Volume 2; Number 2 February 2008
|
|
|
- Jemima Russell
- 5 years ago
- Views:
Transcription
1 Volume 2; Number 2 February 2008 CONTENTS Page 2 Page 3 Page 3 Page 4 Page 6 New Drug Assessment: Rufinamide (Inovelon) New Drug Assessment: Zoledronic acid infusion (Aclasta) New Drug Assessment: Mesalazine (Mezavant XL) NICE Clinical Guideline 57: Atopic Eczema in Children Clostridium difficile reminder This bulletin has been created specifically to convey details of decisions taken at the Prescribing and Clinical Effectiveness Forum (PACEF) to all stakeholders across the Lincolnshire Healthcare Community in both primary and secondary care. Steps will be taken to ensure the widest possible distribution across Lincolnshire PCT and within United Lincolnshire Hospitals Trust and Lincolnshire Partnership Trust. Both paper and electronic copies will be circulated initially with a view to evolving into complete electronic distribution as soon as we are confident that all key stakeholders can access . There are also plans to make all bulletins, guidelines, formularies, new product assessments, care pathways and other PACEF publications available through the LPCT website. SUMMARY OF PACEF DECISIONS: JANUARY UPDATE Drug Indication Traffic Light Status Mesalazine (Mezavant XL) Licensed for the induction GREEN and maintenance of remission in patients with mild to moderate active ulcerative colitis. Pimecrolimus 1% cream (Elidel) Licensed for the second line short term treatment of mild or moderate atopic dermatitis where the use of topical steroids is inadvisable or not possible. For use in adults and children aged 2 and AMBER Rufinamide (Inovelon) Tacrolimus 0.03% ointment and 0.1% ointment (Protopic) over. Licensed as an adjunctive therapy in the treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 4 years and older. Licensed for the second line short term treatment of moderate or severe atopic dermatitis unresponsive or intolerant to topical steroids. For use in adults AMBER subject to specialist initiation, titration and the development of shared care guidelines from tertiary care AMBER 1
2 Zoledronic acid infusion (Aclasta) (0.1% ointment) and children aged 2 and over (0.03% ointment). Licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fracture RED-RED RED-RED: This signifies that a product is not recommended for prescribing in both primary and secondary care. All new products are classified as RED-RED pending assessment by PACEF. RED: This signifies that a product has been approved for use within ULHT and/or LPT only and has no role in primary care. AMBER: This signifies that a drug has been approved for use in primary care subject to specialist initiation; a shared care guideline (SCG) may also be required. The main purpose of the SCG will be to clearly define both specialist and GP responsibilities. Not all AMBER drugs that require SCGs are currently covered by formal documents; in the coming months PACEF will be working to rectify this. GREEN: This signifies a product that is approved for initiation in either primary or secondary care. Specialist initiation and shared care guidelines are not considered necessary. NEW DRUG ASSESSMENTS RUFINAMIDE (INOVELON) Rufinamide is a novel antiepileptic drug (AED) structurally unrelated to other currently available therapies. It is an oral tablet licensed as adjunctive therapy in the treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 4 years and older. The relative rarity of the condition restricts the scale of the trial evidence available. Nonetheless, one small-scale, short-term, placebo controlled double-blind trial of123 patients has been conducted to evaluate rufinamide as adjunctive therapy in patients with LGS. This trial showed a significant reduction in the frequency of tonic-atonic seizures and total seizures, together with a reduction in seizure severity compared to placebo. The most frequent adverse events observed were vomiting (30.6%), pyrexia (25.8%), upper respiratory tract infection (21.8%) and somnolence (21%); possible serious side effects include the development of antiepileptic hypersensitivity syndrome and status epilepticus. Because of the complexity and severity of the condition the prospect of comparative studies versus currently available gold standard therapies are limited and ethically questionable. The most recent NICE guidance on the treatment of LGS (April and October 2004) advocates lamotrigine and topiramate as the most appropriate first line therapies. A Cochrane Collaboration review of the treatment of LGS published in March 2003 concluded that the optimum treatment for LGS remains uncertain; to date no study has shown any one drug to be highly efficacious although lamotrigine, topiramate and felamate may be helpful as add-on therapy. PACEF recognized the rarity and complexity of this condition and the need for alternative adjunct therapies where standard first line treatments fail. Rufinamide was classified as AMBER subject to specialist initiation and titration and the development of appropriate shared care guidelines. The specialist nature of this condition means that the bulk of new initiations will come from tertiary care. 2
3 ZOLEDRONIC ACID INFUSION (ACLASTA) Zoledronic acid (Aclasta) is a bisphosphonate licensed both as a treatment for Paget s disease of bone and as a once yearly intravenous infusion for the treatment of osteoporosis in post menopausal women. Clinical data to support the role in secondary prevention of osteoporosis comes from the HORIZON trial which demonstrated that once yearly zoledronic acid IV infusion was superior to placebo in reducing rates of both vertebral and hip fractures in post menopausal women. However, comparative data against alternative bisphosphonates is lacking. A recent systematic review published early on line suggests that there is good evidence to support the use of zoledronic acid in the prevention of vertebral fractures, but evidence supporting alendronate and risedronate in the prevention of hip fractures is stronger. In addition, further research is required to demonstrate the impact of zoledronic acid on physical function and quality of life, to confirm long term efficacy and to quantify cost effectiveness. Recent comparative safety assessments of bisphosphonates have indicated that the incidence of osteonecrosis of the jaw is higher with zoledronic acid than with other bisphosphonates, although most reports have been in patients with cancer. There was also a statistically significant incidence of serious atrial fibrillation associated with zoledronic acid in the HORIZON study. Recent draft NICE guidance on the secondary and primary prevention of osteoporosis advocated low cost generic oral alendronate 70mg weekly as the first line treatment of choice. Zoledronic acid is not covered by current NICE guidance and also not expected to be featured in updated guidance which is expected to be published shortly. There is currently insufficient evidence to support the use of zoledronic acid IV infusion; concerns remain around clinical effectiveness, cost-effectiveness and safety in comparison to other bisphosphonates. As a result of this, zoledronic acid is designated RED-RED. MESALAZINE (MEZAVANT XL) Mezavant XL is a new sustained-release (SR) formulation of mesalazine; each gastro-resistant SR tablet contains 1200mg of the active drug. The product is licensed for the induction and maintenance of remission in patients with mild to moderate active ulcerative colitis. It is comparably priced to alternatives and has the advantage of once daily dosing. General BNF advice on modified-release mesalazine preparations is that they should not be considered interchangeable. Mezavant XL tablets are designated GREEN on the basis that they are the only once daily mesalazine formulation currently available and may be useful in patients experiencing compliance problems with more frequent dosage regimes. The range of mesalazine preparations advocated by ULHT specialists is currently under review; further advice will be issued shortly. 3
4 NICE UPDATE NICE CLINICAL GUIDELINE 57: ATOPIC ECZEMA IN CHILDREN (DECEMBER 2007) The key recommendations are as follows: Wherever possible potential trigger factors should be identified (e.g. soaps, detergents, shampoos, bubble baths, shower gels, washing up liquids, other irritants, skin infections, contact allergens, food allergens, and inhalant allergens). A diagnosis of food allergy should be considered in children with atopic eczema (AE) who have reacted previously to food with immediate symptoms or in infants and young children with moderate to severe AE that has not been controlled by optimum management (particularly if associated with gut dysmotility or failure to thrive). A diagnosis of inhalant allergy should be considered in children with seasonal flares of AE or where AE is associated with asthma or rhinitis or in children over 3 years with AE on the face. A stepped approach to treatment is recommended. Emollients should form the basis of management and should always be used, even when the AE is clear. Management can be stepped up or down according to the severity of symptoms. Parents or carers should be taught to recognize flares of AE (e.g. increased dryness, itching, redness, swelling and general irritability) and how to manage them. Emollients should be used every day for moisturising, washing and bathing. They should be used on the whole body even when the AE is clear and used concurrently with other treatments. They should be used instead of shampoos for children under 12 months. Patients may be prescribed a combination of products or one product for all purposes depending on their needs and preferences. Leave-on emollients should be prescribed in large quantities ( g weekly). A recent study showed that half of children with eczema treated with a topical steroid were not prescribed a concurrent emollient. In general, emollients tend to be under-prescribed by doctors and under-used by patients. Emollient selection is often driven by patient preference, cosmetic acceptability, severity of the condition, tolerability and packaging. Pump dispensers are popular and convenient, but may be wasteful in terms of residual volume left in the container. A recent study compared currently available pump dispensers and found the Cetraben Cetrapump to be the least wasteful in terms of residual volume left after the pump stops working (2%). Figures recorded for competing products were Diprobase (10.5%), Hydromol (17.6%), Oilatum (20.9%), E45 (21.9%) and Doublebase (30.7%). The potency of topical corticosteroids should be tailored to the severity of the child s AE, which may vary according to body site. Mild potency steroids should be used for mild AE, moderate potency for moderate AE and potent for severe AE. For the face and neck, use mild potency corticosteroids except for the short-term use (3 to 5 days) of moderate potency for severe flares. Use moderate or potent preparations for short periods (7 to 10 days) for flares 4
5 in vulnerable sites such as axillae and groin. Do not use very potent preparations in children without specialist dermatological advice. Topical corticosteroids should only be applied to areas of active AE or eczema that has been active in the past 48 hours. Do not use potent topical corticosteroids on the face or neck or in children under 12 months (without specialist supervision). Prescribe topical corticosteroids for application only once or twice a day. Within the appropriate potency class, prescribe the drug with the lowest acquisition cost, taking into account pack size and frequency of application. For mild topical corticosteroid preparations, the lowest acquisition cost products are: hydrocortisone (Efcortelan) cream and ointment 0.5% (30g, 61p), 1% (30g, 75p) and 2.5% ointment (30g, 1.70). Prescribing hydrocortisone cream or ointment generically as 15g packs can be expensive due to the high price of these packs; ideally 30g packs should be prescribed (either generically or as Efcortelan). For moderate topical steroid preparations, Betnovate RD emerges as the lowest cost option in terms of cost per gram; the product is not recommended for use in children under 1 year and should not be used for longer than 5 days in children over 1 year. Occlusives should not be used. Alternative products with smaller pack sizes are likely to be more cost-effective where patient use is low or irregular. For potent topical corticosteroids, generic betamethasone or Betnovate preparations emerge as the lowest cost options; the products are not recommended for use in children under 1 year and should not be used for longer than 5 days in children over 1 year. Occlusives should not be used. Topical calcineurin inhibitors (such as tacrolimus and pimecrolimus) should not be used for mild AE, as first line for AE of any severity or under bandages or dressings without specialist dermatological advice. Topical calcineurin inhibitors are options for treatment in those patients not controlled by topical corticosteroids or where there is a risk of important adverse effects from topical corticosteroids. Tacrolimus may be used for moderate to severe AE in children aged 2 and over; pimecrolimus may be used for moderate AE on the face and neck in children aged 2 to 16 years. Topical calcineurin inhibitors should only be initiated by physicians with a special interest and experience in dermatology. Both tacrolimus (Protopic) 0.03% ointment (the recommended strength in children) and pimecrolimus (Elidel) 1% cream are designated AMBER on the Traffic Lights List. Antihistamines should not be used routinely. A one month trial of a nonsedating antihistamine may be indicated in a child with severe AE or mild to moderate AE with severe itching or urticaria. Continue if successful while symptoms persist. Sedating antihistamines may be offered as a 7 to 14 day trial for children over 6 months during acute flares if sleep disturbance has a significant impact. Children, parents or carers should be offered information on how to recognize the symptoms and signs of bacterial infection with staphylococcus and/or streptococcus (e.g. weeping, pustules, crusts, AE unresponsive to therapy, rapidly worsening AE, fever and malaise). Information should also be 5
6 provided on how to recognize eczema herpeticum (e.g. rapidly worsening, painful eczema, clustered blisters, punched-out erosions, possible fever, lethargy or distress). A 6-8 week trial of an extensively hydrolysed protein formula or amino acid formula in place of cow s milk formula for bottle-fed infants under 6 months is recommended with uncontrolled moderate or severe AE. Do not use diets based on unmodified proteins of other species milk (e.g. goat s or sheep s milk) or partially hydrolysed formulas for the treatment of suspected cow s milk allergy. Referral for specialist dermatological advice is recommended if: the diagnosis is uncertain; management has not controlled the AE; AE on the face has not responded to appropriate treatment; specialist advice on treatment application is required (e.g. bandaging techniques); contact allergic dermatitis is suspected; the AE is creating significant social or psychological problems; or the AE is associated with severe or recurrent infections. CLOSTRIDIUM DIFFICILE REMINDER Readers will remember from the November 2007 issue of the PACE Bulletin (Vol 1 No 7) that emerging patterns of bacterial resistance to antibiotics and the increasing incidence nationally of Clostridium difficile remain significant problems within the NHS across both primary and secondary care. Prescribers are reminded that particular concerns have been raised by local Microbiologists around the inappropriate use of broad spectrum antibiotics and the increased risk of Clostridium difficile. Specifically, the over-frequent use of broad-spectrum penicillins (amoxicillin, ampicillin, co-amoxiclav and co-fluampicil), cephalosporins (cefalexin, cefaclor, cefradine, cefuroxime, cefpodoxime, cefixime, but particularly second and third generation agents), clarithromycin, quinolones (ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin and ofloxacin) and clindamycin have contributed to the rising incidence of antibiotic associated diarrhoea nationally. Stephen Gibson, Head of Prescribing and Medicines Management, Lincolnshire PCT steve.gibson@lpct.nhs.uk 6
Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57
 Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57
 Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2007. All rights reserved. Contents Introduction... 4 Child-centred care...
Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2007. All rights reserved. Contents Introduction... 4 Child-centred care...
Food Allergy Testing and Guidelines
 Food Allergy Testing and Guidelines Dr Gosia Skibinska Primary Care Allergy Training Day, 15 th October 2011 Food Allergy Testing and Guidelines Food allergy Testing Guidelines Cases Food Allergy NICE
Food Allergy Testing and Guidelines Dr Gosia Skibinska Primary Care Allergy Training Day, 15 th October 2011 Food Allergy Testing and Guidelines Food allergy Testing Guidelines Cases Food Allergy NICE
TCIs are only available on prescription and are usually started by a dermatology specialist.
 (TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
(TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
Atopic Eczema with detail on how to apply wet wraps
 Atopic Eczema with detail on how to apply wet wraps Dr Carol Hlela Consultant Dermatologist Head of Unit, Department of Dermatology, Paediatrics Red Cross Children s Hospital, UCT Red Cross War Memorial
Atopic Eczema with detail on how to apply wet wraps Dr Carol Hlela Consultant Dermatologist Head of Unit, Department of Dermatology, Paediatrics Red Cross Children s Hospital, UCT Red Cross War Memorial
Children s Hospital Of Wisconsin
 Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Food allergies and eczema
 Department of Dermatology Food allergies and eczema Information for parents and carers Eczema, also known as atopic eczema or atopic dermatitis, is a skin condition that causes inflammation and irritation.
Department of Dermatology Food allergies and eczema Information for parents and carers Eczema, also known as atopic eczema or atopic dermatitis, is a skin condition that causes inflammation and irritation.
ATOPIC ECZEMA. What are the aims of this leaflet?
 ATOPIC ECZEMA What are the aims of this leaflet? This leaflet has been written to help you understand more about atopic eczema. It tells you what it is, what causes it, what can be done about it, and where
ATOPIC ECZEMA What are the aims of this leaflet? This leaflet has been written to help you understand more about atopic eczema. It tells you what it is, what causes it, what can be done about it, and where
LRI Children s Hospital
 Atopic Eczema Care LRI Children s Hospital Staff relevant to: Clinical staff working within the UHL Children s Hospital. Team approval date: May 2017 Version: V 4 Revision due: May 2020 Written by: K.
Atopic Eczema Care LRI Children s Hospital Staff relevant to: Clinical staff working within the UHL Children s Hospital. Team approval date: May 2017 Version: V 4 Revision due: May 2020 Written by: K.
Teledermatology Paediatric eczema. Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital
 Teledermatology Paediatric eczema Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital NHS e-referral teledermatology Rapid access to diagnosis / management advice from local integrated
Teledermatology Paediatric eczema Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital NHS e-referral teledermatology Rapid access to diagnosis / management advice from local integrated
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) Title and TA publication number of static topic: Final decision: TA82;
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) Title and TA publication number of static topic: Final decision: TA82;
Lincolnshire Prescribing and Clinical Effectiveness Bulletin
 S Lincolnshire Prescribing and Clinical Effectiveness Bulletin Volume 11 No 12, September 2017 Optum in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services,
S Lincolnshire Prescribing and Clinical Effectiveness Bulletin Volume 11 No 12, September 2017 Optum in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services,
Allergy Medications. Antihistamines. are very safe. Although usually taken as tablets, they may be prescribed as a liquid or syrup for young children
 The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient s health, mood and
The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient s health, mood and
Steroid use in managing your child s Atopic Eczema
 Steroid use in managing your child s Atopic Eczema Clinical Nurse Specialist for Paediatric Dermatology (01284) 713575 Step up step down approach: Addressograph Severe Call your General Practitioner (GP)
Steroid use in managing your child s Atopic Eczema Clinical Nurse Specialist for Paediatric Dermatology (01284) 713575 Step up step down approach: Addressograph Severe Call your General Practitioner (GP)
Recommended management of eczema in older patients
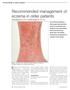 Recommended management of eczema in older patients Victoria Sherman MA, MRCP and Daniel Creamer BSc, MD, FRCP Our series Prescribing in older people gives practical advice for successful management of
Recommended management of eczema in older patients Victoria Sherman MA, MRCP and Daniel Creamer BSc, MD, FRCP Our series Prescribing in older people gives practical advice for successful management of
Horizon Scanning Technology Briefing. Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal. National Horizon Scanning Centre
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
過敏病科中心. Allergy Centre. Eczema. Allergy Centre 過敏病科中心. Allergy Centre. For enquiries and appointments, please contact us at:
 Allergy Centre 過敏病科中心 Eczema For enquiries and appointments, please contact us at: Allergy Centre 9/F, Li Shu Pui Block Hong Kong Sanatorium & Hospital 2 Village Road, Happy Valley, Hong Kong Tel: 2835
Allergy Centre 過敏病科中心 Eczema For enquiries and appointments, please contact us at: Allergy Centre 9/F, Li Shu Pui Block Hong Kong Sanatorium & Hospital 2 Village Road, Happy Valley, Hong Kong Tel: 2835
Medication Policy Manual. Topic: Dupixent, dupilumab Date of Origin: March 10, Committee Approval: March 10, 2017 Next Review Date: May 2018
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
Area Drug and Therapeutics Committee Prescribing Supplement No 59 July 2012
 Area Drug and Therapeutics Committee Prescribing Supplement No 59 In this issue Drugs reviewed by the SMC in June 2012 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Area Drug and Therapeutics Committee Prescribing Supplement No 59 In this issue Drugs reviewed by the SMC in June 2012 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Childhood Eczema Flowchart
 Childhood Eczema Flowchart EXCLUSIONS -Over 15 years of age -Contact dermatitis -Seborrhoeic Eczema -Mild and Moderate Eczema Childhood Eczema Assess Eczema Severity RED FLAGS -Eczema Herpecitum -Severe
Childhood Eczema Flowchart EXCLUSIONS -Over 15 years of age -Contact dermatitis -Seborrhoeic Eczema -Mild and Moderate Eczema Childhood Eczema Assess Eczema Severity RED FLAGS -Eczema Herpecitum -Severe
New Medicine Report. Pimecrolimus. RED- Hospital only Date of Last Revision 6 th March 2003
 New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
Assessing the Current Treatment of Atopic Dermatitis: Unmet Needs
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
RELEVANT DISCLOSURES ATOPIC DERMATITIS / ECZEMA MANAGING ECZEMA IN INFANTS AND CHILDREN
 RELEVANT DISCLOSURES MANAGING ECZEMA IN INFANTS AND CHILDREN Advisory board member - MEDA (Elidel), Speaking honoraria Bayer (Advantan) Advisory board, consultant, speaker: Pfizer, Abbvie, Janssen, Elli
RELEVANT DISCLOSURES MANAGING ECZEMA IN INFANTS AND CHILDREN Advisory board member - MEDA (Elidel), Speaking honoraria Bayer (Advantan) Advisory board, consultant, speaker: Pfizer, Abbvie, Janssen, Elli
Paediatric Eczema. Dr Manjeet Joshi Consultant Dermatologist 16 th May 2012
 Paediatric Eczema Dr Manjeet Joshi Consultant Dermatologist 16 th May 2012 Classification of the principal forms of eczema EXOGENOUS ENDOGENOUS Irritant Allergic contact Photoallergic contact Eczematous
Paediatric Eczema Dr Manjeet Joshi Consultant Dermatologist 16 th May 2012 Classification of the principal forms of eczema EXOGENOUS ENDOGENOUS Irritant Allergic contact Photoallergic contact Eczematous
Volume 4; Number 20 November 2010
 Volume 4; Number 20 November 2010 What s new this month: The PACEF position on agomelatine (Valdoxan), a new antidepressant, has been reviewed. Agomelatine (Valdoxan) remains RED. All prescribing should
Volume 4; Number 20 November 2010 What s new this month: The PACEF position on agomelatine (Valdoxan), a new antidepressant, has been reviewed. Agomelatine (Valdoxan) remains RED. All prescribing should
Appendix 9B. Diagnosis and Management of Infants with Suspected Cow s Milk Protein Allergy.
 Appendix 9B Diagnosis and Management of Infants with Suspected Cow s Milk Protein Allergy. A guide for healthcare professionals working in primary care. This document aims to provide health professionals
Appendix 9B Diagnosis and Management of Infants with Suspected Cow s Milk Protein Allergy. A guide for healthcare professionals working in primary care. This document aims to provide health professionals
The management of chronic urticaria in primary care for adults and children
 The management of chronic urticaria in primary care for adults and children September Version 2.0 This supersedes version 1.0 Review due in September 2019 Document location DOCUMENT CONTROL Copies of this
The management of chronic urticaria in primary care for adults and children September Version 2.0 This supersedes version 1.0 Review due in September 2019 Document location DOCUMENT CONTROL Copies of this
Eczema & Dermatitis Clinical features: Histopathological features: Classification:
 Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Dermatology Literary Review
 Emollients and ageing skin: optimising effectiveness and safety of Nursing, Vol. 25, No.11, pages 596-598. 6 Article outlines the causes of dryness in ageing skin and concludes that emollients are the
Emollients and ageing skin: optimising effectiveness and safety of Nursing, Vol. 25, No.11, pages 596-598. 6 Article outlines the causes of dryness in ageing skin and concludes that emollients are the
What you need to know about ECZEMA
 What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
What you need to know about ECZEMA
 What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
Appropriate prescribing of specialist infant formula feeds
 Appropriate Prescribing of Specialist Infant Formula Feeds Purpose of the guidance These guidelines aim to assist GPs and Health Visitors with information on the appropriate use of infant formula that
Appropriate Prescribing of Specialist Infant Formula Feeds Purpose of the guidance These guidelines aim to assist GPs and Health Visitors with information on the appropriate use of infant formula that
15 minute eczema consultation
 THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
DENOSUMAB SHARED CARE GUIDLINES
 DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
Learning Circle: Jan 26, 2011 Childhood Eczema
 Learning Circle: Jan 26, 2011 Childhood Eczema Wingfield Rehmus, MD MPH BC Children s Hospital Clinical Assistant Professor, UBC Department of Paediatrics Associate Member, UBC Department of Dermatology
Learning Circle: Jan 26, 2011 Childhood Eczema Wingfield Rehmus, MD MPH BC Children s Hospital Clinical Assistant Professor, UBC Department of Paediatrics Associate Member, UBC Department of Dermatology
Volume 2; Number 3 April 2008
 Volume 2; Number 3 April 2008 CONTENTS Page 3 Page 4 Page 5 Page 5 Page 6 Page 9 Page 9 Page 10 Page 10 Page 11 New Drug Assessment: Colesevelam (Cholestagel) Rapid Assessment: Fostair (Beclometasone and
Volume 2; Number 3 April 2008 CONTENTS Page 3 Page 4 Page 5 Page 5 Page 6 Page 9 Page 9 Page 10 Page 10 Page 11 New Drug Assessment: Colesevelam (Cholestagel) Rapid Assessment: Fostair (Beclometasone and
Vulval dermatoses. Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough
 Vulval dermatoses Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough Pigmentation Vulvodynia Ulcers Genetic Pruritus VULVAL
Vulval dermatoses Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough Pigmentation Vulvodynia Ulcers Genetic Pruritus VULVAL
Volume 4; Number 4 April 2010 PRESCRIBING GUIDANCE FOR HAY FEVER: SPRING / SUMMER 2010
 Volume 4; Number 4 April 2010 PRESCRIBING GUIDANCE FOR HAY FEVER: SPRING / SUMMER 2010 PACEF Recommendations Recommended medicines remain the same as in recent years: Non-sedating antihistamines: generic
Volume 4; Number 4 April 2010 PRESCRIBING GUIDANCE FOR HAY FEVER: SPRING / SUMMER 2010 PACEF Recommendations Recommended medicines remain the same as in recent years: Non-sedating antihistamines: generic
PDP SELF-TEST QUESTIONNAIRE LEG ULCERS. Ulcer Full thickness loss of epidermis and some dermis, which will heal with scarring.
 Number 5 CORE TUTORIALS IN DERMATOLOGY FOR PRIMARY CARE PDP SELF-TEST QUESTIONNAIRE METEOR CRATER, ARIZONA, USA LEG ULCERS UPDATED PDP SELF-TEST QUESTIONNAIRE SEPTEMBER 2013 Ulcer Full thickness loss of
Number 5 CORE TUTORIALS IN DERMATOLOGY FOR PRIMARY CARE PDP SELF-TEST QUESTIONNAIRE METEOR CRATER, ARIZONA, USA LEG ULCERS UPDATED PDP SELF-TEST QUESTIONNAIRE SEPTEMBER 2013 Ulcer Full thickness loss of
Volume 7; Number 4 February 2013
 Volume 7; Number 4 February 2013 What s new this month? Following a review of oral mesalazine products, Octasa 400mg MR and 800mg MR tablets emerge as the 400mg and 800mg formulations of choice. ULH gastroenterologists
Volume 7; Number 4 February 2013 What s new this month? Following a review of oral mesalazine products, Octasa 400mg MR and 800mg MR tablets emerge as the 400mg and 800mg formulations of choice. ULH gastroenterologists
Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD)
 Diagnosis: ATOPIC DERMATITIS (AD) Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD) PATIENT ADVICE: Unfortunately, there is no cure for atopic dermatitis, so
Diagnosis: ATOPIC DERMATITIS (AD) Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD) PATIENT ADVICE: Unfortunately, there is no cure for atopic dermatitis, so
Clinical Specialist Statement Template
 Clinical Specialist Statement Template Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can
Clinical Specialist Statement Template Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464
 Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) Routine endoscopic investigation of patients of any age, presenting with dyspepsia
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) Routine endoscopic investigation of patients of any age, presenting with dyspepsia
Contact Allergy Testing (Patch Testing) Information for parents and carers of children up to 12 years of age
 Contact Allergy Testing (Patch Testing) Information for parents and carers of children up to 12 years of age Dermatology Department The aim of this leaflet is to give you information about contact allergy
Contact Allergy Testing (Patch Testing) Information for parents and carers of children up to 12 years of age Dermatology Department The aim of this leaflet is to give you information about contact allergy
Is it allergy? Debbie Shipley
 Is it allergy? Debbie Shipley Topics Food Allergy and Eczema Hand Eczema and Patch Testing Urticaria Tackling Allergy Gell and Coombs classification Skin conditions with possible allergic component Allergy
Is it allergy? Debbie Shipley Topics Food Allergy and Eczema Hand Eczema and Patch Testing Urticaria Tackling Allergy Gell and Coombs classification Skin conditions with possible allergic component Allergy
An Everyday Guide to Eczema
 An Everyday Guide to Eczema By Dr. Kristel Polder, Board-Certified Dermatologist Developed in Partnership with Who is affected by eczema? 32 million people in the US 1 in 5 children 1 in 12 adults *www.eczema.org
An Everyday Guide to Eczema By Dr. Kristel Polder, Board-Certified Dermatologist Developed in Partnership with Who is affected by eczema? 32 million people in the US 1 in 5 children 1 in 12 adults *www.eczema.org
Drug Allergy A Guide to Diagnosis and Management
 Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Derm quiz. Go to this link: goo.gl/forms/kchrhmtzl3vfnlv52. bit.ly/2a8asoy. Scan the QR code with your phone
 Dermatology quiz Derm quiz Go to this link: goo.gl/forms/kchrhmtzl3vfnlv52 OR bit.ly/2a8asoy OR Scan the QR code with your phone Contents Childhood rashes Pigmented lesions Sun damage Pityriasis References
Dermatology quiz Derm quiz Go to this link: goo.gl/forms/kchrhmtzl3vfnlv52 OR bit.ly/2a8asoy OR Scan the QR code with your phone Contents Childhood rashes Pigmented lesions Sun damage Pityriasis References
Dermatology Round Up
 Dermatology Round Up Journal of Family Health Care Live Conference 25 March 2014 Julie Van Onselen Independent Dermatology Nurse, Oxford And Rachael Fagg, Mother Introduction 10.00 10.30hrs: Julie Van
Dermatology Round Up Journal of Family Health Care Live Conference 25 March 2014 Julie Van Onselen Independent Dermatology Nurse, Oxford And Rachael Fagg, Mother Introduction 10.00 10.30hrs: Julie Van
Volume 4; Number 5 May 2010
 Volume 4; Number 5 May 2010 CLINICAL GUIDELINES FOR ANTIDEPRESSANT USE IN PRIMARY AND SECONDARY CARE Lincolnshire Partnership Foundation Trust in conjunction with Lincolnshire PACEF have recently updated
Volume 4; Number 5 May 2010 CLINICAL GUIDELINES FOR ANTIDEPRESSANT USE IN PRIMARY AND SECONDARY CARE Lincolnshire Partnership Foundation Trust in conjunction with Lincolnshire PACEF have recently updated
8-year surveillance 2016 Atopic eczema in under 12s (2007) NICE guideline CG57
 8-year 2016 Atopic eczema in under 12s (2007) NICE guideline CG57 Appendix A: decision matrix year Diagnosis 57 01 What criteria should be used to diagnose atopic eczema in children and how do they vary
8-year 2016 Atopic eczema in under 12s (2007) NICE guideline CG57 Appendix A: decision matrix year Diagnosis 57 01 What criteria should be used to diagnose atopic eczema in children and how do they vary
Doncaster & Bassetlaw Medicines Formulary
 Doncaster & Bassetlaw Medicines Formulary Section 1.5 Chronic Bowel Disorders (including IBD) Aminosalicylates: Mesalazine 400mg and 800mg MR Tablets (Octasa) Mesalazine 1.2g MR Tablets (Mezavant XL) Mesalazine
Doncaster & Bassetlaw Medicines Formulary Section 1.5 Chronic Bowel Disorders (including IBD) Aminosalicylates: Mesalazine 400mg and 800mg MR Tablets (Octasa) Mesalazine 1.2g MR Tablets (Mezavant XL) Mesalazine
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland
 Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland 16:30-17:25 WS #170: Eczema Management 17:35-18:30 WS #182: Eczema Management (Repeated) Managing ECZEMA A/Prof Rohan Ameratunga
Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland 16:30-17:25 WS #170: Eczema Management 17:35-18:30 WS #182: Eczema Management (Repeated) Managing ECZEMA A/Prof Rohan Ameratunga
What is atopic dermatitis?
 What is atopic dermatitis? Complex inflammatory skin disorder intense pruritus cutaneous hyperreactivity immune dysregulation Chronic with exacerbations and remissions Affects all ages, but more common
What is atopic dermatitis? Complex inflammatory skin disorder intense pruritus cutaneous hyperreactivity immune dysregulation Chronic with exacerbations and remissions Affects all ages, but more common
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women
 S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464
 Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
ESCA: Denosumab for the treatment of osteoporosis in postmenopausal women.
 ESCA: Denosumab for the treatment of osteoporosis in postmenopausal women. Specialist details Patient identifier Name Tel: This effective shared care agreement (ESCA) sets out details for the sharing of
ESCA: Denosumab for the treatment of osteoporosis in postmenopausal women. Specialist details Patient identifier Name Tel: This effective shared care agreement (ESCA) sets out details for the sharing of
Eucrisa. Eucrisa (crisaborole) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.25 Subject: Eucrisa Page: 1 of 6 Last Review Date: September 15, 2017 Eucrisa Description Eucrisa
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.25 Subject: Eucrisa Page: 1 of 6 Last Review Date: September 15, 2017 Eucrisa Description Eucrisa
Volume 10; Number 10 July 2016
 Arden and Greater East Midlands Commissioning Support Unit in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services, United Lincolnshire Hospitals Trust and
Arden and Greater East Midlands Commissioning Support Unit in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services, United Lincolnshire Hospitals Trust and
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis. Date: Date:
 Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures
 APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
FACTSHEET CICLOSPORIN. Introduction. How does ciclosporin work? When is ciclosporin used?
 Introduction Ciclosporin is a potent immunosuppressant drug that requires supervision by a specialised doctor such as a dermatologist. It was originally used to prevent organ rejection in transplant patients.
Introduction Ciclosporin is a potent immunosuppressant drug that requires supervision by a specialised doctor such as a dermatologist. It was originally used to prevent organ rejection in transplant patients.
Patient Group Direction for Doxycycline (Tetracycline) Version: 01 Start Date: October 2015 Expiry Date: October 2018
 THIS PATIENT GROUP DIRECTION HAS BEEN AGREED BY THE FOLLOWING ORGANISATIONS: CLINICAL COMMISSIONING GROUP: Doncaster CCG Lancashire North CCG Fylde & Wyre CCG East Lancashire CCG Change history Version
THIS PATIENT GROUP DIRECTION HAS BEEN AGREED BY THE FOLLOWING ORGANISATIONS: CLINICAL COMMISSIONING GROUP: Doncaster CCG Lancashire North CCG Fylde & Wyre CCG East Lancashire CCG Change history Version
Chronic obstructive pulmonary disease (acute exacerbation): antimicrobial prescribing
 National Institute for Health and Care Excellence Final Chronic obstructive pulmonary disease (acute exacerbation): antimicrobial prescribing Evidence review December 2018 Contents Disclaimer The recommendations
National Institute for Health and Care Excellence Final Chronic obstructive pulmonary disease (acute exacerbation): antimicrobial prescribing Evidence review December 2018 Contents Disclaimer The recommendations
PACKAGE LEAFLET: INFORMATION FOR THE USER. Zoledronic Acid IBIGEN 4 mg powder and solvent for solution for infusion Zoledronic acid
 PACKAGE LEAFLET: INFORMATION FOR THE USER Zoledronic Acid IBIGEN 4 mg powder and solvent for solution for infusion Zoledronic acid Read all of this leaflet carefully before you are given Zoledronic Acid
PACKAGE LEAFLET: INFORMATION FOR THE USER Zoledronic Acid IBIGEN 4 mg powder and solvent for solution for infusion Zoledronic acid Read all of this leaflet carefully before you are given Zoledronic Acid
Eucrisa. Eucrisa (crisaborole) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Eucrisa Page: 1 of 7 Last Review Date: June 22, 2018 Eucrisa Description Eucrisa (crisaborole)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Eucrisa Page: 1 of 7 Last Review Date: June 22, 2018 Eucrisa Description Eucrisa (crisaborole)
Title: From zero to comprehensive Fracture Liaison service (FLS) within existing resources
 Best of Health Staff Awards 2010/11 Best of Health Awards 2013 Dr Abhaya Gupta Consultant Physician Hywel Dda Health Board Title: From zero to comprehensive Fracture Liaison service (FLS) within existing
Best of Health Staff Awards 2010/11 Best of Health Awards 2013 Dr Abhaya Gupta Consultant Physician Hywel Dda Health Board Title: From zero to comprehensive Fracture Liaison service (FLS) within existing
Volume 9; Number 3 March 2015 PRESCRIBING AND DISPENSING PREGABALIN FOR NEUROPATHIC PAIN
 Arden and Greater East Midlands Commissioning Support Unit in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services, United Lincolnshire Hospitals Trust and
Arden and Greater East Midlands Commissioning Support Unit in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services, United Lincolnshire Hospitals Trust and
IMMUNODEFICIENCIES PRIMARY ALLERGIES PRIMARY IMMUNODEFICIENCIES AND ALLERGIES
 PRIMARY IMMUNODEFICIENCIES ALLERGIES PRIMARY IMMUNODEFICIENCIES AND ALLERGIES 1 PRIMARY IMMUNODEFICIENCIES KEY ABBREVIATIONS IgE CID Immunoglobulin E Combined Immunodeficiency IL5 Interleukin 5 IPOPI PID
PRIMARY IMMUNODEFICIENCIES ALLERGIES PRIMARY IMMUNODEFICIENCIES AND ALLERGIES 1 PRIMARY IMMUNODEFICIENCIES KEY ABBREVIATIONS IgE CID Immunoglobulin E Combined Immunodeficiency IL5 Interleukin 5 IPOPI PID
TRIGGERS & TREATMENT OF ATOPIC DERMATITIS COA#PCIA0809 CE Activity provided by PCI Journal
 TRIGGERS & TREATMENT OF ATOPIC DERMATITIS COA#PCIA0809 CE Activity provided by PCI Journal INSTRUCTIONS 1. Read the article. 2. Take the test, record your answers in the test answer section (Section B)
TRIGGERS & TREATMENT OF ATOPIC DERMATITIS COA#PCIA0809 CE Activity provided by PCI Journal INSTRUCTIONS 1. Read the article. 2. Take the test, record your answers in the test answer section (Section B)
Zerlinda (MRP DK/H/2265/001)
 Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Area Drug and Therapeutics Committee Prescribing Supplement No 3 November 2003
 Area Drug and Therapeutics Committee Prescribing Supplement No 3 In this issue Drugs currently being considered by the SMC advice due on 8 December 2003. SMC Notice of meeting: Patient power Making a Difference,
Area Drug and Therapeutics Committee Prescribing Supplement No 3 In this issue Drugs currently being considered by the SMC advice due on 8 December 2003. SMC Notice of meeting: Patient power Making a Difference,
Dermatitis (inflammatory skin condition) Nonallergic. dermatitis. Non-atopic eczema (non- IgE mediated)
 Atopic Eczema Dermatitis (inflammatory skin condition) Allergic dermatitis -eczema Nonallergic dermatitis Atopic eczema (IgE mediated) Non-atopic eczema (non- IgE mediated) Pathophysiology of Eczema Allergy
Atopic Eczema Dermatitis (inflammatory skin condition) Allergic dermatitis -eczema Nonallergic dermatitis Atopic eczema (IgE mediated) Non-atopic eczema (non- IgE mediated) Pathophysiology of Eczema Allergy
NHS LINCOLNSHIRE in association with UNITED LINCOLNSHIRE HOSPITALS TRUST
 NHS LINCOLNSHIRE in association with UNITED LINCOLNSHIRE HOSPITALS TRUST SHARED CARE GUIDELINE: CINACALCET in the management of secondary hyperparathyroidism in adult patients with end-stage renal disease
NHS LINCOLNSHIRE in association with UNITED LINCOLNSHIRE HOSPITALS TRUST SHARED CARE GUIDELINE: CINACALCET in the management of secondary hyperparathyroidism in adult patients with end-stage renal disease
Primary Care Guidelines for the Management of Atopic Eczema 10/07100
 4 4 w Primary Care Guidelines for the Management of Atopic Eczema 10/07100 1.0 INTRODUCTION AND OBJECTIVES Primary care manages by far the largest number of patients with atopic eczema, the vast majority
4 4 w Primary Care Guidelines for the Management of Atopic Eczema 10/07100 1.0 INTRODUCTION AND OBJECTIVES Primary care manages by far the largest number of patients with atopic eczema, the vast majority
Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer
 Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer Category: Summary: Guideline Adjuvant Bisphosphonate treatment for Post-Menopausal
Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer Category: Summary: Guideline Adjuvant Bisphosphonate treatment for Post-Menopausal
Food allergy in children. nice bulletin. NICE Bulletin Food Allergy in Chlidren.indd 1
 nice bulletin Food allergy in children NICE provided the content for this booklet which is independent of any company or product advertised NICE Bulletin Food Allergy in Chlidren.indd 1 23/01/2012 11:04
nice bulletin Food allergy in children NICE provided the content for this booklet which is independent of any company or product advertised NICE Bulletin Food Allergy in Chlidren.indd 1 23/01/2012 11:04
Management of eczema in infants and children Assoc Prof David Orchard Director, Department of Dermatology Royal Children s Hospital
 Atopic dermatitis definition Management of eczema in infants and children Assoc Prof David Orchard Director, Department of Dermatology Royal Children s Hospital Atopic dermatitis is long lasting (chronic)
Atopic dermatitis definition Management of eczema in infants and children Assoc Prof David Orchard Director, Department of Dermatology Royal Children s Hospital Atopic dermatitis is long lasting (chronic)
SHARED CARE GUIDELINE: Mycophenolate mofetil or mycophenolic acid for Maintenance of Immunosuppression after Kidney Transplantation in Adults
 NHS LINCOLNSHIRE in association with UNITED LINCOLNSHIRE HOSPITALS TRUST SHARED CARE GUIDELINE: Mycophenolate mofetil or mycophenolic acid for Maintenance of Immunosuppression after Kidney Transplantation
NHS LINCOLNSHIRE in association with UNITED LINCOLNSHIRE HOSPITALS TRUST SHARED CARE GUIDELINE: Mycophenolate mofetil or mycophenolic acid for Maintenance of Immunosuppression after Kidney Transplantation
APPENDIX K Pharmacological Management
 1 2 3 4 APPENDIX K Pharmacological Management Table 1 AED options by seizure type Table 1 AED options by seizure type Seizure type First-line AEDs Adjunctive AEDs Generalised tonic clonic Lamotrigine Oxcarbazepine
1 2 3 4 APPENDIX K Pharmacological Management Table 1 AED options by seizure type Table 1 AED options by seizure type Seizure type First-line AEDs Adjunctive AEDs Generalised tonic clonic Lamotrigine Oxcarbazepine
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Topical Doxepin Prior Authorization with Quantity Limit Program Summary
 Topical Doxepin Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-3 Agent(s) Indication(s) Dosage & Administration Doxepin 5% cream Prudoxin (doxepin) cream 5% KS_PS_Topical_Doxepin_PAQL_ProgSum_AR1018
Topical Doxepin Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-3 Agent(s) Indication(s) Dosage & Administration Doxepin 5% cream Prudoxin (doxepin) cream 5% KS_PS_Topical_Doxepin_PAQL_ProgSum_AR1018
Eczema management: how to control the itch
 ATM - Eczema:Layout 1 24/3/11 3:08 PM Page 24 allergytoday skin Eczema management: how to control the itch Eczema is the most common specific skin disorder encountered but it is very poorly managed and
ATM - Eczema:Layout 1 24/3/11 3:08 PM Page 24 allergytoday skin Eczema management: how to control the itch Eczema is the most common specific skin disorder encountered but it is very poorly managed and
In our patients the cause of seizures can be broadly divided into structural and systemic causes.
 Guidelines for the management of Seizures Amalgamation and update of previous policies 7 (Seizure guidelines, ND, 2015) and 9 (Status epilepticus, KJ, 2011) Seizures can occur in up to 15% of the Palliative
Guidelines for the management of Seizures Amalgamation and update of previous policies 7 (Seizure guidelines, ND, 2015) and 9 (Status epilepticus, KJ, 2011) Seizures can occur in up to 15% of the Palliative
Bisphosphonate treatment break
 Bulletin 110 December 2015 Bisphosphonate treatment break Bisphosphonates have been widely used in the treatment of osteoporosis with robust data demonstrating efficacy in fracture risk reduction over
Bulletin 110 December 2015 Bisphosphonate treatment break Bisphosphonates have been widely used in the treatment of osteoporosis with robust data demonstrating efficacy in fracture risk reduction over
Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults
 Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults Ref: PHARM-0025-v3 Status: FINAL Document type: Guidelines Guidance on Safe Prescribing of Melatonin Page
Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults Ref: PHARM-0025-v3 Status: FINAL Document type: Guidelines Guidance on Safe Prescribing of Melatonin Page
What s Topical About Topicals?
 What s Topical About Topicals? Megha M. Tollefson, MD Associate Professor of Dermatology and Pediatrics July 29, 2017 2015 MFMER 3513105-1 Disclosures None 2015 MFMER 3513105-2 Outline Topical steroids
What s Topical About Topicals? Megha M. Tollefson, MD Associate Professor of Dermatology and Pediatrics July 29, 2017 2015 MFMER 3513105-1 Disclosures None 2015 MFMER 3513105-2 Outline Topical steroids
GASTROINTESTINAL SYSTEM MANAGEMENT OF DYSPEPSIA
 GASTROINTESTINAL SYSTEM MANAGEMENT OF DYSPEPSIA MANAGEMENT Dyspepsia refers to a spectrum of usually intermittent upper gastrointestinal symptoms, including epigastric pain and heartburn. For the majority
GASTROINTESTINAL SYSTEM MANAGEMENT OF DYSPEPSIA MANAGEMENT Dyspepsia refers to a spectrum of usually intermittent upper gastrointestinal symptoms, including epigastric pain and heartburn. For the majority
Seasonal Allergic Rhinitis (Hay Fever)
 Seasonal Allergic Rhinitis (Hay Fever) Link to prescribing guidance: http://www.enhertsccg.nhs.uk/ear-nose-and-oropharynx Clinical Presentation Link to CKS NICE guidance: https://cks.nice.org.uk/allergicrhinitis
Seasonal Allergic Rhinitis (Hay Fever) Link to prescribing guidance: http://www.enhertsccg.nhs.uk/ear-nose-and-oropharynx Clinical Presentation Link to CKS NICE guidance: https://cks.nice.org.uk/allergicrhinitis
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS.
 Appendix I - Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective
Appendix I - Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective
Summary of the risk management plan by product
 Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Produced by Tayside New Medicines Implementation Panel (NMIP) and Tayside Medicines Unit
 TAYSIDE PRESCRIBER Tayside D&TC Supplement No. 26 Produced by Tayside New Medicines Implementation Panel (NMIP) and Tayside Medicines Unit May 2003 In this issue: Scottish Medicines Consortium (SMC) Advice
TAYSIDE PRESCRIBER Tayside D&TC Supplement No. 26 Produced by Tayside New Medicines Implementation Panel (NMIP) and Tayside Medicines Unit May 2003 In this issue: Scottish Medicines Consortium (SMC) Advice
Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level
 Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level Dr Ng Su Yuen Paediatrician and Paediatric Dermatologist Hospital Pulau Pinang Outline Common inflammatory
Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level Dr Ng Su Yuen Paediatrician and Paediatric Dermatologist Hospital Pulau Pinang Outline Common inflammatory
Diagnosis and Treatment of Respiratory Illness in Children and Adults
 Page 1 of 9 Main Algorithm Annotations 1. Patient Reports Some Combination of Symptoms Patients may present for an appointment, call into a provider to schedule an appointment or nurse line presenting
Page 1 of 9 Main Algorithm Annotations 1. Patient Reports Some Combination of Symptoms Patients may present for an appointment, call into a provider to schedule an appointment or nurse line presenting
Changing the way we prescribe in Calderdale. Consultation document
 Changing the way we prescribe in Calderdale Consultation document Who are we? Clinical commissioning groups (CCGs) are responsible for planning and buying (commissioning) healthcare. We are made up of
Changing the way we prescribe in Calderdale Consultation document Who are we? Clinical commissioning groups (CCGs) are responsible for planning and buying (commissioning) healthcare. We are made up of
