs that are not reactions to a medicine
New product information wording Extracts from PRAC recommendations on signals
 12 October 2017 EMA/PRAC/610988/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 25-29 September 2017
12 October 2017 EMA/PRAC/610988/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 25-29 September 2017
건강한성인에서의오진하기쉬운포도구균성열상피부증후군의치험례. Staphylococcal Scalded Skin Syndrome in a Healthy Adult: Easy to Misdiagnose
 Archives of Hand and Microsurgery Arch Hand Microsurg 2018;23(4):271-276. https://doi.org/10.12790/ahm.2018.23.4.271 pissn 2586-3290 eissn 2586-3533 Case Report 건강한성인에서의오진하기쉬운포도구균성열상피부증후군의치험례 김홍일ㆍ곽찬이ㆍ박언주
Archives of Hand and Microsurgery Arch Hand Microsurg 2018;23(4):271-276. https://doi.org/10.12790/ahm.2018.23.4.271 pissn 2586-3290 eissn 2586-3533 Case Report 건강한성인에서의오진하기쉬운포도구균성열상피부증후군의치험례 김홍일ㆍ곽찬이ㆍ박언주
SEIZURE CONTROL * DAY & NIGHT
 SEIZURE CONTROL * DAY & NIGHT Equetro (carbamazepine) Extended-Release Capsules Approved for Use in Treating Epilepsy * *PLEASE SEE FOLLOWING PAGES FOR SPECIFIC INDICATIONS. PLEASE SEE IMPORTANT SAFETY
SEIZURE CONTROL * DAY & NIGHT Equetro (carbamazepine) Extended-Release Capsules Approved for Use in Treating Epilepsy * *PLEASE SEE FOLLOWING PAGES FOR SPECIFIC INDICATIONS. PLEASE SEE IMPORTANT SAFETY
A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash
 CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
Future of Pediatrics: Blisters, Hives and Other Tales from the Emergency Room June 14 th, 2016
 A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
Cutaneous Adverse Drug Reactions in Domestic Animals. Katherine Doerr, DVM, Dip. ACVD. Veterinary Dermatology Center
 Cutaneous Adverse Drug Reactions in Domestic Animals Katherine Doerr, DVM, Dip. ACVD Veterinary Dermatology Center Maitland, Rockledge, Waterford Lakes, FL Not highly studied in veterinary medicine Unknown
Cutaneous Adverse Drug Reactions in Domestic Animals Katherine Doerr, DVM, Dip. ACVD Veterinary Dermatology Center Maitland, Rockledge, Waterford Lakes, FL Not highly studied in veterinary medicine Unknown
AOU Ospedali Riuniti - Ancona
 AOU Ospedali Riuniti - Ancona Ospedale Materno-Infantile di Alta Specializzazione G. Salesi UOC Pediatria Allergia a farmaci e infezioni: tra coesistenza e casualità fabrizio franceschini Drug Hypersensitivity
AOU Ospedali Riuniti - Ancona Ospedale Materno-Infantile di Alta Specializzazione G. Salesi UOC Pediatria Allergia a farmaci e infezioni: tra coesistenza e casualità fabrizio franceschini Drug Hypersensitivity
Cutaneous manifestations and systemic correlation in patients with lupus erythematosus and its subsets: a study of 40 cases
 International Journal of Research in Dermatology Mahajan R et al. Int J Res Dermatol. 2018 Nov;4(4):479-483 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183407
International Journal of Research in Dermatology Mahajan R et al. Int J Res Dermatol. 2018 Nov;4(4):479-483 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183407
A. Erythema multiforme and related diseases
 Go Back to the Top To Order, Visit the Purchasing Page for Details Chapter Erythema, Erythroderma (Exfoliative Dermatitis) Erythema is caused by telangiectasia or hyperemia in the papillary and reticular
Go Back to the Top To Order, Visit the Purchasing Page for Details Chapter Erythema, Erythroderma (Exfoliative Dermatitis) Erythema is caused by telangiectasia or hyperemia in the papillary and reticular
University of Groningen
 University of Groningen Toxic epidermal necrolysis, DRESS, AGEP Bouvresse, Sophie; Valeyrie-Allanore, Laurence; Ortonne, Nicolas; Konstantinou, Marie Pauline; Kardaun, Sylvia H.; Bagot, Martine; Wolkenstein,
University of Groningen Toxic epidermal necrolysis, DRESS, AGEP Bouvresse, Sophie; Valeyrie-Allanore, Laurence; Ortonne, Nicolas; Konstantinou, Marie Pauline; Kardaun, Sylvia H.; Bagot, Martine; Wolkenstein,
Objectives Making CYP450, Drug Interactions, & Pharmacogenetics Easy
 Objectives Making, Drug Interactions, & Pharmacogenetics Easy Anthony J. Busti, MD, PharmD, FNLA, FAHA Describe the differences between phase I and phase II metabolic pathways. Identify the most common
Objectives Making, Drug Interactions, & Pharmacogenetics Easy Anthony J. Busti, MD, PharmD, FNLA, FAHA Describe the differences between phase I and phase II metabolic pathways. Identify the most common
Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection
 Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes
 Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
An unpredictable, dose-independent adverse drug reaction which is immunologically or IgEmediated.
 R H E U M A T I S M D I S O R D E R S A N D A L L E R G I E S APPROACH TO DRUG ALLERGY Dr Bernard Thong DEFINITION OF DRUG ALLERGY An unpredictable, dose-independent adverse drug reaction which is immunologically
R H E U M A T I S M D I S O R D E R S A N D A L L E R G I E S APPROACH TO DRUG ALLERGY Dr Bernard Thong DEFINITION OF DRUG ALLERGY An unpredictable, dose-independent adverse drug reaction which is immunologically
IJBCP International Journal of Basic & Clinical Pharmacology
 Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20171661 Original Research Article A study of cutaneous
Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20171661 Original Research Article A study of cutaneous
Cutaneous drug eruptions are seen commonly
 REVIEW ARTICLE Cutaneous drug reactions in children Sandipan Dhar, Raghubir Banerjee, Rajib Malakar Department of Pediatric Dermatology, Institute of Child Health, Kolkata, West Bengal, India ABSTRACT
REVIEW ARTICLE Cutaneous drug reactions in children Sandipan Dhar, Raghubir Banerjee, Rajib Malakar Department of Pediatric Dermatology, Institute of Child Health, Kolkata, West Bengal, India ABSTRACT
DOSAGE FORMS AND STRENGTHS Tablets: 25 mg, 100 mg, 150 mg, and 200 mg; scored. (3.1, 16)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMOTRIGINE TABLETS safely and effectively. See full prescribing information for LAMOTRIGINE TABLETS.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMOTRIGINE TABLETS safely and effectively. See full prescribing information for LAMOTRIGINE TABLETS.
Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review
 Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Accepted Manuscript. Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need
 Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Subject Outline: Dermatology II
 Subject Outline: Dermatology II Course: Master of Dermatology (Coursework) Subject: Dermatology II (An introduction to clinical dermatology) Credit Points: 3 Year/Semester Delivered: 1/2 Subject Outline:
Subject Outline: Dermatology II Course: Master of Dermatology (Coursework) Subject: Dermatology II (An introduction to clinical dermatology) Credit Points: 3 Year/Semester Delivered: 1/2 Subject Outline:
PRAC recommendations on signals
 25 November 2013 EMA/PRAC/693228/2013 Pharmacovigilance Risk Assessment Committee Adopted at the PRAC meeting of 4-7 November 2013 This document provides an overview of the recommendations adopted by the
25 November 2013 EMA/PRAC/693228/2013 Pharmacovigilance Risk Assessment Committee Adopted at the PRAC meeting of 4-7 November 2013 This document provides an overview of the recommendations adopted by the
PHM142 Autoimmune Disorders + Idiosyncratic Drug Reactions
 PHM142 Autoimmune Disorders + Idiosyncratic Drug Reactions 1 Autoimmune Disorders Auto-reactivity: low physiological levels (e.g. tolerance) vs. pathogenic levels 80+ types of autoimmune diseases affect
PHM142 Autoimmune Disorders + Idiosyncratic Drug Reactions 1 Autoimmune Disorders Auto-reactivity: low physiological levels (e.g. tolerance) vs. pathogenic levels 80+ types of autoimmune diseases affect
INDICATIONS Treatment of Chronic Iron Overload Due to Blood Transfusions (Transfusional Iron Overload) EXJADE (deferasirox) is indicated for the
 INDICATIONS Treatment of Chronic Iron Overload Due to Blood Transfusions (Transfusional Iron Overload) EXJADE (deferasirox) is indicated for the treatment of chronically elevated levels of iron in the
INDICATIONS Treatment of Chronic Iron Overload Due to Blood Transfusions (Transfusional Iron Overload) EXJADE (deferasirox) is indicated for the treatment of chronically elevated levels of iron in the
From. The Department of Pediatrics Dr. Mehtas Hospital
 From The Department of Pediatrics Dr. Mehtas Hospital Case history A 12 yr old girl : Fever 5 days Redness of eyes & erythematous rashes over the body for 2 days Past: Febrile fits at 9 mo. Of age Afebrile
From The Department of Pediatrics Dr. Mehtas Hospital Case history A 12 yr old girl : Fever 5 days Redness of eyes & erythematous rashes over the body for 2 days Past: Febrile fits at 9 mo. Of age Afebrile
Department of Dermatology, Christian Medical College and Hospital, Ludhiana, Punjab, India.
 Bullous pemphigoid mimicking granulomatous inflammation Abhilasha Williams, Emy Abi Thomas. Department of Dermatology, Christian Medical College and Hospital, Ludhiana, Punjab, India. Egyptian Dermatology
Bullous pemphigoid mimicking granulomatous inflammation Abhilasha Williams, Emy Abi Thomas. Department of Dermatology, Christian Medical College and Hospital, Ludhiana, Punjab, India. Egyptian Dermatology
Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015)
 1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
BJD. Summary. British Journal of Dermatology THERAPEUTICS
 THERAPEUTICS BJD British Journal of Dermatology Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of
THERAPEUTICS BJD British Journal of Dermatology Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of
To Study The Adverse Effect of Carbamazepine in A Tertiary Care Teaching Hospital of North East India.
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 5 Ver. VIII (May. 2017), PP 26-31 www.iosrjournals.org To Study The Adverse Effect of Carbamazepine
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 5 Ver. VIII (May. 2017), PP 26-31 www.iosrjournals.org To Study The Adverse Effect of Carbamazepine
Monitoring of adverse drug reactions caused by methotrexate at the dermatology department of a tertiary care centre
 International Journal of Research in Dermatology Sivakumar S et al. Int J Res Dermatol. 2017 Sep;3(3):389-394 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20173078
International Journal of Research in Dermatology Sivakumar S et al. Int J Res Dermatol. 2017 Sep;3(3):389-394 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20173078
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Clindamycin Pharmaceutical form(s)/strength: 300mg/2 ml Ampulle, 600 mg/4 ml Ampulle, 900 mg/6 ml Ampulle, 75 mg/5 ml Granulat für orale Lösung, 150 mg Kapselnn,
M0BCore Safety Profile Active substance: Clindamycin Pharmaceutical form(s)/strength: 300mg/2 ml Ampulle, 600 mg/4 ml Ampulle, 900 mg/6 ml Ampulle, 75 mg/5 ml Granulat für orale Lösung, 150 mg Kapselnn,
TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN
 TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN Nadia Khaldi 1, Alain Miras 1, Sophie Gromb 1,2 1 Forensic Science Laboratory, Pellegrin Hospital, University Teaching Hospital Bordeaux, France, 2 Forensic
TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN Nadia Khaldi 1, Alain Miras 1, Sophie Gromb 1,2 1 Forensic Science Laboratory, Pellegrin Hospital, University Teaching Hospital Bordeaux, France, 2 Forensic
Clinical profile of skin diseases in accident and emergency department attenders
 Hong Kong J. Dermatol. Venereol. (2007) 15, 4-9 Original Article Clinical profile of skin diseases in accident and emergency department attenders CY Chan, KL Kam, CA Graham, TH Rainer, NM Luk Skin problems
Hong Kong J. Dermatol. Venereol. (2007) 15, 4-9 Original Article Clinical profile of skin diseases in accident and emergency department attenders CY Chan, KL Kam, CA Graham, TH Rainer, NM Luk Skin problems
RECENT MAJOR CHANGES Warnings and Precautions, Hemophagocytic
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMICTAL safely and effectively. See full prescribing information for LAMICTAL. LAMICTAL (lamotrigine)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMICTAL safely and effectively. See full prescribing information for LAMICTAL. LAMICTAL (lamotrigine)
cipro loxacin; stevens-johnson syndrome; urinary tract infection; adverse drug reaction
 Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר SUMMARY OF PRODUCT CHARACTERISTICS
 פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT FELDENE GEL 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram contains 5mg
פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT FELDENE GEL 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram contains 5mg
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC
 SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
Analysis of Cutaneous Adverse Drug Reactions at a Tertiary Care Hospital a Prospective Study
 Tropical Journal of Pharmaceutical Research August 2011; 10 (4): 517-522 Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online
Tropical Journal of Pharmaceutical Research August 2011; 10 (4): 517-522 Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online
Early View Article: Online published version of an accepted article before publication in the final form.
 : Online published version of an accepted article before publication in the final form. Journal Name: International Journal of Case Reports and Images (IJCRI) Type of Article: Case Report Title: A Case
: Online published version of an accepted article before publication in the final form. Journal Name: International Journal of Case Reports and Images (IJCRI) Type of Article: Case Report Title: A Case
Correspondence should be addressed to Wanjarus Roongpisuthipong; rr
 Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Danger Signs in Drug Hypersensitivity
 Danger Signs in Drug Hypersensitivity Kathrin Scherer, MD*, Andreas J. Bircher, MD KEYWORDS Drug hypersensitivity Adverse drug reaction Clinical danger signs Immediate-type hypersensitivity Delayed-type
Danger Signs in Drug Hypersensitivity Kathrin Scherer, MD*, Andreas J. Bircher, MD KEYWORDS Drug hypersensitivity Adverse drug reaction Clinical danger signs Immediate-type hypersensitivity Delayed-type
Correlation of Drug Eruption with the Number of CD4 In the HIV-Infected Patient at Haji Adam Malik General Hospital
 International Journal of ChemTech Research CODEN (USA): IJCRGG, ISSN: 0974-4290, ISSN(Online):2455-9555 Vol.11 No.08, pp 297-302, 2018 Correlation of Drug Eruption with the Number of CD4 In the HIV-Infected
International Journal of ChemTech Research CODEN (USA): IJCRGG, ISSN: 0974-4290, ISSN(Online):2455-9555 Vol.11 No.08, pp 297-302, 2018 Correlation of Drug Eruption with the Number of CD4 In the HIV-Infected
Prevalence of Lichen Planus in Hepatitis B Patients Attending Ibn Sina Hospital
 American Journal of Dermatology and Venereology 17, 6(2): 25-29 DOI: 1.5923/j.ajdv.1762.2 Prevalence of Lichen Planus in Hepatitis B Patients Attending Ibn Sina Hospital Sara E. F. E. Ali 1, Ahmed N. Aljarbou
American Journal of Dermatology and Venereology 17, 6(2): 25-29 DOI: 1.5923/j.ajdv.1762.2 Prevalence of Lichen Planus in Hepatitis B Patients Attending Ibn Sina Hospital Sara E. F. E. Ali 1, Ahmed N. Aljarbou
1 Introduction. Kenneth Irungu 1 David Nyamu
 Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Endocarditis. By : Mehrnoush. dianatkhah
 Endocarditis By : Mehrnoush. dianatkhah Case 5.31, 31 years old woman CC : Fever, dyspnea, 3 days postpartum PMH : Mitral prolapse Fever 38.5 WBC : 8900 ESR : 84 CRP : 10.4 Cr : 0.6 NT Pro BNP: 5469 Physical
Endocarditis By : Mehrnoush. dianatkhah Case 5.31, 31 years old woman CC : Fever, dyspnea, 3 days postpartum PMH : Mitral prolapse Fever 38.5 WBC : 8900 ESR : 84 CRP : 10.4 Cr : 0.6 NT Pro BNP: 5469 Physical
Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics
 Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics Maja Mockenhaupt, MD; John Messenheimer, MD; Pat Tennis, PhD; and Juergen Schlingmann Abstract Background:
Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics Maja Mockenhaupt, MD; John Messenheimer, MD; Pat Tennis, PhD; and Juergen Schlingmann Abstract Background:
IJBCP International Journal of Basic & Clinical Pharmacology
 Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20161524 Research Article Prescribing pattern and
Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20161524 Research Article Prescribing pattern and
Successful re-introduction of lamotrigine after initial rash
 Seizure 2000; 9: 282 286 doi: 10.1053/seiz.2000.0394, available online at http://www.idealibrary.com on Successful re-introduction of lamotrigine after initial rash F. M. C. BESAG, G. Y. T. NG & F. POOL
Seizure 2000; 9: 282 286 doi: 10.1053/seiz.2000.0394, available online at http://www.idealibrary.com on Successful re-introduction of lamotrigine after initial rash F. M. C. BESAG, G. Y. T. NG & F. POOL
CASE REPORT. Narin Sriratanaviriyakul 1,2,3*, Lam-Phuong Nguyen 1,2,3, Mark C Henderson 2 and Timothy E Albertson 1,2,3
 Sriratanaviriyakul et al. Journal of Medical Case Reports 2014, 8:332 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Drug reaction with eosinophilia and systemic symptoms syndrome (DRESS) syndrome
Sriratanaviriyakul et al. Journal of Medical Case Reports 2014, 8:332 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Drug reaction with eosinophilia and systemic symptoms syndrome (DRESS) syndrome
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 11/2011
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMICTAL safely and effectively. See full prescribing information for LAMICTAL. LAMICTAL (lamotrigine)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMICTAL safely and effectively. See full prescribing information for LAMICTAL. LAMICTAL (lamotrigine)
LAMICTAL XR (lamotrigine) extended-release tablets, for oral use Initial U.S. Approval: 1994
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMICTAL XR safely and effectively. See full prescribing information for LAMICTAL XR. LAMICTAL XR
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMICTAL XR safely and effectively. See full prescribing information for LAMICTAL XR. LAMICTAL XR
HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review
 HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
Adverse Drug Reactions pattern in a tertiary level teaching hospital: A Retrospective Study
 Original Article Adverse Drug Reactions pattern in a tertiary level teaching hospital: A Retrospective Study Anita Gupta, Anjleen Kaur, Prashant Shukla*, Hema Chhabra Department of Pharmacology, Government
Original Article Adverse Drug Reactions pattern in a tertiary level teaching hospital: A Retrospective Study Anita Gupta, Anjleen Kaur, Prashant Shukla*, Hema Chhabra Department of Pharmacology, Government
DERMATOLOGICAL EMERGENCIES. DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE
 DERMATOLOGICAL EMERGENCIES DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE Dermatological Emergencies INFECTIONS ERYTHRODERMA DRUG ERUPTIONS STEVENS-JOHNSON
DERMATOLOGICAL EMERGENCIES DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE Dermatological Emergencies INFECTIONS ERYTHRODERMA DRUG ERUPTIONS STEVENS-JOHNSON
REVIEW ARTICLES ADVERSE DRUG REACTION: A COMMON DERMATOLOGICAL EMERGENCY
 REVIEW ARTICLES ADVERSE DRUG REACTION: A COMMON DERMATOLOGICAL EMERGENCY LUBNA KHONDKER 1, MD ABDUL WAHAB 2, MD SHIRAJUL ISLAM KHAN 3. Abstract In the evaluation of patients with a history of adverse cutaneous
REVIEW ARTICLES ADVERSE DRUG REACTION: A COMMON DERMATOLOGICAL EMERGENCY LUBNA KHONDKER 1, MD ABDUL WAHAB 2, MD SHIRAJUL ISLAM KHAN 3. Abstract In the evaluation of patients with a history of adverse cutaneous
Personalized Medical Care:Recognition, Management, and Maybe Prevention of Cutaneous Hypersensitivity Reactions
 Personalized Medical Care:Recognition, Management, and Maybe Prevention of Cutaneous Hypersensitivity Reactions Bernard A. Cohen, M.D. Johns Hopkins Children s Center Baltimore, Maryland (NO disclosures)
Personalized Medical Care:Recognition, Management, and Maybe Prevention of Cutaneous Hypersensitivity Reactions Bernard A. Cohen, M.D. Johns Hopkins Children s Center Baltimore, Maryland (NO disclosures)
Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital
 Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital Acknowledgements: KKCTH Dr. Ramkumar Consultant Dermatologist Dr. Ramprakash Consultant Ophthalmologist Dr. Prasad Manne
Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital Acknowledgements: KKCTH Dr. Ramkumar Consultant Dermatologist Dr. Ramprakash Consultant Ophthalmologist Dr. Prasad Manne
