Regional Colorectal Cancer Network Guidelines for the Management of Colorectal Cancer
|
|
|
- Nora Grant
- 6 years ago
- Views:
Transcription
1 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER Regional Colorectal Cancer Network Guidelines for the Management of Colorectal Cancer Document Purpose This guidance has been produced to support: The management of patients with suspected colorectal cancer The management of patients diagnosed with colorectal cancer Treatment decisions are made by weighing a range of factors, which cannot all be accounted for in a single clinical management guideline. This guidance provides a description of the range of treatment options available for a clinical scenario. Individual clinical management strategies arebest discussed at a multidisciplinary meeting (MDM). The Management of patients with suspected and diagnosed Anal Cancer is out with this guidance as is contained within the NICaN Anal Cancer Guidance. Authors Editor Consultation Process Dr Myles Nelson, Consultant Radiologist Marguerite Greenhill, Colorectal Nurse Specialist Annette Mawhinney, Palliative Care Nurse Specialist Mr Kevin McCallion, Consultant Colorectal Surgeon Dr Robert Harte, Consultant Oncologist Dr Maurice Loughrey, Consultant Pathologist DrKiranKaur, Palliative Care Consultant Dr Richard Wilson, Consultant Oncologist Mr Mark Taylor, Consultant HPB Surgeon Mr Roy Maxwell, Consultant Surgeon and Chair, NICaN Regional Colorectal Cancer Group Consultation and review was via the authors and the NICaN Regional Colorectal Cancer Group Version Control Record of Changes Version 1 Ratified 08/12/09 by NICaN Colorectal Regional Group Version 2 CMG Circulated April 2011 issued to NICaN Colorectal Regional Group 29 th September 2011 for discussion 6 th October Version 3 issued to authors 10 th February 2012 Use of Can and Should have been amended to May and Could in light of contradicting evidence Stenting List removed Amendment to page 25 re: Dukes B Palliative Care Section amended by DrKaur Anal Cancer Pathway removed Section 5 Imaging updated by Dr M Nelson Final Version 2 for electronic sign off following discussion at Regional Meeting 13 th March Page 1 of 45
2 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER Section 14 amended by Ms M Greenhill Pathology Section amended by Dr M Loughrey Document History Version Date Issued Brief Summary of Change Draft 1 21 st May 09 Draft for discussion at Regional Colorectal Cancer Group Meeting Draft 2 7 th Sept 09 Draft for discussion at Regional Colorectal Cancer Group Meeting 8 th Sept Draft 3 12 th October Draft for electronic consultation 2009 Draft 4 24 th November 2009 Draft for electronic consultation (amended following last consultation and final benchmark against peer review requirements) Draft 5 12 th December Document formally ratified (subject to amendments agreed at CRC Regional Group meeting). Version 2 Sept 2011 Discussed at October Regional Colorectal Cancer Meeting Version 3 February 2012 Issued to authors following amendments by Lisa McWilliams on 9t h February Further amendments made by Lisa McWilliams. Further amendments made by Maurice Loughrey, Mark Taylor and Richard Wilson and re-issued on 12 th March for discussion at 13 th March Regional Colorectal Cancer Meeting additional minor changes captured at meeting and final version issued for 2 week electronic consultation on xxx Final Version 2 for electronic sign off following discussion at Regional Meeting 13 th March Page 2 of 45
3 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER Contents 1.0 Introduction Network Configuration of Colorectal Services (N08-1A-205d) ReferralGuidelines Primary Care Referral(N08-1C-103C) Referral following surgical emergency(nd_2d_217; see also paragraph 6.6) Agreed secondary to tertiary referral policy (N08-2D-218) Rectal cancer Agreed guidelines on referral between teams for anal cancer (ND08-2D- 233) Agreed guidelines for referral of patients with liver metastases... 9 (ND08_2D_234; see Section 12) Clinical Responsibilities Across the Patient Pathway Imaging Protocol(N08-2D-235) Introduction Examination of the large bowel Staging Surveillance / follow-up Detection of Recurrent or Metastatic Disease Pathology (N08-2D-235) Introduction Blood Tests Specimen Types Specimen Examination Dataset for Reporting Grading And Staging Conventions Use Of Ancillary Laboratory Techniques Audit Referral for Review or Specialist Opinion Management of Surgical Emergencies(N08-2D-217) Clinical Guidelines for the Treatment of Cancers of the Colon(08-2D-230) Introduction Treatment Options Follow-Up Open Access Short-term follow-up Long-term follow-up Follow-up for palliative colorectal cancers Guidelines for the Management of Early Rectal Cancer(08-2D-230) Introduction Staging Treatment Palliative Care Stenting Guideline(N08C-112d) Introduction Stenting for Palliation Bridge to surgery Final Version 2 for electronic sign off following discussion at Regional Meeting 13 th March Page 3 of 45
4 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER 10.4 Service provision and personnel Radiotherapy and Systemic Therapies for Colorectal Cancer(N08-2D-230) Radiotherapy Adjuvant systemic treatments Advanced disease systemic treatments Guidelines For The Management Of Patients With Anal Cancer(08-2D-231 & 08-2D-233) Management Of Patients With Colorectal Metastases (N08-2D-232 & N08-2D-234) Introduction Referral and management Staging protocol Multi Disciplinary Team Involvement Following surgery Neo-adjuvant and adjuvant systemic therapy Follow-up Colorectal Nursing Supportive and Palliative Care Psychosocial Support and Access to Financial Advice Purpose of the pathway Purpose of the pathway Appendix 1: Regional Care pathways..38 Appendix 2: NSSG minutes recording ratification of CMG.43 Final Version 2 for electronic sign off following discussion at Regional Meeting 13 th March Page 4 of 45
5 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER 1.0 Introduction Every year over 39,000 people in the UK are diagnosed with colorectal cancer and around 110 new cases are diagnosed each day. It is the third most common cancer after breast and lung, both in the UK and in the world, and every year it causes over 16,100 deaths in the UK alone 1. The lifetime odds of developing this type of cancer have been calculated as approximately 1in15 for men and 1in19 for women. In Northern Ireland in 2007, there were 755 new cases of colorectal cancer; 388 in men and 367 in women 2. In 2007, it was the second most common male cancer death and the third most common female cancer death; there were 162 male and 145 female deaths from colorectal cancer in the region that year. Surgical operation (including hepatic resection) offers the only proven potential curative prospect. Chemotherapy and Radiotherapy have a significant role in neo-adjuvant, adjuvant and palliative care. Potentially curative operations are defined as those procedures in which metastases have been excluded by pre-operative imaging, and all tumour has been removed at surgery and confirmed by post-operative histological examination of the specimen. Palliative procedures are carried out for relief of symptoms in circumstances where the tumour is not curable because of its local extent or distant spread. The disease is classified according the Dukes pathological staging criteria as: A Tumour limited to the bowel wall and no lymph node metastases (5-year survival 90%) B Tumour extends beyond the muscularispropria of the bowel wall but no lymph node metastases are present (5-year survival 60-70%) C Lymph node metastases are present (5-year survival 35-50%) (Dukes D = Metastatic spread 2 ) TNM (abbr.) 3 Primary Tumour (T) T0 No evidence of primary tumour T1 Tumour extends intosubmucosa T2 Tumour extends intomuscularis propriat3 Tumour extends through muscularispropriainto subserosa on into non-peritonealizedpericolicor perirectal tissues 1 Cancer Research UK, March Northern Ireland Cancer Registry, April 08 3 Association of Coloproctology of Great Britain and Ireland, Guidelines on the management of colorectal cancer, 2007 pp105 3 From Cancer principles and practice of oncology, 5 th Editioni, Vincent T.DeVitaJr, Samuel Hellmen, Steven A.Rosenberg; Lippincott- Raven Final Version 2 for electronic sign off following discussion at Regional Meeting 13 th March Page 5 of 45
6 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER T4 Tumour extends directly into other the organs or tissues or tumour perforates the visceral peritoneum of the specimen Regional Lymph Nodes (N) N0 No LN metastases N1 Metastatic tumour in 1 to 3 pericolic or preirectal lymph nodes N2 Metastatic tumour in 4 or more pericolic or preirectal lymph nodes Distant Metastases (M) M0 No distant metastases M1 Distant metastases present Final Version 2 for electronic sign off following discussion at Regional Meeting 13 th March Page 6 of 45
7 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER 2.0 Network Configuration of Colorectal Services (N08-1A-205d) NICaN has five colorectal cancer MDTs which diagnose and treat colon and rectal cancer. These are held at the following locations: AltnagelvinHospital Western HSS Trust AntrimAreaHospital Northern HSS Trust RoyalVictoriaHospital (RVH) Belfast HSS Trust CraigavonAreaHospital Southern HSS Trust UlsterHospital South Eastern Trust The catchment populations of these MDTs are shown below: Colorectal MDT Catchment 4 AltnagelvinHospital 296,909 AntrimAreaHospital 453,824 CraigavonAreaHospital 348,657 RoyalVictoriaHospital 334,528 UlsterHospital 341,085 Total 1,775,003 Each MDT meets on a weekly basis. All MDTs have named surgeons who deal with early rectal cancer. 4 Catchment populations based on NISRA projected population figures by electoral ward for Final Version 2 for electronic sign off following discussion at Regional Meeting 13 th March Page 7 of 45
8 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER 3.0 ReferralGuidelines 3.1 Primary Care Referral(N08-1C-103C) Patients can be referred to their local hospital as red flags (i.e. suspect cancer) by their GP under the following NICE guidance: Criteria for urgent referral for suspected colorectal cancer 5 1. any age palpable rectal mass (intraluminal and not pelvic; a pelvic mass outside the bowel would warrant an urgent referral to a urologist or gynaecologist) years and above - rectal bleeding with a change in bowel habit to looser stools +/- increased stoolfrequency persisting 6 weeks or more years and above - rectal bleeding persisting for 6 weeks or more without a change in bowel habit and without anal symptoms years and abovechange in bowel habit to looser stools and/or more frequent stools persistent for 6 weeks or more without rectal bleeding 5. Any age with right lower abdominal mass consistent with involvement of the large bowel 6. Men of any age with unexplained iron deficiency anaemia and a Hb of 11g/100ml or below 7. Non menstruating women with unexplained iron deficiency anaemia and Hb of 10g/100ml or below Patients who describe symptoms which do not entirely fulfil the criteria but are a source of concern to the GP can be referred urgently to the colorectal service outwith the red flag system. Referrals should be faxed to local hospital appointment offices within 24 hours. The appointments office will put the patient s details onto PAS on the day that it is received. The CaPPs system receives automatic updates from PAS which alert the patient navigator to the fact that a new red flag referral has been received. Each diagnostic unit has their own internal arrangements to ensure that patients are seen within the agreed waiting times standards. However, in general, the patient is contacted by the appointments office, either by phone or letter, to arrange an appointment. 3.2 Referral following surgical emergency(nd_2d_217; see also paragraph 6.6) Following emergency admission, patients found to have colorectal cancer should be referred to the colorectal team by the end of the first working day. 1. Following discovery of a colorectal carcinoma in the course of investigations by a noncolorectal team, the patient should be referred to a named surgical member of the locality colorectal MDT. Following discussion at the multidisciplinary team meeting (MDM)the patient may be accepted under the care of one of the members which could be an oncologist or palliative care physician for instance. 5 NICaN Referral guidance for suspected cancer, May Final Version 2 for electronic sign off following discussion at Regional Meeting 13 th March Page 8 of 45
9 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER 2. Such referral should occur by the end of the first working day for the patient to be discussed at the next available MDM and to ensure the minimum delay. 3. Following direct access to endoscopy the consultant responsible for the endoscopy list (even if the endoscopy is carried out by a junior doctor or nurse endoscopist) is responsible for ensuring that where appropriate, investigation pathways are immediately commenced (if the tumour is clinically likely to be a carcinoma) and that the patient is discussed at the next appropriate MDM. This may depend on the timing of the meeting relative to biopsy results being available etc. 4. Following discovery of a metastasis by another MDT possibly of CRC origin, a member of the other MDT should either present the patient to the colorectal MDM or ensure a formal referral to a colorectal MDT member who can present the case at the next MDM. 3.3 Agreed secondary to tertiary referral policy (N08-2D-218) 3.31 Rectal cancer Selected early rectal cancers can be treated by local resection. Most trans-anal techniques are performed by MDTs within the Network. The only surgical technique not available locally istems surgery. Patients with early rectal cancer who are considered suitable for TEMS should be referred to an appropriate UKcentre for surgery by day 28 in the pathway. Figure 1 outlines the investigations that would be expected to be completed locally prior to referral. Where the MDT feels a patient may benefit from oncological treatment a referral is made directly to one of the CRC oncologists at the Cancer Centre. Full patient details (i.e. MDT report with management plan, oncology referral proforma, operation notes and diagnostics results) should be forwarded within 24 hours of the MDM Agreed guidelines on referral between teams for anal cancer (ND08-2D-233) See NICaN Anal Cancer Guidelines 3.33 Agreed guidelines for referral of patients with liver metastases (ND08_2D_234; see Section 12) Patients who have potentially resectableliver metastasesshould be referred to the specialist HPB MDT in the Royal Victoria Hospital to evaluate operability and develop an appropriate management plan. Patients who have liver-only colorectal metastases that appear unresectable (but may become potentially suitable for surgery if adequate cytoreduction were achieved with chemotherapy with/without the addition of targeted therapies) should also be referred to the HPB MDT for potential therapeutic action. Final Version 2 for electronic sign off following discussion at Regional Meeting 13 th March Page 9 of 45
10 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER FIGURE 1: INVESTIGATIONS TO BE COMPLETED AT CANCER UNIT TO BE FORWARDED WITH REFERRAL FOR SPECIALIST TREATMENT Rectal Cancer Biopsy Barium Enema or colonoscopy to image the whole bowel CT of chest, abdomen and pelvis MRI and/or Endorectal Ultrasound Liver metastases SpiralCT of the chest, abdomen and pelvis contemporary scan TESLA MRI scan of the liver Colonoscopy (before or after resection of the primary) PET scanning in patients with high risk primary disease (T4(perforated); C2 (apical node)) Immediately the diagnosis has been confirmed, Cancer Units should make arrangements for the results of these investigations to be presented at next MDM in either Belfast or SE Trust. CT Colonography is an acceptable means of combining the CT and the colon imaging in one investigation, but the whole of the chest must be imaged for full cancer staging. If the reason for transfer is not related to the primary tumour e.g. cardiac comorbidity, transfer should be made in sufficient time to allow assessment of that co-morbidity before commencing treatment. Final Version 2 for electronic sign off following discussion at Regional Meeting 13 th March Page 10 of 45
11 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER 4.0 Clinical Responsibilities Across the Patient Pathway (N08-1C-107a) Stage of Clinical Care Prior to First appointment with Secondary care After first hospital visit Primary Surgery (assuming surgical intervention) Primary non-surgical oncological intervention (Radiation and/or Chemotherapy) Post surgery oncology treatment required Follow up will depend on the treatments given Palliative Care required Treatment for metastatic disease Responsible Clinician(s) General Practitioner Hospital consultant Surgeon accepting responsibility at the MDM Oncologist accepting responsibility at the MDM - patient may be passed back to the Surgeon when this phase is concluded Oncologist accepting responsibility at the MDM - patient may be passed back to the Surgeon when this phase is concluded Normally the Consultant Surgeon or Oncologist. [this should be in accordance with the agreed HSCT follow up protocol / holistic assessment as part of the Transforming Cancer Follow UP (TCFU) programme ] Palliative medicine consultant to advise appropriately depending on setting and needs of patient Clinician in the relevant treatment modality e.g. Hepatic / Thoracic surgeon, Oncologist etc General principles 1. All through the patient pathway there should be ongoing access to the Clinical Nurse Specialists and MDT. 2. Decisions can be made determining the care of the patient within a single modality by the responsible clinician. 3. Decisions concerning modalities not covered by the currently responsible clinician should be referred back to an MDM for wider discussion. 4. For endoscopy initial events the consultant responsible for the list must ensure that the patient is entered for the next appropriate MDM and that if they are not going to provide the next stage that the patient has been accepted by another clinician. 5. This policy should not inhibit referral for support from other clinician, e.g. Palliative radiotherapy. 6. Timely and detailed communication with the patient Primary Care colleagues is mandatory at all times. 11
12 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER 5.0 Imaging Protocol(N08-2D-235) 5.1 Introduction The preferred method of establishing a definitive diagnosis of colorectal cancer is with endoscopy (either sigmoidoscopy or colonoscopy) and biopsy. Histological confirmation of a tumour should be sought preoperatively in all tumours if at all feasible. If histology cannot be obtained, the findings of radiological investigations should be discussed at the MDM and a management plan determined on the merits of each individual case. 5.2 Examination of the large bowel Flexible (or rigid) sigmoidoscopy +/- double contrast barium enema (DCBE) or Colonoscopy or CT Colonography If a previously undiagnosed colorectal cancer is made with a high degree of confidence on the basis of radiological imaging, the reporting radiologist should ensure that the examination is reported urgently as per Diagnostic Reporting Turnaround Times (DRTT). The urgent report should be sent to the referring clinician within 48hrs. In addition a copy of the report should be sent to the local MDT coordinator to enable the patient to be discussed at the next MDM. 5.3 Staging The diagnosis of colorectal cancer will usually be made by endoscopic, histopathological or radiological methods, either alone or in combination. All patients who are considered potentially fit for treatment should undergo the appropriate staging investigations as a matter of urgency. If staging is not performed pre-operatively then a formal staging CT should be performed once the patient has recovered from the surgery and prior to any proposed adjuvant therapy. Colonic Cancer All of the colon should be examined for synchronous cancer/polyp. This may be done by colonoscopy or sigmoidoscopy and barium enema or CT colonography. Where obstruction precludes full assessment prior to surgery, the colon should be examined within the next six months. All patients should undergo staging CT examination of Chest, abdomen and pelvis unless contraindicated. Staging for local extent and metastases CT examination of Chest, abdomen and pelvis. Technique Oral contrast and IV contrast multi detector CT acquisition - Chest in arterial phase followed by abdomen/pelvis in portal venous phase. As per RCR guidelines (4) Completion Colonoscopy in patients undergoing initial flexible Sigmoidoscopy where it is judged possible to intubate safely beyond the identified cancer or DCBE or CT Colonography where it would not be safe to do so. 12
13 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER Rectal cancer MRI is used in the pre-operative staging of rectal tumours, to help determine operability vs. neo-adjuvant or palliative treatment MRI of the pelvis is also used in determination of long or short course radiotherapy. Repeat Pelvic MRI and CT chest abdomen and pelvis may be required following long course (chemo)radiotherapy. MRI may also be used at the same time as Pelvic imaging to help distinguish benign from metastatic liver lesions. All patients should under go staging Pelvic MRI examination unless contraindicated. All of the colon should be examined for synchronous lesions as for colon cancer. Local Staging Pelvic MRI examination+/- endoluminal ultrasound Technique IV buscopan Pelvic MRI. As per RCR guidelines (4) Staging for metastates CT examination of Chest, abdomen and pelvis. Technique Oral contrast and IV contrast multi detector CT (MDCT) acquisition - Chest in arterial phase followed by abdomen/pelvis in portal venous phase. As per RCR guidelines (4) Metastatic Disease Confirmation 1. Liver: a. Contrast MRI b. IV Multiphase Contrast MDCT c. Ultrasound ( with or without Ultrasound contrast agent) 2. Lungs a. IV contrast MDCT Chest b. +/- MDCT guided Lung Biopsy ( if required following discussion at MDM) 3. CT PET scan - Where there is suspicion of metastatic disease and after discussion at MDM, a CT PET scan, if required, may be performed either pre- or postoperatively of the primary bowel surgery. 4. If CT examination shows metastatic disease potentially suitable for resection and the patient has no contraindications to further treatment, a specialist MDT (eghepato-biliary, thoracic) should decide if further imaging to confirm surgery is suitable for patient or potentially suitable after further treatment. 5.4 Surveillance / follow-up The aim of follow-up is to identify potentially curable recurrent disease in patients who are otherwise fit to undergo further treatment. Follow-up may increase the probability of longterm survival following surgery for colorectal cancer, particularly in patients with Dukes stage B or C disease. 13
14 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER The liver is the most common site of recurrence after complete excision of the primary tumour and liver resection can lead to long-term survival in a significant sub-group of patients. Research has indicated that surveillance including CT imaging, can detect early recurrence. This allows more prompt treatment and may improve patient survival. Most colorectal cancer recurrence happens in the first 3 years. Effective therapies are emerging, with more intensive intervention have been shown to be effective in selected patients groups with early relapse. However, at the present time there is no robust guidance on a standardised effective approach for follow up. The Association of Coloproctology of Great Britain and Ireland Guidelines on the management of colorectal cancer (2007) recommends that it is reasonable to undertake CT scan in asymptomatic patients at some time in the first two post operative years after curative resection. The Royal College of Radiologists and the SIGN Guideline do not specify the frequency for CT scanning. The following guidance has been agreed: 1. BSG guidelines suggest that patients with an adenoma-free colon should undergo colonoscopy as a minimum every 5 years until age 70. Patients who have ademonomas should be offered follow-up as per BSG guidelines. Patients should be counselled about the potential complications of colonoscopy. 2. As a minimum two CT scans of the chest abdomen and pelvis in otherwise asymptomatic fit patients should be offered during the first three years post resection, in order to detect potentially resectable liver metastases. [ NICE consider 2 CT 3 years!!]. Where judged clinically appropriate and depending on clinical context and staging, annual CT scans of chest abdomen and pelvis in the first three years following surgery may be appropriate. CT scans should be reported as per DRTT. 3. CT PET could be considered in the following cases: Patients with one confirmed elevated CEA level with a negative or equivocal CT. Distinguishing between fibrosis from recurrent disease, particularly within the pelvis. Excluding distant metastases in patients being considered for metastatic disease resection (egliver and/or lung)after case discussion at the hepatobiliary or lung MDM. If patient is being considered for 14
15 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER CT PET then their case should be discussed first at the MDM prior to requesting the CT PET. CT PET scan should be reported as per DRTT 4. Follow up should cease in elderly or frail patients by agreement between the patient and their treating clinician. 5.5 Detection of Recurrent or Metastatic Disease Full Staging CT examination of Chest, abdomen and pelvis. Technique Oral contrast and IV contrast multi detector CT (MDCT) acquisition - Chest in arterial phase followed by abdomen/pelvis in portal venous phase. As per RCR guidelines (4) Hydronephrosis/hydroureter are usually indicative of local pelvic disease recurrence on follow up scans after initial treatment. Patients with steadily increasing CEA levels invariably have recurrent disease, which can often be demonstrated after careful review of the images 6. Patients identified as having recurrent or metastatic disease should have their radiological reports forwarded to the referring clinician and MDT co-ordinator to enable rapid entry into an appropriate management pathway as per DRTT. References 1. Association of Coloproctology of Great Britain and Ireland, Guidelines on the Management of Colorectal cancer, 2007 Investigations 2. British Society of Gastroenterologists. (2002) Guidelines for colorectal cancer screening in high risk groups. 3. NICE (2004) Improving Outcomes in Colorectal Cancer. 4. Royal College of Radiologists Recommendations for Cross-Sectional Imaging in Cancer Management Ref No: RCR (06)1 (published second edition 2006) 5. NICE (2011) Colorectal Cancer: The Diagnosis and Management of colorectal cancer 6 CEA is not specific for colorectal cancer and is only elevated in around 80% of patients with advanced disease. 15
16 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER 6.0 Pathology (N08-2D-235) 6.1 Introduction These guidelines are supplementary to the following national guidance: Dataset for colorectal cancer histopathology reports issued by the Royal College ofpathologists (2007) UKCCCR Handbook for the Clinico-Pathological Assessment and Staging of Colorectal Cancer Improving outcomes in colorectal cancers (NICE, May 2004) Reporting lesions in the NHS bowel Cancer screening programme - Guidelines from the BowelCancer Screening Programme Pathology Group 2007 All colorectal cancer cases should be reviewed by a Colorectal Cancer multidisciplinary team which has a histopathologist as a core member. There should be a nominated Lead colorectal pathologist for the service. The lead and their appointed deputy should participate in colorectal MDMs, in a bowel screening EQA scheme and in local audit (including an assessment of consistency of reporting, as appropriate to the site). All other histopathologists reporting colorectal samples should be enrolled in an EQA scheme that includes a GI pathology component. If there is a significant discrepancy with the clinical/radiological findings the pathological material from diagnostic colorectal specimens should be reviewed, if possible by a second pathologist with an interest in colorectal cancer. Histological proof of colorectal adenocarcinoma should be obtained where possible for all clinically suspicious cases, prior to definitive treatment. All biopsy diagnoses of flat colonic epithelial dysplasia should be confirmed by a second pathologist with a GI interest before issuing a report. Their name will be included as the reviewing pathologist. Polypoid adenomas need not be reviewed routinely. Specimens should be reported to an agreed timeframe so as to allow appropriate clinical decision making at a planned colorectal MDM (usually within 5 working days). 6.2 Blood Tests Haemoglobin Electrolytes, urea and creatinine Liver function tests CEA 6.3 Specimen Types Diagnostic: Colonic and rectal biopsies Needle core biopsies (abdominal masses or liver metastases) 16
17 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER Therapeutic: Colectomy Anterior resection Abdomino-perineal resection (APR) Trans anal resection of tumour (TART) Endoscopic mucosal resection (EMR) specimens Snare polypectomy 6.4Specimen Examination Each pathology service should establish a defined protocol for each type of diagnostic and therapeutic colorectal specimen type received by the laboratory, taking into account the above guidance. The protocols should be regularly reviewed and updated by the Lead colorectal pathologist in consultation with other pathologists who participate in service delivery. Colorectal tissue should only be removed and stored for the purposes of research if it is surplus to the requirements of the diagnostic process. Appropriate patient consent and ethical approval should be obtained. Digital images of all radical surgical resection specimens are desirable, but following TME, eitheranterior resection or abdominoperineal resection, digital images are particularly important for audit purposes. As a minimum, the following images are recommended: An image of the mesorectal excision margin for purposes of surgical audit A cross section slice of tumour showing maximum penetration beyond the muscularispropria for radiological audit If the circumferential margin is involved, an image of showing the site of involvement forcorrelation with pre-operative imaging is desirable 6.5 Dataset for Reporting Cancer resection specimens should be reported according to the guidance issued in the Dataset for Colorectal Cancer of the Royal College of Pathologists, appropriate to the specimen type. Diagnostic specimens: Tumour type Tumour grade Therapeutic resections: Colectomies, Anterior Resection and Abdominoperineal resections Relevant RCPath Dataset with local modifications Pre op radiotherapy (short course or long course chemoradiotherapy) Response to radiotherapy/chemoradiotherapy Specimen type Quality of TME (rectal tumours) i.e. on mesorectal fascia, within mesorectum, or reaching muscularispropria Site of tumour 17
18 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER Length of resection Maximum tumour diameter Distance from nearest cut end Presence of tumour perforation(site of perforation i.eserosal or retroperitoneal) Relation to peritoneal reflection (rectal tumours) Distance from dentate line (APR only) Invasive tumour type Invasive tumour grade (by predominant grade) Local invasion Distance beyond muscularispropria for pt3 and above Peritoneal spread Submucosal / intramural lymphovascular invasion Extramural vascular invasion Number of nodes examined Number of involved nodes Status of apical node Distance to non-peritonealised resection margin Other relevant pathology (adenoma, synchronous carcinoma, FAP, UC or Crohn s) Dukes stage TMN staging system Whether resection complete The dataset items should be reported in a proforma either within or instead of the free text part of the pathology report, or as a separate proforma. Departments and MDTs should work towards recording and storing the dataset items as individually categorised items in a relational database, so as to allow electronic retrieval through the Cancer Patient Pathways System and to facilitate the use of pathology data in clinical audit, service planning and monitoring, research and quality assurance. Local Resections Tumour type Tumour size Tumour grade based on worst grade present Depth of invasion (Haggitt, Kikuchi and/or mm from muscularis mucosa)) Distance of tumour from resection margins Lymphatic or vascular invasion within stalk Haggitt level is appropriate for carcinoma arising within pedunculated polyps: level 0 intramucosal, level 1 confined to head of polyp, level 2 invades the neck of the polyp, level 3 invades the stalk, level 4 invades submucosa below level of stalk Haggitt level cannot always be assessed on polypectomies, in which case depth of invasion in mmshould be given Kikuchi level may be appropriate for some sessile polyp cancers; sm1 superficial third of the submucosa sm2 middle third of the submucosa 18
19 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER sm3 deep third of the submucosa Other Endoscopic Resections (EMR, TART, TEMS) Tumour size Tumour type Tumour grade Depth of invasion Distance from deep margin Distance from lateral margin, including orientation Laboratories should use an agreed diagnostic coding system (eg. SNOMED). All malignancies must be reported to the Northern Ireland Cancer Registry. 6.6Grading And Staging Conventions Dysplasia grading: Revised Vienna classification of gastrointestinal epithelial neoplasia. Dysplasia in polyps should be categorised as either low grade or high grade Tumour grading: WHO invasive carcinoma grade system Tumour staging: TNM classification of malignant tumours. (As agreed by the Royal College of Pathologists and the Pathology section of the British Society of Gastroenterology, staging will continue to be based on the 5th edition and not the 6th) Dukes stage Tumour Regression: The RCPath recommends that for tumour staging following neoadjuvant therapy, only the presence of tumour cells in the surgical specimen is taken to determine the stage. Fibrosis, haemorrhage, necrosis, inflammation and acellular mucus are ignored. Cases with complete regression are therefore recorded as ypt0. The recommended categories are: No residual tumour cells or mucus Mucus lakes (surrounded by a host response) without tumour cells Minimal residual tumour, i.e. only occasional microscopic tumour foci are identified with difficulty No marked regression However, regression based only on the histological appearance may give a false impression ofresponse to chemoradiotherapy. The degree of response clinically may be much greater. MDTs will have to decide locally how best to record overall response as this may affect consideration of future chemotherapy. 6.7 Use Of Ancillary Laboratory Techniques All laboratories providing a Pathology service in the network must have at least 19
20 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER conditional laboratory (eg CPA) accreditation and ensure participation in an appropriate external quality assurance programme which demonstrates satisfactory laboratory performance. Immunohistochemical procedures which may be of value include the following: Diagnostic scenario Intra-abdominal malignancy?primary Neuroendocrine differentiation Gastrointestinal stromal tumour (GIST) Epithelial dysplasia Mismatch repair protein analysis for possible familial colon cancer (Lynch syndrome) Immunohistochemical markers CK7, CK20, CDX2, CEA, CA125,TTF1,S100, PSA, CK19.9 Synaptophysin, Chromogranin A CD117, DOG1, CD34, Desmin, SMA, S100 p53 MLH1, MSH2, MSH6 and PMS2 Notes TTF1 and CDX2 if metastatic cancer being considered Requested by Medical Genetics or by surgeon/oncologist/pathologist via MDM Molecular pathology testing of tumour tissue (from biopsies or resection specimens) for somatic mutations in key genes may inform prognosis and/or response to molecular targeted therapies. As a minimum, KRAS mutation status should be requested in tumours from all patients with unresectable liver-only metastatic colorectal cancer who may be suitable for treatment with cetuximab under NICE Guidance 176. This should be performed in a suitably accredited molecular pathology laboratory. Currently, the KRAS codons that must be tested are codons 12, 13 and 61. The role of testing for KRAS codon 146, BRAF V600 and NRAS codons 12 and 13 mutations is evolving, with increasing evidence that activating mutations in these codons are also both prognostic and predictive. Merck Serono now fully fund KRAS molecular testing in suitably accredited laboratories for all incident and prevalent patients with colorectal cancer. Testing should be considered to allow access in appropriate patients to biological therapies (see section 11). 6.8Audit All pathologists who report colorectal cancer specimens should participate in audit. Audit may take the form of: review of compliance with procedures for specimen examination and reporting completeness of datasets systematic logging of diagnostic agreement/disagreement during review of cases for MDMs review of diagnostic consistency between pathologists using data from cases in EQA circulations or blind circulations number of confirmed cancers discussed at MDM 20
21 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER participation in regional audit as organised by the Network Site Specific Group The results of the audit process should be discussed with all pathologists who participate in service delivery and used to inform the development of reporting protocols. 6.9Referral for Review or Specialist Opinion Referral for treatment All patients referred for treatment at a hospital within the Cancer Network followingdiagnosis elsewhere must be reviewed and discussed at the treating hospital s MDM. The complete diagnostic pathology report must be available at the MDM, and when appropriate, the histological/cytological material should be reviewed prior to the meeting. This is particularly important if there is a significant discrepancy with the clinical/radiological findings. Pathological material should be requested at least 5 working days before and received at least 3 days before the relevant MDM to allow sufficient time for review. A formal report should be issued by the reviewing pathologist to the responsible clinician at the treating hospital. The results of the review should be sent to the original pathologist, either by copy of the review report or by letter. Where patients have been referred for oncology treatment, requests for specialist biomarker studies will be co-ordinated between the treating oncology service, their local pathology service and the referring hospital s pathology service, as appropriate. The oncology service must agree a mechanism for requesting tests and the relevant pathological material with their local pathology service. Requests for pathological material should be made in good time to ensure that results are available at the MDM where the patient is to be discussed Referral for specialist opinion All colorectal lymphomas should be referred to the Haematological Malignancy Diagnostic Service for phenotypic analysis and confirmation of diagnosis. Other colorectal tumours such as GIST, sarcomas and melanomas should be referred to the appropriate specialist MDT. In cases of diagnostic difficulty, referral may be made to the lead pathologist of the relevant specialist MDT in the network, although referral to other specialists within or outwith the network is equally appropriate in individual cases. Cases referred for individual specialist or second opinion will be dealt with by the individual pathologist and a report issued by them. Where relevant, tissue blocks should be made available to allow any further investigations that are deemed appropriate. The result of the review should be communicated to the referring pathologist, either by copy of the review report pr by letter. In instances when the patient is referred for a opinion by a specialist multidisciplinary team the case should be referred to the Lead Pathologist of the appropriate MDT and dealt with according to specialist team/cancer Centre MDT guidelines. 21
22 NICaN GUIDELINES FOR THE MANAGEMENT OF COLORECTAL CANCER References 1. The Royal College of Pathologists (2007)Dataset for colorectal cancer (2 nd Edition). 2. UICC (1998)TNM Classification of Malignant Tumours (5th edition). 3. UICC (2002)TNM Classification of Malignant Tumours (6th edition) L.H. Sobin and C. Wittekind (Eds). 4. WHO (2010) Classification of Tumours of the Digestive System. 5. Dixon, M. F. (2002) Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 2002;51: The Royal College of Pathologists (2004)Guidelines on inter-departmental dispatch of samples from patients sent to another hospital or centre for assessment and/or treatment. 7. NICE (2004)Improving outcomes in colorectal cancer. 8. Mandard, A.M., Dalibard, F, Mandard, J.C., et al (1994) Pathologic assessment of tumor regression afterpreoperative chemoradiotherapy of esophageal carcinoma: Clinicopathologic correlations. Cancer73: , Haggitt, R.C., Glotzbach, R.E., Soffer, E.E., et al. (1985) Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology, 89: Reporting lesions in the NHS bowel Cancer screening programme - Guidelines from the Bowel Cancer Screening Programme Pathology Group
23 7.0 Management of Surgical Emergencies(N08-2D-217) Emergency surgery may by necessity be undertaken by surgeons who are not members of the colorectal MDT. In the absence of (imminent) perforation or life threatening bleeding the patient should be stabilised and referred by the emergency team to a colorectal surgeon by the end of the first working day, if practicable, so that their management can be transferred to the relevant CRC MDT. Where emergency surgery is required, wherever possible preparation for surgery should be completed as below and in all cases antibiotic and DVT prophylaxis should be administered. Informed consent Group & Hold / Cross-matching Thrombo-embolism prophylaxis Antibacterial prophylaxis Provision of stoma information when required The patient should be transferred to the care of the CRC MDT at the earliest possible opportunity to allow the development of an appropriate treatment and care plan. References Guidelines for the management of colorectal cancer, 3 rd Ed. 2007, Association of Coloproctology of Great Britain and Ireland. 23
24 8.0 Clinical Guidelines for the Treatment of Cancers of the Colon(08-2D-230) 8.1 Introduction Local MDTs provide the mechanism for appropriate treatment decisions for patients with a diagnosis of colorectal cancer. MDMs will normally be held on a weekly basis. MDTs will have an operational policy consistent with the agreed Network policies and guidelines. All elective patients with colorectal cancer, regardless of route of referral or diagnosis, should be discussed at the Colorectal MDM to agree treatment plans prior to the commencement of treatment. The colorectal care pathway is outlined in Appendix Treatment Options Scenario No evidence of distant metastases Scenario Resectable distant metastases Extensive distant metastases Treatment Options Radical segmental resection Neo-adjuvant (chemo)radiotherapy if appropriate for initially non-resectable disease. Palliative care if patient unfit for or declines active treatment Treatment Options Radical segmental resection with post-operative chemotherapy and staged resection of metastases Radical segmental resection with simultaneous resection of metastases Neo-adjuvant (chemo)radiotherapy if appropriate for initially non-resectable disease. Palliative segmental resection for symptoms if patient considered unfit for extended treatment plan Palliative care if patient unfit for or declines active treatment Advanced disease chemotherapy and palliative care 24
25 Advanced disease chemotherapy with palliative resection or colonic stent for direct symptoms Palliative care if patient unfit for or declines active treatment Following curative resection Dukes A No additional treatment required Dukes B Referral to oncologist for discussion of potential benefit of adjuvant chemotherapy MDT agreement that additional treatment inappropriate Dukes C Referral to oncologist for discussion of potential benefit of adjuvant chemotherapy MDT agreement that additional treatment inappropriate Patients should be informed about and encouraged to participate in clinical trials of both surgical and medical treatments as appropriate. 8.3 Follow-Up Open Access Colorectal cancer follow-up is the responsibility of the MDT. All patients should be able to access the MDT promptly. Any patient that contacts a member of the MDT with worrying symptoms should be seen within 2 weeks. If necessary, their case should be discussed by the MDT Short-term follow-up Initial follow-up should focus on post-operative problems, planning for possible adjuvant therapy and stoma management. Patients who have not undergone complete colonoscopy prior to surgery should be offered a colonoscopy within 6 months of discharge or upon completion of adjuvant chemotherapy. This is, in effect, completion of the initial diagnostic work up to identify individuals who have adenomas or carcinomas elsewhere in the colon Long-term follow-up The debate continues on the subject of patient follow up after curative treatment for colorectal cancer. However, there is some evidence to suggest that intensive follow-up may increase the probability of long-term survival aftersurgery for colorectal cancer, at least among patients with Dukes stage B/C disease. The aim of follow-up is to identify potentially curable recurrent disease in patients who are fit to undergo further treatment. 25
26 Possible benefits from long-term follow up are: Detection of potentially curable recurrent or metastatic disease. Detection of asymptomatic recurrence when early chemotherapy may improve quality of life and prolong survival. Detection of metachronous tumours. Provision of psychological support by patient / doctor contact. Facilitation of audit, clinical governance and continuing professional development. Improved survival rates Recommendations for long-term follow-up 1. The frequency of follow-up should be based on the risk of recurrence and the patient s individual circumstancesand wishes. Longterm follow-up for colorectal cancer should include regular appointments every 4-6 months for 2 years with review every 6-12 monthsfor a further 3 years. Each visit should include clinical examination, LFT, FBC and CEA. 2. BSG guidelines suggest that patients with an adenoma-free colon should undergo colonoscopy as a minimum every 5 years until age 70. Patients who have adenomas should be offered follow-up in accordance with BSG guidelines. Patients should be counselled about the potential complications of colonoscopy. 3. As a minimum a single CT scan of the chest abdomen and pelvis in otherwise asymptomatic fit patients could be offered during the first two years post resection, in order to detect potentially resectable liver metastases.. Where judged clinically appropriate and depending on clinical context and staging, annual CT scans of chest abdomen and pelvis in the first three years following surgery may be appropriate. CT scans should be reported as per DRTT. 4. CT PET could be considered in the following cases: Patients with one confirmed elevated CEA level with a negative or equivocal CT. Distinguishing between fibrosis from recurrent disease, particularly within the pelvis. Excluding distant metastases in patients being considered for liver and or lung resection after case discussion at the hepatobiliary or lung MDM. 26
27 If patient is being considered for CT PET then their case should be discussed first at the MDM prior to requesting the CT PET. CT PET scan should be reported as per DRTT 5. Follow up should cease in elderly or frail patients by agreement between the patient and their treating clinician Follow-up for palliative colorectal cancers Follow up will be the responsibility of the oncologist and needs to be tailored to individual patient needs in collaboration with specialist palliative care services in hospital or community. References Association of Coloproctology of Great Britain and Ireland (2007)Guidelines on the Management of Colorectal Cancer NICE (2004) Improving Outcomes in Colorectal Cancer. RoyalCollege of Radiologists (2006) Recommendations for Cross-Sectional Imaging in Cancer Management Ref No: RCR (06)1 British Society of Gastroenterologists. (2002) Guidelines for colorectal cancer screening in high risk groups. 27
PATHOLOGY GROUP GUIDELINES FOR THE EXAMINATION AND REPORTING OF COLORECTAL CANCER SPECIMENS
 PATHOLOGY GROUP GUIDELINES FOR THE EXAMINATION AND REPORTING OF COLORECTAL CANCER SPECIMENS Produced by: Address: Yorkshire Cancer Network Pathology Group Arthington House, Cookridge Hospital, Hospital
PATHOLOGY GROUP GUIDELINES FOR THE EXAMINATION AND REPORTING OF COLORECTAL CANCER SPECIMENS Produced by: Address: Yorkshire Cancer Network Pathology Group Arthington House, Cookridge Hospital, Hospital
Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012
 Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012 Version Control This is a controlled document please destroy all previous versions on receipt
Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012 Version Control This is a controlled document please destroy all previous versions on receipt
COLORECTAL CARCINOMA
 QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
ADJUVANT CHEMOTHERAPY...
 Colorectal Pathway Board: Non-Surgical Oncology Guidelines October 2015 Organization» Table of Contents ADJUVANT CHEMOTHERAPY... 2 DUKES C/ TNM STAGE 3... 2 DUKES B/ TNM STAGE 2... 3 LOCALLY ADVANCED
Colorectal Pathway Board: Non-Surgical Oncology Guidelines October 2015 Organization» Table of Contents ADJUVANT CHEMOTHERAPY... 2 DUKES C/ TNM STAGE 3... 2 DUKES B/ TNM STAGE 2... 3 LOCALLY ADVANCED
Colorectal Pathway Board (Clinical Subgroup): Imaging Guidelines September 2015
 Colorectal Pathway Board (Clinical Subgroup): Imaging Guidelines September 2015 1 Contents Page No. 1. Objective 3 2. Imaging Techniques 3 3. Staging of Colorectal Cancer 5 4. Radiological Reporting 6
Colorectal Pathway Board (Clinical Subgroup): Imaging Guidelines September 2015 1 Contents Page No. 1. Objective 3 2. Imaging Techniques 3 3. Staging of Colorectal Cancer 5 4. Radiological Reporting 6
IMAGING GUIDELINES - COLORECTAL CANCER
 IMAGING GUIDELINES - COLORECTAL CANCER DIAGNOSIS The majority of colorectal cancers are diagnosed on colonoscopy, with some being diagnosed on Ba enema, ultrasound or CT. STAGING CT chest, abdomen and
IMAGING GUIDELINES - COLORECTAL CANCER DIAGNOSIS The majority of colorectal cancers are diagnosed on colonoscopy, with some being diagnosed on Ba enema, ultrasound or CT. STAGING CT chest, abdomen and
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases
 Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
Faster Cancer Treatment Indicators: Use cases
 Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abdominal surgery prior as factor in laparoscopic colorectal surgery, 554 555 Abscess(es) CRC presenting as, 539 540 Adenocarcinoma of
Index Note: Page numbers of article titles are in boldface type. A Abdominal surgery prior as factor in laparoscopic colorectal surgery, 554 555 Abscess(es) CRC presenting as, 539 540 Adenocarcinoma of
Greater Manchester & Cheshire Guidelines for Pathology Reporting for Oesophageal and Gastric Malignancy
 Greater Manchester & Cheshire Guidelines for Pathology Reporting for Oesophageal and Gastric Malignancy Authors: Dr Gordon Armstrong, Dr Sue Pritchard 1. General Comments 1.1 Cancer reporting: Biopsies
Greater Manchester & Cheshire Guidelines for Pathology Reporting for Oesophageal and Gastric Malignancy Authors: Dr Gordon Armstrong, Dr Sue Pritchard 1. General Comments 1.1 Cancer reporting: Biopsies
Guideline for the Management of Vulval Cancer
 Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
Cancer of Unknown Primary (CUP) Protocol
 1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
CT PET SCANNING for GIT Malignancies A clinician s perspective
 CT PET SCANNING for GIT Malignancies A clinician s perspective Damon Bizos Head, Surgical Gastroenterology Charlotte Maxeke Johannesburg Academic Hospital Case presentation 54 year old with recent onset
CT PET SCANNING for GIT Malignancies A clinician s perspective Damon Bizos Head, Surgical Gastroenterology Charlotte Maxeke Johannesburg Academic Hospital Case presentation 54 year old with recent onset
Clinical guideline Published: 1 November 2011 nice.org.uk/guidance/cg131
 Colorectal cancer: diagnosis and management Clinical guideline Published: 1 November 2011 nice.org.uk/guidance/cg131 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Colorectal cancer: diagnosis and management Clinical guideline Published: 1 November 2011 nice.org.uk/guidance/cg131 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
11/21/13 CEA: 1.7 WNL
 Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Pathology in Slovenian CRC screening programme:
 Pathology in Slovenian CRC screening programme: Findings, organisation and quality assurance Snježana Frković Grazio University Medical Center Ljubljana, Slovenia Slovenia s population: 2 million Incidence
Pathology in Slovenian CRC screening programme: Findings, organisation and quality assurance Snježana Frković Grazio University Medical Center Ljubljana, Slovenia Slovenia s population: 2 million Incidence
Cancer of Unknown Primary (CUP)
 Cancer of Unknown Primary (CUP) Pathways and Guidelines V1.0 London Cancer September 2013 The following pathways and guidelines document has been compiled by the London Cancer CUP technical subgroup and
Cancer of Unknown Primary (CUP) Pathways and Guidelines V1.0 London Cancer September 2013 The following pathways and guidelines document has been compiled by the London Cancer CUP technical subgroup and
GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY
 Position Statement produced by BSG, AUGIS and ACPGBI GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY Introduction In 2011 the Independent Practice
Position Statement produced by BSG, AUGIS and ACPGBI GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY Introduction In 2011 the Independent Practice
malignant polyp Daily Challenges in Digestive Endoscopy for Endoscopists and Endoscopy Nurses BSGIE Annual Meeting 18/09/2014 Mechelen
 Plan Incidental finding of a malignant polyp 1. What is a polyp malignant? 2. Role of the pathologist and the endoscopist 3. Quantitative and qualitative risk assessment 4. How to decide what to do? Hubert
Plan Incidental finding of a malignant polyp 1. What is a polyp malignant? 2. Role of the pathologist and the endoscopist 3. Quantitative and qualitative risk assessment 4. How to decide what to do? Hubert
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO)
 North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
Audit Report. Colorectal Cancer Quality Performance Indicators. Patients diagnosed April 2014 March Published: July 2016
 NORTH OF SCOTLAND PLANNING GROUP Colorectal Cancer Managed Clinical Network Audit Report Colorectal Cancer Quality Performance Indicators Patients diagnosed April 2014 March 2015 Published: July 2016 Mr
NORTH OF SCOTLAND PLANNING GROUP Colorectal Cancer Managed Clinical Network Audit Report Colorectal Cancer Quality Performance Indicators Patients diagnosed April 2014 March 2015 Published: July 2016 Mr
COLORECTAL CANCER FAISALGHANISIDDIQUI MBBS; FCPS; PGDIP-BIOETHICS; MCPS-HPE
 COLORECTAL CANCER FAISALGHANISIDDIQUI MBBS; FCPS; PGDIP-BIOETHICS; MCPS-HPE PROFESSOR OF SURGERY & DIRECTOR, PROFESSIONAL DEVELOPMENT CENTRE J I N N A H S I N D H M E D I C A L U N I V E R S I T Y faisal.siddiqui@jsmu.edu.pk
COLORECTAL CANCER FAISALGHANISIDDIQUI MBBS; FCPS; PGDIP-BIOETHICS; MCPS-HPE PROFESSOR OF SURGERY & DIRECTOR, PROFESSIONAL DEVELOPMENT CENTRE J I N N A H S I N D H M E D I C A L U N I V E R S I T Y faisal.siddiqui@jsmu.edu.pk
National Breast Cancer Audit next steps. Martin Lee
 National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
Cancer of Unknown Primary Service
 Cancer of Unknown Primary Service Dr Maurice Fernando Consultant In Specialist Palliative Care and CUP lead Doncaster and Bassetlaw Hospitals NHS FT Wakefield meeting -14-07-2016 CUP service CUP MDT
Cancer of Unknown Primary Service Dr Maurice Fernando Consultant In Specialist Palliative Care and CUP lead Doncaster and Bassetlaw Hospitals NHS FT Wakefield meeting -14-07-2016 CUP service CUP MDT
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
Referral Criteria for Direct Access Outpatient Colonoscopy or Computed Tomography Colonography
 Referral Criteria for Direct Access Outpatient Colonoscopy or Computed Tomography Colonography 2019 Released 2019 health.govt.nz Citation: Ministry of Health. 2019. Referral Criteria for Direct Access
Referral Criteria for Direct Access Outpatient Colonoscopy or Computed Tomography Colonography 2019 Released 2019 health.govt.nz Citation: Ministry of Health. 2019. Referral Criteria for Direct Access
05/07/2018. Organisation. The English screening programme what is happening? Organisation. Bowel cancer screening in the UK is:
 Organisation The English screening programme what is happening? Phil Quirke Lead Pathologist Bowel Cancer Screening PHE England Bowel Cancer Screening Pathology Committee Started 2006 with roll out 4 devolved
Organisation The English screening programme what is happening? Phil Quirke Lead Pathologist Bowel Cancer Screening PHE England Bowel Cancer Screening Pathology Committee Started 2006 with roll out 4 devolved
Bladder Cancer Guidelines
 Bladder Cancer Guidelines Agreed by Urology CSG: October 2011 Review Date: September 2013 Bladder Cancer 1. Referral Guidelines The following patients should be considered as potentially having bladder
Bladder Cancer Guidelines Agreed by Urology CSG: October 2011 Review Date: September 2013 Bladder Cancer 1. Referral Guidelines The following patients should be considered as potentially having bladder
Bowel Cancer Information Leaflet THE DIGESTIVE SYSTEM
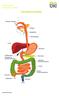 THE DIGESTIVE SYSTEM This factsheet is about bowel cancer Throughout our lives, the lining of the bowel constantly renews itself. This lining contains many millions of tiny cells, which grow, serve their
THE DIGESTIVE SYSTEM This factsheet is about bowel cancer Throughout our lives, the lining of the bowel constantly renews itself. This lining contains many millions of tiny cells, which grow, serve their
Clinical Advice for the Commissioning of the Whole Bowel Cancer Pathway
 Clinical Advice for the Commissioning of the Whole Bowel Cancer Pathway This document was produced by the Colorectal Cancer Clinical Expert Group November 2017 Clinical Advice to Cancer Alliances for the
Clinical Advice for the Commissioning of the Whole Bowel Cancer Pathway This document was produced by the Colorectal Cancer Clinical Expert Group November 2017 Clinical Advice to Cancer Alliances for the
Northern Ireland Bowel Cancer Screening Programme. Pathways. Version 4 1 st October 2013
 Northern Ireland Bowel Cancer Screening Programme Pathways These changes will be version controlled, led by the Quality Assurance Director for the Programme. Any updated versions will be circulated and
Northern Ireland Bowel Cancer Screening Programme Pathways These changes will be version controlled, led by the Quality Assurance Director for the Programme. Any updated versions will be circulated and
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: diagnosis and management of colorectal cancer 1.1 Short title Colorectal cancer 2 The remit The Department
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: diagnosis and management of colorectal cancer 1.1 Short title Colorectal cancer 2 The remit The Department
North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer
 THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
Pathology in Slovenian CRC screening programme: Organisation and quality assurance. Snježana Frković Grazio and Matej Bračko
 Pathology in Slovenian CRC screening programme: Organisation and quality assurance Snježana Frković Grazio and Matej Bračko June 2009 to December 2013 (first three rounds) 33 969 colonoscopies were performed
Pathology in Slovenian CRC screening programme: Organisation and quality assurance Snježana Frković Grazio and Matej Bračko June 2009 to December 2013 (first three rounds) 33 969 colonoscopies were performed
East Midlands Colorectal Cancer Expert Clinical Advisory Group (ECAG) Clinical Guidelines for the Bowel Cancer Care Pathway
 East Midlands Colorectal Cancer Expert Clinical Advisory Group (ECAG) Clinical Guidelines for the Bowel Cancer Care Pathway Reviewed by: Colorectal ECAG August 2014 Ratified by: Colorectal ECAG 2 nd March
East Midlands Colorectal Cancer Expert Clinical Advisory Group (ECAG) Clinical Guidelines for the Bowel Cancer Care Pathway Reviewed by: Colorectal ECAG August 2014 Ratified by: Colorectal ECAG 2 nd March
LCA Lung Clinical Forum. 21 st October 2014
 LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER. Guidelines for the assessment of mismatch. Colorectal Cancer
 COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer March 2017 1 Background Mismatch repair (MMR) deficiency is seen in approximately
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer March 2017 1 Background Mismatch repair (MMR) deficiency is seen in approximately
Neoplastic Colon Polyps. Joyce Au SUNY Downstate Grand Rounds, October 18, 2012
 Neoplastic Colon Polyps Joyce Au SUNY Downstate Grand Rounds, October 18, 2012 CASE 55M with Hepatitis C, COPD (FEV1=45%), s/p vasectomy, knee surgery Meds: albuterol, flunisolide, mometasone, tiotropium
Neoplastic Colon Polyps Joyce Au SUNY Downstate Grand Rounds, October 18, 2012 CASE 55M with Hepatitis C, COPD (FEV1=45%), s/p vasectomy, knee surgery Meds: albuterol, flunisolide, mometasone, tiotropium
8. The polyp in the illustration can be described as (circle all that apply) a. Exophytic b. Pedunculated c. Sessile d. Frank
 Quiz 1 Overview 1. Beginning with the cecum, which is the correct sequence of colon subsites? a. Cecum, ascending, splenic flexure, transverse, hepatic flexure, descending, sigmoid. b. Cecum, ascending,
Quiz 1 Overview 1. Beginning with the cecum, which is the correct sequence of colon subsites? a. Cecum, ascending, splenic flexure, transverse, hepatic flexure, descending, sigmoid. b. Cecum, ascending,
BOWEL CANCER. Causes of bowel cancer
 A cancer is an abnormality in an organ that grows without control. The growth is often quite slow, but will continue unabated until it is detected. It can cause symptoms by its presence in the organ or
A cancer is an abnormality in an organ that grows without control. The growth is often quite slow, but will continue unabated until it is detected. It can cause symptoms by its presence in the organ or
RECTAL CANCER CLINICAL CASE PRESENTATION
 RECTAL CANCER CLINICAL CASE PRESENTATION Francesco Sclafani Medical Oncologist, Clinical Research Fellow The Royal Marsden NHS Foundation Trust, London, UK esmo.org Disclosure I have nothing to declare
RECTAL CANCER CLINICAL CASE PRESENTATION Francesco Sclafani Medical Oncologist, Clinical Research Fellow The Royal Marsden NHS Foundation Trust, London, UK esmo.org Disclosure I have nothing to declare
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician CSCCN PORTSMOUTH HOSPITALS Portsmouth Colorectal MDT (11-2D-1) - 2011/12 Daniel OLeary Compliance Self Assessment COLORECTAL
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician CSCCN PORTSMOUTH HOSPITALS Portsmouth Colorectal MDT (11-2D-1) - 2011/12 Daniel OLeary Compliance Self Assessment COLORECTAL
Audit Report. Colorectal Cancer Quality Performance Indicators. Patients diagnosed April 2016 March Published: March 2018
 Colorectal Cancer Managed Clinical Network Audit Report Colorectal Cancer Quality Performance Indicators Patients diagnosed April 2016 March 2017 Published: March 2018 Mr Michael Walker NOSCAN MCN Clinical
Colorectal Cancer Managed Clinical Network Audit Report Colorectal Cancer Quality Performance Indicators Patients diagnosed April 2016 March 2017 Published: March 2018 Mr Michael Walker NOSCAN MCN Clinical
Colorectal Cancer Structured Pathology Reporting Proforma DD MM YYYY
 Colorectal Cancer Structured Pathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Date of birth DD MM YYYY S1.02 Clinical details
Colorectal Cancer Structured Pathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Date of birth DD MM YYYY S1.02 Clinical details
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER. Guidelines for the assessment of mismatch. Colorectal Cancer
 COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer January 2015 1 Background Mismatch repair (MMR) deficiency is seen in approximately
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer January 2015 1 Background Mismatch repair (MMR) deficiency is seen in approximately
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services. Cancer of Unknown Primary Network Site Specific Group. Clinical Guidelines
 Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Cancer of Unknown Primary Network Site Specific Group Revision due: April 2019 Page 1 of 11 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Cancer of Unknown Primary Network Site Specific Group Revision due: April 2019 Page 1 of 11 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
Colon and Rectum. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition
 Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
LOINC. Clinical information. RCPA code. Record if different to report header Operating surgeon name and contact details. Absent.
 Complete as narrative or use the structured format below 55752-0 17.02.28593 Clinical information 22027-7 17.02.30001 Record if different to report header Operating surgeon name and contact details 52101004
Complete as narrative or use the structured format below 55752-0 17.02.28593 Clinical information 22027-7 17.02.30001 Record if different to report header Operating surgeon name and contact details 52101004
Alison Douglass Gillian Lieberman, MD. November. Colon Cancer. Alison Douglass, Harvard Medical School Year III Gillian Lieberman, MD
 November Colon Cancer Alison Douglass, Harvard Medical School Year III Our Patient Mr. K. is a 67 year old man with no prior medical problems other than hemorrhoids which have caused occasional rectal
November Colon Cancer Alison Douglass, Harvard Medical School Year III Our Patient Mr. K. is a 67 year old man with no prior medical problems other than hemorrhoids which have caused occasional rectal
Guideline for the Diagnosis of Breast Cancer
 Guideline for the Diagnosis of Breast Cancer Version History Version Date Brief Summary of Change Issued 2.0 May 2007 Approved by the Governance Committee 2.0 25.11.08 Discussed at the NSSG 2.1 5.12.08
Guideline for the Diagnosis of Breast Cancer Version History Version Date Brief Summary of Change Issued 2.0 May 2007 Approved by the Governance Committee 2.0 25.11.08 Discussed at the NSSG 2.1 5.12.08
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
Screening & Surveillance Guidelines
 Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs. Gynaecological sarcomas Version 1
 Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Gynaecological sarcomas Version 1 Background This guidance is to provide direction for the management of patients with sarcomas
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Gynaecological sarcomas Version 1 Background This guidance is to provide direction for the management of patients with sarcomas
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2
 Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2 Background Sarcomas that arise in the lung de novo are
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2 Background Sarcomas that arise in the lung de novo are
CLINICAL EFFECTIVENESS
 Re-audit of gastrointestinal tract specimens with respect to compliance with RCPath guidelines Dr Manisha Ram Dr Moina Kadri Background epidemiology and aetiology Over the past 20 years there has been
Re-audit of gastrointestinal tract specimens with respect to compliance with RCPath guidelines Dr Manisha Ram Dr Moina Kadri Background epidemiology and aetiology Over the past 20 years there has been
Structured Follow-Up after Colorectal Cancer Resection: Overrated. R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007
 Structured Follow-Up after Colorectal Cancer Resection: Overrated R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007 Guidelines for Colonoscopy Production: Surveillance US Multi-Society
Structured Follow-Up after Colorectal Cancer Resection: Overrated R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007 Guidelines for Colonoscopy Production: Surveillance US Multi-Society
Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer
 Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer Start date: May 2015 Review date: April 2018 1 Background Mismatch repair (MMR) deficiency is seen in approximately 15%
Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer Start date: May 2015 Review date: April 2018 1 Background Mismatch repair (MMR) deficiency is seen in approximately 15%
Pathway Gynaecology Cancer & Diagnostic Protocol for Inter Trust transfer
 NICaN Pathway Gynaecology Cancer & Diagnostic Protocol for Inter Trust transfer Timed schedules to enable the proactive management of the patient from point of receipt of referral to first definitive treatment
NICaN Pathway Gynaecology Cancer & Diagnostic Protocol for Inter Trust transfer Timed schedules to enable the proactive management of the patient from point of receipt of referral to first definitive treatment
DRAFT SARCOMA MEASURES
 Gateway 15612 Draft Sarcoma Measures Page 1 of 44 DH INFORMATION READER BOX Policy Estates HR / Workforce Commissioning Management IM & T Policy Planning / Finance Clinical Social Care / Partnership Working
Gateway 15612 Draft Sarcoma Measures Page 1 of 44 DH INFORMATION READER BOX Policy Estates HR / Workforce Commissioning Management IM & T Policy Planning / Finance Clinical Social Care / Partnership Working
COLON AND RECTAL CANCER
 COLON AND RECTAL CANCER Mark Sun, MD Clinical Associate Professor of Surgery University of Minnesota No disclosures Objectives 1) Understand the epidemiology, management, and prognosis of colon and rectal
COLON AND RECTAL CANCER Mark Sun, MD Clinical Associate Professor of Surgery University of Minnesota No disclosures Objectives 1) Understand the epidemiology, management, and prognosis of colon and rectal
COLORECTAL CANCER STAGING in 2010
 COLORECTAL CANCER STAGING in 2010 Robert A. Halvorsen, MD, FACR MCV Hospitals / VCU Medical Center Richmond, Virginia I do not have any relevant financial relationships with any commercial interests COLON
COLORECTAL CANCER STAGING in 2010 Robert A. Halvorsen, MD, FACR MCV Hospitals / VCU Medical Center Richmond, Virginia I do not have any relevant financial relationships with any commercial interests COLON
L impatto dell imaging sulla definizione della strategia terapeutica
 GISCoR L impatto dell imaging sulla definizione della strategia terapeutica M. Galeandro U.C. Radioterapia Oncologica ASMN-IRCCS Reggio Emilia 14 Novembre 2014 Rectal Cancer TNM AJCC-7 th edition 2010
GISCoR L impatto dell imaging sulla definizione della strategia terapeutica M. Galeandro U.C. Radioterapia Oncologica ASMN-IRCCS Reggio Emilia 14 Novembre 2014 Rectal Cancer TNM AJCC-7 th edition 2010
Guidelines for the Management of Bladder Cancer
 Guidelines for the Management of Bladder Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Version 3 and 4 Sections 5.2 and 8 updated Page 1 of 9 1. Scope of
Guidelines for the Management of Bladder Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Version 3 and 4 Sections 5.2 and 8 updated Page 1 of 9 1. Scope of
Colorectal Cancer Quality Performance Indicators
 Publication Report Colorectal Cancer Quality Performance Indicators Patients diagnosed between April 2013 and March 2016 Publication date 27th June 2017 An Official Statistics Publication for Scotland
Publication Report Colorectal Cancer Quality Performance Indicators Patients diagnosed between April 2013 and March 2016 Publication date 27th June 2017 An Official Statistics Publication for Scotland
Colorectal Cancer Comparative Audit Report
 SOUTH EAST SCOTLAND CANCER NETWORK (SCAN) PROSPECTIVE CANCER AUDIT Colorectal Cancer 2014 2015 Comparative Audit Report Mr B.J. Mander, NHS Lothian, Lead Colorectal Cancer Clinician, SCAN Group Chair Mr
SOUTH EAST SCOTLAND CANCER NETWORK (SCAN) PROSPECTIVE CANCER AUDIT Colorectal Cancer 2014 2015 Comparative Audit Report Mr B.J. Mander, NHS Lothian, Lead Colorectal Cancer Clinician, SCAN Group Chair Mr
QA Processes. Philip DaCosta BSCP QA Lead, Yorkshire & the Humber September 2013
 QA Processes Philip DaCosta BSCP QA Lead, Yorkshire & the Humber September 2013 Standards QA visits Dashboards BCSP standards Standards for Organisation Service delivery Reporting Data quality Audit and
QA Processes Philip DaCosta BSCP QA Lead, Yorkshire & the Humber September 2013 Standards QA visits Dashboards BCSP standards Standards for Organisation Service delivery Reporting Data quality Audit and
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE
 SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
COLORECTAL CANCER Quality Performance Indicators (QPI) Comparative Report
 SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT COLORECTAL CANCER 2016 2017 Quality Performance Indicators (QPI) Comparative Report Mr S Yalamarthi, NHS Fife, Lead Colorectal Cancer Clinician,
SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT COLORECTAL CANCER 2016 2017 Quality Performance Indicators (QPI) Comparative Report Mr S Yalamarthi, NHS Fife, Lead Colorectal Cancer Clinician,
Early Rectal Cancer Surgical options Organ Preservation? Chinna Reddy Colorectal Surgeon Western General, Edinburgh
 Early Rectal Cancer Surgical options Organ Preservation? Chinna Reddy Colorectal Surgeon Western General, Edinburgh What is Early rectal cancer? pt1t2n0m0 Predictors for LN involvement Size Depth Intramural
Early Rectal Cancer Surgical options Organ Preservation? Chinna Reddy Colorectal Surgeon Western General, Edinburgh What is Early rectal cancer? pt1t2n0m0 Predictors for LN involvement Size Depth Intramural
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician GMCN ROYAL WOLVERHAMPTON HOSPITALS The Royal Wolverhampton Hospitals Trust Lung MDT (11-2C-1) - 2011/12 Dr Angela Morgan
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician GMCN ROYAL WOLVERHAMPTON HOSPITALS The Royal Wolverhampton Hospitals Trust Lung MDT (11-2C-1) - 2011/12 Dr Angela Morgan
performed to help sway the clinician in what the appropriate diagnosis is, which can substantially alter the treatment of management.
 Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Transforming Cancer Services for London
 Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Proposed All Wales Vulval Cancer Guidelines. Dr Amanda Tristram
 Proposed All Wales Vulval Cancer Guidelines Dr Amanda Tristram Previous FIGO staging FIGO Stage Features TNM Ia Lesion confined to vulva with
Proposed All Wales Vulval Cancer Guidelines Dr Amanda Tristram Previous FIGO staging FIGO Stage Features TNM Ia Lesion confined to vulva with
Colorectal Cancer Network Site Specific Group. Clinical Guidelines. June 2017
 Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Colorectal Cancer Network Site Specific Group June 2017 Revision due: April 2019 Page 1 of 31 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Colorectal Cancer Network Site Specific Group June 2017 Revision due: April 2019 Page 1 of 31 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
CHAPTER 7 Concluding remarks and implications for further research
 CONCLUDING REMARKS AND IMPLICATIONS FOR FURTHER RESEARCH CHAPTER 7 Concluding remarks and implications for further research 111 CHAPTER 7 Molecular staging of large sessile rectal tumors In this thesis,
CONCLUDING REMARKS AND IMPLICATIONS FOR FURTHER RESEARCH CHAPTER 7 Concluding remarks and implications for further research 111 CHAPTER 7 Molecular staging of large sessile rectal tumors In this thesis,
Greater Manchester & Cheshire Guidelines for Pathology Reporting of Oesophageal and Gastric Malignancy
 Greater Manchester & Cheshire Guidelines for Pathology Reporting of Oesophageal and Gastric Malignancy Authors: Dr Stephen Hayes, Dr David Bisset, Dr Gordon Armstrong, Dr Sue Pritchard 1. General Comments
Greater Manchester & Cheshire Guidelines for Pathology Reporting of Oesophageal and Gastric Malignancy Authors: Dr Stephen Hayes, Dr David Bisset, Dr Gordon Armstrong, Dr Sue Pritchard 1. General Comments
COLON AND RECTAL CANCER
 No disclosures COLON AND RECTAL CANCER Mark Sun, MD Clinical Assistant Professor of Surgery University of Minnesota Colon and Rectal Cancer Statistics Overall Incidence 2016 134,490 new cases 8.0% of all
No disclosures COLON AND RECTAL CANCER Mark Sun, MD Clinical Assistant Professor of Surgery University of Minnesota Colon and Rectal Cancer Statistics Overall Incidence 2016 134,490 new cases 8.0% of all
Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction
 Version History Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction Version Summary of change Date Issued 2.0 Endorsed by the Governance Committee 20.02.08 2.1 Circulated
Version History Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction Version Summary of change Date Issued 2.0 Endorsed by the Governance Committee 20.02.08 2.1 Circulated
MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER
 MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER (Text update March 2008) A. Stenzl (chairman), N.C. Cowan, M. De Santis, G. Jakse, M. Kuczyk, A.S. Merseburger, M.J. Ribal, A. Sherif, J.A. Witjes Introduction
MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER (Text update March 2008) A. Stenzl (chairman), N.C. Cowan, M. De Santis, G. Jakse, M. Kuczyk, A.S. Merseburger, M.J. Ribal, A. Sherif, J.A. Witjes Introduction
Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 5
 Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 5 Contents 5. Assessment & Management of Liver Metastases 42 5.1. Metachronous
Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 5 Contents 5. Assessment & Management of Liver Metastases 42 5.1. Metachronous
Guideline for the Follow-up of Patients with Gynaecological Malignancies
 Guideline for the Follow-up of Patients with Gynaecological Malignancies Version History Version Date Summary of Change/Process 2.0 20.02.08 Endorsed by the Governance Committee 2.1 18.11.10 Circulated
Guideline for the Follow-up of Patients with Gynaecological Malignancies Version History Version Date Summary of Change/Process 2.0 20.02.08 Endorsed by the Governance Committee 2.1 18.11.10 Circulated
Appendix 4 Urology Care Pathways
 Appendix 4 Urology Care Pathways Cancer Care Pathways outline the steps and stages in the patient journey from referral through to diagnostics, staging, treatment, follow up, rehabilitation and if applicable
Appendix 4 Urology Care Pathways Cancer Care Pathways outline the steps and stages in the patient journey from referral through to diagnostics, staging, treatment, follow up, rehabilitation and if applicable
Colorectal Cancer Care
 Colorectal Cancer Care A Cancer Care Map for Patients Understanding the process of care that a patient goes through in the diagnosis and treatment of colorectal cancer in BC. ROUND2.3_CRCa_20pages_5.5x8.5_FINAL.indd
Colorectal Cancer Care A Cancer Care Map for Patients Understanding the process of care that a patient goes through in the diagnosis and treatment of colorectal cancer in BC. ROUND2.3_CRCa_20pages_5.5x8.5_FINAL.indd
Radiotherapy for Rectal Cancer. Kevin Palumbo Adelaide Radiotherapy Centre
 Radiotherapy for Rectal Cancer Kevin Palumbo Adelaide Radiotherapy Centre Overview CRC are common (3 rd commonest cancer) rectal Ca approx 25-30% of all CRC. Presentation PR bleeding: beware attributing
Radiotherapy for Rectal Cancer Kevin Palumbo Adelaide Radiotherapy Centre Overview CRC are common (3 rd commonest cancer) rectal Ca approx 25-30% of all CRC. Presentation PR bleeding: beware attributing
NICE guideline Published: 24 January 2018 nice.org.uk/guidance/ng83
 Oesophago-gastric cancer: assessment and management in adults NICE guideline Published: 24 January 18 nice.org.uk/guidance/ng83 NICE 18. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Oesophago-gastric cancer: assessment and management in adults NICE guideline Published: 24 January 18 nice.org.uk/guidance/ng83 NICE 18. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
OFCCR CLINICAL DIAGNOSIS AND TREATMENT FORM
 OFCCR CLINICAL DIAGNOSIS AND TREATMENT FORM Name: _, OFCCR # _ OCGN # _ OCR Group # _ HIN# Sex: MALE FEMALE UNKNOWN Date of Birth: DD MMM YYYY BASELINE DIAGNOSIS & TREATMENT 1. Place of Diagnosis: Name
OFCCR CLINICAL DIAGNOSIS AND TREATMENT FORM Name: _, OFCCR # _ OCGN # _ OCR Group # _ HIN# Sex: MALE FEMALE UNKNOWN Date of Birth: DD MMM YYYY BASELINE DIAGNOSIS & TREATMENT 1. Place of Diagnosis: Name
North of Scotland Cancer Network Clinical Management Guideline for Carcinoma of the Uterine Cervix
 THIS DOCUMENT North of Scotland Cancer Network Carcinoma of the Uterine Cervix UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by A Kennedy/AG Macdonald/Others Approved by NOT APPROVED Issue date April
THIS DOCUMENT North of Scotland Cancer Network Carcinoma of the Uterine Cervix UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by A Kennedy/AG Macdonald/Others Approved by NOT APPROVED Issue date April
STANDARDS FOR UPPER GI CANCERS 2004
 STANDARDS FOR UPPER GI CANCERS 2004 Source: CSCG Title: UPPER GI CANCERS STANDARDS Version: 10/CONSULTATION Page 1 of 33 1. INTRODUCTION TO THE CANCER STANDARDS 1.1 These Cancer Standards replace the previous
STANDARDS FOR UPPER GI CANCERS 2004 Source: CSCG Title: UPPER GI CANCERS STANDARDS Version: 10/CONSULTATION Page 1 of 33 1. INTRODUCTION TO THE CANCER STANDARDS 1.1 These Cancer Standards replace the previous
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES. U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease
 PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
SE140. guidelines. Background! Methods!
 SE140 European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition Management of lesions detected in colorectal cancer screening Co-Funded by the Health Programme
SE140 European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition Management of lesions detected in colorectal cancer screening Co-Funded by the Health Programme
Improving Outcomes for People with Sarcoma
 NHS National Institute for Health and Clinical Excellence Guidance on Cancer Services Improving Outcomes for People with Sarcoma List of All Recommendations March 2006 Developed for NICE by the National
NHS National Institute for Health and Clinical Excellence Guidance on Cancer Services Improving Outcomes for People with Sarcoma List of All Recommendations March 2006 Developed for NICE by the National
Navigators Lead the Way
 RN Navigators Their Role in patients with Cancers of the GI tract Navigators Lead the Way Nurse Navigator Defined Nurse Navigator A clinically trained individual responsible for the identification and
RN Navigators Their Role in patients with Cancers of the GI tract Navigators Lead the Way Nurse Navigator Defined Nurse Navigator A clinically trained individual responsible for the identification and
National Oesophago-Gastric Cancer Audit New Patient Registration sheet Patients with Oesophageal High Grade Glandular Dysplasia
 National Oesophago-Gastric Cancer Audit New Patient Registration sheet Patients with Oesophageal High Grade Glandular Dysplasia Patient Details Surname: NHS number: Forename: Postcode: Sex: Male Female
National Oesophago-Gastric Cancer Audit New Patient Registration sheet Patients with Oesophageal High Grade Glandular Dysplasia Patient Details Surname: NHS number: Forename: Postcode: Sex: Male Female
Single Suspected Cancer Pathway Definitions pathway start date
 Single Suspected Cancer Pathway Definitions pathway start date Date: March 2018 Version: 1.2.1 Wales Cancer Owner: Network and Welsh Government Status Published 1 P a g e Purpose of Document This document
Single Suspected Cancer Pathway Definitions pathway start date Date: March 2018 Version: 1.2.1 Wales Cancer Owner: Network and Welsh Government Status Published 1 P a g e Purpose of Document This document
Delivering 62 Day GP Cancer Waits in a Complex Landscape. Hannah Marder Cancer Manager University Hospitals Bristol
 Delivering 62 Day GP Cancer Waits in a Complex Landscape Hannah Marder Cancer Manager University Hospitals Bristol Overview The 62 day GP target Cancer pathways What causes breaches? Good practice and
Delivering 62 Day GP Cancer Waits in a Complex Landscape Hannah Marder Cancer Manager University Hospitals Bristol Overview The 62 day GP target Cancer pathways What causes breaches? Good practice and
Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Disclosure. Acknowledgement. What is the Best Workup for Rectal Cancer Staging: US/MRI/PET? Rectal cancer imaging. None
 What is the Best Workup for Rectal Cancer Staging: US/MRI/PET? Zhen Jane Wang, MD Assistant Professor in Residence UC SF Department of Radiology Disclosure None Acknowledgement Hueylan Chern, MD, Department
What is the Best Workup for Rectal Cancer Staging: US/MRI/PET? Zhen Jane Wang, MD Assistant Professor in Residence UC SF Department of Radiology Disclosure None Acknowledgement Hueylan Chern, MD, Department
