Global Perspective on Donor and Recipient Safety: Biovigilance in Hematopoietic Cell Transplantation
|
|
|
- George Bruce
- 5 years ago
- Views:
Transcription
1 Global Perspective on Donor and Recipient Safety: Biovigilance in Hematopoietic Cell Transplantation John P. Miller, MDPhD Vice President and Senior Medical Director National Marrow Donor Program / Be The Match Public Forum of the Quebec Biovigilance Committee November 16, 2016 Learning Objectives Understand the nature and goals of the NMDP biovigilance system for donors and recipients of hematopoietic progenitor cells (HPC) Global trends in transplantation How are HPC donors different from blood, organ and other tissue donors? Describe the NMDP operational processes that support biovigilance Reporting requirements for donor centers and transplant centers NMDP reporting requirements domestically and internationally Share incidence data on NMDP biovigilance for donors Common adverse events Serious adverse events Marrow compared to PBSC donors Share best practices moving forward: Emerging cellular therapies 2
2 World Marrow Donor Association Transplants by Cell Source Over Time (WMDA)
3 International Donor and Transplant Sharing (WMDA) Differences Between HPC and Blood Donors Annual # of Events Donor Patient Donor Testing Donor Assessment Blood More than 20,000,000 in the US alone 1:1, 1:2, 1:3 whole blood Day of Collection, strict release criteria HHQ, limited physical assessment HPCT 30,000 allohpct/yr worldwide Usually 1:1 Up to 30 days prior to donation, flexible release criteria HHQ, complete H&P, labs and EKG, CXR and extended testing possible Matching ABO/Rh +/- RBC Ag HLA, gender, ABO, KIR, CCR5 etc. Only/best match 6
4 Differences Between HPC and Solid Organ and Tissue Transplantation Tissue/Solid Organ HPCT Annual # of Events More than 1,000,000 in the US alone 30,000 allohpct/yr worldwide Donor Patient 1:many (dozen to 100s) Usually 1:1 Donor Testing Often cadaveric: no retesting possible Alive and well; retesting can be done Donor Assessment Often very time limited (as little as hours) Not severely time limited Matching HLA, lower resolution HLA, allele level 8 loci Product Release/Expiration ASAP/Hours ASAP/Hours or days or cryopreservation 7 Differences Between HPC and Organ, Tissue and Blood Donors HPC donors may be the best or only match for a patient While transplantation may be urgent clinically, there is time to do a complete donor health assessment The emergence of new blood-borne infectious diseases will most likely occur in the setting of the blood and tissue world given the sheer number of transfusion/transplant events Therefore, vigilance efforts should focus on donor and recipient adverse events and product quality issues to enhance donor and recipient safety 8
5 Key Elements in Donor AE Biovigilance Reported via FormsNet Form 701 Medical Quality Assurance Nurses are notified Investigation ensues if appropriate Donor cared for by DC/AC/CC and NMDP NMDP RN staff (TMS/DMS) follow donor to AE resolution Donor advocacy RN involved if prolonged AE Reporting for serious and unexpected AEs plus serious and expected events of interest as determined by medical director review vast majority of events reported are non-serious 9 Key Elements in Recipient AE Biovigilance AE Reporting TC education regarding what to report, timelines AE training associated with protocols (e.g. 10-CBA) National mtgs (Tandem, NMDP Council, CB Symp) Web resources at marrow.org Event Processing Reporting via FormsNet2 (phone or permitted) Events investigated (NMDP or other stakeholder) Confirmed events entered into IMS Tracking and Trending MasterControl tools, reports; staff review 10
6 Recipient Serious Adverse Event (SAE) Reporting for the NMDP Network SAEs associated with PBSC, marrow and cord blood must be reported promptly to NMDP Report using FormsNet Form 3001 Rationale for seriousness (death, life-threatening, hospitalization, birth defect, permanent impairment or disability) Event type / severity using CTCAE Terms and Grading Attribution Some events are not NMDP regulatory responsibility and info will be passed to the appropriate IND holder All Recipient AE and Product Complaint Reporting via FormsNet TM 2 Effective 4/15/12 Cord Blood NMDP IND Other IND, Licensed Mononuclear Cells NMDP Facilitated FormsNet2 Marrow NMDP Facilitated PBSC NMDP Facilitated 12
7 Benefits of Recipient AE Reporting System Single source of event entry in a system (FormsNet 2) with which the Network is familiar Once event is entered, the NMDP provides event notification to the stakeholders (CBBs, IND holders, etc) Enhances ability to comply with all reporting obligations Single source of event submission allows tracking & trending of events producing more timely Network notification Root cause investigation and remedial / corrective interventions 13 What Happens to AE Reports? Investigation by Medical Services RNs and MDs Dissemination of Information: Regulatory reporting by NMDP DPSM (the NMDP s DSMB) and IRB FDA when NMDP is the IND holder Otherwise, NMDP passes through the report to the IND holder HRSA and other US government stakeholders Other stakeholders: Network announcements and PI letters Pharma as applicable (e.g. for mobilizing agents) International Reporting Reporting to World Marrow Donor Association (WMDA) when donor or product-related: S(P)EAR 14
8 Incident Management IMS: MasterControl TM Maintained/administered by NMDP Quality Systems FDA-compliant, configurable software used to report, resolve, monitor, track and trend QIs Multiple quality management functions Incident capture Remedial action Investigation Risk assessment Corrective Action/Preventative Action (CAPA) NMDP SOPs guide actions Definitions of events Process for incident management 15 International Efforts in Biovigilance in HPCT WMDA: S(P)EAR reporting for donor AEs and productrelated issues Consolidates data from independent registries: increases power to detect sentinel AE (Shaw, et al, BMT 2013) Mandatory reporting for accredited registries, standard AE definitions and likely attribution 16
9 WMDA SEAR Reporting Serious/unexpected/medically relevant/previously unknown Any serious event or reaction during anesthesia should be reported. Any serious cardiac complication should be reported. Any serious infection should be reported. Any serious mechanical injury should be reported. Any serious incident in hemostasis should be reported Any serious (late) effect of marrow or PBSC donation should be reported (e.g. autoimmune, malignancy) Any donor death (from 30 post donation; or at any time if the donation is implicated) 17 WMDA S(P)EAR Reporting Processing, labeling, handling and transport errors/problems Wrong stem cell product transfused Wrong stem cell product received Serious problems in transportation Damage to bag Inadequate cell dose in the stem cell product Clotting or other loss of product viability Contamination leading to serious infection in recipient Any serious unpredicted transmissible infection HIV, Hepatitis B, Hepatitis C Any serious unpredicted non-infectious transmissible disease (e.g. malignant) 18
10 WMDA S(P)EAR Committee Review Reporting requirement for accredited WMDA registries Committee has international representation with primary review by non-reporting peers Annual reports to community by category Almost 300 Cases reported and reviewed in 2016 with determination: Donor or product/patient affected Additional information required Attribution: both that reported and as determined by committee Educational value for the transplant community 19 The Case that Sparked GRID GRID will replace the many different methods of identification used across the world today with a standard, consistent format 20
11 Biovigilance Reseach: What Have We Learned and Will Learn About Adverse Events What have we learned: Common Adverse Events (AEs) Marrow vs PBSC donors Serious adverse events (SAE) Related vs. unrelated donors What we will learn: Long term donor follow-up study Malignant, thrombotic and autoimmune diseases Emerging Cellular Therapies Pulsipher: Blood 2008, 2009 and CIBMTR Donor Health and Safety Cmte 21 Common Adverse Events: Frequency of Bone Pain in PBSC Donors
12 Common Adverse Events: Symptom Score During Mobilization The Donor Experience Marrow vs PBSC -1 Bone Pain occurred in 80%, irrespective of donation type Timing of bone pain different, mobilization vs. postcollect Most pain was rated as mild or moderate Other symptoms were similar in both groups Bone marrow donors have more prolonged recovery and lower rates of complete recovery Pulsipher, Blood
13 The Donor Experience Marrow vs PBSC -2 Overweight and obese PBSC donors have higher rates of grade 2-4 pain in the peri-collection period Female donors are more likely to report pain and other symptoms and are less likely to experience full recovery, regardless of donation type Older marrow donors are less likely to experience grades 2-4 skeletal pain in peri-collection period, but they are more likely to have pain at 1 week and 1 month Pulsipher, Blood Probability of Complete Recovery: Marrow vs. PBSC 26
14 What About Serious Adverse Events? FDA Criteria Life-threatening or fatal event Inpatient hospitalization or prolongation of existing hospitalization Persistent or significant disability / incapacity Required intervention to prevent permanent impairment/damage Congenital anomaly / birth defect Other at physician discretion 27 Serious Adverse Events in Marrow and PBSC Donors Methods 5 Physician panel reviewed all events Probable, possible or not AE Classification as serious or not serious (FDA criteria) Attribution as expected or unexpected Marrow attribution to anesthesia, harvest or unrelated PBSC attribution to GCSF, apheresis or unrelated Pulsipher M A et al. Blood 2014;123:
15 Serious Adverse Events in Marrow and PBSC Donors Results: Physician review of Adverse Event Reports 457 AE forms associated with 328 events in 296/2726 marrow donors (10.9%) 1178 AE forms associated with 972 events in 854 PBSC donors (12.6%) Most events were acute and of short duration Pulsipher M A et al. Blood 2014;123: Serious Adverse Events (SAE): NMDP Experience with Unrelated Donors Rates of SAE were 4x higher with bone marrow donation (2.38%) compared to PBSC donation (0.56%) Rates of unexpected SAE were 3x higher with bone marrow donation (0.99%) compared to PBSC donation (0.22%) Life threatening events are rare in both marrow (0.26%) and PBSC donors (0.03%) More life-threatening events, hospitalizations and long term disability with marrow donation The frequency of SAE are two-fold higher in female donors (Odds ratio for men = 0.5) Pulsipher M A et al. Blood 2014;123:
16 Classification of SAEs experienced by BM and PBSC donors. Pulsipher M A et al. Blood 2014;123: by American Society of Hematology
17 Risk of cancer, autoimmunity, and thrombosis in G-CSF treated PBSC donors vs BM donors. A = Cancer, excluding basal cell B = Non-melanoma skin C = Autoimmunity D = Thrombosis Pulsipher M A et al. Blood 2014;123: by American Society of Hematology Risk of Cancer Compared to the General Population Bone Marrow PBSC Observed Cancer Expected Cancer Ratio (obs/exp) P value *
18 Long Term Donor Follow-up Study Primary Objective: To describe the long-term incidence of malignant myeloid hematologic disorders in donors who received and in those who did not receive filgrastim Secondary Objectives: To describe the long-term incidence in donors receiving or not receiving filgrastim: Malignant hematologic disorders Non-hematologic malignant disorders Thrombotic events Autoimmune disorders Long Term Donor Follow-up Study Retrospective and Prospective cohorts Expected Enrollment: 10,956 unstimulated marrow donors 21,172 filgrastim mobilized PBSC donors Enrollment began Oct 2010, now complete Collecting data through 2020 to maximize person-years of follow-up
19 New Frontier in Biovigilance: Emerging Cellular Therapies Cytotoxic T cells: leukemic-antigen or virusspecific (e.g. CMV, EBV, adenovirus) Tumor vaccines Induced pluripotent cells (ipc): regenerate different cell lines Regenerative medicine: cell layers (2-D), tissues and organs 3-D Genomics: screening, diagnosis and treatment Chimeric antigen receptor (CAR) T cells 37 Summary Serious adverse events are rare, but efforts need to be made to minimize the risk of such events Adverse events are more common in bone marrow than PBSC donors Adverse events are more common in female donors and recovery times are longer There appears to be little or no increased risk of malignancies, autoimmune disorders or thrombosis in hematopoietic progenitor cell donors
20 The NATIONAL 39
FormsNet Adverse Event Form
 FormsNet Adverse Event Form Registry Use Only Sequence Number: Date Received: Recipient Identification 1. CIBMTR Recipient ID (CRID): 2. NMDP Recipient ID (RID): (if applicable) - - 3. CIBMTR Center Number
FormsNet Adverse Event Form Registry Use Only Sequence Number: Date Received: Recipient Identification 1. CIBMTR Recipient ID (CRID): 2. NMDP Recipient ID (RID): (if applicable) - - 3. CIBMTR Center Number
BMTCN REVIEW COURSE PRE-TRANSPLANT CARE
 BMTCN REVIEW COURSE PRE-TRANSPLANT CARE Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to edit the Master Experts
BMTCN REVIEW COURSE PRE-TRANSPLANT CARE Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to edit the Master Experts
Donor work up, follow up and ethical issues
 Donor work up, follow up and ethical issues Hans Hägglund MD. PhD. Associate Professor Hematology Center, Karolinska University Hospital, Stockholm, Sweden Outline The donor has been identified as a match
Donor work up, follow up and ethical issues Hans Hägglund MD. PhD. Associate Professor Hematology Center, Karolinska University Hospital, Stockholm, Sweden Outline The donor has been identified as a match
EZER MIZION PROCEDURES FOR TRANSPLANT CENTERS AND COOPERATIVE REGISTRIES
 EZER MIZION PROCEDURES FOR TRANSPLANT CENTERS AND COOPERATIVE REGISTRIES Table of content 1. Purpose and Scope... 2 2. Introduction... 2 3. Procedure for Requesting a Search... 3 4. The Search Process...
EZER MIZION PROCEDURES FOR TRANSPLANT CENTERS AND COOPERATIVE REGISTRIES Table of content 1. Purpose and Scope... 2 2. Introduction... 2 3. Procedure for Requesting a Search... 3 4. The Search Process...
Spanish Donor Registry. Follow-Up Data
 Spanish Donor Registry. Follow-Up Data Javier de la Rubia S. de Hematología Hospital La Fe Valencia 16th Haemovigilance Seminar Barcelona, March 2014 WHO declared 2010 in its Guiding principle Nr.10: Hig-quality,
Spanish Donor Registry. Follow-Up Data Javier de la Rubia S. de Hematología Hospital La Fe Valencia 16th Haemovigilance Seminar Barcelona, March 2014 WHO declared 2010 in its Guiding principle Nr.10: Hig-quality,
OPERATIONS AND PATIENT SERVICES USER GUIDE. Procedures for transplant centres and international establishments
 OPERATIONS AND PATIENT SERVICES USER GUIDE Procedures for transplant centres and international establishments 1. INTRODUCTION 9 1.1. Introduction 9 1.2. User Guide 9 1.3. Services Provided by Anthony Nolan
OPERATIONS AND PATIENT SERVICES USER GUIDE Procedures for transplant centres and international establishments 1. INTRODUCTION 9 1.1. Introduction 9 1.2. User Guide 9 1.3. Services Provided by Anthony Nolan
Regulatory Challenges in Apheresis Global Perspectives - Cell Therapy
 Regulatory Challenges in Apheresis Global Perspectives - Cell Therapy Joseph (Yossi ) Schwartz, MD, MPH Director, Transfusion Medicine & Cellular Therapy Columbia University Medical Center New York Presbyterian
Regulatory Challenges in Apheresis Global Perspectives - Cell Therapy Joseph (Yossi ) Schwartz, MD, MPH Director, Transfusion Medicine & Cellular Therapy Columbia University Medical Center New York Presbyterian
Implementation of the Stem Cell Therapeutic and Research Act of 2005
 Implementation of the Stem Cell Therapeutic and Research Act of 2005 The 6 th Annual Somatic Cell Therapy Symposium Bethesda, MD September 26, 2006 James F. Burdick, M.D. U.S. Department of Health and
Implementation of the Stem Cell Therapeutic and Research Act of 2005 The 6 th Annual Somatic Cell Therapy Symposium Bethesda, MD September 26, 2006 James F. Burdick, M.D. U.S. Department of Health and
USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire. Table of Contents
 Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Hematopoiesis i starts from. HPC differentiates t to sequentially more mature. HPC circulate in very low
 Upstate Cord Blood Bank Hematopoietic Progenitor Cells Hematopoiesis i starts from self renewing HPC HPC differentiates t to sequentially more mature blood cells HPC circulate in very low numbers in adults
Upstate Cord Blood Bank Hematopoietic Progenitor Cells Hematopoiesis i starts from self renewing HPC HPC differentiates t to sequentially more mature blood cells HPC circulate in very low numbers in adults
Back to the Future: The Resurgence of Bone Marrow??
 Back to the Future: The Resurgence of Bone Marrow?? Thomas Spitzer, MD Director. Bone Marrow Transplant Program Massachusetts General Hospital Professor of Medicine, Harvard Medical School Bone Marrow
Back to the Future: The Resurgence of Bone Marrow?? Thomas Spitzer, MD Director. Bone Marrow Transplant Program Massachusetts General Hospital Professor of Medicine, Harvard Medical School Bone Marrow
Role of NMDP Repository in the Evolution of HLA Matching and Typing for Unrelated Donor HCT
 Role of NMDP Repository in the Evolution of HLA Matching and Typing for Unrelated Donor HCT Stephen Spellman, MBS Director, Immunobiology and Observational Research Assistant Scientific Director CIBMTR,
Role of NMDP Repository in the Evolution of HLA Matching and Typing for Unrelated Donor HCT Stephen Spellman, MBS Director, Immunobiology and Observational Research Assistant Scientific Director CIBMTR,
WELCOME TO ONLINE TRAINING FOR CLINICAL RESEARCH COORDINATORS
 UCLA CTSI WELCOME TO ONLINE TRAINING FOR CLINICAL RESEARCH COORDINATORS ROLE OF THE RESEARCH COORDINATOR Adverse Events in Clinical Trials: Definitions and Documentation May 2016 Objectives Recognize the
UCLA CTSI WELCOME TO ONLINE TRAINING FOR CLINICAL RESEARCH COORDINATORS ROLE OF THE RESEARCH COORDINATOR Adverse Events in Clinical Trials: Definitions and Documentation May 2016 Objectives Recognize the
8.0 ADVERSE EVENT HANDLING
 8.0 ADVERSE EVENT HANDLING In accordance with the U.S. Code of Federal Regulations governing IND safety reports (Code of Federal Regulations 21, 312.32), adverse events (AEs) are reported from research
8.0 ADVERSE EVENT HANDLING In accordance with the U.S. Code of Federal Regulations governing IND safety reports (Code of Federal Regulations 21, 312.32), adverse events (AEs) are reported from research
LEGISLATIVE FRAMEWORKS AND ETHICS OF DONATION. Jeff Szer, Royal Melbourne Hospital, Australia
 LEGISLATIVE FRAMEWORKS AND ETHICS OF DONATION Jeff Szer, Royal Melbourne Hospital, Australia Donation of HPC Autologous HPC-A HPC-M Allogeneic Family member Volunteer Unrelated HPC-A HPC-M HPC-C Ethical
LEGISLATIVE FRAMEWORKS AND ETHICS OF DONATION Jeff Szer, Royal Melbourne Hospital, Australia Donation of HPC Autologous HPC-A HPC-M Allogeneic Family member Volunteer Unrelated HPC-A HPC-M HPC-C Ethical
General Terms: Appendix B. National Marrow Donor Program and The Medical College of Wisconsin
 Glossary of Terms This appendix is divided into two sections. The first section, General Terms, defines terms used throughout the CIBMTR data collection forms. The second section, FormsNet TM 2 Terms,
Glossary of Terms This appendix is divided into two sections. The first section, General Terms, defines terms used throughout the CIBMTR data collection forms. The second section, FormsNet TM 2 Terms,
OneMatch : A Closer Look. Gail Morris Principal Case Manager April 2017
 OneMatch : A Closer Look Gail Morris Principal Case Manager April 2017 Donor Recruitment Recruitment Managed by the CBS Donor Relations Team staff across Canada Danny, OneMatch Registrant Canadians register
OneMatch : A Closer Look Gail Morris Principal Case Manager April 2017 Donor Recruitment Recruitment Managed by the CBS Donor Relations Team staff across Canada Danny, OneMatch Registrant Canadians register
The National Marrow Donor Program. Graft Sources for Hematopoietic Cell Transplantation. Simon Bostic, URD Transplant Recipient
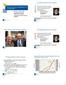 1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire. Table of Contents
 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
CELLULAR THERAPY QUIZ. Correct answers and rationale
 CELLULAR THERAPY QUIZ Correct answers and rationale In January 2018, registered cellular therapy (CT) facilities were asked to participate in the quiz to determine the educational needs of the users regarding
CELLULAR THERAPY QUIZ Correct answers and rationale In January 2018, registered cellular therapy (CT) facilities were asked to participate in the quiz to determine the educational needs of the users regarding
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire. Table of Contents
 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Methods of Administration DHQ Materials - Structure and Content Reformatting
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Methods of Administration DHQ Materials - Structure and Content Reformatting
One Day BMT Course by Thai Society of Hematology. Management of Graft Failure and Relapsed Diseases
 One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
INFORMATION ONLY Changes to Requesting HLA/HPA Selected Apheresis Platelets Customer Letter #
 INFORMATION ONLY Changes to Requesting HLA/HPA Selected Apheresis Platelets Customer Letter # 2015-21 Head Office / Siège social 2015-08-11 Dear Customer, We are writing to inform you of changes to the
INFORMATION ONLY Changes to Requesting HLA/HPA Selected Apheresis Platelets Customer Letter # 2015-21 Head Office / Siège social 2015-08-11 Dear Customer, We are writing to inform you of changes to the
Manual for Expedited Reporting of Adverse Events to DAIDS Version 2.0 January 2010
 Manual for Expedited Reporting of Adverse Events to DAIDS Version 2.0 January 2010 January 2010 Version 2.0 Table of Contents 1. INTRODUCTION...1 1.1 Scope... 1 1.2 Purpose... 1 1.3 Responsibilities...
Manual for Expedited Reporting of Adverse Events to DAIDS Version 2.0 January 2010 January 2010 Version 2.0 Table of Contents 1. INTRODUCTION...1 1.1 Scope... 1 1.2 Purpose... 1 1.3 Responsibilities...
HLA-A * L
 What is Nomenclature? HLA Nomenclature 3.0 Signifies DNA Subtype Differences outside the coding region (introns) HLA-A * 4 0 01 0 L Steve Spellman Sr. Manager, NMDP Asst. Scientific Director, CIBMTR Locus
What is Nomenclature? HLA Nomenclature 3.0 Signifies DNA Subtype Differences outside the coding region (introns) HLA-A * 4 0 01 0 L Steve Spellman Sr. Manager, NMDP Asst. Scientific Director, CIBMTR Locus
2/26/2016. Types of Stem Cells: Agenda. Objectives
 PAMET, March 5, 2016 Umbilical Cord Blood for Public Banking and Research Regenerative Medicine: Curing disease by repairing or replacing organs and systems through cellular and tissue engineering Bladder
PAMET, March 5, 2016 Umbilical Cord Blood for Public Banking and Research Regenerative Medicine: Curing disease by repairing or replacing organs and systems through cellular and tissue engineering Bladder
NATIONAL MARROW DONOR PROGRAM. Creating Connections. Saving Lives. Fran Rabe
 Creating Connections. Saving Lives. National Marrow Donor Program (NMDP) Screening of International Donors Fran Rabe FDA Liaison Meeting January 4, 2007 U.S. Importation of Peripheral Blood Stem Cells
Creating Connections. Saving Lives. National Marrow Donor Program (NMDP) Screening of International Donors Fran Rabe FDA Liaison Meeting January 4, 2007 U.S. Importation of Peripheral Blood Stem Cells
b. Welcome new Co-Chair Bronwen Shaw, MD, PhD; Anthony Nolan Research Institute, London, UK;
 Not for publication or presentation AGENDA CIBMTR WORKING COMMITTEE FOR DONOR HEALTH AND SAFETY Salt Lake City, UT Thursday, February 14, 2013, 2:45 pm - 4:45 pm Co-Chair: David Stroncek, MD, National
Not for publication or presentation AGENDA CIBMTR WORKING COMMITTEE FOR DONOR HEALTH AND SAFETY Salt Lake City, UT Thursday, February 14, 2013, 2:45 pm - 4:45 pm Co-Chair: David Stroncek, MD, National
5/9/2018. Bone marrow failure diseases (aplastic anemia) can be cured by providing a source of new marrow
 5/9/2018 or Stem Cell Harvest Where we are now, and What s Coming AA MDS International Foundation Indianapolis IN Luke Akard MD May 19, 2018 Infusion Transplant Conditioning Treatment 2-7 days STEM CELL
5/9/2018 or Stem Cell Harvest Where we are now, and What s Coming AA MDS International Foundation Indianapolis IN Luke Akard MD May 19, 2018 Infusion Transplant Conditioning Treatment 2-7 days STEM CELL
The TTSN - A Collaborative Biovigilance System
 The TTSN - A Collaborative Biovigilance System 5th World Congress on Tissue Banking 12th International Conference of the APASTB Kuala Lumpur, Malaysia June 4, 2008 Scott A. Brubaker, CTBS American Association
The TTSN - A Collaborative Biovigilance System 5th World Congress on Tissue Banking 12th International Conference of the APASTB Kuala Lumpur, Malaysia June 4, 2008 Scott A. Brubaker, CTBS American Association
Introduction to FACT Immune Effector Cellular Therapy Standards and Accreditation
 Introduction to FACT Immune Effector Cellular Therapy Standards and Accreditation Presented by Ngaire Elwood, PhD Vice President, Foundation for the Accreditation of Cellular Therapy (FACT); Director of
Introduction to FACT Immune Effector Cellular Therapy Standards and Accreditation Presented by Ngaire Elwood, PhD Vice President, Foundation for the Accreditation of Cellular Therapy (FACT); Director of
Blood and Marrow Transplant (BMT) for Sickle Cell Disease
 Blood and Marrow Transplant (BMT) for Sickle Cell Disease Rhiannon is now cured of sickle cell disease after BMT. Blood and marrow transplant (BMT) is a proven cure for sickle cell disease. This handbook
Blood and Marrow Transplant (BMT) for Sickle Cell Disease Rhiannon is now cured of sickle cell disease after BMT. Blood and marrow transplant (BMT) is a proven cure for sickle cell disease. This handbook
Ebola Prevention Vaccine Evaluation in Sierra Leone
 Ebola Prevention Vaccine Evaluation in Sierra Leone Dr. SAS Kargbo Sierra Leone Ministry of Health and Sanitation Sierra Leone CDC Collaboration World Health Organization, Geneva 9 January 2015 Principal
Ebola Prevention Vaccine Evaluation in Sierra Leone Dr. SAS Kargbo Sierra Leone Ministry of Health and Sanitation Sierra Leone CDC Collaboration World Health Organization, Geneva 9 January 2015 Principal
What the Transfusion Scientist should know about Stem Cells. Dr Claire Wiggins BBTS September 2017
 What the Transfusion Scientist should know about Stem Cells Dr Claire Wiggins BBTS September 2017 What is a stem cell? Undifferentiated cell Can divide and self renew for long periods Differentiate into
What the Transfusion Scientist should know about Stem Cells Dr Claire Wiggins BBTS September 2017 What is a stem cell? Undifferentiated cell Can divide and self renew for long periods Differentiate into
AABB Standards and Accreditation. Doug Padley, MT (ASCP) Mayo Clinic Rochester MN USA
 AABB Standards and Accreditation Doug Padley, MT (ASCP) Mayo Clinic Rochester MN USA Objectives Review AABB standard setting process Describe accreditation program What is AABB? International association
AABB Standards and Accreditation Doug Padley, MT (ASCP) Mayo Clinic Rochester MN USA Objectives Review AABB standard setting process Describe accreditation program What is AABB? International association
June 8, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
Adverse Events Monitoring (aka Pharmacovigilance)
 Adverse Events Monitoring (aka Pharmacovigilance) Presentation to American Conference Institute s FDA Boot Camp William W. Vodra May 16, 2007 Agenda What is pharmacovigilance (PV)? How does PV use adverse
Adverse Events Monitoring (aka Pharmacovigilance) Presentation to American Conference Institute s FDA Boot Camp William W. Vodra May 16, 2007 Agenda What is pharmacovigilance (PV)? How does PV use adverse
Factors Influencing Haematopoietic Progenitor cell transplant outcome Optimising donor selection
 Factors Influencing Haematopoietic Progenitor cell transplant outcome Optimising donor selection Alison Logan Transplantation Laboratory Manchester Royal Infirmary Haematopoietic progenitor cell transplants
Factors Influencing Haematopoietic Progenitor cell transplant outcome Optimising donor selection Alison Logan Transplantation Laboratory Manchester Royal Infirmary Haematopoietic progenitor cell transplants
Specimen Collection Requirements. Test Name Specimen Type Storage Time Storage Conditions
 1 Specimen Collection Requirements PURPOSE/PRINCIPLE: To outline proper specimen collection for molecular HLA testing, including tube type, minimum specimen amount, proper tube and requisition labeling,
1 Specimen Collection Requirements PURPOSE/PRINCIPLE: To outline proper specimen collection for molecular HLA testing, including tube type, minimum specimen amount, proper tube and requisition labeling,
ST. MICHAEL S HOSPITAL Guidelines for Reporting Serious Adverse Events / Unanticipated Problems to the SMH Research Ethics Board (REB) July 09, 2014
 ST. MICHAEL S HOSPITAL Guidelines for Reporting Serious Adverse Events / Unanticipated Problems to the SMH Research Ethics Board (REB) July 09, 2014 1. Introduction The St. Michael s Hospital (SMH) REB
ST. MICHAEL S HOSPITAL Guidelines for Reporting Serious Adverse Events / Unanticipated Problems to the SMH Research Ethics Board (REB) July 09, 2014 1. Introduction The St. Michael s Hospital (SMH) REB
21/05/2018. Continuing Education. Presentation Recording. learn.immucor.com
 Transplant Webinar Series: Ep. 6 Donor Selection for Haematopoietic Stem Cell Transplantation Future Webinars The Role of NGS in the Transplant Setting Featuring Dr Sujatha Krishnakumar Sirona Genomics,
Transplant Webinar Series: Ep. 6 Donor Selection for Haematopoietic Stem Cell Transplantation Future Webinars The Role of NGS in the Transplant Setting Featuring Dr Sujatha Krishnakumar Sirona Genomics,
Myelodysplasia/Myeloproliferative Neoplasms (MDS/MPN) Post-HCT Data
 Instructions for Myelodysplasia/Myeloproliferative Neoplasms (MDS/MPN) Post-HCT Data (Form 2114) This section of the CIBMTR Forms Instruction Manual is intended to be a resource for completing the Myelodysplasia/Myeloproliferative
Instructions for Myelodysplasia/Myeloproliferative Neoplasms (MDS/MPN) Post-HCT Data (Form 2114) This section of the CIBMTR Forms Instruction Manual is intended to be a resource for completing the Myelodysplasia/Myeloproliferative
Granix. Granix (tbo-filgrastim) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.16 Subject: Granix 1 of 6 Last Review Date: September 15, 2017 Granix Description Granix (tbo-filgrastim)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.16 Subject: Granix 1 of 6 Last Review Date: September 15, 2017 Granix Description Granix (tbo-filgrastim)
Granix. Granix (tbo-filgrastim) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.16 Subject: Granix 1 of 7 Last Review Date: December 2, 2016 Granix Description Granix (tbo-filgrastim)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.16 Subject: Granix 1 of 7 Last Review Date: December 2, 2016 Granix Description Granix (tbo-filgrastim)
Cord Blood Bankers are. Angie Yost, MT(ASCP) CM 2016 ASCLS-MO CLMA Spring Meeting
 Cord Blood Bankers are Angie Yost, MT(ASCP) CM 2016 ASCLS-MO CLMA Spring Meeting Objectives Cord blood 101 Manufacturing and cryopreservation Characterization testing Why Do We Need Cord Blood? Each year,
Cord Blood Bankers are Angie Yost, MT(ASCP) CM 2016 ASCLS-MO CLMA Spring Meeting Objectives Cord blood 101 Manufacturing and cryopreservation Characterization testing Why Do We Need Cord Blood? Each year,
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
Haplo vs Cord vs URD Debate
 3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
Hematopoietic Stem Cells, Stem Cell Processing, and Transplantation
 Hematopoietic Stem Cells, Stem Cell Processing, and Joseph (Yossi) Schwartz, M irector, Hemotherapy and Stem Cell Processing Facility Bone Marrow Can Cure: Leukemia Lymphoma Multiple Myeloma Genetic iseases:
Hematopoietic Stem Cells, Stem Cell Processing, and Joseph (Yossi) Schwartz, M irector, Hemotherapy and Stem Cell Processing Facility Bone Marrow Can Cure: Leukemia Lymphoma Multiple Myeloma Genetic iseases:
Hematopoietic Stem Cell Therapy
 Hematopoietic Stem Cell Therapy Grace Totoe, MBChB, SBB CME August 2012 Accra-Ghana Hematopoietic Stem Cells Cells capable of self renewal and differentiation into all blood cell lineages Objectives Historical
Hematopoietic Stem Cell Therapy Grace Totoe, MBChB, SBB CME August 2012 Accra-Ghana Hematopoietic Stem Cells Cells capable of self renewal and differentiation into all blood cell lineages Objectives Historical
Voluntary Accreditation of Immune Effector Cell Programs
 Voluntary Accreditation of Immune Effector Cell Programs From Clinical Trials to Licensure and Beyond Phyllis I. Warkentin, MD September 6, 2017 Presentation Outline FACT Standards and Accreditation Program
Voluntary Accreditation of Immune Effector Cell Programs From Clinical Trials to Licensure and Beyond Phyllis I. Warkentin, MD September 6, 2017 Presentation Outline FACT Standards and Accreditation Program
Study Events 2. Consent Information 3. Family History 5. Registry Visit 8. Visit Information 11. Vitals / Measures 13. Clinical History 15
 Study Events 2 Consent Information 3 Family History 5 Registry Visit 8 Visit Information 11 Vitals / Measures 13 Clinical History 15 Immunizations, Infusions, Transfusions 31 Medications & Supplements
Study Events 2 Consent Information 3 Family History 5 Registry Visit 8 Visit Information 11 Vitals / Measures 13 Clinical History 15 Immunizations, Infusions, Transfusions 31 Medications & Supplements
Granix. Granix (tbo-filgrastim) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.10.16 Section: Prescription Drugs Effective Date: April 1, 2014 Subject: Granix 1 of 7 Last Review Date:
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.10.16 Section: Prescription Drugs Effective Date: April 1, 2014 Subject: Granix 1 of 7 Last Review Date:
Rob Wynn RMCH & University of Manchester, UK. HCT in Children
 Rob Wynn RMCH & University of Manchester, UK HCT in Children Summary Indications for HCT in children Donor selection for Paediatric HCT Using cords Achieving engraftment in HCT Conditioning Immune action
Rob Wynn RMCH & University of Manchester, UK HCT in Children Summary Indications for HCT in children Donor selection for Paediatric HCT Using cords Achieving engraftment in HCT Conditioning Immune action
Granix. Granix (tbo-filgrastim) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.10.16 Subject: Granix 1 of 7 Last Review Date: September 18, 2015 Granix Description Granix (tbo-filgrastim)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.10.16 Subject: Granix 1 of 7 Last Review Date: September 18, 2015 Granix Description Granix (tbo-filgrastim)
COHEM Barcellona 2012 Hemoglobinopathies debate
 COHEM Barcellona 2012 Hemoglobinopathies debate September 8, 2012: h. 10:30-12:00 Hall: A Is it justified to perform BMT in hemoglobinopathies using unrelated and/or partially mismatched donors? HSCT indication
COHEM Barcellona 2012 Hemoglobinopathies debate September 8, 2012: h. 10:30-12:00 Hall: A Is it justified to perform BMT in hemoglobinopathies using unrelated and/or partially mismatched donors? HSCT indication
BMTCN Review Course Basic Concepts and Indications for Transplantation. David Rice, PhD, RN, NP
 BMTCN Review Course Basic Concepts and Indications for Transplantation March 16, 2017 David Rice, PhD, RN, NP Director, Professional Practice and Education No disclosures Objectives Describe basic concepts
BMTCN Review Course Basic Concepts and Indications for Transplantation March 16, 2017 David Rice, PhD, RN, NP Director, Professional Practice and Education No disclosures Objectives Describe basic concepts
New Zealand Bone Marrow Donor Registry
 Title: SECTION 4.0 NZBMDR STANDARDS DONOR RECRUITMENT, ENROLMENT & INFORMATION Authorised by: Executive Officer Contributing Authors: ABMDR Dr Hilary Blacklock Raewyn Fisher NZBMDR-Guidelines-004-13 Date
Title: SECTION 4.0 NZBMDR STANDARDS DONOR RECRUITMENT, ENROLMENT & INFORMATION Authorised by: Executive Officer Contributing Authors: ABMDR Dr Hilary Blacklock Raewyn Fisher NZBMDR-Guidelines-004-13 Date
April 7, Introduction
 April 7, 2017 ICD-10 Coordination and Maintenance Committee Department of Health and Human Services Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244-1850 ICDProcedureCodeRequest@cms.hhs.gov
April 7, 2017 ICD-10 Coordination and Maintenance Committee Department of Health and Human Services Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244-1850 ICDProcedureCodeRequest@cms.hhs.gov
SAE håndtering i protokol CC MM-001
 SAE håndtering i protokol CC-92480-MM-001 An SAE is any AE occurring at any dose that: Results in death; Is life-threatening (ie, in the opinion of the Investigator, the subject is at immediate risk of
SAE håndtering i protokol CC-92480-MM-001 An SAE is any AE occurring at any dose that: Results in death; Is life-threatening (ie, in the opinion of the Investigator, the subject is at immediate risk of
Seventy percent of people who are candidates for allogeneic hematopoietic
 274 7th Biennial Symposium on Minorities, the Medically Underserved and Cancer Supplement to Cancer The National Marrow Donor Program Meeting the Needs of the Medically Underserved Dennis L. Confer, M.D.
274 7th Biennial Symposium on Minorities, the Medically Underserved and Cancer Supplement to Cancer The National Marrow Donor Program Meeting the Needs of the Medically Underserved Dennis L. Confer, M.D.
Introduction to EU 'SoHO' Sector Screening Meeting with the Republic of Serbia December 5, 2014 Directorate General for Health and Consumers Unit D4
 Introduction to EU 'SoHO' Sector Screening Meeting with the Republic of Serbia December 5, 2014 Directorate General for Unit D4 1. Introduction: SoHO & the Legal Basis 2. The Legislative Framework 3. Blood
Introduction to EU 'SoHO' Sector Screening Meeting with the Republic of Serbia December 5, 2014 Directorate General for Unit D4 1. Introduction: SoHO & the Legal Basis 2. The Legislative Framework 3. Blood
Inspections, Compliance, Enforcement, and Criminal Investigations. Central Texas Regional Blood & Tissue Center 07-Nov-03
 1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
European actions in the field of transplantation & blood transfusion
 European actions in the field of transplantation & blood transfusion Andrzej Rys Director Directorate Health Systems and Products Madrid, 27 June 2013 1 Blood Tissues and cells Organs 2 Blood transfusion
European actions in the field of transplantation & blood transfusion Andrzej Rys Director Directorate Health Systems and Products Madrid, 27 June 2013 1 Blood Tissues and cells Organs 2 Blood transfusion
MUD SCT. Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University
 MUD SCT Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University Outlines Optimal match criteria for unrelated adult donors Role of ATG in MUD-SCT Post-transplant
MUD SCT Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University Outlines Optimal match criteria for unrelated adult donors Role of ATG in MUD-SCT Post-transplant
Report on the Transplantation Transmission Sentinel Network (TTSN)
 Report on the Transplantation Transmission Sentinel Network (TTSN) CATB Session - AATB s 31st Annual Meeting, Boston September 15, 2007 Scott Brubaker, CTBS AATB Representative, TTSN Advisory Group TTSN
Report on the Transplantation Transmission Sentinel Network (TTSN) CATB Session - AATB s 31st Annual Meeting, Boston September 15, 2007 Scott Brubaker, CTBS AATB Representative, TTSN Advisory Group TTSN
CHAPTER 2 PROTOCOL DESIGN
 CHAPTER 2 PROTOCOL DESIGN CHAPTER 2 PROTOCOL DESIGN 2.1 ELIGIBILITY CRITERIA Participants fulfilling the following criteria will be eligible for enrollment in the protocol: 1. Participant is diagnosed
CHAPTER 2 PROTOCOL DESIGN CHAPTER 2 PROTOCOL DESIGN 2.1 ELIGIBILITY CRITERIA Participants fulfilling the following criteria will be eligible for enrollment in the protocol: 1. Participant is diagnosed
Documentation of Changes to EFI Standards: v 5.6.1
 Modified Standard B - PERSONNEL QUALIFICATIONS B1.000 The laboratory must employ one or more individuals who meet the qualifications and fulfil the responsibilities of the Director/Co-Director, Technical
Modified Standard B - PERSONNEL QUALIFICATIONS B1.000 The laboratory must employ one or more individuals who meet the qualifications and fulfil the responsibilities of the Director/Co-Director, Technical
CHAPTER 3 LABORATORY PROCEDURES
 CHAPTER 3 LABORATORY PROCEDURES CHAPTER 3 LABORATORY PROCEDURES 3.1 HLA TYPING Molecular HLA typing will be performed for all donor cord blood units and patients in the three reference laboratories identified
CHAPTER 3 LABORATORY PROCEDURES CHAPTER 3 LABORATORY PROCEDURES 3.1 HLA TYPING Molecular HLA typing will be performed for all donor cord blood units and patients in the three reference laboratories identified
The role of HLA in Allogeneic Hematopoietic Stem Cell Transplantation and Platelet Refractoriness.
 The role of HLA in Allogeneic Hematopoietic Stem Cell Transplantation and Platelet Refractoriness. Robert Liwski, MD, PhD, FRCPC Medical Director HLA Typing Laboratory Department of Pathology Dalhousie
The role of HLA in Allogeneic Hematopoietic Stem Cell Transplantation and Platelet Refractoriness. Robert Liwski, MD, PhD, FRCPC Medical Director HLA Typing Laboratory Department of Pathology Dalhousie
DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J
 JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
Samples Available for Recipient Only. Samples Available for Recipient and Donor
 Unrelated HCT Research Sample Inventory - Summary for First Allogeneic Transplants in CRF and TED with biospecimens available through the CIBMTR Repository stratified by availability of paired samples,
Unrelated HCT Research Sample Inventory - Summary for First Allogeneic Transplants in CRF and TED with biospecimens available through the CIBMTR Repository stratified by availability of paired samples,
Review of Stem Cell Collection Data 2016 Ronan Foley, Professor, Director Stem Cell Laboratory, McMaster University
 1 Review of Stem Cell Collection Data 2016 Ronan Foley, Professor, Director Stem Cell Laboratory, McMaster University Objectives 2 Review our national apheresis-based stem cell registry To evaluate the
1 Review of Stem Cell Collection Data 2016 Ronan Foley, Professor, Director Stem Cell Laboratory, McMaster University Objectives 2 Review our national apheresis-based stem cell registry To evaluate the
A G E N D A CIBMTR WORKING COMMITTEE FOR DONOR HEALTH AND SAFETY Grapevine, TX Friday, February 28, 2014, 2:45 4:45 pm
 Not for publication or presentation A G E N D A CIBMTR WORKING COMMITTEE FOR DONOR HEALTH AND SAFETY Grapevine, TX Friday, February 28, 2014, 2:45 4:45 pm Co Chair: Steven Goldstein, MD, University of
Not for publication or presentation A G E N D A CIBMTR WORKING COMMITTEE FOR DONOR HEALTH AND SAFETY Grapevine, TX Friday, February 28, 2014, 2:45 4:45 pm Co Chair: Steven Goldstein, MD, University of
About OMICS Group Conferences
 About OMICS Group OMICS Group International is an amalgamation of Open Access publications and worldwide international science conferences and events. Established in the year 2007 with the sole aim of
About OMICS Group OMICS Group International is an amalgamation of Open Access publications and worldwide international science conferences and events. Established in the year 2007 with the sole aim of
APHERESIS PROCEDURES. Worldwide implemented nowadays. Most of Asian Countries also involved. The usage of this procedure varies between countries.
 ASIA COUNTRIES AS APHERESIS PROCEDURES Worldwide implemented nowadays. Most of Asian Countries also involved. The usage of this procedure varies between countries. SPECTRUM OF APHERESIS Therapeutic Apheresis
ASIA COUNTRIES AS APHERESIS PROCEDURES Worldwide implemented nowadays. Most of Asian Countries also involved. The usage of this procedure varies between countries. SPECTRUM OF APHERESIS Therapeutic Apheresis
Protocol Number: 10-CBA National Clinical Trial (NCT) Identified Number: NCT
 A multicenter access and distribution protocol for unlicensed cryopreserved cord blood units (CBUs) for transplantation in pediatric and adult patients with hematologic malignancies and other indications
A multicenter access and distribution protocol for unlicensed cryopreserved cord blood units (CBUs) for transplantation in pediatric and adult patients with hematologic malignancies and other indications
Samples Available for Recipient and Donor
 Unrelated HCT Research Sample Inventory - Summary for First Allogeneic Transplants in CRF and TED with biospecimens available through the CIBMTR Repository stratified by availability of paired samples,
Unrelated HCT Research Sample Inventory - Summary for First Allogeneic Transplants in CRF and TED with biospecimens available through the CIBMTR Repository stratified by availability of paired samples,
Samples Available for Recipient Only. Samples Available for Recipient and Donor
 Unrelated HCT Research Sample Inventory - Summary for First Allogeneic Transplants in CRF and TED with biospecimens available through the CIBMTR Repository stratified by availability of paired samples,
Unrelated HCT Research Sample Inventory - Summary for First Allogeneic Transplants in CRF and TED with biospecimens available through the CIBMTR Repository stratified by availability of paired samples,
D7.1 Report summarising results of survey of EU countries to identify volumes and trends in relation to the import and export of stem cells
 Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
D7.1 Report summarising results of survey of EU countries to identify volumes and trends in relation to the import and export of stem cells
 Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Organ Procurement and Transplantation Network
 OPTN Organ Procurement and Transplantation Network POLICIES This document provides the policy language approved by the OPTN/UNOS Board at its meeting in June 2015 as part of the Operations and Safety Committee
OPTN Organ Procurement and Transplantation Network POLICIES This document provides the policy language approved by the OPTN/UNOS Board at its meeting in June 2015 as part of the Operations and Safety Committee
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/20898 holds various files of this Leiden University dissertation. Author: Jöris, Monique Maria Title: Challenges in unrelated hematopoietic stem cell transplantation.
Cover Page The handle http://hdl.handle.net/1887/20898 holds various files of this Leiden University dissertation. Author: Jöris, Monique Maria Title: Challenges in unrelated hematopoietic stem cell transplantation.
Bone Marrow Transplantation and the Potential Role of Iomab-B
 Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Donatore HLA identico di anni o MUD giovane?
 Donatore HLA identico di 60-70 anni o MUD giovane? Stella Santarone Dipartimento di Ematologia, Medicina Trasfusionale e Biotecnologie Pescara AGENDA 1. Stem Cell Donation: fatalities and severe events
Donatore HLA identico di 60-70 anni o MUD giovane? Stella Santarone Dipartimento di Ematologia, Medicina Trasfusionale e Biotecnologie Pescara AGENDA 1. Stem Cell Donation: fatalities and severe events
HEMATOLOGY AND ONCOLOGY
 x THE POWER OF HEMATOLOGY AND ONCOLOGY Experts. Experience. Execution. A Deeper Dive into Hematology and Oncology Medpace supports our sponsors who are advancing new anti-cancer therapies by providing
x THE POWER OF HEMATOLOGY AND ONCOLOGY Experts. Experience. Execution. A Deeper Dive into Hematology and Oncology Medpace supports our sponsors who are advancing new anti-cancer therapies by providing
OneMatch Stem Cell and Marrow Network. Training Guide
 OneMatch Stem Cell and Marrow Network Training Guide What is OneMatch all about? OneMatch is a Canadian Program that matches and coordinates the collection of stem cells from potential donors to help save
OneMatch Stem Cell and Marrow Network Training Guide What is OneMatch all about? OneMatch is a Canadian Program that matches and coordinates the collection of stem cells from potential donors to help save
Olive J Sturtevant, MHP, MT(ASCP)SBB/SLS, CQA Director, Cellular Therapy Quality Assurance Dana Farber Cancer Institute
 Adverse Events associated with Cell Therapy Products Olive J Sturtevant, MHP, MT(ASCP)SBB/SLS, CQA Director, Cellular Therapy Quality Assurance Dana Farber Cancer Institute 2 Objectives Review the types
Adverse Events associated with Cell Therapy Products Olive J Sturtevant, MHP, MT(ASCP)SBB/SLS, CQA Director, Cellular Therapy Quality Assurance Dana Farber Cancer Institute 2 Objectives Review the types
AETNA BETTER HEALTH Non-Formulary Prior Authorization guideline for Colony Stimulating Factor (CSF)
 AETNA BETTER HEALTH Non-Formulary Prior Authorization guideline for Colony Stimulating Factor (CSF) Colony Stimulating Factor (CSF) Neupogen (filgrastim; G-CSF), Neulasta (peg-filgrastim; G-CSF); Neulasa
AETNA BETTER HEALTH Non-Formulary Prior Authorization guideline for Colony Stimulating Factor (CSF) Colony Stimulating Factor (CSF) Neupogen (filgrastim; G-CSF), Neulasta (peg-filgrastim; G-CSF); Neulasa
Summary of Changes Page BMT CTN 1205 Protocol Amendment #4 (Version 5.0) Dated July 22, 2016
 Page 1 of 8 Date: July 22, 2016 Summary of Changes Page BMT CTN 1205 Protocol #4 Dated July 22, 2016 The following changes, and the rationale for the changes, were made to the attached protocol in this
Page 1 of 8 Date: July 22, 2016 Summary of Changes Page BMT CTN 1205 Protocol #4 Dated July 22, 2016 The following changes, and the rationale for the changes, were made to the attached protocol in this
Guidance for establishing a hematopoietic progenitor cells donor follow-up registry
 Guidance for establishing a hematopoietic progenitor cells donor follow-up registry ARTHIQS Deliverable 8 Work Package 5 ARTHIQS (Grant agreement n. 20132101) has received funding from the European Commission
Guidance for establishing a hematopoietic progenitor cells donor follow-up registry ARTHIQS Deliverable 8 Work Package 5 ARTHIQS (Grant agreement n. 20132101) has received funding from the European Commission
Neutrophil Recovery: The. Posttransplant Recovery. Bus11_1.ppt
 Neutrophil Recovery: The First Step in Posttransplant Recovery No conflicts of interest to disclose Bus11_1.ppt Blood is Made in the Bone Marrow Blood Stem Cell Pre-B White cells B Lymphocyte T Lymphocyte
Neutrophil Recovery: The First Step in Posttransplant Recovery No conflicts of interest to disclose Bus11_1.ppt Blood is Made in the Bone Marrow Blood Stem Cell Pre-B White cells B Lymphocyte T Lymphocyte
Clinical Policy: Pegfilgrastim (Neulasta) Reference Number: CP.CPA.127 Effective Date: Last Review Date: Line of Business: Commercial
 Clinical Policy: (Neulasta) Reference Number: CP.CPA.127 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Neulasta) Reference Number: CP.CPA.127 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for
Blood and Marrow Transplant Patient Information Sheet
 Blood and Marrow Transplant Patient Information Sheet Patient Name MR# DOB Diagnosis Wt. (kg) Ht. (cm) M2 Ethnicity: Sex: UPN # 0.00 BMT Attending: Name Allergies: BMT Coordinator: Name Donor Information:
Blood and Marrow Transplant Patient Information Sheet Patient Name MR# DOB Diagnosis Wt. (kg) Ht. (cm) M2 Ethnicity: Sex: UPN # 0.00 BMT Attending: Name Allergies: BMT Coordinator: Name Donor Information:
BMTCN Review Course Basic Concepts and Indications for Transplantation How the Experts Treat Hematologic Malignancies Las Vegas, NV, March 10, 2016
 BMTCN Review Course Basic Concepts and Indications for Transplantation How the Experts Treat Hematologic Malignancies Las Vegas, NV, March 10, 2016 David Rice, PhD, RN, NP Director, Professional Practice
BMTCN Review Course Basic Concepts and Indications for Transplantation How the Experts Treat Hematologic Malignancies Las Vegas, NV, March 10, 2016 David Rice, PhD, RN, NP Director, Professional Practice
Adverse Events- Love them, hate them, report them!
 Adverse Events- Love them, hate them, report them! Billi Tatum, RN, CCRC Regional Vascular Center Harborview Medical Center and the University of Washington Medical Center Objectives Define and identify
Adverse Events- Love them, hate them, report them! Billi Tatum, RN, CCRC Regional Vascular Center Harborview Medical Center and the University of Washington Medical Center Objectives Define and identify
Unanticipated Problems and Adverse Events
 Unanticipated Problems and Adverse Events David Borasky, MPH, CIP Office of Research Protections RTI International Research Triangle Park, North Carolina, USA Introduction IEC review of unanticipated problems
Unanticipated Problems and Adverse Events David Borasky, MPH, CIP Office of Research Protections RTI International Research Triangle Park, North Carolina, USA Introduction IEC review of unanticipated problems
AETNA BETTER HEALTH Non-Formulary Prior Authorization guideline for Colony Stimulating Factor (CSF)
 AETNA BETTER HEALTH Non-Formulary Prior Authorization guideline for Colony Stimulating Factor (CSF) Colony Stimulating Factor (CSF) Neupogen (filgrastim; G-CSF), Neulasta (peg-filgrastim; G-CSF); Neulasa
AETNA BETTER HEALTH Non-Formulary Prior Authorization guideline for Colony Stimulating Factor (CSF) Colony Stimulating Factor (CSF) Neupogen (filgrastim; G-CSF), Neulasta (peg-filgrastim; G-CSF); Neulasa
XIV. HLA AND TRANSPLANTATION MEDICINE
 XIV. HLA AND TRANSPLANTATION MEDICINE A. Introduction 1. The HLA system includes a complex array of genes and their molecular products that are involved in immune regulation and cellular differentiation.
XIV. HLA AND TRANSPLANTATION MEDICINE A. Introduction 1. The HLA system includes a complex array of genes and their molecular products that are involved in immune regulation and cellular differentiation.
Accreditation of BMT units & EU Regulations
 Accreditation of BMT units & EU Regulations April 1, 2008 EBMT Annual Meeting Workshop: EU regulation/standardisation of cellular therapy Presentation: JACIE: aims and current activities EU regulation
Accreditation of BMT units & EU Regulations April 1, 2008 EBMT Annual Meeting Workshop: EU regulation/standardisation of cellular therapy Presentation: JACIE: aims and current activities EU regulation
