COPYRIGHT AND CITATION CONSIDERATIONS FOR THIS THESIS/ DISSERTATION
|
|
|
- Arthur Freeman
- 5 years ago
- Views:
Transcription
1 COPYRIGHT AND CITATION CONSIDERATIONS FOR THIS THESIS/ DISSERTATION o Attribution You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. o NonCommercial You may not use the material for commercial purposes. o ShareAlike If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original. How to cite this thesis Surname, Initial(s). (2012) Title of the thesis or dissertation. PhD. (Chemistry)/ M.Sc. (Physics)/ M.A. (Philosophy)/M.Com. (Finance) etc. [Unpublished]: University of Johannesburg. Retrieved from: (Accessed: Date).
2 Plasma membrane proteins differentially expressed in response to LPS perception in Arabidopsis thaliana By NWAXIGOMBE MAUREEN BALOYI A dissertation submitted in partial fulfilment for the Degree of Masters in Biochemistry in Biochemistry Faculty of Science UNIVERSITY OF JOHANNESBURG Supervisor: Dr. Lizelle Piater Co-supervisor: Prof. Ian Dubery
3 "Commit to the LORD whatever you do, and your plans will succeed" Proverbs 16:3 2
4 Table of Contents ACKNOWLEDGEMENTS... V ABBREVIATIONS... VI LIST OF FIGURES... IX LIST OF TABLES... XIV SUMMARY... XV CHAPTER 1:... 1 GENERAL INTRODUCTION AND AIMS OF THE STUDY Aims of the study... 2 CHAPTER 2:... 3 LITERATURE REVIEW General introduction Innate immunity Plant-pathogen interactions Non-host resistance Pattern recognition receptors (PRRs) Host-specific resistance Induced resistance Systemic acquired resistance (SAR) Induced systemic resistance (ISR) Lipopolysaccharides (LPS) LPS structure and composition LPS interaction in plants and animals The plant plasma membrane (PM) Membrane structure I
5 2.3.2 Lipid rafts in the model membrane Detergent-resistant membranes (DRMs) Proteomics Defining proteomics The significance of proteomics An overview of expressional proteomics Protein preparation Protein quantification Protein separation Protein staining/visualisation methods Protein identification using mass spectrometry CHAPTER 3: MATERIALS AND METHODS Plant material and treatment Plant growth Lipopolysaccharide (LPS) treatment Plasma membrane (PM) isolation methods Ultracentrifugation-based protocol Microsomal fraction isolation Plasma membrane isolation Detergent resistant membrane (DRM) isolation Small-scale based protocol Microsomal membrane fraction isolation Plasma membrane isolation Quantification and validation of the isolated plasma membrane Protein quantification using the Bradford assay The H + -ATPase assay II
6 3.3.3 Western blot analysis Gel electrophoresis Assembly and electro-blotting of gels Detection of phosphorylated proteins Proteomics Sample preparation One-dimensional (1-D) SDS-PAGE Preparation of 12% resolving and 5% stacking gels Fairbanks Coomassie Brilliant Blue staining Acetone precipitation Two-dimensional gel electrophoresis (2DGE) Rehydration of IPG strips Isoelectrofocusing (IEF) of IPG strips Equilibration of IPG strips Second dimension (2D) separation Protein identification by mass spectrometry (MS) Sample preparation Identification of proteins by MALDI-TOF-MS and LC-MS/MS Extractive in-gel method CHAPTER 4: RESULTS AND DISCUSSION Treatment of Arabidopsis plants with lipopolysaccharide Plasma membrane (PM) isolation and characterisation Ultracentrifugation-based method Small-scale-based protocol Quantification and validation of the isolated plasma membrane Protein quantification using the Bradford assay III
7 4.3.2 The H + -ATPase assay Western blot analysis One-dimensional (1-D) SDS-PAGE Two-dimensional gel electrophoresis (2DGE) Protein identification by MALDI-TOF and LC-MS/MS Functional implication of the identified PM proteins in LPS-perception in Arabidopsis leaves Signalling Membrane trafficking and transporters Membrane structure Defence CHAPTER 5: CONCLUSION REFERENCES APPENDIX IV
8 ACKNOWLEDGEMENTS Firstly, I would like to thank my LORD, Jesus Christ for his surpassing grace and for giving me the strength to help me carry on with my research and keeping the faith until the end. I am deeply grateful and honoured to my parents (Abbie and Tsakani) and siblings (Patience, Wayne, Hope, Miehleketo and Nxalati) for their understanding, encouragements, support and personal guidance that had a significant influence on my whole study career choice. I would like to extend my deep and sincere gratitude to my supervisor, Dr. LA Piater, for her detailed review, constructive comments and exceptional advice during the preparation of my literature review, dissertation and research. For giving me the platform where I could develop my research skills. I would like to extend my gratitude to my co-supervisor, Prof. IA Dubery for allowing me to be part of this dynamic research group and his input in my research work. The financial assistance of the National Research Foundation (NRF) towards this research is hereby acknowledged. Lastly, I would like to thank the whole Molecular Plant-Microbe Interactions Group for their warm welcome and good working relationship as well as the Department of Biochemistry. And also thanks to my friends Cornelius Vilakazi and Thembisile Khosa for helping out with my experiments. V
9 ABBREVIATIONS A APS ammonium persulphate ABC ammonium bicarbonate ACN acetonitrile A. thaliana Arabidopsis thaliana Avr gene avirulent gene B BSA bovine serum albumin BTP Bis-Tris-Propane C CBB Coomassie brilliant blue CDPK calcium-dependent protein kinase CHAPS 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate cyp710a1 cytochrome P450, Family 710, Subfamily A, Polypeptide 1 Ca 2+ calcium ions D dh 2 O distilled water DRMs detergent resistant membranes E EFR elongation factor receptor EF-Tu elongation factor Tu elrrs extracellular leucine-rich repeats ET ethylene F FLS2 flagellin sensitive 2 flg22 22-amino acid sequence of the conserved N-terminal part of flagellin G GPI glycosylphosphatidylinositol H HCl hydrochloric acid HIR hypersensitive-induced response HR hypersensitive response I IEF isoelectric focussing IPG immobilised ph gradient VI
10 pi isoelectric point ISR induced systematic resistance J JA jasmonic acid K KDO 2-keto-3-deoxyoctulosonic acid L LC-MS/MS liquid chromatography-tandem mass spectrometry l d liquid-disordered l o liquid-ordered LOS lipooligosaccharide LPS lipopolysaccharides LPS B.cep LPS from Burkholderia cepacia LPS E.coli LPS from Escherichia coli LRR-RLK leucine-rich repeat receptor-like kinase M MALDI TOF matrix-assisted laser-desorption/ionization time-of-flight MAMP microbe-associated molecular pattern MAPK mitogen activated protein kinase MD-2 myeloid differentiation-2 MS mass spectrometry MTI MAMP-triggered immunity MOWSE molecular weight search MW molecular weight N NB-LRR nucleotide binding leucine-rich repeat NCBInr National Centre for Biotechnology Information non-redundant NPR1 non-expressor of pathogenesis-related genes 1 O OGP n-octyl-β-d-glucopyranoside P PAMP pathogen-associated molecular pattern PGIPs polygalacturonase-inhibiting proteins PGPR plant growth-promoting rhizobacteria PM plasma membrane PMF peptide mass fingerprinting PRR pattern recognition receptor PR genes pathogenesis-related genes PR proteins pathogenesis-related proteins PTI PAMP-triggered immunity VII
11 R R gene resistance gene R proteins resistance proteins ROS reactive oxygen species RLKs receptor-like kinases RLPs receptor-like proteins S SA salicylic acid SAR systematic acquired resistance SNARE soluble N-ethylmaleimide-sensitive factor attachment protein receptors T TIR toll/interleukin-1 receptor TLR-4 toll-like receptor-4 TTSS type III secretion system V VAMP vesicle-associated membrane protein VIII
12 LIST OF FIGURES Figure 2.1: The zigzag model of plant immunity (taken from Jones and Dangl, 2006). PTI - PAMPtriggered immunity, ETS - effector-triggered susceptibility, ETI - effector-triggered immunity, PAMPs pathogen associated molecular patterns, HR - hypersensitive response, Avr-R - avirulence resistant protein Figure 2.2: A schematic illustration of a bacterial PAMP recognised by plant PRRs leading to PAMPtriggered immunity (PTI) and effector-triggered immunity (ETI), which then results in either plant disease or resistance (taken from Zimaro et al., 2011). PRRs - pattern recognition receptors, TTSS - type III secretion system, ETS - effector-triggered susceptibility, R - resistance gene, HR - hypersensitive response Figure 2.3: A schematic diagram of some of the various P/MAMPs and their corresponding receptors (taken from Pel and Pieterse, 2013). EFR - elongation factor receptor, EF-Tu - elongation factor Tu, TLR5 - Toll-like receptors, FLS2 - Flagellin Sensitive 2, LPS - lipopolysaccharides, PGN - peptidoglycan Figure 2.4: A schematic diagram illustrating the compatible and incompatible interactions of a plant specific resistance (R) gene with the corresponding pathogen avirulence (Avr) gene from invading bacteria (taken from Hammond-Kosack and Jones, 2003) Figure 2.5: The different classes of resistance (R) proteins encoded by resistance (R) genes. There are two classes of R proteins, namely NB-LRRs (nucleotide binding leucine-rich repeats) and elrrs (extracellular leucine-rich repeats) that are further categorised into subclasses based on their domain organisation (taken from Chisholm et al., 2006). TIR-NB-LRR - Toll/interleukin-1 receptor nucleotide binding leucine-rich repeat, CC-NB-LRR - coiled-coil nucleotide binding leucine-rich repeat, RLP-LRR - receptor-like protein leucine-rich repeat, RLK-LRR - receptor-like kinases leucine-rich repeat, PGIP-LRR - polygalacturonaseinhibiting proteins leucine-rich repeat, CC - coiled-coil domain Figure 2.6: (A) The activation of SAR by necrotising pathogens at a local site of infection of plant tissue, leading to the accumulation of SA concentrations, which then is released to other uninfected parts of the plant. ISR, on the other hand, is activated by soil-borne microorganisms at the roots of the plant. (B) The chemical regulators or inducers include salicylic acid (SA) which regulates SAR, while jasmonic acid (JA) and ethylene (ET) are regulators of ISR (taken from Pieterse et al., 2009) Figure 2.7: The general structure of LPS consists of the O-antigen, core polysaccharide (outer and inner) and lipid A linked to the inner core polysaccharide by the 2-keto-3-deoxyoctulosonic acid (KDO) molecule (taken from Erbs and Newman, 2011) Figure 2.8: The structure of Gram-negative bacterial cell walls illustrating the inner and outer membranes containing LPS on the outer layer of the outer membrane (taken from Raetz and Whitfield, 2002). LPS - IX
13 lipopolysaccharides, MDO - membrane-derived oligosaccharides, PPEtn - ethanolamine pyrophosphate Figure 2.9: The proposed lipid bilayer structure initially suggested by Gorter and Grendel (1925), illustrating phospholipid hydrophobic tails between the polar head groups that are exposed to water Figure 2.10: The fluid-mosaic model according to Singer and Nicolson (1972), illustrating the lipid bilayer containing both peripheral and transmembrane proteins than are randomly distributed within the membrane Figure 2.11: The proposed mattress model according to Mouritsen and Bloom (1984), illustrating the aggregation and clustering of proteins Figure 2.12: The plasma membrane containing various regions of lipid rafts in a liquid-ordered (l o ) phase and non-raft regions in liquid-disordered (l d ) phase that coexist in the model membrane. Phospholipids, sphingolipids and sterols are the major lipid structures that play a role in the formation of the plasma membrane (taken from Bhat and Panstruga, 2005) Figure 2.13: The endocytosis and exocytosis membrane trafficking pathways in a plant cell (taken from Irani and Russinova, 2009). ER - endoplasmic reticulum, TGN - trans-golgi network, BFA - Brefeldin A, P - phosphate, Tyr - tyrosine, MVB - multivesicular body Figure 2.14: A schematic diagram illustrating the general flow for proteomics analysis in expressional proteomics (taken from Pandey and Mann, 2000) Figure 2.15: The different components that make up the mass spectrometer. The ion source ensures that the protein samples are ionised and enter a gas phase, while a mass analyser separates ions according to their mass-to-charge ratio (m/z) and an ion detector collects the presence of separated ions and stores them in a form of spectrum (taken from Finehout and Lee, 2004) Figure 2.16: A schematic diagram illustrating the tandem mass spectrometry containing two mass analysers (MS1 and MS2) in series with a collision cell in between the analysers according to Finehout and Lee (2004) Figure 3.1: Arabidopsis thaliana plants grown in soil at 22 C under a 9 h light/15 h dark cycle until matured Figure 3.2: Arabidopsis plants treated by pressure-infiltration with 100 µg/ml of LPS E.coli into the lower side of the leaves for 6 and 12 h X
14 Figure 3.3: A step-wise isolation of the plasma membrane achieved by an aqueous two-phase partitioning system. U1, 2 and 3 - upper phases, U3 - upper phase in which the PM is enriched (taken from Larsson and Widell, 1981 cited in Hatti-Kaul, 2000) Figure 3.4: Sucrose density step-gradient centrifugation, where the microsomal membrane fraction was layered on 25% sucrose overlaid on 38% sucrose for isolation of the plasma membrane fraction Figure 3.5: Transfer cassette assembly unit of an immunoblot sandwich for protein transfer (Hoefer, GE Healthcare BioSciences AB) Figure 3.6: A schematic diagram illustrating the lane of a sample excised and divided into 5 pieces Figure 4.1: A comparative 1-D SDS-PAGE analysis of the 0 and 6 h LPS E.coli -treated Arabidopsis leaf proteomes subsequent to the ultracentrifugation-based method. 10 µg protein was loaded per well on a 12% SDS-PAGE gel and bands were visualised with an Aqua-stain solution (Vacutec). L - PageRuler Unstained Low Range Protein Ladder (Thermo Scientific), molecular weight (MW) markers are expressed in kilodalton (kda), HM - homogenate, MF - microsomal fraction and PM - plasma membrane Figure 4.2: A 1-D SDS-PAGE analysis of the 0 and 6 h LPS E.coli -treated Arabidopsis leaf proteomes subsequent to the ultracentrifugation-based method. 10 µg protein was loaded per well on a 12% SDS- PAGE and bands were visualised using the Fairbanks staining protocol. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane Figure 4.3: Plasma membrane isolation from 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf samples. The PM was obtained at 25%/38% sucrose layers in a sucrose-step gradient centrifugation approach using a microcentrifuge Figure 4.4: The standard curve constructed from the absorbance readings of the bovine serum albumin (BSA) standard solutions using the Bradford assay. Error bars represent standard deviation of three technical repeats Figure 4.5: Plasma membrane ATPase activity measured at different time intervals, as calculated from the released inorganic phosphate. Error bars represent standard deviation of three biological repeats Figure 4.6: Representative Western blot analysis of the homogenate (HM), microsomal fraction (MF) and plasma membrane (PM) of 0, 6 and 12 h LPS E.coli -treated Arabidopsis samples. 10 µg protein was loaded on a 12% SDS-PAGE gel and blotted onto a PVDF membrane. Proteins were detected with an anti- MAPK antibody XI
15 Figure 4.7: A comparative 1-D SDS-PAGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes prior- and subsequent to acetone precipitation following the small-scale isolation protocol. 10 µg protein was loaded per well on a 12% SDS-PAGE gels followed by visualisation using the Fairbanks staining solution. L - Prosieve Quad Color Protein marker (Lonza), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane Figure 4.8: Comparative 2DGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes separated on a broad ph range (ph 3-10) using a 7 cm IPG strip following the small-scale isolation protocol. 100 µg protein was first focused and the strip was loaded on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific) and the MW markers are expressed in kda Figure 4.9: Comparative 2DGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes separated on a narrow ph range (ph 4-7) using a 7 cm IPG strip following the small-scale isolation protocol. 100 µg protein was first focused and the strip was loaded on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda. Differences between the gels are highlighted with red bars Figure 4.10: 1-D SDS-PAGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes priorand subsequent to acetone precipitation using the small-scale isolation protocol. 10 µg protein was loaded per well on a 12% SDS-PAGE gel followed by visualisation using the Fairbanks staining solution. L - Prosieve Quad Color Protein marker (Lonza), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. The red blocks indicate where the bands of interest were excised for the identification of proteins by mass spectrometry Figure 4:11: 1-D SDS-PAGE analysis of 0, and 6 h LPS E.coli -treated Arabidopsis leaf proteomes using the ultracentrifugation-based isolation protocol. 10 µg protein was loaded per well on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. The red blocks indicate where the bands of interest were excised for the identification of proteins by mass spectrometry Figure 4.12: 2DGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes separated on a narrow ph range (ph 4-7) using a 7 cm IPG strip following the small-scale isolation protocol. 100 µg protein was first focused and the strip was loaded on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. The arrows with numbers indicate randomly selected differentially expressed protein spots of interest excised for the identification of proteins by mass spectrometry. The MW markers are expressed in kda XII
16 Figure 4.13: 1-D SDS-PAGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes prepared for the extractive in-gel protein identification approach. 10 µg protein was loaded per well on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. L - Prosieve Quad Color Protein marker (Lonza), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. The red blocks indicate where the lanes that were excised for the identification of proteins by mass spectrometry Appendix Figures Figure A1: A 1-D SDS-PAGE analysis of the 0 and 6 h LPS E.coli -treated Arabidopsis leaf proteomes subsequent to the ultracentrifugation-based method. 10 µg protein was loaded per well on a 12% SDS- PAGE gel and bands were visualised with an Aqua-stain solution (Vacutec). L - PageRuler Unstained Low Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane Figure A2: A 1-D SDS-PAGE analysis of the 0 and 6 h LPS E.coli -treated Arabidopsis leaf proteomes subsequent to the ultracentrifugation-based method. 10 µg protein was loaded per well on a 12% SDS- PAGE and bands were visualised using the Fairbanks staining protocol. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane Figure A3: Western blot analysis of the homogenate (HM), microsomal fraction (MF) and plasma membrane (PM) of 0, 6 and 12 h LPS E.coli -treated Arabidopsis samples. 10 µg protein was loaded on a 12% SDS-PAGE gel and blotted onto a PVDF membrane. Proteins were detected with an anti-mapk antibody Figure A4: A comparative 1-D SDS-PAGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes prior- and subsequent to acetone precipitation following the small-scale isolation protocol. 10 µg protein was loaded per well on a 12% SDS-PAGE gels followed by visualisation using the Fairbanks staining solution. L - Prosieve Quad Color Protein marker (Lonza), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane Figure A5: 2DGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes separated on a narrow ph range (ph 4-7) using a 7 cm IPG strip following the small-scale isolation protocol. 100 µg protein was first focused and the strip was loaded on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda XIII
17 LIST OF TABLES Table 3.1: Preparation of 12% resolving and 5% stacking gels Table 3.2: Preparation of protein samples for rehydration Table 3.3: Isoelectricfocusing conditions for 7 cm IPG strips Table 4.1: MALDI-TOF-MS identified Arabidopsis proteins from bands excised from 1-D SDS-PAGE of 6 and 12 h LPS E.coli -treated PM fractions arranged according to functional categories Table 4.2: LC-MS/MS identified Arabidops protein spots excised from 2DGE of 6 and 12 h LPS E.coli - treated PM arranged according to functional categories Table 4.3: LC-MS/MS identified Arabidops proteins from lanes excised from 1-D SDS-PAGE of 0, 6 and 12 h LPS E.coli -treated PM fractions arranged according to functional categories Appendix Tables Table A1: Identified non-pm proteins by MALDI-TOF-MS from bands excised from 1-D SDS-PAGE of 6 and 12 h LPS E.coli -treated Arabidopsis leaves Table A2: Identified Arabidopsis proteins by LC-MS/MS from lanes excised from 1-D SDS-PAGE of 0, 6 and 12 h LPS E.coli -treated PM fractions. Provided on CD XIV
18 SUMMARY Plant innate immunity occurs in two interconnected branches, the first being the recognition of pathogen conserved surface structures known as pathogen- or microbe-associated molecular patterns (P/MAMPs) by the plant plasma membrane pathogen recognition receptors (PRRs), leading to activation of P/MAMP-triggered immunity (P/MTI). The second branch involves the recognition of pathogen avirulence (Avr) genes by the corresponding plant disease resistance (R) genes, known as the gene-for-gene interaction, and results in a more efficient or stronger defence response, namely effector-triggered immunity (ETI). Lipopolysaccharide (LPS) acts as a P/MAMP that induces an innate immune response in both plants and animals. LPS, especially the lipid A component, has been shown to play a vital role in activating immune responses in animals. Other LPS components such as lipooligosaccharide (LOS) and the core-oligosaccharide have also been shown to trigger an immune response in plants such as Arabidopsis thaliana. In mammalian cells, LPS binds to the LPS-binding protein (LBP) forming a LPS-LBP complex, which binds to a Toll-like receptor 4/myeloid differentiation-2 (TLR4/MD-2) complex together with the co-receptor CD14, a glycosylphosphatidylinositol (GPI)-linked protein, and activates an immune response. To date, there is still no knowledge about the LPS receptor(s) in plants. In order to determine the LPS perception mechanism in plants, A. thaliana leaves were treated with 100 µg/ml LPS from Escherichia coli (LPS E.coli ) for 6 and 12 h while non-treated plants served as controls. Two methods were employed in order to isolate the plasma membrane (PM) from the leaves, and included an ultracentrifugation-based method and small-scale, bench-top centrifuge-based approach. The ultracentrifugation-based method was later discontinued due to non-reproducible results. The isolated PM was validated by performing an H + -ATPase assay, Western blotting and identification of PM protein markers. Furthermore, the samples were evaluated using 1-D SDS-PAGE/2DGE gel-based approaches and subsequent matrix-assisted laser desorption/ionization (MALDI) time-of-flight (TOF) and liquid chromatography (LC)-tandem mass spectrometry (MS/MS) analyses. The ATPase activity served as marker enzyme for the protein fraction associated with the PM. In the case of Western blot analysis, an anti-active mitogen activated protein kinase (MAPK) antibody was employed in order to detect corresponding phosphorylated proteins in the different XV
19 fractions [homogenate (HM), microsomal fraction (MF) and plasma membrane (PM)] of 0, 6 and 12 h LPS E.coli -treated Arabidopsis samples. Negligible to no bands were detected in the PM samples in comparison to the other fractions (HM and MF). In addition, PM [or detergent-resistant membranes (DRM)] protein markers were identified and include remorin, aquaporin, glycosylphosphatidylinositol (GPI)-anchored protein and flotillin. Protein bands that were differentially expressed between the LPS E.coli -treated- and untreated PM samples were analysed by MALDI-TOF, where a total of 12 proteins were found to be PM-localised, with a molecular weight search (MOWSE) score lower than 62. In order to increase the score, 13 2DGE protein spots that were differentially expressed between the 6 h and 12 h LPS E.coli -treated PMs were selected and analysed by LC-MS/MS. Only 5 proteins were found to be PM-localised, of which 4 had a score higher than 62. An extractive in-gel method was also included, where the whole lane from each treatment (0 h, 6 and 12 h LPS E.coli -treated samples) was excised and analysed by LC-MS/MS in order to confirm the identified proteins and to increase the identity of low abundant proteins. A total of 88 proteins were found to be PM-localised and classified according to their functional categories. From the identified protein bands, spots and lanes, it is concluded that LPS perception and signal transduction most likely occurs via a PM-localised protein(s) due to those identified being similar to the proteins reported to be involved following treatment with LPS from Burkholderia cepacia (LPS B.cep ) and flagellin, flg22. These include leucine-rich repeat receptor-like kinase (LRR-RLKs), calmodulin/calcium-dependent protein kinase (CDPK), like protein, actin and tubulin. Furthermore, the LRR-RLK flg22 receptor, FLS2, has been reported to be localised within the DRMs of the PM of A. thaliana, and internalised via exo- and endocytosis dependent pathways. Thus, although the identification of the LPS receptor(s) was not fully elucidated, the current results suggest a similar perception mechanism to that of flg22. XVI
20 CHAPTER 1: GENERAL INTRODUCTION AND AIMS OF THE STUDY Many countries worldwide are faced with challenges from both abiotic and biotic factors that have a great impact on crop production by causing instability in plants, affecting production yield and food supply. Biotic factors that affect plants include pathogenic microorganisms such as bacteria, viruses, fungi as well as insect infestation and weed competition, while abiotic factors include drought, salinity, heat, and cold stresses (Campos et al., 2004). These factors can be overcome by studying the mechanisms of how plants interact with stressinducing agents and conditions in order to survive. When it comes to plant-pathogen interactions, plants rely on an innate immune system to defend themselves against the pathogens in order to survive. They do so by recognising the presence of pathogens, thereby triggering defence responses (Dangl and Jones, 2001; Deepak et al., 2006; Spoel and Dong, 2012). A. thaliana, also known as mouse-ear cress or thale cress, is a small flowering plant that belongs to the Brassicaceae family and is used as a model plant in the field of plant research. This is due to the fact that it has a very small genome size of approximately 125 megabase (Mb) that is fully sequenced and annotated and a rapid lifecycle of about 6 weeks from germination to matured seed (Meinke et al., 1998). Plasma membranes (PMs) are composed of both liquid-ordered (l o ) and liquid-disordered (l d ) phases that co-existed in model membranes. These consist of high concentrations of sterols and sphingolipids that allow tight packing of the lipids into a l o phase (Simons and Ikonen, 1997; Brown and London, 1998). Futhermore, PMs contain certain receptors with extracellular domains that are involved in microbe-associated molecular pattern (MAMP) perception and defence responses in Arabidopsis. Therefore, investigations into the protein compositions of these membranes may allow the possible identification of pattern recognition receptors (PRRs) involved in recognition of pathogen-derived or MAMP molecules. 1
21 LPS is found on the outer layer of the membrane of Gram-negative bacteria (Raetz, 1996; Raetz and Whitfield, 2002). As such, LPS acts as a MAMP that triggers an innate immune response in both plants and animals (Bedini et al., 2005; Silipo et al., 2005). In mammalian cells, LPS is recognised by toll-like receptor (TLR) 4, and results in activation of immune responses (Triantafilou et al., 2002; Akira and Takeda, 2004; Nürnberger et al., 2004; Akira et al., 2006), while LPS receptor(s) in plants have not yet been found. Proteomics, which is the analysis of the entire expressed protein component in an organism or tissue, particularly pertaining their structure and function (Blackstock and Weir, 1999; Pandey and Mann, 2000), has thus been employed to analyse the interactions between Arabidopsis and LPS. 1.1 Aims of the study With the above-mentioned background, the hypothesis of the project is that LPS perception and defence responses occur within the PM domains similar to mammalian cells. As such, the objective was to characterise the LPS receptor(s)/binding proteins within the PM in order to assist in gaining insight into the elusive receptor/receptor complex, and could help in improving disease resistance in plants. In order to achieve this, the following aims were addressed: (i) To evaluate and optimise various protocols for isolating the PM from A. thaliana, (ii) To isolate the Arabidopsis PM subsequent to LPS E.coli treatment and, (iii) To analyse Arabidopsis PM proteins with the use of proteomic techniques (1-D SDS- PAGE/2DGE) as well as identification of the proteins using MALDI-TOF MS and LC- MS/MS. 2
22 CHAPTER 2: LITERATURE REVIEW 2.0 General introduction Plants are constantly exposed to attacks by pathogenic microorganisms, known as phytopathogens that include bacteria, viruses and fungi (He et al., 2007; Pieterse et al., 2009). Plants, unlike animals, lack an adaptive immune system and therefore rely on an innate immune system to defend themselves against pathogenic attacks by recognising the presence of pathogens, thereby triggering defence responses (Dangl and Jones, 2001; Deepak et al., 2006; Spoel and Dong, 2012). Plants have interconnected immune responses that enable them to effectively respond to attacks by pathogenic microorganisms to which they are exposed (Chisholm et al., 2006; He et al., 2007). This comprises two defence mechanisms namely preformed and induced defences. Preformed defences include mechanisms/strategies that are constitutive in plants prior to pathogen infection in order to sustain a constant immune response. These include structural or physical barriers such as cell wall lignin, the cuticle and the waxy layer that are found on the plant surface (Reina-Pinto and Yephremov, 2009), as well as chemical barriers including phytoanticipins, which are antimicrobial compounds produced by plants prior to microbial infection and are always present in the plant tissues, thereby inhibiting the growth of pathogens (Dixon and Lamb, 1990; VanEtten et al., 1994; Habib and Fazili, 2007; Lenman et al., 2008; Sels et al., 2008). Successful pathogenic microorganisms are able to infect plants by overcoming these physical and chemical barriers (Kaffarnik et al., 2009), upon which the pathogen is then faced with another guarding system - the plant surface receptors found on the plasma membranes that recognise the presence of the pathogen and induce plant defences (Chisholm et al., 2006). 3
23 2.1 Innate immunity An induced plant defence is activated when the plant recognises surface molecules of a pathogen or detect the presence of pathogen effectors in the cell cytoplasm (Zipfel and Felix, 2005; Lenman et al., 2008). Following infection, a set of defence responses results including generation of reactive oxygen species (ROS), induction of the hypersensitive response (HR), and activation of pathogenesis-related (PR) proteins (Felix et al., 1999; Gómez-Gómez et al., 1999; Chisholm et al., 2006; Fujiwara et al., 2009). This recognition can either be non-host specific, where plant surface receptors recognise conserved surface molecules of the pathogen, or host-specific, where the plant resistance (R) gene recognises the corresponding avirulent (Avr) gene of the pathogen (Dixon et al., 1994; van Loon, 1997; Hammond-Kosack and Parker, 2003) Plant-pathogen interactions Non-host resistance Non-host resistance, also known as basal resistance, is the ability of a wide range of plant species to resist the majority of potential pathogens (Heath, 2000). Jones and Dangl (2006) introduced the zigzag model representing the plant immune system (Figure 2.1). Figure 2.1: The zigzag model of plant immunity (taken from Jones and Dangl, 2006). PTI - PAMPtriggered immunity, ETS - effector-triggered susceptibility, ETI - effector-triggered immunity, PAMPs pathogen associated molecular patterns, HR - hypersensitive response, Avr-R - avirulence resistant protein.1 4
24 Here, innate immunity is activated by direct recognition of conserved cell surface structures or molecules in microorganisms known as pathogen-/microbe-associated molecular patterns (P/MAMPs) by plant-specific plasma membrane-bound extracellular receptors known as pattern recognition receptors (PRRs) (Felix et al., 1993; Jones and Dangl, 2006; Zipfel, 2008; Boller and Felix, 2009; Spoel and Dong, 2012). Since not all the microorganisms that attack plants are pathogenic, the term MAMPs is used in preference to PAMPs (Staal and Dixelius, 2007). These MAMPs, originally known as elicitors (Naito et al., 2008), are regarded as non-host or non-race-specific since they belong to a wide range of microorganisms (Ebel and Cosio, 1994). Included in this category of molecules are lipopolysaccharides (LPS) from the outer membrane of Gram-negative bacteria, peptidoglycan from the cell walls of both Gram-negative and Gram-positive bacteria, flagellin (flg22), glycoproteins as well as fungal chitin, ergosterol and glucan (Felix et al., 1999; Nürnberger et al., 2004; Zipfel and Felix, 2005; Bittel and Robatzek, 2007; Naito et al., 2008; Erbs and Newman, 2011; Klemptner et al., 2014). The recognition of these P/MAMPs leads to the primary immune response known as P/MAMP-triggered immunity (P/MTI) (Chisholm et al., 2006; Jones and Dangl, 2006), resulting in the generation of ROS within minutes after interaction/perception with respective receptors. Additional responses include ion fluxes and protein phosphorylation such as mitogen activated protein kinase (MAPK) signaling (Felix et al., 1999; Gómez-Gómez et al., 1999; Nürnberger et al., 2004; Zipfel et al., 2004; Boller and Felix, 2009). Successful pathogens are able to suppress P/MTI by delivering effectors into the plant cytoplasm using the type III secretion system (TTSS), thereby increasing pathogen virulence or pathogenicity, and resulting in effector-triggered susceptibility (ETS), leading to plant disease (Chisholm et al., 2006; Boller and He, 2009; Cui et al., 2009). In order for plants to overcome this, they have evolved resistance (R) proteins that directly or indirectly recognise the effector, resulting in effector-triggered immunity (ETI), a secondary immune response, which results in an amplified and prolonged generation of ROS known as the oxidative burst. This response is directly associated with programmed cell death known as hypersensitive response (HR) at the site of infection, leading to inhibition of pathogen growth and promoting plant disease resistance (Dangl and Jones, 2001; Zipfel, 2008) as shown in Figure 2.1 and
25 Figure 2.2: A schematic illustration of a bacterial PAMP recognised by plant PRRs leading to PAMPtriggered immunity (PTI) and effector-triggered immunity (ETI), which then results in either plant disease or resistance (taken from Zimaro et al., 2011). PRRs - pattern recognition receptors, TTSS - type III secretion system, ETS - effector-triggered susceptibility, R - resistance gene, HR - hypersensitive response Pattern recognition receptors (PRRs) Pattern recognition receptors (PRRs) are plant-specific plasma membrane-bound receptors that recognise the conserved surface molecules of microorganisms that they come into contact with (Felix et al., 1993; Jones and Dangl, 2006; Zipfel, 2008). These PRRs include receptor-like kinases (RLKs), which are proteins containing a transmembrane (TM) domain with a cytoplasmic kinase or receptor-like proteins (RLPs), which are proteins containing a TM domain lacking a kinase (Chisholm et al., 2006; Boller and Felix, 2009; Monaghan and Zipfel, 2012). Plant PRRs recognise the P/MAMPs resulting in P/MTI, a first line of defence effective against a broad spectrum of pathogens (Jones and Dangl, 2006). The first PRR to be isolated was the rice XA21 (Xanthomonas resistance 21) receptor, that recognises a conserved 17 amino acid sulphated protein Ax21 (activator of XA21-mediated immunity) and confers resistance against the Gram-negative bacteria Xanthomonas oryzae pv. oryzae (Song et al., 1995; Lee et al., 2009). To date, various P/MAMPs have been identified that have the ability to trigger plant defence responses via their corresponding 6
26 PRRs. The two best characterised and well-studied receptors include the Flagellin Sensitive 2 (FLS2) receptor that recognises bacterial flagellin, flg22, a 22 amino acid peptide (Felix et al., 1999; Gómez-Gómez and Boller, 2000), and elongation factor receptor (EFR), which recognises elongation factor Tu (EF-Tu), via the elf18, an 18 amino acid sequence on the N- terminus of EF-Tu (Kunze et al., 2004; Zipfel et al., 2004). FLS2 was originally identified from Arabidopsis mutants as an extracellular region of leucine-rich repeat receptor-like kinase (LRR-RLK) (Boller and Felix, 2009) located on the extracellular domain of the plasma membrane (Robatzek et al., 2006). In mammalian cells, the best studied PRRs are Toll-like receptors (TLRs). There are 13 TLRs that have been identified and are involved in recognising different MAMPs (Kawai and Akira, 2011). LPS, in particular, is recognised by TLR4, while flagellin is recognised by TLR5 (Smith et al., 2003; Nürnberger et al., 2004; Akira et al., 2006). To date, there is still no knowledge with regard to the LPS receptor(s) and how the perception signal transduction pathways function in plants (Bedini et al., 2005; Silipo et al., 2005) as shown in Figure 2.3, but will be discussed in more detail in Section Figure 2.3: A schematic diagram of some of the various P/MAMPs and their corresponding receptors (taken from Pel and Pieterse, 2013). EFR - elongation factor receptor, EF-Tu - elongation factor Tu, TLR5 - Toll-like receptors, FLS2 - Flagellin Sensitive 2, LPS - lipopolysaccharides, PGN - peptidoglycan.1 7
27 Host-specific resistance Host-specific resistance involves the previously-termed gene-for-gene interaction which is the direct or indirect interaction between a plant resistance (R) gene and a corresponding pathogen avirulence (Avr) gene known as an incompatible interaction (Flor, 1971; Keen, 1990; Hammond-Kosack and Jones, 1997; Dangl and Jones, 2001; Hammond-Kosack and Parker, 2003; Chisholm et al., 2006) shown in Figure 2.4B. Plants possessing the R genes produce R proteins that recognise the corresponding Avr proteins of the pathogen known as effectors and results in ETI (Figure 2.2), thereby conferring resistance towards the pathogen. The pathogen is then unable to avoid or suppress ETI as well as to grow, multiply, spread and cause a disease, resulting in a HR (Jones and Dangl, 2006; Zipfel, 2008). This recognition of pathogen Avr genes by the dominant R genes of a plant initiates signal transduction pathways resulting in the activation of defence responses. Prior to the zigzag model, van der Biezen and Jones (1998) proposed the guard hypothesis, suggesting that the R proteins monitor the host components targeted by the pathogen effectors. Pathogen molecules (effectors) achieve this by manipulating or changing the host targets sensed by the R proteins, thereby activating an immune response (Dangl and Jones, 2001; Hammond-Kosack and Parker, 2003; Chisholm et al., 2006) as shown in Figure 2.4B. The absence of the R or Avr gene leads to the pathogen not being recognised by the plant and disease symptoms occur as a consequence (Heath, 2000). Therefore, the plant becomes susceptible to the pathogen, allowing growth and development thereof. This mechanism is known as a compatible interaction (Dangl and Jones, 2001) shown in Figure 2.4A. 8
28 Figure 2.4: A schematic diagram illustrating the compatible and incompatible interactions of a plant specific resistance (R) gene with the corresponding pathogen avirulence (Avr) gene from invading bacteria (taken from Hammond-Kosack and Jones, 2003). 1 The plant R genes encode R proteins that contain a nucleotide binding (NB) site and LRR domains that confer resistance to a variety of unrelated pathogens (Jones et al., 1994; Hammond-Kosack and Jones, 1997; Parker and Coleman, 1997). These NB-LRRs are the most abundant proteins and are classified into two subclasses, namely the coiled-coil (CC) motif and Toll/interleukin-1 receptor (TIR) domains (Feys and Parker, 2000; Hammond- Kosack and Jones, 2003; Eitas and Dangl 2010), and constitute cytoplasmic proteins. There are approximately 125 NB-LRR proteins encoded by R genes in the Arabidopsis genome (Jones and Dangl, 2006). The other class of R proteins encoded by R genes contain extracellular LRRs (elrrs), and these are located in the extracellular domain of the plasma membrane (Buchanan and Gay, 1996). elrrs are classified into three subclasses based on their domain structures, namely receptor-like kinases (RLKs, transmembrane domain containing a cytoplasmic kinase), receptor-like proteins (RLPs, transmembrane domain) and polygalacturonase-inhibiting proteins (PGIPs, cell wall LRR) (Dangl and Jones, 2001; Fritz-Laylin et al., 2005) shown in Figure 2.5. Also, S-domain RLKs that can recognise MAMPs may also function as R genes in certain cases (Sanabria et al., 2008). 9
29 Figure 2.5: The different classes of resistance (R) proteins encoded by resistance (R) genes. There are two classes of R proteins, namely NB-LRRs (nucleotide binding leucine-rich repeats) and elrrs (extracellular leucine-rich repeats) that are further categorised into subclasses based on their domain organisation (taken from Chisholm et al., 2006). TIR-NB-LRR - Toll/interleukin-1 receptor nucleotide binding leucine-rich repeat, CC-NB-LRR - coiled-coil nucleotide binding leucine-rich repeat, RLP-LRR - receptor-like protein leucine-rich repeat, RLK-LRR - receptor-like kinases leucine-rich repeat, PGIP-LRR - polygalacturonase-inhibiting proteins leucine-rich repeat, CC - coiled-coil domain Induced resistance Systemic acquired resistance (SAR) The activation of plant defence at the site of infection leads to the action of systemic defence responses in distant tissue of the plant in order to prevent the spread of disease or infection to other undamaged tissues by the invading pathogen (Ryals et al., 1996; Durrant and Dong, 2004; Pieterse et al., 2009). This mechanism is known as systemic acquired resistance (SAR). The term SAR was coined by Ross in 1961 and is defined as a long-lasting induced disease resistance state against a broad-range of pathogens (Ryals et al., 1994; Durrant and Dong, 10
30 2004; Gautam et al., 2011). SAR is often associated with local and systemic accumulation in endogenous salicylic acid (SA) levels and PR gene activation that encode proteins with antimicrobial activity (Malamy et al., 1990; Ryals et al., 1996; van Loon et al., 2006). SAR is an induced defence response that is triggered by either M/PTI or ETI-mediated pathogen recognition (avr-r gene interaction), as well by necrotising pathogens that cause tissue necrosis, and leads to the initiation of signal transduction pathways and subsequently the HR. This leads to the accumulation of SA levels at the local site of infection. The infected tissue or cells then release SA to other uninfected parts of the plant, initiating signal transduction pathways at distant parts from the infection. This then ultimately results in the production of PR defence proteins in distant tissues, which therefore allows the plant to exhibit resistance to the pathogen infection (Ross, 1961; Malamy et al., 1990; Ryals et al., 1996; Mishina and Zeier, 2007; Tsuda et al., 2008) shown in Figure Induced systemic resistance (ISR) ISR is an induced defence response that is similar to SAR, but is triggered by soil-borne microorganisms such as mycorrhizal fungi or non-pathogenic plant growth-promoting rhizobacteria (PGPR) that belong to the Pseudomonas and Bacillus genera, and is not associated with PR protein activation (Alström, 1991; Kloepper et al., 1992; van Loon, 1997; van Loon et al., 1998). ISR signalling pathways (Figure 2.6A) are regulated by jasmonic acid (JA) and ethylene (ET) (Pieterse et al., 2001; Pozo et al., 2008) shown in Figure 2.6B. The term induced resistance was coined by Kuć et al. (1959) and Ross (1966). Kuć et al. (1959) illustrated the systemic resistance of apple plants to apple scab by infiltrating the lower leaves with amino acids, D-phenylalanine, D-alanine and aminoisobutyric acid (AIB). It was found that there was no inhibition of the Venturia inaequalis in vitro by the amino acids at concentrations that were used for infusion. Ross in 1966 conducted a similar experiment using tobacco plants, where lower leaves were inoculated with a local lesion strain of tobacco mosaic virus (TMV), resulting in an increased systemic resistance of tobacco plants to the same strain of the virus. 11
31 Figure 2.6: (A) The activation of SAR by necrotising pathogens at a local site of infection of plant tissue, leading to the accumulation of SA concentrations, which then is released to other uninfected parts of the plant. ISR, on the other hand, is activated by soil-borne microorganisms at the roots of the plant. (B) The chemical regulators or inducers include salicylic acid (SA) which regulates SAR, while jasmonic acid (JA) and ethylene (ET) are regulators of ISR (taken from Pieterse et al., 2009). 1 Even though the induction of ISR is similar to SAR, there are also major differences such as: (1) ISR is induced by non-pathogenic rhizobacteria, while SAR is induced systemically after infection with necrotising pathogens resulting in HR activation, (2) ISR uses JA and ET for signalling, while SAR uses SA and (3) PR gene activation is often associated with SAR, but absent in ISR, even though both pathways require the NPR1 (non-expressor of pathogenesisrelated genes 1) regulatory protein and are effective against a broad-range of plant pathogens (Kloepper et al., 1992; Delaney et al., 1995; Shah et al., 1997; van Loon, 1997; van Loon et al., 1998; Pieterse et al., 2001; Ton et al., 2002; Pieterse et al., 2009; Tsuda et al., 2009). 12
32 2.2 Lipopolysaccharides (LPS) LPS structure and composition Lipopolysaccharides (LPS) are large amphipathic molecules found only on the outer membrane of Gram-negative bacteria (Albus et al., 2001; Newman et al., 2002; Raetz and Whitfield, 2002; Silipo et al., 2005; Madala et al., 2010; Richer et al., 2010) and composed of three different moieties: (i) the O-antigen known as the O-polysaccharide, (ii) the core oligosaccharide and (iii) the lipid A (Caroff et al., 2002; Erridge et al., 2002; Silipo et al., 2005; Silipo et al., 2010) as shown in Figure 2.7. Figure 2.7: The general structure of LPS consists of the O-antigen, core polysaccharide (outer and inner) and lipid A linked to the inner core polysaccharide by the 2-keto-3-deoxyoctulosonic acid (KDO) molecule (taken from Erbs and Newman, 2011). 1 The O-antigen (O-specific oligosaccharide) is composed of polysaccharide-repeat unit chains and is found on the outermost layer of LPS (Raetz and Whitfield, 2002; Silipo et al., 2010). It consists of varying sugar molecules such as galactose, mannose, glucose and rhamnose (Molinaro et al., 2009). The O-chains composition and arrangement differ from one bacterial strain to another (Braun et al., 2005; Newman et al., 2007). LPS can also be considered as smooth or rough, based on the presence or absence of the O-chains. Bacteria with smooth LPS contains full-length O-chains, while the rough LPS, also known as lipo-oligosaccharide (LOS), have reduced or absence of O-chains (Moriyon and Lopez-Goni, 1998; Caroff et al., 13
33 2002; Silipo et al., 2005), thereby making the lipoglycan more hydrophobic. Furthermore, the O-antigen is attached to the second domain of LPS which is the core-oligosaccharide. The core-oligosaccharide consists of inner and outer oligosaccharides that are linked to the lipid A (Albus et al., 2001) via the characteristic sugar, 2-keto-3-deoxyoctulosonic acid (KDO). The outer core domain consists of sugar molecules such as galactose, N- acetylglucosamine, glucose and heptose, while the inner core domain consists of heptose and KDO (Braun et al., 2005) shown in Figure 2.7. Lipid A is a glucosamine molecule containing multiple acyl chains (fatty acid esters) that allow the LPS to integrate with and attach to the outer layer of the bacterial outer membrane (Raetz, 1996; Albus et al., 2001) shown in Figure 2.8. This component is the hydrophobic and highly conserved part of LPS in Gram-negative bacteria with acyl chain composition that differs in length and number between genera as well as growth conditions of the bacteria (Braun et al., 2005; Newman et al., 2007; Silipo et al., 2008). These variations may, therefore, result in antagonist functions of LPS. Lipid A is, nonetheless, reported to play a major role in triggering innate immune responses in animals (Raetz and Whitfield, 2002). However, this has been debated in plant research since the lipid A and O-antigen has also been shown to induce defence responses and genes/proteins in Arabidopsis (Erbs and Newman, 2012; Madala et al., 2010, 2012). 14
34 Figure 2.8: The structure of Gram-negative bacterial cell walls illustrating the inner and outer membranes containing LPS on the outer layer of the outer membrane (taken from Raetz and Whitfield, 2002). LPS - lipopolysaccharides, MDO - membrane-derived oligosaccharides, PPEtn - ethanolamine pyrophosphate LPS interaction in plants and animals LPS acts as a barrier that prevents the diffusion of host toxic components from entering into the bacterial cell and allows the bacteria to grow in harsh conditions of the plant environment (Dow et al., 1995). It also acts as a MAMP that plays an important role in triggering an innate immune response in both plants and animals (Bedini et al., 2005; Silipo et al., 2005). LPS, especially lipid A, has been shown to play a vital role in activating immune responses in animals (Raetz and Whitfield, 2002; Zeidler et al., 2004). Other LPS components, such as lipo-oligosaccharide (LOS) and the core-oligosaccharide, have also been shown to trigger an immune response in plants such as A. thaliana (Bedini et al., 2005; Silipo et al., 2005; Madala et al., 2010). The LPS from Burkholderia cepacia (LPS B.cep ) have been shown to elicit defence responses at a cellular level such as MAPK-activation, ROS and nitro-oxide (NO) production during the oxidative burst, as well as calcium (Ca 2+ ) flux in tobacco and Arabidopsis (Gerber et al., 2004; Zeidler et al., 2004; Gerber et al., 2006; Silipo et al., 2010). These responses are said to 15
35 be associated with the HR-related cell death, but LPS was not found to trigger programmed cell death in tobacco cells and suppressed the HR (Erbs and Newman, 2003; Zeidler et al., 2004; Silipo et al., 2010). According to Bedini et al. (2005), Arabidopsis recognises the O- chains of LPS, leading to activation of a gene expression (PR gene expression) response and suppression of HR associated with plant defences when using O-antigen polysaccharide elicitation. Similar experiments were conducted using lipo-oligosaccharide (LOS) of Xanthomonas campestris pv. campestris (Xcc). The Xcc LOS was shown to trigger defence responses in Arabidopsis, which includes the activation of defence-related genes (PR1 and PR2) as well as suppression of the HR (Silipo et al., 2005). Madala et al. (2005) extended upon these studies and postulated the existence of different receptors for the carbohydrate and lipid moieties of the LPS lipoglycan. Although LPS or various components thereof have been shown to induce defence responses in plants, the nature of the plant receptor(s) is still unclear, unlike in animals. Mammalians cells are more sensitive to lower concentrations of LPS than plants. In order to elicit defence responses in plants, LPS concentrations ranging from μg/ml can be used, unlike in mammalian cells that respond to low LPS concentrations in the pg to ng/ml range (Alexander and Rietschel, 2001; Zeidler et al., 2004). In mammalian cells, there are 13 TLRs that have been identified and are involved in recognising different MAMPs (Kawai and Akira, 2011). LPS binds to the Toll-like receptor 4 (TLR4), a plasma membrane protein similar to the Drosophila Toll family of receptors. LPS binds to the LPS-binding protein (LBP) forming a LPS-LBP complex, which is transported to the membrane where it interacts with a TLR4/MD-2 complex, which is then recruited to the membrane raft (lipid raft) and activates an immune response. Another co-receptor protein that LPS binds to is CD14, a soluble protein that is constitutively found in the membrane raft microdomains and is involved in innate immunity responses. This interaction results in activation of immune responses and tissue damage or septic shock (Shimazu et al., 1999; Aderem and Ulevitch, 2000; Triantafilou et al., 2002; Akira and Takeda, 2004). Both TLR4 and CD14 consist of multiple LRR domains which may be involved in protein-protein interactions (Kobe and Deisenhofer. 1994). This indicates that the membrane raft microdomains play an important role in LPS-activated innate immunity responses (Nomura et al., 2010). 16
36 2.3 The plant plasma membrane (PM) Plant cells contain specialised membrane systems that carry out particular functions like the plasma membrane (PM) which is composed of various lipids and proteins. It not only functions as a barrier to separate the cytoplasm from the extracellular environment, but is also involved in exchanging substances and information between the two environments (Takahashi et al., 2012). Furthermore, it plays a vital role in signal responses of biotic and abiotic stresses, ion transport, cell differentiation (Simons and Toomre, 2000; Takahashi et al., 2012), and endocytosis and exocytosis regulation (Parton and Richards, 2003; Salaün et al., 2004; Robatzek et al., 2006) Membrane structure In the early 1920s, the plasma membrane was initially hypothesised to consist of phospholipids that assemble to form bilayers, with hydrophobic tails arranged between the polar head groups facing towards the aqueous environment (Gorter and Grendel, 1925; Heller et al., 1993) as shown in Figure 2.9. Figure 2.9: The proposed lipid bilayer structure initially suggested by Gorter and Grendel (1925), illustrating phospholipid hydrophobic tails between the polar head groups that are exposed to water. 1 According to the Singer and Nicolson model, which was published in 1972, it was proposed that the plasma membrane is not only made of lipid bilayers, but contains a mixture of lipids (mainly phospholipids) and proteins (transmembrane and peripheral) that are embedded in 17
37 the membrane to form a fluid-mosaic model (Figure 2.10). This lipid membrane is believed to be a homogeneous fluid mixture that allows lateral diffusion and random distribution of proteins in the membrane, thereby allowing clustered organisation. Figure 2.10: The fluid-mosaic model according to Singer and Nicolson (1972), illustrating the lipid bilayer containing both peripheral and transmembrane proteins than are randomly distributed within the membrane. 1 Further investigations of the membrane were conducted by Mouritsen and Bloom (1984), supporting the fluid-mosaic model with regard to the distribution of lipids and proteins within the membrane. They proposed the mattress model (Figure 2.11), suggesting that the lipids and proteins indeed interact freely in the membrane with different hydrophobic lengths of molecules. The authors also suggested that the lipid bilayer thickness is about 5 nm, therefore, changes in the length of the hydrophobic core of membrane proteins leads to exposure of the hydrophobic proteins or lipid segments to water, resulting in the deformation of the lipid membrane due to unfavourable hydrophobic interactions known as hydrophobic matching. This phenomenon causes tension between lipids and proteins, resulting in certain lipid species accumulating around the proteins due to capillary forces, and leading to protein aggregation. 18
38 Figure 2.11: The proposed mattress model according to Mouritsen and Bloom (1984), illustrating the aggregation and clustering of proteins. 1 When studying biological membranes, one has to consider the phase behaviour of the components since it is temperature-dependent. When lipid membranes are exposed to different temperatures, there is a transition in lipid phase, resulting in changes in lipid distribution and leading to the formation of various domains and clusters. This then influences membrane functions such as regulation and signal transduction (Heerklotz, 2002; Munro, 2003) Lipid rafts in the model membrane The organisation of the lipid bilayer was further investigated through biochemical and biophysical studies of the membrane. As such, the plasma membrane was hypothesised to be composed of both liquid-ordered (l o ) and liquid-disordered (l d ) phases that coexist in model membranes (Zappel and Panstruga, 2008). Simons and Ikonen (1997) speculated that, due the presence of specific lipids such as sphingolipids and sterols that are clustered in high concentrations in various regions of the plasma membrane, those specific regions or domains should be referred to as lipid rafts (Figure 2.12). 19
39 Figure 2.12: The plasma membrane containing various regions of lipid rafts in a liquid-ordered (l o ) phase and non-raft regions in liquid-disordered (l d ) phase that coexist in the model membrane. Phospholipids, sphingolipids and sterols are the major lipid structures that play a role in the formation of the plasma membrane (taken from Bhat and Panstruga, 2005). 1 The term lipid rafts refer to regions in the plasma membrane that are enriched with high concentrations of sphingolipids and sterols, and are heterogeneous and highly dynamic (Simons and Ikonen, 1997; Simons and Toomre, 2000; Pike, 2006). These are organised into microdomains ( nm in diameter) that are not uniformally/randomly distributed but laterally separated and highly organised within various parts of the membrane containing specific proteins that are associated with the raft (Simons and Ikonen, 1997; Pike, 2006). Furthermore, the raft-associated proteins are localised within the rafts due to lipid-protein interactions (Keller and Simons, 1998). 20
40 Lipid rafts (sphingolipid- and sterol-enriched microdomains) are made of saturated fatty acid side chains of phospholipids together with long, saturated acyl chains of sphingolipids and cholesterol that allow tight packing of the lipids into a liquid-ordered phase that is less fluid, thereby conferring resistance to treatment with non-ionic detergents such as Triton X-100 at 4 C (Brown and Rose, 1992; Simons and Ikonen, 1997; Brown and London, 1998; Xu et al., 2001; Mongrand et al., 2004; Veatch and Keller, 2005; Zappel and Panstruga, 2008). Also, the phospholipids present within the rafts have side chains that contain saturated fatty acids in comparison to the phospholipids that are present in the non-raft regions of the membrane (Zappel and Panstruga, 2008) shown in Figure The lipid raft microdomains are formed through lipid-lipid interactions, where the sphingolipids such as glycosphingolipid, found on the exoplasmic or outer leaflet of the membrane bilayer interact with sterols such as cholesterol in mammalian cells and phytosterols in plant cells and function as spacers that fill the voids between sphingolipids (van Meer, 1989; Schroeder et al., 1991; Morrow et al., 1995; Simons and Ikonen, 1997; Brown and London, 1998). The regions between the rafts are occupied by highly unsaturated fatty acids of phospholipids and this region of the membrane forms a liquid-disordered phase that is highly fluid (Simons and Ikonen, 1997; Munro, 2003; Mongrand et al., 2004; Brown, 2006). These small rafts are able to form larger rafts upon stimulation and are stabilised through protein-lipid and protein-protein interactions (Brown and London, 1998; Pike, 2006; Mongrand et al., 2010). Currently, the term lipid raft is no longer used due to the presence of proteins within the membrane that also play a role in the formation and regulation thereof and, as such, the term membrane rafts is preferred (Brown and Rose, 1992; Pike, 2006; Lingwood and Simons, 2010) Detergent-resistant membranes (DRMs) The membrane rafts are more resistant or insoluble than phospholipids when treated with non-ionic detergents such as Triton X-100 at 4 C, due to their tight lipid and protein interactions in a liquid-ordered phase and is therefore referred to as detergent-resistant membranes (DRMs). This characteristic thus allows isolation of the microdomains/lipid rafts 21
41 from the total plasma membrane (Brown and Rose, 1992; Simons and Ikonen, 1997; Mongrand et al., 2004; Brown, 2006). Detergent solubilisation is the most commonly used method for studying microdomains (Brown and Rose, 1992; Lichtenberg et al., 2005), and is used for the isolation and purification of lipid raft microdomains enriched in the membrane through the use of Triton X-100 at 4 C coupled with a sucrose density gradient (Harder and Simons, 1997). The lipid phase behaviour of the lipid raft is also temperature-dependent (Brown, 2006). As such, the detergent concentration and temperature as well as the ratio of detergent to proteins can enhance the formation of an ordered phase of the rafts by reducing sphingolipids and sterols levels present in the liquid-ordered phase, thereby increasing the segregation of membrane components and resulting in changes in the lipid organisation as well as the raft size (Ahmed et al., 1997; Madore et al., 1999; Heerklotz, 2002; Munro, 2003; Simon-Plas et al., 2011; Tanner, 2011). The use of Triton X-100 at a non-physiological temperature of 4 C furthermore prevents complete solubilisation of the raft while causing an aggregation of proteins (Munro, 2003; Morandat and El Kirat, 2006). Other detergents include Brij 96 or - 98 and CHAPS that are used for the isolation of mammalian DRMs at a physiological temperature of 37 C (Campbell et al., 2004). The existence and nature of the microdomains, including their size, still remains unclear due to limitations in the isolation methods used to study the lipid rafts and the misinterpretation of the data generated (Heerklotz, 2002). In order to confirm their existence, biochemical and biophysical studies of the membrane rafts have been performed. This involves detergent solubilisation of the membrane using Triton X-100 at 4 C coupled with a sucrose density gradient as well as sterol-depletion by methyl-β-cyclodextrin (MβCD) (Heerklotz, 2002; Munro, 2003; Bhat and Panstruga, 2005; Nichols, 2005; Calder and Yaqoob, 2007; Keinath et al., 2010). Therefore, to confirm that the isolated fractions are rafts or DRMs, raft markers such as glycosylphosphotidylinositol (GPI)-anchored proteins (GAPs) and flotillin are used (Morrow et al., 1995; Christian et al., 1997; Calder and Yaqoob, 2007). The raft-associated proteins enriched within the DRMs in a l o phase are then identified by their free floating ability on a sucrose density gradient after solubilisation with Triton X-100 at 4 C (Brown and Rose, 1992; Bhat and Panstruga, 2005). The microdomains/lipid rafts can then be visualised using a fluorescence-quenching assay (Ahmed et al., 1997), fluorescence microscopy (Munro, 2003), fluorescence resonance energy transfer (FRET) (Pralle et al., 2000), protein 22
42 immunolocalisation (Oliferenko et al., 1999), fluorescence recovery after photo-bleaching (FRAP) (Kenworthy et al., 2004) and atomic force microscopy (Henderson et al., 2004). The PM microdomains or DRMs play a major role in signal transduction, and are also involved in endocytosis and exocytosis regulation (Figure 2.13), cell adhesion, actin cytoskeleton organisation, cell trafficking and pathogen entry/response (Brown and London, 1998; Simons and Toomre, 2000; Harris and Siu, 2002; Borner et al., 2004; Pike, 2006), but will be discussed in more detail in Section (Results and Discussion). The raft-associated proteins include G-proteins, protein kinase C, Src family kinases as well as GPI-anchored proteins that play a role in signal transduction pathways. These raft-associated proteins are covalently linked to the bilayer via the highly abundant glycosylphosphotidylinositol anchors, which were the first DRM-associated proteins to be identified (Brown and Rose, 1992; Munro, 2003; Borner et al., 2004; Calder and Yaqoob, 2007). Figure 2.13: The endocytosis and exocytosis membrane trafficking pathways in a plant cell (taken from Irani and Russinova, 2009). ER - endoplasmic reticulum, TGN - trans-golgi network, BFA - Brefeldin A, P - phosphate, Tyr - tyrosine, MVB - multivesicular body. 1 23
43 In this regard, PM- or DRM-associated proteins have been isolated from a wide range of plant species such as Arabidopsis thaliana and Nicotiana tabacum, followed by proteomic techniques as well as mass spectrometry for the identification of proteins (Borner et al., 2003; Mongrand et al., 2004; Morel et al., 2006; Laloi et al., 2007; Keinath et al., 2010). 2.4 Proteomics Defining proteomics The term proteomics refers to a complete analysis of proteins expressed in tissue, cells or organisms, particularly pertaining their structure and function. The complete set of expressed proteins at a particular time under defined conditions is known as the proteome. The term was coined by Wilkins et al. (1996) and is a blend between protein and genome, given that proteins play a vital role in the physiological metabolic pathways in cells (Blackstock and Weir, 1999; Pandey and Mann, 2000). Proteomics is comprised of many categories such as expressional, structural and functional proteomics (Blackstock and Weir, 1999; Pandey and Mann, 2000; Ng and Ilag, 2002). Expressional proteomics is the comparative analysis of proteins expressed under certain physiological changes in response to a variety of stresses, using techniques such as electrophoresis, protein chips and mass spectrometry. This approach can be used as a possible application to identify responses to a wide variety of stresses and diseases. Structural proteomics is the study of the three dimensional (3D) structure of proteins using techniques such as high throughput X-ray crystallography and nuclear magnetic resonance (NMR). Functional proteomics, on the other hand, is the study of protein-protein interactions using techniques such as the yeast 2-hybrid system, high throughput assays, ligand chips and motif assays (Blackstock and Weir, 1999; Pandey and Mann, 2000; Ng and Ilag, 2002). For the purpose of this study, the focus will mainly be on expressional proteomics. 24
44 2.4.2 The significance of proteomics Proteomic techniques offer the ability to identify hundreds of expressed proteins involved in the organisation, function and diversity as well as the dynamic range of cells or whole tissue. Such techniques allow one to observe stimulus-related changes in the expression profile of proteins as well as offering adequate information about the quantity of proteins (Zivy and Vienne, 2000). Previously, proteins were identified indirectly through transcriptomics, which is the analysis of mrna expression using the microarray technology (Bohnert et al., 2001; Kreps et al., 2002; Seki et al., 2002). The transcriptomics approach does, however, not give adequate information about the expressed proteins, due to the fact that change in mrna expression and the related protein-product do not always correlate (Gygi et al., 1999a; Thurston et al., 2005). Proteomics reveal more data than what DNA/RNA does, such as posttranslational modifications and isoforms, tissue location, subcompartment of cell, biological activity and sequestration for other functions as well as protein abundance (Futcher et al., 1999; Gygi et al., 1999a; Pandey and Mann, 2000) An overview of expressional proteomics Expressional proteomics is achieved by a series of procedures which begin with sample preparation, protein quantification, and protein separation either by gel-based or gel-free approaches, followed by staining/visualisation of proteins and eventually protein identification by mass spectrometry Protein preparation In proteomics, obtaining and handling of the protein sample is one of the initial vital steps (Pandey and Mann, 2000). The main objectives of sample preparation is to disrupt the cells to release the proteins either by gentle lysis methods such as freezing/thawing, detergents, enzymatic and osmotic means or by vigorous lysis methods such as a mortar and pestle, sonication (Wang et al., 2006). In the case of membrane isolation (specifically DRMs), nonionic detergents such as Triton X-100 are used together with protease inhibitors as well as sonication, while for cytoplasmic proteins isolation, a mortar and pestle can be used (Uemura et al., 1995; Borner et al., 2004; Campbell et al., 2004). 25
45 Protein quantification Protein quantification is used to measure the protein concentration in crude extracted samples, generally expressed in mg/ml or µg/µl, and is important to determine the quantity of the proteins for downstream applications. Methods for protein quantification include the Bradford- (Bradford, 1976), Biuret-, Lowry- and Amido Black assays. When using the Bradford assay, the reagent binds to the proteins, resulting in a colour change of brown to blue. The change in colour density is relative to the concentration of the protein. The absorbance of samples is then read at 595 nm. The Bradford assay is the most commonly used method because it is compatible with reducing agents, and is a simple, fast, cheap, sensitive and reproducible assay for quantifying proteins (Bradford, 1976). The protein samples are then separated by either gel-based or gel-free approaches Protein separation In gel-based proteomics, proteins are commonly separated by one-dimensional sodium dodecyl sulphate-polyacrylamide gel electrophoresis (1-D SDS-PAGE) or two-dimensional gel electrophoresis (2DGE), while in gel-free approaches, techniques such as multiple dimensional protein identification technology (MuDPIT) is used. MuDPIT separates a large number of peptides in 2-D liquid chromatography (Ye at al., 2007), while others include isobaric tags for relative and absolute quantitation (itraq), isotope-coded affinity tags (ICAT), and stable isotope labelling by amino acids in cell culture (SILAC) (Gygi et al., 1999b; Ong et al., 2002; Finehout and Lee, 2004; Ross et al., 2004) to name a few. This section, however, will mainly focus on the gel-based approaches which were applied in the current study. The 1-D SDS-PAGE is a type of gel electrophoresis used for the separation of proteins based on their molecular weight (MW) (Hames and Rickwood, 1990; Coligan et al., 2002). It is useful in evaluating proteins prior to the 2-D gel. The separation of proteins by 1-D SDS- PAGE has advantages in that nearly all proteins are soluble in SDS and allows easy visualisation of proteins that are very acidic or basic (Pandey and Mann, 2000). 26
46 The 2DGE is the most efficient way of separating proteins from complex protein mixtures (Blackstock and Weir, 1999). This is because its separation provides a good protein resolution and detection of post-translational modifications (Carpentier et al., 2008). The protein samples are separated in two stages: first dimension and second dimension. In the first dimension, separation of proteins is based on their isoelectric point (pi), defined as the ph at which a molecule such as protein has a net charge of zero, and achieved by isoelectric focusing (IEF) (Pandey and Mann, 2000; Westermeier, 2005). Protein samples are loaded on immobilised ph gradient (IPG) strips and separated by IEF on the basis of their net charge. As an electric current and ph gradient is applied to the IPG strips, the proteins migrate towards the opposite charge until the respective pi is reached without further migration. In the second dimension, proteins are separated based on their MW by SDS-PAGE (Hames and Rickwood, 1990; Coligan et al., 2002; Gorg et al., 2004) after being equilibrated in SDS equilibration buffer. The first step of equilibration is reduction of proteins by equilibration buffer containing dithiothreitol (DTT), which is used to reduce disulfide bonds of proteins. This is followed by an alkylation step using iodoacetamide equilibration buffer, which is an alkylating agent that covalently binds to cysteine thiol groups in order to prevent the reformation of the disulfide bonds of the proteins (Westermeier, 2005). After the second dimension, the protein gels are stained in order to visualise the protein spots which are then analysed with an image analyser, and those of interest are excised before identification by mass spectrometry (Pandey and Mann, 2000) as shown in Figure Figure 2.14: A schematic diagram illustrating the general flow for proteomics analysis in expressional proteomics (taken from Pandey and Mann, 2000). 1 27
47 In general, the reproducibility and ability to characterise entire proteomes, as well as the analysis and quantification of proteins are difficult (Pandey and Mann, 2000; Molloy and Witzmann, 2002; Baggerman et al., 2005; Lambert et al., 2005; Carpentier et al., 2008). Thus, although 2DGE is widely used for the separation of proteins, it has its own limitations and disadvantages. These include the fact that 2-D is very costly and it is difficult to resolve membrane proteins since many of these are hydrophobic. Furthermore, it is ph range limited and does not allow for the detection of low abundant proteins Protein staining/visualisation methods There are various commonly used staining methods for the visualisation of proteins in both 1- D and 2-D electrophoresis. These include the Coomassie Brilliant Blue (CBB), silver and fluorescent dye stains respectively (Westermeier and Marouga, 2005; Miller et al., 2006; Hurkman and Tanaka, 2007), of which the selection depends on downstream application. The Coomasie Brilliant Blue (CBB) stain is the preferred method of choice for most studies for the visualisation of proteins. This is because the CBB stain is compatible with mass spectrometry (MS) and is inexpensive but is, however, less sensitive compared to other stains such as fluorescent dyes (such as Sypro Ruby, Deep Purple TM ) and silver stain. The latter are very sensitive and easy to use, and are able to identify very low abundant proteins present at low concentrations. Fluorescent dyes are compatible with MS while the silver stain is not since it requires modification in order to avoid cross-linking agents and can also interfere with MS analysis as well as lead to inaccurate determination of protein spots, resulting in poor mass spectrometry results (Berggren et al., 2000; Mortz et al., 2001; Gorg et al., 2004; Wittmann-Liebold et al., 2006; Ball and Karuso, 2007; Chevalier, 2010). Following protein staining and visualisation, the proteins are then identified by MS Protein identification using mass spectrometry MS is an analytical tool used for measuring the mass-to-charge (m/z) ratio of a sample. By using this method, one can obtain qualitative and quantitative information of a sample (Willard et al., 1988; Blackstock and Weir, 1999; Finehout and Lee, 2004). This technique is the method used for the identification of proteins and for characterisation of post-translational 28
48 modifications such as phosphorylation, glycosylation, alkylation and ubiquitination as well as other modifications of proteins (Blackstock and Weir, 1999; Pandey and Mann, 2000). According to Pandey and Mann (2000), MS is very sensitive, has the ability to accommodate protein mixtures and can provide much higher throughput. It also depends on the gelseparated proteins digested with trypsin, a sequence-specific protease that recognises and cleaves proteins at the carboxylic terminus of the amino acids lysine or arginine, resulting in peptides. MS instruments consists of different components namely (1) an ion source such as electrospray ionisation (ESI) and matrix-assisted laser desorption/ionization (MALDI), (2) mass analyser such as time-of-flight (TOF), Fourier transform, magnetic sector, ion trap and quadrupole, and (3) a detector or collector (Mann et al., 2001; Finehout and Lee, 2004) shown in Figure For the purpose of the study, the ion source and mass analyser that will be discussed are MALDI-TOF-MS and tandem-ms. Figure 2.15: The different components that make up the mass spectrometer. The ion source ensures that the protein samples are ionised and enter a gas phase, while a mass analyser separates ions according to their mass-to-charge ratio (m/z) and an ion detector collects the presence of separated ions and stores them in a form of spectrum (taken from Finehout and Lee, 2004).1 MALDI-TOF-MS is a protein identification method coupled with peptide mass fingerprinting (PMF) (Pandey and Mann, 2000). MALDI-TOF can identify low levels of proteins and is suitable for automation. It is a technique used for the identification of protein spots after being excised and digested with a protease enzyme trypsin resulting in peptide fragments (Patterson, 2004; Westermeier, 2005). The peptide fragments are subjected to MALDI-TOF and the resulting peptide masses from the spectra are then submitted to search programs such as MASCOT ( or ProFound along with the enzyme used as well as the protein modifications present. The software then searches against a protein database such 29
49 as Swiss-Prot or National Centre for Biotechnology Information non-redundant (NCBInr), where the peptide masses are measured and correlated with known protein sequences from a database. This then results in a list of possible correctly identified proteins (Zhang and Chait, 2000; Dubey and Grover, 2001; Boeckmann et al., 2003). The positive match of the peptide can be increased by the use of tandem MS. In contrast to MALDI-TOF-MS, tandem MS, also known as MS/MS or MS 2, has two mass analysers in series with a collision cell in between the analysers (Figure 2.16). This technique can isolate individual ions, fragments the ions with the first (MS1) and second analyser (MS2), thereby obtaining structural and sequence information; in the case of a peptide, it will give amino acid sequences of the fragment. These sequences are then submitted to MASCOT where the sequences are compared to the known sequences from the database instead of using peptide masses, and thus increases the chances of positive protein identification due to the fact that it is highly sensitive and compatible with complex mixtures of proteins (Pandey and Mann, 2000; Dubey and Grover, 2001; Mann et al., 2001; Finehout and Lee, 2004; Westermeier, 2005). Figure 2.16: A schematic diagram illustrating the tandem mass spectrometry containing two mass analysers (MS1 and MS2) in series with a collision cell in between the analysers according to Finehout and Lee (2004).1 30
50 CHAPTER 3: MATERIALS AND METHODS 3.1 Plant material and treatment Plant growth Arabidopsis thaliana ecotype Colombia-O (wild-type) seeds were purchased from Lehle Seeds and stored at 4 C until being sowed in plant growth trays containing soil (Germination Mix). The trays were incubated at 22 C under a 9 h light/15 h dark cycle in a growth room and watered with distilled water (dh 2 O) every second day of the week. Seedlings were treated with 1:200 (v/v) dilution of Nitrosol Natural Organic Plant Food (Nitrosol) as required and constantly monitored for any contaminations until they were mature plants and ready to be treated with bacterial LPS (Figure 3.1). Figure 3.1: Arabidopsis thaliana plants grown in soil at 22 C under a 9 h light/15 h dark cycle until matured.1 31
51 3.1.2 Lipopolysaccharide (LPS) treatment After the plants had matured, they were treated by pressure-infiltration with 100 µg/ml lipopolysaccharide from Escherichia coli (LPS E.coli ) (Sigma-Aldrich), solubilised in milliq water, into the lower side of the leaves using a 1 ml syringe and left for 6 and 12 h periods as shown in Figure 3.2. The untreated leaves were used as experimental controls or 0 h. Following the treatment, plant leaves were flash frozen in liquid nitrogen and used for plasma membrane isolation as described in Section 3.2. Figure 3.2: Arabidopsis plants treated by pressure-infiltration with 100 µg/ml of LPS E.coli into the lower side of the leaves and left for 6 and 12 h preiods Plasma membrane (PM) isolation methods Various protocols were tested and adapted (Uemura et al., 1995; Peskan et al., 2000; Mongrand et al., 2004; Morel et al., 2006; Laloi et al., 2007; Zappel and Pantruga, 2008; Minami et al., 2009) in an attempt to isolate the plasma membrane (PM) and detergentresistance membranes (DRMs). Troubleshooting with other plant leaves/material such as tobacco, spinach and broccoli was included to optimise methodologies for subsequent use with Arabidopsis leaves. 32
52 3.2.1 Ultracentrifugation-based protocol Microsomal fraction isolation The isolation procedure was performed according to Uemura et al. (1995). All steps were performed on ice. A total of 50 g of Arabidopsis leaves (treated and untreated) were homogenised separately with a handheld homogeniser (CAT X120 homogeniser, CAT Scientific) in 150 ml homogenisation buffer (0.5 M sorbitol, 5 mm ethylenediaminetetraacetic acid (EDTA), 0.5% (w/v) bovine serum albumin (BSA, fatty acid free), 1.5% (w/v) polyvinylpolypyrrolidone (PVP: added freshly), 50 mm 3-morpholinopropane-1-sulfonic acid (MOPS)/potassium hydroxide (KOH) buffer, ph 7.6) containing complete mini EDTA free protease inhibitor (Roche Applied Biosciences, Mannheim, Germany - added according to manufactures' instrctions) to avoid protease activity. The homogenates were filtered through 2 layers of miracloth membrane (Calbiochem) to remove leaf debris. The filtered homogenates (HM) were centrifuged at xg for 15 min at 4 C to remove cell debris, intact nuclei, chloroplasts and mitochondria. The supernatants were collected and centrifuged using an ultracentrifuge (Beckman Coulter, Optima TM XE-100 Ultracentrifuge) at xg for 60 min at 4 C. The pellets were suspended in suspension medium (0.25 M sucrose, 10 mm KH 2 PO 4 /K 2 HPO 4, ph 7.8) and centrifuged at xg for 60 min at 4 C. The resulting pellets were collected, re-suspended in the suspension medium and used as the microsomal fraction (MF) which was then subjected to a two-phase system for plasma membrane isolation Plasma membrane isolation The isolation procedure was performed according to Uemura et al. (1995). The PM was isolated using an aqueous two-phase partitioning system (6.4 % (w/w) Dextran T-500 (Pharmacia), 6.4 % (w/w) polyethylene glycol (PEG)-3350 (Sigma). Six grams (wet weight) of the MF was loaded onto 24 g (wet weight) two-phase system in a 50 ml Falcon tube. Six millilitres of suspension medium was added into a new two-phase system. Both the systems (with or without MF) were mixed well by shaking the tubes. The systems were then centrifuged at xg for 3 min at 0 C to allow phase separation (step 1). The upper phase from the twophase system without MF was removed without disturbing the interface and the lower phase was saved and stored on ice. The upper phase (U1) from the two-phase system with MF was 33
53 transferred to the saved lower phase (step 2). The systems were mix well and centrifuged at xg for 3 min at 0 C. The procedure was repeated twice (step 3 and 4), resulting in a clear, whitish upper phase, in which the PM is enriched (Figure 3.3). The final upper phase (U3, from step 4) was transferred into ultracentrifuge tubes and at least 3 volumes of wash medium (0.25 M sucrose, 10 mm MOPS/KOH, ph 7.3) were added. The solution was mixed and centrifuged at xg for 60 min at 4 C. The resulting pellet was collected, re-suspended in wash medium and used as the PM fraction. Protein concentrations of all fractions, namely HM, MF and PM, were determined by the Bradford assay (Bradford, 1975). Figure 3.3: A step-wise isolation of the plasma membrane achieved by an aqueous two-phase partitioning system. U1, 2 and 3 - upper phases, U3 - upper phase in which the PM is enriched (taken from Larsson and Widell, 1981 cited in Hatti-Kaul, 2000). 1 34
54 Detergent resistant membrane (DRM) isolation The isolation involved treatment of PM samples with a non-ionic detergent, Triton X-100, followed by a sucrose discontinuous step-gradient or continuous gradient ultracentrifugation. A 2.5 mg (wet weight) PM protein sample was treated with 10% Triton X-100, at a detergent to protein ratio of 15:1, where the final detergent concentration was brought to 1%. The samples were incubated for exactly 30 min on ice, since longer incubation may result in nonspecific protein solubilisation. The detergent-treated samples were then transferred into ultracentrifugation tubes for subsequent isolation of DRMs. After treatment with Triton X-100, the samples were either overlaid with a continuous sucrose gradient [15-45% (w/v) sucrose, uing a peristaltic pump as a gradient mixer] or loaded onto a discontinuous sucrose step-gradient [15, 30 and 35% (w/v) sucrose]. The sucrose solutions were dissolved in TED buffer (1 mm dithiothreitol (DTT), 50 mm tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl), 3 mm EDTA, ph 8). The samples were then centrifuged at xg for 18 h at 4 C in an ultracentrifuge (Beckman Coulter, Optima TM XE-100). DRMs should be visible as a floating opaque ring/band at the low density detergent-resistance phase (at 15/45% or 30/35% interface) and 1 ml fractions, 0.5 ml above and 0.5 ml from below the centre of the band were collected Small-scale based protocol Microsomal membrane fraction isolation The small-scale protocol was adapted from Giannini et al. (1988). Three biological repeats and three independent experiments were conducted. A total of 20 g of Arabidopsis leaves (treated and untreated) were flash frozen in liquid nitrogen and transferred into a beaker. The leaves were homogenised separately with a handheld homogeniser (CAT X120 homogeniser) in 40 ml homogenisation buffer (250 mm sucrose, 3 mm EDTA, 10% (v/v) glycerol, 1% (w/v) BSA, 0.5% (w/v) PVP, 4 mm DTT, 250 mm potassium iodide (KI), 70 mm Tris-HCl, ph 7.8). The homogenates were filtered through 2 layers of miracloth membrane (EMDBiosciences, Merck) and transferred into 2 ml Eppendorf tubes. The homogenates were then centrifuged at xg for 3 min at 4 C in a microcentrifuge (Eppendorf centrifuge 35
55 5424R, Merck). The pellets were discarded and the supernatant was centrifuged at xg for 25 min at 4 C in order to obtain the microsomal membrane pellet, which was suspended in a suspension buffer (250 mm sucrose, 10% glycerol, 1 mm DTT (added freshly), 2 mm Tris-HCl, ph 7.2) for use in PM isolation Plasma membrane isolation The PM was isolated by a sucrose density step-gradient centrifugation approach. A total of 400 µl MF was layered on a discontinuous sucrose density step-gradient consisting of 25% (w/v) sucrose layered over 38% (w/v) sucrose (Figure 3.4). The sucrose solutions were dissolved in Tris-DTT buffer (1 mm DTT, 1 mm Tris-HCl, ph 7.2). The gradients were centrifuged at xg for 60 min at 4 C in a microcentrifuge (Merck). After centrifugation, the plasma membrane preparations were recovered at the 25% and 38% interface. Figure 3.4: Sucrose density step-gradient centrifugation, where the microsomal membrane fraction was layered on 25% sucrose overlaid on 38% sucrose for isolation of the plasma membrane fraction. 1 36
56 3.3 Quantification and validation of the isolated plasma membrane Protein quantification using the Bradford assay Protein concentration was determined using the Bradford assay (Bradford, 1975). A BSA (Sigma Chemical) stock solution of 2 µg/µl was prepared in urea buffer (6 M urea, 2 M thiourea, 4% CHAPS). The 2 µg/µl stock solution was used to prepare a dilution series ranging from to 1.5 µg/µl. The protein dye reagent was prepared by diluting 1 part dye reagent concentrate (BIORAD) with 4 parts dh 2 O. Ten microlitres of the various BSA standards as well as the protein samples (HM, MF and PM) were pipetted into separate wells in triplicate in a 96-well plate. Two hundred microlitres of the diluted dye reagent was added to each well. The samples and reagent were mixed thoroughly. The diluted dye reagent was used as a blank. The solutions were incubated for 5 min at room temperature and the absorbance was measured at 595 nm using a plate reader (BioTek). The absorbance readings of the standard solutions were used to construct a standard curve in order to determine the protein concentrations of the unknown protein samples The H + -ATPase assay The PM H + -ATPase assay was conducted according to Ligaba et al. (2004), in order to evaluate ATPase activity and so validate the isolated PM. Dipotassium phosphate (K 2 HPO 4 ) was used as a standard phosphate solution by preparing stock solutions ranging from nm. The assays were carried out in a 0.5 ml reaction volume (5 mm magnesium sulphate (MgSO 4 ), 50 mm potassium chloride (KCl), and 4 mm Adenosine 5'-triphosphate disodium salt hydrate (Na 2 -ATP), 0.02% (w/v) Brij 58 detergent, 30 mm Bis-Tris-Propane/2-(Nmorpholino)ethanesulfonic acid (BTP/MES), ph 6.5). A total concentration of 2 mg PM protein was added as source of the enzyme in order to initiate the reaction. The reaction was carried out at room temperature and stopped with 1 ml stopping reagent (2% (v/v) sulfuric acid (H 2 SO 4 ), 5% (w/v) sodium dodecyl sulphate (SDS), 0.7% (w/v) sodium molybdate) followed by 50 µl of 10% (v/v) ascorbic acid. The colour development of the phosphomolybdate complex proceeded for 5 to 30 min, and the absorbance was measured at 820 nm with a plate reader (BioTek). The H + -ATPase activity was calculated from the increase in absorbance due to the inorganic phosphate (Pi) liberation and expressed as nmol Pi/min/mg protein. 37
57 3.3.3 Western blot analysis Gel electrophoresis A total concentration of 10 µg protein of each fraction (HM, MF and PM) isolated at different time points (0, 6 and 12 h) from untreated and LPS E.coli -treated samples were electrophoresed per lane including the protein ladder (Spectra Multicolor broad range protein ladder, Thermo Scientific) on a 12% SDS-PAGE gel (Laemmli, 1970) (described in Section 3.4.2). The gels were electrophoresed at 90 V for 3 h and stopped when the samples reached at least 1 cm from the bottom of the gel. The gels were removed from the apparatus, and the stacking gel cut off before blotting Assembly and electro-blotting of gels The resolved gels were equilibrated in 1X transfer buffer (25 mm Tris, 192 mm glycine, 20% (v/v) methanol, ph 8.3). For each gel, two pieces of filter paper and 1 sheet of polyvinylidene difluoride (PVDF) transfer membrane (0.45 µm pore size, Thermo Scientific) were cut to fit each gel. The membranes were blotted in 100% methanol for a minute and then rinsed with dh 2 O. The membranes were then placed in transfer buffer, with care taken to avoid the membranes from drying out. The cassette was packed as described in Figure 3.5, placed in a tank and allowed to blot overnight at 25 V in 1X transfer buffer. 38
58 = cathode side Figure 3.5: Transfer cassette assembly unit of an immunoblot sandwich for protein transfer (Hoefer, GE Healthcare BioSciences AB) Detection of phosphorylated proteins After transfer of the proteins to the membrane, the gels were gently removed from the transfer cassettes, and placed in Fairbanks staining solution (Fairbanks, 1971) to verify the transfer of proteins. The membranes were incubated in 1% blocking solution (SuperBlock Blocking buffer in Tris-buffered saline (TBS), Thermo Scientific) for 1 h before the solution was discarded and the membranes incubated in anti-active MAPK pab, rabbit (ptepy) (Promega) primary antibody (1:5000 dilution) for 60 min. The antibody was discarded and the membranes were washed 3 times in 0.1% TBS-Tween20 (TBS-T: 50 mm Tris, 150 mm sodium chloride (NaCl), 0.1% Tween-20, ph 7.5) for 10 min. The membranes were then incubated in goat anti-rabbit conjugated to horseradish peroxidase (ImmunoPure Antibody, Pierce, Thermo Scientific), used as secondary antibody (1: dilution) for 60 min. The antibody was discarded and the membranes washed 4 times in 0.1% TBS-T for 15 min. Subsequently, membranes were incubated for 5 min in detection solution (A:B ratio of 1:1) (SuperSignal West Pico Chemiluminescent Substrate, Thermo Scientific) with gentle shaking. The rest of the detection procedure was performed in the dark room, using red lights only. Membranes were removed from the detection solution and placed in a plastic bag (care was taken to remove any trapped air bubbles) in an exposure cassette (Okamato PL-B TYPE, 39
59 24x30 cm). The X-ray film (Medical X-ray Blue film, AFGA) was cut to fit the membrane and placed on top, followed by a 30 min exposure. The film was removed and placed in a developer solution (20% (v/v) developer, AFGA) until the bands were visible. The film was then rinsed in stop solution (3% (v/v) acetic acid), followed by fixing in a 20% (v/v) fixer solution (AFGA), and finally rinsed in water for few minutes before being allowed to dry. 3.4 Proteomics Sample preparation Ten micrograms of all protein samples were mixed with 2X sample buffer (30% (v/v) glycerol, 20% (v/v) SDS, 0.1% (w/v) bromophenol blue, 15% β-mercaptoethanol, 1.5 M Tris, ph 6.8, Laemmli, 1970). The samples were pulse spun in a microcentrifuge and then boiled in a heating block at 95 C for 5 min before loading into the gel One-dimensional (1-D) SDS-PAGE Preparation of 12% resolving and 5% stacking gels One-dimensional (1-D) SDS-PAGE gels were electrophoresed using the Hoefer vertical electrophoresis system (9 10 cm gel size, cm dimension, Hoefer minive, Amersham). The gel casting plates were assembled before pouring the gel. The 12% resolving gel was prepared by mixing all the components (Table 3.1) according to Laemmli (1970) into a clean 15 ml Falcon tube. Tetramethylethylenediamine (TEMED) was added to the resolving gel solution and allowed to stand before pouring the gel into the casting plates. The gel was overlaid with water-saturated isopropanol and allowed to polymerise. The isopropanol was discarded and the gel rinsed with dh 2 O before pouring the stacking gel. The 5% stacking gel was prepared by mixing all the components (Table 3.1) into a clean 15 ml Falcon tube. The stacking gel was poured on top of the resolving gel and a 10 well comb (1 mm) was inserted immediately into the gel until polymerisation. 40
60 After the gels were polymerised, a protein ladder (Prosieve Quad Color Protein marker, Lonza) and protein samples were loaded onto the gels and electrophoresed for 3 h at constant voltage of 90 V until the samples reached the bottom of the gel. The gels were removed from the apparatus and placed in a plastic container to be visualised with the Fairbanks staining solution (Fairbanks, 1971) Fairbanks Coomassie Brilliant Blue staining The gels were visualised with Fairbanks staining solution in 4 sequential steps. Firstly, the staining solution A (25% (v/v) ispropanol, 10% (w/v) acetic acid, 0.05% (w/v) Coomassie Brilliant Blue (CBB) R-250) was added to cover the gels, heated in a microwave for 1 min and allowed to shake on a shaker (Gyro-rocker SSL3, Stuart) at room temperature for 15 min. The staining solution A was discarded. The gels were stained again with staining solution B (10% (v/v) isopropanol, 10% (w/v) acetic acid, 0.005% (w/v) CBB R-250), followed by staining solution C (10% (v/v) acetic acid, 0.002% (w/v) CBB R-250) as described above. The staining solution C was discarded. The gels were destained with staining solution D (10% (v/v) acetic acid) until excess dye was removed from the background gel matrix. The staining solution was discarded and the gels were rinsed with distilled water. Table 3.1: Preparation of 12% resolving and 5% stacking gels. 1 Components Volume (ml) for 12% gel Volume (ml) for 5% gel dh 2 O % Acrylamide/Bis-acrylamide solutions (29:1 ratio) 1.5 M Tris-HCl (ph 8.8) M Tris-HCl (ph 6.8) % SDS % Ammonium persulphate (APS) TEMED TOTAL
61 3.4.3 Acetone precipitation Half the volume of samples (HM, MF and PM) was precipitated by mixing 1 part sample and 4 parts absolute acetone (final concentration of 80% (v/v) acetone) for 60 min at -20 C. The samples were vortexed briefly and centrifuged at xg for 10 min at room temperature in a microcentrifuge (Merck). The supernatants were discarded without disturbing the pellet. The pellets were washed three times with 80% (v/v) ice-cold acetone by briefly vortexing and centrifuged at xg for 10 min per wash. The acetone was discarded and pellets air dried for 5 min at room temperature before re-suspending in 2X sample buffer. The samples were then electrophoresed by 1-D SDS-PAGE and 2DGE (Section and 3.4.4) Two-dimensional gel electrophoresis (2DGE) Rehydration of IPG strips A total protein concentration of 100 µg was used for each sample. The components in the preparation of the rehydration step were mixed in a clean 1.5 ml Eppendorf tube to a final volume of 125 µl, as indicated in Table 3.2, with trace amounts of bromophenol blue. The samples were mixed by vortexing and pulse spun in a microcentrifuge prior to loading onto separate slots of the Immobiline TM Reswelling Dry-Strip tray (GE Healthcare, USA). The non-linear IPG strips (7 cm, ph 3-10 or 4-7, ReadyStrip TM Immobilized ph Gradient (IPG) strip, BIO-RAD) were gently placed on top of the samples with the gel side facing down and in contact with the samples, and overlaid with mineral oil (BIO-RAD) to prevent evaporation of the samples and crystallization of urea. The strips were left to rehydrate overnight at room temperature. Table 3.2: Preparation of protein samples for rehydration. 1 Reagents Volume (µl) Protein sample x Ampholytes (BIO-RAD) 1.25 µl 50% (w/v) DTT 2 µl Urea buffer (Section 3.3.1) y Total 125 µl x volume of protein samples y volume of urea buffer 42
62 Isoelectrofocusing (IEF) of IPG strips After the rehydration step, the IPG strips were removed from the Immobiline TM Dry Strip Reswelling tray (GE Healthcare) using forceps and rinsed with dh 2 O in order to remove excess rehydration solution. The IPG strips were briefly blotted by resting the edges of the strips on filter paper to remove excess moisture. For the first-dimension separation, the strips were placed on the IPGphorII electrophoresis unit (Ettan TM IPGphor II TM, GE Healthcare) with the gel side facing up. The wicks were soaked with dh 2 O and placed on the ends of the strips to absorb excess salts and other impurities during the IEF electrophoresis. The IPG strips were overlaid with mineral oil (BIO-RAD) to avoid drying of IPG strips, and the IEF performed at 20 C using the conditions shown in Table 3.3 adapted from Ngara and Ndimba (2011). Table 3.3: Isoelectricfocusing conditions for 7 cm IPG strips. 1 Steps Volts Time Step h Step :00 h Step V/h Equilibration of IPG strips After the IEF was completed, the IPG strips were removed from the instrument and rinsed with dh 2 O to remove excess mineral oil (BIO-RAD). Thereafter, the strips were placed in separate wells on an equilibration tray (BIO-RAD) with the gel side facing up and 2 ml of DTT equilibration buffer (200 mg DTT, 6 M urea, 30% (v/v) glycerol, 2% (w/v) SDS, 0.1% (v/v) bromophenol blue, 50 mm Tris-HCl, ph 8.8) was added. The solution was left to shake gently on a shaker (Gyro-rocker SSL3, Stuart) at room temperature for 10 min. The DTT buffer was discarded and 2 ml of iodoacetamide equilibration buffer (250 mg iodoacetamide, 6 M urea, 30% (v/v) glycerol, 2% (w/v) SDS, 0.1% (w/v) bromophenol blue, 50 mm Tris- HCl, ph 8.8) was added and left to shake for another 10 min. The iodoacetamide was discarded and the strips then rinsed in 1X SDS-PAGE running buffer (25 mm Tris, 192 mm glycine, 0.1% SDS) before loading onto 2DGE. 43
63 Second dimension (2D) separation The gel casting plates were assembled during the IEF run. For each plate, a 12% resolving gel (Laemmli, 1970) was prepared in the same way as described in Section but without the stacking gel (Table 3.1). Four microlitres of protein ladder (Spectra Multicolor broad range protein ladder, Thermo Scientific) was blotted on a piece of filter paper and allowed to dry thoroughly before loading on top of the gel. The IPG strips and the protein ladder (Thermo Scientific) were loaded onto the gels and overlaid with 1% agarose sealing solution. Gels were electrophoresed at a constant voltage of 90 V until the samples reached the bottom of the gel. Following, the gels were removed from the glass sandwich and placed in a plastic container for staining using the Fairbanks protocol (Fairbanks, 1971). After visualisation, the protein spots of interest were further analysed by mass spectrometry. 3.5 Protein identification by mass spectrometry (MS) Sample preparation The Fairbanks-stained 1-D SDS-PAGE gel bands that were differentially expressed between the 0, 6 and 12 h LPS E.coli -treated PM of the precipitated samples, as well as from 6 h LPS E.coli -treated PM of the ultracentrifuge-based method (Sections ) were cut into small 1 x 1 mm cubes with a clean razor, while the 2DGE protein spots that were differentially expressed between the 6 h and 12 h LPS E.coli -treated PM were manually excised with a plastic pipette tip. The gel pieces were transferred into sterile 1.5 ml Eppendorf tubes and rinsed twice with milliq water for 10 min at room temperature. The water was discarded, and 50% (v/v) acetonitrile (ACN) was added to the gel pieces and incubated for 10 min. The ACN was then discarded, and 50 mm ammonium bicarbonate (ABC) was added and incubated for 10 min. The ABC was subsequently discarded, and the destained gel pieces were washed with a wash solution (50 mm ABC/50% (v/v) ACN) for 30 min. The wash solution was discarded and 100% (v/v) ACN was added to the gel pieces, followed by an incubation of 10 min at room temperature until the gel pieces turned white. Finally, the ACN was discarded and the dehydrated gel pieces were further dried under vacuum using a Speed 44
64 Vac centrifugal evaporator (Jouan, RC 10.09) for 20 min at room temperature (Thomas et al., 2010) Identification of proteins by MALDI-TOF-MS and LC-MS/MS All samples were sent to the Centre for Proteomic and Genomic Research (CPGR), University of Cape Town, Cape Town for analysis. The dried gel pieces were analysed by MALDI-TOF-MS, while the protein spots and cut-out lanes (Section 3.5.3) were analysed by liquid chromatography (LC)-tandem mass spectrometry (MS/MS). The analysis by LC- MS/MS was included in order to increase or allow the identification of low-abundant proteins that might not have been identified by MALDI-TOF-MS. For reduction and alkylation of the dried gel pieces, 2 mm TCEP (Tris(2-carboxyethyl) phosphine, Fluka) dissolved in 25 mm ABC was added to the gel pieces and incubated for 15 min at room temperature with agitation. The supernatant was discarded and 20 mm iodoacetamide (Sigma) was added to the gel pieces and incubated for 30 min in the dark. The supernatant was discarded, and in the case of samples run on LC-MS/MS, 0.1% w/v n-octyl-β-d-glucopyranoside (OGP, Sigma) dissolved in 40 mm ABC was added. The gel pieces were washed 3 times with 25 mm ABC for 15 min with agitation. The supernatant was discarded and 100% ACN was added to desiccate the gel pieces. The supernatant was discarded and the gel pieces were dried completely under vacuum using a Speed Vac. The gel pieces were rehydrated (digested) in 0.02 µg/µl trypsin (Promega) reconstituted in 50 mm ABC for peptide mass fingerprint (PMF) or in 0.1% OGP, 40 mm ABC and 10% ACN for LC analysis and incubated for 60 min. Excess trypsin solution was removed and 50 mm ABC was added to cover the gel pieces. The Eppendorf tubes containing the gel pieces were wrapped with parafilm to prevent evaporation and incubated for 18 h at 37 C. The supernatant was removed and transferred to fresh 1.5 ml Eppendorf tube (extract 1). A 0.1% (v/v) trifluoracetic acid (TFA, Sigma) was added to the gel pieces and the Eppendorf tubes were incubated for 60 min at 37 C. The supernatant was removed and combined with extract 1. The combined extracts were dried under vacuum using a Speed Vac, re-suspended in milliq water and again dried. The dried extracts were then re-suspended 0.1% TFA. The samples were purified and concentrated using C₁₈ZipTip according to manufacturer s instructions prior to analysis. One microlitre of the sample was spotted on a MALDI source plate and the remaining sample was stored at -80 C. 45
65 In case of protein spot samples, the peptides were analysed by LC-MS/MS using an Ultimate 3000 nanohplc (Thermo Scientific). Peptides were fractionated on an Acclaim Pepmap RSLC 75 µm X 15 cm, 2 µm particle size C18 column (Thermo Scientific) prior to analysis with MS. Spectra from peptides were searched against the Uniprot Arabidopsis Fasta database ( using MASCOT. The database search parameters were as follows: Deamidated (NQ), Oxidation (M) were used as variable modifications, Carbamidomethyl (C) was used as fixed modifications, maximum number of missed cleavages 1, peptide mass tolerance of 10 ppm and the peptide fragments set to 0.2 Da. For MALDI-TOF samples, the MALDI MS was performed using a 4800 MALDI-TOF system (AB SCIEX) with instrument control through 4000 Series Explorer. Parent spectra were acquired in reflector positive mode at a laser intensity of 3700 arbitrary units using 500 laser shots per spectrum. The scan range was m/z = The resulting peptide mass fingerprint data were searched against the Arabidopsis National Centre for Biotechnology Information non-redundant (NCBInr) protein database using the MASCOT search server (Matrix Science, which measures and correlates the peptide masses of the fragments with known protein sequences from a database. A probability-based MASCOT score was used to evaluate identifications, where only matches with a statistical significance threshold of p < 0.05 and a high molecular weight search (MOWSE) score of 62 were considered significant (further description of MASCOT scores can be found at The search parameters were similar to LC-MS/MS samples, but used a peptide mass tolerance of 150 ppm Extractive in-gel method A second approach for identifying proteins from 1-D gels was performed. An extractive ingel method was used where, instead of cutting out specific bands from a lane, the whole lane from each treatment was cut and divided into 5 pieces of 10 mm each. The gel slices were transferred into separate sterile 1.5 ml Eppendorf tubes (Figure 3.6). This approach was performed in order to improve the chance of identification of low-abundance proteins. The samples were then dried as described in Section and sent away for analysis by LC- MS/MS. 46
66 Figure 3.6: A schematic diagram illustrating the lane of a sample excised and divided into 5 pieces. 1 47
67 CHAPTER 4: RESULTS AND DISCUSSION 4.1 Treatment of Arabidopsis plants with lipopolysaccharide Arabidopsis thaliana plants were treated by pressure-infiltration with 100 µg/ml of LPS from Escherichia coli (LPS E.coli ) into the lower side of the leaves for 6 and 12 h, while the untreated leaves were used as experimental controls or 0 h. After the treatment the leaves were flash frozen in liquid nitrogen for membrane isolation (Section 3.1, Material and Methods). 4.2 Plasma membrane (PM) isolation and characterisation The PM is believed to be contain receptors, and in particular PRRs. For example, Keinath et al. (2010) used bacteria flagellin, flg22, as a MAMP to analyse detergent-resistant membrane (DRM) proteins from Arabidopsis cell suspensions and identified flg22-responsive proteins including the flagellin receptor, FLS2, which is an extracellular LRR-RLK located in the extracellular domain of the plasma membrane (Buchanan and Gay, 1996; Boller and Felix, 2009). In this regard, the DRMs are membrane rafts within the liquid-ordered phase of the PM that are resistant to treatment with non-ionic detergent such as Triton X-100 at 4 C (Simons and Ikonen, 1997; Brown and London, 1998; Mongrand et al., 2004; Zappel and Panstruga, 2008). As such, to address the challenge of elucidating the receptor/binding site for LPS perception in plants, it was deemed feasible to explore the PM and DRM component Ultracentrifugation-based method PM isolation is normally achieved by an aqueous two-phase partition system. In order to obtain a sufficient amount of PM, at least 100 g of starting material is required in the ultracentrifugation method for the isolation of the microsomal membrane fraction (MF) (Giannini et al., 1988) which, in turn, is used to isolate the PM prior to DRM enrichment. Various protocols were adapted (Uemura et al., 1995; Peskan et al., 2000; Mongrand et al., 2004; Morel et al., 2006; Laloi et al., 2007; Zappel and Panstruga, 2008; Minami et al., 2009) 48
68 in an attempt to isolate the PM and DRMs, and troubleshooting with other plant leaves/material such as tobacco, spinach and broccoli was included to optimise methodologies for subsequent use of Arabidopsis leaves. The isolation of DRMs involved treatment of the PM samples with a non-ionic detergent, Triton X-100, followed by a sucrose density discontinuous step-gradient or continuous gradient ultracentrifugation (Section 3.2.1, Materials and Methods). The successful isolation of the DRM fractions should have resulted in identification of a floating opaque ring/band at the low density detergent-resistance phase during sucrose gradient centrifugation (Peskan et al., 2000; Borner et al., 2003). Due to isolation of low amounts of PM, enrichment of DRM fractions was not successful, and therefore aborted due to non-reproducible results (i.e. the floating opaque ring/band was never achieved) (Figure 4.1). Similar results were observed in Figure A1 in the appendix. Figure 4.1: A comparative 1-D SDS-PAGE analysis of the 0 and 6 h LPS E.coli -treated Arabidopsis leaf proteomes subsequent to the ultracentrifugation-based method. 10 µg protein was loaded per well on a 12% SDS-PAGE gel and bands were visualised with an Aqua-stain solution (Vacutec). L - PageRuler Unstained Low Range Protein Ladder (Thermo Scientific), molecular weight (MW) markers are expressed in kilodalton (kda), HM - homogenate, MF - microsomal fraction and PM - plasma membrane.1 Since the attempt to isolate DRMs was never achieved, I then focussed on isolating the PM using a modified protocol (Uemura et al., 1995). A total of 50 g of starting materials from the 0 and 6 h LPS E.coli -treated samples were subjected to differential centrifugation (Section 3.2.1, 49
69 Material and Methods), where the PM was isolated by an aqueous two-phase partition system and separated by 1-D SDS-PAGE (Figure 4.2). The ultracentrifugation-based protocols were, however, discontinued since reproducible results were not obtained and thus the isolation of the PM was attempted by scaling down the starting materials for a small-scale isolation procedure without the use of an ultracentrifuge. Similar results were observed in Figure A2 in the appendix. Figure 4.2: A 1-D SDS-PAGE analysis of the 0 and 6 h LPS E.coli -treated Arabidopsis leaf proteomes subsequent to the ultracentrifugation-based method. 10 µg protein was loaded per well on a 12% SDS- PAGE gel and bands were visualised using the Fairbanks staining protocol. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. 1 50
70 4.2.2 Small-scale-based protocol In this study, the small-scale isolation protocol was the method of choice for PM isolation because it was effective and reproducible unlike the ultracentrifugation method. Here, the PM was isolated from Arabidopsis leaves using a procedure taken from Giannini et al. (1988) with an attainable amount of starting materials. The authors showed that the small-scale isolation method was as efficient as the ultracentrifugation method, and was supported by Abas and Lusching (2010). Thus, the protocol was eventually optimised for Arabidopsis leaves subsequent to troubleshooting with other plant leaves/tissue such as tobacco, spinach and broccoli. A total of 20 g of Arabidopsis leaves from 0, 6 and 12 h LPS E.coli -treated samples were homogenised separately, filtered through 2 layers of miracloth and subjected to differential centrifugation steps. The first centrifugation was to sediment the mitochondria and cell debris, while the second was for obtaining the microsomal membrane fraction (MF) as a pellet. The MF pellets were then resuspended in a suspension buffer (Section 3.2.2, Material and Methods) and used for the isolation of the PM fraction by a sucrose-step gradient centrifugation approach, which was obtained at 25%/38% interface (Figure 4.3) after 60 min at xg. Figure 4.3: Plasma membrane isolation from 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf samples. The PM was obtained at 25%/38% sucrose layers in a sucrose-step gradient centrifugation approach using a microcentrifuge. 1 51
71 4.3 Quantification and validation of the isolated plasma membrane Protein quantification using the Bradford assay The Bradford assay (Bradford, 1976) was the method of choice for this study because of its compatibility with reducing agents such as urea buffer (Westermeier, 2005) used to solubilise the PM. Furthermore, this assay is a simple, fast, cheap, sensitive and reproducible for quantifying proteins. Figure 4.4 illustrates a standard curve constructed using the absorbance readings of BSA standard solutions (0.125 to 1.5 µg/µl) prepared from a stock solution in urea buffer. The R 2 value for this standard curve was The absorbance reading of the unknown protein samples were measured at 595 nm using a spectrophotometer. The concentrations of the unknown samples were estimated from the standard curve prior to further analysis as described in subsequent sections. Also, the standard curve was constructed and the protein content for each of the three biological repeats determined. Figure 4.4: The standard curve constructed from the absorbance readings of the bovine serum albumin (BSA) standard solutions using the Bradford assay. Error bars represent standard deviation of three technical repeats. 1 52
72 4.3.2 The H + -ATPase assay The PM H + -ATPase is activated by variety of factors such as phospholipids, calcium, chaperones such as DNAJ homolog 3, and heat shock protein 40-like protein. H + -ATPase plays a vital role in ion and metabolite transport regulation across the PM (Serrano, 1990; Briskin and Hanson, 1992; Brault et al., 2004; Zhang et al., 2004; Kim et al., 2010). According to Serrano (1989), the PM H + -ATPase belongs to the P-type ATPase family since it undergoes phosphorylation during its catalytic cycle. The interaction between the phosphorylated threonine residue (Thr-947) on the C-terminal region with the protein results in H + -ATPase activation (Camoni et al., 2000; Fuglsang et al., 2007; Gevaudant et al., 2007). In plants, PM H + -ATPases pump protons from the cytosol to the extracellular space by utilising the energy from ATP hydrolysis and thus producing a ph gradient across the membrane. The H + -ATPase activity is generally determined in order to validate the success of the PM isolation from Arabidopsis leaves. The increase in the ATPase activity measured as the release of inorganic phosphate (Pi) as shown in Figure 4.5. According to Tomasi et al. (2009) the increase in PM H + -ATPase activity correlates to the changes in the protein amount that is associated with the PM. The reaction was allowed to continue for 5 to 30 min, after which activity was calculated from the released inorganic phosphate. Figure 4.5: Plasma membrane ATPase activity measured at different time intervals, as calculated from the released inorganic phosphate. Error bars represent standard deviation of three biological repeats. 1 53
73 4.3.3 Western blot analysis During PM isolation contaminants from upstream fractions is often encountered, with mitochondria and chloroplast proteins being the main sources (Widjaja et al., 2009). Western blot analysis was thus performed in order to further validate the success of the PM isolation from LPS E.coli -treated Arabidopsis leaves. Here, an anti-active MAPK (mitogen activated protein kinase) antibody was employed in order to detect any phosphorylated protein in the different fractions. In Figure 4.6, the MAPK activity was detected in both HM and MF fractions across all experimental groups, but not in 6 and 12 h LPS E.coli -treated PM samples. This is due to the fact that the MAPK activity occurs in the cytoplasm (Schaeffer and Weber, 1999), and should therefore not be detected in the PM. Although there are slight bands in the 0 h PM samples and some traces in the 6 h PM sample in comparison the 12 h PM sample, it may be due to non-specific binding or slight contamination when extracting PMs. Looking at the MAPK phosphorylation level across the time points, it is possible that the activity/phosphorylation level decreased after a certain time period. According to Gerber et al. 2004, they conducted a time study using tobacco cell suspensions treated with LPS from Burkholderia cepacia (LPS B.cep ). Here, the level of phosphorylated proteins in LPS B.cep - treated cells increased after 1 h of LPS treatment. In this study, it was observed that after 6 and 12 h, the activity had most likely diminished. Even so, these results, and particularly those of the 12 h samples, shows that the PM fraction has very little contamination compared to the other fractions (HM and MF), and was deemed suitable for downstream application. Similar results were observed in precipitated samples shown in Figure A3. Figure 4.6: Representative Western blot analysis of the homogenate (HM), microsomal fraction (MF) and plasma membrane (PM) of 0, 6 and 12 h LPS E.coli -treated Arabidopsis samples. A total protein concentration of 10 µg was loaded on a 12% SDS-PAGE gel and blotted onto a PVDF membrane. Proteins were detected with an anti-mapk antibody. 1 Another method of validating successful PM isolation from LPS E.coli -treated Arabidopsis leaves was through the identification of PM marker proteins. These markers are proteins that are located in the liquid-ordered phase of the PM microdomains known as lipid 54
74 raft/membrane raft (Figure 2.12, Section 2.3.2), and hence as DRM proteins (Simons and Ikonen, 1997; Brown and London, 1998; Mongrand et al., 2004; Zappel and Panstruga, 2008). The PM or DRM markers include proteins such as remorin, aquaporin, glycosyl phosphatidylinositol (GPI)-anchor protein and flotillin. These proteins were identified and will further be discussed in Section One-dimensional (1-D) SDS-PAGE An equal protein concentration of 10 µg of the HM, MF and PM fractions, respectively, was separated by 1-D SDS-PAGE and visualised using the Fairbanks stain (Fairbanks, 1971). In all cases, three biological repeats and three independent experiments were conducted, but only representative images are shown. The rest of the gels are shown in Figure A4 in the appendix. Figure 4.7 illustrates the results from un-precipitated and precipitated protein bands of control (0 h) and treated samples (6 and 12 h LPS E.coli ). An acetone precipitation step was carried out in an attempt to purify the proteins from a heterogeneous mixture of sucrose and lipids as well as to remove any contaminants such as mitochondrial and chloroplast traces, and to help concentrate the samples. After precipitation, more protein bands were observed, for example, when comparing the protein bands of the PM of all three time points with the un-precipitated samples. In particular, the bands were observed to be more concentrated between kda in the precipitated PM samples. In the case of the HM and MF, similar results were observed and notably in the un-precipitated samples the proteins resolved between kda shows only one band, but after precipitation two bands were clearly observed after Fairbanks CBB staining. 55
75 Figure 4.7: A comparative 1-D SDS-PAGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes prior- and subsequent to acetone precipitation following the small-scale isolation protocol. 10 µg protein was loaded per well on a 12% SDS-PAGE gels followed by visualisation using the Fairbanks staining solution. L - Prosieve Quad Color Protein marker (Lonza), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. 1 56
76 4.5 Two-dimensional gel electrophoresis (2DGE) A total protein concentration of 100 µg of each experimental group was focused using nonlinear 7 cm ReadyStrip TM IPG strips at a ph range of 3-10 (Figure 4.8) then reduced, alkylated and separated by SDS-PAGE. Since the ph range of 3-10 is a broad range, the protein spots were found to be resolved and clustered in the middle of the gel, where it was estimated to be within a ph range of 4-7. Clustering of the protein spots resulted in a reduction of a number of protein spots being visualised. The same protein concentration of each sample was then focused in a narrow ph range of 4-7 (Figure 4.9), after which more protein spots were visualised. In the narrow ph range of 4-7, differentially expressed protein spots can clearly be seen between the 6 and 12 h LPS E.coli -treated PM samples by visual inspection. Some of the differentially expressed spots are highlighted in Figure 4.9. The rest of the gels are shown in Figure A5 in the appendix. 57
77 Figure 4.8: Comparative 2DGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes separated on a broad ph range (ph 3-10) using a 7 cm IPG strip following the small-scale isolation protocol. A 100 µg protein was first focused and the strip was loaded on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific) and the MW markers are expressed in kda. 1 58
78 Figure 4.9: Comparative 2DGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes separated on a narrow ph range (ph 4-7) using a 7 cm IPG strip following the small-scale isolation protocol. A 100 µg protein was first focused and the strip was loaded on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda. Differences between the gels are highlighted with red bars. 1 59
79 4.6 Protein identification by MALDI-TOF and LC-MS/MS MALDI-TOF-MS is a technique used for protein identification coupled with peptide mass fingerprinting (PMF) (Pandey and Mann, 2000). Protein bands that were differentially expressed between the 6 and 12 h LPS E.coli -treated plant samples of PM of the precipitated samples (Figure 4.10), and the 0 and 6 h LPS E.coli -treated PM samples of the ultracentrifuge method (Figure 4.11), were analysed by PMF to see if any PM-related proteins could be identified using the aborted sample preparation approaches. Proten bands visualised by Fairbanks stain were excised and digested with a protease enzyme, trypsin, resulting in peptide mixtures of the proteins (Patterson, 2004; Westermeier, 2005). The resulting peptides were subjected to MALDI-TOF and the peptide masses then submitted to the MASCOT search serve ( and searched against a protein database National Centre for Biotechnology Information non-redundant (NCBInr), which measures and correlates the peptide masses of the fragments with known protein sequences from a database (Dubey and Grover, 2001). A total of 12 proteins out of 14 were found to be PM proteins and classified according to their functional categories. Of all the PM proteins identified, none had a MOWSE score higher than 62 except 2 proteins with unknown/uncharacterised functions (Table 4.1). A higher MOWSE score is used to regard a protein as significant with a statistical significance threshold of p < The rest of the 26 proteins were most likely non- PM contaminants, which also included the identification of MAP kinase protein (see Table A1 in the appendix). The reason for identifying proteins having a MOWSE score lower than the cut off value of 62 may be due to the fact that a mixture of protein bands were analysed, and each band may contain more than one protein. Therefore, the proteins that are highly abundant might have overshadowed the low abundant proteins, thereby having a higher score as compared to the low abundant proteins. Another reason may be that membrane proteins are underrepresented and difficult to resolve since many are hydrophobic as well as due to posttranslational modification of proteins, which may have resulted in a change in functional activity, location, and stability of the protein (Pandey and Mann, 2000; Rais et al., 2004). Nonetheless, these proteins with a low MOWSE score were found to be significant to the study because they were PM proteins triggered by/responsive to LPS E.coli. Therefore, more 2-D gels were electrophoresed and protein spots between the 6 h and 12 h LPS E.coli -treated PMs that were differentially expressed (Figure 4.12), were randomly selected in order to increase the score 60
80 as well as to simplify the identification of the complex mixtures of proteins. The samples were sent away for analysis by LC-MS/MS. Out of the 13 analysed protein spots, only 5 proteins were found to be PM proteins of which 4 proteins had a MOWSE score higher than 62 and were classified according to their functional categories. The remainder of the proteins were most likely contaminants from non-pm fractions (Table 4.2). Selecting Fairbanks CBB visualised protein bands for the identification of proteins resulted in very few PM proteins. Therefore, in order to confirm the identified proteins from the excised protein bands and to identify other low abundant proteins, as well as to validate the successful PM isolation from LPS E.coli -treated Arabidopsis leaves, another approach of identifying proteins from 1-D gels was performed. Using an extractive in-gel method, the whole lane from each treatment (0, 6 and 12 h LPS E.coli -treated samples) was excised and divided separately into 5 pieces instead of selecting certain bands from a lane of each treatment (Section 3.5.3). The samples were then sent away for analysis by LC-MS/MS. Figure 4.13 highlights the lane of each sample that was excised for analysis. Of all the identified proteins from all three samples, a total of 88 proteins were found to be PM proteins and classified according to their functional categories (Table 4.3), while the remainder were most likely non-pm contaminates which were not included in the table (see Table A2 in the appendix). 61
81 Figure 4.10: 1-D SDS-PAGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes prior- and subsequent to acetone precipitation using the small-scale isolation protocol. 10 µg protein was loaded per well on a 12% SDS-PAGE gel followed by visualisation using the Fairbanks staining solution. L - Prosieve Quad Color Protein marker (Lonza), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. The red blocks indicate where the bands of interest were excised for the identification of proteins by mass spectrometry. 1 62
82 Figure 4:11: 1-D SDS-PAGE analysis of 0 and 6 h LPS E.coli -treated Arabidopsis leaf proteomes using the ultracentrifugation-based isolation protocol. 10 µg protein was loaded per well on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. The red blocks indicate where the bands of interest were excised for the identification of proteins by mass spectrometry. 1 63
83 Figure 4.12: 2DGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes separated on a narrow ph range (ph 4-7) using a 7 cm IPG strip following the small-scale isolation protocol. 100 µg protein was first focused and the strip was loaded on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. The arrows with numbers indicate randomly selected differentially expressed protein spots of interest excised for the identification of proteins by mass spectrometry. The MW markers are expressed in kda. 1 64
84 Figure 4.13: 1-D SDS-PAGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes prepared for the extractive in-gel protein identification approach. 10 µg protein was loaded per well on a 12% SDS- PAGE gel. The gels were visualised using the Fairbanks staining solution. L - Prosieve Quad Color Protein marker (Lonza), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. The red blocks indicate where the lanes that were excised for the identification of proteins by mass spectrometry. 1 The identified Arabidopsis proteins are summarised in Table , which include the protein band or spot or lane, accession number, protein names, MOWSE score, peptide mass tolerance, % sequence coverage, theoretical mass and experimental mass. 65
85 Table 4.1: MALDI-TOF-MS identified Arabidopsis proteins from bands excised from 1-D SDS-PAGE of 6 and 12 h LPS E.coli -treated PM fractions arranged according to functional categories. 1 Protein band a Accession no. b Protein name MOWSE score Peptide mass tolerance (ppm) C % Seq. coverage d e MW (kda) Signalling (7) A1 gi Ras-related small GTP-binding protein-like A2 gi Phospholipase D P A4 gi Putative GTP-binding protein gi Serine/threonine-protein kinase gi Contains similarity to protein kinases gi Protein kinase family protein A6 gi Serine/threonine-protein kinase Membrane trafficking and transporters (2) A4 gi Vesicle-associated membrane protein * A6 gi ABC transporter-like protein * Membrane structure (3) A4 gi TPX2 (targeting protein for Xklp2) family protein * A5 gi Actin-binding FH2 (formin homology 2) family protein A6 gi Kinesin * Unknown function (2) A3 gi Unknown protein * A5 gi Uncharacterised protein a Protein band number in Figures 4.10 and 4.11 b National Centre for Biotechnology Information (NCBI) accession number. c Peptide mass tolerance of 150 ppm, where the 150 with an asterix (*) indicates the proteins identified without any modifications. d Percentage (%) sequence coverage. e MW (kda) refers to the molecular weight in kilodaltons. 66
86 Table 4.2: LC-MS/MS identified Arabidopsis protein spots excised from 2DGE of 6 and 12 h LPS E.coli -treated PM arranged according to functional categories.1 Spot no. a Accession no. b Protein name MOWSE score Peptide mass tolerance (ppm) Num. of sequences c MW (kda) d Signalling (1) 12 Q9ZU46 Leucine-rich repeat receptor-like protein kinase Membrane structure (2) 8 Q9SYT0 Annexin D P53492 Actin Defence (1) 13 O04309 Myrosinase-binding protein-like Unknown function (1) 12 Q8H7A6 Putative uncharacterised protein Contaminants/Non-PM proteins 1 Q0WVH4 Ribulose bisphosphate carboxylase small chain 2 Q8LE12 Outer membrane lipoprotein-like Q01667 Chlorophyll a-b binding protein O03042 Ribulose bisphosphate carboxylase large chain 5 Q93ZG8 AT4g39730/T19P19_ (Lipase/lipooxygenase) 6 P23321 Oxygen-evolving enhancer protein Q9S841 Oxygen-evolving enhancer protein B3LF87 At1g30230 (elongation factor 1-beta) P19366 ATP synthase subunit beta P19366 ATP synthase subunit beta a Protein spot number in Figures 4.12 b UniProt accession number. c Number of sequences. d MW (kda) refers to the molecular weight in kilodaltons. 67
87 Table 4.3: LC-MS/MS identified Arabidopsis proteins from lanes excised from 1-D SDS-PAGE of 0, 6 and 12 h LPS E.coli -treated PM fractions arranged according to functional categories. 1 Sample no. a Accession no. b Protein name MW (kda) C Signalling (53) S1, 2, 3 CDPK3_ARATH Calcium-dependent protein kinase 3 59 CDPKW_ARATH Calcium-dependent protein kinase CDPK9_ARATH Calcium-dependent protein kinase 9 60 CDPKL_ARATH Calcium-dependent protein kinase S1, 2 CDPK6_ARATH Calcium-dependent protein kinase 6 61 CDPKD_ARATH Calcium-dependent protein kinase S2, 3 LYM2_ARATH LysM domain-containing GPI-anchored protein 2 38 S1, 2, 3 REMO_ARATH Remorin 21 S1, 2 PLDG1_ARATH Phospholipase D gamma 1 96 S1, 2, 3 PLDA1_ARATH Phospholipase D alpha 1 92 S1, 2, 3 PLCD1_ARATH Phosphoinositide phospholipase C 1 64 S1, 2, 3 NPC3_ARATH Non-specific phospholipase C3 59 S1, 2, _ARATH like protein GF14 upsilon _ARATH like protein GF14 omega _ARATH like protein GF14 nu _ARATH like protein GF14 lambda _ARATH like protein GF14 epsilon 29 S1, 2, 3 PTP1_ARATH Protein-tyrosine-phosphatase PTP1 38 S1, 2, 3 2AAA_ARATH Serine/threonine-protein phosphatase 2A 65 S1, 3 BSL2_ARATH Serine/threonine-protein phosphatase BSL2 109 S1, 2, 3 P2C59_ARATH Probable protein phosphatase 2C S1, 3 P2C17_ARATH Probable protein phosphatase 2C S1 P2C17_ARATH Probable protein phosphatase 2C S1, 2, 3 Y1684_ARATH Probable LRR receptor-like serine/threonine-protein kinase 106 S1, 2, 3 Y5126_ARATH Probable serine/threonine-protein kinase 55 S1, 2, 3 CRK10_ARATH Cysteine-rich receptor-like protein kinase PERK1_ARATH Proline-rich receptor-like protein kinase PERK1 69 S1, 2 Y5838_ARATH Probable leucine-rich repeat receptor-like protein kinase 69 HSL1_ARATH Receptor-like protein kinase HSL1 109 S1, 2, 3 C0LGM6_ARATH Leucine-rich repeat receptor-like protein kinase (Fragment) 68 68
88 Table 4.3 cont. C0LGL2_ARATH Leucine-rich repeat receptor-like protein kinase (Fragment) 72 C0LGT4_ARATH Leucine-rich repeat receptor-like protein kinase (Fragment) 67 C0LGN6_ARATH Leucine-rich repeat receptor-like protein kinase (Fragment) 100 C0LGN3_ARATH Leucine-rich repeat receptor-like protein kinase (Fragment) 70 Y2706_ARATH Probable receptor-like protein kinase 69 S1, 2, 3 PTI12_ARATH PTI1-like tyrosine-protein kinase 2 41 PTI11_ARATH PTI1-like tyrosine-protein kinase 1 40 PTI13_ARATH PTI1-like tyrosine-protein kinase 3 46 S1, 2 SYT5_ARATH Synaptotagmin-5 63 S1 RAA1G_ARATH Ras-related protein RABA1g 24 S1, 2, 3 RAA1F_ARATH Ras-related protein RABA1f 24 S1 RAA1G_ARATH Ras-related protein RABA1g 24 S1, 2, 3 RAA2A_ARATH Ras-related protein RABA2a 24 RAA4B_ARATH Ras-related protein RABA4b 24 RAB1B_ARATH Ras-related protein RABB1b 23 RAE1D_ARATH Ras-related protein RABE1d 24 RABF1_ARATH Ras-related protein RABF1 22 RABG2_ARATH Ras-related protein RABG2 24 RAG3B_ARATH Ras-related protein RABG3b 23 RAG3C_ARATH Ras-related protein RABG3c 23 RAG3E_ARATH Ras-related protein RABG3e 23 RAG3F_ARATH Ras-related protein RABG3f 23 S1, 2, 3 CML12_ARATH Calmodulin-like protein Membrane trafficking and transporters (24) S1, 2, 3 FLOT1_ARATH Flotillin-like protein 1 52 S1, 2, 3 CLC3_ARATH Clathrin light chain 3 29 S1, 2, 3 SY121_ARATH Syntaxin SY122_ARATH Syntaxin S1, 2, 3 VAP11_ARATH Vesicle-associated protein VAP12_ARATH Vesicle-associated protein S1, 2, 3 DRP2A_ARATH Dynamin-2A 99 DRP2B_ARATH Dynamin-2B 100 DRP1A_ARATH Dynamin-related protein 1A 68 DRP1E_ARATH Dynamin-related protein 1E 70 S1, 2, 3 VTI11_ARATH Vesicle transport v-snare
89 Table 4.3 cont. S1, 2, 3 Q0WWE7_ARATH Vesicle-associated membrane protein 7C 25 Q681H0_ARATH Putative vesicle-associated membrane protein, synaptobrevin 7B 25 S1, 2, 3 PATL1_ARATH Patellin-1 64 PATL2_ARATH Patellin-2 72 S1, 2, 3 NSF_ARATH Vesicle-fusing ATPase 81 S1 AB36G_ARATH ABC transporter G family member S1, 2, 3 PIP21_ARATH Aquaporin PIP PIP11_ARATH Aquaporin PIP S1, 2, 3 VATB1_ARATH V-type proton ATPase subunit B1 54 VA0D2_ARATH V-type proton ATPase subunit d2 41 S1, 2, 3 NLTP5_ARATH Non-specific lipid-transfer protein 5 12 S1, 2, 3 ANXD1_ARATH Annexin D1 36 ANXD4_ARATH Annexin D4 36 Membrane structure (6) S1, 2, 3 ACT7_ARATH Actin-7 42 ADF3_ARATH Actin-depolymerising factor 3 16 S1, 2, 3 TBB7_ARATH Tubulin beta-7 chain 51 S1, 2, 3 KCA1_ARATH Kinesin-like protein KCA1 141 S1, 2, 3 FLA2_ARATH Fasciclin-like arabinogalactan protein 2 43 S1, 2 TET8_ARATH Tetraspanin-8 31 Defence (5) S1, 2, 3 MB31_ARATH Myrosinase-binding protein-like 48 S1, 2, 3 HIR1_ARATH Hypersensitive-induced response protein 1 31 HIR2_ARATH Hypersensitive-induced response protein 2 31 HIR3_ARATH Hypersensitive-induced response protein 3 31 HIR4_ARATH Hypersensitive-induced response protein 4 32 a Protein lane number in Figures 4.13, where S1 refers to sample 1 (0 h PM), S2 - sample 2 (6 h LPS E.coli PM) and S3 - sample 3 (12 h LPS E.coli PM). For example: S1, 2, 3 refers to proteins that are found in sample 1, 2 and 3. b UniProt accession number. c MW (kda) refers to the molecular weight in kilodaltons. Proteins highlighted in Blue - are proteins found in all samples. Purple - proteins found in sample 1 and 2. Green - proteins found in sample 1 and 3. Black - proteins found in sample 1 only. Red - proteins found in sample 2 and 3 and also represent PM markers. 70
90 4.7 Functional implication of the identified PM proteins in LPS-perception in Arabidopsis leaves The PM plays a vital role in signal transduction pathways, ion transport, cytoskeleton rearrangement, endocytosis and exocytosis regulation of proteins (Simons and Toomre, 2000; Parton and Richards, 2003; Salaün et al., 2004). A. thaliana plants were treated with LPS E.coli for 0, 6 and 12 h periods in order to identify proteins that are differentially expressed in the PM after treatment with this lipoglycan. The results obtained after proteomic analysis of the PM proteins were found to correlate with the results obtained in other studies (Borner et al., 2003; Borner et al., 2004; Gerber et al., 2004; Mongrand et al., 2004; Shahollari et al., 2004; Bhat and Panstruga, 2005; Morel et al., 2006; Laloi et al., 2007; Minami et al., 2009), and these protein were classified according to their functional categories namely signalling, membrane trafficking and transporters, membrane structure, and defence (Table ). PM isolation often encounters contamination, where mitochondria and chloroplast proteins are the major contributors (Widjaja et al., 2009). The identification of GPI-anchored proteins served to prove the successful isolation of PMs from LPS E.coli -treated Arabidopsis leaves. These proteins, including flotillin as well as remorin and aquaporin, are not only PM proteins but also DRM-associated and thus used as DRM markers (Morrow et al., 2002; Foster et al., 2003; Santoni et al., 2003; Bariola et al., 2004; Shahollari et al., 2004; Borner et al., 2004; Mongrand et al., 2004). The PM (and also DRM) markers are highlighted in red (Table 4.3) and were identified in all samples except for the GPI-anchored protein, which was identified only in samples 2 and 3 (6 and 12 h LPS E.coli -treated PM). Many raft-associated proteins are covalently linked to the bilayer via the highly abundant GPI anchors, which were the first DRM-associated proteins to be identified, hence used as a PM marker (Brown and Rose, 1992; Munro, 2003; Borner et al., 2004; Calder and Yaqoob, 2007). The identification of the other proteins mentioned below not only confirms the isolation of the PM, but hints towards possible involvement in LPS perception. 71
91 4.7.1 Signalling A large number of proteins identified were associated with signalling and included protein kinases, receptors and phosphatases. The protein kinases and receptors identified were calcium-dependent protein kinase (CDPK), serine/threonine-protein kinase, PTI1-like tyrosine-protein kinase and LRR-RLKs. Protein phosphatases include serine/threonineprotein phosphatase and protein-tyrosine-phosphatase, while other signalling proteins comprised GPI-anchored protein, remorin, phospholipase D, like protein, calmodulinlike protein, phosphoinositide phospholipase C, Ras-related small GTP-binding protein-like, and Ras-related proteins. Plants defend themselves against pathogenic organisms by recognising conserved microbial molecules, termed pathogen-/microbe-associated molecular patterns (P/MAMPs), using their PM-bound extracellular pattern recognition receptors (PRRs). This interaction results in a defence response known as P/MAMP-triggered immunity (P/MTI), and subsequently a set of downstream responses such as ion fluxes and protein phosphorylation (Felix et al., 1999; Nürnberger et al., 2004; Zipfel et al., 2004; Chisholm et al., 2006; Jones and Dangl, 2006; Zipfel, 2008; Boller and Felix, 2009). In this study, the bacterial LPS E.coli was used as a MAMP. Plant LRR- RLKs were identified in the PM in response to LPS treatment. These RLKs are transmembrane domains proteins containing a cytoplasmic kinase (Dangl and Jones, 2001; Fritz-Laylin et al., 2005). A plant PRR:MAMP-interaction results in the phosphorylation of proteins, observed by the identification of serine/threonine- and tyrosine-protein kinases, which are involved in transferring a phosphate group to the OH group of a protein for a specific function in a cell, such as signal transduction, as well as MAP kinase activation and calmodulin/calciumdependent protein kinase and synaptotagmin, which are calcium-binding proteins that play a role as calcium ions (Ca 2+ ) sensor. On the other hand, protein phosphatases such as serine/threonine- and tyrosine-phosphatases remove a phosphate group from the OH group of a serine/threonine and tyrosine protein (Manash and Anup, 2004; Harper and Harmon, 2005; Chapman, 2008; Ma et al., 2008; Shi, 2009). The activation of remorin protein, a PM/DRM marker, occurs through phosphorylation at threonine residues by the serine/threonine protein kinases. These proteins are associated with cytoskeleton formation and signalling during 72
92 pathogen attack (Reymond et al., 1996; Bariola et al., 2004; Mongrand et al., 2004). From these results, it is clear that the LPS E.coli resulted in both phosphorylation and dephosphorylation of proteins in response to treatment. This correlates well with the results obtained with previous work (Gerber et al., 2004; Gerber et al., 2006) where a time study was conducted using suspension cultured tobacco cells treated with LPS B.cep. Treatment with LPS B.cep resulted in phosphorylation and dephosphorylation of proteins and calcium ion influxes, where proteins such as calmodulin / calcium-dependent protein kinase, MAP kinase and like protein were identified. Similar results have been reported when bacteria flagellin (flg22), peptidoglycan and elongation factor Tu (EF-Tu) were used as MAMPs in Arabidopsis seedlings and leaves (Robatzek et al., 2006; Zipfel et al., 2004; Boudsocq et al., 2010). LPS E.coli -treatment also resulted in the identification of phospholipase D (PLD) in Arabidopsis leaves. Phospholipids are involved in a variety of functions such as plant defence responses, cytoskeletal rearrangements, signal transduction pathways and lipid degradation (Shukla and Halenda, 1991; Wang et al., 1993; Wang, 2002). PLDs act in the plasma membrane, where they hydrolyse phospholipids to form phosphatidic acid (PA) as a form of defence response after pathogen attack (Dennis et al., 1991). During signalling, PA phosphohydrolase enzyme metabolises PA into diacylglycerol which is involved in activation of G-proteins and protein kinases (Shukla and Halenda, 1991; McPhail et al., 1999). These phospholipase enzymes (PLD and phosphoinositide phospholipase C, Pi-PLC) can both cleave GPI-anchored proteins from the PM resulting in the release of phosphatidylinositol (PI)-linked protein, a soluble protein that is activated for signal transduction. After cleavage by the phospholipases, the protein can be recovered by treatment of the PM by non-ionic detergents, such as Triton X-100/114 at 4 C in the detergent-resistant membrane pellet. The GPI-anchored proteins are extracellular PM proteins located exclusively in the membrane raft. They consist of a phosphatidylinositol group linked to a carbohydrate moiety, glucosamine (Griffith and Ryan, 1999; Sherrier et al., 1999; Ikonen, 2001; Robinson et al., 2002; Chesebro et al., 2005; Watanabe et al., 2007). The GPI-anchored proteins are involved in signalling and cell surface remodelling (Sherrier et al., 1999). In the study conducted by Borner et al. (2003), they identified the GPI-anchored proteins in Triton X-114 phase partitioning treated with phosphatidylinositol-specific phospholipase C from Arabidopsis callus. 73
93 Ras-related small GTP-binding protein-like protein and Ras-related proteins are PMassociated proteins. Phosphorylation of these Ras-related proteins in response to extracellular signals result in Ras stimulating GDP to form GTP which then interacts with several downstream effector proteins, including serine/threonine protein kinase isoforms such as Raf in the cytoplasm, which in turn activate MAPK cascades (Geyer and Wittinghofe, 1997; Campell et al., 1998; Kolch, 2000; Dan et al., 2001) Membrane trafficking and transporters This category consists of proteins that are responsible for transporting cellular substances across the plasma membrane such as aquaporin, ABC transporter G family member, V-type proton ATPase subunit, and ABC transporter-like protein (Higgins, 2001). Membrane trafficking proteins play a role in delivering proteins and lipids to target membranes, and include flotillin-like protein, clathrin, dynamin, annexin, patellin, syntaxin, synaptotagmin, vesicle-fusing ATPase, vesicle-associated protein and vesicle transport v-snare: vesicleassociated membrane protein (VAMP)/synaptobrevin (Sollner et al., 1993; Gerke and Moss, 1997; Rosengarth et al., 2001; Jahn et al., 2003; Chapman, 2008). Treatment of Arabidopsis leaves with LPS E.coli resulted in the phosphorylation of certain proteins. For instance, the phosphorylation of aquaporin allows the diffusion of water across the plasma membranes (Johansson et al., 2000; Maurel et al., 2002). With regard to the trafficking of proteins and lipids from one membrane to another depends on the recognition of the correct target membranes by the vesicles. This movement is facilitated by the SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) which are classified as vesicle transport (v-snare) and target (t-snare). VAMP/synaptobrevin proteins and vesicle-fusing ATPase are classified as v-snare found on the synaptic vesicles and syntaxin proteins are t-snares are found on the pre-synaptic membrane. These v-snare and t- SNARE specifically interact for membrane fusion (Rothman, 1994; Hanson et al., 1997; Jahn and Scheller, 2006). In mammalian cells, during Ca 2+ ion influx, the fusion of syntaxin and synaptobrevin on the PM triggers a synaptic vesicle exocytosis, which allows Ca 2+ ions to pass from one cell to another. The protein involved in the exocytosis of Ca 2+ ions is synaptotagmin, a Ca 2+ sensor/lipid binding protein. Similar mechanisms have been observed in Arabidopsis plants (Sollner et al., 1993; Jahn et al., 2003; Chapman, 2008; Lewis and 74
94 Lazarowitz, 2010). Annexins are Ca 2+ and phospholipid binding proteins involved in membrane organization and membrane trafficking of Ca 2+ ions across membranes during Ca 2+ ion influxes (Gerke and Moss, 1997; Rosengarth et al., 2001). The Arabidopsis flotillin proteins are cholesterol-binding proteins, involved in the uptake of substances via the clathrin-independent endocytosis, which contains raft intermediates such as GPI-anchored proteins for downstream signalling (Glebov et al., 2006; Frick et al., 2007) and are often used as membrane raft markers. Membrane rafts are normally used by MAMPs like in the case of flg22 to enter into the cell through internalisation, because receptors are located/assembled within these rafts during signalling, which suggest a role for rafts in endocytic pathways (Oliferenko et al., 1999; Ikonen 2001; Morrow et al., 2002; Robatzek et al., 2006). These flotillin proteins are said to be involved in endocytic trafficking pathways after internalisation from the PM (Hansen and Nichols, 2009; Pust et al., 2010). In order for the clathrin-coated endocytosis or vesicle membrane must bud off from the PM to form their target membranes. The dynamin proteins assist in the budding off process of the newly formed membrane vesicle through GTP hydrolysis process, where dynamin forms a helix structure around the budding end of the membrane vesicle until the vesicle pitch off from the parent membrane (Marks et al., 2001; Hansen and Nichols, 2009). Robatzek et al. (2006) conducted a study where the FLS2 receptor was labelled with the green fluorescent protein (GFP) with the aim of demonstrating the cell membrane localisation of the FLS2 and its specific ligand-induced internalisation. FLS2 receptor internalisation is via phosphorylation by serine/threonine kinase (Gómez-Gómez et al., 2001). The binding of flg22 to the FLS2 receptor leads to the internalisation of FLS2 through the endocytic pathway facilitated by cytoskeleton proteins like actin that play a role in receptor-mediated endocytosis and dynamin. The endocytosis of FLS2 results in signalling, and after the endocytotic process, the FLS2 was then degraded by proteosomal or lysosomal pathways. This then strongly suggests that the LPS E.coli was internalised for signalling through similar endocytotic processes. In fact, Mgcina et al. (2014) reported on an endo- and exocytosis dependent pathway following LPS-binding studies in Arabidopsis protoplasts. 75
95 4.7.3 Membrane structure Cytoskeletal/microtubule rearrangement is essential in the expression of non-host resistance (Kobayashi et al., 1997). In the study by Kobayashi et al. (1992), barley coleoptile cells were inoculated with the non-pathogenic powdery mildew, Erysiphe pisi, and the authors found that the microtubules had re-arranged at the contact site in order to prevent entry of the organism into the cell. When inoculated with a pathogenic powdery mildew, Erysiphe graminis f. sp. hordei, the reorganisation of the microtubules were found to be much lower than when infected with a non-pathogenic strain. Similar results were observed when cultured parsley cells were attacked by Phytophthora infestans (Gross et al., 1993). In this study, LPS was shown to induce cytoskeletal/microtubule-responsive proteins such as actin, actin-binding / actin-depolymerising factor, tubulin, kinesin 2, kinesin-like protein, TPX2 (Targeting protein for Xklp2), fasciclin-like arabinogalactan protein and tetraspanin. Cytoskeleton rearrangement within the host cells prevents entry of pathogens into the cell during pathogen invasion. Certain pathogenic bacteria such as Clostridium botulinum are able to overcome this by producing toxins which result in cytoskeleton disorganisation. Actindepolymerising factor are microfilament proteins that control the actin assembly (Barth and Stiles, 2008; Van Troys et al., 2008; Lang et al., 2010). Tubulin, TPX2 and kinesin proteins are involved in microtubule rearrangement of the cytoskeleton. The TPX2 is involved in spindle assembly, where microtubules are nucleated in the region of the chromosomes in a Rho-GTP dependent manner. It interacts with the Auro A kinase and targets the kinesis protein 2 to spindle poles resulting in kinase activation (Wittmann-Liebold et al., 2000; Bayliss et al., 2003; Schatz et al., 2003; Sardon et al., 2008). Fasciclin-like arabinogalactan proteins are anchored to the GPI and are involved in cell adhesion and signalling. Furthermore, fasciclin proteins assist in the cell to cell interactions (Elkins et al., 1990; Sherrier et al., 1999; Schultz et al., 2000; Showalter, 2001). The identification of these proteins could indicate that when Arabidopsis leaves were treated with LPS E.coli, these cytoskeletal proteins were activated in order to prevent entry of bacteria (as a result of the recognition of the conserved MAMP) into the cell. Since these proteins reside in the PM, they may also be at the site where the LPS signalling molecules or the 76
96 receptor(s) are situated, which may confirm that the interaction between the plant and LPS represents non-host resistance. Gerber et al. (2006) identified the cytoskeletal tubulin from suspension cultured tobacco cells treated with LPS B.cep, confirming the study conducted by Kobayashi et al. (1997). This cytoskeletal tubulin protein is reported to be associated with non-host resistance and resulted in rearrangement of microtubules Defence Plants defend themselves against pathogenic microorganisms, often by recognition of the pathogen surface molecules (P/MAMPs), in this case LPS, by plant PRRs or effectors by plant R proteins. The recognition initiates signal transduction pathways resulting in the activation of defence responses such as the hypersensitive response (Hammond-Kosack and Jones, 1997; Jones and Dangl, 2006; Zipfel, 2008). Hypersensitive-induced response (HIR) proteins are found on the PM and are involved in programmed cell death known as the HR. During pathogen attack, the HIR proteins interact with LRR proteins in order to trigger a hypersensitive cell death as a form of defence. This interaction was observed in the study by Zhou et al. (2010), where Arabidopsis and rice plants were inoculated with the bacterial Pseudomonas syringae pv. tomato DC3000 pathogen. The interaction between the pathogen and plant was found to be localised on the PM. However, the HR can be triggered by both P/MAMP-triggered immunity and effector-triggered immunity (Section 2.1.2, Literature Review). Myrosinase-binding proteins play a role in plant defence against pathogens. These proteins are activated through disruption of plant tissue. During tissue damage, glucosinolates, which are secondary metabolites, are degraded by thioglucosidases (also known as myrosinases) releasing toxic compounds such as epithionitriles, amines, isothiocyanates, nitriles, oxazolidine-2-thione and thiocyanates. In this regard, there are over 23 different glucosinolates found in Arabidopsis (Rask et al., 2000; Eriksson et al., 2002; de Vos et al., 2008). Exposure of a plant to a P/MAMP can up-regulate the expression of the components involved in its perception (Sanabria et al., 2012). It can therefore be speculated that LPS E.coli perception and signalling occur in the PM (and possibly within DRMs/lipid rafts) due to the proteins induced and identified after LPS E.coli treatment being similar to those identified after treatment with LPS B.cep and flg22 (Gerber et al., 2004; Gerber et al., 2006; Keinath et al., 77
97 2010). The bacterial flg22 receptor, FLS2, was reported within the DRMs of the PM as a LRR-RLK (Keinath et al., 2010). This, then strongly suggests that LPS receptors may be localised within these PM domains. 78
98 CHAPTER 5: CONCLUSION In this study, it was shown that the use of an ultracentrifuge (ultracentrifugation-based method) to successfully isolate the PM, which requires a large amount of starting material and is time consuming, is not necessarily essential. Here, a small-scale isolation protocol was preferred and, with even small amounts of starting material, yielded reproducible results. Both the methods are, however, prone to contamination, with mitochondrial and chloroplast proteins being the major contributors. Using the small-scale protocol, isolation of the PM from LPS E.coli -treated Arabidopsis leaves was successful. This was illustrated by the identification of PM (and also DRM) markers such as GPI-anchored, flotillin, remorin and aquaporin as well as other PM-localised proteins that are involved in variety of functions such as signalling, membrane trafficking and transporting, membrane structure, and defence. These proteins were found to correlate well with previous studies. Isolation of the PM was also confirmed by assaying the H + -ATPase enzyme as a PM-associated marker. The PM proteins were analysed by 1-D SDS-PAGE and 2DGE, and identified by mass spectrometry. MALDI-TOF MS identification of proteins from gel bands resulted in few (12) PM-localised proteins due to the fact that many are intrinsic membrane proteins and underrepresented therefore, difficult to resolve since they are expressed in low abundance as well as the complexity of gel bands. Protein spots from 2-D gels were also analysed, but resulted in few (5) proteins that were PM-localised. An extractive in-gel method was thus employed, where the whole lane instead of selected bands was analysed, and resulted in an increased number (88) of proteins that are PM-localised as identified using LC-MS/MS. This method was both successful in improving the number of identified low abundant proteins and confirming those identified from the excised protein bands/spots. In this study, LPS E.coli was shown to cause changes in PM proteins that are similar to those identified following treatment with LPS B.cep and flg22, as well as the identification of PM (and DRM) markers (Gerber et al., 2004; Gerber et al., 2006; Keinath et al., 2010). Thus, it 79
99 can be concluded that it is most likely that LPS perception and signal transduction occurs within these PM domains, particularly based on the identification of LRR-RLKs which supports Keinath et al. (2010), who identified the flg22 LRR-RLK receptor, FLS2, within the DRMs of the Arabidopsis PM. It is also suggested that the LPS signalling occurs through receptor(s) internalisation via endo- and exocytosis dependent pathways based on the study by Robatzek et al. (2006) and Mgcina et al. (2014). 80
100 REFERENCES Abas, L. and Luschnig, C. (2010) Maximum yields of microsomal-type membranes from small amounts of plant material without requiring ultracentrifugation. Analytical Biochemistry, 401: Aderem, A. and Ulevitch, R.J. (2000) Toll-like receptors in the induction of the innate immune response. Nature, 406: Ahmed, S.N., Brown, D.A. and London, E. (1997) On the origin of sphingolipid-cholesterol rich detergent-insoluble domains in cell membranes: Physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry, 36: Akira, S. and Takeda, K. (2004) Toll-like receptor signalling. Nature, 406: Akira, S., Uematsu, S. and Takeuchi, O. (2006) Pathogen recognition and innate immunity. Cell, 124: Albus, U., Baier, R., Holst, O., Pühler, A. and Niehaus, K. (2001) Suppression of an elicitorinduced oxidative burst reaction in Medicago sativa cell cultures by Sinorhizobium meliloti lipopolysaccharides. New Phytologist, 151: Alexander, C. and Rietschel, E.T. (2001) Bacterial lipopolysaccharides and innate immunity. Journal of Endotoxin Research, 7: Alström, S. (1991) Induction of disease resistance in common bean susceptible to halo blight bacterial pathogen after seed bacterisation with rhizosphere pseudomonads. The Journal of General and Applied Microbiology, 37: Baggerman, G., Vierstraete, E., De Loof, A. and Schoofs, L. (2005) Gel-based versus gelfree proteomics: a review. Combinatorial Chemistry and High Throughput Screening, 8: Ball, M.S. and Karuso, P. (2007) Mass spectral compatibility of four proteomics stains. Journal of Proteome Research, 6: Bariola, P.A., Retelska, D., Stasiak, A., Kammerer, R.A., Fleming, A., Hijri, M., Frank, S and Farmer, E.E. (2004) Remorins form a novel family of coiled coil-forming oligomeric and filamentous proteins associated with apical, vascular and embryonic tissues in plants. Plant Molecular Biology, 55:
101 Barth, H and Stiles, B.G. (2008) Binary actin-adp-ribosylating toxins and their use as molecular Trojan horses for drug delivery into eukaryotic cells. Current Medicinal Chemistry, 15: Bayliss, R., Sardon, T., Vernos, I. and Conti, E. (2003) Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Molecular Cell, 12: Bedini, E., De Castro, C., Erbs, G., Mangoni, L., Dow, J. M., Newman, M-A., Parrilli, M. and Unverzagt, C. (2005) Structure-dependent modulation of a pathogen response in plants by synthetic O-antigen polysaccharides. Journal of the American Chemical Society, 127: Berggren, K., Chernokalskaya, E., Steinberg, T.H., Kemper, C., Lopez, M.F., Diwu, Z., Haugland, R.P. and Patton, W.F. (2000) Background-free, high sensitivity staining of proteins in one- and two-dimensional sodium dodecyl sulphate-polyacrylamide gels using a luminescent ruthenium complex. Electrophoresis, 21: Bhat, R.A. and Panstruga, R. (2005) Lipid rafts in plants. Planta, 223: Bittel, P. and Robatzek, S. (2007) Microbe-associated molecular patterns (MAMPs) probe plant immunity. Current Opinion in Plant Biology, 10: Blackstock, W.P. and Weir, M.P. (1999) Proteomics: quantitative and physical mapping of cellular proteins. Trends in Biotechnology, 17: Boeckmann, B., Bairoch, A., Apweiler, R., Blatter, M. C., Estreicher, A., Gasteiger, E., Martin, M. J., Michoud, K., O Donovan, C., Phan, I., Pilbout, S. and Schneider, M. (2003) The SWISS-PROT protein knowledge base and its supplement TrEMBL in Nucleic Acids Research, 31: Bohnert, H.J., Ayoubi, P., Borchert, C., Bressan, R.A., Burnap, R.L., Cushman, J.C., Cushman, M.A., Deyholos, M., Fischer, R., Galbraith, D.W., Hasegawa, P.M., Jenks, M., Kawasaki, S., Koiwa, H., Kore-eda, S., Lee, B-H., Michalowski, C.B., Misawa, E., Nomura, M., Ozturk, M., Postier, B., Prade R., Song, C-P., Tanaka,Y., Wang, H. and Zhu, J-K. (2001) A genomics approach towards salt stress tolerance. Plant Physiology and Biochemistry, 39: Boller, T. and Felix, G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annual Review of Plant Physiology, 60: Boller, T. and He, S.Y. (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science, 324:
102 Borner, G.H.H., Lilley, K.S., Stevens, T.J. and Dupree, P. (2003) Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A proteomic and genomic analysis. Plant Physiology, 132: Borner, G.H.H., Janine-Sherrier, D., Michaelson, L.V., Hawkins, N.D., MacAskill, A., Napier, J.A., Beale, M.H., Lilley, K.S. and Dupree, P. (2004) Analysis of detergent-resistant membranes in Arabidopsis: evidence for plasma membrane lipid rafts. Plant Physiology, 137: Boudsocq, M., Willmann, M.R., McCormack, M., Lee, H., Shan, L., He, P., Bush, J., Cheng, S.H. and Sheen, J. (2010) Differential innate immune signalling via Ca 2+ sensor protein kinases. Nature, 464: Bradford, M.M. (1975) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: Brault, M., Amiar, Z., Pennarun, A.M., Monestiez, M., Zhang, Z., Cornel, D., Dellis, O., Knight, H., Bouteau, F. and Rona, J.P. (2004) Plasma membrane depolarization induced by abscisic acid in Arabidopsis suspension cells involves reduction of proton pumping in addition to anion channel activation, which are both Ca 2+ dependent. Plant Physiology, 135: Braun, S.G., Meyer, A., Holst, O., Pühler, A. and Niehaus, K. (2005) Characterization of the Xanthomonas campestris pv. campestris lipopolysaccharide substructures essential for elicitation of an oxidative burst in tobacco cells. Molecular Plant-Microbe Interactions, 18: Briskin, D.P. and Hanson, J.B. (1992) How does the plant plasma membrane H + -ATPase pump protons? Journal of Experimental Botany, 43: Brown, D.A. (2006) Lipid rafts, detergent-resistant membranes and raft targeting signals. Physiology, 21: Brown, D.A. and London, E. (1998) Functions of lipid rafts in biological membranes. Annual Review of Cell and Developmental Biology, 14: Brown, D.A. and Rose, J.K. (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell-surface. Cell, 68: Buchanan, S.G. and Gay, N.J. (1996) Structural and functional diversity in the leucine-rich repeat family of proteins. Progress in Biophysics and Molecular Biology, 65: Calder, P.C. and Yaqoob, P. (2007) Lipid rafts-composition, characterization and controversies. Journal of Nutrition, 137:
103 Camoni, L., Iori, V., Marra, M. and Aducci, P. (2000) Phosphorylation-dependent interaction between plant plasma membrane H + -ATPase and proteins. Journal of Biological Chemistry, 275: Campbell, M.A., Hatfield, W.T. and Heyer, L.J. (2004) The raft-promoting property of virion-associated cholesterol, but not the presence of virion-associated Brij 98 rafts, is a determinant of human immunode type 1 infectivity. Journal of Virology, 78: Campos, H., Cooper, M., Habben, J.E., Edmeades, G.O., and Schussler, J.R. (2004). Improving drought tolerance in maize: A view from industry. Field Crops Research, 90: Caroff, M., Karibian, D., Cavaillon, J. and Haeffner-Cavaillon, N. (2002) Structural and functional analyses of bacterial lipopolysaccharides. Microbes and Infection, 4: Carpentier, S.C., Coesmans, B., Podevin, N., Laukens, K., Witters, E., Matsumura, H., Terauchi, R., Swennen, R. and Panis, B. (2008) Functional genomics in a non-model crop: transcriptomics or proteomics? Physiologia Plantarum, 133: Chapman, E.R. (2008) How does synaptotagmin trigger neurotransmitter release? Annual Review of Biochemistry, 77: Chevalier, F. (2010) Highlights on the capacities of "gel-based" proteomics. Proteome Science, 8: Chesebro, B., Trifilo, M., Race, R., Meade-White, K., Teng, C., LaCasse, R., Raymond, L., Favara, C., Baron, G., Priola, S., Caughey, B., Masliah, E. and Oldstone, M. (2005) Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science, 308: Chisholm, S.T., Coaker, G., Day, B. and Staskawicz, B.J. (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell, 124: Christian, A.E., Haynes, M.P., Phillips, M.C. and Rothblat, G.H. (1997) Use of cyclodextrins for manipulating cellular cholesterol content. Journal of Lipid Research, 38: Coligan, J.E., Dunn, B.M., Ploegh, H.L., Speicher, D.W., Wingfield, P.T. and Taylor, G. (2002) Current Protocols in Protein Science. New York: John Wiley and Sons, Inc, pp Cui, H., Xiang, T. and Zhou, J.M. (2009) Plant immunity: a lesson from pathogenic bacterial effector proteins. Cellular Microbiology, 11:
104 Dan, I., Watanabe, N.M. and Kusumi, A. (2001) The Ste20 group kinases as regulators of MAP kinase cascades. Trends in Cell Biology, 11: Dangl, J.L. and Jones, J.D.G. (2001) Plant pathogens and integrated defence responses to infection. Nature, 411: Deepak, S.A., Ishii, H. and Park, P. (2006) Acibenzolar-S-methyl primes cell wall strengthening genes and reactive oxygen species forming/scavenging enzymes in cucumber after fungal pathogen attack. Physiological and Molecular Plant Pathology, 69: Delaney, T. P., Friedrich, L. and Ryals, J. A. (1995) Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proceedings of the National Academy of Sciences of the United States of America, 92: Dennis, E.A., Rhee, S.G., Billah, M.M. and Hannun, Y.A. (1991) Role of phospholipases in generating second messengers in signal transduction. FASEB Journal, 5: de Vos, M., Kriksunov, K.L and Jander, G. (2008) Indole-3-acetonitrile production from indole glucosinolates deters oviposition by Pieris rapae. Plant Physiology, 146: Dixon, R.A. and Lamb, C.J. (1990) Molecular communication in interactions between plants and microbial pathogens. Annual Review of Plant Physiology and Plant Molecular Biology, 41: Dixon, R.A., Harrison, M.J. and Lamb, C.J. (1994) Early Events in the activation of plant defence responses. Annual Review of Phytopathology, 32: Dow, J.M., Osbourn, A.E., Wilson, T.J.G. and Daniels, M.J. (1995) A locus determining pathogenicity of Xanthomonas campestris is involved in lipopolysaccharide biosynthesis. Molecular Plant-Microbe Interactions, 8: Dubey, H. and Grover, A. (2001) Current initiatives in proteomics research: The plant perspective. Current Science, 80: Durrant, W.E. and Dong, X. (2004) Systemic acquired resistance. Annual Review of Phytopathology, 42: Ebel, J. and Cosio, E.G. (1994) Elicitors of plant defence responses. International Review of Cytology, 148: Eitas, T.K. and Dangl, J.L. (2010) NB-LRR proteins: pairs, pieces, perception, partners and pathways. Current Opinion in Plant Biology, 13: 1 6. Elkins, T., Hortsch, M., Bieber, A.J., Snow, PM. and Goodman, C.S. (1990) Drosophila fasciclin I is a novel homophilic adhesion molecule that along with fasciclin III can mediate cell sorting. Journal of Cell Biology, 110:
105 Erbs, G. and Newman, M-A. (2003) The role of lipopolysaccharides in induction of plant defence responses. Molecular Plant Pathology, 4: Erbs, G. and Newman, M-A. (2011) The role of lipopolysaccharide and peptidoglycan, two glycosylated bacterial microbe-associated molecular patterns (MAMPs), in plant innate immunity. Molecular Plant Pathology, 13: Erbs, G. and Newman, M-A. (2012) The role of lipopolysaccharide and peptidoglycan, two glycosylated bacterial microbe-associated molecular patterns (MAMPs), in plant innate immunity. Molecular Plant Pathology, 13: Erridge, C., Bennett-Guerrero, E. and Poxton, I. (2002) Structure and function of lipopolysaccharides. Microbes and Infection, 4: Eriksson, S., Andre asson, E., Ekbom, B., Granér, G., Pontoppidan, B., Taipalensuu, J., Zhang, J., Rask, L. and Meijer, J. (2002) Complex formation of myrosinase isoenzymes in oilseed rape seeds are dependent on the presence of myrosinase-binding proteins. Plant Physiology, 129: Fairbanks, G., Steck, T.L. and Wallach, D.F.H. (1971) Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry, 10: Felix, G., Duran, J.D., Volko, S. and Boller, T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. The Plant Journal, 18: Felix, G., Regenass, M. and Boller, T. (1993) Specific perception of subnanomolar concentrations of chitin fragments by tomato cells. Induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant Journal, 4: Felix, G., Duran, J.D., Volko, S. and Boller, T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. The Plant Journal, 18: Feys, B.J., and Parker, J.E. (2000) Interplay of signalling pathways in plant disease resistance. Trends in Genetics, 16: Finehout, E.J. and Lee, K.H. (2004) An introduction to mass spectrometry applications in biological research. Biochemistry and Molecular Biology Education, 32: Flor, H.H. (1971) Current status of the gene-for-gene concept. Annual Review of Phytopathology, 9: Foster, L.J., de Hoog, C.L. and Mann, M. (2003) Unbiased quantitative proteomics of lipid rafts reveals high specificity for signalling factors. Proceedings of the National Academy of Sciences of the United States of America, 100:
106 Fritz-Laylin, L.K., Krishnamurthy, N., Tor, M., Sjolander, K.V. and Jones, J.D. (2005) Phylogenomic analysis of the receptor-like proteins of rice and Arabidopsis. Plant Physiology, 138: Fuglsang, A.T., Guo, Y., Cuin, T.A., Qiu, Q., Song, C., Kristiansen, K. A., Bych, K., Schulz, A., Shabala, S., Schumaker, K.S., Palmgren, M.G. and Zhu, J.K. (2007) Arabidopsis protein kinase PKS5 inhibits the plasma membrane H + -ATPase by preventing interaction with protein. Plant Cell, 19: Fujiwara, M., Hamada, S., Hiratsuka, M., Fukao, Y., Kawasaki, T. and Shimamoto, K. (2009) Proteome analysis of detergent-resistant membranes (DRMs) associated with OsRac1- mediated innate immunity in rice. Plant Cell Physiology, 50: Futcher, B., Latter, G.I., Monardo, P., McLaughlin C.S. and Garrels, J.I. (1999) A sampling of the yeast proteome. Molecular and Cell Biology, 19: Gautam, P. and Stein, J. (2011) Induction of systemic acquired resistance to Puccinia sorghi in corn. International Journal of Plant Pathology, 2: Gerber, I.B, Zeidler, D., Durner, J. and Dubery, I.A. (2004) Early perception responses of Nicotiana tabacum cells in response to lipopolysaccharides from Burkholderia cepacia. Planta, 218: Gerber, I.B., Laukens, K., Witters, E. and Dubery, I.A. (2006) Lipopolysaccharide responsive phosphoproteins in Nicotiana tabacum cells. Plant Physiology and Biochemistry, 44: Gerke, V. and Moss, S.E. (1997) Annexins and membrane dynamics. Biochimica et Biophysica Acta, 1357: Gevaudant, F., Duby, G., von Stedingk, E., Zhao, R., Morsomme, P. and Boutry, M. (2007) Expression of a constitutively activated plasma membrane H + -ATPase alters plant development and increases salt tolerance. Plant Physiology, 144: Geyer, M. and Wittinghofer, A. (1997) GEFs, GAPs, GDIs and effectors: taking a closer (3D) look at the regulation of Ras-related GTP-binding proteins. Current Opinion in Structural Biology, 7: Giannini, J.L., Ruiz-Cristin, J. and Briskin, D.P. (1988) A small scale procedure for the isolation of transport competent vesicles from plant tissues. Analytical Biochemistry, 174, Glebov, O.O., Bright, N.A. and Nichols, B.J. (2006) Flotillin-1 defines a clathrinindependent endocytic pathway in mammalian cells. Nature Cell Biology, 8:
107 Gómez-Gómez, L., Bauer, Z. and Boller, T. (2001) Both the extracellular leucine-rich repeat domain and the kinase activity of FLS2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell, 13: Gómez-Gómez, L., Felix, G. and Boller, T. (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. The Plant Journal, 18: Gómez-Gómez, L. and Boller, T. (2000) FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Molecular Cell, 5: Gorg, A., Weiss, W. and Dunn, M.J. (2004) Current two-dimensional electrophoresis technology for proteomics. Proteomics, 4: Gorter, E. and Grendel, F. (1925) On bimolecular layers of lipoids on the chromocytes of the blood. Journal of Experimental Medicine, 41: Griffith, O.H. and Ryan, M. (1999) Bacterial phosphatidylinositol-specific phospholipase C: structure, function, and interaction with lipids. Biochimica et Biophysica Acta, 1441: Gross, P., Julius, C., Schmelzer, E. and Hahlbrock, K. (1993) Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defense gene activation in infected, cultured parsley cells. The EMBO Journal, 12: Gygi, S.P., Rochon, Y., Franza, B.R. and Aebersold, R. (1999a) Correlation between protein and mrna abundance in yeast. Molecular and Cell Biology. 19: Gygi, S.P., Rist, B., Gerber, S.A., Turecek, F., Gelb, M.H. and Aebersold, R. (1999b) Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nature Biotechnology, 17: Habib, H. and Fazili, K.M. (2007). Plant protease inhibitors: a defence strategy in plants. Biotechnology and Molecular Biology Reviews, 2: Hames, B.D. and Rickwood, D. (1990) Gel Electrophoresis of Proteins: a practical approach. (2 nd edition). New York: Oxford University Press, pp Hammond-Kosack, K.E. and Jones, J.D.G. (1997) Plant disease resistance genes. Annual Review of Plant Physiology and Plant Molecular Biology, 48: Hammond-Kosack, K.E. and Parker, J.E. (2003) Deciphering plant-pathogen communication: fresh perspectives for molecular resistance breeding. Current Opinion in Biotechnology, 14: Hansen, C.G. and Nichols, B.J. (2009). Molecular mechanisms of clathrin-independent endocytosis. Journal of Cell Science, 122:
108 Hanson, P.I., Heuser, J.E. and Jahn, R. (1997) Neurotransmitter release-four years of SNARE complexes. Current Opinion in Neurobiology, 7: Harder, T. and Simons, K. (1997) Caveolae, DIGs, and the dynamics of sphingolipidcholesterol microdomains. Current Opinion in Cell Biology, 9: Harris, T.J. and Siu, C.H. (2002) Reciprocal raft-receptor interactions and the assembly of adhesion complexes. Bioessays, 24: Harper, J.F. and Harmon, A. (2005) Plants, symbiosis and parasites: a calcium signalling connection. Nature Reviews Molecular Cell Biology, 6: He, P., Shan, L. and Sheen, J. (2007) Elicitation and suppression of microbe-associated molecular pattern-triggered immunity in plant-microbe interactions. Cellular Microbiology, 9: Heath, M. C. (2000) Nonhost resistance and nonspecific plant defences. Current Opinion in Plant Biology, 3: Heerklotz, H. (2002) Triton promotes domain formation in lipid rafts mixtures. Biophysical Journal, 83: Heller, H., Schaefer, M. and Schulten, K. (1993) Molecular dynamics simulation of a bilayer of 200 lipids in the gel and in the liquid-crystal phases. Journal of Physical Chemistry, 97: Henderson, R.M., Edwardson, J.M., Geisse, N.A. and Saslowsky, D.E. (2004) Lipid rafts: feeling is believing. News in Physiological Sciences, 19: Higgins, C.F. (2001) ABC transporters: physiology, structure and mechanism an overview. Research in Microbiology, 152: Hurkman, W.J. and Tanaka, C.K. (2007) High-resolution two-dimensional gel electrophoresis: a cornerstone for plants proteomics. In: J. Samaj, and J.J. Thelen, eds Plant Proteomics. Berlin: Springer, pp Ikonen, E. (2001) Roles of lipid rafts in membrane transport. Current Opinion in Cell Biology, 13: Irani, N.G. and Russinova, E. (2009) Receptor endocytosis and signaling in plants. Current Opinion in Plant Biology, 12: Jahn, R., Lang, T. and Südhof, T.C. (2003) Membrane fusion. Cell, 112: Jahn, R. and Scheller, R.H. (2006) SNAREs engines for membrane fusion. Nature Reviews Molecular Cell Biology, 7:
109 Johansson, I., Karlsson, M., Johanson, U., Larsson, C. and Kjellbom, P. (2000) The role of aquaporins in cellular and whole plant water balance. Biochimica et Biophysica Acta, 1465: Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444: Jones, D.A., Thomas, C.M., Hammond-Kosack. K.E., Balint-Kurti, P.J. and Jones, J.D.G. (1994) Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science, 266: Kaffarnik, F.A.R., Jones, A.M.E., Rathjen, J.P. and Peck, S.C. (2009) Effector proteins of the bacterial pathogen Pseudomonas syringae alter the extracellular proteome of the host plant Arabidopsis thaliana. Molecular and Cellular Proteomics, 8: Kawai, T. and Akira, S. (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity, 34: Keen, N.T. (1990) Gene-for-gene complementarity in plant-pathogen interactions. Annual Review of Genetics, 24: Keinath, N.F., Kierszniowska, S., Lorek, J., Bourdais, G., Kessler, S.A., Shimosato-Asano, H., Grossniklaus, U., Schulze, W.X., Robatzek, S. and Panstruga, R. (2010) PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. Journal of Biological Chemistry, 285: Keller, P. and Simon, K. (1998) Cholesterol is required for surface transport of influenza virus hemagglutinin. Journal of Cell Biology, 140: Kenworthy, A.K., Nichols, B.J., Remmert, C.L., Hendrix, G.M., Kumar, M., Zimmerberg, J. and Lippincott-Schwartz, J. (2004) Dynamics of putative raft-associated proteins at the cell surface. Journal of Cell Biology, 165: Kim, T.H., Bohmer, M., Hu, H., Nishimura, N. and Schroeder, J.I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO 2, and Ca 2+ signaling. Annual Review of Plant Biology, 61: Klemptner, R.L., Sherwood, J.S., Tugizimana, F., Dubery, I.A. and Piater, L.A. (2014) Ergosterol, an orphan fungal microbe-associated molecular pattern (MAMP). Molecular Plant Pathology, 15: Kloepper, J.W., Tuzun, S. and Kuć, J.A. (1992) Proposed definitions related to induced disease resistance. Biocontrol Science and Technology, 2: Kobayashi, I., Kobayashi, Y., Yamaoka, N. and Kunoh, H. (1992) Recognition of a pathogen and a nonpathogen by barley coleoptile cells. III. Responses of microtubules and actin 90
110 filaments in barley coleoptile cells to penetration attempts. Canadian Journal of Botany, 70: Kobayashi, Y., Kobayashi, I., Funaki, Y., Fujimoto, S. and Kunoh, H. (1997) Dynamic reorganization of microfilaments and microtubules is necessary for the expression of non-host resistance in barley coleoptile cells. The Plant Journal, 11: Kobe, B. and Deisenhofer, J. (1994) The leucine-rich repeat: a versatile motif. Trends in Biochemical Sciences, 19: Kolch, W. (2000) Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochemical Journal, 351: Kreps, J., Wu, Y., Chang, H-S., Zhu, T., Wang, X. and Harper, J. (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic and cold. Plant Physiology, 130: Kuć, J., Barnes, E., Daftsios, A. and Williams, E. (1959) The effect of amino acids on susceptibility of apple varieties to scab. Phytopathology, 49: Kunze, G., Zipfel, C., Robatzek, S., Niehaus, K., Boller, T. and Felix, G. (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell, 16: Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: Laloi, M., Perret, A.M., Chatre, L., Melser, S., Cantrel, C., Vaultier, M.N., Zachowski, A., Bathany, K., Schmitter, J.M., Vallet, M., Lessire, R., Hartmann, M.A. and Moreau, P. (2007) Insights into the role of specific lipids in the formation and delivery of lipid microdomains to the plasma membrane of plant cells. Plant Physiology, 143: Lambert, J.P., Ethier, M., Smith, J.C. and Figeys, D. (2005) Proteomics: from gel based to gel free. Analytical Chemistry, 77: Lang, A.E., Schmidt, G., Schlosser, A., Hey, T.D., Larrinua, I.M., Sheets, J.J., Mannhertz, G.H. and Aktories, K. (2010) Photorhabdus luminescenstoxins ADP-ribosylate actin and RhoA to force actin clustering. Science, 327: Larsson, C. and Widell, S. (1981). Isolation of plant plasma membranes and production of inside-out vesicles. In: R. Hatti-Kaul, ed., (2000). Methods in biotechnology: aqueous twophase systems: Methods and Protocols. Totowa, NJ: Humana Press Inc., Ch.13. Lee, S.W., Han, S.W., Sririyanum, M., Park, C.J., Seo, Y.S. and Ronald, P.C. (2009) A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science, 326:
111 Lenman, M., Sorënsson, C. and Andreasson, E. (2008) Enrichment of phosphoproteins and phosphopeptide derivatization identity universal stress proteins in elicitor-treated Arabidopsis. Molecular Plant-Microbe Interactions, 21: Lewis, J.D. and Lazarowitz, S.D. (2010) Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proceedings of the National Academy of Sciences of the United States of America, 107: Lichtenberg, D., Goňi, F.M. and Heerklotz, H. (2005) Detergent-resistant membranes should not be identified with membrane rafts. Trends in Biochemical Sciences, 30: Ligaba, A., Yamaguchi, M., Shen, H., Sasak, T., Yamamoto, Y. and Matsumoto, H. (2004) Phosphorus deficiency enhances plasma membrane H+-ATPase activity and citrate exudation in greater purple lupin (Lupinus pilosus). Functional Plant Biology, 31: Lingwood, D. and Simons, K. (2010) Lipid rafts as a membrane-organisation principle. Science, 327: Ma, W., Smigel, A., Tsai, Y.C., Braam, J. and Berkowitz, G.A. (2008) Innate immunity signaling: cytosolic Ca 2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiology, 148: Madala, N.E., Molinaro, A. and Dubery, I.A. (2010) Distinct carbohydrate and lipid-based molecular patterns within lipopolysaccharides from Burkholderia cepacia contribute to defence-associated differential gene expression in Arabidopsis thaliana. Innate Immunity, 18: Madala, N.E., Molinaro, A. and Dubery, I.A. (2012) Distinct carbohydrate and lipid-based molecular patterns within lipopolysaccharides from Burkholderia cepacia contribute to defence-associated differential gene expression in Arabidopsis thaliana. Innate Immunity, 18: Madore, N., Smith, K.L., Graham, C.H., Jen, A., Brady, K., Hall, S. and Morris, R. (1999) Functionally different GPI proteins are organized in different domains on the neuronal surface. EMBO Journal, 18: Malamy, J., Carr, J.P., Klessig, D.F. and Raskin, I. (1990) Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science, 250: Manash, K.P. and Anup, K.M. (2004) Tyrosine kinase role and significance in cancer, International Journal of Medical Sciences, 1: Mann, M., Hendrickson, R.C. and Pandey, A. (2001) Analysis of proteins and proteomes by mass spectrometry. The Annual Review of Biochemistry, 70:
112 Marks, B., Stowell, M., Vallis, Y., Mills, I., Gibson, A., Hopkins, C.R. and McMahon, H.T. (2001) GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature, 410: Maurel, C., Javot, H., Lauvergeat, V., Gerbeau, P., Tournaire, C., Santoni, V. and Heyes, J. (2002) Molecular physiology of aquaporins in plants. International Review of Cytology, 215: McPhail, L.C., Waite, K.A., Regier, D.S., Nixon, J.B., Qualliotine-Mann, D., Zhang, W-X., Wallin R. and Sergeant, S. (1999) A novel protein kinase target for the lipid second messenger phosphatidic acid. Biochimica et Biophysica Acta, 1439: Meinke, D.W., Cherry, J.M., Dean, C., Rounsley, S.D. and Koornneef, M. (1998). Arabidopsis thaliana: A model plant for genome analysis. Science, 282: Mgcina, L.S., Dubery, I.A. and Piater, L.A. (2014) Comparative conventional- and quantum dot-labelling strategies of LPS shows specific binding sites in Arabidopsis thaliana mesophyll protoplasts. Submitted to New Phytologist. Miller, I., Crawford, J. and Gianazza, E. (2006) Protein stains for proteomic applications: which, when, why? Proteomics, 6: Minami, A., Fujiwara, M., Furuto, A., Fukao, Y., Yamashita, T., Kamo, M., Kawamura, M. and Uemura, M. (2009) Alterations in detergent-resistant plasma membrane microdomains in Arabidopsis thaliana during cold acclimation. Plant and Cell Physiology, 50: Mishina, T.E. and Zeier, J. (2007) Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. The Plant Journal, 50: Molinaro, A., Newman, M-A., Lanzetta, R. and Parrilli, M. (2009) The structures of lipopolysaccharides from plant-associated Gram-negative bacteria. European Journal of Organic Chemistry, 2009: Molloy, M.P. and Witzmann, F.A. (2002) Proteomics: technologies and applications. Briefings in Functional Genomics, 1: Monaghan, J. and Zipfel, C. (2012) Plant pattern recognition receptor complexes at the plasma membrane. Current Opinion in Plant Biology, 15: Mongrand, S., Morel, J., Laroche, J., Claverol, S., Carde, J.P., Hartmann, M-A., Bonneu, M., Simon-Plas, F., Lessire, R. and Bessoule, J.J. (2004) Lipid rafts in higher plant cells: purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. Journal of Biological Chemistry, 279:
113 Mongrand, S., Stanislas, T., Bayer, E.M.F., Lherminier, J. and Simon-Plas, F. (2010) Membrane rafts in plant cells. Trends in Plant Science, 12: Morandat, S. and El Kirat, K. (2006) Membrane resistance to Triton X-100 explored by realtime atomic force microscopy. Langmuir: The American Chemical Society Journal of Surfaces and Colloids, 22: Morel, J., Claverol, S., Mongrand, S., Furt, F., Fromentin, J., Bessoule, J.J., Blein, J.B. and Simon-Plas, F. (2006) Proteomics of plant detergent-resistant membranes. Molecular and Cellular Proteomics, 5: Moriyon, I. and Lopez-Goni, I. (1998) Structure and properties of the outer membranes of Brucella abortus and Brucella melitensis. International Microbiology, 1: Mortz, E., Krogh, T.N., Vorum, H. and Gorg, A. (2001) Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionizationtime of flight analysis. Proteomics, 1: Mouritsen, O.G. and Bloom, M. (1984) Mattress model of lipid-protein interactions in membranes. Biophysical Journal, 46: Morrow, M.R., Singh, D., Lu, D. and Grant C.W. (1995) Glycosphingolipid fatty acid arrangement in phospholipid bilayers: cholesterol effects. Biophysical Journal, 68: Morrow, I.C., Rea, S., Martin, S., Prior, I.A., Prohaska, R., Hancock, J.F., James, D.E. and Parton, R.G. (2002) Flotillin-1/reggie-2 traffics to surface raft domains via a novel Golgiindependent pathway. Identification of a novel membrane targeting domain and a role for palmitoylation. Journal of Biological Chemistry, 277: Munro, S. (2003) Lipid rafts: elusive or illusive. Cell, 115: Naito, K., Taguchi, F., Suzuki, T., Inagaki, Y., Toyoda, K., Shiraishi, T. and Ichinose, Y. (2008) Amino acid sequence of bacterial microbe-associated molecular pattern flg22 is required for virulence. Molecular Plant-Microbe Interactions, 21: Newman, M-A., von Roepenack-Lahaye, E., Parr, A., Daniels, M.J. and Dow, J.M. (2002) Prior exposure to lipopolysaccharide potentiates expression of plant defences in response to bacteria. The Plant Journal, 29: Newman, M-A., Dow, J.M., Molinaro, A. and Parrilli, M. (2007) Priming, induction and modulation of plant defence responses by bacterial lipopolysaccharides. Journal of Endotoxin Research, 13: Ng, J.H. and Ilag, L.L. (2002) Functional proteomics: separating the substances from the hype. Drug Discovery Today, 7:
114 Ngara, R and Ndimba, B.K. (2011) Mapping and characterisation of the sorghum cell suspension culture secretome. African Journal of Biotechnology, 10: Nichols, B. (2005) Cell biology: without a raft. Nature, 436: Nomura, K., Maeda, M., Sugase, K. and Kusumoto, S. (2010) Lipopolysaccharide induces raft domain expansion in membrane composed of a phospholipid cholesterol sphingomyelin ternary system. Innate Immunity, 17: Nürnberger, T., Brunner, F., Kemmerling, B. and Piater, L. (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunological Reviews, 198: Oliferenko, S., Paiha, K., Harder, T., Gerke, V., Schwärzler, C., Schwarz, H., Beug, H., Günthert, U. and Huber, L.A. (1999) Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. Journal of Cell Biology, 146: Ong, S.E., Blagoev, B., Kratchmarova, I., Kristensen, D. B., Steen, H., Pandey, A. and Mann, M. (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Molecular and Cellular Proteomics, 1: Pandey, A. and Mann, M. (2000) Proteomics study genes and genomes. Nature, 405: Parker, J.E. and Coleman, M.J. (1997) Molecular intimacy between proteins specifying plant-pathogen recognition. Trends in Biochemical Sciences, 22: Parton, R.G. and Richards, A.A. (2003) Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic, 4: Patterson, S.D. (2004) How much of the proteome do we see with discovery-based proteomics methods and how much do we need to see? Current Proteomics, 1: Pel, M.J.C. and Pieterse, C.M.J. (2013) Microbial recognition and evasion of host immunity. Journal of Experimental Botany, 64: Peskan, T., Westermann, M. and Oelmüller, R. (2000) Identification of low-density Triton X-100-insoluble plasma membrane microdomains in higher plants. European Journal of Biochemistry, 267: Pieterse, C.M.J., Ton, J. and van Loon, L.C. (2001) Cross-talk between plant defence signalling pathways: boost or burden? AgBiotechNet, 3: Pieterse, C.M.J., Leon-Reyes, A., van der Ent, S. and van Wees, S.C.M. (2009) Networking by small-molecule hormones in plant immunity. Nature Chemical Biology, 5: Pike, L.J. (2006) Rafts defined: a report on the Keystone symposium on lipid rafts and cell function. Journal of Lipid Research, 47:
115 Pozo, M.J., van der Ent, S., van Loon, L.C. and Pieterse, C.M.J. (2008) Transcription factor MYC2 is involved in priming for enhanced defence during rhizobacteria-induced systemic resistance in Arabidopsis thaliana. New Phytologist, 180: Pralle, A., Keller, P., Florin, E.L., Simons, K. and Horber, J.K.H. (2000) Sphingolipidcholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. Journal of Cell Biology, 148: Pust, S., Dyve, A.B., Torgersen, M. L., van Deurs, B. and Sandvig, K. (2010) Interplay between toxin transport and flotillin localization. PLoS ONE, 5: Raetz, C.R.H. (1996). Escherichia coli and Salmonella: Cellular and Molecular Biology. (2 nd edition). Washington, D.C: American Society of Microbiology, pp Raetz, C.R.H. and Whitfield, C. (2002) Lipopolysaccharide endotoxins. Annual Review of Biochemistry, 71: Rais, I., Karas, M. and Schägger, H. (2004) Two-dimensional electrophoresis for the isolation of integral membrane proteins and mass spectrometric identification. Proteomics, 4: Rask, L., Andréasson, E., Ekbom, B., Eriksson, S., Pontoppidan, B. and Meijer, J. (2000) Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Molecular Biology, 42: Reina-Pinto, J.J. and Yephremov, A. (2009) Surface lipids and plant defences. Plant Physiology and Biochemistry, 47: Reymond, P., Kunz, B., Paul-Pletzer, K., Grimm, R., Eckerskorn, C. and Farmer, E.E. (1996) Cloning of a cdna encoding a plasma membrane-associated, uronide binding phosphoprotein with physical properties similar to viral movement proteins. Plant Cell, 8: Richter, W., Vogel V., Howe, J., Steiniger, F., Brauser, A., Koch, M.H.J., Roessle, M., Gutsmann, T., Garidel, P., Mäntele, W. and Brandenburg, K. (2010) Morphology, size distribution and aggregate structure of lipopolysaccharide and lipid A dispersions from enterobacterial origin. Innate Immunity, 17: Robatzek, S., Chinchilla, D. and Boller, T. (2006) Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes and Development, 20: Robinson, P.N., Booms, P., Katzke, S., Ladewig, M., Neumann, L., Palz, M., Pregla, R., Tiecke, F. and Rosenberg, T. (2002) Mutations of FBN1 and genotype-phenotype correlations in Marfan syndrome and related fibrillinopathies. Human Mutation, 20:
116 Ross, A. (1966). Systemic effects of local lesion formation. In: A.B.R. Belmster and J. Dijkstra, eds. Viruses of Plants. Amsterdam: North Holland Publication Company, pp Ross, A.F. (1961) Systemic acquired resistance induced by localized virus infections in plants. Virology, 14: Ross, R. L., Huang, Y. N., Marchese, J. N., Williamson, B., Parker, K., Hattan, S., Khainovski, N., Pillai, S., Dey, S., Daniels, S., Purkayastha, S., Juhasz, P., Martin, S., Bartlet- Jones, M., He, F., Jacobson, A. and Pappin, D. J. (2004) Multiplex protein quantification in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Molecular and Cellular Proteomics, 3: Rosengarth, A., Gerke, V. and Luecke, H. (2001) X-ray structure of full-length annexin I and implications for membrane aggregation. Journal of Molecular Biology, 306: Rothman, J.E. (1994) Mechanisms of intracellular protein transport. Nature, 372: Ryals, J.A., Uknes, S. and Ward, E. (1994) Systemic acquired resistance. Plant Physiology, 104: Ryals, J.A., Neuenschwander, U.H., Willits, M.G., Molina, A., Steiner, H-Y. and Hunt, M.D. (1996) Systemic acquired resistance. The Plant Cell, 8: Salaün, C., James, D.J. and Chamberlain, L.H. (2004) Lipid rafts and the regulation of exocytosis. Traffic, 5: Sanabria, N., Goring, D., Nürnberger, T. and Dubery, I. (2008) Self/nonself perception and recognition mechanisms in plants: a comparison of self-incompatibility and innate immunity. New Phytologist, 178: Santoni, V., Vinh, J., Pflieger, D., Sommerer, N. and Maurel, C. (2003) A proteomic study reveals novel insights into the diversity of aquaporin forms expressed in the plasma membrane of plant roots. Biochemical Journal, 373: Sardon, T., Peset, I., Petrova, B. and Vernos, I. (2008) Dissecting the role of Aurora A during spindle assembly. The EMBO Journal, 27: Schaeffer, H.J. and Weber, M.J. (1999) Mitogen-Activated Protein Kinases: specific messages from ubiquitous messengers. Molecular and Cellular Biology, 19: Schatz, C.A., Santarella, R., Hoenger, A., Karsenti, E., Mattaj, I.W., Gruss, O.J. and Carazo- Salas, R.E. (2003) Importin alpha-regulated nucleation of microtubules by TPX2. The EMBO Journal, 22:
117 Schroeder, F., Jefferson, J.R., Kier, A.B., Knittel, J., Scallen, T.J., Wood. W.G. and Habala, I. (1991) Membrane cholesterol dynamics: cholesterol domains and kinetic pools. Proceedings of the Society for Experimental Biology and Medicine, 196: Schultz, C.J., Johnson, K.L., Currie, G. and Bacic, A. (2000) The classical arabinogalactan protein gene family of Arabidopsis. Plant Cell, 12: Seki, M., Narusaka, M., Ishida, J., Nanjo, T., Fujita, M., Oono, Y., Kamiya, A., Nakajima, M., Enju, A., Sakurai, T., Satou, M., Akiyama, K., Taji, T., Yamaguchi-Shinozaki, K., Carninci, P., Kawai, J., Hayashizaki, Y. and Shinozaki, K. (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold, and high-salinity stresses using a full-length cdna microarray. Plant Physiology, 31: Sels, J., Mathys, J., De Coninck, B.M.A., Cammue, B.P.A. and De Bolle, M.F.C. (2008) Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiology and Biochemistry, 46: Serrano, R. (1989) Structure and function of plasma membrane ATPase. Annual Review of Plant Physiology and Plant Molecular Biology, 40: Serrano, R. (1990) Plasma membrane H + -ATPase. In: C. Larsson, and I.M. Møller. The plasma membrane. Berlin: Springer-Verlag, pp Shah, J., Tsui, F. and Klessig, D. F. (1997) Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA inducible expression of the tms2 gene. Molecular Plant-Microbe Interactions, 10: Shahollari, B., Peskan-Berghöfer, T. and Oelmuller, R. (2004) Receptor kinases with leucine-rich repeats are enriched in Triton X-100 insoluble plasma membrane microdomains from plants. Physiologia Plantarum, 122: Sherrier, D.J, Prime, T.A. and Dupree, P. (1999) Glycosylphosphatidylinositol-anchored cell-surface proteins from Arabidopsis. Electrophoresis, 20: Shi, Y. (2009) Serine/threonine phosphatases: mechanism through structure. Cell, 139: Shimazu, R., Akashi, S., Ogata H., Nagai, Y., Fukudome, K., Miyake, K. and Kimoto, M. (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. Journal of Experimental Medicine, 189: Showalter, A.M. (2001) Arabinogalactan-proteins: structure, expression and function. Cellular and Molecular Life Sciences, 58: Shukla, S.D. and Halenda, S.P. (1991) Phospholipase D in cell signaling and its relationship to phospholipase C. Life Science, 48:
118 Silipo, A., Molinaro, A., Sturiale, L., Dow, J.M., Erbs, J., Lanzetta, R., Newman, M-A. and Parrilli, M. (2005) The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris. Journal of Biological Chemistry, 280: Silipo, A., Sturiale, L., Garozzo, D., Erbs, G., Jensen, T.T., Lanzetta, R., Dow, J.M., Parrilli, M., Newman, M-A. and Molinaro, A. (2008) The acylation and phosphorylation patterns of Lipid A from Xanthomonas campestris strongly influence its ability to trigger the innate immune response in Arabidopsis. ChemBioChem, 9: Silipo, A., Erbs, G., Shinya, T., Dow, J.M., Parrilli, M., Lanzetta, R., Shibuya, N., Newman M-A. and Molinaro, A. (2010) Glyco-conjugates as elicitors or suppressors of plant innate immunity. Glycobiology, 20: Simons, K. and Ikonen, I. (1997) Functional rafts in cell membranes. Nature, 387: Simons, K. and Toomre, D. (2000) Lipid rafts and signal transduction. Nature Reviews Molecular Cell Biology, 1: Simon-Plas, F., Perraki, A., Bayer, E., Gerbeau-Pissot, P. and Mongrand, S. (2011). An update on plant membrane rafts. Current Opinion in Plant Biology, 14: 1 8. Singer, S.J. and Nicolson G.L. (1972) The fluid-mosaic model of the structure of cell membranes. Science, 175: Smith, K.D., Andersen-Nissen, E., Hayashi, F., Strobe, K., Bergman, M,A., Barrett, S.L.R., Cookson, B.T. and Aderem A. (2003) Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nature Immunology, 4: Sollner, T., Whiteheart, S.W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P. and Rothman, J.E. (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature, 362: Song, W.Y., Wang, G.L., Chen,.LL., Kim, H.S., Pi, L.Y., Holsten, T., Gardner, J., Wang, B., Zhai, W.X., Zhu, L.H., Fauquet, C. and Ronald, P. (1995) A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science, 270: Spoel, S.H. and Dong, X. (2012) How do plants achieve immunity? Defence without specialized immune cells. Nature Reviews Immunology, 12: Staal, J. and Dixelius, C. (2007) Tracing the ancient origins of plant innate immunity. Trends in Plant Science, 12: Takahashi, D., Kawamura, Y., Yamashita, T. and Uemura, M. (2012) Detergent-resistant plasma membrane proteome in oat and rye: similarities and dissimilarities between two monocotyledonous plants. Journal of Proteome Research, 11:
119 Tanner, W. (2011) In plant and animal cells, detergent-resistant membranes do not define functional membrane rafts. The Plant Cell, 23: Thomas, L.A., Sehata, M.J., du Preez, M.G., Rees, J.G. and Ndimba, B.K. (2010) Establishment of proteome spot profiles and comparative analysis of the red and green phenotypes of Bon Rouge pear (Pyrus communis) leaves. African Journal of Biotechnology, 9: Thurston, G., Regan, S., Rampitsch, C. and Xing, T. (2005) Proteomic and phosphoproteomic approaches to understand plant-pathogen interactions. Physiological and Molecular Plant Pathology, 66: Tomasi, N., Kretzschmar, T., Espen, L., Weisskopf, Fuglsang, A.T., Palmgren, M.J., Neumann, G., Varanini, Z., Pinton, R., Martinoia, E. and Cesco, S. (2010) Plasma membrane H + -ATPase-dependent citrate exudation from cluster roots of phosphate-deficient white lupin. Plant, Cell and Environment, 32: Ton, J., van Pelt, J.A., van Loon, L.C. and Pieterse, C.M. (2002) Differential effectiveness of salicylate-dependent and jasmonate/ethylene-dependent induced resistance in Arabidopsis. Molecular Plant-Microbe Interactions, 15: Triantafilou, M., Miyake, K., Golenbock, D.T. and Triantafilou, K. (2002) Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. Journal of Cell Science, 115: Tsuda, K., Sato, M., Glazebrook, J., Cohen, J.D. and Katagiri, F. (2008) Interplay between MAMP-triggered and SA-mediated defence responses. The Plant Journal, 53: Tsuda, K., Sato, M., Stoddard, T., Glazebrook, J. and Katagiri, F. (2009) Network properties of robust immunity in plants. PLoS Genetics, 5: Uemura, M., Joseph, R.A. and Steponkus, P.L. (1995) Cold Acclimation of Arabidopsis thaliana: effect on plasma membrane lipid composition and freeze-induced Lesions. Plant Physiology, 109: VanEtten, H.D., Mansfield, J.W., Bailey, J.A. and Farmer, E.E. (1994) Two classes of plant antibiotics: Phytoalexins versus Phytoanticipins. The Plant Cell, 6: Van der Biezen, E.A. and Jones, J.D.G. (1998) Plant disease-resistance proteins and the gene-for-gene concept. Trends in Biochemical Sciences, 23: van Loon, L.C. (1997) Induced resistance in plants and the role of pathogenesis-related proteins. European Journal of Plant Pathology, 103: van Loon, L.C., Bakker, P.A.H.M. and Pieterse, C.M.J. (1998) Systemic resistance induced by rhizosphere bacteria. Annual Review of Phytopathology, 36:
120 van Loon, L.C., Rep, M. and Pieterse, C.M.J. (2006) Significance of inducible defencerelated proteins in infected plants. Annual Review of Phytopathology, 44: van Meer, G. (1989) Lipid traffic in animal cells. Annual Review of Cell and Developmental Biology, 5: Van Troys, M., Huyck, L., Leyman, S., Dhaese, S., Vandekerkhove, J. and Ampe, C. (2008) Ins and outs of ADF/cofilin activity and regulation. European Journal of Cell Biology, 87: Veatch, S.L. and Keller, S.L. (2005) Seeing spots: complex phase behavior in simple membranes. Biochimica et Biophysica Acta, 1746: Wang, X. (2002) Phospholipase D in hormonal and stress signaling. Current Opinion in Plant Biology, 5: Wang, X., Dyer, J.H. and Zheng, L. (1993) Purification and immunological analysis of phospholipase D from germinating castor bean endosperm. Archives of Biochemistry and Biophysics, 306: Wang, W., Vignani, R., Scali, M. and Cresti, M. (2006) A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis, 27: Watanabe, K., Bianco, C., Strizzi, L., Hamada, S., Mancino, M., Bailly, V., Mo, W., Wen, D., Miatkowski, K., Gonzales, M., Sanicola, M., Seno, M. and Salomon, D.D. (2007) Growth factor induction of Cripto-1 shedding by glycosylphosphatidylinositol phospholipase D and enhancement of endothelial cell migration. Journal of Biological Chemistry, 282: Westermeier, R. (2005) Electrophoresis in practice. (4 th edition). Weinheim: Wiley-VCH, pp Westermeier, R. and Marouga, R. (2005) Protein detection methods in proteomics research. Bioscience Reports, 25: Widjaja, I., Naumann, K., Roth, U., Wolf, N., Mackey, D., Dangl, J.L., Scheel, D. and Lee, J. (2009) Combining subproteome enrichment and rubisco depletion enables identification of low abundance proteins differentially regulated during plant defense. Proteomics, 9: Wilkins, M.R., Sanchez, J.C., Gooley, A.A., Appel, R.D., Humphery-Smith, I., Hochstrasser, D.F. and Williams, K.L. (1996) Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnology and Genetic Engineering Reviews, 13:
121 Willard, H. H., Merritt, Jr., L. L., Dean, J. A. and Settle, Jr. F. A. (1988) Instrumental Methods of Analysis. (6 th edition). Belmont, CA: Wadsworth Publishing Co, pp Wittmann-Liebold, B., Graack, H.R. and Pohl, T. (2006) Two-dimensional gel electrophoresis as tool for proteomics studies in combination with protein identification by mass spectrometry. Proteomics, 6: Xu, X., Bittman, R., Duportail, G., Heissler, D., Vilcheze, C. and London, E. (2001) Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and diseaseassociated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. Journal of Biological Chemistry, 276: Ye, M., Jiang, X., Feng, S., Tian, R. and Zou, H. (2007) Advances in chromatographic techniques and methods in shotgun proteome analysis. Trends in Analytical Chemistry, 26: Zappel, N.F. and Panstruga, R. (2008) Heterogeneity and lateral compartmentalization of plant plasma membrane. Current Opinion in Plant Biology, 11: Zhang, W. and Chait, B.T. (2000) ProFound: An expert system for protein identification using mass spectrometric peptide mapping information. Analytical Chemistry, 72: Zhang, X., Wang, H., Takemiya, A., Song, C.P., Kinoshita, T. and Shimazaki, K. (2004) Inhibition of blue light-dependent H + pumping by abscisic acid through hydrogen peroxideinduced dephosphorylation of the plasma membrane H + -ATPase in guard cell protoplasts. Plant Physiology, 136: Zeidler, D., Zahringer, U., Gerber, I.B., Dubery, I.A., Hartung, T., Bors, W., Hutzler, P. and Durner, J. (2004) Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defence genes. Proceedings of the National Academy of Sciences of the United States of America, 101: Zimaro, T., Gottig, N., Garavaglia, B.S., Gehring, C. and Ottado, J. (2011) Unraveling plant responses to bacterial pathogens through proteomics. Journal of Biomedicine and Biotechnology, 2011: Zipfel, C. (2008) Pattern-recognition receptors in plant innate immunity. Current Opinion in Immunology, 20: Zipfel, C. and Felix, G. (2005) Plants and animals: a different taste for microbes? Current Opinion in Plant Biology, 8:
122 Zipfel, C., Robatzek, S., Navarro, L., Oakeley, E.J., Jones, J.D., Felix, G. and Boller, T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature, 428: Zivy, M. and de Vienne, D. (2000) Proteomics: a link between genomics, genetics and physiology. Plant Molecular Biology, 44: Zhou, L., Cheung, M.Y., Li, M-W., Fu, Y., Sun, Z., Sun, S-M. and Lam, H-M. (2010) Rice hypersensitive induced reaction protein 1 (OsHIR1) associates with plasma membrane and triggers hypersensitive cell death. BMC Plant Biology, 10:
123 APPENDIX Figure A1: A 1-D SDS-PAGE analysis of the 0 and 6 h LPS E.coli -treated Arabidopsis leaf proteomes subsequent to the ultracentrifugation-based method. 10 µg protein was loaded per well on a 12% SDS- PAGE gel and bands were visualised with an Aqua-stain solution (Vacutec). L - PageRuler Unstained Low Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. 104
124 Figure A2: A 1-D SDS-PAGE analysis of the 0 and 6 h LPS E.coli -treated Arabidopsis leaf proteomes subsequent to the ultracentrifugation-based method. 10 µg protein was loaded per well on a 12% SDS- PAGE and bands were visualised using the Fairbanks staining protocol. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. Figure A3: Western blot analysis of the homogenate (HM), microsomal fraction (MF) and plasma membrane (PM) of 0, 6 and 12 h LPS E.coli -treated Arabidopsis samples. 10 µg protein was loaded per well on a 12% SDS-PAGE gel and blotted onto a PVDF membrane. Proteins were detected with an anti-mapk antibody. 105
125 Figure A4: A comparative 1-D SDS-PAGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes prior- and subsequent to acetone precipitation following the small-scale isolation protocol. 10 µg protein was loaded per well on a 12% SDS-PAGE gels followed by visualisation using the Fairbanks staining solution. L - Prosieve Quad Color Protein marker (Lonza), MW markers are expressed in kda, HM - homogenate, MF - microsomal fraction and PM - plasma membrane. 106
126 Figure A5: 2DGE analysis of 0, 6 and 12 h LPS E.coli -treated Arabidopsis leaf proteomes separated on a narrow ph range (ph 4-7) using a 7 cm IPG strip following the small-scale isolation protocol. 100 µg protein was first focused and the strip was loaded on a 12% SDS-PAGE gel. The gels were visualised using the Fairbanks staining solution. L - Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific), MW markers are expressed in kda. 107
Getting to PTI of bacterial RNAs: Triggering plant innate immunity by extracellular RNAs from bacteria
 Plant Signaling & Behavior ISSN: (Print) 1559-2324 (Online) Journal homepage: http://www.tandfonline.com/loi/kpsb20 Getting to PTI of bacterial RNAs: Triggering plant innate immunity by extracellular RNAs
Plant Signaling & Behavior ISSN: (Print) 1559-2324 (Online) Journal homepage: http://www.tandfonline.com/loi/kpsb20 Getting to PTI of bacterial RNAs: Triggering plant innate immunity by extracellular RNAs
Toll-like Receptor Signaling
 Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
Membrane Structure and Membrane Transport of Small Molecules. Assist. Prof. Pinar Tulay Faculty of Medicine
 Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Cell Structure. Morphology of Prokaryotic Cell. Cytoplasmic Membrane 4/6/2011. Chapter 3. Cytoplasmic membrane
 Cell Structure Chapter 3 Morphology of Prokaryotic Cell Cytoplasmic membrane Delicate thin fluid structure Surrounds cytoplasm of cell Defines boundary Defines boundary Serves as a selectively permeable
Cell Structure Chapter 3 Morphology of Prokaryotic Cell Cytoplasmic membrane Delicate thin fluid structure Surrounds cytoplasm of cell Defines boundary Defines boundary Serves as a selectively permeable
Copyright 2014 Edmentum - All rights reserved. 2. In plants, which characteristic or behavior is typically independent of the plant's environment?
 Copyright 2014 Edmentum - All rights reserved. AP Biology Living System and Free Energy Blizzard Bag 2014 2015 1. How is cellular respiration useful to the cell? A. producing ATP, which provides the nucleotides
Copyright 2014 Edmentum - All rights reserved. AP Biology Living System and Free Energy Blizzard Bag 2014 2015 1. How is cellular respiration useful to the cell? A. producing ATP, which provides the nucleotides
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
Cell Membranes. Dr. Diala Abu-Hassan School of Medicine Cell and Molecular Biology
 Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Chapter 3 The Induced Responses of Innate Immunity
 Chapter 3 The Induced Responses of Innate Immunity Pattern recognition by cells of the innate immune system Pattern recognition by cells of the innate immune system 4 main pattern recognition receptors
Chapter 3 The Induced Responses of Innate Immunity Pattern recognition by cells of the innate immune system Pattern recognition by cells of the innate immune system 4 main pattern recognition receptors
The Cell Membrane (Ch. 7)
 The Cell Membrane (Ch. 7) Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those structure
The Cell Membrane (Ch. 7) Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those structure
Rama Abbady. Odai Bani-Monia. Diala Abu-Hassan
 5 Rama Abbady Odai Bani-Monia Diala Abu-Hassan Lipid Rafts Lipid rafts are aggregates (accumulations) of sphingolipids. They re semisolid clusters (10-200 nm) of cholesterol and sphingolipids (sphingomyelin
5 Rama Abbady Odai Bani-Monia Diala Abu-Hassan Lipid Rafts Lipid rafts are aggregates (accumulations) of sphingolipids. They re semisolid clusters (10-200 nm) of cholesterol and sphingolipids (sphingomyelin
Chapter 4: Cell Membrane Structure and Function
 Chapter 4: Cell Membrane Structure and Function Plasma Membrane: Thin barrier separating inside of cell (cytoplasm) from outside environment Function: 1) Isolate cell s contents from outside environment
Chapter 4: Cell Membrane Structure and Function Plasma Membrane: Thin barrier separating inside of cell (cytoplasm) from outside environment Function: 1) Isolate cell s contents from outside environment
Innate Immunity. Chapter 3. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples. Antimicrobial peptide psoriasin
 Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Prokaryotic Cell Structure
 Prokaryotic Cell Structure Chapter 3 Prokaryotes vs Eukaryotes DNA Prokaryotes Eukaryotes Organelles Size & Organization Kingdoms 1 Where do viruses fit in? Acellular microorganisms Cannot reproduce outside
Prokaryotic Cell Structure Chapter 3 Prokaryotes vs Eukaryotes DNA Prokaryotes Eukaryotes Organelles Size & Organization Kingdoms 1 Where do viruses fit in? Acellular microorganisms Cannot reproduce outside
Prokaryotic Cell Structure
 Prokaryotic Cell Structure Chapter 3 Prokaryotes vs Eukaryotes DNA Prokaryotes Eukaryotes Organelles Size & Organization Kingdoms Where do viruses fit in? Acellular microorganisms Cannot reproduce outside
Prokaryotic Cell Structure Chapter 3 Prokaryotes vs Eukaryotes DNA Prokaryotes Eukaryotes Organelles Size & Organization Kingdoms Where do viruses fit in? Acellular microorganisms Cannot reproduce outside
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Diffusion, Osmosis and Active Transport
 Diffusion, Osmosis and Active Transport Particles like atoms, molecules and ions are always moving Movement increases with temperature (affects phases of matter - solid, liquid, gas) Solids - atoms, molecules
Diffusion, Osmosis and Active Transport Particles like atoms, molecules and ions are always moving Movement increases with temperature (affects phases of matter - solid, liquid, gas) Solids - atoms, molecules
An aldose contains an aldehyde functionality A ketose contains a ketone functionality
 RCT Chapter 7 Aldoses and Ketoses; Representative monosaccharides. (a)two trioses, an aldose and a ketose. The carbonyl group in each is shaded. An aldose contains an aldehyde functionality A ketose contains
RCT Chapter 7 Aldoses and Ketoses; Representative monosaccharides. (a)two trioses, an aldose and a ketose. The carbonyl group in each is shaded. An aldose contains an aldehyde functionality A ketose contains
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
CELLS. Cells. Basic unit of life (except virus)
 Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
(d) are made mainly of lipids and of proteins that lie like thin sheets on the membrane surface
 Which of the following statements is no true? Biological membranes (a) are composed partly of amphipathic lipids (b) have hydrophobic and hydrophilic regions (c) are typically in a fluid state (d) are
Which of the following statements is no true? Biological membranes (a) are composed partly of amphipathic lipids (b) have hydrophobic and hydrophilic regions (c) are typically in a fluid state (d) are
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 5 Cellular Membranes
 Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
A. Membrane Composition and Structure. B. Animal Cell Adhesion. C. Passive Processes of Membrane Transport. D. Active Transport
 Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Models of the plasma membrane - from the fluid mosaic to the picket fence model. Mario Schelhaas Institute of Cellular Virology
 Models of the plasma membrane - from the fluid mosaic to the picket fence model Mario Schelhaas Institute of Cellular Virology Today s lecture Central Question: How does the plasma membrane fulfil its
Models of the plasma membrane - from the fluid mosaic to the picket fence model Mario Schelhaas Institute of Cellular Virology Today s lecture Central Question: How does the plasma membrane fulfil its
Cell Membranes Valencia college
 6 Cell Membranes Valencia college 6 Cell Membranes Chapter objectives: The Structure of a Biological Membrane The Plasma Membrane Involved in Cell Adhesion and Recognition Passive Processes of Membrane
6 Cell Membranes Valencia college 6 Cell Membranes Chapter objectives: The Structure of a Biological Membrane The Plasma Membrane Involved in Cell Adhesion and Recognition Passive Processes of Membrane
PLASMA MEMBRANE. Submitted by:- DR.Madhurima Sharma PGGCG-II,Chandigarh
 PLASMA MEMBRANE Submitted by:- DR.Madhurima Sharma PGGCG-II,Chandigarh LIPID COMPONENTS OF THE PLASMA MEMBRANE The outer leaflet consists predominantly of phosphatidylcholine, sphingomyelin, and glycolipids,
PLASMA MEMBRANE Submitted by:- DR.Madhurima Sharma PGGCG-II,Chandigarh LIPID COMPONENTS OF THE PLASMA MEMBRANE The outer leaflet consists predominantly of phosphatidylcholine, sphingomyelin, and glycolipids,
Chapter 5. The Working Cell. Lecture by Richard L. Myers
 Chapter 5 The Working Cell PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Lecture by Richard L. Myers MEMBRANE STRUCTURE AND FUNCTION
Chapter 5 The Working Cell PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Lecture by Richard L. Myers MEMBRANE STRUCTURE AND FUNCTION
BIOLOGY 111. CHAPTER 3: The Cell: The Fundamental Unit of Life
 BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
Explain the reason for this difference in resolving power.
 1. (a) An electron microscope has a much greater resolving power than an optical microscope. (i) Explain the meaning of the term resolving power. Explain the reason for this difference in resolving power.
1. (a) An electron microscope has a much greater resolving power than an optical microscope. (i) Explain the meaning of the term resolving power. Explain the reason for this difference in resolving power.
The Cell. Biology 105 Lecture 4 Reading: Chapter 3 (pages 47 62)
 The Cell Biology 105 Lecture 4 Reading: Chapter 3 (pages 47 62) Outline I. Prokaryotic vs. Eukaryotic II. Eukaryotic A. Plasma membrane transport across B. Main features of animal cells and their functions
The Cell Biology 105 Lecture 4 Reading: Chapter 3 (pages 47 62) Outline I. Prokaryotic vs. Eukaryotic II. Eukaryotic A. Plasma membrane transport across B. Main features of animal cells and their functions
1.4 Page 1 Cell Membranes S. Preston 1
 AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
Double charge of 33kD peak A1 A2 B1 B2 M2+ M/z. ABRF Proteomics Research Group - Qualitative Proteomics Study Identifier Number 14146
 Abstract The 2008 ABRF Proteomics Research Group Study offers participants the chance to participate in an anonymous study to identify qualitative differences between two protein preparations. We used
Abstract The 2008 ABRF Proteomics Research Group Study offers participants the chance to participate in an anonymous study to identify qualitative differences between two protein preparations. We used
Zool 3200: Cell Biology Exam 4 Part I 2/3/15
 Name: Key Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name: Key Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Introduction. Biochemistry: It is the chemistry of living things (matters).
 Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins
 Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Organelles. copyright cmassengale 1
 Organelles copyright cmassengale 1 Organelles Very small (Microscopic) Perform various functions for a cell Found in the cytoplasm May or may not be membrane-bound 2 Animal Cell Organelles Nucleolus Nucleus
Organelles copyright cmassengale 1 Organelles Very small (Microscopic) Perform various functions for a cell Found in the cytoplasm May or may not be membrane-bound 2 Animal Cell Organelles Nucleolus Nucleus
Membranes 9/15/2016. Phospholipids. Phospholipid bilayer
 Membranes Phospholipids Type of complex lipid that forms biological membranes. Have a polar hydrophilic head and two nonpolar hydrophobic tails. Amphipathic. This causes the tails to cluster together in
Membranes Phospholipids Type of complex lipid that forms biological membranes. Have a polar hydrophilic head and two nonpolar hydrophobic tails. Amphipathic. This causes the tails to cluster together in
The Cell Membrane. Usman Sumo Friend Tambunan Arli Aditya Parikesit. Bioinformatics Group Faculty of Mathematics and Science University of Indonesia
 The Cell Membrane Usman Sumo Friend Tambunan Arli Aditya Parikesit Bioinformatics Group Faculty of Mathematics and Science University of Indonesia Overview Cell membrane separates living cell from nonliving
The Cell Membrane Usman Sumo Friend Tambunan Arli Aditya Parikesit Bioinformatics Group Faculty of Mathematics and Science University of Indonesia Overview Cell membrane separates living cell from nonliving
Innate Immunity. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples Chapter 3. Antimicrobial peptide psoriasin
 Know Differences and Provide Examples Chapter * Innate Immunity * kin and Epithelial Barriers * Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive
Know Differences and Provide Examples Chapter * Innate Immunity * kin and Epithelial Barriers * Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive
Lecture 15. Membrane Proteins I
 Lecture 15 Membrane Proteins I Introduction What are membrane proteins and where do they exist? Proteins consist of three main classes which are classified as globular, fibrous and membrane proteins. A
Lecture 15 Membrane Proteins I Introduction What are membrane proteins and where do they exist? Proteins consist of three main classes which are classified as globular, fibrous and membrane proteins. A
Cytoskeleton. Provide shape and support for the cell. Other functions of the cytoskeleton. Nucleolus. Nucleus
 Chapter 4: Cell Structure and Function Cytoskeleton The cytoskeleton is a network of fibers that organizes structures and activities in the cell. Microtubules (the largest) Intermediate fibers Microfilaments
Chapter 4: Cell Structure and Function Cytoskeleton The cytoskeleton is a network of fibers that organizes structures and activities in the cell. Microtubules (the largest) Intermediate fibers Microfilaments
LIVING SYSTEMS APPEAR COMPLEX BUT THERE IS AN UNDERLYING SIMPLICITY AND ELEGANCE:
 CHM333 LECTURE 1: 8/26/09 FALL 2009 Professor Christine Hrycyna What is Biochemistry? Simplest definition: Chemistry of the living cell Uses basic laws of chemistry, biology and physics to explain processes
CHM333 LECTURE 1: 8/26/09 FALL 2009 Professor Christine Hrycyna What is Biochemistry? Simplest definition: Chemistry of the living cell Uses basic laws of chemistry, biology and physics to explain processes
MEMBRANE STRUCTURE. Lecture 8. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 1 MEMBRANE STRUCTURE Lecture 8 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Plasma Membrane 2 Plasma membrane: The outer boundary of the cell that separates it from the world
1 MEMBRANE STRUCTURE Lecture 8 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Plasma Membrane 2 Plasma membrane: The outer boundary of the cell that separates it from the world
ISSN: Bogatzevska & Stoyanova J. BioSci. Biotechnol. 2015, SE/ONLINE: RESEARCH ARTICLE. Nevena Bogatzevska Mariya Stoyanova
 Nevena Bogatzevska Mariya Stoyanova Induction of systemic acquired resistance in pepper to X. vesicatoria pepper pathotype after treatment with X. vesicatoria tomato pathotype and Pseudomonas syringae
Nevena Bogatzevska Mariya Stoyanova Induction of systemic acquired resistance in pepper to X. vesicatoria pepper pathotype after treatment with X. vesicatoria tomato pathotype and Pseudomonas syringae
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Cell Membranes and Signaling
 5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
Cellular Physiology (PHSI3009) Contents:
 Cellular Physiology (PHSI3009) Contents: Cell membranes and communication 2 nd messenger systems G-coupled protein signalling Calcium signalling Small G-protein signalling o RAS o MAPK o PI3K RHO GTPases
Cellular Physiology (PHSI3009) Contents: Cell membranes and communication 2 nd messenger systems G-coupled protein signalling Calcium signalling Small G-protein signalling o RAS o MAPK o PI3K RHO GTPases
Comprehensive and Easy Course Notes for BIOL1040 Exams and Assessment
 Comprehensive and Easy Course Notes for BIOL1040 Exams and Assessment MODULE 1: PRINCIPLES OF CELL FUNCTION Membrane Structure & Function Cellular membranes are fluid mosaics of lipids and proteins Phospholipids
Comprehensive and Easy Course Notes for BIOL1040 Exams and Assessment MODULE 1: PRINCIPLES OF CELL FUNCTION Membrane Structure & Function Cellular membranes are fluid mosaics of lipids and proteins Phospholipids
Cell Membrane Structure (1.3) IB Diploma Biology
 Cell Membrane Structure (1.3) IB Diploma Biology Essential idea: The structure of biological membranes makes them fluid and dynamic http://www.flickr.com/photos/edsweeney/6346198056/ 1.3.1 Phospholipids
Cell Membrane Structure (1.3) IB Diploma Biology Essential idea: The structure of biological membranes makes them fluid and dynamic http://www.flickr.com/photos/edsweeney/6346198056/ 1.3.1 Phospholipids
MEMBRANE STRUCTURE & FUNCTION
 MEMBRANE STRUCTURE & FUNCTION Chapter 8 KEY CONCEPTS Cellular s are fluid mosaics of lipids and proteins Membrane structure results in selective permeability Passive transport is diffusion of a substance
MEMBRANE STRUCTURE & FUNCTION Chapter 8 KEY CONCEPTS Cellular s are fluid mosaics of lipids and proteins Membrane structure results in selective permeability Passive transport is diffusion of a substance
Membranes. Chapter 5. Membrane Structure
 Membranes Chapter 5 Membrane Structure Lipid Bilayer model: - double phospholipid layer - Gorter & Grendel: 1925 Fluid Mosaic model: consist of -phospholipids arranged in a bilayer -globular proteins inserted
Membranes Chapter 5 Membrane Structure Lipid Bilayer model: - double phospholipid layer - Gorter & Grendel: 1925 Fluid Mosaic model: consist of -phospholipids arranged in a bilayer -globular proteins inserted
Glycosaminoglycans: Anionic polysaccharide chains made of repeating disaccharide units
 Glycosaminoglycans: Anionic polysaccharide chains made of repeating disaccharide units Glycosaminoglycans present on the animal cell surface and in the extracellular matrix. Glycoseaminoglycans (mucopolysaccharides)
Glycosaminoglycans: Anionic polysaccharide chains made of repeating disaccharide units Glycosaminoglycans present on the animal cell surface and in the extracellular matrix. Glycoseaminoglycans (mucopolysaccharides)
The Cell Membrane AP Biology
 The Cell Membrane AP Biology! 2007-2008 Overview! Cell membrane separates living cell from nonliving surroundings " thin barrier = 8nm thick! Controls traffic in & out of the cell " selectively permeable
The Cell Membrane AP Biology! 2007-2008 Overview! Cell membrane separates living cell from nonliving surroundings " thin barrier = 8nm thick! Controls traffic in & out of the cell " selectively permeable
Supplementary material: Materials and suppliers
 Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Membrane Structure and Function
 Chapter 7 Membrane Structure and Function PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Chapter 7 Membrane Structure and Function PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Key Concepts - All Cells Use Energy Energy Conversions - Reactions Absorb or Release Energy Endergonic, Exergonic - ATP is Cellular Energy
 Key Concepts - All Cells Use Energy Energy Conversions - Reactions Absorb or Release Energy Endergonic, Exergonic - ATP is Cellular Energy ATP Cycle - Enzymes Speed Up Reactions Enzyme Function, Factors
Key Concepts - All Cells Use Energy Energy Conversions - Reactions Absorb or Release Energy Endergonic, Exergonic - ATP is Cellular Energy ATP Cycle - Enzymes Speed Up Reactions Enzyme Function, Factors
Chapter 1 Plasma membranes
 1 of 5 TEXTBOOK ANSWERS Chapter 1 Plasma membranes Recap 1.1 1 The plasma membrane: keeps internal contents of the cell confined to one area keeps out foreign molecules that damage or destroy the cell
1 of 5 TEXTBOOK ANSWERS Chapter 1 Plasma membranes Recap 1.1 1 The plasma membrane: keeps internal contents of the cell confined to one area keeps out foreign molecules that damage or destroy the cell
Chapter 8 Cells and Their Environment
 Chapter Outline Chapter 8 Cells and Their Environment Section 1: Cell Membrane KEY IDEAS > How does the cell membrane help a cell maintain homeostasis? > How does the cell membrane restrict the exchange
Chapter Outline Chapter 8 Cells and Their Environment Section 1: Cell Membrane KEY IDEAS > How does the cell membrane help a cell maintain homeostasis? > How does the cell membrane restrict the exchange
TECHNICAL BULLETIN. R 2 GlcNAcβ1 4GlcNAcβ1 Asn
 GlycoProfile II Enzymatic In-Solution N-Deglycosylation Kit Product Code PP0201 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description Glycosylation is one of the most common posttranslational
GlycoProfile II Enzymatic In-Solution N-Deglycosylation Kit Product Code PP0201 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description Glycosylation is one of the most common posttranslational
1. endoplasmic reticulum This is the location where N-linked oligosaccharide is initially synthesized and attached to glycoproteins.
 Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
3- Cell Structure and Function How do things move in and out of cells? A Quick Review Taft College Human Physiology
 3- Cell Structure and Function How do things move in and out of cells? A Quick Review Taft College Human Physiology How do things move in and out of cells? Things may move through cell membranes by Passive
3- Cell Structure and Function How do things move in and out of cells? A Quick Review Taft College Human Physiology How do things move in and out of cells? Things may move through cell membranes by Passive
CWDHS Mr. Winch Grade 12 Biology
 The Cell Membrane Overview Cell separates living cell from nonliving surroundings thin barrier = 8nm thick Controls traffic in & out of the cell selectively permeable allows some substances to cross more
The Cell Membrane Overview Cell separates living cell from nonliving surroundings thin barrier = 8nm thick Controls traffic in & out of the cell selectively permeable allows some substances to cross more
Cells and Their Environment Chapter 8. Cell Membrane Section 1
 Cells and Their Environment Chapter 8 Cell Membrane Section 1 Homeostasis Key Idea: One way that a cell maintains homeostasis is by controlling the movement of substances across the cell membrane. Homeostasis
Cells and Their Environment Chapter 8 Cell Membrane Section 1 Homeostasis Key Idea: One way that a cell maintains homeostasis is by controlling the movement of substances across the cell membrane. Homeostasis
Cellular Biochemistry
 Cellular Biochemistry Fall Semester 2013 Sept. 23 Benoit Kornmann Institute of Biochemistry Introduction to biological membranes General functions and properties Membrane lipids Physical properties Distribution/asymmetry
Cellular Biochemistry Fall Semester 2013 Sept. 23 Benoit Kornmann Institute of Biochemistry Introduction to biological membranes General functions and properties Membrane lipids Physical properties Distribution/asymmetry
Main differences between plant and animal cells: Plant cells have: cell walls, a large central vacuole, plastids and turgor pressure.
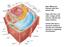 Main differences between plant and animal cells: Plant cells have: cell walls, a large central vacuole, plastids and turgor pressure. Animal cells have a lysosome (related to vacuole) and centrioles (function
Main differences between plant and animal cells: Plant cells have: cell walls, a large central vacuole, plastids and turgor pressure. Animal cells have a lysosome (related to vacuole) and centrioles (function
Homeostasis, Transport & The Cell Membrane. Chapter 4-2 (pg 73 75) Chapter 5
 Homeostasis, Transport & The Cell Membrane Chapter 4-2 (pg 73 75) Chapter 5 Unit 5: Lecture 1 Topic: The Cell Membrane Covers: Chapter 5, pages 95-96 Chapter 4, pages 73-75 The Cell Membrane The chemistry
Homeostasis, Transport & The Cell Membrane Chapter 4-2 (pg 73 75) Chapter 5 Unit 5: Lecture 1 Topic: The Cell Membrane Covers: Chapter 5, pages 95-96 Chapter 4, pages 73-75 The Cell Membrane The chemistry
Cell wall components:
 Main differences between plant and animal cells: Plant cells have: cell walls, a large central vacuole, plastids and turgor pressure. The Cell Wall The primary cell wall is capable of rapid expansion during
Main differences between plant and animal cells: Plant cells have: cell walls, a large central vacuole, plastids and turgor pressure. The Cell Wall The primary cell wall is capable of rapid expansion during
Membrane Structure and Function
 Membrane Structure and Function Chapter 7 Objectives Define the following terms: amphipathic molecules, aquaporins, diffusion Distinguish between the following pairs or sets of terms: peripheral and integral
Membrane Structure and Function Chapter 7 Objectives Define the following terms: amphipathic molecules, aquaporins, diffusion Distinguish between the following pairs or sets of terms: peripheral and integral
AP Biology Cells: Chapters 4 & 5
 AP Biology Cells: Chapters 4 & 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The was the first unifying principle of biology. a. spontaneous generation
AP Biology Cells: Chapters 4 & 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The was the first unifying principle of biology. a. spontaneous generation
5 Identification of Binding Partners of the Annexin A2 / P11 Complex by Chemical Cross-Linking
 5 Identification of Binding Partners of the Annexin A2 / P11 Complex by Chemical Cross-Linking In the quest of the omics sciences for holistic schemes, the identification of binding partners of proteins
5 Identification of Binding Partners of the Annexin A2 / P11 Complex by Chemical Cross-Linking In the quest of the omics sciences for holistic schemes, the identification of binding partners of proteins
Elements & Macromolecules in Organisms
 Name: Period: Date: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Period: Date: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
BIOL 158: BIOLOGICAL CHEMISTRY II
 BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 1: Membranes Lecturer: Christopher Larbie, PhD Introduction Introduction Cells and Organelles have membranes Membranes contain lipids, proteins and polysaccharides
BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 1: Membranes Lecturer: Christopher Larbie, PhD Introduction Introduction Cells and Organelles have membranes Membranes contain lipids, proteins and polysaccharides
Cell Walls, the Extracellular Matrix, and Cell Interactions (part 1)
 14 Cell Walls, the Extracellular Matrix, and Cell Interactions (part 1) Introduction Many cells are embedded in an extracellular matrix which is consist of insoluble secreted macromolecules. Cells of bacteria,
14 Cell Walls, the Extracellular Matrix, and Cell Interactions (part 1) Introduction Many cells are embedded in an extracellular matrix which is consist of insoluble secreted macromolecules. Cells of bacteria,
1. This is the location where N-linked oligosaccharide is initially synthesized and attached to glycoproteins.
 Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
Chapters 2 and 3. Pages and Pages Prayer Attendance Homework
 Chapters 2 and 3 Pages 44-45 and Pages 59-62 Prayer Attendance Homework The Cell The cell is the basic unit of life on Earth, separated from its environment by a membrane and sometimes an outer wall. Prokaryotic
Chapters 2 and 3 Pages 44-45 and Pages 59-62 Prayer Attendance Homework The Cell The cell is the basic unit of life on Earth, separated from its environment by a membrane and sometimes an outer wall. Prokaryotic
PTM Discovery Method for Automated Identification and Sequencing of Phosphopeptides Using the Q TRAP LC/MS/MS System
 Application Note LC/MS PTM Discovery Method for Automated Identification and Sequencing of Phosphopeptides Using the Q TRAP LC/MS/MS System Purpose This application note describes an automated workflow
Application Note LC/MS PTM Discovery Method for Automated Identification and Sequencing of Phosphopeptides Using the Q TRAP LC/MS/MS System Purpose This application note describes an automated workflow
Significance and Functions of Carbohydrates. Bacterial Cell Walls
 Biochemistry 462a - Carbohydrate Function Reading - Chapter 9 Practice problems - Chapter 9: 2, 4a, 4b, 6, 9, 10, 13, 14, 15, 16a, 17; Carbohydrate extra problems Significance and Functions of Carbohydrates
Biochemistry 462a - Carbohydrate Function Reading - Chapter 9 Practice problems - Chapter 9: 2, 4a, 4b, 6, 9, 10, 13, 14, 15, 16a, 17; Carbohydrate extra problems Significance and Functions of Carbohydrates
Practice Exam 2 MCBII
 1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
Ch7: Membrane Structure & Function
 Ch7: Membrane Structure & Function History 1915 RBC membranes studied found proteins and lipids 1935 membrane mostly phospholipids 2 layers 1950 electron microscopes supported bilayer idea (Sandwich model)
Ch7: Membrane Structure & Function History 1915 RBC membranes studied found proteins and lipids 1935 membrane mostly phospholipids 2 layers 1950 electron microscopes supported bilayer idea (Sandwich model)
Ch. 7 Cell Membrane BIOL 222
 Ch. 7 Cell Membrane BIOL 222 Overview: Plasma Membrane Plasma membrane boundary that separates the living cell from its surroundings Selec4ve permeability Allowance of some substances to cross more easily
Ch. 7 Cell Membrane BIOL 222 Overview: Plasma Membrane Plasma membrane boundary that separates the living cell from its surroundings Selec4ve permeability Allowance of some substances to cross more easily
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10962 Supplementary Figure 1. Expression of AvrAC-FLAG in protoplasts. Total protein extracted from protoplasts described in Fig. 1a was subjected to anti-flag immunoblot to detect AvrAC-FLAG
doi:10.1038/nature10962 Supplementary Figure 1. Expression of AvrAC-FLAG in protoplasts. Total protein extracted from protoplasts described in Fig. 1a was subjected to anti-flag immunoblot to detect AvrAC-FLAG
Lipids are macromolecules, but NOT polymers. They are amphipathic composed of a phosphate head and two fatty acid tails attached to a glycerol
 d 1 2 Lipids are macromolecules, but NOT polymers. They are amphipathic composed of a phosphate head and two fatty acid tails attached to a glycerol backbone. The phosphate head group is hydrophilic water
d 1 2 Lipids are macromolecules, but NOT polymers. They are amphipathic composed of a phosphate head and two fatty acid tails attached to a glycerol backbone. The phosphate head group is hydrophilic water
Chapter 9 - Biological Membranes. Membranes form a semi-permeable boundary between a cell and its environment.
 Chapter 9 - Biological Membranes www.gsbs.utmb.edu/ microbook/ch037.htmmycoplasma Membranes form a semi-permeable boundary between a cell and its environment. Membranes also permit subcellular organization
Chapter 9 - Biological Membranes www.gsbs.utmb.edu/ microbook/ch037.htmmycoplasma Membranes form a semi-permeable boundary between a cell and its environment. Membranes also permit subcellular organization
Tree defense against pathogens
 Tree defense against pathogens Pathogens penetrate and feed on trees differently than insects Insects ingest tree foliage or stem tissue and digest it internally; they usually don t invade host cells Pathogens
Tree defense against pathogens Pathogens penetrate and feed on trees differently than insects Insects ingest tree foliage or stem tissue and digest it internally; they usually don t invade host cells Pathogens
Division Ave High School Ms. Foglia AP Biology
 The Cell Membrane Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water 2007-2008 Aaaah, one of those structure
The Cell Membrane Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water 2007-2008 Aaaah, one of those structure
The Cell Membrane. Cell membrane separates living cell from nonliving surroundings. Controls traffic in & out of the cell
 The Cell Membrane 1 Overview Cell membrane separates living cell from nonliving surroundings thin barrier = 8nm thick Controls traffic in & out of the cell selectively permeable allows some substances
The Cell Membrane 1 Overview Cell membrane separates living cell from nonliving surroundings thin barrier = 8nm thick Controls traffic in & out of the cell selectively permeable allows some substances
EDUCATIONAL OBJECTIVES
 EDUCATIONAL OBJECTIVES The lectures and reading assignments of BIS 2A are designed to convey a large number of facts and concepts that have evolved from modern studies of living organisms. In order to
EDUCATIONAL OBJECTIVES The lectures and reading assignments of BIS 2A are designed to convey a large number of facts and concepts that have evolved from modern studies of living organisms. In order to
Identification of Microbes
 Identification of Microbes Recognition by PRR (pattern recognition receptors) Recognize conserved molecular patterns on microbes called pathogen associated molecular patterns (PAMPs) which are not present
Identification of Microbes Recognition by PRR (pattern recognition receptors) Recognize conserved molecular patterns on microbes called pathogen associated molecular patterns (PAMPs) which are not present
Chapter 7: Membranes
 Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
AP Biology. Overview. The Cell Membrane. Phospholipids. Phospholipid bilayer. More than lipids. Fatty acid tails. Phosphate group head
 Overview The Cell Membrane Cell separates living cell from nonliving surroundings thin barrier = 8nm thick Controls traffic in & out of the cell selectively permeable allows some substances to cross more
Overview The Cell Membrane Cell separates living cell from nonliving surroundings thin barrier = 8nm thick Controls traffic in & out of the cell selectively permeable allows some substances to cross more
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Biological membranes are composed of lipid bilayers
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Biological membranes are composed of lipid bilayers
Study Guide for Biology Chapter 5
 Class: Date: Study Guide for Biology Chapter 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following led to the discovery of cells? a.
Class: Date: Study Guide for Biology Chapter 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following led to the discovery of cells? a.
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 11 T-Cell Activation, Differentiation, and Memory Copyright 2013 by W. H. Freeman and
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 11 T-Cell Activation, Differentiation, and Memory Copyright 2013 by W. H. Freeman and
UNIT 2: Cells Chapter 3: Cell Structure and Function
 UNIT 2: Cells Chapter 3: Cell Structure and Function I. Cell Theory (3.1) A. Early studies led to the development of the cell theory 1. Discovery of Cells a. Robert Hooke (1665)-Used compound microscope
UNIT 2: Cells Chapter 3: Cell Structure and Function I. Cell Theory (3.1) A. Early studies led to the development of the cell theory 1. Discovery of Cells a. Robert Hooke (1665)-Used compound microscope
Oxi1 mutant plays an important role in Arabidopsis resistance against aphid (Myzus persicae)
 Oxi1 mutant plays an important role in Arabidopsis resistance against aphid (Myzus persicae) Dr. Tahsin Shoala Assistant professor - College of Biotechnology - Misr University for Science and Technology
Oxi1 mutant plays an important role in Arabidopsis resistance against aphid (Myzus persicae) Dr. Tahsin Shoala Assistant professor - College of Biotechnology - Misr University for Science and Technology
Reading Packet 2- Cells Unit. Chapter 6: A Tour of the Cell 1. What is resolving power?
 AP Biology Reading Packet 2- Cells Unit Name Chapter 6: A Tour of the Cell 1. What is resolving power? 2. How is an electron microscope different from a light microscope and what is the difference between
AP Biology Reading Packet 2- Cells Unit Name Chapter 6: A Tour of the Cell 1. What is resolving power? 2. How is an electron microscope different from a light microscope and what is the difference between
1. Overview of Adaptive Immunity
 Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
A Bacterial Virulence Protein Suppresses Host Innate Immunity to Cause Plant Disease
 A Bacterial Virulence Protein Suppresses Host Innate Immunity to Cause Plant Disease Nomura, K., Debroy, S., Lee, Y.H., Pumplin, N., Jones, J., and He, S.Y. (2006). Science 313, 220-223. Presented by:
A Bacterial Virulence Protein Suppresses Host Innate Immunity to Cause Plant Disease Nomura, K., Debroy, S., Lee, Y.H., Pumplin, N., Jones, J., and He, S.Y. (2006). Science 313, 220-223. Presented by:
