F - No Eligible G - No Patients. D - Sponsor delays. C - Closed by Sponsor. E - Staff Availability issues. patient seen)
|
|
|
- Evelyn Marshall
- 5 years ago
- Views:
Transcription
1 No REC Reference Number IRAS No Title Valid Research Application Date of First Patient Recruitment A - Permissions delayed/deni ed B - Suspended by C - Closed by D - delays E - Staff Availability issues F - No Eligible G - No Patients Patients (No Consented patient seen) H - Contracting Delays I-Rare disease J-Other Comments Source of delay INFORM: A Multi-Centred, Randomised Trial to compare 1-Stage with 2-Stage Revision 1 14/SW/ Surgery for Prosthetic Hip Joint Infection. 08/04/ /06/2015 Patients screened but no eligible patients identified. A pilot study for developing and evaluating a care pathway for cognitive problems after 2 12/WM/ stroke (OCS-care) 13/04/ /06/ /LO/0649 Exercise training as a novel primary therapy for men with localised prostate cancer: the PANTERA trial (Prostate cancer Novel ThERApy) 28/04/ /05/ /H/0005 A Phase 3 Confirmatory Study Investigating The Efficacy And Safety Of Dupilumab Monotherapy Administered To Adult Patients With Moderate -To severe Atopic Dermatitis 05/05/ /06/2015 Using remote telemonitoring to detect early decline in lung function & streamline clinics 5 15/H/ in adults with cystic fibrosis 07/05/ /05/ /NW/1294 A Phase III, multi-center, double-blind, placebo controlled, randomized withdrawal study of LCI699 following a 24 week, singlearm, open-label dose titration and treatment to evaluate the safety and efficacy of LCI699 for the treatment of patients with Cushing s disease who are candidates for medical therapy. 08/05/ /07/2015 Rare disease group. 7 14/WM/1083 Cognitive Rehabilitation of Attention and Memory for people with Multiple Sclerosis (CRAMMS): A pragmatic randomised controlled trial 12/05/ /06/ /LO/1043 Multicentre Phase III Double-masked Randomised Controlled Non-Inferiority Trial comparing the clinical and cost effectiveness of intravitreal therapy with ranibizumab (Lucentis) vs aflibercept (Eylea) vs bevacizumab (Avastin) for Macular Oedema due to Central Retinal Vein Occlusion (CRVO). Short Title: LEAVO. 14/05/ /08/2015 Patients screened but no eligible patients identified. The RAPID-CTCA trial (Rapid Assessment of Potential Ischaemic Heart Disease with CTCA) The role of early CT Coronary Angiography in the evaluation, intervention and outcome of patients presenting to the Emergency Department with suspected or confirmed Acute Coronary Syndrome 9 14/SS/ /05/ /06/ /SS/0007 MS-SMART: A Multi-Arm Phase IIb Randomised, Double Blind Placebo- Controlled Clinical Trial Comparing The Efficacy of 3 Neuroprotective Drugs in Secondary Progressive Multiple Sclerosis 19/05/ /07/ /H/1178 ETOP 5-12/ EORTC (SPLENDOUR): A randomised, open-label phase III trial evaluating the addition of denosumab to standard first-line anticancer treatment in advanced NSCLC 05/06/ /07/2015
2 12 14/WM/1190 Drug eluting stent for percutaneous coronary intervention of the left main artery in a real world all-comers population (IDEAL LM Study) 08/06/ /01/2016 delay in provision of equipment 13 13/EM/0230 A Phase II Randomised, Double Blind, Placebo Controlled, Multicentre Study of VS in Subjects With Malignant Pleural Mesothelioma 09/06/ /08/ /H/1274 Study of two regimens of TicagrElor compared to clopidogrel in patients undergoing ELective Percutaneous Coronary Intervention - STEEL PCI 09/06/ /06/ /LO/0673 A Phase 3, Randomized, Placebo- Controlled, Double-Blind Study of Oral MLN9708 Maintenance Therapy in Patients with Multiple Myeloma Following Autologous Stem Cell Transplant - Millennium 12/06/ /11/2015 Patients screened but no eligible patients identified. Strict patient eligibility criteria; patients must be 75 days posttransplant before they become eligible to enter screening. Acceptability study on Nutricomp Drink 16 15/H/ Plus in adult patients 15/06/ /07/ /EM/0014 Behavioural activation therapy for treating post-stroke depression: a feasibility randomised controlled trial 17/06/ /07/ /H/0394 Multicentre Randomised Controlled Trial of Angiotensin Converting Enzyme inhibitor (ACEi) / Angiotensin Receptor Blocker (ARB) withdrawal in advanced renal disease; The STOPACEi Trial 25/06/ /07/ /H/0128 SaFaRI: Sacral nerve stimulation versus the FENIXTM magnetic sphincter augmentation for adult faecal incontinence: a Randomised Investigation 25/06/ /10/2015 ESPAC5F: European Study Group for 20 14/NW/ Pancreatic Cancer Trial 5F 29/06/ /11/2015 delayed green light for 2 weeks at start of study. Patients screened but no eligible patients identified to date since being given green light by. Eligible patients seen chose not to participant in study; patients prefer standard treatment. Rare disease group /LO/1786 CapaCiT study 1: Randomised trial of Habit Training Vs. Habit Training with Direct Visual Biofeedback in Adults with Chronic Constipation. 01/07/ /10/2015 Eligible patients seen chose not to participate in study Developing electrical impedance myography of the tongue for motor system disorders 22 15/H/ EIM 06/07/ /09/2015 Simple Hysterectomy And Pelvic node 23 14/H/ dissection in Early cervix cancer (SHAPE) 08/07/ /07/2015 Understanding the early pathological 24 10/H0505/ pathways in Parkinson s Disease 09/07/ /08/2015 Abdominal massage for neurogenic bowel 25 14/WS/ dysfunction in people with multiple sclerosis 09/07/ /09/ /H/0003 ZINN - Randomized, Doubleblind, Multicenter, Phase III Study Comparing the Efficacy and Safety of Retosiban Versus Atosiban Therapy for Women in Spontaneous Preterm Labor 09/07/ /01/2016 delayed site initiation visit. delayed confirmation of study open to recruitment at site. delay in provision of IMP and equipment. International randomised controlled trial of chemotherapy for the treatment of recurrent 27 14/NW/ and primary refractory Ewing Sarcoma 14/07/2015 Patients screened but no eligible patients identified. Rare disease group.
3 The Efficacy and Mechanism Evaluation of Treating Idiopathic Pulmonary Fibrosis with 28 14/LO/ the addition of Co-trimoxazole (EME-TIPAC) 15/07/ /08/ /LO/0684 Effects of ODM-109 on respiratory function in patients with ALS. A randomised, double blind, placebo-controlled, cross-over, 3, multicentre study with open-label follow-up extension. 17/07/ /08/ /H/1131 Analysis of the immune response to Fendrix as compared to double-dose Engerix B in HIV-infected non-responders to standard Hepatitis B vaccination courses. 20/07/ /09/ /SC/1345 The effectiveness of progesterone to prevent miscarriage in women with early pregnancy bleeding. A randomised placebocontrolled trial: PRISM /07/ /08/ /EE/0011 A Phase III Randomised Trial of Gemcitabine plus Docetaxel followed by Doxorubicin versus observation for uteruslimited high grade uterine leiomyosarcoma 28/07/2015 Randomised, double-blind, multicentre Phase III trial evaluating the safety and benefit of adding Everolimus to adjuvant hormone therapy in women with poor prognosis, ER+ and HER2- primary breast cancer who remain free of disease after receiving at least 1 year of adjuvant 33 14/SS/ hormone therapy 31/07/ /10/2015 Rare disease group. Patients screened but no eligible patients identified Eligible patients seen chose not to participate in study PREVAIL: PREVenting infection using 34 14/H/ Antimicrobial Impregnated Long lines 31/07/ /09/ /NW/1531 Comparative testing of Transfix Vacuum blood collection tubes and Cytochex blood collection tubes for FDA 510 (k) submission 04/08/ /09/2015 Investigating the Energy Requirements of 36 15/SW/ Spinal Cord Injured Patients 05/08/ /09/ /LO/0075 A Phase 3 Multicenter, Double-Blind, Randomized, Active Comparator-Controlled Clinical Trial to Evaluate the Safety and Efficacy of Doravirine (MK-1439) 100 mg Once Daily Versus Darunavir 800 mg Once Daily plus Ritonavir 100 mg Once Daily, Each in Combination with TRUVADA or EPZICOM/KIVEXA, in Treatment-Naïve HIV Infected Subjects 05/08/2015 Screening and recruitment closed by to all patients internationally shortly after site was opened 38 14/SC/1366 EPOCH: Protocol TS-102: A phase 3 clinical trial evaluating Therasphere in patients with metastatic colorectal carcinoma of the liver who have failed first line chemotherapy 06/08/ /11/2015 delayed confirmation of study open to recruitment at site until after 70 day benchmark had elapsed /LO/2182 STOP-HCC TS-103 A phase 3 clinical trial of intra-arterial TheraSphere in the treatment of patients with unresectable hepatocellular carcinoma 06/08/2015 delayed confirmation of study open to recruitment at site until approx 60 days after VRA and NHS Permission causing main delay to recruitment. Also rare disease group /SS/0050 A Phase 2, Dose-Ranging, Randomized, Double-Blind, Placebo-Controlled Study of GS-4997 in Subjects with Pulmonary Arterial Hypertension 12/08/ /10/2015 select-d: Anticoagulation Therapy in SELECTeD Cancer Patients at Risk of 41 13/WM/ Recurrence of Venous Thromboembolism 13/08/ /12/2015 Patients screened but no eligible patients identified
4 42 14/H/1141 A phase II randomized, double-blind, placebo-controlled trial of radium-223 dichloride versus placebo when administered to metastatic HER2 negative hormone receptor positive breast cancer with bone metastases treated with hormonal treatment background therapy 17/08/2015 delayed confirmation of study open to recruitment at site until approx 50 days after VRA and NHS Permission causing main delay to recruitment. Also rare disease group /LO/0040 Hypertrophic Cardiomyopathy Study - Phase 2 study to evaluate the safety and efficacy of a novel compound, GS-6615 on Exercise Capacity in Subjects with Symptomatic Hypertrophic Cardiomyopathy 18/08/ /10/ /H/0259 Pulmonary Rehabilitation and ACTIvity after COPD Exacerbations: A multi-centre, randomised, pilot, factorial (2x2: in-hospital exercise versus no in-hospital exercise and in-home rehabilitation plus usual care versus usual care alone), parallel arm (allocation 1:1 for each factor) trial to evaluate the feasibility of a full scale trial in terms of patients recruited in a 7 months window) randomised controlled trial. 20/08/ /10/ /LO/0337 A randomized, double-blind, placebocontrolled, prospective, multicenter, parallel group study to assess the safety and efficacy of macitentan in patients with portopulmonary hypertension 21/08/2015 Rare disease group. Strict patient eligibility criteria /LO/0413 Safety and Efficacy of Brimonidine Posterior Segment Drug Delivery System in Patients with Geographic Atrophy Secondary to Agerelated Macular Degeneration /08/2015 delayed confirmation of study open to recruitment at site. NHS Provider staff availability issues. Patients screened but no eligible patients identified Both 47 15/NW/0593 The effectiveness of mindfulness based cognitive group therapy for social anxiety symptoms in people living with visible skin conditions: A case series of a group intervention 24/08/ /11/2015 Patients screened but no eligible patients identified 48 15/H/0127 An open label extension study to investigate the long term safety, tolerability and efficacy of PF in subjects with Huntington's disease who previously completed study A /08/ /09/ /LO/1074 ExplorerTM3 A multi-centre, randomised, placebo controlled, double blinded, multiple dose trial investigating safety, pharmacokinetics and pharmacodynamics of concizumab administered subcutaneously to haemophilia A subjects. Trial ID: NN /09/2015 Eligible patients chose not to participate in study. GALACTIC: GA-101 (obinutuzumab) monoclonal Antibody as Consolidation 50 14/H/ Therapy In CLL 04/09/ /02/2016 Eligible patients seen chose not to participate in study. The feasibility of employing ambulatory fetal heart rate monitoring in small for gestational 51 15/NW/ age foetuses at risk of stillbirth. 04/09/ /09/ /LO/0448 A Randomized, Double-Blind, Placebo- Controlled Phase 3 Study of Capecitabine and Cisplatin With or Without Ramucirumab as First-line Therapy in Patients With Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma (RAINFALL) 10/09/ /11/2015 Patients screened but no eligible patients identified.
5 53 15/H/0300 Advanced inertial sensors to quantify disability progression in patients with secondary progressive Multiple Sclerosis taking part in the MS SMART clinical trial. 11/09/ /10/2015 The effect of acupuncture on preoperative anxiety in neurosurgical patients: a 54 15/NS/ randomised control trial. 14/09/ /10/ /H/0243 A phase 3, randomized, double-blind, placebo-controlled study investigating the efficacy and safety of multiple Dupilumab dose regimens administered as monotherapy for maintaining treatment response in patients with atopic dermatitis. 17/09/ /10/ /WA/0230 ROCS: Palliative radiotherapy in addition to self-expanding metal stent for improving outcomes of dysphagia and survival in advanced oesophageal cancer: (Radiotherapy after Oesophageal Cancer Stenting) 23/09/ /11/2015 PLATFORM: Planning Treatment for Oesophago-Gastric Cancer: A randomised 57 14/LO/ maintenance therapy trial 23/09/ /10/ /SC/1203 A phase 3 randomized, double-blind study assessing the efficacy and safety of pf and adalimumab in combination with methotrexate in subjects with moderately to severely active rheumatoid arthritis who have had an inadequate response to methotrexate 25/09/2015 Eligible patients seen chose not to participate in study 59 09/H0706/90 DEPICT: A phase 1/ 2 multi-centre dose escalation study of simultaneous boost Intensity-modulated radiotherapy for locally advanced cervical cancer 28/09/ /02/ /H/0397 Feasibility and Acceptability of Transcutaneous Vagal Nerve Stimulation in Recovery of Upper Limb function Post Stroke. 15/10/ /10/ /WS/1146 Multicenter, randomized, double-blind, double-dummy, active-comparator, eventdriven, superiority phase III study of secondary prevention of stroke and prevention of systemic embolism in patients with a recent Embolic Stroke of Undetermined Source (ESUS), comparing rivaroxaban 15 mg once daily with aspirin mg (NAVIGATE ESUS) 16/10/ /03/2016 Change in free vitamin D after high dose cholecalciferol administered to vitamin D 62 15/EM/ deficient postmenopausal women. 19/10/ /10/ /SC/0523 Phase II Study Of The Impact Of AZD4017, A Selective 11b-HSD1 Inhibitor, On Biochemical Markers Of Bone Turnover In Post-Menopausal Osteopaenia 21/10/ /11/2015 Rare disease group. Patients screened but no eligible patients identified. Eligible patients seen chose not to participate in study. Hysterectomy or Endometrial AbLaTion for 64 13/NS/ Heavy menstrual bleeding 21/10/ /H/0287 An open-label study of Dupilumab in patients with atopic dermatitis who participated in previous Dupilumab clinical trials. 22/10/ /11/ /NW/0592 A randomised, double-blind, double-dummy, placebo-controlled, parallel-group multicentre clinical proof-of-principle trial in adult subjects with newly diagnosed type 1 diabetes mellitus investigating the effect of NNC and liraglutide on preservation of beta-cell function. 26/10/2015 Patients screened but no eligible patients identified Eligible patients chose not to participate in study.
6 67 14/SC/1223 CALIBER - A phase II randomised feasibility study of chemoresection and surgical management in low risk non muscle invasive bladder cancer. 27/10/ /12/ /LO/0691 An Open-Label, Multicenter Clinical Trial with Nivolumab (BMS ) Monotherapy in Subjects with Advanced or Metastatic Squamous Cell (Sq) Non-Small Cell Lung Cancer (NSCLC) who Have Received at Least One Prior Systemic Regimen for the Treatment of Stage IIIb/IV SqNSCLC 27/10/ /12/ /WA/1075 The HeadPost study is an investigator initiated, international collaborative, multicentred, cluste randomised controlled trial to establish the effects of head positioning in patients with acute stroke 28/10/ /11/2015 TRACERx - TRAcking non-small cell lung 70 13/LO/ Cancer Evolution through therapy (Rx) 29/10/ /02/2016 Eligible patients seen chose not to participate in study 71 15/NW/0319 A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-Group, Multicenter Study to Evaluate the Efficacy, Safety and Tolerability of LX4211 as Adjunct Therapy in Adults Patients with Type 1 Diabetes Mellitus Who Have Inadequate Glycemic Control with Insulin Therapy. 29/10/ /12/ /SC/0171 Add-Aspirin Trial: A phase III double-blind placebo-controlled randomised trial assessing the addition of aspirin after standard primary therapy in early stage common solid 02/11/ /01/2016 Eligible patients seen chose not to participate in study. Left Atrial Appendage Occlusion Study III 73 15/LO/ (LAAOS III) 03/11/ /03/ /WA/0106 PRotective Ventilation with Higher versus Lower PEEP during General Anesthesia for Surgery in OBESE Patients The PROBESE Randomized Controlled Trial 03/11/2015 Eligible patients chose not to participate in study. Eligible patients seen chose not to participate in study 75 14/LO/1090 A Multi-center, Prospective, Randomized, Controlled Trial of Endobronchial Valve (EBV) Therapy vs. Standard of Care (SoC) in Heterogeneous Emphysema 05/11/ /11/ /H/0317 The 4AT as a triage test for delirium: a validation study in acutely hospitalised older patients 06/11/ /11/2015 Clinical Post-Market Surveillance Study at the University of Z on the Safety and Performance of K-188 when used for Direct Class II Restorations /NS/ /11/ /11/ /H/0117 A prospective, multicenter, single-arm, openlabel, Phase 4 study to evaluate the effects of macitentan on Right ventricular remodeling in Pulmonary ArterIal hypertension assessed by cardiac magnetic resonance imaging - REPAIR 11/11/2015 EuroHP-1: European multicentre, randomised, phase III clinical trial of therapeutic hypothermia plus best medical treatment versus best medical treatment 79 13/NE/ alone for acute ischaemic stroke 13/11/2015 Patients screened but no eligible patients identified Strict patient eligibility criteria /LO/0443 OPTIMISSM: Safety and Efficacy of Pomalidomide, Bortezomib and Low-dose Dexamethasone in Subjects With Relapsed or Refractory Multiple Myeloma. 13/11/ /02/2016 Patients screened but no eligible patients identified. Rare disease group /SC/1376 PART - A randomised controlled trial of Partial prostate Ablation versus Radical prostatectomy (PART) in intermediate risk, unilateral clinically localised prostate cancer 16/11/ /12/2015
7 82 13/NW/0153 CiPHER Phase II multicentre study assessing the efficacy of Cabazitaxel in patients with HER2-negative metastatic breast cancer and having unresectable brain metastases 18/11/2015 Patients screened but no eligible patients identified. Rare disease group /NW/1097 A Phase II Trial of PLX3397 in the Treatment of KIT Mutated Advanced Acral and Mucosal Melanoma (the PIANO trial) 18/11/2015 An open-label, multi-center, non-randomized Phase Ib study to investigate safety and tolerability of radium Ra 223 dichloride (BA ) administered in combination with paclitaxel in cancer subjects with bone 84 15/LO/ lesions. 24/11/ /01/2016 Rare disease group Ablation Verses Anti-arrhythmic Therapy for Reducing All Hospital Episodes from 85 14/LO/ Recurrent Atrial Fibrillation (AVATAR AF) 25/11/ /12/2015 Effect of Remote Ischaemic Conditioning in 86 15/LO/ PPCI patients (ERIC-PPCI study) 02/12/ /01/ /H/0295 Multi-center, Open-Label, Randomized, Two- Arm, Parallel-Group Study to Assess Efficacy and Safety of Envarsus Compared with Tacrolimus Used as Per Current Clinical Practice in the Initial Maintenance Setting in De Novo Kidney Transplant Patients 08/12/ /03/2016 delayed confirmation of study open to recruitment at site /NE/0086 A phase iii, multicenter, randomized, doublemasked, sham-controlled study to assess the efficacy and safety of Lampalizumab administered intravitreally to patients with Geographic atrophy secondary to Agerelated macular degeneration /12/ /02/ /LO/1370 Long term, multicenter, single-arm, openlabel extension study of the MERIT-1 study, to assess the safety, tolerability and efficacy of macitentan in subjects with inoperable chronic thromboembolic pulmonary hypertension (CTEPH) 09/12/ /01/ /NI/1120 EDNA: Early Detection of Neovascular Agerelated macular degeneration (AMD) /12/ /01/ /NW/0022 Early treatment of Class III malocclusion using Bone Anchored Maxillary Protraction, Multicentre RCT 15/12/ /01/ /H/0319 Advanced proton and phosphorous spectroscopy in multiple sclerosis: An pilot study aiming to quantify the putative pathology leading to disease progression in vivo 17/12/2015 PI had to re-evaluate parameters for proton and phosphorous spectroscopy which caused a delay in recruitment commencing /NE/0417 Bladder Sphericity: A pilot study for the ultrasonographic assessment of bladder shape in women with overactive bladder (BLAST Study) 18/12/ /03/2016 An international, multicenter, randomized, double-blind, placebo-controlled phase 3 trial investigating the efficacy and safety of rivaroxaban to reduce the risk of major thrombotic vascular events in patients with symptomatic peripheral artery disease undergoing lower extremity revascularization 94 15/LO/ procedures. 07/01/ /02/2016 Study authorised around Christmas, at which time there was reduced clinical activity. Diffuse Optical Tomography and 95 15/H/ Microvascular Function 08/01/ /02/2016 Multi-drug, genetic marker-directed, noncomparative, multi-centre, multi-arm 96 14/SC/ phase II trial in non-small cell lung cancer 12/01/2016 Patients screened but no eligible patients identified. Rare disease group.
8 97 15/WM/0207 A Randomized, Open-label, Phase 3 Study Assessing Safety and Efficacy in Subjects with Relapsed and Refractory Multiple Myeloma Receiving Once-weekly Carfilzomib Compared to Twice-weekly Carfilzomib in Combination with Dexamethasone. 15/01/ /03/ /NW/0324 Does early referral of patients with metastastic non-small cell lung CA to UK specialist palliative care services make a difference to their quality of life or survival SPECIAL 19/01/ /02/ /H/0116 CULPRIT-SHOCK - Prospective randomised multicentre study comparing immediate multivessel revascularisation by PCI versus culprit lesion PCI with staged non-culprit lesion revascularisation in patients with acute myocardial infarction complicated by cardiogenic shock 20/01/2016 A Phase 3 study of Efficacy, Safety and Tolerability of Chronocort compared with Standard Glucocorticoid Replacement Therapy in the treatment of Congenital Adrenal Hyperplasia /NW/ /01/ /02/2016 Patients screened but no eligible patients identified /EM/1267 An open-label, multicenter study to provide patients with chronic heart failure and reduced ejection fraction access to LCZ following participation in PARADIGM-HF 21/01/ /02/ /H/0458 Use of the SMART COPD physical activity app in pulmonary rehabilitation: a randomised feasibility study. 21/01/ /H/0477 Feasibility Study to Investigate the Effects of Theta Burst Stimulation (TBS) to Treat Upper Limb Spasticity and Dysfunction in Spinal Cord Injured Patients 22/01/ /02/ /EM/0569 Monitoring and promoting effectiveness and adherence to non-invasive ventilation in motor neurone disease using EncoreAnywhere telemonitoring. 22/01/ /02/ /SC/0411 Physiotherapy Rehabilitation for Osteoporotic Vertebral Fracture (PROVE) 27/01/2016 ROSCO Response to optimal selection of neo-adjuvant chemotherapy in operable breast cancer: A randomised Phase III, stratified biomarker trial of neo-adjuvant 5- Fluorouracil, Epirubicin and Cyclophosphamide vs Docetaxel and Cyclophosphamide chemotherapy /WM/ /02/ /03/ /H/0004 ARIOS: Follow-up of infants born to mothers in retosiban studies 03/02/2016 NeSST2: A multi-stage pharmacokinetic and clinical study to develop a non-invasive Short Synacthen Test (SST) with nasally administered Synacthen and salivary cortisol, establish normative data in children and delineate risks for adrenal suppression in asthmatic children /LO/ /02/ /02/2016 A Multi Center, Open Label Study Evaluating the Safety, Tolerability, and Efficacy of Pridopidine in Patients with /EM/ Huntington s Disease (Open PRIDE HD) 04/02/ /02/2016 A randomised, double blind, placebocontrolled trial of a two-week course of dexamethasone for adult patients with a symptomatic Chronic Subdural Haematoma (Dex-CSDH trial) /NW/ /02/2016
9 111 15/LO/0609 CapaCiT Study 3 - Stepped wedge randomised trial of laparoscopic ventral mesh rectopexy in adults with chronic constipation. 10/02/ /H/0538 Phase 3 Study Investigating the Efficacy, Safety and Tolerability of Dupilumab Monotherapy Administered to Adult Patients With Atopic Dermatitis (AD) Who Are Not Adequately Controlled With or Are Intolerant to Oral Cyclosporine or When This Treatment is Not Medically Advisable. 15/02/ /03/ /WM/1170 COMPARE: Phase III randomised controlled trial Comparing Alternative Regimens for escalating treatment of intermediate and high-risk oropharyngeal cancer 16/02/ /H/1259 Comparison of ALitretinoin with PUVA as the first line treatment in patients with severe chronic HAnd eczema ALPHA 16/02/2016 SELPAC: A Randomised three-arm, open label, Phase II study of continuous Selumetinib versus continuous or interrupted Selumetinib in combination with weekly Paclitaxel in metastatic Uveal /LO/ Melanoma. 16/02/ /ES/0130 Physiotherapy Management of Lumbar Radicular Syndrome; Does Early Intervention Improve Outcomes? POLAR 16/02/ /02/ /EM/0062 Clinical Pilot Study of: Astringent Retraction Paste, ImprintTM 4 VPS, Imprint 4TM Preliminary, Intra-oral syringes, 3M ESPE New Temporary restoration (SuPro 100 and Temporary cement), RelyXTM UnicemTM 2 Automix Cement, LavaTM Plus Zirconia crowns 16/02/2016 High Intensity Interval Training for People with Mild Multiple Sclerosis: A Feasibility /H/ Study. 17/02/2016 Anticoagulants for Living FoEtuses in women with recurrent miscarriage and /WM/ inherited thrombophilia : ALIFE 2. 18/02/2016 Multi-centre randomised controlled trial of clinical and cost-effectiveness of drug coated balloons, drug eluting stents and plain balloon angioplasty with bail-out bare metal stent revascularisation strategies for severe limb ischaemia due to femoropopliteal /NS/0070 disease /02/2016 PROgressive Supranuclear Palsy CorTico- Basal Syndrome Multiple System Atrophy /LO/ Longitudinal Study UK. 23/02/ /EM/0055 Rapid Intervention with GTN in Hypertensive Stroke Trial RIGHT2. 24/02/2016 Development of a MR Scanner Capable of Being Sited in a Neonatal Intensive Care /WM/ Unit 25/02/ /03/2016 A Phase II Proof of Concept (PoC), Double- Blind, Randomised, Placebo-controlled Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of VSN16R for the Treatment of Spasticity in Subjects with /LO/ Multiple Sclerosis 03/03/ /H/0366 Lowering Intracranial Pressure in Idiopathic Intracranial Hypertension: Assessing the therapeutic efficacy and safety of an 11ßhydroxysteroid dehydrogenase type inhibitor (AZD4017) (IIH:DT) 04/03/2016
10 Investigation of bacteriophage as potential /H/ source of oral antimicrobials. 07/03/2016 Pilot Study: The salivary glycome as a novel diagnostic marker in oral cancer- glycan /LO/ fingerprinting. 07/03/ /H/0527 Next-generation sequencing to identify biomarkers of radiotherapy resistance in locally-advanced pharyngeal cancer (NSeRRP). 07/03/2016 MUK7: Pomalidomide specific targeting in /EE/ relapsed and refractory myeloma 07/03/2016 The NAtional Trial of Tonsillectomy IN Adults: a clinical and cost effectiveness /NE/ study (NATTINA). 14/03/ /EE/0260 MULTICENTER, OPEN-LABEL (PART A) FOLLOWED B A RANDOMIZED, DOUBLE- BLIND, PARALLEL-GROUP, PLACEBO CONTROLLED STUD (PART B) TO EVALUATE MAINTENANCE OF REMISSION IN SUBJECTS WITH ACTIVE AXIAL SPONDLOARTHRITIS (AXSPA) RECEIVING EITHER CERTOLIZUMAB PEGOL 200MG Q2W OR 200MG Q4W AS COMPARED TO PLACEBO. 14/03/ /NS/0077 A-GLOVES: The effects of arthritis gloves on people with Rheumatoid Arthritis or Early Inflammatory Arthritis with hand pain: a multicentre randomised controlled trial /03/2016 A randomised, double-blind, placebocontrolled, phase 3 trial of doxorubicin plus olaratumab versus doxorubicin plus placebo in patients with advanced or metastatic soft /SC/ tissue sarcoma. 21/03/ /NW/0466 A Comparison of a new with standard child and family primary care service to reduce the re-occurrence of childhood dental caries (DENTALRECUR Trial). 22/03/ /SC/1231 VANCE01: A randomized phase I study to determine the safety and immunogenicity of ChAd-MVA vaccination compared to MVA alone with and without low dose cyclophosphamide in low and intermediate risk localised prostate cancer 22/03/2016 A randomised, phase 3 trial with anti-pd-1 monoclonal antibody pembrolizumab (MK- 3475) versus placebo for patients with early stage NSCLC after resection and completion of standard adjuvant therapy /LO/ (PEARLS). 23/03/ /H/0398 Magnetic Resonance Imaging to Enhance the Diagnosis of Foetal Brain Abnormalities in Utero year follow up study (MERIDIAN 2) 24/03/2016 Safety and Efficacy of Abicipar Pegol in Patients with Neovascular Age-related /SC/ Macular Degeneration. 24/03/ /LO/0564 ARMOR3-SV A Phase 3, randomised, open label, multicentre, controlled study of Galeterone compared to Enzalutamide in men expressing androgen receptor splice variant-7 mrna (AR-V7) metastatic (M1) castrate resistant prostate cancer (CRPC). 29/03/2016
Target date to recruit patients agreed? Target range minimum. Target range maximum
 No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date Total no of patients recruited
No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date Total no of patients recruited
Target date to recruit patients agreed? Target range minimum. Target range maximum. Agreed 1 1 Agreed 01/01/ /01/2016 Recruitment Finished
 No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date No of patients recruited
No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date No of patients recruited
Target date to recruit patients agreed? Target range. maximum. minimum. Date. 5 Agreed 26/01/ /11/2015 Recruitment Finished.
 No REC Reference IRAS No Title Target number of agreed? Target range minimum Target range maximum Target date to recruit agreed? Planned Recruitment end date Total no of recruited at the agreed target
No REC Reference IRAS No Title Target number of agreed? Target range minimum Target range maximum Target date to recruit agreed? Planned Recruitment end date Total no of recruited at the agreed target
Performance in Initiating Clinical Research
 Performance in Initiating Clinical Research January 2016 December 2016 NHS Permissions Site approval Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial
Performance in Initiating Clinical Research January 2016 December 2016 NHS Permissions Site approval Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial
Studies proceeding under pre HRA-Approval system (NHS Permission)
 15/NW/0004 162679 Studies proceeding under pre HRA-roval system (NHS Permission) A MULTI-CENTRE RANDOMISED CLINICAL TRIAL OF BIOMARKER-DRIVEN MAINTENANCE TREATMENT FOR FIRST- LINE METASTATIC COLORECTAL
15/NW/0004 162679 Studies proceeding under pre HRA-roval system (NHS Permission) A MULTI-CENTRE RANDOMISED CLINICAL TRIAL OF BIOMARKER-DRIVEN MAINTENANCE TREATMENT FOR FIRST- LINE METASTATIC COLORECTAL
Performance in Initiating Clinical Research Q4 2015/16
 Performance in Initiating Clinical Research Q4 2015/16 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Date of Receipt of Valid Research Application
Performance in Initiating Clinical Research Q4 2015/16 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Date of Receipt of Valid Research Application
Performance in Initiating Clinical Research Q2 2016/17
 Performance in Initiating Clinical Research Q2 2016/17 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Date of Receipt of Valid Research Application
Performance in Initiating Clinical Research Q2 2016/17 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Date of Receipt of Valid Research Application
Date of NHS Permission. Date of First Patient Recruited. Date Site Invited
 Reference of Receipt of of NHS of First 14/WA/1075 150715 NHS HeadPoST Version 2.1 23/11/2015 05/01/2016 11/01/2016 N/A 12/YH/0452 106985 NHS INCA 10/12/2015 15/01/2016 17/03/2016 N/A Excess Treatment
Reference of Receipt of of NHS of First 14/WA/1075 150715 NHS HeadPoST Version 2.1 23/11/2015 05/01/2016 11/01/2016 N/A 12/YH/0452 106985 NHS INCA 10/12/2015 15/01/2016 17/03/2016 N/A Excess Treatment
Date of First Patient Recruitment. Benchm ark Met. A - Permissions delayed/denied
 No REC Ref Title Authorisation Valid Research Date Application 1 13/EE/0011 2 14/SS/1043 Date of First Patient Recruitment A Phase III Randomised Trial of Gemcitabine plus Docetaxel followed by Doxorubicin
No REC Ref Title Authorisation Valid Research Date Application 1 13/EE/0011 2 14/SS/1043 Date of First Patient Recruitment A Phase III Randomised Trial of Gemcitabine plus Docetaxel followed by Doxorubicin
The Pennine Acute Hospitals NHS Trust Performance in initiating clinical research Reporting period 1 October 2017 to 30 September 2018
 The Pennine Acute Hospitals NHS Trust Performance in initiating clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial First patient recruited?
The Pennine Acute Hospitals NHS Trust Performance in initiating clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial First patient recruited?
Perfomance in Delivering (Commercial Trials)
 Perfomance in Delivering (Commercial Trials) REC Ref IRAS ID Name of Trial Target Of? Minimum Of Maximum Of Target Date To Recruit? Total Date Of to recruit target number Recruited At of patients The Target
Perfomance in Delivering (Commercial Trials) REC Ref IRAS ID Name of Trial Target Of? Minimum Of Maximum Of Target Date To Recruit? Total Date Of to recruit target number Recruited At of patients The Target
The Pennine Acute Hospitals NHS Trust Performance in delivering clinical research Reporting period 1 October 2017 to 30 September 2018
 The Pennine Acute Hospitals NHS Trust Performance in delivering clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial Target number of? Minimum
The Pennine Acute Hospitals NHS Trust Performance in delivering clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial Target number of? Minimum
Performance in Initiating and Delivering Clinical Research Why are we doing this?
 Performance in Initiating and Delivering Clinical Research Why are we doing this? Through the NIHR (National Institute for Health Research) the Government wishes to see a dramatic and sustained improvement
Performance in Initiating and Delivering Clinical Research Why are we doing this? Through the NIHR (National Institute for Health Research) the Government wishes to see a dramatic and sustained improvement
Performance in Delivering Q4. Target number of patients
 Performance in Delivering Q4 Research Ethics Committee Reference Number Name of Trial Target number of patients Date Agreed to recruit target number of patients Trial Status Target met within the agreed
Performance in Delivering Q4 Research Ethics Committee Reference Number Name of Trial Target number of patients Date Agreed to recruit target number of patients Trial Status Target met within the agreed
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT
 NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including accrual, and
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including accrual, and
Summary of Strategic Competitive Analysis and Publication Planning
 Summary of Strategic Competitive Analysis and Publication Planning Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Briefing
Summary of Strategic Competitive Analysis and Publication Planning Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Briefing
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT
 NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including
First Patient Recruited? 17/07/2015 Yes 07/09/2015 Yes. 17/07/2015 Yes 11/09/2015 Yes. 31/07/2015 Yes 06/08/2015 Yes
 Research Ethics Committee Reference Number Name of Trial Date of Receipt of Valid Research Application First Patient Recruited? Date of First Patient Recruited Benchmark Met Reasons for delay correspond
Research Ethics Committee Reference Number Name of Trial Date of Receipt of Valid Research Application First Patient Recruited? Date of First Patient Recruited Benchmark Met Reasons for delay correspond
Summary of Research and Writing Activities In Cardiovascular Disease
 Summary of Research and Writing Activities In Cardiovascular Disease Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts
Summary of Research and Writing Activities In Cardiovascular Disease Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts
National Cancer Drugs Fund List - Approved
 National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
Performance in delivering Clinical Research at PHT Q3 14/15 study met the 70 day target study within 70 day target study not met 70 day target
 Performance in delivering Clinical Research at PHT Q3 14/15 study met the 70 day target study within 70 day target study not met 70 day target Research Ethics Committee 14/ES/0004 ALA-BCC-CT008 - A randomized,
Performance in delivering Clinical Research at PHT Q3 14/15 study met the 70 day target study within 70 day target study not met 70 day target Research Ethics Committee 14/ES/0004 ALA-BCC-CT008 - A randomized,
Performance in Initiating and Delivering Clinical Research
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
OPEN TRIALS Accruals counted until 30-April Current Accrual
 Disease group Breast OPEN TRIALS Trial Title Accrual Target IBCSG 48-14 POSITIVE IBCSG 50-14 OLYMPIA IBCSG 52-15 PALLAS SAKK 21/12 A study evaluating the pregnancy outcomes and safety of interrupting endocrine
Disease group Breast OPEN TRIALS Trial Title Accrual Target IBCSG 48-14 POSITIVE IBCSG 50-14 OLYMPIA IBCSG 52-15 PALLAS SAKK 21/12 A study evaluating the pregnancy outcomes and safety of interrupting endocrine
Performance in Initiating and Delivering Clinical Research
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many as possible the opportunity to get involved
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many as possible the opportunity to get involved
2017 Eligible Measure Applicability (EMA) for Registry Data Submission of Individual Quality Measures
 2017 Eligible Measure Applicability (EMA) for Registry Data Submission of Individual Quality Measures 07/17/2017 Page 1 of 10 QPP Clinically Related Measure Analysis Used in EMA Clinical Relation including
2017 Eligible Measure Applicability (EMA) for Registry Data Submission of Individual Quality Measures 07/17/2017 Page 1 of 10 QPP Clinically Related Measure Analysis Used in EMA Clinical Relation including
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 October September 2013
 Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 October 2012-30 September 2013 Research Ethics Committee reference number Name of Trial 1 12/NW/0321
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 October 2012-30 September 2013 Research Ethics Committee reference number Name of Trial 1 12/NW/0321
Etudes cliniques Service d Oncologie - Radiothérapie
 Etudes cliniques Service d Oncologie - Radiothérapie Décembre 2017 SEIN NEO ADJUVANT WO39392 (Impassion 031) : A phase III randomized study to investigate the efficacy and safety of Atezolizumab in combination
Etudes cliniques Service d Oncologie - Radiothérapie Décembre 2017 SEIN NEO ADJUVANT WO39392 (Impassion 031) : A phase III randomized study to investigate the efficacy and safety of Atezolizumab in combination
Clinical indications for positron emission tomography
 Clinical indications for positron emission tomography Oncology applications Brain and spinal cord Parotid Suspected tumour recurrence when anatomical imaging is difficult or equivocal and management will
Clinical indications for positron emission tomography Oncology applications Brain and spinal cord Parotid Suspected tumour recurrence when anatomical imaging is difficult or equivocal and management will
List of Qualifying Conditions
 List of Qualifying Conditions Cancer Conditions 1) Adrenal cancer 2) Bladder cancer 3) Bone cancer all forms 4) Brain cancer 5) Breast cancer 6) Cervical cancer 7) Colon cancer 8) Colorectal cancer 9)
List of Qualifying Conditions Cancer Conditions 1) Adrenal cancer 2) Bladder cancer 3) Bone cancer all forms 4) Brain cancer 5) Breast cancer 6) Cervical cancer 7) Colon cancer 8) Colorectal cancer 9)
CLINICAL TRIALS ACC. Jul 2016
 CLINICAL TRIALS ACC Jul 2016 Glioblastoma BRAIN A071102 A Phase II/III Randomized Trial of Veliparib or Placebo in Combination With Temozolomide in Newly Diagnosed Glioblastoma With MGMT Promoter Hypermethylation
CLINICAL TRIALS ACC Jul 2016 Glioblastoma BRAIN A071102 A Phase II/III Randomized Trial of Veliparib or Placebo in Combination With Temozolomide in Newly Diagnosed Glioblastoma With MGMT Promoter Hypermethylation
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction 1.1
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction 1.1
Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD
 CLINICAL TRIALS Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer A PHASE II PROTOCOL FOR PATIENTS
CLINICAL TRIALS Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer A PHASE II PROTOCOL FOR PATIENTS
Open clinical uro-oncology trials in Canada
 Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer A PHASE II PROTOCOL FOR PATIENTS WITH STAGE T1
Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer A PHASE II PROTOCOL FOR PATIENTS WITH STAGE T1
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY NFORMATON n format provided by Melero et al. (AUGUST 2015) Supplementary nformation S3 Combinations including two or more immunotherapy agents based on PD-1/PD-L1 blockade. (Source: https://clinicaltrials.gov/
SUPPLEMENTARY NFORMATON n format provided by Melero et al. (AUGUST 2015) Supplementary nformation S3 Combinations including two or more immunotherapy agents based on PD-1/PD-L1 blockade. (Source: https://clinicaltrials.gov/
Directorate General of Health Services Office of Drugs Controller General (India) (Biological Division)
 Directorate General of Health Services Office of Drugs Controller General (India) (Biological Division) List of r-dna drug products approved in the country (Form 45 and 45A) from 1 st Jan 2015 to 30 th
Directorate General of Health Services Office of Drugs Controller General (India) (Biological Division) List of r-dna drug products approved in the country (Form 45 and 45A) from 1 st Jan 2015 to 30 th
Epidemiology, aetiology and the patient pathway in oesophageal and pancreatic cancers
 Epidemiology, aetiology and the patient pathway in oesophageal and pancreatic cancers Dr Ian Chau Consultant Medical Oncologist Women's cancers Breast cancer introduction 3 What profession are you in?
Epidemiology, aetiology and the patient pathway in oesophageal and pancreatic cancers Dr Ian Chau Consultant Medical Oncologist Women's cancers Breast cancer introduction 3 What profession are you in?
PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM
 PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM International Consensus Statement 2013 Guidelines According to Scientific Evidence Developed under the auspices of the: Cardiovascular Disease Educational
PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM International Consensus Statement 2013 Guidelines According to Scientific Evidence Developed under the auspices of the: Cardiovascular Disease Educational
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 July June 2013
 Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 July 2012-30 June 2013 Name of trial Note, some study titles are shortened 1 Long-Term Study
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 July 2012-30 June 2013 Name of trial Note, some study titles are shortened 1 Long-Term Study
Avastin Sample Coding
 First- and Second-line Metastatic Colorectal Cancer C18.0 Malignant neoplasm of the cecum C18.1 Malignant neoplasm of appendix C18.2-C18.9 C19 C20 C21.8 Malignant neoplasm of the colon, various sites Malignant
First- and Second-line Metastatic Colorectal Cancer C18.0 Malignant neoplasm of the cecum C18.1 Malignant neoplasm of appendix C18.2-C18.9 C19 C20 C21.8 Malignant neoplasm of the colon, various sites Malignant
Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD
 Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A MULTICENTRE, RANDOMIZED PLACEBO-CONTROLLED, DOUBLE-BLIND
Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A MULTICENTRE, RANDOMIZED PLACEBO-CONTROLLED, DOUBLE-BLIND
CLINICAL TRIALS Open clinical uro-oncology trials in Canada George Rodrigues, MD, Eric Winquist, MD
 Open clinical uro-oncology trials in Canada George Rodrigues, MD, Eric Winquist, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer AN OPEN-LABEL, MULTICENTER, RANDOMIZED PHASE II
Open clinical uro-oncology trials in Canada George Rodrigues, MD, Eric Winquist, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer AN OPEN-LABEL, MULTICENTER, RANDOMIZED PHASE II
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abdominal drainage, after hepatic resection, 159 160 Ablation, radiofrequency, for hepatocellular carcinoma, 160 161 Adenocarcinoma, pancreatic.
Index Note: Page numbers of article titles are in boldface type. A Abdominal drainage, after hepatic resection, 159 160 Ablation, radiofrequency, for hepatocellular carcinoma, 160 161 Adenocarcinoma, pancreatic.
National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) Trial design:
 Open clinical uro-oncology trials in Canada Eric Winquist, MD, Mary J. Mackenzie, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A PHASE III STUDY OF IRESSA
Open clinical uro-oncology trials in Canada Eric Winquist, MD, Mary J. Mackenzie, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A PHASE III STUDY OF IRESSA
Open Trials as of end of March 2016
 EORTC 10085 PRO EORTC 10085 prospective part, Clinical and biological characterization of Male Breast Cancer: an international EORTC, BIG and NABCG inter study 30 30-Jun-2016 estelle.cassoly@sakk.ch IBCSG
EORTC 10085 PRO EORTC 10085 prospective part, Clinical and biological characterization of Male Breast Cancer: an international EORTC, BIG and NABCG inter study 30 30-Jun-2016 estelle.cassoly@sakk.ch IBCSG
Performance in Initiating and Delivering Clinical Research
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Avastin (bevacizumab) DRUG.00028, CG-DRUG-68
 Avastin (bevacizumab) DRUG.00028, CG-DRUG-68 Override(s) Prior Authorization Approval Duration 1 year Medications Avastin (bevacizumab) APPROVAL CRITERIA Requests for Avastin (bevacizumab) may be approved
Avastin (bevacizumab) DRUG.00028, CG-DRUG-68 Override(s) Prior Authorization Approval Duration 1 year Medications Avastin (bevacizumab) APPROVAL CRITERIA Requests for Avastin (bevacizumab) may be approved
2018 MIPS Reporting Family Medicine
 2018 MIPS Reporting Family Medicine Quality Reporting Requirements: Report on 6 quality measures or a specialty measure set Include at least ONE outcome or high-priority measure Report on patients of All-Payers
2018 MIPS Reporting Family Medicine Quality Reporting Requirements: Report on 6 quality measures or a specialty measure set Include at least ONE outcome or high-priority measure Report on patients of All-Payers
MEDICAL PRIOR AUTHORIZATION
 MEDICAL PRIOR AUTHORIZATION TAXOTERE (docetaxel) DOCEFREZ(docetaxel) docetaxel (generic) POLICY I. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
MEDICAL PRIOR AUTHORIZATION TAXOTERE (docetaxel) DOCEFREZ(docetaxel) docetaxel (generic) POLICY I. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
Manuel Castella MD PhD Hospital Clínic, University of
 Manuel Castella MD PhD Hospital Clínic, University of Barcelona mcaste@clinic.ub.es @mcastellamd www.escardio.org/guidelines European Heart Journal - doi:10.1093/eurheartj/ehw210 Providing integrated care
Manuel Castella MD PhD Hospital Clínic, University of Barcelona mcaste@clinic.ub.es @mcastellamd www.escardio.org/guidelines European Heart Journal - doi:10.1093/eurheartj/ehw210 Providing integrated care
29 August 2016 Page 1 of 7. How does the NHS board decide which new medicines to make available for patients?
 NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available for use in an NHS board only after it has been: accepted for use in the NHSScotland
NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available for use in an NHS board only after it has been: accepted for use in the NHSScotland
HRA Approval Date Site Date Site Sponsor. Non- Confirmation Status. Date Site Ready To Start
 Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial Date of Receipt of Date of Valid Research Application First Patient Recruited? Date
Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial Date of Receipt of Date of Valid Research Application First Patient Recruited? Date
Open clinical uro-oncology trials in Canada
 Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer A PHASE II PROTOCOL FOR PATIENTS WITH STAGE T1
Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer A PHASE II PROTOCOL FOR PATIENTS WITH STAGE T1
proposed set to a required subset of 3 to 5 measures based on the availability of electronic
 CMS-0033-P 143 proposed set to a required subset of 3 to 5 measures based on the availability of electronic measure specifications and comments received. We propose to require for 2011 and 2012 that EP's
CMS-0033-P 143 proposed set to a required subset of 3 to 5 measures based on the availability of electronic measure specifications and comments received. We propose to require for 2011 and 2012 that EP's
Open clinical uro-oncology trials in Canada George Rodrigues, MD, Mary J. Mackenzie, MD, Eric Winquist, MD
 Open clinical uro-oncology trials in Canada George Rodrigues, MD, Mary J. Mackenzie, MD, Eric Winquist, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A MULTICENTRE, RANDOMIZED
Open clinical uro-oncology trials in Canada George Rodrigues, MD, Mary J. Mackenzie, MD, Eric Winquist, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A MULTICENTRE, RANDOMIZED
National Horizon Scanning Centre. Sunitinib (Sutent) for advanced and/or metastatic breast cancer. December 2007
 Sunitinib (Sutent) for advanced and/or metastatic breast cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not
Sunitinib (Sutent) for advanced and/or metastatic breast cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not
Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD
 Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD Hanahan and Weinberg, 2000 Acquired Capabilities of Cancer Clinical Trials When should I consider a clinical trial? How do I find the right
Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD Hanahan and Weinberg, 2000 Acquired Capabilities of Cancer Clinical Trials When should I consider a clinical trial? How do I find the right
Current Clinical Trials for Stroke Survivors in NJ and Philadelphia Areas
 Current Clinical Trials for Survivors in NJ and Philadelphia Areas For more information go to https://clinicaltrials.gov/ and search for the title in search box Condition / Disease 1. Spatial Neglect and
Current Clinical Trials for Survivors in NJ and Philadelphia Areas For more information go to https://clinicaltrials.gov/ and search for the title in search box Condition / Disease 1. Spatial Neglect and
Clinical Research in Rare Cancers. Friday 10 th February Matt Seymour & Nicola Keat
 Clinical Research in Rare Cancers Friday 10 th February 2012 Matt Seymour & Nicola Keat Rare cancer is a common disease Rare Cancer : [prevalence
Clinical Research in Rare Cancers Friday 10 th February 2012 Matt Seymour & Nicola Keat Rare cancer is a common disease Rare Cancer : [prevalence
It is a malignancy originating from breast tissue
 59 Breast cancer 1 It is a malignancy originating from breast tissue including both early stages which are potentially curable, and metastatic breast cancer (MBC) which is usually incurable. Most breast
59 Breast cancer 1 It is a malignancy originating from breast tissue including both early stages which are potentially curable, and metastatic breast cancer (MBC) which is usually incurable. Most breast
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Locally advanced or metastatic bladder cancer Bladder cancer is the sixth most common cancer. It is more common in men than in
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Locally advanced or metastatic bladder cancer Bladder cancer is the sixth most common cancer. It is more common in men than in
2016 General Practice/Family Practice Preferred Specialty Measure Set
 1 0059 5 0081 41 N/A 50 N/A 65 0069, EHR 66 0002, EHR Effective Clinical Care Effective Clinical Care Effective Clinical Care Diabetes: Hemoglobin A1c (HbA1c) Poor Control (>9%): Percentage of patients
1 0059 5 0081 41 N/A 50 N/A 65 0069, EHR 66 0002, EHR Effective Clinical Care Effective Clinical Care Effective Clinical Care Diabetes: Hemoglobin A1c (HbA1c) Poor Control (>9%): Percentage of patients
Clinical Research Institute HUCH Sponsored, fully signed Clinical Trial Agreements (except grants)
 , fully signed Clinical Trial Agreements (except grants) Sponsor Full name of the Hospital clinic where the is details of the January x x January x NightstaRx Ltd A Randomised, Open Label, Outcomes-Assessor
, fully signed Clinical Trial Agreements (except grants) Sponsor Full name of the Hospital clinic where the is details of the January x x January x NightstaRx Ltd A Randomised, Open Label, Outcomes-Assessor
Date of HRA. Date Site Invited. Date Site Selected. Approval. Recruited. Date
 14/LO/0259 134883 RE-AKT: A randomised Phase II study of enzalutamide (MDV3100) in combination with AZD5363 in s with Metastatic Castration - Resistant Prostate Cancer Yes 08/01/2018 01/07/2016 01/07/2017
14/LO/0259 134883 RE-AKT: A randomised Phase II study of enzalutamide (MDV3100) in combination with AZD5363 in s with Metastatic Castration - Resistant Prostate Cancer Yes 08/01/2018 01/07/2016 01/07/2017
WASHINGTON. Spokane Cheney. Olympia. Tri-Cities. Walla Walla. Portland North Central Oregon City OREGON. Mt. Vernon. Port Angeles
 2 3 Mt. Vernon Port Angeles Bremerton Tacoma 5 5 Olympia Everett Edmonds Seattle 90 WASHINGTON 90 Spokane Cheney Pullman Longview 82 Tri-Cities Portland North Central Oregon City 5 84 OREGON Walla Walla
2 3 Mt. Vernon Port Angeles Bremerton Tacoma 5 5 Olympia Everett Edmonds Seattle 90 WASHINGTON 90 Spokane Cheney Pullman Longview 82 Tri-Cities Portland North Central Oregon City 5 84 OREGON Walla Walla
HEALTH MATTERS, INC. SUMMARY OF PROJECTS AND KEY ACCOMPLISHMENTS TO 2016
 Selected 2016 Highlights Epidemiologic research on chronic kidney disease and fistula patency, solid and hematologic malignancies Health economics and outcomes research on beta3-agonist treatment for overactive
Selected 2016 Highlights Epidemiologic research on chronic kidney disease and fistula patency, solid and hematologic malignancies Health economics and outcomes research on beta3-agonist treatment for overactive
Open clinical uro-oncology trials in Canada
 CLINICAL TRIALS Open clinical uro-oncology trials in Canada Eric Winquist, MD, Mary J. Mackenzie, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada ADRENOCORTICAL MALIGNANCIES
CLINICAL TRIALS Open clinical uro-oncology trials in Canada Eric Winquist, MD, Mary J. Mackenzie, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada ADRENOCORTICAL MALIGNANCIES
Quality Payment Program: Cardiology Specialty Measure Set
 Quality Payment Program: Cardiology Specialty Set Title Number CMS Reporting Method(s) Heart Failure (HF): Angiotensin- Converting Enzyme (ACE) Inhibitor or Angiotensin Receptor Blocker (ARB) Therapy for
Quality Payment Program: Cardiology Specialty Set Title Number CMS Reporting Method(s) Heart Failure (HF): Angiotensin- Converting Enzyme (ACE) Inhibitor or Angiotensin Receptor Blocker (ARB) Therapy for
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Proposed Health Technology Appraisal Radium-223 chloride for the treatment of bone metastases in castrate resistant prostate cancer Draft scope Draft
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Proposed Health Technology Appraisal Radium-223 chloride for the treatment of bone metastases in castrate resistant prostate cancer Draft scope Draft
Quality Payment Program: Cardiology Specialty Measure Set
 Measure Title * Reportable via PINNACLE α Reportable via Diabetes Collaborative CQMC v1.0 Measure High Priority Measure Cross Cutting Measure Heart Failure (HF): Angiotensin- Converting Enzyme (ACE) Inhibitor
Measure Title * Reportable via PINNACLE α Reportable via Diabetes Collaborative CQMC v1.0 Measure High Priority Measure Cross Cutting Measure Heart Failure (HF): Angiotensin- Converting Enzyme (ACE) Inhibitor
Selected tables standardised to Segi population
 Selected tables standardised to Segi population LIST OF TABLES Table 4.2S: Selected causes of death, all-ages, 2000 2004 (Segi Standard) Table 5.3S: Public hospitalisations by major cause of admission
Selected tables standardised to Segi population LIST OF TABLES Table 4.2S: Selected causes of death, all-ages, 2000 2004 (Segi Standard) Table 5.3S: Public hospitalisations by major cause of admission
Title Cancer Drug Phase Status
 Clinical Trial Identifier Title Cancer Drug Phase Status NCT01164995 Study With Wee-1 Inhibitor MK-1775 and Carboplatin to Treat p53 Mutated Refractory and Resistant Ovarian Cancer Epithelial Ovarian Cancer
Clinical Trial Identifier Title Cancer Drug Phase Status NCT01164995 Study With Wee-1 Inhibitor MK-1775 and Carboplatin to Treat p53 Mutated Refractory and Resistant Ovarian Cancer Epithelial Ovarian Cancer
All Studies by Indication
 Therapeutic Area Protocol Number Drug/Device Phase Description Advanced malignancies 337 20101132 AMG 337 I " A Phase 1, First-in-Human Study Evaluating the Safety, Tolerability, and Pharmacokinetics of
Therapeutic Area Protocol Number Drug/Device Phase Description Advanced malignancies 337 20101132 AMG 337 I " A Phase 1, First-in-Human Study Evaluating the Safety, Tolerability, and Pharmacokinetics of
including prevention, healthy lifestyle behaviors, populations at risk & disparities (age, race/ ethnicity, gender, geographic & socioeconomic)
 Endorsement Maintenance 2010 Identification of Gap Areas for which Evidence-based Surgery-related Measures are Needed Cardiac, General, Other Surgical Subspecialties The table below is a tool that identifies
Endorsement Maintenance 2010 Identification of Gap Areas for which Evidence-based Surgery-related Measures are Needed Cardiac, General, Other Surgical Subspecialties The table below is a tool that identifies
Etudes cliniques Service d Oncologie - Radiothérapie
 Etudes cliniques Service d Oncologie - Radiothérapie Juillet 2017 SEIN NEO ADJUVANT Neo-RHEA : NEOadjuvant Biomarker ResearcH Study of Palbociclib in Estrogen Receptor Positive HER2 Negative Breast CAncer
Etudes cliniques Service d Oncologie - Radiothérapie Juillet 2017 SEIN NEO ADJUVANT Neo-RHEA : NEOadjuvant Biomarker ResearcH Study of Palbociclib in Estrogen Receptor Positive HER2 Negative Breast CAncer
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
Reasons for not achieving the 70 day target from receipt of valid research application to 1st patient recruited. E - Staff availability issues
 Research Ethics Committee Reference Number 13/LO/0145 14/LO/0203 Full Name of Trial Date of Receipt of Valid Research Application Date of First Patient Recruited A - Permissions delayed/ denied B - Suspended
Research Ethics Committee Reference Number 13/LO/0145 14/LO/0203 Full Name of Trial Date of Receipt of Valid Research Application Date of First Patient Recruited A - Permissions delayed/ denied B - Suspended
Improving outcomes as rapidly as possible for patients. Multi-arm, multi stage platform, umbrella and basket protocols
 Improving outcomes as rapidly as possible for patients Multi-arm, multi stage platform, umbrella and basket protocols Mahesh Parmar MRC Clinical Trials Unit at UCL Institute of Clinical Trials and Methdology
Improving outcomes as rapidly as possible for patients Multi-arm, multi stage platform, umbrella and basket protocols Mahesh Parmar MRC Clinical Trials Unit at UCL Institute of Clinical Trials and Methdology
Index. cardiology.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Acute myocardial infarction contemporary DES platforms in patients with, 290 AF. See Atrial fibrillation (AF) African Americans dietary factors
Note: Page numbers of article titles are in boldface type. A Acute myocardial infarction contemporary DES platforms in patients with, 290 AF. See Atrial fibrillation (AF) African Americans dietary factors
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 April March 2014
 Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 April 2013-31 March 2014 Research Ethics Committee Name of Trial reference number 1 08/H0906/140
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 April 2013-31 March 2014 Research Ethics Committee Name of Trial reference number 1 08/H0906/140
A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines
 A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction 1
A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction 1
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Meaningful Use Clinical Quality Measures for Eligible Professionals
 Meaningful Use Clinical Quality Measures for Eligible Professionals Measure Type NQF ID CMS ID Description Title: Adult Weight Screening and Follow-Up 1 NQF 0421 PQRI 128 calculated BMI in the past six
Meaningful Use Clinical Quality Measures for Eligible Professionals Measure Type NQF ID CMS ID Description Title: Adult Weight Screening and Follow-Up 1 NQF 0421 PQRI 128 calculated BMI in the past six
Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014
 Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014 Indications for cardiac catheterization Before a decision to perform an invasive procedure such
Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014 Indications for cardiac catheterization Before a decision to perform an invasive procedure such
HRA Approval Date. confirmed by sponsor 07/04/ /04/ /04/ /04/ /04/ /04/ /05/2016
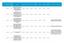 Reference Reason for delay in recruiting first 1 15/NE/0144 179243 AN OPEN-LABEL EXTENSION AND SAFETY MONITORING STUDY OF PATIENTS WITH MODERATELY TO SEVERELY ACTIVE CROHN S DISEASE PREVIOUSLY ENROLLED
Reference Reason for delay in recruiting first 1 15/NE/0144 179243 AN OPEN-LABEL EXTENSION AND SAFETY MONITORING STUDY OF PATIENTS WITH MODERATELY TO SEVERELY ACTIVE CROHN S DISEASE PREVIOUSLY ENROLLED
The Royal Marsden NHS Foundation Trust Medicines Formulary. NICE register for medicines initiated at the trust. Type and code. Publication.
 NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
Dr. Rami M. Adil Al-Hayali Assistant Professor in Medicine
 Dr. Rami M. Adil Al-Hayali Assistant Professor in Medicine Venous thromboembolism: pulmonary embolism (PE) deep vein thrombosis (DVT) 1% of all patients admitted to hospital 5% of in-hospital mortality
Dr. Rami M. Adil Al-Hayali Assistant Professor in Medicine Venous thromboembolism: pulmonary embolism (PE) deep vein thrombosis (DVT) 1% of all patients admitted to hospital 5% of in-hospital mortality
Integrated Research Application System Number. Total Number Of Study Participants Recruited. Research Ethics Committee Reference Number
 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Minimum Number Of Patients Agreed Maximum Number Of Patients Agreed Date That The Trial Closed To
Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Minimum Number Of Patients Agreed Maximum Number Of Patients Agreed Date That The Trial Closed To
8th Emirates Cardiac Society Congress in collaboration with ACC Middle East Conference Dubai: October Acute Coronary Syndromes
 8th Emirates Cardiac Society Congress in collaboration with ACC Middle East Conference 2017 Dubai: 19-21 October 2017 Acute Coronary Syndromes Antonio Colombo Centro Cuore Columbus and S. Raffaele Scientific
8th Emirates Cardiac Society Congress in collaboration with ACC Middle East Conference 2017 Dubai: 19-21 October 2017 Acute Coronary Syndromes Antonio Colombo Centro Cuore Columbus and S. Raffaele Scientific
Subject Index. Androgen antiandrogen therapy, see Hormone ablation therapy, prostate cancer synthesis and metabolism 49
 OOOOOOOOOOOOOOOOOOOOOOOOOOOOOO Subject Index Androgen antiandrogen therapy, see Hormone ablation therapy, synthesis and metabolism 49 Bacillus Calmette-Guérin adjunct therapy with transurethral resection
OOOOOOOOOOOOOOOOOOOOOOOOOOOOOO Subject Index Androgen antiandrogen therapy, see Hormone ablation therapy, synthesis and metabolism 49 Bacillus Calmette-Guérin adjunct therapy with transurethral resection
STEPHEN P. NONN OFFICE OF THE CORONER MADISON COUNTY, ILLINOIS 157 MAIN STREET SUITE 354 EDWARDSVILLE, IL
 MAIN OFFICE: (618) 692-7478 MORGUE: (618) 296-4525 FAX: (618) 692-6042 FAX: (618) 692-9304 STEPHEN P. NONN OFFICE OF THE CORONER MADISON COUNTY, ILLINOIS 157 MAIN STREET SUITE 354 EDWARDSVILLE, IL. 62025-1962
MAIN OFFICE: (618) 692-7478 MORGUE: (618) 296-4525 FAX: (618) 692-6042 FAX: (618) 692-9304 STEPHEN P. NONN OFFICE OF THE CORONER MADISON COUNTY, ILLINOIS 157 MAIN STREET SUITE 354 EDWARDSVILLE, IL. 62025-1962
Policy. Medical Policy Manual Approved Revised: Do Not Implement until 6/30/2019. Nivolumab
 Medical Manual Approved Revised: Do Not Implement until 6/30/2019 Nivolumab NDC CODE(S) 00003-3772-XX Opdivo 40 MG/4ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3774-XX Opdivo 100 MG/10ML SOLN (B-M SQUIBB
Medical Manual Approved Revised: Do Not Implement until 6/30/2019 Nivolumab NDC CODE(S) 00003-3772-XX Opdivo 40 MG/4ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3774-XX Opdivo 100 MG/10ML SOLN (B-M SQUIBB
Avastin (bevacizumab)
 Avastin (bevacizumab) Policy Number: 5.02.502 Last Review: 04/2018 Origination: 03/2017 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Avastin
Avastin (bevacizumab) Policy Number: 5.02.502 Last Review: 04/2018 Origination: 03/2017 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Avastin
WARFARIN: PERI OPERATIVE MANAGEMENT
 WARFARIN: PERI OPERATIVE MANAGEMENT OBJECTIVE: To provide an approach to the perioperative management of warfarin treated patients who require an elective or urgent surgery/procedure. To provide an approach
WARFARIN: PERI OPERATIVE MANAGEMENT OBJECTIVE: To provide an approach to the perioperative management of warfarin treated patients who require an elective or urgent surgery/procedure. To provide an approach
Technology appraisal guidance Published: 15 March 2012 nice.org.uk/guidance/ta249
 Dabigatran an etexilate for the preventionention of stroke and systemic embolism in atrial fibrillation Technology appraisal guidance Published: 15 March 2012 nice.org.uk/guidance/ta249 NICE 2012. All
Dabigatran an etexilate for the preventionention of stroke and systemic embolism in atrial fibrillation Technology appraisal guidance Published: 15 March 2012 nice.org.uk/guidance/ta249 NICE 2012. All
A - Nonconfirmation Suspended by Sponsor B - D - Date site. C - Closed ready to start. Permissions. Sponsor delays ied. delayed/den.
 No REC Reference Number IRAS No Title Submission Type Valid Research Application Date of First Patient Recruitment Benchmark Met invited selected Approval date confirmed by sponsor confirmed A - Nonconfirmation
No REC Reference Number IRAS No Title Submission Type Valid Research Application Date of First Patient Recruitment Benchmark Met invited selected Approval date confirmed by sponsor confirmed A - Nonconfirmation
Acute myocardial infarction. Cardiovascular disorders. main/0202_new 02/03/06. Search date August 2004 Nicholas Danchin and Eric Durand
 main/0202_new 02/03/06 Acute myocardial infarction Search date August 2004 Nicholas Danchin and Eric Durand QUESTIONS Which treatments improve outcomes in acute myocardial infarction?...4 Which treatments
main/0202_new 02/03/06 Acute myocardial infarction Search date August 2004 Nicholas Danchin and Eric Durand QUESTIONS Which treatments improve outcomes in acute myocardial infarction?...4 Which treatments
trial update clinical
 trial update clinical by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UPMC Cancer Centers The treatment outcome for patients with relapsed or refractory cervical carcinoma remains dismal.
trial update clinical by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UPMC Cancer Centers The treatment outcome for patients with relapsed or refractory cervical carcinoma remains dismal.
Ontario s Referral and Listing Criteria for Adult Pancreas-After- Kidney Transplantation
 Ontario s Referral and Listing Criteria for Adult Pancreas-After- Kidney Transplantation Version 2.0 Trillium Gift of Life Network Adult Pancreas-After-Kidney Transplantation Referral & Listing Criteria
Ontario s Referral and Listing Criteria for Adult Pancreas-After- Kidney Transplantation Version 2.0 Trillium Gift of Life Network Adult Pancreas-After-Kidney Transplantation Referral & Listing Criteria
