A - Permissions delayed/den ied. Date site ready to start. B - Suspended by Sponsor. C - Closed D - Sponsor
|
|
|
- Tamsin Chandler
- 5 years ago
- Views:
Transcription
1 No REC Reference IRAS No Number Title Date of First Patient Recruitment Benchmark Met invited selected HRA Approval date confirmed by sponsor confirmed ready to start A - Permissions delayed/den ied B - Suspended by C - Closed D - by delays E - Staff Availability issues F - No Eligible Patients (No patient seen) G - No Patients Consented H - Contracting Delays I-Rare disease J-Other Comments Sources of delay 1 15/EM/0551 Effectiveness of Intravenous iron treatment vs standard care in patients with heart failure and iron deficiency: a randomised, open-label multicentre trial (IRONMAN) N/A 26/08/ /06/ /07/ /05/ /06/ /06/2017 Three versus five years of adjuvant imatinib as treatment of patients with operable GIST with a high risk for recurrence: A randomised phase III 2 16/EE/ study. N/A 15/06/ /05/ /09/ /05/ /05/ /EE/0370 MesoTRAP: A feasibility study comparing video-assisted thoracoscopic partial pleurectomy/decortication with indwelling pleural catheter in patients with trapped lung and pleural effusion due to malignant pleural mesothelioma designed to address recruitment and randomisation uncertainties and sample size requirements for a phase III trial. N/A 16/12/ /05/ /01/ /05/ /05/ /LO/1992 Beyond education: A Hypoglycaemia Awareness Restoration Programme for people with type 1 diabetes and problematic hypoglycaemia persisting despite optimised self-care (HARPdoc) N/A 18/01/ /05/ /01/ /05/ /05/ /05/ /NW/0517 Accord Myeloma XII: A phase 3 study to determine the role of ixazomib as an augmented conditioning therapy in salvage autologous stem cell transplant (ASCT) and as a post-asct consolidation and maintenance strategy in patients with relapsed multiple myeloma. N/A 10/05/ /06/ /10/ /06/ /06/ /06/ /SC/0484 An Open Label, Randomized, Two Arm Phase III Study of Nivolumab in Combination with Ipilimumab versus Extreme Study Regimen (cetuximab + cisplatin/carboplatin + fluorouracil) as First Line Therapy in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (SCCHN) CheckMate 651 N/A 04/01/ /05/ /02/ /06/ /06/ /06/2017 Accuracy of Detection using ENdocuff Optimisation of Mucosal Abnormalities: The B-ADENOMA 7 16/WM/ Study N/A 17/01/ /06/ /01/ /06/ /06/ /06/ /EM/0014 Prospective Clinical Investigation for a Randomized, Controlled, Multicenter Non-inferiority Study Comparing Standard Wound Closure Technique with Drains (control) to Standard Wound Closure Techniques with TissuGlu and No Drains (test) in Mastectomy. N/A 13/04/ /06/ /03/ /06/ /06/ /06/ /LO/0243 A randomized, double-blind, multidose, placebo-controlled study to evaluate the efficacy, safety and tolerability of GSK administration for the treatment of pruritus in patients with primary biliary cholangitis. (GLIMMER: GSK trial of Ibat inhibition with Multidose Measurement for Evaluation of Response) N/A 09/03/ /05/ /03/ /05/ /05/2017
2 Using Virtual Reality as a distraction technique to reduce pain during burn 10 17/LO/ treatments. N/A 07/02/ /05/ /03/ /05/ /05/ /LO/0380 A phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group, efficacy and safety study of Crenezumab in patients with prodromal to mild Alzheimer s disease - Cread 2 N/A 05/01/ /06/ /05/ /06/ /06/ /06/ /NE/0115 Multi-center, international, doubleblind, two-arm, randomized, placebocontrolled phase II trial of pirfenidone in patients with unclassifiable progressive fibrosing ILD N/A 07/12/ /06/ /05/ /06/ /06/ /06/ /YH/0083 A randomised controlled trial of routine massage with a new lotion for the enhancement of skin health and cognitive development in infants N/A 01/02/ /06/ /06/ /06/ /06/ /SS/0225 SYSTEMS-2: A Randomised Phase II trial of standard versus dose escalated radiotherapy in the treatment of pain in malignant pleural mesothelioma. 22/02/2017 No 18/01/ /12/ /10/ /12/ /02/ /02/2017 Y Contracting / costing delays with NHS Provider support service (Radiotherapy Physics) NHS Provider 15 16/EE/0243 A 6-month, multicenter, Phase 3, open-label extension safety study of OTO-104 given at 3-month intervals by intratympanic injection in subjects with unilateral Meniere s disease 06/02/2017 No 21/07/ /11/ /08/ /11/ /12/ /02/2017 Y 16 16/EE/0356 A Phase 2b, Randomised, Doubleblind, Placebo-controlled, Parallelgroup, Multicenter Protocol to Evaluate the Safety and Efficacy of JNJ in Subjects with Moderately to Severely Active Crohn's Disease. No 27/10/ /02/ /11/ /02/ /03/ /05/2017 Y delayed confirmation of study open to delayed confirmation of study open to 17 16/EM/0193 A phase III, double-blind, randomized placebo-controlled study to evaluate the effects of dalcetrapib on cardiovascular (CV) risk in a genetically defined population with a recent Acute Coronary Syndrome (ACS): The dal-gene trial. 17/02/2017 No 11/03/ /09/ /06/ /09/ /09/ /09/2016 Y Patients screened but no eligible patients identified 18 16/EM/0376 A 12-week, randomized, multicenter, double-blind, placebocontrolled, 3-arm, parallel-group, phase 3 trial to evaluate the efficacy and safety of 2 doses of AQX-1125 targeting the SHIP1 pathway in subjects with interstitial cystitits/bladder pain syndrome followed by a 2-arm, 14 or 40 week open label extension 09/05/2017 No 12/10/ /01/ /12/ /02/ /02/ /02/2017 Y Patients screened but no eligible patients identified until after 70 days had passed 19 16/EM/0384 CV : An Open-label, 2x2 Fractorial, Randomized Controlled, Clinical Trial to Evaluate the Safety of Apixaban vs. Vitamin K Antagonist and Aspirin vs. Aspirin Placebo in Patients with Atrial Fibrilation and Acute Coronary Syndrome or Percutaneous Coronary Intervention 27/06/2017 No 25/08/ /01/ /11/ /01/ /02/ /02/2017 Y Strict patient eligibility criteria 20 16/LO/0913 Corneal cross-linking versus standard care in children with keratoconus; a randomised, multicentre, observer-masked trial of efficacy and safety. 01/03/2017 No 25/08/ /09/ /08/ /11/ /11/ /12/2016 Y Y Modulation of immune response and outcomes in ALS using IL /LO/ (MIROCALS-IL2) No 09/09/ /03/ /10/ /03/ /04/2017 Y Costing delays by NHS Provider. Patients screened but no eligible patients identified. delayed confirmation of study open to NHS Provider
3 22 16/LO/1138 A Phase II, randomized, doubleblind, placebo-controlled, parallelgroup, multicenter trial to evaluate the efficacy and safety of abituzumab in subjects with systemic sclerosis-associated interstitial lung disease (SSc-ILD). No 25/05/ /11/ /08/ /11/ /11/ /11/2016 Y 23 16/LO/1386 Phase 2 Study: A Phase 2 Randomized, Double-Blinded, Placebo-Controlled Study to Evaluate the Cardiac and Renal Effects of 7 Day Treatment with Elamipretide in Patients Hospitalized with Severe Congestion due to Heart Failure. No 07/06/ /02/ /11/ /02/ /02/ /03/2017 Y Y Patients screened but no eligible patients identified. Strict patient eligibility criteria. Patients screened but no eligible patients identified. In addition, sponsor delayed confirmation of study open to 24 16/LO/1697 A Phase III randomised, controlled clinical trial of Pembrolizumab with or without platinum-based combination chemotherapy versus chemotherapy in subjects with advanced or metastatic urothelial carcinoma. No 28/07/ /11/ /11/ /11/ /11/2016 Y The British Heart Foundation older patients with non-st Segment elevation myocardial infarction randomized interventional treatment 25 16/NE/ trial 07/03/2017 No 01/09/ /09/ /08/ /10/ /10/ /10/2016 Y delayed confirmation of study open to delayed confirmation of study open to 26 16/NE/0253 Rituximab - a potential cure for the young patient with Graves' disease No 07/04/ /10/ /09/ /02/ /02/ /05/2017 Y A COMbined programme of exercise and dietary ADvice in men with castrate resistant prostate 27 16/NE/ Contracting delays by, further excaberated by incomplete funding information from. cancer - COMRADE trial 15/03/2017 No 31/10/ /12/ /01/ /01/ /01/ /02/2017 Y NHS Provider staff availability NHS Provider 28 16/NI/0145 Diabetic Macular Oedema and Diode Subthreshhold Micropulse Laser (DIAMONDS): A pragmatic, multicentre, allocation concealed, prospective, randomised, noninferiority double masked trial /03/2017 No 23/09/ /12/ /11/ /01/ /02/ /02/2017 Y Y Contracting delays by and Lead Site, with participating sites. delayed confirmation of study open to 29 16/NW/0305 A Phase III, Multicentre, Randomised, Double Blind, Parallel Group, Placebo Controlled Study to Assess the Efficacy and Safety of one or more Intradetrusor Treatments of 600 or 800 Units of DYSPORT for the Treatment of Urinary Incontinence in Subjects with Neurogenic Detrusor Overactivity due to Spinal Cord Injury or Multiple Sclerosis. No 03/05/ /10/ /07/ /10/ /10/ /10/2016 Y NHS Provider staff availability NHS Provider 30 16/NW/0396 Very Low Dose Dexamethasone versus Placebo For the Treatment of Ventilator Dependent Preterm Babies. No 22/09/ /10/ /06/ /02/ /02/ /03/2017 Y Contracting delays by sponsor /NW/0606 A Phase II single-arm, open-label monotherapy clinical trial of Pembrolizumab (MK-3475) in locally advanced/metastatic renal cell carcinoma (mrcc) (KEYNOTE-427) No 03/08/ /11/ /09/ /02/ /02/ /06/2017 Y delay in provision of study documentation. Trust provided budget feedback to sponsor in October 2016 and did not respond to this until January /NW/0620 Phase 3, Randomized, Open-Label, Active-Controlled Study Evaluating The Efficacy And Safety Of Oral Vadadustat For The Correction Of Anemia In Subjects With Non- Dialysis-Dependent Chronic Kidney Disease (NDD-CKD) (PRO2TECT CORRECTION) No 13/10/ /03/ /11/ /03/ /03/ /05/2017 Y delayed confirmation of study open to until after 70 days had passed
4 33 16/SC/0147 Randomised double-blind cross-over study of a DPP4 inhibitor, SGLT2 inhibitor and thiazolidinedione as third line therapy in patients with type 2 diabetes who have suboptimal glycaemic control on dual therapy with metformin and a sulphonylurea. 22/03/2017 No 08/03/ /12/ /07/ /12/ /01/ /02/2017 Y delayed confirmation of study open to 34 16/SC/0382 Effect of the Prevena Incision Management System on Sternal Wound Edge Perfusion in Patients undergoing CABG with Bilateral Mammary Artery grafts 15/02/2017 No 27/06/ /08/ /08/ /08/ /08/ /09/2016 Y Delay in delivery of equipment from supplier /SC/0439 ACL Surgery Necessity in Non Acute Patients Comparison of the clinical and cost effectiveness of two management strategies for nonacute Anterior Ligament (ACL) injury: Rehabilitation versus surgical Reconstruction No 23/02/ /03/ /11/ /03/ /04/ /06/2017 Y Patients screened but no eligible patients identified Acceptability and tolerability of magnetic assisted capsule 36 16/SC/ endoscopy compared to gastroscopy 09/03/2017 No 24/10/ /11/ /01/ /01/ /01/ /03/2017 Y Y Costing delays by. Also, delays with manufacturer in Korea visiting STH to train staff /WM/0197 A multi-center, randomized, doubleblind, placebo controlled, parallel group phase 2a study to assess the efficacy of RO in patients with primary Sjogren s syndrome. No 04/04/ /07/ /07/ /06/ /07/ /08/2016 Y Patients screened but no eligible patients identified 38 16/WM/0359 A Phase 2/3 Multi-center Study to Evaluate the Safety and Efficacy of Blinatumomab in Subjects with Relapsed/Refractory Aggressive B Cell Non Hodgkin Lymphoma. 23/02/2017 No 29/07/ /10/ /10/ /10/ /11/ /11/2016 Y Y delay in provision of equipment (scanner). Patients screened but no eligible patients identified /YH/0186 A phase 4 double-blind, randomized, placebo-controlled, multi-center study to evaluate the efficacy, safety and tolerability of mirabegron in men with overactive bladder (OAB) symptoms while taking the alphablocker tamsulosin hydrochloride for lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH). No 29/04/ /10/ /08/ /10/ /10/ /11/2016 Y 40 16/YH/0212 Unilateral and Bilateral Neurodynamic Sliding Techniques as a Means of Treating Noncompressive Sciatic Leg Pain: A Pilot Study for a Randomised Controlled Trial 05/01/2017 No 27/06/ /10/ /10/ /10/ /10/ /10/2016 Y Treatment of poor-grade Subarachnoid Haemorrhage Trial /YH/ TOPSAT2 No 08/09/ /10/ /07/ /11/ /12/ /12/2016 Y Y Y delayed confirmation to study open to delay as a result of a protocol amendment - change of venue of intervention. The main source of delay was due to rare disease group so not expected to recruit within 70 day benchmark. Other delays included contracting / costing delays with NHS Provider support services. delayed confirmation of study open to 42 16/YH/ : A Phase 2 Open-label Study Investigating the Safety and Efficacy of Blinatumomab After Frontline R-Chemotherapy in Adult Subjects With Newly Diagnosed High-risk Diffuse Large B-Cell Lymphoma (DLBCL) No 25/01/ /01/ /01/ /02/ /02/ /02/2017 Y Patients screened but no eligible patients identified.
5 43 16/YH/0454 A prospective multi-centre observational study to evaluate the capabilities of a portable magnetocardiograph (MCG) device to rule-out acute coronary syndrome (ACS) in patients who present to the emergency department with chest pain symptoms consistent with ACS. 27/02/2017 No 01/02/ /12/ /12/ /01/ /02/ /02/2017 Y 44 17/LO/0046 Evaluation of safety following Immune Tolerance Induction treatment with turoctocog alfa in patients with haemophilia A following inhibitor development in NN trial No 10/02/ /02/ /02/ /04/ /04/ /04/2017 Y Y Contracting delays. delayed returning signed contracts Main source of delay was delay in study set up as site was to be a later additional site. Patients screened but no eligible patients identified /SC/0023 A Phase 2 study to investigate the efficacy, safety, and tolerability of six weeks treatment with V565 in subjects with active Crohn s disease No 11/01/ /04/ /02/ /04/ /04/ /05/2017 Y Y delayed confirmation of study open to Patients screened but no eligible patients identified /EE/0235 A 24-week, double-blind, randomized, parallel-group study evaluating the efficacy and safety of oral nintedanib co-administered with oral sildenafil, compared to treatment with nintedanib alone, in patients with idiopathic pulmonary fibrosis (IPF) and advanced lung function impairment 21/12/2016 Yes 13/06/ /10/ /10/ /10/ /11/ /11/ /EE/0463 COMPLEEMENT-1: An open-label, multicentre, Phase IIIb study to assess the safety and efficacy of ribociclib (LEE011) in combination with letrozole for the treatment of men and postmenopausal women with hormone receptor-positive (HR+) HER2-negative (HER2-) advanced breast cancer (abc) with no prior hormonal therapy for advanced disease 26/04/2017 Yes 19/10/ /02/ /01/ /02/ /02/ /03/ /EM/0279 PIONEER-5: Efficacy and safety of oral semaglutide versus placebo in subjects with Type 2 diabetes and moderate renal impairment. 22/11/2016 Yes 05/08/ /10/ /09/ /10/ /10/ /11/ /EM/0320 A Randomized, Multicentre, Double- Blind, Placebo-Controlled Phase II Study of the Efficacy and Safety of Trastuzumab Emtansine in combination with Atezolizumab or Atezolizumab-Placebo in Patients with HER2-Positive Locally Advanced or Metastatic Breast Cancer who have Received Prior Trastuzumab and Taxane Base Therapy [KATE-2] 13/02/2017 Yes 24/01/ /01/ /11/ /01/ /02/ /02/ /LO/1024 Safety and efficacy of Belimumab After B cell depletion therapy in systemic LUPUS erythematosus [BEAT LUPUS] 02/02/2017 Yes 20/10/ /12/ /09/ /01/ /01/ /01/ /LO/1385 The PodPAD Project - To evaluate the introduction of a public health approach to Peripheral Arterial Disease (PAD) using National Centre for Sport and Exercise Medicine facilities. 04/11/2016 Yes 12/07/ /09/ /09/ /09/ /09/ /09/ /LO/1794 Safety, tolerability, and pharmacokinetics study of single and multiple subcutaneous doses of turoctocog alfa pegol in patients with haemophilia A 20/04/2017 Yes 17/01/ /03/ /12/ /03/ /03/ /03/2017
6 53 16/NE/0418 A phase III multicentre randomised controlled trial to compare the efficacy of Robotically Assisted Radical Cystectomy (RARC) and intracorporeal urinary diversion with Open Radical Cystectomy (ORC) in patients with bladder cancer 06/06/2017 Yes 08/02/ /05/ /01/ /05/ /05/ /05/ /NS/0056 Comparing the usability of an electronic bladder diary and a paper bladder diary: a two-way crossover study 02/11/2016 Yes 07/06/ /08/ /08/ /08/ /08/ /08/ /NW/0242 A Phase 1, randomised, open-label, 2-cohort, cross-over study to evaluate the pharmacokinetics, safety, and tolerability of a native oral testosterone formulation (DITEST) in the fed and fasted state and compared to testosterone undecanoate in adult men with primary or secondary hypogonadism 03/11/2016 Yes 12/02/ /09/ /07/ /10/ /10/ /10/ /SC/0351 A Phase III, open-label, multicenter, three-arm, randomized study to investigate the efficacy and safety of cobimetinib plus atezolizumab and atezolizumab monotherapy vs. regorafenib in patients with previously treated unresectable locally advanced or metastatic colorectal adenocarcinoma. 09/12/2016 Yes 19/07/ /10/ /08/ /11/ /11/ /11/ /SC/0391 A randomized, double-blind, doubledummy, parallel-group study comparing the efficacy and safety of ofatumumab versus teriflunomide in patients with relapsing multiple sclerosis. 12/04/2017 Yes 05/09/ /02/ /10/ /02/ /03/ /03/ /WM/0391 The reality of myoelectric prostheses: Understanding the impact of skill, uncertainties and delays on the control of myoelectric prostheses 07/06/2017 Yes 24/01/ /03/ /12/ /04/ /04/ /04/2017 Occupational Therapy Environmental 59 16/WS/ assessment and falls [OTIS] 02/02/2017 Yes 15/09/ /01/ /09/ /01/ /01/ /01/ /YH/0169 Multi-channel Stimulation for Post Stroke Spasticity (MUSTS): A randomised controlled cross over feasibility trial. 11/11/2016 Yes 01/04/ /09/ /05/ /09/ /09/ /09/ /YH/0268 BRAVO study 2: High grade Bladder cancer: A randomised controlled trial of Radical Cystectomy against intravesical immunotherapy A feasibility study 27/10/2016 Yes 08/06/ /09/ /08/ /09/ /09/ /10/ /YH/0284 Feasibility Study to Investigate the Potential Reduction of Bone Mineral Density Loss in Spinal Cord Injured Patients Using Ekso Therapy 08/12/2016 Yes 26/06/ /10/ /10/ /10/ /10/ /10/ /YH/0304 Is it feasible to conduct a randomised controlled trial of pre-transplant exercise (prehabilitation) for multiple myeloma patients awaiting autologous stem cell transplantation? 14/10/2016 Yes 05/07/ /09/ /09/ /09/ /09/ /09/ /YH/0315 Elastography in the diagnosis of chronic pancreatitis. 07/12/2016 Yes 22/07/ /10/ /11/ /11/ /11/ /11/ /YH/0377 The use of a virtual reality headset for the treatment of visual vertigo symptoms. 25/01/2017 Yes 12/08/ /11/ /11/ /01/ /01/ /01/2017
7 66 16/YH/0393 The Effect of Selenium Supplementation on Musculoskeletal Health in Older Women Doubleblind, randomised, placebocontrolled trial /01/2017 Yes 24/08/ /12/ /12/ /01/ /01/ /01/ /YH/0506 A Randomized, Double-Blind, Parallel Group, Multi-Centre Study to Assess the Efficacy and Safety of PT009 compared to PT005 on COPD Exacerbations over a 52- Week Treatment Period in Subjects With Moderate to Very Severe COPD 22/05/2017 Yes 10/01/ /03/ /02/ /04/ /04/ /05/2017 Double blind randomised controlled trial of Remote Ischemic 68 17/NS/ preconditioning in Multiple sclerosis 02/05/2017 Yes 28/12/ /03/ /03/ /04/ /04/ /04/ /YH/0103 Prolonged ENoxapariN in primary Percutaneous Coronary Intervention; a Pilot Pharmacodynamic study (PENNY PCI) 14/06/2017 Yes 09/03/ /05/ /05/ /06/ /06/ /06/2017
A - Nonconfirmation Suspended by Sponsor B - D - Date site. C - Closed ready to start. Permissions. Sponsor delays ied. delayed/den.
 No REC Reference Number IRAS No Title Submission Type Valid Research Application Date of First Patient Recruitment Benchmark Met invited selected Approval date confirmed by sponsor confirmed A - Nonconfirmation
No REC Reference Number IRAS No Title Submission Type Valid Research Application Date of First Patient Recruitment Benchmark Met invited selected Approval date confirmed by sponsor confirmed A - Nonconfirmation
Perfomance in Delivering (Commercial Trials)
 Perfomance in Delivering (Commercial Trials) REC Ref IRAS ID Name of Trial Target Of? Minimum Of Maximum Of Target Date To Recruit? Total Date Of to recruit target number Recruited At of patients The Target
Perfomance in Delivering (Commercial Trials) REC Ref IRAS ID Name of Trial Target Of? Minimum Of Maximum Of Target Date To Recruit? Total Date Of to recruit target number Recruited At of patients The Target
Performance in Initiating Clinical Research
 Performance in Initiating Clinical Research January 2016 December 2016 NHS Permissions Site approval Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial
Performance in Initiating Clinical Research January 2016 December 2016 NHS Permissions Site approval Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial
The Pennine Acute Hospitals NHS Trust Performance in initiating clinical research Reporting period 1 October 2017 to 30 September 2018
 The Pennine Acute Hospitals NHS Trust Performance in initiating clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial First patient recruited?
The Pennine Acute Hospitals NHS Trust Performance in initiating clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial First patient recruited?
Target date to recruit patients agreed? Target range minimum. Target range maximum. Agreed 1 1 Agreed 01/01/ /01/2016 Recruitment Finished
 No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date No of patients recruited
No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date No of patients recruited
Studies proceeding under pre HRA-Approval system (NHS Permission)
 15/NW/0004 162679 Studies proceeding under pre HRA-roval system (NHS Permission) A MULTI-CENTRE RANDOMISED CLINICAL TRIAL OF BIOMARKER-DRIVEN MAINTENANCE TREATMENT FOR FIRST- LINE METASTATIC COLORECTAL
15/NW/0004 162679 Studies proceeding under pre HRA-roval system (NHS Permission) A MULTI-CENTRE RANDOMISED CLINICAL TRIAL OF BIOMARKER-DRIVEN MAINTENANCE TREATMENT FOR FIRST- LINE METASTATIC COLORECTAL
Table 1 Salford Royal NHS Foundation Trust: Performance in initiating clinical research (HRA Approval) Reporting period 1 April March 2017
 Table 1 Salford Royal NHS Foundation Trust: Performance in initiating clinical research (HRA Approval) Reporting period 1 April 2016 31 March 2017 REC reference IRAS number Study title 16/NW/0428 207062
Table 1 Salford Royal NHS Foundation Trust: Performance in initiating clinical research (HRA Approval) Reporting period 1 April 2016 31 March 2017 REC reference IRAS number Study title 16/NW/0428 207062
Target date to recruit patients agreed? Target range minimum. Target range maximum
 No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date Total no of patients recruited
No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date Total no of patients recruited
The Pennine Acute Hospitals NHS Trust Performance in delivering clinical research Reporting period 1 October 2017 to 30 September 2018
 The Pennine Acute Hospitals NHS Trust Performance in delivering clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial Target number of? Minimum
The Pennine Acute Hospitals NHS Trust Performance in delivering clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial Target number of? Minimum
Performance in Initiating Clinical Research Q2 2016/17
 Performance in Initiating Clinical Research Q2 2016/17 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Date of Receipt of Valid Research Application
Performance in Initiating Clinical Research Q2 2016/17 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Date of Receipt of Valid Research Application
Clinical Research Institute HUCH Sponsored, fully signed Clinical Trial Agreements (except grants)
 , fully signed Clinical Trial Agreements (except grants) Sponsor Full name of the Hospital clinic where the is details of the January x x January x NightstaRx Ltd A Randomised, Open Label, Outcomes-Assessor
, fully signed Clinical Trial Agreements (except grants) Sponsor Full name of the Hospital clinic where the is details of the January x x January x NightstaRx Ltd A Randomised, Open Label, Outcomes-Assessor
Target date to recruit patients agreed? Target range. maximum. minimum. Date. 5 Agreed 26/01/ /11/2015 Recruitment Finished.
 No REC Reference IRAS No Title Target number of agreed? Target range minimum Target range maximum Target date to recruit agreed? Planned Recruitment end date Total no of recruited at the agreed target
No REC Reference IRAS No Title Target number of agreed? Target range minimum Target range maximum Target date to recruit agreed? Planned Recruitment end date Total no of recruited at the agreed target
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT
 NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including accrual, and
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including accrual, and
Performance in Delivering Q4. Target number of patients
 Performance in Delivering Q4 Research Ethics Committee Reference Number Name of Trial Target number of patients Date Agreed to recruit target number of patients Trial Status Target met within the agreed
Performance in Delivering Q4 Research Ethics Committee Reference Number Name of Trial Target number of patients Date Agreed to recruit target number of patients Trial Status Target met within the agreed
Min number of patients agreed to be recruited. Max number of patients agreed to be recruited
 be 12/SC/0139 98125 A PHASE III PROSPECTIVE, TWO-COHORT NON-RANDOMIZED, MULTI- CENTRE, MULTINATIONAL, OPEN LABEL STUDY TO ASSESS THE SAFETY OF ASSISTED- AND SELF-ADMINISTERED SUBCUTANEOUS TRASTUZUMAB AS
be 12/SC/0139 98125 A PHASE III PROSPECTIVE, TWO-COHORT NON-RANDOMIZED, MULTI- CENTRE, MULTINATIONAL, OPEN LABEL STUDY TO ASSESS THE SAFETY OF ASSISTED- AND SELF-ADMINISTERED SUBCUTANEOUS TRASTUZUMAB AS
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT
 NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 April March 2014
 Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 April 2013-31 March 2014 Research Ethics Committee Name of Trial reference number 1 08/H0906/140
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 April 2013-31 March 2014 Research Ethics Committee Name of Trial reference number 1 08/H0906/140
Performance in Initiating and Delivering Clinical Research Why are we doing this?
 Performance in Initiating and Delivering Clinical Research Why are we doing this? Through the NIHR (National Institute for Health Research) the Government wishes to see a dramatic and sustained improvement
Performance in Initiating and Delivering Clinical Research Why are we doing this? Through the NIHR (National Institute for Health Research) the Government wishes to see a dramatic and sustained improvement
University Hospitals of Leicester NHS Trust Research & Innovation Directorate Performance in Delivering Research 01/04/2015 to 31/03/2016
 University Hospitals of Leicester NHS Trust Research & Innovation Directorate Performance in Delivering Research 01/04/2015 to 31/03/2016 REC Date 10/H0808/109 58307 EXCEL Clinical Trial 1.0 8th June 2010
University Hospitals of Leicester NHS Trust Research & Innovation Directorate Performance in Delivering Research 01/04/2015 to 31/03/2016 REC Date 10/H0808/109 58307 EXCEL Clinical Trial 1.0 8th June 2010
Performance in delivering Clinical Research at PHT Q3 14/15 study met the 70 day target study within 70 day target study not met 70 day target
 Performance in delivering Clinical Research at PHT Q3 14/15 study met the 70 day target study within 70 day target study not met 70 day target Research Ethics Committee 14/ES/0004 ALA-BCC-CT008 - A randomized,
Performance in delivering Clinical Research at PHT Q3 14/15 study met the 70 day target study within 70 day target study not met 70 day target Research Ethics Committee 14/ES/0004 ALA-BCC-CT008 - A randomized,
Performance in Initiating Clinical Research Q4 2015/16
 Performance in Initiating Clinical Research Q4 2015/16 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Date of Receipt of Valid Research Application
Performance in Initiating Clinical Research Q4 2015/16 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Date of Receipt of Valid Research Application
Date Site Confirmed By Sponsor. Non- Confirmation Status. HRA Approval Date. Date Site Confirmed
 Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial First Patient Recruited? Date of First Patient Recruited Invited Selected Approval
Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial First Patient Recruited? Date of First Patient Recruited Invited Selected Approval
Date of HRA. Date Site Invited. Date Site Selected. Approval. Recruited. Date
 14/LO/0259 134883 RE-AKT: A randomised Phase II study of enzalutamide (MDV3100) in combination with AZD5363 in s with Metastatic Castration - Resistant Prostate Cancer Yes 08/01/2018 01/07/2016 01/07/2017
14/LO/0259 134883 RE-AKT: A randomised Phase II study of enzalutamide (MDV3100) in combination with AZD5363 in s with Metastatic Castration - Resistant Prostate Cancer Yes 08/01/2018 01/07/2016 01/07/2017
CLINICAL TRIALS ACC. Jul 2016
 CLINICAL TRIALS ACC Jul 2016 Glioblastoma BRAIN A071102 A Phase II/III Randomized Trial of Veliparib or Placebo in Combination With Temozolomide in Newly Diagnosed Glioblastoma With MGMT Promoter Hypermethylation
CLINICAL TRIALS ACC Jul 2016 Glioblastoma BRAIN A071102 A Phase II/III Randomized Trial of Veliparib or Placebo in Combination With Temozolomide in Newly Diagnosed Glioblastoma With MGMT Promoter Hypermethylation
HRA Approval Date. confirmed by sponsor 07/04/ /04/ /04/ /04/ /04/ /04/ /05/2016
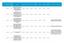 Reference Reason for delay in recruiting first 1 15/NE/0144 179243 AN OPEN-LABEL EXTENSION AND SAFETY MONITORING STUDY OF PATIENTS WITH MODERATELY TO SEVERELY ACTIVE CROHN S DISEASE PREVIOUSLY ENROLLED
Reference Reason for delay in recruiting first 1 15/NE/0144 179243 AN OPEN-LABEL EXTENSION AND SAFETY MONITORING STUDY OF PATIENTS WITH MODERATELY TO SEVERELY ACTIVE CROHN S DISEASE PREVIOUSLY ENROLLED
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 October September 2013
 Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 October 2012-30 September 2013 Research Ethics Committee reference number Name of Trial 1 12/NW/0321
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 October 2012-30 September 2013 Research Ethics Committee reference number Name of Trial 1 12/NW/0321
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 July June 2013
 Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 July 2012-30 June 2013 Name of trial Note, some study titles are shortened 1 Long-Term Study
Table 1: Salford Royal NHS Foundation Trust: Performance in initiating clinical research: Reporting period 1 July 2012-30 June 2013 Name of trial Note, some study titles are shortened 1 Long-Term Study
Performance in Initiating and Delivering Clinical Research
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
David Henry Jablonski, M.D North Mills Avenue Orlando, FL 32803
 David Henry Jablonski, M.D. 1812 North Mills Avenue Orlando, FL 32803 EDUCATION: University of Florida College of Medicine, Gainesville, Florida Division of Urology Residency Training Program July 1994-June
David Henry Jablonski, M.D. 1812 North Mills Avenue Orlando, FL 32803 EDUCATION: University of Florida College of Medicine, Gainesville, Florida Division of Urology Residency Training Program July 1994-June
Date of NHS Permission. Date of First Patient Recruited. Date Site Invited
 Reference of Receipt of of NHS of First 14/WA/1075 150715 NHS HeadPoST Version 2.1 23/11/2015 05/01/2016 11/01/2016 N/A 12/YH/0452 106985 NHS INCA 10/12/2015 15/01/2016 17/03/2016 N/A Excess Treatment
Reference of Receipt of of NHS of First 14/WA/1075 150715 NHS HeadPoST Version 2.1 23/11/2015 05/01/2016 11/01/2016 N/A 12/YH/0452 106985 NHS INCA 10/12/2015 15/01/2016 17/03/2016 N/A Excess Treatment
Clinical Policy: Nivolumab (Opdivo) Reference Number: CP.PHAR.121 Effective Date: Last Review Date: Line of Business: Medicaid
 Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07.15 Last Review Date: 01.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07.15 Last Review Date: 01.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
HRA Approval Date Site Date Site Sponsor. Non- Confirmation Status. Date Site Ready To Start
 Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial Date of Receipt of Date of Valid Research Application First Patient Recruited? Date
Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial Date of Receipt of Date of Valid Research Application First Patient Recruited? Date
Performance of Initiating Q South London and Maudsley NHS Foundation Trust:
 Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial Date of Receipt of Valid Research Application Date of NHS First Patient Recruited?
Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial Date of Receipt of Valid Research Application Date of NHS First Patient Recruited?
Jeffrey Richard Thill, M.D North Mills Avenue Orlando, FL 32803
 Jeffrey Richard Thill, M.D. 1812 North Mills Avenue Orlando, FL 32803 CURRENT POSITION AFFILIATIONS BIRTHPLACE BOARD CERTIFIED UROLOGIST WINTER PARK UROLOGY ASSOCIATES, P.A. 1812 NORTH MILLS AVENUE, ORLANDO,
Jeffrey Richard Thill, M.D. 1812 North Mills Avenue Orlando, FL 32803 CURRENT POSITION AFFILIATIONS BIRTHPLACE BOARD CERTIFIED UROLOGIST WINTER PARK UROLOGY ASSOCIATES, P.A. 1812 NORTH MILLS AVENUE, ORLANDO,
Open and Pending Trials Listing For Arizona Oncology Associates P.C. HOPE Division/ Tucson, Arizona
 Open and Pending Trials Listing For Arizona Oncology Associates P.C. HOPE Division/ Tucson, Arizona Solid Tumor: USON 15180 / A Multicenter, Open-Label Phase 1b/2 Trial of Lenvatinib (E7080) Plus Pembrolizumab
Open and Pending Trials Listing For Arizona Oncology Associates P.C. HOPE Division/ Tucson, Arizona Solid Tumor: USON 15180 / A Multicenter, Open-Label Phase 1b/2 Trial of Lenvatinib (E7080) Plus Pembrolizumab
Performance in Initiating and Delivering Clinical Research
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Gary Scott Friedlander, M.D. Urological Consultants, P.A. A Division of Chesapeake Urology Associates, LLC
 Gary Scott Friedlander, M.D. Urological Consultants, P.A. A Division of Chesapeake Urology Associates, LLC 9420 Key West Ave, Suite 420, Rockville, MD 20850 2730 University Blvd, West, Suite 516, Wheaton,
Gary Scott Friedlander, M.D. Urological Consultants, P.A. A Division of Chesapeake Urology Associates, LLC 9420 Key West Ave, Suite 420, Rockville, MD 20850 2730 University Blvd, West, Suite 516, Wheaton,
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December
 Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
National Cancer Drugs Fund List - Approved
 National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
Hu J, Gonsahn MD, Nerenz DR. Socioeconomic status and readmissions: evidence from an urban teaching hospital. Health Aff (Millwood). 2014;33(5).
 Appendix Definitions of Index Admission and Readmission Definitions of index admission and readmission follow CMS hospital-wide all-cause unplanned readmission (HWR) measure as far as data are available.
Appendix Definitions of Index Admission and Readmission Definitions of index admission and readmission follow CMS hospital-wide all-cause unplanned readmission (HWR) measure as far as data are available.
Policy. Medical Policy Manual Approved Revised: Do Not Implement until 6/30/2019. Nivolumab
 Medical Manual Approved Revised: Do Not Implement until 6/30/2019 Nivolumab NDC CODE(S) 00003-3772-XX Opdivo 40 MG/4ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3774-XX Opdivo 100 MG/10ML SOLN (B-M SQUIBB
Medical Manual Approved Revised: Do Not Implement until 6/30/2019 Nivolumab NDC CODE(S) 00003-3772-XX Opdivo 40 MG/4ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3774-XX Opdivo 100 MG/10ML SOLN (B-M SQUIBB
Policy. Medical Policy Manual Approved Revised: Do Not Implement Until 3/2/19. Nivolumab (Intravenous)
 Nivolumab (Intravenous) NDC CODE(S) 00003-3772-XX Opdivo 40 MG/4ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3774-XX Opdivo 100 MG/10ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3734-XX Opdivo 240
Nivolumab (Intravenous) NDC CODE(S) 00003-3772-XX Opdivo 40 MG/4ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3774-XX Opdivo 100 MG/10ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3734-XX Opdivo 240
Horizon Scanning Technology Briefing. Sutent (Sunitinib) for first-line and adjuvant treatment of renal cell carcinoma
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Sutent (Sunitinib) for first-line and adjuvant treatment of renal cell carcinoma August 2006: Updated October 2006 This technology
Horizon Scanning Technology Briefing National Horizon Scanning Centre Sutent (Sunitinib) for first-line and adjuvant treatment of renal cell carcinoma August 2006: Updated October 2006 This technology
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN BY PRODUCT
 PAGE 175 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN BY PRODUCT Summary of risk management plan for pembrolizumab This is a summary of the risk management plan (RMP) for pembrolizumab. The RMP details
PAGE 175 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN BY PRODUCT Summary of risk management plan for pembrolizumab This is a summary of the risk management plan (RMP) for pembrolizumab. The RMP details
Clinical Policy: Nivolumab (Opdivo) Reference Number: CP.PHAR.121
 Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07/15 Last Review Date: 04/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07/15 Last Review Date: 04/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Summary of Strategic Competitive Analysis and Publication Planning
 Summary of Strategic Competitive Analysis and Publication Planning Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Briefing
Summary of Strategic Competitive Analysis and Publication Planning Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Briefing
Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2
 Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2 1 The Male Health Centre, Oakville, Ontario, Canada 2 Humber River Regional Hospital, University of Toronto, Toronto,
Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2 1 The Male Health Centre, Oakville, Ontario, Canada 2 Humber River Regional Hospital, University of Toronto, Toronto,
Non-QPP Measures 3 AQUA12. 6 AQUA15 Stones: Urinalysis documented 30 days before
 Non-QPP Measures 1 Measure ID Measure Title Definition Type Domain AQUA3 (inverse) Cryptorchidism: Inappropriate use of scrotal/groin ultrasound on boys Percentage of patients (boys) =< 18 years of age
Non-QPP Measures 1 Measure ID Measure Title Definition Type Domain AQUA3 (inverse) Cryptorchidism: Inappropriate use of scrotal/groin ultrasound on boys Percentage of patients (boys) =< 18 years of age
Annual Flash Report (unaudited) Fiscal Year ended March 31, 2017
 Annual Flash Report (unaudited) Fiscal Year ended March 31, 2017 Supplemental nation Status of Development Pipeline as of May 8, 2017. Main Status of Development Pipelines (Oncology) 1. Development Status
Annual Flash Report (unaudited) Fiscal Year ended March 31, 2017 Supplemental nation Status of Development Pipeline as of May 8, 2017. Main Status of Development Pipelines (Oncology) 1. Development Status
Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies. Eric H. Rubin, MD Merck Research Laboratories
 Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies Eric H. Rubin, MD Merck Research Laboratories Outline Pembrolizumab P001 study - example of multiple expansion
Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies Eric H. Rubin, MD Merck Research Laboratories Outline Pembrolizumab P001 study - example of multiple expansion
Performance in Initiating and Delivering Clinical Research
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
All Studies by Indication
 Therapeutic Area Protocol Number Drug/Device Phase Description Advanced malignancies 337 20101132 AMG 337 I " A Phase 1, First-in-Human Study Evaluating the Safety, Tolerability, and Pharmacokinetics of
Therapeutic Area Protocol Number Drug/Device Phase Description Advanced malignancies 337 20101132 AMG 337 I " A Phase 1, First-in-Human Study Evaluating the Safety, Tolerability, and Pharmacokinetics of
Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD
 Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD Hanahan and Weinberg, 2000 Acquired Capabilities of Cancer Clinical Trials When should I consider a clinical trial? How do I find the right
Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD Hanahan and Weinberg, 2000 Acquired Capabilities of Cancer Clinical Trials When should I consider a clinical trial? How do I find the right
6/22/2017 TARGETING THE TARGETS IN 2017 TARGETING THE TARGETS IN 2017
 TARGETING THE TARGETS IN 2017 Primary Care Focus Symposium July 1, 2017 Grace Wang MD I do not have any relevant financial relationships to disclose at this time TARGETING THE TARGETS IN 2017 What are
TARGETING THE TARGETS IN 2017 Primary Care Focus Symposium July 1, 2017 Grace Wang MD I do not have any relevant financial relationships to disclose at this time TARGETING THE TARGETS IN 2017 What are
Developmental Therapeutics for HCC, Colorectal Cancer, and Pancreatic Cancer. Manish Sharma, MD Developmental Therapeutics Symposium April 20, 2018
 Developmental Therapeutics for HCC, Colorectal Cancer, and Pancreatic Cancer Manish Sharma, MD Developmental Therapeutics Symposium April 20, 2018 Disclosure Information 23 rd Annual Developmental Therapeutics
Developmental Therapeutics for HCC, Colorectal Cancer, and Pancreatic Cancer Manish Sharma, MD Developmental Therapeutics Symposium April 20, 2018 Disclosure Information 23 rd Annual Developmental Therapeutics
Highlights from AACR 2015: The Emerging Potential of Immunotherapeutic Approaches in Non-Small Cell Lung Cancer
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Histology independent indications in Oncology
 CHMP Oncology Working Party Workshop Histology independent indications in Oncology What have we learnt from the anti PD1- PDL1 story? J Camarero (CHMP alternate ES, OncWP) Disclaimers the views presented
CHMP Oncology Working Party Workshop Histology independent indications in Oncology What have we learnt from the anti PD1- PDL1 story? J Camarero (CHMP alternate ES, OncWP) Disclaimers the views presented
Table 2: Salford Royal NHS Foundation Trust: Performance in delivering clinical research: Reporting period 1 January December 2014
 Table 2: Salford Royal HS Foundation Trust: Performance in delivering clinical research: Reporting period 1 January 2014 1 December 2014 Research Ethics Committee reference number 1 12/W/0321 2 11/LO/1986
Table 2: Salford Royal HS Foundation Trust: Performance in delivering clinical research: Reporting period 1 January 2014 1 December 2014 Research Ethics Committee reference number 1 12/W/0321 2 11/LO/1986
Clinical indications for positron emission tomography
 Clinical indications for positron emission tomography Oncology applications Brain and spinal cord Parotid Suspected tumour recurrence when anatomical imaging is difficult or equivocal and management will
Clinical indications for positron emission tomography Oncology applications Brain and spinal cord Parotid Suspected tumour recurrence when anatomical imaging is difficult or equivocal and management will
Supplementary Online Content
 Supplementary Online Content Dharmarajan K, Wang Y, Lin Z, et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. doi:10.1001/jama.2017.8444 etable
Supplementary Online Content Dharmarajan K, Wang Y, Lin Z, et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. doi:10.1001/jama.2017.8444 etable
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
2016 Updates in Oncology & Malignant Hematology Brendan Curley, DO
 2016 Updates in Oncology & Malignant Hematology Brendan Curley, DO Disclosures I received final support from ASCO Conquer Cancer foundation in two Merit Awards and one travel award I am on the speakers
2016 Updates in Oncology & Malignant Hematology Brendan Curley, DO Disclosures I received final support from ASCO Conquer Cancer foundation in two Merit Awards and one travel award I am on the speakers
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 10/11/2013. ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 10/11/2013 ClinicalTrials.gov ID: NCT00168454 Study Identification Unique Protocol ID: 191622-077 Brief Title: A
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 10/11/2013 ClinicalTrials.gov ID: NCT00168454 Study Identification Unique Protocol ID: 191622-077 Brief Title: A
OPEN TRIALS Accruals counted until 30-April Current Accrual
 Disease group Breast OPEN TRIALS Trial Title Accrual Target IBCSG 48-14 POSITIVE IBCSG 50-14 OLYMPIA IBCSG 52-15 PALLAS SAKK 21/12 A study evaluating the pregnancy outcomes and safety of interrupting endocrine
Disease group Breast OPEN TRIALS Trial Title Accrual Target IBCSG 48-14 POSITIVE IBCSG 50-14 OLYMPIA IBCSG 52-15 PALLAS SAKK 21/12 A study evaluating the pregnancy outcomes and safety of interrupting endocrine
S2 File. Clinical Classifications Software (CCS). The CCS is a
 S2 File. Clinical Classifications Software (CCS). The CCS is a diagnosis categorization scheme based on the ICD-9-CM that aggregates all diagnosis codes into 262 mutually exclusive, clinically homogeneous
S2 File. Clinical Classifications Software (CCS). The CCS is a diagnosis categorization scheme based on the ICD-9-CM that aggregates all diagnosis codes into 262 mutually exclusive, clinically homogeneous
Summary of risk management plan for OPDIVO (nivolumab)
 Summary of risk management plan for OPDIVO (nivolumab) This is a summary of the risk management plan (RMP) for OPDIVO. The RMP details important risks of OPDIVO, how these risks can be minimized, and how
Summary of risk management plan for OPDIVO (nivolumab) This is a summary of the risk management plan (RMP) for OPDIVO. The RMP details important risks of OPDIVO, how these risks can be minimized, and how
Summary of risk management plan for OPDIVO (nivolumab)
 Summary of risk management plan for OPDIVO (nivolumab) This is a summary of the risk management plan (RMP) for OPDIVO. The RMP details important risks of OPDIVO, how these risks can be minimized, and how
Summary of risk management plan for OPDIVO (nivolumab) This is a summary of the risk management plan (RMP) for OPDIVO. The RMP details important risks of OPDIVO, how these risks can be minimized, and how
How to carry out health technology appraisals and guidance. Learning from the Scottish experience Richard Clark, Principal Pharmaceutical
 The Managed Introduction of New Medicines How to carry out health technology appraisals and guidance. Learning from the Scottish experience Richard Clark, Principal Pharmaceutical Analyst July 10 th 2009,
The Managed Introduction of New Medicines How to carry out health technology appraisals and guidance. Learning from the Scottish experience Richard Clark, Principal Pharmaceutical Analyst July 10 th 2009,
Summary of Research and Writing Activities in Oncology
 Summary of Research and Writing Activities in Oncology Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts, Posters,
Summary of Research and Writing Activities in Oncology Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts, Posters,
PRESCRIPTION PAD. The Newsletter of the Cumbria Area Prescribing Committee. February 2012 No. 18. Click here to find more. News from the MHRA
 PRESCRIPTION PAD The Newsletter of the Cumbria Area Prescribing Committee February 2012 No. 18 Click here to find more Clinical policy and Formulary news Lothian changes Targeted Medication Use Reviews
PRESCRIPTION PAD The Newsletter of the Cumbria Area Prescribing Committee February 2012 No. 18 Click here to find more Clinical policy and Formulary news Lothian changes Targeted Medication Use Reviews
London Cancer New Drugs Group. February London Cancer New Drugs Group (LCNDG) Work Plan for the London Cancer Drugs Fund list.
 February 2013 London Cancer New s Group (LCNDG) Work Plan for the London Cancer s Fund London Cancer s Fund List This Cancer s Fund (CDF) list of medicines and s is in two parts. 1. The standard list of
February 2013 London Cancer New s Group (LCNDG) Work Plan for the London Cancer s Fund London Cancer s Fund List This Cancer s Fund (CDF) list of medicines and s is in two parts. 1. The standard list of
Metastatic NSCLC: Expanding Role of Immunotherapy. Evan W. Alley, MD, PhD Abramson Cancer Center at Penn Presbyterian
 Metastatic NSCLC: Expanding Role of Immunotherapy Evan W. Alley, MD, PhD Abramson Cancer Center at Penn Presbyterian Disclosures: No relevant disclosures Please note that some of the studies reported in
Metastatic NSCLC: Expanding Role of Immunotherapy Evan W. Alley, MD, PhD Abramson Cancer Center at Penn Presbyterian Disclosures: No relevant disclosures Please note that some of the studies reported in
ENROLLMENT : Line of Business Summary
 ENROLLMENT : Line of Business Summary Date Range : JAN 2017 through DEC 2017 COMPREHENSIVE MAJOR MEDICAL Print Date : 1/19/2018 9:43:49AM Page 1 of 1 Month Year Single 2 Person : Emp/Spouse 2 Person :
ENROLLMENT : Line of Business Summary Date Range : JAN 2017 through DEC 2017 COMPREHENSIVE MAJOR MEDICAL Print Date : 1/19/2018 9:43:49AM Page 1 of 1 Month Year Single 2 Person : Emp/Spouse 2 Person :
South London and Maudsley NHS Foundation Trust: FY1819 Q2 Performance in Initiating
 of First 17/YH/0195 228739 17/YH/0196 228435 17/LO/0987 215947 17/LO/1314 224111 17/WM/0441 224759 17/LO/1867 229005 A Randomized, Double-Blind, Placebo Controlled, Two-Period Cross-Over, Proof of Activity
of First 17/YH/0195 228739 17/YH/0196 228435 17/LO/0987 215947 17/LO/1314 224111 17/WM/0441 224759 17/LO/1867 229005 A Randomized, Double-Blind, Placebo Controlled, Two-Period Cross-Over, Proof of Activity
Development status of OPDIVO (nivolumab) 1
 November 2, 2018 Development status of OPDIVO (nivolumab) 1 Target disease JAPAN US/EU KR/TW Melanoma (1 st ) Approved Approved Approved Melanoma (Adjuvant Therapy) Approved Approved(EU) Non-small cell
November 2, 2018 Development status of OPDIVO (nivolumab) 1 Target disease JAPAN US/EU KR/TW Melanoma (1 st ) Approved Approved Approved Melanoma (Adjuvant Therapy) Approved Approved(EU) Non-small cell
Current Clinical Trials for Stroke Survivors in NJ and Philadelphia Areas
 Current Clinical Trials for Survivors in NJ and Philadelphia Areas For more information go to https://clinicaltrials.gov/ and search for the title in search box Condition / Disease 1. Spatial Neglect and
Current Clinical Trials for Survivors in NJ and Philadelphia Areas For more information go to https://clinicaltrials.gov/ and search for the title in search box Condition / Disease 1. Spatial Neglect and
Keytruda (pembrolizumab)
 Keytruda (pembrolizumab) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 07/24/2017TBD03/01/2018 POLICY A. INDICATIONS The
Keytruda (pembrolizumab) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 07/24/2017TBD03/01/2018 POLICY A. INDICATIONS The
MEDICAL PRIOR AUTHORIZATION
 MEDICAL PRIOR AUTHORIZATION TAXOTERE (docetaxel) DOCEFREZ(docetaxel) docetaxel (generic) POLICY I. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
MEDICAL PRIOR AUTHORIZATION TAXOTERE (docetaxel) DOCEFREZ(docetaxel) docetaxel (generic) POLICY I. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
CENTER FOR CLINICAL TRIALS St. Luke s Medical Center. Ongoing Clinical Trials CANCER INSTITUTE (36)
 CENTER FOR CLINICAL TRIALS St. Luke s Medical Center Ongoing Clinical Trials CANCER INSTITUTE (36) BLOOD TITLE : A Randomized, Two By Two Arm, Multicenter, Open-Label Phase III Study Of BMS-354825 Administered
CENTER FOR CLINICAL TRIALS St. Luke s Medical Center Ongoing Clinical Trials CANCER INSTITUTE (36) BLOOD TITLE : A Randomized, Two By Two Arm, Multicenter, Open-Label Phase III Study Of BMS-354825 Administered
(generic name: ipilimumab) Injection 50 mg ( Yervoy ), a human anti-human CTLA-4 monoclonal. August 21, 2018
 August 21, 2018 Opdivo Approved for Supplemental Applications for Expanded Indications of Malignant Pleural Mesothelioma and Adjuvant Treatment of Melanoma, Change in Dosage and Administration (D&A) of
August 21, 2018 Opdivo Approved for Supplemental Applications for Expanded Indications of Malignant Pleural Mesothelioma and Adjuvant Treatment of Melanoma, Change in Dosage and Administration (D&A) of
National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) Trial design:
 Open clinical uro-oncology trials in Canada Eric Winquist, MD, Mary J. Mackenzie, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A PHASE III STUDY OF IRESSA
Open clinical uro-oncology trials in Canada Eric Winquist, MD, Mary J. Mackenzie, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A PHASE III STUDY OF IRESSA
Clinical Policy: Nivolumab (Opdivo) Reference Number: CP.HNMC.27 Effective Date: Last Review Date: Line of Business: Medicaid - HNMC
 Clinical Policy: (Opdivo) Reference Number: CP.HNMC.27 Effective Date: 07.01.17 Last Review Date: 02.18 Line of Business: Medicaid - HNMC Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Opdivo) Reference Number: CP.HNMC.27 Effective Date: 07.01.17 Last Review Date: 02.18 Line of Business: Medicaid - HNMC Revision Log See Important Reminder at the end of this policy for
Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer
 Find Studies About Studies Submit Studies Resources About Site Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer The safety and scientific validity of this study is the responsibility
Find Studies About Studies Submit Studies Resources About Site Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer The safety and scientific validity of this study is the responsibility
Nintedanib in Oncology Backgrounder
 For media outside the US, UK and Canada only Nintedanib in Oncology Backgrounder 1. What is nintedanib? 2. How does nintedanib work? 3. Data overview 4. Additional clinical data 5. Nintedanib approval
For media outside the US, UK and Canada only Nintedanib in Oncology Backgrounder 1. What is nintedanib? 2. How does nintedanib work? 3. Data overview 4. Additional clinical data 5. Nintedanib approval
Supplementary Online Content
 Supplementary Online Content Chacón MR, Enrico DH, Burton J, Waisberg FD, Videla VM. Incidence of placebo adverse events in randomized clinical trials of targeted and immunotherapy cancer drugs in the
Supplementary Online Content Chacón MR, Enrico DH, Burton J, Waisberg FD, Videla VM. Incidence of placebo adverse events in randomized clinical trials of targeted and immunotherapy cancer drugs in the
Opdivo. Opdivo (nivolumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.53 Subsection: Antineoplastic Agents Original Policy Date: January 16, 2015 Subject: Opdivo Page:
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.53 Subsection: Antineoplastic Agents Original Policy Date: January 16, 2015 Subject: Opdivo Page:
Development Pipeline Progress Status. February 1, 2019
 Development Pipeline Progress Status February 1, 2019 /9 Development status of OPDIVO (nivolumab) (1) Target disease JAPAN US/EU KR/TW Melanoma (1 st ) Approved Approved Approved Melanoma (Adjuvant Therapy)
Development Pipeline Progress Status February 1, 2019 /9 Development status of OPDIVO (nivolumab) (1) Target disease JAPAN US/EU KR/TW Melanoma (1 st ) Approved Approved Approved Melanoma (Adjuvant Therapy)
LIST OF ADVANCED TREATMENTS COVERABLE UNDER MEDICAL BENEFITS. Bladder cancer. Classical Hodgkin lymphoma. Head and neck cancer
 LIST OF ADVANCED TREATMENTS COVERABLE UNDER MEDICAL BENEFITS ADVANCE TREATMENT COVERABLE CONDITIONS 1 Cryoablation Primary therapy alternative to surgery for individuals with localized disease or as a
LIST OF ADVANCED TREATMENTS COVERABLE UNDER MEDICAL BENEFITS ADVANCE TREATMENT COVERABLE CONDITIONS 1 Cryoablation Primary therapy alternative to surgery for individuals with localized disease or as a
FOR IMMEDIATE RELEASE
 FOR IMMEDIATE RELEASE FOR UK MEDICAL AND TRADE MEDIA ONLY Takeda Presents Data from TOURMALINE-MM1 Study for Ixazomib, the First and Only Once-Weekly Oral Proteasome Inhibitor Studied in Phase III Clinical
FOR IMMEDIATE RELEASE FOR UK MEDICAL AND TRADE MEDIA ONLY Takeda Presents Data from TOURMALINE-MM1 Study for Ixazomib, the First and Only Once-Weekly Oral Proteasome Inhibitor Studied in Phase III Clinical
CEDR 2018 QCDR Measures for CMS 2018 MIPS Performance Year Reporting
 ACEP19 Emergency Department Utilization of CT for Minor Blunt Head Trauma for Aged 18 Years and Older Percentage of visits for aged 18 years and older who presented with a minor blunt head trauma who had
ACEP19 Emergency Department Utilization of CT for Minor Blunt Head Trauma for Aged 18 Years and Older Percentage of visits for aged 18 years and older who presented with a minor blunt head trauma who had
EACTS Adult Cardiac Database
 EACTS Adult Cardiac Database Quality Improvement Programme List of changes to Version 2.0, 13 th Dec 2018, compared to version 1.0, 1 st May 2014. INTRODUCTORY NOTES This document s purpose is to list
EACTS Adult Cardiac Database Quality Improvement Programme List of changes to Version 2.0, 13 th Dec 2018, compared to version 1.0, 1 st May 2014. INTRODUCTORY NOTES This document s purpose is to list
Crosswalk File of ICD9 Diagnosis Codes to Risk Group Assignment 1-Apr-15
 1 1500 MALIGNANT NEOPLASM OF CERVICAL ESOPHAGUS 1 1501 MALIGNANT NEOPLASM OF THORACIC ESOPHAGUS 1 1502 MALIGNANT NEOPLASM OF ABDOMINAL ESOPHAGUS 1 1503 MALIGNANT NEOPLASM OF UPPER THIRD OF ESOPHAGUS 1
1 1500 MALIGNANT NEOPLASM OF CERVICAL ESOPHAGUS 1 1501 MALIGNANT NEOPLASM OF THORACIC ESOPHAGUS 1 1502 MALIGNANT NEOPLASM OF ABDOMINAL ESOPHAGUS 1 1503 MALIGNANT NEOPLASM OF UPPER THIRD OF ESOPHAGUS 1
Directorate General of Health Services Office of Drugs Controller General (India) (Biological Division)
 Directorate General of Health Services Office of Drugs Controller General (India) (Biological Division) List of r-dna drug products approved in the country (Form 45 and 45A) from 1 st Jan 2015 to 30 th
Directorate General of Health Services Office of Drugs Controller General (India) (Biological Division) List of r-dna drug products approved in the country (Form 45 and 45A) from 1 st Jan 2015 to 30 th
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center
 Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Etudes cliniques Service d Oncologie - Radiothérapie
 Etudes cliniques Service d Oncologie - Radiothérapie Juillet 2017 SEIN NEO ADJUVANT Neo-RHEA : NEOadjuvant Biomarker ResearcH Study of Palbociclib in Estrogen Receptor Positive HER2 Negative Breast CAncer
Etudes cliniques Service d Oncologie - Radiothérapie Juillet 2017 SEIN NEO ADJUVANT Neo-RHEA : NEOadjuvant Biomarker ResearcH Study of Palbociclib in Estrogen Receptor Positive HER2 Negative Breast CAncer
Our Specified Illness Benefit Is Now Even Better.
 Our Specified Illness Benefit Is Now Even Better. Now Covering 47 Specified Illnesses 20 Partial Payment Illnesses Enhancements to our Specified Illness Benefit We have made enhancements to our Specified
Our Specified Illness Benefit Is Now Even Better. Now Covering 47 Specified Illnesses 20 Partial Payment Illnesses Enhancements to our Specified Illness Benefit We have made enhancements to our Specified
