Clinical Medicine Insights: Gastroenterology. Once-Daily MMX Mesalamine in the Management of Ulcerative Colitis
|
|
|
- Esmond Fisher
- 5 years ago
- Views:
Transcription
1 Clinical Medicine Insights: Gastroenterology Review Open Access Full open access to this and thousands of other papers at Once-Daily MMX Mesalamine in the Management of Ulcerative Colitis Wojciech Blonski 1,2, Anna M. Buchner 1 and Gary R. Lichtenstein 1 1 Division of Gastroenterology, University of Pennsylvania, Philadelphia, PA, USA. 2 Department of Gastroenterology, Medical University, Wroclaw, Poland. Corresponding author grl@uphs.upenn.edu Abstract: Mesalamine (5-ASA) has been the mainstay therapy of mild to moderate active ulcerative colitis both as an induction and maintenance treatment. Multimatrix system (MMX) 5-ASA is a recently developed formulation of 5-ASA allowing for slow and gradual release of 5-ASA throughout the entire colon. This review article discusses the structure, pharmacokinetics, efficacy and safety of this new 5-ASA formulation. Keywords: MMX-5ASA, ulcerative colitis Clinical Medicine Insights: Gastroenterology 2011: doi: /CGast.S4276 This article is available from the author(s), publisher and licensee Libertas Academica Ltd. This is an open access article. Unrestricted non-commercial use is permitted provided the original work is properly cited. Clinical Medicine Insights: Gastroenterology 2011:4 7
2 Blonski et al Introduction The 5-aminosalicylic acid agents (5-ASA, mesalamine) have been recommended as the first-line of therapy in patients with mild-to-moderate ulcerative colitis (UC) for induction and maintaining remission. 1,2 Currently there are several delayed release formulations of mesalamine available in the treatment of UC. Although the main focus of our article is to review the efficacy and tolerability of MMX-5ASA, it is worth acknowledging that among these 5-ASA agents of a delayed release mode are mesalamine delayed- release (Asacol HD 800), mesalamine pellets (Pentasa), mesalamine (micro) pellets (Salofalk Granustix), mesalamine granules (Apriso) and the Multi Matrix System (MMX). Their unique formulations have been designed to maximize drug delivery to the colon and minimize their systemic absorption. Asacol 800 with EUDRAGIT S-coated tablets release with ph. 7 have delayed mode of the release until terminal ileum and cecum. 3 Mesalamine pellets (Pentasa) with ethylcellulose coated microgranules (time dependent release) have prolonged release throughout small bowel delivering approximately 50% to the small bowel and the remaining to the colon. 4,5 Mesalamine (micro) pellets (Salofalk Granustix) with Eudragit L-coated pellets contain additionally delaying polymer which leads to their delayed and sustained release. 6 Mesalamine granules (MG, Apriso) contain delayed release enteric coating and extended release polymer matrix which lead to their delayed and gradual release beginning at ph 6 starting in the terminal ileum and throughout colon. 7 Several randomized placebo controlled trials have demonstrated their efficacy compared to placebo in the treatment of UC However the only head to head controlled trial directly comparing delayed release formulations of mesalamine was performed for MMX-mesalamine and Asacol as the maintenance treatment in UC and it will be discussed in the later section of this review. 14 The Multi Matrix System (MMX) 5-ASA (previous name SPD476, currently marketed as Lialda (United States) and Mezavant (Canada and European Union); Shire Pharmaceuticals Inc., Wayne PA) is a new highstrength formulation of 5-ASA (1.2 gram per tablet). 15 It is the first oral mesalamine formulation that has been approved by the US Food and Drug Administration in once daily dosing fashion in patients presenting with with mild to moderately active UC. 15,16 The MMX-5ASA system tablet consists of the core containing mesalamine incorporated in microparticles of lipophilic matrix dispersed within hydrophilic matrix that is covered with ph-dependent coating. 15,17 The MMX coating enables the tablet to release mesalamine when the ph $ 7 ie, within terminal ileum. 15,18,19 There is an interaction between intestinal fluids and hydrophilic matrix upon disintegration of the coating of the tablet which in turn causes swelling of the tablet leading to formation of outer viscous gel mass. 18 This slows diffusion of the 5-ASA from the core of the tablet into the lumen of the colon. 18 There is a slow and gradual release of 5-ASA throughout the colon providing homogenous release of the medication within entire colon. 15,17,18 Based on results of the plasma kinetics study performed in 12 healthy volunteers it was suggested that MMX-mesalamine starts releasing 5-ASA during transit in small intestine and ileum. 20 Gamma-scintigraphy study using Samarium 153-labelled MMX-mesalamine observed homogenous spread of radioactivity throughout the colon indicating instant release of 5-ASA within colon. 20 Release of 5-ASA from MMX-mesalamine was also assessed in an experimental model using in vitro gastrointestinal tract system (TNO Gastrointestinal Model). 18 It was determined that most of 5-ASA from 1.2 g dose was released in the stimulated colon (78% and 68.5% in fasted and fed state, respectively) whereas less than 1% of 5-ASA was released in stimulated stomach and small intestine. 18 The release of 5-ASA in the simulated colon was prolonged and achieved a peak rate of 67.5 mg/h within 5 to 6 hours in stimulated fed state and 73.3 mg/h within 6 to 8 hours in the stimulated fasted state with subsequent decline afterwards. 18 On the other hand, within 8 to 18 hours 5-ASA release rate was still substantial with a rate of 49.6 mg/h in fasted state and 40.7 mg/h in fed state. 18 Pharmacokinetics Phase I study Evaluation of plasma kinetics of MMX-5ASA (single dose of 1.2 g) performed in 12 healthy male volunteers determined that initial absorption of 5-ASA occurred in 8 Clinical Medicine Insights: Gastroenterology 2011:4
3 Treatment of UC with MMX mesalamine the small intestine and ileum with the mean maximum plasma concentration of ± ng/ml in the ileo-cecal part and a mean time to reach this concentration of 8.3 ± 6.2 hours ASA had mean relative absorption of 80.1% in the colon and 19.9% in the small intestine and ileum. 20 There was 8.8% of the administered dose of 5-ASA excreted in the urine and urinary excretion was not complete after 24 hours post dosing. 20 Steady state concentrations were achieved after 4 5 days following administration of MMX-5ASA for 7 days at a daily dose of 2.4 g given in two divided doses. 20 Maximum plasma concentration of 5-ASA at steady state was ± ng/ml and occurred after 5.5 ± 2.9 hours. 20 The average plasma concentration of 5-ASA at steady state was ± ng/ml. 20 Mean half time of 5-ASA was 7.1 ± 6.3 hours and its metabolite Acetyl- 5-ASA was 11.4 ± 10.1 hours after last dose of medication. 20 An average urinary elimination rate of 5-ASA and Acetyl-5-ASA was 4.1 ± 5.6 mg/h and 23.6 ± 15.9 mg/h measured on the last day of drug administration. 20 The calculated at steady state cumulative daily urinary elimination rate of a total dose of 2.4 g 5-ASA was 3.6% ± 3.8% for 5-ASA and 20% ± 12.1% for Acetyl-5-ASA. 20 Phase II study A phase II dose ranging study of MMX-5ASA described the pharmacokinetics parameters of this formulation in 38 patients with mild to moderately active UC. 21 The observed geometric mean plasma concentrations of 5-ASA (283.6, and 1926 ng/ml) and its metabolite Acetyl-5-ASA (783, and ng/ml) increased with increasing dose of the medication (1.2 g/day, 2.4 g/day and 4.8 g/day). 21 Patients who received MMX-5ASA at a daily dose of 4.8 g had higher geometric mean mucosal 5-ASA (48.8 vs.11.2 vs. 6.9 ng/mg) and Acetyl- 5-ASA (29.7 vs. 8.6 vs. 7.0 ng/mg) concentrations than those who received a daily dose of 1.2 g and 2.4 g. 21 In addition, at week 8 patients who received the highest dose of MMX-5ASA were found to have the best median histology and endoscopy scores vs. those who received lower doses. 21 Patients receiving MMX-5ASA at the daily dose of 4.8 g experienced median 3 point and 4.5 point improvement in their rectal and sigmoid histology scores whereas patients receiving MMX-5ASA at the daily dose of 2.4 g experienced lower 0.5 point and 3 point improvement in their respective rectal and sigmoid histology scores at week 8 when compared to baseline. 21 Patients receiving the lowest daily dose of MMX-5ASA (1.2 g) had no change in their median histology score and median 1 point worsening in their sigmoid histology score at week 8 when compared to baseline. 21 Patients treated with the highest dose of MMX-5ASA had also the highest median improvement in their sigmidoscopy score of 2 points, whereas those treated with lower doses had only 1 point improvement in their median score at week 8 when compared to baseline. 21 There was no strong relationship observed between mucosal and plasma levels of 5-ASA or its metabolite. 21 Likewise, there was no strong relationship between mucosal and plasma levels of 5-ASA or its metabolite and disease activity score. 21 Induction of Response and Remission A preliminary multicenter double blind, doubledummy trial from Italy comparing 8-week treatment with MMX-5ASA (1.2 g taken three times daily) and placebo-enema (n = 40) to 5-ASA enema (mesalamine 4 g/100 ml enema) and oral placebo (n = 39) in patients with active left-sided UC demonstrated similar clinical remission rates between both treatment arms at week 8 (primary endpoint) (MMX-5-ASA: 24/40 (60%) patients, 95% CI vs. 5-ASA enema: 19/38 (50%) patients, 95% CI ). 17 A pilot phase II randomized multicenter, double blind, parallel-group and dose ranging trial of 38 patients with mild-to moderately active UC assessed efficacy of MMX-5ASA administered daily at 1.2 g, 2.4 g and 4.8 g for 8 weeks. 21 Primary outcome were the remission rates at the end of treatment. 21 It was defined as UC-Disease Activity Index (UC-DAI) score #1 with accompanying rectal bleeding score of 0 and reduction in endoscopy score $1 point when compared to baseline score. 21 Although there was no significant difference in the remission rates at week 8 between treatment arms (1.2 g: 0%, 2.4 g: 30.8%, 4.8 g: 18.2%, P = 0.130), MMX-mesalamine given at 2.4 g and 4.8 g daily resulted in greater response to treatment measured by greater median improvement in UC-DAI score at week 8 (secondary outcome) (5.7 points Clinical Medicine Insights: Gastroenterology 2011:4 9
4 Blonski et al for 4.8 g and 3.3 points for 2.4 g) when compared to MMX-mesalamine given at the lowest daily dose of 1.2 g (1 point). 21 Likewise, there was observed a clinically relevant median 1-point improvement in Physician Global Assessment (PGA) score at week 8 in patients treated with MMX-mesalamine 2.4 g and 4.8 g suggesting improvement from moderate to mild activity of UC and no improvement in 1.2 g MMX-mesalamine treated patients. 21 There were two large multicenter, randomized, double-blind, placebo controlled, phase III trials that assessed efficacy of MMX-mesalamine in inducing remission in patients with mild to moderately active UC. 22,23 The first of these trials, performed by Lichtenstein et al enrolled 280 patients with mild to moderately active UC (score 4 10 according to modified UC-DAI with endoscopy score $1 and PGA score #2) who were randomly assigned to receive 8 week treatment with MMX-mesalamine at a daily dose of either 2.4 g given twice daily (n = 93), 4.8 g given once daily (n = 94) or placebo (n = 93). 22 The primary endopoint was combined clinical and endoscopic remission at week 8 that was defined as a modified UC-DAI score #1 with accompanying rectal bleeding and stool frequency score of 0, absence of mucosal friability and a reduction in endoscopic score of at least 1 point from baseline score. 22 At the conclusion of the trial there were significantly higher rates of combined clinical and endoscopic remission among patients treated with higher (29.2%, P = 0.009) and lower (34.1%, P, 0.001) dose of MMXmesalamine when compared to placebo (12.9%). 22 In other words, patients treated with higher or lower dose of MMX-mesalamine were respectively nearly 3-fold (OR = 2.78, 95% CI , P = 0.009) or more than 3-fold (OR = 3.48, 97.5% CI , P = 0.001) more likely to achieve the primary end point of the trial than thos receiving placebo. 22 There was no significant difference between active treatment arms with respect to meeting the primary end point (P = 0.485, OR = 1.25, 95% CI ). 22 Proportions of patients who experienced treatment failure at week 8 (no change, worsening or missing modified UC-DAI scores) were significantly (P, 0.001) lower in those treated with active drug (OR = 0.34, 95% CI for lower dose and OR = 0.28, 95% CI ) than placebo. 22 The rates of clinical improvement (decrease of at least 3 points in modified UC-DAI score from baseline) at week 8 were also significantly higher in patients treated with either lower (55.7%, P, 0.001) or higher (59.6%, P, 0.001) dose of MMX-mesalamine when compared to placebo (25.9%). 22 The second trial was performed by Kamm et al 23 who enrolled 343 patients with mild to moderately active UC that was defined according to the same criteria used in the trial by Lichtenstein et al. 22 Patients were randomly assigned to receive for 8 weeks either MMX-mesalamine given once daily at 2.4 g (n = 84) or 4.8 g (n = 85), delayed-release oral 5-ASA at a daily dose of 2.4 g given three times daily (Asacol, Procter and Gamble, Cincinnatti, OH) (n = 86) or placebo (n = 86). 23 The primary endpoint was clinical and endoscopic remission at week 8 defined according to the same criteria used in the trial by Lichtenstein et al. 22,23 The combined rates of clinical and endoscopic remission at week 8 were significantly greater among patients treated with MMX-mesalamine regarless of daily dose (41.2% for lower dose, P = 0.007, 40.5% for higher dose, P = 0.01) when compared to placebo (22.1%). 23 On the other hand, there was no significant difference in combined clinical and endoscopic remission rates at week 8 between patients treated with Asacol and placebo (32.6% vs. 22.1%, P = 0.124). 23 On the other hand, the rates of endoscopic remission (69%, P = 0.003; 77.6%, P, 0.001; 61.6%, P = vs. 46.5%) and clinical improvement (60.7%, P = 0.006; 64.7%, P, 0.001; 55.8%, P = vs. 39.5%) at week 8 were significantly greater among patients treated with lower or greater dose of MMXmesalamine or Asacol when compared to placebo, respectively. 23 On the other hand, the rates of treatment failure at week 8 were significantly lower for patients receiving active treatment when compared to placebo (47.7%) with 21.4% (P, 0.001), 20% (P, 0.001) and 27.9% (P = 0.008) patients receiving either MMX-mesalamine 2.4 g daily, MMX-mesalamine 4.8 g daily or Asacol 2.4 g daily and experiencing treatment failure, respectively. 23 It should be also noted that while 8-week clinical remission rates were significantly greater in patients treated with MMXmesalamine (41.7% for lower dose, P = 0.006; 41.2%, P = for higher dose) when compared to placebo (22.1%), there was no significant difference 10 Clinical Medicine Insights: Gastroenterology 2011:4
5 Treatment of UC with MMX mesalamine in clinical remission rates between patients receiving Asacol and placebo (33.7% vs. 22.1%, P = 0.089). 23 A combined analysis of these two phase III trials of MMX-mesalamine (Lichtenstein et al 22 and Kamm et al) 23 was performed by Sandborn et al. 24 It was demonstrated that among a total of 517 patients who received either MMX-5ASA (2.4 g once daily, 1.2 g twice daily or 4.8 g once daily) or placebo the remission rates at week 8 were significantly higher (P, 0.001) in those who received active drug (37.2% for 2.4 g/day and 35.1% for 4.8 g/day) than those receiving placebo (17.5%). 24 In addition, patients treated with MMX-5ASA had two-fold higher rates of complete mucosal healing than those treated with placebo (33% vs. 16%, p-not reported). 24 Further analysis of data from two aforementioned phase III trials suggested the superiority of MMX-5ASA over placebo in active UC regardless of disease extent, severity, patient s gender or prior therapy with low dose oral 5-ASA formulations (#2 g/day). 25 Among patients who were switched directly (within 5 days prior to baseline) from low-dose oral 5-ASA to MMX-5ASA the significant effectiveness of MMX-5ASA over placebo was demonstrated only among those who were switched directly to the highest dose of MMX-5ASA (4.8 g daily) with clinical and endoscopic remission rates at week 8 of 37.5% vs. 20.9% (P, 0.05). 25 This effect was not observed for those switching directly to MMX-5 ASA at lower daily dose (2.4 g) with combined clinical remission rate of 31.8% vs. 20.9% for placebo (P = 0.09). 25 On the other hand, among patients who either had discontinued prior low dose oral 5-ASA treatment within more than 5 days prior to baseline or had not been treated with oral 5-ASA formulation within 6 weeks prior to baseline or were just diagnosed with UC prior to baseline the clinical and endoscopic remission rates were significantly higher at week 8 when compared to placebo regardless of MMX-5ASA dosing (42.9% for 2.4 g/day, 33% for 4.8 g/day vs. 13.8% for placebo (P, vs. placebo). 25 It has been suggested that patients with active disease on low dose oral 5-ASA may benefit from switching to dose escalation of 5-ASA given as MMX-mesalamine formulation. 25 An open-label extension trial of 304 patients who did not achieve remission in two previous phase III trials by Lichtenstein et al 22 and Kamm et al 23 demonstrated that additional 8-week administration of MMX-5ASA at the dose of 2.4 g given twice daily (total daily dose 4.8 g) resulted in 59.5% clinical and endoscopic remission rates suggesting that some patients with active UC may require additional longer treatment with MMX-5ASA with either increased dose of MMX-5ASA if they previously did not respond to lower dose or the same high dose to achieve remission. 26 Because not all patients had received split dosing of MMX-mesalamine prior to entering an open label extension, the possibility that administration of MMX-mesalamine in two daily doses of 2.4 g during an open label extension might have caused induction of remission in patients who previously did not respond to treatment with once daily dosing of MMX-mesalamine should also be considered. Maintenance of Remission There have been two randomized trials assessing the efficacy of MMX-5 ASA in maintaining remission in patients with UC. 14,27 A multicenter randomized open-label trial that directly enrolled 451 UC patients in clinical and endoscopic remission from either Lichtenstein et al trial, 22 Kamm et al trial 23 or an openlabel 8-week extension study 26 and who were randomly assigned to a daily dose of 2.4 g of MMX-5ASA given either in one or two divided doses for 12 months observed comparable remission rates at week 12 (64.4% vs. 68.5%, P = 0.351) and relapse free rates at week 12 (88.9% vs. 93.2%, P-value not reported) in the once-daily and twice daily arms, respectively. 27 A randomized, double-blind, double-dummy and parallel-group trial of 331 UC patients in remission (UC-DAI score #1) for $1 month compared the efficacy of MMX-5ASA given at single daily dose of 2.4 g to Asacol delayed-release mesalamine formulation given at the total daily dose of 2.4 g (given in two daily doses of 1.6 g and 0.8 g) in maintaining remission for 12 months. 14 MMX-5ASA given once daily was found to be equally effective as Asacol given twice daily in maintaining clinical remission (68% vs. 65.9%, P = 0.69) and both clinical and endoscopic remission (60.9% vs. 61.7%, P = 0.89) after 12 months of treatment. 14 Safety A pooled analysis of two large clinical trials 22,23 performed by Sandborn et al 24 provides the most robust Clinical Medicine Insights: Gastroenterology 2011:4 11
6 Blonski et al data on safety profile of MMX-5ASA formulation. MMX-5ASA has demonstrated a favorable safety profile with majority of adverse events being mild or moderate in their intensity. 24 The most commonly occurring adverse events in all treatment arms were gastrointestinal disorders such as deterioration of UC, abdominal pain, diarrhea, flatulence and nausea that were observed among patients treated either with MMX-5ASA at 2.4 g/day, 4.8 g/day or placebo with rates of 18.1%, 11.7% and 24%, respectively. 24 Treatment with MMX-5ASA was associated with the occurrence of headache (5.6% in 2.4 g/day arm and 3.4% in 4.8 g/day arm vs. 0.6% in placebo arm) and flatulence (4% in 2.4 g/day arm, 2.8% in 4.8 g/day arm vs. 2.8% in placebo arm). 24 Among 13 individual serious adverse events, two cases of pancreatitis were associated with the study medication that occurred in both MMX-5-ASA treatment arms (one in each arm) and they were considered a hypersensitivity reactions to 5-ASA. 24 Conclusions In summary, therapy with MMX-5ASA 2.4 or 4.8 g once daily have been demonstrated to be superior to placebo for induction in patients with mild to moderate ulcerative colitis. Furthermore, MMX-5ASA was also shown to be effective as maintenance therapy based on the available 12- months maintenance therapy trials. Therefore in the light of presented data from several clinical trials we conclude that once daily administration of MMX-5ASA is an efficacious and relatively safe treatment of patients with mild to moderate active UC for both induction and maintenance treatment. In addition once daily administration of MMX-5ASA may lead to better adherence to therapy among patients and thus better treatment outcomes. Since head to head comparative trials between MMX-5ASA and other delayed-release formulations of mesalamine are limited only to one previously discussed maintenance trial by Prantera et al, 14 such trials are warranted to determine fully the efficacy of various delayed-release oral 5-ASA agents in treatment of UC. Disclosure This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material. The authors declare the following potential conflicts of interest. Dr. Gary Lichtenstein: Abbott Corporation, Consultant; Alaven, Consultant, Research; Bristol-Myers Squibb, Research; Centocor Orthobiotech, Consultant, Research; Elan, Consultant; Ferring, Consultant, Research; Meda Pharmaceuticals, Consultant; Millenium Pharmaceuticals, Consultant; Pfizer Pharmaceuticals, Consultant; Proctor and Gamble, Consultant, Research; Prometheus Laboratories, Inc., Consultant, Research; Salix Pharmaceuticals, Consultant, Research; Santarus, Consultant; Schering-Plough Corporation, Consultant; Shire Pharmaceuticals, Consultant, Research; UCB, Consultant, Research; Warner Chilcotte, Consultant, Research; Wyeth, Consultant. References 1. Baron JH, Connell AM, Lennard-Jones JE, Jones FA. Sulphasalazine and salicylazosulphadimidine in ulcerative colitis. Lancet. 1962;1: Misiewicz J, Lennard-Jones J, Conell A, et al. Controlled trial of sulphasaalazine in maintenance therapy for ulcerative colitis. Lancet. 1965; i: Asacol HD (mesalamine) delayed-release tablets. Prescribing information. Medeva Pharma Suisse AG, used under license by Warner Chilcott Pharmaceuticals Inc. Mason, OH, USA; Rasmussen SN, Bondesen S, Hvidberg EF, et al. 5-aminosalicylic acid in a slow-release preparation: bioavailability, plasma level, and excretion in humans. Gastroenterology. 1982;83: Wilding IR, Kenyon CJ, Hooper G. Gastrointestinal spread of oral prolongedrelease mesalazine microgranules (Pentasa) dosed as either tablets or sachet. Aliment Pharmacol Ther. 2000;14: Wilding IR, Behrens C, Tardif SJ, Wray H, Bias P, Albrecht W. Combined scintigraphic and pharmacokinetic investigation of enteric-coated mesalazine micropellets in healthy subjects. Aliment Pharmacol Ther. 2003;17: APRISO (mesalamine) extended-release capsules. Prescribing information. Salix Pharmaceuticals, Inc., Morrisville, NC. USA; Lichtenstein GR, Gordon GL, Zakko S, et al. Clinical trial: once-daily mesalamine granules for maintenance of remission of ulcerative colitis a 6-month placebo-controlled trial. Aliment Pharmacol Ther. 2010;32: Farup PG, Hinterleitner TA, Lukas M, et al. Mesalazine 4 g daily given as prolonged-release granules twice daily and four times daily is at least as effective as prolonged-release tablets four times daily in patients with ulcerative colitis. Inflamm Bowel Dis. 2001;7: Hanauer SB, Sandborn WJ, Kornbluth A, et al. Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II trial. Am J Gastroenterol. 2005;100: Kruis W, Bar-Meir S, Feher J, et al. The optimal dose of 5-aminosalicylic acid in active ulcerative colitis: a dose-finding study with newly developed mesalamine. Clin Gastroenterol Hepatol. 2003;1: Gibson PR, Fixa B, Pekarkova B, et al. Comparison of the efficacy and safety of Eudragit-L-coated mesalazine tablets with ethylcellulose-coated mesalazine tablets in patients with mild to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2006;23: Clinical Medicine Insights: Gastroenterology 2011:4
7 Treatment of UC with MMX mesalamine 13. Marakhouski Y, Fixa B, Holoman J, et al. A double-blind dose-escalating trial comparing novel mesalazine pellets with mesalazine tablets in active ulcerative colitis. Aliment Pharmacol Ther. 2005;21: Prantera C, Kohn A, Campieri M, et al. Clinical trial: ulcerative colitis maintenance treatment with 5-ASA: a 1-year, randomized multicentre study comparing MMX with Asacol. Aliment Pharmacol Ther. 2009;30: Lialda (mesalamine) Delayed Release Tablets. Prescribing information. Shire US Inc., Wayne, PA, USA; (Accessed Jan 13, 2011, at php.). 17. Prantera C, Viscido A, Biancone L, Francavilla A, Giglio L, Campieri M. A new oral delivery system for 5-ASA: preliminary clinical findings for MMx. Inflamm Bowel Dis. 2005;11: Tenjarla S, Romasanta V, Zeijdner E, Villa R, Moro L. Release of 5-aminosalicylate from an MMX mesalamine tablet during transit through a simulated gastrointestinal tract system. Adv Ther. 2007;24: Tenjarla S, Abinusawa A. In-vitro characterization of 5-aminosalicylic acid release from MMX mesalamine tablets and determination of tablet coating thickness. Adv Ther. 2011;28: Brunner M, Assandri R, Kletter K, et al. Gastrointestinal transit and 5-ASA release from a new mesalazine extended-release formulation. Aliment Pharmacol Ther. 2003;17: D Haens G, Hommes D, Engels L, et al. Once daily MMX mesalazine for the treatment of mild-to-moderate ulcerative colitis: a phase II, dose-ranging study. Aliment Pharmacol Ther. 2006;24: Lichtenstein GR, Kamm MA, Boddu P, et al. Effect of once- or twice-daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2007;5: Kamm MA, Sandborn WJ, Gassull M, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132: 66 75; quiz Sandborn WJ, Kamm MA, Lichtenstein GR, Lyne A, Butler T, Joseph RE. MMX Multi Matrix System mesalazine for the induction of remission in patients with mild-to-moderate ulcerative colitis: a combined analysis of two randomized, double-blind, placebo-controlled trials. Aliment Pharmacol Ther. 2007;26: Lichtenstein GR, Kamm MA, Sandborn WJ, Lyne A, Joseph RE. MMX mesalazine for the induction of remission of mild-to-moderately active ulcerative colitis: efficacy and tolerability in specific patient subpopulations. Aliment Pharmacol Ther. 2008;27: Kamm MA, Lichtenstein GR, Sandborn WJ, et al. Effect of extended MMX mesalamine therapy for acute, mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2009;15: Kamm MA, Lichtenstein GR, Sandborn WJ, et al. Randomised trial of onceor twice-daily MMX mesalazine for maintenance of remission in ulcerative colitis. Gut. 2008;57: Publish with Libertas Academica and every scientist working in your field can read your article I would like to say that this is the most author-friendly editing process I have experienced in over 150 publications. Thank you most sincerely. The communication between your staff and me has been terrific. Whenever progress is made with the manuscript, I receive notice. Quite honestly, I ve never had such complete communication with a journal. LA is different, and hopefully represents a kind of scientific publication machinery that removes the hurdles from free flow of scientific thought. Your paper will be: Available to your entire community free of charge Fairly and quickly peer reviewed Yours! You retain copyright Clinical Medicine Insights: Gastroenterology 2011:4 13
5-aminosalicylic acid (5-ASA) is the mainstay of first-line therapy
 MMX Mesalamine for Induction and Maintenance Therapy in Mild-to-Moderate Ulcerative Colitis Stephen B. Hanauer, MD 1 ; Gary R. Lichtenstein, MD 2 ; Michael A. Kamm, MD 3 ; William J. Sandborn, MD 4 ; Kirstin
MMX Mesalamine for Induction and Maintenance Therapy in Mild-to-Moderate Ulcerative Colitis Stephen B. Hanauer, MD 1 ; Gary R. Lichtenstein, MD 2 ; Michael A. Kamm, MD 3 ; William J. Sandborn, MD 4 ; Kirstin
Release of 5-Aminosalicylic Acid (5-ASA) from Mesalamine Formulations at Various ph Levels
 Adv Ther (2015) 32:477 484 DOI 10.1007/s12325-015-0206-4 ORIGINAL RESEARCH Release of 5-Aminosalicylic Acid (5-ASA) from Mesalamine Formulations at Various ph Levels Adeyinka Abinusawa. Srini Tenjarla
Adv Ther (2015) 32:477 484 DOI 10.1007/s12325-015-0206-4 ORIGINAL RESEARCH Release of 5-Aminosalicylic Acid (5-ASA) from Mesalamine Formulations at Various ph Levels Adeyinka Abinusawa. Srini Tenjarla
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 20 October 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 October 2010 MEZAVANT LP 1200 mg, prolonged-release gastro-resistant tablets B/60 (CIP code: 378 689-2) Applicant
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 October 2010 MEZAVANT LP 1200 mg, prolonged-release gastro-resistant tablets B/60 (CIP code: 378 689-2) Applicant
Randomised clinical trial: delayed-release oral mesalazine 4.8 g day vs. 2.4 g day in endoscopic mucosal healing ASCEND I and II combined analysis
 Alimentary Pharmacology and Therapeutics Randomised clinical trial: delayed-release oral mesalazine 4.8 g day vs. 2.4 g day in endoscopic mucosal healing ASCEND I and II combined analysis G. R. Lichtenstein*,
Alimentary Pharmacology and Therapeutics Randomised clinical trial: delayed-release oral mesalazine 4.8 g day vs. 2.4 g day in endoscopic mucosal healing ASCEND I and II combined analysis G. R. Lichtenstein*,
Review article: induction therapy for patients with active ulcerative colitis
 Alimentary Pharmacology & Therapeutics Review article: induction therapy for patients with active ulcerative colitis S. P. L. TRAVIS John Radcliffe Hospital and Linacre College, Oxford, UK Correspondence
Alimentary Pharmacology & Therapeutics Review article: induction therapy for patients with active ulcerative colitis S. P. L. TRAVIS John Radcliffe Hospital and Linacre College, Oxford, UK Correspondence
Novel Formulations and Dosing Strategies for 5-ASA
 D e c e m b e r 2 0 0 8 w w w. c l i n i c a l a d v a n c e s. c o m V o l u m e 4, I s s u e 1 2, S u p p l e m e n t 2 8 Novel Formulations and Dosing Strategies for 5-ASA A Summary of Selected Recent
D e c e m b e r 2 0 0 8 w w w. c l i n i c a l a d v a n c e s. c o m V o l u m e 4, I s s u e 1 2, S u p p l e m e n t 2 8 Novel Formulations and Dosing Strategies for 5-ASA A Summary of Selected Recent
Month/Year of Review: September 2012 Date of Last Review: September 2010
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
5-ASA Therapy, Steroids and Antibiotics in Inflammatory Bowel Disease
 5-ASA Therapy, Steroids and Antibiotics in Inflammatory Bowel Disease David T. Rubin, MD Associate Professor of Medicine Co-Director, Inflammatory Bowel Disease Center University it of Chicago Medical
5-ASA Therapy, Steroids and Antibiotics in Inflammatory Bowel Disease David T. Rubin, MD Associate Professor of Medicine Co-Director, Inflammatory Bowel Disease Center University it of Chicago Medical
Once Daily Dosing for Induction and Maintenance of Remission in Ulcerative Colitis
 Once Daily Dosing for Induction and Maintenance of Remission in Ulcerative Colitis John K. Marshall MD MSc FRCPC AGAF Division of Gastroenterology McMaster University JKM 2014 Svartz N. Acta Med Scand
Once Daily Dosing for Induction and Maintenance of Remission in Ulcerative Colitis John K. Marshall MD MSc FRCPC AGAF Division of Gastroenterology McMaster University JKM 2014 Svartz N. Acta Med Scand
Literature Review: CORE I & II: COlonic RElease Budesonide for the Induction of Remission for Mild- Moderate Ulcerative Colitis
 VOLUME 13, ISSUE 1, YEAR 2014 Literature Review: CORE I & II: COlonic RElease Budesonide for the Induction of Remission for Mild- Moderate Ulcerative Colitis Peter R. McNally, DO, MACG, MSRF Center for
VOLUME 13, ISSUE 1, YEAR 2014 Literature Review: CORE I & II: COlonic RElease Budesonide for the Induction of Remission for Mild- Moderate Ulcerative Colitis Peter R. McNally, DO, MACG, MSRF Center for
Oral mesalamine formulations are first-line treatments
 COCHRANE REVIEW Once Daily Oral Mesalamine Compared to Conventional Dosing for Induction and Maintenance of Remission in Ulcerative Colitis: A Systematic Review and Meta-Analysis Brian G. Feagan, MD, and
COCHRANE REVIEW Once Daily Oral Mesalamine Compared to Conventional Dosing for Induction and Maintenance of Remission in Ulcerative Colitis: A Systematic Review and Meta-Analysis Brian G. Feagan, MD, and
A Review of Delayed-Release MMX Mesalamine: It s Use in the Treatment of Ulcerative Colitis
 A Review of Delayed-Release MMX Mesalamine: It s Use in the Treatment of Ulcerative Colitis Reem Sharaiha 1 and Arun Swaminath 2 REVIEW 1 Fellow, Division of Digestive and Liver Diseases, Columbia University
A Review of Delayed-Release MMX Mesalamine: It s Use in the Treatment of Ulcerative Colitis Reem Sharaiha 1 and Arun Swaminath 2 REVIEW 1 Fellow, Division of Digestive and Liver Diseases, Columbia University
Ulcerative colitis (UC) is a chronic disease that is commonly
 ORIGINAL ARTICLE Voting with Their Feet (VWF) Endpoint: A Meta-Analysis of an Alternative Endpoint in Clinical Trials, Using 5-ASA Induction Studies in Ulcerative Colitis Sujal C. Rangwalla, DO,* Akbar
ORIGINAL ARTICLE Voting with Their Feet (VWF) Endpoint: A Meta-Analysis of an Alternative Endpoint in Clinical Trials, Using 5-ASA Induction Studies in Ulcerative Colitis Sujal C. Rangwalla, DO,* Akbar
FERRING PHARMACEUTICALS. Enjoy The simple COR/939/2014/CH3
 Enjoy The simple pleasures of life COR/939/2014/CH3 Ulcerative colitis disrupts the simple pleasures in life Patients with ulcerative colitis may live with a considerable symptom burden despite medical
Enjoy The simple pleasures of life COR/939/2014/CH3 Ulcerative colitis disrupts the simple pleasures in life Patients with ulcerative colitis may live with a considerable symptom burden despite medical
Synopsis. Clinical Report Synopsis for Protocol Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc.
 Synopsis Clinical Report Synopsis for Protocol 197-02-220 Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc. Name of Product: Tetomilast (OPC-6535) Study Title: A Phase 3, Multicenter,
Synopsis Clinical Report Synopsis for Protocol 197-02-220 Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc. Name of Product: Tetomilast (OPC-6535) Study Title: A Phase 3, Multicenter,
How to Optimize Induction and Maintenance Responses: Definitions and Dosing Advances in Inflammatory Bowel Disease December 6, 2009
 How to Optimize Induction and Maintenance Responses: Definitions and Dosing 2009 Advances in Inflammatory Bowel Disease December 6, 2009 Fernando Velayos MD MPH University of California, San Francisco
How to Optimize Induction and Maintenance Responses: Definitions and Dosing 2009 Advances in Inflammatory Bowel Disease December 6, 2009 Fernando Velayos MD MPH University of California, San Francisco
Ulcerative Colitis: Refining our Management and Incorporating Newer Concepts
 Ulcerative Colitis: Refining our Management and Incorporating Newer Concepts Asher Kornbluth, MD Clinical Professor of Medicine The Henry D. Janowitz The Mt. Sinai School of Medicine Refining our Management
Ulcerative Colitis: Refining our Management and Incorporating Newer Concepts Asher Kornbluth, MD Clinical Professor of Medicine The Henry D. Janowitz The Mt. Sinai School of Medicine Refining our Management
Oxford Inflammatory Bowel Disease MasterClass The mesalazine chamaeleon
 Oxford Inflammatory Bowel Disease MasterClass The mesalazine chamaeleon Ailsa Hart Lead IBD Unit, St Mark s Hospital Senior Clinical lecturer Imperial College, London Oxford Inflammatory Bowel Disease
Oxford Inflammatory Bowel Disease MasterClass The mesalazine chamaeleon Ailsa Hart Lead IBD Unit, St Mark s Hospital Senior Clinical lecturer Imperial College, London Oxford Inflammatory Bowel Disease
How do I choose amongst medicines for inflammatory bowel disease. Maria T. Abreu, MD
 How do I choose amongst medicines for inflammatory bowel disease Maria T. Abreu, MD Overview of IBD Pathogenesis Bacterial Products Moderately Acutely Inflamed Chronic Inflammation = IBD Normal Gut Mildly
How do I choose amongst medicines for inflammatory bowel disease Maria T. Abreu, MD Overview of IBD Pathogenesis Bacterial Products Moderately Acutely Inflamed Chronic Inflammation = IBD Normal Gut Mildly
Title: Authors: Journal:
 IMPORTANT COPYRIGHT NOTICE: This electronic article is provided to you by courtesy of Ferring Pharmaceuticals. The document is provided for personal usage only. Further reproduction and/or distribution
IMPORTANT COPYRIGHT NOTICE: This electronic article is provided to you by courtesy of Ferring Pharmaceuticals. The document is provided for personal usage only. Further reproduction and/or distribution
Clinical trial: once-daily mesalamine granules for maintenance of remission of ulcerative colitis a 6-month placebo-controlled trial
 Alimentary Pharmacology and Therapeutics Clinical trial: once-daily mesalamine granules for maintenance of remission of ulcerative colitis a 6-month placebo-controlled trial G. R. Lichtenstein*, G. L.
Alimentary Pharmacology and Therapeutics Clinical trial: once-daily mesalamine granules for maintenance of remission of ulcerative colitis a 6-month placebo-controlled trial G. R. Lichtenstein*, G. L.
Mild-moderate Ulcerative Colitis Sequential & Combined treatments need to be tested. Philippe Marteau, Paris, France
 Mild-moderate Ulcerative Colitis Sequential & Combined treatments need to be tested Philippe Marteau, Paris, France Sequential vs combined treatments When should one switch? Sequential vs combined treatments
Mild-moderate Ulcerative Colitis Sequential & Combined treatments need to be tested Philippe Marteau, Paris, France Sequential vs combined treatments When should one switch? Sequential vs combined treatments
Title: Authors: Journal:
 IMPORTANT COPYRIGHT NOTICE: This electronic article is provided to you by courtesy of Ferring Pharmaceuticals. The document is provided for personal usage only. Further reproduction and/or distribution
IMPORTANT COPYRIGHT NOTICE: This electronic article is provided to you by courtesy of Ferring Pharmaceuticals. The document is provided for personal usage only. Further reproduction and/or distribution
As clinicians we would all agree that the goal for our
 CURRENT CONTROVERSIES: PRO, CON, AND BALANCE Controversies in Mucosal Healing in Ulcerative Colitis Sunanda Kane, MD,* Frances Lu, MD, Asher Kornbluth, MD, Dahlia Awais, MD, and Peter D.R. Higgins, MD,
CURRENT CONTROVERSIES: PRO, CON, AND BALANCE Controversies in Mucosal Healing in Ulcerative Colitis Sunanda Kane, MD,* Frances Lu, MD, Asher Kornbluth, MD, Dahlia Awais, MD, and Peter D.R. Higgins, MD,
Moderately to severely active ulcerative colitis
 Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
National Institute for Health and Care Excellence
 National Institute for Health and Care Excellence 4-year surveillance (2017) Ulcerative colitis: management (2013) NICE guideline CG166 Appendix A.2: Summary of new evidence from surveillance Patient information
National Institute for Health and Care Excellence 4-year surveillance (2017) Ulcerative colitis: management (2013) NICE guideline CG166 Appendix A.2: Summary of new evidence from surveillance Patient information
Budesonide Multi-matrix for the Treatment of Patients with Ulcerative Colitis
 Dig Dis Sci (2016) 61:358 370 DOI 10.1007/s10620-015-3897-0 REVIEW Budesonide Multi-matrix for the Treatment of Patients with Ulcerative Colitis Gary R. Lichtenstein 1 Received: 19 August 2015 / Accepted:
Dig Dis Sci (2016) 61:358 370 DOI 10.1007/s10620-015-3897-0 REVIEW Budesonide Multi-matrix for the Treatment of Patients with Ulcerative Colitis Gary R. Lichtenstein 1 Received: 19 August 2015 / Accepted:
Gastrointestinal transit and release of 5-aminosalicylic acid from. volunteers. 153 Sm-labelled mesalazine pellets vs. tablets in male healthy
 Aliment Pharmacol Ther 2003; 17: 1163 1169. doi: 10.1046/j.0269-2813.2003.01564.x Gastrointestinal transit and release of 5-aminosalicylic acid from 153 Sm-labelled mesalazine pellets vs. tablets in male
Aliment Pharmacol Ther 2003; 17: 1163 1169. doi: 10.1046/j.0269-2813.2003.01564.x Gastrointestinal transit and release of 5-aminosalicylic acid from 153 Sm-labelled mesalazine pellets vs. tablets in male
Clinical Trials in IBD. Bruce Yacyshyn MD Professor of Medicine Division of Digestive Diseases
 Clinical Trials in IBD Bruce Yacyshyn MD Professor of Medicine Division of Digestive Diseases Objectives Today s discussion will address the following topics: Similarities and differences between Crohn
Clinical Trials in IBD Bruce Yacyshyn MD Professor of Medicine Division of Digestive Diseases Objectives Today s discussion will address the following topics: Similarities and differences between Crohn
Position of Biologics in IBD Circa 2006: Top Down vs. Step Up Therapy
 Position of Biologics in IBD Circa 2006: Top Down vs. Step Up Therapy Stephen B. Hanauer, MD University of Chicago Potential Conflicts: Centocor/Schering, Abbott, UCB, Elan, Berlex, PDL Goals of Treatment
Position of Biologics in IBD Circa 2006: Top Down vs. Step Up Therapy Stephen B. Hanauer, MD University of Chicago Potential Conflicts: Centocor/Schering, Abbott, UCB, Elan, Berlex, PDL Goals of Treatment
Dr. Elmer Schabel, MD. Bundesinstitut für Arzneimittel und Medizinprodukte, Bonn, Germany (No conflicts of interest)
 EMA workshop on the development of new medicinal products for the treatment of ulcerative colitis and Crohn s disease Overview of authorised medicines for IBD in Europe - previous regulatory positions
EMA workshop on the development of new medicinal products for the treatment of ulcerative colitis and Crohn s disease Overview of authorised medicines for IBD in Europe - previous regulatory positions
Efficacy and Safety of Treatment for Pediatric IBD
 Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Associate Professor of Clinical Pediatrics Division of Gastroenterology,
Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Associate Professor of Clinical Pediatrics Division of Gastroenterology,
Azathioprine for Induction and Maintenance of Remission in Crohn s Disease
 Azathioprine for Induction and Maintenance of Remission in Crohn s Disease William J. Sandborn, MD Chief, Division of Gastroenterology Director, UCSD IBD Center Objectives Azathioprine as induction and
Azathioprine for Induction and Maintenance of Remission in Crohn s Disease William J. Sandborn, MD Chief, Division of Gastroenterology Director, UCSD IBD Center Objectives Azathioprine as induction and
Clinical Medicine Reviews in Therapeutics. Pharmacotherapy of Ulcerative Colitis: What Role for Mesalamine?
 Clinical Medicine Reviews in Therapeutics R e v i e w Pharmacotherapy of Ulcerative Colitis: What Role for Mesalamine? Adam Harris 1, Mitul Patel 1 and Samir A. Shah 2 1 Warren Alpert School of Medicine
Clinical Medicine Reviews in Therapeutics R e v i e w Pharmacotherapy of Ulcerative Colitis: What Role for Mesalamine? Adam Harris 1, Mitul Patel 1 and Samir A. Shah 2 1 Warren Alpert School of Medicine
Crohn's disease. Appendix J. Clinical Guideline < > 10 October Review of Cochrane ASA review
 Crohn's disease Clinical Guideline < > Review of Cochrane ASA review 0 October 202 Commissioned by the National Institute for Health and Clinical Excellence Review of Cochrane 5-ASA review Contents Published
Crohn's disease Clinical Guideline < > Review of Cochrane ASA review 0 October 202 Commissioned by the National Institute for Health and Clinical Excellence Review of Cochrane 5-ASA review Contents Published
The following slides are provided as presented by the author during the live educa7onal ac7vity and are intended for reference purposes only.
 The following slides are provided as presented by the author during the live educa7onal ac7vity and are intended for reference purposes only. If you have any ques7ons, please contact Imedex via email at:
The following slides are provided as presented by the author during the live educa7onal ac7vity and are intended for reference purposes only. If you have any ques7ons, please contact Imedex via email at:
Oral mesalamine formulations are widely used for the
 ORIGINAL ARTICLE Direct Comparison of Two Different Mesalamine Formulations for the Induction of Remission in Patients with Ulcerative Colitis: A Double-blind, Randomized Study Hiroaki Ito, MD,* Mitsuo
ORIGINAL ARTICLE Direct Comparison of Two Different Mesalamine Formulations for the Induction of Remission in Patients with Ulcerative Colitis: A Double-blind, Randomized Study Hiroaki Ito, MD,* Mitsuo
Old and new steroids: hitting the target
 Oxford Inflammatory Bowel Disease & Hepatology MasterClass Old and new steroids: hitting the target Silvio Danese, MD, PhD IBD Center Division of Gastroenterology Istituto Clinico Humanitas, Milan, Italy
Oxford Inflammatory Bowel Disease & Hepatology MasterClass Old and new steroids: hitting the target Silvio Danese, MD, PhD IBD Center Division of Gastroenterology Istituto Clinico Humanitas, Milan, Italy
Review article: balsalazide therapy in ulcerative colitis
 Aliment Pharmacol Ther 2001; 15: 1549±1554. Review article: balsalazide therapy in ulcerative colitis K. RAGUNATH & J. G. WILLIAMS Centre for Digestive Diseases and Nutrition, Morriston Hospital, Swansea,
Aliment Pharmacol Ther 2001; 15: 1549±1554. Review article: balsalazide therapy in ulcerative colitis K. RAGUNATH & J. G. WILLIAMS Centre for Digestive Diseases and Nutrition, Morriston Hospital, Swansea,
Article: Min, T and Ford, AC (2016) Efficacy of mesalazine in IBS. Gut, 65 (1). pp ISSN
 This is a repository copy of Efficacy of mesalazine in IBS. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/97338/ Version: Accepted Version Article: Min, T and Ford, AC (2016)
This is a repository copy of Efficacy of mesalazine in IBS. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/97338/ Version: Accepted Version Article: Min, T and Ford, AC (2016)
New treatment options in UC. Rob Bryant IBD Consultant Royal Adelaide Hospital
 New treatment options in UC Rob Bryant IBD Consultant Royal Adelaide Hospital Talk Outline 1. Raising expectations 2. Optimising UC therapy 3. Clinical trials 4. What s new on the PBS? 5. Questions 1.
New treatment options in UC Rob Bryant IBD Consultant Royal Adelaide Hospital Talk Outline 1. Raising expectations 2. Optimising UC therapy 3. Clinical trials 4. What s new on the PBS? 5. Questions 1.
Ulcerative colitis (UC) is a chronic relapsing and remitting inflammatory
 TREATMENT UPDATE The Efficacy of Oral 5-ASAs in the Treatment of Active Ulcerative Colitis: A Systematic Review Sunanda V. Kane, MD, MSPH,* David J. Bjorkman, MD, MSPH, SM (Epid) *University of Chicago,
TREATMENT UPDATE The Efficacy of Oral 5-ASAs in the Treatment of Active Ulcerative Colitis: A Systematic Review Sunanda V. Kane, MD, MSPH,* David J. Bjorkman, MD, MSPH, SM (Epid) *University of Chicago,
AVX-470, an Orally Delivered Anti-TNF Antibody for Treatment of Acute Ulcerative Colitis: Results of a First-in- Human Trial
 AVX-470, an Orally Delivered Anti-TNF Antibody for Treatment of Acute Ulcerative Colitis: Results of a First-in- Human Trial M Scott Harris 1, Deborah Hartman 1, Sharon Spence 1, Sally Kennedy 1, Theodore
AVX-470, an Orally Delivered Anti-TNF Antibody for Treatment of Acute Ulcerative Colitis: Results of a First-in- Human Trial M Scott Harris 1, Deborah Hartman 1, Sharon Spence 1, Sally Kennedy 1, Theodore
Doncaster & Bassetlaw Medicines Formulary
 Doncaster & Bassetlaw Medicines Formulary Section 1.5 Chronic Bowel Disorders (including IBD) Aminosalicylates: Mesalazine 400mg and 800mg MR Tablets (Octasa) Mesalazine 1.2g MR Tablets (Mezavant XL) Mesalazine
Doncaster & Bassetlaw Medicines Formulary Section 1.5 Chronic Bowel Disorders (including IBD) Aminosalicylates: Mesalazine 400mg and 800mg MR Tablets (Octasa) Mesalazine 1.2g MR Tablets (Mezavant XL) Mesalazine
Aminosalicylates in Inflammatory Bowel Disease in Adults (Review date: July 2020) Page 1
 Full Title of Guideline: Author (include email and role): Aminosalicylates in Inflammatory Bowel Disease in Adults Natalie Tse- Senior Clinical Pharmacist (natalie.tse@nuh.nhs.uk) Dr. Nina Lewis- Consultant
Full Title of Guideline: Author (include email and role): Aminosalicylates in Inflammatory Bowel Disease in Adults Natalie Tse- Senior Clinical Pharmacist (natalie.tse@nuh.nhs.uk) Dr. Nina Lewis- Consultant
Efficacy of 5-Aminosalicylates in Ulcerative Colitis: Systematic Review and Meta-Analysis
 CLINICAL AND SYSTEMATIC S nature publishing group 601 Efficacy of 5-Aminosalicylates in Ulcerative Colitis: Systematic Review and Meta-Analysis Alexander C. Ford, MBChB, MD, MRCP 1, 2, Je an - Pau l Ach
CLINICAL AND SYSTEMATIC S nature publishing group 601 Efficacy of 5-Aminosalicylates in Ulcerative Colitis: Systematic Review and Meta-Analysis Alexander C. Ford, MBChB, MD, MRCP 1, 2, Je an - Pau l Ach
Positioning New Therapies
 Positioning New Therapies Stephen Hanauer, MD Professor of Medicine Medical Director, Digestive Disease Center Northwestern Medicine Chicago, Illinois Speaker Disclosure Stephen Hanauer, MD has disclosed
Positioning New Therapies Stephen Hanauer, MD Professor of Medicine Medical Director, Digestive Disease Center Northwestern Medicine Chicago, Illinois Speaker Disclosure Stephen Hanauer, MD has disclosed
Clinical Policy: Budesonide (Uceris) Reference Number: CP.PCH.11 Effective Date: Last Review Date: Line of Business: Commercial, HIM*
 Clinical Policy: (Uceris) Reference Number: CP.PCH.11 Effective Date: 08.14.18 Last Review Date: 11.18 Line of Business: Commercial, HIM* Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Uceris) Reference Number: CP.PCH.11 Effective Date: 08.14.18 Last Review Date: 11.18 Line of Business: Commercial, HIM* Revision Log See Important Reminder at the end of this policy for
Update on the management of ulcerative colitis: treatment and maintenance approaches focused on MMX mesalamine
 Update on the management of ulcerative colitis: treatment and maintenance approaches focused on MMX mesalamine The Harvard community has made this article openly available. Please share how this access
Update on the management of ulcerative colitis: treatment and maintenance approaches focused on MMX mesalamine The Harvard community has made this article openly available. Please share how this access
Update on the Optimization of Mesalamine-Based Therapy in the Treatment of Ulcerative Colitis
 M e s a l a m i n e - B a s e d T h e r a p y i n t h e T r e a t m e n t o f U l c e r a t i v e C o l i t i s M a r c h 2 0 0 8 w w w. c l i n i c a l a d v a n c e s. c o m V o l u m e 4, I s s u e
M e s a l a m i n e - B a s e d T h e r a p y i n t h e T r e a t m e n t o f U l c e r a t i v e C o l i t i s M a r c h 2 0 0 8 w w w. c l i n i c a l a d v a n c e s. c o m V o l u m e 4, I s s u e
INTESTINAL DISEASE MEETING BERLIN Topical steroids rectal application
 INTESTINAL DISEASE MEETING BERLIN 2006 Topical steroids rectal application Prof. Dr. med. T. Andus Department of Internal Medicine, Gastroenterology, Hepatology, and Oncology Krankenhaus Bad Cannstatt,
INTESTINAL DISEASE MEETING BERLIN 2006 Topical steroids rectal application Prof. Dr. med. T. Andus Department of Internal Medicine, Gastroenterology, Hepatology, and Oncology Krankenhaus Bad Cannstatt,
Efficacy and Safety of Treatment for Pediatric IBD
 Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Assistant Professor of Clinical Pediatrics Division of Gastroenterology,
Efficacy and Safety of Treatment for Pediatric IBD Andrew B. Grossman MD Co-Director, Center for Pediatric Inflammatory Bowel Disease Assistant Professor of Clinical Pediatrics Division of Gastroenterology,
Biologic therapy with antagonists to tumor necrosis factor
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2010;8:696 702 Reinduction With Certolizumab Pegol in Patients With Relapsed Crohn s Disease: Results From the PRECiSE 4 Study WILLIAM J. SANDBORN,* STEFAN SCHREIBER,
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2010;8:696 702 Reinduction With Certolizumab Pegol in Patients With Relapsed Crohn s Disease: Results From the PRECiSE 4 Study WILLIAM J. SANDBORN,* STEFAN SCHREIBER,
Treatment of Ulcerative Colitis in the Elderly: A Systematic
 Medicine Insights: Geriatrics Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Treatment of Ulcerative Colitis in the Elderly: A Systematic Review Brooke
Medicine Insights: Geriatrics Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Treatment of Ulcerative Colitis in the Elderly: A Systematic Review Brooke
The Liver and Right Atrium Hepatic Cyst as a Cause of Arrhythmia
 Clinical Medicine Insights: Cardiology Case report Open Access Full open access to this and thousands of other papers at http://www.la-press.com. The Liver and Right Atrium Hepatic Cyst as a Cause of Arrhythmia
Clinical Medicine Insights: Cardiology Case report Open Access Full open access to this and thousands of other papers at http://www.la-press.com. The Liver and Right Atrium Hepatic Cyst as a Cause of Arrhythmia
Treating to Achieve a Target and Disease Monitoring in 2015: State of the Art
 Treating to Achieve a Target and Disease Monitoring in 2015: State of the Art David T. Rubin, MD The Joseph B. Kirsner Professor of Medicine Chief, Section of Gastroenterology, Hepatology and Nutrition
Treating to Achieve a Target and Disease Monitoring in 2015: State of the Art David T. Rubin, MD The Joseph B. Kirsner Professor of Medicine Chief, Section of Gastroenterology, Hepatology and Nutrition
Medical Management of Inflammatory Bowel Disease
 Medical Management of Inflammatory Bowel Disease John K. Marshall MD MSc FRCPC AGAF Division of Gastroenterology McMaster University John K. Marshall: Conflicts of Interest Speaker: AbbVie, Allergan, Ferring,
Medical Management of Inflammatory Bowel Disease John K. Marshall MD MSc FRCPC AGAF Division of Gastroenterology McMaster University John K. Marshall: Conflicts of Interest Speaker: AbbVie, Allergan, Ferring,
Public Assessment Report Scientific discussion. Mesalazin ESPL (mesalazine) SE/H/1654/01/DC
 Public Assessment Report Scientific discussion Mesalazin ESPL (mesalazine) SE/H/1654/01/DC This module reflects the scientific discussion for the approval of Mesalazin ESPL. The procedure was finalised
Public Assessment Report Scientific discussion Mesalazin ESPL (mesalazine) SE/H/1654/01/DC This module reflects the scientific discussion for the approval of Mesalazin ESPL. The procedure was finalised
FOR UK NURSING MEDIA Embargoed until: 00:01 GMT, Friday 13 March 2015
 Contact: Ross Selby Takeda UK Ltd Email ross.selby@takeda.com News Release FOR UK NURSING MEDIA Embargoed until: 00:01 GMT, Friday 13 March 2015 World s first gut-selective treatment for ulcerative colitis
Contact: Ross Selby Takeda UK Ltd Email ross.selby@takeda.com News Release FOR UK NURSING MEDIA Embargoed until: 00:01 GMT, Friday 13 March 2015 World s first gut-selective treatment for ulcerative colitis
Randomised clinical trial: once- vs. twice-daily prolongedrelease mesalazine for active ulcerative colitis
 Alimentary Pharmacology and Therapeutics Randomised clinical trial: once- vs. twice-daily prolongedrelease mesalazine for active ulcerative colitis B. Flourie*, H. Hagege, G. Tucat, D. Maetz,X.Hebuterne,
Alimentary Pharmacology and Therapeutics Randomised clinical trial: once- vs. twice-daily prolongedrelease mesalazine for active ulcerative colitis B. Flourie*, H. Hagege, G. Tucat, D. Maetz,X.Hebuterne,
The future of IBD therapeutic research
 The future of IBD therapeutic research Jean-Frederic Colombel, MD Director Susan and Leonard Feinstein IBD Clinical Center Icahn School of Medicine, Mount Sinai Hospital New York J-F Colombel has served
The future of IBD therapeutic research Jean-Frederic Colombel, MD Director Susan and Leonard Feinstein IBD Clinical Center Icahn School of Medicine, Mount Sinai Hospital New York J-F Colombel has served
A Bad Outcome. Drugs don t work in patients who don t take them. Does Compliance Really Matter? Why Should I Care about This?
 Does Compliance Really Matter? Sunanda Kane MD, MSPH, FACG, FACP, AGAF Professor of Medicine Mayo Clinic Drugs don t work in patients who don t take them C. Everett Koop A Bad Outcome Why Should I Care
Does Compliance Really Matter? Sunanda Kane MD, MSPH, FACG, FACP, AGAF Professor of Medicine Mayo Clinic Drugs don t work in patients who don t take them C. Everett Koop A Bad Outcome Why Should I Care
Latest Treatment Updates for Ulcerative Colitis: Evolving Treatment Goals
 Latest Treatment Updates for Ulcerative Colitis: Evolving Treatment Goals Stephen Hanauer, MD Professor of Medicine Medical Director, Digestive Disease Center Northwestern Medicine Chicago, Illinois Speaker
Latest Treatment Updates for Ulcerative Colitis: Evolving Treatment Goals Stephen Hanauer, MD Professor of Medicine Medical Director, Digestive Disease Center Northwestern Medicine Chicago, Illinois Speaker
5-ASA for the treatment of Crohn s disease DR. STEPHEN HANAUER FEINBERG SCHOOL OF MEDICINE, NORTHWESTERN UNIVERSITY, CHICAGO, IL, USA
 5-ASA for the treatment of Crohn s disease DR. STEPHEN HANAUER FEINBERG SCHOOL OF MEDICINE, NORTHWESTERN UNIVERSITY, CHICAGO, IL, USA Background RCTs investigating the efficacy of aminosalicylates for
5-ASA for the treatment of Crohn s disease DR. STEPHEN HANAUER FEINBERG SCHOOL OF MEDICINE, NORTHWESTERN UNIVERSITY, CHICAGO, IL, USA Background RCTs investigating the efficacy of aminosalicylates for
DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE
 1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
D. Gillespie, K. Hood, A. Williams, R. Stenson, C.S.J. Probert and A.B. Hawthorne Clinical Trials Methodology Conference, Bristol 2011
 Use of the Medication Event Monitoring System for assessing medication adherence for chronic conditions: Results from a 12 month trial of patients in remission with ulcerative colitis D. Gillespie, K.
Use of the Medication Event Monitoring System for assessing medication adherence for chronic conditions: Results from a 12 month trial of patients in remission with ulcerative colitis D. Gillespie, K.
Aminosalicylates. Giovanni Barbara. Department of Medical and Surgical Sciences Alma Mater Studiorum, University of Bologna, Italy ( )
 Aminosalicylates Giovanni Barbara Department of Medical and Surgical Sciences Alma Mater Studiorum, University of Bologna, Italy (1088 2016) Colonic Diverticula, a Painless Ageing Change? 20% develop symptoms
Aminosalicylates Giovanni Barbara Department of Medical and Surgical Sciences Alma Mater Studiorum, University of Bologna, Italy (1088 2016) Colonic Diverticula, a Painless Ageing Change? 20% develop symptoms
Treatment Guidelines and Clinical Practice: Optimizing Foundational Therapies for Ulcerative Colitis
 O c t o b e r 2 0 0 8 w w w. c l i n i c a l a d v a n c e s. c o m V o l u m e 4, I s s u e 1 0, S u p p l e m e n t 2 2 Faculty Stephen B. Hanauer, MD Program Chair Professor of Medicine and Clinical
O c t o b e r 2 0 0 8 w w w. c l i n i c a l a d v a n c e s. c o m V o l u m e 4, I s s u e 1 0, S u p p l e m e n t 2 2 Faculty Stephen B. Hanauer, MD Program Chair Professor of Medicine and Clinical
Which is the Safest Strategy to Treat Moderate to Severe IBD?
 Which is the Safest Strategy to Treat Moderate to Severe IBD? David G. Binion, M.D. Co-Director, Inflammatory Bowel Disease Center Director, Translational Inflammatory Bowel Disease Research Visiting Professor
Which is the Safest Strategy to Treat Moderate to Severe IBD? David G. Binion, M.D. Co-Director, Inflammatory Bowel Disease Center Director, Translational Inflammatory Bowel Disease Research Visiting Professor
Activity and Endoscopic measures : Crohn s disease. Jean-Frederic COLOMBEL Justin Cote-Daigneault Icahn Medical School at Mount Sinai, New York
 Activity and Endoscopic measures : Crohn s disease Jean-Frederic COLOMBEL Justin Cote-Daigneault Icahn Medical School at Mount Sinai, New York J-F Colombel has served as consultant or advisory board member
Activity and Endoscopic measures : Crohn s disease Jean-Frederic COLOMBEL Justin Cote-Daigneault Icahn Medical School at Mount Sinai, New York J-F Colombel has served as consultant or advisory board member
NON INVASIVE MONITORING OF MUCOSAL HEALING IN IBD. THE ROLE OF BOWEL ULTRASOUND. Fabrizio Parente
 NON INVASIVE MONITORING OF MUCOSAL HEALING IN IBD. THE ROLE OF BOWEL ULTRASOUND Fabrizio Parente Gastrointestinal Unit, A.Manzoni Hospital, Lecco & L.Sacco School of Medicine,University of Milan - Italy
NON INVASIVE MONITORING OF MUCOSAL HEALING IN IBD. THE ROLE OF BOWEL ULTRASOUND Fabrizio Parente Gastrointestinal Unit, A.Manzoni Hospital, Lecco & L.Sacco School of Medicine,University of Milan - Italy
Full title of guideline: Ulcerative colitis: the management of ulcerative colitis
 Economic Plan This document identifies the priorities for economic analysis and the proposed methods for addressing these questions as described in section 7.1.3 of the Guidelines Manual (2009). 1 Guideline
Economic Plan This document identifies the priorities for economic analysis and the proposed methods for addressing these questions as described in section 7.1.3 of the Guidelines Manual (2009). 1 Guideline
Speaker Introduction
 Speaker Introduction Stephen B. Hanauer, MD Professor of Medicine and Clinical Pharmacology University of Chicago Pritzker School of Medicine Chief of Gastroenterology, Hepatology, and Nutrition University
Speaker Introduction Stephen B. Hanauer, MD Professor of Medicine and Clinical Pharmacology University of Chicago Pritzker School of Medicine Chief of Gastroenterology, Hepatology, and Nutrition University
Treatment Goals. Current Therapeutic Pyramids Crohn s Disease Ulcerative Colitis 11/14/10
 Current Management of IBD: From Conventional Agents to Biologics Stephen B. Hanauer, M.D. University of Chicago Treatment Goals Induce and maintain response/ remission Prevent complications Improve quality
Current Management of IBD: From Conventional Agents to Biologics Stephen B. Hanauer, M.D. University of Chicago Treatment Goals Induce and maintain response/ remission Prevent complications Improve quality
IBD Updates. Themes in IBD IBD management journey. New tools for therapeutic monitoring. First-line treatment in IBD
 IBD Updates Maria T. Abreu, MD University of Miami Miller School of Medicine Miami, Florida Themes in IBD 213 First-line treatment in IBD New tools for therapeutic monitoring Biologic therapy for CD and
IBD Updates Maria T. Abreu, MD University of Miami Miller School of Medicine Miami, Florida Themes in IBD 213 First-line treatment in IBD New tools for therapeutic monitoring Biologic therapy for CD and
Mesalazine in inflammatory bowel disease: A trendy topic once again?
 review Mesalazine in inflammatory bowel disease: A trendy topic once again? Marietta Iacucci MD 1, Shanika de Silva MRCP 2, Subrata Ghosh MD FRCPC FRCP FRCPE 2 M Iacucci, S de Silva, S Ghosh. Mesalazine
review Mesalazine in inflammatory bowel disease: A trendy topic once again? Marietta Iacucci MD 1, Shanika de Silva MRCP 2, Subrata Ghosh MD FRCPC FRCP FRCPE 2 M Iacucci, S de Silva, S Ghosh. Mesalazine
Title: Author: Journal:
 IMPORTANT COPYRIGHT NOTICE: This electronic article is provided to you by courtesy of Ferring Pharmaceuticals. The document is provided for personal usage only. Further reproduction and/or distribution
IMPORTANT COPYRIGHT NOTICE: This electronic article is provided to you by courtesy of Ferring Pharmaceuticals. The document is provided for personal usage only. Further reproduction and/or distribution
Ulcerative colitis (UC) is a relatively common inflammatory
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2012;10:513 519 Efficacy of Topical 5-Aminosalicylates in Preventing Relapse of Quiescent Ulcerative Colitis: A Meta-analysis ALEXANDER C. FORD,*, KHURRAM J. KHAN,
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2012;10:513 519 Efficacy of Topical 5-Aminosalicylates in Preventing Relapse of Quiescent Ulcerative Colitis: A Meta-analysis ALEXANDER C. FORD,*, KHURRAM J. KHAN,
IBD Understanding Your Medications. Thomas V. Aguirre, MD Santa Barbara GI Consultants
 IBD Understanding Your Medications Thomas V. Aguirre, MD Santa Barbara GI Consultants IBD Understanding Your Medications (& Your Doctor) Thomas V. Aguirre, MD Santa Barbara GI Consultants Disclosure I
IBD Understanding Your Medications Thomas V. Aguirre, MD Santa Barbara GI Consultants IBD Understanding Your Medications (& Your Doctor) Thomas V. Aguirre, MD Santa Barbara GI Consultants Disclosure I
SUMMARY INTRODUCTION. Accepted for publication 10 September 2004
 Aliment Pharmacol Ther 2004; 20: 1353 1363. doi: 10.1111/j.1365-2036.2004.02282.x Mesalazine (5-aminosalicylic acid) micropellets show similar efficacy and tolerability to mesalazine tablets in patients
Aliment Pharmacol Ther 2004; 20: 1353 1363. doi: 10.1111/j.1365-2036.2004.02282.x Mesalazine (5-aminosalicylic acid) micropellets show similar efficacy and tolerability to mesalazine tablets in patients
APDW 2016 Poster No. a90312
 APDW 2016 Poster No. a90312 SYN-010, a Proprietary Modified-Release Formulation of Lovastatin Lactone, Lowered Breath Methane and Improved Stool Frequency in Patients with IBS-C Results of a multi-center,
APDW 2016 Poster No. a90312 SYN-010, a Proprietary Modified-Release Formulation of Lovastatin Lactone, Lowered Breath Methane and Improved Stool Frequency in Patients with IBS-C Results of a multi-center,
Medical Therapy for Pediatric IBD: Efficacy and Safety
 Medical Therapy for Pediatric IBD: Efficacy and Safety Betsy Maxwell, MD Assistant Professor of Clinical Pediatrics Division of Gastroenterology, Hepatology, and Nutrition Pediatric IBD: Defining Remission
Medical Therapy for Pediatric IBD: Efficacy and Safety Betsy Maxwell, MD Assistant Professor of Clinical Pediatrics Division of Gastroenterology, Hepatology, and Nutrition Pediatric IBD: Defining Remission
Implementation of disease and safety predictors during disease management in UC
 Implementation of disease and safety predictors during disease management in UC DR ARIELLA SHITRIT DIGESTIVE DISEASES INSTITUTE SHAARE ZEDEK MEDICAL CENTER JERUSALEM Case presentation A 52 year old male
Implementation of disease and safety predictors during disease management in UC DR ARIELLA SHITRIT DIGESTIVE DISEASES INSTITUTE SHAARE ZEDEK MEDICAL CENTER JERUSALEM Case presentation A 52 year old male
SHIRE v FERRING. Promotion of Pentasa CASE AUTH/2299/2/10
 CASE AUTH/2299/2/10 SHIRE v FERRING Promotion of Pentasa Shire complained about the promotion of Pentasa (mesalazine) by Ferring. The items at issue were a 'power of five' booklet, an A4 sheet and an advertisement
CASE AUTH/2299/2/10 SHIRE v FERRING Promotion of Pentasa Shire complained about the promotion of Pentasa (mesalazine) by Ferring. The items at issue were a 'power of five' booklet, an A4 sheet and an advertisement
Positioning Biologics in Ulcerative Colitis
 Positioning Biologics in Ulcerative Colitis Bruce E. Sands, MD, MS Acting Chief, Gastrointestinal Unit Massachusetts General Hospital Associate Professor of Medicine Harvard Medical School Sequential Therapies
Positioning Biologics in Ulcerative Colitis Bruce E. Sands, MD, MS Acting Chief, Gastrointestinal Unit Massachusetts General Hospital Associate Professor of Medicine Harvard Medical School Sequential Therapies
There is a well-established association between inflammatory
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:1346 1350 Aminosalicylate Therapy in the Prevention of Dysplasia and Colorectal Cancer in Ulcerative Colitis DAVID T. RUBIN, ANDELKA LOSAVIO, NICOLE YADRON,
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:1346 1350 Aminosalicylate Therapy in the Prevention of Dysplasia and Colorectal Cancer in Ulcerative Colitis DAVID T. RUBIN, ANDELKA LOSAVIO, NICOLE YADRON,
Until recently, treatment of active ulcerative colitis
 ORIGINAL ARTICLE Prognostic Significance of Endoscopic Remission in Patients with Active Ulcerative Colitis Treated with Oral and Topical Mesalazine: A Prospective, Multicenter Study Gianmichele Meucci,
ORIGINAL ARTICLE Prognostic Significance of Endoscopic Remission in Patients with Active Ulcerative Colitis Treated with Oral and Topical Mesalazine: A Prospective, Multicenter Study Gianmichele Meucci,
Dr David Epstein Vincent Pallotti Hospital and University of Cape Town
 Inflammatory Bowel Disease Management in South Africa in 2016 Pharmaceutical Care Management Association Dr David Epstein Vincent Pallotti Hospital and University of Cape Town Inflammatory Bowel Disease
Inflammatory Bowel Disease Management in South Africa in 2016 Pharmaceutical Care Management Association Dr David Epstein Vincent Pallotti Hospital and University of Cape Town Inflammatory Bowel Disease
An Update on the Biologic Treatment for Patients with Inflammatory Bowel Disease. David A. Schwartz, MD
 An Update on the Biologic Treatment for Patients with Inflammatory Bowel Disease David A. Schwartz, MD Director, Inflammatory Bowel Disease Center Associate Professor of Medicine Vanderbilt University
An Update on the Biologic Treatment for Patients with Inflammatory Bowel Disease David A. Schwartz, MD Director, Inflammatory Bowel Disease Center Associate Professor of Medicine Vanderbilt University
Review article: the long-term management of ulcerative colitis
 Aliment Pharmacol Ther 2004; 20 (Suppl. 4): 97 101. Review article: the long-term management of ulcerative colitis S. B. HANAUER Section of Gastroenterology, University of Chicago, Chicago, IL, USA SUMMARY
Aliment Pharmacol Ther 2004; 20 (Suppl. 4): 97 101. Review article: the long-term management of ulcerative colitis S. B. HANAUER Section of Gastroenterology, University of Chicago, Chicago, IL, USA SUMMARY
Mucosal Healing in Crohn s Disease. Geert D Haens MD, PhD University Hospital Gasthuisberg University of Leuven Leuven, Belgium
 Mucosal Healing in Crohn s Disease Geert D Haens MD, PhD University Hospital Gasthuisberg University of Leuven Leuven, Belgium Mucosal Lesions in CD: General Features CD can affect the entire GI tract
Mucosal Healing in Crohn s Disease Geert D Haens MD, PhD University Hospital Gasthuisberg University of Leuven Leuven, Belgium Mucosal Lesions in CD: General Features CD can affect the entire GI tract
Anti tumor necrosis factor (TNF) agents have
 Achieving Clinical Response and Remission in Moderate-to-Severe Ulcerative Colitis With Golimumab Sandborn WJ, Feagan BG, Marano C, et al; PURSUIT-SC Study Group. Subcutaneous golimumab induces clinical
Achieving Clinical Response and Remission in Moderate-to-Severe Ulcerative Colitis With Golimumab Sandborn WJ, Feagan BG, Marano C, et al; PURSUIT-SC Study Group. Subcutaneous golimumab induces clinical
Top Clinical Tips for Inflammatory Bowel Disease
 Job bag number: UK/AS/0165/07-13 Date of preparation: July 2013 Top Clinical Tips for Inflammatory Bowel Disease Dr Ayesha Akbar Consultant Gastroenterologist Honorary Senior Lecturer Contents-focus on
Job bag number: UK/AS/0165/07-13 Date of preparation: July 2013 Top Clinical Tips for Inflammatory Bowel Disease Dr Ayesha Akbar Consultant Gastroenterologist Honorary Senior Lecturer Contents-focus on
Prevalence of Nonadherence With Maintenance Mesalamine in Quiescent Ulcerative Colitis
 THE AMERICAN JOURNAL OF GASTROENTEROLOGY Vol. 96, No. 10, 2001 2001 by Am. Coll. of Gastroenterology ISSN 0002-9270/01/$20.00 Published by Elsevier Science Inc. PII S0002-9270(01)03245-2 Prevalence of
THE AMERICAN JOURNAL OF GASTROENTEROLOGY Vol. 96, No. 10, 2001 2001 by Am. Coll. of Gastroenterology ISSN 0002-9270/01/$20.00 Published by Elsevier Science Inc. PII S0002-9270(01)03245-2 Prevalence of
September 12, 2015 Millie D. Long MD, MPH, FACG
 Update on Biologic Therapy in 2015 September 12, 2015 Millie D. Long MD, MPH, FACG Assistant Professor of Medicine Inflammatory Bowel Disease Center University of North Carolina-Chapel Hill Outline Crohn
Update on Biologic Therapy in 2015 September 12, 2015 Millie D. Long MD, MPH, FACG Assistant Professor of Medicine Inflammatory Bowel Disease Center University of North Carolina-Chapel Hill Outline Crohn
Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort
 Alimentary Pharmacology and Therapeutics Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort N. Gies, K. I. Kroeker, K. Wong & R. N. Fedorak Division
Alimentary Pharmacology and Therapeutics Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort N. Gies, K. I. Kroeker, K. Wong & R. N. Fedorak Division
Medical therapies and IBD
 Medical therapies and IBD Although there is no cure for IBD, there are many treatment options available. There is no standard treatment for IBD that is effective in all situations or for all patients,
Medical therapies and IBD Although there is no cure for IBD, there are many treatment options available. There is no standard treatment for IBD that is effective in all situations or for all patients,
Ulcerative Colitis. ulcerative colitis usually only affects the colon.
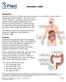 Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
Treating inflammatory bowel disease
 Treating inflammatory bowel disease Topical mesalazine (Enema and Suppository) Information for patients Gastroenterology page 2 of 8 What is my medicine? Mesalazine enemas and suppositories are used in
Treating inflammatory bowel disease Topical mesalazine (Enema and Suppository) Information for patients Gastroenterology page 2 of 8 What is my medicine? Mesalazine enemas and suppositories are used in
Mucosal healing: does it really matter?
 Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does it really matter? Professor Jean-Frédéric Colombel, New York, USA Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does
Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does it really matter? Professor Jean-Frédéric Colombel, New York, USA Oxford Inflammatory Bowel Disease MasterClass Mucosal healing: does
