VULVAR INTRAEPITHELIAL NEOPLASIA (VIN)
|
|
|
- Charlotte Jennings
- 5 years ago
- Views:
Transcription
1 SPANISH ASSOCIATION OF CERVICAL PATHOLOGY AND COLPOSCOPY (ASOCIACIÓN ESPAÑOLA DE PATOLOGÍA CERVICAL Y COLPOSCOPIA) VULVAR INTRAEPITHELIAL NEOPLASIA (VIN)
2 For the citation of the present AEPCC-Guide it will be stated: AEPCC-Guía: Neoplasia vulvar intraepitelial (VIN). Coordinadora: Ramírez, M. Autores: Andía, D., Bosch, J.M., Cararach, M., Coronado, P., de Sanjosé, S., López, J.A., Martínez J.C., Puig-Tintoré, L.M., Vidart, J.A. Revisores-editores: del Pino M., Torné, A. Publicaciones AEPCC. 2015; 39p. ISBN Copyright AEPCC 2015
3 1. RATIONALE AND OBJECTIVES The main purpose of the Spanish Association of Cervix Pathologies and Colposcopy (Asociación Española de Patología Cervical y Colposcopia AEPCC) is to promote knowledge and investigation of the lower genital tract in women. Therefore, the AEPCC has developed the AEPCC-Guidelines (AEPCC-guías) in order to guide decision making and to provide criteria for the diagnosis, management and treatment of lower genital lower genital tract pathologies. These scientific evidence-based guidelines are systematically developed and cover specific areas of knowledge of lower genital tract diseases, being characterized by their relevance and important impact on clinical practice. The specific objectives of the AEPCC-guidelines are to: Promote standardized lines of action based on the current scientific evidence and on reliable information and scientific consensus. Ensure the equality of patients receiving health care, regardless of their location, thus promoting good practice. Improve the effectiveness of interventions and the quality of health care. Favor the implementation of quality control or clinical efficacy indicators. Facilitate decision-making among administrative managers and planners of health resources. Lastly, the methodological rigor established for the preparation of the AEPCC-guidelines is aimed at the development of documents of outstanding scientific quality which will allow better clinical practice and greater knowledge of lower genital tract diseases. 2. METHODOLOGY The specific methodology followed for the preparation of AEPCC-guidelines includes the following aspects: The AEPCC Steering Committee appoints a coordinator responsible for the preparation of the AEPCC-guidelines. In accordance with the Steering Committee, the Coordinator appoints the Writing Committee consisting of him/herself, a Secretary and the participants. The members are professional experts who are members of the AEPCC or other scientific societies with recognized prestige in this topic. Consensual development of the index. Critical review of the available literature and assignment of levels of evidence. Discussion and consensus among the committee members for assigning the grade of recommendation. Writing the document. Final analysis of the document by a Review and Editing Committee. Printed and online format of the final version. Dissemination of the AEPCC-guidelines in congresses, courses and seminars organized by the AEPCC. Development of online courses on the content of the AEPCC-guidelines to provide detailed knowledge of these guidelines and facilitate their implementation in daily clinical practice. (training credits). Update of the AEPCC-guidelines.
4 Assessment of the scientific evidence and extent and strength of the recommendations. The GRADE System. Clinical practice guidelines consist of recommendations addressed to health care professionals to help them care for patients with a particular clinical condition. They are based on the most important literature on a certain topic (systematic reviews of the medical literature and identification of studies with greater scientific evidence) and on clinical experience. In general, prospective randomized studies are granted the highest level of consideration, whereas data obtained from the opinion of experts receive the lowest level. In this way it is possible to assess the quality of the evidence associated with the outcomes of a particular strategy. All the recommendations of the AEPCC-guidelines take into account the quality of the current scientific literature. The strength of each recommendation is agreed upon by the Writing Committee of the AEPCC-guidelines depending on the quality of the studies available. The GRADE (Grading of Recommendations Assessment, Development and Evaluation Working Group) system ( is used for the classification of scientific evidence and to determine the extent and strength of the recommendations. To do this the following steps are followed: 1. The PICO (patient, intervention, comparison, outcomes) questions are established and outcome variables (in terms of benefit and risk) for each of the intervention questions asked are defined. 2. Outcome variables are scored from 1 to 9. Outcome variables considered key for decision making are scored from 7 to 9; important variables (but not key) from 4 to 6, and less important variables are scored from 1 to 3. The Working Group identifies, assesses and agrees upon the importance of the outcome variables. 3. Evaluation of the quality of the evidence for each of the key outcome variables. Search strategies have been designed to identify systematic reviews and randomized clinical trials and other studies published. The GRADE system assesses the quality of the evidence for each variable as high, moderate, low and very low. Randomized clinical trials and systematic reviews of randomized clinical trials have a high quality of evidence while observational studies and systematic reviews of observational studies have a low quality of evidence. Table 1 shows the different aspects which decrease or increase the quality of evidence. 4. Assessment of the overall quality of evidence. The overall quality of evidence was based across outcomes based on the lowest quality of evidence achieved for key outcome variables. In cases in which evidence for all the key variables favors the same alternative and there is evidence of high quality for some but not for all variables, the overall quality is considered as high. Low quality evidence on unimportant benefits and risks does not decrease the overall grade of evidence. 5. Designating strength to the recommendation. The GRADE system differentiates between strong and weak recommendations and makes explicit judgments about the factors that may affect the strength of the recommendation: balance between benefits and risks, overall quality of evidence, values and preferences of the population and costs. Both categories, strong and weak, may be for or against a particular intervention. Table II describes the meaning of the strong and weak categories.
5 QUALITY OF THE EVIDENCE Table 1.- GRADE system for assigning the quality of evidence Study designo Initial quality of evidence In clinical trials, decrease if * In observational studies increase if Final quality of evidence Randomized clinical trials High Critical (- 1) or very important (- 2) limitation to study quality Important inconsistency (- 1) Some (- 1) or major (- 2) uncertainty about directness of evidence Consistent and direct strong association** without confusing factors Very strong evidence** without major threats to validity (no biases) and direct evidence (+2). High Moderate Imprecise or sparse data Dose response gradient (+1) Low Observational study Low High probability of reporting bias (- 1) All plausible confounders could have reduced the observed effect (+1) Very low * Increase (+1) or decrease (-1) a level (e.g. from high to moderate); 2, increase (+2) or decrease (-2) two levels (e.g. from high to low) ** A statistically significant relative risk > 2 (< 0.5) based on evidences consisting in two or more observational studies without plausible confounders. *** a statistically significant relative risk > 5 (< 0.2) based on direct evidence and without major threats to validity. Source: Adapted from: Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64: STRENGTH OF RECOMMENDATIONS Table 2.- GRADE system for designating strength to recommendations Patients Clinicians Managers/policy makers Strong Most people would agree on the recommended course of action and only a small number would not. Most patients should receive the intervention recommended the recommendation can be adopted as a policy in most situations Weak Most informed people would choose the recommended course of action, but a substantial number would not. Recognizes that there are different options for different patients and health care professionals must help each patient to make the decision which is most consistent with their values and preferences. Policy making will require substantial debate and involvement of many stakeholders Source: Adapted from: Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations J Clin Epidemiol. 2013;66 (7): doi: /j.jclinepi
6 AEPCC-Guidelines Participants VULVAR INTRAEPITHELIAL NEOPLASIA (VIN) COORDINATOR Dra. Mar Ramírez Mena Gynaecological Oncology Unit. Instituto de Salud de la Mujer José Botella Llusiá. Hospital Clínico San Carlos. Facultad de Medicina, Universidad Complutense, Madrid. Spain. REVIEWERS - EDITORS Dr. Aureli Torné Bladé Gynaecological Oncology Unit, Instituto Clínico de Ginecología y Obstetricia y Neonatología (ICGON), Hospital Clínic. Instituto de Investigaciones Biomédicas August Pi i Sunyer (IDIBAPS), Facultad de Medicina, Universidad de Barcelona, Barcelona, Spain. Dra. Marta del Pino Saladrigues Gynaecological Oncology Unit, Instituto Clínico de Ginecología y Obstetricia y Neonatología (ICGON), Hospital Clínic. Instituto de Investigaciones Biomédicas August Pi i Sunyer (IDIBAPS), Facultad de Medicina, Universidad de Barcelona, Barcelona, Spain. 6
7 Vulvar Intraepithelial Neoplasia (VIN) AUTHORS Dra. Montserrat Cararach Tur Servicio de Ginecología y Obstetricia. Institut Riera Bartra. Clínica Sagrada Familia. Barcelona Dr. Jaume Ordi Maja Servicio de Anatomía Patológica, CRESIB (Centre de Recerca en Salut Internacional de Barcelona). Hospital Clínic. Universitat de Barcelona, Barcelona, España Dr. Luis M Puig-Tintoré. Facultad de Medicina, Universidad de Barcelona, Barcelona, España Dr. Daniel Andía Ortiz Servicio de Ginecología. Hospital Universitario Basurto. Facultad de Medicina. Universidad del País Vasco. Bilbao. España Dra. Silvia de Sanjosé Llongueras Research Programme, IDIBELL, Institut Català d Oncologia, CIBER Epidemiología y Salud Pública, L Hospitalet de Llobregat, Barcelona, España Dr. Juan Carlos Martínez Escoriza Servicio de Obstetricia y Ginecología. Hospital General Universitario de Alicante. Facultad de Medicina, Universidad de Alicante, Alicante, España. Dr. Pluvio Coronado Martín Unidad de Ginecología Oncológica. Instituto de Salud de la Mujer José Botella Llusiá. Hospital Clínico San Carlos. Facultad de Medicina, Universidad Complutense, Madrid. España. Dr. José Antonio López Fernández Servicio de Obstetricia y Ginecología. Hospital General Universitario de Alicante. Facultad de Medicina, Universidad de Alicante, Alicante, España. Dr. Jose Manuel Bosch Martí Unidad de Ginecología Oncológica. Instituto Valenciano Oncología. Valencia Dr. José Antonio Vidart Aragón Unidad de Ginecología Oncológica. Instituto de Salud de la Mujer José Botella Llusiá. Hospital Clínico San Carlos. Facultad de Medicina, Universidad Complutense, Madrid. España. 7
8 AEPCC-Guidelines VULVAR INTRAEPITHELIAL NEOPLASIA (VIN) TABLE OF CONTENTS 1. INTRODUCTION CLINICAL CHARACTERISTICS TERMINOLOGY AND CLASSIFICATION ISSVD Classification WHO Classification and 2015 ISSVD Classification EPIDEMIOLOGY ETIOPATHOGENESIS HSIL (usual-type VIN) Differentiated-type VIN NATURAL HISTORY OF VIN HISTOLOGY HSIL (usual-type VIN) Differentiated-type VIN DIAGNOSIS Clinical Examination and Vulvoscopy Biopsy (Indications and Methodology) Suspected Occult Invasion Differential Diagnosis TREATMENT General Principles Excision Simple local excision Partial or Total Skinning Vulvectomy Simple Vulvectomy Ablation C02 Laser Vaporization Other destructive therapies under investigation Topical Treatment
9 Vulvar Intraepithelial Neoplasia (VIN) Fluorouracil (5-FU) Imiquimod Cidofovir Combined Treatments Observation without treatment Treatment under special circumstances Pregnancy Immunosuppression Investigational Therapies: Therapeutic vaccines Impact of VIN and its treatment in patients 27 quality of life Therapeutic Algorithm PRIMARY PREVENTION OF VIN. ROLE OF PROPHILACTIC VACCINES AGAINST HPV RECOMENDATIONS REFERENCES FOLLOW-UP Recurrences after treatment Risk of Progression Follow-up guidelines Follow-up Algorithm
10 AEPCC-Guidelines 1. Introduction Vulvar intraepithelial neoplasia (VIN) is considered the precursor lesion for vulvar squamous cell carcinoma. Early diagnosis and adequate treatment of VIN is the only method of secondary prevention currently available to prevent the development of this neoplasia. However, in terms of efficiency, the low incidence of vulvar cancer does not justify the establishment of population screening programs. Vulvar squamous cell carcinoma encompasses two different etiopathogenic entities. Infection by the human papillomavirus (HPV) is causally associated with a proportion of carcinomas of the vulva, while the remaining carcinomas develop from precursor lesions secondary to chronic skin diseases. Knowledge of this dual etiopathogenic pattern for vulvar cancer and its precursor lesions is based on the recently approved classification of the 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) which establishes two VIN lesion patterns: HSIL (usualtype VIN) and differential VIN, with clearly differentiated epidemiologic factors, histological characteristics, clinical behavior and potential to progress to cancer. Determination of the course of action to be followed in patients diagnosed with VIN is an important challenge in health care practice. The great variability in the clinical presentation, the high risk of occult invasion at the time of diagnosis, the multiple therapeutic options available without a defined optimal treatment, and the high percentage of relapse after treatment, establishes the complexity of this pathology. Therefore, it is essential that all specialists involved in the diagnosis and treatment of these lesions have thorough and detailed knowledge of all aspects related to VIN. The aim of the AEPCC-guidelines is to provide a detailed updated review of each of the most relevant aspects of VIN to support and aid in the decision making process and allow standardized management of this disease based on the scientific evidence available and state-of-the-art knowledge. 10
11 Vulvar Intraepithelial Neoplasia (VIN) 2. Terminology and classification The term VIN (from the Anglo-Saxon acronym, Vulvar Intraepithelial Neoplasia) was first introduced in the literature by Richart and was subsequently extended by Crum1. This terminology was an adaptation of the much more frequent and better known terminology used for uterine cervical intraepithelial neoplasm (CIN). This parallelism was established on the basis of the clinical, etiologic, pathogenic and histological similarities between the two lesions. The term VIN has been widely accepted by the leading societies and international organizations, including the World Health Organization (WHO), the International Society of Gynaecological Pathologists (ISGYP), the International Federation of Gynaecology and Obstetrics (FIGO) and the ISSVD. In 1986, the ISSVD, established 3 degrees of VIN based on their severity: VIN 1, 2 and 3, depending on the alteration of epithelial maturation, in analogy to the criteria used for cervical injuries. In this first classification, a distinct form of VIN called differentiated-type VIN was determined, making clear the clinical and histological differences between the two types of VIN ISSVD CLASSIFICATION Since 1986, knowledge of the natural history of precursor lesions of lower genital tract cancers has significantly advanced3. Indeed, in recent years there have been important modifications in the classification of VIN. First, several studies have described the presence of two types of VIN with clearly differentiated etiopathogenic and clinical-pathological features: usual-type VIN, related to HPV, which generally presents a basaloid, warty or mixed (basaloid/ warty) morphology, and differentiated-type VIN, related to inflammatory dermatoses such as lichen sclerosus and lichen simplex chronicus. Secondly, it was agreed to limit the classification of VIN to squamous intraepithelial neoplasia, thereby discarding in situ melanoma and vulvar Paget s disease. Finally, VIN1 was excluded as a precancerous lesion of the vulva since it represents a process without oncogenic potential secondary to HPV infection. The VIN 2 and VIN 3 categories, which are difficult to pathologically differentiate and have doubtful clinical difference, were grouped into a single category designated as VIN. Thus, VIN was considered the only precursor lesion of squamous vulvar cancer 4. It is difficult to include some very rare histological forms of VIN such as pagetoid VIN within the aforementioned categories, and therefore, these lesions are included in a new category of unclassifiable VIN. The 2004 ISSVD classification is shown in Table 3. Table ISSVD Classification of Vulvar Intraepithelial Neoplasia5 VIN Characteristics Basaloid type Usual-type VIN HPV-related Warty type Mixed type Differentiated-type VIN Non HPV-related Unclassifiable VIN It cannot be included in the prior categories. It may include rare types such as Pagetoid VIN 11
12 AEPCC-Guidelines WHO CLASSIFICATION AND 2015 ISSVD CLASSIFICATION In 2012, the American College of Pathologists and the American Society of Cervical Pathology and Colposcopy published the conclusions reached by the Consensus Committee of Lower Anogenital Squamous Terminology (LAST) Standardization Project for HPV-Associated Lesions. This aim of this classification was to standardize the nomenclature of the lesions associated with HPV infection. The LAST classification does not refer to differentiated or unclassifiable VIN but rather recognizes the existence of two basic forms of HPV infection: 1) productive infection, with minimal risk of oncogenic transformation and a high capacity of regression, and 2) infection with the capacity to induce cell transformation able to generate precursor lesions with a high risk of progression to cancer. Finally, the LAST classification combines all intraepithelial lesions associated with HPV infection into a single terminology regardless of their location in the anogenital tract (cervix, vagina, vulva, anus, perianal area or penis) 6. The LAST terminology recommends using the same terms as those in cervical cytology (Bethesda system) for histological diagnosis. Lesions previously called -IN 1 (CIN 1, vaginal intraepithelial neoplasia [VaIN]1, etc.) are now called low-grade squamous intraepithelial lesions (LSIL), and lesions previously called -IN2 or -IN3 (CIN 2-3, VaIN 2-3, etc.) are now known as high-grade squamous intraepithelial lesions (HSIL). In addition, this terminology recommends specifying in parentheses the anatomic location where the intraepithelial lesion is located (vulvar VIN, vaginal VaIN, anal AIN, etc.). In the 2014 classification of gynecologic tumors7 the World Health Organization (WHO) incorporated the LAST terminology to include HPV-associated vulvar squamous intraepithelial lesions and recommended the use of LSIL and HSIL specifying the term usual-type VIN in parentheses. This terminology is not suitable for non-hpv-related lesions, and thus, the term differentiated-type VIN remains the same following the criteria established in the 2004 classification. In July 2015, the ISSVD Terminology Committee proposed some changes to the 2014 WHO classification. Accordingly, LSIL (VIN1) should not be strictly considered as a precursor lesion, but a skin reaction secondary to HPV infection without oncogenic potential. The effect of HPV infection on the vulva is not biologically equivalent to that produced in the cervix or anus since the vulva consists of keratinized epithelium and lacks a transformation zone, and therefore, LSIL (VIN1) should not be considered or treated as a potential neoplastic lesion. Ultimately, the latest edition of the ISSVD classification recommends not to use the term VIN in parentheses with LSIL, but the term condyloma or HPV-related changes. Therefore, only HSIL (usual-type VIN) and differentiated-type VIN are considered true precursor lesions 8-9 (Table 4). This new classification has implications in the clinical course of action since the treatment for LSIL is not justified as prophylaxis for vulvar cancer but as a symptomatic treatment of HPV-related lesions. Tabla 4. Clasificación neoplasia vulvar intraepitelial ISSVD Lesión intraepitelial escamosa de alto grado: H-SIL (VIN tipo común) Neoplasia intraepitelial vulvar tipo diferenciado: VIN tipo diferenciado 12
13 Vulvar Intraepithelial Neoplasia (VIN) 3. Epidemiology VIN is a clearly under-diagnosed and under-reported entity. Population-based epidemiological data on VIN are virtually non-existent and most of the information available is based on studies in patients attended for medical evaluation. A standard population-based recommendation for the detection of VIN has not been established. It is difficulty to determine the actual incidence of VIN since the lesions are often asymptomatic and go unnoticed if a thorough and systematic exploration of the vulva is not made during gynecological examination. In addition, population-based cancer registries do not usually include the incidence of these lesions. The incidence of VIN registered in Nordic countries is between 2.5 and 3.1 cases per 100,000 women/year, increasing after the age of In an international series of 587 consecutive cases of VIN, the mean age at the time of diagnosis was 50 years 11. Figure 1 shows the incidence rates of the precursor lesions of cervical, vulvar and vaginal cancer by country and age group. The reported incidence of VIN is below that of CIN (A) and above vagina intraepithelial lesions (C). In a pooled analysis of three clinical trials on HPV vaccines, the incidence of HPV16/18-related VIN was 0.04 per 100 people/year in the placebo group (7785 women). However, it is difficult to extrapolate the data related to the incidence of VIN in these clinical trials to the general population due to the bias induced by the population included (young age, HPV infection related to vaccine types, among others). In recent years, the incidence of VIN has increased, especially at younger ages. In addition to the increase in the number of lesions, detection is also greater due to greater awareness of HPV infection and better knowledge about the virus, precursor lesions and lower genital tract neoplasias 13. Data from the United States seem to confirm this increase in diagnosis Figure 2 shows the incidence rates of vulvar carcinoma in situ (equivalent to the current concept of VIN) between 1973 and , demonstrating a clear rise in the incidence in the last period with a peak at age 40. In Spain, a multicenter study including 5,665 women attended by 385 gynaecologists, found that VIN was diagnosed in 2% of all HPV-associated lower genital tract pathologies 15. Another study reported no differences in the frequency of VIN among the various age groups 16. In addition, epidemiological features differentiate HSIL (usual-type VIN) and differentiated-type VIN. HSIL lesions (usual-type VIN) tend to affect women aged around 40-45, with a second peak after the age of In contrast, differentiated-type VIN is most frequently found in older women usually over the age of 60. The incidence of VIN is clearly higher in HIV-positive patients compared to the general population. The results of a study evaluating a cohort of 2,791 women over 13 years reported an incidence of VIN of 0.42 vs per 100 women/year, respectively
14 AEPCC-Guidelines Figure 1 - Comparison of the incidence rates of VIN, CIN and VAIN by age in Nordic countries during Obtained from SanJosé S et al. Eur J Cancer 2013; 49(16): Figure 2 - Trends of vulvar carcinoma in situ by age and diagnostic period. Obtained from SanJosé S et al. Eur J Cancer 2013; 49(16):
15 Vulvar Intraepithelial Neoplasia (VIN) 4. Etiopathogenesis The genesis of VIN involves two types of etiopathogenesis, which are the basis for the development of two distinct entities: HSIL (usual-type VIN) and differentiated-type VIN. 4.1 HSIL (USUAL-TYPE VIN) Persistent infection and the oncogenic activity of the E6 and E7 proteins are crucial for the development of HSIL (usual-type VIN). Most HSIL cases (usual-type VIN) are positive for p16 and p14 (reflecting the interaction of the E6 and E7 oncoproteins in the cell cycle) and negative for p The causative agents of this entity are oncogenic HPV genotypes. In most cases, HPV-16 is involved. Data from a multicenter study carried out by the HPV-VVAP working group which included 587 cases of VIN and 1,709 cases of vulvar squamous cell carcinoma highlighted that HPV is present in 86.7% of VIN cases compared with 25.1% of invasive vulvar lesions. The most common genotypes in HSIL (usual-type VIN) were HPV-16 (77.3%) followed by HPV-33 (10.6%) and HPV-18 (2.5%) 11. Similar to what occurs with precursor lesions related to HPV in other anatomical locations, infections are transitory in 90% of cases and resolve by immune response within 2 years after presentation 19. Therefore, immunosuppression is frequently associated with viral persistence and the development of intraepithelial lesions Smoking is frequently associated with HSIL (usual-type VIN) DIFFERENTIATED-TYPE VIN Differentiated-type VIN is not causally related to HPV and is much less frequent than HSIL (usual-type VIN). This entity is associated with chronic inflammatory dermatoses such as lichen sclerosus and lichen simplex chronicus. The exact pathogenic mechanism of the development of differentiated-type VIN is unknown. Chronic inflammatory processes constitute the main factors involved in the development of this disease. From a molecular point of view, more than 80% of the cases show overexpression of p53 and negativity to p16 24,25. 15
16 AEPCC-Guidelines 5. Natural History Of Vin The natural history of VIN is largely unknown. The risk of progression of these lesions is not uniform and is mainly determined by the type of VIN. In general, the risk of progression of VIN to vulvar squamous cell carcinoma is 7-10% 26. One of the main difficulties in assessing the true potential of progression to vulvar cancer is that most studies assess the risk of progression after VIN treatment. On the other hand, the presence of occult invasion at the time of VIN diagnosis is relatively frequent. A systematic review of 3,322 patients with VIN found that 6.5% of patients developed vulvar squamous cell carcinoma, half of which were occult carcinomas detected during the diagnostic study and treatment and the other half were discovered during follow-up 27. Other studies have reported that in 3% of differentiated-type VIN there is an underlying occult carcinoma at the time of diagnosis 8 (Table 5). If no treatment is administered, VIN lesions may persist, progress or return to normal epithelium. In non-treated patients, VIN progression is estimated to be around 10% over a period of 1 to 8 years 27. Nonetheless, in spite of treatment, up to 8% of patients with VIN can develop vulvar squamous cell carcinoma 27,28. Differentiated-type VIN has a higher risk of progression to vulvar squamous cell carcinoma than HSIL (usual-type VIN) (33% versus 6%, respectively) and within a shorter period of time. Immunosuppression, advanced age, or extensive or ulcerated lesions are associated with an increased risk of progression26. Immunosuppression facilitates maintenance, recurrence and progression of these lesions, especially in HSIL (usual-type VIN) 29,30. Spontaneous complete remission has been described in around 1% of patients. In a review of cases, Van Seters et al.. found that remissions were seen especially in women under 35 years of age (mean age, 20 years) and were related to pregnancy in 17 out of 41 cases (Table 5). Along 10 months of follow-up, Jones et al. observed that spontaneous regression is possible in young people in 12% of cases 31. Table 5 - Natural history of VIN n % Progression to cancer Occult cancer Progression during follow-up Spontaneous regression Pregnant women (from the remission group) Systemic review of 97 series, 3,322 patients 27 16
17 Vulvar Intraepithelial Neoplasia (VIN) 6. Histology 6.1. HSIL (USUAL-TYPE VIN) Histologically, the epidermis in HSIL (usual-type VIN) shows acanthosis (dermal thickening) hyperkeratosis (hypertrophy of the stratum corneum of the epidermis) and parakeratosis (a cell maturation disorder on the stratum corneum of the epidermis). Loss of cell maturation, chromatin, increased mitotic figures, pleomorphism and increased nuclearcytoplasmic relationship are present. HSIL (usual-type VIN) lesions are divided into two subtypes: basaloid (undifferentiated) and condylomatose or bowenoid or so-called warty lesions. Basaloid lesions are typically flat and composed of small, uniform cells similar to basal cells, with an increased nuclear-cytoplasmic ratio and little or no evident koilocytic changes which replace the whole thickness of the epidermis. Warty lesions are characterized by acanthosis, with marked papilomatosis with thickened interpapillary ridges, marked cellular pleomorphism and prominent koilocytic changes. Basaloid and warty patterns are frequently combined in the same lesion. In one third of cases, the atypical cells extend to cutaneous annexes (pilosebaceous follicles and excretory ducts of the sweat glands). This may cause problems in the differential diagnosis with incipient invasion 32. differentiated-type VIN. Although these lesions may occur in young patients, they are generally diagnosed in women over 60 years of age. Differentiated-type VIN is often developed in chronic dermatological lesions such as lichen sclerosus and lichen simplex chronicus. Mutations of the p53 gene, a frequent phenomenon in HPV-negative vulvar squamous cell carcinomas, seem to be an early event in the development of lesions iin this type of VIN 22. Histological findings of differentiated-type VIN are subtle and difficult to recognize making diagnosis extremely difficult leading to little agreement among pathologists on the diagnosis of this entity 24. Consequently, up to 40% of these lesions are erroneously diagnosed as benign dermatoses 34. Differentiated-type VIN lesions are characterized by thickening of the epithelium, elongated and anastomosed interpapillary ridges and parakeratosis. Cellular atypia, which is often important, is confined to the basal and parabasal layers of the epidermis. Atypical mitosis is frequently identified in these layers. Basal layer keratinocytes characteristically show abundant and eosinophilic cytoplasm, very prominent intercellular bridges and prominent eosinophilic nucleoli. Immunohistochemically, HSIL (usual-type VIN) lesions are characterized by a continuous basal and parabasal band of positive cells showing intense nuclear and cytoplasmic staining for p16. Positivity for p16 is often extended to high strata of the epidermis. The Ki67 marker shows a notable increase in proliferative activity with positive cells spreading into the upper two-thirds of the epithelium. LSIL (HPV lesions) lesions secondary to transitory HPV infections may present staining patterns ranging from completely negative cases to others showing diffuse positivity similar to HSIL (usual-type VIN) DIFFERENTIATED-TYPE VIN Many HPV-negative keratinizing vulvar squamous cell carcinomas develop in intraepithelial lesions called Diskeratosic cells with premature maturation, cytoplasmic eosinophilia and premature keratinization in all epidermic layers are frequently observed. The epithelial cells of the upper layers have correct differentiation and marked maturation. These keratinocytes in the middle and upper layers do not show signs of atypia or show only focal atypia. Changes in lichen simplex chronicus or lichen sclerosus adjacent to lesions of differentiated-type VIN or other areas of the vulva are frequently identified. Five histological criteria have proven to be particularly useful in the diagnosis of this lesion: atypical mitosis in the stratum basale, atypia of basal cells, dyskeratosis, prominent nucleoli, and elongation and anastomosis of interpapillary ridges. The differential diagnosis of differentiated-type VIN includes reactive lesions such as pseudo-epitheliomatous hyperplasia, and some inflammatory skin lesions such as 17
18 AEPCC-Guidelines 7. Clinical characteristics The clinical presentation of VIN is very heterogeneous and can manifest with different symptoms in each patient. Only 50% of VIN lesions are symptomatic. Pruritus is the most common symptom followed by pain, stinging, dyspareunia or dysuria 37. In asymptomatic patients, the lesions are usually diagnosed unexpectedly during a gynecologic examination. This fact reinforces the importance of conducting systematic and thorough vulvar examinations 38. There is no characteristic lesion pattern of VIN, and clinical findings are very heterogeneous regarding color, surface, and topography. Lesions may be single or multiple, white, red or pigmented, and the surface can be completely flat or raised. HSIL (usual-type VIN) and differentiated-type VIN differ in not only their epidemiologic characteristics but also in their clinical presentation 9. HSIL (usual-type VIN): lesions tend to be polymorphic (often elevated or papillomatous and pigmented), and multifocal, located in the mucous areas devoid of hair, preferably in the lower third of the vulva. They frequently compromise several areas (vulvar and perineal). Association with intraepithelial lesions in other anatomical regions of the anogenital tract is frequent (either synchronously or metachronically). HSIL (usual-type VIN) is frequently associated with multifocal and multicentric lesions in the cervix and vagina 39. Between 30-70% of women with HSIL (usualtype VIN) have a synchronous HSIL (CIN2-3)40. The frequent presence of a multicentric pattern in women diagnosed with HSIL (usual-type VIN) justifies the need for thorough examination of the whole anogenital tract 41. Differentiated-type VIN: clinical and macroscopic manifestations of these lesions are nonspecific. The most outstanding feature of these lesions is often the association with other vulvar skin lesions such as lichen sclerosus and lichen simplex chronicus. Differentiatedtype VIN lesions tend to be single, small, white (due to hyperkeratoris) or reddish, ill-defined and frequently located in hairy areas. Patients frequently report longterm vulvar itching. The clinical features that characterize HSIL (usual-type VIN) and differentiated-type VIN are shown in Table 6. Table 6 - Clinical-pathological types of Vulvar Intraepithelial Neoplasia (VIN) VIN histological type HPV Non HPV HSIL (usual-type VIN) Differentiated-type VIN Age years > 40 years Presence of HPV Warts + - Abnormal cytology + - Smokers ++ +/- Immunosuppression, HIV + - Lesional foci Multiple Single TGI-related neoplasms ++ - Related with inflammatory dermatosis - ++ Prognosis Favourable Unfavourable Molecular markers Integrates HPV p16 and p16 p53 mutation 18
19 Vulvar Intraepithelial Neoplasia (VIN) 8. Diagnosis 8.1. CLINICAL EXAMINATION AND VULVOSCOPY 8.2. BIOPSY (INDICATIONS AND METHODOLOGY) Vulvar examination should be systematically performed in all women who visit the gynecologist, and especially in those with HPV-related lesions in other locations of the anogenital tract, vulvar skin diseases, or reporting any symptoms. Vulvar examination should include general and perianal inspection. The examination should be meticulous and with the help of adequate lighting to facilitate the identification of various macroscopic aspects of the lesions. Examination with a colposcope and acetic acid (vulvoscopy) allows magnified examination and more in depth inspection, and it is useful for the identification of suspicious lesions and for directing the biopsy 41. An acetic acid 5% solution should be applied for several minutes to be effective in the areas of keratinized epithelium. Bleaching of certain areas after applying the acetic acid allows the diagnosis of specific lesions that otherwise would not be identified. However, this procedure has a low specificity, since the vulvar epithelium, mainly at the introitus level, often reacts to the acetic acid in a diffuse manner in the absence of pathology. A low-power magnified inspection is used for general vulvar tracking and a high-power magnified inspection is carried out for in depth examination of small lesions. In addition to the acetowhite areas, abnormal mosaic and dotted vascular patterns may also occasionally be seen, although there is no analogy between the colposcopic changes described in the cervix and those found in the vulva. Vulvar examination or vulvoscopy can identify suspicious lesions but is not useful for diagnostic purposes. Since VIN has no pathognomonic macroscopic characteristics, confirmation of the diagnosis requires histological study of a sample obtained by biopsy. Table 7 describes the Indications for performing a vulvar biopsy. Table 7 - Indications for biopsy of a vulvar lesion 27 Pigmented lesions Condylomas / verrucous lesions in menopausal women Clinically non-filiated and doubtful vulvar lesion Suspected invasion Prior to destructive/medical treatment Biopsy is performed after infiltration of a local anesthetic forming a small wheal below the lesion. A punch clamp or Keyes dermal punch, a scalpel or scissors may be used. The sample should include subcutaneous tissue or stroma and allow proper orientation of the sample. Scant bleeding caused by the biopsy procedure can be easily controlled with perchloride of iron or Monsel s solution (ferric sulphate), silver nitrate, electrocoagulation, and exceptionally, stitches. When lesions are multifocal or diffuse it is recommended to take biopsies in different locations SUSPECTED OCCULT INVASION After the examination, all clinical findings (color, surface and vascularisation) as well as the topography and exact location should be described in detail using descriptive schemas or ideally photographs, vulvophotographs, or videos can be taken 43. Occult stromal invasion in patients with VIN is described in a high percentage of cases (2-22%, although the results in the literature are highly variable) and it is a primary target of the diagnosis. Clinical factors associated with a high risk of occult invasion are shown in Table 8 44,45. The capacity of vulvoscopy to detect occult invasion has been questioned. However, some authors argue that a careful and comprehensive colposcopic examination of the vulva along with liberal practice of a directed biopsy, greatly reduces the possibility of an invasive lesion going unnoticed
20 AEPCC-Guidelines 8.4. DIFFERENTIAL DIAGNOSIS Given the great heterogeneity in the clinical presentation of VIN, many vulvar disorders can be confused with this entity. In clinically borderline cases, the histological study will allow differentiating this lesion from condyloma acuminata seborrheic keratosis, psoriasis, lichen simplex chronicus and lichen sclerosus 46. Table 8 - Factors associated to the risk of occult invasion 44,45 Lesional factors Clinical factors Ulcerated, nodular or extent lesions Lesions with necrosis Lesions with indurated base Hyperkeratosic areas Multifocal lesions Atypic vascularisation Rapid growth of the lesion Advanced age (> 50 years) Immunosuppression Smoking 20
21 Vulvar Intraepithelial Neoplasia (VIN) 9. Treatment GENERAL PRINCIPLES There is no ideal treatment for patients with VIN, although there are specific recommendations to guide the most appropriate therapeutic course of action depending on specific clinical features 37. The main objectives of treatment are: 1. To prevent progression to invasive carcinoma 2. Cure or alleviate symptoms 3. Avoid relapses, and 4. To preserve vulvar anatomy and functionality. The increased incidence of VIN in young women described in recent years has contributed to the search for more conservative treatments. Before selecting a particular therapeutic option, it is essential to assess the risk of progression to cancer. This is conditioned by the characteristics of the patient (age, immunological status, associated pathology) and the lesion (location, extension, involvement of hairy/non-hairy uni/multifocality areas, and multicentricity) 47,48. There are multiple treatment options (excision, ablation, medical) that can be administered alone or in combination. In general, treatment is recommended in all patients with VIN. Excision is necessary in differentiated-type VIN and HSIL (usual-type VIN) with a high risk of occult invasion. Ablation and/or combined therapies may be used in cases in which invasion is ruled out 37. Expectant behavior by observation can be an alternative in very selected cases with a low risk of progression 27. Quality of evidence: moderate; Grade of recommendation: strongly in favor EXCISION Excision is the treatment of choice for differential VIN and HSIL (usual-type VIN) with no secondary lesions from ablation or topical treatments 37. Quality of evidence: moderate; Grade of recommendation: strongly in favor Simple local excision Procedure Excision of the whole lesion with a safety margin of 0.5 cm around the visible lesion (or less for lesions in which other anatomical structures such as the anus, urethra, or clitoris may be compromised) and a minimum depth of 3 mm in hairy areas and 1 mm in non-hairy areas. Indication Isolated unifocal or multifocal VIN lesions. Quality of evidence: moderate; Grade of recommendation: strongly in favor. Rationale Treatment with simple local excision can confirm the diagnosis of VIN, exclude the existence of invasion, and assess the status of the margins of the treated lesion 37,49. Compromised margins have shown to be an independent factor of lesion recurrence 37, but they are not associated with an increase in the risk of progression to invasive lesions The analysis of a series of 405 VIN patients treated with excision or C02 laser vaporization followed for 14 years showed, that half of the patients with positive margins and only 15% of patients with negative margins required a second treatment. Relapses occurred especially within the first 5 years after treatment 26. Another study in 73 cases of VIN treated with excision showed a clear relationship between recurrence and the presence of positive margins (46% and 17% for positive and negative margins respectively; p < 0.005) 49. In a review of 12 series including 296 patients, 23.9% of the cases undergoing local excision presented recurrence Partial or Total Skinning Vulvectomy Procedure Excision of the whole thickness of the vulvar skin includes hairy skin and adjacent follicles, with preservation of the subcutaneous tissue. This method includes the preservation of the clitoris although lesions on the surface of the gland or cap can be removed. The closure of the defect is usually 21
22 AEPCC-Guidelines primary and a free graft is used only exceptionally. In the case of lesions located in the perineum or perianal area, plastic surgery techniques (such as plasty by displacement and Dufourmentel LLL plasty) are frequently used to close the defect 52. Indication Widespread VIN lesions involving most of the vulvar tissue. Quality of evidence: low; Grade of recommendation: strongly in favor. Rationale Widespread VIN lesions involving most of the vulva require skinning vulvectomy. In a review of 9 series including 157 patients undergoing skinning vulvectomy, 22.3% presented recurrence Simple Vulvectomy Procedure Vulvectomy consists of the removal of all the vulvar skin including the labia majora, labia minora and clitoris, resulting in major deformity of the external genitalia. Indication This procedure is currently not indicated in any case of VIN. Quality of evidence: low; Grade of recommendation: strongly against. Rationale Vulvectomy is considered an excessive and unacceptable treatment for an intraepithelial lesion. Despite the invasiveness of this procedure, it is not exempt from recurrence, which has been described in up to 15% in some series Ablation Ablation consists of removing the entire lesion with various methods of tissue destruction without obtaining any surgical specimen for histological study. Before this treatment, it is mandatory to rule out the presence of invasive lesions by taking multiple biopsies. The main advantage of this procedure is the lesser invasiveness and better anatomical and functional conservation of the vulva compared with excisional procedures. These advantages are very important taking into account that VIN is increasingly diagnosed in younger women, in whom the most frequent histological type is HSIL (usual-type VIN), with a lower risk of progression to invasive lesions C02 Laser Vaporization Procedure CO2 laser vaporization involves the destruction of affected tissue. The laser beam may be directed using a manual or colposcope-connected device, which allows accuracy. The laser is applied in continuous mode at 10-20w. This procedure requires anesthesia (local for small lesions and general or regional for more extensive lesions). The depth of vaporization ablation depends on the area affected: 3-4 mm in hairy areas and mm in non-hairy areas 52 Indication HSIL (usual-type VIN), preferably located at the introitus or non-hairy areas, after ruling out invasion. This procedure is especially recommended in multifocal and extensive lesions 50,52,54. Quality of evidence: moderate; Grade of recommendation: strongly in favor. Rationale CO2 laser vaporization is one of the main ablative treatments, and it is characterized by its high accuracy and low aggressiveness. The minimum residual damage to the tissue significantly reduces the formation of scar tissue. The cosmetic advantages of surface vaporization (1 mm deep) over other excisional procedures are lost in cases in which hairy areas are involved since these areas require deeper vaporization (more than 2-3 mm). It is better to use other methods for the treatment of these surfaces Other destructive therapies under investigation Photodynamic therapy Procedure This treatment is based on the interaction between a photosensitizing substance which finds tumor cells (5-aminolevulinic acid) in combination with non-thermal light at wavelengths matched to the absorption characteristics of the photosensitizer. When the photosensitizer contacts 22
23 Vulvar Intraepithelial Neoplasia (VIN) the light, the formation of toxic singlet oxygen is induced generating free radicals. Within a few hours this procedure induces coagulative necrosis, apoptosis and microthrombosis of new tumor vessels and severe inflammation of the area by releasing vasoactive and procoagulant factors and the destruction of malignant cells 50. Indication HSIL (usual-type VIN), only in research. Quality of evidence: low; Grade of recommendation: strongly against. Rationale To date there is little scientific evidence for the clinical application of this treatment. The studies available only include small series with short follow-up periods, reporting response rates of around 55% with 48% of relapses Taking into account the mechanism of action of this procedure, its role could be limited to pigmented hyperkeratotic lesions Cavitational Ultrasonic Surgical Aspiration Procedure This treatment is based on the use of ultrasound to produce tissue destruction (cavitation), followed by aspiration to remove cell debris and blood. Indication HSIL (usual-type VIN), only in research. Quality of evidence: low; Grade of recommendation: strongly against. Rationale In general, there is little evidence in the literature on the use of this therapy in the treatment of these lesions. However, several studies have concluded that ultrasonic surgical aspiration is a useful and safe technique for tissue removal. The advantage of this treatment over others is based on rapid healing with good esthetic and functional results, and it allows material to be obtained for histological study 57. Data from a prospective randomized trial including 110 patients showed a recurrence rate of 25% TOPICAL TREATMENT Topical treatments have emerged as an alternative to surgery in VIN, although the majority of studies on this type of therapy describe isolated cases or short series. Although at present the FDA has not approved any topical treatment for VIN, its use is recommended and accepted by scientific societies in certain situations 37,38,50. Topical therapy alone or in combination with destructive or excisional therapies is indicated in the treatment of HSIL (usual-type VIN) in unifocal or multifocal isolated lesions after having ruled out occult invasion. Topical therapy is not indicated under any circumstance for differentiated-type VIN 59. Quality of evidence: low; Grade of recommendation: weakly in favor Fluorouracil (5-FU) Procedure The mechanism of action of 5-FU is based on producing a chemical peeling of VIN. The most commonly used dosage consists on the application of a thin layer of 5-FU 5% on the lesion (avoiding the rest of the vulva) 1-2 times a day for 6-10 weeks. Intense inflammatory response is observed in 2 weeks. Monitoring every 4-6 weeks during treatment is recommended. Local tissue response such as erythema, edema, skin desquamation and significant pain may be presented. In case of important local effects, the application may be reduced to once a week. Following completion of the treatment, complete tissue healing occurs within 4-6 weeks. Indication HSIL (usual-type VIN) after ruling out occult invasion and in the absence of other excisional or ablative treatments. Quality of evidence: low; Grade of recommendation: weakly in favor. Rationale The use of 5-FU has shown to be effective in 75% of cases. The main drawback limiting the use of this treatment is poor tolerance due to burns, pain, swelling, edema, and the development of vulvar ulcers. Given these disadvantages, the use of 5-FU is very limited
24 AEPCC-Guidelines Imiquimod Procedure Imiquimod is an immune response modulator with antitumor effects. Its activity is due to the stimulation of local cytokines and cellular immunity. The dosage most commonly used consists of the application of a thin layer of imiquimod 5% cream on the lesion (avoiding the rest of the vulva) before bedtime 2-3 times a week for a maximum of weeks, with follow-up every 4-6 weeks during treatment. This treatment may cause itching, redness and a severe inflammatory reaction. If important local effects develop, cream administration may be reduced to once a week. To treat a local reaction, non-steroidal antiinflammatory drugs (NSAIDs) may be used since it has been demonstrated that these antiinflammatories do not interfere with the immunomodulatory effect of imiquimod 54,60,61. Indication HSIL (usual-type VIN) for isolated unifocal or multifocal lesions alone or in combination after having ruled out occult invasion. Quality of evidence: high; Grade of recommendation: weakly in favor. Rationale Imiquimod is considered an effective treatment for VIN. However, studies comparing imiquimod with other therapies are needed 62. The first double-blind placebo-controlled studies showed imiquimod to be significantly more effective than placebo in the treatment of VIN 27,63. Data from a more recent observational study showed a complete response rate of 66.6% at 30 months of follow-up, being more effective in pigmented lesions. Similarly, a Cochrane meta-analysis found that imiquimod had an 11-fold higher efficacy compared to placebo in the treatment of these lesions 62 (Table 9). The best results were obtained in patients aged <65 and when severe local reactions were present 60. Surgical treatment may be required if residual lesions or lesional persistence develops following treatment with imiquimod Cidofovir Procedure Cidofovir is an acyclic nucleoside analog with antiviral activity. It acts by inducing apoptosis of HPV-infected cells and may thereby be effective for the treatment of HSIL?? (usual-type VIN). A topical formulation of 1% or 3% (gel, cream or intralesional injection) applied once or twice a day, up to a maximum of 10 weeks is generally used, although this Indication is not included in the summary of product characteristics. Indication HSIL (usual-type VIN), only in research. Quality of evidence: low; Grade of recommendation: weakly against. Rationale There are few studies on the use of cidofovir in VIN. A recent randomized multicenter study compared the efficacy of cidofovir 1% gel with imiquimod 5% in 180 patients diagnosed with VIN and reported a complete response rate of 46% in both groups after 6 weeks of treatment COMBINED TREATMENTS Procedure Combined treatment approaches involve the combination of more than one primary treatment for VIN. Excisional therapy combined with ablative or topical treatment in the residual lesion is the general approach. The most frequent combined procedure is excision + CO2 laser vaporization or excision + imiquimod. Indication Complex widespread or multifocal VIN lesions with: 1) excision of areas at risk of occult infiltration or hairy areas in which ablative treatment is more difficult, and 2) ablation or topical treatment in muco-cutaneous lesional areas. Quality of evidence: low; Grade of recommendation: strongly in favor. Rationale In the treatment of complex widespread lesions or multifocal lesions possible occult invasion must be ruled out while preserving the anatomical and functionality of the vulva. There are very few studies on combined 24
25 Vulvar Intraepithelial Neoplasia (VIN) Table 9. Efficacy of imiquimod in the treatment of VIN Author/year Study design Number of patients Quality of evidence Total response to Imiquimod Mathiesen Randomized controlled trial 21 High 81% Tien Le Cohort Study 36 Moderate 77% Van Seeters Randomized Study 52 High 35% Westermann Observational study 62 Low 76% Terlou Randomized controlled trial 24 High 35% Frega Tristam Randomized controlled trial comparing Imiquimod vs surgery Randomized controlled trial comparing Cidofovir vs Imiquimod 18 High 31% 180 total High 46% Kim Observational 9 Moderate 66% treatments in VIN. One study showed that the combination of excisional treatment and CO2 laser vaporization induced lower disease-free interval rates and a higher number of recurrences. Nonetheless, the results of this study may have been significantly biased by the inclusion of patients with complex lesions69. In a comparative study by Gentile et al. on the treatment of 80 patients with VIN (40 women treated with surgery alone and 40 undergoing surgery + imiquimod), the 5-year follow-up showed a recurrence rate of 44.8% versus 48.4%, respectively OBSERVATION WITHOUT TREATMENT Procedure This approach involves clinical observation without treatment at variable time intervals (every 6 months maximum). Observation without treatment is discontinued in cases presenting clear progression or suspicion of infiltration. Indication Observation without treatment is rarely carried out in HSIL (usual-type VIN), except in very limited lesions or pregnant women. Quality of evidence: low; Grade of recommendation: weakly in favor. There is no Indication for observation without treatment for differentiated-type VIN. Quality of evidence: low; Grade of recommendation: strongly in favor. Rationale Observation without treatment is only justified in cases with a low risk of progression or a high probability of spontaneous regression. For this reason, treatment is mandatory in women with differentiated-type VIN lesions and in elderly patients due to the high rate of progression and the risk of occult invasive carcinoma. In a very select group of HSIL (usual-type VIN), some authors advise an observational period without treatment of between 6 months and one year taking into account the possibility of spontaneous regression. Several studies have reported regression in: 1) patients under 35 years of age, 2) the presence of limited unifocal lesions, 3) the presence of isolated multifocal lesions, 4) pregnancy, and 5) cases with transitory immunosuppression. In all these cases, follow-up must be carried out 27,53. 25
26 AEPCC-Guidelines In this sense, a systematic review including 97 articles and 3,322 cases found that only 1.2% of the patients showed spontaneous and complete remission (most within 10 months after diagnosis, 41% were pregnant, and all were under 35 years of age). In the subgroup of 88 non-treated patients, 9 (10.2%) progressed to cancer within a variable period of between 12 and 96 months 27. Another study reported regression at between 3 and 30 months (mean, 9.5 months) in 14 young women (4 of whom were pregnant) with multifocal and pigmented VIN lesions associated with condylomas TREATMENT UNDER SPECIAL CIRCUMSTANCES Pregnancy Indication Observation without treatment is carried out in all cases provided an invasive lesion is previously ruled out 27,53. Quality of evidence: low; Grade of recommendation: strongly in favor. In cases requiring treatment, C02 laser vaporization is recommended 27,53. Rationale Small series have described the potential of lesion regression, especially in women under 30 years of age with pigmented lesions 71. There is currently little data on the use of medical therapies during pregnancy. Imiquimod is considered class C, and its safety in pregnancy has not been clearly established. Neither is there any evidence regarding its effectiveness during gestation Immunosuppression Indication The treatment of choice is lesion excision. In selected cases with widespread lesions or with specific compromised areas, ablative or combined treatments with C02 laser vaporization may be used. Quality of evidence: low; Grade of recommendation: moderately in favor. Rationale The treatment of VIN in immunosuppressed patients is justified by the increased severity of the lesions, less likelihood of regression, an increased risk of progression to invasive cancer and a higher recurrence rate. A retrospective study in 224 patients who received immunosuppressive therapy for renal transplantation reported an increased risk of developing a malignant tumor in the lower genital tract (2- to 6-fold for CIN, 3-fold for cervical carcinoma and 50-fold for vulvar cancer) 73. There are few data about the use of topical therapies in immunosuppressed patients. These treatments are used in cases in which ablative or surgical treatment compromises vulvar anatomy or functionality as in cases in which the lesions include anatomical areas such as the urethra, clitoris and non-hairy areas. 9.8 INVESTIGATIONAL THERAPIES: THERAPEUTIC VACCINES Therapeutic vaccines in patients with VIN are intended to stimulate cell immunity with the aim of achieving lesion regression. After the excellent results obtained in the prevention of VIN with prophylactic vaccines against HPV in recent years, phase II trials have been conducted to assess the role of therapeutic vaccines designed to stimulate response against cells expressing the viral E6 and E7 oncogenes and with a proliferative phenotype. Along this line, a study assessing the efficacy of a specific vaccine against HPV-16 E6 and E7 oncoproteins in 20 patients with HSIL (usual-type VIN) and HPV-16 infection yielded a response of 60% at 3 months and 79% at 12 months 74. The role of vaccines developed against oncogenes of HPV-16 and HPV-18 has also been evaluated, obtaining response rates (assessed as a reduction in lesion size) of 40-42% 75,76. These promising preliminary results, as well as those obtained with therapeutic vaccines for the treatment of CIN, must be validated in phase III clinical trials. 26
27 Vulvar Intraepithelial Neoplasia (VIN) 9.9 IMPACT OF VIN AND ITS TREATMENT ON PATIENT QUALITY OF LIFE In recent years the treatment of VIN has evolved to more conservative forms with the aim of decreasing the psychosexual impact on patients and increasing their quality of life 52. A recent multicenter study in 842 women evaluated the impact of HPV-related lesions in the lower genital tract on sexual function, general health and psychosocial burden using questionnaires. The results obtained were compared with those from women attended for a routine gynecological check-up. Patients with HPV lesions showed an increasing negative impact, proportional to the severity of the diagnosis. VIN patients showed a significant negative impact on sexual function compared with the general population 77. In general, the studies undertaken to date have shown a negative impact of the treatment on the quality of life of patients treated for VIN or vulvar cancer. A study of 58 patients with VIN and vulvar cancer treated with surgery or C02 laser vaporization showed that sexual dysfunction was independent of the type of treatment and increased with age 78. However, another study in patients with vulvar excision for VIN found a greater incidence of sexual and psychosomatic problems in women with vulvectomy compared to women with partial excision of the vulva 79. Data on the impact on sexual function and quality of life of patients with VIN are not very consistent. Indeed, the presence of genital lesions and symptoms associated with VIN represents aggression to the personal and sexual identity of the patient which goes beyond the treatment itself. Despite this, from the information available in the literature, it can be concluded that: 1) the diagnosis and treatment of VIN has a significant psychosexual impact on women, 2) the older the patient the greater the degree of involvement, and 3) the impact on the quality of life of patients is directly proportional to the aggressiveness of the treatment and the mutilation incurred. Therefore, in order to choose the most appropriate therapy for each patient it is essential to find the most effective and conservative options to preserve vulvar anatomy and functionality as well as the psychosexual dimension of the patients. Follow-up of treated VIN patients is imperative to detect possible recurrence and prevent progression to cancer
28 AEPCC-Guidelines 9.10 THERAPEUTIC ALGORITHM Differentiated-type VIN Excisional therapy: simple local excision Usual-type VIN Attempt to preserve vulvar anatomy and functionality Occult invasion ruled out Excisional therapies Ablative therapies Topical therapies Combined therapies Simple local excision Partial/total skinning vulvectomy C02 laser vaporization Imiquimod Excision + C02 laser Topical + Excision/C02 laser Isolated unifocal or multifocal lesions Confluent widespread multifocal lesions Isolated unifocal or multifocal lesions Confluent widespread multifocal lesions C02 laser lesions in introitum, clítoris, periurethral, non-hairy areas 28
29 Vulvar Intraepithelial Neoplasia (VIN) 10. Follow-Up 10.1 RECURRENCE AFTER TREATMENT According to the literature, the recurrence rate of VIN patients varies greatly from 20-50%, regardless of the therapeutic modality used 69,27,80. Risk factors for recurrence are: 1) widespread lesions, 2) positive margins at the excision site, 3) multifocal lesion, 4) immunosuppression, and 5) smoking. Similar to what occurs in other pre-malignant lesions of the lower genital tract, widespread lesions are associated with an increased risk of recurrence. In addition, these lesions are more difficult to treat, with incomplete or suboptimal treatments frequently being administered. Another factor clearly related to the risk of recurrence is the state of the surgical margins. Jones et at. described a recurrence rate of 50% in cases with affected margins compared with 15% in patients with disease-free margins 26. Other authors have reported similar findings 27. However, there are scarce studies including the state of the margins, and this may significantly bias the results. Some studies have questioned the association between affected margins and recurrence in HSIL (usual-type VIN) 27 and concluded that only multifocality is related to the risk of recurrence, postulating that the affected margin, considered as minimal residual disease, is able to trigger an activation of the immune system that may be effective in the resolution of the lesion. Evaluation of recurrence based on treatment is very complicated since the type of treatment is not an independent factor, being conditioned by multiple factors including lesion extension or multifocality. The few studies assessing treatment-related recurrence concluded that most of the therapeutic interventions for VIN have a similar efficacy with comparable recurrence rates. After a mean follow-up of 39 months, Van Seters et al. observed a recurrence rate of 19% after vulvectomy, 18% after partial vulvectomy, 22% after local excision and 23% after C02 laser vaporization 27. A review including 16 non-randomized cohorts series including 504 VIN cases treated with CO2 laser vaporization reported a mean recurrence rate of 12.8% (0-33%) 80. A retrospective study analyzing 93 VIN cases treated with different methods, with a mean follow-up of 54 months, showed a significantly higher risk of recurrence in cases with multifocal VIN (p=0.012) and positive HPV (p<0.001). In a multivariate analysis, the only risk factor was the presence of HPV (p=0.012) and multifocality in negative HPV cases (p=0.03) 56. Finally, a recently published multivariate study showed that smoking plays a key role in the risk of recurrence and progression of VIN. Compared to non-smokers smokers have a 1.6-fold and 3-fold greater risk of recurrence and progression, respectively83. Wallbillich et al. also found a significant association between smoking and relapse (p<0.001), extension of the lesion (p=0.016), and involvement of the margins (p=0.005) RISK OF PROGRESSION VIN is considered a precursor lesion for vulvar squamous cell carcinoma based on the histological and clinical association between these two entities. In addition, the presence of foci of invasion in surgical specimens of patients previously diagnosed with VIN (occult invasion) is confirmed in 3-20% of cases21. A systematic review of 3,322 VIN patients showed that 6.5% of cases developed vulvar squamous cell carcinoma, half of which were occult carcinomas detected during diagnostic studies 27. This same review also found that over a follow-up of 1-8 years, untreated patients had a 9% rate of progression to invasive cancer. Several studies have shown that the risk of progression differs depending on the type of VIN. However, there is no specific test to determine the behavior of VIN in each case. The limited data available in the literature do not allow the risk of malignancy in patients with differentiated-type VIN to be clearly established, but it is recognized to be considerably greater than that of HSIL (usual-type VIN). This increased 29
30 AEPCC-Guidelines risk may be explained by histological changes and the older age of the patients with differentiated-type VIN. Indeed, an analysis including 67 patients with differentiated-type VIN reported a rate of progression of 33% in non-treated patients 44. The mean time to progression to vulvar squamous cell cancer in VIN patients varies (3.9 years in non-treated women, 2.4 years in cases with incomplete treatment and 13.5 years in patients adequately treated) 26. Quantitatively, the risk of developing vulvar cancer after treatment for VIN is at least 10-fold higher than the risk of cervical cancer after treatment for HSIL/CIN347. Among cases with progression, two different situations are of note: cases with early progression (mean 2.4 years) in which the lesion is located at the same site as the previous lesion, probably related to incomplete excision, and late invasive lesions (average 13.8 years) located at a site different from previous lesions that have been completely resected 26. Advances in the knowledge of the role of the immune status of patients in the evolution of HSIL (usual-type VIN) will help to better understand the conditions favoring the persistence of HPV infection and the progression of VIN to invasive lesions. These advances could also help to determine immunological markers capable of predicting response to immunotherapy 50. Finally, the limited knowledge of recurrence and progression of VIN should be taken into account since most of the studies published to date are retrospective, with a small number of cases and with different follow-up periods, leading to non-significant results. Therefore, prospective, multicenter, randomized studies with a sufficient number of cases are needed 81. early diagnosis of recurrence. Currently, there are no clinical guidelines defining the most adequate follow-up period of women treated for VIN. In 2011, the recommendations on VIN of the American College of Obstetricians and Gynecologists and the American Society of Cervical Pathology and Colposcopy stated that women with complete response to treatment should be monitored at six months and then at 12 months post-treatment. Subsequently, monitoring should be done annually for a minimum of 10 years (see follow-up algorithm) 37. In the review conducted in Up-to-date biannual follow-up was recommended during the first five years followed by annual controls 23. Quality of evidence: low; Grade of recommendation: weakly in favor. Closer monitoring is required in immunocompromised women in whom the risk of recurrence or progression is high, with quarterly follow-up being advisable during the first two years followed by biannually thereafter. Quality of evidence: low; Grade of recommendation: weakly in favor. The coexistence of HSIL (usual-type VIN) with other HPVrelated diseases has been clearly described 82. Therefore, follow-up of patients with VIN should include careful examination of the entire anogenital tract. Finally, it is important to underline the need for these patients to be treated and followed by teams specialized in lower genital tract pathology. This criterion has been reinforced by the results of a study published in Northern Ireland which reported a rate of progression of VIN to cancer of greater than 20% in women treated by general gynecologists not following established protocols FOLLOW-UP GUIDELINES The high risk of recurrence and progression of VIN lesions makes long-term follow-up after treatment necessary (up to 35% of relapses occur within the first 5 years posttreatment). However, follow-up data of VIN patients are very limited and prospective studies have not shown the value of self-examination and regular visits after treatment. Despite this, it is logical to think that follow-up will allow 30
31 Vulvar Intraepithelial Neoplasia (VIN) 10.4 FOLLOW-UP ALGORITHM VIN post-treatment follow-up Detailed inspection of vulva +/- vulvoscopy In depth examination of the lower genital tract First follow-up at 6 months* Second follow-up at 12 months Annual follow-up for 10 years *Immunocompromised patients: quarterly follow-ups for the first two years followed by biannually thereafter 31
32 AEPCC-Guidelines 11. Primary prevention of VIN. role of prophilactic vaccines against HPV. For years, the primary prevention of HSIL (usual-type VIN) has been based on the prevention of HPV infection and the avoidance of factors involved in its persistence, such as smoking 26. Currently, prophylactic vaccination against HPV is the mainstay in the prevention of these lesions. Although the role of vaccines in the prevention of invasive vulvar cancer has not been directly demonstrated, the decrease in the incidence of pre-malignant HSIL lesions (usual-type VIN) as a surrogate marker suggests that a reduction in the rates of HPV-related invasive vulvar cancer can be foreseen 4. There are currently two prophylactic vaccines against HPV. The results of phase II and III clinical trials have shown that both the bivalent and quadrivalent vaccines are effective in the prevention of VIN lesions, with figures ranging between % depending on the population group analyzed. Based on these data one of the indications of both vaccines is the prevention VIN, as stated in the summary of the product characteristics of these vaccines 85,86. The marketing of HPV vaccines has evolved around clinical trials designed to determine the impact of these vaccines on the development of cervical lesions, leading to the recruitment of a small number of VIN lesions and thereby impeding the analysis of other types of data such as costeffectiveness. The low incidence of VIN requires large multicenter studies to reliably determine both the future effectiveness (effectiveness in real life) and the cost-benefit ratio of prophylactic vaccines in all HPV-related lesions 50. However, although post-hoc studies do not exclusively address VIN lesions, the results of these studies indicate the need to consider the overall benefit that vaccination may have in high grade vulvar lesions with a positive impact. Several pharmacoeconomic studies have analyzed the economic impact of prophylactic vaccines on the prevention of VIN and have shown that vaccines against HPV are costeffective in cohorts of pre-adolescent girls 87. Prophylactic vaccines against HPV are the only method of primary prevention of HSIL (usual-type VIN) available. In the near future, primary and secondary prevention based on early diagnosis and adequate treatment of these lesions will likely significantly reduce the incidence of vulvar cancer which, albeit rare, causes great patient morbidity and mortality. 32
33 Vulvar Intraepithelial Neoplasia (VIN) 12. Recommendations 1. The diagnosis and treatment of VIN aims to prevent vulvar squamous cell carcinoma. 2. Given the heterogeneity of the clinical presentation of VIN, histological study of all suspicious and non-filiated vulvar lesions is essential. 3. Treatment is recommended in all cases of VIN. 4. The treatment of choice for differentiated-type VIN is excision. Ablative or topical therapies may be used in HSIL (usual-type VIN) after ruling out occult invasion. 5. Therapeutic strategies as conservative as possible are needed in order to preserve vulvar anatomy and functionality, while always ensuring satisfactory results in terms of efficacy. 6. The high percentage of recurrence, regardless of the therapeutic approach, as well as the risk of progression to invasive lesions despite treatment, makes close monitoring of these patients necessary, especially in immunocompromised subjects. Follow-up is recommended at 6 and 12 months post-treatment and annually thereafter. 33
34 AEPCC-Guidelines 13. References 1. Crum CP, Fu YS, Levine RU, Richart RM, Townsend DE, Fenoglio CM. Intraepithelial squamous lesions of the vulva: Biologic and histologic criteria for the distinction of condyloma from vulvar intraepithelial neoplasia. Am J Obstet Gynecol 1982;144:77Y83, PMID: Wilkinson EJ1, Cox JT, Selim MA, O Connor DM. Evolution of terminology for human-papillomavirus-infection-related vulvar squamous intraepithelial lesions. J Low Genit Tract Dis Jan;19(1): Scurry J, Wilkinson EJ. Review of terminology of precursors of vulvar squamous cell carcinoma. J Low Genit Tract Dis Jul;10(3): Review. 4. Wilkinson EJ, Teixeira MR. Tumors of the vulva. In: Tavassoli FA, Deville T, eds. Pathology & Genetics, Tumours of the Breast and Female Genital Organs, In: World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2003:316Y Sideri M1, Jones RW, Wilkinson EJ, Preti M, Heller DS, Scurry J, Haefner H, Neill S. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med Nov;50(11): Darragh TM, Colgan TJ, Thomas Cox J, Heller DS, Henry MR, Luff RD, McCalmont T, Nayar R, Palefsky JM, Stoler MH, Wilkinson EJ, Zaino RJ, Wilbur DC; Members of the LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol Jan;32(1): ISSVD Terminology of Vulvar Squamous Intraepithelial Lesions. Accepted in New York World Congress, July 28, Terminology Committee: Bornstein J, Bogliatto F, Bohl T, Coady D, Haefner H, Preti M, Reutter J, Selva-Nayagam P, Stockdale C, Van-Beurden M. In preparation for publication Nygård M, Hansen BT, Dillner J, Munk C, Oddsson K, Tryggvadottir L, et al. Targeting human papillomavirus to reduce the burden of cervical, vulvar and vaginal cancer and pre-invasive neoplasia: establishing the baseline for surveillance. PloS One. 2014;9(2):e de Sanjosé S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesionsofthevulva. EurJCancer.2013Nov;49(16): Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Garland SM, Harper DM, Tang GW, Ferris DG, Steben M, Jones RW, Bryan J, Taddeo FJ, Bautista OM, Esser MT, Sings HL, Nelson M, Boslego JW, Sattler C, Barr E, Paavonen J. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet.2007 May 19;369(9574): Hampl M, Sarajuuri H, Wentzensen N, Bender HG, Kueppers V. Effect of human papillomavirus vaccines on vulvar, vaginal, and anal intraepithelial lesions and vulvar cancer. Obstet Gynecol Dec;108(6): Crum CP, Herrington CS, McCluggage WG, Regauer S, Wilkinson EJ. Tumours of the vulva; epithelial tumors. In: Kurman RJ et al. ed. WHO Classification of Tumours of Female Reproductive Organs. 4th Edition. IARC Press, Lyon Bogliatto F, Bohl T, Reutter J, Sideri M, Bornstein J. LAST terminology applied to the vulva: the challengue of VIN continues. J Low Genit Tract Dis 2015;19:e Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol May;107(5): Cortés J, Castellsagué X, Torné A, Gil Á, San-Martín M. Patología del tracto genital inferior asociada al virus del papiloma humano en mujeres españolas. Prog Obstet Ginecol Jul;54(7):
35 Vulvar Intraepithelial Neoplasia (VIN) 16. Gil-Prieto R, Cortés J, Manuel Ramón Y Cajal J, Ester PV, Gil de Miguel A. Hospitalization in Spain associated with malignant neoplasias of the vulva and vagina ( ). HumVaccin.2011Aug1;7(8): Joura EA, Lösch A, Haider-Angeler MG, Breitenecker G, Leodolter S. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. J Reprod Med Aug;45(8): Massad LS, Xie X, Darragh T, Minkoff H, Levine AM, Watts DH, et al; Women s Interagency HIV Study Collaborative Study Group. Genital warts and vulvar intraepithelial neoplasia: natural history and effects of treatment and human immunodeficiency virus infection. Obstet Gynecol Oct;118(4): Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med Feb 12; 338(7): van Seters M, Beckmann I, Heijmans-Antonissen C, van Beurden M, Ewing PC, Zijlstra FJ, et al. Disturbed patterns of immunocompetent cells in usual-type vulvar intraepithelial neoplasia. Cancer Res Aug 15;68(16): van Esch EM, Welters MJ, Jordanova ES, Trimbos JB, van der Burg SH, van Poelgeest MI. Treatment failure in patients with HPV 16-induced vulvar intraepithelial neoplasia: understanding different clinical responses to immunotherapy. Expert Rev Vaccines Jul;11(7): Santos M, Montagut C, Mellado B, García A, Ramón y Cajal S, Cardesa A, Puig-Tintoré LM, Ordi J. Immunohistochemical staining for p16 and p53 in premalignant and malignant epithelial lesions of the vulva. Int J Gynecol Pathol. 2004;23: Holschneider CH, Goff B, Garcia RL, Falk SJ. Vulvar intraepithelial neoplasia. UpToDate Ordi J, Alejo M, Fusté V, Lloveras B, Del Pino M, Alonso I, Torné A. HPV-negative vulvar intraepithelial neoplasia (VIN) with basaloid histologic pattern: an unrecognized variant ofsimplex (differentiated) VIN. Am J Surg Pathol Nov;33(11): Yang B, Hart WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: A clinicopathologic study including analysis of HPV and p53 expression. Am J Surg Pathol 2000;24: Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol Dec;106(6): van Seters M, van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005;97: Reyes MC, Cooper K. An update on vulvar intraepithelial neoplasia: terminology and a practical approach to diagnosis. J Clin Pathol ;67 : Chiasson MA, Ellerbrock TV, Bush TJ, Sun XW, Wright TC Jr. Increased prevalence of vulvovaginal condyloma and vulvar intraepithelial neoplasia in women infected with the human immunodeficiency virus. Obstet Gynecol. 1997;89(5 Pt 1): Coronado PJ, Fasero M, Ramírez M, Arab C, Bellón M, García- Santos J, Vidart JA. La inmunosupresión es un factor mayor de riesgo de la recidiva de las lesiones del tracto genital inferior asociadas al virus del papiloma humano. Prog Obstet Ginecol 2010;53(5): Jones RW, Rowan DM. Vulvar intraepithelial neoplasia III: a clinical study of the outcome in 113 cases with relation to the later development of invasive vulvar carcinoma. Obstet Gynecol. 1994;84(5): del Pino M, Rodriguez-Carunchio L, Ordi J. Pathways of vulvar intraepiteliales neoplasia y escamosas cell carcinoma. Histopathology. 2013;62: Santos M, Landolfi S, Olivella A, et al. p16 overexpression identifies VPH-positive vulvar escamosas cell carcinomas. Am J Surg Pathol. 2006;30: van de Nieuwenhof HP, Bulten J, Hollema H, Dommerholt RG, Massuger LF, van der Zee AG, de Hullu JA, van Kempen LC. Differentiated vulvar intraepithelial neoplasia is often found in lesions, previously diagnosed as lichen sclerosus, which have progressed to vulvar squamous cell carcinoma. Mod Pathol Feb;24(2): Saglam O, Samayoa E, Somasekar S, et al. No viral association found in a set of differentiated vulvar intraepiteliales neoplasia cases by human papillomavirus y pan-viral microarray testing. PLoS One. 2015;10:e
36 AEPCC-Guidelines 36. van den Einden LC, de Hullu JA, Massuger LF, et al. Interobserver variability y the effect of education in the histopathological diagnosis of differentiated vulvar intraepiteliales neoplasia. Mod Pathol. 2013;26: Jones RW. Letter to van Seters: Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol p ACOG. Management of vulvar intraepithelial neoplasia. Committee Opinion Nº.509. American College of Obstetricians and Gynecologists. Obstet Gynecol 2011; 118: Van de Nieuwenhof HP, van der Avoort IAM, de Hullu JA. Review of squamous premalignant vulvar lesions. Critical Reviews in Oncology/Hematology 2008;68: Comino R, Coronado P, Cararach M, Nieto A, Martunez Escoriza JC, Salamanca A, Torres LM, Vidart JA, Mendoza N, Torné A, Sanchez Borrego R. Spanish Consensus on vulvar disorders in postmenopausal women. Maturitas 2015;80: Preti M, Igidbashian S, Costa S, Cristoforoni P, Mariani L, Origoni M, et al. VIN usual type-from the past to the future. Ecancermedicalscience Apr 29;9: Hørding U, Junge J, Poulsen H, Lundvall F. Vulvar intraepithelial neoplasia III: a viral disease of undetermined progressive potential. Gynecol Oncol. 1995;56(2): Modesitt SC et al. Vulvar intraepithelial neoplasia III: occult cancer and the impact of margin status on recurrence Obstet Gynecol 1998; 92: Preti M, Igidbashian S, Costa S, Cristoforoni P, Mariani L, Origoni M, et al. VIN usual type-from the past to the future. ecancer 2015, 9: Puig-Tintoré LM, Jou Collel P, Casanova Domenech L, Lejarcegui Fort JA,. Neoplasia Intraepitelial y cáncer de vulva. En: González-Merlo et al. Oncología Ginecológica. Barcelona: Salvat 1991; pag Cararach M, Dexeus D. Preinvasive lesions of the vulva. CME J Gynecol Oncol 2007; 12: Eva LJ, Ganesan R, Chan KK, Honest H, Luesley DM. Differentiated-type vulval intraepithelial neoplasia has a high-risk association with vulval squamous cell carcinoma. Int J Gybecol Cancer 2009; 19: Bornstein J, Tuma R. Metodología diagnóstica en la neoplasia intraepitelial vulvar. En: Tatti, Fleider, Maldonado, Suzuki (Editors). Enfermedades de la vulva, la vagina y la región anal. Cap 21.1 ( ). Ed Panamericana Puig-Tintoré LM, Ordi J, Torné A, Jou P, Pahisa J, Lejárcegui J a. Neoplasia vulvar intraepitelial (VIN). Progresos Obstet y Ginecol Jan;45(11): Puig-Tintoré LM. Neoplasias intraepiteliales de vulva, vagina y ano. En Pahisa J. y Torné A. Directores. Ginecología Oncológica. Cursos CLINIC de Formación Continuada en Obstetricia y Ginecología Ergon. 54. Preti M, Scurry J, Marchitelli CE, Micheletti L. Vulvar intraepithelial neoplasia. Best practice & research Clinical obstetrics & gynaecology. 2014;28(7): van de Nieuwenhof HP, Massuger LFAG,, van der Avorrt IAM, Bekkers RLM, Casparine M, Abma W, et al. Vulvar squamous cell carcinoma development after diagnosis of VIN invreases with age. Eur J Cancer 2009; 45: Bornstein J, Sideri M, Tatti S, Walker P, Prendiville W, Haefner H. For the Nomenclature Committee of the International Federation for Cervical Pathology and Colposcopy. J Lower Gen Tract Dis 2012; 16(3): Holschneider CH. Vulvar intraepithelial neoplasia. Rev. UpToDate Winters U, Daayana S, Lear JT, Tomlinson AE, Elkord E, Stern PL, et al. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(16): Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO(2) laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecologic oncology. 2006;100(2):
37 Vulvar Intraepithelial Neoplasia (VIN) 57. Miller BE. Vulvar intraepithelial neoplasia treated with cavitational ultrasonic surgical aspiration. Gynecologic oncology. 2002;85(1): von Gruenigen VE, Gibbons HE, Gibbins K, Jenison EL, Hopkins MP. Surgical treatments for vulvar and vaginal dysplasia: a randomized controlled trial. Obstetrics and gynecology. 2007;109(4): Regauer S. Etiology of vulvar cancer will impact on treatment options and therapy outcome: two major pathways of vulvar cancer. Gynecol Oncol 2013;131: Westermann C, Fisher A, Clad A. Treatment of vulvar intraepithelial neoplasia with topical 5% Imiquimod cream. Int J Obstet Gynecol 2013;120: Terlou A, Kleinjan A, Beckman I, Heijmans-Antonissen C, van Seeters M, Santegoets LA, van Beurden M, Helmershorst TJ, Blok LJ. Nonsteroidal anti-inflammatory drugs do not interfere with imiquimod treatment for usual type vulvar intraepithelial neoplasia. Int J of Cancer 2011;128: Tristam A, Hurt CN, Madden T, Powell N, Man S, Hibbits S, Dutton P, Jones S, Nordin A, Naik R, Fiander A, Griffiths G. Activity, Safety and feasibility of cidofovir and imiquimod for treatment of vulvar intarepithelial neoplasia (RT3VIN): a multicentre phase 2 trial. Vol 15 nov Kim JM, Lee HJ, Kim SH, Kim HS, Ko HC, KIM BS, KIM MB, Song M. Efficacy of 5% imiquimod cream on vulvar intraepithelial neoplasia in Korea: pilot study. Annals of Dermatol 2015;27: Wallbilich JJ, Rhodes HE, Milbourne AM, Munsell MF, Frumovitz M, Brown J, Trimble CL, Schmeler KM. Vulvar intraepithelial neoplasia (VIN2/3): comparing clinical outcomes and evaluating risks factors for recurrence. Gynecol Oncol 2012; 127: Gentile M, Bianchi P, sesti F, Sopracordevole F, Biamonti A, Scirpa P, Schinbermi M, Cozza G, Marziani R, di Martino G, Catalano A, Milazzo GN, Zinna M, Caserta D, Frega A. Adjuvant topical reatment with imiquimod 5% after excisional surgery for VIN 2/3. Europ Rew Med Pharmacol Sc 2014;18: Jones RW, Rowan DM. Spontaneous regression of vulvar intraepithelial neoplasia 2-3. Obstet Gynecol 2000;96: Pepas L, Kaushik S, Bryant A, et al. Medical interventions for high grade vulvar intraepithelial neoplasia. CochraneDatabase Syst Rev 2011;(4):CD Mathiesen O, Buus SK, Cramers M. Topical Imiquimod can reverse vulvar intraepithelial neoplasia: a randomised doubleblinded study. Gynecol Oncol 2007;107: Food and Drug Administration. Highlights of prescribing information: Aldara (Imiquimod cream) Accesed Meeuwis KA, van Rossum MM, van de Kerkhof PC, Hoitsma AJ, Massuger LF, de Hullu JA. Skin cancer and (pre)malignancies of the female genital tract in renal transplant recipients.transpl Int Feb;23(2): Le T, Menard C, Hicks-Boucher W, Hopkins L, Weberpas J, Fung-Kee-Fung M. Final results of a phase 2 study using 5% Imiquimod cream application in the primary treatment of high grade vulva intraepithelial neoplasia. Gynecol Oncol 2007;106: van Seeters M, van Beurden M, ten Kate FJ, Beckman I, Ewing PC, Eijkemnas MJ, Kagie MJ, Meijer CJ, Aaronson NK, Kleinjan A, Heimans-antonissen C, Zijlstra FJ, Burger MP, Helmerhorst TJ. Treatment of vulvar intraepithelila neoplasia with imiquimod. New E J Med 2008;358: Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berendsvan der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. The New England journal of medicine. 2009;361(19): Baldwin PJ, van der Burg SH, Boswell CM, Offringa R, Hickling JK, Dobson J, et al. Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9(14): Frega A, Sesti F, Sopracordevole F, Biamonti A, Scirpa P et al. Imiquimod 5% versus cold knije excision for treatment of VIN3/3: a five year follow up. European Review for Med Pharmacol Sciences 2013;17: Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer research. 2003;63(18):
38 AEPCC-Guidelines 77. Dominiak-Felden G, Cohet C, Atrux-Tallau S, Gilet H, Tristram A, Fiander A. Impact of human papillomavirus-related genital diseases on quality of life and psychosocial wellbeing: results of an observational, health-related quality of life study in the UK. BMC public health. 2013;13: Grimm D, Eulenburg C, Brummer O, Schliedermann AK, Trillsch F, Prieske K, et al. Sexual activity and function after surgical treatment in patients with (pre)invasive vulvar lesions. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer Thuesen B, Andreasson B, Bock JE. Sexual function and somatopsychic reactions after local excision of vulvar intraepithelial neoplasia. Acta obstetricia et gynecologica Scandinavica. 1992;71(2): Epub 1992/02/ Comino R. Infección por Papilomavirus en Ginecología. Delgado RC, editor. Madrid Athavale R, Naik R, Godfrey KA, Cross P, Hatem MH, de Barros Lopes A. Vulvar intraepithelial neoplasia--the need for auditable measures of management Eur J Obstet Gynecol Reprod Biol Mar;137(1): Goffin F, Mayrand MH, Gauthier P, et al. High-risk human papillomavirus infection of the genital tract of women with a previous history or current high-grade vulvar intraepithelial neoplasia. J Med Virol. 2006;78: Zawislak AA, Price JH, Dobbs SP, McClelland HR, McCluggage WG.The management of vulval intraepithelial neoplasia in Northern Ireland. Int J Gynecol Cancer 2006;16(2): Jemal A, Simard EP, Dorell C, Noone AM, Markowitx LE, Kholer B et al. Annual report to the nation on the status of cancer, , featuring the burden and trends in human papillomavirus(hpv)-associated cancers and HPV vaccination coverage levels J Natl Cancer Inst 2013; Ficha Técnica de Cervarix [consultado Julio 2015]. Disponible en: Ficha Técnica de Gardasil [consultado Julio 2015]. Disponible en: Giraldi G, Martinoli L, De Luca d Alessandro E. The human papillomavirus vaccination: a review of the cost-effectiveness studies. Clin Ter 2014; 165(6) 38
39 Vulvar Intraepithelial Neoplasia (VIN) 39
40
Disorders of the vulva
 Vulval lesions Disorders of the vulva Terminology standardised by the International Society for the Study of Vulvovaginal Disease(ISSVD) Classification 1.Nonneoplastic epithelial disorders of vulva Lichen
Vulval lesions Disorders of the vulva Terminology standardised by the International Society for the Study of Vulvovaginal Disease(ISSVD) Classification 1.Nonneoplastic epithelial disorders of vulva Lichen
Diseases of the vulva
 Diseases of the vulva 1. Bartholin Cyst - Infection of the Bartholin gland produces an acute inflammation within the gland (adenitis) and may result in an abscess. Bartholin duct cysts - Are relatively
Diseases of the vulva 1. Bartholin Cyst - Infection of the Bartholin gland produces an acute inflammation within the gland (adenitis) and may result in an abscess. Bartholin duct cysts - Are relatively
Clinically Microscopically Pathogenesis: autoimmune not lifetime
 Vulvar Diseases: Can be divided to non-neoplastic and neoplastic diseases. The neoplastic diseases are much less common. Of those, squamous cell carcinoma is the most common. most common in postmenopausal
Vulvar Diseases: Can be divided to non-neoplastic and neoplastic diseases. The neoplastic diseases are much less common. Of those, squamous cell carcinoma is the most common. most common in postmenopausal
VULVAR CARCINOMA. Page 1 of 5
 VULVAR CARCINOMA EXAMPLE OF A VULVAR CARCINOMA USING PROPOSED TEMPLATE Case: Invasive squamous cell carcinoma arising in D-VIN Tumor in left labia major Left partial vaginectomy and sentinel lymph node
VULVAR CARCINOMA EXAMPLE OF A VULVAR CARCINOMA USING PROPOSED TEMPLATE Case: Invasive squamous cell carcinoma arising in D-VIN Tumor in left labia major Left partial vaginectomy and sentinel lymph node
Treatment of Bowenoid and Basaloid Vulvar Intraepithelial Neoplasia 2/3 with Imiquimod 5% Cream DO NOT DUPLICATE
 The Journal of Reproductive Medicine Treatment of Bowenoid and Basaloid Vulvar Intraepithelial Neoplasia 2/3 with Imiquimod 5% Cream Claudia Marchitelli, M.D., Graciela Secco, M.D., Myriam Perrotta, M.D.,
The Journal of Reproductive Medicine Treatment of Bowenoid and Basaloid Vulvar Intraepithelial Neoplasia 2/3 with Imiquimod 5% Cream Claudia Marchitelli, M.D., Graciela Secco, M.D., Myriam Perrotta, M.D.,
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER VULVAR
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER VULVAR Last Revision Date July 2015 1 Site Group: Gynecologic Cancer Vulvar Author: Dr. Stephane Laframboise 1. INTRODUCTION
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER VULVAR Last Revision Date July 2015 1 Site Group: Gynecologic Cancer Vulvar Author: Dr. Stephane Laframboise 1. INTRODUCTION
Appropriate Use of Cytology and HPV Testing in the New Cervical Cancer Screening Guidelines
 Appropriate Use of Cytology and HPV Testing in the New Cervical Cancer Screening Guidelines Tim Kremer, MD Ralph Anderson, MD 1 Objectives Describe the natural history of HPV particularly as it relates
Appropriate Use of Cytology and HPV Testing in the New Cervical Cancer Screening Guidelines Tim Kremer, MD Ralph Anderson, MD 1 Objectives Describe the natural history of HPV particularly as it relates
VIN/VAIN O C T O B E R 3 RD J M O R G A N
 VIN/VAIN O C T O B E R 3 RD 2 0 1 8 J M O R G A N Vaginal Intraepithelial Neoplasia VAIN I, II, III Incidence 0.1/100,000 women in US Mean age 50s (J Womens Health (Larchmt) 2009:18:1731) (J Obstet Gynaecol
VIN/VAIN O C T O B E R 3 RD 2 0 1 8 J M O R G A N Vaginal Intraepithelial Neoplasia VAIN I, II, III Incidence 0.1/100,000 women in US Mean age 50s (J Womens Health (Larchmt) 2009:18:1731) (J Obstet Gynaecol
Vaginal intraepithelial neoplasia
 Vaginal intraepithelial neoplasia The terminology and pathology of VAIN are analogous to those of CIN (VAIN I-III). The main difference is that vaginal epithelium does not normally have crypts, so the
Vaginal intraepithelial neoplasia The terminology and pathology of VAIN are analogous to those of CIN (VAIN I-III). The main difference is that vaginal epithelium does not normally have crypts, so the
Human Papillomavirus
 Human Papillomavirus Dawn Palaszewski, MD Assistant Professor of Obstetrics and Gynecology University of February 18, 2018 9:40 am Dawn Palaszewski, MD Assistant Professor Department of Obstetrics and
Human Papillomavirus Dawn Palaszewski, MD Assistant Professor of Obstetrics and Gynecology University of February 18, 2018 9:40 am Dawn Palaszewski, MD Assistant Professor Department of Obstetrics and
HPV-related papillomatous-condylomatous lesions in female anogenital area
 HPV-related papillomatous-condylomatous lesions in female anogenital area Theo Panoskaltsis MD, FRCOG, CCST (UK) Epidemiology Anal cancer is increasing in both men and women Groups at risk: - HIV (+)
HPV-related papillomatous-condylomatous lesions in female anogenital area Theo Panoskaltsis MD, FRCOG, CCST (UK) Epidemiology Anal cancer is increasing in both men and women Groups at risk: - HIV (+)
Vulvar intraepithelial neoplasia: Current concepts
 Vulvar intraepithelial neoplasia: Current concepts L. Stewart Massad, M.D. Dept. of Obstetrics & Gynecology Washington University School of Medicine St. Louis, MO Disclosure I have no financial ties to
Vulvar intraepithelial neoplasia: Current concepts L. Stewart Massad, M.D. Dept. of Obstetrics & Gynecology Washington University School of Medicine St. Louis, MO Disclosure I have no financial ties to
Cervical Dysplasia and HPV
 Cervical Dysplasia and HPV J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse HPV Double stranded DNA virus The HPV infect epithelial cells of the skin and mucous membranes Highest risk
Cervical Dysplasia and HPV J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse HPV Double stranded DNA virus The HPV infect epithelial cells of the skin and mucous membranes Highest risk
Vulvar squamous cell carcinoma
 The Clinical Significance of Stratifying Vulval Squamous Carcinoma into HPV and Non-HPV Related Variants C. BLAKE GILKS MD FRCPC Dept of Pathology, University of British Columbia Vulvar squamous cell carcinoma
The Clinical Significance of Stratifying Vulval Squamous Carcinoma into HPV and Non-HPV Related Variants C. BLAKE GILKS MD FRCPC Dept of Pathology, University of British Columbia Vulvar squamous cell carcinoma
!"#$%&'(#)*$+&,$-&.#,$/#0()1-$ ),1')$2(%&,2#,%$%(0'#$34567$
 !"#$%&'(#)*$+&,$-&.#,$/#0()1-$ ),1')$2(%&,2#,%$%(0'#$34567$ Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
!"#$%&'(#)*$+&,$-&.#,$/#0()1-$ ),1')$2(%&,2#,%$%(0'#$34567$ Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
When Immunostains Can Get You in Trouble: Gynecologic Pathology p16: Panacea or Pandora s Box?
 When Immunostains Can Get You in Trouble: Gynecologic Pathology p16: Panacea or Pandora s Box? Teri A. Longacre, MD Stanford Medicine Stanford California pi6 in Gynecologic Pathology: Panacea or Pandora
When Immunostains Can Get You in Trouble: Gynecologic Pathology p16: Panacea or Pandora s Box? Teri A. Longacre, MD Stanford Medicine Stanford California pi6 in Gynecologic Pathology: Panacea or Pandora
The society for lower genital tract disorders since 1964.
 The society for lower genital tract disorders since 1964. Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
The society for lower genital tract disorders since 1964. Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
Faculty Pap Smear Guidelines: Family Planning Update 2008 Part Two
 Faculty Pap Smear Guidelines: Family Planning Update 2008 Part Two Seshu P. Sarma, MD, FAAP Emory University Regional Training Center Atlanta, Georgia Produced by the Alabama Department of Public Health
Faculty Pap Smear Guidelines: Family Planning Update 2008 Part Two Seshu P. Sarma, MD, FAAP Emory University Regional Training Center Atlanta, Georgia Produced by the Alabama Department of Public Health
chapter 4. The effect of oncogenic HPV on transformation zone epithelium
 chapter 4. The effect of oncogenic HPV on transformation zone epithelium CHAPTER 1 All squamous cervical cancer (and probably all cervical adenocarcinoma) is associated with oncogenic HPV, and the absence
chapter 4. The effect of oncogenic HPV on transformation zone epithelium CHAPTER 1 All squamous cervical cancer (and probably all cervical adenocarcinoma) is associated with oncogenic HPV, and the absence
Clinical Scoring System to Detect Malignant and Premalignant Vulval Lesions
 The Journal of Obstetrics and Gynecology of India (January February 2014) 64(1):41 46 DOI 10.1007/s13224-013-0458-3 ORIGINAL ARTICLE Clinical Scoring System to Detect Malignant and Premalignant Vulval
The Journal of Obstetrics and Gynecology of India (January February 2014) 64(1):41 46 DOI 10.1007/s13224-013-0458-3 ORIGINAL ARTICLE Clinical Scoring System to Detect Malignant and Premalignant Vulval
Carcinomas escamosos de vulva y vagina. Relación con HPV
 Carcinomas escamosos de vulva y vagina. Relación con HPV Dr. Jaume Ordi Servei d Anatomia Patològica Hospital Clínic. Barcelona jordi@clinic.ub.es - 1 - Carcinoma de cérvix y VPH International Biological
Carcinomas escamosos de vulva y vagina. Relación con HPV Dr. Jaume Ordi Servei d Anatomia Patològica Hospital Clínic. Barcelona jordi@clinic.ub.es - 1 - Carcinoma de cérvix y VPH International Biological
HPV infections and potential outcomes
 CONTENTS Preface by Silvia de Sanjosé... 33 Preface by Jacob Bornstein... 37 Author s note... 39 Acknowledgments... 45 CHAPTER 1 HPV infections and potential outcomes HPV: What it is, where it is and what
CONTENTS Preface by Silvia de Sanjosé... 33 Preface by Jacob Bornstein... 37 Author s note... 39 Acknowledgments... 45 CHAPTER 1 HPV infections and potential outcomes HPV: What it is, where it is and what
Histopathology: skin pathology
 Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
1.Acute and Chronic Cervicitis - At the onset of menarche, the production of estrogens by the ovary stimulates maturation of the cervical and vaginal
 Diseases of cervix I. Inflammations 1.Acute and Chronic Cervicitis - At the onset of menarche, the production of estrogens by the ovary stimulates maturation of the cervical and vaginal squamous mucosa
Diseases of cervix I. Inflammations 1.Acute and Chronic Cervicitis - At the onset of menarche, the production of estrogens by the ovary stimulates maturation of the cervical and vaginal squamous mucosa
Making Sense of Cervical Cancer Screening
 Making Sense of Cervical Cancer Screening New Guidelines published November 2012 Tammie Koehler DO, FACOG The incidence of cervical cancer in the US has decreased more than 50% in the past 30 years because
Making Sense of Cervical Cancer Screening New Guidelines published November 2012 Tammie Koehler DO, FACOG The incidence of cervical cancer in the US has decreased more than 50% in the past 30 years because
Dysplasia, Mimics and Other Controversies
 Dysplasia, Mimics and Other Controversies Mary S. Richardson, MD Dept. of Pathology Medical University of South Carolina Charleston, SC Notice of Faculty Disclosure In accordance with ACGME guidelines,
Dysplasia, Mimics and Other Controversies Mary S. Richardson, MD Dept. of Pathology Medical University of South Carolina Charleston, SC Notice of Faculty Disclosure In accordance with ACGME guidelines,
Vulvar Carcinoma. Definition: Cases should be classified as carsinoma of the vulva when the primary site growth is in the vulva Malignant melanoma sho
 Carcinoma Vulva & Vagina Subdivisi Onkologi Ginekologi Bagian Obgin FK USU Vulvar Carcinoma. Definition: Cases should be classified as carsinoma of the vulva when the primary site growth is in the vulva
Carcinoma Vulva & Vagina Subdivisi Onkologi Ginekologi Bagian Obgin FK USU Vulvar Carcinoma. Definition: Cases should be classified as carsinoma of the vulva when the primary site growth is in the vulva
Basal cell carcinoma 5/28/2011
 Goal of this Presentation A practical approach to the diagnosis of cutaneous carcinomas and their mimics Thaddeus Mully, MD University of California San Francisco To review common non-melanoma skin cancers
Goal of this Presentation A practical approach to the diagnosis of cutaneous carcinomas and their mimics Thaddeus Mully, MD University of California San Francisco To review common non-melanoma skin cancers
Histopathology: Cervical HPV and neoplasia
 Histopathology: Cervical HPV and neoplasia These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you need to learn about
Histopathology: Cervical HPV and neoplasia These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you need to learn about
Benign and malignant epithelial lesions: Seborrheic keratosis: A common benign pigmented epidermal tumor occur in middle-aged or older persons more
 Benign and malignant epithelial lesions: Seborrheic keratosis: A common benign pigmented epidermal tumor occur in middle-aged or older persons more common on the trunk; but extremities, head and neck are
Benign and malignant epithelial lesions: Seborrheic keratosis: A common benign pigmented epidermal tumor occur in middle-aged or older persons more common on the trunk; but extremities, head and neck are
Objectives. Atypical Glandular Cells. Atypical Endocervical Cells. Reactive Endocervical Cells
 2013 California Society of Pathologists 66 th Annual Meeting San Francisco, CA Atypical Glandular Cells to Early Invasive Adenocarcinoma: Cervical Cytology and Histology Christina S. Kong, MD Associate
2013 California Society of Pathologists 66 th Annual Meeting San Francisco, CA Atypical Glandular Cells to Early Invasive Adenocarcinoma: Cervical Cytology and Histology Christina S. Kong, MD Associate
Cervical Cancer Screening for the Primary Care Physician for Average Risk Individuals Clinical Practice Guidelines. June 2013
 Cervical Cancer Screening for the Primary Care Physician for Average Risk Individuals Clinical Practice Guidelines General Principles: Since its introduction in 1943, Papanicolaou (Pap) smear is widely
Cervical Cancer Screening for the Primary Care Physician for Average Risk Individuals Clinical Practice Guidelines General Principles: Since its introduction in 1943, Papanicolaou (Pap) smear is widely
FORUM 044 VULVAR DISEASE: WHAT DO YOU KNOW? AN OVERVIEW
 FORUM 044 VULVAR DISEASE: WHAT DO YOU KNOW? AN OVERVIEW Vulvar Neoplasms: Benign and Malignant Jill Allbritton, MD March 2, 2019 NO DISCLOSURES WITH INDUSTRY Jill Allbritton, MD Forum 044 Vulvar Disease:
FORUM 044 VULVAR DISEASE: WHAT DO YOU KNOW? AN OVERVIEW Vulvar Neoplasms: Benign and Malignant Jill Allbritton, MD March 2, 2019 NO DISCLOSURES WITH INDUSTRY Jill Allbritton, MD Forum 044 Vulvar Disease:
Chapter 6 Squamous Cell Carcinoma: Variants and Challenges
 Chapter 6 Squamous Cell Carcinoma: Variants and Challenges Michael B. Morgan EPIDEMIOLOGY: Second most common skin cancer, rare in the dark-skinned races. ETIOLOGY: Ultraviolet light, HPV infection. PATHOGENESIS:
Chapter 6 Squamous Cell Carcinoma: Variants and Challenges Michael B. Morgan EPIDEMIOLOGY: Second most common skin cancer, rare in the dark-skinned races. ETIOLOGY: Ultraviolet light, HPV infection. PATHOGENESIS:
Done by khozama jehad. Neoplasia of the cervix
 Done by khozama jehad Neoplasia of the cervix An overview of cervical neoplasia very import. Most tumors of the cervix are of epithelial origin and are caused by oncogenic strains of human papillomavirus
Done by khozama jehad Neoplasia of the cervix An overview of cervical neoplasia very import. Most tumors of the cervix are of epithelial origin and are caused by oncogenic strains of human papillomavirus
Cervical Conization. 1
 Cervical Conization www.zohrehyousefi.com 1 Cone Biopsy is a surgical procedure with removal of a cone shaped portion of the cervix The extent of involvement of epithelium on the ectocervix has been clearly
Cervical Conization www.zohrehyousefi.com 1 Cone Biopsy is a surgical procedure with removal of a cone shaped portion of the cervix The extent of involvement of epithelium on the ectocervix has been clearly
Prevalence Of Precancerous Lesions Of The Uterine Cervix
 Prevalence Of Precancerous Lesions Of The Uterine Cervix 1 / 6 2 / 6 3 / 6 Prevalence Of Precancerous Lesions Of INTRODUCTION. Gastric intestinal metaplasia is an intermediate precancerous gastric lesion
Prevalence Of Precancerous Lesions Of The Uterine Cervix 1 / 6 2 / 6 3 / 6 Prevalence Of Precancerous Lesions Of INTRODUCTION. Gastric intestinal metaplasia is an intermediate precancerous gastric lesion
3/25/2019. J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse
 J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse 1 4 th most common GYN cancer 5% of malignancies of GYN type. 4850 new cases annually 1030 deaths Cigarette smoking Vulvar dystrophy (Lichen
J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse 1 4 th most common GYN cancer 5% of malignancies of GYN type. 4850 new cases annually 1030 deaths Cigarette smoking Vulvar dystrophy (Lichen
HOW TO DIAGNOSE VULVAR DISEASES:
 HOW TO DIAGNOSE VULVAR DISEASES: Diagnostic approach. Normal findings. Maria Sol Peremateu, MD A wide spectrum of benign, premalignant, and malignant lesions may occur on the vulva. Some of the disorders
HOW TO DIAGNOSE VULVAR DISEASES: Diagnostic approach. Normal findings. Maria Sol Peremateu, MD A wide spectrum of benign, premalignant, and malignant lesions may occur on the vulva. Some of the disorders
The Case of Mrs. Virginia Jones* Asst. Professor Division of Gyne-Oncology University of British Columbia, Department of Gynecology Vancouver, Canada
 Case title: Case authors: Case synopsis: The Case of Mrs. Virginia Jones* Dr. Leslie A. Sadownik Asst. Professor Division of Gyne-Oncology University of British Columbia, Department of Gynecology Vancouver,
Case title: Case authors: Case synopsis: The Case of Mrs. Virginia Jones* Dr. Leslie A. Sadownik Asst. Professor Division of Gyne-Oncology University of British Columbia, Department of Gynecology Vancouver,
MECHANISMS OF HUMAN DISEASE: LABORATORY SESSION PATHOLOGY OF THE SKIN LAB. Friday, February 12, :30 am 11:00 am
 MECHANISMS OF HUMAN DISEASE: LABORATORY SESSION PATHOLOGY OF THE SKIN LAB Friday, February 12, 2012 9:30 am 11:00 am FACULTY COPY GOALS: Describe the basic clinical and morphologic features of various
MECHANISMS OF HUMAN DISEASE: LABORATORY SESSION PATHOLOGY OF THE SKIN LAB Friday, February 12, 2012 9:30 am 11:00 am FACULTY COPY GOALS: Describe the basic clinical and morphologic features of various
HPV and Cervical Cancer, Screening and Prevention. John Ragsdale, MD July 12, 2018 CME Lecture Series
 HPV and Cervical Cancer, Screening and Prevention John Ragsdale, MD July 12, 2018 CME Lecture Series We have come a long Way Prevalence HPV in Young Adults in U.S HPV genotypes 55-60% of All cancers 20%
HPV and Cervical Cancer, Screening and Prevention John Ragsdale, MD July 12, 2018 CME Lecture Series We have come a long Way Prevalence HPV in Young Adults in U.S HPV genotypes 55-60% of All cancers 20%
HIV-infected men and women. Joel Palefsky, M.D. University of California, San Francisco
 Anal cytology and anal cancer in HIV-infected men and women. Joel Palefsky, M.D. University of California, San Francisco April 10, 2010 Disclosures Merck and Co: Research grant support, advisory boards
Anal cytology and anal cancer in HIV-infected men and women. Joel Palefsky, M.D. University of California, San Francisco April 10, 2010 Disclosures Merck and Co: Research grant support, advisory boards
I have no financial interests in any product I will discuss today.
 Cervical Cancer Screening Update and Implications for Annual Exams George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics
Cervical Cancer Screening Update and Implications for Annual Exams George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics
Human Papillomavirus (HPV) in Patients with HIV.
 Human Papillomavirus (HPV) in Patients with HIV www.hivguidelines.org Purpose of the Guideline Increase the numbers of NYS residents with HIV who are screened for HPV-related dysplasia and managed effectively.
Human Papillomavirus (HPV) in Patients with HIV www.hivguidelines.org Purpose of the Guideline Increase the numbers of NYS residents with HIV who are screened for HPV-related dysplasia and managed effectively.
Clinical Pathological Conference. Malignant Melanoma of the Vulva
 Clinical Pathological Conference Malignant Melanoma of the Vulva History F/48 Chinese Married Para 1 Presented in September 2004 Vulval mass for 2 months Associated with watery and blood stained discharge
Clinical Pathological Conference Malignant Melanoma of the Vulva History F/48 Chinese Married Para 1 Presented in September 2004 Vulval mass for 2 months Associated with watery and blood stained discharge
Pathology of the skin. 2nd Department of Pathology, Semmelweis University
 Pathology of the skin 2nd Department of Pathology, Semmelweis University Histology of the skin Epidermis: Stratum corneum Stratum granulosum Stratum spinosum Stratum basale Dermis: papillary and reticular
Pathology of the skin 2nd Department of Pathology, Semmelweis University Histology of the skin Epidermis: Stratum corneum Stratum granulosum Stratum spinosum Stratum basale Dermis: papillary and reticular
WOMEN S INTERAGENCY HIV STUDY LABORATORY - PELVIC EXAM STUDIES TREATMENT FORM FORM L16
 WOMEN S INTERAGENCY HIV STUDY LABORATORY - PELVIC EXAM STUDIES TREATMENT FORM FORM L16 ID LABEL HERE ---> - - - VISIT #: FORM COMPLETED BY: VERSION DATE: 05/01/95 ANY MISSING OR INCOMPLETE TEST RESULTS
WOMEN S INTERAGENCY HIV STUDY LABORATORY - PELVIC EXAM STUDIES TREATMENT FORM FORM L16 ID LABEL HERE ---> - - - VISIT #: FORM COMPLETED BY: VERSION DATE: 05/01/95 ANY MISSING OR INCOMPLETE TEST RESULTS
Genital Human Papillomavirus (HPV) Infections
 Genital Human Papillomavirus (HPV) Infections January 2008 Etiology... 1 Epidemiology... 1 Prevention... 2 Diagnosis... 3 Management... 6 Treatment... 7 Consideration for Other STIs... 9 Reporting and
Genital Human Papillomavirus (HPV) Infections January 2008 Etiology... 1 Epidemiology... 1 Prevention... 2 Diagnosis... 3 Management... 6 Treatment... 7 Consideration for Other STIs... 9 Reporting and
Human Papillomaviruses and Cancer: Questions and Answers. Key Points. 1. What are human papillomaviruses, and how are they transmitted?
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Human Papillomaviruses
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Human Papillomaviruses
New Diagnoses Need New Approaches: A Glimpse into the Near Future of Gynecologic Pathology
 New Diagnoses Need New Approaches: A Glimpse into the Near Future of Gynecologic Pathology United States and Canadian Academy of Pathology 102 nd Annual Meeting Baltimore, Maryland Christina S. Kong, M.D.
New Diagnoses Need New Approaches: A Glimpse into the Near Future of Gynecologic Pathology United States and Canadian Academy of Pathology 102 nd Annual Meeting Baltimore, Maryland Christina S. Kong, M.D.
Update of the role of Human Papillomavirus in Head and Neck Cancer
 Update of the role of Human Papillomavirus in Head and Neck Cancer 2013 International & 12 th National Head and Neck Tumour Conference Shanghai, 11 13 Oct 2013 Prof. Paul KS Chan Department of Microbiology
Update of the role of Human Papillomavirus in Head and Neck Cancer 2013 International & 12 th National Head and Neck Tumour Conference Shanghai, 11 13 Oct 2013 Prof. Paul KS Chan Department of Microbiology
Prepared By Jocelyn Palao and Layla Faqih
 Prepared By Jocelyn Palao and Layla Faqih The structure of the suspected atypical cell should always be compared to the structure of other similar, benign, cells which are present in the smears. The diagnosis
Prepared By Jocelyn Palao and Layla Faqih The structure of the suspected atypical cell should always be compared to the structure of other similar, benign, cells which are present in the smears. The diagnosis
Meet the ISSVD. President ISSVD. Jacob Bornstein, MD
 Meet the ISSVD Jacob Bornstein, MD President ISSVD Chairman, Department of Obstetrics and Gynecology Western Galilee Hospital Bar-Ilan University Faculty of Medicine Nahariya, Israel ISSVD Symposium: The
Meet the ISSVD Jacob Bornstein, MD President ISSVD Chairman, Department of Obstetrics and Gynecology Western Galilee Hospital Bar-Ilan University Faculty of Medicine Nahariya, Israel ISSVD Symposium: The
Cervical Cancer Screening. David Quinlan December 2013
 Cervical Cancer Screening David Quinlan December 2013 Cervix Cervical Cancer Screening Modest variation provincially WHO and UK begin at 25 stop at 60 Finland begin at 30 stop at 60 Rationale for
Cervical Cancer Screening David Quinlan December 2013 Cervix Cervical Cancer Screening Modest variation provincially WHO and UK begin at 25 stop at 60 Finland begin at 30 stop at 60 Rationale for
Colposcopy. Attila L Major, MD, PhD
 Colposcopy Attila L Major, MD, PhD Histology Colposcopy Cytology It has been estimated that annual Pap smear testing reduces a woman s chance of dying of cervical cancer from 4 in 1000 to about 5 in 10,000
Colposcopy Attila L Major, MD, PhD Histology Colposcopy Cytology It has been estimated that annual Pap smear testing reduces a woman s chance of dying of cervical cancer from 4 in 1000 to about 5 in 10,000
PRE TEST CERVICAL SCREENING MANAGEMENT COLPOSCOPY PATHOLOGIC DIAGNOSIS AND TREATMENT
 PRE TEST CERVICAL SCREENING MANAGEMENT COLPOSCOPY PATHOLOGIC DIAGNOSIS AND TREATMENT QUESTION #1 WHICH OF THE FOLLOWING IS NOT A RISK FACTOR FOR CERVICAL CANCER? A. HIGH RISK HPV B. CIGARETTE SMOKING C.
PRE TEST CERVICAL SCREENING MANAGEMENT COLPOSCOPY PATHOLOGIC DIAGNOSIS AND TREATMENT QUESTION #1 WHICH OF THE FOLLOWING IS NOT A RISK FACTOR FOR CERVICAL CANCER? A. HIGH RISK HPV B. CIGARETTE SMOKING C.
Case Report A Rare Cutaneous Adnexal Tumor: Malignant Proliferating Trichilemmal Tumor
 Case Reports in Medicine Volume 2015, Article ID 742920, 4 pages http://dx.doi.org/10.1155/2015/742920 Case Report A Rare Cutaneous Adnexal Tumor: Malignant Proliferating Trichilemmal Tumor Omer Alici,
Case Reports in Medicine Volume 2015, Article ID 742920, 4 pages http://dx.doi.org/10.1155/2015/742920 Case Report A Rare Cutaneous Adnexal Tumor: Malignant Proliferating Trichilemmal Tumor Omer Alici,
PAP SMEAR by Dr.Shantha Krishnamurthy MD Senior Consultant Pathology Fortis Hospitals
 PAP SMEAR by Dr.Shantha Krishnamurthy MD Senior Consultant Pathology Fortis Hospitals Historical Named after George Papanicolaou, a Greek American Studied cervical epithelium in menstrual cycle of guinea
PAP SMEAR by Dr.Shantha Krishnamurthy MD Senior Consultant Pathology Fortis Hospitals Historical Named after George Papanicolaou, a Greek American Studied cervical epithelium in menstrual cycle of guinea
Clinical Guidance: Recommended Best Practices for Delivery of Colposcopy Services in Ontario Best Practice Pathway Summary
 Clinical Guidance: Recommended Best Practices for Delivery of Colposcopy Services in Ontario Best Practice Pathway Summary Glossary of Terms Colposcopy is the examination of the cervix, vagina and, in
Clinical Guidance: Recommended Best Practices for Delivery of Colposcopy Services in Ontario Best Practice Pathway Summary Glossary of Terms Colposcopy is the examination of the cervix, vagina and, in
Cervical Screening for Dysplasia and Cancer in Patients with HIV
 Cervical Screening for Dysplasia and Cancer in Patients with HIV Adult Clinical Guideline from the New York State Department of Health AIDS Institute w w w.hivg uidelines.org Purpose of the Guideline Increase
Cervical Screening for Dysplasia and Cancer in Patients with HIV Adult Clinical Guideline from the New York State Department of Health AIDS Institute w w w.hivg uidelines.org Purpose of the Guideline Increase
Management Algorithms for Abnormal Cervical Cytology and Colposcopy
 Management Algorithms for Abnormal Cervical Cytology and Colposcopy Table of Contents Standard Colposcopic Definitions... 1 Guidelines for the Assessment of Abnormal Cervical Cytology... 2 Ia: Persistent
Management Algorithms for Abnormal Cervical Cytology and Colposcopy Table of Contents Standard Colposcopic Definitions... 1 Guidelines for the Assessment of Abnormal Cervical Cytology... 2 Ia: Persistent
LABORATORY - PELVIC EXAM STUDIES COLPOSCOPY RESULTS FORM L14
 LABORATORY - PELVIC EXAM STUDIES COLPOSCOPY RESULTS FORM L4 ID LABEL HERE ---> - - - VISIT #: FORM COMPLETED BY: VERSION DATE: 08/5/94 ANY MISSING OR INCOMPLETE TEST RESULTS MUST BE EXPLAINED ON THIS FORM.
LABORATORY - PELVIC EXAM STUDIES COLPOSCOPY RESULTS FORM L4 ID LABEL HERE ---> - - - VISIT #: FORM COMPLETED BY: VERSION DATE: 08/5/94 ANY MISSING OR INCOMPLETE TEST RESULTS MUST BE EXPLAINED ON THIS FORM.
Clinical Practice Guidelines June 2013
 Clinical Practice Guidelines June 2013 General Principles: The Papanicolaou (Pap) smear is widely credited with reducing mortality from cervical cancer, and remains the single best method for the early
Clinical Practice Guidelines June 2013 General Principles: The Papanicolaou (Pap) smear is widely credited with reducing mortality from cervical cancer, and remains the single best method for the early
Management of Abnormal Cervical Cytology and Histology
 Management of Abnormal Cervical Cytology and Histology Assoc. Prof. Gökhan Tulunay Etlik Zübeyde Hanım Women s Diseases Teaching & Research Hospital Gynecologic Oncology Clinic Universally accepted guideline
Management of Abnormal Cervical Cytology and Histology Assoc. Prof. Gökhan Tulunay Etlik Zübeyde Hanım Women s Diseases Teaching & Research Hospital Gynecologic Oncology Clinic Universally accepted guideline
Diagnostic difficulties with lesions of the oral mucosa
 BDIAP London, November 2010 School of Clinical Dentistry University of Sheffield Diagnostic difficulties with lesions of the oral mucosa Paul M Speight Dept Oral & Maxillofacial Pathology University of
BDIAP London, November 2010 School of Clinical Dentistry University of Sheffield Diagnostic difficulties with lesions of the oral mucosa Paul M Speight Dept Oral & Maxillofacial Pathology University of
CASE 4 21/07/2017. Ectopic Prostatic Tissue in Cervix. Female 31. LLETZ for borderline nuclear abnormalities
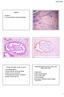 Female 31 CASE 4 LLETZ for borderline nuclear abnormalities PSA Ectopic Prostatic Tissue in Cervix AJSP 2006;30;209-215 usually incidental microscopic finding usually in ectocervical stroma? developmental
Female 31 CASE 4 LLETZ for borderline nuclear abnormalities PSA Ectopic Prostatic Tissue in Cervix AJSP 2006;30;209-215 usually incidental microscopic finding usually in ectocervical stroma? developmental
Swiss Federal Office of Public Health
 SGPath - Bern May 9, 2015 CIN3+ Study of the Swiss Federal Office of Public Health Joachim Diebold Luzerner Kantonsspital Pathologisches Institut HPV - vaccination: Australia as good example Anderson LA
SGPath - Bern May 9, 2015 CIN3+ Study of the Swiss Federal Office of Public Health Joachim Diebold Luzerner Kantonsspital Pathologisches Institut HPV - vaccination: Australia as good example Anderson LA
Penile cancer teams in UK. Common variants. Penile cancer teams. Basaloid squamous carcinoma. The Pathology of Penile Tumours
 The Pathology of Penile Tumours Dr Jonathan H Shanks The Christie NHS Foundation Trust, Manchester, UK Penile cancer teams in UK 12 centres for penile cancer work (10 in England and Wales, 2 in Scotland)
The Pathology of Penile Tumours Dr Jonathan H Shanks The Christie NHS Foundation Trust, Manchester, UK Penile cancer teams in UK 12 centres for penile cancer work (10 in England and Wales, 2 in Scotland)
Postmenopausal Vulvar Pruritus - Colposcopic Diagnosis and Treatment
 Postmenopausal Vulvar Pruritus - Colposcopic Diagnosis and Treatment Pages with reference to book, From 315 To 317 A Bilge Sener, Neslihan C. Seckin, Oya Gokmen ( Departments of Menopause, Dr. Z.T.B. Women
Postmenopausal Vulvar Pruritus - Colposcopic Diagnosis and Treatment Pages with reference to book, From 315 To 317 A Bilge Sener, Neslihan C. Seckin, Oya Gokmen ( Departments of Menopause, Dr. Z.T.B. Women
Cervical Skills. Dr Margaret Laing Queen Elizabeth University Hospital
 Cervical Skills Dr Margaret Laing Queen Elizabeth University Hospital What is screening? Screening is a test offered to an apparently well person with the possibility of detecting a serious disease before
Cervical Skills Dr Margaret Laing Queen Elizabeth University Hospital What is screening? Screening is a test offered to an apparently well person with the possibility of detecting a serious disease before
MECHANISMS OF HUMAN DISEASE: LABORATORY SESSION PATHOLOGY OF THE SKIN LAB. Friday, February 13, :30 am 11:00 am
 MECHANISMS OF HUMAN DISEASE: LABORATORY SESSION PATHOLOGY OF THE SKIN LAB Friday, February 13, 2009 9:30 am 11:00 am FACULTY COPY GOALS: Describe the basic clinical and morphologic features of various
MECHANISMS OF HUMAN DISEASE: LABORATORY SESSION PATHOLOGY OF THE SKIN LAB Friday, February 13, 2009 9:30 am 11:00 am FACULTY COPY GOALS: Describe the basic clinical and morphologic features of various
I have no financial interests in any product I will discuss today.
 Cervical Cancer Screening Update and Implications for Annual Exams George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics
Cervical Cancer Screening Update and Implications for Annual Exams George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics
Dysplasia: layer of the cervical CIN. Intraepithelial Neoplasia. p16 immunostaining. 1, Cervical. Higher-risk, requires CIN.
 CLINICAL PRACTICE GUIDELINE Guideline Number: DHMP_DHMC_PG1015 Guideline Subject: Routine Cervical Cancer Screening Effective Date: 9/2018 Revision Date: 9/2019 Pages: 2 of 2 Quality Management Committee
CLINICAL PRACTICE GUIDELINE Guideline Number: DHMP_DHMC_PG1015 Guideline Subject: Routine Cervical Cancer Screening Effective Date: 9/2018 Revision Date: 9/2019 Pages: 2 of 2 Quality Management Committee
TISSUE TUMOR MARKER EXPRESSION IN
 TISSUE TUMOR MARKER EXPRESSION IN NORMAL CERVICAL TISSUE AND IN CERVICAL INTRAEPITHELIAL NEOPLASIA, FOR WOMEN WHO ARE AT HIGH RISK OF HPV (HUMAN PAPILLOMA VIRUS INFECTION). Raghad Samir MD PhD Verksamhet
TISSUE TUMOR MARKER EXPRESSION IN NORMAL CERVICAL TISSUE AND IN CERVICAL INTRAEPITHELIAL NEOPLASIA, FOR WOMEN WHO ARE AT HIGH RISK OF HPV (HUMAN PAPILLOMA VIRUS INFECTION). Raghad Samir MD PhD Verksamhet
Cervical cancer presentation
 Carcinoma of the cervix: Carcinoma of the cervix is the second commonest cancer among women worldwide, with only breast cancer occurring more commonly. Worldwide, cervical cancer accounts for about 500,000
Carcinoma of the cervix: Carcinoma of the cervix is the second commonest cancer among women worldwide, with only breast cancer occurring more commonly. Worldwide, cervical cancer accounts for about 500,000
ASCCP 2013 Guidelines for Managing Abnormal Cervical Cancer Screening Tests
 ASCCP 2013 Guidelines for Managing Abnormal Cervical Cancer Screening Tests www.treatmentok.com Barbara S. Apgar, MD, MS Professor of Family Medicine University of Michigan Ann Arbor, Michigan Disclosures
ASCCP 2013 Guidelines for Managing Abnormal Cervical Cancer Screening Tests www.treatmentok.com Barbara S. Apgar, MD, MS Professor of Family Medicine University of Michigan Ann Arbor, Michigan Disclosures
Understanding Your Pap Test Results
 Understanding Your Pap Test Results Most laboratories in the United States use a standard set of terms called the Bethesda System to report pap test results. Normal: Pap samples that have no cell abnormalities
Understanding Your Pap Test Results Most laboratories in the United States use a standard set of terms called the Bethesda System to report pap test results. Normal: Pap samples that have no cell abnormalities
CERVICAL CANCER FACTSHEET. What is cervical cancer?
 CERVICAL CANCER FACTSHEET What is cervical cancer? ENGAGe is releasing a series of factsheets to raise awareness of gynaecological cancers and to support its network to work at a grassroots level. Take-up
CERVICAL CANCER FACTSHEET What is cervical cancer? ENGAGe is releasing a series of factsheets to raise awareness of gynaecological cancers and to support its network to work at a grassroots level. Take-up
Cytology/Biopsy/Leep Gynecologic Correlation: Practical Considerations and Approaches.
 Cytology/Biopsy/Leep Gynecologic Correlation: Practical Considerations and Approaches. Fadi W. Abdul-Karim MD MEd. Professor of Pathology. Vice chair for education. Robert Tomsich Pathology and Lab Med
Cytology/Biopsy/Leep Gynecologic Correlation: Practical Considerations and Approaches. Fadi W. Abdul-Karim MD MEd. Professor of Pathology. Vice chair for education. Robert Tomsich Pathology and Lab Med
Vulva Cancer Histopathology Reporting Proforma
 Vulva Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Ethnicity Unknown AboriginalTorres
Vulva Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Ethnicity Unknown AboriginalTorres
LARYNGEAL DYSPLASIA. Tomas Fernandez M; 3 rd year ENT resident, Son Espases University Hospital
 LARYNGEAL DYSPLASIA Tomas Fernandez M; 3 rd year ENT resident, Son Espases University Hospital INTRODUCTION Laryngeal cancer constitutes 1-2% of all malignancies diagnosed worldwide Survival is related
LARYNGEAL DYSPLASIA Tomas Fernandez M; 3 rd year ENT resident, Son Espases University Hospital INTRODUCTION Laryngeal cancer constitutes 1-2% of all malignancies diagnosed worldwide Survival is related
III CONGRESO INTERNACIONAL EN GINECOESTÉTICA Y CIRUGÍA ÍNTIMA
 28 OCTUBRE 2017 MADRID, ESPAÑA III CONGRESO INTERNACIONAL EN GINECOESTÉTICA Y CIRUGÍA ÍNTIMA PLASTIA DE LOS LABIOS MAYORES DRA. ANA LÚCIA NOGUEIRA www.clinicasabeanas.pt ABOUT ME BOUT Dra. Ana Lúcia Nogueira,
28 OCTUBRE 2017 MADRID, ESPAÑA III CONGRESO INTERNACIONAL EN GINECOESTÉTICA Y CIRUGÍA ÍNTIMA PLASTIA DE LOS LABIOS MAYORES DRA. ANA LÚCIA NOGUEIRA www.clinicasabeanas.pt ABOUT ME BOUT Dra. Ana Lúcia Nogueira,
I have no financial interests to disclose.
 Workshop: Case Management of Abnormal Pap Smears and Colposcopies Rebecca Jackson, MD Professor Obstetrics, Gynecology & Reproductive Sciences and Epidemiology & Biostatistics I have no financial interests
Workshop: Case Management of Abnormal Pap Smears and Colposcopies Rebecca Jackson, MD Professor Obstetrics, Gynecology & Reproductive Sciences and Epidemiology & Biostatistics I have no financial interests
High Resolution Anoscopy Overview. Naomi Jay, RN, NP, PhD University of California San Francisco
 High Resolution Anoscopy Overview Naomi Jay, RN, NP, PhD University of California San Francisco Email: naomi.jay@ycsf.edy Disclosures No Disclosures Definition of HRA Examination of the anus, anal canal
High Resolution Anoscopy Overview Naomi Jay, RN, NP, PhD University of California San Francisco Email: naomi.jay@ycsf.edy Disclosures No Disclosures Definition of HRA Examination of the anus, anal canal
I have no financial interests in any product I will discuss today.
 Cervical Cancer Prevention: 2012 and Beyond George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics University of California,
Cervical Cancer Prevention: 2012 and Beyond George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics University of California,
Vulval intraepithelial neoplasia: clinical review
 Genitourin Med 1987;63:147-152 Review article Vulval intraepithelial neoplasia: clinical review Nomenclature Preinvasive neoplasia ofthe vulva has been recognised for over 75 years, but terminology to
Genitourin Med 1987;63:147-152 Review article Vulval intraepithelial neoplasia: clinical review Nomenclature Preinvasive neoplasia ofthe vulva has been recognised for over 75 years, but terminology to
CPC on Cervical Pathology
 CPC on Cervical Pathology Dr. W.K. Ng Senior Medical Officer Department of Clinical Pathology Pamela Youde Nethersole Eastern Hospital Cervical Smear: High Grade SIL (CIN III) Cervical Smear: High Grade
CPC on Cervical Pathology Dr. W.K. Ng Senior Medical Officer Department of Clinical Pathology Pamela Youde Nethersole Eastern Hospital Cervical Smear: High Grade SIL (CIN III) Cervical Smear: High Grade
CERVICAL INTRAEPITHELIAL NEOPLASIA (CIN)
 CERVICAL INTRAEPITHELIAL NEOPLASIA (CIN) The cervix constitutes the lower third of the uterus. It is in two parts, the endocervix and the ectocervix. Ectocervix is covered with squamous epithelium. Endocervix
CERVICAL INTRAEPITHELIAL NEOPLASIA (CIN) The cervix constitutes the lower third of the uterus. It is in two parts, the endocervix and the ectocervix. Ectocervix is covered with squamous epithelium. Endocervix
Jean Anderson, MD Catherine Sewell, MD, MPH
 Jean Anderson, MD Catherine Sewell, MD, MPH To review diagnosis and management of HPV disease in the setting of HIV infection and address controversies HIV-infected women have: higher prevalence and incidence
Jean Anderson, MD Catherine Sewell, MD, MPH To review diagnosis and management of HPV disease in the setting of HIV infection and address controversies HIV-infected women have: higher prevalence and incidence
Cervical Precancer: Evaluation and Management
 TAJ June 2002; Volume 15 Number 1 ISSN 1019-8555 The Journal of Teachers Association RMC, Rajshahi Review fam Cervical Precancer: Evaluation and Management SM Khodeza Nahar Begum 1 Abstract Carcinoma of
TAJ June 2002; Volume 15 Number 1 ISSN 1019-8555 The Journal of Teachers Association RMC, Rajshahi Review fam Cervical Precancer: Evaluation and Management SM Khodeza Nahar Begum 1 Abstract Carcinoma of
HPV and PREGNANCY Arsenio Spinillo,, MD
 HPV and PREGNANCY Arsenio Spinillo,, MD University of Pavia, Department of Obstetrics and Gynecology, IRCCS Fondazione Policlinico San Matteo di Pavia Human Papilloma Virus HPV is a member of the Papovaviridae
HPV and PREGNANCY Arsenio Spinillo,, MD University of Pavia, Department of Obstetrics and Gynecology, IRCCS Fondazione Policlinico San Matteo di Pavia Human Papilloma Virus HPV is a member of the Papovaviridae
HPV and Lower Genital Tract Disease. Simon Herrington University of Edinburgh, UK Royal Infirmary of Edinburgh, UK
 HPV and Lower Genital Tract Disease Simon Herrington University of Edinburgh, UK Royal Infirmary of Edinburgh, UK Conflict of interest/funding X None Company: Product royalties Paid consultant Research
HPV and Lower Genital Tract Disease Simon Herrington University of Edinburgh, UK Royal Infirmary of Edinburgh, UK Conflict of interest/funding X None Company: Product royalties Paid consultant Research
Cytology Update M Laing QEUH
 Cytology Update M Laing QEUH Age change to 25 to 65 Age 25 to 50 Three yearly smear invitation Age 50 to 65 Five yearly smear invitation Women on non routine screening will be invited up to age 70 OUTCOME
Cytology Update M Laing QEUH Age change to 25 to 65 Age 25 to 50 Three yearly smear invitation Age 50 to 65 Five yearly smear invitation Women on non routine screening will be invited up to age 70 OUTCOME
Advanced Training Skills Module August Vulval Disease
 Introduction Vulval Disease This module is designed to provide training in all aspects of vulval disease. Successful completion of the ATSM will equip an individual to develop this aspect of care in their
Introduction Vulval Disease This module is designed to provide training in all aspects of vulval disease. Successful completion of the ATSM will equip an individual to develop this aspect of care in their
Dermatopathology: The tumor is composed of keratinocytes which show atypia, increase mitoses and abnormal mitoses.
 Squamous cell carcinoma (SCC): A common malignant tumor of keratinocytes arising in the epidermis, usually from a precancerous condition: 1- UV induced actinic keratosis, usually of low grade malignancy.
Squamous cell carcinoma (SCC): A common malignant tumor of keratinocytes arising in the epidermis, usually from a precancerous condition: 1- UV induced actinic keratosis, usually of low grade malignancy.
News. Laboratory NEW GUIDELINES DEMONSTRATE GREATER ROLE FOR HPV TESTING IN CERVICAL CANCER SCREENING TIMOTHY UPHOFF, PHD, DABMG, MLS (ASCP) CM
 Laboratory News Inside This Issue NEW GUIDELINES DEMONSTRATE GREATER ROLE FOR HPV TESTING IN CERVICAL CANCER SCREENING...1 NEW HPV TEST METHODOLOGY PROVIDES BETTER SPECIFICITY FOR CERVICAL CANCER...4 BEYOND
Laboratory News Inside This Issue NEW GUIDELINES DEMONSTRATE GREATER ROLE FOR HPV TESTING IN CERVICAL CANCER SCREENING...1 NEW HPV TEST METHODOLOGY PROVIDES BETTER SPECIFICITY FOR CERVICAL CANCER...4 BEYOND
Histopathology of Melanoma
 THE YALE JOURNAL OF BIOLOGY AND MEDICINE 48, 409-416 (1975) Histopathology of Melanoma G. J. WALKER SMITH Department ofpathology, Yale University School ofmedicine, 333 Cedar Street, New Haven, Connecticut
THE YALE JOURNAL OF BIOLOGY AND MEDICINE 48, 409-416 (1975) Histopathology of Melanoma G. J. WALKER SMITH Department ofpathology, Yale University School ofmedicine, 333 Cedar Street, New Haven, Connecticut
Salivary Glands 3/7/2017
 Salivary Glands 3/7/2017 Goals and objectives Focus on the entities unique to H&N Common board type facts Information for your future practice Salivary Glands Salivary Glands Major gland. Paratid. Submandibular.
Salivary Glands 3/7/2017 Goals and objectives Focus on the entities unique to H&N Common board type facts Information for your future practice Salivary Glands Salivary Glands Major gland. Paratid. Submandibular.
