Factorial Study Design 07/18/12
|
|
|
- Charla Goodwin
- 6 years ago
- Views:
Transcription
1 Disclaimer: The following information is fictional and is only intended for the purposes of illustrating key concepts for results data entry in the Protocol Registration System (PRS). Example Factorial Study Design (A Phase III Double-Blind, -Controlled, Randomized, Factorial Design Trial of Two Doses of and Supplement in Patients with Heart Failure) Methods Study Design This multicenter, double-blind (subject/investigator), randomized, placebocontrolled interventional, factorial design study enrolled patients hospitalized with Heart Failure from research sites in the United States: Brigham and Women's Hospital at Harvard Medical School (Boston, MA), Children's Hospital Montefiore (Bronx, NY), Duke University Medical Center (Durham, NC), Thomas Jefferson University Hospital (Philadelphia, PA), University of Texas Medical Branch at Galveston (Galveston, TX). Patients entered a run-in period during which they received mg tablet once daily and placebo Softgel Supplement for 2 months. Eligible patients who completed run-in were then randomized in a 2x2 factorial blinded design between 80 mg tablet once daily versus mg tablet once daily and Softgel Supplement (900 mg EPA, g DHA) once daily versus placebo Softgel Supplement once daily (See Table 1). The protocol and informed consent documents were reviewed and approved by a recognized ethics review board at each study facility. The study was performed in accordance with the Declaration of Helsinki. Table 1. 2x2 Factorial Design Randomization mg 80 mg Total Supplement 100 participants a 100 participants b participants c 100 participants d 200 Total a Reasons for drop out: 2 Lack of Efficacy; 1 Physician Decision; 1 Pregnancy; 2 Did Not Follow Protocol; 10 Died; 17 Adverse Event b Reasons for drop out: 1 Lack of Efficacy; 9 Died; 16 Adverse Event c Reasons for drop out: 3 Lack of Efficacy; 1 Physician Decision; 1 Moved Out of Country; 10 Died; 16 Adverse Event d Reasons for drop out: 1 Lack of Efficacy; 1 Did Not Follow Protocol; 8 Died; 16 Adverse Event 1
2 Patients Inclusion Criteria Patients, regardless of gender, at least 18 years of age and hospitalized for the management of Class III or IV Heart Failure (HF) using the New York Heart Association (NYHA) classification 1 or diagnosed with Class III or IV Heart Failure within 72 hours of hospitalization for another reason were eligible for the trial. Patients were also required to have a sufficient level of education to understand study procedures and be able to communicate with site personnel. Exclusion Criteria Patients having received an antihistamine for more than 2 days prior to randomization or those unable to be treated by were excluded. Additional exclusion criteria included history of acute liver injury (e.g., hepatitis) or severe cirrhosis; pregnancy or breast-feeding; allergy to or Supplement; and participation in a study of an investigational medication within the past 30 days. Primary Endpoint The motivation for this study came from indications in the literature that supplements may have a protective and/or ameliorative clinical effect on heart failure. Since statins are frequently prescribed for certain patients with heart failure, it was a primary goal to see if had any short-term protective clinical effect for patients receiving statins and secondarily whether the effect, if any, had an interaction with the statin dose. The primary composite endpoint was rehospitalization for heart failure or death from any cause during the period from randomization to day 30 by intervention, summing all participants who received each intervention regardless of the paired combination (i.e., mg; 80 mg; Supplement; and ). Rehospitalization and fatal events within 30 days after randomization were reviewed and categorized by an independent, blinded clinical-events committee. The following criteria were required for rehospitalization events to be classified as due to heart failure: typical clinical manifestations of worsening heart failure and the addition of (or increase in) interventions specifically for worsening heart failure with an intravenous pharmacologic agent, or mechanical or surgical intervention or ultrafiltration, hemofiltration, or dialysis specifically for management of persistent or worsening heart failure. Hospitalized patients who remained in the hospital at 30 days because of heart failure were counted as being rehospitalized for heart failure in the analysis of the primary composite end point. Secondary Endpoints Secondary endpoints included the composite endpoint of rehospitalization for heart failure and death from any cause during the period from randomization to day 30 by randomization group; and safety. Safety Safety was assessed by the number of adverse events (AEs). AEs were collected by systematic assessment using terms from the Medical Dictionary for Regulatory Activities (MedDRA), version.1. Results Of the 600 patients screened during the run-in period between July 1998 and September 2007, 67% (N = 400) were randomized to the four intervention groups (Table 1). The last patient completed in May Participant characteristics by randomization and by intervention are shown in Tables 2 and 3, respectively. 2
3 Table 2. Demographic Characteristics of Participants by Randomization Group Age in Years Mean (SD) Gender Male Female NYHA HF Class Class III Class IV Heart Failure Diagnosis Pre-hospitalization During hospitalization SD = Standard Deviation mg + 80 mg + mg + 80 mg (4.7) 64. (.0) 64.0 (4.8) 64.6 (.1) Total n = (4.9) Table 3. Demographic Characteristics of Participants by Intervention Age in Years Mean (SD) Gender Male Female NYHA HF Class Class III Class IV Heart Failure Diagnosis Pre-hospitalization During hospitalization mg 80 mg Total n = (4.7) 64.6 (.2) 64.2 (4.9) 64.3 (.0) 64.2 (4.9) SD = Standard Deviation; NYHA HF = New York Heart Association Heart Failure
4 The primary and secondary clinical endpoints are reported in Table 4. Statistical analysis was performed with chi square, and a p-value<0.0 was considered statistically significant. There was no significant improvement for rehospitalization or death when analyzed by intervention (p = 0.96) or by randomization group (p = 0.97). Cumulative probabilities for the primary clinical endpoint for the and analysis populations were estimated using Kaplan-Meier product-limit method and Greenwood s formula for 9% confidence intervals. For, the estimated cumulative probability of rehospitalization or death at 30 days was 0.28 (9% CI: 0.17 to 0.39). For, the cumulative probability was 0.26 (9% CI: 0.1 to 0.37) Adverse events are shown in Table. If a participant experienced the same serious adverse event more than once, then each event would have been recorded as a distinct event. However, no participant experienced the same serious adverse event more than once. If a participant experienced the same non-serious adverse event more than once, then it was only recorded as one adverse event. References 1 eartfailure/aboutheartfailure/classes-of-heart- Failure_UCM_306328_Article.jsp#.T1eG2vW- 32k Table 4. Primary and Secondary Clinical Endpoints from Randomization through Day 30: Rehospitalization for Heart Failure and Death from Any Cause Primary mg: no./total no. (%) 3/200 (26.) 80 mg: no./total no. (%) 49/200 (24.) : no./total no. (%) 2/200 (26.0) : no./total no. (%) 0/200 (2.0) Secondary mg + : no./total no. (%) 27/100 (27.0) 80 mg + : no./total no. (%) 2/100 (2.0) mg + : no./total no. (%) 26/100 (26) 80 mg + : no./total no. (%) 24/100 (24) 4
5 Table. Adverse Events through Day 30* mg + 80 mg + mg + 80 mg + Total**/Total Other 30/20 26/22 27/27 27/28 Myocardial Infarction** Death** Palpitations 8 1 Ventricular tachycardia Chest pain Hyperglycemia Hyperlipidemia Hemorrhagic stroke** Hemorrhagic transformation stroke** Dizziness Headache Dyspnea Hypertension Ischemia *If a participant experienced the same serious adverse event more than once, then each event was recorded as a distinct event. If a participant experienced the same non-serious adverse event more than once, then it was only recorded as one adverse event. **Event classified as Serious (i.e., Death or Resulting in Death, requiring either inpatient hospitalization or the prolongation of hospitalization, are life-threatening, result in a persistent or significant disability/incapacity or result in a congenital anomaly/birth defect).
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
Hydrocodone/Acetaminophen Extended-Release Tablets M Clinical Study Report R&D/09/1109
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-712 Volume: Hydrocodone/Acetaminophen Extended-Release Name
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-712 Volume: Hydrocodone/Acetaminophen Extended-Release Name
2.0 Synopsis. Adalimumab M Clinical Study Report R&D/04/900. (For National Authority Use Only) Referring to Part of Dossier: Volume:
 2. Synopsis Abbott Laboratories Name of Study Drug: Name of Active Ingredient: Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Phase
2. Synopsis Abbott Laboratories Name of Study Drug: Name of Active Ingredient: Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Phase
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
2.0 Synopsis. Adalimumab R&D/04/118. (For National Authority Use Only) Referring to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
2.0 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
egfr > 50 (n = 13,916)
 Saxagliptin and Cardiovascular Risk in Patients with Type 2 Diabetes Mellitus and Moderate or Severe Renal Impairment: Observations from the SAVOR-TIMI 53 Trial Supplementary Table 1. Characteristics according
Saxagliptin and Cardiovascular Risk in Patients with Type 2 Diabetes Mellitus and Moderate or Severe Renal Impairment: Observations from the SAVOR-TIMI 53 Trial Supplementary Table 1. Characteristics according
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Allergan Not Applicable AGN A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Multiple Dose, Parallel
 Peripheral Neuropathy Design, Dose Ranging Study of the Safety and Efficacy of AGN 203818 in Patients with Painful Diabetic 203818-004. A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Multiple
Peripheral Neuropathy Design, Dose Ranging Study of the Safety and Efficacy of AGN 203818 in Patients with Painful Diabetic 203818-004. A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Multiple
ClinicalTrials.gov Identifier: NCT Sponsor/company: Sanofi-Aventis. Date: 08/02/ 2008
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Sanofi-Aventis ClinicalTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Sanofi-Aventis ClinicalTrials.gov
ASCEND Randomized placebo-controlled trial of aspirin 100 mg daily in 15,480 patients with diabetes and no baseline cardiovascular disease
 ASCEND Randomized placebo-controlled trial of aspirin 100 mg daily in 15,480 patients with diabetes and no baseline cardiovascular disease Jane Armitage and Louise Bowman on behalf of the ASCEND Study
ASCEND Randomized placebo-controlled trial of aspirin 100 mg daily in 15,480 patients with diabetes and no baseline cardiovascular disease Jane Armitage and Louise Bowman on behalf of the ASCEND Study
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
SYNOPSIS 2/198 CSR_BDY-EFC5825-EN-E02. Name of company: TABULAR FORMAT (For National Authority Use only)
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
SYNOPSIS. Publications No publications at the time of writing this report.
 Drug product: TOPROL-XL Drug substance(s): Metoprolol succinate Study code: D4020C00033 (307A) Date: 8 February 2006 SYNOPSIS Dose Ranging, Safety and Tolerability of TOPROL-XL (metoprolol succinate) Extended-release
Drug product: TOPROL-XL Drug substance(s): Metoprolol succinate Study code: D4020C00033 (307A) Date: 8 February 2006 SYNOPSIS Dose Ranging, Safety and Tolerability of TOPROL-XL (metoprolol succinate) Extended-release
DEMOGRAPHICS PHYSICAL ATTRIBUTES VITAL SIGNS. Protocol: ABC-123 SCREENING. Subject ID. Subject Initials. Visit Date: / / [ YYYY/MM/DD]
![DEMOGRAPHICS PHYSICAL ATTRIBUTES VITAL SIGNS. Protocol: ABC-123 SCREENING. Subject ID. Subject Initials. Visit Date: / / [ YYYY/MM/DD] DEMOGRAPHICS PHYSICAL ATTRIBUTES VITAL SIGNS. Protocol: ABC-123 SCREENING. Subject ID. Subject Initials. Visit Date: / / [ YYYY/MM/DD]](/thumbs/78/78699510.jpg) SCREENING Visit Date: DEMOGRAPHICS / / [YYYY/MM/DD] Consent Signed Date and time / / [YYYY/MM/DD] : Has written assent been obtained? If no, why not? YES NO Gender: Male Female Birthdate: Permission given
SCREENING Visit Date: DEMOGRAPHICS / / [YYYY/MM/DD] Consent Signed Date and time / / [YYYY/MM/DD] : Has written assent been obtained? If no, why not? YES NO Gender: Male Female Birthdate: Permission given
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
HANDOUT #1 CONCEPT INTRODUCTION PRESENTATION: PERFUSION
 HANDOUT #1 CONCEPT INTRODUCTION PRESENTATION: PERFUSION Topic Definition of Perfusion Description The passage of oxygenated capillary blood through body tissues. Peripheral perfusion is passage (flow)
HANDOUT #1 CONCEPT INTRODUCTION PRESENTATION: PERFUSION Topic Definition of Perfusion Description The passage of oxygenated capillary blood through body tissues. Peripheral perfusion is passage (flow)
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Vicodin CR Name of Active Ingredient: Page: Hydrocodone/Acetaminophen
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Vicodin CR Name of Active Ingredient: Page: Hydrocodone/Acetaminophen
SYNOPSIS. Risperidone-R064766: Clinical Study Report RIS-USA-232 (FOR NATIONAL AUTHORITY USE ONLY)
 SYNOPSIS Protocol No.: RIS-USA-232 Title of Study: Efficacy and Safety of a Flexible Dose of Risperidone Versus Placebo in the Treatment of Psychosis of Alzheimer's Disease Principal Investigator: M.D.
SYNOPSIS Protocol No.: RIS-USA-232 Title of Study: Efficacy and Safety of a Flexible Dose of Risperidone Versus Placebo in the Treatment of Psychosis of Alzheimer's Disease Principal Investigator: M.D.
Clinical Trial Synopsis TL-OPI-516, NCT#
 Clinical Trial Synopsis, NCT#00225277 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl Versus
Clinical Trial Synopsis, NCT#00225277 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl Versus
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Study No: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
ASCEND A randomized trial of omega-3 fatty acids (fish oil) versus placebo for primary cardiovascular prevention in 15,480 patients with diabetes
 ASCEND A randomized trial of omega-3 fatty acids (fish oil) versus placebo for primary cardiovascular prevention in 15,480 patients with diabetes Jane Armitage and Louise Bowman on behalf of the ASCEND
ASCEND A randomized trial of omega-3 fatty acids (fish oil) versus placebo for primary cardiovascular prevention in 15,480 patients with diabetes Jane Armitage and Louise Bowman on behalf of the ASCEND
Vest Prevention of Early Sudden Death Trial (VEST)
 ACC Late Breaking Clinical Trials 2018 Vest Prevention of Early Sudden Death Trial (VEST) Jeffrey Olgin, MD, FACC Division of Cardiology, UCSF On behalf of the VEST Investigators Disclosures ClinicalTrials.gov
ACC Late Breaking Clinical Trials 2018 Vest Prevention of Early Sudden Death Trial (VEST) Jeffrey Olgin, MD, FACC Division of Cardiology, UCSF On behalf of the VEST Investigators Disclosures ClinicalTrials.gov
PFIZER INC. Study Initiation Date and Completion Dates: 09 March 2000 to 09 August 2001.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Study No.:MPX Title: Rationale: Phase: IIB Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Safety Assessment in Clinical Trials and Beyond
 Safety Assessment in Clinical Trials and Beyond Yuliya Yasinskaya, MD Medical Team Leader Division of Anti-Infective Products Center for Drug Evaluation and Research FDA Clinical Investigator Training
Safety Assessment in Clinical Trials and Beyond Yuliya Yasinskaya, MD Medical Team Leader Division of Anti-Infective Products Center for Drug Evaluation and Research FDA Clinical Investigator Training
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
E2804 The BeST Trial
 E2804 The BeST Trial A randomized Phase II Study of VEGF, RAF Kinase and MTOR Combination Targeted Therapy with Bevacizumab, Sorafenib and Temsirolimus in Advanced Renal Cell Carcinoma Investigators Keith
E2804 The BeST Trial A randomized Phase II Study of VEGF, RAF Kinase and MTOR Combination Targeted Therapy with Bevacizumab, Sorafenib and Temsirolimus in Advanced Renal Cell Carcinoma Investigators Keith
Study No.: LOV Title: Rationale: Phase: IIB Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary-
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Individual Study Table Referring to Part of Dossier: Volume: Page:
 Synopsis Abbott Laboratories Name of Study Drug: Paricalcitol Capsules (ABT-358) (Zemplar ) Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For
Synopsis Abbott Laboratories Name of Study Drug: Paricalcitol Capsules (ABT-358) (Zemplar ) Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For
Synopsis (C1034T02) CNTO 95 Module 5.3 Clinical Study Report C1034T02
 Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
Product: Omecamtiv Mecarbil Clinical Study Report: Date: 02 April 2014 Page 1
 Date: 02 April 2014 Page 1. 2. SYNOPSIS Name of Sponsor: Amgen Inc. Name of Finished Product: Omecamtiv mecarbil injection Name of Active Ingredient: Omecamtiv mecarbil (AMG 423) Title of Study: A double-blind,
Date: 02 April 2014 Page 1. 2. SYNOPSIS Name of Sponsor: Amgen Inc. Name of Finished Product: Omecamtiv mecarbil injection Name of Active Ingredient: Omecamtiv mecarbil (AMG 423) Title of Study: A double-blind,
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Niaspan Name of Active Ingredient: Page: Niacin extended-release
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Niaspan Name of Active Ingredient: Page: Niacin extended-release
Learning Objectives 9/9/2013. Hypothesis Testing. Conflicts of Interest. Descriptive statistics: Numerical methods Measures of Central Tendency
 Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Last-Chance Ambulatory Care Webinar Thursday, September 5, 2013 Learning Objectives For
Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Last-Chance Ambulatory Care Webinar Thursday, September 5, 2013 Learning Objectives For
9/4/2013. Decision Errors. Hypothesis Testing. Conflicts of Interest. Descriptive statistics: Numerical methods Measures of Central Tendency
 Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Pharmacotherapy Webinar Review Course Tuesday, September 3, 2013 Descriptive statistics:
Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Pharmacotherapy Webinar Review Course Tuesday, September 3, 2013 Descriptive statistics:
mg 25 mg mg 25 mg mg 100 mg 1
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
ABC/3TC/ZDV ABC PBO/3TC/ZDV
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Sponsor/Company: sanofi-aventis Drug substance: Elitek/Fasturtec (rasburicase, SR29142)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: sanofi-aventis Drug
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: sanofi-aventis Drug
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: ABT-335 Name of Active Ingredient: Page: ABT-335, A-7770335.115
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: ABT-335 Name of Active Ingredient: Page: ABT-335, A-7770335.115
Sponsor Novartis. Generic Drug Name Vildagliptin/Metformin. Therapeutic Area of Trial Type 2 diabetes. Approved Indication Type 2 diabetes
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: June 25, ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: June 25, 2014 ClinicalTrials.gov ID: NCT00372385 Study Identification Unique Protocol ID: VX05-950-104EU Brief Title:
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: June 25, 2014 ClinicalTrials.gov ID: NCT00372385 Study Identification Unique Protocol ID: VX05-950-104EU Brief Title:
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Quality ID #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care
 Quality ID #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Quality ID #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Full Novartis CTRD Results Template
 Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Final Clinical Study Report. to the Dossier SYNOPSIS. Final Clinical Study Report for Study AI463110
 BMS-475 AI463 Name of Sponsor/Company: Bristol-Myers Squibb Individual Study Table Referring to the Dossier For National Authority Use Only) Name of Finished Product: Baraclude Name of Active Ingredient:
BMS-475 AI463 Name of Sponsor/Company: Bristol-Myers Squibb Individual Study Table Referring to the Dossier For National Authority Use Only) Name of Finished Product: Baraclude Name of Active Ingredient:
Study No.: Title: Rationale: Phase: Study Period: Study Design: Center: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Piazza G, Creager M. Thromboangiitis Obliterans. Circulation Apr 27; 121(16):
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Thromboangiitis Obliterans (TAO) 12 TAO, also called Buerger disease, is an inflammatory disease that most commonly affects the
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Thromboangiitis Obliterans (TAO) 12 TAO, also called Buerger disease, is an inflammatory disease that most commonly affects the
Title of Study: Evaluation of Efficacy and Safety of Galantamine in Patients With Dementia of Alzheimer's Type Who Failed to Benefit From Donepezil
 SYNOPSIS Name of Sponsor/Company Name of Finished Product REMINYL Name of Active Ingredient(s) Galantamine hydrobromide Issue Date: 18 October 2013 Protocol No.: Title of Study: Evaluation of Efficacy
SYNOPSIS Name of Sponsor/Company Name of Finished Product REMINYL Name of Active Ingredient(s) Galantamine hydrobromide Issue Date: 18 October 2013 Protocol No.: Title of Study: Evaluation of Efficacy
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Adults With Diagnosed Diabetes
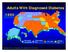 Adults With Diagnosed Diabetes 1990 No data available Less than 4% 4%-6% Above 6% Mokdad AH, et al. Diabetes Care. 2000;23(9):1278-1283. Adults With Diagnosed Diabetes 2000 4%-6% Above 6% Mokdad AH, et
Adults With Diagnosed Diabetes 1990 No data available Less than 4% 4%-6% Above 6% Mokdad AH, et al. Diabetes Care. 2000;23(9):1278-1283. Adults With Diagnosed Diabetes 2000 4%-6% Above 6% Mokdad AH, et
From PARADIGM-HF to Clinical Practice. Waleed AlHabeeb, MD, MHA Associate Professor of Medicine President of the Saudi Heart Failure Group
 From PARADIGM-HF to Clinical Practice Waleed AlHabeeb, MD, MHA Associate Professor of Medicine President of the Saudi Heart Failure Group PARADIGM-HF: Inclusion Criteria Chronic HF NYHA FC II IV with LVEF
From PARADIGM-HF to Clinical Practice Waleed AlHabeeb, MD, MHA Associate Professor of Medicine President of the Saudi Heart Failure Group PARADIGM-HF: Inclusion Criteria Chronic HF NYHA FC II IV with LVEF
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Chantix Label Update 2018
 Chantix Label Update 2018 Chantix (varenicline) Prescribing Information Chantix Prescribing Info URL and Disclaimer Please refer to the full Prescribing Information on important treatment considerations
Chantix Label Update 2018 Chantix (varenicline) Prescribing Information Chantix Prescribing Info URL and Disclaimer Please refer to the full Prescribing Information on important treatment considerations
Study Day 1 Study Days 2 to 9 Sequence 1 Placebo for moxifloxacin Study Days 2 to 8: placebo for pazopanib (placebopaz) 800 mg;
 The study listed may include approved non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Mortality as an Efficacy or Safety Endpoint : Lessons Learned from the Heart Failure Trials
 Mortality as an Efficacy or Safety Endpoint : Lessons Learned from the Heart Failure Trials Christopher M. O Connor, MD Professor of Medicine Director, Duke Heart Center Acting Chief, Division of Cardiology
Mortality as an Efficacy or Safety Endpoint : Lessons Learned from the Heart Failure Trials Christopher M. O Connor, MD Professor of Medicine Director, Duke Heart Center Acting Chief, Division of Cardiology
Study No. 178-CL-008 Report Final Version, 14 Dec 2006 Reissued Version, 18 Jul 2011 Astellas Pharma Europe B.V. Page 13 of 122
 Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
Synopsis. Clinical Report Synopsis for Protocol Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc.
 Synopsis Clinical Report Synopsis for Protocol 197-02-220 Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc. Name of Product: Tetomilast (OPC-6535) Study Title: A Phase 3, Multicenter,
Synopsis Clinical Report Synopsis for Protocol 197-02-220 Name of Company: Otsuka Pharmaceutical Development & Commercialization, Inc. Name of Product: Tetomilast (OPC-6535) Study Title: A Phase 3, Multicenter,
Study No.: Title: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Adalimumab M Clinical Study Report Final R&D/14/1263. Page:
 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
Previous Study Return to List Next Study
 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: 42603ATT3013 Previous Study Return to List Next Study A Study to Evaluate Effectiveness and Safety of Prolonged Release OROS
A service of the U.S. National Institutes of Health Trial record 1 of 1 for: 42603ATT3013 Previous Study Return to List Next Study A Study to Evaluate Effectiveness and Safety of Prolonged Release OROS
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Lack of Effect of Beta-blocker Therapy in Patients with ST-elevation Acute Myocardial Infarction in PCI Era
 Lack of Effect of Beta-blocker Therapy in Patients with ST-elevation Acute Myocardial Infarction in PCI Era B. Bao 1, N. Ozasa 1, T. Morimoto 2, Y. Furukawa 3, M. Shirotani 4, H. Ogawa 5, C. Tei 6, H.
Lack of Effect of Beta-blocker Therapy in Patients with ST-elevation Acute Myocardial Infarction in PCI Era B. Bao 1, N. Ozasa 1, T. Morimoto 2, Y. Furukawa 3, M. Shirotani 4, H. Ogawa 5, C. Tei 6, H.
A factorial randomized trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes
 A factorial randomized trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Hypotheses: Among individuals with type 2 diabetes, the risks of major microvascular
A factorial randomized trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Hypotheses: Among individuals with type 2 diabetes, the risks of major microvascular
Nitrate s Effect on Activity Tolerance in Heart Failure with Preserved Ejection Fraction (NEAT) A Randomized Clinical Trial
 Nitrate s Effect on Activity Tolerance in Heart Failure with Preserved Ejection Fraction (NEAT) A Randomized Clinical Trial Margaret M Redfield On behalf of the NHLBI Heart Failure Clinical Research Network
Nitrate s Effect on Activity Tolerance in Heart Failure with Preserved Ejection Fraction (NEAT) A Randomized Clinical Trial Margaret M Redfield On behalf of the NHLBI Heart Failure Clinical Research Network
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
2.0 Synopsis. ABT-711 M Clinical Study Report R&D/06/573. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex sodium (ABT-711) Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex sodium (ABT-711) Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
Polypharmacy - arrhythmic risks in patients with heart failure
 Influencing sudden cardiac death by pharmacotherapy Polypharmacy - arrhythmic risks in patients with heart failure Professor Dan Atar Head, Dept. of Cardiology Oslo University Hospital Ullevål Norway 27.8.2012
Influencing sudden cardiac death by pharmacotherapy Polypharmacy - arrhythmic risks in patients with heart failure Professor Dan Atar Head, Dept. of Cardiology Oslo University Hospital Ullevål Norway 27.8.2012
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Clinical Trial Synopsis TL-OPI-525, NCT#
 Clinical Trial Synopsis, NCT#00762736 Title of Study: A Phase II, Double-Blind, Randomized, Placebo-Controlled, Proof-of-Concept Study of the Efficacy, Safety, and Tolerability of Pioglitazone HCl (ACTOS
Clinical Trial Synopsis, NCT#00762736 Title of Study: A Phase II, Double-Blind, Randomized, Placebo-Controlled, Proof-of-Concept Study of the Efficacy, Safety, and Tolerability of Pioglitazone HCl (ACTOS
SYNOPSIS THIS IS A PRINTED COPY OF AN ELECTRONIC DOCUMENT. PLEASE CHECK ITS VALIDITY BEFORE USE.
 Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Immediate-release Hydrocodone/Acetaminophen M Abbreviated Clinical Study Report R&D/08/1020
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Hydrocodone Bitartrate- Acetaminophen (NORCO ) Name of
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Hydrocodone Bitartrate- Acetaminophen (NORCO ) Name of
Search for studies: ClinicalTrials.gov Identifier: NCT
 ClinicalTrials.gov A service of the U.S. National Institutes of Health Search for studies: Example. "Heart attack" AND "Los Angeles" Advanced Search Help Studies by Topic Glossary Find Studies About Clinical
ClinicalTrials.gov A service of the U.S. National Institutes of Health Search for studies: Example. "Heart attack" AND "Los Angeles" Advanced Search Help Studies by Topic Glossary Find Studies About Clinical
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 Study No.: 29060/717 Title: A Double-Blind, Placebo-Controlled, 3-Arm, Fixed-Dose Study of CR Intermittent Dosing (12.5 mg and 25 mg) for Premenstrual Dysphoric Disorder Rationale: In most trials investigating
Study No.: 29060/717 Title: A Double-Blind, Placebo-Controlled, 3-Arm, Fixed-Dose Study of CR Intermittent Dosing (12.5 mg and 25 mg) for Premenstrual Dysphoric Disorder Rationale: In most trials investigating
a) Is it reasonable to compute the relative risk for endometrial cancer? Explain. b) Can we estimate relative risk for heart attacks? Explain.
 1. A 1980 study investigated the relationship between the use of oral contraceptives and the development of endometrial cancer. It was found that of 117 endometrial cancer patients, 6 had used oral contraceptives
1. A 1980 study investigated the relationship between the use of oral contraceptives and the development of endometrial cancer. It was found that of 117 endometrial cancer patients, 6 had used oral contraceptives
SYNOPSIS. Study center(s) This study was conducted in the United States (128 centers).
 Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Prasugrel vs. Ticagrelor in ACS/PCI Which one to choose? V. Voudris MD FESC FACC 2 nd Cardiology Division Onassis Cardiac Surgery Center
 Prasugrel vs. Ticagrelor in ACS/PCI Which one to choose? V. Voudris MD FESC FACC 2 nd Cardiology Division Onassis Cardiac Surgery Center Hospitalizations in the U.S. Due to ACS Acute Coronary Syndromes
Prasugrel vs. Ticagrelor in ACS/PCI Which one to choose? V. Voudris MD FESC FACC 2 nd Cardiology Division Onassis Cardiac Surgery Center Hospitalizations in the U.S. Due to ACS Acute Coronary Syndromes
Using DOACs in CAD Patients in Sinus Ryhthm Results of the ATLAS ACS 2, COMPASS and COMMANDER-HF Trials
 Using DOACs in CAD Patients in Sinus Ryhthm Results of the ATLAS ACS 2, COMPASS and COMMANDER-HF Trials 19 th Annual San Diego Heart Failure Symposium for Primary Care Physicians January 11-12, 2019 La
Using DOACs in CAD Patients in Sinus Ryhthm Results of the ATLAS ACS 2, COMPASS and COMMANDER-HF Trials 19 th Annual San Diego Heart Failure Symposium for Primary Care Physicians January 11-12, 2019 La
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Neprilysin Inhibitor (Entresto ) Prior Authorization and Quantity Limit Program Summary
 Neprilysin Inhibitor (Entresto ) Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Indication Entresto Reduce the risk of cardiovascular (sacubitril/valsartan) death
Neprilysin Inhibitor (Entresto ) Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Indication Entresto Reduce the risk of cardiovascular (sacubitril/valsartan) death
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
Study No.: ADF Title: Phase III study of adefovir dipivoxil (ADV) tablets in patients with compensated chronic hepatitis B -comparative study
 Study No.: ADF105220 Title: Phase III study of adefovir dipivoxil () tablets in patients with compensated chronic hepatitis B -comparative study against lamivudine ()- Rationale: This study wass a confirmatory
Study No.: ADF105220 Title: Phase III study of adefovir dipivoxil () tablets in patients with compensated chronic hepatitis B -comparative study against lamivudine ()- Rationale: This study wass a confirmatory
BRL /RSD-101RLL/1/CPMS-716. Report Synopsis
 Report Synopsis Study Title: A Multicenter, Open-label, Six-Month Extension Study to Assess the Long-term Safety of Paroxetine in Children and Adolescents with Major Depressive Disorder (MDD) or Obsessive-Compulsive
Report Synopsis Study Title: A Multicenter, Open-label, Six-Month Extension Study to Assess the Long-term Safety of Paroxetine in Children and Adolescents with Major Depressive Disorder (MDD) or Obsessive-Compulsive
Study Center(s): The study was conducted at 39 study sites in Japan.
 SYNOPSIS Issue Date: 20 NOVEMBER 2012 Name of Sponsor/Company Janssen Pharmaceutical K. K. Name of Finished Product CONCERTA Name of Active Ingredient(s) Methylphenidate HCl Protocol No.: JNS001-JPN-A01
SYNOPSIS Issue Date: 20 NOVEMBER 2012 Name of Sponsor/Company Janssen Pharmaceutical K. K. Name of Finished Product CONCERTA Name of Active Ingredient(s) Methylphenidate HCl Protocol No.: JNS001-JPN-A01
Blood Pressure Management in Acute Ischemic Stroke
 Blood Pressure Management in Acute Ischemic Stroke Kimberly Clark, PharmD, BCCCP Clinical Pharmacy Specialist Critical Care, Greenville Health System Adjunct Assistant Professor, South Carolina College
Blood Pressure Management in Acute Ischemic Stroke Kimberly Clark, PharmD, BCCCP Clinical Pharmacy Specialist Critical Care, Greenville Health System Adjunct Assistant Professor, South Carolina College
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
