NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE. Final Appraisal Determination. Frequency of application of topical corticosteroids for atopic eczema
|
|
|
- Sharlene Newman
- 5 years ago
- Views:
Transcription
1 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE Frequency of application of topical corticosteroids for atopic eczema 1 Guidance This appraisal relates to the frequency of application of topical corticosteroids in the treatment of atopic eczema. It does not include the use of topical agents that combine corticosteroids with other active agents (for example, antimicrobials or salicylic acid). 1.1 It is recommended that topical corticosteroids for atopic eczema should be prescribed for application only once or twice. 1.2 It is recommended that where more than one alternative topical corticosteroid is considered clinically appropriate within a potency class, the drug with the lowest acquisition cost should be prescribed, taking into account pack size and frequency of application. 2 Clinical need and practice 2.1 Atopic eczema (synonymous with atopic dermatitis) is a chronic relapsing skin condition characterised by intense itching, dry skin, redness, inflammation and exudation. It affects mainly the flexor surfaces of the elbows and knees, as well as the face and neck. 2.2 The term atopic refers to the association with atopy (a state of hypersensitivity to common environmental allergens that may be inherited), and differentiates atopic eczema from other forms of eczema such as irritant, allergic contact, discoid, venous, seborrhoeic and photosensitive eczema, which have different disease patterns and aetiologies. Issue date: April 2004 Page 1 of 34
2 2.3 Estimates of prevalence vary but suggest that the condition may affect as many as 15 20% of school-age children and 2 10% of adults. Most people with atopic eczema (over 80%) experience mild disease; only around 2 4% of people with eczema have a severe form of the disease. Despite the lower prevalence, the presentation of disease in adults is often more severe and chronic in nature. 2.4 In most people with atopic eczema, the condition begins in early childhood often in the first year of life when it can be particularly severe. Findings from the National Child Development Study (NCDS), developed from the birth cohort of 1958, suggested an incidence of around 50 cases per 1000 in the first year of life, falling to 5 new cases per 1000 per year for the rest of childhood. In around 60% of children, the condition clears by the time they reach their teens. However, the tendency towards dry and irritable skin generally persists and later recurrences are common. 2.5 The aetiology of atopic eczema is complex and not fully understood. Genetic factors are important but environmental factors, such as house dust mites, pollen, tobacco, air pollution and low humidity, may cause its onset and/or exacerbate existing symptoms. More persistent disease has been consistently linked with early disease onset, severe widespread disease in early life, concomitant asthma or hay fever, and a family history of atopic eczema. The condition is exacerbated by soap and detergents, clothes containing wool or certain synthetic fibres, and extremes of temperature. 2.6 The severity of atopic eczema varies enormously, from an occasional dry, scaly patch to a debilitating disease, where much of the body is covered by excoriated, bleeding and infected lesions. Its course may be continuous for prolonged periods or of a relapsing remitting nature, characterised by acute flare-ups. 2.7 Itching skin (pruritus) is a major symptom of atopic eczema. A vicious circle can occur, where itching and scratching damage the skin and increase inflammation, which in turn increases the itch. Damage to the skin from Issue date: April 2004 Page 2 of 34
3 scratching can cause bleeding, secondary infection and thickening of the skin (lichenification). 2.8 The impact of atopic eczema on quality of life can be considerable, and varies according to disease severity. In addition to the burden imposed by treatment, studies have shown not only that the condition affects everyday activities such as work or school and social relationships, but also that people with atopic eczema may also experience anxiety, depression and other psychological problems. Sleep disturbance is common, especially during flare-ups, which in turn can lead to problems with irritability and lack of concentration. Severe atopic eczema in children can also have a significant impact on family life, with parents/carers having to cope with the demands associated with caring for a child with a chronic illness. 2.9 Historically, there have been variations over the clinical definition and diagnosis of atopic eczema. A UK Working Party has developed criteria for use in epidemiological studies, and these are now commonly used, although further validation is required. To qualify as a case of atopic eczema using these criteria, the person must have had an itchy skin condition in the last 12 months, plus three or more of the following: a history of flexural involvement (that is, affecting the bends of the elbows or behind the knees) a history of a generally dry skin a personal history of other atopic disease (in children under 4 years, a history of atopic disease in a first-degree relative may be included) visible flexural dermatitis as defined by a photographic protocol onset below the age of 2 years (not used in children under 4 years) There is uncertainty and a lack of standardisation around clinical assessment of disease severity, both in practice and in trial settings. Although a number of scoring systems have been used to categorise the disease as mild, moderate or severe, usually by aggregating scores from a range of symptoms and Issue date: April 2004 Page 3 of 34
4 disease characteristics, none of these scoring systems has been accepted as a gold standard and there remains general debate over their use Atopic eczema in childhood shows a reverse social class gradient, with higher rates in socioeconomically advantaged groups and smaller families. There is also evidence of variation in prevalence by region, with the highest rates recorded in the South East and industrialised Midlands, and the lowest rates in Wales and Scotland Management of atopic eczema takes place predominantly in primary care, and aims to relieve symptoms and prevent complications such as infections until remission occurs. This management involves the identification and avoidance of exacerbating factors, skin care and anti-inflammatory treatment. Providing people with good-quality information about these issues is essential to successfully managing and treating atopic eczema. Referral to secondary care is advised only if the condition is severe and has not responded to appropriate therapy Emollients are a first-line therapy for atopic eczema and aim to retain the skin s barrier function (keeping water in and irritants or pathogens out) and to prevent painful cracking. Frequent and continuous use is recommended even in the absence of symptoms. Preparations available include bath oils, soap substitutes and moisturisers; generally the greasier the preparation, the better the effect, although people using very greasy products may consider them unacceptable Topical corticosteroids are the first-line treatment for flare-ups of atopic eczema. In order to reduce exposure to topical corticosteroids, they are used only intermittently to control exacerbations. Treatment regimens for topical steroids vary with disease severity, with clinicians usually recommending use of the mildest potency products possible to treat the condition, in order to minimise the potential adverse effects. Emollients are used together with the topical corticosteroids. Issue date: April 2004 Page 4 of 34
5 2.15 Where there are associated bacterial or fungal infections, corticosteroids are combined with other substances (such as antimicrobials or salicylic acid) in topical preparations Other treatments for atopic eczema include antihistamines, topical immunomodulators (see Section 8), and wet wraps (when a layer of emollients with or without corticosteroids is applied to the skin and wrapped in wet bandages, followed by dry bandages, and left overnight), which may be used in an attempt to maximise the effect of treatment Treatments of last resort in resistant severe cases include systemic corticosteroids, phototherapy and systemic use of immunosuppressants. 3 The technology 3.1 Topical corticosteroids have anti-inflammatory and immunosuppressive effects. The mechanism of the anti-inflammatory activity of topical steroids in general is unclear, although various symptomatic components of the inflammatory pathway are known to be suppressed. 3.2 Thirty preparations of topical corticosteroids are included in this appraisal (see Appendix D). Topical corticosteroids are classified according to their potency. This is determined by the amount of vasoconstriction a topical corticosteroid produces and the degree to which it inhibits inflammation (a more potent product increases suppression to the inflammatory pathway). In the UK, four potencies are recognised: mild, moderately potent, potent and very potent. Across the different potencies, products have different formulations and different strengths (for example, 0.025%, 0.1%, 0.5%) and are available in various preparations (for example, ointment, cream, lotion, foam). 3.3 The most widespread side effect of topical corticosteroid treatment is skin atrophy, where the skin becomes thin and may become easily bruised. This is more likely to occur on areas where the skin is already thin, such as the face or flexures. Absorption is greatest in these areas and therefore the use of Issue date: April 2004 Page 5 of 34
6 potent steroids on these sites should generally be avoided. The skin may recover gradually after stopping treatment, but the original structure may never return. Prolonged or excessive use of potent steroids causes the dermis to lose its elasticity and stretch marks (striae) to appear, which are permanent. Children, especially babies, are particularly susceptible to side effects. The more potent corticosteroids are contraindicated for infants less than 1 year old. For full details of side effects and contraindications, see the Summaries of Product Characteristics (SmPCs) for the topical corticosteroids. 3.4 Guidelines from the British Association of Dermatologists suggest that the best way of using topical corticosteroids is probably twice for days when the eczema is active, followed by a holiday period of emollients only. The National Prescribing Centre recommends that, in general practice, topical corticosteroids be used in short bursts (for 3 7 days) to treat exacerbations of disease. 3.5 There are varying recommendations about the frequency of application. The British National Formulary (BNF) states that corticosteroid preparations should normally be applied once or twice. It is not necessary to apply them more frequently. Although there are few empirical data to assess the patterns of prescribing with respect to frequency of application, it appears that a twice- regimen is the most widespread approach to the use of topical corticosteroids in atopic eczema. However, the SmPCs for some of the topical corticosteroids indicate that some are licensed for more frequent use (up to four times a day), and two products are licensed for use only once a day in atopic eczema. For individual posologies, see Appendix D. 4 Evidence and interpretation The Appraisal Committee (Appendix A) considered evidence from a number of sources (Appendix B). The remit given to NICE by the Department of Health/Welsh Assembly Government was to advise on the clinical and cost effectiveness of once- use compared with more frequent use of same- Issue date: April 2004 Page 6 of 34
7 potency topical corticosteroids in the treatment of people with atopic eczema. The evidence appraised was restricted to comparisons of topical corticosteroids for atopic eczema within the same potency class. 4.1 Clinical effectiveness The Assessment Report reviewed data from one systematic review and ten randomised controlled trials (RCTs) that examined frequency of application of topical corticosteroids of the same potency. No RCTs or clinical controlled trials of mild topical corticosteroids were identified. One RCT examined moderately potent corticosteroids, eight RCTs examined potent corticosteroids and one RCT examined very potent corticosteroids The study setting was hospital or secondary care for four of the ten trials but was not reported in the remaining studies. The duration of treatment for the trials ranged from 7 days to 4 weeks. Quality of life and patient preference were not reported by any of the included trials The Assessment Group concluded the systematic review to be of good methodological quality. The systematic review included three RCTs (two trials examining potent topical corticosteroids and one examining very potent topical corticosteroids), all of which were included in the Assessment Report. The authors of the systematic review found that in none of the studies was more frequent application superior to once- application. They concluded that point estimates suggest that a small difference in favour of more frequent application cannot be excluded The Assessment Group did not consider meta-analysis to be appropriate because of the clinical and statistical heterogeneity of the trials. Moderately potent preparations One RCT was identified that examined the frequency of application in moderately potent topical corticosteroids for atopic eczema; the study population was children. The Assessment Report stated that the study was Issue date: April 2004 Page 7 of 34
8 small and the duration of treatment was 7 days; the study did not report the setting, how allocation to treatment groups occurred, blinding of either outcome assessors or patients, or the number of patients responding to the treatment. There was no statistically significant difference in severity of symptoms following treatment with once- versus twice- application of topical corticosteroids. Adverse effects were not reported. Potent preparations Eight RCTs were identified that examined frequency of application in potent topical corticosteroids for atopic eczema. Five of these compared the same active compound administered once and twice (four of these trials examined fluticasone propionate [ointment and cream]). Three trials investigating potent topical corticosteroids compared different active compounds; these all compared a once--only product, mometasone furoate, with other topical corticosteroids administered twice Apart from two trials within this potency class, the Assessment Report considered the quality of reporting and the methodology of the included RCTs to be generally poor For four of the studies, the study setting was hospital or secondary care but the setting was not reported in the remaining studies. Duration of treatment in the studies was up to either 3 or 4 weeks. Where reported, the studies included people who had moderate to severe atopic eczema, apart from one study that included adults with mild to moderate eczema. One other study did not report the minimum severity of eczema of the study population Studies included children and adults, people aged over 12 years or 16 years, or adults only. Subgroup analyses of children aged 12 years or younger were reported for two trials. Response to treatment The studies measured effectiveness of the treatments using a variety of different outcome measures, most of which were subjective assessments by Issue date: April 2004 Page 8 of 34
9 the investigator and/or patient. All studies apart from one reported the number of patients responding to treatment. However, response to treatment was defined in different ways by the studies. Two outcomes were considered in the Assessment Report: the number of patients with at least a good response or 50% improvement, and the number of patients whose eczema was rated cleared or controlled Seven studies reported the number of patients with at least a good response, assessed by the investigator and/or patient, or at least 50% improvement by the end of 3 or 4 weeks. Six studies reported the number of patients with eczema that was rated as cleared/controlled or excellent after 3 or 4 weeks Overall, studies found little difference in response to treatment between once and twice- application of potent corticosteroids. Some statistically significant differences favouring twice- treatment were identified, but these were inconsistent between outcome assessors (physicians versus patients) and outcomes selected for analysis. Subgroup analysis of patients aged 12 years or younger produced similar findings to the main analysis One study compared success rates between morning and evening application in the once- group (67% versus 78%, difference 11.3%, 95% confidence interval [CI] 4.6 to 27.2, p = 0.17). Despite finding a statistically significant difference between once- and twice- application, when assessed by the physician (but not when assessed by the patient), the difference between once- evening treatment and twice- application was not statistically significant (78% versus 84%, difference 5.9%, 95% CI 6.6 to 18.4, p = 0.33). Severity of signs and symptoms None of the studies reported the use of a validated severity scale, and the clinical relevance of a change in severity is not clear. However, in one study, once- use of mometasone furoate, which is a once--only product, was found to result in a greater percentage improvement in total atopic eczema scores than twice- betamethasone valerate at each assessment, Issue date: April 2004 Page 9 of 34
10 (p < 0.01). Another study found an improvement in pruritus (p = 0.007) only, following mometasone furoate, compared with twice- hydrocortisone 17- butyrate. A third study comparing once- use of mometasone furoate with betamethasone dipropionate found no statistically significant differences in percentage reduction of severity for erythema, induration or pruritus. However, the Assessment Group stated that these three trials were all of poor quality because they were described as single-blind (investigators blinded), but the trials did not give details of methods or procedures, or use of placebo treatment in the once- group. Two of these trials also failed to report whether comparison groups were similar at baseline A greater reduction in severity scores demonstrated at 2 weeks (p = 0.04) for twice- compared with once- use of hydrocortisone 17-butyrate was not maintained at 4 weeks (p = 0.08) in one trial, and although the twice- group showed more pronounced reductions in rating for erythema at 4 weeks (p = 0.03), this was not the case for the other symptoms assessed. No confidence intervals were available for these trials One trial found total severity scores to be similar between once- and twice- application of fluticasone propionate ointment at each visit, although logistic regression analysis of total severity score (adjusting for age and baseline total severity score) favoured twice- application at the last visit attended (odds ratio [OR] 1.72, 95% CI 1.05 to 2.82, p = 0.033). However, the odds ratio for the treatment effect in the subgroup analysis of patients aged 12 years or younger was not statistically significant (OR 1.85, 95% CI 0.88 to 3.89, p = 1.03) None of the other studies comparing potent topical corticosteroids found a statistically significant difference in severity of atopic eczema following once application compared with more frequent applications. Issue date: April 2004 Page 10 of 34
11 Adverse effects The quality and extent of reporting of adverse effects was variable among studies. There appeared to be little difference in the frequency or severity of adverse events between once- and twice- application of topical corticosteroids, although data were limited because of the short duration of the studies One study did report potential differences in sleep disturbance, finding sleep to be as good as ever has been or better by 37% of patients following once application of fluticasone propionate compared with 55% of patients following twice- application. No p value or confidence intervals were available for this outcome. Very potent preparations One RCT compared once- application of halcinonide cream (0.1%) with three-times- application of the same product The trial was double-blind, but the concealment of allocation was not reported. The duration of the study was a maximum of 3 weeks, or shorter if complete remission was obtained. The age range of patients, the study setting and the minimum severity of eczema for the included patients were not reported in the study The study compared the response of similar lesions on each side of the patient. A better response (slightly superior or markedly superior) was observed following three-times- application. Overall, 32% of patients had a better clinical response to three-times- application, 21% had a better clinical response to once- application, and 47% had an equal response (p < 0.05), but no statistically significant difference was found in the number of patients with at least a good absolute therapeutic response The authors of the study stated that the side effects were generally of a mild nature, the most common being burning, pruritus and erythema, with no difference in incidence between once- and three-times- regimens, Issue date: April 2004 Page 11 of 34
12 and that no systemic effects were observed. However, the Assessment Report pointed out that no data were presented on adverse effects. Summary Overall, the Assessment Report did not identify any clear differences for any of the potency classes in outcomes between once- and more frequent application of topical corticosteroids. For potent preparations, one study indicated a statistically significant difference in favour of the twice- application of fluticasone propionate (ointment) in response rates between the different regimens (at least a good response rate or 50% improvement), when patients were assessed by physicians; however, this was not the case for patient assessment. For a response of cleared or controlled atopic eczema, one trial indicated a significant difference in favour of twice- treatment of hydrocortisone 17-butyrate when patients were assessed by a physician. Two studies, considered by the Assessment Group to be of poor quality (as described in Section ), favoured once- treatment of mometasone furoate over twice- use of other products (depending on severity of certain symptoms). The trial of a very potent corticosteroid reported a statistically significant difference in clinical response, favouring more frequent application, but no significant difference in the number of patients with at least a good response. 4.2 Cost effectiveness The Assessment Group did not identify any published economic evaluations that examined frequency of use of same-potency topical corticosteroids No economic evaluations were identified or submitted by the manufacturers or other consultees. No quality-of-life or patient preference outcomes were included in any of the studies in the systematic review The Assessment Group concluded that there was no basis to draw firm conclusions over the relative effectiveness of once- versus more frequent use of same-potency topical corticosteroids for atopic eczema. Consequently, Issue date: April 2004 Page 12 of 34
13 the economic analysis assumes equivalent effectiveness of once- application and more frequent application of topical corticosteroids, and costminimisation analysis was undertaken The cost per application of topical corticosteroids varies depending on the quantity used per application. Evidence was derived from two of the included RCTs and four additional studies that were identified. The Assessment Group stated that, although it would be reasonable to assume that the actual amount of topical corticosteroid used in a once- regimen is less than that used for more frequent applications (especially when referring to the same product), it is not possible to estimate accurately the quantity of medication used according to frequency of application. Another consideration was that topical corticosteroids are applied when people experience flare-ups, rather than continuously over time. Consequently, extrapolation over longer periods of time was not straightforward The Assessment Group provided a cost-minimisation analysis for nine of the ten included clinical trials. In this, once- use was the least costly option on six occasions and twice- use the least costly on three occasions. The wide range of topical corticosteroid products available and their varied prices means that there are many possible prescribing scenarios. The availability of specifically marketed once- topical corticosteroids, which are priced much higher than other generic and proprietary products, makes a once- regimen more costly when these products are used. For example, where fluticasone propionate cream ( 4.59) or mometasone furoate ( 4.22) once is substituted for betamethasone valerate ( 1.31), betamethasone dipropionate ( 2.05) or hydrocortisone butyrate ( 2.38) twice, the once regimen would be expected to cost more than the twice- regimen The trial examining fluticasone propionate (ointment) showed a benefit associated with twice- use in terms of physician assessment (but not for patient assessment) of patients target area of atopic eczema. Consequently, a simple estimate of cost effectiveness was made. This found the additional Issue date: April 2004 Page 13 of 34
14 cost per treatment success to be The assumptions underlying this analysis were generous and a more realistic estimate of the treatment cost per additional successfully treated flare-up would probably be half that value. The Assessment Group concluded that the greater likelihood of treatment success (that is, successfully treated flare-up) would be of sufficient value (in terms of patient benefit, and avoided GP consultations, referrals to specialists or prescribing of more expensive products) to regard twice- application as cost effective. 4.3 Consideration of the evidence The Committee reviewed the data available on the clinical and cost effectiveness of the frequency of application of topical corticosteroids for atopic eczema, having considered evidence on the nature of the condition and the value placed on the benefits of different frequencies of application of topical corticosteroids by people with atopic eczema, those who represent them, and clinical experts. It was also mindful of the need to take account of the effective use of NHS resources The Committee considered the various factors that might influence the frequency of application of topical corticosteroids for atopic eczema. These included the clinical presentation, factors influencing concordance with treatment, and patient choice. It heard from the experts that the potency of corticosteroid was not a relevant factor in determining the frequency of application Additionally, the Committee appreciated that people with eczema may have considerable fear of the use of corticosteroids, and also need to use a number of other measures to manage their condition on a basis. On the basis of expert testimony, concordance with once- or twice- application of topical corticosteroids is not of particular concern to patients because of the fact that they have to apply emollients regularly to manage their condition. The Committee was informed that good-quality patient education on the use of topical corticosteroids was a significant factor in ensuring the success of Issue date: April 2004 Page 14 of 34
15 therapy. The Committee was also informed that there was a clear need for continuing education of healthcare professionals to ensure that correct advice on the use of topical corticosteroids is given to people with atopic eczema The Committee reviewed the evidence related to the frequency of application of topical corticosteroids in atopic eczema. It considered that the RCTs available were, in general, of poor methodological quality, and it was advised by the experts that longer follow-up months, not weeks would be required of trials to assess fully any potential differences in long-term efficacy and adverse effects between once- and more frequent applications of topical corticosteroids. The Committee additionally appreciated that there may be differences in the pharmacokinetics of the individual topical corticosteroids, but it was persuaded that these differences, if of clinical significance, would be reflected in the clinical effectiveness evidence The Committee was informed that differences exist in clinical practice, between clinicians, in the prescription of once- or more frequent use of topical corticosteroids. However, it was agreed by the experts that, where once- application of a topical corticosteroid was initially advised, clinicians would have to increase either the potency or the frequency of the topical corticosteroid, if there was no improvement in the condition. Alternatively, if twice- application was advised initially for a flare-up, it would be expected that people would reduce the frequency of application of the same product once their condition began to improve Having considered the results from the RCTs, as well as the testimony from the expert witnesses, the Committee concluded that there was no compelling evidence of a clinically significant difference between once- application and more frequent application of topical corticosteroids in terms of their effectiveness, patient satisfaction, adverse events, concordance with therapy or the number of follow-up visits required. It was persuaded that current clinical practice would therefore support a recommendation for the use of topical corticosteroids no more frequently than twice. Issue date: April 2004 Page 15 of 34
16 4.3.7 The Committee concluded that, on the basis of the consideration in Section 4.3.6, where more than one alternative topical corticosteroid is considered clinically appropriate within a potency class, the product with the lowest acquisition cost (taking into account pack size and frequency of application) should be used in preference to more expensive alternatives. From the costminimisation analysis presented, the Committee noted that because of the acquisition cost of some products licensed solely for once- application, in some product comparisons, twice- application of other products was less costly than once- application. 5 Recommendations for further research 5.1 The trial literature is dominated by comparisons of differing frequency of use of fluticasone propionate (four trials) and comparisons of mometasone furoate with more traditional twice- treatment options (three trials). Trials are needed to establish whether once- use of the older (twice-) products is equivalent to more frequent use. Trials are also required to establish whether once- use of the older twice products is equivalent to the once--only products. Trials are required for all the potency classes, in particular for mild potency preparations, as no trials examining frequency of application of topical corticosteroids exist for this group. 5.2 Robust trials are required that report quality-of-life data and patient preferences. 5.3 Long-term follow-up is required in trials to assess adverse effects such as skin atrophy. 5.4 The experts informed the Committee that there was a lack of support for people with the condition and inadequate information about the management of atopic eczema and the risks associated with the use of topical corticosteroids. Research should therefore be conducted to establish the most Issue date: April 2004 Page 16 of 34
17 suitable method of conveying high-quality information to people with atopic eczema. 6 Implications for the NHS 6.1 Information is not readily available on current prescribing patterns of topical corticosteroids in patients with atopic eczema. There is also limited information on the quantity of product used per treatment regimen. Consequently, it is not possible, with any certainty, to establish baseline information on which to base estimates of the resource impact of changes in prescribing between preparations of different acquisition costs. Furthermore, such cost savings will be relatively small at the patient level, and issues related to pack size and product waste can easily erode any potential cost saving. However, given the large patient group with atopic eczema, there may be opportunities for significant savings to the NHS on products prescribed, particularly at a primary care level, as this is where most prescribing of topical corticosteroids is likely to occur. 6.2 An illustrative scenario is explored below. The estimate is based on a number of assumptions used in the calculations, and so should be interpreted cautiously. The underlying assumptions are that patients have two to four flare-ups a year, that they throw away any unused products after each flareup, and that patients applying topical corticosteroids once would use either 50% or 75% of the amount they would use if they were applying the product twice. These potential savings assume that all the patient prescription costs are met by the NHS. In practice, however, many patients may receive only one prescription per year, as they may not discard their unused products. Consequently, the figures in the scenario below are likely to be an estimate of the maximum cost savings to the NHS. 6.3 Where a prescribing practice of one of the newer once--only products can appropriately be altered to twice- use of one of the older, cheaper topical corticosteroids of the same potency, cost savings have been estimated Issue date: April 2004 Page 17 of 34
18 to range from 300,000 to 600,000 (excluding VAT) for a patient group of 100,000 people with atopic eczema. 7 Implementation and audit 7.1 All clinicians who care for people with atopic eczema should review their current practice and policies to take account of the guidance set out in Section Local guidelines or care pathways for people with atopic eczema should incorporate the guidance. 7.3 To measure compliance locally with the guidance, the following criteria could be used. Further details on suggestions for audit are presented in Appendix C Topical corticosteroids for atopic eczema are prescribed for application only once or twice If more than one alternative topical corticosteroid is considered clinically appropriate within a potency class, the drug with the lowest acquisition cost is prescribed. 7.4 Local clinical audits could also include measurement of compliance with recognised guidelines for the management of atopic eczema and the effectiveness of patient education on the use of topical corticosteroids. 8 Related guidance 8.1 The clinical and cost effectiveness of tacrolimus and pimecrolimus compared with topical corticosteroids is currently being appraised by NICE, and guidance is due to be published in September Issue date: April 2004 Page 18 of 34
19 9 Review of guidance 9.1 The review date for a technology appraisal refers to the month and year in which the Guidance Executive will consider any new evidence on the technology, in the form of an updated Assessment Report, and decide whether the technology should be referred to the Appraisal Committee for review. 9.2 The guidance on this technology will be reviewed in July David Barnett Chair, Appraisal Committee April 2004 Issue date: April 2004 Page 19 of 34
20 Appendix A. Appraisal Committee members NOTE The Appraisal Committee is a standing advisory committee of the Institute. Its members are appointed for a 3-year term. A list of the Committee members who took part in the discussions for this appraisal appears below. The Appraisal Committee meets twice a month except in December, when there are no meetings. The Committee membership is split into three branches, with the chair, vice-chair and a number of other members attending meetings of all branches. Each branch considers its own list of technologies and ongoing topics are not moved between the branches. Committee members are asked to declare any interests in the technology to be appraised. If it is considered there is a conflict of interest, the member is excluded from participating further in that appraisal. The minutes of each Appraisal Committee meeting, which include the names of the members who attended and their declarations of interests, are posted on the NICE website. Dr A E Ades Senior Scientist, MRC Health Services Research Collaboration, University of Bristol Dr Tom Aslan General Practitioner, Stockwell, London Professor David Barnett (Chair) Professor of Clinical Pharmacology, University of Leicester Professor Rosamund Bryar Professor of Community & Primary Care Nursing, St Bartholomew School of Nursing and Midwifery Dr Rodney Burnham Consultant Physician & Gastroenterologist, Oldchurch Hospital, Romford Dr Gary Butler Consultant Paediatrician/Endocrinologist, Leeds Teaching Hospitals NHS Trust Issue date: April 2004 Page 20 of 34
21 Dr Karl Claxton Health Economist, University of York Dr Christopher Eccleston Director, Pain Management Unit, Department of Psychology, University of Bath Ms Bethan George Interface Liaison Pharmacist, Mile End Hospital, London Mr John Goulston Director of Finance, Barts and the London NHS Trust Mr Adrian Griffin Health Outcomes Manager, Johnson & Johnson Medical Ltd Judith Paget Chief Executive, Caerphilly Local Health Board Dr Katherine Payne Research Fellow, Health Economics, University of Manchester Mrs Kathryn Roberts Nurse Practitioner, Hyde, Cheshire Ms Anne Smith Lay Representative; Trustee, Long-Term Medical Conditions Alliance Professor Andrew Stevens (Vice-Chair) Professor of Public Health, University of Birmingham Dr Cathryn Thomas General Practitioner, and Senior Lecturer, Department of Primary Care & General Practice, University of Birmingham Dr Norman Vetter Reader, Department of Epidemiology, Statistics and Public Health, College of Medicine, University of Wales, Cardiff Dr Paul Watson Medical Director, Essex Strategic Health Authority Issue date: April 2004 Page 21 of 34
22 NICE Project Team Each appraisal of a technology is assigned to a Health Technology Analyst and a Technology Appraisal Project Manager within the Institute. Joanna Richardson, Health Technology Analyst Kathleen Dalby, Technology Appraisal Project Manager Issue date: April 2004 Page 22 of 34
23 Appendix B. Sources of evidence considered by the Committee The following documentation and opinions were made available to the Committee: A The Assessment Report for this appraisal was prepared by Southampton Health Technology Assessment Centre. Green C, Colquitt JL, Kirby J et al. Clinical and cost-effectiveness of once versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation, November B The following organisations accepted the invitation to participate in this appraisal. They were invited to make submissions and comment on the draft scope, Assessment Report and the Appraisal Consultation Document (ACD). Consultee organisations are provided with the opportunity to appeal against the. I Manufacturer/sponsors: Dermal Laboratories GlaxoSmithKline Novartis Pliva Pharma Schering-Plough Stiefel Laboratories (UK) Typharm Waymade Healthcare Yamanouchi II Professional/specialist, patient/carer and other groups: British Association of Dermatologists British Generic Manufacturers' Association Department of Health Issue date: April 2004 Page 23 of 34
24 Hounslow Primary Care Trust National Eczema Society Primary Care Dermatology Society Royal College of General Practitioners Royal College of Nursing Royal College of Physicians Royal Pharmaceutical Society of Great Britain Skin Care Campaign Welsh Assembly Government III Commentator organisations (without the right of appeal): NHS Confederation NHS Purchasing and Supply Agency NHS Quality Improvement Scotland Skin Treatment and Research Trust C The following individuals were selected from clinical expert and patient advocate nominations from the professional/specialist and patient/carer groups. They participated in the Appraisal Committee discussions and provided evidence to inform the Appraisal Committee s deliberations. They gave their expert personal view on the frequency of application of topical corticosteroids for atopic eczema by attending the initial Committee discussion and/or providing written evidence to the Committee. They were also invited to comment on the ACD. Ms Sandra Lawton, Nurse Consultant Dermatology, Queen s Medical Centre, Nottingham Dr Celia Moss, Consultant Dermatologist, Birmingham Children s Hospital Ms Sue Ward, Information and Education Manager, National Eczema Society Issue date: April 2004 Page 24 of 34
25 Appendix C. Detail on criteria for audit of the frequency of application of topical corticosteroids for atopic eczema Possible objectives for an audit An audit could be carried out to ensure the appropriateness of prescription of topical corticosteroids for atopic eczema. Possible patients to be included in the audit An audit could be carried out on all patients seen for atopic eczema in a reasonable period for audit, for example, 6 months, who are prescribed topical corticosteroids but not those topical agents that combine corticosteroids with other active agents (for example, antimicrobials or salicylic acid). Measures that could be used as a basis for an audit The measures that could be used in an audit of the prescription of topical corticosteroids for atopic eczema are as follows. Issue date: April 2004 Page 25 of 34
26 Criterion Standard Exception Definition of terms 1. Topical corticosteroids for atopic eczema are prescribed for application only once or twice 2. If more than one alternative topical corticosteroid is considered clinically appropriate within a potency class, the drug with the lowest acquisition cost is prescribed 100% of people for whom topical corticosteroids for atopic eczema are prescribed 100% of people who are prescribed topical corticosteroids for atopic eczema None None The diagnosis of atopic eczema is established by the person having an itchy skin condition in the last 12 months plus three or more of the following: history of flexural involvement (that is, affecting the bends of the elbow or behind the knees); history of a generally dry skin; personal history of other atopic disease (in children under 4 years, history of atopic disease in a first-degree relative may be included); visible flexural dermatitis as defined by a photographic protocol; and onset below the age of 2 years (not used in children under 4 years). For a list of preparations of topical corticosteroids that are relevant for this measure, see Appendix D. Clinicians will need to agree locally on how the lowest acquisition cost is determined for audit purposes, taking into account pack size and frequency of application. See Appendix D for a list of preparations by potency class. Calculation of compliance Compliance (%) with each measure described in the table above is calculated as follows. Issue date: April 2004 Page 26 of 34
27 Number of patients whose care is consistent with the criterion plus number of patients who meet any exception listed Number of patients to whom the measure applies 100 Clinicians should review the findings of measurement, identify whether practice can be improved, agree on a plan to achieve any desired improvement and repeat the measurement of actual practice to confirm that the desired improvement is being achieved. Issue date: April 2004 Page 27 of 34
28 Appendix D. Topical corticosteroids for the treatment of atopic eczema, grouped by potency BNF chemical name Manufacturer Product Name ( ) Posology from SmPCs, where available Mild potency Hydrocortisone Alpharma BCM Specials Bell Sons & Co. (Druggists) Bioglan Laboratories Bioglan Pharmaceuticals Biorex Laboratories Co-Pharma Diomed Developments Galpharm Healthcare Lagap Pharmaceuticals Norton Pharmaceuticals Novartis Pinewood Laboratories Reckitt Benckiser Healthcare (UK) Roussel Laboratories Thornton & Ross Waymade Generic hydrocortisone cream 0.5%, 1%, ointment 0.5%, 1% Hydrocortisone GlaxoSmithKline Efcortelan cream/ointment 0.5%, 1%, 2.5% Hydrocortisone Yamanouchi Mildison Lipocream 1% Hydrocortisone Dermal Dioderm cream 0.1% Fluocinolone acetonide GP Pharma Synalar cream 1/10, % Net cost a per 30 g/30 ml b for each strength (does not include VAT or dispensing fee) N/A 0.66, 0.74, 0.65, times 2 3 times 0.66, 0.81, Twice times 1.15 Moderate potency Alclometasone dipropionate Pliva Modrasone cream/ointment 0.05% 2 3 times 1.69 Issue date: April 2004 Page 28 of 34
29 Betamethasone valerate GlaxoSmithKline Betnovate RD cream/ointment 0.025% Clobetasone butyrate GlaxoSmithKline Eumovate cream/ointment 0.05% Desoximetasone Stiefel Stiedex LP oily cream 0.05% Fluocinolone acetonide GP Pharma Synalar cream/ointment 1/4, % Fluocortolone Meadow Ultralanum Plain cream/ointment 0.25% Fludroxycortide Typharm Haelan cream/ointment % Potent Beclometasone dipropionate Betamethasone dipropionate Betamethasone valerate Betamethasone valerate Betamethasone valerate Betamethasone valerate GlaxoSmithKline Schering Plough GlaxoSmithKline Celltech Dermal Dowelhurst, Futuna Propaderm cream/ointment 0.025% Diprosone cream/ointment 0.05%, lotion 0.05% Betnovate cream/ointment 0.1%, lotion 0.1%, scalp application 0.1% Bettamousse foam 0.12% Betacap scalp application 0.1% Generic betamethasone valerate cream 0.1%, ointment 0.1% Diflucortolone valerate Meadow Nerisone cream/ointment 0.1%, oily cream 0.1% Fluocinolone acetonide GP Pharma Synalar cream/ointment 0.025%, gel 0.025% Fluocinonide GP Pharma Metosyn FAPG cream 0.05%, ointment 0.05% Fluticasone propionate GlaxoSmithKline Cutivate cream 0.05% 2 3 times Up to 4 times 2 3 times 2 3 times N/A times 1.63 Twice times 2 3 times Twice 2.25 Twice , , 1.57, 1.71 N/A 1.54, 1.69 N/A 1.59, times 3 4 times Once , , 1.52 Issue date: April 2004 Page 29 of 34
30 Fluticasone propionate GlaxoSmithKline Cutivate ointment 0.05% Twice 4.59 Hydrocortisone butyrate Hydrocortisone butyrate Hydrocortisone butyrate Yamanouchi Yamanouchi Locoid Lipocream 0.1% Locoid cream/ointment 0.1%, scalp lotion 0.1% 2 3 times 2 4 times Yamanouchi Locoid Crelo 0.1% 2 3 times Mometasone furoate Schering Plough Elocon cream/ointment 0.1%, scalp lotion 0.1% Very potent Clobetasol propionate GlaxoSmithKline Dermovate cream/ointment 0.05%, scalp application 0.05% Diflucortolone valerate Schering Health/Meadow Nerisone Forte ointment/oily cream 0.3% Halcinonide Bristol-Myers Squibb Halciderm cream 0.1% a Taken from BNF 47 (March 2004) , Once 4.22, times N/A times 2.48, b Using largest pack sizes available (for example, where 100 g is the largest pack size, the cost is calculated using the 100 g price multiplied by 0.3) N/A not available Issue date: April 2004 Page 30 of 34
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) report Title and TA publication number of static topic: Final decision:
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) report Title and TA publication number of static topic: Final decision:
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Review proposal of NICE guidance no. 82; on the use of pimecrolimus and tacrolimus for atopic eczema Provisional matrix
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Review proposal of NICE guidance no. 82; on the use of pimecrolimus and tacrolimus for atopic eczema Provisional matrix
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Review Proposal of NICE Technology Appraisal no. 81; on the frequency of application of topical Provisional matrix of consultees
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Review Proposal of NICE Technology Appraisal no. 81; on the frequency of application of topical Provisional matrix of consultees
Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82
 Tacrolimus and pimecrolimus for atopic eczema Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Tacrolimus and pimecrolimus for atopic eczema Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Children s Hospital Of Wisconsin
 Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Medication Policy Manual. Topic: Dupixent, dupilumab Date of Origin: March 10, Committee Approval: March 10, 2017 Next Review Date: May 2018
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
RELEVANT DISCLOSURES ATOPIC DERMATITIS / ECZEMA MANAGING ECZEMA IN INFANTS AND CHILDREN
 RELEVANT DISCLOSURES MANAGING ECZEMA IN INFANTS AND CHILDREN Advisory board member - MEDA (Elidel), Speaking honoraria Bayer (Advantan) Advisory board, consultant, speaker: Pfizer, Abbvie, Janssen, Elli
RELEVANT DISCLOSURES MANAGING ECZEMA IN INFANTS AND CHILDREN Advisory board member - MEDA (Elidel), Speaking honoraria Bayer (Advantan) Advisory board, consultant, speaker: Pfizer, Abbvie, Janssen, Elli
Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD)
 Diagnosis: ATOPIC DERMATITIS (AD) Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD) PATIENT ADVICE: Unfortunately, there is no cure for atopic dermatitis, so
Diagnosis: ATOPIC DERMATITIS (AD) Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD) PATIENT ADVICE: Unfortunately, there is no cure for atopic dermatitis, so
15 minute eczema consultation
 THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
The safety and effectiveness of Dupixent in pediatric patients have not been established (1).
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.30 Subject: Dupixent Page: 1 of 6 Last Review Date: September 15, 2017 Dupixent Description Dupixent
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.30 Subject: Dupixent Page: 1 of 6 Last Review Date: September 15, 2017 Dupixent Description Dupixent
New Medicine Report. Pimecrolimus. RED- Hospital only Date of Last Revision 6 th March 2003
 New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
TCIs are only available on prescription and are usually started by a dermatology specialist.
 (TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
(TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) Title and TA publication number of static topic: Final decision: TA82;
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) Title and TA publication number of static topic: Final decision: TA82;
Dupixent (dupilumab)
 Dupixent (dupilumab) Line(s) of Business: HMO; PPO; QUEST Integration Effective Date: TBD POLICY A. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
Dupixent (dupilumab) Line(s) of Business: HMO; PPO; QUEST Integration Effective Date: TBD POLICY A. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
Rituximab for aggressive non- Hodgkin's lymphoma
 Rituximab for aggressive non- Hodgkin's lymphoma Issued: September 2003 NICE technology appraisal guidance 65 guidance.nice.org.uk/ta65 NICE 2003 Contents 1 Guidance... 3 2 Clinical need and practice...
Rituximab for aggressive non- Hodgkin's lymphoma Issued: September 2003 NICE technology appraisal guidance 65 guidance.nice.org.uk/ta65 NICE 2003 Contents 1 Guidance... 3 2 Clinical need and practice...
Guidance on the use of zaleplon, zolpidem and zopiclone for the short-term management of insomnia
 Guidance on the use of zaleplon, zolpidem and zopiclone for the Issued: April 2004 guidance.nice.org.uk/ta77 NICE 2004 Contents 1 Guidance... 3 2 Clinical need and practice... 4 3 The technologies... 8
Guidance on the use of zaleplon, zolpidem and zopiclone for the Issued: April 2004 guidance.nice.org.uk/ta77 NICE 2004 Contents 1 Guidance... 3 2 Clinical need and practice... 4 3 The technologies... 8
ATOPIC ECZEMA. What are the aims of this leaflet?
 ATOPIC ECZEMA What are the aims of this leaflet? This leaflet has been written to help you understand more about atopic eczema. It tells you what it is, what causes it, what can be done about it, and where
ATOPIC ECZEMA What are the aims of this leaflet? This leaflet has been written to help you understand more about atopic eczema. It tells you what it is, what causes it, what can be done about it, and where
Royal College of Radiologists (RCR) Referral guidelines. Final Accreditation Report. Guidance producer: Guidance product: Date: 29 June 2010
 Guidance producer: Royal College of Radiologists (RCR) Guidance product: Referral guidelines Date: 29 June 2010 Final Accreditation Report Contents Introduction... 3 Accreditation recommendation... 3 Implementation...
Guidance producer: Royal College of Radiologists (RCR) Guidance product: Referral guidelines Date: 29 June 2010 Final Accreditation Report Contents Introduction... 3 Accreditation recommendation... 3 Implementation...
Eucrisa. Eucrisa (crisaborole) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.25 Subject: Eucrisa Page: 1 of 6 Last Review Date: September 15, 2017 Eucrisa Description Eucrisa
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.25 Subject: Eucrisa Page: 1 of 6 Last Review Date: September 15, 2017 Eucrisa Description Eucrisa
Eucrisa. Eucrisa (crisaborole) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Eucrisa Page: 1 of 7 Last Review Date: June 22, 2018 Eucrisa Description Eucrisa (crisaborole)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Eucrisa Page: 1 of 7 Last Review Date: June 22, 2018 Eucrisa Description Eucrisa (crisaborole)
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2116-3 Program Prior Authorization/Medical Necessity Medications Dupixent (dupilumab) P&T Approval Date 1/2017, 5/2017, 7/2017
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2116-3 Program Prior Authorization/Medical Necessity Medications Dupixent (dupilumab) P&T Approval Date 1/2017, 5/2017, 7/2017
Technology appraisal guidance Published: 23 August 2006 nice.org.uk/guidance/ta105
 Laparoscopic surgery for colorectal cancer Technology appraisal guidance Published: 23 August 2006 nice.org.uk/guidance/ta105 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Laparoscopic surgery for colorectal cancer Technology appraisal guidance Published: 23 August 2006 nice.org.uk/guidance/ta105 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
RECLASSIFICATION SUBMISSION. EUMOVATE Eczema and Dermatitis Cream. (Clobetasone butyrate 0.05%) From Prescription Only Medicine
 RECLASSIFICATION SUBMISSION EUMOVATE Eczema and Dermatitis Cream (Clobetasone butyrate 0.05%) From Prescription Only Medicine To Pharmacist Only Medicine NOVEMBER 2000 MEETING PART A 1. International non-proprietary
RECLASSIFICATION SUBMISSION EUMOVATE Eczema and Dermatitis Cream (Clobetasone butyrate 0.05%) From Prescription Only Medicine To Pharmacist Only Medicine NOVEMBER 2000 MEETING PART A 1. International non-proprietary
Atopic Eczema with detail on how to apply wet wraps
 Atopic Eczema with detail on how to apply wet wraps Dr Carol Hlela Consultant Dermatologist Head of Unit, Department of Dermatology, Paediatrics Red Cross Children s Hospital, UCT Red Cross War Memorial
Atopic Eczema with detail on how to apply wet wraps Dr Carol Hlela Consultant Dermatologist Head of Unit, Department of Dermatology, Paediatrics Red Cross Children s Hospital, UCT Red Cross War Memorial
Technology appraisal guidance Published: 12 July 2017 nice.org.uk/guidance/ta456
 Ustekinumab for moderately to severelyerely active Crohn s disease after previous treatment Technology appraisal guidance Published: 12 July 2017 nice.org.uk/guidance/ta456 NICE 2017. All rights reserved.
Ustekinumab for moderately to severelyerely active Crohn s disease after previous treatment Technology appraisal guidance Published: 12 July 2017 nice.org.uk/guidance/ta456 NICE 2017. All rights reserved.
過敏病科中心. Allergy Centre. Eczema. Allergy Centre 過敏病科中心. Allergy Centre. For enquiries and appointments, please contact us at:
 Allergy Centre 過敏病科中心 Eczema For enquiries and appointments, please contact us at: Allergy Centre 9/F, Li Shu Pui Block Hong Kong Sanatorium & Hospital 2 Village Road, Happy Valley, Hong Kong Tel: 2835
Allergy Centre 過敏病科中心 Eczema For enquiries and appointments, please contact us at: Allergy Centre 9/F, Li Shu Pui Block Hong Kong Sanatorium & Hospital 2 Village Road, Happy Valley, Hong Kong Tel: 2835
LRI Children s Hospital
 Atopic Eczema Care LRI Children s Hospital Staff relevant to: Clinical staff working within the UHL Children s Hospital. Team approval date: May 2017 Version: V 4 Revision due: May 2020 Written by: K.
Atopic Eczema Care LRI Children s Hospital Staff relevant to: Clinical staff working within the UHL Children s Hospital. Team approval date: May 2017 Version: V 4 Revision due: May 2020 Written by: K.
Dermatology Round Up
 Dermatology Round Up Journal of Family Health Care Live Conference 25 March 2014 Julie Van Onselen Independent Dermatology Nurse, Oxford And Rachael Fagg, Mother Introduction 10.00 10.30hrs: Julie Van
Dermatology Round Up Journal of Family Health Care Live Conference 25 March 2014 Julie Van Onselen Independent Dermatology Nurse, Oxford And Rachael Fagg, Mother Introduction 10.00 10.30hrs: Julie Van
Final appraisal determination. Peginterferon alfa and ribavirin for the treatment of mild chronic hepatitis C
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Final appraisal determination Peginterferon alfa and ribavirin for the treatment of mild chronic hepatitis C NICE issued guidance on the use of interferon
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Final appraisal determination Peginterferon alfa and ribavirin for the treatment of mild chronic hepatitis C NICE issued guidance on the use of interferon
Steroid use in managing your child s Atopic Eczema
 Steroid use in managing your child s Atopic Eczema Clinical Nurse Specialist for Paediatric Dermatology (01284) 713575 Step up step down approach: Addressograph Severe Call your General Practitioner (GP)
Steroid use in managing your child s Atopic Eczema Clinical Nurse Specialist for Paediatric Dermatology (01284) 713575 Step up step down approach: Addressograph Severe Call your General Practitioner (GP)
MALE GENITAL (PENIS) LICHEN SCLEROSUS
 MALE GENITAL (PENIS) LICHEN SCLEROSUS What are the aims of this leaflet? This leaflet has been written to help you understand more about male genital lichen sclerosus (also known as balanitis xerotica
MALE GENITAL (PENIS) LICHEN SCLEROSUS What are the aims of this leaflet? This leaflet has been written to help you understand more about male genital lichen sclerosus (also known as balanitis xerotica
Ustekinumab for the treatment of moderate to severe psoriasis
 DOI: 10.3310/hta13suppl3/10 Health Technology Assessment 2009; Vol. 13: Suppl. 3 Ustekinumab for the treatment of moderate to severe psoriasis E Gospodarevskaya, J Picot, K Cooper, E Loveman* and A Takeda
DOI: 10.3310/hta13suppl3/10 Health Technology Assessment 2009; Vol. 13: Suppl. 3 Ustekinumab for the treatment of moderate to severe psoriasis E Gospodarevskaya, J Picot, K Cooper, E Loveman* and A Takeda
Ustekinumab for the treatment of adults with moderate to severe psoriasis
 Ustekinumab for the treatment of adults with moderate to severe psoriasis Issued: September 2009 guidance.nice.org.uk/ta180 NICE has accredited the process used by the Centre for Health Technology Evaluation
Ustekinumab for the treatment of adults with moderate to severe psoriasis Issued: September 2009 guidance.nice.org.uk/ta180 NICE has accredited the process used by the Centre for Health Technology Evaluation
Technology appraisal guidance Published: 24 January 2007 nice.org.uk/guidance/ta116
 Gemcitabine for the treatment of metastatic breast cancer Technology appraisal guidance Published: 24 January 2007 nice.org.uk/guidance/ta116 NICE 2018. All rights reserved. Subject to Notice of rights
Gemcitabine for the treatment of metastatic breast cancer Technology appraisal guidance Published: 24 January 2007 nice.org.uk/guidance/ta116 NICE 2018. All rights reserved. Subject to Notice of rights
Pharmacy Benefit Determination Policy
 Policy Subject: Atopic Dermatitis Agents Policy Number: SHS PBD18 Category: Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual HMO/POS PPO ASO s:
Policy Subject: Atopic Dermatitis Agents Policy Number: SHS PBD18 Category: Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual HMO/POS PPO ASO s:
Pharmacologic Treatment of Atopic Dermatitis
 J KMA Pharmacotherapeutics Pharmacologic Treatment of Atopic Dermatitis Chun Wook Park, MD Department of Dermatology, Hallym University College of Medicine E mail : dermap@paran.com J Korean Med Assoc
J KMA Pharmacotherapeutics Pharmacologic Treatment of Atopic Dermatitis Chun Wook Park, MD Department of Dermatology, Hallym University College of Medicine E mail : dermap@paran.com J Korean Med Assoc
Peginterferon alfa and ribavirin for the treatment of mild chronic hepatitis C
 Issue date: August 2006 Review date: November 2007 Peginterferon alfa and ribavirin for the treatment of mild chronic hepatitis C This is an extension of the guidance given in NICE technology appraisal
Issue date: August 2006 Review date: November 2007 Peginterferon alfa and ribavirin for the treatment of mild chronic hepatitis C This is an extension of the guidance given in NICE technology appraisal
Technology appraisal guidance Published: 27 October 2004 nice.org.uk/guidance/ta86
 Imatinib for the treatment of unresectable and/or metastatic gastro- intestinal stromal tumours Technology appraisal guidance Published: 27 October 2004 nice.org.uk/guidance/ta86 NICE 2018. All rights
Imatinib for the treatment of unresectable and/or metastatic gastro- intestinal stromal tumours Technology appraisal guidance Published: 27 October 2004 nice.org.uk/guidance/ta86 NICE 2018. All rights
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE. Final Appraisal Determination
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE of acute mania associated with bipolar I disorder 1 1 Guidance 1.1 Olanzapine and valproate semisodium, within their licensed indications, are recommended as
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE of acute mania associated with bipolar I disorder 1 1 Guidance 1.1 Olanzapine and valproate semisodium, within their licensed indications, are recommended as
Technology appraisal guidance Published: 23 August 2006 nice.org.uk/guidance/ta106
 Peginterferon alfa and ribavirin for the treatment of mild chronic hepatitis C Technology appraisal guidance Published: 23 August 2006 nice.org.uk/guidance/ta106 NICE 2017. All rights reserved. Subject
Peginterferon alfa and ribavirin for the treatment of mild chronic hepatitis C Technology appraisal guidance Published: 23 August 2006 nice.org.uk/guidance/ta106 NICE 2017. All rights reserved. Subject
Teledermatology Paediatric eczema. Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital
 Teledermatology Paediatric eczema Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital NHS e-referral teledermatology Rapid access to diagnosis / management advice from local integrated
Teledermatology Paediatric eczema Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital NHS e-referral teledermatology Rapid access to diagnosis / management advice from local integrated
An Everyday Guide to Eczema
 An Everyday Guide to Eczema By Dr. Kristel Polder, Board-Certified Dermatologist Developed in Partnership with Who is affected by eczema? 32 million people in the US 1 in 5 children 1 in 12 adults *www.eczema.org
An Everyday Guide to Eczema By Dr. Kristel Polder, Board-Certified Dermatologist Developed in Partnership with Who is affected by eczema? 32 million people in the US 1 in 5 children 1 in 12 adults *www.eczema.org
Primary Care Guidelines for the Management of Atopic Eczema 10/07100
 4 4 w Primary Care Guidelines for the Management of Atopic Eczema 10/07100 1.0 INTRODUCTION AND OBJECTIVES Primary care manages by far the largest number of patients with atopic eczema, the vast majority
4 4 w Primary Care Guidelines for the Management of Atopic Eczema 10/07100 1.0 INTRODUCTION AND OBJECTIVES Primary care manages by far the largest number of patients with atopic eczema, the vast majority
Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57
 Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2007. All rights reserved. Contents Introduction... 4 Child-centred care...
Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2007. All rights reserved. Contents Introduction... 4 Child-centred care...
Alteplase for the treatment of acute ischaemic stroke. This guidance was developed using the single technology appraisal process
 Issue date: June 2007 Review date: April 2010 Alteplase for the treatment of acute ischaemic stroke This guidance was developed using the single technology appraisal process NICE technology appraisal guidance
Issue date: June 2007 Review date: April 2010 Alteplase for the treatment of acute ischaemic stroke This guidance was developed using the single technology appraisal process NICE technology appraisal guidance
Technology appraisal guidance Published: 26 August 2009 nice.org.uk/guidance/ta177
 Alitretinoin for the treatment of severeere chronic hand eczema Technology appraisal guidance Published: 26 August 2009 nice.org.uk/guidance/ta177 NICE 2018. All rights reserved. Subject to Notice of rights
Alitretinoin for the treatment of severeere chronic hand eczema Technology appraisal guidance Published: 26 August 2009 nice.org.uk/guidance/ta177 NICE 2018. All rights reserved. Subject to Notice of rights
Dermatology Literary Review
 Emollients and ageing skin: optimising effectiveness and safety of Nursing, Vol. 25, No.11, pages 596-598. 6 Article outlines the causes of dryness in ageing skin and concludes that emollients are the
Emollients and ageing skin: optimising effectiveness and safety of Nursing, Vol. 25, No.11, pages 596-598. 6 Article outlines the causes of dryness in ageing skin and concludes that emollients are the
Technology appraisal guidance Published: 28 March 2018 nice.org.uk/guidance/ta516
 Cabozantinib for treating medullary thyroid cancer Technology appraisal guidance Published: 28 March 2018 nice.org.uk/guidance/ta516 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Cabozantinib for treating medullary thyroid cancer Technology appraisal guidance Published: 28 March 2018 nice.org.uk/guidance/ta516 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Technology appraisal guidance Published: 23 September 2009 nice.org.uk/guidance/ta180
 Ustekinumab for the treatment of adults with moderate to severe ere psoriasis Technology appraisal guidance Published: 23 September 2009 nice.org.uk/guidance/ta180 NICE 2017. All rights reserved. Subject
Ustekinumab for the treatment of adults with moderate to severe ere psoriasis Technology appraisal guidance Published: 23 September 2009 nice.org.uk/guidance/ta180 NICE 2017. All rights reserved. Subject
CHAPTER 1. Eczema Basics
 CHAPTER 1 Eczema Basics Definition Eczema is an inflammatory skin condition, characterised by ichtyosis (dry skin), erythema (redness), excoriation (interruption of the skin), scratching lesions, lichenification
CHAPTER 1 Eczema Basics Definition Eczema is an inflammatory skin condition, characterised by ichtyosis (dry skin), erythema (redness), excoriation (interruption of the skin), scratching lesions, lichenification
Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211
 Prucalopride for the treatment of chronic constipation in women Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211 NICE 2018. All rights reserved. Subject to Notice of
Prucalopride for the treatment of chronic constipation in women Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211 NICE 2018. All rights reserved. Subject to Notice of
Allergy Medications. Antihistamines. are very safe. Although usually taken as tablets, they may be prescribed as a liquid or syrup for young children
 The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient s health, mood and
The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient s health, mood and
Technology appraisal guidance Published: 6 September 2017 nice.org.uk/guidance/ta476
 Paclitaxel as albumin-bound nanoparticles with gemcitabine for untreated metastatic pancreatic cancer Technology appraisal guidance Published: 6 September 2017 nice.org.uk/guidance/ta476 NICE 2018. All
Paclitaxel as albumin-bound nanoparticles with gemcitabine for untreated metastatic pancreatic cancer Technology appraisal guidance Published: 6 September 2017 nice.org.uk/guidance/ta476 NICE 2018. All
PACKAGE LEAFLET: INFORMATION FOR THE USER. Flutarzole 0,05% w/w cream, Fluticasone propionate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
What s Topical About Topicals?
 What s Topical About Topicals? Megha M. Tollefson, MD Associate Professor of Dermatology and Pediatrics July 29, 2017 2015 MFMER 3513105-1 Disclosures None 2015 MFMER 3513105-2 Outline Topical steroids
What s Topical About Topicals? Megha M. Tollefson, MD Associate Professor of Dermatology and Pediatrics July 29, 2017 2015 MFMER 3513105-1 Disclosures None 2015 MFMER 3513105-2 Outline Topical steroids
Technology appraisal guidance Published: 26 April 2017 nice.org.uk/guidance/ta442
 Ixekizumab for treating moderate to severe ere plaque psoriasis Technology appraisal guidance Published: 26 April 2017 nice.org.uk/guidance/ta442 NICE 2017. All rights reserved. Subject to Notice of rights
Ixekizumab for treating moderate to severe ere plaque psoriasis Technology appraisal guidance Published: 26 April 2017 nice.org.uk/guidance/ta442 NICE 2017. All rights reserved. Subject to Notice of rights
TOPCORT Cream/Ointment (Mometasone furoate 0.1%)
 Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
Oseltamivir, amantadine (review) and zanamivir for the prophylaxis of influenza
 Oseltamivir, amantadine (review) and zanamivir for the prophylaxis of influenza Issued: September 2008 guidance.nice.org.uk/ta158 NICE has accredited the process used by the Centre for Health Technology
Oseltamivir, amantadine (review) and zanamivir for the prophylaxis of influenza Issued: September 2008 guidance.nice.org.uk/ta158 NICE has accredited the process used by the Centre for Health Technology
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Consideration of consultation responses on review proposal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Consideration of consultation responses on review proposal Review of TA114 Methadone and buprenorphine for the management of
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Consideration of consultation responses on review proposal Review of TA114 Methadone and buprenorphine for the management of
Technology appraisal guidance Published: 28 October 2009 nice.org.uk/guidance/ta183
 Topotecan for the treatment of recurrent and stage IVB cervical cancer Technology appraisal guidance Published: 28 October 2009 nice.org.uk/guidance/ta183 NICE 2018. All rights reserved. Subject to Notice
Topotecan for the treatment of recurrent and stage IVB cervical cancer Technology appraisal guidance Published: 28 October 2009 nice.org.uk/guidance/ta183 NICE 2018. All rights reserved. Subject to Notice
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Consideration of consultation responses on review proposal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Consideration of consultation responses on review proposal Review of TA96; Adefovir dipivoxil and peginterferon alfa-2a for
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Consideration of consultation responses on review proposal Review of TA96; Adefovir dipivoxil and peginterferon alfa-2a for
Area Drug and Therapeutics Committee Prescribing Supplement No 3 November 2003
 Area Drug and Therapeutics Committee Prescribing Supplement No 3 In this issue Drugs currently being considered by the SMC advice due on 8 December 2003. SMC Notice of meeting: Patient power Making a Difference,
Area Drug and Therapeutics Committee Prescribing Supplement No 3 In this issue Drugs currently being considered by the SMC advice due on 8 December 2003. SMC Notice of meeting: Patient power Making a Difference,
Appendix D Clinical specialist statement template
 Appendix D Colistimethate sodium powder and tobramycin powder for inhalation for the treatment of pseudomonas lung infection in cystic fibrosis Thank you for agreeing to give us a statement on your organisation
Appendix D Colistimethate sodium powder and tobramycin powder for inhalation for the treatment of pseudomonas lung infection in cystic fibrosis Thank you for agreeing to give us a statement on your organisation
Ezetimibe for the treatment of primary (heterozygousfamilial. hypercholesterolaemia. NICE technology appraisal guidance 132. Issue date: November 2007
 Issue date: November 2007 Review date: August 2010 Ezetimibe for the treatment of primary (heterozygousfamilial and non-familial) hypercholesterolaemia NICE technology appraisal guidance 132 NICE technology
Issue date: November 2007 Review date: August 2010 Ezetimibe for the treatment of primary (heterozygousfamilial and non-familial) hypercholesterolaemia NICE technology appraisal guidance 132 NICE technology
Royal College of Physicians (RCP) National Clinical Guidelines for Stroke. Final Accreditation Report. Guidance producer: Guidance product:
 Guidance producer: Royal College of Physicians (RCP) Guidance product: National Clinical Guidelines for Stroke Date: 29 June 2010 Final Accreditation Report Contents Contents... 2 Introduction... 3 Implementation...
Guidance producer: Royal College of Physicians (RCP) Guidance product: National Clinical Guidelines for Stroke Date: 29 June 2010 Final Accreditation Report Contents Contents... 2 Introduction... 3 Implementation...
Technology appraisal guidance Published: 24 February 2016 nice.org.uk/guidance/ta385
 Ezetimibe for treating primary heterozygous-familial and non-familial hypercholesterolaemia Technology appraisal guidance Published: 24 February 2016 nice.org.uk/guidance/ta385 NICE 2016. All rights reserved.
Ezetimibe for treating primary heterozygous-familial and non-familial hypercholesterolaemia Technology appraisal guidance Published: 24 February 2016 nice.org.uk/guidance/ta385 NICE 2016. All rights reserved.
Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57
 Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Surveillance report Published: 8 June 2017 nice.org.uk. NICE All rights reserved.
 Surveillance report 2017 Antenatal and postnatal mental health: clinical management and service guidance (2014) NICE guideline CG192 Surveillance report Published: 8 June 2017 nice.org.uk NICE 2017. All
Surveillance report 2017 Antenatal and postnatal mental health: clinical management and service guidance (2014) NICE guideline CG192 Surveillance report Published: 8 June 2017 nice.org.uk NICE 2017. All
Eczema & Dermatitis Clinical features: Histopathological features: Classification:
 Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Prescribing Information
 Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
The clinical effectiveness and costeffectiveness. treatment of chronic asthma in children under the age of 12 years
 The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
Professional organisation statement template
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Recommended management of eczema in older patients
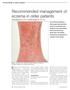 Recommended management of eczema in older patients Victoria Sherman MA, MRCP and Daniel Creamer BSc, MD, FRCP Our series Prescribing in older people gives practical advice for successful management of
Recommended management of eczema in older patients Victoria Sherman MA, MRCP and Daniel Creamer BSc, MD, FRCP Our series Prescribing in older people gives practical advice for successful management of
Single Technology Appraisal (STA) Tildrakizumab for treating moderate to severe plaque psoriasis
 Single Technology Appraisal (STA) Tildrakizumab for treating moderate to severe plaque psoriasis Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please
Single Technology Appraisal (STA) Tildrakizumab for treating moderate to severe plaque psoriasis Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please
Learning Circle: Jan 26, 2011 Childhood Eczema
 Learning Circle: Jan 26, 2011 Childhood Eczema Wingfield Rehmus, MD MPH BC Children s Hospital Clinical Assistant Professor, UBC Department of Paediatrics Associate Member, UBC Department of Dermatology
Learning Circle: Jan 26, 2011 Childhood Eczema Wingfield Rehmus, MD MPH BC Children s Hospital Clinical Assistant Professor, UBC Department of Paediatrics Associate Member, UBC Department of Dermatology
Inhaled corticosteroids for the treatment of chronic asthma in adults and in children aged 12 years and over
 Issue date: March 2008 Review date: November 2012 Inhaled corticosteroids for the treatment of chronic asthma in adults and in children aged 12 years and over NICE technology appraisal guidance 138 NICE
Issue date: March 2008 Review date: November 2012 Inhaled corticosteroids for the treatment of chronic asthma in adults and in children aged 12 years and over NICE technology appraisal guidance 138 NICE
Technology appraisal guidance Published: 28 November 2018 nice.org.uk/guidance/ta547
 Tofacitinib for moderately to severelyerely active ulcerative colitis Technology appraisal guidance Published: 28 November 2018 nice.org.uk/guidance/ta547 NICE 2019. All rights reserved. Subject to Notice
Tofacitinib for moderately to severelyerely active ulcerative colitis Technology appraisal guidance Published: 28 November 2018 nice.org.uk/guidance/ta547 NICE 2019. All rights reserved. Subject to Notice
OBSOLETE: REPLACED BY TA385
 Ezetimibe for the treatment of primary (heterozygous-familial and non-familial) hypercholesterolaemia Technology appraisal guidance Published: 28 November 2007 nice.org.uk/guidance/ta132 NICE 2007. All
Ezetimibe for the treatment of primary (heterozygous-familial and non-familial) hypercholesterolaemia Technology appraisal guidance Published: 28 November 2007 nice.org.uk/guidance/ta132 NICE 2007. All
Peginterferon alfa and ribavirin for the treatment of chronic hepatitis C. Part review of NICE technology appraisal guidance 75 and 106
 Issue date: September 2010 Peginterferon alfa and ribavirin for the treatment of chronic hepatitis C Part review of NICE technology appraisal guidance 75 and 106 National Institute for Health and Clinical
Issue date: September 2010 Peginterferon alfa and ribavirin for the treatment of chronic hepatitis C Part review of NICE technology appraisal guidance 75 and 106 National Institute for Health and Clinical
Managing and Minimizing Flare-ups in Atopic Dermatitis
 Managing and Minimizing Flare-ups in Atopic Dermatitis Importance of the skin barrier & how commonly used drugs are impacting it Dr. Benjamin Barankin, MD FRCPC Medical Director & Founder of Toronto Dermatology
Managing and Minimizing Flare-ups in Atopic Dermatitis Importance of the skin barrier & how commonly used drugs are impacting it Dr. Benjamin Barankin, MD FRCPC Medical Director & Founder of Toronto Dermatology
Technology appraisal guidance Published: 22 September 2010 nice.org.uk/guidance/ta200
 Peginterferon alfa and ribavirin for the treatment of chronic hepatitis C Technology appraisal guidance Published: 22 September 2010 nice.org.uk/guidance/ta200 NICE 2018. All rights reserved. Subject to
Peginterferon alfa and ribavirin for the treatment of chronic hepatitis C Technology appraisal guidance Published: 22 September 2010 nice.org.uk/guidance/ta200 NICE 2018. All rights reserved. Subject to
Case studies of children and adults with atopic eczema being treated under the Aron regime
 Case studies of children and adults with atopic eczema being treated under the Prepared by: Parents of patients of Dr Richard Aron (GMC ref no. 3151727) May 2015 Dr Aron s website: www.draron.com Dr Aron
Case studies of children and adults with atopic eczema being treated under the Prepared by: Parents of patients of Dr Richard Aron (GMC ref no. 3151727) May 2015 Dr Aron s website: www.draron.com Dr Aron
Skin disorders. Seborrhoeic dermatitis Search date April 2010 Luigi Naldi ...
 Seborrhoeic Search date April 21 Luigi Naldi.................................................. ABSTRACT INTRODUCTION: Seborrhoeic affects at least 1% of the population. Malassezia (Pityrosporum) ovale
Seborrhoeic Search date April 21 Luigi Naldi.................................................. ABSTRACT INTRODUCTION: Seborrhoeic affects at least 1% of the population. Malassezia (Pityrosporum) ovale
Guidance on the use of paclitaxel in the treatment of ovarian cancer
 Guidance on the use of paclitaxel in the treatment of Issued: January 2003 last modified: May 2005 guidance.nice.org.uk/ta55 NICE 2003 Contents 1 Guidance... 3 2 Clinical need and practice... 4 3 The technology...
Guidance on the use of paclitaxel in the treatment of Issued: January 2003 last modified: May 2005 guidance.nice.org.uk/ta55 NICE 2003 Contents 1 Guidance... 3 2 Clinical need and practice... 4 3 The technology...
Technology appraisal guidance Published: 25 July 2007 nice.org.uk/guidance/ta123
 Varenicline for smoking cessation Technology appraisal guidance Published: 25 July 2007 nice.org.uk/guidance/ta123 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Varenicline for smoking cessation Technology appraisal guidance Published: 25 July 2007 nice.org.uk/guidance/ta123 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
KEY MESSAGES. Psoriasis patients are more prone to cardiovascular diseases, stroke, lymphoma and non-melanoma skin cancers, and increased mortality.
 KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
NOVOLIZER BUDESONIDE. Corticosteroids for the treatment of chronic asthma in children under the age of 12 years
 NOVOLIZER BUDESONIDE Corticosteroids for the treatment of chronic asthma in children under the age of 12 years Response to the assessment report produced by the Peninsula Technology Assessment group and
NOVOLIZER BUDESONIDE Corticosteroids for the treatment of chronic asthma in children under the age of 12 years Response to the assessment report produced by the Peninsula Technology Assessment group and
Constitutional eczema
 Patient information Constitutional eczema What is constitutional eczema? Constitutional eczema, also called atopic eczema, is a form of eczema that mainly occurs in childhood. Eczema usually starts before
Patient information Constitutional eczema What is constitutional eczema? Constitutional eczema, also called atopic eczema, is a form of eczema that mainly occurs in childhood. Eczema usually starts before
Assessing the Current Treatment of Atopic Dermatitis: Unmet Needs
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Technology appraisal guidance Published: 22 July 2009 nice.org.uk/guidance/ta174
 Rituximab for the first-line treatment of chronic lymphocytic leukaemia Technology appraisal guidance Published: 22 July 2009 nice.org.uk/guidance/ta174 NICE 2018. All rights reserved. Subject to Notice
Rituximab for the first-line treatment of chronic lymphocytic leukaemia Technology appraisal guidance Published: 22 July 2009 nice.org.uk/guidance/ta174 NICE 2018. All rights reserved. Subject to Notice
Kent and Medway Policy Recommendation and Guidance Committee. Policy Recommendation PR : Hyperhidrosis
 Kent and Medway Policy Recommendation and Guidance Committee. Policy Recommendation PR 2014-06: Hyperhidrosis Recommendation The Kent and Medway Policy Recommendation and Guidance Committee (PRGC) considered
Kent and Medway Policy Recommendation and Guidance Committee. Policy Recommendation PR 2014-06: Hyperhidrosis Recommendation The Kent and Medway Policy Recommendation and Guidance Committee (PRGC) considered
Technology appraisal guidance Published: 26 March 2008 nice.org.uk/guidance/ta138
 Inhaled corticosteroids for the treatment of chronic asthma in adults and in children aged 12 years and over Technology appraisal guidance Published: 26 March 2008 nice.org.uk/guidance/ta138 NICE 2018.
Inhaled corticosteroids for the treatment of chronic asthma in adults and in children aged 12 years and over Technology appraisal guidance Published: 26 March 2008 nice.org.uk/guidance/ta138 NICE 2018.
Technology appraisal guidance Published: 6 December 2017 nice.org.uk/guidance/ta493
 Cladribine tablets for treating relapsing remitting multiple sclerosis Technology appraisal guidance Published: 6 December 2017 nice.org.uk/guidance/ta493 NICE 2018. All rights reserved. Subject to Notice
Cladribine tablets for treating relapsing remitting multiple sclerosis Technology appraisal guidance Published: 6 December 2017 nice.org.uk/guidance/ta493 NICE 2018. All rights reserved. Subject to Notice
ECZEMA SOCIETY OF CANADA ATOPIC DERMATITIS: PATIENT INSIGHTS REPORT MILD-TO-MODERATE DISEASE
 ECZEMA SOCIETY OF CANADA ATOPIC DERMATITIS: PATIENT INSIGHTS REPORT MILD-TO-MODERATE DISEASE 1 OVERVIEW OF SURVEY RESULTS INTRODUCTION PATIENT INSIGHTS BY THE NUMBERS: Adult Atopic Dermatitis (AD) In the
ECZEMA SOCIETY OF CANADA ATOPIC DERMATITIS: PATIENT INSIGHTS REPORT MILD-TO-MODERATE DISEASE 1 OVERVIEW OF SURVEY RESULTS INTRODUCTION PATIENT INSIGHTS BY THE NUMBERS: Adult Atopic Dermatitis (AD) In the
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Psoriasis (excluding widespread plaque psoriasis) Psoriasis is a common chronic skin disorder. Estimates of the prevalence (proportion
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Psoriasis (excluding widespread plaque psoriasis) Psoriasis is a common chronic skin disorder. Estimates of the prevalence (proportion
Final Accreditation Report
 Guidance producer: Uveal Melanoma Guideline Development Group Guidance product: Uveal Melanoma National Guidelines Date: 24 November 2014 Version: 1.3 Final Accreditation Report Uveal Melanoma Guideline
Guidance producer: Uveal Melanoma Guideline Development Group Guidance product: Uveal Melanoma National Guidelines Date: 24 November 2014 Version: 1.3 Final Accreditation Report Uveal Melanoma Guideline
Guideline Ulcerative colitis: management
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline Ulcerative colitis: management Draft for consultation, December 0 This guideline covers the care and treatment of adults, children and young
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline Ulcerative colitis: management Draft for consultation, December 0 This guideline covers the care and treatment of adults, children and young
Contact Allergy Testing (Patch Testing) Information for parents and carers of children up to 12 years of age
 Contact Allergy Testing (Patch Testing) Information for parents and carers of children up to 12 years of age Dermatology Department The aim of this leaflet is to give you information about contact allergy
Contact Allergy Testing (Patch Testing) Information for parents and carers of children up to 12 years of age Dermatology Department The aim of this leaflet is to give you information about contact allergy
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Single Technology Appraisal (STA)
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE. Final Appraisal Determination. Continuous subcutaneous insulin infusion for diabetes
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guidance 1.1 Continuous subcutaneous insulin infusion (CSII or insulin pump therapy') is recommended as an option for people with type 1 diabetes provided that:
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guidance 1.1 Continuous subcutaneous insulin infusion (CSII or insulin pump therapy') is recommended as an option for people with type 1 diabetes provided that:
