CONTOUR PROFILE TISSUE EXPANDER WITH BUFFERZONE AREA
|
|
|
- Harold Grant
- 6 years ago
- Views:
Transcription
1 1 ENGLISH Product Insert Data Sheet CONTOUR PROFILE TISSUE EXPANDER WITH BUFFERZONE AREA CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician or properly licensed practitioner Rev. B Effective June 2003 DESCRIPTION The Contour Profile Tissue Expander is used for breast reconstruction following mastectomy. In order to provide a Tissue Expander with elasticity and integrity, the shell is made with successive cross-linked layers of silicone elastomer. Superior and anterior reinforcement allows for directional expansion in the lower pole of the device. The device has an integral, silicone elastomer, magnetically detected, injection site, and incorporates a BufferZone area with self-sealing technology (containing silicone oil) to the front patch of the device in order to minimize and/or prevent device leakage in the event of an accidental needle puncture. The Siltex textured shell provides a disruptive surface for collagen interface. The expander is intended for temporary subcutaneous or submuscular implantation and is not intended for use beyond six months. Identification of the injection port site can be accomplished by use of either the Centerscope magnetic detection device provided with the Tissue Expander, The Detector TM, or by palpation of the raised outer ring of the injection port. To use the Centerscope magnetic detection device or The Detector, follow the instructions provided with the specific port detection device. Injections must be made using sterile, pyrogen-free Sodium Chloride U.S.P. Solution for Injection and into the area enclosed within the palpation ring. If injections are made on or outside this palpation ring, leakage can occur. INDICATIONS It is the responsibility of the surgeon to advise the prospective patients or their representatives, prior to surgery, of the indications associated with the use of this product. The Contour Profile Tissue Expander can be utilized for breast reconstruction after mastectomy, correction of an underdeveloped breast, scar revision and tissue defect procedures. The device is intended for temporary subcutaneous or submuscular implantation and is not intended for use beyond six months. CONTRAINDICATIONS It is the responsibility of the surgeon to advise the prospective patients or their representatives, prior to surgery, of the contraindications associated with the use of this product. Patient Groups in which the product is contraindicated: The use of this expander is contraindicated in patients who have any of the following conditions: Implanted devices such as pacemakers, drug infusion devices, artificial sensing devices, etc., that would be affected by a magnetic field. Active infection anywhere in the body. Existing malignant or pre-malignant breast cancer without adequate treatment Surgical practices in which product use is contraindicated due to compromise of product integrity: Do not alter the expander shell or valve. Do not place drugs or substances inside the implant other than sterile saline for injection. Do not allow the device to come into contact with Betadine.
2 2 NOTE: The satisfactory use of this Tissue Expander for tissue replacement following mastectomy or trauma may require special reconstructive procedures. WARNINGS It is the responsibility of the surgeon to advise the prospective patients or their representatives, prior to surgery, of the possible warnings associated with the use of this product. 1. Magnetic Fields DO NOT use the Contour Profile Tissue Expander in patients that have a previously implanted device that could be affected by a magnetic field. MRI is not to be used on a patient implanted with this device because movement of the device could occur causing patient pain or lead to displacement of the tissue expander which could require revision surgery. 2. Radiation Therapy Mentor has not tested the in-vivo effects of radiation therapy with the device and cannot warrant the safety of such use. The decision regarding the use of the device in patients about to undergo radiation therapy should be made by the surgeon and the radiation oncologist. 3. Extrusion of the Device The incidence of extrusion of the expander has been shown to increase when the expander has been placed in injured areas: scarred, heavily irradiated or burned tissue, crushed bone areas or where severe surgical reduction of the area has previously been performed. 4. This device is a temporary expander. The expander is not intended for use beyond six months. 5. Reuse Tissue Expanders are for single use only. Do not resterilize. 6. Avoid Damage During Surgery Care should be taken to prevent damaging the device with surgical instruments. Do not insert or repair a damaged expander. Use care in subsequent procedures such as tissue expansion, open capsulotomy, breast pocket revision, hematoma/seroma aspiration, and biopsy/lumpectomy to avoid damage to the implant shell or valve. Do not contact the implant with disposable, capacitor-type cautery devices. 7. Proper Filling Surgeons should ensure themselves of the position of the fill dome prior to adding or withdrawing fluid. Needle punctures on or outside the palpation ring of the device may penetrate the shell causing deflation or compromise the fill dome and necessitate replacement of the device. Although the device has a self-sealing BufferZone area around the injection dome, DO NOT ATTEMPT TO INJECT INTO THE AREA AROUND THE DOME, as damage to the device may occur. Mentor relies on the surgeon to select the optimum incision and pocket size for the chosen expander design and projected volume.
3 3 The injection dome should not be penetrated with a needle larger than 21 gauge as it may not reseal. Injections should be made only into the top of the injection dome, perpendicular to ± 30 to the base and within the raised palpation ring. Excessive inflation of the device may result in tissue necrosis/thrombosis. Failure of the device to inflate may be due to leakage or injections which do not penetrate the injection dome. Leakage from the injection dome can result from the use of an improper size of injection needle, injections outside of the palpation ring or excessive pressure on the overlying tissue at the expander site, resulting in backpressure directed to the injection site. Instructions to Patient The patient should be advised that vigorous body movement (e.g., physical exercise) or excessive manipulation or trauma in the region of the expander may cause stress to the device and result in subsequent deflation. PRECAUTIONS It is the responsibility of the surgeon to advise the prospective patients or their representatives, prior to surgery, of the possible complications associated with the use of this product. Pre-existing infection should be treated and resolved before implantation of the Tissue Expander. Any surgeon performing reconstructive mammoplasty with Tissue Expanders should be familiar with the currently available techniques for measuring the patient, determining the Tissue Expander size and performing the surgery. Lint, dust, talc, surgical glove powder, drape and sponge lint, fingerprints, skin oils and other surface contaminants deposited on a Tissue Expander by improper handling may cause foreign body reactions. Strict adherence to clean, aseptic techniques should be maintained to prevent contamination of the Tissue Expander and possible complications. Surgical instruments and gloves should be rinsed clean of any impurities before handling the Tissue Expander. The silicone elastomer shell may easily be cut by a scalpel or ruptured by excessive stress, manipulation with blunt instruments or penetration by a needle. Subsequent deflation and/or rupture will result. All prostheses should be carefully inspected for structural integrity prior to and during implantation. Any subsequent surgical procedures in the area of the Tissue Expander should be undertaken with extreme caution as damage to the Tissue Expander could occur. In the event that the Tissue Expander is damaged, it must be removed. Each device should be checked for patency prior to surgery and continuously monitored throughout the surgical procedure to ensure the structural integrity of the device is not compromised in any way. A standby Tissue Expander should be available at the time of surgery. Potential for contamination exists when fluid is added or removed from the device. Use aseptic technique in the introduction of saline into the Tissue Expander; a single-use, sterile saline container is recommended. ADVERSE REACTIONS Any patient undergoing a surgical procedure is subject to possible unforeseen operative and postoperative complications. Potential reactions and complications associated with the use of the Tissue Expander should be discussed with and understood by the patient prior to surgery. It is the responsibility of the surgeon, and Mentor relies on the surgeon, to provide the patient with this information and to weigh the risk/benefit potential for each patient.
4 4 Complications which may result from the use of this product include the risks associated with the medication and methods used in the surgical procedures as well as the patient s degree of intolerance to any foreign object placed in the body. The complications may include, but are not limited to, the following: Additional Surgeries Additional surgery will be required to either replace a deflated tissue expander and/or to complete the breast reconstruction procedure. Cancer Published studies indicate that breast cancer is no more common in women with implants than those without implants. Capsule Formation and Contracture Postoperative formation of a fibrous tissue capsule around an implanted device is a normal physiologic response to the implantation of a foreign object. Capsule formation occurs in all patients in varying degrees. Capsules range from thin to heavily-thickened. Contracture of the fibrous capsule may occur, independent of its thickness. Discomfort, pain, excessive tissue firmness and misshapen expanded tissue, deflation, increased palpability and wrinkling and/or displacement of the expander may occur and may require surgical intervention. In some patients, tissue firmness may recur subsequent to corrective surgical procedures. Complications of Tissue Expansion Tissue thinning. Sloughing of poorly vascularized tissue. Gross postoperative hematoma, manifested by enlargement, tenderness and discoloration leading, if untreated, to extrusion of the device. Undue pressure on the tissue located over the device or trauma to surrounding tissues which may lead to venous thrombosis, the breakdown of skin over the device and subsequent extrusion. Deflation or removal of the device may be necessary for tissue repair. Connective Tissue Disease Concern over the association of breast implants to the development of autoimmune or connective tissue diseases, such as lupus, scleroderma, or rheumatoid arthritis, was raised because of cases reported in the literature with small numbers of women with implants. A review of several large epidemiological studies of women with and without implants indicates that these diseases are no more common in women with implants than those in women without implants. Deflation/Rupture/Leakage Saline-filled tissue expanders deflate when saline solution leaks through an unsealed or damaged valve or through a break in the implant shell. Implant deflation can occur immediately or progressively over a period of days and is noticed by loss of size or shape of the implant. Additional surgery is needed to remove deflated implants.
5 5 Dissatisfaction With Cosmetic Results Incorrect expander size, inappropriate scar location or appearance and misplacement or migration of expanders may interfere with a satisfactory cosmetic result. These complications are generally associated with the surgical procedure and technique. Extrusion of Expander/Interruption of Wound Healing Skin necrosis and/or sloughing may result from undue tension on the skin overlying the expander, trauma to the skin during surgical procedures or inadequate tissue thickness inhibiting circulation. Subsequent exposure and/or extrusion of the expander may occur. Displacement, twisting, fracture or extrusion may occur from improper expander sizing and/or placement, e.g., when the expander is too large or the pocket is too small or when there has been inadequate preoperative assessment of stresses causing movement of the expander. The incidence of extrusion of the expander has been shown to increase when the expander has been placed in injured areas: scarred, heavily irradiated or burned tissue or crushed bone areas; where severe surgical reduction of the area has been performed; and where steroids are used in the surgical pocket. Fluid Accumulation Excessive postoperative fluid accumulation and transient reaccumulation of fluid around the expander as a result of trauma and after vigorous exercise have been reported. Hematoma Careful hemostasis is important to prevent postoperative hematoma formation. Should excessive bleeding persist, it is recommended that the device not be used until bleeding is controlled. Gross postoperative hematoma, manifested by enlargement, tenderness and discoloration of tissue may, if untreated, lead to extrusion of the device. Infection Infection, manifested by swelling, tenderness, pain and fever, may appear in the immediate postoperative period or at any time after insertion of the device. In the absence of classic symptoms, subacute or chronic infections may be difficult to diagnose. If infection does not subside promptly with the appropriate treatment, removal of the Tissue Expander is indicated. Toxic Shock Syndrome has been reported as a complication of both augmentation and reconstructive mammaplasty. Pain Pain may be felt of varying severity (degrees) and duration (length of time) during the tissue expansion process. Wrinkling of the Tissue Expander Surgeons have reported that in some patients, visible or palpable wrinkling of the envelope, usually associated with textured prostheses, has occurred. Folds in the envelope can be visible beneath the overlying skin. This is reported to occur more frequently with: thin-skinned patients with little or no subcutaneous fat; subglandular rather than submuscular placement; a Tissue Expander that is too large relative to the pocket size or frame of the patient; overlying tissue that is minimal or of poor quality; or where there is contracture and/or insufficient fill volume.
6 6 RECORDING PROCEDURE FOR TISSUE EXPANDERS Each device is supplied with a patient record label showing the catalog number and lot number for that unit. One of these pressure sensitive labels should be attached directly to the patient s chart. The date of placement, expansion data (date and volume), and date of explant should be indicated on the label. Sterilization Tissue expanders are provided sterile. The product is dry heat sterilized and is for single use only. Do not resterilize. Implant Selection Some of the important surgical and implant sizing variables that have been identified include the following: The expander should not be too small or too large in comparison to the patient s chest wall dimensions. Available tissue must provide adequate coverage of the device. Submuscular placement of the expander may be preferable in patients with thin or poor quality tissue. A well-defined, dry pocket of adequate size and symmetry must be created to allow the implant to be placed flat on a smooth surface. Avoid too small of an incision. TESTING PROCEDURE FOR TISSUE EXPANDERS The device should be tested for patency and shell integrity immediately prior to use. This can be accomplished by the following steps: 1. Using a needle smaller than 21 gauge, partially inflate the device with air through the injection port. 2. Submerge the air-filled prosthesis in sterile, pyrogen-free testing fluid (water or saline). 3. Apply mild pressure and check for possible punctures or leaks. MAINTAINING HEMOSTASIS/AVOIDING FLUID ACCUMULATION Careful hemostasis is important to prevent postoperative hematoma formation. Should excessive bleeding persist, the implantation of the device should be delayed until bleeding is controlled. Postoperative evacuation of hematoma or seroma must be conducted with care to avoid expander contamination, or damage from sharp instruments. MENTOR INJECTION PORT DETECTOR Instructions For Use Using sterile technique, remove the Centerscope port detector from the sterile pouch. Grip the detector with either hand, ensuring that the free-swinging magnetic arm is pointing away from the hand holding it. Place the base of the unit (the flat side) on the patient. Move the unit in a circular motion until the magnetic arm detects (points towards) the location of the injection dome. Follow the direction that the arm points towards until the arm is pointing straight towards the hole in the base of the detector (the target). When the arm is centered perfectly in the target, the injection site has been located. To mark the site for injection, follow one of these three options:
7 7 Option 1: With the magnetic arm centered in the target, make a mark with an ink pen in each of the three notches around the anterior perimeter of the base. Next, make a fourth mark in the hole located behind the magnetic arm that runs through the center base of the detector. After all four marks have been made, lift the device from the patient. Using the same pen, carefully connect the opposing dots with a line, creating an injection crosshair. Then, make a clear mark at the point where the two perpendicular lines intersect. This point is your injection site. Option 2: With the magnetic arm centered in the target, gently but firmly press the device against the patient. Hold for several seconds. Lift the device from the patient. The raised X on the bottom of the detector will leave a clearly imprinted crosshair mark on the patient s skin. Mark the center of the crosshair with a pen. This point is your injection site. Option 3: Utilize a combination of options 1 and 2 for further confirmation of the best point for injection. TISSUE EXPANDER FILLING PROCEDURE To inflate the tissue expander; 1. Identify the injection port site using either the Centerscope magnetic detection device included with the expander, The Detector, or by palpating the raised outer ring of the injection port. 2. Once the center of the port has been identified, a skin marker can be used to identify the area of injection. 3. Inflation is accomplished by premarking the skin, inserting a 21 gauge (or smaller) standard bevel or Huber-tip needle into the top of the injection site, perpendicular ±30 to the base (a 21 gauge butterfly needle may also be used), and filling the device using sterile, pyrogen-free Sodium Chloride U.S.P. Solution for Injection. 4. Injections must be made into the area enclosed within the palpation ring. If injections are made on or outside the palpation ring, leakage can occur. Although the device has a self sealing feature around the area of the injection dome, DO NOT ATTEMPT TO INJECT OUTSIDE THE DOME AS LEAKAGE MAY OCCUR. POSTOPERATIVE CARE Mentor recommends that the patient be wrapped superiorly with an elastic (Ace) bandage, taped laterally, and wear a surgical bra 24 hours a day to help prevent shifting of the implant. DEVICE RETRIEVAL EFFORTS Mentor requests that any explanted devices be sent to Mentor Corporation, Product Evaluation Department, 3041 Skyway Circle North, Irving, TX USA for examination and analysis. PRODUCT EVALUATION Mentor requires that any complications or explantation resulting from the use of this device be brought to the immediate attention of the Product Evaluation Department at Mentor, 3041 Skyway Circle North, Irving, TX USA. 30 Injection Area 30
8 8 RETURNED GOODS AUTHORIZATION U.S. Customers Merchandise returned must have all manufacturer s seals intact and be returned within 60 days from date of invoice to be eligible for credit or replacement. Please contact the Mentor Customer Service Department for details. Returned products may be subject to restocking charges. International Customers Authorization for return of merchandise should be obtained from your local Mentor representative. Other conditions noted above also apply. PRODUCT ORDER INFORMATION U.S. Customers To order directly in the USA, please contact the Mentor Customer Service Department at Mentor, 201 Mentor Drive, Santa Barbara, CA 93111; Toll free telephone (800) , FAX (805) International Customers For product information or to order directly, contact your local Mentor representative. REFERENCES Literature references are available upon request from: Mentor Marketing Services, Literature Department 201 Mentor Drive Santa Barbara, CA USA For customer service or to return product, please call (800) in USA; outside of USA contact your local Mentor representative. Manufacturer MENTOR Irving, TX USA European Representative Mentor Medical Systems B.V. Zernikedreef CL, Leiden The Netherlands
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
INFORMED-CONSENT AUGMENTATION MAMMAPLASTY
 INFORMED-CONSENT AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks and alternative treatments.
INFORMED-CONSENT AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks and alternative treatments.
INFORMED-CONSENT- AUGMENTATION MAMMOPLASTY
 INFORMED-CONSENT- AUGMENTATION MAMMOPLASTY Instructions This is an informed-consent document that has been prepared to help inform you about augmentation mammoplasty, its risks, and alternative treatments.
INFORMED-CONSENT- AUGMENTATION MAMMOPLASTY Instructions This is an informed-consent document that has been prepared to help inform you about augmentation mammoplasty, its risks, and alternative treatments.
INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY
 INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein
INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein
MICHAEL J. BROWN, M.D., P.L.L.C.
 MICHAEL J. BROWN, M.D., P.L.L.C. Aesthetic Cosmetic Plastic Surgery INFORMED-CONSENT OPEN CAPSULECTOMY WITH BREAST IMPLANT EXCHANGE INSTRUCTIONS This is an informed-consent document that has been prepared
MICHAEL J. BROWN, M.D., P.L.L.C. Aesthetic Cosmetic Plastic Surgery INFORMED-CONSENT OPEN CAPSULECTOMY WITH BREAST IMPLANT EXCHANGE INSTRUCTIONS This is an informed-consent document that has been prepared
SALINE-FILLED & SPECTRUM MAMMARY PROSTHESES
 Product Insert Data Sheet SALINE-FILLED & SPECTRUM MAMMARY PROSTHESES DIRECTIONS FOR USE 102876-001 Rev. A Effective January 2005 CAUTION: Federal (USA) law restricts this device to sale by or on the order
Product Insert Data Sheet SALINE-FILLED & SPECTRUM MAMMARY PROSTHESES DIRECTIONS FOR USE 102876-001 Rev. A Effective January 2005 CAUTION: Federal (USA) law restricts this device to sale by or on the order
Breast Augmentation - Silicone Implants
 Breast Augmentation - Silicone Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics,
Breast Augmentation - Silicone Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics,
Breast Augmentation - Saline Implants
 Breast Augmentation - Saline Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy,
Breast Augmentation - Saline Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy,
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
-AESTETICA- Plastic Surgery Clinic JACEK JARLINSKI, MD, PhD plastic surgeon
 -AESTETICA- Plastic Surgery Clinic JACEK JARLINSKI, MD, PhD plastic surgeon www.aestetica.pl Contact: Jacek Jarlinski, MD, PhD tel. +48 600 208 208 jarlinski@aestetica.pl Piotr Jarlinski, MD tel. +48 601
-AESTETICA- Plastic Surgery Clinic JACEK JARLINSKI, MD, PhD plastic surgeon www.aestetica.pl Contact: Jacek Jarlinski, MD, PhD tel. +48 600 208 208 jarlinski@aestetica.pl Piotr Jarlinski, MD tel. +48 601
Directions for Use. INAMED Style 410 Silicone-Filled Breast Implants. and TM owned by Allergan Inc Allergan Inc. L037-02
 Directions for Use INAMED Style 410 Silicone-Filled Breast Implants w w w a l l e r g a n c o m 110 Cochrane Drive Markham, ON L3R 9S1 8009628728 5540 Ekwill Street Santa Barbara, CA 93111 8006244261 and
Directions for Use INAMED Style 410 Silicone-Filled Breast Implants w w w a l l e r g a n c o m 110 Cochrane Drive Markham, ON L3R 9S1 8009628728 5540 Ekwill Street Santa Barbara, CA 93111 8006244261 and
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Vaxcel Implantable Ports Valved and Non-Valved. A Patient s Guide
 Vaxcel Implantable Ports Valved and Non-Valved A Patient s Guide Vaxcel Implantable Port This pamphlet provides some answers to questions you may have about your implantable port and how to care for it
Vaxcel Implantable Ports Valved and Non-Valved A Patient s Guide Vaxcel Implantable Port This pamphlet provides some answers to questions you may have about your implantable port and how to care for it
Based on my discussions with Dr Gutowski, I agree with, and choose to have the following options as part of my breast augmentation:
 INFORMED CONSENT FOR BREAST AUGMENTATION PLEASE REVIEW AND BRING WITH YOU ON THE DAY OF YOUR PROCEDURE PATIENT NAME Based on my discussions with Dr Gutowski, I agree with, and choose to have the following
INFORMED CONSENT FOR BREAST AUGMENTATION PLEASE REVIEW AND BRING WITH YOU ON THE DAY OF YOUR PROCEDURE PATIENT NAME Based on my discussions with Dr Gutowski, I agree with, and choose to have the following
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP
 INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
INFORMED CONSENT - PLACEMENT OF PERMANENT BREAST IMPLANT FOLLOWING BREAST RECONSTRUCTION BY TISSUE EXPANSION INSTRUCTIONS
 INSTRUCTIONS This informed-consent document has been prepared to help inform you about placement of permanent breast implant following tissue expansion breast reconstruction, its risks, and alternative
INSTRUCTIONS This informed-consent document has been prepared to help inform you about placement of permanent breast implant following tissue expansion breast reconstruction, its risks, and alternative
INFORMED CONSENT BREAST RECONSTRUCTION WITH TISSUE EXPANDER
 Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only. American
Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only. American
NATRELLE 410 HIGHLY COHESIVE ANATOMICALLY SHAPED SILICONE-FILLED BREAST IMPLANTS
 NATRELLE 410 HIGHLY COHESIVE ANATOMICALLY SHAPED SILICONE-FILLED BREAST IMPLANTS Breast Augmentation and Reconstruction Patients Should Consider Introduction Allergan has prepared this brochure to provide
NATRELLE 410 HIGHLY COHESIVE ANATOMICALLY SHAPED SILICONE-FILLED BREAST IMPLANTS Breast Augmentation and Reconstruction Patients Should Consider Introduction Allergan has prepared this brochure to provide
SYNPOR POROUS POLYETHYLENE IMPLANTS. For craniofacial and orbital augmentation and reconstruction
 SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN
 MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC ( Mentor ) Product Replacement Policy
MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC ( Mentor ) Product Replacement Policy
Important Information. about Mentor MemoryGel Silicone Gel-Filled Breast Implants
 Important Information for Augmentation Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Augmentation Patients about Mentor MemoryGel Silicone gel-filled
Important Information for Augmentation Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Augmentation Patients about Mentor MemoryGel Silicone gel-filled
GEL-FILLED BREAST IMPLANT SURGERY
 GEL-FILLED BREAST IMPLANT SURGERY Making an Informed Decision 10647-03 1 Gel-Filled Breast Implant Surgery Making an Informed Decision Updated November 2017 TABLE OF CONTENTS 1 1. INTRODUCTION 5 Purpose
GEL-FILLED BREAST IMPLANT SURGERY Making an Informed Decision 10647-03 1 Gel-Filled Breast Implant Surgery Making an Informed Decision Updated November 2017 TABLE OF CONTENTS 1 1. INTRODUCTION 5 Purpose
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
INFORMED CONSENT AUGMENTATION MAMMAPLASTY WITH SILICONE GEL-FILLED IMPLANTS INSTRUCTIONS
 AUGMENTATION MAMMAPLASTY WITH SILICONE GEL-FILLED IMPLANTS INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty surgery with silicone
AUGMENTATION MAMMAPLASTY WITH SILICONE GEL-FILLED IMPLANTS INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty surgery with silicone
Body Contouring Implants - Calf
 Body Contouring Implants - Calf The body you were born with may or may not have the musculature you desire and it may be difficult to improve upon certain areas with exercise alone. In some cases, damage
Body Contouring Implants - Calf The body you were born with may or may not have the musculature you desire and it may be difficult to improve upon certain areas with exercise alone. In some cases, damage
IMPORTANT INFORMATION FOR AUGMENTATION PATIENTS ABOUT MENTOR MEMORYGEL SILICONE GEL-FILLED BREAST IMPLANTS August 2006
 1 Canadian IMPORTANT INFORMATION FOR AUGMENTATION PATIENTS ABOUT MENTOR MEMORYGEL SILICONE GEL-FILLED BREAST IMPLANTS August 2006 2 11859-00 1 Important Information for Augmentation Patients About Mentor
1 Canadian IMPORTANT INFORMATION FOR AUGMENTATION PATIENTS ABOUT MENTOR MEMORYGEL SILICONE GEL-FILLED BREAST IMPLANTS August 2006 2 11859-00 1 Important Information for Augmentation Patients About Mentor
GEL-FILLED BREAST IMPLANT SURGERY
 GEL-FILLED BREAST IMPLANT SURGERY Making an Informed Decision 10647-01 1 Gel-Filled Breast Implant Surgery Making an Informed Decision Updated September 2013 TABLE OF CONTENTS 1 1. INTRODUCTION 5 Purpose
GEL-FILLED BREAST IMPLANT SURGERY Making an Informed Decision 10647-01 1 Gel-Filled Breast Implant Surgery Making an Informed Decision Updated September 2013 TABLE OF CONTENTS 1 1. INTRODUCTION 5 Purpose
INFORMED CONSENT BREAST IMPLANT EXPLORATION
 . Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
INFORMED CONSENT AUGMENTATION MAMMAPLASTY WITH SALINE-FILLED IMPLANTS
 INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty surgery with saline-filled implants, its risks, as well as alternative treatment(s).
INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty surgery with saline-filled implants, its risks, as well as alternative treatment(s).
Technique Guide. SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction.
 Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
inding the fit that s right for you. Your Surgery Planner For Breast Augmentation or Reconstruction with NATRELLE Saline-Filled Breast Implants
 inding the fit that s right for you. Your Surgery Planner For Breast Augmentation or Reconstruction with NATRELLE Saline-Filled Breast Implants L Place Your Device Identification Card(s) Here R INTRODUCTION
inding the fit that s right for you. Your Surgery Planner For Breast Augmentation or Reconstruction with NATRELLE Saline-Filled Breast Implants L Place Your Device Identification Card(s) Here R INTRODUCTION
GEL-FILLED BREAST IMPLANT SURGERY Making an Informed Decision
 GEL-FILLED BREAST IMPLANT SURGERY Making an Informed Decision GEL-FILLED BREAST IMPLANT SURGERY Making an Informed Decision Updated September 2013 TABLE OF CONTENTS 1. INTRODUCTION 3 Purpose of the Brochure
GEL-FILLED BREAST IMPLANT SURGERY Making an Informed Decision GEL-FILLED BREAST IMPLANT SURGERY Making an Informed Decision Updated September 2013 TABLE OF CONTENTS 1. INTRODUCTION 3 Purpose of the Brochure
Considering Breast Enhancement
 Consent Form While every effort has been made by Allergan to ensure the accuracy of the information contained in this booklet, Allergan accepts no responsibility and/or liability for errors or omissions.
Consent Form While every effort has been made by Allergan to ensure the accuracy of the information contained in this booklet, Allergan accepts no responsibility and/or liability for errors or omissions.
Directions for Use (DFU) Natrelle. Saline-Filled Breast Implants. Physician Labeling
 Directions for Use (DFU) Natrelle Saline-Filled Breast Implants Physician Labeling Table of Contents Page Introduction... 1 Directions to the Physician... 1 Device Description... 2 Indications... 3 Contraindications...
Directions for Use (DFU) Natrelle Saline-Filled Breast Implants Physician Labeling Table of Contents Page Introduction... 1 Directions to the Physician... 1 Device Description... 2 Indications... 3 Contraindications...
Ideal Implant Incorporated
 Ideal Implant Incorporated IDEAL IMPLANT STRUCTURED BREAST IMPLANTS Instructions for Use October 2015 Caution: Federal law restricts this device to sale by or on the order of a licensed physician. IDEAL
Ideal Implant Incorporated IDEAL IMPLANT STRUCTURED BREAST IMPLANTS Instructions for Use October 2015 Caution: Federal law restricts this device to sale by or on the order of a licensed physician. IDEAL
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Directions for Use. NATRELLE Saline-Filled Breast Implants. Smooth & BIOCELL Texture. Directions for Use
 Directions for Use Directions for Use NATRELLE Saline-Filled Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician.
Directions for Use Directions for Use NATRELLE Saline-Filled Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician.
CONSENT FOR BREAST IMPLANT REMOVAL AND REPLACEMENT SURGERY
 CONSENT FOR BREAST IMPLANT REMOVAL AND REPLACEMENT SURGERY Breast Augmentation is a surgical procedure performed to enlarge the breasts for a number of reasons: To enhance the body contour of a woman,
CONSENT FOR BREAST IMPLANT REMOVAL AND REPLACEMENT SURGERY Breast Augmentation is a surgical procedure performed to enlarge the breasts for a number of reasons: To enhance the body contour of a woman,
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Breast Augmentation with NATRELLE Silicone-Filled Breast Implants
 Breast Augmentation with NATRELLE Silicone-Filled Breast Implants AUG Patient Labeling Rev 4/6/09 page 1 TABLE OF CONTENTS Page GLOSSARY... 3 1.0 CONSIDERING SILICONE GEL-FILLED BREAST IMPLANT SURGERY...
Breast Augmentation with NATRELLE Silicone-Filled Breast Implants AUG Patient Labeling Rev 4/6/09 page 1 TABLE OF CONTENTS Page GLOSSARY... 3 1.0 CONSIDERING SILICONE GEL-FILLED BREAST IMPLANT SURGERY...
INFORMED CONSENT AUGMENTATION MAMMAPLASTY WITH SALINE-FILLED IMPLANTS
 Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only. All
Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only. All
INFORMED- CONSENT- BREAST IMPLANT REMOVAL SURGERY
 INFORMED- CONSENT- BREAST IMPLANT REMOVAL SURGERY INSTRUCTIONS This is an informed- consent document that has been prepared to help your plastic surgeon inform you concerning breast implant removal, its
INFORMED- CONSENT- BREAST IMPLANT REMOVAL SURGERY INSTRUCTIONS This is an informed- consent document that has been prepared to help your plastic surgeon inform you concerning breast implant removal, its
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
INFORMED CONSENT AUGMENTATION MAMMAPLASTY WITH SALINE-FILLED IMPLANTS 2005 American Society of Plastic Surgeons. Purchasers of the Patient
 2005 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use
2005 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use
Augmentation BREAST AUGMENTATION WITH NATRELLE SILICONE-FILLED BREAST IMPLANTS AND NATRELLE INSPIRA BREAST IMPLANTS
 Augmentation BREAST AUGMENTATION WITH NATRELLE SILICONE-FILLED BREAST IMPLANTS AND NATRELLE INSPIRA BREAST IMPLANTS Table of Contents Glossary... 2 1. Considering Silicone Gel-Filled Breast Implant Surgery...11
Augmentation BREAST AUGMENTATION WITH NATRELLE SILICONE-FILLED BREAST IMPLANTS AND NATRELLE INSPIRA BREAST IMPLANTS Table of Contents Glossary... 2 1. Considering Silicone Gel-Filled Breast Implant Surgery...11
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
BREAST AUGMENTATION TECHNIQUES
 BREAST AUGMENTATION TECHNIQUES Breast Augmentation Top Surgical Procedure in 2015 (Worldwide) Surgical Procedure : Breast Augmentation Rank : 1 Total : 1,488,992 Percent of Total Surgical Procedures :
BREAST AUGMENTATION TECHNIQUES Breast Augmentation Top Surgical Procedure in 2015 (Worldwide) Surgical Procedure : Breast Augmentation Rank : 1 Total : 1,488,992 Percent of Total Surgical Procedures :
NovoSorb BTM. A unique synthetic biodegradable wound scaffold. Regenerating tissue. Changing lives.
 NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
INFORMED CONSENT AUGMENTATION MAMMAPLASTY WITH SALINE-FILLED IMPLANTS
 Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only. All
Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only. All
INFORMED CONSENT - PLACEMENT OF BREAST IMPLANT FOR STAGED BREAST RECONSTRUCTION
 INFORMED CONSENT - PLACEMENT OF BREAST IMPLANT FOR STAGED Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified
INFORMED CONSENT - PLACEMENT OF BREAST IMPLANT FOR STAGED Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified
OptiFix Absorbable Fixation System
 OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
Pre-pectoral Breast Reconstruction in Nipple Sparing Mastectomy
 September 2017 Issue 9 Pre-pectoral Breast Reconstruction in Nipple Sparing Mastectomy Aldona J. Spiegel, MD Director and Founder of the Center for Breast Restoration at the Institute for Reconstructive
September 2017 Issue 9 Pre-pectoral Breast Reconstruction in Nipple Sparing Mastectomy Aldona J. Spiegel, MD Director and Founder of the Center for Breast Restoration at the Institute for Reconstructive
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER
 INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER Introduction The following instructions explain how to prepare and inject Enbrel powder for injection. Please read the instructions carefully
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER Introduction The following instructions explain how to prepare and inject Enbrel powder for injection. Please read the instructions carefully
Information For Women AMERICAN SOCIETYOF PLASTIC SURGEONS
 Information For Women AMERICAN SOCIETYOF PLASTIC SURGEONS CONTENTS What are silicone implants?.............................................................4 Risks related to silicone gel-filled implants.................................................5
Information For Women AMERICAN SOCIETYOF PLASTIC SURGEONS CONTENTS What are silicone implants?.............................................................4 Risks related to silicone gel-filled implants.................................................5
INFORMED CONSENT OPEN CAPSULOTOMY WITH BREAST IMPLANT REPLACEMENT USING SALINE-FILLED IMPLANTS
 INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about open capsulotomy and breast implant exchange using saline-filled implants, its risks, and alternative treatments.
INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about open capsulotomy and breast implant exchange using saline-filled implants, its risks, and alternative treatments.
Mommy Makeover
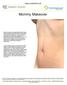 Mommy Makeover Many women experience significant physical changes following pregnancy and breast-feeding, many of which can be persistent and difficult to correct with diet and exercise alone. Changes
Mommy Makeover Many women experience significant physical changes following pregnancy and breast-feeding, many of which can be persistent and difficult to correct with diet and exercise alone. Changes
MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN
 MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS, MEMORYGEL XTRA BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC
MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS, MEMORYGEL XTRA BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
CASEY. Paralegal NATRELLE Style 15. YOUR SURGERY PLANNER Breast Augmentation With Natrelle Silicone-Filled Breast Implants
 The Right Time. The Right Fit. CASEY Paralegal NATRELLE Style 15 YOUR SURGERY PLANNER Breast Augmentation With Natrelle Silicone-Filled Breast Implants Dear Patient, Allergan has developed this AUGMENTATION
The Right Time. The Right Fit. CASEY Paralegal NATRELLE Style 15 YOUR SURGERY PLANNER Breast Augmentation With Natrelle Silicone-Filled Breast Implants Dear Patient, Allergan has developed this AUGMENTATION
Strattice Reconstructive Tissue Matrix used in the repair of rippling
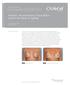 Clinical case study Strattice Tissue Matrix Strattice Reconstructive Tissue Matrix used in the repair of rippling Steven Teitelbaum, MD* Santa Monica, CA Case summary A 48-year-old woman with a history
Clinical case study Strattice Tissue Matrix Strattice Reconstructive Tissue Matrix used in the repair of rippling Steven Teitelbaum, MD* Santa Monica, CA Case summary A 48-year-old woman with a history
with Blom-Singer Gel Cap Insertion System
 with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
Aus Artificial Uretheral Sphincter Port System
 NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
Jadelle Contraceptive Implants up to 5 years Insertion and Removal
 Contraceptive Implants up to 5 years Bayer AG Global HealthCare Programs Family Planning 13342 Berlin, Germany www.bayer.com September 2017 Global HealthCare Programs Family Planning Contraceptive Independence»»
Contraceptive Implants up to 5 years Bayer AG Global HealthCare Programs Family Planning 13342 Berlin, Germany www.bayer.com September 2017 Global HealthCare Programs Family Planning Contraceptive Independence»»
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
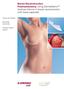 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Restoring a body. Restoring a life. Mentor Breast Reconstruction Product Portfolio NOT FOR DISTRIBUTION IN USA. APPROVED FOR EMEA REGION.
 Restoring a body Restoring a life Mentor Breast Reconstruction Product Portfolio NOT FOR DISTRIBUTION IN USA. APPROVED FOR EMEA REGION. Rebuilding beauty. Breast cancer is a reality in our world today
Restoring a body Restoring a life Mentor Breast Reconstruction Product Portfolio NOT FOR DISTRIBUTION IN USA. APPROVED FOR EMEA REGION. Rebuilding beauty. Breast cancer is a reality in our world today
Directions for Use. NATRELLE Silicone-Filled Breast Implants and NATRELLE INSPIRA Breast Implants
 Directions for Use Directions for Use NATRELLE Silicone-Filled Breast Implants and NATRELLE INSPIRA Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by
Directions for Use Directions for Use NATRELLE Silicone-Filled Breast Implants and NATRELLE INSPIRA Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by
inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with NATRELLE Silicone-Filled Breast Implants
 inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with NATRELLE Silicone-Filled Breast Implants L Place Your Device Identification Card(s) Here R ACCEPTANCE OF RISK AND
inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with NATRELLE Silicone-Filled Breast Implants L Place Your Device Identification Card(s) Here R ACCEPTANCE OF RISK AND
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
INFORMED CONSENT OPEN CAPSULOTOMY WITH BREAST IMPLANT REPLACEMENT USING SILICONE GEL-FILLED IMPLANTS
 INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about open capsulotomy and breast implant exchange using silicone gel-filled implants, its risks, and alternative
INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about open capsulotomy and breast implant exchange using silicone gel-filled implants, its risks, and alternative
Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial
 Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial How do I prepare and give an injection with Enbrel multiple-dose vial? A multiple-dose vial contains
Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial How do I prepare and give an injection with Enbrel multiple-dose vial? A multiple-dose vial contains
Goals of Care. Restore shape and function after cancer
 Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
INFORMED CONSENT AUGMENTATION MAMMAPLASTY WITH SILICONE GEL-FILLED IMPLANTS
 Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only. All
Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only. All
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Doc no: IFU/CPL Issue date: Rev no: 00 Rev date: 1
 FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Plain. Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Plain. Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
Application Guide for Full-Thickness Wounds
 Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
FLEXIC ATH LTD. Peripherally Inserted. Instructions n For Use.
 FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons
 Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
BREAST AUGMENTATION WITH MOTIVA IMPLANTS INFORMATION FOR THE PATIENT
 Page 1 of 14 1. Overview BREAST AUGMENTATION WITH MOTIVA IMPLANTS INFORMATION FOR THE PATIENT Breast augmentation/reconstruction is an elective surgical procedure for enhancing the breast area in women
Page 1 of 14 1. Overview BREAST AUGMENTATION WITH MOTIVA IMPLANTS INFORMATION FOR THE PATIENT Breast augmentation/reconstruction is an elective surgical procedure for enhancing the breast area in women
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe
 Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe How do I prepare and give an injection with Enbrel Single-dose Prefilled Syringe? There
Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe How do I prepare and give an injection with Enbrel Single-dose Prefilled Syringe? There
SYNTHECEL Dura Repair. Assurance and Versatility for Dura Reconstruction.
 SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
Individual Women. Individual Choices. onsidering Breast CEnhancement
 While every effort has been made to ensure the accuracy of the information contained in this booklet, Allergan accepts no responsibility and/or liability for errors or omissions. The information contained
While every effort has been made to ensure the accuracy of the information contained in this booklet, Allergan accepts no responsibility and/or liability for errors or omissions. The information contained
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
Tackling challenging revision breast augmentation cases
 the BREAST Careful preoperative consultations can reduce the need for revision breast surgery. Second Time Around Tackling challenging revision breast augmentation cases By Adam D. Schaffner, MD, FACS
the BREAST Careful preoperative consultations can reduce the need for revision breast surgery. Second Time Around Tackling challenging revision breast augmentation cases By Adam D. Schaffner, MD, FACS
