Development and regeneration of the neonatal digit tip in mice
|
|
|
- Patience Hunter
- 6 years ago
- Views:
Transcription
1 Available online at Developmental Biology 315 (2008) Development and regeneration of the neonatal digit tip in mice Manjong Han a, Xiaodong Yang a, Jangwoo Lee a, Christopher H. Allan c, Ken Muneoka a,b, a Division of Developmental Biology, Department of Cell and Molecular Biology, Tulane University, New Orleans, LA 70118, USA b The Center for Bioenvironmental Research, Tulane University, New Orleans, LA, USA c Department of Orthopaedics and Sports Medicine, University of Washington, Seattle, WA, USA Received for publication 31 October 2007; revised 13 December 2007; accepted 13 December 2007 Available online 27 December 2007 Abstract The digit tips of children and rodents are known to regenerate following amputation. The skeletal structure that regenerates is the distal region of the terminal phalangeal bone that is associated with the nail organ. The terminal phalanx forms late in gestation by endochondral ossification and continues to elongate until sexual maturity (8 weeks of age). Postnatal elongation at its distal end occurs by appositional ossification, i.e. direct ossification on the surface of the terminal phalanx, whereas proximal elongation results from an endochondral growth plate. Amputation through the middle of the terminal phalanx regenerates whereas regenerative failure is observed following amputation to remove the distal 2/3 of the bone. Regeneration is characterized by the formation of a blastema of proliferating cells that appear undifferentiated and express Bmp4. Using chondrogenic and osteogenic markers we show that redifferentiation does not occur by endochondral ossification but by the direct ossification of blastema cells that form the rudiment of the digit tip. Once formed the rudiment elongates by appositional ossification in parallel with unamputated control digits. Regenerated digits are consistently shorter than unamputated control digits. Finally, we present a case study of a child who suffered an amputation injury at a proximal level of the terminal phalanx, but failed to regenerate despite conservative treatment and the presence of the nail organ. These clinical and experimental findings expand on previously published observations and initiate a molecular assessment of a mammalian regeneration model Elsevier Inc. All rights reserved. Keywords: Regeneration; Mammal; Digit; Finger; Blastema; Ossification Introduction Fingertip regeneration in children has been reported in the clinical literature when amputation injuries are conservatively managed and allowed to heal by secondary intention (Stevenson, 1992). Conservatively managed amputation injuries restore the digit contour, the fingerprint, normal sensibility and digit function and heal with minimal scarring. Lengthening of the finger is described in some cases; however, it is not always clear whether finger elongation results from regeneration of the terminal phalangeal bone and/or by distal growth of granulation tissue (Douglas, 1972; Vidal and Dickson, 1993). Observations on the healing of fingertip amputation injuries make it difficult Corresponding author. Division of Developmental Biology, Department of Cell and Molecular Biology, Tulane University, New Orleans, LA 70118, USA. Fax: address: kmuneoka@tulane.edu (K. Muneoka). to distinguish between a wound healing response that gives excellent cosmetic and functional repair of soft tissues, from a regeneration response that, in addition, completely or partially restores skeletal structure. Regeneration responses in lower vertebrates, such as the salamander, involve complete skeletal replacement (Bryant et al., 2002), thus whether or not there is skeletal regrowth following fingertip amputation is key to establishing whether a regeneration response is stimulated. We are aware of a limited number of documented cases of bone regrowth following an amputation injury in humans (Lee et al., 1995; Vidal and Dickson, 1993). Based on the clinical literature we can conclude that the human fingertip possesses some regenerative capacity; however there is insufficient documentation to predict whether or not a regenerative response will occur for any given amputation injury. Digit tip regeneration in rodents has been used as an experimental model to study regenerative mechanisms in mammals, in particular, as it relates to fingertip regeneration /$ - see front matter 2008 Elsevier Inc. All rights reserved. doi: /j.ydbio
2 126 M. Han et al. / Developmental Biology 315 (2008) in children. In both neonate and adult rodents, digit tip regeneration involves wound healing with regrowth of the terminal phalangeal bone (Neufeld and Zhao, 1995). The regeneration response is level specific and restricted to amputation through the terminal phalangeal bone (Borgens, 1982; Neufeld and Zhao, 1995; Reginelli et al., 1995). Regeneration in both children and rodents is noted to be associated with the presence of the nail organ at the wound site, and ablation/transplantation studies in rodents suggest that the nail organ plays a crucial inductive role in the response (Mohammad et al., 1999; Neufeld and Zhao, 1995). During digit development in mice and humans, the nail organ forms in association with distal mesenchymal cells that prominently express transcripts for Msx1 and Bmp4, and these genes have been shown to be functionally required for the embryonic regeneration response (Allan et al., 2006; Han et al., 2003; Reginelli et al., 1995). Thus, the evidence implicates either the nail organ itself and/or cells associated with the nail organ as playing a critical role in digit tip regeneration. In higher vertebrates, the terminal phalangeal bone is the only bone known to have the capacity to fully regenerate following an amputation injury. Like other long bones of the limb, the terminal phalanx initially forms by endochondral ossification, first forming a chondrogenic template that is later replaced by bone. Unlike classically studied long bones, the terminal phalanx possesses only a single growth plate located at its proximal end, so it is best described as equivalent to the proximal half of a typical long bone (Dixey, ). Typical long bones increase in length by endochondral growth occurring at the epiphyseal plates and involve cartilaginous growth with bone replacement. In contrast, long bones increase in diameter by appositional ossification that occurs along the diaphysis and involves the progressive laying down of new bone directly onto the surface of existing bone, a process that does not involve cartilage formation. In the case of the terminal phalanx, bone elongation appears to occur by endochondral growth proximally and appositional ossification distally. Since the terminal phalanx is the only bone of the mammalian limb that has retained regenerative ability, we have carried out studies to characterizing its formation, elongation and regeneration in the neonatal mouse. We developed the postnatal day 3 (PN3) digit tip as a model for regeneration and we show that amputation through the distal region of the terminal phalanx results in a regeneration response whereas amputation through the proximal region does not. We find that when neonatal regeneration occurs it is not perfect; the regenerated bone is anatomically correct but never attains the length of unamputated digits. Our studies provide evidence that neonatal digit tip regeneration involves the formation of a blastema of proliferating cells expressing developmentally relevant genes, and the differentiation of bone tissue by direct ossification. Finally, to provide a link between experimental and clinical findings, we present a case report of a proximal amputation injury to the fingertip of a 28 month old child that was conservatively treated and resulted in a wound healing response without evidence of bone regrowth. This case report identifies a fingertip amputation level in children, similar to a proximal amputation in neonatal mice, which is unable to mount a regeneration response. Materials and methods Digit tip amputation and tissue collection All mice used in this study were outbred CD#1 strain supplied by Charles River Laboratories (Wilmington, MA). Following anesthetizing with an intraperitoneal injection of Nembutal (Pentobarbital; 50 mg per kg body weight) or Ketamine/Xylazine (Ketamine 80 mg and Xylazine 8 mg per kg body weight), we utilized the central three digits (digits 2, 3, 4) of the hindlimb of postnatal day 3 (PN3) neonates for digit formation and regeneration studies. Amputations were carried out using microdissection scissors. For distal amputations the terminal phalangeal bone was transected at its midpoint so that 50% of the bone remained in the stump tissue. For proximal amputations the transaction level removed between 67% and 75% of the terminal phalangeal bone leaving 25 33% of the bone in the stump tissue. At PN3 the distal amputation level transects forming bone tissue while proximal amputations transect cartilage (Figs. 2A and B). For all of our studies we treated the central three digits of the hindlimb as equivalent. For histological and in situ hybridization analyses digit tissues were obtained from mice at embryonic day 17.5 (E17.5), E18.5 (birth), PN3, PN7, PN14 and PN21. Procedures for care and use of mice for this study were in compliance with standard operating procedures (SOPs) approved by the Institutional Animal Care & Use Committee (IACUC) of Tulane University Health Science Center or the University of California Irvine. Histological analysis Differential whole-mount bone staining of developing or regenerated digits was performed essentially as described by McLeod (1980) with minor modification. Briefly, digits of neonates (younger than 3 weeks) were fixed with 100% EtOH, skinned, delipidated in acetone and stained with Alcian Blue 8XG/Alizarin Red S in 5% acetic acid, 95% EtOH. Stained tissues were treated in 1% KOH and cleared by glycerol. Mice older than 3 weeks were stained simply with Alizarin Red S because cartilage tissue is absent in the digit tips. Adult digit samples were stained with 0.002% Alizarin Red S solution in 2% KOH. Samples were washed with 2% KOH and Mall's solution and stored in glycerol. For paraffin sectioning tissues were fixed in 4% paraformaldehyde, dekeratinized in 1% KOH, decalcified in Decalcifier II (Surgipath, Richmond, IL) and stained with Mallory's triple stain (Humason, 1962). Bone length and ossification To assess growth rate we directly measured the proximo-distal length of the terminal phalanx in whole-mount preparations (n=8 per time point). Postnatal ossification was determined by analyzing calcein labeled whole-mount samples. To determine new bone formation, calcein was injected intraperitoneal (10 mg/ kg body weight) and digit tissue analyzed 1 day later using fluorescence microscopy (Suzuki and Mathews, 1966). In other experiments calcein was used as a vital marker to label existing bone and non-labeled bone distal to the calcein label was measured to estimate subsequent ossification. In some cases we used a double labeling technique in which new bone was labeled with Alizarin Red (Neufeld and Mohammad, 2000). In situ hybridization Section in situ hybridization was performed to characterize gene expression during digit tip development and regeneration as previously described (Han et al., 2003). In situ probes for type II collagen (Kosher et al., 1986; Nah et al., 1988), Indian hedgehog (Bitgood and McMahon, 1995), type X collagen (Schmid and Linsenmayer, 1985), type I collagen (Kosher et al., 1986) and osteocalcin (Komori et al., 1997) were used to characterize differentiation of skeletal elements. In situ probes for Msx1, Msx2 and Bmp4 were used to characterize the expression of developmental genes during regeneration (Han et al., 2003).
3 M. Han et al. / Developmental Biology 315 (2008) Cell proliferation For cell proliferation study during digit tip regeneration, 10 mm of BrdU (Roche) was injected into the intraperitoneum (20 μl/g body weight) 4 days and 7 days postamputation. After a 2-hour labeling period, digit samples were fixed with Carnoy's fixative, dekeratinized in 1% KOH and decalcified with 10% EDTA. To explore direct ossification of the terminal phalangeal bone during development, BrdU was injected at birth and detected 3 weeks after labeling. Detection of labeled cells was performed using Roche BrdU Detection Kit II (Roche) on paraffin-sectioned tissue samples following the manufacturer's recommended protocol. Results A case of regenerative failure in children Finger tip regeneration in children has been described following conservative management of the amputation wound. The regeneration response is not described as being level specific but extends proximally to a level below the base of the nail (Illingworth, 1974; King, 1979). The data clearly indicate that conservative management of fingertip amputation injuries results in an excellent healing response; however what remains uncertain is defining a level of injury that results in a true regenerative response (i.e. bone regrowth). One of us (CHA) treated a fingertip amputation injury of the terminal phalanx that resulted in what might be considered a successful wound healing response; however lengthening of the phalangeal bone was not observed. Case report A 28 month old child was treated for an amputation injury of the left long finger. The amputation transected the terminal phalanx at a level that was proximal to the nail plate but included proximal tissue of the nail organ. The terminal phalanx was exposed but it did not protrude from the wound and was not trimmed. X-rays at the time of injury verified the amputation level and indicated that approximately 70% of the phalangeal bone was lost leaving about 30% of the bone at the wound stump (Fig. 1A). The wound was treated conservatively with dressing changes. The final follow-up visit 10 months after the injury showed that the wound had healed to form a smooth, rounded tip with normal contour and sensibility, but there was no elongation of the finger (Figs. 1B and C). A rudimentary nail was present and was slightly hooked, thus verifying the presence of the nail organ in the amputation injury (Fig. 1D). X-rays at 10 months indicated that the terminal phalanx had not elongated, but in fact appeared to have shortened (Fig. 1E). These observations suggest that amputation injury in the proximal third of the terminal phalanx undergoes excellent wound healing but is unable to mount a regenerative response. Regeneration of the terminal phalanx in neonatal mice To determine whether a mouse model of digit tip regeneration displayed a similar level-specific regenerative response we carried out amputations at two different levels (distal and proximal) of the terminal phalanx (Figs. 2A and B). The distal amputation removed the distal half of the terminal phalanx, whereas the proximal amputation level removed between twothirds and three-fourths of the terminal phalanx. The regenerative response was assessed after 6 weeks using Alcian Blue/ Alizarin Red whole-mount skeletal staining and paraffin histology using the unamputated digit tip (Figs. 2C and F) as a reference for completeness of the regenerative response. Distal level amputations resulted in a morphologically normal terminal phalanx (Figs. 2D and G), whereas we observed no measurable response following a proximal level amputation (Figs. 2E and H). These findings are consistent with previous reports on older stage neonates (Neufeld and Zhao, 1995). The failure of the proximal amputation level to regenerate is similar to that observed in the case report (Fig. 1). While regenerates from distal amputation of the terminal phalanx appeared morphologically normal, they seemed shorter when compared to control unamputated digit tips (Chadwick et al., 2007). To explore this further we carried out distal level amputations of PN3 digit tips collecting samples weekly beginning at 2 weeks postamputation. Digits were processed for whole-mount skeletal staining and measurements were made to determine the proximal distal length of the terminal phalanx. Measurements of control unamputated digits from the same animals were used for comparison. Our analysis of terminal phalanx elongation during neonatal digit tip regeneration showed that the rate of elongation during regeneration paralleled the rate of normal digit tip elongation and that the regenerated terminal phalanx never reached the length of control unamputated digits (Fig. 2I). We confirmed this finding by carrying out similar studies following amputation at PN9 and PN14 (not shown) with the same result. Our data show that the regeneration of the terminal phalanx following digit tip Fig. 1. A case of regenerative failure after proximal amputation injury of the fingertip (*) of a 2 year old child that was conservatively treated. A: Radiograph at the time of injury indicated about 70% of the terminal phalangeal bone was lost. B E: Fingertip 10 months after injury. B: Dorsal view, C: ventral view and D: lateral view. The wound healed to form a small bump with normal contour and sensibility (B D), but there was no elongation of the terminal phalangeal bone (E).
4 128 M. Han et al. / Developmental Biology 315 (2008) Fig. 2. Regeneration of the terminal phalanx of digit tips in neonatal mice. Amputations were carried out at a distal level through bone (A) and a proximal level through cartilage (B) at postnatal day 3 (PN3). Control unamputated digits were used for comparison (C, F). After 6 weeks, digits were analyzed using whole-mount bone stain with Alizarin Red S (C E) and histological analysis with Mallory's triple stain (F H). Proximal amputations show no signs of regeneration (E, H). Distal amputations regenerate anatomically normal digit tips (D, G); however the length of the terminal phalanx of these digit tips never reaches that of unamputated control digits (I). Scale bars: A, B 200 μm; C E 300 μm; F H 400 μm. amputation involves a process that does not catch up with endogenous digit tip elongation. The elongation dynamics following amputation injury raised the possibility that digit tip regeneration was simply a healing process in which the amputated phalanx was elongated by an ongoing process intrinsic to normal digit formation. In other words, the elongation response following amputation was not a regenerative response. To investigate this possibility we first needed to understand the normal elongation process of the terminal phalanx. Ossification during formation and elongation of the terminal phalanx The terminal phalanx is a triangular shaped bone that develops by endochondral ossification, but unlike typical long bones, possesses a single growth plate at its proximal end. Based on whole-mount skeletal staining, the terminal phalanx at E17.5 is entirely chondrogenic and ossification is first evident at the apex at E18.5 (Figs. 3A and B). Following birth, ossification extends proximally with the distal 2/3 ossified by PN7 and by PN14 the entire terminal phalanx stains positive for bone (Figs. 3C and D). During this period the terminal phalanx has a growth plate that is histologically well-defined at the proximal end (Fig. 3E); however the growth plate is closed by PN21 (Fig. 3F). The initiation of ossification at the apex between E17.5 and E18.5 is associated with the onset of osteogenic marker genes and a change in expression of chondrogenic genes. Type II collagen (ColII) is expressed throughout the terminal phalanx at E17.5 (Fig. 4A) but expression at the apex is down-regulated by E18.5 (Fig. 4B). The prehypertrophic chondrocyte marker Ihh is strongly expressed at the digit apex at E17.5 (Fig. 4C) but its expression is restricted to a central band of chondrocytes by
5 M. Han et al. / Developmental Biology 315 (2008) Fig. 3. Differentiation and elongation of the terminal phalangeal bone. A D: Whole-mount differential bone staining of developing hindlimb digits with Alizarin Red S and Alcian Blue. Ossification of the terminal phalangeal bone is initiated at the distal tip between E17.5 and E18.5 (A, B). Ossification progresses in a proximal direction after birth (C), and the entire bone is Alizarin Red S positive by PN14 (D). E, F: Mallory's triple stained sections of neonatal digits. At PN7 an endochondral growth plate is clearly evident in the proximal region of the terminal phalangeal bone (arrowheads in E); however this growth plate is absent by PN21 (F). Scale bars: A, B 150 μm; C F 300 μm. E18.5 (Fig. 4D). The hypertrophic chondrocyte marker type X collagen (Col X) which is weakly expressed at E17.5 (Fig. 4E) is up-regulated at the digit apex at E18.5 (Fig. 4F). Evidence of ossification at the tip of the terminal phalanx is first found between E17.5 and E18.5. Histological staining demonstrates the formation of bone tissue along the dorsal surface of the terminal phalanx between E17.5 and E18.5 (Figs. 4G and H). Expression of type I collagen (ColI) is initiated at the apex of the digit tip on E17.5 (Fig. 4I) and spreads proximally along the dorsal phalangeal surface by E18.5 (Fig. 4J). At E17.5 we found no evidence of osteocalcin expression (Fig. 4K), but by E18.5 transcripts are up-regulated in cells on the distal-dorsal surface of the terminal phalanx (Fig. 4L). These results show that the terminal phalanx is formed by endochondral ossification and that ossification is initiated at the distal tip of the digit and progresses in a proximal direction. Our measurements of the terminal phalanx during postnatal growth indicate that the bone triples its length between birth and maturity (8 weeks of age, see Fig. 2I), yet our histological analysis of the proximal growth plate of the terminal phalanx indicates that it is closed by PN21. To characterize postnatal bone growth we used the vital fluorescent bone marker calcein which specifically labels calcium salt released into newly formed bone matrix and thus identifies sites of new bone deposition. In addition, calcein is very stable once it is incorporated, and free calcein is rapidly cleared from the circulation, making it a useful vital marker to characterize the temporal sequence of bone formation (Suzuki and Mathews, 1966). We carried out two types of experiments using calcein. First, we characterized regions of new bone formation during postnatal elongation of the terminal phalanx by characterizing calcein incorporation, and second, we introduced calcein as a label for existing bone to determine rates of new (unlabeled) bone formation. Calcein incorporation during the first week of postnatal elongation showed new bone deposition distributed throughout the terminal phalanx with the strongest staining at the base of the bone (Fig. 5A). By 3 weeks new bone deposition is largely restricted proximally and along the distal-dorsal surface of the terminal phalanx (Fig. 5B). There is also a low level of new bone deposited around the central region that is associated with an increase in bone diameter. At 5 weeks there is an absence of new bone deposition proximally; however there remains strong staining at the distal tip and along the dorsal surface of the terminal phalanx (Fig. 5C). By 7 weeks of age the terminal phalanx has reached its maximum length (see Fig. 2I) and at this time there is little calcein incorporation at either the proximal or distal ends of the bone (Fig. 5D). These data identify distal and proximal regions of bone growth that contribute to the final length and shape of the terminal phalanx. We investigated further the distal region of ossification because it appears to be most relevant to ossification associated with the regeneration response. To characterize the contribution of distal ossification to the elongation of the terminal phalanx we labeled the new born bone by injecting calcein at PN1 and analyzed the deposition of new unlabeled distal bone during the elongation period (Figs. 5E G). Such measurements show a continuous increase in bone length that is attributed to a distal ossification center identified by our gene expression and calcein incorporation studies during digit tip elongation. Based on these measurements we estimate that 55% of the postnatal elongation of bone can be attributed to ossification occurring at the distal tip of the terminal phalanx. We have also carried out birthday studies by introducing BrdU into neonates and analyzing the terminal phalanx at PN21. The incorporation of BrdU labeled cells into bone at the distal tip of the phalanx confirms the involvement of a distal ossification center in postnatal bone elongation (Fig. 5H). Ossification during digit regeneration We carried out calcein labeling studies during the regeneration response associated with distal amputation at PN3. At 1 week postamputation, the proximal bone stump was labeled with calcein. At this stage of regeneration, calcein incorporation demonstrates that the regeneration of bone tissue has not commenced (Fig. 5I). By 2 weeks postamputation, the distal tip of the terminal phalanx is clearly defined and the general shape of the terminal phalanx is reestablished. Calcein labeling at this stage indicates enhanced bone deposition throughout the regenerate (Fig. 5J). By the third week postamputation, the terminal phalanx is enlarging and general pattern of calcein staining is similar to that of the unamputated digit tip (see Fig. 5B) with enhanced calcein incorporation proximally and along
6 130 M. Han et al. / Developmental Biology 315 (2008) Fig. 4. Expression of cartilage-specific (A F) and bone-specific (I L) transcripts in the E17.5 and E18.5 terminal phalanx at the onset of ossification. Type II collagen (ColII; A, B) is a marker for proliferating chondrocytes, Ihh (C, D) is a prehypertrophic chondrocyte marker, and Type X collagen (Col X; E, F) is a hypertrophic chondrocyte marker. G, H: Mallory's triple stained section showing the deposition of collagen along the dorsal tip of the terminal phalangeal bone. Collagen staining in G and H correlates with the expression of Type I collagen transcripts (ColI; I and J). The osteoblast-specific marker, Osteocalcin (K, L), identifies a distal ossification center during the maturation of the terminal phalangeal bone. Scale bars: 100 μm. the distal-dorsal surface (Fig. 5K). These studies identified the period between 1 week and 2 weeks postamputation as a time when the amputated digit reestablishes the skeletal pattern during regeneration. To verify this observation we carried out a pulse labeling study in which the terminal phalanx was calcein labeled at 1 week postamputation to label the stump bone and collected regenerates 1 week later. In these samples new bone formation distal to the amputation plane was labeled with Alizarin Red (Neufeld and Mohammad, 2000). As can be seen in Fig. 5L the structure of the amputated terminal phalanx regenerates between 7 and 14 days postamputation. These findings demonstrate that digit tip elongation following amputation cannot be simply interpreted as the continuation of endogenous postnatal bone elongation and that a true regeneration response does indeed occur. In addition, these studies identify a 14-day window following PN3 digit tip amputation as the critical period during which regeneration occurs. Blastema formation during digit regeneration The salient features of mouse digit tip regeneration include the formation of a blastema or a blastema-like structure and the redifferentiation of the terminal phalanx by direct ossification (Neufeld, 1992; Revardel and Chebouki, 1987). A blastema can be defined as a population of proliferating undifferentiated cells from which an organ or body part is restored. We have carried out histological and BrdU incorporation studies that demonstrate the formation of a blastema during the regeneration of a mouse PN3 digit. The terminal phalanx of the PN3 digit at the time of amputation is undergoing ossification distally and is chondrogenic at the base of the digit (see Fig. 3). Amputation through the distal region transects ossifying tissues and creates a gaping wound (Fig. 6A). Four days postamputation (PA4) the epidermis has yet to completely close the amputation wound and what appear to be undifferentiated cells begin to accumulate just distal to the bone (Fig. 6B). By PA6 the epidermis covering the wound is thickened in the ventral region relative to the dorsal, and a blastema-like population of undifferentiated cells has accumulated distal to the amputated bone (Fig. 6C). The bone stump is undergoing some erosion, most prominent in the ventral region (see also Fig. 5I), and the regenerating cells are contiguous with cells of the forming bone marrow. The distal region of the PA8 blastema-like structure contains cells that appear undifferentiated, and we notice numerous blood vessels that appear to be contiguous with the marrow vasculature. There is an enhanced level of collagen-specific staining at the proximal interface with the bone stump suggestive that bone differentiation is initiated at this time (Fig. 6D). Consistent with previous reports, we find
7 M. Han et al. / Developmental Biology 315 (2008) Fig. 5. Ossification and elongation of the terminal phalangeal bone. A D and I K: Calcein incorporation into the terminal phalangeal bone identifies new bone deposition. E G and L: Calcein incorporation into the terminal phalangeal bone is used as a vital marker to mark existing bone so subsequent bone deposition (unlabeled) can be identified. During postnatal digit elongation new bone is deposited throughout the terminal phalangeal bone at 1 week of age (A). At 3 weeks new bone deposition is restricted to the proximal (*) and dorsal-distal (arrow) regions of the bone (B). At 5 weeks new bone deposition is only observed at the dorsal-distal (arrow) region of the bone (C). At 7 weeks new bone deposition is not localized but occurs throughout the bone (D). E G: The terminal phalangeal bone was labeled with calcein at PN1 (E) to characterize regions of new bone formation during digit elongation. Unlabeled bone distal to the calcein label (double arrow lines) identifies progressive deposition of new bone at the digit tip in mice 4 weeks (F) and 7 weeks (G) of age. H: BrdU, introduced at birth, labels bone cells at the distal tip of the terminal phalanx 3 weeks later (arrow). I L. Calcein labeling during digit tip regeneration. Calcein was injected 1 day before collecting samples of regenerating digits 1 week (I), 2 weeks (J) and 3 weeks (K) postamputation. Note that the general morphology of the digit tip is regenerated by 2 weeks postamputation (J) and that by 3 weeks postamputation the normal dorsal-distal bone deposition pattern (arrow) is observed in the regenerating digit tip (K). To demonstrate that the bulk of the regeneration response occurs between 1 and 2 weeks postamputation, calcein incorporation at 1 week postamputation labeled the stump bone and digits collected at 2 weeks postamputation were collected and stained with Alizarin Red S to label all bone (L). The double arrow line in L shows the extent of regenerated new bone during this 1 week period. Scale bars: 250 μm. no histological evidence of chondrogenesis during this redifferentiation process (Neufeld, 1992). One characteristic of a blastema is that it contains proliferating cells. We carried out BrdU incorporation studies to determine the extent of cell proliferation during the regeneration process. We compared regenerating PN3 amputated digits at PA4 and PA7 to unamputated control digits. In the terminal phalangeal region of the unamputated control digits at PN7 and PN10 there are three distinct regions where cells were actively proliferating: the dorsal nail matrix, the ventral epidermis associated with the digit fat pad and the bone marrow of the terminal phalanx (Figs. 6E and G, respectively). In addition there are few BrdU labeled cells scattered in other epidermal regions and in the loose connective tissue surrounding the bone. In the PA4 regenerate we observed enhanced proliferation in the epidermis involved in wound closure, but not among cells at the wound edge. In addition, we see enhance proliferation in connective tissue cells at the interface with the amputated bone stump (Fig. 6F). By PA7 epidermal cell proliferation has largely subsided; however there is enhanced proliferation in the region between the bone marrow and blastema-like structure, and within the loose connective tissues surrounding the bone (Fig. 6H). These data demonstrate that there is a localized proliferative response associated with digit tip regeneration. We investigated the expression of three developmental genes, Msx1, Msx2 and Bmp4, known to be important for digit tip regeneration in the embryo. Msx1 is required for embryonic digit tip regeneration, and Bmp4 expression is downstream of Msx1 and Msx2 (Han et al., 2003). In the postnatal digit Msx1 is expressed by loose connective tissue cells between the nail organ and the terminal phalangeal bone (Han et al., 2003; Reginelli et al., 1995). Following amputation of the PN3 digit tip we observed no qualitative change in Msx1 expression at PA4, although we did notice higher levels of expression in cells associated with the amputation wound (not shown). This apparent up-regulation of Msx1 expression was transiently restricted to PA4 and similar to Msx2 (see below). At PA8 Msx1 expression was present in the loose connective tissue cells between the nail and the terminal phalangeal bone in the digit stump but was not detectable in regenerating digit blastema cells (Fig. 6I). Msx2 expression is normally restricted to the nail epidermis but is up-regulated in the dorsal loose connective tissue cells associated with the amputation wound at PA4 (Fig. 6J). However, its expression is not detected in the PA8 regenerate (Fig. 6K); thus Msx2 is transiently upregulated during the early stages of the regeneration response. Bmp4 is normally expressed in the bone marrow and in chondrocytes at the base of the terminal phalangeal bone, and there is no expression associated with the loose connective tissue of the
8 132 M. Han et al. / Developmental Biology 315 (2008) Fig. 6. Histological and in situ hybridization analyses of digit tip regeneration. A D: Histological sections of regenerating digit tips stained with Mallory's triple stain at the time of amputation (A), 4 days postamputation (B), 6 days postamputation (C) and 8 days postamputation (D). A blastema-like structure is evident at PA6 (C) and collagen deposition (c) is evident between the bone stump and the blastema-like structure at PA8 (D). E H: BrdU incorporation was used to identify regions of cell proliferation in the PN7 (E) and PN10 unamputated control digit (G), and in the regenerating digit at PA4 (F; correspondent to PN7), and PA7 (H; correspondent to PN10). Regions of proliferation in the unamputated digits (E and G) include the epidermis (arrowheads) and the bone marrow (*). At PA4 (F) epidermal proliferation appears more widespread, extending distalward in association with epidermal closure, but not including the leading edge (*). In addition, a region of enhanced proliferation is found at the interface between the forming blastema-like structure and the bone marrow (arrow). At PA7 (H) many cells in the connective tissue between the nail bed and the bone are proliferating (*), as are the cells at the interface of the bone marrow and the blastema-like structure. I L: In situ hybridization analyses documenting the expression of developmental genes during regeneration. Transcripts for Msx1 (I) are absent from the blastema-like structure but are localized in the dorsal connective tissue between the nail bed and the terminal phalangeal bone. This pattern is similar to that observed in the unamputated digit (not shown). Msx2 expression at PA4 (J) indicates a transient up-regulation in the connective tissue cells associated with the amputation wound (arrow); however Msx2 expression is absent in the blastema-like structure at PA8 (K). At PA8 Bmp4 transcripts (L) are present throughout the blastema-like structure (*) and also in connective tissue cells just proximal to the regenerate (arrow). Scale bars: 100 μm. digit tip (not shown). In the PA8 regenerate some Bmp4 expressing cells are found in the loose connective tissue and throughout the regenerating blastema (Fig. 6L). These observations show that of the genes known to be critical for embryonic digit tip regeneration, only Bmp4 expression correlates with postnatal regeneration. We have carried out in situ hybridization studies to characterize early events associated with bone redifferentiation during regeneration. Histological studies suggest that redifferentiation of regenerated bone proceeds by direct ossification (Muller et al., 1999; Neufeld, 1992). We used three cartilage specific markers to identify different stages of chondrocyte differentiation (Type II Collagen, ColII; Indian Hedgehog, Ihh; Type X Collagen, ColX) and one bone-specific marker to identify osteoblasts, Osteocalcin. Transcripts for the proliferating chondrocyte marker, ColII, is normally restricted to the endochondral growth plate of the terminal phalanx (not shown), and during digit tip regeneration ColII expression is unaltered (Figs. 7A and B). The prehypertrophic marker, Ihh, is normally expressed in a transverse band of cells distal to the growth plate, and during regeneration this pattern of expression is unaltered (Figs. 7C and D). Similarly, ColX, a marker for hypertrophic chondrocytes, is expressed during regeneration in a manner identical to non-regenerating digits (Figs. 7E and F). On the other hand, Osteocalcin expression, which identifies osteoblasts, is significantly modified during digit tip regeneration. Osteocalcin is normally expressed along the periphery of the forming bone and also in the marrow region (not shown). Following digit tip amputation, Osteocalcin expression in both the periphery and marrow is down-regulated at PA4 (Fig. 7G) then up-regulated in the distal region at PA8 (Fig. 7H). We find many Osteocalcin positive cells at the interface between the distal blastema-like structure and the proximal bone stump (Fig. 7H). These observations demonstrate that regenerative ossification following digit tip amputation occurs by direct ossification. Discussion We have initiated studies on amputated PN3 neonatal digits as a model for level-specific regeneration. In this report we confirmed and extended a number of observations from previous regeneration studies using either rat or mouse, and
9 M. Han et al. / Developmental Biology 315 (2008) Third, we confirm histological observations that redifferentiation of the terminal phalanx is accomplished by direct ossification (Muller et al., 1999; Neufeld, 1992), and we demonstrate this by using chondrogenic and osteogenic markers to show that ossification of the digit tip does not involve chondrogenic cells. A mammalian blastema? Fig. 7. Cartilage-specific and bone-specific gene expression during regeneration. A, C, E, G: 4 days after distal amputation, B, D, F, H: 8 days after distal amputation. The cartilage-specific marker, Type II Collagen (ColII), is expressed in the proximal region of the terminal phalanx but not in the regenerating distal region (A, B). The prehypertrophic cartilage-specific marker, Ihh, (C, D) and the hypertrophic cartilage-specific marker, Type X Collagen (ColX), (E, F) are expressed in the proximal bone stump but not distally. The osteoblast-specific marker, Osteocalcin, is expressed proximally at PA4 (G), and both proximally and at the interface between the bone stump and the blastema-like structure at PA8 (H, arrows). Scale bars: 100 μm. carrying out digit amputations at different neonatal stages (Borgens, 1982; Chadwick et al., 2007; Muller et al., 1999; Neufeld, 1992; Neufeld and Zhao, 1995; Reginelli et al., 1995; Revardel and Chebouki, 1987; Zhao and Neufeld, 1995). First, we confirmed that the regeneration response is level-specific within the terminal phalangeal bone, and we report that terminal phalanx regeneration is morphologically normal but shorter than unamputated controls. Our results are consistent with the hypothesis that the anatomical response is specific to the digit stage at the time of amputation (i.e. PN3), which regenerated within a 2-week period, after which normal postnatal lengthening of the digit ensues. Second, we confirm that distal amputation results in the formation of a blastema-like structure containing undifferentiated cells (Chadwick et al., 2007; Neufeld, 1992; Revardel and Chebouki, 1987), and we show that these cells are proliferating and express Bmp4, a developmentally regulated gene known to be involved in digit formation and embryonic digit regeneration (Han et al., 2003). One of the hallmarks of limb regeneration in urodele amphibians is the formation of a blastema of undifferentiated cells that proliferate, go through morphogenesis, and differentiate to replace structures lost by amputation (Brockes and Kumar, 2005; Bryant et al., 2002). The blastema is a transient phase in regeneration that has been described in terms of the characteristics of cells during this phase with respect to both their tissue of origin as well as their ultimate fate in regeneration. Thus, for example, we know that blastema cells (1) arise from either dedifferentiation of, or from stem cells present in, mature tissues, (2) appear undifferentiated and express developmental genes during the blastema phase, (3) proliferate and (4) differentiate in either a homotypic or heterotypic (metaplastic) manner (Brockes and Kumar, 2002; Han et al., 2005; Morrison et al., 2006). There is currently no mammalian counterpart to the urodele blastema, and because there exists a growing interest in developing strategies to induce regenerative responses in mammals, particularly humans, it is both necessary and important to identify parallels with, as well as deviations from, the best characterized regenerating systems. In this initial study we provide evidence that the cells involved in the mouse digit tip regeneration response are proliferating and appear undifferentiated based on histological staining. Our in situ hybridization studies demonstrate that Bmp4 expression is up-regulated during regeneration; thus we provide evidence that a developmental gene is induced during the response. However, unlike the regenerating embryonic digit (Allan et al., 2006; Han et al., 2003), we find that Msx1 is not expressed in the blastema but is expressed in the connective tissue of the stump and that both Msx1 and Msx2 are transiently up-regulated in the connective tissue during early stages of wound healing. Thus, the molecular regulation of postnatal regeneration appears to be distinct from the embryonic response. Nevertheless, our evidence is consistent with the idea that digit tip regeneration in neonatal mice involves the formation of a blastema that mediates the regenerative response. Regeneration occurs in distinct phases The regenerating neonatal mouse digit tip represents a novel model for exploring an inherent regenerative response in a mammal that has clear parallels with a similar human response. The regeneration process can be broken down into 3 phases. The first phase involves a wound healing response that ends with wound closure and the formation of a wound epithelium. In PN3 digits this phase is quite variable and relatively long with completed wound closure taking between 6 and 8 days following amputation (J. Lee unpublished). The wound epithelium in the
10 134 M. Han et al. / Developmental Biology 315 (2008) regenerating limbs of salamanders is known to play a critical role in regulating regenerative outgrowth, and it has been proposed that differences between the amphibian and mammalian wound epidermis are responsible for regenerative failure in mammals (Tassava and Olsen, 1982). A clear difference is that in the amphibian regeneration response wound closure is completed very rapidly (Carlson et al., 1998) whereas in the mouse digit tip it takes an extraordinarily long time. Since both healing modes are associated with a regenerative response it is reasonable to conclude that healing rate per se is not likely to be a limiting factor in the control of regeneration. The second phase is the formation of a blastema of proliferating cells subjacent to the wound epithelium. The process of blastema formation occurs concurrent with wound closure since the blastema is well formed at 8 days postamputation; thus we can conclude that wound closure per se is not required for the generation of blastema cells. The source of blastema cells remains unknown, although histological evidence suggests that connective tissue cells migrate distally during wound closure to cover the amputated bone stump (Neufeld et al., 2004). A connective tissue contribution to the blastema is also supported by our BrdU incorporation studies showing enhanced proliferation in the loose connective tissue. An alternative, but not mutually exclusive, possibility is that blastema cells arise from stem cells present in the bone marrow. We find that the bone marrow is contiguous with the forming blastema during the early stages of regeneration and that this region is associated with enhanced cell proliferation and osteoblast differentiation. Identification of the cellular origins of the digit tip blastema will provide important clues about the interface between wound healing and regeneration versus wound healing and scar deposition. The final phase of regeneration involves the redifferentiation of the distal digit tip tissues, i.e. bone and loose connective tissue. Bone differentiation is initiated at the base of the blastema and new bone forms contiguous with existing bone. The new bone is highly vascularized and has the histological appearance of trabecular bone, suggesting that bone marrow derived osteoblasts may be involved in the regeneration response. During regeneration new bone begins forming at 7 days postamputation, the general morphology of the distal phalange is restored by 14 days postamputation, and elongation of the regenerated bone is largely completed by 21 days postamputation. Thereafter terminal phalanx appears to elongate by appositional ossification, forming cortical bone at a rate that parallels normal postnatal growth. Our findings show that terminal phalanx elongation terminates in unamputated and regenerating digits at the same time and is independent of the final bone length. The reduced final size of the regenerated terminal phalanx can therefore be attributed to a systemic control of postnatal bone growth coupled with a developmental stage-specific regenerative response. Clinical implications for mouse digit regeneration In this study we present clinical evidence and experimental evidence that the regeneration of the terminal phalangeal element is level specific in children and neonatal mice. The clinical literature on fingertip regeneration in children suggests that the ability to regenerate is age-related and linked to the presence of the nail organ in stump tissue (Illingworth, 1974; King, 1979; Rosenthal et al., 1979). Similarly, experimental evidence that the nail organ stimulates some level of regenerative bone growth in mice suggests that the nail organ plays a critical role in the regeneration response (Mohammad et al., 1999; Zhao and Neufeld, 1995). On the other hand, our clinical and experimental data fail to demonstrate a relationship between the nail organ and regeneration of the terminal phalanx and thus suggest that the nail organ might be necessary but not sufficient for a successful regeneration response. Since regenerative responses in general are known to be complex and regulated at multiple levels (Gardiner, 2005; Han et al., 2005), it is therefore not surprising that the nail organ is not the sole regulator of digit tip regeneration. Conservatively managed fingertip amputation injuries in children and adults result in a wound healing response that restores cosmetic appearance and functionality. While there is limited documentation of bone regrowth (Vidal and Dickson, 1993), there is considerable evidence that the wound healing response is near perfect with the recovery of the digit contour, sensibility and fingerprint (Douglas, 1972). Thus, amputation of fingertips in humans and digit tips in mice represent situations where large wounds heal with minimal formation of scar tissue. In the mouse amputation through the sub-terminal phalanx results in wound healing with the formation of scar tissue (Muller et al., 1999); thus scar-free healing appears to be a response restricted to the terminal digit injuries. Studies demonstrating scar-free healing of embryonic wounds (Martin, 1997) coupled with embryonic regeneration studies (Reginelli et al., 1995; Wanek et al., 1989) indicate that scar-free wound healing may be necessary but is not sufficient for regeneration; thus it is plausible that the nail organ may be associated with scar-free healing rather than regeneration itself. Acknowledgments We thank members of the Muneoka laboratory, David Gardiner and Susan Bryant for many thoughtful discussions. We are especially thankful to the Department of Developmental and Cell Biology at the University of California Irvine for providing laboratory space and housing during a 3-month period following hurricane Katrina and to the NIH for providing supplemental funding to aid in rebuilding the laboratory. This work was funded by R01-HD and P01-HD (project 3). References Allan, C.H., Fleckman, P., Fernandes, R.J., Hager, B., James, J., Wisecarver, Z., Satterstrom, F.K., Gutierrez, A., Norman, A., Pirrone, A., Underwood, R.A., Rubin, B.P., Zhang, M., Ramay, H.R., Clark, J.M., Tissue response and Msx1 expression after human fetal digit tip amputation in vitro. Wound Repair Regen. 14, Bitgood, M.J., McMahon, A.P., Hedgehog and Bmp genes are coexpressed at many diverse sites of cell cell interaction in the mouse embryo. Dev. Biol. 172,
Supplemental Data. Wnt/β-Catenin Signaling in Mesenchymal Progenitors. Controls Osteoblast and Chondrocyte
 Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Abstract. capable only of partial regeneration of the terminal phalanx (P3), i.e., amputation midway
 Abstract Humans and mice lack the broad regenerative capacity of Urodele amphibians, capable only of partial regeneration of the terminal phalanx (P3), i.e., amputation midway through P3 results in essentially
Abstract Humans and mice lack the broad regenerative capacity of Urodele amphibians, capable only of partial regeneration of the terminal phalanx (P3), i.e., amputation midway through P3 results in essentially
FORMATION OF BONE. Intramembranous Ossification. Bone-Lec-10-Prof.Dr.Adnan Albideri
 FORMATION OF BONE All bones are of mesodermal origin. The process of bone formation is called ossification. We have seen that formation of most bones is preceded by the formation of a cartilaginous model,
FORMATION OF BONE All bones are of mesodermal origin. The process of bone formation is called ossification. We have seen that formation of most bones is preceded by the formation of a cartilaginous model,
Bone (2) Chapter 8. The bone is surrounded by the periosteum, the periosteum consists of two layers: a fibrous outer layer and an innercellular layer.
 Bone (2) Chapter 8 The bone is surrounded by the periosteum, the periosteum consists of two layers: a fibrous outer layer and an innercellular layer. The innercellular layer contains osteoprogenitor cells,
Bone (2) Chapter 8 The bone is surrounded by the periosteum, the periosteum consists of two layers: a fibrous outer layer and an innercellular layer. The innercellular layer contains osteoprogenitor cells,
Bone. Development. Tim Arnett. University College London. Department of Anatomy and Developmental Biology
 Bone Development Tim Arnett Department of Anatomy and Developmental Biology University College London Bone development Outline Bone composition matrix + mineral Bone formation - intramembranous & endochondral
Bone Development Tim Arnett Department of Anatomy and Developmental Biology University College London Bone development Outline Bone composition matrix + mineral Bone formation - intramembranous & endochondral
Skeletal Tissues. Skeletal tissues. Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs.
 Skeletal Tissues Functions 1) support 2) protection 3) movement Skeletal tissues Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs. Aids muscle contraction; generate
Skeletal Tissues Functions 1) support 2) protection 3) movement Skeletal tissues Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs. Aids muscle contraction; generate
The Skeletal System:Bone Tissue
 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
Skeletal Development Multiple Cellular Origins. Intramembranous Bone. Endochondrial Bone. Cartilage template of the limb in the Chick wing
 Skeletal Development Multiple Cellular Origins 1 - Paraxial Mesoderm Somite, Sclerotome Axial Skeleton (e.g. vertebra) 2 - Lateral Plate Mesoderm Appendicular Skeleton (e.g. limb) 3 - Neural Crest Head
Skeletal Development Multiple Cellular Origins 1 - Paraxial Mesoderm Somite, Sclerotome Axial Skeleton (e.g. vertebra) 2 - Lateral Plate Mesoderm Appendicular Skeleton (e.g. limb) 3 - Neural Crest Head
NOTES: Skeletal System (Ch 5, part 1)
 NOTES: Skeletal System (Ch 5, part 1) Individual bones are the organs of the skeletal system. A bone contains very active tissues. BONE STRUCTURE: *Bone structure reflects its function. Parts of a long
NOTES: Skeletal System (Ch 5, part 1) Individual bones are the organs of the skeletal system. A bone contains very active tissues. BONE STRUCTURE: *Bone structure reflects its function. Parts of a long
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
Cartilage Engineering From Autologous Fat For Oro-Facial Deformity Reconstruction - Focus On The Nose
 Cartilage Engineering From Autologous Fat For Oro-Facial Deformity Reconstruction - Focus On The Nose 12-month progress report submitted to AOMS Mr Atheer Ujam PhD student Primary supervisor: Professor
Cartilage Engineering From Autologous Fat For Oro-Facial Deformity Reconstruction - Focus On The Nose 12-month progress report submitted to AOMS Mr Atheer Ujam PhD student Primary supervisor: Professor
Chapter 6: Skeletal System: Bones and Bone Tissue
 Chapter 6: Skeletal System: Bones and Bone Tissue I. Functions A. List and describe the five major functions of the skeletal system: 1. 2. 3.. 4. 5.. II. Cartilage A. What do chondroblasts do? B. When
Chapter 6: Skeletal System: Bones and Bone Tissue I. Functions A. List and describe the five major functions of the skeletal system: 1. 2. 3.. 4. 5.. II. Cartilage A. What do chondroblasts do? B. When
o~ r;'c' - OSTEOARTHRITIS
 Osteoarthritis and Cartilage (2001) 9, Supplement A, S102-S108 2001 OsteoArthritis Research Society International doi:10.1053/joca.2001.0451, available online at http://www.idealibrary.com on IDE~l Osteoarthritis
Osteoarthritis and Cartilage (2001) 9, Supplement A, S102-S108 2001 OsteoArthritis Research Society International doi:10.1053/joca.2001.0451, available online at http://www.idealibrary.com on IDE~l Osteoarthritis
The Skeletal System:Bone Tissue
 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
Skeletal System. The skeletal System... Components
 Skeletal System The skeletal System... What are the general components of the skeletal system? What does the skeletal system do for you & how does it achieve these functions? Components The skeletal system
Skeletal System The skeletal System... What are the general components of the skeletal system? What does the skeletal system do for you & how does it achieve these functions? Components The skeletal system
Supplementary Figure 1 The ability to regenerate an ear hole is discontinuous with wound healing. Ear-hole closure at D85 for each sex within each
 Supplementary Figure 1 The ability to regenerate an ear hole is discontinuous with wound healing. Ear-hole closure at D85 for each sex within each species observed. Data show a binary response to a 4 mm
Supplementary Figure 1 The ability to regenerate an ear hole is discontinuous with wound healing. Ear-hole closure at D85 for each sex within each species observed. Data show a binary response to a 4 mm
Pathophysiology of fracture healing
 Pathophysiology of fracture healing Bone anatomy and biomechanics Fracture patterns Bone healing and blood supply Influence of implants 1 What is the structure of bone? 2 Bone structure Four levels: Chemical
Pathophysiology of fracture healing Bone anatomy and biomechanics Fracture patterns Bone healing and blood supply Influence of implants 1 What is the structure of bone? 2 Bone structure Four levels: Chemical
Supplemental Tables and Figures. The metalloproteinase-proteoglycans ADAMTS7 and ADAMTS12 provide an innate,
 Supplemental Tables and Figures The metalloproteinase-proteoglycans ADAMTS7 and ADAMTS12 provide an innate, tendon-specific protective mechanism against heterotopic ossification Timothy Mead et al Supplemental
Supplemental Tables and Figures The metalloproteinase-proteoglycans ADAMTS7 and ADAMTS12 provide an innate, tendon-specific protective mechanism against heterotopic ossification Timothy Mead et al Supplemental
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BONE TISSUE. Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology
 BONE TISSUE Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology BONE FUNCTION Support Protection (protect internal organs) Movement (provide leverage system for skeletal muscles, tendons, ligaments
BONE TISSUE Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology BONE FUNCTION Support Protection (protect internal organs) Movement (provide leverage system for skeletal muscles, tendons, ligaments
For more information about how to cite these materials visit
 Author(s): University of Michigan Medical School, Department of Cell and Developmental Biology License: Unless otherwise noted, the content of this course material is licensed under a Creative Commons
Author(s): University of Michigan Medical School, Department of Cell and Developmental Biology License: Unless otherwise noted, the content of this course material is licensed under a Creative Commons
BONE LABORATORY DEMONSTRATIONS. These demonstrations are found on the bulletin boards outside the MCO Bookstore.
 BONE LABORATORY DEMONSTRATIONS These demonstrations are found on the bulletin boards outside the MCO Bookstore. COMPACT & TRABECULAR BONE - LM When viewed under the polarizing light microscope, the layering
BONE LABORATORY DEMONSTRATIONS These demonstrations are found on the bulletin boards outside the MCO Bookstore. COMPACT & TRABECULAR BONE - LM When viewed under the polarizing light microscope, the layering
Compact bone; Many parallel Haversian canals contain: small blood vessels. very small nerve. Interconnected by Volkmann s canals.
 Special characteristics of COMPACT BONE (dense bone) Thick; well vascularized Osteocytes and lamellae Concentric rings around blood vessels Most bones: outer compact bone inner spongy bone Marrow cavity
Special characteristics of COMPACT BONE (dense bone) Thick; well vascularized Osteocytes and lamellae Concentric rings around blood vessels Most bones: outer compact bone inner spongy bone Marrow cavity
Dr. Heba Kalbouneh. Saba Alfayoumi. Heba Kalbouneh
 11 Dr. Heba Kalbouneh Saba Alfayoumi Heba Kalbouneh 2- Bone Bone tissue is also classified into primary bone and secondary bone. In the beginning, the first bone that is deposited by the osteoblasts is
11 Dr. Heba Kalbouneh Saba Alfayoumi Heba Kalbouneh 2- Bone Bone tissue is also classified into primary bone and secondary bone. In the beginning, the first bone that is deposited by the osteoblasts is
2 PROCESSES OF BONE OSSIFICATION
 2 PROCESSES OF BONE OSSIFICATION ENDOCHONDRAL OSSIFICATION 6 STEPS 1. CARTILAGE ENLARGES, BY APPOSITIONAL GROWTH; CHONDROCYTES AT CENTER OF CARTILAGE GROW IN SIZE; MATRIX REDUCES IN SIZE & SPICULES CALCIFY;
2 PROCESSES OF BONE OSSIFICATION ENDOCHONDRAL OSSIFICATION 6 STEPS 1. CARTILAGE ENLARGES, BY APPOSITIONAL GROWTH; CHONDROCYTES AT CENTER OF CARTILAGE GROW IN SIZE; MATRIX REDUCES IN SIZE & SPICULES CALCIFY;
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Lecture 2: Skeletogenesis
 Jilin University School of Stomatology Skeletogenesis Lecture 2: Skeletogenesis Aug. 18, 2015 Yuji Mishina, Ph.D. mishina@umich.edu Student will describe Development of Bone - the general anatomy of bone
Jilin University School of Stomatology Skeletogenesis Lecture 2: Skeletogenesis Aug. 18, 2015 Yuji Mishina, Ph.D. mishina@umich.edu Student will describe Development of Bone - the general anatomy of bone
Ossification = Osteogenesis
 Ossification = Osteogenesis Ossification = Osteogenesis Parts of the fetal skeleton form during the first few weeks after conception By the end of the 8 th week, the skeletal pattern is formed : cartilage
Ossification = Osteogenesis Ossification = Osteogenesis Parts of the fetal skeleton form during the first few weeks after conception By the end of the 8 th week, the skeletal pattern is formed : cartilage
Rama Nada. - Mousa Al-Abbadi. 1 P a g e
 - 1 - Rama Nada - - Mousa Al-Abbadi 1 P a g e Bones, Joints and Soft tissue tumors Before we start: the first 8 minutes was recalling to Dr.Mousa s duties, go over them in the slides. Wherever you see
- 1 - Rama Nada - - Mousa Al-Abbadi 1 P a g e Bones, Joints and Soft tissue tumors Before we start: the first 8 minutes was recalling to Dr.Mousa s duties, go over them in the slides. Wherever you see
Histopathology: healing
 Histopathology: healing These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information that
Histopathology: healing These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information that
The role of mechanical stimuli induced by prenatal movements in skeletal development
 The role of mechanical stimuli induced by prenatal movements in skeletal development Niamh Nowlan, PhD Department of Bioengineering, Imperial College London Mechanobiology of Bone Mechanoregulation of
The role of mechanical stimuli induced by prenatal movements in skeletal development Niamh Nowlan, PhD Department of Bioengineering, Imperial College London Mechanobiology of Bone Mechanoregulation of
Diseases of the skeleton
 Diseases of the skeleton Flexibility Protection of vital organs Strength Skeletal defects impact human health Developmental diseases Degenerative diseases Fins regenerate and grow rapidly following amputation
Diseases of the skeleton Flexibility Protection of vital organs Strength Skeletal defects impact human health Developmental diseases Degenerative diseases Fins regenerate and grow rapidly following amputation
Joint and Epiphyseal Progenitor Cells Revitalize Tendon Graft and Form Mineralized Insertion Sites in Murine ACL Reconstruction Model
 Joint and Epiphyseal Progenitor Cells Revitalize Tendon Graft and Form Mineralized Insertion Sites in Murine ACL Reconstruction Model Yusuke Hagiwara 1,2, Nathaniel A. Dyment 3, Douglas J. Adams 3, Shinro
Joint and Epiphyseal Progenitor Cells Revitalize Tendon Graft and Form Mineralized Insertion Sites in Murine ACL Reconstruction Model Yusuke Hagiwara 1,2, Nathaniel A. Dyment 3, Douglas J. Adams 3, Shinro
Osteology. Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College
 Osteology Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Functions of the Skeletal System: Support Movement Protection Hemopoiesis Electrolyte balance (Ca ++ /PO -3 4 ) Acid-base balance Storage
Osteology Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Functions of the Skeletal System: Support Movement Protection Hemopoiesis Electrolyte balance (Ca ++ /PO -3 4 ) Acid-base balance Storage
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Generation of a conditional allele of the Kindlin-2 gene. (A) A restriction map of the relevant genomic region of Kindlin-2 (top), the targeting construct
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Generation of a conditional allele of the Kindlin-2 gene. (A) A restriction map of the relevant genomic region of Kindlin-2 (top), the targeting construct
CHAPTER 6 LECTURE OUTLINE
 CHAPTER 6 LECTURE OUTLINE I. INTRODUCTION A. Bone is made up of several different tissues working together: bone, cartilage, dense connective tissue, epithelium, various blood forming tissues, adipose
CHAPTER 6 LECTURE OUTLINE I. INTRODUCTION A. Bone is made up of several different tissues working together: bone, cartilage, dense connective tissue, epithelium, various blood forming tissues, adipose
Fractures of the Hand in Children Which are simple? And Which have pitfalls??
 Fractures of the Hand in Children Which are simple? And Which have pitfalls?? Kaye E Wilkins DVM, MD Professor of Orthopedics and Pediatrics Departments of Orthopedics and Pediatrics University of Texas
Fractures of the Hand in Children Which are simple? And Which have pitfalls?? Kaye E Wilkins DVM, MD Professor of Orthopedics and Pediatrics Departments of Orthopedics and Pediatrics University of Texas
Lecture 3: Skeletogenesis and diseases
 Jilin University School of Stomatology Skeletogenesis Lecture 3: Skeletogenesis and diseases Aug. 21, 2015 Yuji Mishina, Ph.D. mishina@umich.edu Bone Development Mouse embryo, E14.5 Mouse embryo, E18.0
Jilin University School of Stomatology Skeletogenesis Lecture 3: Skeletogenesis and diseases Aug. 21, 2015 Yuji Mishina, Ph.D. mishina@umich.edu Bone Development Mouse embryo, E14.5 Mouse embryo, E18.0
Fig Articular cartilage. Epiphysis. Red bone marrow Epiphyseal line. Marrow cavity. Yellow bone marrow. Periosteum. Nutrient foramen Diaphysis
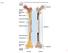 Fig. 7.1 Articular cartilage Epiphysis Red bone marrow Epiphyseal line Marrow cavity Yellow bone marrow Nutrient foramen Diaphysis Site of endosteum Compact bone Spongy bone Epiphyseal line Epiphysis Articular
Fig. 7.1 Articular cartilage Epiphysis Red bone marrow Epiphyseal line Marrow cavity Yellow bone marrow Nutrient foramen Diaphysis Site of endosteum Compact bone Spongy bone Epiphyseal line Epiphysis Articular
Skeletal System. Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section
 Skeletal System Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section Learning objectives At the end of this lecture, the medical student will be able to: State the embryonic origin of skeletal
Skeletal System Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section Learning objectives At the end of this lecture, the medical student will be able to: State the embryonic origin of skeletal
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 6 The Skeletal System: Bone Tissue Introduction The skeletal system has 6 important functions: Provides support Protects the internal organs (brain,
Principles of Anatomy and Physiology 14 th Edition CHAPTER 6 The Skeletal System: Bone Tissue Introduction The skeletal system has 6 important functions: Provides support Protects the internal organs (brain,
Sheets 16&17. Dr. Heba Kalbouneh. Dr. Heba Kalbouneh. Dr. Heba Kalbouneh
 Sheets 16&17 Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Ossification (formation of bone) - Osteoblasts are responsible for producing the extracellular matrix of the bone and these osteoblasts
Sheets 16&17 Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Ossification (formation of bone) - Osteoblasts are responsible for producing the extracellular matrix of the bone and these osteoblasts
Skeleton. Flexibility. Protection of vital organs. Strength
 Skeleton Flexibility Protection of vital organs Strength Skeletal defects Developmental defects Degenerative diseases Fibrous dysplasia (fibrous tissue develops in place of normal bones), Cleidocranial
Skeleton Flexibility Protection of vital organs Strength Skeletal defects Developmental defects Degenerative diseases Fibrous dysplasia (fibrous tissue develops in place of normal bones), Cleidocranial
Chapter 7. Skeletal System
 Chapter 7 Skeletal System 1 Introduction: A. Bones are very active, living tissues B. Each bone is made up of several types of tissues and so is an organ. C. Bone functions include: muscle attachment,
Chapter 7 Skeletal System 1 Introduction: A. Bones are very active, living tissues B. Each bone is made up of several types of tissues and so is an organ. C. Bone functions include: muscle attachment,
Regulation of the skeletal mass through the life span
 Regulation of the skeletal mass through the life span Functions of the skeletal system Mechanical protection skull Movement leverage for muscles Mineral metabolism calcium store Erythropoiesis red blood
Regulation of the skeletal mass through the life span Functions of the skeletal system Mechanical protection skull Movement leverage for muscles Mineral metabolism calcium store Erythropoiesis red blood
Finite-element study of vibration effect to fracture healing of a human tibia
 Finite-element study of vibration effect to fracture healing of a human tibia Leonid Maslov 1, Jean-Baptiste Etheve 2, Nikolay Sabaneev 3 Ivanovo State Power Engineering University, Ivanovo, Russia 1 Corresponding
Finite-element study of vibration effect to fracture healing of a human tibia Leonid Maslov 1, Jean-Baptiste Etheve 2, Nikolay Sabaneev 3 Ivanovo State Power Engineering University, Ivanovo, Russia 1 Corresponding
ODONTOGENESIS- A HIGHLY COMPLEX CELL-CELL INTERACTION PROCESS
 ODONTOGENESIS- A HIGHLY COMPLEX CELL-CELL INTERACTION PROCESS AMBRISH KAUSHAL, MALA KAMBOJ Department of Oral and Maxillofacial Pathology Career Post Graduate Institute of Dental Sciences and Hospital
ODONTOGENESIS- A HIGHLY COMPLEX CELL-CELL INTERACTION PROCESS AMBRISH KAUSHAL, MALA KAMBOJ Department of Oral and Maxillofacial Pathology Career Post Graduate Institute of Dental Sciences and Hospital
From the Orthopaedic Department, St. George's Hospital Medical School, London S.W.I.
 TRANSPLANTATION OF THE NAIL: A CASE REPORT By NICHOLAS P. PAPAVASSlI.IOU, M.D. 1 From the Orthopaedic Department, St. George's Hospital Medical School, London S.W.I. THE loss of a finger nail may be of
TRANSPLANTATION OF THE NAIL: A CASE REPORT By NICHOLAS P. PAPAVASSlI.IOU, M.D. 1 From the Orthopaedic Department, St. George's Hospital Medical School, London S.W.I. THE loss of a finger nail may be of
Blood. Hematopoietic Tissue
 Blood Hematopoietic Tissue Is a type of connective tissue in which its cells are suspended in a circulating fluid. Erythrocytes+ leukocytes + platelets (thrombocytes) =formed elements of blood. These formed
Blood Hematopoietic Tissue Is a type of connective tissue in which its cells are suspended in a circulating fluid. Erythrocytes+ leukocytes + platelets (thrombocytes) =formed elements of blood. These formed
Presidency General Hospital, Calcutta
 CONGENITAL ANOMALY OF HAND: " MIRROR HAND " By M. MUKERJI, F.R.C.S. Presidency General Hospital, Calcutta Case Report.--S. B., aged 4 months, was born with eight fingers and no thumb on the left hand and
CONGENITAL ANOMALY OF HAND: " MIRROR HAND " By M. MUKERJI, F.R.C.S. Presidency General Hospital, Calcutta Case Report.--S. B., aged 4 months, was born with eight fingers and no thumb on the left hand and
Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signalling pathway
 Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signalling pathway Jieyuan Zhang, Xiaolin Liu, Haiyan Li, Chunyuan Chen, Bin Hu, Xin Niu, Qing
Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signalling pathway Jieyuan Zhang, Xiaolin Liu, Haiyan Li, Chunyuan Chen, Bin Hu, Xin Niu, Qing
Bone Development. V. Gilsanz and O. Ratib, Hand Bone Age, DOI / _2, Springer-Verlag Berlin Heidelberg 2012
 Bone Development 2 Skeletal maturity is a measure of development incorporating the size, shape and degree of mineralization of bone to define its proximity to full maturity. The assessment of skeletal
Bone Development 2 Skeletal maturity is a measure of development incorporating the size, shape and degree of mineralization of bone to define its proximity to full maturity. The assessment of skeletal
Functions of the Skeletal System. Chapter 6: Osseous Tissue and Bone Structure. Classification of Bones. Bone Shapes
 Chapter 6: Osseous Tissue and Bone Structure Functions of the Skeletal System 1. Support 2. Storage of minerals (calcium) 3. Storage of lipids (yellow marrow) 4. Blood cell production (red marrow) 5. Protection
Chapter 6: Osseous Tissue and Bone Structure Functions of the Skeletal System 1. Support 2. Storage of minerals (calcium) 3. Storage of lipids (yellow marrow) 4. Blood cell production (red marrow) 5. Protection
Bone Tissue- Chapter 5 5-1
 Bone Tissue- Chapter 5 5-1 Bone Functions Support Protection Assistance in movement Mineral storage and release Blood cell production Triglyceride storage 5-2 Bone Chemistry Water (25%) Organic Constituent
Bone Tissue- Chapter 5 5-1 Bone Functions Support Protection Assistance in movement Mineral storage and release Blood cell production Triglyceride storage 5-2 Bone Chemistry Water (25%) Organic Constituent
Re-growing the Skeleton: Approaches in Tissue Engineering and Regenerative Medicine
 Re-growing the Skeleton: Approaches in Tissue Engineering and Regenerative Medicine How we fix things now Total Knee Replacements Fracture Plates Fusing Joints Defining Regenerative Medicine restore form
Re-growing the Skeleton: Approaches in Tissue Engineering and Regenerative Medicine How we fix things now Total Knee Replacements Fracture Plates Fusing Joints Defining Regenerative Medicine restore form
Ossification and Bone Remodeling
 Ossification and Bone Remodeling Pre-natal Ossification Embryonic skeleton: fashioned from fibrous membranes or cartilage to accommodate mitosis. 2 types of pre-natal ossification (bone formation) 1.
Ossification and Bone Remodeling Pre-natal Ossification Embryonic skeleton: fashioned from fibrous membranes or cartilage to accommodate mitosis. 2 types of pre-natal ossification (bone formation) 1.
KEY CONCEPTS Unit 6 THE SKELETAL SYSTEM
 ANATOMY & PHYSIOLOGY 1 (101-805 - AB) PAUL ANDERSON 2011 KEY CONCEPTS Unit 6 THE SKELETAL SYSTEM A Overview of The Skeletal System 1. Definition: Anatomically the SKELETAL SYSTEM consists of bones, cartilages,
ANATOMY & PHYSIOLOGY 1 (101-805 - AB) PAUL ANDERSON 2011 KEY CONCEPTS Unit 6 THE SKELETAL SYSTEM A Overview of The Skeletal System 1. Definition: Anatomically the SKELETAL SYSTEM consists of bones, cartilages,
Lecture 9 - Wound Healing
 Lecture 9 - Wound Healing A wound is any disruption to tissue caused by injury The injury is usually traumatic, i.e. physical, mechanical damage to tissue: Wound healing is a special case of acute inflammation
Lecture 9 - Wound Healing A wound is any disruption to tissue caused by injury The injury is usually traumatic, i.e. physical, mechanical damage to tissue: Wound healing is a special case of acute inflammation
SKELETAL TISSUES CHAPTER 7 INTRODUCTION TO THE SKELETAL SYSTEM TYPES OF BONES
 SKELETAL TISSUES CHAPTER 7 By John McGill Supplement Outlines: Beth Wyatt Original PowerPoint: Jack Bagwell INTRODUCTION TO THE SKELETAL SYSTEM STRUCTURE Organs: Bones Related Tissues: Cartilage and Ligaments
SKELETAL TISSUES CHAPTER 7 By John McGill Supplement Outlines: Beth Wyatt Original PowerPoint: Jack Bagwell INTRODUCTION TO THE SKELETAL SYSTEM STRUCTURE Organs: Bones Related Tissues: Cartilage and Ligaments
The Regeneration of Brachial Nerves of Contralateral Origin into Denervated Fused Newt Forelimbs ~ 3
 The Regeneration of Brachial Nerves of Contralateral Origin into Denervated Fused Newt Forelimbs 1 7 2 ~ 3 HARRY G. GOSHGARIAN 4 Department of Anatomy, University of Michigan, Ann Arbor, Michigan 48104
The Regeneration of Brachial Nerves of Contralateral Origin into Denervated Fused Newt Forelimbs 1 7 2 ~ 3 HARRY G. GOSHGARIAN 4 Department of Anatomy, University of Michigan, Ann Arbor, Michigan 48104
Supplemental Figure 1. Egr1 expression in adult Achilles tendons. (A,B) Achilles tendons were isolated from 2 month-old Egr1 +/- mice and stained for
 Supplemental Figure 1. Egr1 expression in adult Achilles tendons. (A,B) Achilles tendons were isolated from 2 month-old Egr1 +/- mice and stained for LacZ activity, which reflects Egr1 expression. (A)
Supplemental Figure 1. Egr1 expression in adult Achilles tendons. (A,B) Achilles tendons were isolated from 2 month-old Egr1 +/- mice and stained for LacZ activity, which reflects Egr1 expression. (A)
Growth and repair: Cartilage is a vascular tissues that receives nutrients by diffusion through its matrix, cartilage grow by 2 mechanisms:
 Skeletal connective tissues: (cartilage and bone): Cartilage and bone are specialized connective tissues both adapted to serve as skeletal framework in most vertebrates the presence of solid inter cellular
Skeletal connective tissues: (cartilage and bone): Cartilage and bone are specialized connective tissues both adapted to serve as skeletal framework in most vertebrates the presence of solid inter cellular
Tetrapod Limb Development
 IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
Tissue renewal and Repair. Nisamanee Charoenchon, PhD Department of Pathobiology, Faculty of Science
 Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Do Now: 1. Where, specifically, is blood created? Which part of the long bone? 2. Which structures are primarily associated with growth? 3.
 Do Now: 1. Where, specifically, is blood created? Which part of the long bone? 2. Which structures are primarily associated with growth? 3. How could damage to these areas impact bone growth? WRITE AND
Do Now: 1. Where, specifically, is blood created? Which part of the long bone? 2. Which structures are primarily associated with growth? 3. How could damage to these areas impact bone growth? WRITE AND
The formation of blood cells is called. hemopoiesis. What does our bone store? Where do our bones store fat? yellow marrow.
 What are the 5/6 functions of the skeletal system? support, protection, movement, blood cell formation, storage, homeostasis The formation of blood cells is called hemopoiesis What does our bone store?
What are the 5/6 functions of the skeletal system? support, protection, movement, blood cell formation, storage, homeostasis The formation of blood cells is called hemopoiesis What does our bone store?
Lab Animal Tissue. LEARNING OBJECTIVES: To understand the relationship between the structure and function of different animal tissues
 Name: Bio A.P. PURPOSE: HYPOTHESIS: NONE Lab Animal Tissue BACKGROUND: In animals, groups of closely related cells specialized to perform the same function are called tissues. There are four general classes
Name: Bio A.P. PURPOSE: HYPOTHESIS: NONE Lab Animal Tissue BACKGROUND: In animals, groups of closely related cells specialized to perform the same function are called tissues. There are four general classes
Standard Operating Procedure
 1.0 Purpose: 1.1 Relaxation, dissection, weighing and fixation of heart for histological analysis. Changes in heart weight and wall thickness are linked to cardiovascular phenotypes. This protocol describes
1.0 Purpose: 1.1 Relaxation, dissection, weighing and fixation of heart for histological analysis. Changes in heart weight and wall thickness are linked to cardiovascular phenotypes. This protocol describes
BONE HISTOLOGY SLIDE PRESENTATION
 BONE HISTOLOGY SLIDE PRESENTATION PRESENTED BY: SKELETECH, INC. Clients and Friends: SkeleTech invites you to use these complimentary images for your own presentations or as teaching slides for bone biology.
BONE HISTOLOGY SLIDE PRESENTATION PRESENTED BY: SKELETECH, INC. Clients and Friends: SkeleTech invites you to use these complimentary images for your own presentations or as teaching slides for bone biology.
Experiment Note the locations of the epidermis, dermis, dermal papillae, and the sweat glands. Note that fat cells that comprise the
 Experiment 1 Examining Skin, Bones and Muscle Histology Experiment Inventory Skin Digital Slide Images Cortical (Compact) Bone Digital Slide Image Trabecular (Spongy) Bone Digital Slide Image Cardiac Muscle
Experiment 1 Examining Skin, Bones and Muscle Histology Experiment Inventory Skin Digital Slide Images Cortical (Compact) Bone Digital Slide Image Trabecular (Spongy) Bone Digital Slide Image Cardiac Muscle
Bone Development. Two Types of OssificaDon 10/18/14. Osteogenesis ( ) bone Dssue formadon Stages. Bones and Skeletal Tissues: Part B
 Bone Development 6 Bones and Skeletal Tissues: Part B Osteogenesis ( ) bone Dssue formadon Stages Bone formadon begins in the 2nd month of development Postnatal bone growth undl early adulthood Bone remodeling
Bone Development 6 Bones and Skeletal Tissues: Part B Osteogenesis ( ) bone Dssue formadon Stages Bone formadon begins in the 2nd month of development Postnatal bone growth undl early adulthood Bone remodeling
Cells and Tissues 3PART D. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Cells and Tissues 3PART D Connective Tissue Found everywhere in the body Includes the most abundant
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Cells and Tissues 3PART D Connective Tissue Found everywhere in the body Includes the most abundant
Bones. The division of bones anatomically is : long, short, irregular, flat and sesamoid.
 Bones Osteocytes : Are responsible for maintenance of bones Present in lacunae, and send processes. Unable to divide. The division of bones anatomically is : long, short, irregular, flat and sesamoid.
Bones Osteocytes : Are responsible for maintenance of bones Present in lacunae, and send processes. Unable to divide. The division of bones anatomically is : long, short, irregular, flat and sesamoid.
International Graduate Research Programme in Cardiovascular Science
 1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over 3 Weeks. A SEPARATE WORKSHEET WILL BE PROVIDED.
 BIO 211; Anatomy and Physiology I REFERENCE: CHAPTER 07 1 Dr. Lawrence Altman Naugatuck Valley Community College LECTURE TOPICS OUTLINE SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over
BIO 211; Anatomy and Physiology I REFERENCE: CHAPTER 07 1 Dr. Lawrence Altman Naugatuck Valley Community College LECTURE TOPICS OUTLINE SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over
Skeletal System worksheet
 Skeletal System worksheet Name Section A: Intro to Skeletal System The skeletal system performs vital functions that enable us to move through our daily lives. Support - The skeleton provides support and
Skeletal System worksheet Name Section A: Intro to Skeletal System The skeletal system performs vital functions that enable us to move through our daily lives. Support - The skeleton provides support and
Module 2:! Functional Musculoskeletal Anatomy A! Semester 1! !!! !!!! Hard Tissues, Distal Upper Limb & Neurovascular Supply of Upper Limb!
 Functional Musculoskeletal Anatomy A Module 2: Hard Tissues, Distal Upper Limb & Neurovascular Supply of Upper Limb Semester 1 1 18. Bone Tissue & Growth of Bones 18.1 Describe the structure of bone tissue
Functional Musculoskeletal Anatomy A Module 2: Hard Tissues, Distal Upper Limb & Neurovascular Supply of Upper Limb Semester 1 1 18. Bone Tissue & Growth of Bones 18.1 Describe the structure of bone tissue
Genetics contributes to size. Bundschu et al., 2005, JBC Shingleton et al., 2005, PLOS
 Matters of size Genetics contributes to size Bundschu et al., 2005, JBC Shingleton et al., 2005, PLOS Advantages to examining growth mechanisms in the zebrafish fin The fin and body are easy to measure
Matters of size Genetics contributes to size Bundschu et al., 2005, JBC Shingleton et al., 2005, PLOS Advantages to examining growth mechanisms in the zebrafish fin The fin and body are easy to measure
Outline. Skeletal System. Functions of Bone. Bio 105: Skeletal System 3/17/2016. The material from this lecture packet will be on the lecture exam
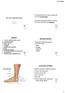 Bio 105: Skeletal System Lecture 8 Chapter 5 The material from this lecture packet will be on the lecture exam The identification that you do after this lecture will be on the lab exam Outline I. Overview
Bio 105: Skeletal System Lecture 8 Chapter 5 The material from this lecture packet will be on the lecture exam The identification that you do after this lecture will be on the lab exam Outline I. Overview
Chapter 6 Bones and Bone Tissue Chapter Outline
 Chapter 6 Bones and Bone Tissue Chapter Outline Module 6.1: Introduction to Bones as Organs (Figures 6.1, 6.2, 6.3, 6.4) A. The skeletal system includes the bones, joints, and their associated supporting
Chapter 6 Bones and Bone Tissue Chapter Outline Module 6.1: Introduction to Bones as Organs (Figures 6.1, 6.2, 6.3, 6.4) A. The skeletal system includes the bones, joints, and their associated supporting
Development of the Axial Skeleton and Limbs. Professor Alfred Cuschieri Department of Anatomy University of Malta
 Development of the Axial Skeleton and Limbs Professor Alfred Cuschieri Department of Anatomy University of Malta During the Fourth Week the Embryo Is Segmented. Each segment consists of: a segment of neural
Development of the Axial Skeleton and Limbs Professor Alfred Cuschieri Department of Anatomy University of Malta During the Fourth Week the Embryo Is Segmented. Each segment consists of: a segment of neural
Supplementary Figure 1
 Supplementary Figure 1 Genetic labeling of microglia Male and female 2-3 month-old CreERT2;R26-tdTomato mice or CreERT2;R26-tdTomato;Iba1-eGFP transgenic mice were treated with 1x, 2x (48 h apart), or
Supplementary Figure 1 Genetic labeling of microglia Male and female 2-3 month-old CreERT2;R26-tdTomato mice or CreERT2;R26-tdTomato;Iba1-eGFP transgenic mice were treated with 1x, 2x (48 h apart), or
Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair
 Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair Chapter 04 Boek 1_Gie.indb 55 21-05-2007 12:27:33 Chapter 04 Abstract Goal: TGF-b and IGF-I
Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair Chapter 04 Boek 1_Gie.indb 55 21-05-2007 12:27:33 Chapter 04 Abstract Goal: TGF-b and IGF-I
Biology. Dr. Khalida Ibrahim
 Biology Dr. Khalida Ibrahim BONE TISSUE Bone tissue is a specialized form of connective tissue and is the main element of the skeletal tissues. It is composed of cells and an extracellular matrix in which
Biology Dr. Khalida Ibrahim BONE TISSUE Bone tissue is a specialized form of connective tissue and is the main element of the skeletal tissues. It is composed of cells and an extracellular matrix in which
Skin: The Body s Protection
 Ch 34: Protection, Support and Locomotion 34.1 - Skin: The Body s Protection Inside This Section... The Structure of Skin The Function of Skin Response to Injury Structure and Function of the skin 4 tissue
Ch 34: Protection, Support and Locomotion 34.1 - Skin: The Body s Protection Inside This Section... The Structure of Skin The Function of Skin Response to Injury Structure and Function of the skin 4 tissue
LRI Emergency Department
 LRI Emergency Department Guideline for the management of: Finger Tip Injuries in Children In the Paediatric Emergency Department (UHL Category C Guidance) Staff relevant to: ED Medical and Nursing staff
LRI Emergency Department Guideline for the management of: Finger Tip Injuries in Children In the Paediatric Emergency Department (UHL Category C Guidance) Staff relevant to: ED Medical and Nursing staff
Chapter 5 The Skeletal System:Bone Tissue. Functions of Bone. Bones
 Chapter 5 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose,
Chapter 5 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose,
Technique Guide Hammertoe Correction System
 Technique Guide Hammertoe Correction System TM The ToeMATE Hammertoe Correction System is an easy to implant bone screw system intended for the correction of hammertoe deformity. It is provided in a complete
Technique Guide Hammertoe Correction System TM The ToeMATE Hammertoe Correction System is an easy to implant bone screw system intended for the correction of hammertoe deformity. It is provided in a complete
NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM
 LECTURE 4 SKULL NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM CRANIUM NEUROCRANIUM (protective case around brain) VISCEROCRANIUM (skeleton of face) NASOMAXILLARY COMPLEX MANDIBLE (DESMOCRANIUM)
LECTURE 4 SKULL NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM CRANIUM NEUROCRANIUM (protective case around brain) VISCEROCRANIUM (skeleton of face) NASOMAXILLARY COMPLEX MANDIBLE (DESMOCRANIUM)
ANATOMY & PHYSIOLOGY - CLUTCH CH. 8 - BONE AND CARTILAGE.
 !! www.clutchprep.com CONCEPT: BONE CLASSIFICATIONS There are four classifications of bones based on their 1. Long bones are greater in length than in width - Found in the upper and lower limbs (ex: arm,
!! www.clutchprep.com CONCEPT: BONE CLASSIFICATIONS There are four classifications of bones based on their 1. Long bones are greater in length than in width - Found in the upper and lower limbs (ex: arm,
What s New in Fingertip Injuries. Gordon A. Brody, MD SOAR Redwood City
 What s New in Fingertip Injuries Gordon A. Brody, MD SOAR Redwood City Goals of Treatment Durable Sensate Aesthetic Preserve Length Preserve Mobility Goals of Treatment Pain and Worker s Compensation are
What s New in Fingertip Injuries Gordon A. Brody, MD SOAR Redwood City Goals of Treatment Durable Sensate Aesthetic Preserve Length Preserve Mobility Goals of Treatment Pain and Worker s Compensation are
PROCHONDRIX CARTILAGE RESTORATION MATRIX CONTAINS GROWTH FACTORS NECESSARY FOR HYALINE CARTILAGE REGENERATION
 A L L O S O U R C E PROCHONDRIX CARTILAGE RESTORATION MATRIX CONTAINS GROWTH FACTORS NECESSARY FOR HYALINE CARTILAGE REGENERATION Ryan Delaney MS; Carolyn Barrett BS, MBA; Peter Stevens PhD, MBA AlloSource,
A L L O S O U R C E PROCHONDRIX CARTILAGE RESTORATION MATRIX CONTAINS GROWTH FACTORS NECESSARY FOR HYALINE CARTILAGE REGENERATION Ryan Delaney MS; Carolyn Barrett BS, MBA; Peter Stevens PhD, MBA AlloSource,
Skeletal Tissues Dr. Ali Ebneshahidi
 Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection: Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection: Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
Effects of Delayed Stabilization on Fracture Healing
 Effects of Delayed Stabilization on Fracture Healing Theodore Miclau, 1 Chuanyong Lu, 1 Zachary Thompson, 1 Paul Choi, 1 Christian Puttlitz, 2 Ralph Marcucio, 1 Jill A. Helms 3 1 Department of Orthopaedic
Effects of Delayed Stabilization on Fracture Healing Theodore Miclau, 1 Chuanyong Lu, 1 Zachary Thompson, 1 Paul Choi, 1 Christian Puttlitz, 2 Ralph Marcucio, 1 Jill A. Helms 3 1 Department of Orthopaedic
Introduction and Terminology
 Introduction and Terminology Histology - study of tissues (histo = tissues). Tissue Epithelial Tissue Covers and protects exposed surfaces. Muscle Tissue Skeletal muscle, smooth muscle and cardiac muscle.
Introduction and Terminology Histology - study of tissues (histo = tissues). Tissue Epithelial Tissue Covers and protects exposed surfaces. Muscle Tissue Skeletal muscle, smooth muscle and cardiac muscle.
Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen. Yusuke Murasawa, Pi-chao Wang
 Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen Yusuke Murasawa, Pi-chao Wang Abstract Although tissue engineering of artificial organs such as skin or cartilage
Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen Yusuke Murasawa, Pi-chao Wang Abstract Although tissue engineering of artificial organs such as skin or cartilage
Human Anatomy & Physiology
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 6 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 6 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
Two Separated Protocols with the Most Important Comments for Skeletal Staining in Embryonic and Adulthood Period in Laboratory Animals
 May 2014, Volume 11, Number 2 Two Separated Protocols with the Most Important Comments for Skeletal Staining in Embryonic and Adulthood Period in Laboratory Animals Farzane Sadeghi * PhD in Anatomical
May 2014, Volume 11, Number 2 Two Separated Protocols with the Most Important Comments for Skeletal Staining in Embryonic and Adulthood Period in Laboratory Animals Farzane Sadeghi * PhD in Anatomical
Name Date Score. Skeletal System. Indicate if the following statements are true or false. Correct false statements
 Name Date Score Skeletal System True/False Indicate if the following statements are true or false. Correct false statements 1. Bones surround vital organs to protect them. 2. Bones store most of the calcium
Name Date Score Skeletal System True/False Indicate if the following statements are true or false. Correct false statements 1. Bones surround vital organs to protect them. 2. Bones store most of the calcium
