Colorectal Cancer Network Site Specific Group. Clinical Guidelines. June 2017
|
|
|
- Doris Neal
- 5 years ago
- Views:
Transcription
1 Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Colorectal Cancer Network Site Specific Group June 2017 Revision due: April 2019 Page 1 of 31
2 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT. PLEASE DESTROY ALL PREVIOUS VERSIONS ON RECEIPT OF A NEW VERSION. Please check the SWSCN website for the latest version available here. VERSION DATE ISSUED SUMMARY OF CHANGE OWNER S NAME Draft 0.1 April 2015 First draft SWAG Colorectal SSG Draft th April 2015 Pathology guideline N Wong update Draft nd June 2015 Addition of M Williams Chemotherapy for Liver Mets Guidelines Draft nd July 2015 Removal of RAS flow diagram and points 1-5 N Wong / H Dunderdale from section 4.4 and retrospective update of version control st July 2015 Finalised Colorectal MDT Leads 1.1 April 2017 Biennial Review Colorectal MDT Leads nd May 2017 Change funding of RAS N Wong test th June 2017 Finalised H Dunderdale Page 2 of 31
3 This document has been edited by: Mike Williamson, Chair of the SWAG Colorectal SSG, Consultant Colorectal Surgeon, Royal United Hospital Bath NHS Foundation Trust Newton Wong, Consultant Histopathologist, North Bristol NHS Trust Helen Dunderdale, SWAG Cancer Network SSG Support Manager These clinical guidelines have been agreed by: Name Position Trust Date agreed Nader Francis Consultant Colorectal Yeovil District June 2017 Surgeon Hospital NHS Louise Hunt Michael Thomas Krishna Kandaswamy Ann Lyons Neil Borley Consultant Colorectal Surgeon Consultant Colorectal Surgeon Consultant Colorectal Surgeon Consultant Colorectal Surgeon Consultant Colorectal Surgeon Foundation Trust Taunton and Somerset NHS Foundation Trust University Hospitals Bristol NHS Foundation Trust Weston Area Health NHS Trust North Bristol NHS Trust Gloucestershire Hospitals NHS Foundation Trust June 2017 June 2017 June 2017 June 2017 June 2017 Page 3 of 31
4 Colorectal NSSG Contents Section 1 Contents Measures Page Introduction 2 The NSSG Agreed Clinical Guidelines for Colorectal Cancer 2.1 Investigations for Patients Suspected of having Colorectal Cancer NS/SCS/CC Imaging Guidelines Histopathology Guidelines Management of Rectal Cancer Investigation and Staging Treatment Options Surgery Neo-adjuvant Chemotherapy 13 and Radiotherapy 2.5 Management of Early Rectal 14 Cancer Investigation and Staging Post-operative MDT Management of Colon Cancer Diagnosis Investigation and Staging Surgery Post-Operative 16 Multidisciplinary Team (MDT) Meeting Postoperative Adjuvant Chemotherapy Page 4 of 31
5 Palliative Chemotherapy 16 3 The NSSG agreed Clinical 14-1C-108d 17 Guidelines for Anal Cancer 3.1 Diagnosis Investigation and Staging Multidisciplinary Team 17 meeting (MDT) Meeting, UH Bristol 3.4 Treatment Options 17 4 Management of Liver 18 Metastases 4.1 Staging Investigations at 18 Presentation of Primary Cancer 4.2 Synchronous Liver Metastases Metachronous Liver 19 Metastases 4.4 Chemotherapy Ablative Therapy Follow up after Liver 20 Resection 5 Management of Advanced 20 Colorectal Cancer 5.1 Presentation Treatment Metastatic Disease Chemotherapy Radiotherapy Palliative Care Referral for Specialist 23 Palliative Assessment 6 Follow up Guidelines NICE Guidance: Follow-up after Apparently Curative Resection 6.2 Follow-up of Patients with Anal Cancer Page 5 of 31
6 6.3 Risk Assessment and Clinical 24 Surveillance in patients with a Family History of Colorectal Cancer 7 Appendices Appendix 1: Preparation for 25 Surgical Procedures 7.2 Appendix 2: Pulmonary 25 Metastasectomy in Colorectal Cancer- Guidelines for Referral and Eligibility for the PulMiCC trial 7.3 PulMiCC Trial Design Introduction The following guidelines pertain to the local management of colorectal malignancies for the Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Network Colorectal Oncology Site Specific Group (NSSG). The NSSG refers to the National Institute for Health and Care Excellence (NICE) Colorectal Cancer clinical guidelines (December 2014): Primary care clinicians should refer to the NICE guidelines Suspected Cancer: recognition and management of suspected cancer in children, young people and adults (2015) for the signs and symptoms relevant when referring to colorectal oncology services. Further details on the two week wait referral process can be found in the NSSG constitution. The NSSG is committed to offering all eligible patients entry into clinical trials where available. Consent to provide tissue for research purposes will also be sought wherever appropriate. 2. The NSSG Agreed for Colorectal Cancer (NS/SCS/CC ) 2.1 Investigations for patients suspected of having colorectal cancer Patients with high-risk symptoms are fast tracked to an urgent two-week clinic appointment or straight to a diagnostic test where appropriate. Investigations for patients suspected of having colorectal cancer will vary according to the symptomatic presentation. The investigations should be performed as expediently as possible to allow treatment within recognised target times. Page 6 of 31
7 Change of Bowel Habit Colonoscopy is the recommended investigation and allows biopsy to better confirm the diagnosis, remove polyps and to extend the diagnostic yield for non-cancer diseases CT colonography is an alternative investigation, especially when colonoscopy has already failed or in elderly and comorbid patients using the minimal prep versions If colorectal cancer is confirmed, a CT of the chest, abdomen and pelvis will be requested. Bleeding Flexible sigmoidoscopy to splenic flexure; full colonoscopy if appropriate If colorectal cancer is confirmed, a full colonoscopy or CT colonogram will be requested to exclude disease in unseen colon. If colorectal cancer is confirmed a CT of the chest, abdomen and pelvis will be requested. Iron-deficiency Anaemia Colonoscopy and gastroscopy with D2 duodenal biopsy Minimal prep CT colonography is an alternative in elderly and comorbid patients If colorectal cancer is confirmed a CT of the chest, abdomen and pelvis will be requested. Abdominal Mass Two-week wait outpatient for clinical examination/confirmation Abdominal & pelvic (consider chest) CT scan Colonoscopy if the mass is likely to be related to the colon or after confirmation on CT. Rectal Mass Two-week wait outpatient for clinical examination/confirmation If suspected rectal cancer, a colonoscopy and biopsy or CT colonography (after biopsy in clinic) will be requested to exclude synchronous disease If suspected rectal cancer, a CT of the Chest/Abdomen/Pelvis will be requested If suspected rectal cancer, an MR Pelvis will be requested. Page 7 of 31
8 2.2 Imaging Guidelines Colonoscopy: The endoscopist should biopsy / remove lesions as appropriate. Patients will be informed about the possibility of discomfort, risks of perforation and bleeding. Doctors performing colonoscopies must audit their results and aim to have high completion (>90% caecal intubation) and low perforation (0.1%) rates. CT colonography: Allows diagnosis of colon lesions with similar specificity and sensitivity to colonoscopy but does not allow biopsy. It is particularly useful in cases where colonoscopy cannot be completed. It is a useful and safer alternative to colonoscopy in patients who cannot tolerate full bowel prep and is best performed using long oral prep. CT Chest, abdomen & pelvis: All non-allergenic patients should receive oral and intra venous contrast. Examinations should be performed to the highest specificity of the local machine. Images will be taken of the liver in the portal venous phase of contrast enhancement. Barium Enema: This is no longer regarded as a good alternative to colonoscopy or even CT colonography due to the relative lack of sensitivity and specificity compared to alternatives. It may be used when there is no alternative because of local issues with supply and demand in the interests of meeting targets. MRI Rectum: Scanning protocols should comprise sagittal T2 3mm and axial T2 5mm slices of the whole pelvis with additional axial-oblique T2 high resolution slices perpendicular to the plane of the rectal tumour. 2.3 Histopathology guidelines The NSSG guidelines for the examination and reporting of colorectal cancer specimens refer to the following publications: Dataset for colorectal cancer histopathology reports (3rd edition). The Royal College of Pathologists, orectaldataset_july14.pdf TNM classification of malignant tumours (7th edition). UICC, 2009 WHO Classification of Tumours of the Digestive System. IARC Press, 2010 Reporting lesions in the NHS bowel cancer screening programme. Guidelines from the Bowel Cancer Screening Programme Pathology Group. NHS Cancer Screening Programmes, Specimen Types Colon: Endoscopic biopsies, Polypectomy specimens, Colectomy specimens. Rectum: Endoscopic biopsies, Polypectomy specimens, TEM specimens, Anterior Resection specimens, Abdomino-perineal Resection specimens. Page 8 of 31
9 Colorectal Cancer Specimen Examination Refer to: Dataset for colorectal cancer histopathology reports (3rd edition). The Royal College of Pathologists, ectaldataset_july14.pdf Grading and Staging of Colorectal Cancers Refer to: Dataset for colorectal cancer histopathology reports (3rd edition). The Royal College of Pathologists, ectaldataset_july14.pdf In particular, the Colorectal Lead Pathologists should ensure that completed staging data is reported for all cancer polyps, i.e. invasive adenocarcinoma arising within a locally resected adenoma/polyp. This data (which includes minimum clearance from resection margins, and Kikuchi and/or Haggitt levels) are outlined in the Royal College of Pathologists dataset for colorectal cancer histopathology reports (3rd edition), Clinical Governance Pathologists reporting colorectal cancer resection specimens will undertake the following tasks: Participate in relevant EQA schemes Have access to and be familiar with the abovementioned publications 1)-3) and, if participating in the Bowel Cancer Screening Programme, publication 4). Bowel Cancer Screening Programme (BCSP) Pathologists examine, dissect and report specimens derived from BCSP patients according to the Guidelines from the Bowel Cancer Screening Programme Pathology Group, NHS Cancer Screening Programmes BCSP pathologists should participate in the National BCSP EQA scheme. Proforma for Reporting of Colorectal Cancer Resections Refer to the Proforma for Colorectal Cancer Resections as published by the Royal College of Pathologists. Dataset_July14.pdf Proforma for Reporting of Colorectal Local Excision Specimens Refer to the Proforma for Local Excision Specimens as published by the Royal College of Pathologists. Dataset_July14.pdf Page 9 of 31
10 Standard operating protocol for reflex mismatch repair immunohistochemistry of colorectal carcinoma This standard operating protocol addresses the guidance issued in the 2014 Royal College of Pathologists dataset for colorectal carcinoma (CRC): "In summary, MMR immunohistochemistry is currently considered a core dataset item for patients under 50 years at the time of diagnosis and for patients, in whom an assessment of prognosis is appropriate, with adenocarcinomas classified as poorly differentiated morphologically or tumours showing other morphological features of MMR deficiency". 1. Reflex mismatch repair (MMR) immunohistochemistry should be performed on CRCs resected from patients less than 50 years of age at time of diagnosis. By contrast, MMR immunohistochemistry for assessment of prognosis should be an on-demand process; it is anticipated that these requests will come from oncologists. 2. The reflex MMR testing should be organised by the Histopathology Department that has received and reported the CRC resection specimen. 3. When the histopathology of the CRC resection specimen is presented at the local Lower GI MDT meeting, the MDT should be informed that MMR immunohistochemistry data is awaited for the patient s CRC. 4. The local Lower GI MDT should take responsibility for chasing up this data. 5. Once the completed MMR data is presented to the local Lower GI MDT and if there is evidence of MMR deficiency, the MDT should refer the patient to its local Clinical Genetics service. 6. This reflex testing does not include microsatellite instability (MSI), BRAF mutation or MLH1 hypermethylation analyses. 7. This reflex testing does not replace pre-existing local MDT protocols for identifying potential Lynch syndrome patients for referral to Clinical Genetics. MMR CRC SOP v1.0 (Newton Wong, 6 May 2015) 2.4 Management of Rectal Cancer Defined as tumours below the sacral promontory/within 18 cms of anal verge Investigation and staging: Assessment of patient s general health and operative risk: ASA and PPOSSUM score if available. Record ASA and WHO Performance Status for NBOCAP database Digital examination (consider EUA in difficult cases) Bloods (FBC, U&E, LFT), baseline CEA Page 10 of 31
11 Colonoscopy or CT colonography to visualise the colon (or within 6 months of potentially curative surgery performed as an emergency) CT chest, abdomen & pelvis to exclude metastatic disease MR rectum to assess operability / at risk CRM MR liver if liver metastases to assess suitability for hepatic resection. All patients with liver metastases will be referred to a regional hepatobiliary MDT for their opinion about optimal management All patients with potentially resectable lung metastases on CT will be referred to the regional lung MDT for their opinion about optimal management Early rectal cancer assessed by high quality MRI and EUS as stage T1 should be considered for local resection. Patients considered suitable for Transanal Endoscopic Microsurgery (TEMs) should be referred to UH Bristol or Cheltenham. All surgeons undertaking TEMS are accredited to perform this procedure PET will be considered when there is a high clinical or biochemical suspicion of systemic, metastatic or local recurrence in the context of negative or equivocal CT where there are further treatment options All investigations and histopathology results are discussed at the local MDT before treatment Treatment Options Patients with rectal cancer are managed by surgeons with appropriate training who have completed the national TME, MDT Development Programme or equivalent, LOREC, and have fully audited outcome measures, and are working within a multidisciplinary team. Preoperative long course chemo-radiotherapy is indicated if clinical examination (fixed, tethered) and/or MR indicate that circumferential margins are potentially at risk i.e. R1 or R2 resections. Preoperative short course radiotherapy should be considered if MR indicates that margins are likely to be clear but there is a higher than usual risk of loco-regional recurrence i.e. major lymph node involvement. Careful consideration should be given to the relative merits of palliative resection for rectal cancer, as should treatment with endoluminal stenting and palliative radiotherapy. When a stoma is required or considered a possibility, all patients should see an accredited stoma nurse, ideally prior to admission, to allow adequate time for preparation. The first definitive treatment is normally the first intervention intended to remove or shrink the tumour. Where there is no anti-cancer treatment, palliative intervention/best supportive care will be recorded. Page 11 of 31
12 2.4.3 Surgery Preparation for Surgery refer to Appendix 1 Upper third rectal cancer: Anterior Resection Excision outside mesorectum with pelvic autonomic nerve preservation aiming for minimum of 5cms clearance below the lower edge of the cancer Cytocidal washout of rectal stump & peritoneal cavity is recommended Covering stoma only when necessary. Middle & lower third rectal cancer: Low Anterior resection Total Mesorectal Excision (TME) with pelvic autonomic nerve preservation Cytocidal washout of rectal stump & peritoneal cavity is recommended Consider a colonic pouch Temporary covering stoma should be considered in most cases. Low rectal cancer: Where distal clearance <1cm, sphincter involvement, poor anal tone Intersphincteric Abdomino-Perineal Excision (APERi): For low but early rectal cancer where the anal sphincter cannot be preserved (consider entering patients into a trial of local excision). For rectal cancer in patients with poor anal tone as an alternative to TME Hartmann s (see below). Standard abdomino-perineal Excision (APERs): For low rectal cancer within or just beyond the rectal wall in whom intersphincteric dissection would risk an involved margin. Extra-Levator Abdominal Perineal Excision (ELAPE): For locally advanced low rectal cancers (within 6cm from the anal verge) there is a national drive to promote extra-levator technique of abdominal perineal excision (ELAPE). This process has gained popularity because of the associated low intra-operative tumour perforation rates and subsequent local recurrence. It involves excision of the anus outside the levator plate to ensure enough clear tissue around the tumour. TME Hartmann s: Frail, elderly or incontinent patients with rectal cancer technically suitable for restorative surgery in whom there is a desire to avoid restoration of bowel continuity. An alternative to APERi. Metastatic / Disseminated Disease: Unexpected/suspicious metastases within the abdomen should be biopsied. Biopsy/FNA liver lesions within the liver substance should not be undertaken without prior discussion with regional hepatobiliary surgeons. Page 12 of 31
13 Early rectal cancer - local excision see 2.5 below Following pre-operative T1 stage assessment by EUS & MR Performed by an adequately trained surgeon after discussion at MDT Neo-adjuvant chemotherapy and radiotherapy Discussion about the use of neo-adjuvant chemo/radiotherapy takes place at the MDT meeting. Preoperative Long course chemo-radiotherapy will be considered when: MR predictive of positive circumferential margin (R2) or threatened <1mm margin (R1) Schedule: Gy in fractions in 5 weeks given to a planned volume using a 3-4 field technique covering the tumour and an adequate margin Chemotherapy (5 Fluorouracil and folinic acid) is given daily in the first and fifth weeks of treatment Patients are reassessed after 2-4 weeks with further imaging ± PR or EUA. Short-course radiotherapy will be considered when: MRI predictive of R0 resection but advanced T3 tumour / major lymph node involvement Schedule: 25 Gy in 5 fractions in 1 week. Surgery is scheduled within 7 days of completion. Post-Operative Multidisciplinary Team (MDT) Meeting Surgical treatment must be judged as curative or palliative. Consider the place of post-operative radiotherapy or chemotherapy: Postoperative Radiotherapy MDT to consider long-course chemo-radiotherapy when: Circumferential margin is +ve (R2) or threatened <1mm (R1) The schedule is a 5 week course of Gy and 25 fractions using a 3-4 field technique covering the posterior pelvis Chemotherapy (5 Fluorouracil and folinic acid) is given daily in the first and fifth weeks of treatment Patients with co-morbidity may receive radiotherapy as above or as a short course, both without chemotherapy. Page 13 of 31
14 Postoperative adjuvant chemotherapy Adjuvant chemotherapy usually recommended for all T4 NX tumours and TX N1-2, also for high risk T3 N0 tumours as judged by extramural vascular invasion. Patients should be counselled on the relative risks and benefits of treatment and understand that a "no chemotherapy approach is an option Patients with favourable T3 tumours less than 80 years of age may be offered a discussion with the oncologist to discuss the potential but small benefits of therapy Chemotherapy may not be appropriate for patients with significant co-morbidity (WHO performance status), high frailty index or extremes of age when unacceptable toxicity a high probability, or their likelihood of death from those diseases affects any reduction in the risk of cancer recurrence or death. Palliative chemotherapy Patients who have un-resectable disease or undergo palliative surgery should be considered for palliative chemotherapy and offered the opportunity to meet with an oncologist. Patients unfit for chemotherapy should receive best supportive care 2.5 Management of Early Rectal Cancer Investigation and Staging Preoperative staging includes: Digital rectal examination Endoscopic evaluation with biopsy CT chest / abdomen / pelvis MR rectum Endoscopic ultrasound is highly recommended. Accurate staging will determine which patients may be candidates for local excision. In fit patients local excision by TART or preferably TEMS (or equivalent technique) may be considered for early rectal cancers with good prognostic features: T1 (sm1) cancer on TRUS Small (<4 cm) tumours Good histological prognostic features (well to moderately differentiated adenocarcinoma) Mobile In patients unfit for major resection local excision of more advanced early rectal cancers, with or without radiotherapy, may be considered to try and achieve local control. Page 14 of 31
15 2.5.2 Post-operative MDT Discovery of poor prognostic features on histopathology, including deeper or margin involvement, poor differentiation, and or vascular or perineural invasion, should lead to a discussion about the merits of a formal resection or post-operative chemo-radiotherapy. Most early rectal cancers are diagnosed when a local excision is performed for a benign lesion and incidental cancer is found. If TEMS / TART or EMR has achieved a clear margin with good prognostic histological features, no further intervention will be necessary. Mr Mike Thomas (UH Bristol) and Mr Neil Borley (Cheltenham) provide the Network TEMS service. 2.6 Management of Colon Cancer Diagnosis Diagnosis usually occurs after a colonoscopy and a biopsy. Histological confirmation of radiologically diagnosed cancer is desirable but not essential; however, every effort should be made when radiology is equivocal and especially for right-sided cancers Investigation and Staging Patient s general health and operative risk (ASA, WHO PS & PPOSSUM) Bloods (FBC, U&E, LFT) Baseline CEA Colonoscopy or CT colonography to visualise the non-visualised colon prior to surgery (or within 6 months of potentially curative surgery performed as an emergency) CT scan chest, abdomen & pelvis to exclude metastatic disease MRI liver if liver metastases to assess suitability for hepatic resection. All patients with liver metastases will be referred to the regional hepatobiliary MDT for their opinion about optimal management All patients with potentially resectable lung metastases will be referred to the regional lung MDT for their opinion about optimal management PET will be considered when there is a high clinical or biochemical suspicion of systemic, metastatic or local recurrence in the context of negative or equivocal CT where there are further treatment options All investigations and histopathology results are discussed at the local MDT before treatment. Page 15 of 31
16 2.6.3 Surgery Preparation for surgery refer to Appendix 1 Colonic resection Laparoscopy is offered to every suitable patient in the NSSG in accordance with NICE Guidelines For most fit patients, excision will be by segmental resection to include high ligation of feeding arterial supply with associated lymph nodes Biopsy any suspicious lesions within the abdomen Do not biopsy / FNA liver lesions within liver substance unless discussed with the regional hepatobiliary MDT De-functioning stomas may be required (consider if cancer not resectable or bypass not possible). Colonic Stenting (SEMS) Consider this option to ameliorate obstructive symptoms where disseminated disease is present or the patient is too unfit for surgical resection SEMS as a bridge-to-surgery in patients with acute bowel obstruction by probable cancer should be discussed with the patient as an alternative to Hartmann s procedure and you are encouraged to enter patients in an accredited randomised trial Post-Operative Multidisciplinary Team (MDT) Meeting Surgical treatment must be judged as curative or palliative. Consider the place of post-operative chemotherapy: Postoperative adjuvant chemotherapy Adjuvant chemotherapy usually recommended for all T4 NX tumours and TX N1-2, also high risk T3 N0 tumours as judged by extra-mural vascular invasion. Patients should be counselled on the relative risks and benefits of treatment and understand that a "no chemotherapy approach is an option Patients with favourable T3 tumours less than 80 years of age may be offered a discussion with the oncologist to discuss the potential but small benefits of therapy Chemotherapy may not be appropriate for patients with significant co-morbidity (WHO performance status), high frailty index or extremes of age when unacceptable toxicity is a high probability, or their likelihood of death from those diseases affects any reduction in the risk of cancer recurrence or death. Page 16 of 31
17 Palliative chemotherapy Patients who have un-resectable disease or undergo palliative surgery should be considered for palliative chemotherapy and offered the opportunity to meet with an oncologist. Patients unfit for chemotherapy should receive best supportive care. 3. The NSSG agreed for Anal Cancer This service is centralised at University Hospitals Bristol NHS Foundation Trust and Gloucestershire Royal Hospitals NHS Foundation Trust. 3.1 Diagnosis Achieved by biopsy - histological confirmation is essential Consider examination under anaesthesia in difficult cases FNAC any palpable or radiologically enlarged inguinal lymph nodes. 3.2 Investigation and Staging Patient s general health (WHO performance status) CT scan chest, abdomen & pelvis Consider the role of pelvic MRI to aid staging. 3.3 Multidisciplinary Team meeting (MDT) Meeting, UH Bristol Radiotherapists and surgeons who are part of the specialist MDT at UH Bristol and GLOS treat anal cancer. The SWAG NSSG recognises that the principle management of anal squamous cell carcinoma (SCC) is chemo-radiotherapy rather than resectional surgery. Individual Trust based MDTs should confirm the histological diagnosis and stage of the disease, and then refer on to the Network Anal Cancer MDT (Consultant Clinical Oncologist and /or his Deputy) for discussion about the various options (radical chemo-radiotherapy or palliative radiotherapy) in light of the local extent of the tumour, the patient s co-morbidities and wishes. Patients requiring pre-treatment stomas are to be referred back to the referring surgeon and CNS. 3.4 Treatment Options Histologically confirmed complete excision of an early lesion may be the sole treatment Radical chemo-radiotherapy will be the standard treatment Consider palliative radiotherapy for advanced disease / comorbid patients Consider palliative defunctioning stoma for advanced disease / comorbid patients Eligibility of entry into the national portfolio of clinical trials Salvage surgery: consider other specialities e.g. plastic surgery, urology and gynaecology Where palliative treatment is performed or best supportive care advised consider further supportive therapies and follow-up by the Palliative Care Team Page 17 of 31
18 MDT decision and follow-up plan to be documented in patient s notes & conveyed to the patient/carers, referring surgical team/cns, and the patient s GP. 4. Management of Liver Metastases Colorectal MDTs should refer patients with liver metastases, selected according to the network guidelines, to the liver resection MDT. All patients with potentially curable liver metastases from colorectal cancer will be considered for liver resection, and referred for discussion to the regional hepatobiliary MDT at UHB (Basingstoke from RUH Bath, Birmingham from Cheltenham/Gloucester) Unsuitable cases for referral include patients with uncontrollable extra-hepatic disease defined as: Non-treatable primary tumour Wide-spread pulmonary disease Loco-regional recurrence Peritoneal disease Retroperitoneal, mediastinal or portal nodes Bone or CNS metastases. Or, the patient is unwilling to have further treatment or is unfit for further treatment. Whilst patients with more than 5 multifocal metastases are less likely to benefit from surgery, each case will be looked at individually. All patients with liver metastases with a performance status appropriate for chemotherapy +/- surgery will be referred to the regional hepatobiliary MDT. Patients with unresectable liver disease should be considered for chemotherapy +/- cetuximab as per the NICE Guidelines. The decision to undertake RAS testing will usually be made at the local MDT. 4.1 Staging investigations at presentation of primary cancer All patients will have undergone a staging CT scan of the chest, abdomen and pelvis with intravenous contrast (ideally at a maximum collimation of 5mm), the whole colon visualised (barium enema or colonoscopy) and a baseline CEA measurement performed. Increasingly, PET scanning is requested and can be requested by the HPB MDT or the local MDT if the indication is obvious. 4.2 Synchronous liver metastases Colorectal cancer and liver resection are not normally performed synchronously. Patients found to have synchronous liver metastases at the time of their initial presentation should undergo a contrast enhanced liver MRI scan and be referred to/discussed at the designated Network HPB MDT (Bristol Royal Infirmary / Basingstoke / Birmingham). Patients with potentially resectable disease, who have undergone radical resection of their primary bowel cancer, should be considered for 3 months of adjuvant chemotherapy (FOLFOX 4) prior to Page 18 of 31
19 any liver resection. These patients will be restaged at 3 months by MRI. A further 3 months of chemotherapy will be considered post any potentially curative liver resection. 4.3 Metachronous liver metastases Follow-up after resection of the primary colorectal cancer will be according to local protocol. The NSSG recommends that a CT scan of the chest, abdomen and pelvis are performed as a minimum in the 2 years following completion of treatment of the primary tumour. Patients found to have liver metastases during follow up will have a treatment plan discussed at the Network HPB MDT. If there is no extrahepatic disease on axial CT scanning, all patients will have a contrast enhanced MRI scan. Biopsy of liver lesions would not be performed without prior discussion at the Network HPB MDT. Patients should be considered for 3 months of neoadjuvant chemotherapy before any liver resection. 4.4 Chemotherapy All patients with liver only metastases with a performance status appropriate for surgery +/- chemotherapy will be referred to the regional hepatobiliary MDT. In all cases of metastatic colorectal cancer, molecular analyses to determine the presence of mutations in KRAS and NRAS (collectively known as RAS) should be performed as soon as the diagnosis is made and treatment is being considered. RAS Mutation Anti-EGFR targeted therapy should not be incorporated into the treatment algorithm of patients with RAS mutations RAS Wild type Anti-EGFR targeted therapy can be incorporated into the treatment algorithm of patients with wild-type RAS The decision to undertake RAS testing will usually be taken at the CRC MDT. Patients with potentially resectable liver metastases without unresectable extrahepatic disease should be considered for neoadjuvant chemotherapy (+/- cetuximab as per the NICE Technology Appraisal 176 Guidelines). See management Algorithmhttp:// Patients with unresectable liver metastases +/- extrahepatic disease should be considered for palliative chemotherapy. RAS mutation testing is requested via the CDF for these patients by an oncologist. The following metastatic colorectal cancer patient treatment algorithms are routinely funded by NHS England via the cancer drug fund (12 March 2015 V4.0 ) Algorithm Page 19 of 31
20 RAS Testing Overview RAS Testing Guidelines for mcrc In order to guide molecularly targeted therapy, and in line with the product licenses of currently approved treatments, NICE TAG 176 and CDF treatment access criteria, RAS testing requests should be made at the earliest opportunity to inform the 1 st line treatment clinical decision making process. RAS test requests will typically be initiated by oncologists at the CRC MDT. Systematic and robust testing pathways should ensure that every patient who qualifies for a test is offered one in a timely manner, with results being accessible by the treating oncologist at the time of the patient s first line treatment consultation Clear and unambiguous local responsibilities for RAS test requests, tissue sample handing, molecular analysis and reporting of the results should exist within each local Trust to ensure that necessary tests are requested timely and avoid unnecessary delays to patient access to treatment. Where RAS testing is performed on demand, rapid turnaround times (from test request to result) are critical to avoid treatment delays. Molecular genetics laboratory turnaround times for RAS testing should be 7 working days from receipt of the specimen in the testing laboratory to issuing of the final report, for >90% of specimens Pathology laboratories should ensure that their RAS diagnostic testing covers all relevant genes that appear in the Summary of Product Characteristics published of the available treatment options. For RAS testing of CRC patients, these should include analysis of at least KRAS codons 12, 13, 59, 61, 117 and 146 and NRAS codons 12, 13, 59 and 61. When integrated into pathology reports, RAS test results should accurately convey the information the clinician needs to treat the patient on whom the test was performed, with sufficient information to allow correct interpretation of the results. RAS tests are funded by NHS England. 4.5 Ablative therapy Patients not suitable for liver resection (e.g. extensive co-morbidity, patient choice, irresectable disease) may be offered ablative treatment (radiofrequency ablation). This would be discussed at the HPB MDT. 4.6 Follow up after liver resection Follow up will be agreed in collaboration with the HPB MDT according to HPB and Colorectal MDT Page 20 of 31
21 guidelines, in order to direct follow up for both liver and colorectal disease. CEA levels should be monitored at least 6 monthly. Six monthly reviews should be considered for 3 years and then on an annual basis thereafter. Follow up will be shared between either the HPB team and the oncology team or base colorectal team. In patients who develop a local liver recurrence it may be appropriate to consider referral for re-resection and/or ablation as well as chemotherapy. Patients will remain under the care of the local colorectal team for surveillance colonoscopy according to local protocol. 5. Management of Advanced Colorectal Cancer 5.1 Presentation Inoperable primary disease Inoperable metastatic disease Loco-regional recurrence. 5.2 Treatment For patients with inoperable rectal cancer, (fixed or tethered on clinical examination and/or MRI indicates that margins are potentially at risk i.e. R1 or R2 resections) without evidence of metastatic spread, preoperative long course chemo-radiotherapy should be offered to downstage the cancer in the hope of making it resectable for potential cure. Although there is less good evidence, for patients with apparently inoperable colonic cancer on CT scanning, without evidence of metastatic spread, preoperative chemotherapy may be offered to downstage the cancer in the hope of making it resectable for cure or could become palliative. Page 21 of 31
22 5.3 Metastatic Disease Colorectal MDTs should refer patients with liver metastases, selected according to the network guidelines, to the liver resection MDT (see above) Colorectal MDTs should refer patients with lung metastases, selected according to the network guidelines, to the appropriate chest MDT. Lung resection to be considered in patients where: Primary disease has been radically removed Resectable colorectal metastases confined to the lung and/or liver. 5.4 Chemotherapy Patients with incurable disease, and who are fit enough, should be offered the opportunity to discuss the benefits and disadvantages of chemotherapy with palliative intent. 5.5 Radiotherapy Radiotherapy can be an effective form of treatment for palliation of rectal bleeding, tenesmus and rectal pain. It should be considered for patients with inoperable advanced disease and recurrent disease (not suitable for resection). 5.6 Palliative Care Palliative care is an integral component of the management of incurable cancer and should be practised by all the members of the MDT. The key components are: A focus on quality of life, which includes good symptom control A whole person approach, taking into account past experiences and current situation Respect for patient autonomy and choice Open and sensitive communication between patients, carers and professional colleagues. Page 22 of 31
23 5.7 Referral for Specialist Palliative Assessment The patient should meet one or both of the following criteria: Incurable disease with the focus being on quality of life The patient has one or more of the following needs, which cannot be met by the referring team: Uncontrolled / complicated symptoms Complex psychological / emotional issues Complex social / family issues Difficult decision making about appropriate future carers. 6. Follow up Guidelines Colon Cancer & Rectal Cancer It is expected that most Trusts in the SWAG Network will adhere to the NICE guidelines on follow-up after resection of colon and rectal cancer. There is a national move towards remote surveillance programmes minimising the need for patient attendance at clinic. Consideration may be given to specialist nurse lead clinics and telephone follow-up. 6.1 NICE Guidance: Follow-up after apparently curative resection Offer follow-up to all patients with primary colorectal cancer undergoing treatment with curative intent. Start follow-up at a clinic visit 4 6 weeks after potentially curative treatment. Offer patients regular surveillance with: a minimum of two CTs of the chest, abdomen, and pelvis in the first 3 years and regular serum carcinoembryonic antigen tests (at least every 6 months in the first 3 years). Offer a surveillance colonoscopy at 1 year after initial treatment. If this investigation is normal consider further colonoscopic follow-up after 5 years, and thereafter as determined by cancer networks. The timing of surveillance for patients with subsequent adenomas should be determined by the risk status of the adenoma. Start reinvestigation if there is any clinical, radiological or biochemical suspicion of recurrent disease. Stop regular follow-up: when the patient and the healthcare professional have discussed and agreed that the likely benefits no longer outweigh the risks of further tests or when the patient cannot tolerate further treatments. 6.2 Follow up of Patients with Anal Cancer Page 23 of 31
24 Follow-up of patients with anal cancer is the responsibility of the anal cancer MDT at UH Bristol. The majority of these patients will be under the follow-up of the Lead Clinical Oncologist. Patients with disease relapse will be discussed at the UH Bristol Colorectal Cancer MDT (de facto Anal Cancer MDT) for the consideration of salvage surgery. The NSSG salvage surgery service is run by Mr M Thomas and his colleagues in UH Bristol. 6.3 Risk Assessment and Clinical Surveillance in patients with a Family History of colorectal cancer Referral for screening colonoscopy and / or specialist genetic counselling will be considered for the following patients: CRC in single first degree relative (FDR) <50 years or 2 FDR of any age. Risk 1 in 12. Once-only colonoscopy at age 55 CRC in 2 FDR mean age <60 years or 3 FDR with first degree kinship* Risk between 1 in 6 and 1 in 10 suggest referral to geneticist Colonoscopy age 50 then 5 yearly colonoscopy to age 75 Less strong family history of CRC. Risk >1 in 12. No colonoscopy offered (UK background population risk for colorectal cancer is 1 in 18) N.B. first degree kinship* - affected relatives who are first-degree relatives of each other AND at least one is a first degree relative of the patient Colorectal cancer associated with other characteristic tumours in same individual/ family (ovary, urothelium, small bowel, biliary tree) Surveillance and genetic testing should be offered to all Familial Adenomatous Polyposis (FAP) families and Hereditary Non-polyposis Colorectal Cancer (HNPCC) / Lynch families that either meet the Amsterdam or Amsterdam II criteria (3 affected relatives inn 2 successive generations of which one is under the age of 50) or have confirmed mismatch repair gene mutations. Page 24 of 31
25 7. Appendices Preparation for Surgical Procedures 7.1 Appendix 1 Informed consent, detailing the benefits and risks of the procedure versus alternatives including no surgery, will be obtained by the operating surgeon or delegated trainee with sufficient knowledge and training Appropriate treatment options will be discussed fully with all patients including laparoscopic intervention Consider iron infusion to restore iron deficiency anaemia prior to elective surgery Bowel preparation according to the local surgeon s preference / mechanical bowel preparation provided there is no incipient obstruction Anti-thrombotic & antibiotic prophylaxis according to local policy Group and save will be sufficient for most colorectal cancer resections dependent upon local transfusion facilities. Consider cross-match for pelvic resections and pre-operative transfusion if haemoglobin level (Hb) < 8 or if the patient is symptomatic) Counselling, guidance and support Stoma marking if applicable HDU / ITU facilities if required. 7.2 Appendix 2 Pulmonary Metastasectomy in Colorectal Cancer - Guidelines for referral and eligibility for the PulMiCC trial (currently being updated) Authors: Tim Batchelor, Consultant Thoracic Surgeon (UH Bristol) Katrina Hurley, Research Nurse (UH Bristol) Introduction There is an absence of good quality evidence to support the role of pulmonary metastasectomy in colorectal cancer. Subsequently, there is great variation in clinicians' practices regarding selection of patients suitable for the procedure. From the multiple case series in the literature we know that multiple pulmonary metastases, a raised CEA and a short disease-free interval are poor prognostic factors. Conversely, almost all patients with a solitary pulmonary nodule, a normal CEA and a long disease-free interval would be considered for surgery. Page 25 of 31
26 A randomised trial of pulmonary metastasectomy in colorectal cancer (PulMiCC) has opened in the UK, initially as a feasibility study. Bristol is the sixth centre to start recruiting. The null hypothesis is that pulmonary metastasectomy does not alter the natural history of the disease. The PulMiCC study has allowed us to formulate selection criteria for pulmonary metastasectomy whether a patient wishes to be enrolled in the study or whether they would prefer to proceed with surgery (or indeed no surgery) off trial. Consequently, those patients with one nodule should be referred for surgery while those with 5 or more nodules are not eligible for surgical management. Those patients with 2-4 nodules (the so-called group of uncertainty ) should be considered for the PulMiCC trial. If a patient does not wish to be randomised they should be referred for surgery. The solitary pulmonary nodule Patients on routine follow up following resection of primary colorectal cancer may have a solitary pulmonary nodule discovered on routine surveillance CT scans or as a result of some specific trigger such as symptoms or a raised CEA. The possibility that this is a primary lung cancer must be considered by the lung multidisciplinary team (MDT). The patient should then be referred for surgical resection. Eligibility for surgery: Patients with primary colorectal cancer who have undergone resection of the primary cancer with intent to cure Local control has been confirmed and no clinical indications of other active colorectal cancer other than the known pulmonary nodule (PET-CT required) Pulmonary function adequate to sustain good performance after the largest likely loss of parenchyma (spirometry required) ECOG performance status 0-1 Any recommended systemic or other non- surgical treatment has been completed. 5 or more pulmonary metastases Those patients who are discovered to have 5 or more pulmonary metastases will not be considered for pulmonary metastasectomy. Furthermore, they will not be considered eligible for the PulMiCC trial. They should be treated as per local practice guidelines. Referral to thoracic surgery for a lung biopsy should be considered if the diagnosis is in doubt and a diagnosis cannot be achieved by less invasive procedures. The group of uncertainty : 2 to 4 pulmonary metastases Patients on routine follow up following resection of primary colorectal cancer may have pulmonary metastases discovered on routine surveillance CT scans, or as a result of some specific trigger such as symptoms or a raised CEA. The results should be discussed in the colorectal MDT meeting where radiological evidence of pulmonary metastases will be first presented by the radiologist. If there is agreement within the colorectal MDT that the multiple pulmonary nodules most likely represent metastatic colorectal cancer then the patient s case does not need to be discussed at a lung MDT meeting. Page 26 of 31
27 Patients fulfilling the following criteria will be eligible for registration for evaluation for the PulMiCC trial. Even if a patient does not want to be considered for the trial they should be encouraged to register. Inclusion criteria for surgery or registration for PulMiCC: Patients with primary colorectal cancer who have undergone resection of the primary cancer with intent to cure Local control has been confirmed No clinical indications of other active colorectal cancer other than the known lung metastases (PET-CT required) Pulmonary function adequate to sustain good performance after the largest likely loss of parenchyma (spirometry required) ECOG performance status 0-1. Exclusion criteria: Previous malignancy likely to interfere with protocol treatment or measurement of endpoints Any concurrent illness which could interfere with the treatment protocol or confound survival Unavailable for follow up and assessment according to protocol Psychiatric or mental incapacity that precludes fully informed consent. Exceptions Surgery will not normally be considered for 5 or more pulmonary metastases. However, rare cases where the nodules are unilateral and preferably within one lobe will be considered after specific discussion with the thoracic surgeons The PulMiCC trial Informed consent for registration The clinical team member at the base hospital designated to inform potentially eligible patients of the MDT findings will explain the situation, indicating explicitly the uncertainty surrounding the management of pulmonary metastases. The trial will be introduced and interested patients will be given a patient information leaflet and DVD to take home. Arrangements will be made for a follow up discussion in the thoracic surgical clinic in Bristol when patients who express an interest in the study will be invited to discuss the trial further and ask questions. Patients who confirm that they are willing to join stage 1 of the trial will be asked to sign a registration for evaluation consent form. Patients who do not wish to be considered for registration will be offered surgery off trial. Evaluation following registration Patients should be evaluated according to local practice but investigations should also include the following: Page 27 of 31
28 ECOG performance status assessment PET-CT (half body) Histology/cytology to confirm the nature of the nodules if there is clinical uncertainty concerning their nature (this is not mandatory) Full blood count, serum biochemistry and liver function tests CEA measurement Lung spirometry Further lung function tests if there is uncertainty about fitness for surgery according to BTS guidelines Weight. Evaluation for randomisation Following completion of investigations all patients should be evaluated according to local practice guidelines. Some teams may offer systemic treatment. This can be given and the patients re-evaluated according to the template below following treatment. If the best course of action with respect to surgery of the lung metastases seems clear (i.e. no surgery if 5 or more metastases or unfit for lung surgery; or surgery if only one nodule) at this point, these patients will not take any further part in the trial. However, the status of these patients at one year after registration will be recorded by the centre using information from the MDT. Patients for whom uncertainty exists whether surgery would improve survival and/or quality of life are eligible for the second stage of the trial. Patients fulfilling the following criteria will be eligible for randomisation. Inclusion criteria for randomisation: One or more nodules histologically/cytologically confirmed as metastases from colorectal cancer OR >90% likelihood of being metastases from colorectal cancer Pulmonary function adequate to sustain good performance after the largest likely loss of parenchyma (calculated as the predicted postoperative FEV1 according to BTS guidelines) ECOG performance status 0-1 Any recommended systemic or other non-surgical treatment has been completed Available for trial assessments and follow up Consent form has been signed. Exclusion criteria: Patients with a nodule which is proven or is likely to be lung cancer Concurrent disease that may interfere with protocol treatment or measurement of endpoints. Informed consent for randomisation and accept or decline questionnaire Page 28 of 31
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases
 Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
ADJUVANT CHEMOTHERAPY...
 Colorectal Pathway Board: Non-Surgical Oncology Guidelines October 2015 Organization» Table of Contents ADJUVANT CHEMOTHERAPY... 2 DUKES C/ TNM STAGE 3... 2 DUKES B/ TNM STAGE 2... 3 LOCALLY ADVANCED
Colorectal Pathway Board: Non-Surgical Oncology Guidelines October 2015 Organization» Table of Contents ADJUVANT CHEMOTHERAPY... 2 DUKES C/ TNM STAGE 3... 2 DUKES B/ TNM STAGE 2... 3 LOCALLY ADVANCED
COLORECTAL CARCINOMA
 QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services. Cancer of Unknown Primary Network Site Specific Group. Clinical Guidelines
 Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Cancer of Unknown Primary Network Site Specific Group Revision due: April 2019 Page 1 of 11 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Cancer of Unknown Primary Network Site Specific Group Revision due: April 2019 Page 1 of 11 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
Clinical guideline Published: 1 November 2011 nice.org.uk/guidance/cg131
 Colorectal cancer: diagnosis and management Clinical guideline Published: 1 November 2011 nice.org.uk/guidance/cg131 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Colorectal cancer: diagnosis and management Clinical guideline Published: 1 November 2011 nice.org.uk/guidance/cg131 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012
 Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012 Version Control This is a controlled document please destroy all previous versions on receipt
Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012 Version Control This is a controlled document please destroy all previous versions on receipt
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
RECTAL CANCER APPARENT COMPLETE RESPONSE (acr) AFTER LONG COURSE CHEMORADIOTHERAPY
 COLORECTAL CLINICAL SUBGROUP RECTAL CANCER APPARENT COMPLETE RESPONSE (acr) AFTER LONG COURSE CHEMORADIOTHERAPY Finalised by: Dr Simon Gollins Mr Andrew Renehan Dr Mark Saunders Mr Nigel Scott Dr Shabbir
COLORECTAL CLINICAL SUBGROUP RECTAL CANCER APPARENT COMPLETE RESPONSE (acr) AFTER LONG COURSE CHEMORADIOTHERAPY Finalised by: Dr Simon Gollins Mr Andrew Renehan Dr Mark Saunders Mr Nigel Scott Dr Shabbir
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: diagnosis and management of colorectal cancer 1.1 Short title Colorectal cancer 2 The remit The Department
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: diagnosis and management of colorectal cancer 1.1 Short title Colorectal cancer 2 The remit The Department
RECTAL CANCER CLINICAL CASE PRESENTATION
 RECTAL CANCER CLINICAL CASE PRESENTATION Francesco Sclafani Medical Oncologist, Clinical Research Fellow The Royal Marsden NHS Foundation Trust, London, UK esmo.org Disclosure I have nothing to declare
RECTAL CANCER CLINICAL CASE PRESENTATION Francesco Sclafani Medical Oncologist, Clinical Research Fellow The Royal Marsden NHS Foundation Trust, London, UK esmo.org Disclosure I have nothing to declare
IMAGING GUIDELINES - COLORECTAL CANCER
 IMAGING GUIDELINES - COLORECTAL CANCER DIAGNOSIS The majority of colorectal cancers are diagnosed on colonoscopy, with some being diagnosed on Ba enema, ultrasound or CT. STAGING CT chest, abdomen and
IMAGING GUIDELINES - COLORECTAL CANCER DIAGNOSIS The majority of colorectal cancers are diagnosed on colonoscopy, with some being diagnosed on Ba enema, ultrasound or CT. STAGING CT chest, abdomen and
PATHOLOGY GROUP GUIDELINES FOR THE EXAMINATION AND REPORTING OF COLORECTAL CANCER SPECIMENS
 PATHOLOGY GROUP GUIDELINES FOR THE EXAMINATION AND REPORTING OF COLORECTAL CANCER SPECIMENS Produced by: Address: Yorkshire Cancer Network Pathology Group Arthington House, Cookridge Hospital, Hospital
PATHOLOGY GROUP GUIDELINES FOR THE EXAMINATION AND REPORTING OF COLORECTAL CANCER SPECIMENS Produced by: Address: Yorkshire Cancer Network Pathology Group Arthington House, Cookridge Hospital, Hospital
Bladder Cancer Guidelines
 Bladder Cancer Guidelines Agreed by Urology CSG: October 2011 Review Date: September 2013 Bladder Cancer 1. Referral Guidelines The following patients should be considered as potentially having bladder
Bladder Cancer Guidelines Agreed by Urology CSG: October 2011 Review Date: September 2013 Bladder Cancer 1. Referral Guidelines The following patients should be considered as potentially having bladder
Colorectal Pathway Board (Clinical Subgroup): Imaging Guidelines September 2015
 Colorectal Pathway Board (Clinical Subgroup): Imaging Guidelines September 2015 1 Contents Page No. 1. Objective 3 2. Imaging Techniques 3 3. Staging of Colorectal Cancer 5 4. Radiological Reporting 6
Colorectal Pathway Board (Clinical Subgroup): Imaging Guidelines September 2015 1 Contents Page No. 1. Objective 3 2. Imaging Techniques 3 3. Staging of Colorectal Cancer 5 4. Radiological Reporting 6
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abdominal surgery prior as factor in laparoscopic colorectal surgery, 554 555 Abscess(es) CRC presenting as, 539 540 Adenocarcinoma of
Index Note: Page numbers of article titles are in boldface type. A Abdominal surgery prior as factor in laparoscopic colorectal surgery, 554 555 Abscess(es) CRC presenting as, 539 540 Adenocarcinoma of
Cancer of Unknown Primary (CUP) Protocol
 1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
Audit Report. Colorectal Cancer Quality Performance Indicators. Patients diagnosed April 2016 March Published: March 2018
 Colorectal Cancer Managed Clinical Network Audit Report Colorectal Cancer Quality Performance Indicators Patients diagnosed April 2016 March 2017 Published: March 2018 Mr Michael Walker NOSCAN MCN Clinical
Colorectal Cancer Managed Clinical Network Audit Report Colorectal Cancer Quality Performance Indicators Patients diagnosed April 2016 March 2017 Published: March 2018 Mr Michael Walker NOSCAN MCN Clinical
Audit Report. Colorectal Cancer Quality Performance Indicators. Patients diagnosed April 2014 March Published: July 2016
 NORTH OF SCOTLAND PLANNING GROUP Colorectal Cancer Managed Clinical Network Audit Report Colorectal Cancer Quality Performance Indicators Patients diagnosed April 2014 March 2015 Published: July 2016 Mr
NORTH OF SCOTLAND PLANNING GROUP Colorectal Cancer Managed Clinical Network Audit Report Colorectal Cancer Quality Performance Indicators Patients diagnosed April 2014 March 2015 Published: July 2016 Mr
BOWEL CANCER. Causes of bowel cancer
 A cancer is an abnormality in an organ that grows without control. The growth is often quite slow, but will continue unabated until it is detected. It can cause symptoms by its presence in the organ or
A cancer is an abnormality in an organ that grows without control. The growth is often quite slow, but will continue unabated until it is detected. It can cause symptoms by its presence in the organ or
Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer
 Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer Start date: May 2015 Review date: April 2018 1 Background Mismatch repair (MMR) deficiency is seen in approximately 15%
Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer Start date: May 2015 Review date: April 2018 1 Background Mismatch repair (MMR) deficiency is seen in approximately 15%
Guideline for the Management of Vulval Cancer
 Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
Bowel Cancer Information Leaflet THE DIGESTIVE SYSTEM
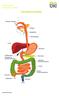 THE DIGESTIVE SYSTEM This factsheet is about bowel cancer Throughout our lives, the lining of the bowel constantly renews itself. This lining contains many millions of tiny cells, which grow, serve their
THE DIGESTIVE SYSTEM This factsheet is about bowel cancer Throughout our lives, the lining of the bowel constantly renews itself. This lining contains many millions of tiny cells, which grow, serve their
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER. Guidelines for the assessment of mismatch. Colorectal Cancer
 COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer March 2017 1 Background Mismatch repair (MMR) deficiency is seen in approximately
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer March 2017 1 Background Mismatch repair (MMR) deficiency is seen in approximately
LCA Lung Clinical Forum. 21 st October 2014
 LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
11/21/13 CEA: 1.7 WNL
 Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Cancer of Unknown Primary Service
 Cancer of Unknown Primary Service Dr Maurice Fernando Consultant In Specialist Palliative Care and CUP lead Doncaster and Bassetlaw Hospitals NHS FT Wakefield meeting -14-07-2016 CUP service CUP MDT
Cancer of Unknown Primary Service Dr Maurice Fernando Consultant In Specialist Palliative Care and CUP lead Doncaster and Bassetlaw Hospitals NHS FT Wakefield meeting -14-07-2016 CUP service CUP MDT
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER. Guidelines for the assessment of mismatch. Colorectal Cancer
 COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer January 2015 1 Background Mismatch repair (MMR) deficiency is seen in approximately
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer January 2015 1 Background Mismatch repair (MMR) deficiency is seen in approximately
Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
COLON AND RECTAL CANCER
 COLON AND RECTAL CANCER Mark Sun, MD Clinical Associate Professor of Surgery University of Minnesota No disclosures Objectives 1) Understand the epidemiology, management, and prognosis of colon and rectal
COLON AND RECTAL CANCER Mark Sun, MD Clinical Associate Professor of Surgery University of Minnesota No disclosures Objectives 1) Understand the epidemiology, management, and prognosis of colon and rectal
Rectal Cancer. GI Practice Guideline
 Rectal Cancer GI Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Dr. Francisco Perera MD, FRCPC (Radiation Oncologist) Dr. Jay Engel MD, FRCPC (Surgical Oncologist) Approval Date: 2006 This guideline
Rectal Cancer GI Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Dr. Francisco Perera MD, FRCPC (Radiation Oncologist) Dr. Jay Engel MD, FRCPC (Surgical Oncologist) Approval Date: 2006 This guideline
Colorectal Cancer Quality Performance Indicators
 Publication Report Colorectal Cancer Quality Performance Indicators Patients diagnosed between April 2013 and March 2016 Publication date 27th June 2017 An Official Statistics Publication for Scotland
Publication Report Colorectal Cancer Quality Performance Indicators Patients diagnosed between April 2013 and March 2016 Publication date 27th June 2017 An Official Statistics Publication for Scotland
North of Scotland Cancer Network Clinical Management Guideline for Carcinoma of the Uterine Cervix
 THIS DOCUMENT North of Scotland Cancer Network Carcinoma of the Uterine Cervix UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by A Kennedy/AG Macdonald/Others Approved by NOT APPROVED Issue date April
THIS DOCUMENT North of Scotland Cancer Network Carcinoma of the Uterine Cervix UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by A Kennedy/AG Macdonald/Others Approved by NOT APPROVED Issue date April
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO)
 North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
COLON AND RECTAL CANCER
 No disclosures COLON AND RECTAL CANCER Mark Sun, MD Clinical Assistant Professor of Surgery University of Minnesota Colon and Rectal Cancer Statistics Overall Incidence 2016 134,490 new cases 8.0% of all
No disclosures COLON AND RECTAL CANCER Mark Sun, MD Clinical Assistant Professor of Surgery University of Minnesota Colon and Rectal Cancer Statistics Overall Incidence 2016 134,490 new cases 8.0% of all
Delivering 62 Day GP Cancer Waits in a Complex Landscape. Hannah Marder Cancer Manager University Hospitals Bristol
 Delivering 62 Day GP Cancer Waits in a Complex Landscape Hannah Marder Cancer Manager University Hospitals Bristol Overview The 62 day GP target Cancer pathways What causes breaches? Good practice and
Delivering 62 Day GP Cancer Waits in a Complex Landscape Hannah Marder Cancer Manager University Hospitals Bristol Overview The 62 day GP target Cancer pathways What causes breaches? Good practice and
South West Strategic Clinical Network Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services
 Meeting of the SWAG Network Colorectal Site Specific Group (SSG) 10:00 16:00, Wednesday, 22nd April 2015, South West House, Blackbrook Park Avenue, Taunton, TA1 2PX Chair: Mr Michael Williamson (MW) This
Meeting of the SWAG Network Colorectal Site Specific Group (SSG) 10:00 16:00, Wednesday, 22nd April 2015, South West House, Blackbrook Park Avenue, Taunton, TA1 2PX Chair: Mr Michael Williamson (MW) This
Laparoscopic Resection Of Colon & Rectal Cancers. R Sim Centre for Advanced Laparoscopic Surgery, TTSH
 Laparoscopic Resection Of Colon & Rectal Cancers R Sim Centre for Advanced Laparoscopic Surgery, TTSH Feasibility and safety Adequacy - same radical surgery as open op. Efficacy short term benefits and
Laparoscopic Resection Of Colon & Rectal Cancers R Sim Centre for Advanced Laparoscopic Surgery, TTSH Feasibility and safety Adequacy - same radical surgery as open op. Efficacy short term benefits and
Faster Cancer Treatment Indicators: Use cases
 Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 5
 Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 5 Contents 5. Assessment & Management of Liver Metastases 42 5.1. Metachronous
Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 5 Contents 5. Assessment & Management of Liver Metastases 42 5.1. Metachronous
East Midlands Colorectal Cancer Expert Clinical Advisory Group (ECAG) Clinical Guidelines for the Bowel Cancer Care Pathway
 East Midlands Colorectal Cancer Expert Clinical Advisory Group (ECAG) Clinical Guidelines for the Bowel Cancer Care Pathway Reviewed by: Colorectal ECAG August 2014 Ratified by: Colorectal ECAG 2 nd March
East Midlands Colorectal Cancer Expert Clinical Advisory Group (ECAG) Clinical Guidelines for the Bowel Cancer Care Pathway Reviewed by: Colorectal ECAG August 2014 Ratified by: Colorectal ECAG 2 nd March
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES. U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease
 PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
COLORECTAL CANCER FAISALGHANISIDDIQUI MBBS; FCPS; PGDIP-BIOETHICS; MCPS-HPE
 COLORECTAL CANCER FAISALGHANISIDDIQUI MBBS; FCPS; PGDIP-BIOETHICS; MCPS-HPE PROFESSOR OF SURGERY & DIRECTOR, PROFESSIONAL DEVELOPMENT CENTRE J I N N A H S I N D H M E D I C A L U N I V E R S I T Y faisal.siddiqui@jsmu.edu.pk
COLORECTAL CANCER FAISALGHANISIDDIQUI MBBS; FCPS; PGDIP-BIOETHICS; MCPS-HPE PROFESSOR OF SURGERY & DIRECTOR, PROFESSIONAL DEVELOPMENT CENTRE J I N N A H S I N D H M E D I C A L U N I V E R S I T Y faisal.siddiqui@jsmu.edu.pk
8. The polyp in the illustration can be described as (circle all that apply) a. Exophytic b. Pedunculated c. Sessile d. Frank
 Quiz 1 Overview 1. Beginning with the cecum, which is the correct sequence of colon subsites? a. Cecum, ascending, splenic flexure, transverse, hepatic flexure, descending, sigmoid. b. Cecum, ascending,
Quiz 1 Overview 1. Beginning with the cecum, which is the correct sequence of colon subsites? a. Cecum, ascending, splenic flexure, transverse, hepatic flexure, descending, sigmoid. b. Cecum, ascending,
Cancer of Unknown Primary (CUP)
 Cancer of Unknown Primary (CUP) Pathways and Guidelines V1.0 London Cancer September 2013 The following pathways and guidelines document has been compiled by the London Cancer CUP technical subgroup and
Cancer of Unknown Primary (CUP) Pathways and Guidelines V1.0 London Cancer September 2013 The following pathways and guidelines document has been compiled by the London Cancer CUP technical subgroup and
Colorectal Cancer Comparative Audit Report
 SOUTH EAST SCOTLAND CANCER NETWORK (SCAN) PROSPECTIVE CANCER AUDIT Colorectal Cancer 2014 2015 Comparative Audit Report Mr B.J. Mander, NHS Lothian, Lead Colorectal Cancer Clinician, SCAN Group Chair Mr
SOUTH EAST SCOTLAND CANCER NETWORK (SCAN) PROSPECTIVE CANCER AUDIT Colorectal Cancer 2014 2015 Comparative Audit Report Mr B.J. Mander, NHS Lothian, Lead Colorectal Cancer Clinician, SCAN Group Chair Mr
South West Strategic Clinical Network Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services
 SWAG Cancer of Unknown Primary (CUP) Referral Processes The SWAG network site specific groups refer any patient with metastatic carcinoma of unknown origin on for discussion to the specialist carcinoma
SWAG Cancer of Unknown Primary (CUP) Referral Processes The SWAG network site specific groups refer any patient with metastatic carcinoma of unknown origin on for discussion to the specialist carcinoma
Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
CT PET SCANNING for GIT Malignancies A clinician s perspective
 CT PET SCANNING for GIT Malignancies A clinician s perspective Damon Bizos Head, Surgical Gastroenterology Charlotte Maxeke Johannesburg Academic Hospital Case presentation 54 year old with recent onset
CT PET SCANNING for GIT Malignancies A clinician s perspective Damon Bizos Head, Surgical Gastroenterology Charlotte Maxeke Johannesburg Academic Hospital Case presentation 54 year old with recent onset
Radiotherapy for Rectal Cancer. Kevin Palumbo Adelaide Radiotherapy Centre
 Radiotherapy for Rectal Cancer Kevin Palumbo Adelaide Radiotherapy Centre Overview CRC are common (3 rd commonest cancer) rectal Ca approx 25-30% of all CRC. Presentation PR bleeding: beware attributing
Radiotherapy for Rectal Cancer Kevin Palumbo Adelaide Radiotherapy Centre Overview CRC are common (3 rd commonest cancer) rectal Ca approx 25-30% of all CRC. Presentation PR bleeding: beware attributing
Navigators Lead the Way
 RN Navigators Their Role in patients with Cancers of the GI tract Navigators Lead the Way Nurse Navigator Defined Nurse Navigator A clinically trained individual responsible for the identification and
RN Navigators Their Role in patients with Cancers of the GI tract Navigators Lead the Way Nurse Navigator Defined Nurse Navigator A clinically trained individual responsible for the identification and
Bowel Cancer in England and Wales A summary report about the management and outcomes of people with bowel cancer
 Bowel Cancer in England and Wales A summary report about the management and outcomes of people with bowel cancer Based on findings from the National Bowel Cancer Audit Background How are patients diagnosed?
Bowel Cancer in England and Wales A summary report about the management and outcomes of people with bowel cancer Based on findings from the National Bowel Cancer Audit Background How are patients diagnosed?
Colorectal Cancer Structured Pathology Reporting Proforma DD MM YYYY
 Colorectal Cancer Structured Pathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Date of birth DD MM YYYY S1.02 Clinical details
Colorectal Cancer Structured Pathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Date of birth DD MM YYYY S1.02 Clinical details
The NIHCE guidelines for the management of colorectal cancer
 The NIHCE guidelines for the management of colorectal cancer Graeme Poston Chair Colorectal Cancer Guideline Development Group and Colorectal Cancer Quality Standards Committee National Institute of Health
The NIHCE guidelines for the management of colorectal cancer Graeme Poston Chair Colorectal Cancer Guideline Development Group and Colorectal Cancer Quality Standards Committee National Institute of Health
By: Tania Cortas, MD Arizona Oncology 03/10/2015
 By: Tania Cortas, MD Arizona Oncology 03/10/2015 Epidemiology In the United States, CRC incidence rates have declined about 2 to 3 percent per year over the last 15 years Death rates from CRC have declined
By: Tania Cortas, MD Arizona Oncology 03/10/2015 Epidemiology In the United States, CRC incidence rates have declined about 2 to 3 percent per year over the last 15 years Death rates from CRC have declined
Screening & Surveillance Guidelines
 Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Colorectal cancer: the diagnosis and management of colorectal cancer
 Clinical Guideline Colorectal cancer: the diagnosis and management of colorectal cancer Full Guideline Update information Since original publication this guideline has been partially updated: July 2018:
Clinical Guideline Colorectal cancer: the diagnosis and management of colorectal cancer Full Guideline Update information Since original publication this guideline has been partially updated: July 2018:
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services. Lung Cancer Network Site Specific Group. Clinical Guidelines.
 Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Lung Cancer Network Site Specific Group June 2017 Revision due: April 2019 Page 1 of 15 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Lung Cancer Network Site Specific Group June 2017 Revision due: April 2019 Page 1 of 15 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
National Breast Cancer Audit next steps. Martin Lee
 National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
Intended for use by Clinicians and Health Care Providers involved in the Management or Referral of adult patients with pancreatic
 Intended for use by Clinicians and Health Care Providers involved in the Management or Referral of adult patients with pancreatic cancer Section AA Cancer Centre Referrals In the absence of metastatic
Intended for use by Clinicians and Health Care Providers involved in the Management or Referral of adult patients with pancreatic cancer Section AA Cancer Centre Referrals In the absence of metastatic
Upper Gastro-Intestinal (UGI) and Hepato-Pancreato Biliary (HPB) Cancer Network Site Specific Group. Clinical Guidelines.
 Upper Gastro-Intestinal (UGI) and Hepato-Pancreato Biliary (HPB) Cancer Network Site Specific Group June 2015 Revision due: April 2016 Page 1 of 40 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT. PLEASE
Upper Gastro-Intestinal (UGI) and Hepato-Pancreato Biliary (HPB) Cancer Network Site Specific Group June 2015 Revision due: April 2016 Page 1 of 40 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT. PLEASE
Hepatobiliary Malignancies Retrospective Study at Truman Medical Center
 Hepatobiliary Malignancies 206-207 Retrospective Study at Truman Medical Center Brandon Weckbaugh MD, Prarthana Patel & Sheshadri Madhusudhana MD Introduction: Hepatobiliary malignancies are cancers which
Hepatobiliary Malignancies 206-207 Retrospective Study at Truman Medical Center Brandon Weckbaugh MD, Prarthana Patel & Sheshadri Madhusudhana MD Introduction: Hepatobiliary malignancies are cancers which
Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
PANCREATIC CANCER GUIDELINES
 PANCREATIC CANCER GUIDELINES North-East London Cancer Network & Barts and the London HPB Centre PROTOCOL FOR MANAGEMENT OF PANCREATIC CANCER (SEPTEMBER 2010) I. PRE-REFERRAL GUIDELINES Screening 1. Offer
PANCREATIC CANCER GUIDELINES North-East London Cancer Network & Barts and the London HPB Centre PROTOCOL FOR MANAGEMENT OF PANCREATIC CANCER (SEPTEMBER 2010) I. PRE-REFERRAL GUIDELINES Screening 1. Offer
COLON CANCER CARE GUIDELINES NON-METASTATIC DISEASE
 COLON CANCER CARE GUIDELINES NON-METASTATIC DISEASE Guideline Authors: Todd S. Crocenzi, M.D.; Mark Whiteford, M.D.; Matthew Solhjem, M.D.; Carlo Bifulco, M.D.; Melissa Li, M.D.; Christopher Cai, M.D.;
COLON CANCER CARE GUIDELINES NON-METASTATIC DISEASE Guideline Authors: Todd S. Crocenzi, M.D.; Mark Whiteford, M.D.; Matthew Solhjem, M.D.; Carlo Bifulco, M.D.; Melissa Li, M.D.; Christopher Cai, M.D.;
05/07/2018. Organisation. The English screening programme what is happening? Organisation. Bowel cancer screening in the UK is:
 Organisation The English screening programme what is happening? Phil Quirke Lead Pathologist Bowel Cancer Screening PHE England Bowel Cancer Screening Pathology Committee Started 2006 with roll out 4 devolved
Organisation The English screening programme what is happening? Phil Quirke Lead Pathologist Bowel Cancer Screening PHE England Bowel Cancer Screening Pathology Committee Started 2006 with roll out 4 devolved
Clinical Advice for the Commissioning of the Whole Bowel Cancer Pathway
 Clinical Advice for the Commissioning of the Whole Bowel Cancer Pathway This document was produced by the Colorectal Cancer Clinical Expert Group November 2017 Clinical Advice to Cancer Alliances for the
Clinical Advice for the Commissioning of the Whole Bowel Cancer Pathway This document was produced by the Colorectal Cancer Clinical Expert Group November 2017 Clinical Advice to Cancer Alliances for the
COLORECTAL CANCER Quality Performance Indicators (QPI) Comparative Report
 SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT COLORECTAL CANCER 2016 2017 Quality Performance Indicators (QPI) Comparative Report Mr S Yalamarthi, NHS Fife, Lead Colorectal Cancer Clinician,
SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT COLORECTAL CANCER 2016 2017 Quality Performance Indicators (QPI) Comparative Report Mr S Yalamarthi, NHS Fife, Lead Colorectal Cancer Clinician,
North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer
 THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
Resource impact report: Molecular testing strategies for Lynch syndrome in people with colorectal cancer (DG27)
 Putting NICE guidance into practice Resource impact report: Molecular testing strategies for Lynch syndrome in people with colorectal cancer (DG27) Published: February 2017 Summary Molecular testing strategies
Putting NICE guidance into practice Resource impact report: Molecular testing strategies for Lynch syndrome in people with colorectal cancer (DG27) Published: February 2017 Summary Molecular testing strategies
COLORECTAL CANCER COMPARATIVE AUDIT REPORT SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT. Mr B.J. Mander SCAN Group Chair
 SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT COLORECTAL CANCER 2013-2014 COMPARATIVE AUDIT REPORT Mr B.J. Mander SCAN Group Chair Mr K Pal, NHS Borders Mr S Whitelaw, NHS Dumfries & Galloway
SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT COLORECTAL CANCER 2013-2014 COMPARATIVE AUDIT REPORT Mr B.J. Mander SCAN Group Chair Mr K Pal, NHS Borders Mr S Whitelaw, NHS Dumfries & Galloway
Index. Surg Oncol Clin N Am 14 (2005) Note: Page numbers of article titles are in boldface type.
 Surg Oncol Clin N Am 14 (2005) 433 439 Index Note: Page numbers of article titles are in boldface type. A Abdominosacral resection, of recurrent rectal cancer, 202 215 Ablative techniques, image-guided,
Surg Oncol Clin N Am 14 (2005) 433 439 Index Note: Page numbers of article titles are in boldface type. A Abdominosacral resection, of recurrent rectal cancer, 202 215 Ablative techniques, image-guided,
Ghosts in the Machine: Jonathan B. Koea MD; FRACS. Department of Surgery Auckland Hospital Auckland New Zealand
 Ghosts in the Machine: Patient Journeys Through Cancer Treatment Jonathan B. Koea MD; FRACS. Department of Surgery Auckland Hospital Auckland New Zealand Age-Standardised Cancer Incidence (100,000 population)
Ghosts in the Machine: Patient Journeys Through Cancer Treatment Jonathan B. Koea MD; FRACS. Department of Surgery Auckland Hospital Auckland New Zealand Age-Standardised Cancer Incidence (100,000 population)
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Malignant melanoma: assessment and management of malignant melanoma 1.1 Short title Malignant Melanoma 2 The remit The Department
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Malignant melanoma: assessment and management of malignant melanoma 1.1 Short title Malignant Melanoma 2 The remit The Department
NICE guideline Published: 24 January 2018 nice.org.uk/guidance/ng83
 Oesophago-gastric cancer: assessment and management in adults NICE guideline Published: 24 January 18 nice.org.uk/guidance/ng83 NICE 18. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Oesophago-gastric cancer: assessment and management in adults NICE guideline Published: 24 January 18 nice.org.uk/guidance/ng83 NICE 18. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
NICE BULLETIN Diagnosis & treatment of prostate cancer
 Diagnosis & treatment of prostate cancer NICE provided the content for this booklet which is independent of any company or product advertised Diagnosis and treatment of prostate cancer Introduction In
Diagnosis & treatment of prostate cancer NICE provided the content for this booklet which is independent of any company or product advertised Diagnosis and treatment of prostate cancer Introduction In
Structured Follow-Up after Colorectal Cancer Resection: Overrated. R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007
 Structured Follow-Up after Colorectal Cancer Resection: Overrated R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007 Guidelines for Colonoscopy Production: Surveillance US Multi-Society
Structured Follow-Up after Colorectal Cancer Resection: Overrated R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007 Guidelines for Colonoscopy Production: Surveillance US Multi-Society
Guidelines for Laparoscopic Resection of Curable Colon and Rectal Cancer
 SAGES Society of American Gastrointestinal and Endoscopic Surgeons http://www.sages.org Guidelines for Laparoscopic Resection of Curable Colon and Rectal Cancer Author : SAGES Webmaster PREAMBLE The following
SAGES Society of American Gastrointestinal and Endoscopic Surgeons http://www.sages.org Guidelines for Laparoscopic Resection of Curable Colon and Rectal Cancer Author : SAGES Webmaster PREAMBLE The following
Disclosure. Acknowledgement. What is the Best Workup for Rectal Cancer Staging: US/MRI/PET? Rectal cancer imaging. None
 What is the Best Workup for Rectal Cancer Staging: US/MRI/PET? Zhen Jane Wang, MD Assistant Professor in Residence UC SF Department of Radiology Disclosure None Acknowledgement Hueylan Chern, MD, Department
What is the Best Workup for Rectal Cancer Staging: US/MRI/PET? Zhen Jane Wang, MD Assistant Professor in Residence UC SF Department of Radiology Disclosure None Acknowledgement Hueylan Chern, MD, Department
Colorectal Cancer. Mark Chapman. MA MS FRCS EBSQ(coloproct) 21 st March 2018 Consultant Coloproctologist
 Colorectal Cancer Mark Chapman MA MS FRCS EBSQ(coloproct) 21 st March 2018 Consultant Coloproctologist Overview Epidemiology of colorectal cancer Adenoma carcinoma sequence Tumour diagnosis & staging Treatment
Colorectal Cancer Mark Chapman MA MS FRCS EBSQ(coloproct) 21 st March 2018 Consultant Coloproctologist Overview Epidemiology of colorectal cancer Adenoma carcinoma sequence Tumour diagnosis & staging Treatment
Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction
 Version History Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction Version Summary of change Date Issued 2.0 Endorsed by the Governance Committee 20.02.08 2.1 Circulated
Version History Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction Version Summary of change Date Issued 2.0 Endorsed by the Governance Committee 20.02.08 2.1 Circulated
Appendix 4 Urology Care Pathways
 Appendix 4 Urology Care Pathways Cancer Care Pathways outline the steps and stages in the patient journey from referral through to diagnostics, staging, treatment, follow up, rehabilitation and if applicable
Appendix 4 Urology Care Pathways Cancer Care Pathways outline the steps and stages in the patient journey from referral through to diagnostics, staging, treatment, follow up, rehabilitation and if applicable
Rectal cancer with synchroneous liver mets: A challenging clinical case
 ESMO Preceptorship Programme Rectal cancer Singapur November 2017 Rectal cancer with synchroneous liver mets: A challenging clinical case Andrés Cervantes Disclosures Consulting and advisory services,
ESMO Preceptorship Programme Rectal cancer Singapur November 2017 Rectal cancer with synchroneous liver mets: A challenging clinical case Andrés Cervantes Disclosures Consulting and advisory services,
L impatto dell imaging sulla definizione della strategia terapeutica
 GISCoR L impatto dell imaging sulla definizione della strategia terapeutica M. Galeandro U.C. Radioterapia Oncologica ASMN-IRCCS Reggio Emilia 14 Novembre 2014 Rectal Cancer TNM AJCC-7 th edition 2010
GISCoR L impatto dell imaging sulla definizione della strategia terapeutica M. Galeandro U.C. Radioterapia Oncologica ASMN-IRCCS Reggio Emilia 14 Novembre 2014 Rectal Cancer TNM AJCC-7 th edition 2010
Treatment strategy of metastatic rectal cancer
 35.Schweizerische Koloproktologie-Tagung Treatment strategy of metastatic rectal cancer Gilles Mentha University hospital of Geneva Bern, January 18th, 2014 Colorectal cancer is the third most frequent
35.Schweizerische Koloproktologie-Tagung Treatment strategy of metastatic rectal cancer Gilles Mentha University hospital of Geneva Bern, January 18th, 2014 Colorectal cancer is the third most frequent
Colorectal cancer: pathology
 UK NEQAS for Molecular Pathology Colorectal cancer: pathology Nick West Pathology & Tumour Biology May 2013 Colorectal cancer (CRC) 40,695 new cases in 2010 15,708 deaths Management of CRC Surgery Main
UK NEQAS for Molecular Pathology Colorectal cancer: pathology Nick West Pathology & Tumour Biology May 2013 Colorectal cancer (CRC) 40,695 new cases in 2010 15,708 deaths Management of CRC Surgery Main
Follow up The way ahead. John Griffith
 Follow up The way ahead John Griffith Key Emerging Principles Risk stratified pathways of care Personalised care plan and treatment summary with a hand held record Information and education Remote monitoring
Follow up The way ahead John Griffith Key Emerging Principles Risk stratified pathways of care Personalised care plan and treatment summary with a hand held record Information and education Remote monitoring
Preoperative adjuvant radiotherapy
 Preoperative adjuvant radiotherapy Dr John Hay Radiation Oncology Program BC Cancer Agency Vancouver Cancer Centre The key question for the surgeon Do you think that this tumour can be resected with clear
Preoperative adjuvant radiotherapy Dr John Hay Radiation Oncology Program BC Cancer Agency Vancouver Cancer Centre The key question for the surgeon Do you think that this tumour can be resected with clear
MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER
 MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER (Text update March 2008) A. Stenzl (chairman), N.C. Cowan, M. De Santis, G. Jakse, M. Kuczyk, A.S. Merseburger, M.J. Ribal, A. Sherif, J.A. Witjes Introduction
MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER (Text update March 2008) A. Stenzl (chairman), N.C. Cowan, M. De Santis, G. Jakse, M. Kuczyk, A.S. Merseburger, M.J. Ribal, A. Sherif, J.A. Witjes Introduction
COLORECTAL CANCER STAGING in 2010
 COLORECTAL CANCER STAGING in 2010 Robert A. Halvorsen, MD, FACR MCV Hospitals / VCU Medical Center Richmond, Virginia I do not have any relevant financial relationships with any commercial interests COLON
COLORECTAL CANCER STAGING in 2010 Robert A. Halvorsen, MD, FACR MCV Hospitals / VCU Medical Center Richmond, Virginia I do not have any relevant financial relationships with any commercial interests COLON
Pathway Gynaecology Cancer & Diagnostic Protocol for Inter Trust transfer
 NICaN Pathway Gynaecology Cancer & Diagnostic Protocol for Inter Trust transfer Timed schedules to enable the proactive management of the patient from point of receipt of referral to first definitive treatment
NICaN Pathway Gynaecology Cancer & Diagnostic Protocol for Inter Trust transfer Timed schedules to enable the proactive management of the patient from point of receipt of referral to first definitive treatment
National Optimal Lung Cancer Pathway
 National Optimal Lung Cancer Pathway This document was produced by the Lung Clinical Expert Group 2017 Document Title: National Optimal Lung Cancer Pathway and Implementation Guide Date of issue: August
National Optimal Lung Cancer Pathway This document was produced by the Lung Clinical Expert Group 2017 Document Title: National Optimal Lung Cancer Pathway and Implementation Guide Date of issue: August
Colorectal Cancer Care
 Colorectal Cancer Care A Cancer Care Map for Patients Understanding the process of care that a patient goes through in the diagnosis and treatment of colorectal cancer in BC. ROUND2.3_CRCa_20pages_5.5x8.5_FINAL.indd
Colorectal Cancer Care A Cancer Care Map for Patients Understanding the process of care that a patient goes through in the diagnosis and treatment of colorectal cancer in BC. ROUND2.3_CRCa_20pages_5.5x8.5_FINAL.indd
Rectal cancer management: a team sport The role of radiology and the multidisciplinary conference
 Rectal cancer management: a team sport The role of radiology and the multidisciplinary conference W. Donald Buie MD MSc FRCSC Professor of Surgery and Oncology Department of Surgery University of Calgary
Rectal cancer management: a team sport The role of radiology and the multidisciplinary conference W. Donald Buie MD MSc FRCSC Professor of Surgery and Oncology Department of Surgery University of Calgary
Early Rectal Cancer Surgical options Organ Preservation? Chinna Reddy Colorectal Surgeon Western General, Edinburgh
 Early Rectal Cancer Surgical options Organ Preservation? Chinna Reddy Colorectal Surgeon Western General, Edinburgh What is Early rectal cancer? pt1t2n0m0 Predictors for LN involvement Size Depth Intramural
Early Rectal Cancer Surgical options Organ Preservation? Chinna Reddy Colorectal Surgeon Western General, Edinburgh What is Early rectal cancer? pt1t2n0m0 Predictors for LN involvement Size Depth Intramural
Soft Tissue Tumour & Sarcoma Imaging Guidelines 2012
 Soft Tissue Tumour & Sarcoma Imaging Guidelines 2012 Version Control This is a controlled document please destroy all previous versions on receipt of a new version. Date Approved: March 2011 reissued April
Soft Tissue Tumour & Sarcoma Imaging Guidelines 2012 Version Control This is a controlled document please destroy all previous versions on receipt of a new version. Date Approved: March 2011 reissued April
Guideline for the Follow-up of Patients with Gynaecological Malignancies
 Guideline for the Follow-up of Patients with Gynaecological Malignancies Version History Version Date Summary of Change/Process 2.0 20.02.08 Endorsed by the Governance Committee 2.1 18.11.10 Circulated
Guideline for the Follow-up of Patients with Gynaecological Malignancies Version History Version Date Summary of Change/Process 2.0 20.02.08 Endorsed by the Governance Committee 2.1 18.11.10 Circulated
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2
 Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2 Background Sarcomas that arise in the lung de novo are
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2 Background Sarcomas that arise in the lung de novo are
Transforming Cancer Services for London
 Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
MDT IMPROVEMENT PROJECT. Professor Muntzer Mughal, UCLH
 MDT IMPROVEMENT PROJECT Professor Muntzer Mughal, UCLH 1995..assessment and management of rare cancers in multidisciplinary teams.. 2000 the care of all patients with cancer should be formally reviewed
MDT IMPROVEMENT PROJECT Professor Muntzer Mughal, UCLH 1995..assessment and management of rare cancers in multidisciplinary teams.. 2000 the care of all patients with cancer should be formally reviewed
