Management of sharps injuries in the healthcare setting
|
|
|
- Marilyn McBride
- 5 years ago
- Views:
Transcription
1 Link to this article online for CPD/CME credits Management of sharps injuries in the healthcare setting Anna Riddell, 1 Ioana Kennedy, 2 C Y William Tong Department of Infection, Barts Health NHS Trust, London E1 2ES, UK 2 Occupational Health Service, Guy s and St Thomas NHS Foundation Trust, London, UK 3 Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK Correspondence to: C Y William Tong william.tong@nhs.net Cite this as: BMJ 2015;351:h3733 doi: /bmj.h3733 thebmj.com Previous articles in this series ЖЖAcute rheumatic fever (BMJ 2015;351:h3443) ЖЖrmal lower limb variants in children (BMJ 2015;351:h3394) ЖЖDementia: timely diagnosis and early intervention (BMJ 2015;350:h3029) ЖЖSepsis in children (BMJ 2015;350:h3017) ЖЖThe diagnosis and management of hypocalcaemia (BMJ 2015;350:h2723) Sharps injuries are common in the healthcare setting. Between 2004 and 2013 a total of 4830 healthcare associated occupational s to body fluid were reported in the UK, 71% of these for percutaneous injuries. 1 As the reporting system is likely to have recorded only cases with an important, the actual burden of sharps injuries is likely to be much higher. Healthcare workers need to be familiar with immediate management both for themselves if they become injured and for assisting injured colleagues. Many healthcare workers do not know how to manage a sharps injury, 2 particularly if this occurs out of hours. This review presents a summary of the immediate management of sharps injuries and outlines the risk assessment and management strategies to prevent the transmission of HIV, hepatitis B virus, and hepatitis C virus. What is a sharps injury? A sharps injury occurs when a sharp object such as a needle, a scalpel, bone fragments, or teeth penetrate(s) the skin. A splash of body fluid to mucous membrane or non-intact skin is another form of to body fluids that could have a similar consequence. Where do sharps injuries occur? Healthcare related sharps injuries are not confined to hospitals, with 3-7% occurring outside. 1 The most commonly reported injuries are associated with venepuncture. Injuries to nurses and healthcare assistants accounted for 42% of all reports, whereas doctors and dental professions accounted for 41% and 5%, respectively. 1 Worryingly, ancillary healthcare workers without direct patient contact were also injured by inappropriate disposal of sharps. What are the risks associated with sharps injuries? Apart from the trauma of the injury itself, a major concern with sharps injuries is the risk of infection. In Western countries the three most common blood borne infections usually associated with transmission through sharps injuries are HIV, hepatitis B virus, and hepatitis C virus. Rarely, other SOURCES AND SELECTION CRITERIA We searched PubMed and the Cochrane Library for articles published over the past 20 years using the terms sharps injury, needle stick injury, and body fluid and hand selected the most relevant and appropriate articles. To search for relevant UK national guidelines we also accessed the UK Department of Health and Public Health England (formerly Health Protection Agency) websites. We consulted guidelines from the World Health Organization, Centers for Disease Control and Prevention, British HIV Association, and British Society for Sexual Health and HIV. infections such as malaria, 3 human T cell leukaemia viruses (types I and II), 4 and haemorrhagic fever viruses, such as Ebola virus, 5 may be implicated. The risks of transmission of hepatitis B virus (when positive for HB e antigen), hepatitis C virus, and HIV through sharps injuries are often quoted as 1:3, 1:30, and 1:300, respectively. 6 7 Mucosal to body fluid carries a much lower risk (<1:1000 for HIV). 7 The actual risk of transmission during an incident depends on several factors, such as the type of injury, the viral load of the source patient, the immune status of the recipient, and risk reduction strategies implemented in the healthcare setting. Since 1997 there has only been one documented case in the UK of HIV seroconversion in a healthcare worker after an occupational. 8 Despite hepatitis B virus being highly infectious, no transmission by sharps injuries has been reported in the UK in the past 10 years. This probably relates to the high percentage of healthcare workers who are immunised against hepatitis B virus. Hepatitis C virus is most commonly associated with sharps injuries, with the virus involved in 50% of all reported cases. Since 1997 a total of 21 hepatitis C virus seroconversions in healthcare workers have been reported in the UK. 1 As these infections have a relatively long incubation period, of as much as 3-6 months, the psychological impact and associated anxiety of potential infection during the follow-up period should not be underestimated. 9 THE BOTTOM LINE First aid should be undertaken as soon as possible and a risk assessment needs to be carried out urgently by an appropriately trained individual If post- prophylaxis is deemed necessary this should begin as soon as possible without waiting for the test results of the source patient Post- prophylaxis using antiretroviral drugs within the hour after injury can considerably reduce the risk of HIV transmission Hepatitis B vaccine is highly effective in the prevention of hepatitis B; all healthcare workers should be immunised against the virus Despite the lack of post- prophylaxis to hepatitis C, such should be followed up vigorously as treatment has a high success rate Box 1 Immediate first aid after to body fluid (based on UK guidelines) 7 Gently encourage bleeding in the puncture site Wash the injured area with soap and water Do not scrub the site or use antiseptic agents Cover the wound with an impermeable dressing after cleansing In the case of mucosal, wash the exposed area copiously with water or normal saline If contact lenses are worn, wash the eyes with water or normal saline both before and after removing the lenses 26 1 August 2015 the bmj
2 Box 2 Risk assessment based on injury type (adapted from UK guidelines 6 and case-control studies ) High risk s Deep percutaneous injury Freshly used sharps Visible blood on sharps Needle used on source s blood vessels Low risk s Superficial injury, through broken skin, mucosal Old discarded sharps visible blood on sharps Needle not used on blood vessels for example, suturing, subcutaneous injection needles Exposures with no or minimal risk Skin not breached Contact of body fluid with intact skin Contact with saliva (nondental), urine, vomit, or faeces that is not visibly blood stained Needle not used on a patient before injury What should be done immediately after a sharps injury? First aid should be performed on-site immediately after a sharps injury (box 1). How is a risk assessment performed? Prompt reporting of injuries is necessary so that a risk assessment can be carried out urgently by an appropriately trained individual (other than the exposed worker) who is familiar with the local management pathway. The arrangement for the provision of post- advice varies between hospitals and time of day. All healthcare workers should be familiar with local policy. The first step in risk assessment is to establish the type of injury (box 2). In the case of bites, it is important to establish whether the source patient has been bleeding from the mouth for example, from a fight. The next step is to consider the body fluid involved (box 3). An is considered clinically important if the injury carries a risk and the body fluid is considered high risk. Where the injury does not carry a risk or the body fluid is not high risk, no further action is required other than a review of vaccine history for hepatitis B virus and the offer of vaccination if indicated. 12 The risk of transmission of a blood borne virus is related to the volume of blood transferred; thus hollow bore needles carry more risk than solid instruments. A case-control study identified high risk factors for transmission of HIV from an infected source patient after a sharps injury as a device visibly contaminated with blood, a cannula that has been inserted in the source patient s artery or vein, a deep injury, and a source patient with a high plasma viral load (for example, at the time of seroconversion) or in the advanced stages of untreated HIV infection (box 4). 10 What blood tests are required for source patients and recipients? If the risk assessment indicates that a clinically important to body fluid has occurred, the status of the source patient s blood borne viruses should be established. In some cases it may be possible to ascertain this from the source patient s medical records. If the blood borne virus status is not known, appropriate arrangements should be made, with the consent of the source patient, either to test an existing blood sample or to take a fresh sample for testing. 13 Box 5 lists the recommended tests. Immediate management and prophylaxis should be offered based on the initial risk assessment and should not be delayed while waiting the results of blood tests. A baseline serum sample should be taken from the recipient and stored for potential retrospective testing. If the hepatitis B virus immunity status of the recipient is not already known, the baseline sample can be tested for antihepatitis B surface antibody to guide further immunisation against hepatitis B virus. Further blood borne virus testing of the recipient at this stage is unnecessary, as this only reflects the status of the recipient at the time of testing and not whether transmission has occurred. What consent is required? In addition to obtaining the source patient s consent for blood borne virus testing, consent should also be sought for disclosure of the test results to the occupational health ser- vice and the injured healthcare worker. If the source patient is deemed not to have capacity to consent, the tests cannot be performed, as this is for the benefit of a third party and not in the patient s own best interests. 13 Next of kin cannot give consent on behalf of a patient, unless the patient is deceased, or a child, in which case the parents or guardians may give consent. The recipient of the sharps injury should not approach the source patient for consent as this may influence the source patient s decision and could invalidate the consent. If the incident happened during a procedure where sedation or anaesthesia was given, the source patient should be given sufficient time to recover capacity. If there are practical obstacles to obtaining consent promptly, the decision for starting post- prophylaxis should be based on the information available at the time. When should post- prophylaxis for HIV be started? The evidence for efficacy of post- prophylaxis in preventing transmission of HIV is limited. 15 Transmission of simian immunodeficiency virus in macaques was shown to be prevented by tenofovir when given within 24 hours of inoculation and continued for four weeks. 16 Treatment efficacy was reduced if there was a delay between inoculation and treatment and if the duration of treatment was shortened. The use of post- prophylaxis in an occupational health setting was based on an observational study, which showed that the use of zidovudine reduced the risk of transmission after by 80%. 10 Table 1 summarises the recommended use of post- prophylaxis based on risk assessment. Although the risk of HIV transmission is increased if patients have a high viral load, it is not clear what the risk of transmission is if the viral load is undetectable. In such cases the risk is thought to be low although not zero. 17 Guidelines from the United States recommend that post- prophylaxis should be offered even when source patients have undetectable viral loads. 18 In the UK a discussion with Box 3 Body fluids and risk for transmission of blood borne viruses (in alphabetical order, based on UK guidelines 6 7 ) High risk body fluids Amniotic fluid Blood Cerebrospinal fluid Exudative or other tissue fluid from burns or skin lesions Human breast milk Pericardial fluid Peritoneal fluid Pleural fluid Saliva in association with dentistry (likely to be contaminated with blood, even when not visibly so) Semen Synovial fluid Unfixed human tissues and organs Vaginal secretions Low risk body fluids (unless visibly blood stained) Saliva (non-dentistry associated) Stool Urine Vomit the bmj 1 August
3 the recipient about the balance between the risk of transmission and the side effects of post- prophylaxis is recommended. Post- prophylaxis is generally not recommended if the viral load is less than 200 copies/ml but could be offered if the recipient is anxious about the risk. 19 Thus the final decision on whether or not post- prophylaxis should be used should be made with full engagement of the recipient. The antiretroviral agents recommended for post- prophylaxis differ between the guidelines from different countries. Table 2 summarises the recommended agents and their possible side effects. The current agents recommended in the UK are well tolerated, with few side effects, can be taken at any time of the day, and can be stored at room temperature. Pregnancy is not a contraindication, although the possible risk and benefits to the fetus should be discussed with the recipient. Advice from a specialist experienced in the management of HIV in pregnancy should be sought. UK guidelines recommend starting post- prophylaxis as soon as possible and no later than 72 hours after, and to continue for 28 days. 7 If the source patient s HIV test result is negative, post- prophylaxis can be discontinued. Before starting post- prophylaxis a full drug history should be obtained from the recipient because of potential interactions between antiretroviral agents and other drugs. An excellent resource for checking drug interactions is available online. 22 Post- prophylaxis may need to be adjusted if the source patient is suspected of having or known to have resistance against one or more components of the standard post- prophylaxis. Such problems should be discussed with local HIV experts or the HIV doctor treating the source patient, although this should not delay post- prophylaxis. How can the transmission of hepatitis B virus be prevented? The vaccine against hepatitis B virus can be given shortly after either as the first dose of a primary course or as a booster. Table 3 shows the strategy for offering vaccination against hepatitis B virus after a sharps injury based on vaccination history, previous response to vaccination, type of, and the hepatitis B virus status of the source patient. The additional use of hepatitis B immunoglobulin aims to provide passive immunity if the source patient is known to be at high risk of hepatitis B virus infection and the recipient has not been previously adequately immunised or is a known non-responder to the vaccine that is, those with a documented absence of hepatitis B surface antibodies after a full course of hepatitis B vaccination. 12 The ideal time frame for use of post- hepatitis B immunoglobulin is within 48 hours of, although it can be considered up to one week. 12 What can be done about to hepatitis C virus? A case-control study found that the risk of hepatitis C virus transmission after percutaneous increased with deep injuries and procedures involving hollow bore needles placed in a source patient s blood vessel. 11 Hepatitis C virus has also been found to have prolonged survival in syringes with a high residual void volume. 23 The risk of hepatitis C virus transmission increases significantly if Box 4 Risk assessment of source patient (based on UK guidelines 7 and case-control studies ) High risk source Known to be infected with one or more blood borne viruses (viral load and treatment status unknown) Known to have a detectable viral load for one or more blood borne viruses Unknown viral load but known to have advanced or untreated blood borne virus infection Blood borne virus status unknown but had known risk factors* Low risk source Ongoing risk factors for blood borne viruses and recent blood test results were negative for all three blood borne viruses Infected with a blood borne virus but known to have a fully suppressed viral load Unknown viral load but receiving long term antiviral treatment for blood borne virus with good adherence and known to be stable Blood borne virus status unknown but had no known risk factors for such viruses Source with no or minimal risk A recent blood test result was negative for all three blood borne viruses *Examples of risk factors: intravenous drug use, men who have sex with men, commercial sex workers, origin from high prevalence areas for HIV, hepatitis B virus, or hepatitis C virus. Can be arranged from source patient after consent if no recent results for blood borne viruses are available. However, management should not be delayed while waiting for results. Box 5 Recommended investigations in source patient after consent (based on expert opinion) Combined HIV antigen and antibody (fourth generation HIV immunoassay) Hepatitis B surface antigen Hepatitis C antibody* Other additional investigations could be added if a specific transmissible infectious condition is suspected for example, malaria, human T cell leukaemia virus *Testing for hepatitis C virus RNA or antigen should also be considered if source patient is at high risk for hepatitis C virus. This is because hepatitis C virus antibody may be negative during acute infection and may remain negative for more than 12 months in immunocompromised patients. 14 Table 1 Recommendation for HIV post- prophylaxis based on HIV status of source patient and nature of incident (based on UK guidelines 7 ) Incident risk and nature of Status of source patient* or low risk for HIV High risk or known to be HIV positive Minimal risk incident or low risk Post- prophylaxis t recommended t recommended Follow-up t required t required Low risk incident and high risk Post- prophylaxis t recommended Considered Follow-up t required Advisable High risk incident and high risk Post- prophylaxis t recommended Recommended Follow-up t required Required *Where it is not possible to identify the source patient, a risk assessment should be conducted, including circumstances of and epidemiological likelihood of HIV being present. Use of post- prophylaxis is unlikely to be justified in most such s. Could be offered after a thorough discussion of risk. Table 2 Recommended antiretroviral agents for HIV post- prophylaxis after sharps injuries (based on US, 18 UK, 20 and WHO 21 guidelines) Source of guidelines and recommendations UK and USA Truvada (245 mg tenofovir disoproxil fumarate and 200 mg emtricitabine) one tablet daily Raltegravir 400 mg twice daily World Health Organization 2 nucleoside reverse transcriptase inhibitors: tenofovir+lamivudine or emtricitabine Kaletra (200 mg lopinavir and 50 mg ritonavir) or other ritonavir boosted protease inhibitor Potential side effects Rare, but important side effects include acute renal failure and proximal renal tubolopathy (Fanconi s syndrome) Rare, but include insomnia, diarrhoea, and nausea and vomiting Rare, but important side effects of tenofovir include acute renal failure and proximal renal tubolopathy (Fanconi s syndrome) Rare, but include rash, diarrhoea, nausea and vomiting, and abnormal liver function test results 28 1 August 2015 the bmj
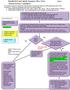
4 Managing sharps injuries Gently encourage bleeding Wash with soap p and water Don t scrub wound Don t use antiseptic illustrations by Katie Jessop 1 First aid Mucosal wash copiously with water or saline Wash eyes before and after removing contact lenses Cover with impermeable dressing 2 Assess incident risk Incident risk: Injury Needle use Age of sharps Blood on sharps High Low Minimal Deep Superficial skin breach Intravascular Recently used Visible Suturing/ Subcutaneous Old, discarded t visible t used on patient Contact with low risk fluids: Saliva Urine Vomit Faeces 3 Assess source patient for blood borne viruses. HIV, hepatitis B (HBV) and hepatitis C (HCV) are the most common. Post- prophylaxis (PEP) and/or follow up treatment may be required. Detectable Untreated / advanced infection Is the source patient known to be infected with one or more blood borne viruses? Viral load Unknown Treatment status Unknown HIV Begin immediate PEP with antiretroviral agents High risk Fully suppressed Long term treatment, good adherence HBV Check vaccine status further action required Do risk factors apply? e.g. Injecting drug users, men who have sex with men, sex workers HCV Follow up and treat if necessary See table 2 See table 3 See table 4 Recent blood test negative for HIV, HBV & HCV Low risk further action required WILL STAHL-TIMMINS the bmj 1 August
5 Table 3 Hepatitis B management algorithm based on vaccination history of recipient (based on UK immunisation guidelines 12 ) Hepatitis B vaccination history of recipient 1 dose of vaccine or uncertain vaccination history 2 doses of vaccine Hepatitis B immunity status Unknown Unknown Known vaccine responder Known vaccine non-responder High risk, source patient positive for hepatitis B surface antigen High risk, hepatitis B surface antigen status of source patient unknown Source patient negative for hepatitis B surface antigen or low risk (regardless of hepatitis B surface antigen status) *Doses spaced at 0, 1, and 2 months with booster dose at 12 months. Hepatitis B immunoglobulin 500 units intramuscularly per dose. Accelerated vaccine* course+1 dose of hepatitis B immunoglobulin 2 doses of vaccine at 0 and 1 month Consider booster Accelerated vaccine* course 1 dose of vaccine Consider booster Initiate vaccine course Complete vaccine course Consider booster 2 doses of hepatitis B immunoglobulin at 0 and 1 month; consider booster 2 doses of hepatitis B immunoglobulin at 0 and 1 month; consider booster hepatitis B immunoglobulin; consider booster Table 4 Suggested follow-up schedules after high risk sharps injuries (based on UK guidelines 7 for HIV and expert opinions for hepatitis B virus and hepatitis C virus) Blood borne virus risk in source patient Within first 12 weeks Week 12* Week 24 HIV Hepatitis B virus If post- prophylaxis is started, review within seven days to monitor any side effects. Carry out tests for full blood count, urea and electrolytes, liver function, and bone profile, and carry out urine analysis Attendance for hepatitis B vaccination with or without second dose of hepatitis B immunoglobulin according to recommended schedule Test for combined HIV antigen and antibody (fourth generation HIV immunoassay) Test for hepatitis B surface antigen and hepatitis B surface antibody Hepatitis C virus Test for hepatitis C virus RNA at week 6 Test for hepatitis C virus antibody and hepatitis C virus RNA *If HIV post- prophylaxis has been started, week 12 is calculated from end of post- prophylaxis. t routinely recommended t routinely recommended unless hepatitis B immunoglobulin was given t routinely recommended unless risk of hepatitis C virus transmission is high ADDITIONAL EDUCATIONAL RESOURCES Resources for healthcare professionals Health and Safety Executive. Sharps injuries what you need to do ( provides a perspective from the regulatory aspect of sharps injury NHS Choices. What should I do if I injure myself with a used needle? ( provides a concise and practical approach to needlestick injury; also useful for nonhealthcare workers Royal College of Nursing. Needlestick and sharps injuries ( sharps_injuries) has a link to the Royal College of Nursing guidance on sharps safety Patient.co.uk. Needlestick injury ( provides good tips on how to prevent sharps injury Health Education England. e-learning for Healthcare ( several e-learning modules under Pathology (PATH)/e-Path 07-Virology provide useful information on prevention and management of sharps injury: 07_052 Sharps Injuries; 07_060 HBV; 07_104 HIV prevention Medscape ( a comprehensive overview of the American approach to management of to body fluids HIV-drug interaction.org ( maintained by the University of Liverpool, which provides a clinically useful, up to date and evidence based drug-drug interaction resource, freely available to healthcare workers, patients, and researchers the source has a high viral load, 11 whereas those with an undetectable viral load are unlikely to be infectious. 24 Currently no vaccine or post- prophylaxis is effective in the prevention of hepatitis C virus transmission. However, treatment of acute hepatitis C infection is known to be highly effective. 25 Early detection of hepatitis C virus transmission and referral to an appropriate specialist for assessment and treatment is therefore essential. How is care accessed in different healthcare settings? Local policy should clearly identify which department to contact during and out of normal working hours. The emergency department is usually the location for immediate access to advice, medicines, and vaccines, although in some hospitals additional post- prophylaxis packs are stored in strategic locations such as operating theatres or delivery suites. In the community setting or in dental practices, the initial management of the injury has to be started on-site, immediately after the incident. A system to enable injured healthcare workers to access urgent expert advice should be locally agreed. As this occurs in an outpatient setting, it is important that source patients should be assessed before discharge and consent obtained for any potential blood tests. Hepatitis B virus vaccines are widely available in general practice, but access to HIV post- prophylaxis would require a visit to the local emergency department. Because of the need to start these drugs early, attendance at an emergency department should not be delayed if this is deemed necessary after risk assessment. How should injured healthcare workers be followed up? After important to body fluid, recipients should be followed up for at least 12 weeks. Table 4 summarises the testing required and timing of follow-up. Healthcare workers who have sustained a high risk injury and receive post- prophylaxis should not be considered infectious and should be reassured that it is safe for them to return to clinical work, including performing procedures that are prone to. 26 They should, however, be advised to use barrier contraception and to avoid blood or tissue donations, pregnancy, and breast feeding, especially during the first six to 12 weeks after August 2015 the bmj
Clinical Education Initiative OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS. Antonio E. Urbina, MD
 Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
Contamination Incidents Frequently Asked Questions
 Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis).
 LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
Sunny Smiles Clinical Guidelines
 Sunny Smiles Clinical Guidelines Accidental Inoculation Injury - Guidance for healthcare professionals on dealing with needle-stick injuries to members Date Written: April 2010 Contents 1 Local Contacts...3
Sunny Smiles Clinical Guidelines Accidental Inoculation Injury - Guidance for healthcare professionals on dealing with needle-stick injuries to members Date Written: April 2010 Contents 1 Local Contacts...3
Guidance for management of exposure events where there is a risk of transmission of blood borne viruses
 Guidance for management of exposure events where there is a risk of transmission of blood borne viruses (HIV, Hepatitis B and Hepatitis C) in the community SUMMARY Where a child is thought to have had
Guidance for management of exposure events where there is a risk of transmission of blood borne viruses (HIV, Hepatitis B and Hepatitis C) in the community SUMMARY Where a child is thought to have had
EXPOSURE (HIV/HEPATITIS) BLOOD & BODY FLUIDS
 Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
One of your office personnel
 Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Management of Exposure to Needlestick Injuries & Body Fluids
 Management of Exposure to Needlestick Injuries & Body Fluids Clinical S.O.P. No.:28 Compiled by: Approved by: Review date: November 2016 DOCUMENT HISTORY Version Detail of purpose / change Author / edited
Management of Exposure to Needlestick Injuries & Body Fluids Clinical S.O.P. No.:28 Compiled by: Approved by: Review date: November 2016 DOCUMENT HISTORY Version Detail of purpose / change Author / edited
43. Guidelines on Needle stick Injury
 43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE
 Sandyford Protocols MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE www.hiv-druginteractions.org If you require information on occupational exposure to blood borne viruses, including HIV, please refer to the
Sandyford Protocols MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE www.hiv-druginteractions.org If you require information on occupational exposure to blood borne viruses, including HIV, please refer to the
HIV Occupational Transmission and Exposure. Marsh Gelbart 2010
 HIV Occupational Transmission and Exposure Marsh Gelbart 2010 Rationale for Post Exposure Prophylaxis (PEP) It was estimated that the risk for HIV transmission after percutaneous exposures involving larger
HIV Occupational Transmission and Exposure Marsh Gelbart 2010 Rationale for Post Exposure Prophylaxis (PEP) It was estimated that the risk for HIV transmission after percutaneous exposures involving larger
Bloodborne Pathogens Across the Continuum of Care Sue Sebazco, Arlington, Texas A Webber Training Teleclass
 Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Blood/Body Fluid Exposure Option
 Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Management of Workplace Exposure to Blood-borne Pathogens
 Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
INITIAL EVALUATION AND MANAGEMENT FOLLOWING EXPOSURE TO BLOOD OR BODY FLUIDS
 Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Blood-Borne Pathogens and Post-Exposure Prophylaxis
 Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison
 SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
Bloodborne Pathogens Exposure Procedure
 Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Needlestick and Splash Exposure Flow Chart Page 1 Clinical Practice Guidelines
 Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Peninsula Dental Social Enterprise (PDSE)
 Peninsula Dental Social Enterprise (PDSE) Inoculation Incidents and Blood Borne Viruses Version 1.0 Date approved: October 2013 Approved by: The Board Review due: March 2016 Contents Section Topic Page
Peninsula Dental Social Enterprise (PDSE) Inoculation Incidents and Blood Borne Viruses Version 1.0 Date approved: October 2013 Approved by: The Board Review due: March 2016 Contents Section Topic Page
Village By Village HIV/AIDS Policy
 The Old Sweet Shop Teme Street, Tenbury Wells, Worcs. WR15 8BB Phone: 07887 870090 Web: villagebyvillage.org.uk Email: neil@villagebyvillage.org.uk Registered Charity Number 1116952 18/9/07 Village By
The Old Sweet Shop Teme Street, Tenbury Wells, Worcs. WR15 8BB Phone: 07887 870090 Web: villagebyvillage.org.uk Email: neil@villagebyvillage.org.uk Registered Charity Number 1116952 18/9/07 Village By
SHARPS MANAGEMENT AND INOCULATION INJURIES
 Community Infection Prevention and Control Guidance for Health and Social Care Sharps Management and Inoculation Injuries Version 1.01 May 15 Harrogate and District NHS Foundation Trust Sharps Management
Community Infection Prevention and Control Guidance for Health and Social Care Sharps Management and Inoculation Injuries Version 1.01 May 15 Harrogate and District NHS Foundation Trust Sharps Management
Post Exposure Prophylaxis (PEP)
 Post Exposure Prophylaxis (PEP) Occupational exposure Occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV and HCV) that may occur in healthcare settings during performance
Post Exposure Prophylaxis (PEP) Occupational exposure Occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV and HCV) that may occur in healthcare settings during performance
MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES
 NHS GREATER GLASGOW CONTROL OF INFECTION COMMITTEE GUIDELINE MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES Effective
NHS GREATER GLASGOW CONTROL OF INFECTION COMMITTEE GUIDELINE MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES Effective
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS
 NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
Exposures at Non-MUSC Clinical Sites
 BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Exposure. Blood. Department of Health & Human Services
 Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital
 HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital HIV continues to be a major global public health issue, Approximately
HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital HIV continues to be a major global public health issue, Approximately
Definitions. Appendix A
 Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
SHARPS PROCEDURE PROCEDURE FOR THE SAFE USE & DISPOSAL OF SHARPS AND THE MANAGEMENT OF SHARPS INJURIES AND BLOOD EXPOSURE INCIDENTS.
 SHARPS PROCEDURE PROCEDURE FOR THE SAFE USE & DISPOSAL OF SHARPS AND THE MANAGEMENT OF SHARPS INJURIES AND BLOOD EXPOSURE INCIDENTS February 2012 Version 1.0 1 CONTENTS Section Page 1.0 Introduction 3
SHARPS PROCEDURE PROCEDURE FOR THE SAFE USE & DISPOSAL OF SHARPS AND THE MANAGEMENT OF SHARPS INJURIES AND BLOOD EXPOSURE INCIDENTS February 2012 Version 1.0 1 CONTENTS Section Page 1.0 Introduction 3
Blood and Body Fluid Exposure
 Blood and Body Fluid Exposure Infection Prevention and Control Contents Policy... 1 Purpose... 1 Scope/Audience... 1 Definitions... 2 Associated documents... 2 1.1 Indications for BBFE reporting... 2 1.2
Blood and Body Fluid Exposure Infection Prevention and Control Contents Policy... 1 Purpose... 1 Scope/Audience... 1 Definitions... 2 Associated documents... 2 1.1 Indications for BBFE reporting... 2 1.2
TRUST POLICY AND PROCEDURES FOR THE MANAGEMENT OF INOCULATION INCIDENTS
 TRUST POLICY AND PROCEDURES FOR THE MANAGEMENT OF INOCULATION INCIDENTS Reference Number CL-RM 2014 022 Version: 2.6 Status Final Authors: Dr D.Gnanarajah, Consultant Microbiologist Dr Apoola Consultant
TRUST POLICY AND PROCEDURES FOR THE MANAGEMENT OF INOCULATION INCIDENTS Reference Number CL-RM 2014 022 Version: 2.6 Status Final Authors: Dr D.Gnanarajah, Consultant Microbiologist Dr Apoola Consultant
HEPATITIS B INFECTION and Pregnancy. Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011
 HEPATITIS B INFECTION and Pregnancy Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011 HEPATITIS B 26/07/2011 What is Hepatitis B? It is inflammation (infection) of the liver
HEPATITIS B INFECTION and Pregnancy Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011 HEPATITIS B 26/07/2011 What is Hepatitis B? It is inflammation (infection) of the liver
The Bloodborne Pathogen Standard. An Overview
 The Bloodborne Pathogen Standard An Overview The Standard l In 1990, OSHA (Occupational Safety and Health Administration), developed the Bloodborne Pathogen Standard to protect workers by limiting occupational
The Bloodborne Pathogen Standard An Overview The Standard l In 1990, OSHA (Occupational Safety and Health Administration), developed the Bloodborne Pathogen Standard to protect workers by limiting occupational
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1
 PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
Bloodborne Pathogens and Exposure Control
 Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
A. Background for Trainer: B. What OSHA Requires: Bloodborne Pathogens. Lesson Plan 6080a
 Lesson Plan 6080a This training session outline is designed to follow the accompanying booklet, OSHA s Bloodborne Pathogens Standard. The booklet reviews what employees who are potentially exposed to the
Lesson Plan 6080a This training session outline is designed to follow the accompanying booklet, OSHA s Bloodborne Pathogens Standard. The booklet reviews what employees who are potentially exposed to the
Sharps and Blood/Body Fluid Contamination Injury Immediate Actions
 Infection Prevention and Control Assurance - Standard Operating Procedure 8 (IPC SOP 8) Sharps and Blood/Body Fluid Contamination Injury Immediate Actions Why we have a procedure? The Health and Social
Infection Prevention and Control Assurance - Standard Operating Procedure 8 (IPC SOP 8) Sharps and Blood/Body Fluid Contamination Injury Immediate Actions Why we have a procedure? The Health and Social
Dr Helena Nixon Specialist Occupational Physician University Hospital Birmingham
 A Study in Scarlet Dr Helena Nixon Specialist Occupational Physician University Hospital Birmingham World Map April 2006 World developing nations World life expectancy Why the interest in BBVs? Estimated
A Study in Scarlet Dr Helena Nixon Specialist Occupational Physician University Hospital Birmingham World Map April 2006 World developing nations World life expectancy Why the interest in BBVs? Estimated
Sharps injuries and exposure to blood and high risk body fluids SOP:
 Clinical Sharps injuries and exposure to blood and high risk body fluids SOP: Document Control Summary Status: Replacement. Replaces: Management of clinical sharps injuries and exposure to blood and high
Clinical Sharps injuries and exposure to blood and high risk body fluids SOP: Document Control Summary Status: Replacement. Replaces: Management of clinical sharps injuries and exposure to blood and high
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS
 NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
OVERVIEW OF PRESENTATION
 OVERVIEW OF PRESENTATION This training is required by the Texas Department of Health Ch. 96, Bloodborne Pathogen Control. Every employee of the district will be required to have some training on bloodborne
OVERVIEW OF PRESENTATION This training is required by the Texas Department of Health Ch. 96, Bloodborne Pathogen Control. Every employee of the district will be required to have some training on bloodborne
Needles and Sharps Exposure: How do We Proceed?
 Needles and Sharps Exposure: How do We Proceed? UMAYYA MUSHARRAFIEH,MD AMERICAN UNIVERSITY OF BEIRUT MEDICAL CENTER JUNE 14, 2013 Health care workers who use or may be exposed to needles are at increased
Needles and Sharps Exposure: How do We Proceed? UMAYYA MUSHARRAFIEH,MD AMERICAN UNIVERSITY OF BEIRUT MEDICAL CENTER JUNE 14, 2013 Health care workers who use or may be exposed to needles are at increased
Greater Glasgow and Clyde. Blood Borne Viruses: Some important basic facts
 Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts A programme developed by Greater Glasgow and Clyde Health
Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts A programme developed by Greater Glasgow and Clyde Health
HIV and PEP. LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED
 HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
3/23/2016. Managing Bloodborne Pathogen Exposures. Managing Bloodborne Pathogen Exposures
 This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
Introduction to Blood Borne Pathogens
 Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
EU Directive for Safer Sharps. Anna Pronyszyn Infection Prevention Nurse Conusultant
 EU Directive for Safer Sharps Anna Pronyszyn Infection Prevention Nurse Conusultant Regulation and Legislation Health and Social Care Act 2008 Occupier s Liability Act 1957 Health and Safety at Work Act
EU Directive for Safer Sharps Anna Pronyszyn Infection Prevention Nurse Conusultant Regulation and Legislation Health and Social Care Act 2008 Occupier s Liability Act 1957 Health and Safety at Work Act
Goldenrod Hills Community Action. Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR
 Goldenrod Hills Community Action Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR 1910.1030 Welcome to GHCA s Bloodborne Pathogen Training based upon the Occupational Safety and Health
Goldenrod Hills Community Action Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR 1910.1030 Welcome to GHCA s Bloodborne Pathogen Training based upon the Occupational Safety and Health
Bloodbourne Pathogens (BBP) Occupational Post-Exposure Chemophrophylaxis
 Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
June 4, Page 1 of 5 POLICY STATEMENT
 POLICY STATEMENT This policy has been written to inform all staff, clients, relatives and other visitors to the homes of service users about the risks associated with MRSA, AIDS and HIV hazards in the
POLICY STATEMENT This policy has been written to inform all staff, clients, relatives and other visitors to the homes of service users about the risks associated with MRSA, AIDS and HIV hazards in the
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS
 STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
19/08/2014. What is Injection Safety?
 Infection Prevention and Control A Foundation Course SAFE INJECTION PRACTICES AND SHARPS MANAGEMENT Fiona Barry IPCN Mercy University Hospital, Cork 2014 Links to CDC Materials http://www.youtube.com/watch%3fv%3d6d0stmoz80k
Infection Prevention and Control A Foundation Course SAFE INJECTION PRACTICES AND SHARPS MANAGEMENT Fiona Barry IPCN Mercy University Hospital, Cork 2014 Links to CDC Materials http://www.youtube.com/watch%3fv%3d6d0stmoz80k
Universal Precautions
 Universal Precautions emphasizes the need for workers and students to consider all blood and body fluids as potentially infected with HIV, HBV, and / or other blood-borne pathogens, and to adhere rigorously
Universal Precautions emphasizes the need for workers and students to consider all blood and body fluids as potentially infected with HIV, HBV, and / or other blood-borne pathogens, and to adhere rigorously
Occupational Exposure to Blood Borne Viruses
 Occupational Exposure to Blood Borne Viruses Marissa Herron Occupational Health Support Nurse [BBV] 2014 Aim To raise awareness of the key information and procedures in NHS Lanarkshire's Control of Infection
Occupational Exposure to Blood Borne Viruses Marissa Herron Occupational Health Support Nurse [BBV] 2014 Aim To raise awareness of the key information and procedures in NHS Lanarkshire's Control of Infection
Information for Primary Care: Managing patients who require assessment for Ebola virus disease Updated 17 Oct 2014
 Information for Primary Care: Managing patients who require assessment for Ebola virus This guidance is aimed at clinical staff undertaking direct patient care in primary care, including GP surgeries,
Information for Primary Care: Managing patients who require assessment for Ebola virus This guidance is aimed at clinical staff undertaking direct patient care in primary care, including GP surgeries,
Sharps Policy: Safe Handling and Disposal of Sharps
 Sharps Policy: Safe Handling and Disposal of Sharps Document Control Sheet Name of document: Sharps Policy: Safe Handling and Disposal of Sharps Version: 8 Status: Owner: In review Infection Prevention
Sharps Policy: Safe Handling and Disposal of Sharps Document Control Sheet Name of document: Sharps Policy: Safe Handling and Disposal of Sharps Version: 8 Status: Owner: In review Infection Prevention
Needle Stick. Mr. Fadi J. Zaben RN MSN IMET 2000, Ramallah IMET 2000
 Needle Stick Mr. Fadi J. Zaben RN MSN, Ramallah 1 Objectives: Define Needle Stick. Mention the sharps. Discus the rate of incidence. Identify the person who is at risk. Discus Needle stick and infectious
Needle Stick Mr. Fadi J. Zaben RN MSN, Ramallah 1 Objectives: Define Needle Stick. Mention the sharps. Discus the rate of incidence. Identify the person who is at risk. Discus Needle stick and infectious
Module 1: HIV epidemiology, transmission and prevention
 Session 2 Module goals Module 1 Participants will be able to: -offer an insight into the epidemiological situation in the country and worldwide -present the HIV transmission modes and the broad approaches
Session 2 Module goals Module 1 Participants will be able to: -offer an insight into the epidemiological situation in the country and worldwide -present the HIV transmission modes and the broad approaches
Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May Contains information for:
 APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet. Instructions for Employees/Students:
 MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
HEPATITIS HEPATITIS A. The Hepatitis Alphabet HOW DOES ONE GET HEPATITIS A? THE SYMPTOMS of HEPATITIS A
 HEPATITIS Hepatitis is an inflammation of the liver that can be caused by viruses, chemicals or drugs. The two most common types of viral hepatitis are Hepatitis A (also called infectious hepatitis ) and
HEPATITIS Hepatitis is an inflammation of the liver that can be caused by viruses, chemicals or drugs. The two most common types of viral hepatitis are Hepatitis A (also called infectious hepatitis ) and
Colgate University. Bloodborne Pathogens Exposure Control Plan
 Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland. An update for registered practitioners September 2017
 HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland An update for registered practitioners September 2017 Key Messages HIV is a major public health challenge for Scotland with an annual average of 359
HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland An update for registered practitioners September 2017 Key Messages HIV is a major public health challenge for Scotland with an annual average of 359
Blood Borne Pathogens (BBP)
 Blood Borne Pathogens (BBP) Healthcare facilities are high-risk areas for exposure to bloodborne pathogens, so protect yourself and remind others to do the same. There are three bloodborne pathogens of
Blood Borne Pathogens (BBP) Healthcare facilities are high-risk areas for exposure to bloodborne pathogens, so protect yourself and remind others to do the same. There are three bloodborne pathogens of
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK)
 POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
Post-Sexual Exposure Prophylaxis (npep)
 Projeto Praça Onze Universidade Federal do Rio de Janeiro Post-Sexual Exposure Prophylaxis (npep) Mauro Schechter Principal Investigator, Projeto Praça Onze Professor of Infectious Diseases Universidade
Projeto Praça Onze Universidade Federal do Rio de Janeiro Post-Sexual Exposure Prophylaxis (npep) Mauro Schechter Principal Investigator, Projeto Praça Onze Professor of Infectious Diseases Universidade
Revised April WGH ER #5 Hospital Rd. Whitehorse Phone: (867) Outside of Whitehorse: Local Community Health Centre/Clinic/ER
 Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid
Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid
The Prevention of Infection with Blood Borne Viruses [BBV]
![The Prevention of Infection with Blood Borne Viruses [BBV] The Prevention of Infection with Blood Borne Viruses [BBV]](/thumbs/93/112976583.jpg) The Prevention of Infection with Blood Borne Viruses [BBV] Control of Infection Manual Section G Study Morning 25 th November 2014 Marissa Herron Occupational Health Support Nurse [BBV] Aim To raise awareness
The Prevention of Infection with Blood Borne Viruses [BBV] Control of Infection Manual Section G Study Morning 25 th November 2014 Marissa Herron Occupational Health Support Nurse [BBV] Aim To raise awareness
Blood borne Pathogen
 Blood borne Pathogen Training For Certified Nursing Assistants Meets the Blood borne Pathogens & Infection Control Update (Formerly HIV/AIDS) 1 0 In-service Hour Meets the Blood borne Pathogens & Infection
Blood borne Pathogen Training For Certified Nursing Assistants Meets the Blood borne Pathogens & Infection Control Update (Formerly HIV/AIDS) 1 0 In-service Hour Meets the Blood borne Pathogens & Infection
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Management
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
Hepatitis C Virus (HCV)
 Clinical Practice Guidelines Hepatitis C Virus (HCV) OBJECTIVE The purpose is to guide the appropriate diagnosis and management of Hepatitis C Virus (HCV). GUIDELINE These are only guidelines, and are
Clinical Practice Guidelines Hepatitis C Virus (HCV) OBJECTIVE The purpose is to guide the appropriate diagnosis and management of Hepatitis C Virus (HCV). GUIDELINE These are only guidelines, and are
Blood Borne Pathogens. Becky Walch, R.N. Micheel Valdez, L.V.N.
 Blood Borne Pathogens Becky Walch, R.N. Micheel Valdez, L.V.N. Examples of Blood Borne Pathogens Hepatitis B Hepatitis C Other Hepatitis HIV Hepatitis Hepatitis means inflammation of the liver. Hepatitis
Blood Borne Pathogens Becky Walch, R.N. Micheel Valdez, L.V.N. Examples of Blood Borne Pathogens Hepatitis B Hepatitis C Other Hepatitis HIV Hepatitis Hepatitis means inflammation of the liver. Hepatitis
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: F:
 University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
HEPATITIS B GUIDANCE FOR THE PROTECTION OF HEALTH CARE WORKERS AND PATIENTS. All clinical areas of the Trust. Prevent spread of Hepatitis B virus
 Trust Policy and Procedure PP(15)027 Document Ref No: HEPATITIS B GUIDANCE FOR THE PROTECTION OF HEALTH CARE WORKERS AND PATIENTS For use in: For use by: For use for: Document owner: Status: All clinical
Trust Policy and Procedure PP(15)027 Document Ref No: HEPATITIS B GUIDANCE FOR THE PROTECTION OF HEALTH CARE WORKERS AND PATIENTS For use in: For use by: For use for: Document owner: Status: All clinical
PROTECTION FROM OCCUPATIONAL ACQUIRED/TRANSMITTED COMMUNICABLE DISEASES Policy
 PROTECTION FROM OCCUPATIONAL ACQUIRED/TRANSMITTED COMMUNICABLE DISEASES Policy 1 Policy title Protection from Occupational Acquired /Transmitted Communicable Diseases Policy COR 60 reference Policy category
PROTECTION FROM OCCUPATIONAL ACQUIRED/TRANSMITTED COMMUNICABLE DISEASES Policy 1 Policy title Protection from Occupational Acquired /Transmitted Communicable Diseases Policy COR 60 reference Policy category
UCP BloodBorne Pathogens Recertification
 UCP BloodBorne Pathogens Recertification 1 OSHA Occupational Safety and Health Administration: a. In 1991 OSHA established Bloodborne Pathogen Standard 29 CFR 1910.1030. b. Set code of conduct / limit
UCP BloodBorne Pathogens Recertification 1 OSHA Occupational Safety and Health Administration: a. In 1991 OSHA established Bloodborne Pathogen Standard 29 CFR 1910.1030. b. Set code of conduct / limit
Sharps Injuries Management
 Sharps Injuries Management and other blood or body fluid exposure incidents This procedural document supersedes: PAT/IC 14 v.5 Sharps Injuries Management and other blood or body fluid exposure incidents
Sharps Injuries Management and other blood or body fluid exposure incidents This procedural document supersedes: PAT/IC 14 v.5 Sharps Injuries Management and other blood or body fluid exposure incidents
CMC Annual Review of BLOODBORNE DISEASES. Prevention of Transmission for School Staff
 CMC Annual Review of BLOODBORNE DISEASES Prevention of Transmission for School Staff Standard on Bloodborne Pathogens OSHA sets the standard of care We must have standards to follow in schools for everyone
CMC Annual Review of BLOODBORNE DISEASES Prevention of Transmission for School Staff Standard on Bloodborne Pathogens OSHA sets the standard of care We must have standards to follow in schools for everyone
Sharps Management, Needle-Stick Injuries and Exposure to Blood Borne Viruses Procedure ICPr005
 Sharps Management, Needle-Stick Injuries and Exposure to Blood Borne Viruses Procedure ICPr005 Version Date Date of Next Reason for Change (eg. full rewrite, No. Ratified/ Implementation Review amendment
Sharps Management, Needle-Stick Injuries and Exposure to Blood Borne Viruses Procedure ICPr005 Version Date Date of Next Reason for Change (eg. full rewrite, No. Ratified/ Implementation Review amendment
Gwynedd Mercy University Bloodborne Pathogen Safety and Awareness Training
 Gwynedd Mercy University Bloodborne Pathogen Safety and Awareness Training Education is the KEY Here are Gwynedd Mercy University, we recognize the importance of providing a safe working environment for
Gwynedd Mercy University Bloodborne Pathogen Safety and Awareness Training Education is the KEY Here are Gwynedd Mercy University, we recognize the importance of providing a safe working environment for
ACS BLOOD BORNE PATHOGEN TRAINING
 ACS BLOOD BORNE PATHOGEN TRAINING OBJECTIVE Define Blood borne pathogens Instruct how to recognize exposure to BBP Prevent or reduce risk of BBP exposure Identify high risk groups Review ACS exposure protocol
ACS BLOOD BORNE PATHOGEN TRAINING OBJECTIVE Define Blood borne pathogens Instruct how to recognize exposure to BBP Prevent or reduce risk of BBP exposure Identify high risk groups Review ACS exposure protocol
POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV
 POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV DISCLOSURE Relevant relationships with commercial entities none Potential for conflicts of interest within this presentation none
POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV DISCLOSURE Relevant relationships with commercial entities none Potential for conflicts of interest within this presentation none
Chapter 2 Hepatitis B Overview
 Chapter 2 Hepatitis B Overview 23 24 This page intentionally left blank. HEPATITIS B OVERVIEW Hepatitis B Virus The hepatitis B virus (HBV) belongs to the Hepadnaviridae family and is known to cause both
Chapter 2 Hepatitis B Overview 23 24 This page intentionally left blank. HEPATITIS B OVERVIEW Hepatitis B Virus The hepatitis B virus (HBV) belongs to the Hepadnaviridae family and is known to cause both
Commonly Asked Questions About Chronic Hepatitis C
 Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
DEPARTMENT OF GENITO-URINARY MEDICINE HEPATITIS B
 DEPARTMENT OF GENITO-URINARY MEDICINE MAIN TRANSMISSION ROUTES HEPATITIS B Sexual transmission Occurs in unvaccinated/non immune men who have sex with men and correlates with multiple partners, unprotected
DEPARTMENT OF GENITO-URINARY MEDICINE MAIN TRANSMISSION ROUTES HEPATITIS B Sexual transmission Occurs in unvaccinated/non immune men who have sex with men and correlates with multiple partners, unprotected
Bloodborne Pathogens. Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
Infection Control Standard Precautions. CDC Recommendations: Application of Standard Precautions for All Patients
 Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
What does the HIV Pharmacy Team need to know about PEP
 What does the HIV Pharmacy Team need to know about PEP Rosy Weston Senior Lead Pharmacist Sexual Health and HIV St. Mary s Hospital Imperial College Healthcare NHS Trust London November 2008 Objectives
What does the HIV Pharmacy Team need to know about PEP Rosy Weston Senior Lead Pharmacist Sexual Health and HIV St. Mary s Hospital Imperial College Healthcare NHS Trust London November 2008 Objectives
Blood Borne Pathogens
 Bloomer School District Blood Borne Pathogens Developed by: Tammy Kornesczuk, RN Act Rather Than Re-act School Staff tend to be nurturing and care-taking people Don t rush to help without putting on gloves
Bloomer School District Blood Borne Pathogens Developed by: Tammy Kornesczuk, RN Act Rather Than Re-act School Staff tend to be nurturing and care-taking people Don t rush to help without putting on gloves
Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV
 Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV By Dr. Sinan Butrus F.I.C.M.S Clinical Standards & Guidelines Kurdistan Board For Medical Specialties An exposure that might place health
Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV By Dr. Sinan Butrus F.I.C.M.S Clinical Standards & Guidelines Kurdistan Board For Medical Specialties An exposure that might place health
HEPATITIS B VIRUS: PROTECTING EMPLOYEES AND PATIENTS
 HUMAN RESOURCES (WITH OCCUPATIONAL HEALTH) HEPATITIS B VIRUS: PROTECTING EMPLOYEES AND PATIENTS DOCUMENT REF: PHRMHEPAB (Version No. 5.0) Name and designation of policy author(s) Approved by (committee,
HUMAN RESOURCES (WITH OCCUPATIONAL HEALTH) HEPATITIS B VIRUS: PROTECTING EMPLOYEES AND PATIENTS DOCUMENT REF: PHRMHEPAB (Version No. 5.0) Name and designation of policy author(s) Approved by (committee,
Prevention and Management of Occupational Exposure to Blood Borne Viruses, Post Exposure Prophylaxis and Prevention of Sharps Injuries Policy
 Prevention and Management of Occupational Exposure to Blood Borne Viruses, Post Exposure Prophylaxis and Prevention of Sharps Injuries Policy Author(s) & Designation Lead Clinician if appropriate In consultation
Prevention and Management of Occupational Exposure to Blood Borne Viruses, Post Exposure Prophylaxis and Prevention of Sharps Injuries Policy Author(s) & Designation Lead Clinician if appropriate In consultation
For. Correctional. Officers. Your. Health Risks. in Correctional Settings. Facts. About HIV
 For Correctional Officers Your Health Risks in Correctional Settings Facts About HIV Working in close quarters with inmates in a correctional facility puts correctional officers (COs) at increased risk
For Correctional Officers Your Health Risks in Correctional Settings Facts About HIV Working in close quarters with inmates in a correctional facility puts correctional officers (COs) at increased risk
Prof.Pavitra Ganguly M.S., FMAS, FIAGES Prof & HOD Surgery HIMSR & HAHC Hospital Jamia Hamdard,Delhi
 Prof.Pavitra Ganguly M.S., FMAS, FIAGES Prof & HOD Surgery HIMSR & HAHC Hospital Jamia Hamdard,Delhi Universal precaution are control guidelines designed to protect workers from exposure to Diseases spread
Prof.Pavitra Ganguly M.S., FMAS, FIAGES Prof & HOD Surgery HIMSR & HAHC Hospital Jamia Hamdard,Delhi Universal precaution are control guidelines designed to protect workers from exposure to Diseases spread
Safety Services Guidance. Guidance on working with blood and body fluids
 Guidance on working with blood and body fluids Key word(s): Blood, body fluids, sputum, blood borne viruses, BBV Target audience: Laboratory personnel Contents Introduction... 2 Assessing the risks...
Guidance on working with blood and body fluids Key word(s): Blood, body fluids, sputum, blood borne viruses, BBV Target audience: Laboratory personnel Contents Introduction... 2 Assessing the risks...
May Safety Subject. Bloodborne Pathogens
 May Safety Subject Bloodborne Pathogens Everyone is at risk to contact bloodborne pathogens. Some more than others. Universal precautions means treating all objects as potentially contaminated Personal
May Safety Subject Bloodborne Pathogens Everyone is at risk to contact bloodborne pathogens. Some more than others. Universal precautions means treating all objects as potentially contaminated Personal
